User login
Adult-Onset Asymmetrical Lipomatosis
To the Editor:
An 85-year-old woman presented with extra growth of subcutaneous fat at the left anterior infradiaphragm that expanded circumferentially to the left back over the last 4 years. Two years prior to the current presentation, the left thigh became visibly thicker than the right. Diffuse subtle lipomatosis affecting the ipsilateral face, neck, arms, calf, and foot was noted at that time. Additionally, the patient had hyperlipidemia, gastroesophageal reflux disease, osteoporosis, and scoliosis, all beginning in her late 60s. She reported no alcohol or tobacco use and was taking rosuvastatin, esomeprazole, calcium, vitamin D, and glucosamine. There was no reported family history of asymmetric growth or bony deformities, and her children were healthy.
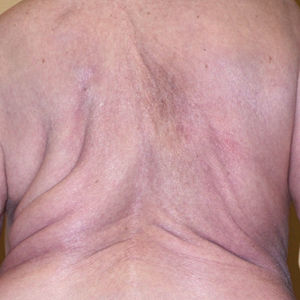
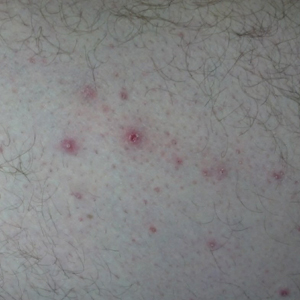
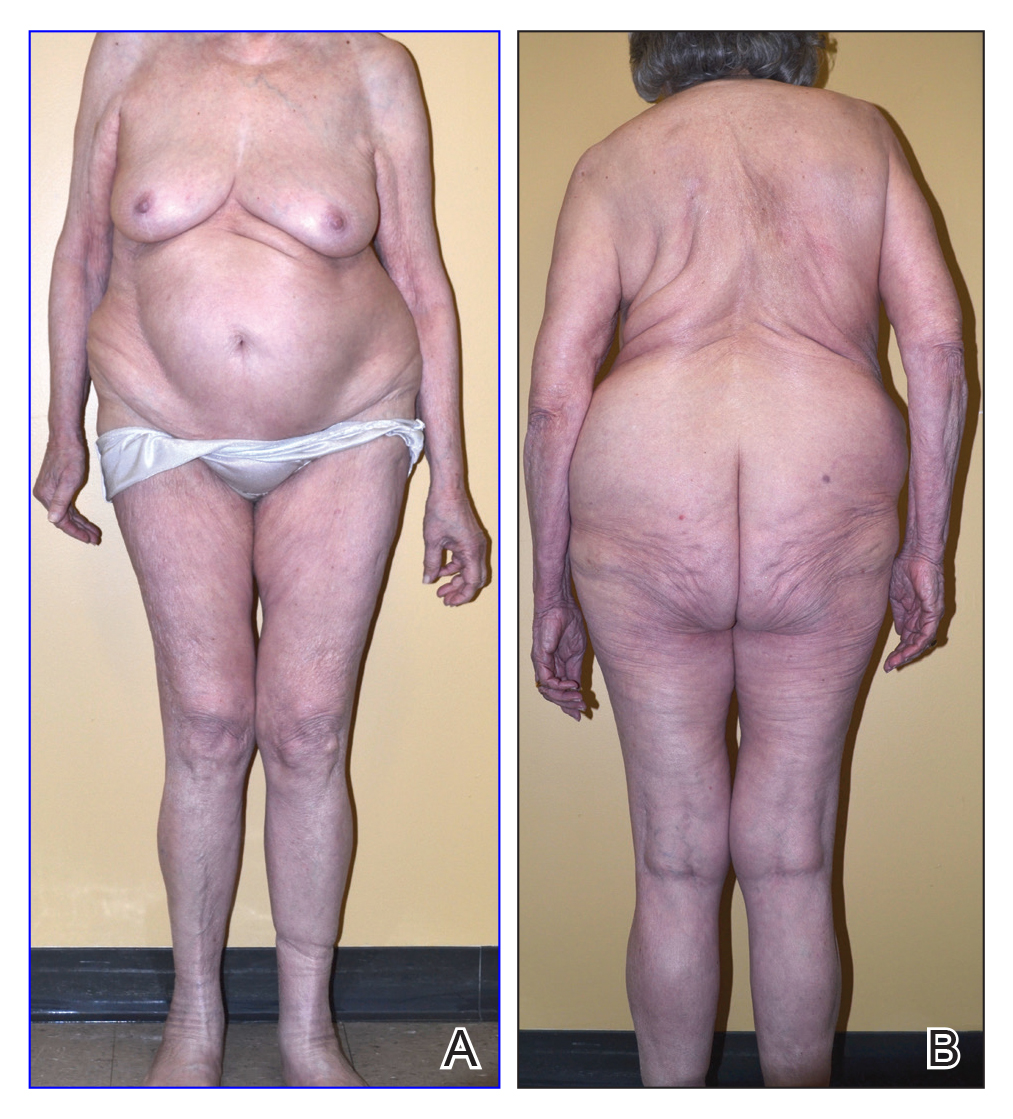
On physical examination, the lipomatosis affected the entire left side, most prominently around the abdomen, back, and thighs. The affected side was nontender and nonpruritic; there was no atrophy of the unaffected side (Figure). Maximum thigh circumference was 55.1 cm on the affected side and 52.6 cm on the unaffected side. There were no differences in power, reflex, or sensation between the 2 sides, and no hyperhidrosis or vascular malformations were present. Laboratory investigations, including complete blood cell count, complete metabolic panel, lipids, and thyroid-stimulating and sex hormone panels all were within reference range.
Enzi et al1 reported 2 women who developed asymmetrical lipomatosis between the ages of 13 and 20 years. Acquired asymmetrical lipomatosis should be differentiated from the asymmetrical overgrowth diagnosed in neonates and infants.
Proteus syndrome (PS) is a progressive disease involving a combination of overgrowth in a mosaic distribution, connective tissue and epidermal nevi, ovarian cysts, parotid gland tumor, dysregulated adipose tissue, lymphovascular malformation, and certain facial phenotypes.2,3 The average age of onset is 6 to 18 months, and half of cases present at birth.3,4 Hemihyperplasia-multiple lipomatosis syndrome (HHML) describes a mild and nonprogressive variant that does not satisfy the diagnostic criteria of PS; it typically is diagnosed at birth.5 One case of mild and delayed-onset PS was described in a woman who started developing signs at 15 years of age.6 In comparison, asymmetrical lipomatosis and scoliosis were the only abnormal clinical signs present in our patient, and the lipomatosis developed diffusely, as opposed to the typical mosaic distribution found in PS and HHML. Scoliosis can be found in PS and HHML secondary to hemihypertrophy of vertebra or infiltrative intraspinal lipomatosis.7,8 Our patient’s scoliosis was diagnosed more than 10 years prior to the onset of lipomatosis, likely representing degenerative joint disease.9
Prior reported cases of asymmetrical lipomatosis did not describe treatment.1 Ultrasound-guided or conventional liposuction and lipectomy are mainstream therapies for multiple symmetrical lipomatosis, an acquired lipomatosis typically affecting alcoholics in the fourth decade of life. However, recurrence rates are high for surgical treatment of unencapsulated lipomatosis, likely due to incomplete removal of the adipose tissue.10 Alternative treatments found in case reports, including oral salbutamol, mesotherapy using phosphatidylcholine, and fenofibrate (200 mg/d), require further study.11-13 Our patient was not aesthetically bothered by her lipomatosis; therefore, imaging and treatment options were not pursued. In conclusion, we report a patient with acquired asymmetrical lipomatosis with onset in late adulthood, unique from the existing syndromes of asymmetrical hemihyperplasia.1,14
- Enzi G, Digito M, Enzi GB, et al. Asymmetrical lipomatosis: report of two cases. Postgrad Med J. 1985;61:797-800.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Cohen MM Jr. Proteus syndrome: an update. Am J Med Genet C Semin Med Genet. 2005;137C:38-52.
- Biesecker LG, Peters KF, Darling TN, et al. Clinical differentiation between Proteus syndrome and hemihyperplasia: description of a distinct form of hemihyperplasia. Am J Med Genet. 1998;79:311-318.
- Luo S, Feng Y, Zheng Y, et al. Mild and delayed-onset Proteus syndrome. Eur J Dermatol. 2007;17:172-173.
- Takebayashi T, Yamashita T, Yokogushi K, et al. Scoliosis in Proteus syndrome: case report. Spine. 2001;26:E395-E398.
- Schulte TL, Liljenqvist U, Görgens H, et al. Hemihyperplasia-multiple lipomatosis syndrome (HHML): a challenge in spinal care. Acta Orthop Belg. 2008;74:714-719.
- Robin GC, Span Y, Steinberg R, et al. Scoliosis in the elderly: a follow-up study. Spine. 1982;7:355-359.
- Brea-García B, Cameselle-Teijeiro J, Couto-González I, et al. Madelung’s disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthet Plast Surg. 2013;37:409-416.
- Hasegawa T, Matsukura T, Ikeda S. Mesotherapy for benign symmetric lipomatosis. Aesthet Plast Surg. 2010;34:153-156.
- Zeitler H, Ulrich-Merzenich G, Richter DF, et al. Multiple benign symmetric lipomatosis—a differential diagnosis of obesity. is there a rationale for fibrate treatment? Obes Surg. 2008;18:1354-1356.
- Leung N, Gaer J, Beggs D, et al. Multiple symmetric lipomatosis (Launois‐Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol. 1987;27:601-606.
- Craiglow BG, Ko CJ, Antaya RJ. Two cases of hemihyperplasia-multiple lipomatosis syndrome and review of asymmetric hemihyperplasia syndromes. Pediatr Dermatol. 2014;31:507-510.
To the Editor:
An 85-year-old woman presented with extra growth of subcutaneous fat at the left anterior infradiaphragm that expanded circumferentially to the left back over the last 4 years. Two years prior to the current presentation, the left thigh became visibly thicker than the right. Diffuse subtle lipomatosis affecting the ipsilateral face, neck, arms, calf, and foot was noted at that time. Additionally, the patient had hyperlipidemia, gastroesophageal reflux disease, osteoporosis, and scoliosis, all beginning in her late 60s. She reported no alcohol or tobacco use and was taking rosuvastatin, esomeprazole, calcium, vitamin D, and glucosamine. There was no reported family history of asymmetric growth or bony deformities, and her children were healthy.
On physical examination, the lipomatosis affected the entire left side, most prominently around the abdomen, back, and thighs. The affected side was nontender and nonpruritic; there was no atrophy of the unaffected side (Figure). Maximum thigh circumference was 55.1 cm on the affected side and 52.6 cm on the unaffected side. There were no differences in power, reflex, or sensation between the 2 sides, and no hyperhidrosis or vascular malformations were present. Laboratory investigations, including complete blood cell count, complete metabolic panel, lipids, and thyroid-stimulating and sex hormone panels all were within reference range.

Enzi et al1 reported 2 women who developed asymmetrical lipomatosis between the ages of 13 and 20 years. Acquired asymmetrical lipomatosis should be differentiated from the asymmetrical overgrowth diagnosed in neonates and infants.
Proteus syndrome (PS) is a progressive disease involving a combination of overgrowth in a mosaic distribution, connective tissue and epidermal nevi, ovarian cysts, parotid gland tumor, dysregulated adipose tissue, lymphovascular malformation, and certain facial phenotypes.2,3 The average age of onset is 6 to 18 months, and half of cases present at birth.3,4 Hemihyperplasia-multiple lipomatosis syndrome (HHML) describes a mild and nonprogressive variant that does not satisfy the diagnostic criteria of PS; it typically is diagnosed at birth.5 One case of mild and delayed-onset PS was described in a woman who started developing signs at 15 years of age.6 In comparison, asymmetrical lipomatosis and scoliosis were the only abnormal clinical signs present in our patient, and the lipomatosis developed diffusely, as opposed to the typical mosaic distribution found in PS and HHML. Scoliosis can be found in PS and HHML secondary to hemihypertrophy of vertebra or infiltrative intraspinal lipomatosis.7,8 Our patient’s scoliosis was diagnosed more than 10 years prior to the onset of lipomatosis, likely representing degenerative joint disease.9
Prior reported cases of asymmetrical lipomatosis did not describe treatment.1 Ultrasound-guided or conventional liposuction and lipectomy are mainstream therapies for multiple symmetrical lipomatosis, an acquired lipomatosis typically affecting alcoholics in the fourth decade of life. However, recurrence rates are high for surgical treatment of unencapsulated lipomatosis, likely due to incomplete removal of the adipose tissue.10 Alternative treatments found in case reports, including oral salbutamol, mesotherapy using phosphatidylcholine, and fenofibrate (200 mg/d), require further study.11-13 Our patient was not aesthetically bothered by her lipomatosis; therefore, imaging and treatment options were not pursued. In conclusion, we report a patient with acquired asymmetrical lipomatosis with onset in late adulthood, unique from the existing syndromes of asymmetrical hemihyperplasia.1,14
To the Editor:
An 85-year-old woman presented with extra growth of subcutaneous fat at the left anterior infradiaphragm that expanded circumferentially to the left back over the last 4 years. Two years prior to the current presentation, the left thigh became visibly thicker than the right. Diffuse subtle lipomatosis affecting the ipsilateral face, neck, arms, calf, and foot was noted at that time. Additionally, the patient had hyperlipidemia, gastroesophageal reflux disease, osteoporosis, and scoliosis, all beginning in her late 60s. She reported no alcohol or tobacco use and was taking rosuvastatin, esomeprazole, calcium, vitamin D, and glucosamine. There was no reported family history of asymmetric growth or bony deformities, and her children were healthy.
On physical examination, the lipomatosis affected the entire left side, most prominently around the abdomen, back, and thighs. The affected side was nontender and nonpruritic; there was no atrophy of the unaffected side (Figure). Maximum thigh circumference was 55.1 cm on the affected side and 52.6 cm on the unaffected side. There were no differences in power, reflex, or sensation between the 2 sides, and no hyperhidrosis or vascular malformations were present. Laboratory investigations, including complete blood cell count, complete metabolic panel, lipids, and thyroid-stimulating and sex hormone panels all were within reference range.

Enzi et al1 reported 2 women who developed asymmetrical lipomatosis between the ages of 13 and 20 years. Acquired asymmetrical lipomatosis should be differentiated from the asymmetrical overgrowth diagnosed in neonates and infants.
Proteus syndrome (PS) is a progressive disease involving a combination of overgrowth in a mosaic distribution, connective tissue and epidermal nevi, ovarian cysts, parotid gland tumor, dysregulated adipose tissue, lymphovascular malformation, and certain facial phenotypes.2,3 The average age of onset is 6 to 18 months, and half of cases present at birth.3,4 Hemihyperplasia-multiple lipomatosis syndrome (HHML) describes a mild and nonprogressive variant that does not satisfy the diagnostic criteria of PS; it typically is diagnosed at birth.5 One case of mild and delayed-onset PS was described in a woman who started developing signs at 15 years of age.6 In comparison, asymmetrical lipomatosis and scoliosis were the only abnormal clinical signs present in our patient, and the lipomatosis developed diffusely, as opposed to the typical mosaic distribution found in PS and HHML. Scoliosis can be found in PS and HHML secondary to hemihypertrophy of vertebra or infiltrative intraspinal lipomatosis.7,8 Our patient’s scoliosis was diagnosed more than 10 years prior to the onset of lipomatosis, likely representing degenerative joint disease.9
Prior reported cases of asymmetrical lipomatosis did not describe treatment.1 Ultrasound-guided or conventional liposuction and lipectomy are mainstream therapies for multiple symmetrical lipomatosis, an acquired lipomatosis typically affecting alcoholics in the fourth decade of life. However, recurrence rates are high for surgical treatment of unencapsulated lipomatosis, likely due to incomplete removal of the adipose tissue.10 Alternative treatments found in case reports, including oral salbutamol, mesotherapy using phosphatidylcholine, and fenofibrate (200 mg/d), require further study.11-13 Our patient was not aesthetically bothered by her lipomatosis; therefore, imaging and treatment options were not pursued. In conclusion, we report a patient with acquired asymmetrical lipomatosis with onset in late adulthood, unique from the existing syndromes of asymmetrical hemihyperplasia.1,14
- Enzi G, Digito M, Enzi GB, et al. Asymmetrical lipomatosis: report of two cases. Postgrad Med J. 1985;61:797-800.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Cohen MM Jr. Proteus syndrome: an update. Am J Med Genet C Semin Med Genet. 2005;137C:38-52.
- Biesecker LG, Peters KF, Darling TN, et al. Clinical differentiation between Proteus syndrome and hemihyperplasia: description of a distinct form of hemihyperplasia. Am J Med Genet. 1998;79:311-318.
- Luo S, Feng Y, Zheng Y, et al. Mild and delayed-onset Proteus syndrome. Eur J Dermatol. 2007;17:172-173.
- Takebayashi T, Yamashita T, Yokogushi K, et al. Scoliosis in Proteus syndrome: case report. Spine. 2001;26:E395-E398.
- Schulte TL, Liljenqvist U, Görgens H, et al. Hemihyperplasia-multiple lipomatosis syndrome (HHML): a challenge in spinal care. Acta Orthop Belg. 2008;74:714-719.
- Robin GC, Span Y, Steinberg R, et al. Scoliosis in the elderly: a follow-up study. Spine. 1982;7:355-359.
- Brea-García B, Cameselle-Teijeiro J, Couto-González I, et al. Madelung’s disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthet Plast Surg. 2013;37:409-416.
- Hasegawa T, Matsukura T, Ikeda S. Mesotherapy for benign symmetric lipomatosis. Aesthet Plast Surg. 2010;34:153-156.
- Zeitler H, Ulrich-Merzenich G, Richter DF, et al. Multiple benign symmetric lipomatosis—a differential diagnosis of obesity. is there a rationale for fibrate treatment? Obes Surg. 2008;18:1354-1356.
- Leung N, Gaer J, Beggs D, et al. Multiple symmetric lipomatosis (Launois‐Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol. 1987;27:601-606.
- Craiglow BG, Ko CJ, Antaya RJ. Two cases of hemihyperplasia-multiple lipomatosis syndrome and review of asymmetric hemihyperplasia syndromes. Pediatr Dermatol. 2014;31:507-510.
- Enzi G, Digito M, Enzi GB, et al. Asymmetrical lipomatosis: report of two cases. Postgrad Med J. 1985;61:797-800.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Cohen MM Jr. Proteus syndrome: an update. Am J Med Genet C Semin Med Genet. 2005;137C:38-52.
- Biesecker LG, Peters KF, Darling TN, et al. Clinical differentiation between Proteus syndrome and hemihyperplasia: description of a distinct form of hemihyperplasia. Am J Med Genet. 1998;79:311-318.
- Luo S, Feng Y, Zheng Y, et al. Mild and delayed-onset Proteus syndrome. Eur J Dermatol. 2007;17:172-173.
- Takebayashi T, Yamashita T, Yokogushi K, et al. Scoliosis in Proteus syndrome: case report. Spine. 2001;26:E395-E398.
- Schulte TL, Liljenqvist U, Görgens H, et al. Hemihyperplasia-multiple lipomatosis syndrome (HHML): a challenge in spinal care. Acta Orthop Belg. 2008;74:714-719.
- Robin GC, Span Y, Steinberg R, et al. Scoliosis in the elderly: a follow-up study. Spine. 1982;7:355-359.
- Brea-García B, Cameselle-Teijeiro J, Couto-González I, et al. Madelung’s disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthet Plast Surg. 2013;37:409-416.
- Hasegawa T, Matsukura T, Ikeda S. Mesotherapy for benign symmetric lipomatosis. Aesthet Plast Surg. 2010;34:153-156.
- Zeitler H, Ulrich-Merzenich G, Richter DF, et al. Multiple benign symmetric lipomatosis—a differential diagnosis of obesity. is there a rationale for fibrate treatment? Obes Surg. 2008;18:1354-1356.
- Leung N, Gaer J, Beggs D, et al. Multiple symmetric lipomatosis (Launois‐Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol. 1987;27:601-606.
- Craiglow BG, Ko CJ, Antaya RJ. Two cases of hemihyperplasia-multiple lipomatosis syndrome and review of asymmetric hemihyperplasia syndromes. Pediatr Dermatol. 2014;31:507-510.
Practice Points
- Acquired asymmetrical lipomatosis is a rare condition that can develop at any age; it should be differentiated from existing syndromes of asymmetrical hemihyperplasia.
- Acquired asymmetrical lipomatosis is a clinical diagnosis with no laboratory changes. If the patient is clinically stable and asymptomatic, no further investigation or management is required.
Asboe-Hansen Sign in Toxic Epidermal Necrolysis
To the Editor:
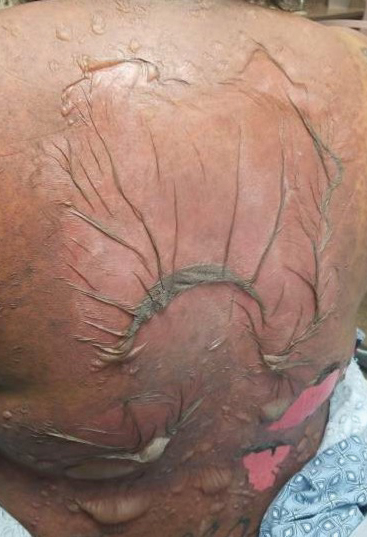
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.
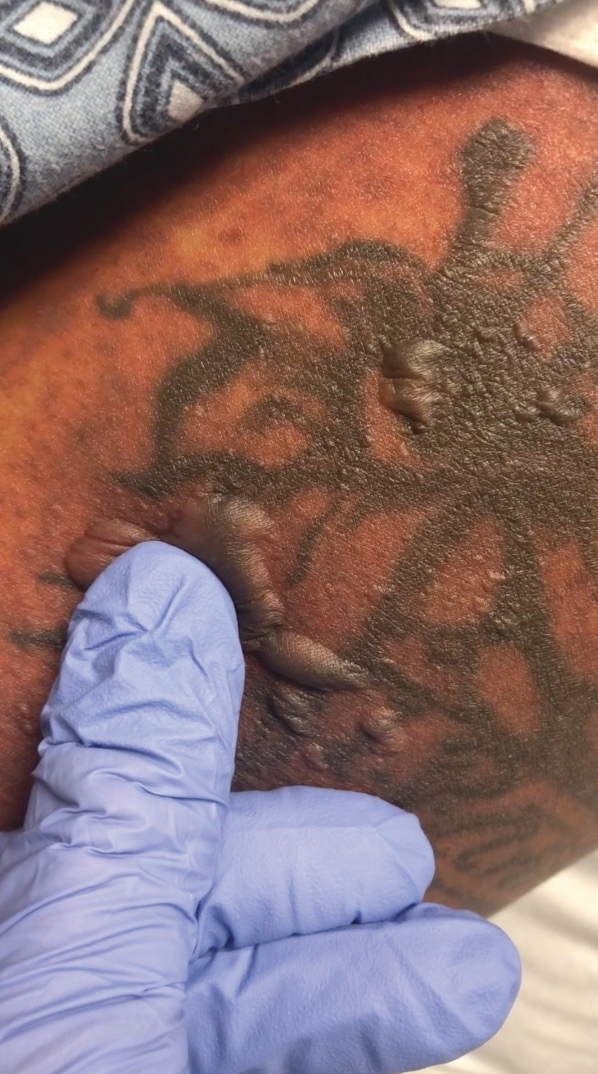
applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
To the Editor:
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.


applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
To the Editor:
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.


applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
Practice Points
- Asboe-Hansen sign is a useful clinical tool for diagnosing toxic epidermal necrolysis (TEN).
- Asboe-Hansen sign can be employed to generate a fresh bulla for lesional skin biopsy in the evaluation of TEN.
Symmetric Lichen Amyloidosis: An Atypical Location on the Bilateral Extensor Surfaces of the Arms
To the Editor:
Lichen amyloidosis (LA) classically presents as a pruritic, hyperkeratotic, papular eruption localized to the pretibial surface of the legs.1 Nonpruritic and generalized variants have been reported but are rare.2 Although it is the most common subtype of primary localized cutaneous amyloidosis, LA is a benign condition but is difficult to eradicate.1 The precise pathophysiology is poorly understood, but chronic frictional irritation is closely associated with the eruption. We present a nongeneralized case of LA in an atypical location.
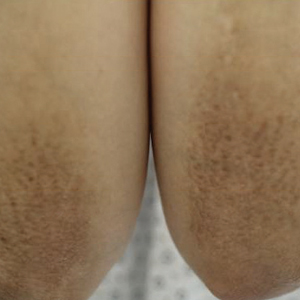
A healthy 30-year-old woman presented with an intermittent itchy rash on the elbows and knees of 2 years’ duration. The patient was first diagnosed with lichen simplex chronicus (LSC) and initially responded well to treatment with fluocinonide ointment 0.05%. Nearly 2 years after the initial presentation, she developed recurrent symptoms and sought further treatment. She reported frequent scratching in association with episodes of anxiety. Examination revealed numerous 1- to 3-mm, flesh-colored to light brown, monomorphic, dome-shaped papules over the extensor surfaces of the bilateral arms and left pretibial surface (Figure 1).
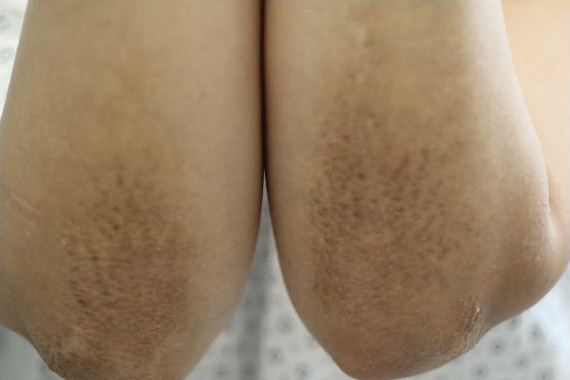
papules (1–3 mm) over the extensor surfaces of the bilateral arms.
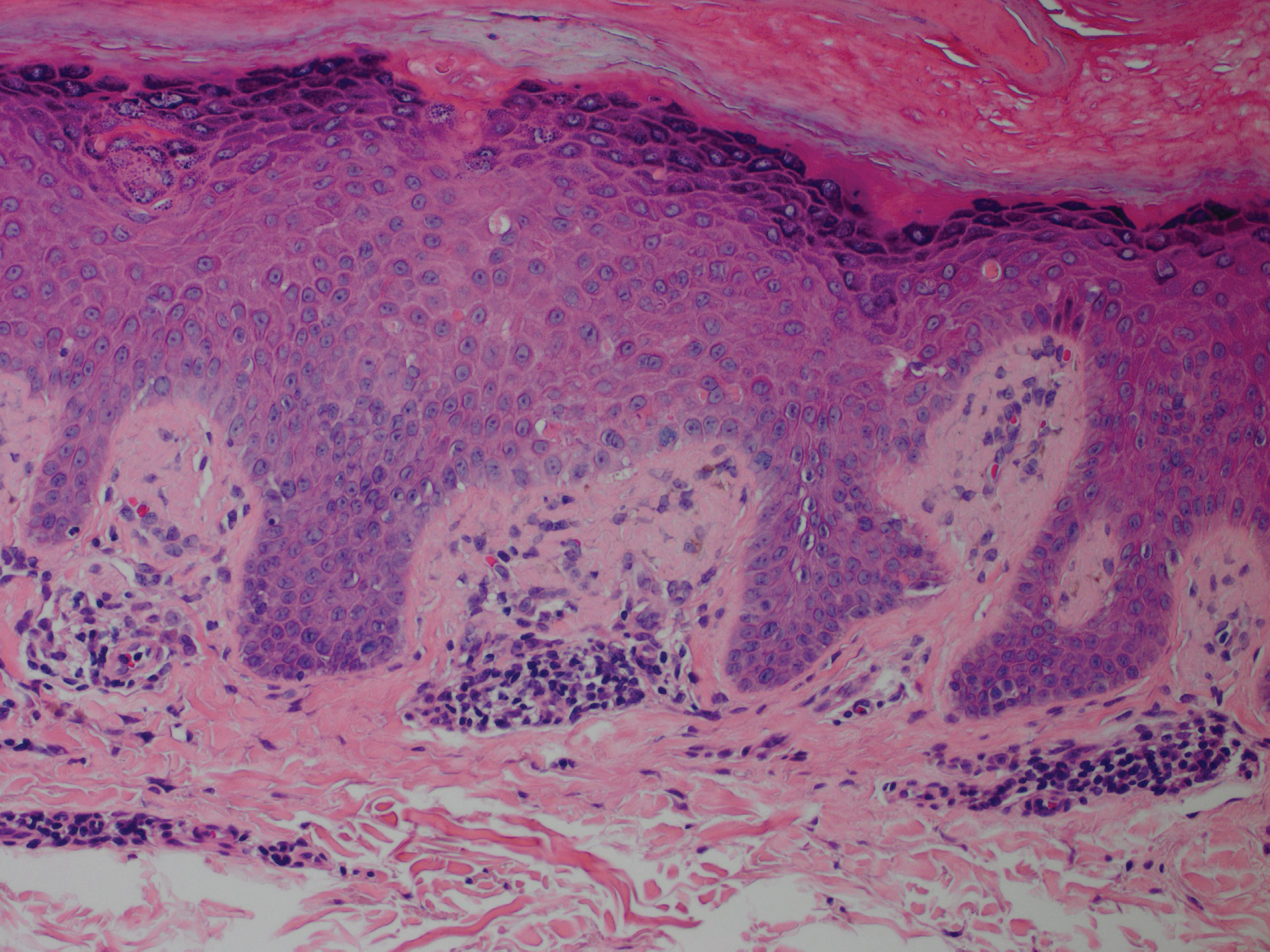
Although in an atypical location, LA was clinically suspected due to the morphology, and a biopsy was performed given the evolving nature of the lesions. The differential diagnosis included LSC, hypertrophic lichen planus, papular mucinosis, prurigo nodularis, and pretibial myxedema. Pathology revealed small eosinophilic globules in the papillary dermis (Figure 2), and cytokeratin 5/6 immunostaining showed amorphous papillary dermal deposits consistent with keratin-derived amyloid deposition (Figure 3). The deposits stained positive for Congo red and displayed apple green birefringence under polarized light. Thus, the diagnosis of LA was confirmed. After limited success with triamcinolone ointment 0.1%, the patient was transitioned to clobetasol cream 0.05% with notable physical and symptomatic improvement.
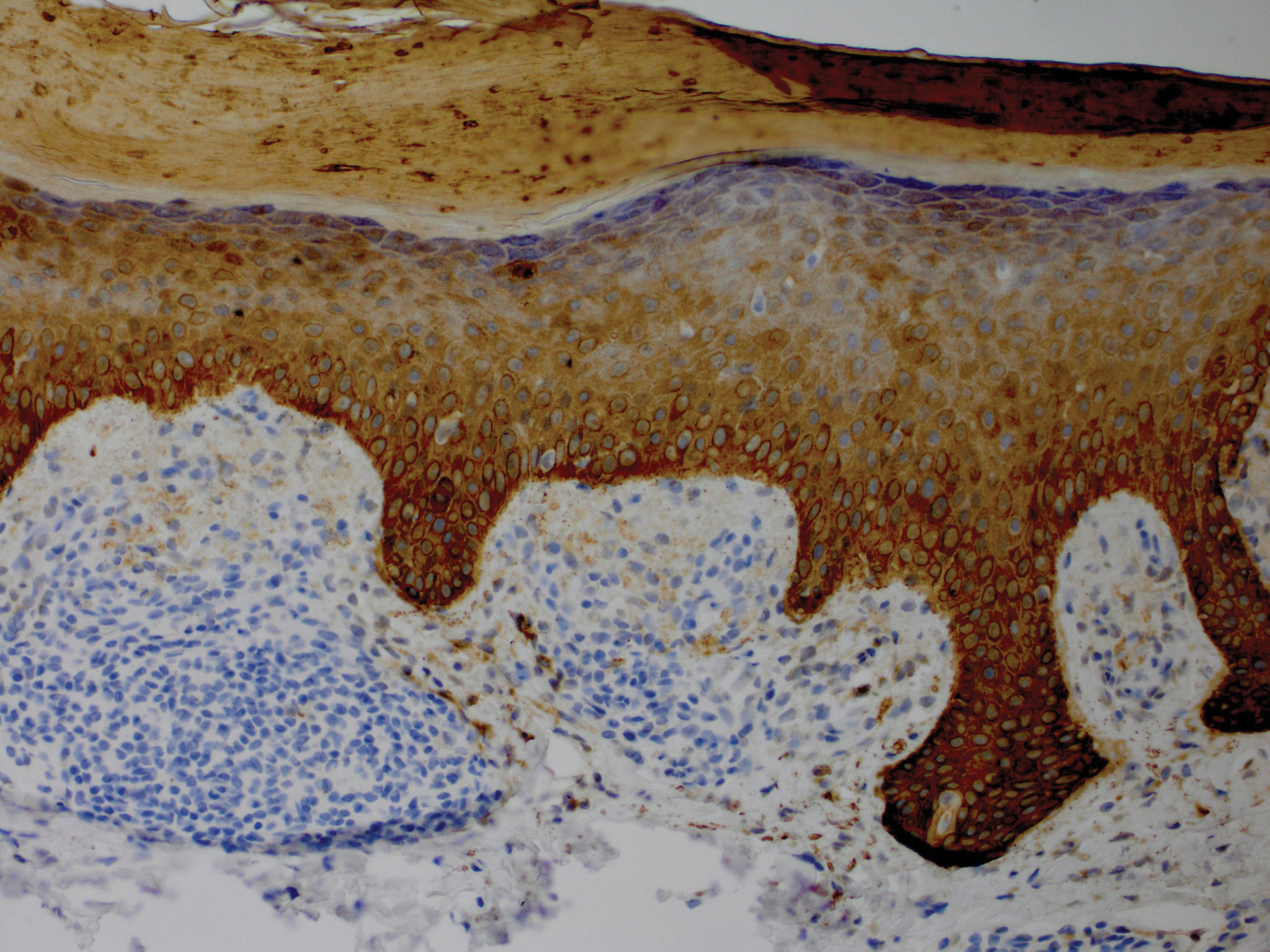
Amyloidosis is histopathologically characterized by extracellular deposits of amyloid, a polypeptide that polymerizes to form cross-β sheets.3 It is believed that the deposits seen in localized amyloidosis result from local production of amyloid, as opposed to the deposition of circulating light chains that is characteristic of systemic amyloidosis.3 Lichen amyloidosis is the most common subtype of primary localized cutaneous amyloidosis.1 The amyloid in this condition has been found to react immunohistochemically with antikeratin antibody, leading to the conclusion that the amyloid is formed by degeneration of keratinocytes locally due to chronic rubbing and scratching.
4-6
The possibility remains that this patient first presented with LSC 2 years prior and secondarily developed LA due to chronic trauma. Indeed, LA has been proposed as a variant of LSC. In both conditions, scratching seems to be the most important factor in the development of lesions. It has been proposed that treatment should primarily focus on the amelioration of pruritus.5
Five percent to 10% of cases of LA have been found to have some form of upper extremity involvement.7 However, these cases typically are associated with a generalized presentation involving the trunk and arms.2,7 Our patient had no evidence of disease elsewhere. When evaluating a localized, pruritic, monomorphic, papular eruption on the extensor surfaces of the arms, LA may be an important consideration.
- Tay CH, Dacosta JL. Lichen amyloidosis. clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Kandhari R, Ramesh V, Singh A. A generalized, non-pruritic variant of lichen amyloidosis: a case report and a brief review. Indian J Dermatol. 2013;58:328.
- Biewend ML, Menke DM, Calamia KT. The spectrum of localized amyloidosis: a case series of 20 patients and review of the literature. Amyloid. 2006;13:135-142.
- Jambrosic J, From L, Hanna W. Lichen amyloidosus. ultrastructure and pathogenesis. Am J Dermatopathol. 1984;6:151-158.
- Weyers W, Weyers I, Bonczkowitz M, et al. Lichen amyloidosis: a consequence of scratching. J Am Acad Dermatol. 1997;37:923-928.
- Kumakiri M, Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J Invest Dermatol. 1979;73:150-162.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosus: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
To the Editor:
Lichen amyloidosis (LA) classically presents as a pruritic, hyperkeratotic, papular eruption localized to the pretibial surface of the legs.1 Nonpruritic and generalized variants have been reported but are rare.2 Although it is the most common subtype of primary localized cutaneous amyloidosis, LA is a benign condition but is difficult to eradicate.1 The precise pathophysiology is poorly understood, but chronic frictional irritation is closely associated with the eruption. We present a nongeneralized case of LA in an atypical location.
A healthy 30-year-old woman presented with an intermittent itchy rash on the elbows and knees of 2 years’ duration. The patient was first diagnosed with lichen simplex chronicus (LSC) and initially responded well to treatment with fluocinonide ointment 0.05%. Nearly 2 years after the initial presentation, she developed recurrent symptoms and sought further treatment. She reported frequent scratching in association with episodes of anxiety. Examination revealed numerous 1- to 3-mm, flesh-colored to light brown, monomorphic, dome-shaped papules over the extensor surfaces of the bilateral arms and left pretibial surface (Figure 1).

papules (1–3 mm) over the extensor surfaces of the bilateral arms.
Although in an atypical location, LA was clinically suspected due to the morphology, and a biopsy was performed given the evolving nature of the lesions. The differential diagnosis included LSC, hypertrophic lichen planus, papular mucinosis, prurigo nodularis, and pretibial myxedema. Pathology revealed small eosinophilic globules in the papillary dermis (Figure 2), and cytokeratin 5/6 immunostaining showed amorphous papillary dermal deposits consistent with keratin-derived amyloid deposition (Figure 3). The deposits stained positive for Congo red and displayed apple green birefringence under polarized light. Thus, the diagnosis of LA was confirmed. After limited success with triamcinolone ointment 0.1%, the patient was transitioned to clobetasol cream 0.05% with notable physical and symptomatic improvement.


Amyloidosis is histopathologically characterized by extracellular deposits of amyloid, a polypeptide that polymerizes to form cross-β sheets.3 It is believed that the deposits seen in localized amyloidosis result from local production of amyloid, as opposed to the deposition of circulating light chains that is characteristic of systemic amyloidosis.3 Lichen amyloidosis is the most common subtype of primary localized cutaneous amyloidosis.1 The amyloid in this condition has been found to react immunohistochemically with antikeratin antibody, leading to the conclusion that the amyloid is formed by degeneration of keratinocytes locally due to chronic rubbing and scratching.
4-6
The possibility remains that this patient first presented with LSC 2 years prior and secondarily developed LA due to chronic trauma. Indeed, LA has been proposed as a variant of LSC. In both conditions, scratching seems to be the most important factor in the development of lesions. It has been proposed that treatment should primarily focus on the amelioration of pruritus.5
Five percent to 10% of cases of LA have been found to have some form of upper extremity involvement.7 However, these cases typically are associated with a generalized presentation involving the trunk and arms.2,7 Our patient had no evidence of disease elsewhere. When evaluating a localized, pruritic, monomorphic, papular eruption on the extensor surfaces of the arms, LA may be an important consideration.
To the Editor:
Lichen amyloidosis (LA) classically presents as a pruritic, hyperkeratotic, papular eruption localized to the pretibial surface of the legs.1 Nonpruritic and generalized variants have been reported but are rare.2 Although it is the most common subtype of primary localized cutaneous amyloidosis, LA is a benign condition but is difficult to eradicate.1 The precise pathophysiology is poorly understood, but chronic frictional irritation is closely associated with the eruption. We present a nongeneralized case of LA in an atypical location.
A healthy 30-year-old woman presented with an intermittent itchy rash on the elbows and knees of 2 years’ duration. The patient was first diagnosed with lichen simplex chronicus (LSC) and initially responded well to treatment with fluocinonide ointment 0.05%. Nearly 2 years after the initial presentation, she developed recurrent symptoms and sought further treatment. She reported frequent scratching in association with episodes of anxiety. Examination revealed numerous 1- to 3-mm, flesh-colored to light brown, monomorphic, dome-shaped papules over the extensor surfaces of the bilateral arms and left pretibial surface (Figure 1).

papules (1–3 mm) over the extensor surfaces of the bilateral arms.
Although in an atypical location, LA was clinically suspected due to the morphology, and a biopsy was performed given the evolving nature of the lesions. The differential diagnosis included LSC, hypertrophic lichen planus, papular mucinosis, prurigo nodularis, and pretibial myxedema. Pathology revealed small eosinophilic globules in the papillary dermis (Figure 2), and cytokeratin 5/6 immunostaining showed amorphous papillary dermal deposits consistent with keratin-derived amyloid deposition (Figure 3). The deposits stained positive for Congo red and displayed apple green birefringence under polarized light. Thus, the diagnosis of LA was confirmed. After limited success with triamcinolone ointment 0.1%, the patient was transitioned to clobetasol cream 0.05% with notable physical and symptomatic improvement.


Amyloidosis is histopathologically characterized by extracellular deposits of amyloid, a polypeptide that polymerizes to form cross-β sheets.3 It is believed that the deposits seen in localized amyloidosis result from local production of amyloid, as opposed to the deposition of circulating light chains that is characteristic of systemic amyloidosis.3 Lichen amyloidosis is the most common subtype of primary localized cutaneous amyloidosis.1 The amyloid in this condition has been found to react immunohistochemically with antikeratin antibody, leading to the conclusion that the amyloid is formed by degeneration of keratinocytes locally due to chronic rubbing and scratching.
4-6
The possibility remains that this patient first presented with LSC 2 years prior and secondarily developed LA due to chronic trauma. Indeed, LA has been proposed as a variant of LSC. In both conditions, scratching seems to be the most important factor in the development of lesions. It has been proposed that treatment should primarily focus on the amelioration of pruritus.5
Five percent to 10% of cases of LA have been found to have some form of upper extremity involvement.7 However, these cases typically are associated with a generalized presentation involving the trunk and arms.2,7 Our patient had no evidence of disease elsewhere. When evaluating a localized, pruritic, monomorphic, papular eruption on the extensor surfaces of the arms, LA may be an important consideration.
- Tay CH, Dacosta JL. Lichen amyloidosis. clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Kandhari R, Ramesh V, Singh A. A generalized, non-pruritic variant of lichen amyloidosis: a case report and a brief review. Indian J Dermatol. 2013;58:328.
- Biewend ML, Menke DM, Calamia KT. The spectrum of localized amyloidosis: a case series of 20 patients and review of the literature. Amyloid. 2006;13:135-142.
- Jambrosic J, From L, Hanna W. Lichen amyloidosus. ultrastructure and pathogenesis. Am J Dermatopathol. 1984;6:151-158.
- Weyers W, Weyers I, Bonczkowitz M, et al. Lichen amyloidosis: a consequence of scratching. J Am Acad Dermatol. 1997;37:923-928.
- Kumakiri M, Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J Invest Dermatol. 1979;73:150-162.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosus: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
- Tay CH, Dacosta JL. Lichen amyloidosis. clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Kandhari R, Ramesh V, Singh A. A generalized, non-pruritic variant of lichen amyloidosis: a case report and a brief review. Indian J Dermatol. 2013;58:328.
- Biewend ML, Menke DM, Calamia KT. The spectrum of localized amyloidosis: a case series of 20 patients and review of the literature. Amyloid. 2006;13:135-142.
- Jambrosic J, From L, Hanna W. Lichen amyloidosus. ultrastructure and pathogenesis. Am J Dermatopathol. 1984;6:151-158.
- Weyers W, Weyers I, Bonczkowitz M, et al. Lichen amyloidosis: a consequence of scratching. J Am Acad Dermatol. 1997;37:923-928.
- Kumakiri M, Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J Invest Dermatol. 1979;73:150-162.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosus: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
Practice Points
- Lichen amyloidosis (LA) classically presents as a pruritic and papular eruption localized to the pretibial surface of the legs.
- Nonpruritic and generalized variants are rare.
- This case represents a pruritic and nongeneralized
case located on the arms; LA should be considered
for any localized and pruritic eruption on the arms.
Bothersome Blisters: Localized Epidermolysis Bullosa Simplex
To the Editor:
Epidermolysis bullosa (EB) was first described in 1886, with the first classification scheme proposed in 1962 utilizing transmission electron microscopy (TEM) findings to delineate categories: epidermolytic (EB simplex [EBS]), lucidolytic (junctional EB), and dermolytic (dystrophic EB).1 Localized EBS (EBS-loc) is an autosomal-dominant disorder caused by negative mutations in keratin-5 and keratin-14, proteins expressed in the intermediate filaments of basal keratinocytes, which result in fragility of the skin in response to minor trauma.2 The incidence of EBS-loc is approximately 10 to 30 cases per million live births, with the age of presentation typically between the first and third decades of life.3,4 Because EBS-loc is the most common and often mildest form of EB, not all patients present for medical evaluation and true prevalence may be underestimated.4 We report a case of EBS-loc.
A 26-year-old woman with no notable medical history presented to the dermatology clinic for evaluation of skin blisters that had been intermittently present since infancy. The blisters primarily occurred on the feet, but she did occasionally develop blisters on the hands, knees, and elbows and at sites of friction or trauma (eg, bra line, medial thighs) following exercise. The blisters were worsened by heat and tight-fitting shoes. Because of the painful nature of the blisters, she would lance them with a needle. On the medial thighs, she utilized nonstick and gauze bandage roll dressings to minimize friction. A review of systems was positive for hyperhidrosis. Her family history revealed multiple family members with blisters involving the feet and areas of friction or trauma for 4 generations with no known diagnosis.
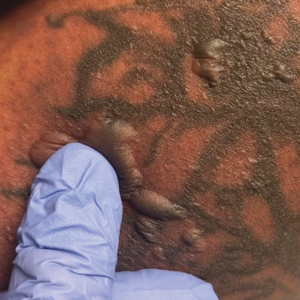
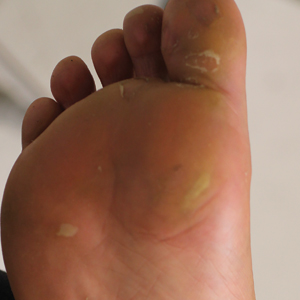
Physical examination revealed multiple tense bullae and calluses scattered over the bilateral plantar and distal dorsal feet with a few healing, superficially eroded, erythematous papules and plaques on the bilateral medial thighs (Figure 1). A biopsy from an induced blister on the right dorsal second toe was performed and sent in glutaraldehyde to the Epidermolysis Bullosa Clinic at Stanford University (Redwood City, California) for electron microscopy, which revealed lysis within the basal keratinocytes through the tonofilaments with continuous and intact lamina densa and lamina lucida (Figure 2). In this clinical context with the relevant family history, the findings were consistent with the diagnosis of EBS-loc (formerly Weber-Cockayne syndrome).2

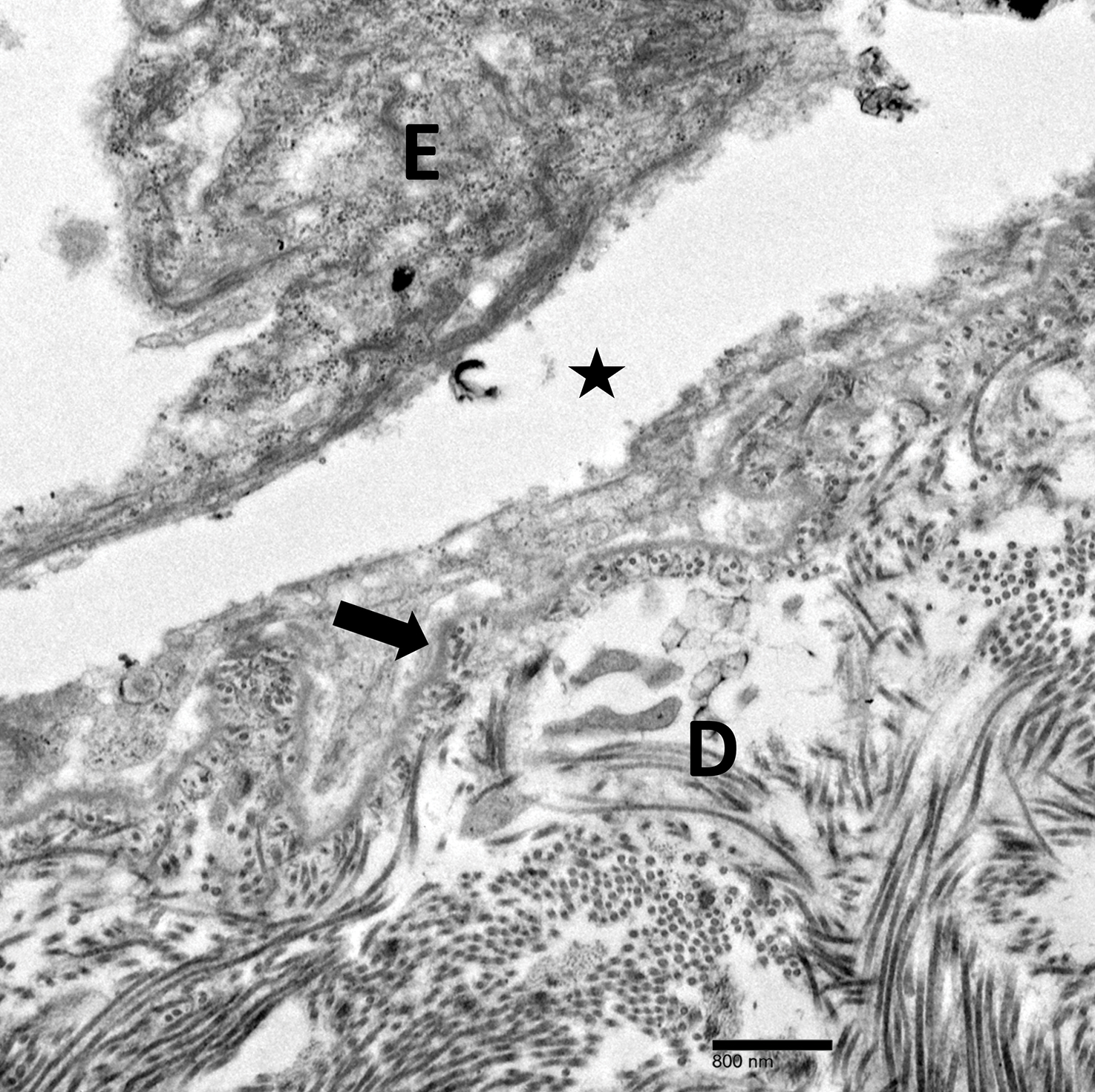
Skin manifestations of EBS-loc typically consist of friction-induced blisters, erosions, and calluses primarily on the palms and soles, often associated with hyperhidrosis and worsening of symptoms in summer months and hot temperatures.3 Milia, atrophic scarring, and dystrophic nails are uncommon.1 Extracutaneous involvement is rare with the exception of oral cavity erosions, which typically are asymptomatic and usually are only seen during infancy.1
Light microscopy does not have a notable role in diagnosis of classic forms of inherited EB unless another autoimmune blistering disorder is suspected.2,5 Both TEM and immunofluorescence mapping are used to diagnose EB.1 DNA mutational analysis is not considered a first-line diagnostic test for EB given it is a costly labor-intensive technique with limited access at present, but it may be considered in settings of prenatal diagnosis or in vitro fertilization.1 Biopsy of a freshly induced blister should be performed, as early reepithelialization of an existing blister makes it difficult to establish the level of cleavage.5 Applying firm pressure using a pencil eraser and rotating it on intact skin induces a subclinical blister. Two punch biopsies (4 mm) at the edge of the blister with one-third lesional and two-thirds perilesional skin should be obtained, with one biopsy sent for immunofluorescence mapping in Michel fixative and the other for TEM in glutaraldehyde.3,5 Transmission electron microscopy of an induced blister in EBS-loc shows cleavage within the most inferior portion of the basilar keratinocyte.2 Immunofluorescence mapping with anti–epidermal basement membrane monoclonal antibodies can distinguish between EB subtypes and assess expression of specific skin-associated proteins on both a qualitative or semiquantitative basis, providing insight on which structural protein is mutated.1,5
No specific treatments are available for EBS-loc. Mainstays of treatment include prevention of mechanical trauma and secondary infection. Hyperhidrosis of thepalms and soles may be treated with topical aluminum chloride hexahydrate or injections of botulinum toxin type A.2,6 Patients have normal life expectancy, though some cases may have complications with substantial morbidity.1 Awareness of this disease, its clinical course, and therapeutic options will allow physicians to more appropriately counsel patients on the disease process.
Localized EBS may be more common than previously thought, as not all patients seek medical care. Given its impact on patient quality of life, it is important for clinicians to recognize EBS-loc. Although no specific treatments are available, wound care counseling and explanation of the genetics of the disease should be provided to patients.
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931-950.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Limited; 2012.
- Eichenfield LF, Frieden IJ, Mathes EF, et al, eds. Neonatal and Infant Dermatology. 3rd ed. New York, NY: Elsevier Health Sciences; 2015.
- Spitz JL. Genodermatoses: A Clinical Guide to Genetic Skin Disorders. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Epidermolysis bullosa. Stanford Medicine website. http://med.stanford.edu/dermatopathology/dermpath-services/epiderm.html. Accessed April 3, 2019.
- Abitbol RJ, Zhou LH. Treatment of epidermolysis bullosa simplex, Weber-Cockayne type, with botulinum toxin type A. Arch Dermatol. 2009;145:13-15.
To the Editor:
Epidermolysis bullosa (EB) was first described in 1886, with the first classification scheme proposed in 1962 utilizing transmission electron microscopy (TEM) findings to delineate categories: epidermolytic (EB simplex [EBS]), lucidolytic (junctional EB), and dermolytic (dystrophic EB).1 Localized EBS (EBS-loc) is an autosomal-dominant disorder caused by negative mutations in keratin-5 and keratin-14, proteins expressed in the intermediate filaments of basal keratinocytes, which result in fragility of the skin in response to minor trauma.2 The incidence of EBS-loc is approximately 10 to 30 cases per million live births, with the age of presentation typically between the first and third decades of life.3,4 Because EBS-loc is the most common and often mildest form of EB, not all patients present for medical evaluation and true prevalence may be underestimated.4 We report a case of EBS-loc.
A 26-year-old woman with no notable medical history presented to the dermatology clinic for evaluation of skin blisters that had been intermittently present since infancy. The blisters primarily occurred on the feet, but she did occasionally develop blisters on the hands, knees, and elbows and at sites of friction or trauma (eg, bra line, medial thighs) following exercise. The blisters were worsened by heat and tight-fitting shoes. Because of the painful nature of the blisters, she would lance them with a needle. On the medial thighs, she utilized nonstick and gauze bandage roll dressings to minimize friction. A review of systems was positive for hyperhidrosis. Her family history revealed multiple family members with blisters involving the feet and areas of friction or trauma for 4 generations with no known diagnosis.
Physical examination revealed multiple tense bullae and calluses scattered over the bilateral plantar and distal dorsal feet with a few healing, superficially eroded, erythematous papules and plaques on the bilateral medial thighs (Figure 1). A biopsy from an induced blister on the right dorsal second toe was performed and sent in glutaraldehyde to the Epidermolysis Bullosa Clinic at Stanford University (Redwood City, California) for electron microscopy, which revealed lysis within the basal keratinocytes through the tonofilaments with continuous and intact lamina densa and lamina lucida (Figure 2). In this clinical context with the relevant family history, the findings were consistent with the diagnosis of EBS-loc (formerly Weber-Cockayne syndrome).2


Skin manifestations of EBS-loc typically consist of friction-induced blisters, erosions, and calluses primarily on the palms and soles, often associated with hyperhidrosis and worsening of symptoms in summer months and hot temperatures.3 Milia, atrophic scarring, and dystrophic nails are uncommon.1 Extracutaneous involvement is rare with the exception of oral cavity erosions, which typically are asymptomatic and usually are only seen during infancy.1
Light microscopy does not have a notable role in diagnosis of classic forms of inherited EB unless another autoimmune blistering disorder is suspected.2,5 Both TEM and immunofluorescence mapping are used to diagnose EB.1 DNA mutational analysis is not considered a first-line diagnostic test for EB given it is a costly labor-intensive technique with limited access at present, but it may be considered in settings of prenatal diagnosis or in vitro fertilization.1 Biopsy of a freshly induced blister should be performed, as early reepithelialization of an existing blister makes it difficult to establish the level of cleavage.5 Applying firm pressure using a pencil eraser and rotating it on intact skin induces a subclinical blister. Two punch biopsies (4 mm) at the edge of the blister with one-third lesional and two-thirds perilesional skin should be obtained, with one biopsy sent for immunofluorescence mapping in Michel fixative and the other for TEM in glutaraldehyde.3,5 Transmission electron microscopy of an induced blister in EBS-loc shows cleavage within the most inferior portion of the basilar keratinocyte.2 Immunofluorescence mapping with anti–epidermal basement membrane monoclonal antibodies can distinguish between EB subtypes and assess expression of specific skin-associated proteins on both a qualitative or semiquantitative basis, providing insight on which structural protein is mutated.1,5
No specific treatments are available for EBS-loc. Mainstays of treatment include prevention of mechanical trauma and secondary infection. Hyperhidrosis of thepalms and soles may be treated with topical aluminum chloride hexahydrate or injections of botulinum toxin type A.2,6 Patients have normal life expectancy, though some cases may have complications with substantial morbidity.1 Awareness of this disease, its clinical course, and therapeutic options will allow physicians to more appropriately counsel patients on the disease process.
Localized EBS may be more common than previously thought, as not all patients seek medical care. Given its impact on patient quality of life, it is important for clinicians to recognize EBS-loc. Although no specific treatments are available, wound care counseling and explanation of the genetics of the disease should be provided to patients.
To the Editor:
Epidermolysis bullosa (EB) was first described in 1886, with the first classification scheme proposed in 1962 utilizing transmission electron microscopy (TEM) findings to delineate categories: epidermolytic (EB simplex [EBS]), lucidolytic (junctional EB), and dermolytic (dystrophic EB).1 Localized EBS (EBS-loc) is an autosomal-dominant disorder caused by negative mutations in keratin-5 and keratin-14, proteins expressed in the intermediate filaments of basal keratinocytes, which result in fragility of the skin in response to minor trauma.2 The incidence of EBS-loc is approximately 10 to 30 cases per million live births, with the age of presentation typically between the first and third decades of life.3,4 Because EBS-loc is the most common and often mildest form of EB, not all patients present for medical evaluation and true prevalence may be underestimated.4 We report a case of EBS-loc.
A 26-year-old woman with no notable medical history presented to the dermatology clinic for evaluation of skin blisters that had been intermittently present since infancy. The blisters primarily occurred on the feet, but she did occasionally develop blisters on the hands, knees, and elbows and at sites of friction or trauma (eg, bra line, medial thighs) following exercise. The blisters were worsened by heat and tight-fitting shoes. Because of the painful nature of the blisters, she would lance them with a needle. On the medial thighs, she utilized nonstick and gauze bandage roll dressings to minimize friction. A review of systems was positive for hyperhidrosis. Her family history revealed multiple family members with blisters involving the feet and areas of friction or trauma for 4 generations with no known diagnosis.
Physical examination revealed multiple tense bullae and calluses scattered over the bilateral plantar and distal dorsal feet with a few healing, superficially eroded, erythematous papules and plaques on the bilateral medial thighs (Figure 1). A biopsy from an induced blister on the right dorsal second toe was performed and sent in glutaraldehyde to the Epidermolysis Bullosa Clinic at Stanford University (Redwood City, California) for electron microscopy, which revealed lysis within the basal keratinocytes through the tonofilaments with continuous and intact lamina densa and lamina lucida (Figure 2). In this clinical context with the relevant family history, the findings were consistent with the diagnosis of EBS-loc (formerly Weber-Cockayne syndrome).2


Skin manifestations of EBS-loc typically consist of friction-induced blisters, erosions, and calluses primarily on the palms and soles, often associated with hyperhidrosis and worsening of symptoms in summer months and hot temperatures.3 Milia, atrophic scarring, and dystrophic nails are uncommon.1 Extracutaneous involvement is rare with the exception of oral cavity erosions, which typically are asymptomatic and usually are only seen during infancy.1
Light microscopy does not have a notable role in diagnosis of classic forms of inherited EB unless another autoimmune blistering disorder is suspected.2,5 Both TEM and immunofluorescence mapping are used to diagnose EB.1 DNA mutational analysis is not considered a first-line diagnostic test for EB given it is a costly labor-intensive technique with limited access at present, but it may be considered in settings of prenatal diagnosis or in vitro fertilization.1 Biopsy of a freshly induced blister should be performed, as early reepithelialization of an existing blister makes it difficult to establish the level of cleavage.5 Applying firm pressure using a pencil eraser and rotating it on intact skin induces a subclinical blister. Two punch biopsies (4 mm) at the edge of the blister with one-third lesional and two-thirds perilesional skin should be obtained, with one biopsy sent for immunofluorescence mapping in Michel fixative and the other for TEM in glutaraldehyde.3,5 Transmission electron microscopy of an induced blister in EBS-loc shows cleavage within the most inferior portion of the basilar keratinocyte.2 Immunofluorescence mapping with anti–epidermal basement membrane monoclonal antibodies can distinguish between EB subtypes and assess expression of specific skin-associated proteins on both a qualitative or semiquantitative basis, providing insight on which structural protein is mutated.1,5
No specific treatments are available for EBS-loc. Mainstays of treatment include prevention of mechanical trauma and secondary infection. Hyperhidrosis of thepalms and soles may be treated with topical aluminum chloride hexahydrate or injections of botulinum toxin type A.2,6 Patients have normal life expectancy, though some cases may have complications with substantial morbidity.1 Awareness of this disease, its clinical course, and therapeutic options will allow physicians to more appropriately counsel patients on the disease process.
Localized EBS may be more common than previously thought, as not all patients seek medical care. Given its impact on patient quality of life, it is important for clinicians to recognize EBS-loc. Although no specific treatments are available, wound care counseling and explanation of the genetics of the disease should be provided to patients.
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931-950.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Limited; 2012.
- Eichenfield LF, Frieden IJ, Mathes EF, et al, eds. Neonatal and Infant Dermatology. 3rd ed. New York, NY: Elsevier Health Sciences; 2015.
- Spitz JL. Genodermatoses: A Clinical Guide to Genetic Skin Disorders. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Epidermolysis bullosa. Stanford Medicine website. http://med.stanford.edu/dermatopathology/dermpath-services/epiderm.html. Accessed April 3, 2019.
- Abitbol RJ, Zhou LH. Treatment of epidermolysis bullosa simplex, Weber-Cockayne type, with botulinum toxin type A. Arch Dermatol. 2009;145:13-15.
- Fine JD, Eady RA, Bauer EA, et al. The classification of inherited epidermolysis bullosa (EB): report of the Third International Consensus Meeting on Diagnosis and Classification of EB. J Am Acad Dermatol. 2008;58:931-950.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Limited; 2012.
- Eichenfield LF, Frieden IJ, Mathes EF, et al, eds. Neonatal and Infant Dermatology. 3rd ed. New York, NY: Elsevier Health Sciences; 2015.
- Spitz JL. Genodermatoses: A Clinical Guide to Genetic Skin Disorders. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Epidermolysis bullosa. Stanford Medicine website. http://med.stanford.edu/dermatopathology/dermpath-services/epiderm.html. Accessed April 3, 2019.
- Abitbol RJ, Zhou LH. Treatment of epidermolysis bullosa simplex, Weber-Cockayne type, with botulinum toxin type A. Arch Dermatol. 2009;145:13-15.
Practice Points
- Localized epidermolysis bullosa simplex (formerly Weber-Cockayne syndrome) presents with flaccid bullae and erosions predominantly on the hands and feet, most commonly related to mechanical friction and heat.
- It is inherited in an autosomal-dominant fashion with defects in keratin-5 and keratin-14.
- Biopsy of a freshly induced blister should be examined by transmission electron microscopy or immunofluorescence mapping.
- Treatment is focused on wound management and infection control of the blisters.
Scurvy Masquerading as Reactive Arthritis
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.

Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.

Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.

Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
Practice Points
- Patients with scurvy often pose a diagnostic dilemma because their presenting symptoms can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups.
- The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency with rapid resolution upon vitamin C supplementation.
Pigmented Fungiform Papillae of the Tongue in an Indian Male
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.
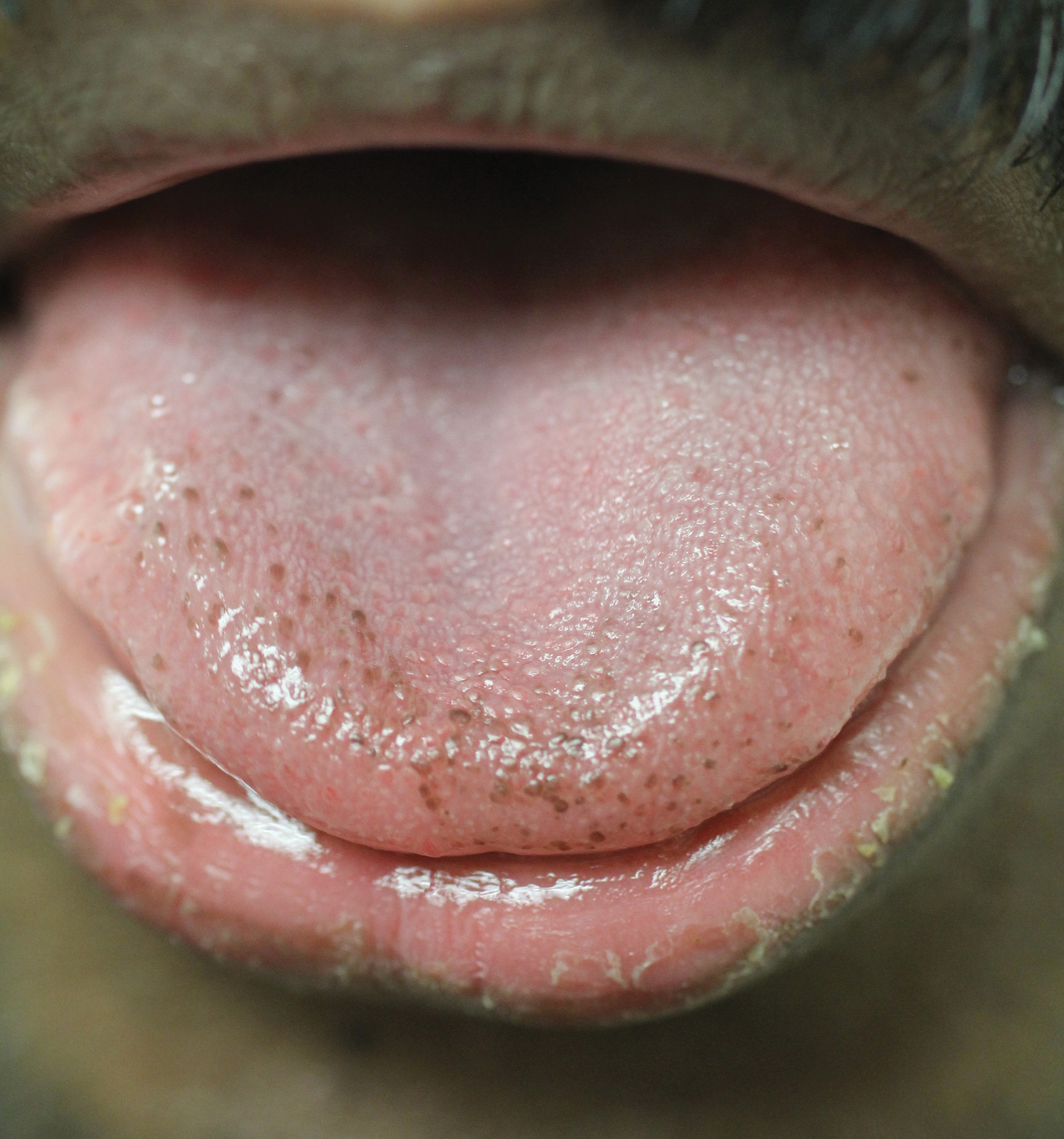
Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.

Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.

Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
Practice Points
- Pigmented fungiform papillae of the tongue are common lingual hyperpigmented macules in patients with skin of color.
- It is important to be aware of this benign entity to provide reassurance to patients and avoid unnecessary testing.
Prurigo Pigmentosa Induced by Ketosis: Resolution Through Dietary Modification
To the Editor:
A 40-year-old white woman presented with a waxing and waning erythematous pruritic rash on the chest, back, and axillae of 3 years’ duration. The appearance of the rash coincided with an intentional weight loss of more than 100 lb, achieved through various diets, most recently a Paleolithic (paleo) diet that was high in protein; low in carbohydrates; and specifically restricted dairy, cereal grains, refined sugars, processed foods, white potatoes, salt, refined oils, and legumes.1 The patient had been monitoring blood glucose and ketone levels. Prior to presentation, she received various treatments including clotrimazole cream and topical steroids with no improvement.
On physical examination, there were scaly, pink-red, reticulated papules and plaques coexisting with tan reticulated patches that were symmetrically distributed on the central back, lateral and central chest (Figure 1A), breasts, and inframammary areas. During the most severe flare-up, the blood ketones measured 1 mmol/L. There was no relevant medical history. She was of Spanish and Italian descent.
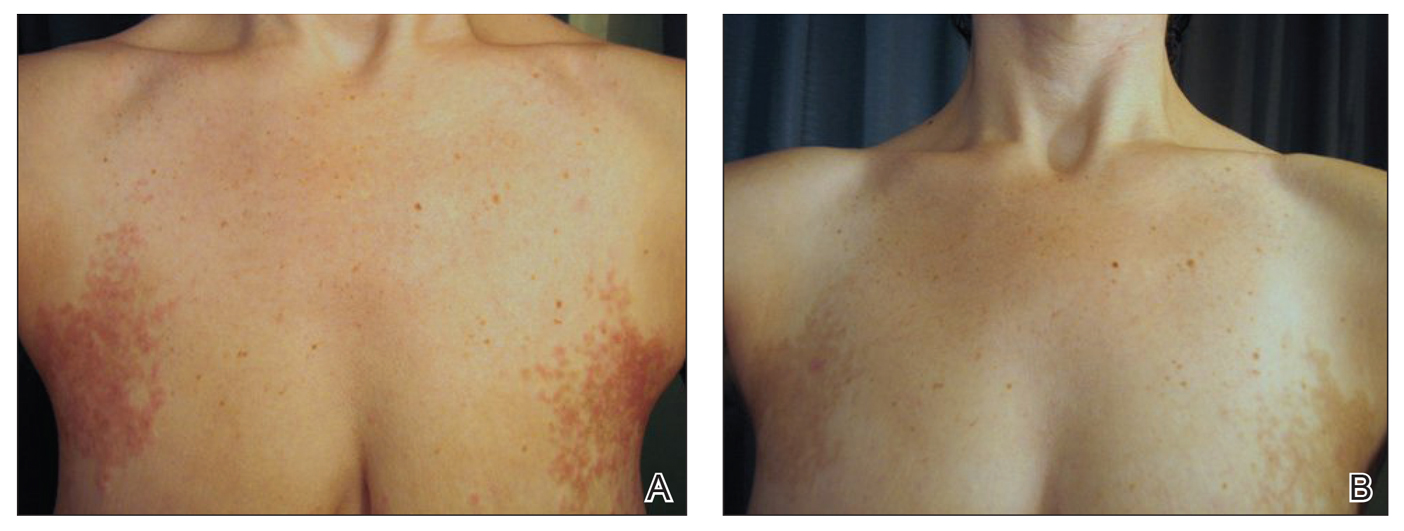
Histologic sections showed a sparse infiltrate of lymphocytes surrounding superficial dermal vessels and a mildly acanthotic epidermis with a focally parakeratotic stratum corneum (Figure 2A). Pigmentary incontinence and subtle interface changes were apparent, including rare necrotic keratinocytes (Figure 2B). No eosinophils or neutrophils were present.
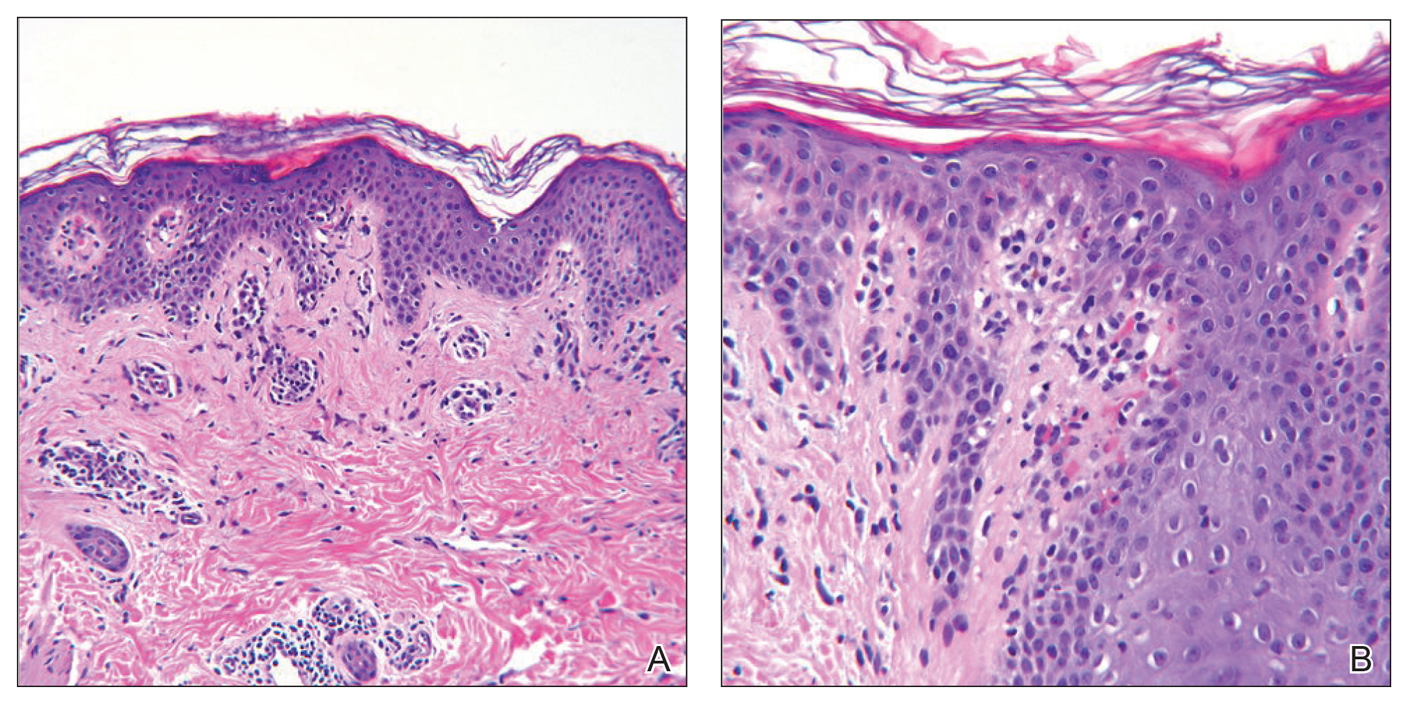
After the initial presentation, carbohydrates were added back into her diet and both the ketosis and eruption remarkably resolved. When carbohydrate restriction was rechallenged, she again entered ketosis (0.5 mmol/L), followed by subsequent recurrence of the pruritic lesions. With re-introduction of carbohydrates, the eruption and ketosis once more resolved, leaving only postinflammatory reticulated hyperpigmentation (Figure 1B). Based on the clinical presentation, supportive histopathologic findings, and interesting response to ketones and diet modification, the patient was diagnosed with prurigo pigmentosa (PP).
Prurigo pigmentosa is a rare inflammatory dermatosis that was initially described in 1971 as “a peculiar pruriginous dermatosis with gross reticular pigmentation” by Nagashima et al.2 Prurigo pigmentosa is most frequently diagnosed in Japan, and since its discovery, it has been reported in more than 300 cases worldwide.2-4
Fewer than 50 non-Japanese cases have been reported, with the possibility of an additional ethnic predisposition among the Turkish and Sicilian populations, though only 6 cases have been reported in the United States.3-6 Prurigo pigmentosa tends to occur in the spring and summer months and is most common among young females, with a mean age of 24 years. The typical lesions of PP are symmetrically distributed on the trunk with a tendency to localize on the upper back, nape of the neck, and intermammary and inframammary regions. Eruptions have been reported to occur on additional areas; however, mucus membranes are always spared.6
Individual lesions differ in appearance depending on the stage of presentation and are categorized as early, fully developed, resolving, and late lesions.6 Pruritic macules and papules are present early in the disease state and resolve into crusted and/or scaly papules followed by pigmented macules. Early lesions tend to be intensely pruritic with signs of excoriation, while resolving lesions lack symptoms. Lesions last approximately 1 week but tend to reappear at the site where they were previously present, which allows for lesions of different ages to coexist, appearing in a reticular arrangement with hyperpigmented mottling lasting from a few weeks to months.6
Just as the clinical picture transpires rapidly within 1 week, so do the histopathologic findings.6 Early lesions are categorized by a superficial perivascular and interstitial infiltrate of neutrophils, spongiosis, ballooning, and necrotic keratinocytes. These early lesions are present for less than 48 hours, and these histopathologic findings are diagnostic of PP. Within 2 days, lymphocytes predominate in the dermal infiltrate, and a patchy lichenoid aspect is established in the fully developed lesion along with reticular and vacuolar alterations. Late lesions show a parakeratotic and hyperpigmented epidermis with melanophages present in the papillary and reticular dermis. At this last stage, the histopathologic features of PP are indistinguishable from any other disease that results in postinflammatory hyperpigmentation, making diagnosis difficult.6
A variety of therapeutic options are used in the treatment of PP, with the most effective agents being oral antibiotics including dapsone, minocycline, and doxycycline, all of which limit the local tissue inflammatory response and cytotoxic effects. Topical and systemic antihistamines as well as corticosteroids are ineffective and have not been shown to prevent the postinflammatory reticular pigmentation.6-10
Various underlying factors have been associated with PP, including friction, heat, sunlight, sweating, allergic contact sensitization, and ketosis due to nutritional deficiency or diabetes mellitus; however; the exact etiology remains ambiguous.2-7 The association with ketosis and nutrition is of particular interest in this case. Onset of PP has been reported to coincide with dieting, fasting, weight loss, anorexia nervosa, and diabetes mellitus.3,6-9 Roughly 50 patients with PP had ketosis subsequent to these metabolic disturbances.3,6-10 As of now, the only reported correlation between ketosis and PP is that upon diet modification, lesions resolved following ketone normalization, as was observed in our patient.3,6-8 Reports of PP in diabetic patients while in ketoacidosis describe resolution of lesions with insulin administration.6-9 The pathophysiology of ketosis and its association with PP is unclear; however, the similarities seen in the immune response of PP and that stimulated by ketosis may expose an associated mechanism.
Ketosis is a temporary condition characterized by elevated serum ketones that are used as an alternative energy source when blood glucose is low or insulin is deficient.11 The most common causes of ketosis are the physiologic responses to fasting, prolonged exercise, or a high-protein/low-carbohydrate diet, though pathologic causes include insulin-dependent diabetes mellitus, alcoholism, and salicylate overdose.11 In healthy individuals, blood ketone levels rarely approach 0.5 mmol/L. Prolonged fasting or restricting intake of carbohydrates to less than 40 g daily can induce mild ketosis that resolves with re-introduction of carbohydrates.11
Ketone bodies pass from the circulating blood into tissues or remain near the blood vessels, inducing cytotoxic effects and perivascular inflammation.10,11 Increased ketone bodies have been shown to upregulate intercellular adhesion molecule 1 (ICAM-1) and leukocyte function-associated antigen 1 (LFA-1), a phenomenon also seen in lesional keratinocytes of PP.12,13 Teraki et al13 observed that epidermal keratinocytes exhibited increased expression of ICAM-1 as well as intense expression of LFA-1 on dermal and epidermotropic leukocytes, which was thought to be due to cell-mediated cytotoxicity. Not only do increased ketone bodies upregulate ICAM-1 and LFA-1, but they also are involved in increasing many proinflammatory mediators that may be capable of inducing the response seen in PP keratinocytes.12,13
Intercellular adhesion molecule 1 is important in initiating cellular interactions in the immune response and is the ligand for LFA-1 found on most leukocytes.14 Increased ICAM-1/LFA-1 interaction is thought to be the major pathway by which leukocytes are able to attach to keratinocytes and endothelial cells, allowing for leukocyte tissue migration and specific immunologic reactions, including leukocyte-mediated cytotoxicity. Interestingly, glucocorticoids are ineffective in reducing the expression of ICAM-1 in cultured keratinocytes.14 This connection between ketosis and inflammation that results in leukocyte migration and ultimately keratinocyte cytotoxicity may well be fundamental to the pathophysiology of PP and may provide a possible explanation for the ineffectiveness of corticosteroid treatment.
Middleton and Norris15 observed that individual keratinocyte strains show considerable variability in ICAM-1 expression that was found to be attributable to genetic polymorphisms. The presence of a particular polymorphism affecting ICAM-1 expression on human keratinocytes may explain the apparent ethnogeographic predisposition of PP as well as the ease at which ICAM-1 is expressed in the presence of ketones.
We describe a case of a 40-year-old white woman who was diagnosed with PP that was prompted by a 100-lb weight loss and self-induced ketosis while following a paleo diet with carbohydrate restriction. Successful treatment was attained through diet modification alone. This interesting case was another instance in which the pathophysiology of PP was attributed to ketosis. Because not all patients that are in ketosis have PP, larger prospective cohort studies are needed to further elucidate the association of PP and ketosis.
- What is the paleo diet? The Paleo Diet website. http://thepaleodiet.com/the-paleo-diet-premise. Accessed March 9, 2019.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation [in Japanese]. Japanese J Dermatol. 1971;81:38-39.
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet [published online December 30, 2013]. Pediatr Dermatol. 2015;32:248-251.
- Baykal C, Buyukbabani N, Akinturk S, et al. Prurigo pigmentosa: not an uncommon disease in the Turkish population. Int J Dermatol. 2006;45:1164-1168.
- Whang T, Kirkorian Y, Krishtul A, et al. Prurigo pigmentosa: report of two cases in the United States and review of the literature. Dermatology Online J. 2011;17:2.
- Böer A, Ackerman AB. Prurigo Pigmentosa (Nagashima Disease): Textbook and Atlas of a Distinctive Inflammatory Disease of the Skin. New York, NY: Ardor Scribendi Ltd; 2004.
- Teraki Y, Teraki E, Kawashima M, at al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511.
- Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2011;26:1149-1153.
- Yokozeki M, Watanabe J, Hotsubo T, et al. Prurigo pigmentosa disappeared following improvement of diabetic ketosis by insulin. J Dermatol. 2003;30:257-258.
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
- VanItallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Annu Rev Nutr. 2003;61:327-341.
- Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:e298-e306.
- Teraki Y, Shiohara T, Nagashima M, et al. Prurigo pigmentosa: role of ICAM-1 in the localization of the eruption. Br J Dermatol. 1991;125:360-363.
- Kashihara-Sawami M, Norris DA. The state of differentiation of cultured human keratinocytes determines the level of intercellular adhesion molecule-1 (ICAM-1) expression induced by gamma interferon. J Invest Dermatol. 1992;98:741-747.
- Middleton MH, Norris DA. Cytokine-induced ICAM-1 expression in human keratinocytes is highly variable in keratinocyte strains from different donors. J Invest Dermatol. 1995;104:489-496.
To the Editor:
A 40-year-old white woman presented with a waxing and waning erythematous pruritic rash on the chest, back, and axillae of 3 years’ duration. The appearance of the rash coincided with an intentional weight loss of more than 100 lb, achieved through various diets, most recently a Paleolithic (paleo) diet that was high in protein; low in carbohydrates; and specifically restricted dairy, cereal grains, refined sugars, processed foods, white potatoes, salt, refined oils, and legumes.1 The patient had been monitoring blood glucose and ketone levels. Prior to presentation, she received various treatments including clotrimazole cream and topical steroids with no improvement.
On physical examination, there were scaly, pink-red, reticulated papules and plaques coexisting with tan reticulated patches that were symmetrically distributed on the central back, lateral and central chest (Figure 1A), breasts, and inframammary areas. During the most severe flare-up, the blood ketones measured 1 mmol/L. There was no relevant medical history. She was of Spanish and Italian descent.

Histologic sections showed a sparse infiltrate of lymphocytes surrounding superficial dermal vessels and a mildly acanthotic epidermis with a focally parakeratotic stratum corneum (Figure 2A). Pigmentary incontinence and subtle interface changes were apparent, including rare necrotic keratinocytes (Figure 2B). No eosinophils or neutrophils were present.

After the initial presentation, carbohydrates were added back into her diet and both the ketosis and eruption remarkably resolved. When carbohydrate restriction was rechallenged, she again entered ketosis (0.5 mmol/L), followed by subsequent recurrence of the pruritic lesions. With re-introduction of carbohydrates, the eruption and ketosis once more resolved, leaving only postinflammatory reticulated hyperpigmentation (Figure 1B). Based on the clinical presentation, supportive histopathologic findings, and interesting response to ketones and diet modification, the patient was diagnosed with prurigo pigmentosa (PP).
Prurigo pigmentosa is a rare inflammatory dermatosis that was initially described in 1971 as “a peculiar pruriginous dermatosis with gross reticular pigmentation” by Nagashima et al.2 Prurigo pigmentosa is most frequently diagnosed in Japan, and since its discovery, it has been reported in more than 300 cases worldwide.2-4
Fewer than 50 non-Japanese cases have been reported, with the possibility of an additional ethnic predisposition among the Turkish and Sicilian populations, though only 6 cases have been reported in the United States.3-6 Prurigo pigmentosa tends to occur in the spring and summer months and is most common among young females, with a mean age of 24 years. The typical lesions of PP are symmetrically distributed on the trunk with a tendency to localize on the upper back, nape of the neck, and intermammary and inframammary regions. Eruptions have been reported to occur on additional areas; however, mucus membranes are always spared.6
Individual lesions differ in appearance depending on the stage of presentation and are categorized as early, fully developed, resolving, and late lesions.6 Pruritic macules and papules are present early in the disease state and resolve into crusted and/or scaly papules followed by pigmented macules. Early lesions tend to be intensely pruritic with signs of excoriation, while resolving lesions lack symptoms. Lesions last approximately 1 week but tend to reappear at the site where they were previously present, which allows for lesions of different ages to coexist, appearing in a reticular arrangement with hyperpigmented mottling lasting from a few weeks to months.6
Just as the clinical picture transpires rapidly within 1 week, so do the histopathologic findings.6 Early lesions are categorized by a superficial perivascular and interstitial infiltrate of neutrophils, spongiosis, ballooning, and necrotic keratinocytes. These early lesions are present for less than 48 hours, and these histopathologic findings are diagnostic of PP. Within 2 days, lymphocytes predominate in the dermal infiltrate, and a patchy lichenoid aspect is established in the fully developed lesion along with reticular and vacuolar alterations. Late lesions show a parakeratotic and hyperpigmented epidermis with melanophages present in the papillary and reticular dermis. At this last stage, the histopathologic features of PP are indistinguishable from any other disease that results in postinflammatory hyperpigmentation, making diagnosis difficult.6
A variety of therapeutic options are used in the treatment of PP, with the most effective agents being oral antibiotics including dapsone, minocycline, and doxycycline, all of which limit the local tissue inflammatory response and cytotoxic effects. Topical and systemic antihistamines as well as corticosteroids are ineffective and have not been shown to prevent the postinflammatory reticular pigmentation.6-10
Various underlying factors have been associated with PP, including friction, heat, sunlight, sweating, allergic contact sensitization, and ketosis due to nutritional deficiency or diabetes mellitus; however; the exact etiology remains ambiguous.2-7 The association with ketosis and nutrition is of particular interest in this case. Onset of PP has been reported to coincide with dieting, fasting, weight loss, anorexia nervosa, and diabetes mellitus.3,6-9 Roughly 50 patients with PP had ketosis subsequent to these metabolic disturbances.3,6-10 As of now, the only reported correlation between ketosis and PP is that upon diet modification, lesions resolved following ketone normalization, as was observed in our patient.3,6-8 Reports of PP in diabetic patients while in ketoacidosis describe resolution of lesions with insulin administration.6-9 The pathophysiology of ketosis and its association with PP is unclear; however, the similarities seen in the immune response of PP and that stimulated by ketosis may expose an associated mechanism.
Ketosis is a temporary condition characterized by elevated serum ketones that are used as an alternative energy source when blood glucose is low or insulin is deficient.11 The most common causes of ketosis are the physiologic responses to fasting, prolonged exercise, or a high-protein/low-carbohydrate diet, though pathologic causes include insulin-dependent diabetes mellitus, alcoholism, and salicylate overdose.11 In healthy individuals, blood ketone levels rarely approach 0.5 mmol/L. Prolonged fasting or restricting intake of carbohydrates to less than 40 g daily can induce mild ketosis that resolves with re-introduction of carbohydrates.11
Ketone bodies pass from the circulating blood into tissues or remain near the blood vessels, inducing cytotoxic effects and perivascular inflammation.10,11 Increased ketone bodies have been shown to upregulate intercellular adhesion molecule 1 (ICAM-1) and leukocyte function-associated antigen 1 (LFA-1), a phenomenon also seen in lesional keratinocytes of PP.12,13 Teraki et al13 observed that epidermal keratinocytes exhibited increased expression of ICAM-1 as well as intense expression of LFA-1 on dermal and epidermotropic leukocytes, which was thought to be due to cell-mediated cytotoxicity. Not only do increased ketone bodies upregulate ICAM-1 and LFA-1, but they also are involved in increasing many proinflammatory mediators that may be capable of inducing the response seen in PP keratinocytes.12,13
Intercellular adhesion molecule 1 is important in initiating cellular interactions in the immune response and is the ligand for LFA-1 found on most leukocytes.14 Increased ICAM-1/LFA-1 interaction is thought to be the major pathway by which leukocytes are able to attach to keratinocytes and endothelial cells, allowing for leukocyte tissue migration and specific immunologic reactions, including leukocyte-mediated cytotoxicity. Interestingly, glucocorticoids are ineffective in reducing the expression of ICAM-1 in cultured keratinocytes.14 This connection between ketosis and inflammation that results in leukocyte migration and ultimately keratinocyte cytotoxicity may well be fundamental to the pathophysiology of PP and may provide a possible explanation for the ineffectiveness of corticosteroid treatment.
Middleton and Norris15 observed that individual keratinocyte strains show considerable variability in ICAM-1 expression that was found to be attributable to genetic polymorphisms. The presence of a particular polymorphism affecting ICAM-1 expression on human keratinocytes may explain the apparent ethnogeographic predisposition of PP as well as the ease at which ICAM-1 is expressed in the presence of ketones.
We describe a case of a 40-year-old white woman who was diagnosed with PP that was prompted by a 100-lb weight loss and self-induced ketosis while following a paleo diet with carbohydrate restriction. Successful treatment was attained through diet modification alone. This interesting case was another instance in which the pathophysiology of PP was attributed to ketosis. Because not all patients that are in ketosis have PP, larger prospective cohort studies are needed to further elucidate the association of PP and ketosis.
To the Editor:
A 40-year-old white woman presented with a waxing and waning erythematous pruritic rash on the chest, back, and axillae of 3 years’ duration. The appearance of the rash coincided with an intentional weight loss of more than 100 lb, achieved through various diets, most recently a Paleolithic (paleo) diet that was high in protein; low in carbohydrates; and specifically restricted dairy, cereal grains, refined sugars, processed foods, white potatoes, salt, refined oils, and legumes.1 The patient had been monitoring blood glucose and ketone levels. Prior to presentation, she received various treatments including clotrimazole cream and topical steroids with no improvement.
On physical examination, there were scaly, pink-red, reticulated papules and plaques coexisting with tan reticulated patches that were symmetrically distributed on the central back, lateral and central chest (Figure 1A), breasts, and inframammary areas. During the most severe flare-up, the blood ketones measured 1 mmol/L. There was no relevant medical history. She was of Spanish and Italian descent.

Histologic sections showed a sparse infiltrate of lymphocytes surrounding superficial dermal vessels and a mildly acanthotic epidermis with a focally parakeratotic stratum corneum (Figure 2A). Pigmentary incontinence and subtle interface changes were apparent, including rare necrotic keratinocytes (Figure 2B). No eosinophils or neutrophils were present.

After the initial presentation, carbohydrates were added back into her diet and both the ketosis and eruption remarkably resolved. When carbohydrate restriction was rechallenged, she again entered ketosis (0.5 mmol/L), followed by subsequent recurrence of the pruritic lesions. With re-introduction of carbohydrates, the eruption and ketosis once more resolved, leaving only postinflammatory reticulated hyperpigmentation (Figure 1B). Based on the clinical presentation, supportive histopathologic findings, and interesting response to ketones and diet modification, the patient was diagnosed with prurigo pigmentosa (PP).
Prurigo pigmentosa is a rare inflammatory dermatosis that was initially described in 1971 as “a peculiar pruriginous dermatosis with gross reticular pigmentation” by Nagashima et al.2 Prurigo pigmentosa is most frequently diagnosed in Japan, and since its discovery, it has been reported in more than 300 cases worldwide.2-4
Fewer than 50 non-Japanese cases have been reported, with the possibility of an additional ethnic predisposition among the Turkish and Sicilian populations, though only 6 cases have been reported in the United States.3-6 Prurigo pigmentosa tends to occur in the spring and summer months and is most common among young females, with a mean age of 24 years. The typical lesions of PP are symmetrically distributed on the trunk with a tendency to localize on the upper back, nape of the neck, and intermammary and inframammary regions. Eruptions have been reported to occur on additional areas; however, mucus membranes are always spared.6
Individual lesions differ in appearance depending on the stage of presentation and are categorized as early, fully developed, resolving, and late lesions.6 Pruritic macules and papules are present early in the disease state and resolve into crusted and/or scaly papules followed by pigmented macules. Early lesions tend to be intensely pruritic with signs of excoriation, while resolving lesions lack symptoms. Lesions last approximately 1 week but tend to reappear at the site where they were previously present, which allows for lesions of different ages to coexist, appearing in a reticular arrangement with hyperpigmented mottling lasting from a few weeks to months.6
Just as the clinical picture transpires rapidly within 1 week, so do the histopathologic findings.6 Early lesions are categorized by a superficial perivascular and interstitial infiltrate of neutrophils, spongiosis, ballooning, and necrotic keratinocytes. These early lesions are present for less than 48 hours, and these histopathologic findings are diagnostic of PP. Within 2 days, lymphocytes predominate in the dermal infiltrate, and a patchy lichenoid aspect is established in the fully developed lesion along with reticular and vacuolar alterations. Late lesions show a parakeratotic and hyperpigmented epidermis with melanophages present in the papillary and reticular dermis. At this last stage, the histopathologic features of PP are indistinguishable from any other disease that results in postinflammatory hyperpigmentation, making diagnosis difficult.6
A variety of therapeutic options are used in the treatment of PP, with the most effective agents being oral antibiotics including dapsone, minocycline, and doxycycline, all of which limit the local tissue inflammatory response and cytotoxic effects. Topical and systemic antihistamines as well as corticosteroids are ineffective and have not been shown to prevent the postinflammatory reticular pigmentation.6-10
Various underlying factors have been associated with PP, including friction, heat, sunlight, sweating, allergic contact sensitization, and ketosis due to nutritional deficiency or diabetes mellitus; however; the exact etiology remains ambiguous.2-7 The association with ketosis and nutrition is of particular interest in this case. Onset of PP has been reported to coincide with dieting, fasting, weight loss, anorexia nervosa, and diabetes mellitus.3,6-9 Roughly 50 patients with PP had ketosis subsequent to these metabolic disturbances.3,6-10 As of now, the only reported correlation between ketosis and PP is that upon diet modification, lesions resolved following ketone normalization, as was observed in our patient.3,6-8 Reports of PP in diabetic patients while in ketoacidosis describe resolution of lesions with insulin administration.6-9 The pathophysiology of ketosis and its association with PP is unclear; however, the similarities seen in the immune response of PP and that stimulated by ketosis may expose an associated mechanism.
Ketosis is a temporary condition characterized by elevated serum ketones that are used as an alternative energy source when blood glucose is low or insulin is deficient.11 The most common causes of ketosis are the physiologic responses to fasting, prolonged exercise, or a high-protein/low-carbohydrate diet, though pathologic causes include insulin-dependent diabetes mellitus, alcoholism, and salicylate overdose.11 In healthy individuals, blood ketone levels rarely approach 0.5 mmol/L. Prolonged fasting or restricting intake of carbohydrates to less than 40 g daily can induce mild ketosis that resolves with re-introduction of carbohydrates.11
Ketone bodies pass from the circulating blood into tissues or remain near the blood vessels, inducing cytotoxic effects and perivascular inflammation.10,11 Increased ketone bodies have been shown to upregulate intercellular adhesion molecule 1 (ICAM-1) and leukocyte function-associated antigen 1 (LFA-1), a phenomenon also seen in lesional keratinocytes of PP.12,13 Teraki et al13 observed that epidermal keratinocytes exhibited increased expression of ICAM-1 as well as intense expression of LFA-1 on dermal and epidermotropic leukocytes, which was thought to be due to cell-mediated cytotoxicity. Not only do increased ketone bodies upregulate ICAM-1 and LFA-1, but they also are involved in increasing many proinflammatory mediators that may be capable of inducing the response seen in PP keratinocytes.12,13
Intercellular adhesion molecule 1 is important in initiating cellular interactions in the immune response and is the ligand for LFA-1 found on most leukocytes.14 Increased ICAM-1/LFA-1 interaction is thought to be the major pathway by which leukocytes are able to attach to keratinocytes and endothelial cells, allowing for leukocyte tissue migration and specific immunologic reactions, including leukocyte-mediated cytotoxicity. Interestingly, glucocorticoids are ineffective in reducing the expression of ICAM-1 in cultured keratinocytes.14 This connection between ketosis and inflammation that results in leukocyte migration and ultimately keratinocyte cytotoxicity may well be fundamental to the pathophysiology of PP and may provide a possible explanation for the ineffectiveness of corticosteroid treatment.
Middleton and Norris15 observed that individual keratinocyte strains show considerable variability in ICAM-1 expression that was found to be attributable to genetic polymorphisms. The presence of a particular polymorphism affecting ICAM-1 expression on human keratinocytes may explain the apparent ethnogeographic predisposition of PP as well as the ease at which ICAM-1 is expressed in the presence of ketones.
We describe a case of a 40-year-old white woman who was diagnosed with PP that was prompted by a 100-lb weight loss and self-induced ketosis while following a paleo diet with carbohydrate restriction. Successful treatment was attained through diet modification alone. This interesting case was another instance in which the pathophysiology of PP was attributed to ketosis. Because not all patients that are in ketosis have PP, larger prospective cohort studies are needed to further elucidate the association of PP and ketosis.
- What is the paleo diet? The Paleo Diet website. http://thepaleodiet.com/the-paleo-diet-premise. Accessed March 9, 2019.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation [in Japanese]. Japanese J Dermatol. 1971;81:38-39.
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet [published online December 30, 2013]. Pediatr Dermatol. 2015;32:248-251.
- Baykal C, Buyukbabani N, Akinturk S, et al. Prurigo pigmentosa: not an uncommon disease in the Turkish population. Int J Dermatol. 2006;45:1164-1168.
- Whang T, Kirkorian Y, Krishtul A, et al. Prurigo pigmentosa: report of two cases in the United States and review of the literature. Dermatology Online J. 2011;17:2.
- Böer A, Ackerman AB. Prurigo Pigmentosa (Nagashima Disease): Textbook and Atlas of a Distinctive Inflammatory Disease of the Skin. New York, NY: Ardor Scribendi Ltd; 2004.
- Teraki Y, Teraki E, Kawashima M, at al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511.
- Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2011;26:1149-1153.
- Yokozeki M, Watanabe J, Hotsubo T, et al. Prurigo pigmentosa disappeared following improvement of diabetic ketosis by insulin. J Dermatol. 2003;30:257-258.
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
- VanItallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Annu Rev Nutr. 2003;61:327-341.
- Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:e298-e306.
- Teraki Y, Shiohara T, Nagashima M, et al. Prurigo pigmentosa: role of ICAM-1 in the localization of the eruption. Br J Dermatol. 1991;125:360-363.
- Kashihara-Sawami M, Norris DA. The state of differentiation of cultured human keratinocytes determines the level of intercellular adhesion molecule-1 (ICAM-1) expression induced by gamma interferon. J Invest Dermatol. 1992;98:741-747.
- Middleton MH, Norris DA. Cytokine-induced ICAM-1 expression in human keratinocytes is highly variable in keratinocyte strains from different donors. J Invest Dermatol. 1995;104:489-496.
- What is the paleo diet? The Paleo Diet website. http://thepaleodiet.com/the-paleo-diet-premise. Accessed March 9, 2019.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation [in Japanese]. Japanese J Dermatol. 1971;81:38-39.
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet [published online December 30, 2013]. Pediatr Dermatol. 2015;32:248-251.
- Baykal C, Buyukbabani N, Akinturk S, et al. Prurigo pigmentosa: not an uncommon disease in the Turkish population. Int J Dermatol. 2006;45:1164-1168.
- Whang T, Kirkorian Y, Krishtul A, et al. Prurigo pigmentosa: report of two cases in the United States and review of the literature. Dermatology Online J. 2011;17:2.
- Böer A, Ackerman AB. Prurigo Pigmentosa (Nagashima Disease): Textbook and Atlas of a Distinctive Inflammatory Disease of the Skin. New York, NY: Ardor Scribendi Ltd; 2004.
- Teraki Y, Teraki E, Kawashima M, at al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511.
- Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2011;26:1149-1153.
- Yokozeki M, Watanabe J, Hotsubo T, et al. Prurigo pigmentosa disappeared following improvement of diabetic ketosis by insulin. J Dermatol. 2003;30:257-258.
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
- VanItallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Annu Rev Nutr. 2003;61:327-341.
- Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:e298-e306.
- Teraki Y, Shiohara T, Nagashima M, et al. Prurigo pigmentosa: role of ICAM-1 in the localization of the eruption. Br J Dermatol. 1991;125:360-363.
- Kashihara-Sawami M, Norris DA. The state of differentiation of cultured human keratinocytes determines the level of intercellular adhesion molecule-1 (ICAM-1) expression induced by gamma interferon. J Invest Dermatol. 1992;98:741-747.
- Middleton MH, Norris DA. Cytokine-induced ICAM-1 expression in human keratinocytes is highly variable in keratinocyte strains from different donors. J Invest Dermatol. 1995;104:489-496.
Practice Points
- Ketosis can be associated with a specific rash known as prurigo pigmentosa (PP).
- Resolution of PP is related to re-introduction of carbohydrates into the diet.
- Consider asking about dietary modifications in patients presenting with a new rash.
Radiographic Changes of Osteomyelitis in a Patient With Periungual Lichen Planus
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.
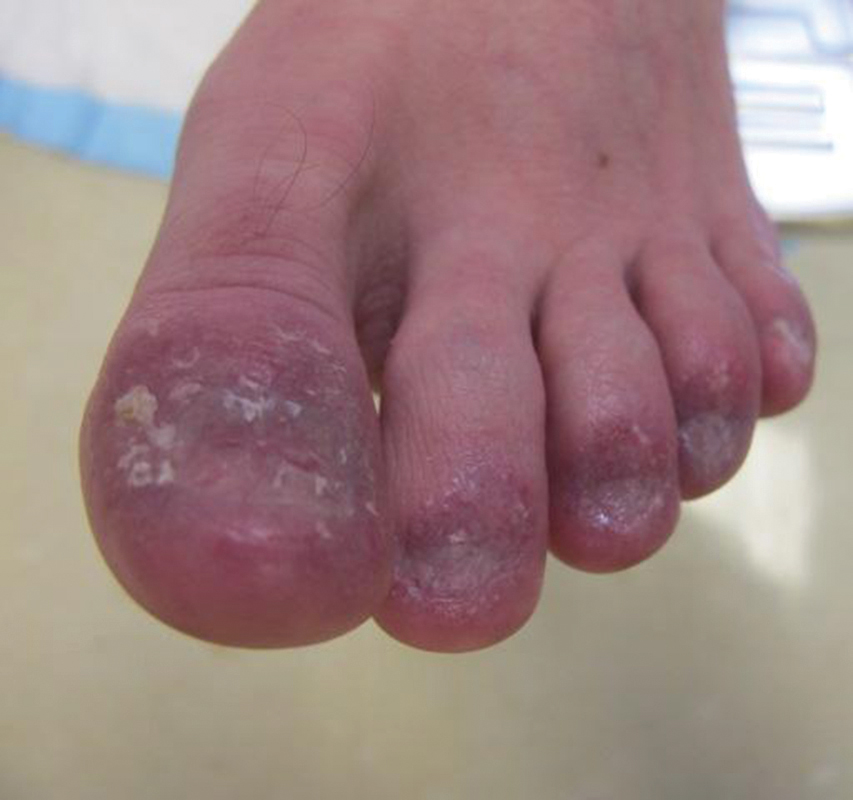
Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.
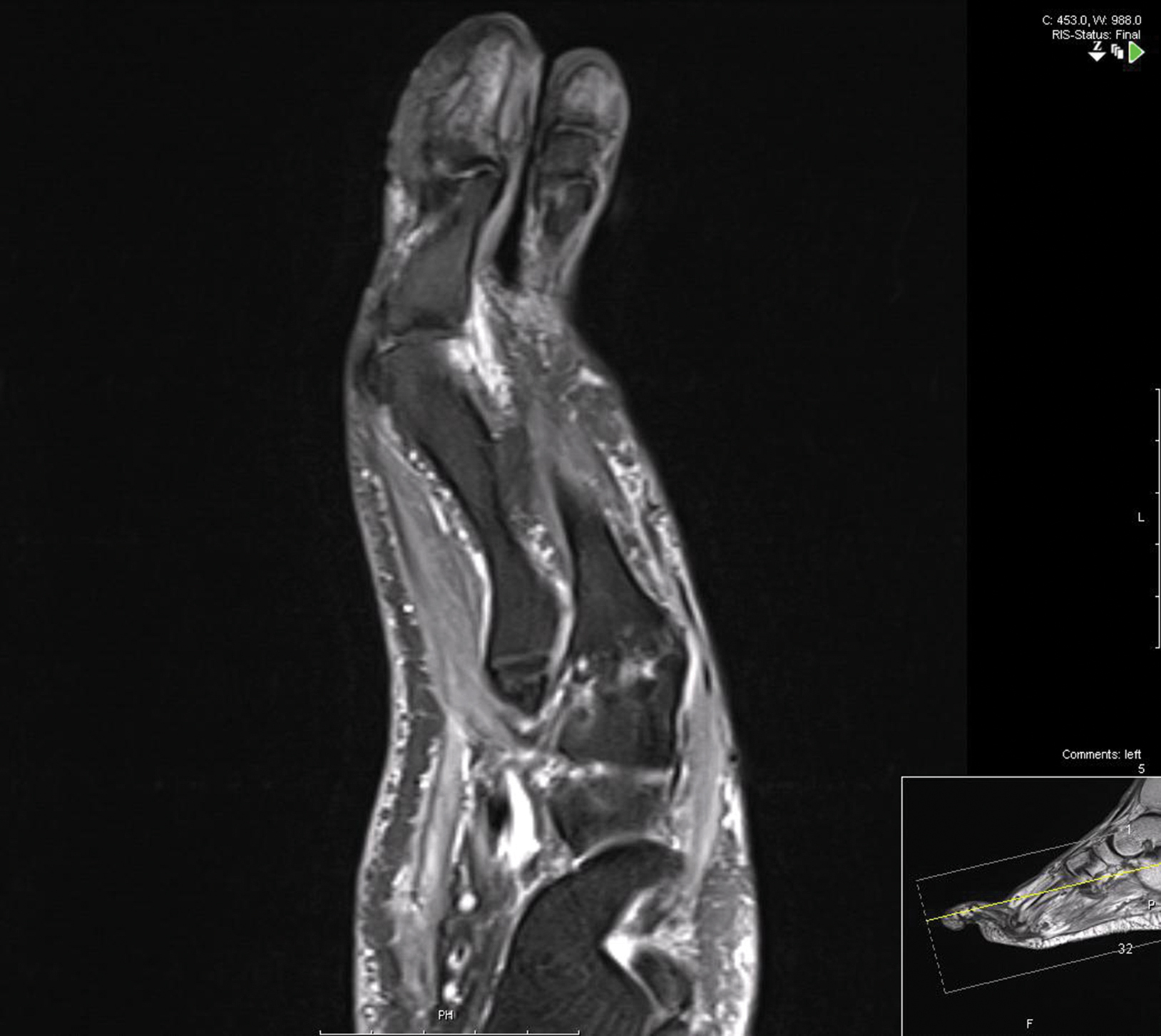
On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.

Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.

On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
To the Editor:
A 60-year-old woman presented for evaluation of a 1-year history of left hallux nail plate dystrophy and proximal nail fold inflammation. Her medical history included Cushing disease with associated uncontrolled diabetes mellitus (DM) and a remote history of cutaneous lichen planus (LP) that resolved 15 years prior to presentation. She noted improvement during intravenous courses of antibiotics for other infections.
Examination of the left hallux revealed onycholysis, loss of the nail plate, and a yellow fibrinous base alongside erosion, erythema, and edema of the proximal toenail fold (Figure 1). The left second toe pad was markedly tender to palpation with scant exudate expressed from underneath the nail bed. Two biopsies of the hallux were performed. The proximal nail fold specimen revealed mild epidermal hyperplasia, and the nail bed demonstrated a nonspecific ulcer that was negative for acid-fast bacilli and fungi.

Treatment over 2 months with cephalexin yielded improvement in both erythema and edema. Initial and repeat nail plate cultures grew ampicillin- and penicillin-sensitive Enterococcus faecalis. Magnetic resonance imaging was performed to evaluate for osteomyelitis because of lack of resolution. Results demonstrated osteomyelitis of the distal tuft of the left hallux and the distal phalanx of the second toe (Figure 2). Vascular surgery evaluation revealed no evidence of large vessel arterial insufficiency. She was started on amoxicillin for superficial Enterococcus and ciprofloxacin for underlying enteric bacilli. The persistence of infection was attributed to microvascular disease secondary to the patient's associated DM. Months later, due to suspected worsening of osteomyelitis, she underwent treatment with oral fluconazole to cover potential fungal co-infection and intravenous vancomycin and piperacillin-tazobactam for broad-spectrum antibacterial coverage. She was eventually transitioned to antimicrobial agents including amoxicillin-clavulanate potassium and topical mupirocin with improvement in periungual erythema and edema.

On subsequent dermatologic evaluation after 1 month, she presented with pterygium and loss of all nail plates on the left foot. The nail bed now had a violaceous color and was studded with milia. The clinical findings were suggestive of LP, consistent with her history of LP. In light of these new findings, both topical corticosteroids and retinoids were utilized for treatment without remarkable benefit. The patient declined further management with systemic medications.
We report a case of nail LP associated with underlying radiographic osteomyelitis. Erosive nail LP has been associated with underlying osteomyelitis of the phalanx.1 Our patient developed these manifestations in the setting of Cushing disease, a unique finding given that many report improvement of LP with systemic corticosteroids.2,3 Tacrolimus, a calcineurin inhibitor, has been used in oral or topical formulations for lower extremity ulcers caused by LP as well as nail LP.1,4 Long-term prognosis of nail LP is poor, with high relapse rates and permanent damage to the nail unit.2 It is important to be aware that LP of the nail unit may cause radiographic changes of osteomyelitis that are not infectious in nature.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
- Miller S. The effect of tacrolimus on lower extremity ulcers: a case study and review of the literature. Ostomy Wound Manage. 2008;54:36-42.
- Goettmann S, Zaraa I, Moulonguet I. Nail lichen planus: epidemiological, clinical, pathological, therapeutic and prognosis study of 67 cases. Eur Acad Dermatol Venereol. 2012;26:1304-1309.
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496.
- Ujiie H, Shibaki A, Akiyama M, et al. Successful treatment of nail lichen planus with topical tacrolimus. Acta Derm Venereol. 2010;90:218-219.
Practice Points
- Lichen planus (LP) is an inflammatory mucocutaneous disorder with variable presentations.
- With extensive nail involvement, nail LP may impart radiographic findings suggestive of osteomyelitis.
Concurrent Keratoacanthomas and Nonsarcoidal Granulomatous Reactions in New and Preexisting Tattoos
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.
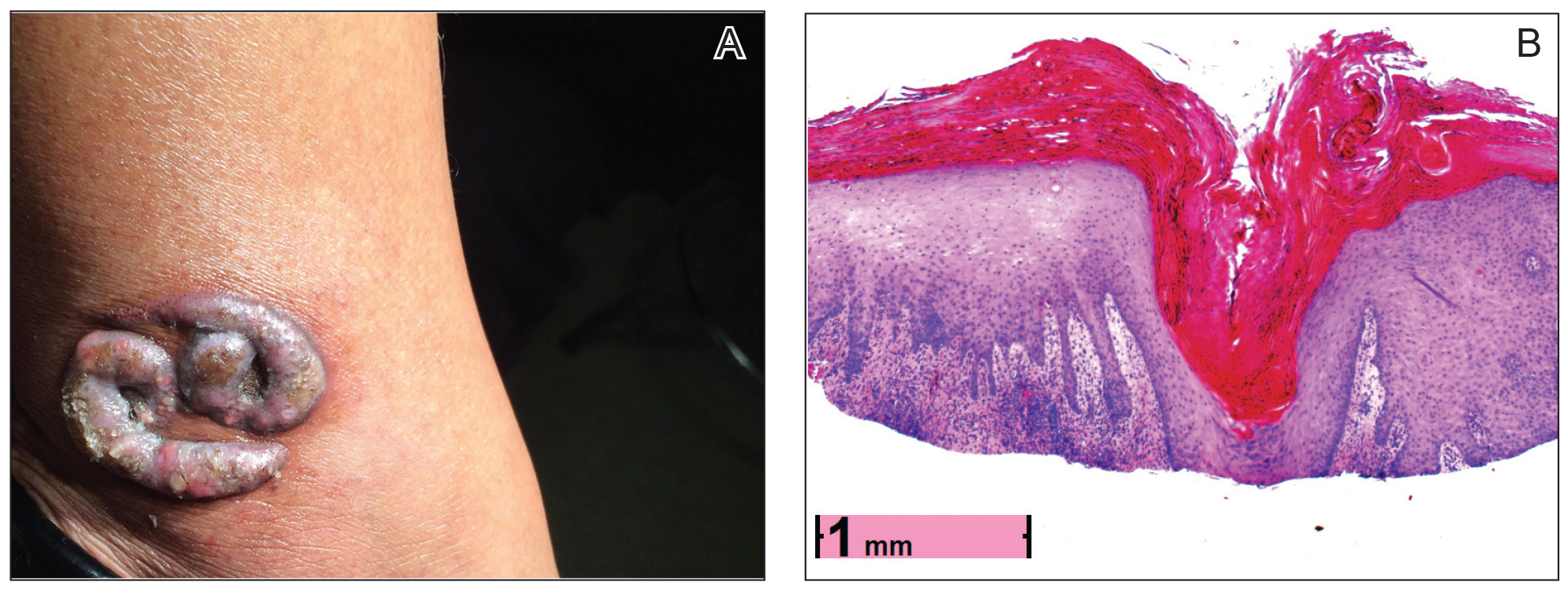

contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).
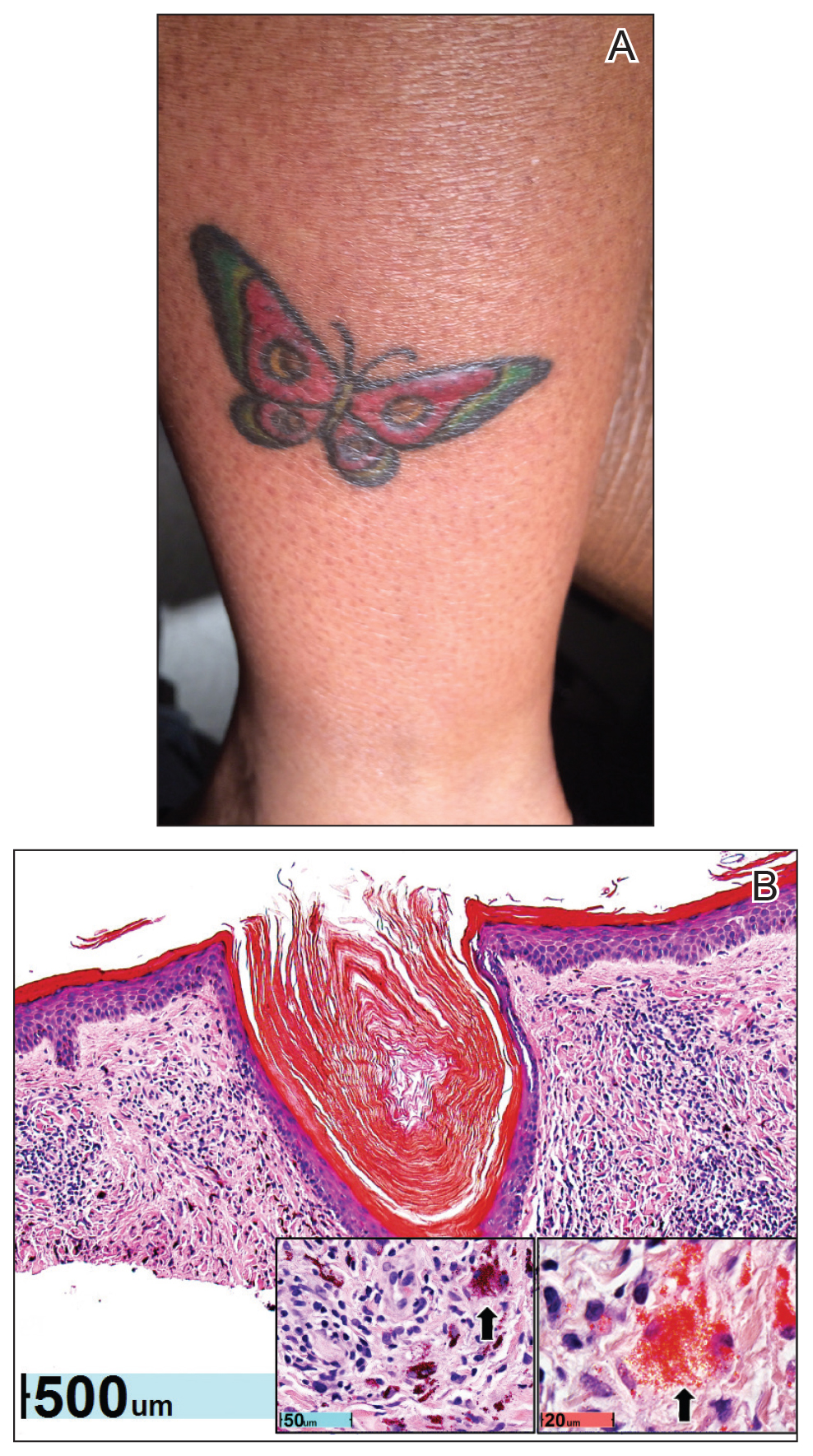
The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.


contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).

The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.


contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).

The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
Practice Points
- Keratoacanthomas (KAs) are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution.
- The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, scarring disorders, and trauma (including tattoos).
- Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Our case adds additional weight to the idea that some KAs are primarily reactive phenomena sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
Chronic Lymphocytic Leukemia and Infiltrates Seen During Excision of Nonmelanoma Skin Cancer
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.
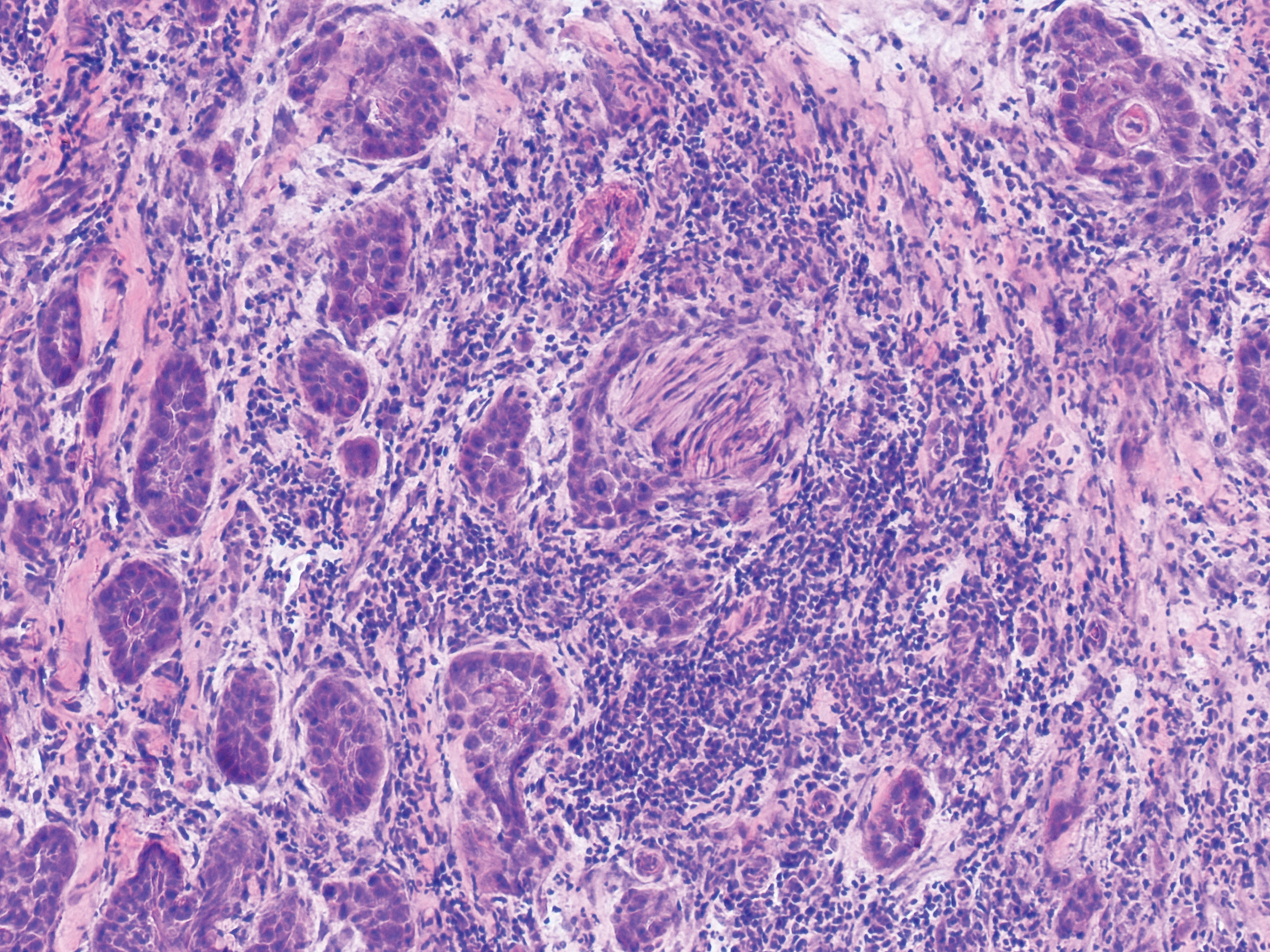
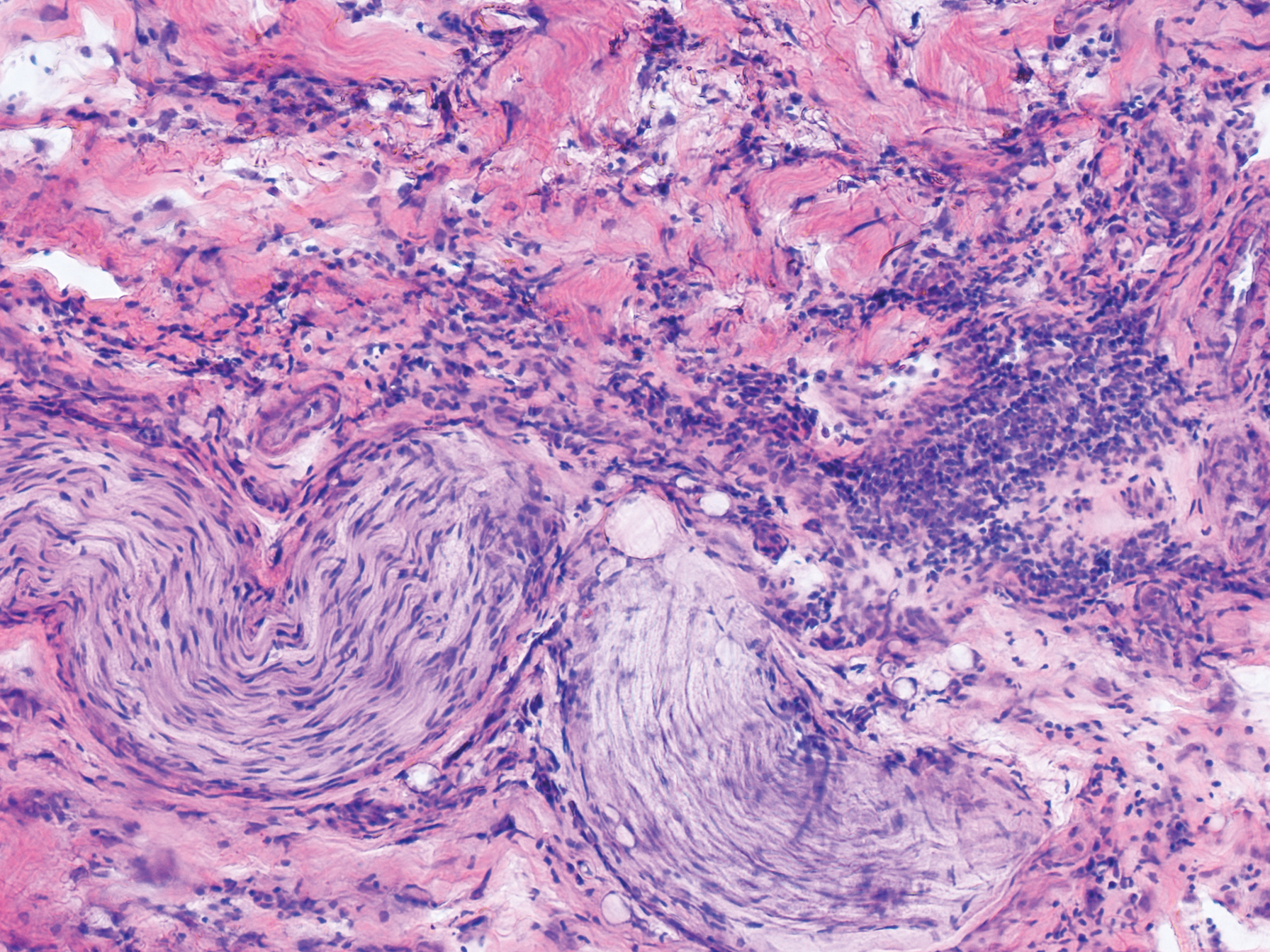
An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7
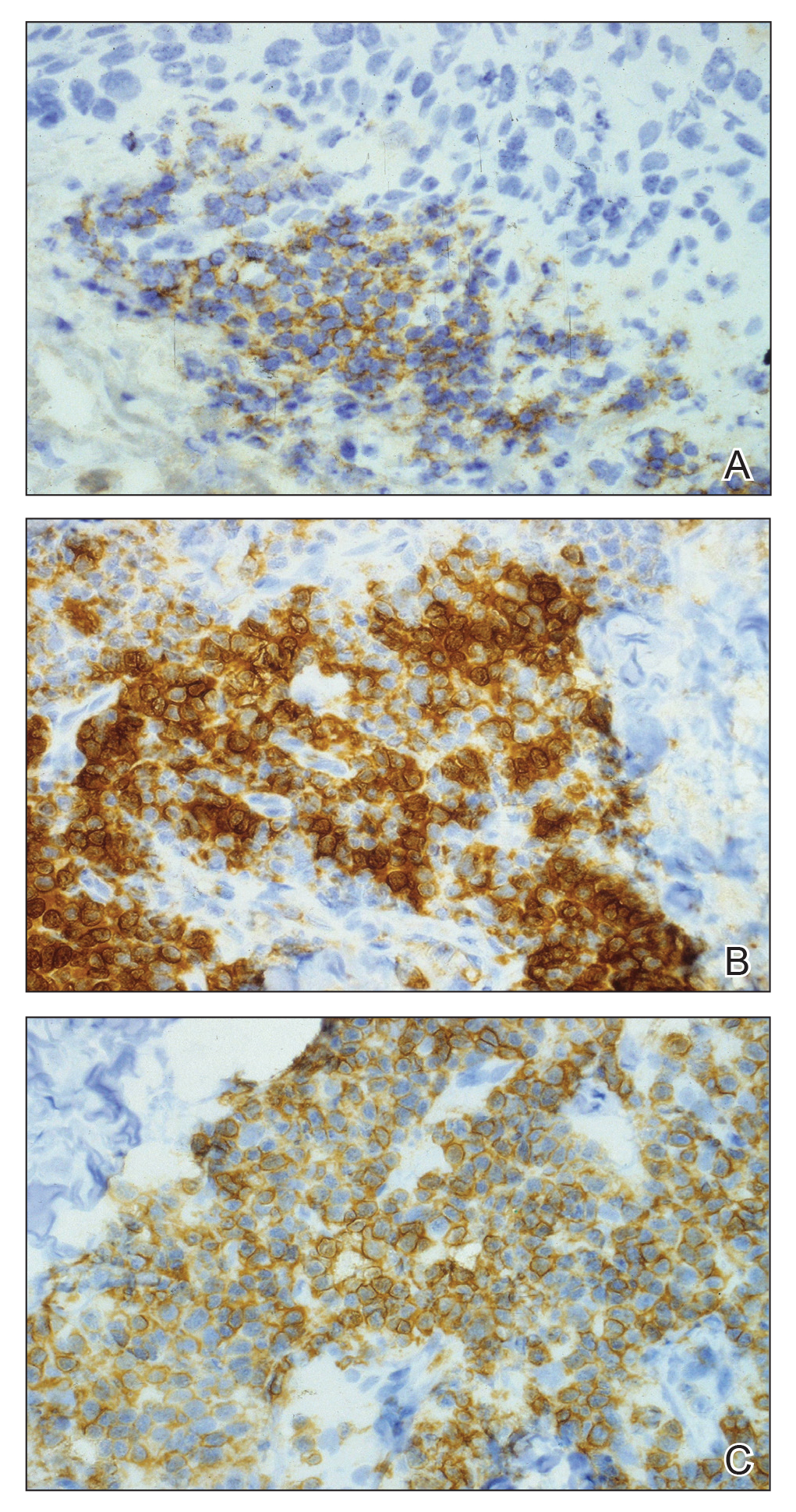

The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.


An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7


The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.


An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7


The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
Practice Points
- Chronic lymphocytic leukemia (CLL) may be seen during histologic examination of specimens during Mohs micrographic surgery as a monomorphic infiltrate of small mature lymphocytes with dense nuclei. Patients may be unaware of their diagnosis, which can be the presenting feature.
- An infiltrate of CLL may mimic aggressive behavior of nonmelanoma skin cancers including perineural invasion. A leukemic infiltrate may appear more dense and monomorphic. If needed, immunohistochemical staining of leukemic cells will show CD5 and CD23 positivity.
- Anecdotally, patients with CLL may not remember this pertinent medical history. Whether due to its asymptomatic nature or lack of treatment in early stages, direct questioning about CLL may be warranted if this characteristic infiltrate is encountered.