User login
Children bearing the brunt of declining flu activity
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
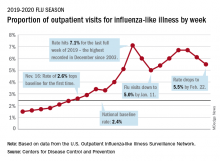
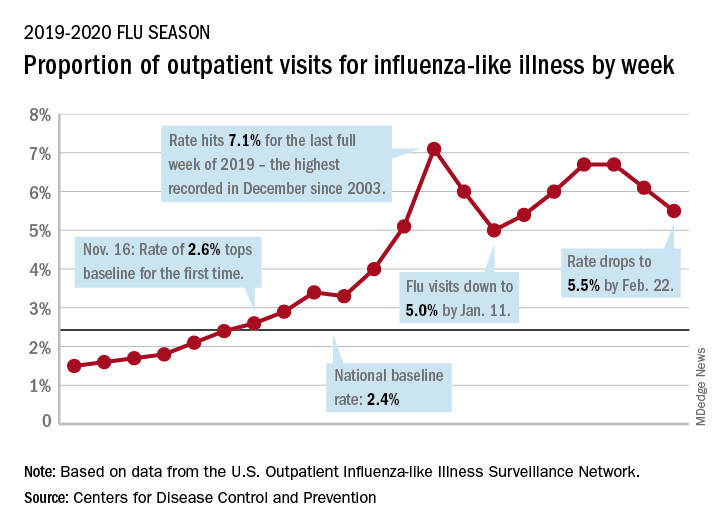
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
What hospitalists need to know about COVID-19
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
This article last updated 4/8/20. (Disclaimer: The information in this article may not be updated regularly. For more COVID-19 coverage, bookmark our COVID-19 updates page. The editors of The Hospitalist encourage clinicians to also review information on the CDC website and on the AHA website.)
An infectious disease outbreak that began in December 2019 in Wuhan (Hubei Province), China, was found to be caused by the seventh strain of coronavirus, initially called the novel (new) coronavirus. The virus was later labeled as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The disease caused by SARS-CoV-2 is named COVID-19. Until 2019, only six strains of human coronaviruses had previously been identified.
As of April 8, 2020, according to the U.S. Centers for Disease Control and Prevention, COVID-19 has been detected in at least 209 countries and has spread to every contintent except Antarctica. More than 1,469,245 people have become infected globally, and at least 86,278 have died. Based on the cases detected and tested in the United States through the U.S. public health surveillance systems, we have had 406,693 confirmed cases and 13,089 deaths.
On March 11, 2020, the World Health Organization formally declared the COVID-19 outbreak to be a pandemic.
As the number of cases increases in the United States, we hope to provide answers about some common questions regarding COVID-19. The information summarized in this article is obtained and modified from the CDC.
What are the clinical features of COVID-19?
Ranges from asymptomatic infection, a mild disease with nonspecific signs and symptoms of acute respiratory illness, to severe pneumonia with respiratory failure and septic shock.
Who is at risk for COVID-19?
Persons who have had prolonged, unprotected close contact with a patient with symptomatic, confirmed COVID-19, and those with recent travel to China, especially Hubei Province.
Who is at risk for severe disease from COVID-19?
Older adults and persons who have underlying chronic medical conditions, such as immunocompromising conditions.
How is COVID-19 spread?
Person-to-person, mainly through respiratory droplets. SARS-CoV-2 has been isolated from upper respiratory tract specimens and bronchoalveolar lavage fluid.
When is someone infectious?
Incubation period may range from 2 to 14 days. Detection of viral RNA does not necessarily mean that infectious virus is present, as it may be detectable in the upper or lower respiratory tract for weeks after illness onset.
Can someone who has been quarantined for COVID-19 spread the illness to others?
For COVID-19, the period of quarantine is 14 days from the last date of exposure, because 14 days is the longest incubation period seen for similar coronaviruses. Someone who has been released from COVID-19 quarantine is not considered a risk for spreading the virus to others because they have not developed illness during the incubation period.
Can a person test negative and later test positive for COVID-19?
Yes. In the early stages of infection, it is possible the virus will not be detected.
Do patients with confirmed or suspected COVID-19 need to be admitted to the hospital?
Not all patients with COVID-19 require hospital admission. Patients whose clinical presentation warrants inpatient clinical management for supportive medical care should be admitted to the hospital under appropriate isolation precautions. The decision to monitor these patients in the inpatient or outpatient setting should be made on a case-by-case basis.
What should you do if you suspect a patient for COVID-19?
Immediately notify both infection control personnel at your health care facility and your local or state health department. State health departments that have identified a person under investigation (PUI) should immediately contact CDC’s Emergency Operations Center (EOC) at 770-488-7100 and complete a COVID-19 PUI case investigation form.
CDC’s EOC will assist local/state health departments to collect, store, and ship specimens appropriately to CDC, including during after-hours or on weekends/holidays.
What type of isolation is needed for COVID-19?
Airborne Infection Isolation Room (AIIR) using Standard, Contact, and Airborne Precautions with eye protection.
How should health care personnel protect themselves when evaluating a patient who may have COVID-19?
Standard Precautions, Contact Precautions, Airborne Precautions, and use eye protection (e.g., goggles or a face shield).
What face mask do health care workers wear for respiratory protection?
A fit-tested NIOSH-certified disposable N95 filtering facepiece respirator should be worn before entry into the patient room or care area. Disposable respirators should be removed and discarded after exiting the patient’s room or care area and closing the door. Perform hand hygiene after discarding the respirator.
If reusable respirators (e.g., powered air purifying respirator/PAPR) are used, they must be cleaned and disinfected according to manufacturer’s reprocessing instructions prior to re-use.
What should you tell the patient if COVID-19 is suspected or confirmed?
Patients with suspected or confirmed COVID-19 should be asked to wear a surgical mask as soon as they are identified, to prevent spread to others.
Should any diagnostic or therapeutic interventions be withheld because of concerns about the transmission of COVID-19?
No.
How do you test a patient for SARS-CoV-2, the virus that causes COVID-19?
At this time, diagnostic testing for COVID-19 can be conducted only at CDC.
The CDC recommends collecting and testing multiple clinical specimens from different sites, including two specimen types – lower respiratory and upper respiratory (nasopharyngeal and oropharyngeal aspirates or washes, nasopharyngeal and oropharyngeal swabs, bronchioalveolar lavage, tracheal aspirates, sputum, and serum) using a real-time reverse transcription PCR (rRT-PCR) assay for SARS-CoV-2. Specimens should be collected as soon as possible once a PUI is identified regardless of the time of symptom onset. Turnaround time for the PCR assay testing is about 24-48 hours.
Testing for other respiratory pathogens should not delay specimen shipping to CDC. If a PUI tests positive for another respiratory pathogen, after clinical evaluation and consultation with public health authorities, they may no longer be considered a PUI.
Will existing respiratory virus panels detect SARS-CoV-2, the virus that causes COVID-19?
No.
How is COVID-19 treated?
Symptomatic management. Corticosteroids are not routinely recommended for viral pneumonia or acute respiratory distress syndrome and should be avoided unless they are indicated for another reason (e.g., COPD exacerbation, refractory septic shock following Surviving Sepsis Campaign Guidelines). There are currently no antiviral drugs licensed by the U.S. Food and Drug Administration to treat COVID-19.
What is considered ‘close contact’ for health care exposures?
Being within approximately 6 feet (2 meters), of a person with COVID-19 for a prolonged period of time (such as caring for or visiting the patient, or sitting within 6 feet of the patient in a health care waiting area or room); or having unprotected direct contact with infectious secretions or excretions of the patient (e.g., being coughed on, touching used tissues with a bare hand). However, until more is known about transmission risks, it would be reasonable to consider anything longer than a brief (e.g., less than 1-2 minutes) exposure as prolonged.
What happens if the health care personnel (HCP) are exposed to confirmed COVID-19 patients? What’s the protocol for HCP exposed to persons under investigation (PUI) if test results are delayed beyond 48-72 hours?
Management is similar in both these scenarios. CDC categorized exposures as high, medium, low, and no identifiable risk. High- and medium-risk exposures are managed similarly with active monitoring for COVID-19 until 14 days after last potential exposure and exclude from work for 14 days after last exposure. Active monitoring means that the state or local public health authority assumes responsibility for establishing regular communication with potentially exposed people to assess for the presence of fever or respiratory symptoms (e.g., cough, shortness of breath, sore throat). For HCP with high- or medium-risk exposures, CDC recommends this communication occurs at least once each day. For full details, please see www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html.
Should postexposure prophylaxis be used for people who may have been exposed to COVID-19?
None available.
COVID-19 test results are negative in a symptomatic patient you suspected of COVID-19? What does it mean?
A negative test result for a sample collected while a person has symptoms likely means that the COVID-19 virus is not causing their current illness.
What if your hospital does not have an Airborne Infection Isolation Room (AIIR) for COVID-19 patients?
Transfer the patient to a facility that has an available AIIR. If a transfer is impractical or not medically appropriate, the patient should be cared for in a single-person room and the door should be kept closed. The room should ideally not have an exhaust that is recirculated within the building without high-efficiency particulate air (HEPA) filtration. Health care personnel should still use gloves, gown, respiratory and eye protection and follow all other recommended infection prevention and control practices when caring for these patients.
What if your hospital does not have enough Airborne Infection Isolation Rooms (AIIR) for COVID-19 patients?
Prioritize patients for AIIR who are symptomatic with severe illness (e.g., those requiring ventilator support).
When can patients with confirmed COVID-19 be discharged from the hospital?
Patients can be discharged from the health care facility whenever clinically indicated. Isolation should be maintained at home if the patient returns home before the time period recommended for discontinuation of hospital transmission-based precautions.
Considerations to discontinue transmission-based precautions include all of the following:
- Resolution of fever, without the use of antipyretic medication.
- Improvement in illness signs and symptoms.
- Negative rRT-PCR results from at least two consecutive sets of paired nasopharyngeal and throat swabs specimens collected at least 24 hours apart (total of four negative specimens – two nasopharyngeal and two throat) from a patient with COVID-19 are needed before discontinuing transmission-based precautions.
Should people be concerned about pets or other animals and COVID-19?
To date, CDC has not received any reports of pets or other animals becoming sick with COVID-19.
Should patients avoid contact with pets or other animals if they are sick with COVID-19?
Patients should restrict contact with pets and other animals while they are sick with COVID-19, just like they would around other people.
Does CDC recommend the use of face masks in the community to prevent COVID-19?
CDC does not recommend that people who are well wear a face mask to protect themselves from respiratory illnesses, including COVID-19. A face mask should be used by people who have COVID-19 and are showing symptoms to protect others from the risk of getting infected.
Should medical waste or general waste from health care facilities treating PUIs and patients with confirmed COVID-19 be handled any differently or need any additional disinfection?
No. CDC’s guidance states that management of laundry, food service utensils, and medical waste should be performed in accordance with routine procedures.
Can people who recover from COVID-19 be infected again?
Unknown. The immune response to COVID-19 is not yet understood.
What is the mortality rate of COVID-19, and how does it compare to the mortality rate of influenza (flu)?
The average 10-year mortality rate for flu, using CDC data, is found to be around 0.1%. Even though this percentage appears to be small, influenza is estimated to be responsible for 30,000 to 40,000 deaths annually in the U.S.
According to statistics released by the Chinese Center for Disease Control and Prevention on Feb. 17, the mortality rate of COVID-19 is estimated to be around 2.3%. This calculation was based on cases reported through Feb. 11, and calcuated by dividing the number of coronavirus-related deaths at the time (1,023) by the number of confirmed cases (44,672) of COVID-19 infection. However, this report has its limitations, since Chinese officials have a vague way of defining who has COVID-19 infection.
The World Health Organization (WHO) currently estimates the mortality rate for COVID-19 to be between 2% and 4%.
Dr. Sitammagari is a co-medical director for quality and assistant professor of internal medicine at Atrium Health, Charlotte, N.C. He is also a physician advisor. He currently serves as treasurer for the NC-Triangle Chapter of the Society of Hospital Medicine and as an editorial board member of The Hospitalist.
Dr. Skandhan is a hospitalist and member of the Core Faculty for the Internal Medicine Residency Program at Southeast Health (SEH), Dothan Ala., and an assistant professor at the Alabama College of Osteopathic Medicine. He serves as the medical director/physician liaison for the Clinical Documentation Program at SEH and also as the director for physician integration for Southeast Health Statera Network, an Accountable Care Organization. Dr. Skandhan was a cofounder of the Wiregrass chapter of SHM and currently serves on the Advisory board. He is also a member of the editorial board of The Hospitalist.
Dr. Dahlin is a second-year internal medicine resident at Southeast Health, Dothan, Ala. She serves as her class representative and is the cochair/resident liaison for the research committee at SEH. Dr. Dahlin also serves as a resident liaison for the Wiregrass chapter of SHM.
Hospitalist profile: Vineet Chopra, MD, MSc, FHM
Vineet Chopra, MD, MSc, FHM, is associate professor of medicine and chief of the Division of Hospital Medicine at Michigan Medicine and the VA Ann Arbor (Michigan) Health System. A career hospitalist, Dr. Chopra’s research is dedicated to improving the safety of hospitalized patients through prevention of hospital-acquired complications. His work focuses on identifying and preventing complications associated with central venous catheters with a particular emphasis on peripherally inserted central catheters (PICCs).
Dr. Chopra is the recipient of numerous teaching and research awards including the 2016 Kaiser Permanente Award for Clinical Teaching, the Jerome W. Conn Award for Outstanding Research in the Department of Medicine, the 2016 Society of Hospital Medicine Award for Excellence in Research, and the 2014 McDevitt Award for Research Excellence. He has published over 100 peer-reviewed articles and has served as associate editor for the American Journal of Medicine and Journal of Hospital Medicine.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
I think I knew very early – toward the middle of my intern year – that I wanted to be a hospitalist. There was much that drew me to the field. First, I loved being in the inpatient setting. The tempo of work, the unexpected nature of what may come next, and the opportunity to truly have an impact on a patients life at their time of greatest need appealed to me. I wasn’t as inclined towards the procedural fields and also loved the cognitive aspects of general medicine – doing the work up on a difficult diagnosis or medically managing a patient with acute coronary syndrome came naturally. I found myself loving the work so much so that it didn’t feel like work. And the rest was history!
What is your current role at Michigan Medicine?
I started at Michigan Medicine in 2008 as a full-time clinician taking care of patients on direct care and resident services. After 3 years of clinical work, I decided it was time to hone in on a specific skill set and went back to a research fellowship.
I become Michigan’s first fellow in hospital medicine – the guinea pig – for what would turn out to be one of the best decisions in my life. After finishing fellowship, I switched my focus from clinical work to research and rose up the ranks to receive tenure as an associate professor of medicine. After attaining tenure, I was among a handful of people in the nation who had success in both the research and the clinical arenas and leadership opportunities began to come my way.
I was fortunate to be recruited as the inaugural division chief of hospital medicine at Michigan Medicine in 2017. The Division of hospital medicine is the 13th in the department of medicine and the first one to be created in over 60 years. As division chief, I oversee all of our clinical, academic, research, and educational endeavors. Currently, we have approximately 130 hospitalists in our group and about 30 advanced practice providers (APPs) with a support and research staff of about 15 individuals. So I like to say we have a big family!
What are your favorite areas of clinical practice and/or research?
I am fortunate to have the ability to enjoy all that hospital medicine has to offer. I still appreciate the challenges that direct care brings, and I continue to do as much as I can in this area. I also enjoy working with residents and medical students at the university and at our VA site – where much of my focus is devoted to making sure all learners on the team are growing while they provide excellent patient care. To meet a new patient and work to develop a therapeutic relationship with them such that we can make positive changes in their disease trajectory remains my favorite part of clinical work.
My research work remains closely linked to my clinical interests around preventing patient harm and improving patient safety – so studying hospital-acquired infections, coming up with new ideas and strategies, and then implementing them when on clinical service represents the perfect blend of the two. My research is largely focused on intravenous devices and catheters, and I focus my work on preventing harms such as bloodstream infection, venous thrombosis, and related adverse events. I have been fortunate to receive national and international attention for my research, including adoption of my work into guidelines and changes to national policies. I am honored to serve on the most important federal advisory committee that advises the government on health care infections (the committee is called HICPAC – Healthcare Infection Control Practice Advisory Committee).
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
For me, the most challenging aspects are also the most rewarding. First and foremost, making a connection with a patient and their family to understand their concerns and define a therapeutic alliance is both challenging and rewarding. Second, ensuring that we have the ability to work efficiently and effectively to manage patient care is sometimes challenging but also the most rewarding aspect of the job. I am fortunate to work in a health system where I am surrounded by smart colleagues, important resources, advanced technology, and the support of nurses and advanced practice providers who share this zeal of patient care with me.
Finally, one the greatest challenges and rewards remains time. Our work is hard and grueling, and it is often very challenging to get things done at different times of the day. But the ability to make a diagnosis or see a patient improve makes it all worth it!
How will hospital medicine change in the next decade or two?
I predict our work will shift from a model that is reactive – taking care of patients that are sick and need hospitalization – to a proactive approach where the focus will remain on keeping people out of the hospital. This doesn’t necessarily mean that we will be out of a job – but I see the model of our work shifting to ensure that patients who are discharged remain healthy and well. This means we will need to embrace extensivist models, hospital at home care, and aspects such as bridge clinics.
I also think our work will evolve to harness some of the incredible technology that surrounds us outside health care, but has not yet permeated our work flow. To that end, aspects such as virtual consultations and patient assessments, and remote monitoring that includes biometrics, will all fall into our workflow. And of course, lets not forget about the mighty electronic medical record and how that will affect our experience and work. I see much more of our work shifting toward becoming digital experts, harnessing the power of big data and predictive analytics to provide better care for patients. These are skills that are emerging in our field, but we have not yet mastered the art of managing data.
Do you have any advice for students and residents interested in hospital medicine?
I would highly recommend taking on a rotation with a hospitalist, carrying the pager and working side-by-side with someone who truly loves what they do. Many students and residents just see the on/off nature of the work, but that is truly skin deep in terms of attraction.
The beauty of hospital medicine is that you can be everything for a patient – their doctor, their health care navigator, their friend, and their partner during their hospital stay. Find that joy – you will not regret it!
Vineet Chopra, MD, MSc, FHM, is associate professor of medicine and chief of the Division of Hospital Medicine at Michigan Medicine and the VA Ann Arbor (Michigan) Health System. A career hospitalist, Dr. Chopra’s research is dedicated to improving the safety of hospitalized patients through prevention of hospital-acquired complications. His work focuses on identifying and preventing complications associated with central venous catheters with a particular emphasis on peripherally inserted central catheters (PICCs).
Dr. Chopra is the recipient of numerous teaching and research awards including the 2016 Kaiser Permanente Award for Clinical Teaching, the Jerome W. Conn Award for Outstanding Research in the Department of Medicine, the 2016 Society of Hospital Medicine Award for Excellence in Research, and the 2014 McDevitt Award for Research Excellence. He has published over 100 peer-reviewed articles and has served as associate editor for the American Journal of Medicine and Journal of Hospital Medicine.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
I think I knew very early – toward the middle of my intern year – that I wanted to be a hospitalist. There was much that drew me to the field. First, I loved being in the inpatient setting. The tempo of work, the unexpected nature of what may come next, and the opportunity to truly have an impact on a patients life at their time of greatest need appealed to me. I wasn’t as inclined towards the procedural fields and also loved the cognitive aspects of general medicine – doing the work up on a difficult diagnosis or medically managing a patient with acute coronary syndrome came naturally. I found myself loving the work so much so that it didn’t feel like work. And the rest was history!
What is your current role at Michigan Medicine?
I started at Michigan Medicine in 2008 as a full-time clinician taking care of patients on direct care and resident services. After 3 years of clinical work, I decided it was time to hone in on a specific skill set and went back to a research fellowship.
I become Michigan’s first fellow in hospital medicine – the guinea pig – for what would turn out to be one of the best decisions in my life. After finishing fellowship, I switched my focus from clinical work to research and rose up the ranks to receive tenure as an associate professor of medicine. After attaining tenure, I was among a handful of people in the nation who had success in both the research and the clinical arenas and leadership opportunities began to come my way.
I was fortunate to be recruited as the inaugural division chief of hospital medicine at Michigan Medicine in 2017. The Division of hospital medicine is the 13th in the department of medicine and the first one to be created in over 60 years. As division chief, I oversee all of our clinical, academic, research, and educational endeavors. Currently, we have approximately 130 hospitalists in our group and about 30 advanced practice providers (APPs) with a support and research staff of about 15 individuals. So I like to say we have a big family!
What are your favorite areas of clinical practice and/or research?
I am fortunate to have the ability to enjoy all that hospital medicine has to offer. I still appreciate the challenges that direct care brings, and I continue to do as much as I can in this area. I also enjoy working with residents and medical students at the university and at our VA site – where much of my focus is devoted to making sure all learners on the team are growing while they provide excellent patient care. To meet a new patient and work to develop a therapeutic relationship with them such that we can make positive changes in their disease trajectory remains my favorite part of clinical work.
My research work remains closely linked to my clinical interests around preventing patient harm and improving patient safety – so studying hospital-acquired infections, coming up with new ideas and strategies, and then implementing them when on clinical service represents the perfect blend of the two. My research is largely focused on intravenous devices and catheters, and I focus my work on preventing harms such as bloodstream infection, venous thrombosis, and related adverse events. I have been fortunate to receive national and international attention for my research, including adoption of my work into guidelines and changes to national policies. I am honored to serve on the most important federal advisory committee that advises the government on health care infections (the committee is called HICPAC – Healthcare Infection Control Practice Advisory Committee).
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
For me, the most challenging aspects are also the most rewarding. First and foremost, making a connection with a patient and their family to understand their concerns and define a therapeutic alliance is both challenging and rewarding. Second, ensuring that we have the ability to work efficiently and effectively to manage patient care is sometimes challenging but also the most rewarding aspect of the job. I am fortunate to work in a health system where I am surrounded by smart colleagues, important resources, advanced technology, and the support of nurses and advanced practice providers who share this zeal of patient care with me.
Finally, one the greatest challenges and rewards remains time. Our work is hard and grueling, and it is often very challenging to get things done at different times of the day. But the ability to make a diagnosis or see a patient improve makes it all worth it!
How will hospital medicine change in the next decade or two?
I predict our work will shift from a model that is reactive – taking care of patients that are sick and need hospitalization – to a proactive approach where the focus will remain on keeping people out of the hospital. This doesn’t necessarily mean that we will be out of a job – but I see the model of our work shifting to ensure that patients who are discharged remain healthy and well. This means we will need to embrace extensivist models, hospital at home care, and aspects such as bridge clinics.
I also think our work will evolve to harness some of the incredible technology that surrounds us outside health care, but has not yet permeated our work flow. To that end, aspects such as virtual consultations and patient assessments, and remote monitoring that includes biometrics, will all fall into our workflow. And of course, lets not forget about the mighty electronic medical record and how that will affect our experience and work. I see much more of our work shifting toward becoming digital experts, harnessing the power of big data and predictive analytics to provide better care for patients. These are skills that are emerging in our field, but we have not yet mastered the art of managing data.
Do you have any advice for students and residents interested in hospital medicine?
I would highly recommend taking on a rotation with a hospitalist, carrying the pager and working side-by-side with someone who truly loves what they do. Many students and residents just see the on/off nature of the work, but that is truly skin deep in terms of attraction.
The beauty of hospital medicine is that you can be everything for a patient – their doctor, their health care navigator, their friend, and their partner during their hospital stay. Find that joy – you will not regret it!
Vineet Chopra, MD, MSc, FHM, is associate professor of medicine and chief of the Division of Hospital Medicine at Michigan Medicine and the VA Ann Arbor (Michigan) Health System. A career hospitalist, Dr. Chopra’s research is dedicated to improving the safety of hospitalized patients through prevention of hospital-acquired complications. His work focuses on identifying and preventing complications associated with central venous catheters with a particular emphasis on peripherally inserted central catheters (PICCs).
Dr. Chopra is the recipient of numerous teaching and research awards including the 2016 Kaiser Permanente Award for Clinical Teaching, the Jerome W. Conn Award for Outstanding Research in the Department of Medicine, the 2016 Society of Hospital Medicine Award for Excellence in Research, and the 2014 McDevitt Award for Research Excellence. He has published over 100 peer-reviewed articles and has served as associate editor for the American Journal of Medicine and Journal of Hospital Medicine.
At what point in your education/training did you decide to practice hospital medicine? What about hospital medicine appealed to you?
I think I knew very early – toward the middle of my intern year – that I wanted to be a hospitalist. There was much that drew me to the field. First, I loved being in the inpatient setting. The tempo of work, the unexpected nature of what may come next, and the opportunity to truly have an impact on a patients life at their time of greatest need appealed to me. I wasn’t as inclined towards the procedural fields and also loved the cognitive aspects of general medicine – doing the work up on a difficult diagnosis or medically managing a patient with acute coronary syndrome came naturally. I found myself loving the work so much so that it didn’t feel like work. And the rest was history!
What is your current role at Michigan Medicine?
I started at Michigan Medicine in 2008 as a full-time clinician taking care of patients on direct care and resident services. After 3 years of clinical work, I decided it was time to hone in on a specific skill set and went back to a research fellowship.
I become Michigan’s first fellow in hospital medicine – the guinea pig – for what would turn out to be one of the best decisions in my life. After finishing fellowship, I switched my focus from clinical work to research and rose up the ranks to receive tenure as an associate professor of medicine. After attaining tenure, I was among a handful of people in the nation who had success in both the research and the clinical arenas and leadership opportunities began to come my way.
I was fortunate to be recruited as the inaugural division chief of hospital medicine at Michigan Medicine in 2017. The Division of hospital medicine is the 13th in the department of medicine and the first one to be created in over 60 years. As division chief, I oversee all of our clinical, academic, research, and educational endeavors. Currently, we have approximately 130 hospitalists in our group and about 30 advanced practice providers (APPs) with a support and research staff of about 15 individuals. So I like to say we have a big family!
What are your favorite areas of clinical practice and/or research?
I am fortunate to have the ability to enjoy all that hospital medicine has to offer. I still appreciate the challenges that direct care brings, and I continue to do as much as I can in this area. I also enjoy working with residents and medical students at the university and at our VA site – where much of my focus is devoted to making sure all learners on the team are growing while they provide excellent patient care. To meet a new patient and work to develop a therapeutic relationship with them such that we can make positive changes in their disease trajectory remains my favorite part of clinical work.
My research work remains closely linked to my clinical interests around preventing patient harm and improving patient safety – so studying hospital-acquired infections, coming up with new ideas and strategies, and then implementing them when on clinical service represents the perfect blend of the two. My research is largely focused on intravenous devices and catheters, and I focus my work on preventing harms such as bloodstream infection, venous thrombosis, and related adverse events. I have been fortunate to receive national and international attention for my research, including adoption of my work into guidelines and changes to national policies. I am honored to serve on the most important federal advisory committee that advises the government on health care infections (the committee is called HICPAC – Healthcare Infection Control Practice Advisory Committee).
What are the most challenging aspects of practicing hospital medicine? What are the most rewarding?
For me, the most challenging aspects are also the most rewarding. First and foremost, making a connection with a patient and their family to understand their concerns and define a therapeutic alliance is both challenging and rewarding. Second, ensuring that we have the ability to work efficiently and effectively to manage patient care is sometimes challenging but also the most rewarding aspect of the job. I am fortunate to work in a health system where I am surrounded by smart colleagues, important resources, advanced technology, and the support of nurses and advanced practice providers who share this zeal of patient care with me.
Finally, one the greatest challenges and rewards remains time. Our work is hard and grueling, and it is often very challenging to get things done at different times of the day. But the ability to make a diagnosis or see a patient improve makes it all worth it!
How will hospital medicine change in the next decade or two?
I predict our work will shift from a model that is reactive – taking care of patients that are sick and need hospitalization – to a proactive approach where the focus will remain on keeping people out of the hospital. This doesn’t necessarily mean that we will be out of a job – but I see the model of our work shifting to ensure that patients who are discharged remain healthy and well. This means we will need to embrace extensivist models, hospital at home care, and aspects such as bridge clinics.
I also think our work will evolve to harness some of the incredible technology that surrounds us outside health care, but has not yet permeated our work flow. To that end, aspects such as virtual consultations and patient assessments, and remote monitoring that includes biometrics, will all fall into our workflow. And of course, lets not forget about the mighty electronic medical record and how that will affect our experience and work. I see much more of our work shifting toward becoming digital experts, harnessing the power of big data and predictive analytics to provide better care for patients. These are skills that are emerging in our field, but we have not yet mastered the art of managing data.
Do you have any advice for students and residents interested in hospital medicine?
I would highly recommend taking on a rotation with a hospitalist, carrying the pager and working side-by-side with someone who truly loves what they do. Many students and residents just see the on/off nature of the work, but that is truly skin deep in terms of attraction.
The beauty of hospital medicine is that you can be everything for a patient – their doctor, their health care navigator, their friend, and their partner during their hospital stay. Find that joy – you will not regret it!
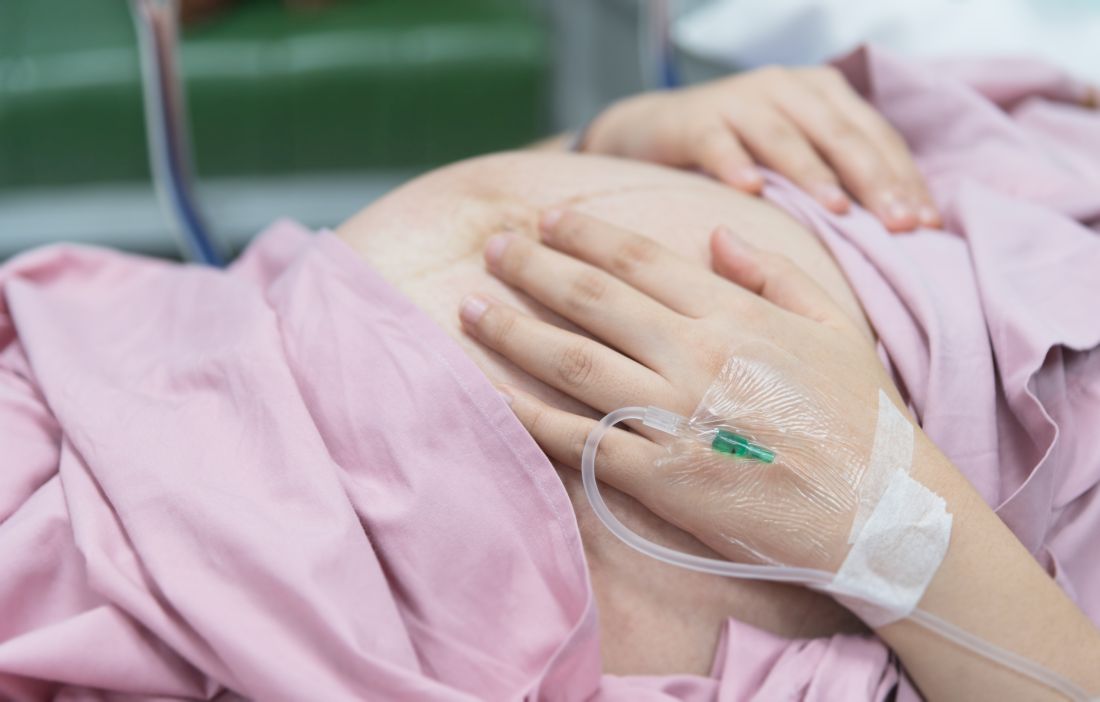
Labor & Delivery: An overlooked entry point for the spread of viral infection
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
OB hospitalists have a key role to play
OB hospitalists have a key role to play
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
A novel coronavirus originating in Wuhan, China, has killed more than 2,800 people and infected more than 81,000 individuals globally. Public health officials around the world and in the United States are working together to contain the outbreak.
There are 57 confirmed cases in the United States, including 18 people evacuated from the Diamond Princess, a cruise ship docked in Yokohama, Japan.1 But the focus on coronavirus, even in early months of the epidemic, serves as an opportunity to revisit the spread of viral disease in hospital settings.
Multiple points of viral entry
In truth, most hospitals are well prepared for the coronavirus, starting with the same place they prepare for most infectious disease epidemics – the emergency department. Patients who seek treatment for early onset symptoms may start with their primary care physicians, but increasing numbers of patients with respiratory concerns and/or infection-related symptoms will first seek medical attention in an emergency care setting.2
Many experts have acknowledged the ED as a viral point of entry, including the American College of Emergency Physicians (ACEP), which produced an excellent guide for management of influenza that details prevention, diagnoses, and treatment protocols in an ED setting.3
But another important, and often forgotten, point of entry in a hospital setting is the obstetrical (OB) Labor & Delivery (L&D) department. Although triage for most patients begins in the main ED, in almost every hospital in the United States, women who present with pregnancy-related issues are sent directly to and triaged in L&D, where – when the proper protocols are not in place – they may transmit viral infection to others.
Pregnancy imparts higher risk
“High risk” is often associated with older, immune-compromised adults. But pregnant women who may appear “healthy” are actually in a state that a 2015 study calls “immunosuppressed” whereby the “… pregnant woman actually undergoes an immunological transformation, where the immune system is necessary to promote and support the pregnancy and growing fetus.”4 Pregnant women, or women with newborns or babies, are at higher risk when exposed to viral infection, with a higher mortality risk than the general population.5 In the best cases, women who contract viral infections are treated carefully and recover fully. In the worst cases, they end up on ventilators and can even die as a result.
Although we are still learning about the Wuhan coronavirus, we already know it is a respiratory illness with a lot of the same characteristics as the influenza virus, and that it is transmitted through droplets (such as a sneeze) or via bodily secretions. Given the extreme vulnerability and physician exposure of women giving birth – in which not one, but two lives are involved – viruses like coronavirus can pose extreme risk. What’s more, public health researchers are still learning about potential transmission of coronavirus from mothers to babies. In the international cases of infant exposure to coronavirus, the newborn showed symptoms within 36 hours of being born, but it is unclear if exposure happened in utero or was vertical transmission after birth.6
Role of OB hospitalists in identifying risk and treating viral infection
Regardless of the type of virus, OB hospitalists are key to screening for viral exposure and care for women, fetuses, and newborns. Given their 24/7 presence and experience with women in L&D, they must champion protocols and precautions that align with those in an ED.
For coronavirus, if a woman presents in L&D with a cough, difficulty breathing, or signs of pneumonia, clinicians should be accustomed to asking about travel to China within the last 14 days and whether the patient has been around someone who has recently traveled to China. If the answer to either question is yes, the woman needs to be immediately placed in a single patient room at negative pressure relative to the surrounding areas, with a minimum of six air changes per hour.
Diagnostic testing should immediately follow. The U.S. Food and Drug Administration just issued Emergency Use Authorization (EUA) for the first commercially-available coronavirus diagnostic test, allowing the use of the test at any lab across the country qualified by the Centers for Disease Control and Prevention.7
If exposure is suspected, containment is paramount until definitive results of diagnostic testing are received. The CDC recommends “Standard Precautions,” which assume that every person is potentially infected or colonized with a pathogen that could be transmitted in the health care setting. These precautions include hand hygiene and personal protective equipment (PPE) to ensure health care workers are not exposed.8
In short, protocols in L&D should mirror those of the ED. But in L&D, clinicians and staff haven’t necessarily been trained to look for or ask for these conditions. Hospitalists can educate their peers and colleagues and advocate for changes at the administrative level.
Biggest current threat: The flu
The coronavirus may eventually present a threat in the United States, but as yet, it is a largely unrealized one. From the perspective of an obstetrician, more immediately concerning is the risk of other viral infections. Although viruses like Ebola and Zika capture headlines, influenza remains the most serious threat to pregnant women in the United States.
According to an article by my colleague, Dr. Mark Simon, “pregnant women and their unborn babies are especially vulnerable to influenza and are more likely to develop serious complications from it … pregnant women who develop the flu are more likely to give birth to children with birth defects of the brain and spine.”9
As of Feb. 1, 2020, the CDC estimates there have been at least 22 million flu illnesses, 210,000 hospitalizations, and 12,000 deaths from flu in the 2019-2020 flu season.10 But the CDC data also suggest that only 54% of pregnant women were vaccinated for influenza in 2019 before or during their pregnancy.11 Hospitalists should ensure that patients diagnosed with flu are quickly and safely treated with antivirals at all stages of their pregnancy to keep them and their babies safe, as well as keep others safe from infection.
Hospitalists can also advocate for across-the-board protocols for the spread of viral illness. The same protocols that protect us from the flu will also protect against coronavirus and viruses that will emerge in the future. Foremost, pregnant women, regardless of trimester, need to receive a flu shot. Women who are pregnant and receive a flu shot can pass on immunity in vitro, and nursing mothers can deliver immunizing agents in their breast milk to their newborn.
Given that hospitalists serve in roles as patient-facing physicians, we should be doing more to protect the public from viral spread, whether coronavirus, influenza, or whatever new viruses the future may hold.
Dr. Dimino is a board-certified ob.gyn. and a Houston-based OB hospitalist with Ob Hospitalist Group. She serves as a faculty member of the TexasAIM Plus Obstetric Hemorrhage Learning Collaborative and currently serves on the Texas Medical Association Council of Science and Public Health.
References
1. The New York Times. Tracking the Coronavirus Map: Tracking the Spread of the Outbreak. Accessed Feb 24, 2020.
2. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Accessed Feb 10, 2020.
3. Influenza Emergency Department Best Practices. ACEP Public Health & Injury Prevention Committee, Epidemic Expert Panel, https://www.acep.org/globalassets/uploads/uploaded-files/acep/by-medical-focus/influenza-emergency-department-best-practices.pdf.
4. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199-213.
5. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387-390.
6. BBC. Coronavirus: Newborn becomes youngest person diagnosed with virus. Accessed Feb 10, 2020.
7. FDA press release. FDA Takes Significant Step in Coronavirus Response Efforts, Issues Emergency Use Authorization for the First 2019 Novel Coronavirus Diagnostic. Feb 4, 2020.
8. CDC. Interim Infection Prevention and Control Recommendations for Patients with Confirmed 2019 Novel Coronavirus (2019-nCoV) or Persons Under Investigation for 2019-nCoV in Healthcare Settings. Accessed Feb 10, 2020.
9. STAT First Opinion. Two-thirds of pregnant women aren’t getting the flu vaccine. That needs to change. Jan 18, 2018.
10. CDC. Weekly U.S. Influenza Surveillance Report, Key Updates for Week 5, ending February 1, 2020.
11. CDC. Vaccinating Pregnant Women Protects Moms and Babies. Accessed Feb 10, 2020.
Pence named COVID-19 point person as CDC reports possible community spread
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
Vice President Mike Pence will be the White House point person quarterbacking the administration’s response to COVID-19, although President Donald Trump was quick to dismiss the notion that he is a so-called coronavirus “czar.”
President Trump introduced Vice President Pence in this role during a Feb. 26 press conference. The same night, officials at the Centers for Disease Control and Prevention announced the first case of possible community spread of the novel coronavirus in the United States.
“I am going to be putting our vice president, Mike Pence, in charge, and Mike will be working with the professionals, the doctors, and everybody else that is working” on this, President Trump said.
“Mike is going to be in charge and Mike will report back to me, but he’s got a certain talent for this,” President Trump continued, noting that while Vice President Pence was governor of Indiana, his was the first state to have a patient affected by the 2014 Middle East Respiratory Syndrome coronavirus (MERS-CoV) outbreak, so he has experience in a similar situation.
“I know full well the importance of presidential leadership, the importance of administration leadership, and the vital role of partnerships of state and local governments and health authorities in responding to the potential threat of dangerous infectious diseases,” Vice President Pence said.
He said that his role will be to continue to meet with the Coronavirus Task Force and bring to the president “the best options for action and to see to the safety and well being and health of the American people. I will also be continuing to reach out to governors [and] state and local officials.”
Vice President Pence said he will also be working with Congress to ensure that resources are available.
It was noted during the press conference that some members of Congress consider the $2.5 billion in emergency appropriations requested by the White House to be inadequate and that the legislative branch is working to provide more funding.
Vice President Pence’s new role does not change the command structure of the Coronavirus Task Force, which is currently led by Department of Health & Human Services Secretary Alex Azar.
Speaking at the press conference, Secretary Azar noted that he is still chairman of the task force. “Having the vice president gives me the biggest stick one can have in the government on this whole-of-government approach.”
He emphatically stated, “not in the least,” in response to a question about whether he felt he was being replaced. “When this was mentioned to me, I said I was delighted that I get to have the vice president helping in this way. Delighted.”
The announcement came as President Trump continued to downplay the threat of the coronavirus to U.S. citizens, going so far as to contradict CDC officials who have stated that it is a matter of when, not if, there will be community spread in the United States.
“I don’t think it’s inevitable,” President Trump said. “I think that there’s a chance that it could get worse. There’s a chance it could get fairly substantially worse, but nothing’s inevitable.”
Immediately after President Trump wrapped up his statement, however, the CDC formally announced the first case of possible community spread of the coronavirus. In a statement issued to the press, the agency announced the 15th confirmed case in the United States, a person in California “who reportedly did not have relevant travel history or exposure to another known patient” with the coronavirus.
“This case was detected through the U.S. public health system – picked up by astute clinicians,” CDC added, noting it will continue to provide updates on the evolving situation.
AI algorithm finds diagnostic AFib signatures in normal ECGs
NATIONAL HARBOR, MD. – Researchers have created an artificial intelligence algorithm that can evaluate a 10-second ECG recording of a person in normal sinus rhythm and tell with a sensitivity and specificity of almost 80% whether or not that person ever had atrial fibrillation episodes some time in the past or will have a first arrhythmia episode in the near future.
Although this algorithm – derived from and then validated with a dataset of nearly 650,000 ECG recordings from more than 180,000 patients – still needs prospective validation, it offers the prospect for a potential revolution in screening for atrial fibrillation (AFib), Paul A. Friedman, MD, cautioned at the annual International AF Symposium. If initial clinical findings are confirmed, it would show that a 10-second, 12-lead ECG recording can provide the same screening scope as what otherwise takes weeks of ambulatory ECG recording with a Holter monitor or an implanted device, explained Dr. Friedman, professor of medicine and chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
This finding “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” Dr. Friedman and his associates noted in the published report of their study (Lancet. 2019 Sep 7;394[10201]:861-7). “We’re still working to define the window of ECG” recording time that provides the optimal assessment for a history of asymptomatic AFib, but the “possibilities this opens are huge,” Dr. Friedman said in his talk at the symposium. This work sprang from the premise that “subtle signatures” in a brief, apparently normal sinus rhythm ECG tracing can harbor reliable clues about AFib history or an imminent episode.
The 2019 report by Dr. Friedman and associates documented that in the validation phase of their study, the trained artificial intelligence (AI) program identified patients with a history of AFib or an impending arrhythmia event from a single, 10-second ECG that to the naked eye seemed to show normal sinus rhythm with a sensitivity of 79.0%, a specificity of 79.5%, and an accuracy of 79.4%. It also showed an area under a receiver operating characteristic curve of 0.87, meaning that screening for AFib by this method compared favorably with the area-under-the-curve (AUC) results tallied by several widely accepted screening tools, including Pap smears for cervical cancer (AUC of 0.70), mammograms for breast cancer (AUC of 0.85), and CHA2DS2-VASc scoring for estimating stroke risk in AFib patients (AUC of 0.57-0.72), Dr. Friedman said.
The researchers developed the AI algorithm with more than 450,000 10-second ECG tracings collected from roughly 126,000 patients who underwent at least one ECG recording as part of their routine care at the Mayo Clinic during 1993-2017. The goal was for the program to find and validate recurring characteristics in the ECG that consistently linked with a history of or an impending AFib episode and that did not appear in ECG recordings from people without any AFib history. The program this effort produced then underwent further adjustment with the use of more than 64,340 ECGs from an additional 18,116 patients, and then the final product underwent validation testing with a further 130,802 ECGs collected from an additional 36,280 people, the study phase that resulted in the reported sensitivity and specificity estimates.
It’s currently unclear to Dr. Friedman and associates what specific features the program uses to classify patients. It’s an important question, but if the results are reproducible and reliable, this uncertainty shouldn’t slow clinical adoption, he said in an interview.
While “this particular algorithm needs prospective vetting,” a similar algorithm developed by Dr. Friedman and the same research team that uses a 10-second ECG to identify patients with a left ventricular ejection fraction of 35% or less is further advanced in development, and a device that uses this algorithm will soon receive Food and Drug Administration review under a fast track designation that the agency approved in late 2019.
The researchers developed this algorithm for estimating left ventricular function using a strategy similar to their development of a tool for diagnosing AFib (Nat Med. 2019 Jan 7;25[1]:70-4), and results from 100 patients prospectively studied with this approach to ECG analysis and reported at the American Heart Association scientific sessions in November 2019 showed that the algorithm identified substantial left ventricular dysfunction with an AUC of 0.906 (Circulation. 2019 Nov 19;140[suppl 1]:A13447). The same team of investigators has developed an AI algorithm that can calculate a person’s physiologic age based on the ECG recording (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]: 10.1161/CIRCEP.119.007284).
The study received no commercial funding, and Dr. Friedman and coauthors had no relevant disclosures. The Mayo Clinic has licensed a related artificial intelligence algorithm to EKO, and Dr. Friedman may benefit financially from this arrangement.
NATIONAL HARBOR, MD. – Researchers have created an artificial intelligence algorithm that can evaluate a 10-second ECG recording of a person in normal sinus rhythm and tell with a sensitivity and specificity of almost 80% whether or not that person ever had atrial fibrillation episodes some time in the past or will have a first arrhythmia episode in the near future.
Although this algorithm – derived from and then validated with a dataset of nearly 650,000 ECG recordings from more than 180,000 patients – still needs prospective validation, it offers the prospect for a potential revolution in screening for atrial fibrillation (AFib), Paul A. Friedman, MD, cautioned at the annual International AF Symposium. If initial clinical findings are confirmed, it would show that a 10-second, 12-lead ECG recording can provide the same screening scope as what otherwise takes weeks of ambulatory ECG recording with a Holter monitor or an implanted device, explained Dr. Friedman, professor of medicine and chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
This finding “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” Dr. Friedman and his associates noted in the published report of their study (Lancet. 2019 Sep 7;394[10201]:861-7). “We’re still working to define the window of ECG” recording time that provides the optimal assessment for a history of asymptomatic AFib, but the “possibilities this opens are huge,” Dr. Friedman said in his talk at the symposium. This work sprang from the premise that “subtle signatures” in a brief, apparently normal sinus rhythm ECG tracing can harbor reliable clues about AFib history or an imminent episode.
The 2019 report by Dr. Friedman and associates documented that in the validation phase of their study, the trained artificial intelligence (AI) program identified patients with a history of AFib or an impending arrhythmia event from a single, 10-second ECG that to the naked eye seemed to show normal sinus rhythm with a sensitivity of 79.0%, a specificity of 79.5%, and an accuracy of 79.4%. It also showed an area under a receiver operating characteristic curve of 0.87, meaning that screening for AFib by this method compared favorably with the area-under-the-curve (AUC) results tallied by several widely accepted screening tools, including Pap smears for cervical cancer (AUC of 0.70), mammograms for breast cancer (AUC of 0.85), and CHA2DS2-VASc scoring for estimating stroke risk in AFib patients (AUC of 0.57-0.72), Dr. Friedman said.
The researchers developed the AI algorithm with more than 450,000 10-second ECG tracings collected from roughly 126,000 patients who underwent at least one ECG recording as part of their routine care at the Mayo Clinic during 1993-2017. The goal was for the program to find and validate recurring characteristics in the ECG that consistently linked with a history of or an impending AFib episode and that did not appear in ECG recordings from people without any AFib history. The program this effort produced then underwent further adjustment with the use of more than 64,340 ECGs from an additional 18,116 patients, and then the final product underwent validation testing with a further 130,802 ECGs collected from an additional 36,280 people, the study phase that resulted in the reported sensitivity and specificity estimates.
It’s currently unclear to Dr. Friedman and associates what specific features the program uses to classify patients. It’s an important question, but if the results are reproducible and reliable, this uncertainty shouldn’t slow clinical adoption, he said in an interview.
While “this particular algorithm needs prospective vetting,” a similar algorithm developed by Dr. Friedman and the same research team that uses a 10-second ECG to identify patients with a left ventricular ejection fraction of 35% or less is further advanced in development, and a device that uses this algorithm will soon receive Food and Drug Administration review under a fast track designation that the agency approved in late 2019.
The researchers developed this algorithm for estimating left ventricular function using a strategy similar to their development of a tool for diagnosing AFib (Nat Med. 2019 Jan 7;25[1]:70-4), and results from 100 patients prospectively studied with this approach to ECG analysis and reported at the American Heart Association scientific sessions in November 2019 showed that the algorithm identified substantial left ventricular dysfunction with an AUC of 0.906 (Circulation. 2019 Nov 19;140[suppl 1]:A13447). The same team of investigators has developed an AI algorithm that can calculate a person’s physiologic age based on the ECG recording (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]: 10.1161/CIRCEP.119.007284).
The study received no commercial funding, and Dr. Friedman and coauthors had no relevant disclosures. The Mayo Clinic has licensed a related artificial intelligence algorithm to EKO, and Dr. Friedman may benefit financially from this arrangement.
NATIONAL HARBOR, MD. – Researchers have created an artificial intelligence algorithm that can evaluate a 10-second ECG recording of a person in normal sinus rhythm and tell with a sensitivity and specificity of almost 80% whether or not that person ever had atrial fibrillation episodes some time in the past or will have a first arrhythmia episode in the near future.
Although this algorithm – derived from and then validated with a dataset of nearly 650,000 ECG recordings from more than 180,000 patients – still needs prospective validation, it offers the prospect for a potential revolution in screening for atrial fibrillation (AFib), Paul A. Friedman, MD, cautioned at the annual International AF Symposium. If initial clinical findings are confirmed, it would show that a 10-second, 12-lead ECG recording can provide the same screening scope as what otherwise takes weeks of ambulatory ECG recording with a Holter monitor or an implanted device, explained Dr. Friedman, professor of medicine and chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
This finding “could have important implications for atrial fibrillation screening and for the management of patients with unexplained stroke,” Dr. Friedman and his associates noted in the published report of their study (Lancet. 2019 Sep 7;394[10201]:861-7). “We’re still working to define the window of ECG” recording time that provides the optimal assessment for a history of asymptomatic AFib, but the “possibilities this opens are huge,” Dr. Friedman said in his talk at the symposium. This work sprang from the premise that “subtle signatures” in a brief, apparently normal sinus rhythm ECG tracing can harbor reliable clues about AFib history or an imminent episode.
The 2019 report by Dr. Friedman and associates documented that in the validation phase of their study, the trained artificial intelligence (AI) program identified patients with a history of AFib or an impending arrhythmia event from a single, 10-second ECG that to the naked eye seemed to show normal sinus rhythm with a sensitivity of 79.0%, a specificity of 79.5%, and an accuracy of 79.4%. It also showed an area under a receiver operating characteristic curve of 0.87, meaning that screening for AFib by this method compared favorably with the area-under-the-curve (AUC) results tallied by several widely accepted screening tools, including Pap smears for cervical cancer (AUC of 0.70), mammograms for breast cancer (AUC of 0.85), and CHA2DS2-VASc scoring for estimating stroke risk in AFib patients (AUC of 0.57-0.72), Dr. Friedman said.
The researchers developed the AI algorithm with more than 450,000 10-second ECG tracings collected from roughly 126,000 patients who underwent at least one ECG recording as part of their routine care at the Mayo Clinic during 1993-2017. The goal was for the program to find and validate recurring characteristics in the ECG that consistently linked with a history of or an impending AFib episode and that did not appear in ECG recordings from people without any AFib history. The program this effort produced then underwent further adjustment with the use of more than 64,340 ECGs from an additional 18,116 patients, and then the final product underwent validation testing with a further 130,802 ECGs collected from an additional 36,280 people, the study phase that resulted in the reported sensitivity and specificity estimates.
It’s currently unclear to Dr. Friedman and associates what specific features the program uses to classify patients. It’s an important question, but if the results are reproducible and reliable, this uncertainty shouldn’t slow clinical adoption, he said in an interview.
While “this particular algorithm needs prospective vetting,” a similar algorithm developed by Dr. Friedman and the same research team that uses a 10-second ECG to identify patients with a left ventricular ejection fraction of 35% or less is further advanced in development, and a device that uses this algorithm will soon receive Food and Drug Administration review under a fast track designation that the agency approved in late 2019.
The researchers developed this algorithm for estimating left ventricular function using a strategy similar to their development of a tool for diagnosing AFib (Nat Med. 2019 Jan 7;25[1]:70-4), and results from 100 patients prospectively studied with this approach to ECG analysis and reported at the American Heart Association scientific sessions in November 2019 showed that the algorithm identified substantial left ventricular dysfunction with an AUC of 0.906 (Circulation. 2019 Nov 19;140[suppl 1]:A13447). The same team of investigators has developed an AI algorithm that can calculate a person’s physiologic age based on the ECG recording (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]: 10.1161/CIRCEP.119.007284).
The study received no commercial funding, and Dr. Friedman and coauthors had no relevant disclosures. The Mayo Clinic has licensed a related artificial intelligence algorithm to EKO, and Dr. Friedman may benefit financially from this arrangement.
THE AF SYMPOSIUM 2020
Innovations to expect at HM20
Course director Dr. Benji Mathews offers highlights
Benji K. Mathews, MD, SFHM, CLHM, chief of hospital medicine at Regions Hospital, HealthPartners, in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners, is the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), which will be held April 16-18 in San Diego.
Dr. Mathews, also an associate professor of medicine at the University of Minnesota, Minneapolis, sat down with the Hospitalist to discuss the role of the course director in formulating the HM20 agenda, as well as highlighting some exciting educational sessions, workshops, and other events during the annual conference.
In your role as course director for HM20, did you have a particular theme you wanted to emphasize?
We did not go with a single theme, because we’re trying to provide a comprehensive educational and networking opportunity, so trying to focus the conference on a single theme a year in advance did not seem very prudent. There are multiple themes, from health disparities to technology to education. For a field like hospital medicine that’s rapidly evolving, we thought it best to keep it open and instead further develop the conference tracks: What new tracks can be created, what older tracks can be maintained because they have been highly successful, and which tracks do we retire?
Can you discuss some of the tracks at HM20?
The new track we have this year is the Technology track. That track will examine current and future technology that will impact care delivery, including telehealth, wearables, apps for digital learning, and for clinicians at the bedside. Innovation is at the core of hospital medicine, and we’re constantly exploring how to deliver efficient, timely, and effective care. “Future-casting” is important, and this track speaks to that.
There are some old standards that I would also recommend. The “Great Debate” is one of the hardest to finalize, because while you can create a great session topic and title, we need to find two talented speakers for a debate, as that is very different than a presentation. The speakers take opposing sides on clinical decisions, the latest literature reviews, best practices, and the audience gets to vote. Topics we’re using this year include “Procalcitonin: Friend or Foe,” “Guidelines Controversies in Inpatient Care,” and “POCUS vs. Physical Exam – Tech vs. Tradition.” Some of the debaters include Carrie Herzke, MD, of Johns Hopkins University, Baltimore; Daniel Dressler, MD, of Emory University, Atlanta; Jordan Messler, MD, of Morton Plant Hospital in Clearwater, Fla.; and Michelle Guidry, MD, of the Southeast Louisiana Veterans Health Care System and Tulane University, both in New Orleans; Ria Dancel, MD, from the University of North Carolina, and Michael Janjigian, MD, from NYU Langone Health.
One of the highlights this year is that we’re trying to bring more gender equity into our speaker lineup. Rarely will we have only two male speakers at a session, and I don’t think we have any all-male panels, jokingly called “manels” in the past.
Are there some “tried-and-true” tracks or sessions that are returning in HM20?
I’d like to highlight the Clinical Mastery track. That was a new track last year, and has returned this year. That track is focused on helping hospitalists become expert diagnosticians at the bedside. “Pitfalls, Myths and Pearls in Diagnostic Reasoning” is one session to note in that track, with Dr. Gopi Astik, Dr. Andrew Olson, and Dr. Reza Manesh. Another special focus this year within Clinical Mastery will be on using the rational clinical exam to augment your diagnostic skills.
When programming the annual conference, how do you balance the needs of community hospitalists with academic hospitalists?
The value we have on the annual conference committee is that there are a fair number of community hospitalists, advance practice clinicians, representation from med-peds, and family practice, for instance. Generally, there is a wide sampling of the decision makers from across the specialty helping to program the conference – we have great academic institutions, but we have representation from the larger impressive community as well. That said, it is hard to curate content that is solely for a specific subset of hospitalists without marginalizing other subsets. We don’t want to isolate people. A lot of our Rapid Fire topics are geared toward frontline hospitalists. This is content that will directly impact hospitalists as they care for patients. And some of the content that we’re bringing in this year with more emphasis are in health equity and disparities. Academic groups study this, however frontline clinicians from both academic and community settings deal with this every day, relating to both patients and staff. For example, in regard to patients, we have content focused on caring for the LGBTQ community, sessions on refugee health, as well as hospitalists and global health. We have an emphasis on diversity and inclusion in the workplace, with speakers from both community and academic settings. There will be good sessions with gender equity themes, practical tips in promotion and hiring practices. There are a couple workshops on gender equity; one to note is “Top 10 Ways for Men + Women to Engage in Gender Equity.”
Can you speak to the content that is targeted at nurse practitioners and physician assistants?
This is near and dear to my heart as I’m from an institution that has a positive history of strong partnerships with our advance practice clinician colleagues. Our goal this year was to continue to highlight nurse practitioners and physician assistants in a track dedicated to them. We have a core session called “Training Day: How to Onboard and Operationalize an Advanced Practice Provider Workforce” – this is a “bread-and-butter” session presented by speakers who have built programs from the ground up. Other important sessions address how to advance the careers of NPs and PAs – “Professional Development for NP/PAs” – and on mentorship, which emphasizes a culture of partnership on projects like providing high quality, safe care.
Are there any workshops that attendees should take note of?
One I would like to highlight is “Survive! The POCUS Apocalypse Adventure.” This highly anticipated offering is preregistration required, hosted for the first time on day 1 of the main conference. The workshop will introduce the gamification of POCUS to hospitalists. Each participant will be expected to perform ultrasound examinations and interpret their findings in order to gather clues that will lead to the cure for a zombie apocalypse! There are a lot of innovations this year in programming the Annual Conference, and gamification might be considered risky but I think it has a very good chance of success with entertainment and learning combined into one amazing workshop.
What are some other innovations that the annual conference committee has planned for 2020?
Another exciting innovation is what we call “Breakfast with an Expert.” This is a new rapid-fire didactic session format where we have three experts speak on different hot topics, such as “Nutritional Counseling” (led by Kate Shafto, MD), “Things I Wish I Knew Earlier in my Career” (Brad Sharpe, MD), and “Case-Based Controversies in Ethics” (Hannah Lipman, MD). These take place on the very first day of the conference, before the opening general session. Attendees can grab their breakfast and listen to any of these sessions before they head into the plenary. Hospitalists have asked for more content, so we’re adding these as a response to that hunger for more educational content. This format is supposed to be a bit cozier, with more Q&A.
Another aspect of HM20 to highlight is the Simulation Center. The Sim Center is a space that hosts a variety of hospital medicine skill development areas. This is an interactive center where attendees can learn to perform bedside procedures and learn hands-on skills with diagnostic point-of-care ultrasound during the first 2 days of the conference. The Sim Center is slightly different than the precourses, in that we are offering 1-hour blocks of small-group instruction for which attendees preregister. This aligns with larger SHM efforts to encourage hospitalists to be more confident with bedside procedures, and engage with SHM’s ultrasound offerings, including the certificate of completion program.
To register for the 2020 Annual Conference, including precourses, visit https://shmannualconference.org/register/.
Course director Dr. Benji Mathews offers highlights
Course director Dr. Benji Mathews offers highlights
Benji K. Mathews, MD, SFHM, CLHM, chief of hospital medicine at Regions Hospital, HealthPartners, in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners, is the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), which will be held April 16-18 in San Diego.
Dr. Mathews, also an associate professor of medicine at the University of Minnesota, Minneapolis, sat down with the Hospitalist to discuss the role of the course director in formulating the HM20 agenda, as well as highlighting some exciting educational sessions, workshops, and other events during the annual conference.
In your role as course director for HM20, did you have a particular theme you wanted to emphasize?
We did not go with a single theme, because we’re trying to provide a comprehensive educational and networking opportunity, so trying to focus the conference on a single theme a year in advance did not seem very prudent. There are multiple themes, from health disparities to technology to education. For a field like hospital medicine that’s rapidly evolving, we thought it best to keep it open and instead further develop the conference tracks: What new tracks can be created, what older tracks can be maintained because they have been highly successful, and which tracks do we retire?
Can you discuss some of the tracks at HM20?
The new track we have this year is the Technology track. That track will examine current and future technology that will impact care delivery, including telehealth, wearables, apps for digital learning, and for clinicians at the bedside. Innovation is at the core of hospital medicine, and we’re constantly exploring how to deliver efficient, timely, and effective care. “Future-casting” is important, and this track speaks to that.
There are some old standards that I would also recommend. The “Great Debate” is one of the hardest to finalize, because while you can create a great session topic and title, we need to find two talented speakers for a debate, as that is very different than a presentation. The speakers take opposing sides on clinical decisions, the latest literature reviews, best practices, and the audience gets to vote. Topics we’re using this year include “Procalcitonin: Friend or Foe,” “Guidelines Controversies in Inpatient Care,” and “POCUS vs. Physical Exam – Tech vs. Tradition.” Some of the debaters include Carrie Herzke, MD, of Johns Hopkins University, Baltimore; Daniel Dressler, MD, of Emory University, Atlanta; Jordan Messler, MD, of Morton Plant Hospital in Clearwater, Fla.; and Michelle Guidry, MD, of the Southeast Louisiana Veterans Health Care System and Tulane University, both in New Orleans; Ria Dancel, MD, from the University of North Carolina, and Michael Janjigian, MD, from NYU Langone Health.
One of the highlights this year is that we’re trying to bring more gender equity into our speaker lineup. Rarely will we have only two male speakers at a session, and I don’t think we have any all-male panels, jokingly called “manels” in the past.
Are there some “tried-and-true” tracks or sessions that are returning in HM20?
I’d like to highlight the Clinical Mastery track. That was a new track last year, and has returned this year. That track is focused on helping hospitalists become expert diagnosticians at the bedside. “Pitfalls, Myths and Pearls in Diagnostic Reasoning” is one session to note in that track, with Dr. Gopi Astik, Dr. Andrew Olson, and Dr. Reza Manesh. Another special focus this year within Clinical Mastery will be on using the rational clinical exam to augment your diagnostic skills.
When programming the annual conference, how do you balance the needs of community hospitalists with academic hospitalists?
The value we have on the annual conference committee is that there are a fair number of community hospitalists, advance practice clinicians, representation from med-peds, and family practice, for instance. Generally, there is a wide sampling of the decision makers from across the specialty helping to program the conference – we have great academic institutions, but we have representation from the larger impressive community as well. That said, it is hard to curate content that is solely for a specific subset of hospitalists without marginalizing other subsets. We don’t want to isolate people. A lot of our Rapid Fire topics are geared toward frontline hospitalists. This is content that will directly impact hospitalists as they care for patients. And some of the content that we’re bringing in this year with more emphasis are in health equity and disparities. Academic groups study this, however frontline clinicians from both academic and community settings deal with this every day, relating to both patients and staff. For example, in regard to patients, we have content focused on caring for the LGBTQ community, sessions on refugee health, as well as hospitalists and global health. We have an emphasis on diversity and inclusion in the workplace, with speakers from both community and academic settings. There will be good sessions with gender equity themes, practical tips in promotion and hiring practices. There are a couple workshops on gender equity; one to note is “Top 10 Ways for Men + Women to Engage in Gender Equity.”
Can you speak to the content that is targeted at nurse practitioners and physician assistants?
This is near and dear to my heart as I’m from an institution that has a positive history of strong partnerships with our advance practice clinician colleagues. Our goal this year was to continue to highlight nurse practitioners and physician assistants in a track dedicated to them. We have a core session called “Training Day: How to Onboard and Operationalize an Advanced Practice Provider Workforce” – this is a “bread-and-butter” session presented by speakers who have built programs from the ground up. Other important sessions address how to advance the careers of NPs and PAs – “Professional Development for NP/PAs” – and on mentorship, which emphasizes a culture of partnership on projects like providing high quality, safe care.
Are there any workshops that attendees should take note of?
One I would like to highlight is “Survive! The POCUS Apocalypse Adventure.” This highly anticipated offering is preregistration required, hosted for the first time on day 1 of the main conference. The workshop will introduce the gamification of POCUS to hospitalists. Each participant will be expected to perform ultrasound examinations and interpret their findings in order to gather clues that will lead to the cure for a zombie apocalypse! There are a lot of innovations this year in programming the Annual Conference, and gamification might be considered risky but I think it has a very good chance of success with entertainment and learning combined into one amazing workshop.
What are some other innovations that the annual conference committee has planned for 2020?
Another exciting innovation is what we call “Breakfast with an Expert.” This is a new rapid-fire didactic session format where we have three experts speak on different hot topics, such as “Nutritional Counseling” (led by Kate Shafto, MD), “Things I Wish I Knew Earlier in my Career” (Brad Sharpe, MD), and “Case-Based Controversies in Ethics” (Hannah Lipman, MD). These take place on the very first day of the conference, before the opening general session. Attendees can grab their breakfast and listen to any of these sessions before they head into the plenary. Hospitalists have asked for more content, so we’re adding these as a response to that hunger for more educational content. This format is supposed to be a bit cozier, with more Q&A.
Another aspect of HM20 to highlight is the Simulation Center. The Sim Center is a space that hosts a variety of hospital medicine skill development areas. This is an interactive center where attendees can learn to perform bedside procedures and learn hands-on skills with diagnostic point-of-care ultrasound during the first 2 days of the conference. The Sim Center is slightly different than the precourses, in that we are offering 1-hour blocks of small-group instruction for which attendees preregister. This aligns with larger SHM efforts to encourage hospitalists to be more confident with bedside procedures, and engage with SHM’s ultrasound offerings, including the certificate of completion program.
To register for the 2020 Annual Conference, including precourses, visit https://shmannualconference.org/register/.
Benji K. Mathews, MD, SFHM, CLHM, chief of hospital medicine at Regions Hospital, HealthPartners, in St. Paul, Minn., and director of point of care ultrasound (POCUS) for hospital medicine at HealthPartners, is the course director for the Society of Hospital Medicine’s 2020 Annual Conference (HM20), which will be held April 16-18 in San Diego.
Dr. Mathews, also an associate professor of medicine at the University of Minnesota, Minneapolis, sat down with the Hospitalist to discuss the role of the course director in formulating the HM20 agenda, as well as highlighting some exciting educational sessions, workshops, and other events during the annual conference.
In your role as course director for HM20, did you have a particular theme you wanted to emphasize?
We did not go with a single theme, because we’re trying to provide a comprehensive educational and networking opportunity, so trying to focus the conference on a single theme a year in advance did not seem very prudent. There are multiple themes, from health disparities to technology to education. For a field like hospital medicine that’s rapidly evolving, we thought it best to keep it open and instead further develop the conference tracks: What new tracks can be created, what older tracks can be maintained because they have been highly successful, and which tracks do we retire?
Can you discuss some of the tracks at HM20?
The new track we have this year is the Technology track. That track will examine current and future technology that will impact care delivery, including telehealth, wearables, apps for digital learning, and for clinicians at the bedside. Innovation is at the core of hospital medicine, and we’re constantly exploring how to deliver efficient, timely, and effective care. “Future-casting” is important, and this track speaks to that.
There are some old standards that I would also recommend. The “Great Debate” is one of the hardest to finalize, because while you can create a great session topic and title, we need to find two talented speakers for a debate, as that is very different than a presentation. The speakers take opposing sides on clinical decisions, the latest literature reviews, best practices, and the audience gets to vote. Topics we’re using this year include “Procalcitonin: Friend or Foe,” “Guidelines Controversies in Inpatient Care,” and “POCUS vs. Physical Exam – Tech vs. Tradition.” Some of the debaters include Carrie Herzke, MD, of Johns Hopkins University, Baltimore; Daniel Dressler, MD, of Emory University, Atlanta; Jordan Messler, MD, of Morton Plant Hospital in Clearwater, Fla.; and Michelle Guidry, MD, of the Southeast Louisiana Veterans Health Care System and Tulane University, both in New Orleans; Ria Dancel, MD, from the University of North Carolina, and Michael Janjigian, MD, from NYU Langone Health.
One of the highlights this year is that we’re trying to bring more gender equity into our speaker lineup. Rarely will we have only two male speakers at a session, and I don’t think we have any all-male panels, jokingly called “manels” in the past.
Are there some “tried-and-true” tracks or sessions that are returning in HM20?
I’d like to highlight the Clinical Mastery track. That was a new track last year, and has returned this year. That track is focused on helping hospitalists become expert diagnosticians at the bedside. “Pitfalls, Myths and Pearls in Diagnostic Reasoning” is one session to note in that track, with Dr. Gopi Astik, Dr. Andrew Olson, and Dr. Reza Manesh. Another special focus this year within Clinical Mastery will be on using the rational clinical exam to augment your diagnostic skills.
When programming the annual conference, how do you balance the needs of community hospitalists with academic hospitalists?
The value we have on the annual conference committee is that there are a fair number of community hospitalists, advance practice clinicians, representation from med-peds, and family practice, for instance. Generally, there is a wide sampling of the decision makers from across the specialty helping to program the conference – we have great academic institutions, but we have representation from the larger impressive community as well. That said, it is hard to curate content that is solely for a specific subset of hospitalists without marginalizing other subsets. We don’t want to isolate people. A lot of our Rapid Fire topics are geared toward frontline hospitalists. This is content that will directly impact hospitalists as they care for patients. And some of the content that we’re bringing in this year with more emphasis are in health equity and disparities. Academic groups study this, however frontline clinicians from both academic and community settings deal with this every day, relating to both patients and staff. For example, in regard to patients, we have content focused on caring for the LGBTQ community, sessions on refugee health, as well as hospitalists and global health. We have an emphasis on diversity and inclusion in the workplace, with speakers from both community and academic settings. There will be good sessions with gender equity themes, practical tips in promotion and hiring practices. There are a couple workshops on gender equity; one to note is “Top 10 Ways for Men + Women to Engage in Gender Equity.”
Can you speak to the content that is targeted at nurse practitioners and physician assistants?
This is near and dear to my heart as I’m from an institution that has a positive history of strong partnerships with our advance practice clinician colleagues. Our goal this year was to continue to highlight nurse practitioners and physician assistants in a track dedicated to them. We have a core session called “Training Day: How to Onboard and Operationalize an Advanced Practice Provider Workforce” – this is a “bread-and-butter” session presented by speakers who have built programs from the ground up. Other important sessions address how to advance the careers of NPs and PAs – “Professional Development for NP/PAs” – and on mentorship, which emphasizes a culture of partnership on projects like providing high quality, safe care.
Are there any workshops that attendees should take note of?
One I would like to highlight is “Survive! The POCUS Apocalypse Adventure.” This highly anticipated offering is preregistration required, hosted for the first time on day 1 of the main conference. The workshop will introduce the gamification of POCUS to hospitalists. Each participant will be expected to perform ultrasound examinations and interpret their findings in order to gather clues that will lead to the cure for a zombie apocalypse! There are a lot of innovations this year in programming the Annual Conference, and gamification might be considered risky but I think it has a very good chance of success with entertainment and learning combined into one amazing workshop.
What are some other innovations that the annual conference committee has planned for 2020?
Another exciting innovation is what we call “Breakfast with an Expert.” This is a new rapid-fire didactic session format where we have three experts speak on different hot topics, such as “Nutritional Counseling” (led by Kate Shafto, MD), “Things I Wish I Knew Earlier in my Career” (Brad Sharpe, MD), and “Case-Based Controversies in Ethics” (Hannah Lipman, MD). These take place on the very first day of the conference, before the opening general session. Attendees can grab their breakfast and listen to any of these sessions before they head into the plenary. Hospitalists have asked for more content, so we’re adding these as a response to that hunger for more educational content. This format is supposed to be a bit cozier, with more Q&A.
Another aspect of HM20 to highlight is the Simulation Center. The Sim Center is a space that hosts a variety of hospital medicine skill development areas. This is an interactive center where attendees can learn to perform bedside procedures and learn hands-on skills with diagnostic point-of-care ultrasound during the first 2 days of the conference. The Sim Center is slightly different than the precourses, in that we are offering 1-hour blocks of small-group instruction for which attendees preregister. This aligns with larger SHM efforts to encourage hospitalists to be more confident with bedside procedures, and engage with SHM’s ultrasound offerings, including the certificate of completion program.
To register for the 2020 Annual Conference, including precourses, visit https://shmannualconference.org/register/.
COVID-19: Time to ‘take the risk of scaring people’
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
It’s past time to call the novel coronavirus, COVID-19, a pandemic and “time to push people to prepare, and guide their prep,” according to risk communication experts.
Medical messaging about containing or stopping the spread of the virus is doing more harm than good, write Peter Sandman, PhD, and Jody Lanard, MD, both based in New York City, in a recent blog post.
“We are near-certain that the desperate-sounding last-ditch containment messaging of recent days is contributing to a massive global misperception,” they warn.
“The most crucial (and overdue) risk communication task … is to help people visualize their communities when ‘keeping it out’ – containment – is no longer relevant.”
That message is embraced by several experts who spoke to Medscape Medical News.
“I’m jealous of what [they] have written: It is so clear, so correct, and so practical,” said David Fisman, MD, MPH, professor of epidemiology at the University of Toronto, Canada. “I think WHO [World Health Organization] is shying away from the P word,” he continued, referring to the organization’s continuing decision not to call the outbreak a pandemic.
“I fully support exactly what [Sandman and Lanard] are saying,” said Michael Osterholm, PhD, MPH, professor of environmental health sciences and director of the Center for Infectious Disease Research and Policy (CIDRAP) at the University of Minnesota in Minneapolis.
Sandman and Lanard write. “Hardly any officials are telling civil society and the general public how to get ready for this pandemic.”
Effective communication should inform people of what to expect now, they continue: “[T]he end of most quarantines, travel restrictions, contact tracing, and other measures designed to keep ‘them’ from infecting ‘us,’ and the switch to measures like canceling mass events designed to keep us from infecting each other.”
Among the new messages that should be delivered are things like:
- Stockpiling nonperishable food and prescription meds.
- Considering care of sick family members.
- Cross-training work personnel so one person’s absence won’t derail an organization’s ability to function.
“We hope that governments and healthcare institutions are using this time wisely,” Sandman and Lanard continue. “We know that ordinary citizens are not being asked to do so. In most countries … ordinary citizens have not been asked to prepare. Instead, they have been led to expect that their governments will keep the virus from their doors.”
This article first appeared on Medscape.com.
CDC expects eventual community spread of coronavirus in U.S.
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
“We have for many weeks been saying that, while we hope this is not going to be severe, we are planning as if it is,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC, said during a Feb. 25, 2020, telebriefing with reporters. “The data over the last week and the spread in other countries has certainly raised our level of concern and raised our level expectation that we are going to have community spread here.”
Dr. Messonnier noted that the coronavirus is now showing signs of community spread without a known source of exposure in a number of countries, including in Hong Kong, Iran, Italy, Japan, Singapore, South Korea, Taiwan, and Thailand. This has now raised the belief that there will be more widespread outbreaks in the United States.
“What we still don’t know is what that will look like,” she said. “As many of you know, we can have community spread in the United States and have it be reasonably mild. We can have community spread in the U.S. and have it be very severe. That is what we don’t completely know yet and we certainly also don’t exactly know when it is going to happen.”
She reiterated the number of actions being taken to slow the potential spread in the United States, including detecting, tracking, and isolating all cases, as well as restricting travel into the United States and issuing travel advisories for countries where coronavirus outbreaks are known.
“We are doing this with the goal of slowing the introduction of this new virus into the U.S. and buying us more time to prepare,” Dr. Messonnier said, noting the containment strategies have been largely successful, though it will be more difficult as more countries experience community spread of the virus.
Dr. Messonnier also reiterated that at this time there are no vaccines and no medicines to treat the coronavirus. She stressed the need to adhere to nonpharmaceutical interventions (NPIs), as they will be “the most important tools in our response to this virus.”
She said the NPIs will vary based on the severity of the outbreak in any given local community and include personal protective measures that individuals can take every day (many of which mirror the recommendations for preventing the spread of the seasonal flu virus), community NPIs that involve social distancing measures designed to keep people away from others, and environmental NPIs such as surface cleaning measures.
CDC’s latest warning comes as parent agency the Department of Health & Human Services is seeking $2.5 billion in funds from Congress to address the coronavirus outbreak.
During a separate press conference on the same day, HHS Secretary Alex Azar noted that there are five major priorities related to those funds, which would be used in the current year, including expansion of surveillance work within the influenza surveillance network; supporting public health preparedness and response for state and local governments; support the development of therapeutics and the development of vaccines; and the purchase of personal protective equipment for national stockpiles.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Disease at the National Institutes of Health, added during the press conference that vaccine work is in progress and could be ready for phase 1 testing within a month and a half. If all goes well, it would still be at least 12 - 18 months following the completion of a phase 2 trial before it could be produced for mass consumption.
“It is certainly conceivable that this issue with this coronavirus will go well beyond this season into next season,” Dr. Fauci said. “So a vaccine may not solve the problems of the next couple of months, but it certainly would be an important tool that we would have and we will keep you posted on that.”
He also mentioned that NIAID is looking at a number of candidates for therapeutic treatment of coronavirus. He highlighted Gilead’s remdesivir, a nucleotide analog, as one which undergoing two trials – a randomized controlled trial in China and a copy of that trial in Nebraska among patients with the coronavirus who were taken from the Diamond Princess cruise line in Japan.
“I am optimistic that we will at least get an answer if we do have do have a therapy that really is a gamechanger because then we could do something from the standpoint of intervention for those who are sick,” Dr. Fauci said.
UPDATE: This story was updated 2/25 at 4:51 p.m. ET
Guidance defines vaping-related respiratory syndrome
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
REPORTING FROM CCC49