User login
Physician burnout: Signs and solutions
CASE
Dr. Peter D is a mid-career family physician in a group practice that recently adopted an electronic health record system. Although he realizes he is now competent at computerized medicine, he has far less of the one-on-one patient contact that he once found so gratifying about the field of medicine.
Others in the practice have similar concerns, but they suggest that everyone ought to “go along to get along.” To manage the increasing demands of his case load and the required documentation, Dr. D has begun staying late to finish charting, which is negatively impacting his family life.
Dr. D finds himself burdened by record keeping that is increasingly complicated and insurance company demands that are onerous. Pharmaceutical prior authorizations that previously had been mildly bothersome are now a full-on burden. More often than not, he finds himself becoming irritable over extra requests and administrative demands, impatient with some patients and staff, and extremely fatigued at the end of workdays. Simply put, he finds that practicing medicine is far less enjoyable than it once was. He takes the Maslach Burnout Inventory, and his score indicates that he has moderate burnout.

Physician burnout has been a growing concern in recent decades.1 Characterized by varying degrees of job dissatisfaction, cynicism, emotional exhaustion, clinical inefficiency, and depression, physician burnout can impede effective patient care, cause significant health issues among physicians, diminish professional gratification and feelings of accomplishment, and financially burden society as a whole. Here we present the information you need to recognize burnout in yourself and colleagues and address the problem on personal, organizational, and legislative levels.
A problem that affects physicians of all ages
Physician burnout has been recognized to present anywhere on a spectrum, manifesting as ineffectiveness, overextension, disengagement, and/or an inability to practice.2 Such features may lead to feelings of professional inadequacy among even the highest functioning physicians.
Burnout occurs in all stages of medical life—as students, residents, and practicing physicians.3-6 Due to pressures in excess of coping capacity, some physicians will suffer from alcohol or other drug abuse, depression, and/or suicidal thinking.7 Stress and burnout can also result in musculoskeletal disorders, immune system dysfunction, cardiac pathology, and a shorter lifespan.8
Not only do individual practitioners suffer consequences from burnout, but it also compromises health care delivery. In 2018, the Medscape National Physician Burnout and Depression Report surveyed 15,000 physicians from 29 specialties; 33% of the respondents said that they were more easily frustrated by patients, and 32% reported less personal engagement.9 Burnout adversely impacts care, patient satisfaction, productivity, physician retention, retirement, and income, as well.6 Safety during clinical practice deteriorates because of an increase in medical error rates.10 Resultant emotional distress for physicians creates a vicious cycle.10
[polldaddy:10427848]
Continue to: These issues negatively impact...
These issues negatively impact practice enthusiasm and may engender self-doubt.11 They may lead to absenteeism or, worse, to abandoning the profession, further contributing to physician shortages.12 The financial impact of physician burnout in lost revenue in 2018 was about $17 billion, according to the National Taskforce for Humanity in Medicine.13
How prevalent is physician burnout?
Between October 2012 and March 2013, the American Society of Clinical Oncology surveyed US oncologists and found that 45% had evidence of burnout.14 In another survey of US physicians from all specialties conducted in 2011, at least 1 symptom of burnout was documented in nearly 46% of respondents.15 By 2014, this percentage increased to 54%.16
In 2018, the Medscape National Physician Burnout and Depression Report indicated that 42% of physicians admitted to some burnout, while 12% said they were unhappy at work, and 3% reported being clinically depressed.9 About 48% of female practitioners reported burnout vs 38% of male peers.9 Work-related distress varies between specialties, with internists, family physicians, intensivists, neurologists, and gynecologists more affected than those from other specialties.9
Causes and contributing factors
Job stress generally increases with changes in the workplace. This can be heightened in the health care workplace, which demands perfection and leaves little room for emotional issues. Loss of autonomy, time constraints associated with clinical care, electronic health record (EHR) documentation, and disorganized workflow tend to contribute to provider dissatisfaction and stress, as do ethical disagreements about patient care between physicians and leadership.10,17 Fear of reprisal for speaking up about such issues can further exacerbate the problem. Some older physicians may have difficulty with technology and computerized record keeping. Reduced patient contact due to increasing reliance on computers can diminish physicians’ job satisfaction. And managing recurrent or difficult-to-treat ailments can result in compassion fatigue, diminished empathy, and emotional disengagement.
Burnout in the health care workplace is inconsistently addressed, despite negative professional and personal ramifications. The reasons include denial, uncertainty about monetary implications, and lack of corrective programs by decision-making organizations and/or employers.6 American medicine has lacked the political and financial will to implement strategies to mitigate burnout. Improvement requires changes on the part of government, physician groups, and the population at large.
The answer?
A multipronged approach
Identifying burnout is the first step in management. The 22-item Maslach Burnout Inventory (MBI) is a self-reporting questionnaire, reliable at detecting and assessing burnout severity.18 It screens 3 main domains: emotional exhaustion, depersonalization, and diminished feelings of accomplishment. The American Medical Association recommends the 10-item Zero Burnout Program—the “Mini Z Survey”—as being quicker and more convenient.19
Once the problem is recognized, experts suggest adopting a multipronged approach to prevention and intervention by using personal, organizational, and legislative strategies.20
Continue to: On a personal level...
On a personal level, it’s important to identify stressors and employ stress-reduction and coping skills, such as mindfulness and/or reflection.21 Mindfulness programs may help to minimize exhaustion, increase compassion, and improve understanding of other people’s feelings.22 Such programs are widely available and may be accessed through the Internet, mental health centers, or by contacting psychiatric or psychological services.
Other self-care methods include ensuring adequate sleep, nutrition, exercise, and enjoyable activities. If a physician who is suffering from burnout is taking any prescription or over-the-counter drugs or supplements, it is important to be self-aware of the potential for misuse of medications. Of course, one should never self-prescribe controlled drugs, such as opiates and sedatives. Consumption of alcohol must be well-controlled, without excesses, and drinking near bedtime is ill-advised. The use of illegal substances should be avoided.
Pursuing aspects of health care that are meaningful and that increase patient contact time can boost enthusiasm, as can focusing on the positives aspects of one’s career.23 Continuing medical education can enhance self-esteem and promote a sense of purpose.24
Peer support. Practice partners may assist their colleagues by alerting them to signs of burnout, offering timely intervention suggestions, and monitoring the effectiveness of strategies. Physicians should discuss stress and burnout with their peers; camaraderie within a practice group is helpful.
Professional coaches or counselors may be engaged to mitigate workplace distress. Coaching is best instituted collegially with pre-identified goals in order to minimize stigmatization.
Continue to: Professional societies and medical boards
Professional societies and medical boards. Reporting requirements by medical boards tend to stigmatize those seeking professional assistance. But that could change if all of us—through our participation in these organizations—pursue change.
Specifically, organizations and related societies could assist with better guidance and policy adjustment (see “Resources”). State medical boards could, for example, increase education of, and outreach to, physicians about mental health issues, while maintaining confidentiality.25 Medical organizations could regularly survey their membership to identify burnout early and identify personal, social, and institutional shortcomings that contribute to physician burnout. In addition, hospital quality improvement committees that monitor health care delivery appropriateness could take steps toward change as well.
SIDEBAR
Resources to help combat burnout
- National Academy of Medicine Action Collaborative on Clinician Well- Being and Resilience: https://nam.edu/initiatives/clinician-resilience-and-well-being/
- The Schwartz Center for Compassionate Healthcare: http://www.theschwartzcenter.org
- NEJM Catalyst: http://catalyst.nejm.org/posts?q=burnout
- The American Medical Association, Joy in Medicine: https://www.ama-assn.org/search?search=joy+in+medicine
- Agency for Healthcare Research and Quality: https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
- Accreditation Council for Graduate Medical Education Tools and Resources for Resident and Faculty Member Well-Being: https://www.acgme.org/What-We-Do/Initiatives/Physician-Well-Being/Resources
The American Medical Association (AMA) just recently announced that they are launching a new effort to fight the causes of physician burnout. The AMA’s Practice Transformation Inititative26 seeks to fill the knowledge gaps regarding effective interventions to reduce burnout. AMA’s leadership indicates that the initiative will focus on “improving joy in medicine by using validated assessment tools to measure burnout; field-testing interventions that are designed to improve workflows, applying practice science research methodology to evaluate impact, and sharing best practices within an AMA-facilitated learning community.”26
Stanford’s example. Stanford University instituted a ‘time bank’ program, to help their academic medical faculty balance work and life and reduce stress. They essentially offer services, such as home food delivery and house cleaning, in return for hours spent in the clinic.27
Reorganizing and reprioritizing. Prioritizing physician wellness as a quality indicator and instituting a committee to advocate for wellness can help attenuate burnout.28,29 Specific measures include minimizing rushed, overloaded scheduling and allowing more clinical contact time with patients. Using nursing and office staff to streamline workflow is also helpful.29 The University of Colorado’s “Ambulatory Process Excellence Model” strives to assist doctors by increasing the medical assistant-to-clinician ratio, yielding better productivity.23 Medical assistants are increasingly handling tasks such as data entry, medication reconciliation, and preventive care, to allow physicians more time to focus on medical decision-making.23
Continue to: The role of the EHR
The role of the EHR. One important way to boost professional morale is to simplify and shorten the EHR. The complexity of and reduced patient contact caused by today’s record-keeping systems is the source of great frustration among many physicians. In addition, many patients dislike the disproportionate attention paid by physicians to the computer during office visits, further compromising physician-patient relationships. Improving documentation methodology and/or employing medical assistant scribes can be helpful.30,31 (See “Advanced team-based care: How we made it work” at http://bit.ly/2lNaB5Q.)
Legislation with physician input can mandate policies for more appropriate work environments. A good way to initiate improvement and reform strategies is to contact local medical societies and political representatives. Federal and state collaboration to reduce physician shortages in selected specialties or geographic regions can improve work-related stress. This might be attained by expanding residency programs, using telemedicine in underserved regions, and employing more physician assistants.32
Health insurance. Enhancing universal access to affordable medical care, including pharmaceutical coverage, would alleviate stress for physicians and patients alike.33 Health insurance regulation to decrease paperwork and simplify coverage would decrease physician workload. Standardized policy requirements, fewer exclusionary rules, and simplified prescribing guidelines (including having less cumbersome prescription pre-authorizations and greater standardization of drug formularies by different payer sources or insurance plans) would facilitate better clinical management.
CASE
Dr. D begins by discussing his concerns with his colleagues in the group practice and finds he is not alone. Many of the concerns of the group center around brief, rushed appointments that diminish relationships with patients, a lack of autonomy, and the fear of medical malpractice. Several older physicians acknowledge that they just want to retire.
To address the patient contact and documentation issues, the group decides to hire scribes. They also decide to bring their concerns to the next county medical society meeting. The end result: They petitioned their state medical association to host presentations about mitigating burnout, to hold roundtable discussions, and to establish panels focused on remedying the situation.
Continue to: With this accomplished...
With this accomplished, Dr. D’s anxieties lessened. He surveyed relevant literature and shared tips for improving professional time management with his partners. In a hopeful mood, he volunteered to address burnout prevention at the next statewide medical meeting. He felt it was a good start.
CORRESPONDENCE
Steven Lippmann, MD, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Ramirez AJ, Graham J, Richards MA, et al. Burnout and psychiatric disorder among cancer clinicians. Br J Cancer. 1995;71:1263-1269.
2. Leiter MP, Maslach C. Latent burnout profiles: a new approach to understanding the burnout experience. Burnout Research. 2016;3:89-100.
3. Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among U.S. medical students. Ann Int Med. 2008;149:334-341.
4. West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
5. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250:463-471.
6. Shanafelt TD, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
7. Cottler LB, Ajinkya S, Merlo LJ, et al. Lifetime psychiatric and substance use disorders among impaired physicians in a physicians health program. J Addict Med. 2013;7:108-112.
8. Consiglio C. Interpersonal strain at work: a new burnout facet relevant for the health of hospital staff. Burnout Res. 2014;1:69-75.
9. Peckham C. Medscape National Physician Burnout and Depression Report 2018. January 12, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Accessed October 4, 2019.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174:527-533.
12. Suñer-Soler R, Grau-Martin A, Flichtentrei D, et al. The consequences of burnout syndrome among healthcare professionals in Spain and Spanish speaking Latin American countries. Burnout Research. 2014;1:82-89.
13. National Taskforce for Humanity in Healthcare. Position paper: The business case for humanity in healthcare. April 2018. https://www.vocera.com/public/pdf/NTHBusinessCase_final003.pdf. Accessed October 4, 2019.
14. Shanafelt TD, Gradishar WJ, Kosty M, et al. Burnout and career satisfaction among US oncologists. J Clin Oncol. 2014;32:678-686.
15. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Int Med. 2012;172:1377-1385.
16. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90;1600-1613.
17. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
18. Maslach C, Jackson SE. The measurement of experienced burnout. J Occcup Behav. 1981;2:99-113.
19. Linzer M, Guzman-Corrales L, Poplau S. Physician Burnout: improve physician satisfaction and patient outcomes. June 5, 2015. https://www.stepsforward.org/modules/physician-burnout. Accessed October 4, 2019.
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388:2272-2281.
21. Nedrow A, Steckler NA, Hardman J. Physician resilience and burnout: can you make the switch? Fam Prac Manag. 2013;20:25-30.
22. Verweij H, van Ravesteijn H, van Hooff MLM, et al. Mindfulness-based stress reduction for residents: a randomized controlled trial. J Gen Intern Med. 2018;33:429-436.
23. Wright AA, Katz IT. Beyond burnout – redesigning care to restore meaning and sanity for physicians. N Eng J Med. 2018;378:309-311.
24. Shanafelt TD, Gorringe G, Menaker R, et. al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90:432-440.
25. Hengerer A, Kishore S. 2017. Breaking a culture of silence: the role of state medical boards. National Academy of Medicine, Washington DC. https://nam.edu/breaking-a-culture-of-silence-the-role-of-state-medical-boards/. Accessed October 4, 2019.
26. American Medical Association. AMA fights burnout with new practice transformation initiative. September 5, 2019. https://www.ama-assn.org/press-center/press-releases/ama-fights-burnout-new-practice-transformation-initiative. Accessed September 5, 2019.
27. Schulte B. Time in the bank: a Stanford plan to save doctors from burnout. The Washington Post. https://www.washingtonpost.com/news/inspired-life/wp/2015/08/20/the-innovative-stanford-program-thats-saving-emergency-room-doctors-from-burnout/?utm_term=.838c930e8de7. Accessed October 4, 2019.
28. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374:1714-1721.
29. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
30. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Info Assoc. 2014;21:E100-E106.
31. Bodenheimer T, Willard-Grace R, Ghorob A. Expanding the roles of medical assistants: who does what in primary care? JAMA Intern Med. 2014;174:1025-1026.
32. Mangiofico G. Physician shortage requires multi-prong solution. January 26, 2018. Am J Manag Care. https://www.ajmc.com/contributor/dr-gary-mangiofico/2018/01/physician-shortage-requires-multiprong-solution. Accessed October 4, 2019.
33. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
CASE
Dr. Peter D is a mid-career family physician in a group practice that recently adopted an electronic health record system. Although he realizes he is now competent at computerized medicine, he has far less of the one-on-one patient contact that he once found so gratifying about the field of medicine.
Others in the practice have similar concerns, but they suggest that everyone ought to “go along to get along.” To manage the increasing demands of his case load and the required documentation, Dr. D has begun staying late to finish charting, which is negatively impacting his family life.
Dr. D finds himself burdened by record keeping that is increasingly complicated and insurance company demands that are onerous. Pharmaceutical prior authorizations that previously had been mildly bothersome are now a full-on burden. More often than not, he finds himself becoming irritable over extra requests and administrative demands, impatient with some patients and staff, and extremely fatigued at the end of workdays. Simply put, he finds that practicing medicine is far less enjoyable than it once was. He takes the Maslach Burnout Inventory, and his score indicates that he has moderate burnout.

Physician burnout has been a growing concern in recent decades.1 Characterized by varying degrees of job dissatisfaction, cynicism, emotional exhaustion, clinical inefficiency, and depression, physician burnout can impede effective patient care, cause significant health issues among physicians, diminish professional gratification and feelings of accomplishment, and financially burden society as a whole. Here we present the information you need to recognize burnout in yourself and colleagues and address the problem on personal, organizational, and legislative levels.
A problem that affects physicians of all ages
Physician burnout has been recognized to present anywhere on a spectrum, manifesting as ineffectiveness, overextension, disengagement, and/or an inability to practice.2 Such features may lead to feelings of professional inadequacy among even the highest functioning physicians.
Burnout occurs in all stages of medical life—as students, residents, and practicing physicians.3-6 Due to pressures in excess of coping capacity, some physicians will suffer from alcohol or other drug abuse, depression, and/or suicidal thinking.7 Stress and burnout can also result in musculoskeletal disorders, immune system dysfunction, cardiac pathology, and a shorter lifespan.8
Not only do individual practitioners suffer consequences from burnout, but it also compromises health care delivery. In 2018, the Medscape National Physician Burnout and Depression Report surveyed 15,000 physicians from 29 specialties; 33% of the respondents said that they were more easily frustrated by patients, and 32% reported less personal engagement.9 Burnout adversely impacts care, patient satisfaction, productivity, physician retention, retirement, and income, as well.6 Safety during clinical practice deteriorates because of an increase in medical error rates.10 Resultant emotional distress for physicians creates a vicious cycle.10
[polldaddy:10427848]
Continue to: These issues negatively impact...
These issues negatively impact practice enthusiasm and may engender self-doubt.11 They may lead to absenteeism or, worse, to abandoning the profession, further contributing to physician shortages.12 The financial impact of physician burnout in lost revenue in 2018 was about $17 billion, according to the National Taskforce for Humanity in Medicine.13
How prevalent is physician burnout?
Between October 2012 and March 2013, the American Society of Clinical Oncology surveyed US oncologists and found that 45% had evidence of burnout.14 In another survey of US physicians from all specialties conducted in 2011, at least 1 symptom of burnout was documented in nearly 46% of respondents.15 By 2014, this percentage increased to 54%.16
In 2018, the Medscape National Physician Burnout and Depression Report indicated that 42% of physicians admitted to some burnout, while 12% said they were unhappy at work, and 3% reported being clinically depressed.9 About 48% of female practitioners reported burnout vs 38% of male peers.9 Work-related distress varies between specialties, with internists, family physicians, intensivists, neurologists, and gynecologists more affected than those from other specialties.9
Causes and contributing factors
Job stress generally increases with changes in the workplace. This can be heightened in the health care workplace, which demands perfection and leaves little room for emotional issues. Loss of autonomy, time constraints associated with clinical care, electronic health record (EHR) documentation, and disorganized workflow tend to contribute to provider dissatisfaction and stress, as do ethical disagreements about patient care between physicians and leadership.10,17 Fear of reprisal for speaking up about such issues can further exacerbate the problem. Some older physicians may have difficulty with technology and computerized record keeping. Reduced patient contact due to increasing reliance on computers can diminish physicians’ job satisfaction. And managing recurrent or difficult-to-treat ailments can result in compassion fatigue, diminished empathy, and emotional disengagement.
Burnout in the health care workplace is inconsistently addressed, despite negative professional and personal ramifications. The reasons include denial, uncertainty about monetary implications, and lack of corrective programs by decision-making organizations and/or employers.6 American medicine has lacked the political and financial will to implement strategies to mitigate burnout. Improvement requires changes on the part of government, physician groups, and the population at large.
The answer?
A multipronged approach
Identifying burnout is the first step in management. The 22-item Maslach Burnout Inventory (MBI) is a self-reporting questionnaire, reliable at detecting and assessing burnout severity.18 It screens 3 main domains: emotional exhaustion, depersonalization, and diminished feelings of accomplishment. The American Medical Association recommends the 10-item Zero Burnout Program—the “Mini Z Survey”—as being quicker and more convenient.19
Once the problem is recognized, experts suggest adopting a multipronged approach to prevention and intervention by using personal, organizational, and legislative strategies.20
Continue to: On a personal level...
On a personal level, it’s important to identify stressors and employ stress-reduction and coping skills, such as mindfulness and/or reflection.21 Mindfulness programs may help to minimize exhaustion, increase compassion, and improve understanding of other people’s feelings.22 Such programs are widely available and may be accessed through the Internet, mental health centers, or by contacting psychiatric or psychological services.
Other self-care methods include ensuring adequate sleep, nutrition, exercise, and enjoyable activities. If a physician who is suffering from burnout is taking any prescription or over-the-counter drugs or supplements, it is important to be self-aware of the potential for misuse of medications. Of course, one should never self-prescribe controlled drugs, such as opiates and sedatives. Consumption of alcohol must be well-controlled, without excesses, and drinking near bedtime is ill-advised. The use of illegal substances should be avoided.
Pursuing aspects of health care that are meaningful and that increase patient contact time can boost enthusiasm, as can focusing on the positives aspects of one’s career.23 Continuing medical education can enhance self-esteem and promote a sense of purpose.24
Peer support. Practice partners may assist their colleagues by alerting them to signs of burnout, offering timely intervention suggestions, and monitoring the effectiveness of strategies. Physicians should discuss stress and burnout with their peers; camaraderie within a practice group is helpful.
Professional coaches or counselors may be engaged to mitigate workplace distress. Coaching is best instituted collegially with pre-identified goals in order to minimize stigmatization.
Continue to: Professional societies and medical boards
Professional societies and medical boards. Reporting requirements by medical boards tend to stigmatize those seeking professional assistance. But that could change if all of us—through our participation in these organizations—pursue change.
Specifically, organizations and related societies could assist with better guidance and policy adjustment (see “Resources”). State medical boards could, for example, increase education of, and outreach to, physicians about mental health issues, while maintaining confidentiality.25 Medical organizations could regularly survey their membership to identify burnout early and identify personal, social, and institutional shortcomings that contribute to physician burnout. In addition, hospital quality improvement committees that monitor health care delivery appropriateness could take steps toward change as well.
SIDEBAR
Resources to help combat burnout
- National Academy of Medicine Action Collaborative on Clinician Well- Being and Resilience: https://nam.edu/initiatives/clinician-resilience-and-well-being/
- The Schwartz Center for Compassionate Healthcare: http://www.theschwartzcenter.org
- NEJM Catalyst: http://catalyst.nejm.org/posts?q=burnout
- The American Medical Association, Joy in Medicine: https://www.ama-assn.org/search?search=joy+in+medicine
- Agency for Healthcare Research and Quality: https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
- Accreditation Council for Graduate Medical Education Tools and Resources for Resident and Faculty Member Well-Being: https://www.acgme.org/What-We-Do/Initiatives/Physician-Well-Being/Resources
The American Medical Association (AMA) just recently announced that they are launching a new effort to fight the causes of physician burnout. The AMA’s Practice Transformation Inititative26 seeks to fill the knowledge gaps regarding effective interventions to reduce burnout. AMA’s leadership indicates that the initiative will focus on “improving joy in medicine by using validated assessment tools to measure burnout; field-testing interventions that are designed to improve workflows, applying practice science research methodology to evaluate impact, and sharing best practices within an AMA-facilitated learning community.”26
Stanford’s example. Stanford University instituted a ‘time bank’ program, to help their academic medical faculty balance work and life and reduce stress. They essentially offer services, such as home food delivery and house cleaning, in return for hours spent in the clinic.27
Reorganizing and reprioritizing. Prioritizing physician wellness as a quality indicator and instituting a committee to advocate for wellness can help attenuate burnout.28,29 Specific measures include minimizing rushed, overloaded scheduling and allowing more clinical contact time with patients. Using nursing and office staff to streamline workflow is also helpful.29 The University of Colorado’s “Ambulatory Process Excellence Model” strives to assist doctors by increasing the medical assistant-to-clinician ratio, yielding better productivity.23 Medical assistants are increasingly handling tasks such as data entry, medication reconciliation, and preventive care, to allow physicians more time to focus on medical decision-making.23
Continue to: The role of the EHR
The role of the EHR. One important way to boost professional morale is to simplify and shorten the EHR. The complexity of and reduced patient contact caused by today’s record-keeping systems is the source of great frustration among many physicians. In addition, many patients dislike the disproportionate attention paid by physicians to the computer during office visits, further compromising physician-patient relationships. Improving documentation methodology and/or employing medical assistant scribes can be helpful.30,31 (See “Advanced team-based care: How we made it work” at http://bit.ly/2lNaB5Q.)
Legislation with physician input can mandate policies for more appropriate work environments. A good way to initiate improvement and reform strategies is to contact local medical societies and political representatives. Federal and state collaboration to reduce physician shortages in selected specialties or geographic regions can improve work-related stress. This might be attained by expanding residency programs, using telemedicine in underserved regions, and employing more physician assistants.32
Health insurance. Enhancing universal access to affordable medical care, including pharmaceutical coverage, would alleviate stress for physicians and patients alike.33 Health insurance regulation to decrease paperwork and simplify coverage would decrease physician workload. Standardized policy requirements, fewer exclusionary rules, and simplified prescribing guidelines (including having less cumbersome prescription pre-authorizations and greater standardization of drug formularies by different payer sources or insurance plans) would facilitate better clinical management.
CASE
Dr. D begins by discussing his concerns with his colleagues in the group practice and finds he is not alone. Many of the concerns of the group center around brief, rushed appointments that diminish relationships with patients, a lack of autonomy, and the fear of medical malpractice. Several older physicians acknowledge that they just want to retire.
To address the patient contact and documentation issues, the group decides to hire scribes. They also decide to bring their concerns to the next county medical society meeting. The end result: They petitioned their state medical association to host presentations about mitigating burnout, to hold roundtable discussions, and to establish panels focused on remedying the situation.
Continue to: With this accomplished...
With this accomplished, Dr. D’s anxieties lessened. He surveyed relevant literature and shared tips for improving professional time management with his partners. In a hopeful mood, he volunteered to address burnout prevention at the next statewide medical meeting. He felt it was a good start.
CORRESPONDENCE
Steven Lippmann, MD, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
CASE
Dr. Peter D is a mid-career family physician in a group practice that recently adopted an electronic health record system. Although he realizes he is now competent at computerized medicine, he has far less of the one-on-one patient contact that he once found so gratifying about the field of medicine.
Others in the practice have similar concerns, but they suggest that everyone ought to “go along to get along.” To manage the increasing demands of his case load and the required documentation, Dr. D has begun staying late to finish charting, which is negatively impacting his family life.
Dr. D finds himself burdened by record keeping that is increasingly complicated and insurance company demands that are onerous. Pharmaceutical prior authorizations that previously had been mildly bothersome are now a full-on burden. More often than not, he finds himself becoming irritable over extra requests and administrative demands, impatient with some patients and staff, and extremely fatigued at the end of workdays. Simply put, he finds that practicing medicine is far less enjoyable than it once was. He takes the Maslach Burnout Inventory, and his score indicates that he has moderate burnout.

Physician burnout has been a growing concern in recent decades.1 Characterized by varying degrees of job dissatisfaction, cynicism, emotional exhaustion, clinical inefficiency, and depression, physician burnout can impede effective patient care, cause significant health issues among physicians, diminish professional gratification and feelings of accomplishment, and financially burden society as a whole. Here we present the information you need to recognize burnout in yourself and colleagues and address the problem on personal, organizational, and legislative levels.
A problem that affects physicians of all ages
Physician burnout has been recognized to present anywhere on a spectrum, manifesting as ineffectiveness, overextension, disengagement, and/or an inability to practice.2 Such features may lead to feelings of professional inadequacy among even the highest functioning physicians.
Burnout occurs in all stages of medical life—as students, residents, and practicing physicians.3-6 Due to pressures in excess of coping capacity, some physicians will suffer from alcohol or other drug abuse, depression, and/or suicidal thinking.7 Stress and burnout can also result in musculoskeletal disorders, immune system dysfunction, cardiac pathology, and a shorter lifespan.8
Not only do individual practitioners suffer consequences from burnout, but it also compromises health care delivery. In 2018, the Medscape National Physician Burnout and Depression Report surveyed 15,000 physicians from 29 specialties; 33% of the respondents said that they were more easily frustrated by patients, and 32% reported less personal engagement.9 Burnout adversely impacts care, patient satisfaction, productivity, physician retention, retirement, and income, as well.6 Safety during clinical practice deteriorates because of an increase in medical error rates.10 Resultant emotional distress for physicians creates a vicious cycle.10
[polldaddy:10427848]
Continue to: These issues negatively impact...
These issues negatively impact practice enthusiasm and may engender self-doubt.11 They may lead to absenteeism or, worse, to abandoning the profession, further contributing to physician shortages.12 The financial impact of physician burnout in lost revenue in 2018 was about $17 billion, according to the National Taskforce for Humanity in Medicine.13
How prevalent is physician burnout?
Between October 2012 and March 2013, the American Society of Clinical Oncology surveyed US oncologists and found that 45% had evidence of burnout.14 In another survey of US physicians from all specialties conducted in 2011, at least 1 symptom of burnout was documented in nearly 46% of respondents.15 By 2014, this percentage increased to 54%.16
In 2018, the Medscape National Physician Burnout and Depression Report indicated that 42% of physicians admitted to some burnout, while 12% said they were unhappy at work, and 3% reported being clinically depressed.9 About 48% of female practitioners reported burnout vs 38% of male peers.9 Work-related distress varies between specialties, with internists, family physicians, intensivists, neurologists, and gynecologists more affected than those from other specialties.9
Causes and contributing factors
Job stress generally increases with changes in the workplace. This can be heightened in the health care workplace, which demands perfection and leaves little room for emotional issues. Loss of autonomy, time constraints associated with clinical care, electronic health record (EHR) documentation, and disorganized workflow tend to contribute to provider dissatisfaction and stress, as do ethical disagreements about patient care between physicians and leadership.10,17 Fear of reprisal for speaking up about such issues can further exacerbate the problem. Some older physicians may have difficulty with technology and computerized record keeping. Reduced patient contact due to increasing reliance on computers can diminish physicians’ job satisfaction. And managing recurrent or difficult-to-treat ailments can result in compassion fatigue, diminished empathy, and emotional disengagement.
Burnout in the health care workplace is inconsistently addressed, despite negative professional and personal ramifications. The reasons include denial, uncertainty about monetary implications, and lack of corrective programs by decision-making organizations and/or employers.6 American medicine has lacked the political and financial will to implement strategies to mitigate burnout. Improvement requires changes on the part of government, physician groups, and the population at large.
The answer?
A multipronged approach
Identifying burnout is the first step in management. The 22-item Maslach Burnout Inventory (MBI) is a self-reporting questionnaire, reliable at detecting and assessing burnout severity.18 It screens 3 main domains: emotional exhaustion, depersonalization, and diminished feelings of accomplishment. The American Medical Association recommends the 10-item Zero Burnout Program—the “Mini Z Survey”—as being quicker and more convenient.19
Once the problem is recognized, experts suggest adopting a multipronged approach to prevention and intervention by using personal, organizational, and legislative strategies.20
Continue to: On a personal level...
On a personal level, it’s important to identify stressors and employ stress-reduction and coping skills, such as mindfulness and/or reflection.21 Mindfulness programs may help to minimize exhaustion, increase compassion, and improve understanding of other people’s feelings.22 Such programs are widely available and may be accessed through the Internet, mental health centers, or by contacting psychiatric or psychological services.
Other self-care methods include ensuring adequate sleep, nutrition, exercise, and enjoyable activities. If a physician who is suffering from burnout is taking any prescription or over-the-counter drugs or supplements, it is important to be self-aware of the potential for misuse of medications. Of course, one should never self-prescribe controlled drugs, such as opiates and sedatives. Consumption of alcohol must be well-controlled, without excesses, and drinking near bedtime is ill-advised. The use of illegal substances should be avoided.
Pursuing aspects of health care that are meaningful and that increase patient contact time can boost enthusiasm, as can focusing on the positives aspects of one’s career.23 Continuing medical education can enhance self-esteem and promote a sense of purpose.24
Peer support. Practice partners may assist their colleagues by alerting them to signs of burnout, offering timely intervention suggestions, and monitoring the effectiveness of strategies. Physicians should discuss stress and burnout with their peers; camaraderie within a practice group is helpful.
Professional coaches or counselors may be engaged to mitigate workplace distress. Coaching is best instituted collegially with pre-identified goals in order to minimize stigmatization.
Continue to: Professional societies and medical boards
Professional societies and medical boards. Reporting requirements by medical boards tend to stigmatize those seeking professional assistance. But that could change if all of us—through our participation in these organizations—pursue change.
Specifically, organizations and related societies could assist with better guidance and policy adjustment (see “Resources”). State medical boards could, for example, increase education of, and outreach to, physicians about mental health issues, while maintaining confidentiality.25 Medical organizations could regularly survey their membership to identify burnout early and identify personal, social, and institutional shortcomings that contribute to physician burnout. In addition, hospital quality improvement committees that monitor health care delivery appropriateness could take steps toward change as well.
SIDEBAR
Resources to help combat burnout
- National Academy of Medicine Action Collaborative on Clinician Well- Being and Resilience: https://nam.edu/initiatives/clinician-resilience-and-well-being/
- The Schwartz Center for Compassionate Healthcare: http://www.theschwartzcenter.org
- NEJM Catalyst: http://catalyst.nejm.org/posts?q=burnout
- The American Medical Association, Joy in Medicine: https://www.ama-assn.org/search?search=joy+in+medicine
- Agency for Healthcare Research and Quality: https://www.ahrq.gov/prevention/clinician/ahrq-works/burnout/index.html
- Accreditation Council for Graduate Medical Education Tools and Resources for Resident and Faculty Member Well-Being: https://www.acgme.org/What-We-Do/Initiatives/Physician-Well-Being/Resources
The American Medical Association (AMA) just recently announced that they are launching a new effort to fight the causes of physician burnout. The AMA’s Practice Transformation Inititative26 seeks to fill the knowledge gaps regarding effective interventions to reduce burnout. AMA’s leadership indicates that the initiative will focus on “improving joy in medicine by using validated assessment tools to measure burnout; field-testing interventions that are designed to improve workflows, applying practice science research methodology to evaluate impact, and sharing best practices within an AMA-facilitated learning community.”26
Stanford’s example. Stanford University instituted a ‘time bank’ program, to help their academic medical faculty balance work and life and reduce stress. They essentially offer services, such as home food delivery and house cleaning, in return for hours spent in the clinic.27
Reorganizing and reprioritizing. Prioritizing physician wellness as a quality indicator and instituting a committee to advocate for wellness can help attenuate burnout.28,29 Specific measures include minimizing rushed, overloaded scheduling and allowing more clinical contact time with patients. Using nursing and office staff to streamline workflow is also helpful.29 The University of Colorado’s “Ambulatory Process Excellence Model” strives to assist doctors by increasing the medical assistant-to-clinician ratio, yielding better productivity.23 Medical assistants are increasingly handling tasks such as data entry, medication reconciliation, and preventive care, to allow physicians more time to focus on medical decision-making.23
Continue to: The role of the EHR
The role of the EHR. One important way to boost professional morale is to simplify and shorten the EHR. The complexity of and reduced patient contact caused by today’s record-keeping systems is the source of great frustration among many physicians. In addition, many patients dislike the disproportionate attention paid by physicians to the computer during office visits, further compromising physician-patient relationships. Improving documentation methodology and/or employing medical assistant scribes can be helpful.30,31 (See “Advanced team-based care: How we made it work” at http://bit.ly/2lNaB5Q.)
Legislation with physician input can mandate policies for more appropriate work environments. A good way to initiate improvement and reform strategies is to contact local medical societies and political representatives. Federal and state collaboration to reduce physician shortages in selected specialties or geographic regions can improve work-related stress. This might be attained by expanding residency programs, using telemedicine in underserved regions, and employing more physician assistants.32
Health insurance. Enhancing universal access to affordable medical care, including pharmaceutical coverage, would alleviate stress for physicians and patients alike.33 Health insurance regulation to decrease paperwork and simplify coverage would decrease physician workload. Standardized policy requirements, fewer exclusionary rules, and simplified prescribing guidelines (including having less cumbersome prescription pre-authorizations and greater standardization of drug formularies by different payer sources or insurance plans) would facilitate better clinical management.
CASE
Dr. D begins by discussing his concerns with his colleagues in the group practice and finds he is not alone. Many of the concerns of the group center around brief, rushed appointments that diminish relationships with patients, a lack of autonomy, and the fear of medical malpractice. Several older physicians acknowledge that they just want to retire.
To address the patient contact and documentation issues, the group decides to hire scribes. They also decide to bring their concerns to the next county medical society meeting. The end result: They petitioned their state medical association to host presentations about mitigating burnout, to hold roundtable discussions, and to establish panels focused on remedying the situation.
Continue to: With this accomplished...
With this accomplished, Dr. D’s anxieties lessened. He surveyed relevant literature and shared tips for improving professional time management with his partners. In a hopeful mood, he volunteered to address burnout prevention at the next statewide medical meeting. He felt it was a good start.
CORRESPONDENCE
Steven Lippmann, MD, 401 E. Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. Ramirez AJ, Graham J, Richards MA, et al. Burnout and psychiatric disorder among cancer clinicians. Br J Cancer. 1995;71:1263-1269.
2. Leiter MP, Maslach C. Latent burnout profiles: a new approach to understanding the burnout experience. Burnout Research. 2016;3:89-100.
3. Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among U.S. medical students. Ann Int Med. 2008;149:334-341.
4. West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
5. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250:463-471.
6. Shanafelt TD, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
7. Cottler LB, Ajinkya S, Merlo LJ, et al. Lifetime psychiatric and substance use disorders among impaired physicians in a physicians health program. J Addict Med. 2013;7:108-112.
8. Consiglio C. Interpersonal strain at work: a new burnout facet relevant for the health of hospital staff. Burnout Res. 2014;1:69-75.
9. Peckham C. Medscape National Physician Burnout and Depression Report 2018. January 12, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Accessed October 4, 2019.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174:527-533.
12. Suñer-Soler R, Grau-Martin A, Flichtentrei D, et al. The consequences of burnout syndrome among healthcare professionals in Spain and Spanish speaking Latin American countries. Burnout Research. 2014;1:82-89.
13. National Taskforce for Humanity in Healthcare. Position paper: The business case for humanity in healthcare. April 2018. https://www.vocera.com/public/pdf/NTHBusinessCase_final003.pdf. Accessed October 4, 2019.
14. Shanafelt TD, Gradishar WJ, Kosty M, et al. Burnout and career satisfaction among US oncologists. J Clin Oncol. 2014;32:678-686.
15. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Int Med. 2012;172:1377-1385.
16. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90;1600-1613.
17. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
18. Maslach C, Jackson SE. The measurement of experienced burnout. J Occcup Behav. 1981;2:99-113.
19. Linzer M, Guzman-Corrales L, Poplau S. Physician Burnout: improve physician satisfaction and patient outcomes. June 5, 2015. https://www.stepsforward.org/modules/physician-burnout. Accessed October 4, 2019.
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388:2272-2281.
21. Nedrow A, Steckler NA, Hardman J. Physician resilience and burnout: can you make the switch? Fam Prac Manag. 2013;20:25-30.
22. Verweij H, van Ravesteijn H, van Hooff MLM, et al. Mindfulness-based stress reduction for residents: a randomized controlled trial. J Gen Intern Med. 2018;33:429-436.
23. Wright AA, Katz IT. Beyond burnout – redesigning care to restore meaning and sanity for physicians. N Eng J Med. 2018;378:309-311.
24. Shanafelt TD, Gorringe G, Menaker R, et. al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90:432-440.
25. Hengerer A, Kishore S. 2017. Breaking a culture of silence: the role of state medical boards. National Academy of Medicine, Washington DC. https://nam.edu/breaking-a-culture-of-silence-the-role-of-state-medical-boards/. Accessed October 4, 2019.
26. American Medical Association. AMA fights burnout with new practice transformation initiative. September 5, 2019. https://www.ama-assn.org/press-center/press-releases/ama-fights-burnout-new-practice-transformation-initiative. Accessed September 5, 2019.
27. Schulte B. Time in the bank: a Stanford plan to save doctors from burnout. The Washington Post. https://www.washingtonpost.com/news/inspired-life/wp/2015/08/20/the-innovative-stanford-program-thats-saving-emergency-room-doctors-from-burnout/?utm_term=.838c930e8de7. Accessed October 4, 2019.
28. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374:1714-1721.
29. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
30. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Info Assoc. 2014;21:E100-E106.
31. Bodenheimer T, Willard-Grace R, Ghorob A. Expanding the roles of medical assistants: who does what in primary care? JAMA Intern Med. 2014;174:1025-1026.
32. Mangiofico G. Physician shortage requires multi-prong solution. January 26, 2018. Am J Manag Care. https://www.ajmc.com/contributor/dr-gary-mangiofico/2018/01/physician-shortage-requires-multiprong-solution. Accessed October 4, 2019.
33. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
1. Ramirez AJ, Graham J, Richards MA, et al. Burnout and psychiatric disorder among cancer clinicians. Br J Cancer. 1995;71:1263-1269.
2. Leiter MP, Maslach C. Latent burnout profiles: a new approach to understanding the burnout experience. Burnout Research. 2016;3:89-100.
3. Dyrbye LN, Thomas MR, Massie FS, et al. Burnout and suicidal ideation among U.S. medical students. Ann Int Med. 2008;149:334-341.
4. West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
5. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250:463-471.
6. Shanafelt TD, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
7. Cottler LB, Ajinkya S, Merlo LJ, et al. Lifetime psychiatric and substance use disorders among impaired physicians in a physicians health program. J Addict Med. 2013;7:108-112.
8. Consiglio C. Interpersonal strain at work: a new burnout facet relevant for the health of hospital staff. Burnout Res. 2014;1:69-75.
9. Peckham C. Medscape National Physician Burnout and Depression Report 2018. January 12, 2018. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Accessed October 4, 2019.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251:995-1000.
11. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174:527-533.
12. Suñer-Soler R, Grau-Martin A, Flichtentrei D, et al. The consequences of burnout syndrome among healthcare professionals in Spain and Spanish speaking Latin American countries. Burnout Research. 2014;1:82-89.
13. National Taskforce for Humanity in Healthcare. Position paper: The business case for humanity in healthcare. April 2018. https://www.vocera.com/public/pdf/NTHBusinessCase_final003.pdf. Accessed October 4, 2019.
14. Shanafelt TD, Gradishar WJ, Kosty M, et al. Burnout and career satisfaction among US oncologists. J Clin Oncol. 2014;32:678-686.
15. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Int Med. 2012;172:1377-1385.
16. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90;1600-1613.
17. Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28-36.
18. Maslach C, Jackson SE. The measurement of experienced burnout. J Occcup Behav. 1981;2:99-113.
19. Linzer M, Guzman-Corrales L, Poplau S. Physician Burnout: improve physician satisfaction and patient outcomes. June 5, 2015. https://www.stepsforward.org/modules/physician-burnout. Accessed October 4, 2019.
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to prevent and reduce physician burnout: a systematic review and meta-analysis. Lancet. 2016;388:2272-2281.
21. Nedrow A, Steckler NA, Hardman J. Physician resilience and burnout: can you make the switch? Fam Prac Manag. 2013;20:25-30.
22. Verweij H, van Ravesteijn H, van Hooff MLM, et al. Mindfulness-based stress reduction for residents: a randomized controlled trial. J Gen Intern Med. 2018;33:429-436.
23. Wright AA, Katz IT. Beyond burnout – redesigning care to restore meaning and sanity for physicians. N Eng J Med. 2018;378:309-311.
24. Shanafelt TD, Gorringe G, Menaker R, et. al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90:432-440.
25. Hengerer A, Kishore S. 2017. Breaking a culture of silence: the role of state medical boards. National Academy of Medicine, Washington DC. https://nam.edu/breaking-a-culture-of-silence-the-role-of-state-medical-boards/. Accessed October 4, 2019.
26. American Medical Association. AMA fights burnout with new practice transformation initiative. September 5, 2019. https://www.ama-assn.org/press-center/press-releases/ama-fights-burnout-new-practice-transformation-initiative. Accessed September 5, 2019.
27. Schulte B. Time in the bank: a Stanford plan to save doctors from burnout. The Washington Post. https://www.washingtonpost.com/news/inspired-life/wp/2015/08/20/the-innovative-stanford-program-thats-saving-emergency-room-doctors-from-burnout/?utm_term=.838c930e8de7. Accessed October 4, 2019.
28. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374:1714-1721.
29. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
30. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Info Assoc. 2014;21:E100-E106.
31. Bodenheimer T, Willard-Grace R, Ghorob A. Expanding the roles of medical assistants: who does what in primary care? JAMA Intern Med. 2014;174:1025-1026.
32. Mangiofico G. Physician shortage requires multi-prong solution. January 26, 2018. Am J Manag Care. https://www.ajmc.com/contributor/dr-gary-mangiofico/2018/01/physician-shortage-requires-multiprong-solution. Accessed October 4, 2019.
33. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
Advanced team-based care: How we made it work
Leaders in health care and practicing physicians recognize the need for changes in how health care is delivered.1-3 Despite this awareness, though, barriers to meaningful change persist and the current practice environment wherein physicians must routinely spend 2 hours on electronic health records (EHRs) and desk work for every hour of direct face time with patients4 is driving trainees away from ambulatory specialties and is contributing to physicians’ decisions to reduce their practices to part-time, retire early, or leave medicine altogether.5,6 Those who persevere in this environment with heavy administrative burdens run the increasing risk of burnout.7
Some physicians and practices are responding by taking creative measures to reform the way patient care is delivered. Bellin Health—a 160-provider, multispecialty health system in northeast Wisconsin where one of the authors (JJ) works—introduced an advanced team-based care (aTBC) model between November 2014 and November 2018, starting with our primary care providers. The development and introduction of this new model arose from an iterative, multidisciplinary process driven by the desire to transform the Triple Aim—enhancing patient experience, improving population health, and reducing costs—into a Quadruple Aim8 by additionally focusing on improving the work life of health care providers, which, in turn, will help achieve the first 3 goals. In introducing an aTBC model, Bellin Health focused on 3 elements: office visit redesign, in-basket management redesign, and the use of extended care team members and system and community resources to assist in the care of complex and high-risk patients.
Herein we describe the 3 components of our aTBC model,1,9 identify the barriers that existed in the minds of multiple stakeholders (from patients to clinicians and Bellin executives), and describe the strategies that enabled us to overcome these barriers.
The impetus behind our move to aTBC
Bellin Health considered a move to an aTBC model to be critical in light of factors in the health care environment, in general, and at Bellin, in particular. The factors included
- an industry-wide shift to value-based payments, which requires new models for long-term financial viability.
- recognition that physician and medical staff burnout leads to lower productivity and, in some cases, workforce losses.5,6 Replacing a physician in a practice can be difficult and expensive, with cost estimates of $500,000 to more than $1 million per physician.10,11
- a belief that aTBC could help the Bellin Health leadership team meet its organizational goals of improved patient satisfaction, achieve gains in quality measures, enhance engagement and loyalty among patients and employees, and lower recruitment costs.
A 3-part aTBC initiative
■ Part 1: Redesign the office visit
We redesigned staffing and workflow for office visits to maximize the core skills of physicians, which required distributing ancillary tasks among support staff. We up-trained certified medical assistants (CMAs) and licensed practical nurses (LPNs) to take on the new role of care team coordinator (CTC) and optimized the direct clinical support ratio for busier physicians. For physicians who were seeing 15 to 19 patients a day, a ratio of 3 CTCs to 2 physicians was implemented; for those seeing 20 or more patients a day, we used a support ratio of 2:1.
The role of CTC was designed so that he or she would accompany a patient throughout the entire appointment. Responsibilities were broken out as follows:
Pre-visit. Before the physician enters the room, the CTC would now perform expanded rooming functions including pending orders, refill management, care gap closure using standing orders, agenda setting, and preliminary documentation.12
Visit. The CTC would now hand off the patient to the physician and stay in the room to document details of the visit and record new orders for consults, x-ray films, referrals, or prescriptions.13 This intensive EHR support was established to ensure that the physician could focus directly on the patient without the distraction of the computer.
Continue to: Post-visit
Post-visit. After a physician leaves a room, the CTC was now charged with finishing the pending orders, setting up the patient’s next appointment and pre-visit labs, reviewing details of the after-visit summary, and doing any basic health coaching with the patient. During this time, the physician would use the co-location space to review and edit the documentation, cosign the orders and prescriptions submitted by the CTC, and close the chart before going into the next room with the second CTC. The need to revisit these details after clinic hours was eliminated.
Another change … The role of our phone triage registered nurses (RN) was expanded. Care team RNs began providing diabetes counseling, blood pressure checks, annual wellness visits (AWV), and follow-up through the Centers for Medicare and Medicaid Services (CMS)'s Chronic Care Management and Transitional Care Management programs.
■ Part 2: Redesign between-visit in-basket management
Responding to an increasing number of inbox messages had become overwhelming for our physicians. Bellin Health’s management was aware that strategic delegation of inbox messages could save an hour or more of a physician’s time each day.14 Bellin implemented a procedure whereby inbox test results would be handled by the same CTC who saw the patient, thereby extending continuity. If the results were normal, the CTC would contact the patient. If the results were abnormal, the physician and the CTC would discuss them and develop a plan. Co-location of the RN, the CTC, and the physician would leverage face-to-face communication and make in-basket management more efficient.
■ Part 3: Redesign population health management
We developed an Extended Care Team (ECT), including social workers, clinical pharmacists, RN care coordinators, and diabetes educators, to assist with the care of patients with high-risk disorders or otherwise complex issues. These team members would work closely with the CTC, care team RN, and physician to review patients, develop plans of care, optimize management, and improve outcomes. Patients would be identified as candidates for potential ECT involvement based on the physician’s judgment in consultation with an EHR-based risk score for hospitalization or emergency department visit.
As we developed new processes, such as screening for determinants of health, we engaged additional system and community resources to help meet the needs of our patients.
Continue to: A look at stakeholder concerns and overcoming the barriers
A look at stakeholder concerns and overcoming the barriers
Critical to our success was being attentive to the concerns of our stakeholders and addressing them. Along the way, we gained valuable implementation insights, which we share here along with some specifics about how, exactly, we did things at Bellin.
Patients
Some patients expressed hesitation at having a person other than their physician in the exam room. They worried that the intimacy and privacy with their physician would be lost. In light of this, we gave patients the option not to have the CTC remain in the room. However, patients quickly saw the value of this team-based care approach and seldom asked to be seen without the CTC.
Throughout the process, we surveyed patients for feedback on their experiences. Comments indicated that the presence of the CTC in our team-based model led to positive patient experiences:
My physician is fully attentive. Patients appreciated that physicians were not distracted by the computer in the exam room. “I feel like I’ve got my doctor back” has been a common refrain.
The office staff is more responsive. The CTC, having been present during the appointment, has a deeper understanding of the care plan and can respond to calls or emails between visits, thereby reducing the time patients must wait for answers. One patient commented that, “I love [the doctor’s] team; his nurses are willing to answer every question I have.”
Continue to: I increasingly feel that I'm understood
I increasingly feel that I’m understood. We have seen patients develop meaningful relationships with other team members, confiding in them in ways that they hadn’t always done with physicians and advanced practice clinicians (APCs). Team members, in turn, have added valuable insights that help optimize patients’ care. In particular, the care of patients with multiple needs has been enhanced with the addition of ECT members who work with the core team and use their expertise to optimize the care of these patients.
Certified medical assistants and licensed practical nurses
Bellin’s leadership knew that team documentation could cause stress for the CMA, who, acting as a CTC, wanted to avoid misrepresenting details of the clinical encounter.13 Adding to the stress were other duties that would need to be learned, including agenda setting, refill management, care gap closure, and health coaching. With thorough training and preparation, many—but not all—of our CMAs and LPNs were able to successfully make the transition and flourish.
Implementation strategies
Provide thorough training. Our training process started 8 weeks before it was time to “go live.” There were weekly hour-long training sessions in population health basics, team culture and change management, documentation basics, and new roles and responsibilities. In the final week, the entire aTBC team sat together for 3 days of EHR training. All new teams shadowed existing teams to get a clear picture of the new processes.
Create a community of support. As our CMAs adapted to their new CTC roles, it was critical that they had support from experienced CTCs. Encouragement and patience from physicians were—and are—essential for CTCs to develop confidence in their new roles.
Enable ongoing feedback. We introduced weekly team meetings to enhance team communication and dynamics. Forums for all roles are held periodically to facilitate discussion, share learning, and enable support between teams.
Continue to: Use EHR tools to facilitate this work
Use EHR tools to facilitate this work. Using standard templates and documentation tools helped CTCs develop the confidence needed to thrive in their new role. Knowing these tools were available helped CTCs become effective in helping the team manage the between-visit work.
Monitor workload. As we developed more workflows and processes, we took care to monitor the amount of additional work for those in this role. We offloaded work whenever possible. For example, coordinated refill management at time of service, coupled with a back-up centralized refill system, can significantly decrease the number of refill requests made to CTCs. We continue to adjust staffing, where appropriate, to provide adequate support for those in this valuable role.
Be prepared for turnover. As CTCs became empowered in their new roles, some decided to advance their training into other roles. We developed a plan for replacing and training new staff. Higher pay can also be used to help attract and retain these staff members. Bellin uses LPNs in this role to ensure adequate staffing. Other health systems have developed a tier system for CMAs to improve retention.
Registered nurses
Before our move to an aTBC model, our office RNs primarily managed phone triage. Now the nurses were enlisted to play a more active role in patient care and team leadership. Although it was a dramatic departure from prior responsibilities, the majority of Bellin’s RNs have found increased satisfaction in taking on direct patient care.
Implementation strategies
Define new roles and provide training. In addition to participating in acute patient visits, consider ways that care team RNs can expand responsibilities as they pertain to disease counseling, population health management, and team leadership.15 At Bellin, the expanded role of the RN is evident in diabetes education and Medicare AWVs. Specifically, RNs now provide diabetes education to appropriate patients following a warm handoff from the physician at the time of the visit. RNs now also complete Medicare AWVs, which frees up physicians for other tasks and helps ensure sustainability for the new RN roles. Rates of completed AWVs at Bellin are now more than 70%, compared with reported national rates of less than 30%.16
Continue to: Maximize co-location
Maximize co-location. It is helpful to have the team members whose work is closely related—such as the CTCs and the RN for the team—to be situated near each other, rather than down a hall or in separate offices. Since the RN is co-located with the core teams at Bellin, there is now greater opportunity for verbal interaction, rather than just electronic communications, for matters such as triage calls and results management. RNs also provide a valuable resource for CMAs and LPNs, as well as help oversee team management of the in-basket.
Evaluate sustainability. Additional roles for the RNs required additional RN staffing. We assessed the new workload duties and balanced that against potential revenue from RN visits. This analysis indicated that an optimal ratio was 1 RN to every 3000 patients. This would allow an adequate number of RNs to fulfill additional roles and was financially sustainable with the goal of 4 billable RN visits per day.
Physicians
Bellin’s leadership recognized that some physicians might perceive team-based care as eroding their primary responsibility for patients’ care. Physicians have historically been trained in a model based on the primacy of the individual physician and that can be a hurdle to embracing team culture as a new paradigm of care. Several strategies helped us and can help others, too.
Implementation strategies
Cultivate trust. Thorough training of CTCs and RNs is critical to helping physicians develop trust and reliance in the team. The physician retains final authority over the team for cosigning orders, editing and finalizing documentation, and overseeing results management. Physicians invested in training and educating their staff will reap the rewards of a highly functioning, more satisfied team.
Encourage leadership. This can be a cultural shift for physicians, yet it is critical that they take a leadership role in this transformation.17 Physicians and their team leaders attended training sessions in team culture and change management. Prior to the go-live date, team leaders also met with the physician individually to explore their concerns and discuss ways to effectively lead and support their teams.
Continue to: Urge acceptance of support
Urge acceptance of support. The complexity of patient care today makes it difficult for a physician to manage all of a patient’s needs single-handedly. Complexity arises from the variety of plan co-pays and deductibles, the number of patients with chronic diseases, and the increased emphasis on improving quality measures.18 Enhanced support during any office visit and the extra support of an ECT for complex patients improves the ability of the physician to more effectively meet the needs of the patient.
Emphasize the benefit of an empowered team. The demands of the EHR on physicians and the resultant frustrations are well chronicled.4,19-22 Strategically delegating much of this work to other team members allows the physician to focus on the patient and perform physician-level work. At Bellin, we observed that our most successful care teams were those in which the physician fully accepted team-based care principles and empowered the staff to work at the top of their skill set.
Advanced practice clinicians
APCs in our system had traditionally practiced in 1 of 3 ways: independently handling defined panels with physician supervision; handling overflow or acute visits; or working collaboratively with a supervising physician to share a larger “team panel.” The third approach has become our preferred model. aTBC provides opportunities for APCs to thrive and collaborate with the physician to provide excellent care for patients.
APCs underwent the same process changes as physicians, including appropriate CTC support. Implementation strategies for APCs were similar to those that were useful for physicians.
Risk management professionals
At Bellin, we found that risk-management professionals had concerns about the scope of practice assigned to various team members, particularly regarding documentation. CMS allows for elements of a patient visit to be documented by CMAs and other members of the care team in real time as authorized by the physician.23,24 CTCs at Bellin also have other clinical duties in patient and EHR management. aTBC practices generally prefer the term team documentation over scribing, since it more accurately reflects the scope of the CTC’s work.
Continue to: Implementation strategies
Implementation strategies
Clarify regulatory issues. Extensive use of standing orders and protocols allowed us to increase involvement of various team members. State laws vary in what functions CMAs and LPNs are allowed to perform, so it is important to check your state guidelines.25 There is a tendency for some risk managers to overinterpret regulations. Challenge them to provide exact documentation from regulatory agencies to support their decisions.
Give assurances of physician oversight and processes. The physician assumes responsibility for standing orders, protocols, and documentation. We made sure that we had clear and consistent processes in place and worked closely with our risk managers as we developed our model. aTBC provides checks and balances to ensure accurate records, since team members are able to contribute and check for accuracy. A recent study suggested that CMAs perform documentation that is of equal or higher quality than that performed by the physician.26
Financial leadership
Like any organization adopting aTBC, Bellin’s leadership was concerned about the expense of adopting this approach. However, the leadership also recognized that the transition to aTBC could increase revenue by more than the increased staffing costs. In addition, we expected that capacity, access, continuity, and financial margins would increase.2,3,27,28 We also anticipated a decrease in downstream services, such as unnecessary tests, emergency department visits, and hospitalizations—a benefit of accountable care payment models.
Our efforts have been successful from a financial point of view. We attribute the financial sustainability that we have experienced to 4 factors:
1. Increased productivity. We knew that the increased efficiency of team-based care enables physicians to see 1 to 2 more patients per half day, and sometimes more.3,28,29 An increase of at least 1 patient visit per half-day was expected of our physicians and APCs on aTBC. In addition, they were expected to support the care team RN in achieving at least 4 billable visits per day. Our current level of RN visits is at 3.5 per nurse per day. There is significant variability in the increase of patients seen by a physician per day, ranging from 1 to 4 additional patients. These increased visits have helped us achieve financial viability, even in a predominantly fee-for-service environment.
2. More thorough service. The ability to keep patients in primary care and to focus on the patient’s full range of needs has led to higher levels of service and, consequently, to appropriately higher levels of billing codes. For example, Bellin’s revenue from billing increased by $724 per patient, related (in part) to higher rates of immunizations, cancer screenings with mammography, and colonoscopies.
Continue to: 3. New billable services
3. New billable services. Billing for RN blood pressure checks, AWVs, and extended care team services have helped make aTBC at Bellin financially feasible. Revenue from RN visits, for example, was $630,000 in 2018.
4. Improved access for patients. Of the 130 primary care providers now on aTBC, 15 (11.5%) had closed their practices to new patients before aTBC. Now, all of their practices are open to new patients, which has improved access to care. In a 2018 patient access survey, 96.6% of patients obtained an appointment as soon as they thought it was needed, compared with 70.7% of patients before the transition to aTBC.
Greater opportunity for financial sustainability. The combination of improved quality measures and decreased cost of care in the Bellin aTBC bodes well for future success in a value-based world. We have realized a significant increase in value-based payments for improved quality, and in our Next Gen Accountable Care Organization (ACO) patients, we have seen a decrease of $29 in per-member-per-month costs, likely due to the use of nonphysicians in expanded roles. In addition, hospital admissions have decreased by 5% due to the ability of ambulatory teams to manage more complex patients in the office setting. This model has also allowed physicians and APCs to increase their panel size, another key value-based metric. From 2016 to 2018, panel size for primary care providers increased by an average of 8%.
Enhanced ability to retain and recruit. Several of Bellin’s primary care recruits indicated that they had interviewed only at practices incorporating team-based care. This trend may increase as residencies transition to team-based models of care.
So how did we do?
Metrics of Bellin’s aTBC success
By the end of 2018, all 130 primary care physicians and APCs at Bellin had made the transition to this model, representing family medicine, internal medicine, and pediatrics. We have now begun the transition of our non-primary care specialties to team-based care.
Continue to: In the aTBC model...
In the aTBC model, the percentage of patients receiving age-appropriate screening is higher than before in every domain we measure (FIGURE 1). There has also been improvement in major quality metrics (FIGURE 2).
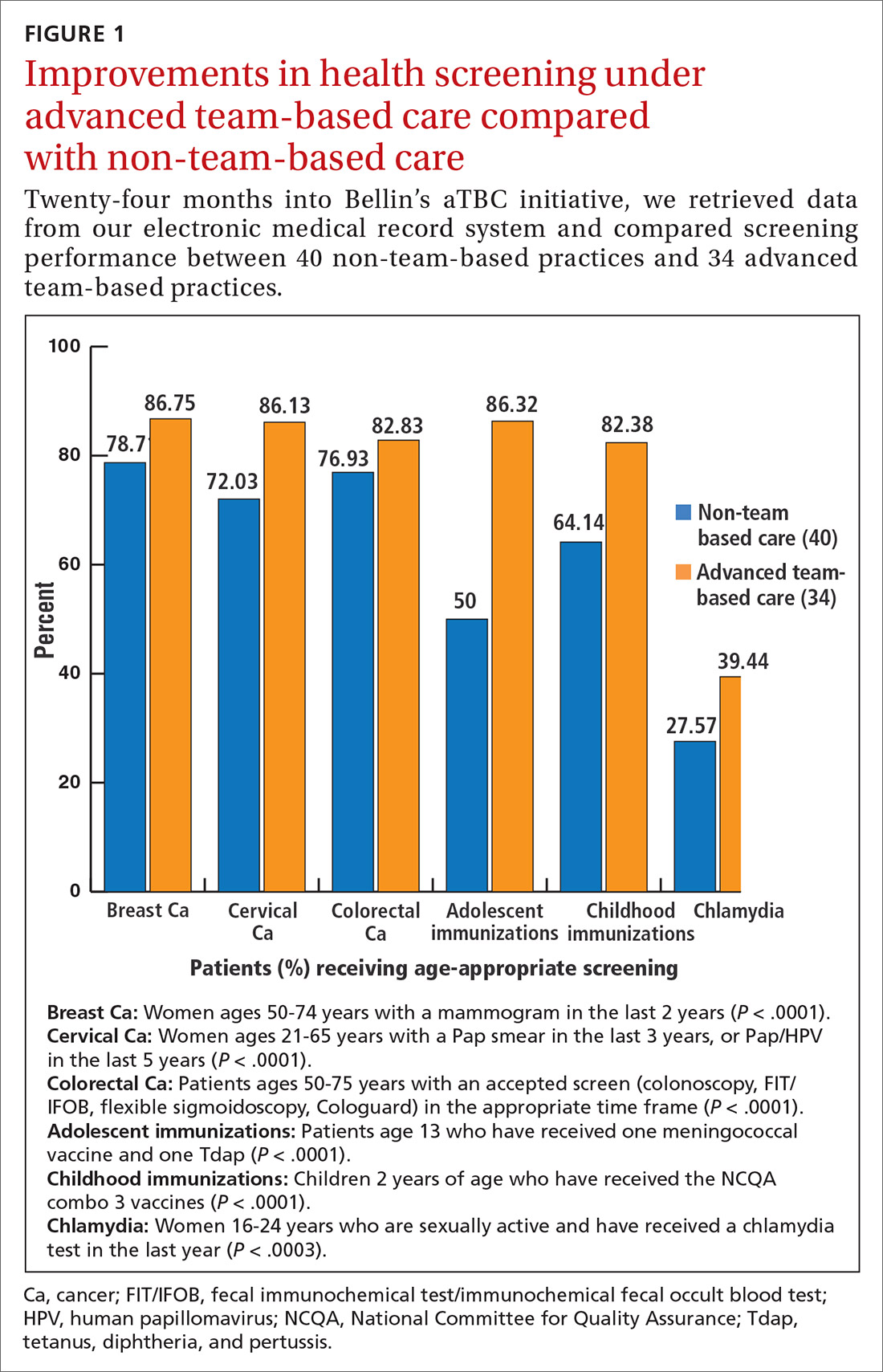
In a survey done in Spring 2018 by St. Norbert College Strategic Research Center, provider satisfaction increased, with 83% of physicians having made the transition to an aTBC practice moderately or very satisfied with their Bellin Health experience, compared with 70% in the traditional model. More recent 2019 survey data show a satisfaction rate of 90% for team-based care providers. Finally, in our aTBC model—in CMS’s Next-Gen ACO initiative—the cost per patient per month is significantly less than for those in a non-team-based care model ($796 vs $940).

CORRESPONDENCE
James Jerzak, MD, 1630 Commanche Ave, Green Bay, WI 54313; [email protected].
ACKNOWLEDGEMENTS
The authors would like to thank Lindsey E. Carlasare, MBA, from the American Medical Association, and Brad Wozney, MD, Kathy Kerscher, and Christopher Elfner from Bellin Health, for their contributions to the content and review of this manuscript.
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
3. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
4. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
5. Shanafelt TD, Mungo M, Schmitgen J, et al. Longitudinal study evaluating the association between physician burnout and changes in professional work effort. Mayo Clin Proc. 2016;91:422-431.
6. Sinsky CA, Dyrbye LN, West CP, et al. Professional satisfaction and the career plans of US physicians. Mayo Clin Proc. 2017;92:1625-1635.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90:1600-1613.
8. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
9. Sinsky CA, Sinsky TA, Althaus D, et al. Practice profile. ‘Core teams’: nurse-physician partnerships provide patient-centered care at an Iowa practice. Health Aff (Millwood). 2010;29:966-968.
10. Shanafelt T, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
11. Association for Advancing Physician and Provider Recruitment. Schutte L. What you don’t know can cost you: building a business case for recruitment and retention best practices. 2012. https://member.aappr.org/general/custom.asp?page=696. Accessed June 20, 2019.
12. American Medical Association. AMA STEPS Forward. Expanded rooming and discharge protocols. https://edhub.ama-assn.org/steps-forward/module/2702600. Accessed June 20, 2019.
13. American Medical Association. AMA STEPS Forward. Team documentation. https://edhub.ama-assn.org/steps-forward/module/2702598?resultClick=3&bypassSolrId=J_2702598. Accessed June 20, 2019.
14. American Medical Association. AMA STEPS Forward. EHR in-basket restructuring for improved efficiency. https://edhub.ama-assn.org/steps-forward/module/2702694?resultClick=3&bypassSolrId=J_2702694. Accessed June 20, 2019.
15. California Health Care Foundation. Bodenheimer T, Bauer L, Olayiwola JN. RN role reimagined: how empowering registered nurses can improve primary care. https://www.chcf.org/publication/rn-role-reimagined-how-empowering-registered-nurses-can-improve-primary-care/. Accessed June 20, 2019.
16. Chung S, Lesser LI, Lauderdale DS, et al. Medicare annual preventive care visits: use increased among fee-for-service patients, but many do not participate. Health Aff (Millwood). 2015;34:11-20.
17. American Medical Association. AMA Policy H-160.912. The structure and function of interprofessional health care teams. https://policysearch.ama-assn.org/policyfinder/detail/The%20Structure%20and%20Function%20of%20Interprofessional%20Health%20Care%20Teams?uri=%2FAMADoc%2FHOD.xml-0-727.xml. Accessed June 20, 2019.
18. Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128:337-343.
19. Hill RG Jr, Sears LM, Melanson SW. 4000 clicks: a productivity analysis of electronic medical records in a community hospital ED. Am J Emerg Med. 2013;31:1591-1594.
20. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21:e100-e106.
21. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
22. RAND Corporation. Friedberg MW, Chen PG, Ban Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. https://www.rand.org/pubs/research_reports/RR439.html. Accessed June 20, 2019.
23. Evaluation and Management (E/M) visit frequently asked questions (FAQs): physician fee schedule (PPS). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/E-M-Visit-FAQs-PFS.pdf. Accessed August 27, 2019.
24. Centers for Medicare & Medicaid Services. Scribe services signature requirements. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017-Transmittals-Items/R713PI.html. Accessed June 20, 2019.
25. American Association of Medical Assistants. State scope of practice laws. http://www.aama-ntl.org/employers/state-scope-of-practice-laws. Accessed June 20, 2019.
26. Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65:155-159.
27. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
28. Bank AJ, Obetz C, Konrardy A, et al. Impact of scribes on patient interaction, productivity, and revenue in a cardiology clinic: a prospective study. Clinicoecon Outcomes Res. 2013;5:399-406.
29. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
Leaders in health care and practicing physicians recognize the need for changes in how health care is delivered.1-3 Despite this awareness, though, barriers to meaningful change persist and the current practice environment wherein physicians must routinely spend 2 hours on electronic health records (EHRs) and desk work for every hour of direct face time with patients4 is driving trainees away from ambulatory specialties and is contributing to physicians’ decisions to reduce their practices to part-time, retire early, or leave medicine altogether.5,6 Those who persevere in this environment with heavy administrative burdens run the increasing risk of burnout.7
Some physicians and practices are responding by taking creative measures to reform the way patient care is delivered. Bellin Health—a 160-provider, multispecialty health system in northeast Wisconsin where one of the authors (JJ) works—introduced an advanced team-based care (aTBC) model between November 2014 and November 2018, starting with our primary care providers. The development and introduction of this new model arose from an iterative, multidisciplinary process driven by the desire to transform the Triple Aim—enhancing patient experience, improving population health, and reducing costs—into a Quadruple Aim8 by additionally focusing on improving the work life of health care providers, which, in turn, will help achieve the first 3 goals. In introducing an aTBC model, Bellin Health focused on 3 elements: office visit redesign, in-basket management redesign, and the use of extended care team members and system and community resources to assist in the care of complex and high-risk patients.
Herein we describe the 3 components of our aTBC model,1,9 identify the barriers that existed in the minds of multiple stakeholders (from patients to clinicians and Bellin executives), and describe the strategies that enabled us to overcome these barriers.
The impetus behind our move to aTBC
Bellin Health considered a move to an aTBC model to be critical in light of factors in the health care environment, in general, and at Bellin, in particular. The factors included
- an industry-wide shift to value-based payments, which requires new models for long-term financial viability.
- recognition that physician and medical staff burnout leads to lower productivity and, in some cases, workforce losses.5,6 Replacing a physician in a practice can be difficult and expensive, with cost estimates of $500,000 to more than $1 million per physician.10,11
- a belief that aTBC could help the Bellin Health leadership team meet its organizational goals of improved patient satisfaction, achieve gains in quality measures, enhance engagement and loyalty among patients and employees, and lower recruitment costs.
A 3-part aTBC initiative
■ Part 1: Redesign the office visit
We redesigned staffing and workflow for office visits to maximize the core skills of physicians, which required distributing ancillary tasks among support staff. We up-trained certified medical assistants (CMAs) and licensed practical nurses (LPNs) to take on the new role of care team coordinator (CTC) and optimized the direct clinical support ratio for busier physicians. For physicians who were seeing 15 to 19 patients a day, a ratio of 3 CTCs to 2 physicians was implemented; for those seeing 20 or more patients a day, we used a support ratio of 2:1.
The role of CTC was designed so that he or she would accompany a patient throughout the entire appointment. Responsibilities were broken out as follows:
Pre-visit. Before the physician enters the room, the CTC would now perform expanded rooming functions including pending orders, refill management, care gap closure using standing orders, agenda setting, and preliminary documentation.12
Visit. The CTC would now hand off the patient to the physician and stay in the room to document details of the visit and record new orders for consults, x-ray films, referrals, or prescriptions.13 This intensive EHR support was established to ensure that the physician could focus directly on the patient without the distraction of the computer.
Continue to: Post-visit
Post-visit. After a physician leaves a room, the CTC was now charged with finishing the pending orders, setting up the patient’s next appointment and pre-visit labs, reviewing details of the after-visit summary, and doing any basic health coaching with the patient. During this time, the physician would use the co-location space to review and edit the documentation, cosign the orders and prescriptions submitted by the CTC, and close the chart before going into the next room with the second CTC. The need to revisit these details after clinic hours was eliminated.
Another change … The role of our phone triage registered nurses (RN) was expanded. Care team RNs began providing diabetes counseling, blood pressure checks, annual wellness visits (AWV), and follow-up through the Centers for Medicare and Medicaid Services (CMS)'s Chronic Care Management and Transitional Care Management programs.
■ Part 2: Redesign between-visit in-basket management
Responding to an increasing number of inbox messages had become overwhelming for our physicians. Bellin Health’s management was aware that strategic delegation of inbox messages could save an hour or more of a physician’s time each day.14 Bellin implemented a procedure whereby inbox test results would be handled by the same CTC who saw the patient, thereby extending continuity. If the results were normal, the CTC would contact the patient. If the results were abnormal, the physician and the CTC would discuss them and develop a plan. Co-location of the RN, the CTC, and the physician would leverage face-to-face communication and make in-basket management more efficient.
■ Part 3: Redesign population health management
We developed an Extended Care Team (ECT), including social workers, clinical pharmacists, RN care coordinators, and diabetes educators, to assist with the care of patients with high-risk disorders or otherwise complex issues. These team members would work closely with the CTC, care team RN, and physician to review patients, develop plans of care, optimize management, and improve outcomes. Patients would be identified as candidates for potential ECT involvement based on the physician’s judgment in consultation with an EHR-based risk score for hospitalization or emergency department visit.
As we developed new processes, such as screening for determinants of health, we engaged additional system and community resources to help meet the needs of our patients.
Continue to: A look at stakeholder concerns and overcoming the barriers
A look at stakeholder concerns and overcoming the barriers
Critical to our success was being attentive to the concerns of our stakeholders and addressing them. Along the way, we gained valuable implementation insights, which we share here along with some specifics about how, exactly, we did things at Bellin.
Patients
Some patients expressed hesitation at having a person other than their physician in the exam room. They worried that the intimacy and privacy with their physician would be lost. In light of this, we gave patients the option not to have the CTC remain in the room. However, patients quickly saw the value of this team-based care approach and seldom asked to be seen without the CTC.
Throughout the process, we surveyed patients for feedback on their experiences. Comments indicated that the presence of the CTC in our team-based model led to positive patient experiences:
My physician is fully attentive. Patients appreciated that physicians were not distracted by the computer in the exam room. “I feel like I’ve got my doctor back” has been a common refrain.
The office staff is more responsive. The CTC, having been present during the appointment, has a deeper understanding of the care plan and can respond to calls or emails between visits, thereby reducing the time patients must wait for answers. One patient commented that, “I love [the doctor’s] team; his nurses are willing to answer every question I have.”
Continue to: I increasingly feel that I'm understood
I increasingly feel that I’m understood. We have seen patients develop meaningful relationships with other team members, confiding in them in ways that they hadn’t always done with physicians and advanced practice clinicians (APCs). Team members, in turn, have added valuable insights that help optimize patients’ care. In particular, the care of patients with multiple needs has been enhanced with the addition of ECT members who work with the core team and use their expertise to optimize the care of these patients.
Certified medical assistants and licensed practical nurses
Bellin’s leadership knew that team documentation could cause stress for the CMA, who, acting as a CTC, wanted to avoid misrepresenting details of the clinical encounter.13 Adding to the stress were other duties that would need to be learned, including agenda setting, refill management, care gap closure, and health coaching. With thorough training and preparation, many—but not all—of our CMAs and LPNs were able to successfully make the transition and flourish.
Implementation strategies
Provide thorough training. Our training process started 8 weeks before it was time to “go live.” There were weekly hour-long training sessions in population health basics, team culture and change management, documentation basics, and new roles and responsibilities. In the final week, the entire aTBC team sat together for 3 days of EHR training. All new teams shadowed existing teams to get a clear picture of the new processes.
Create a community of support. As our CMAs adapted to their new CTC roles, it was critical that they had support from experienced CTCs. Encouragement and patience from physicians were—and are—essential for CTCs to develop confidence in their new roles.
Enable ongoing feedback. We introduced weekly team meetings to enhance team communication and dynamics. Forums for all roles are held periodically to facilitate discussion, share learning, and enable support between teams.
Continue to: Use EHR tools to facilitate this work
Use EHR tools to facilitate this work. Using standard templates and documentation tools helped CTCs develop the confidence needed to thrive in their new role. Knowing these tools were available helped CTCs become effective in helping the team manage the between-visit work.
Monitor workload. As we developed more workflows and processes, we took care to monitor the amount of additional work for those in this role. We offloaded work whenever possible. For example, coordinated refill management at time of service, coupled with a back-up centralized refill system, can significantly decrease the number of refill requests made to CTCs. We continue to adjust staffing, where appropriate, to provide adequate support for those in this valuable role.
Be prepared for turnover. As CTCs became empowered in their new roles, some decided to advance their training into other roles. We developed a plan for replacing and training new staff. Higher pay can also be used to help attract and retain these staff members. Bellin uses LPNs in this role to ensure adequate staffing. Other health systems have developed a tier system for CMAs to improve retention.
Registered nurses
Before our move to an aTBC model, our office RNs primarily managed phone triage. Now the nurses were enlisted to play a more active role in patient care and team leadership. Although it was a dramatic departure from prior responsibilities, the majority of Bellin’s RNs have found increased satisfaction in taking on direct patient care.
Implementation strategies
Define new roles and provide training. In addition to participating in acute patient visits, consider ways that care team RNs can expand responsibilities as they pertain to disease counseling, population health management, and team leadership.15 At Bellin, the expanded role of the RN is evident in diabetes education and Medicare AWVs. Specifically, RNs now provide diabetes education to appropriate patients following a warm handoff from the physician at the time of the visit. RNs now also complete Medicare AWVs, which frees up physicians for other tasks and helps ensure sustainability for the new RN roles. Rates of completed AWVs at Bellin are now more than 70%, compared with reported national rates of less than 30%.16
Continue to: Maximize co-location
Maximize co-location. It is helpful to have the team members whose work is closely related—such as the CTCs and the RN for the team—to be situated near each other, rather than down a hall or in separate offices. Since the RN is co-located with the core teams at Bellin, there is now greater opportunity for verbal interaction, rather than just electronic communications, for matters such as triage calls and results management. RNs also provide a valuable resource for CMAs and LPNs, as well as help oversee team management of the in-basket.
Evaluate sustainability. Additional roles for the RNs required additional RN staffing. We assessed the new workload duties and balanced that against potential revenue from RN visits. This analysis indicated that an optimal ratio was 1 RN to every 3000 patients. This would allow an adequate number of RNs to fulfill additional roles and was financially sustainable with the goal of 4 billable RN visits per day.
Physicians
Bellin’s leadership recognized that some physicians might perceive team-based care as eroding their primary responsibility for patients’ care. Physicians have historically been trained in a model based on the primacy of the individual physician and that can be a hurdle to embracing team culture as a new paradigm of care. Several strategies helped us and can help others, too.
Implementation strategies
Cultivate trust. Thorough training of CTCs and RNs is critical to helping physicians develop trust and reliance in the team. The physician retains final authority over the team for cosigning orders, editing and finalizing documentation, and overseeing results management. Physicians invested in training and educating their staff will reap the rewards of a highly functioning, more satisfied team.
Encourage leadership. This can be a cultural shift for physicians, yet it is critical that they take a leadership role in this transformation.17 Physicians and their team leaders attended training sessions in team culture and change management. Prior to the go-live date, team leaders also met with the physician individually to explore their concerns and discuss ways to effectively lead and support their teams.
Continue to: Urge acceptance of support
Urge acceptance of support. The complexity of patient care today makes it difficult for a physician to manage all of a patient’s needs single-handedly. Complexity arises from the variety of plan co-pays and deductibles, the number of patients with chronic diseases, and the increased emphasis on improving quality measures.18 Enhanced support during any office visit and the extra support of an ECT for complex patients improves the ability of the physician to more effectively meet the needs of the patient.
Emphasize the benefit of an empowered team. The demands of the EHR on physicians and the resultant frustrations are well chronicled.4,19-22 Strategically delegating much of this work to other team members allows the physician to focus on the patient and perform physician-level work. At Bellin, we observed that our most successful care teams were those in which the physician fully accepted team-based care principles and empowered the staff to work at the top of their skill set.
Advanced practice clinicians
APCs in our system had traditionally practiced in 1 of 3 ways: independently handling defined panels with physician supervision; handling overflow or acute visits; or working collaboratively with a supervising physician to share a larger “team panel.” The third approach has become our preferred model. aTBC provides opportunities for APCs to thrive and collaborate with the physician to provide excellent care for patients.
APCs underwent the same process changes as physicians, including appropriate CTC support. Implementation strategies for APCs were similar to those that were useful for physicians.
Risk management professionals
At Bellin, we found that risk-management professionals had concerns about the scope of practice assigned to various team members, particularly regarding documentation. CMS allows for elements of a patient visit to be documented by CMAs and other members of the care team in real time as authorized by the physician.23,24 CTCs at Bellin also have other clinical duties in patient and EHR management. aTBC practices generally prefer the term team documentation over scribing, since it more accurately reflects the scope of the CTC’s work.
Continue to: Implementation strategies
Implementation strategies
Clarify regulatory issues. Extensive use of standing orders and protocols allowed us to increase involvement of various team members. State laws vary in what functions CMAs and LPNs are allowed to perform, so it is important to check your state guidelines.25 There is a tendency for some risk managers to overinterpret regulations. Challenge them to provide exact documentation from regulatory agencies to support their decisions.
Give assurances of physician oversight and processes. The physician assumes responsibility for standing orders, protocols, and documentation. We made sure that we had clear and consistent processes in place and worked closely with our risk managers as we developed our model. aTBC provides checks and balances to ensure accurate records, since team members are able to contribute and check for accuracy. A recent study suggested that CMAs perform documentation that is of equal or higher quality than that performed by the physician.26
Financial leadership
Like any organization adopting aTBC, Bellin’s leadership was concerned about the expense of adopting this approach. However, the leadership also recognized that the transition to aTBC could increase revenue by more than the increased staffing costs. In addition, we expected that capacity, access, continuity, and financial margins would increase.2,3,27,28 We also anticipated a decrease in downstream services, such as unnecessary tests, emergency department visits, and hospitalizations—a benefit of accountable care payment models.
Our efforts have been successful from a financial point of view. We attribute the financial sustainability that we have experienced to 4 factors:
1. Increased productivity. We knew that the increased efficiency of team-based care enables physicians to see 1 to 2 more patients per half day, and sometimes more.3,28,29 An increase of at least 1 patient visit per half-day was expected of our physicians and APCs on aTBC. In addition, they were expected to support the care team RN in achieving at least 4 billable visits per day. Our current level of RN visits is at 3.5 per nurse per day. There is significant variability in the increase of patients seen by a physician per day, ranging from 1 to 4 additional patients. These increased visits have helped us achieve financial viability, even in a predominantly fee-for-service environment.
2. More thorough service. The ability to keep patients in primary care and to focus on the patient’s full range of needs has led to higher levels of service and, consequently, to appropriately higher levels of billing codes. For example, Bellin’s revenue from billing increased by $724 per patient, related (in part) to higher rates of immunizations, cancer screenings with mammography, and colonoscopies.
Continue to: 3. New billable services
3. New billable services. Billing for RN blood pressure checks, AWVs, and extended care team services have helped make aTBC at Bellin financially feasible. Revenue from RN visits, for example, was $630,000 in 2018.
4. Improved access for patients. Of the 130 primary care providers now on aTBC, 15 (11.5%) had closed their practices to new patients before aTBC. Now, all of their practices are open to new patients, which has improved access to care. In a 2018 patient access survey, 96.6% of patients obtained an appointment as soon as they thought it was needed, compared with 70.7% of patients before the transition to aTBC.
Greater opportunity for financial sustainability. The combination of improved quality measures and decreased cost of care in the Bellin aTBC bodes well for future success in a value-based world. We have realized a significant increase in value-based payments for improved quality, and in our Next Gen Accountable Care Organization (ACO) patients, we have seen a decrease of $29 in per-member-per-month costs, likely due to the use of nonphysicians in expanded roles. In addition, hospital admissions have decreased by 5% due to the ability of ambulatory teams to manage more complex patients in the office setting. This model has also allowed physicians and APCs to increase their panel size, another key value-based metric. From 2016 to 2018, panel size for primary care providers increased by an average of 8%.
Enhanced ability to retain and recruit. Several of Bellin’s primary care recruits indicated that they had interviewed only at practices incorporating team-based care. This trend may increase as residencies transition to team-based models of care.
So how did we do?
Metrics of Bellin’s aTBC success
By the end of 2018, all 130 primary care physicians and APCs at Bellin had made the transition to this model, representing family medicine, internal medicine, and pediatrics. We have now begun the transition of our non-primary care specialties to team-based care.
Continue to: In the aTBC model...
In the aTBC model, the percentage of patients receiving age-appropriate screening is higher than before in every domain we measure (FIGURE 1). There has also been improvement in major quality metrics (FIGURE 2).

In a survey done in Spring 2018 by St. Norbert College Strategic Research Center, provider satisfaction increased, with 83% of physicians having made the transition to an aTBC practice moderately or very satisfied with their Bellin Health experience, compared with 70% in the traditional model. More recent 2019 survey data show a satisfaction rate of 90% for team-based care providers. Finally, in our aTBC model—in CMS’s Next-Gen ACO initiative—the cost per patient per month is significantly less than for those in a non-team-based care model ($796 vs $940).

CORRESPONDENCE
James Jerzak, MD, 1630 Commanche Ave, Green Bay, WI 54313; [email protected].
ACKNOWLEDGEMENTS
The authors would like to thank Lindsey E. Carlasare, MBA, from the American Medical Association, and Brad Wozney, MD, Kathy Kerscher, and Christopher Elfner from Bellin Health, for their contributions to the content and review of this manuscript.
Leaders in health care and practicing physicians recognize the need for changes in how health care is delivered.1-3 Despite this awareness, though, barriers to meaningful change persist and the current practice environment wherein physicians must routinely spend 2 hours on electronic health records (EHRs) and desk work for every hour of direct face time with patients4 is driving trainees away from ambulatory specialties and is contributing to physicians’ decisions to reduce their practices to part-time, retire early, or leave medicine altogether.5,6 Those who persevere in this environment with heavy administrative burdens run the increasing risk of burnout.7
Some physicians and practices are responding by taking creative measures to reform the way patient care is delivered. Bellin Health—a 160-provider, multispecialty health system in northeast Wisconsin where one of the authors (JJ) works—introduced an advanced team-based care (aTBC) model between November 2014 and November 2018, starting with our primary care providers. The development and introduction of this new model arose from an iterative, multidisciplinary process driven by the desire to transform the Triple Aim—enhancing patient experience, improving population health, and reducing costs—into a Quadruple Aim8 by additionally focusing on improving the work life of health care providers, which, in turn, will help achieve the first 3 goals. In introducing an aTBC model, Bellin Health focused on 3 elements: office visit redesign, in-basket management redesign, and the use of extended care team members and system and community resources to assist in the care of complex and high-risk patients.
Herein we describe the 3 components of our aTBC model,1,9 identify the barriers that existed in the minds of multiple stakeholders (from patients to clinicians and Bellin executives), and describe the strategies that enabled us to overcome these barriers.
The impetus behind our move to aTBC
Bellin Health considered a move to an aTBC model to be critical in light of factors in the health care environment, in general, and at Bellin, in particular. The factors included
- an industry-wide shift to value-based payments, which requires new models for long-term financial viability.
- recognition that physician and medical staff burnout leads to lower productivity and, in some cases, workforce losses.5,6 Replacing a physician in a practice can be difficult and expensive, with cost estimates of $500,000 to more than $1 million per physician.10,11
- a belief that aTBC could help the Bellin Health leadership team meet its organizational goals of improved patient satisfaction, achieve gains in quality measures, enhance engagement and loyalty among patients and employees, and lower recruitment costs.
A 3-part aTBC initiative
■ Part 1: Redesign the office visit
We redesigned staffing and workflow for office visits to maximize the core skills of physicians, which required distributing ancillary tasks among support staff. We up-trained certified medical assistants (CMAs) and licensed practical nurses (LPNs) to take on the new role of care team coordinator (CTC) and optimized the direct clinical support ratio for busier physicians. For physicians who were seeing 15 to 19 patients a day, a ratio of 3 CTCs to 2 physicians was implemented; for those seeing 20 or more patients a day, we used a support ratio of 2:1.
The role of CTC was designed so that he or she would accompany a patient throughout the entire appointment. Responsibilities were broken out as follows:
Pre-visit. Before the physician enters the room, the CTC would now perform expanded rooming functions including pending orders, refill management, care gap closure using standing orders, agenda setting, and preliminary documentation.12
Visit. The CTC would now hand off the patient to the physician and stay in the room to document details of the visit and record new orders for consults, x-ray films, referrals, or prescriptions.13 This intensive EHR support was established to ensure that the physician could focus directly on the patient without the distraction of the computer.
Continue to: Post-visit
Post-visit. After a physician leaves a room, the CTC was now charged with finishing the pending orders, setting up the patient’s next appointment and pre-visit labs, reviewing details of the after-visit summary, and doing any basic health coaching with the patient. During this time, the physician would use the co-location space to review and edit the documentation, cosign the orders and prescriptions submitted by the CTC, and close the chart before going into the next room with the second CTC. The need to revisit these details after clinic hours was eliminated.
Another change … The role of our phone triage registered nurses (RN) was expanded. Care team RNs began providing diabetes counseling, blood pressure checks, annual wellness visits (AWV), and follow-up through the Centers for Medicare and Medicaid Services (CMS)'s Chronic Care Management and Transitional Care Management programs.
■ Part 2: Redesign between-visit in-basket management
Responding to an increasing number of inbox messages had become overwhelming for our physicians. Bellin Health’s management was aware that strategic delegation of inbox messages could save an hour or more of a physician’s time each day.14 Bellin implemented a procedure whereby inbox test results would be handled by the same CTC who saw the patient, thereby extending continuity. If the results were normal, the CTC would contact the patient. If the results were abnormal, the physician and the CTC would discuss them and develop a plan. Co-location of the RN, the CTC, and the physician would leverage face-to-face communication and make in-basket management more efficient.
■ Part 3: Redesign population health management
We developed an Extended Care Team (ECT), including social workers, clinical pharmacists, RN care coordinators, and diabetes educators, to assist with the care of patients with high-risk disorders or otherwise complex issues. These team members would work closely with the CTC, care team RN, and physician to review patients, develop plans of care, optimize management, and improve outcomes. Patients would be identified as candidates for potential ECT involvement based on the physician’s judgment in consultation with an EHR-based risk score for hospitalization or emergency department visit.
As we developed new processes, such as screening for determinants of health, we engaged additional system and community resources to help meet the needs of our patients.
Continue to: A look at stakeholder concerns and overcoming the barriers
A look at stakeholder concerns and overcoming the barriers
Critical to our success was being attentive to the concerns of our stakeholders and addressing them. Along the way, we gained valuable implementation insights, which we share here along with some specifics about how, exactly, we did things at Bellin.
Patients
Some patients expressed hesitation at having a person other than their physician in the exam room. They worried that the intimacy and privacy with their physician would be lost. In light of this, we gave patients the option not to have the CTC remain in the room. However, patients quickly saw the value of this team-based care approach and seldom asked to be seen without the CTC.
Throughout the process, we surveyed patients for feedback on their experiences. Comments indicated that the presence of the CTC in our team-based model led to positive patient experiences:
My physician is fully attentive. Patients appreciated that physicians were not distracted by the computer in the exam room. “I feel like I’ve got my doctor back” has been a common refrain.
The office staff is more responsive. The CTC, having been present during the appointment, has a deeper understanding of the care plan and can respond to calls or emails between visits, thereby reducing the time patients must wait for answers. One patient commented that, “I love [the doctor’s] team; his nurses are willing to answer every question I have.”
Continue to: I increasingly feel that I'm understood
I increasingly feel that I’m understood. We have seen patients develop meaningful relationships with other team members, confiding in them in ways that they hadn’t always done with physicians and advanced practice clinicians (APCs). Team members, in turn, have added valuable insights that help optimize patients’ care. In particular, the care of patients with multiple needs has been enhanced with the addition of ECT members who work with the core team and use their expertise to optimize the care of these patients.
Certified medical assistants and licensed practical nurses
Bellin’s leadership knew that team documentation could cause stress for the CMA, who, acting as a CTC, wanted to avoid misrepresenting details of the clinical encounter.13 Adding to the stress were other duties that would need to be learned, including agenda setting, refill management, care gap closure, and health coaching. With thorough training and preparation, many—but not all—of our CMAs and LPNs were able to successfully make the transition and flourish.
Implementation strategies
Provide thorough training. Our training process started 8 weeks before it was time to “go live.” There were weekly hour-long training sessions in population health basics, team culture and change management, documentation basics, and new roles and responsibilities. In the final week, the entire aTBC team sat together for 3 days of EHR training. All new teams shadowed existing teams to get a clear picture of the new processes.
Create a community of support. As our CMAs adapted to their new CTC roles, it was critical that they had support from experienced CTCs. Encouragement and patience from physicians were—and are—essential for CTCs to develop confidence in their new roles.
Enable ongoing feedback. We introduced weekly team meetings to enhance team communication and dynamics. Forums for all roles are held periodically to facilitate discussion, share learning, and enable support between teams.
Continue to: Use EHR tools to facilitate this work
Use EHR tools to facilitate this work. Using standard templates and documentation tools helped CTCs develop the confidence needed to thrive in their new role. Knowing these tools were available helped CTCs become effective in helping the team manage the between-visit work.
Monitor workload. As we developed more workflows and processes, we took care to monitor the amount of additional work for those in this role. We offloaded work whenever possible. For example, coordinated refill management at time of service, coupled with a back-up centralized refill system, can significantly decrease the number of refill requests made to CTCs. We continue to adjust staffing, where appropriate, to provide adequate support for those in this valuable role.
Be prepared for turnover. As CTCs became empowered in their new roles, some decided to advance their training into other roles. We developed a plan for replacing and training new staff. Higher pay can also be used to help attract and retain these staff members. Bellin uses LPNs in this role to ensure adequate staffing. Other health systems have developed a tier system for CMAs to improve retention.
Registered nurses
Before our move to an aTBC model, our office RNs primarily managed phone triage. Now the nurses were enlisted to play a more active role in patient care and team leadership. Although it was a dramatic departure from prior responsibilities, the majority of Bellin’s RNs have found increased satisfaction in taking on direct patient care.
Implementation strategies
Define new roles and provide training. In addition to participating in acute patient visits, consider ways that care team RNs can expand responsibilities as they pertain to disease counseling, population health management, and team leadership.15 At Bellin, the expanded role of the RN is evident in diabetes education and Medicare AWVs. Specifically, RNs now provide diabetes education to appropriate patients following a warm handoff from the physician at the time of the visit. RNs now also complete Medicare AWVs, which frees up physicians for other tasks and helps ensure sustainability for the new RN roles. Rates of completed AWVs at Bellin are now more than 70%, compared with reported national rates of less than 30%.16
Continue to: Maximize co-location
Maximize co-location. It is helpful to have the team members whose work is closely related—such as the CTCs and the RN for the team—to be situated near each other, rather than down a hall or in separate offices. Since the RN is co-located with the core teams at Bellin, there is now greater opportunity for verbal interaction, rather than just electronic communications, for matters such as triage calls and results management. RNs also provide a valuable resource for CMAs and LPNs, as well as help oversee team management of the in-basket.
Evaluate sustainability. Additional roles for the RNs required additional RN staffing. We assessed the new workload duties and balanced that against potential revenue from RN visits. This analysis indicated that an optimal ratio was 1 RN to every 3000 patients. This would allow an adequate number of RNs to fulfill additional roles and was financially sustainable with the goal of 4 billable RN visits per day.
Physicians
Bellin’s leadership recognized that some physicians might perceive team-based care as eroding their primary responsibility for patients’ care. Physicians have historically been trained in a model based on the primacy of the individual physician and that can be a hurdle to embracing team culture as a new paradigm of care. Several strategies helped us and can help others, too.
Implementation strategies
Cultivate trust. Thorough training of CTCs and RNs is critical to helping physicians develop trust and reliance in the team. The physician retains final authority over the team for cosigning orders, editing and finalizing documentation, and overseeing results management. Physicians invested in training and educating their staff will reap the rewards of a highly functioning, more satisfied team.
Encourage leadership. This can be a cultural shift for physicians, yet it is critical that they take a leadership role in this transformation.17 Physicians and their team leaders attended training sessions in team culture and change management. Prior to the go-live date, team leaders also met with the physician individually to explore their concerns and discuss ways to effectively lead and support their teams.
Continue to: Urge acceptance of support
Urge acceptance of support. The complexity of patient care today makes it difficult for a physician to manage all of a patient’s needs single-handedly. Complexity arises from the variety of plan co-pays and deductibles, the number of patients with chronic diseases, and the increased emphasis on improving quality measures.18 Enhanced support during any office visit and the extra support of an ECT for complex patients improves the ability of the physician to more effectively meet the needs of the patient.
Emphasize the benefit of an empowered team. The demands of the EHR on physicians and the resultant frustrations are well chronicled.4,19-22 Strategically delegating much of this work to other team members allows the physician to focus on the patient and perform physician-level work. At Bellin, we observed that our most successful care teams were those in which the physician fully accepted team-based care principles and empowered the staff to work at the top of their skill set.
Advanced practice clinicians
APCs in our system had traditionally practiced in 1 of 3 ways: independently handling defined panels with physician supervision; handling overflow or acute visits; or working collaboratively with a supervising physician to share a larger “team panel.” The third approach has become our preferred model. aTBC provides opportunities for APCs to thrive and collaborate with the physician to provide excellent care for patients.
APCs underwent the same process changes as physicians, including appropriate CTC support. Implementation strategies for APCs were similar to those that were useful for physicians.
Risk management professionals
At Bellin, we found that risk-management professionals had concerns about the scope of practice assigned to various team members, particularly regarding documentation. CMS allows for elements of a patient visit to be documented by CMAs and other members of the care team in real time as authorized by the physician.23,24 CTCs at Bellin also have other clinical duties in patient and EHR management. aTBC practices generally prefer the term team documentation over scribing, since it more accurately reflects the scope of the CTC’s work.
Continue to: Implementation strategies
Implementation strategies
Clarify regulatory issues. Extensive use of standing orders and protocols allowed us to increase involvement of various team members. State laws vary in what functions CMAs and LPNs are allowed to perform, so it is important to check your state guidelines.25 There is a tendency for some risk managers to overinterpret regulations. Challenge them to provide exact documentation from regulatory agencies to support their decisions.
Give assurances of physician oversight and processes. The physician assumes responsibility for standing orders, protocols, and documentation. We made sure that we had clear and consistent processes in place and worked closely with our risk managers as we developed our model. aTBC provides checks and balances to ensure accurate records, since team members are able to contribute and check for accuracy. A recent study suggested that CMAs perform documentation that is of equal or higher quality than that performed by the physician.26
Financial leadership
Like any organization adopting aTBC, Bellin’s leadership was concerned about the expense of adopting this approach. However, the leadership also recognized that the transition to aTBC could increase revenue by more than the increased staffing costs. In addition, we expected that capacity, access, continuity, and financial margins would increase.2,3,27,28 We also anticipated a decrease in downstream services, such as unnecessary tests, emergency department visits, and hospitalizations—a benefit of accountable care payment models.
Our efforts have been successful from a financial point of view. We attribute the financial sustainability that we have experienced to 4 factors:
1. Increased productivity. We knew that the increased efficiency of team-based care enables physicians to see 1 to 2 more patients per half day, and sometimes more.3,28,29 An increase of at least 1 patient visit per half-day was expected of our physicians and APCs on aTBC. In addition, they were expected to support the care team RN in achieving at least 4 billable visits per day. Our current level of RN visits is at 3.5 per nurse per day. There is significant variability in the increase of patients seen by a physician per day, ranging from 1 to 4 additional patients. These increased visits have helped us achieve financial viability, even in a predominantly fee-for-service environment.
2. More thorough service. The ability to keep patients in primary care and to focus on the patient’s full range of needs has led to higher levels of service and, consequently, to appropriately higher levels of billing codes. For example, Bellin’s revenue from billing increased by $724 per patient, related (in part) to higher rates of immunizations, cancer screenings with mammography, and colonoscopies.
Continue to: 3. New billable services
3. New billable services. Billing for RN blood pressure checks, AWVs, and extended care team services have helped make aTBC at Bellin financially feasible. Revenue from RN visits, for example, was $630,000 in 2018.
4. Improved access for patients. Of the 130 primary care providers now on aTBC, 15 (11.5%) had closed their practices to new patients before aTBC. Now, all of their practices are open to new patients, which has improved access to care. In a 2018 patient access survey, 96.6% of patients obtained an appointment as soon as they thought it was needed, compared with 70.7% of patients before the transition to aTBC.
Greater opportunity for financial sustainability. The combination of improved quality measures and decreased cost of care in the Bellin aTBC bodes well for future success in a value-based world. We have realized a significant increase in value-based payments for improved quality, and in our Next Gen Accountable Care Organization (ACO) patients, we have seen a decrease of $29 in per-member-per-month costs, likely due to the use of nonphysicians in expanded roles. In addition, hospital admissions have decreased by 5% due to the ability of ambulatory teams to manage more complex patients in the office setting. This model has also allowed physicians and APCs to increase their panel size, another key value-based metric. From 2016 to 2018, panel size for primary care providers increased by an average of 8%.
Enhanced ability to retain and recruit. Several of Bellin’s primary care recruits indicated that they had interviewed only at practices incorporating team-based care. This trend may increase as residencies transition to team-based models of care.
So how did we do?
Metrics of Bellin’s aTBC success
By the end of 2018, all 130 primary care physicians and APCs at Bellin had made the transition to this model, representing family medicine, internal medicine, and pediatrics. We have now begun the transition of our non-primary care specialties to team-based care.
Continue to: In the aTBC model...
In the aTBC model, the percentage of patients receiving age-appropriate screening is higher than before in every domain we measure (FIGURE 1). There has also been improvement in major quality metrics (FIGURE 2).

In a survey done in Spring 2018 by St. Norbert College Strategic Research Center, provider satisfaction increased, with 83% of physicians having made the transition to an aTBC practice moderately or very satisfied with their Bellin Health experience, compared with 70% in the traditional model. More recent 2019 survey data show a satisfaction rate of 90% for team-based care providers. Finally, in our aTBC model—in CMS’s Next-Gen ACO initiative—the cost per patient per month is significantly less than for those in a non-team-based care model ($796 vs $940).

CORRESPONDENCE
James Jerzak, MD, 1630 Commanche Ave, Green Bay, WI 54313; [email protected].
ACKNOWLEDGEMENTS
The authors would like to thank Lindsey E. Carlasare, MBA, from the American Medical Association, and Brad Wozney, MD, Kathy Kerscher, and Christopher Elfner from Bellin Health, for their contributions to the content and review of this manuscript.
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
3. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
4. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
5. Shanafelt TD, Mungo M, Schmitgen J, et al. Longitudinal study evaluating the association between physician burnout and changes in professional work effort. Mayo Clin Proc. 2016;91:422-431.
6. Sinsky CA, Dyrbye LN, West CP, et al. Professional satisfaction and the career plans of US physicians. Mayo Clin Proc. 2017;92:1625-1635.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90:1600-1613.
8. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
9. Sinsky CA, Sinsky TA, Althaus D, et al. Practice profile. ‘Core teams’: nurse-physician partnerships provide patient-centered care at an Iowa practice. Health Aff (Millwood). 2010;29:966-968.
10. Shanafelt T, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
11. Association for Advancing Physician and Provider Recruitment. Schutte L. What you don’t know can cost you: building a business case for recruitment and retention best practices. 2012. https://member.aappr.org/general/custom.asp?page=696. Accessed June 20, 2019.
12. American Medical Association. AMA STEPS Forward. Expanded rooming and discharge protocols. https://edhub.ama-assn.org/steps-forward/module/2702600. Accessed June 20, 2019.
13. American Medical Association. AMA STEPS Forward. Team documentation. https://edhub.ama-assn.org/steps-forward/module/2702598?resultClick=3&bypassSolrId=J_2702598. Accessed June 20, 2019.
14. American Medical Association. AMA STEPS Forward. EHR in-basket restructuring for improved efficiency. https://edhub.ama-assn.org/steps-forward/module/2702694?resultClick=3&bypassSolrId=J_2702694. Accessed June 20, 2019.
15. California Health Care Foundation. Bodenheimer T, Bauer L, Olayiwola JN. RN role reimagined: how empowering registered nurses can improve primary care. https://www.chcf.org/publication/rn-role-reimagined-how-empowering-registered-nurses-can-improve-primary-care/. Accessed June 20, 2019.
16. Chung S, Lesser LI, Lauderdale DS, et al. Medicare annual preventive care visits: use increased among fee-for-service patients, but many do not participate. Health Aff (Millwood). 2015;34:11-20.
17. American Medical Association. AMA Policy H-160.912. The structure and function of interprofessional health care teams. https://policysearch.ama-assn.org/policyfinder/detail/The%20Structure%20and%20Function%20of%20Interprofessional%20Health%20Care%20Teams?uri=%2FAMADoc%2FHOD.xml-0-727.xml. Accessed June 20, 2019.
18. Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128:337-343.
19. Hill RG Jr, Sears LM, Melanson SW. 4000 clicks: a productivity analysis of electronic medical records in a community hospital ED. Am J Emerg Med. 2013;31:1591-1594.
20. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21:e100-e106.
21. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
22. RAND Corporation. Friedberg MW, Chen PG, Ban Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. https://www.rand.org/pubs/research_reports/RR439.html. Accessed June 20, 2019.
23. Evaluation and Management (E/M) visit frequently asked questions (FAQs): physician fee schedule (PPS). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/E-M-Visit-FAQs-PFS.pdf. Accessed August 27, 2019.
24. Centers for Medicare & Medicaid Services. Scribe services signature requirements. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017-Transmittals-Items/R713PI.html. Accessed June 20, 2019.
25. American Association of Medical Assistants. State scope of practice laws. http://www.aama-ntl.org/employers/state-scope-of-practice-laws. Accessed June 20, 2019.
26. Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65:155-159.
27. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
28. Bank AJ, Obetz C, Konrardy A, et al. Impact of scribes on patient interaction, productivity, and revenue in a cardiology clinic: a prospective study. Clinicoecon Outcomes Res. 2013;5:399-406.
29. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
1. Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
2. Reuben DB, Knudsen J, Senelick W, et al. The effect of a physician partner program on physician efficiency and patient satisfaction. JAMA Intern Med. 2014;174:1190-1193.
3. Hopkins K, Sinsky CA. Team-based care: saving time and improving efficiency. Fam Pract Manag. 2014;21:23-29.
4. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760.
5. Shanafelt TD, Mungo M, Schmitgen J, et al. Longitudinal study evaluating the association between physician burnout and changes in professional work effort. Mayo Clin Proc. 2016;91:422-431.
6. Sinsky CA, Dyrbye LN, West CP, et al. Professional satisfaction and the career plans of US physicians. Mayo Clin Proc. 2017;92:1625-1635.
7. Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015;90:1600-1613.
8. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12:573-576.
9. Sinsky CA, Sinsky TA, Althaus D, et al. Practice profile. ‘Core teams’: nurse-physician partnerships provide patient-centered care at an Iowa practice. Health Aff (Millwood). 2010;29:966-968.
10. Shanafelt T, Goh J, Sinsky C. The business case for investing in physician well-being. JAMA Intern Med. 2017;177:1826-1832.
11. Association for Advancing Physician and Provider Recruitment. Schutte L. What you don’t know can cost you: building a business case for recruitment and retention best practices. 2012. https://member.aappr.org/general/custom.asp?page=696. Accessed June 20, 2019.
12. American Medical Association. AMA STEPS Forward. Expanded rooming and discharge protocols. https://edhub.ama-assn.org/steps-forward/module/2702600. Accessed June 20, 2019.
13. American Medical Association. AMA STEPS Forward. Team documentation. https://edhub.ama-assn.org/steps-forward/module/2702598?resultClick=3&bypassSolrId=J_2702598. Accessed June 20, 2019.
14. American Medical Association. AMA STEPS Forward. EHR in-basket restructuring for improved efficiency. https://edhub.ama-assn.org/steps-forward/module/2702694?resultClick=3&bypassSolrId=J_2702694. Accessed June 20, 2019.
15. California Health Care Foundation. Bodenheimer T, Bauer L, Olayiwola JN. RN role reimagined: how empowering registered nurses can improve primary care. https://www.chcf.org/publication/rn-role-reimagined-how-empowering-registered-nurses-can-improve-primary-care/. Accessed June 20, 2019.
16. Chung S, Lesser LI, Lauderdale DS, et al. Medicare annual preventive care visits: use increased among fee-for-service patients, but many do not participate. Health Aff (Millwood). 2015;34:11-20.
17. American Medical Association. AMA Policy H-160.912. The structure and function of interprofessional health care teams. https://policysearch.ama-assn.org/policyfinder/detail/The%20Structure%20and%20Function%20of%20Interprofessional%20Health%20Care%20Teams?uri=%2FAMADoc%2FHOD.xml-0-727.xml. Accessed June 20, 2019.
18. Milani RV, Lavie CJ. Health care 2020: reengineering health care delivery to combat chronic disease. Am J Med. 2015;128:337-343.
19. Hill RG Jr, Sears LM, Melanson SW. 4000 clicks: a productivity analysis of electronic medical records in a community hospital ED. Am J Emerg Med. 2013;31:1591-1594.
20. Babbott S, Manwell LB, Brown R, et al. Electronic medical records and physician stress in primary care: results from the MEMO Study. J Am Med Inform Assoc. 2014;21:e100-e106.
21. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848.
22. RAND Corporation. Friedberg MW, Chen PG, Ban Busum KR, et al. Factors affecting physician professional satisfaction and their implications for patient care, health systems, and health policy. https://www.rand.org/pubs/research_reports/RR439.html. Accessed June 20, 2019.
23. Evaluation and Management (E/M) visit frequently asked questions (FAQs): physician fee schedule (PPS). https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/E-M-Visit-FAQs-PFS.pdf. Accessed August 27, 2019.
24. Centers for Medicare & Medicaid Services. Scribe services signature requirements. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2017-Transmittals-Items/R713PI.html. Accessed June 20, 2019.
25. American Association of Medical Assistants. State scope of practice laws. http://www.aama-ntl.org/employers/state-scope-of-practice-laws. Accessed June 20, 2019.
26. Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65:155-159.
27. Arya R, Salovich DM, Ohman-Strickland P, et al. Impact of scribes on performance indicators in the emergency department. Acad Emerg Med. 2010;17:490-494.
28. Bank AJ, Obetz C, Konrardy A, et al. Impact of scribes on patient interaction, productivity, and revenue in a cardiology clinic: a prospective study. Clinicoecon Outcomes Res. 2013;5:399-406.
29. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
PRACTICE RECOMMENDATIONS
› Up-train staff to provide enhanced support for physicians during the office visit, such as handling most electronic health record work, including documentation. C
› Take a team approach to between-visit work, leveraging principles of team-based care (such as co-location) to optimize efficiency. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How best to address breast pain in nonbreastfeeding women
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).
Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15
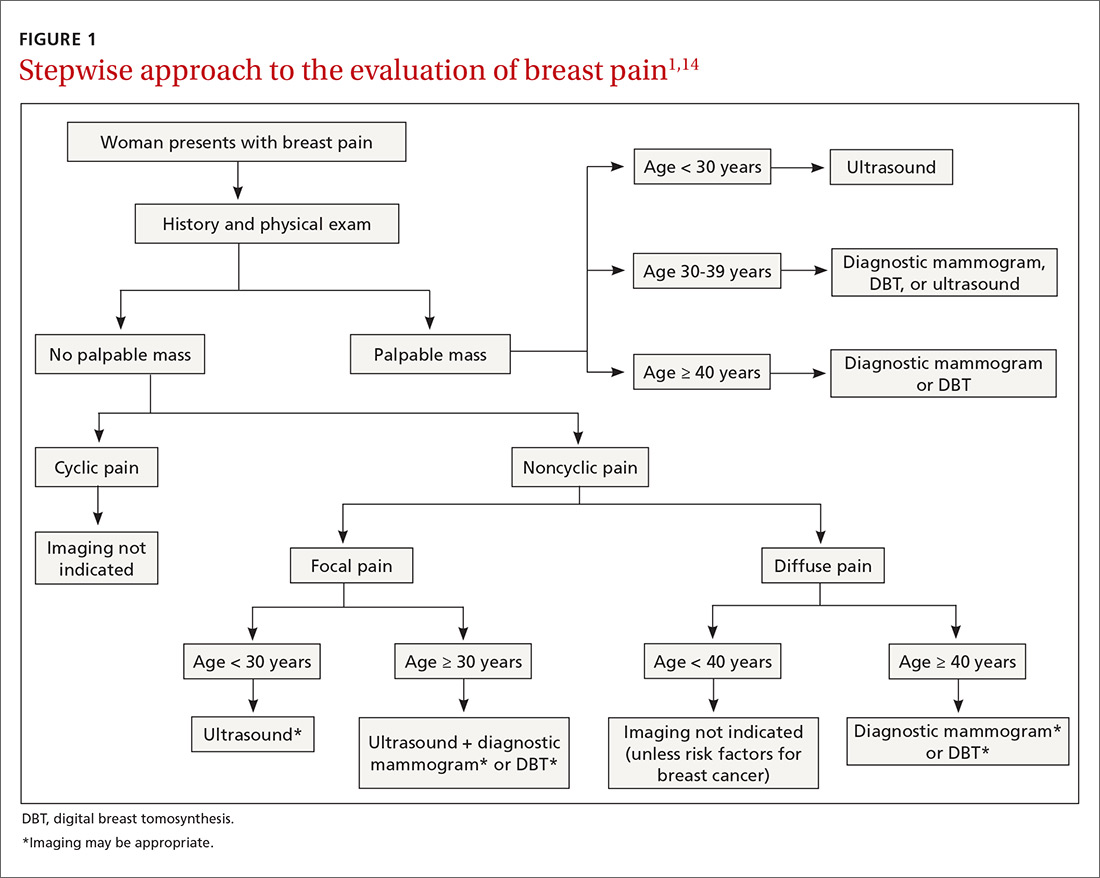
Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15
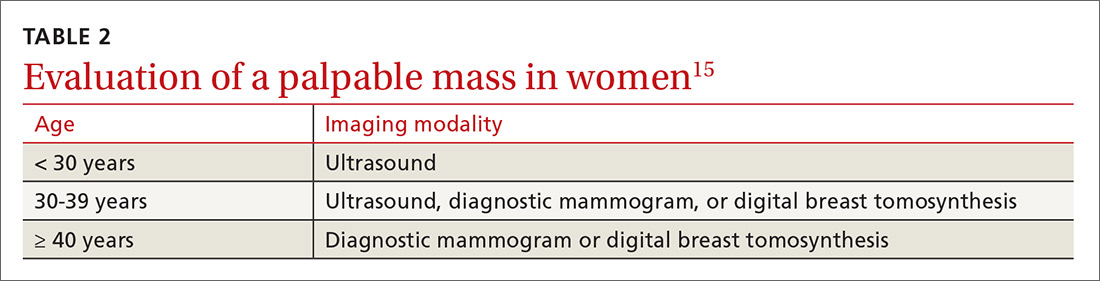
Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).
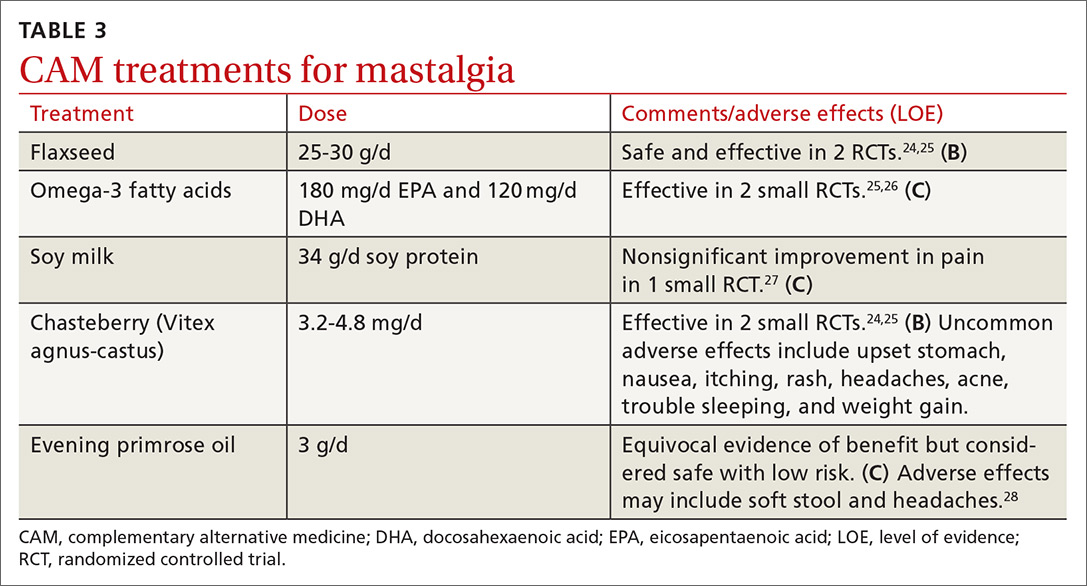
Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38
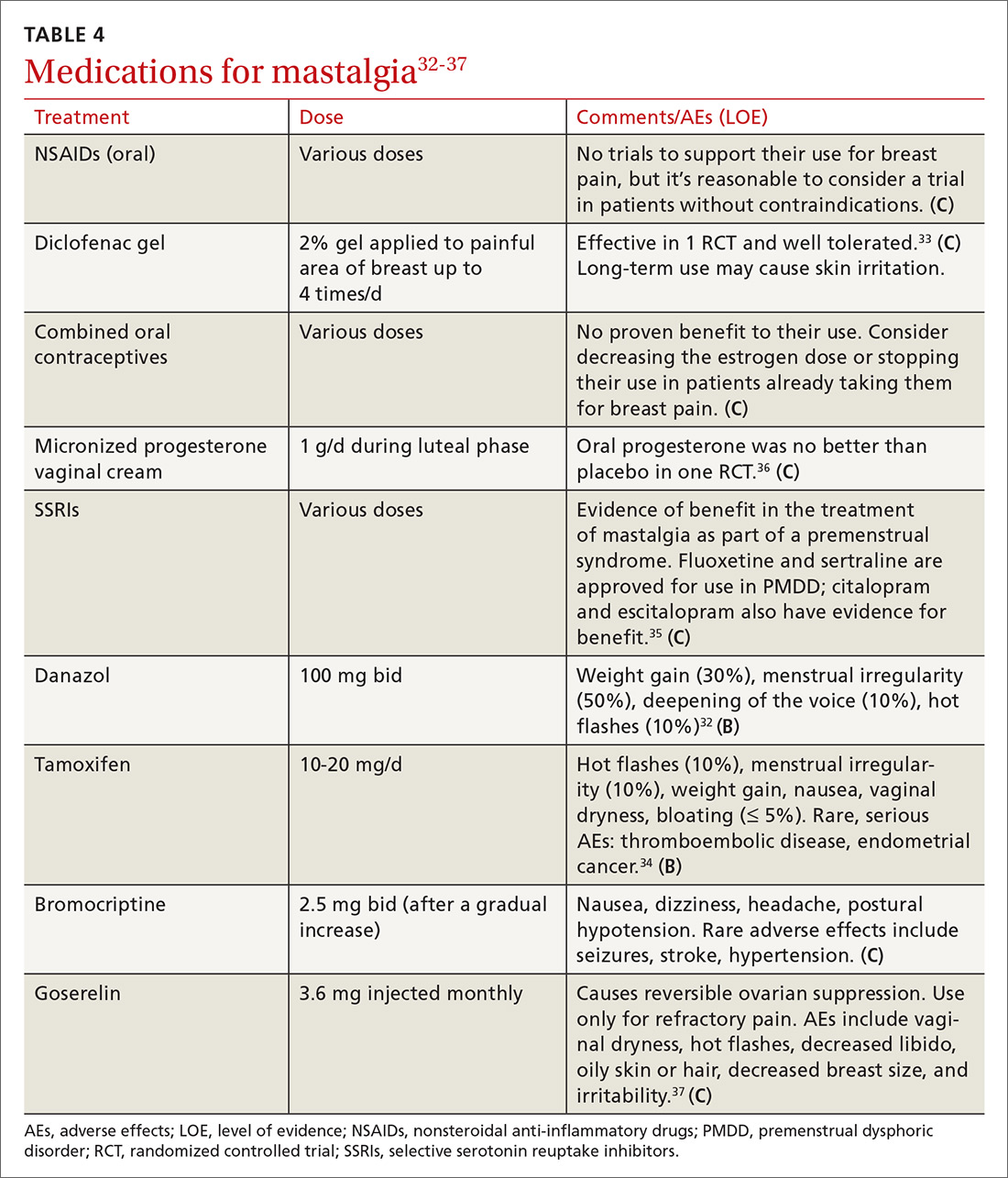
SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35
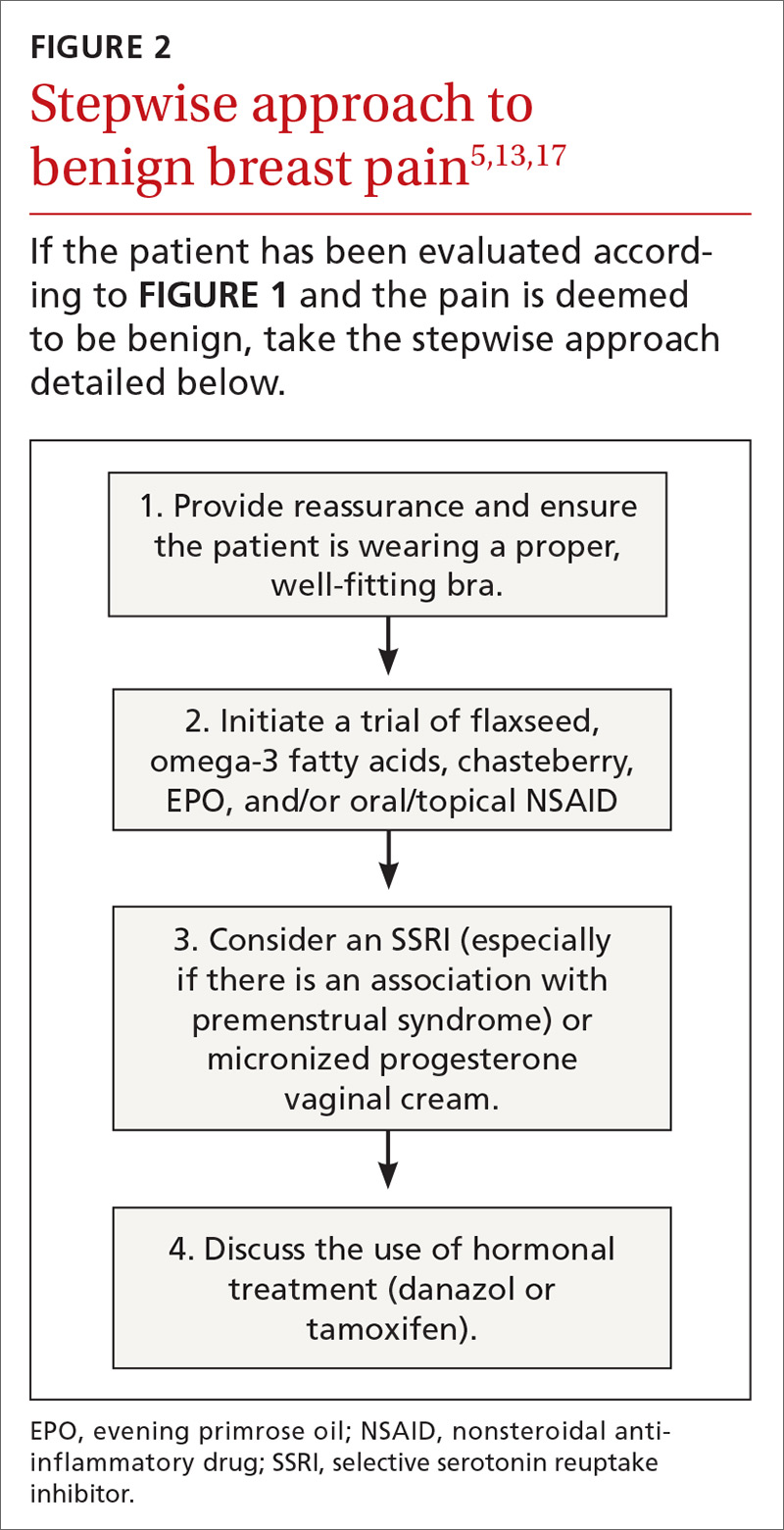
Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).

Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15

Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15

Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).

Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38

SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35

Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).

Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15

Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15

Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).

Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38

SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35

Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
PRACTICE RECOMMENDATIONS
› Instruct patients to maintain a pain diary, which, along with a careful history and physical examination, helps to determine the cause of breast pain and the type of evaluation needed. C
› Treat cyclic, bilateral breast pain with chasteberry and flaxseed. B
› Consider short-term treatment with danazol or tamoxifen for women with severe pain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Medical Cannabis: A guide to the clinical and legal landscapes
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used
A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20

Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected].
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used

A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20

Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected].
CASE
Barry S, a 45-year-old man with a new diagnosis of non-Hodgkin’s lymphoma, recently started induction chemotherapy. He has struggled with nausea, profound gustatory changes, and poor appetite; various antiemetics have provided only minimal relief. He tells you that he is hesitant to try “yet another pill” but has heard and read that marijuana (genus Cannabis) is used to alleviate disruptive chemotherapy-induced adverse effects. He asks if this is a treatment you’d recommend for him.
As Mr. S’s physician, how do you respond?
Understandably, some family physicians are hesitant to recommend an unregulated, federally illegal substance characterized by conflicting or absent evidence of safety and effectiveness.1 Nevertheless, throughout history and in the current court of public opinion, medical Cannabis has overwhelming support,2 leading to legalization in most of the United States.
As with many traditionally accepted therapies (whether they are or are not supported by substantial evidence), physicians are expected to provide individualized guidance regarding minimizing risk and maximizing benefit of the therapeutic use of Cannabis. The rapidly growing scientific and commercial fields of medical Cannabis guarantee that information on this topic will constantly be changing—and will often be contradictory. In this article, we review the most common concerns about medical Cannabis and provide up-to-date evidence on its use.
The pharmacology of cannabis
Cannabis sativa was among the earliest plants cultivated by man, with the first evidence of its use in China, approximately 4000 BC, to make twine and rope from its fibers.3 Records of medicinal Cannabis date back to the world’s oldest pharmacopoeia, a written summary of what was known about herbal medicine through the late 16th century.4
The 2 principal species of Cannabis are sativa and indica. There is no good medical evidence to separate the impacts of either strain; however, a staggering amount of lay information exists about the reported differing effects of each strain.5
Chemical constituents. Phytocannabinoids derived from C sativa are the plant’s best-known proteins, constituting a complex lipid-signaling network involved in numerous physiological processes. There are more than 100 known phytocannabinoids, the most well-recognized being Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Additional sources of cannabinoids include endogenous cannabinoids, or endocannabinoids, and synthetic cannabinoids.
The endocannabinoid system, comprising cannabinoid receptors, endocannabinoids, and their specific enzymes, is a potential therapeutic target for a variety of pathologic processes.6,7 The 2 most well-studied targets for cannabinoids in the human body are the cannabinoid receptors CB1 and CB2, found throughout the body: CB1, predominantly in the central and peripheral nervous system, and CB2 in a more limited distribution in the immune and hematopoietic systems. Other pathways activated or antagonized by THC and CBD exist, but are less well-mapped than CB1 and CB2.
[polldaddy:10402702]
Continue to: Botanical or synthetic?
Botanical or synthetic? It is important to distinguish between synthetic and plant-based cannabinoids, for you and your patients' benefit. Pharmaceutical (synthetic) THC is just that: THC alone. Whole-plant Cannabis, on the other hand, has hundreds of additional chemicals—most notably, phytocannabinoids and terpenoids. Data on the mechanisms of action and interactions of these additional chemicals are limited.
Although clinical trials have been undertaken with synthetic cannabinoids, there is increasing understanding and interest in the medical community of whole-plant Cannabis as a distinct entity. For example, nabiximols is a novel development in plant-based Cannabis products. Available as an oromucosal spray, a dose provides THC and CBD at 2.7 mg/100 mcL. Nabiximols is not approved by the US Food and Drug Administration (FDA) but is widely used

A third class of Cannabis comprises nonregulated synthetic cannabinoids that have no medically recognized benefit. They are solely a drug of abuse; common names include “K2” and “Spice.” These cannabinoids are outside of the scope of our discussion, but patients and providers should be aware of these cannabinoids because they are street-available. Unsuspecting patients might not know the difference between abusive and therapeutic formulations.8
Delivery and strength. Common forms of plant-based Cannabis include leaf that is smoked or vaporized, oral tincture, pill, and oil concentrate that can be vaporized. All forms come in a range of THC:CBD ratios—from as high as 90% THC content to 0% THC and all CBD-based content. Patients who are naïve to Cannabis might be concerned about formulations with a high THC concentration because of the psychoactive effects of this substance. Given the minimal CNS activity of CBD, a tolerable therapeutic starting point often is a THC:CBD ratio of 1:1, which contains a lower percentage of THC.4
Physiologic effects. THC is a partial agonist of CB1 and CB2 receptors; CBD functions as an antagonist at both receptors. The primary effects of THC result from activation of CB1 receptors, which exist in various areas of the cerebrum and cerebellum, as well as in the spinal cord.7 THC exerts its psychotropic effects at CB1 sites in the central nervous system; CBD can antagonize these THC effects at CB1 receptors. CBD also has anti-inflammatory and other effects that are mediated through peripherally distributed CB2 receptors.9
Continue to: THC has tremendously...
THC has tremendously complex capacity for activation and inhibition within various neuronal circuits, resulting in effects on mood, appetite, and movement.1,7 Adverse effects associated with Cannabis are wide-ranging: Most commonly, nausea, drowsiness, fatigue, dry mouth, and dizziness are reported alongside cognitive effects. Rarely, tachycardia, hypotension, hyperemesis, and depression can be seen.
Clinical implications and indications
Clinical indications for legal medical Cannabis vary by state; typically, indications include human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS), cachexia, cancer, glaucoma, epilepsy and other seizure disorders, severe and chronic pain, spasticity from neurodegenerative disorders, and irritable bowel syndrome and Crohn’s disease, as well as a wide range of less-universal diagnoses. A patient may have a so-called qualifying diagnosis (ie, having the potential to allow the patient to be certified to purchase and use Cannabis) in one state but not have the same standing in a neighboring state, posing a complex legal issue. Given the significant complexities of performing medical research with plant-based Cannabis in the United States, little research has been done. The result? Policymakers are grappling with questions that only scientific research can answer:
- For which conditions does Cannabis provide medicinal benefit equal to or superior to alternatives?
- What are the appropriate dosages (or CBD:THC ratios), formulations (plant-derived or synthetic), and routes of administration (smoked, ingested, or topical) for various conditions?
Bird’s-eye view of clinical research. A meta-analysis of isolated synthetic and plant-based cannabinoids for medical use was published in 2015.10 The analysis included more than 6000 patients in 79 trials, most of which assessed whether dronabinol or nabilone (both synthetic isolates) were effective compared to placebo or alternative non-Cannabis-based therapy. The studies examined chemotherapy-induced nausea and vomiting, appetite stimulation in HIV and AIDS, chronic pain, spasticity, depression and anxiety, sleep disorders, and psychosis.
Twenty-eight studies assessed chemotherapy-induced nausea and vomiting. All of these studies indicated a greater benefit from cannabinoids than from alternative antiemetic regimens and placebo; however, that finding did not reach statistical significance across all studies.
There was moderate evidence to suggest the use of Cannabis for neuropathic and nonneuropathic cancer-related pain. However, there is an increased short-term risk of adverse events with synthetic isolates dronabinol (when used for pain) and nabilone (when used for nausea and vomiting).
Continue to: The primary conclusion...
The primary conclusion of the meta-analysis is that further study is required because little evidence exists on the effects and the adverse events of plant-based Cannabis.
HIV infection. Data on Cannabis for the treatment of refractory neuropathy and appetite stimulation in HIV infection is mixed.10,11 Smoked Cannabis for medically refractory neuropathy was examined in several trials:
- In a randomized crossover trial, researchers found statistically significant subjective improvement in neuropathic pain, with minimal intolerable adverse effects, in the 28 HIV-infected participants who completed the trial.11
- In another study,Cannabis ingested in various forms resulted in appetite stimulation in late-stage HIV infection but did not produce statistically significant weight gain.10
Pediatric epilepsy. Research on pediatric patients who have epilepsy characterized by refractory seizures has shown that the impact of Cannabis on their disease is promising. Specifically, CBD has shown tremendous potential impact: Patients experienced a statistically significant reduction in the number of seizures.9 In 2018, the FDA approved the first plant-based derivative of Cannabis: an oral cannabidiol (marketed as Epidiolex [Greenwich Biosciences, Inc.]) for the treatment of intractable seizures associated with Lennox-Gastaut syndrome and Dravet syndrome, rare and severe forms of epilepsy. Epidiolex is the first FDA-approved drug that contains a purified drug substance derived from marijuana.
CASE
Mr. S’s diagnosis of cancer is broadly included in the list of Cannabis-qualifying illnesses in all 34 states that certify patients for medical Cannabis. He qualifies both because (1) he is a cancer patient and (2) he has not found relief from chemotherapy-induced nausea and vomiting with several targeted therapies, including 5-hydroxytryptamine-receptor antagonists, steroids, and antipsychotics. Evidence supports CB1 and CB2 as potential targets for antiemetic treatment.
Given Mr. S’s consequent anorexia, his frustration with taking an increasing number of medications, and possible adverse effects of additional therapy, Cannabis is a reasonable course of action to treat nausea and vomiting. He would be able to use oral tincture or vaporization of oil to further limit his pill burden—likely, with a THC:CBD ratio of 1:1 or similar.
Continue to: Based on recent observational data...
Based on recent observational data from New York Cannabis dispensaries, cancer patients pursing Cannabis to treat chemotherapy-induced symptoms report that (1) either products with a high concentration of THC or products that contain THC and CBD in a 1:1 ratio are most effective and (2) products in 1:1 ratio of THC and CBD are most tolerable.
A legal system at oddsover the status of medical Cannabis
The core legal issue underlying medical Cannabis is a contradiction between federal and state laws.
At the federal level. The federal government regulates the lawful production, possession, and distribution of controlled substances through the Controlled Substances Act (CSA).12 The CSA is the basis for categorizing certain plants, drugs, and chemicals into 5 schedules, based on the substance’s medical use, potential for abuse, and safety or dependence liability.13 Under the CSA, marijuana (along with substances such as heroin and methamphetamine) is categorized as Schedule I14; ie, the substance
- has high potential for abuse,
- has no accepted therapeutic medical use in the United States, and
- lacks acceptable safety for use under medical supervision.
Despite waxing and waning efforts to protect states from federal prosecution, any use of a Schedule-1 substance violates federal law.15
In June 2018, a bipartisan group of federal lawmakers introduced a bill designed to amend the CSA and guarantee the rights of states and territories to self-determine marijuana regulation. The bill established a so-called STATES (Strengthening the Tenth Amendment Through Entrusting States) Act that “amends the Controlled Substances Act (21 U.S.C. § 801 et seq.) so that—as states and tribes comply with a few basic protections—its provisions no longer apply to any person acting in compliance with state or tribal laws relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marijuana.”15
Continue to: The bill was referred to the Senate...
The bill was referred to the Senate and House Judiciary Committees but, ultimately, the STATES Act was blocked from debate in 2018.
On April 4, 2019, the Act was reintroduced in the House (H.R. 2093) and Senate (S. 1028) of the 116th Congress. Although there is bipartisan support for this bill, the timeline for moving it forward is unclear.16,17
At the state level. Thirty-four states have comprehensive public medical marijuana and Cannabis programs. The National Conference of State Legislatures18 (www.ncsl.org) designates a program “comprehensive” if it
- includes protection from criminal penalties for using marijuana for a medical purpose,
- allows access to marijuana through home cultivation, dispensaries, or other system,
- permits a variety of strains, including those more potent than what is labeled “low-THC,” and
- allows smoking or vaporization of marijuana products, plant-based material, or extract.
An additional 14 states allow for “low-THC, high-CBD” products for medical reasons, in limited situations, or as a legal defense. Regulation in these states varies widely, however: Some states allow industrialized hemp products only; others do not provide for any in-state production.18
Last, many states have some form of so-called “affirmative-defense” statutes that allow people charged with marijuana possession to mention use of marijuana for medical purposes as a possible defense.
Continue to: Physician shield
Physician shield. Despite inconsistent and evolving state and federal laws, physicians are protected, based on the Conant v Walters decision, from prosecution or revocation of their prescriptive authority for the professional “recommendation” of the use of medical marijuana.19 In 2002, the US Ninth Circuit Court of Appeals upheld the permanent injunction, based on a physician’s First Amendment right to discuss medical marijuana with patients.
CASE
Mr. S is amenable to trial of Cannabis to relieve nausea and anorexia. He asks you if he is allowed to use Cannabis at work, were he to return to an office-based desk job—even part-time—during treatment for cancer.
How would you answer Mr. S? Patients are legally protected from workplace penalties and dismissal for using and consuming Cannabis in states with a medical Cannabis law (including the state in which Mr. S resides). However, all employers have some variability in corporate policy, especially if a person works in a federally supported or regulated occupation. It’s always helpful to advise patients who will be using medical Cannabis to be proactive and speak with a human resources or employee health department staff member before beginning a course of medical Cannabis. Additionally, Cannabis with any amount of THC has the ability to alter focus, concentration, and perceptions of time. Thus, if a patient using medical Cannabis with THC asks about driving to work, he should be given the same advice one would offer about driving after consuming alcohol or ingesting opioids.
Common concerns
Ignorance of legal status. Theoretically, the Conant v Walters decision protects physicians from investigation for recommending medical Cannabis even in states where it is illegal. However, you should adhere closely to procedures set out by your state. The National Council of State Legislatures provides up-to-date information on each state’s procedures and programs,18 and the American Society of Addiction Medicine (www.asam.org) has established standards of professionalism for physicians who discuss medical Cannabis with patients (TABLE).20

Exposure to smoke. Cannabis smoke carries many of the same carcinogens found in tobacco smoke; furthermore, use of Cannabis and tobacco are highly correlated, confounding many population-based studies. The manner of inhalation of Cannabis can result in significantly higher levels of tar and carbon dioxide than with tobacco smoking. Because the effects of Cannabis last longer, however, people who smoke Cannabis may smoke it less often than tobacco smokers smoke tobacco.21
Continue to: Large cross-sectional...
Large cross-sectional and longitudinal studies have not found a link between Cannabis smoking and long-term pulmonary consequences, such as chronic obstructive pulmonary disease and lung cancer.22,23 The technology of Cannabis delivery systems has progressed far more rapidly than the clinical evidence for or against such technology.
“Vaping” is an informal term for inhalation of aerosolized Cannabis components and water vapor. Vaporizers do not heat Cannabis to the point of combustion; therefore, they provide less exposure to smoke-related toxicants while providing similar time of onset.
Neuropsychiatric adverse effects. Data regarding the relationship between Cannabis use and psychiatric disorders are incompletely understood, in conflict, and related to cannabinoid type. Consider Pennsylvania’s addition of anxiety disorder as a “serious medical condition” covered under the Pennsylvania Medical Marijuana Act.24 Although patients often report the use of medical Cannabis to treat anxiety,25 panic attacks are often associated with Cannabis use.26
While there is a clear association between Cannabis use and psychotic disorder, a causal link has yet to be unequivocally established. However, the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily.27
We recommend, therefore, that physicians screen patients for serious mental health concerns before recommending or certifying them to use medical Cannabis.
Continue to: Overconsumption of edibles
Overconsumption of edibles. Cannabis edibles (ie, food products infused with Cannabis extract) are distinct from inhaled Cannabis in regard to onset, duration, and potential for adverse effects. Cannabis edibles might be more popular than inhaled products among older medical Cannabis users.28
Edible Cannabis has a reported onset of 1 to 3 hours (compared to 5-10 minutes with inhaled Cannabis) and a duration of effect of 6 to 8 hours (compared with 2-4 hours for inhaled products).29 These qualities might render Cannabis edibles preferable to inhaled formulations for controlling chronic symptoms and conditions. However, delayed onset of edible products and wide variation in the concentration of THC also increase the risk of overconsumption, which can lead to overdose and self-limited Cannabis-induced psychosis. We recommend providing patient education about the effects of the physiologically active therapeutic compounds tetrahydrocannabinol and cannabidiol, to prevent overconsumption of high-THC products.30
CASE
Mr. S returns to your office after a trial of Cannabis as vaporized oil and reports some relief of nausea and a mild increase in appetite, but no weight gain. He is concerned about overconsumption or overdose, and asks you what the risks of these problems are.
How should you counsel Mr. S? Explain that ingestion of Cannabis has a prolonged onset of action; vaporization has a more rapid onset of action; therefore, he could more easily self-regulate ingestion with the vehicle he has chosen. In states where edible Cannabis products are legal, education is necessary so that patients know how much of the edible to consume and how long they will wait to feel the full impact of the effects of THC.30
Cannabis use disorder in the context of medical marijuana
Cannabis use disorder (CUD) incorporates general diagnostic features of a substance use disorder, including behavioral, cognitive, and physiologic symptoms such as cravings, tolerance, and withdrawal, in the setting of persistent use despite significant substance-related problems.31 Features of Cannabis withdrawal syndrome include irritability, anger or aggression, anxiety, depressed mood, restlessness, sleep difficulty, and decreased appetite or weight loss.31 Cannabis use disorder can develop in people who use medical Cannabis; however, physiologic symptoms of tolerance and withdrawal can also develop in the setting of appropriate medical use and do not, in isolation, represent CUD.
Continue to: A recent study...
A recent study considered nationwide cross-sectional survey data from the US National Survey of Drug Use and Health to examine the relationship between medical marijuana laws and CUD.32 Study findings did not show an increase in the prevalence of CUD or marijuana use among adults in states with a legalized medical marijuana program. Importantly, when researchers looked at marijuana use among adolescents and young adults, they found no increase in measured outcomes (eg, active [ie, past-month] marijuana use, heavy [> 300 d/yr] use, and a diagnosis of CUD) after medical marijuana laws were passed.32
A paucity of pediatric data
The adolescent brain might be more vulnerable to the adverse long-term effects of Cannabis; there is potential significant harm associated with Cannabis in children and adolescence. However, accurate data concerning risk and benefit are limited.
The most recent policy statement of the American Academy of Pediatrics (AAP) reflects this paucity of data.33 The AAP opposes the use of medical Cannabis outside regulation by the FDA, although the organization allows for consideration of compassionate use of medical Cannabis for children who have life-threatening or severely disabling conditions. The AAP does support (1) additional research into pharmaceutical cannabinoids and (2) changing Cannabis from Schedule I to Schedule II to facilitate this process. Since the publication of the policy statement, Pediatrics, the official journal of the AAP, has published a review of medical cannabinoids and found (1) strong evidence for benefit in chemotherapy-induced nausea and vomiting and (2) accumulating evidence of benefit in epilepsy.34
Recognized risk: Not supporting medical Cannabis
As with all medical decisions, the risks and benefits of certifying patients for medical Cannabis must be balanced against the risks and benefits of not doing so. The risks that accompany failure to certify a patient for medical marijuana fall into 3 categories:
Blocking access to a substance that has potential therapeutic benefit. More data regarding the potential benefits and risks of medical Cannabis will, undoubtedly, dispel some of the uncertainty regarding the decision to certify a patient for medical Cannabis. When you recommend medical Cannabis and certify patients for its use, you do so with the certainty that the Cannabis safety index (ie, risk of overdose or serious adverse effects) is exceedingly low.35
Continue to: Limiting patients to other medications
Limiting patients to other medications that, potentially, carry a risk of more or greater harmful effects. An example is the decision to prescribe an opioid for chronic pain instead of certifying a patient for medical Cannabis. For certain other conditions, including chemotherapy-induced nausea and vomiting, FDA-approved pharmaceuticals might have more reported serious adverse events and interactions than medical Cannabis.36
Resigning patients to obtain Cannabis from an illegal source. This speaks to harm reduction and social justice, because obtaining Cannabis from an illegal source carries health and legal risks:
- Increased health risks result from lacing or cutting botanical or synthetic Cannabis products with potentially toxic substances. Cocaine, the rodenticide brodifacoum, methamphetamine, and phencyclidine are all known, or have been reported, to be added to botanical and synthetic Cannabis.37
- Legal repercussions of Cannabis possession are disproportionately racially based, with a significantly higher arrest rate among people of color, even in states where medical Cannabis has been legalized.38
CORRESPONDENCE
Lara Carson Weinstein, MD, MPH, DrPH, Department of Family and Community Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, PA 19107; [email protected].
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
1. College of Family Physicians of Canada. Authorizing Dried Cannabis for Chronic Pain or Anxiety: Preliminary Guidance from the College of Family Physicians of Canada. Mississauga, Ontario: College of Family Physicians of Canada; 2014. www.cfpc.ca/uploadedFiles/Resources/_PDFs/Authorizing%20Dried%20Cannabis%20for%20Chronic%20Pain%20or%20Anxiety.pdf. Accessed July 10, 2019.
2. Hartig H, Geiger AW. About six-in-ten Americans support marijuana legalization. Pew Research Center Web site. www.pewresearch.org/fact-tank/2018/10/08/americans-support-marijuana-legalization/. Published October 8, 2018. Accessed July 10, 2019.
3. Li H-L. An archaeological and historical account of cannabis in China. Econ Bot. 1974:28:437-448.
4. Zuardi AW. History of cannabis as a medicine: a review. Braz J Psychiatry. 2006;28:153-157.
5. Marijuana strains and infused products. Leafly Web site. www.leafly.com/start-exploring. Accessed July 10, 2019.
6. Fraguas-Sánchez AI, Torres-Suárez AI. Medical use of cannabinoids. Drugs. 2018;78:1665-1703.
7. Maurya N, Velmurugan BK. Therapeutic applications of cannabinoids. Chem Biol Interact. 2018;293:77-88.
8. Kelkar AH, Smith NA, Martial A, et al. An outbreak of synthetic cannabinoid-associated coagulopathy in Illinois. N Engl J Med. 2018;379:1216-1223.
9. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199-215.
10. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
11. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2008;34:672-680.
12. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part A—Introductory Provisions. §801. Congressional findings and declarations: controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/801.htm. Accessed July 10, 2019.
13. Yeh BT. The Controlled Substances Act: regulatory requirements. Congressional Research Service 7-5700. https://fas.org/sgp/crs/misc/RL34635.pdf. Published December 13, 2012. Accessed July 10, 2019.
14. US Department of Justice, Drug Enforcement Administration, Diversion Control Division. Title 21 United States Code (USC) Controlled Substances Act. Subchapter I—Control and Enforcement. Part B—Authority to Control; Standards and Schedules. §812. Schedules of controlled substances. www.deadiversion.usdoj.gov/21cfr/21usc/812.htm. Accessed July 10, 2019.
15. United States Senate. The STATES Act. Senator Elizabeth Warren and Senator Cory Gardner. 2018. www.warren.senate.gov/imo/media/doc/STATES%20Act%20One%20Pager.pdf. Accessed July 10, 2019.
16. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, HR 2093. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/house-bill/2093/text. Accessed July 20, 2019.
17. Strengthening the Tenth Amendment Through Entrusting States (STATES) Act of 2019, S 1028. 116th Cong, 1st Session (2019). www.congress.gov/bill/116th-congress/senate-bill/1028/all-info?r=3&s=6. Accessed August 8, 2019.
18. State medical marijuana laws. National Conference of State Legislatures Web site. www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#3. Published July 2, 2019. Accessed July 10, 2019.
19. Conant v Walters. 309 F.3d 629 (9th cir. 2002).
20. American Society of Addiction Medicine. The role of the physician in “medical” marijuana. www.asam.org/docs/publicy-policy-statements/1role_of_phys_in_med_mj_9-10.pdf?sfvrsn=0. Published September 2010. Accessed July 12, 2019.
21. What are marijuana’s effects on lung health? National Institute on Drug Abuse Web site. www.drugabuse.gov/publications/research-reports/marijuana/what-are-marijuanas-effects-lung-health. Updated July 2019. Accessed July 10, 2019.
22. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239-247.
23. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136:894-903.
24. Getting medical marijuana. Commonwealth of Pennsylvania Web site. www.pa.gov/guides/pennsylvania-medical-marijuana-program/. Accessed July 20, 2019.
25. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: systematic review and meta-analysis. Soc Sci Med. 2019;233:181-192.
26. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol. 2009;24:515-523.
27. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319-328.
28. Barrus DG, Capogrossi KL, Cates S, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Publication No. OP-0035-1611. Research Triangle Park, NC: RTI Press; 2016. www.rti.org/sites/default/files/resources/rti-publication-file-6ff047d7-3fa4-41ad-90ed-9fb11663bc89.pdf. Accessed July 10, 2019.
29. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12-19.
30. MacCoun RJ, Mello MM. Half-baked—the retail promotion of marijuana edibles. N Engl J Med. 2015;372:989-991.
31. Cannabis use disorder [305.20, 304.30]. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013:509-516.
32. Williams AR, Santaella-Tenorio J, Mauro CM, et al. Loose regulation of medical marijuana programs associated with higher rates of adult marijuana use but not cannabis use disorder. Addiction. 2017;112:1985-1991.
33. American Academy of Pediatrics Committee on Substance Abuse, American Academy of Pediatrics Committee on Adolescents. The impact of marijuana policies on youth: clinical, research, and legal update. Pediatrics. 2015;135:584-587.
34. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: a systematic review. Pediatrics. 2017;140. pii: e20171818.
35. Drug Enforcement Administration. Drugs of abuse: a DEA resource guide. www.dea.gov/sites/default/files/drug_of_abuse.pdf. Published 2017. Accessed July 10, 2019.
36. National Academies of Science, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. www.nap.edu/read/24625/chapter/12017:2017-2019. Published 2017. Accessed July 10, 2019.
37. Emerging trend and alerts. National Institute on Drug Abuse Web site. www.drugabuse.gov/drugs-abuse/emerging-trends-alerts. Accessed July 10, 2019.
38. Drug Policy Alliance. From prohibition to progress: a status report on marijuana legalization. www.drugpolicy.org/sites/default/files/dpa_marijuana_legalization_report_feb14_2018_0.pdf. Published January 2018. Accessed July 10, 2019.
PRACTICE RECOMMENDATIONS
› Educate patients about the effects of the physiologically active therapeutic compounds in Cannabis; this is critical to prevent overconsumption of products with high levels of tetrahydrocannabinol. B
› Screen patients for serious mental health concerns before recommending or certifying medical Cannabis; this is essential because the rate of psychiatric hospitalization is increased in bipolar disorder and schizophrenia patients who use Cannabis heavily. B
› You can recommend medical Cannabis and certify patients for its use with the certainty that the risk of overdose or serious adverse effects is exceedingly low. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Managing dermatologic changes of targeted cancer therapy
Advances in cancer therapy have improved survival, such that many cancers have been transformed from a terminal illness to a chronic disease, and the population of patients living with cancer or who are disease-free has grown. However, these patients face complex medical problems because of the systemic effects of their treatment and many endure a constellation of treatment-emergent adverse effects that require ongoing care and support.1
Primary care physicians have been called on to take a larger role in the care of these adverse effects as the growing number of treatments has meant more affected patients. In addition, an urgent, unmet need has developed for better coordination between specialists and family physicians for providing this supportive care.2
In this article, we (1) describe the most commonly encountered cancer treatment–related skin toxicities, paying particular attention to the effects of epidermal growth factor receptor (EGFR)–targeting therapies, and (2) review up-to-date management recommendations in an area of practice where established clinical guidance from the scientific literature is limited.
Biggest culprit: Targeted cancer therapies
Skin rash and dermatologic adverse effects are commonplace in patients undergoing cancer treatment; timely management can often prevent long-term skin damage.3 Dermatologic effects have been associated with various therapeutic agents, but are most commonly associated with targeted therapies—specifically, agents targeting EGFR.
Why the attention to EGFR inhibition? EGFR is overexpressed or mutated in a multitude of solid tumors; as such, agents have been developed that target this aberrant signaling pathway. EGFR is highly expressed in the skin and dermal tissue, where it plays a number of roles, including protection against ultraviolet radiation damage.4
Blockade of the EGFR molecule leads to dermal changes, however, presenting as acneiform rash, skin fissure and xerosis, and pruritus.5 In extreme instances, toxic effects can manifest as paronychia, facial hypertrichosis, and trichomegaly. These skin changes can be deforming as well as painful, and can have physiological and psychological consequences.6
In turn, a decrease in quality of life (as reported by patients suffering from skin toxicity) can affect cancer treatment adherence and efficacy,7 and severe skin changes can result in the need to reduce the dosage of anti-cancer therapies.8 Skillful evaluation and appropriate management of skin eruptions in patients undergoing cancer therapy is therefore vital to an overall satisfactory outcome.
Continue to: How common a problem?
How common a problem? The incidence of EGFR inhibitor (EGFRI)–related rash is noteworthy: Overall incidence ranges from 45% to 100% of treated patients, with 10% experiencing Grade 3 to 4 changes (covering > 30% of body surface, restricting activities of daily living, severe itching).9 Monoclonal antibody therapies that target EGFR, such as cetuximab, have a reported 90% risk of skin rash, with 10% also being of Grade 3 to 4.10 Risk factors for rash include skin phototype, male gender, and younger age.11,12 Common cancer therapies with known skin effects are listed in the TABLE.13
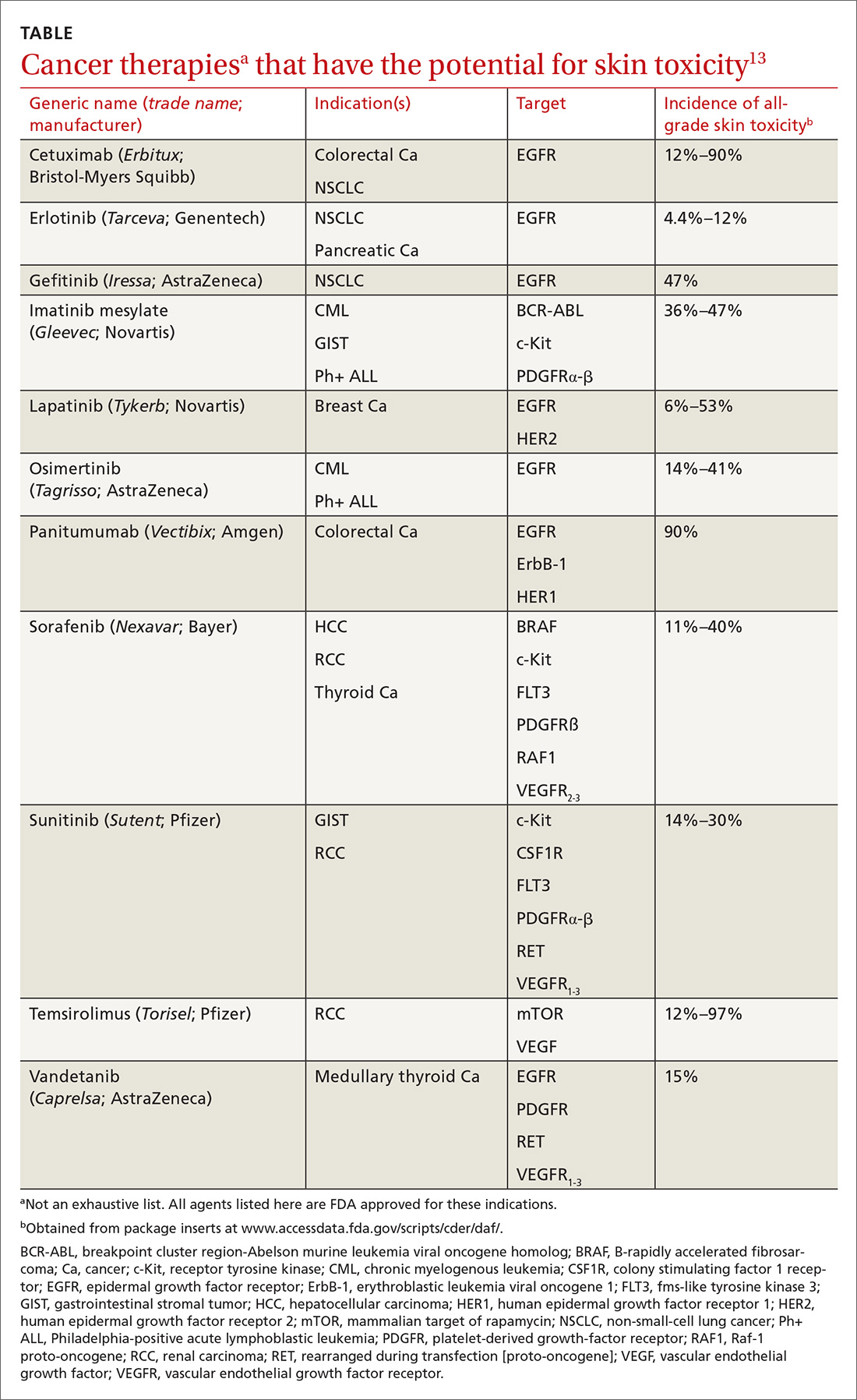
What should you look for? The most common clinical manifestation of dermatologic toxicity is an acneiform, or papulopustular, rash marked by eruptions characterized as “acne-like” pustules with monotonous lesion morphology (Figure 1a). A hallmark of these lesions that can be used to help distinguish them from acne vulgaris is the absence of comedones on eruptions.
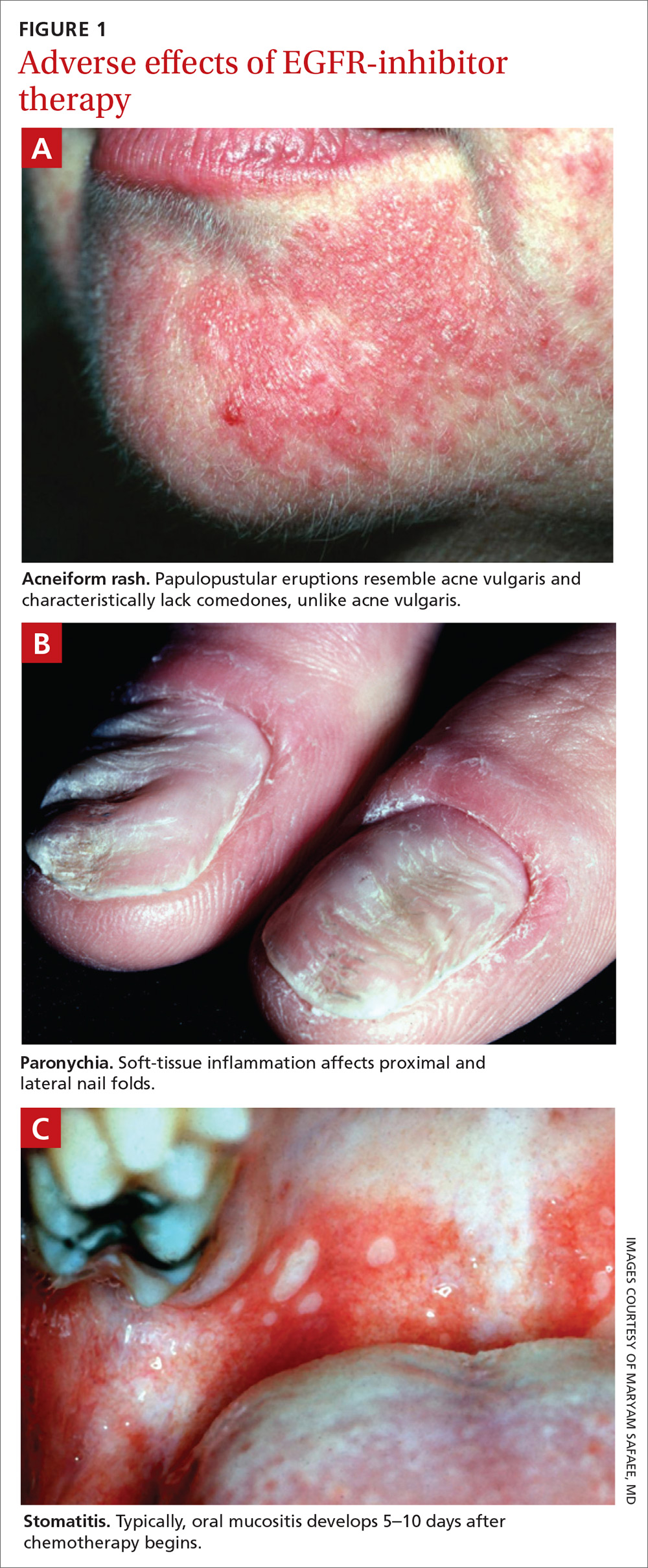
The timeline of the rash has been well characterized and is another tool that you can use to guide management:
- During Week 1 of cancer treatment, the patient often experiences sensory disturbances, with erythema and edema.14
- Throughout Weeks 2 and 3, erythematous skin evolves into papulopustular eruptions.
- By Week 4, eruptions typically crust over and leave persistently dry skin for weeks.15,16
Of note, the rash is dosage related; we recommend scrupulous vigilance when a patient is receiving a high dosage of a targeted therapy agent.
Controlling a rash
Treatment of EGFRI-associated skin changes stems from recommendations from a number of individual investigators and studies; however, few consensus guidelines exist to guide practice. Understanding of the underlying pathophysiological mechanism of skin changes has evolved, but preventive and treatment modalities remain unchanged—and limited.
Continue to: Always counsel patients...
Always counsel patients before a rash develops (and, ideally, before chemotherapy begins) that they should report a rash early in its development, to you or their oncologist, so that timely treatment can occur. Early recognition and intervention have proven benefits and can prevent the rash and its symptoms from becoming worse17; if the rash remains uncontrolled, dosage reduction of the chemotherapeutic agent is an inevitable reality, and the clinical outcome of the primary disease might therefore not be ideal.18
Prophylaxis. Daily application of an alcohol-free emollient cream is highly recommended as a preventive measure. Patients should be counseled to avoid activities and skin products that lead to dry skin, including long and hot showers; perfumes or other alcohol-based products; and soaps marketed for treating acne, which have a profound skin-drying effect.
Cornerstones of treatment include topical moisturizers, steroids, and antihistamines for symptom control. Once an identifiable skin rash has developed, a topical steroid cream is first-line treatment. Successful control has been reported with 1% hydrocortisone lotion applied daily to the affected area.15
Second- and third-line Tx. If the rash progresses in size or severity, we recommend switching to 2% hydrocortisone valerate cream, applied twice daily. For a moderate-to-severe rash, an oral tetracycline is a valid option for its anti-inflammatory effects and, possibly, to prevent secondary infection. In the event of progression, refer the patient to an oncologist, who can consider suspending the anti-EGRF drug temporarily until the rash improves. If disease persists, consultation with a dermatologist is appropriate for consideration of systemic prednisolone.
Alleviating discomfort. Patients commonly report pruritus and mild-to-moderate pain with the rash; standard analgesic therapy is appropriate.19 Severe pain might indicate secondary infection; in that case, consider antibiotic therapy for presumed cellulitis. Moreover, because of the risk of thrombosis in the cancer population, underlying deep-vein thrombosis must always remain in the differential diagnosis of an erythematous rash.
Continue to: A short course...
A short course of systemic steroids might be beneficial for pain control; however, no data from clinical trials suggest that this is beneficial. Dermatology consultation is recommended before prescribing a systemic steroid.
Regrettably, treatment options for pruritus are limited. Antihistamines, such as diphenhydramine and hydroxyzine, can be considered, but their effectiveness is marginal.20 If a patient reports a painful rash, we recommend that you collaborate with the dermatologist and oncologist to make adjustments to the cancer treatment plan.
Retinoids: Caution is advised. Several case reports and a small investigational study describe a potential role for retinoids such as isotretinoin, a 13-cis retinoic acid, in the treatment of chemotherapy-related skin changes.21,22 Isotretinoin is available under several trade names in pill and cream formulations.
Retinoids exert their effect at the level of DNA transcription, and act as a transcription factor in keratinocytes. Their downstream signaling pathway includes EGFR signaling ligands; introduction of exogenous retinoids has been shown to deter development of EGFRI-associated skin toxicity.23 Given the lack of clinical data, retinoid-based medications should be used at the discretion of a dermatologist; thorough discussion is encouraged among the dermatologist, oncologist, and primary care physician before employing a retinoid.
Recommend a sunscreen? Given the endogenous role of EGFR in protecting skin from ultraviolet B damage, some clinicians have recommended that patients use a sunscreen. However, randomized, controlled trials have failed to demonstrate any benefit to their use with regard to incidence or severity of rash or patient-reported discomfort.24 We do not recommend routine use of sunscreen to prevent chemotherapy-induced skin changes, although sensible use during periods of prolonged sun exposure is encouraged.
Continue to: Risk of infection and the role of antibiotics
Risk of infection and the role of antibiotics
Skin damage can lead to further complications—namely, leaving the skin vulnerable to bacterial overgrowth and serious infection.14 The primary acneiform eruption is believed to be inflammatory in nature, with most cases being sterile and lacking bacterial growth.25 However, rash-associated infections are a common complication and leave the immunocompromised patient at risk of systemic infection: Harandi et al26 reported a 35% rate of secondary infection. Viral or bacterial growth (the primary pathogen is Staphylococcus aureus) within the wound can aggravate the severity of the rash, prohibit effective healing, and exacerbate the disfiguring appearance of the rash.
The use of a prophylactic antibiotic for treating a rash in this setting has been an active area of discussion and research, although no guidelines or recommendations exist that can be routinely employed. A comprehensive systematic review and meta-analysis demonstrated that, in patients undergoing EGFR-based therapy, those who received a prophylactic antibiotic had a lower risk of developing folliculitis than those who did not (odds ratio = 0.53; 95% confidence interval, 0.39-0.72; P < .01). 27
A consensus agreement on the use of prophylactic antibiotics has yet to be reached. An emerging clinical practice entails the use of oral minocycline (100 mg/d) during the first 4 weeks of EGFRI-based therapy because studies have shown a benefit from this regimen in reducing eruptions.28
Other adverse dermatologic effects to watch for
Paronychia is common in patients undergoing EGFRI therapy but, unlike the acneiform rash that typically occurs within 1 week of treatment, paronychia can occur weeks or months after initiation of therapy. Careful examination of the nail beds is important in patients undergoing EGFRI therapy (FIGURE 1B). Paronychia can affect the nail beds of the fingers and toes—most often, the first digits.29
No evidence-based trials have been conducted to evaluate treatment options; recommendations provided are drawn from the literature and expert opinion. Patients are encouraged to apply petroleum jelly or an emollient daily both as a preventive measure and for mild cases. Patient counseling on the importance of nail hygiene and avoidance of aggressive manicures and pedicures is encouraged.30
Continue to: In the general population...
In the general population, acute and chronic paronychia entail infection with S aureus and Candida spp, respectively. To this end, there is a role for antibacterial and antifungal intervention. As is the case of the EGFRI-associated acneiform rash, inflammation in paronychia is sterile, with only rare pathogen involvement.
There is no role for topical or systemic antibiotics in the cancer population suffering from paronychia. A viable treatment option for moderate lesions is betamethasone valerate, applied 2 or 3 times daily; if there is no resolution, clobetasol cream, applied 2 or 3 times daily, can be prescribed.30 The role of tetracyclines as anti-inflammatory agents in paronychia has not been studied to the extent it has been for acneiform rash; however, studies have shown a protective effect in small patient samples.31 In severe disease, the patient can be instructed to temporarily discontinue the drug and you can provide a referral to a dermatologist.
Stomatitis is also an area of concern in this patient population (FIGURE 1c). Prior to initiating treatment, a thorough examination of the patient’s oral cavity and oropharynx should be conducted. Loose or improperly fitting dentures should be adjusted because they can prohibit effective healing after ulceration develops.
Stomatitis initially presents as erythematous or aphthous-like lesions, and can develop into acutely painful, large, continuous lesions.29 Timely management of stomatitis is beneficial to patient outcomes because it can lead to severe pain and interference in oral intake; uncontrolled disease requires interruption and dosage-reduction of cancer therapy.14,32
Patients should be encouraged to use soft-bristle toothbrushes and rinse with normal saline, not with commercial mouthwashes that typically contain alcohol. Grade 1 stomatitis (ie, pain and erythema) can be treated with triamcinolone dental paste, which can reduce inflammation caused by the ulcers. If disease progresses to Grade 2 to 3 stomatitis (erythema; ulceration; difficulty swallowing, or inability to swallow food), oral erythromycin (250-350 mg/d) or minocycline (50 mg/d) should be prescribed and the patient referred to a dermatologist.30
Continue to: Does rash correlate with cancer treatment efficacy?
Does rash correlate with cancer treatment efficacy?
Despite troubling dermatologic effects of cancer therapies, a retrospective analysis of several clinical trials has revealed another side to this coin: namely, the appearance, and the severity, of a rash correlates positively with objective tumor response.14 That correlation allows the oncologist to use a rash as a surrogate marker of treatment efficacy20 (although, notably, there remains a lack of prospective trials that would validate a rash as such a marker). Epidermal growth factor receptor-tyrosine kinase inhibitors are mainly prescribed in patients who harbor an activating EGFR mutation; no studies have stratified patients by EGFR mutation and incidence of rash.33
The upshot? Although there are gaps in our understanding of the relationship between a rash and overall survival, we are nevertheless presented with this paradigm: A patient who is taking an EGFR-tyrosine kinase inhibitor and who develops a rash should be continued on that treatment for as long as can be tolerated, because the rash is presumed to be a sign that the patient is deriving the greatest clinical benefit from therapy.14,20,33
CORRESPONDENCE
Kevin Zarrabi, MD, MSc, Department of Medicine, Health Science Center T16, Room 020, Stony Brook, NY 11790-8160; [email protected]
ACKNOWLEDGMENT
Ali John Zarrabi, MD, provided skillful editing of the manuscript of this article.
1. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian. 2010;17:47-50.
2. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029-1036.
3. Agha R, Kinahan K, Bennett CL, et al. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21:1462-1472; discussion 1473,1476,1481 passim.
4. Mitchell EP, Pérez-Soler R, Van Cutsem, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park). 2007;21(11 suppl 5):4-9.
5. Liu S, Kurzrock R. Understanding toxicities of targeted agents: implications for anti-tumor activity and management. Semin Oncol. 2015;42:863-875.
6. Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer. 2010;18:329-334.
7. Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913-3921.
8. Chou LS, Garey J, Oishi K, et al. Managing dermatologic toxicities of epidermal growth factor receptor inhibitors. Clin Lung Cancer. 2006;8(suppl 1):S15-S22.
9. Li T, Pérez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-119.
10. Su X, Lacouture ME, Jia Y, et al. Risk of high-grade skin rash in cancer patients treated with cetuximab—an antibody against epidermal growth factor receptor: systemic review and meta- analysis. Oncology. 2009;77:124-133.
11. Luu M, Boone SL, Patel J, et al. Higher severity grade of erlotinib-induced rash is associated with lower skin phototype. Clin Exp Dermatol. 2011;36:733-738.
12. Jatoi A, Green EM, Rowland KM Jr, et al. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in North Central Cancer Treatment Group study N0147. Oncology. 2009;77:120-123.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed June 4, 2019.
14. Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16-26.
15. Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007; 12:1-5.
16. Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008; 12:283-290.
17. Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126-138.
18. Reguiai Z, Bachet JB, Bachmeyer C, et al. Management of cuta- neous adverse events induced by anti-EGFR (epidermal growth factor receptor): a French interdisciplinary therapeutic algo- rithm. Support Care Cancer. 2012;20:1395-1404.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
20. Pérez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005;10:345-356.
21. Bidoli P, Cortinovis DL, Colombo I, et al. Isotretinoin plus clindamycin seem highly effective against severe erlotinib-induced skin rash in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1662-1663.
22. Vezzoli P, Marzano AV, Onida F, et al. Cetuximab-induced ac - neiform eruption and the response to isotretinoin. Acta Derm Venereol. 2008;88:84-86.
23. Rittié L, Varani J, Kang S, et al. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739.
24. Jatoi A, Thrower A, Sloan JA, et al. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist. 2010; 15:1016-1022.
25. Lynch TJ Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610-621.
26. Harandi A, Zaidi AS, Stocker AM, et al. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009:567486.
27. Petrelli F, Borgonovo K, Cabiddu M, et al. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol. 2016;175:1166-1174.
28. Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390-5396.
29. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al; MASCC Skin Toxicity Study Group. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19:1079-1095.
30. Melosky B, Leighl NB, Rothenstein J, et al. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015; 22:123-132.
31. Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88:282-288.
32. Saito H, Watanabe Y, Sato K, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22:2935-2940.
33. Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291-298.
Advances in cancer therapy have improved survival, such that many cancers have been transformed from a terminal illness to a chronic disease, and the population of patients living with cancer or who are disease-free has grown. However, these patients face complex medical problems because of the systemic effects of their treatment and many endure a constellation of treatment-emergent adverse effects that require ongoing care and support.1
Primary care physicians have been called on to take a larger role in the care of these adverse effects as the growing number of treatments has meant more affected patients. In addition, an urgent, unmet need has developed for better coordination between specialists and family physicians for providing this supportive care.2
In this article, we (1) describe the most commonly encountered cancer treatment–related skin toxicities, paying particular attention to the effects of epidermal growth factor receptor (EGFR)–targeting therapies, and (2) review up-to-date management recommendations in an area of practice where established clinical guidance from the scientific literature is limited.
Biggest culprit: Targeted cancer therapies
Skin rash and dermatologic adverse effects are commonplace in patients undergoing cancer treatment; timely management can often prevent long-term skin damage.3 Dermatologic effects have been associated with various therapeutic agents, but are most commonly associated with targeted therapies—specifically, agents targeting EGFR.
Why the attention to EGFR inhibition? EGFR is overexpressed or mutated in a multitude of solid tumors; as such, agents have been developed that target this aberrant signaling pathway. EGFR is highly expressed in the skin and dermal tissue, where it plays a number of roles, including protection against ultraviolet radiation damage.4
Blockade of the EGFR molecule leads to dermal changes, however, presenting as acneiform rash, skin fissure and xerosis, and pruritus.5 In extreme instances, toxic effects can manifest as paronychia, facial hypertrichosis, and trichomegaly. These skin changes can be deforming as well as painful, and can have physiological and psychological consequences.6
In turn, a decrease in quality of life (as reported by patients suffering from skin toxicity) can affect cancer treatment adherence and efficacy,7 and severe skin changes can result in the need to reduce the dosage of anti-cancer therapies.8 Skillful evaluation and appropriate management of skin eruptions in patients undergoing cancer therapy is therefore vital to an overall satisfactory outcome.
Continue to: How common a problem?
How common a problem? The incidence of EGFR inhibitor (EGFRI)–related rash is noteworthy: Overall incidence ranges from 45% to 100% of treated patients, with 10% experiencing Grade 3 to 4 changes (covering > 30% of body surface, restricting activities of daily living, severe itching).9 Monoclonal antibody therapies that target EGFR, such as cetuximab, have a reported 90% risk of skin rash, with 10% also being of Grade 3 to 4.10 Risk factors for rash include skin phototype, male gender, and younger age.11,12 Common cancer therapies with known skin effects are listed in the TABLE.13

What should you look for? The most common clinical manifestation of dermatologic toxicity is an acneiform, or papulopustular, rash marked by eruptions characterized as “acne-like” pustules with monotonous lesion morphology (Figure 1a). A hallmark of these lesions that can be used to help distinguish them from acne vulgaris is the absence of comedones on eruptions.

The timeline of the rash has been well characterized and is another tool that you can use to guide management:
- During Week 1 of cancer treatment, the patient often experiences sensory disturbances, with erythema and edema.14
- Throughout Weeks 2 and 3, erythematous skin evolves into papulopustular eruptions.
- By Week 4, eruptions typically crust over and leave persistently dry skin for weeks.15,16
Of note, the rash is dosage related; we recommend scrupulous vigilance when a patient is receiving a high dosage of a targeted therapy agent.
Controlling a rash
Treatment of EGFRI-associated skin changes stems from recommendations from a number of individual investigators and studies; however, few consensus guidelines exist to guide practice. Understanding of the underlying pathophysiological mechanism of skin changes has evolved, but preventive and treatment modalities remain unchanged—and limited.
Continue to: Always counsel patients...
Always counsel patients before a rash develops (and, ideally, before chemotherapy begins) that they should report a rash early in its development, to you or their oncologist, so that timely treatment can occur. Early recognition and intervention have proven benefits and can prevent the rash and its symptoms from becoming worse17; if the rash remains uncontrolled, dosage reduction of the chemotherapeutic agent is an inevitable reality, and the clinical outcome of the primary disease might therefore not be ideal.18
Prophylaxis. Daily application of an alcohol-free emollient cream is highly recommended as a preventive measure. Patients should be counseled to avoid activities and skin products that lead to dry skin, including long and hot showers; perfumes or other alcohol-based products; and soaps marketed for treating acne, which have a profound skin-drying effect.
Cornerstones of treatment include topical moisturizers, steroids, and antihistamines for symptom control. Once an identifiable skin rash has developed, a topical steroid cream is first-line treatment. Successful control has been reported with 1% hydrocortisone lotion applied daily to the affected area.15
Second- and third-line Tx. If the rash progresses in size or severity, we recommend switching to 2% hydrocortisone valerate cream, applied twice daily. For a moderate-to-severe rash, an oral tetracycline is a valid option for its anti-inflammatory effects and, possibly, to prevent secondary infection. In the event of progression, refer the patient to an oncologist, who can consider suspending the anti-EGRF drug temporarily until the rash improves. If disease persists, consultation with a dermatologist is appropriate for consideration of systemic prednisolone.
Alleviating discomfort. Patients commonly report pruritus and mild-to-moderate pain with the rash; standard analgesic therapy is appropriate.19 Severe pain might indicate secondary infection; in that case, consider antibiotic therapy for presumed cellulitis. Moreover, because of the risk of thrombosis in the cancer population, underlying deep-vein thrombosis must always remain in the differential diagnosis of an erythematous rash.
Continue to: A short course...
A short course of systemic steroids might be beneficial for pain control; however, no data from clinical trials suggest that this is beneficial. Dermatology consultation is recommended before prescribing a systemic steroid.
Regrettably, treatment options for pruritus are limited. Antihistamines, such as diphenhydramine and hydroxyzine, can be considered, but their effectiveness is marginal.20 If a patient reports a painful rash, we recommend that you collaborate with the dermatologist and oncologist to make adjustments to the cancer treatment plan.
Retinoids: Caution is advised. Several case reports and a small investigational study describe a potential role for retinoids such as isotretinoin, a 13-cis retinoic acid, in the treatment of chemotherapy-related skin changes.21,22 Isotretinoin is available under several trade names in pill and cream formulations.
Retinoids exert their effect at the level of DNA transcription, and act as a transcription factor in keratinocytes. Their downstream signaling pathway includes EGFR signaling ligands; introduction of exogenous retinoids has been shown to deter development of EGFRI-associated skin toxicity.23 Given the lack of clinical data, retinoid-based medications should be used at the discretion of a dermatologist; thorough discussion is encouraged among the dermatologist, oncologist, and primary care physician before employing a retinoid.
Recommend a sunscreen? Given the endogenous role of EGFR in protecting skin from ultraviolet B damage, some clinicians have recommended that patients use a sunscreen. However, randomized, controlled trials have failed to demonstrate any benefit to their use with regard to incidence or severity of rash or patient-reported discomfort.24 We do not recommend routine use of sunscreen to prevent chemotherapy-induced skin changes, although sensible use during periods of prolonged sun exposure is encouraged.
Continue to: Risk of infection and the role of antibiotics
Risk of infection and the role of antibiotics
Skin damage can lead to further complications—namely, leaving the skin vulnerable to bacterial overgrowth and serious infection.14 The primary acneiform eruption is believed to be inflammatory in nature, with most cases being sterile and lacking bacterial growth.25 However, rash-associated infections are a common complication and leave the immunocompromised patient at risk of systemic infection: Harandi et al26 reported a 35% rate of secondary infection. Viral or bacterial growth (the primary pathogen is Staphylococcus aureus) within the wound can aggravate the severity of the rash, prohibit effective healing, and exacerbate the disfiguring appearance of the rash.
The use of a prophylactic antibiotic for treating a rash in this setting has been an active area of discussion and research, although no guidelines or recommendations exist that can be routinely employed. A comprehensive systematic review and meta-analysis demonstrated that, in patients undergoing EGFR-based therapy, those who received a prophylactic antibiotic had a lower risk of developing folliculitis than those who did not (odds ratio = 0.53; 95% confidence interval, 0.39-0.72; P < .01). 27
A consensus agreement on the use of prophylactic antibiotics has yet to be reached. An emerging clinical practice entails the use of oral minocycline (100 mg/d) during the first 4 weeks of EGFRI-based therapy because studies have shown a benefit from this regimen in reducing eruptions.28
Other adverse dermatologic effects to watch for
Paronychia is common in patients undergoing EGFRI therapy but, unlike the acneiform rash that typically occurs within 1 week of treatment, paronychia can occur weeks or months after initiation of therapy. Careful examination of the nail beds is important in patients undergoing EGFRI therapy (FIGURE 1B). Paronychia can affect the nail beds of the fingers and toes—most often, the first digits.29
No evidence-based trials have been conducted to evaluate treatment options; recommendations provided are drawn from the literature and expert opinion. Patients are encouraged to apply petroleum jelly or an emollient daily both as a preventive measure and for mild cases. Patient counseling on the importance of nail hygiene and avoidance of aggressive manicures and pedicures is encouraged.30
Continue to: In the general population...
In the general population, acute and chronic paronychia entail infection with S aureus and Candida spp, respectively. To this end, there is a role for antibacterial and antifungal intervention. As is the case of the EGFRI-associated acneiform rash, inflammation in paronychia is sterile, with only rare pathogen involvement.
There is no role for topical or systemic antibiotics in the cancer population suffering from paronychia. A viable treatment option for moderate lesions is betamethasone valerate, applied 2 or 3 times daily; if there is no resolution, clobetasol cream, applied 2 or 3 times daily, can be prescribed.30 The role of tetracyclines as anti-inflammatory agents in paronychia has not been studied to the extent it has been for acneiform rash; however, studies have shown a protective effect in small patient samples.31 In severe disease, the patient can be instructed to temporarily discontinue the drug and you can provide a referral to a dermatologist.
Stomatitis is also an area of concern in this patient population (FIGURE 1c). Prior to initiating treatment, a thorough examination of the patient’s oral cavity and oropharynx should be conducted. Loose or improperly fitting dentures should be adjusted because they can prohibit effective healing after ulceration develops.
Stomatitis initially presents as erythematous or aphthous-like lesions, and can develop into acutely painful, large, continuous lesions.29 Timely management of stomatitis is beneficial to patient outcomes because it can lead to severe pain and interference in oral intake; uncontrolled disease requires interruption and dosage-reduction of cancer therapy.14,32
Patients should be encouraged to use soft-bristle toothbrushes and rinse with normal saline, not with commercial mouthwashes that typically contain alcohol. Grade 1 stomatitis (ie, pain and erythema) can be treated with triamcinolone dental paste, which can reduce inflammation caused by the ulcers. If disease progresses to Grade 2 to 3 stomatitis (erythema; ulceration; difficulty swallowing, or inability to swallow food), oral erythromycin (250-350 mg/d) or minocycline (50 mg/d) should be prescribed and the patient referred to a dermatologist.30
Continue to: Does rash correlate with cancer treatment efficacy?
Does rash correlate with cancer treatment efficacy?
Despite troubling dermatologic effects of cancer therapies, a retrospective analysis of several clinical trials has revealed another side to this coin: namely, the appearance, and the severity, of a rash correlates positively with objective tumor response.14 That correlation allows the oncologist to use a rash as a surrogate marker of treatment efficacy20 (although, notably, there remains a lack of prospective trials that would validate a rash as such a marker). Epidermal growth factor receptor-tyrosine kinase inhibitors are mainly prescribed in patients who harbor an activating EGFR mutation; no studies have stratified patients by EGFR mutation and incidence of rash.33
The upshot? Although there are gaps in our understanding of the relationship between a rash and overall survival, we are nevertheless presented with this paradigm: A patient who is taking an EGFR-tyrosine kinase inhibitor and who develops a rash should be continued on that treatment for as long as can be tolerated, because the rash is presumed to be a sign that the patient is deriving the greatest clinical benefit from therapy.14,20,33
CORRESPONDENCE
Kevin Zarrabi, MD, MSc, Department of Medicine, Health Science Center T16, Room 020, Stony Brook, NY 11790-8160; [email protected]
ACKNOWLEDGMENT
Ali John Zarrabi, MD, provided skillful editing of the manuscript of this article.
Advances in cancer therapy have improved survival, such that many cancers have been transformed from a terminal illness to a chronic disease, and the population of patients living with cancer or who are disease-free has grown. However, these patients face complex medical problems because of the systemic effects of their treatment and many endure a constellation of treatment-emergent adverse effects that require ongoing care and support.1
Primary care physicians have been called on to take a larger role in the care of these adverse effects as the growing number of treatments has meant more affected patients. In addition, an urgent, unmet need has developed for better coordination between specialists and family physicians for providing this supportive care.2
In this article, we (1) describe the most commonly encountered cancer treatment–related skin toxicities, paying particular attention to the effects of epidermal growth factor receptor (EGFR)–targeting therapies, and (2) review up-to-date management recommendations in an area of practice where established clinical guidance from the scientific literature is limited.
Biggest culprit: Targeted cancer therapies
Skin rash and dermatologic adverse effects are commonplace in patients undergoing cancer treatment; timely management can often prevent long-term skin damage.3 Dermatologic effects have been associated with various therapeutic agents, but are most commonly associated with targeted therapies—specifically, agents targeting EGFR.
Why the attention to EGFR inhibition? EGFR is overexpressed or mutated in a multitude of solid tumors; as such, agents have been developed that target this aberrant signaling pathway. EGFR is highly expressed in the skin and dermal tissue, where it plays a number of roles, including protection against ultraviolet radiation damage.4
Blockade of the EGFR molecule leads to dermal changes, however, presenting as acneiform rash, skin fissure and xerosis, and pruritus.5 In extreme instances, toxic effects can manifest as paronychia, facial hypertrichosis, and trichomegaly. These skin changes can be deforming as well as painful, and can have physiological and psychological consequences.6
In turn, a decrease in quality of life (as reported by patients suffering from skin toxicity) can affect cancer treatment adherence and efficacy,7 and severe skin changes can result in the need to reduce the dosage of anti-cancer therapies.8 Skillful evaluation and appropriate management of skin eruptions in patients undergoing cancer therapy is therefore vital to an overall satisfactory outcome.
Continue to: How common a problem?
How common a problem? The incidence of EGFR inhibitor (EGFRI)–related rash is noteworthy: Overall incidence ranges from 45% to 100% of treated patients, with 10% experiencing Grade 3 to 4 changes (covering > 30% of body surface, restricting activities of daily living, severe itching).9 Monoclonal antibody therapies that target EGFR, such as cetuximab, have a reported 90% risk of skin rash, with 10% also being of Grade 3 to 4.10 Risk factors for rash include skin phototype, male gender, and younger age.11,12 Common cancer therapies with known skin effects are listed in the TABLE.13

What should you look for? The most common clinical manifestation of dermatologic toxicity is an acneiform, or papulopustular, rash marked by eruptions characterized as “acne-like” pustules with monotonous lesion morphology (Figure 1a). A hallmark of these lesions that can be used to help distinguish them from acne vulgaris is the absence of comedones on eruptions.

The timeline of the rash has been well characterized and is another tool that you can use to guide management:
- During Week 1 of cancer treatment, the patient often experiences sensory disturbances, with erythema and edema.14
- Throughout Weeks 2 and 3, erythematous skin evolves into papulopustular eruptions.
- By Week 4, eruptions typically crust over and leave persistently dry skin for weeks.15,16
Of note, the rash is dosage related; we recommend scrupulous vigilance when a patient is receiving a high dosage of a targeted therapy agent.
Controlling a rash
Treatment of EGFRI-associated skin changes stems from recommendations from a number of individual investigators and studies; however, few consensus guidelines exist to guide practice. Understanding of the underlying pathophysiological mechanism of skin changes has evolved, but preventive and treatment modalities remain unchanged—and limited.
Continue to: Always counsel patients...
Always counsel patients before a rash develops (and, ideally, before chemotherapy begins) that they should report a rash early in its development, to you or their oncologist, so that timely treatment can occur. Early recognition and intervention have proven benefits and can prevent the rash and its symptoms from becoming worse17; if the rash remains uncontrolled, dosage reduction of the chemotherapeutic agent is an inevitable reality, and the clinical outcome of the primary disease might therefore not be ideal.18
Prophylaxis. Daily application of an alcohol-free emollient cream is highly recommended as a preventive measure. Patients should be counseled to avoid activities and skin products that lead to dry skin, including long and hot showers; perfumes or other alcohol-based products; and soaps marketed for treating acne, which have a profound skin-drying effect.
Cornerstones of treatment include topical moisturizers, steroids, and antihistamines for symptom control. Once an identifiable skin rash has developed, a topical steroid cream is first-line treatment. Successful control has been reported with 1% hydrocortisone lotion applied daily to the affected area.15
Second- and third-line Tx. If the rash progresses in size or severity, we recommend switching to 2% hydrocortisone valerate cream, applied twice daily. For a moderate-to-severe rash, an oral tetracycline is a valid option for its anti-inflammatory effects and, possibly, to prevent secondary infection. In the event of progression, refer the patient to an oncologist, who can consider suspending the anti-EGRF drug temporarily until the rash improves. If disease persists, consultation with a dermatologist is appropriate for consideration of systemic prednisolone.
Alleviating discomfort. Patients commonly report pruritus and mild-to-moderate pain with the rash; standard analgesic therapy is appropriate.19 Severe pain might indicate secondary infection; in that case, consider antibiotic therapy for presumed cellulitis. Moreover, because of the risk of thrombosis in the cancer population, underlying deep-vein thrombosis must always remain in the differential diagnosis of an erythematous rash.
Continue to: A short course...
A short course of systemic steroids might be beneficial for pain control; however, no data from clinical trials suggest that this is beneficial. Dermatology consultation is recommended before prescribing a systemic steroid.
Regrettably, treatment options for pruritus are limited. Antihistamines, such as diphenhydramine and hydroxyzine, can be considered, but their effectiveness is marginal.20 If a patient reports a painful rash, we recommend that you collaborate with the dermatologist and oncologist to make adjustments to the cancer treatment plan.
Retinoids: Caution is advised. Several case reports and a small investigational study describe a potential role for retinoids such as isotretinoin, a 13-cis retinoic acid, in the treatment of chemotherapy-related skin changes.21,22 Isotretinoin is available under several trade names in pill and cream formulations.
Retinoids exert their effect at the level of DNA transcription, and act as a transcription factor in keratinocytes. Their downstream signaling pathway includes EGFR signaling ligands; introduction of exogenous retinoids has been shown to deter development of EGFRI-associated skin toxicity.23 Given the lack of clinical data, retinoid-based medications should be used at the discretion of a dermatologist; thorough discussion is encouraged among the dermatologist, oncologist, and primary care physician before employing a retinoid.
Recommend a sunscreen? Given the endogenous role of EGFR in protecting skin from ultraviolet B damage, some clinicians have recommended that patients use a sunscreen. However, randomized, controlled trials have failed to demonstrate any benefit to their use with regard to incidence or severity of rash or patient-reported discomfort.24 We do not recommend routine use of sunscreen to prevent chemotherapy-induced skin changes, although sensible use during periods of prolonged sun exposure is encouraged.
Continue to: Risk of infection and the role of antibiotics
Risk of infection and the role of antibiotics
Skin damage can lead to further complications—namely, leaving the skin vulnerable to bacterial overgrowth and serious infection.14 The primary acneiform eruption is believed to be inflammatory in nature, with most cases being sterile and lacking bacterial growth.25 However, rash-associated infections are a common complication and leave the immunocompromised patient at risk of systemic infection: Harandi et al26 reported a 35% rate of secondary infection. Viral or bacterial growth (the primary pathogen is Staphylococcus aureus) within the wound can aggravate the severity of the rash, prohibit effective healing, and exacerbate the disfiguring appearance of the rash.
The use of a prophylactic antibiotic for treating a rash in this setting has been an active area of discussion and research, although no guidelines or recommendations exist that can be routinely employed. A comprehensive systematic review and meta-analysis demonstrated that, in patients undergoing EGFR-based therapy, those who received a prophylactic antibiotic had a lower risk of developing folliculitis than those who did not (odds ratio = 0.53; 95% confidence interval, 0.39-0.72; P < .01). 27
A consensus agreement on the use of prophylactic antibiotics has yet to be reached. An emerging clinical practice entails the use of oral minocycline (100 mg/d) during the first 4 weeks of EGFRI-based therapy because studies have shown a benefit from this regimen in reducing eruptions.28
Other adverse dermatologic effects to watch for
Paronychia is common in patients undergoing EGFRI therapy but, unlike the acneiform rash that typically occurs within 1 week of treatment, paronychia can occur weeks or months after initiation of therapy. Careful examination of the nail beds is important in patients undergoing EGFRI therapy (FIGURE 1B). Paronychia can affect the nail beds of the fingers and toes—most often, the first digits.29
No evidence-based trials have been conducted to evaluate treatment options; recommendations provided are drawn from the literature and expert opinion. Patients are encouraged to apply petroleum jelly or an emollient daily both as a preventive measure and for mild cases. Patient counseling on the importance of nail hygiene and avoidance of aggressive manicures and pedicures is encouraged.30
Continue to: In the general population...
In the general population, acute and chronic paronychia entail infection with S aureus and Candida spp, respectively. To this end, there is a role for antibacterial and antifungal intervention. As is the case of the EGFRI-associated acneiform rash, inflammation in paronychia is sterile, with only rare pathogen involvement.
There is no role for topical or systemic antibiotics in the cancer population suffering from paronychia. A viable treatment option for moderate lesions is betamethasone valerate, applied 2 or 3 times daily; if there is no resolution, clobetasol cream, applied 2 or 3 times daily, can be prescribed.30 The role of tetracyclines as anti-inflammatory agents in paronychia has not been studied to the extent it has been for acneiform rash; however, studies have shown a protective effect in small patient samples.31 In severe disease, the patient can be instructed to temporarily discontinue the drug and you can provide a referral to a dermatologist.
Stomatitis is also an area of concern in this patient population (FIGURE 1c). Prior to initiating treatment, a thorough examination of the patient’s oral cavity and oropharynx should be conducted. Loose or improperly fitting dentures should be adjusted because they can prohibit effective healing after ulceration develops.
Stomatitis initially presents as erythematous or aphthous-like lesions, and can develop into acutely painful, large, continuous lesions.29 Timely management of stomatitis is beneficial to patient outcomes because it can lead to severe pain and interference in oral intake; uncontrolled disease requires interruption and dosage-reduction of cancer therapy.14,32
Patients should be encouraged to use soft-bristle toothbrushes and rinse with normal saline, not with commercial mouthwashes that typically contain alcohol. Grade 1 stomatitis (ie, pain and erythema) can be treated with triamcinolone dental paste, which can reduce inflammation caused by the ulcers. If disease progresses to Grade 2 to 3 stomatitis (erythema; ulceration; difficulty swallowing, or inability to swallow food), oral erythromycin (250-350 mg/d) or minocycline (50 mg/d) should be prescribed and the patient referred to a dermatologist.30
Continue to: Does rash correlate with cancer treatment efficacy?
Does rash correlate with cancer treatment efficacy?
Despite troubling dermatologic effects of cancer therapies, a retrospective analysis of several clinical trials has revealed another side to this coin: namely, the appearance, and the severity, of a rash correlates positively with objective tumor response.14 That correlation allows the oncologist to use a rash as a surrogate marker of treatment efficacy20 (although, notably, there remains a lack of prospective trials that would validate a rash as such a marker). Epidermal growth factor receptor-tyrosine kinase inhibitors are mainly prescribed in patients who harbor an activating EGFR mutation; no studies have stratified patients by EGFR mutation and incidence of rash.33
The upshot? Although there are gaps in our understanding of the relationship between a rash and overall survival, we are nevertheless presented with this paradigm: A patient who is taking an EGFR-tyrosine kinase inhibitor and who develops a rash should be continued on that treatment for as long as can be tolerated, because the rash is presumed to be a sign that the patient is deriving the greatest clinical benefit from therapy.14,20,33
CORRESPONDENCE
Kevin Zarrabi, MD, MSc, Department of Medicine, Health Science Center T16, Room 020, Stony Brook, NY 11790-8160; [email protected]
ACKNOWLEDGMENT
Ali John Zarrabi, MD, provided skillful editing of the manuscript of this article.
1. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian. 2010;17:47-50.
2. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029-1036.
3. Agha R, Kinahan K, Bennett CL, et al. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21:1462-1472; discussion 1473,1476,1481 passim.
4. Mitchell EP, Pérez-Soler R, Van Cutsem, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park). 2007;21(11 suppl 5):4-9.
5. Liu S, Kurzrock R. Understanding toxicities of targeted agents: implications for anti-tumor activity and management. Semin Oncol. 2015;42:863-875.
6. Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer. 2010;18:329-334.
7. Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913-3921.
8. Chou LS, Garey J, Oishi K, et al. Managing dermatologic toxicities of epidermal growth factor receptor inhibitors. Clin Lung Cancer. 2006;8(suppl 1):S15-S22.
9. Li T, Pérez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-119.
10. Su X, Lacouture ME, Jia Y, et al. Risk of high-grade skin rash in cancer patients treated with cetuximab—an antibody against epidermal growth factor receptor: systemic review and meta- analysis. Oncology. 2009;77:124-133.
11. Luu M, Boone SL, Patel J, et al. Higher severity grade of erlotinib-induced rash is associated with lower skin phototype. Clin Exp Dermatol. 2011;36:733-738.
12. Jatoi A, Green EM, Rowland KM Jr, et al. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in North Central Cancer Treatment Group study N0147. Oncology. 2009;77:120-123.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed June 4, 2019.
14. Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16-26.
15. Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007; 12:1-5.
16. Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008; 12:283-290.
17. Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126-138.
18. Reguiai Z, Bachet JB, Bachmeyer C, et al. Management of cuta- neous adverse events induced by anti-EGFR (epidermal growth factor receptor): a French interdisciplinary therapeutic algo- rithm. Support Care Cancer. 2012;20:1395-1404.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
20. Pérez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005;10:345-356.
21. Bidoli P, Cortinovis DL, Colombo I, et al. Isotretinoin plus clindamycin seem highly effective against severe erlotinib-induced skin rash in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1662-1663.
22. Vezzoli P, Marzano AV, Onida F, et al. Cetuximab-induced ac - neiform eruption and the response to isotretinoin. Acta Derm Venereol. 2008;88:84-86.
23. Rittié L, Varani J, Kang S, et al. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739.
24. Jatoi A, Thrower A, Sloan JA, et al. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist. 2010; 15:1016-1022.
25. Lynch TJ Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610-621.
26. Harandi A, Zaidi AS, Stocker AM, et al. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009:567486.
27. Petrelli F, Borgonovo K, Cabiddu M, et al. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol. 2016;175:1166-1174.
28. Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390-5396.
29. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al; MASCC Skin Toxicity Study Group. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19:1079-1095.
30. Melosky B, Leighl NB, Rothenstein J, et al. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015; 22:123-132.
31. Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88:282-288.
32. Saito H, Watanabe Y, Sato K, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22:2935-2940.
33. Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291-298.
1. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian. 2010;17:47-50.
2. Klabunde CN, Ambs A, Keating NL, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029-1036.
3. Agha R, Kinahan K, Bennett CL, et al. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21:1462-1472; discussion 1473,1476,1481 passim.
4. Mitchell EP, Pérez-Soler R, Van Cutsem, et al. Clinical presentation and pathophysiology of EGFRI dermatologic toxicities. Oncology (Williston Park). 2007;21(11 suppl 5):4-9.
5. Liu S, Kurzrock R. Understanding toxicities of targeted agents: implications for anti-tumor activity and management. Semin Oncol. 2015;42:863-875.
6. Romito F, Giuliani F, Cormio C, et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer. 2010;18:329-334.
7. Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913-3921.
8. Chou LS, Garey J, Oishi K, et al. Managing dermatologic toxicities of epidermal growth factor receptor inhibitors. Clin Lung Cancer. 2006;8(suppl 1):S15-S22.
9. Li T, Pérez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107-119.
10. Su X, Lacouture ME, Jia Y, et al. Risk of high-grade skin rash in cancer patients treated with cetuximab—an antibody against epidermal growth factor receptor: systemic review and meta- analysis. Oncology. 2009;77:124-133.
11. Luu M, Boone SL, Patel J, et al. Higher severity grade of erlotinib-induced rash is associated with lower skin phototype. Clin Exp Dermatol. 2011;36:733-738.
12. Jatoi A, Green EM, Rowland KM Jr, et al. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in North Central Cancer Treatment Group study N0147. Oncology. 2009;77:120-123.
13. Drugs@FDA: FDA approved drug products. US Food and Drug Administration Web site. https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed June 4, 2019.
14. Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16-26.
15. Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007; 12:1-5.
16. Eaby B, Culkin A, Lacouture ME. An interdisciplinary consensus on managing skin reactions associated with human epidermal growth factor receptor inhibitors. Clin J Oncol Nurs. 2008; 12:283-290.
17. Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18:126-138.
18. Reguiai Z, Bachet JB, Bachmeyer C, et al. Management of cuta- neous adverse events induced by anti-EGFR (epidermal growth factor receptor): a French interdisciplinary therapeutic algo- rithm. Support Care Cancer. 2012;20:1395-1404.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
20. Pérez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR Inhibitor Rash Management Forum. Oncologist. 2005;10:345-356.
21. Bidoli P, Cortinovis DL, Colombo I, et al. Isotretinoin plus clindamycin seem highly effective against severe erlotinib-induced skin rash in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1662-1663.
22. Vezzoli P, Marzano AV, Onida F, et al. Cetuximab-induced ac - neiform eruption and the response to isotretinoin. Acta Derm Venereol. 2008;88:84-86.
23. Rittié L, Varani J, Kang S, et al. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739.
24. Jatoi A, Thrower A, Sloan JA, et al. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist. 2010; 15:1016-1022.
25. Lynch TJ Jr, Kim ES, Eaby B, et al. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610-621.
26. Harandi A, Zaidi AS, Stocker AM, et al. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009:567486.
27. Petrelli F, Borgonovo K, Cabiddu M, et al. Antibiotic prophylaxis for skin toxicity induced by antiepidermal growth factor receptor agents: a systematic review and meta-analysis. Br J Dermatol. 2016;175:1166-1174.
28. Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390-5396.
29. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al; MASCC Skin Toxicity Study Group. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19:1079-1095.
30. Melosky B, Leighl NB, Rothenstein J, et al. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015; 22:123-132.
31. Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88:282-288.
32. Saito H, Watanabe Y, Sato K, et al. Effects of professional oral health care on reducing the risk of chemotherapy-induced oral mucositis. Support Care Cancer. 2014;22:2935-2940.
33. Kozuki T. Skin problems and EGFR-tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291-298.
PRACTICE RECOMMENDATIONS
› Counsel patients about their risk of rash before epidermal growth factor receptor–targeting treatment is initiated; early recognition of rash and intervention lead to milder symptoms. A
› Encourage daily skin care with an alcohol-free emollient cream. Instruct patients to avoid products that can cause skin drying, prolonged hot showers, perfumes, and soaps marketed for treating acne. B
› Instruct patients that oral hygiene to lower their risk of stomatitis should include a soft-bristle toothbrush and oral rinsing with normal saline—not with an alcohol-based commercial mouthwash. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What we know—and don’t—about non-nutritive sweeteners
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.
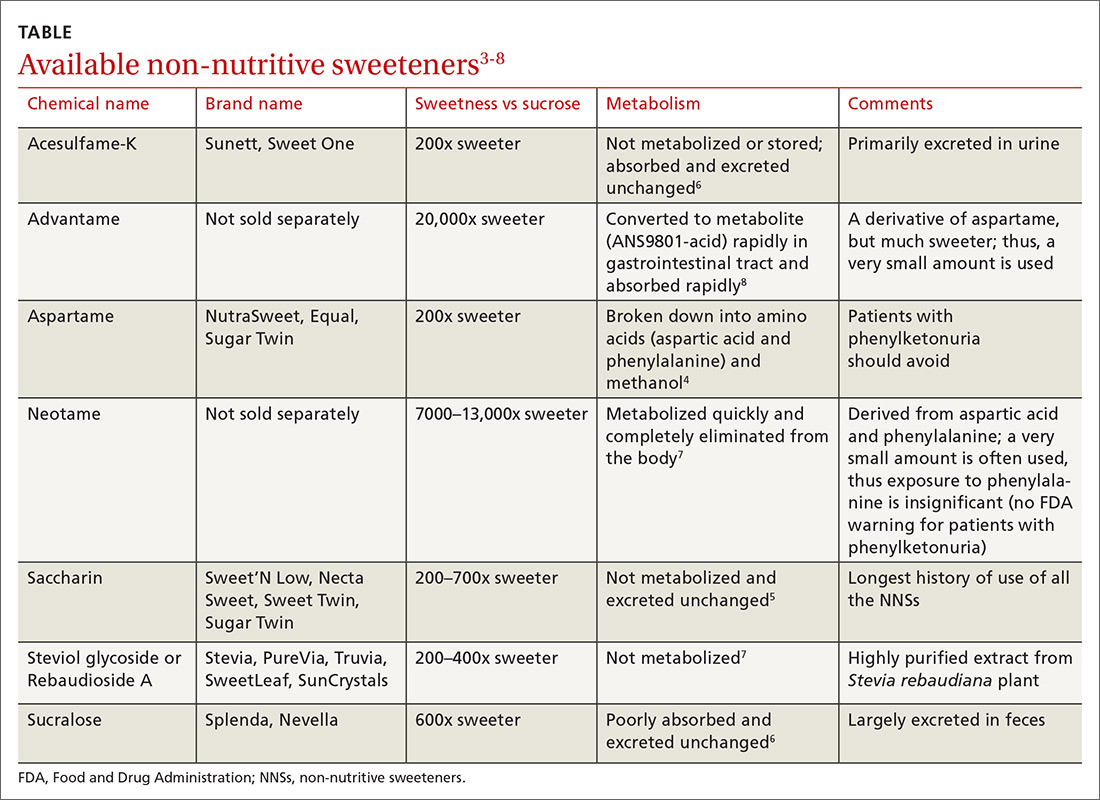
Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected].
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.

Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected].
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.

Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected].
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
PRACTICE RECOMMENDATIONS
› Advise patients who are trying to lose weight that non-nutritive sweeteners (NNSs) are not beneficial for weight loss. A
› Reassure patients that NNSs do not appear to cause, or increase the risk of, developing type 2 diabetes mellitus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Is it time to taper that opioid? (And how best to do it)
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
PRACTICE RECOMMENDATIONS
› Continue opioid therapy only when it has brought clinically meaningful improvement in pain and function and when the benefits outweigh adverse events or risks. C
› Review the selected opioid tapering plan in detail with the patient and provide close follow-up monitoring of ongoing or emerging risks. C
› Be vigilant: Enacting an opioid-tapering plan can unmask opioid use disorder, which can cause the patient to seek alternative forms of opioids, including illicit, potentially lethal fentanyl analogues. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series