User login
Transgender Care in the Primary Care Setting: A Review of Guidelines and Literature
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
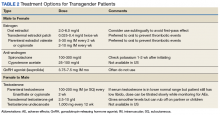
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
Screening and Treating Hepatitis C in the VA: Achieving Excellence Using Lean and System Redesign
Hepatitis C virus (HCV) infection is a major public health problem in the US. Following the 2010 report of the Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) on hepatitis and liver cancer, the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan in 2011 with subsequent action plan updates for 2014-2016 and 2017-2020.1-3 A NASEM phase 2 report and the 2017-2020 HHS action plan outline a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.3,4 The Department of Veterans Affairs (VA) is the single largest HCV care provider in the US with about 165,000 veterans in care diagnosed with HCV in the beginning of 2014 and is a national leader in the testing and treatment of HCV.5,6
The VA’s recommendations for screening for HCV infection are in alignment with the United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommendations to test all veterans born between 1945 and 1965 and anyone with risk factors such as injection drug use.7-9 As of January 1, 2018, the VA had screened more than 80% of veterans in care within this highest risk birth cohort. As of January 1, 2018, more than 100,000 veterans in VA care have initiated treatment for HCV with direct-acting antivirals (DAAs) (Figure 1).
Several critical factors contributed to the VA success with HCV testing and treatment, including congressional appropriation of funding from fiscal year (FY) 2016 through FY 2018, unrestricted access to interferon-free DAA HCV treatments, and dedicated resources from the VA National Viral Hepatitis Program within the HIV, Hepatitis, and Related Conditions Programs (HHRC) in the Office of Specialty Care Services.5 In 2014, HHRC created and supported the Hepatitis Innovation Team (HIT) Collaborative, a VA process improvement initiative enabling
Veterans Integrated Service Network (VISN) -based, multidisciplinary teams to increase veterans’ access to HCV testing and treatment.
As the VA makes consistent progress toward eliminating HCV in veterans in VA care, it has become clear that achieving a cure is only a starting point in improving HCV care. Many patients with HCV infection also have advanced liver disease (ALD), or cirrhosis, which is a condition of permanent liver fibrosis that remains after the patient has been cured of HCV infection. In addition to hepatitis C, ALD also can be caused by excessive alcohol use, hepatitis B virus (HBV) infection, nonalcoholic fatty liver diseases, and several other inherited diseases. Advanced liver disease affects more than 80,000 veterans in VA care, and the HIT infrastructure provides an excellent framework to better understand and address facility-level and systemwide challenges in diagnosing, caring for, and treating veterans with ALD across the Veterans Health Administration (VHA) system.
This report will describe the elements that contributed to the success of the HIT Collaborative in redesigning care for patients affected by HCV in the VA and how these elements can be applied to improve the system of care for VHA ALD care.
Hepatitis Innovation Teams Collaborative Leadership
After the US Food and Drug Administration (FDA) approved new DAA medications to treat HCV, the VA recognized the need to mobilize the health care system quickly and allocate resources for these new, minimally toxic, and highly effective medications. Early in 2014, HHRC established the National Hepatitis C Resource Center (NHCRC), a successor program to the 4 regional hepatitis C resource centers that had addressed HCV care across the system.10 The NHCRC was charged with developing an operational strategy for VA to respond rapidly to the availability of DAAs. In collaboration with representatives from the Office of Strategic Integration | Veterans Engineering Resource Center (OSI|VERC), the NHCRC formed the HIT Collaborative Leadership Team (CLT).
The HIT CLT is responsible for executing the HIT Collaborative and uses a Lean process improvement framework focused on eliminating waste and maximizing value. Members of the CLT with expertise in facilitation, Lean process improvement, leadership, clinical knowledge, and population health management act as coaches for the VISN HITs. The CLT works to build and support the VISN HITs, identify opportunities for individual teams to improve and assist in finding the right local mix of “players” to be successful. The HIT CLT ensures all teams are functioning and working toward achieving their goals. The CLT obtains data from VA national databases, which are provided to the VISN HITs to inform and encourage continuous improvement of their strategies. Annual VA-wide aspirational goals are developed and disseminated to encourage a unified mission.
Catchment areas for each VISN include between 6 and 10 medical centers as well as outpatient and ambulatory care centers. Multidisciplinary HITs are composed of physicians, nurses, pharmacists, nurse practitioners, physician assistants, social workers, mental health and substance use providers, peer support specialists, administrators, information technology experts, and systems redesign professionals from medical centers within each VISN. Teams develop strong relationships across medical centers, implement context-specific strategies applicable to rural
and urban centers, and share expertise. In addition to intra-VISN process improvement, HITs collaborate monthly across VISNs via a virtual platform. They share strong practices, seek advice from one another, and compare outcomes on an established set of goals.
The HITs use process improvement tools to systematically assess the current steps involved in care. At the close of each year, the HITs analyze the current state of operations and set goals to improve over the following year guided by a target state map. Seed funding is provided to every VISN HIT annually to launch change initiatives. Many VISN HITs use these funds to support a VISN HIT coordinator, and HITs also use this financial support to conduct 2- to 3-day process improvement workshops and to purchase supplies, such as point-of-care testing kits. The HIT communication and work are predominantly executed virtually.
Each year, teams worked toward achieving goals set nationally. These included increasing HCV birth cohort testing and improving the percentage of patients who had SVR12 testing
(Table).
the percentage of patients who received SVR12 testing posttreatment completion was not included in the HIT Collaborative’s annual goals for the first year of the program. Recognizing this as a critical area for improvement, the HIT CLT set a goal to test 80% of all patients who completed treatment. The HITs applied Lean tools to identify and overcome gaps in the SVR12 testing process. By the end of the second year, 84% of all patients who completed treatment had been tested for SVR12.
The HITs also set specific local VISN and medical center goals, prioritizing projects that could have the greatest impact on local patient access and quality of care and build on existing strengths and address barriers. These projects encompass a wide range of areas that contribute to the overall national goals.
Focus on Lean
Lean process improvement is based on 2 key pillars: respect for people (those seeking service as customers and patients and those providing service as frontline staff and stakeholders) and continuous improvement. With Lean, personnel providing care should work to identify and eliminate waste in the system and to streamline care delivery to maximize process steps that are most valued by patients (eg, interaction with a clinical provider) and minimize those that are not valued (eg, time spent waiting to see a provider). With the knowledge that HHRC fully supports their work, HITs were encouraged to innovate based on local resources, context, and culture.
Teams receive basic training in Lean from the HIT CLT and local systems redesign specialists if available. The HITs apply the A3 structured approach to problem solving.11 The HITs follow prescribed problemsolving steps that help identify where to focus process improvement efforts, including analyzing the current state of care, outlining the target state, and prioritizing solution
approaches based on what will have the highest impact for patients.
to accommodate the outcomes they observe (Figure 2).
Innovations
Over the course of the HIT Collaborative, numerous innovations have emerged to address and mitigate barriers to HCV screening and treatment. Examples of successful innovations include the following:
- To address transportation issues, several teams developed programs specific to patients with HCV in rural locations or with limited mobility. Mobile vans and units traditionally used as mobile cardiology clinics were transformed into HCV clinics, bringing testing and treatment services directly to veterans;
- Pharmacists and social workers developed outreach strategies to locate homeless veterans, provide point-of-care testing and utilize mobile technology to concurrently enroll and link veterans to care; and
- Many liver care teams partnered with inpatient and outpatient substance use treatment clinics to provide patient education and coordinate HCV treatment.
Inter-VISN working groups developed systemwide tools to address common needs. In the program’s first year, a few medical facilities across a handful of VISNs shared local population health management systems, programming, and best practices. Over time, this working group combined the virtual networking capacity of the HIT Collaborative with technical expertise to promote rapid dissemination and uptake of a population health management system. Providers at medical centers across VA use the tools to identify veterans who should be screened and treated for HCV with the ability to continuously update information, identifying patients who do not respond to treatment or patients overdue for SVR12 testing.
Providers with experience using telehepatology formed another inter-VISN working group. These subject matter experts provided guidance to care teams interested in implementing telehealth in areas where limited local resources or knowledge had prevented them from moving forward. The ability to build a strong coalition across content areas fostered a collaborative learning environment, adaptable to implementing new processes and technologies.
In 2017, the VA made significant efforts to reach out to veterans eligible for VA care who had not yet been screened or remained untreated. In May, Hepatitis Awareness Month, HITs held HCV testing and community outreach events and participated in veteran stand-downs and veteran service organization activities.
Evaluation
Since 2014, the VA has increased its HCV treatment and screening rates. To assess the components contributing to these achievements and the role of the HIT Collaborative in driving this success, a team of implementation scientists have been working with the CLT to conduct a HIT program evaluation. The goal of the evaluation is to establish the impact of the HIT Collaborative. The evaluation team catalogs the activities of the Collaborative and the HITs and assesses implementation strategies (use of specific techniques) to increase the uptake of evidence-based practices specifically related to HCV treatment.12
At the close of each FY, HCV providers and members of the HIT Collaborative are queried through an online survey to determine which strategies have been used to improve HCV care and how these strategies were associated with the HIT Collaborative. The use of more strategies was associated with more HCV treatment initiations.13 All utilized strategies were identified whether or not they were associated with treatment starts. These data are being used to understand which combinations of strategies are most effective at increasing treatment for HCV in the VA and to inform future initiatives.
Expanding the Scope
Inspired by the successful results of the HIT work in HCV and in the spirit of continuously improving health care delivery, HHRC expanded the scope of the HIT Collaborative in FY 2018 to include ALD. There are about 80,000 veterans in VA care with advanced scarring of the liver and between 10,000 to 15,000 new diagnoses each year. In addition to HCV as an etiology for ALD, cases of cirrhosis are projected to increase among veterans in care due to metabolic syndrome and alcohol use. A recent review of VA data from fiscal year 2016 found that 88.6% of ALD patients had been seen in primary care within the past 2 years, with about half (51%) seen in a gastroenterology (GI) or hepatology clinic (Personal communication, HIV, Hepatitis, and Related Conditions Program Office March 16, 2018). For patients in VA care with ALD, GI visits are associated with a lower 5-year mortality.14 Annual mortality for all ALD patients in VA is 6.2%, and of those with a hospital admission, mortality rises to 31%.15 In FY 2016, there were about 52,000 ALD-related discharges (more than 2 per patient). Of those discharges, 24% were readmitted within 30 days, with an average length of stay of 1.9 days and an estimated cost per patient of $47,000 over 3 years.16
Hepatologists from across the VA convened to identify critical opportunities for improvement for patients with ALD. Base on available evidence presented in the literature and their clinical expertise, these subject matter experts identified several areas for quality improvement, with the overarching goal to improve identification of patients with early cirrhosis and ensure appropriate linkage to care for all cirrhotic patients, thus improving quality of life and reducing mortality. Although not finalized, candidate improvement targets include consistent linkage to care and treatment for HCV and HBV, comprehensive case management, post-discharge patient follow-up, and adherence to evidence-based standards of care.
Conclusion
The VA has made great strides in nearly eliminating HCV among veterans in VA care. The national effort to redesign hepatitis care using Lean management strategies and develop local and regional teams and centralized support allowed VA to maximize available resources to achieve higher rates of HCV birth cohort testing and treatment of patients infected with HCV than has any other health care system in the US.
The HIT Collaborative has been a unique and innovative mechanism to promote directed, patient-outcome driven change in a large and dynamic health care system. It has allowed rural and urban providers to work together to develop and spread quality improvement innovations and as an integrated system to achieve national priorities. The focus of this foundational HIT structure is expanding to identifying, treat, and care for VA’s ALD population.
1. Colvin HM, Mitchell AE, eds; and the Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National Academies Press; 2010.
2. US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care and treatment of viral hepatitis. https://www.hhs.gov/sites/default/files/action-plan-viral-hepatitis-2011.pdf. Accessed April 27, 2018.
3. Wolitski R. National viral hepatitis action plan: 2017-2020. https://www.hhs.gov/hepatitis/action-plan/national-viralhepatitis-action-plan-overview/index.html. Updated February
21, 2018. Accessed May 8, 2018.
4. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press; 2017.
5. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C infection: best practices from the Department of Veterans Affairs. Ann of Intern Med. 2017;167(7):499-504.
6. Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol. 2015;63(3):586-592.
7. US Department of Veterans Affairs, Veteran Health Administration. National Clinical Preventive Service Guidance Statements: Screening for Hepatitis C. http://www.prevention.va.gov/CPS/Screening_for_Hepatitis_C.asp. Published on June 20, 2017. [Nonpubic document; source not verified.]
8. Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
9. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
10. Garrard J, Choudary V, Groom H, et al. Organizational change in management of hepatitis C: evaluation of a CME program. J Contin Educ Health Prof. 2006;26(2):145-160.
11. Shook J. Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor, and Lead. Cambridge, MA: Lean Enterprise Institute; 2010.
12. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
13. Rogal SS, Yakovchenko V, Waltz TJ, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci.
2017;12(1):60.
14. Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111(6):838-844.
15. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in the burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans from 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
16. Kaplan DE, Chapko MK, Mehta R, et al; VOCAL Study Group. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018;16(1):106-114.
Hepatitis C virus (HCV) infection is a major public health problem in the US. Following the 2010 report of the Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) on hepatitis and liver cancer, the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan in 2011 with subsequent action plan updates for 2014-2016 and 2017-2020.1-3 A NASEM phase 2 report and the 2017-2020 HHS action plan outline a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.3,4 The Department of Veterans Affairs (VA) is the single largest HCV care provider in the US with about 165,000 veterans in care diagnosed with HCV in the beginning of 2014 and is a national leader in the testing and treatment of HCV.5,6
The VA’s recommendations for screening for HCV infection are in alignment with the United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommendations to test all veterans born between 1945 and 1965 and anyone with risk factors such as injection drug use.7-9 As of January 1, 2018, the VA had screened more than 80% of veterans in care within this highest risk birth cohort. As of January 1, 2018, more than 100,000 veterans in VA care have initiated treatment for HCV with direct-acting antivirals (DAAs) (Figure 1).
Several critical factors contributed to the VA success with HCV testing and treatment, including congressional appropriation of funding from fiscal year (FY) 2016 through FY 2018, unrestricted access to interferon-free DAA HCV treatments, and dedicated resources from the VA National Viral Hepatitis Program within the HIV, Hepatitis, and Related Conditions Programs (HHRC) in the Office of Specialty Care Services.5 In 2014, HHRC created and supported the Hepatitis Innovation Team (HIT) Collaborative, a VA process improvement initiative enabling
Veterans Integrated Service Network (VISN) -based, multidisciplinary teams to increase veterans’ access to HCV testing and treatment.
As the VA makes consistent progress toward eliminating HCV in veterans in VA care, it has become clear that achieving a cure is only a starting point in improving HCV care. Many patients with HCV infection also have advanced liver disease (ALD), or cirrhosis, which is a condition of permanent liver fibrosis that remains after the patient has been cured of HCV infection. In addition to hepatitis C, ALD also can be caused by excessive alcohol use, hepatitis B virus (HBV) infection, nonalcoholic fatty liver diseases, and several other inherited diseases. Advanced liver disease affects more than 80,000 veterans in VA care, and the HIT infrastructure provides an excellent framework to better understand and address facility-level and systemwide challenges in diagnosing, caring for, and treating veterans with ALD across the Veterans Health Administration (VHA) system.
This report will describe the elements that contributed to the success of the HIT Collaborative in redesigning care for patients affected by HCV in the VA and how these elements can be applied to improve the system of care for VHA ALD care.
Hepatitis Innovation Teams Collaborative Leadership
After the US Food and Drug Administration (FDA) approved new DAA medications to treat HCV, the VA recognized the need to mobilize the health care system quickly and allocate resources for these new, minimally toxic, and highly effective medications. Early in 2014, HHRC established the National Hepatitis C Resource Center (NHCRC), a successor program to the 4 regional hepatitis C resource centers that had addressed HCV care across the system.10 The NHCRC was charged with developing an operational strategy for VA to respond rapidly to the availability of DAAs. In collaboration with representatives from the Office of Strategic Integration | Veterans Engineering Resource Center (OSI|VERC), the NHCRC formed the HIT Collaborative Leadership Team (CLT).
The HIT CLT is responsible for executing the HIT Collaborative and uses a Lean process improvement framework focused on eliminating waste and maximizing value. Members of the CLT with expertise in facilitation, Lean process improvement, leadership, clinical knowledge, and population health management act as coaches for the VISN HITs. The CLT works to build and support the VISN HITs, identify opportunities for individual teams to improve and assist in finding the right local mix of “players” to be successful. The HIT CLT ensures all teams are functioning and working toward achieving their goals. The CLT obtains data from VA national databases, which are provided to the VISN HITs to inform and encourage continuous improvement of their strategies. Annual VA-wide aspirational goals are developed and disseminated to encourage a unified mission.
Catchment areas for each VISN include between 6 and 10 medical centers as well as outpatient and ambulatory care centers. Multidisciplinary HITs are composed of physicians, nurses, pharmacists, nurse practitioners, physician assistants, social workers, mental health and substance use providers, peer support specialists, administrators, information technology experts, and systems redesign professionals from medical centers within each VISN. Teams develop strong relationships across medical centers, implement context-specific strategies applicable to rural
and urban centers, and share expertise. In addition to intra-VISN process improvement, HITs collaborate monthly across VISNs via a virtual platform. They share strong practices, seek advice from one another, and compare outcomes on an established set of goals.
The HITs use process improvement tools to systematically assess the current steps involved in care. At the close of each year, the HITs analyze the current state of operations and set goals to improve over the following year guided by a target state map. Seed funding is provided to every VISN HIT annually to launch change initiatives. Many VISN HITs use these funds to support a VISN HIT coordinator, and HITs also use this financial support to conduct 2- to 3-day process improvement workshops and to purchase supplies, such as point-of-care testing kits. The HIT communication and work are predominantly executed virtually.
Each year, teams worked toward achieving goals set nationally. These included increasing HCV birth cohort testing and improving the percentage of patients who had SVR12 testing
(Table).
the percentage of patients who received SVR12 testing posttreatment completion was not included in the HIT Collaborative’s annual goals for the first year of the program. Recognizing this as a critical area for improvement, the HIT CLT set a goal to test 80% of all patients who completed treatment. The HITs applied Lean tools to identify and overcome gaps in the SVR12 testing process. By the end of the second year, 84% of all patients who completed treatment had been tested for SVR12.
The HITs also set specific local VISN and medical center goals, prioritizing projects that could have the greatest impact on local patient access and quality of care and build on existing strengths and address barriers. These projects encompass a wide range of areas that contribute to the overall national goals.
Focus on Lean
Lean process improvement is based on 2 key pillars: respect for people (those seeking service as customers and patients and those providing service as frontline staff and stakeholders) and continuous improvement. With Lean, personnel providing care should work to identify and eliminate waste in the system and to streamline care delivery to maximize process steps that are most valued by patients (eg, interaction with a clinical provider) and minimize those that are not valued (eg, time spent waiting to see a provider). With the knowledge that HHRC fully supports their work, HITs were encouraged to innovate based on local resources, context, and culture.
Teams receive basic training in Lean from the HIT CLT and local systems redesign specialists if available. The HITs apply the A3 structured approach to problem solving.11 The HITs follow prescribed problemsolving steps that help identify where to focus process improvement efforts, including analyzing the current state of care, outlining the target state, and prioritizing solution
approaches based on what will have the highest impact for patients.
to accommodate the outcomes they observe (Figure 2).
Innovations
Over the course of the HIT Collaborative, numerous innovations have emerged to address and mitigate barriers to HCV screening and treatment. Examples of successful innovations include the following:
- To address transportation issues, several teams developed programs specific to patients with HCV in rural locations or with limited mobility. Mobile vans and units traditionally used as mobile cardiology clinics were transformed into HCV clinics, bringing testing and treatment services directly to veterans;
- Pharmacists and social workers developed outreach strategies to locate homeless veterans, provide point-of-care testing and utilize mobile technology to concurrently enroll and link veterans to care; and
- Many liver care teams partnered with inpatient and outpatient substance use treatment clinics to provide patient education and coordinate HCV treatment.
Inter-VISN working groups developed systemwide tools to address common needs. In the program’s first year, a few medical facilities across a handful of VISNs shared local population health management systems, programming, and best practices. Over time, this working group combined the virtual networking capacity of the HIT Collaborative with technical expertise to promote rapid dissemination and uptake of a population health management system. Providers at medical centers across VA use the tools to identify veterans who should be screened and treated for HCV with the ability to continuously update information, identifying patients who do not respond to treatment or patients overdue for SVR12 testing.
Providers with experience using telehepatology formed another inter-VISN working group. These subject matter experts provided guidance to care teams interested in implementing telehealth in areas where limited local resources or knowledge had prevented them from moving forward. The ability to build a strong coalition across content areas fostered a collaborative learning environment, adaptable to implementing new processes and technologies.
In 2017, the VA made significant efforts to reach out to veterans eligible for VA care who had not yet been screened or remained untreated. In May, Hepatitis Awareness Month, HITs held HCV testing and community outreach events and participated in veteran stand-downs and veteran service organization activities.
Evaluation
Since 2014, the VA has increased its HCV treatment and screening rates. To assess the components contributing to these achievements and the role of the HIT Collaborative in driving this success, a team of implementation scientists have been working with the CLT to conduct a HIT program evaluation. The goal of the evaluation is to establish the impact of the HIT Collaborative. The evaluation team catalogs the activities of the Collaborative and the HITs and assesses implementation strategies (use of specific techniques) to increase the uptake of evidence-based practices specifically related to HCV treatment.12
At the close of each FY, HCV providers and members of the HIT Collaborative are queried through an online survey to determine which strategies have been used to improve HCV care and how these strategies were associated with the HIT Collaborative. The use of more strategies was associated with more HCV treatment initiations.13 All utilized strategies were identified whether or not they were associated with treatment starts. These data are being used to understand which combinations of strategies are most effective at increasing treatment for HCV in the VA and to inform future initiatives.
Expanding the Scope
Inspired by the successful results of the HIT work in HCV and in the spirit of continuously improving health care delivery, HHRC expanded the scope of the HIT Collaborative in FY 2018 to include ALD. There are about 80,000 veterans in VA care with advanced scarring of the liver and between 10,000 to 15,000 new diagnoses each year. In addition to HCV as an etiology for ALD, cases of cirrhosis are projected to increase among veterans in care due to metabolic syndrome and alcohol use. A recent review of VA data from fiscal year 2016 found that 88.6% of ALD patients had been seen in primary care within the past 2 years, with about half (51%) seen in a gastroenterology (GI) or hepatology clinic (Personal communication, HIV, Hepatitis, and Related Conditions Program Office March 16, 2018). For patients in VA care with ALD, GI visits are associated with a lower 5-year mortality.14 Annual mortality for all ALD patients in VA is 6.2%, and of those with a hospital admission, mortality rises to 31%.15 In FY 2016, there were about 52,000 ALD-related discharges (more than 2 per patient). Of those discharges, 24% were readmitted within 30 days, with an average length of stay of 1.9 days and an estimated cost per patient of $47,000 over 3 years.16
Hepatologists from across the VA convened to identify critical opportunities for improvement for patients with ALD. Base on available evidence presented in the literature and their clinical expertise, these subject matter experts identified several areas for quality improvement, with the overarching goal to improve identification of patients with early cirrhosis and ensure appropriate linkage to care for all cirrhotic patients, thus improving quality of life and reducing mortality. Although not finalized, candidate improvement targets include consistent linkage to care and treatment for HCV and HBV, comprehensive case management, post-discharge patient follow-up, and adherence to evidence-based standards of care.
Conclusion
The VA has made great strides in nearly eliminating HCV among veterans in VA care. The national effort to redesign hepatitis care using Lean management strategies and develop local and regional teams and centralized support allowed VA to maximize available resources to achieve higher rates of HCV birth cohort testing and treatment of patients infected with HCV than has any other health care system in the US.
The HIT Collaborative has been a unique and innovative mechanism to promote directed, patient-outcome driven change in a large and dynamic health care system. It has allowed rural and urban providers to work together to develop and spread quality improvement innovations and as an integrated system to achieve national priorities. The focus of this foundational HIT structure is expanding to identifying, treat, and care for VA’s ALD population.
Hepatitis C virus (HCV) infection is a major public health problem in the US. Following the 2010 report of the Institute of Medicine/National Academies of Sciences, Engineering, and Medicine (NASEM) on hepatitis and liver cancer, the US Department of Health and Human Services (HHS) released the first National Viral Hepatitis Action Plan in 2011 with subsequent action plan updates for 2014-2016 and 2017-2020.1-3 A NASEM phase 2 report and the 2017-2020 HHS action plan outline a national strategy to prevent new viral hepatitis infections; reduce deaths and improve the health of people living with viral hepatitis; reduce viral hepatitis health disparities; and coordinate, monitor, and report on implementation of viral hepatitis activities.3,4 The Department of Veterans Affairs (VA) is the single largest HCV care provider in the US with about 165,000 veterans in care diagnosed with HCV in the beginning of 2014 and is a national leader in the testing and treatment of HCV.5,6
The VA’s recommendations for screening for HCV infection are in alignment with the United States Preventive Services Task Force (USPSTF) and Centers for Disease Control and Prevention (CDC) recommendations to test all veterans born between 1945 and 1965 and anyone with risk factors such as injection drug use.7-9 As of January 1, 2018, the VA had screened more than 80% of veterans in care within this highest risk birth cohort. As of January 1, 2018, more than 100,000 veterans in VA care have initiated treatment for HCV with direct-acting antivirals (DAAs) (Figure 1).
Several critical factors contributed to the VA success with HCV testing and treatment, including congressional appropriation of funding from fiscal year (FY) 2016 through FY 2018, unrestricted access to interferon-free DAA HCV treatments, and dedicated resources from the VA National Viral Hepatitis Program within the HIV, Hepatitis, and Related Conditions Programs (HHRC) in the Office of Specialty Care Services.5 In 2014, HHRC created and supported the Hepatitis Innovation Team (HIT) Collaborative, a VA process improvement initiative enabling
Veterans Integrated Service Network (VISN) -based, multidisciplinary teams to increase veterans’ access to HCV testing and treatment.
As the VA makes consistent progress toward eliminating HCV in veterans in VA care, it has become clear that achieving a cure is only a starting point in improving HCV care. Many patients with HCV infection also have advanced liver disease (ALD), or cirrhosis, which is a condition of permanent liver fibrosis that remains after the patient has been cured of HCV infection. In addition to hepatitis C, ALD also can be caused by excessive alcohol use, hepatitis B virus (HBV) infection, nonalcoholic fatty liver diseases, and several other inherited diseases. Advanced liver disease affects more than 80,000 veterans in VA care, and the HIT infrastructure provides an excellent framework to better understand and address facility-level and systemwide challenges in diagnosing, caring for, and treating veterans with ALD across the Veterans Health Administration (VHA) system.
This report will describe the elements that contributed to the success of the HIT Collaborative in redesigning care for patients affected by HCV in the VA and how these elements can be applied to improve the system of care for VHA ALD care.
Hepatitis Innovation Teams Collaborative Leadership
After the US Food and Drug Administration (FDA) approved new DAA medications to treat HCV, the VA recognized the need to mobilize the health care system quickly and allocate resources for these new, minimally toxic, and highly effective medications. Early in 2014, HHRC established the National Hepatitis C Resource Center (NHCRC), a successor program to the 4 regional hepatitis C resource centers that had addressed HCV care across the system.10 The NHCRC was charged with developing an operational strategy for VA to respond rapidly to the availability of DAAs. In collaboration with representatives from the Office of Strategic Integration | Veterans Engineering Resource Center (OSI|VERC), the NHCRC formed the HIT Collaborative Leadership Team (CLT).
The HIT CLT is responsible for executing the HIT Collaborative and uses a Lean process improvement framework focused on eliminating waste and maximizing value. Members of the CLT with expertise in facilitation, Lean process improvement, leadership, clinical knowledge, and population health management act as coaches for the VISN HITs. The CLT works to build and support the VISN HITs, identify opportunities for individual teams to improve and assist in finding the right local mix of “players” to be successful. The HIT CLT ensures all teams are functioning and working toward achieving their goals. The CLT obtains data from VA national databases, which are provided to the VISN HITs to inform and encourage continuous improvement of their strategies. Annual VA-wide aspirational goals are developed and disseminated to encourage a unified mission.
Catchment areas for each VISN include between 6 and 10 medical centers as well as outpatient and ambulatory care centers. Multidisciplinary HITs are composed of physicians, nurses, pharmacists, nurse practitioners, physician assistants, social workers, mental health and substance use providers, peer support specialists, administrators, information technology experts, and systems redesign professionals from medical centers within each VISN. Teams develop strong relationships across medical centers, implement context-specific strategies applicable to rural
and urban centers, and share expertise. In addition to intra-VISN process improvement, HITs collaborate monthly across VISNs via a virtual platform. They share strong practices, seek advice from one another, and compare outcomes on an established set of goals.
The HITs use process improvement tools to systematically assess the current steps involved in care. At the close of each year, the HITs analyze the current state of operations and set goals to improve over the following year guided by a target state map. Seed funding is provided to every VISN HIT annually to launch change initiatives. Many VISN HITs use these funds to support a VISN HIT coordinator, and HITs also use this financial support to conduct 2- to 3-day process improvement workshops and to purchase supplies, such as point-of-care testing kits. The HIT communication and work are predominantly executed virtually.
Each year, teams worked toward achieving goals set nationally. These included increasing HCV birth cohort testing and improving the percentage of patients who had SVR12 testing
(Table).
the percentage of patients who received SVR12 testing posttreatment completion was not included in the HIT Collaborative’s annual goals for the first year of the program. Recognizing this as a critical area for improvement, the HIT CLT set a goal to test 80% of all patients who completed treatment. The HITs applied Lean tools to identify and overcome gaps in the SVR12 testing process. By the end of the second year, 84% of all patients who completed treatment had been tested for SVR12.
The HITs also set specific local VISN and medical center goals, prioritizing projects that could have the greatest impact on local patient access and quality of care and build on existing strengths and address barriers. These projects encompass a wide range of areas that contribute to the overall national goals.
Focus on Lean
Lean process improvement is based on 2 key pillars: respect for people (those seeking service as customers and patients and those providing service as frontline staff and stakeholders) and continuous improvement. With Lean, personnel providing care should work to identify and eliminate waste in the system and to streamline care delivery to maximize process steps that are most valued by patients (eg, interaction with a clinical provider) and minimize those that are not valued (eg, time spent waiting to see a provider). With the knowledge that HHRC fully supports their work, HITs were encouraged to innovate based on local resources, context, and culture.
Teams receive basic training in Lean from the HIT CLT and local systems redesign specialists if available. The HITs apply the A3 structured approach to problem solving.11 The HITs follow prescribed problemsolving steps that help identify where to focus process improvement efforts, including analyzing the current state of care, outlining the target state, and prioritizing solution
approaches based on what will have the highest impact for patients.
to accommodate the outcomes they observe (Figure 2).
Innovations
Over the course of the HIT Collaborative, numerous innovations have emerged to address and mitigate barriers to HCV screening and treatment. Examples of successful innovations include the following:
- To address transportation issues, several teams developed programs specific to patients with HCV in rural locations or with limited mobility. Mobile vans and units traditionally used as mobile cardiology clinics were transformed into HCV clinics, bringing testing and treatment services directly to veterans;
- Pharmacists and social workers developed outreach strategies to locate homeless veterans, provide point-of-care testing and utilize mobile technology to concurrently enroll and link veterans to care; and
- Many liver care teams partnered with inpatient and outpatient substance use treatment clinics to provide patient education and coordinate HCV treatment.
Inter-VISN working groups developed systemwide tools to address common needs. In the program’s first year, a few medical facilities across a handful of VISNs shared local population health management systems, programming, and best practices. Over time, this working group combined the virtual networking capacity of the HIT Collaborative with technical expertise to promote rapid dissemination and uptake of a population health management system. Providers at medical centers across VA use the tools to identify veterans who should be screened and treated for HCV with the ability to continuously update information, identifying patients who do not respond to treatment or patients overdue for SVR12 testing.
Providers with experience using telehepatology formed another inter-VISN working group. These subject matter experts provided guidance to care teams interested in implementing telehealth in areas where limited local resources or knowledge had prevented them from moving forward. The ability to build a strong coalition across content areas fostered a collaborative learning environment, adaptable to implementing new processes and technologies.
In 2017, the VA made significant efforts to reach out to veterans eligible for VA care who had not yet been screened or remained untreated. In May, Hepatitis Awareness Month, HITs held HCV testing and community outreach events and participated in veteran stand-downs and veteran service organization activities.
Evaluation
Since 2014, the VA has increased its HCV treatment and screening rates. To assess the components contributing to these achievements and the role of the HIT Collaborative in driving this success, a team of implementation scientists have been working with the CLT to conduct a HIT program evaluation. The goal of the evaluation is to establish the impact of the HIT Collaborative. The evaluation team catalogs the activities of the Collaborative and the HITs and assesses implementation strategies (use of specific techniques) to increase the uptake of evidence-based practices specifically related to HCV treatment.12
At the close of each FY, HCV providers and members of the HIT Collaborative are queried through an online survey to determine which strategies have been used to improve HCV care and how these strategies were associated with the HIT Collaborative. The use of more strategies was associated with more HCV treatment initiations.13 All utilized strategies were identified whether or not they were associated with treatment starts. These data are being used to understand which combinations of strategies are most effective at increasing treatment for HCV in the VA and to inform future initiatives.
Expanding the Scope
Inspired by the successful results of the HIT work in HCV and in the spirit of continuously improving health care delivery, HHRC expanded the scope of the HIT Collaborative in FY 2018 to include ALD. There are about 80,000 veterans in VA care with advanced scarring of the liver and between 10,000 to 15,000 new diagnoses each year. In addition to HCV as an etiology for ALD, cases of cirrhosis are projected to increase among veterans in care due to metabolic syndrome and alcohol use. A recent review of VA data from fiscal year 2016 found that 88.6% of ALD patients had been seen in primary care within the past 2 years, with about half (51%) seen in a gastroenterology (GI) or hepatology clinic (Personal communication, HIV, Hepatitis, and Related Conditions Program Office March 16, 2018). For patients in VA care with ALD, GI visits are associated with a lower 5-year mortality.14 Annual mortality for all ALD patients in VA is 6.2%, and of those with a hospital admission, mortality rises to 31%.15 In FY 2016, there were about 52,000 ALD-related discharges (more than 2 per patient). Of those discharges, 24% were readmitted within 30 days, with an average length of stay of 1.9 days and an estimated cost per patient of $47,000 over 3 years.16
Hepatologists from across the VA convened to identify critical opportunities for improvement for patients with ALD. Base on available evidence presented in the literature and their clinical expertise, these subject matter experts identified several areas for quality improvement, with the overarching goal to improve identification of patients with early cirrhosis and ensure appropriate linkage to care for all cirrhotic patients, thus improving quality of life and reducing mortality. Although not finalized, candidate improvement targets include consistent linkage to care and treatment for HCV and HBV, comprehensive case management, post-discharge patient follow-up, and adherence to evidence-based standards of care.
Conclusion
The VA has made great strides in nearly eliminating HCV among veterans in VA care. The national effort to redesign hepatitis care using Lean management strategies and develop local and regional teams and centralized support allowed VA to maximize available resources to achieve higher rates of HCV birth cohort testing and treatment of patients infected with HCV than has any other health care system in the US.
The HIT Collaborative has been a unique and innovative mechanism to promote directed, patient-outcome driven change in a large and dynamic health care system. It has allowed rural and urban providers to work together to develop and spread quality improvement innovations and as an integrated system to achieve national priorities. The focus of this foundational HIT structure is expanding to identifying, treat, and care for VA’s ALD population.
1. Colvin HM, Mitchell AE, eds; and the Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National Academies Press; 2010.
2. US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care and treatment of viral hepatitis. https://www.hhs.gov/sites/default/files/action-plan-viral-hepatitis-2011.pdf. Accessed April 27, 2018.
3. Wolitski R. National viral hepatitis action plan: 2017-2020. https://www.hhs.gov/hepatitis/action-plan/national-viralhepatitis-action-plan-overview/index.html. Updated February
21, 2018. Accessed May 8, 2018.
4. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press; 2017.
5. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C infection: best practices from the Department of Veterans Affairs. Ann of Intern Med. 2017;167(7):499-504.
6. Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol. 2015;63(3):586-592.
7. US Department of Veterans Affairs, Veteran Health Administration. National Clinical Preventive Service Guidance Statements: Screening for Hepatitis C. http://www.prevention.va.gov/CPS/Screening_for_Hepatitis_C.asp. Published on June 20, 2017. [Nonpubic document; source not verified.]
8. Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
9. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
10. Garrard J, Choudary V, Groom H, et al. Organizational change in management of hepatitis C: evaluation of a CME program. J Contin Educ Health Prof. 2006;26(2):145-160.
11. Shook J. Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor, and Lead. Cambridge, MA: Lean Enterprise Institute; 2010.
12. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
13. Rogal SS, Yakovchenko V, Waltz TJ, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci.
2017;12(1):60.
14. Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111(6):838-844.
15. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in the burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans from 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
16. Kaplan DE, Chapko MK, Mehta R, et al; VOCAL Study Group. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018;16(1):106-114.
1. Colvin HM, Mitchell AE, eds; and the Committee on the Prevention and Control of Viral Hepatitis Infections Board on Population Health and Public Health Practice. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. Washington, DC: The National Academies Press; 2010.
2. US Department of Health and Human Services. Combating the silent epidemic of viral hepatitis: action plan for the prevention, care and treatment of viral hepatitis. https://www.hhs.gov/sites/default/files/action-plan-viral-hepatitis-2011.pdf. Accessed April 27, 2018.
3. Wolitski R. National viral hepatitis action plan: 2017-2020. https://www.hhs.gov/hepatitis/action-plan/national-viralhepatitis-action-plan-overview/index.html. Updated February
21, 2018. Accessed May 8, 2018.
4. National Academies of Sciences, Engineering, and Medicine. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. Washington, DC: The National Academies Press; 2017.
5. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C infection: best practices from the Department of Veterans Affairs. Ann of Intern Med. 2017;167(7):499-504.
6. Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: prevalence, risk factors, and outcomes. J Hepatol. 2015;63(3):586-592.
7. US Department of Veterans Affairs, Veteran Health Administration. National Clinical Preventive Service Guidance Statements: Screening for Hepatitis C. http://www.prevention.va.gov/CPS/Screening_for_Hepatitis_C.asp. Published on June 20, 2017. [Nonpubic document; source not verified.]
8. Moyer VA; US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
9. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
10. Garrard J, Choudary V, Groom H, et al. Organizational change in management of hepatitis C: evaluation of a CME program. J Contin Educ Health Prof. 2006;26(2):145-160.
11. Shook J. Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor, and Lead. Cambridge, MA: Lean Enterprise Institute; 2010.
12. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
13. Rogal SS, Yakovchenko V, Waltz TJ, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci.
2017;12(1):60.
14. Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111(6):838-844.
15. Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in the burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US Veterans from 2001-2013. Gastroenterology. 2015;149(6):1471-1482.e5.
16. Kaplan DE, Chapko MK, Mehta R, et al; VOCAL Study Group. Healthcare costs related to treatment of hepatocellular carcinoma among veterans with cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018;16(1):106-114.
Best Practices: Life-Threatening Allergy Prevention
This supplement is sponsored by kaléo.
Click here to read the article
This supplement to Pediatric News discusses life-threatening allergy prevention, including the importance of early introduction of peanut foods to certain infants.
About the Author
Vivian Hernandez-Trujillo, MD
Allergy and Immunology Care Center of South Florida
This supplement is sponsored by kaléo.
Click here to read the article
This supplement to Pediatric News discusses life-threatening allergy prevention, including the importance of early introduction of peanut foods to certain infants.
About the Author
Vivian Hernandez-Trujillo, MD
Allergy and Immunology Care Center of South Florida
This supplement is sponsored by kaléo.
Click here to read the article
This supplement to Pediatric News discusses life-threatening allergy prevention, including the importance of early introduction of peanut foods to certain infants.
About the Author
Vivian Hernandez-Trujillo, MD
Allergy and Immunology Care Center of South Florida
Treatment and Management of Patients With Prostate Cancer (FULL)
PSA Screening
William J. Aronson, MD. I’m very encouraged that the U.S. Preventive Services Task Force (USPSTF) has recently drafted revised guidelines for screening men for prostate cancer in which they now are proposing a C grade for prostate specific antigen (PSA) screening in men aged < 70 years. In this age group they now propose an informed discussion with the patient regarding the pros and cons of screening (shared decision making). The USPSTF recommended against PSA screening in men aged ≥ 75 years in 2008 (D grade), and they recommended against PSA screening in all men in 2012 (D grade). Previously the USPSTF put a great deal of emphasis on the PLCO (Prostate, Lung, Colorectal, and Ovarian Screening Trial). In that trial, there was no difference in prostate cancer mortality between the study groups, but, it appears that up to 90% of men in the control group received PSA screening, therefore, invalidating the studies findings.
I still have serious concerns about giving a D grade for men aged > 70 years. Dr. Jim Hu from Cornell University recently published a study in JAMA Oncology and reported that men aged > 74 years now have twice the rate (12%) of presenting with metastatic disease at the time of diagnosis compared with men aged > 74 years prior to the 2008 USPSTF recommendations. In my view, otherwise healthy men with a good life expectancy, even if they’re aged > 70 years, should still have an informed discussion with their physician about getting PSA screening.
Julie N. Graff, MD. I completely agree with Dr. Aronson, and I would add that our veterans are a special group of patients who have risk factors that aren’t seen in the general population. For example, Agent Orange exposure, and I think the VA has not necessarily embraced those recommendations. I’d also add that people are living longer, and most of the men who die of prostate cancer are over the age of 80 years. We need to consider each patient individually and his life expectancy. It’s okay to diagnose someone with prostate cancer, and it’s important to have a conversation about how likely that cancer is to shorten his life and not just turn a blind eye to it.
Nicholas G. Nickols, MD, PhD. I don’t think there’s really anything clinically meaningful about PSA screening that can be gleaned from the PLCO trial. However, there was another trial that looked at PSA screening, the ERSPC (European Randomized Study of Screening for Prostate Cancer) trial, and had less contamination in the nonscreened arm and actually did ultimately show a 27% reduction in prostate cancer mortality in the screened men. We also know that local treatment in men with high-risk prostate cancer actually improves survival. By not screening, men with high-risk disease are going to miss out on potentially curative therapy.
Dr. Aronson. I think other endpoints are crucial to consider beyond just survival. Once patients have metastatic disease that can markedly impact their quality of life. Also, patients who are starting androgen deprivation therapy (ADT) have very significant issues with quality of life as well. I believe these other endpoints should also be considered by the USPSTF.
Jenna M. Houranieh, PharmD, BCOP. The American Cancer Society, ASCO (American Society of Clinical Oncology), NCCN (National Comprehensive Cancer Network), and the American Urological Association all had a different view on screening compared with the USPSTF that I think go more in line with some of the ways that we practice, because they take into consideration life expectancy, patients’ risks, and the age of screening as well.
Active Surveillance
Dr. Aronson. Active surveillance is now a wellestablished, reasonable approach to managing patients with low-risk prostate cancer. When we talk about the various treatment options, we always include a discussion of active surveillance and watchful waiting. Certainly, patients who have a Gleason score of 3+3, a low PSA (< 10) and low volume disease are ideal candidates for active surveillance. There is no established protocol for active surveillance, though there are a number of large series that report specific ways to go about doing it. The key issue for patients is to deemphasize the importance of the PSA, which is a very poor tool for monitoring progression of prostate cancer in men on active surveillance, and to focus on periodically obtaining prostate biopsies.
For patients with prostate cancer who have multiple medical problems and limited life expectancy, there is no reason to do biopsies on a regular basis. Watchful waiting would be more appropriate for these patients. One key issue, which is challenging right now, is that probably the best way to do active surveillance is with the more sophisticated biopsy technology that is now available. That includes both fusing magnetic resonance imaging (MRI) of the prostate into the ultrasound unit we are using to perform transrectal prostate biopsies. The more advanced biopsy units also provide the ability to perform same-site biopsies. There are specific coordinates at each site where a biopsy is performed so that we can go back to that same site on subsequent biopsies. Due to cost issues, these advanced biopsy units are not yet being used at a high frequency.
Dr. Nickols. The large ProtecT trial in the UK randomized men diagnosed with prostate cancer out of a PSA screening cohort to an active surveillance arm, a radical prostatectomy arm, and a radical radiation arm, and has a median of 10 years’ followup. Importantly, the endpoints of overall survival and prostate cancer specific survival were actually the same for all 3 arms, and were quite high. A little more than half of the patients who were on surveillance ended up getting delayed radical therapy of some kind within 10 years.
There was, however, a difference in metastasis-free survival and clinical progression, which were both higher in the active surveillance arm as compared to the treatment arms. Progression to metastatic disease was more than twice as high in the active surveillance arm than the other 2. Most of the patients who had progressed on the active surveillance arm were Gleason 7, and probably were not ideal candidates for active surveillance by today’s standards and would not normally be recommended active surveillance.
Androgen Deprivation Therapies
Dr. Houranieh. Androgen deprivation therapy plays a large role in prostate cancer management and is used in several areas of prostate cancer care. Androgen deprivation therapy can given be before, during or after radiation or alone in the metastatic setting. It's also continued along with chemotherapy in the more advanced stages. It's use is generally guided by our urologists and the duration of therapy is determined by the risk and stage of the cancer. It can be used for as little as a few months for lower risk, early stage disease or for a few years for higher risk disease. It can also be continued indefinitely for metastatic disease. Androgen deprivation therapy is a combination of 2 types of therapies, injectable LHRH (luteinizing hormone-releasing hormone) agonists and oral antiandrogens.
A number of products are available. The most commonly used LHRH agonist, at least at the Lexington VAMC in Kentucky, is leuprolide, which comes either in an intramuscular or subcutaneous formulation and can be given at different frequencies either monthly, every 3 months, or every 6 months.
There are also a number of antiandrogens available on the market. The most commonly used one is bicalutamide. It is generally the best tolerated and given once daily, as opposed to the other 2, which are either given twice or 3 times daily.
Dr. Nickols. We typically add ADT to radiation for patients with high-risk prostate cancer, defined as any of the following: A PSA ≥ 20, clinical T3 or higher disease, or Gleason score of ≥ 8. The addition of ADT to radiation in high-risk patients improves overall survival, prostate cancer survival, and biochemical recurrence-free survival. The backbone of this hormone therapy is usually a GnRH analogue like leuprolide.
The data are extensive, including many large phase 3 randomized trials of patients with highrisk prostate cancer treated with radiation plus or minus androgen suppression. Many of these trials were led by the big cooperative trial groups: the Radiation Therapy Oncology Group (RTOG), the European Organisation for Research and Treatment of Cancer (EORTC), and others. Looking
at all of the data, the answer to the general question of whether hormone therapy is beneficial is yes. The unknown question is what is the optimal duration for this concurrent and adjuvant hormone therapy. The optimal duration is probably somewhere between 1 and 3 years. That’s a large range, and clearly preferences of the patient and the comorbidities play a role in the decision of duration. The radiation doses were considerably lower than what is considered standard of care at this time in the trials that have established the use of concurrent and adjuvant hormone therapy with radiation, which needs to be taken in context.
For patients with localized intermediate-risk prostate cancer, a shorter course of hormone therapy is reasonable. The RTOG 9408 and DFCI 95096 trials showed that a 4- to 6-month course of ADT with RT in mostly intermediate-risk patients was better than RT alone. However, studies looking at the different comorbidities present in these patients showed that patients with less comorbidity actually benefit more from the addition of hormone therapy, which needs to be taken into account.
The benefit to the intermediate-risk patients is probably driven by the patients with unfavorable intermediate-risk disease, for example, with the primary Gleason 4 patterns, such as Gleason 4+3 patients rather than Gleason 3+4, patients with higher volume of prostate cancer, patients with multiple intermediate-risk features, etc. For the truly favorable intermediate-risk patients, low-volume disease, low PSA, and Gleason 3+4 pattern, the added value of concurrent ADT may be small.
The mechanism of why ADT may contribute to radiation efficacy may be explained by direct radio-sensitization: the transcription factor androgen receptor activates expression of many genes involved in DNA repair. Interfere with that, and you sensitize to radiation.
Dr. Graff. Of note, we don’t use ADT as the primary treatment for localized prostate cancer. This is for use in combination with radiation, in people with positive lymph nodes after surgery and in people with incurable prostate cancer.
Dr. Nickols. The question of whether or not to treat patients with localized high-risk prostate cancer with hormone therapy alone has been answered: The SPCG-7 and NCIC CTG PR3/MRC PR07 trials proved that adding radiation to long-term ADT improved survival in these patients.
The DFCI 95096 trial also showed that patients with a high level of comorbidities benefitted the least from concurrent hormone therapy; cardiovascular risks from the hormone therapy can offset the anticancer effect in these patients.
Analyses of the large randomized trials of radiation with or without hormones looking at the question of whether or not there was increased cardiovascular mortality in the patients that had hormone therapy did not show more cardiovascular mortality. Importantly, those trials were not enriched for patients with comorbidities that would set them up for this risk. One needs to weigh the benefits of adding hormones to radiation against the risks on a patient-to-patient basis.
Dr. Aronson. Another scenario where we used ADT is for patients whose cancer progressed after primary therapy; for example, when radical prostatectomy and RT are not successful for a patient. We see patients on a regular basis with a rising PSA after primary therapy. Our main goal is to avoid giving ADT to these patients as long as possible and only use it when it is clearly indicated.
The best measure that we typically use is the PSA doubling time. If the PSA doubling time, for example, is > 1 year, than we feel more confident in holding off on starting ADT and instead just monitoring the PSA. Adverse effects (AEs) of ADT are dramatic. We know that patients can get significant fatigue, gain weight, lose muscle mass, have an increased risk of diabetes mellitus, get hot flashes, and develop impotence and loss of libido. And now there are emerging data on an increased risk of Alzheimer disease. We use ADT but only when clearly indicated.
When I start patients on ADT, in addition to explaining the AEs, I also strongly suggest that, if their health allows, they walk at a brisk rate for at least 30 minutes daily and get on a regular weight training or a resistance training program to try to maintain muscle mass. They need to watch their diet more carefully because they are at increased risk for weight gain. And if they also can do balancing exercises, that would also be ideal. Typically, we also start patients on calcium and vitamin D as there is a risk for bone loss and osteoporosis, and we monitor their bone density.
Dr. Nickols. There’s another role for ADT. In patients who have a PSA recurrence after surgery, RT directed to the prostate bed and/or pelvic nodes is a potential curative therapy. There’s now some emerging evidence that analogous to the definitive radiation setting, the addition of hormone therapy to salvage radiation may be of value.
There were 2 recent trials published. The RTOG-9601 trial showed a benefit to the addition of bicalutamide for patients who had rising PSA after a surgery and were randomized to radiation that was directed to the prostate bed plus or minus 2 years of bicalutamide. The second trial, GETUG-AFU 16, was similar except that in this case the hormone therapy used was 6 months of the GnRH analogue leuprolide. The RTOG-9601 trial had positive outcomes in multiple endpoints, including survival. The GETUG trial is not as mature but had a biochemical improvement.
I don’t think the interpretation of this should be to use hormones with salvage radiation all the time. Importantly, in the RTOG 9601 trial, the patients that had the greatest benefit to the addition of concurrent hormone therapy were those that had a PSA of higher than 0.5 or 0.7. Most patients who get salvage radiation now get it at a much lower PSA, so we probably don’t want to overinterpret that data. And, of course, we have to wait for the GETUG-AFU data to mature more to see if there’s any hard clinical endpoints met
there. Notably, the incidence of gynecomastia in the bicalutamide arm of RTOG 9601 was near 70%. I discuss the addition of hormones with my patients who are getting salvage radiation and usually recommend it to the ones who have the high-risk features, those who would have gotten the concurrent hormones in the definitive setting, those with a PSA greater than 0.5 at the time of salvage, and those with a rapid PSA doubling time.
Dr. Houranieh. Androgen deprivation therapy includes use of LHRH agonists, like leuprolide and antiandrogens like bicalutamide. Some of the short-term AEs from androgen deprivation that we counsel patients on are things like tumor flare, hot flashes, erectile dysfunction, and injection site reactions. Some of the more long-term complications that we touch upon are osteoporosis, obesity, insulin resistance, increased risk of diabetes mellitus and cardiovascular events. We counsel patients on these adverse reactions and do our best with monitoring and prevention.
Dr. Nickols. The ProtecT trial also had some valuable patient-reported outcomes that were very carefully tracked. It confirmed what we already believe. The patients that had primary RT had the greatest negative impact on bowel function and on urinary irritative and obstructive symptoms. The patients who had surgery had the greatest negative impact on sexual function and on urinary incontinence. Obviously, active surveillance had the benefit of avoiding or postponing AEs of local therapy.
You can break up RT for localized disease, into 2 general approaches. The first is external beam radiation. This can be delivered as intensity-modulated radiotherapy (IMRT), which is the most common approach right now, typically stretched over more than 2 months of daily treatments. In addition, there is a newer technique called stereotactic body radiation therapy (SBRT), which has been applied to localized prostate cancer now for more than a decade. It’s efficacy was demonstrated first in low-risk patients as normally is the case. It has the advantage of convenience; it is just 5 treatment days total, which can be accomplished in a couple of weeks. And its convenient for patients who are commuting some distance. That’s really important for veterans, as radiotherapy is not available at all VAs.
At the West LA VAMC, we offer SBRT as a standard treatment for men with low and favorable intermediate risk prostate cancer. In addition, we offer it in the context of a clinical trial for patients with unfavorable intermediate- and high-risk prostate cancer.
The other type of radiation therapy is brachytherapy in which the radiation is temporarily or permanently inserted into the target, the prostate. It is a good stand-alone option for men with low- or intermediate-risk prostate cancer. It has the advantage of being relatively fast in that it is done in a day, although it is more invasive than IMRT or SBRT, and certain anatomic features of the prostate and the patient’s baseline urinary function can limit its appropriateness in some patients.
There are some recent data of interest for the combination of brachytherapy and externalbeam radiation therapy (EBRT). The recently reported ASCENDE-RT trial randomized mostly high-risk patients to either EBRT with 1 year of androgen suppression or EBRT with a boost of brachytherapy to the prostate and 1 year of ADT.
The arm that got the brachytherapy boost actually had half the biochemical recurrence of the EBRT alone but had double the rate of grade 2 acute genitourinary toxicity and triple the rate of grade 3. Metastasis-free survival and other hard clinical endpoints will need longer follow-up, but the biochemical control was quite high: It was about 80% at 10 years out.
Dr. Aronson. For surgical approaches, many VAs now have the da Vinci robot system (Sunnyvale, CA). When we look at the key results, which examine cancer care and AEs, such as incontinence and impotence, there actually is no clear advantage over the open procedure that we previously used. That being said, with the robotic surgery, because we do it laparoscopically, there’s significantly less blood loss. The magnification is such that it is much easier to do the surgery. It’s also much easier on the surgeon’s body, given that you’re in an anatomically, ergonomically good position, and patients go home much sooner, typically on postoperative day 1 or postoperative day 2 with less morbidity following the procedure and a much quicker recovery.
Precision Medicine
Dr. Graff. Prostate cancer may not be cured, even after the best attempts at surgery or radiation. The medical oncologist is probably most utilized with people with incurable prostate cancer. Once it’s incurable, it develops tumors in the bones and lymph nodes most commonly, and we call it metastatic prostate cancer.
Right now we use mostly a once-size-fits-all approach. Everyone initially gets some form of castration therapy, usually medical castration with LHRH agonists. However, prostate cancer invariably becomes resistant to those maneuvers. We call that castration-resistant prostate cancer. That opens the door to 6 other treatments that can prolong survival in prostate cancer. Two of the treatments are hormonal (enzalutamide and abiraterone), 2 are chemotherapy (docetaxel and cabazitaxel), 1 is IV radiation with radium-223, and 1 is an immunotherapy (sipuleucel-T).
At this point, there’s not a lot of guidance about what to use when except that each of these therapies has unique AEs, so we may not use one of the therapies because it causes a lot of fatigue or it could cause seizures, for example, in a patient at risk for those. Sometimes the therapies are inappropriate. For example, with radium, you wouldn’t give it to a patient with a tumor in the liver.
We don’t have readily available companion diagnostics to help us narrow the selection. In 2015, there was an article in Cell that looked at men with metastatic castration-resistant prostate cancer. The tumors were biopsied and analyzed, and we found some surprising things, including certain mutations called DNA repair defects that could make them susceptible to a drug already approved in ovarian cancer, such as olaparib and rucaparib.
A subsequent study in the New England Journal of Medicine looked at patients with advanced prostate cancer whose cancers have these DNA repair defects. Those cancers were susceptible to the PARP (poly ADP ribose polymerase) inhibitor olaparib. That’s an example of where looking and sequencing a tumor could lead to a treatment selection. The PARP inhibitors are not yet approved in prostate cancer, but the Prostate Cancer Foundation is interested in supporting research that could help deliver appropriate therapies to veterans in particular whose cancers have certain markers.
So, we are biopsying patients’ tumors, looking at the mutations in their germline DNA, and matching patients to treatments and vice versa. The DNA repair defects is the one that’s probably under most active evaluation right now. Another example of a biomarker is the AR-V7, which is a mutation in the androgen receptor that renders the cancer resistant to enzalutamide and abiraterone.
Also, I have a study of pembrolizumab which is a PD-1 inhibitor, and I’ve seen some very good responses to that therapy. And we’re not yet sure how to identify prospectively those patients who are likely to respond.
Use of Imaging
Dr. Nickols. The sensitivity of technetium-99m bone scans and CT (computed tomography) scans is not good enough. Many patients that we classify as M0, but with clear evidence of disease with a rising PSA, will be more accurately classified as M1 when the imaging allows this to be the case.
I think prostate-specific membrane antigen positron emission tomography (PET), which is not approved at this time, is going to be of value. A lot of data are coming out of Europe and in the recurrence setting show that PET imaging can detect metastatic sites at PSA values as low as 0.2 with the per lesion sensitivity around 80% and a specificity upward of 97%. This is clearly far and away much better than anything we have now.
There’s going to be a whole cohort of patients that we literally can’t see now, patients with essentially minimally metastatic disease, and they will be revealed when the imaging gets there. And the question is what to do for these patients. Treating patients with a heavy metastatic disease burden is much different from treating patients who may have just one or a few areas of disease outside of their prostate. And we need new strategies for these patients. We are now looking at new treatment regimens for patients with limited metastatic disease burden. I think this is going to be important going forward.
It’s also worth asking: What is the role of local therapy in patients with advanced prostate cancer, patients with metastatic disease? If you look at the patients who were in a lot of the old trials, for example, the NCIC trial, that was adding radiation to hormone therapy for high-risk patients, about 25% of patients in that trial had a PSA > 50. That’s a lot. Many of those patients probably had occult metastases. And there are trials now looking at the role of local therapy in metastatic patients.
Another area of interest is precision oncology, which Dr. Graff touched on, is starting to play a big role in the metastatic setting, but what about the local setting? There are now genomic classifiers available to help with risk assessments, but we don’t yet have much in the way of predictive tools that help guide specific therapies in the localized setting. We know that patients, for example, who have germline BRCA1 or 2 mutations have a worse outcome, period, after local therapy; and right now it may play some into treatment decisions, but we don’t have tailored therapy yet in the localized setting at the molecular level. And I think this is something that we need to start looking at.
Dr. Aronson. The VA is a very rich environment for performing clinical research as well as translational research (bench to bedside). And for example, at the West Los Angeles VAMC, I think one of the key steps that we have taken, moving forward is now our urology, radiation oncology, and hematology-oncology research groups have now merged together. This allows us to not only combine our administrative resources but to really improve the ability for us to perform highquality research in our veterans. And so that’s a model which I think other VAs might consider pursuing, depending upon their circumstances.
Author Disclosures
Dr. Graff has received research support from Sanofi, Astellas, Merck, Janssen, and Bristol Myers Squibb; an honorarium from Astellas; travel support from Clovis and Sanofi; and has consulted for Bayer and Dendreon. No other authors report actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
PSA Screening
William J. Aronson, MD. I’m very encouraged that the U.S. Preventive Services Task Force (USPSTF) has recently drafted revised guidelines for screening men for prostate cancer in which they now are proposing a C grade for prostate specific antigen (PSA) screening in men aged < 70 years. In this age group they now propose an informed discussion with the patient regarding the pros and cons of screening (shared decision making). The USPSTF recommended against PSA screening in men aged ≥ 75 years in 2008 (D grade), and they recommended against PSA screening in all men in 2012 (D grade). Previously the USPSTF put a great deal of emphasis on the PLCO (Prostate, Lung, Colorectal, and Ovarian Screening Trial). In that trial, there was no difference in prostate cancer mortality between the study groups, but, it appears that up to 90% of men in the control group received PSA screening, therefore, invalidating the studies findings.
I still have serious concerns about giving a D grade for men aged > 70 years. Dr. Jim Hu from Cornell University recently published a study in JAMA Oncology and reported that men aged > 74 years now have twice the rate (12%) of presenting with metastatic disease at the time of diagnosis compared with men aged > 74 years prior to the 2008 USPSTF recommendations. In my view, otherwise healthy men with a good life expectancy, even if they’re aged > 70 years, should still have an informed discussion with their physician about getting PSA screening.
Julie N. Graff, MD. I completely agree with Dr. Aronson, and I would add that our veterans are a special group of patients who have risk factors that aren’t seen in the general population. For example, Agent Orange exposure, and I think the VA has not necessarily embraced those recommendations. I’d also add that people are living longer, and most of the men who die of prostate cancer are over the age of 80 years. We need to consider each patient individually and his life expectancy. It’s okay to diagnose someone with prostate cancer, and it’s important to have a conversation about how likely that cancer is to shorten his life and not just turn a blind eye to it.
Nicholas G. Nickols, MD, PhD. I don’t think there’s really anything clinically meaningful about PSA screening that can be gleaned from the PLCO trial. However, there was another trial that looked at PSA screening, the ERSPC (European Randomized Study of Screening for Prostate Cancer) trial, and had less contamination in the nonscreened arm and actually did ultimately show a 27% reduction in prostate cancer mortality in the screened men. We also know that local treatment in men with high-risk prostate cancer actually improves survival. By not screening, men with high-risk disease are going to miss out on potentially curative therapy.
Dr. Aronson. I think other endpoints are crucial to consider beyond just survival. Once patients have metastatic disease that can markedly impact their quality of life. Also, patients who are starting androgen deprivation therapy (ADT) have very significant issues with quality of life as well. I believe these other endpoints should also be considered by the USPSTF.
Jenna M. Houranieh, PharmD, BCOP. The American Cancer Society, ASCO (American Society of Clinical Oncology), NCCN (National Comprehensive Cancer Network), and the American Urological Association all had a different view on screening compared with the USPSTF that I think go more in line with some of the ways that we practice, because they take into consideration life expectancy, patients’ risks, and the age of screening as well.
Active Surveillance
Dr. Aronson. Active surveillance is now a wellestablished, reasonable approach to managing patients with low-risk prostate cancer. When we talk about the various treatment options, we always include a discussion of active surveillance and watchful waiting. Certainly, patients who have a Gleason score of 3+3, a low PSA (< 10) and low volume disease are ideal candidates for active surveillance. There is no established protocol for active surveillance, though there are a number of large series that report specific ways to go about doing it. The key issue for patients is to deemphasize the importance of the PSA, which is a very poor tool for monitoring progression of prostate cancer in men on active surveillance, and to focus on periodically obtaining prostate biopsies.
For patients with prostate cancer who have multiple medical problems and limited life expectancy, there is no reason to do biopsies on a regular basis. Watchful waiting would be more appropriate for these patients. One key issue, which is challenging right now, is that probably the best way to do active surveillance is with the more sophisticated biopsy technology that is now available. That includes both fusing magnetic resonance imaging (MRI) of the prostate into the ultrasound unit we are using to perform transrectal prostate biopsies. The more advanced biopsy units also provide the ability to perform same-site biopsies. There are specific coordinates at each site where a biopsy is performed so that we can go back to that same site on subsequent biopsies. Due to cost issues, these advanced biopsy units are not yet being used at a high frequency.
Dr. Nickols. The large ProtecT trial in the UK randomized men diagnosed with prostate cancer out of a PSA screening cohort to an active surveillance arm, a radical prostatectomy arm, and a radical radiation arm, and has a median of 10 years’ followup. Importantly, the endpoints of overall survival and prostate cancer specific survival were actually the same for all 3 arms, and were quite high. A little more than half of the patients who were on surveillance ended up getting delayed radical therapy of some kind within 10 years.
There was, however, a difference in metastasis-free survival and clinical progression, which were both higher in the active surveillance arm as compared to the treatment arms. Progression to metastatic disease was more than twice as high in the active surveillance arm than the other 2. Most of the patients who had progressed on the active surveillance arm were Gleason 7, and probably were not ideal candidates for active surveillance by today’s standards and would not normally be recommended active surveillance.
Androgen Deprivation Therapies
Dr. Houranieh. Androgen deprivation therapy plays a large role in prostate cancer management and is used in several areas of prostate cancer care. Androgen deprivation therapy can given be before, during or after radiation or alone in the metastatic setting. It's also continued along with chemotherapy in the more advanced stages. It's use is generally guided by our urologists and the duration of therapy is determined by the risk and stage of the cancer. It can be used for as little as a few months for lower risk, early stage disease or for a few years for higher risk disease. It can also be continued indefinitely for metastatic disease. Androgen deprivation therapy is a combination of 2 types of therapies, injectable LHRH (luteinizing hormone-releasing hormone) agonists and oral antiandrogens.
A number of products are available. The most commonly used LHRH agonist, at least at the Lexington VAMC in Kentucky, is leuprolide, which comes either in an intramuscular or subcutaneous formulation and can be given at different frequencies either monthly, every 3 months, or every 6 months.
There are also a number of antiandrogens available on the market. The most commonly used one is bicalutamide. It is generally the best tolerated and given once daily, as opposed to the other 2, which are either given twice or 3 times daily.
Dr. Nickols. We typically add ADT to radiation for patients with high-risk prostate cancer, defined as any of the following: A PSA ≥ 20, clinical T3 or higher disease, or Gleason score of ≥ 8. The addition of ADT to radiation in high-risk patients improves overall survival, prostate cancer survival, and biochemical recurrence-free survival. The backbone of this hormone therapy is usually a GnRH analogue like leuprolide.
The data are extensive, including many large phase 3 randomized trials of patients with highrisk prostate cancer treated with radiation plus or minus androgen suppression. Many of these trials were led by the big cooperative trial groups: the Radiation Therapy Oncology Group (RTOG), the European Organisation for Research and Treatment of Cancer (EORTC), and others. Looking
at all of the data, the answer to the general question of whether hormone therapy is beneficial is yes. The unknown question is what is the optimal duration for this concurrent and adjuvant hormone therapy. The optimal duration is probably somewhere between 1 and 3 years. That’s a large range, and clearly preferences of the patient and the comorbidities play a role in the decision of duration. The radiation doses were considerably lower than what is considered standard of care at this time in the trials that have established the use of concurrent and adjuvant hormone therapy with radiation, which needs to be taken in context.
For patients with localized intermediate-risk prostate cancer, a shorter course of hormone therapy is reasonable. The RTOG 9408 and DFCI 95096 trials showed that a 4- to 6-month course of ADT with RT in mostly intermediate-risk patients was better than RT alone. However, studies looking at the different comorbidities present in these patients showed that patients with less comorbidity actually benefit more from the addition of hormone therapy, which needs to be taken into account.
The benefit to the intermediate-risk patients is probably driven by the patients with unfavorable intermediate-risk disease, for example, with the primary Gleason 4 patterns, such as Gleason 4+3 patients rather than Gleason 3+4, patients with higher volume of prostate cancer, patients with multiple intermediate-risk features, etc. For the truly favorable intermediate-risk patients, low-volume disease, low PSA, and Gleason 3+4 pattern, the added value of concurrent ADT may be small.
The mechanism of why ADT may contribute to radiation efficacy may be explained by direct radio-sensitization: the transcription factor androgen receptor activates expression of many genes involved in DNA repair. Interfere with that, and you sensitize to radiation.
Dr. Graff. Of note, we don’t use ADT as the primary treatment for localized prostate cancer. This is for use in combination with radiation, in people with positive lymph nodes after surgery and in people with incurable prostate cancer.
Dr. Nickols. The question of whether or not to treat patients with localized high-risk prostate cancer with hormone therapy alone has been answered: The SPCG-7 and NCIC CTG PR3/MRC PR07 trials proved that adding radiation to long-term ADT improved survival in these patients.
The DFCI 95096 trial also showed that patients with a high level of comorbidities benefitted the least from concurrent hormone therapy; cardiovascular risks from the hormone therapy can offset the anticancer effect in these patients.
Analyses of the large randomized trials of radiation with or without hormones looking at the question of whether or not there was increased cardiovascular mortality in the patients that had hormone therapy did not show more cardiovascular mortality. Importantly, those trials were not enriched for patients with comorbidities that would set them up for this risk. One needs to weigh the benefits of adding hormones to radiation against the risks on a patient-to-patient basis.
Dr. Aronson. Another scenario where we used ADT is for patients whose cancer progressed after primary therapy; for example, when radical prostatectomy and RT are not successful for a patient. We see patients on a regular basis with a rising PSA after primary therapy. Our main goal is to avoid giving ADT to these patients as long as possible and only use it when it is clearly indicated.
The best measure that we typically use is the PSA doubling time. If the PSA doubling time, for example, is > 1 year, than we feel more confident in holding off on starting ADT and instead just monitoring the PSA. Adverse effects (AEs) of ADT are dramatic. We know that patients can get significant fatigue, gain weight, lose muscle mass, have an increased risk of diabetes mellitus, get hot flashes, and develop impotence and loss of libido. And now there are emerging data on an increased risk of Alzheimer disease. We use ADT but only when clearly indicated.
When I start patients on ADT, in addition to explaining the AEs, I also strongly suggest that, if their health allows, they walk at a brisk rate for at least 30 minutes daily and get on a regular weight training or a resistance training program to try to maintain muscle mass. They need to watch their diet more carefully because they are at increased risk for weight gain. And if they also can do balancing exercises, that would also be ideal. Typically, we also start patients on calcium and vitamin D as there is a risk for bone loss and osteoporosis, and we monitor their bone density.
Dr. Nickols. There’s another role for ADT. In patients who have a PSA recurrence after surgery, RT directed to the prostate bed and/or pelvic nodes is a potential curative therapy. There’s now some emerging evidence that analogous to the definitive radiation setting, the addition of hormone therapy to salvage radiation may be of value.
There were 2 recent trials published. The RTOG-9601 trial showed a benefit to the addition of bicalutamide for patients who had rising PSA after a surgery and were randomized to radiation that was directed to the prostate bed plus or minus 2 years of bicalutamide. The second trial, GETUG-AFU 16, was similar except that in this case the hormone therapy used was 6 months of the GnRH analogue leuprolide. The RTOG-9601 trial had positive outcomes in multiple endpoints, including survival. The GETUG trial is not as mature but had a biochemical improvement.
I don’t think the interpretation of this should be to use hormones with salvage radiation all the time. Importantly, in the RTOG 9601 trial, the patients that had the greatest benefit to the addition of concurrent hormone therapy were those that had a PSA of higher than 0.5 or 0.7. Most patients who get salvage radiation now get it at a much lower PSA, so we probably don’t want to overinterpret that data. And, of course, we have to wait for the GETUG-AFU data to mature more to see if there’s any hard clinical endpoints met
there. Notably, the incidence of gynecomastia in the bicalutamide arm of RTOG 9601 was near 70%. I discuss the addition of hormones with my patients who are getting salvage radiation and usually recommend it to the ones who have the high-risk features, those who would have gotten the concurrent hormones in the definitive setting, those with a PSA greater than 0.5 at the time of salvage, and those with a rapid PSA doubling time.
Dr. Houranieh. Androgen deprivation therapy includes use of LHRH agonists, like leuprolide and antiandrogens like bicalutamide. Some of the short-term AEs from androgen deprivation that we counsel patients on are things like tumor flare, hot flashes, erectile dysfunction, and injection site reactions. Some of the more long-term complications that we touch upon are osteoporosis, obesity, insulin resistance, increased risk of diabetes mellitus and cardiovascular events. We counsel patients on these adverse reactions and do our best with monitoring and prevention.
Dr. Nickols. The ProtecT trial also had some valuable patient-reported outcomes that were very carefully tracked. It confirmed what we already believe. The patients that had primary RT had the greatest negative impact on bowel function and on urinary irritative and obstructive symptoms. The patients who had surgery had the greatest negative impact on sexual function and on urinary incontinence. Obviously, active surveillance had the benefit of avoiding or postponing AEs of local therapy.
You can break up RT for localized disease, into 2 general approaches. The first is external beam radiation. This can be delivered as intensity-modulated radiotherapy (IMRT), which is the most common approach right now, typically stretched over more than 2 months of daily treatments. In addition, there is a newer technique called stereotactic body radiation therapy (SBRT), which has been applied to localized prostate cancer now for more than a decade. It’s efficacy was demonstrated first in low-risk patients as normally is the case. It has the advantage of convenience; it is just 5 treatment days total, which can be accomplished in a couple of weeks. And its convenient for patients who are commuting some distance. That’s really important for veterans, as radiotherapy is not available at all VAs.
At the West LA VAMC, we offer SBRT as a standard treatment for men with low and favorable intermediate risk prostate cancer. In addition, we offer it in the context of a clinical trial for patients with unfavorable intermediate- and high-risk prostate cancer.
The other type of radiation therapy is brachytherapy in which the radiation is temporarily or permanently inserted into the target, the prostate. It is a good stand-alone option for men with low- or intermediate-risk prostate cancer. It has the advantage of being relatively fast in that it is done in a day, although it is more invasive than IMRT or SBRT, and certain anatomic features of the prostate and the patient’s baseline urinary function can limit its appropriateness in some patients.
There are some recent data of interest for the combination of brachytherapy and externalbeam radiation therapy (EBRT). The recently reported ASCENDE-RT trial randomized mostly high-risk patients to either EBRT with 1 year of androgen suppression or EBRT with a boost of brachytherapy to the prostate and 1 year of ADT.
The arm that got the brachytherapy boost actually had half the biochemical recurrence of the EBRT alone but had double the rate of grade 2 acute genitourinary toxicity and triple the rate of grade 3. Metastasis-free survival and other hard clinical endpoints will need longer follow-up, but the biochemical control was quite high: It was about 80% at 10 years out.
Dr. Aronson. For surgical approaches, many VAs now have the da Vinci robot system (Sunnyvale, CA). When we look at the key results, which examine cancer care and AEs, such as incontinence and impotence, there actually is no clear advantage over the open procedure that we previously used. That being said, with the robotic surgery, because we do it laparoscopically, there’s significantly less blood loss. The magnification is such that it is much easier to do the surgery. It’s also much easier on the surgeon’s body, given that you’re in an anatomically, ergonomically good position, and patients go home much sooner, typically on postoperative day 1 or postoperative day 2 with less morbidity following the procedure and a much quicker recovery.
Precision Medicine
Dr. Graff. Prostate cancer may not be cured, even after the best attempts at surgery or radiation. The medical oncologist is probably most utilized with people with incurable prostate cancer. Once it’s incurable, it develops tumors in the bones and lymph nodes most commonly, and we call it metastatic prostate cancer.
Right now we use mostly a once-size-fits-all approach. Everyone initially gets some form of castration therapy, usually medical castration with LHRH agonists. However, prostate cancer invariably becomes resistant to those maneuvers. We call that castration-resistant prostate cancer. That opens the door to 6 other treatments that can prolong survival in prostate cancer. Two of the treatments are hormonal (enzalutamide and abiraterone), 2 are chemotherapy (docetaxel and cabazitaxel), 1 is IV radiation with radium-223, and 1 is an immunotherapy (sipuleucel-T).
At this point, there’s not a lot of guidance about what to use when except that each of these therapies has unique AEs, so we may not use one of the therapies because it causes a lot of fatigue or it could cause seizures, for example, in a patient at risk for those. Sometimes the therapies are inappropriate. For example, with radium, you wouldn’t give it to a patient with a tumor in the liver.
We don’t have readily available companion diagnostics to help us narrow the selection. In 2015, there was an article in Cell that looked at men with metastatic castration-resistant prostate cancer. The tumors were biopsied and analyzed, and we found some surprising things, including certain mutations called DNA repair defects that could make them susceptible to a drug already approved in ovarian cancer, such as olaparib and rucaparib.
A subsequent study in the New England Journal of Medicine looked at patients with advanced prostate cancer whose cancers have these DNA repair defects. Those cancers were susceptible to the PARP (poly ADP ribose polymerase) inhibitor olaparib. That’s an example of where looking and sequencing a tumor could lead to a treatment selection. The PARP inhibitors are not yet approved in prostate cancer, but the Prostate Cancer Foundation is interested in supporting research that could help deliver appropriate therapies to veterans in particular whose cancers have certain markers.
So, we are biopsying patients’ tumors, looking at the mutations in their germline DNA, and matching patients to treatments and vice versa. The DNA repair defects is the one that’s probably under most active evaluation right now. Another example of a biomarker is the AR-V7, which is a mutation in the androgen receptor that renders the cancer resistant to enzalutamide and abiraterone.
Also, I have a study of pembrolizumab which is a PD-1 inhibitor, and I’ve seen some very good responses to that therapy. And we’re not yet sure how to identify prospectively those patients who are likely to respond.
Use of Imaging
Dr. Nickols. The sensitivity of technetium-99m bone scans and CT (computed tomography) scans is not good enough. Many patients that we classify as M0, but with clear evidence of disease with a rising PSA, will be more accurately classified as M1 when the imaging allows this to be the case.
I think prostate-specific membrane antigen positron emission tomography (PET), which is not approved at this time, is going to be of value. A lot of data are coming out of Europe and in the recurrence setting show that PET imaging can detect metastatic sites at PSA values as low as 0.2 with the per lesion sensitivity around 80% and a specificity upward of 97%. This is clearly far and away much better than anything we have now.
There’s going to be a whole cohort of patients that we literally can’t see now, patients with essentially minimally metastatic disease, and they will be revealed when the imaging gets there. And the question is what to do for these patients. Treating patients with a heavy metastatic disease burden is much different from treating patients who may have just one or a few areas of disease outside of their prostate. And we need new strategies for these patients. We are now looking at new treatment regimens for patients with limited metastatic disease burden. I think this is going to be important going forward.
It’s also worth asking: What is the role of local therapy in patients with advanced prostate cancer, patients with metastatic disease? If you look at the patients who were in a lot of the old trials, for example, the NCIC trial, that was adding radiation to hormone therapy for high-risk patients, about 25% of patients in that trial had a PSA > 50. That’s a lot. Many of those patients probably had occult metastases. And there are trials now looking at the role of local therapy in metastatic patients.
Another area of interest is precision oncology, which Dr. Graff touched on, is starting to play a big role in the metastatic setting, but what about the local setting? There are now genomic classifiers available to help with risk assessments, but we don’t yet have much in the way of predictive tools that help guide specific therapies in the localized setting. We know that patients, for example, who have germline BRCA1 or 2 mutations have a worse outcome, period, after local therapy; and right now it may play some into treatment decisions, but we don’t have tailored therapy yet in the localized setting at the molecular level. And I think this is something that we need to start looking at.
Dr. Aronson. The VA is a very rich environment for performing clinical research as well as translational research (bench to bedside). And for example, at the West Los Angeles VAMC, I think one of the key steps that we have taken, moving forward is now our urology, radiation oncology, and hematology-oncology research groups have now merged together. This allows us to not only combine our administrative resources but to really improve the ability for us to perform highquality research in our veterans. And so that’s a model which I think other VAs might consider pursuing, depending upon their circumstances.
Author Disclosures
Dr. Graff has received research support from Sanofi, Astellas, Merck, Janssen, and Bristol Myers Squibb; an honorarium from Astellas; travel support from Clovis and Sanofi; and has consulted for Bayer and Dendreon. No other authors report actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
PSA Screening
William J. Aronson, MD. I’m very encouraged that the U.S. Preventive Services Task Force (USPSTF) has recently drafted revised guidelines for screening men for prostate cancer in which they now are proposing a C grade for prostate specific antigen (PSA) screening in men aged < 70 years. In this age group they now propose an informed discussion with the patient regarding the pros and cons of screening (shared decision making). The USPSTF recommended against PSA screening in men aged ≥ 75 years in 2008 (D grade), and they recommended against PSA screening in all men in 2012 (D grade). Previously the USPSTF put a great deal of emphasis on the PLCO (Prostate, Lung, Colorectal, and Ovarian Screening Trial). In that trial, there was no difference in prostate cancer mortality between the study groups, but, it appears that up to 90% of men in the control group received PSA screening, therefore, invalidating the studies findings.
I still have serious concerns about giving a D grade for men aged > 70 years. Dr. Jim Hu from Cornell University recently published a study in JAMA Oncology and reported that men aged > 74 years now have twice the rate (12%) of presenting with metastatic disease at the time of diagnosis compared with men aged > 74 years prior to the 2008 USPSTF recommendations. In my view, otherwise healthy men with a good life expectancy, even if they’re aged > 70 years, should still have an informed discussion with their physician about getting PSA screening.
Julie N. Graff, MD. I completely agree with Dr. Aronson, and I would add that our veterans are a special group of patients who have risk factors that aren’t seen in the general population. For example, Agent Orange exposure, and I think the VA has not necessarily embraced those recommendations. I’d also add that people are living longer, and most of the men who die of prostate cancer are over the age of 80 years. We need to consider each patient individually and his life expectancy. It’s okay to diagnose someone with prostate cancer, and it’s important to have a conversation about how likely that cancer is to shorten his life and not just turn a blind eye to it.
Nicholas G. Nickols, MD, PhD. I don’t think there’s really anything clinically meaningful about PSA screening that can be gleaned from the PLCO trial. However, there was another trial that looked at PSA screening, the ERSPC (European Randomized Study of Screening for Prostate Cancer) trial, and had less contamination in the nonscreened arm and actually did ultimately show a 27% reduction in prostate cancer mortality in the screened men. We also know that local treatment in men with high-risk prostate cancer actually improves survival. By not screening, men with high-risk disease are going to miss out on potentially curative therapy.
Dr. Aronson. I think other endpoints are crucial to consider beyond just survival. Once patients have metastatic disease that can markedly impact their quality of life. Also, patients who are starting androgen deprivation therapy (ADT) have very significant issues with quality of life as well. I believe these other endpoints should also be considered by the USPSTF.
Jenna M. Houranieh, PharmD, BCOP. The American Cancer Society, ASCO (American Society of Clinical Oncology), NCCN (National Comprehensive Cancer Network), and the American Urological Association all had a different view on screening compared with the USPSTF that I think go more in line with some of the ways that we practice, because they take into consideration life expectancy, patients’ risks, and the age of screening as well.
Active Surveillance
Dr. Aronson. Active surveillance is now a wellestablished, reasonable approach to managing patients with low-risk prostate cancer. When we talk about the various treatment options, we always include a discussion of active surveillance and watchful waiting. Certainly, patients who have a Gleason score of 3+3, a low PSA (< 10) and low volume disease are ideal candidates for active surveillance. There is no established protocol for active surveillance, though there are a number of large series that report specific ways to go about doing it. The key issue for patients is to deemphasize the importance of the PSA, which is a very poor tool for monitoring progression of prostate cancer in men on active surveillance, and to focus on periodically obtaining prostate biopsies.
For patients with prostate cancer who have multiple medical problems and limited life expectancy, there is no reason to do biopsies on a regular basis. Watchful waiting would be more appropriate for these patients. One key issue, which is challenging right now, is that probably the best way to do active surveillance is with the more sophisticated biopsy technology that is now available. That includes both fusing magnetic resonance imaging (MRI) of the prostate into the ultrasound unit we are using to perform transrectal prostate biopsies. The more advanced biopsy units also provide the ability to perform same-site biopsies. There are specific coordinates at each site where a biopsy is performed so that we can go back to that same site on subsequent biopsies. Due to cost issues, these advanced biopsy units are not yet being used at a high frequency.
Dr. Nickols. The large ProtecT trial in the UK randomized men diagnosed with prostate cancer out of a PSA screening cohort to an active surveillance arm, a radical prostatectomy arm, and a radical radiation arm, and has a median of 10 years’ followup. Importantly, the endpoints of overall survival and prostate cancer specific survival were actually the same for all 3 arms, and were quite high. A little more than half of the patients who were on surveillance ended up getting delayed radical therapy of some kind within 10 years.
There was, however, a difference in metastasis-free survival and clinical progression, which were both higher in the active surveillance arm as compared to the treatment arms. Progression to metastatic disease was more than twice as high in the active surveillance arm than the other 2. Most of the patients who had progressed on the active surveillance arm were Gleason 7, and probably were not ideal candidates for active surveillance by today’s standards and would not normally be recommended active surveillance.
Androgen Deprivation Therapies
Dr. Houranieh. Androgen deprivation therapy plays a large role in prostate cancer management and is used in several areas of prostate cancer care. Androgen deprivation therapy can given be before, during or after radiation or alone in the metastatic setting. It's also continued along with chemotherapy in the more advanced stages. It's use is generally guided by our urologists and the duration of therapy is determined by the risk and stage of the cancer. It can be used for as little as a few months for lower risk, early stage disease or for a few years for higher risk disease. It can also be continued indefinitely for metastatic disease. Androgen deprivation therapy is a combination of 2 types of therapies, injectable LHRH (luteinizing hormone-releasing hormone) agonists and oral antiandrogens.
A number of products are available. The most commonly used LHRH agonist, at least at the Lexington VAMC in Kentucky, is leuprolide, which comes either in an intramuscular or subcutaneous formulation and can be given at different frequencies either monthly, every 3 months, or every 6 months.
There are also a number of antiandrogens available on the market. The most commonly used one is bicalutamide. It is generally the best tolerated and given once daily, as opposed to the other 2, which are either given twice or 3 times daily.
Dr. Nickols. We typically add ADT to radiation for patients with high-risk prostate cancer, defined as any of the following: A PSA ≥ 20, clinical T3 or higher disease, or Gleason score of ≥ 8. The addition of ADT to radiation in high-risk patients improves overall survival, prostate cancer survival, and biochemical recurrence-free survival. The backbone of this hormone therapy is usually a GnRH analogue like leuprolide.
The data are extensive, including many large phase 3 randomized trials of patients with highrisk prostate cancer treated with radiation plus or minus androgen suppression. Many of these trials were led by the big cooperative trial groups: the Radiation Therapy Oncology Group (RTOG), the European Organisation for Research and Treatment of Cancer (EORTC), and others. Looking
at all of the data, the answer to the general question of whether hormone therapy is beneficial is yes. The unknown question is what is the optimal duration for this concurrent and adjuvant hormone therapy. The optimal duration is probably somewhere between 1 and 3 years. That’s a large range, and clearly preferences of the patient and the comorbidities play a role in the decision of duration. The radiation doses were considerably lower than what is considered standard of care at this time in the trials that have established the use of concurrent and adjuvant hormone therapy with radiation, which needs to be taken in context.
For patients with localized intermediate-risk prostate cancer, a shorter course of hormone therapy is reasonable. The RTOG 9408 and DFCI 95096 trials showed that a 4- to 6-month course of ADT with RT in mostly intermediate-risk patients was better than RT alone. However, studies looking at the different comorbidities present in these patients showed that patients with less comorbidity actually benefit more from the addition of hormone therapy, which needs to be taken into account.
The benefit to the intermediate-risk patients is probably driven by the patients with unfavorable intermediate-risk disease, for example, with the primary Gleason 4 patterns, such as Gleason 4+3 patients rather than Gleason 3+4, patients with higher volume of prostate cancer, patients with multiple intermediate-risk features, etc. For the truly favorable intermediate-risk patients, low-volume disease, low PSA, and Gleason 3+4 pattern, the added value of concurrent ADT may be small.
The mechanism of why ADT may contribute to radiation efficacy may be explained by direct radio-sensitization: the transcription factor androgen receptor activates expression of many genes involved in DNA repair. Interfere with that, and you sensitize to radiation.
Dr. Graff. Of note, we don’t use ADT as the primary treatment for localized prostate cancer. This is for use in combination with radiation, in people with positive lymph nodes after surgery and in people with incurable prostate cancer.
Dr. Nickols. The question of whether or not to treat patients with localized high-risk prostate cancer with hormone therapy alone has been answered: The SPCG-7 and NCIC CTG PR3/MRC PR07 trials proved that adding radiation to long-term ADT improved survival in these patients.
The DFCI 95096 trial also showed that patients with a high level of comorbidities benefitted the least from concurrent hormone therapy; cardiovascular risks from the hormone therapy can offset the anticancer effect in these patients.
Analyses of the large randomized trials of radiation with or without hormones looking at the question of whether or not there was increased cardiovascular mortality in the patients that had hormone therapy did not show more cardiovascular mortality. Importantly, those trials were not enriched for patients with comorbidities that would set them up for this risk. One needs to weigh the benefits of adding hormones to radiation against the risks on a patient-to-patient basis.
Dr. Aronson. Another scenario where we used ADT is for patients whose cancer progressed after primary therapy; for example, when radical prostatectomy and RT are not successful for a patient. We see patients on a regular basis with a rising PSA after primary therapy. Our main goal is to avoid giving ADT to these patients as long as possible and only use it when it is clearly indicated.
The best measure that we typically use is the PSA doubling time. If the PSA doubling time, for example, is > 1 year, than we feel more confident in holding off on starting ADT and instead just monitoring the PSA. Adverse effects (AEs) of ADT are dramatic. We know that patients can get significant fatigue, gain weight, lose muscle mass, have an increased risk of diabetes mellitus, get hot flashes, and develop impotence and loss of libido. And now there are emerging data on an increased risk of Alzheimer disease. We use ADT but only when clearly indicated.
When I start patients on ADT, in addition to explaining the AEs, I also strongly suggest that, if their health allows, they walk at a brisk rate for at least 30 minutes daily and get on a regular weight training or a resistance training program to try to maintain muscle mass. They need to watch their diet more carefully because they are at increased risk for weight gain. And if they also can do balancing exercises, that would also be ideal. Typically, we also start patients on calcium and vitamin D as there is a risk for bone loss and osteoporosis, and we monitor their bone density.
Dr. Nickols. There’s another role for ADT. In patients who have a PSA recurrence after surgery, RT directed to the prostate bed and/or pelvic nodes is a potential curative therapy. There’s now some emerging evidence that analogous to the definitive radiation setting, the addition of hormone therapy to salvage radiation may be of value.
There were 2 recent trials published. The RTOG-9601 trial showed a benefit to the addition of bicalutamide for patients who had rising PSA after a surgery and were randomized to radiation that was directed to the prostate bed plus or minus 2 years of bicalutamide. The second trial, GETUG-AFU 16, was similar except that in this case the hormone therapy used was 6 months of the GnRH analogue leuprolide. The RTOG-9601 trial had positive outcomes in multiple endpoints, including survival. The GETUG trial is not as mature but had a biochemical improvement.
I don’t think the interpretation of this should be to use hormones with salvage radiation all the time. Importantly, in the RTOG 9601 trial, the patients that had the greatest benefit to the addition of concurrent hormone therapy were those that had a PSA of higher than 0.5 or 0.7. Most patients who get salvage radiation now get it at a much lower PSA, so we probably don’t want to overinterpret that data. And, of course, we have to wait for the GETUG-AFU data to mature more to see if there’s any hard clinical endpoints met
there. Notably, the incidence of gynecomastia in the bicalutamide arm of RTOG 9601 was near 70%. I discuss the addition of hormones with my patients who are getting salvage radiation and usually recommend it to the ones who have the high-risk features, those who would have gotten the concurrent hormones in the definitive setting, those with a PSA greater than 0.5 at the time of salvage, and those with a rapid PSA doubling time.
Dr. Houranieh. Androgen deprivation therapy includes use of LHRH agonists, like leuprolide and antiandrogens like bicalutamide. Some of the short-term AEs from androgen deprivation that we counsel patients on are things like tumor flare, hot flashes, erectile dysfunction, and injection site reactions. Some of the more long-term complications that we touch upon are osteoporosis, obesity, insulin resistance, increased risk of diabetes mellitus and cardiovascular events. We counsel patients on these adverse reactions and do our best with monitoring and prevention.
Dr. Nickols. The ProtecT trial also had some valuable patient-reported outcomes that were very carefully tracked. It confirmed what we already believe. The patients that had primary RT had the greatest negative impact on bowel function and on urinary irritative and obstructive symptoms. The patients who had surgery had the greatest negative impact on sexual function and on urinary incontinence. Obviously, active surveillance had the benefit of avoiding or postponing AEs of local therapy.
You can break up RT for localized disease, into 2 general approaches. The first is external beam radiation. This can be delivered as intensity-modulated radiotherapy (IMRT), which is the most common approach right now, typically stretched over more than 2 months of daily treatments. In addition, there is a newer technique called stereotactic body radiation therapy (SBRT), which has been applied to localized prostate cancer now for more than a decade. It’s efficacy was demonstrated first in low-risk patients as normally is the case. It has the advantage of convenience; it is just 5 treatment days total, which can be accomplished in a couple of weeks. And its convenient for patients who are commuting some distance. That’s really important for veterans, as radiotherapy is not available at all VAs.
At the West LA VAMC, we offer SBRT as a standard treatment for men with low and favorable intermediate risk prostate cancer. In addition, we offer it in the context of a clinical trial for patients with unfavorable intermediate- and high-risk prostate cancer.
The other type of radiation therapy is brachytherapy in which the radiation is temporarily or permanently inserted into the target, the prostate. It is a good stand-alone option for men with low- or intermediate-risk prostate cancer. It has the advantage of being relatively fast in that it is done in a day, although it is more invasive than IMRT or SBRT, and certain anatomic features of the prostate and the patient’s baseline urinary function can limit its appropriateness in some patients.
There are some recent data of interest for the combination of brachytherapy and externalbeam radiation therapy (EBRT). The recently reported ASCENDE-RT trial randomized mostly high-risk patients to either EBRT with 1 year of androgen suppression or EBRT with a boost of brachytherapy to the prostate and 1 year of ADT.
The arm that got the brachytherapy boost actually had half the biochemical recurrence of the EBRT alone but had double the rate of grade 2 acute genitourinary toxicity and triple the rate of grade 3. Metastasis-free survival and other hard clinical endpoints will need longer follow-up, but the biochemical control was quite high: It was about 80% at 10 years out.
Dr. Aronson. For surgical approaches, many VAs now have the da Vinci robot system (Sunnyvale, CA). When we look at the key results, which examine cancer care and AEs, such as incontinence and impotence, there actually is no clear advantage over the open procedure that we previously used. That being said, with the robotic surgery, because we do it laparoscopically, there’s significantly less blood loss. The magnification is such that it is much easier to do the surgery. It’s also much easier on the surgeon’s body, given that you’re in an anatomically, ergonomically good position, and patients go home much sooner, typically on postoperative day 1 or postoperative day 2 with less morbidity following the procedure and a much quicker recovery.
Precision Medicine
Dr. Graff. Prostate cancer may not be cured, even after the best attempts at surgery or radiation. The medical oncologist is probably most utilized with people with incurable prostate cancer. Once it’s incurable, it develops tumors in the bones and lymph nodes most commonly, and we call it metastatic prostate cancer.
Right now we use mostly a once-size-fits-all approach. Everyone initially gets some form of castration therapy, usually medical castration with LHRH agonists. However, prostate cancer invariably becomes resistant to those maneuvers. We call that castration-resistant prostate cancer. That opens the door to 6 other treatments that can prolong survival in prostate cancer. Two of the treatments are hormonal (enzalutamide and abiraterone), 2 are chemotherapy (docetaxel and cabazitaxel), 1 is IV radiation with radium-223, and 1 is an immunotherapy (sipuleucel-T).
At this point, there’s not a lot of guidance about what to use when except that each of these therapies has unique AEs, so we may not use one of the therapies because it causes a lot of fatigue or it could cause seizures, for example, in a patient at risk for those. Sometimes the therapies are inappropriate. For example, with radium, you wouldn’t give it to a patient with a tumor in the liver.
We don’t have readily available companion diagnostics to help us narrow the selection. In 2015, there was an article in Cell that looked at men with metastatic castration-resistant prostate cancer. The tumors were biopsied and analyzed, and we found some surprising things, including certain mutations called DNA repair defects that could make them susceptible to a drug already approved in ovarian cancer, such as olaparib and rucaparib.
A subsequent study in the New England Journal of Medicine looked at patients with advanced prostate cancer whose cancers have these DNA repair defects. Those cancers were susceptible to the PARP (poly ADP ribose polymerase) inhibitor olaparib. That’s an example of where looking and sequencing a tumor could lead to a treatment selection. The PARP inhibitors are not yet approved in prostate cancer, but the Prostate Cancer Foundation is interested in supporting research that could help deliver appropriate therapies to veterans in particular whose cancers have certain markers.
So, we are biopsying patients’ tumors, looking at the mutations in their germline DNA, and matching patients to treatments and vice versa. The DNA repair defects is the one that’s probably under most active evaluation right now. Another example of a biomarker is the AR-V7, which is a mutation in the androgen receptor that renders the cancer resistant to enzalutamide and abiraterone.
Also, I have a study of pembrolizumab which is a PD-1 inhibitor, and I’ve seen some very good responses to that therapy. And we’re not yet sure how to identify prospectively those patients who are likely to respond.
Use of Imaging
Dr. Nickols. The sensitivity of technetium-99m bone scans and CT (computed tomography) scans is not good enough. Many patients that we classify as M0, but with clear evidence of disease with a rising PSA, will be more accurately classified as M1 when the imaging allows this to be the case.
I think prostate-specific membrane antigen positron emission tomography (PET), which is not approved at this time, is going to be of value. A lot of data are coming out of Europe and in the recurrence setting show that PET imaging can detect metastatic sites at PSA values as low as 0.2 with the per lesion sensitivity around 80% and a specificity upward of 97%. This is clearly far and away much better than anything we have now.
There’s going to be a whole cohort of patients that we literally can’t see now, patients with essentially minimally metastatic disease, and they will be revealed when the imaging gets there. And the question is what to do for these patients. Treating patients with a heavy metastatic disease burden is much different from treating patients who may have just one or a few areas of disease outside of their prostate. And we need new strategies for these patients. We are now looking at new treatment regimens for patients with limited metastatic disease burden. I think this is going to be important going forward.
It’s also worth asking: What is the role of local therapy in patients with advanced prostate cancer, patients with metastatic disease? If you look at the patients who were in a lot of the old trials, for example, the NCIC trial, that was adding radiation to hormone therapy for high-risk patients, about 25% of patients in that trial had a PSA > 50. That’s a lot. Many of those patients probably had occult metastases. And there are trials now looking at the role of local therapy in metastatic patients.
Another area of interest is precision oncology, which Dr. Graff touched on, is starting to play a big role in the metastatic setting, but what about the local setting? There are now genomic classifiers available to help with risk assessments, but we don’t yet have much in the way of predictive tools that help guide specific therapies in the localized setting. We know that patients, for example, who have germline BRCA1 or 2 mutations have a worse outcome, period, after local therapy; and right now it may play some into treatment decisions, but we don’t have tailored therapy yet in the localized setting at the molecular level. And I think this is something that we need to start looking at.
Dr. Aronson. The VA is a very rich environment for performing clinical research as well as translational research (bench to bedside). And for example, at the West Los Angeles VAMC, I think one of the key steps that we have taken, moving forward is now our urology, radiation oncology, and hematology-oncology research groups have now merged together. This allows us to not only combine our administrative resources but to really improve the ability for us to perform highquality research in our veterans. And so that’s a model which I think other VAs might consider pursuing, depending upon their circumstances.
Author Disclosures
Dr. Graff has received research support from Sanofi, Astellas, Merck, Janssen, and Bristol Myers Squibb; an honorarium from Astellas; travel support from Clovis and Sanofi; and has consulted for Bayer and Dendreon. No other authors report actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Use of Urinalysis to Assess Adherence to Prescribed Cardiovascular Medications: Focus on Hypertension
Click here to read the supplement
Topics include:
- Common problem of nonadherence to CV medications
- Nonadherence as a contributor to poor CV outcomes
- Improvement of adherence in meeting quality payment program criteria
- Urinalysis as a tool to support adherence
- Benefits of using the KardiAssure™ urinalysis test
Click here to read the supplement
Click here to read the supplement
Topics include:
- Common problem of nonadherence to CV medications
- Nonadherence as a contributor to poor CV outcomes
- Improvement of adherence in meeting quality payment program criteria
- Urinalysis as a tool to support adherence
- Benefits of using the KardiAssure™ urinalysis test
Click here to read the supplement
Click here to read the supplement
Topics include:
- Common problem of nonadherence to CV medications
- Nonadherence as a contributor to poor CV outcomes
- Improvement of adherence in meeting quality payment program criteria
- Urinalysis as a tool to support adherence
- Benefits of using the KardiAssure™ urinalysis test
Click here to read the supplement
Distress Screening and Management in an Outpatient VA Cancer Clinic: A Pilot Project Involving Ambulatory Patients Across the Disease Trajectory (FULL)
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
A diagnosis of cancer, its treatment, and surveillance are fraught with distress. Distress is defined by the National Comprehensive Cancer Network® (NCCN®) as “a multifactorial unpleasant emotional experience of a psychological (cognitive, behavioral, emotional), social, and/or spiritual nature that may interfere with the ability to cope effectively with cancer, its physical symptoms, and its treatment.”1 Distress is known to occur at any point along the cancer-disease trajectory: during diagnosis, during treatment, at the end of treatment, at pivotal treatment decision points, from survivorship through to end of life.2 The severity of the distress can range from “common normal feelings of vulnerability, sadness, and fears to problems that can become disabling, such as depression, anxiety, panic, social isolation, and existential and spiritual crisis.”1 Most important, the impact of distress has been associated with reduced quality of life (QOL) and potentially reduced survival.3,4
About 33% of all persons with cancer experience severe distress.5,6 As a result of the prevalence and severity of distress, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Distress Management recommend that all patients with cancer should be screened for distress, using a standardized tool, at their initial visit, at appropriate intervals, and as clinically indicated.1 The time line for longitudinal screening of “appropriate intervals” has not been firmly established.2 However, it is well recognized that appropriate intervals include times of vulnerability such as remission, recurrence, termination of treatment, and progression.1,7 Despite efforts to improve distress screening and intervention, many institutions struggle to adhere to the NCCN Guidelines®.8,9
In 2012, the American College of Surgeons Commission on Cancer (ACoS CoC) identified distress screening as an essential accreditation standard by 2015.10 The standard mandates that patients be screened a minimum of 1 time at a “pivotal” medical visit (such as time of diagnosis, transitions in cancer treatment, recurrence, completion of cancer treatment, and progression of disease). In practice, most institutions typically screen at diagnosis.2 According to the ACoS CoC, 41 VAMCs are accredited sites that will be impacted by the implementation of this standard.10
Distress Screening Tools
A major challenge and barrier to integrating distress screening in cancer clinics is the lack of consensus on the best measurement tool in a busy ambulatory clinic. Although a number of screening tools are available for measuring cancer-related distress, they vary in efficacy and feasibility. According to Zabora and Macmurray, the perfect screening instrument for distress in persons with cancer does not exist.6 Brief screening tools demonstrate high sensitivity in identifying very distressed patients but lack specificity, resulting in false positives.8,11 More extensive screening instruments, such as the Hospital Anxiety and Depression Scale (HADS), the Brief Symptom Inventory (BSI)-18, and the Psycho-Oncology Screening Tool (POST), have lower rates of false positives but may be more burdensome for providers, especially when considering copyright and cost.6
Ambulatory cancer care requires a rapid screening method with high sensitivity and minimal burden.12 The NCCN Distress Thermometer (DT) has face validity and allows for rapid screening; however, its psychometric properties are not as robust as other instru ments, such as the Center for Epidemiological Studies Depression Scale, the Hospital Anxiety and Depression Scale, Psychological Distress Inventory, or Brief Symptom Inventory.13 Although the DT has been shown to identify clinically significant anxiety, it is not as sensitive in identifying depression.4
The NCCN DT has 2 parts to the screening: (1) an overall distressintensity score within the past week, including the current day; and (2) an accompanying problem list, grouped into 5 categories, addressing QOL domains.14 The quantitative score ranges from 0 (no distress) to 10 (extreme distress). The problem list complements the quantitative score by providing information about the source of distress and can help to tailor the intervention (Figure 1). Access to the NCCN Guideline and DT is free for clinical and personal use.
According to the NCCN Guideline, scores of ≥ 4 require distress-management intervention.1 Mild distress (score < 4) usually can be managed by the primary oncology team.15 However, if the patient’s score is moderate (4-7) or severe (8-10), urgent intervention is necessary. Depending on the source of the distress, the patient should be seen by the appropriate discipline. For patients with practical problems, such as transportation, finances, and housing issues, a referral to social work is needed. For those with distress related to mental health issues, psychology, psychiatry, or social work may be appropriate.
Patients with distressing physical symptoms should be seen by the physician or advanced practice registered nurse (APRN) from the oncology or palliative care team. With limited psychosocial resources available at many cancer clinics, identification and triage for those with the highest levels of distress are critical.5 Triage must incorporate both the total distress score and the components of the distress so that the appropriate disciplines are accessed for the plan of care. More than one discipline may be needed to address multifactorial distress.
Despite strong recommendations from NCCN, ACoS, and many other professional and accrediting agencies, numerous cancer programs face challenges implementing routine screening. This article reports on a large, inner city ambulatory clinic’s pilot project to distress screen all patients at every appointment in the Cancer Center of Excellence (CoE) at Louis Stokes Cleveland VAMC (LSCVAMC) between May 2012 and May 2014 and to provide immediate intervention from the appropriate discipline for patients scoring ≥ 4 on a 0 to 10 DT scale. Results of the screenings, feasibility of screening in an ambulatory VA cancer clinic, and impact on psychosocial resources are presented.
Center of Excellence Project
The LSCVAMC CoE Cancer Care Clinic began as a 3-year grant-funded project from the VA Offices of Specialty Care and Academic Affiliations with 2 major objectives: (1) to deliver quality patient-centered cancer care as measured by implementation of a process for distress screening and management, and development and implementation of a survivorship care plan for patients who have completed cancer treatment; and (2) to provide interprofessional education for the interdisciplinary health care professionals who participate in the clinic as part of their training experience.
Patients in this unique CoE cancer clinic have sameday access to all members of the interdisciplinary and interprofessional team. The ambulatory cancer care CoE team was originally composed of a surgical oncologist, a medical oncologist, a clinical nurse specialist (CNS) patient navigator, a nurse practitioner (NP) in survivorship care, a registered nurse (RN), a psychologist, and an oncology social worker. The project’s patient population included patients with a cancer concern (positive family history and suspicious scans) or a diagnosis of breast cancer, melanoma, sarcoma, or hematologic malignancies. The patient population for the project was based on the CoE team expertise and feasibility of implementation, with plans to roll out the model of care for all patients with any cancer diagnosis across the VAMC at the completion of the project.
The CoE made distress screening and management the leading priority for quality patient-centered care at the start of the project. The purpose of this emphasis on distress screening was to develop a process at LSCVAMC that would meet the 2015 CoC standards and to teach health care professional trainees (NP students, residents, social work students, and fellows in psychology and medical oncology) about distress screening and intervention.
A plan-do-act model of quality improvement (QI) was used to support the development and implementation of the distress-screening process. At the beginning of the project, the institutional review board (IRB) reviewed the protocol and determined that informed consent was not necessary because a QI project for a new standard of care did not require IRB approval. The CoE team met for about 4 months to develop a policy and procedure for the process, based on evidence from national guidelines, a review of the literature, and a discussion of the benefits and burdens of implementation within the current practice.
Limiting initial implementation to a single clinic day made the process more manageable. Descriptive methods analyzed the incidence and percentage of overall distress in this veteran population and quantified the incidences and percentages of each DT component. Feedback from patients and staff offered information on the feasibility of and satisfaction with the process.
From May 2012 to May 2014, all patients who attended the Monday outpatient CoE clinic with a diagnosis of cancer or a cancer concern were given the NCCN, 2.2013 DT instrument by the registration desk clerk at the time they registered for their clinic appointment. 16 Veterans who had difficulty filling out the DT or who had diminished capacity were assisted in completing the instrument by a designated family member and/or the clinic RN.
The completed instrument was evaluated by the CNS patient navigator, and any patient with a score ≥ 4 received an automatic referral to the behavioral health psychologist, social worker, NP, or all team members and their trainees, depending on the areas of distress (eg, practical, family, and emotional problems, spiritual/religious concerns, and/or physical problems) endorsed by the patient.
A psychiatrist was not embedded into the team but worked closely with the team’s oncology psychologist. The psychologist communicated directly with the psychiatrist, and the plan was shared with the team through the electronic medical record (EMR). The appropriate team member(s) and trainee(s) saw the patient at the visit to address needs in real time. Access to palliative care support and spiritual care was readily available if needed.
Distress screenings were recorded in a templated note in the patient’s EMR, which allowed the team to follow the distress scores on an individual basis across the cancer disease trajectory and to assess response to interventions. Multiple screenings of individuals resulted from the fact that many of the patients were seen monthly or every 3/6/9 months, depending on their disease and treatment status. Because levels of distress can fluctuate, distress was assessed at every visit to determine whether an intervention was needed at that visit. Once distress screenings were recorded in the patient’s EMR, the DT instrument was de-identified and given to the CoE research consultant to enter into a database file for analysis.
Trainees were educated about the use of the DT at time of diagnosis and across the disease trajectory. The 4-week CoE curriculum included 2 weeks of conference time to teach about the roles of psychologist, oncology social worker, and survivorship NP in assessing and initiating interventions to address the multidimensional components of the DT. Trainees working with a veteran who was distressed participated in the assessment(s) and intervention(s) for all components of distress that were endorsed.
Results
A total of 866 distress screenings were performed during the first 2 years of the project. Since all patients were screened at all visits, the 866 distress screenings reflect multiple screenings for 445 unique patients. Of the 866 screenings, 290 (33%) had distress scores of ≥ 4, meeting the criteria for intervention. Screenings reflected patient visits at any point in the disease trajectory. Because this was a new standard of care QI project rather than a research project, additional data, such as diagnosis or staging, were not collected, and IRB approval was not needed.
Because the NCCN Guideline recommendation for intervention is a score of ≥ 4, the descriptive statistics focused on those with moderate-to-severe distress. However, there were numerous occasions when the veteran would report a score of 0 to 3 and still endorse a number of the problems on the DT. The CNS and RN on the team discussed these findings with the appropriate discipline. For example, if the veteran reported a score of 1 but endorsed all 6 components on the emotional problem list, the nurses discussed the patient with the social worker or psychologist to determine whether behavioral health intervention was needed.
The mean distress score for the 290 screenings ≥ 4 was 6.3 on a 0 to 10 scale; median was 6.0 and mode 5.0. Two hundred ten of these screenings (72%) were categorized as moderate distress (4-7), and 80 patients (28%) reported severe distress (Figure 2). If the veteran left a box empty on the problem list, itwas recorded as missing. The frequency that patients reported each type of distress are reported in Figure 3.
The incidence of each component of distress, from those screenings with a score of ≥ 4 is described below, along with case study examples for each component. Team members involved in patient interventions provided these case studies to demonstrate clinical examples of the veteran’s distress from the problem list on the DT.
Practical/Family Distress
Practical issues were reported in 38% of the screenings (109/290). Intervention for moderate-to-severe distress associated with practical problems and family issues was provided by the team social worker. The social worker frequently addressed transportationrelated distress. Providing transportation was essential for adherence to clinic appointments, follow-up testing, treatments, and ultimately, disease management. Housing was also a problem for many veterans. It is critical that patients have access to electricity, heating, food, and water to be able to safely undergo adjuvant therapy. Thus, treatments decisions could be impacted by the veterans’ housing and transportation issues; immediate access to social work support is essential for quality cancer care.
Twenty-six percent of patients that were screened expressed concerns with practical and/or family problems (75/289). Issues of domestic violence, difficulties dealing with a significant other, and concerns about children were referred to social work (Table 1).
Case Study
Ms. S. is a veteran aged 71 years with recently diagnosed breast cancer. She is being seen in the clinic for a postoperative visit following partial mastectomy and is anticipating beginning radiation therapy within the next 3 weeks. She reports a distress score of 7 and identifies concerns about work and transportation to the clinic as the sources of distress. The social worker meets with the patient and learns that she fears losing her job because of the daily travel time to and from radiation and that she cannot afford to travel 65 miles daily to LSCVAMC for radiation. The social worker listens to her concerns and assists her with a plan for short-term disability and VA housing during her radiation therapy treatments. Ms. S. was able to complete radiation at LSCVAMC with temporary housing and to return to work after therapy.
Emotional Distress
Patients who identified that their moderate-to-severe distress was related to emotional problems received same-day intervention from a psychologist skilled in providing emotional support, cognitive behavioral strategies, and assessing the need for referral to either a psychiatrist or oncology social worker. Seventy-one percent of patients reported emotional problems, such as worry, depression, and nervousness (Table 2).
Case Study
Mr. K. is a veteran aged 71 years with a new diagnosis of breast cancer. He lives on his own but has family and a few friends nearby. He reports that he doesn’t like to share his problems with others and has not told anyone of his new diagnosis. Mr. K. rates his distress a 7 and endorses worry, fear, and depression. At a treatment-planning visit, he agrees to see the psychologist for help in dealing with his distress. Treatment involves a mastectomy followed by hormonal therapy.
Mr. K. was scared about having cancer; some of his veteran colleagues have developed cancer recently, and 2 have died. He told the psychologist that he feels worthless and that this disease just makes him more of a burden on society. He has had thoughts of taking his life so that he doesn’t have to deal with cancer, but he does not have a plan. The team formulated a plan to address his anxiety and depression. Mr. K. started a serotonin reuptake inhibitor, and he met with the VA psychiatrist weekly to help develop coping strategies. The team’s psychologist worked closely with Mr. K.’s psychiatrist, and he successfully completed surgery and chemotherapy. He is now being seen in survivorship clinic, continuing care with the team and his psychologist.
Spiritual Distress
Although spiritual/religious concerns are part of DT screening, it is only a single item on the DT. Just 8% of patients (21/276) reported moderate-to-severe spiritual distress. However, there was access to a chaplain at LSCVAMC.
Case Study
Mr. H., a 63-year-old veteran with stage IV melanoma, was seen in the clinic for severe pain in his left hip and ribs (8 on a 10-point scale); he was unresponsive to escalating doses of oxycodone. During the visit, he reported that his distress level is a 10, and in addition to identifying pain as a source of distress, he indicated that he has spiritual distress. When questioned further about spiritual distress, Mr. H. reported that he deserves this pain since he caused so many others pain during his time in Vietnam. The chaplain was contacted, and the patient was seen in clinic at this visit. The chaplain gave him the opportunity to share his feelings of guilt. The importance of spiritual care when the patient is experiencing “total pain” is essential to pain management. Within 3 days, his pain score decreased to an acceptable level of 3 with no additional pharmacologic intervention.
Physical Distress
Physical problems associated with the distress scores were addressed by the surgical and medical oncologists and the APRNs (CNS patient navigator and survivorship NP). When the clinic opened, the team used the Memorial Symptom Assessment Scale to assess physical and psychological symptoms.16 However, patients reported experiencing distress at having to complete 2 tools that had a great deal of overlap. The team determined that the DT could be used as the sole screening tool for all QOL domains.
It is important to note that 92% of patients with moderate-to-severe distress reported physical symptoms as a source of distress (Table 3).
Case Study
Ms. L. is a Vietnam War veteran aged 64 years who was seen in the survivorship clinic. She was diagnosed with estrogen-receptor (ER) and progesterone-receptor (PR) breast cancer 1 year previously, had a lumpectomy followed by radiation therapy, and was on hormonal therapy. She recorded her distress score as a 6 and indicated that multiple physical symptoms were her major concern. She had difficulty with insomnia, fatigue, and hot flashes. The survivorship NP talked with Ms. L. about her symptoms and made nonpharmacologic recommendations for improving sleep, provided an exercise plan for fatigue, and initiated venlafaxine to manage the hot flashes. Ms. L. continued to be seen by the team in survivorship clinic, and during her 3-month follow-up visit, she reported improvement in sleep as the hot flashes diminished.
Multifactoral Distress
Many patients endorsed ≥ 1 component of distress. This required a team approach to intervene for the multifactorial nature of the distress.
Case Study
Mr. K. is a veteran aged 82 years who had been a farmer most of his life. He was cared for at the VA for an advancedstage squamous cell skin cancer of his scalp, which he had allowed to go untreated. The cancer has completely eroded beneath his scalp, and he wore a hat to cover the foul-smelling wound. He lived in rural Ohio with his wife of 55 years; 3 adult daughters lived in the Cleveland area. His daughters served as primary caregivers when Mr. K. came to Cleveland for daily radiation and weekly chemotherapy treatments. He had not been away from his wife since the war and misses her terribly, returning home only on weekends during the 6-week course of radiation.
His primary goal was to return home in time to harvest his farm’s produce 2 months later. He was aware that he has < 6 months to live but wanted chemotherapy and radiation to control the growth of the cancer. During this visit to the ambulatory clinic, he reported a distress score of 5 andidentified family concerns (eg, living away from his wife most of every week) and endorsed emotional concerns of fear, worry, and sadness, and reported pain, fatigue, insomnia, and constipation as physical concerns.
Mr. K. received support from the social worker, the psychologist, and the APRN for symptom management during this visit. The social worker was able to advocate for limited palliative radiation therapy treatments rather than a 6-week course; the psychologist spent 45 minutes talking with him about his fears of a painful death, worries about his wife, and sadness at not being alive for another planting season. The APRN recommended both pharmacologic and nonpharmacologic interventions for his fatigue and insomnia and initiated a pain and bowel pharmacologic regimen. The team respected Mr. K.’s wish to reconsider hospice care at the following visit after he had talked with his wife. Mr. K. died peacefully in his home with his wife and family just before the start of planting season.
Clinical Implications
Distress screening and intervention is essential for quality cancer care. While a great deal of controversy exists about the best time to screen for distress, the LSCVAMC CoE has taken on the challenge of screening and intervening in real time at every patient visit across the disease trajectory. The model of distress screening all veterans at CoE clinic visits has been rolled out to other cancer clinics at LSCVAMC.
Distress screening at each visit is not time intensive. Patients are willing to fill out the instrument while waiting for their clinic visit, and most patients find that it takes less than 5 minutes to complete. The major challenge for institutions considering screening with each visit is not the screening but access to appropriate providers able to provide timely intervention. The success of this model results, in part, because the clinic RN assesses the responses to the DT and refers to the appropriate discipline, utilizing precious resources of social work and psychology appropriately. The VA system is already committed to improving the psychosocial well-being of veterans and has established social work and psychology resources specifically for the cancer clinics.
Many patients reported to the authors that they might not have been able or willing to return to LSCVAMC to see the behavioral health specialists on another day. In addition, scheduling behavioral health appointments at another time would not allow for attending to the distress in real time. Also, from a systems standpoint, it would have been an added cost to the VA and/or the veteran for transportation for additional appointments on different days.
Finally, although the impact of the CoE project on health professional trainees has been reported elsewhere, the distress screening and intervention process were valued as being very positive for all trainees who participated in CoE clinic.17 The trainees were able to stay with the patient for the entire clinic visit, including the visits made by disciplines other than their own. For example, the family medicine residents stayed with the patient they examined to observe the distress assessments and interventions offered by the social worker and/or psychologist for the patients who scored ≥ 4 on the DT.
At the end of their rotations in the CoE, trainees reported an increased awareness of the importance of distress screening in a cancer clinic. Many were not aware of the NCCN guidelines and the ACoS CoC mandate for distress screening as a standard of cancer care. Interdisciplinary trainees rated the CoE curriculum and the conference teaching/learning sessions on distress management highly. However, observing the role of the social worker and psychologist were the most valuable to trainees, regardless of the area of practice they enter.
Conclusion
Addressing practical, psychosocial, physical, and spiritual needs will help decrease distress, support patients’ ability to tolerate treatment, and improve veterans’ QOL across the cancer-disease trajectory. Screening all patients at an outpatient cancer clinic at LSCVAMC is feasible and does not seem to be a burden for patients or providers. This pilot project has become standard of care across the LSCVAMC cancer clinics, demonstrating its sustainability.
Screening with the DT provides information about the intensity of the distress and the components contributing to the distress. The most important aspect of the screening is assessing the components of the distress and providing real-time intervention from the appropriate discipline. It is critical that the oncology team refer to the appropriate discipline based on the source of the distress rather than on only the intensity. Findings from this project indicate that physical symptoms are frequently the source of distress and may not require behavioral health intervention. However, for patients with psychosocial needs, rapid access to behavioral health care services is critical for quality veteran-centered cancer care.
Since 2015, all VA cancer centers are required to have implemented distress screening. According to the CoC, at least 1 screening must be done on every patient.10 Many institutions have begun to screen at diagnosis, but it is well known that there are many points along the cancer trajectory when patients may experience an increase in distress. Simple screening with the DT at every cancer clinic visit helps identify the veterans’ needs at any point along the disease spectrum.
At LSCVAMC, the CoE was designed as an interdisciplinary cancer clinic. With the conclusion of funding in FY 2015, the clinic has continued to function. The rollout into other clinics has continued with movement toward use of formal consult requests and continual, real-time evaluation of the process. Work on accurate, timely identification of new cancer patients and identifying pivotal cancer visits is underway. The LSCVAMC is committed to improving care and access to its veterans with cancer to ensure appropriate and adequate services across the cancer trajectory.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
1. Holand JC, Jacobsen PB, Anderson A, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management 2.2016. © 2014 National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Updated July 25, 2016. Accessed January 13, 2017.
2. Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol. 2012;30(11):1160-1177.
3. Hamer M, Chida Y, Molloy G. Psychological distress and cancer mortality. J Psychosom Res. 2009;66(3):255-258.
4. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2013;8(4):487-494.
5. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19-28.
6. Zabora JR, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2012;30(6):625-635.
7. Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(91):2946-2954.
8. Parry C, Padgett LS, Zebrack B. Now what? Toward an integrated research and practice agenda in distress screening. J Psychosoc Oncol. 2012;30(6):715-727.
9. Wagner LI, Spiegel D, Pearman T. Using the science of psychosocial care to implement the new American College of Surgeons Commission on Cancer distress screening standard. J Natl Compr Canc Netw. 2013;11(2):214-221.
10. American College of Surgeons Commision on Cancer. https://www.facs.org/quality-programs/cancer/coc Published 1996. Updated January 19, 2017. Accessed April 16, 2016.
11. Rohan EA. Removing the stress from selecting instruments: arming social workers to take leadership in routine distress screening implementation. J Psychosoc Oncol. 2012;30(6):667-678.
12. Merport A, Bober SL, Grose A, Recklitis CJ. Can the distress thermometer (DT) identify significant psychological distress in long-term cancer survivors? A comparison with the Brief Symptom Inventory-18 (BSI-18). Support Care Cancer. 2012;20(1):195-198.
13. Carlson LE, Bultz BD. Cancer distress screening: Needs, models, and methods. J Psychosom Res. 2003;55(5):403-409.
14. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010:8(1):4-12.
15. Jacobsen P, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494-1502.
16. Portenoy R, Thaler HT, Korblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A(9):1326-1336.
17. Arfons L, Mazanec P, Smith J, et al. Training health care professionals in interprofessional collaborative cancer care. Health Interprof Pract. 2015;2(3):eP1073.
Ketogenic Diets and Cancer: Emerging Evidence
As early as 500 bc , fasting was used as an effective treatment for many medical ailments. Fasting continued into modern times, and in 1910, Guelpa and Marie proposed fasting as an antiepilepsy treatment. In 1921, Woodyatt noted that starvation or use of high-fat, low-carbohydrate diets in individuals with no significant medical comorbidities yielded acetone and β-hydroxybutyrate, 2 energy sources produced by the liver in the absence of glucose. A low-carbohydrate, high-fat diet was thought to be an alternative to fasting or starvation, having many of the same desired effects while continuing to nourish healthy cells. The term ketogenic diet (KD) was later coined by Wilder and Peterman, who formulated the fat-to-carbohydrate ratio that is still used today: 1 g protein per kg of body weight in children and 10 to 15 g carbohydrates daily, and fat for the remainder of calories. Both investigators reported that this diet improved their patients’ mentation and cognition.1
Use of the KD as an adjuvant to cancer therapy also began to emerge. In 1922, Braunstein noted that glucose disappeared from the urine of patients with diabetes after they were diagnosed with cancer, suggesting that glucose is recruited to cancerous areas where it is consumed at higher than normal rates. During that same time, Nobel laureate Otto Warburg found that cancer cells thrive on glycolysis, producing high lactate levels, even in the presence of abundant oxygen. Warburg conducted many in vitro and animal experiments demonstrating this outcome, known as the Warburg effect.
By the mid-20th century, KD use in epilepsy treatment and cancer research had waned. However, in the mid-to-late 1990s, with the establishment of the Charlie Foundation, the diet slowly started regaining recognition.1 Results of many in vitro and animal studies were reported, and human data also began to accumulate.
Mechanism of Action
Glucose normally stimulates pancreatic β cells to release insulin, which allows glucose to enter cells and provide energy. With high carbohydrate and glucose intake, the pancreas increasingly secretes more insulin, which promotes the interaction of growth hormone receptors and growth hormones to produce insulin-like growth factor 1 (IGF-1) in the liver—promoting cell growth and proliferation, which can be detrimental to patients with cancer. Overexpression of glucose transporters 1 and 3 (Glut-1, Glut-3) also occurs in many cancers and corresponds to the degree of glucose uptake in aggressive tumors, as seen on positron emission tomography (PET).2 Overexpression of hexokinase, the rate-limiting enzyme of glycolysis, further drives production of pyruvate and lactate, which cause reactive oxygen species damage. Translocation of the hexokinase rate-limiting enzyme from the cytosol into the outer mitochondrial membrane, where it interacts with voltage-dependent anion channels, can disrupt caspasedependent cytochrome release, which suppresses the apoptotic pathways of cancer cells and makes cancer more resistant to chemotherapy.3
When glucose is scarce, the body senses the need to make an alternative form of energy for cells. The liver then produces ketones and fatty acids, which provide for normal cells but do not benefit cancer cells. Cancer cells have dysfunctional mitochondria and possibly electron transport chain defects, which disrupt normal adenosine triphosphate (ATP) production from the mitochondria. The result is that the cancer cells become heavily dependent on ATP coming from the less efficient process of glycolysis (Figure 1).
Ketogenic diets mimic the fasting state, wherein the body responds to the lack of glucose by producing ketones for energy. Excess lactate production, which is part of the Warburg effect, compensates for ATP production defects caused by dysfunctional mitochondrial oxidative phosphorylation.2,4 The resulting tumor dependence on glucose can be exploited with KD use. Ketogenic diets selectively starve tumors by providing the fat and protein that otherwise could not be used by glucose-dependent tumor cells.
In KDs, the 4:1 ratio of high fat to low carbohydrates mimics the metabolic effects of starvation (Figure 2). These diets slow cancer by inhibiting insulin/IGF and downstream intracellular signaling pathways, such as
Ketogenic Diet Benefits
There are concerns about providing protein to patients who are at risk for renal problems. However, mouse models of diabetic nephropathy showed improved renal function with KD use. The hypothesis was that KD use, which produces prolonged elevated 3-β-hydroxybutyric acid levels, also reduces molecular responses to glucose and consequently reduces renal damage.6 Use of the diet also reduced pain and inflammation in both juvenile and adult rats. Mechanisms of action were thought to be reduced reactive oxygen species and increased central adenosine levels.7,8
Adverse Effects
Dieting is of concern to cancer patients worried about additional weight loss. The standard diet is made up predominantly of carbohydrates and is high in caloric value (Figure 3). Beck and Tisdale investigated the effect of KD use on delaying cachexia in mouse models of colon carcinoma. They found that dieting was more effective than insulin in reversing weight loss and had the added effect of reducing tumor size.7 Moreover, Tisdale and colleagues found that KD use in cachectic cancer patients could promote weight gain.8
A possible explanation is that healthy tissue nutrition selectively delays tumor growth, while cancer cells are deprived of nutrition (carbohydrates). A therapeutic weight plateau should follow initial weight loss with KD, in contrast to pathologic rapid weight loss in non-KD patients.9 Kidney stones, gout, and symptomatic hypoglycemia were also potential expected adverse effects (AEs).
Case Reports
In 1962, the New York Department of Mental Hygiene published an article about 2 women whose metastatic cancers disappeared after a series of daily hypoglycemiainduced insulin comas (brief and reversible). These patients could not undergo conventional electric shock therapy, hence, the medically induced psychotherapy. Not only did their psychotic and depressive symptoms resolve, but their cancers (grossly visible cervical cancer and metastatic melanoma) became undetectable as early as 2 months into treatment.10 Zuccoli and colleagues reported on a glioblastoma that was effectively treated with temozolomide oral chemotherapy after the patient was weaned off steroids. The patient had a radiographic response and good tumor control for about a year, before discontinuing the diet. She transitioned to chemotherapy, which included bevacizumab (antivascular endothelial growth factor), but the disease progressed and she died.11 In addition, during 8 weeks of ketogenic dieting, 2 pediatric female astrocytoma patients experienced improved mood and showed decreased glucose uptake on PET-computed tomography (PET-CT) of their tumor sites. One of these patients continued the diet and remained disease-free another 12 months.12 These early case reports provide compelling evidence for further research into the role of glucose metabolism in cancer
treatment.
Current Research
Compared with normal mice, tumor-bearing mice placed on a low-carbohydrate diet had lower glucose, insulin, and lactic acid levels.4 An in vivo microdialysis study of patients with head and neck cancers found decreased lactic acid levels in the tumor tissues after a 4-day KD.13 Most early studies and case reports involving KD in cancer focused on brain tumors.11,14-16 Human clinical studies on this diet in cancer care were limited to small, nonrandomized, brief trials (4-12 weeks) or single case studies (Table).11,17-20
Fine and colleagues conducted a 4-week feasibility study of the low-carbohydrate modified Atkins KD (≤ 20 g of carbohydrates daily) in PET-positive advanced cancer patients with solid tumors. There was a correlation between insulin levels and ketosis but not IGF. Stable disease or partial remission (measured standardized uptake value) on PET-CT correlated with 3-fold higher ketosis (but not weight loss or reduced caloric intake) relative to patients with progressive disease.17 In the ERGO trial, Rieger and colleagues studied 20 relapsed glioblastoma patients who were on KD supplemented with plant oils.18 Calories were unlimited. The primary endpoint was percentage of patients who discontinued the diet. Mean weight loss was significant, but quality of life (QOL) was maintained. The investigators also studied the effects of KD alone or combined with bevacizumab in a mouse glioblastoma model. In this mouse study, KD alone had no effect; however, it increased median survival from 52 to 58 days (P < .05) with bevacizumab.
In a pilot study, Schmidt and colleagues provided KD plus oil-protein shakes as snacks to 7 of 16 patients with a variety of advanced metastatic cancers. Mean weight loss was statistically significant, blood lipid or cholesterol levels remained stable, some QOL measures improved, and there were no severe AEs.19 Schwartz and colleagues reviewed the cases of 32 glioma patients treated with energy restricted KDs —5 from case reports, 19 from a clinical trial by Rieger and colleagues, and 8 from Champ.18,20,21 They also reported on 2 of their own glioma cases treated with the energy-restricted KD and studied tissues for expression of key ketolytic enzymes. Prolonged remission was noted from 4 months to more than 5 years in these case reports.20
The VA Pittsburgh Healthcare System safety that trial enrolled 17 patients, 11 of whom were evaluated. Mean weight loss was significant, and weight loss of ≥ 10% was noted in responders (stable or improved disease) compared with nonresponders. Three patients dieted longer than 16 weeks (survival, 80-116 weeks). One of these patients was alive at 121 weeks.22
The safety and feasibility data suggest that cancer patients can tolerate KD use. Investigators should consider combining the KD approach with standard treatment modalities, including chemotherapy and radiation.
Already investigators have conducted in vitro studies of the effect of gene expression of ketolytic and glycolytic enzymes within tumor tissue on KD response. Tumors expressing mutations in key mitochondrial oxidative phosphorylation enzymes were more likely to respond to KD use than were tumors without mutations.16,23
Ongoing Clinical Trials
Duke University has initiated a randomized study (NCT00932672) of the Atkins diet and androgen deprivation therapy for patients with prostate cancer. Tel Aviv Sourasky Medical Center in Israel is recruiting previously treated chemoradiation patients with high-grade glial tumors for an open-label study (NCT01092247) of the efficacy of KD in preventing tumor growth and recurrence. St. Joseph’s Hospital and Medical Center (Phoenix, AZ) is recruiting newly diagnosed glioblastoma patients for a phase 1/2 prospective trial (NCT02046187) involving upfront resection followed by KD with radiotherapy and concurrent temozolomide, followed by adjuvant temozolomide chemotherapy. The primary endpoint is number of patients with AEs and the secondary endpoints are overall survival, time to progression, and QOL. The University of Iowa is recruiting patients with prostate cancer and non-small cell lung cancer for 2 phase 1 studies (NCT01419483 and NCT01419587, respectively) involving KD using Nutritia KetoCal 4:1 (Gaithersburg, MD).
Conclusion
Data from case reports and trials suggest KD use is safe and tolerable for patients with cancer. Although it would be ideal to conduct a larger trial using a randomized therapeutic approach, the current emphasis on drug-based trials is a formidable obstacle. Other major obstacles are patient initiative and adherence. For now, investigators must work with anecdotal data. Examination of gene expression patterns in mitochondria and mutations in ketolytic and glycolytic enzymes may prove useful in selecting potentially responsive patients. Combining this dietary approach with standard chemotherapeutic and radiotherapeutic options may help improve tumor response, and further research is desperately needed.
Click to read the digital edition.
1. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(suppl 8):3-5.
2. Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31(1):5-12.
3. Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40(3):171-182.
4. Ho VW, Leung K, Hsu A, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484-4493.
5. Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711-1720.
6. Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One. 2011;6(4):e18604.
7. Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59(5):677-681.
8. Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39-43.
9. Tan-Shalaby J, Seyfried T. Ketogenic diet in advanced cancer: a pilot feasibility and safety trial in the Veterans Affairs cancer patient population. J Clin Trials. 2013;3(4):149.
10. Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36:261-270.
11. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). 2010;7:33.
12. Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202-208.
13. Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993-1001.
14. Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28(8):1002-1008.
15. Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer. 2013;65(6):843-849.
16. Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 2013;10(1):47.
17. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028-1035.
18. Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843-1852. [published correction appears in Int J Oncol. 2014;45(6):2605.
19. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54.
20. Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3.
21. Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125-131.
22. Tan-Shalaby J, Carrick J, Edinger K, et al. Modified ketogenic diet in advanced malignancies—final results of a safety and feasibility trial within the Veterans Affairs Healthcare System. J Clin Oncol. 2016;34(suppl): Abstract e23173.
23. Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578-585.
As early as 500 bc , fasting was used as an effective treatment for many medical ailments. Fasting continued into modern times, and in 1910, Guelpa and Marie proposed fasting as an antiepilepsy treatment. In 1921, Woodyatt noted that starvation or use of high-fat, low-carbohydrate diets in individuals with no significant medical comorbidities yielded acetone and β-hydroxybutyrate, 2 energy sources produced by the liver in the absence of glucose. A low-carbohydrate, high-fat diet was thought to be an alternative to fasting or starvation, having many of the same desired effects while continuing to nourish healthy cells. The term ketogenic diet (KD) was later coined by Wilder and Peterman, who formulated the fat-to-carbohydrate ratio that is still used today: 1 g protein per kg of body weight in children and 10 to 15 g carbohydrates daily, and fat for the remainder of calories. Both investigators reported that this diet improved their patients’ mentation and cognition.1
Use of the KD as an adjuvant to cancer therapy also began to emerge. In 1922, Braunstein noted that glucose disappeared from the urine of patients with diabetes after they were diagnosed with cancer, suggesting that glucose is recruited to cancerous areas where it is consumed at higher than normal rates. During that same time, Nobel laureate Otto Warburg found that cancer cells thrive on glycolysis, producing high lactate levels, even in the presence of abundant oxygen. Warburg conducted many in vitro and animal experiments demonstrating this outcome, known as the Warburg effect.
By the mid-20th century, KD use in epilepsy treatment and cancer research had waned. However, in the mid-to-late 1990s, with the establishment of the Charlie Foundation, the diet slowly started regaining recognition.1 Results of many in vitro and animal studies were reported, and human data also began to accumulate.
Mechanism of Action
Glucose normally stimulates pancreatic β cells to release insulin, which allows glucose to enter cells and provide energy. With high carbohydrate and glucose intake, the pancreas increasingly secretes more insulin, which promotes the interaction of growth hormone receptors and growth hormones to produce insulin-like growth factor 1 (IGF-1) in the liver—promoting cell growth and proliferation, which can be detrimental to patients with cancer. Overexpression of glucose transporters 1 and 3 (Glut-1, Glut-3) also occurs in many cancers and corresponds to the degree of glucose uptake in aggressive tumors, as seen on positron emission tomography (PET).2 Overexpression of hexokinase, the rate-limiting enzyme of glycolysis, further drives production of pyruvate and lactate, which cause reactive oxygen species damage. Translocation of the hexokinase rate-limiting enzyme from the cytosol into the outer mitochondrial membrane, where it interacts with voltage-dependent anion channels, can disrupt caspasedependent cytochrome release, which suppresses the apoptotic pathways of cancer cells and makes cancer more resistant to chemotherapy.3
When glucose is scarce, the body senses the need to make an alternative form of energy for cells. The liver then produces ketones and fatty acids, which provide for normal cells but do not benefit cancer cells. Cancer cells have dysfunctional mitochondria and possibly electron transport chain defects, which disrupt normal adenosine triphosphate (ATP) production from the mitochondria. The result is that the cancer cells become heavily dependent on ATP coming from the less efficient process of glycolysis (Figure 1).
Ketogenic diets mimic the fasting state, wherein the body responds to the lack of glucose by producing ketones for energy. Excess lactate production, which is part of the Warburg effect, compensates for ATP production defects caused by dysfunctional mitochondrial oxidative phosphorylation.2,4 The resulting tumor dependence on glucose can be exploited with KD use. Ketogenic diets selectively starve tumors by providing the fat and protein that otherwise could not be used by glucose-dependent tumor cells.
In KDs, the 4:1 ratio of high fat to low carbohydrates mimics the metabolic effects of starvation (Figure 2). These diets slow cancer by inhibiting insulin/IGF and downstream intracellular signaling pathways, such as
Ketogenic Diet Benefits
There are concerns about providing protein to patients who are at risk for renal problems. However, mouse models of diabetic nephropathy showed improved renal function with KD use. The hypothesis was that KD use, which produces prolonged elevated 3-β-hydroxybutyric acid levels, also reduces molecular responses to glucose and consequently reduces renal damage.6 Use of the diet also reduced pain and inflammation in both juvenile and adult rats. Mechanisms of action were thought to be reduced reactive oxygen species and increased central adenosine levels.7,8
Adverse Effects
Dieting is of concern to cancer patients worried about additional weight loss. The standard diet is made up predominantly of carbohydrates and is high in caloric value (Figure 3). Beck and Tisdale investigated the effect of KD use on delaying cachexia in mouse models of colon carcinoma. They found that dieting was more effective than insulin in reversing weight loss and had the added effect of reducing tumor size.7 Moreover, Tisdale and colleagues found that KD use in cachectic cancer patients could promote weight gain.8
A possible explanation is that healthy tissue nutrition selectively delays tumor growth, while cancer cells are deprived of nutrition (carbohydrates). A therapeutic weight plateau should follow initial weight loss with KD, in contrast to pathologic rapid weight loss in non-KD patients.9 Kidney stones, gout, and symptomatic hypoglycemia were also potential expected adverse effects (AEs).
Case Reports
In 1962, the New York Department of Mental Hygiene published an article about 2 women whose metastatic cancers disappeared after a series of daily hypoglycemiainduced insulin comas (brief and reversible). These patients could not undergo conventional electric shock therapy, hence, the medically induced psychotherapy. Not only did their psychotic and depressive symptoms resolve, but their cancers (grossly visible cervical cancer and metastatic melanoma) became undetectable as early as 2 months into treatment.10 Zuccoli and colleagues reported on a glioblastoma that was effectively treated with temozolomide oral chemotherapy after the patient was weaned off steroids. The patient had a radiographic response and good tumor control for about a year, before discontinuing the diet. She transitioned to chemotherapy, which included bevacizumab (antivascular endothelial growth factor), but the disease progressed and she died.11 In addition, during 8 weeks of ketogenic dieting, 2 pediatric female astrocytoma patients experienced improved mood and showed decreased glucose uptake on PET-computed tomography (PET-CT) of their tumor sites. One of these patients continued the diet and remained disease-free another 12 months.12 These early case reports provide compelling evidence for further research into the role of glucose metabolism in cancer
treatment.
Current Research
Compared with normal mice, tumor-bearing mice placed on a low-carbohydrate diet had lower glucose, insulin, and lactic acid levels.4 An in vivo microdialysis study of patients with head and neck cancers found decreased lactic acid levels in the tumor tissues after a 4-day KD.13 Most early studies and case reports involving KD in cancer focused on brain tumors.11,14-16 Human clinical studies on this diet in cancer care were limited to small, nonrandomized, brief trials (4-12 weeks) or single case studies (Table).11,17-20
Fine and colleagues conducted a 4-week feasibility study of the low-carbohydrate modified Atkins KD (≤ 20 g of carbohydrates daily) in PET-positive advanced cancer patients with solid tumors. There was a correlation between insulin levels and ketosis but not IGF. Stable disease or partial remission (measured standardized uptake value) on PET-CT correlated with 3-fold higher ketosis (but not weight loss or reduced caloric intake) relative to patients with progressive disease.17 In the ERGO trial, Rieger and colleagues studied 20 relapsed glioblastoma patients who were on KD supplemented with plant oils.18 Calories were unlimited. The primary endpoint was percentage of patients who discontinued the diet. Mean weight loss was significant, but quality of life (QOL) was maintained. The investigators also studied the effects of KD alone or combined with bevacizumab in a mouse glioblastoma model. In this mouse study, KD alone had no effect; however, it increased median survival from 52 to 58 days (P < .05) with bevacizumab.
In a pilot study, Schmidt and colleagues provided KD plus oil-protein shakes as snacks to 7 of 16 patients with a variety of advanced metastatic cancers. Mean weight loss was statistically significant, blood lipid or cholesterol levels remained stable, some QOL measures improved, and there were no severe AEs.19 Schwartz and colleagues reviewed the cases of 32 glioma patients treated with energy restricted KDs —5 from case reports, 19 from a clinical trial by Rieger and colleagues, and 8 from Champ.18,20,21 They also reported on 2 of their own glioma cases treated with the energy-restricted KD and studied tissues for expression of key ketolytic enzymes. Prolonged remission was noted from 4 months to more than 5 years in these case reports.20
The VA Pittsburgh Healthcare System safety that trial enrolled 17 patients, 11 of whom were evaluated. Mean weight loss was significant, and weight loss of ≥ 10% was noted in responders (stable or improved disease) compared with nonresponders. Three patients dieted longer than 16 weeks (survival, 80-116 weeks). One of these patients was alive at 121 weeks.22
The safety and feasibility data suggest that cancer patients can tolerate KD use. Investigators should consider combining the KD approach with standard treatment modalities, including chemotherapy and radiation.
Already investigators have conducted in vitro studies of the effect of gene expression of ketolytic and glycolytic enzymes within tumor tissue on KD response. Tumors expressing mutations in key mitochondrial oxidative phosphorylation enzymes were more likely to respond to KD use than were tumors without mutations.16,23
Ongoing Clinical Trials
Duke University has initiated a randomized study (NCT00932672) of the Atkins diet and androgen deprivation therapy for patients with prostate cancer. Tel Aviv Sourasky Medical Center in Israel is recruiting previously treated chemoradiation patients with high-grade glial tumors for an open-label study (NCT01092247) of the efficacy of KD in preventing tumor growth and recurrence. St. Joseph’s Hospital and Medical Center (Phoenix, AZ) is recruiting newly diagnosed glioblastoma patients for a phase 1/2 prospective trial (NCT02046187) involving upfront resection followed by KD with radiotherapy and concurrent temozolomide, followed by adjuvant temozolomide chemotherapy. The primary endpoint is number of patients with AEs and the secondary endpoints are overall survival, time to progression, and QOL. The University of Iowa is recruiting patients with prostate cancer and non-small cell lung cancer for 2 phase 1 studies (NCT01419483 and NCT01419587, respectively) involving KD using Nutritia KetoCal 4:1 (Gaithersburg, MD).
Conclusion
Data from case reports and trials suggest KD use is safe and tolerable for patients with cancer. Although it would be ideal to conduct a larger trial using a randomized therapeutic approach, the current emphasis on drug-based trials is a formidable obstacle. Other major obstacles are patient initiative and adherence. For now, investigators must work with anecdotal data. Examination of gene expression patterns in mitochondria and mutations in ketolytic and glycolytic enzymes may prove useful in selecting potentially responsive patients. Combining this dietary approach with standard chemotherapeutic and radiotherapeutic options may help improve tumor response, and further research is desperately needed.
Click to read the digital edition.
As early as 500 bc , fasting was used as an effective treatment for many medical ailments. Fasting continued into modern times, and in 1910, Guelpa and Marie proposed fasting as an antiepilepsy treatment. In 1921, Woodyatt noted that starvation or use of high-fat, low-carbohydrate diets in individuals with no significant medical comorbidities yielded acetone and β-hydroxybutyrate, 2 energy sources produced by the liver in the absence of glucose. A low-carbohydrate, high-fat diet was thought to be an alternative to fasting or starvation, having many of the same desired effects while continuing to nourish healthy cells. The term ketogenic diet (KD) was later coined by Wilder and Peterman, who formulated the fat-to-carbohydrate ratio that is still used today: 1 g protein per kg of body weight in children and 10 to 15 g carbohydrates daily, and fat for the remainder of calories. Both investigators reported that this diet improved their patients’ mentation and cognition.1
Use of the KD as an adjuvant to cancer therapy also began to emerge. In 1922, Braunstein noted that glucose disappeared from the urine of patients with diabetes after they were diagnosed with cancer, suggesting that glucose is recruited to cancerous areas where it is consumed at higher than normal rates. During that same time, Nobel laureate Otto Warburg found that cancer cells thrive on glycolysis, producing high lactate levels, even in the presence of abundant oxygen. Warburg conducted many in vitro and animal experiments demonstrating this outcome, known as the Warburg effect.
By the mid-20th century, KD use in epilepsy treatment and cancer research had waned. However, in the mid-to-late 1990s, with the establishment of the Charlie Foundation, the diet slowly started regaining recognition.1 Results of many in vitro and animal studies were reported, and human data also began to accumulate.
Mechanism of Action
Glucose normally stimulates pancreatic β cells to release insulin, which allows glucose to enter cells and provide energy. With high carbohydrate and glucose intake, the pancreas increasingly secretes more insulin, which promotes the interaction of growth hormone receptors and growth hormones to produce insulin-like growth factor 1 (IGF-1) in the liver—promoting cell growth and proliferation, which can be detrimental to patients with cancer. Overexpression of glucose transporters 1 and 3 (Glut-1, Glut-3) also occurs in many cancers and corresponds to the degree of glucose uptake in aggressive tumors, as seen on positron emission tomography (PET).2 Overexpression of hexokinase, the rate-limiting enzyme of glycolysis, further drives production of pyruvate and lactate, which cause reactive oxygen species damage. Translocation of the hexokinase rate-limiting enzyme from the cytosol into the outer mitochondrial membrane, where it interacts with voltage-dependent anion channels, can disrupt caspasedependent cytochrome release, which suppresses the apoptotic pathways of cancer cells and makes cancer more resistant to chemotherapy.3
When glucose is scarce, the body senses the need to make an alternative form of energy for cells. The liver then produces ketones and fatty acids, which provide for normal cells but do not benefit cancer cells. Cancer cells have dysfunctional mitochondria and possibly electron transport chain defects, which disrupt normal adenosine triphosphate (ATP) production from the mitochondria. The result is that the cancer cells become heavily dependent on ATP coming from the less efficient process of glycolysis (Figure 1).
Ketogenic diets mimic the fasting state, wherein the body responds to the lack of glucose by producing ketones for energy. Excess lactate production, which is part of the Warburg effect, compensates for ATP production defects caused by dysfunctional mitochondrial oxidative phosphorylation.2,4 The resulting tumor dependence on glucose can be exploited with KD use. Ketogenic diets selectively starve tumors by providing the fat and protein that otherwise could not be used by glucose-dependent tumor cells.
In KDs, the 4:1 ratio of high fat to low carbohydrates mimics the metabolic effects of starvation (Figure 2). These diets slow cancer by inhibiting insulin/IGF and downstream intracellular signaling pathways, such as
Ketogenic Diet Benefits
There are concerns about providing protein to patients who are at risk for renal problems. However, mouse models of diabetic nephropathy showed improved renal function with KD use. The hypothesis was that KD use, which produces prolonged elevated 3-β-hydroxybutyric acid levels, also reduces molecular responses to glucose and consequently reduces renal damage.6 Use of the diet also reduced pain and inflammation in both juvenile and adult rats. Mechanisms of action were thought to be reduced reactive oxygen species and increased central adenosine levels.7,8
Adverse Effects
Dieting is of concern to cancer patients worried about additional weight loss. The standard diet is made up predominantly of carbohydrates and is high in caloric value (Figure 3). Beck and Tisdale investigated the effect of KD use on delaying cachexia in mouse models of colon carcinoma. They found that dieting was more effective than insulin in reversing weight loss and had the added effect of reducing tumor size.7 Moreover, Tisdale and colleagues found that KD use in cachectic cancer patients could promote weight gain.8
A possible explanation is that healthy tissue nutrition selectively delays tumor growth, while cancer cells are deprived of nutrition (carbohydrates). A therapeutic weight plateau should follow initial weight loss with KD, in contrast to pathologic rapid weight loss in non-KD patients.9 Kidney stones, gout, and symptomatic hypoglycemia were also potential expected adverse effects (AEs).
Case Reports
In 1962, the New York Department of Mental Hygiene published an article about 2 women whose metastatic cancers disappeared after a series of daily hypoglycemiainduced insulin comas (brief and reversible). These patients could not undergo conventional electric shock therapy, hence, the medically induced psychotherapy. Not only did their psychotic and depressive symptoms resolve, but their cancers (grossly visible cervical cancer and metastatic melanoma) became undetectable as early as 2 months into treatment.10 Zuccoli and colleagues reported on a glioblastoma that was effectively treated with temozolomide oral chemotherapy after the patient was weaned off steroids. The patient had a radiographic response and good tumor control for about a year, before discontinuing the diet. She transitioned to chemotherapy, which included bevacizumab (antivascular endothelial growth factor), but the disease progressed and she died.11 In addition, during 8 weeks of ketogenic dieting, 2 pediatric female astrocytoma patients experienced improved mood and showed decreased glucose uptake on PET-computed tomography (PET-CT) of their tumor sites. One of these patients continued the diet and remained disease-free another 12 months.12 These early case reports provide compelling evidence for further research into the role of glucose metabolism in cancer
treatment.
Current Research
Compared with normal mice, tumor-bearing mice placed on a low-carbohydrate diet had lower glucose, insulin, and lactic acid levels.4 An in vivo microdialysis study of patients with head and neck cancers found decreased lactic acid levels in the tumor tissues after a 4-day KD.13 Most early studies and case reports involving KD in cancer focused on brain tumors.11,14-16 Human clinical studies on this diet in cancer care were limited to small, nonrandomized, brief trials (4-12 weeks) or single case studies (Table).11,17-20
Fine and colleagues conducted a 4-week feasibility study of the low-carbohydrate modified Atkins KD (≤ 20 g of carbohydrates daily) in PET-positive advanced cancer patients with solid tumors. There was a correlation between insulin levels and ketosis but not IGF. Stable disease or partial remission (measured standardized uptake value) on PET-CT correlated with 3-fold higher ketosis (but not weight loss or reduced caloric intake) relative to patients with progressive disease.17 In the ERGO trial, Rieger and colleagues studied 20 relapsed glioblastoma patients who were on KD supplemented with plant oils.18 Calories were unlimited. The primary endpoint was percentage of patients who discontinued the diet. Mean weight loss was significant, but quality of life (QOL) was maintained. The investigators also studied the effects of KD alone or combined with bevacizumab in a mouse glioblastoma model. In this mouse study, KD alone had no effect; however, it increased median survival from 52 to 58 days (P < .05) with bevacizumab.
In a pilot study, Schmidt and colleagues provided KD plus oil-protein shakes as snacks to 7 of 16 patients with a variety of advanced metastatic cancers. Mean weight loss was statistically significant, blood lipid or cholesterol levels remained stable, some QOL measures improved, and there were no severe AEs.19 Schwartz and colleagues reviewed the cases of 32 glioma patients treated with energy restricted KDs —5 from case reports, 19 from a clinical trial by Rieger and colleagues, and 8 from Champ.18,20,21 They also reported on 2 of their own glioma cases treated with the energy-restricted KD and studied tissues for expression of key ketolytic enzymes. Prolonged remission was noted from 4 months to more than 5 years in these case reports.20
The VA Pittsburgh Healthcare System safety that trial enrolled 17 patients, 11 of whom were evaluated. Mean weight loss was significant, and weight loss of ≥ 10% was noted in responders (stable or improved disease) compared with nonresponders. Three patients dieted longer than 16 weeks (survival, 80-116 weeks). One of these patients was alive at 121 weeks.22
The safety and feasibility data suggest that cancer patients can tolerate KD use. Investigators should consider combining the KD approach with standard treatment modalities, including chemotherapy and radiation.
Already investigators have conducted in vitro studies of the effect of gene expression of ketolytic and glycolytic enzymes within tumor tissue on KD response. Tumors expressing mutations in key mitochondrial oxidative phosphorylation enzymes were more likely to respond to KD use than were tumors without mutations.16,23
Ongoing Clinical Trials
Duke University has initiated a randomized study (NCT00932672) of the Atkins diet and androgen deprivation therapy for patients with prostate cancer. Tel Aviv Sourasky Medical Center in Israel is recruiting previously treated chemoradiation patients with high-grade glial tumors for an open-label study (NCT01092247) of the efficacy of KD in preventing tumor growth and recurrence. St. Joseph’s Hospital and Medical Center (Phoenix, AZ) is recruiting newly diagnosed glioblastoma patients for a phase 1/2 prospective trial (NCT02046187) involving upfront resection followed by KD with radiotherapy and concurrent temozolomide, followed by adjuvant temozolomide chemotherapy. The primary endpoint is number of patients with AEs and the secondary endpoints are overall survival, time to progression, and QOL. The University of Iowa is recruiting patients with prostate cancer and non-small cell lung cancer for 2 phase 1 studies (NCT01419483 and NCT01419587, respectively) involving KD using Nutritia KetoCal 4:1 (Gaithersburg, MD).
Conclusion
Data from case reports and trials suggest KD use is safe and tolerable for patients with cancer. Although it would be ideal to conduct a larger trial using a randomized therapeutic approach, the current emphasis on drug-based trials is a formidable obstacle. Other major obstacles are patient initiative and adherence. For now, investigators must work with anecdotal data. Examination of gene expression patterns in mitochondria and mutations in ketolytic and glycolytic enzymes may prove useful in selecting potentially responsive patients. Combining this dietary approach with standard chemotherapeutic and radiotherapeutic options may help improve tumor response, and further research is desperately needed.
Click to read the digital edition.
1. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(suppl 8):3-5.
2. Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31(1):5-12.
3. Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40(3):171-182.
4. Ho VW, Leung K, Hsu A, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484-4493.
5. Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711-1720.
6. Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One. 2011;6(4):e18604.
7. Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59(5):677-681.
8. Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39-43.
9. Tan-Shalaby J, Seyfried T. Ketogenic diet in advanced cancer: a pilot feasibility and safety trial in the Veterans Affairs cancer patient population. J Clin Trials. 2013;3(4):149.
10. Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36:261-270.
11. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). 2010;7:33.
12. Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202-208.
13. Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993-1001.
14. Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28(8):1002-1008.
15. Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer. 2013;65(6):843-849.
16. Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 2013;10(1):47.
17. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028-1035.
18. Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843-1852. [published correction appears in Int J Oncol. 2014;45(6):2605.
19. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54.
20. Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3.
21. Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125-131.
22. Tan-Shalaby J, Carrick J, Edinger K, et al. Modified ketogenic diet in advanced malignancies—final results of a safety and feasibility trial within the Veterans Affairs Healthcare System. J Clin Oncol. 2016;34(suppl): Abstract e23173.
23. Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578-585.
1. Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49(suppl 8):3-5.
2. Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31(1):5-12.
3. Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40(3):171-182.
4. Ho VW, Leung K, Hsu A, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71(13):4484-4493.
5. Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP. Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int J Cancer. 2014;135(7):1711-1720.
6. Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One. 2011;6(4):e18604.
7. Beck SA, Tisdale MJ. Effect of insulin on weight loss and tumour growth in a cachexia model. Br J Cancer. 1989;59(5):677-681.
8. Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56(1):39-43.
9. Tan-Shalaby J, Seyfried T. Ketogenic diet in advanced cancer: a pilot feasibility and safety trial in the Veterans Affairs cancer patient population. J Clin Trials. 2013;3(4):149.
10. Koroljow S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatr Q. 1962;36:261-270.
11. Zuccoli G, Marcello N, Pisanello A, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond). 2010;7:33.
12. Nebeling LC, Miraldi F, Shurin SB, Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. J Am Coll Nutr. 1995;14(2):202-208.
13. Masino SA, Ruskin DN. Ketogenic diets and pain. J Child Neurol. 2013;28(8):993-1001.
14. Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28(8):1002-1008.
15. Schroeder U, Himpe B, Pries R, Vonthein R, Nitsch S, Wollenberg B. Decline of lactate in tumor tissue after ketogenic diet: in vivo microdialysis study in patients with head and neck cancer. Nutr Cancer. 2013;65(6):843-849.
16. Chang HT, Olson LK, Schwartz KA. Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab (Lond). 2013;10(1):47.
17. Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028-1035.
18. Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;44(6):1843-1852. [published correction appears in Int J Oncol. 2014;45(6):2605.
19. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54.
20. Schwartz K, Chang HT, Nikolai M, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab. 2015;3:3.
21. Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125-131.
22. Tan-Shalaby J, Carrick J, Edinger K, et al. Modified ketogenic diet in advanced malignancies—final results of a safety and feasibility trial within the Veterans Affairs Healthcare System. J Clin Oncol. 2016;34(suppl): Abstract e23173.
23. Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer. 2006;94(4):578-585.
Treatment and Management of Multiple Myeloma (FULL)
Early Treatment and Diagnosis
Dr. Ascensão. An area that is becoming very important is identifying and separating smoldering multiple myeloma (SMM) from multiple myeloma (MM) and determining when to start treatment. At the Washington DC VAMC (DCVAMC) we started early on bisphosphonates and thalidomide without much benefit, but perhaps we were treating the wrong disease.
Dr. Mehta. Identifying patients as early as possible is often the best way to start. Treating early disease is easier than treating late disease, and it avoids all the complications. The problem is we don’t want to treat too many people because some of the people with SMM will never develop overt MM and, therefore, may not need treatment. We don’t have benign treatment yet. Whatever treatment we decide to use is going to carry adverse effects and toxicity.
So the trick is identifying those patients with SMM who are likely to progress in a finite period and, therefore, can be helped by treating early to avoid the complications of late diagnosis. We know that early treatment for patients with high-risk SMM helps. In a report from Lancet Oncology, early treatment with lenalidomide and dexamethasone reduces time to progression.1 There are other reports that treating early reduces time to progression.
So how do we identify those patients who are going to progress? We have a few clues. We know that patients who have a myeloma spike of > 1.5 g/dL are more likely to progress than others…The more discordant the / ratio from 1:1, the higher the risk for progression. And if that ratio is 1:100 or more, that would be a risk factor for progression.
We know from the work of Mayo Clinic researchers that if there are ≥ 60% of plasma cells in the bone marrow then it is a risk factor for progression. And we know from early studies that magnetic resonance imaging (MRI) detection of bone lesions, even long before they become detectable by X-ray, also is a risk factor for rapid development to myeloma.
...Methods such as genotyping, which we do here at the University of Arkansas for Medical Sciences, even in patients with MGUS (monoclonal gammopathy of undetermined significance), can identify high-risk patients, but that is not the standard of care yet. But it may become the standard of care in the days to come.
Another thing to think about for MGUS patients: Are there ways to identify what causes MGUS patients to evolve to SMM and then to overt myeloma, and to develop means of interrupting the progression cascade? There have been clinical trials on treatments (eg, bisphosphonates, thalidomide, aspirin, and cyclooxygenase inhibitors), but we haven’t found any safe, good treatment to prevent progression yet. With better technologies, we may be able to do that.
Dr. Ascensão. At the DCVAMC often we receive consults for a patient who had a little anemia, diabetes, renal disease, and the serum protein electrophoresis reveals a very small peak. How often should you follow patients? Do you do a complete workup the moment you see an MGUS or do you wait until they reach SMM?
Dr. Mehta. I don’t think every patient needs a complete workup. If you have obviously identifiable reasons for the anemia or the renal failure, then it’s less likely to be suspicious for myeloma. But patients with M spikes > 1 g/dL deserve a workup with a bone marrow aspirate and biopsy and at least bone X-rays, although MRIs would be even better.
I would differentiate based on the amount of M protein. Higher M protein patients deserve to have at least a bone marrow aspirate and bone study. Patients
with M protein > 1g/dL deserve to be seen every 3 to 4 months. I see patients with tiny little peaks every 6 months. And then, after 1 or 2 years, I turn over their care to the primary care doctor to follow. If we had research protocols to look at those patients and find the methods for progression, which I had at one point, then of course, we could see them more often and try to unravel the mystery.
Use of Imaging
Dr. Ascensão. That’s pretty close to what we do at DCVAMC. What do you think is the role for a bone survey as opposed to MRIs and positron emission tomography (PET) scans in this setting?
Dr. Mehta. In the real world X-rays are more accessible and much less expensive. So for the patient with very low risk who doesn’t have any complaints and
who has a low M spike, I think a bone survey is adequate. But you need about 30% to 40% bone destruction before you’re going to find anything on the X-ray.
MRIs are much more sensitive, plus they tell you about bone marrow involvement, but that should be reserved for the patient who has symptoms or a high
M protein. At Central Arkansas Veterans Healthcare System we simply can’t get PET scans for myeloma patients. At the myeloma center across the street from us, PET scans are used for routine evaluations.
Dr. Chauncey. I agree with Dr. Mehta. At VA Puget Sound Healthcare System (VAPSHCS) there isn’t a problem getting PET scans, but we probably get far fewer
scans than Arkansas. I still like the skeletal survey because it directs you where to look for potential pathologic fracture. It’s definitely not as sensitive as the dedicated myeloma MRI, but it’s a lot easier to get at VAPSHCS, especially as a screening tool.
Dr. Ascensão. Right, I believe there are some issues about the number of osteolytic lesions that may drive diagnosis.
Dr. Mehta. For patients with high M protein, I always request MRI. But the correlation is poorer in patients who have lower M protein. I try to limit it to the patients who have symptoms or high M protein, but I don’t have any evidence-based data to prove that’s the right way.
Dr. Ascensão. If you were going to start treatment of SMM that you believe is evolving to a more regular myeloma, do you do anything different than you would for any of the patients that you have identified as having active myeloma? Do you have different protocols for those patients as opposed to patients who present de novo with active myeloma?
Dr. Mehta. Those patients should be treated with the same drugs, an IMiD and a steroid. And the question is plus or minus a proteasome inhibitor. Studies have shown that an IMiD with a steroid gets much better results than using observation alone. Whether you would get even better results with the proteasome inhibitor remains to be seen. Maybe we can do that study.
Dr. Chauncey. We strive to identify high-risk SMM patients and treat them accordingly. Alternatively, physicians are pulling the trigger for therapy earlier and earlier and when they come for transplant with a diagnosis of MM, it is critical to review the initial diagnostic information. Most transplant centers have experience with this phenomena and know that they don’t want to transplant a non-high-risk SMM or any MGUS. However, by the time the patient is referred for transplantation, the initial clinical data are sometimes obscured or inaccessible.
Dr. Ascensão. We also look into the bone bearing areas, which allows us to make sure that if the patient has hip problems, we can work on how to approach them, whether we want to radiate those patients to prevent fractures.
Use of Bisphosphonates
Dr. Cosgriff. Myeloma metastasizes to bone, and it is one of the common sites of metastatic disease. It poses some interesting complications, whether it is from hypercalcemia due to metastatic sites, or pain syndromes. Bisphosphonates are indicated for myeloma, and they have been for years. Interestingly, unlike some of the other disease, the use of bisphosphonates induces apoptosis in myeloma. So we have seen some disease control with these agents.
The 2 bisphosphonates that are available for use are pamidronate and zoledronic acid. At the VA Portland Health Care System (VAPORHCS), we have been
using pamidronate exclusively for individuals with myeloma. There was a 2003 paper that evaluated the use of bisphosphonates for skeletal-related events in myeloma and in patients with metastatic breast cancer.2 In the subset analysis of myeloma patients with the bisphosphonates, there was no difference between pamidronate and zoledronic acid.
At the time, zoledronic acid was significantly more expensive than pamidronate, and so VAPORHCS opted to use pamidronate as a cost-saving measure. But there are the other reasons for picking pamidronate: Zoledronic acid has some dose recommendations and guidelines for individuals with renal failure, which is often a significant problem in patients with myeloma as well. To get around dose adjustments that need to be made for zoledronic acid, VAPORHCS switched to pamidronate, which is looser with the recommendations on renal failure.
Earlier use criteria, like the National Comprehensive Cancer Network guidelines, stated that if the renal failure was due to the disease itself and not some other outlying factor, a full 90-mg dose of pamidronate could still be used. That comment has since been removed. We still pay attention to it and reduce pamidronate dosing to 60 mg for patients with renal failure.
The prices for zoledronic acid have dropped significantly since it became a generic. The nice thing about zoledronic acid is that it has a short infusion time of 15 minutes. As chair space becomes a problem—VAPHCS has significant issues with that—zoledronic acid looks more and more attractive. The FDA label states that pamidronate should be infused over 4 hours, but VAPHCS typically has been infusing it for 3 hours.
It should be noted that denosumab (XGEVA), a monoclonal antibody that also is targeted for hypercalcemia, has been specifically excluded for myeloma. It
has no FDA indication for myeloma. It does have an indication for hypercalcemia. Whether or not you can state that the patient with myeloma is hypercalcemic, and that’s the reason you want to use it, it starts crossing into some gray area. The drug is still significantly more expensive and it seems to have similar efficacy rates compared with both pamidronate and zoledronic acid, so VAPHCS limits its use to individuals who would otherwise be contraindicated to zoledronic acid or pamidronate due to renal failure.
Dr. Ascensão. How often do you give it, every month, every 3 months?
Dr. Cosgriff. Currently, VAPORHCS is giving bisphosphonates every month whether in the chemotherapy unit or in the short stay unit. We are starting to reevaluate that. I have heard some emerging data that suggest we can use it once a quarter and get the same results. Those data are still emerging. It would be nice to be able to reduce the infusion frequency. But bisphosphonates adhere to bone and get incorporated into the bone matrix and stay there for an extended period of time, upwards of 6 months to a year, as with zoledronic acid.
Osteonecrosis
Dr. Ascensão. Do you require dental clearance prior to first dose?
Dr. Cosgriff. Bisphosphonates have a warning for 2% incidence of osteonecrosis of the jaw. Risk factors for the development of osteonecrosis of the jaw include poor dentition or major dental work, like extractions and illfitting dentures but not necessarily root canals. Ill-fitting dentures tend to rub on the gums and irritate the bone layer underneath. It’s the irritation of the bone that’s the biggest risk factor for osteonecrosis of the jaw.
We require that patients see the dentist because we’ve had individuals develop osteonecrosis eventhough we thought they had good dentition. If a patient is seeing a dentist outside of the VA system, we ask them to notify their dentist that they’re receiving bisphosphonates. Because of the risk and because we’ve had some individuals with good dentition develop it, VAPORHCS requires all patients, particularly those who are receiving zoledronic acid, to have dental evaluations. Denosumab also has a listed 2% incidence of osteonecrosis of the jaw, so those individuals also need to be evaluated by our dental service.
Dr. Ascensão. The DCVAMC has the same problem. I have a patient that presented primarily with a plasmacytoma, and we tried to get him to see the dentist. The dentist said, ‘You’ve got to get your teeth pulled.’ The patient has tried to see outside dentists and is finding all kinds of excuses because he would like to have implants.
Dr. Cosgriff. Anytime that you somehow damage or irritate that bone, that becomes a risk factor for the development of osteonecrosis. And for those individuals, we delay the bisphosphonate. If they’re having pain syndrome, we try to support them with opiates. We would love to be able to use nonsteroidal anti-inflammatory drugs—they have really good efficacy against bone pain—but renal function and renal failures prevent the use of those in a majority of patients. We start bisphosphonates as soon as dental clears them.
Dr. Mehta. Isn’t there a contraindication for denosumab and some evidence that it may worsen MM outcomes?
Dr. Cosgriff. When the drug first came on the market, it specifically stated in the package insert that it is not to be used in MM (it doesn’t state it specifically anymore). There is a thought that maybe some underlying mechanism exists that might stimulate some of the myeloma problems, which is why I get a little concerned when people say, “Well, I’m using it for hypercalcemia, I’m not using it to treat or to prevent a skeletal-related event in patients with myeloma.” That becomes a gray area and in that type of situation, I would recommend treating the hypercalcemia with a single dose and then switching the
patient to a bisphosphonate.
Dr. Mehta. And of course, bisphosphonates also lower calcium. They can be used to treat hypercalcemia.
Dr. Cosgriff. Yes. Zoledronic acid does have limitations in renal failure, though pamidronate doesn’t have quite the same limitations. The VAPORHCS tries to
use exclusively for hypercalcemia as well. The data show that when using zoledronic acid compared with pamidronate, you end up with the same outcomes as far as hypercalcemia. The zoledronic acid onset of action is a little faster, around 12 to 24 hours vs 48 to 72 hours with pamidronate, but you can get around that by using calcitonin over a short period; 48 hours is typically the maximum efficacy for calcitonin in treating hypercalcemia. So we use pamidronate in place of that, supplementing with calcitonin.
The result is that at 7 days, pamidronate and zoledronic acid show the same efficacy rates for treating hypercalcemia. But the renal function sometimes prevents us from doing that. Denosumab does become an option for hypercalcemia, but again, I caution against its use for treating hypercalcemia in patients with myeloma due to the risk of advancing the myeloma.
Bone Marrow Transplant
Dr. Ascensão. Do you transplant for 1 or 2 bone marrows? What’s the best maintenance regimen postallograft, and when do you start? Do you use lenalidomide the first month of the transplant or do you wait until day 100?
Dr. Chauncey. From my perspective, hematopoietic stem cell transplantation has never really lost prominence. It is true that the concept of marrow transplantation for MM has been around for more than 20 years for those patients with first best response (Note that I’ll use best response rather than first remission). The concept was developed in an era when we had much less effective therapy, and in comparative trials, progressionfree survival was consistently superior and occasionally, overall survival was better with transplantation. As treatments got better, responses got better, and there were regular questions as to whether we still needed transplantation. But the data show that as responses got better, the progression-free survivals continued to improve, and transplantation still adds something to initial therapy.
Probably the most current data are from the Dana Farber- IFM trial for which Nikhil Munshi, MD, is an investigator. The trial includes induction with lenalidomide/bortezomib/dexamethasone, which is one of the more aggressive induction regimens. When upfront transplant vs delayed transplant are compared, it seems the preliminary data still favor having an upfront transplant after initial induction therapy.
The consensus is that autologous transplantation adds to the better response that we see with better induction therapy. Overall survival has become a less accessible endpoint since the initial trials, and that’s really a consequence of having better salvage therapy, and the confounding effects of subsequent treatments. We have so many options for salvage therapy that it’s now very hard to look at overall survival as an endpoint in trials of initial therapy.
A sometimes contentious question when it comes to payers, and less so in the VA, is how many transplants to do as part of initial therapy? Little Rock and the French did some of the pioneering work on tandem transplants. The BMT CTN 0702–StaMINA trial looks at this directly, and is mature and should be presented soon [Editorial Note: Preliminary results were presented at the American Society of Hematology meeting on December 6, 2016].
The approach at VAPSHCS and most other transplant centers has typically been to harvest a sufficient quantity of peripheral blood stem cells to do 2 transplants. If less than a very good partial response is achieved after the first transplant, then we do a second transplant in tandem fashion.
One exception would be for plasma cell leukemia, which is very aggressive. In that case, we would routinely perform tandem transplantation. We are unlikely to ever have a randomized trial that compares 1 vs 2 transplants in that particular setting.
Another question is whether a second autologous transplantation can be useful in a nontandem fashion, and there is a large amount of retrospective data about its use as salvage treatment. In eras when there were not as many effective therapies, salvage autologous transplant was more attractive. As new therapies came along, its use has somewhat waned, but there’s been renewed interest because dose-intensive melphalan with autologous rescue is relatively safe and not cross-resistant to other therapies. It also offers the option of a drug holiday after the transplant, whereas salvage drug therapy is typically continuous.
There is no universal agreement on nontandem second transplantation, there are no consistent algorithms to say when it is appropriate, but it’s worth discussing with the transplant programs, especially if there is a lot of toxicity with current salvage therapy.
The last question is the role of allogeneic transplantation, and while I’m generally a proponent of allogeneic transplantation for many diseases, in spite of some really significant efforts, the majority of allogeneic data for MM has not been very positive. The large BMT-CTN 0102 trial compared tandem autologous transplant at first response to a single autologous transplantation followed by reduced-intensity autologous transplantation from a matched sibling. This study was limited in part by enrollment bias, but the published results did not favor an allogeneic approach.3 Although there was less relapse in the allogeneic setting, the mortality of allogeneic transplant was not overcome by the decrease in relapse. Neither progression-free or overall survivals at 3 years were better in the allogeneic group.
Despite small studies showing feasibility and promising results, it’s currently very hard to advocate for allogeneic transplantation in MM. There are certainly centers that continue to have their own approach, with some in the U.S. that are pioneering tweaks on allogeneic regimens and graft engineering, but the data are typically small and anecdotal. That doesn’t mean that there won’t ultimately be a better way to do allogeneic transplantation in MM, but rather that we don’t currently know the best way to approach this strategy.
Next Steps in Myeloma Treatment
Dr. Ascensão. There are some people who are now starting to talk about a cure for myeloma. I’m not sure we’re there yet. Certainly, it’s a chronic disease that, if we can take care of the complications and maybe by starting treatment early. I’m not sure Agent Orange-exposed patients do better or worse. That’s something that needs to be researched if we can find a way to compare within this group and within the type of treatment that patients get.
Is it reasonable to start looking for minimal residual disease in cells? Should we shoot for the best response? I think one of the points that Dr. Chauncey made a number of times, and I agree, is that our patient population may not be able to tolerate some of the more aggressive therapies. Perhaps we need to find a slightly different version of this algorithm for VA patients.
Dr. Chauncey. There’s a diverse biology for both veterans and nonveterans alike. There are patients for whom a deeper response will lead to longer remission and better survival, and there are others whose disease will smolder with a lower tumor burden and not progress quickly. A lot of the early gene expression profiling data on this comes from Little Rock. Unfortunately, determination of an individual’s biology is not readily accessible in the clinic, and we are typically unable to clearly define each patient’s inherent disease biology.
Dr. Mehta. We just don’t have the answers as to exactly what to do with the information that we get except watch more closely and treat a little bit earlier. We don’t even know the significance of minimal residue disease and how often to test for it and if it correlates truly with longer-term survival. These are great research questions. We need to accumulate the data and try to analyze it. We need to participate in the big data programs.
Dr. Ascensão. The other thing, of course, is now we have new immunotherapy approaches beyond transplant, which includes some of the checkpoint inhibitors and there’s some exciting data coming out. So I think the future looks good.
We all are committed to treating our patients, our veterans, to the best of our abilities. And I think the VA has done a very good job in allowing us to do this for our patients and allowing us to provide the best treatments available out there.
Click here to read the digital edition.
1. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136.
2. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735-1744.
3. Krishnan A, Pasquini MC, Logan B, et al; Blood Marrow Transplant Clinical Trials Network (BMT CTN). Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195-11203.
Early Treatment and Diagnosis
Dr. Ascensão. An area that is becoming very important is identifying and separating smoldering multiple myeloma (SMM) from multiple myeloma (MM) and determining when to start treatment. At the Washington DC VAMC (DCVAMC) we started early on bisphosphonates and thalidomide without much benefit, but perhaps we were treating the wrong disease.
Dr. Mehta. Identifying patients as early as possible is often the best way to start. Treating early disease is easier than treating late disease, and it avoids all the complications. The problem is we don’t want to treat too many people because some of the people with SMM will never develop overt MM and, therefore, may not need treatment. We don’t have benign treatment yet. Whatever treatment we decide to use is going to carry adverse effects and toxicity.
So the trick is identifying those patients with SMM who are likely to progress in a finite period and, therefore, can be helped by treating early to avoid the complications of late diagnosis. We know that early treatment for patients with high-risk SMM helps. In a report from Lancet Oncology, early treatment with lenalidomide and dexamethasone reduces time to progression.1 There are other reports that treating early reduces time to progression.
So how do we identify those patients who are going to progress? We have a few clues. We know that patients who have a myeloma spike of > 1.5 g/dL are more likely to progress than others…The more discordant the / ratio from 1:1, the higher the risk for progression. And if that ratio is 1:100 or more, that would be a risk factor for progression.
We know from the work of Mayo Clinic researchers that if there are ≥ 60% of plasma cells in the bone marrow then it is a risk factor for progression. And we know from early studies that magnetic resonance imaging (MRI) detection of bone lesions, even long before they become detectable by X-ray, also is a risk factor for rapid development to myeloma.
...Methods such as genotyping, which we do here at the University of Arkansas for Medical Sciences, even in patients with MGUS (monoclonal gammopathy of undetermined significance), can identify high-risk patients, but that is not the standard of care yet. But it may become the standard of care in the days to come.
Another thing to think about for MGUS patients: Are there ways to identify what causes MGUS patients to evolve to SMM and then to overt myeloma, and to develop means of interrupting the progression cascade? There have been clinical trials on treatments (eg, bisphosphonates, thalidomide, aspirin, and cyclooxygenase inhibitors), but we haven’t found any safe, good treatment to prevent progression yet. With better technologies, we may be able to do that.
Dr. Ascensão. At the DCVAMC often we receive consults for a patient who had a little anemia, diabetes, renal disease, and the serum protein electrophoresis reveals a very small peak. How often should you follow patients? Do you do a complete workup the moment you see an MGUS or do you wait until they reach SMM?
Dr. Mehta. I don’t think every patient needs a complete workup. If you have obviously identifiable reasons for the anemia or the renal failure, then it’s less likely to be suspicious for myeloma. But patients with M spikes > 1 g/dL deserve a workup with a bone marrow aspirate and biopsy and at least bone X-rays, although MRIs would be even better.
I would differentiate based on the amount of M protein. Higher M protein patients deserve to have at least a bone marrow aspirate and bone study. Patients
with M protein > 1g/dL deserve to be seen every 3 to 4 months. I see patients with tiny little peaks every 6 months. And then, after 1 or 2 years, I turn over their care to the primary care doctor to follow. If we had research protocols to look at those patients and find the methods for progression, which I had at one point, then of course, we could see them more often and try to unravel the mystery.
Use of Imaging
Dr. Ascensão. That’s pretty close to what we do at DCVAMC. What do you think is the role for a bone survey as opposed to MRIs and positron emission tomography (PET) scans in this setting?
Dr. Mehta. In the real world X-rays are more accessible and much less expensive. So for the patient with very low risk who doesn’t have any complaints and
who has a low M spike, I think a bone survey is adequate. But you need about 30% to 40% bone destruction before you’re going to find anything on the X-ray.
MRIs are much more sensitive, plus they tell you about bone marrow involvement, but that should be reserved for the patient who has symptoms or a high
M protein. At Central Arkansas Veterans Healthcare System we simply can’t get PET scans for myeloma patients. At the myeloma center across the street from us, PET scans are used for routine evaluations.
Dr. Chauncey. I agree with Dr. Mehta. At VA Puget Sound Healthcare System (VAPSHCS) there isn’t a problem getting PET scans, but we probably get far fewer
scans than Arkansas. I still like the skeletal survey because it directs you where to look for potential pathologic fracture. It’s definitely not as sensitive as the dedicated myeloma MRI, but it’s a lot easier to get at VAPSHCS, especially as a screening tool.
Dr. Ascensão. Right, I believe there are some issues about the number of osteolytic lesions that may drive diagnosis.
Dr. Mehta. For patients with high M protein, I always request MRI. But the correlation is poorer in patients who have lower M protein. I try to limit it to the patients who have symptoms or high M protein, but I don’t have any evidence-based data to prove that’s the right way.
Dr. Ascensão. If you were going to start treatment of SMM that you believe is evolving to a more regular myeloma, do you do anything different than you would for any of the patients that you have identified as having active myeloma? Do you have different protocols for those patients as opposed to patients who present de novo with active myeloma?
Dr. Mehta. Those patients should be treated with the same drugs, an IMiD and a steroid. And the question is plus or minus a proteasome inhibitor. Studies have shown that an IMiD with a steroid gets much better results than using observation alone. Whether you would get even better results with the proteasome inhibitor remains to be seen. Maybe we can do that study.
Dr. Chauncey. We strive to identify high-risk SMM patients and treat them accordingly. Alternatively, physicians are pulling the trigger for therapy earlier and earlier and when they come for transplant with a diagnosis of MM, it is critical to review the initial diagnostic information. Most transplant centers have experience with this phenomena and know that they don’t want to transplant a non-high-risk SMM or any MGUS. However, by the time the patient is referred for transplantation, the initial clinical data are sometimes obscured or inaccessible.
Dr. Ascensão. We also look into the bone bearing areas, which allows us to make sure that if the patient has hip problems, we can work on how to approach them, whether we want to radiate those patients to prevent fractures.
Use of Bisphosphonates
Dr. Cosgriff. Myeloma metastasizes to bone, and it is one of the common sites of metastatic disease. It poses some interesting complications, whether it is from hypercalcemia due to metastatic sites, or pain syndromes. Bisphosphonates are indicated for myeloma, and they have been for years. Interestingly, unlike some of the other disease, the use of bisphosphonates induces apoptosis in myeloma. So we have seen some disease control with these agents.
The 2 bisphosphonates that are available for use are pamidronate and zoledronic acid. At the VA Portland Health Care System (VAPORHCS), we have been
using pamidronate exclusively for individuals with myeloma. There was a 2003 paper that evaluated the use of bisphosphonates for skeletal-related events in myeloma and in patients with metastatic breast cancer.2 In the subset analysis of myeloma patients with the bisphosphonates, there was no difference between pamidronate and zoledronic acid.
At the time, zoledronic acid was significantly more expensive than pamidronate, and so VAPORHCS opted to use pamidronate as a cost-saving measure. But there are the other reasons for picking pamidronate: Zoledronic acid has some dose recommendations and guidelines for individuals with renal failure, which is often a significant problem in patients with myeloma as well. To get around dose adjustments that need to be made for zoledronic acid, VAPORHCS switched to pamidronate, which is looser with the recommendations on renal failure.
Earlier use criteria, like the National Comprehensive Cancer Network guidelines, stated that if the renal failure was due to the disease itself and not some other outlying factor, a full 90-mg dose of pamidronate could still be used. That comment has since been removed. We still pay attention to it and reduce pamidronate dosing to 60 mg for patients with renal failure.
The prices for zoledronic acid have dropped significantly since it became a generic. The nice thing about zoledronic acid is that it has a short infusion time of 15 minutes. As chair space becomes a problem—VAPHCS has significant issues with that—zoledronic acid looks more and more attractive. The FDA label states that pamidronate should be infused over 4 hours, but VAPHCS typically has been infusing it for 3 hours.
It should be noted that denosumab (XGEVA), a monoclonal antibody that also is targeted for hypercalcemia, has been specifically excluded for myeloma. It
has no FDA indication for myeloma. It does have an indication for hypercalcemia. Whether or not you can state that the patient with myeloma is hypercalcemic, and that’s the reason you want to use it, it starts crossing into some gray area. The drug is still significantly more expensive and it seems to have similar efficacy rates compared with both pamidronate and zoledronic acid, so VAPHCS limits its use to individuals who would otherwise be contraindicated to zoledronic acid or pamidronate due to renal failure.
Dr. Ascensão. How often do you give it, every month, every 3 months?
Dr. Cosgriff. Currently, VAPORHCS is giving bisphosphonates every month whether in the chemotherapy unit or in the short stay unit. We are starting to reevaluate that. I have heard some emerging data that suggest we can use it once a quarter and get the same results. Those data are still emerging. It would be nice to be able to reduce the infusion frequency. But bisphosphonates adhere to bone and get incorporated into the bone matrix and stay there for an extended period of time, upwards of 6 months to a year, as with zoledronic acid.
Osteonecrosis
Dr. Ascensão. Do you require dental clearance prior to first dose?
Dr. Cosgriff. Bisphosphonates have a warning for 2% incidence of osteonecrosis of the jaw. Risk factors for the development of osteonecrosis of the jaw include poor dentition or major dental work, like extractions and illfitting dentures but not necessarily root canals. Ill-fitting dentures tend to rub on the gums and irritate the bone layer underneath. It’s the irritation of the bone that’s the biggest risk factor for osteonecrosis of the jaw.
We require that patients see the dentist because we’ve had individuals develop osteonecrosis eventhough we thought they had good dentition. If a patient is seeing a dentist outside of the VA system, we ask them to notify their dentist that they’re receiving bisphosphonates. Because of the risk and because we’ve had some individuals with good dentition develop it, VAPORHCS requires all patients, particularly those who are receiving zoledronic acid, to have dental evaluations. Denosumab also has a listed 2% incidence of osteonecrosis of the jaw, so those individuals also need to be evaluated by our dental service.
Dr. Ascensão. The DCVAMC has the same problem. I have a patient that presented primarily with a plasmacytoma, and we tried to get him to see the dentist. The dentist said, ‘You’ve got to get your teeth pulled.’ The patient has tried to see outside dentists and is finding all kinds of excuses because he would like to have implants.
Dr. Cosgriff. Anytime that you somehow damage or irritate that bone, that becomes a risk factor for the development of osteonecrosis. And for those individuals, we delay the bisphosphonate. If they’re having pain syndrome, we try to support them with opiates. We would love to be able to use nonsteroidal anti-inflammatory drugs—they have really good efficacy against bone pain—but renal function and renal failures prevent the use of those in a majority of patients. We start bisphosphonates as soon as dental clears them.
Dr. Mehta. Isn’t there a contraindication for denosumab and some evidence that it may worsen MM outcomes?
Dr. Cosgriff. When the drug first came on the market, it specifically stated in the package insert that it is not to be used in MM (it doesn’t state it specifically anymore). There is a thought that maybe some underlying mechanism exists that might stimulate some of the myeloma problems, which is why I get a little concerned when people say, “Well, I’m using it for hypercalcemia, I’m not using it to treat or to prevent a skeletal-related event in patients with myeloma.” That becomes a gray area and in that type of situation, I would recommend treating the hypercalcemia with a single dose and then switching the
patient to a bisphosphonate.
Dr. Mehta. And of course, bisphosphonates also lower calcium. They can be used to treat hypercalcemia.
Dr. Cosgriff. Yes. Zoledronic acid does have limitations in renal failure, though pamidronate doesn’t have quite the same limitations. The VAPORHCS tries to
use exclusively for hypercalcemia as well. The data show that when using zoledronic acid compared with pamidronate, you end up with the same outcomes as far as hypercalcemia. The zoledronic acid onset of action is a little faster, around 12 to 24 hours vs 48 to 72 hours with pamidronate, but you can get around that by using calcitonin over a short period; 48 hours is typically the maximum efficacy for calcitonin in treating hypercalcemia. So we use pamidronate in place of that, supplementing with calcitonin.
The result is that at 7 days, pamidronate and zoledronic acid show the same efficacy rates for treating hypercalcemia. But the renal function sometimes prevents us from doing that. Denosumab does become an option for hypercalcemia, but again, I caution against its use for treating hypercalcemia in patients with myeloma due to the risk of advancing the myeloma.
Bone Marrow Transplant
Dr. Ascensão. Do you transplant for 1 or 2 bone marrows? What’s the best maintenance regimen postallograft, and when do you start? Do you use lenalidomide the first month of the transplant or do you wait until day 100?
Dr. Chauncey. From my perspective, hematopoietic stem cell transplantation has never really lost prominence. It is true that the concept of marrow transplantation for MM has been around for more than 20 years for those patients with first best response (Note that I’ll use best response rather than first remission). The concept was developed in an era when we had much less effective therapy, and in comparative trials, progressionfree survival was consistently superior and occasionally, overall survival was better with transplantation. As treatments got better, responses got better, and there were regular questions as to whether we still needed transplantation. But the data show that as responses got better, the progression-free survivals continued to improve, and transplantation still adds something to initial therapy.
Probably the most current data are from the Dana Farber- IFM trial for which Nikhil Munshi, MD, is an investigator. The trial includes induction with lenalidomide/bortezomib/dexamethasone, which is one of the more aggressive induction regimens. When upfront transplant vs delayed transplant are compared, it seems the preliminary data still favor having an upfront transplant after initial induction therapy.
The consensus is that autologous transplantation adds to the better response that we see with better induction therapy. Overall survival has become a less accessible endpoint since the initial trials, and that’s really a consequence of having better salvage therapy, and the confounding effects of subsequent treatments. We have so many options for salvage therapy that it’s now very hard to look at overall survival as an endpoint in trials of initial therapy.
A sometimes contentious question when it comes to payers, and less so in the VA, is how many transplants to do as part of initial therapy? Little Rock and the French did some of the pioneering work on tandem transplants. The BMT CTN 0702–StaMINA trial looks at this directly, and is mature and should be presented soon [Editorial Note: Preliminary results were presented at the American Society of Hematology meeting on December 6, 2016].
The approach at VAPSHCS and most other transplant centers has typically been to harvest a sufficient quantity of peripheral blood stem cells to do 2 transplants. If less than a very good partial response is achieved after the first transplant, then we do a second transplant in tandem fashion.
One exception would be for plasma cell leukemia, which is very aggressive. In that case, we would routinely perform tandem transplantation. We are unlikely to ever have a randomized trial that compares 1 vs 2 transplants in that particular setting.
Another question is whether a second autologous transplantation can be useful in a nontandem fashion, and there is a large amount of retrospective data about its use as salvage treatment. In eras when there were not as many effective therapies, salvage autologous transplant was more attractive. As new therapies came along, its use has somewhat waned, but there’s been renewed interest because dose-intensive melphalan with autologous rescue is relatively safe and not cross-resistant to other therapies. It also offers the option of a drug holiday after the transplant, whereas salvage drug therapy is typically continuous.
There is no universal agreement on nontandem second transplantation, there are no consistent algorithms to say when it is appropriate, but it’s worth discussing with the transplant programs, especially if there is a lot of toxicity with current salvage therapy.
The last question is the role of allogeneic transplantation, and while I’m generally a proponent of allogeneic transplantation for many diseases, in spite of some really significant efforts, the majority of allogeneic data for MM has not been very positive. The large BMT-CTN 0102 trial compared tandem autologous transplant at first response to a single autologous transplantation followed by reduced-intensity autologous transplantation from a matched sibling. This study was limited in part by enrollment bias, but the published results did not favor an allogeneic approach.3 Although there was less relapse in the allogeneic setting, the mortality of allogeneic transplant was not overcome by the decrease in relapse. Neither progression-free or overall survivals at 3 years were better in the allogeneic group.
Despite small studies showing feasibility and promising results, it’s currently very hard to advocate for allogeneic transplantation in MM. There are certainly centers that continue to have their own approach, with some in the U.S. that are pioneering tweaks on allogeneic regimens and graft engineering, but the data are typically small and anecdotal. That doesn’t mean that there won’t ultimately be a better way to do allogeneic transplantation in MM, but rather that we don’t currently know the best way to approach this strategy.
Next Steps in Myeloma Treatment
Dr. Ascensão. There are some people who are now starting to talk about a cure for myeloma. I’m not sure we’re there yet. Certainly, it’s a chronic disease that, if we can take care of the complications and maybe by starting treatment early. I’m not sure Agent Orange-exposed patients do better or worse. That’s something that needs to be researched if we can find a way to compare within this group and within the type of treatment that patients get.
Is it reasonable to start looking for minimal residual disease in cells? Should we shoot for the best response? I think one of the points that Dr. Chauncey made a number of times, and I agree, is that our patient population may not be able to tolerate some of the more aggressive therapies. Perhaps we need to find a slightly different version of this algorithm for VA patients.
Dr. Chauncey. There’s a diverse biology for both veterans and nonveterans alike. There are patients for whom a deeper response will lead to longer remission and better survival, and there are others whose disease will smolder with a lower tumor burden and not progress quickly. A lot of the early gene expression profiling data on this comes from Little Rock. Unfortunately, determination of an individual’s biology is not readily accessible in the clinic, and we are typically unable to clearly define each patient’s inherent disease biology.
Dr. Mehta. We just don’t have the answers as to exactly what to do with the information that we get except watch more closely and treat a little bit earlier. We don’t even know the significance of minimal residue disease and how often to test for it and if it correlates truly with longer-term survival. These are great research questions. We need to accumulate the data and try to analyze it. We need to participate in the big data programs.
Dr. Ascensão. The other thing, of course, is now we have new immunotherapy approaches beyond transplant, which includes some of the checkpoint inhibitors and there’s some exciting data coming out. So I think the future looks good.
We all are committed to treating our patients, our veterans, to the best of our abilities. And I think the VA has done a very good job in allowing us to do this for our patients and allowing us to provide the best treatments available out there.
Click here to read the digital edition.
Early Treatment and Diagnosis
Dr. Ascensão. An area that is becoming very important is identifying and separating smoldering multiple myeloma (SMM) from multiple myeloma (MM) and determining when to start treatment. At the Washington DC VAMC (DCVAMC) we started early on bisphosphonates and thalidomide without much benefit, but perhaps we were treating the wrong disease.
Dr. Mehta. Identifying patients as early as possible is often the best way to start. Treating early disease is easier than treating late disease, and it avoids all the complications. The problem is we don’t want to treat too many people because some of the people with SMM will never develop overt MM and, therefore, may not need treatment. We don’t have benign treatment yet. Whatever treatment we decide to use is going to carry adverse effects and toxicity.
So the trick is identifying those patients with SMM who are likely to progress in a finite period and, therefore, can be helped by treating early to avoid the complications of late diagnosis. We know that early treatment for patients with high-risk SMM helps. In a report from Lancet Oncology, early treatment with lenalidomide and dexamethasone reduces time to progression.1 There are other reports that treating early reduces time to progression.
So how do we identify those patients who are going to progress? We have a few clues. We know that patients who have a myeloma spike of > 1.5 g/dL are more likely to progress than others…The more discordant the / ratio from 1:1, the higher the risk for progression. And if that ratio is 1:100 or more, that would be a risk factor for progression.
We know from the work of Mayo Clinic researchers that if there are ≥ 60% of plasma cells in the bone marrow then it is a risk factor for progression. And we know from early studies that magnetic resonance imaging (MRI) detection of bone lesions, even long before they become detectable by X-ray, also is a risk factor for rapid development to myeloma.
...Methods such as genotyping, which we do here at the University of Arkansas for Medical Sciences, even in patients with MGUS (monoclonal gammopathy of undetermined significance), can identify high-risk patients, but that is not the standard of care yet. But it may become the standard of care in the days to come.
Another thing to think about for MGUS patients: Are there ways to identify what causes MGUS patients to evolve to SMM and then to overt myeloma, and to develop means of interrupting the progression cascade? There have been clinical trials on treatments (eg, bisphosphonates, thalidomide, aspirin, and cyclooxygenase inhibitors), but we haven’t found any safe, good treatment to prevent progression yet. With better technologies, we may be able to do that.
Dr. Ascensão. At the DCVAMC often we receive consults for a patient who had a little anemia, diabetes, renal disease, and the serum protein electrophoresis reveals a very small peak. How often should you follow patients? Do you do a complete workup the moment you see an MGUS or do you wait until they reach SMM?
Dr. Mehta. I don’t think every patient needs a complete workup. If you have obviously identifiable reasons for the anemia or the renal failure, then it’s less likely to be suspicious for myeloma. But patients with M spikes > 1 g/dL deserve a workup with a bone marrow aspirate and biopsy and at least bone X-rays, although MRIs would be even better.
I would differentiate based on the amount of M protein. Higher M protein patients deserve to have at least a bone marrow aspirate and bone study. Patients
with M protein > 1g/dL deserve to be seen every 3 to 4 months. I see patients with tiny little peaks every 6 months. And then, after 1 or 2 years, I turn over their care to the primary care doctor to follow. If we had research protocols to look at those patients and find the methods for progression, which I had at one point, then of course, we could see them more often and try to unravel the mystery.
Use of Imaging
Dr. Ascensão. That’s pretty close to what we do at DCVAMC. What do you think is the role for a bone survey as opposed to MRIs and positron emission tomography (PET) scans in this setting?
Dr. Mehta. In the real world X-rays are more accessible and much less expensive. So for the patient with very low risk who doesn’t have any complaints and
who has a low M spike, I think a bone survey is adequate. But you need about 30% to 40% bone destruction before you’re going to find anything on the X-ray.
MRIs are much more sensitive, plus they tell you about bone marrow involvement, but that should be reserved for the patient who has symptoms or a high
M protein. At Central Arkansas Veterans Healthcare System we simply can’t get PET scans for myeloma patients. At the myeloma center across the street from us, PET scans are used for routine evaluations.
Dr. Chauncey. I agree with Dr. Mehta. At VA Puget Sound Healthcare System (VAPSHCS) there isn’t a problem getting PET scans, but we probably get far fewer
scans than Arkansas. I still like the skeletal survey because it directs you where to look for potential pathologic fracture. It’s definitely not as sensitive as the dedicated myeloma MRI, but it’s a lot easier to get at VAPSHCS, especially as a screening tool.
Dr. Ascensão. Right, I believe there are some issues about the number of osteolytic lesions that may drive diagnosis.
Dr. Mehta. For patients with high M protein, I always request MRI. But the correlation is poorer in patients who have lower M protein. I try to limit it to the patients who have symptoms or high M protein, but I don’t have any evidence-based data to prove that’s the right way.
Dr. Ascensão. If you were going to start treatment of SMM that you believe is evolving to a more regular myeloma, do you do anything different than you would for any of the patients that you have identified as having active myeloma? Do you have different protocols for those patients as opposed to patients who present de novo with active myeloma?
Dr. Mehta. Those patients should be treated with the same drugs, an IMiD and a steroid. And the question is plus or minus a proteasome inhibitor. Studies have shown that an IMiD with a steroid gets much better results than using observation alone. Whether you would get even better results with the proteasome inhibitor remains to be seen. Maybe we can do that study.
Dr. Chauncey. We strive to identify high-risk SMM patients and treat them accordingly. Alternatively, physicians are pulling the trigger for therapy earlier and earlier and when they come for transplant with a diagnosis of MM, it is critical to review the initial diagnostic information. Most transplant centers have experience with this phenomena and know that they don’t want to transplant a non-high-risk SMM or any MGUS. However, by the time the patient is referred for transplantation, the initial clinical data are sometimes obscured or inaccessible.
Dr. Ascensão. We also look into the bone bearing areas, which allows us to make sure that if the patient has hip problems, we can work on how to approach them, whether we want to radiate those patients to prevent fractures.
Use of Bisphosphonates
Dr. Cosgriff. Myeloma metastasizes to bone, and it is one of the common sites of metastatic disease. It poses some interesting complications, whether it is from hypercalcemia due to metastatic sites, or pain syndromes. Bisphosphonates are indicated for myeloma, and they have been for years. Interestingly, unlike some of the other disease, the use of bisphosphonates induces apoptosis in myeloma. So we have seen some disease control with these agents.
The 2 bisphosphonates that are available for use are pamidronate and zoledronic acid. At the VA Portland Health Care System (VAPORHCS), we have been
using pamidronate exclusively for individuals with myeloma. There was a 2003 paper that evaluated the use of bisphosphonates for skeletal-related events in myeloma and in patients with metastatic breast cancer.2 In the subset analysis of myeloma patients with the bisphosphonates, there was no difference between pamidronate and zoledronic acid.
At the time, zoledronic acid was significantly more expensive than pamidronate, and so VAPORHCS opted to use pamidronate as a cost-saving measure. But there are the other reasons for picking pamidronate: Zoledronic acid has some dose recommendations and guidelines for individuals with renal failure, which is often a significant problem in patients with myeloma as well. To get around dose adjustments that need to be made for zoledronic acid, VAPORHCS switched to pamidronate, which is looser with the recommendations on renal failure.
Earlier use criteria, like the National Comprehensive Cancer Network guidelines, stated that if the renal failure was due to the disease itself and not some other outlying factor, a full 90-mg dose of pamidronate could still be used. That comment has since been removed. We still pay attention to it and reduce pamidronate dosing to 60 mg for patients with renal failure.
The prices for zoledronic acid have dropped significantly since it became a generic. The nice thing about zoledronic acid is that it has a short infusion time of 15 minutes. As chair space becomes a problem—VAPHCS has significant issues with that—zoledronic acid looks more and more attractive. The FDA label states that pamidronate should be infused over 4 hours, but VAPHCS typically has been infusing it for 3 hours.
It should be noted that denosumab (XGEVA), a monoclonal antibody that also is targeted for hypercalcemia, has been specifically excluded for myeloma. It
has no FDA indication for myeloma. It does have an indication for hypercalcemia. Whether or not you can state that the patient with myeloma is hypercalcemic, and that’s the reason you want to use it, it starts crossing into some gray area. The drug is still significantly more expensive and it seems to have similar efficacy rates compared with both pamidronate and zoledronic acid, so VAPHCS limits its use to individuals who would otherwise be contraindicated to zoledronic acid or pamidronate due to renal failure.
Dr. Ascensão. How often do you give it, every month, every 3 months?
Dr. Cosgriff. Currently, VAPORHCS is giving bisphosphonates every month whether in the chemotherapy unit or in the short stay unit. We are starting to reevaluate that. I have heard some emerging data that suggest we can use it once a quarter and get the same results. Those data are still emerging. It would be nice to be able to reduce the infusion frequency. But bisphosphonates adhere to bone and get incorporated into the bone matrix and stay there for an extended period of time, upwards of 6 months to a year, as with zoledronic acid.
Osteonecrosis
Dr. Ascensão. Do you require dental clearance prior to first dose?
Dr. Cosgriff. Bisphosphonates have a warning for 2% incidence of osteonecrosis of the jaw. Risk factors for the development of osteonecrosis of the jaw include poor dentition or major dental work, like extractions and illfitting dentures but not necessarily root canals. Ill-fitting dentures tend to rub on the gums and irritate the bone layer underneath. It’s the irritation of the bone that’s the biggest risk factor for osteonecrosis of the jaw.
We require that patients see the dentist because we’ve had individuals develop osteonecrosis eventhough we thought they had good dentition. If a patient is seeing a dentist outside of the VA system, we ask them to notify their dentist that they’re receiving bisphosphonates. Because of the risk and because we’ve had some individuals with good dentition develop it, VAPORHCS requires all patients, particularly those who are receiving zoledronic acid, to have dental evaluations. Denosumab also has a listed 2% incidence of osteonecrosis of the jaw, so those individuals also need to be evaluated by our dental service.
Dr. Ascensão. The DCVAMC has the same problem. I have a patient that presented primarily with a plasmacytoma, and we tried to get him to see the dentist. The dentist said, ‘You’ve got to get your teeth pulled.’ The patient has tried to see outside dentists and is finding all kinds of excuses because he would like to have implants.
Dr. Cosgriff. Anytime that you somehow damage or irritate that bone, that becomes a risk factor for the development of osteonecrosis. And for those individuals, we delay the bisphosphonate. If they’re having pain syndrome, we try to support them with opiates. We would love to be able to use nonsteroidal anti-inflammatory drugs—they have really good efficacy against bone pain—but renal function and renal failures prevent the use of those in a majority of patients. We start bisphosphonates as soon as dental clears them.
Dr. Mehta. Isn’t there a contraindication for denosumab and some evidence that it may worsen MM outcomes?
Dr. Cosgriff. When the drug first came on the market, it specifically stated in the package insert that it is not to be used in MM (it doesn’t state it specifically anymore). There is a thought that maybe some underlying mechanism exists that might stimulate some of the myeloma problems, which is why I get a little concerned when people say, “Well, I’m using it for hypercalcemia, I’m not using it to treat or to prevent a skeletal-related event in patients with myeloma.” That becomes a gray area and in that type of situation, I would recommend treating the hypercalcemia with a single dose and then switching the
patient to a bisphosphonate.
Dr. Mehta. And of course, bisphosphonates also lower calcium. They can be used to treat hypercalcemia.
Dr. Cosgriff. Yes. Zoledronic acid does have limitations in renal failure, though pamidronate doesn’t have quite the same limitations. The VAPORHCS tries to
use exclusively for hypercalcemia as well. The data show that when using zoledronic acid compared with pamidronate, you end up with the same outcomes as far as hypercalcemia. The zoledronic acid onset of action is a little faster, around 12 to 24 hours vs 48 to 72 hours with pamidronate, but you can get around that by using calcitonin over a short period; 48 hours is typically the maximum efficacy for calcitonin in treating hypercalcemia. So we use pamidronate in place of that, supplementing with calcitonin.
The result is that at 7 days, pamidronate and zoledronic acid show the same efficacy rates for treating hypercalcemia. But the renal function sometimes prevents us from doing that. Denosumab does become an option for hypercalcemia, but again, I caution against its use for treating hypercalcemia in patients with myeloma due to the risk of advancing the myeloma.
Bone Marrow Transplant
Dr. Ascensão. Do you transplant for 1 or 2 bone marrows? What’s the best maintenance regimen postallograft, and when do you start? Do you use lenalidomide the first month of the transplant or do you wait until day 100?
Dr. Chauncey. From my perspective, hematopoietic stem cell transplantation has never really lost prominence. It is true that the concept of marrow transplantation for MM has been around for more than 20 years for those patients with first best response (Note that I’ll use best response rather than first remission). The concept was developed in an era when we had much less effective therapy, and in comparative trials, progressionfree survival was consistently superior and occasionally, overall survival was better with transplantation. As treatments got better, responses got better, and there were regular questions as to whether we still needed transplantation. But the data show that as responses got better, the progression-free survivals continued to improve, and transplantation still adds something to initial therapy.
Probably the most current data are from the Dana Farber- IFM trial for which Nikhil Munshi, MD, is an investigator. The trial includes induction with lenalidomide/bortezomib/dexamethasone, which is one of the more aggressive induction regimens. When upfront transplant vs delayed transplant are compared, it seems the preliminary data still favor having an upfront transplant after initial induction therapy.
The consensus is that autologous transplantation adds to the better response that we see with better induction therapy. Overall survival has become a less accessible endpoint since the initial trials, and that’s really a consequence of having better salvage therapy, and the confounding effects of subsequent treatments. We have so many options for salvage therapy that it’s now very hard to look at overall survival as an endpoint in trials of initial therapy.
A sometimes contentious question when it comes to payers, and less so in the VA, is how many transplants to do as part of initial therapy? Little Rock and the French did some of the pioneering work on tandem transplants. The BMT CTN 0702–StaMINA trial looks at this directly, and is mature and should be presented soon [Editorial Note: Preliminary results were presented at the American Society of Hematology meeting on December 6, 2016].
The approach at VAPSHCS and most other transplant centers has typically been to harvest a sufficient quantity of peripheral blood stem cells to do 2 transplants. If less than a very good partial response is achieved after the first transplant, then we do a second transplant in tandem fashion.
One exception would be for plasma cell leukemia, which is very aggressive. In that case, we would routinely perform tandem transplantation. We are unlikely to ever have a randomized trial that compares 1 vs 2 transplants in that particular setting.
Another question is whether a second autologous transplantation can be useful in a nontandem fashion, and there is a large amount of retrospective data about its use as salvage treatment. In eras when there were not as many effective therapies, salvage autologous transplant was more attractive. As new therapies came along, its use has somewhat waned, but there’s been renewed interest because dose-intensive melphalan with autologous rescue is relatively safe and not cross-resistant to other therapies. It also offers the option of a drug holiday after the transplant, whereas salvage drug therapy is typically continuous.
There is no universal agreement on nontandem second transplantation, there are no consistent algorithms to say when it is appropriate, but it’s worth discussing with the transplant programs, especially if there is a lot of toxicity with current salvage therapy.
The last question is the role of allogeneic transplantation, and while I’m generally a proponent of allogeneic transplantation for many diseases, in spite of some really significant efforts, the majority of allogeneic data for MM has not been very positive. The large BMT-CTN 0102 trial compared tandem autologous transplant at first response to a single autologous transplantation followed by reduced-intensity autologous transplantation from a matched sibling. This study was limited in part by enrollment bias, but the published results did not favor an allogeneic approach.3 Although there was less relapse in the allogeneic setting, the mortality of allogeneic transplant was not overcome by the decrease in relapse. Neither progression-free or overall survivals at 3 years were better in the allogeneic group.
Despite small studies showing feasibility and promising results, it’s currently very hard to advocate for allogeneic transplantation in MM. There are certainly centers that continue to have their own approach, with some in the U.S. that are pioneering tweaks on allogeneic regimens and graft engineering, but the data are typically small and anecdotal. That doesn’t mean that there won’t ultimately be a better way to do allogeneic transplantation in MM, but rather that we don’t currently know the best way to approach this strategy.
Next Steps in Myeloma Treatment
Dr. Ascensão. There are some people who are now starting to talk about a cure for myeloma. I’m not sure we’re there yet. Certainly, it’s a chronic disease that, if we can take care of the complications and maybe by starting treatment early. I’m not sure Agent Orange-exposed patients do better or worse. That’s something that needs to be researched if we can find a way to compare within this group and within the type of treatment that patients get.
Is it reasonable to start looking for minimal residual disease in cells? Should we shoot for the best response? I think one of the points that Dr. Chauncey made a number of times, and I agree, is that our patient population may not be able to tolerate some of the more aggressive therapies. Perhaps we need to find a slightly different version of this algorithm for VA patients.
Dr. Chauncey. There’s a diverse biology for both veterans and nonveterans alike. There are patients for whom a deeper response will lead to longer remission and better survival, and there are others whose disease will smolder with a lower tumor burden and not progress quickly. A lot of the early gene expression profiling data on this comes from Little Rock. Unfortunately, determination of an individual’s biology is not readily accessible in the clinic, and we are typically unable to clearly define each patient’s inherent disease biology.
Dr. Mehta. We just don’t have the answers as to exactly what to do with the information that we get except watch more closely and treat a little bit earlier. We don’t even know the significance of minimal residue disease and how often to test for it and if it correlates truly with longer-term survival. These are great research questions. We need to accumulate the data and try to analyze it. We need to participate in the big data programs.
Dr. Ascensão. The other thing, of course, is now we have new immunotherapy approaches beyond transplant, which includes some of the checkpoint inhibitors and there’s some exciting data coming out. So I think the future looks good.
We all are committed to treating our patients, our veterans, to the best of our abilities. And I think the VA has done a very good job in allowing us to do this for our patients and allowing us to provide the best treatments available out there.
Click here to read the digital edition.
1. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136.
2. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735-1744.
3. Krishnan A, Pasquini MC, Logan B, et al; Blood Marrow Transplant Clinical Trials Network (BMT CTN). Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195-11203.
1. Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136.
2. Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735-1744.
3. Krishnan A, Pasquini MC, Logan B, et al; Blood Marrow Transplant Clinical Trials Network (BMT CTN). Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195-11203.
Timeliness of Lung Cancer Diagnosis and Treatment (FULL)
Lung cancer is the leading cause of cancer-related deaths worldwide and causes more deaths than do colorectal, breast, and prostate cancers combined.1 An estimated 155,870 Americans are expected to die of lung cancer in 2017, and these deaths account for about 26% of all cancer deaths.1 The overall 5-year survival rate for patients with lung cancer is 16.8%.2 However, this rate varies considerably, from 54% for those with early-stage cancer to 26.5% for those with locally advanced cancer and 4% for those with distant metastases.2
The Institute of Medicine’s Committee on Quality Health Care in America recognized timeliness of care as 1 of 6 important dimensions of health care quality.3 Delays in timely diagnosis and treatment of cancer, especially lung cancer, can result in significant emotional distress, impaired quality of life, increased use of health care resources, and, arguably, increased cost of care.4 In addition, delayed diagnosis of cancer can lead to negligence litigation.4
In the U.S., there are no federal standardized guidelines regarding timeliness of lung cancer care. In 2000, the RAND Corporation, a research organization, published several quality indicators recommending lung cancer diagnoses be established within 2 months after initial abnormal chest radiographs and treatment be offered within 6 weeks after diagnosis.5
Using these recommendations as benchmarks, a quality improvement study was conducted to determine the time lines of comprehensive lung cancer care at the Dayton VAMC in Ohio. The primary aim of the study was to evaluate adherence to the RAND criteria (the only U.S.- based guidelines) for the diagnosis and treatment of lung cancer in Dayton VAMC patients. The secondary aim was to assess the effect of preoperative cardiopulmonary rehabilitation on timeliness of treatment. The authors plan to use the results of the study to guide and improve cancer practices at the Dayton VAMC.
Methods
The authors conducted a retrospective study of a series of 121 consecutive patients who had lung cancer that was confirmed at the Dayton VAMC with a cytohistologic diagnosis between January 2011 and December 2013. The study was approved by the Dayton VAMC Research and Development committee and the Wright State University Institutional Review Board. After data collection and review, all patient identifiers were replaced with sequential numbering.
The Dayton VAMC is a 356-bed facility serving 16 counties and > 50,000 patients. Lung cancer diagnosis and management are collaboratively undertaken by various Dayton VAMC departments, including pulmonology, radiology, interventional radiology, pathology, thoracic surgery, medical oncology, radiation oncology, and palliative care. The facility, fully equipped with scanners for positron emission tomography and magnetic resonance imaging, provides comprehensive cancer care without the need for referrals to outside facilities for any part of care from diagnosis to end of life.
The study patients were identified from the Dayton VAMC tumor registry. Patients with only biopsy-confirmed malignancy were included in the study. Patients who did not follow up before biopsy or did not pursue treatment after biopsy confirmation were excluded from analysis where appropriate.
Patient data collected included age, sex, presenting symptom, histology, cancer stage, treatment modality, cardiopulmonary rehabilitation, and if applicable,
tumor size. Patients were retrospectively followed for 3 years. Charts reviewed did not include outcomes information. Historically, delays have been categorized as provider delays, patient delays, or system delays. Provider delay stems from the primary care provider’s (PCP) failure to investigate a presenting symptom further, patient delay from the patient’s failure to seek medical care or to follow through on medical advice in a timely manner, and system delay from the health care organization’s failure to obtain imaging or biopsy results in a timely manner. Assessment of system delay is focused on quality improvement at a treatment center.
In the present study, the primary aim was to assess system delay. The authors analyzed delay during 3 different periods: time to diagnosis (interval from date an abnormality was found on chest radiograph or computed tomography scan to date of tissue diagnosis); time to treatment initiation (interval from date of histopathologic diagnosis to date of treatment initiation); and time from date of initial abnormal imaging to date of treatment initiation. With RAND criteria applied, time to diagnosis longer than 60 days was considered diagnostic delay, time to treatment longer than 42 days was considered treatment delay, and the sum of these periods (102 days) was considered total delay.5 Patients with diagnosis and treatment intervals that fell within these criteria were considered in adherence with the RAND criteria.
Means and standard deviations were reported for continuous variables and counts and percentages for categoric variables. Calculations were performed with IBM SPSS 21.0 (Armonk, NY).
Results
Of the 121 patients, 118 (97.5%) were men, and 3 (2.5%) were women. Mean (SD) age was 68.5 (8.9) years (range, 50-89 years). Of the 121 patients, 88 (73%) opted to be treated at Dayton VAMC, and the other 33 opted to receive palliative care only (20) or to be treated at an outside facility (13). The group of 33 patients was included in the analyses of diagnostic delay but not treatment delay (Table 1).
Mean (SD) time to diagnosis was 35.5 (31.6) days (n = 111), mean (SD) time to treatment was 55.9 (46.3) days (n = 87), and mean (SD) total time was 92.7 (62.1) days (n = 82). Table 2 lists data regarding adherence to RAND guidelines for diagnostic delay (diagnostic timeliness), treatment delay (treatment timeliness), and total delay (total timeliness) for 3 groups of patients: all patients, and those who did and did not participate in cardiopulmonary rehabilitation. Of all patients, 82.9% met the RAND diagnostic time standard, 51.7% met the treatment time standard, and 61.0% met the total time standard. As expected, the proportions of patients meeting the RAND standards were higher for the group that participated in cardiopulmonary rehabilitation: 89.2%, 58.6%, and 72.3% for diagnostic, treatment, and total time, respectively.
Table 3 lists data regarding adherence to RAND guidelines by treatment modality, excluding the patients who participated in cardiopulmonary rehabilitation. With the exception of surgery only, all other primary treatment modalities were marked by 90% or higher adherence in meeting diagnostic timeliness. However, treatment initiation adherence was lower: 40% to 76.2% in the nonsurgical groups and 25% in the surgery group.
The cardiopulmonary rehabilitation group was analyzed separately (Table 4).
For diagnostic time, patients with advancedstage (IIIB/IV) disease and patients with small cell lung cancer (SCLC) had adherence of at least 87.5%, and patients with stage II/IIIA disease had adherence of at least 80% (Table 5). However, only 62.5% of patients with stage I disease were adherent to the diagnostic guideline. Patients with stage IIIA/IV disease and patients with SCLC had the best performance for the treatment guideline, with no group < 60% adherent.
Discussion
Several international study groups have recommended establishing standards for timely care of patients with known or suspected lung cancer.5-10 According to a study in Brazil, an application interval exceeding 30 days is considered patient delay.6 The Swedish Lung Cancer Study Group recommended that diagnostic tests be completed within 4 weeks in 80% of all patients and that treatment be started within 2 weeks thereafter.7 The recommendations from Canada are a maximum of 4 weeks between first PCP visit and diagnosis and 2 weeks for surgery.8 The British Thoracic Society recommended that all patients have completed diagnostic tests within 2 weeks of request with specific time intervals for treatment initiation based on treatment modality.9
Numerous studies10-27 and 2 meta-analyses28,29 have addressed timeliness of care or associations between timeliness and clinical outcomes, and 1 study27 tested an intervention to improve timeliness of care in patients with lung cancer. These studies varied in important ways because of the complexities inherent in the diagnosis and management of lung cancer, patient- and system-specific factors, and the definitions used for “delays.”
For this study, the authors examined Dayton VAMC adherence to RAND guidelines regarding time from imaging to diagnosis, time from diagnosis to treatment initiation, and time from abnormal imaging to treatment initiation. Separately, the authors examined the impact of cardiopulmonary rehabilitation on delay.
The 89.2% adherence to RAND diagnostic time guidelines (avoiding diagnostic delay) in this study’s population (excluding patients who participated in cardiopulmonary rehabilitation) was better than the 59% and 68.8% found in 2 larger VAMC studies.24,26 In addition, adherence to the RAND time standard for the interval from diagnosis to treatment initiation (avoiding treatment delay) was similar between this study (58.6%) and one of those studies (62.2%), which was a multicenter investigation.26 The other VAMC study, a singleinstitution investigation, was superior to the present study with respect to avoiding treatment delay (adherence, 76% vs 58.6%).24 These overtly similar results suggest that system delay is accompanied by patient delay involving time for decision making, acute illness, missed appointments, and so forth.
In this study, timeliness was most disappointing for the patients who underwent primary surgical resection. Surgery patients’ poor diagnostic timeliness rate (14.3%) was likely multifactorial, involving additional pretissue procurement staging workup, including more imaging scans, invasive procedures (mediastinoscopy), and repeat biopsy in cases of negative initial biopsy results. In addition, patients who initially qualified for definitive surgical resection of early-stage lung cancer likely underwent extensive postdiagnostic workup that included pulmonary function testing, split-function studies, and preoperative assessment for cardiac clearance. In a single-center prospective study, O’Rourke and Edwards found that progression of early-stage lung cancer after a median system delay of 94 days resulted in decreased candidacy for curative therapy in 21% of patients.22
Surgical resection was previously thought to be the best curative option for early-stage lung cancer. However, recent data on use of stereotactic ablative radiotherapy (SABR) in early-stage non-SCLC (NSCLC) showed equivalent outcomes. In a pooled study, Chang and colleagues found 3-year overall survival of 95% in their SABR group and 79% in their surgery group.30 Given these data, findings from this study, and significant delays experienced by surgery patients, it is worth considering whether SABR should be used more often.
The benefits of preoperative cardiopulmonary rehabilitation in the surgical outcomes of patients with lung cancer have been well described.31-36 Bobbio and colleagues noted that shortterm cardiopulmonary rehabilitation might improve the surgical candidacy of patients with chronic obstructive pulmonary disease.34 Moreover, Benzo and colleagues reported that 10 rehabilitation sessions resulted in shorter chest tube time and decreased length of stay, both of which lower postoperative morbidity and cost.33
In this study, although patients who had preoperative cardiopulmonary rehabilitation experienced diagnostic delays for reasons similar to those found for patients who did not have cardiopulmonary surgery, rehabilitation led to significant delays in treatment initiation, and more than three-fourths of patients experienced delay. This delay was hardly unexpected, but only 11 of the 18 patients who had preoperative cardiopulmonary rehabilitation underwent surgical resection. As anticipated, rehabilitation did not improve the surgical candidacy of the other patients.
Regarding staging, this study is consistent with international studies in which advanced-stage NSCLC and SCLC cases were diagnosed earlier, presumably because of the associated symptom burden.15,20,23 These results are also comparable to those of previous VAMC studies.24-26
The authors of this quality improvement study will apply its findings when they appoint a cancer care coordinator (nurse coordinator or clinical nurse specialist) at Dayton VAMC. The services of a cancer care coordinator have significantly reduced system delay elsewhere. The VA Connecticut Healthcare System added a cancer care coordinator in 2007, and by 2010, time from lung cancer suspicion to treatment was reduced to 55 days from 136 days in 2003.27
Limitations
First, the study was retrospective and used a small sample from a single institution; therefore, the results may not be generalizable to other health care settings. Second, the study included a small but significant number of patients who underwent serial imaging for asymptomatic pulmonary nodules; including this subgroup in the analyses of diagnostic delay negatively affected the results. Third, the effect of delay on survival was not evaluated.
Conclusion
This quality improvement lung cancer delay study examined adherence to the diagnostic and treatment time intervals recommended by the RAND Corporation in 2000.5 Although most of its patients received histopathologic confirmation within prespecified parameters, significant delays occurred for surgical patients, presumably as a result of extensive preoperative testing and optimization. Without improved surgical candidacy for most patients enrolled in preoperative cardiopulmonary rehabilitation, the authors urge facilities to consider alternatives to surgery. Given recent advances in SABR outcomes in early-stage NSCLC, SABR is worth considering as an upfront option in cases of equivocal performance status or early-stage NSCLC.
The authors will use information from this study as a baseline at the Dayton VAMC. Planned changes include appointment of a cancer care coordinator and increased awareness of system delay. Already under way is a follow-up study of the utility of this intervention.
Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Acknowledgments
The authors thank the Dayton VAMC, Nicholas McCray and the Dayton VAMC IRB/R&D, and Brenda Duncan in the Dayton VAMC tumor registry office.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures 2017. Atlanta, GA: American Cancer Society. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf. Published 2017. Accessed January 16, 2017.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012. Published April 2015. Updated November 18, 2015. Accessed January 12, 2017.
3. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
4. Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129(4):397-403.
5. Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA, eds. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. https://www.rand.org/content/dam/rand/pubs/monograph_reports/2007/MR1281.pdf. Published 2000. Accessed November 18, 2016.
6. Silva PP, Pereira JR, Ikari FK, Minamoto H. Lung cancer and the delay in the diagnosis: analysis of 300 cases [in Portuguese]. Rev Assoc Med Bras. 1992;38(3):145-149.
7. Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45-49.
8. Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165(4):421-425.
9. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1-S8.
10. Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113(3):287-296.
11. Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of
primary lung cancer. Acta Oncol. 2002;41(2):147-152.
12. Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108-113.
13. Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34(2):243-252.
14. Salomaa ER, Sä llinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128(4):2282-2288.
15. González-Barcala FJ, García-Prim JM, Álvarez-Dobaño JM, et al. Effect of delays
on survival in patients with lung cancer. Clin Transl Oncol. 2010;12(12):836-842.
16. Shin DW, Cho J, Kim SY, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20(8):2468-2476.
17. Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung
cancer and their prognostic implications. J Thorac Oncol. 2011;6(7):1254-1259.
18. Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. In: Pokorski M, ed. Neurobiology of Respiration. Dordrecht, Netherlands: Springer Science+Business Media; 2013:355-362.
19. Sawicki M, Szczyrek M, Krawczyk P, Rybojad P, Jabłonka A, Milanowski J. Reasons for delay in diagnosis and treatment of lung cancer among patients in Lublin Voivodeship who were consulted in thoracic surgery department. Ann Agric Environ Med. 2013;20(1):72-76.
20. Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8): 238-239.
21. Leveque N, Brouchet L, Lepage B, et al. An analysis of treatment delays of thoracic cancers: a prospective study [in French]. Rev Mal Respir. 2014;31(3):208-213.
22. O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol). 2000;12(3):141-144.
23. Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, et al. Timeliness of care and prognosis in patients with lung cancer. Ir J Med Sci. 2014;183(3):383-390.
24. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
25. Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer. J Thorac Oncol. 2008;3(9):951-957.
26. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):595-600.
27. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
28. Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749-756.
29. Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2014;10(3):267-271.
30. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637.
31. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94(1):53-58.
32. Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13(3):300-302.
33. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441-445.
34. Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95-98.
35. Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10(1):68-75.
36. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43(2):293-296.
Lung cancer is the leading cause of cancer-related deaths worldwide and causes more deaths than do colorectal, breast, and prostate cancers combined.1 An estimated 155,870 Americans are expected to die of lung cancer in 2017, and these deaths account for about 26% of all cancer deaths.1 The overall 5-year survival rate for patients with lung cancer is 16.8%.2 However, this rate varies considerably, from 54% for those with early-stage cancer to 26.5% for those with locally advanced cancer and 4% for those with distant metastases.2
The Institute of Medicine’s Committee on Quality Health Care in America recognized timeliness of care as 1 of 6 important dimensions of health care quality.3 Delays in timely diagnosis and treatment of cancer, especially lung cancer, can result in significant emotional distress, impaired quality of life, increased use of health care resources, and, arguably, increased cost of care.4 In addition, delayed diagnosis of cancer can lead to negligence litigation.4
In the U.S., there are no federal standardized guidelines regarding timeliness of lung cancer care. In 2000, the RAND Corporation, a research organization, published several quality indicators recommending lung cancer diagnoses be established within 2 months after initial abnormal chest radiographs and treatment be offered within 6 weeks after diagnosis.5
Using these recommendations as benchmarks, a quality improvement study was conducted to determine the time lines of comprehensive lung cancer care at the Dayton VAMC in Ohio. The primary aim of the study was to evaluate adherence to the RAND criteria (the only U.S.- based guidelines) for the diagnosis and treatment of lung cancer in Dayton VAMC patients. The secondary aim was to assess the effect of preoperative cardiopulmonary rehabilitation on timeliness of treatment. The authors plan to use the results of the study to guide and improve cancer practices at the Dayton VAMC.
Methods
The authors conducted a retrospective study of a series of 121 consecutive patients who had lung cancer that was confirmed at the Dayton VAMC with a cytohistologic diagnosis between January 2011 and December 2013. The study was approved by the Dayton VAMC Research and Development committee and the Wright State University Institutional Review Board. After data collection and review, all patient identifiers were replaced with sequential numbering.
The Dayton VAMC is a 356-bed facility serving 16 counties and > 50,000 patients. Lung cancer diagnosis and management are collaboratively undertaken by various Dayton VAMC departments, including pulmonology, radiology, interventional radiology, pathology, thoracic surgery, medical oncology, radiation oncology, and palliative care. The facility, fully equipped with scanners for positron emission tomography and magnetic resonance imaging, provides comprehensive cancer care without the need for referrals to outside facilities for any part of care from diagnosis to end of life.
The study patients were identified from the Dayton VAMC tumor registry. Patients with only biopsy-confirmed malignancy were included in the study. Patients who did not follow up before biopsy or did not pursue treatment after biopsy confirmation were excluded from analysis where appropriate.
Patient data collected included age, sex, presenting symptom, histology, cancer stage, treatment modality, cardiopulmonary rehabilitation, and if applicable,
tumor size. Patients were retrospectively followed for 3 years. Charts reviewed did not include outcomes information. Historically, delays have been categorized as provider delays, patient delays, or system delays. Provider delay stems from the primary care provider’s (PCP) failure to investigate a presenting symptom further, patient delay from the patient’s failure to seek medical care or to follow through on medical advice in a timely manner, and system delay from the health care organization’s failure to obtain imaging or biopsy results in a timely manner. Assessment of system delay is focused on quality improvement at a treatment center.
In the present study, the primary aim was to assess system delay. The authors analyzed delay during 3 different periods: time to diagnosis (interval from date an abnormality was found on chest radiograph or computed tomography scan to date of tissue diagnosis); time to treatment initiation (interval from date of histopathologic diagnosis to date of treatment initiation); and time from date of initial abnormal imaging to date of treatment initiation. With RAND criteria applied, time to diagnosis longer than 60 days was considered diagnostic delay, time to treatment longer than 42 days was considered treatment delay, and the sum of these periods (102 days) was considered total delay.5 Patients with diagnosis and treatment intervals that fell within these criteria were considered in adherence with the RAND criteria.
Means and standard deviations were reported for continuous variables and counts and percentages for categoric variables. Calculations were performed with IBM SPSS 21.0 (Armonk, NY).
Results
Of the 121 patients, 118 (97.5%) were men, and 3 (2.5%) were women. Mean (SD) age was 68.5 (8.9) years (range, 50-89 years). Of the 121 patients, 88 (73%) opted to be treated at Dayton VAMC, and the other 33 opted to receive palliative care only (20) or to be treated at an outside facility (13). The group of 33 patients was included in the analyses of diagnostic delay but not treatment delay (Table 1).
Mean (SD) time to diagnosis was 35.5 (31.6) days (n = 111), mean (SD) time to treatment was 55.9 (46.3) days (n = 87), and mean (SD) total time was 92.7 (62.1) days (n = 82). Table 2 lists data regarding adherence to RAND guidelines for diagnostic delay (diagnostic timeliness), treatment delay (treatment timeliness), and total delay (total timeliness) for 3 groups of patients: all patients, and those who did and did not participate in cardiopulmonary rehabilitation. Of all patients, 82.9% met the RAND diagnostic time standard, 51.7% met the treatment time standard, and 61.0% met the total time standard. As expected, the proportions of patients meeting the RAND standards were higher for the group that participated in cardiopulmonary rehabilitation: 89.2%, 58.6%, and 72.3% for diagnostic, treatment, and total time, respectively.
Table 3 lists data regarding adherence to RAND guidelines by treatment modality, excluding the patients who participated in cardiopulmonary rehabilitation. With the exception of surgery only, all other primary treatment modalities were marked by 90% or higher adherence in meeting diagnostic timeliness. However, treatment initiation adherence was lower: 40% to 76.2% in the nonsurgical groups and 25% in the surgery group.
The cardiopulmonary rehabilitation group was analyzed separately (Table 4).
For diagnostic time, patients with advancedstage (IIIB/IV) disease and patients with small cell lung cancer (SCLC) had adherence of at least 87.5%, and patients with stage II/IIIA disease had adherence of at least 80% (Table 5). However, only 62.5% of patients with stage I disease were adherent to the diagnostic guideline. Patients with stage IIIA/IV disease and patients with SCLC had the best performance for the treatment guideline, with no group < 60% adherent.
Discussion
Several international study groups have recommended establishing standards for timely care of patients with known or suspected lung cancer.5-10 According to a study in Brazil, an application interval exceeding 30 days is considered patient delay.6 The Swedish Lung Cancer Study Group recommended that diagnostic tests be completed within 4 weeks in 80% of all patients and that treatment be started within 2 weeks thereafter.7 The recommendations from Canada are a maximum of 4 weeks between first PCP visit and diagnosis and 2 weeks for surgery.8 The British Thoracic Society recommended that all patients have completed diagnostic tests within 2 weeks of request with specific time intervals for treatment initiation based on treatment modality.9
Numerous studies10-27 and 2 meta-analyses28,29 have addressed timeliness of care or associations between timeliness and clinical outcomes, and 1 study27 tested an intervention to improve timeliness of care in patients with lung cancer. These studies varied in important ways because of the complexities inherent in the diagnosis and management of lung cancer, patient- and system-specific factors, and the definitions used for “delays.”
For this study, the authors examined Dayton VAMC adherence to RAND guidelines regarding time from imaging to diagnosis, time from diagnosis to treatment initiation, and time from abnormal imaging to treatment initiation. Separately, the authors examined the impact of cardiopulmonary rehabilitation on delay.
The 89.2% adherence to RAND diagnostic time guidelines (avoiding diagnostic delay) in this study’s population (excluding patients who participated in cardiopulmonary rehabilitation) was better than the 59% and 68.8% found in 2 larger VAMC studies.24,26 In addition, adherence to the RAND time standard for the interval from diagnosis to treatment initiation (avoiding treatment delay) was similar between this study (58.6%) and one of those studies (62.2%), which was a multicenter investigation.26 The other VAMC study, a singleinstitution investigation, was superior to the present study with respect to avoiding treatment delay (adherence, 76% vs 58.6%).24 These overtly similar results suggest that system delay is accompanied by patient delay involving time for decision making, acute illness, missed appointments, and so forth.
In this study, timeliness was most disappointing for the patients who underwent primary surgical resection. Surgery patients’ poor diagnostic timeliness rate (14.3%) was likely multifactorial, involving additional pretissue procurement staging workup, including more imaging scans, invasive procedures (mediastinoscopy), and repeat biopsy in cases of negative initial biopsy results. In addition, patients who initially qualified for definitive surgical resection of early-stage lung cancer likely underwent extensive postdiagnostic workup that included pulmonary function testing, split-function studies, and preoperative assessment for cardiac clearance. In a single-center prospective study, O’Rourke and Edwards found that progression of early-stage lung cancer after a median system delay of 94 days resulted in decreased candidacy for curative therapy in 21% of patients.22
Surgical resection was previously thought to be the best curative option for early-stage lung cancer. However, recent data on use of stereotactic ablative radiotherapy (SABR) in early-stage non-SCLC (NSCLC) showed equivalent outcomes. In a pooled study, Chang and colleagues found 3-year overall survival of 95% in their SABR group and 79% in their surgery group.30 Given these data, findings from this study, and significant delays experienced by surgery patients, it is worth considering whether SABR should be used more often.
The benefits of preoperative cardiopulmonary rehabilitation in the surgical outcomes of patients with lung cancer have been well described.31-36 Bobbio and colleagues noted that shortterm cardiopulmonary rehabilitation might improve the surgical candidacy of patients with chronic obstructive pulmonary disease.34 Moreover, Benzo and colleagues reported that 10 rehabilitation sessions resulted in shorter chest tube time and decreased length of stay, both of which lower postoperative morbidity and cost.33
In this study, although patients who had preoperative cardiopulmonary rehabilitation experienced diagnostic delays for reasons similar to those found for patients who did not have cardiopulmonary surgery, rehabilitation led to significant delays in treatment initiation, and more than three-fourths of patients experienced delay. This delay was hardly unexpected, but only 11 of the 18 patients who had preoperative cardiopulmonary rehabilitation underwent surgical resection. As anticipated, rehabilitation did not improve the surgical candidacy of the other patients.
Regarding staging, this study is consistent with international studies in which advanced-stage NSCLC and SCLC cases were diagnosed earlier, presumably because of the associated symptom burden.15,20,23 These results are also comparable to those of previous VAMC studies.24-26
The authors of this quality improvement study will apply its findings when they appoint a cancer care coordinator (nurse coordinator or clinical nurse specialist) at Dayton VAMC. The services of a cancer care coordinator have significantly reduced system delay elsewhere. The VA Connecticut Healthcare System added a cancer care coordinator in 2007, and by 2010, time from lung cancer suspicion to treatment was reduced to 55 days from 136 days in 2003.27
Limitations
First, the study was retrospective and used a small sample from a single institution; therefore, the results may not be generalizable to other health care settings. Second, the study included a small but significant number of patients who underwent serial imaging for asymptomatic pulmonary nodules; including this subgroup in the analyses of diagnostic delay negatively affected the results. Third, the effect of delay on survival was not evaluated.
Conclusion
This quality improvement lung cancer delay study examined adherence to the diagnostic and treatment time intervals recommended by the RAND Corporation in 2000.5 Although most of its patients received histopathologic confirmation within prespecified parameters, significant delays occurred for surgical patients, presumably as a result of extensive preoperative testing and optimization. Without improved surgical candidacy for most patients enrolled in preoperative cardiopulmonary rehabilitation, the authors urge facilities to consider alternatives to surgery. Given recent advances in SABR outcomes in early-stage NSCLC, SABR is worth considering as an upfront option in cases of equivocal performance status or early-stage NSCLC.
The authors will use information from this study as a baseline at the Dayton VAMC. Planned changes include appointment of a cancer care coordinator and increased awareness of system delay. Already under way is a follow-up study of the utility of this intervention.
Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Acknowledgments
The authors thank the Dayton VAMC, Nicholas McCray and the Dayton VAMC IRB/R&D, and Brenda Duncan in the Dayton VAMC tumor registry office.
Click here to read the digital edition.
Lung cancer is the leading cause of cancer-related deaths worldwide and causes more deaths than do colorectal, breast, and prostate cancers combined.1 An estimated 155,870 Americans are expected to die of lung cancer in 2017, and these deaths account for about 26% of all cancer deaths.1 The overall 5-year survival rate for patients with lung cancer is 16.8%.2 However, this rate varies considerably, from 54% for those with early-stage cancer to 26.5% for those with locally advanced cancer and 4% for those with distant metastases.2
The Institute of Medicine’s Committee on Quality Health Care in America recognized timeliness of care as 1 of 6 important dimensions of health care quality.3 Delays in timely diagnosis and treatment of cancer, especially lung cancer, can result in significant emotional distress, impaired quality of life, increased use of health care resources, and, arguably, increased cost of care.4 In addition, delayed diagnosis of cancer can lead to negligence litigation.4
In the U.S., there are no federal standardized guidelines regarding timeliness of lung cancer care. In 2000, the RAND Corporation, a research organization, published several quality indicators recommending lung cancer diagnoses be established within 2 months after initial abnormal chest radiographs and treatment be offered within 6 weeks after diagnosis.5
Using these recommendations as benchmarks, a quality improvement study was conducted to determine the time lines of comprehensive lung cancer care at the Dayton VAMC in Ohio. The primary aim of the study was to evaluate adherence to the RAND criteria (the only U.S.- based guidelines) for the diagnosis and treatment of lung cancer in Dayton VAMC patients. The secondary aim was to assess the effect of preoperative cardiopulmonary rehabilitation on timeliness of treatment. The authors plan to use the results of the study to guide and improve cancer practices at the Dayton VAMC.
Methods
The authors conducted a retrospective study of a series of 121 consecutive patients who had lung cancer that was confirmed at the Dayton VAMC with a cytohistologic diagnosis between January 2011 and December 2013. The study was approved by the Dayton VAMC Research and Development committee and the Wright State University Institutional Review Board. After data collection and review, all patient identifiers were replaced with sequential numbering.
The Dayton VAMC is a 356-bed facility serving 16 counties and > 50,000 patients. Lung cancer diagnosis and management are collaboratively undertaken by various Dayton VAMC departments, including pulmonology, radiology, interventional radiology, pathology, thoracic surgery, medical oncology, radiation oncology, and palliative care. The facility, fully equipped with scanners for positron emission tomography and magnetic resonance imaging, provides comprehensive cancer care without the need for referrals to outside facilities for any part of care from diagnosis to end of life.
The study patients were identified from the Dayton VAMC tumor registry. Patients with only biopsy-confirmed malignancy were included in the study. Patients who did not follow up before biopsy or did not pursue treatment after biopsy confirmation were excluded from analysis where appropriate.
Patient data collected included age, sex, presenting symptom, histology, cancer stage, treatment modality, cardiopulmonary rehabilitation, and if applicable,
tumor size. Patients were retrospectively followed for 3 years. Charts reviewed did not include outcomes information. Historically, delays have been categorized as provider delays, patient delays, or system delays. Provider delay stems from the primary care provider’s (PCP) failure to investigate a presenting symptom further, patient delay from the patient’s failure to seek medical care or to follow through on medical advice in a timely manner, and system delay from the health care organization’s failure to obtain imaging or biopsy results in a timely manner. Assessment of system delay is focused on quality improvement at a treatment center.
In the present study, the primary aim was to assess system delay. The authors analyzed delay during 3 different periods: time to diagnosis (interval from date an abnormality was found on chest radiograph or computed tomography scan to date of tissue diagnosis); time to treatment initiation (interval from date of histopathologic diagnosis to date of treatment initiation); and time from date of initial abnormal imaging to date of treatment initiation. With RAND criteria applied, time to diagnosis longer than 60 days was considered diagnostic delay, time to treatment longer than 42 days was considered treatment delay, and the sum of these periods (102 days) was considered total delay.5 Patients with diagnosis and treatment intervals that fell within these criteria were considered in adherence with the RAND criteria.
Means and standard deviations were reported for continuous variables and counts and percentages for categoric variables. Calculations were performed with IBM SPSS 21.0 (Armonk, NY).
Results
Of the 121 patients, 118 (97.5%) were men, and 3 (2.5%) were women. Mean (SD) age was 68.5 (8.9) years (range, 50-89 years). Of the 121 patients, 88 (73%) opted to be treated at Dayton VAMC, and the other 33 opted to receive palliative care only (20) or to be treated at an outside facility (13). The group of 33 patients was included in the analyses of diagnostic delay but not treatment delay (Table 1).
Mean (SD) time to diagnosis was 35.5 (31.6) days (n = 111), mean (SD) time to treatment was 55.9 (46.3) days (n = 87), and mean (SD) total time was 92.7 (62.1) days (n = 82). Table 2 lists data regarding adherence to RAND guidelines for diagnostic delay (diagnostic timeliness), treatment delay (treatment timeliness), and total delay (total timeliness) for 3 groups of patients: all patients, and those who did and did not participate in cardiopulmonary rehabilitation. Of all patients, 82.9% met the RAND diagnostic time standard, 51.7% met the treatment time standard, and 61.0% met the total time standard. As expected, the proportions of patients meeting the RAND standards were higher for the group that participated in cardiopulmonary rehabilitation: 89.2%, 58.6%, and 72.3% for diagnostic, treatment, and total time, respectively.
Table 3 lists data regarding adherence to RAND guidelines by treatment modality, excluding the patients who participated in cardiopulmonary rehabilitation. With the exception of surgery only, all other primary treatment modalities were marked by 90% or higher adherence in meeting diagnostic timeliness. However, treatment initiation adherence was lower: 40% to 76.2% in the nonsurgical groups and 25% in the surgery group.
The cardiopulmonary rehabilitation group was analyzed separately (Table 4).
For diagnostic time, patients with advancedstage (IIIB/IV) disease and patients with small cell lung cancer (SCLC) had adherence of at least 87.5%, and patients with stage II/IIIA disease had adherence of at least 80% (Table 5). However, only 62.5% of patients with stage I disease were adherent to the diagnostic guideline. Patients with stage IIIA/IV disease and patients with SCLC had the best performance for the treatment guideline, with no group < 60% adherent.
Discussion
Several international study groups have recommended establishing standards for timely care of patients with known or suspected lung cancer.5-10 According to a study in Brazil, an application interval exceeding 30 days is considered patient delay.6 The Swedish Lung Cancer Study Group recommended that diagnostic tests be completed within 4 weeks in 80% of all patients and that treatment be started within 2 weeks thereafter.7 The recommendations from Canada are a maximum of 4 weeks between first PCP visit and diagnosis and 2 weeks for surgery.8 The British Thoracic Society recommended that all patients have completed diagnostic tests within 2 weeks of request with specific time intervals for treatment initiation based on treatment modality.9
Numerous studies10-27 and 2 meta-analyses28,29 have addressed timeliness of care or associations between timeliness and clinical outcomes, and 1 study27 tested an intervention to improve timeliness of care in patients with lung cancer. These studies varied in important ways because of the complexities inherent in the diagnosis and management of lung cancer, patient- and system-specific factors, and the definitions used for “delays.”
For this study, the authors examined Dayton VAMC adherence to RAND guidelines regarding time from imaging to diagnosis, time from diagnosis to treatment initiation, and time from abnormal imaging to treatment initiation. Separately, the authors examined the impact of cardiopulmonary rehabilitation on delay.
The 89.2% adherence to RAND diagnostic time guidelines (avoiding diagnostic delay) in this study’s population (excluding patients who participated in cardiopulmonary rehabilitation) was better than the 59% and 68.8% found in 2 larger VAMC studies.24,26 In addition, adherence to the RAND time standard for the interval from diagnosis to treatment initiation (avoiding treatment delay) was similar between this study (58.6%) and one of those studies (62.2%), which was a multicenter investigation.26 The other VAMC study, a singleinstitution investigation, was superior to the present study with respect to avoiding treatment delay (adherence, 76% vs 58.6%).24 These overtly similar results suggest that system delay is accompanied by patient delay involving time for decision making, acute illness, missed appointments, and so forth.
In this study, timeliness was most disappointing for the patients who underwent primary surgical resection. Surgery patients’ poor diagnostic timeliness rate (14.3%) was likely multifactorial, involving additional pretissue procurement staging workup, including more imaging scans, invasive procedures (mediastinoscopy), and repeat biopsy in cases of negative initial biopsy results. In addition, patients who initially qualified for definitive surgical resection of early-stage lung cancer likely underwent extensive postdiagnostic workup that included pulmonary function testing, split-function studies, and preoperative assessment for cardiac clearance. In a single-center prospective study, O’Rourke and Edwards found that progression of early-stage lung cancer after a median system delay of 94 days resulted in decreased candidacy for curative therapy in 21% of patients.22
Surgical resection was previously thought to be the best curative option for early-stage lung cancer. However, recent data on use of stereotactic ablative radiotherapy (SABR) in early-stage non-SCLC (NSCLC) showed equivalent outcomes. In a pooled study, Chang and colleagues found 3-year overall survival of 95% in their SABR group and 79% in their surgery group.30 Given these data, findings from this study, and significant delays experienced by surgery patients, it is worth considering whether SABR should be used more often.
The benefits of preoperative cardiopulmonary rehabilitation in the surgical outcomes of patients with lung cancer have been well described.31-36 Bobbio and colleagues noted that shortterm cardiopulmonary rehabilitation might improve the surgical candidacy of patients with chronic obstructive pulmonary disease.34 Moreover, Benzo and colleagues reported that 10 rehabilitation sessions resulted in shorter chest tube time and decreased length of stay, both of which lower postoperative morbidity and cost.33
In this study, although patients who had preoperative cardiopulmonary rehabilitation experienced diagnostic delays for reasons similar to those found for patients who did not have cardiopulmonary surgery, rehabilitation led to significant delays in treatment initiation, and more than three-fourths of patients experienced delay. This delay was hardly unexpected, but only 11 of the 18 patients who had preoperative cardiopulmonary rehabilitation underwent surgical resection. As anticipated, rehabilitation did not improve the surgical candidacy of the other patients.
Regarding staging, this study is consistent with international studies in which advanced-stage NSCLC and SCLC cases were diagnosed earlier, presumably because of the associated symptom burden.15,20,23 These results are also comparable to those of previous VAMC studies.24-26
The authors of this quality improvement study will apply its findings when they appoint a cancer care coordinator (nurse coordinator or clinical nurse specialist) at Dayton VAMC. The services of a cancer care coordinator have significantly reduced system delay elsewhere. The VA Connecticut Healthcare System added a cancer care coordinator in 2007, and by 2010, time from lung cancer suspicion to treatment was reduced to 55 days from 136 days in 2003.27
Limitations
First, the study was retrospective and used a small sample from a single institution; therefore, the results may not be generalizable to other health care settings. Second, the study included a small but significant number of patients who underwent serial imaging for asymptomatic pulmonary nodules; including this subgroup in the analyses of diagnostic delay negatively affected the results. Third, the effect of delay on survival was not evaluated.
Conclusion
This quality improvement lung cancer delay study examined adherence to the diagnostic and treatment time intervals recommended by the RAND Corporation in 2000.5 Although most of its patients received histopathologic confirmation within prespecified parameters, significant delays occurred for surgical patients, presumably as a result of extensive preoperative testing and optimization. Without improved surgical candidacy for most patients enrolled in preoperative cardiopulmonary rehabilitation, the authors urge facilities to consider alternatives to surgery. Given recent advances in SABR outcomes in early-stage NSCLC, SABR is worth considering as an upfront option in cases of equivocal performance status or early-stage NSCLC.
The authors will use information from this study as a baseline at the Dayton VAMC. Planned changes include appointment of a cancer care coordinator and increased awareness of system delay. Already under way is a follow-up study of the utility of this intervention.
Disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Acknowledgments
The authors thank the Dayton VAMC, Nicholas McCray and the Dayton VAMC IRB/R&D, and Brenda Duncan in the Dayton VAMC tumor registry office.
Click here to read the digital edition.
1. American Cancer Society. Cancer facts & figures 2017. Atlanta, GA: American Cancer Society. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf. Published 2017. Accessed January 16, 2017.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012. Published April 2015. Updated November 18, 2015. Accessed January 12, 2017.
3. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
4. Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129(4):397-403.
5. Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA, eds. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. https://www.rand.org/content/dam/rand/pubs/monograph_reports/2007/MR1281.pdf. Published 2000. Accessed November 18, 2016.
6. Silva PP, Pereira JR, Ikari FK, Minamoto H. Lung cancer and the delay in the diagnosis: analysis of 300 cases [in Portuguese]. Rev Assoc Med Bras. 1992;38(3):145-149.
7. Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45-49.
8. Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165(4):421-425.
9. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1-S8.
10. Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113(3):287-296.
11. Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of
primary lung cancer. Acta Oncol. 2002;41(2):147-152.
12. Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108-113.
13. Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34(2):243-252.
14. Salomaa ER, Sä llinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128(4):2282-2288.
15. González-Barcala FJ, García-Prim JM, Álvarez-Dobaño JM, et al. Effect of delays
on survival in patients with lung cancer. Clin Transl Oncol. 2010;12(12):836-842.
16. Shin DW, Cho J, Kim SY, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20(8):2468-2476.
17. Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung
cancer and their prognostic implications. J Thorac Oncol. 2011;6(7):1254-1259.
18. Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. In: Pokorski M, ed. Neurobiology of Respiration. Dordrecht, Netherlands: Springer Science+Business Media; 2013:355-362.
19. Sawicki M, Szczyrek M, Krawczyk P, Rybojad P, Jabłonka A, Milanowski J. Reasons for delay in diagnosis and treatment of lung cancer among patients in Lublin Voivodeship who were consulted in thoracic surgery department. Ann Agric Environ Med. 2013;20(1):72-76.
20. Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8): 238-239.
21. Leveque N, Brouchet L, Lepage B, et al. An analysis of treatment delays of thoracic cancers: a prospective study [in French]. Rev Mal Respir. 2014;31(3):208-213.
22. O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol). 2000;12(3):141-144.
23. Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, et al. Timeliness of care and prognosis in patients with lung cancer. Ir J Med Sci. 2014;183(3):383-390.
24. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
25. Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer. J Thorac Oncol. 2008;3(9):951-957.
26. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):595-600.
27. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
28. Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749-756.
29. Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2014;10(3):267-271.
30. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637.
31. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94(1):53-58.
32. Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13(3):300-302.
33. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441-445.
34. Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95-98.
35. Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10(1):68-75.
36. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43(2):293-296.
1. American Cancer Society. Cancer facts & figures 2017. Atlanta, GA: American Cancer Society. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf. Published 2017. Accessed January 16, 2017.
2. Howlader N, Noone AM, Krapcho M, et al, eds. SEER Cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012. Published April 2015. Updated November 18, 2015. Accessed January 12, 2017.
3. Committee on Quality Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
4. Kern KA. Medicolegal analysis of the delayed diagnosis of cancer in 338 cases in the United States. Arch Surg. 1994;129(4):397-403.
5. Asch SM, Kerr EA, Hamilton EG, Reifel JL, McGlynn EA, eds. Quality of Care for Oncologic Conditions and HIV: A Review of the Literature and Quality Indicators. https://www.rand.org/content/dam/rand/pubs/monograph_reports/2007/MR1281.pdf. Published 2000. Accessed November 18, 2016.
6. Silva PP, Pereira JR, Ikari FK, Minamoto H. Lung cancer and the delay in the diagnosis: analysis of 300 cases [in Portuguese]. Rev Assoc Med Bras. 1992;38(3):145-149.
7. Myrdal G, Lambe M, Hillerdal G, Lamberg K, Agustsson T, Ståhle E. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59(1):45-49.
8. Simunovic M, Gagliardi A, McCready D, Coates A, Levine M, DePetrillo D. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165(4):421-425.
9. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53(suppl 1):S1-S8.
10. Yilmaz A, Damadoglu E, Salturk C, Okur E, Tuncer LY, Halezeroglu S. Delays in the diagnosis and treatment of primary lung cancer: are longer delays associated with advanced pathological stage? Ups J Med Sci. 2008;113(3):287-296.
11. Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of
primary lung cancer. Acta Oncol. 2002;41(2):147-152.
12. Quarterman RL, McMillan A, Ratcliffe MB, Block MI. Effect of preoperative delay on prognosis for patients with early stage non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;125(1):108-113.
13. Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34(2):243-252.
14. Salomaa ER, Sä llinen S, Hiekkanen H, Liippo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128(4):2282-2288.
15. González-Barcala FJ, García-Prim JM, Álvarez-Dobaño JM, et al. Effect of delays
on survival in patients with lung cancer. Clin Transl Oncol. 2010;12(12):836-842.
16. Shin DW, Cho J, Kim SY, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013;20(8):2468-2476.
17. Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung
cancer and their prognostic implications. J Thorac Oncol. 2011;6(7):1254-1259.
18. Radzikowska E, Roszkowski-Sliz K, Chabowski M, Glaz P. Influence of delays in diagnosis and treatment on survival in small cell lung cancer patients. In: Pokorski M, ed. Neurobiology of Respiration. Dordrecht, Netherlands: Springer Science+Business Media; 2013:355-362.
19. Sawicki M, Szczyrek M, Krawczyk P, Rybojad P, Jabłonka A, Milanowski J. Reasons for delay in diagnosis and treatment of lung cancer among patients in Lublin Voivodeship who were consulted in thoracic surgery department. Ann Agric Environ Med. 2013;20(1):72-76.
20. Comber H, Cronin DP, Deady S, Lorcain PO, Riordan P. Delays in treatment in the cancer services: impact on cancer stage and survival. Ir Med J. 2005;98(8): 238-239.
21. Leveque N, Brouchet L, Lepage B, et al. An analysis of treatment delays of thoracic cancers: a prospective study [in French]. Rev Mal Respir. 2014;31(3):208-213.
22. O’Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol). 2000;12(3):141-144.
23. Gonzalez-Barcala FJ, Falagan JA, Garcia-Prim JM, et al. Timeliness of care and prognosis in patients with lung cancer. Ir J Med Sci. 2014;183(3):383-390.
24. Gould MK, Ghaus SJ, Olsson JK, Schultz EM. Timeliness of care in veterans with non-small cell lung cancer. Chest. 2008;133(5):1167-1173.
25. Powell AA, Schultz EM, Ordin DL, et al. Timeliness across the continuum of care in veterans with lung cancer. J Thorac Oncol. 2008;3(9):951-957.
26. Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179(7):595-600.
27. Hunnibell LS, Rose MG, Connery DM, et al. Using nurse navigation to improve timeliness of lung cancer care at a veterans hospital. Clin J Oncol Nurs. 2012;16(1):29-36.
28. Olsson JK, Schultz EM, Gould MK. Timeliness of care in patients with lung cancer: a systematic review. Thorax. 2009;64(9):749-756.
29. Vinas F, Ben Hassen I, Jabot L, Monnet I, Chouaid C. Delays for diagnosis and treatment of lung cancers: a systematic review. Clin Respir J. 2014;10(3):267-271.
30. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630-637.
31. Morano MT, Araújo AS, Nascimento FB, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2013;94(1):53-58.
32. Nagarajan K, Bennett A, Agostini P, Naidu B. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg. 2011;13(3):300-302.
33. Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer. 2011;74(3):441-445.
34. Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33(1):95-98.
35. Mujovic N, Mujovic N, Subotic D, et al. Preoperative pulmonary rehabilitation in patients with non-small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci. 2014;10(1):68-75.
36. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43(2):293-296.
A Road Map for Creating a CPRS Template for a Cancer Survivorship Treatment Summary and Care Plan (FULL)
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
In 2012, staff at the Comprehensive Cancer Center of VA Connecticut Healthcare System in West Haven (VACHS) decided to create a template for a Cancer Survivorship Treatment Summary and Care Plan (Survivorship Care Plan [SCP] and treatment summary are used interchangeably in this article and refer to the same document) in the VACHS Computerized Patient Record System (CPRS) to be used as one component of a Multidisciplinary Cancer Survivorship Clinic. The clinic’s providers would be advanced practice registered nurses (APRNs), based in the Comprehensive Cancer Center of VACHS. This quality improvement project was created in response to the American College of Surgeons (ACoS) Commission on Cancer (CoC) Standard 3.3, effective January 1, 2012, which mandated that the cancer committee “develops and implements a process to disseminate a comprehensive care summary and follow-up plan to patients with cancer who are completing cancer treatment.”1 According to ACoS CoC the process should be monitored, evaluated, presented, and documented at least annually to the cancer committee.
Creating the CPRS template took 9 months before the first SCPs were provided to patients in July 2013. Since that time, 210 SCPs have been provided to VACHS patients. Patient response was positive. Since implementation, patients have told their provider that they found the SCP’s list of signs and symptoms of cancer recurrence a helpful and reassuring resource.
Objective
This project is designed to be road map for other VA providers to follow by offering a review of the processes and resources that VACHS used and to share lessons learned.
The SCP is an important component of the survivorship standard of care. The CoC Standard 3.3 (version 2016) mandated that SCPs must be provided during an in-person meeting to an annually increasing percentage of patients initially diagnosed and treated for stage I, II, or III cancer in a given year—10% for those diagnosed and treated in 2015 and 25% for those diagnosed and treated in 2016 with increases in the required percentage each year thereafter. The mandated increase from 10% in 2015 to 25% in 2016 is significant and requires substantial resources to meet. Cancer centers seeking to achieve or maintain ACoS accreditation must fulfill this standard.2
It is important to establish a robust SCP process proactively. The percentage of SCPs provided that is mandated by the CoC continues to rise annually and the rate of survivorship also is expected to rise. The January 2016 CoC update clarified the phase-in of this standard over 4 years: (1) 2015: Implement a process to provide treatment summaries to at least 10% of patients treated for stage I-III cancer; (2) By end of 2016: Provide treatment summaries to at least 25% of eligible patients; (3) By end of 2017: Provide treatment summaries to at least 50% of eligible patients; and (4) By end of 2018: Provide treatment summaries to at least 75% of eligible patients.2
Background
In the fall of 2012, the project began with listening to survivors. The VA Survivorship Special Interest Group (SSIG) already had done significant work throughout the national VA system.3 The VACHS staff participated in monthly SSIG conference calls and reviewed the extensive resources created by its members, which is available through an internal VA website (Figure).
The VACHS staff reviewed the experiences of 2 VA sites using a draft CPRS survivorship care plan template. They also spent a day observing an established survivorship clinic at the VACHS academic affiliate Yale-New Haven Hospital (YNHH) in November of 2012. At that time, the YNHH clinic format was a 2-visit model for patients who had completed treatment. During their first visit to the YNHH clinic, patients meet with 4 members of an interdisciplinary team: a medical provider, a dietician, a physical therapist, and a social worker. At the end of the visit, the patient receives a SCP, a comprehensive document based on a template from the Livestrong organization.4 The second visit is scheduled 3 months later to follow up with patients and address any ongoing concerns. Patients then would be discharged from the survivorship clinic.
Given the complicated needs of the VA patient population, VACHS staff wanted to create a survivorship clinic that would provide regular, close followup by a multidisciplinary team within the existing hematology/oncology outpatient clinic. This design was believed to better serve veteran cancer survivors than a stand-alone clinic.
Clinic Creation
The VACHS chief of oncology, the cancer registrar, and the cancer care coordinator met in October 2012 to review Standard 3.3 and determine the best approach for VACHS patients. A plan to phase survivorship care into the existing hematology/oncology clinic was established. The group identified appropriate cancer survivor patients who would be followed by an APRN and a medical doctor. After reviewing the most common cancers treated at VACHS, it was decided to start the survivorship clinic with patients who had been treated for stage I lung cancer, stage I or II colorectal cancer, and/or stage I melanoma. These patients are not usually treated with chemotherapy, are less likely to relapse given early stage, and generally would be expected to be less complicated medically than would patients with more advanced disease. Patients previously treated for more advanced cancers would continue to be followed by medical doctors unless determined to be appropriate for migration to this new clinic.
The VACHS staff chose to embed this new clinic within existing APRN hematology/oncology clinics. Survivorship clinic visits were not restricted to a particular date or time in order to maximize efficiency as the workload associated with this clinic was not initially known. To track patient volume in the clinic, VACHS staff created the following note titles for patients being followed in the survivorship clinic: (1) Hem/Onc APRN Survivorship Clinic Initial Consult; (2) Hem/Onc APRN Cancer Survivorship Note; and (3) Cancer Survivor Treatment Summary. Unique note titles can be searched in VistA to create real-time reporting, thereby enabling staff to monitor the size, demographics, and workload associated with this clinic.
Vision for Care
The goals of survivorship care at VACHS were (1) to prevent, detect early, and treat complications from cancer treatment through regular clinic visits and ongoing education and support; (2) to provide holistic, individualized medical care and psychosocial support for veterans who are cancer survivors; (3) to maximize health, quality of life, and longevity; and (4) to facilitate appropriate referrals.
The VACHS clinic model incorporated: (1) regular clinic visits and follow-up with laboratories and imaging for 5 years, based on National Comprehensive Cancer Network (NCCN) guidelines; (2) monitoring for psychosocial distress at each visit, using a modified version of the NCCN Distress Thermometer, and a registered nurse or health tech documenting scores in CPRS with a templated Distress Screening note; (3) referrals to nutrition, health psychology, social work, physical therapy, smoking cessation, and the palliative care team as appropriate; (4) education about diagnosis, risk factors, and healthy living; and (5) reviewing the SCP to each patient.
The VACHS approach was designed to be patientcentered by incorporating individualized surveillance and screening guidelines, wellness education tailored to cancer type and treatment history, psychosocial support for survivors and their families through individual therapy and support groups for patients and families, and individual exercise and fitness recommendations through physical therapy and pulmonary rehabilitation referrals.
Needs Assessment
The VACHS cancer care coordinator worked with the tumor registrar to generate an initial referral patient list. Patients were identified in the tumor registry who met these criteria: diagnosed with stage I lung cancer or stage I or II colorectal cancer and treated at VACHS between 2008 and 2012. Initial research showed that there were 117 patients followed in the VACHS hematology/oncology clinic in the fall of 2012 who met the criteria. At that time, 80% of the patients identified were being followed by an attending oncology physician and 20% by an oncology APRN (Table).
Based on this initial analysis, the projected patient load for this clinic was anticipated to be 100 patients annually with between 2 and 4 annual visits each. Of note, in 2013 VACHS started a low-dose chest computed tomography screening program for patients who met high-risk criteria. The number of patients diagnosed and treated for stage I non-small cell lung cancer at VACHS during 2014 and 2015 was more than double the number treated in 2012.
Partnering With IT
The VACHS cancer care coordinator contacted the VISN 1 clinical application coordinator (CAC) in October 2012 who then reached out to her counterpart in Boston, where the sample CPRS template from the survivorship toolkit (toolkit template) was being tested. In November 2012, the VACHS CAC loaded the toolkit template into the test folder of the template drawer of VACHS CPRS. Changes were made over time to shorten the toolkit template to include the relevant information using the shortest number of words.
The template was modified by deleting 3 sections. The appointment list and the medication list were eliminated from the template as it was felt that these change with each visit. The psychosocial distress assessment also was eliminated as this score was known to change from visit to visit.
A separate initiative was undertaken simultaneously to address the need to monitor oncology patients for psychosocial distress regularly. Health psychology providers at VACHS used the NCCN Distress Thermometer to monitor distress in oncology patients. The team elected to take the distress assessment language out of the SCP and create a separate note template to record the distress scores for VACHS patients. The group chose the note titles Cancer Survivor Treatment Summary and Patient Distress Screening to clarify the purpose of the notes and to make searching CPRS or VistA for the note titles easier for providers or researchers.
CPRS Template Format
The VACHS team decided to create a short, clear, document that included diagnosis (date, location, pathology, staging), treatment (types, dates, locations, complications), disease-specific plan for surveillance, healthy living guidelines, and contact information for the survivorship provider.
The template was designed to be relatively simple for a provider to create, using check boxes that would populate the template with disease-specific care plans based on NCCN guidelines.
Review Process
The VACHS team’s original goal was to provide the first treatment summary to a patient on January 1, 2013, 10 weeks after the initial meeting with the CAC. This turned out to be overly optimistic. The VACHS Forms Committee meets once a month at VACHS. There is a formal review process for CPRS templates and all the decision makers must be present (Review Process Time Line).
The changes requested by the VACHS Forms Committee included taking out medications/appointments sections; spelling out all abbreviations; asking the educational coordinator to review educational portion of the template to make sure it complies with guidelines and reading level; and adding fields next to date of diagnosis field and, in every instance of treatment, dates for author to indicate where the diagnosis was made and where the treatment occurred.
Implementation
Starting with the list originally generated by the tumor registrar, the VACHS team set a goal of about 100 patients to be followed in an APRN survivorship clinic to focus on stage I lung cancer, stage I and II colorectal cancer, and stage I melanoma patients. As appropriate, patients were transitioned from being followed by a fellow or attending MD at the VACHS hematology/oncology clinic to the APRN survivorship clinic. As patients were seen in clinic for scheduled surveillance visits, the APRN survivorship clinic provider reviewed the process of creating a treatment summary with each patient and family as appropriate and reviewed their history with them in person to make sure that any complications related to treatment were identified. During each survivorship clinic visit, the provider verbally reviewed the plan for surveillance and signs and symptoms of recurrence to report to their clinician before providing the SCP to the patient.
Over the past 3 years, the VACHS Cancer Center has incorporated a hematology/oncology dietician, a health psychologist, a social worker, and a physical therapist into the outpatient clinic; all are usually available for sameday referrals. During regular survivorship visits, the survivorship APRN reviews any needs the veteran has and makes appropriate referrals. Palliative care personnel also are available in the cancer center during outpatient clinics for same-day consults. Survivorship patients are not automatically scheduled to see members of the team; rather, appropriate referrals are made via consults in CPRS after meeting with patients and assessing their needs.
In the past 3 years, 2 support groups were created, one for VACHS cancer center patients and one for caregivers. These groups are well attended by oncology and survivorship patients.
As part of the patient’s initial visit, the survivorship APRN reviews the patient’s information in CPRS and systematically reviews the original pathology, surgery, and tumor board notes as well as any notes related to treatments both within the VA system and in the community and creates the treatment summary CPRS note. In cases in which the patient had treatment at an outside facility, the patient signs a release of information form and original documentation of that treatment is requested. The completion of the SCP depends on the timing of when all appropriate information is available to be reviewed. As with all templates, minor editing is done to create the final note.
Once the survivorship APRN completes and signs the SCP, the patient’s primary care provider is added as a cosigner to the note. The patient receives the signed SCP at the visit. The January 2016 CoC Standard 3.3 update specifies that the SCP must be provided to the patient at an in-person visit and not mailed. As the SCP is a signed note in CPRS, it is easy to keep track of the date on which the information was reviewed and documented. If there are changes, such as a new cancer diagnoses or subsequent treatments, it is clear when the original information was documented. After providing the SCP to the patient and reviewing the document at an in-person meeting with the patient, the survivorship APRN documents the date that the SCP was provided to the patient in the progress note in CPRS. Once signed, the SCP is available to all providers within VACHS and able to be printed.
To date, 210 treatment summaries have been created for and provided to patients. Only 1 provider, the cancer care coordinator, is currently using the template, but use is not restricted. Patient feedback has been favorable: Patients state that the list of symptoms included in the treatment summary is useful. Patients report sharing the document with outside providers. The treatment summary also provides patients and families with a predictable plan for surveillance and regular in-person follow-up.
Patient Satsfaction Survey
In March 2015, VACHS conducted a patient satisfaction survey of 98 patients who had been provided with treatment summaries to better understand the impact on patients. This survey assessed quality measures, including patient’s confidence in their understanding of their cancer diagnosis, stage, treatment history, and plan for surveillance. Patient satisfaction with the resources available to them for healthy living also was measured, as was patient satisfaction with their survivorship and oncology providers and awareness that they had received a care plan.
Surveys were mailed to all VACHS survivorship patients for whom the treatment summaries were created who were still living and had not experienced a recurrence. The list of survey recipients was generated by searching VistA for the unique note title: Cancer Survivor Treatment Summary.
Sixty-six patients responded, a 67% response rate. The primary cancer diagnoses of the 66 study participants were lung (62.5%), colorectal (21.9%), melanoma (7.8%), head and neck (3.1%), and more than 1 malignancy (15.6%). Of the 66 respondents, 36.5% acknowledged receiving a treatment summary (23 patients).
Of those who acknowledge receiving a treatment summary, two-thirds stated that they have referred to the treatment summary for details about their diagnosis, treatment, plan for surveillance, and symptoms to report to practitioner. Between 73% and 76% were highly confident and between 22% and 25% were somewhat confident in their knowledge of their type of cancer, stage, treatment history and surveillance plan (> 90% positive response). The majority (66%) of patients were highly confident, and 32% were somewhat confident that there are resources available at VA to support their healthy lifestyle (98% positive response).
The survey also noted that 86% report being highly satisfied with care, and 92.4% are highly confident that their caregiver will provide compassionate care. Participants state that they have used the nutrition consults (38.5%), physical therapy (23%), health psychology (15.5%), smoking cessation (15.3%), and social work (10%). Of note, almost all those patients who reported using these services responded that they would recommend them.
Challenges
Despite the progress made at VACHS, there are significant challenges to meeting the CoC revised standard 3.3, which requires that 25% of patients treated with a stage I, II, or III cancer receive a Cancer Survivor Treatment Summary at an in-person visit in 2016. These relate primarily to multiple competing demands on provider time. In addition, 63.5% of patients who had been provided with a SCP at an in-person visit and responded to a satisfaction survey said they had not received the SCP. More research is needed to inform practice changes to optimize ongoing education and post-treatment care for veterans who are cancer survivors.
Conclusion
VA cancer centers seeking to ACoS CoC accreditation are required to provide a written summary of cancer treatment and plan for survivorship care to patients
diagnosed with at stage I, II, or III malignancy and treated at their facility. This requirement necessitates a significant ongoing investment in clinician and administrative workload to comply with the standard. The Comprehensive Cancer Center at VACHS, building on work by the SSIG, developed a concise note template in CPRS that enables oncology clinicians to create a treatment summary for each patient who meets criteria. During this process, VACHS has developed resources that may be useful to other VA cancer centers who are working to create this process. Clinicians interested in trialing the VACHS Cancer Survivor Treatment Summary template are encouraged to contact the author for additional information.
Visit www.fedprac.com/avahoupdates for an exclusive video interview with the author.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Acknowledgments
This project was a team effort. The author thanks the following VACHS colleagues for their input and support for this project: Michal Rose, MD, cancer center director; Donna Connery, tumor registrar; Renee Midgett, former clinical applications coordinator; Robert Troy Nall, health systems specialist; Forms Committee; Clarice Grens, APRN; and Jessica Barber, PhD, clinical psychologist as well as Members of VA Survivorship special interest group.
Click here to read the digital edition.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.
1. American College of Surgeons Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed January 18, 2017.
2. American College of Surgeons Commission on Cancer. Cancer program standards: ensuring patient-centered care 2016 Edition. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/2016%20coc%20standards%20manual_interactive%20pdf.ashx. Accessed January 18, 2017.
3. Smith J, Arfons L, Cmolik B, Moye J, Ballard E, Haggstrom D. Development and implementation of a veterans’ cancer survivorship program. Fed Pract. 2015;32(suppl 1):42S-48S..
4. National Comprehensive Cancer Network. NCCN distress thermometer and problem list for patients. http://www.nccn.org/patients/resources/life_with_cancer/pdf/nccn_distress_thermometer.pdf. Updated May 6, 2016. Accessed January 18, 2016.