User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Asthma: Easy strategy reduces exacerbations, improves control
PHOENIX – In a 15-month phase 4 trial, an inexpensive intervention that can be explained in a single office visit reduced severe exacerbations and improved asthma control in patient populations that suffer disproportionately from the disease. This easy-to-implement strategy achieved benefits similar to those from previous studies that prompted new treatment recommendations for moderate-to-severe asthma.
The findings were reported Feb. 26 in the Late-Breaking Oral Abstracts session at the American Academy of Allergy, Asthma, and Immunology (AAAAI) 2022 Meeting, coinciding with publication in the New England Journal of Medicine.
Black and Latino patients are under-represented in asthma research trials yet visit the emergency room and die from asthma-related complications at more than twice the rates of their White counterparts. Prior efforts to reduce this burden “have been expensive, difficult, and mostly unsuccessful,” Juan-Carlos Cardet, MD, MPH, assistant professor of internal medicine at the University of South Florida, Tampa, told attendees.
Dr. Cardet and his colleagues, led by principal investigator Elliot Israel, MD, of Brigham & Women’s Hospital, Boston, Mass., designed a study with input and financial support from the Patient-Centered Outcomes Research Institute (PCORI). The trial recruited 603 Black and 598 Latino adults with moderate-to-severe asthma. About a fifth were current or former smokers, and many lived in smoking environments. All had poorly controlled asthma or at least one severe asthma attack in the previous year. Each participant held prescriptions for daily inhaled corticosteroids (ICS) with or without long-acting beta-agonists.
Current guidelines recommend daily ICS in all but the mildest asthma cases, yet adherence is poor. Patients generally take medicine when they perceive a need, and since asthma is episodic, “most people don’t like to take controller therapy for asthma,” Dr. Cardet told this news organization in advance of his meeting presentation. Rather, many asthma patients use quick-relief therapies, such as albuterol or nebulizers, on an as-needed basis.
Prior research showed that clinical outcomes can improve with a strategy called Single Maintenance and Reliever Therapy (SMART). In this approach, an ICS (budesonide) is combined with a long-acting beta-agonist (formoterol) into a single inhaler so that patients automatically receive inhaled steroids whenever they treat their symptoms with quick-relief medication. The ICS-formoterol strategy looked promising in studies published more than a decade ago, and those results have prompted an update in national treatment guidelines, but “it’s been difficult to get [the strategy] into the clinic,” Dr. Cardet told this news organization. “FDA cautions against as-needed use of ICS-formoterol. That’s a big reason. Insurance companies won’t pay for it.”
Unlike the SMART studies, which asked participants to replace their usual controller and rescue therapies with the all-in-one inhaler, Black and Latino patients in the new trial were told to continue with their usual asthma care. On top of usual care, half of the participants were randomly assigned to receive one-time instruction around use of a controller medication (beclomethasone; Qvar) supplied by study investigators. “Essentially we told them to keep doing what your doctor tells you to do, but whenever you use a puff of rescue therapy, puff yourself with this Qvar, and if you use the nebulizer, puff yourself five times with the Qvar,” Dr. Cardet said.
This approach, called Patient Activated Reliever-Triggered Inhaled Corticosteroid (PARTICS), was explained to patients through a video in English and Spanish. “All of this we instructed in a single study visit,” Dr. Cardet said.
The PARTICS intervention reduced severe asthma exacerbations by 15% (0.13 exacerbations per patient per year) – on par with the estimated 0.12 exacerbations per patient annualized reduction with SMART. In addition, the PARTICS group had:
- better asthma control (3.4-point increase on the Asthma Control Test, vs. a 2.5-point increase in the usual-care group);
- improved quality of life (0.12-point increase on the Asthma Symptom Utility Index, vs. a 0.08-point increase in the usual-care group);
- fewer self-reported days lost from work, school, and usual activities (13.4 days, vs. 16.8 days in the usual-care group).
Addressing long-standing challenges with controller therapy compliance, this was a real-world strategy “to get more inhaled steroids in [asthma patients] on a regular basis,” Brian Vickery, MD, director of the Food Allergy Center at Emory University + Children’s Healthcare of Atlanta, said during the meeting session Q&A. Dr. Vickery was not involved in the study. “And you see an effect size that rivaled previous studies, which suggests to me that the improvement is in the inhaled steroid component and not necessarily the long-acting beta-agonist.”
The study team hopes these results can be implemented on a health care system level. “If it stays just in a journal, it’s not going to do anything. We want to help people. We want to bring it to clinic,” Dr. Cardet said in an interview.
The study was supported by a Patient-Centered Outcomes Research Institute (PCORI) award to Israel and by grants from the National Institute of Allergy and Infectious Diseases and the American Lung Association–American Academy of Allergy, Asthma, and Immunology to Dr. Cardet. QVAR and QVAR RediHaler inhalers were provided free of charge, and funding for the AssistRx pharmacy was provided by Teva Pharmaceuticals. NIOX VERO devices for measuring exhaled nitric oxide were provided free of charge by Circassia Pharmaceuticals. Dr. Cardet reported honoraria from AstraZeneca and Genentech for work in advisory boards and from GlaxoSmithKline for educational lectures on asthma, all unrelated to the AAAAI presentation. Dr. Vickery has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHOENIX – In a 15-month phase 4 trial, an inexpensive intervention that can be explained in a single office visit reduced severe exacerbations and improved asthma control in patient populations that suffer disproportionately from the disease. This easy-to-implement strategy achieved benefits similar to those from previous studies that prompted new treatment recommendations for moderate-to-severe asthma.
The findings were reported Feb. 26 in the Late-Breaking Oral Abstracts session at the American Academy of Allergy, Asthma, and Immunology (AAAAI) 2022 Meeting, coinciding with publication in the New England Journal of Medicine.
Black and Latino patients are under-represented in asthma research trials yet visit the emergency room and die from asthma-related complications at more than twice the rates of their White counterparts. Prior efforts to reduce this burden “have been expensive, difficult, and mostly unsuccessful,” Juan-Carlos Cardet, MD, MPH, assistant professor of internal medicine at the University of South Florida, Tampa, told attendees.
Dr. Cardet and his colleagues, led by principal investigator Elliot Israel, MD, of Brigham & Women’s Hospital, Boston, Mass., designed a study with input and financial support from the Patient-Centered Outcomes Research Institute (PCORI). The trial recruited 603 Black and 598 Latino adults with moderate-to-severe asthma. About a fifth were current or former smokers, and many lived in smoking environments. All had poorly controlled asthma or at least one severe asthma attack in the previous year. Each participant held prescriptions for daily inhaled corticosteroids (ICS) with or without long-acting beta-agonists.
Current guidelines recommend daily ICS in all but the mildest asthma cases, yet adherence is poor. Patients generally take medicine when they perceive a need, and since asthma is episodic, “most people don’t like to take controller therapy for asthma,” Dr. Cardet told this news organization in advance of his meeting presentation. Rather, many asthma patients use quick-relief therapies, such as albuterol or nebulizers, on an as-needed basis.
Prior research showed that clinical outcomes can improve with a strategy called Single Maintenance and Reliever Therapy (SMART). In this approach, an ICS (budesonide) is combined with a long-acting beta-agonist (formoterol) into a single inhaler so that patients automatically receive inhaled steroids whenever they treat their symptoms with quick-relief medication. The ICS-formoterol strategy looked promising in studies published more than a decade ago, and those results have prompted an update in national treatment guidelines, but “it’s been difficult to get [the strategy] into the clinic,” Dr. Cardet told this news organization. “FDA cautions against as-needed use of ICS-formoterol. That’s a big reason. Insurance companies won’t pay for it.”
Unlike the SMART studies, which asked participants to replace their usual controller and rescue therapies with the all-in-one inhaler, Black and Latino patients in the new trial were told to continue with their usual asthma care. On top of usual care, half of the participants were randomly assigned to receive one-time instruction around use of a controller medication (beclomethasone; Qvar) supplied by study investigators. “Essentially we told them to keep doing what your doctor tells you to do, but whenever you use a puff of rescue therapy, puff yourself with this Qvar, and if you use the nebulizer, puff yourself five times with the Qvar,” Dr. Cardet said.
This approach, called Patient Activated Reliever-Triggered Inhaled Corticosteroid (PARTICS), was explained to patients through a video in English and Spanish. “All of this we instructed in a single study visit,” Dr. Cardet said.
The PARTICS intervention reduced severe asthma exacerbations by 15% (0.13 exacerbations per patient per year) – on par with the estimated 0.12 exacerbations per patient annualized reduction with SMART. In addition, the PARTICS group had:
- better asthma control (3.4-point increase on the Asthma Control Test, vs. a 2.5-point increase in the usual-care group);
- improved quality of life (0.12-point increase on the Asthma Symptom Utility Index, vs. a 0.08-point increase in the usual-care group);
- fewer self-reported days lost from work, school, and usual activities (13.4 days, vs. 16.8 days in the usual-care group).
Addressing long-standing challenges with controller therapy compliance, this was a real-world strategy “to get more inhaled steroids in [asthma patients] on a regular basis,” Brian Vickery, MD, director of the Food Allergy Center at Emory University + Children’s Healthcare of Atlanta, said during the meeting session Q&A. Dr. Vickery was not involved in the study. “And you see an effect size that rivaled previous studies, which suggests to me that the improvement is in the inhaled steroid component and not necessarily the long-acting beta-agonist.”
The study team hopes these results can be implemented on a health care system level. “If it stays just in a journal, it’s not going to do anything. We want to help people. We want to bring it to clinic,” Dr. Cardet said in an interview.
The study was supported by a Patient-Centered Outcomes Research Institute (PCORI) award to Israel and by grants from the National Institute of Allergy and Infectious Diseases and the American Lung Association–American Academy of Allergy, Asthma, and Immunology to Dr. Cardet. QVAR and QVAR RediHaler inhalers were provided free of charge, and funding for the AssistRx pharmacy was provided by Teva Pharmaceuticals. NIOX VERO devices for measuring exhaled nitric oxide were provided free of charge by Circassia Pharmaceuticals. Dr. Cardet reported honoraria from AstraZeneca and Genentech for work in advisory boards and from GlaxoSmithKline for educational lectures on asthma, all unrelated to the AAAAI presentation. Dr. Vickery has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHOENIX – In a 15-month phase 4 trial, an inexpensive intervention that can be explained in a single office visit reduced severe exacerbations and improved asthma control in patient populations that suffer disproportionately from the disease. This easy-to-implement strategy achieved benefits similar to those from previous studies that prompted new treatment recommendations for moderate-to-severe asthma.
The findings were reported Feb. 26 in the Late-Breaking Oral Abstracts session at the American Academy of Allergy, Asthma, and Immunology (AAAAI) 2022 Meeting, coinciding with publication in the New England Journal of Medicine.
Black and Latino patients are under-represented in asthma research trials yet visit the emergency room and die from asthma-related complications at more than twice the rates of their White counterparts. Prior efforts to reduce this burden “have been expensive, difficult, and mostly unsuccessful,” Juan-Carlos Cardet, MD, MPH, assistant professor of internal medicine at the University of South Florida, Tampa, told attendees.
Dr. Cardet and his colleagues, led by principal investigator Elliot Israel, MD, of Brigham & Women’s Hospital, Boston, Mass., designed a study with input and financial support from the Patient-Centered Outcomes Research Institute (PCORI). The trial recruited 603 Black and 598 Latino adults with moderate-to-severe asthma. About a fifth were current or former smokers, and many lived in smoking environments. All had poorly controlled asthma or at least one severe asthma attack in the previous year. Each participant held prescriptions for daily inhaled corticosteroids (ICS) with or without long-acting beta-agonists.
Current guidelines recommend daily ICS in all but the mildest asthma cases, yet adherence is poor. Patients generally take medicine when they perceive a need, and since asthma is episodic, “most people don’t like to take controller therapy for asthma,” Dr. Cardet told this news organization in advance of his meeting presentation. Rather, many asthma patients use quick-relief therapies, such as albuterol or nebulizers, on an as-needed basis.
Prior research showed that clinical outcomes can improve with a strategy called Single Maintenance and Reliever Therapy (SMART). In this approach, an ICS (budesonide) is combined with a long-acting beta-agonist (formoterol) into a single inhaler so that patients automatically receive inhaled steroids whenever they treat their symptoms with quick-relief medication. The ICS-formoterol strategy looked promising in studies published more than a decade ago, and those results have prompted an update in national treatment guidelines, but “it’s been difficult to get [the strategy] into the clinic,” Dr. Cardet told this news organization. “FDA cautions against as-needed use of ICS-formoterol. That’s a big reason. Insurance companies won’t pay for it.”
Unlike the SMART studies, which asked participants to replace their usual controller and rescue therapies with the all-in-one inhaler, Black and Latino patients in the new trial were told to continue with their usual asthma care. On top of usual care, half of the participants were randomly assigned to receive one-time instruction around use of a controller medication (beclomethasone; Qvar) supplied by study investigators. “Essentially we told them to keep doing what your doctor tells you to do, but whenever you use a puff of rescue therapy, puff yourself with this Qvar, and if you use the nebulizer, puff yourself five times with the Qvar,” Dr. Cardet said.
This approach, called Patient Activated Reliever-Triggered Inhaled Corticosteroid (PARTICS), was explained to patients through a video in English and Spanish. “All of this we instructed in a single study visit,” Dr. Cardet said.
The PARTICS intervention reduced severe asthma exacerbations by 15% (0.13 exacerbations per patient per year) – on par with the estimated 0.12 exacerbations per patient annualized reduction with SMART. In addition, the PARTICS group had:
- better asthma control (3.4-point increase on the Asthma Control Test, vs. a 2.5-point increase in the usual-care group);
- improved quality of life (0.12-point increase on the Asthma Symptom Utility Index, vs. a 0.08-point increase in the usual-care group);
- fewer self-reported days lost from work, school, and usual activities (13.4 days, vs. 16.8 days in the usual-care group).
Addressing long-standing challenges with controller therapy compliance, this was a real-world strategy “to get more inhaled steroids in [asthma patients] on a regular basis,” Brian Vickery, MD, director of the Food Allergy Center at Emory University + Children’s Healthcare of Atlanta, said during the meeting session Q&A. Dr. Vickery was not involved in the study. “And you see an effect size that rivaled previous studies, which suggests to me that the improvement is in the inhaled steroid component and not necessarily the long-acting beta-agonist.”
The study team hopes these results can be implemented on a health care system level. “If it stays just in a journal, it’s not going to do anything. We want to help people. We want to bring it to clinic,” Dr. Cardet said in an interview.
The study was supported by a Patient-Centered Outcomes Research Institute (PCORI) award to Israel and by grants from the National Institute of Allergy and Infectious Diseases and the American Lung Association–American Academy of Allergy, Asthma, and Immunology to Dr. Cardet. QVAR and QVAR RediHaler inhalers were provided free of charge, and funding for the AssistRx pharmacy was provided by Teva Pharmaceuticals. NIOX VERO devices for measuring exhaled nitric oxide were provided free of charge by Circassia Pharmaceuticals. Dr. Cardet reported honoraria from AstraZeneca and Genentech for work in advisory boards and from GlaxoSmithKline for educational lectures on asthma, all unrelated to the AAAAI presentation. Dr. Vickery has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiac arrest survival lower in COVID-19 inpatients
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
Survival after in-hospital cardiac arrest was roughly one-third lower in patients with COVID-19 infections compared to uninfected patients, based on data from nearly 25,000 individuals.
Survival rates of less than 3% were reported in the United States and China for patients who suffered in-hospital cardiac arrest (IHCA) while infected with COVID-19 early in the pandemic, but the data came from small, single-center studies in overwhelmed hospitals, wrote Saket Girotra, MD, of the University of Iowa, Iowa City, and fellow American Heart Association Get With the Guidelines–Resuscitation Investigators. Whether these early reports reflect the broader experience of patients with COVID-19 in hospitals in the United States remains unknown.
In a study published as a research letter in JAMA Network Open, the researchers reviewed data from the American Heart Association Get With the Guidelines–Resuscitation registry. The registry collects detailed information on patients aged 18 years and older who experience cardiac arrest at participating hospitals in the United States. The study population included 24,915 patients aged 18 years and older from 286 hospitals who experienced IHCA during March–December 2020. The mean age of the patients was 64.7 years; 61.1% were White, 24.8% were Black, 3.8% were of other race or ethnicity, and 10.3% were of unknown race or ethnicity.
The primary outcomes were survival to discharge and return of spontaneous circulation (ROSC) for at least 20 minutes.
A total of 5,916 patients (23.7%) had suspected or confirmed COVID-19 infections, and infected patients were more likely to be younger, male, and Black. Patients with COVID-19 infections also were significantly more likely than noninfected patients to have nonshockable rhythm, pneumonia, respiratory insufficiency, or sepsis, and to be on mechanical ventilation or vasopressors when the IHCA occurred, the researchers noted.
Survival rates to hospital discharge were 11.9% for COVID-19 patients, compared with 23.5% for noninfected patients (adjusted relative risk, 0.65; P < .001). ROSC was 53.7% and 63.6%, for infected and noninfected patients, respectively (aRR, 0.86; P < .001).
COVID-19 patients also were more likely than noninfected patients to receive delayed defibrillation, the researchers said. “Although delays in resuscitation, especially defibrillation, may have contributed to lower survival, the negative association of COVID-19 with survival in this study was consistent across subgroups, including patients who received timely treatment with defibrillation and epinephrine.”
The extremely low survival rate in early pandemic studies likely reflected the overwhelming burden on health systems at the time, the researchers said in their discussion.
The study findings were limited by several factors, including potential confounding from unmeasured variables, the use of a quality improvement registry that may not reflect nonparticipating hospitals, and potential false-positive COVID-19 cases. However, the result support findings from recent studies of multiple centers and extend clinical knowledge by comparing infected and noninfected patients from a larger group of hospitals than previously studied, the researchers said.
“We believe that these data will be relevant to health care providers and hospital administrators as the COVID-19 pandemic continues,” they concluded.
Think beyond COVID-19 for cardiac care
“Early during the pandemic, questions were raised whether COVID-19 patients should be treated with CPR,” Dr. Girotra said in an interview. “This was because initial studies had found a dismal survival of 0%-3% in COVID patients treated with CPR. The potential of transmitting the virus to health care professionals during CPR further heightened these concerns. We wanted to know whether the poor survival reported in these initial studies were broadly representative.”
Dr. Girotra said that some of the study findings were surprising. “We found that of all patients with IHCA in 2020 in our study, one in four were suspected or confirmed to have COVID-19 infection. We were surprised by the magnitude of COVID’s impact on the cardiac arrest incidence.”
The implications for clinical decision-making are to think outside of COVID-19 infection, said Dr. Girotra. In the current study, “Although overall survival of cardiac arrest in COVID-positive patients was 30% lower, compared to non-COVID patients, it was not as poor as previously reported. COVID-19 infection alone should not be considered the sole factor for making decisions regarding CPR.
“Over the past 2 decades, we have experienced large gains in survival for in-hospital cardiac arrest. However, the COVID-19 pandemic has eroded these gains,” said Dr. Girotra. “Future studies are needed to monitor the impact of any new variants on cardiac arrest care,” as well as studies “to see whether we return to the prepandemic levels of IHCA survival once the pandemic recedes.”
Dr. Girotra has no relevant financial disclosures.
FROM JAMA NETWORK OPEN
Analysis questions tocilizumab in ventilated COVID patients
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
A new statistical analysis of an existing meta-analysis reaffirms a finding that hospitalized patients with COVID-19 who are on simple oxygen or noninvasive ventilation can benefit from treatment with the arthritis drug tocilizumab (Actemra) in conjunction with corticosteroids. But the report also casts doubt on the effectiveness of tocilizumab in patients who are on ventilators.
“Clinicians should prescribe steroids and tocilizumab for hospitalized patients needing simple oxygen or noninvasive ventilation,” epidemiologist and study coauthor James (Jay) Brophy, MD, PhD, of McGill University, Montreal, said in an interview. “Further research is required to answer the question of whether tocilizumab is beneficial in patients requiring invasive ventilation, and consideration of participation in further tocilizumab studies seems reasonable.”
The new analysis was published Feb. 28, 2022, in JAMA Network Open.
The initial meta-analysis, published in 2021 in JAMA, was conducted by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group. It analyzed the results of 27 randomized trials that explored the use of interleukin-6 antagonists, including tocilizumab, and found that “28-day all-cause mortality was lower among patients who received IL-6 antagonists, compared with those who received usual care or placebo (summary odds ratio, 0.86). The summary ORs for the association of IL-6 antagonist treatment with 28-day all-cause mortality were 0.78 with concomitant administration of corticosteroids versus 1.09 without administration of corticosteroids.”
For the new report, researchers conducted a Bayesian statistical analysis of 15 studies within the meta-analysis that specifically examined the use of the rheumatoid arthritis drug tocilizumab. “Bayesian analysis allows one to make direct probability statements regarding the exact magnitude and the certainty of any benefit,” Dr. Brophy said. “This provides clinicians with the information they require to make well-informed decisions.”
The analysis estimated that the probability of a “clinically meaningful association” (absolute mortality risk difference, >1%) because of use of tocilizumab was higher than 95% in patients receiving simple oxygen and higher than 90% in those receiving noninvasive ventilation. But the probability was only about 67% higher in those receiving invasive mechanical ventilation.
Also, the researchers estimated that about 72% of future tocilizumab studies in patients on invasive mechanical ventilation would show a benefit.
The new analysis findings don’t add much to existing knowledge, said nephrologist David E. Leaf, MD, MMSc, of Harvard Medical School, Boston, who’s studied tocilizumab in COVID-19.
“The signal seems to be consistent that there is a greater benefit of tocilizumab in less ill patients than those who are more ill – e.g., those who are receiving invasive mechanical ventilation,” Dr. Leaf said in an interview. “This is interesting because in clinical practice the opposite approach is often undertaken, with tocilizumab use only being used in the sickest patients, even though the patients most likely to benefit seem to be those who are less ill.”
Clinically, he said, “hospitalized patients with COVID-19 should receive tocilizumab unless they have a clear contraindication and assuming it can be administered relatively early in their disease course. Earlier administration, before the onset of irreversible organ injury, is likely to have greater benefit.”
Dr. Leaf also noted it’s unknown whether the drug is helpful in several groups – patients presenting later in the course of COVID-19 illness, patients with additional infections, and immunocompromised patients.
It’s also not clear if tocilizumab benefits patients with lower levels of C-reactive protein, Shruti Gupta, MD, MPH, a nephrologist at Brigham and Women’s Hospital in Boston, said in an interview. The RECOVERY trial, for example, limited subjects to those with C-reactive protein of at least 75 mg/L.
Dr. Leaf and Dr. Gupta coauthored a 2021 cohort study analyzing mortality rates in patients with COVID-19 who were treated with tocilizumab versus those who were not.
No study funding was reported. Dr. Brophy, Dr. Leaf, and Dr. Gupta disclosed no relevant financial relationships. One study author reported participating in one of the randomized clinical trials included in the analysis.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Nasal microbiota show promise as polyp predictor
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
A study of the nasal microbiome helped researchers predict recurrent polyps in chronic rhinosinusitis patients with more than 90% accuracy, based on data from 85 individuals.
Chronic rhinosinusitis with nasal polyps (CRSwNP) has a significant impact on patient quality of life, but the underlying mechanism of the disease has not been well studied, and treatment options remain limited, wrote Yan Zhao, MD, of Capital Medical University, Beijing, and study coauthors.
Previous research has shown that nasal microbiome composition differs in patients with and without asthma, and some studies suggest that changes in microbiota could contribute to CRSwNP, the authors wrote. The researchers wondered if features of the nasal microbiome can predict the recurrence of nasal polyps after endoscopic sinus surgery and serve as a potential treatment target.
In a study in Allergy, the researchers examined nasal swab samples from 85 adults with CRSwNP who underwent endoscopic sinus surgery between August 2014 and March 2016 at a single center in China. The researchers performed bacterial analysis and gene sequencing on all samples.
The patients ranged in age from 18-73 years, with a mean age of 46 years, and included 64 men and 21 women. The primary outcome was recurrence of polyps. Of the total, 39 individuals had recurrence, and 46 did not.
When the researchers compared microbiota from swab samples of recurrent and nonrecurrent patients, they found differences in composition based on bacterial genus abundance. “Campylobacter, Bdellovibrio, and Aggregatibacter, among others, were more abundant in swabs from CRSwNP recurrence samples, whereas Actinobacillus, Gemella, and Moraxella were more abundant in non-recurrence samples,” they wrote.
The researchers then tested their theory that distinct nasal microbiota could be a predictive marker of risk for future nasal polyp recurrence. They used a training set of 48 samples and constructed models from nasal microbiota alone, clinical features alone, and both together.
The regression model identified Porphyromonas, Bacteroides, Moryella, Aggregatibacter, Butyrivibrio, Shewanella, Pseudoxanthomonas, Friedmanniella, Limnobacter, and Curvibacter as the most important taxa that distinguished recurrence from nonrecurrence in the specimens. When the model was validated, the area under the curve was 0.914, yielding a predictor of nasal polyp recurrence with 91.4% accuracy.
“It is highly likely that proteins, nucleic acids, and other small molecules produced by nasal microbiota are associated with the progression of CRSwNP,” the researchers noted in their discussion of the findings. “Further, the nasal microbiota could maintain a stable community environment through the secretion of various chemical compounds and/or inflammatory factors, thus playing a central role in the development of CRSwNP.”
The study findings were limited by several factors, including the analysis of nasal flora only at the genus level in the screening phase, the use only of bioinformatic analysis for recurrence prediction, and the inclusion only of subjects from a single center, the researchers noted. Future studies should combine predictors to increase accuracy and include deeper sequencing, they said. However, the results support data from previous studies and suggest a strategy to meet the need for predictors of recurrence in CRSwNP, they concluded.
“There is a critical need to understand the role of the upper airway microbiome in different phenotypes of CRS,” said Emily K. Cope, PhD, assistant director at the Pathogen and Microbiome Institute, Northern Arizona University, Flagstaff, in an interview. “This was one of the first studies to evaluate the predictive power of the microbiome in recurrence of a common CRS phenotype – CRS with nasal polyps,” she said. “Importantly, the researchers were able to predict recurrence of polyps prior to the disease manifestation,” she noted.
“Given the nascent state of current upper airway microbiome research, I was surprised that they were able to predict polyp recurrence prior to disease manifestation,” Dr. Cope said. “This is exciting, and I can imagine a future where we use microbiome data to understand risk for disease.”
What is the take-home message for clinicians? Although the immediate clinical implications are limited, Dr. Cope expressed enthusiasm for additional research. “At this point, there’s not a lot we can do without validation studies, but this study is promising. I hope we can understand the mechanism that an altered microbiome might drive (or be a result of) polyposis,” she said.
The study was supported by the National Natural Science Foundation of China, the program for the Changjiang scholars and innovative research team, the Beijing Bai-Qian-Wan talent project, the Public Welfare Development and Reform Pilot Project, the National Science and Technology Major Project, and the CAMS Innovation Fund for Medical Sciences. The researchers and Dr. Cope disclosed no financial conflicts.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease shows steady increase in U.S. over 15+ years
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Legionnaires’ disease (LD) in the United States appears to be on an upswing that started in 2003, according to a study from the Centers for Disease Control and Prevention.
The reasons for this increased incidence are unclear, the researchers write in Emerging Infectious Diseases.
“The findings revealed a rising national trend in cases, widening racial disparities between Black or African American persons and White persons, and an increasing geographic focus in the Middle Atlantic, the East North Central, and New England,” lead author Albert E. Barskey, MPH, an epidemiologist in CDC’s Division of Bacterial Diseases, Atlanta, said in an email.
“Legionnaires’ disease cannot be diagnosed based on clinical features alone, and studies estimate that it is underdiagnosed, perhaps by 50%,” he added. “Our findings may serve to heighten clinicians’ awareness of this severe pneumonia’s etiology, so with an earlier correct diagnosis, appropriate treatment can be rendered sooner.”
Mr. Barskey and his coauthors at CDC – mathematical statistician Gordana Derado, PhD, and epidemiologist Chris Edens, PhD – used surveillance data to investigate the incidence of LD in the U.S. over time. They compared LD incidence in 2018 with average incidence between 1992 and 2002. The incidence data, from over 80,000 LD cases, were age-standardized using the 2005 U.S. standard population as the reference.
The researchers analyzed LD data reported to CDC by the 50 states, New York City, and Washington, D.C., through the National Notifiable Diseases Surveillance System. They performed regression analysis to identify the optimal year when population parameters changed, and for most analyses, they compared 1992-2002 data with 2003-2018 data.
Legionnaires’ disease up in various groups
- The overall age-standardized average incidence grew from 0.48 per 100,000 people during 1992-2002 to 2.71 per 100,000 in 2018 (incidence risk ratio, 5.67; 95% confidence interval, 5.52-5.83).
- LD incidence more than quintupled for people over 34 years of age, with the largest relative increase in those over 85 (RR, 6.50; 95% CI, 5.82-7.27).
- Incidence in men increased slightly more (RR, 5.86; 95% CI, 5.67-6.05) than in women (RR, 5.29; 95% CI, 5.06-5.53).
- Over the years, the racial disparity in incidence grew markedly. Incidence in Black persons increased from 0.47 to 5.21 per 100,000 (RR, 11.04; 95% CI, 10.39-11.73), compared with an increase from 0.37 to 1.99 per 100,000 in White persons (RR, 5.30; 95% CI, 5.12-5.49).
- The relative increase in incidence was highest in the Northeast (RR, 7.04; 95% CI, 6.70-7.40), followed by the Midwest (RR, 6.13; 95% CI, 5.85-6.42), the South (RR, 5.97; 95% CI, 5.67-6.29), and the West (RR, 3.39; 95% CI, 3.11-3.68).
Most LD cases occurred in summer or fall, and the seasonal pattern became more pronounced over time. The average of 57.8% of cases between June and November during 1992-2002 grew to 68.9% in 2003-2018.
Although the study “was hindered by incomplete race and ethnicity data,” Mr. Barskey said, “its breadth was a strength.”
Consider legionella in your diagnosis
In an interview, Paul G. Auwaerter, MD, a professor of medicine and the clinical director of the Division of Infectious Diseases at Johns Hopkins University School of Medicine, Baltimore, said he was not surprised by the results. “CDC has been reporting increased incidence of Legionnaires’ disease from water source outbreaks over the years. As a clinician, I very much depend on epidemiologic trends to help me understand the patient in front of me.
“The key point is that there’s more of it around, so consider it in your diagnosis,” he advised.
“Physicians are increasingly beginning to consider Legionella. Because LD is difficult to diagnose by traditional methods such as culture, they may use a PCR test,” said Dr. Auwaerter, who was not involved in the study. “Legionella needs antibiotics that differ a bit from traditional antibiotics used to treat bacterial pneumonia, so a correct diagnosis can inform a more directed therapy.”
“Why the incidence is increasing is the big question, and the authors nicely outline a litany of things,” he said.
The authors and Dr. Auwaerter proposed a number of possible contributing factors to the increased incidence:
- an aging population
- aging municipal and residential water sources that may harbor more organisms
- racial disparities and poverty
- underlying conditions, including diabetes, end-stage renal disease, and some cancers
- occupations in transportation, repair, cleaning services, and construction
- weather patterns
- improved surveillance and reporting
“Why Legionella appears in some locations more than others has not been explained,” Dr. Auwaerter added. “For example, Pittsburgh always seemed to have much more Legionella than Baltimore.”
Mr. Barskey and his team are planning further research into racial disparities and links between weather and climate and Legionnaires’ disease.
The authors are employees of CDC. Dr. Auwaerter has disclosed no relevant financial realtionships.
A version of this article first appeared on Medscape.com.
Ivermectin does not stop progression to severe COVID: randomized trial
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
Ivermectin treatment given to high-risk patients with mild-to-moderate COVID-19 during the first week of illness did not prevent progression to severe disease, according to results from a randomized clinical trial.
“The study findings do not support the use of ivermectin for patients with COVID-19,” researchers conclude in the paper published online in JAMA Internal Medicine.
The open-label trial was conducted at 20 public hospitals and a COVID-19 quarantine center in Malaysia between May 31 and Oct. 25, 2021. It was led by Steven Chee Loon Lim, MRCP, department of medicine, Raja Permaisuri Bainun Hospital, Perak, Malaysia.
Among 490 patients in the primary analysis, 52 of 241 patients (21.6%) in the ivermectin group and 43 of 249 patients (17.3%) in the control group progressed to severe disease (relative risk, 1.25; 95% confidence interval, 0.87-1.80; P = .25). All major ethnic groups in Malaysia were well represented, the researchers write.
Participants (average age 62.5 and 54.5% women) were randomly assigned 1:1 to receive either a 5-day course of oral ivermectin (0.4 mg/kg body weight daily for 5 days) plus standard of care (n = 241) or standard of care alone (n = 249). Standard of care included symptomatic therapy and monitoring for early deterioration based on clinical findings, laboratory tests, and chest imaging.
Secondary outcomes
Secondary outcomes included rates of mechanical ventilation, intensive care unit (ICU) admission, 28-day in-hospital mortality, and side effects.
In all the secondary outcomes, there were no significant differences between groups.
Mechanical ventilation occurred in four patients on the ivermectin protocol (1.7%) versus 10 patients in the control group (4.0%) (RR, 0.41; 95% CI, 0.13-1.30; P = .17); ICU admission occurred in six (2.4%) versus eight (3.2%) (RR, 0.78; 95% CI, 0.27-2.20; P = .79); and 28-day in-hospital death occurred in three (1.2%) versus 10 (4.0%) (RR, 0.31; 95% CI, 0.09-1.11; P = .09).
The most common adverse event was diarrhea, reported by 5.8% in the ivermectin group and 1.6% in the control group.
No difference by vaccine status
The researchers conducted a subgroup analysis to evaluate any differences in whether participants were vaccinated. They said that analysis was “unremarkable.”
Just more than half of participants (51.8%) were fully vaccinated, with two doses of COVID-19 vaccines. Among the fully vaccinated patients, 17.7% in the ivermectin group and 9.2% in the control group developed severe disease (RR, 1.92; 95% CI, 0.99-3.71; P = .06).
Ivermectin, an inexpensive and widely available antiparasitic drug, is prescribed to treat COVID-19 but has not been approved by the U.S. Food and Drug Administration for that purpose. Evidence-based data for or against use has been sparse.
The authors write that “although some early clinical studies suggested the potential efficacy of ivermectin in the treatment and prevention of COVID-19, these studies had methodologic weaknesses.”
Dr. Lim and colleagues point out that their findings are consistent with those of the IVERCOR-COVID19 trial, which found ivermectin ineffective in reducing hospitalization risk.
Previous randomized trials of ivermectin for COVID-19 patients that have included at least 400 patients have focused on outpatients.
In the current study, the authors note, patients were hospitalized, which allowed investigators to observe administration of ivermectin with a high adherence rate. Additionally, the researchers used clearly defined criteria for determining progression to severe disease.
Limitations of the current study include that the open-label design might lead to under-reporting of adverse events in the control group while overestimating the drug effects of ivermectin. The study was also not designed to assess the effects of ivermectin on mortality from COVID-19.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICNE
New stroke risk score developed for COVID patients
Researchers have developed a quick and easy scoring system to predict which hospitalized COVID-19 patients are more at risk for stroke.
“The system is simple. You can calculate the points in 5 seconds and then predict the chances the patient will have a stroke,” Alexander E. Merkler, MD, assistant professor of neurology at Weill Cornell Medical College/NewYork-Presbyterian Hospital, and lead author of a study of the system, told this news organization.
The new system will allow clinicians to stratify patients and lead to closer monitoring of those at highest risk for stroke, said Dr. Merkler.
The study was presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Some, but not all, studies suggest COVID-19 increases the risk of stroke and worsens stroke outcomes, and the association isn’t clear, investigators note.
Researchers used the American Heart Association Get With the Guidelines COVID-19 cardiovascular disease registry for this analysis. They evaluated 21,420 adult patients (mean age 61 years, 54% men), who were hospitalized with COVID-19 at 122 centers from March 2020 to March 2021.
Investigators tapped into the vast amounts of data in this registry on different variables, including demographics, comorbidities, and lab values.
The outcome was a cerebrovascular event, defined as any ischemic or hemorrhagic stroke, transient ischemic attack (TIA), or cerebral vein thrombosis. Of the total hospitalized COVID-19 population, 312 (1.5%) had a cerebrovascular event.
Researchers first used standard statistical models to determine which risk factors are most associated with the development of stroke. They identified six such factors:
- history of stroke
- no fever at the time of hospital admission
- no history of pulmonary disease
- high white blood cell count
- history of hypertension
- high systolic blood pressure at the time of hospital admission
That the list of risk factors included absence of fever and no history of pulmonary disease was somewhat surprising, said Dr. Merkler, but there may be possible explanations, he added.
A high fever is an inflammatory response, and perhaps patients who aren’t responding appropriately “could be sicker in general and have a poor immune system, and thereby be at increased risk for stroke,” said Dr. Merkler.
In the case of pulmonary disease, patients without a history who are admitted for COVID “may have an extremely high burden of COVID, or are extremely sick, and that’s why they’re at higher risk for stroke.”
The scoring system assigns points for each variable, with more points conferring a higher risk of stroke. For example, someone who has 0-1 points has 0.2% risk of having a stroke, and someone with 4-6 points has 2% to 3% risk, said Dr. Merkler.
“So, we’re talking about a 10- to 15-fold increased risk of having a stroke with 4 to 6 versus 0 to 1 variables.”
The accuracy of the risk stratification score (C-statistic of 0.66; 95% confidence interval, 0.60-0.72) is “fairly good or modestly good,” said Dr. Merkler.
A patient with a score of 5 or 6 may need more vigilant monitoring to make sure symptoms are caught early and therapies such as thrombolytics and thrombectomy are readily available, he added.
Researchers also used a sophisticated machine-learning approach where a computer takes all the variables and identifies the best algorithm to predict stroke.
“The machine-learning algorithm was basically just as good as our standard model; it was almost identical,” said Dr. Merkler.
Outside of COVID, other scoring systems are used to predict stroke. For example, the ABCD2 score uses various factors to predict risk of recurrent stroke.
Philip B. Gorelick, MD, adjunct professor, Northwestern University Feinberg School of Medicine, Chicago, said the results are promising, as they may lead to identifying modifiable factors to prevent stroke.
Dr. Gorelick noted that the authors identified risk factors to predict risk of stroke “after an extensive analysis of baseline factors that included an internal validation process.”
The finding that no fever and no history of pulmonary disease were included in those risk factors was “unexpected,” said Dr. Gorelick, who is also medical director of the Hauenstein Neuroscience Center in Grand Rapids, Michigan. “This may reflect the baseline timing of data collection.”
He added further validation of the results in other data sets “will be useful to determine the consistency of the predictive model and its potential value in general practice.”
Louise D. McCullough, MD, PhD, professor and chair of neurology, McGovern Medical School, The University of Texas Health Science Center, Houston, said the association between stroke risk and COVID exposure “has been very unclear.”
“Some people find a very strong association between stroke and COVID, some do not,” said Dr. McCullough, who served as the chair of the ISC 2022 meeting.
This new study looking at a risk stratification model for COVID patients was “very nicely done,” she added.
“They used the American Heart Association Get With The Guidelines COVID registry, which was an amazing feat that was done very quickly by the AHA to establish COVID reporting in the Get With The Guidelines data, allowing us to really look at other factors related to stroke that are in this unique database.”
The study received funding support from the American Stroke Association. Dr. Merkler has received funding from the American Heart Association and the Leon Levy Foundation. Dr. Gorelick was not involved in the study and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have developed a quick and easy scoring system to predict which hospitalized COVID-19 patients are more at risk for stroke.
“The system is simple. You can calculate the points in 5 seconds and then predict the chances the patient will have a stroke,” Alexander E. Merkler, MD, assistant professor of neurology at Weill Cornell Medical College/NewYork-Presbyterian Hospital, and lead author of a study of the system, told this news organization.
The new system will allow clinicians to stratify patients and lead to closer monitoring of those at highest risk for stroke, said Dr. Merkler.
The study was presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Some, but not all, studies suggest COVID-19 increases the risk of stroke and worsens stroke outcomes, and the association isn’t clear, investigators note.
Researchers used the American Heart Association Get With the Guidelines COVID-19 cardiovascular disease registry for this analysis. They evaluated 21,420 adult patients (mean age 61 years, 54% men), who were hospitalized with COVID-19 at 122 centers from March 2020 to March 2021.
Investigators tapped into the vast amounts of data in this registry on different variables, including demographics, comorbidities, and lab values.
The outcome was a cerebrovascular event, defined as any ischemic or hemorrhagic stroke, transient ischemic attack (TIA), or cerebral vein thrombosis. Of the total hospitalized COVID-19 population, 312 (1.5%) had a cerebrovascular event.
Researchers first used standard statistical models to determine which risk factors are most associated with the development of stroke. They identified six such factors:
- history of stroke
- no fever at the time of hospital admission
- no history of pulmonary disease
- high white blood cell count
- history of hypertension
- high systolic blood pressure at the time of hospital admission
That the list of risk factors included absence of fever and no history of pulmonary disease was somewhat surprising, said Dr. Merkler, but there may be possible explanations, he added.
A high fever is an inflammatory response, and perhaps patients who aren’t responding appropriately “could be sicker in general and have a poor immune system, and thereby be at increased risk for stroke,” said Dr. Merkler.
In the case of pulmonary disease, patients without a history who are admitted for COVID “may have an extremely high burden of COVID, or are extremely sick, and that’s why they’re at higher risk for stroke.”
The scoring system assigns points for each variable, with more points conferring a higher risk of stroke. For example, someone who has 0-1 points has 0.2% risk of having a stroke, and someone with 4-6 points has 2% to 3% risk, said Dr. Merkler.
“So, we’re talking about a 10- to 15-fold increased risk of having a stroke with 4 to 6 versus 0 to 1 variables.”
The accuracy of the risk stratification score (C-statistic of 0.66; 95% confidence interval, 0.60-0.72) is “fairly good or modestly good,” said Dr. Merkler.
A patient with a score of 5 or 6 may need more vigilant monitoring to make sure symptoms are caught early and therapies such as thrombolytics and thrombectomy are readily available, he added.
Researchers also used a sophisticated machine-learning approach where a computer takes all the variables and identifies the best algorithm to predict stroke.
“The machine-learning algorithm was basically just as good as our standard model; it was almost identical,” said Dr. Merkler.
Outside of COVID, other scoring systems are used to predict stroke. For example, the ABCD2 score uses various factors to predict risk of recurrent stroke.
Philip B. Gorelick, MD, adjunct professor, Northwestern University Feinberg School of Medicine, Chicago, said the results are promising, as they may lead to identifying modifiable factors to prevent stroke.
Dr. Gorelick noted that the authors identified risk factors to predict risk of stroke “after an extensive analysis of baseline factors that included an internal validation process.”
The finding that no fever and no history of pulmonary disease were included in those risk factors was “unexpected,” said Dr. Gorelick, who is also medical director of the Hauenstein Neuroscience Center in Grand Rapids, Michigan. “This may reflect the baseline timing of data collection.”
He added further validation of the results in other data sets “will be useful to determine the consistency of the predictive model and its potential value in general practice.”
Louise D. McCullough, MD, PhD, professor and chair of neurology, McGovern Medical School, The University of Texas Health Science Center, Houston, said the association between stroke risk and COVID exposure “has been very unclear.”
“Some people find a very strong association between stroke and COVID, some do not,” said Dr. McCullough, who served as the chair of the ISC 2022 meeting.
This new study looking at a risk stratification model for COVID patients was “very nicely done,” she added.
“They used the American Heart Association Get With The Guidelines COVID registry, which was an amazing feat that was done very quickly by the AHA to establish COVID reporting in the Get With The Guidelines data, allowing us to really look at other factors related to stroke that are in this unique database.”
The study received funding support from the American Stroke Association. Dr. Merkler has received funding from the American Heart Association and the Leon Levy Foundation. Dr. Gorelick was not involved in the study and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers have developed a quick and easy scoring system to predict which hospitalized COVID-19 patients are more at risk for stroke.
“The system is simple. You can calculate the points in 5 seconds and then predict the chances the patient will have a stroke,” Alexander E. Merkler, MD, assistant professor of neurology at Weill Cornell Medical College/NewYork-Presbyterian Hospital, and lead author of a study of the system, told this news organization.
The new system will allow clinicians to stratify patients and lead to closer monitoring of those at highest risk for stroke, said Dr. Merkler.
The study was presented during the International Stroke Conference, presented by the American Stroke Association, a division of the American Heart Association.
Some, but not all, studies suggest COVID-19 increases the risk of stroke and worsens stroke outcomes, and the association isn’t clear, investigators note.
Researchers used the American Heart Association Get With the Guidelines COVID-19 cardiovascular disease registry for this analysis. They evaluated 21,420 adult patients (mean age 61 years, 54% men), who were hospitalized with COVID-19 at 122 centers from March 2020 to March 2021.
Investigators tapped into the vast amounts of data in this registry on different variables, including demographics, comorbidities, and lab values.
The outcome was a cerebrovascular event, defined as any ischemic or hemorrhagic stroke, transient ischemic attack (TIA), or cerebral vein thrombosis. Of the total hospitalized COVID-19 population, 312 (1.5%) had a cerebrovascular event.
Researchers first used standard statistical models to determine which risk factors are most associated with the development of stroke. They identified six such factors:
- history of stroke
- no fever at the time of hospital admission
- no history of pulmonary disease
- high white blood cell count
- history of hypertension
- high systolic blood pressure at the time of hospital admission
That the list of risk factors included absence of fever and no history of pulmonary disease was somewhat surprising, said Dr. Merkler, but there may be possible explanations, he added.
A high fever is an inflammatory response, and perhaps patients who aren’t responding appropriately “could be sicker in general and have a poor immune system, and thereby be at increased risk for stroke,” said Dr. Merkler.
In the case of pulmonary disease, patients without a history who are admitted for COVID “may have an extremely high burden of COVID, or are extremely sick, and that’s why they’re at higher risk for stroke.”
The scoring system assigns points for each variable, with more points conferring a higher risk of stroke. For example, someone who has 0-1 points has 0.2% risk of having a stroke, and someone with 4-6 points has 2% to 3% risk, said Dr. Merkler.
“So, we’re talking about a 10- to 15-fold increased risk of having a stroke with 4 to 6 versus 0 to 1 variables.”
The accuracy of the risk stratification score (C-statistic of 0.66; 95% confidence interval, 0.60-0.72) is “fairly good or modestly good,” said Dr. Merkler.
A patient with a score of 5 or 6 may need more vigilant monitoring to make sure symptoms are caught early and therapies such as thrombolytics and thrombectomy are readily available, he added.
Researchers also used a sophisticated machine-learning approach where a computer takes all the variables and identifies the best algorithm to predict stroke.
“The machine-learning algorithm was basically just as good as our standard model; it was almost identical,” said Dr. Merkler.
Outside of COVID, other scoring systems are used to predict stroke. For example, the ABCD2 score uses various factors to predict risk of recurrent stroke.
Philip B. Gorelick, MD, adjunct professor, Northwestern University Feinberg School of Medicine, Chicago, said the results are promising, as they may lead to identifying modifiable factors to prevent stroke.
Dr. Gorelick noted that the authors identified risk factors to predict risk of stroke “after an extensive analysis of baseline factors that included an internal validation process.”
The finding that no fever and no history of pulmonary disease were included in those risk factors was “unexpected,” said Dr. Gorelick, who is also medical director of the Hauenstein Neuroscience Center in Grand Rapids, Michigan. “This may reflect the baseline timing of data collection.”
He added further validation of the results in other data sets “will be useful to determine the consistency of the predictive model and its potential value in general practice.”
Louise D. McCullough, MD, PhD, professor and chair of neurology, McGovern Medical School, The University of Texas Health Science Center, Houston, said the association between stroke risk and COVID exposure “has been very unclear.”
“Some people find a very strong association between stroke and COVID, some do not,” said Dr. McCullough, who served as the chair of the ISC 2022 meeting.
This new study looking at a risk stratification model for COVID patients was “very nicely done,” she added.
“They used the American Heart Association Get With The Guidelines COVID registry, which was an amazing feat that was done very quickly by the AHA to establish COVID reporting in the Get With The Guidelines data, allowing us to really look at other factors related to stroke that are in this unique database.”
The study received funding support from the American Stroke Association. Dr. Merkler has received funding from the American Heart Association and the Leon Levy Foundation. Dr. Gorelick was not involved in the study and has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ISC 2022
‘Substantial’ CVD risks, burden up to a year after COVID-19
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who have had COVID-19 have an increased risk for, and 12-month burden of, cardiovascular disease (CVD) that is substantial and spans an array of cardiovascular disorders, a deep dive into federal data suggests.
“I went into this thinking that this is most likely happening in people to start with who have a higher risk of cardiovascular disorders, smokers, people with high BMI, diabetes, but what we found is something different,” Ziyad Al-Aly, MD, said in an interview. “It’s evident in people at high risk, but it was also as clear as the sun even in people who have no cardiovascular risk whatsoever.”
Rates were increased in younger adults, never smokers, White and Black people, and males and females, he said. “So the risk confirmed by the SARS-CoV-2 virus seems to spare almost no one.”
Although cardiovascular outcomes increased with the severity of the acute infection, the excess risks and burdens were also evident in those who never required hospitalization, a group that represents the majority of people with COVID-19, observed Dr. Al-Aly, who directs the Clinical Epidemiology Center at the Veterans Affairs St. Louis Health Care System.
“This study is very important because it underscores not just the acute cardiovascular risk associated with COVID but the increased risk of chronic cardiovascular outcomes as well,” cardiologist C. Michael Gibson, MD, professor of medicine, Harvard Medical School, Boston, said in an interview. “Given the number of patients in the U.S. who have been infected with COVID, this could represent a significant chronic burden on the health care system, particularly as health care professionals leave the profession.”
For the study, the investigators used national VA databases to build a cohort of 153,760 veterans who were alive 30 days after testing positive for COVID-19 between March 1, 2020, and January 2021. They were compared with a contemporary cohort of 5.6 million veterans with no evidence of SARS-CoV-2 infection and a historical cohort of 5.8 million veterans using the system in 2017 prior to the pandemic. Median follow-up was 347, 348, and 347 days, respectively.
As reported in Nature Medicine, the risk for a major adverse cardiovascular event, a composite of myocardial infarction, stroke, and all-cause mortality, was 4% higher in people who had been infected with COVID-19 than in those who had not.
“People say 4% is small, but actually it’s really, really big if you think about it in the context of the huge number of people who have had COVID-19 in the United States, and also globally,” Dr. Al-Aly said.
Compared with the contemporary control group, people who had COVID-19 had an increased risk (hazard ratio [HR]) and burden per 1,000 people at 1 year for the following cardiovascular outcomes:
- Stroke: HR, 1.52; burden, 4.03
- Transient ischemic attack: HR, 1.49; burden, 1.84
- Dysrhythmias: HR, 1.69; burden, 19.86
- Ischemic heart disease: HR, 1.66; burden, 7.28
- Heart failure: HR, 1.72; burden, 11.61
- Nonischemic cardiomyopathy: HR, 1.62; burden 3.56
- Pulmonary embolism: HR, 2.93; burden, 5.47
- Deep vein thrombosis: HR, 2.09; burden, 4.18
- Pericarditis: HR, 1.85, burden, 0.98
- Myocarditis: HR, 5.38; burden, 0.31
Recent reports have raised concerns about an association between COVID-19 vaccines and myocarditis and pericarditis, particularly in young males. Although very few of the participants were vaccinated prior to becoming infected, as vaccines were not yet widely available, the researchers performed two analyses censoring participants at the time of the first dose of any COVID-19 vaccine and adjusting for vaccination as a time-varying covariate.
The absolute numbers of myocarditis and pericarditis were still higher than the contemporary and historical cohorts. These numbers are much larger than those reported for myocarditis after vaccines, which are generally around 40 cases per 1 million people, observed Dr. Al-Aly.
The overall results were also consistent when compared with the historical control subjects.
“What we’re seeing in our report and others is that SARS-CoV-2 can leave a sort of scar or imprint on people, and some of these conditions are likely chronic conditions,” Dr. Al-Aly said. “So you’re going to have a generation of people who will bear the scar of COVID for their lifetime and I think that requires recognition and attention, so we’re aware of the magnitude of the problem and prepared to deal with it.”
With more than 76 million COVID-19 cases in the United States, that effort will likely have to be at the federal level, similar to President Joe Biden’s recent relaunch of the “Cancer Moonshot,” he added. “We need a greater and broader recognition at the federal level to try and recognize that when you have an earthquake, you don’t just deal with the earthquake when the earth is shaking, but you also need to deal with the aftermath.”
Dr. Gibson pointed out that this was a study of predominantly males and, thus, it’s unclear if the results can be extended to females. Nevertheless, he added, “long COVID may include outcomes beyond the central nervous system and we should educate patients about the risk of late cardiovascular outcomes.”
The authors noted the largely White, male cohort may limit generalizability of the findings. Other limitations include the possibility that some people may have had COVID-19 but were not tested, the datasets lacked information on cause of death, and possible residual confounding not accounted for in the adjusted analyses.
The research was funded by the U.S. Department of Veterans Affairs and two American Society of Nephrology and Kidney Cure fellowship awards. The authors declared no competing interests. Dr. Gibson reports having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Hong Kong, U.S., Israeli data illuminate COVID vaccine myocarditis
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.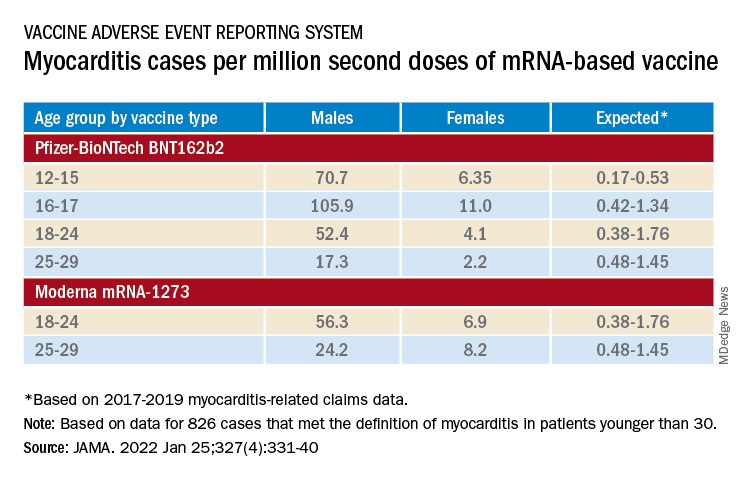
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Cardiac inflammation can be present after mild COVID infection
Myocardial inflammation is present in a small proportion of patients who have recovered from relatively mild cases of COVID-19 infection, a new study shows.
“Our findings suggest that even in patients who have had relatively mild cases of COVID-19, some will have inflammatory changes to the heart, and these changes can be present without any cardiac symptoms,” senior author, Paaladinesh Thavendiranathan, MD, University of Toronto, told this news organization.
“While our data suggest that this inflammation improves over time, and the outcomes seem positive, we don’t know if there will be any long-term consequences,” he added.
Noting that even a short period of inflammation in the heart may be associated with symptoms or arrhythmias in the longer term, Dr. Thavendiranathan said: “I would recommend that it is best to avoid getting the infection if there is any chance of heart inflammation.”
The study was published online in JAMA Cardiology on Jan. 12.
The authors explain that among patients hospitalized with COVID, early studies suggested that approximately one in four experience cardiovascular injury, defined as an elevation in troponin levels, which was associated with a 5- to 10-fold increase in the risk for death. But there is limited information on cardiac injury in patients who do not require hospitalization.
Although a broad range of abnormal myocardial tissue has been reported in several cardiac MRI studies of patients recovered from COVID infection, there is little understanding of persistent changes in myocardial metabolism in recovered patients, which is a potential concern, given that COVID-19 is associated with systemic inflammation during the acute illness, they say.
For the current study, the researchers examined myocardial inflammation measured using two different methods – cardiac MRI and fluorodeoxyglucose–positron emission tomography (FDG-PET) – in individuals who had recovered from COVID-19 infection and looked at how this related to changes in inflammatory blood markers.
Lead author Kate Hanneman, MD, also from the University of Toronto, explained that FDG-PET imaging is more sensitive than MRI in detecting active inflammation. “Inflammatory cells have a higher uptake of glucose, and FDG-PET imaging is used to look for metabolically active inflammatory tissue that takes up glucose. It gives complementary information to MRI. Cardiac MRI shows structural or functional changes, such as scarring or edema, whereas FDG-PET imaging directly measures metabolic activity related to inflammatory cells.”
The study involved 47 individuals, 51% female, with a mean age of 43 years, who had recently recovered from COVID-19 infection. Of these, the majority had had relatively mild COVID disease, with 85% not requiring hospitalization.
Cardiac imaging was performed a mean of 67 days after the diagnosis of COVID-19. At the time of imaging, 19 participants (40%) reported at least one cardiac symptom, including palpitations, chest pain, and shortness of breath.
Results showed that eight patients (17%) had focal FDG uptake on PET consistent with myocardial inflammation. Compared with those without FDG uptake, patients with focal FDG uptake had higher regional T2, T1, and extracellular volume (colocalizing with focal FDG uptake), higher prevalence of late gadolinium enhancement indicating fibrosis, lower left ventricular ejection fraction, worse global longitudinal and circumferential strain, and higher systemic inflammatory blood markers, including interleukin (IL)-6, IL- 8, an high-sensitivity C-reactive protein.
Of the 47 patients in the study, 13 had received at least one dose of a COVID-19 vaccine. There was no significant difference in the proportion of patients who were PET-positive among those who had received a COVID-19 vaccine and those who had not.
There was also no difference in inflammation in patients who had been hospitalized with COVID-19 and those who had managed their infection at home.
Among patients with focal FDG uptake, PET, MRI, and inflammatory blood markers improved at follow-up imaging performed a mean of 52 days after the first imaging. The authors say this suggests that these abnormalities were not related to pre-existing cardiovascular disease.
Of the eight patients with positive FDG-PET results, two did not show any MRI abnormalities. These two patients also had elevated inflammatory biomarkers. “PET is a more sensitive method of measuring cardiac inflammation, and our results show that these changes may not always translate into functional changes seen on MRI,” Dr. Thavendiranathan noted.
The only cardiac risk factor that was more common in participants with FDG uptake was hypertension. Although cardiac symptoms were nearly twice as common in participants with focal FDG uptake, this difference was not statistically significant.
“Given the growing number of survivors with similar symptoms, these interesting findings warrant further investigation,” the authors say.
Noting that FDG uptake correlated with elevations in systemic inflammatory biomarkers, the researchers suggest that “a more intense systemic inflammatory process may be contributing to cardiac inflammation and the consequential alteration to regional and global myocardial function in PET-positive participants.”
On repeat imaging 2 months later, all eight patients who showed FDG uptake showed improvement or resolution of inflammation without any treatment, although two patients still had some signs of inflammation. Blood biomarkers also improved on follow-up.
“This is encouraging information, but we need longer-term data to see if there are any long-term repercussions of this inflammation,” Dr. Hanneman said.
“Overall, the study findings suggest an imaging phenotype that is expected to have good prognosis. However, longer-term follow-up studies are required to understand the need for ongoing cardiac surveillance, relationship to cardiac symptoms, guidance for safe return to exercise and sports participation, and long-term cardiovascular disease risk,” the researchers state.
This study was funded by grants from the Joint Department of Medical Imaging Academic Incentive Fund, Peter Munk Cardiac Center Innovation Committee, and Ted Rogers Center for Heart Research. Dr. Hanneman reports personal fees from Sanofi Genzyme, Amicus, and Medscape outside the submitted work.
A version of this article first appeared on Medscape.com.
Myocardial inflammation is present in a small proportion of patients who have recovered from relatively mild cases of COVID-19 infection, a new study shows.
“Our findings suggest that even in patients who have had relatively mild cases of COVID-19, some will have inflammatory changes to the heart, and these changes can be present without any cardiac symptoms,” senior author, Paaladinesh Thavendiranathan, MD, University of Toronto, told this news organization.
“While our data suggest that this inflammation improves over time, and the outcomes seem positive, we don’t know if there will be any long-term consequences,” he added.
Noting that even a short period of inflammation in the heart may be associated with symptoms or arrhythmias in the longer term, Dr. Thavendiranathan said: “I would recommend that it is best to avoid getting the infection if there is any chance of heart inflammation.”
The study was published online in JAMA Cardiology on Jan. 12.
The authors explain that among patients hospitalized with COVID, early studies suggested that approximately one in four experience cardiovascular injury, defined as an elevation in troponin levels, which was associated with a 5- to 10-fold increase in the risk for death. But there is limited information on cardiac injury in patients who do not require hospitalization.
Although a broad range of abnormal myocardial tissue has been reported in several cardiac MRI studies of patients recovered from COVID infection, there is little understanding of persistent changes in myocardial metabolism in recovered patients, which is a potential concern, given that COVID-19 is associated with systemic inflammation during the acute illness, they say.
For the current study, the researchers examined myocardial inflammation measured using two different methods – cardiac MRI and fluorodeoxyglucose–positron emission tomography (FDG-PET) – in individuals who had recovered from COVID-19 infection and looked at how this related to changes in inflammatory blood markers.
Lead author Kate Hanneman, MD, also from the University of Toronto, explained that FDG-PET imaging is more sensitive than MRI in detecting active inflammation. “Inflammatory cells have a higher uptake of glucose, and FDG-PET imaging is used to look for metabolically active inflammatory tissue that takes up glucose. It gives complementary information to MRI. Cardiac MRI shows structural or functional changes, such as scarring or edema, whereas FDG-PET imaging directly measures metabolic activity related to inflammatory cells.”
The study involved 47 individuals, 51% female, with a mean age of 43 years, who had recently recovered from COVID-19 infection. Of these, the majority had had relatively mild COVID disease, with 85% not requiring hospitalization.
Cardiac imaging was performed a mean of 67 days after the diagnosis of COVID-19. At the time of imaging, 19 participants (40%) reported at least one cardiac symptom, including palpitations, chest pain, and shortness of breath.
Results showed that eight patients (17%) had focal FDG uptake on PET consistent with myocardial inflammation. Compared with those without FDG uptake, patients with focal FDG uptake had higher regional T2, T1, and extracellular volume (colocalizing with focal FDG uptake), higher prevalence of late gadolinium enhancement indicating fibrosis, lower left ventricular ejection fraction, worse global longitudinal and circumferential strain, and higher systemic inflammatory blood markers, including interleukin (IL)-6, IL- 8, an high-sensitivity C-reactive protein.
Of the 47 patients in the study, 13 had received at least one dose of a COVID-19 vaccine. There was no significant difference in the proportion of patients who were PET-positive among those who had received a COVID-19 vaccine and those who had not.
There was also no difference in inflammation in patients who had been hospitalized with COVID-19 and those who had managed their infection at home.
Among patients with focal FDG uptake, PET, MRI, and inflammatory blood markers improved at follow-up imaging performed a mean of 52 days after the first imaging. The authors say this suggests that these abnormalities were not related to pre-existing cardiovascular disease.
Of the eight patients with positive FDG-PET results, two did not show any MRI abnormalities. These two patients also had elevated inflammatory biomarkers. “PET is a more sensitive method of measuring cardiac inflammation, and our results show that these changes may not always translate into functional changes seen on MRI,” Dr. Thavendiranathan noted.
The only cardiac risk factor that was more common in participants with FDG uptake was hypertension. Although cardiac symptoms were nearly twice as common in participants with focal FDG uptake, this difference was not statistically significant.
“Given the growing number of survivors with similar symptoms, these interesting findings warrant further investigation,” the authors say.
Noting that FDG uptake correlated with elevations in systemic inflammatory biomarkers, the researchers suggest that “a more intense systemic inflammatory process may be contributing to cardiac inflammation and the consequential alteration to regional and global myocardial function in PET-positive participants.”
On repeat imaging 2 months later, all eight patients who showed FDG uptake showed improvement or resolution of inflammation without any treatment, although two patients still had some signs of inflammation. Blood biomarkers also improved on follow-up.
“This is encouraging information, but we need longer-term data to see if there are any long-term repercussions of this inflammation,” Dr. Hanneman said.
“Overall, the study findings suggest an imaging phenotype that is expected to have good prognosis. However, longer-term follow-up studies are required to understand the need for ongoing cardiac surveillance, relationship to cardiac symptoms, guidance for safe return to exercise and sports participation, and long-term cardiovascular disease risk,” the researchers state.
This study was funded by grants from the Joint Department of Medical Imaging Academic Incentive Fund, Peter Munk Cardiac Center Innovation Committee, and Ted Rogers Center for Heart Research. Dr. Hanneman reports personal fees from Sanofi Genzyme, Amicus, and Medscape outside the submitted work.
A version of this article first appeared on Medscape.com.
Myocardial inflammation is present in a small proportion of patients who have recovered from relatively mild cases of COVID-19 infection, a new study shows.
“Our findings suggest that even in patients who have had relatively mild cases of COVID-19, some will have inflammatory changes to the heart, and these changes can be present without any cardiac symptoms,” senior author, Paaladinesh Thavendiranathan, MD, University of Toronto, told this news organization.
“While our data suggest that this inflammation improves over time, and the outcomes seem positive, we don’t know if there will be any long-term consequences,” he added.
Noting that even a short period of inflammation in the heart may be associated with symptoms or arrhythmias in the longer term, Dr. Thavendiranathan said: “I would recommend that it is best to avoid getting the infection if there is any chance of heart inflammation.”
The study was published online in JAMA Cardiology on Jan. 12.
The authors explain that among patients hospitalized with COVID, early studies suggested that approximately one in four experience cardiovascular injury, defined as an elevation in troponin levels, which was associated with a 5- to 10-fold increase in the risk for death. But there is limited information on cardiac injury in patients who do not require hospitalization.
Although a broad range of abnormal myocardial tissue has been reported in several cardiac MRI studies of patients recovered from COVID infection, there is little understanding of persistent changes in myocardial metabolism in recovered patients, which is a potential concern, given that COVID-19 is associated with systemic inflammation during the acute illness, they say.
For the current study, the researchers examined myocardial inflammation measured using two different methods – cardiac MRI and fluorodeoxyglucose–positron emission tomography (FDG-PET) – in individuals who had recovered from COVID-19 infection and looked at how this related to changes in inflammatory blood markers.
Lead author Kate Hanneman, MD, also from the University of Toronto, explained that FDG-PET imaging is more sensitive than MRI in detecting active inflammation. “Inflammatory cells have a higher uptake of glucose, and FDG-PET imaging is used to look for metabolically active inflammatory tissue that takes up glucose. It gives complementary information to MRI. Cardiac MRI shows structural or functional changes, such as scarring or edema, whereas FDG-PET imaging directly measures metabolic activity related to inflammatory cells.”
The study involved 47 individuals, 51% female, with a mean age of 43 years, who had recently recovered from COVID-19 infection. Of these, the majority had had relatively mild COVID disease, with 85% not requiring hospitalization.
Cardiac imaging was performed a mean of 67 days after the diagnosis of COVID-19. At the time of imaging, 19 participants (40%) reported at least one cardiac symptom, including palpitations, chest pain, and shortness of breath.
Results showed that eight patients (17%) had focal FDG uptake on PET consistent with myocardial inflammation. Compared with those without FDG uptake, patients with focal FDG uptake had higher regional T2, T1, and extracellular volume (colocalizing with focal FDG uptake), higher prevalence of late gadolinium enhancement indicating fibrosis, lower left ventricular ejection fraction, worse global longitudinal and circumferential strain, and higher systemic inflammatory blood markers, including interleukin (IL)-6, IL- 8, an high-sensitivity C-reactive protein.
Of the 47 patients in the study, 13 had received at least one dose of a COVID-19 vaccine. There was no significant difference in the proportion of patients who were PET-positive among those who had received a COVID-19 vaccine and those who had not.
There was also no difference in inflammation in patients who had been hospitalized with COVID-19 and those who had managed their infection at home.
Among patients with focal FDG uptake, PET, MRI, and inflammatory blood markers improved at follow-up imaging performed a mean of 52 days after the first imaging. The authors say this suggests that these abnormalities were not related to pre-existing cardiovascular disease.
Of the eight patients with positive FDG-PET results, two did not show any MRI abnormalities. These two patients also had elevated inflammatory biomarkers. “PET is a more sensitive method of measuring cardiac inflammation, and our results show that these changes may not always translate into functional changes seen on MRI,” Dr. Thavendiranathan noted.
The only cardiac risk factor that was more common in participants with FDG uptake was hypertension. Although cardiac symptoms were nearly twice as common in participants with focal FDG uptake, this difference was not statistically significant.
“Given the growing number of survivors with similar symptoms, these interesting findings warrant further investigation,” the authors say.
Noting that FDG uptake correlated with elevations in systemic inflammatory biomarkers, the researchers suggest that “a more intense systemic inflammatory process may be contributing to cardiac inflammation and the consequential alteration to regional and global myocardial function in PET-positive participants.”
On repeat imaging 2 months later, all eight patients who showed FDG uptake showed improvement or resolution of inflammation without any treatment, although two patients still had some signs of inflammation. Blood biomarkers also improved on follow-up.
“This is encouraging information, but we need longer-term data to see if there are any long-term repercussions of this inflammation,” Dr. Hanneman said.
“Overall, the study findings suggest an imaging phenotype that is expected to have good prognosis. However, longer-term follow-up studies are required to understand the need for ongoing cardiac surveillance, relationship to cardiac symptoms, guidance for safe return to exercise and sports participation, and long-term cardiovascular disease risk,” the researchers state.
This study was funded by grants from the Joint Department of Medical Imaging Academic Incentive Fund, Peter Munk Cardiac Center Innovation Committee, and Ted Rogers Center for Heart Research. Dr. Hanneman reports personal fees from Sanofi Genzyme, Amicus, and Medscape outside the submitted work.
A version of this article first appeared on Medscape.com.

