User login
AGA clinical practice update: Extraesophageal gastroesophageal reflux disease
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.
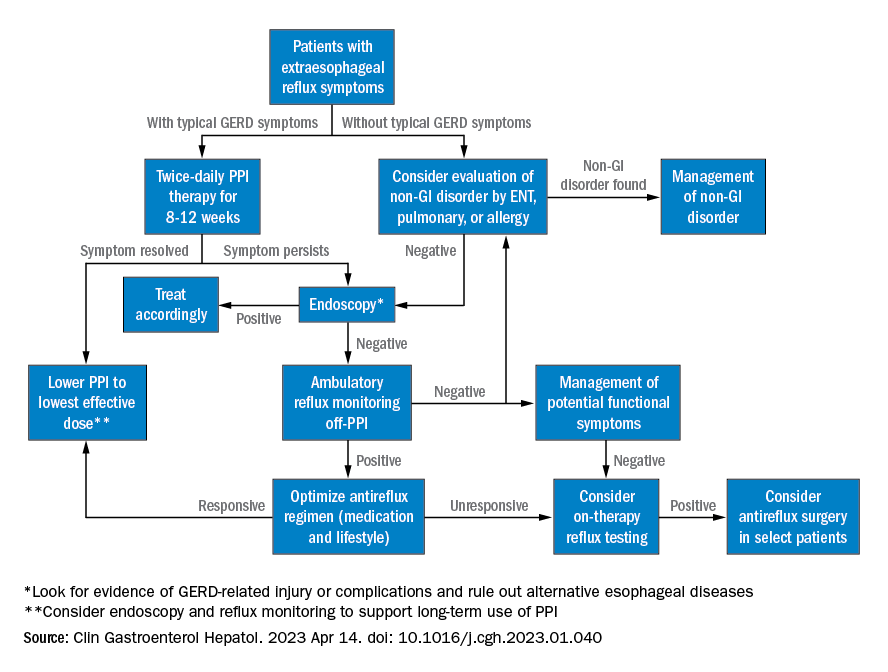
Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.

Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
Extraesophageal reflux (EER) symptoms are a subset of gastroesophageal reflux disease (GERD) that can be difficult to diagnose because of its heterogeneous nature and symptoms that overlap with other conditions.
That puts the onus on physicians to take all symptoms into account and work across disciplines to diagnose, manage, and treat the condition, according to a new clinical practice update from the American Gastroenterological Association, which was published in Clinical Gastroenterology and Hepatology.
GERD is becoming increasingly common, which in turn has led to greater awareness and consideration of EER symptoms. EER symptoms can present a challenge because they may vary considerably and are not unique to GERD. The symptoms often do not respond well to proton pump inhibitor (PPI) therapy.
EER symptoms can include cough, laryngeal hoarseness, dysphonia, pulmonary fibrosis, asthma, dental erosions/caries, sinus disease, ear disease, postnasal drip, and throat clearing. Some patients with EER symptoms do not report heartburn or regurgitation, which leaves it up to the physician to determine if acid reflux is present and contributing to symptoms.
“The concept of extraesophageal symptoms secondary to GERD is complex and often controversial, leading to diagnostic and therapeutic challenges. Several extraesophageal symptoms have been associated with GERD, although the strength of evidence to support a causal relation varies,” wrote the authors, who were led by Joan W. Chen, MD, MS, a gastroenterologist with the University of Michigan, Ann Arbor.
There is also debate over whether fluid refluxate is the source of damage that causes EER symptoms, and if so, whether it is sufficient that the fluid be acidic or that pepsin be present, or if the cause is related to neurogenic signaling and resulting inflammation. Because of these questions, a PPI trial will not necessarily provide insight into the role of acid reflux in EER symptoms.

Best practice advice 1: The authors emphasized that gastroenterologists need to be aware of the potential extraesophageal symptoms of GERD. They should inquire with GERD patients to determine if laryngitis, chronic cough, asthma, and dental erosions are present.
Best practice advice 2: Consider a multidisciplinary approach to EER manifestations. Cases may require input from non-GI specialties. Tests performed by other specialists, such as bronchoscopy, thoracic imaging, or laryngoscopy, should be taken into account, since patients will also seek out multiple specialists to address their symptoms.
Best practice advice 3: There is no specific diagnostic test available to determine if GER is the cause of EER symptoms. Instead, physicians should interpret patient symptoms, response to GER therapy, and input from endoscopy and reflux tests.
Best practice advice 4: Rather than subject the patient to the cost and potential for even rare adverse events of a PPI trial, physicians should first consider conducting reflux testing. A PPI trial has clinical value but is insufficient on its own to help diagnose or manage EER. Initial single-dose PPI trial, titrating up to twice daily in those with typical GERD symptoms, is reasonable.
Best practice advice 5: The inconsistent therapeutic response to PPI therapy means that positive effects of PPI therapy on EER symptoms can’t confirm a GERD diagnosis because a placebo effect may be involved, and because symptom improvement can occur through mechanisms other than acid suppression. A meta-analysis found that a PPI trial has a sensitivity of 71%-78% and a specificity of 41%-54% with typical symptoms of heartburn and regurgitation. “Considering the greater variation expected with PPI response for extraesophageal symptoms, the diagnostic performance of empiric PPI trial for a diagnosis of EER would be anticipated to be substantially lower,” the authors wrote.
Best practice advice 6: When EER symptoms related to GERD are suspected and a PPI trial of up to 12 weeks does not lead to adequate improvement, the physician should consider testing for pathologic GER. Additional trials employing other PPIs are unlikely to succeed.
Best practice advice 7: Initial testing to evaluate for reflux should be tailored to patients’ clinical presentation. Potential methods to evaluate reflux include upper endoscopy and ambulatory reflux monitoring studies of acid suppressive therapy, which can assist with a GERD diagnosis, particularly when nonerosive reflux is present.
Best practice advice 8: About 50%-60% of patients with EER symptoms will not have GERD. Testing can be considered for those with an established objective diagnosis of GERD who do not respond well to high doses of acid suppression. Cost-effectiveness studies have confirmed the value of starting with ambulatory reflux monitoring, which can include a catheter-based pH sensor, pH impedance, or wireless pH capsule.
Ambulatory esophageal pH monitoring can also assist in making a GERD diagnosis, but it does not indicate whether GERD may be contributing to EER symptoms.
“Whichever the reflux testing modality, the strongest confidence for EER is achieved after ambulatory reflux testing showing pathologic acid exposure and a positive symptom-reflux association for EER symptoms,” the authors wrote. They also pointed out that ambulatory reflux monitoring in EER patients should be done in the absence of acid suppression unless there is already objective evidence for the presence of GERD.
Best practice advice 9: Aside from acid suppression, EER symptoms can also be managed through other means, including lifestyle modifications, such as eating avoidance prior to lying down, elevation of the head of the bed, sleeping on the left side, and weight loss. Or, alginate containing antacids, external upper esophageal sphincter compression device, cognitive behavioral therapy, and neuromodulators.
Best practice advice 10: In cases where the EER patient has objectively defined evidence of GERD, physicians should employ shared decision-making before considering anti-reflux surgery. If the patient did not respond to PPI therapy, this predicts a lack of response to antireflux surgery.
All four authors reported financial ties to multiple pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Joint symposium addresses exocrine pancreatic insufficiency
Based on discussions during PancreasFest 2021, , according to a recent report in Gastro Hep Advances
Due to its complex and individualized nature, EPI requires multidisciplinary approaches to therapy, as well as better pancreas function tests and biomarkers for diagnosis and treatment, wrote researchers who were led by David C. Whitcomb, MD, PhD, AGAF, emeritus professor of medicine in the division of gastroenterology, hepatology and nutrition at the University of Pittsburgh.
“This condition remains challenging even to define, and serious limitations in diagnostic testing and therapeutic options lead to clinical confusion and frequently less than optimal patient management,” the authors wrote.
EPI is clinically defined as inadequate delivery of pancreatic digestive enzymes to meet nutritional needs, which is typically based on a physician’s assessment of a patient’s maldigestion. However, there’s not a universally accepted definition or a precise threshold of reduced pancreatic digestive enzymes that indicates “pancreatic insufficiency” in an individual patient.
Current guidelines also don’t clearly outline the role of pancreatic function tests, the effects of different metabolic needs and nutrition intake, the timing of pancreatic enzyme replacement therapy (PERT), or the best practices for monitoring or titrating multiple therapies.
In response, Dr. Whitcomb and colleagues proposed a new mechanistic definition of EPI, including the disorder’s physiologic effects and impact on health. First, they said, EPI is a disorder caused by failure of the pancreas to deliver a minimum or threshold level of specific pancreatic digestive enzymes to the intestine in concert with ingested nutrients, followed by enzymatic digestion of individual meals over time to meet certain nutritional and metabolic needs. In addition, the disorder is characterized by variable deficiencies in micronutrients and macronutrients, especially essential fats and fat-soluble vitamins, as well as gastrointestinal symptoms of nutrient maldigestion.
The threshold for EPI should consider the nutritional needs of the patient, dietary intake, residual exocrine pancreas function, and the absorptive capacity of the intestine based on anatomy, mucosal function, motility, inflammation, the microbiome, and physiological adaptation, the authors wrote.
Due to challenges in diagnosing EPI and its common chronic symptoms such as abdominal pain, bloating, and diarrhea, several conditions may mimic EPI, be present concomitantly with EPI, or hinder PERT response. These include celiac disease, small intestinal bacterial overgrowth, disaccharidase deficiencies, inflammatory bowel disease (IBD), bile acid diarrhea, giardiasis, diabetes mellitus, and functional conditions such as irritable bowel syndrome. These conditions should be considered to address underlying pathology and PERT diagnostic challenges.
Although there is consensus that exocrine pancreatic function testing (PFT) is important to diagnosis EPI, no optimal test exists, and pancreatic function is only one aspect of digestion and absorption that should be considered. PFT may be needed to make an objective EPI diagnosis related to acute pancreatitis, pancreatic cancer, pancreatic resection, gastric resection, cystic fibrosis, or IBD. Direct or indirect PFTs may be used, which typically differs by center.
“The medical community still awaits a clinically useful pancreas function test that is easy to perform, well tolerated by patients, and allows personalized dosing of PERT,” the authors wrote.
After diagnosis, a general assessment should include information about symptoms, nutritional status, medications, diet, and lifestyle. This information can be used for a multifaceted treatment approach, with a focus on lifestyle changes, concomitant disease treatment, optimized diet, dietary supplements, and PERT administration.
PERT remains a mainstay of EPI treatment and has shown improvements in steatorrhea, postprandial bloating and pain, nutrition, and unexplained weight loss. The Food and Drug Administration has approved several formulations in different strengths. The typical starting dose is based on age and weight, which is derived from guidelines for EPI treatment in patients with cystic fibrosis. However, the recommendations don’t consider many of the variables discussed above and simply provide an estimate for the average subject with severe EPI, so the dose should be titrated as needed based on age, weight, symptoms, and the holistic management plan.
For optimal results, regular follow-up is necessary to monitor compliance and treatment response. A reduction in symptoms can serve as a reliable indicator of effective EPI management, particularly weight stabilization, improved steatorrhea and diarrhea, and reduced postprandial bloating, pain, and flatulence. Physicians may provide patients with tracking tools to record their PERT compliance, symptom frequency, and lifestyle changes.
For patients with persistent concerns, PERT can be increased as needed. Although many PERT formulations are enteric coated, a proton pump inhibitor or H2 receptor agonist may improve their effectiveness. If EPI symptoms persist despite increased doses, other causes of malabsorption should be considered, such as the concomitant conditions mentioned above.
“As EPI escalates, a lower fat diet may become necessary to alleviate distressing gastrointestinal symptoms,” the authors wrote. “A close working relationship between the treating provider and the [registered dietician] is crucial so that barriers to optimum nutrient assimilation can be identified, communicated, and overcome. Frequent monitoring of the nutritional state with therapy is also imperative.”
PancreasFest 2021 received no specific funding for this event. The authors declared grant support, adviser roles, and speaking honoraria from several pharmaceutical and medical device companies and health care foundations, including the National Pancreas Foundation.
Recognition of recent advances and unaddressed gaps can clarify key issues around exocrine pancreatic insufficiency (EPI).
The loss of pancreatic digestive enzymes and bicarbonate is caused by exocrine pancreatic and proximal small intestine disease. EPI’s clinical impact has been expanded by reports that 30% of subjects can develop EPI after a bout of acute pancreatitis. Diagnosing and treating EPI challenges clinicians and investigators.
The contribution on EPI by Whitcomb and colleagues provides state-of-the-art content relating to diagnosing EPI, assessing its metabolic impact, enzyme replacement, nutritional considerations, and how to assess the effectiveness of therapy.
Though the diagnosis and treatment of EPI have been examined for over 50 years, a consensus for either is still needed. Assessment of EPI with luminal tube tests and endoscopic collections of pancreatic secretion are the most accurate, but they are invasive, limited in availability, and time-consuming. Indirect assays of intestinal activities of pancreatic enzymes by the hydrolysis of substrates or stool excretion are frequently used to diagnose EPI. However, they need to be more insensitive and specific to meet clinical and investigative needs.
Indeed, all tests of exocrine secretion are surrogates of unclear value for the critical endpoint of EPI, its nutritional impact. An unmet need is the development of nutritional standards for assessing EPI and measures for the adequacy of pancreatic enzyme replacement therapy. In this context, a patient’s diet, and other factors, such as the intestinal microbiome, can affect pancreatic digestive enzyme activity and must be considered in designing the best EPI treatments. The summary concludes with a thoughtful and valuable road map for moving forward.
Fred Sanford Gorelick, MD, is the Henry J. and Joan W. Binder Professor of Medicine (Digestive Diseases) and of Cell Biology for Yale School of Medicine, New Haven, Conn. He also serves as director of the Yale School of Medicine NIH T32-funded research track in gastroenterology; and as deputy director of Yale School of Medicine MD-PhD program.
Potential conflicts: Dr. Gorelick serves as chair of NIH NIDDK DSMB for Stent vs. Indomethacin for Preventing Post-ERCP Pancreatitis (SVI) study. He also holds grants for research on mechanisms of acute pancreatitis from the U.S. Department of Veterans Affairs and the Department of Defense.
Recognition of recent advances and unaddressed gaps can clarify key issues around exocrine pancreatic insufficiency (EPI).
The loss of pancreatic digestive enzymes and bicarbonate is caused by exocrine pancreatic and proximal small intestine disease. EPI’s clinical impact has been expanded by reports that 30% of subjects can develop EPI after a bout of acute pancreatitis. Diagnosing and treating EPI challenges clinicians and investigators.
The contribution on EPI by Whitcomb and colleagues provides state-of-the-art content relating to diagnosing EPI, assessing its metabolic impact, enzyme replacement, nutritional considerations, and how to assess the effectiveness of therapy.
Though the diagnosis and treatment of EPI have been examined for over 50 years, a consensus for either is still needed. Assessment of EPI with luminal tube tests and endoscopic collections of pancreatic secretion are the most accurate, but they are invasive, limited in availability, and time-consuming. Indirect assays of intestinal activities of pancreatic enzymes by the hydrolysis of substrates or stool excretion are frequently used to diagnose EPI. However, they need to be more insensitive and specific to meet clinical and investigative needs.
Indeed, all tests of exocrine secretion are surrogates of unclear value for the critical endpoint of EPI, its nutritional impact. An unmet need is the development of nutritional standards for assessing EPI and measures for the adequacy of pancreatic enzyme replacement therapy. In this context, a patient’s diet, and other factors, such as the intestinal microbiome, can affect pancreatic digestive enzyme activity and must be considered in designing the best EPI treatments. The summary concludes with a thoughtful and valuable road map for moving forward.
Fred Sanford Gorelick, MD, is the Henry J. and Joan W. Binder Professor of Medicine (Digestive Diseases) and of Cell Biology for Yale School of Medicine, New Haven, Conn. He also serves as director of the Yale School of Medicine NIH T32-funded research track in gastroenterology; and as deputy director of Yale School of Medicine MD-PhD program.
Potential conflicts: Dr. Gorelick serves as chair of NIH NIDDK DSMB for Stent vs. Indomethacin for Preventing Post-ERCP Pancreatitis (SVI) study. He also holds grants for research on mechanisms of acute pancreatitis from the U.S. Department of Veterans Affairs and the Department of Defense.
Recognition of recent advances and unaddressed gaps can clarify key issues around exocrine pancreatic insufficiency (EPI).
The loss of pancreatic digestive enzymes and bicarbonate is caused by exocrine pancreatic and proximal small intestine disease. EPI’s clinical impact has been expanded by reports that 30% of subjects can develop EPI after a bout of acute pancreatitis. Diagnosing and treating EPI challenges clinicians and investigators.
The contribution on EPI by Whitcomb and colleagues provides state-of-the-art content relating to diagnosing EPI, assessing its metabolic impact, enzyme replacement, nutritional considerations, and how to assess the effectiveness of therapy.
Though the diagnosis and treatment of EPI have been examined for over 50 years, a consensus for either is still needed. Assessment of EPI with luminal tube tests and endoscopic collections of pancreatic secretion are the most accurate, but they are invasive, limited in availability, and time-consuming. Indirect assays of intestinal activities of pancreatic enzymes by the hydrolysis of substrates or stool excretion are frequently used to diagnose EPI. However, they need to be more insensitive and specific to meet clinical and investigative needs.
Indeed, all tests of exocrine secretion are surrogates of unclear value for the critical endpoint of EPI, its nutritional impact. An unmet need is the development of nutritional standards for assessing EPI and measures for the adequacy of pancreatic enzyme replacement therapy. In this context, a patient’s diet, and other factors, such as the intestinal microbiome, can affect pancreatic digestive enzyme activity and must be considered in designing the best EPI treatments. The summary concludes with a thoughtful and valuable road map for moving forward.
Fred Sanford Gorelick, MD, is the Henry J. and Joan W. Binder Professor of Medicine (Digestive Diseases) and of Cell Biology for Yale School of Medicine, New Haven, Conn. He also serves as director of the Yale School of Medicine NIH T32-funded research track in gastroenterology; and as deputy director of Yale School of Medicine MD-PhD program.
Potential conflicts: Dr. Gorelick serves as chair of NIH NIDDK DSMB for Stent vs. Indomethacin for Preventing Post-ERCP Pancreatitis (SVI) study. He also holds grants for research on mechanisms of acute pancreatitis from the U.S. Department of Veterans Affairs and the Department of Defense.
Based on discussions during PancreasFest 2021, , according to a recent report in Gastro Hep Advances
Due to its complex and individualized nature, EPI requires multidisciplinary approaches to therapy, as well as better pancreas function tests and biomarkers for diagnosis and treatment, wrote researchers who were led by David C. Whitcomb, MD, PhD, AGAF, emeritus professor of medicine in the division of gastroenterology, hepatology and nutrition at the University of Pittsburgh.
“This condition remains challenging even to define, and serious limitations in diagnostic testing and therapeutic options lead to clinical confusion and frequently less than optimal patient management,” the authors wrote.
EPI is clinically defined as inadequate delivery of pancreatic digestive enzymes to meet nutritional needs, which is typically based on a physician’s assessment of a patient’s maldigestion. However, there’s not a universally accepted definition or a precise threshold of reduced pancreatic digestive enzymes that indicates “pancreatic insufficiency” in an individual patient.
Current guidelines also don’t clearly outline the role of pancreatic function tests, the effects of different metabolic needs and nutrition intake, the timing of pancreatic enzyme replacement therapy (PERT), or the best practices for monitoring or titrating multiple therapies.
In response, Dr. Whitcomb and colleagues proposed a new mechanistic definition of EPI, including the disorder’s physiologic effects and impact on health. First, they said, EPI is a disorder caused by failure of the pancreas to deliver a minimum or threshold level of specific pancreatic digestive enzymes to the intestine in concert with ingested nutrients, followed by enzymatic digestion of individual meals over time to meet certain nutritional and metabolic needs. In addition, the disorder is characterized by variable deficiencies in micronutrients and macronutrients, especially essential fats and fat-soluble vitamins, as well as gastrointestinal symptoms of nutrient maldigestion.
The threshold for EPI should consider the nutritional needs of the patient, dietary intake, residual exocrine pancreas function, and the absorptive capacity of the intestine based on anatomy, mucosal function, motility, inflammation, the microbiome, and physiological adaptation, the authors wrote.
Due to challenges in diagnosing EPI and its common chronic symptoms such as abdominal pain, bloating, and diarrhea, several conditions may mimic EPI, be present concomitantly with EPI, or hinder PERT response. These include celiac disease, small intestinal bacterial overgrowth, disaccharidase deficiencies, inflammatory bowel disease (IBD), bile acid diarrhea, giardiasis, diabetes mellitus, and functional conditions such as irritable bowel syndrome. These conditions should be considered to address underlying pathology and PERT diagnostic challenges.
Although there is consensus that exocrine pancreatic function testing (PFT) is important to diagnosis EPI, no optimal test exists, and pancreatic function is only one aspect of digestion and absorption that should be considered. PFT may be needed to make an objective EPI diagnosis related to acute pancreatitis, pancreatic cancer, pancreatic resection, gastric resection, cystic fibrosis, or IBD. Direct or indirect PFTs may be used, which typically differs by center.
“The medical community still awaits a clinically useful pancreas function test that is easy to perform, well tolerated by patients, and allows personalized dosing of PERT,” the authors wrote.
After diagnosis, a general assessment should include information about symptoms, nutritional status, medications, diet, and lifestyle. This information can be used for a multifaceted treatment approach, with a focus on lifestyle changes, concomitant disease treatment, optimized diet, dietary supplements, and PERT administration.
PERT remains a mainstay of EPI treatment and has shown improvements in steatorrhea, postprandial bloating and pain, nutrition, and unexplained weight loss. The Food and Drug Administration has approved several formulations in different strengths. The typical starting dose is based on age and weight, which is derived from guidelines for EPI treatment in patients with cystic fibrosis. However, the recommendations don’t consider many of the variables discussed above and simply provide an estimate for the average subject with severe EPI, so the dose should be titrated as needed based on age, weight, symptoms, and the holistic management plan.
For optimal results, regular follow-up is necessary to monitor compliance and treatment response. A reduction in symptoms can serve as a reliable indicator of effective EPI management, particularly weight stabilization, improved steatorrhea and diarrhea, and reduced postprandial bloating, pain, and flatulence. Physicians may provide patients with tracking tools to record their PERT compliance, symptom frequency, and lifestyle changes.
For patients with persistent concerns, PERT can be increased as needed. Although many PERT formulations are enteric coated, a proton pump inhibitor or H2 receptor agonist may improve their effectiveness. If EPI symptoms persist despite increased doses, other causes of malabsorption should be considered, such as the concomitant conditions mentioned above.
“As EPI escalates, a lower fat diet may become necessary to alleviate distressing gastrointestinal symptoms,” the authors wrote. “A close working relationship between the treating provider and the [registered dietician] is crucial so that barriers to optimum nutrient assimilation can be identified, communicated, and overcome. Frequent monitoring of the nutritional state with therapy is also imperative.”
PancreasFest 2021 received no specific funding for this event. The authors declared grant support, adviser roles, and speaking honoraria from several pharmaceutical and medical device companies and health care foundations, including the National Pancreas Foundation.
Based on discussions during PancreasFest 2021, , according to a recent report in Gastro Hep Advances
Due to its complex and individualized nature, EPI requires multidisciplinary approaches to therapy, as well as better pancreas function tests and biomarkers for diagnosis and treatment, wrote researchers who were led by David C. Whitcomb, MD, PhD, AGAF, emeritus professor of medicine in the division of gastroenterology, hepatology and nutrition at the University of Pittsburgh.
“This condition remains challenging even to define, and serious limitations in diagnostic testing and therapeutic options lead to clinical confusion and frequently less than optimal patient management,” the authors wrote.
EPI is clinically defined as inadequate delivery of pancreatic digestive enzymes to meet nutritional needs, which is typically based on a physician’s assessment of a patient’s maldigestion. However, there’s not a universally accepted definition or a precise threshold of reduced pancreatic digestive enzymes that indicates “pancreatic insufficiency” in an individual patient.
Current guidelines also don’t clearly outline the role of pancreatic function tests, the effects of different metabolic needs and nutrition intake, the timing of pancreatic enzyme replacement therapy (PERT), or the best practices for monitoring or titrating multiple therapies.
In response, Dr. Whitcomb and colleagues proposed a new mechanistic definition of EPI, including the disorder’s physiologic effects and impact on health. First, they said, EPI is a disorder caused by failure of the pancreas to deliver a minimum or threshold level of specific pancreatic digestive enzymes to the intestine in concert with ingested nutrients, followed by enzymatic digestion of individual meals over time to meet certain nutritional and metabolic needs. In addition, the disorder is characterized by variable deficiencies in micronutrients and macronutrients, especially essential fats and fat-soluble vitamins, as well as gastrointestinal symptoms of nutrient maldigestion.
The threshold for EPI should consider the nutritional needs of the patient, dietary intake, residual exocrine pancreas function, and the absorptive capacity of the intestine based on anatomy, mucosal function, motility, inflammation, the microbiome, and physiological adaptation, the authors wrote.
Due to challenges in diagnosing EPI and its common chronic symptoms such as abdominal pain, bloating, and diarrhea, several conditions may mimic EPI, be present concomitantly with EPI, or hinder PERT response. These include celiac disease, small intestinal bacterial overgrowth, disaccharidase deficiencies, inflammatory bowel disease (IBD), bile acid diarrhea, giardiasis, diabetes mellitus, and functional conditions such as irritable bowel syndrome. These conditions should be considered to address underlying pathology and PERT diagnostic challenges.
Although there is consensus that exocrine pancreatic function testing (PFT) is important to diagnosis EPI, no optimal test exists, and pancreatic function is only one aspect of digestion and absorption that should be considered. PFT may be needed to make an objective EPI diagnosis related to acute pancreatitis, pancreatic cancer, pancreatic resection, gastric resection, cystic fibrosis, or IBD. Direct or indirect PFTs may be used, which typically differs by center.
“The medical community still awaits a clinically useful pancreas function test that is easy to perform, well tolerated by patients, and allows personalized dosing of PERT,” the authors wrote.
After diagnosis, a general assessment should include information about symptoms, nutritional status, medications, diet, and lifestyle. This information can be used for a multifaceted treatment approach, with a focus on lifestyle changes, concomitant disease treatment, optimized diet, dietary supplements, and PERT administration.
PERT remains a mainstay of EPI treatment and has shown improvements in steatorrhea, postprandial bloating and pain, nutrition, and unexplained weight loss. The Food and Drug Administration has approved several formulations in different strengths. The typical starting dose is based on age and weight, which is derived from guidelines for EPI treatment in patients with cystic fibrosis. However, the recommendations don’t consider many of the variables discussed above and simply provide an estimate for the average subject with severe EPI, so the dose should be titrated as needed based on age, weight, symptoms, and the holistic management plan.
For optimal results, regular follow-up is necessary to monitor compliance and treatment response. A reduction in symptoms can serve as a reliable indicator of effective EPI management, particularly weight stabilization, improved steatorrhea and diarrhea, and reduced postprandial bloating, pain, and flatulence. Physicians may provide patients with tracking tools to record their PERT compliance, symptom frequency, and lifestyle changes.
For patients with persistent concerns, PERT can be increased as needed. Although many PERT formulations are enteric coated, a proton pump inhibitor or H2 receptor agonist may improve their effectiveness. If EPI symptoms persist despite increased doses, other causes of malabsorption should be considered, such as the concomitant conditions mentioned above.
“As EPI escalates, a lower fat diet may become necessary to alleviate distressing gastrointestinal symptoms,” the authors wrote. “A close working relationship between the treating provider and the [registered dietician] is crucial so that barriers to optimum nutrient assimilation can be identified, communicated, and overcome. Frequent monitoring of the nutritional state with therapy is also imperative.”
PancreasFest 2021 received no specific funding for this event. The authors declared grant support, adviser roles, and speaking honoraria from several pharmaceutical and medical device companies and health care foundations, including the National Pancreas Foundation.
FROM GASTRO HEP ADVANCES
Pediatric Crohn’s disease: Adalimumab plus methotrexate offers strong benefit
Children initiating treatment with adalimumab plus a low dose of methotrexate experienced a twofold reduction in treatment failure, note the authors of the largest, double-blind, randomized trial to date in pediatric Crohn’s disease. However, children initiating infliximab, another TNFi, had similar outcomes with or without methotrexate.
“We believe these results are practice-changing,” said principal investigator Michael Kappelman, MD, MPH, professor of pediatrics at University of North Carolina, Chapel Hill.
All patients with pediatric Crohn’s disease starting on adalimumab and their parents should be informed that combining the drug with low-dose oral methotrexate improves treatment effectiveness, he said.
“Those without contraindications should be offered combination therapy, and shared decision-making should be incorporated into final treatment decisions. In contrast, most patients starting infliximab are not likely to experience added benefits from low-dose oral methotrexate,” Dr. Kappelman added.
The study was published online in Gastroenterology and will be presented in early May at Digestive Disease Week® 2023.
Impactful study
“This is an important study, published in a very high-ranking journal, that will have a huge impact on how we practice,” said Jacob Kurowski, MD, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s, who wasn’t involved in the study.
Treatment with a TNFi, including infliximab and adalimumab, is a mainstay of pediatric Crohn’s disease therapy. However, not all patients achieve remission, and many lose response over time.
The current trial compared the effectiveness and safety of adding a low-dose of oral methotrexate to adalimumab or infliximab versus TNFi therapy alone in 297 children with Crohn’s disease.
The mean age was 13.9 years, and about two-thirds were boys. None had a prior history of TNFi therapy.
Participants initiating infliximab or adalimumab were randomly allocated (1:1) to oral methotrexate or placebo. Of them, 110 infliximab initiators and 46 adalimumab initiators received methotrexate, while 102 infliximab initiators and 39 adalimumab initiators were given placebo. Methotrexate was administered as a weekly dose of 15 mg for children weighing 40 kg or more, 12.5 mg for children 30 to less than 40 kg, and 10 mg for children 20 to less than 30 kg. All participants received pretreatment with ondansetron 4 mg (or placebo) to prevent nausea and folic acid (1 mg per day). Participants were followed for 12-36 months.
The primary outcome was a failure to achieve or maintain steroid-free remission defined by occurrence of any of the following.
- Short Pediatric Crohn’s Disease Activity Index score of less than 15 by week 26
- Failure to complete a steroid taper by week 16
- SPCDAI score of 15 or higher as a result of active Crohn’s disease at two or more consecutive visits beyond week 26
- Hospitalization or surgery for Crohn’s disease beyond week 26
- Use of corticosteroids for Crohn’s disease for 10 or more weeks cumulatively beyond week 16
- Discontinuation of anti-TNF and/or study drug for lack of effectiveness or toxicity
Overall, 88 of 297 children (30%) experienced treatment failure, including 57 of 212 (27%) on infliximab and 31 of 85 (36%) on adalimumab. Overall, 40 of 156 children (26%) on combination therapy and 48 of 141 (34%) on monotherapy experienced treatment failure.
Kaplan Meier analysis of the overall population showed a nonsignificant trend toward lower event rates with combination therapy (hazard ratio, 0.69; 95% confidence interval, 0.45-1.05; P = .08).
After stratification by TNFi, there was no difference in time to treatment failure among infliximab initiators between combination and monotherapy (HR, 0.93; 95% CI, 0.55-1.56; P = .78). In contrast, among adalimumab initiators, combination therapy was significantly associated with a longer time to treatment failure (HR, 0.40; 95% CI, 0.19-0.81; P = .01).
There was a nonsignificant trend toward lower development of anti-drug antibodies with combination therapy (risk ratio, 0.72 with infliximab and 0.71 with adalimumab). This trend is in line with adult studies and adds substantially to the pediatric literature on this topic, the researchers noted.
No differences in patient-reported outcomes were observed. There were slightly more adverse events with combination therapy, as expected, but fewer serious adverse events.
Shared decision-making
Dr. Kappelman noted that the study was not designed to answer the question of which is better – adalimumab plus methotrexate or infliximab alone. “This is an area for future research. At this point, we believe it is an individualized decision, and appropriate counseling is needed to support shared decision-making,” he said.
Nor was the trial designed to evaluate the role of proactive therapeutic drug monitoring. However, proactive TDM is endorsed in the ImproveCareNow Model IBD Care guidelines and was considered standard of care at the 35 study sites.
The findings “suggest strong consideration of using combination therapy for pediatric Crohn’s disease patients initiating adalimumab but not infliximab,” Dr. Kappelman and colleagues said.
“Dissemination and implementation of these findings should lead to improved outcomes in this patient population, including consideration of de-implementation of combination therapy in infliximab treated patients,” they added.
The decision about which approach to use is still very dependent on patients and their providers, Dr. Kurowski said.
“The study shows that you can safely use infliximab as monotherapy, with low risk of antibody formation, while utilizing proactive therapeutic drug monitoring and dose optimization. The study also shows that adalimumab in combination with low-dose methotrexate can be strongly considered when needed.”
The researchers’ standardization of methotrexate doses by weight “is another significant contribution and provides a guide for clinicians,” Dr. Kurowski added.
The study was funded by grants from the Patient-Centered Outcomes Research Institute, the Helmsley Charitable Trust, and National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kappelman has consulted for AbbVie, Janssen, Pfizer, Takeda, and Lilly; holds shares in Johnson & Johnson; and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol-Myers Squibb, Celtrion, and Arena Pharmaceuticals. Dr. Kurowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children initiating treatment with adalimumab plus a low dose of methotrexate experienced a twofold reduction in treatment failure, note the authors of the largest, double-blind, randomized trial to date in pediatric Crohn’s disease. However, children initiating infliximab, another TNFi, had similar outcomes with or without methotrexate.
“We believe these results are practice-changing,” said principal investigator Michael Kappelman, MD, MPH, professor of pediatrics at University of North Carolina, Chapel Hill.
All patients with pediatric Crohn’s disease starting on adalimumab and their parents should be informed that combining the drug with low-dose oral methotrexate improves treatment effectiveness, he said.
“Those without contraindications should be offered combination therapy, and shared decision-making should be incorporated into final treatment decisions. In contrast, most patients starting infliximab are not likely to experience added benefits from low-dose oral methotrexate,” Dr. Kappelman added.
The study was published online in Gastroenterology and will be presented in early May at Digestive Disease Week® 2023.
Impactful study
“This is an important study, published in a very high-ranking journal, that will have a huge impact on how we practice,” said Jacob Kurowski, MD, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s, who wasn’t involved in the study.
Treatment with a TNFi, including infliximab and adalimumab, is a mainstay of pediatric Crohn’s disease therapy. However, not all patients achieve remission, and many lose response over time.
The current trial compared the effectiveness and safety of adding a low-dose of oral methotrexate to adalimumab or infliximab versus TNFi therapy alone in 297 children with Crohn’s disease.
The mean age was 13.9 years, and about two-thirds were boys. None had a prior history of TNFi therapy.
Participants initiating infliximab or adalimumab were randomly allocated (1:1) to oral methotrexate or placebo. Of them, 110 infliximab initiators and 46 adalimumab initiators received methotrexate, while 102 infliximab initiators and 39 adalimumab initiators were given placebo. Methotrexate was administered as a weekly dose of 15 mg for children weighing 40 kg or more, 12.5 mg for children 30 to less than 40 kg, and 10 mg for children 20 to less than 30 kg. All participants received pretreatment with ondansetron 4 mg (or placebo) to prevent nausea and folic acid (1 mg per day). Participants were followed for 12-36 months.
The primary outcome was a failure to achieve or maintain steroid-free remission defined by occurrence of any of the following.
- Short Pediatric Crohn’s Disease Activity Index score of less than 15 by week 26
- Failure to complete a steroid taper by week 16
- SPCDAI score of 15 or higher as a result of active Crohn’s disease at two or more consecutive visits beyond week 26
- Hospitalization or surgery for Crohn’s disease beyond week 26
- Use of corticosteroids for Crohn’s disease for 10 or more weeks cumulatively beyond week 16
- Discontinuation of anti-TNF and/or study drug for lack of effectiveness or toxicity
Overall, 88 of 297 children (30%) experienced treatment failure, including 57 of 212 (27%) on infliximab and 31 of 85 (36%) on adalimumab. Overall, 40 of 156 children (26%) on combination therapy and 48 of 141 (34%) on monotherapy experienced treatment failure.
Kaplan Meier analysis of the overall population showed a nonsignificant trend toward lower event rates with combination therapy (hazard ratio, 0.69; 95% confidence interval, 0.45-1.05; P = .08).
After stratification by TNFi, there was no difference in time to treatment failure among infliximab initiators between combination and monotherapy (HR, 0.93; 95% CI, 0.55-1.56; P = .78). In contrast, among adalimumab initiators, combination therapy was significantly associated with a longer time to treatment failure (HR, 0.40; 95% CI, 0.19-0.81; P = .01).
There was a nonsignificant trend toward lower development of anti-drug antibodies with combination therapy (risk ratio, 0.72 with infliximab and 0.71 with adalimumab). This trend is in line with adult studies and adds substantially to the pediatric literature on this topic, the researchers noted.
No differences in patient-reported outcomes were observed. There were slightly more adverse events with combination therapy, as expected, but fewer serious adverse events.
Shared decision-making
Dr. Kappelman noted that the study was not designed to answer the question of which is better – adalimumab plus methotrexate or infliximab alone. “This is an area for future research. At this point, we believe it is an individualized decision, and appropriate counseling is needed to support shared decision-making,” he said.
Nor was the trial designed to evaluate the role of proactive therapeutic drug monitoring. However, proactive TDM is endorsed in the ImproveCareNow Model IBD Care guidelines and was considered standard of care at the 35 study sites.
The findings “suggest strong consideration of using combination therapy for pediatric Crohn’s disease patients initiating adalimumab but not infliximab,” Dr. Kappelman and colleagues said.
“Dissemination and implementation of these findings should lead to improved outcomes in this patient population, including consideration of de-implementation of combination therapy in infliximab treated patients,” they added.
The decision about which approach to use is still very dependent on patients and their providers, Dr. Kurowski said.
“The study shows that you can safely use infliximab as monotherapy, with low risk of antibody formation, while utilizing proactive therapeutic drug monitoring and dose optimization. The study also shows that adalimumab in combination with low-dose methotrexate can be strongly considered when needed.”
The researchers’ standardization of methotrexate doses by weight “is another significant contribution and provides a guide for clinicians,” Dr. Kurowski added.
The study was funded by grants from the Patient-Centered Outcomes Research Institute, the Helmsley Charitable Trust, and National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kappelman has consulted for AbbVie, Janssen, Pfizer, Takeda, and Lilly; holds shares in Johnson & Johnson; and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol-Myers Squibb, Celtrion, and Arena Pharmaceuticals. Dr. Kurowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children initiating treatment with adalimumab plus a low dose of methotrexate experienced a twofold reduction in treatment failure, note the authors of the largest, double-blind, randomized trial to date in pediatric Crohn’s disease. However, children initiating infliximab, another TNFi, had similar outcomes with or without methotrexate.
“We believe these results are practice-changing,” said principal investigator Michael Kappelman, MD, MPH, professor of pediatrics at University of North Carolina, Chapel Hill.
All patients with pediatric Crohn’s disease starting on adalimumab and their parents should be informed that combining the drug with low-dose oral methotrexate improves treatment effectiveness, he said.
“Those without contraindications should be offered combination therapy, and shared decision-making should be incorporated into final treatment decisions. In contrast, most patients starting infliximab are not likely to experience added benefits from low-dose oral methotrexate,” Dr. Kappelman added.
The study was published online in Gastroenterology and will be presented in early May at Digestive Disease Week® 2023.
Impactful study
“This is an important study, published in a very high-ranking journal, that will have a huge impact on how we practice,” said Jacob Kurowski, MD, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s, who wasn’t involved in the study.
Treatment with a TNFi, including infliximab and adalimumab, is a mainstay of pediatric Crohn’s disease therapy. However, not all patients achieve remission, and many lose response over time.
The current trial compared the effectiveness and safety of adding a low-dose of oral methotrexate to adalimumab or infliximab versus TNFi therapy alone in 297 children with Crohn’s disease.
The mean age was 13.9 years, and about two-thirds were boys. None had a prior history of TNFi therapy.
Participants initiating infliximab or adalimumab were randomly allocated (1:1) to oral methotrexate or placebo. Of them, 110 infliximab initiators and 46 adalimumab initiators received methotrexate, while 102 infliximab initiators and 39 adalimumab initiators were given placebo. Methotrexate was administered as a weekly dose of 15 mg for children weighing 40 kg or more, 12.5 mg for children 30 to less than 40 kg, and 10 mg for children 20 to less than 30 kg. All participants received pretreatment with ondansetron 4 mg (or placebo) to prevent nausea and folic acid (1 mg per day). Participants were followed for 12-36 months.
The primary outcome was a failure to achieve or maintain steroid-free remission defined by occurrence of any of the following.
- Short Pediatric Crohn’s Disease Activity Index score of less than 15 by week 26
- Failure to complete a steroid taper by week 16
- SPCDAI score of 15 or higher as a result of active Crohn’s disease at two or more consecutive visits beyond week 26
- Hospitalization or surgery for Crohn’s disease beyond week 26
- Use of corticosteroids for Crohn’s disease for 10 or more weeks cumulatively beyond week 16
- Discontinuation of anti-TNF and/or study drug for lack of effectiveness or toxicity
Overall, 88 of 297 children (30%) experienced treatment failure, including 57 of 212 (27%) on infliximab and 31 of 85 (36%) on adalimumab. Overall, 40 of 156 children (26%) on combination therapy and 48 of 141 (34%) on monotherapy experienced treatment failure.
Kaplan Meier analysis of the overall population showed a nonsignificant trend toward lower event rates with combination therapy (hazard ratio, 0.69; 95% confidence interval, 0.45-1.05; P = .08).
After stratification by TNFi, there was no difference in time to treatment failure among infliximab initiators between combination and monotherapy (HR, 0.93; 95% CI, 0.55-1.56; P = .78). In contrast, among adalimumab initiators, combination therapy was significantly associated with a longer time to treatment failure (HR, 0.40; 95% CI, 0.19-0.81; P = .01).
There was a nonsignificant trend toward lower development of anti-drug antibodies with combination therapy (risk ratio, 0.72 with infliximab and 0.71 with adalimumab). This trend is in line with adult studies and adds substantially to the pediatric literature on this topic, the researchers noted.
No differences in patient-reported outcomes were observed. There were slightly more adverse events with combination therapy, as expected, but fewer serious adverse events.
Shared decision-making
Dr. Kappelman noted that the study was not designed to answer the question of which is better – adalimumab plus methotrexate or infliximab alone. “This is an area for future research. At this point, we believe it is an individualized decision, and appropriate counseling is needed to support shared decision-making,” he said.
Nor was the trial designed to evaluate the role of proactive therapeutic drug monitoring. However, proactive TDM is endorsed in the ImproveCareNow Model IBD Care guidelines and was considered standard of care at the 35 study sites.
The findings “suggest strong consideration of using combination therapy for pediatric Crohn’s disease patients initiating adalimumab but not infliximab,” Dr. Kappelman and colleagues said.
“Dissemination and implementation of these findings should lead to improved outcomes in this patient population, including consideration of de-implementation of combination therapy in infliximab treated patients,” they added.
The decision about which approach to use is still very dependent on patients and their providers, Dr. Kurowski said.
“The study shows that you can safely use infliximab as monotherapy, with low risk of antibody formation, while utilizing proactive therapeutic drug monitoring and dose optimization. The study also shows that adalimumab in combination with low-dose methotrexate can be strongly considered when needed.”
The researchers’ standardization of methotrexate doses by weight “is another significant contribution and provides a guide for clinicians,” Dr. Kurowski added.
The study was funded by grants from the Patient-Centered Outcomes Research Institute, the Helmsley Charitable Trust, and National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Kappelman has consulted for AbbVie, Janssen, Pfizer, Takeda, and Lilly; holds shares in Johnson & Johnson; and has received research support from Pfizer, Takeda, Janssen, AbbVie, Lilly, Genentech, Boehringer Ingelheim, Bristol-Myers Squibb, Celtrion, and Arena Pharmaceuticals. Dr. Kurowski reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
Refined incidence rate of HCC with alcohol-associated cirrhosis encourages surveillance
Incidence rates were higher for cohorts that underwent HCC surveillance versus those that did not undergo surveillance, suggesting that such programs offer significant benefit, lead author Daniel Q. Huang, MBBS, of the University of California, San Diego, and colleagues reported.
“A systematic review of the incidence of HCC among patients with alcohol-associated cirrhosis has not been reported,” the investigators wrote in Clinical Gastroenterology and Hepatology, prompting the present research.
Previous studies have described a broad range of annual incidence findings for HCC in this population, from 0.6% to 5.6%, suggesting that a systematic approach was needed.
To this end, Dr. Huang and colleagues analyzed data from 18 studies that involved 148,333 patients with alcohol-associated cirrhosis. The primary analysis aimed to determine cumulative incidence rates over time, while the secondary analysis characterized the impact of participation in HCC surveillance programs.
“This meta-analysis used reconstructed individual participant data, which is considered to be the gold standard for reporting survival data because it accounts for censoring of events,” the investigators noted. “The current study provides important data that are useful for clinical practice and clinical trial design.”
The cumulative incidence rates of HCC were 1%, 3%, and 9% at 1 year, 5 years, and 10 years, respectively. Among 12 of the risk factors studied, smoking, diabetes, and decompensation were all significantly associated with rate of HCC.
“Therefore, patients with alcohol-associated cirrhosis should be screened for diabetes to identify the patients at high risk for HCC development,” the investigators wrote. “In addition, patients with alcohol-associated cirrhosis should be advised to stop smoking, while patients with hepatic decompensation should be monitored carefully for the development of HCC if clinically appropriate.”
The secondary analysis showed that HCC incidence rates were higher among patients participating in HCC surveillance programs than those who did not participate (18.6 vs. 4.8 per 1,000 person-years; P = .001).
“Patients with alcohol-associated cirrhosis are known to have lower HCC surveillance rates, which may be related to poor disease awareness, clinic time constraints caused by other active medical issues, and provider beliefs regarding the likelihood of adherence,” the investigators noted.
Increased efforts are needed to promote surveillance in this population, they added, suggesting a range of communication pathways, including social media, traditional news outlets, and direct mailing.
Dr. Huang and colleagues also suggested that the findings should be validated in large prospective studies.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, and others. Dr. Huang disclosed funding from the Singapore Ministry of Health’s National Medical Research Council.
The association between cirrhosis and hepatocellular carcinoma (HCC) risk is well known and therefore routine surveillance is recommended by the American Association for the Study of Liver Diseases. More recent data has shown alcohol use to be an independent risk factor for HCC along with various other cancers.
Quite frequently, the focus of management in patients with alcohol-associated liver disease is alcohol cessation to prevent further decompensation, with screening often being overlooked. Previous studies have shown, however, that earlier detection is associated with improved survival. Another interesting finding of this study was that those patients who had concomitant smoking use, diabetes, and hepatic decompensation were more likely to develop HCC. When managing patients with alcohol related liver disease, confounding risk factors should be mitigated (that is, encouragement of smoking cessation, enhanced screening for diabetes, and more rigorous screening in decompensated patients).
This study brings to light the need for improved screening and concomitant risk factor mitigation for hepatocellular carcinoma given higher rates of detection in those undergoing surveillance. Larger, prospective studies are needed, however, to validate the findings in this study given the recent overall increase in rates of alcohol-associated liver disease.
Priya Maddur MD, is a visiting clinical associate professor of medicine, University of Arizona, Tucson. Dr. Maddur has no relevant disclosures.
The association between cirrhosis and hepatocellular carcinoma (HCC) risk is well known and therefore routine surveillance is recommended by the American Association for the Study of Liver Diseases. More recent data has shown alcohol use to be an independent risk factor for HCC along with various other cancers.
Quite frequently, the focus of management in patients with alcohol-associated liver disease is alcohol cessation to prevent further decompensation, with screening often being overlooked. Previous studies have shown, however, that earlier detection is associated with improved survival. Another interesting finding of this study was that those patients who had concomitant smoking use, diabetes, and hepatic decompensation were more likely to develop HCC. When managing patients with alcohol related liver disease, confounding risk factors should be mitigated (that is, encouragement of smoking cessation, enhanced screening for diabetes, and more rigorous screening in decompensated patients).
This study brings to light the need for improved screening and concomitant risk factor mitigation for hepatocellular carcinoma given higher rates of detection in those undergoing surveillance. Larger, prospective studies are needed, however, to validate the findings in this study given the recent overall increase in rates of alcohol-associated liver disease.
Priya Maddur MD, is a visiting clinical associate professor of medicine, University of Arizona, Tucson. Dr. Maddur has no relevant disclosures.
The association between cirrhosis and hepatocellular carcinoma (HCC) risk is well known and therefore routine surveillance is recommended by the American Association for the Study of Liver Diseases. More recent data has shown alcohol use to be an independent risk factor for HCC along with various other cancers.
Quite frequently, the focus of management in patients with alcohol-associated liver disease is alcohol cessation to prevent further decompensation, with screening often being overlooked. Previous studies have shown, however, that earlier detection is associated with improved survival. Another interesting finding of this study was that those patients who had concomitant smoking use, diabetes, and hepatic decompensation were more likely to develop HCC. When managing patients with alcohol related liver disease, confounding risk factors should be mitigated (that is, encouragement of smoking cessation, enhanced screening for diabetes, and more rigorous screening in decompensated patients).
This study brings to light the need for improved screening and concomitant risk factor mitigation for hepatocellular carcinoma given higher rates of detection in those undergoing surveillance. Larger, prospective studies are needed, however, to validate the findings in this study given the recent overall increase in rates of alcohol-associated liver disease.
Priya Maddur MD, is a visiting clinical associate professor of medicine, University of Arizona, Tucson. Dr. Maddur has no relevant disclosures.
Incidence rates were higher for cohorts that underwent HCC surveillance versus those that did not undergo surveillance, suggesting that such programs offer significant benefit, lead author Daniel Q. Huang, MBBS, of the University of California, San Diego, and colleagues reported.
“A systematic review of the incidence of HCC among patients with alcohol-associated cirrhosis has not been reported,” the investigators wrote in Clinical Gastroenterology and Hepatology, prompting the present research.
Previous studies have described a broad range of annual incidence findings for HCC in this population, from 0.6% to 5.6%, suggesting that a systematic approach was needed.
To this end, Dr. Huang and colleagues analyzed data from 18 studies that involved 148,333 patients with alcohol-associated cirrhosis. The primary analysis aimed to determine cumulative incidence rates over time, while the secondary analysis characterized the impact of participation in HCC surveillance programs.
“This meta-analysis used reconstructed individual participant data, which is considered to be the gold standard for reporting survival data because it accounts for censoring of events,” the investigators noted. “The current study provides important data that are useful for clinical practice and clinical trial design.”
The cumulative incidence rates of HCC were 1%, 3%, and 9% at 1 year, 5 years, and 10 years, respectively. Among 12 of the risk factors studied, smoking, diabetes, and decompensation were all significantly associated with rate of HCC.
“Therefore, patients with alcohol-associated cirrhosis should be screened for diabetes to identify the patients at high risk for HCC development,” the investigators wrote. “In addition, patients with alcohol-associated cirrhosis should be advised to stop smoking, while patients with hepatic decompensation should be monitored carefully for the development of HCC if clinically appropriate.”
The secondary analysis showed that HCC incidence rates were higher among patients participating in HCC surveillance programs than those who did not participate (18.6 vs. 4.8 per 1,000 person-years; P = .001).
“Patients with alcohol-associated cirrhosis are known to have lower HCC surveillance rates, which may be related to poor disease awareness, clinic time constraints caused by other active medical issues, and provider beliefs regarding the likelihood of adherence,” the investigators noted.
Increased efforts are needed to promote surveillance in this population, they added, suggesting a range of communication pathways, including social media, traditional news outlets, and direct mailing.
Dr. Huang and colleagues also suggested that the findings should be validated in large prospective studies.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, and others. Dr. Huang disclosed funding from the Singapore Ministry of Health’s National Medical Research Council.
Incidence rates were higher for cohorts that underwent HCC surveillance versus those that did not undergo surveillance, suggesting that such programs offer significant benefit, lead author Daniel Q. Huang, MBBS, of the University of California, San Diego, and colleagues reported.
“A systematic review of the incidence of HCC among patients with alcohol-associated cirrhosis has not been reported,” the investigators wrote in Clinical Gastroenterology and Hepatology, prompting the present research.
Previous studies have described a broad range of annual incidence findings for HCC in this population, from 0.6% to 5.6%, suggesting that a systematic approach was needed.
To this end, Dr. Huang and colleagues analyzed data from 18 studies that involved 148,333 patients with alcohol-associated cirrhosis. The primary analysis aimed to determine cumulative incidence rates over time, while the secondary analysis characterized the impact of participation in HCC surveillance programs.
“This meta-analysis used reconstructed individual participant data, which is considered to be the gold standard for reporting survival data because it accounts for censoring of events,” the investigators noted. “The current study provides important data that are useful for clinical practice and clinical trial design.”
The cumulative incidence rates of HCC were 1%, 3%, and 9% at 1 year, 5 years, and 10 years, respectively. Among 12 of the risk factors studied, smoking, diabetes, and decompensation were all significantly associated with rate of HCC.
“Therefore, patients with alcohol-associated cirrhosis should be screened for diabetes to identify the patients at high risk for HCC development,” the investigators wrote. “In addition, patients with alcohol-associated cirrhosis should be advised to stop smoking, while patients with hepatic decompensation should be monitored carefully for the development of HCC if clinically appropriate.”
The secondary analysis showed that HCC incidence rates were higher among patients participating in HCC surveillance programs than those who did not participate (18.6 vs. 4.8 per 1,000 person-years; P = .001).
“Patients with alcohol-associated cirrhosis are known to have lower HCC surveillance rates, which may be related to poor disease awareness, clinic time constraints caused by other active medical issues, and provider beliefs regarding the likelihood of adherence,” the investigators noted.
Increased efforts are needed to promote surveillance in this population, they added, suggesting a range of communication pathways, including social media, traditional news outlets, and direct mailing.
Dr. Huang and colleagues also suggested that the findings should be validated in large prospective studies.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Environmental Health Sciences, the National Center for Advancing Translational Sciences, and others. Dr. Huang disclosed funding from the Singapore Ministry of Health’s National Medical Research Council.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Antibiotic pretreatment reduces liver ischemia/reperfusion injury
Antibiotic pretreatment may protect against liver ischemia/reperfusion (I/R) injury through altered gut microbiota, glutamine levels, and glutamine downstream products in circulation, according to a recent study in Cellular and Molecular Gastroenterology and Hepatology.
The findings show that , wrote Tianfei Lu, with the Abdominal Transplant Surgery Center at Ruijin Hospital and Shanghai Jiao Tong University, China, and colleagues.
“Potential therapies that target macrophage metabolism, including antibiotic therapies and novel immunometabolism modulators, can be exploited for the treatment of liver I/R injury,” the authors wrote.
Liver I/R injury is a common complication of liver resection, transplantation, trauma, and hemorrhagic shock. Previous studies have noted the important role of gut microbiota in liver disease progression, yet the mechanisms in liver I/R injury remain unknown.
The researchers pretreated mice with an antibiotic cocktail to modify the gut microbiome. They found that the pretreatment showed protective effects against hepatic I/R injury, with reductions in serum alanine aminotransferase (ALT), interleukin-1 beta, tumor necrosis factor–alpha, IL-6, IL-12b, and CXCL10.
Through histologic analysis of liver tissues, they also found that the area of necrosis, the degree of congestion and edema, and the presence of vacuole-like lesions were alleviated in the preconditioned mice. Inflammation and necrosis of the liver were also lower, according to both qualitative and quantitative data.
Then, through fecal microbiota transplantation into germ-free mice, they found that the protection from I/R injury was transferable. This finding indicated that the altered gut microbiome, rather than the antibiotic treatment itself, exerted the protective effect.
Because altered gut microbiota can cause changes in metabolites, the researchers used ultra-performance liquid chromatography coupled to tandem mass spectrometry to explore the changes of gut microbiota and metabolites in both feces and portal blood, as well as analyze the mechanisms underlying their protective effects in liver I/R injury.
The researchers found that glutamine and its downstream product called alpha-ketoglutarate (AKG) were present in higher concentrations in feces and blood in the mice with antibiotic pretreatment. Glutamate levels were significantly lower, indicating that glutamine is converted into AKG through glutamate after entering the blood.
In addition, there were increased levels of intermediate products of the tricarboxylic acid (TCA) cycle, as well as pyruvate produced by glycolysis. That led to an increase in M2 macrophages, which are responsible for anti-inflammatory processes and tissue repair.
The authors concluded that elevated glutamine levels in the intestine cause an increase in AKG levels in the blood, and AKG can promote M2 macrophage polarization by fueling the TCA cycle. In turn, the increased number of M2 macrophages can repair hepatic I/R injury.
Finally, the researchers tested oligomycin A, which can block the OXPHOS metabolic pathway and inhibit the mitochondrial ATP synthase. As expected, they wrote, the protective effect of antibiotic pretreatment reversed, M2 macrophages decreased, and serum ALT levels increased.
“The immunometabolism and polarization of macrophages play an important role in host homeostasis and the development of various diseases,” the authors wrote. “The relationship between antibiotics treatment, altered gut microbiota, and liver I/R injury are complex and worthy of further study.”
The study was supported by the China National Science and Technology Major Project, National Natural Science Foundation of China, and Natural Science Foundation exploration project of Zhejiang Province. The authors disclosed no conflicts.
In modern clinical practice, multiple conditions can cause ischemia and reperfusion injury to the liver, including surgical liver resection, liver transplantation, and physical trauma to the organ. Liver damage due to hypoxia is followed by reperfusion injury, resulting in a pre-proinflammatory environment. Liver resident macrophages called Kupffer cells are major mediators of this response, initiating a signaling cascade that leads to recruitment of neutrophils, natural killer cells, and circulating macrophages, which attack sinusoidal endothelial cells and hepatocytes.
In the current issue of CMGH, Lu and colleagues address the question of to what extent do the gut microbiome and its metabolite products, which reach the liver via the portal circulation, play a role in the severity of ischemia and reperfusion injury (Cell Mol Gastroenterol Hepatol. 2023 Jan 24. doi: 10.1016/j.jcmgh.2023.01.004). This topic is of clinical relevance, as the microbial load of the gut lumen can be easily reduced by several orders of magnitude using non-absorbed antibiotics. Thus, it is important to establish if pretreatment of patients scheduled for liver resection or transplantation might benefit from preprocedure antibiotic treatment.
Remarkably, Lu and colleagues find that antibiotic preconditioning significantly reduces ischemia and reperfusion injury in an animal model. Mechanistically, they linked the protective effects to a shift of macrophage polarization to the protective M phenotype, which is known to promote tissue repair. These findings suggest that the antibiotic preconditioning of patients who are undergoing procedures with significant ischemia and reperfusion injury should be evaluated in future clinical trials.
Klaus H. Kaestner, PhD, MS, is the Thomas and Evelyn Suor Butterworth Professor in Genetics and associate director of the Penn Diabetes Research Center at the University of Pennsylvania, Philadelphia. He has no relevant financial relationships.
In modern clinical practice, multiple conditions can cause ischemia and reperfusion injury to the liver, including surgical liver resection, liver transplantation, and physical trauma to the organ. Liver damage due to hypoxia is followed by reperfusion injury, resulting in a pre-proinflammatory environment. Liver resident macrophages called Kupffer cells are major mediators of this response, initiating a signaling cascade that leads to recruitment of neutrophils, natural killer cells, and circulating macrophages, which attack sinusoidal endothelial cells and hepatocytes.
In the current issue of CMGH, Lu and colleagues address the question of to what extent do the gut microbiome and its metabolite products, which reach the liver via the portal circulation, play a role in the severity of ischemia and reperfusion injury (Cell Mol Gastroenterol Hepatol. 2023 Jan 24. doi: 10.1016/j.jcmgh.2023.01.004). This topic is of clinical relevance, as the microbial load of the gut lumen can be easily reduced by several orders of magnitude using non-absorbed antibiotics. Thus, it is important to establish if pretreatment of patients scheduled for liver resection or transplantation might benefit from preprocedure antibiotic treatment.
Remarkably, Lu and colleagues find that antibiotic preconditioning significantly reduces ischemia and reperfusion injury in an animal model. Mechanistically, they linked the protective effects to a shift of macrophage polarization to the protective M phenotype, which is known to promote tissue repair. These findings suggest that the antibiotic preconditioning of patients who are undergoing procedures with significant ischemia and reperfusion injury should be evaluated in future clinical trials.
Klaus H. Kaestner, PhD, MS, is the Thomas and Evelyn Suor Butterworth Professor in Genetics and associate director of the Penn Diabetes Research Center at the University of Pennsylvania, Philadelphia. He has no relevant financial relationships.
In modern clinical practice, multiple conditions can cause ischemia and reperfusion injury to the liver, including surgical liver resection, liver transplantation, and physical trauma to the organ. Liver damage due to hypoxia is followed by reperfusion injury, resulting in a pre-proinflammatory environment. Liver resident macrophages called Kupffer cells are major mediators of this response, initiating a signaling cascade that leads to recruitment of neutrophils, natural killer cells, and circulating macrophages, which attack sinusoidal endothelial cells and hepatocytes.
In the current issue of CMGH, Lu and colleagues address the question of to what extent do the gut microbiome and its metabolite products, which reach the liver via the portal circulation, play a role in the severity of ischemia and reperfusion injury (Cell Mol Gastroenterol Hepatol. 2023 Jan 24. doi: 10.1016/j.jcmgh.2023.01.004). This topic is of clinical relevance, as the microbial load of the gut lumen can be easily reduced by several orders of magnitude using non-absorbed antibiotics. Thus, it is important to establish if pretreatment of patients scheduled for liver resection or transplantation might benefit from preprocedure antibiotic treatment.
Remarkably, Lu and colleagues find that antibiotic preconditioning significantly reduces ischemia and reperfusion injury in an animal model. Mechanistically, they linked the protective effects to a shift of macrophage polarization to the protective M phenotype, which is known to promote tissue repair. These findings suggest that the antibiotic preconditioning of patients who are undergoing procedures with significant ischemia and reperfusion injury should be evaluated in future clinical trials.
Klaus H. Kaestner, PhD, MS, is the Thomas and Evelyn Suor Butterworth Professor in Genetics and associate director of the Penn Diabetes Research Center at the University of Pennsylvania, Philadelphia. He has no relevant financial relationships.
Antibiotic pretreatment may protect against liver ischemia/reperfusion (I/R) injury through altered gut microbiota, glutamine levels, and glutamine downstream products in circulation, according to a recent study in Cellular and Molecular Gastroenterology and Hepatology.
The findings show that , wrote Tianfei Lu, with the Abdominal Transplant Surgery Center at Ruijin Hospital and Shanghai Jiao Tong University, China, and colleagues.
“Potential therapies that target macrophage metabolism, including antibiotic therapies and novel immunometabolism modulators, can be exploited for the treatment of liver I/R injury,” the authors wrote.
Liver I/R injury is a common complication of liver resection, transplantation, trauma, and hemorrhagic shock. Previous studies have noted the important role of gut microbiota in liver disease progression, yet the mechanisms in liver I/R injury remain unknown.
The researchers pretreated mice with an antibiotic cocktail to modify the gut microbiome. They found that the pretreatment showed protective effects against hepatic I/R injury, with reductions in serum alanine aminotransferase (ALT), interleukin-1 beta, tumor necrosis factor–alpha, IL-6, IL-12b, and CXCL10.
Through histologic analysis of liver tissues, they also found that the area of necrosis, the degree of congestion and edema, and the presence of vacuole-like lesions were alleviated in the preconditioned mice. Inflammation and necrosis of the liver were also lower, according to both qualitative and quantitative data.
Then, through fecal microbiota transplantation into germ-free mice, they found that the protection from I/R injury was transferable. This finding indicated that the altered gut microbiome, rather than the antibiotic treatment itself, exerted the protective effect.
Because altered gut microbiota can cause changes in metabolites, the researchers used ultra-performance liquid chromatography coupled to tandem mass spectrometry to explore the changes of gut microbiota and metabolites in both feces and portal blood, as well as analyze the mechanisms underlying their protective effects in liver I/R injury.
The researchers found that glutamine and its downstream product called alpha-ketoglutarate (AKG) were present in higher concentrations in feces and blood in the mice with antibiotic pretreatment. Glutamate levels were significantly lower, indicating that glutamine is converted into AKG through glutamate after entering the blood.
In addition, there were increased levels of intermediate products of the tricarboxylic acid (TCA) cycle, as well as pyruvate produced by glycolysis. That led to an increase in M2 macrophages, which are responsible for anti-inflammatory processes and tissue repair.
The authors concluded that elevated glutamine levels in the intestine cause an increase in AKG levels in the blood, and AKG can promote M2 macrophage polarization by fueling the TCA cycle. In turn, the increased number of M2 macrophages can repair hepatic I/R injury.
Finally, the researchers tested oligomycin A, which can block the OXPHOS metabolic pathway and inhibit the mitochondrial ATP synthase. As expected, they wrote, the protective effect of antibiotic pretreatment reversed, M2 macrophages decreased, and serum ALT levels increased.
“The immunometabolism and polarization of macrophages play an important role in host homeostasis and the development of various diseases,” the authors wrote. “The relationship between antibiotics treatment, altered gut microbiota, and liver I/R injury are complex and worthy of further study.”
The study was supported by the China National Science and Technology Major Project, National Natural Science Foundation of China, and Natural Science Foundation exploration project of Zhejiang Province. The authors disclosed no conflicts.
Antibiotic pretreatment may protect against liver ischemia/reperfusion (I/R) injury through altered gut microbiota, glutamine levels, and glutamine downstream products in circulation, according to a recent study in Cellular and Molecular Gastroenterology and Hepatology.
The findings show that , wrote Tianfei Lu, with the Abdominal Transplant Surgery Center at Ruijin Hospital and Shanghai Jiao Tong University, China, and colleagues.
“Potential therapies that target macrophage metabolism, including antibiotic therapies and novel immunometabolism modulators, can be exploited for the treatment of liver I/R injury,” the authors wrote.
Liver I/R injury is a common complication of liver resection, transplantation, trauma, and hemorrhagic shock. Previous studies have noted the important role of gut microbiota in liver disease progression, yet the mechanisms in liver I/R injury remain unknown.
The researchers pretreated mice with an antibiotic cocktail to modify the gut microbiome. They found that the pretreatment showed protective effects against hepatic I/R injury, with reductions in serum alanine aminotransferase (ALT), interleukin-1 beta, tumor necrosis factor–alpha, IL-6, IL-12b, and CXCL10.
Through histologic analysis of liver tissues, they also found that the area of necrosis, the degree of congestion and edema, and the presence of vacuole-like lesions were alleviated in the preconditioned mice. Inflammation and necrosis of the liver were also lower, according to both qualitative and quantitative data.
Then, through fecal microbiota transplantation into germ-free mice, they found that the protection from I/R injury was transferable. This finding indicated that the altered gut microbiome, rather than the antibiotic treatment itself, exerted the protective effect.
Because altered gut microbiota can cause changes in metabolites, the researchers used ultra-performance liquid chromatography coupled to tandem mass spectrometry to explore the changes of gut microbiota and metabolites in both feces and portal blood, as well as analyze the mechanisms underlying their protective effects in liver I/R injury.
The researchers found that glutamine and its downstream product called alpha-ketoglutarate (AKG) were present in higher concentrations in feces and blood in the mice with antibiotic pretreatment. Glutamate levels were significantly lower, indicating that glutamine is converted into AKG through glutamate after entering the blood.
In addition, there were increased levels of intermediate products of the tricarboxylic acid (TCA) cycle, as well as pyruvate produced by glycolysis. That led to an increase in M2 macrophages, which are responsible for anti-inflammatory processes and tissue repair.
The authors concluded that elevated glutamine levels in the intestine cause an increase in AKG levels in the blood, and AKG can promote M2 macrophage polarization by fueling the TCA cycle. In turn, the increased number of M2 macrophages can repair hepatic I/R injury.
Finally, the researchers tested oligomycin A, which can block the OXPHOS metabolic pathway and inhibit the mitochondrial ATP synthase. As expected, they wrote, the protective effect of antibiotic pretreatment reversed, M2 macrophages decreased, and serum ALT levels increased.
“The immunometabolism and polarization of macrophages play an important role in host homeostasis and the development of various diseases,” the authors wrote. “The relationship between antibiotics treatment, altered gut microbiota, and liver I/R injury are complex and worthy of further study.”
The study was supported by the China National Science and Technology Major Project, National Natural Science Foundation of China, and Natural Science Foundation exploration project of Zhejiang Province. The authors disclosed no conflicts.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Clinical Practice Update: Alpha-gal syndrome often causes GI issues without anaphylaxis, skin changes
according to an American Gastroenterological Association clinical practice update.
Although the allergic response is best known for a combination of anaphylaxis, skin changes, and gastrointestinal symptoms that occurs within hours of consuming mammalian-derived food products, health care providers should know that many patients experience gastrointestinal distress in the absence of other clinical signs, lead author Sarah K. McGill, MD, MSc, of the University of North Carolina at Chapel Hill, and colleagues reported.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” the investigators wrote in Clinical Gastroenterology and Hepatology.
To this end, Dr. McGill and colleagues drafted the present clinical practice update, covering pathogenesis, clinical manifestations, diagnosis, and management.
“The allergy in alpha-gal syndrome is to galactose alpha-1,3-galactose, an oligosaccharide on the cells of all nonprimate mammals,” the investigators wrote. “Surprisingly, sensitization to alpha-gal, that is, the process by which human beings develop IgE antibodies to the sugar, is understood to occur after the bite of a tick or parasitic infection. In the United States, the Lone Star tick, an ectoparasite whose principal host is deer, is strongly implicated.”
Gastrointestinal focused clinical research is scarce, the investigators noted, citing two observational studies involving 375 patients positive for alpha-gal IgE. Almost half of these patients (40.7%) had gastrointestinal symptoms alone. Across the entire population, the most common gastrointestinal symptoms were abdominal pain (71%) and vomiting (22%). About three out of four patients reported improvement on an alpha-gal avoidance diet.
“Clinicians should consider alpha-gal syndrome in the differential diagnosis of patients with unexplained gastrointestinal symptoms of abdominal pain, diarrhea, nausea, and vomiting, particularly those who live or have lived in an alpha-gal–prevalent area,” the investigators wrote.
In the United States, these areas span the domain of the Lone Star tick, including most of the East Coast, the central Midwest, the South, and all of Texas. Overseas, alpha-gal syndrome has been reported in Japan, Australia, Western Europe, and South Africa.
Clinical suspicion should be increased in patients with a history of tick bite, engagement in outdoor activities, and awakening in the night with gastrointestinal distress (because of the delay between allergen ingestion and symptom onset). Workup should include serum testing for alpha-gal IgE antibodies, according to the update. Serum positivity alone, however, is not sufficient for diagnosis. Alpha-gal syndrome must be confirmed by symptom resolution or improvement upon adherence to an alpha-gal avoidance diet for at least a month.
“During this time, patients may want to avoid eating at restaurants, which can easily cross-contaminate food, and processed food, which may contain alpha-gal in additives,” Dr. McGill and colleagues wrote.
Patients with alpha-gal syndrome who accidentally consume alpha-gal should take 25-50 mg of diphenhydramine and ensure access to a self-injectable epinephrine if symptoms progress, particularly if respiratory compromise occurs, they added.
The coauthors are Jana G. Hasash, MD, and Thomas A. Platts-Mills, MD, PhD.
The investigators disclosed relationships with Olympus America, Exact Sciences, Guardant Health, Finch Therapeutics, and others.
according to an American Gastroenterological Association clinical practice update.
Although the allergic response is best known for a combination of anaphylaxis, skin changes, and gastrointestinal symptoms that occurs within hours of consuming mammalian-derived food products, health care providers should know that many patients experience gastrointestinal distress in the absence of other clinical signs, lead author Sarah K. McGill, MD, MSc, of the University of North Carolina at Chapel Hill, and colleagues reported.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” the investigators wrote in Clinical Gastroenterology and Hepatology.
To this end, Dr. McGill and colleagues drafted the present clinical practice update, covering pathogenesis, clinical manifestations, diagnosis, and management.
“The allergy in alpha-gal syndrome is to galactose alpha-1,3-galactose, an oligosaccharide on the cells of all nonprimate mammals,” the investigators wrote. “Surprisingly, sensitization to alpha-gal, that is, the process by which human beings develop IgE antibodies to the sugar, is understood to occur after the bite of a tick or parasitic infection. In the United States, the Lone Star tick, an ectoparasite whose principal host is deer, is strongly implicated.”
Gastrointestinal focused clinical research is scarce, the investigators noted, citing two observational studies involving 375 patients positive for alpha-gal IgE. Almost half of these patients (40.7%) had gastrointestinal symptoms alone. Across the entire population, the most common gastrointestinal symptoms were abdominal pain (71%) and vomiting (22%). About three out of four patients reported improvement on an alpha-gal avoidance diet.
“Clinicians should consider alpha-gal syndrome in the differential diagnosis of patients with unexplained gastrointestinal symptoms of abdominal pain, diarrhea, nausea, and vomiting, particularly those who live or have lived in an alpha-gal–prevalent area,” the investigators wrote.
In the United States, these areas span the domain of the Lone Star tick, including most of the East Coast, the central Midwest, the South, and all of Texas. Overseas, alpha-gal syndrome has been reported in Japan, Australia, Western Europe, and South Africa.
Clinical suspicion should be increased in patients with a history of tick bite, engagement in outdoor activities, and awakening in the night with gastrointestinal distress (because of the delay between allergen ingestion and symptom onset). Workup should include serum testing for alpha-gal IgE antibodies, according to the update. Serum positivity alone, however, is not sufficient for diagnosis. Alpha-gal syndrome must be confirmed by symptom resolution or improvement upon adherence to an alpha-gal avoidance diet for at least a month.
“During this time, patients may want to avoid eating at restaurants, which can easily cross-contaminate food, and processed food, which may contain alpha-gal in additives,” Dr. McGill and colleagues wrote.
Patients with alpha-gal syndrome who accidentally consume alpha-gal should take 25-50 mg of diphenhydramine and ensure access to a self-injectable epinephrine if symptoms progress, particularly if respiratory compromise occurs, they added.
The coauthors are Jana G. Hasash, MD, and Thomas A. Platts-Mills, MD, PhD.
The investigators disclosed relationships with Olympus America, Exact Sciences, Guardant Health, Finch Therapeutics, and others.
according to an American Gastroenterological Association clinical practice update.
Although the allergic response is best known for a combination of anaphylaxis, skin changes, and gastrointestinal symptoms that occurs within hours of consuming mammalian-derived food products, health care providers should know that many patients experience gastrointestinal distress in the absence of other clinical signs, lead author Sarah K. McGill, MD, MSc, of the University of North Carolina at Chapel Hill, and colleagues reported.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” the investigators wrote in Clinical Gastroenterology and Hepatology.
To this end, Dr. McGill and colleagues drafted the present clinical practice update, covering pathogenesis, clinical manifestations, diagnosis, and management.
“The allergy in alpha-gal syndrome is to galactose alpha-1,3-galactose, an oligosaccharide on the cells of all nonprimate mammals,” the investigators wrote. “Surprisingly, sensitization to alpha-gal, that is, the process by which human beings develop IgE antibodies to the sugar, is understood to occur after the bite of a tick or parasitic infection. In the United States, the Lone Star tick, an ectoparasite whose principal host is deer, is strongly implicated.”
Gastrointestinal focused clinical research is scarce, the investigators noted, citing two observational studies involving 375 patients positive for alpha-gal IgE. Almost half of these patients (40.7%) had gastrointestinal symptoms alone. Across the entire population, the most common gastrointestinal symptoms were abdominal pain (71%) and vomiting (22%). About three out of four patients reported improvement on an alpha-gal avoidance diet.
“Clinicians should consider alpha-gal syndrome in the differential diagnosis of patients with unexplained gastrointestinal symptoms of abdominal pain, diarrhea, nausea, and vomiting, particularly those who live or have lived in an alpha-gal–prevalent area,” the investigators wrote.
In the United States, these areas span the domain of the Lone Star tick, including most of the East Coast, the central Midwest, the South, and all of Texas. Overseas, alpha-gal syndrome has been reported in Japan, Australia, Western Europe, and South Africa.
Clinical suspicion should be increased in patients with a history of tick bite, engagement in outdoor activities, and awakening in the night with gastrointestinal distress (because of the delay between allergen ingestion and symptom onset). Workup should include serum testing for alpha-gal IgE antibodies, according to the update. Serum positivity alone, however, is not sufficient for diagnosis. Alpha-gal syndrome must be confirmed by symptom resolution or improvement upon adherence to an alpha-gal avoidance diet for at least a month.
“During this time, patients may want to avoid eating at restaurants, which can easily cross-contaminate food, and processed food, which may contain alpha-gal in additives,” Dr. McGill and colleagues wrote.
Patients with alpha-gal syndrome who accidentally consume alpha-gal should take 25-50 mg of diphenhydramine and ensure access to a self-injectable epinephrine if symptoms progress, particularly if respiratory compromise occurs, they added.
The coauthors are Jana G. Hasash, MD, and Thomas A. Platts-Mills, MD, PhD.
The investigators disclosed relationships with Olympus America, Exact Sciences, Guardant Health, Finch Therapeutics, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA clinical practice update addresses role of endoscopic ultrasound–guided gallbladder drainage in acute cholecystitis
according to a recent clinical practice update by the American Gastroenterological Association.
The update, written by Shayan S. Irani, MD, of Virginia Mason Medical Center, Seattle, and colleagues, also covers techniques and outcomes of EUS-GBD and provides suggestions for training and patient selection.
“In this clinical practice update, we comment on the role of EUS-GBD (compared with ET-GBD [endoscopic treatment via transpapillary gallbladder drainage] and PT [percutaneous transhepatic]-GBD) in the management of acute cholecystitis, and describe its indications, contraindications, procedural considerations, and associated adverse events,” the authors wrote in Clinical Gastroenterology and Hepatology.
Dr. Irani and colleagues noted that EUS-GBD is a valuable alternative to PT-GBD, which can have a significant morbidity, and ET-GBD has been associated with relatively lower technical and clinical success rates in the presence of obstructing pathology of the cystic duct. Advances in lumen-apposing metal stents have further improved outcomes in EUS-GBD, as demonstrated by multiple case series and comparative trials.
According to the update, EUS-GBD is suggested in three scenarios: for draining the gallbladder in patients with acute cholecystitis who are at high risk for surgery, for removing percutaneous cholecystostomy drains in patients who cannot undergo cholecystectomy, and for draining malignant biliary obstruction in patients who have not responded to other treatments. EUS-GBD is contraindicated in patients with significant coagulopathy, large-volume uncontrolled ascites, or gallbladder perforation.
Dr. Irani and colleagues also noted that, between the three main techniques mentioned above, EUS-GBD has the lowest risk of recurrent cholecystitis, whereas ET-GBD and PT-GBD present slightly lower mortality rates.
While the update provides technical guidance on performing EUS-GBD, Dr. Irani and colleagues make clear that EUS-GBD is a highly specialized procedure that requires sufficient training to optimal results.
“Performing the procedure has an associated learning curve and requires advanced EUS training,” they wrote. “Two recent publications have suggested that the minimum number of procedures to gain competency should be approximately 19-25 procedures.”
Addressing unmet needs, Dr. Irani and colleagues suggested that more research is needed to standardize patient selection, procedure technique, and stent follow-up evaluation.
Ongoing studies aim to address whether endoscopic management of cholecystitis and symptomatic gallstones could become a mainstream treatment in the future, they wrote, but “we are still a long way from abandoning standard of care with cholecystectomy.”
This clinical practice update was commissioned by the AGA. Dr. Irani is a consultant for Boston Scientific, ConMed, and GORE; one coauthor received research support from Boston Scientific and Olympus and is a consultant and speaker for Boston Scientific, Cook, Medtronic, Olympus and ConMed. The remaining coauthor disclosed no conflicts.
according to a recent clinical practice update by the American Gastroenterological Association.
The update, written by Shayan S. Irani, MD, of Virginia Mason Medical Center, Seattle, and colleagues, also covers techniques and outcomes of EUS-GBD and provides suggestions for training and patient selection.
“In this clinical practice update, we comment on the role of EUS-GBD (compared with ET-GBD [endoscopic treatment via transpapillary gallbladder drainage] and PT [percutaneous transhepatic]-GBD) in the management of acute cholecystitis, and describe its indications, contraindications, procedural considerations, and associated adverse events,” the authors wrote in Clinical Gastroenterology and Hepatology.
Dr. Irani and colleagues noted that EUS-GBD is a valuable alternative to PT-GBD, which can have a significant morbidity, and ET-GBD has been associated with relatively lower technical and clinical success rates in the presence of obstructing pathology of the cystic duct. Advances in lumen-apposing metal stents have further improved outcomes in EUS-GBD, as demonstrated by multiple case series and comparative trials.
According to the update, EUS-GBD is suggested in three scenarios: for draining the gallbladder in patients with acute cholecystitis who are at high risk for surgery, for removing percutaneous cholecystostomy drains in patients who cannot undergo cholecystectomy, and for draining malignant biliary obstruction in patients who have not responded to other treatments. EUS-GBD is contraindicated in patients with significant coagulopathy, large-volume uncontrolled ascites, or gallbladder perforation.
Dr. Irani and colleagues also noted that, between the three main techniques mentioned above, EUS-GBD has the lowest risk of recurrent cholecystitis, whereas ET-GBD and PT-GBD present slightly lower mortality rates.
While the update provides technical guidance on performing EUS-GBD, Dr. Irani and colleagues make clear that EUS-GBD is a highly specialized procedure that requires sufficient training to optimal results.
“Performing the procedure has an associated learning curve and requires advanced EUS training,” they wrote. “Two recent publications have suggested that the minimum number of procedures to gain competency should be approximately 19-25 procedures.”
Addressing unmet needs, Dr. Irani and colleagues suggested that more research is needed to standardize patient selection, procedure technique, and stent follow-up evaluation.
Ongoing studies aim to address whether endoscopic management of cholecystitis and symptomatic gallstones could become a mainstream treatment in the future, they wrote, but “we are still a long way from abandoning standard of care with cholecystectomy.”
This clinical practice update was commissioned by the AGA. Dr. Irani is a consultant for Boston Scientific, ConMed, and GORE; one coauthor received research support from Boston Scientific and Olympus and is a consultant and speaker for Boston Scientific, Cook, Medtronic, Olympus and ConMed. The remaining coauthor disclosed no conflicts.
according to a recent clinical practice update by the American Gastroenterological Association.
The update, written by Shayan S. Irani, MD, of Virginia Mason Medical Center, Seattle, and colleagues, also covers techniques and outcomes of EUS-GBD and provides suggestions for training and patient selection.
“In this clinical practice update, we comment on the role of EUS-GBD (compared with ET-GBD [endoscopic treatment via transpapillary gallbladder drainage] and PT [percutaneous transhepatic]-GBD) in the management of acute cholecystitis, and describe its indications, contraindications, procedural considerations, and associated adverse events,” the authors wrote in Clinical Gastroenterology and Hepatology.
Dr. Irani and colleagues noted that EUS-GBD is a valuable alternative to PT-GBD, which can have a significant morbidity, and ET-GBD has been associated with relatively lower technical and clinical success rates in the presence of obstructing pathology of the cystic duct. Advances in lumen-apposing metal stents have further improved outcomes in EUS-GBD, as demonstrated by multiple case series and comparative trials.
According to the update, EUS-GBD is suggested in three scenarios: for draining the gallbladder in patients with acute cholecystitis who are at high risk for surgery, for removing percutaneous cholecystostomy drains in patients who cannot undergo cholecystectomy, and for draining malignant biliary obstruction in patients who have not responded to other treatments. EUS-GBD is contraindicated in patients with significant coagulopathy, large-volume uncontrolled ascites, or gallbladder perforation.
Dr. Irani and colleagues also noted that, between the three main techniques mentioned above, EUS-GBD has the lowest risk of recurrent cholecystitis, whereas ET-GBD and PT-GBD present slightly lower mortality rates.
While the update provides technical guidance on performing EUS-GBD, Dr. Irani and colleagues make clear that EUS-GBD is a highly specialized procedure that requires sufficient training to optimal results.
“Performing the procedure has an associated learning curve and requires advanced EUS training,” they wrote. “Two recent publications have suggested that the minimum number of procedures to gain competency should be approximately 19-25 procedures.”
Addressing unmet needs, Dr. Irani and colleagues suggested that more research is needed to standardize patient selection, procedure technique, and stent follow-up evaluation.
Ongoing studies aim to address whether endoscopic management of cholecystitis and symptomatic gallstones could become a mainstream treatment in the future, they wrote, but “we are still a long way from abandoning standard of care with cholecystectomy.”
This clinical practice update was commissioned by the AGA. Dr. Irani is a consultant for Boston Scientific, ConMed, and GORE; one coauthor received research support from Boston Scientific and Olympus and is a consultant and speaker for Boston Scientific, Cook, Medtronic, Olympus and ConMed. The remaining coauthor disclosed no conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
PNPLA3 genotype predicts cirrhosis in NAFLD
while those with this genotype who also have diabetes and indeterminate Fibrosis-4 (FIB4) scores may have the same risk of cirrhosis as patients with a high FIB4, according to investigators.
These findings suggest that NAFLD patients with indeterminate FIB4 and metabolic risk factors should routinely undergo PNPLA3 genotyping, lead author Vincent L. Chen, MD, of the University of Michigan, Ann Arbor, and colleagues reported.
“Whether incorporating genetics into risk stratification results in meaningful improvement over clinical predictors, such as FIB4, diabetes, and obesity status, is unknown,” the investigators wrote in Gastroenterology. “Improved understanding of how genetics influences the rate of disease progression and how it interacts with established risk factors for advanced liver disease is crucial for genetic testing to be applicable in clinical practice.”
To evaluate the risk presented by the PNPLA3 p.I148M variant, Dr. Chen and colleagues analyzed data from two independent cohorts with 7,893 patients and 46,880 patients each.
They first characterized the relationship between PNPLA3 genotype and cirrhosis via univariable and multivariable analyses. These efforts revealed that the genotype predicted cirrhosis in both cohorts, with associations also detected for well-documented clinical risk factors, including diabetes, obesity, and high ALT. Of note, PNPLA3 genotype demonstrated an additive effect for cirrhosis when detected in conjunction with these risks.
Further analysis revealed that homozygous carriers of PNPLA3 p.I148M with indeterminate FIB4 scores (1.3-2.67) and diabetes had an incidence rate of cirrhosis on par with patients who had high-risk FIB4 (greater than 2.67).
The effects of the risk allele were also made evident by comparing patients with diabetes and indeterminate FIB4 based on presence or absence of the marker – those testing positive for h PNPLA3 p.I148M had 2.9-4.8 times greater risk of cirrhosis. Conversely, patients with FIB4 scores less than 1.3, regardless of other risk factors, had little change in cirrhosis rate regardless of PNPLA3 status.
“We found that PNPLA3 genotyping in conjunction with clinical risk factors may improve risk stratification in patients with NAFLD,” the investigators concluded. “Although it may be possible to develop more complex polygenic risk scores for cirrhosis, these findings suggest that genotyping of PNPLA3 alone, which is less expensive than genomewide genotyping and easier to understand, may have similar clinical applicability for NAFLD.”
Dr. Chen and colleagues therefore recommended that NAFLD patients with metabolic risk factors (particularly diabetes) and indeterminate FIB4 routinely undergo PNPLA3 genotyping, with referral to hepatology if positive for two risk alleles.
The study was supported by the American Association for the Study of Liver Diseases, National Institutes of Health, and the University of Michigan department of internal medicine. The investigators disclosed no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) is becoming globally a leading cause of cirrhosis and related complications, namely decompensation and hepatocellular carcinoma. Since NAFLD affects a large fraction of the population, and especially people with obesity, type 2 diabetes and metabolic comorbidities, it is difficult to identify those at risk of cirrhosis and liver-related versus more frequent cardiometabolic events. The first step in risk stratification is based on the calculation of simple liver fibrosis scores, such as the FIB4, but this too often leads to indeterminate results requiring additional testing.
These results will contribute to establish referral pathways to identify persons at high risk of liver disease, even at a young age. This may enable preventive programs based on intensified lifestyle and diabetes management, specific treatments for fibrotic NAFLD once these become available, and close surveillance for complications. What’s more, therapeutic approaches directly targeting liver PNPLA3 p.I148M are already under clinical evaluation to prevent disease progression specifically in this high-risk group.
Luca Valenti, MD, is an associate professor of internal medicine in pathophysiology and transplantation at the Università degli Studi di Milano. He is head of the Precision Lab and Biological Resource Center Unit. Dr. Valenti has no relevant disclosures.
Nonalcoholic fatty liver disease (NAFLD) is becoming globally a leading cause of cirrhosis and related complications, namely decompensation and hepatocellular carcinoma. Since NAFLD affects a large fraction of the population, and especially people with obesity, type 2 diabetes and metabolic comorbidities, it is difficult to identify those at risk of cirrhosis and liver-related versus more frequent cardiometabolic events. The first step in risk stratification is based on the calculation of simple liver fibrosis scores, such as the FIB4, but this too often leads to indeterminate results requiring additional testing.
These results will contribute to establish referral pathways to identify persons at high risk of liver disease, even at a young age. This may enable preventive programs based on intensified lifestyle and diabetes management, specific treatments for fibrotic NAFLD once these become available, and close surveillance for complications. What’s more, therapeutic approaches directly targeting liver PNPLA3 p.I148M are already under clinical evaluation to prevent disease progression specifically in this high-risk group.
Luca Valenti, MD, is an associate professor of internal medicine in pathophysiology and transplantation at the Università degli Studi di Milano. He is head of the Precision Lab and Biological Resource Center Unit. Dr. Valenti has no relevant disclosures.
Nonalcoholic fatty liver disease (NAFLD) is becoming globally a leading cause of cirrhosis and related complications, namely decompensation and hepatocellular carcinoma. Since NAFLD affects a large fraction of the population, and especially people with obesity, type 2 diabetes and metabolic comorbidities, it is difficult to identify those at risk of cirrhosis and liver-related versus more frequent cardiometabolic events. The first step in risk stratification is based on the calculation of simple liver fibrosis scores, such as the FIB4, but this too often leads to indeterminate results requiring additional testing.
These results will contribute to establish referral pathways to identify persons at high risk of liver disease, even at a young age. This may enable preventive programs based on intensified lifestyle and diabetes management, specific treatments for fibrotic NAFLD once these become available, and close surveillance for complications. What’s more, therapeutic approaches directly targeting liver PNPLA3 p.I148M are already under clinical evaluation to prevent disease progression specifically in this high-risk group.
Luca Valenti, MD, is an associate professor of internal medicine in pathophysiology and transplantation at the Università degli Studi di Milano. He is head of the Precision Lab and Biological Resource Center Unit. Dr. Valenti has no relevant disclosures.
while those with this genotype who also have diabetes and indeterminate Fibrosis-4 (FIB4) scores may have the same risk of cirrhosis as patients with a high FIB4, according to investigators.
These findings suggest that NAFLD patients with indeterminate FIB4 and metabolic risk factors should routinely undergo PNPLA3 genotyping, lead author Vincent L. Chen, MD, of the University of Michigan, Ann Arbor, and colleagues reported.
“Whether incorporating genetics into risk stratification results in meaningful improvement over clinical predictors, such as FIB4, diabetes, and obesity status, is unknown,” the investigators wrote in Gastroenterology. “Improved understanding of how genetics influences the rate of disease progression and how it interacts with established risk factors for advanced liver disease is crucial for genetic testing to be applicable in clinical practice.”
To evaluate the risk presented by the PNPLA3 p.I148M variant, Dr. Chen and colleagues analyzed data from two independent cohorts with 7,893 patients and 46,880 patients each.
They first characterized the relationship between PNPLA3 genotype and cirrhosis via univariable and multivariable analyses. These efforts revealed that the genotype predicted cirrhosis in both cohorts, with associations also detected for well-documented clinical risk factors, including diabetes, obesity, and high ALT. Of note, PNPLA3 genotype demonstrated an additive effect for cirrhosis when detected in conjunction with these risks.
Further analysis revealed that homozygous carriers of PNPLA3 p.I148M with indeterminate FIB4 scores (1.3-2.67) and diabetes had an incidence rate of cirrhosis on par with patients who had high-risk FIB4 (greater than 2.67).
The effects of the risk allele were also made evident by comparing patients with diabetes and indeterminate FIB4 based on presence or absence of the marker – those testing positive for h PNPLA3 p.I148M had 2.9-4.8 times greater risk of cirrhosis. Conversely, patients with FIB4 scores less than 1.3, regardless of other risk factors, had little change in cirrhosis rate regardless of PNPLA3 status.
“We found that PNPLA3 genotyping in conjunction with clinical risk factors may improve risk stratification in patients with NAFLD,” the investigators concluded. “Although it may be possible to develop more complex polygenic risk scores for cirrhosis, these findings suggest that genotyping of PNPLA3 alone, which is less expensive than genomewide genotyping and easier to understand, may have similar clinical applicability for NAFLD.”
Dr. Chen and colleagues therefore recommended that NAFLD patients with metabolic risk factors (particularly diabetes) and indeterminate FIB4 routinely undergo PNPLA3 genotyping, with referral to hepatology if positive for two risk alleles.
The study was supported by the American Association for the Study of Liver Diseases, National Institutes of Health, and the University of Michigan department of internal medicine. The investigators disclosed no conflicts of interest.
while those with this genotype who also have diabetes and indeterminate Fibrosis-4 (FIB4) scores may have the same risk of cirrhosis as patients with a high FIB4, according to investigators.
These findings suggest that NAFLD patients with indeterminate FIB4 and metabolic risk factors should routinely undergo PNPLA3 genotyping, lead author Vincent L. Chen, MD, of the University of Michigan, Ann Arbor, and colleagues reported.
“Whether incorporating genetics into risk stratification results in meaningful improvement over clinical predictors, such as FIB4, diabetes, and obesity status, is unknown,” the investigators wrote in Gastroenterology. “Improved understanding of how genetics influences the rate of disease progression and how it interacts with established risk factors for advanced liver disease is crucial for genetic testing to be applicable in clinical practice.”
To evaluate the risk presented by the PNPLA3 p.I148M variant, Dr. Chen and colleagues analyzed data from two independent cohorts with 7,893 patients and 46,880 patients each.
They first characterized the relationship between PNPLA3 genotype and cirrhosis via univariable and multivariable analyses. These efforts revealed that the genotype predicted cirrhosis in both cohorts, with associations also detected for well-documented clinical risk factors, including diabetes, obesity, and high ALT. Of note, PNPLA3 genotype demonstrated an additive effect for cirrhosis when detected in conjunction with these risks.
Further analysis revealed that homozygous carriers of PNPLA3 p.I148M with indeterminate FIB4 scores (1.3-2.67) and diabetes had an incidence rate of cirrhosis on par with patients who had high-risk FIB4 (greater than 2.67).
The effects of the risk allele were also made evident by comparing patients with diabetes and indeterminate FIB4 based on presence or absence of the marker – those testing positive for h PNPLA3 p.I148M had 2.9-4.8 times greater risk of cirrhosis. Conversely, patients with FIB4 scores less than 1.3, regardless of other risk factors, had little change in cirrhosis rate regardless of PNPLA3 status.
“We found that PNPLA3 genotyping in conjunction with clinical risk factors may improve risk stratification in patients with NAFLD,” the investigators concluded. “Although it may be possible to develop more complex polygenic risk scores for cirrhosis, these findings suggest that genotyping of PNPLA3 alone, which is less expensive than genomewide genotyping and easier to understand, may have similar clinical applicability for NAFLD.”
Dr. Chen and colleagues therefore recommended that NAFLD patients with metabolic risk factors (particularly diabetes) and indeterminate FIB4 routinely undergo PNPLA3 genotyping, with referral to hepatology if positive for two risk alleles.
The study was supported by the American Association for the Study of Liver Diseases, National Institutes of Health, and the University of Michigan department of internal medicine. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
AI helps predict ulcerative colitis remission/activity, flare-ups
The AI tool predicted UC disease activity with 89% accuracy and inflammation at the biopsy site with 80% accuracy. Its ability to stratify risk of UC flare was on par with human pathologists.
“This tool in the near future will speed up, simplify, and standardize histological assessment of ulcerative colitis and provide the clinician with accurate prognostic information in real time,” co–lead author Marietta Iacucci, MD, PhD, from the University of Birmingham (England), and University College Cork (Ireland), said in an interview.
“The tool needs to be refined and further validated before it is ready for daily clinical practice. That work is ongoing now,” Dr. Iacucci said.
The researchers describe their advanced AI-based computer-aided detection tool in a study published online in Gastroenterology.
‘Strong’ performance
They used 535 digitized biopsies from 273 patients with UC (mean age, 48 years; 41% women) to develop and test the tool. They used a subset of 118 to train it to distinguish remission from activity, 42 to calibrate it, and 375 to test it. An additional 154 biopsies from 58 patients with UC were used to externally validate the tool.
The model also was tested to predict the corresponding endoscopic assessment and occurrence of flares at 12 months.
UC disease activity was defined by three different histologic indices: the Robarts Histopathology Index (RHI), the Nancy Histological Index (NHI), and the newly developed PICaSSO Histologic Remission Index (PHRI).
The AI tool had “strong diagnostic performance to detect disease activity” (PHRI > 0) with an overall area under the receiver operating characteristic curve of 0.87 and sensitivity and specificity of 89% and 85%, respectively.
The researchers note that, while the AI tool was trained for the PHRI, its sensitivity for RHI and NHI histologic remission/activity was also high (94% and 89%, respectively).
Despite the different mix of severity grades, the AI model “maintained a good diagnostic performance, proving its applicability outside the original development setting,” they reported.
The AI tool could also predict the presence of endoscopic inflammation in the biopsy area with about 80% accuracy.
“Though imperfect, this result is consistent with human-assessed correlation between endoscopy and histology,” the researchers noted.
The model predicted the corresponding endoscopic remission/activity with 79% and 82% accuracy for UCEIS and PICaSSO, respectively.
The hazard ratios for disease flare-up between the AI system and pathologists assessed by PHRI was similar (4.64 and 3.56, respectively), “demonstrating the ability of the computer to stratify the risk of flare comparably well to pathologists,” they added.
Both histology and outcome prediction were confirmed in the external validation cohort.
The AI system delivered results in an average of 9.8 seconds per slide.
Potential ‘game changer’
UC is a “complex condition to predict, and developing machine learning–derived systems to make this diagnostic job quicker and more accurate could be a game changer,” Dr. Iacucci said in a news release.
With refinement, the AI tool will have an impact on both clinical trials and daily practice, the researchers wrote. In clinical practice, histological reporting remains “largely descriptive and nonstandard, thus would greatly benefit from a quick and objective assessment. Similarly, clinical trials in UC could efficiently overcome costly central readings.”
Assessing and measuring improvement in endoscopy and histology are difficult parts of treating UC, said David Hudesman, MD, codirector of the Inflammatory Bowel Disease Center at New York University Langone Health.
“We do not know how much improvement is associated with improved long-term outcomes,” Dr. Hudesman said in an interview. “For example, does a patient need complete healing or is 50% better enough?” Dr. Hudesman was not involved with the current research.
“This study showed that AI can predict – with good accuracy – endoscopy and histology scores, as well as 1-year patient outcomes. If this is validated in larger studies, AI can help determine if we should adjust/change therapies or continue, which is very important,” he said.
This research was supported by the National Institute for Health Research Birmingham Biomedical Research Centre. Dr. Iacucci and Dr. Hudesman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The AI tool predicted UC disease activity with 89% accuracy and inflammation at the biopsy site with 80% accuracy. Its ability to stratify risk of UC flare was on par with human pathologists.
“This tool in the near future will speed up, simplify, and standardize histological assessment of ulcerative colitis and provide the clinician with accurate prognostic information in real time,” co–lead author Marietta Iacucci, MD, PhD, from the University of Birmingham (England), and University College Cork (Ireland), said in an interview.
“The tool needs to be refined and further validated before it is ready for daily clinical practice. That work is ongoing now,” Dr. Iacucci said.
The researchers describe their advanced AI-based computer-aided detection tool in a study published online in Gastroenterology.
‘Strong’ performance
They used 535 digitized biopsies from 273 patients with UC (mean age, 48 years; 41% women) to develop and test the tool. They used a subset of 118 to train it to distinguish remission from activity, 42 to calibrate it, and 375 to test it. An additional 154 biopsies from 58 patients with UC were used to externally validate the tool.
The model also was tested to predict the corresponding endoscopic assessment and occurrence of flares at 12 months.
UC disease activity was defined by three different histologic indices: the Robarts Histopathology Index (RHI), the Nancy Histological Index (NHI), and the newly developed PICaSSO Histologic Remission Index (PHRI).
The AI tool had “strong diagnostic performance to detect disease activity” (PHRI > 0) with an overall area under the receiver operating characteristic curve of 0.87 and sensitivity and specificity of 89% and 85%, respectively.
The researchers note that, while the AI tool was trained for the PHRI, its sensitivity for RHI and NHI histologic remission/activity was also high (94% and 89%, respectively).
Despite the different mix of severity grades, the AI model “maintained a good diagnostic performance, proving its applicability outside the original development setting,” they reported.
The AI tool could also predict the presence of endoscopic inflammation in the biopsy area with about 80% accuracy.
“Though imperfect, this result is consistent with human-assessed correlation between endoscopy and histology,” the researchers noted.
The model predicted the corresponding endoscopic remission/activity with 79% and 82% accuracy for UCEIS and PICaSSO, respectively.
The hazard ratios for disease flare-up between the AI system and pathologists assessed by PHRI was similar (4.64 and 3.56, respectively), “demonstrating the ability of the computer to stratify the risk of flare comparably well to pathologists,” they added.
Both histology and outcome prediction were confirmed in the external validation cohort.
The AI system delivered results in an average of 9.8 seconds per slide.
Potential ‘game changer’
UC is a “complex condition to predict, and developing machine learning–derived systems to make this diagnostic job quicker and more accurate could be a game changer,” Dr. Iacucci said in a news release.
With refinement, the AI tool will have an impact on both clinical trials and daily practice, the researchers wrote. In clinical practice, histological reporting remains “largely descriptive and nonstandard, thus would greatly benefit from a quick and objective assessment. Similarly, clinical trials in UC could efficiently overcome costly central readings.”
Assessing and measuring improvement in endoscopy and histology are difficult parts of treating UC, said David Hudesman, MD, codirector of the Inflammatory Bowel Disease Center at New York University Langone Health.
“We do not know how much improvement is associated with improved long-term outcomes,” Dr. Hudesman said in an interview. “For example, does a patient need complete healing or is 50% better enough?” Dr. Hudesman was not involved with the current research.
“This study showed that AI can predict – with good accuracy – endoscopy and histology scores, as well as 1-year patient outcomes. If this is validated in larger studies, AI can help determine if we should adjust/change therapies or continue, which is very important,” he said.
This research was supported by the National Institute for Health Research Birmingham Biomedical Research Centre. Dr. Iacucci and Dr. Hudesman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The AI tool predicted UC disease activity with 89% accuracy and inflammation at the biopsy site with 80% accuracy. Its ability to stratify risk of UC flare was on par with human pathologists.
“This tool in the near future will speed up, simplify, and standardize histological assessment of ulcerative colitis and provide the clinician with accurate prognostic information in real time,” co–lead author Marietta Iacucci, MD, PhD, from the University of Birmingham (England), and University College Cork (Ireland), said in an interview.
“The tool needs to be refined and further validated before it is ready for daily clinical practice. That work is ongoing now,” Dr. Iacucci said.
The researchers describe their advanced AI-based computer-aided detection tool in a study published online in Gastroenterology.
‘Strong’ performance
They used 535 digitized biopsies from 273 patients with UC (mean age, 48 years; 41% women) to develop and test the tool. They used a subset of 118 to train it to distinguish remission from activity, 42 to calibrate it, and 375 to test it. An additional 154 biopsies from 58 patients with UC were used to externally validate the tool.
The model also was tested to predict the corresponding endoscopic assessment and occurrence of flares at 12 months.
UC disease activity was defined by three different histologic indices: the Robarts Histopathology Index (RHI), the Nancy Histological Index (NHI), and the newly developed PICaSSO Histologic Remission Index (PHRI).
The AI tool had “strong diagnostic performance to detect disease activity” (PHRI > 0) with an overall area under the receiver operating characteristic curve of 0.87 and sensitivity and specificity of 89% and 85%, respectively.
The researchers note that, while the AI tool was trained for the PHRI, its sensitivity for RHI and NHI histologic remission/activity was also high (94% and 89%, respectively).
Despite the different mix of severity grades, the AI model “maintained a good diagnostic performance, proving its applicability outside the original development setting,” they reported.
The AI tool could also predict the presence of endoscopic inflammation in the biopsy area with about 80% accuracy.
“Though imperfect, this result is consistent with human-assessed correlation between endoscopy and histology,” the researchers noted.
The model predicted the corresponding endoscopic remission/activity with 79% and 82% accuracy for UCEIS and PICaSSO, respectively.
The hazard ratios for disease flare-up between the AI system and pathologists assessed by PHRI was similar (4.64 and 3.56, respectively), “demonstrating the ability of the computer to stratify the risk of flare comparably well to pathologists,” they added.
Both histology and outcome prediction were confirmed in the external validation cohort.
The AI system delivered results in an average of 9.8 seconds per slide.
Potential ‘game changer’
UC is a “complex condition to predict, and developing machine learning–derived systems to make this diagnostic job quicker and more accurate could be a game changer,” Dr. Iacucci said in a news release.
With refinement, the AI tool will have an impact on both clinical trials and daily practice, the researchers wrote. In clinical practice, histological reporting remains “largely descriptive and nonstandard, thus would greatly benefit from a quick and objective assessment. Similarly, clinical trials in UC could efficiently overcome costly central readings.”
Assessing and measuring improvement in endoscopy and histology are difficult parts of treating UC, said David Hudesman, MD, codirector of the Inflammatory Bowel Disease Center at New York University Langone Health.
“We do not know how much improvement is associated with improved long-term outcomes,” Dr. Hudesman said in an interview. “For example, does a patient need complete healing or is 50% better enough?” Dr. Hudesman was not involved with the current research.
“This study showed that AI can predict – with good accuracy – endoscopy and histology scores, as well as 1-year patient outcomes. If this is validated in larger studies, AI can help determine if we should adjust/change therapies or continue, which is very important,” he said.
This research was supported by the National Institute for Health Research Birmingham Biomedical Research Centre. Dr. Iacucci and Dr. Hudesman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
Patient-based mouse MVID model hints at mechanism
Researchers have identified a novel mutation in a patient with microvillus inclusion disease (MVID) and rapidly developed a specific mouse model to find insights into the disease process.
MVID is characterized by severe diarrhea, generally beginning within a few hours of birth. The condition is caused by inactivating mutations in the gene myosin VB (MYO5B). Affected individuals usually require lifetime use of total parenteral nutrition or small-bowel transplantation.
More than 100 MYO5B mutations have been identified in MVID patients, most of whom inherit two unique mutant alleles. This can lead to variability in phenotypes, which single-mutation animal models have been unable to mimic. Generally, patients have atrophy of microvilli on enterocytes as well as inclusion bodies within enterocytes that contain microvilli.
In the study, published in Cellular and Molecular Gastroenterology and Hepatology, researchers describe genetic sequencing of MYO5B mutations in a patient and both parents. One mutation is predicted to lead to protein truncation (c.1821delG), and the other (c.1555G>A) appeared to be inherited from the patient’s mother. The patient suffered from severe diarrhea after birth and was intermittently feeding intolerant.
The researchers conducted a range of diagnostic tests and biopsies. One particularly interesting finding was expression of the A-kinase anchoring protein 350 in the vicinity of the inclusion, wrote Andreanna Burman and her coauthors at Vanderbilt University, Nashville, Tenn. This protein takes part in a protein-mediating scaffolding pathway, suggesting that it could play a role in the development of the inclusions.
The researchers used multiplexed immunofluorescence (MxIF) staining of a biopsy of the duodenum to look for expression of a range of proteins. They found a striking decrease in enterocytes that express glucose transporter 2, implying that malabsorption might be due at least in part to enterocytes that fail to mature.
The patient also had internalization of apical nutrient transporters, which has been reported in other MVID patients. The researchers also developed a mouse model that incorporated the patient’s novel compound heterozygous genotype, which they cross-bred to produce a tamoxifen-inducible mouse model.
After the tamoxifen injection, the patient-mimicking animals exhibited a severe watery diarrhea, losing 19% of their body weight by day 4. The animals’ intestines showed a similar phenotype to that of the patient.
The apical sodium transporters sodium-glucose cotransporter 1 (SGLT1), apical sodium-dependent bile transporter, and NHE3 were internalized away from the apical membrane of the enterocytes in the patient-mimicking model animals. This structural difference may explain the limited absorption of sodium and water and the resultant watery diarrhea. The researchers also noted disruption of the actinin-4+ terminal web structure, as well as increased SGLT1 localization with lysosome-associated membrane protein 1+ lysosomes within the mouse model enterocytes. This association may indicate degradation of mislocalized proteins, the authors noted, which has also been seen in expanded RAB7+ vesicles within MVID patient tissues.
Other signs pointed to the expansion of immature cells in the upper crypt and lower villus, as well as faster shedding of enterocytes in the villus than in control animals. Scanning electron microscope images also showed immature and disorganized microvilli in the enterocytes of the patient-mimicking mice.
Taken together, “these findings are consistent with a deficit in enterocyte maturation in Myo5b(G519R) mice and in the patient with the MYO5B(G519R) mutation,” the authors wrote.
The authors suggest that their approach could be used more generally to quickly create mouse models of patient-specific monogenic congenital disorders.
The authors disclosed no conflicts of interest. The research was funded by the National Foundation of Science, National Institutes of Health, Vanderbilt Digestive Diseases Research Center Pilot and Feasibility grant, an American Physiological Society John F. Perkins, Jr. Research Career Enhancement Award, and a gift from the Christine Volpe Fund.
Genetically engineered mouse models (GEMMs) have offered tremendous insight into the genetics, biology, and pathobiology of the gastrointestinal tract. Yet, in the past, the time necessary to generate these GEMMs has presented challenges for modeling human diseases. With the ongoing identification of disease-associated polymorphisms or mutations, including through approaches like genomewide association studies (GWAS), defining the function of these specific genetic alterations in disease pathogenesis is of great importance.
Overall, this study demonstrates the value of both patient-mimicking mouse models and multiplexed staining to define the molecular mechanisms of congenital diseases in vivo.
Dr. Jonathan P. Katz is associate professor of medicine, department of medicine, gastroenterology division, University of Pennsylvania Perelman School of Medicine, Philadelphia. He has no relevant conflicts of interest.
Genetically engineered mouse models (GEMMs) have offered tremendous insight into the genetics, biology, and pathobiology of the gastrointestinal tract. Yet, in the past, the time necessary to generate these GEMMs has presented challenges for modeling human diseases. With the ongoing identification of disease-associated polymorphisms or mutations, including through approaches like genomewide association studies (GWAS), defining the function of these specific genetic alterations in disease pathogenesis is of great importance.
Overall, this study demonstrates the value of both patient-mimicking mouse models and multiplexed staining to define the molecular mechanisms of congenital diseases in vivo.
Dr. Jonathan P. Katz is associate professor of medicine, department of medicine, gastroenterology division, University of Pennsylvania Perelman School of Medicine, Philadelphia. He has no relevant conflicts of interest.
Genetically engineered mouse models (GEMMs) have offered tremendous insight into the genetics, biology, and pathobiology of the gastrointestinal tract. Yet, in the past, the time necessary to generate these GEMMs has presented challenges for modeling human diseases. With the ongoing identification of disease-associated polymorphisms or mutations, including through approaches like genomewide association studies (GWAS), defining the function of these specific genetic alterations in disease pathogenesis is of great importance.
Overall, this study demonstrates the value of both patient-mimicking mouse models and multiplexed staining to define the molecular mechanisms of congenital diseases in vivo.
Dr. Jonathan P. Katz is associate professor of medicine, department of medicine, gastroenterology division, University of Pennsylvania Perelman School of Medicine, Philadelphia. He has no relevant conflicts of interest.
Researchers have identified a novel mutation in a patient with microvillus inclusion disease (MVID) and rapidly developed a specific mouse model to find insights into the disease process.
MVID is characterized by severe diarrhea, generally beginning within a few hours of birth. The condition is caused by inactivating mutations in the gene myosin VB (MYO5B). Affected individuals usually require lifetime use of total parenteral nutrition or small-bowel transplantation.
More than 100 MYO5B mutations have been identified in MVID patients, most of whom inherit two unique mutant alleles. This can lead to variability in phenotypes, which single-mutation animal models have been unable to mimic. Generally, patients have atrophy of microvilli on enterocytes as well as inclusion bodies within enterocytes that contain microvilli.
In the study, published in Cellular and Molecular Gastroenterology and Hepatology, researchers describe genetic sequencing of MYO5B mutations in a patient and both parents. One mutation is predicted to lead to protein truncation (c.1821delG), and the other (c.1555G>A) appeared to be inherited from the patient’s mother. The patient suffered from severe diarrhea after birth and was intermittently feeding intolerant.
The researchers conducted a range of diagnostic tests and biopsies. One particularly interesting finding was expression of the A-kinase anchoring protein 350 in the vicinity of the inclusion, wrote Andreanna Burman and her coauthors at Vanderbilt University, Nashville, Tenn. This protein takes part in a protein-mediating scaffolding pathway, suggesting that it could play a role in the development of the inclusions.
The researchers used multiplexed immunofluorescence (MxIF) staining of a biopsy of the duodenum to look for expression of a range of proteins. They found a striking decrease in enterocytes that express glucose transporter 2, implying that malabsorption might be due at least in part to enterocytes that fail to mature.
The patient also had internalization of apical nutrient transporters, which has been reported in other MVID patients. The researchers also developed a mouse model that incorporated the patient’s novel compound heterozygous genotype, which they cross-bred to produce a tamoxifen-inducible mouse model.
After the tamoxifen injection, the patient-mimicking animals exhibited a severe watery diarrhea, losing 19% of their body weight by day 4. The animals’ intestines showed a similar phenotype to that of the patient.
The apical sodium transporters sodium-glucose cotransporter 1 (SGLT1), apical sodium-dependent bile transporter, and NHE3 were internalized away from the apical membrane of the enterocytes in the patient-mimicking model animals. This structural difference may explain the limited absorption of sodium and water and the resultant watery diarrhea. The researchers also noted disruption of the actinin-4+ terminal web structure, as well as increased SGLT1 localization with lysosome-associated membrane protein 1+ lysosomes within the mouse model enterocytes. This association may indicate degradation of mislocalized proteins, the authors noted, which has also been seen in expanded RAB7+ vesicles within MVID patient tissues.
Other signs pointed to the expansion of immature cells in the upper crypt and lower villus, as well as faster shedding of enterocytes in the villus than in control animals. Scanning electron microscope images also showed immature and disorganized microvilli in the enterocytes of the patient-mimicking mice.
Taken together, “these findings are consistent with a deficit in enterocyte maturation in Myo5b(G519R) mice and in the patient with the MYO5B(G519R) mutation,” the authors wrote.
The authors suggest that their approach could be used more generally to quickly create mouse models of patient-specific monogenic congenital disorders.
The authors disclosed no conflicts of interest. The research was funded by the National Foundation of Science, National Institutes of Health, Vanderbilt Digestive Diseases Research Center Pilot and Feasibility grant, an American Physiological Society John F. Perkins, Jr. Research Career Enhancement Award, and a gift from the Christine Volpe Fund.
Researchers have identified a novel mutation in a patient with microvillus inclusion disease (MVID) and rapidly developed a specific mouse model to find insights into the disease process.
MVID is characterized by severe diarrhea, generally beginning within a few hours of birth. The condition is caused by inactivating mutations in the gene myosin VB (MYO5B). Affected individuals usually require lifetime use of total parenteral nutrition or small-bowel transplantation.
More than 100 MYO5B mutations have been identified in MVID patients, most of whom inherit two unique mutant alleles. This can lead to variability in phenotypes, which single-mutation animal models have been unable to mimic. Generally, patients have atrophy of microvilli on enterocytes as well as inclusion bodies within enterocytes that contain microvilli.
In the study, published in Cellular and Molecular Gastroenterology and Hepatology, researchers describe genetic sequencing of MYO5B mutations in a patient and both parents. One mutation is predicted to lead to protein truncation (c.1821delG), and the other (c.1555G>A) appeared to be inherited from the patient’s mother. The patient suffered from severe diarrhea after birth and was intermittently feeding intolerant.
The researchers conducted a range of diagnostic tests and biopsies. One particularly interesting finding was expression of the A-kinase anchoring protein 350 in the vicinity of the inclusion, wrote Andreanna Burman and her coauthors at Vanderbilt University, Nashville, Tenn. This protein takes part in a protein-mediating scaffolding pathway, suggesting that it could play a role in the development of the inclusions.
The researchers used multiplexed immunofluorescence (MxIF) staining of a biopsy of the duodenum to look for expression of a range of proteins. They found a striking decrease in enterocytes that express glucose transporter 2, implying that malabsorption might be due at least in part to enterocytes that fail to mature.
The patient also had internalization of apical nutrient transporters, which has been reported in other MVID patients. The researchers also developed a mouse model that incorporated the patient’s novel compound heterozygous genotype, which they cross-bred to produce a tamoxifen-inducible mouse model.
After the tamoxifen injection, the patient-mimicking animals exhibited a severe watery diarrhea, losing 19% of their body weight by day 4. The animals’ intestines showed a similar phenotype to that of the patient.
The apical sodium transporters sodium-glucose cotransporter 1 (SGLT1), apical sodium-dependent bile transporter, and NHE3 were internalized away from the apical membrane of the enterocytes in the patient-mimicking model animals. This structural difference may explain the limited absorption of sodium and water and the resultant watery diarrhea. The researchers also noted disruption of the actinin-4+ terminal web structure, as well as increased SGLT1 localization with lysosome-associated membrane protein 1+ lysosomes within the mouse model enterocytes. This association may indicate degradation of mislocalized proteins, the authors noted, which has also been seen in expanded RAB7+ vesicles within MVID patient tissues.
Other signs pointed to the expansion of immature cells in the upper crypt and lower villus, as well as faster shedding of enterocytes in the villus than in control animals. Scanning electron microscope images also showed immature and disorganized microvilli in the enterocytes of the patient-mimicking mice.
Taken together, “these findings are consistent with a deficit in enterocyte maturation in Myo5b(G519R) mice and in the patient with the MYO5B(G519R) mutation,” the authors wrote.
The authors suggest that their approach could be used more generally to quickly create mouse models of patient-specific monogenic congenital disorders.
The authors disclosed no conflicts of interest. The research was funded by the National Foundation of Science, National Institutes of Health, Vanderbilt Digestive Diseases Research Center Pilot and Feasibility grant, an American Physiological Society John F. Perkins, Jr. Research Career Enhancement Award, and a gift from the Christine Volpe Fund.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
















