User login
A multicenter trial of vena cava filters in severely injured patients
Background: Venous thromboembolism and pulmonary embolism are common after major trauma. Anticoagulant prophylaxis usually is not considered because of the increased risk of bleeding. Despite the limited data, many trauma centers use inferior vena cava (IVC) filters as a primary means to prevent pulmonary embolism.
Study design: Randomized, controlled, and multicenter trial.
Setting: Four tertiary hospitals in Australia.
Synopsis: 240 major trauma patients were randomly assigned to receive either IVC filter or no IVC filter within 72 hours after admission. The primary endpoint was a composite of 90-day mortality or symptomatic pulmonary embolism confirmed on imaging. There was no difference in the rate of composite outcome in those with IVC filter, compared with those with no IVC filter.
Bottom line: After major trauma, early prophylactic placement of IVC filter did not reduce the 90-day mortality or incidence of symptomatic pulmonary embolism.
Citation: Ho KM et al. A multicenter trial of vena cava filters in severely injured patients. N Engl J Med. 2019 Jul 25;381:328-37.
Dr. Hoque Sharmy is a hospitalist and assistant professor of medicine in the division of hospital medicine at St. Louis University School of Medicine.
Background: Venous thromboembolism and pulmonary embolism are common after major trauma. Anticoagulant prophylaxis usually is not considered because of the increased risk of bleeding. Despite the limited data, many trauma centers use inferior vena cava (IVC) filters as a primary means to prevent pulmonary embolism.
Study design: Randomized, controlled, and multicenter trial.
Setting: Four tertiary hospitals in Australia.
Synopsis: 240 major trauma patients were randomly assigned to receive either IVC filter or no IVC filter within 72 hours after admission. The primary endpoint was a composite of 90-day mortality or symptomatic pulmonary embolism confirmed on imaging. There was no difference in the rate of composite outcome in those with IVC filter, compared with those with no IVC filter.
Bottom line: After major trauma, early prophylactic placement of IVC filter did not reduce the 90-day mortality or incidence of symptomatic pulmonary embolism.
Citation: Ho KM et al. A multicenter trial of vena cava filters in severely injured patients. N Engl J Med. 2019 Jul 25;381:328-37.
Dr. Hoque Sharmy is a hospitalist and assistant professor of medicine in the division of hospital medicine at St. Louis University School of Medicine.
Background: Venous thromboembolism and pulmonary embolism are common after major trauma. Anticoagulant prophylaxis usually is not considered because of the increased risk of bleeding. Despite the limited data, many trauma centers use inferior vena cava (IVC) filters as a primary means to prevent pulmonary embolism.
Study design: Randomized, controlled, and multicenter trial.
Setting: Four tertiary hospitals in Australia.
Synopsis: 240 major trauma patients were randomly assigned to receive either IVC filter or no IVC filter within 72 hours after admission. The primary endpoint was a composite of 90-day mortality or symptomatic pulmonary embolism confirmed on imaging. There was no difference in the rate of composite outcome in those with IVC filter, compared with those with no IVC filter.
Bottom line: After major trauma, early prophylactic placement of IVC filter did not reduce the 90-day mortality or incidence of symptomatic pulmonary embolism.
Citation: Ho KM et al. A multicenter trial of vena cava filters in severely injured patients. N Engl J Med. 2019 Jul 25;381:328-37.
Dr. Hoque Sharmy is a hospitalist and assistant professor of medicine in the division of hospital medicine at St. Louis University School of Medicine.
FDA safety alert: Face masks with metal can burn during MRI
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
After a patient’s face was burned in the outline of a mask worn during a 3-Tesla MRI neck scan, the US Food and Drug Administration (FDA) cautioned that face masks containing metal can heat to unsafe temperatures during scanning.
Clinicians have known for years to ask patients to remove all metal jewelry and other objects prior to an MRI. The widespread wearing of face masks during the COVID-19 pandemic, however, adds one more consideration to the list.
The FDA’s December 7 safety communication applies to surgical and nonsurgical face masks and respirators.
The injury risk relates to rapid heating of metal components. Many face masks contain a nose wire or metal clip that helps the product conform to the face. Some masks contain metal nanoparticles, while others feature antimicrobial coatings with silver or copper. Each of these products should be avoided during MRI scanning. Also watch out for staples on headbands, the FDA warned.
If the metal content of a face mask is unknown, the FDA suggests providing the patient with a facial covering that is known not to contain any metal.
Robert E. Watson Jr, MD, PhD, chair of the American College of Radiology (ACR) Committee on MR Safety, agreed. He recommended that facilities “provide patients with masks known to be MRI-safe and not permit patient-owned masks in the MRI.”
Watson suggested this strategy at a time when face masks are required.
“COVID-19 safety protocols require that patients wear masks when being scanned, to decrease infection risk to MRI staff, decrease risk of contaminating the MRI scanner, and to protect themselves from infection,” he told Medscape Medical News. “Any conducting metal that enters the MRI machine is at risk of heating due to the radiofrequency fields inherent to image generation.”
Adverse events related to the metal components of a face mask should be reported to the FDA using the MedWatch voluntary reporting form. In addition, healthcare providers subject to the FDA user facility reporting requirements should follow procedures at their facilities to report such events.
This article first appeared on Medscape.com.
New child COVID-19 cases down in last weekly count
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 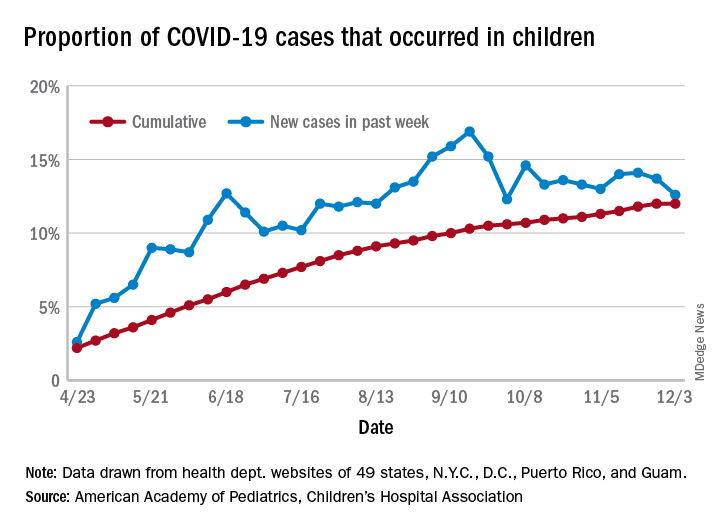
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
A tiny bit of light may have broken though the COVID-19 storm clouds.
The number of new cases in children in the United States did not set a new weekly high for the first time in months and the cumulative proportion of COVID-19 cases occurring in children did not go up for the first time since the pandemic started, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. 
which is the first time since late September that the weekly total has fallen in the United States, the AAP/CHA data show.
Another measure, the cumulative proportion of infected children among all COVID-19 cases, stayed at 12.0% for the second week in a row, and that is the first time there was no increase since the AAP and CHA started tracking health department websites in 49 states (not New York), the District of Columbia, New York City, Puerto Rico, and Guam in April.
For the week ending Dec. 3, those 123,688 children represented 12.6% of all U.S. COVID-19 cases, marking the second consecutive weekly drop in that figure, which has been as high as 16.9% in the previous 3 months, based on data in the AAP/CHA weekly report.
The total number of reported COVID-19 cases in children is now up to 1.46 million, and the overall rate is 1,941 per 100,000 children. Comparable figures for states show that California has the most cumulative cases at over 139,000 and that North Dakota has the highest rate at over 6,800 per 100,000 children. Vermont, the state with the smallest child population, has the fewest cases (687) and the lowest rate (511 per 100,000), the report said.
The total number of COVID-19–related deaths in children has reached 154 in the 44 jurisdictions (43 states and New York City) reporting such data. That number represents 0.06% of all coronavirus deaths, a proportion that has changed little – ranging from 0.04% to 0.07% – over the course of the pandemic, the AAP and CHA said.
COVID-19 and risk of clotting: ‘Be proactive about prevention’
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
The risk of arterial and venous thrombosis in patients with COVID-19 has been a major issue throughout the pandemic, and how best to manage this risk is the subject of a new review article.
The article, by Gregory Dr. Piazza, MD, and David A. Morrow, MD, Brigham and Women’s Hospital, Boston, was published online in JAMA on Nov. 23.
“Basically we’re saying: ‘Be proactive about prevention,’” Dr. Piazza told this news organization.
There is growing recognition among those on the frontline that there is an increased risk of thrombosis in COVID-19 patients, Dr. Piazza said. The risk is highest in patients in the intensive care unit, but the risk is also increased in patients hospitalized with COVID-19, even those not in ICU.
“We don’t really know what the risk is in nonhospitalized COVID-19 patients, but we think it’s much lower than in those who are hospitalized,” he said. “We are waiting for data on the optimal way of managing this increased risk of thrombosis in COVID patients, but for the time being, we believe a systematic way of addressing this risk is best, with every patient hospitalized with COVID-19 receiving some type of thromboprophylaxis. This would mainly be with anticoagulation, but in patients in whom anticoagulation is contraindicated, then mechanical methods could be used, such as pneumatic compression boots or compression stockings.”
The authors report thrombotic complication rates of 2.6% in noncritically ill hospitalized patients with COVID-19 and 35.3% in critically ill patients from a recent U.S. registry study.
Autopsy findings of microthrombi in multiple organ systems, including the lungs, heart, and kidneys, suggest that thrombosis may contribute to multisystem organ dysfunction in severe COVID-19, they note. Although the pathophysiology is not fully defined, prothrombotic abnormalities have been identified in patients with COVID-19, including elevated levels of D-dimer, fibrinogen, and factor VIII, they add.
“There are several major questions about which COVID-19 patients to treat with thromboprophylaxis, how to treat them in term of levels of anticoagulation, and there are many ongoing clinical trials to try and answer these questions,” Dr. Piazza commented. “We need results from these randomized trials to provide a better compass for COVID-19 patients at risk of clotting.”
At present, clinicians can follow two different sets of guidelines on the issue, one from the American College of Chest Physicians and the other from the International Society on Thrombosis and Hemostasis, the authors note.
“The ACCP guidelines are very conservative and basically follow the evidence base for medical patients, while the ISTH guidelines are more aggressive and recommend increased levels of anticoagulation in both ICU and hospitalized non-ICU patients and also extend prophylaxis after discharge,” Dr. Piazza said.
“There is quite a difference between the two sets of guidelines, which can be a point of confusion,” he added.
Dr. Piazza notes that at his center every hospitalized COVID patient who does not have a contraindication to anticoagulation receives a standard prophylactic dose of a once-daily low-molecular-weight heparin (for example, enoxaparin 40 mg). A once-daily product is used to minimize infection risk to staff.
While all COVID patients in the ICU should automatically receive some anticoagulation, the optimal dose is an area of active investigation, he explained. “There were several early reports of ICU patients developing blood clots despite receiving standard thromboprophylaxis so perhaps we need to use higher doses. There are trials underway looking at this, and we would advise enrolling patients into these trials.”
If patients can’t be enrolled into trials, and clinicians feel higher anticoagulation levels are needed, Dr. Piazza advises following the ISTH guidance, which allows an intermediate dose of low-molecular-weight heparin (up to 1 mg/kg enoxaparin).
“Some experts are suggesting even higher doses may be needed in some ICU patients, such as the full therapeutic dose, but I worry about the risk of bleeding with such a strategy,” he said.
Dr. Piazza says they do not routinely give anticoagulation after discharge, but if this is desired then patients could be switched to an oral agent, and some of the direct-acting oral anticoagulants are approved for prophylactic use in medically ill patients.
Dr. Piazza points out that whether thromboprophylaxis should be used for nonhospitalized COVID patients who have risk factors for clotting such as a prior history of thrombosis or obesity is a pressing question, and he encourages clinicians to enroll these patients in clinical trials evaluating this issue, such as the PREVENT-HD trial.
“If they can’t enroll patents in a trial, then they have to make a decision whether the patient is high-enough risk to justify off-label use of anticoagulant. There is a case to be made for this, but there is no evidence for or against such action at present,” he noted.
At this time, neither the ISTH nor ACCP recommend measuring D-dimer to screen for venous thromboembolism or to determine intensity of prophylaxis or treatment, the authors note.
“Ongoing investigation will determine optimal preventive regimens in COVID-19 in the intensive care unit, at hospital discharge, and in nonhospitalized patients at high risk for thrombosis,” they conclude.
Dr. Piazza reported grants from Bayer, Bristol Myers Squibb, Boston Scientific, Janssen, and Portola, and personal fees from Agile, Amgen, Pfizer, and the Prairie Education and Research Cooperative outside the submitted work. Dr. Morrow reported grants from Abbott Laboratories, Amgen, Anthos Therapeutics, Esai, GlaxoSmithKline, Takeda, and The Medicines Company; grants and personal fees from AstraZeneca, Merck, Novartis, and Roche Diagnostics; and personal fees from Bayer Pharma and InCarda outside the submitted work.
A version of this article originally appeared on Medscape.com.
Excess antibiotics and adverse events in patients with pneumonia
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Past surveys of providers revealed a tendency to select longer durations of antibiotics to reduce disease recurrence, but recent studies have shown that shorter courses of antibiotics are safe and equally effective in treatment for pneumonia. In addition, there has been a renewed focus on reducing unnecessary use of antibiotics to decrease adverse effects.
Study design: Retrospective cohort study.
Setting: 43 hospitals in the Michigan Hospital Medicine Safety Consortium.
Synopsis: A retrospective chart review of 6,481 patients hospitalized with pneumonia revealed that 67.8% of patients received excessive days of antibiotic treatment. On average, patients received 2 days of excessive treatment and 93.2% of the additional days came in the form of antibiotics prescribed at discharge.
Excessive treatment was defined as more than 5 days for community-acquired pneumonia (CAP) and more than 7 days for health care–associated pneumonia, methicillin-resistant Staphylococcus aureus, or gram-negative organisms. The authors adjusted for time to clinical stability when defining the expected duration of treatment.
After statistical adjustment, excess antibiotic days were not associated with increased rates of C. diff infection, emergency department visits, readmission, or 30-day mortality. Additional treatment was associated with increased patient-reported adverse effects including diarrhea, gastrointestinal distress, and mucosal candidiasis.
The impact of this study is limited by a few factors. The study was observational and relied on provider documentation and patient reporting of adverse events. Also, it was published prior to updates to the Infectious Diseases Society of America CAP guidelines, which may affect how it will be interpreted once those guidelines are released.
Bottom line: Adherence to the shortest effective duration of antibiotic treatment for pneumonia may lead to a reduction in the rates of patient reported adverse effects while not impacting treatment success.
Citation: Vaughn VM et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019 Aug 6;171(3):153-63.
Dr. Purdy is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Rounding to make the hospital go ‘round
Hospitalists and performance incentive measures
No matter how you spin it, hospitalists are key to making the world of the hospital go ‘round, making their daily work of paramount interest to both hospitals and health systems.
Hospitalists are the primary attending physicians for patients in the hospital while also bridging the patient and their needs to the services of other subspecialists, allied health professionals, and when needed, postacute services. In this way, patients are efficiently moved along the acute care experience with multiple process and outcome measures being recorded along the way.
Some of these common performance incentive measures are determined by the Centers for Medicare and Medicaid Services while others may be of interest to third party payers. Often surrogate markers of process metrics (i.e. order set usage for certain diagnoses) are measured and incentivized as a way of directionally measuring small steps that each hospitalist can reliably control toward a presumably associated improvement in mortality or readmissions, for instance. Still other measures such as length of stay or timely completion of documentation have more to do with hospital operations, regulatory governance, and finance.
There are a variety of performance incentive metrics reported in the 2020 SoHM Report. Survey respondents could choose all measures that applied as compensation measures for their group in the past year. The most common metrics reported include patient satisfaction (48.7%), citizenship (45.8%), accuracy or timeliness of documentation (32.8%), and clinical process measures (30.7%).
It is important to acknowledge that most of these metrics are objective measurements and can be measured down to the individual physician. However, some of the objective measures, such as patient satisfaction data, must rely on agreed upon methods of attribution – which can include anything from attributing based on admitting physician, discharging attending, or the attending with the greatest number of days (i.e. daily charges) seeing the patient. Because of challenges with attribution, groups may opt for group measurement of metrics for some of the compensation metrics where attribution is most muddy.
For performance incentive metrics that may be more subjective, such as citizenship, it is important for hospitalist leaders to consider having a method of determining a person’s contribution with a rubric as well as some shared decision making among a committee of leaders or team members to promote fairness in compensation.
Hospital leaders must also recognize that what is measured will lead to “performance” in that area. The perfect example here is the “early morning discharge time/orders” which is a compensation metric in 27.6% of hospitalist groups. Most agree that having some early discharges, up to maybe 25%-30% of the total number of discharges before noon, can be helpful with hospital throughput. The trick here is that if a patient can be discharged that early, it is likely that some of those patients could have gone home the evening prior. It is important for hospitalist physician leaders and administrators to think about the behaviors that are incentivized in compensation metrics to ensure that the result is indeed helpful.
Other hospitalist compensation metrics such as readmissions are most effectively addressed if there are multiple physician teams working toward the same metric. Hospitalist work does effect readmissions within the first 7 days of discharge based on available evidence.1 Preventing readmissions from days 8-30 following discharge are more amenable to outpatient and home-based interventions. Also, effective readmission work involves collaboration among the emergency physician team, surgeons, primary care, and subspecialty physicians. So while having this as a compensation metric will gain the attention of hospitalist physicians, the work will be most effective when it is shared with other teams.
Overall, performance incentive metrics for hospitalists can be effective in allowing hospitals and hospitalist groups to partner toward achieving important outcomes for patients. Easy and frequent sharing of data on meaningful metrics with hospitalists is important to effect change. Also, hospital leadership can facilitate collaboration among nursing and multiple physician groups to promote a team culture with hospitalists in achieving goals related to performance incentive metrics.
Dr. McNeal is the division director of inpatient medicine at Baylor Scott & White Medical Center in Temple, Tex.
Reference
1. Graham, et al. Preventability of Early Versus Late Hospital Readmissions in a National Cohort of General Medicine Patients. Ann Intern Med. 2018 Jun 5;168(11):766-74.
Hospitalists and performance incentive measures
Hospitalists and performance incentive measures
No matter how you spin it, hospitalists are key to making the world of the hospital go ‘round, making their daily work of paramount interest to both hospitals and health systems.
Hospitalists are the primary attending physicians for patients in the hospital while also bridging the patient and their needs to the services of other subspecialists, allied health professionals, and when needed, postacute services. In this way, patients are efficiently moved along the acute care experience with multiple process and outcome measures being recorded along the way.
Some of these common performance incentive measures are determined by the Centers for Medicare and Medicaid Services while others may be of interest to third party payers. Often surrogate markers of process metrics (i.e. order set usage for certain diagnoses) are measured and incentivized as a way of directionally measuring small steps that each hospitalist can reliably control toward a presumably associated improvement in mortality or readmissions, for instance. Still other measures such as length of stay or timely completion of documentation have more to do with hospital operations, regulatory governance, and finance.
There are a variety of performance incentive metrics reported in the 2020 SoHM Report. Survey respondents could choose all measures that applied as compensation measures for their group in the past year. The most common metrics reported include patient satisfaction (48.7%), citizenship (45.8%), accuracy or timeliness of documentation (32.8%), and clinical process measures (30.7%).
It is important to acknowledge that most of these metrics are objective measurements and can be measured down to the individual physician. However, some of the objective measures, such as patient satisfaction data, must rely on agreed upon methods of attribution – which can include anything from attributing based on admitting physician, discharging attending, or the attending with the greatest number of days (i.e. daily charges) seeing the patient. Because of challenges with attribution, groups may opt for group measurement of metrics for some of the compensation metrics where attribution is most muddy.
For performance incentive metrics that may be more subjective, such as citizenship, it is important for hospitalist leaders to consider having a method of determining a person’s contribution with a rubric as well as some shared decision making among a committee of leaders or team members to promote fairness in compensation.
Hospital leaders must also recognize that what is measured will lead to “performance” in that area. The perfect example here is the “early morning discharge time/orders” which is a compensation metric in 27.6% of hospitalist groups. Most agree that having some early discharges, up to maybe 25%-30% of the total number of discharges before noon, can be helpful with hospital throughput. The trick here is that if a patient can be discharged that early, it is likely that some of those patients could have gone home the evening prior. It is important for hospitalist physician leaders and administrators to think about the behaviors that are incentivized in compensation metrics to ensure that the result is indeed helpful.
Other hospitalist compensation metrics such as readmissions are most effectively addressed if there are multiple physician teams working toward the same metric. Hospitalist work does effect readmissions within the first 7 days of discharge based on available evidence.1 Preventing readmissions from days 8-30 following discharge are more amenable to outpatient and home-based interventions. Also, effective readmission work involves collaboration among the emergency physician team, surgeons, primary care, and subspecialty physicians. So while having this as a compensation metric will gain the attention of hospitalist physicians, the work will be most effective when it is shared with other teams.
Overall, performance incentive metrics for hospitalists can be effective in allowing hospitals and hospitalist groups to partner toward achieving important outcomes for patients. Easy and frequent sharing of data on meaningful metrics with hospitalists is important to effect change. Also, hospital leadership can facilitate collaboration among nursing and multiple physician groups to promote a team culture with hospitalists in achieving goals related to performance incentive metrics.
Dr. McNeal is the division director of inpatient medicine at Baylor Scott & White Medical Center in Temple, Tex.
Reference
1. Graham, et al. Preventability of Early Versus Late Hospital Readmissions in a National Cohort of General Medicine Patients. Ann Intern Med. 2018 Jun 5;168(11):766-74.
No matter how you spin it, hospitalists are key to making the world of the hospital go ‘round, making their daily work of paramount interest to both hospitals and health systems.
Hospitalists are the primary attending physicians for patients in the hospital while also bridging the patient and their needs to the services of other subspecialists, allied health professionals, and when needed, postacute services. In this way, patients are efficiently moved along the acute care experience with multiple process and outcome measures being recorded along the way.
Some of these common performance incentive measures are determined by the Centers for Medicare and Medicaid Services while others may be of interest to third party payers. Often surrogate markers of process metrics (i.e. order set usage for certain diagnoses) are measured and incentivized as a way of directionally measuring small steps that each hospitalist can reliably control toward a presumably associated improvement in mortality or readmissions, for instance. Still other measures such as length of stay or timely completion of documentation have more to do with hospital operations, regulatory governance, and finance.
There are a variety of performance incentive metrics reported in the 2020 SoHM Report. Survey respondents could choose all measures that applied as compensation measures for their group in the past year. The most common metrics reported include patient satisfaction (48.7%), citizenship (45.8%), accuracy or timeliness of documentation (32.8%), and clinical process measures (30.7%).
It is important to acknowledge that most of these metrics are objective measurements and can be measured down to the individual physician. However, some of the objective measures, such as patient satisfaction data, must rely on agreed upon methods of attribution – which can include anything from attributing based on admitting physician, discharging attending, or the attending with the greatest number of days (i.e. daily charges) seeing the patient. Because of challenges with attribution, groups may opt for group measurement of metrics for some of the compensation metrics where attribution is most muddy.
For performance incentive metrics that may be more subjective, such as citizenship, it is important for hospitalist leaders to consider having a method of determining a person’s contribution with a rubric as well as some shared decision making among a committee of leaders or team members to promote fairness in compensation.
Hospital leaders must also recognize that what is measured will lead to “performance” in that area. The perfect example here is the “early morning discharge time/orders” which is a compensation metric in 27.6% of hospitalist groups. Most agree that having some early discharges, up to maybe 25%-30% of the total number of discharges before noon, can be helpful with hospital throughput. The trick here is that if a patient can be discharged that early, it is likely that some of those patients could have gone home the evening prior. It is important for hospitalist physician leaders and administrators to think about the behaviors that are incentivized in compensation metrics to ensure that the result is indeed helpful.
Other hospitalist compensation metrics such as readmissions are most effectively addressed if there are multiple physician teams working toward the same metric. Hospitalist work does effect readmissions within the first 7 days of discharge based on available evidence.1 Preventing readmissions from days 8-30 following discharge are more amenable to outpatient and home-based interventions. Also, effective readmission work involves collaboration among the emergency physician team, surgeons, primary care, and subspecialty physicians. So while having this as a compensation metric will gain the attention of hospitalist physicians, the work will be most effective when it is shared with other teams.
Overall, performance incentive metrics for hospitalists can be effective in allowing hospitals and hospitalist groups to partner toward achieving important outcomes for patients. Easy and frequent sharing of data on meaningful metrics with hospitalists is important to effect change. Also, hospital leadership can facilitate collaboration among nursing and multiple physician groups to promote a team culture with hospitalists in achieving goals related to performance incentive metrics.
Dr. McNeal is the division director of inpatient medicine at Baylor Scott & White Medical Center in Temple, Tex.
Reference
1. Graham, et al. Preventability of Early Versus Late Hospital Readmissions in a National Cohort of General Medicine Patients. Ann Intern Med. 2018 Jun 5;168(11):766-74.
Assessing the impact of glucocorticoids on COVID-19 mortality
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
Clinical question: Is early glucocorticoid therapy associated with reduced mortality or need for mechanical ventilation in hospitalized patients with SARS-CoV-2 infection?
Background: Glucocorticoids have been used as adjunctive treatment in some infections with inflammatory responses, but their efficacy in COVID-19 infections had not been entirely clear. The RECOVERY trial found a subset of patients with COVID-19 who may benefit from treatment with glucocorticoids. The ideal role of steroids in this infection, and who the subset of patients might be for whom they would benefit, is so far unclear.
Study design: Retrospective cohort analysis.
Setting: Large academic health center in New York.
Synopsis: Researchers analyzed admissions of COVID-19 positive patients hospitalized between March 11, 2020 and April 13, 2020 who did not die or become mechanically ventilated within the first 48 hours of admission. Patients treated with glucocorticoids within 48 hours of admission were compared with patients who were not treated with glucocorticoids during this time frame. In total, 2,998 patients were examined, of whom 1,806 met inclusion criteria, and 140 (7.7%) were treated with glucocorticoids within 48 hours of admission. These treated patients were more likely to have an underlying pulmonary or rheumatologic comorbidity. Early use of glucocorticoids was not associated with in-hospital mortality or mechanical ventilation in either adjusted or unadjusted models. However, if the initial C-reactive protein (CRP) was >20mg/dL, this was associated with a reduced risk of mortality or mechanical ventilation in unadjusted (odds ratio, 0.23; 95% confidence interval, 0.08-0.70) and adjusted analyses for clinical characteristics (adjusted OR, 0.20; 95% CI, 0.06-0.67). Conversely, treatment in patients with CRP <10mg/dL was associated with significantly increased risk of mortality or ventilation during analysis.
Bottom line: Glucocorticoids can benefit patients with significantly elevated CRP but may be harmful to those with lower CRPs.
Citation: Keller MJ et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;8;489-493. Published online first. 2020 Jul 22. doi:10.12788/jhm.3497.
Dr. Halpern is a med-peds hospitalist at Brigham and Women’s Hospital in Boston.
FROM THE JOURNAL OF HOSPITAL MEDICINE
Biden chooses California Attorney General Xavier Becerra to head HHS
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
PPE shortage crisis continues at most hospitals, survey shows
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
A majority of hospitals and health care facilities surveyed report operating according to “crisis standards of care” as they struggle to provide sufficient personal protective equipment (PPE).
For example, in a national survey, 73% of 1,083 infection prevention experts said respirator shortages related to care for patients with COVID-19 drove their facility to move beyond conventional standards of care. Furthermore, 69% of facilities are using crisis standards of care (CSC) to provide masks, and 76% are apportioning face shields or eye protection.
Almost 76% of respondents who report reusing respirators said their facility allows them to use each respirator either five times or as many times as possible before replacement; 74% allow similar reuse of masks.
Although the majority of institutions remain in this crisis mode, many health care providers have better access to PPE than they did in the spring 2020, the Association for Professionals in Infection Control and Epidemiology (APIC) noted in its latest national survey.
“It is disheartening to see our healthcare system strained and implementing PPE crisis standards of care more than eight months into the pandemic,” APIC President Connie Steed, MSN, RN, said in a December 3 news release.
The association surveyed experts online between Oct. 22 and Nov. 5. The survey was timed to gauge the extent of resource shortages as COVID-19 cases increase and the 2020-2021 flu season begins.
“Many of us on the front lines are waiting for the other shoe to drop. With the upcoming flu season, we implore people to do what they can to keep safe, protect our healthcare personnel, and lessen the strain on our health care system,” Ms. Steed said.
COVID-19 linked to more infections, too
APIC also asked infection prevention specialists about changes in health care–associated infection rates since the onset of the pandemic. The experts reported an almost 28% increase in central line–associated bloodstream infections and 21% more catheter-associated urinary tract infections. They also reported an 18% rise in ventilator-associated pneumonia or ventilator-associated events, compared with before the COVID-19 pandemic.
This is the second PPE survey the APIC has conducted during the pandemic. The organization first reported a dire situation in March. For example, the initial survey found that 48% of facilities were almost out or were out of respirators used to care for patients with COVID-19.
This article first appeared on Medscape.com.
Higher 10-day mortality of lower-acuity patients during times of increased ED crowding
Background: Studies have assessed mortality effect from ED crowding on high-acuity patients, but limited evidence exists for how this affects lower-acuity patients who are discharged home.
Study design: Retrospective cohort study.
Setting: Emergency department, Karolinska University Hospital, Solna, Sweden.
Synopsis: During 2009-2016, 705,813 encounters seen in the ED, triaged to lower-acuity levels 3-5 and discharged without further hospitalization needs were identified. A total of 623 patients died within 10 days of the initial ED visit (0.09%). The study evaluated the association of 10-day mortality with mean ED length of stay and ED-occupancy ratio.
The study demonstrated an increased 10-day mortality for mean ED length of stay of 8 hours or more vs. less than 2 hours (adjusted odds ratio, 5.86; 95% CI, 2.15-15.94). It also found an increased mortality rate for occupancy ratio quartiles with an aOR for quartiles 2, 3, and 4 vs. quartile 1 of 1.48 (95% CI, 1.14-1.92), 1.63 (95% CI, 1.24-2.14), and 1.53 (95% CI, 1.15-2.03), respectively.
While this suggests increased 10-day mortality in this patient population, additional studies should be conducted to determine if this risk is caused by ED crowding and length of stay or by current limitations in triage scoring.
Bottom line: There is an increased 10-day mortality rate for lower-acuity triaged patients who were discharged from the ED without hospitalization experiencing increased ED length of stay and during times of ED crowding.
Citation: Berg L et al. Associations between crowding and 10-day mortality among patients allocated lower triage acuity levels without need of acute hospital care on departure from the emergency department. Ann Emerg Med. 2019 Sep;74(3):345-56.
Dr. Merando is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Studies have assessed mortality effect from ED crowding on high-acuity patients, but limited evidence exists for how this affects lower-acuity patients who are discharged home.
Study design: Retrospective cohort study.
Setting: Emergency department, Karolinska University Hospital, Solna, Sweden.
Synopsis: During 2009-2016, 705,813 encounters seen in the ED, triaged to lower-acuity levels 3-5 and discharged without further hospitalization needs were identified. A total of 623 patients died within 10 days of the initial ED visit (0.09%). The study evaluated the association of 10-day mortality with mean ED length of stay and ED-occupancy ratio.
The study demonstrated an increased 10-day mortality for mean ED length of stay of 8 hours or more vs. less than 2 hours (adjusted odds ratio, 5.86; 95% CI, 2.15-15.94). It also found an increased mortality rate for occupancy ratio quartiles with an aOR for quartiles 2, 3, and 4 vs. quartile 1 of 1.48 (95% CI, 1.14-1.92), 1.63 (95% CI, 1.24-2.14), and 1.53 (95% CI, 1.15-2.03), respectively.
While this suggests increased 10-day mortality in this patient population, additional studies should be conducted to determine if this risk is caused by ED crowding and length of stay or by current limitations in triage scoring.
Bottom line: There is an increased 10-day mortality rate for lower-acuity triaged patients who were discharged from the ED without hospitalization experiencing increased ED length of stay and during times of ED crowding.
Citation: Berg L et al. Associations between crowding and 10-day mortality among patients allocated lower triage acuity levels without need of acute hospital care on departure from the emergency department. Ann Emerg Med. 2019 Sep;74(3):345-56.
Dr. Merando is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.
Background: Studies have assessed mortality effect from ED crowding on high-acuity patients, but limited evidence exists for how this affects lower-acuity patients who are discharged home.
Study design: Retrospective cohort study.
Setting: Emergency department, Karolinska University Hospital, Solna, Sweden.
Synopsis: During 2009-2016, 705,813 encounters seen in the ED, triaged to lower-acuity levels 3-5 and discharged without further hospitalization needs were identified. A total of 623 patients died within 10 days of the initial ED visit (0.09%). The study evaluated the association of 10-day mortality with mean ED length of stay and ED-occupancy ratio.
The study demonstrated an increased 10-day mortality for mean ED length of stay of 8 hours or more vs. less than 2 hours (adjusted odds ratio, 5.86; 95% CI, 2.15-15.94). It also found an increased mortality rate for occupancy ratio quartiles with an aOR for quartiles 2, 3, and 4 vs. quartile 1 of 1.48 (95% CI, 1.14-1.92), 1.63 (95% CI, 1.24-2.14), and 1.53 (95% CI, 1.15-2.03), respectively.
While this suggests increased 10-day mortality in this patient population, additional studies should be conducted to determine if this risk is caused by ED crowding and length of stay or by current limitations in triage scoring.
Bottom line: There is an increased 10-day mortality rate for lower-acuity triaged patients who were discharged from the ED without hospitalization experiencing increased ED length of stay and during times of ED crowding.
Citation: Berg L et al. Associations between crowding and 10-day mortality among patients allocated lower triage acuity levels without need of acute hospital care on departure from the emergency department. Ann Emerg Med. 2019 Sep;74(3):345-56.
Dr. Merando is a hospitalist and assistant professor of internal medicine at St. Louis University School of Medicine.











