User login
Catheter ablation of AF in patients with heart failure decreases mortality and HF admissions
Background: Rhythm control with medical therapy has been shown to not be superior to rate control for patients with both heart failure and AF. Rhythm control by ablation has been associated with positive outcomes in this same population, but its effectiveness, compared with medical therapy for patient-centered outcomes, has not been demonstrated.
Study design: Multicenter, open-label, randomized, controlled superiority trial.
Setting: 33 hospitals from Europe, Australia, and the United States during 2008-2016.
Synopsis: A total of 363 patients with HF with LVEF less than 35%, New York Heart Association II-IV symptoms, and permanent or paroxysmal AF who had previously failed or declined antiarrhythmic medications were randomly assigned to undergo ablation by pulmonary vein isolation or to medical therapy. The primary outcome – a composite of death or hospitalization for heart failure – was significantly lower in the ablation group, compared with the medical therapy group (28.5% vs. 44.6%; P = .006) with a number needed to treat of 8.3. The secondary outcomes of all-cause mortality and heart failure admissions were also significantly lower in the ablation group (13.4% vs. 25%; P = .01 and 20.7% vs. 35.9%; P = .004 respectively). The burden of AF, as identified by patient implantable devices was significantly lower in the ablation group, suggesting the likely mechanism of ablation benefit. Limitations of this study include its small sample size and lack of physician or patient blinding to treatment assignment.
Bottom line: Compared with medical therapy, catheter ablation of atrial fibrillation for patients with symptomatic heart failure with LVEF less than 35% was associated with significantly decreased mortality and heart failure admissions.
Citation: Marrouche N et al. Catheter ablation for atrial fibrillation with heart failure. N Eng J Med. 2018 Feb 1; 378:417-27.
Dr. Salber is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: Rhythm control with medical therapy has been shown to not be superior to rate control for patients with both heart failure and AF. Rhythm control by ablation has been associated with positive outcomes in this same population, but its effectiveness, compared with medical therapy for patient-centered outcomes, has not been demonstrated.
Study design: Multicenter, open-label, randomized, controlled superiority trial.
Setting: 33 hospitals from Europe, Australia, and the United States during 2008-2016.
Synopsis: A total of 363 patients with HF with LVEF less than 35%, New York Heart Association II-IV symptoms, and permanent or paroxysmal AF who had previously failed or declined antiarrhythmic medications were randomly assigned to undergo ablation by pulmonary vein isolation or to medical therapy. The primary outcome – a composite of death or hospitalization for heart failure – was significantly lower in the ablation group, compared with the medical therapy group (28.5% vs. 44.6%; P = .006) with a number needed to treat of 8.3. The secondary outcomes of all-cause mortality and heart failure admissions were also significantly lower in the ablation group (13.4% vs. 25%; P = .01 and 20.7% vs. 35.9%; P = .004 respectively). The burden of AF, as identified by patient implantable devices was significantly lower in the ablation group, suggesting the likely mechanism of ablation benefit. Limitations of this study include its small sample size and lack of physician or patient blinding to treatment assignment.
Bottom line: Compared with medical therapy, catheter ablation of atrial fibrillation for patients with symptomatic heart failure with LVEF less than 35% was associated with significantly decreased mortality and heart failure admissions.
Citation: Marrouche N et al. Catheter ablation for atrial fibrillation with heart failure. N Eng J Med. 2018 Feb 1; 378:417-27.
Dr. Salber is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Background: Rhythm control with medical therapy has been shown to not be superior to rate control for patients with both heart failure and AF. Rhythm control by ablation has been associated with positive outcomes in this same population, but its effectiveness, compared with medical therapy for patient-centered outcomes, has not been demonstrated.
Study design: Multicenter, open-label, randomized, controlled superiority trial.
Setting: 33 hospitals from Europe, Australia, and the United States during 2008-2016.
Synopsis: A total of 363 patients with HF with LVEF less than 35%, New York Heart Association II-IV symptoms, and permanent or paroxysmal AF who had previously failed or declined antiarrhythmic medications were randomly assigned to undergo ablation by pulmonary vein isolation or to medical therapy. The primary outcome – a composite of death or hospitalization for heart failure – was significantly lower in the ablation group, compared with the medical therapy group (28.5% vs. 44.6%; P = .006) with a number needed to treat of 8.3. The secondary outcomes of all-cause mortality and heart failure admissions were also significantly lower in the ablation group (13.4% vs. 25%; P = .01 and 20.7% vs. 35.9%; P = .004 respectively). The burden of AF, as identified by patient implantable devices was significantly lower in the ablation group, suggesting the likely mechanism of ablation benefit. Limitations of this study include its small sample size and lack of physician or patient blinding to treatment assignment.
Bottom line: Compared with medical therapy, catheter ablation of atrial fibrillation for patients with symptomatic heart failure with LVEF less than 35% was associated with significantly decreased mortality and heart failure admissions.
Citation: Marrouche N et al. Catheter ablation for atrial fibrillation with heart failure. N Eng J Med. 2018 Feb 1; 378:417-27.
Dr. Salber is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Patent foramen ovale may be associated with increased risk of perioperative ischemic stroke
Clinical question: Are patients with patent foramen ovale (PFO) at increased risk of perioperative ischemic stroke?
Background: Prior research has identified an association between PFO and risk of stroke. However, little is known about the effect of a preoperatively diagnosed PFO on perioperative stroke risk.
Study design: Retrospective cohort study.
Setting: Three Massachusetts hospitals, from January 2007 to December 2015.
Synopsis: The charts of 150,198 adult patients who underwent noncardiac surgery were reviewed for ICD codes for PFO. The primary outcome was perioperative ischemic stroke within 30 days of surgery, as identified via ICD code and subsequent chart review. After they adjusted for confounding variables, the study authors found that patients with PFO had an increased risk of perioperative ischemic stroke (odds ratio, 2.66; 95% confidence interval, 1.96-3.63; P less than .001) compared with patients without PFO. These findings were replicated in a propensity score–matched cohort to adjust for baseline differences between PFO and non-PFO groups. Patients with PFO also had a significantly increased risk of large-vessel territory ischemia and more severe neurologic deficits.
Given the observational design, this study could not establish a causal relationship between presence of a PFO and perioperative stroke. While the results support the consideration of PFO as a risk factor for perioperative stroke, research into whether this risk can be mitigated is needed.
Bottom line: Patients with PFO undergoing noncardiac surgery may be at increased risk of perioperative ischemic stroke.
Citation: Ng PY et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA. 2018 Feb 6;319(5):452-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Are patients with patent foramen ovale (PFO) at increased risk of perioperative ischemic stroke?
Background: Prior research has identified an association between PFO and risk of stroke. However, little is known about the effect of a preoperatively diagnosed PFO on perioperative stroke risk.
Study design: Retrospective cohort study.
Setting: Three Massachusetts hospitals, from January 2007 to December 2015.
Synopsis: The charts of 150,198 adult patients who underwent noncardiac surgery were reviewed for ICD codes for PFO. The primary outcome was perioperative ischemic stroke within 30 days of surgery, as identified via ICD code and subsequent chart review. After they adjusted for confounding variables, the study authors found that patients with PFO had an increased risk of perioperative ischemic stroke (odds ratio, 2.66; 95% confidence interval, 1.96-3.63; P less than .001) compared with patients without PFO. These findings were replicated in a propensity score–matched cohort to adjust for baseline differences between PFO and non-PFO groups. Patients with PFO also had a significantly increased risk of large-vessel territory ischemia and more severe neurologic deficits.
Given the observational design, this study could not establish a causal relationship between presence of a PFO and perioperative stroke. While the results support the consideration of PFO as a risk factor for perioperative stroke, research into whether this risk can be mitigated is needed.
Bottom line: Patients with PFO undergoing noncardiac surgery may be at increased risk of perioperative ischemic stroke.
Citation: Ng PY et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA. 2018 Feb 6;319(5):452-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Are patients with patent foramen ovale (PFO) at increased risk of perioperative ischemic stroke?
Background: Prior research has identified an association between PFO and risk of stroke. However, little is known about the effect of a preoperatively diagnosed PFO on perioperative stroke risk.
Study design: Retrospective cohort study.
Setting: Three Massachusetts hospitals, from January 2007 to December 2015.
Synopsis: The charts of 150,198 adult patients who underwent noncardiac surgery were reviewed for ICD codes for PFO. The primary outcome was perioperative ischemic stroke within 30 days of surgery, as identified via ICD code and subsequent chart review. After they adjusted for confounding variables, the study authors found that patients with PFO had an increased risk of perioperative ischemic stroke (odds ratio, 2.66; 95% confidence interval, 1.96-3.63; P less than .001) compared with patients without PFO. These findings were replicated in a propensity score–matched cohort to adjust for baseline differences between PFO and non-PFO groups. Patients with PFO also had a significantly increased risk of large-vessel territory ischemia and more severe neurologic deficits.
Given the observational design, this study could not establish a causal relationship between presence of a PFO and perioperative stroke. While the results support the consideration of PFO as a risk factor for perioperative stroke, research into whether this risk can be mitigated is needed.
Bottom line: Patients with PFO undergoing noncardiac surgery may be at increased risk of perioperative ischemic stroke.
Citation: Ng PY et al. Association of preoperatively diagnosed patent foramen ovale with perioperative ischemic stroke. JAMA. 2018 Feb 6;319(5):452-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Documentation and billing: Tips for hospitalists
Is it AMS, Delirium, or Encephalopathy?
During residency, physicians are trained to care for patients and write notes that are clinically useful. However, physicians are often not taught about how documentation affects reimbursement and quality measures. Our purpose here, and in articles to follow, is to give readers tools to enable them to more accurately reflect the complexity and work that is done for accurate reimbursements.
If you were to get in a car accident, the body shop would document the damage done and submit it to the insurance company. It’s the body shop’s responsibility to record the damage, not the insurance company’s. So while documentation can seem onerous, the insurance company is not going to scour the chart to find diagnoses missed in the note. That would be like the body shop doing repair work without documenting the damage but then somehow expecting to get paid.
For the insurance company, “If you didn’t document it, it didn’t happen.” The body shop should not underdocument and say there were only a few scratches on the right rear panel if it was severely damaged. Likewise, it should not overbill and say the front bumper was damaged if it was not. The goal is not to bill as much as possible but rather to document appropriately.
Terminology
The expected length of stay (LOS) and the expected mortality for a particular patient is determined by how sick the patient appears to be based on the medical record documentation. So documenting all the appropriate diagnoses makes the LOS index (actual LOS divided by expected LOS) and mortality index more accurate as well. It is particularly important to document when a condition is (or is not) “present on admission”.

While physician payments can be based on evaluation and management coding, the hospital’s reimbursement is largely determined by physician documentation. Hospitals are paid by Medicare on a capitated basis according to the Acute Inpatient Prospective Payment System. The amount paid is determined by the base rate of the hospital multiplied by the relative weight (RW) of the Medicare Severity Diagnosis Related Group (MS-DRG).
The base rate is adjusted by the wage index of the hospital location. Hospitals that serve a high proportion of low income patients receive a Disproportionate Share Hospital adjustment. The base rate is not something hospitalists have control over.
The RW, however, is determined by the primary diagnosis (reason for admission) and whether or not there are complications or comorbidities (CCs) or major complications or comorbidities (MCCs). The more CCs and MCCs a patient has, the higher the severity of illness and expected increased resources needed to care for that patient.
Diagnoses are currently coded using ICD-10 used by the World Health Organization. The ICD-10 of the primary diagnosis is mapped to an MS-DRG. Many, but not all, MS-DRGs have increasing reimbursements for CCs and MCCs. Coders map the ICD-10 of the principal diagnosis along with any associated CCs or MCCs to the MS-DRG code. The relative weights for different DRGs can found on table 5 of the Medicare website (see reference 1).
Altered mental status versus delirium versus encephalopathy
As an example, let’s look at the difference in RW, LOS, and reimbursement in an otherwise identical patient based on documenting altered mental status (AMS), delirium, or encephalopathy. (see Table 1)
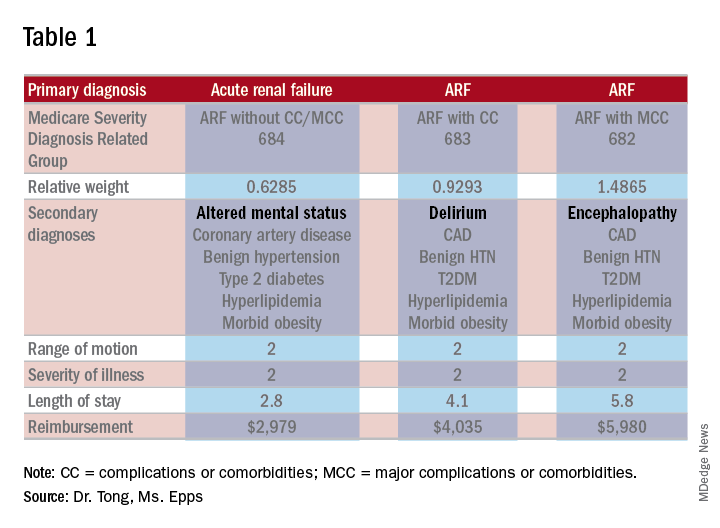
As one can see, RW, estimated LOS, and reimbursement would significantly increase for the patient with delirium (CC) or encephalopathy (MCC) versus AMS (no CC/MCC). A list of which diagnoses are considered CC’s versus MCC’s are on tables 6J and 6I, respectively, on the same Medicare website as table 5.
The difference between AMS, delirium, and encephalopathy
AMS is a sign/symptom complex similar to shortness of breath before an etiology is found. AMS can be the presenting symptom; when a specific etiology is found, however, a more specific diagnosis should be used such as delirium or encephalopathy.
Delirium, according to the DSM-5, is an acute change in the level of attention, cognition, or perception from baseline that developed over hours or days and tends to fluctuate during the course of a day. The change described is not better explained by a preexisting or evolving neurocognitive disorder and does not occur in the context of a severely reduced level of arousal, such as coma. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct consequence of a general medical condition, substance intoxication or withdrawal, exposure to a toxin, or more than one cause.
The National Institute of Neurological Diseases and Stroke defines encephalopathy as “any diffuse disease of the brain that alters brain function or structure. Encephalopathy may be caused by an infectious agent, metabolic or mitochondrial dysfunction, brain tumor or increased intracranial pressure, prolonged exposure to toxic elements, chronic progressive trauma, poor nutrition, or lack of oxygen or blood flow to the brain. The hallmark of encephalopathy is an altered mental state.”
It is confusing since there is a lot of overlap in the definitions of delirium and encephalopathy. One way to tease this out conceptually is noting that delirium is listed under mental, behavioral, and neurodevelopmental disorders, while encephalopathy appears under disorders of the nervous system. One can think of delirium as more of a “mental/psychiatric” diagnosis, while encephalopathy is caused by more “medical” causes.
If a patient who is normally not altered presents with confusion because of an infection or metabolic derangement, one can diagnose and document the cause of an acute encephalopathy. However, let’s say a patient is admitted in the morning with an infection, is started on treatment, but is not initially confused. If he/she later becomes confused at night, one could err conservatively and document delirium caused by sundowning.
Differentiating delirium and encephalopathy can be especially difficult in patients who have dementia with episodic confusion when they present with an infection and confusion. If the confusion is within what family members/caretakers say is “normal,” then one shouldn’t document encephalopathy. As a provider, one shouldn’t focus on all the rules and exceptions, just document as specifically and accurately as possible and the coders should take care of the rest.
Dr. Tong is an assistant professor of hospital medicine and an assistant director of the clinical research program at Emory University, Atlanta. Ms. Epps is director of clinical documentation improvement at Emory Healthcare, Atlanta.
References
1. “Acute Inpatient PPS.” Centers for Medicare and Medicaid Services. Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013.
3. “Details for title: FY 2018 Final Rule and Correction Notice Tables.” Centers for Medicare and Medicaid Services Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page-Items/FY2018-IPPS-Final-Rule-Tables.html.
4. “Encephalopathy Information Page.” National Institute of Neurologic Disorders and Stroke. Accessed on 2/17/18. https://www.ninds.nih.gov/Disorders/All-Disorders/Encephalopathy-Information-Page.
5. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. http://apps.who.int/iris/handle/10665/37958.
Is it AMS, Delirium, or Encephalopathy?
Is it AMS, Delirium, or Encephalopathy?
During residency, physicians are trained to care for patients and write notes that are clinically useful. However, physicians are often not taught about how documentation affects reimbursement and quality measures. Our purpose here, and in articles to follow, is to give readers tools to enable them to more accurately reflect the complexity and work that is done for accurate reimbursements.
If you were to get in a car accident, the body shop would document the damage done and submit it to the insurance company. It’s the body shop’s responsibility to record the damage, not the insurance company’s. So while documentation can seem onerous, the insurance company is not going to scour the chart to find diagnoses missed in the note. That would be like the body shop doing repair work without documenting the damage but then somehow expecting to get paid.
For the insurance company, “If you didn’t document it, it didn’t happen.” The body shop should not underdocument and say there were only a few scratches on the right rear panel if it was severely damaged. Likewise, it should not overbill and say the front bumper was damaged if it was not. The goal is not to bill as much as possible but rather to document appropriately.
Terminology
The expected length of stay (LOS) and the expected mortality for a particular patient is determined by how sick the patient appears to be based on the medical record documentation. So documenting all the appropriate diagnoses makes the LOS index (actual LOS divided by expected LOS) and mortality index more accurate as well. It is particularly important to document when a condition is (or is not) “present on admission”.

While physician payments can be based on evaluation and management coding, the hospital’s reimbursement is largely determined by physician documentation. Hospitals are paid by Medicare on a capitated basis according to the Acute Inpatient Prospective Payment System. The amount paid is determined by the base rate of the hospital multiplied by the relative weight (RW) of the Medicare Severity Diagnosis Related Group (MS-DRG).
The base rate is adjusted by the wage index of the hospital location. Hospitals that serve a high proportion of low income patients receive a Disproportionate Share Hospital adjustment. The base rate is not something hospitalists have control over.
The RW, however, is determined by the primary diagnosis (reason for admission) and whether or not there are complications or comorbidities (CCs) or major complications or comorbidities (MCCs). The more CCs and MCCs a patient has, the higher the severity of illness and expected increased resources needed to care for that patient.
Diagnoses are currently coded using ICD-10 used by the World Health Organization. The ICD-10 of the primary diagnosis is mapped to an MS-DRG. Many, but not all, MS-DRGs have increasing reimbursements for CCs and MCCs. Coders map the ICD-10 of the principal diagnosis along with any associated CCs or MCCs to the MS-DRG code. The relative weights for different DRGs can found on table 5 of the Medicare website (see reference 1).
Altered mental status versus delirium versus encephalopathy
As an example, let’s look at the difference in RW, LOS, and reimbursement in an otherwise identical patient based on documenting altered mental status (AMS), delirium, or encephalopathy. (see Table 1)

As one can see, RW, estimated LOS, and reimbursement would significantly increase for the patient with delirium (CC) or encephalopathy (MCC) versus AMS (no CC/MCC). A list of which diagnoses are considered CC’s versus MCC’s are on tables 6J and 6I, respectively, on the same Medicare website as table 5.
The difference between AMS, delirium, and encephalopathy
AMS is a sign/symptom complex similar to shortness of breath before an etiology is found. AMS can be the presenting symptom; when a specific etiology is found, however, a more specific diagnosis should be used such as delirium or encephalopathy.
Delirium, according to the DSM-5, is an acute change in the level of attention, cognition, or perception from baseline that developed over hours or days and tends to fluctuate during the course of a day. The change described is not better explained by a preexisting or evolving neurocognitive disorder and does not occur in the context of a severely reduced level of arousal, such as coma. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct consequence of a general medical condition, substance intoxication or withdrawal, exposure to a toxin, or more than one cause.
The National Institute of Neurological Diseases and Stroke defines encephalopathy as “any diffuse disease of the brain that alters brain function or structure. Encephalopathy may be caused by an infectious agent, metabolic or mitochondrial dysfunction, brain tumor or increased intracranial pressure, prolonged exposure to toxic elements, chronic progressive trauma, poor nutrition, or lack of oxygen or blood flow to the brain. The hallmark of encephalopathy is an altered mental state.”
It is confusing since there is a lot of overlap in the definitions of delirium and encephalopathy. One way to tease this out conceptually is noting that delirium is listed under mental, behavioral, and neurodevelopmental disorders, while encephalopathy appears under disorders of the nervous system. One can think of delirium as more of a “mental/psychiatric” diagnosis, while encephalopathy is caused by more “medical” causes.
If a patient who is normally not altered presents with confusion because of an infection or metabolic derangement, one can diagnose and document the cause of an acute encephalopathy. However, let’s say a patient is admitted in the morning with an infection, is started on treatment, but is not initially confused. If he/she later becomes confused at night, one could err conservatively and document delirium caused by sundowning.
Differentiating delirium and encephalopathy can be especially difficult in patients who have dementia with episodic confusion when they present with an infection and confusion. If the confusion is within what family members/caretakers say is “normal,” then one shouldn’t document encephalopathy. As a provider, one shouldn’t focus on all the rules and exceptions, just document as specifically and accurately as possible and the coders should take care of the rest.
Dr. Tong is an assistant professor of hospital medicine and an assistant director of the clinical research program at Emory University, Atlanta. Ms. Epps is director of clinical documentation improvement at Emory Healthcare, Atlanta.
References
1. “Acute Inpatient PPS.” Centers for Medicare and Medicaid Services. Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013.
3. “Details for title: FY 2018 Final Rule and Correction Notice Tables.” Centers for Medicare and Medicaid Services Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page-Items/FY2018-IPPS-Final-Rule-Tables.html.
4. “Encephalopathy Information Page.” National Institute of Neurologic Disorders and Stroke. Accessed on 2/17/18. https://www.ninds.nih.gov/Disorders/All-Disorders/Encephalopathy-Information-Page.
5. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. http://apps.who.int/iris/handle/10665/37958.
During residency, physicians are trained to care for patients and write notes that are clinically useful. However, physicians are often not taught about how documentation affects reimbursement and quality measures. Our purpose here, and in articles to follow, is to give readers tools to enable them to more accurately reflect the complexity and work that is done for accurate reimbursements.
If you were to get in a car accident, the body shop would document the damage done and submit it to the insurance company. It’s the body shop’s responsibility to record the damage, not the insurance company’s. So while documentation can seem onerous, the insurance company is not going to scour the chart to find diagnoses missed in the note. That would be like the body shop doing repair work without documenting the damage but then somehow expecting to get paid.
For the insurance company, “If you didn’t document it, it didn’t happen.” The body shop should not underdocument and say there were only a few scratches on the right rear panel if it was severely damaged. Likewise, it should not overbill and say the front bumper was damaged if it was not. The goal is not to bill as much as possible but rather to document appropriately.
Terminology
The expected length of stay (LOS) and the expected mortality for a particular patient is determined by how sick the patient appears to be based on the medical record documentation. So documenting all the appropriate diagnoses makes the LOS index (actual LOS divided by expected LOS) and mortality index more accurate as well. It is particularly important to document when a condition is (or is not) “present on admission”.

While physician payments can be based on evaluation and management coding, the hospital’s reimbursement is largely determined by physician documentation. Hospitals are paid by Medicare on a capitated basis according to the Acute Inpatient Prospective Payment System. The amount paid is determined by the base rate of the hospital multiplied by the relative weight (RW) of the Medicare Severity Diagnosis Related Group (MS-DRG).
The base rate is adjusted by the wage index of the hospital location. Hospitals that serve a high proportion of low income patients receive a Disproportionate Share Hospital adjustment. The base rate is not something hospitalists have control over.
The RW, however, is determined by the primary diagnosis (reason for admission) and whether or not there are complications or comorbidities (CCs) or major complications or comorbidities (MCCs). The more CCs and MCCs a patient has, the higher the severity of illness and expected increased resources needed to care for that patient.
Diagnoses are currently coded using ICD-10 used by the World Health Organization. The ICD-10 of the primary diagnosis is mapped to an MS-DRG. Many, but not all, MS-DRGs have increasing reimbursements for CCs and MCCs. Coders map the ICD-10 of the principal diagnosis along with any associated CCs or MCCs to the MS-DRG code. The relative weights for different DRGs can found on table 5 of the Medicare website (see reference 1).
Altered mental status versus delirium versus encephalopathy
As an example, let’s look at the difference in RW, LOS, and reimbursement in an otherwise identical patient based on documenting altered mental status (AMS), delirium, or encephalopathy. (see Table 1)

As one can see, RW, estimated LOS, and reimbursement would significantly increase for the patient with delirium (CC) or encephalopathy (MCC) versus AMS (no CC/MCC). A list of which diagnoses are considered CC’s versus MCC’s are on tables 6J and 6I, respectively, on the same Medicare website as table 5.
The difference between AMS, delirium, and encephalopathy
AMS is a sign/symptom complex similar to shortness of breath before an etiology is found. AMS can be the presenting symptom; when a specific etiology is found, however, a more specific diagnosis should be used such as delirium or encephalopathy.
Delirium, according to the DSM-5, is an acute change in the level of attention, cognition, or perception from baseline that developed over hours or days and tends to fluctuate during the course of a day. The change described is not better explained by a preexisting or evolving neurocognitive disorder and does not occur in the context of a severely reduced level of arousal, such as coma. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct consequence of a general medical condition, substance intoxication or withdrawal, exposure to a toxin, or more than one cause.
The National Institute of Neurological Diseases and Stroke defines encephalopathy as “any diffuse disease of the brain that alters brain function or structure. Encephalopathy may be caused by an infectious agent, metabolic or mitochondrial dysfunction, brain tumor or increased intracranial pressure, prolonged exposure to toxic elements, chronic progressive trauma, poor nutrition, or lack of oxygen or blood flow to the brain. The hallmark of encephalopathy is an altered mental state.”
It is confusing since there is a lot of overlap in the definitions of delirium and encephalopathy. One way to tease this out conceptually is noting that delirium is listed under mental, behavioral, and neurodevelopmental disorders, while encephalopathy appears under disorders of the nervous system. One can think of delirium as more of a “mental/psychiatric” diagnosis, while encephalopathy is caused by more “medical” causes.
If a patient who is normally not altered presents with confusion because of an infection or metabolic derangement, one can diagnose and document the cause of an acute encephalopathy. However, let’s say a patient is admitted in the morning with an infection, is started on treatment, but is not initially confused. If he/she later becomes confused at night, one could err conservatively and document delirium caused by sundowning.
Differentiating delirium and encephalopathy can be especially difficult in patients who have dementia with episodic confusion when they present with an infection and confusion. If the confusion is within what family members/caretakers say is “normal,” then one shouldn’t document encephalopathy. As a provider, one shouldn’t focus on all the rules and exceptions, just document as specifically and accurately as possible and the coders should take care of the rest.
Dr. Tong is an assistant professor of hospital medicine and an assistant director of the clinical research program at Emory University, Atlanta. Ms. Epps is director of clinical documentation improvement at Emory Healthcare, Atlanta.
References
1. “Acute Inpatient PPS.” Centers for Medicare and Medicaid Services. Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/index.html.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing, 2013.
3. “Details for title: FY 2018 Final Rule and Correction Notice Tables.” Centers for Medicare and Medicaid Services Accessed 2/17/18. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page-Items/FY2018-IPPS-Final-Rule-Tables.html.
4. “Encephalopathy Information Page.” National Institute of Neurologic Disorders and Stroke. Accessed on 2/17/18. https://www.ninds.nih.gov/Disorders/All-Disorders/Encephalopathy-Information-Page.
5. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. http://apps.who.int/iris/handle/10665/37958.
Positive change through advocacy
SHM seen as an ‘honest broker’ on Capitol Hill
Editor’s note: The “Legacies of Hospital Medicine” is a recurring opinion column submitted by some of the best and brightest hospitalists in the field, who have helped shape our specialty into what it is today. It is a series of articles that reflect on Hospital Medicine and its evolution over time, from a variety of unique and innovative perspectives.
Medical professional societies have many goals and serve numerous functions. Some of these include education and training, professional development, and shaping the perception of their specialty both in the medical world and the public arena. Advocacy and governmental affairs are also on that list. SHM is no exception to that rule, although we have taken what is clearly an unorthodox approach to those efforts and our strategy has resulted in an unusual amount of success for a society of our size and age.
As my contribution to the “Legacies” series, I am calling upon my 20-year history of participation in SHM’s advocacy and policy efforts to describe that approach, recount some of the history of our efforts, and to talk a bit about our current activities, goals, and strategies.
In 1999 the leadership of SHM decided to create the Public Policy Committee and to provide resources for what was, at the time, a single dedicated staff position to support the work of the committee. As nascent as our efforts were, the strategy for entering into the Washington fray was clear. We decided our priorities were first and foremost to educate our “targets” on exactly what a hospitalist was and on the increasing role hospitalists were playing in the American health care system.
The target audience was (and has remained) Congress, the Centers for Medicare and Medicaid Services, and the Medicare Payment Advisory Committee, which is the advisory board tasked to recommend to Congress how Medicare should spend its resources. The goal of this education was to establish our credibility and to advance the notion that we were the experts on care design for acutely ill patients in the inpatient setting. To this end, we decided that, when we met with folks on the Hill, we would ask for nothing for ourselves or our members, an approach that was virtually unheard of in the halls of Congress.
When responding to questions as to why we were not bringing “asks” to our Hill meetings, we would simply comment that we were only offering our services. And whenever they decided to try to make the health care system better and expertise was required regarding redesign of care in the hospital, they should think about us. Our stated goal: improve the delivery system and provide better and more cost-effective care for our patients.
We also exercised what I will call “issue discipline.” With very limited resources it was critical that we limit our issues to ones on which we could have significant impact, and had enough expertise to shape an effective argument. In addition, as we were going to be operating within a highly partisan system and representing members with varying political views, it was highly important that we did not approach issues in a way that resulted in our appearing politically motivated.
That approach took a lot of time and patience. But as a small and relatively under-resourced organization, we saw it as the only way that we could eventually have our message heard. So for many years the small contingent of SHM staff and the members of the Public Policy Committee (PPC) worked quietly to have our specialty and society recognized by policy makers in Washington and Baltimore (where CMS resides). But in the years just prior to and since the passage of the Affordable Care Act, when serious redesign of the American health care system began, our patience started to pay dividends and policy makers actually reached out for our input on issues related to the care of patients admitted to acute care hospitals. In addition, our advocacy efforts started to gain more traction.
Today, our specialty and society are well known by the key health care policymakers at CMS, MedPAC, and the Center for Medicare and Medicaid Innovation (CMMI), the latter of which was created by the ACA and whose role is to test the new alternative payment models (like accountable care organizations and bundled payments) to find out if they actually lead to better outcomes and lower costs. In the halls of Congress, especially with the health care staff for the committees of jurisdiction for federal health care legislation, our society is seen as an “honest broker” and as an organization committed not just to the issues that impact our members, but one that has the improvement of the entire health care system at the top of its priority list. We have been told that this perception gives us a voice that is much more influential than would be expected for a society of our age, size, and resources.
Along the way, the PPC has grown to a committee of 20 select members led by committee chair Joshua Lenchus, DO, RPh, SFHM. The committee is known to be among the most difficult committees to get on, and members commit to hours of work monthly to support our efforts. Our government relations staff in Philadelphia is still small at just three, but they are extremely bright and productive. Director Josh Boswell serves as their extremely capable leader. Josh Lapps and Ellen Boyer round out the incredibly strong team. Recently, my role evolved from being the long-term chairman of the PPC to one of volunteer staff, as the senior advisor for government relations. In this role I hope to support our full time staff, especially in our Washington-facing efforts.
The SHM staff has brought several systemic improvements to our advocacy work, including execution of several highly successful “Hill Days” and, more recently, the establishment of our “Grassroots Network” that allows a wider swath of our membership to get involved in the field. The Hill Days occur during years when the SHM Annual Conference is in Washington, and one of the days includes busing hundreds of hospitalists to Capitol Hill for meetings with their representatives to discuss our advocacy issues. Our next Hill Day will be at the 2019 annual conference, and we will be signing up volunteer members for this unique experience.
The success of our advocacy can be seen in several high-level “wins” over the last few years. Some of the more notable include:
- Successful application to CMS for a specialty code for Hospital Medicine (the C6 designation), so that performance data for hospitalists will be fairly compared with other hospitalists and not with our outpatient colleagues’ performance.
- Successful support of risk adjustment of readmission rates for safety net hospitals.
- Creation of a hardship exemption of Meaningful Use penalties for hospitalists, an initiative that saved our membership approximately $37 million of unfair penalties per year; this ensured a permanent exemption from these penalties within the Medicare Access and CHIP Reauthorization Act.
- Implementation of Advanced Care Planning CPT codes to encourage appropriate use of “end of life” discussions.
- Establishment of a Hospitalist Measure set with CMS.
- Repeal of the Independent Advisory Board earlier this year.
- Creation of the “Facility Based Option” to replace Merit-Based Incentive Payment System reporting for hospital-based physicians including hospitalists. This voluntary method to replace MIPS reporting was first suggested to CMS by SHM, was developed in partnership with CMS, and will be available in 2019.
SHM continues to take the lead on issues that impact the U.S. health care system and our patients. For several years we have been explaining to CMS and Congress the complete dysfunction of observation status, and its negative impact on elderly patients and hospitals. We have taken advantage of the expertise of several members of the PPC, including research currently being done by member Ann Sheehy, MD, SFHM, to publish two iterations of a white paper on the subject, which was widely read by Hill staff and resulted in Dr. Sheehy testifying on the subject to Congress.
More recently, SHM released a consensus statement on the use of opioids in the inpatient setting, along with a policy statement on opioid abuse, both of which have been widely lauded after being distributed to key committees of both chambers of Congress. Our recommendations will undoubtedly be addressed in an opioid bill which, at the time of this writing, is moving to a vote on the Hill.
As the U.S. health care system undergoes a necessary transformation to one in which value creation is tantamount, hospitalists – by the nature of our work – are in a propitious position to guide the development of better federal policy. We still must be judicious in the use of our limited resources and circumspect in our selection of issues. And we must jealously guard the reputation we have cultivated as a medical society that is looking out for the entire health care system and its patients, while we also support our members and their work.
We want to continue to be an organization that, rather than resisting change, is focused on driving positive change through better ideas and intelligent advocacy.
Dr. Greeno is senior advisor for government affairs and past president of the Society of Hospital Medicine.
SHM seen as an ‘honest broker’ on Capitol Hill
SHM seen as an ‘honest broker’ on Capitol Hill
Editor’s note: The “Legacies of Hospital Medicine” is a recurring opinion column submitted by some of the best and brightest hospitalists in the field, who have helped shape our specialty into what it is today. It is a series of articles that reflect on Hospital Medicine and its evolution over time, from a variety of unique and innovative perspectives.
Medical professional societies have many goals and serve numerous functions. Some of these include education and training, professional development, and shaping the perception of their specialty both in the medical world and the public arena. Advocacy and governmental affairs are also on that list. SHM is no exception to that rule, although we have taken what is clearly an unorthodox approach to those efforts and our strategy has resulted in an unusual amount of success for a society of our size and age.
As my contribution to the “Legacies” series, I am calling upon my 20-year history of participation in SHM’s advocacy and policy efforts to describe that approach, recount some of the history of our efforts, and to talk a bit about our current activities, goals, and strategies.
In 1999 the leadership of SHM decided to create the Public Policy Committee and to provide resources for what was, at the time, a single dedicated staff position to support the work of the committee. As nascent as our efforts were, the strategy for entering into the Washington fray was clear. We decided our priorities were first and foremost to educate our “targets” on exactly what a hospitalist was and on the increasing role hospitalists were playing in the American health care system.
The target audience was (and has remained) Congress, the Centers for Medicare and Medicaid Services, and the Medicare Payment Advisory Committee, which is the advisory board tasked to recommend to Congress how Medicare should spend its resources. The goal of this education was to establish our credibility and to advance the notion that we were the experts on care design for acutely ill patients in the inpatient setting. To this end, we decided that, when we met with folks on the Hill, we would ask for nothing for ourselves or our members, an approach that was virtually unheard of in the halls of Congress.
When responding to questions as to why we were not bringing “asks” to our Hill meetings, we would simply comment that we were only offering our services. And whenever they decided to try to make the health care system better and expertise was required regarding redesign of care in the hospital, they should think about us. Our stated goal: improve the delivery system and provide better and more cost-effective care for our patients.
We also exercised what I will call “issue discipline.” With very limited resources it was critical that we limit our issues to ones on which we could have significant impact, and had enough expertise to shape an effective argument. In addition, as we were going to be operating within a highly partisan system and representing members with varying political views, it was highly important that we did not approach issues in a way that resulted in our appearing politically motivated.
That approach took a lot of time and patience. But as a small and relatively under-resourced organization, we saw it as the only way that we could eventually have our message heard. So for many years the small contingent of SHM staff and the members of the Public Policy Committee (PPC) worked quietly to have our specialty and society recognized by policy makers in Washington and Baltimore (where CMS resides). But in the years just prior to and since the passage of the Affordable Care Act, when serious redesign of the American health care system began, our patience started to pay dividends and policy makers actually reached out for our input on issues related to the care of patients admitted to acute care hospitals. In addition, our advocacy efforts started to gain more traction.
Today, our specialty and society are well known by the key health care policymakers at CMS, MedPAC, and the Center for Medicare and Medicaid Innovation (CMMI), the latter of which was created by the ACA and whose role is to test the new alternative payment models (like accountable care organizations and bundled payments) to find out if they actually lead to better outcomes and lower costs. In the halls of Congress, especially with the health care staff for the committees of jurisdiction for federal health care legislation, our society is seen as an “honest broker” and as an organization committed not just to the issues that impact our members, but one that has the improvement of the entire health care system at the top of its priority list. We have been told that this perception gives us a voice that is much more influential than would be expected for a society of our age, size, and resources.
Along the way, the PPC has grown to a committee of 20 select members led by committee chair Joshua Lenchus, DO, RPh, SFHM. The committee is known to be among the most difficult committees to get on, and members commit to hours of work monthly to support our efforts. Our government relations staff in Philadelphia is still small at just three, but they are extremely bright and productive. Director Josh Boswell serves as their extremely capable leader. Josh Lapps and Ellen Boyer round out the incredibly strong team. Recently, my role evolved from being the long-term chairman of the PPC to one of volunteer staff, as the senior advisor for government relations. In this role I hope to support our full time staff, especially in our Washington-facing efforts.
The SHM staff has brought several systemic improvements to our advocacy work, including execution of several highly successful “Hill Days” and, more recently, the establishment of our “Grassroots Network” that allows a wider swath of our membership to get involved in the field. The Hill Days occur during years when the SHM Annual Conference is in Washington, and one of the days includes busing hundreds of hospitalists to Capitol Hill for meetings with their representatives to discuss our advocacy issues. Our next Hill Day will be at the 2019 annual conference, and we will be signing up volunteer members for this unique experience.
The success of our advocacy can be seen in several high-level “wins” over the last few years. Some of the more notable include:
- Successful application to CMS for a specialty code for Hospital Medicine (the C6 designation), so that performance data for hospitalists will be fairly compared with other hospitalists and not with our outpatient colleagues’ performance.
- Successful support of risk adjustment of readmission rates for safety net hospitals.
- Creation of a hardship exemption of Meaningful Use penalties for hospitalists, an initiative that saved our membership approximately $37 million of unfair penalties per year; this ensured a permanent exemption from these penalties within the Medicare Access and CHIP Reauthorization Act.
- Implementation of Advanced Care Planning CPT codes to encourage appropriate use of “end of life” discussions.
- Establishment of a Hospitalist Measure set with CMS.
- Repeal of the Independent Advisory Board earlier this year.
- Creation of the “Facility Based Option” to replace Merit-Based Incentive Payment System reporting for hospital-based physicians including hospitalists. This voluntary method to replace MIPS reporting was first suggested to CMS by SHM, was developed in partnership with CMS, and will be available in 2019.
SHM continues to take the lead on issues that impact the U.S. health care system and our patients. For several years we have been explaining to CMS and Congress the complete dysfunction of observation status, and its negative impact on elderly patients and hospitals. We have taken advantage of the expertise of several members of the PPC, including research currently being done by member Ann Sheehy, MD, SFHM, to publish two iterations of a white paper on the subject, which was widely read by Hill staff and resulted in Dr. Sheehy testifying on the subject to Congress.
More recently, SHM released a consensus statement on the use of opioids in the inpatient setting, along with a policy statement on opioid abuse, both of which have been widely lauded after being distributed to key committees of both chambers of Congress. Our recommendations will undoubtedly be addressed in an opioid bill which, at the time of this writing, is moving to a vote on the Hill.
As the U.S. health care system undergoes a necessary transformation to one in which value creation is tantamount, hospitalists – by the nature of our work – are in a propitious position to guide the development of better federal policy. We still must be judicious in the use of our limited resources and circumspect in our selection of issues. And we must jealously guard the reputation we have cultivated as a medical society that is looking out for the entire health care system and its patients, while we also support our members and their work.
We want to continue to be an organization that, rather than resisting change, is focused on driving positive change through better ideas and intelligent advocacy.
Dr. Greeno is senior advisor for government affairs and past president of the Society of Hospital Medicine.
Editor’s note: The “Legacies of Hospital Medicine” is a recurring opinion column submitted by some of the best and brightest hospitalists in the field, who have helped shape our specialty into what it is today. It is a series of articles that reflect on Hospital Medicine and its evolution over time, from a variety of unique and innovative perspectives.
Medical professional societies have many goals and serve numerous functions. Some of these include education and training, professional development, and shaping the perception of their specialty both in the medical world and the public arena. Advocacy and governmental affairs are also on that list. SHM is no exception to that rule, although we have taken what is clearly an unorthodox approach to those efforts and our strategy has resulted in an unusual amount of success for a society of our size and age.
As my contribution to the “Legacies” series, I am calling upon my 20-year history of participation in SHM’s advocacy and policy efforts to describe that approach, recount some of the history of our efforts, and to talk a bit about our current activities, goals, and strategies.
In 1999 the leadership of SHM decided to create the Public Policy Committee and to provide resources for what was, at the time, a single dedicated staff position to support the work of the committee. As nascent as our efforts were, the strategy for entering into the Washington fray was clear. We decided our priorities were first and foremost to educate our “targets” on exactly what a hospitalist was and on the increasing role hospitalists were playing in the American health care system.
The target audience was (and has remained) Congress, the Centers for Medicare and Medicaid Services, and the Medicare Payment Advisory Committee, which is the advisory board tasked to recommend to Congress how Medicare should spend its resources. The goal of this education was to establish our credibility and to advance the notion that we were the experts on care design for acutely ill patients in the inpatient setting. To this end, we decided that, when we met with folks on the Hill, we would ask for nothing for ourselves or our members, an approach that was virtually unheard of in the halls of Congress.
When responding to questions as to why we were not bringing “asks” to our Hill meetings, we would simply comment that we were only offering our services. And whenever they decided to try to make the health care system better and expertise was required regarding redesign of care in the hospital, they should think about us. Our stated goal: improve the delivery system and provide better and more cost-effective care for our patients.
We also exercised what I will call “issue discipline.” With very limited resources it was critical that we limit our issues to ones on which we could have significant impact, and had enough expertise to shape an effective argument. In addition, as we were going to be operating within a highly partisan system and representing members with varying political views, it was highly important that we did not approach issues in a way that resulted in our appearing politically motivated.
That approach took a lot of time and patience. But as a small and relatively under-resourced organization, we saw it as the only way that we could eventually have our message heard. So for many years the small contingent of SHM staff and the members of the Public Policy Committee (PPC) worked quietly to have our specialty and society recognized by policy makers in Washington and Baltimore (where CMS resides). But in the years just prior to and since the passage of the Affordable Care Act, when serious redesign of the American health care system began, our patience started to pay dividends and policy makers actually reached out for our input on issues related to the care of patients admitted to acute care hospitals. In addition, our advocacy efforts started to gain more traction.
Today, our specialty and society are well known by the key health care policymakers at CMS, MedPAC, and the Center for Medicare and Medicaid Innovation (CMMI), the latter of which was created by the ACA and whose role is to test the new alternative payment models (like accountable care organizations and bundled payments) to find out if they actually lead to better outcomes and lower costs. In the halls of Congress, especially with the health care staff for the committees of jurisdiction for federal health care legislation, our society is seen as an “honest broker” and as an organization committed not just to the issues that impact our members, but one that has the improvement of the entire health care system at the top of its priority list. We have been told that this perception gives us a voice that is much more influential than would be expected for a society of our age, size, and resources.
Along the way, the PPC has grown to a committee of 20 select members led by committee chair Joshua Lenchus, DO, RPh, SFHM. The committee is known to be among the most difficult committees to get on, and members commit to hours of work monthly to support our efforts. Our government relations staff in Philadelphia is still small at just three, but they are extremely bright and productive. Director Josh Boswell serves as their extremely capable leader. Josh Lapps and Ellen Boyer round out the incredibly strong team. Recently, my role evolved from being the long-term chairman of the PPC to one of volunteer staff, as the senior advisor for government relations. In this role I hope to support our full time staff, especially in our Washington-facing efforts.
The SHM staff has brought several systemic improvements to our advocacy work, including execution of several highly successful “Hill Days” and, more recently, the establishment of our “Grassroots Network” that allows a wider swath of our membership to get involved in the field. The Hill Days occur during years when the SHM Annual Conference is in Washington, and one of the days includes busing hundreds of hospitalists to Capitol Hill for meetings with their representatives to discuss our advocacy issues. Our next Hill Day will be at the 2019 annual conference, and we will be signing up volunteer members for this unique experience.
The success of our advocacy can be seen in several high-level “wins” over the last few years. Some of the more notable include:
- Successful application to CMS for a specialty code for Hospital Medicine (the C6 designation), so that performance data for hospitalists will be fairly compared with other hospitalists and not with our outpatient colleagues’ performance.
- Successful support of risk adjustment of readmission rates for safety net hospitals.
- Creation of a hardship exemption of Meaningful Use penalties for hospitalists, an initiative that saved our membership approximately $37 million of unfair penalties per year; this ensured a permanent exemption from these penalties within the Medicare Access and CHIP Reauthorization Act.
- Implementation of Advanced Care Planning CPT codes to encourage appropriate use of “end of life” discussions.
- Establishment of a Hospitalist Measure set with CMS.
- Repeal of the Independent Advisory Board earlier this year.
- Creation of the “Facility Based Option” to replace Merit-Based Incentive Payment System reporting for hospital-based physicians including hospitalists. This voluntary method to replace MIPS reporting was first suggested to CMS by SHM, was developed in partnership with CMS, and will be available in 2019.
SHM continues to take the lead on issues that impact the U.S. health care system and our patients. For several years we have been explaining to CMS and Congress the complete dysfunction of observation status, and its negative impact on elderly patients and hospitals. We have taken advantage of the expertise of several members of the PPC, including research currently being done by member Ann Sheehy, MD, SFHM, to publish two iterations of a white paper on the subject, which was widely read by Hill staff and resulted in Dr. Sheehy testifying on the subject to Congress.
More recently, SHM released a consensus statement on the use of opioids in the inpatient setting, along with a policy statement on opioid abuse, both of which have been widely lauded after being distributed to key committees of both chambers of Congress. Our recommendations will undoubtedly be addressed in an opioid bill which, at the time of this writing, is moving to a vote on the Hill.
As the U.S. health care system undergoes a necessary transformation to one in which value creation is tantamount, hospitalists – by the nature of our work – are in a propitious position to guide the development of better federal policy. We still must be judicious in the use of our limited resources and circumspect in our selection of issues. And we must jealously guard the reputation we have cultivated as a medical society that is looking out for the entire health care system and its patients, while we also support our members and their work.
We want to continue to be an organization that, rather than resisting change, is focused on driving positive change through better ideas and intelligent advocacy.
Dr. Greeno is senior advisor for government affairs and past president of the Society of Hospital Medicine.
PE is rare in patients presenting to the ED with syncope
Clinical question: What is the prevalence of pulmonary embolism (PE) in patients presenting to the ED with syncope?
Study design: Retrospective, observational study.
Setting: Canada, Denmark, Italy, and the United States, from January 2010 to September 2016.
Synopsis: Longitudinal administrative databases were used to identify patients with ICD codes for syncope at discharge from the ED or hospital. Those with an ICD code for PE were included to calculate the prevalence of PE in this population (primary outcome).
The prevalence of PE in all patients ranged from 0.06% (95% confidence interval, 0.05%-0.06%) to 0.55% (95% CI, 0.50%-0.61%); and in hospitalized patients from 0.15% (95% CI, 0.14%-0.16%) to 2.10% (95% CI, 1.84%-2.39%). This is a much lower than the estimated 17.3% prevalence of PE in patients presenting with syncope estimated by the PESIT study published in the New England Journal of Medicine in 2016. Further definitive research is needed to better characterize prevalence rates.
Limitations of this study include the potential for information bias: The inclusion criteria of patients coded for syncope at discharge likely omits some patients who initially presented with syncope but were coded for a primary diagnosis that caused syncope.
Bottom line: PE in patients presenting to the ED with syncope may be rare.
Citation: Costantino G et al. Prevalence of pulmonary embolism in patients with syncope. JAMA. 2018;178(3):356-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What is the prevalence of pulmonary embolism (PE) in patients presenting to the ED with syncope?
Study design: Retrospective, observational study.
Setting: Canada, Denmark, Italy, and the United States, from January 2010 to September 2016.
Synopsis: Longitudinal administrative databases were used to identify patients with ICD codes for syncope at discharge from the ED or hospital. Those with an ICD code for PE were included to calculate the prevalence of PE in this population (primary outcome).
The prevalence of PE in all patients ranged from 0.06% (95% confidence interval, 0.05%-0.06%) to 0.55% (95% CI, 0.50%-0.61%); and in hospitalized patients from 0.15% (95% CI, 0.14%-0.16%) to 2.10% (95% CI, 1.84%-2.39%). This is a much lower than the estimated 17.3% prevalence of PE in patients presenting with syncope estimated by the PESIT study published in the New England Journal of Medicine in 2016. Further definitive research is needed to better characterize prevalence rates.
Limitations of this study include the potential for information bias: The inclusion criteria of patients coded for syncope at discharge likely omits some patients who initially presented with syncope but were coded for a primary diagnosis that caused syncope.
Bottom line: PE in patients presenting to the ED with syncope may be rare.
Citation: Costantino G et al. Prevalence of pulmonary embolism in patients with syncope. JAMA. 2018;178(3):356-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: What is the prevalence of pulmonary embolism (PE) in patients presenting to the ED with syncope?
Study design: Retrospective, observational study.
Setting: Canada, Denmark, Italy, and the United States, from January 2010 to September 2016.
Synopsis: Longitudinal administrative databases were used to identify patients with ICD codes for syncope at discharge from the ED or hospital. Those with an ICD code for PE were included to calculate the prevalence of PE in this population (primary outcome).
The prevalence of PE in all patients ranged from 0.06% (95% confidence interval, 0.05%-0.06%) to 0.55% (95% CI, 0.50%-0.61%); and in hospitalized patients from 0.15% (95% CI, 0.14%-0.16%) to 2.10% (95% CI, 1.84%-2.39%). This is a much lower than the estimated 17.3% prevalence of PE in patients presenting with syncope estimated by the PESIT study published in the New England Journal of Medicine in 2016. Further definitive research is needed to better characterize prevalence rates.
Limitations of this study include the potential for information bias: The inclusion criteria of patients coded for syncope at discharge likely omits some patients who initially presented with syncope but were coded for a primary diagnosis that caused syncope.
Bottom line: PE in patients presenting to the ED with syncope may be rare.
Citation: Costantino G et al. Prevalence of pulmonary embolism in patients with syncope. JAMA. 2018;178(3):356-62.
Dr. Roy is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Multifaceted pharmacist intervention may reduce postdischarge ED visits and readmissions
Clinical question: Can a multifaceted intervention by a clinical pharmacist reduce the rate of ED visits and readmission over the subsequent 180 days?
Background: The period following an inpatient admission contains many potential risks for patients, among them the risk for adverse drug events. Approximately 45% of readmissions from adverse drug reactions are thought to be avoidable.
Study design: Multicentered, single-blinded, randomized, control trial, from September 2013 to April 2015.
Setting: Four acute inpatient hospitals in Denmark.
Synopsis: 1,467 adult patients being admitted for an acute hospitalization on a minimum of five medications were randomized to receive usual care, a basic intervention (medication review by a clinical pharmacist), or an extended intervention (medication review, three motivational interviews, and follow-up with the primary care physician, pharmacy and, if appropriate, nursing home by a clinical pharmacist). The primary endpoints were readmission within 30 days or 180 days, ED visits within 180 days, and a composite endpoint of readmission or ED visit within 180 days post discharge. For these endpoints, the basic intervention group had no statistically significant difference from the usual-care group. The extended intervention group had significantly lower rates of readmission within 30 days and 180 days, as well as the primary composite endpoint compared to the usual-care group (P less than .05 for all comparisons). For the extended intervention, the number needed to treat for the main composite endpoint was 12.
Bottom line: For patients admitted to the hospital, an extended intervention by a clinical pharmacist resulted in a significant reduction in readmissions.
Citation: Ravn-Nielsen LV et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission. JAMA Intern Med. 2018;178(3):375-82.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Can a multifaceted intervention by a clinical pharmacist reduce the rate of ED visits and readmission over the subsequent 180 days?
Background: The period following an inpatient admission contains many potential risks for patients, among them the risk for adverse drug events. Approximately 45% of readmissions from adverse drug reactions are thought to be avoidable.
Study design: Multicentered, single-blinded, randomized, control trial, from September 2013 to April 2015.
Setting: Four acute inpatient hospitals in Denmark.
Synopsis: 1,467 adult patients being admitted for an acute hospitalization on a minimum of five medications were randomized to receive usual care, a basic intervention (medication review by a clinical pharmacist), or an extended intervention (medication review, three motivational interviews, and follow-up with the primary care physician, pharmacy and, if appropriate, nursing home by a clinical pharmacist). The primary endpoints were readmission within 30 days or 180 days, ED visits within 180 days, and a composite endpoint of readmission or ED visit within 180 days post discharge. For these endpoints, the basic intervention group had no statistically significant difference from the usual-care group. The extended intervention group had significantly lower rates of readmission within 30 days and 180 days, as well as the primary composite endpoint compared to the usual-care group (P less than .05 for all comparisons). For the extended intervention, the number needed to treat for the main composite endpoint was 12.
Bottom line: For patients admitted to the hospital, an extended intervention by a clinical pharmacist resulted in a significant reduction in readmissions.
Citation: Ravn-Nielsen LV et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission. JAMA Intern Med. 2018;178(3):375-82.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Can a multifaceted intervention by a clinical pharmacist reduce the rate of ED visits and readmission over the subsequent 180 days?
Background: The period following an inpatient admission contains many potential risks for patients, among them the risk for adverse drug events. Approximately 45% of readmissions from adverse drug reactions are thought to be avoidable.
Study design: Multicentered, single-blinded, randomized, control trial, from September 2013 to April 2015.
Setting: Four acute inpatient hospitals in Denmark.
Synopsis: 1,467 adult patients being admitted for an acute hospitalization on a minimum of five medications were randomized to receive usual care, a basic intervention (medication review by a clinical pharmacist), or an extended intervention (medication review, three motivational interviews, and follow-up with the primary care physician, pharmacy and, if appropriate, nursing home by a clinical pharmacist). The primary endpoints were readmission within 30 days or 180 days, ED visits within 180 days, and a composite endpoint of readmission or ED visit within 180 days post discharge. For these endpoints, the basic intervention group had no statistically significant difference from the usual-care group. The extended intervention group had significantly lower rates of readmission within 30 days and 180 days, as well as the primary composite endpoint compared to the usual-care group (P less than .05 for all comparisons). For the extended intervention, the number needed to treat for the main composite endpoint was 12.
Bottom line: For patients admitted to the hospital, an extended intervention by a clinical pharmacist resulted in a significant reduction in readmissions.
Citation: Ravn-Nielsen LV et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission. JAMA Intern Med. 2018;178(3):375-82.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Opioid use has not declined meaningfully
Opioid use has not significantly declined over the past 10 years despite efforts to educate prescribers about the risks of opioid abuse, with over half of disabled Medicare beneficiaries using opioids each year, according to a recent retrospective cohort study published in the BMJ.
“We found very high prevalence of opioid use and opioid doses in disabled Medicare beneficiaries, most likely reflecting the high burden of illness in this population,” Molly M. Jeffery, PhD, from the Mayo Clinic, Rochester, Minn., and her associates wrote in their study.
The investigators evaluated pharmaceutical and medical claims data from 48 million individuals who were commercially insured or were Medicare Advantage recipients (both those eligible because they were older than 65 years and those under 65 years old who still were eligible because of disability). The researchers found that 52% of disabled Medicare patients, 26% of aged Medicare patients, and 14% of commercially insured patients used opioids annually within the study period.
In the commercially insured group, there was little fluctuation in patient opioid prevalence by quarter, with an average daily dose of 17 mg morphine equivalents (MME) during 2011-2016; 6% of patients used opioids quarterly at the beginning and end of the study. There was an increase of quarterly opioid prevalence in the aged Medicare group from 11% to 14% at the beginning and end of the study period. Average daily dose also increased during this period for the aged Medicare group from 18 MME in 2011 to 20 MME in 2016.
Researchers said commercial beneficiaries between 45 years and 54 years old had the highest prevalence of opioid use. The disabled Medicare group saw the greatest increase among groups in opioid prevalence and average daily dose, with a 26% prevalence in 2007 and 53 MME average daily dose, which increased to a prevalence of 39% and an average daily dose of 56 MME in 2016.
“Doctors and patients should consider whether long-term opioid use is improving the patient’s ability to function and, if not, should consider other treatments either as an addition or replacement to opioid use,” Dr. Jeffery and her colleagues wrote. “Evidence-based approaches are needed to improve both the safety of opioid use and patient outcomes including pain management and ability to function.”
The researchers noted limitations in the study, such as not including people with Medicaid, fee-for-service Medicare, or the uninsured. In addition, the data reviewed did not indicate the prevalence of chronic pain or pain duration in the patient population studied, they said.
The authors reported no relevant financial disclosures.
SOURCE: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
Opioid use has not significantly declined over the past 10 years despite efforts to educate prescribers about the risks of opioid abuse, with over half of disabled Medicare beneficiaries using opioids each year, according to a recent retrospective cohort study published in the BMJ.
“We found very high prevalence of opioid use and opioid doses in disabled Medicare beneficiaries, most likely reflecting the high burden of illness in this population,” Molly M. Jeffery, PhD, from the Mayo Clinic, Rochester, Minn., and her associates wrote in their study.
The investigators evaluated pharmaceutical and medical claims data from 48 million individuals who were commercially insured or were Medicare Advantage recipients (both those eligible because they were older than 65 years and those under 65 years old who still were eligible because of disability). The researchers found that 52% of disabled Medicare patients, 26% of aged Medicare patients, and 14% of commercially insured patients used opioids annually within the study period.
In the commercially insured group, there was little fluctuation in patient opioid prevalence by quarter, with an average daily dose of 17 mg morphine equivalents (MME) during 2011-2016; 6% of patients used opioids quarterly at the beginning and end of the study. There was an increase of quarterly opioid prevalence in the aged Medicare group from 11% to 14% at the beginning and end of the study period. Average daily dose also increased during this period for the aged Medicare group from 18 MME in 2011 to 20 MME in 2016.
Researchers said commercial beneficiaries between 45 years and 54 years old had the highest prevalence of opioid use. The disabled Medicare group saw the greatest increase among groups in opioid prevalence and average daily dose, with a 26% prevalence in 2007 and 53 MME average daily dose, which increased to a prevalence of 39% and an average daily dose of 56 MME in 2016.
“Doctors and patients should consider whether long-term opioid use is improving the patient’s ability to function and, if not, should consider other treatments either as an addition or replacement to opioid use,” Dr. Jeffery and her colleagues wrote. “Evidence-based approaches are needed to improve both the safety of opioid use and patient outcomes including pain management and ability to function.”
The researchers noted limitations in the study, such as not including people with Medicaid, fee-for-service Medicare, or the uninsured. In addition, the data reviewed did not indicate the prevalence of chronic pain or pain duration in the patient population studied, they said.
The authors reported no relevant financial disclosures.
SOURCE: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
Opioid use has not significantly declined over the past 10 years despite efforts to educate prescribers about the risks of opioid abuse, with over half of disabled Medicare beneficiaries using opioids each year, according to a recent retrospective cohort study published in the BMJ.
“We found very high prevalence of opioid use and opioid doses in disabled Medicare beneficiaries, most likely reflecting the high burden of illness in this population,” Molly M. Jeffery, PhD, from the Mayo Clinic, Rochester, Minn., and her associates wrote in their study.
The investigators evaluated pharmaceutical and medical claims data from 48 million individuals who were commercially insured or were Medicare Advantage recipients (both those eligible because they were older than 65 years and those under 65 years old who still were eligible because of disability). The researchers found that 52% of disabled Medicare patients, 26% of aged Medicare patients, and 14% of commercially insured patients used opioids annually within the study period.
In the commercially insured group, there was little fluctuation in patient opioid prevalence by quarter, with an average daily dose of 17 mg morphine equivalents (MME) during 2011-2016; 6% of patients used opioids quarterly at the beginning and end of the study. There was an increase of quarterly opioid prevalence in the aged Medicare group from 11% to 14% at the beginning and end of the study period. Average daily dose also increased during this period for the aged Medicare group from 18 MME in 2011 to 20 MME in 2016.
Researchers said commercial beneficiaries between 45 years and 54 years old had the highest prevalence of opioid use. The disabled Medicare group saw the greatest increase among groups in opioid prevalence and average daily dose, with a 26% prevalence in 2007 and 53 MME average daily dose, which increased to a prevalence of 39% and an average daily dose of 56 MME in 2016.
“Doctors and patients should consider whether long-term opioid use is improving the patient’s ability to function and, if not, should consider other treatments either as an addition or replacement to opioid use,” Dr. Jeffery and her colleagues wrote. “Evidence-based approaches are needed to improve both the safety of opioid use and patient outcomes including pain management and ability to function.”
The researchers noted limitations in the study, such as not including people with Medicaid, fee-for-service Medicare, or the uninsured. In addition, the data reviewed did not indicate the prevalence of chronic pain or pain duration in the patient population studied, they said.
The authors reported no relevant financial disclosures.
SOURCE: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
FROM THE BMJ
Key clinical point: The highest prevalence was seen among disabled patients with Medicare Advantage.
Major finding: Of those studied, annual prevalence of opioid use was 14% for commercial beneficiaries, 26% for aged Medicare beneficiaries, and 52% for disabled Medicare beneficiaries.
Study details: An observational cohort study of claims data from 48 million people who had commercial insurance or Medicare Advantage between January 2007 and December 2016.
Disclosures: The authors reported no relevant financial disclosures.
Source: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
ED key to reducing pediatric asthma x-rays
ATLANTA – but accomplishing this goal takes more than a new clinical practice guideline, according to a quality improvement team at the Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn.
The team eventually reduced the chest x-ray rate for pediatric asthma exacerbations from 30% to 15% without increasing 3-day all-cause readmissions, but it took some sleuthing in the ED and good relations with staff. “We were way out in left field when we started this. Working in silos is never ideal,” said senior project member David Johnson, MD, a pediatric hospitalist and assistant professor of pediatrics at Vanderbilt.
It’s been known for a while that chest x-rays are almost always a waste of time and money for asthma exacerbations, and national guidelines recommend against them. X-rays don’t improve outcomes and needlessly expose children to radiation.
In 2014, some of the providers at Vanderbilt, which has about 1,700 asthma encounters a year, realized that the institution’s 30% x-ray rate was a problem. The quality improvement team hoped a new guideline would address the issue, but that didn’t happen. “We roll out clinical practice guidelines” from on high, “and think people will magically change their behavior,” but they don’t, Dr. Johnson said at the annual Pediatric Hospital Medicine meeting.
The guideline was not being fully implemented. So the team asked the ED what was the standard procedure for a child presenting with asthma exacerbation. It turned out that the ED had a dyspnea order set that the team ”had no idea existed.” Chest x-rays were at the top of the list; next came blood gases, ventilation-perfusion scans, and leg Dopplers, he said.
The investigators tried to get rid of the whole order set but were unsuccessful. The ED department did, however, let the team eliminate chest x-rays in the default order set in July 2015. That helped, but more changes were needed.
The next conversation was to figure out why x-rays were being ordered in the first place. ED staff said they were worried about missing something, especially pneumonia. They also thought they were helping hospitalists by getting x-rays before sending kids to the ward even though, in reality, it didn’t matter whether x-rays were done a few hours later on the floor. ED providers also said that ill-appearing children often got better after a few hours but were kept back from discharge because x-ray results were still pending and that sometimes these results revealed problems at 3 a.m. that had nothing to do with why the patients were in the ED but still required a work-up.
This discussion opened a door. The ED staff didn’t want to order unnecessary x-rays, either. That led to talks about letting kids declare themselves a bit before x-rays were ordered. ED staff liked the idea, so the guidelines were updated in early 2016 to say that chest x-rays should only be ordered if there is persistent severe respiratory distress with hypoxia, there are focal findings that don’t improve after 12 hours of treatment, or there were concerns for pneumomediastinum or collapsed lung. The updated guidelines were posted in work areas and brought home by resident education. A reminder was added to the electronic medical record system that popped up when someone tried to order a chest x-ray for an child with asthma.
It worked. Chest x-ray rates in asthma fell to 15%, and have remained there since.
“We gave them permission to take their foot off the throttle and wait a little bit, and we don’t have more kids bouncing back from reduced x-rays.” The approach is “probably generalizable everywhere,” Dr. Johnson said.
It was essential that an ED fellow, Caroline Watnick, MD, led the effort and eventually bridged the gap between hospitalists and ED providers. In the end, “the change wasn’t something from the outside,” Dr. Johnson said.
There was no industry funding, and Dr. Johnson didn’t have any disclosures. The Pediatric Hospital Medicine meeting is sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
ATLANTA – but accomplishing this goal takes more than a new clinical practice guideline, according to a quality improvement team at the Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn.
The team eventually reduced the chest x-ray rate for pediatric asthma exacerbations from 30% to 15% without increasing 3-day all-cause readmissions, but it took some sleuthing in the ED and good relations with staff. “We were way out in left field when we started this. Working in silos is never ideal,” said senior project member David Johnson, MD, a pediatric hospitalist and assistant professor of pediatrics at Vanderbilt.
It’s been known for a while that chest x-rays are almost always a waste of time and money for asthma exacerbations, and national guidelines recommend against them. X-rays don’t improve outcomes and needlessly expose children to radiation.
In 2014, some of the providers at Vanderbilt, which has about 1,700 asthma encounters a year, realized that the institution’s 30% x-ray rate was a problem. The quality improvement team hoped a new guideline would address the issue, but that didn’t happen. “We roll out clinical practice guidelines” from on high, “and think people will magically change their behavior,” but they don’t, Dr. Johnson said at the annual Pediatric Hospital Medicine meeting.
The guideline was not being fully implemented. So the team asked the ED what was the standard procedure for a child presenting with asthma exacerbation. It turned out that the ED had a dyspnea order set that the team ”had no idea existed.” Chest x-rays were at the top of the list; next came blood gases, ventilation-perfusion scans, and leg Dopplers, he said.
The investigators tried to get rid of the whole order set but were unsuccessful. The ED department did, however, let the team eliminate chest x-rays in the default order set in July 2015. That helped, but more changes were needed.
The next conversation was to figure out why x-rays were being ordered in the first place. ED staff said they were worried about missing something, especially pneumonia. They also thought they were helping hospitalists by getting x-rays before sending kids to the ward even though, in reality, it didn’t matter whether x-rays were done a few hours later on the floor. ED providers also said that ill-appearing children often got better after a few hours but were kept back from discharge because x-ray results were still pending and that sometimes these results revealed problems at 3 a.m. that had nothing to do with why the patients were in the ED but still required a work-up.
This discussion opened a door. The ED staff didn’t want to order unnecessary x-rays, either. That led to talks about letting kids declare themselves a bit before x-rays were ordered. ED staff liked the idea, so the guidelines were updated in early 2016 to say that chest x-rays should only be ordered if there is persistent severe respiratory distress with hypoxia, there are focal findings that don’t improve after 12 hours of treatment, or there were concerns for pneumomediastinum or collapsed lung. The updated guidelines were posted in work areas and brought home by resident education. A reminder was added to the electronic medical record system that popped up when someone tried to order a chest x-ray for an child with asthma.
It worked. Chest x-ray rates in asthma fell to 15%, and have remained there since.
“We gave them permission to take their foot off the throttle and wait a little bit, and we don’t have more kids bouncing back from reduced x-rays.” The approach is “probably generalizable everywhere,” Dr. Johnson said.
It was essential that an ED fellow, Caroline Watnick, MD, led the effort and eventually bridged the gap between hospitalists and ED providers. In the end, “the change wasn’t something from the outside,” Dr. Johnson said.
There was no industry funding, and Dr. Johnson didn’t have any disclosures. The Pediatric Hospital Medicine meeting is sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
ATLANTA – but accomplishing this goal takes more than a new clinical practice guideline, according to a quality improvement team at the Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn.
The team eventually reduced the chest x-ray rate for pediatric asthma exacerbations from 30% to 15% without increasing 3-day all-cause readmissions, but it took some sleuthing in the ED and good relations with staff. “We were way out in left field when we started this. Working in silos is never ideal,” said senior project member David Johnson, MD, a pediatric hospitalist and assistant professor of pediatrics at Vanderbilt.
It’s been known for a while that chest x-rays are almost always a waste of time and money for asthma exacerbations, and national guidelines recommend against them. X-rays don’t improve outcomes and needlessly expose children to radiation.
In 2014, some of the providers at Vanderbilt, which has about 1,700 asthma encounters a year, realized that the institution’s 30% x-ray rate was a problem. The quality improvement team hoped a new guideline would address the issue, but that didn’t happen. “We roll out clinical practice guidelines” from on high, “and think people will magically change their behavior,” but they don’t, Dr. Johnson said at the annual Pediatric Hospital Medicine meeting.
The guideline was not being fully implemented. So the team asked the ED what was the standard procedure for a child presenting with asthma exacerbation. It turned out that the ED had a dyspnea order set that the team ”had no idea existed.” Chest x-rays were at the top of the list; next came blood gases, ventilation-perfusion scans, and leg Dopplers, he said.
The investigators tried to get rid of the whole order set but were unsuccessful. The ED department did, however, let the team eliminate chest x-rays in the default order set in July 2015. That helped, but more changes were needed.
The next conversation was to figure out why x-rays were being ordered in the first place. ED staff said they were worried about missing something, especially pneumonia. They also thought they were helping hospitalists by getting x-rays before sending kids to the ward even though, in reality, it didn’t matter whether x-rays were done a few hours later on the floor. ED providers also said that ill-appearing children often got better after a few hours but were kept back from discharge because x-ray results were still pending and that sometimes these results revealed problems at 3 a.m. that had nothing to do with why the patients were in the ED but still required a work-up.
This discussion opened a door. The ED staff didn’t want to order unnecessary x-rays, either. That led to talks about letting kids declare themselves a bit before x-rays were ordered. ED staff liked the idea, so the guidelines were updated in early 2016 to say that chest x-rays should only be ordered if there is persistent severe respiratory distress with hypoxia, there are focal findings that don’t improve after 12 hours of treatment, or there were concerns for pneumomediastinum or collapsed lung. The updated guidelines were posted in work areas and brought home by resident education. A reminder was added to the electronic medical record system that popped up when someone tried to order a chest x-ray for an child with asthma.
It worked. Chest x-ray rates in asthma fell to 15%, and have remained there since.
“We gave them permission to take their foot off the throttle and wait a little bit, and we don’t have more kids bouncing back from reduced x-rays.” The approach is “probably generalizable everywhere,” Dr. Johnson said.
It was essential that an ED fellow, Caroline Watnick, MD, led the effort and eventually bridged the gap between hospitalists and ED providers. In the end, “the change wasn’t something from the outside,” Dr. Johnson said.
There was no industry funding, and Dr. Johnson didn’t have any disclosures. The Pediatric Hospital Medicine meeting is sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
REPORTING FROM PHM 2018
Key clinical point: Reduction of chest x-rays for routine pediatric asthma exacerbations in the ED can be accomplished with a team effort.
Major finding: A team project reduced x-rays for pediatric asthma exacerbations from 30% to 15% without increasing 3-day, all-cause readmissions.
Study details: Pre/post quality improvement analysis of asthma encounters in the Monroe Carell Jr. Children’s Hospital, Nashville, Tenn., starting in 2014.
Disclosures: There was no industry funding, and the presenter didn’t have any disclosures.
Does supplemental oxygen help COPD patients who have chronic stable moderate hypoxia?
New study a departure from previous research
Case
An 85-year-old man with long-standing chronic obstructive pulmonary disease (COPD) has a witnessed aspiration event while undergoing an outpatient procedure requiring conscious sedation. He is admitted to the hospital for observation overnight. The next morning, he feels well, but his oxygen saturation dips to 85% with ambulation. He reports this is not new for him, but he vehemently does not want supplemental oxygen.
Background
Patients with COPD and severe resting hypoxemia – arterial oxygen partial pressure less than or equal to 55 mm Hg or peripheral capillary oxygen saturation (SpO2) less than or equal to 88% – commonly are prescribed supplemental oxygen. The evidence supporting this practice is limited to two small trials from the 1970s that showed a survival benefit of long-term oxygen therapy (LTOT) in this population,1,2 but these trials may not be generalizable to patients today.
For patients with COPD and mild to moderate resting hypoxemia (SpO2, 89%-93%) or patients with exercise-induced hypoxemia, LTOT has not been shown to improve survival, although it may improve symptoms of dyspnea, exercise tolerance, and other patient reported outcomes. Given the costs, risks, and burdens associated with LTOT, a high-quality clinical trial assessing the effects of LTOT on clinically meaningful outcomes, such as survival or hospitalization, in patients with COPD and moderate hypoxemia has been long overdue.
Overview of the data
The utility of long-term treatment with supplemental oxygen in patients with stable COPD and moderate resting or exercise-induced desaturation was examined by the Long-Term Oxygen Treatment Trial (LOTT) Research Group. Results were published in the New England Journal of Medicine in October 2016 in the article, “A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation.”3
The study was initially designed to test whether the use of supplemental oxygen would lead to longer time until death as compared with no supplemental oxygen in the subgroup of COPD patients with stable disease and moderate resting desaturation (defined as resting SpO2 of 89%-93%). However, because of an enrollment of only 34 patients after 7 months, the trial was redesigned to include exercise-induced desaturation (defined as SpO2 of greater than or equal to 80% for at least 5 minutes, and less than 90% for at least 10 seconds, on a 6-minute walk test) and the secondary outcome of all-cause hospitalization. Hospitalization for any cause was combined with mortality into a new composite primary outcome.
This study was a randomized, controlled trial which enrolled patients at a total of 14 regional clinical centers and their associated sites for a total of 42 centers in the United States. The experimental arm consisted of a long term supplemental oxygen group, and the control group did not receive long term supplemental oxygen. Patients were assigned to groups in a 1:1 ratio and the study was not blinded. Patients with moderate resting desaturation were prescribed 24 hour oxygen at 2 L/min, and patients with moderate exercise-induced desaturation were prescribed oxygen at 2 L/min during exercise and sleep only. The primary outcome was a composite outcome of time until death or time until first hospitalization for any cause. There were multiple secondary outcomes, including incidence of COPD exacerbation, incidence of severe resting desaturation and severe exercise-induced desaturation, quality of life, sleep quality, depression and anxiety, adherence to regimen, 6-minute walk distance, spirometric measurements, risk of cardiovascular disease, and neurocognitive function.
Data were gathered via yearly visits, biannual telephone interviews, and questionnaires mailed at 4 months and 16 months. Adherence was assessed by inquiring about oxygen use every 4 months. If patients in the supplemental oxygen group used stationary oxygen concentrators, logs of meter readings were kept as well. The necessary final sample size was calculated using a time to composite event survival model with the use of the log-rank test statistic.
A total of 738 patients were enrolled in the trial between January 2009 and September 2015 and were followed for 1-6 years. A total of 97% of participants had at least 1 year of follow-up. Out of the 738 randomized patients, 133 (18%) had only resting desaturations, 319 (43%) had only exercise-induced desaturations, and 286 (39%) had both resting and exercise-induced desaturations. Baseline characteristics including age, sex, race, smoking status, quality of life scores, resting SpO2, and nadir SpO2 during the 6-minute walk test were similar between the two groups. The only significant difference noted by the authors between the two groups was a lower BODE (body mass index, airflow obstruction, dyspnea, and exercise) index, which was lower in the group with no supplemental oxygen.
In the time-to-event analysis, there was no significant difference between the two groups in the time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval, 0.79-1.12; P = .52). There were no significant differences in the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91-1.13), COPD exacerbations (RR 1.08; 95% CI, 0.98-1.19), and COPD related hospitalizations (RR, 0.99; 95% CI, 0.83-1.17). There were also no differences between the experimental and control groups in quality of life, lung function, and 6-minute walk distance. There were no significant differences in the subgroups classified by desaturation profile, sex, race, nadir SpO2 during the 6-minute walk test, and forced expiratory volume in 1 second.
The findings in this study show that, in the subgroup of chronic obstructive pulmonary disease patients with stable COPD and moderate resting or exercise-induced desaturation, supplemental oxygen did not affect the time to death or first hospitalization, time to death, time to first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, rate of all hospitalizations, rate of all COPD exacerbations, or changes in metrics surrounding quality of life, anxiety/depression, or functional status. This supports earlier studies that demonstrated that long-term treatment with oxygen does not result in longer survival than does no long-term treatment with oxygen in patients with COPD and resting SpO2 of more than 88%.
The results of this study are a departure from previous studies that had shown improved mortality in patients with COPD and severe desaturation who were treated with LTOT. The authors hypothesized that this may have been caused by physiological effects of oxygen saturation on pulmonary vasoconstriction, release of mediators, and ventilator drive, which occur at an O2 saturation of 88% or less and may be more significant in patients with chronic hypoxemia. This trial also contrasted previous studies that had shown that oxygen therapy may reduce dyspnea in COPD patients with mild or no hypoxia because the LOTT trial showed no improvement in quality of life, anxiety, and depression measures in patients treated with long-term oxygen as compared with those treated with no oxygen.
Some limitations of the study included the absence of highly symptomatic patients or patients who the providers believed were too ill to participate, the effect of the unblinded nature of the study on outcomes that were patient reported, the lack of assessment of immediate effects of oxygen on exercise performance or symptoms, possible variability in amount of oxygen delivered, and the fact that patients may have overestimated their oxygen use.
In patients with stable COPD and moderate resting or exercise induced desaturation, long-term supplemental oxygen did not provide any benefit in regard to time until death or first hospitalization or any of the other measured outcomes.
Application of data to the case
Our patient has stable COPD and had only moderate exercise-induced desaturation. Long-term supplemental oxygen would not produce a benefit for him.
This study shows us that it would not increase his survival at this point; however, if he were to have worsening exercise-induced or new resting desaturation at some point in the future, supplemental oxygen would then be beneficial. At this point supplemental oxygen would not even affect his rate of hospitalization for COPD- or non-COPD–related reasons. Perhaps most importantly, adding oxygen therapy would not affect his overall quality of life, including his functional status and mood.
Bottom line
The addition of supplemental oxygen is not helpful for patients with COPD who have chronic stable moderate hypoxia.
Dr. Farber is a medical instructor in the Duke University Health System in Durham, N.C. Dr. Sata is a medical instructor in the Duke University Hospital. Dr. Wachter is an assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
References
1. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann Intern Med. 1980 Sep;93(3):391-8.
2. Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981 Mar 28;1(8222):681-6.
3. Long-term oxygen treatment trial research group et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016 Oct 27;375(17):1617-27.
Additional reading
Stoller JK et al. Oxygen therapy for patients with COPD: Current evidence and the Long-term Oxygen Treatment Trial. Chest. 2010 July;138:179-87.
Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179-91.
Ameer F et al. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev. 2014 Jun 24;(6):CD000238.
Vestbo J et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013 Feb 15;187(4):347-65.
Quiz: Does this patient need oxygen?
You are caring for a 72-year-old man with stable COPD who was admitted for cellulitis. He is improving clinically on appropriate antibiotics, and he has been stable on room air every time you examine him. The nurse pages you on the day of discharge – a Sunday – informing you that his oxygen saturation dropped to 88% while he was walking the halls this morning. She asks whether he needs to stay in the hospital so you can arrange home supplemental oxygen therapy. What should you do?
A. Keep him in the hospital until you can arrange home oxygen therapy.
B. Discharge him home Sunday but have the oxygen company go out to his house first thing on Monday.
C. Discharge him home without supplemental oxygen therapy.
D. Check an arterial blood gas to help decide if you should set up oxygen therapy.
The answer is C. He meets the description of stable COPD with mild to moderate exercise-induced desaturation. The LOTT trial supports our clinical decision that he would not benefit from supplemental oxygen therapy at this point.
Key Points
- Long-term oxygen therapy (LTOT) is beneficial in patients with COPD and severe resting hypoxemia (arterial oxygen partial pressure ≤ 55 mm Hg or SpO2 ≤ 88%) and should be prescribed to improve survival in this population.
- Patients with COPD and mild to moderate resting hypoxemia or exercised-induced hypoxemia should not be routinely prescribed LTOT given the associated costs, risks, and burdens and the lack of evidence of benefit.
New study a departure from previous research
New study a departure from previous research
Case
An 85-year-old man with long-standing chronic obstructive pulmonary disease (COPD) has a witnessed aspiration event while undergoing an outpatient procedure requiring conscious sedation. He is admitted to the hospital for observation overnight. The next morning, he feels well, but his oxygen saturation dips to 85% with ambulation. He reports this is not new for him, but he vehemently does not want supplemental oxygen.
Background
Patients with COPD and severe resting hypoxemia – arterial oxygen partial pressure less than or equal to 55 mm Hg or peripheral capillary oxygen saturation (SpO2) less than or equal to 88% – commonly are prescribed supplemental oxygen. The evidence supporting this practice is limited to two small trials from the 1970s that showed a survival benefit of long-term oxygen therapy (LTOT) in this population,1,2 but these trials may not be generalizable to patients today.
For patients with COPD and mild to moderate resting hypoxemia (SpO2, 89%-93%) or patients with exercise-induced hypoxemia, LTOT has not been shown to improve survival, although it may improve symptoms of dyspnea, exercise tolerance, and other patient reported outcomes. Given the costs, risks, and burdens associated with LTOT, a high-quality clinical trial assessing the effects of LTOT on clinically meaningful outcomes, such as survival or hospitalization, in patients with COPD and moderate hypoxemia has been long overdue.
Overview of the data
The utility of long-term treatment with supplemental oxygen in patients with stable COPD and moderate resting or exercise-induced desaturation was examined by the Long-Term Oxygen Treatment Trial (LOTT) Research Group. Results were published in the New England Journal of Medicine in October 2016 in the article, “A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation.”3
The study was initially designed to test whether the use of supplemental oxygen would lead to longer time until death as compared with no supplemental oxygen in the subgroup of COPD patients with stable disease and moderate resting desaturation (defined as resting SpO2 of 89%-93%). However, because of an enrollment of only 34 patients after 7 months, the trial was redesigned to include exercise-induced desaturation (defined as SpO2 of greater than or equal to 80% for at least 5 minutes, and less than 90% for at least 10 seconds, on a 6-minute walk test) and the secondary outcome of all-cause hospitalization. Hospitalization for any cause was combined with mortality into a new composite primary outcome.
This study was a randomized, controlled trial which enrolled patients at a total of 14 regional clinical centers and their associated sites for a total of 42 centers in the United States. The experimental arm consisted of a long term supplemental oxygen group, and the control group did not receive long term supplemental oxygen. Patients were assigned to groups in a 1:1 ratio and the study was not blinded. Patients with moderate resting desaturation were prescribed 24 hour oxygen at 2 L/min, and patients with moderate exercise-induced desaturation were prescribed oxygen at 2 L/min during exercise and sleep only. The primary outcome was a composite outcome of time until death or time until first hospitalization for any cause. There were multiple secondary outcomes, including incidence of COPD exacerbation, incidence of severe resting desaturation and severe exercise-induced desaturation, quality of life, sleep quality, depression and anxiety, adherence to regimen, 6-minute walk distance, spirometric measurements, risk of cardiovascular disease, and neurocognitive function.
Data were gathered via yearly visits, biannual telephone interviews, and questionnaires mailed at 4 months and 16 months. Adherence was assessed by inquiring about oxygen use every 4 months. If patients in the supplemental oxygen group used stationary oxygen concentrators, logs of meter readings were kept as well. The necessary final sample size was calculated using a time to composite event survival model with the use of the log-rank test statistic.
A total of 738 patients were enrolled in the trial between January 2009 and September 2015 and were followed for 1-6 years. A total of 97% of participants had at least 1 year of follow-up. Out of the 738 randomized patients, 133 (18%) had only resting desaturations, 319 (43%) had only exercise-induced desaturations, and 286 (39%) had both resting and exercise-induced desaturations. Baseline characteristics including age, sex, race, smoking status, quality of life scores, resting SpO2, and nadir SpO2 during the 6-minute walk test were similar between the two groups. The only significant difference noted by the authors between the two groups was a lower BODE (body mass index, airflow obstruction, dyspnea, and exercise) index, which was lower in the group with no supplemental oxygen.
In the time-to-event analysis, there was no significant difference between the two groups in the time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval, 0.79-1.12; P = .52). There were no significant differences in the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91-1.13), COPD exacerbations (RR 1.08; 95% CI, 0.98-1.19), and COPD related hospitalizations (RR, 0.99; 95% CI, 0.83-1.17). There were also no differences between the experimental and control groups in quality of life, lung function, and 6-minute walk distance. There were no significant differences in the subgroups classified by desaturation profile, sex, race, nadir SpO2 during the 6-minute walk test, and forced expiratory volume in 1 second.
The findings in this study show that, in the subgroup of chronic obstructive pulmonary disease patients with stable COPD and moderate resting or exercise-induced desaturation, supplemental oxygen did not affect the time to death or first hospitalization, time to death, time to first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, rate of all hospitalizations, rate of all COPD exacerbations, or changes in metrics surrounding quality of life, anxiety/depression, or functional status. This supports earlier studies that demonstrated that long-term treatment with oxygen does not result in longer survival than does no long-term treatment with oxygen in patients with COPD and resting SpO2 of more than 88%.
The results of this study are a departure from previous studies that had shown improved mortality in patients with COPD and severe desaturation who were treated with LTOT. The authors hypothesized that this may have been caused by physiological effects of oxygen saturation on pulmonary vasoconstriction, release of mediators, and ventilator drive, which occur at an O2 saturation of 88% or less and may be more significant in patients with chronic hypoxemia. This trial also contrasted previous studies that had shown that oxygen therapy may reduce dyspnea in COPD patients with mild or no hypoxia because the LOTT trial showed no improvement in quality of life, anxiety, and depression measures in patients treated with long-term oxygen as compared with those treated with no oxygen.
Some limitations of the study included the absence of highly symptomatic patients or patients who the providers believed were too ill to participate, the effect of the unblinded nature of the study on outcomes that were patient reported, the lack of assessment of immediate effects of oxygen on exercise performance or symptoms, possible variability in amount of oxygen delivered, and the fact that patients may have overestimated their oxygen use.
In patients with stable COPD and moderate resting or exercise induced desaturation, long-term supplemental oxygen did not provide any benefit in regard to time until death or first hospitalization or any of the other measured outcomes.
Application of data to the case
Our patient has stable COPD and had only moderate exercise-induced desaturation. Long-term supplemental oxygen would not produce a benefit for him.
This study shows us that it would not increase his survival at this point; however, if he were to have worsening exercise-induced or new resting desaturation at some point in the future, supplemental oxygen would then be beneficial. At this point supplemental oxygen would not even affect his rate of hospitalization for COPD- or non-COPD–related reasons. Perhaps most importantly, adding oxygen therapy would not affect his overall quality of life, including his functional status and mood.
Bottom line
The addition of supplemental oxygen is not helpful for patients with COPD who have chronic stable moderate hypoxia.
Dr. Farber is a medical instructor in the Duke University Health System in Durham, N.C. Dr. Sata is a medical instructor in the Duke University Hospital. Dr. Wachter is an assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
References
1. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann Intern Med. 1980 Sep;93(3):391-8.
2. Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981 Mar 28;1(8222):681-6.
3. Long-term oxygen treatment trial research group et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016 Oct 27;375(17):1617-27.
Additional reading
Stoller JK et al. Oxygen therapy for patients with COPD: Current evidence and the Long-term Oxygen Treatment Trial. Chest. 2010 July;138:179-87.
Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179-91.
Ameer F et al. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev. 2014 Jun 24;(6):CD000238.
Vestbo J et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013 Feb 15;187(4):347-65.
Quiz: Does this patient need oxygen?
You are caring for a 72-year-old man with stable COPD who was admitted for cellulitis. He is improving clinically on appropriate antibiotics, and he has been stable on room air every time you examine him. The nurse pages you on the day of discharge – a Sunday – informing you that his oxygen saturation dropped to 88% while he was walking the halls this morning. She asks whether he needs to stay in the hospital so you can arrange home supplemental oxygen therapy. What should you do?
A. Keep him in the hospital until you can arrange home oxygen therapy.
B. Discharge him home Sunday but have the oxygen company go out to his house first thing on Monday.
C. Discharge him home without supplemental oxygen therapy.
D. Check an arterial blood gas to help decide if you should set up oxygen therapy.
The answer is C. He meets the description of stable COPD with mild to moderate exercise-induced desaturation. The LOTT trial supports our clinical decision that he would not benefit from supplemental oxygen therapy at this point.
Key Points
- Long-term oxygen therapy (LTOT) is beneficial in patients with COPD and severe resting hypoxemia (arterial oxygen partial pressure ≤ 55 mm Hg or SpO2 ≤ 88%) and should be prescribed to improve survival in this population.
- Patients with COPD and mild to moderate resting hypoxemia or exercised-induced hypoxemia should not be routinely prescribed LTOT given the associated costs, risks, and burdens and the lack of evidence of benefit.
Case
An 85-year-old man with long-standing chronic obstructive pulmonary disease (COPD) has a witnessed aspiration event while undergoing an outpatient procedure requiring conscious sedation. He is admitted to the hospital for observation overnight. The next morning, he feels well, but his oxygen saturation dips to 85% with ambulation. He reports this is not new for him, but he vehemently does not want supplemental oxygen.
Background
Patients with COPD and severe resting hypoxemia – arterial oxygen partial pressure less than or equal to 55 mm Hg or peripheral capillary oxygen saturation (SpO2) less than or equal to 88% – commonly are prescribed supplemental oxygen. The evidence supporting this practice is limited to two small trials from the 1970s that showed a survival benefit of long-term oxygen therapy (LTOT) in this population,1,2 but these trials may not be generalizable to patients today.
For patients with COPD and mild to moderate resting hypoxemia (SpO2, 89%-93%) or patients with exercise-induced hypoxemia, LTOT has not been shown to improve survival, although it may improve symptoms of dyspnea, exercise tolerance, and other patient reported outcomes. Given the costs, risks, and burdens associated with LTOT, a high-quality clinical trial assessing the effects of LTOT on clinically meaningful outcomes, such as survival or hospitalization, in patients with COPD and moderate hypoxemia has been long overdue.
Overview of the data
The utility of long-term treatment with supplemental oxygen in patients with stable COPD and moderate resting or exercise-induced desaturation was examined by the Long-Term Oxygen Treatment Trial (LOTT) Research Group. Results were published in the New England Journal of Medicine in October 2016 in the article, “A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation.”3
The study was initially designed to test whether the use of supplemental oxygen would lead to longer time until death as compared with no supplemental oxygen in the subgroup of COPD patients with stable disease and moderate resting desaturation (defined as resting SpO2 of 89%-93%). However, because of an enrollment of only 34 patients after 7 months, the trial was redesigned to include exercise-induced desaturation (defined as SpO2 of greater than or equal to 80% for at least 5 minutes, and less than 90% for at least 10 seconds, on a 6-minute walk test) and the secondary outcome of all-cause hospitalization. Hospitalization for any cause was combined with mortality into a new composite primary outcome.
This study was a randomized, controlled trial which enrolled patients at a total of 14 regional clinical centers and their associated sites for a total of 42 centers in the United States. The experimental arm consisted of a long term supplemental oxygen group, and the control group did not receive long term supplemental oxygen. Patients were assigned to groups in a 1:1 ratio and the study was not blinded. Patients with moderate resting desaturation were prescribed 24 hour oxygen at 2 L/min, and patients with moderate exercise-induced desaturation were prescribed oxygen at 2 L/min during exercise and sleep only. The primary outcome was a composite outcome of time until death or time until first hospitalization for any cause. There were multiple secondary outcomes, including incidence of COPD exacerbation, incidence of severe resting desaturation and severe exercise-induced desaturation, quality of life, sleep quality, depression and anxiety, adherence to regimen, 6-minute walk distance, spirometric measurements, risk of cardiovascular disease, and neurocognitive function.
Data were gathered via yearly visits, biannual telephone interviews, and questionnaires mailed at 4 months and 16 months. Adherence was assessed by inquiring about oxygen use every 4 months. If patients in the supplemental oxygen group used stationary oxygen concentrators, logs of meter readings were kept as well. The necessary final sample size was calculated using a time to composite event survival model with the use of the log-rank test statistic.
A total of 738 patients were enrolled in the trial between January 2009 and September 2015 and were followed for 1-6 years. A total of 97% of participants had at least 1 year of follow-up. Out of the 738 randomized patients, 133 (18%) had only resting desaturations, 319 (43%) had only exercise-induced desaturations, and 286 (39%) had both resting and exercise-induced desaturations. Baseline characteristics including age, sex, race, smoking status, quality of life scores, resting SpO2, and nadir SpO2 during the 6-minute walk test were similar between the two groups. The only significant difference noted by the authors between the two groups was a lower BODE (body mass index, airflow obstruction, dyspnea, and exercise) index, which was lower in the group with no supplemental oxygen.
In the time-to-event analysis, there was no significant difference between the two groups in the time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval, 0.79-1.12; P = .52). There were no significant differences in the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91-1.13), COPD exacerbations (RR 1.08; 95% CI, 0.98-1.19), and COPD related hospitalizations (RR, 0.99; 95% CI, 0.83-1.17). There were also no differences between the experimental and control groups in quality of life, lung function, and 6-minute walk distance. There were no significant differences in the subgroups classified by desaturation profile, sex, race, nadir SpO2 during the 6-minute walk test, and forced expiratory volume in 1 second.
The findings in this study show that, in the subgroup of chronic obstructive pulmonary disease patients with stable COPD and moderate resting or exercise-induced desaturation, supplemental oxygen did not affect the time to death or first hospitalization, time to death, time to first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, rate of all hospitalizations, rate of all COPD exacerbations, or changes in metrics surrounding quality of life, anxiety/depression, or functional status. This supports earlier studies that demonstrated that long-term treatment with oxygen does not result in longer survival than does no long-term treatment with oxygen in patients with COPD and resting SpO2 of more than 88%.
The results of this study are a departure from previous studies that had shown improved mortality in patients with COPD and severe desaturation who were treated with LTOT. The authors hypothesized that this may have been caused by physiological effects of oxygen saturation on pulmonary vasoconstriction, release of mediators, and ventilator drive, which occur at an O2 saturation of 88% or less and may be more significant in patients with chronic hypoxemia. This trial also contrasted previous studies that had shown that oxygen therapy may reduce dyspnea in COPD patients with mild or no hypoxia because the LOTT trial showed no improvement in quality of life, anxiety, and depression measures in patients treated with long-term oxygen as compared with those treated with no oxygen.
Some limitations of the study included the absence of highly symptomatic patients or patients who the providers believed were too ill to participate, the effect of the unblinded nature of the study on outcomes that were patient reported, the lack of assessment of immediate effects of oxygen on exercise performance or symptoms, possible variability in amount of oxygen delivered, and the fact that patients may have overestimated their oxygen use.
In patients with stable COPD and moderate resting or exercise induced desaturation, long-term supplemental oxygen did not provide any benefit in regard to time until death or first hospitalization or any of the other measured outcomes.
Application of data to the case
Our patient has stable COPD and had only moderate exercise-induced desaturation. Long-term supplemental oxygen would not produce a benefit for him.
This study shows us that it would not increase his survival at this point; however, if he were to have worsening exercise-induced or new resting desaturation at some point in the future, supplemental oxygen would then be beneficial. At this point supplemental oxygen would not even affect his rate of hospitalization for COPD- or non-COPD–related reasons. Perhaps most importantly, adding oxygen therapy would not affect his overall quality of life, including his functional status and mood.
Bottom line
The addition of supplemental oxygen is not helpful for patients with COPD who have chronic stable moderate hypoxia.
Dr. Farber is a medical instructor in the Duke University Health System in Durham, N.C. Dr. Sata is a medical instructor in the Duke University Hospital. Dr. Wachter is an assistant professor of medicine at Duke University. Dr. Sharma is associate medical director for clinical education in hospital medicine at Duke Regional Hospital and an assistant professor of medicine at Duke University.
References
1. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: A clinical trial. Ann Intern Med. 1980 Sep;93(3):391-8.
2. Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981 Mar 28;1(8222):681-6.
3. Long-term oxygen treatment trial research group et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016 Oct 27;375(17):1617-27.
Additional reading
Stoller JK et al. Oxygen therapy for patients with COPD: Current evidence and the Long-term Oxygen Treatment Trial. Chest. 2010 July;138:179-87.
Qaseem A et al. Diagnosis and management of stable chronic obstructive pulmonary disease: A clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011 Aug 2;155(3):179-91.
Ameer F et al. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev. 2014 Jun 24;(6):CD000238.
Vestbo J et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013 Feb 15;187(4):347-65.
Quiz: Does this patient need oxygen?
You are caring for a 72-year-old man with stable COPD who was admitted for cellulitis. He is improving clinically on appropriate antibiotics, and he has been stable on room air every time you examine him. The nurse pages you on the day of discharge – a Sunday – informing you that his oxygen saturation dropped to 88% while he was walking the halls this morning. She asks whether he needs to stay in the hospital so you can arrange home supplemental oxygen therapy. What should you do?
A. Keep him in the hospital until you can arrange home oxygen therapy.
B. Discharge him home Sunday but have the oxygen company go out to his house first thing on Monday.
C. Discharge him home without supplemental oxygen therapy.
D. Check an arterial blood gas to help decide if you should set up oxygen therapy.
The answer is C. He meets the description of stable COPD with mild to moderate exercise-induced desaturation. The LOTT trial supports our clinical decision that he would not benefit from supplemental oxygen therapy at this point.
Key Points
- Long-term oxygen therapy (LTOT) is beneficial in patients with COPD and severe resting hypoxemia (arterial oxygen partial pressure ≤ 55 mm Hg or SpO2 ≤ 88%) and should be prescribed to improve survival in this population.
- Patients with COPD and mild to moderate resting hypoxemia or exercised-induced hypoxemia should not be routinely prescribed LTOT given the associated costs, risks, and burdens and the lack of evidence of benefit.
Balanced fluid resuscitation vs. saline does not decrease hospital-free days
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does balanced crystalloid fluid improve outcomes versus saline in noncritically ill patients who are hospitalized?
Background: Prior research has raised concerns about a connection between intravenous saline administration and adverse outcomes. However, this work has been limited to patients in the ICU and operative room settings.
Study design: Single-center, unblinded, multiple crossover (clustered randomization) trial.
Setting: A tertiary-care, academic medical center, from January 2016 to April 2017.
Synopsis: This study enrolled 13,347 adult patients receiving a minimum of 500 cc of intravenous fluid in the emergency department. Participants were randomized to receive either normal saline or balanced crystalloid fluid (lactated Ringer’s solution or Plasma-Lyte A). The study authors found no significant difference between the two groups in the primary outcome of hospital-free days (P = .41), or in several of the secondary outcomes including acute kidney injury stage 2 or higher (P = .14) and in-hospital mortality (P = .36). The balanced crystalloid fluid group did have a significantly lower incidence of a composite secondary outcome of major adverse kidney events (P = .01). However, given the primary and other secondary outcome findings, and concerns that composite outcomes lack patient centeredness, an accompanying editorial urged caution against changing clinical practice based on this finding.
Bottom line: There was no significant difference in hospital-free days for noncritically ill patients receiving IV fluids in the ED between those treated with saline and balanced crystalloid fluid.
Citation: Self WH et al. Balanced crystalloids versus saline in noncritically ill adults. N Eng J Med. 2018;378:819-28.
Dr. Biddick is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.


















