User login
Activating the Immune System to Treat Multiple Myeloma
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
Assessment of Free Flap Breast Reconstructions
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
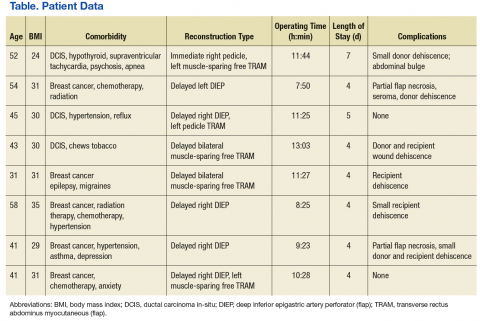
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
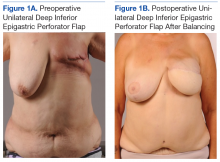
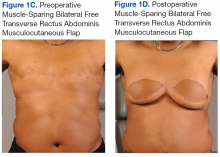
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
Free flap autologous breast reconstruction is an excellent surgical option for breast reconstruction in select patients. A free flap involves moving skin, fat, and/or muscle from a distant part of the body, based on a named blood supply (pedicle), and attaching it to another blood supply adjacent to the acquired defect. This procedure is particularly useful in areas where local tissue supply is lacking in volume or is damaged due to trauma or radiation. These reconstructions are performed largely in high-volume centers outside the VA because of the required specialized level of surgical training, manpower, and nursing support.1 The Malcom Randall VAMC in Gainesville, Florida, started offering autologous free flap breast reconstruction as an option to select patients in October 2012.
The Malcom Randall VAMC operating room (OR) does not operate 24/7, and the system has limited available OR time and surgical staff compared with the volume of patients requesting care.2 Operative planning for free flap autologous breast reconstruction must occur months ahead of surgery to balance the system limitations with the ability to offer the highest level of care. Planning includes strict patient selection, preoperative imaging, practice runs with OR staff, use of venous couplers, and frequent intensive care unit (ICU) staff in-services. Planning also includes the need to keep surgeries within the allocated OR time to avoid shift changes during critical periods. Frequent and early communication occurs between the surgical scheduler, OR nurses, and the anesthesia and critical care teams.
Studies have found that the best chance of flap salvage in the event of a thrombotic event is a rapid return to the OR.3 It is essential to minimize the risk of emergent returns to the OR because it is not staffed throughout the night. Patient risk factors for perioperative vascular complications include hypercoagulable disorders, peripheral vascular disease, use of the superficial epigastric system, and smoking.4-7
A PubMed search for free flap reconstruction solely within the VA over the past 20 years found 1 article discussing the use of free flaps in head and neck reconstruction which demonstrated an impressive success rate of 93%.8
The object of this study was to assess free flap breast reconstruction results at the Malcolm Randall VAMC to determine whether it is a realistic treatment to offer in the federal system.
Methods
The Malcolm Randall Institutional Review Board approved a retrospective chart review of all autologous free flap breast reconstructions using CPT code 19364, performed from October 2012 to June 2016. Medical records of patients who had a free flap breast reconstruction were queried during that period. Patient age; comorbidities listed on the electronic medical record “problem list;” body mass index (BMI); type of reconstruction (delayed vs immediate); length of surgery; length of stay; and complications over a 30-day period were recorded (Table). The authors looked for documentation of preoperative imaging and unplanned returns to the OR within the 30-day period.
Of 3 full-time VA plastic surgeons on staff during the study period, 2 surgeons had advanced fellowship training in either microsurgery or hand and microsurgery. Plastic surgery fellows and general surgery interns participated in the surgeries and postoperative care. The service had 1 dedicated advanced practice registered nurse involved in the surgical scheduling and perioperative care.
Results
A total of 11 abdominally based free flap breast reconstructions—6 muscle-sparing transverse rectus abdominus musculocutaneous (TRAM) and 5 deep inferior epigastric perforator (DIEP) flaps—were performed in 8 patients during the study period (Figures 1A, 1B, 1C, and 1D). Patient ages ranged from 31 to 58 years with a mean of 45.6 years. Six patients had preoperative computer tomography angiography (CTA) to define the location of the abdominal wall perforators. One muscle-sparing free flap was performed immediately after mastectomy; the other free flaps were performed as delayed reconstructions. Body mass index ranged from 24 to 35, with a mean of 30. All patients reported no tobacco use during the consultation; however, 1 patient later admitted to chewing tobacco. No urinary cotinine confirmation was requested. Two patients had 1 free flap reconstruction and 1 pedicle TRAM. This bilateral combination has been recently described in the literature and was chosen as a reasonable option to balance limited resources with abdominal wall morbidity.9 Operating room time ranged from 7 hours 50 minutes to 13 hours 3 minutes. All patients went to the ICU for hourly flap monitoring.
Length of stay ranged from 4 to 7 days, with a mean of 4.5 days. The longest stay was for a patient who had immediate reconstruction using a pedicle TRAM and muscle-sparing free TRAM. She was not a DIEP candidate because poor perforator quality had been noted during preoperative imaging.
Six patients had documentation of postoperative wound complications. One patient returned to the OR on the elective schedule 3 weeks postoperatively for a partial flap debridement. Her tissue transfer was > 1,000 g, and she required a matching reduction on the other side. There were no complete flap losses or postoperative thrombotic events; no cases went back to the OR emergently.
Discussion
With the number of women veterans steadily increasing, the number of patients in need of breast cancer surgery, including reconstruction, will rise in the VA.10 Fortunately, breast reconstruction is an elective procedure. Immediate breast reconstruction is a popular option because patients can combine surgeries and potentially avoid 2 recovery periods, and a better aesthetic outcome is possible because the skin does not have time to contract. Although immediate reconstruction has been increasing in popularity, it is associated with a higher complication rate.11 Further, reconstruction can be jeopardized if the oncologic plan is changed in the early postoperative period.
Positive margins found after an autologous reconstruction result in a more complicated postoperative course and a higher rate of wound complications.12 Unexpected radiation therapy after autologous reconstruction can severely distort a tissue flap because of fat necrosis, fibrosis, and contraction.13,14 From a practical perspective in the federal system, it is very difficult to coordinate 2 surgeons’ schedules when the system is already struggling to keep up with demand. Splitting the ablative and reconstructive surgery allows the urgent problem (cancer) to be addressed first, ensuring clear margins and allowing the patient to recover and consider all reconstructive options without feeling time pressure.
A large tertiary care center will have staff and equipment redundancy, but this study had to consider limitations in resources. The preoperative lead time allows the ICU to arrange a bed for hourly flap checks and for in-servicing new nursing staff on free flap monitoring. This was well received, and patients gave positive feedback on the staff. The OR schedulers can schedule nurses and techs who are familiar with the microscope and microsurgery instruments. The micro sets were opened, and the microscope powered on for practice runs a week before the procedures to insure no broken or missing instruments.
High-procedure volume would logically improve efficiency. Although the VA is not likely to become a tertiary center for breast reconstruction, the findings of other high-volume microsurgeons can be applied to improve speed and limit complications. Efforts to limit the OR time included use of preoperative imaging and intraoperative venous couplers. Venous couplers can result in shorter OR time, fewer returns to the OR, and excellent patency rates.15,16 One microsurgeon performed his surgery using only loupe-assisted vision (x 3.5), without use of the microscope. Pannucci and colleagues have recommended this as a way to improve access and OR efficiency.17 Use of the CTA has been found to decrease the rate of partial flap necrosis and improve speed of surgery.18-20
Careful patient selection allowed a hospital stay that averaged 4.5 days and minimized risks for return to the OR. Only patients who were nonsmokers were offered the surgery. Average BMI was 30 to prevent the known operative risks in breast surgery patients who are morbidly obese.21-23 No patients had a history of thromboembolic disease. Most patients were discharged home from the ICU. They eventually returned for elective revisions, second stages, and balancing procedures.
Conclusion
Free flap breast reconstruction can be offered as a treatment option with appropriate patient selection and planning. The most efficient way to provide this procedure within the federal system and to minimize the risk of flap loss and complications is by offering delayed reconstruction, obtaining preoperative CTA imaging, utilizing venous couplers, and frequently communicating with all involved practitioners from the OR to the ICU. This small study provides a good starting point to illustrate that tertiary-care reconstructive surgery can be offered to veterans within the federal system.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida/South Georgia Veterans Health System, Gainesville, Florida.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
1. Tuggle CT, Patel A, Broer N, Persing JA, Sosa JA, Au AF. Increased hospital volume is associated with improved outcomes following abdominal-based breast reconstruction. J Plast Surg Hand Surg. 2014;48(6):382-388.
2. Shulkin DJ. Beyond the VA crisis — becoming a high-performance network. N Engl J Med. 2016;374(11):1003-1005.
3. Novakovic D, Patel RS, Goldstein DP, Gullane PJ. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009;1:33.
4. Davison SP, Kessler CM, Al-Attar A. Microvascular free flap failure caused by unrecognized hypercoagulability. Plast Reconstr Surg. 2009;124(2):490-495.
5. Masoomi H, Clark EG, Paydar KZ, et al. Predictive risk factors of free flap thrombosis in breast reconstructive surgery. Microsurgery. 2014;34(8):589-594.
6. O’Neill AC, Haykal S, Bagher S, Zhong T, Hofer S. Predictors and consequences of intraoperative microvascular problems in autologous breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(10):1349-1355.
7. Sanati-Mehrizy P, Massengburg BB, Rozehnal JM, Ignargiola MJ, Hernandez Rosa J, Taub PJ. Risk factors leading to free flap failure: analysis from the national surgical quality improvement program database. J Craniofac Surg. 2016;27(8):1956-1964.
8. Myers LL, Sumer BD, Defatta RJ, Minhajuddin A. Free tissue transfer reconstruction of the head and neck at a Veterans Affairs hospital. Head Neck. 2008;30(8):1007-1011.
9. Roslan EJ, Kelly EG, Zain MA, Basiron NH, Imran FH. Immediate simultaneous bilateral breast reconstruction with deep inferior epigastric (DIEP) flap and pedicled transverse rectus abdominis musculocutaneous (TRAM) pedicle flap. Med J Malaysia. 2017;72(1):85-87.
10. Leong M, Chike-Obi CJ, Basu CB, Lee EL, Albo D, Netscher DT. Effective breast reconstruction in female veterans. Am J Surg. 2009;198(5):658-663.
11. Kwok AC, Goodwin IA, Ying J, Agarwal JP. National trends and complication rates after bilateral mastectomy and immediate breast reconstruction from 2005 to 2012. Am J Surg. 2015;210(3):512-516.
12. Ochoa O, Theoharis C, Pisano S, et al. Positive margin re-excision following immediate autologous breast reconstruction: morbidity, cosmetic outcome, and oncologic significance. Aesthet Surg J. 2017; [Epub ahead of print.]
13. Garvey PB, Clemens MW, Hoy AE, et al. Muscle-sparing TRAM flap does not protect breast reconstruction from post-mastectomy radiation damage compared to DIEP flap. Plast Reconstr Surg. 2014;133(2):223-233.
14. Kronowitz SJ. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282-292.
15. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, et al. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland Surg. 2016;5(2):88-92.
16. Jandali S, Wu LC, Vega SJ, Kovach SJ, Serletti JM. 1000 consecutive venous anastomoses using the microvascular anastomotic coupler in breast reconstruction. Plast Reconstr Surg. 2010;125(3):792-798.
17. Pannucci CJ, Basta MN, Kovach SJ, Kanchwala SK, Wu LC, Serletti JM. Loupes-only microsurgery is a safe alternative to the operating microscope: an analysis of 1,649 consecutive free flap breast reconstruction. J Reconstr Microsurg. 2015;31(9):636-642.
18. Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap reconstruction: a systemic review and meta-analysis. Microsurgery. 2013;33(6):496-502.
19. Fitzgerald O’Connor E, Rozen WM, Chowdhry M, Band B, Ramakrishnan VV, Griffiths M. Preoperative computed tomography angiography for planning DIEP flap breast reconstruction reduces operative time and overall complications. Gland Surgery. 2016;5(2):93-98.
20. Malhotra A, Chhaya N, Nsiah-Sarbeng P, Mosahebi A. CT-guided deep inferior epigastric perforator (DIEP) flap localization—better for the patient, the surgeon, and the hospital. Clin Radiol. 2013;68(2):131-138.
21. Ilonzo N, Tsang A, Tsantes S, Estabrook A, Thu Ma AM. Breast reconstruction after mastectomy: a ten-year analysis of trends and immediate postoperative outcomes. Breast. 2017;32:7-12.
22. McAllister P, Teo L, Chin K, Makubate B, Alexander Munnoch D. Bilateral breast reconstruction with abdominal free flaps: a single centre, single surgeon retrospective review of 55 consecutive patients. Plast Surg Int. 2016;2016:6085624.
23. Myung Y, Heo CY. Relationship between obesity and surgical complications after reduction mammoplasty: a systemic literature review and meta-analysis. Aesthet Surg J. 2017;37(3):308-315.
Is Your Practice Biosimilar Ready?
Click Here to Read the Full Supplement.
Topics include:
- The Pathway for Biosimilar Approval
- The Difference between Biosimilars and Generics
- Draft Interchangeablity Guidelines
- ACR Position Statement on Biosimilars
- Effect of the Reimbursement Model on Biosimilars
Click Here to Read the Full Supplement.
Topics include:
- The Pathway for Biosimilar Approval
- The Difference between Biosimilars and Generics
- Draft Interchangeablity Guidelines
- ACR Position Statement on Biosimilars
- Effect of the Reimbursement Model on Biosimilars
Click Here to Read the Full Supplement.
Topics include:
- The Pathway for Biosimilar Approval
- The Difference between Biosimilars and Generics
- Draft Interchangeablity Guidelines
- ACR Position Statement on Biosimilars
- Effect of the Reimbursement Model on Biosimilars
Best Practices: In the Management of Hemophilia
The treatment of patients with hemophilia has rapidly evolved from one-size-fits-all factor replacement strategies to highly individualized, patient-specific care.
Faculty:
Erik Berntorp, MD, PhD
Malmö Centre for Thrombosis and Haemostasis
Lund University
Malmö, Sweden
Faculty Disclosures:
This sponsored content was prepared by Dr. Berntorp and reviewed by Shire. Dr. Berntorp discloses that he is a consultant and on the advisory boards and speakers’ bureaus for Bayer, CSL Behring, Octapharma, Shire, and Sobi. The production of this section did not involve the news or editorial staff of Frontline Medical Communications.
S28001
2/17
Click here to read the supplement
The treatment of patients with hemophilia has rapidly evolved from one-size-fits-all factor replacement strategies to highly individualized, patient-specific care.
Faculty:
Erik Berntorp, MD, PhD
Malmö Centre for Thrombosis and Haemostasis
Lund University
Malmö, Sweden
Faculty Disclosures:
This sponsored content was prepared by Dr. Berntorp and reviewed by Shire. Dr. Berntorp discloses that he is a consultant and on the advisory boards and speakers’ bureaus for Bayer, CSL Behring, Octapharma, Shire, and Sobi. The production of this section did not involve the news or editorial staff of Frontline Medical Communications.
S28001
2/17
Click here to read the supplement
The treatment of patients with hemophilia has rapidly evolved from one-size-fits-all factor replacement strategies to highly individualized, patient-specific care.
Faculty:
Erik Berntorp, MD, PhD
Malmö Centre for Thrombosis and Haemostasis
Lund University
Malmö, Sweden
Faculty Disclosures:
This sponsored content was prepared by Dr. Berntorp and reviewed by Shire. Dr. Berntorp discloses that he is a consultant and on the advisory boards and speakers’ bureaus for Bayer, CSL Behring, Octapharma, Shire, and Sobi. The production of this section did not involve the news or editorial staff of Frontline Medical Communications.
S28001
2/17
Click here to read the supplement
Military Sexual Trauma and Sexual Health: Practice and Future Research for Mental Health Professionals
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
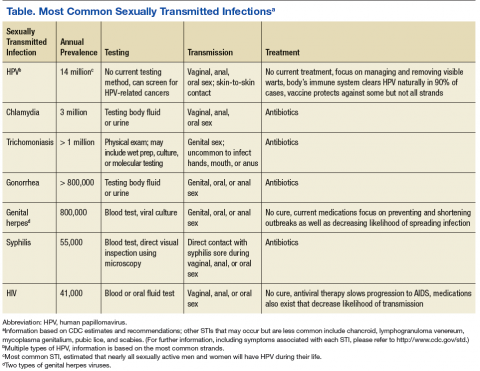
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
About 24% of women and 1% of men will experience military sexual trauma (MST) during their service.1 Despite the higher percentage of women reporting MST, the estimated number of men (55,491) and women (72,497) who endorse MST is relatively similar. Military sexual trauma is associated with negative psychosocial (eg, decreased quality of life) and psychiatric (eg, posttraumatic stress disorder [PTSD], depression) sequelae. Surís and colleagues provided a full review of sequelae, with PTSD being the most discussed consequence of MST.2 However, sexually transmitted infections (STIs) during or after MST are a consequence of growing concern.
Sexually Transmitted Infections
The link between sexual trauma and increased incidence of STIs is well established. Survivors of rape are at a higher risk of exposure to STIs due to unprotected sexual contact that may occur during the assault(s).3 Numerous studies have demonstrated that sexual trauma is directly related to greater engagement in risky sexual behaviors (eg, more sexual partners, unprotected sex, and “sex trading”).2,4
This relationship is particularly concerning given that individuals in the military tend to report sexual trauma with greater propensity than that reported in civilian populations.4 Additionally, military personnel and veteran populations tend to engage in high-risk behaviors (eg, alcohol and drug use) more often than their civilian counterparts, increasing their potential susceptibility to predatory sexual trauma(s) and victimization.2,5 Taken in aggregate, military personnel and veterans may be at increased risk for STIs compared with the civilian population due to the increased incidence of risky sexual behavior and sexual traumatization during military service.
Sexually transmitted infections potentially lead to immediate-term (eg, physical discomfort, sexual dysfunction) and long-term (eg, cancer, infertility) adverse health consequences.6 Early detection is crucial in the treatment of STIs because it can aid in preventing STI transmission and allow for early intervention. See Table for a list of common STIs and their prevalence, testing method, method of sexual transmission, and treatment. Early detection is also important because many STIs may be asymptomatic (eg, HIV, human papillomavirus [HPV]), which decreases the likelihood of seeking testing or treatment as well as increases the likelihood of transmission.7
Current Research
To date, only 1 study has explicitly examined the relationship between MST and STIs. In 2011, researchers analyzed a large national database of 420,725 male and female Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) veterans.6 In the study, both male and female OEF/OIF veterans who endorsed MST were significantly more likely than those who did not endorse MST to have a STI diagnosis. The researchers noted that this finding underscored the necessity for sexual health assessment in survivors of MST, to facilitate early detection and treatment.
STI Risk Assessment
Because military personnel and veterans often initially disclose their MST to a mental health provider (MHP), these providers operate in a unique circumstance where they may be the individual’s first point of contact for determining STI risk. In these circumstances, the MHP should consider the utility of briefly assessing the patient’s sexual health and making subsequent medical referrals as necessary.
To accurately assess a patient’s STI risk, the MHP should gather information regarding both current/acute risk (eg, “Have you had unprotected sexual contact, for instance, genital contact without a condom or oral sex without a dental dam, in the past month?”) as well as longer standing (eg, “Have you had unprotected sexual contact... in the past year?”) STI risk. Providers also should consider oral, anal, and genital modes of sexual contact as well as common STI symptoms (eg, warts, sores, genital discharge, and/or pain or burning sensation when peeing or during sex). Additionally, psychoeducation should be provided, especially information regarding the asymptomatic nature of certain STIs, such as HIV and HPV, and the risk of transmission in all forms of sexual contact, including nongenital anal contact. Appropriate referrals to sexual health education, including safe sex practices, also should be considered in order to minimize risk of future STI transmission.
Mental health providers also should determine the date of patient’s most recent STI test. Not all STIs can be detected via a blood test or routine yearly Papanicolaou (Pap test) physicals. Additionally, MHPs should be aware that risky practices, including substance misuse, are more common in survivors of MST and that there is an association between substance misuse and STIs.2,6 If testing has not occurred recently, MHPs should strongly encourage the individual to access STI testing and provide resources as necessary, such as access to low-cost STI testing. Further, if the MHP has reason to suspect the presence of an STI after the brief assessment (eg, individual endorses unprotected sexual contact or risky behaviors, including substance misuse, sex trading, and STI symptomatology; a positive STI test but the patient has not accessed treatment), an appropriate sexual health referral should be made.
During the assessment and psychoeducation processes, terminology and language plays an integral role. If a MHP assumes an individual has sexual contact only with opposite sex partners (eg, asking a male “How many women have you had sexual contact with in the past 30 days?” vs “How many partners have you had sexual contact with in the past 30 days?”), the MHP will not accurately assess the individual’s level of current risk. Additionally, it is important to remember that sexual behavior does not always align with sexual identity: A man who identifies as heterosexual may still have sexual contact with men. Due to the sensitive nature of sexual health, MHPs should be careful to use nonjudgmental language, such as using the term sex work rather than the more pejorative term prostitution, to avoid offending patients and to increase their likelihood to disclose sexual health information.
Nonjudgmental language is especially relevant when working with gender-minority veterans (eg, transgender, gender nonconforming, gender transitioning), because this clinical population has a higher risk of victimization and lower rates of help-seeking health behavior.7 In particular, a sizable portion of individuals who identify as transgender do not seek services out of fear that they will be discriminated against, humiliated, or misunderstood.7 To assuage these concerns, MHPs should ensure they refer to the veteran with the veteran’s preferred pronouns. For example, a MHP could ask “I would like to be respectful, how would you like to be addressed?” or “What name and pronoun would you like me/us to use?” Providers also should consider nonbinary pronouns when appropriate (eg, singular: ze/hir/hirs; plural: they/them/theirs). Providers also should recognize that making a mistake is not uncommon, and they should apologize to maintain rapport and maximize the patient’s comfort during this distressing process. Further, MHPs should consider additional training, education, and/or consultation if they feel uncomfortable or ill prepared when working with gender-minority veterans.
Future Research
Research has attempted to understand the consequences of MST on sexual health; however, despite these efforts, more research is necessary. The majority of published studies have focused on females even though a similar number of males have reported MST.2 This dearth of published studies likely is due to hesitation by male active-duty personnel and veterans to disclose or seek treatment for MST and because the percentage of females reporting MST is much higher. Males are less likely to report or seek treatment for MST because of stigma-based concerns (eg, shame, self-blame, privacy concerns).8,9 Therefore, it is difficult to acquire a sizable research sample to study. As previously noted, a single study has specifically examined MST and STI risk. Although this study included a sizable population of male OEF/OIF veterans, results have yet to be replicated in other clinical populations of interest, such as male military personnel and male veterans of other service eras.
Research is even more limited regarding lesbian, gay, bisexual, transgender, and other gender-minority military personnel and veterans. Although researchers propose that these populations may experience a similar, or even heightened, likelihood of MST during their service, no empirical research yet exists to fully examine this hypothesis.10,11 It is important to note that the paucity of research attention may be related to the Don’t Ask, Don’t Tell (DADT) policy, which obstructed the open discussion and empirical examination of sexual and gender minorities within military populations.11 The DADT policy led to limited awareness and greater stigmatization among sexual- and gender-minority personnel, resulting in poorer sexual health outcomes in these populations.10,11 With the end of DADT in 2011, it is now imperative for future research to examine the prevalence and associated consequences of MST in sexual- and gender-minority military personnel and veterans.
Conclusion
The DoD and VA should be commended for their continued focus on understanding the health consequences of MST. These efforts have yielded substantial information regarding the negative effects of MST on sexual health; in particular, increased risk for STIs. These findings suggest that MHPs may, at times, be the first point of contact for MST-related sexual health concerns. These providers should be aware of their ability to assess for STI risk and make appropriate referrals to facilitate early detection and access to treatment. Despite the presence of MST-related sexual health research, continued research remains necessary. In particular, a broader focus that includes other genders (eg, male, transgender) and sexual minorities would further inform research and clinical practice.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
1. Military Sexual Trauma Support Team. Military sexual trauma (MST) screening report fiscal year 2012. Washington DC: U.S. Department of Veterans Affairs, Office of Patient Care Services, Mental Health Services; 2013.
2. Surís A, Holliday R, Weitlauf JC, North CS; the Veteran Safety Initiative Writing Collaborative. Military sexual trauma in the context of veterans’ life experiences. Fed Pract. 2013;30(suppl 3):16S-20S.
3. Jenny C, Hooton TM, Bowers A, et al. Sexually transmitted diseases in victims of rape. N Eng J Med. 1990;322(11):713-716.
4. Senn TE, Carey MP, Vanable PA, Coury-Doniger P, Urban MA. Childhood sexual abuse and sexual risk behavior among men and women attending a sexually transmitted disease clinic. J Consult Clin Psychol. 2006;74(4):720-731.
5. Schultz JR, Bell KM, Naugle AE, Polusny MA. Child sexual abuse and adult sexual assault among military veteran and civilian women. Mil Med. 2006;171(8):723-728.
6. Turchik JA, Pavao J, Nazarian D, Iqbal S, McLean C, Kimerling R. Sexually transmitted infections and sexual dysfunctions among newly returned veterans with and without military sexual trauma. Int J Sex Health. 2012;24(1):45-59.
7. National LGBT Health Education Center. Affirmative care for transgender and gender non-conforming people: best practices for front-line health care staff. https://www.lgbthealth education.org/wp-content/uploads/2016/12/Affirmative-Care-for-Transgender -and-Gender-Non-conforming-People-Best-Practices -for-Frontline-Health-Care-Staff.pdf. Published 2016. Accessed February 16, 2017.
8. Morris EE, Smith JC, Farooqui SY, Surís AM. Unseen battles: the recognition, assessment, and treatment issues of men with military sexual trauma (MST). Trauma Violence Abuse. 2014;15(2):94-101.
9. Turchik JA, Edwards KM. Myths about male rape: a literature review. Psychol Men Masc. 2012;13(2):211-226.
10. Mattocks KM, Kauth MR, Sandfort T, Matza AR, Sullivan JC, Shipherd J. Understanding health-care needs of sexual and gender minority veterans: how targeted research and policy can improve health. LGBT Health. 2014;1(1):50-57.
11. Burks DJ. Lesbian, gay, and bisexual victimization in the military: an unintended consequence of “Don’t Ask, Don’t Tell”? Am Psychol. 2011;66(7):604-613.
BEST PRACTICES: Reinforced Reload Stapling in the Thoracic Space
Air leaks have been reported to be frequent and difficult-to-manage sequelae in some lung resection procedures. Click here to learn about a new device being used in the thoracic space
Author:
Mark Ellis Ginsburg,
MD, FACS
Associate Clinical Professor of Surgery
Division of Cardiothoracic Surgery
Columbia University Medical Center
New York, NY
Faculty Disclosure: Dr. Ginsburg has received compensation from Medtronic Inc. for his participation in this article. He has no other conflicts of interest to disclose.
Air leaks have been reported to be frequent and difficult-to-manage sequelae in some lung resection procedures. Click here to learn about a new device being used in the thoracic space
Author:
Mark Ellis Ginsburg,
MD, FACS
Associate Clinical Professor of Surgery
Division of Cardiothoracic Surgery
Columbia University Medical Center
New York, NY
Faculty Disclosure: Dr. Ginsburg has received compensation from Medtronic Inc. for his participation in this article. He has no other conflicts of interest to disclose.
Air leaks have been reported to be frequent and difficult-to-manage sequelae in some lung resection procedures. Click here to learn about a new device being used in the thoracic space
Author:
Mark Ellis Ginsburg,
MD, FACS
Associate Clinical Professor of Surgery
Division of Cardiothoracic Surgery
Columbia University Medical Center
New York, NY
Faculty Disclosure: Dr. Ginsburg has received compensation from Medtronic Inc. for his participation in this article. He has no other conflicts of interest to disclose.
Development and Implementation of a Veterans’ Cancer Survivorship Program
The aging of the U.S. population has led to an increase in the number of patients diagnosed with cancer each year. Fortunately, advances in screening, detection, and treatments have contributed to an improvement in cancer survival rates during the past few decades. More than 1.6 million new cases of cancer are expected to be diagnosed in 2014. It is estimated that there are currently 14 million cancer survivors, and the number of survivors by 2022 is expected to be 18 million.1,2
The growing number of cancer survivors is exceeding the ability of the cancer care system to meet the demand.3 Many primary care providers (PCPs) lack the confidence to provide cancer surveillance for survivors, but at the same time, patients and physicians continue to expect that PCPs will play a substantial role in general preventive health and in treating other medical problems.4 These conditions make it critical that at a minimum, survivorship care is integrated between oncology and primary care teams through a systematic, coordinated plan.5 This integration is especially important for the vulnerable population of veterans who are cancer survivors, as they have additional survivorship needs.
The purpose of this article is to assist other VA health care providers in establishing a cancer survivorship program to address the unique needs of veterans not only during active treatment, but after their initial treatment is completed. Described are the unique needs of veterans who are cancer survivors and the development and implementation of a cancer survivorship program at a large metropolitan VAMC, which is grounded in VA and national guidelines and evidence-based cancer care. Lessons learned and recommendations for other VA programs seeking to improve coordination of care for veteran cancer survivors are presented.
Cancer Survivorship
The Institute of Medicine (IOM) report, From Cancer Patient to Cancer Survivor: Lost in Transition, identified the importance of providing quality survivorship care to those “living with, through, and beyond a diagnosis of cancer.”6,7 The period of survivorship extends from the time of diagnosis, through treatment, long-term survival, and end-of-life.8,9 Although there are several definitions of cancer survivor, the most widely accepted definition is one who has been diagnosed with cancer, regardless of their position on the disease trajectory.8
The complex needs of cancer survivors encompass physical, psychological, social, and spiritual concerns across the disease trajectory.3 Cancer survivors who are also veterans have additional needs and risk factors related to their service that can make survivorship care more challenging.10 Veterans tend to be older compared with the age of the general population, have more comorbid conditions, and many have combat-related posttraumatic stress disorder (PTSD), all of which can complicate the survivorship experience.11
The first challenge for veteran cancer survivors is in the term cancer survivor, which may take on a different meaning for a veteran when compared with a civilian. For some civilians and veterans, survivor is a constant reminder of having had cancer. There are some veterans who prefer not to be called survivors, because they do not feel worthy of this terminology. They believe they have not struggled enough to self-identify as a survivor and that survivorship is “something to be earned, following a physically grueling experience.”12
The meaning of the word survivor may even be culturally linked to the population of veterans who have survived a life-threatening combat experience. More research is needed to understand the veteran cancer survivorship experience. The meaning of survivorship must be explored with each veteran, as it may influence his or her adherence to a survivorship plan of care.
Veterans make up a unique subset of cancer survivors, in part because of risk factors associated with their service. Many veterans developed cancer as a result of their military exposure to toxic chemicals and radiation. To date, VA recognizes that chronic B-cell leukemias, Hodgkin disease, multiple myeloma, non-Hodgkin lymphomas, prostate cancer, respiratory cancers, and soft tissue sarcomas are all presumptive diseases related to Agent Orange exposure.13 There are other substances also presumed to increase the risk of certain cancers in veterans who have had ionizing radiation exposure.14 There is still much to learn regarding veterans who served during the Gulf War, Operation Enduring Freedom, and Operation Iraqi Freedom.15,16
In a comparison of VA data files with U.S. SEER data files from 2007, researchers identified differences in characteristics between veteran cancer survivors and civilian cancer survivors.17 In addition to increased exposure risks, the veteran cancer survivor population is older than the general cancer survivorship population and is mostly male.17 Veterans’ comorbid conditions, such as type 2 diabetes, ischemic heart disease, Parkinson disease, and peripheral neuropathy, which may be service related, complicate survivorship.17 These characteristics (age, gender, exposure risks, and comorbid conditions) influence the type of cancer diagnosed and treatment options, and they may ultimately impact survivorship needs
(Table 1).
The prevalence of mental health issues in the veteran population is significant.18 Posttraumatic stress disorder affects 7% to 8% of the general population at some point during their lifetime and as many as 16% of those returning from military deployment.19 In a predominantly
male veteran study correlating combat PTSD with cancerrelated PTSD, about half the participants (n = 170) met PTSD Criterion A, viewing their cancer as a traumatic experience.20 Posttraumatic stress disorder, depression, anxiety, and addictive disease all must be addressed in the survivorship plan of care.
Poor mental health has been linked to increased morbidity and mortality and can limit the veteran’s ability to participate in health promotion and medical care.21 Distress related to cancer is well recognized in the civilian population.22,23 Veterans are at risk for moderate-tosevere disabling distress, especially when the cancer is associated with their military service. Vietnam veterans who have a diagnosis of cancer report that they have already served their time and are now serving it again, having to wage a battle on cancer and undergo difficult treatments and associated adverse effects (AEs).24 It is important to note, however, that some veterans have developed strong coping skills, which gives them strength and resilience for the survivorship experience.25
Other factors also contribute to veterans’ unique survivorship needs. Many veterans have limited social and/or economic resources, making it difficult to receive cancer treatment and follow recommendations for a healthful lifestyle as a cancer survivor. Demographics from the VA have illustrated that many veterans have a limited support system (65% do not have a spouse), and many have low incomes.26 Although veterans comprise about 11% of the general population, they make up 26% of the homeless population.26 It is estimated that 260,000 veterans are homeless at some time during the course of a year, and of these, 45% have mental health issues and 70% have substance abuse problems.27 Basic needs such as housing, running water, heat and electricity, and nutrition must be met in order to prevent infection during treatment, maximize the benefit, and reduce the risks associated with treatment. Transportation issues can make it challenging to travel to medical centers for cancer surveillance following treatment.
Models of Care
As defined in the aforementioned IOM report, multiple models of survivorship care have surfaced over the years.6 Much that was originally seen and implemented in adult cancer survivorship was known from pediatric cancer care. Early models that surfaced included shared care models, nurse-led models, and tertiary survivorship clinics. Each model has its strengths and disadvantages.
The shared care model of survivorship involves a sharing of the responsibility for the survivor among different specialties, potentially at different facilities, and the primary care team. Typically, the PCP refers the patient to the oncologist when cancer is suspected or diagnosed. The primary care team continues to provide routine health maintenance and manages other health problems while the oncology team provides cancer care. The patient is transitioned back to the primary care team with a survivorship care plan (SCP) at 1 to 2 years after completion of cancer therapy or at the discretion of the oncology team.28 For
this model to work, the PCP must be willing to take on this responsibility, and there must be a coordinated effort for seamless communication between teams, which can be potentially challenging.
Nurse-led programs emerged in the pediatric populations. Pediatric nurse-led clinics assume care of the patient after active treatment to manage long-term AEs of cancer treatments, symptom management, care planning, and education. A comprehensive review of the literature identified that “nurse-led follow-up services are acceptable, appropriate, and effective.”6 Barriers to this model of care include a shortage of trained oncology nurses and a preference for physician follow-up by some cancer survivors who want the security of their oncologist for ongoing, long-term care.6
Survivorship follow-up clinics, a tertiary model of care, have been implemented at some larger academic centers. These clinics focus on cancer survivorship and are often separate from other routine health care visits. Typically, these clinics include multiple specialties and are often disease-specific. These types of clinics pose a different set of challenges regarding duplication of services and reimbursement issues.
As of yet, no model has been proven more effective than the others. Each institution and patient population may not lend themselves to a one-size-fits-all model. There may be different models of care needed, based on patient population. Regardless of the model selected, individualized survivorship care plans are an essential component of quality cancer survivorship care.
Addessing Survivorship Care
In 2009, 5 interdisciplinary leaders in VA cancer care (Ellen Ballard, RN; David Haggstrom, MD, MAS; Veronica Reis, PhD; Mark Detzer, PhD; and Tina Gill, MA) attended a breakout session on psychosocial oncology at the Association of VA Hematology and Oncology (AVAHO) meeting in Minneapolis, Minnesota, and most members of this team participated in the 2009-2012 VHA Cancer Care Collaboratives to improve the timeliness and quality of care for veterans who were cancer patients. Dr. Haggstrom and Ms. Ballard developed a SharePoint site for the Survivorship Special Interest Group (SIG) members through the Loma Linda VAMC in California. The SIG workgroup then built the Cancer Survivorship Toolkit, composed of
5 critical tools (Figure).
In July 2012, the VA Cancer Survivorship Toolkit content was disseminated at AVAHO and launched behind the VA firewall. It subsequently received accolades from the national program director for VHA Oncology and was listed on the American College of Surgeons Commission on Cancer (CoC) Best Practices website. The toolkit is accessible to all VA programs, and suggestions for new content can be submitted directly on the site (Figure).
The development of a SCP began in late 2011 when SIG members collected examples of SCPs from leading organizations. The members compared this content with the IOM recommendations for SCPs and developed a template. The template was programmed for the VHA computerized patient record system (CPRS) and placed on the internal VA toolkit website. The template included the treatment summary and care plan. The treatment summary portion included the diagnosis and tumor characteristics, diagnostic tests used, dates and types of treatment, chemoprevention or maintenance treatments, supportive services required, the surveillance plan, and signs of recurrence. The care plan portion provided information on the likely course of recovery and a checklist for common long-term AEs in the areas of psychological distress, financial and practical effects, and physical effects. Also included was information about referral, health behaviors, late effects that may develop, contact information, and general resource information.
The computer applications coordinator at any VA can download the template from the toolkit onto their CPRS, and the template can then be brought into any progress note. Individual sites may also edit the template to suit specific needs. The SCP can be completed by any clinician with the appropriate clinical competencies. To date, > 50 sites have downloaded the SCP template for use.
Cancer Survivorship Clinic
At the Louis Stokes Cleveland (LSC) VAMC, a nurse-led model of a cancer survivorship clinic was established with an expert nurse practitioner (NP). A major catalyst for the development of this clinic was the receipt of a Specialty Care Education Center of Excellence, funded by the Offices of Specialty Care and Academic Affiliations. A priority of this project was the implementation of survivorship care for every veteran with a cancer diagnosis. A system redesign was implemented to deliver quality, cost-effective, patient-centered cancer care within an interprofessional, team-based practice. This clinic is imbedded within an interdisciplinary clinic setting where the NP works in close collaboration with the medical and surgical oncologists as well as providers from mental health, social work, nutrition, physical therapy, and others.
The first patients to receive survivorship care in this new model from the time of their diagnosis were veterans with breast cancer, sarcoma, melanoma, and lymphomas. Veterans are followed jointly by the NP and the medical and surgical oncologists during active treatment. The NP provides physical symptom assessment and management for patients both during and after treatment.
At the end of active treatment, patient visits are alternated between oncology physicians and the survivorship NP for 5 years. The timeline for follow-up visits is based on National Comprehensive Cancer Network guidelines for each cancer type but then individualized based on patient need.29 During this 5-year time period, patients under active surveillance whose conditions have been stable are seen by the NP. Any concerning symptoms are immediately relayed to the primary oncologist or surgical oncologist, often the same day, and patients can be seen the same day if necessary, to improve coordination and access to services.
A unique focus of the clinic is the integration of health promotion and risk reduction that coincides with the active surveillance plan. This transition of active surveillance patients to the NP-led survivorship clinic not only opens access to newly diagnosed cancer patients to be seen by the oncologist, but also allows for seamless transition and coordination to active surveillance. Within the clinic structure, patients receive patient navigation beginning with a cancer concern; patients also receive screening for psychosocial distress at the time of diagnosis and at every visit. Patient navigation and distress screening are both considered essential elements to survivorship care in the most recent CoC guidelines.30 The survivorship NP keeps the primary care team up-to-date regarding patient care across the disease trajectory by alerting them to updates electronically in the CPRS in real time.
Survivorship Care Plan
A focus of the clinic has also been on the implementation of a formal SCP to be completed 3 months after the conclusion of active treatment. The formal SCP was downloaded from the Cancer Survivorship Toolkit and is composed of a 3-part summary. The 3 parts consist of the treatment summary, the plan for rehabilitation, and the plan for the future. The first section of the SCP is completed by the medical oncologist as a summary of treatment received by the veteran. The summary of treatment section is reviewed and discussed with the veteran survivor at the visit, and the second and third sections are completed during the 3-month follow-up visit with the veteran.
Success and Areas for Improvement
The survivorship clinic has been well received by veterans. Patient satisfaction scores have been overwhelmingly positive. Veterans appreciate and feel comfortable knowing their providers from the beginning of diagnosis along the entire disease trajectory. They know that if problems arise, the survivorship NP has direct access to the medical or surgical oncologist for immediate review.
The difficult challenge for the cancer care providers is to know when is the right time to transition care back to the PCP. Transitions of care often come with high anxiety and a sense of loss for the veteran. The 5-year survival mark is not always the appropriate transition time for some veterans. Those with extensive physical and mental health issues may need continuity of care and continued support from the oncology team.
The SCP has presented challenges in terms of when to complete and who should complete the form. There has also been concern over the length of the summary, how long it will take to complete the document, and which summary template to use. Areas for improvement with the template could potentially be to automate population of the chemotherapy and radiation summaries. Some software packages are available, but they are costly. Another issue with external software is getting it accepted by VHA and incorporated into the CPRS.
Recommendations
Many cancer programs are struggling to provide highquality survivorship care. The CoC, recognizing the challenges programs are having implementing survivorship care, has extended the accreditation requirement for full implementation from 2015 to 2019.31
The following recommendations should be considered for the successful implementation of a new survivorship program:
- Collect information from multiple resources to guide the establishment of the survivorship clinic;
- Become familiar with the IOM From Cancer Patient to Cancer Survivor: Lost in Transition;6
- Understand local issues and barriers specific to your care delivery system;
- Collaborate with key stakeholders from multiple specialties to gain momentum and buy-in;
- Hold regular meetings with stakeholders as well as leadership to identify and remove barriers to the clinic success;
- Join the VA Survivorship SIG to collaborate with other sites who have already started to pilot survivorship programs and discuss barriers to and successes of programs so as to not reinvent the wheel;
- Utilize the Cancer Survivorship Toolkit;
- Download the SCP;
- Establish a close partnership with your local cancer committee; and
- Collect and report data to show effectiveness and need.
All these strategies were vital to the success of the LSCVAMC survivorship program.
Summary
The VA is uniquely positioned to be a leader in highquality, comprehensive, and veteran-centered cancer survivorship care in the years ahead. The close relationship between specialty and primary care allows for smooth continuity of care and easy transitions between oncology and primary care. The comprehensive CPRS allows easy accessibility to information for the entire health care team. The Cancer Survivorship Toolkit provides a template of the survivorship care plan for the veteran and his or her health care providers.
The LSCVAMC is one of many VA institutions implementing quality care for cancer survivors and can serve as a role model for other VA programs initiating the survivorship care process (Table 2).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Levit LA, Balough EP, Nass SJ, Ganz PA, eds. Washington, DC: The National Academies Press; 2013.
3. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;(suppl 18):15-22.
4. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
5. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
6. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Hewitt M, Greenfield S, Stovall E, eds. Washington, DC: The National Academies Press; 2006.
7. Clark EJ, Stovall EL, Leigh S, Siu AL, Austin DK, Rowland JH, eds. Imperatives for Quality Cancer Care: Access, Advocacy, Action, and Accountability. Silver Spring, MD: National Coalition for Cancer Survivorship; 1996.
8. National Cancer Institute. Survivorship. NCI Dictionary of Cancer Terms Website. http://www.cancer.gov/dictionary?CdrID=445089. Accessed December 3, 2014.
9. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
10. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
11. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: Protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93.
12. Beehler GP, Rodriques AE, Kay MA, Kiviniemi MT, Steinbrenner L. Lasting impact: Understanding the psychosocial implications of cancer among military veterans. J Psychosoc Oncol. 2013:31(4):430-450.
13. U.S. Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Public Health Website. http://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp. Updated December 30, 2013. Accessed December3, 2014.
14. U.S. Department of Veterans Affairs. Radiation. Public Health Website. http://www.publichealth.va.gov/exposures/radiation/index.asp. Updated December 31, 2013. Accessed December 3, 2014.
15. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
16. U.S. Department of Veterans Affairs. Gulf War veterans’ illnesses. Public Health Website. http://www.publichealth.va.gov/exposures/gulfwar/index.asp. Updated November 7, 2014. Accessed December 3, 2014.
17. National Cancer Institute. Cancer query systems. Surveillance, Epidemiology, and End Results Program Website. http://seer.cancer.gov/canques/index.html. Accessed December 3, 2014.
18. Suicide in the military: Army-NIH funded study points to risk and protective factors [news release]. Washington, DC: National Institute of Mental Health; March 3, 2014. http://www.nimh.nih.gov/news/science-news/2014/suicide-in-the-military-army-nih-funded-study-points-to-risk-and-protective-factors.shtml. Accessed December 3, 2014.
19. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
20. Mulligan EA, Schuster Wachen J, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: Prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
21. Musuuza JS, Sherman ME, Knudsen KJ, Sweeney HA, Tyler CV, Koroukian SM. Analyzing excess mortality from cancer among individuals with mental illness. Cancer. 2013;119(13):2469-2476.
22. Zabora J, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2013:30(6):625-635.
23. Holland JC, Andersen B, Breitbart WS, et al. Distress management: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2010:8(4):448-485.
24. Grassman DL. The Hero Within. St. Petersburg, FL: Vandamere Press; 2012.
25. Jahn AL, Herman L, Schuster J, Naik A, Moye J. Distress and resilience after cancer
in veterans. Res Hum Dev. 2012;9(3):229-247.
26. National Association of Social Workers. Social workers speak on veterans issues June 2009. National Association of Social Workers Website. http://www.naswdc.org/pressroom/2009/Social%20Work%20Veterans%20Fact%20Sheet.pdf. Accessed December 3, 2014.
27. Homeless. U.S. Department of Veterans Affairs Website. http://www.va.gov/homeless. Accessed December 3, 2014.
28. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117-5124.
29. NCCN Guidelines. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed December 3, 2014.
30. American College of Surgeons, Commission on Cancer. Cancer program standards 2012, version 1.2.1: Ensuring patient-centered care. https://www.facs.org/~/media/file/quality%20programs/cancer/coc/programstandards2012.ashx. Published January 21, 2014. Accessed December 3, 2014.
31. Accreditation committee clarifications for standard 3.3 survivorship care plan. American College of Surgeons Website. https://www.facs.org/publications/newsletters/coc-source/special-source/standard33. Published September 9, 2014. Accessed December 3, 2014.
The aging of the U.S. population has led to an increase in the number of patients diagnosed with cancer each year. Fortunately, advances in screening, detection, and treatments have contributed to an improvement in cancer survival rates during the past few decades. More than 1.6 million new cases of cancer are expected to be diagnosed in 2014. It is estimated that there are currently 14 million cancer survivors, and the number of survivors by 2022 is expected to be 18 million.1,2
The growing number of cancer survivors is exceeding the ability of the cancer care system to meet the demand.3 Many primary care providers (PCPs) lack the confidence to provide cancer surveillance for survivors, but at the same time, patients and physicians continue to expect that PCPs will play a substantial role in general preventive health and in treating other medical problems.4 These conditions make it critical that at a minimum, survivorship care is integrated between oncology and primary care teams through a systematic, coordinated plan.5 This integration is especially important for the vulnerable population of veterans who are cancer survivors, as they have additional survivorship needs.
The purpose of this article is to assist other VA health care providers in establishing a cancer survivorship program to address the unique needs of veterans not only during active treatment, but after their initial treatment is completed. Described are the unique needs of veterans who are cancer survivors and the development and implementation of a cancer survivorship program at a large metropolitan VAMC, which is grounded in VA and national guidelines and evidence-based cancer care. Lessons learned and recommendations for other VA programs seeking to improve coordination of care for veteran cancer survivors are presented.
Cancer Survivorship
The Institute of Medicine (IOM) report, From Cancer Patient to Cancer Survivor: Lost in Transition, identified the importance of providing quality survivorship care to those “living with, through, and beyond a diagnosis of cancer.”6,7 The period of survivorship extends from the time of diagnosis, through treatment, long-term survival, and end-of-life.8,9 Although there are several definitions of cancer survivor, the most widely accepted definition is one who has been diagnosed with cancer, regardless of their position on the disease trajectory.8
The complex needs of cancer survivors encompass physical, psychological, social, and spiritual concerns across the disease trajectory.3 Cancer survivors who are also veterans have additional needs and risk factors related to their service that can make survivorship care more challenging.10 Veterans tend to be older compared with the age of the general population, have more comorbid conditions, and many have combat-related posttraumatic stress disorder (PTSD), all of which can complicate the survivorship experience.11
The first challenge for veteran cancer survivors is in the term cancer survivor, which may take on a different meaning for a veteran when compared with a civilian. For some civilians and veterans, survivor is a constant reminder of having had cancer. There are some veterans who prefer not to be called survivors, because they do not feel worthy of this terminology. They believe they have not struggled enough to self-identify as a survivor and that survivorship is “something to be earned, following a physically grueling experience.”12
The meaning of the word survivor may even be culturally linked to the population of veterans who have survived a life-threatening combat experience. More research is needed to understand the veteran cancer survivorship experience. The meaning of survivorship must be explored with each veteran, as it may influence his or her adherence to a survivorship plan of care.
Veterans make up a unique subset of cancer survivors, in part because of risk factors associated with their service. Many veterans developed cancer as a result of their military exposure to toxic chemicals and radiation. To date, VA recognizes that chronic B-cell leukemias, Hodgkin disease, multiple myeloma, non-Hodgkin lymphomas, prostate cancer, respiratory cancers, and soft tissue sarcomas are all presumptive diseases related to Agent Orange exposure.13 There are other substances also presumed to increase the risk of certain cancers in veterans who have had ionizing radiation exposure.14 There is still much to learn regarding veterans who served during the Gulf War, Operation Enduring Freedom, and Operation Iraqi Freedom.15,16
In a comparison of VA data files with U.S. SEER data files from 2007, researchers identified differences in characteristics between veteran cancer survivors and civilian cancer survivors.17 In addition to increased exposure risks, the veteran cancer survivor population is older than the general cancer survivorship population and is mostly male.17 Veterans’ comorbid conditions, such as type 2 diabetes, ischemic heart disease, Parkinson disease, and peripheral neuropathy, which may be service related, complicate survivorship.17 These characteristics (age, gender, exposure risks, and comorbid conditions) influence the type of cancer diagnosed and treatment options, and they may ultimately impact survivorship needs
(Table 1).
The prevalence of mental health issues in the veteran population is significant.18 Posttraumatic stress disorder affects 7% to 8% of the general population at some point during their lifetime and as many as 16% of those returning from military deployment.19 In a predominantly
male veteran study correlating combat PTSD with cancerrelated PTSD, about half the participants (n = 170) met PTSD Criterion A, viewing their cancer as a traumatic experience.20 Posttraumatic stress disorder, depression, anxiety, and addictive disease all must be addressed in the survivorship plan of care.
Poor mental health has been linked to increased morbidity and mortality and can limit the veteran’s ability to participate in health promotion and medical care.21 Distress related to cancer is well recognized in the civilian population.22,23 Veterans are at risk for moderate-tosevere disabling distress, especially when the cancer is associated with their military service. Vietnam veterans who have a diagnosis of cancer report that they have already served their time and are now serving it again, having to wage a battle on cancer and undergo difficult treatments and associated adverse effects (AEs).24 It is important to note, however, that some veterans have developed strong coping skills, which gives them strength and resilience for the survivorship experience.25
Other factors also contribute to veterans’ unique survivorship needs. Many veterans have limited social and/or economic resources, making it difficult to receive cancer treatment and follow recommendations for a healthful lifestyle as a cancer survivor. Demographics from the VA have illustrated that many veterans have a limited support system (65% do not have a spouse), and many have low incomes.26 Although veterans comprise about 11% of the general population, they make up 26% of the homeless population.26 It is estimated that 260,000 veterans are homeless at some time during the course of a year, and of these, 45% have mental health issues and 70% have substance abuse problems.27 Basic needs such as housing, running water, heat and electricity, and nutrition must be met in order to prevent infection during treatment, maximize the benefit, and reduce the risks associated with treatment. Transportation issues can make it challenging to travel to medical centers for cancer surveillance following treatment.
Models of Care
As defined in the aforementioned IOM report, multiple models of survivorship care have surfaced over the years.6 Much that was originally seen and implemented in adult cancer survivorship was known from pediatric cancer care. Early models that surfaced included shared care models, nurse-led models, and tertiary survivorship clinics. Each model has its strengths and disadvantages.
The shared care model of survivorship involves a sharing of the responsibility for the survivor among different specialties, potentially at different facilities, and the primary care team. Typically, the PCP refers the patient to the oncologist when cancer is suspected or diagnosed. The primary care team continues to provide routine health maintenance and manages other health problems while the oncology team provides cancer care. The patient is transitioned back to the primary care team with a survivorship care plan (SCP) at 1 to 2 years after completion of cancer therapy or at the discretion of the oncology team.28 For
this model to work, the PCP must be willing to take on this responsibility, and there must be a coordinated effort for seamless communication between teams, which can be potentially challenging.
Nurse-led programs emerged in the pediatric populations. Pediatric nurse-led clinics assume care of the patient after active treatment to manage long-term AEs of cancer treatments, symptom management, care planning, and education. A comprehensive review of the literature identified that “nurse-led follow-up services are acceptable, appropriate, and effective.”6 Barriers to this model of care include a shortage of trained oncology nurses and a preference for physician follow-up by some cancer survivors who want the security of their oncologist for ongoing, long-term care.6
Survivorship follow-up clinics, a tertiary model of care, have been implemented at some larger academic centers. These clinics focus on cancer survivorship and are often separate from other routine health care visits. Typically, these clinics include multiple specialties and are often disease-specific. These types of clinics pose a different set of challenges regarding duplication of services and reimbursement issues.
As of yet, no model has been proven more effective than the others. Each institution and patient population may not lend themselves to a one-size-fits-all model. There may be different models of care needed, based on patient population. Regardless of the model selected, individualized survivorship care plans are an essential component of quality cancer survivorship care.
Addessing Survivorship Care
In 2009, 5 interdisciplinary leaders in VA cancer care (Ellen Ballard, RN; David Haggstrom, MD, MAS; Veronica Reis, PhD; Mark Detzer, PhD; and Tina Gill, MA) attended a breakout session on psychosocial oncology at the Association of VA Hematology and Oncology (AVAHO) meeting in Minneapolis, Minnesota, and most members of this team participated in the 2009-2012 VHA Cancer Care Collaboratives to improve the timeliness and quality of care for veterans who were cancer patients. Dr. Haggstrom and Ms. Ballard developed a SharePoint site for the Survivorship Special Interest Group (SIG) members through the Loma Linda VAMC in California. The SIG workgroup then built the Cancer Survivorship Toolkit, composed of
5 critical tools (Figure).
In July 2012, the VA Cancer Survivorship Toolkit content was disseminated at AVAHO and launched behind the VA firewall. It subsequently received accolades from the national program director for VHA Oncology and was listed on the American College of Surgeons Commission on Cancer (CoC) Best Practices website. The toolkit is accessible to all VA programs, and suggestions for new content can be submitted directly on the site (Figure).
The development of a SCP began in late 2011 when SIG members collected examples of SCPs from leading organizations. The members compared this content with the IOM recommendations for SCPs and developed a template. The template was programmed for the VHA computerized patient record system (CPRS) and placed on the internal VA toolkit website. The template included the treatment summary and care plan. The treatment summary portion included the diagnosis and tumor characteristics, diagnostic tests used, dates and types of treatment, chemoprevention or maintenance treatments, supportive services required, the surveillance plan, and signs of recurrence. The care plan portion provided information on the likely course of recovery and a checklist for common long-term AEs in the areas of psychological distress, financial and practical effects, and physical effects. Also included was information about referral, health behaviors, late effects that may develop, contact information, and general resource information.
The computer applications coordinator at any VA can download the template from the toolkit onto their CPRS, and the template can then be brought into any progress note. Individual sites may also edit the template to suit specific needs. The SCP can be completed by any clinician with the appropriate clinical competencies. To date, > 50 sites have downloaded the SCP template for use.
Cancer Survivorship Clinic
At the Louis Stokes Cleveland (LSC) VAMC, a nurse-led model of a cancer survivorship clinic was established with an expert nurse practitioner (NP). A major catalyst for the development of this clinic was the receipt of a Specialty Care Education Center of Excellence, funded by the Offices of Specialty Care and Academic Affiliations. A priority of this project was the implementation of survivorship care for every veteran with a cancer diagnosis. A system redesign was implemented to deliver quality, cost-effective, patient-centered cancer care within an interprofessional, team-based practice. This clinic is imbedded within an interdisciplinary clinic setting where the NP works in close collaboration with the medical and surgical oncologists as well as providers from mental health, social work, nutrition, physical therapy, and others.
The first patients to receive survivorship care in this new model from the time of their diagnosis were veterans with breast cancer, sarcoma, melanoma, and lymphomas. Veterans are followed jointly by the NP and the medical and surgical oncologists during active treatment. The NP provides physical symptom assessment and management for patients both during and after treatment.
At the end of active treatment, patient visits are alternated between oncology physicians and the survivorship NP for 5 years. The timeline for follow-up visits is based on National Comprehensive Cancer Network guidelines for each cancer type but then individualized based on patient need.29 During this 5-year time period, patients under active surveillance whose conditions have been stable are seen by the NP. Any concerning symptoms are immediately relayed to the primary oncologist or surgical oncologist, often the same day, and patients can be seen the same day if necessary, to improve coordination and access to services.
A unique focus of the clinic is the integration of health promotion and risk reduction that coincides with the active surveillance plan. This transition of active surveillance patients to the NP-led survivorship clinic not only opens access to newly diagnosed cancer patients to be seen by the oncologist, but also allows for seamless transition and coordination to active surveillance. Within the clinic structure, patients receive patient navigation beginning with a cancer concern; patients also receive screening for psychosocial distress at the time of diagnosis and at every visit. Patient navigation and distress screening are both considered essential elements to survivorship care in the most recent CoC guidelines.30 The survivorship NP keeps the primary care team up-to-date regarding patient care across the disease trajectory by alerting them to updates electronically in the CPRS in real time.
Survivorship Care Plan
A focus of the clinic has also been on the implementation of a formal SCP to be completed 3 months after the conclusion of active treatment. The formal SCP was downloaded from the Cancer Survivorship Toolkit and is composed of a 3-part summary. The 3 parts consist of the treatment summary, the plan for rehabilitation, and the plan for the future. The first section of the SCP is completed by the medical oncologist as a summary of treatment received by the veteran. The summary of treatment section is reviewed and discussed with the veteran survivor at the visit, and the second and third sections are completed during the 3-month follow-up visit with the veteran.
Success and Areas for Improvement
The survivorship clinic has been well received by veterans. Patient satisfaction scores have been overwhelmingly positive. Veterans appreciate and feel comfortable knowing their providers from the beginning of diagnosis along the entire disease trajectory. They know that if problems arise, the survivorship NP has direct access to the medical or surgical oncologist for immediate review.
The difficult challenge for the cancer care providers is to know when is the right time to transition care back to the PCP. Transitions of care often come with high anxiety and a sense of loss for the veteran. The 5-year survival mark is not always the appropriate transition time for some veterans. Those with extensive physical and mental health issues may need continuity of care and continued support from the oncology team.
The SCP has presented challenges in terms of when to complete and who should complete the form. There has also been concern over the length of the summary, how long it will take to complete the document, and which summary template to use. Areas for improvement with the template could potentially be to automate population of the chemotherapy and radiation summaries. Some software packages are available, but they are costly. Another issue with external software is getting it accepted by VHA and incorporated into the CPRS.
Recommendations
Many cancer programs are struggling to provide highquality survivorship care. The CoC, recognizing the challenges programs are having implementing survivorship care, has extended the accreditation requirement for full implementation from 2015 to 2019.31
The following recommendations should be considered for the successful implementation of a new survivorship program:
- Collect information from multiple resources to guide the establishment of the survivorship clinic;
- Become familiar with the IOM From Cancer Patient to Cancer Survivor: Lost in Transition;6
- Understand local issues and barriers specific to your care delivery system;
- Collaborate with key stakeholders from multiple specialties to gain momentum and buy-in;
- Hold regular meetings with stakeholders as well as leadership to identify and remove barriers to the clinic success;
- Join the VA Survivorship SIG to collaborate with other sites who have already started to pilot survivorship programs and discuss barriers to and successes of programs so as to not reinvent the wheel;
- Utilize the Cancer Survivorship Toolkit;
- Download the SCP;
- Establish a close partnership with your local cancer committee; and
- Collect and report data to show effectiveness and need.
All these strategies were vital to the success of the LSCVAMC survivorship program.
Summary
The VA is uniquely positioned to be a leader in highquality, comprehensive, and veteran-centered cancer survivorship care in the years ahead. The close relationship between specialty and primary care allows for smooth continuity of care and easy transitions between oncology and primary care. The comprehensive CPRS allows easy accessibility to information for the entire health care team. The Cancer Survivorship Toolkit provides a template of the survivorship care plan for the veteran and his or her health care providers.
The LSCVAMC is one of many VA institutions implementing quality care for cancer survivors and can serve as a role model for other VA programs initiating the survivorship care process (Table 2).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The aging of the U.S. population has led to an increase in the number of patients diagnosed with cancer each year. Fortunately, advances in screening, detection, and treatments have contributed to an improvement in cancer survival rates during the past few decades. More than 1.6 million new cases of cancer are expected to be diagnosed in 2014. It is estimated that there are currently 14 million cancer survivors, and the number of survivors by 2022 is expected to be 18 million.1,2
The growing number of cancer survivors is exceeding the ability of the cancer care system to meet the demand.3 Many primary care providers (PCPs) lack the confidence to provide cancer surveillance for survivors, but at the same time, patients and physicians continue to expect that PCPs will play a substantial role in general preventive health and in treating other medical problems.4 These conditions make it critical that at a minimum, survivorship care is integrated between oncology and primary care teams through a systematic, coordinated plan.5 This integration is especially important for the vulnerable population of veterans who are cancer survivors, as they have additional survivorship needs.
The purpose of this article is to assist other VA health care providers in establishing a cancer survivorship program to address the unique needs of veterans not only during active treatment, but after their initial treatment is completed. Described are the unique needs of veterans who are cancer survivors and the development and implementation of a cancer survivorship program at a large metropolitan VAMC, which is grounded in VA and national guidelines and evidence-based cancer care. Lessons learned and recommendations for other VA programs seeking to improve coordination of care for veteran cancer survivors are presented.
Cancer Survivorship
The Institute of Medicine (IOM) report, From Cancer Patient to Cancer Survivor: Lost in Transition, identified the importance of providing quality survivorship care to those “living with, through, and beyond a diagnosis of cancer.”6,7 The period of survivorship extends from the time of diagnosis, through treatment, long-term survival, and end-of-life.8,9 Although there are several definitions of cancer survivor, the most widely accepted definition is one who has been diagnosed with cancer, regardless of their position on the disease trajectory.8
The complex needs of cancer survivors encompass physical, psychological, social, and spiritual concerns across the disease trajectory.3 Cancer survivors who are also veterans have additional needs and risk factors related to their service that can make survivorship care more challenging.10 Veterans tend to be older compared with the age of the general population, have more comorbid conditions, and many have combat-related posttraumatic stress disorder (PTSD), all of which can complicate the survivorship experience.11
The first challenge for veteran cancer survivors is in the term cancer survivor, which may take on a different meaning for a veteran when compared with a civilian. For some civilians and veterans, survivor is a constant reminder of having had cancer. There are some veterans who prefer not to be called survivors, because they do not feel worthy of this terminology. They believe they have not struggled enough to self-identify as a survivor and that survivorship is “something to be earned, following a physically grueling experience.”12
The meaning of the word survivor may even be culturally linked to the population of veterans who have survived a life-threatening combat experience. More research is needed to understand the veteran cancer survivorship experience. The meaning of survivorship must be explored with each veteran, as it may influence his or her adherence to a survivorship plan of care.
Veterans make up a unique subset of cancer survivors, in part because of risk factors associated with their service. Many veterans developed cancer as a result of their military exposure to toxic chemicals and radiation. To date, VA recognizes that chronic B-cell leukemias, Hodgkin disease, multiple myeloma, non-Hodgkin lymphomas, prostate cancer, respiratory cancers, and soft tissue sarcomas are all presumptive diseases related to Agent Orange exposure.13 There are other substances also presumed to increase the risk of certain cancers in veterans who have had ionizing radiation exposure.14 There is still much to learn regarding veterans who served during the Gulf War, Operation Enduring Freedom, and Operation Iraqi Freedom.15,16
In a comparison of VA data files with U.S. SEER data files from 2007, researchers identified differences in characteristics between veteran cancer survivors and civilian cancer survivors.17 In addition to increased exposure risks, the veteran cancer survivor population is older than the general cancer survivorship population and is mostly male.17 Veterans’ comorbid conditions, such as type 2 diabetes, ischemic heart disease, Parkinson disease, and peripheral neuropathy, which may be service related, complicate survivorship.17 These characteristics (age, gender, exposure risks, and comorbid conditions) influence the type of cancer diagnosed and treatment options, and they may ultimately impact survivorship needs
(Table 1).
The prevalence of mental health issues in the veteran population is significant.18 Posttraumatic stress disorder affects 7% to 8% of the general population at some point during their lifetime and as many as 16% of those returning from military deployment.19 In a predominantly
male veteran study correlating combat PTSD with cancerrelated PTSD, about half the participants (n = 170) met PTSD Criterion A, viewing their cancer as a traumatic experience.20 Posttraumatic stress disorder, depression, anxiety, and addictive disease all must be addressed in the survivorship plan of care.
Poor mental health has been linked to increased morbidity and mortality and can limit the veteran’s ability to participate in health promotion and medical care.21 Distress related to cancer is well recognized in the civilian population.22,23 Veterans are at risk for moderate-tosevere disabling distress, especially when the cancer is associated with their military service. Vietnam veterans who have a diagnosis of cancer report that they have already served their time and are now serving it again, having to wage a battle on cancer and undergo difficult treatments and associated adverse effects (AEs).24 It is important to note, however, that some veterans have developed strong coping skills, which gives them strength and resilience for the survivorship experience.25
Other factors also contribute to veterans’ unique survivorship needs. Many veterans have limited social and/or economic resources, making it difficult to receive cancer treatment and follow recommendations for a healthful lifestyle as a cancer survivor. Demographics from the VA have illustrated that many veterans have a limited support system (65% do not have a spouse), and many have low incomes.26 Although veterans comprise about 11% of the general population, they make up 26% of the homeless population.26 It is estimated that 260,000 veterans are homeless at some time during the course of a year, and of these, 45% have mental health issues and 70% have substance abuse problems.27 Basic needs such as housing, running water, heat and electricity, and nutrition must be met in order to prevent infection during treatment, maximize the benefit, and reduce the risks associated with treatment. Transportation issues can make it challenging to travel to medical centers for cancer surveillance following treatment.
Models of Care
As defined in the aforementioned IOM report, multiple models of survivorship care have surfaced over the years.6 Much that was originally seen and implemented in adult cancer survivorship was known from pediatric cancer care. Early models that surfaced included shared care models, nurse-led models, and tertiary survivorship clinics. Each model has its strengths and disadvantages.
The shared care model of survivorship involves a sharing of the responsibility for the survivor among different specialties, potentially at different facilities, and the primary care team. Typically, the PCP refers the patient to the oncologist when cancer is suspected or diagnosed. The primary care team continues to provide routine health maintenance and manages other health problems while the oncology team provides cancer care. The patient is transitioned back to the primary care team with a survivorship care plan (SCP) at 1 to 2 years after completion of cancer therapy or at the discretion of the oncology team.28 For
this model to work, the PCP must be willing to take on this responsibility, and there must be a coordinated effort for seamless communication between teams, which can be potentially challenging.
Nurse-led programs emerged in the pediatric populations. Pediatric nurse-led clinics assume care of the patient after active treatment to manage long-term AEs of cancer treatments, symptom management, care planning, and education. A comprehensive review of the literature identified that “nurse-led follow-up services are acceptable, appropriate, and effective.”6 Barriers to this model of care include a shortage of trained oncology nurses and a preference for physician follow-up by some cancer survivors who want the security of their oncologist for ongoing, long-term care.6
Survivorship follow-up clinics, a tertiary model of care, have been implemented at some larger academic centers. These clinics focus on cancer survivorship and are often separate from other routine health care visits. Typically, these clinics include multiple specialties and are often disease-specific. These types of clinics pose a different set of challenges regarding duplication of services and reimbursement issues.
As of yet, no model has been proven more effective than the others. Each institution and patient population may not lend themselves to a one-size-fits-all model. There may be different models of care needed, based on patient population. Regardless of the model selected, individualized survivorship care plans are an essential component of quality cancer survivorship care.
Addessing Survivorship Care
In 2009, 5 interdisciplinary leaders in VA cancer care (Ellen Ballard, RN; David Haggstrom, MD, MAS; Veronica Reis, PhD; Mark Detzer, PhD; and Tina Gill, MA) attended a breakout session on psychosocial oncology at the Association of VA Hematology and Oncology (AVAHO) meeting in Minneapolis, Minnesota, and most members of this team participated in the 2009-2012 VHA Cancer Care Collaboratives to improve the timeliness and quality of care for veterans who were cancer patients. Dr. Haggstrom and Ms. Ballard developed a SharePoint site for the Survivorship Special Interest Group (SIG) members through the Loma Linda VAMC in California. The SIG workgroup then built the Cancer Survivorship Toolkit, composed of
5 critical tools (Figure).
In July 2012, the VA Cancer Survivorship Toolkit content was disseminated at AVAHO and launched behind the VA firewall. It subsequently received accolades from the national program director for VHA Oncology and was listed on the American College of Surgeons Commission on Cancer (CoC) Best Practices website. The toolkit is accessible to all VA programs, and suggestions for new content can be submitted directly on the site (Figure).
The development of a SCP began in late 2011 when SIG members collected examples of SCPs from leading organizations. The members compared this content with the IOM recommendations for SCPs and developed a template. The template was programmed for the VHA computerized patient record system (CPRS) and placed on the internal VA toolkit website. The template included the treatment summary and care plan. The treatment summary portion included the diagnosis and tumor characteristics, diagnostic tests used, dates and types of treatment, chemoprevention or maintenance treatments, supportive services required, the surveillance plan, and signs of recurrence. The care plan portion provided information on the likely course of recovery and a checklist for common long-term AEs in the areas of psychological distress, financial and practical effects, and physical effects. Also included was information about referral, health behaviors, late effects that may develop, contact information, and general resource information.
The computer applications coordinator at any VA can download the template from the toolkit onto their CPRS, and the template can then be brought into any progress note. Individual sites may also edit the template to suit specific needs. The SCP can be completed by any clinician with the appropriate clinical competencies. To date, > 50 sites have downloaded the SCP template for use.
Cancer Survivorship Clinic
At the Louis Stokes Cleveland (LSC) VAMC, a nurse-led model of a cancer survivorship clinic was established with an expert nurse practitioner (NP). A major catalyst for the development of this clinic was the receipt of a Specialty Care Education Center of Excellence, funded by the Offices of Specialty Care and Academic Affiliations. A priority of this project was the implementation of survivorship care for every veteran with a cancer diagnosis. A system redesign was implemented to deliver quality, cost-effective, patient-centered cancer care within an interprofessional, team-based practice. This clinic is imbedded within an interdisciplinary clinic setting where the NP works in close collaboration with the medical and surgical oncologists as well as providers from mental health, social work, nutrition, physical therapy, and others.
The first patients to receive survivorship care in this new model from the time of their diagnosis were veterans with breast cancer, sarcoma, melanoma, and lymphomas. Veterans are followed jointly by the NP and the medical and surgical oncologists during active treatment. The NP provides physical symptom assessment and management for patients both during and after treatment.
At the end of active treatment, patient visits are alternated between oncology physicians and the survivorship NP for 5 years. The timeline for follow-up visits is based on National Comprehensive Cancer Network guidelines for each cancer type but then individualized based on patient need.29 During this 5-year time period, patients under active surveillance whose conditions have been stable are seen by the NP. Any concerning symptoms are immediately relayed to the primary oncologist or surgical oncologist, often the same day, and patients can be seen the same day if necessary, to improve coordination and access to services.
A unique focus of the clinic is the integration of health promotion and risk reduction that coincides with the active surveillance plan. This transition of active surveillance patients to the NP-led survivorship clinic not only opens access to newly diagnosed cancer patients to be seen by the oncologist, but also allows for seamless transition and coordination to active surveillance. Within the clinic structure, patients receive patient navigation beginning with a cancer concern; patients also receive screening for psychosocial distress at the time of diagnosis and at every visit. Patient navigation and distress screening are both considered essential elements to survivorship care in the most recent CoC guidelines.30 The survivorship NP keeps the primary care team up-to-date regarding patient care across the disease trajectory by alerting them to updates electronically in the CPRS in real time.
Survivorship Care Plan
A focus of the clinic has also been on the implementation of a formal SCP to be completed 3 months after the conclusion of active treatment. The formal SCP was downloaded from the Cancer Survivorship Toolkit and is composed of a 3-part summary. The 3 parts consist of the treatment summary, the plan for rehabilitation, and the plan for the future. The first section of the SCP is completed by the medical oncologist as a summary of treatment received by the veteran. The summary of treatment section is reviewed and discussed with the veteran survivor at the visit, and the second and third sections are completed during the 3-month follow-up visit with the veteran.
Success and Areas for Improvement
The survivorship clinic has been well received by veterans. Patient satisfaction scores have been overwhelmingly positive. Veterans appreciate and feel comfortable knowing their providers from the beginning of diagnosis along the entire disease trajectory. They know that if problems arise, the survivorship NP has direct access to the medical or surgical oncologist for immediate review.
The difficult challenge for the cancer care providers is to know when is the right time to transition care back to the PCP. Transitions of care often come with high anxiety and a sense of loss for the veteran. The 5-year survival mark is not always the appropriate transition time for some veterans. Those with extensive physical and mental health issues may need continuity of care and continued support from the oncology team.
The SCP has presented challenges in terms of when to complete and who should complete the form. There has also been concern over the length of the summary, how long it will take to complete the document, and which summary template to use. Areas for improvement with the template could potentially be to automate population of the chemotherapy and radiation summaries. Some software packages are available, but they are costly. Another issue with external software is getting it accepted by VHA and incorporated into the CPRS.
Recommendations
Many cancer programs are struggling to provide highquality survivorship care. The CoC, recognizing the challenges programs are having implementing survivorship care, has extended the accreditation requirement for full implementation from 2015 to 2019.31
The following recommendations should be considered for the successful implementation of a new survivorship program:
- Collect information from multiple resources to guide the establishment of the survivorship clinic;
- Become familiar with the IOM From Cancer Patient to Cancer Survivor: Lost in Transition;6
- Understand local issues and barriers specific to your care delivery system;
- Collaborate with key stakeholders from multiple specialties to gain momentum and buy-in;
- Hold regular meetings with stakeholders as well as leadership to identify and remove barriers to the clinic success;
- Join the VA Survivorship SIG to collaborate with other sites who have already started to pilot survivorship programs and discuss barriers to and successes of programs so as to not reinvent the wheel;
- Utilize the Cancer Survivorship Toolkit;
- Download the SCP;
- Establish a close partnership with your local cancer committee; and
- Collect and report data to show effectiveness and need.
All these strategies were vital to the success of the LSCVAMC survivorship program.
Summary
The VA is uniquely positioned to be a leader in highquality, comprehensive, and veteran-centered cancer survivorship care in the years ahead. The close relationship between specialty and primary care allows for smooth continuity of care and easy transitions between oncology and primary care. The comprehensive CPRS allows easy accessibility to information for the entire health care team. The Cancer Survivorship Toolkit provides a template of the survivorship care plan for the veteran and his or her health care providers.
The LSCVAMC is one of many VA institutions implementing quality care for cancer survivors and can serve as a role model for other VA programs initiating the survivorship care process (Table 2).
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Levit LA, Balough EP, Nass SJ, Ganz PA, eds. Washington, DC: The National Academies Press; 2013.
3. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;(suppl 18):15-22.
4. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
5. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
6. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Hewitt M, Greenfield S, Stovall E, eds. Washington, DC: The National Academies Press; 2006.
7. Clark EJ, Stovall EL, Leigh S, Siu AL, Austin DK, Rowland JH, eds. Imperatives for Quality Cancer Care: Access, Advocacy, Action, and Accountability. Silver Spring, MD: National Coalition for Cancer Survivorship; 1996.
8. National Cancer Institute. Survivorship. NCI Dictionary of Cancer Terms Website. http://www.cancer.gov/dictionary?CdrID=445089. Accessed December 3, 2014.
9. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
10. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
11. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: Protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93.
12. Beehler GP, Rodriques AE, Kay MA, Kiviniemi MT, Steinbrenner L. Lasting impact: Understanding the psychosocial implications of cancer among military veterans. J Psychosoc Oncol. 2013:31(4):430-450.
13. U.S. Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Public Health Website. http://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp. Updated December 30, 2013. Accessed December3, 2014.
14. U.S. Department of Veterans Affairs. Radiation. Public Health Website. http://www.publichealth.va.gov/exposures/radiation/index.asp. Updated December 31, 2013. Accessed December 3, 2014.
15. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
16. U.S. Department of Veterans Affairs. Gulf War veterans’ illnesses. Public Health Website. http://www.publichealth.va.gov/exposures/gulfwar/index.asp. Updated November 7, 2014. Accessed December 3, 2014.
17. National Cancer Institute. Cancer query systems. Surveillance, Epidemiology, and End Results Program Website. http://seer.cancer.gov/canques/index.html. Accessed December 3, 2014.
18. Suicide in the military: Army-NIH funded study points to risk and protective factors [news release]. Washington, DC: National Institute of Mental Health; March 3, 2014. http://www.nimh.nih.gov/news/science-news/2014/suicide-in-the-military-army-nih-funded-study-points-to-risk-and-protective-factors.shtml. Accessed December 3, 2014.
19. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
20. Mulligan EA, Schuster Wachen J, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: Prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
21. Musuuza JS, Sherman ME, Knudsen KJ, Sweeney HA, Tyler CV, Koroukian SM. Analyzing excess mortality from cancer among individuals with mental illness. Cancer. 2013;119(13):2469-2476.
22. Zabora J, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2013:30(6):625-635.
23. Holland JC, Andersen B, Breitbart WS, et al. Distress management: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2010:8(4):448-485.
24. Grassman DL. The Hero Within. St. Petersburg, FL: Vandamere Press; 2012.
25. Jahn AL, Herman L, Schuster J, Naik A, Moye J. Distress and resilience after cancer
in veterans. Res Hum Dev. 2012;9(3):229-247.
26. National Association of Social Workers. Social workers speak on veterans issues June 2009. National Association of Social Workers Website. http://www.naswdc.org/pressroom/2009/Social%20Work%20Veterans%20Fact%20Sheet.pdf. Accessed December 3, 2014.
27. Homeless. U.S. Department of Veterans Affairs Website. http://www.va.gov/homeless. Accessed December 3, 2014.
28. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117-5124.
29. NCCN Guidelines. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed December 3, 2014.
30. American College of Surgeons, Commission on Cancer. Cancer program standards 2012, version 1.2.1: Ensuring patient-centered care. https://www.facs.org/~/media/file/quality%20programs/cancer/coc/programstandards2012.ashx. Published January 21, 2014. Accessed December 3, 2014.
31. Accreditation committee clarifications for standard 3.3 survivorship care plan. American College of Surgeons Website. https://www.facs.org/publications/newsletters/coc-source/special-source/standard33. Published September 9, 2014. Accessed December 3, 2014.
1. American Cancer Society. Cancer Facts & Figures 2014. Atlanta, GA: American Cancer Society; 2014.
2. Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Levit LA, Balough EP, Nass SJ, Ganz PA, eds. Washington, DC: The National Academies Press; 2013.
3. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;(suppl 18):15-22.
4. Cowens-Alvarado R, Sharpe K, Pratt-Chapman M, et al. Advancing survivorship care through the National Cancer Survivorship Resource Center: Developing American Cancer Society guidelines for primary care providers. CA Cancer J Clin. 2013;63(3):147-150.
5. Cheung WY, Neville BA, Cameron DB, Cook EF, Earle CC. Comparisons of patient and physician expectations for cancer survivorship care. J Clin Oncol. 2009;27(15):2489-2495.
6. Institute of Medicine. From Cancer Patient to Cancer Survivor: Lost in Transition. Hewitt M, Greenfield S, Stovall E, eds. Washington, DC: The National Academies Press; 2006.
7. Clark EJ, Stovall EL, Leigh S, Siu AL, Austin DK, Rowland JH, eds. Imperatives for Quality Cancer Care: Access, Advocacy, Action, and Accountability. Silver Spring, MD: National Coalition for Cancer Survivorship; 1996.
8. National Cancer Institute. Survivorship. NCI Dictionary of Cancer Terms Website. http://www.cancer.gov/dictionary?CdrID=445089. Accessed December 3, 2014.
9. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
10. Moye J, Schuster JL, Latini DM, Naik AD. The future of cancer survivorship care for veterans. Fed Pract. 2010;27(3):36-43.
11. Naik AD, Martin LA, Karel M, et al. Cancer survivor rehabilitation and recovery: Protocol for the Veterans Cancer Rehabilitation Study (Vet-CaRes). BMC Health Serv Res. 2013;13:93.
12. Beehler GP, Rodriques AE, Kay MA, Kiviniemi MT, Steinbrenner L. Lasting impact: Understanding the psychosocial implications of cancer among military veterans. J Psychosoc Oncol. 2013:31(4):430-450.
13. U.S. Department of Veterans Affairs. Veterans’ diseases associated with Agent Orange. Public Health Website. http://www.publichealth.va.gov/exposures/agentorange/conditions/index.asp. Updated December 30, 2013. Accessed December3, 2014.
14. U.S. Department of Veterans Affairs. Radiation. Public Health Website. http://www.publichealth.va.gov/exposures/radiation/index.asp. Updated December 31, 2013. Accessed December 3, 2014.
15. Cohen BE, Gima K, Bertenthal D, Kim S, Marmar CR, Seal KH. Mental health diagnoses and utilization of VA non-mental health medical services among returning Iraq and Afghanistan veterans. J Gen Intern Med. 2010;25(1):18-24.
16. U.S. Department of Veterans Affairs. Gulf War veterans’ illnesses. Public Health Website. http://www.publichealth.va.gov/exposures/gulfwar/index.asp. Updated November 7, 2014. Accessed December 3, 2014.
17. National Cancer Institute. Cancer query systems. Surveillance, Epidemiology, and End Results Program Website. http://seer.cancer.gov/canques/index.html. Accessed December 3, 2014.
18. Suicide in the military: Army-NIH funded study points to risk and protective factors [news release]. Washington, DC: National Institute of Mental Health; March 3, 2014. http://www.nimh.nih.gov/news/science-news/2014/suicide-in-the-military-army-nih-funded-study-points-to-risk-and-protective-factors.shtml. Accessed December 3, 2014.
19. Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: Epidemiology, screening, and case recognition. Psychol Serv. 2012;9(4):361-382.
20. Mulligan EA, Schuster Wachen J, Naik AD, Gosian J, Moye J. Cancer as a criterion a traumatic stressor for veterans: Prevalence and correlates. Psychol Trauma. 2014;6(suppl 1):S73-S81.
21. Musuuza JS, Sherman ME, Knudsen KJ, Sweeney HA, Tyler CV, Koroukian SM. Analyzing excess mortality from cancer among individuals with mental illness. Cancer. 2013;119(13):2469-2476.
22. Zabora J, Macmurray L. The history of psychosocial screening among cancer patients. J Psychosoc Oncol. 2013:30(6):625-635.
23. Holland JC, Andersen B, Breitbart WS, et al. Distress management: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2010:8(4):448-485.
24. Grassman DL. The Hero Within. St. Petersburg, FL: Vandamere Press; 2012.
25. Jahn AL, Herman L, Schuster J, Naik A, Moye J. Distress and resilience after cancer
in veterans. Res Hum Dev. 2012;9(3):229-247.
26. National Association of Social Workers. Social workers speak on veterans issues June 2009. National Association of Social Workers Website. http://www.naswdc.org/pressroom/2009/Social%20Work%20Veterans%20Fact%20Sheet.pdf. Accessed December 3, 2014.
27. Homeless. U.S. Department of Veterans Affairs Website. http://www.va.gov/homeless. Accessed December 3, 2014.
28. Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117-5124.
29. NCCN Guidelines. National Comprehensive Cancer Network Website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed December 3, 2014.
30. American College of Surgeons, Commission on Cancer. Cancer program standards 2012, version 1.2.1: Ensuring patient-centered care. https://www.facs.org/~/media/file/quality%20programs/cancer/coc/programstandards2012.ashx. Published January 21, 2014. Accessed December 3, 2014.
31. Accreditation committee clarifications for standard 3.3 survivorship care plan. American College of Surgeons Website. https://www.facs.org/publications/newsletters/coc-source/special-source/standard33. Published September 9, 2014. Accessed December 3, 2014.
Dispensing and Monitoring Oral Anticancer Therapy
The availability and popularity of orally administered anticancer therapy has drastically increased in recent years. Currently, there are more than 40 oral anticancer medications on the market in the U.S.; and about 40% of all newly FDA-approved anticancer agents in 2013 and 2014 have been oral agents.1
The use of these agents is often driven by patients. In a review of 103 patients, an overwhelming 90% of patients who were to receive palliative chemotherapy chose oral chemotherapy over IV chemotherapy, assuming equivalent efficacy, toxicity, clinic visits, and blood work schedules. However, 70% of these patients were unwilling to sacrifice any efficacy between IV and oral chemotherapy.2 Several other factors influenced the preference of oral chemotherapy for patients, including convenience, avoidance of central venous catheter placement or need for other IV access, control of the environment in which they receive chemotherapy, and travel considerations.2 In addition to these practical benefits, patients reported a great sense of freedom with oral chemotherapy.3
Although patients may prefer oral anticancer therapies, for providers, several issues exist surrounding the shift in delivery of anticancer therapies from IV to oral therapies. The most significant concern is patient adherence, defined as “the extent to which patients take medications as prescribed by their health care providers.”4
Adherence rates in clinical trials are often excellent; however, real-life adherence rates tend to be less optimal.5 In a study of women receiving 5 years of adjuvant tamoxifen for breast cancer, the researchers determined that patients filled their prescription 87% of the time the first year of treatment. This rate of adherence dramatically decreased to only 50% by year 4.6
These results suggest that a longer duration of treatment can adversely affect adherence. Duration of treatment is of great concern for providers specifically when considering the need for indefinite duration of use of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. In 2011, Ibrahim and colleagues showed that imatinib adherence rates of 85% have been directly correlated to the loss of complete cytogenetic response (26.8% vs 1.5%, P = .0002) and lower probability of continuing imatinib (64.5% vs 90.6%, P = .006).7 Whereas several factors are known to influence adherence rates, Marin and colleagues identified the 2 main risk factors for poor adherence to imatinib: younger age and adverse effects (AEs). The median age for patients with adherence rates of 90% was 43.8 years compared with 53.8 years for patients with > 90% adherence rate. Imatinib AEs, such as asthenia, nausea, muscle cramps, and bone or joint pains, also significantly decreased imatinib adherence.8
In addition to concerns for poor therapeutic outcomes and suboptimal toxicity management, lack of adherence to oral anticancer regimens can result in significant waste of medication and increased health care costs. In most situations, IV anticancer treatment cycles are repeated every 1 to 3 weeks and allow the patient more frequent face-to-face interaction with the oncology team. Oral chemotherapy, on the other hand, is traditionally dispensed as a 28- to 30-day supply. This practice often limits the patient’s access to the oncology team for full evaluation of adherence and toxicity, which can lead to oral anticancer therapy waste.
Khandelwal and colleagues investigated the utility of a split-fill to decrease health care costs. In the splitfill process, patients were dispensed only days 1 to 16 of their oral anticancer medication at the initial fill. If the medication was tolerated and the prescribing provider deemed no changes in treatment necessary, the remaining 12 to 14 days of the cycle were then dispensed. Unfortunately, all insurance companies did not authorize the split-fill plan, thus preventing some patients in the study to participate in this cost savings strategy. However, it was determined for the patients who discontinued therapy, about 34% could have reduced wastage had they been on the split-fill plan, resulting in an average direct savings of $934.20 per patient who discontinued use.9
In 2011, the Hematology/Oncology and Pharmacy divisions at the VA Pittsburgh Healthcare System (VAPHS) examined the issues surrounding dispensing and monitoring of oral anticancer therapy. Higher utilization of oral anticancer therapy was identified and in parallel, increasing rates of patient nonadherence, toxicity, and wasted medication. Originally, most providers dispensed oral anticancer therapy as a 1-month supply. However, in efforts to increase adherence, limit toxicity, and avoid medication waste, some oncologists began only dispensing a 1- to 2-week supply of medication per visit. This shift in practice led to a pilot study evaluating the utility of limiting all oral chemotherapy to a 7- to 14-day supply during the first 3 months of treatment.
Pilot Study
The goal of the pilot study was to increase adherence, decrease toxicity, and avoid medication waste. Patients who initiated a new oral anticancer therapy between August 15, 2011, and February 15, 2012, were enrolled in the pilot study. Each patient was to be provided only a 14-day supply of medication at each visit. Patients on concurrent chemoradiotherapy with capecitabine were dispensed only a 7-day supply (as they were at VAPHS daily for radiation) of medication. A pillbox designated for oral anticancer therapy was provided and filled by the clinical pharmacist before leaving the hematology/oncology clinic.
Patients were provided a calendar to record the time and date of their oral anticancer therapy selfadministration. Patients were also asked to record any missed doses and the reasons they missed taking the medication. In addition, patients were counseled on the importance of medication adherence, food-drug and drug-drug interaction, proper storage and administration of medications, and when/who to notify if AEs occurred.
Patients were asked to return the pillboxes to the hematology/oncology clinic for the next refill and meet with the clinical pharmacist. A pill count was performed at each visit in addition to screening for toxicity. If a toxicity was identified, the prescribing provider was contacted for further orders. If no changes were needed, the remaining 14-day supply was dispensed to the patient at that time. Adherence and toxicity were documented in the electronic medical record (EMR) at each visit.
Thirty patients were started on 32 different oral anticancer therapies (Table 1) over the 6 months between August 15, 2011, and February 15, 2012. Patients already initiated on oral anticancer therapy before the start date were not included in this analysis. This number also did not include patients on lenalidomide, because this medication is mailed directly to the patient from a specialty pharmacy. All patients were male; average age was 68 (45-89) years; 83.3% of patients were white; and 83% of patients had a stage IV disease.
Adherence assessments using pill counts and medication calendars demonstrated that 6% (121/2,037) of doses that were dispensed were not taken. Overall adherence rate was 94%. The average patient adherence rate was 93.2%. Adverse events contributed to 62.8% of doses omitted (Table 2). Some AEs (eg, nausea, vomiting, and hypertension) were deemed preventable or modifiable with better symptom management. However, the majority of AEs that led to dose omission were not preventable.
Ten patients had their treatments discontinued midcycle, leading to 24.7% of missed doses. Adverse events led to 70% of discontinuation, whereas 20% resulted from disease progression. In both cases of disease progression, the patient was given a 30-day supply before the restaging scan, and in both cases this led to oral anticancer therapy waste. An additional 12.3% of doses were omitted due to hospitalization of patients.
Over a 6-month period, an estimated $32,314 was saved under the 14-day dosing pilot. This number was reached by subtracting the number of pills actually dispensed under pilot protocol from the number of pills that would have been dispensed under old dispensing standards (usually 28- to 30-day supply), then multiplying the difference by the cost per pill.
The results of this study were presented to the Pharmacy and Therapeutics Committee and led to the approval to continue with the 14-day dispensing protocol at VAPHS in March 2012. In addition, the pilot served as the backbone for the VHA Guidance on Oral Chemotherapy Dispensing and Monitoring.10 As part of the guidance, a monitoring guide for all the FDA-approved oral anticancer therapies is maintained and available for all VA practitioners to access on the PBM website under the Clinical Guidance subheading.
Current Practice
From the time the original pilot was conducted, the number of available oral anticancer therapies has increased along with the patient volume. Due to these factors and the lack of a dedicated outpatient oncology clinical pharmacist, oncology nurses in the outpatient clinic now direct the education, dispensing, and monitoring of patients on oral chemotherapy.
Treatment Plan
An oral anticancer treatment plan is developed by the oncology physician and entered in the EMR as a progress note titled Treatment Plan. The treatment plan includes, disease, stage, curative vs palliative intent, premedications, oral anticancer medication, dose, route and frequency, cycle length and number of cycles, baseline and continuous monitoring parameters, follow-up with provider, and staging follow-up. Once the oncology clinical pharmacist approves a treatment plan, the oncology nursing staff ensures that all the prechemotherapy laboratory tests are ordered and helps arrange any additional tests needed (echocardiogram, electrocardiogram, etc). After all the prechemotherapy testing is complete, the oncology nurse phones the patientto schedule a date for chemotherapy education and to pick up the first 14-day supply.
Initial Visit
The oncology nurse meets with each patient receiving oral anticancer therapy and provides them with an oncology clinic information packet, which includes chemotherapy education, a medication sheet, questions and answers about chemotherapy, common AEs and ways to manage them, as well as tips for meeting with the nurse and physician. The oncology nurse then reviews the oral anticancer treatment the patient is to receive, including how to administer the medication and timing, whether to take with or without food, common AEs, storage, safe handling, contact name if a toxicity arises, and importance of adherence.
The patient is provided with a pillbox and encouraged to track any missed doses. The oncology nurse then reschedules the patient for the next appointment at the clinic no more than 14 days later. Some treatments require more frequent monitoring and therefore are only dispensed 7 days at a time.
First Follow-up Visit (7-14 days)
At the first follow-up visit, the oncology nurse reviews adherence and toxicity with the patients. If any toxicity is identified, the oncology nurse contacts the oncology physician for additional assessment and orders. If the patient demonstrates adherence and tolerability, an additional 7- to 14-day supply is dispensed and the next appointment is scheduled 7 to 14 days later.
Subsequent Follow-up Visits
The patient continues to follow up at least every 28 days after cycle 1. The oncology nurse practices veterancentered care when trying to determine the appropriate follow-up for each patient. Continuous monitoring of toxicity and adherence occurs at each visit. If toxicity develops, monitoring may be increased at the discretion of the oncology nurse or physician.
Conclusions
Patients at VAPHS have been very receptive to the oral anticancer therapy protocol. Few patients have refused the initial biweekly visits, and many patients appreciate the special attention being focused on their treatment. The facility hopes to be able to expand its oral anticancer monitoring protocol to a telehealth clinic to help reduce the travel time of many patients. Additionally, as the program continues to expand, it is hoped it will be able to support a full-time outpatient oncology clinical pharmacist with a scope of practice to help manage toxicity and continue to improve adherence rates.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Center Watch. FDA approved drugs. Center Watch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/year/2014. Accessed October 24, 2014.
2. Liu G, Franssen E, Fitch Mi, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110-115.
3. Catania C, Didier F, Leon ME, et al. Perception that oral anticancer treatments are less efficacious: Development of a questionnaire to assess the possible prejudices of patients with cancer. Breast Cancer Res Treat. 2005;92(3):265-272.
4. Kelly A, Agius CR. Improving adherence to endocrine therapies: The role of advanced practice nurses. Oncology (Williston Park). 2006;20(10 Nurse Ed):50-54.
5. Prasad V, Massey PR, Fojo T. Oral anticancer drugs: How limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol. 2014;32(15):1620-1629.
6. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606.
7. Ibrahim A, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on longterm therapy. Blood. 2011;117(14):3733-3736.
8. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
9. Khandelwal N, Duncan I, Ahmed T, Rubinstein E, Pegus C. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admission. J Natl Compr Canc Netw. 2012;10(5):618-625.
10. Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. VHA guidance on oral anticancer drugs dispensing and monitoring. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; September 2012.
The availability and popularity of orally administered anticancer therapy has drastically increased in recent years. Currently, there are more than 40 oral anticancer medications on the market in the U.S.; and about 40% of all newly FDA-approved anticancer agents in 2013 and 2014 have been oral agents.1
The use of these agents is often driven by patients. In a review of 103 patients, an overwhelming 90% of patients who were to receive palliative chemotherapy chose oral chemotherapy over IV chemotherapy, assuming equivalent efficacy, toxicity, clinic visits, and blood work schedules. However, 70% of these patients were unwilling to sacrifice any efficacy between IV and oral chemotherapy.2 Several other factors influenced the preference of oral chemotherapy for patients, including convenience, avoidance of central venous catheter placement or need for other IV access, control of the environment in which they receive chemotherapy, and travel considerations.2 In addition to these practical benefits, patients reported a great sense of freedom with oral chemotherapy.3
Although patients may prefer oral anticancer therapies, for providers, several issues exist surrounding the shift in delivery of anticancer therapies from IV to oral therapies. The most significant concern is patient adherence, defined as “the extent to which patients take medications as prescribed by their health care providers.”4
Adherence rates in clinical trials are often excellent; however, real-life adherence rates tend to be less optimal.5 In a study of women receiving 5 years of adjuvant tamoxifen for breast cancer, the researchers determined that patients filled their prescription 87% of the time the first year of treatment. This rate of adherence dramatically decreased to only 50% by year 4.6
These results suggest that a longer duration of treatment can adversely affect adherence. Duration of treatment is of great concern for providers specifically when considering the need for indefinite duration of use of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. In 2011, Ibrahim and colleagues showed that imatinib adherence rates of 85% have been directly correlated to the loss of complete cytogenetic response (26.8% vs 1.5%, P = .0002) and lower probability of continuing imatinib (64.5% vs 90.6%, P = .006).7 Whereas several factors are known to influence adherence rates, Marin and colleagues identified the 2 main risk factors for poor adherence to imatinib: younger age and adverse effects (AEs). The median age for patients with adherence rates of 90% was 43.8 years compared with 53.8 years for patients with > 90% adherence rate. Imatinib AEs, such as asthenia, nausea, muscle cramps, and bone or joint pains, also significantly decreased imatinib adherence.8
In addition to concerns for poor therapeutic outcomes and suboptimal toxicity management, lack of adherence to oral anticancer regimens can result in significant waste of medication and increased health care costs. In most situations, IV anticancer treatment cycles are repeated every 1 to 3 weeks and allow the patient more frequent face-to-face interaction with the oncology team. Oral chemotherapy, on the other hand, is traditionally dispensed as a 28- to 30-day supply. This practice often limits the patient’s access to the oncology team for full evaluation of adherence and toxicity, which can lead to oral anticancer therapy waste.
Khandelwal and colleagues investigated the utility of a split-fill to decrease health care costs. In the splitfill process, patients were dispensed only days 1 to 16 of their oral anticancer medication at the initial fill. If the medication was tolerated and the prescribing provider deemed no changes in treatment necessary, the remaining 12 to 14 days of the cycle were then dispensed. Unfortunately, all insurance companies did not authorize the split-fill plan, thus preventing some patients in the study to participate in this cost savings strategy. However, it was determined for the patients who discontinued therapy, about 34% could have reduced wastage had they been on the split-fill plan, resulting in an average direct savings of $934.20 per patient who discontinued use.9
In 2011, the Hematology/Oncology and Pharmacy divisions at the VA Pittsburgh Healthcare System (VAPHS) examined the issues surrounding dispensing and monitoring of oral anticancer therapy. Higher utilization of oral anticancer therapy was identified and in parallel, increasing rates of patient nonadherence, toxicity, and wasted medication. Originally, most providers dispensed oral anticancer therapy as a 1-month supply. However, in efforts to increase adherence, limit toxicity, and avoid medication waste, some oncologists began only dispensing a 1- to 2-week supply of medication per visit. This shift in practice led to a pilot study evaluating the utility of limiting all oral chemotherapy to a 7- to 14-day supply during the first 3 months of treatment.
Pilot Study
The goal of the pilot study was to increase adherence, decrease toxicity, and avoid medication waste. Patients who initiated a new oral anticancer therapy between August 15, 2011, and February 15, 2012, were enrolled in the pilot study. Each patient was to be provided only a 14-day supply of medication at each visit. Patients on concurrent chemoradiotherapy with capecitabine were dispensed only a 7-day supply (as they were at VAPHS daily for radiation) of medication. A pillbox designated for oral anticancer therapy was provided and filled by the clinical pharmacist before leaving the hematology/oncology clinic.
Patients were provided a calendar to record the time and date of their oral anticancer therapy selfadministration. Patients were also asked to record any missed doses and the reasons they missed taking the medication. In addition, patients were counseled on the importance of medication adherence, food-drug and drug-drug interaction, proper storage and administration of medications, and when/who to notify if AEs occurred.
Patients were asked to return the pillboxes to the hematology/oncology clinic for the next refill and meet with the clinical pharmacist. A pill count was performed at each visit in addition to screening for toxicity. If a toxicity was identified, the prescribing provider was contacted for further orders. If no changes were needed, the remaining 14-day supply was dispensed to the patient at that time. Adherence and toxicity were documented in the electronic medical record (EMR) at each visit.
Thirty patients were started on 32 different oral anticancer therapies (Table 1) over the 6 months between August 15, 2011, and February 15, 2012. Patients already initiated on oral anticancer therapy before the start date were not included in this analysis. This number also did not include patients on lenalidomide, because this medication is mailed directly to the patient from a specialty pharmacy. All patients were male; average age was 68 (45-89) years; 83.3% of patients were white; and 83% of patients had a stage IV disease.
Adherence assessments using pill counts and medication calendars demonstrated that 6% (121/2,037) of doses that were dispensed were not taken. Overall adherence rate was 94%. The average patient adherence rate was 93.2%. Adverse events contributed to 62.8% of doses omitted (Table 2). Some AEs (eg, nausea, vomiting, and hypertension) were deemed preventable or modifiable with better symptom management. However, the majority of AEs that led to dose omission were not preventable.
Ten patients had their treatments discontinued midcycle, leading to 24.7% of missed doses. Adverse events led to 70% of discontinuation, whereas 20% resulted from disease progression. In both cases of disease progression, the patient was given a 30-day supply before the restaging scan, and in both cases this led to oral anticancer therapy waste. An additional 12.3% of doses were omitted due to hospitalization of patients.
Over a 6-month period, an estimated $32,314 was saved under the 14-day dosing pilot. This number was reached by subtracting the number of pills actually dispensed under pilot protocol from the number of pills that would have been dispensed under old dispensing standards (usually 28- to 30-day supply), then multiplying the difference by the cost per pill.
The results of this study were presented to the Pharmacy and Therapeutics Committee and led to the approval to continue with the 14-day dispensing protocol at VAPHS in March 2012. In addition, the pilot served as the backbone for the VHA Guidance on Oral Chemotherapy Dispensing and Monitoring.10 As part of the guidance, a monitoring guide for all the FDA-approved oral anticancer therapies is maintained and available for all VA practitioners to access on the PBM website under the Clinical Guidance subheading.
Current Practice
From the time the original pilot was conducted, the number of available oral anticancer therapies has increased along with the patient volume. Due to these factors and the lack of a dedicated outpatient oncology clinical pharmacist, oncology nurses in the outpatient clinic now direct the education, dispensing, and monitoring of patients on oral chemotherapy.
Treatment Plan
An oral anticancer treatment plan is developed by the oncology physician and entered in the EMR as a progress note titled Treatment Plan. The treatment plan includes, disease, stage, curative vs palliative intent, premedications, oral anticancer medication, dose, route and frequency, cycle length and number of cycles, baseline and continuous monitoring parameters, follow-up with provider, and staging follow-up. Once the oncology clinical pharmacist approves a treatment plan, the oncology nursing staff ensures that all the prechemotherapy laboratory tests are ordered and helps arrange any additional tests needed (echocardiogram, electrocardiogram, etc). After all the prechemotherapy testing is complete, the oncology nurse phones the patientto schedule a date for chemotherapy education and to pick up the first 14-day supply.
Initial Visit
The oncology nurse meets with each patient receiving oral anticancer therapy and provides them with an oncology clinic information packet, which includes chemotherapy education, a medication sheet, questions and answers about chemotherapy, common AEs and ways to manage them, as well as tips for meeting with the nurse and physician. The oncology nurse then reviews the oral anticancer treatment the patient is to receive, including how to administer the medication and timing, whether to take with or without food, common AEs, storage, safe handling, contact name if a toxicity arises, and importance of adherence.
The patient is provided with a pillbox and encouraged to track any missed doses. The oncology nurse then reschedules the patient for the next appointment at the clinic no more than 14 days later. Some treatments require more frequent monitoring and therefore are only dispensed 7 days at a time.
First Follow-up Visit (7-14 days)
At the first follow-up visit, the oncology nurse reviews adherence and toxicity with the patients. If any toxicity is identified, the oncology nurse contacts the oncology physician for additional assessment and orders. If the patient demonstrates adherence and tolerability, an additional 7- to 14-day supply is dispensed and the next appointment is scheduled 7 to 14 days later.
Subsequent Follow-up Visits
The patient continues to follow up at least every 28 days after cycle 1. The oncology nurse practices veterancentered care when trying to determine the appropriate follow-up for each patient. Continuous monitoring of toxicity and adherence occurs at each visit. If toxicity develops, monitoring may be increased at the discretion of the oncology nurse or physician.
Conclusions
Patients at VAPHS have been very receptive to the oral anticancer therapy protocol. Few patients have refused the initial biweekly visits, and many patients appreciate the special attention being focused on their treatment. The facility hopes to be able to expand its oral anticancer monitoring protocol to a telehealth clinic to help reduce the travel time of many patients. Additionally, as the program continues to expand, it is hoped it will be able to support a full-time outpatient oncology clinical pharmacist with a scope of practice to help manage toxicity and continue to improve adherence rates.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The availability and popularity of orally administered anticancer therapy has drastically increased in recent years. Currently, there are more than 40 oral anticancer medications on the market in the U.S.; and about 40% of all newly FDA-approved anticancer agents in 2013 and 2014 have been oral agents.1
The use of these agents is often driven by patients. In a review of 103 patients, an overwhelming 90% of patients who were to receive palliative chemotherapy chose oral chemotherapy over IV chemotherapy, assuming equivalent efficacy, toxicity, clinic visits, and blood work schedules. However, 70% of these patients were unwilling to sacrifice any efficacy between IV and oral chemotherapy.2 Several other factors influenced the preference of oral chemotherapy for patients, including convenience, avoidance of central venous catheter placement or need for other IV access, control of the environment in which they receive chemotherapy, and travel considerations.2 In addition to these practical benefits, patients reported a great sense of freedom with oral chemotherapy.3
Although patients may prefer oral anticancer therapies, for providers, several issues exist surrounding the shift in delivery of anticancer therapies from IV to oral therapies. The most significant concern is patient adherence, defined as “the extent to which patients take medications as prescribed by their health care providers.”4
Adherence rates in clinical trials are often excellent; however, real-life adherence rates tend to be less optimal.5 In a study of women receiving 5 years of adjuvant tamoxifen for breast cancer, the researchers determined that patients filled their prescription 87% of the time the first year of treatment. This rate of adherence dramatically decreased to only 50% by year 4.6
These results suggest that a longer duration of treatment can adversely affect adherence. Duration of treatment is of great concern for providers specifically when considering the need for indefinite duration of use of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. In 2011, Ibrahim and colleagues showed that imatinib adherence rates of 85% have been directly correlated to the loss of complete cytogenetic response (26.8% vs 1.5%, P = .0002) and lower probability of continuing imatinib (64.5% vs 90.6%, P = .006).7 Whereas several factors are known to influence adherence rates, Marin and colleagues identified the 2 main risk factors for poor adherence to imatinib: younger age and adverse effects (AEs). The median age for patients with adherence rates of 90% was 43.8 years compared with 53.8 years for patients with > 90% adherence rate. Imatinib AEs, such as asthenia, nausea, muscle cramps, and bone or joint pains, also significantly decreased imatinib adherence.8
In addition to concerns for poor therapeutic outcomes and suboptimal toxicity management, lack of adherence to oral anticancer regimens can result in significant waste of medication and increased health care costs. In most situations, IV anticancer treatment cycles are repeated every 1 to 3 weeks and allow the patient more frequent face-to-face interaction with the oncology team. Oral chemotherapy, on the other hand, is traditionally dispensed as a 28- to 30-day supply. This practice often limits the patient’s access to the oncology team for full evaluation of adherence and toxicity, which can lead to oral anticancer therapy waste.
Khandelwal and colleagues investigated the utility of a split-fill to decrease health care costs. In the splitfill process, patients were dispensed only days 1 to 16 of their oral anticancer medication at the initial fill. If the medication was tolerated and the prescribing provider deemed no changes in treatment necessary, the remaining 12 to 14 days of the cycle were then dispensed. Unfortunately, all insurance companies did not authorize the split-fill plan, thus preventing some patients in the study to participate in this cost savings strategy. However, it was determined for the patients who discontinued therapy, about 34% could have reduced wastage had they been on the split-fill plan, resulting in an average direct savings of $934.20 per patient who discontinued use.9
In 2011, the Hematology/Oncology and Pharmacy divisions at the VA Pittsburgh Healthcare System (VAPHS) examined the issues surrounding dispensing and monitoring of oral anticancer therapy. Higher utilization of oral anticancer therapy was identified and in parallel, increasing rates of patient nonadherence, toxicity, and wasted medication. Originally, most providers dispensed oral anticancer therapy as a 1-month supply. However, in efforts to increase adherence, limit toxicity, and avoid medication waste, some oncologists began only dispensing a 1- to 2-week supply of medication per visit. This shift in practice led to a pilot study evaluating the utility of limiting all oral chemotherapy to a 7- to 14-day supply during the first 3 months of treatment.
Pilot Study
The goal of the pilot study was to increase adherence, decrease toxicity, and avoid medication waste. Patients who initiated a new oral anticancer therapy between August 15, 2011, and February 15, 2012, were enrolled in the pilot study. Each patient was to be provided only a 14-day supply of medication at each visit. Patients on concurrent chemoradiotherapy with capecitabine were dispensed only a 7-day supply (as they were at VAPHS daily for radiation) of medication. A pillbox designated for oral anticancer therapy was provided and filled by the clinical pharmacist before leaving the hematology/oncology clinic.
Patients were provided a calendar to record the time and date of their oral anticancer therapy selfadministration. Patients were also asked to record any missed doses and the reasons they missed taking the medication. In addition, patients were counseled on the importance of medication adherence, food-drug and drug-drug interaction, proper storage and administration of medications, and when/who to notify if AEs occurred.
Patients were asked to return the pillboxes to the hematology/oncology clinic for the next refill and meet with the clinical pharmacist. A pill count was performed at each visit in addition to screening for toxicity. If a toxicity was identified, the prescribing provider was contacted for further orders. If no changes were needed, the remaining 14-day supply was dispensed to the patient at that time. Adherence and toxicity were documented in the electronic medical record (EMR) at each visit.
Thirty patients were started on 32 different oral anticancer therapies (Table 1) over the 6 months between August 15, 2011, and February 15, 2012. Patients already initiated on oral anticancer therapy before the start date were not included in this analysis. This number also did not include patients on lenalidomide, because this medication is mailed directly to the patient from a specialty pharmacy. All patients were male; average age was 68 (45-89) years; 83.3% of patients were white; and 83% of patients had a stage IV disease.
Adherence assessments using pill counts and medication calendars demonstrated that 6% (121/2,037) of doses that were dispensed were not taken. Overall adherence rate was 94%. The average patient adherence rate was 93.2%. Adverse events contributed to 62.8% of doses omitted (Table 2). Some AEs (eg, nausea, vomiting, and hypertension) were deemed preventable or modifiable with better symptom management. However, the majority of AEs that led to dose omission were not preventable.
Ten patients had their treatments discontinued midcycle, leading to 24.7% of missed doses. Adverse events led to 70% of discontinuation, whereas 20% resulted from disease progression. In both cases of disease progression, the patient was given a 30-day supply before the restaging scan, and in both cases this led to oral anticancer therapy waste. An additional 12.3% of doses were omitted due to hospitalization of patients.
Over a 6-month period, an estimated $32,314 was saved under the 14-day dosing pilot. This number was reached by subtracting the number of pills actually dispensed under pilot protocol from the number of pills that would have been dispensed under old dispensing standards (usually 28- to 30-day supply), then multiplying the difference by the cost per pill.
The results of this study were presented to the Pharmacy and Therapeutics Committee and led to the approval to continue with the 14-day dispensing protocol at VAPHS in March 2012. In addition, the pilot served as the backbone for the VHA Guidance on Oral Chemotherapy Dispensing and Monitoring.10 As part of the guidance, a monitoring guide for all the FDA-approved oral anticancer therapies is maintained and available for all VA practitioners to access on the PBM website under the Clinical Guidance subheading.
Current Practice
From the time the original pilot was conducted, the number of available oral anticancer therapies has increased along with the patient volume. Due to these factors and the lack of a dedicated outpatient oncology clinical pharmacist, oncology nurses in the outpatient clinic now direct the education, dispensing, and monitoring of patients on oral chemotherapy.
Treatment Plan
An oral anticancer treatment plan is developed by the oncology physician and entered in the EMR as a progress note titled Treatment Plan. The treatment plan includes, disease, stage, curative vs palliative intent, premedications, oral anticancer medication, dose, route and frequency, cycle length and number of cycles, baseline and continuous monitoring parameters, follow-up with provider, and staging follow-up. Once the oncology clinical pharmacist approves a treatment plan, the oncology nursing staff ensures that all the prechemotherapy laboratory tests are ordered and helps arrange any additional tests needed (echocardiogram, electrocardiogram, etc). After all the prechemotherapy testing is complete, the oncology nurse phones the patientto schedule a date for chemotherapy education and to pick up the first 14-day supply.
Initial Visit
The oncology nurse meets with each patient receiving oral anticancer therapy and provides them with an oncology clinic information packet, which includes chemotherapy education, a medication sheet, questions and answers about chemotherapy, common AEs and ways to manage them, as well as tips for meeting with the nurse and physician. The oncology nurse then reviews the oral anticancer treatment the patient is to receive, including how to administer the medication and timing, whether to take with or without food, common AEs, storage, safe handling, contact name if a toxicity arises, and importance of adherence.
The patient is provided with a pillbox and encouraged to track any missed doses. The oncology nurse then reschedules the patient for the next appointment at the clinic no more than 14 days later. Some treatments require more frequent monitoring and therefore are only dispensed 7 days at a time.
First Follow-up Visit (7-14 days)
At the first follow-up visit, the oncology nurse reviews adherence and toxicity with the patients. If any toxicity is identified, the oncology nurse contacts the oncology physician for additional assessment and orders. If the patient demonstrates adherence and tolerability, an additional 7- to 14-day supply is dispensed and the next appointment is scheduled 7 to 14 days later.
Subsequent Follow-up Visits
The patient continues to follow up at least every 28 days after cycle 1. The oncology nurse practices veterancentered care when trying to determine the appropriate follow-up for each patient. Continuous monitoring of toxicity and adherence occurs at each visit. If toxicity develops, monitoring may be increased at the discretion of the oncology nurse or physician.
Conclusions
Patients at VAPHS have been very receptive to the oral anticancer therapy protocol. Few patients have refused the initial biweekly visits, and many patients appreciate the special attention being focused on their treatment. The facility hopes to be able to expand its oral anticancer monitoring protocol to a telehealth clinic to help reduce the travel time of many patients. Additionally, as the program continues to expand, it is hoped it will be able to support a full-time outpatient oncology clinical pharmacist with a scope of practice to help manage toxicity and continue to improve adherence rates.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Center Watch. FDA approved drugs. Center Watch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/year/2014. Accessed October 24, 2014.
2. Liu G, Franssen E, Fitch Mi, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110-115.
3. Catania C, Didier F, Leon ME, et al. Perception that oral anticancer treatments are less efficacious: Development of a questionnaire to assess the possible prejudices of patients with cancer. Breast Cancer Res Treat. 2005;92(3):265-272.
4. Kelly A, Agius CR. Improving adherence to endocrine therapies: The role of advanced practice nurses. Oncology (Williston Park). 2006;20(10 Nurse Ed):50-54.
5. Prasad V, Massey PR, Fojo T. Oral anticancer drugs: How limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol. 2014;32(15):1620-1629.
6. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606.
7. Ibrahim A, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on longterm therapy. Blood. 2011;117(14):3733-3736.
8. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
9. Khandelwal N, Duncan I, Ahmed T, Rubinstein E, Pegus C. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admission. J Natl Compr Canc Netw. 2012;10(5):618-625.
10. Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. VHA guidance on oral anticancer drugs dispensing and monitoring. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; September 2012.
1. Center Watch. FDA approved drugs. Center Watch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/year/2014. Accessed October 24, 2014.
2. Liu G, Franssen E, Fitch Mi, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110-115.
3. Catania C, Didier F, Leon ME, et al. Perception that oral anticancer treatments are less efficacious: Development of a questionnaire to assess the possible prejudices of patients with cancer. Breast Cancer Res Treat. 2005;92(3):265-272.
4. Kelly A, Agius CR. Improving adherence to endocrine therapies: The role of advanced practice nurses. Oncology (Williston Park). 2006;20(10 Nurse Ed):50-54.
5. Prasad V, Massey PR, Fojo T. Oral anticancer drugs: How limited dosing options and dose reductions may affect outcomes in comparative trials and efficacy in patients. J Clin Oncol. 2014;32(15):1620-1629.
6. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602-606.
7. Ibrahim A, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on longterm therapy. Blood. 2011;117(14):3733-3736.
8. Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388.
9. Khandelwal N, Duncan I, Ahmed T, Rubinstein E, Pegus C. Oral chemotherapy program improves adherence and reduces medication wastage and hospital admission. J Natl Compr Canc Netw. 2012;10(5):618-625.
10. Pharmacy Benefits Management Services, Medical Advisory Panel and VISN Pharmacist Executives. VHA guidance on oral anticancer drugs dispensing and monitoring. Washington, DC: Veterans Health Administration, Department of Veterans Affairs; September 2012.
Controlling the Cost of Oncology Drugs Within the VA: A National Perspective
The VA National Formulary has existed since 1995. Before the development of a single national formulary, each VA facility managed its pharmacy benefit plan through its pharmacy and therapeutics committees. In other words, 173 formulary processes correlating with 173 facilities managed the pharmacy benefit across the entire VA system. This system served > 4 million veterans, providing > 108 million prescriptions per year.
Variations in provision of the pharmacy benefit were commonplace, including veteran access to drug therapy. Formulary processes for a particular drug that were already established in one facility might not have been developed in another facility. This variation among locations oftentimes limited drug availability. The purpose of developing a single National Formulary was twofold: (1) provide a uniform pharmacy benefit to all veterans by reducing variation in access to drugs among the facilities; and (2) obtain leverage in contract pricing for drugs across the entire VA system.
Pharmacy Benefits Management Capabilities
In 1995, VA Under Secretary for Health Kenneth Kizer,MD, established the VA Pharmacy Benefits Management (PBM) Services division. Pharmacy Benefits Management was assigned the tasks of developing a national formulary, creating pharmacologic guidelines, and managing drug costs and utilization. The VA Drug Product and Pharmaceuticals Management Division, based in Hines, Illinois, which already managed and monitored drug usage and purchasing for each VA Medical Center (VAMC) facility, expanded its services by hiring clinical pharmacists. These clinical pharmacists collaborated with field-based physicians to form the VA Medical Advisory Panel (MAP).
The VA Healthcare System is currently divided into 21 geographically defined VISN (Veteran Integrated System Network) regions. Each VISN has a designated VISN Pharmacist Executive (VPE), formerly known as a VISN Formulary Leader. The VPE serves as a pharmacy liaison between the VA health care facilities within the VISN and the national PBM. This collaboration allows open communication and a sharing of ideas and issues regarding drug therapy within the VA system. Collectively, this physician-pharmacist-based group became known as the Veterans Affairs Pharmacy Benefits Management Services division.
The National Acquisition Center (NAC) is another important collaborator with the PBM. Opportunities for pharmaceutical contracting are sought through the NAC. This contracting mechanism offers the VA opportunities for price reductions on bulk purchases, ready access to needed drugs, and a streamlined drug inventory process that reduces inventory management costs. In addition, with pharmaceutical contracting, the VA can provide identical drugs via multiple sources to minimize confusion for the patient. The NAC obtains optimized pricing through various techniques, such as competitive bidding among branded products within drug classes, the Federal Supply Schedule (FSS) program, and performance-based incentive agreements. These techniques allow the VA to maintain stability with regard to average acquisition costs per 30-day-equivalent prescriptions.1,2
National PBM Clinical Program
The primary function of the National PBM Clinical Pharmacy Program Managers (NPBM-CPPMs) is to maintain the National Formulary. In addition, PBM functions to support VA field practitioners with promoting the safe and effective use of all medications, with the ultimate goal of helping veterans achieve optimal therapeutic outcomes.
The Clinical Program includes 12 NPBM-CPPMs. This group is composed of clinical pharmacists with advanced training and education in specialty therapeutic areas who serve as pharmaceutical subject matter experts within their specialty. It is the responsibility of this group to author drug monographs that summarize clinical data about the safety and efficacy of newly approved drugs (new molecular entities). These drug monographs serve as a tool to assist in determining the formulary status of a drug. The documents are evidence based and extensive, providing the necessary information for considerations related to formulary status.
A major role of the NPBM-CPPM group involves clinical document development, which is inclusive of the monograph-style documents used for formulary decision making. These clinical documents can be found stored on the PBM intranet sites, and most are under the Clinical Guidance subhead. Included among these documents are Drug Monographs used for formulary consideration, Criteria for Use (CFU), Abbreviated Reviews, Clinical Recommendations, and Drug Class Reviews. The various documents are designed to serve as resources for field practitioners to help optimize drug therapy for veterans.
The focus of the NPBM-CPPMs is to optimize pharmacotherapy from a population-based perspective. This focus is in contrast to the clinical pharmacy specialists who function at the facility level and focus primarily on patients in their particular geographic region. The NPBMCPPMs need to be familiar with the VA population as a whole. Although recognizing that every patient is different, NPBM-CPPMs develop clinical guidance documents that pertain to as many veterans as possible—typically about 80% of the population. About 20% of veterans may not possess the most common characteristics of an individual with a particular condition. If a common thread can be identified among this minority, then the focus of clinical guidance can expand to help improve the outcomes for this group, as well as educate VA providers.
Oncology NPBM-CPPMs
The field of oncology pharmacy has seen tremendous growth since it was originally recognized as a specialized field of pharmacy practice in 1998. At the same time, the FDA has approved many new drugs designated for oncologic conditions.3 This expansion of drugs has led to an increase in the NPBM-CPPMs oncology workforce, allowing the CPPMs to “divide and conquer” their responsibilities with respect to the oncologic diseases and pharmacotherapeutic agents used to treat these specific conditions.
The FDA approval of an oncology drug means that an NPBM-CPPM needs to first determine the role and value of this drug to the veteran population. Knowing the most common oncologic conditions that afflict veterans helps to understand a drug’s importance to the VA. A number of common cancers among veterans include conditions associated with exposure to Agent Orange or other herbicides during military service and include chronic B-cell leukemias, Hodgkin lymphoma, non-Hodgkin lymphoma, multiple myeloma, and prostate cancer.4 Aside from exposures related to military service, demographic and personal characteristics of the veteran population help determine the malignancies that put veterans at risk (eg, age and smoking history). It is apparent that colorectal cancer and lung cancer are among the most frequent tumor types detected among veterans.
Malignancies that are seen with less frequency in the VA are still important to the NPBM-CPPM. Breast cancer, for example, is a malignancy that afflicts a relatively small proportion of veterans, yet FDA-approved breast cancer drugs are reviewed for formulary consideration under the same national process.
Evidence-Based Determinations
The evidence-based drug monographs prepared for formulary consideration are approached in a consistent manner that takes into account clinical trial data published in peer-reviewed journals. In situations when peer-reviewed evidence is lacking, as in FDA-approval of a drug given Breakthrough Therapy designation, FDA Medical Review transcripts and abstracts from major meetings, such as the American Society of Clinical Oncology (ASCO), may be considered until published evidence is available.
The focus of the monograph is on efficacy and safety of the product and its potential impact on the veteran population. Cost-effective analyses are considered when available, although they are not commonplace at the time
of product launch.5 Authoritative reviews from other national public health providers (eg, National Institute for Health and Care Excellence) are sought to provide a perspective on a drug therapy’s impact on other health care systems.
Criteria for Use documents are tools to help direct therapy to the appropriate veterans, emphasizing the considerations for safe and optimal use. Criteria for Use are not developed for every drug under review. Instead, CFUs are focused on only those drugs that may be considered a high risk for inappropriate use or may raise safety concerns. The documents developed by the NPBM-CPPM, whether they are monographs for formulary consideration or CFUs, undergo peer review by the Medical Advisory Panel (MAP), VISN VPEs, and fieldbased experts that include Field Advisory Committees (FACs) and other field practitioners.
Cost Issues
The stimulus to develop clinical guidance is not solely based on FDA approval of a new molecular entity. Many times, there are drug-related issues, identified by practitioners in the field, that call for resolution. Some of these issues are not exclusive to VA practice but impact VA practitioners just as they would impact non-VA practitioners. It is the role of the PBM to help address those drugrelated issues.
The high cost of oncology drugs is one such issue that impacts clinicians and patients both inside and outside the VA system. The Oncology FAC recognizes the impact of high-cost drugs on the VA system as a whole. They had been tasked with the goal of providing guidance to the field on the use of high-cost oncology drugs. The oncology-focused NPBM-CPPMs has helped the Oncology FAC address this issue. The plan was to develop guidance documents that focus on minimizing the cost to both veterans and VA facilities. The strategy was to first develop
general, broad-based guidance documents that can be used by any site or VISN, especially those sites without oncology-trained pharmacists, to aid in making decisions about high-cost oncology drugs. The second step was to focus on the nuances of select drugs or diseases and provide drug-specific or disease-specific guidance to help manage cost issues within the identified areas.
Under the auspices of the Oncology FAC, the oncology-focused NPBM-CPPMs convened the High Cost Oncology Drug Workgroup to help tackle this concern. The workgroup included oncology-specialized VA physicians and pharmacists who were divided into subgroups to address areas where recognition and subsequent intervention had the greatest potential to reduce facility drug expenditures.
These interventions previously have been identified as best practices within the VA and were thought to be applicable as broad-based guidance to serve as the first step of the cost control strategy. The work of the subgroups resulted in the following guidance documents:
- Dose Rounding in Oncology
- Oral Anticancer Drugs Dispensing and Monitoring
- Oral Anticancer Drugs: Recommended Dispensing and Monitoring
- Chemotherapy Review Committee Process
- Determining Clinical Benefit of High Cost Oncology Drugs
The Oncology FAC approved these guidance documents with subsequent review under the national PBM approval process. They are not mandatory for decision making but are encouraged for use at the facility or VISN level and can be found at the PBM website.
Clinical Pathways
Prostate cancer is one of the common malignancies that afflicts veterans. It is a disease with treatments involving multiple high-cost oncology drugs and as such is an ideal therapeutic area for possible intervention. Prostate cancer provides an opportunity for the second step of this project. As there are multiple therapies available for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) that have been evaluated in the clinical trial setting for similar indications among comparable patient populations and are high cost items, providers find it difficult to choose among them.
A clinical pathway (CP) is a visual care map that provides direction for treatment options.6-8 Brief annotations are provided throughout the map to help provide rationale along with a rating of the clinical evidence that supports that decision. The ultimate goal of the CP is to improve patient outcomes by providing uniformity of care. Uniformity can lead to increased efficiencies, reduced chance of medication errors, and proactive management of expected toxicities. Clinical pathway development is an extensive process.
The oncology-focused NPBM-CPPMs serve as facilitators for the development of the prostate cancer pathway. This involved the creation of a database of pertinent prostate cancer literature, including national consensus guidelines (ie, National Comprehensive Cancer Network, American Urology Association). This database is available for reference and discussion throughout the process. Key VA oncologists with expertise in prostate cancer management were identified to serve as stakeholders and critically review the literature, providing input regarding each step throughout the pathway process.
Similar to previously described documents, the CP for mCRPC (CP-mCRPC) will undergo peer review by the Oncology FAC with subsequent review under the national PBM approval process. The intent of the CPmCRPC is not to mandate decision making regarding treatment but to encourage consistent treatment and ultimately to minimize variance in practice and optimize patient outcomes. Clinical pathways are dependent on the current evidence and, therefore, are documents that require evaluation and regular updates. The CP process for prostate cancer
will serve as a model for the development of subsequent pathways for other diseases.
Prior Authorization
Many commercial insurers use prior authorization (PA) solely for drug coverage decision making. The PBM has recently adopted an expanded variation of the PA process for a few select medications at both the national and VISN level. The VA PBM PA is a thorough review process to ensure that select patients are appropriate for a particular therapy in an attempt to optimize outcomes. In the process, providing drug therapy to those veterans most likely to benefit will minimize the impact of drug cost.
Drugs selected for PA review are those that meet the following characteristics: (1) Drug has demonstrated limited clinical benefit in a select subpopulation of patients; (2) Drug has a high potential for off-label use; and (3) Drug is considered a high-cost item. The potential benefits of this process are not limited just to ensuring that the appropriate patient receives the appropriate therapy. Prior authorization at the national and VISN levels promotes consistent health care delivery throughout the VA.
Similar to the aforementioned CP process, consistency and minimization of variance in practice are desirable to improve veteran outcomes. As more experience is obtained with the PA process, its role within the VA will be reviewed and evaluated.
Conclusion
The task of addressing the high cost of today’s anticancer therapies is not one that can be addressed with a single initiative. The ASCO Cost of Care Task Force has been focusing on various initiatives that promote evidence-based decision making aimed at addressing the cost of cancer care.9 Consistent with this approach, the VA PBM division has been working with key stakeholders at the VISN and local levels to develop interventions aimed at optimizing therapeutic outcomes for the veteran.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Sales MM, Cunningham FE, Glassman PA, Valentino MA, Good CB. Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104-122.
2. Good CB, Valentino M. Access to affordable medications: The Department of Veterans Affairs pharmacy plan as a national model. Am J Public Health. 2007; 97(12):2129-2131.
3. CenterWatch. FDA approved drugs by therapeutic area. CenterWatch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/therapeuticarea/ 12/oncology. Accessed November 26, 2014.
4. Department of Veterans Affairs. Veterans’ disease associated with Agent Orange. Department of Veterans Affairs Website. http://www.publichealth.va.gov/exposures/agentorang/conditions/index.asp. Last Updated December 30, 2013. Accessed November 26, 2014.
5. Aspinall SL, Good CB, Glassman PA, Valentino MA. The evolving use of cost-effectiveness analysis in formulary management within the Department of Veterans Affairs. Med Care. 2005;43(suppl 7):20-26.
6. Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: Do pathways work? Int J Qual Health Care. 2003;15(6):509-521.
7. Kinsman L, Rotter T, James E, Snow P, Willis J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31.
8. Gesme DH, Wiseman M. Strategic use of clinical pathways. J Oncol Pract. 2011;7(1):54-56.
9. Meropol NJ, Schrag D, Smith TJ, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol. 2009;27(23):3868-3874.
The VA National Formulary has existed since 1995. Before the development of a single national formulary, each VA facility managed its pharmacy benefit plan through its pharmacy and therapeutics committees. In other words, 173 formulary processes correlating with 173 facilities managed the pharmacy benefit across the entire VA system. This system served > 4 million veterans, providing > 108 million prescriptions per year.
Variations in provision of the pharmacy benefit were commonplace, including veteran access to drug therapy. Formulary processes for a particular drug that were already established in one facility might not have been developed in another facility. This variation among locations oftentimes limited drug availability. The purpose of developing a single National Formulary was twofold: (1) provide a uniform pharmacy benefit to all veterans by reducing variation in access to drugs among the facilities; and (2) obtain leverage in contract pricing for drugs across the entire VA system.
Pharmacy Benefits Management Capabilities
In 1995, VA Under Secretary for Health Kenneth Kizer,MD, established the VA Pharmacy Benefits Management (PBM) Services division. Pharmacy Benefits Management was assigned the tasks of developing a national formulary, creating pharmacologic guidelines, and managing drug costs and utilization. The VA Drug Product and Pharmaceuticals Management Division, based in Hines, Illinois, which already managed and monitored drug usage and purchasing for each VA Medical Center (VAMC) facility, expanded its services by hiring clinical pharmacists. These clinical pharmacists collaborated with field-based physicians to form the VA Medical Advisory Panel (MAP).
The VA Healthcare System is currently divided into 21 geographically defined VISN (Veteran Integrated System Network) regions. Each VISN has a designated VISN Pharmacist Executive (VPE), formerly known as a VISN Formulary Leader. The VPE serves as a pharmacy liaison between the VA health care facilities within the VISN and the national PBM. This collaboration allows open communication and a sharing of ideas and issues regarding drug therapy within the VA system. Collectively, this physician-pharmacist-based group became known as the Veterans Affairs Pharmacy Benefits Management Services division.
The National Acquisition Center (NAC) is another important collaborator with the PBM. Opportunities for pharmaceutical contracting are sought through the NAC. This contracting mechanism offers the VA opportunities for price reductions on bulk purchases, ready access to needed drugs, and a streamlined drug inventory process that reduces inventory management costs. In addition, with pharmaceutical contracting, the VA can provide identical drugs via multiple sources to minimize confusion for the patient. The NAC obtains optimized pricing through various techniques, such as competitive bidding among branded products within drug classes, the Federal Supply Schedule (FSS) program, and performance-based incentive agreements. These techniques allow the VA to maintain stability with regard to average acquisition costs per 30-day-equivalent prescriptions.1,2
National PBM Clinical Program
The primary function of the National PBM Clinical Pharmacy Program Managers (NPBM-CPPMs) is to maintain the National Formulary. In addition, PBM functions to support VA field practitioners with promoting the safe and effective use of all medications, with the ultimate goal of helping veterans achieve optimal therapeutic outcomes.
The Clinical Program includes 12 NPBM-CPPMs. This group is composed of clinical pharmacists with advanced training and education in specialty therapeutic areas who serve as pharmaceutical subject matter experts within their specialty. It is the responsibility of this group to author drug monographs that summarize clinical data about the safety and efficacy of newly approved drugs (new molecular entities). These drug monographs serve as a tool to assist in determining the formulary status of a drug. The documents are evidence based and extensive, providing the necessary information for considerations related to formulary status.
A major role of the NPBM-CPPM group involves clinical document development, which is inclusive of the monograph-style documents used for formulary decision making. These clinical documents can be found stored on the PBM intranet sites, and most are under the Clinical Guidance subhead. Included among these documents are Drug Monographs used for formulary consideration, Criteria for Use (CFU), Abbreviated Reviews, Clinical Recommendations, and Drug Class Reviews. The various documents are designed to serve as resources for field practitioners to help optimize drug therapy for veterans.
The focus of the NPBM-CPPMs is to optimize pharmacotherapy from a population-based perspective. This focus is in contrast to the clinical pharmacy specialists who function at the facility level and focus primarily on patients in their particular geographic region. The NPBMCPPMs need to be familiar with the VA population as a whole. Although recognizing that every patient is different, NPBM-CPPMs develop clinical guidance documents that pertain to as many veterans as possible—typically about 80% of the population. About 20% of veterans may not possess the most common characteristics of an individual with a particular condition. If a common thread can be identified among this minority, then the focus of clinical guidance can expand to help improve the outcomes for this group, as well as educate VA providers.
Oncology NPBM-CPPMs
The field of oncology pharmacy has seen tremendous growth since it was originally recognized as a specialized field of pharmacy practice in 1998. At the same time, the FDA has approved many new drugs designated for oncologic conditions.3 This expansion of drugs has led to an increase in the NPBM-CPPMs oncology workforce, allowing the CPPMs to “divide and conquer” their responsibilities with respect to the oncologic diseases and pharmacotherapeutic agents used to treat these specific conditions.
The FDA approval of an oncology drug means that an NPBM-CPPM needs to first determine the role and value of this drug to the veteran population. Knowing the most common oncologic conditions that afflict veterans helps to understand a drug’s importance to the VA. A number of common cancers among veterans include conditions associated with exposure to Agent Orange or other herbicides during military service and include chronic B-cell leukemias, Hodgkin lymphoma, non-Hodgkin lymphoma, multiple myeloma, and prostate cancer.4 Aside from exposures related to military service, demographic and personal characteristics of the veteran population help determine the malignancies that put veterans at risk (eg, age and smoking history). It is apparent that colorectal cancer and lung cancer are among the most frequent tumor types detected among veterans.
Malignancies that are seen with less frequency in the VA are still important to the NPBM-CPPM. Breast cancer, for example, is a malignancy that afflicts a relatively small proportion of veterans, yet FDA-approved breast cancer drugs are reviewed for formulary consideration under the same national process.
Evidence-Based Determinations
The evidence-based drug monographs prepared for formulary consideration are approached in a consistent manner that takes into account clinical trial data published in peer-reviewed journals. In situations when peer-reviewed evidence is lacking, as in FDA-approval of a drug given Breakthrough Therapy designation, FDA Medical Review transcripts and abstracts from major meetings, such as the American Society of Clinical Oncology (ASCO), may be considered until published evidence is available.
The focus of the monograph is on efficacy and safety of the product and its potential impact on the veteran population. Cost-effective analyses are considered when available, although they are not commonplace at the time
of product launch.5 Authoritative reviews from other national public health providers (eg, National Institute for Health and Care Excellence) are sought to provide a perspective on a drug therapy’s impact on other health care systems.
Criteria for Use documents are tools to help direct therapy to the appropriate veterans, emphasizing the considerations for safe and optimal use. Criteria for Use are not developed for every drug under review. Instead, CFUs are focused on only those drugs that may be considered a high risk for inappropriate use or may raise safety concerns. The documents developed by the NPBM-CPPM, whether they are monographs for formulary consideration or CFUs, undergo peer review by the Medical Advisory Panel (MAP), VISN VPEs, and fieldbased experts that include Field Advisory Committees (FACs) and other field practitioners.
Cost Issues
The stimulus to develop clinical guidance is not solely based on FDA approval of a new molecular entity. Many times, there are drug-related issues, identified by practitioners in the field, that call for resolution. Some of these issues are not exclusive to VA practice but impact VA practitioners just as they would impact non-VA practitioners. It is the role of the PBM to help address those drugrelated issues.
The high cost of oncology drugs is one such issue that impacts clinicians and patients both inside and outside the VA system. The Oncology FAC recognizes the impact of high-cost drugs on the VA system as a whole. They had been tasked with the goal of providing guidance to the field on the use of high-cost oncology drugs. The oncology-focused NPBM-CPPMs has helped the Oncology FAC address this issue. The plan was to develop guidance documents that focus on minimizing the cost to both veterans and VA facilities. The strategy was to first develop
general, broad-based guidance documents that can be used by any site or VISN, especially those sites without oncology-trained pharmacists, to aid in making decisions about high-cost oncology drugs. The second step was to focus on the nuances of select drugs or diseases and provide drug-specific or disease-specific guidance to help manage cost issues within the identified areas.
Under the auspices of the Oncology FAC, the oncology-focused NPBM-CPPMs convened the High Cost Oncology Drug Workgroup to help tackle this concern. The workgroup included oncology-specialized VA physicians and pharmacists who were divided into subgroups to address areas where recognition and subsequent intervention had the greatest potential to reduce facility drug expenditures.
These interventions previously have been identified as best practices within the VA and were thought to be applicable as broad-based guidance to serve as the first step of the cost control strategy. The work of the subgroups resulted in the following guidance documents:
- Dose Rounding in Oncology
- Oral Anticancer Drugs Dispensing and Monitoring
- Oral Anticancer Drugs: Recommended Dispensing and Monitoring
- Chemotherapy Review Committee Process
- Determining Clinical Benefit of High Cost Oncology Drugs
The Oncology FAC approved these guidance documents with subsequent review under the national PBM approval process. They are not mandatory for decision making but are encouraged for use at the facility or VISN level and can be found at the PBM website.
Clinical Pathways
Prostate cancer is one of the common malignancies that afflicts veterans. It is a disease with treatments involving multiple high-cost oncology drugs and as such is an ideal therapeutic area for possible intervention. Prostate cancer provides an opportunity for the second step of this project. As there are multiple therapies available for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) that have been evaluated in the clinical trial setting for similar indications among comparable patient populations and are high cost items, providers find it difficult to choose among them.
A clinical pathway (CP) is a visual care map that provides direction for treatment options.6-8 Brief annotations are provided throughout the map to help provide rationale along with a rating of the clinical evidence that supports that decision. The ultimate goal of the CP is to improve patient outcomes by providing uniformity of care. Uniformity can lead to increased efficiencies, reduced chance of medication errors, and proactive management of expected toxicities. Clinical pathway development is an extensive process.
The oncology-focused NPBM-CPPMs serve as facilitators for the development of the prostate cancer pathway. This involved the creation of a database of pertinent prostate cancer literature, including national consensus guidelines (ie, National Comprehensive Cancer Network, American Urology Association). This database is available for reference and discussion throughout the process. Key VA oncologists with expertise in prostate cancer management were identified to serve as stakeholders and critically review the literature, providing input regarding each step throughout the pathway process.
Similar to previously described documents, the CP for mCRPC (CP-mCRPC) will undergo peer review by the Oncology FAC with subsequent review under the national PBM approval process. The intent of the CPmCRPC is not to mandate decision making regarding treatment but to encourage consistent treatment and ultimately to minimize variance in practice and optimize patient outcomes. Clinical pathways are dependent on the current evidence and, therefore, are documents that require evaluation and regular updates. The CP process for prostate cancer
will serve as a model for the development of subsequent pathways for other diseases.
Prior Authorization
Many commercial insurers use prior authorization (PA) solely for drug coverage decision making. The PBM has recently adopted an expanded variation of the PA process for a few select medications at both the national and VISN level. The VA PBM PA is a thorough review process to ensure that select patients are appropriate for a particular therapy in an attempt to optimize outcomes. In the process, providing drug therapy to those veterans most likely to benefit will minimize the impact of drug cost.
Drugs selected for PA review are those that meet the following characteristics: (1) Drug has demonstrated limited clinical benefit in a select subpopulation of patients; (2) Drug has a high potential for off-label use; and (3) Drug is considered a high-cost item. The potential benefits of this process are not limited just to ensuring that the appropriate patient receives the appropriate therapy. Prior authorization at the national and VISN levels promotes consistent health care delivery throughout the VA.
Similar to the aforementioned CP process, consistency and minimization of variance in practice are desirable to improve veteran outcomes. As more experience is obtained with the PA process, its role within the VA will be reviewed and evaluated.
Conclusion
The task of addressing the high cost of today’s anticancer therapies is not one that can be addressed with a single initiative. The ASCO Cost of Care Task Force has been focusing on various initiatives that promote evidence-based decision making aimed at addressing the cost of cancer care.9 Consistent with this approach, the VA PBM division has been working with key stakeholders at the VISN and local levels to develop interventions aimed at optimizing therapeutic outcomes for the veteran.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The VA National Formulary has existed since 1995. Before the development of a single national formulary, each VA facility managed its pharmacy benefit plan through its pharmacy and therapeutics committees. In other words, 173 formulary processes correlating with 173 facilities managed the pharmacy benefit across the entire VA system. This system served > 4 million veterans, providing > 108 million prescriptions per year.
Variations in provision of the pharmacy benefit were commonplace, including veteran access to drug therapy. Formulary processes for a particular drug that were already established in one facility might not have been developed in another facility. This variation among locations oftentimes limited drug availability. The purpose of developing a single National Formulary was twofold: (1) provide a uniform pharmacy benefit to all veterans by reducing variation in access to drugs among the facilities; and (2) obtain leverage in contract pricing for drugs across the entire VA system.
Pharmacy Benefits Management Capabilities
In 1995, VA Under Secretary for Health Kenneth Kizer,MD, established the VA Pharmacy Benefits Management (PBM) Services division. Pharmacy Benefits Management was assigned the tasks of developing a national formulary, creating pharmacologic guidelines, and managing drug costs and utilization. The VA Drug Product and Pharmaceuticals Management Division, based in Hines, Illinois, which already managed and monitored drug usage and purchasing for each VA Medical Center (VAMC) facility, expanded its services by hiring clinical pharmacists. These clinical pharmacists collaborated with field-based physicians to form the VA Medical Advisory Panel (MAP).
The VA Healthcare System is currently divided into 21 geographically defined VISN (Veteran Integrated System Network) regions. Each VISN has a designated VISN Pharmacist Executive (VPE), formerly known as a VISN Formulary Leader. The VPE serves as a pharmacy liaison between the VA health care facilities within the VISN and the national PBM. This collaboration allows open communication and a sharing of ideas and issues regarding drug therapy within the VA system. Collectively, this physician-pharmacist-based group became known as the Veterans Affairs Pharmacy Benefits Management Services division.
The National Acquisition Center (NAC) is another important collaborator with the PBM. Opportunities for pharmaceutical contracting are sought through the NAC. This contracting mechanism offers the VA opportunities for price reductions on bulk purchases, ready access to needed drugs, and a streamlined drug inventory process that reduces inventory management costs. In addition, with pharmaceutical contracting, the VA can provide identical drugs via multiple sources to minimize confusion for the patient. The NAC obtains optimized pricing through various techniques, such as competitive bidding among branded products within drug classes, the Federal Supply Schedule (FSS) program, and performance-based incentive agreements. These techniques allow the VA to maintain stability with regard to average acquisition costs per 30-day-equivalent prescriptions.1,2
National PBM Clinical Program
The primary function of the National PBM Clinical Pharmacy Program Managers (NPBM-CPPMs) is to maintain the National Formulary. In addition, PBM functions to support VA field practitioners with promoting the safe and effective use of all medications, with the ultimate goal of helping veterans achieve optimal therapeutic outcomes.
The Clinical Program includes 12 NPBM-CPPMs. This group is composed of clinical pharmacists with advanced training and education in specialty therapeutic areas who serve as pharmaceutical subject matter experts within their specialty. It is the responsibility of this group to author drug monographs that summarize clinical data about the safety and efficacy of newly approved drugs (new molecular entities). These drug monographs serve as a tool to assist in determining the formulary status of a drug. The documents are evidence based and extensive, providing the necessary information for considerations related to formulary status.
A major role of the NPBM-CPPM group involves clinical document development, which is inclusive of the monograph-style documents used for formulary decision making. These clinical documents can be found stored on the PBM intranet sites, and most are under the Clinical Guidance subhead. Included among these documents are Drug Monographs used for formulary consideration, Criteria for Use (CFU), Abbreviated Reviews, Clinical Recommendations, and Drug Class Reviews. The various documents are designed to serve as resources for field practitioners to help optimize drug therapy for veterans.
The focus of the NPBM-CPPMs is to optimize pharmacotherapy from a population-based perspective. This focus is in contrast to the clinical pharmacy specialists who function at the facility level and focus primarily on patients in their particular geographic region. The NPBMCPPMs need to be familiar with the VA population as a whole. Although recognizing that every patient is different, NPBM-CPPMs develop clinical guidance documents that pertain to as many veterans as possible—typically about 80% of the population. About 20% of veterans may not possess the most common characteristics of an individual with a particular condition. If a common thread can be identified among this minority, then the focus of clinical guidance can expand to help improve the outcomes for this group, as well as educate VA providers.
Oncology NPBM-CPPMs
The field of oncology pharmacy has seen tremendous growth since it was originally recognized as a specialized field of pharmacy practice in 1998. At the same time, the FDA has approved many new drugs designated for oncologic conditions.3 This expansion of drugs has led to an increase in the NPBM-CPPMs oncology workforce, allowing the CPPMs to “divide and conquer” their responsibilities with respect to the oncologic diseases and pharmacotherapeutic agents used to treat these specific conditions.
The FDA approval of an oncology drug means that an NPBM-CPPM needs to first determine the role and value of this drug to the veteran population. Knowing the most common oncologic conditions that afflict veterans helps to understand a drug’s importance to the VA. A number of common cancers among veterans include conditions associated with exposure to Agent Orange or other herbicides during military service and include chronic B-cell leukemias, Hodgkin lymphoma, non-Hodgkin lymphoma, multiple myeloma, and prostate cancer.4 Aside from exposures related to military service, demographic and personal characteristics of the veteran population help determine the malignancies that put veterans at risk (eg, age and smoking history). It is apparent that colorectal cancer and lung cancer are among the most frequent tumor types detected among veterans.
Malignancies that are seen with less frequency in the VA are still important to the NPBM-CPPM. Breast cancer, for example, is a malignancy that afflicts a relatively small proportion of veterans, yet FDA-approved breast cancer drugs are reviewed for formulary consideration under the same national process.
Evidence-Based Determinations
The evidence-based drug monographs prepared for formulary consideration are approached in a consistent manner that takes into account clinical trial data published in peer-reviewed journals. In situations when peer-reviewed evidence is lacking, as in FDA-approval of a drug given Breakthrough Therapy designation, FDA Medical Review transcripts and abstracts from major meetings, such as the American Society of Clinical Oncology (ASCO), may be considered until published evidence is available.
The focus of the monograph is on efficacy and safety of the product and its potential impact on the veteran population. Cost-effective analyses are considered when available, although they are not commonplace at the time
of product launch.5 Authoritative reviews from other national public health providers (eg, National Institute for Health and Care Excellence) are sought to provide a perspective on a drug therapy’s impact on other health care systems.
Criteria for Use documents are tools to help direct therapy to the appropriate veterans, emphasizing the considerations for safe and optimal use. Criteria for Use are not developed for every drug under review. Instead, CFUs are focused on only those drugs that may be considered a high risk for inappropriate use or may raise safety concerns. The documents developed by the NPBM-CPPM, whether they are monographs for formulary consideration or CFUs, undergo peer review by the Medical Advisory Panel (MAP), VISN VPEs, and fieldbased experts that include Field Advisory Committees (FACs) and other field practitioners.
Cost Issues
The stimulus to develop clinical guidance is not solely based on FDA approval of a new molecular entity. Many times, there are drug-related issues, identified by practitioners in the field, that call for resolution. Some of these issues are not exclusive to VA practice but impact VA practitioners just as they would impact non-VA practitioners. It is the role of the PBM to help address those drugrelated issues.
The high cost of oncology drugs is one such issue that impacts clinicians and patients both inside and outside the VA system. The Oncology FAC recognizes the impact of high-cost drugs on the VA system as a whole. They had been tasked with the goal of providing guidance to the field on the use of high-cost oncology drugs. The oncology-focused NPBM-CPPMs has helped the Oncology FAC address this issue. The plan was to develop guidance documents that focus on minimizing the cost to both veterans and VA facilities. The strategy was to first develop
general, broad-based guidance documents that can be used by any site or VISN, especially those sites without oncology-trained pharmacists, to aid in making decisions about high-cost oncology drugs. The second step was to focus on the nuances of select drugs or diseases and provide drug-specific or disease-specific guidance to help manage cost issues within the identified areas.
Under the auspices of the Oncology FAC, the oncology-focused NPBM-CPPMs convened the High Cost Oncology Drug Workgroup to help tackle this concern. The workgroup included oncology-specialized VA physicians and pharmacists who were divided into subgroups to address areas where recognition and subsequent intervention had the greatest potential to reduce facility drug expenditures.
These interventions previously have been identified as best practices within the VA and were thought to be applicable as broad-based guidance to serve as the first step of the cost control strategy. The work of the subgroups resulted in the following guidance documents:
- Dose Rounding in Oncology
- Oral Anticancer Drugs Dispensing and Monitoring
- Oral Anticancer Drugs: Recommended Dispensing and Monitoring
- Chemotherapy Review Committee Process
- Determining Clinical Benefit of High Cost Oncology Drugs
The Oncology FAC approved these guidance documents with subsequent review under the national PBM approval process. They are not mandatory for decision making but are encouraged for use at the facility or VISN level and can be found at the PBM website.
Clinical Pathways
Prostate cancer is one of the common malignancies that afflicts veterans. It is a disease with treatments involving multiple high-cost oncology drugs and as such is an ideal therapeutic area for possible intervention. Prostate cancer provides an opportunity for the second step of this project. As there are multiple therapies available for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) that have been evaluated in the clinical trial setting for similar indications among comparable patient populations and are high cost items, providers find it difficult to choose among them.
A clinical pathway (CP) is a visual care map that provides direction for treatment options.6-8 Brief annotations are provided throughout the map to help provide rationale along with a rating of the clinical evidence that supports that decision. The ultimate goal of the CP is to improve patient outcomes by providing uniformity of care. Uniformity can lead to increased efficiencies, reduced chance of medication errors, and proactive management of expected toxicities. Clinical pathway development is an extensive process.
The oncology-focused NPBM-CPPMs serve as facilitators for the development of the prostate cancer pathway. This involved the creation of a database of pertinent prostate cancer literature, including national consensus guidelines (ie, National Comprehensive Cancer Network, American Urology Association). This database is available for reference and discussion throughout the process. Key VA oncologists with expertise in prostate cancer management were identified to serve as stakeholders and critically review the literature, providing input regarding each step throughout the pathway process.
Similar to previously described documents, the CP for mCRPC (CP-mCRPC) will undergo peer review by the Oncology FAC with subsequent review under the national PBM approval process. The intent of the CPmCRPC is not to mandate decision making regarding treatment but to encourage consistent treatment and ultimately to minimize variance in practice and optimize patient outcomes. Clinical pathways are dependent on the current evidence and, therefore, are documents that require evaluation and regular updates. The CP process for prostate cancer
will serve as a model for the development of subsequent pathways for other diseases.
Prior Authorization
Many commercial insurers use prior authorization (PA) solely for drug coverage decision making. The PBM has recently adopted an expanded variation of the PA process for a few select medications at both the national and VISN level. The VA PBM PA is a thorough review process to ensure that select patients are appropriate for a particular therapy in an attempt to optimize outcomes. In the process, providing drug therapy to those veterans most likely to benefit will minimize the impact of drug cost.
Drugs selected for PA review are those that meet the following characteristics: (1) Drug has demonstrated limited clinical benefit in a select subpopulation of patients; (2) Drug has a high potential for off-label use; and (3) Drug is considered a high-cost item. The potential benefits of this process are not limited just to ensuring that the appropriate patient receives the appropriate therapy. Prior authorization at the national and VISN levels promotes consistent health care delivery throughout the VA.
Similar to the aforementioned CP process, consistency and minimization of variance in practice are desirable to improve veteran outcomes. As more experience is obtained with the PA process, its role within the VA will be reviewed and evaluated.
Conclusion
The task of addressing the high cost of today’s anticancer therapies is not one that can be addressed with a single initiative. The ASCO Cost of Care Task Force has been focusing on various initiatives that promote evidence-based decision making aimed at addressing the cost of cancer care.9 Consistent with this approach, the VA PBM division has been working with key stakeholders at the VISN and local levels to develop interventions aimed at optimizing therapeutic outcomes for the veteran.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Sales MM, Cunningham FE, Glassman PA, Valentino MA, Good CB. Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104-122.
2. Good CB, Valentino M. Access to affordable medications: The Department of Veterans Affairs pharmacy plan as a national model. Am J Public Health. 2007; 97(12):2129-2131.
3. CenterWatch. FDA approved drugs by therapeutic area. CenterWatch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/therapeuticarea/ 12/oncology. Accessed November 26, 2014.
4. Department of Veterans Affairs. Veterans’ disease associated with Agent Orange. Department of Veterans Affairs Website. http://www.publichealth.va.gov/exposures/agentorang/conditions/index.asp. Last Updated December 30, 2013. Accessed November 26, 2014.
5. Aspinall SL, Good CB, Glassman PA, Valentino MA. The evolving use of cost-effectiveness analysis in formulary management within the Department of Veterans Affairs. Med Care. 2005;43(suppl 7):20-26.
6. Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: Do pathways work? Int J Qual Health Care. 2003;15(6):509-521.
7. Kinsman L, Rotter T, James E, Snow P, Willis J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31.
8. Gesme DH, Wiseman M. Strategic use of clinical pathways. J Oncol Pract. 2011;7(1):54-56.
9. Meropol NJ, Schrag D, Smith TJ, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol. 2009;27(23):3868-3874.
1. Sales MM, Cunningham FE, Glassman PA, Valentino MA, Good CB. Pharmacy benefits management in the Veterans Health Administration: 1995 to 2003. Am J Manag Care. 2005;11(2):104-122.
2. Good CB, Valentino M. Access to affordable medications: The Department of Veterans Affairs pharmacy plan as a national model. Am J Public Health. 2007; 97(12):2129-2131.
3. CenterWatch. FDA approved drugs by therapeutic area. CenterWatch Website. http://www.centerwatch.com/drug-information/fda-approved-drugs/therapeuticarea/ 12/oncology. Accessed November 26, 2014.
4. Department of Veterans Affairs. Veterans’ disease associated with Agent Orange. Department of Veterans Affairs Website. http://www.publichealth.va.gov/exposures/agentorang/conditions/index.asp. Last Updated December 30, 2013. Accessed November 26, 2014.
5. Aspinall SL, Good CB, Glassman PA, Valentino MA. The evolving use of cost-effectiveness analysis in formulary management within the Department of Veterans Affairs. Med Care. 2005;43(suppl 7):20-26.
6. Panella M, Marchisio S, Di Stanislao F. Reducing clinical variations with clinical pathways: Do pathways work? Int J Qual Health Care. 2003;15(6):509-521.
7. Kinsman L, Rotter T, James E, Snow P, Willis J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31.
8. Gesme DH, Wiseman M. Strategic use of clinical pathways. J Oncol Pract. 2011;7(1):54-56.
9. Meropol NJ, Schrag D, Smith TJ, et al; American Society of Clinical Oncology. American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol. 2009;27(23):3868-3874.
Treating Patients With Multiple Myeloma in the VA
The following is a lightly edited transcript of a portion of a teleconference discussion on treating patients with multiple myeloma in the VHA. The conclusion will be published in the August special issue. For more information and to listen to the conversation, visit FedPrac.com/AVAHOupdates.
Biology of Multiple Myeloma
Dr. Munshi. There are many new advances in understanding of basic molecular and genomic changes in multiple myeloma (MM) that involve signaling pathways that drive MM. We have many drugs that target signaling pathways and the number of newer mutational changes that are identified could have therapeutic as well as prognostic significance.
One of the important findings is the lack of specific myeloma-related mutations. Unlike Waldenström macroglobulinemia, which has about 90% patients with Myd88 mutation, in myeloma we do not see that. The mutation frequency, at the maximum, is in the range of 20% for any one gene, and the 3 to 4 most common genes mutated are KRAS, NRAS, BRAF, and P53.
Importantly, if we look at RAS and RAF combined, they target the MEK pathway. So, almost 45% patients have a mutation affecting the MEK pathway, and potentially we can use drugs in the future to see if MEK inhibitors will provide some benefit. And there are anecdotal reports and 1 medium-sized study that has used either a pure MEK inhibitor or a BRAF inhibitor with responses in MM patients. This is a very exciting new area of development.
The second important biological feature in myeloma is clonal shifts. You see multiple clones even at the time of diagnosis. The clonal complexity increases and different clones shift over time with treatment, various other interventions, and changes in myeloma growth patterns. In the future, we cannot just do genomic or cytogenetic analysis one time and sit on it. Over the course of the disease, we may have to repeat it to see if a new clone has evolved. Low-grade or low-risk disease can become a high-grade disease over time.
What is becoming apparent is that sometimes a clone that has almost disappeared reappears after 3, 4, or 5 years. The patient can become resistant to a drug that he or she previously was responsive to with the emergenceof a resistant clone. Three or 4 years later the old sensitive clone can reappear and again be sensitive to the drug that it was not responsive to. The patient may be able to use the same drug again down the line and/or consider similar pathways for targeting. This is one of the major advances that is happening now and is going to inform how we treat patients and how we evaluate patients.
Dr. Mehta. Dr. Munshi, is this going to become “big data?” Are we going to get a lot of information about the molecular changes and findings in our patients not only over time with the evolution of the disease, but also with treatment with various combinations and single agents? Do you think that we at the VA would be able to contribute to some type of banking of material with reference labs that can help to interpret all of the data that we’re going to be able to generate?
Tissue Banking
Dr. Munshi. I think that’s a very important and interesting question. Some mechanisms should be developed so we could not only bank, but also study these genomic patterns. From the VA point of view, there would be some peculiarities that we should understand. One is the age of the patient population—veterans usually end up being older. Number 2, we know the disease is more common in the African American population, and we need to understand why. Tissue banking may help us compare the genomic differences and similarities to understand who may be predisposed to increased frequency of myeloma.
Finally, we still have to keep Agent Orange in mind. Although it is becoming quite an old exposure, a lot of times myeloma occurs. There is a recent paper in JAMA Oncology that showed that incidence of MGUS [monoclonal gammopathy of undetermined significance] is higher in people who are exposed to Agent Orange. Tissue banking to understand that also would be extremely important, and the banked tissue should be processed to understand what’s happening at the molecular level.
Dr. Ascensão. What’s interesting is that there are some DoD specimens (most of it is serology is my understanding), but we may be able to get other material. Having predisease tissue and watching as the patient develops MGUS, smoldering MM, or MM would allow us to see whether there were already mutational changes in the individual even before Agent Orange exposure or, perhaps, was a result of the exposure.
Dr. Mehta. If we could do that, we could even develop protocols to prevent progression of disease. We could imagine a day when we can see the first event occurring and do something to prevent the progression to MGUS and then to smoldering and overt myeloma. Now, we’re in a particularly good position having a national network to propose this type of national bank.
Dr. Ascensão. We have some advantages as a group with a high prevalence of African American patients, as Dr. Munshi mentioned. And we have the biologic components of pathogenesis with Agent Orange. I think it’s something we can afford. Dr. Roodman, what do people in the field need to know about the biology of myeloma that’s going to help them?
Dr. Roodman. People still don’t understand some of the mechanisms underlying support of myeloma growth by the microenvironment. There are multiple targets that have been examined, and none of them work especially well except for what we already use, proteasome antagonists and immunomodulatory agents. In terms of the biology of myeloma bone disease, healing myeloma bone lesions is still a major issue that needs to be addressed. The question is how to do that. I have a VA grant to look at that question and other groups are actively studying the problem.
How particular myeloma clones become dominant is still a wide-open question. Some researchers are pursuing how the microenvironment selectively allows more aggressive clones to become dominant. Currently, the major research focus is on intrinsic changes in the myeloma cell but the microenvironment may also be contributing to the process.
Relapse
Dr. Ascensão. Years ago, people who relapsed, relapsed with bone disease, which may not be necessarily how people are relapsing these days. We are seeing testicular relapses, hepatic relapses, or pulmonary relapses in individuals who are exposed to some of the new agents. There may be interesting developments in terms of interactions in the hepatocellular microenvironment component and the myeloma cells at that level.
Dr. Roodman. These types of relapses are by myeloma cells that can grow independent of the bone marrow micro-environment and these myeloma cells are behaving more like a lymphoma than a myeloma. Several groups have been studying these types of relapses and are examining the expression of adhesion molecules and loss of expression of adhesion molecules to understand why the myeloma cells aren’t anchored in the marrow. This is just my opinion, but we really need to decide on something that could be done within the VA and ask questions similar to the 2 VA clinical trials Dr. Munshi developed. Those were doable in the VA, and we were able to get support for these trials. I think we have to ask questions that allow us to take advantage of the unique features of the VA patient population.
Dr. Chauncey. I would offer a comment from the clinical perspective. You mentioned that this is an observation with newer therapies, and it’s certainly been an observation in the marrow transplantation setting that the pattern of relapse changes. As treatments become more effective, the pattern of relapse can change. When we first started performing autologous transplantation, the pattern of relapse changed from the chemotherapy used at the time. When we started performing allogeneic transplantation, and to the extent that we use that option, we see a different pattern of relapse with substantially more extramedullary disease. This is really a polyclonal or oligoclonal disease, and as different clones evolve over time, whether it’s immunologically mediated or cytotoxic suppression of the initially dominant clone, you see clonal evolution with a different clinical presentation.
Immune System
Dr. Ascensão. Dr. Munshi, what do you think about the immunologic aspects of the disease in terms of its evolution?
Dr. Munshi. They are both aspects of the impact of myeloma on the immune system as Dr. Chauncey mentioned with progression similar to what Dr. Roodman described in the bone, but with greater impact on immune functions. With both pro- and antifunctions you get more TH17 responses, increased T regulatory cell responses, but also more microenvironmental immune cells change.
The second effect is that the immune cell also affects the myeloma growth. For example, proinflammatory cytokine produce interleukin (IL)6, IL17, IL21, and IL23 that affect myeloma or provide myeloma cell growth and signaling mechanism. Also, the PDC (plasmacytoid dendritic cell) is one of the best bone marrow components that induces and supports myeloma growth. With progression, some of these microenvironmental elements actually play a greater role in having the disease function or progress growth more aggressively than otherwise.
The second important aspect that comes into the picture in the immune and bone marrow microenvironment is the role of selecting the clone. There are literally hundreds of clones in a given patient. Certain clones would be supported preferentially by the immune cells, and in some cases, these aggressive clones become independent and grow without the need of support. That’s when they end up becoming extramedullary disease, which also determines how the myeloma cell is growing with these molecular changes.
Dr. Mehta. Another point of evidence for the immune system dysregulation in allowing growth of myeloma is the impact of some of the new trials. So the KEYSTONE 023 trial, for example, showed that pembrolizumab, the programmed death 1 protein (PD1) inhibitor, helped to prevent progression of disease and actually increased survival. The CAR T [chimeric antigen receptor T-cell] studies, some of which were presented at ASH [American Society of Hematology annual meeting] last December, also are beginning to show promise, almost as well as in chronic lymphocytic leukemia. We could look at the therapeutic response as an indicator of biologic pathogenesis and say, “likely the immune system is a major determinant.”
Standard of Therapy
Dr. Ascensão. The Spanish group published some interesting data on high-risk MGUS and smoldering MM. What do you as investigators and clinicians look at to make a diagnosis of MGUS, and what kind of test would you do in order to separate these different groups? How would you define current standard of therapy, and do you assign specific therapies for specific groups of patients?
Dr. Munshi. The current standard is a 3-drug regimen in the U.S. The most common 3-drug regimen that we all usually use, definitely in younger people, but also in older people with some dose modification, was a proteasome inhibitor and immunomodulator with dexamethasone—commonly lenalidomide, bortezomib, and dexamethasone (RVD). One can use carfilzomib also. Now we are beginning to switch to ixazomib, an oral proteasome inhibitor, and it can become an all-oral regimen that could be very convenient. We will have a VA study utilizing a similar agent to make an all-oral regimen for treatment.
An alternative people use that has a financial difference is to use a proteasome inhibitor with cyclophosphamide and dexamethasone, a VCD-like regimen. The study is going to use ixazomib, cyclophosphamide, and dexamethasone followed by ixazomib maintenance. That’s the usual induction regimen.
The question is do we do this differently whether the patient is high risk or low risk? My personal bias in the answer is, not really. At the beginning, we will present the best treatment for the patient’s health. If they are older patients with a lot of comorbidities, which is more important, we can use weekly bortezomib instead of using the full dose. We can use subcutaneous weekly bortezomib or a similar 3-drug regimen. And so high risk or low risk doesn’t change how we’re doing the beginning.
The place where the risk stratification comes into the picture is if patients get a transplant. We are beginning to do posttransplant consolidation, and that’s where we would add 2 cycles of consolidation. If consolidation is not done, for high-risk patients one may do maintenance with 2 drugs like bortezomib and lenalidomide alone; whereas in a low-risk patient, you could do lenalidomide only and not use bortezomib with it. This is the impact of risk stratification as far as what we do. It comes more at the later time rather than earlier time point.
Dr. Mehta. There are still a lot of unanswered questions. One area where I think the VA is very good at becoming involved, if we choose to do so, would be some of the drug dosings. For example, dexamethasone used to be used in high dose and the ECOG study showed that you can use it in lower dose weekly rather than 4 days on, 4 days off. The Myeloma In VA (MIVA) group led by Dr. Munshi did a study looking at different doses of lenalidomide. There’s still unanswered questions even in this standard of care regimen that we use where we could try to make the regimen more tolerable for patients.
Dr. Roodman. RVd (modified RVD) has been piloted by Dr. Noopur Raje at Mass General, but I don’t think it’s a large study. She uses it in older patients. To follow-up on what you said, which I think is a really great idea, we could look at RVd as well as 2 doses of dexamethasone, or use oral proteasome antagonists instead.
Dr. Mehta. Yes, to reduce the neurotoxicity.
Dr. Roodman. That is correct. But I think the VA is set up to look at those kinds of questions because most of our patients are older.
Dr. Mehta. They have all the comorbidities.
Dr. Chauncey. We have what we think is an optimal regimen for an optimal patient, and then we have what we often see in the clinic. I’m not sure of the quantitative VA demographics, but if you look at the U.S. myeloma population, the median age is 70 years. While a triplet like
RVd or a carfilzomib-based triplet is probably optimal based on depth of response and the theoretical aspect of suppression of all subclones, it’s not really an accessible regimen for many patients that I see in clinic who are not transplant eligible.
For the nontransplant patients and more frail patients, the doublets or the cyclophosphamide/bortezomib/dexamethasone are reasonable options. I know there are proponents saying that everybody should get RVd or dose-attenuated RVd; I don’t think that’s practical for many of the patients that we see in our clinics.
Dr. Mehta. Why is it not practical in most of your patients? Because they have to come once a week to the clinic?
Dr. Chauncey. That’s part of it, but I find that it’s often too intense with excessive toxicity. The patients are older with comorbidities and they have more limited physiologic reserve. Part of it is coming to clinic, and that’s where all oral regimens such as Rd are useful. I don’t know that if you have an older patient and you see a good response that you’re tracking in terms of their Mspike (monoclonal protein) and their CRAB criteria, that everybody requires the aggressive approach of RVd or a similar triplet with a proteasome inhibitor, an IMiD [immunomodulator drug], and a steroid as induction therapy.
This is true even for some of the older patients who come to autologous transplantation. The data we talk about are in a younger group of patients who can tolerate the full dose of those regimens. The all-oral regimens are attractive, but it’s also quite a financial burden, maybe not to an individual patient, but certainly to the whole health care system. I’m not sure if there is a depth or durability of response advantage for the oral proteasome inhibitor over those available as IV or subcutaneous dosing.
Pricing
Dr. Ascensão. It’s interesting you bring that up because at the Washington DC VAMC, ixazomib may be priced similarly to bortezomib.
Dr. Chauncey. Well that would be very good and very interesting. It’s possible the federal pricing would make it a lot more attractive. It’s not the case outside of the VA, but there the cost burden for oral medication is often shifted to the patient.
Dr. Cosgriff. The price of carfilzomib is about $7,000 to $9,000 per month or per cycle. When we priced ixazomib here in Portland, it actually was more expensive than bortezomib, but it was cheaper than carfilzomib.
Dr. Mehta. If we can do a drug company-sponsored study, the ixazomib people would surely be interested in its usefulness as a triplet in VA patients. It also would be good for the VA patient because you don’t have to come once a week and it has much less neurotoxicity compared to the other proteasome inhibitors, which is a big problem.
Dr. Chauncey. That would be a great study to offer the right patient. I see a lot of myeloma patients in clinic that aren’t really eligible for transplantation. Many of those patients do well on doublet therapy, though they will often require dose adjustments, both down and up, as well as tracking disease status.
Dr. Cosgriff. In Portland, we’re using a lenalidomidebased regimen as a first-line therapy. We’re starting to see more and more individuals starting RVD upfront. There are some select individuals who have diabetic neuropathy or something like that, who may go on just
a lenalidomide plus dexamethasone, leaving out the bortezomib because of neuropathy issues.
Our second-line choice at this point in time has been carfilzomib, but with ixazomib being a cheaper option, we may actually consider switching over to that as a second-line choice. When you look at the clinical trials they cite in the product literature, the grade 1 neuropathy is about 18% with ixazomib and 14% with the placebo arm, so it does add some peripheral neuropathy. Maybe not as much as we would think.
While these oral agents and all-oral regimens are nice to have, we still bring in the patients for clinic and for monitoring of white blood count. It doesn’t necessarily decrease the burden of clinic visits. We still have to get lab draws. Yes, the patient is not sitting in a chair waiting for the bortezomib infusion to be mixed by the pharmacy, but we still have considerations for travel and those types of things.
We treat the entire state of Oregon and parts of southwest Washington. Fortunately, we have community-based outpatient clinics (CBOCs) and VAMCs in other areas of the state, and we also utilize places like Walla Walla and Spokane, Washington, where we can draw and check labs remotely, which decreases the travel burden, but it still is a burden to the patient to actually go in for lab tests. We’re not looking at ixazomib as a first line currently.
We’re going to wait for data to be published on ixazomib in the first line. In a couple of presentations, researchers have suggested that if you tease the data, ixazomib may be inferior to bortezomib in the first-line setting. It will be interesting to see what the data say for that as far as a first-line setting choice.
Dr. Chauncey. I second what Dr. Cosgriff said. You can’t just give patients 3 bottles of pills and say, “See you in 3 months.” Here in the Northwest we cover large rural areas, and there are many CBOCs whose labs feed directly into our CPRS (Computerized Patient Record System) so it’s very accessible and easy to follow patients that come in less frequently, but that doesn’t change the need for regular clinical follow-up. The other thing I’d say is the noncomparative data of carfilzomib/lenalidomide/dexamethasone (CRD) is quite compelling in terms of depth and duration of response. We usually don’t want to accept convenience over efficacy. Whether that durability translates long term, we don’t really know, but I think right now, CRD is the best available regimen, again, with the caveat that it’s not really accessible to patients of all ages and performance status.
Dr. Ascensão. At the Washington DC VAMC in our first cycle it is day 1, 4, 8, and 11 with bortezomib, but after that we go pretty much to weekly bortezomib. We also tend to use what I would call lower dose 20 mg dexamethasone in patients over the age of 70 years. And we feel that it is a lot less toxic. We use subcutaneous bortezomib for pretty much everybody else.
Managing Adverse Effects
Dr. Chauncey. I think we’re all using subcutaneous bortezomib at this point. Dexamethasone doesn’t get a lot of independent attention, but there’s no question that, as Dr. Mehta mentioned, the older regimen that we used, the dose-dense dexamethasone of the VAD regimen, was
quite disabling. In addition to obvious hyperglycemia, there were psychiatric problems and, ultimately, profound steroid myopathy that seemed to affect patients in variable fashion. Different patients seem to be more or less susceptible, but after a couple of cycles, it starts to kick in and is progressive.
So we’ve since abandoned those massive doses. But when you look at the ECOG study (E4A03) that really defined the lower dose (40 mg weekly), there’s no question the higher dose was more toxic but also more effective in terms of disease response. While there are many older patients that I would start with a lower dose of dexamethasone, whether it’s with lenalidomide or bortezomib, I keep in mind there can be a steep dose response curve for dexamethasone. If you’re giving 20 mg and you’re not getting the response you need, then you increase the dose. There is definitely a dose response, but the higher doses are just not as well tolerated.
Dr. Cosgriff. I don’t know if other institutions are doing it, but instead of doing 40 mg as a single dose because of patient performance status, providers in Portland will prescribe 20 mg on days 1, 2, 8, 9, 15, and 16. They’ll break up that 40 mg dose and give it that way.
Dr. Chauncey. I don’t know if that strategy is biologically equivalent in terms of antimyeloma activity or less toxic in terms of myopathy. There’s almost always some disease marker to track, so that whether you’re using the serum free-light chain assay or serum protein electrophoresis, you can see if the strategy you’re using is working in real time.
Dr. Cosgriff. I’ve never known whether it’s been shown to be more efficacious or if it’s just a way of getting around some of the adverse effects. However, it does pose some alternate challenges. With higher doses of steroids, you’re looking at 2 days where the patient can become hyperglycemic, if not, a little bit longer.
The other thing with it is that adding on that extra day of dexamethasone can interfere with some other drugs and some other therapies. In individuals who have had a deep vein thrombosis for whatever reason and they’re on warfarin, now we have an agent that really screws up our warfarin monitoring. We would have to consider switching them to another agent.
Thrombosis
Dr. Mehta. It’s also prothrombotic.
Dr. Chauncey. If you actually ask the patient, independent of the hyperglycemia, independent of the myopathy, independent of psychosis, but just quality of life, they’ll typically tell you that the on-and-off of steroids is the worst part of the regimen. It’s often the roller coaster ride of short-term hypomania followed by dysphoria.
Dr. Mehta. And the lack of sleep. They describe it as being out of their skin.
Dr. Chauncey. They are. And as soon as they stop, often there is a depression.
Dr. Mehta. It is very, very difficult. And some actually develop psychosis.
Dr. Ascensão. We all use some form of acyclovir or its derivative for the prevention of shingles in patients exposed to the proteasome inhibitors. We use aspirin, usually low dose (81 mg), for deep vein thrombosis prophylaxis. But is anybody using other anticoagulants or putting everybody prophylactically on proton-pump inhibitors (PPIs) or just seeing how people do first and then adjusting?
Dr. Chauncey. I typically use conventional dose aspirin, and if there’s breakthrough thrombosis, the first response should be that it is not the best regimen for this patient. Sometimes you have to go back to it, and if someone’s an anticoagulation candidate, then full anticoagulation is
needed if that’s the best regimen. Usually if there’s a breakthrough thrombosis, it is a deal breaker, and you’re ready to move on to a nonthrombogenic regimen.
There has been an observation (there is some biological basis to back this up) that if you give bortezomib with an IMiD, the regimen became less thrombogenic than with the IMiD and dexamethasone alone.
Dr. Ascensão. On aspirin, even if they’re on an IMiD plus a proteasome inhibitor, I just don’t know that the data are good enough for us to avoid it at this point in time. And I don’t necessarily put people on a PPI unless they’ve got added gastrointestinal problems and unless they have associated heartburn or dyspepsia symptoms.
Dr. Mehta. I use low-dose aspirin in every patient. And if they breakthrough, they go on full anticoagulation usually with a new oral anticoagulant. I use PPIs only if needed, although most of them do need it, and, of course, bisphosphonates so the bone protective aspect.
Click to read the digital edition.
The following is a lightly edited transcript of a portion of a teleconference discussion on treating patients with multiple myeloma in the VHA. The conclusion will be published in the August special issue. For more information and to listen to the conversation, visit FedPrac.com/AVAHOupdates.
Biology of Multiple Myeloma
Dr. Munshi. There are many new advances in understanding of basic molecular and genomic changes in multiple myeloma (MM) that involve signaling pathways that drive MM. We have many drugs that target signaling pathways and the number of newer mutational changes that are identified could have therapeutic as well as prognostic significance.
One of the important findings is the lack of specific myeloma-related mutations. Unlike Waldenström macroglobulinemia, which has about 90% patients with Myd88 mutation, in myeloma we do not see that. The mutation frequency, at the maximum, is in the range of 20% for any one gene, and the 3 to 4 most common genes mutated are KRAS, NRAS, BRAF, and P53.
Importantly, if we look at RAS and RAF combined, they target the MEK pathway. So, almost 45% patients have a mutation affecting the MEK pathway, and potentially we can use drugs in the future to see if MEK inhibitors will provide some benefit. And there are anecdotal reports and 1 medium-sized study that has used either a pure MEK inhibitor or a BRAF inhibitor with responses in MM patients. This is a very exciting new area of development.
The second important biological feature in myeloma is clonal shifts. You see multiple clones even at the time of diagnosis. The clonal complexity increases and different clones shift over time with treatment, various other interventions, and changes in myeloma growth patterns. In the future, we cannot just do genomic or cytogenetic analysis one time and sit on it. Over the course of the disease, we may have to repeat it to see if a new clone has evolved. Low-grade or low-risk disease can become a high-grade disease over time.
What is becoming apparent is that sometimes a clone that has almost disappeared reappears after 3, 4, or 5 years. The patient can become resistant to a drug that he or she previously was responsive to with the emergenceof a resistant clone. Three or 4 years later the old sensitive clone can reappear and again be sensitive to the drug that it was not responsive to. The patient may be able to use the same drug again down the line and/or consider similar pathways for targeting. This is one of the major advances that is happening now and is going to inform how we treat patients and how we evaluate patients.
Dr. Mehta. Dr. Munshi, is this going to become “big data?” Are we going to get a lot of information about the molecular changes and findings in our patients not only over time with the evolution of the disease, but also with treatment with various combinations and single agents? Do you think that we at the VA would be able to contribute to some type of banking of material with reference labs that can help to interpret all of the data that we’re going to be able to generate?
Tissue Banking
Dr. Munshi. I think that’s a very important and interesting question. Some mechanisms should be developed so we could not only bank, but also study these genomic patterns. From the VA point of view, there would be some peculiarities that we should understand. One is the age of the patient population—veterans usually end up being older. Number 2, we know the disease is more common in the African American population, and we need to understand why. Tissue banking may help us compare the genomic differences and similarities to understand who may be predisposed to increased frequency of myeloma.
Finally, we still have to keep Agent Orange in mind. Although it is becoming quite an old exposure, a lot of times myeloma occurs. There is a recent paper in JAMA Oncology that showed that incidence of MGUS [monoclonal gammopathy of undetermined significance] is higher in people who are exposed to Agent Orange. Tissue banking to understand that also would be extremely important, and the banked tissue should be processed to understand what’s happening at the molecular level.
Dr. Ascensão. What’s interesting is that there are some DoD specimens (most of it is serology is my understanding), but we may be able to get other material. Having predisease tissue and watching as the patient develops MGUS, smoldering MM, or MM would allow us to see whether there were already mutational changes in the individual even before Agent Orange exposure or, perhaps, was a result of the exposure.
Dr. Mehta. If we could do that, we could even develop protocols to prevent progression of disease. We could imagine a day when we can see the first event occurring and do something to prevent the progression to MGUS and then to smoldering and overt myeloma. Now, we’re in a particularly good position having a national network to propose this type of national bank.
Dr. Ascensão. We have some advantages as a group with a high prevalence of African American patients, as Dr. Munshi mentioned. And we have the biologic components of pathogenesis with Agent Orange. I think it’s something we can afford. Dr. Roodman, what do people in the field need to know about the biology of myeloma that’s going to help them?
Dr. Roodman. People still don’t understand some of the mechanisms underlying support of myeloma growth by the microenvironment. There are multiple targets that have been examined, and none of them work especially well except for what we already use, proteasome antagonists and immunomodulatory agents. In terms of the biology of myeloma bone disease, healing myeloma bone lesions is still a major issue that needs to be addressed. The question is how to do that. I have a VA grant to look at that question and other groups are actively studying the problem.
How particular myeloma clones become dominant is still a wide-open question. Some researchers are pursuing how the microenvironment selectively allows more aggressive clones to become dominant. Currently, the major research focus is on intrinsic changes in the myeloma cell but the microenvironment may also be contributing to the process.
Relapse
Dr. Ascensão. Years ago, people who relapsed, relapsed with bone disease, which may not be necessarily how people are relapsing these days. We are seeing testicular relapses, hepatic relapses, or pulmonary relapses in individuals who are exposed to some of the new agents. There may be interesting developments in terms of interactions in the hepatocellular microenvironment component and the myeloma cells at that level.
Dr. Roodman. These types of relapses are by myeloma cells that can grow independent of the bone marrow micro-environment and these myeloma cells are behaving more like a lymphoma than a myeloma. Several groups have been studying these types of relapses and are examining the expression of adhesion molecules and loss of expression of adhesion molecules to understand why the myeloma cells aren’t anchored in the marrow. This is just my opinion, but we really need to decide on something that could be done within the VA and ask questions similar to the 2 VA clinical trials Dr. Munshi developed. Those were doable in the VA, and we were able to get support for these trials. I think we have to ask questions that allow us to take advantage of the unique features of the VA patient population.
Dr. Chauncey. I would offer a comment from the clinical perspective. You mentioned that this is an observation with newer therapies, and it’s certainly been an observation in the marrow transplantation setting that the pattern of relapse changes. As treatments become more effective, the pattern of relapse can change. When we first started performing autologous transplantation, the pattern of relapse changed from the chemotherapy used at the time. When we started performing allogeneic transplantation, and to the extent that we use that option, we see a different pattern of relapse with substantially more extramedullary disease. This is really a polyclonal or oligoclonal disease, and as different clones evolve over time, whether it’s immunologically mediated or cytotoxic suppression of the initially dominant clone, you see clonal evolution with a different clinical presentation.
Immune System
Dr. Ascensão. Dr. Munshi, what do you think about the immunologic aspects of the disease in terms of its evolution?
Dr. Munshi. They are both aspects of the impact of myeloma on the immune system as Dr. Chauncey mentioned with progression similar to what Dr. Roodman described in the bone, but with greater impact on immune functions. With both pro- and antifunctions you get more TH17 responses, increased T regulatory cell responses, but also more microenvironmental immune cells change.
The second effect is that the immune cell also affects the myeloma growth. For example, proinflammatory cytokine produce interleukin (IL)6, IL17, IL21, and IL23 that affect myeloma or provide myeloma cell growth and signaling mechanism. Also, the PDC (plasmacytoid dendritic cell) is one of the best bone marrow components that induces and supports myeloma growth. With progression, some of these microenvironmental elements actually play a greater role in having the disease function or progress growth more aggressively than otherwise.
The second important aspect that comes into the picture in the immune and bone marrow microenvironment is the role of selecting the clone. There are literally hundreds of clones in a given patient. Certain clones would be supported preferentially by the immune cells, and in some cases, these aggressive clones become independent and grow without the need of support. That’s when they end up becoming extramedullary disease, which also determines how the myeloma cell is growing with these molecular changes.
Dr. Mehta. Another point of evidence for the immune system dysregulation in allowing growth of myeloma is the impact of some of the new trials. So the KEYSTONE 023 trial, for example, showed that pembrolizumab, the programmed death 1 protein (PD1) inhibitor, helped to prevent progression of disease and actually increased survival. The CAR T [chimeric antigen receptor T-cell] studies, some of which were presented at ASH [American Society of Hematology annual meeting] last December, also are beginning to show promise, almost as well as in chronic lymphocytic leukemia. We could look at the therapeutic response as an indicator of biologic pathogenesis and say, “likely the immune system is a major determinant.”
Standard of Therapy
Dr. Ascensão. The Spanish group published some interesting data on high-risk MGUS and smoldering MM. What do you as investigators and clinicians look at to make a diagnosis of MGUS, and what kind of test would you do in order to separate these different groups? How would you define current standard of therapy, and do you assign specific therapies for specific groups of patients?
Dr. Munshi. The current standard is a 3-drug regimen in the U.S. The most common 3-drug regimen that we all usually use, definitely in younger people, but also in older people with some dose modification, was a proteasome inhibitor and immunomodulator with dexamethasone—commonly lenalidomide, bortezomib, and dexamethasone (RVD). One can use carfilzomib also. Now we are beginning to switch to ixazomib, an oral proteasome inhibitor, and it can become an all-oral regimen that could be very convenient. We will have a VA study utilizing a similar agent to make an all-oral regimen for treatment.
An alternative people use that has a financial difference is to use a proteasome inhibitor with cyclophosphamide and dexamethasone, a VCD-like regimen. The study is going to use ixazomib, cyclophosphamide, and dexamethasone followed by ixazomib maintenance. That’s the usual induction regimen.
The question is do we do this differently whether the patient is high risk or low risk? My personal bias in the answer is, not really. At the beginning, we will present the best treatment for the patient’s health. If they are older patients with a lot of comorbidities, which is more important, we can use weekly bortezomib instead of using the full dose. We can use subcutaneous weekly bortezomib or a similar 3-drug regimen. And so high risk or low risk doesn’t change how we’re doing the beginning.
The place where the risk stratification comes into the picture is if patients get a transplant. We are beginning to do posttransplant consolidation, and that’s where we would add 2 cycles of consolidation. If consolidation is not done, for high-risk patients one may do maintenance with 2 drugs like bortezomib and lenalidomide alone; whereas in a low-risk patient, you could do lenalidomide only and not use bortezomib with it. This is the impact of risk stratification as far as what we do. It comes more at the later time rather than earlier time point.
Dr. Mehta. There are still a lot of unanswered questions. One area where I think the VA is very good at becoming involved, if we choose to do so, would be some of the drug dosings. For example, dexamethasone used to be used in high dose and the ECOG study showed that you can use it in lower dose weekly rather than 4 days on, 4 days off. The Myeloma In VA (MIVA) group led by Dr. Munshi did a study looking at different doses of lenalidomide. There’s still unanswered questions even in this standard of care regimen that we use where we could try to make the regimen more tolerable for patients.
Dr. Roodman. RVd (modified RVD) has been piloted by Dr. Noopur Raje at Mass General, but I don’t think it’s a large study. She uses it in older patients. To follow-up on what you said, which I think is a really great idea, we could look at RVd as well as 2 doses of dexamethasone, or use oral proteasome antagonists instead.
Dr. Mehta. Yes, to reduce the neurotoxicity.
Dr. Roodman. That is correct. But I think the VA is set up to look at those kinds of questions because most of our patients are older.
Dr. Mehta. They have all the comorbidities.
Dr. Chauncey. We have what we think is an optimal regimen for an optimal patient, and then we have what we often see in the clinic. I’m not sure of the quantitative VA demographics, but if you look at the U.S. myeloma population, the median age is 70 years. While a triplet like
RVd or a carfilzomib-based triplet is probably optimal based on depth of response and the theoretical aspect of suppression of all subclones, it’s not really an accessible regimen for many patients that I see in clinic who are not transplant eligible.
For the nontransplant patients and more frail patients, the doublets or the cyclophosphamide/bortezomib/dexamethasone are reasonable options. I know there are proponents saying that everybody should get RVd or dose-attenuated RVd; I don’t think that’s practical for many of the patients that we see in our clinics.
Dr. Mehta. Why is it not practical in most of your patients? Because they have to come once a week to the clinic?
Dr. Chauncey. That’s part of it, but I find that it’s often too intense with excessive toxicity. The patients are older with comorbidities and they have more limited physiologic reserve. Part of it is coming to clinic, and that’s where all oral regimens such as Rd are useful. I don’t know that if you have an older patient and you see a good response that you’re tracking in terms of their Mspike (monoclonal protein) and their CRAB criteria, that everybody requires the aggressive approach of RVd or a similar triplet with a proteasome inhibitor, an IMiD [immunomodulator drug], and a steroid as induction therapy.
This is true even for some of the older patients who come to autologous transplantation. The data we talk about are in a younger group of patients who can tolerate the full dose of those regimens. The all-oral regimens are attractive, but it’s also quite a financial burden, maybe not to an individual patient, but certainly to the whole health care system. I’m not sure if there is a depth or durability of response advantage for the oral proteasome inhibitor over those available as IV or subcutaneous dosing.
Pricing
Dr. Ascensão. It’s interesting you bring that up because at the Washington DC VAMC, ixazomib may be priced similarly to bortezomib.
Dr. Chauncey. Well that would be very good and very interesting. It’s possible the federal pricing would make it a lot more attractive. It’s not the case outside of the VA, but there the cost burden for oral medication is often shifted to the patient.
Dr. Cosgriff. The price of carfilzomib is about $7,000 to $9,000 per month or per cycle. When we priced ixazomib here in Portland, it actually was more expensive than bortezomib, but it was cheaper than carfilzomib.
Dr. Mehta. If we can do a drug company-sponsored study, the ixazomib people would surely be interested in its usefulness as a triplet in VA patients. It also would be good for the VA patient because you don’t have to come once a week and it has much less neurotoxicity compared to the other proteasome inhibitors, which is a big problem.
Dr. Chauncey. That would be a great study to offer the right patient. I see a lot of myeloma patients in clinic that aren’t really eligible for transplantation. Many of those patients do well on doublet therapy, though they will often require dose adjustments, both down and up, as well as tracking disease status.
Dr. Cosgriff. In Portland, we’re using a lenalidomidebased regimen as a first-line therapy. We’re starting to see more and more individuals starting RVD upfront. There are some select individuals who have diabetic neuropathy or something like that, who may go on just
a lenalidomide plus dexamethasone, leaving out the bortezomib because of neuropathy issues.
Our second-line choice at this point in time has been carfilzomib, but with ixazomib being a cheaper option, we may actually consider switching over to that as a second-line choice. When you look at the clinical trials they cite in the product literature, the grade 1 neuropathy is about 18% with ixazomib and 14% with the placebo arm, so it does add some peripheral neuropathy. Maybe not as much as we would think.
While these oral agents and all-oral regimens are nice to have, we still bring in the patients for clinic and for monitoring of white blood count. It doesn’t necessarily decrease the burden of clinic visits. We still have to get lab draws. Yes, the patient is not sitting in a chair waiting for the bortezomib infusion to be mixed by the pharmacy, but we still have considerations for travel and those types of things.
We treat the entire state of Oregon and parts of southwest Washington. Fortunately, we have community-based outpatient clinics (CBOCs) and VAMCs in other areas of the state, and we also utilize places like Walla Walla and Spokane, Washington, where we can draw and check labs remotely, which decreases the travel burden, but it still is a burden to the patient to actually go in for lab tests. We’re not looking at ixazomib as a first line currently.
We’re going to wait for data to be published on ixazomib in the first line. In a couple of presentations, researchers have suggested that if you tease the data, ixazomib may be inferior to bortezomib in the first-line setting. It will be interesting to see what the data say for that as far as a first-line setting choice.
Dr. Chauncey. I second what Dr. Cosgriff said. You can’t just give patients 3 bottles of pills and say, “See you in 3 months.” Here in the Northwest we cover large rural areas, and there are many CBOCs whose labs feed directly into our CPRS (Computerized Patient Record System) so it’s very accessible and easy to follow patients that come in less frequently, but that doesn’t change the need for regular clinical follow-up. The other thing I’d say is the noncomparative data of carfilzomib/lenalidomide/dexamethasone (CRD) is quite compelling in terms of depth and duration of response. We usually don’t want to accept convenience over efficacy. Whether that durability translates long term, we don’t really know, but I think right now, CRD is the best available regimen, again, with the caveat that it’s not really accessible to patients of all ages and performance status.
Dr. Ascensão. At the Washington DC VAMC in our first cycle it is day 1, 4, 8, and 11 with bortezomib, but after that we go pretty much to weekly bortezomib. We also tend to use what I would call lower dose 20 mg dexamethasone in patients over the age of 70 years. And we feel that it is a lot less toxic. We use subcutaneous bortezomib for pretty much everybody else.
Managing Adverse Effects
Dr. Chauncey. I think we’re all using subcutaneous bortezomib at this point. Dexamethasone doesn’t get a lot of independent attention, but there’s no question that, as Dr. Mehta mentioned, the older regimen that we used, the dose-dense dexamethasone of the VAD regimen, was
quite disabling. In addition to obvious hyperglycemia, there were psychiatric problems and, ultimately, profound steroid myopathy that seemed to affect patients in variable fashion. Different patients seem to be more or less susceptible, but after a couple of cycles, it starts to kick in and is progressive.
So we’ve since abandoned those massive doses. But when you look at the ECOG study (E4A03) that really defined the lower dose (40 mg weekly), there’s no question the higher dose was more toxic but also more effective in terms of disease response. While there are many older patients that I would start with a lower dose of dexamethasone, whether it’s with lenalidomide or bortezomib, I keep in mind there can be a steep dose response curve for dexamethasone. If you’re giving 20 mg and you’re not getting the response you need, then you increase the dose. There is definitely a dose response, but the higher doses are just not as well tolerated.
Dr. Cosgriff. I don’t know if other institutions are doing it, but instead of doing 40 mg as a single dose because of patient performance status, providers in Portland will prescribe 20 mg on days 1, 2, 8, 9, 15, and 16. They’ll break up that 40 mg dose and give it that way.
Dr. Chauncey. I don’t know if that strategy is biologically equivalent in terms of antimyeloma activity or less toxic in terms of myopathy. There’s almost always some disease marker to track, so that whether you’re using the serum free-light chain assay or serum protein electrophoresis, you can see if the strategy you’re using is working in real time.
Dr. Cosgriff. I’ve never known whether it’s been shown to be more efficacious or if it’s just a way of getting around some of the adverse effects. However, it does pose some alternate challenges. With higher doses of steroids, you’re looking at 2 days where the patient can become hyperglycemic, if not, a little bit longer.
The other thing with it is that adding on that extra day of dexamethasone can interfere with some other drugs and some other therapies. In individuals who have had a deep vein thrombosis for whatever reason and they’re on warfarin, now we have an agent that really screws up our warfarin monitoring. We would have to consider switching them to another agent.
Thrombosis
Dr. Mehta. It’s also prothrombotic.
Dr. Chauncey. If you actually ask the patient, independent of the hyperglycemia, independent of the myopathy, independent of psychosis, but just quality of life, they’ll typically tell you that the on-and-off of steroids is the worst part of the regimen. It’s often the roller coaster ride of short-term hypomania followed by dysphoria.
Dr. Mehta. And the lack of sleep. They describe it as being out of their skin.
Dr. Chauncey. They are. And as soon as they stop, often there is a depression.
Dr. Mehta. It is very, very difficult. And some actually develop psychosis.
Dr. Ascensão. We all use some form of acyclovir or its derivative for the prevention of shingles in patients exposed to the proteasome inhibitors. We use aspirin, usually low dose (81 mg), for deep vein thrombosis prophylaxis. But is anybody using other anticoagulants or putting everybody prophylactically on proton-pump inhibitors (PPIs) or just seeing how people do first and then adjusting?
Dr. Chauncey. I typically use conventional dose aspirin, and if there’s breakthrough thrombosis, the first response should be that it is not the best regimen for this patient. Sometimes you have to go back to it, and if someone’s an anticoagulation candidate, then full anticoagulation is
needed if that’s the best regimen. Usually if there’s a breakthrough thrombosis, it is a deal breaker, and you’re ready to move on to a nonthrombogenic regimen.
There has been an observation (there is some biological basis to back this up) that if you give bortezomib with an IMiD, the regimen became less thrombogenic than with the IMiD and dexamethasone alone.
Dr. Ascensão. On aspirin, even if they’re on an IMiD plus a proteasome inhibitor, I just don’t know that the data are good enough for us to avoid it at this point in time. And I don’t necessarily put people on a PPI unless they’ve got added gastrointestinal problems and unless they have associated heartburn or dyspepsia symptoms.
Dr. Mehta. I use low-dose aspirin in every patient. And if they breakthrough, they go on full anticoagulation usually with a new oral anticoagulant. I use PPIs only if needed, although most of them do need it, and, of course, bisphosphonates so the bone protective aspect.
Click to read the digital edition.
The following is a lightly edited transcript of a portion of a teleconference discussion on treating patients with multiple myeloma in the VHA. The conclusion will be published in the August special issue. For more information and to listen to the conversation, visit FedPrac.com/AVAHOupdates.
Biology of Multiple Myeloma
Dr. Munshi. There are many new advances in understanding of basic molecular and genomic changes in multiple myeloma (MM) that involve signaling pathways that drive MM. We have many drugs that target signaling pathways and the number of newer mutational changes that are identified could have therapeutic as well as prognostic significance.
One of the important findings is the lack of specific myeloma-related mutations. Unlike Waldenström macroglobulinemia, which has about 90% patients with Myd88 mutation, in myeloma we do not see that. The mutation frequency, at the maximum, is in the range of 20% for any one gene, and the 3 to 4 most common genes mutated are KRAS, NRAS, BRAF, and P53.
Importantly, if we look at RAS and RAF combined, they target the MEK pathway. So, almost 45% patients have a mutation affecting the MEK pathway, and potentially we can use drugs in the future to see if MEK inhibitors will provide some benefit. And there are anecdotal reports and 1 medium-sized study that has used either a pure MEK inhibitor or a BRAF inhibitor with responses in MM patients. This is a very exciting new area of development.
The second important biological feature in myeloma is clonal shifts. You see multiple clones even at the time of diagnosis. The clonal complexity increases and different clones shift over time with treatment, various other interventions, and changes in myeloma growth patterns. In the future, we cannot just do genomic or cytogenetic analysis one time and sit on it. Over the course of the disease, we may have to repeat it to see if a new clone has evolved. Low-grade or low-risk disease can become a high-grade disease over time.
What is becoming apparent is that sometimes a clone that has almost disappeared reappears after 3, 4, or 5 years. The patient can become resistant to a drug that he or she previously was responsive to with the emergenceof a resistant clone. Three or 4 years later the old sensitive clone can reappear and again be sensitive to the drug that it was not responsive to. The patient may be able to use the same drug again down the line and/or consider similar pathways for targeting. This is one of the major advances that is happening now and is going to inform how we treat patients and how we evaluate patients.
Dr. Mehta. Dr. Munshi, is this going to become “big data?” Are we going to get a lot of information about the molecular changes and findings in our patients not only over time with the evolution of the disease, but also with treatment with various combinations and single agents? Do you think that we at the VA would be able to contribute to some type of banking of material with reference labs that can help to interpret all of the data that we’re going to be able to generate?
Tissue Banking
Dr. Munshi. I think that’s a very important and interesting question. Some mechanisms should be developed so we could not only bank, but also study these genomic patterns. From the VA point of view, there would be some peculiarities that we should understand. One is the age of the patient population—veterans usually end up being older. Number 2, we know the disease is more common in the African American population, and we need to understand why. Tissue banking may help us compare the genomic differences and similarities to understand who may be predisposed to increased frequency of myeloma.
Finally, we still have to keep Agent Orange in mind. Although it is becoming quite an old exposure, a lot of times myeloma occurs. There is a recent paper in JAMA Oncology that showed that incidence of MGUS [monoclonal gammopathy of undetermined significance] is higher in people who are exposed to Agent Orange. Tissue banking to understand that also would be extremely important, and the banked tissue should be processed to understand what’s happening at the molecular level.
Dr. Ascensão. What’s interesting is that there are some DoD specimens (most of it is serology is my understanding), but we may be able to get other material. Having predisease tissue and watching as the patient develops MGUS, smoldering MM, or MM would allow us to see whether there were already mutational changes in the individual even before Agent Orange exposure or, perhaps, was a result of the exposure.
Dr. Mehta. If we could do that, we could even develop protocols to prevent progression of disease. We could imagine a day when we can see the first event occurring and do something to prevent the progression to MGUS and then to smoldering and overt myeloma. Now, we’re in a particularly good position having a national network to propose this type of national bank.
Dr. Ascensão. We have some advantages as a group with a high prevalence of African American patients, as Dr. Munshi mentioned. And we have the biologic components of pathogenesis with Agent Orange. I think it’s something we can afford. Dr. Roodman, what do people in the field need to know about the biology of myeloma that’s going to help them?
Dr. Roodman. People still don’t understand some of the mechanisms underlying support of myeloma growth by the microenvironment. There are multiple targets that have been examined, and none of them work especially well except for what we already use, proteasome antagonists and immunomodulatory agents. In terms of the biology of myeloma bone disease, healing myeloma bone lesions is still a major issue that needs to be addressed. The question is how to do that. I have a VA grant to look at that question and other groups are actively studying the problem.
How particular myeloma clones become dominant is still a wide-open question. Some researchers are pursuing how the microenvironment selectively allows more aggressive clones to become dominant. Currently, the major research focus is on intrinsic changes in the myeloma cell but the microenvironment may also be contributing to the process.
Relapse
Dr. Ascensão. Years ago, people who relapsed, relapsed with bone disease, which may not be necessarily how people are relapsing these days. We are seeing testicular relapses, hepatic relapses, or pulmonary relapses in individuals who are exposed to some of the new agents. There may be interesting developments in terms of interactions in the hepatocellular microenvironment component and the myeloma cells at that level.
Dr. Roodman. These types of relapses are by myeloma cells that can grow independent of the bone marrow micro-environment and these myeloma cells are behaving more like a lymphoma than a myeloma. Several groups have been studying these types of relapses and are examining the expression of adhesion molecules and loss of expression of adhesion molecules to understand why the myeloma cells aren’t anchored in the marrow. This is just my opinion, but we really need to decide on something that could be done within the VA and ask questions similar to the 2 VA clinical trials Dr. Munshi developed. Those were doable in the VA, and we were able to get support for these trials. I think we have to ask questions that allow us to take advantage of the unique features of the VA patient population.
Dr. Chauncey. I would offer a comment from the clinical perspective. You mentioned that this is an observation with newer therapies, and it’s certainly been an observation in the marrow transplantation setting that the pattern of relapse changes. As treatments become more effective, the pattern of relapse can change. When we first started performing autologous transplantation, the pattern of relapse changed from the chemotherapy used at the time. When we started performing allogeneic transplantation, and to the extent that we use that option, we see a different pattern of relapse with substantially more extramedullary disease. This is really a polyclonal or oligoclonal disease, and as different clones evolve over time, whether it’s immunologically mediated or cytotoxic suppression of the initially dominant clone, you see clonal evolution with a different clinical presentation.
Immune System
Dr. Ascensão. Dr. Munshi, what do you think about the immunologic aspects of the disease in terms of its evolution?
Dr. Munshi. They are both aspects of the impact of myeloma on the immune system as Dr. Chauncey mentioned with progression similar to what Dr. Roodman described in the bone, but with greater impact on immune functions. With both pro- and antifunctions you get more TH17 responses, increased T regulatory cell responses, but also more microenvironmental immune cells change.
The second effect is that the immune cell also affects the myeloma growth. For example, proinflammatory cytokine produce interleukin (IL)6, IL17, IL21, and IL23 that affect myeloma or provide myeloma cell growth and signaling mechanism. Also, the PDC (plasmacytoid dendritic cell) is one of the best bone marrow components that induces and supports myeloma growth. With progression, some of these microenvironmental elements actually play a greater role in having the disease function or progress growth more aggressively than otherwise.
The second important aspect that comes into the picture in the immune and bone marrow microenvironment is the role of selecting the clone. There are literally hundreds of clones in a given patient. Certain clones would be supported preferentially by the immune cells, and in some cases, these aggressive clones become independent and grow without the need of support. That’s when they end up becoming extramedullary disease, which also determines how the myeloma cell is growing with these molecular changes.
Dr. Mehta. Another point of evidence for the immune system dysregulation in allowing growth of myeloma is the impact of some of the new trials. So the KEYSTONE 023 trial, for example, showed that pembrolizumab, the programmed death 1 protein (PD1) inhibitor, helped to prevent progression of disease and actually increased survival. The CAR T [chimeric antigen receptor T-cell] studies, some of which were presented at ASH [American Society of Hematology annual meeting] last December, also are beginning to show promise, almost as well as in chronic lymphocytic leukemia. We could look at the therapeutic response as an indicator of biologic pathogenesis and say, “likely the immune system is a major determinant.”
Standard of Therapy
Dr. Ascensão. The Spanish group published some interesting data on high-risk MGUS and smoldering MM. What do you as investigators and clinicians look at to make a diagnosis of MGUS, and what kind of test would you do in order to separate these different groups? How would you define current standard of therapy, and do you assign specific therapies for specific groups of patients?
Dr. Munshi. The current standard is a 3-drug regimen in the U.S. The most common 3-drug regimen that we all usually use, definitely in younger people, but also in older people with some dose modification, was a proteasome inhibitor and immunomodulator with dexamethasone—commonly lenalidomide, bortezomib, and dexamethasone (RVD). One can use carfilzomib also. Now we are beginning to switch to ixazomib, an oral proteasome inhibitor, and it can become an all-oral regimen that could be very convenient. We will have a VA study utilizing a similar agent to make an all-oral regimen for treatment.
An alternative people use that has a financial difference is to use a proteasome inhibitor with cyclophosphamide and dexamethasone, a VCD-like regimen. The study is going to use ixazomib, cyclophosphamide, and dexamethasone followed by ixazomib maintenance. That’s the usual induction regimen.
The question is do we do this differently whether the patient is high risk or low risk? My personal bias in the answer is, not really. At the beginning, we will present the best treatment for the patient’s health. If they are older patients with a lot of comorbidities, which is more important, we can use weekly bortezomib instead of using the full dose. We can use subcutaneous weekly bortezomib or a similar 3-drug regimen. And so high risk or low risk doesn’t change how we’re doing the beginning.
The place where the risk stratification comes into the picture is if patients get a transplant. We are beginning to do posttransplant consolidation, and that’s where we would add 2 cycles of consolidation. If consolidation is not done, for high-risk patients one may do maintenance with 2 drugs like bortezomib and lenalidomide alone; whereas in a low-risk patient, you could do lenalidomide only and not use bortezomib with it. This is the impact of risk stratification as far as what we do. It comes more at the later time rather than earlier time point.
Dr. Mehta. There are still a lot of unanswered questions. One area where I think the VA is very good at becoming involved, if we choose to do so, would be some of the drug dosings. For example, dexamethasone used to be used in high dose and the ECOG study showed that you can use it in lower dose weekly rather than 4 days on, 4 days off. The Myeloma In VA (MIVA) group led by Dr. Munshi did a study looking at different doses of lenalidomide. There’s still unanswered questions even in this standard of care regimen that we use where we could try to make the regimen more tolerable for patients.
Dr. Roodman. RVd (modified RVD) has been piloted by Dr. Noopur Raje at Mass General, but I don’t think it’s a large study. She uses it in older patients. To follow-up on what you said, which I think is a really great idea, we could look at RVd as well as 2 doses of dexamethasone, or use oral proteasome antagonists instead.
Dr. Mehta. Yes, to reduce the neurotoxicity.
Dr. Roodman. That is correct. But I think the VA is set up to look at those kinds of questions because most of our patients are older.
Dr. Mehta. They have all the comorbidities.
Dr. Chauncey. We have what we think is an optimal regimen for an optimal patient, and then we have what we often see in the clinic. I’m not sure of the quantitative VA demographics, but if you look at the U.S. myeloma population, the median age is 70 years. While a triplet like
RVd or a carfilzomib-based triplet is probably optimal based on depth of response and the theoretical aspect of suppression of all subclones, it’s not really an accessible regimen for many patients that I see in clinic who are not transplant eligible.
For the nontransplant patients and more frail patients, the doublets or the cyclophosphamide/bortezomib/dexamethasone are reasonable options. I know there are proponents saying that everybody should get RVd or dose-attenuated RVd; I don’t think that’s practical for many of the patients that we see in our clinics.
Dr. Mehta. Why is it not practical in most of your patients? Because they have to come once a week to the clinic?
Dr. Chauncey. That’s part of it, but I find that it’s often too intense with excessive toxicity. The patients are older with comorbidities and they have more limited physiologic reserve. Part of it is coming to clinic, and that’s where all oral regimens such as Rd are useful. I don’t know that if you have an older patient and you see a good response that you’re tracking in terms of their Mspike (monoclonal protein) and their CRAB criteria, that everybody requires the aggressive approach of RVd or a similar triplet with a proteasome inhibitor, an IMiD [immunomodulator drug], and a steroid as induction therapy.
This is true even for some of the older patients who come to autologous transplantation. The data we talk about are in a younger group of patients who can tolerate the full dose of those regimens. The all-oral regimens are attractive, but it’s also quite a financial burden, maybe not to an individual patient, but certainly to the whole health care system. I’m not sure if there is a depth or durability of response advantage for the oral proteasome inhibitor over those available as IV or subcutaneous dosing.
Pricing
Dr. Ascensão. It’s interesting you bring that up because at the Washington DC VAMC, ixazomib may be priced similarly to bortezomib.
Dr. Chauncey. Well that would be very good and very interesting. It’s possible the federal pricing would make it a lot more attractive. It’s not the case outside of the VA, but there the cost burden for oral medication is often shifted to the patient.
Dr. Cosgriff. The price of carfilzomib is about $7,000 to $9,000 per month or per cycle. When we priced ixazomib here in Portland, it actually was more expensive than bortezomib, but it was cheaper than carfilzomib.
Dr. Mehta. If we can do a drug company-sponsored study, the ixazomib people would surely be interested in its usefulness as a triplet in VA patients. It also would be good for the VA patient because you don’t have to come once a week and it has much less neurotoxicity compared to the other proteasome inhibitors, which is a big problem.
Dr. Chauncey. That would be a great study to offer the right patient. I see a lot of myeloma patients in clinic that aren’t really eligible for transplantation. Many of those patients do well on doublet therapy, though they will often require dose adjustments, both down and up, as well as tracking disease status.
Dr. Cosgriff. In Portland, we’re using a lenalidomidebased regimen as a first-line therapy. We’re starting to see more and more individuals starting RVD upfront. There are some select individuals who have diabetic neuropathy or something like that, who may go on just
a lenalidomide plus dexamethasone, leaving out the bortezomib because of neuropathy issues.
Our second-line choice at this point in time has been carfilzomib, but with ixazomib being a cheaper option, we may actually consider switching over to that as a second-line choice. When you look at the clinical trials they cite in the product literature, the grade 1 neuropathy is about 18% with ixazomib and 14% with the placebo arm, so it does add some peripheral neuropathy. Maybe not as much as we would think.
While these oral agents and all-oral regimens are nice to have, we still bring in the patients for clinic and for monitoring of white blood count. It doesn’t necessarily decrease the burden of clinic visits. We still have to get lab draws. Yes, the patient is not sitting in a chair waiting for the bortezomib infusion to be mixed by the pharmacy, but we still have considerations for travel and those types of things.
We treat the entire state of Oregon and parts of southwest Washington. Fortunately, we have community-based outpatient clinics (CBOCs) and VAMCs in other areas of the state, and we also utilize places like Walla Walla and Spokane, Washington, where we can draw and check labs remotely, which decreases the travel burden, but it still is a burden to the patient to actually go in for lab tests. We’re not looking at ixazomib as a first line currently.
We’re going to wait for data to be published on ixazomib in the first line. In a couple of presentations, researchers have suggested that if you tease the data, ixazomib may be inferior to bortezomib in the first-line setting. It will be interesting to see what the data say for that as far as a first-line setting choice.
Dr. Chauncey. I second what Dr. Cosgriff said. You can’t just give patients 3 bottles of pills and say, “See you in 3 months.” Here in the Northwest we cover large rural areas, and there are many CBOCs whose labs feed directly into our CPRS (Computerized Patient Record System) so it’s very accessible and easy to follow patients that come in less frequently, but that doesn’t change the need for regular clinical follow-up. The other thing I’d say is the noncomparative data of carfilzomib/lenalidomide/dexamethasone (CRD) is quite compelling in terms of depth and duration of response. We usually don’t want to accept convenience over efficacy. Whether that durability translates long term, we don’t really know, but I think right now, CRD is the best available regimen, again, with the caveat that it’s not really accessible to patients of all ages and performance status.
Dr. Ascensão. At the Washington DC VAMC in our first cycle it is day 1, 4, 8, and 11 with bortezomib, but after that we go pretty much to weekly bortezomib. We also tend to use what I would call lower dose 20 mg dexamethasone in patients over the age of 70 years. And we feel that it is a lot less toxic. We use subcutaneous bortezomib for pretty much everybody else.
Managing Adverse Effects
Dr. Chauncey. I think we’re all using subcutaneous bortezomib at this point. Dexamethasone doesn’t get a lot of independent attention, but there’s no question that, as Dr. Mehta mentioned, the older regimen that we used, the dose-dense dexamethasone of the VAD regimen, was
quite disabling. In addition to obvious hyperglycemia, there were psychiatric problems and, ultimately, profound steroid myopathy that seemed to affect patients in variable fashion. Different patients seem to be more or less susceptible, but after a couple of cycles, it starts to kick in and is progressive.
So we’ve since abandoned those massive doses. But when you look at the ECOG study (E4A03) that really defined the lower dose (40 mg weekly), there’s no question the higher dose was more toxic but also more effective in terms of disease response. While there are many older patients that I would start with a lower dose of dexamethasone, whether it’s with lenalidomide or bortezomib, I keep in mind there can be a steep dose response curve for dexamethasone. If you’re giving 20 mg and you’re not getting the response you need, then you increase the dose. There is definitely a dose response, but the higher doses are just not as well tolerated.
Dr. Cosgriff. I don’t know if other institutions are doing it, but instead of doing 40 mg as a single dose because of patient performance status, providers in Portland will prescribe 20 mg on days 1, 2, 8, 9, 15, and 16. They’ll break up that 40 mg dose and give it that way.
Dr. Chauncey. I don’t know if that strategy is biologically equivalent in terms of antimyeloma activity or less toxic in terms of myopathy. There’s almost always some disease marker to track, so that whether you’re using the serum free-light chain assay or serum protein electrophoresis, you can see if the strategy you’re using is working in real time.
Dr. Cosgriff. I’ve never known whether it’s been shown to be more efficacious or if it’s just a way of getting around some of the adverse effects. However, it does pose some alternate challenges. With higher doses of steroids, you’re looking at 2 days where the patient can become hyperglycemic, if not, a little bit longer.
The other thing with it is that adding on that extra day of dexamethasone can interfere with some other drugs and some other therapies. In individuals who have had a deep vein thrombosis for whatever reason and they’re on warfarin, now we have an agent that really screws up our warfarin monitoring. We would have to consider switching them to another agent.
Thrombosis
Dr. Mehta. It’s also prothrombotic.
Dr. Chauncey. If you actually ask the patient, independent of the hyperglycemia, independent of the myopathy, independent of psychosis, but just quality of life, they’ll typically tell you that the on-and-off of steroids is the worst part of the regimen. It’s often the roller coaster ride of short-term hypomania followed by dysphoria.
Dr. Mehta. And the lack of sleep. They describe it as being out of their skin.
Dr. Chauncey. They are. And as soon as they stop, often there is a depression.
Dr. Mehta. It is very, very difficult. And some actually develop psychosis.
Dr. Ascensão. We all use some form of acyclovir or its derivative for the prevention of shingles in patients exposed to the proteasome inhibitors. We use aspirin, usually low dose (81 mg), for deep vein thrombosis prophylaxis. But is anybody using other anticoagulants or putting everybody prophylactically on proton-pump inhibitors (PPIs) or just seeing how people do first and then adjusting?
Dr. Chauncey. I typically use conventional dose aspirin, and if there’s breakthrough thrombosis, the first response should be that it is not the best regimen for this patient. Sometimes you have to go back to it, and if someone’s an anticoagulation candidate, then full anticoagulation is
needed if that’s the best regimen. Usually if there’s a breakthrough thrombosis, it is a deal breaker, and you’re ready to move on to a nonthrombogenic regimen.
There has been an observation (there is some biological basis to back this up) that if you give bortezomib with an IMiD, the regimen became less thrombogenic than with the IMiD and dexamethasone alone.
Dr. Ascensão. On aspirin, even if they’re on an IMiD plus a proteasome inhibitor, I just don’t know that the data are good enough for us to avoid it at this point in time. And I don’t necessarily put people on a PPI unless they’ve got added gastrointestinal problems and unless they have associated heartburn or dyspepsia symptoms.
Dr. Mehta. I use low-dose aspirin in every patient. And if they breakthrough, they go on full anticoagulation usually with a new oral anticoagulant. I use PPIs only if needed, although most of them do need it, and, of course, bisphosphonates so the bone protective aspect.