User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Children rarely transmit SARS-CoV-2 within households
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
“Unlike with other viral respiratory infections, children do not seem to be a major vector of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, with most pediatric cases described inside familial clusters and no documentation of child-to-child or child-to-adult transmission,” said Klara M. Posfay-Barbe, MD, of the University of Geneva, Switzerland, and colleagues.
In a study published in Pediatrics, the researchers analyzed data from all COVID-19 patients younger than 16 years who were identified between March 10, 2020, and April 10, 2020, through a hospital surveillance network. Parents and household contacts were called for contact tracing.
In 31 of 39 (79%) households, at least one adult family member had a suspected or confirmed SARS-CoV-2 infection before onset of symptoms in the child. These findings support data from previous studies suggesting that children mainly become infected from adult family members rather than transmitting the virus to them, the researchers said
In only 3 of 39 (8%) households was the study child the first to develop symptoms. “Surprisingly, in 33% of households, symptomatic HHCs [household contacts] tested negative despite belonging to a familial cluster with confirmed SARS-CoV-2 cases, suggesting an underreporting of cases,” Dr. Posfay-Barbe and associates noted.
The findings were limited by several factors including potential underreporting of cases because those with mild or atypical presentations may not have sought medical care, and the inability to confirm child-to-adult transmission. The results were strengthened by the extensive contact tracing and very few individuals lost to follow-up, they said; however, more diagnostic screening and contact tracing are needed to improve understanding of household transmission of SARS-CoV-2, they concluded.
Resolving the issue of how much children contribute to transmission of SARS-CoV-2 is essential to making informed decisions about public health, including how to structure schools and child-care facility reopening, Benjamin Lee, MD, and William V. Raszka Jr., MD, both of the University of Vermont, Burlington, said in an accompanying editorial (Pediatrics. 2020 Jul 10. doi: 10.1542/peds/2020-004879).
The data in the current study support other studies of transmission among household contacts in China suggesting that, in most cases of childhood infections, “the child was not the source of infection and that children most frequently acquire COVID-19 from adults, rather than transmitting it to them,” they wrote.
In addition, the limited data on transmission of SARS-CoV-2 by children outside of the household show few cases of secondary infection from children identified with SARS-CoV-2 in school settings in studies from France and Australia, Dr. Lee and Dr. Raszka noted.
the editorialists wrote. “This would be another manner by which SARS-CoV2 differs drastically from influenza, for which school-based transmission is well recognized as a significant driver of epidemic disease and forms the basis for most evidence regarding school closures as public health strategy.”
“Therefore, serious consideration should be paid toward strategies that allow schools to remain open, even during periods of COVID-19 spread,” the editorialists concluded. “In doing so, we could minimize the potentially profound adverse social, developmental, and health costs that our children will continue to suffer until an effective treatment or vaccine can be developed and distributed or, failing that, until we reach herd immunity,” Dr. Lee and Dr. Raszka emphasized.
The study received no outside funding. The researchers and editorialists had no financial conflicts to disclose.
SOURCE: Posfay-Barbe KM et al. Pediatrics. 2020 Jul 10. doi: 10.1542/peds.2020-1576.
FROM PEDIATRICS
Myocarditis in COVID-19: An elusive cardiac complication
The COVID-19 literature has been peppered with reports about myocarditis accompanying the disease. If true, this could, in part, explain some of the observed cardiac injury and arrhythmias in seriously ill patients, but also have implications for prognosis.
But endomyocardial biopsies and autopsies, the gold-standard confirmation tests, have been few and far between.
Predictors of death in COVID-19 are older age, cardiovascular comorbidities, and elevated troponin or NT-proBNP – none of which actually fit well with the epidemiology of myocarditis due to other causes, Alida L.P. Caforio, MD, of Padua (Italy) University said in an interview. Myocarditis is traditionally a disease of the young, and most cases are immune-mediated and do not release troponin.
Moreover, myocarditis is a diagnosis of exclusion. For it to be made with any certainty requires proof, by biopsy or autopsy, of inflammatory infiltrates within the myocardium with myocyte necrosis not typical of myocardial infarction, said Dr. Caforio, who chaired the European Society of Cardiology’s writing committee for its 2013 position statement on myocardial and pericardial diseases.
“We have one biopsy-proven case, and in this case there were no viruses in the myocardium, including COVID-19,” she said. “There’s no proof that we have COVID-19 causing myocarditis because it has not been found in the cardiomyocytes.”
Emerging evidence
The virus-negative case from Lombardy, Italy, followed an early case series suggesting fulminant myocarditis was involved in 7% of COVID-related deaths in Wuhan, China.
Other case reports include cardiac magnetic resonance (CMR) findings typical of acute myocarditis in a man with no lung involvement or fever but a massive troponin spike, and myocarditis presenting as reverse takotsubo syndrome in a woman undergoing CMR and endomyocardial biopsy.
A CMR analysis in May said acute myocarditis, by 2018 Lake Louise Criteria, was present in eight of 10 patients with “myocarditis-like syndrome,” and a study just out June 30 said the coronavirus can infect heart cells in a lab dish.
Among the few autopsy series, a preprint on 12 patients with COVID-19 in the Seattle area showed coronavirus in the heart tissue of 1 patient.
“It was a low level, so there’s the possibility that it could be viremia, but the fact we do see actual cardiomyocyte injury associated with inflammation, that’s a myocarditis pattern. So it could be related to the SARS-CoV-2 virus,” said Desiree Marshall, MD, director of autopsy and after-death services, University of Washington Medical Center, Seattle.
The “waters are a little bit muddy,” however, because the patient had a coinfection clinically with influenza and methicillin-susceptible Staphylococcus aureus, which raises the specter that influenza could also have contributed, she said.
Data pending publication from two additional patients show no coronavirus in the heart. Acute respiratory distress syndrome pathology was common in all patients, but there was no evidence of vascular inflammation, such as endotheliitis, Dr. Marshall said.
SARS-CoV-2 cell entry depends on the angiotensin-converting enzyme 2 (ACE2) receptor, which is widely expressed in the heart and on endothelial cells and is linked to inflammatory activation. Autopsy data from three COVID-19 patients showed endothelial cell infection in the heart and diffuse endothelial inflammation, but no sign of lymphocytic myocarditis.
Defining myocarditis
“There are some experts who believe we’re likely still dealing with myocarditis but with atypical features, while others suggest there is no myocarditis by strict classic criteria,” said Peter Liu, MD, chief scientific officer/vice president of research, University of Ottawa Heart Institute.
“I don’t think either extreme is accurate,” he said. “The truth is likely somewhere in between, with evidence of both cardiac injury and inflammation. But nothing in COVID-19, as we know today, is classic; it’s a new disease, so we need to be more open minded as new data emerge.”
Part of the divide may indeed stem from the way myocarditis is defined. “Based on traditional Dallas criteria, classic myocarditis requires evidence of myocyte necrosis, which we have, but also inflammatory cell infiltrate, which we don’t consistently have,” he said. “But on the other hand, there is evidence of inflammation-induced cardiac damage, often aggregated around blood vessels.”
The situation is evolving in recent days, and new data under review demonstrated inflammatory infiltrates, which fits the traditional myocarditis criteria, Dr. Liu noted. Yet the viral etiology for the inflammation is still elusive in definitive proof.
In traditional myocarditis, there is an abundance of lymphocytes and foci of inflammation in the myocardium, but COVID-19 is very unusual, in that these lymphocytes are not as exuberant, he said. Lymphopenia or low lymphocyte counts occur in up to 80% of patients. Also, older patients, who initially made up the bulk of the severe COVID-19 cases, are less T-lymphocyte responsive.
“So the lower your lymphocyte count, the worse your outcome is going to be and the more likely you’re going to get cytokine storm,” Dr. Liu said. “And that may be the reason the suspected myocarditis in COVID-19 is atypical because the lymphocytes, in fact, are being suppressed and there is instead more vasculitis.”
Recent data from myocardial gene expression analysis showed that the viral receptor ACE2 is present in the myocardium, and can be upregulated in conditions such as heart failure, he said. However, the highest ACE2 expression is found in pericytes around blood vessels, not myocytes. “This may explain the preferential vascular involvement often observed.”
Cardiac damage in the young
Evidence started evolving in early April that young COVID-19 patients without lung disease, generally in their 20s and 30s, can have very high troponin peaks and a form of cardiac damage that does not appear to be related to sepsis, systemic shock, or cytokine storm.
“That’s the group that I do think has some myocarditis, but it’s different. It’s not lymphocytic myocarditis, like enteroviral myocarditis,” Leslie T. Cooper Jr., MD, a myocarditis expert at Mayo Clinic, Jacksonville, Florida, said in an interview.
“The data to date suggest that most SARS cardiac injury is related to stress or high circulating cytokine levels. However, myocarditis probably does affect some patients, he added. “The few published cases suggest a role for macrophages or endothelial cells, which could affect cardiac myocyte function. This type of injury could cause the ST-segment elevation MI-like patterns we have seen in young people with normal epicardial coronary arteries.”
Dr. Cooper, who coauthored a report on the management of COVID-19 cardiovascular syndrome, pointed out that it’s been hard for researchers to isolate genome from autopsy samples because of RNA degradation prior to autopsy and the use of formalin fixation for tissues prior to RNA extraction.
“Most labs are not doing next-generation sequencing, and even with that, RNA protection and fresh tissue may be required to detect viral genome,” he said.
No proven therapy
Although up to 50% of acute myocarditis cases undergo spontaneous healing, recognition and multidisciplinary management of clinically suspected myocarditis is important. The optimal treatment remains unclear.
An early case report suggested use of methylprednisolone and intravenous immunoglobulin helped spare the life of a 37-year-old with clinically suspected fulminant myocarditis with cardiogenic shock.
In a related commentary, Dr. Caforio and colleagues pointed out that the World Health Organization considers the use of IV corticosteroids controversial, even in pneumonia due to COVID-19, because it may reduce viral clearance and increase sepsis risk. Intravenous immunoglobulin is also questionable because there is no IgG response to COVID-19 in the plasma donors’ pool.
“Immunosuppression should be reserved for only virus-negative non-COVID myocarditis,” Dr. Caforio said in an interview. “There is no appropriate treatment nowadays for clinically suspected COVID-19 myocarditis. There is no proven therapy for COVID-19, even less for COVID-19 myocarditis.”
Although definitive publication of the RECOVERY trial is still pending, the benefits of dexamethasone – a steroid that works predominantly through its anti-inflammatory effects – appear to be in the sickest patients, such as those requiring ICU admission or respiratory support.
“Many of the same patients would have systemic inflammation and would have also shown elevated cardiac biomarkers,” Dr. Liu observed. “Therefore, it is conceivable that a subset who had cardiac inflammation also benefited from the treatment. Further data, possibly through subgroup analysis and eventually meta-analysis, may help us to understand if dexamethasone also benefited patients with dominant cardiac injury.”
Dr. Caforio, Dr. Marshall, Dr. Liu, and Dr. Cooper reported having no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
The COVID-19 literature has been peppered with reports about myocarditis accompanying the disease. If true, this could, in part, explain some of the observed cardiac injury and arrhythmias in seriously ill patients, but also have implications for prognosis.
But endomyocardial biopsies and autopsies, the gold-standard confirmation tests, have been few and far between.
Predictors of death in COVID-19 are older age, cardiovascular comorbidities, and elevated troponin or NT-proBNP – none of which actually fit well with the epidemiology of myocarditis due to other causes, Alida L.P. Caforio, MD, of Padua (Italy) University said in an interview. Myocarditis is traditionally a disease of the young, and most cases are immune-mediated and do not release troponin.
Moreover, myocarditis is a diagnosis of exclusion. For it to be made with any certainty requires proof, by biopsy or autopsy, of inflammatory infiltrates within the myocardium with myocyte necrosis not typical of myocardial infarction, said Dr. Caforio, who chaired the European Society of Cardiology’s writing committee for its 2013 position statement on myocardial and pericardial diseases.
“We have one biopsy-proven case, and in this case there were no viruses in the myocardium, including COVID-19,” she said. “There’s no proof that we have COVID-19 causing myocarditis because it has not been found in the cardiomyocytes.”
Emerging evidence
The virus-negative case from Lombardy, Italy, followed an early case series suggesting fulminant myocarditis was involved in 7% of COVID-related deaths in Wuhan, China.
Other case reports include cardiac magnetic resonance (CMR) findings typical of acute myocarditis in a man with no lung involvement or fever but a massive troponin spike, and myocarditis presenting as reverse takotsubo syndrome in a woman undergoing CMR and endomyocardial biopsy.
A CMR analysis in May said acute myocarditis, by 2018 Lake Louise Criteria, was present in eight of 10 patients with “myocarditis-like syndrome,” and a study just out June 30 said the coronavirus can infect heart cells in a lab dish.
Among the few autopsy series, a preprint on 12 patients with COVID-19 in the Seattle area showed coronavirus in the heart tissue of 1 patient.
“It was a low level, so there’s the possibility that it could be viremia, but the fact we do see actual cardiomyocyte injury associated with inflammation, that’s a myocarditis pattern. So it could be related to the SARS-CoV-2 virus,” said Desiree Marshall, MD, director of autopsy and after-death services, University of Washington Medical Center, Seattle.
The “waters are a little bit muddy,” however, because the patient had a coinfection clinically with influenza and methicillin-susceptible Staphylococcus aureus, which raises the specter that influenza could also have contributed, she said.
Data pending publication from two additional patients show no coronavirus in the heart. Acute respiratory distress syndrome pathology was common in all patients, but there was no evidence of vascular inflammation, such as endotheliitis, Dr. Marshall said.
SARS-CoV-2 cell entry depends on the angiotensin-converting enzyme 2 (ACE2) receptor, which is widely expressed in the heart and on endothelial cells and is linked to inflammatory activation. Autopsy data from three COVID-19 patients showed endothelial cell infection in the heart and diffuse endothelial inflammation, but no sign of lymphocytic myocarditis.
Defining myocarditis
“There are some experts who believe we’re likely still dealing with myocarditis but with atypical features, while others suggest there is no myocarditis by strict classic criteria,” said Peter Liu, MD, chief scientific officer/vice president of research, University of Ottawa Heart Institute.
“I don’t think either extreme is accurate,” he said. “The truth is likely somewhere in between, with evidence of both cardiac injury and inflammation. But nothing in COVID-19, as we know today, is classic; it’s a new disease, so we need to be more open minded as new data emerge.”
Part of the divide may indeed stem from the way myocarditis is defined. “Based on traditional Dallas criteria, classic myocarditis requires evidence of myocyte necrosis, which we have, but also inflammatory cell infiltrate, which we don’t consistently have,” he said. “But on the other hand, there is evidence of inflammation-induced cardiac damage, often aggregated around blood vessels.”
The situation is evolving in recent days, and new data under review demonstrated inflammatory infiltrates, which fits the traditional myocarditis criteria, Dr. Liu noted. Yet the viral etiology for the inflammation is still elusive in definitive proof.
In traditional myocarditis, there is an abundance of lymphocytes and foci of inflammation in the myocardium, but COVID-19 is very unusual, in that these lymphocytes are not as exuberant, he said. Lymphopenia or low lymphocyte counts occur in up to 80% of patients. Also, older patients, who initially made up the bulk of the severe COVID-19 cases, are less T-lymphocyte responsive.
“So the lower your lymphocyte count, the worse your outcome is going to be and the more likely you’re going to get cytokine storm,” Dr. Liu said. “And that may be the reason the suspected myocarditis in COVID-19 is atypical because the lymphocytes, in fact, are being suppressed and there is instead more vasculitis.”
Recent data from myocardial gene expression analysis showed that the viral receptor ACE2 is present in the myocardium, and can be upregulated in conditions such as heart failure, he said. However, the highest ACE2 expression is found in pericytes around blood vessels, not myocytes. “This may explain the preferential vascular involvement often observed.”
Cardiac damage in the young
Evidence started evolving in early April that young COVID-19 patients without lung disease, generally in their 20s and 30s, can have very high troponin peaks and a form of cardiac damage that does not appear to be related to sepsis, systemic shock, or cytokine storm.
“That’s the group that I do think has some myocarditis, but it’s different. It’s not lymphocytic myocarditis, like enteroviral myocarditis,” Leslie T. Cooper Jr., MD, a myocarditis expert at Mayo Clinic, Jacksonville, Florida, said in an interview.
“The data to date suggest that most SARS cardiac injury is related to stress or high circulating cytokine levels. However, myocarditis probably does affect some patients, he added. “The few published cases suggest a role for macrophages or endothelial cells, which could affect cardiac myocyte function. This type of injury could cause the ST-segment elevation MI-like patterns we have seen in young people with normal epicardial coronary arteries.”
Dr. Cooper, who coauthored a report on the management of COVID-19 cardiovascular syndrome, pointed out that it’s been hard for researchers to isolate genome from autopsy samples because of RNA degradation prior to autopsy and the use of formalin fixation for tissues prior to RNA extraction.
“Most labs are not doing next-generation sequencing, and even with that, RNA protection and fresh tissue may be required to detect viral genome,” he said.
No proven therapy
Although up to 50% of acute myocarditis cases undergo spontaneous healing, recognition and multidisciplinary management of clinically suspected myocarditis is important. The optimal treatment remains unclear.
An early case report suggested use of methylprednisolone and intravenous immunoglobulin helped spare the life of a 37-year-old with clinically suspected fulminant myocarditis with cardiogenic shock.
In a related commentary, Dr. Caforio and colleagues pointed out that the World Health Organization considers the use of IV corticosteroids controversial, even in pneumonia due to COVID-19, because it may reduce viral clearance and increase sepsis risk. Intravenous immunoglobulin is also questionable because there is no IgG response to COVID-19 in the plasma donors’ pool.
“Immunosuppression should be reserved for only virus-negative non-COVID myocarditis,” Dr. Caforio said in an interview. “There is no appropriate treatment nowadays for clinically suspected COVID-19 myocarditis. There is no proven therapy for COVID-19, even less for COVID-19 myocarditis.”
Although definitive publication of the RECOVERY trial is still pending, the benefits of dexamethasone – a steroid that works predominantly through its anti-inflammatory effects – appear to be in the sickest patients, such as those requiring ICU admission or respiratory support.
“Many of the same patients would have systemic inflammation and would have also shown elevated cardiac biomarkers,” Dr. Liu observed. “Therefore, it is conceivable that a subset who had cardiac inflammation also benefited from the treatment. Further data, possibly through subgroup analysis and eventually meta-analysis, may help us to understand if dexamethasone also benefited patients with dominant cardiac injury.”
Dr. Caforio, Dr. Marshall, Dr. Liu, and Dr. Cooper reported having no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
The COVID-19 literature has been peppered with reports about myocarditis accompanying the disease. If true, this could, in part, explain some of the observed cardiac injury and arrhythmias in seriously ill patients, but also have implications for prognosis.
But endomyocardial biopsies and autopsies, the gold-standard confirmation tests, have been few and far between.
Predictors of death in COVID-19 are older age, cardiovascular comorbidities, and elevated troponin or NT-proBNP – none of which actually fit well with the epidemiology of myocarditis due to other causes, Alida L.P. Caforio, MD, of Padua (Italy) University said in an interview. Myocarditis is traditionally a disease of the young, and most cases are immune-mediated and do not release troponin.
Moreover, myocarditis is a diagnosis of exclusion. For it to be made with any certainty requires proof, by biopsy or autopsy, of inflammatory infiltrates within the myocardium with myocyte necrosis not typical of myocardial infarction, said Dr. Caforio, who chaired the European Society of Cardiology’s writing committee for its 2013 position statement on myocardial and pericardial diseases.
“We have one biopsy-proven case, and in this case there were no viruses in the myocardium, including COVID-19,” she said. “There’s no proof that we have COVID-19 causing myocarditis because it has not been found in the cardiomyocytes.”
Emerging evidence
The virus-negative case from Lombardy, Italy, followed an early case series suggesting fulminant myocarditis was involved in 7% of COVID-related deaths in Wuhan, China.
Other case reports include cardiac magnetic resonance (CMR) findings typical of acute myocarditis in a man with no lung involvement or fever but a massive troponin spike, and myocarditis presenting as reverse takotsubo syndrome in a woman undergoing CMR and endomyocardial biopsy.
A CMR analysis in May said acute myocarditis, by 2018 Lake Louise Criteria, was present in eight of 10 patients with “myocarditis-like syndrome,” and a study just out June 30 said the coronavirus can infect heart cells in a lab dish.
Among the few autopsy series, a preprint on 12 patients with COVID-19 in the Seattle area showed coronavirus in the heart tissue of 1 patient.
“It was a low level, so there’s the possibility that it could be viremia, but the fact we do see actual cardiomyocyte injury associated with inflammation, that’s a myocarditis pattern. So it could be related to the SARS-CoV-2 virus,” said Desiree Marshall, MD, director of autopsy and after-death services, University of Washington Medical Center, Seattle.
The “waters are a little bit muddy,” however, because the patient had a coinfection clinically with influenza and methicillin-susceptible Staphylococcus aureus, which raises the specter that influenza could also have contributed, she said.
Data pending publication from two additional patients show no coronavirus in the heart. Acute respiratory distress syndrome pathology was common in all patients, but there was no evidence of vascular inflammation, such as endotheliitis, Dr. Marshall said.
SARS-CoV-2 cell entry depends on the angiotensin-converting enzyme 2 (ACE2) receptor, which is widely expressed in the heart and on endothelial cells and is linked to inflammatory activation. Autopsy data from three COVID-19 patients showed endothelial cell infection in the heart and diffuse endothelial inflammation, but no sign of lymphocytic myocarditis.
Defining myocarditis
“There are some experts who believe we’re likely still dealing with myocarditis but with atypical features, while others suggest there is no myocarditis by strict classic criteria,” said Peter Liu, MD, chief scientific officer/vice president of research, University of Ottawa Heart Institute.
“I don’t think either extreme is accurate,” he said. “The truth is likely somewhere in between, with evidence of both cardiac injury and inflammation. But nothing in COVID-19, as we know today, is classic; it’s a new disease, so we need to be more open minded as new data emerge.”
Part of the divide may indeed stem from the way myocarditis is defined. “Based on traditional Dallas criteria, classic myocarditis requires evidence of myocyte necrosis, which we have, but also inflammatory cell infiltrate, which we don’t consistently have,” he said. “But on the other hand, there is evidence of inflammation-induced cardiac damage, often aggregated around blood vessels.”
The situation is evolving in recent days, and new data under review demonstrated inflammatory infiltrates, which fits the traditional myocarditis criteria, Dr. Liu noted. Yet the viral etiology for the inflammation is still elusive in definitive proof.
In traditional myocarditis, there is an abundance of lymphocytes and foci of inflammation in the myocardium, but COVID-19 is very unusual, in that these lymphocytes are not as exuberant, he said. Lymphopenia or low lymphocyte counts occur in up to 80% of patients. Also, older patients, who initially made up the bulk of the severe COVID-19 cases, are less T-lymphocyte responsive.
“So the lower your lymphocyte count, the worse your outcome is going to be and the more likely you’re going to get cytokine storm,” Dr. Liu said. “And that may be the reason the suspected myocarditis in COVID-19 is atypical because the lymphocytes, in fact, are being suppressed and there is instead more vasculitis.”
Recent data from myocardial gene expression analysis showed that the viral receptor ACE2 is present in the myocardium, and can be upregulated in conditions such as heart failure, he said. However, the highest ACE2 expression is found in pericytes around blood vessels, not myocytes. “This may explain the preferential vascular involvement often observed.”
Cardiac damage in the young
Evidence started evolving in early April that young COVID-19 patients without lung disease, generally in their 20s and 30s, can have very high troponin peaks and a form of cardiac damage that does not appear to be related to sepsis, systemic shock, or cytokine storm.
“That’s the group that I do think has some myocarditis, but it’s different. It’s not lymphocytic myocarditis, like enteroviral myocarditis,” Leslie T. Cooper Jr., MD, a myocarditis expert at Mayo Clinic, Jacksonville, Florida, said in an interview.
“The data to date suggest that most SARS cardiac injury is related to stress or high circulating cytokine levels. However, myocarditis probably does affect some patients, he added. “The few published cases suggest a role for macrophages or endothelial cells, which could affect cardiac myocyte function. This type of injury could cause the ST-segment elevation MI-like patterns we have seen in young people with normal epicardial coronary arteries.”
Dr. Cooper, who coauthored a report on the management of COVID-19 cardiovascular syndrome, pointed out that it’s been hard for researchers to isolate genome from autopsy samples because of RNA degradation prior to autopsy and the use of formalin fixation for tissues prior to RNA extraction.
“Most labs are not doing next-generation sequencing, and even with that, RNA protection and fresh tissue may be required to detect viral genome,” he said.
No proven therapy
Although up to 50% of acute myocarditis cases undergo spontaneous healing, recognition and multidisciplinary management of clinically suspected myocarditis is important. The optimal treatment remains unclear.
An early case report suggested use of methylprednisolone and intravenous immunoglobulin helped spare the life of a 37-year-old with clinically suspected fulminant myocarditis with cardiogenic shock.
In a related commentary, Dr. Caforio and colleagues pointed out that the World Health Organization considers the use of IV corticosteroids controversial, even in pneumonia due to COVID-19, because it may reduce viral clearance and increase sepsis risk. Intravenous immunoglobulin is also questionable because there is no IgG response to COVID-19 in the plasma donors’ pool.
“Immunosuppression should be reserved for only virus-negative non-COVID myocarditis,” Dr. Caforio said in an interview. “There is no appropriate treatment nowadays for clinically suspected COVID-19 myocarditis. There is no proven therapy for COVID-19, even less for COVID-19 myocarditis.”
Although definitive publication of the RECOVERY trial is still pending, the benefits of dexamethasone – a steroid that works predominantly through its anti-inflammatory effects – appear to be in the sickest patients, such as those requiring ICU admission or respiratory support.
“Many of the same patients would have systemic inflammation and would have also shown elevated cardiac biomarkers,” Dr. Liu observed. “Therefore, it is conceivable that a subset who had cardiac inflammation also benefited from the treatment. Further data, possibly through subgroup analysis and eventually meta-analysis, may help us to understand if dexamethasone also benefited patients with dominant cardiac injury.”
Dr. Caforio, Dr. Marshall, Dr. Liu, and Dr. Cooper reported having no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
‘Doc, can I get a mask exemption?’
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
As more jurisdictions mandate facial coverings in public, questions have arisen about whether it’s safe for everyone – including those with lung disease – to wear masks.
To address these issues, Medscape spoke with the chief medical officer of the American Lung Association, Dr. Albert Rizzo.
The CDC recommendations on mask wearing say, “Cloth face coverings should not be placed on young children under age 2, anyone who has trouble breathing, or is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.” Does this language suggest that there indeed is a subset of the adult population with lung disease who shouldn’t wear masks?
It makes sense to say that if it makes you uncomfortable to wear a mask because it affects your breathing, you should think twice about getting in a situation where you would have to wear a mask.
I’ve told many of my high-risk patients, “The best way to avoid getting COVID-19 is to stay home and stay away from sick people, especially if you feel that you are not going to be able to wear a mask or facial covering of some sort.”
The reason that some people have trouble with a mask is that they haven’t tried the right style of mask – by that I mean how tightly it fits and the material it’s made out of. Sometimes it really is just that people with lung disease don’t like to have anything covering their faces. Many of these patients feel better where there is air blowing across their faces – they will have a fan blowing even in the middle of winter because they feel more comfortable.
I won’t say it’s all in their heads, but sometimes it’s a matter of desensitizing themselves to wearing a mask. I liken it to people who have sleep apnea. We often have to desensitize them to wearing a mask for sleeping. We tell them to put it on while they are watching TV — don’t hook it up to anything yet, just get used to having something on your face.
I’ve told my patients the same thing about masks for COVID-19. Put on the mask, see how it feels. If you become uncomfortable breathing with it on, take it off, but maybe you can handle it for a half hour or 45 minutes. Find out how much time you have for a trip to the grocery store based on how comfortable you are wearing it at home.
It’s a matter of training the patient, giving them options of how to get comfortable with it, and then making them realize that they have to weigh the benefits and risks of wearing the mask and feeling out of breath versus going out in public and being potentially exposed to coronavirus. And the bottom line is, anybody who is wearing a mask and starts to feel uncomfortable, they can take the mask off.
You mentioned different types of masks. Is there a type of mask that is typically more breathable that clinicians can recommend to patients with lung disease?
First, I remind patients who think they will have trouble breathing with a mask on that they are choosing a mask not so much to protect themselves – that would take an N95 mask to filter out the virus. The mask is worn so that when they cough or drink or speak, they aren’t sending respiratory droplets out into the environment. Even when we speak, respiratory droplets can easily go out as far as 6 feet, or further with coughing or sneezing. With facial coverings, we try to keep those respiratory droplets from getting out and infecting others.
So when choosing a mask, you don’t have to worry as much about a tight-fitting mask. I recommend a loose-fitting mask that covers the nose and mouth and isn’t going to fall off but isn’t so tight around the ears and neck to make them feel uncomfortable. Even though it doesn’t really protect the wearer, it is cutting down on the ability to breathe in droplets – maybe not microscopic particles, but it’s better than nothing.
Is a face shield a reasonable alternative for someone who feels they can’t breathe with a mask on?
Yes. I’m surprised that face shields don’t get more attention. I’ve tried them out, and they are actually more comfortable than masks. They do impede the spilling out of droplets into the public, but they are not as close fitting to the face as a mask. If you want to protect others, the face shield should be adequate. It is not as good at preventing you from breathing in viral particles.
Some people have claimed that wearing a mask makes them hyperventilate and feel like they are going to pass out, or the mask causes them to become hypoxic. Are these valid concerns?
We get two questions about masks from patients who feel that they are short of breath or are worried about wearing a mask. One is whether their oxygen level is dropping. It’s usually not that. It’s usually because they feel that the mask is an impediment to getting air in. Their oxygen levels are stable.
The other question is whether the mask causes CO2 retention. For the mask to trap enough exhaled CO2 and for us to breathe enough of that CO2 back in to raise our CO2 level, it has to be a pretty tight-fitting mask. With the type of masks we are suggesting that people wear, that’s very unlikely to occur.
What can clinicians do to reassure patients with some type of lung disease that they can safely wear masks?
There are a few things they can do right in the office. Have them put the mask on for a few minutes and make sure they feel comfortable with it. With an oximeter, patients can see that their oxygen levels don’t change when they are breathing through the mask for a period of time.
You can’t really measure CO2 retention that easily, but most patients with chronic obstructive pulmonary disease or pulmonary fibrosis don’t have an elevated CO2 at baseline. A little more education is helpful in those situations. In most cases, they aren’t going to retain enough CO2 to have problems wearing a mask.
Only a small percentage of patients with lung disease are CO2 retainers, and many of those patients are being seen by pulmonary specialists. Those are the patients you might want to be more cautious with, to make sure they aren’t wearing anything that is tight fitting or that makes them work harder to breathe. It’s not that the mask is causing CO2 retention, but the increased work of breathing may make it harder to exhale the CO2.
Does a mask interfere with supplemental oxygen in any way?
Supplemental oxygen is typically supplied through a nasal cannula, so 100% oxygen is still getting to the nasal passages and entrained down into the airway, so it shouldn’t be a problem.
Some of the resistance to wearing masks has come from people with asthma. Is it safe for patients with asthma to wear masks, or should these patients be exempt from wearing masks?
In general, the breathing of people with mild asthma, both young and old, should not be impeded by the wearing of facial coverings. The concerns about oxygen and carbon dioxide among patients with more severe lung disease should not play a role in asthma.
Since younger adults with COVID-19 seem to have fewer or no symptoms and may actually be carrying the virus unknowingly, this should be the main population who should wear masks to prevent transmission to others.
Exemptions for mask wearing for mild asthma should be discouraged and dealt with on a case-by-case basis if there is a particular concern for that individual.
How do you respond if a patient asks you for a formal medical exemption to wearing a mask?
We’ve been asked to do a lot of letter writing for patients around going back to work, as well as the issue of wearing masks. The discussion usually revolves around trying to avoid going somewhere where you would have to wear a mask if it makes you feel uncomfortable.
I do not recommend automatically exempting individuals from wearing masks, even many of my pulmonary patients. There needs to be an understanding by the patient regarding the purpose of the mask and the overall advice to stay out of situations where social distancing is not being practiced. If you can take the time to discuss options as mentioned above – mask styles, desensitization, etc – the patient usually understands and will try wearing a mask.
On a case-by-case basis, some individuals may need to be exempted, but I feel this is a small number. I prefer my high-risk (older, chronic disease, etc) patients do everything they can to avoid infection – handwashing, mask wearing, and socially distancing.
They should also realize that even with a note, it is not going to help if they are in the middle of the grocery store and someone confronts them about not wearing a mask. It may help as they enter a store that says “masks required” and they can show it to someone monitoring the door. But I’m not really sure in what situations having that note is going to be helpful if confrontations occur.
Patients are also asking how safe is it for them to go back to work and be out in public. I tell them, nothing is going to be 100% safe. Until we have an effective vaccine, we are all going to have to weigh the potential risks of going to an area where social distancing isn’t maintained, people aren’t wearing face masks, and you can’t wash your hands as much as you’d like to. That’s going to be a struggle for all of us to get back out into situations where people interact socially.
Albert A. Rizzo, MD, is chief medical officer for the American Lung Association, chief of the Section of Pulmonary and Critical Care Medicine at the Christiana Care Health System in Newark, Delaware, and a member of Christiana Care Pulmonary Associates. He is board certified in internal medicine, pulmonary medicine, critical care medicine, and sleep medicine and is a clinical assistant professor of medicine at Thomas Jefferson University Medical School, Philadelphia.
This article first appeared on Medscape.com.
Brensocatib reduced bronchiectasis exacerbations
Brensocatib, an experimental small-molecule inhibitor targeted to inflammation-regulating neutrophil serine proteases, may be a novel, nonantibiotic option for reducing exacerbations in patients with bronchiectasis, investigators in the phase 2 WILLOW study said.
Among 256 adults with a recent history of bronchiectasis exacerbations, oral brensocatib at doses of both 10 mg and 25 mg daily for 24 weeks was associated with significantly longer time to first exacerbation than placebo, and the 10-mg dose was associated with a significant reduction in the annualized rate of exacerbations, reported James Chalmers, MB, ChB, PhD of Ninewells Hospital and Medical School in Dundee (England).
“We also observed a dose-dependent reduction in neutrophil elastase levels in sputum, which supports the mechanism of action of this drug, and importantly showed a link between reducing neutrophil serine protease activity and clinical benefits in people with bronchiectasis,” he said in at the American Thoracic Society’s virtual clinical trial session.
“This is a very important trial, a landmark trial for people with bronchiectasis, because this is a drug that for the first time appears to be able to target directly neutrophilic inflammation, resulting in clinical benefit,” he said.
Pulmonologist Jennifer L. Taylor-Cousar, MD, MSCS, of National Jewish Health in Denver, who was facilitator for the online presentation but was not involved in the study, said that it offered welcome news.
“For those of us who treat bronchiectasis, a safe and effective anti-inflammatory has really been the Holy Grail, so this is really exciting,” she said.
Novel mechanism of action
Frequent exacerbations in bronchiectasis are related to uncontrolled neutrophilic inflammation, and proinflammatory neutrophil serine proteases (NSPs), including neutrophil elastase, are seen at increased levels in sputum of patients with bronchiectasis. In addition, the presence in sputum of elevated NSPs are associated with exacerbations and poor quality of life, Dr. Chalmers said.
Brensocatib is an inhibitor of dipeptidyl peptidase 1 (DPP1), a lysosomal cysteine protease that is responsible for NSP activation in bone marrow during the neutrophil maturation cycle.
In phase 1 trials, brensocatib was associated with a dose-dependent reduction in neutrophil elastase in healthy volunteers.
Three WILLOW branches
In the phase 2 WILLOW trial, patients with bronchiectasis not related to cystic fibrosis were screened and stratified by Pseudomonas aeruginosa on sputum culture and use of macrolide antibiotics and then randomized in equal proportions to receive either brensocatib at daily oral doses of 25 mg or 10 mg, or placebo for 24 weeks, followed by a 4-week off-treatment period.
Both doses of brensocatib met the primary endpoint of time to first exacerbation, compared with placebo. The hazard ratio (HR) for the 10-mg brensocatib dose, compared with placebo was 0.58 (P = .029), and the HR for the 25-mg dose was 0.62 (P = .046).
The exacerbation rate over 24 weeks among patients on placebo was 48.3%, compared with 31.7% of patients on 10 mg brensocatib (P = .033) and 33.3% of patients on the 25 mg dose (P = .038).
The annualized exacerbation rate was 1.37 for patients on placebo, compared with 0.88 with 10 mg brensocatib (P = .041) and 1.03 with 25 mg brensocatib (nonsignificant).
In both brensocatib groups there were significant reductions from baseline neutrophil elastase concentrations in sputum, compared with placebo (P = .034 for 10 mg and .021 for 25 mg). During the 4-week period following treatment neutrophil elastase levels in both active drug arms rose rapidly and returned to baseline.
The importance of these reductions was reflected in pooled data from the two brensocatib cohorts, which showed that patients who achieved neutrophil elastase levels below the limit of quantification had a significantly lower incidence of bronchiectasis exacerbations (HR 0.28, P < .0001).
Although the study was not powered to compare changes in postbronchodilator forced expiratory volume in 1 second (FEV1) levels, placebo-treated patients had a numerically larger decline in lung function from baseline, compared with brensocatib-treated patients.
Safety
Expected adverse events with brensocatib included those associated with Papillon-Lefèvre syndrome, a rare congenital condition caused by the absence of the gene coding for DPP1, resulting in keratinization leading to redness, thickening of soles and palms, and severe, destructive periodontal disease, as well as reduced immune response to bacterial infection.
Treatment-emergent adverse events (TEAEs) resulting in study discontinuation occurred in only three patients on placebo and 10 mg brensocatib, and four on the 25-mg dose. TEAEs resulting in treatment discontinuation were more common in the placebo arm, occurring in nine patients compared with six each in the brensocatib arms.
Serious TEAEs occurring in more than 3% of patients in any group included infective exacerbations in three patients on placebo, none on the 10-mg dose, and four on the 25-mg dose of brensocatib. Respective numbers of patients with treatment-emergent pneumonia were three, zero, and four.
Other TEAEs included cough, headache, sputum increase, dyspnea, and diarrhea.
Adverse events of special interest included skin events in 10 patients on placebo, 12 on the 10-mg dose, and 21 on the 25-mg brensocatib dose. Dental changes occurred in 3, 13, and 9 patients, and infections in 9, 12, and 14 patients, respectively.
A phase 3 study to confirm efficacy and establish the optimal dose of brensocatib is planned for the end of 2020, “COVID willing,” Dr. Chalmers said.
Dr. Chalmers disclosed consultancy with and research funding from Insmed, which funded the study. Dr. Taylor-Cousar has disclosed grants and/or personal fees from various companies.
Brensocatib, an experimental small-molecule inhibitor targeted to inflammation-regulating neutrophil serine proteases, may be a novel, nonantibiotic option for reducing exacerbations in patients with bronchiectasis, investigators in the phase 2 WILLOW study said.
Among 256 adults with a recent history of bronchiectasis exacerbations, oral brensocatib at doses of both 10 mg and 25 mg daily for 24 weeks was associated with significantly longer time to first exacerbation than placebo, and the 10-mg dose was associated with a significant reduction in the annualized rate of exacerbations, reported James Chalmers, MB, ChB, PhD of Ninewells Hospital and Medical School in Dundee (England).
“We also observed a dose-dependent reduction in neutrophil elastase levels in sputum, which supports the mechanism of action of this drug, and importantly showed a link between reducing neutrophil serine protease activity and clinical benefits in people with bronchiectasis,” he said in at the American Thoracic Society’s virtual clinical trial session.
“This is a very important trial, a landmark trial for people with bronchiectasis, because this is a drug that for the first time appears to be able to target directly neutrophilic inflammation, resulting in clinical benefit,” he said.
Pulmonologist Jennifer L. Taylor-Cousar, MD, MSCS, of National Jewish Health in Denver, who was facilitator for the online presentation but was not involved in the study, said that it offered welcome news.
“For those of us who treat bronchiectasis, a safe and effective anti-inflammatory has really been the Holy Grail, so this is really exciting,” she said.
Novel mechanism of action
Frequent exacerbations in bronchiectasis are related to uncontrolled neutrophilic inflammation, and proinflammatory neutrophil serine proteases (NSPs), including neutrophil elastase, are seen at increased levels in sputum of patients with bronchiectasis. In addition, the presence in sputum of elevated NSPs are associated with exacerbations and poor quality of life, Dr. Chalmers said.
Brensocatib is an inhibitor of dipeptidyl peptidase 1 (DPP1), a lysosomal cysteine protease that is responsible for NSP activation in bone marrow during the neutrophil maturation cycle.
In phase 1 trials, brensocatib was associated with a dose-dependent reduction in neutrophil elastase in healthy volunteers.
Three WILLOW branches
In the phase 2 WILLOW trial, patients with bronchiectasis not related to cystic fibrosis were screened and stratified by Pseudomonas aeruginosa on sputum culture and use of macrolide antibiotics and then randomized in equal proportions to receive either brensocatib at daily oral doses of 25 mg or 10 mg, or placebo for 24 weeks, followed by a 4-week off-treatment period.
Both doses of brensocatib met the primary endpoint of time to first exacerbation, compared with placebo. The hazard ratio (HR) for the 10-mg brensocatib dose, compared with placebo was 0.58 (P = .029), and the HR for the 25-mg dose was 0.62 (P = .046).
The exacerbation rate over 24 weeks among patients on placebo was 48.3%, compared with 31.7% of patients on 10 mg brensocatib (P = .033) and 33.3% of patients on the 25 mg dose (P = .038).
The annualized exacerbation rate was 1.37 for patients on placebo, compared with 0.88 with 10 mg brensocatib (P = .041) and 1.03 with 25 mg brensocatib (nonsignificant).
In both brensocatib groups there were significant reductions from baseline neutrophil elastase concentrations in sputum, compared with placebo (P = .034 for 10 mg and .021 for 25 mg). During the 4-week period following treatment neutrophil elastase levels in both active drug arms rose rapidly and returned to baseline.
The importance of these reductions was reflected in pooled data from the two brensocatib cohorts, which showed that patients who achieved neutrophil elastase levels below the limit of quantification had a significantly lower incidence of bronchiectasis exacerbations (HR 0.28, P < .0001).
Although the study was not powered to compare changes in postbronchodilator forced expiratory volume in 1 second (FEV1) levels, placebo-treated patients had a numerically larger decline in lung function from baseline, compared with brensocatib-treated patients.
Safety
Expected adverse events with brensocatib included those associated with Papillon-Lefèvre syndrome, a rare congenital condition caused by the absence of the gene coding for DPP1, resulting in keratinization leading to redness, thickening of soles and palms, and severe, destructive periodontal disease, as well as reduced immune response to bacterial infection.
Treatment-emergent adverse events (TEAEs) resulting in study discontinuation occurred in only three patients on placebo and 10 mg brensocatib, and four on the 25-mg dose. TEAEs resulting in treatment discontinuation were more common in the placebo arm, occurring in nine patients compared with six each in the brensocatib arms.
Serious TEAEs occurring in more than 3% of patients in any group included infective exacerbations in three patients on placebo, none on the 10-mg dose, and four on the 25-mg dose of brensocatib. Respective numbers of patients with treatment-emergent pneumonia were three, zero, and four.
Other TEAEs included cough, headache, sputum increase, dyspnea, and diarrhea.
Adverse events of special interest included skin events in 10 patients on placebo, 12 on the 10-mg dose, and 21 on the 25-mg brensocatib dose. Dental changes occurred in 3, 13, and 9 patients, and infections in 9, 12, and 14 patients, respectively.
A phase 3 study to confirm efficacy and establish the optimal dose of brensocatib is planned for the end of 2020, “COVID willing,” Dr. Chalmers said.
Dr. Chalmers disclosed consultancy with and research funding from Insmed, which funded the study. Dr. Taylor-Cousar has disclosed grants and/or personal fees from various companies.
Brensocatib, an experimental small-molecule inhibitor targeted to inflammation-regulating neutrophil serine proteases, may be a novel, nonantibiotic option for reducing exacerbations in patients with bronchiectasis, investigators in the phase 2 WILLOW study said.
Among 256 adults with a recent history of bronchiectasis exacerbations, oral brensocatib at doses of both 10 mg and 25 mg daily for 24 weeks was associated with significantly longer time to first exacerbation than placebo, and the 10-mg dose was associated with a significant reduction in the annualized rate of exacerbations, reported James Chalmers, MB, ChB, PhD of Ninewells Hospital and Medical School in Dundee (England).
“We also observed a dose-dependent reduction in neutrophil elastase levels in sputum, which supports the mechanism of action of this drug, and importantly showed a link between reducing neutrophil serine protease activity and clinical benefits in people with bronchiectasis,” he said in at the American Thoracic Society’s virtual clinical trial session.
“This is a very important trial, a landmark trial for people with bronchiectasis, because this is a drug that for the first time appears to be able to target directly neutrophilic inflammation, resulting in clinical benefit,” he said.
Pulmonologist Jennifer L. Taylor-Cousar, MD, MSCS, of National Jewish Health in Denver, who was facilitator for the online presentation but was not involved in the study, said that it offered welcome news.
“For those of us who treat bronchiectasis, a safe and effective anti-inflammatory has really been the Holy Grail, so this is really exciting,” she said.
Novel mechanism of action
Frequent exacerbations in bronchiectasis are related to uncontrolled neutrophilic inflammation, and proinflammatory neutrophil serine proteases (NSPs), including neutrophil elastase, are seen at increased levels in sputum of patients with bronchiectasis. In addition, the presence in sputum of elevated NSPs are associated with exacerbations and poor quality of life, Dr. Chalmers said.
Brensocatib is an inhibitor of dipeptidyl peptidase 1 (DPP1), a lysosomal cysteine protease that is responsible for NSP activation in bone marrow during the neutrophil maturation cycle.
In phase 1 trials, brensocatib was associated with a dose-dependent reduction in neutrophil elastase in healthy volunteers.
Three WILLOW branches
In the phase 2 WILLOW trial, patients with bronchiectasis not related to cystic fibrosis were screened and stratified by Pseudomonas aeruginosa on sputum culture and use of macrolide antibiotics and then randomized in equal proportions to receive either brensocatib at daily oral doses of 25 mg or 10 mg, or placebo for 24 weeks, followed by a 4-week off-treatment period.
Both doses of brensocatib met the primary endpoint of time to first exacerbation, compared with placebo. The hazard ratio (HR) for the 10-mg brensocatib dose, compared with placebo was 0.58 (P = .029), and the HR for the 25-mg dose was 0.62 (P = .046).
The exacerbation rate over 24 weeks among patients on placebo was 48.3%, compared with 31.7% of patients on 10 mg brensocatib (P = .033) and 33.3% of patients on the 25 mg dose (P = .038).
The annualized exacerbation rate was 1.37 for patients on placebo, compared with 0.88 with 10 mg brensocatib (P = .041) and 1.03 with 25 mg brensocatib (nonsignificant).
In both brensocatib groups there were significant reductions from baseline neutrophil elastase concentrations in sputum, compared with placebo (P = .034 for 10 mg and .021 for 25 mg). During the 4-week period following treatment neutrophil elastase levels in both active drug arms rose rapidly and returned to baseline.
The importance of these reductions was reflected in pooled data from the two brensocatib cohorts, which showed that patients who achieved neutrophil elastase levels below the limit of quantification had a significantly lower incidence of bronchiectasis exacerbations (HR 0.28, P < .0001).
Although the study was not powered to compare changes in postbronchodilator forced expiratory volume in 1 second (FEV1) levels, placebo-treated patients had a numerically larger decline in lung function from baseline, compared with brensocatib-treated patients.
Safety
Expected adverse events with brensocatib included those associated with Papillon-Lefèvre syndrome, a rare congenital condition caused by the absence of the gene coding for DPP1, resulting in keratinization leading to redness, thickening of soles and palms, and severe, destructive periodontal disease, as well as reduced immune response to bacterial infection.
Treatment-emergent adverse events (TEAEs) resulting in study discontinuation occurred in only three patients on placebo and 10 mg brensocatib, and four on the 25-mg dose. TEAEs resulting in treatment discontinuation were more common in the placebo arm, occurring in nine patients compared with six each in the brensocatib arms.
Serious TEAEs occurring in more than 3% of patients in any group included infective exacerbations in three patients on placebo, none on the 10-mg dose, and four on the 25-mg dose of brensocatib. Respective numbers of patients with treatment-emergent pneumonia were three, zero, and four.
Other TEAEs included cough, headache, sputum increase, dyspnea, and diarrhea.
Adverse events of special interest included skin events in 10 patients on placebo, 12 on the 10-mg dose, and 21 on the 25-mg brensocatib dose. Dental changes occurred in 3, 13, and 9 patients, and infections in 9, 12, and 14 patients, respectively.
A phase 3 study to confirm efficacy and establish the optimal dose of brensocatib is planned for the end of 2020, “COVID willing,” Dr. Chalmers said.
Dr. Chalmers disclosed consultancy with and research funding from Insmed, which funded the study. Dr. Taylor-Cousar has disclosed grants and/or personal fees from various companies.
FROM ATS 2020
WHO plans to address airborne COVID-19 transmission
WHO will likely address airborne transmission of the virus after a commentary from almost 240 multidisciplinary scientists raised the alarm that virus particles could remain airborne longer that previously appreciated, particularly in poorly ventilated indoor spaces.
“Airborne route of infection transmission is significant, but so far completely undermined, and not recognized by the decision makers and bodies responsible for infection control,” lead commentary author Lidia Morawska, PhD, told Medscape Medical News.
“This means that no control measures are taken to mitigate airborne transmission and, as a consequence, people are infected and can die,” said Morawska, director of the International Laboratory for Air Quality and Health at Queensland University of Technology in Brisbane, Australia. “We wanted to bring this to the attention of the world to prevent this from happening.”
The commentary was published July 6 in Clinical Infectious Diseases.
WHO leaders defended their progress in announcing any changes regarding how COVID-19 can be transmitted during a virtual press briefing today. They have collaborated since April with some of the scientists who coauthored the commentary, for example, said Maria Van Kerkhove, PhD, WHO technical lead on COVID-19.
“We have been working on a scientific brief ... to consolidate knowledge around transmission,” she added.
One focus will be on how masks protect healthcare workers. “We are also looking at the possible role of airborne transmission in other settings,” Van Kerkhove said. “We will be releasing our brief in the coming days.”
“We acknowledge there is emerging evidence in this field,” Benedetta Allegranzi, MD, WHO technical lead on COVID-19, said during the briefing from Geneva. “Therefore, we believe we have to be open to this evidence and its implications.”
WHO participated in an international research meeting last week that addressed means for controlling modes of COVID-19 transmission, Allegranzi said. “Our group and others really highlighted importance of research on different modes of transmission, including droplets of different sizes and their relative importance,” she said. Another aim was determining the dose of the virus required for airborne transmission.
“These fields of research are really growing but not definitive. More evidence needs to be gathered and evaluated,” she explained.
In the meantime, Allegranzi said, “the possibility of airborne transmission in public settings – especially closed, poorly ventilated settings – cannot be ruled out.”
Morawska said the evidence already exists. “A continuous surprise is that it takes the world such a long time to accept this, while this has such solid scientific foundation.” As an example, she cited an April report she coauthored in the journal Environment International. She and colleagues call for “national authorities to acknowledge the reality that the virus spreads through air and recommend that adequate control measures be implemented to prevent further spread of the SARS-CoV-2 virus, in particularly removal of the virus-laden droplets from indoor air by ventilation.”
The take-home message from the commentary, Morawska said, is a call to action. The authors state there is a need “to provide sufficient and effective ventilation (supply clean outdoor air, minimize recirculating air) particularly in public buildings, workplace environments, schools, hospitals, and aged care homes.”
WHO Chief Scientist Soumya Swaminathan, MD, explained why the organization remains cautious about making premature pronouncements regarding airborne transmission. “Any guidance we put out has implications for billions of people around the world, so we want to be as careful as possible,” she said during the press briefing. “We have to consider the weight of the evidence.”
“We are constantly looking for information on how we can do better,” Swaminathan added. WHO officials are reviewing hundreds of scientific reports every day, she said, and not all are of good quality. For this reason, she and other scientists at WHO perform a “living systematic review” – updating the consensus of evidence on a weekly basis.
“This process on COVID-19 will, I am sure, continue for the weeks and months to come,” she added.
This article first appeared on Medscape.com.
WHO will likely address airborne transmission of the virus after a commentary from almost 240 multidisciplinary scientists raised the alarm that virus particles could remain airborne longer that previously appreciated, particularly in poorly ventilated indoor spaces.
“Airborne route of infection transmission is significant, but so far completely undermined, and not recognized by the decision makers and bodies responsible for infection control,” lead commentary author Lidia Morawska, PhD, told Medscape Medical News.
“This means that no control measures are taken to mitigate airborne transmission and, as a consequence, people are infected and can die,” said Morawska, director of the International Laboratory for Air Quality and Health at Queensland University of Technology in Brisbane, Australia. “We wanted to bring this to the attention of the world to prevent this from happening.”
The commentary was published July 6 in Clinical Infectious Diseases.
WHO leaders defended their progress in announcing any changes regarding how COVID-19 can be transmitted during a virtual press briefing today. They have collaborated since April with some of the scientists who coauthored the commentary, for example, said Maria Van Kerkhove, PhD, WHO technical lead on COVID-19.
“We have been working on a scientific brief ... to consolidate knowledge around transmission,” she added.
One focus will be on how masks protect healthcare workers. “We are also looking at the possible role of airborne transmission in other settings,” Van Kerkhove said. “We will be releasing our brief in the coming days.”
“We acknowledge there is emerging evidence in this field,” Benedetta Allegranzi, MD, WHO technical lead on COVID-19, said during the briefing from Geneva. “Therefore, we believe we have to be open to this evidence and its implications.”
WHO participated in an international research meeting last week that addressed means for controlling modes of COVID-19 transmission, Allegranzi said. “Our group and others really highlighted importance of research on different modes of transmission, including droplets of different sizes and their relative importance,” she said. Another aim was determining the dose of the virus required for airborne transmission.
“These fields of research are really growing but not definitive. More evidence needs to be gathered and evaluated,” she explained.
In the meantime, Allegranzi said, “the possibility of airborne transmission in public settings – especially closed, poorly ventilated settings – cannot be ruled out.”
Morawska said the evidence already exists. “A continuous surprise is that it takes the world such a long time to accept this, while this has such solid scientific foundation.” As an example, she cited an April report she coauthored in the journal Environment International. She and colleagues call for “national authorities to acknowledge the reality that the virus spreads through air and recommend that adequate control measures be implemented to prevent further spread of the SARS-CoV-2 virus, in particularly removal of the virus-laden droplets from indoor air by ventilation.”
The take-home message from the commentary, Morawska said, is a call to action. The authors state there is a need “to provide sufficient and effective ventilation (supply clean outdoor air, minimize recirculating air) particularly in public buildings, workplace environments, schools, hospitals, and aged care homes.”
WHO Chief Scientist Soumya Swaminathan, MD, explained why the organization remains cautious about making premature pronouncements regarding airborne transmission. “Any guidance we put out has implications for billions of people around the world, so we want to be as careful as possible,” she said during the press briefing. “We have to consider the weight of the evidence.”
“We are constantly looking for information on how we can do better,” Swaminathan added. WHO officials are reviewing hundreds of scientific reports every day, she said, and not all are of good quality. For this reason, she and other scientists at WHO perform a “living systematic review” – updating the consensus of evidence on a weekly basis.
“This process on COVID-19 will, I am sure, continue for the weeks and months to come,” she added.
This article first appeared on Medscape.com.
WHO will likely address airborne transmission of the virus after a commentary from almost 240 multidisciplinary scientists raised the alarm that virus particles could remain airborne longer that previously appreciated, particularly in poorly ventilated indoor spaces.
“Airborne route of infection transmission is significant, but so far completely undermined, and not recognized by the decision makers and bodies responsible for infection control,” lead commentary author Lidia Morawska, PhD, told Medscape Medical News.
“This means that no control measures are taken to mitigate airborne transmission and, as a consequence, people are infected and can die,” said Morawska, director of the International Laboratory for Air Quality and Health at Queensland University of Technology in Brisbane, Australia. “We wanted to bring this to the attention of the world to prevent this from happening.”
The commentary was published July 6 in Clinical Infectious Diseases.
WHO leaders defended their progress in announcing any changes regarding how COVID-19 can be transmitted during a virtual press briefing today. They have collaborated since April with some of the scientists who coauthored the commentary, for example, said Maria Van Kerkhove, PhD, WHO technical lead on COVID-19.
“We have been working on a scientific brief ... to consolidate knowledge around transmission,” she added.
One focus will be on how masks protect healthcare workers. “We are also looking at the possible role of airborne transmission in other settings,” Van Kerkhove said. “We will be releasing our brief in the coming days.”
“We acknowledge there is emerging evidence in this field,” Benedetta Allegranzi, MD, WHO technical lead on COVID-19, said during the briefing from Geneva. “Therefore, we believe we have to be open to this evidence and its implications.”
WHO participated in an international research meeting last week that addressed means for controlling modes of COVID-19 transmission, Allegranzi said. “Our group and others really highlighted importance of research on different modes of transmission, including droplets of different sizes and their relative importance,” she said. Another aim was determining the dose of the virus required for airborne transmission.
“These fields of research are really growing but not definitive. More evidence needs to be gathered and evaluated,” she explained.
In the meantime, Allegranzi said, “the possibility of airborne transmission in public settings – especially closed, poorly ventilated settings – cannot be ruled out.”
Morawska said the evidence already exists. “A continuous surprise is that it takes the world such a long time to accept this, while this has such solid scientific foundation.” As an example, she cited an April report she coauthored in the journal Environment International. She and colleagues call for “national authorities to acknowledge the reality that the virus spreads through air and recommend that adequate control measures be implemented to prevent further spread of the SARS-CoV-2 virus, in particularly removal of the virus-laden droplets from indoor air by ventilation.”
The take-home message from the commentary, Morawska said, is a call to action. The authors state there is a need “to provide sufficient and effective ventilation (supply clean outdoor air, minimize recirculating air) particularly in public buildings, workplace environments, schools, hospitals, and aged care homes.”
WHO Chief Scientist Soumya Swaminathan, MD, explained why the organization remains cautious about making premature pronouncements regarding airborne transmission. “Any guidance we put out has implications for billions of people around the world, so we want to be as careful as possible,” she said during the press briefing. “We have to consider the weight of the evidence.”
“We are constantly looking for information on how we can do better,” Swaminathan added. WHO officials are reviewing hundreds of scientific reports every day, she said, and not all are of good quality. For this reason, she and other scientists at WHO perform a “living systematic review” – updating the consensus of evidence on a weekly basis.
“This process on COVID-19 will, I am sure, continue for the weeks and months to come,” she added.
This article first appeared on Medscape.com.
Despite guidelines, children receive opioids and steroids for pneumonia and sinusitis
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
A significant percentage of children receive opioids and systemic corticosteroids for pneumonia and sinusitis despite guidelines, according to an analysis of 2016 Medicaid data from South Carolina.
Prescriptions for these drugs were more likely after visits to EDs than after ambulatory visits, researchers reported in Pediatrics.
“Each of the 828 opioid and 2,737 systemic steroid prescriptions in the data set represent a potentially inappropriate prescription,” wrote Karina G. Phang, MD, MPH, of Geisinger Medical Center in Danville, Pa., and colleagues. “These rates appear excessive given that the use of these medications is not supported by available research or recommended in national guidelines.”
To compare the frequency of opioid and corticosteroid prescriptions for children with pneumonia or sinusitis in ED and ambulatory care settings, the investigators studied 2016 South Carolina Medicaid claims, examining data for patients aged 5-18 years with pneumonia or sinusitis. They excluded children with chronic conditions and acute secondary diagnoses with potentially appropriate indications for steroids, such as asthma. They also excluded children seen at more than one type of clinical location or hospitalized within a week of the visit. Only the primary diagnosis of pneumonia or sinusitis during the first visit of the year for each patient was included.
The researchers included data from 31,838 children in the study, including 2,140 children with pneumonia and 29,698 with sinusitis.
Pneumonia was linked to an opioid prescription in 6% of ED visits (34 of 542) and 1.5% of ambulatory visits (24 of 1,590) (P ≤ .0001). Pneumonia was linked to a steroid prescription in 20% of ED visits (106 of 542) and 12% of ambulatory visits (196 of 1,590) (P ≤ .0001).
Sinusitis was linked to an opioid prescription in 7.5% of ED visits (202 of 2,705) and 2% of ambulatory visits (568 of 26,866) (P ≤ .0001). Sinusitis was linked to a steroid prescription in 19% of ED visits (510 of 2,705) and 7% of ambulatory visits (1,922 of 26,866) (P ≤ .0001).
In logistic regression analyses, ED visits for pneumonia or sinusitis were more than four times more likely to result in children receiving opioids, relative to ambulatory visits (adjusted odds ratio, 4.69 and 4.02, respectively). ED visits also were more likely to result in steroid prescriptions, with aORs of 1.67 for pneumonia and 3.05 for sinusitis.
“I was disappointed to read of these results, although not necessarily surprised,” Michael E. Pichichero, MD, a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital, said in an interview.
The data suggest that improved prescribing practices may be needed, “especially in the ED,” wrote Dr. Phang and colleagues. “Although more children who are acutely ill may be seen in the ED, national practice guidelines and research remain relevant for these patients.”
Repeated or prolonged courses of systemic corticosteroids put children at risk for adrenal suppression and hypothalamic-pituitary-adrenal axis dysfunction. “Providers for children must also be aware of the trends in opioid abuse and diversion and must mitigate those risks while still providing adequate analgesia and symptom control,” they wrote.
The use of Medicaid data from 1 year in one state limits the generalizability of the findings. Nevertheless, the visits occurred “well after publication of relevant guidelines and after concerns of opioid prescribing had become widespread,” according to Dr. Phang and colleagues.
A post hoc evaluation identified one patient with a secondary diagnosis of fracture and 24 patients with a secondary diagnosis of pain, but none of these patients had received an opioid. “Thus, the small subset of patients who may have had secondary diagnoses that would warrant an opioid prescription would not have changed the overall results,” they wrote.
The study was funded by the National Institutes of Health. The authors had no relevant financial disclosures.
SOURCE: Phang KG et al. Pediatrics. 2020 Jul 2. doi: 10.1542/peds.2019-3690.
FROM PEDIATRICS
Diagnostic criteria may miss some MIS-C cases, experts say
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
New data from active surveillance of the severe inflammatory condition associated with COVID-19 in previously healthy children provide further insight into the prevalence and course of the rare syndrome, but experts are concerned that current diagnostic criteria may not capture the true scope of the problem.
In separate reports published online June 29 in the New England Journal of Medicine, researchers from the New York State Department of Health and the Centers for Disease Control and Prevention (CDC) describe the epidemiology and clinical features of multisystem inflammatory syndrome in children (MIS-C) on the basis of information derived from targeted surveillance programs in New York State and across the country.
For the New York study, Elizabeth M. Dufort, MD, from the New York Department of Health in Albany and colleagues analyzed MIS-C surveillance data from 106 hospitals across the state. Of 191 suspected MIS-C cases reported to the Department of Health from March 1 through May 10, 99 met the state’s interim case definition of the condition and were included in the analysis.
The incidence rate for MIS-C was two cases per 100,000 individuals younger than 21 years, whereas the incidence rate of confirmed COVID-19 cases in this age group was 322 per 100,000. Most cases occurred approximately 1 month after the state’s COVID-19 peak.
“Among our patients, predominantly from the New York Metropolitan Region, 40% were black and 36% were Hispanic. This may be a reflection of the well-documented elevated incidence of SARS-CoV-2 infection among black and Hispanic communities,” the authors report.
All children presented with fever or chills, and most had tachycardia (97%) and gastrointestinal symptoms (80%). Rash (60%), conjunctival infection (56%), hypotension (32%), and mucosal changes (27%) were reported. Among all of the children, levels of inflammatory markers were elevated, including levels of C-reactive protein (100%), D-dimer (91%), and troponin (71%). More than one third of the patients (36%) were diagnosed with myocarditis, and an additional 16% had clinical myocarditis.
Of the full cohort, 80% of the children required intensive care, 62% received vasopressor support, and two children died.
The high prevalence of cardiac dysfunction or depression, coagulopathy, gastrointestinal symptoms, mild respiratory symptoms, and indications for supplemental oxygen in patients with MIS-C stands in contrast to the clinical picture observed in most acute cases of COVID-19 in hospitalized children, the authors write.
“Although most children have mild or no illness from SARS-CoV-2 infection, MIS-C may follow Covid-19 or asymptomatic SARS-CoV-2 infection. Recognition of the syndrome and early identification of children with MIS-C, including early monitoring of blood pressure and electrocardiographic and echocardiographic evaluation, could inform appropriate supportive care and other potential therapeutic options,” they continue.
The incidence of MIS-C among children infected with SARS-CoV-2 is unclear because children with COVID-19 often have mild or no symptoms and because children are not tested as frequently, the authors state. For this reason, “[i]t is crucial to establish surveillance for MIS-C cases, particularly in communities with higher levels of SARS-CoV-2 transmission.”
Important Differences From Kawasaki Disease
In a separate study, Leora R. Feldstein, MD, of the CDC, and colleagues report 186 cases of MIS-C collected through targeted surveillance of pediatric health centers in 26 US states from March 15 to May 20, 2020. As with the New York cohort, a disproportionate number of children in this cohort were black (25%) and Hispanic or Latino (31%).
Similar to the New York cohort, 80% of the children in this group required intensive care, 48% received vasoactive support, 20% required invasive mechanical ventilation, and four children died. Skin rashes, gastrointestinal symptoms, cardiovascular and hematologic effects, mucous changes, and elevations of inflammatory biomarkers were also similarly observed.
The researchers note that, although many of the features of MIS-C overlap with Kawasaki disease, there are some important differences, particularly with respect to the nature of cardiovascular involvement. “Approximately 5% of children with Kawasaki’s disease in the United States present with cardiovascular shock leading to vasopressor or inotropic support, as compared with 50% of the patients in our series,” the authors write.
In addition, coronary-artery aneurysms affect approximately one quarter of Kawasaki disease patients within 21 days of disease onset. “In our series, a maximum z score of 2.5 or higher in the left anterior descending or right coronary artery was reported in 8% of the patients overall and in 9% of patients with echocardiograms,” they report.
Additional differentiating features include patient age and race/ethnicity. Kawasaki disease occurs most commonly in children younger than 5 years. The median age in the multistate study was 8.3 years, and nearly half of the children in the New York cohort were in the 6- to 12-year age group. Further, Kawasaki disease is disproportionately prevalent in children of Asian descent.
Despite the differences, “until more is known about long-term cardiac sequelae of MIS-C, providers could consider following Kawasaki’s disease guidelines for follow-up, which recommend repeat echocardiographic imaging at 1 to 2 weeks.”
As was the case in the New York series, treatment in the multistate cohort most commonly included intravenous immunoglobulin and systemic glucocorticoids. Optimal management, however, will require a better understanding of the pathogenesis of MIS-C, Feldstein and colleagues write.
Questions Remain
With the accumulating data on this syndrome, the MIS-C picture seems to be getting incrementally clearer, but there is still much uncertainty, according to Michael Levin, FMedSci, PhD, from the Department of Infectious Disease, Imperial College London, United Kingdom.
“The recognition and description of new diseases often resemble the parable of the blind men and the elephant, with each declaring that the part of the beast they have touched fully defines it,” he writes in an accompanying editorial.
“As the coronavirus disease 2019 (Covid-19) pandemic has evolved, case reports have appeared describing children with unusual febrile illnesses that have features of Kawasaki’s disease, toxic shock syndrome, acute abdominal conditions, and encephalopathy, along with other reports of children with fever, elevated inflammatory markers, and multisystem involvement. It is now apparent that these reports were describing different clinical presentations of a new childhood inflammatory disorder.”
Although a consistent clinical picture is emerging, “[t]he published reports have used a variety of hastily developed case definitions based on the most severe cases, possibly missing less serious cases,” Levin writes. In particular, both the CDC and World Health Organization definitions require evidence of SARS-CoV-2 infection or exposure, which might contribute to underrecognition and underreporting because asymptomatic infections are common and antibody testing is not universally available.
“There is concern that children meeting current diagnostic criteria for MIS-C are the ‘tip of the iceberg,’ and a bigger problem may be lurking below the waterline,” Levin states. With approximately 1000 cases of the syndrome reported worldwide, “do we now have a clear picture of the new disorder, or as in the story of the blind men and the elephant, has only part of the beast been described?”
Adrienne Randolph, MD, of Boston Children’s Hospital, who is a coauthor of the multistate report, agrees that there is still much to learn about MIS-C before the whole beast can be understood. In an interview with Medscape Medical News, she listed the following key questions that have yet to be answered:
- Why do some children get MIS-C and not others?
- What is the long-term outcome of children with MIS-C?
- How can we differentiate MIS-C from acute COVID-19 infection in children with respiratory failure?
- Does MIS-C occur in young adults?
Randolph said her team is taking the best path forward toward answering these questions, including conducting a second study to identify risk factors for MIS-C and longer-term follow-up studies with the National Institutes of Health. “We are also getting consent to collect blood samples and look at other tests to help distinguish MIS-C from acute COVID-19 infection,” she said. She encouraged heightened awareness among physicians who care for young adults to consider MIS-C in patients aged 21 years and older who present with similar signs and symptoms.
On the basis of the answers to these and additional questions, the case definitions for MIS-C may need refinement to capture the wider spectrum of illness, Levin writes in his editorial. “The challenges of this new condition will now be to understand its pathophysiological mechanisms, to develop diagnostics, and to define the best treatment.”
Kleinman has received grants from the Health Services Resources Administration outside the submitted work. Maddux has received grants from the NIH/NICHD and the Francis Family Foundation outside the submitted work. Randolph has received grants from Genentech and personal fees from La Jolla Pharma outside the submitted work and others from the CDC during the conduct of the study.
This article first appeared on Medscape.com.
Captopril questioned for diabetes patients in COVID-19 setting
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Captopril appears to be associated with a higher rate of pulmonary adverse reactions in patients with diabetes than that of other ACE inhibitors or angiotensin receptor blockers (ARBs) and therefore may not be the best choice for patients with diabetes and COVID-19, a new study suggests.
The study was published online in the Journal of the American Pharmacists Association.
The authors, led by Emma G. Stafford, PharmD, University of Missouri-Kansas City School of Pharmacy, note that diabetes seems to confer a higher risk of adverse outcomes in COVID-19 infection and there is conflicting data on the contribution of ACE inhibitors and ARBs, commonly used medications in diabetes, on the mortality and morbidity of COVID-19.
“In light of the recent COVID-19 outbreak, more research is needed to understand the effects that diabetes (and its medications) may have on the respiratory system and how that could affect the management of diseases such as COVID-19,” they say.
“Although ACE inhibitors and ARBs are generally considered to have similar adverse event profiles, evaluation of postmarketing adverse events may shed light on minute differences that could have important clinical impacts,” they add.
For the current study, the researchers analyzed data from multiple publicly available data sources on adverse drug reactions in patients with diabetes taking ACE inhibitors or ARBs. The data included all adverse drug events (ADEs) reported nationally to the US Food and Drug Administration and internationally to the Medical Dictionary for Regulatory Activities (MedDRA).
Results showed that captopril, the first ACE inhibitor approved back in 1981, has a higher incidence of pulmonary ADEs in patients with diabetes as compared with other ACE-inhibitor drugs (P = .005) as well as a statistically significant difference in pulmonary events compared with ARBs (P = .012).
“These analyses suggest that pharmacists and clinicians will need to consider the specific medication’s adverse event profile, particularly captopril, on how it may affect infections and other acute disease states that alter pulmonary function, such as COVID-19,” the authors conclude.
They say that the high incidence of pulmonary adverse drug effects with captopril “highlights the fact that the drugs belonging in one class are not identical and that its pharmacokinetics and pharmacodynamics can affect the patients’ health especially during acute processes like COVID-19.”
“This is especially important as current observational studies of COVID-19 patients tend to group drugs within a class and are not analyzing the potential differences within each class,” they add.
They note that ACE inhibitors can be broadly classified into 3 structural classes: sulfhydryl-, dicarboxyl-, and phosphorous- containing molecules. Notably, captopril is the only currently available ACE inhibitor belonging to the sulfhydryl-containing class and may explain the higher incidence of adverse drug effects observed, they comment.
“Health care providers have been left with many questions when treating patients with COVID-19, including how ACE inhibitors or ARBs may affect their clinical course. Results from this study may be helpful when prescribing or continuing ACE inhibitors or ARBs for patients with diabetes and infections or illnesses that may affect pulmonary function, such as COVID-19,” they conclude.
Questioning safety in COVID-19 an “overreach”
Commenting for Medscape Medical News, Michael A. Weber, MD, professor of medicine at State University of New York, said he thought the current article appears to overreach in questioning captopril’s safety in the COVID-19 setting.
“Captopril was the first ACE inhibitor available for clinical use. In early prescribing its dosage was not well understood and it might have been administered in excessive amounts,” Weber notes.
“There were some renal and other adverse effects reported that at first were attributed to the fact that captopril, unlike any other popular ACE inhibitors, contained a sulfhydryl (SH) group in its molecule,” he said. “It is not clear whether this feature could be responsible for the increased pulmonary side effects and potential danger to COVID-19 patients now reported with captopril in this new pharmacy article.”
But he adds: “The article contains no evidence that the effect of captopril or any other ACE inhibitor on the pulmonary ACE-2 enzyme has a deleterious effect on outcomes of COVID-19 disease. In any case, captopril — which should be prescribed in a twice-daily dose — is not frequently prescribed these days since newer ACE inhibitors are effective with just once-daily dosing.”
This article first appeared on Medscape.com.
Lifestyle changes may explain skin lesions in pandemic-era patients
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
such as lockdown conditions, which may be clarified with additional research.
Lindy P. Fox, MD, professor of dermatology at the University of California, San Francisco, who was not an author of either study, urged caution in interpreting these results. Data from the American Academy of Dermatology and a recent paper from the British Journal of Dermatology suggest a real association exists, at in least some patients. “It’s going to be true that most patients with toe lesions are PCR [polymerase chain reaction]-negative because it tends to be a late phenomenon when patients are no longer shedding virus,” Dr. Fox said in an interview.
Reports about chickenpox-like vesicles, urticaria, and other skin lesions in SARS-CoV-2 patients have circulated in the clinical literature and the media. Acute acro-ischemia has been cited as a potential sign of infection in adolescents and children.
One of the European studies, which was published in JAMA Dermatology, explored this association in 20 patients aged 1-18 years (mean age, 12.3 years), who presented with new-onset acral inflammatory lesions in their hands and feet at La Fe University Hospital, in Valencia, during the country’s peak quarantine period in April. Investigators conducted blood tests and reverse transcriptase–PCR (RT-PCR) for SARS-CoV-2, and six patients had skin biopsies.
Juncal Roca-Ginés, MD, of the department of dermatology, at the Hospital Universitario y Politécnico in La Fe, and coauthors, identified acral erythema in 6 (30%) of the cases, dactylitis in 4 (20%), purpuric maculopapules in 7 (35%), and a mixed pattern in 3 (15%). Serologic and viral testing yielded no positive results for SARS-CoV-2 or other viruses, and none of the patients exhibited COVID-19 symptoms such as fever, dry cough, sore throat, myalgia, or taste or smell disorders. In other findings, 45% of the patients had a history of vascular reactive disease of the hands, and 75% reported walking barefoot in their homes while staying at home. Only two patients reported taking medications.
In the six patients who had a biopsy, the findings were characteristic of chillblains, “confirming the clinical impression,” the authors wrote. Concluding that they could not show a relationship between acute acral skin changes and COVID-19, they noted that “other studies with improved microbiologic tests or molecular techniques aimed at demonstrating the presence of SARS-CoV-2 in the skin may help to clarify this problem.”
The other case series, which was also published in JAMA Dermatology and included 31 adults at a hospital in Brussels, who had recently developed chillblains, also looked for a connection between SARS-CoV-2 and chilblains, in April. Most of the participants were in their teens or 20s. Lesions had appeared on hands, feet, or on both extremities within 1-30 days of consultation, presenting as erythematous or purplish erythematous macules, occasionally with central vesicular or bullous lesions or necrotic areas. Patients reported pain, burning, and itching.
Skin biopsies were obtained in 22 patients and confirmed the diagnosis of chilblains; of the 15 with immunofluorescence analyses, 7 patients were found to have vasculitis of small-diameter vessels.
Of the 31 patients, 20 (64%) reported mild symptoms consistent with SARS-CoV-2, yet none of the RT-PCR or serologic test results showed signs of the virus in all 31 patients. “Because some patients had experienced chilblains for more than 15 days [under 30 days or less] at the time of inclusion, we can reasonably exclude the possibility that serologic testing was done too soon,” observed the authors. They also didn’t find eosinopenia, lymphopenia, and hyperferritinemia, which have been associated with COVID-19, they added.
Changes in lifestyle conditions during the pandemic may explain the appearance of these lesions, according to the authors of both studies, who mentioned that walking around in socks or bare feet and reduced physical activity could have indirectly led to the development of skin lesions.
It’s also possible that young people have less severe disease and a delayed reaction to the virus, Ignacio Torres-Navarro, MD, a dermatologist with La Fe University and the Spanish study’s corresponding author, said in an interview. Their feet may lack maturity in neurovascular regulation and/or the eccrine glands, which can happen in other diseases such as neutrophilic idiopathic eccrine hidradenitis. “In this context, perhaps there was an observational bias of the parents to the children when this manifestation was reported in the media. However, nothing has been demonstrated,” he said.
In an accompanying editor’s note, Claudia Hernandez, MD, of the departments of dermatology and pediatrics, Rush University Medical Center, Chicago, and Anna L. Bruckner, MD, of the departments of dermatology and pediatrics at the University of Colorado, Aurora, wrote that “it is still unclear whether a viral cytopathic process vs a viral reaction pattern or other mechanism is responsible for ‘COVID toes.’ ” Lack of confirmatory testing and reliance on indirect evidence of infection complicates this further, they noted, adding that “dermatologists must be aware of the protean cutaneous findings that are possibly associated with COVID-19, even if our understanding of their origins remains incomplete.”
In an interview, Dr. Fox, a member of the AAD’s’s COVID-19 Registry task force, offered other possible reasons for the negative antibody tests in the studies. The assay might not have been testing the correct antigen, or the timing of the test might not have been optimal. “More studies will help this become less controversial,” she said.
The authors of the two case series acknowledged potential limitations of their studies. Neither was large in scope: Both took place over a week’s time and included small cohorts. The Belgian study had no control group or long-term follow-up. Little is still known about the clinical manifestations and detection methods for SARS-CoV-2, noted the authors of the Spanish study.
The Spanish study received funding La Fe University Hospital’s department of dermatology, and the authors had no disclosures. The Belgian study received support from the Fondation Saint-Luc, which provided academic funding for its lead author, Marie Baeck, MD, PhD. Another author of this study received personal fees from the Fondation Saint-Luc and personal fees and nonfinancial support from Bioderma. The authors of the editor’s note had no disclosures.
SOURCES: Roca-Ginés J et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2340; Herman A et al. JAMA Dermatol. 2020 Jun 25. doi: 10.1001/jamadermatol.2020.2368.
Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
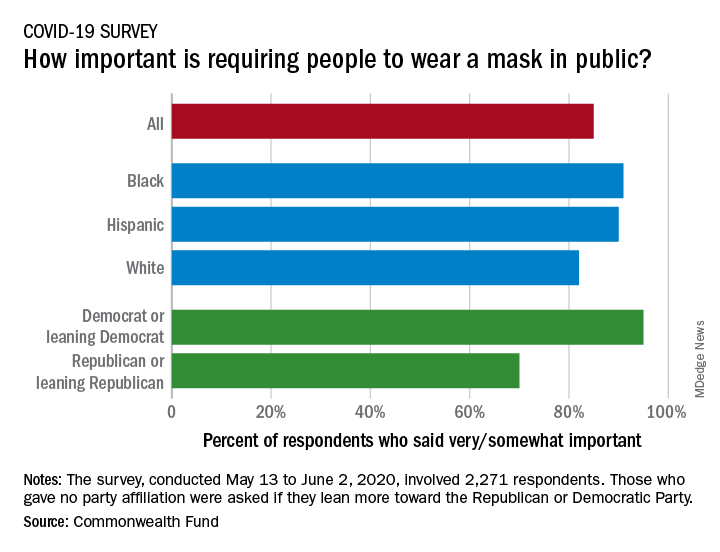
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.

Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.