User login
Histiocytoid Pyoderma Gangrenosum: A Challenging Case With Features of Sweet Syndrome
To the Editor:
Neutrophilic dermatoses—a group of inflammatory cutaneous conditions—include acute febrile neutrophilic dermatosis (Sweet syndrome), pyoderma gangrenosum, and neutrophilic dermatosis of the dorsal hands. Histopathology shows a dense dermal infiltrate of mature neutrophils. In 2005, the histiocytoid subtype of Sweet syndrome was introduced with histopathologic findings of a dermal infiltrate composed of immature myeloid cells that resemble histiocytes in appearance but stain strongly with neutrophil markers on immunohistochemistry.1 We present a case of histiocytoid pyoderma gangrenosum with histopathology that showed a dense dermal histiocytoid infiltrate with strong positivity for neutrophil markers on immunohistochemistry.
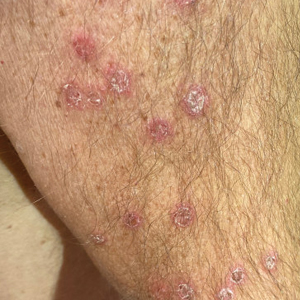
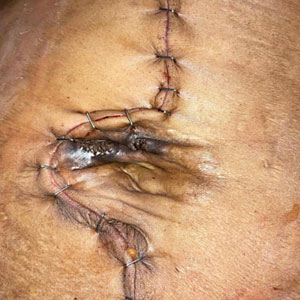
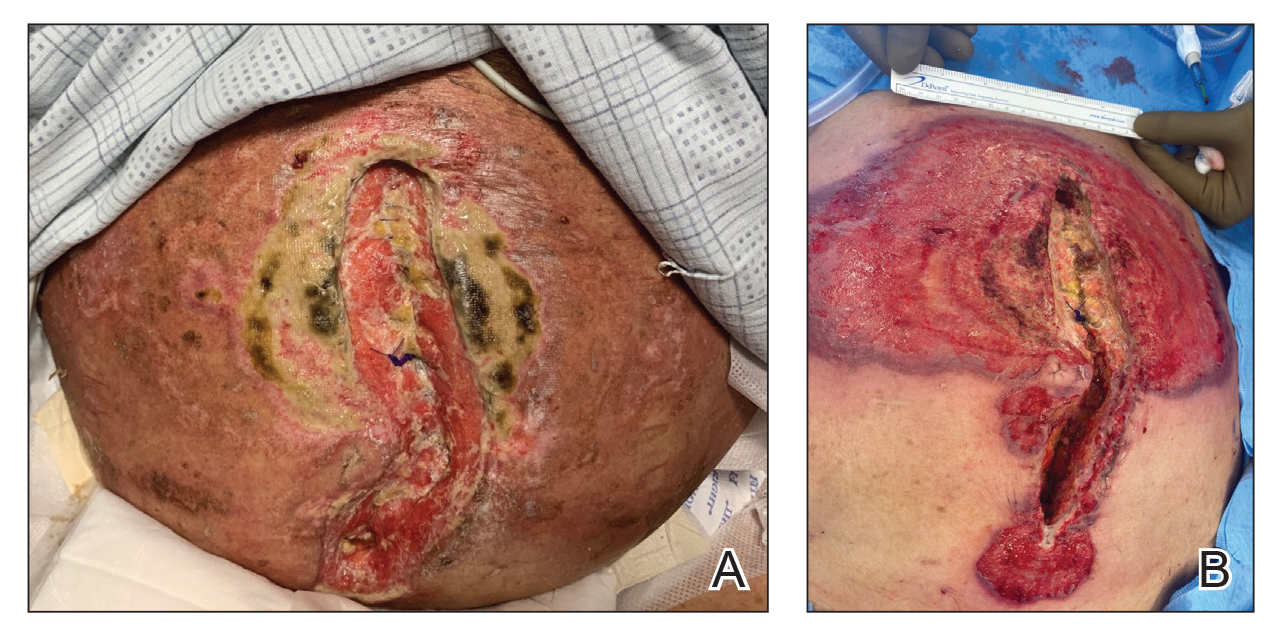
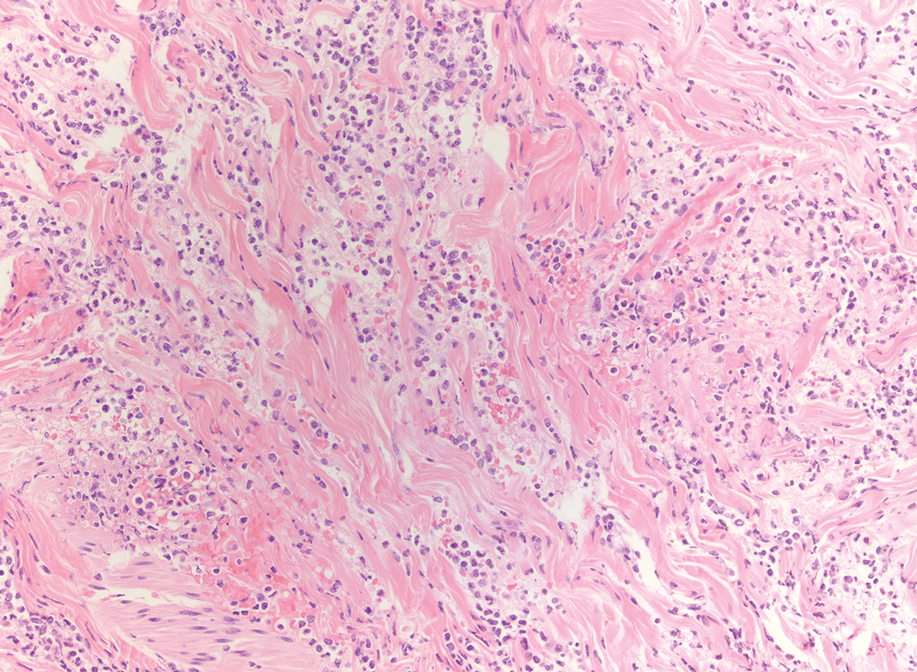
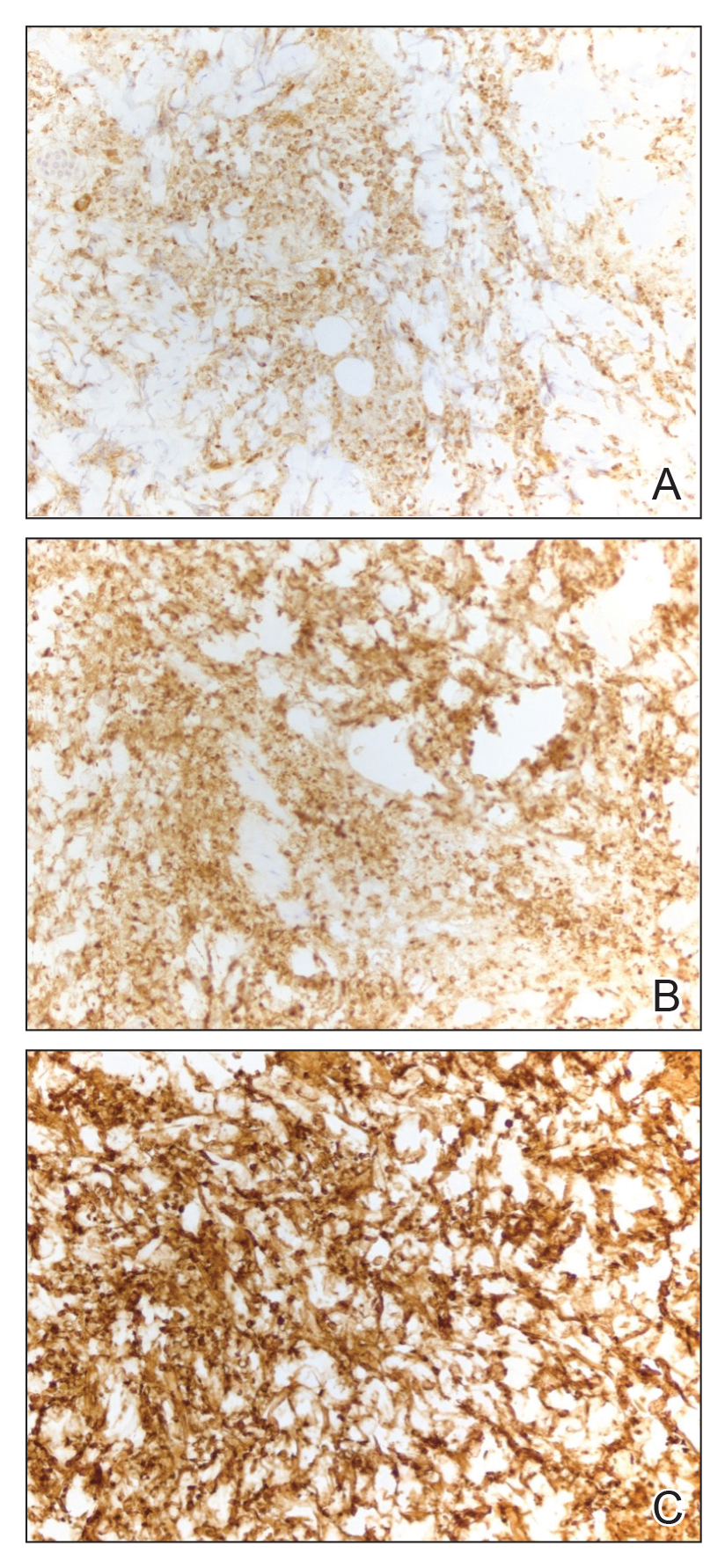
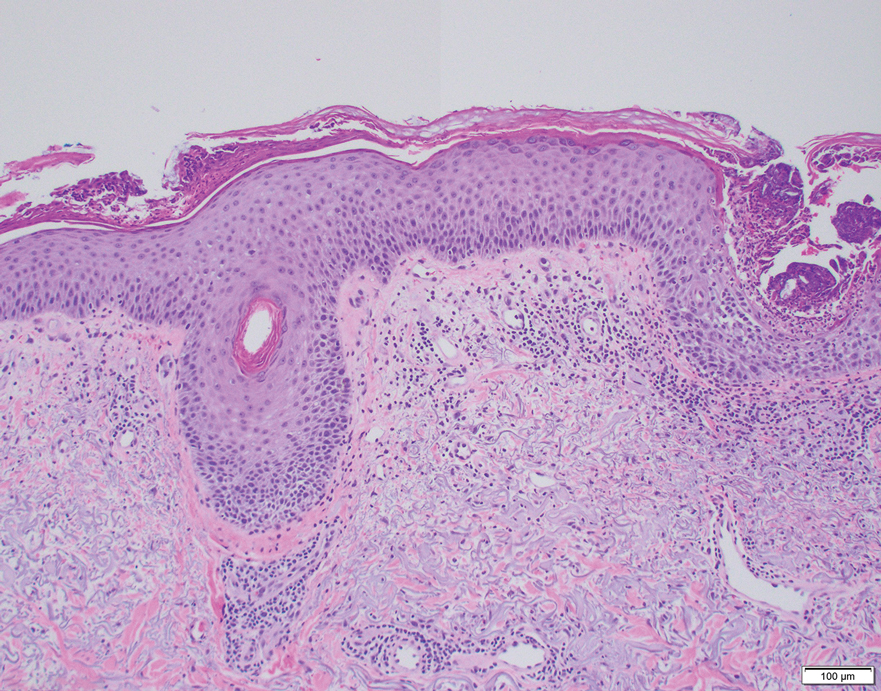
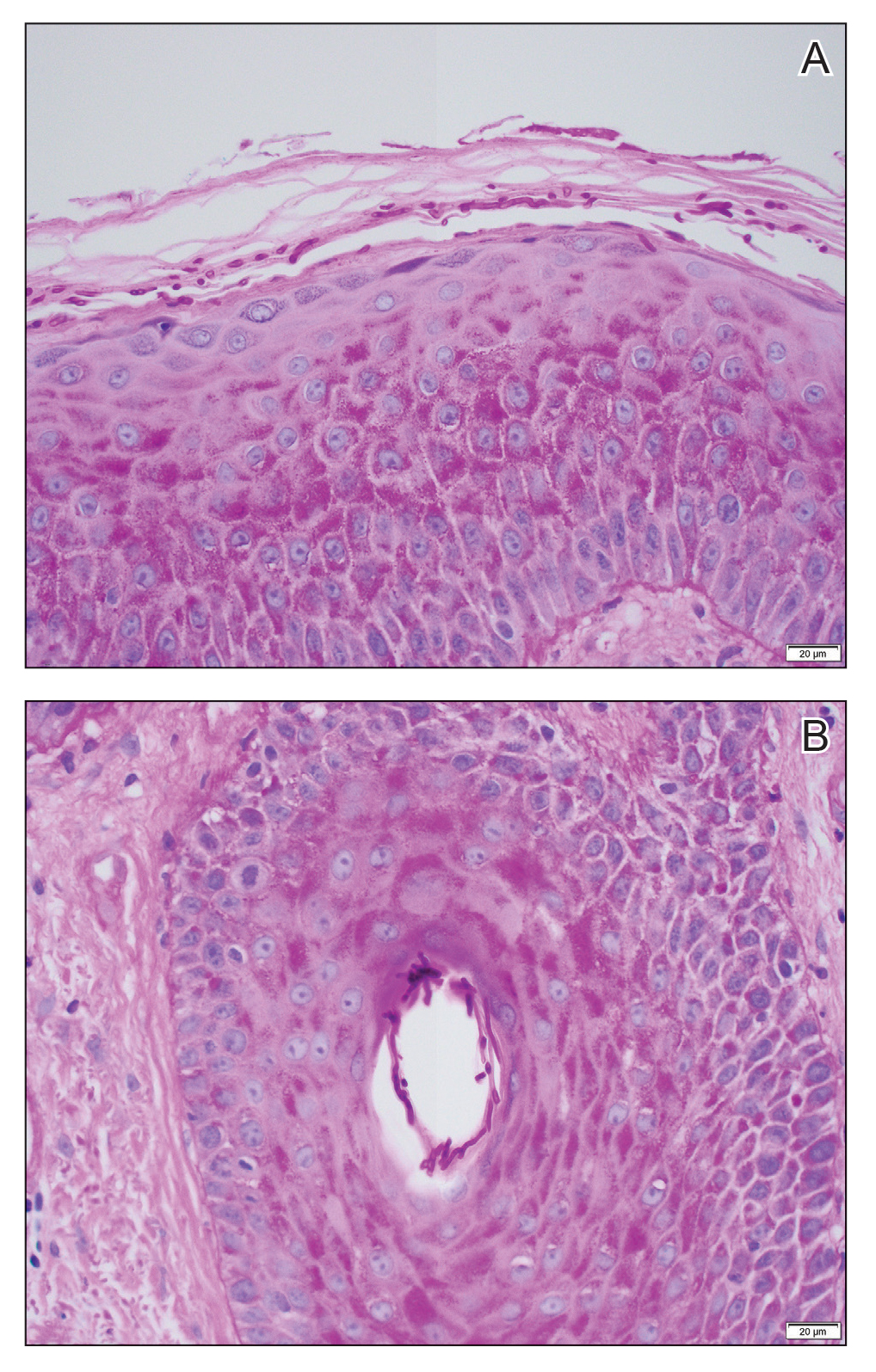
An 85-year-old man was seen by dermatology in the inpatient setting for a new-onset painful abdominal wound. He had a medical history of myelodysplastic syndrome (MDS), high-grade invasive papillary urothelial carcinoma of the bladder, and a recent diagnosis of low-grade invasive ascending colon adenocarcinoma. Ten days prior he underwent a right colectomy without intraoperative complications that was followed by septic shock. Workup with urinalysis and urine culture showed minimal pyuria with Pseudomonas aeruginosa. Additional studies, including blood cultures, abdominal wound cultures, computed tomography of the abdomen and pelvis, renal ultrasound, and chest radiographs, were unremarkable and showed no signs of surgical site infection, intra-abdominal or pelvic abscess formation, or pulmonary embolism. Broad-spectrum antibiotics—vancomycin and piperacillin-tazobactam—were started. Persistent fever (Tmax of 102.3 °F [39.1 °C]) and leukocytosis (45.3×109/L [4.2–10×109/L]) despite antibiotic therapy, increasing pressor requirements, and progressive painful erythema and purulence at the abdominal surgical site led to debridement of the wound by the general surgery team on day 9 following the initial surgery due to suspected necrotizing infection. Within 24 hours, dermatology was consulted for continued rapid expansion of the wound. Physical examination of the abdomen revealed a large, well-demarcated, pink-red, indurated, ulcerated plaque with clear to purulent exudate and superficial erosions with violaceous undermined borders extending centrifugally from the abdominal surgical incision line (Figure 1A). Two punch biopsies sent for histopathologic evaluation and tissue culture showed dermal edema with a dense histiocytic infiltrate with nodular foci and admixed mature neutrophils to a lesser degree (Figure 2). Special staining was negative for bacteria, fungi, and mycobacteria. Immunohistochemistry revealed positive staining of the dermal inflammatory infiltrate with CD68, myeloperoxidase, and lysozyme, as well as negative staining with CD34 (Figure 3). These findings were suggestive of a histiocytoid neutrophilic dermatosis such as Sweet syndrome or pyoderma gangrenosum. Due to the morphology of the solitary lesion and the abrupt exacerbation shortly after surgical intervention, the patient was diagnosed with histiocytoid pyoderma gangrenosum. At the same time, the patient’s septic shock was treated with intravenous hydrocortisone (100 mg 3 times daily) for 2 days and also achieved a prompt response in the cutaneous symptoms (Figure 1B).
Sweet syndrome and pyoderma gangrenosum are considered distinct neutrophilic dermatoses that rarely coexist but share several clinical and histopathologic features, which can become a diagnostic challenge.2 Both conditions can manifest clinically as abrupt-onset, tender, erythematous papules; vesiculopustular lesions; or bullae with ulcerative changes. They also exhibit pathergy; present with systemic symptoms such as pyrexia, malaise, and joint pain; are associated with underlying systemic conditions such as infections and/or malignancy; demonstrate a dense neutrophilic infiltrate in the dermis on histopathology; and respond promptly to systemic corticosteroids.2-6 Bullous Sweet syndrome, which can present as vesicles, pustules, or bullae that progress to superficial ulcerations, may represent a variant of neutrophilic dermatosis characterized by features seen in both Sweet syndrome and pyoderma gangrenosum, suggesting that these 2 conditions may be on a spectrum.5Clinical features such as erythema with a blue, gray, or purple hue; undermined and ragged borders; and healing of skin lesions with atrophic or cribriform scarring may favor pyoderma gangrenosum, whereas a dull red or plum color and resolution of lesions without scarring may support the diagnosis of Sweet syndrome.7 Although both conditions can exhibit pathergy secondary to minor skin trauma such as venipuncture and biopsies,2,3,5,8 Sweet syndrome rarely has been described to develop after surgery in a patient without a known history of the condition.9 In contrast, postsurgical pyoderma gangrenosum has been well described as secondary to the pathergy phenomenon.5
Our patient was favored to have pyoderma gangrenosum given the solitary lesion, its abrupt development after surgery, and the morphology of the lesion that exhibited a large violaceous to red ulcerative and exudative plaque with undermined borders with atrophic scarring. In patients with skin disease that cannot be distinguished with certainty as either Sweet syndrome or pyoderma gangrenosum, it is essential to recognize that, as neutrophilic dermatoses, both conditions can be managed with either the first-line treatment option of high-dose systemic steroids or one of the shared alternative first-line or second-line steroid-sparing treatments, such as dapsone and cyclosporine.2
Although the exact pathogenesis of pyoderma gangrenosum remains to be fully understood, paraneoplastic pyoderma gangrenosum is a frequently described phenomenon.10,11 Our patient’s history of multiple malignancies, both solid and hematologic, supports the likelihood of malignancy-induced pyoderma gangrenosum; however, given his history of MDS, several other conditions were ruled out prior to making the diagnosis of pyoderma gangrenosum.
Classically, neutrophilic dermatoses such as pyoderma gangrenosum have a dense dermal neutrophilic infiltrate. Concurrent myeloproliferative disorders can alter the maturation of leukocytes, subsequently leading to an atypical appearance of the inflammatory cells on histopathology. Further, in the setting of myeloproliferative disorders, conditions such as leukemia cutis, in which there can be a cutaneous infiltrate of immature or mature myeloid or lymphocytic cells, must be considered. To ensure our patient’s abdominal skin changes were not a cutaneous manifestation of hematologic malignancy, immunohistochemical staining with CD20 and CD3 was performed and showed only the rare presence of B and T lymphocytes, respectively. Staining with CD34 for lymphocytic and myeloid progenitor cells was negative in the dermal infiltrate and further reduced the likelihood of leukemia cutis. Alternatively, patients can have aleukemic cutaneous myeloid sarcoma or leukemia cutis without an underlying hematologic condition or with latent peripheral blood or bone marrow myeloproliferative disorder, but our patient’s history of MDS eliminated this possibility.12 After exclusion of cutaneous infiltration by malignant leukocytes, our patient was diagnosed with histiocytoid neutrophilic dermatosis.
Multiple reports have described histiocytoid Sweet syndrome, in which there is a dense dermal histiocytoid infiltrate on histopathology that demonstrates myeloid lineage with immunologic staining.1,13 The typical pattern of histiocytoid Sweet syndrome includes a predominantly unaffected epidermis with papillary dermal edema, an absence of vasculitis, and a dense dermal infiltrate primarily composed of immature histiocytelike mononuclear cells with a basophilic elongated, twisted, or kidney-shaped nucleus and pale eosinophilic cytoplasm.1,13 In an analogous manner, Morin et al12 described a patient with congenital hypogammaglobulinemia who presented with lesions that clinically resembled pyoderma gangrenosum but revealed a dense dermal infiltrate mostly made of large immature histiocytoid mononuclear cells on histopathology, consistent with the histopathologic features observed in histiocytoid Sweet syndrome. The patient ultimately was diagnosed with histiocytoid pyoderma gangrenosum. Similarly, we believe that our patient also developed histiocytoid pyoderma gangrenosum. As with histiocytoid Sweet syndrome, this diagnosis is based on histopathologic and immunohistochemical findings of a dense dermal infiltrate composed of histiocyte-resembling immature neutrophils.
Typically, pyoderma gangrenosum responds promptly to treatment with systemic corticosteroids.4 Steroid-sparing agents such as cyclosporine, azathioprine, dapsone, and tumor necrosis factor α inhibitors also may be used.4,10 In the setting of MDS, clearance of pyoderma gangrenosum has been reported upon treatment of the underlying malignancy,14 high-dose systemic corticosteroids,11,15 cyclosporine with systemic steroids,16 thalidomide,17 combination therapy with thalidomide and interferon alfa-2a,18 and ustekinumab with vacuum-assisted closure therapy.19 Our patient’s histiocytoid pyoderma gangrenosum in the setting of solid and hematologic malignancy cleared rapidly with high-dose systemic hydrocortisone.
In the setting of malignancy, as in our patient, neutrophilic dermatoses may develop from an aberrant immune system or tumor-induced cytokine dysregulation that leads to increased neutrophil production or dysfunction.4,10,11 Although our patient’s MDS may have contributed to the atypical appearance of the dermal inflammatory infiltrate, it is unclear whether the hematologic disorder increased his risk for the histiocytoid variant of neutrophilic dermatoses. Alegría-Landa et al13 reported that histiocytoid Sweet syndrome is associated with hematologic malignancy at a similar frequency as classic Sweet syndrome. It is unknown if histiocytoid pyoderma gangrenosum would have a strong association with hematologic malignancy. Future reports may elucidate a better understanding of the histiocytoid subtype of pyoderma gangrenosum and its clinical implications.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10:301-312.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Wallach D, Vignon-Pennamen MD. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Lear JT, Atherton MT, Byrne JP. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet’s syndrome. Postgrad Med. 1997;73:65-68.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:E74-E76.
- Shah M, Sachdeva M, Gefri A, et al. Paraneoplastic pyoderma gangrenosum in solid organ malignancy: a literature review. Int J Dermatol. 2020;59:154-158.
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359.
- Morin CB, Côté B, Belisle A. An interesting case of pyoderma gangrenosum with immature histiocytoid neutrophils. J Cutan Pathol. 2018;45:63-66.
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Saleh MFM, Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep. 2017;5:2025-2027.
- Yamauchi R, Ishida K, Iwashima Y, et al. Successful treatment of pyoderma gangrenosum that developed in a patient with myelodysplastic syndrome. J Infect Chemother. 2003;9:268-271.
- Ha JW, Hahm JE, Kim KS, et al. A case of pyoderma gangrenosum with myelodysplastic syndrome. Ann Dermatol. 2018;30:392-393.
- Malkan UY, Gunes G, Eliacik E, et al. Treatment of pyoderma gangrenosum with thalidomide in a myelodysplastic syndrome case. Int J Med Case Rep. 2016;9:61-64.
- Koca E, Duman AE, Cetiner D, et al. Successful treatment of myelodysplastic syndrome-induced pyoderma gangrenosum. Neth J Med. 2006;64:422-424.
- Nieto D, Sendagorta E, Rueda JM, et al. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: case report and literature review. Clin Exp Dermatol. 2019;44:116-119.
To the Editor:
Neutrophilic dermatoses—a group of inflammatory cutaneous conditions—include acute febrile neutrophilic dermatosis (Sweet syndrome), pyoderma gangrenosum, and neutrophilic dermatosis of the dorsal hands. Histopathology shows a dense dermal infiltrate of mature neutrophils. In 2005, the histiocytoid subtype of Sweet syndrome was introduced with histopathologic findings of a dermal infiltrate composed of immature myeloid cells that resemble histiocytes in appearance but stain strongly with neutrophil markers on immunohistochemistry.1 We present a case of histiocytoid pyoderma gangrenosum with histopathology that showed a dense dermal histiocytoid infiltrate with strong positivity for neutrophil markers on immunohistochemistry.
An 85-year-old man was seen by dermatology in the inpatient setting for a new-onset painful abdominal wound. He had a medical history of myelodysplastic syndrome (MDS), high-grade invasive papillary urothelial carcinoma of the bladder, and a recent diagnosis of low-grade invasive ascending colon adenocarcinoma. Ten days prior he underwent a right colectomy without intraoperative complications that was followed by septic shock. Workup with urinalysis and urine culture showed minimal pyuria with Pseudomonas aeruginosa. Additional studies, including blood cultures, abdominal wound cultures, computed tomography of the abdomen and pelvis, renal ultrasound, and chest radiographs, were unremarkable and showed no signs of surgical site infection, intra-abdominal or pelvic abscess formation, or pulmonary embolism. Broad-spectrum antibiotics—vancomycin and piperacillin-tazobactam—were started. Persistent fever (Tmax of 102.3 °F [39.1 °C]) and leukocytosis (45.3×109/L [4.2–10×109/L]) despite antibiotic therapy, increasing pressor requirements, and progressive painful erythema and purulence at the abdominal surgical site led to debridement of the wound by the general surgery team on day 9 following the initial surgery due to suspected necrotizing infection. Within 24 hours, dermatology was consulted for continued rapid expansion of the wound. Physical examination of the abdomen revealed a large, well-demarcated, pink-red, indurated, ulcerated plaque with clear to purulent exudate and superficial erosions with violaceous undermined borders extending centrifugally from the abdominal surgical incision line (Figure 1A). Two punch biopsies sent for histopathologic evaluation and tissue culture showed dermal edema with a dense histiocytic infiltrate with nodular foci and admixed mature neutrophils to a lesser degree (Figure 2). Special staining was negative for bacteria, fungi, and mycobacteria. Immunohistochemistry revealed positive staining of the dermal inflammatory infiltrate with CD68, myeloperoxidase, and lysozyme, as well as negative staining with CD34 (Figure 3). These findings were suggestive of a histiocytoid neutrophilic dermatosis such as Sweet syndrome or pyoderma gangrenosum. Due to the morphology of the solitary lesion and the abrupt exacerbation shortly after surgical intervention, the patient was diagnosed with histiocytoid pyoderma gangrenosum. At the same time, the patient’s septic shock was treated with intravenous hydrocortisone (100 mg 3 times daily) for 2 days and also achieved a prompt response in the cutaneous symptoms (Figure 1B).
Sweet syndrome and pyoderma gangrenosum are considered distinct neutrophilic dermatoses that rarely coexist but share several clinical and histopathologic features, which can become a diagnostic challenge.2 Both conditions can manifest clinically as abrupt-onset, tender, erythematous papules; vesiculopustular lesions; or bullae with ulcerative changes. They also exhibit pathergy; present with systemic symptoms such as pyrexia, malaise, and joint pain; are associated with underlying systemic conditions such as infections and/or malignancy; demonstrate a dense neutrophilic infiltrate in the dermis on histopathology; and respond promptly to systemic corticosteroids.2-6 Bullous Sweet syndrome, which can present as vesicles, pustules, or bullae that progress to superficial ulcerations, may represent a variant of neutrophilic dermatosis characterized by features seen in both Sweet syndrome and pyoderma gangrenosum, suggesting that these 2 conditions may be on a spectrum.5Clinical features such as erythema with a blue, gray, or purple hue; undermined and ragged borders; and healing of skin lesions with atrophic or cribriform scarring may favor pyoderma gangrenosum, whereas a dull red or plum color and resolution of lesions without scarring may support the diagnosis of Sweet syndrome.7 Although both conditions can exhibit pathergy secondary to minor skin trauma such as venipuncture and biopsies,2,3,5,8 Sweet syndrome rarely has been described to develop after surgery in a patient without a known history of the condition.9 In contrast, postsurgical pyoderma gangrenosum has been well described as secondary to the pathergy phenomenon.5
Our patient was favored to have pyoderma gangrenosum given the solitary lesion, its abrupt development after surgery, and the morphology of the lesion that exhibited a large violaceous to red ulcerative and exudative plaque with undermined borders with atrophic scarring. In patients with skin disease that cannot be distinguished with certainty as either Sweet syndrome or pyoderma gangrenosum, it is essential to recognize that, as neutrophilic dermatoses, both conditions can be managed with either the first-line treatment option of high-dose systemic steroids or one of the shared alternative first-line or second-line steroid-sparing treatments, such as dapsone and cyclosporine.2
Although the exact pathogenesis of pyoderma gangrenosum remains to be fully understood, paraneoplastic pyoderma gangrenosum is a frequently described phenomenon.10,11 Our patient’s history of multiple malignancies, both solid and hematologic, supports the likelihood of malignancy-induced pyoderma gangrenosum; however, given his history of MDS, several other conditions were ruled out prior to making the diagnosis of pyoderma gangrenosum.
Classically, neutrophilic dermatoses such as pyoderma gangrenosum have a dense dermal neutrophilic infiltrate. Concurrent myeloproliferative disorders can alter the maturation of leukocytes, subsequently leading to an atypical appearance of the inflammatory cells on histopathology. Further, in the setting of myeloproliferative disorders, conditions such as leukemia cutis, in which there can be a cutaneous infiltrate of immature or mature myeloid or lymphocytic cells, must be considered. To ensure our patient’s abdominal skin changes were not a cutaneous manifestation of hematologic malignancy, immunohistochemical staining with CD20 and CD3 was performed and showed only the rare presence of B and T lymphocytes, respectively. Staining with CD34 for lymphocytic and myeloid progenitor cells was negative in the dermal infiltrate and further reduced the likelihood of leukemia cutis. Alternatively, patients can have aleukemic cutaneous myeloid sarcoma or leukemia cutis without an underlying hematologic condition or with latent peripheral blood or bone marrow myeloproliferative disorder, but our patient’s history of MDS eliminated this possibility.12 After exclusion of cutaneous infiltration by malignant leukocytes, our patient was diagnosed with histiocytoid neutrophilic dermatosis.


Multiple reports have described histiocytoid Sweet syndrome, in which there is a dense dermal histiocytoid infiltrate on histopathology that demonstrates myeloid lineage with immunologic staining.1,13 The typical pattern of histiocytoid Sweet syndrome includes a predominantly unaffected epidermis with papillary dermal edema, an absence of vasculitis, and a dense dermal infiltrate primarily composed of immature histiocytelike mononuclear cells with a basophilic elongated, twisted, or kidney-shaped nucleus and pale eosinophilic cytoplasm.1,13 In an analogous manner, Morin et al12 described a patient with congenital hypogammaglobulinemia who presented with lesions that clinically resembled pyoderma gangrenosum but revealed a dense dermal infiltrate mostly made of large immature histiocytoid mononuclear cells on histopathology, consistent with the histopathologic features observed in histiocytoid Sweet syndrome. The patient ultimately was diagnosed with histiocytoid pyoderma gangrenosum. Similarly, we believe that our patient also developed histiocytoid pyoderma gangrenosum. As with histiocytoid Sweet syndrome, this diagnosis is based on histopathologic and immunohistochemical findings of a dense dermal infiltrate composed of histiocyte-resembling immature neutrophils.

Typically, pyoderma gangrenosum responds promptly to treatment with systemic corticosteroids.4 Steroid-sparing agents such as cyclosporine, azathioprine, dapsone, and tumor necrosis factor α inhibitors also may be used.4,10 In the setting of MDS, clearance of pyoderma gangrenosum has been reported upon treatment of the underlying malignancy,14 high-dose systemic corticosteroids,11,15 cyclosporine with systemic steroids,16 thalidomide,17 combination therapy with thalidomide and interferon alfa-2a,18 and ustekinumab with vacuum-assisted closure therapy.19 Our patient’s histiocytoid pyoderma gangrenosum in the setting of solid and hematologic malignancy cleared rapidly with high-dose systemic hydrocortisone.
In the setting of malignancy, as in our patient, neutrophilic dermatoses may develop from an aberrant immune system or tumor-induced cytokine dysregulation that leads to increased neutrophil production or dysfunction.4,10,11 Although our patient’s MDS may have contributed to the atypical appearance of the dermal inflammatory infiltrate, it is unclear whether the hematologic disorder increased his risk for the histiocytoid variant of neutrophilic dermatoses. Alegría-Landa et al13 reported that histiocytoid Sweet syndrome is associated with hematologic malignancy at a similar frequency as classic Sweet syndrome. It is unknown if histiocytoid pyoderma gangrenosum would have a strong association with hematologic malignancy. Future reports may elucidate a better understanding of the histiocytoid subtype of pyoderma gangrenosum and its clinical implications.
To the Editor:
Neutrophilic dermatoses—a group of inflammatory cutaneous conditions—include acute febrile neutrophilic dermatosis (Sweet syndrome), pyoderma gangrenosum, and neutrophilic dermatosis of the dorsal hands. Histopathology shows a dense dermal infiltrate of mature neutrophils. In 2005, the histiocytoid subtype of Sweet syndrome was introduced with histopathologic findings of a dermal infiltrate composed of immature myeloid cells that resemble histiocytes in appearance but stain strongly with neutrophil markers on immunohistochemistry.1 We present a case of histiocytoid pyoderma gangrenosum with histopathology that showed a dense dermal histiocytoid infiltrate with strong positivity for neutrophil markers on immunohistochemistry.
An 85-year-old man was seen by dermatology in the inpatient setting for a new-onset painful abdominal wound. He had a medical history of myelodysplastic syndrome (MDS), high-grade invasive papillary urothelial carcinoma of the bladder, and a recent diagnosis of low-grade invasive ascending colon adenocarcinoma. Ten days prior he underwent a right colectomy without intraoperative complications that was followed by septic shock. Workup with urinalysis and urine culture showed minimal pyuria with Pseudomonas aeruginosa. Additional studies, including blood cultures, abdominal wound cultures, computed tomography of the abdomen and pelvis, renal ultrasound, and chest radiographs, were unremarkable and showed no signs of surgical site infection, intra-abdominal or pelvic abscess formation, or pulmonary embolism. Broad-spectrum antibiotics—vancomycin and piperacillin-tazobactam—were started. Persistent fever (Tmax of 102.3 °F [39.1 °C]) and leukocytosis (45.3×109/L [4.2–10×109/L]) despite antibiotic therapy, increasing pressor requirements, and progressive painful erythema and purulence at the abdominal surgical site led to debridement of the wound by the general surgery team on day 9 following the initial surgery due to suspected necrotizing infection. Within 24 hours, dermatology was consulted for continued rapid expansion of the wound. Physical examination of the abdomen revealed a large, well-demarcated, pink-red, indurated, ulcerated plaque with clear to purulent exudate and superficial erosions with violaceous undermined borders extending centrifugally from the abdominal surgical incision line (Figure 1A). Two punch biopsies sent for histopathologic evaluation and tissue culture showed dermal edema with a dense histiocytic infiltrate with nodular foci and admixed mature neutrophils to a lesser degree (Figure 2). Special staining was negative for bacteria, fungi, and mycobacteria. Immunohistochemistry revealed positive staining of the dermal inflammatory infiltrate with CD68, myeloperoxidase, and lysozyme, as well as negative staining with CD34 (Figure 3). These findings were suggestive of a histiocytoid neutrophilic dermatosis such as Sweet syndrome or pyoderma gangrenosum. Due to the morphology of the solitary lesion and the abrupt exacerbation shortly after surgical intervention, the patient was diagnosed with histiocytoid pyoderma gangrenosum. At the same time, the patient’s septic shock was treated with intravenous hydrocortisone (100 mg 3 times daily) for 2 days and also achieved a prompt response in the cutaneous symptoms (Figure 1B).
Sweet syndrome and pyoderma gangrenosum are considered distinct neutrophilic dermatoses that rarely coexist but share several clinical and histopathologic features, which can become a diagnostic challenge.2 Both conditions can manifest clinically as abrupt-onset, tender, erythematous papules; vesiculopustular lesions; or bullae with ulcerative changes. They also exhibit pathergy; present with systemic symptoms such as pyrexia, malaise, and joint pain; are associated with underlying systemic conditions such as infections and/or malignancy; demonstrate a dense neutrophilic infiltrate in the dermis on histopathology; and respond promptly to systemic corticosteroids.2-6 Bullous Sweet syndrome, which can present as vesicles, pustules, or bullae that progress to superficial ulcerations, may represent a variant of neutrophilic dermatosis characterized by features seen in both Sweet syndrome and pyoderma gangrenosum, suggesting that these 2 conditions may be on a spectrum.5Clinical features such as erythema with a blue, gray, or purple hue; undermined and ragged borders; and healing of skin lesions with atrophic or cribriform scarring may favor pyoderma gangrenosum, whereas a dull red or plum color and resolution of lesions without scarring may support the diagnosis of Sweet syndrome.7 Although both conditions can exhibit pathergy secondary to minor skin trauma such as venipuncture and biopsies,2,3,5,8 Sweet syndrome rarely has been described to develop after surgery in a patient without a known history of the condition.9 In contrast, postsurgical pyoderma gangrenosum has been well described as secondary to the pathergy phenomenon.5
Our patient was favored to have pyoderma gangrenosum given the solitary lesion, its abrupt development after surgery, and the morphology of the lesion that exhibited a large violaceous to red ulcerative and exudative plaque with undermined borders with atrophic scarring. In patients with skin disease that cannot be distinguished with certainty as either Sweet syndrome or pyoderma gangrenosum, it is essential to recognize that, as neutrophilic dermatoses, both conditions can be managed with either the first-line treatment option of high-dose systemic steroids or one of the shared alternative first-line or second-line steroid-sparing treatments, such as dapsone and cyclosporine.2
Although the exact pathogenesis of pyoderma gangrenosum remains to be fully understood, paraneoplastic pyoderma gangrenosum is a frequently described phenomenon.10,11 Our patient’s history of multiple malignancies, both solid and hematologic, supports the likelihood of malignancy-induced pyoderma gangrenosum; however, given his history of MDS, several other conditions were ruled out prior to making the diagnosis of pyoderma gangrenosum.
Classically, neutrophilic dermatoses such as pyoderma gangrenosum have a dense dermal neutrophilic infiltrate. Concurrent myeloproliferative disorders can alter the maturation of leukocytes, subsequently leading to an atypical appearance of the inflammatory cells on histopathology. Further, in the setting of myeloproliferative disorders, conditions such as leukemia cutis, in which there can be a cutaneous infiltrate of immature or mature myeloid or lymphocytic cells, must be considered. To ensure our patient’s abdominal skin changes were not a cutaneous manifestation of hematologic malignancy, immunohistochemical staining with CD20 and CD3 was performed and showed only the rare presence of B and T lymphocytes, respectively. Staining with CD34 for lymphocytic and myeloid progenitor cells was negative in the dermal infiltrate and further reduced the likelihood of leukemia cutis. Alternatively, patients can have aleukemic cutaneous myeloid sarcoma or leukemia cutis without an underlying hematologic condition or with latent peripheral blood or bone marrow myeloproliferative disorder, but our patient’s history of MDS eliminated this possibility.12 After exclusion of cutaneous infiltration by malignant leukocytes, our patient was diagnosed with histiocytoid neutrophilic dermatosis.


Multiple reports have described histiocytoid Sweet syndrome, in which there is a dense dermal histiocytoid infiltrate on histopathology that demonstrates myeloid lineage with immunologic staining.1,13 The typical pattern of histiocytoid Sweet syndrome includes a predominantly unaffected epidermis with papillary dermal edema, an absence of vasculitis, and a dense dermal infiltrate primarily composed of immature histiocytelike mononuclear cells with a basophilic elongated, twisted, or kidney-shaped nucleus and pale eosinophilic cytoplasm.1,13 In an analogous manner, Morin et al12 described a patient with congenital hypogammaglobulinemia who presented with lesions that clinically resembled pyoderma gangrenosum but revealed a dense dermal infiltrate mostly made of large immature histiocytoid mononuclear cells on histopathology, consistent with the histopathologic features observed in histiocytoid Sweet syndrome. The patient ultimately was diagnosed with histiocytoid pyoderma gangrenosum. Similarly, we believe that our patient also developed histiocytoid pyoderma gangrenosum. As with histiocytoid Sweet syndrome, this diagnosis is based on histopathologic and immunohistochemical findings of a dense dermal infiltrate composed of histiocyte-resembling immature neutrophils.

Typically, pyoderma gangrenosum responds promptly to treatment with systemic corticosteroids.4 Steroid-sparing agents such as cyclosporine, azathioprine, dapsone, and tumor necrosis factor α inhibitors also may be used.4,10 In the setting of MDS, clearance of pyoderma gangrenosum has been reported upon treatment of the underlying malignancy,14 high-dose systemic corticosteroids,11,15 cyclosporine with systemic steroids,16 thalidomide,17 combination therapy with thalidomide and interferon alfa-2a,18 and ustekinumab with vacuum-assisted closure therapy.19 Our patient’s histiocytoid pyoderma gangrenosum in the setting of solid and hematologic malignancy cleared rapidly with high-dose systemic hydrocortisone.
In the setting of malignancy, as in our patient, neutrophilic dermatoses may develop from an aberrant immune system or tumor-induced cytokine dysregulation that leads to increased neutrophil production or dysfunction.4,10,11 Although our patient’s MDS may have contributed to the atypical appearance of the dermal inflammatory infiltrate, it is unclear whether the hematologic disorder increased his risk for the histiocytoid variant of neutrophilic dermatoses. Alegría-Landa et al13 reported that histiocytoid Sweet syndrome is associated with hematologic malignancy at a similar frequency as classic Sweet syndrome. It is unknown if histiocytoid pyoderma gangrenosum would have a strong association with hematologic malignancy. Future reports may elucidate a better understanding of the histiocytoid subtype of pyoderma gangrenosum and its clinical implications.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10:301-312.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Wallach D, Vignon-Pennamen MD. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Lear JT, Atherton MT, Byrne JP. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet’s syndrome. Postgrad Med. 1997;73:65-68.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:E74-E76.
- Shah M, Sachdeva M, Gefri A, et al. Paraneoplastic pyoderma gangrenosum in solid organ malignancy: a literature review. Int J Dermatol. 2020;59:154-158.
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359.
- Morin CB, Côté B, Belisle A. An interesting case of pyoderma gangrenosum with immature histiocytoid neutrophils. J Cutan Pathol. 2018;45:63-66.
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Saleh MFM, Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep. 2017;5:2025-2027.
- Yamauchi R, Ishida K, Iwashima Y, et al. Successful treatment of pyoderma gangrenosum that developed in a patient with myelodysplastic syndrome. J Infect Chemother. 2003;9:268-271.
- Ha JW, Hahm JE, Kim KS, et al. A case of pyoderma gangrenosum with myelodysplastic syndrome. Ann Dermatol. 2018;30:392-393.
- Malkan UY, Gunes G, Eliacik E, et al. Treatment of pyoderma gangrenosum with thalidomide in a myelodysplastic syndrome case. Int J Med Case Rep. 2016;9:61-64.
- Koca E, Duman AE, Cetiner D, et al. Successful treatment of myelodysplastic syndrome-induced pyoderma gangrenosum. Neth J Med. 2006;64:422-424.
- Nieto D, Sendagorta E, Rueda JM, et al. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: case report and literature review. Clin Exp Dermatol. 2019;44:116-119.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10:301-312.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Wallach D, Vignon-Pennamen MD. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Lear JT, Atherton MT, Byrne JP. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet’s syndrome. Postgrad Med. 1997;73:65-68.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:E74-E76.
- Shah M, Sachdeva M, Gefri A, et al. Paraneoplastic pyoderma gangrenosum in solid organ malignancy: a literature review. Int J Dermatol. 2020;59:154-158.
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359.
- Morin CB, Côté B, Belisle A. An interesting case of pyoderma gangrenosum with immature histiocytoid neutrophils. J Cutan Pathol. 2018;45:63-66.
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Saleh MFM, Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep. 2017;5:2025-2027.
- Yamauchi R, Ishida K, Iwashima Y, et al. Successful treatment of pyoderma gangrenosum that developed in a patient with myelodysplastic syndrome. J Infect Chemother. 2003;9:268-271.
- Ha JW, Hahm JE, Kim KS, et al. A case of pyoderma gangrenosum with myelodysplastic syndrome. Ann Dermatol. 2018;30:392-393.
- Malkan UY, Gunes G, Eliacik E, et al. Treatment of pyoderma gangrenosum with thalidomide in a myelodysplastic syndrome case. Int J Med Case Rep. 2016;9:61-64.
- Koca E, Duman AE, Cetiner D, et al. Successful treatment of myelodysplastic syndrome-induced pyoderma gangrenosum. Neth J Med. 2006;64:422-424.
- Nieto D, Sendagorta E, Rueda JM, et al. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: case report and literature review. Clin Exp Dermatol. 2019;44:116-119.
Practice Points:
- Dermatologists and dermatopathologists should be aware of the histiocytoid variant of pyoderma gangrenosum, which can clinical and histologic features that overlap with histiocytoid Sweet syndrome.
- When considering a diagnosis of histiocytoid neutrophilic dermatoses, leukemia cutis or aleukemic cutaneous myeloid sarcoma should be ruled out.
- Similar to histiocytoid Sweet syndrome and neutrophilic dermatoses in the setting of hematologic or solid organ malignancy, histiocytoid pyoderma gangrenosum may respond well to high-dose systemic corticosteroids.
Extensive Multidrug-Resistant Dermatophytosis From Trichophyton indotineae
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
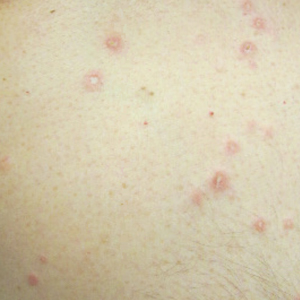
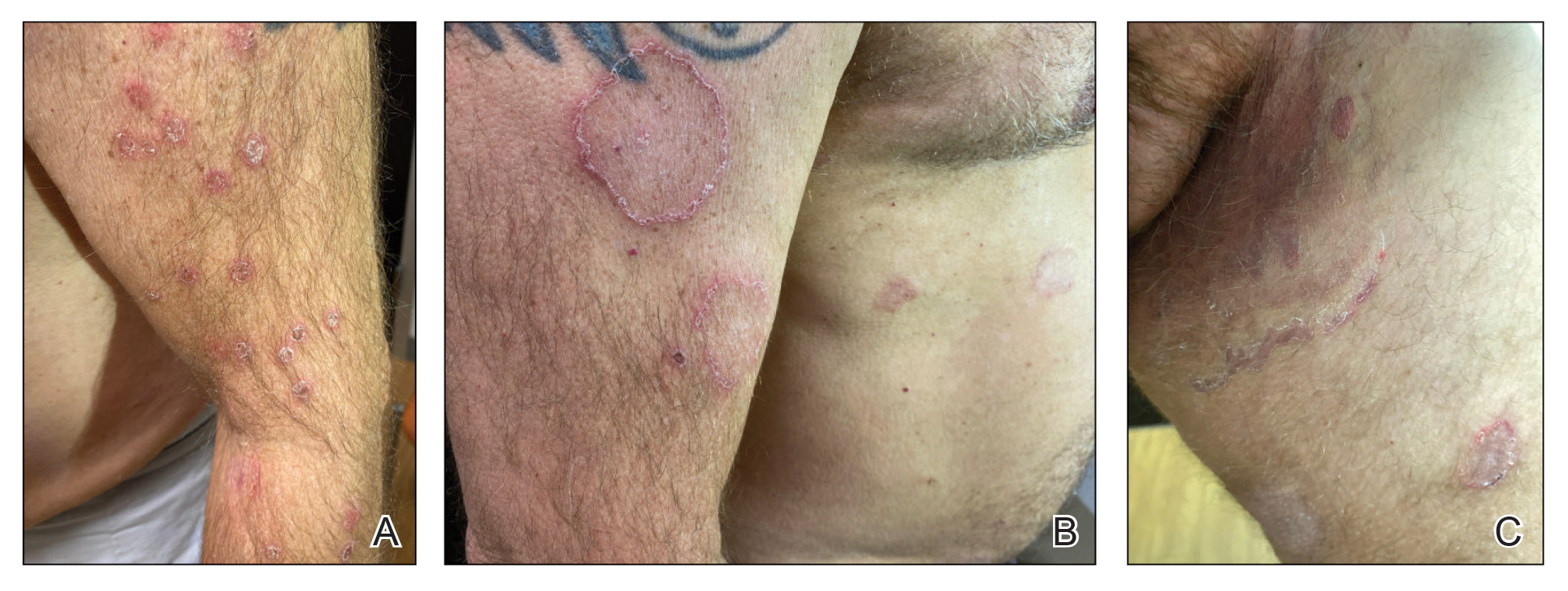
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
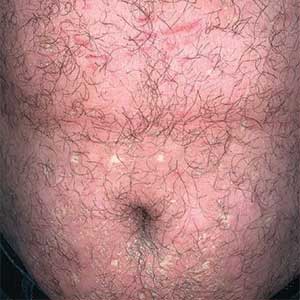
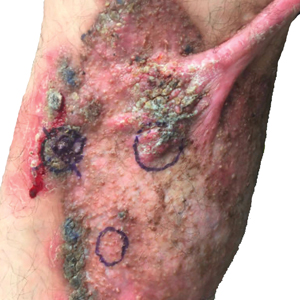
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.
The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.
Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8 Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.

The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.


Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8 Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.

The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.


Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8 Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
Practice Points
- Trichophyton indotineae can cause extensive dermatophytosis that often is resistant to terbinafine and in some cases to other antifungals.
- Only molecular testing, which is not widely available, can distinguish T indotineae from other dermatophytes.
- Suspected or confirmed cases of T indotineae dermatophytosis should be reported to public health agencies to provide assistance with testing, as well as surveillance, prevention, and control of infection.
Treatment of Infantile Hemangiomas in Concomitant Tuberous Sclerosis Complex Should Prompt Evaluation for Cardiac Rhabdomyomas Prior to Initiation of Propranolol
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.
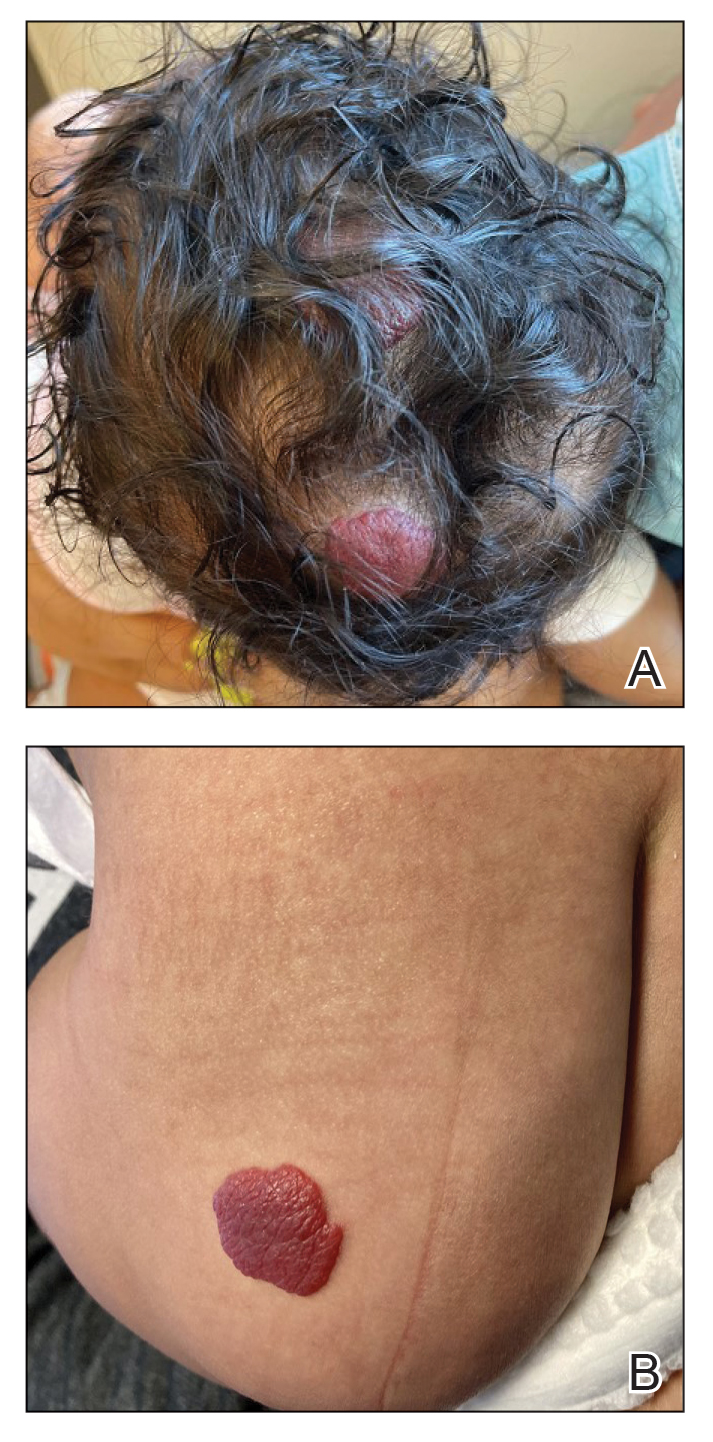
Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.
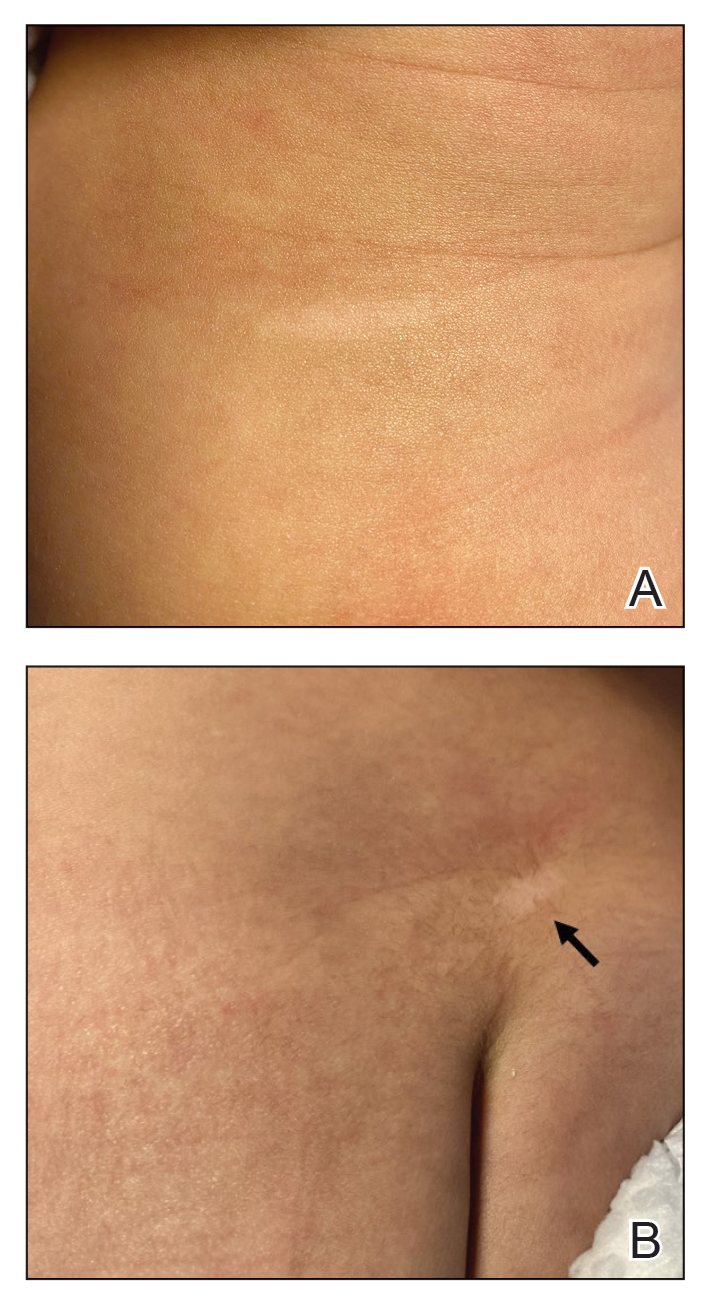
Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
Practice Points
- Dermatologists may see patients with infantile hemangiomas (IHs) and tuberous sclerosis complex (TSC); therefore, they should be familiar with TSC diagnostic criteria to reach a prompt diagnosis and make appropriate referrals.
- Cardiologic evaluation is not routinely required prior to systemic treatment of IH, but knowledge of cardiac findings in TSC should prompt cardiologic clearance prior to β-blocker initiation.
Hypopigmented Cutaneous Langerhans Cell Histiocytosis in a Hispanic Infant
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.
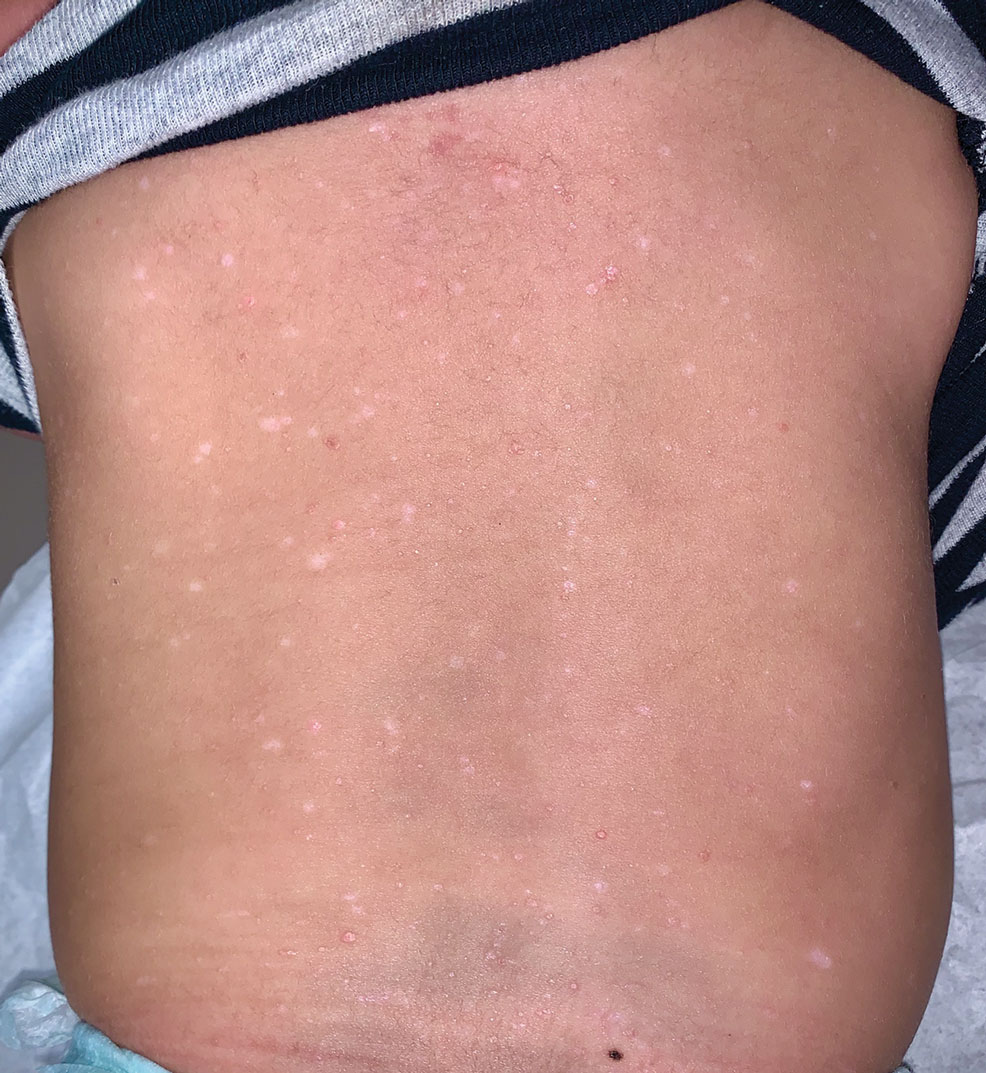
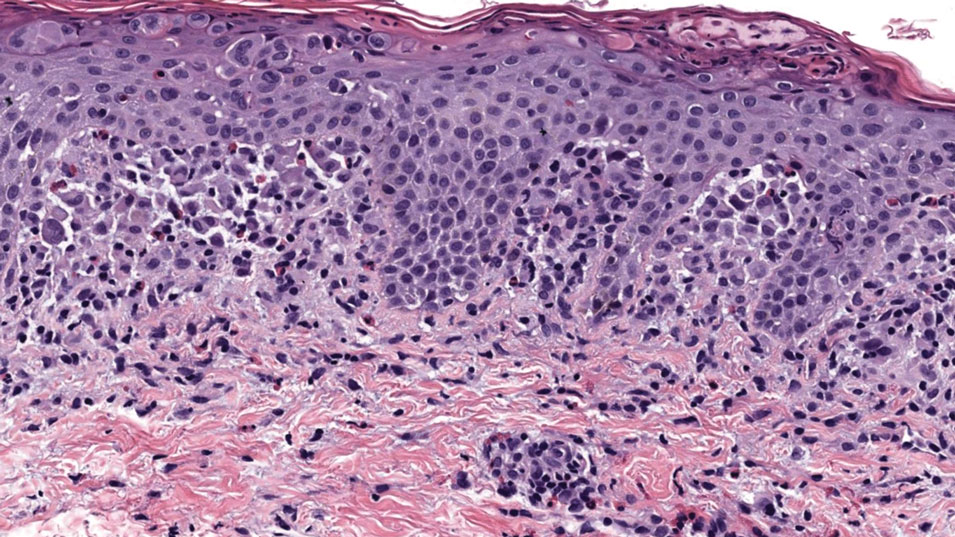
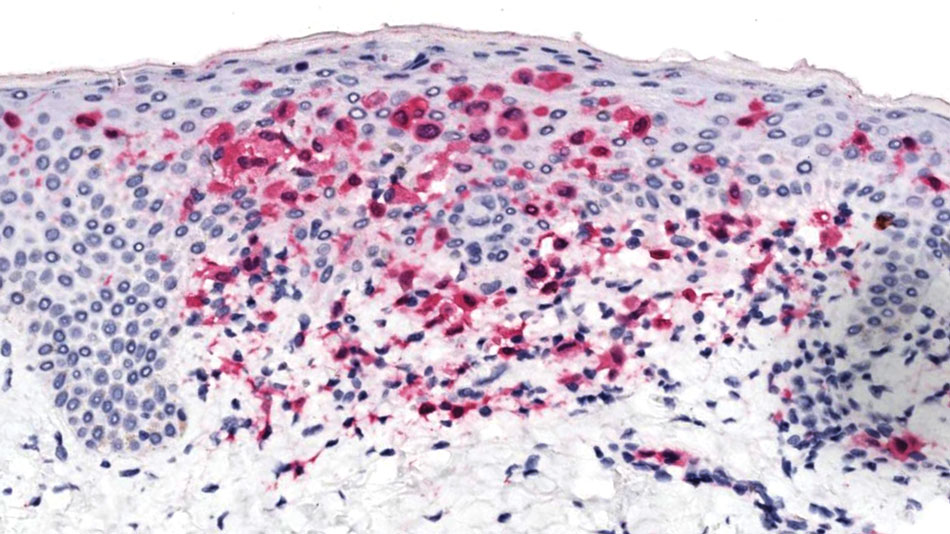
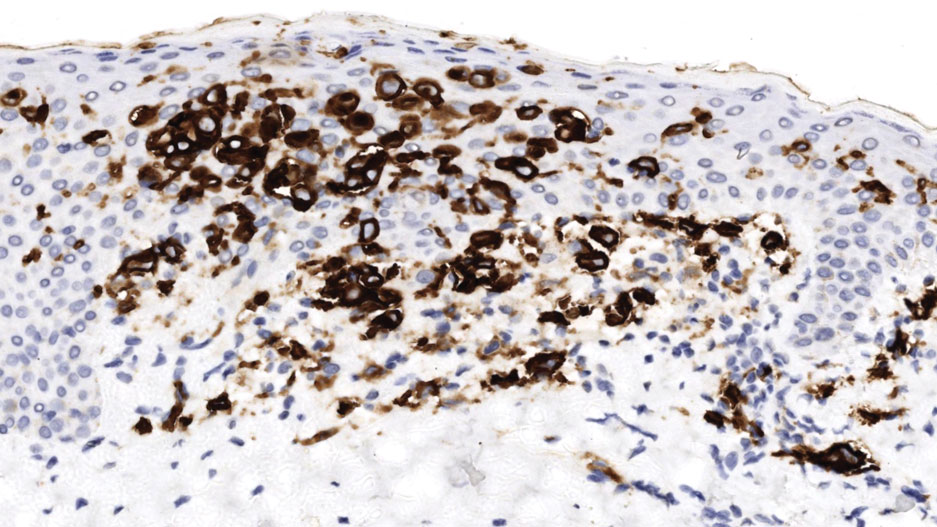
The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
To the Editor:
Langerhans cell histiocytosis (LCH) is a rare inflammatory neoplasia caused by accumulation of clonal Langerhans cells in 1 or more organs. The clinical spectrum is diverse, ranging from mild, single-organ involvement that may resolve spontaneously to severe progressive multisystem disease that can be fatal. It is most prevalent in children, affecting an estimated 4 to 5 children for every 1 million annually, with male predominance.1 The pathogenesis is driven by activating mutations in the mitogen-activated protein kinase pathway, with the BRAF V600E mutation detected in most LCH patients, resulting in proliferation of pathologic Langerhans cells and dysregulated expression of inflammatory cytokines in LCH lesions.2 A biopsy of lesional tissue is required for definitive diagnosis. Histopathology reveals a mixed inflammatory infiltrate and characteristic mononuclear cells with reniform nuclei that are positive for CD1a and CD207 proteins on immunohistochemical staining.3
Langerhans cell histiocytosis is categorized by the extent of organ involvement. It commonly affects the bones, skin, pituitary gland, liver, lungs, bone marrow, and lymph nodes.4 Single-system LCH involves a single organ with unifocal or multifocal lesions; multisystem LCH involves 2 or more organs and has a worse prognosis if risk organs (eg, liver, spleen, bone marrow) are involved.4
Skin lesions are reported in more than half of LCH cases and are the most common initial manifestation in patients younger than 2 years.4 Cutaneous findings are highly variable, which poses a diagnostic challenge. Common morphologies include erythematous papules, pustules, papulovesicles, scaly plaques, erosions, and petechiae. Lesions can be solitary or widespread and favor the trunk, head, and face.4 We describe an atypical case of hypopigmented cutaneous LCH and review the literature on this morphology in patients with skin of color.
A 7-month-old Hispanic male infant who was otherwise healthy presented with numerous hypopigmented macules and pink papules on the trunk and groin that had progressed since birth. A review of systems was unremarkable. Physical examination revealed 1- to 3-mm, discrete, hypopigmented macules intermixed with 1- to 2-mm pearly pink papules scattered on the back, chest, abdomen, and inguinal folds (Figure 1). Some lesions appeared koebnerized; however, the parents denied a history of scratching or trauma.
Histopathology of a lesion in the inguinal fold showed aggregates of mononuclear cells with reniform nuclei and abundant amphophilic cytoplasm in the papillary dermis, with focal extension into the epidermis. Scattered eosinophils and multinucleated giant cells were present in the dermal inflammatory infiltrate (Figure 2). Immunohistochemical staining was positive for CD1a (Figure 3) and S-100 protein (Figure 4). Although epidermal Langerhans cell collections also can be seen in allergic contact dermatitis,5 predominant involvement of the papillary dermis and the presence of multinucleated giant cells are characteristic of LCH.4 Given these findings, which were consistent with LCH, the dermatopathology deemed BRAF V600E immunostaining unnecessary for diagnostic purposes.





The patient was referred to the hematology and oncology department to undergo thorough evaluation for extracutaneous involvement. The workup included a complete blood cell count, liver function testing, electrolyte assessment, skeletal survey, chest radiography, and ultrasonography of the liver and spleen. All results were negative, suggesting a diagnosis of single-system cutaneous LCH.
Three months later, the patient presented to dermatology with spontaneous regression of all skin lesions. Continued follow-up—every 6 months for 5 years—was recommended to monitor for disease recurrence or progression to multisystem disease.
Cutaneous LCH is a clinically heterogeneous disease with the potential for multisystem involvement and long-term sequelae; therefore, timely diagnosis is paramount to optimize outcomes. However, delayed diagnosis is common because of the spectrum of skin findings that can mimic common pediatric dermatoses, such as seborrheic dermatitis, atopic dermatitis, and diaper dermatitis.4 In one study, the median time from onset of skin lesions to diagnostic biopsy was longer than 3 months (maximum, 5 years).6 Our patient was referred to dermatology 7 months after onset of hypopigmented macules, a rarely reported cutaneous manifestation of LCH.
A PubMed search of articles indexed for MEDLINE from 1994 to 2019 using the terms Langerhans cell histiocytotis and hypopigmented yielded 17 cases of LCH presenting as hypopigmented skin lesions (Table).7-22 All cases occurred in patients with skin of color (ie, patients of Asian, Hispanic, or African descent). Hypopigmented macules were the only cutaneous manifestation in 10 (59%) cases. Lesions most commonly were distributed on the trunk (16/17 [94%]) and extremities (8/17 [47%]). The median age of onset was 1 month; 76% (13/17) of patients developed skin lesions before 1 year of age, indicating that this morphology may be more common in newborns. In most patients, the diagnosis was single-system cutaneous LCH; they exhibited spontaneous regression by 8 months of age on average, suggesting that this variant may be associated with a better prognosis. Mori and colleagues21 hypothesized that hypopigmented lesions may represent the resolving stage of active LCH based on histopathologic findings of dermal pallor and fibrosis in a hypopigmented LCH lesion. However, systemic involvement was reported in 7 cases of hypopigmented LCH, highlighting the importance of assessing for multisystem disease regardless of cutaneous morphology.21Langerhans cell histiocytosis should be considered in the differential diagnosis when evaluating hypopigmented skin eruptions in infants with darker skin types. Prompt diagnosis of this atypical variant requires a higher index of suspicion because of its rarity and the polymorphic nature of cutaneous LCH. This morphology may go undiagnosed in the setting of mild or spontaneously resolving disease; notwithstanding, accurate diagnosis and longitudinal surveillance are necessary given the potential for progressive systemic involvement.
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
1. Guyot-Goubin A, Donadieu J, Barkaoui M, et al. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000–2004. Pediatr Blood Cancer. 2008;51:71-75. doi:10.1002/pbc.21498
2. Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
3. Haupt R, Minkov M, Astigarraga I, et al; Euro Histio Network. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work‐up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
4. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. 2018;78:1035-1044. doi:10.1016/j.jaad.2017.05.059
5. Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504. doi:10.1111/cup.12707
6. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996. doi:10.1016/j.jpeds.2014.07.063
7. Longaker MA, Frieden IJ, LeBoit PE, et al. Congenital “self-healing” Langerhans cell histiocytosis: the need for long-term follow-up. J Am Acad Dermatol. 1994;31(5, pt 2):910-916. doi:10.1016/s0190-9622(94)70258-6
8. Feroze K, Unni M, Jayasree MG, et al. Langerhans cell histiocytosis presenting with hypopigmented macules. Indian J Dermatol Venereol Leprol. 2008;74:670-672. doi:10.4103/0378-6323.45128
9. Satter EK, High WA. Langerhans cell histiocytosis: a case report and summary of the current recommendations of the Histiocyte Society. Dermatol Online J. 2008;14:3.
10. Chang SL, Shih IH, Kuo TT, et al. Congenital self-healing reticulohistiocytosis presenting as hypopigmented macules and papules in a neonate. Dermatologica Sinica 2008;26:80-84.
11. Aggarwal V, Seth A, Jain M, et al. Congenital Langerhans cell histiocytosis with skin and lung involvement: spontaneous regression. Indian J Pediatr. 2010;77:811-812.
12. Battistella M, Fraitag S, Teillac DH, et al. Neonatal and early infantile cutaneous Langerhans cell histiocytosis: comparison of self-regressive and non-self-regressive forms. Arch Dermatol. 2010;146:149-156. doi:10.1001/archdermatol.2009.360
13. Kaddu S, Mulyowa G, Kovarik C. Hypopigmented scaly, scalp and facial lesions and disfiguring exopthalmus. Clin Exp Dermatol. 2010;3:E52-E53. doi:10.1111/j.1365-2230.2009.03336.x
14. Mehta B, Amladi S. Langerhans cell histiocytosis presenting as hypopigmented papules. Pediatr Dermatol. 2010;27:215-217. doi:10.1111/j.1525-1470.2010.01104.x
15. Shetty S, Monappa V, Pai K, et al. Congenital self-healing reticulohistiocytosis: a case report. Our Dermatol Online. 2014;5:264-266.
16. Uaratanawong R, Kootiratrakarn T, Sudtikoonaseth P, et al. Congenital self-healing reticulohistiocytosis presented with multiple hypopigmented flat-topped papules: a case report and review of literatures. J Med Assoc Thai. 2014;97:993-997.
17. Tan Q, Gan LQ, Wang H. Congenital self-healing Langerhans cell histiocytosis in a male neonate. Indian J Dermatol Venereol Leprol. 2015;81:75-77. doi:10.4103/0378-6323.148587
18. Lozano Masdemont B, Gómez‐Recuero Muñoz L, Villanueva Álvarez‐Santullano A, et al. Langerhans cell histiocytosis mimicking lichen nitidus with bone involvement. Australas J Dermatol. 2017;58:231-233. doi:10.1111/ajd.12467
19. Parimi LR, You J, Hong L, et al. Congenital self-healing reticulohistiocytosis with spontaneous regression. An Bras Dermatol. 2017;92:553-555. doi:10.1590/abd1806-4841.20175432
20. Bishnoi A, De D, Khullar G, et al. Hypopigmented and acneiform lesions: an unusual initial presentation of adult-onset multisystem Langerhans cell histiocytosis. Indian J Dermatol Venereol Leprol. 2018;84:621-626. doi:10.4103/ijdvl.IJDVL_639_17
21. Mori S, Adar T, Kazlouskaya V, et al. Cutaneous Langerhans cell histiocytosis presenting with hypopigmented lesions: report of two cases and review of literature. Pediatr Dermatol. 2018;35:502-506. doi:10.1111/pde.13509
22. Wu X, Huang J, Jiang L, et al. Congenital self‐healing reticulohistiocytosis with BRAF V600E mutation in an infant. Clin Exp Dermatol. 2019;44:647-650. doi:10.1111/ced.13880
Practice Points
- Dermatologists should be aware of the hypopigmented variant of cutaneous Langerhans cell histiocytosis (LCH), which has been reported exclusively in patients with skin of color.
- Langerhans cell histiocytosis should be included in the differential diagnosis of hypopigmented macules, which may be the only cutaneous manifestation or may coincide with typical lesions of LCH.
- Hypopigmented cutaneous LCH may be more common in newborns and associated with a better prognosis.
Reactive Granulomatous Dermatitis: Variability of the Predominant Inflammatory Cell Type
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.
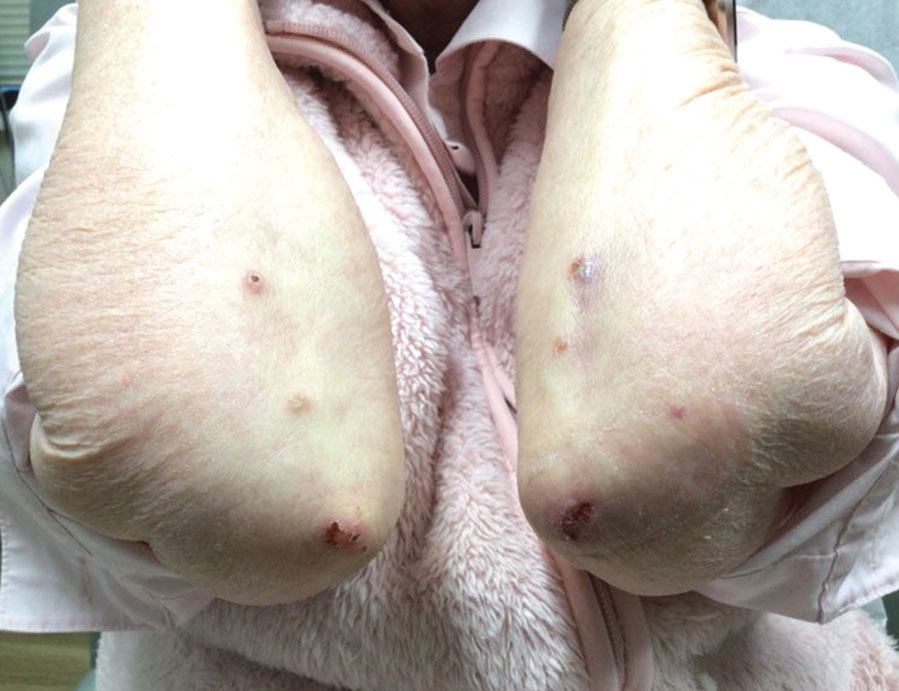
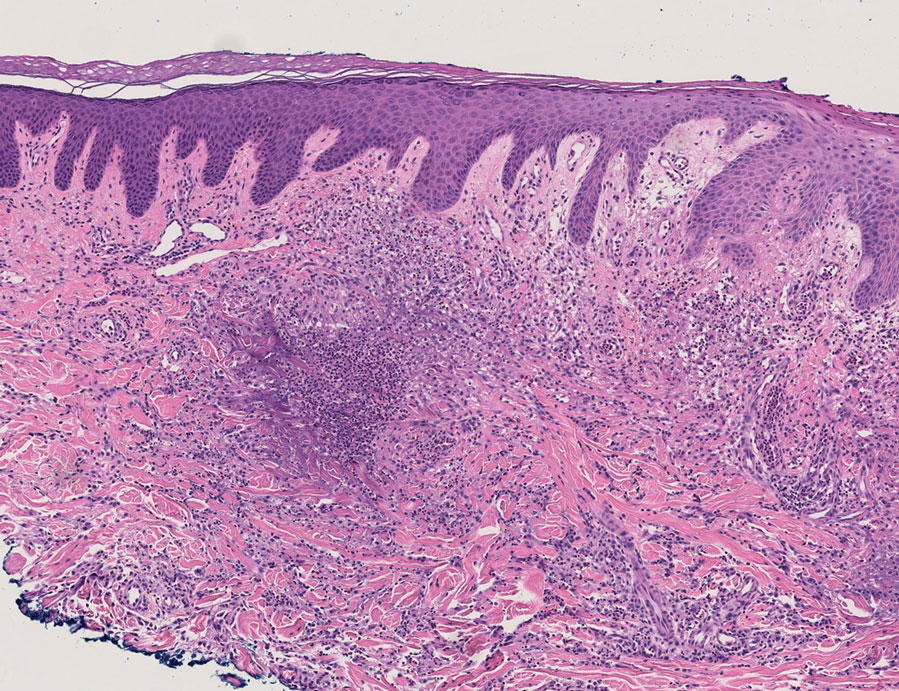
The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.
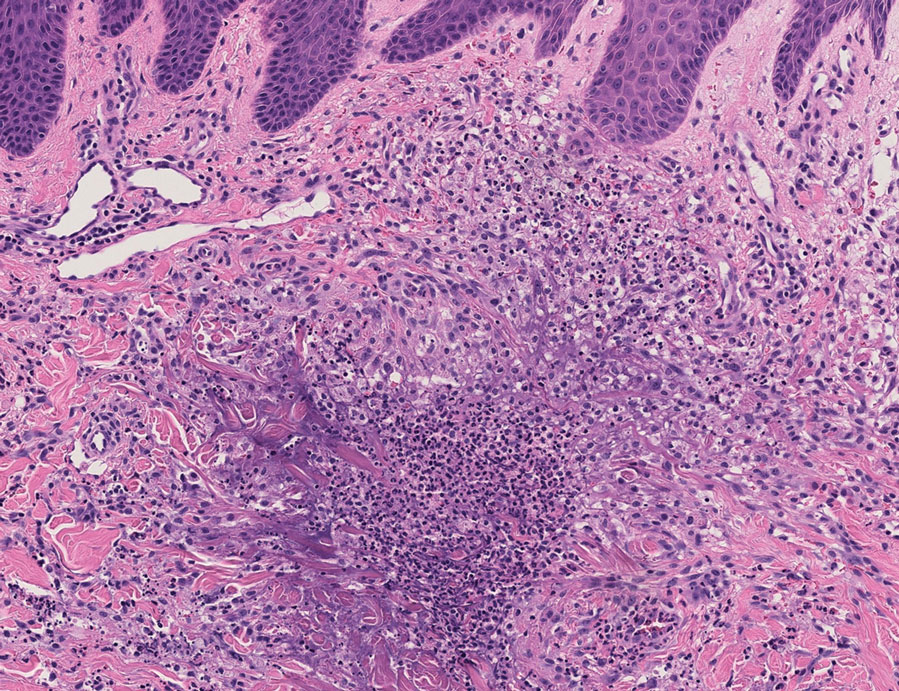
In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.
The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
To the Editor:
The term palisaded neutrophilic and granulomatous dermatitis (PNGD) has been proposed to encompass various conditions, including Winkelmann granuloma and superficial ulcerating rheumatoid necrobiosis. More recently, PNGD has been classified along with interstitial granulomatous dermatitis and interstitial granulomatous drug reaction under a unifying rubric of reactive granulomatous dermatitis (RGD).1-4 The diagnosis of RGD can be challenging because of a range of clinical and histopathologic features as well as variable nomenclature.1-3,5
Palisaded neutrophilic and granulomatous dermatitis classically manifests with papules and small plaques on the extensor extremities, with histopathology showing characteristic necrobiosis with both neutrophils and histiocytes.1,2,6 We report 6 cases of RGD, including an index case in which a predominance of neutrophils in the infiltrate impeded the diagnosis.
An 85-year-old woman (the index patient) presented with a several-week history of asymmetric crusted papules on the right upper extremity—3 lesions on the elbow and forearm and 1 lesion on a finger. She was an avid gardener with severe rheumatoid arthritis treated with Janus kinase (JAK) inhibitor therapy. An initial biopsy of the elbow revealed a dense infiltrate of neutrophils and sparse eosinophils within the dermis. Special stains for bacterial, fungal, and acid-fast organisms were negative.
Because infection with sporotrichoid spread remained high in the differential diagnosis, the JAK inhibitor was discontinued and an antifungal agent was initiated. Given the persistence of the lesions, a subsequent biopsy of the right finger revealed scarce neutrophils and predominant histiocytes with rare foci of degenerated collagen. Sporotrichosis remained the leading diagnosis for these unilateral lesions. The patient subsequently developed additional crusted papules on the left arm (Figure 1). A biopsy of a left elbow lesion revealed palisades of histiocytes around degenerated collagen and collections of neutrophils compatible with RGD (Figures 2 and 3). Incidentally, the patient also presented with bilateral lower extremity palpable purpura, with a biopsy showing leukocytoclastic vasculitis. Antifungal therapy was discontinued and JAK inhibitor therapy resumed, with partial resolution of both the arm and right finger lesions and complete resolution of the lower extremity palpable purpura over several months.


The dense neutrophilic infiltrate and asymmetric presentation seen in our index patient’s initial biopsy hindered categorization of the cutaneous findings as RGD in association with her rheumatoid arthritis rather than as an infectious process. To ascertain whether diagnosis also was difficult in other cases of RGD, we conducted a search of the Yale Dermatopathology database for the diagnosis palisaded neutrophilic and granulomatous dermatitis, a term consistently used at our institution over the past decade. This study was approved by the institutional review board of Yale University (New Haven, Connecticut), and informed consent was waived. The search covered a 10-year period; 13 patients were found. Eight patients were eliminated because further clinical information or follow-up could not be obtained, leaving 5 additional cases (Table). The 8 eliminated cases were consultations submitted to the laboratory by outside pathologists from other institutions.

In one case (patient 5), the diagnosis of RGD was delayed for 7 years from first documentation of an RGD-compatible neutrophil-predominant infiltrate (Table). In 3 other cases, PNGD was in the clinical differential diagnosis. In patient 6 with known eosinophilic granulomatosis with polyangiitis, biopsy findings included a mixed inflammatory infiltrate with eosinophils, and the clinical and histopathologic findings were deemed compatible with RGD by group consensus at Grand Rounds.
In practice, a consistent unifying nomenclature has not been achieved for RGD and the diseases it encompasses—PNGD, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction. In this small series, a diagnosis of PNGD was given in the dermatopathology report only when biopsy specimens were characterized by histiocytes, neutrophils, and necrobiosis. Histopathology reports for neutrophil-predominant, histiocyte-predominant, and eosinophil-predominant cases did not mention PNGD or RGD, though potential association with systemic disease generally was noted.
Given the variability in the predominant inflammatory cell type in these patients, adding a qualifier to the histopathologic diagnosis—“RGD, eosinophil rich,” “RGD, histiocyte rich,” or “RGD, neutrophil rich”1—would underscore the range of inflammatory cells in this entity. Employing this terminology rather than stating a solely descriptive diagnosis such as neutrophilic infiltrate, which may bias clinicians toward an infectious process, would aid in the association of a given rash with systemic disease and may prevent unnecessary tissue sampling. Indeed, 3 patients in this small series underwent more than 2 biopsies; multiple procedures might have been avoided had there been better communication about the spectrum of inflammatory cells compatible with RGD.

The inflammatory infiltrate in biopsy specimens of RGD can be solely neutrophil or histiocyte predominant or even have prominent eosinophils depending on the stage of disease. Awareness of variability in the predominant inflammatory cell in RGD may facilitate an accurate diagnosis as well as an association with any underlying autoimmune process, thereby allowing better management and treatment.1
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
- Rosenbach M, English JC. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387. doi:10.1016/j.det.2015.03.005
- Wanat KA, Caplan A, Messenger E, et al. Reactive granulomatous dermatitis: a useful and encompassing term. JAAD Intl. 2022;7:126-128. doi:10.1016/j.jdin.2022.03.004
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283. doi:10.1001/archderm.1994.01690100062010
- Dykman CJ, Galens GJ, Good AE. Linear subcutaneous bands in rheumatoid arthritis: an unusual form of rheumatoid granuloma. Ann Intern Med. 1965;63:134-140. doi:10.7326/0003-4819-63-1-134
- Rodríguez-Garijo N, Bielsa I, Mascaró JM Jr, et al. Reactive granulomatous dermatitis as a histological pattern including manifestations of interstitial granulomatous dermatitis and palisaded neutrophilic and granulomtous dermatitis: a study of 52 patients. J Eur Acad Dermatol Venereol. 2021;35:988-994. doi:10.1111/jdv.17010
- Kalen JE, Shokeen D, Ramos-Caro F, et al. Palisaded neutrophilic granulomatous dermatitis: spectrum of histologic findings in a single patient. JAAD Case Rep. 2017;3:425. doi:10.1016/j.jdcr.2017.06.010
Practice Points
- The term reactive granulomatous dermatitis (RGD) provides a unifying rubric for palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, and interstitial granulomatous drug reaction.
- Reactive granulomatous dermatitis can have a variable infiltrate that includes neutrophils, histiocytes, and/or eosinophils.
- Awareness of the variability in inflammatory cell type is important for the diagnosis of RGD.
Growing Periumbilical Plaque: A Case of Perforating Calcific Elastosis
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.
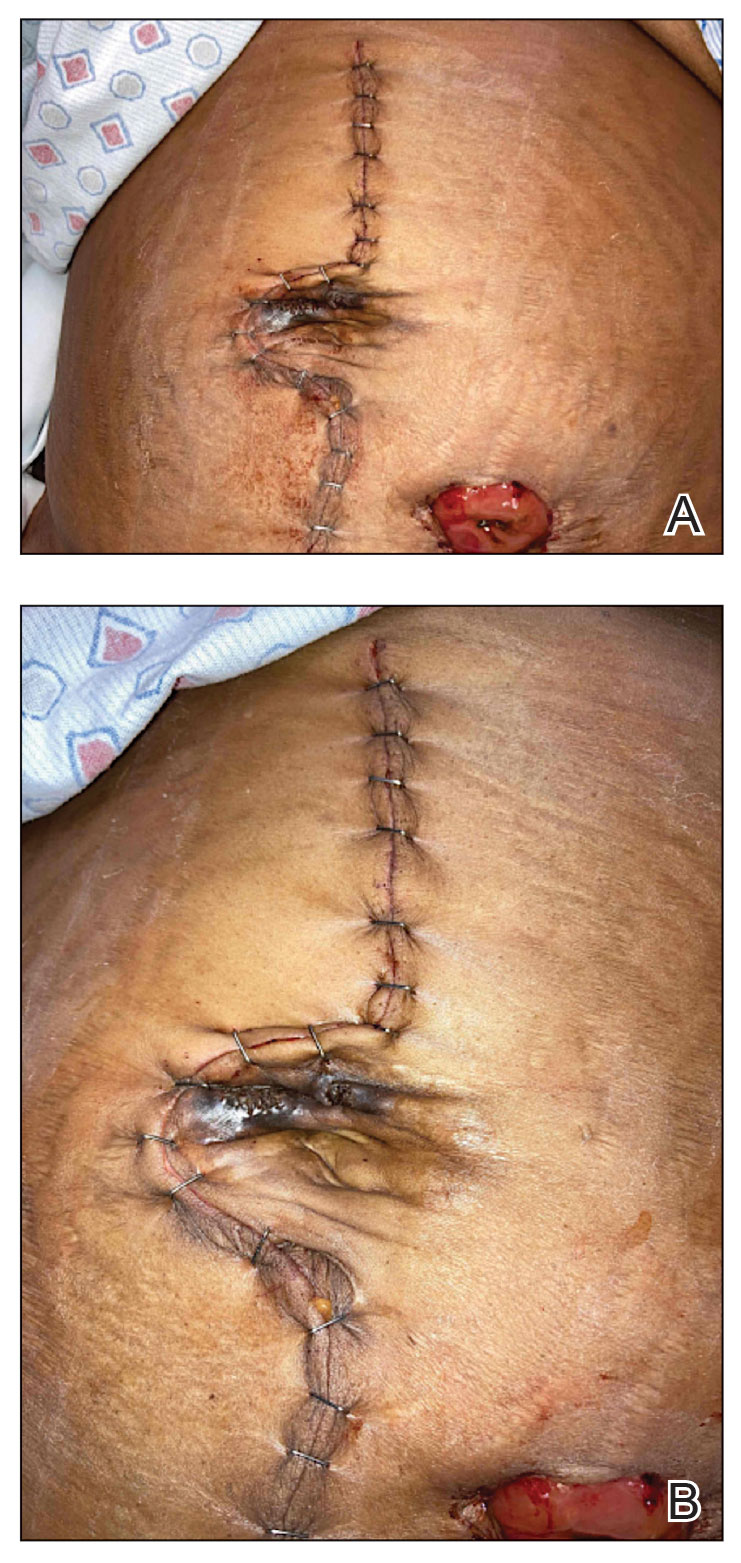
A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.
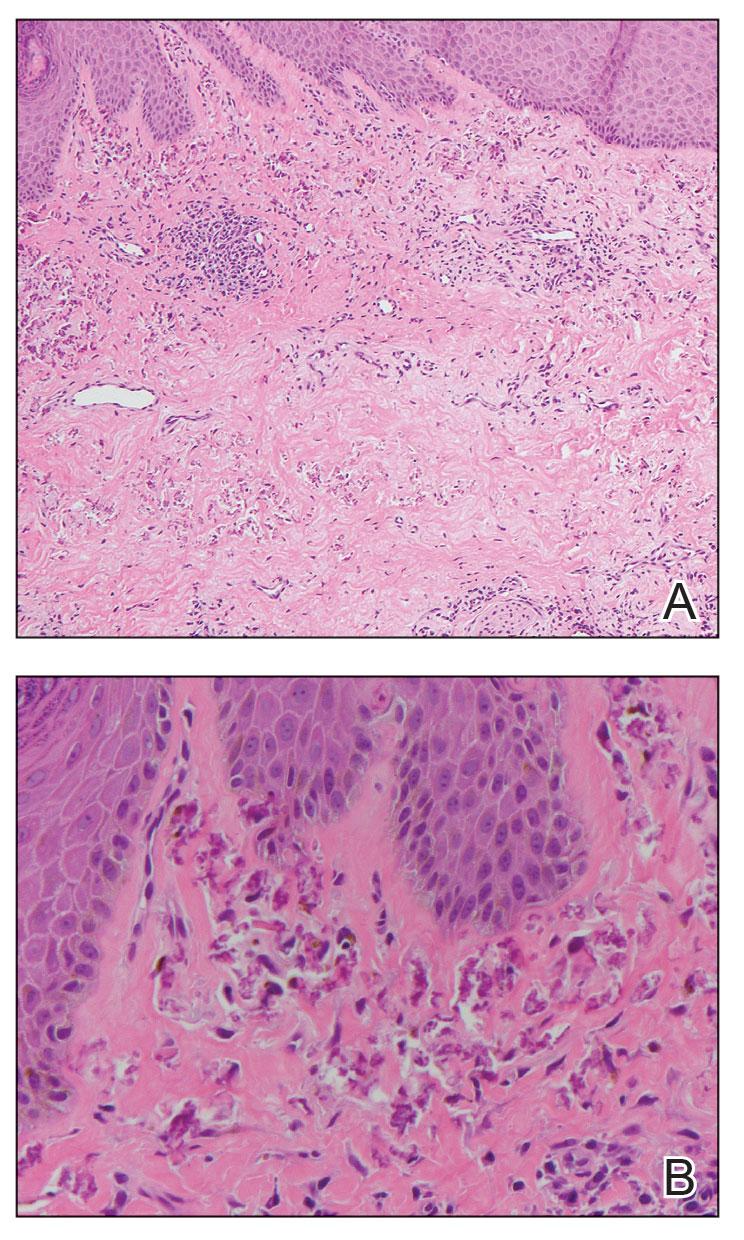
Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6
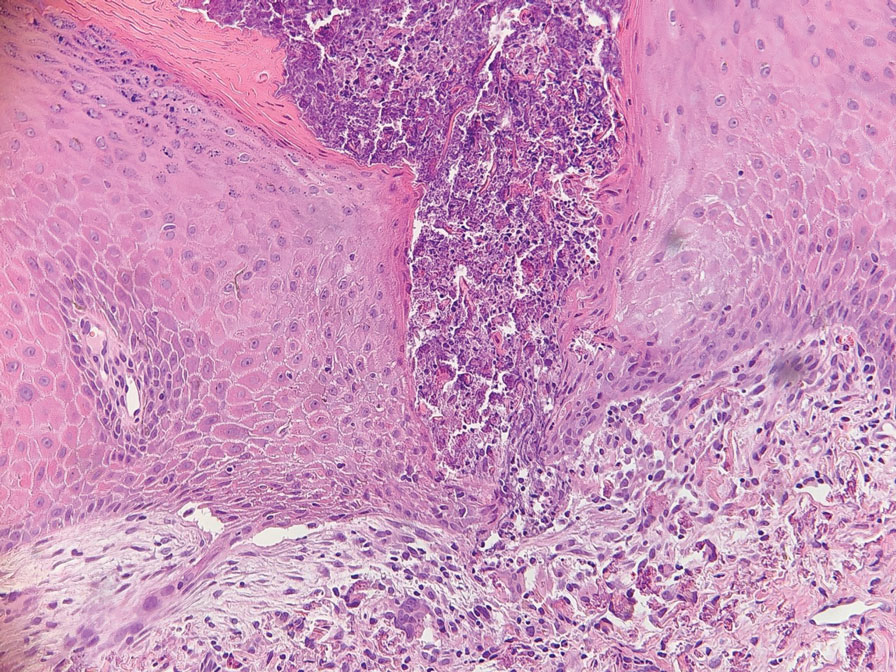
The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
PRACTICE POINTS
- Perforating calcific elastosis (PCE) is a rare, localized, acquired variant of the inherited connective tissue disorder pseudoxanthoma elasticum (PXE).
- Histopathologic findings are identical for PCE and PXE, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history.
- Although there are no definitive treatments, most cases of PCE resolve spontaneously.
- Dermatologists should be aware of the importance of clinically differentiating PCE from PXE to prevent extensive workup, which can lead to unnecessary testing and increased morbidity in patients.
Early Treatment of Lyme Disease Prompted by Histopathologic Analysis of the Abdomen of an Engorged Tick
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.
Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
PRACTICE POINTS
- Lyme disease is increasingly common in the United States.
- Lyme disease can cause debilitating sequelae if left untreated, including arthritis, neurologic deficits, and heart block.
- Diagnostic methods for identifying early Lyme disease have limited sensitivity and specificity, necessitating alternative strategies for making an accurate diagnosis and initiating treatment.
Clinical Manifestation of Degos Disease: Painful Penile Ulcers
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.
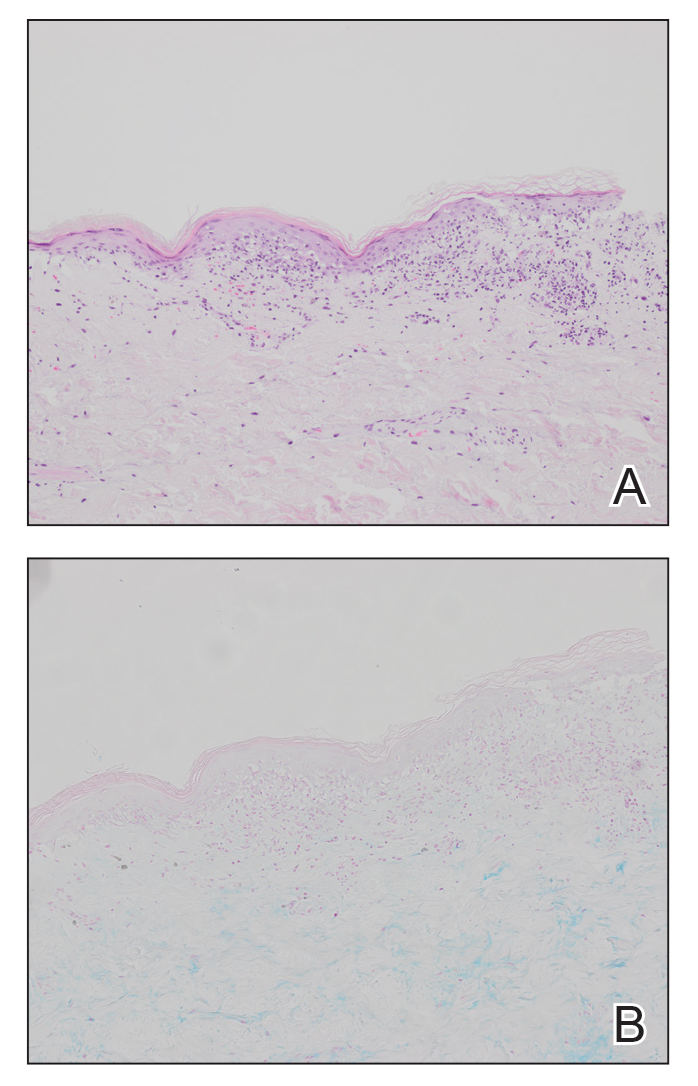
At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).
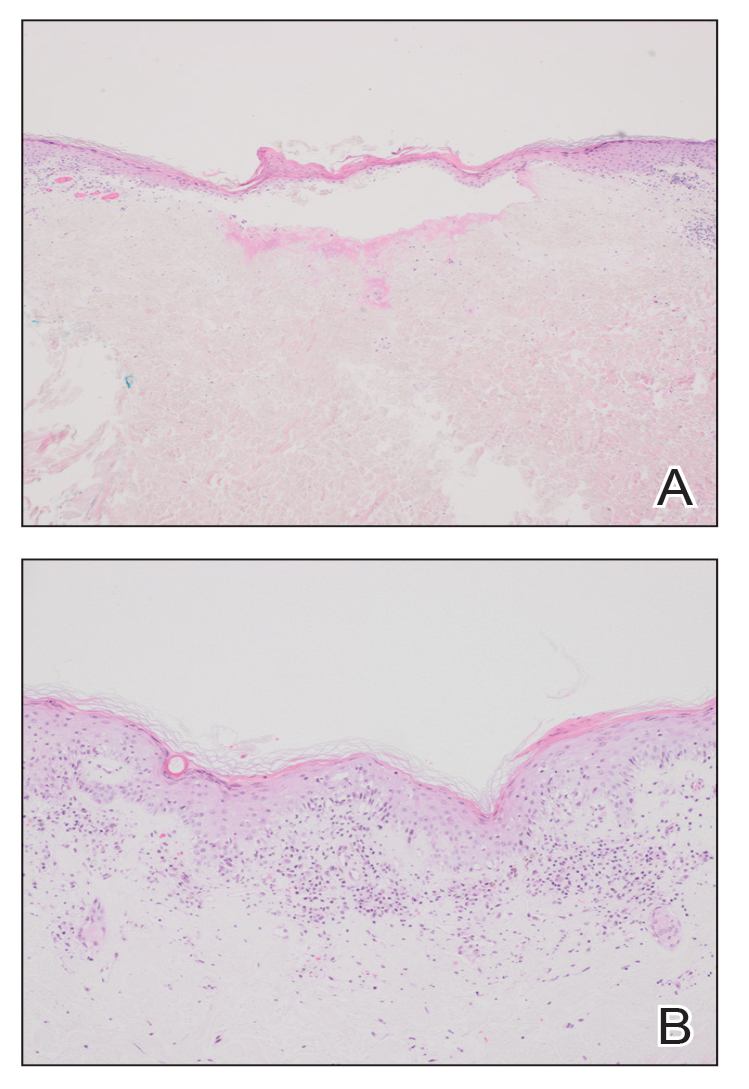
Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.

At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).

Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.

At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).

Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
PRACTICE POINTS
- Papules with atrophic white centers and erythematous telangiectatic rims are the characteristic skin lesions found in Degos disease.
- A painful penile ulceration also may occur in Degos disease, though it is uncommon.
- The histopathology of skin lesions changes as the lesions evolve. Early lesions may resemble connective tissue disease. Late lesions show the classic pathology of wedge-shaped dermal sclerosis.
Erythrodermic Pityriasis Rubra Pilaris Following COVID-19 Vaccination
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed
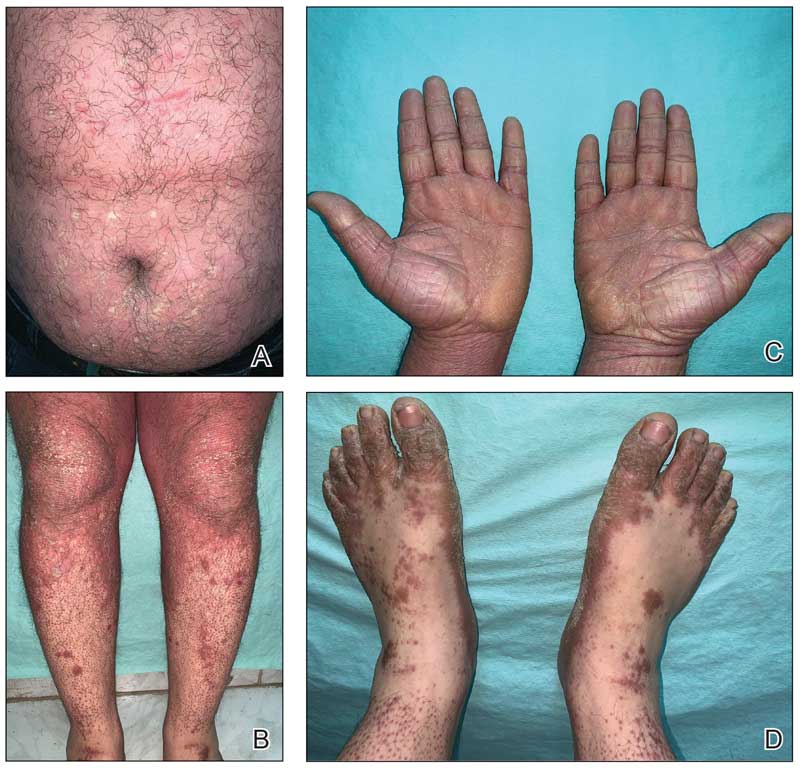
Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed
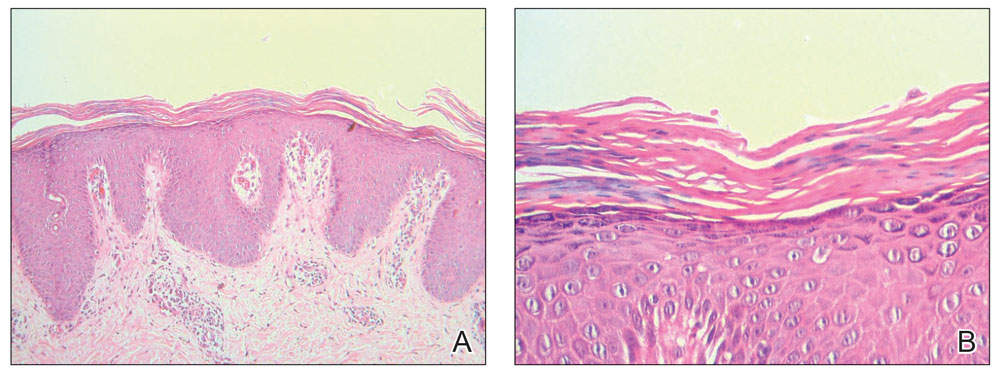
Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
To the Editor:
A 32-year-old man presented to our clinic with acute-onset erythroderma associated with severe itching of 1 month’s duration. The patient developed the eruption after receiving the second dose of the Sinopharm BBIBP COVID-19 vaccine (BBIBP-CorV) 2 weeks prior to presentation. His medical history was unremarkable. There was no personal or family history of skin disease and no history of drug intake. Physical examination revealed

Histopathology of two 4-mm punch biopsies of the skin on the trunk and lower limb showed

Pityriasis rubra pilaris is a rare papulosquamous skin disease of unknown etiology with several theories including genetic factors, aberrant metabolism of vitamin A, infection, drug reaction, autoimmune disease, and malignancy.1 Clinically, there are 6 types of PRP: type I (classical adult), type II (atypical adult), type III (classical juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (HIV associated). Classic features include orange-red keratotic follicular papules that coalesce into plaques with characteristic islands of sparing.1
Pityriasis rubra pilaris is a rare sequela following administration of certain vaccines, including diphtheria, pertussis, and tetanus; measles-mumps-rubella; and polio vaccines.2,3 Among the various skin reactions that have been reported following COVID-19 vaccination, PRP has been reported in 19 patients: 7 (36.8%) after AstraZeneca vaccination, 3 (15.8%) after CoronaVac, 3 (15.8%) after Moderna, 5 (26.3%) after Pfizer-BioNTech,4 and 1 (5.3%) after Sinopharm.5 Our patient represents an additional case of a reaction after the Sinopharm vaccine. The condition developed after the first dose of vaccine in 11 patients, after the second dose in 6 patients, and after the third dose in 2 patients.
Other papulosquamous skin reactions have been reported after
Pityriasis rubra pilaris can be self-limited in some cases and may not require treatment. Topical therapies such as keratolytics, emollients, and vitamin D may be utilized, especially for localized disease. Systemic therapy may be needed for refractory cases, including retinoids or immunosuppressive medications such as methotrexate, which is considered a second-line treatment for refractory PRP (after retinoids) and was used in our case. Azathioprine and cyclosporine also may be used. Phototherapy may play a role in PRP treatment, but the response is variable.7
Pityriasis rubra pilaris should be added to the list of cutaneous adverse reactions that can occur following vaccination with the Sinopharm BBIBP-CorV vaccine. Dermatologists must be aware of the possibility of vaccine-induced PRP, especially in de novo cases.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
- Wang D, Chong VC-L, Chong W-S, et al. A review on pityriasis rubra pilaris. Am J Clin Dermatol. 2018;19:377-390. doi:10.1007/s40257-017-0338-1
- Mohamed M, Belhadjali H, Hammedi F, et al. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines [letter]. Indian J Dermatol Venereol Leprol. 2015;81:618-620. doi:10.4103/0378-6323.168326
- Naciri Bennani B, Cheikh Rouhou H, Waton J, et al. Pityriasis rubra pilaris after vaccination. Ann Dermatol Venereol. 2011;138:753-756. doi:10.1016/j.annder.2011.01.049
- Liu YA, Dai J, Nagarajan P, et al. Pityriasis rubra pilaris after Moderna COVID-19 vaccination: a case report and literature review. Am J Dermatopathol. 2023;45:185-188. doi:10.1097/DAD.0000000000002369.
- Samarasinghe KH, Janani T, Gunasekera CN. Pityriasis rubra pilaris like eruption following Sinopharm-SARS COVID-19 vaccine. Sri Lanka J Dermatol. 2021;22:99-100.
- Shakoei S, Kalantari Y, Nasimi M, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. 2022;35:E15651. doi:10.1111/dth.15651
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351.
Practice Points
- Dermatologists must be aware of the possibility of COVID-19 vaccine–induced pityriasis rubra pilaris (PRP), especially in de novo cases.
- Management of these cases usually follows similar standards for PRP cases.
Centrifugally Spreading Lymphocutaneous Sporotrichosis: A Rare Cutaneous Manifestation
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).
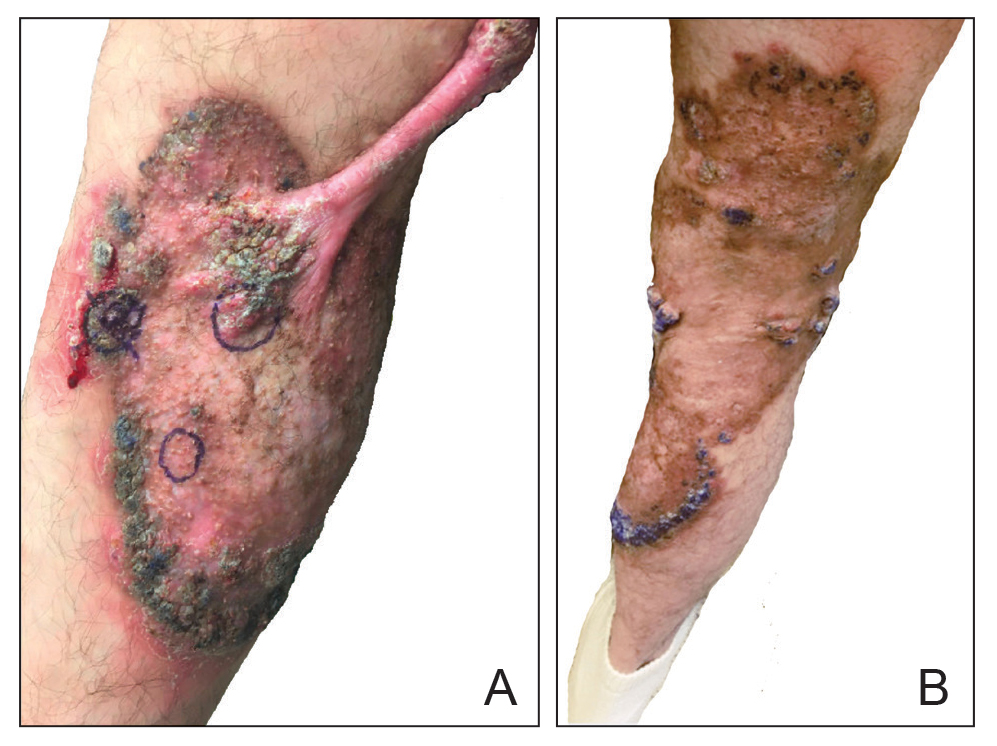
Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.
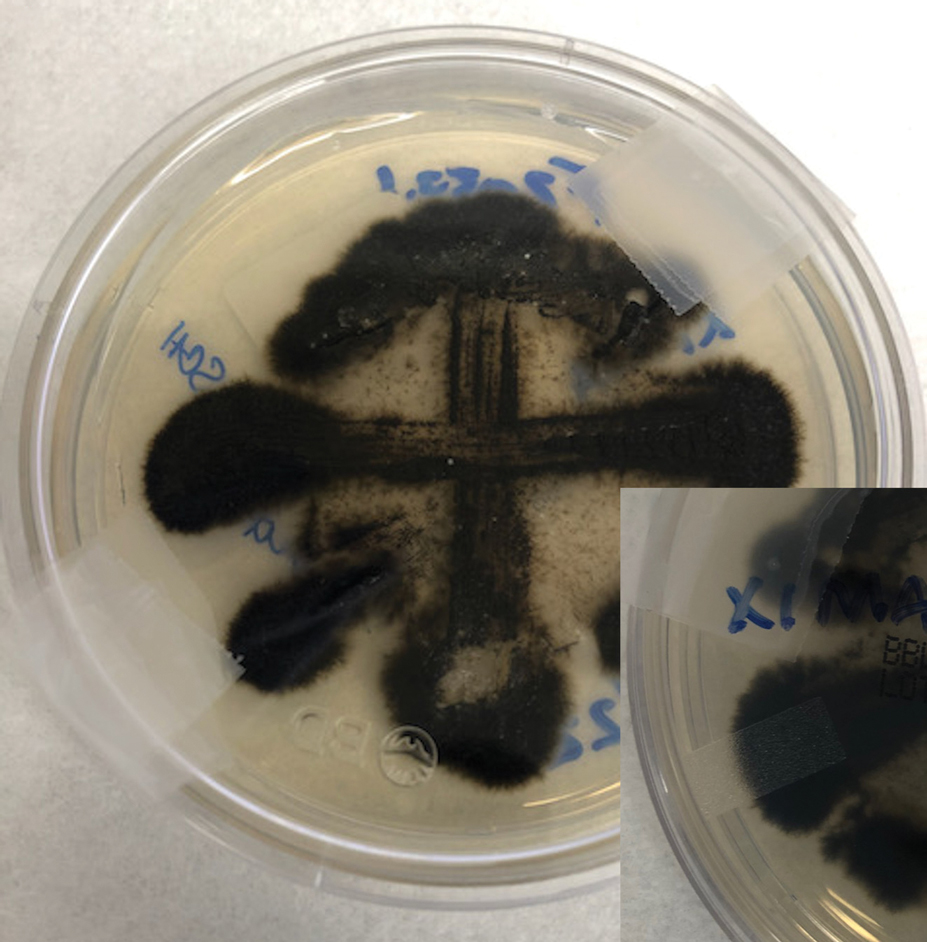
The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.
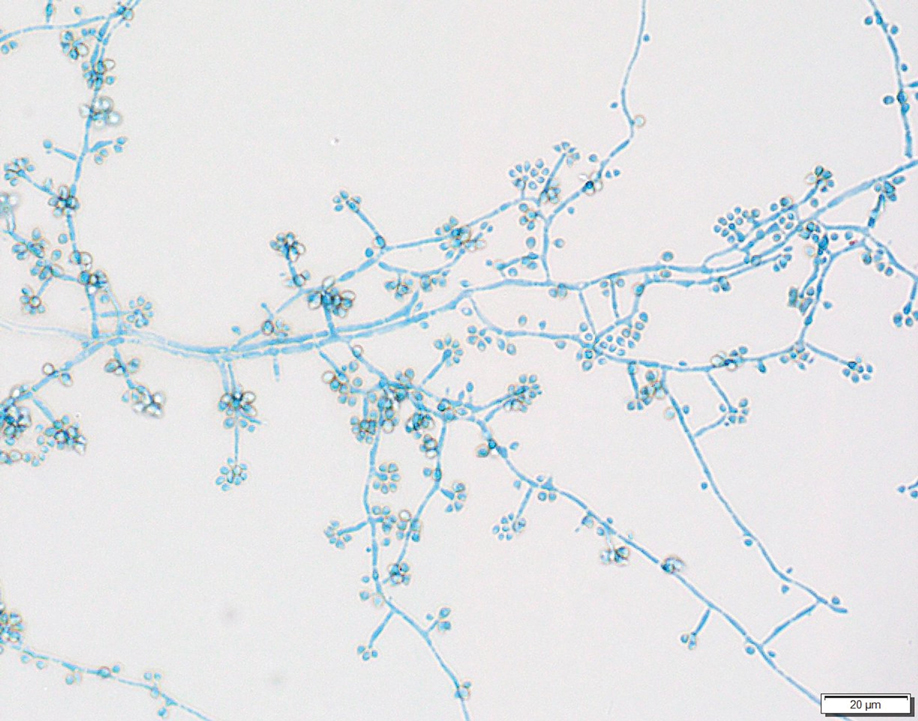
Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4
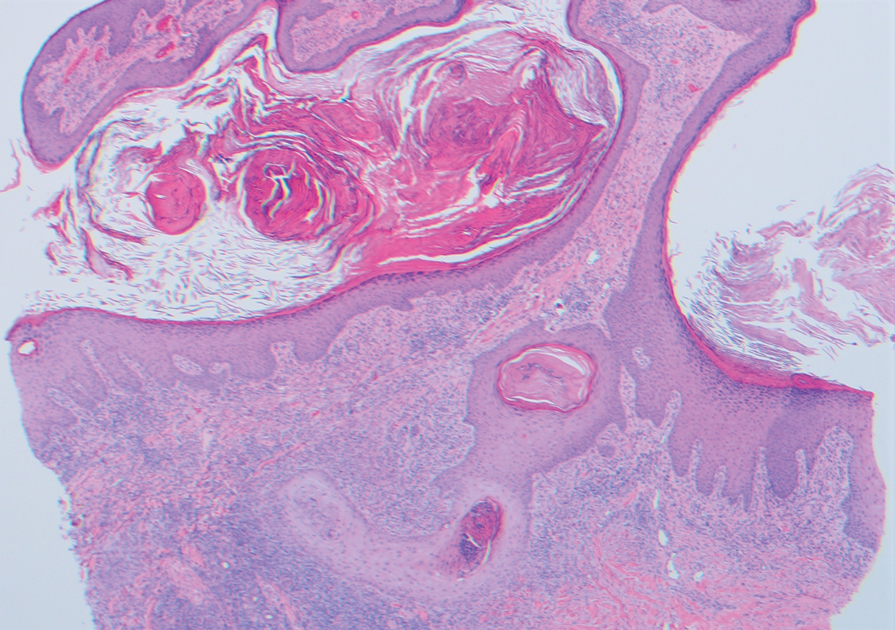
Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.
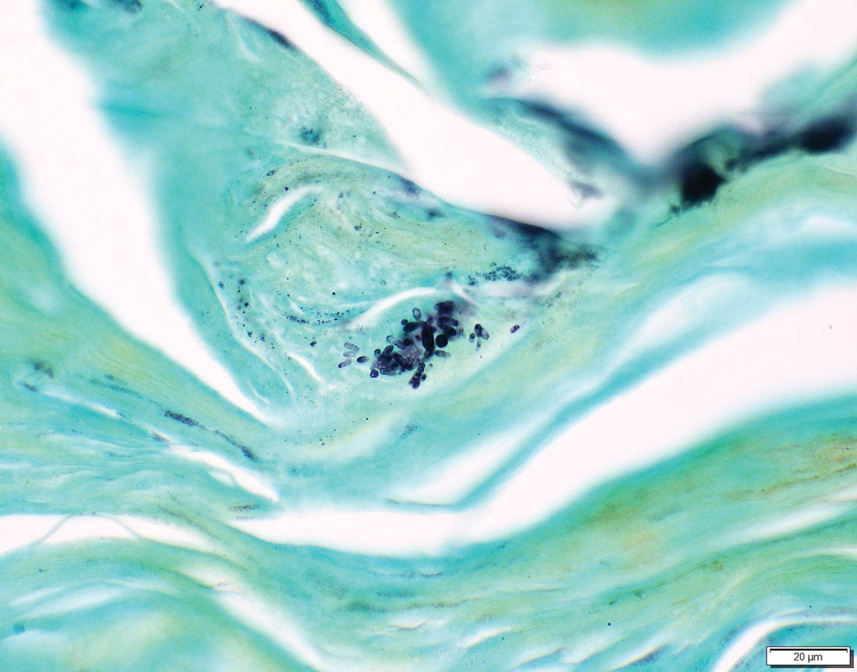
In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
Practice Points
- An atypical presentation of lymphocutaneous sporotrichosis may pose challenges to timely diagnosis and treatment.
- Although lymphocutaneous sporotrichosis spreads most commonly in a linear fashion along lymphatic channels, an annular configuration is possible.
- Initial tissue cultures and histopathology of lymphocutaneous sporotrichosis may not yield a diagnosis, necessitating repeat biopsies when clinical suspicion is high.