User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
When You and Your Malpractice Insurer Disagree on Your Case
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
You’ve been sued for medical malpractice. If you are a physician in the United States, that is not an unlikely scenario.
An analysis by the American Medical Association shows that almost half of all physicians are sued by the time they reach 54. In some specialties, such as ob.gyn., one is almost guaranteed to be sued at some point.
But that’s what medical malpractice insurance is for, right? Your medical malpractice insurer will assign an attorney to take care of you and help you through this situation. Won’t they?
Maybe so, but the attorney and the claims representative your insurer assigns to your case may have a different idea about how to proceed than you do. Though the defense attorney assigned to you represents you, he or she gets paid by the insurance carrier.
This can create a conflict when your defense counsel and your insurance claims representative aim to take your case in a direction you don’t like.
Disagreements might include:
- Choice of expert witnesses
- Tactical decisions related to trial strategy
- Public relations considerations
- Admissions of liability
- Allocation of resources
To Settle or Not?
One of the most challenging — and common — disagreements is whether to settle the case.
Sometimes a malpractice insurer wants to settle the case against the defendant doctor’s wishes. Or the doctor wants to settle but is pushed into going to trial. In the following case, one doctor had to face the consequences of a decision he didn’t even make.
The Underlying Medical Malpractice Case
Dr. D was sued by a patient who had allegedly called Dr. D’s office six times in 2 days complaining of intermittent chest pain.
Dr. D had been swamped with patients and couldn’t squeeze this patient in for an office visit, but he did call back. The patient later claimed that during the call he told the doctor he was suffering from chest pain. The doctor recalled that the patient had complained of abdominal discomfort that began after he had exercised.
The physician wrote a prescription for an ECG at the local hospital and called to ensure that the patient could just walk in. The ECG was allegedly abnormal but was not read as representing an impending or current heart attack. Later that evening, however, the patient went to the emergency department of another hospital where it was confirmed that he had suffered a heart attack. The patient underwent cardiac catheterization and stent placement to address a blockage in his left anterior descending artery.
The patient subsequently sued Dr. D and the hospital where he had the original ECG. Dr. D contacted his medical malpractice insurance company. The insurance company assigned an attorney to represent Dr. D. Discovery in the case began.
The plaintiff’s own medical expert testified in a deposition that there was no way for the heart attack to have been prevented and that the treatment would have been the same either way. But Dr. D could not find a record of the phone calls with the patient, and he had not noted his conversation the patient in their medical records.
Dr. D held a policy for $1 million, and his state had a fund that would kick in an additional $1 million. But the plaintiffs demanded $4 million to settle.
A month before trial, the plaintiff’s attorney sent a threatening letter to Dr. D’s attorney warning him that Dr. D was underinsured and suggesting that it would be in the physician’s best interests to settle.
“I want to stress to you that it is not my desire to harm your client’s reputation or to destroy his business,” wrote the plaintiff’s attorney. “However, now is the time to avoid consequences such as these by making a good faith effort to get this case resolved.”
The letter went on to note that the defense attorney should give Dr. D a copy of the letter so that everyone would be aware of the potential consequences of an award against Dr. D in excess of his limits of insurance coverage. The plaintiff’s attorney even suggested that Dr. D should retain personal counsel.
Dr. D’s defense attorney downplayed the letter and assured him that there was no reason to worry.
Meanwhile the case inched closer to trial.
The codefendant hospital settled with the plaintiff on the night before jury selection, leaving Dr. D in the uncomfortable position of being the only defendant in the case. At this point, Dr. D decided he would like to settle, and he sent his attorney an email telling him so. But the attorney instead referred him to an insurance company claims.
Just days before the trial was to start, Dr. D repeatedly told the claims representative assigned to his claim that he did not want to go to trial but rather wanted to settle. The representative told Dr. D that he had no choice in whether the action settled.
A committee at the insurance company had decided to proceed with the trial rather than settle.
The trial proved a painful debacle for Dr. D. His attorney’s idea of showing a “gotcha” video of the allegedly permanently injured plaintiff carrying a large, heavy box backfired when the jury was shown by the plaintiff that the box actually contained ice cream cones and weighed very little.
Prior to trial, the plaintiff offered to settle for $1 million. On the first day of trial, they lowered that amount to $750,000, yet the defense attorney did not settle the case, and it proceeded to a jury verdict. The jury awarded the plaintiff over $4 million — well in excess of Dr. D’s policy limits.
The Follow-up
Dr. D was horrified, but the insurance company claims representative said the insurer would promptly offer $2 million in available insurance coverage to settle the case post verdict. This did not happen. Instead, the insurer chose to appeal the verdict against Dr. D’s wishes.
Ultimately, Dr. D was forced to hire his own lawyer. He ultimately sued the insurance company for breach of contract and bad faith.
The insurance company eventually attempted to settle with the plaintiffs’ counsel, but the plaintiff refused to accept the available insurance coverage. The insurance carrier still has not posted the entire appeal bond. The case is still pending.
Protecting Yourself
The lesson from Dr. D’s experience: Understand that the insurance company is not your friend. It’s a business looking out for its own interests.
The plaintiff’s attorney was absolutely correct in suggesting that Dr. D retain his own attorney to represent his own interests. You should hire your own lawyer when:
- You disagree with your insurer on how to proceed in a case.
- You receive a demand that exceeds your available insurance coverage or for damages that may not be covered by your policy, such as punitive damages.
- Your insurance carrier attempts to deny insurance coverage for your claim or sends you a letter stating that it is “reserving its rights” not to cover or to limit coverage for your claim.
Retaining independent counsel protects your interests, not those of your insurance company.
Independent counsel can give you a second opinion on the strengths and weaknesses of your claim, help you prepare for your deposition, and attend court dates with you to ensure that you are completely protected.
Independent counsel can challenge your insurance company’s decision to deny or limit your insurance coverage and ensure that you receive all of the benefits to which you are entitled under your insurance policy. Some policies may include an independent lawyer to be paid for by your insurance carrier in case of a conflicts.
The most important takeaway? Your medical malpractice insurance carrier is not your friend, so act accordingly in times of conflict.
A version of this article first appeared on Medscape.com.
Incidence and Risk Factors Associated With Switching Between b/tsDMARD in PsA
Key clinical point: Switching between biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) was common due to treatment inefficacy in patients with psoriatic arthritis (PsA), with concomitant therapies and multiple prior treatments being significant risk factors.
Major finding: Overall, 40% of patients switched between b/tsDMARD, with 85.1% switches due to treatment inefficacy. The risk for switching was not affected by b/tsDMARD type (P > .05) but increased with multiple b/tsDMARD courses (adjusted hazard ratio [aHR] 1.22; P = .010), concomitant glucocorticoids (aHR 2.05; P = .001), and sulfalazine use (aHR 2.25; P = .006). Women and those with inflammatory back pain also faced an increased risk for switching.
Study details: This longitudinal retrospective study included 141 patients with PsA (age ≥ 16 years) who were treated with b/tsDMARD.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Ministry of Health, Spain, and Red de Enfermedades Inflamatorias, with co-funding from el Fondo Europeo de Desarrollo Regional. The authors declared no conflicts of interest.
Source: Freites-Nuñez D, Leon L, Toledano E, et al. Switching related to inefficacy in biologics and targeted synthetic therapies for psoriatic arthritis: A comparative real-life study. Ther Adv Musculoskelet Dis. 2024 (Aug 31). doi:10.1177/1759720X241273083 Source
Key clinical point: Switching between biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) was common due to treatment inefficacy in patients with psoriatic arthritis (PsA), with concomitant therapies and multiple prior treatments being significant risk factors.
Major finding: Overall, 40% of patients switched between b/tsDMARD, with 85.1% switches due to treatment inefficacy. The risk for switching was not affected by b/tsDMARD type (P > .05) but increased with multiple b/tsDMARD courses (adjusted hazard ratio [aHR] 1.22; P = .010), concomitant glucocorticoids (aHR 2.05; P = .001), and sulfalazine use (aHR 2.25; P = .006). Women and those with inflammatory back pain also faced an increased risk for switching.
Study details: This longitudinal retrospective study included 141 patients with PsA (age ≥ 16 years) who were treated with b/tsDMARD.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Ministry of Health, Spain, and Red de Enfermedades Inflamatorias, with co-funding from el Fondo Europeo de Desarrollo Regional. The authors declared no conflicts of interest.
Source: Freites-Nuñez D, Leon L, Toledano E, et al. Switching related to inefficacy in biologics and targeted synthetic therapies for psoriatic arthritis: A comparative real-life study. Ther Adv Musculoskelet Dis. 2024 (Aug 31). doi:10.1177/1759720X241273083 Source
Key clinical point: Switching between biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) was common due to treatment inefficacy in patients with psoriatic arthritis (PsA), with concomitant therapies and multiple prior treatments being significant risk factors.
Major finding: Overall, 40% of patients switched between b/tsDMARD, with 85.1% switches due to treatment inefficacy. The risk for switching was not affected by b/tsDMARD type (P > .05) but increased with multiple b/tsDMARD courses (adjusted hazard ratio [aHR] 1.22; P = .010), concomitant glucocorticoids (aHR 2.05; P = .001), and sulfalazine use (aHR 2.25; P = .006). Women and those with inflammatory back pain also faced an increased risk for switching.
Study details: This longitudinal retrospective study included 141 patients with PsA (age ≥ 16 years) who were treated with b/tsDMARD.
Disclosures: This study was supported by the Instituto de Salud Carlos III, Ministry of Health, Spain, and Red de Enfermedades Inflamatorias, with co-funding from el Fondo Europeo de Desarrollo Regional. The authors declared no conflicts of interest.
Source: Freites-Nuñez D, Leon L, Toledano E, et al. Switching related to inefficacy in biologics and targeted synthetic therapies for psoriatic arthritis: A comparative real-life study. Ther Adv Musculoskelet Dis. 2024 (Aug 31). doi:10.1177/1759720X241273083 Source
Apremilast Effective in Early PsA With Limited Joint Involvement
Key clinical point: Patients with early oligoarticular psoriatic arthritis (PsA) treated with apremilast vs placebo showed greater disease control and minimal disease activity response with a maximum of one swollen joint and one tender joint count (MDA-Joints).
Major finding: At week 16, a higher proportion of patients receiving apremilast vs placebo achieved MDA-Joints response based on sentinel joints (33.9% vs 16.0%; P = .0008) and total joints (21.3% vs 7.9%; nominal P = .0028). No new safety signals were reported.
Study details: This phase 4 FOREMOST trial included 308 patients with early oligoarticular PsA previously treated with non-steroidal anti-inflammatory drugs or ≥2 conventional synthetic disease-modifying antirheumatic drugs and were randomly assigned to receive apremilast (n = 203) or placebo (n = 105).
Disclosures: This study was funded by Amgen. Five authors declared being employees and owning stocks of Amgen. Several authors have declared other ties with Amgen and other sources.
Source: Gossec L, Coates LC, Gladman DD, et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: Primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann Rheum Dis. 2024 (Aug 20). doi: 10.1136/ard-2024-225833 Source
Key clinical point: Patients with early oligoarticular psoriatic arthritis (PsA) treated with apremilast vs placebo showed greater disease control and minimal disease activity response with a maximum of one swollen joint and one tender joint count (MDA-Joints).
Major finding: At week 16, a higher proportion of patients receiving apremilast vs placebo achieved MDA-Joints response based on sentinel joints (33.9% vs 16.0%; P = .0008) and total joints (21.3% vs 7.9%; nominal P = .0028). No new safety signals were reported.
Study details: This phase 4 FOREMOST trial included 308 patients with early oligoarticular PsA previously treated with non-steroidal anti-inflammatory drugs or ≥2 conventional synthetic disease-modifying antirheumatic drugs and were randomly assigned to receive apremilast (n = 203) or placebo (n = 105).
Disclosures: This study was funded by Amgen. Five authors declared being employees and owning stocks of Amgen. Several authors have declared other ties with Amgen and other sources.
Source: Gossec L, Coates LC, Gladman DD, et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: Primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann Rheum Dis. 2024 (Aug 20). doi: 10.1136/ard-2024-225833 Source
Key clinical point: Patients with early oligoarticular psoriatic arthritis (PsA) treated with apremilast vs placebo showed greater disease control and minimal disease activity response with a maximum of one swollen joint and one tender joint count (MDA-Joints).
Major finding: At week 16, a higher proportion of patients receiving apremilast vs placebo achieved MDA-Joints response based on sentinel joints (33.9% vs 16.0%; P = .0008) and total joints (21.3% vs 7.9%; nominal P = .0028). No new safety signals were reported.
Study details: This phase 4 FOREMOST trial included 308 patients with early oligoarticular PsA previously treated with non-steroidal anti-inflammatory drugs or ≥2 conventional synthetic disease-modifying antirheumatic drugs and were randomly assigned to receive apremilast (n = 203) or placebo (n = 105).
Disclosures: This study was funded by Amgen. Five authors declared being employees and owning stocks of Amgen. Several authors have declared other ties with Amgen and other sources.
Source: Gossec L, Coates LC, Gladman DD, et al. Treatment of early oligoarticular psoriatic arthritis with apremilast: Primary outcomes at week 16 from the FOREMOST randomised controlled trial. Ann Rheum Dis. 2024 (Aug 20). doi: 10.1136/ard-2024-225833 Source
ANCA-Associated Vasculitis Has Five Unique Patient Clusters
TOPLINE:
A data-driven subclassification of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis has identified five distinct clusters with varying degrees of kidney involvement and systemic inflammation, offering insights into improved patient stratification and treatment approaches.
METHODOLOGY:
- ANCA-associated vasculitis is a rare and complex autoimmune disease that is traditionally classified into granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
- Researchers employed advanced artificial intelligence and big data techniques to identify phenotypically distinct subgroups of ANCA-associated vasculitis and developed a classification system using real-world patient data from the Federated Vasculitis Registry consortium.
- They included 3868 patients diagnosed with ANCA-associated vasculitis between November 1, 1966, and March 1, 2023 (mean age at diagnosis, 57.2 years; 51.9% men), across six European vasculitis registries; while a majority of patients (62.9%) were diagnosed with GPA, the remaining 37.1% were diagnosed with MPA.
- Overall, 17 clinical and demographic variables such as the age at diagnosis, gender, serum creatinine and C-reactive protein levels, the type of ANCA, and the involvement of various organ systems were used to create a model for categorizing patients into different clusters.
- The median follow-up duration was 4.2 years.
TAKEAWAY:
- Five distinct clusters were identified in ANCA-associated vasculitis; three had significant kidney involvement (the severe kidney cluster, myeloperoxidase-ANCA-positive kidney cluster, and proteinase 3-ANCA-positive kidney cluster) and two had minimal kidney involvement (young respiratory cluster and inflammatory multisystem cluster).
- The clusters with significant kidney involvement were associated with poorer outcomes, including a higher risk for kidney failure and death. The severe kidney cluster had the poorest prognosis, with mortality and the rate of end-stage kidney failure being 30.5% and 41.6%, respectively.
- The young respiratory cluster, characterized by predominant ear-nose-throat involvement and low systemic inflammation, showed the best prognostic outcomes.
- This cluster membership model showed a greater predictive accuracy for patient and kidney survival than traditional methods based on clinical diagnosis or ANCA specificity.
IN PRACTICE:
“These findings highlight the necessity of recognizing severe kidney disease at the time of diagnosis as an indicator of poor outcome, thereby necessitating intensified treatment approaches,” experts from the Department of Internal Medicine IV (Nephrology and Hypertension), Medical University of Innsbruck, Austria, wrote in an accompanying editorial published online on August 22, 2024, in The Lancet Rheumatology.
SOURCE:
This study was led by Karl Gisslander, Department of Clinical Sciences, Lund University, Lund, Sweden, and was published online on August 22, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Data on estimated glomerular filtration rate recovery in clusters with kidney disease were lacking. Populations from East Asia, where myeloperoxidase-ANCA positivity is more prevalent, were not included.
DISCLOSURES:
This study received funding from the European Union’s Horizon 2020 research and innovation program under the European Joint Programme on Rare Diseases. Some authors declared serving on advisory boards or receiving grants, contracts, travel support, consulting fees, payments, or honoraria from various pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A data-driven subclassification of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis has identified five distinct clusters with varying degrees of kidney involvement and systemic inflammation, offering insights into improved patient stratification and treatment approaches.
METHODOLOGY:
- ANCA-associated vasculitis is a rare and complex autoimmune disease that is traditionally classified into granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
- Researchers employed advanced artificial intelligence and big data techniques to identify phenotypically distinct subgroups of ANCA-associated vasculitis and developed a classification system using real-world patient data from the Federated Vasculitis Registry consortium.
- They included 3868 patients diagnosed with ANCA-associated vasculitis between November 1, 1966, and March 1, 2023 (mean age at diagnosis, 57.2 years; 51.9% men), across six European vasculitis registries; while a majority of patients (62.9%) were diagnosed with GPA, the remaining 37.1% were diagnosed with MPA.
- Overall, 17 clinical and demographic variables such as the age at diagnosis, gender, serum creatinine and C-reactive protein levels, the type of ANCA, and the involvement of various organ systems were used to create a model for categorizing patients into different clusters.
- The median follow-up duration was 4.2 years.
TAKEAWAY:
- Five distinct clusters were identified in ANCA-associated vasculitis; three had significant kidney involvement (the severe kidney cluster, myeloperoxidase-ANCA-positive kidney cluster, and proteinase 3-ANCA-positive kidney cluster) and two had minimal kidney involvement (young respiratory cluster and inflammatory multisystem cluster).
- The clusters with significant kidney involvement were associated with poorer outcomes, including a higher risk for kidney failure and death. The severe kidney cluster had the poorest prognosis, with mortality and the rate of end-stage kidney failure being 30.5% and 41.6%, respectively.
- The young respiratory cluster, characterized by predominant ear-nose-throat involvement and low systemic inflammation, showed the best prognostic outcomes.
- This cluster membership model showed a greater predictive accuracy for patient and kidney survival than traditional methods based on clinical diagnosis or ANCA specificity.
IN PRACTICE:
“These findings highlight the necessity of recognizing severe kidney disease at the time of diagnosis as an indicator of poor outcome, thereby necessitating intensified treatment approaches,” experts from the Department of Internal Medicine IV (Nephrology and Hypertension), Medical University of Innsbruck, Austria, wrote in an accompanying editorial published online on August 22, 2024, in The Lancet Rheumatology.
SOURCE:
This study was led by Karl Gisslander, Department of Clinical Sciences, Lund University, Lund, Sweden, and was published online on August 22, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Data on estimated glomerular filtration rate recovery in clusters with kidney disease were lacking. Populations from East Asia, where myeloperoxidase-ANCA positivity is more prevalent, were not included.
DISCLOSURES:
This study received funding from the European Union’s Horizon 2020 research and innovation program under the European Joint Programme on Rare Diseases. Some authors declared serving on advisory boards or receiving grants, contracts, travel support, consulting fees, payments, or honoraria from various pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A data-driven subclassification of antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis has identified five distinct clusters with varying degrees of kidney involvement and systemic inflammation, offering insights into improved patient stratification and treatment approaches.
METHODOLOGY:
- ANCA-associated vasculitis is a rare and complex autoimmune disease that is traditionally classified into granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA).
- Researchers employed advanced artificial intelligence and big data techniques to identify phenotypically distinct subgroups of ANCA-associated vasculitis and developed a classification system using real-world patient data from the Federated Vasculitis Registry consortium.
- They included 3868 patients diagnosed with ANCA-associated vasculitis between November 1, 1966, and March 1, 2023 (mean age at diagnosis, 57.2 years; 51.9% men), across six European vasculitis registries; while a majority of patients (62.9%) were diagnosed with GPA, the remaining 37.1% were diagnosed with MPA.
- Overall, 17 clinical and demographic variables such as the age at diagnosis, gender, serum creatinine and C-reactive protein levels, the type of ANCA, and the involvement of various organ systems were used to create a model for categorizing patients into different clusters.
- The median follow-up duration was 4.2 years.
TAKEAWAY:
- Five distinct clusters were identified in ANCA-associated vasculitis; three had significant kidney involvement (the severe kidney cluster, myeloperoxidase-ANCA-positive kidney cluster, and proteinase 3-ANCA-positive kidney cluster) and two had minimal kidney involvement (young respiratory cluster and inflammatory multisystem cluster).
- The clusters with significant kidney involvement were associated with poorer outcomes, including a higher risk for kidney failure and death. The severe kidney cluster had the poorest prognosis, with mortality and the rate of end-stage kidney failure being 30.5% and 41.6%, respectively.
- The young respiratory cluster, characterized by predominant ear-nose-throat involvement and low systemic inflammation, showed the best prognostic outcomes.
- This cluster membership model showed a greater predictive accuracy for patient and kidney survival than traditional methods based on clinical diagnosis or ANCA specificity.
IN PRACTICE:
“These findings highlight the necessity of recognizing severe kidney disease at the time of diagnosis as an indicator of poor outcome, thereby necessitating intensified treatment approaches,” experts from the Department of Internal Medicine IV (Nephrology and Hypertension), Medical University of Innsbruck, Austria, wrote in an accompanying editorial published online on August 22, 2024, in The Lancet Rheumatology.
SOURCE:
This study was led by Karl Gisslander, Department of Clinical Sciences, Lund University, Lund, Sweden, and was published online on August 22, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Data on estimated glomerular filtration rate recovery in clusters with kidney disease were lacking. Populations from East Asia, where myeloperoxidase-ANCA positivity is more prevalent, were not included.
DISCLOSURES:
This study received funding from the European Union’s Horizon 2020 research and innovation program under the European Joint Programme on Rare Diseases. Some authors declared serving on advisory boards or receiving grants, contracts, travel support, consulting fees, payments, or honoraria from various pharmaceutical companies and other institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).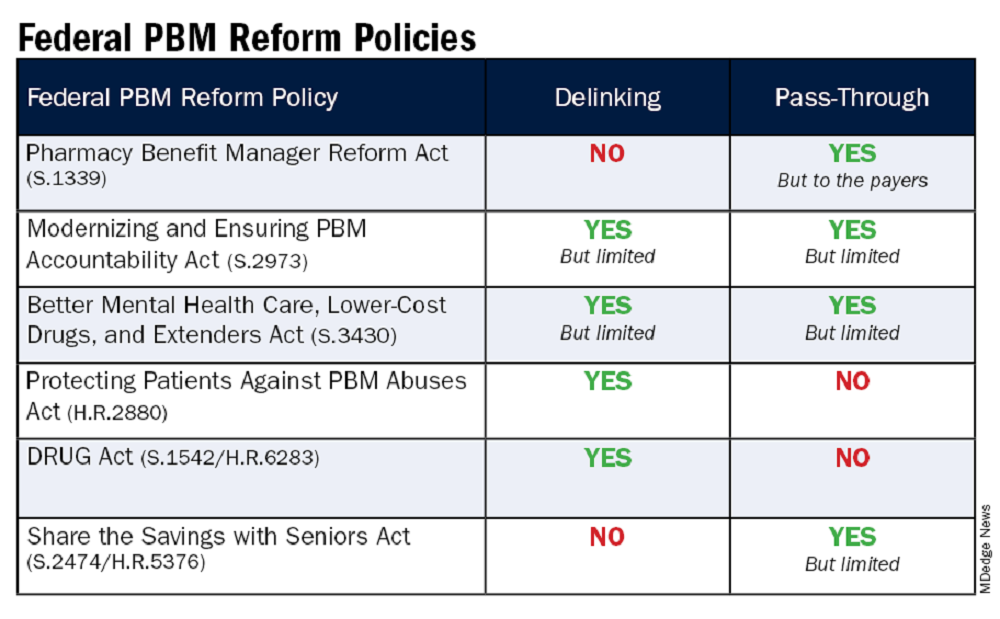
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at [email protected].
Belimumab Hits Newer Remission, Low Disease Activity Metrics
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
A greater proportion of patients with active systemic lupus erythematosus (SLE) treated with belimumab plus standard therapy achieved the newest definitions for remission and low disease activity compared with those treated with placebo plus standard therapy, with benefits observed as early as week 28 for remission and week 8 for disease activity, according to pooled results from five clinical trials.
METHODOLOGY:
- Researchers conducted an integrated post hoc analysis of five randomized phase 3 clinical trials to evaluate the attainment of remission and low disease activity in adult patients with active, autoantibody-positive SLE.
- A total of 3086 patients (median age, 36 years; 94% women) were randomly assigned to receive standard therapy with intravenous belimumab 10 mg/kg monthly or subcutaneous belimumab 200 mg weekly (n = 1869) or placebo (n = 1217).
- The proportion of patients who achieved definitions of remission in SLE (DORIS) remission and lupus low disease activity state (LLDAS) by visit up to week 52 was assessed.
- The analysis also evaluated the time taken to achieve sustained (at least two consecutive visits) and maintained (up to week 52) DORIS remission and LLDAS.
TAKEAWAY:
- At week 52, a higher proportion of patients receiving belimumab vs placebo achieved DORIS remission (8% vs 6%; risk ratio [RR], 1.51; P = .0055) and LLDAS (17% vs 10%; RR, 1.74; P < .0001).
- The earliest observed significant benefit of belimumab over placebo in patients with a higher baseline disease activity was at week 20 for DORIS remission (RR, 2.09; P = .043) and at week 16 for LLDAS (RR, 1.46; P = .034), with both maintained through week 52.
- The proportion of patients who attained DORIS remission and LLDAS as early as week 28 and week 8, respectively, was higher in the belimumab group than in the placebo group, with both maintained through week 52.
- Patients on belimumab were more likely to have a sustained and maintained DORIS remission (hazard ratio [HR], 1.53; P = .013) and LLDAS (HR, 1.79; P < .0001) at any timepoint.
IN PRACTICE:
“The data clearly support that belimumab is a valuable addition toward accomplishing and maintaining remission or LLDAS,” George Bertsias, MD, PhD, University of Crete Medical School, Heraklion, Greece, and Jinoos Yazdany, MD, University of California San Francisco, wrote in a related comment.
SOURCE:
This study, led by Ioannis Parodis, MD, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden, was published online on August 26, 2024, in The Lancet Rheumatology.
LIMITATIONS:
Due to the post hoc nature of the analysis, the trials were not specifically designed to have adequate statistical power to demonstrate the difference between patients who did or did not achieve DORIS remission or LLDAS. The analysis was limited to patients who met the eligibility criteria, and the outcomes are not generalizable to populations outside a clinical trial setting. The study population had high disease activity, which made it challenging to attain the treatment targets.
DISCLOSURES:
The five trials included in this analysis were funded by GSK. The study was supported by the Swedish Rheumatism Association, King Gustaf V’s 80-year Foundation, the Swedish Society of Medicine, Nyckelfonden, Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, Region Stockholm, and Karolinska Institutet. Some authors reported receiving grants, speaker honoraria, or consulting fees from various pharmaceutical companies. Some authors reported being employees and owning stocks and shares of GSK.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
AI-Powered Clinical Documentation Tool Reduces EHR Time for Clinicians
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An artificial intelligence (AI)-powered clinical documentation tool helped reduce time spent on electronic health records (EHR) at home for almost 48% physicians, and nearly 45% reported less weekly time spent on EHR tasks outside of normal work hours.
METHODOLOGY:
- Researchers recruited 112 clinicians from family medicine, internal medicine, and general pediatrics in North Carolina and Georgia.
- Patients were divided into an intervention group (n = 85) and control group (n = 55), with the intervention group receiving a 1-hour training program on a commercially available AI tool.
- A seven-question survey was administered to participants before and 5 weeks after the intervention to evaluate their experience.
TAKEAWAY:
- The researchers found 47.1% of clinicians in the intervention group reported spending less time on the EHR at home compared with 14.5% in the control group (P < .001); 44.7% reported decreased weekly time on the EHR outside normal work hours compared with 20% in the control group (P = .003).
- The study revealed 43.5% of physicians who used the AI instrument reported spending less time on documentation after visits compared with 18.2% in the control group (P = .002).
- Further, 44.7% reported less frustration when using the EHR compared with 14.5% in the control group (P < .001).
IN PRACTICE:
“Approximately half of clinicians using the AI-powered clinical documentation tool based on interest reported a positive outcome, potentially reducing burnout. However, a significant subset did not find time-saving benefits or improved EHR experience,” the authors of the study wrote.
SOURCE:
The study was led by Tsai-Ling Liu, PhD, Center for Health System Sciences, Atrium Health in Charlotte, North Carolina. It was published online in JAMA Network Open.
LIMITATIONS:
The researchers reported potential selection and recall bias in both groups. Additional research is needed to find areas of improvement and assess the effects on clinician groups and health systems, they said.
DISCLOSURES:
Andrew McWilliams, MD, MPH, reported receiving grants from the Agency for Healthcare Research Quality, the National Institutes of Health, and the Duke Endowment unrelated to this work. Ajay Dharod, MD, reported his role as an electronic health record consultant for the Association of American Medical College CORE program. Jeffrey Cleveland, MD, disclosed his participation on the Executive Client Council, a noncompensated advisory group, for Nuance/Microsoft.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Are Pharmacy Deserts Worsening Health Disparities?
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Pharmacy closures in the United States are creating “pharmacy deserts,” disproportionately affecting socially vulnerable communities. High social vulnerability and low primary care practitioner (PCP) density are linked to increased pharmacy desert density.
METHODOLOGY:
- Data through 2020 on communities located 10 or more miles from the nearest retail pharmacy were sourced from TelePharm Map.
- Counties were stratified as having a high pharmacy desert density if the number of pharmacy deserts per 1000 inhabitants was in the 80th percentile or higher.
- Social vulnerability index and healthcare practitioner data were obtained from the Agency for Toxic Substances and Disease Registry and the Area Health Resources Files.
- PCP density was calculated as the number of PCPs per 10,000 inhabitants.
- A total of 3143 counties were analyzed, with 1447 (46%) having at least one pharmacy desert.
TAKEAWAY:
- Counties with a high pharmacy desert density had a higher social vulnerability index than those with a low pharmacy desert density (P = .006).
- Areas with a high pharmacy desert density had lower median PCP density than those with low or no pharmacy desert density (P < .001).
- High social vulnerability index (odds ratio [OR], 1.35; 95% CI, 1.07-1.70; P = .01) and low PCP density (OR, 2.27; 95% CI, 1.80-2.86; P < .001) were associated with a higher likelihood for a county to have a high pharmacy desert density.
- Pharmacy closures are leaving more individuals without easy access to medications, with disproportionate consequences for certain communities.
IN PRACTICE:
“As high pharmacy desert density counties also have a lower PCP density, patients residing in these regions face increased barriers to accessing primary healthcare needs,” wrote the authors of the study.
SOURCE:
The study was led by Giovanni Catalano, MD, Muhammad Muntazir Mehdi Khan, MBBS, and Timothy M. Pawlik, MD, PhD, MPH, MTS, MBA, Department of Surgery, The Ohio State University Wexner Medical Center in Columbus, Ohio. It was published online in JAMA Network Open.
LIMITATIONS:
The cross-sectional design of the study limited the ability to draw causal inferences. The study relied on public county-level data, which may not have captured all relevant variables. The use of the social vulnerability index and PCP density as proxies did not fully represent the complexity of pharmacy access issues. The study’s findings were not generalizable to regions outside the United States.
DISCLOSURES:
No relevant conflicts of interest were disclosed by the authors. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
UVA Defends Medical School Dean, Hospital CEO After Docs Call for Their Removal
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
The University of Virginia (UVA) is defending the CEO of its health system and its medical school dean in the wake of a very public call for their removal.
At least 128 members of the University of Virginia faculty who are employed by both the medical school and the UVA Physicians Group wrote to the UVA Board of Visitors and its peer-elected faculty leaders, expressing no confidence in K. Craig Kent, MD, CEO of UVA Health and executive vice president for health affairs, and Melina Kibbe, MD, dean of the medical school and chief health affairs officer.
Dr. Kibbe, a vascular surgeon and researcher, is also the editor in chief of JAMA Surgery.
“We call for the immediate removal of Craig Kent and Melina Kibbe,” wrote the physicians.
The letter alleged that patient safety was compromised because doctors, nurses, and other staff were pressured to abstain from reporting safety concerns and that physicians had been hired “despite concerns regarding integrity and quality.” Those who raised safety concerns faced “explicit and implicit threats and retaliation,” including delays and denials of promotion and tenure, said the letter.
The September 5 letter did not include signatures. The authors said that names were being protected, but that they would share the names with a limited audience.
UVA President Jim Ryan took issue with the notion that the signees were anonymous. He said in his own letter to medical school faculty that some of the accusations were about matters that had already been addressed or that were being worked on. As far as allegations that he was not previously aware of, “we will do our best to investigate,” he said.
The faculty who signed the letter “have besmirched the reputations of not just Melina and Craig,” wrote Mr. Ryan. “They have unfairly — and I trust unwittingly — cast a shadow over the great work of the entire health system and medical school.”
The authors claimed that reports about bullying and harassment of trainees had been “suppressed, minimized, and subsequently altered.”
And they said that spending on leadership was prioritized over addressing clinical and technical staff shortages. Whistleblowers who reported fraud were not protected, and clinicians were pressured to modify patient records to “obfuscate adverse outcomes and boost productivity metrics,” they wrote.
The 128 members of the UVA Physicians Group who signed the letter represent about 10% of the 1400 medical school faculty members.
It is not the first time that Dr. Kent has been given a vote of no confidence. In 2017, when he was the dean of the College of Medicine at the Ohio State University, Dr. Kent was accused in a “no confidence” letter from 25 physicians and faculty of helping to undermine the school’s mission and taking actions that led to resignations and early retirements of many staff, the Columbus Dispatch reported.
William G. Crutchfield Jr., a member of the UVA Health System Board, defended Dr. Kent and Dr. Kibbe in a lengthy statement shared with this news organization. He said that UVA Health’s four hospitals had received “A” ratings for safety, and that the system has a 5.1% turnover rate compared with a national average of 8.3%.
Dr. Kent and Dr. Kibbe have recruited faculty from top academic medical centers, Mr. Crutchfield wrote.
“If our work environment were so toxic, these people would not have joined our faculty,” he wrote.
Mr. Crutchfield credited Dr. Kent and Dr. Kibbe with crafting a new 10-year strategic plan and for hiring a chief strategy officer to lead the plan — a move that replaced “expensive outside consultants.”
Mr. Ryan said in his letter that his inbox “is overflowing with testimonials from some of the 1200-plus faculty who did not sign the letter, who attest that the health system today — under Melina and Craig’s leadership — is in the best shape it has ever been in, and that they have addressed changes that have needed to be made for more than two decades.”
A request to see some of these positive testimonials was not answered by press time.
Mr. Crutchfield, like Mr. Ryan, said that the letter writers were doing more harm than good.
“If a small cabal of people hiding behind anonymity can force outstanding leaders out of UVA, it will make it extremely difficult to recruit outstanding new physicians, nurses, technicians, and administrators,” he wrote.
A version of this article first appeared on Medscape.com.
Early Use of Steroids Linked to Prolonged Treatment in Early Rheumatoid Arthritis
TOPLINE:
A substantial proportion of older adults with early rheumatoid arthritis (RA) initiate glucocorticoids before receiving care from a rheumatologist. The early initiation of glucocorticoids in this group is associated with prolonged use.
METHODOLOGY:
- Researchers analyzed data from Medicare claims and Rheumatology Informatics System for Effectiveness registry of the American College of Rheumatology from 2016 to 2018 to assess the relationship between the timing of glucocorticoid initiation and the subsequent duration of glucocorticoid use in older adults with early RA in the United States.
- They included 1733 patients aged ≥ 65 years (mean age, 76 years; 67% women) with early RA.
- Glucocorticoid initiation was defined as the first use between 3 months before and 6 months after entrance into rheumatology care.
- The continuous administration of glucocorticoid therapy was monitored for all individuals who initiated glucocorticoid treatment for up to 12 months after entering rheumatology care.
- The primary outcome was the duration of continuous glucocorticoid use after entering rheumatology care.
TAKEAWAY:
- Glucocorticoids were initiated in 41% of patients, with 65% starting them before the initial RA diagnosis by a rheumatologist. The median duration of glucocorticoid use was 157 days.
- Patients with early RA who initiated glucocorticoids before entering rheumatology care showed a significantly longer duration of glucocorticoid use than those who initiated it later (median, 186 vs 97 days; P < .0001).
- Patients who initiated glucocorticoids before entering rheumatology care were 39% less likely to stop its use within 1 year (hazard ratio, 0.61; 95% CI, 0.51-0.74).
- The mean daily dose of glucocorticoids was < 5 mg/d for patients who received them for at least 3 months, indicating a trend toward low-dose, long-term use.
IN PRACTICE:
“Initiatives to reduce GC [glucocorticoid] exposure among patients with eRA [early RA] will likely require attention to rheumatology workforce shortages and close collaboration between rheumatologists and primary care clinicians to expedite referrals to rheumatology care,” the authors wrote.
SOURCE:
This study was led by Andriko Palmowski, MD, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin in Germany, and was published online in Seminars in Arthritis and Rheumatism.
LIMITATIONS:
The observational nature of this study limited the ability to establish causality between early glucocorticoid initiation and the prolonged use of glucocorticoids. Moreover, the study population was limited to older adults, which affected the generalizability of the findings to younger populations. It also did not account for the fact that patients with more severe early RA were more likely to start glucocorticoid therapy early and continue it for longer durations.
DISCLOSURES:
The study was supported by research grants from the Deutsche Autoimmun-Stiftung and other sources. Some authors declared receiving grant support, consultancy fees, honoraria, and travel expenses and had other ties with various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A substantial proportion of older adults with early rheumatoid arthritis (RA) initiate glucocorticoids before receiving care from a rheumatologist. The early initiation of glucocorticoids in this group is associated with prolonged use.
METHODOLOGY:
- Researchers analyzed data from Medicare claims and Rheumatology Informatics System for Effectiveness registry of the American College of Rheumatology from 2016 to 2018 to assess the relationship between the timing of glucocorticoid initiation and the subsequent duration of glucocorticoid use in older adults with early RA in the United States.
- They included 1733 patients aged ≥ 65 years (mean age, 76 years; 67% women) with early RA.
- Glucocorticoid initiation was defined as the first use between 3 months before and 6 months after entrance into rheumatology care.
- The continuous administration of glucocorticoid therapy was monitored for all individuals who initiated glucocorticoid treatment for up to 12 months after entering rheumatology care.
- The primary outcome was the duration of continuous glucocorticoid use after entering rheumatology care.
TAKEAWAY:
- Glucocorticoids were initiated in 41% of patients, with 65% starting them before the initial RA diagnosis by a rheumatologist. The median duration of glucocorticoid use was 157 days.
- Patients with early RA who initiated glucocorticoids before entering rheumatology care showed a significantly longer duration of glucocorticoid use than those who initiated it later (median, 186 vs 97 days; P < .0001).
- Patients who initiated glucocorticoids before entering rheumatology care were 39% less likely to stop its use within 1 year (hazard ratio, 0.61; 95% CI, 0.51-0.74).
- The mean daily dose of glucocorticoids was < 5 mg/d for patients who received them for at least 3 months, indicating a trend toward low-dose, long-term use.
IN PRACTICE:
“Initiatives to reduce GC [glucocorticoid] exposure among patients with eRA [early RA] will likely require attention to rheumatology workforce shortages and close collaboration between rheumatologists and primary care clinicians to expedite referrals to rheumatology care,” the authors wrote.
SOURCE:
This study was led by Andriko Palmowski, MD, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin in Germany, and was published online in Seminars in Arthritis and Rheumatism.
LIMITATIONS:
The observational nature of this study limited the ability to establish causality between early glucocorticoid initiation and the prolonged use of glucocorticoids. Moreover, the study population was limited to older adults, which affected the generalizability of the findings to younger populations. It also did not account for the fact that patients with more severe early RA were more likely to start glucocorticoid therapy early and continue it for longer durations.
DISCLOSURES:
The study was supported by research grants from the Deutsche Autoimmun-Stiftung and other sources. Some authors declared receiving grant support, consultancy fees, honoraria, and travel expenses and had other ties with various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
A substantial proportion of older adults with early rheumatoid arthritis (RA) initiate glucocorticoids before receiving care from a rheumatologist. The early initiation of glucocorticoids in this group is associated with prolonged use.
METHODOLOGY:
- Researchers analyzed data from Medicare claims and Rheumatology Informatics System for Effectiveness registry of the American College of Rheumatology from 2016 to 2018 to assess the relationship between the timing of glucocorticoid initiation and the subsequent duration of glucocorticoid use in older adults with early RA in the United States.
- They included 1733 patients aged ≥ 65 years (mean age, 76 years; 67% women) with early RA.
- Glucocorticoid initiation was defined as the first use between 3 months before and 6 months after entrance into rheumatology care.
- The continuous administration of glucocorticoid therapy was monitored for all individuals who initiated glucocorticoid treatment for up to 12 months after entering rheumatology care.
- The primary outcome was the duration of continuous glucocorticoid use after entering rheumatology care.
TAKEAWAY:
- Glucocorticoids were initiated in 41% of patients, with 65% starting them before the initial RA diagnosis by a rheumatologist. The median duration of glucocorticoid use was 157 days.
- Patients with early RA who initiated glucocorticoids before entering rheumatology care showed a significantly longer duration of glucocorticoid use than those who initiated it later (median, 186 vs 97 days; P < .0001).
- Patients who initiated glucocorticoids before entering rheumatology care were 39% less likely to stop its use within 1 year (hazard ratio, 0.61; 95% CI, 0.51-0.74).
- The mean daily dose of glucocorticoids was < 5 mg/d for patients who received them for at least 3 months, indicating a trend toward low-dose, long-term use.
IN PRACTICE:
“Initiatives to reduce GC [glucocorticoid] exposure among patients with eRA [early RA] will likely require attention to rheumatology workforce shortages and close collaboration between rheumatologists and primary care clinicians to expedite referrals to rheumatology care,” the authors wrote.
SOURCE:
This study was led by Andriko Palmowski, MD, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin in Germany, and was published online in Seminars in Arthritis and Rheumatism.
LIMITATIONS:
The observational nature of this study limited the ability to establish causality between early glucocorticoid initiation and the prolonged use of glucocorticoids. Moreover, the study population was limited to older adults, which affected the generalizability of the findings to younger populations. It also did not account for the fact that patients with more severe early RA were more likely to start glucocorticoid therapy early and continue it for longer durations.
DISCLOSURES:
The study was supported by research grants from the Deutsche Autoimmun-Stiftung and other sources. Some authors declared receiving grant support, consultancy fees, honoraria, and travel expenses and had other ties with various pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.

