User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
U.S. tops 500,000 COVID-19 cases in children
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
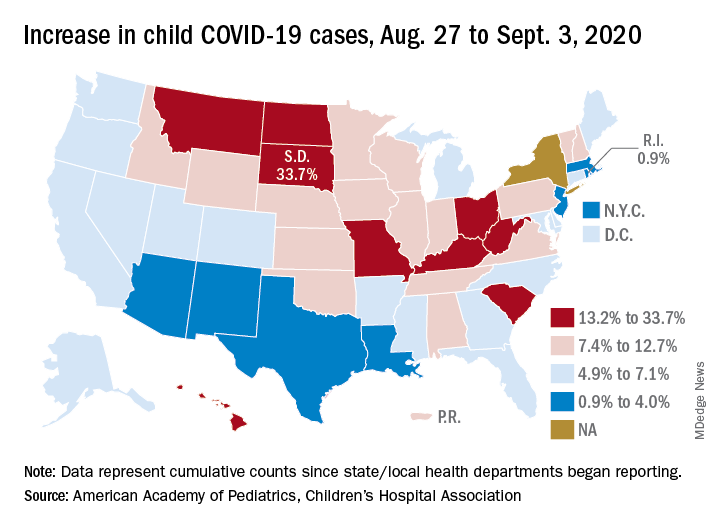
States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

States have reported 513,415 cases of COVID-19 in children since the beginning of the pandemic, with almost 37,000 coming in the last week, the AAP and the CHA said Sept. 8 in the weekly report. That figure includes New York City – the rest of New York State is not reporting ages for COVID-19 patients – as well as Puerto Rico, the District of Columbia, and Guam.
“These numbers are a chilling reminder of why we need to take this virus seriously,” AAP President Sara Goza, MD, said in a written statement.
Children now represent 9.8% of the almost 5.3 million cases that have been reported in Americans of all ages. The proportion of child cases has continued to increase as the pandemic has progressed – it was 8.0% as of mid-July and 5.2% in early June, the data show.
“Throughout the summer, surges in the virus have occurred in Southern, Western, and Midwestern states,” the AAP statement said.
The latest AAP/CHA report shows that, from Aug. 27 to Sept. 3, the total number of child cases jumped by 33.7% in South Dakota, more than any other state. North Dakota was next at 22.7%, followed by Hawaii (18.1%), Missouri (16.8%), and Kentucky (16.4%).
“This rapid rise in positive cases occurred over the summer, and as the weather cools, we know people will spend more time indoors,” said Sean O’Leary, MD, MPH, vice chair of the AAP Committee on Infectious Diseases. “The goal is to get children back into schools for in-person learning, but in many communities, this is not possible as the virus spreads unchecked.”
The smallest increase over the last week, just 0.9%, came in Rhode Island, with Massachusetts just a bit higher at 1.0%. Also at the low end of the increase scale are Arizona (3.3%) and Louisiana (4.0%), two states that have very high rates of cumulative cases: 1,380 per 100,000 children for Arizona and 1,234 per 100,000 for Louisiana, the report said.
To give those figures some context, Tennessee has the highest cumulative count of any state at 1,553 cases per 100,000 children and Vermont has the lowest at 151, based on the data gathered by the AAP and CHA.
“While much remains unknown about COVID-19, we do know that the spread among children reflects what is happening in the broader communities. A disproportionate number of cases are reported in Black and Hispanic children and in places where there is high poverty. We must work harder to address societal inequities that contribute to these disparities,” Dr. Goza said.
Deaths sky high in hospitalized COVID patients with kidney injury
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
More evidence indicates that the development of acute kidney injury
“This ... is the first study in the United States to report the persistence of kidney dysfunction (lack of recovery) in survivors of COVID-19–associated AKI [and] this is in marked contrast to other forms of AKI where over 80% of patients recover their renal function by 10 days,” Lili Chan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues observed.
The research is a retrospective, observational cohort study published online Sept. 3 in the Journal of the American Society of Nephrology
“We may be facing an epidemic of post–COVID-19 kidney disease and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants,” said senior author Girish Nadkarni, MD, a nephrologist, in a statement from Mount Sinai.
Nephrologists will need to prepare for a significant uptick in patients with chronic kidney disease as a result of exposure to the SARS-CoV-2 virus that causes COVID-19, the researchers warned.
“These findings may help centers with resource planning and preparing for the increased load resulting from survivors of COVID-19–associated AKI who do not experience recovery of kidney function,” they added.
Analysis of patients from February to end of May 2020
“AKI among hospitalized patients with COVID-19 in the United States is not well described,” they noted in their article.
And so they analyzed data from five major hospitals in the Mount Sinai Health System between Feb. 27 and May 30 of this year, during which 3,993 patients were hospitalized within the system for COVID-19. The MSHS has a patient population of racially and ethnically diverse citizens from New York.
AKI was defined using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. AKI occurred in 46% of the overall cohort of patients, 19% of whom required dialysis.
However, among those patients who required admission to the ICU, over three-quarters (76%) developed AKI and almost one-third of ICU patients required dialysis, the investigators said.
“The median time from hospital admission until AKI diagnoses was 1 day and the median time from AKI diagnosis to dialysis was 3 days,” they explain.
The proportion of patients with stages 1, 2, or 3 AKI among those admitted to hospital were 39%, 19%, and 42%, respectively. In patients requiring admission to ICU, 28% had stage 1 AKI, 17% had stage 2, and 56% had stage 3.
And among those who required dialysis for AKI, the median peak serum creatinine was 8.2 mg/dL, compared with 2.2 mg/dL for those who did not require dialysis.
Predictors of AKI: male sex, potassium levels, and preexisting CKD
Almost two thirds of patients (65%) had recovered from their kidney injury by the time they left hospital but 35% had acute kidney disease. Of this latter group, on follow-up, 36% had recovered from it, the investigators noted.
Conversely, of those patients who had recovered from AKI by hospital discharge, 14% went on to develop acute kidney disease at the time of follow-up.
And 30% of patients who had required dialysis at some point during their hospital care required dialysis again within 72 hours of being discharged, the investigators noted.
Predictors of severe AKI included male sex (adjusted odds ratio, 1.46), potassium levels on admission (aOR, 1.7), and preexisting chronic kidney disease (CKD) (aOR, 2.8).
Most compellingly, “in-hospital mortality in patients who experienced AKI was 50% [versus] 8% in patients without AKI (P < .001),” Dr. Nadkarni and colleagues reported.
Among those who required ICU care, 42% of patients with AKI died, compared with 7% of those in ICU who did not develop AKI, while in patients cared for outside of ICU, 62% with AKI died compared with only 13% of those who did not develop AKI.
And after adjusting for demographics, comorbidities, and laboratory values, the aOR for death was 11.4 times higher for ICU patients with AKI, compared with ICU patients without AKI, the authors emphasize.
In all patients who developed AKI, the aOR for mortality was 9.2, compared with patients who did not develop AKI, they added.
Perhaps predictably, the risk of death rose with increasing stage of AKI, and patients with stage 3 AKI who required dialysis were at highest risk of death, the authors observe.
Sheer number of AKI cases, need for dialysis unprecedented
“The sheer number of AKI cases and the overwhelming need for dialysis that we are seeing in the context of COVID-19 is unprecedented,” Dr. Nadkarni said.
“These findings bring clinical evidence to the hypothesis of lingering organ dysfunction among patients recovering from COVID-19 and serve as a reminder to hospitals around the country to be very strategic in the allocation of resources to care for patients who experience AKI,” he cautioned.
“We are grappling with a great deal of uncertainty as to how the virus will impact the kidneys in the long haul,” Dr. Nadkarni added. “We may be facing an epidemic of post–COVID-19 kidney disease, and that, in turn, could mean much greater numbers of patients who require kidney dialysis and even transplants.”
Dr. Nadkarni reported serving as a consultant and advisory board member for RenalytixAI and owns equity in the company.
This article first appeared on Medscape.com.
Could these old drugs help fight COVID-19 and save lives?
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
Early in the COVID-19 pandemic, entrepreneur and philanthropist Steve Kirsch realized that until we have a vaccine against SARS-CoV-2, we would be at the mercy of this virus. He realized that the fastest and most effective way to reduce COVID-19 fatalities would be to leverage existing drugs to treat patients at the onset of infection — before they become sick.
Medscape spoke with CETF’s chief medical advisor, Lisa Danzig, MD, about the organization’s aim to fund promising research on repurposed drugs to treat COVID-19.
What is CETF trying to do?
Two things: save lives, and get control of this pandemic.
We are facing perhaps the greatest crisis of our lifetime. Doctors who have taken care of patients with COVID are really frustrated about not having anything to offer; they just watch patients die. We want to change that. CETF was founded to find treatments that, when given early, could improve outcomes and avoid catastrophic complications in patients suffering from COVID-19. That means reducing hospitalizations, which can reduce mortality, but it also can mean reducing viral load, and that can have a profound impact on transmission within communities. We are a funding organization — a Band-Aid. We shouldn’t exist, but we do, aiming to close gaps until a coordinated response can get set up.
Tell us about drug repurposing and why you think existing drugs might have a role in mitigating COVID-19 or slowing its transmission.
This disease has two components — the viral infection, and the immunopathology. So the two promising categories of drugs are classical antivirals (or repurposed drugs with antiviral activity), and the immunomodulators. We are mechanism-agnostic. It doesn’t matter what kind of drug it is if it keeps people out of the hospital and prevents chronic morbidity and mortality.
Repurposed drugs are sort of the low-hanging fruit of clinical drugs. The QBI Coronavirus Research Group identified 69 compounds that have theoretical activity against SARS-CoV-2, 29 of which are already FDA-approved drugs. We thought, why don’t we start testing them?
Some people might call this a long shot. Does drug repurposing really work?
Drugmakers don’t test their drugs on every disease they might be effective for. Drug repurposing can work, but if we don’t look, we definitely won’t find anything. The classic repurposed drug is Viagra, a failed hypertension drug. When the studies ended because it didn’t work, the drug company asked patients to send back the unused drugs. The women all returned the drugs, but the men didn’t. And the rest is history.
There’s a long list of potential drugs that can be repurposed, but few are being tested. The famous poster child of a repurposed drug — hydroxychloroquine — has been the subject of more than 250 clinical trials, but the others weren’t getting much attention.
The beauty of a repurposed drug is that if you can get funding and start enrolling patients, you could potentially find out fairly quickly, as early as a few months, if that drug has an antiviral effect or not. These data would help prioritize drugs to be tested in larger confirmatory studies.
Your focus is on early treatment. What’s the rationale for that?
We are focusing on early treatment because it has been overlooked. The attention has been on vaccines and therapeutics for hospitalized patients. But if you are spending $20 billion on potential vaccines and billions more on diagnostics, we need to give proportional resources toward drugs that might actually work, when given early, in preventing severe disease and death.
Early treatment, if successful, would allow us to avoid the severe complications that we are seeing now. If we can find an early treatment with an existing drug, it would be the fastest, most clinically- and cost-effective way to mitigate the impact of COVID-19 and get us on the road to recovery.
How do you get from a potential repurposed drug for COVID-19 to having a therapeutic agent that will save lives?
Most of the studies we are funding are smaller outpatient studies with virologic endpoints. We are looking for a signal that the drug has antiviral activity. We want to know whether a drug works before we spend the money on questions that take a much larger sample size to answer, for example, a big postexposure prophylaxis study. We’d like to see a meaningful signal in proof-of-concept studies, so we can look at a small group of patients with positive tests and see whether their viral load dropped by more than half if they got the drug compared with those who took the placebo. If the drug had an impact on the viral load and shortened the period of infectivity and was safe, these findings would provide justification to spend a lot of money on a large clinical trial. That would probably encourage the NIH and ACTIV [Accelerating COVID-19 Therapeutic Interventions and Vaccines] collaboration to prioritize the drug for one of their big platform trials. That›s what we are aiming for.
CETF isn’t a drug developer — we are a funder for a good proposal to study a repurposed drug. We want to help move the dial — can we get an early yes or an early no? In drug development, we say, “fail fast and fail early.” It’s a numbers game. Only 10% of early candidates will become approved drugs. The value is in the data, whether they are positive or negative — it doesn’t matter. If the study is a definitive “no,” that is just as helpful as a definitive “yes.” Of course, we all want the definitive “yes,” but there are so many things to look at, the “no’s” will help us redirect resources toward what may really help.
You first announced these funding opportunities in April. How is it going so far?
As soon as the website went up, we got 40 applications. Our scientific advisory board, which has expertise from medicinal chemistry and coronavirology to translational and clinical trial expertise, reviewed the applications and prioritized 11 fundable proposals. We are using milestone-based funding; in other words, funding those who are ready to go.
Which drugs are being tested in the funded studies?
One of the earliest grants we supported was Dr David Boulaware’s randomized controlled trial of hydroxychloroquine (NCT 04308668) in 821 asymptomatic patients within 4 days after a high-risk or moderate-risk exposure. That trial did not show any benefit of hydroxychloroquine as postexposure prophylaxis against COVID-19. This trial was important for another reason. It proved the feasibility of a no-contact trial design in the setting of COVID-19, and participants enrolled themselves through a secure Internet-based survey using the Research Electronic Data Capture (REDCap) system.
Camostat, a transmembrane serine protease (TMPRSS2) inhibitor licensed for use in Japan to treat pancreatitis and esophagitis, combined with the antiandrogen bicalutamide, is being explored for early COVID-19 treatment. TMPRSS2 primes the SARS-CoV-2 spike protein to bind to the ACE2 receptor and gain entry to the cell, and has been shown to have antiviral activity. CETF has provided funding support to ongoing trials of Camostat at Yale University and Aarhus University in Denmark.
Another outpatient trial for fluvoxamine, a drug approved in the United States and routinely prescribed for depression, was also partially funded by a CETF grant to Washington University in St. Louis. Fluvoxamine is a serotonin regulator but also activates the sigma-1 receptor, which reduces the body’s immune response to prevent an overactive immune response or cytokine storm, a major cause of clinical deterioration, serious organ damage, and even death from COVID. This trial was recently completed, and the results have been submitted for publication.
Other promising drugs include niclosamide, doxazosin, favipiravir, leronlimab, interferon beta, interferon lambda, and other monoclonal antibodies. New compounds considered to have potential against COVID include a flu drug (MK-4482/EIDD-2801) and GS-441524, a metabolite of the antiviral drug, remdesivir.
Why not just put all of our resources into vaccine development?
We absolutely need a vaccine to control the outbreak and stop the pandemic. However, it’s a long road to finding an effective vaccine, and in the meantime, we need tools to keep people alive. If we can find an antiviral drug that acts early, we can reduce transmission and contribute to outbreak control. All these tools help us get back to normal while we are waiting for a vaccine. The vaccine is only good if we can give it to every susceptible person in the world — which will take longer than 3 years. And there are no guarantees. Remember, we are still waiting for an HIV vaccine.
You are calling on Americans to help. What do you want them to do?
Everyone must participate in the behavioral changes designed to control the outbreak — physical distancing, face-covering, and paying attention to case counts in local areas to enable them to take appropriate precautions. I know people are bored of that message, but we are going to repeat it until we have a vaccine or herd immunity.
This organism is ripping like wildfire through our unimmunized population. Personal behaviors might slow it down, but finding a drug that can be given to people after they’ve been exposed and test positive will have a meaningful impact on helping us get back to normal.
There’s a great spirit of volunteerism — people are constantly asking how they can help. Through us at CETF, we offer three ways that people can help. They can participate as subjects in clinical trials, many of which are ongoing, including clinical trials, surveillance studies, and follow-up studies. They can donate to our fund and help support the research needed to find an effective early treatment. We have a link on our website, TreatEarly.org. And finally, researchers can apply for funding. We think everybody can help in one of these ways by participating in trials, donating, or applying for funding. It’s an all-hands-on-deck moment for our country.
Danzig is the chief medical advisor of the COVID-19 Early Treatment Fund. She has spent more than 20 years in the pharmaceutical industry developing vaccines, diagnostics, and drugs and is currently advising companies and investors.
This article first appeared on Medscape.com.
MIS-C cardiac evaluation requires more than EF
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
Patients with multisystem inflammatory syndrome caused by COVID-19 typically seem to avoid coronary artery dilation early on, but they may be prone to cardiac injury and dysfunction longer term that requires a more discerning diagnostic approach to sort out.
The findings were revealed in a study of 28 children with COVID-19–related multisystem inflammatory syndrome (MIS-C) at Children’s Hospital of Philadelphia. The study reported that cardiac injury and dysfunction are common in these patients – even those who have preserved ejection fraction – and that diastolic dysfunction is persistent. For comparison, the study also included 20 healthy controls and 20 patients with classic Kawasaki disease (KD).
The study analyzed echocardiography findings in the patients, reporting left ventricular (LV) systolic and diastolic function were worse than in classic Kawasaki disease (KD), which MIS-C mimics. Lead author Daisuke Matsubara, MD, PhD, and colleagues reported that four markers – LV global longitudinal strain, LV circumferential strain rate, right ventricular strain, and left atrial strain – were the strongest predictors of myocardial injury in these patients. After the acute phase, systolic function tended to recover, but diastolic dysfunction persisted.
‘Strain’ measurement boosts accuracy
While echocardiography has been reported to be valuable in evaluating coronary artery function in MIS-C patients, Dr. Matsubara of the division of cardiology at CHOP, said in an interview that study is the first to use the newer echocardiography indexes, known as “strain,” to assess heart function.
“Strain is a more sensitive tool than more conventional indexes and can detect subtle decrease in heart function, even when ejection fraction is preserved,” he said. “Numerous publications have reached conclusions that strain improves the prognostic and diagnostic accuracy of echocardiography in a wide variety of cardiac pathologies causing LV dysfunction.”
Dr. Matsubara noted that the coronary arteries were mostly unaffected in the acute stage of MIS-C, as only one patient in their MIS-C cohort had coronary artery involvement, which normalized during early follow-up. “On the other hand, 20% of our classic KD patients had coronary abnormalities, including two with aneurysms.”
By using positive troponin I or elevated brain natriuretic peptide (BNP) to assess cardiac injury, they found a “high” (60%) incidence of myocardial injury in their MIS-C cohort. During early follow-up, most of the MIS-C patients showed normalization of systolic function, although diastolic dysfunction persisted.
When compared with the classic KD group, MIS-C patients had higher rates of mitral regurgitation (46% vs. 15%, P = .06), more pericardial effusion (32% vs. 15%, P = 0.46), and more pleural effusion (39% vs. 0%, P = .004). MIS-C patients with suspected myocardial injury show these findings more frequently than those with actual myocardial injury.
Compared with the healthy controls, the MIS-C patients showed both LV systolic and diastolic dysfunction as well as significantly lower left atrium (LA) strain and peak right ventricle (RV) free-wall longitudinal strain.
“In addition to the left ventricle, two other chambers of the heart, the LA and the RV that are often labeled as the ‘forgotten chambers’ of the heart, were also affected by MIS-C,” Dr. Matsubara said. “Both LA and RV strains were markedly reduced in MIS-C patients, compared to normal and KD patients.”
The study also indicates that elevated troponin I levels may not be as dire in children as they are in adults. Dr. Matsubara cited a study of more than 2,700 adult COVID-19 patients that found that even mild increases in troponin I level were associated with increased death during hospitalization (J Am Coll Cardiol. 2020;76:533-46).
However, most of the patients in the CHOP study, even those with elevated troponin I levels, recovered systolic function quickly. “We speculate that the elevation in cardiac troponins may have less dire implications in children, likely due to a more transient type of cardiac injury and less comorbidities in children,” he said. “Clearly further studies are needed before a definitive statement can be made.”
Dr. Matsubara added that recovered COVID-19 patients may be able to participate in sports as some schools reopen. “We are not saying restrict sport participation, but we are merely urging caution.”
Comprehensive LV evaluation needed
The findings reinforce that myocardial involvement is more frequent and sometimes more severe in MIS-C than previously thought, said Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital. “We are underestimating it by using just traditional measures like ejection fraction. It requires a comprehensive evaluation of left ventricular function; it really affects all aspects of the ventricle, both the systolic function and the diastolic function.”
This study supports that MIS-C patients should have a more detailed analysis than EF on echocardiography, including strain imaging. “Probably these patients should all be followed at centers where they can evaluate a more detailed analysis of the LV and RV function,” he said. Patients with ongoing CA enlargement and LV dysfunction should have follow-up cardiac care indefinitely. Patients who have no cardiac symptoms during the acute phase probably don’t need long-term follow-up.
“We’re just trying to learn more about this disease, and it’s certainly concerning that so many kids are having cardiac involvement,” Dr. Friedman said. “Fortunately they’re getting better; we’re just trying to find out what this means for the long term.”
Dr. Matsubara and Dr. Friedman have no relevant financial disclosures.
SOURCE: Matsubara D et al. J Am Coll Cardiol. 2020 Sep 2. doi: 10.1016/j.jacc.2020.08.056.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Unexpected results in new COVID-19 ‘cytokine storm’ data
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
The immune system overactivation known as a “cytokine storm” does not play a major role in more severe COVID-19 outcomes, according to unexpected findings in new research. The findings stand in direct contrast to many previous reports.
“We were indeed surprised by the results of our study,” senior study author Peter Pickkers, MD, PhD, said in an interview.
In a unique approach, Dr. Pickkers and colleagues compared cytokine levels in critically ill people with COVID-19 with those in patients with bacterial sepsis, trauma, and after cardiac arrest.
“For the first time, we measured the cytokines in different diseases using the same methods. Our results convincingly show that the circulating cytokine concentrations are not higher, but lower, compared to other diseases,” said Dr. Pickkers, who is affiliated with the department of intensive care medicine at Radboud University Medical Center in Nijmegen, the Netherlands.
The team’s research was published online on Sept. 3 in a letter in JAMA.
Cytokines lower than expected
Normally, cytokines trigger inflammation and promote healing after trauma, infection, or other conditions.
Although a cytokine storm remains ill defined, the authors noted, many researchers have implicated a hyperinflammatory response involving these small proteins in the pathophysiology of COVID-19.
The question remains, however, whether all cytokine storms strike people with different conditions the same way.
Dr. Pickkers, lead author Matthijs Kox, PhD, and colleagues studied 46 people with COVID-19 and acute respiratory distress syndrome (ARDS) who were admitted to the ICU at Radboud University Medical Center. All participants underwent mechanical ventilation and were treated between March 11 and April 27, 2020.
The investigators measured plasma levels of cytokines, including tumor necrosis factor (TNF), interleukin-6, and IL-8. They compared results in this group with those in 51 patients who experienced septic shock and ARDS, 15 patients with septic shock without ARDS, 30 people with out-of-hospital cardiac arrest, and 62 people who experienced multiple traumas. They used historical data for the non–COVID-19 cohorts.
Conditional findings
Compared with patients with septic shock and ARDS, the COVID-19 cohort had lower levels of TNF, IL-6, and IL-8. The differences were statistically significant for TNF (P < .01), as well as for IL-6 and IL-8 concentrations (for both, P < .001).
In addition, the COVID-19 group had significantly lower IL-6 and IL-8 concentrations compared with the patients who had septic shock without ARDS.
The researchers likewise found lower concentrations of IL-8 in patients with COVID-19, compared with the out-of-hospital cardiac arrest patients. IL-8 levels did not differ between the COVID-19 and trauma groups.
Furthermore, the researchers found no differences in IL-6 concentrations between patients with COVID-19 and those who experienced out-of-hospital cardiac arrest or trauma.
However, levels of TNF in people with COVID-19 were higher than in trauma patients.
The small sample sizes and single-center study design are limitations.
“The findings of this preliminary analysis suggest COVID-19 may not be characterized by cytokine storm,” the researchers noted. However, they added, “whether anticytokine therapies will benefit patients with COVID-19 remains to be determined.”
Going forward, Dr. Pickkers and colleagues are investigating the effectiveness of different treatments to lower cytokine levels. They are treating people with COVID-19, for example, with the IL-1 cytokine inhibitor anakinra and steroids.
They also plan to assess the long-term effects of COVID-19 on the immune system. “Following an infection, it is known that the immune system may be suppressed for a longer period of time, and we are determining to what extent this is also present in COVID-19 patients,” Dr. Pickkers said.
Enough to cause a storm?
The study “is quite interesting, and data in this paper are consistent with our data,” Tadamitsu Kishimoto, MD, PhD, of the department of immune regulation at the Immunology Frontier Research Center at Osaka (Japan) University, said in an interview.
His study, published online August 21 in PNAS, also revealed lower serum IL-6 levels among people with COVID-19, compared with patients with bacterial ARDS or sepsis.
Dr. Kishimoto drew a distinction, however: COVID-19 patients can develop severe respiratory failure, suggesting a distinct immune reaction, compared with patients with bacterial sepsis. SARS-CoV-2 directly infects and activates endothelial cells rather than macrophages, as occurs in sepsis.
For this reason, Dr. Kishimoto said, “SARS-CoV-2 infection causes critical illness and severe dysfunction in respiratory organs and induces a cytokine storm,” even in the setting of lower but still elevated serum IL-6 levels.
Dr. Pickkers and Dr. Kishimoto reported no relevant financial relationships.
This story first appeared on Medscape.com.
Asymptomatic children may transmit COVID-19 in communities
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
About 22% of children with COVID-19 infections were asymptomatic, and 66% of the symptomatic children had unrecognized symptoms at the time of diagnosis, based on data from a case series of 91 confirmed cases.
Although recent reports suggest that COVID-19 infections in children are generally mild, data on the full spectrum of illness and duration of viral RNA in children are limited, wrote Mi Seon Han, MD, PhD, of Seoul (South Korea) Metropolitan Government–Seoul National University Boramae Medical Center, and colleagues.
To examine the full clinical course and duration of COVID-19 RNA detectability in children with confirmed infections, the researchers reviewed data from 91 individuals with confirmed infections. The children ranged in age from 27 days to 18 years, and 58% were male. The children were monitored at 20 hospitals and 2 isolation facilities for a mean 21.9 days. The findings were published in JAMA Pediatrics.
Overall, COVID-19 viral RNA was present in the study population for a mean 17.6 days, with testing done at a median interval of 3 days. A total of 20 children (22%) were asymptomatic throughout the study period. In these children, viral RNA was detected for a mean 14 days.
“The major hurdle implicated in this study in diagnosing and treating children with COVID-19 is that the researchers noted.
Of the 71 symptomatic children, 47 (66%) had unrecognized symptoms prior to diagnosis, 18 (25%) developed symptoms after diagnosis, and 6 (9%) were diagnosed at the time of symptom onset. The symptomatic children were symptomatic for a median of 11 days; 43 (61%) remained symptomatic at 7 days’ follow-up after the study period, 27 (38%) were symptomatic at 14 days, and 7 (10%) were symptomatic at 21 days.
A total of 41 children had upper respiratory infections (58%) and 22 children (24%) had lower respiratory tract infections. No difference in the duration of virus RNA was detected between children with upper respiratory tract infections and lower respiratory tract infections (average, 18.7 days vs. 19.9 days).
Among the symptomatic children, 46 (65%) had mild cases and 20 (28%) had moderate cases.
For treatment, 14 children (15%) received lopinavir-ritonavir and/or hydroxychloroquine. Two patients had severe illness and received oxygen via nasal prong, without the need for mechanical ventilation. All the children in the case series recovered from their infections with no fatalities.
The study’s main limitation was the inability to analyze the transmission potential of the children because of the quarantine and isolation policies in Korea, the researchers noted. In addition, the researchers did not perform follow-up testing at consistent intervals, so the duration of COVID-19 RNA detection may be inexact.
However, the results suggest “that suspecting and diagnosing COVID-19 in children based on their symptoms without epidemiologic information and virus testing is very challenging,” the researchers emphasized.
“Most of the children with COVID-19 have silent disease, but SARS-CoV-2 RNA can still be detected in the respiratory tract for a prolonged period,” they wrote. More research is needed to explore the potential for disease transmission by children in the community, and increased surveillance with laboratory screening can help identify children with unrecognized infections.
The study is the first known to focus on the frequency of asymptomatic infection in children and the duration of symptoms in both asymptomatic and symptomatic children, Roberta L. DeBiasi, MD, and Meghan Delaney, DO, both affiliated with Children’s National Hospital and Research Institute, Washington, and George Washington University, Washington, wrote in an accompanying editorial. The structure of the Korean public health system “allowed for the sequential observation, testing (median testing interval of every 3 days), and comparison of 91 asymptomatic, presymptomatic, and symptomatic children with mild to moderate upper and lower respiratory tract infection, identified primarily by contact tracing from laboratory-proven cases.”
Two take-home points from the study are that not all infected children are symptomatic, and the duration of symptoms in those who are varies widely, they noted. “Interestingly, this study aligns with adult data in which up to 40% of adults may remain asymptomatic in the face of infection.”
However, “The third and most important take-home point from this study relates to the duration of viral shedding in infected pediatric patients,” Dr. DeBiasi and Dr. Delaney said (JAMA Pediatr. 2020 Aug 28. doi: 10.1001/jamapediatrics.2020.3996).
“Fully half of symptomatic children with both upper and lower tract disease were still shedding virus at 21 days. These are striking data, particularly since 86 of 88 diagnosed children (98%) either had no symptoms or mild or moderate disease,” they explained. The results highlight the need for improvements in qualitative molecular testing and formal studies to identify differences in results from different testing scenarios, such as hospital entry, preprocedure screening, and symptomatic testing. In addition, “these findings are highly relevant to the development of public health strategies to mitigate and contain spread within communities, particularly as affected communities begin their recovery phases.”
The study is important because “schools are opening, and we don’t know what is going to happen,” Michael E. Pichichero, MD, of Rochester General Hospital, N.Y., said in an interview.
“Clinicians, parents, students, school administrators and politicians are worried,” he said. “This study adds to others recently published, bringing into focus the challenges to several suppositions that existed when the COVID-19 pandemic began and over the summer.”
“This study of 91 Korean children tells us that taking a child’s temperature as a screening tool to decide if they may enter school will not be a highly successful strategy,” he said. “Many children are without fever and asymptomatic when infected and contagious. The notion that children shed less virus or shed it for shorter lengths of time we keep learning from this type of research is not true. In another recent study the authors found that children shed as much of the SARS-CoV-2 virus as an adult in the ICU on a ventilator.”
Dr. Pichichero said he was not surprised by the study findings. “A similar paper was published last week in the Journal of Pediatrics from Massachusetts General Hospital, so the findings in the JAMA paper are similar to what has been reported in the United States.”
“Availability of testing will continue to be a challenge in some communities,” said Dr. Pichichero. “Here in the Rochester, New York, area we will use a screening questionnaire based on the CDC [Centers for Disease Control and Prevention] symptom criteria of SARS-CoV-2 infections to decide whom to test.”
As for additional research, “We have so much more to learn about SARS-CoV-2 in children,” he emphasized. “The focus has been on adults because the morbidity and mortality has been greatest in adults, especially the elderly and those with compromised health.”
“The National Institutes of Health has issued a call for more research in children to characterize the spectrum of SARS-CoV-2 illness, including the multisystem inflammatory syndrome in children [MIS-C] and try to identify biomarkers and/or biosignatures for a prognostic algorithm to predict the longitudinal risk of disease severity after a child is exposed to and may be infected with SARS-CoV-2,” said Dr. Pichichero. “NIH has asked researchers to answer the following questions.”
- Why do children have milder illness?
- Are there differences in childhood biology (e.g., gender, puberty, etc.) that contribute to illness severity?
- Are there genetic host differences associated with different disease severity phenotypes, including MIS-C?
- Are there innate mucosal, humoral, cellular and other adaptive immune profiles that are associated with reduced or increased risk of progressive disease, including previous coronavirus infections?
- Will SARS-CoV-2 reinfection cause worse disease as seen with antibody-dependent enhancement (ADE) in other viral infections (e.g., dengue)? Will future vaccines carry a risk of the ADE phenomenon?
- Does substance use (e.g., nicotine, marijuana) exacerbate or trigger MIS-C through immune activation?
“We have no knowledge yet about SARS-CoV-2 vaccination of children, especially young children,” Dr. Pichichero emphasized. “There are different types of vaccines – messenger RNA, adenovirus vector and purified spike proteins of the virus – among others, but questions remain: Will the vaccines work in children? What about side effects? Will the antibodies and cellular immunity protect partially or completely?”
The researchers and editorialists had no financial conflicts to disclose. Dr. Pichichero had no financial conflicts to disclose.
SOURCE: Han MS et al. JAMA Pediatr. 2020 Aug 28. doi:10.1001/jamapediatrics.2020.3988.
FROM JAMA PEDIATRICS
High mortality rates reported in large COVID-19 study
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Factors including older age and certain comorbidities have been linked to more serious COVID-19 outcomes in previous research, and now a large dataset collected from hundreds of hospitals nationwide provides more detailed data regarding risk for mechanical ventilation and death.
History of pulmonary disease or smoking, interestingly, were not.
One expert urges caution when interpreting the results, however. Although the study found a number of risk factors for ventilation and mortality, she says the dataset lacks information on race and disease severity, and the sample may not be nationally representative.
The investigators hope their level of granularity will further assist researchers searching for effective treatments and clinicians seeking to triage patients during the COVID-19 pandemic.
The study was published online August 28 in Clinical Infectious Diseases.
COVID-19 and comorbidities
“What I found most illuminating was this whole concept of comorbid conditions. This provides suggestive data about who we need to worry about most and who we may need to worry about less,” study author Robert S. Brown Jr, MD, MPH, told Medscape Medical News.
Comorbid conditions included hypertension in 47% of patients, diabetes in 28%, and cardiovascular disease in 19%. Another 16% were obese and 12% had chronic kidney disease. People with comorbid obesity, chronic kidney disease, and cardiovascular disease were more likely to receive mechanical ventilation compared to those without a history of these conditions in an adjusted, multivariable logistic analysis.
With the exception of obesity, the same factors were associated with risk for death during hospitalization.
In contrast, hypertension, history of smoking, and history of pulmonary disease were associated with a lower risk of needing mechanical ventilation and/or lower risk for mortality.
Furthermore, people with liver disease, gastrointestinal diseases, and even autoimmune diseases – which are likely associated with immunosuppression – “are not at that much of an increased risk that we noticed it in our data,” Brown said.
“As I tell many of my patients who have mild liver disease, for example, I would rather have mild liver disease and be on immunosuppressant therapy than be an older, obese male,” he added.
Assessing data for people in 38 U.S. states, and not limiting outcomes to patients in a particular COVID-19 hot spot, was a unique aspect of the research, said Brown, clinical chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine in New York City.
Brown, lead author Michael W. Fried, MD, from TARGET PharmaSolutions in Durham, North Carolina, and colleagues studied adults from a commercially available Target Real-World Evidence (RWE) dataset of nearly 70,000 patients. They examined hospital chargemaster data and ICD-10 codes for COVID-19 inpatients between February 15 and April 20.
This population tended to be older, with 60% older than 60 years. A little more than half of participants, 53%, were men.
Key findings
A total of 21% of patients died after a median hospital length of stay of 8 days.
Older patients were significantly more likely to die, particularly those older than 60 years (P < .0001).
“This confirms some of the things we know about age and its impact on outcome,” Brown said.
The risk for mortality among patients older than 60 years was 7.2 times that of patients between 18 and 40 years in an adjusted multivariate analysis. The risk for death for those between 41 and 60 years of age was lower (odds ratio [OR], 2.6), compared with the youngest cohort.
Men were more likely to die than women (OR, 1.5).
When asked if he was surprised by the high mortality rates, Brown said, “Having worked here in New York? No, I was not.”
Mechanical ventilation and mortality
Male sex, age older than 40 years, obesity, and presence of cardiovascular or chronic kidney disease were risk factors for mechanical ventilation.
Among the nearly 2,000 hospitalized adults requiring mechanical ventilation in the current report, only 27% were discharged alive. “The outcomes of people who are mechanically ventilated are really quite sobering,” Brown said.
People who ever required mechanical ventilation were 32 times more likely to die compared with others whose highest level of oxygenation was low-flow, high-flow, or no-oxygen therapy in an analysis that controlled for demographics and comorbidities.
Furthermore, patients placed on mechanical ventilation earlier – within 24 hours of admission – tended to experience better outcomes.
COVID-19 therapies?
Brown and colleagues also evaluated outcomes in patients who were taking either remdesivir or hydroxychloroquine. A total of 48 people were treated with remdesivir.
The four individuals receiving remdesivir who died were among 11 who were taking remdesivir and also on mechanical ventilation.
“The data for remdesivir is very encouraging,” Brown said.
Many more participants were treated with hydroxychloroquine, more than 4,200 or 36% of the total study population.
A higher proportion of people treated with hydroxychloroquine received mechanical ventilation, at 25%, versus 12% not treated with hydroxychloroquine.
The unadjusted mortality rate was also higher among those treated with the agent, at 25%, compared to 20% not receiving hydroxychloroquine.
The data with hydroxychloroquine can lead to two conclusions, Brown said: “One, it doesn’t work. Or two, it doesn’t work in the way that we use it.”
The researchers cautioned that their hydroxychloroquine findings must be interpreted carefully because those treated with the agent were also more likely to have comorbidities and greater COVID-19 disease severity.
“This study greatly contributes to understanding the natural course of COVID-19 infection by describing characteristics and outcomes of patients with COVID-19 hospitalized throughout the US,” the investigators note. “It identified categories of patients at greatest risk for poor outcomes, which should be used to prioritize prevention and treatment strategies in the future.”
Some limitations
“The findings that patients with hypertension and who were smokers had lower ventilation rates, and patients with hypertension, pulmonary disease, who were smokers had lower mortality risks was very surprising,” Ninez A. Ponce, PhD, MPP, told Medscape Medical News when asked to comment on the study.
Although the study identified multiple risk factors for ventilation and mortality, “unfortunately the dataset did not have race available or disease severity,” said Ponce, director of the UCLA Center for Health Policy Research and professor in the Department of Health Policy and Management at the UCLA Fielding School of Public Health.
“These omitted variables could have a considerable effect on the significance, magnitude, and direction of point estimates provided, so I would be cautious in interpreting the results as a picture of a nationally representative sample,” she said.
On a positive note, the study and dataset could illuminate the utility of medications used to treat COVID-19, Ponce said. In addition, as the authors note, “the data will expand over time.”
Brown has reported receiving grants and consulting for Gilead. Ponce has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Interstitial lung abnormalities linked to COPD exacerbations
Patients with chronic obstructive pulmonary disease who also had certain interstitial lung abnormalities experienced more exacerbations and reduced lung function than those without such abnormalities, findings from a retrospective study has shown.
Interstitial lung abnormalities (ILA) are considered precursor lesions of interstitial lung disease and previous studies suggested an association with poor outcomes among chronic obstructive pulmonary disease (COPD) patients, but data on long-term clinical relevance are limited, wrote Tae Seung Lee, MD, of Seoul (South Korea) National University Hospital, and colleagues.
In a study published in Chest, the researchers reviewed data from 363 COPD patients including 44 with equivocal ILA and 103 with definite ILA. Overall, the ILA patients were older and had poorer lung function than non-ILA patients. Patients received chest CT scan and longitudinal pulmonary function tests between January 2013 and December 2018.
Over an average follow-up period of 5.4 years, patients with ILA experienced significantly more acute COPD exacerbations than did those without ILA (adjusted odds ratio, 2.03). The percentages of frequent exacerbators among patients with no ILA, equivocal ILA, and definite ILA were 8.3%, 15.9%, and 20.4%, respectively.
“Acute exacerbation is an important event during the clinical course of COPD, because it is associated with temporary or persistent reductions in lung function, lower quality of life, hospitalization, and mortality,” the researchers noted.
In a multivariate analysis, the annual decline in lung function (FEV1) was –35.7 in patients with equivocal ILA, compared with –28.0 in patients with no ILA and –15.9 in those with definite ILA.
“This may be due to the distribution of the spirometric stages in each group, and to the resulting changes in lung function,” the researchers wrote. In this study, “the equivocal ILA group had a significantly lower baseline FEV1 than the other groups. In our study population, the lower the spirometric stage, the faster the annual decline in FEV1, consistent with the results of a prior prospective study of a COPD cohort.”
The findings were limited by several factors including the retrospective design and relatively small number of ILA patients, as well as the limited evaluation of ILA and potential for selection bias, the researchers noted. However, the result support the impact of ILA on exacerbations and accelerated decline in lung function in COPD patients.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Lee TS et al. Chest. 2020 Aug 13. doi: 10.1016/j.chest.2020.08.017.
Patients with chronic obstructive pulmonary disease who also had certain interstitial lung abnormalities experienced more exacerbations and reduced lung function than those without such abnormalities, findings from a retrospective study has shown.
Interstitial lung abnormalities (ILA) are considered precursor lesions of interstitial lung disease and previous studies suggested an association with poor outcomes among chronic obstructive pulmonary disease (COPD) patients, but data on long-term clinical relevance are limited, wrote Tae Seung Lee, MD, of Seoul (South Korea) National University Hospital, and colleagues.
In a study published in Chest, the researchers reviewed data from 363 COPD patients including 44 with equivocal ILA and 103 with definite ILA. Overall, the ILA patients were older and had poorer lung function than non-ILA patients. Patients received chest CT scan and longitudinal pulmonary function tests between January 2013 and December 2018.
Over an average follow-up period of 5.4 years, patients with ILA experienced significantly more acute COPD exacerbations than did those without ILA (adjusted odds ratio, 2.03). The percentages of frequent exacerbators among patients with no ILA, equivocal ILA, and definite ILA were 8.3%, 15.9%, and 20.4%, respectively.
“Acute exacerbation is an important event during the clinical course of COPD, because it is associated with temporary or persistent reductions in lung function, lower quality of life, hospitalization, and mortality,” the researchers noted.
In a multivariate analysis, the annual decline in lung function (FEV1) was –35.7 in patients with equivocal ILA, compared with –28.0 in patients with no ILA and –15.9 in those with definite ILA.
“This may be due to the distribution of the spirometric stages in each group, and to the resulting changes in lung function,” the researchers wrote. In this study, “the equivocal ILA group had a significantly lower baseline FEV1 than the other groups. In our study population, the lower the spirometric stage, the faster the annual decline in FEV1, consistent with the results of a prior prospective study of a COPD cohort.”
The findings were limited by several factors including the retrospective design and relatively small number of ILA patients, as well as the limited evaluation of ILA and potential for selection bias, the researchers noted. However, the result support the impact of ILA on exacerbations and accelerated decline in lung function in COPD patients.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Lee TS et al. Chest. 2020 Aug 13. doi: 10.1016/j.chest.2020.08.017.
Patients with chronic obstructive pulmonary disease who also had certain interstitial lung abnormalities experienced more exacerbations and reduced lung function than those without such abnormalities, findings from a retrospective study has shown.
Interstitial lung abnormalities (ILA) are considered precursor lesions of interstitial lung disease and previous studies suggested an association with poor outcomes among chronic obstructive pulmonary disease (COPD) patients, but data on long-term clinical relevance are limited, wrote Tae Seung Lee, MD, of Seoul (South Korea) National University Hospital, and colleagues.
In a study published in Chest, the researchers reviewed data from 363 COPD patients including 44 with equivocal ILA and 103 with definite ILA. Overall, the ILA patients were older and had poorer lung function than non-ILA patients. Patients received chest CT scan and longitudinal pulmonary function tests between January 2013 and December 2018.
Over an average follow-up period of 5.4 years, patients with ILA experienced significantly more acute COPD exacerbations than did those without ILA (adjusted odds ratio, 2.03). The percentages of frequent exacerbators among patients with no ILA, equivocal ILA, and definite ILA were 8.3%, 15.9%, and 20.4%, respectively.
“Acute exacerbation is an important event during the clinical course of COPD, because it is associated with temporary or persistent reductions in lung function, lower quality of life, hospitalization, and mortality,” the researchers noted.
In a multivariate analysis, the annual decline in lung function (FEV1) was –35.7 in patients with equivocal ILA, compared with –28.0 in patients with no ILA and –15.9 in those with definite ILA.
“This may be due to the distribution of the spirometric stages in each group, and to the resulting changes in lung function,” the researchers wrote. In this study, “the equivocal ILA group had a significantly lower baseline FEV1 than the other groups. In our study population, the lower the spirometric stage, the faster the annual decline in FEV1, consistent with the results of a prior prospective study of a COPD cohort.”
The findings were limited by several factors including the retrospective design and relatively small number of ILA patients, as well as the limited evaluation of ILA and potential for selection bias, the researchers noted. However, the result support the impact of ILA on exacerbations and accelerated decline in lung function in COPD patients.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Lee TS et al. Chest. 2020 Aug 13. doi: 10.1016/j.chest.2020.08.017.
FROM CHEST
First randomized trial reassures on ACEIs, ARBs in COVID-19
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The first randomized study to compare continuing versus stopping ACE inhibitors or angiotensin receptor blockers (ARBs) for patients with COVID-19 has shown no difference in key outcomes between the two approaches.
The BRACE CORONA trial – conducted in patients had been taking an ACE inhibitor or an ARB on a long-term basis and who were subsequently hospitalized with COVID-19 – showed no difference in the primary endpoint of number of days alive and out of hospital among those whose medication was suspended for 30 days and those who continued undergoing treatment with these agents.
“Because these data indicate that there is no clinical benefit from routinely interrupting these medications in hospitalized patients with mild to moderate COVID-19, they should generally be continued for those with an indication,” principal investigator Renato Lopes, MD, of Duke Clinical Research Institute, Durham, N.C., concluded.
The BRACE CORONA trial was presented at the European Society of Cardiology Congress 2020 on Sept. 1.
Dr. Lopes explained that there are two conflicting hypotheses about the role of ACE inhibitors and ARBs in COVID-19.
One hypothesis suggests that use of these drugs could be harmful by increasing the expression of ACE2 receptors (which the SARS-CoV-2 virus uses to gain entry into cells), thus potentially enhancing viral binding and viral entry. The other suggests that ACE inhibitors and ARBs could be protective by reducing production of angiotensin II and enhancing the generation of angiotensin 1-7, which attenuates inflammation and fibrosis and therefore could attenuate lung injury.
The BRACE CORONA trial was an academic-led randomized study that tested two strategies: temporarily stopping the ACE inhibitor/ARB for 30 days or continuing these drugs for patients who had been taking these medications on a long-term basis and were hospitalized with a confirmed diagnosis of COVID-19.
The primary outcome was the number of days alive and out of hospital at 30 days. Patients who were using more than three antihypertensive drugs or sacubitril/valsartan or who were hemodynamically unstable at presentation were excluded from the study.
The trial enrolled 659 patients from 29 sites in Brazil. The mean age of patients was 56 years, 40% were women, and 52% were obese. ACE inhibitors were being taken by 15% of the trial participants; ARBs were being taken by 85%. The median duration of ACE inhibitor/ARB treatment was 5 years.
Patients were a median of 6 days from COVID-19 symptom onset. For 30% of the patients, oxygen saturation was below 94% at entry. In terms of COVID-19 symptoms, 57% were classified as mild, and 43% as moderate.
Those with severe COVID-19 symptoms who needed intubation or vasoactive drugs were excluded. Antihypertensive therapy would generally be discontinued in these patients anyway, Dr. Lopes said.
Results showed that the average number of days alive and out of hospital was 21.9 days for patients who stopped taking ACE inhibitors/ARBs and 22.9 days for patients who continued taking these medications. The average difference between groups was –1.1 days.
The average ratio of days alive and out of hospital between the suspending and continuing groups was 0.95 (95% CI, 0.90-1.01; P = .09).
The proportion of patients alive and out of hospital by the end of 30 days in the suspending ACE inhibitor/ARB group was 91.8% versus 95% in the continuing group.
A similar 30-day mortality rate was seen for patients who continued and those who suspended ACE inhibitor/ARB therapy, at 2.8% and 2.7%, respectively (hazard ratio, 0.97). The median number of days that patients were alive and out of hospital was 25 in both groups.
Dr. Lopes said that there was no difference between the two groups with regard to many other secondary outcomes. These included COVID-19 disease progression (need for intubation, ventilation, need for vasoactive drugs, or imaging results) and cardiovascular endpoints (MI, stroke, thromboembolic events, worsening heart failure, myocarditis, or hypertensive crisis).
“Our results endorse with reliable and more definitive data what most medical and cardiovascular societies are recommending – that patients do not stop ACE inhibitor or ARB medication. This has been based on observational data so far, but BRACE CORONA now provides randomized data to support this recommendation,” Dr. Lopes concluded.
Dr. Lopes noted that several subgroups had been prespecified for analysis. Factors included age, obesity, difference between ACE inhibitors/ARBs, difference in oxygen saturation at presentation, time since COVID-19 symptom onset, degree of lung involvement on CT, and symptom severity on presentation.
“We saw very consistent effects of our main findings across all these subgroups, and we plan to report more details of these in the near future,” he said.
Protective for older patients?
The discussant of the study at the ESC Hotline session, Gianfranco Parati, MD, University of Milan-Bicocca and San Luca Hospital, Milan, congratulated Lopes and his team for conducting this important trial at such a difficult time.
He pointed out that patients in the BRACE CORONA trial were quite young (average age, 56 years) and that observational data so far suggest that ACE inhibitors and ARBs have a stronger protective effect in older COVID-19 patients.
He also noted that the percentage of patients alive and out of hospital at 30 days was higher for the patients who continued on treatment in this study (95% vs. 91.8%), which suggested an advantage in maintaining the medication.
Dr. Lopes replied that one-quarter of the population in the BRACE CORONA trial was older than 65 years, which he said was a “reasonable number.”
“Subgroup analysis by age did not show a significant interaction, but the effect of continuing treatment does seem to be more favorable in older patients and also in those who were sicker and had more comorbidities,” he added.
Dr. Parati also suggested that it would have been difficult to discern differences between ACE inhibitors and ARBs in the BRACE CORONA trial, because so few patents were taking ACE inhibitors; the follow-up period of 30 days was relatively short, inasmuch as these drugs may have long-term effects; and it would have been difficult to show differences in the main outcomes used in the study – mortality and time out of hospital – in these patients with mild to moderate disease.
Franz H. Messerli, MD, and Christoph Gräni, MD, University of Bern (Switzerland), said in a joint statement: “The BRACE CORONA trial provides answers to what we know from retrospective studies: if you have already COVID, don’t stop renin-angiotensin system blocker medication.”
But they added that the study does not answer the question about the risk/benefit of ACE inhibitors or ARBs with regard to possible enhanced viral entry through the ACE2 receptor. “What about all those on these drugs who are not infected with COVID? Do they need to stop them? We simply don’t know yet,” they said.
Dr. Messerli and Dr. Gräni added that they would like to see a study that compared patients before SARS-CoV-2 infection who were without hypertension, patients with hypertension who were taking ACE inhibitors or ARBs, and patients with hypertension taking other antihypertensive drugs.
The BRACE CORONA trial was sponsored by D’Or Institute for Research and Education and the Brazilian Clinical Research Institute. Dr. Lopes has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Obesity boosts risks in COVID-19 from diagnosis to death
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
FROM OBESITY REVIEWS









