User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
FDA expands remdesivir use for all COVID-19 hospitalized patients
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
An EUA of remdesivir issued in May allowed the drug to be used only for patients with severe COVID-19, specifically, COVID-19 patients with low blood oxygen levels or who need oxygen therapy or mechanical ventilation.
“Today, based on the Agency’s ongoing review of the EUA, including its review of the totality of scientific information now available, the FDA has determined that it is reasonable to believe Veklury may be effective for the treatment of suspected or laboratory-confirmed COVID-19 in all hospitalized adult and pediatric patients,” the FDA news release about the expanded EUA said. “The Agency’s review has also concluded that the known and potential benefits of Veklury outweigh the known and potential risks for these uses.”
‘Further evaluation’ needed
The EUA expansion is partially based on the results of a randomized, open-label trial that Gilead Sciences, remdesivir’s manufacturer, conducted at multiple sites.
The trial showed that a 5-day course of remdesivir was associated with statistically significant improvement among patients hospitalized with moderate COVID-19 in comparison with those receiving standard care. However, patients who were randomly assigned to a receive longer, 10-day remdesivir course had not improved significantly 11 days after treatment started, compared with those who received standard care.
Results with remdesivir in this trial and in two previously reported randomized trials varied, “raising the question of whether the discrepancies are artifacts of study design choices, including patient populations, or whether the drug is less efficacious than hoped,” wrote Erin K. McCreary, PharmD, and Derek C. Angus, MD, MPH, with the University of Pittsburgh School of Medicine, in an editorial that accompanied publication of the trials in JAMA.
Angus previously expressed concern that expanding remdesivir’s EUA could “interrupt or thwart efforts to execute the needed RCTs [randomized controlled trials].
“We think there really needs to be further evaluation of remdesivir in large-scale RCTs adequately powered to understand in which patients, at which dose, given at which point in the course of illness leads to what concrete and tangible improvement in clinical outcomes,” he told Medscape Medical News.
“At this point, remdesivir definitely holds promise, but given the cost to produce and distribute the drug, it seems crucial to know with more certainty how best to use it,” Angus said.
The EUA expansion is also partially based on results from a randomized, double-blind, placebo-controlled clinical trial that the National Institutes of Allergy and Infectious Diseases conducted. In that trial, there was a statistically significant reduction in median recovery time and higher odds of clinical improvement after 2 weeks for hospitalized patients who received remdesivir.
For hospitalized patients with mild to moderate disease, the results were consistent with the overall study results but were not statistically significant.
This article first appeared on Medscape.com.
Two PR employees at FDA fired after plasma therapy controversy
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
The US Food and Drug Administration has removed two senior public relations employees, one of whom advised the agency against unbridled promotion of convalescent blood plasma as a treatment for people with COVID-19, multiple media outlets reported Aug. 28.
Officials claim the dismissals are coincidental and are not related to a controversy about whether claims regarding convalescent plasma therapy that were put forth by President Donald Trump and FDA Commissioner Stephen M. Hahn, MD, were exaggerated, according to reports from The New York Times , CNN, and elsewhere.
One of the PR employees, Emily Miller, was on the job less than 2 weeks. The White House named her FDA chief spokeswoman 11 days ago, but Hahn removed her from that post Aug. 28.
On Aug. 27, the US Department of Health and Human Services terminated the contract for Wayne L. Pines, a PR consultant to the FDA. Pines reportedly advised Hahn to apologize for making misleading claims about the therapeutic benefits of convalescent plasma therapy for COVID-19.
The FDA did not respond to multiple requests for comment.
The controversy stems from comments Hahn made about the announcement of the emergency use authorization for convalescent plasma for patients with COVID-19. He said that plasma had been found to save the lives of 35 out of every 100 people who were treated. That statement was later found to be erroneous because he presented a relative risk reduction as an absolute decrease in risk. He later apologized via Twitter.
Researchers running clinical trials to evaluate the efficacy of convalescent plasma for COVID-19 are concerned that the emergency use authorization could thwart efforts to recruit participants for their studies.
This article first appeared on Medscape.com.
NYC public hospitals rose to the demands of the COVID-19 crisis
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
Hospitalists at the center of the storm
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
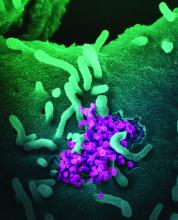
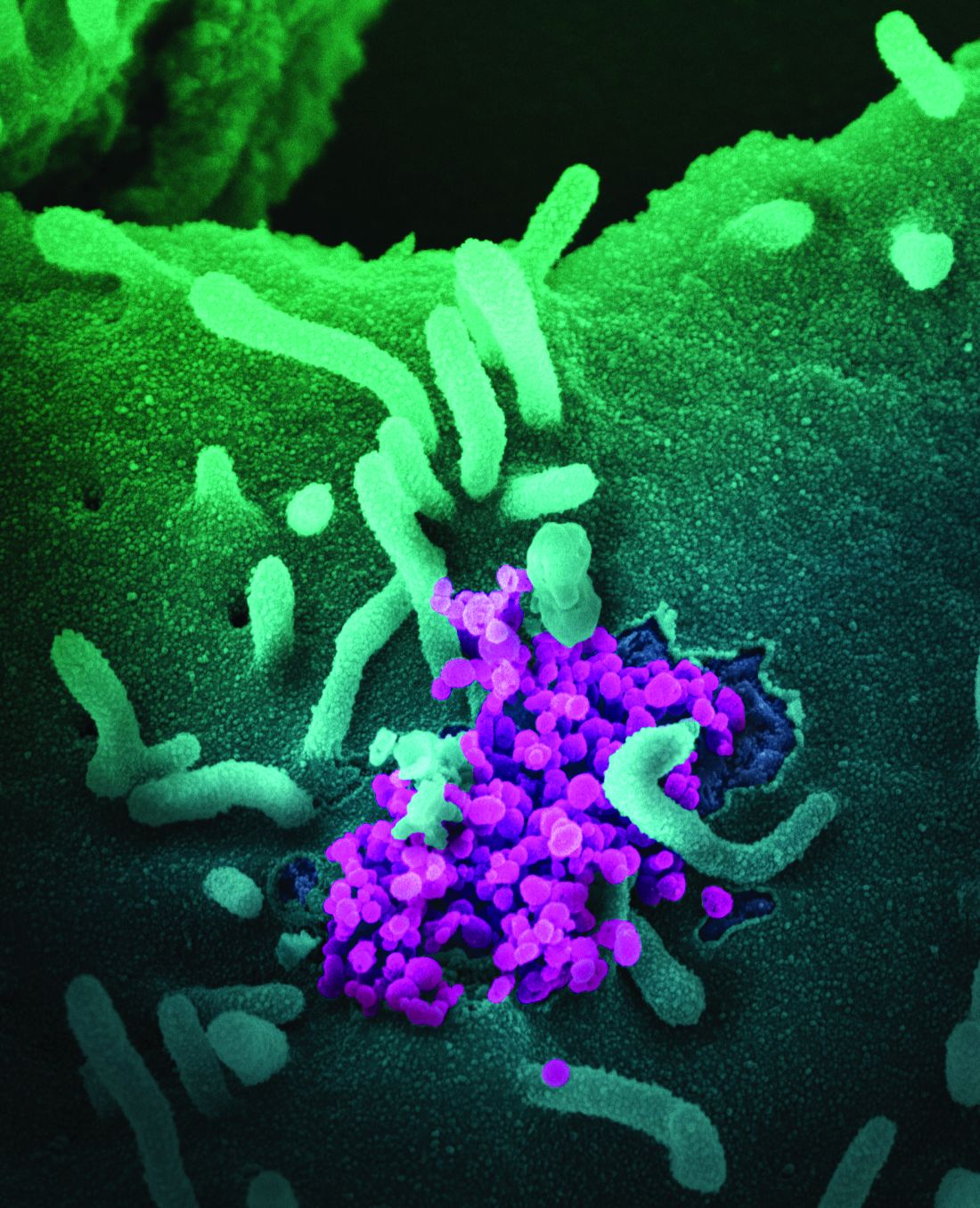
SARS-CoV-2 appears unlikely to pass through breast milk
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
Breast milk is an unlikely source of transmission of SARS-CoV-2 from mothers to infants, according to data from case reports and breast milk samples from 18 women.
“To date, SARS-CoV-2 has not been isolated from breast milk, and there are no documented cases of transmission of infectious virus to the infant through breast milk,” but the potential for transmission remains a concern among women who want to breastfeed, wrote Christina Chambers, PhD, of the University of California, San Diego, and colleagues.
In a research letter published in JAMA, the investigators identified 18 women with confirmed SARS-CoV-2 infections (all but 1 of the women had symptomatic COVID-19 disease) and infants aged 0-19 months between March 27 and May 6, 2020. The average age of the mothers was 34 years, and 78% were non-Hispanic White. The women provided 1-12 samples of breast milk for a total of 64 samples collected before and after positive COVID-19 tests.
One sample yielded detectable RNA from SARS-CoV-2 and was collected on the day of the woman’s symptom onset. However, one sample taken 2 days prior to symptom onset and two samples collected 12 and 41 days later tested negative for viral RNA, the researchers said. In addition, no replication-competent virus was identified in the positive sample or any of the other samples.
The researchers spiked two stored milk samples collected prior to the pandemic with replication-competent SARS-CoV-2. Virus was not detected by culture in the samples after Holder pasteurization, but was detected by culture in nonpasteurized aliquots of the same samples.
“These data suggest that SARS-CoV-2 RNA does not represent replication-competent virus and that breast milk may not be a source of infection for the infant,” Dr. Chambers and associates said.
The results were limited by several factors including the small sample size and potential for selection bias, as well as the use of self-reports of positive tests and self-collection of breast milk, the researchers noted. However, the findings are reassuring in light of the known benefits of breastfeeding and the use of milk banks.
“This research is important because the pandemic is ongoing and has far-reaching consequences: as the authors indicate, the potential for viral transmission through breast milk remains a critical question for women infected with SARS-CoV-2 who wish to breastfeed,” Janet R. Hardy, PhD, MPH, MSc, a consultant on global maternal-child health and pharmacoepidemiology, said in an interview.
“This virus has everyone on a rapid learning track, and all information that helps build evidence to support women’s decision-making in the care of their children is valuable,” she said. “These findings suggest that breast milk may not be a source of SARS-CoV-2 infection for the infant. They provide some reassurance given the recognized benefits of breastfeeding and human milk.”
However, “This study is very specific to breast milk,” she emphasized. “In advising women infected with SARS-CoV-2, clinicians may want to include a discussion of protection methods to prevent maternal transmission of the virus through respiratory droplets.”
Although the data are preliminary, “the investigators established and validated an RT-PCR [reverse transcription polymerase chain reaction] assay and developed tissue culture methods for replication-competent SARS-CoV-2 in breast milk, both valuable tools for further studies. Next steps will include controlled studies of greater sample size with independent verification of RT-PCR positivity,” said Dr. Hardy, a consultant to Biohaven Pharmaceuticals, New Haven, Conn.
The study was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health. Medela Corporation provided milk sample collection materials. The Family Larsson-Rosenquist Foundation provided an unrestricted COVID19 emergency gift fund. The Mothers’ Milk Bank at Austin paid for shipping costs.
SOURCE: Chambers C et al. JAMA. 2020 Aug 19. doi: 10.1001/jama.2020.15580.
FROM JAMA
Mitigating psychiatric disorder relapse in pregnancy during pandemic
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
In a previous column, I addressed some of the issues that quickly arose in the context of the COVID-19 pandemic and their implications for reproductive psychiatry. These issues ranged from the importance of sustaining well-being in pregnant and postpartum women during the pandemic, to temporary restrictions that were in place during the early part of the pandemic with respect to performing infertility procedures, to the practical issues of limiting the number of people who could attend to women during labor and delivery in the hospital.
Five months later, we’ve learned a great deal about trying to sustain emotional well-being among pregnant women during COVID-19. There is a high rate of anxiety among women who are pregnant and women who have particularly young children around the various issues of juggling activities of daily living during the pandemic, including switching to remote work and homeschooling children. There is fear of contracting COVID-19 during pregnancy, the exact effects of which are still somewhat unknown. We have seen a shift to telemedicine for prenatal and postpartum obstetrics visits, and a change with respect to visitors and even in-home nurses that would help during the first weeks of life for some couples.
We wondered whether we would see a falloff in the numbers of women presenting to our clinic with questions about the reproductive safety of taking psychiatric medications during pregnancy. We were unclear as to whether women would defer plans to get pregnant given some of the uncertainties that have come with COVID-19. What we’ve seen, at least early on in the pandemic in Massachusetts, has been the opposite. More women during the first 4 months of the pandemic have been seen in our center compared with the same corresponding period over the last 5 years. The precise reasons for this are unclear, but one reason may be that shifting the practice of reproductive psychiatry and pregnancy planning for reproductive-age women to full virtual care has dropped the number of missed appointments to essentially zero. Women perhaps feel an urgency to have a plan for using psychiatric medication during pregnancy. They may also see the benefit of being able to have extended telemedicine consultations that frequently involve their partners, a practice we have always supported, but posed logistical challenges for some.
As our colleagues learned that we had shifted our clinical rounds at the Center for Women’s Mental Health, which we’ve been doing for 25 years, to a virtual format, we began offering a free 1-hour forum to discuss relevant issues around caring for psychiatrically ill women, with a focus on some of the issues that were particularly relevant during the pandemic. The most common reasons for consultation on our service are the appropriate, safest use of antidepressants and mood stabilizers during pregnancy, and that continues to be the case.
If there has been one guiding principle in treating perinatal depression during pregnancy, it has been our long-standing, laser-like focus on keeping women emotionally well during pregnancy, and to highlight the importance of this with women during consultations prior to and during pregnancy. Relapse of psychiatric disorder during pregnancy is one the strongest predictors of postpartum depression, and the impact of untreated depression during pregnancy has been described in the literature and over the years in this column. However, where we want to minimize, if possible, severe onset of illness requiring hospitalization or emergent attention considering it may make social distancing and some of the other mitigating factors vis-à-vis COVID-19 more challenging.
Despite the accumulated data over the last 2 decades on the reproductive safety of antidepressants, women continue to have questions about the safety of these medications during pregnancy. Studies show now that many women would prefer, if at all possible, to defer treatment with antidepressants, and so they come to us with questions about their reproductive safety, the potential of switching to nonpharmacologic interventions, and the use of alternative interventions that might be used to treat their underlying mood disorder.
Investigators at the University of British Columbia recently have tried to inform the field with still another look, not at reproductive safety per se, but at risk of relapse of depression if women discontinue those medicines during pregnancy.1 There is a timeliness to this investigation, which was a systematic review and meta-analysis of studies that met a priori criteria for inclusion. Since some of our own group’s early work over 15 years ago on relapse of psychiatric disorder during pregnancy,2 which indicated a substantial difference in risk of relapse between women who continued versus who discontinued antidepressants, other investigators have showed the difference in risk for relapse is not as substantial, and that continuation of medication did not appear to mitigate risk for relapse. In fact, in the systematic review, the investigators demonstrated that as a group, maintaining medicine did not appear to confer particular benefit to patients relative to risk for relapse compared to discontinuation of antidepressants.
However, looking more closely, Bayrampour and colleagues note for women with histories of more severe recurrent, major depression, relapse did in fact appear to be greater in women who discontinued compared with those with cases of mild to moderate depression. It is noteworthy that in both our early and later work, and certainly dovetailing with our clinical practice, we have noted severity of illness does not appear to correlate with the actual decisions women ultimately make regarding what they will do with antidepressants. Specifically, some women with very severe illness histories will discontinue antidepressants regardless of their risk for relapse. Alternatively, women with mild to moderate illness will sometimes elect to stay on antidepressant therapy. With all the information that we have about fetal exposure to antidepressants on one hand, the “unknown unknowns” are an understandable concern to both patients and clinicians. Clinicians are faced with the dilemma of how to best counsel women on continuing or discontinuing antidepressants as they plan to conceive or during pregnancy and in the postpartum period.
The literature cited and clinical experience over the last 3 decades suggests rather strongly that there is a relatively low likelihood women with histories of severe recurrent disease will be able to successfully discontinue antidepressants in the absence of relapse. A greater question is, what is the best way to proceed for women who have been on maintenance therapy and had more moderate symptoms?
I am inspired by some of the more recent literature that has tried to elucidate the role of nonpharmacologic interventions such as mindfulness-based cognitive therapy (MBCT) in an effort to mitigate risk for depressive relapse in pregnant women who are well with histories of depression. To date, data do not inform the question as to whether MBCT can be used to mitigate risk of depressive relapse in pregnant women who continue or discontinue antidepressants. That research question is actively being studied by several investigators, including ourselves.
Of particular interest is whether the addition of mindfulness practices such as MBCT in treatment could mitigate risk for depressive relapse in pregnant women who continue or discontinue antidepressant treatment, as that would certainly be a no-harm intervention that could mitigate risk even in a lower risk sample of patients. The question of how to “thread the needle” during the pandemic and best approach woman with a history of recurrent major depression on antidepressants is particularly timely and critical.
Regardless, we make clinical decisions collaboratively with patients based on their histories and individual wishes, and perhaps what we have learned over the last 5 months is the use of telemedicine does afford us the opportunity, regardless of the decisions that patients make, to more closely follow the clinical trajectory of women during pregnancy and the postpartum period so that regardless of treatment, we have an opportunity to intervene early when needed and to ascertain changes in clinical status early to mitigate the risk of frank relapse. From a reproductive psychiatric point of view, that is a silver lining with respect to the associated challenges that have come along with the pandemic.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
References
1. J Clin Psychiatry 2020;81(4):19r13134.
2. JAMA. 2006 Feb 1;295(5):499-507.
FDA approves point-of-care COVID-19 antigen test
The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.
COVID-19 vaccine supply will be limited at first, ACIP says
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
Asymptomatic SARS-CoV-2 infections in kids tied to local rates
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Convalescent plasma actions spark trial recruitment concerns
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
Prognosis for rural hospitals worsens with pandemic
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.