User login
Pustular Tinea Id Reaction
To the Editor:
A 17-year-old adolescent girl presented to the dermatology clinic with a tender pruritic rash on the left wrist that was spreading to the bilateral arms and legs of several years’ duration. An area of a prior biopsy on the left wrist was healing well with use of petroleum jelly and halcinonide cream. The patient denied any constitutional symptoms.
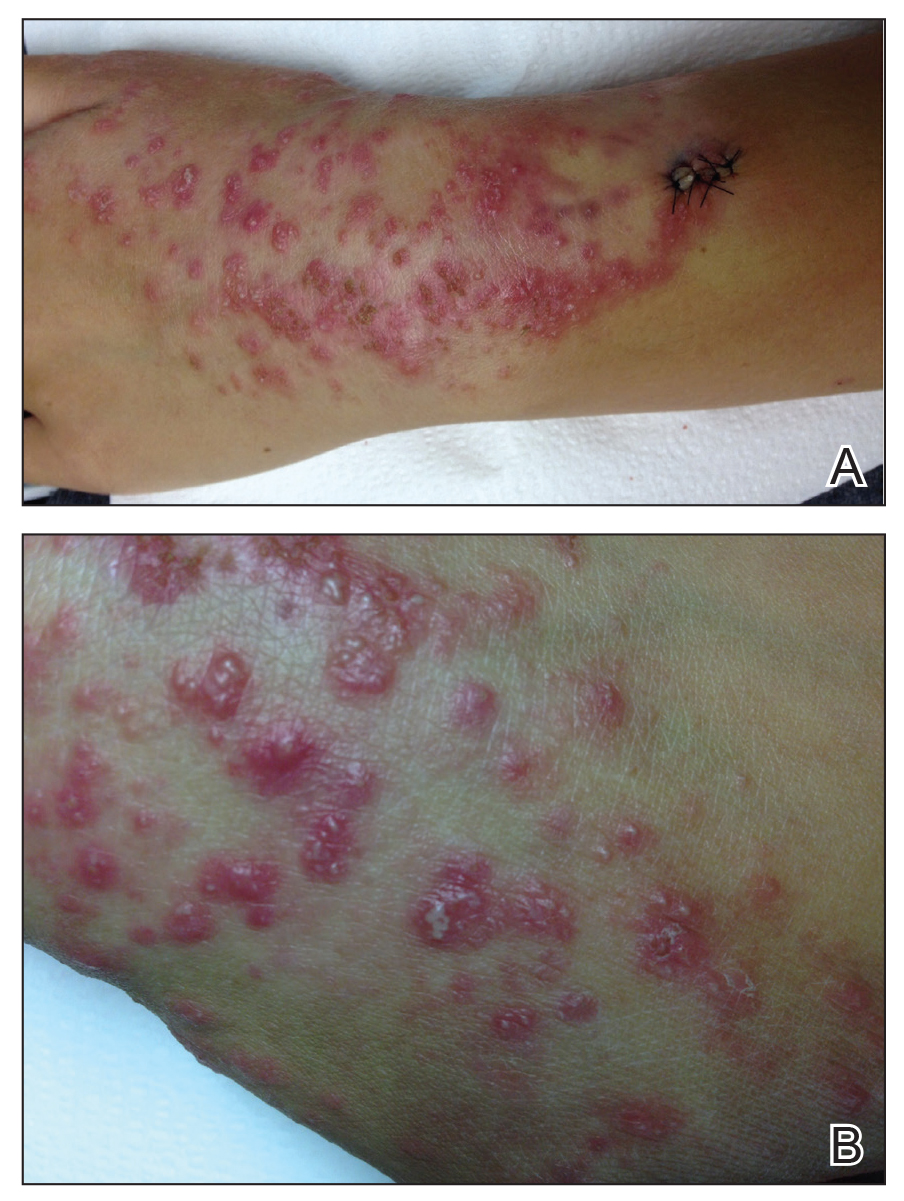
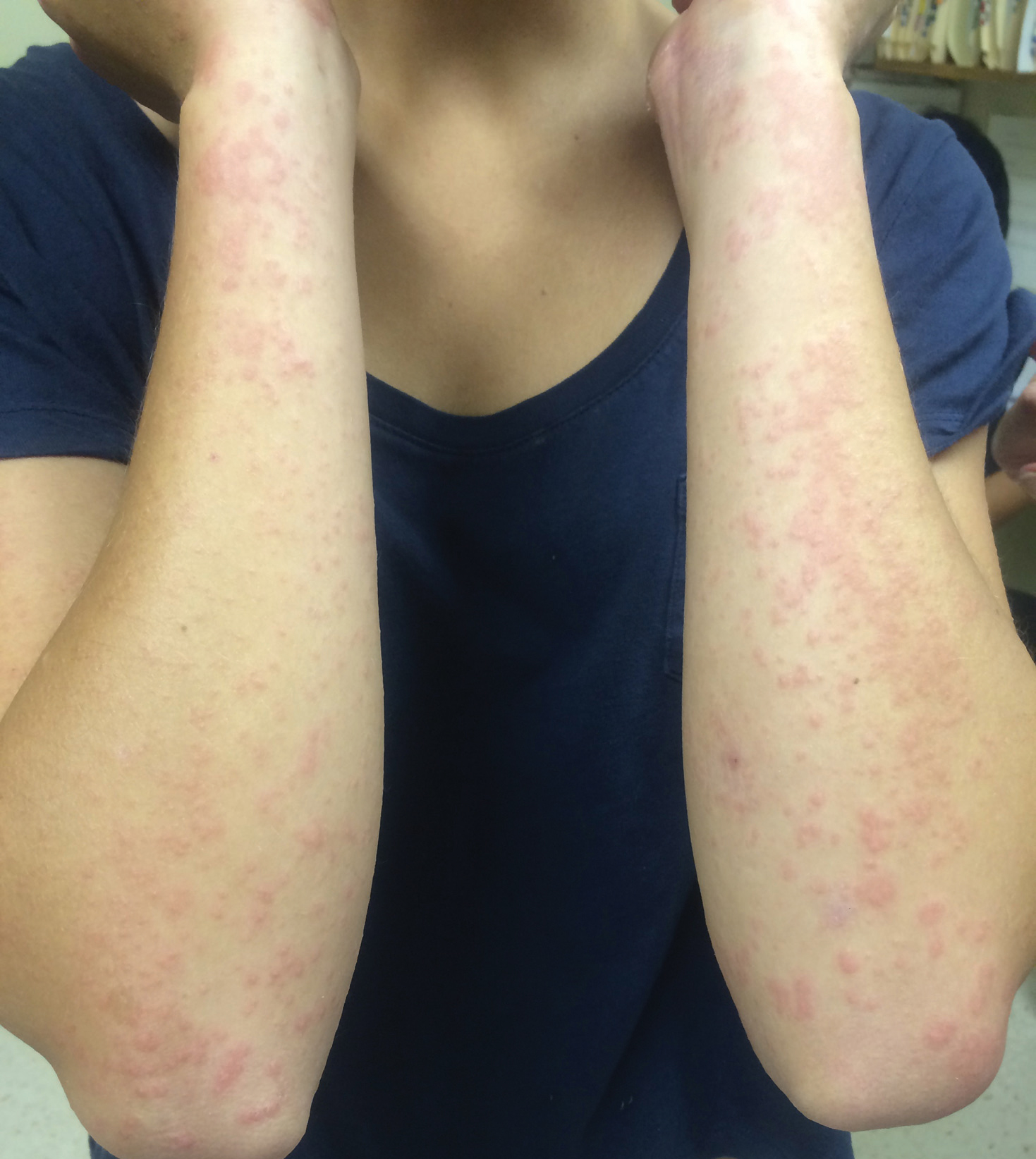
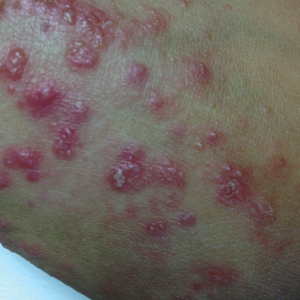
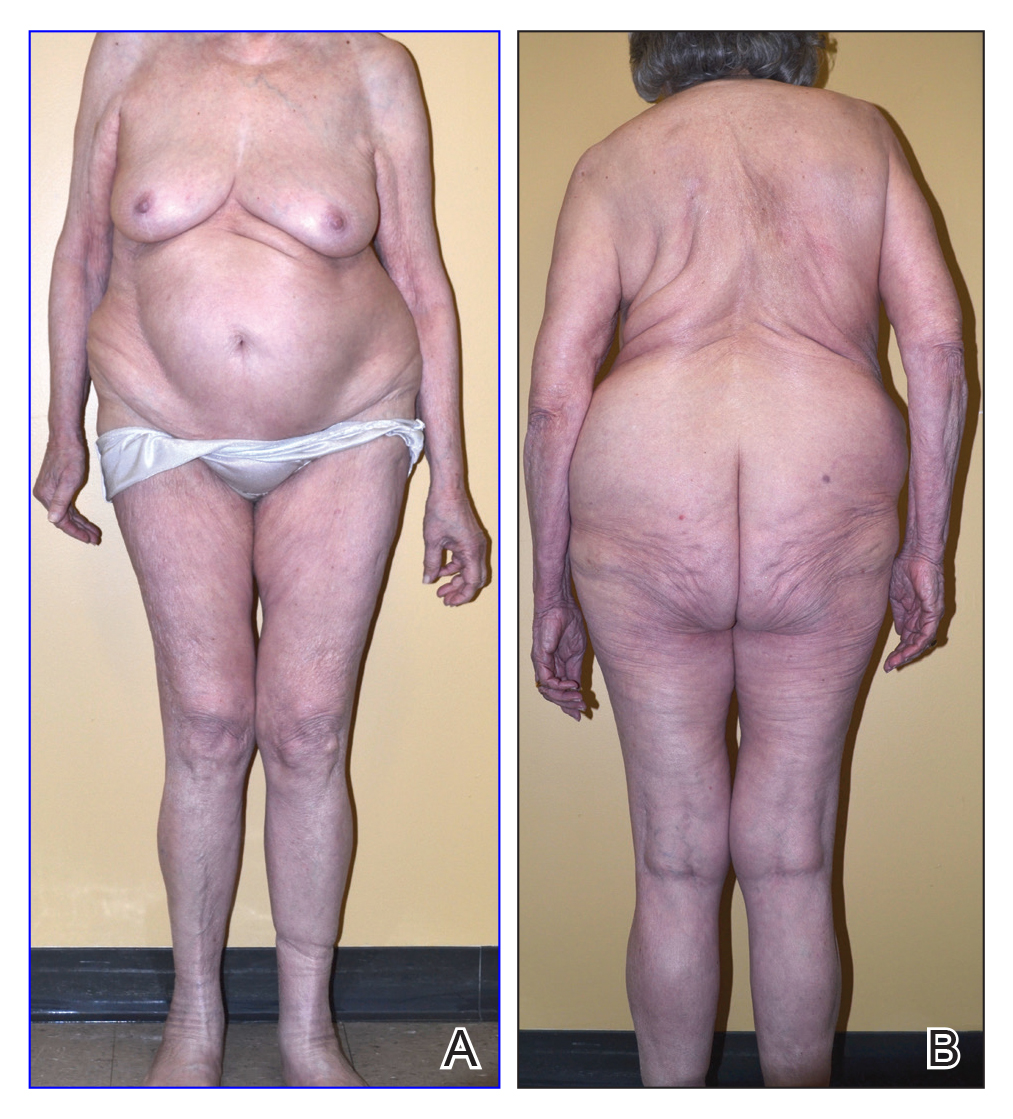
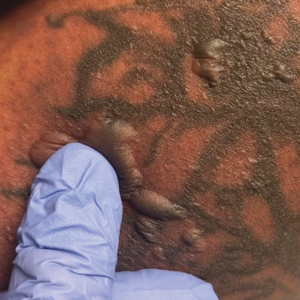
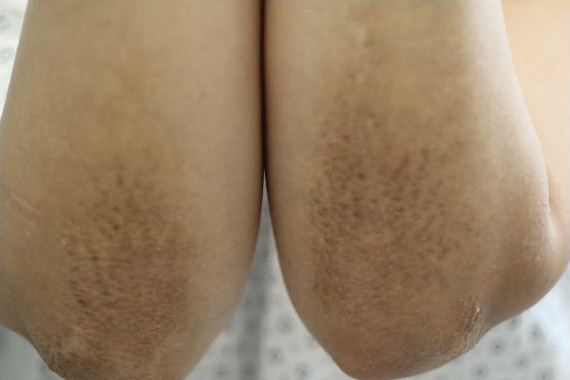
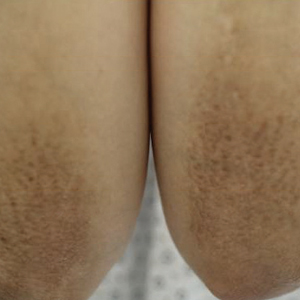
Physical examination revealed numerous erythematous papules coalescing into plaques on the bilateral anterior and posterior arms and legs, including some erythematous macules and papules on the palms and soles. The original area of involvement on the left dorsal medial wrist demonstrated a background of erythema with overlying peripheral scaling and resolving violaceous to erythematous papules with signs of serosanguineous crusting (Figure 1). Scattered perifollicular erythema was present on the posterior aspects of the bilateral thighs and arms (Figure 2). Baseline complete blood cell count and complete metabolic panel were within reference range.
Clinical histopathology showed evidence of a pustular superficial dermatophyte infection, and Grocott-Gomori methenamine-silver stain demonstrated numerous fungal hyphae within subcorneal pustules, indicating pustular tinea. Based on the clinicopathologic correlation, the initial presentation was diagnosed as pustular tinea of the entire left wrist, followed by a generalized id reaction 1 week later.
The patient was prescribed oral terbinafine 250 mg once daily to treat the diffuse involvement of the pustular tinea as well as once-daily oral cetirizine, once-daily oral diphenhydramine, a topical emollient, and a topical nonsteroidal antipruritic gel.
Tinea is a superficial fungal infection commonly caused by the dermatophytes Epidermophyton, Trichophyton, and Microsporum. It has a variety of clinical presentations based on the anatomic location, including tinea capitis (hair/scalp), tinea pedis (feet), tinea corporis (face/trunk/extremities), tinea cruris (groin), and tinea unguium (nails).1 Tinea infections occur in the stratum corneum, hair, and nails, thriving on dead keratin in these areas.2 Tinea corporis usually appears as an erythematous ring-shaped lesion with a scaly border, but atypical cases presenting with vesicles, pustules, and bullae also have been reported.3 Additionally, secondary eruptions called id reactions, or autoeczematization, can present in the setting of dermatophyte infections. Such outbreaks may be due to a delayed hypersensitivity reaction to the fungal antigens. Id reactions can manifest in many forms of tinea with patients generally exhibiting pruritic papulovesicular lesions that can present far from the site of origin.4
Patients with id reactions can have atypical and varied presentations. In a case of id reaction due to tinea corporis, a patient presented with vesicles and pustules that grew in number and coalesced to form annular lesions.5 A case of an id reaction caused by tinea pedis also noted the presence of pustules, which are atypical in this form of tinea.6 In another case of tinea pedis, a generalized id reaction was noted, illustrating that such eruptions do not necessarily appear at the original site of infection.7 Additionally, in a rare presentation of tinea invading the nares, a patient developed an erythema multiforme id reaction.8 Id reactions also were noted in 14 patients with refractory otitis externa, illustrating the ability of this fungal infection to persist and infect distant locations.9
Because the differential diagnoses for tinea infection are extensive, pathology or laboratory confirmation is necessary for diagnosis, and potassium hydroxide preparation often is used to diagnose dermatophyte infections.1,2 Additionally, the possibility of a hypersensitivity drug rash should remain in the differential if the patient received allergy-inducing medications prior to the outbreak, which may in turn complicate the diagnosis.
Tinea infections typically can be treated with topical antifungals such as terbinafine, butenafine,1 and luliconazole10; however, more involved cases may require oral antifungal treatment.1 Systemic treatment of tinea corporis includes itraconazole, terbinafine, and fluconazole,11 all of which exhibit fewer side effects and greater efficacy when compared to griseofulvin.12-15
Treatment of id reactions centers on the proper clearance of the dermatophyte infection, and treatment with oral antifungals generally is sufficient. In the cases of id reaction in patients with refractory otitis, some success was achieved with treatment involving immunotherapy with dermatophyte and dust mite allergen extracts coupled with a yeast elimination diet.9 In acute id reactions, topical corticosteroids and antipruritic agents can be applied.4 Rarely, systemic glucocorticoids are required, such as in cases in which the id reaction persists despite proper treatment of the primary infection.16
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Hanover, NH: Elsevier, Inc; 2010.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50(suppl 2):31-35.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications [published online July 4, 2011]. Pediatrics. 2011;128:e453-e457.
- Ohno S, Tanabe H, Kawasaki M, et al. Tinea corporis with acute inflammation caused by Trichophyton tonsurans. J Dermatol. 2008;35:590-593.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Iglesias ME, España A, Idoate MA, et al. Generalized skin reaction following tinea pedis (dermatophytids). J Dermatol. 1994;21:31-34.
- Atzori L, Pau M, Aste M. Erythema multiforme ID reaction in atypical dermatophytosis: a case report. J Eur Acad Dermatol Venereol. 2003;17:699-701.
- Derebery J, Berliner KI. Foot and ear disease—the dermatophytid reaction in otology. Laryngoscope. 1996;106(2 Pt 1):181-186.
- Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: an evidence-based review. Core Evid. 2014;9:113-124.
- Korting HC, Schöllmann C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. J Dtsch Dermatol Ges. 2009;7:11-20.
- Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. UpToDate website. https://www.uptodate.com/contents/dermatophyte-tinea-infections. Updated December 28, 2018. Accessed April 24, 2019.
- Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537.
- Panagiotidou D, Kousidou T, Chaidemenos G, et al. A comparison of itraconazole and griseofulvin in the treatment of tinea corporis and tinea cruris: a double-blind study. J Int Med Res. 1992;20:392-400.
- Faergemann J, Mörk NJ, Haglund A, et al. A multicentre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol. 1997;136:575-577.
- Ilkit M, Durdu M, Karakas M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
To the Editor:
A 17-year-old adolescent girl presented to the dermatology clinic with a tender pruritic rash on the left wrist that was spreading to the bilateral arms and legs of several years’ duration. An area of a prior biopsy on the left wrist was healing well with use of petroleum jelly and halcinonide cream. The patient denied any constitutional symptoms.
Physical examination revealed numerous erythematous papules coalescing into plaques on the bilateral anterior and posterior arms and legs, including some erythematous macules and papules on the palms and soles. The original area of involvement on the left dorsal medial wrist demonstrated a background of erythema with overlying peripheral scaling and resolving violaceous to erythematous papules with signs of serosanguineous crusting (Figure 1). Scattered perifollicular erythema was present on the posterior aspects of the bilateral thighs and arms (Figure 2). Baseline complete blood cell count and complete metabolic panel were within reference range.
Clinical histopathology showed evidence of a pustular superficial dermatophyte infection, and Grocott-Gomori methenamine-silver stain demonstrated numerous fungal hyphae within subcorneal pustules, indicating pustular tinea. Based on the clinicopathologic correlation, the initial presentation was diagnosed as pustular tinea of the entire left wrist, followed by a generalized id reaction 1 week later.
The patient was prescribed oral terbinafine 250 mg once daily to treat the diffuse involvement of the pustular tinea as well as once-daily oral cetirizine, once-daily oral diphenhydramine, a topical emollient, and a topical nonsteroidal antipruritic gel.
Tinea is a superficial fungal infection commonly caused by the dermatophytes Epidermophyton, Trichophyton, and Microsporum. It has a variety of clinical presentations based on the anatomic location, including tinea capitis (hair/scalp), tinea pedis (feet), tinea corporis (face/trunk/extremities), tinea cruris (groin), and tinea unguium (nails).1 Tinea infections occur in the stratum corneum, hair, and nails, thriving on dead keratin in these areas.2 Tinea corporis usually appears as an erythematous ring-shaped lesion with a scaly border, but atypical cases presenting with vesicles, pustules, and bullae also have been reported.3 Additionally, secondary eruptions called id reactions, or autoeczematization, can present in the setting of dermatophyte infections. Such outbreaks may be due to a delayed hypersensitivity reaction to the fungal antigens. Id reactions can manifest in many forms of tinea with patients generally exhibiting pruritic papulovesicular lesions that can present far from the site of origin.4
Patients with id reactions can have atypical and varied presentations. In a case of id reaction due to tinea corporis, a patient presented with vesicles and pustules that grew in number and coalesced to form annular lesions.5 A case of an id reaction caused by tinea pedis also noted the presence of pustules, which are atypical in this form of tinea.6 In another case of tinea pedis, a generalized id reaction was noted, illustrating that such eruptions do not necessarily appear at the original site of infection.7 Additionally, in a rare presentation of tinea invading the nares, a patient developed an erythema multiforme id reaction.8 Id reactions also were noted in 14 patients with refractory otitis externa, illustrating the ability of this fungal infection to persist and infect distant locations.9
Because the differential diagnoses for tinea infection are extensive, pathology or laboratory confirmation is necessary for diagnosis, and potassium hydroxide preparation often is used to diagnose dermatophyte infections.1,2 Additionally, the possibility of a hypersensitivity drug rash should remain in the differential if the patient received allergy-inducing medications prior to the outbreak, which may in turn complicate the diagnosis.
Tinea infections typically can be treated with topical antifungals such as terbinafine, butenafine,1 and luliconazole10; however, more involved cases may require oral antifungal treatment.1 Systemic treatment of tinea corporis includes itraconazole, terbinafine, and fluconazole,11 all of which exhibit fewer side effects and greater efficacy when compared to griseofulvin.12-15
Treatment of id reactions centers on the proper clearance of the dermatophyte infection, and treatment with oral antifungals generally is sufficient. In the cases of id reaction in patients with refractory otitis, some success was achieved with treatment involving immunotherapy with dermatophyte and dust mite allergen extracts coupled with a yeast elimination diet.9 In acute id reactions, topical corticosteroids and antipruritic agents can be applied.4 Rarely, systemic glucocorticoids are required, such as in cases in which the id reaction persists despite proper treatment of the primary infection.16
To the Editor:
A 17-year-old adolescent girl presented to the dermatology clinic with a tender pruritic rash on the left wrist that was spreading to the bilateral arms and legs of several years’ duration. An area of a prior biopsy on the left wrist was healing well with use of petroleum jelly and halcinonide cream. The patient denied any constitutional symptoms.
Physical examination revealed numerous erythematous papules coalescing into plaques on the bilateral anterior and posterior arms and legs, including some erythematous macules and papules on the palms and soles. The original area of involvement on the left dorsal medial wrist demonstrated a background of erythema with overlying peripheral scaling and resolving violaceous to erythematous papules with signs of serosanguineous crusting (Figure 1). Scattered perifollicular erythema was present on the posterior aspects of the bilateral thighs and arms (Figure 2). Baseline complete blood cell count and complete metabolic panel were within reference range.
Clinical histopathology showed evidence of a pustular superficial dermatophyte infection, and Grocott-Gomori methenamine-silver stain demonstrated numerous fungal hyphae within subcorneal pustules, indicating pustular tinea. Based on the clinicopathologic correlation, the initial presentation was diagnosed as pustular tinea of the entire left wrist, followed by a generalized id reaction 1 week later.
The patient was prescribed oral terbinafine 250 mg once daily to treat the diffuse involvement of the pustular tinea as well as once-daily oral cetirizine, once-daily oral diphenhydramine, a topical emollient, and a topical nonsteroidal antipruritic gel.
Tinea is a superficial fungal infection commonly caused by the dermatophytes Epidermophyton, Trichophyton, and Microsporum. It has a variety of clinical presentations based on the anatomic location, including tinea capitis (hair/scalp), tinea pedis (feet), tinea corporis (face/trunk/extremities), tinea cruris (groin), and tinea unguium (nails).1 Tinea infections occur in the stratum corneum, hair, and nails, thriving on dead keratin in these areas.2 Tinea corporis usually appears as an erythematous ring-shaped lesion with a scaly border, but atypical cases presenting with vesicles, pustules, and bullae also have been reported.3 Additionally, secondary eruptions called id reactions, or autoeczematization, can present in the setting of dermatophyte infections. Such outbreaks may be due to a delayed hypersensitivity reaction to the fungal antigens. Id reactions can manifest in many forms of tinea with patients generally exhibiting pruritic papulovesicular lesions that can present far from the site of origin.4
Patients with id reactions can have atypical and varied presentations. In a case of id reaction due to tinea corporis, a patient presented with vesicles and pustules that grew in number and coalesced to form annular lesions.5 A case of an id reaction caused by tinea pedis also noted the presence of pustules, which are atypical in this form of tinea.6 In another case of tinea pedis, a generalized id reaction was noted, illustrating that such eruptions do not necessarily appear at the original site of infection.7 Additionally, in a rare presentation of tinea invading the nares, a patient developed an erythema multiforme id reaction.8 Id reactions also were noted in 14 patients with refractory otitis externa, illustrating the ability of this fungal infection to persist and infect distant locations.9
Because the differential diagnoses for tinea infection are extensive, pathology or laboratory confirmation is necessary for diagnosis, and potassium hydroxide preparation often is used to diagnose dermatophyte infections.1,2 Additionally, the possibility of a hypersensitivity drug rash should remain in the differential if the patient received allergy-inducing medications prior to the outbreak, which may in turn complicate the diagnosis.
Tinea infections typically can be treated with topical antifungals such as terbinafine, butenafine,1 and luliconazole10; however, more involved cases may require oral antifungal treatment.1 Systemic treatment of tinea corporis includes itraconazole, terbinafine, and fluconazole,11 all of which exhibit fewer side effects and greater efficacy when compared to griseofulvin.12-15
Treatment of id reactions centers on the proper clearance of the dermatophyte infection, and treatment with oral antifungals generally is sufficient. In the cases of id reaction in patients with refractory otitis, some success was achieved with treatment involving immunotherapy with dermatophyte and dust mite allergen extracts coupled with a yeast elimination diet.9 In acute id reactions, topical corticosteroids and antipruritic agents can be applied.4 Rarely, systemic glucocorticoids are required, such as in cases in which the id reaction persists despite proper treatment of the primary infection.16
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Hanover, NH: Elsevier, Inc; 2010.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50(suppl 2):31-35.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications [published online July 4, 2011]. Pediatrics. 2011;128:e453-e457.
- Ohno S, Tanabe H, Kawasaki M, et al. Tinea corporis with acute inflammation caused by Trichophyton tonsurans. J Dermatol. 2008;35:590-593.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Iglesias ME, España A, Idoate MA, et al. Generalized skin reaction following tinea pedis (dermatophytids). J Dermatol. 1994;21:31-34.
- Atzori L, Pau M, Aste M. Erythema multiforme ID reaction in atypical dermatophytosis: a case report. J Eur Acad Dermatol Venereol. 2003;17:699-701.
- Derebery J, Berliner KI. Foot and ear disease—the dermatophytid reaction in otology. Laryngoscope. 1996;106(2 Pt 1):181-186.
- Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: an evidence-based review. Core Evid. 2014;9:113-124.
- Korting HC, Schöllmann C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. J Dtsch Dermatol Ges. 2009;7:11-20.
- Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. UpToDate website. https://www.uptodate.com/contents/dermatophyte-tinea-infections. Updated December 28, 2018. Accessed April 24, 2019.
- Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537.
- Panagiotidou D, Kousidou T, Chaidemenos G, et al. A comparison of itraconazole and griseofulvin in the treatment of tinea corporis and tinea cruris: a double-blind study. J Int Med Res. 1992;20:392-400.
- Faergemann J, Mörk NJ, Haglund A, et al. A multicentre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol. 1997;136:575-577.
- Ilkit M, Durdu M, Karakas M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 5th ed. Hanover, NH: Elsevier, Inc; 2010.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50(suppl 2):31-35.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications [published online July 4, 2011]. Pediatrics. 2011;128:e453-e457.
- Ohno S, Tanabe H, Kawasaki M, et al. Tinea corporis with acute inflammation caused by Trichophyton tonsurans. J Dermatol. 2008;35:590-593.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Iglesias ME, España A, Idoate MA, et al. Generalized skin reaction following tinea pedis (dermatophytids). J Dermatol. 1994;21:31-34.
- Atzori L, Pau M, Aste M. Erythema multiforme ID reaction in atypical dermatophytosis: a case report. J Eur Acad Dermatol Venereol. 2003;17:699-701.
- Derebery J, Berliner KI. Foot and ear disease—the dermatophytid reaction in otology. Laryngoscope. 1996;106(2 Pt 1):181-186.
- Khanna D, Bharti S. Luliconazole for the treatment of fungal infections: an evidence-based review. Core Evid. 2014;9:113-124.
- Korting HC, Schöllmann C. The significance of itraconazole for treatment of fungal infections of skin, nails and mucous membranes. J Dtsch Dermatol Ges. 2009;7:11-20.
- Goldstein AO, Goldstein BG. Dermatophyte (tinea) infections. UpToDate website. https://www.uptodate.com/contents/dermatophyte-tinea-infections. Updated December 28, 2018. Accessed April 24, 2019.
- Cole GW, Stricklin G. A comparison of a new oral antifungal, terbinafine, with griseofulvin as therapy for tinea corporis. Arch Dermatol. 1989;125:1537.
- Panagiotidou D, Kousidou T, Chaidemenos G, et al. A comparison of itraconazole and griseofulvin in the treatment of tinea corporis and tinea cruris: a double-blind study. J Int Med Res. 1992;20:392-400.
- Faergemann J, Mörk NJ, Haglund A, et al. A multicentre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol. 1997;136:575-577.
- Ilkit M, Durdu M, Karakas M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
Practice Points
• Id reactions, or autoeczematization, can occur secondary to dermatophyte infections, possibly due to a hypersensitivity reaction to the fungus. These eruptions can occur in many forms of tinea and in a variety of clinical presentations.
• Treatment is based on clearance of the original dermatophyte infection.
Dupilumab for Treatment of Severe Atopic Dermatitis in a Heart Transplant Recipient
To the Editor:
Solid-organ transplant recipients can develop a range of dermatologic consequences due to chronic immunosuppression, including frequent skin infections and malignancies. Atopic dermatitis (AD) and psoriasis are relatively rare in this population because many immunosuppressive therapies, such as mycophenolate mofetil and tacrolimus, also are used to treat inflammatory dermatoses.1 In a large renal transplant population, the prevalence of AD was 1.3%.2 The pathogenesis of posttransplantation AD is poorly understood, and standard treatment regimens have not been defined. Dupilumab is a novel biologic medication that has demonstrated efficacy in the treatment of AD.3 Reports of dupilumab use for AD management in solid-organ transplant recipients are limited in the literature.
A 29-year-old woman with a history of a heart transplant 4 years prior presented to our dermatology clinic with an itchy rash over the entire body. Since the transplant, she had been on long-term immunosuppression with prednisone, mycophenolate mofetil, and tacrolimus. The rash appeared after she switched from brand-name to generic versions of the medications. Physical examination revealed erythematous scaly plaques on the lateral face, back, chest, arms, and legs covering approximately 10% of the body surface area. The patient’s total serum IgE level was elevated at 711,500 µg/L (reference range, 0–1500 µg/L). Outside biopsies revealed changes consistent with spongiotic dermatitis, and patch testing performed by an outside physician was positive for sensitivity to the preservative bronopol.
The patient was switched back to brand-name tacrolimus, but the rash did not improve. Topical steroids, phototherapy, and omalizumab were ineffective. The itching was primarily managed with desoximetasone spray, mometasone cream, and loratidine. With approval from the patient’s transplant team outside of our hospital system, she was started on dupilumab 300 mg once every 14 days. Complete clearance of the rash was noted within 3 months of treatment. Besides bilateral conjunctivitis, which was treated with ophthalmic prednisolone and moxifloxacin solutions, dupilumab was well tolerated. No issues related to immunosuppressant levels or graft-related issues, including rejection, were reported at 6-, 12-, and 18-month follow-up visits.
Atopic dermatitis is characterized by activation of type 2 immune responses, skin barrier defects, and increased Staphylococcus aureus colonization.4 A potential mechanism for the development of AD in transplant recipients relates to their use of tacrolimus for chronic immunosuppression. Tacrolimus increases intestinal permeability and therefore allows greater absorption of allergens. This influx of allergens promotes hypersensitivity reactions, resulting in elevated IgE levels and eosinophilia. Tacrolimus also facilitates predominance of helper T cells (TH2 cytokines) through selective inhibition of the TH1 cytokine IL-2.5
Dupilumab is a human monoclonal antibody that blocks IL-4 and IL-13, which are key drivers of TH2-mediated inflammation. In addition to downregulation of inflammatory mediators, dupilumab also increases production of epidermal barrier proteins, resulting in skin repair. It has demonstrated rapid, dose-dependent efficacy in patients with moderate to severe AD.6 Dupilumab boasts a good safety profile with no increase in risk for skin infections compared to placebo6; however, its safety has not yet been verified in transplant recipients.
Our case is notable for the severity of the patient’s AD despite considerable immunosuppression with transplant medications. Development of AD was associated with a switch from brand-name to generic drugs, which is not commonly reported. Her condition was refractory to a litany of treatments prior to a trial of dupilumab. The rapid clearance observed with this novel biologic medication highlights its potential to provide relief to patients who have particularly tenacious cases of AD. Prior to starting dupilumab, we do recommend more extensive laboratory testing in immunosuppressed patients including transplant recipients and patients with human immunodeficiency virus. We illustrate that a history of solid-organ transplant need not exclude patients from consideration for dupilumab therapy.
- Savoia P, Cavaliere G, Zavattaro E, et al. Inflammatory cutaneous diseases in renal transplant recipients [published online August 19, 2016]. Int J Mol Sci. doi:10.3390/ijms17081362.
- Lally A, Casabonne D, Imko-Walczuk B, et al. Prevalence of benign cutaneous disease among Oxford renal transplant recipients. J Eur Acad Dermatol Venereol. 2011;25:462-470.
- Beck L, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Machura E, Chodór B, Kleszyk M, et al. Atopic allergy and chronic inflammation of the oral mucosa in a 3-year-old boy after heart transplantation—diagnostic and therapeutic difficulties. Kardiochir Torakochirurgia Pol. 2015;12:176-180.
- Beck L, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
To the Editor:
Solid-organ transplant recipients can develop a range of dermatologic consequences due to chronic immunosuppression, including frequent skin infections and malignancies. Atopic dermatitis (AD) and psoriasis are relatively rare in this population because many immunosuppressive therapies, such as mycophenolate mofetil and tacrolimus, also are used to treat inflammatory dermatoses.1 In a large renal transplant population, the prevalence of AD was 1.3%.2 The pathogenesis of posttransplantation AD is poorly understood, and standard treatment regimens have not been defined. Dupilumab is a novel biologic medication that has demonstrated efficacy in the treatment of AD.3 Reports of dupilumab use for AD management in solid-organ transplant recipients are limited in the literature.
A 29-year-old woman with a history of a heart transplant 4 years prior presented to our dermatology clinic with an itchy rash over the entire body. Since the transplant, she had been on long-term immunosuppression with prednisone, mycophenolate mofetil, and tacrolimus. The rash appeared after she switched from brand-name to generic versions of the medications. Physical examination revealed erythematous scaly plaques on the lateral face, back, chest, arms, and legs covering approximately 10% of the body surface area. The patient’s total serum IgE level was elevated at 711,500 µg/L (reference range, 0–1500 µg/L). Outside biopsies revealed changes consistent with spongiotic dermatitis, and patch testing performed by an outside physician was positive for sensitivity to the preservative bronopol.
The patient was switched back to brand-name tacrolimus, but the rash did not improve. Topical steroids, phototherapy, and omalizumab were ineffective. The itching was primarily managed with desoximetasone spray, mometasone cream, and loratidine. With approval from the patient’s transplant team outside of our hospital system, she was started on dupilumab 300 mg once every 14 days. Complete clearance of the rash was noted within 3 months of treatment. Besides bilateral conjunctivitis, which was treated with ophthalmic prednisolone and moxifloxacin solutions, dupilumab was well tolerated. No issues related to immunosuppressant levels or graft-related issues, including rejection, were reported at 6-, 12-, and 18-month follow-up visits.
Atopic dermatitis is characterized by activation of type 2 immune responses, skin barrier defects, and increased Staphylococcus aureus colonization.4 A potential mechanism for the development of AD in transplant recipients relates to their use of tacrolimus for chronic immunosuppression. Tacrolimus increases intestinal permeability and therefore allows greater absorption of allergens. This influx of allergens promotes hypersensitivity reactions, resulting in elevated IgE levels and eosinophilia. Tacrolimus also facilitates predominance of helper T cells (TH2 cytokines) through selective inhibition of the TH1 cytokine IL-2.5
Dupilumab is a human monoclonal antibody that blocks IL-4 and IL-13, which are key drivers of TH2-mediated inflammation. In addition to downregulation of inflammatory mediators, dupilumab also increases production of epidermal barrier proteins, resulting in skin repair. It has demonstrated rapid, dose-dependent efficacy in patients with moderate to severe AD.6 Dupilumab boasts a good safety profile with no increase in risk for skin infections compared to placebo6; however, its safety has not yet been verified in transplant recipients.
Our case is notable for the severity of the patient’s AD despite considerable immunosuppression with transplant medications. Development of AD was associated with a switch from brand-name to generic drugs, which is not commonly reported. Her condition was refractory to a litany of treatments prior to a trial of dupilumab. The rapid clearance observed with this novel biologic medication highlights its potential to provide relief to patients who have particularly tenacious cases of AD. Prior to starting dupilumab, we do recommend more extensive laboratory testing in immunosuppressed patients including transplant recipients and patients with human immunodeficiency virus. We illustrate that a history of solid-organ transplant need not exclude patients from consideration for dupilumab therapy.
To the Editor:
Solid-organ transplant recipients can develop a range of dermatologic consequences due to chronic immunosuppression, including frequent skin infections and malignancies. Atopic dermatitis (AD) and psoriasis are relatively rare in this population because many immunosuppressive therapies, such as mycophenolate mofetil and tacrolimus, also are used to treat inflammatory dermatoses.1 In a large renal transplant population, the prevalence of AD was 1.3%.2 The pathogenesis of posttransplantation AD is poorly understood, and standard treatment regimens have not been defined. Dupilumab is a novel biologic medication that has demonstrated efficacy in the treatment of AD.3 Reports of dupilumab use for AD management in solid-organ transplant recipients are limited in the literature.
A 29-year-old woman with a history of a heart transplant 4 years prior presented to our dermatology clinic with an itchy rash over the entire body. Since the transplant, she had been on long-term immunosuppression with prednisone, mycophenolate mofetil, and tacrolimus. The rash appeared after she switched from brand-name to generic versions of the medications. Physical examination revealed erythematous scaly plaques on the lateral face, back, chest, arms, and legs covering approximately 10% of the body surface area. The patient’s total serum IgE level was elevated at 711,500 µg/L (reference range, 0–1500 µg/L). Outside biopsies revealed changes consistent with spongiotic dermatitis, and patch testing performed by an outside physician was positive for sensitivity to the preservative bronopol.
The patient was switched back to brand-name tacrolimus, but the rash did not improve. Topical steroids, phototherapy, and omalizumab were ineffective. The itching was primarily managed with desoximetasone spray, mometasone cream, and loratidine. With approval from the patient’s transplant team outside of our hospital system, she was started on dupilumab 300 mg once every 14 days. Complete clearance of the rash was noted within 3 months of treatment. Besides bilateral conjunctivitis, which was treated with ophthalmic prednisolone and moxifloxacin solutions, dupilumab was well tolerated. No issues related to immunosuppressant levels or graft-related issues, including rejection, were reported at 6-, 12-, and 18-month follow-up visits.
Atopic dermatitis is characterized by activation of type 2 immune responses, skin barrier defects, and increased Staphylococcus aureus colonization.4 A potential mechanism for the development of AD in transplant recipients relates to their use of tacrolimus for chronic immunosuppression. Tacrolimus increases intestinal permeability and therefore allows greater absorption of allergens. This influx of allergens promotes hypersensitivity reactions, resulting in elevated IgE levels and eosinophilia. Tacrolimus also facilitates predominance of helper T cells (TH2 cytokines) through selective inhibition of the TH1 cytokine IL-2.5
Dupilumab is a human monoclonal antibody that blocks IL-4 and IL-13, which are key drivers of TH2-mediated inflammation. In addition to downregulation of inflammatory mediators, dupilumab also increases production of epidermal barrier proteins, resulting in skin repair. It has demonstrated rapid, dose-dependent efficacy in patients with moderate to severe AD.6 Dupilumab boasts a good safety profile with no increase in risk for skin infections compared to placebo6; however, its safety has not yet been verified in transplant recipients.
Our case is notable for the severity of the patient’s AD despite considerable immunosuppression with transplant medications. Development of AD was associated with a switch from brand-name to generic drugs, which is not commonly reported. Her condition was refractory to a litany of treatments prior to a trial of dupilumab. The rapid clearance observed with this novel biologic medication highlights its potential to provide relief to patients who have particularly tenacious cases of AD. Prior to starting dupilumab, we do recommend more extensive laboratory testing in immunosuppressed patients including transplant recipients and patients with human immunodeficiency virus. We illustrate that a history of solid-organ transplant need not exclude patients from consideration for dupilumab therapy.
- Savoia P, Cavaliere G, Zavattaro E, et al. Inflammatory cutaneous diseases in renal transplant recipients [published online August 19, 2016]. Int J Mol Sci. doi:10.3390/ijms17081362.
- Lally A, Casabonne D, Imko-Walczuk B, et al. Prevalence of benign cutaneous disease among Oxford renal transplant recipients. J Eur Acad Dermatol Venereol. 2011;25:462-470.
- Beck L, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Machura E, Chodór B, Kleszyk M, et al. Atopic allergy and chronic inflammation of the oral mucosa in a 3-year-old boy after heart transplantation—diagnostic and therapeutic difficulties. Kardiochir Torakochirurgia Pol. 2015;12:176-180.
- Beck L, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Savoia P, Cavaliere G, Zavattaro E, et al. Inflammatory cutaneous diseases in renal transplant recipients [published online August 19, 2016]. Int J Mol Sci. doi:10.3390/ijms17081362.
- Lally A, Casabonne D, Imko-Walczuk B, et al. Prevalence of benign cutaneous disease among Oxford renal transplant recipients. J Eur Acad Dermatol Venereol. 2011;25:462-470.
- Beck L, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Simpson EL, Bieber T, Guttman-Yassky E, et al; SOLO 1 and SOLO 2 Investigators. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348.
- Machura E, Chodór B, Kleszyk M, et al. Atopic allergy and chronic inflammation of the oral mucosa in a 3-year-old boy after heart transplantation—diagnostic and therapeutic difficulties. Kardiochir Torakochirurgia Pol. 2015;12:176-180.
- Beck L, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
Practice Points
- Chronic tacrolimus use in solid-organ transplant recipients may increase intestinal permeability to allergens and is a potential cause for development of atopic dermatitis (AD).
- Dupilumab has the potential to provide relief from particularly tenacious cases of AD.
- History of solid-organ transplant should not be cause for exclusion from consideration for dupilumab therapy.
Graham-Little-Piccardi-Lassueur Syndrome
To the Editor:
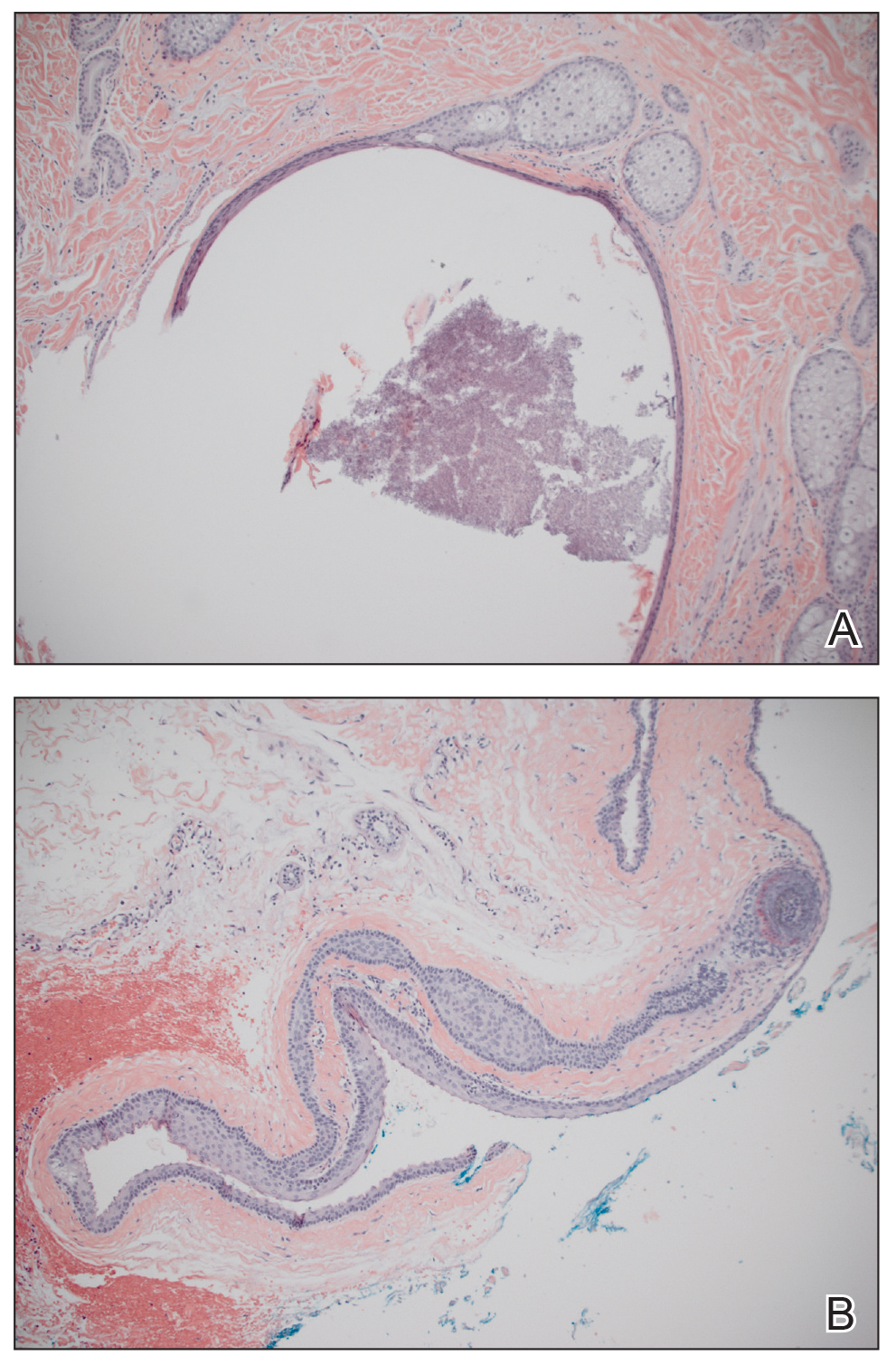
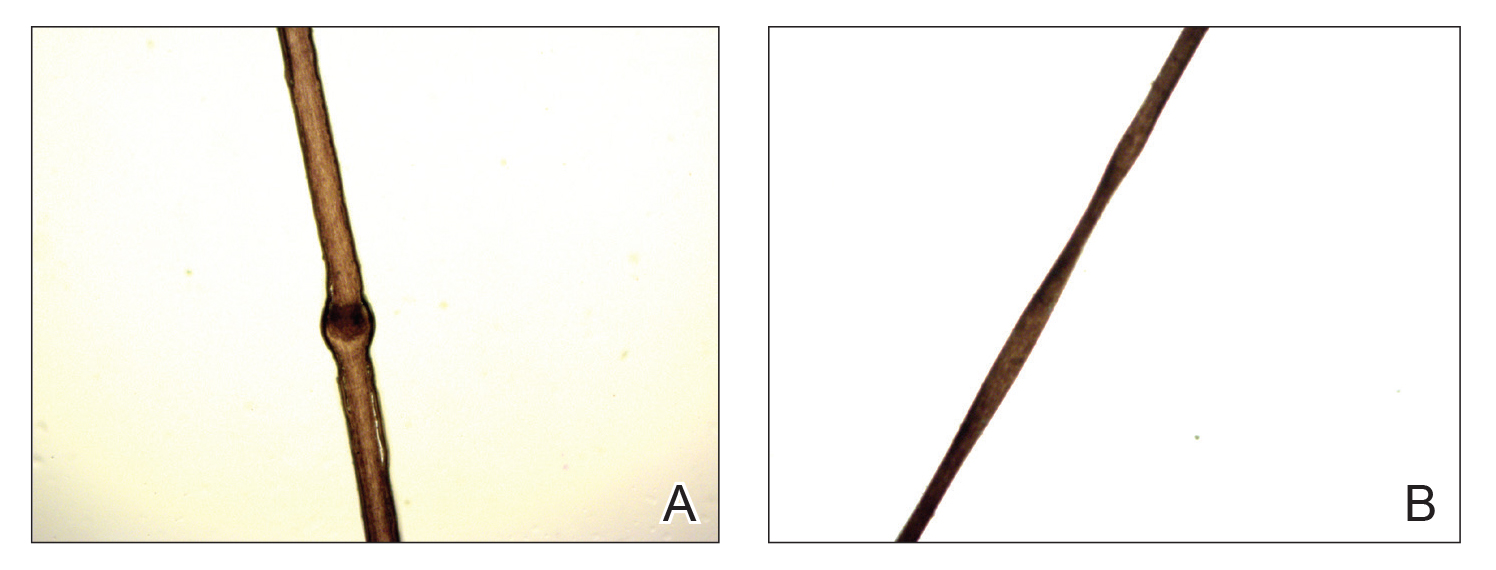
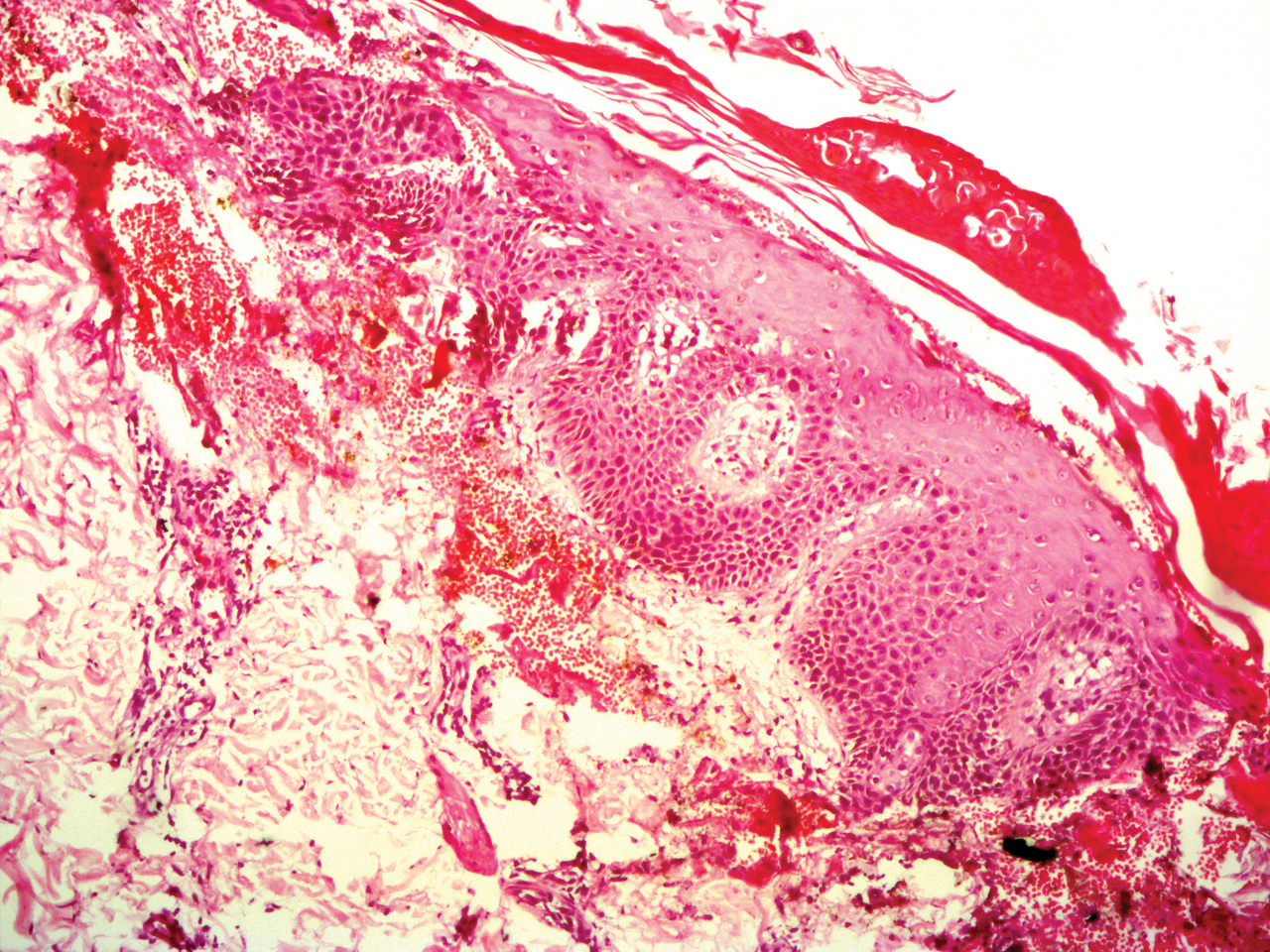
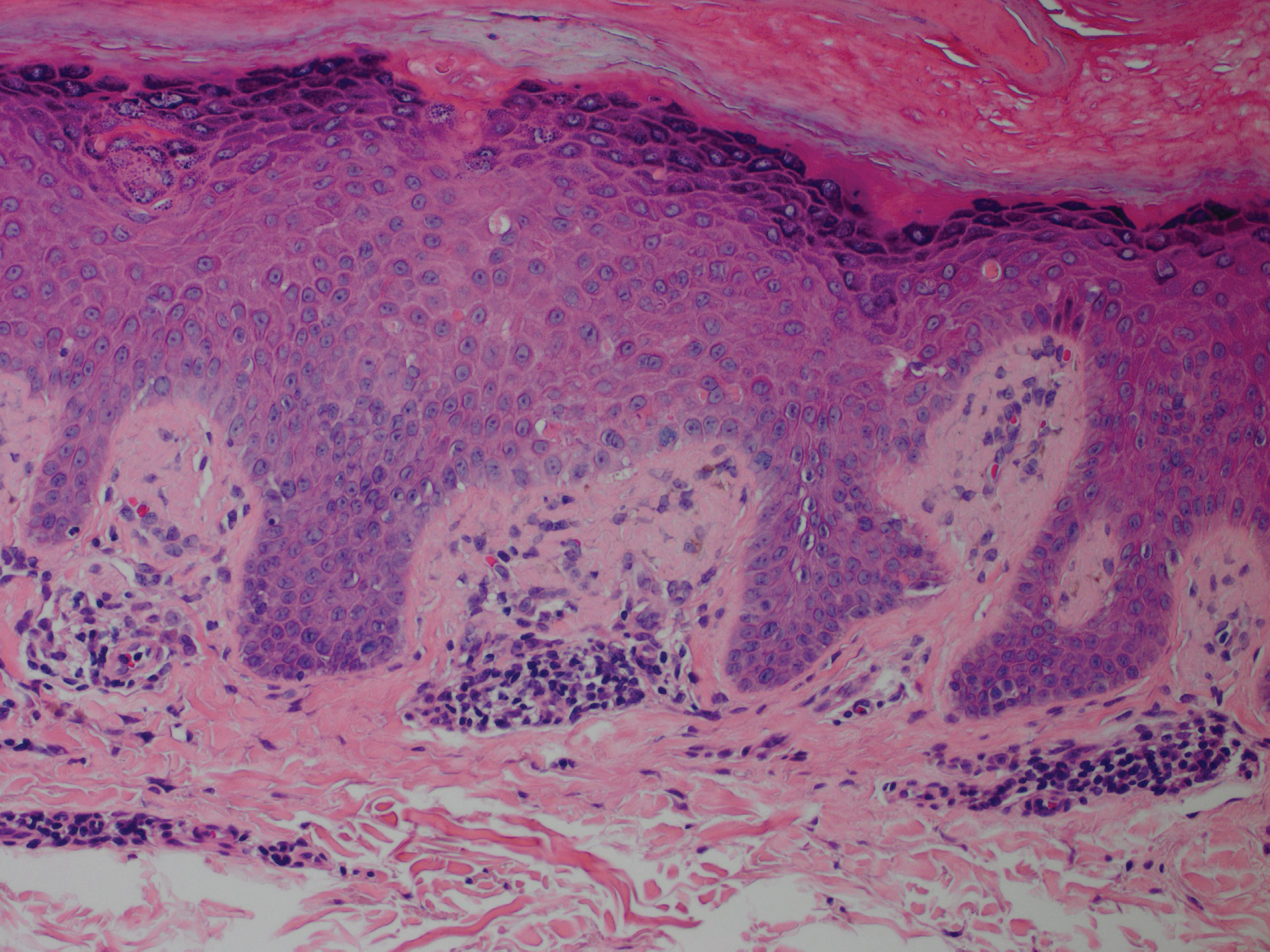
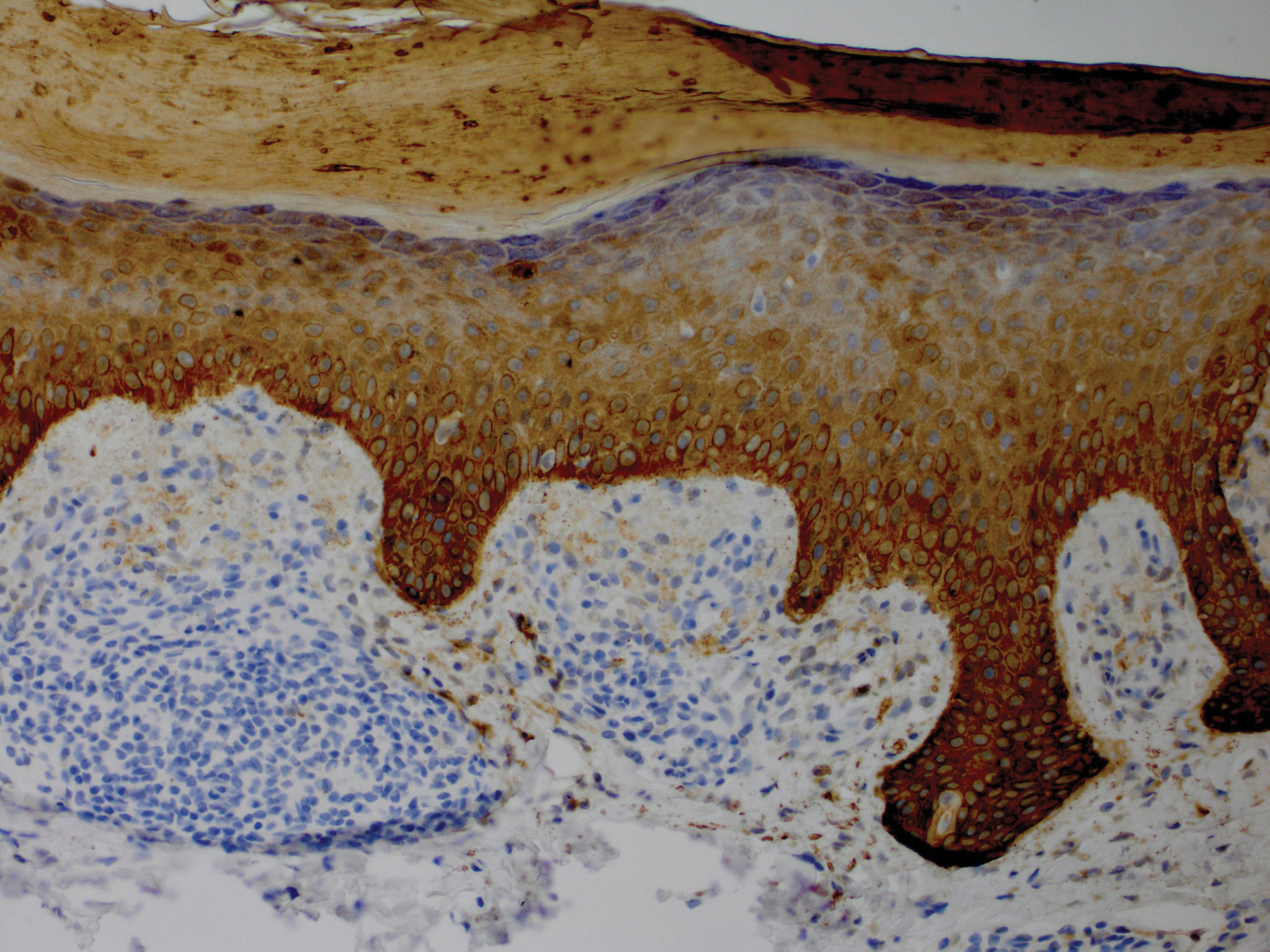
A 56-year-old white woman with a history of melanoma and hypertension presented for evaluation of progressive hair loss of more than 1 year’s duration with associated pruritis. Scalp examination revealed diffuse erythema and scarring alopecia of the bilateral parietal and temporal regions. Physical examination also revealed nonscarring alopecia of the bilateral axillae, with associated thinning of the pubic hair, eyebrows, and eyelashes, as well as keratosis pilaris on the upper arms. Biopsy of the parietal scalp revealed mild scarring alopecia with isthmic fibroplasia consistent with early lichen planopilaris (LPP)(Figure). These histologic features combined with the patient’s clinical presentation were consistent with a diagnosis of Graham-Little-Piccardi-Lassueur syndrome (GLPL).
Graham-Little-Piccardi-Lassueur syndrome was first described by Piccardi in 1913.A second case was then described by Graham-Little in 1915 in a patient referred by Lassueur, resulting in the name it bears today.1,2 The condition presents most commonly in middle-aged white women and is characterized by a triad of cicatricial alopecia of the scalp, nonscarring alopecia of the axillae and/or groin, and a rough follicular eruption on the body and/or scalp. Symptoms may not be present simultaneously. In GLPL, scarring alopecia of the scalp often precedes follicular eruptions of the trunk, arms, and legs by as much as years,2 and the inverse also has been reported.1 The inflammatory lesions of the scalp eventually resolve spontaneously, but the hair loss is by definition irreversible.
This rare condition is considered one of the 3 clinical variants of LPP. Other variants include classic LPP, also known as follicular lichen planus, and frontal fibrosing alopecia.3 More recently, fibrosing alopecia in a pattern distribution has gained some popularity as a fourth variant of LPP.4 All variants of LPP, including GLPL, result in a scarring alopecia. The classic scalp finding is an erythematous to violaceous, perifollicular, hyperkeratotic scale at the base of the terminal hairs. The population of inflamed follicles spreads outward, leaving behind a round to oval, central, atrophic scar that often is devoid of follicles. Few hairs may persist within zones of alopecia at presentation; however, these hairs are affected by inflammation and also will likely shed. A hair pull test will be positive at the margins during active disease, consisting of mostly anagen hairs on trichogram examination.1,5 Patients may develop only a single foci of hair loss, but much more commonly, a patchy multifocal alopecia is noted.6 Sites often will coalesce. Onset of scalp alopecia may be insidious or fulminant.
The nonscarring alopecia of the axillae and groin may be described as subtle thinning to complete hair loss with no signs of atrophy or inflammation. Although not commonly reported, a case of nonscarring alopecia located on the shoulders has been seen.7
The follicular eruption that can be present on the trunk, arms, or legs in GLPL is most often but not limited to keratosis pilaris, as was seen in our patient. One reported case also described lichen spinulosus as a potential variant.8 Lichen planopilaris is separate from lichen planus (LP) because of its selective follicular involvement vs the nonselective mucocutaneous distribution of LP. The 2 processes also are histologically distinct; however, estimations have shown that more than 50% of patients with GLPL experience at least 1 episode of mucosal or cutaneous LP in their lifetime.9 Rarely, coexistence of GLPL and LP lesions has been described. One reported case of GLPL and concomitant hypertrophic LP could represent a severe form of the disease.9 Additionally, lichen planus pigmentosus, an uncommon variant of LP characterized by hyperpigmented brown macules in sun-exposed areas and flexural folds, was identified in a case report of an Asian woman with GLPL.10
As a general rule, the variants of LPP most commonly are seen in postmenopausal women aged 40 to 60 years; however, rare cases in a child and a teenager have been reported.11 The GLPL variant of LPP is reported up to 4 times more frequently in females.5 Pruritus and pain are inconsistent findings, and there are no systemic signs of illness. A case of androgen insensitivity syndrome associated with GLPL suggested a potential influence of hormones in LPP.12 Stress, vitamin A deficiency, and autoimmunity also have been proposed as triggers of GLPL.13 Furthermore, familial GLPL was described in a mother and daughter, though the association was uncertain.14 Our patient had no relevant family history.
Workups to reveal the etiology of GLPL have been inconclusive. Reports of laboratory testing including complete blood cell count, basic metabolic panel, liver function tests, testosterone and dehydroepiandrosterone levels, and chest radiograph have been normal.2 Additional workup for viral triggers also has been negative.15 A case series of 29 patients with LPP and its variants, including GLPL, revealed positive antinuclear antibodies in 10% of patients and a thyroid disorder in 24% of patients, with Hashimoto thyroiditis being the most prevalent in 7% of cases.16 There may be a strong association between the comorbidities of thyroid dysfunction and GLPL, as documented in other studies.10,17 A case-control study by Mesinkovska et al17 revealed a considerable increase in the prevalence of thyroid gland disease among patients with LPP vs controls. Human leukocyte antigen DR1 was found in a familial case of GLPL,4 and a case of GLPL following hepatitis B vaccination also has been described.18
Graham-Little-Piccardi-Lassueur syndrome most likely is a T-cell mediated autoimmune condition associated with one or multiple unknown keratinocyte antigens. Autoantibodies to the inner centromere protein were identified in a case that was positive on direct immunofluorescence, which may provide more insight into the disease pathophysiology.13 Interestingly, a study comparing the concentrations of inflammatory cells in LPP and traction alopecia found an elevation in the ratio of Langerhans cells to T lymphocytes within the follicular inflammatory infiltrate of LPP.19
Histologically, cicatricial alopecia of the scalp is characterized by an interface dermatitis and a lichenoid lymphocytic infiltrate of the isthmus and infundibulum of the hair follicle sparing the bulb (Figure). A follicular plug is present in the active border. The increased pressure from the keratinous plug from above and the pressure from the infiltrate from the sides has been proposed to decrease the blood supply to the follicle and result in its death.2 Late-stage disease is notable for fibrotic longitudinal tracks of the hair follicle, perifollicular lamellar fibrosis, and adjacent epidermal atrophy.20 Direct immunofluorescence in GLPL generally is negative. A trichogram performed in a 29-year-old woman with GLPL was normal, with 84% anagen, 2% catagen, and 14% telogen hairs. It was noted that 10% of the sampled hairs were classified as dystrophical dysplastic hairs.12 Despite the lack of fibrosis on physical examination in patients with GLPL, nonscarring alopecia of the axilla and groin may show follicular destruction on microscopic examination.1 The pathology of the papules present on the trunk and extremities—whether that of keratosis pilaris or lichen spinulosus—demonstrates similar hyperkeratosis, hypergranulosis, and follicular plugging with a possible superficial, perivascular, lymphocytic infiltrate.
The differential diagnosis of GLPL includes other variants of LPP as well as discoid lupus erythematous (DLE), pseudopelade of Brocq, pityriasis rubra pilaris, sarcoidosis, acne keloidalis, central centrifugal scarring alopecia, follicular mucinosis, and folliculitis decalvans.14 Differentiation of LPP from DLE is difficult. Clinical clues include lack of central erythema and telangiectases within the lesions. Histologically, the lymphocytic dermatitis and folliculitis can be indistinguishable, but subtle findings suggesting DLE may be present, such as increased mucin in the reticular dermis, a focally thinned epidermis, and less severe dermal sclerosis when compared to cases of LPP.2 Direct immunofluorescence with IgG and C3 revealing linear granular deposits at the dermoepidermal junction is characteristic of DLE.20 Pseudopelade of Brocq is best thought of as an end-stage clinical pattern of hair loss in LPP rather than a separate condition. It is considered to be the end point of GLPL as well as DLE and others when the inflammation has subsided and the cicatricial alopecia is stable. For the duration of active disease, GLPL is classified as an unstable cicatricial alopecia that has a tendency to progress and recur periodically.20 Folliculitis decalvans also can mimic GLPL during a period when the pustules have resolved; however, a neutrophilic infiltrate will be present.
The goal of treatment in GLPL as well as other scarring alopecias is to stop the progression of hair loss. Early diagnosis is imperative if control is to be gained before considerable hair loss has occurred. Once follicular destruction has occurred as a result of the inflammation, there is minimal potential for hair rejuvenation.21 To date, treatment has been mostly fruitless, except in the management of keratosis pilaris that accompanies GLPL. First-line therapy often includes topical corticosteroids with or without intralesional corticosteroids. Systemic corticosteroids, retinoids, and psoralen plus UVA therapy also are frequently employed.1,2 Success in treating GLPL with cyclosporine A at a dosage of 4 mg/kg daily was described in several studies.1,2,15 Treatment resulted in reduction of perifollicular erythema and follicular hyperkeratotic papules as well as mild hair regrowth within the scarring patches.15 Nonetheless, cyclosporine A may prove useful in the initial inflammatory phase of GLPL. Consequently, cyclosporine A also is associated with a high relapse rate.1,2
Because the number of patients with GLPL is so few, therapy should mirror advances being made in treatments for other variants of LPP. More recent studies of LPP treatment with hydroxychloroquine showed opposing results, though the safety profile of this agent makes it an enticing treatment option.22,23 Tetracyclines showed improvement in 4 of 15 (26.7%) patients in a retrospective study by Spencer et al.24 Another retrospective study showed promising results with the potent 5-alpha reductase inhibitor dutasteride with 7 of 10 (70%) postmenopausal patients reporting stabilization over a mean duration of 28 months with no reported side effects.25 Antimalarial medications also have been implemented as adjunct therapies with mixed results.5 A case of a 26-year-old man with GLPL from South India showed systemic disease improvement following treatment with pulsed systemic steroids, isotretinoin, and anxiolytics.7 Chloroquine phosphate at a daily dose of 150 mg for 3 to 9 months yielded a transient response in one postmenopausal patient with frontal fibrosing alopecia.6 Stabilization of hair loss was achieved with a combination of hydroxychloroquine and doxycycline in a woman with GLPL who was previously unresponsive to tacrolimus ointment.10 Thalidomide showed early promise in an isolated report claiming successful treatment of LPP,26 but there is contradictory evidence, as thalidomide showed no benefit in a series of 4 patients with LPP.27
Peroxisome proliferator–activated receptor gamma (PPAR-γ), a transcription factor that regulates genes, is downregulated in LPP.28 Deletion of PPAR-γ within follicular stem cells in mice results in a phenotype similar to cicatricial alopecia. Data have supported the role of PPAR-γ in maintaining the pilosebaceous unit. A case report of pioglitazone (PPAR-γ agonist) therapy used at 15 mg daily for 8 months was successful in treating a patient with LPP.28 Further investigation must be conducted to evaluate these treatments since early attenuation of the disease process is crucial to the reduction of permanent hair loss.
Advances in the early recognition and successful treatment of GLPL are dependent on continued research in all variants of LPP. Randomized controlled trials are necessary to establish standard of care. Further studies should target the association of GLPL and other autoimmune phenomena. Moreover, research into the etiology will provide direction in understanding disease progression and outcome.
- Zegarska B, Kallas D, Schwartz RA, et al. Graham-Little syndrome. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:39-42.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Olsen EA, Bergfield WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)–sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
- Zinkernagel MS, Trueb RM. Fibrosing alopecia in a pattern distribution: patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch Dermatol. 2000;136:205-211.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: WB Saunders Company; 2016.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Pai VV, Kikkeri NN, Sori T, et al. Graham-Little Piccardi Lassueur syndrome: an unusual variant of follicular lichen planus. Int J Trichology. 2011;3:28-30.
- Srivastava M, Mikkilineni R, Konstadt J. Lassueur-Graham-Little-Piccardi syndrome. Dermatol Online J. 2007;13:12.
- Brar BK, Khanna E, Mahajan BB. Graham Little Piccardi Lasseur syndrome: a rare case report with concomitant hypertrophic lichen planus. Int J Trichology. 2011;5:199-200.
- Vashi N, Newlove T, Chu J, et al. Graham-Little-Piccardi-Lassueur syndrome. Dermatol Online J. 2011;17:30.
- Chieregato C, Zini A, Barba A, et al. Lichen planopilaris: report of 30 cases and review of the literature. Int J Dermatol. 2003;42:342-345.
- Vega Gutierrez J, Miranda-Romera A, Perez Milan F, et al. Graham Little-Piccardi-Lassueur syndrome associated with androgen insensitivity syndrome (testicular feminization). J Eur Acad Dermatol Venereol. 2004;18:463-466.
- Rodríguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Viglizzo G, Verrini A, Rongioletti F. Familial Lassueur-Graham-Little-Piccardi syndrome. Dermatology. 2004;208:142-144.
- Bianchi L, Paro Vidolin A, Piemonte P, et al. Graham Little-Piccardi-Lassueur syndrome: effective treatment with cyclosporin A. Clin Exp Dermatol. 2001;26:518-520.
- Cevasco NC, Bergfeld WF, Remzi BK, et al. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. 2007;57:47-53.
- Mesinkovska NA, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Bardazzi F, Landi C, Orlandi C, et al. Graham Little-Piccardi-Lasseur syndrome following HBV vaccination. Acta Derm Venereol. 1999;79:93.
- Hutchens KA, Balfour EM, Smoller BR. Comparison between Langerhans cell concentration in lichen planopilaris and traction alopecia with possible immunologic implications. Am J Dermatopathol. 2011;33:277-280.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-590.
- Daoud MS, Pittelkow MR. Lichen planus. In: Wolff K, Goldsmith LA, Katz Si, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: Mc Graw Hill; 2008:463-477.
- Donati A, Assouly P, Matard B, et al. Clinical and photographic assessment of lichen planopilaris treatment efficacy. J Am Acad Dermatol. 2011;64:597-599.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Spencer LA, Hawryluk EB, English JC. Lichen planopilaris: retrospective study and stepwise therapeutic approach. Arch Dermatol. 2009;145:333-334.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755
- George SJ, Hsu SJ. Lichen planopilaris treated with thalidomide. J Am Acad Dermatol. 2001;45:965-966.
- Jouanique C, Reygagne P, Bachelez H, et al. Thalidomide is ineffective in the treatment of lichen planopilaris. J Am Acad Dermatol. 2004;51:480-481.
- Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator–activated receptor γ agonist. Arch Dermatol. 2009;145:1363-1366.
To the Editor:
A 56-year-old white woman with a history of melanoma and hypertension presented for evaluation of progressive hair loss of more than 1 year’s duration with associated pruritis. Scalp examination revealed diffuse erythema and scarring alopecia of the bilateral parietal and temporal regions. Physical examination also revealed nonscarring alopecia of the bilateral axillae, with associated thinning of the pubic hair, eyebrows, and eyelashes, as well as keratosis pilaris on the upper arms. Biopsy of the parietal scalp revealed mild scarring alopecia with isthmic fibroplasia consistent with early lichen planopilaris (LPP)(Figure). These histologic features combined with the patient’s clinical presentation were consistent with a diagnosis of Graham-Little-Piccardi-Lassueur syndrome (GLPL).
Graham-Little-Piccardi-Lassueur syndrome was first described by Piccardi in 1913.A second case was then described by Graham-Little in 1915 in a patient referred by Lassueur, resulting in the name it bears today.1,2 The condition presents most commonly in middle-aged white women and is characterized by a triad of cicatricial alopecia of the scalp, nonscarring alopecia of the axillae and/or groin, and a rough follicular eruption on the body and/or scalp. Symptoms may not be present simultaneously. In GLPL, scarring alopecia of the scalp often precedes follicular eruptions of the trunk, arms, and legs by as much as years,2 and the inverse also has been reported.1 The inflammatory lesions of the scalp eventually resolve spontaneously, but the hair loss is by definition irreversible.
This rare condition is considered one of the 3 clinical variants of LPP. Other variants include classic LPP, also known as follicular lichen planus, and frontal fibrosing alopecia.3 More recently, fibrosing alopecia in a pattern distribution has gained some popularity as a fourth variant of LPP.4 All variants of LPP, including GLPL, result in a scarring alopecia. The classic scalp finding is an erythematous to violaceous, perifollicular, hyperkeratotic scale at the base of the terminal hairs. The population of inflamed follicles spreads outward, leaving behind a round to oval, central, atrophic scar that often is devoid of follicles. Few hairs may persist within zones of alopecia at presentation; however, these hairs are affected by inflammation and also will likely shed. A hair pull test will be positive at the margins during active disease, consisting of mostly anagen hairs on trichogram examination.1,5 Patients may develop only a single foci of hair loss, but much more commonly, a patchy multifocal alopecia is noted.6 Sites often will coalesce. Onset of scalp alopecia may be insidious or fulminant.
The nonscarring alopecia of the axillae and groin may be described as subtle thinning to complete hair loss with no signs of atrophy or inflammation. Although not commonly reported, a case of nonscarring alopecia located on the shoulders has been seen.7
The follicular eruption that can be present on the trunk, arms, or legs in GLPL is most often but not limited to keratosis pilaris, as was seen in our patient. One reported case also described lichen spinulosus as a potential variant.8 Lichen planopilaris is separate from lichen planus (LP) because of its selective follicular involvement vs the nonselective mucocutaneous distribution of LP. The 2 processes also are histologically distinct; however, estimations have shown that more than 50% of patients with GLPL experience at least 1 episode of mucosal or cutaneous LP in their lifetime.9 Rarely, coexistence of GLPL and LP lesions has been described. One reported case of GLPL and concomitant hypertrophic LP could represent a severe form of the disease.9 Additionally, lichen planus pigmentosus, an uncommon variant of LP characterized by hyperpigmented brown macules in sun-exposed areas and flexural folds, was identified in a case report of an Asian woman with GLPL.10
As a general rule, the variants of LPP most commonly are seen in postmenopausal women aged 40 to 60 years; however, rare cases in a child and a teenager have been reported.11 The GLPL variant of LPP is reported up to 4 times more frequently in females.5 Pruritus and pain are inconsistent findings, and there are no systemic signs of illness. A case of androgen insensitivity syndrome associated with GLPL suggested a potential influence of hormones in LPP.12 Stress, vitamin A deficiency, and autoimmunity also have been proposed as triggers of GLPL.13 Furthermore, familial GLPL was described in a mother and daughter, though the association was uncertain.14 Our patient had no relevant family history.
Workups to reveal the etiology of GLPL have been inconclusive. Reports of laboratory testing including complete blood cell count, basic metabolic panel, liver function tests, testosterone and dehydroepiandrosterone levels, and chest radiograph have been normal.2 Additional workup for viral triggers also has been negative.15 A case series of 29 patients with LPP and its variants, including GLPL, revealed positive antinuclear antibodies in 10% of patients and a thyroid disorder in 24% of patients, with Hashimoto thyroiditis being the most prevalent in 7% of cases.16 There may be a strong association between the comorbidities of thyroid dysfunction and GLPL, as documented in other studies.10,17 A case-control study by Mesinkovska et al17 revealed a considerable increase in the prevalence of thyroid gland disease among patients with LPP vs controls. Human leukocyte antigen DR1 was found in a familial case of GLPL,4 and a case of GLPL following hepatitis B vaccination also has been described.18
Graham-Little-Piccardi-Lassueur syndrome most likely is a T-cell mediated autoimmune condition associated with one or multiple unknown keratinocyte antigens. Autoantibodies to the inner centromere protein were identified in a case that was positive on direct immunofluorescence, which may provide more insight into the disease pathophysiology.13 Interestingly, a study comparing the concentrations of inflammatory cells in LPP and traction alopecia found an elevation in the ratio of Langerhans cells to T lymphocytes within the follicular inflammatory infiltrate of LPP.19
Histologically, cicatricial alopecia of the scalp is characterized by an interface dermatitis and a lichenoid lymphocytic infiltrate of the isthmus and infundibulum of the hair follicle sparing the bulb (Figure). A follicular plug is present in the active border. The increased pressure from the keratinous plug from above and the pressure from the infiltrate from the sides has been proposed to decrease the blood supply to the follicle and result in its death.2 Late-stage disease is notable for fibrotic longitudinal tracks of the hair follicle, perifollicular lamellar fibrosis, and adjacent epidermal atrophy.20 Direct immunofluorescence in GLPL generally is negative. A trichogram performed in a 29-year-old woman with GLPL was normal, with 84% anagen, 2% catagen, and 14% telogen hairs. It was noted that 10% of the sampled hairs were classified as dystrophical dysplastic hairs.12 Despite the lack of fibrosis on physical examination in patients with GLPL, nonscarring alopecia of the axilla and groin may show follicular destruction on microscopic examination.1 The pathology of the papules present on the trunk and extremities—whether that of keratosis pilaris or lichen spinulosus—demonstrates similar hyperkeratosis, hypergranulosis, and follicular plugging with a possible superficial, perivascular, lymphocytic infiltrate.
The differential diagnosis of GLPL includes other variants of LPP as well as discoid lupus erythematous (DLE), pseudopelade of Brocq, pityriasis rubra pilaris, sarcoidosis, acne keloidalis, central centrifugal scarring alopecia, follicular mucinosis, and folliculitis decalvans.14 Differentiation of LPP from DLE is difficult. Clinical clues include lack of central erythema and telangiectases within the lesions. Histologically, the lymphocytic dermatitis and folliculitis can be indistinguishable, but subtle findings suggesting DLE may be present, such as increased mucin in the reticular dermis, a focally thinned epidermis, and less severe dermal sclerosis when compared to cases of LPP.2 Direct immunofluorescence with IgG and C3 revealing linear granular deposits at the dermoepidermal junction is characteristic of DLE.20 Pseudopelade of Brocq is best thought of as an end-stage clinical pattern of hair loss in LPP rather than a separate condition. It is considered to be the end point of GLPL as well as DLE and others when the inflammation has subsided and the cicatricial alopecia is stable. For the duration of active disease, GLPL is classified as an unstable cicatricial alopecia that has a tendency to progress and recur periodically.20 Folliculitis decalvans also can mimic GLPL during a period when the pustules have resolved; however, a neutrophilic infiltrate will be present.
The goal of treatment in GLPL as well as other scarring alopecias is to stop the progression of hair loss. Early diagnosis is imperative if control is to be gained before considerable hair loss has occurred. Once follicular destruction has occurred as a result of the inflammation, there is minimal potential for hair rejuvenation.21 To date, treatment has been mostly fruitless, except in the management of keratosis pilaris that accompanies GLPL. First-line therapy often includes topical corticosteroids with or without intralesional corticosteroids. Systemic corticosteroids, retinoids, and psoralen plus UVA therapy also are frequently employed.1,2 Success in treating GLPL with cyclosporine A at a dosage of 4 mg/kg daily was described in several studies.1,2,15 Treatment resulted in reduction of perifollicular erythema and follicular hyperkeratotic papules as well as mild hair regrowth within the scarring patches.15 Nonetheless, cyclosporine A may prove useful in the initial inflammatory phase of GLPL. Consequently, cyclosporine A also is associated with a high relapse rate.1,2
Because the number of patients with GLPL is so few, therapy should mirror advances being made in treatments for other variants of LPP. More recent studies of LPP treatment with hydroxychloroquine showed opposing results, though the safety profile of this agent makes it an enticing treatment option.22,23 Tetracyclines showed improvement in 4 of 15 (26.7%) patients in a retrospective study by Spencer et al.24 Another retrospective study showed promising results with the potent 5-alpha reductase inhibitor dutasteride with 7 of 10 (70%) postmenopausal patients reporting stabilization over a mean duration of 28 months with no reported side effects.25 Antimalarial medications also have been implemented as adjunct therapies with mixed results.5 A case of a 26-year-old man with GLPL from South India showed systemic disease improvement following treatment with pulsed systemic steroids, isotretinoin, and anxiolytics.7 Chloroquine phosphate at a daily dose of 150 mg for 3 to 9 months yielded a transient response in one postmenopausal patient with frontal fibrosing alopecia.6 Stabilization of hair loss was achieved with a combination of hydroxychloroquine and doxycycline in a woman with GLPL who was previously unresponsive to tacrolimus ointment.10 Thalidomide showed early promise in an isolated report claiming successful treatment of LPP,26 but there is contradictory evidence, as thalidomide showed no benefit in a series of 4 patients with LPP.27
Peroxisome proliferator–activated receptor gamma (PPAR-γ), a transcription factor that regulates genes, is downregulated in LPP.28 Deletion of PPAR-γ within follicular stem cells in mice results in a phenotype similar to cicatricial alopecia. Data have supported the role of PPAR-γ in maintaining the pilosebaceous unit. A case report of pioglitazone (PPAR-γ agonist) therapy used at 15 mg daily for 8 months was successful in treating a patient with LPP.28 Further investigation must be conducted to evaluate these treatments since early attenuation of the disease process is crucial to the reduction of permanent hair loss.
Advances in the early recognition and successful treatment of GLPL are dependent on continued research in all variants of LPP. Randomized controlled trials are necessary to establish standard of care. Further studies should target the association of GLPL and other autoimmune phenomena. Moreover, research into the etiology will provide direction in understanding disease progression and outcome.
To the Editor:
A 56-year-old white woman with a history of melanoma and hypertension presented for evaluation of progressive hair loss of more than 1 year’s duration with associated pruritis. Scalp examination revealed diffuse erythema and scarring alopecia of the bilateral parietal and temporal regions. Physical examination also revealed nonscarring alopecia of the bilateral axillae, with associated thinning of the pubic hair, eyebrows, and eyelashes, as well as keratosis pilaris on the upper arms. Biopsy of the parietal scalp revealed mild scarring alopecia with isthmic fibroplasia consistent with early lichen planopilaris (LPP)(Figure). These histologic features combined with the patient’s clinical presentation were consistent with a diagnosis of Graham-Little-Piccardi-Lassueur syndrome (GLPL).
Graham-Little-Piccardi-Lassueur syndrome was first described by Piccardi in 1913.A second case was then described by Graham-Little in 1915 in a patient referred by Lassueur, resulting in the name it bears today.1,2 The condition presents most commonly in middle-aged white women and is characterized by a triad of cicatricial alopecia of the scalp, nonscarring alopecia of the axillae and/or groin, and a rough follicular eruption on the body and/or scalp. Symptoms may not be present simultaneously. In GLPL, scarring alopecia of the scalp often precedes follicular eruptions of the trunk, arms, and legs by as much as years,2 and the inverse also has been reported.1 The inflammatory lesions of the scalp eventually resolve spontaneously, but the hair loss is by definition irreversible.
This rare condition is considered one of the 3 clinical variants of LPP. Other variants include classic LPP, also known as follicular lichen planus, and frontal fibrosing alopecia.3 More recently, fibrosing alopecia in a pattern distribution has gained some popularity as a fourth variant of LPP.4 All variants of LPP, including GLPL, result in a scarring alopecia. The classic scalp finding is an erythematous to violaceous, perifollicular, hyperkeratotic scale at the base of the terminal hairs. The population of inflamed follicles spreads outward, leaving behind a round to oval, central, atrophic scar that often is devoid of follicles. Few hairs may persist within zones of alopecia at presentation; however, these hairs are affected by inflammation and also will likely shed. A hair pull test will be positive at the margins during active disease, consisting of mostly anagen hairs on trichogram examination.1,5 Patients may develop only a single foci of hair loss, but much more commonly, a patchy multifocal alopecia is noted.6 Sites often will coalesce. Onset of scalp alopecia may be insidious or fulminant.
The nonscarring alopecia of the axillae and groin may be described as subtle thinning to complete hair loss with no signs of atrophy or inflammation. Although not commonly reported, a case of nonscarring alopecia located on the shoulders has been seen.7
The follicular eruption that can be present on the trunk, arms, or legs in GLPL is most often but not limited to keratosis pilaris, as was seen in our patient. One reported case also described lichen spinulosus as a potential variant.8 Lichen planopilaris is separate from lichen planus (LP) because of its selective follicular involvement vs the nonselective mucocutaneous distribution of LP. The 2 processes also are histologically distinct; however, estimations have shown that more than 50% of patients with GLPL experience at least 1 episode of mucosal or cutaneous LP in their lifetime.9 Rarely, coexistence of GLPL and LP lesions has been described. One reported case of GLPL and concomitant hypertrophic LP could represent a severe form of the disease.9 Additionally, lichen planus pigmentosus, an uncommon variant of LP characterized by hyperpigmented brown macules in sun-exposed areas and flexural folds, was identified in a case report of an Asian woman with GLPL.10
As a general rule, the variants of LPP most commonly are seen in postmenopausal women aged 40 to 60 years; however, rare cases in a child and a teenager have been reported.11 The GLPL variant of LPP is reported up to 4 times more frequently in females.5 Pruritus and pain are inconsistent findings, and there are no systemic signs of illness. A case of androgen insensitivity syndrome associated with GLPL suggested a potential influence of hormones in LPP.12 Stress, vitamin A deficiency, and autoimmunity also have been proposed as triggers of GLPL.13 Furthermore, familial GLPL was described in a mother and daughter, though the association was uncertain.14 Our patient had no relevant family history.
Workups to reveal the etiology of GLPL have been inconclusive. Reports of laboratory testing including complete blood cell count, basic metabolic panel, liver function tests, testosterone and dehydroepiandrosterone levels, and chest radiograph have been normal.2 Additional workup for viral triggers also has been negative.15 A case series of 29 patients with LPP and its variants, including GLPL, revealed positive antinuclear antibodies in 10% of patients and a thyroid disorder in 24% of patients, with Hashimoto thyroiditis being the most prevalent in 7% of cases.16 There may be a strong association between the comorbidities of thyroid dysfunction and GLPL, as documented in other studies.10,17 A case-control study by Mesinkovska et al17 revealed a considerable increase in the prevalence of thyroid gland disease among patients with LPP vs controls. Human leukocyte antigen DR1 was found in a familial case of GLPL,4 and a case of GLPL following hepatitis B vaccination also has been described.18
Graham-Little-Piccardi-Lassueur syndrome most likely is a T-cell mediated autoimmune condition associated with one or multiple unknown keratinocyte antigens. Autoantibodies to the inner centromere protein were identified in a case that was positive on direct immunofluorescence, which may provide more insight into the disease pathophysiology.13 Interestingly, a study comparing the concentrations of inflammatory cells in LPP and traction alopecia found an elevation in the ratio of Langerhans cells to T lymphocytes within the follicular inflammatory infiltrate of LPP.19
Histologically, cicatricial alopecia of the scalp is characterized by an interface dermatitis and a lichenoid lymphocytic infiltrate of the isthmus and infundibulum of the hair follicle sparing the bulb (Figure). A follicular plug is present in the active border. The increased pressure from the keratinous plug from above and the pressure from the infiltrate from the sides has been proposed to decrease the blood supply to the follicle and result in its death.2 Late-stage disease is notable for fibrotic longitudinal tracks of the hair follicle, perifollicular lamellar fibrosis, and adjacent epidermal atrophy.20 Direct immunofluorescence in GLPL generally is negative. A trichogram performed in a 29-year-old woman with GLPL was normal, with 84% anagen, 2% catagen, and 14% telogen hairs. It was noted that 10% of the sampled hairs were classified as dystrophical dysplastic hairs.12 Despite the lack of fibrosis on physical examination in patients with GLPL, nonscarring alopecia of the axilla and groin may show follicular destruction on microscopic examination.1 The pathology of the papules present on the trunk and extremities—whether that of keratosis pilaris or lichen spinulosus—demonstrates similar hyperkeratosis, hypergranulosis, and follicular plugging with a possible superficial, perivascular, lymphocytic infiltrate.
The differential diagnosis of GLPL includes other variants of LPP as well as discoid lupus erythematous (DLE), pseudopelade of Brocq, pityriasis rubra pilaris, sarcoidosis, acne keloidalis, central centrifugal scarring alopecia, follicular mucinosis, and folliculitis decalvans.14 Differentiation of LPP from DLE is difficult. Clinical clues include lack of central erythema and telangiectases within the lesions. Histologically, the lymphocytic dermatitis and folliculitis can be indistinguishable, but subtle findings suggesting DLE may be present, such as increased mucin in the reticular dermis, a focally thinned epidermis, and less severe dermal sclerosis when compared to cases of LPP.2 Direct immunofluorescence with IgG and C3 revealing linear granular deposits at the dermoepidermal junction is characteristic of DLE.20 Pseudopelade of Brocq is best thought of as an end-stage clinical pattern of hair loss in LPP rather than a separate condition. It is considered to be the end point of GLPL as well as DLE and others when the inflammation has subsided and the cicatricial alopecia is stable. For the duration of active disease, GLPL is classified as an unstable cicatricial alopecia that has a tendency to progress and recur periodically.20 Folliculitis decalvans also can mimic GLPL during a period when the pustules have resolved; however, a neutrophilic infiltrate will be present.
The goal of treatment in GLPL as well as other scarring alopecias is to stop the progression of hair loss. Early diagnosis is imperative if control is to be gained before considerable hair loss has occurred. Once follicular destruction has occurred as a result of the inflammation, there is minimal potential for hair rejuvenation.21 To date, treatment has been mostly fruitless, except in the management of keratosis pilaris that accompanies GLPL. First-line therapy often includes topical corticosteroids with or without intralesional corticosteroids. Systemic corticosteroids, retinoids, and psoralen plus UVA therapy also are frequently employed.1,2 Success in treating GLPL with cyclosporine A at a dosage of 4 mg/kg daily was described in several studies.1,2,15 Treatment resulted in reduction of perifollicular erythema and follicular hyperkeratotic papules as well as mild hair regrowth within the scarring patches.15 Nonetheless, cyclosporine A may prove useful in the initial inflammatory phase of GLPL. Consequently, cyclosporine A also is associated with a high relapse rate.1,2
Because the number of patients with GLPL is so few, therapy should mirror advances being made in treatments for other variants of LPP. More recent studies of LPP treatment with hydroxychloroquine showed opposing results, though the safety profile of this agent makes it an enticing treatment option.22,23 Tetracyclines showed improvement in 4 of 15 (26.7%) patients in a retrospective study by Spencer et al.24 Another retrospective study showed promising results with the potent 5-alpha reductase inhibitor dutasteride with 7 of 10 (70%) postmenopausal patients reporting stabilization over a mean duration of 28 months with no reported side effects.25 Antimalarial medications also have been implemented as adjunct therapies with mixed results.5 A case of a 26-year-old man with GLPL from South India showed systemic disease improvement following treatment with pulsed systemic steroids, isotretinoin, and anxiolytics.7 Chloroquine phosphate at a daily dose of 150 mg for 3 to 9 months yielded a transient response in one postmenopausal patient with frontal fibrosing alopecia.6 Stabilization of hair loss was achieved with a combination of hydroxychloroquine and doxycycline in a woman with GLPL who was previously unresponsive to tacrolimus ointment.10 Thalidomide showed early promise in an isolated report claiming successful treatment of LPP,26 but there is contradictory evidence, as thalidomide showed no benefit in a series of 4 patients with LPP.27
Peroxisome proliferator–activated receptor gamma (PPAR-γ), a transcription factor that regulates genes, is downregulated in LPP.28 Deletion of PPAR-γ within follicular stem cells in mice results in a phenotype similar to cicatricial alopecia. Data have supported the role of PPAR-γ in maintaining the pilosebaceous unit. A case report of pioglitazone (PPAR-γ agonist) therapy used at 15 mg daily for 8 months was successful in treating a patient with LPP.28 Further investigation must be conducted to evaluate these treatments since early attenuation of the disease process is crucial to the reduction of permanent hair loss.
Advances in the early recognition and successful treatment of GLPL are dependent on continued research in all variants of LPP. Randomized controlled trials are necessary to establish standard of care. Further studies should target the association of GLPL and other autoimmune phenomena. Moreover, research into the etiology will provide direction in understanding disease progression and outcome.
- Zegarska B, Kallas D, Schwartz RA, et al. Graham-Little syndrome. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:39-42.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Olsen EA, Bergfield WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)–sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
- Zinkernagel MS, Trueb RM. Fibrosing alopecia in a pattern distribution: patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch Dermatol. 2000;136:205-211.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: WB Saunders Company; 2016.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Pai VV, Kikkeri NN, Sori T, et al. Graham-Little Piccardi Lassueur syndrome: an unusual variant of follicular lichen planus. Int J Trichology. 2011;3:28-30.
- Srivastava M, Mikkilineni R, Konstadt J. Lassueur-Graham-Little-Piccardi syndrome. Dermatol Online J. 2007;13:12.
- Brar BK, Khanna E, Mahajan BB. Graham Little Piccardi Lasseur syndrome: a rare case report with concomitant hypertrophic lichen planus. Int J Trichology. 2011;5:199-200.
- Vashi N, Newlove T, Chu J, et al. Graham-Little-Piccardi-Lassueur syndrome. Dermatol Online J. 2011;17:30.
- Chieregato C, Zini A, Barba A, et al. Lichen planopilaris: report of 30 cases and review of the literature. Int J Dermatol. 2003;42:342-345.
- Vega Gutierrez J, Miranda-Romera A, Perez Milan F, et al. Graham Little-Piccardi-Lassueur syndrome associated with androgen insensitivity syndrome (testicular feminization). J Eur Acad Dermatol Venereol. 2004;18:463-466.
- Rodríguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Viglizzo G, Verrini A, Rongioletti F. Familial Lassueur-Graham-Little-Piccardi syndrome. Dermatology. 2004;208:142-144.
- Bianchi L, Paro Vidolin A, Piemonte P, et al. Graham Little-Piccardi-Lassueur syndrome: effective treatment with cyclosporin A. Clin Exp Dermatol. 2001;26:518-520.
- Cevasco NC, Bergfeld WF, Remzi BK, et al. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. 2007;57:47-53.
- Mesinkovska NA, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Bardazzi F, Landi C, Orlandi C, et al. Graham Little-Piccardi-Lasseur syndrome following HBV vaccination. Acta Derm Venereol. 1999;79:93.
- Hutchens KA, Balfour EM, Smoller BR. Comparison between Langerhans cell concentration in lichen planopilaris and traction alopecia with possible immunologic implications. Am J Dermatopathol. 2011;33:277-280.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-590.
- Daoud MS, Pittelkow MR. Lichen planus. In: Wolff K, Goldsmith LA, Katz Si, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: Mc Graw Hill; 2008:463-477.
- Donati A, Assouly P, Matard B, et al. Clinical and photographic assessment of lichen planopilaris treatment efficacy. J Am Acad Dermatol. 2011;64:597-599.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Spencer LA, Hawryluk EB, English JC. Lichen planopilaris: retrospective study and stepwise therapeutic approach. Arch Dermatol. 2009;145:333-334.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755
- George SJ, Hsu SJ. Lichen planopilaris treated with thalidomide. J Am Acad Dermatol. 2001;45:965-966.
- Jouanique C, Reygagne P, Bachelez H, et al. Thalidomide is ineffective in the treatment of lichen planopilaris. J Am Acad Dermatol. 2004;51:480-481.
- Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator–activated receptor γ agonist. Arch Dermatol. 2009;145:1363-1366.
- Zegarska B, Kallas D, Schwartz RA, et al. Graham-Little syndrome. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:39-42.
- Assouly P, Reygagne P. Lichen planopilaris: update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3-10.
- Olsen EA, Bergfield WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)–sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110.
- Zinkernagel MS, Trueb RM. Fibrosing alopecia in a pattern distribution: patterned lichen planopilaris or androgenetic alopecia with a lichenoid tissue reaction pattern? Arch Dermatol. 2000;136:205-211.
- James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 12th ed. Philadelphia, PA: WB Saunders Company; 2016.
- Kossard S, Lee MS, Wilkinson B. Postmenopausal frontal fibrosing alopecia: a frontal variant of lichen planopilaris. J Am Acad Dermatol. 1997;36:59-66.
- Pai VV, Kikkeri NN, Sori T, et al. Graham-Little Piccardi Lassueur syndrome: an unusual variant of follicular lichen planus. Int J Trichology. 2011;3:28-30.
- Srivastava M, Mikkilineni R, Konstadt J. Lassueur-Graham-Little-Piccardi syndrome. Dermatol Online J. 2007;13:12.
- Brar BK, Khanna E, Mahajan BB. Graham Little Piccardi Lasseur syndrome: a rare case report with concomitant hypertrophic lichen planus. Int J Trichology. 2011;5:199-200.
- Vashi N, Newlove T, Chu J, et al. Graham-Little-Piccardi-Lassueur syndrome. Dermatol Online J. 2011;17:30.
- Chieregato C, Zini A, Barba A, et al. Lichen planopilaris: report of 30 cases and review of the literature. Int J Dermatol. 2003;42:342-345.
- Vega Gutierrez J, Miranda-Romera A, Perez Milan F, et al. Graham Little-Piccardi-Lassueur syndrome associated with androgen insensitivity syndrome (testicular feminization). J Eur Acad Dermatol Venereol. 2004;18:463-466.
- Rodríguez-Bayona B, Ruchaud S, Rodriguez C, et al. Autoantibodies against the chromosomal passenger protein INCENP found in a patient with Graham Little-Piccardi-Lassueur syndrome. J Autoimmune Dis. 2007;4:1.
- Viglizzo G, Verrini A, Rongioletti F. Familial Lassueur-Graham-Little-Piccardi syndrome. Dermatology. 2004;208:142-144.
- Bianchi L, Paro Vidolin A, Piemonte P, et al. Graham Little-Piccardi-Lassueur syndrome: effective treatment with cyclosporin A. Clin Exp Dermatol. 2001;26:518-520.
- Cevasco NC, Bergfeld WF, Remzi BK, et al. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. 2007;57:47-53.
- Mesinkovska NA, Brankov N, Piliang M, et al. Association of lichen planopilaris with thyroid disease: a retrospective case-control study. J Am Acad Dermatol. 2014;70:889-892.
- Bardazzi F, Landi C, Orlandi C, et al. Graham Little-Piccardi-Lasseur syndrome following HBV vaccination. Acta Derm Venereol. 1999;79:93.
- Hutchens KA, Balfour EM, Smoller BR. Comparison between Langerhans cell concentration in lichen planopilaris and traction alopecia with possible immunologic implications. Am J Dermatopathol. 2011;33:277-280.
- Dogra S, Sarangal R. What’s new in cicatricial alopecia? Indian J Dermatol Venereol Leprol. 2013;79:576-590.
- Daoud MS, Pittelkow MR. Lichen planus. In: Wolff K, Goldsmith LA, Katz Si, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: Mc Graw Hill; 2008:463-477.
- Donati A, Assouly P, Matard B, et al. Clinical and photographic assessment of lichen planopilaris treatment efficacy. J Am Acad Dermatol. 2011;64:597-599.
- Samrao A, Chew AL, Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296-1300.
- Spencer LA, Hawryluk EB, English JC. Lichen planopilaris: retrospective study and stepwise therapeutic approach. Arch Dermatol. 2009;145:333-334.
- Ladizinski B, Bazakas A, Selim MA, et al. Frontal fibrosing alopecia: a retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749-755
- George SJ, Hsu SJ. Lichen planopilaris treated with thalidomide. J Am Acad Dermatol. 2001;45:965-966.
- Jouanique C, Reygagne P, Bachelez H, et al. Thalidomide is ineffective in the treatment of lichen planopilaris. J Am Acad Dermatol. 2004;51:480-481.
- Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator–activated receptor γ agonist. Arch Dermatol. 2009;145:1363-1366.
Practice Points
- Graham-Little-Piccardi-Lassueur syndrome (GLPL) is characterized by a triad of cicatricial alopecia of the scalp, nonscarring alopecia of the axillae and/or groin, and a rough follicular eruption on the body and/or scalp.
- Graham-Little-Piccardi-Lassueur syndrome is considered one of the 3 clinical variants of lichen planopilaris.
- Potential therapies for GLPL include hydroxychloroquine, cyclosporine, tetracyclines, and pioglitazone.
Multiple Subcutaneous Dermoid Cysts
To the Editor:
A 30-year-old man with no notable medical history presented to the dermatology clinic with multiple subcutaneous nodules on the forehead of 5 years’ duration. He reported no history of forehead trauma or manipulation of the lesions, and there was no accompanying pruritis, pain, erythema, or purulent discharge. There was no family history of skin or gastrointestinal tract tumors. On physical examination, the patient had 5 firm, flesh-colored to yellow nodules measuring approximately 0.2 to 1.5 cm in diameter without central punctae scattered over the central forehead (Figure 1). Due to cosmetic concerns, the patient elected to pursue surgical excision of the lesions, which occurred over several office visits. During surgical excision, the lesions were found to be smooth, encapsulated, and mobile, and they were excised without surgical complication. Histopathologic examination showed subcutaneous cysts lined by squamous epithelium with associated sebaceous glands (Figure 2A) and hair follicles in the cyst lumen (Figure 2B). These findings confirmed the diagnosis of multiple subcutaneous dermoid cysts.
Dermoid cysts are relatively uncommon, benign tumors consisting of tissue derived from ectodermal and mesodermal germ cell layers. Dermoid cysts may be distinguished from teratomas, which may contain tissues derived from all 3 germ cell layers and typically consist of types of tissues foreign to the site of origin, such as dental, thyroid, gastrointestinal, or neural tissue.1,2 The majority of dermoid cysts are congenitally developed along the lines of embryologic fusion due to an error in the division of the ectoderm and mesoderm3,4; however, some dermoid cysts may be acquired from epidermal elements being traumatically implanted into the dermis.5
Our patient’s presentation with multiple dermoid cysts was atypical, as dermoid cysts are almost always solitary tumors. A similar case was reported in a 41-year-old man who developed multiple dermoid cysts on the forehead over a 20-year period.This patient also was otherwise healthy, denied prior trauma to the forehead, and reported no family history of skin or gastrointestinal tract tumors.5
Another unusual feature in our case was the location of the dermoid cysts on the central forehead. The most common location for dermoid cysts is the lateral third of the eyebrows (47%–70% of cases).1,4,6-10 These cysts occur because of sequestration of the surface ectoderm during fusion along the naso-optic groove.2 Dermoid cysts also have been noted in other anatomical areas such as the scalp, nose, anterior neck, and trunk.6
Dermoid cysts tend to be small, round, smooth, and slowly growing until sudden enlargement prompts surgical evaluation.4,6 During surgical excision, they often are fixed to the underlying bone but also may be freely mobile, as in our patient.6 Histopathologic examination reveals a stratified squamous epithelium with associated adnexal structures such as sebaceous glands or hair follicles.1 Smooth muscle fibers, prominent vascular stroma, small nerves, and collagen and elastic fibers also may be found within the lumen of dermoid cysts.2
In some cases, dermoid cysts may be invasive and carry the risk of bony erosion, intracranial extension, osteomyelitis, meningitis, or cerebral abscess. Imaging studies sometimes are needed to rule out intracranial or intraspinal extension, particularly for midline dermoid cysts.6 The standard of treatment for dermoid cysts is surgical excision and complete enucleation without disruption of the cyst wall; however, invasive dermoid cysts may require endoscopic excision, orbitotomy, or craniotomy.4,6
- Brownstein MH, Helwig EB. Subcutaneous dermoid cysts. Arch Dermatol. 1973;107:237-239.
- Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437-1455.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Yamaki T, Higuchi R, Sasaki K, et al. Multiple dermoid cysts on the forehead. case report. Scand J Plast Reconstr Surg Hand Surg. 1996;30:321-324.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Al-Khateeb TH, Al-Masri NM, Al-Zoubi F. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2009;67:52-57.
- McAvoy JM, Zuckerbraun L. Dermoid cysts of the head and neck in children. Arch Otolaryngol. 1976;102:529-531.
- Taylor BW, Erich JB, Dockerty MB. Dermoids of the head and neck. Minnesota Med. 1966;49:1535-1540.
- Golden BA, Zide MF. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2005;63:1613-1619.
To the Editor:
A 30-year-old man with no notable medical history presented to the dermatology clinic with multiple subcutaneous nodules on the forehead of 5 years’ duration. He reported no history of forehead trauma or manipulation of the lesions, and there was no accompanying pruritis, pain, erythema, or purulent discharge. There was no family history of skin or gastrointestinal tract tumors. On physical examination, the patient had 5 firm, flesh-colored to yellow nodules measuring approximately 0.2 to 1.5 cm in diameter without central punctae scattered over the central forehead (Figure 1). Due to cosmetic concerns, the patient elected to pursue surgical excision of the lesions, which occurred over several office visits. During surgical excision, the lesions were found to be smooth, encapsulated, and mobile, and they were excised without surgical complication. Histopathologic examination showed subcutaneous cysts lined by squamous epithelium with associated sebaceous glands (Figure 2A) and hair follicles in the cyst lumen (Figure 2B). These findings confirmed the diagnosis of multiple subcutaneous dermoid cysts.
Dermoid cysts are relatively uncommon, benign tumors consisting of tissue derived from ectodermal and mesodermal germ cell layers. Dermoid cysts may be distinguished from teratomas, which may contain tissues derived from all 3 germ cell layers and typically consist of types of tissues foreign to the site of origin, such as dental, thyroid, gastrointestinal, or neural tissue.1,2 The majority of dermoid cysts are congenitally developed along the lines of embryologic fusion due to an error in the division of the ectoderm and mesoderm3,4; however, some dermoid cysts may be acquired from epidermal elements being traumatically implanted into the dermis.5
Our patient’s presentation with multiple dermoid cysts was atypical, as dermoid cysts are almost always solitary tumors. A similar case was reported in a 41-year-old man who developed multiple dermoid cysts on the forehead over a 20-year period.This patient also was otherwise healthy, denied prior trauma to the forehead, and reported no family history of skin or gastrointestinal tract tumors.5
Another unusual feature in our case was the location of the dermoid cysts on the central forehead. The most common location for dermoid cysts is the lateral third of the eyebrows (47%–70% of cases).1,4,6-10 These cysts occur because of sequestration of the surface ectoderm during fusion along the naso-optic groove.2 Dermoid cysts also have been noted in other anatomical areas such as the scalp, nose, anterior neck, and trunk.6
Dermoid cysts tend to be small, round, smooth, and slowly growing until sudden enlargement prompts surgical evaluation.4,6 During surgical excision, they often are fixed to the underlying bone but also may be freely mobile, as in our patient.6 Histopathologic examination reveals a stratified squamous epithelium with associated adnexal structures such as sebaceous glands or hair follicles.1 Smooth muscle fibers, prominent vascular stroma, small nerves, and collagen and elastic fibers also may be found within the lumen of dermoid cysts.2
In some cases, dermoid cysts may be invasive and carry the risk of bony erosion, intracranial extension, osteomyelitis, meningitis, or cerebral abscess. Imaging studies sometimes are needed to rule out intracranial or intraspinal extension, particularly for midline dermoid cysts.6 The standard of treatment for dermoid cysts is surgical excision and complete enucleation without disruption of the cyst wall; however, invasive dermoid cysts may require endoscopic excision, orbitotomy, or craniotomy.4,6
To the Editor:
A 30-year-old man with no notable medical history presented to the dermatology clinic with multiple subcutaneous nodules on the forehead of 5 years’ duration. He reported no history of forehead trauma or manipulation of the lesions, and there was no accompanying pruritis, pain, erythema, or purulent discharge. There was no family history of skin or gastrointestinal tract tumors. On physical examination, the patient had 5 firm, flesh-colored to yellow nodules measuring approximately 0.2 to 1.5 cm in diameter without central punctae scattered over the central forehead (Figure 1). Due to cosmetic concerns, the patient elected to pursue surgical excision of the lesions, which occurred over several office visits. During surgical excision, the lesions were found to be smooth, encapsulated, and mobile, and they were excised without surgical complication. Histopathologic examination showed subcutaneous cysts lined by squamous epithelium with associated sebaceous glands (Figure 2A) and hair follicles in the cyst lumen (Figure 2B). These findings confirmed the diagnosis of multiple subcutaneous dermoid cysts.
Dermoid cysts are relatively uncommon, benign tumors consisting of tissue derived from ectodermal and mesodermal germ cell layers. Dermoid cysts may be distinguished from teratomas, which may contain tissues derived from all 3 germ cell layers and typically consist of types of tissues foreign to the site of origin, such as dental, thyroid, gastrointestinal, or neural tissue.1,2 The majority of dermoid cysts are congenitally developed along the lines of embryologic fusion due to an error in the division of the ectoderm and mesoderm3,4; however, some dermoid cysts may be acquired from epidermal elements being traumatically implanted into the dermis.5
Our patient’s presentation with multiple dermoid cysts was atypical, as dermoid cysts are almost always solitary tumors. A similar case was reported in a 41-year-old man who developed multiple dermoid cysts on the forehead over a 20-year period.This patient also was otherwise healthy, denied prior trauma to the forehead, and reported no family history of skin or gastrointestinal tract tumors.5
Another unusual feature in our case was the location of the dermoid cysts on the central forehead. The most common location for dermoid cysts is the lateral third of the eyebrows (47%–70% of cases).1,4,6-10 These cysts occur because of sequestration of the surface ectoderm during fusion along the naso-optic groove.2 Dermoid cysts also have been noted in other anatomical areas such as the scalp, nose, anterior neck, and trunk.6
Dermoid cysts tend to be small, round, smooth, and slowly growing until sudden enlargement prompts surgical evaluation.4,6 During surgical excision, they often are fixed to the underlying bone but also may be freely mobile, as in our patient.6 Histopathologic examination reveals a stratified squamous epithelium with associated adnexal structures such as sebaceous glands or hair follicles.1 Smooth muscle fibers, prominent vascular stroma, small nerves, and collagen and elastic fibers also may be found within the lumen of dermoid cysts.2
In some cases, dermoid cysts may be invasive and carry the risk of bony erosion, intracranial extension, osteomyelitis, meningitis, or cerebral abscess. Imaging studies sometimes are needed to rule out intracranial or intraspinal extension, particularly for midline dermoid cysts.6 The standard of treatment for dermoid cysts is surgical excision and complete enucleation without disruption of the cyst wall; however, invasive dermoid cysts may require endoscopic excision, orbitotomy, or craniotomy.4,6
- Brownstein MH, Helwig EB. Subcutaneous dermoid cysts. Arch Dermatol. 1973;107:237-239.
- Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437-1455.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Yamaki T, Higuchi R, Sasaki K, et al. Multiple dermoid cysts on the forehead. case report. Scand J Plast Reconstr Surg Hand Surg. 1996;30:321-324.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Al-Khateeb TH, Al-Masri NM, Al-Zoubi F. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2009;67:52-57.
- McAvoy JM, Zuckerbraun L. Dermoid cysts of the head and neck in children. Arch Otolaryngol. 1976;102:529-531.
- Taylor BW, Erich JB, Dockerty MB. Dermoids of the head and neck. Minnesota Med. 1966;49:1535-1540.
- Golden BA, Zide MF. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2005;63:1613-1619.
- Brownstein MH, Helwig EB. Subcutaneous dermoid cysts. Arch Dermatol. 1973;107:237-239.
- Smirniotopoulos JG, Chiechi MV. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics. 1995;15:1437-1455.
- Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolaryngol Head Neck Surg. 2005;132:938-942.
- Yamaki T, Higuchi R, Sasaki K, et al. Multiple dermoid cysts on the forehead. case report. Scand J Plast Reconstr Surg Hand Surg. 1996;30:321-324.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
- Al-Khateeb TH, Al-Masri NM, Al-Zoubi F. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2009;67:52-57.
- McAvoy JM, Zuckerbraun L. Dermoid cysts of the head and neck in children. Arch Otolaryngol. 1976;102:529-531.
- Taylor BW, Erich JB, Dockerty MB. Dermoids of the head and neck. Minnesota Med. 1966;49:1535-1540.
- Golden BA, Zide MF. Cutaneous cysts of the head and neck. J Oral Maxillofac Surg. 2005;63:1613-1619.
Practice Points
- The majority of dermoid cysts are congenital; however, they may be acquired from traumatic implantation of epidermal elements into the dermis.
- The most common location for dermoid cysts is the lateral third of the eyebrows; however, they also may occur on the mid forehead, scalp, nose, anterior neck, and trunk.
- Imaging studies may be needed to rule out intracranial or intraspinal extension of dermoid cysts, particularly for those presenting in the midline.
Multiple Eruptive Syringomas on the Penis
To the Editor:
Syringomas are small, benign, asymptomatic eccrine or apocrine tumors that present as multiple discrete flesh-colored papules. They are more common in females than males.1 The etiology of eruptive syringomas is unclear, though an inflammatory process has been implicated in the abnormal proliferation of sweat glands.2 However, a minority of tumors have been known to have an autosomal-dominant mode of transmission. Multiple or eruptive syringomas are associated with Down syndrome, Marfan syndrome, Ehlers-Danlos syndrome, and Blau syndrome.3 The clear cell variant has been found to be associated with diabetes mellitus.4 Syringomas most commonly appear on the lower eyelids, upper cheeks, neck, and upper chest; presentation on the penis is rare.5 We report a case of multiple eruptive syringomas located exclusively on the penis mimicking a sexually transmitted condition.
A 53-year-old man who was otherwise healthy presented with multiple flesh-colored papules on the penis that initially began to develop 30 years prior, but increased crops of lesions appeared 4 to 6 weeks prior to presentation. The patient described the lesions as rashlike, nonpruritic, and sensitive to the touch. He denied any discharge, oozing, crusting, or bleeding from the lesions. He did not report any high-risk sexual behaviors and stated that he was in a monogamous relationship with his wife. He had a medical history of molluscum contagiosum that was diagnosed and treated with cryotherapy 30 years prior; however, he did not have a history of any other sexually transmitted diseases. He also did not have a history of diabetes mellitus or thyroid disease.
Physical examination revealed multiple pink papules on the dorsal and ventral shaft of the penis, measuring 2 to 4 mm in diameter, with koebnerization (Figure 1). Based on clinical examination, the differential included condyloma, inflamed seborrheic keratosis, bowenoid papulosis, atypical molluscum contagiosum, or lichen planus. Consequently, a punch biopsy of the penile shaft was performed and histopathologic examination revealed proliferation of ducts focally that were tadpole shaped and embedded in a sclerotic stroma. The lining of the ducts was composed of cuboidal cells, some with clear cell change. The microscopic findings were consistent with penile syringomas (Figure 2). Laboratory results revealed the patient was negative for human immunodeficiency virus, hepatitis B, hepatitis C, and syphilis. The patient was given topical hydrocortisone butyrate and tacrolimus for symptomatic treatment. He declined further aggressive treatment.
Due to the rarity of syringomas on the penis, presentation of these benign eccrine tumors can be commonly mistaken for lichen planus, molluscum contagiosum, genital warts, or bowenoid papulosis.5 The characteristic histopathology of syringomas consists of multiple, small, tadpole or paisley tie–shaped ducts within an eosinophilic stroma. Often, the findings can be histologically confused with desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma. Although the histopathology of our patient’s biopsy showed clear cell change, the patient did not report a history of diabetes mellitus, which is a disease that can be associated with the clear cell variant of syringoma. Because syringomas are benign tumors, treatment is not medically necessary unless the lesions are symptomatic. Treatment often is regarded as challenging, as lesions often recur and scarring is a consideration. Possible treatments for removal of the benign papules include surgical excision, electrodesiccation and curettage, shave removal, chemical peels, liquid nitrogen cryotherapy, and CO2 laser vaporization.6
To prevent misdiagnosis and unnecessary treatment, it is important to have syringomas as part of the differential diagnosis when patients present with multiple small flesh-colored papules on the penis. The lesions should be biopsied for accurate diagnosis and to provide reassurance to patients who usually come in for evaluation for fear of having acquired a sexually transmitted disease.
- Yalisove B, Stolar EEH, Williams CM. Multiple penile papules. syringoma. Arch Dermatol. 1987;123:1391-1396.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Yoshimi N, Kurokawa I, Kakuno A, et al. Case of generalized eruptive clear cell syringoma with diabetes mellitus. J Dermatol. 2012;39:744-745.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Wu CY. Multifocal penile syringoma masquerading as genital warts. Clin Exp Dermatol. 2009;34:e290-e291.
- Lipshutz RL, Kantor GR, Vonderheid EC. Multiple penile syringomas mimicking verrucae. Int J Dermatol. 1991;30:69.
To the Editor:
Syringomas are small, benign, asymptomatic eccrine or apocrine tumors that present as multiple discrete flesh-colored papules. They are more common in females than males.1 The etiology of eruptive syringomas is unclear, though an inflammatory process has been implicated in the abnormal proliferation of sweat glands.2 However, a minority of tumors have been known to have an autosomal-dominant mode of transmission. Multiple or eruptive syringomas are associated with Down syndrome, Marfan syndrome, Ehlers-Danlos syndrome, and Blau syndrome.3 The clear cell variant has been found to be associated with diabetes mellitus.4 Syringomas most commonly appear on the lower eyelids, upper cheeks, neck, and upper chest; presentation on the penis is rare.5 We report a case of multiple eruptive syringomas located exclusively on the penis mimicking a sexually transmitted condition.
A 53-year-old man who was otherwise healthy presented with multiple flesh-colored papules on the penis that initially began to develop 30 years prior, but increased crops of lesions appeared 4 to 6 weeks prior to presentation. The patient described the lesions as rashlike, nonpruritic, and sensitive to the touch. He denied any discharge, oozing, crusting, or bleeding from the lesions. He did not report any high-risk sexual behaviors and stated that he was in a monogamous relationship with his wife. He had a medical history of molluscum contagiosum that was diagnosed and treated with cryotherapy 30 years prior; however, he did not have a history of any other sexually transmitted diseases. He also did not have a history of diabetes mellitus or thyroid disease.
Physical examination revealed multiple pink papules on the dorsal and ventral shaft of the penis, measuring 2 to 4 mm in diameter, with koebnerization (Figure 1). Based on clinical examination, the differential included condyloma, inflamed seborrheic keratosis, bowenoid papulosis, atypical molluscum contagiosum, or lichen planus. Consequently, a punch biopsy of the penile shaft was performed and histopathologic examination revealed proliferation of ducts focally that were tadpole shaped and embedded in a sclerotic stroma. The lining of the ducts was composed of cuboidal cells, some with clear cell change. The microscopic findings were consistent with penile syringomas (Figure 2). Laboratory results revealed the patient was negative for human immunodeficiency virus, hepatitis B, hepatitis C, and syphilis. The patient was given topical hydrocortisone butyrate and tacrolimus for symptomatic treatment. He declined further aggressive treatment.
Due to the rarity of syringomas on the penis, presentation of these benign eccrine tumors can be commonly mistaken for lichen planus, molluscum contagiosum, genital warts, or bowenoid papulosis.5 The characteristic histopathology of syringomas consists of multiple, small, tadpole or paisley tie–shaped ducts within an eosinophilic stroma. Often, the findings can be histologically confused with desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma. Although the histopathology of our patient’s biopsy showed clear cell change, the patient did not report a history of diabetes mellitus, which is a disease that can be associated with the clear cell variant of syringoma. Because syringomas are benign tumors, treatment is not medically necessary unless the lesions are symptomatic. Treatment often is regarded as challenging, as lesions often recur and scarring is a consideration. Possible treatments for removal of the benign papules include surgical excision, electrodesiccation and curettage, shave removal, chemical peels, liquid nitrogen cryotherapy, and CO2 laser vaporization.6
To prevent misdiagnosis and unnecessary treatment, it is important to have syringomas as part of the differential diagnosis when patients present with multiple small flesh-colored papules on the penis. The lesions should be biopsied for accurate diagnosis and to provide reassurance to patients who usually come in for evaluation for fear of having acquired a sexually transmitted disease.
To the Editor:
Syringomas are small, benign, asymptomatic eccrine or apocrine tumors that present as multiple discrete flesh-colored papules. They are more common in females than males.1 The etiology of eruptive syringomas is unclear, though an inflammatory process has been implicated in the abnormal proliferation of sweat glands.2 However, a minority of tumors have been known to have an autosomal-dominant mode of transmission. Multiple or eruptive syringomas are associated with Down syndrome, Marfan syndrome, Ehlers-Danlos syndrome, and Blau syndrome.3 The clear cell variant has been found to be associated with diabetes mellitus.4 Syringomas most commonly appear on the lower eyelids, upper cheeks, neck, and upper chest; presentation on the penis is rare.5 We report a case of multiple eruptive syringomas located exclusively on the penis mimicking a sexually transmitted condition.
A 53-year-old man who was otherwise healthy presented with multiple flesh-colored papules on the penis that initially began to develop 30 years prior, but increased crops of lesions appeared 4 to 6 weeks prior to presentation. The patient described the lesions as rashlike, nonpruritic, and sensitive to the touch. He denied any discharge, oozing, crusting, or bleeding from the lesions. He did not report any high-risk sexual behaviors and stated that he was in a monogamous relationship with his wife. He had a medical history of molluscum contagiosum that was diagnosed and treated with cryotherapy 30 years prior; however, he did not have a history of any other sexually transmitted diseases. He also did not have a history of diabetes mellitus or thyroid disease.
Physical examination revealed multiple pink papules on the dorsal and ventral shaft of the penis, measuring 2 to 4 mm in diameter, with koebnerization (Figure 1). Based on clinical examination, the differential included condyloma, inflamed seborrheic keratosis, bowenoid papulosis, atypical molluscum contagiosum, or lichen planus. Consequently, a punch biopsy of the penile shaft was performed and histopathologic examination revealed proliferation of ducts focally that were tadpole shaped and embedded in a sclerotic stroma. The lining of the ducts was composed of cuboidal cells, some with clear cell change. The microscopic findings were consistent with penile syringomas (Figure 2). Laboratory results revealed the patient was negative for human immunodeficiency virus, hepatitis B, hepatitis C, and syphilis. The patient was given topical hydrocortisone butyrate and tacrolimus for symptomatic treatment. He declined further aggressive treatment.
Due to the rarity of syringomas on the penis, presentation of these benign eccrine tumors can be commonly mistaken for lichen planus, molluscum contagiosum, genital warts, or bowenoid papulosis.5 The characteristic histopathology of syringomas consists of multiple, small, tadpole or paisley tie–shaped ducts within an eosinophilic stroma. Often, the findings can be histologically confused with desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma. Although the histopathology of our patient’s biopsy showed clear cell change, the patient did not report a history of diabetes mellitus, which is a disease that can be associated with the clear cell variant of syringoma. Because syringomas are benign tumors, treatment is not medically necessary unless the lesions are symptomatic. Treatment often is regarded as challenging, as lesions often recur and scarring is a consideration. Possible treatments for removal of the benign papules include surgical excision, electrodesiccation and curettage, shave removal, chemical peels, liquid nitrogen cryotherapy, and CO2 laser vaporization.6
To prevent misdiagnosis and unnecessary treatment, it is important to have syringomas as part of the differential diagnosis when patients present with multiple small flesh-colored papules on the penis. The lesions should be biopsied for accurate diagnosis and to provide reassurance to patients who usually come in for evaluation for fear of having acquired a sexually transmitted disease.
- Yalisove B, Stolar EEH, Williams CM. Multiple penile papules. syringoma. Arch Dermatol. 1987;123:1391-1396.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Yoshimi N, Kurokawa I, Kakuno A, et al. Case of generalized eruptive clear cell syringoma with diabetes mellitus. J Dermatol. 2012;39:744-745.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Wu CY. Multifocal penile syringoma masquerading as genital warts. Clin Exp Dermatol. 2009;34:e290-e291.
- Lipshutz RL, Kantor GR, Vonderheid EC. Multiple penile syringomas mimicking verrucae. Int J Dermatol. 1991;30:69.
- Yalisove B, Stolar EEH, Williams CM. Multiple penile papules. syringoma. Arch Dermatol. 1987;123:1391-1396.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Yoshimi N, Kurokawa I, Kakuno A, et al. Case of generalized eruptive clear cell syringoma with diabetes mellitus. J Dermatol. 2012;39:744-745.
- Petersson F, Mjornberg PA, Kazakov DV, et al. Eruptive syringoma of the penis. a report of 2 cases and a review of the literature. Am J Dermatopathol. 2009;31:436-438.
- Wu CY. Multifocal penile syringoma masquerading as genital warts. Clin Exp Dermatol. 2009;34:e290-e291.
- Lipshutz RL, Kantor GR, Vonderheid EC. Multiple penile syringomas mimicking verrucae. Int J Dermatol. 1991;30:69.
Practice Points
- Penile syringoma can mimic sexually transmitted disease such as condyloma acuminatum or molluscum contagiosum.
- Penile syringomas can be long-standing and require biopsy to differentiate from other conditions.
Apremilast and Phototherapy for Treatment of Psoriasis in a Patient With Human Immunodeficiency Virus
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
To the Editor:
A 50-year old man with Fitzpatrick skin type IV, human immunodeficiency virus (HIV), fatty liver disease, and moderate psoriasis (10% body surface area [BSA] affected) currently treated with clobetasol spray and calcitriol ointment presented with persistent psoriatic lesions on the trunk, arms, legs, and buttocks. His CD4 count was 460 and his HIV RNA count was 48 copies/mL on polymerase chain reaction 2 months prior to the current presentation. He had been undergoing phototherapy 3 times weekly for the last 5 months for treatment of psoriasis.
At the current presentation, he was started on an apremilast starter pack with the dosage titrated from 10 mg to 30 mg over the course of 1 week. He was maintained on a dose of 30 mg twice daily after 1 week and continued clobetasol spray, calcitriol ointment, and phototherapy 3 times weekly with the intent to reduce the frequency after adequate control of psoriasis was achieved. After 3 months of treatment, the affected BSA was 0%. He continued apremilast, and phototherapy was reduced to once weekly. Phototherapy was discontinued after 7 months of concomitant treatment with apremilast after clearance was maintained. It was reinitiated twice weekly after a mild flare (3% BSA affected). After 20 total months of treatment, the patient was no longer able to afford apremilast treatment and presented with a severe psoriasis flare (40% BSA affected). He was switched to acitretin with a plan to apply for apremilast financial assistance programs.
Psoriasis treatment in the HIV population poses a challenge given the immunosuppressed state of these patients, the risk of reactivation of latent infections, and the refractory nature of psoriasis in the setting of HIV. Two of the authors (S.P.R. and J.J.W.) previously reported a case of moderate to severe psoriasis in a patient with HIV and hepatitis C who demonstrated treatment success with apremilast until it was discontinued due to financial implications.1 Currently, apremilast is not widely used to treat psoriasis in the HIV population. The National Psoriasis Foundation 2010 guidelines recommended UV light therapy for treatment of moderate to severe psoriasis in HIV-positive patients, with oral retinoids as the second-line treatment.2 There remains a need for updated guidelines on the use of systemic agents for psoriasis treatment in the HIV population.
Apremilast, a phosphodiesterase 4 inhibitor, is an oral therapy that restores the balance of proinflammatory and anti-inflammatory cytokines by inhibiting inflammatory cytokine (eg, tumor necrosis factor α, IFN-γ, IL-2, IL-12, IL-23) secretion and stimulating anti-inflammatory cytokine (eg, IL-6, IL-10) production. In 2015, the phase 3 ESTEEM 13 and ESTEEM 24 trials demonstrated the efficacy of apremilast 30 mg twice daily for treatment of psoriasis. In both trials, the psoriasis area and severity index 75 response rate at week 16 was significantly
Use of other systemic agents such as tumor necrosis factor α inhibitors and ustekinumab has been reported in HIV-positive patients.5-7 There is no current data on IL-17 and IL-23 inhibitors. Acitretin generally is recommended as a second-line agent in HIV patients given its lack of immunosuppression2; however, methotrexate and cyclosporine should be avoided given the risk of opportunistic infections.8
Apremilast is a promising therapy with a favorable safety profile that should be considered as an adjuvant treatment to first-line agents such as phototherapy in HIV-positive patients. Apremilast has been successfully used in an HIV patient with a concomitant chronic hepatitis C infection.1 Systemic medications such as apremilast should be managed in coordination with infectious disease specialists with close monitoring of CD4 levels and viral loads as well as prophylactic agents.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation [published online July 31, 2009]. J Am Acad Dermatol. 2010;62:291-299.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Lindsey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871.
- Saeki H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655.
- Paparizos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatolog Treat. 2012;23:398-399.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections [published online July 11, 2018]. J Am Acad Dermatol. 2019;80:43-53.
Practice Point
- Apremilast may be considered as a first-line therapy in the human immunodeficiency virus population due to decreased immunosuppression.
Netherton Syndrome: An Atypical Presentation
To the Editor:
Netherton syndrome (NS) is a rare autosomal-recessive ichthyosiform disease.1 The incidence is estimated to be 1 in 200,000 individuals.2 Netherton syndrome presents with generalized erythroderma and scaling, characteristic hair shaft abnormalities, and dysregulation of the immune system. Treatment is largely symptomatic and includes fragrance-free emollients, keratolytics, tretinoin, and corticosteroids, either alone or in combination. We report a case of NS in a man with congenital erythroderma, pili torti, and elevated IgE levels.
A 23-year-old man presented with generalized scaly skin that was present since birth. He was the first child born of nonconsanguineous parents. His medical history was suggestive of atopic diatheses such as allergic rhinitis and recurrent urticaria. The patient was of thin build and had widespread erythematous, annular, and polycyclic scaly lesions (Figure 1A), some with characteristic double-edged scale (Figure 1B). The skin was dry due to anhidrosis that was present since birth. Flexural lichenification was present at the cubital fossa of both arms. Scalp hairs were easily pluckable and had generalized thinning of hair density. Hair mount examination showed characteristic features of both trichorrhexis invaginata (Figure 2A) and pili torti (Figure 2B).
hair shaft known as bamboo hair or trichorrhexis invaginata. B, Features of pili torti; the hair
shaft twisted at irregular intervals.
Potassium hydroxide mount from a lesion was negative for fungal elements. Complete hematologic workup showed moderate anemia at 8.0 g/dL (reference range, 8.0–10.9 g/dL) and peripheral eosinophilia at 12% (reference range, 0%–6%). His IgE level was markedly elevated at6331 IU/mL (reference range, 150–1000 IU/mL) when tested with fully automated bidirectionally interfaced chemiluminescent immunoassay. Histopathologic examination of a lesion biopsy showed psoriasiform epidermal hyperplasia, papillomatosis, and acanthosis, consistent with ichthyosis linearis circumflexa (ILC)(Figure 3). Clinicopathologic correlation led to a diagnosis of ILC, trichorrhexis invaginata/pili torti, and atopic diathesis, which is a constellation of disorders related to NS.
We prescribed oral acitretin 25 mg once daily and instructed the patient to apply petroleum jelly; however, the patient returned after 2 weeks due to aggravation of the skin condition with increased scaling and redness. Because the patient showed signs of acute skin failure and erythroderma, we stopped acitretin treatment and managed his condition conservatively with the application of petroleum jelly.
Netherton syndrome is caused by mutation of the SPINK5 gene, serine protease inhibitor Kazal type 5; the corresponding gene is located on the long arm of chromosome 5.3 The gene encodes a serine protease inhibitor proprotein LEKTI (lymphoepithelial Kazal type inhibitor).4 The product of the gene is thought to be necessary for epidermal cell growth and differentiation. The classic clinical triad of NS includes ichthyosiform dermatosis with double-edged scale, hair shaft abnormalities, and atopy or elevated IgE levels.5 Generalized (congenital) erythroderma usually becomes evident at birth or shortly thereafter. Half of patients develop lesions of ILC on the trunk and limbs during childhood.6 A typical ILC lesion is characterized by an erythematous scaly patch that may be annular or polycyclic with double-edged scale at the advancing border. The ability to sweat is impaired, which may cause episodes of hyperpyrexia, especially during humid weather. Patients with hyperpyrexia may be incorrectly diagnosed with bacterial infection and treated with antipyretic drugs or a prolonged course of antibiotics. Trichorrhexis invaginata, also referred to as bamboo hair or ball-and-socket defect, is the pathognomonic hair shaft abnormality seen in NS.7 Other hair shaft abnormalities in this syndrome include trichorrhexis nodosa and pili torti.8 Our patient had hair shaft abnormalities of trichorrhexis invaginata and pili torti, which are rare findings. The third component of this syndrome is atopy, which generally manifests as angioedema, urticaria, allergic rhinitis, peripheral eosinophilia, atopic dermatitis–like skin lesions, asthma, and elevated IgE levels.9
Treatment with emollients, topical steroids, tacrolimus, and psoralen plus UVA does not elicit a satisfactory response. The Table highlights the clinical features and management of NS.
Generally, systemic retinoid therapy is helpful in cases of erythrodermic ichthyosis, but a unique feature of NS is that erythroderma may worsen with systemic retinoid therapy, as retinoids aggravate atopic dermatitis by worsening existing xerosis.4 Our case highlights the rare association of trichorrhexis invaginata with pili torti as well as acitretin treatment worsening our patient’s condition. This paradoxical effect of retinoid therapy further confirmed the diagnosis of NS.
- Suhaila O, Muzhirah A. Netherton syndrome: a case report. Malaysian J Pediatr Child Health. 2010;16:26.
- Emre S, Metin A, Demirseren D, et al. Two siblings with Netherton syndrome. Turk J Med Sci. 2010;40:819-823.
- Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141-142.
- Judge MR, Mclean WH, Munro CS. Disorders of keratinization. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 8th ed. Singapore: Wiley-Blackwell; 2010:19.1-19.122.
- Greene SL, Muller SA. Netherton’s syndrome. report of a case and review of the literature. J Am Acad Dermatol. 1985;13:329-337.
- Khan I-U, Chaudhary R. Netherton’s syndrome, an uncommon genodermatosis. J Pakistan Assoc Dermatol. 2006;16.
- Boskabadi H, Maamouri G, Mafinejad S. Netherton syndrome, a case report and review of literature. Iran J Pediatr. 2013;23:611-612.
- Hurwitz S. Hereditary skin disorders: the genodermatoses. In: Hurwitz, ed. Clinical Pediatric Dermatology. Philadelphia, PA: WB Saunders; 1993:173.
- Judge MR, McLean WH, Munro CS. Disorders of keratinization. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 7th ed. Vol 2. Oxford, England: Blackwell Science; 2004:34.35.
To the Editor:
Netherton syndrome (NS) is a rare autosomal-recessive ichthyosiform disease.1 The incidence is estimated to be 1 in 200,000 individuals.2 Netherton syndrome presents with generalized erythroderma and scaling, characteristic hair shaft abnormalities, and dysregulation of the immune system. Treatment is largely symptomatic and includes fragrance-free emollients, keratolytics, tretinoin, and corticosteroids, either alone or in combination. We report a case of NS in a man with congenital erythroderma, pili torti, and elevated IgE levels.
A 23-year-old man presented with generalized scaly skin that was present since birth. He was the first child born of nonconsanguineous parents. His medical history was suggestive of atopic diatheses such as allergic rhinitis and recurrent urticaria. The patient was of thin build and had widespread erythematous, annular, and polycyclic scaly lesions (Figure 1A), some with characteristic double-edged scale (Figure 1B). The skin was dry due to anhidrosis that was present since birth. Flexural lichenification was present at the cubital fossa of both arms. Scalp hairs were easily pluckable and had generalized thinning of hair density. Hair mount examination showed characteristic features of both trichorrhexis invaginata (Figure 2A) and pili torti (Figure 2B).
hair shaft known as bamboo hair or trichorrhexis invaginata. B, Features of pili torti; the hair
shaft twisted at irregular intervals.
Potassium hydroxide mount from a lesion was negative for fungal elements. Complete hematologic workup showed moderate anemia at 8.0 g/dL (reference range, 8.0–10.9 g/dL) and peripheral eosinophilia at 12% (reference range, 0%–6%). His IgE level was markedly elevated at6331 IU/mL (reference range, 150–1000 IU/mL) when tested with fully automated bidirectionally interfaced chemiluminescent immunoassay. Histopathologic examination of a lesion biopsy showed psoriasiform epidermal hyperplasia, papillomatosis, and acanthosis, consistent with ichthyosis linearis circumflexa (ILC)(Figure 3). Clinicopathologic correlation led to a diagnosis of ILC, trichorrhexis invaginata/pili torti, and atopic diathesis, which is a constellation of disorders related to NS.
We prescribed oral acitretin 25 mg once daily and instructed the patient to apply petroleum jelly; however, the patient returned after 2 weeks due to aggravation of the skin condition with increased scaling and redness. Because the patient showed signs of acute skin failure and erythroderma, we stopped acitretin treatment and managed his condition conservatively with the application of petroleum jelly.
Netherton syndrome is caused by mutation of the SPINK5 gene, serine protease inhibitor Kazal type 5; the corresponding gene is located on the long arm of chromosome 5.3 The gene encodes a serine protease inhibitor proprotein LEKTI (lymphoepithelial Kazal type inhibitor).4 The product of the gene is thought to be necessary for epidermal cell growth and differentiation. The classic clinical triad of NS includes ichthyosiform dermatosis with double-edged scale, hair shaft abnormalities, and atopy or elevated IgE levels.5 Generalized (congenital) erythroderma usually becomes evident at birth or shortly thereafter. Half of patients develop lesions of ILC on the trunk and limbs during childhood.6 A typical ILC lesion is characterized by an erythematous scaly patch that may be annular or polycyclic with double-edged scale at the advancing border. The ability to sweat is impaired, which may cause episodes of hyperpyrexia, especially during humid weather. Patients with hyperpyrexia may be incorrectly diagnosed with bacterial infection and treated with antipyretic drugs or a prolonged course of antibiotics. Trichorrhexis invaginata, also referred to as bamboo hair or ball-and-socket defect, is the pathognomonic hair shaft abnormality seen in NS.7 Other hair shaft abnormalities in this syndrome include trichorrhexis nodosa and pili torti.8 Our patient had hair shaft abnormalities of trichorrhexis invaginata and pili torti, which are rare findings. The third component of this syndrome is atopy, which generally manifests as angioedema, urticaria, allergic rhinitis, peripheral eosinophilia, atopic dermatitis–like skin lesions, asthma, and elevated IgE levels.9
Treatment with emollients, topical steroids, tacrolimus, and psoralen plus UVA does not elicit a satisfactory response. The Table highlights the clinical features and management of NS.
Generally, systemic retinoid therapy is helpful in cases of erythrodermic ichthyosis, but a unique feature of NS is that erythroderma may worsen with systemic retinoid therapy, as retinoids aggravate atopic dermatitis by worsening existing xerosis.4 Our case highlights the rare association of trichorrhexis invaginata with pili torti as well as acitretin treatment worsening our patient’s condition. This paradoxical effect of retinoid therapy further confirmed the diagnosis of NS.
To the Editor:
Netherton syndrome (NS) is a rare autosomal-recessive ichthyosiform disease.1 The incidence is estimated to be 1 in 200,000 individuals.2 Netherton syndrome presents with generalized erythroderma and scaling, characteristic hair shaft abnormalities, and dysregulation of the immune system. Treatment is largely symptomatic and includes fragrance-free emollients, keratolytics, tretinoin, and corticosteroids, either alone or in combination. We report a case of NS in a man with congenital erythroderma, pili torti, and elevated IgE levels.
A 23-year-old man presented with generalized scaly skin that was present since birth. He was the first child born of nonconsanguineous parents. His medical history was suggestive of atopic diatheses such as allergic rhinitis and recurrent urticaria. The patient was of thin build and had widespread erythematous, annular, and polycyclic scaly lesions (Figure 1A), some with characteristic double-edged scale (Figure 1B). The skin was dry due to anhidrosis that was present since birth. Flexural lichenification was present at the cubital fossa of both arms. Scalp hairs were easily pluckable and had generalized thinning of hair density. Hair mount examination showed characteristic features of both trichorrhexis invaginata (Figure 2A) and pili torti (Figure 2B).
hair shaft known as bamboo hair or trichorrhexis invaginata. B, Features of pili torti; the hair
shaft twisted at irregular intervals.
Potassium hydroxide mount from a lesion was negative for fungal elements. Complete hematologic workup showed moderate anemia at 8.0 g/dL (reference range, 8.0–10.9 g/dL) and peripheral eosinophilia at 12% (reference range, 0%–6%). His IgE level was markedly elevated at6331 IU/mL (reference range, 150–1000 IU/mL) when tested with fully automated bidirectionally interfaced chemiluminescent immunoassay. Histopathologic examination of a lesion biopsy showed psoriasiform epidermal hyperplasia, papillomatosis, and acanthosis, consistent with ichthyosis linearis circumflexa (ILC)(Figure 3). Clinicopathologic correlation led to a diagnosis of ILC, trichorrhexis invaginata/pili torti, and atopic diathesis, which is a constellation of disorders related to NS.
We prescribed oral acitretin 25 mg once daily and instructed the patient to apply petroleum jelly; however, the patient returned after 2 weeks due to aggravation of the skin condition with increased scaling and redness. Because the patient showed signs of acute skin failure and erythroderma, we stopped acitretin treatment and managed his condition conservatively with the application of petroleum jelly.
Netherton syndrome is caused by mutation of the SPINK5 gene, serine protease inhibitor Kazal type 5; the corresponding gene is located on the long arm of chromosome 5.3 The gene encodes a serine protease inhibitor proprotein LEKTI (lymphoepithelial Kazal type inhibitor).4 The product of the gene is thought to be necessary for epidermal cell growth and differentiation. The classic clinical triad of NS includes ichthyosiform dermatosis with double-edged scale, hair shaft abnormalities, and atopy or elevated IgE levels.5 Generalized (congenital) erythroderma usually becomes evident at birth or shortly thereafter. Half of patients develop lesions of ILC on the trunk and limbs during childhood.6 A typical ILC lesion is characterized by an erythematous scaly patch that may be annular or polycyclic with double-edged scale at the advancing border. The ability to sweat is impaired, which may cause episodes of hyperpyrexia, especially during humid weather. Patients with hyperpyrexia may be incorrectly diagnosed with bacterial infection and treated with antipyretic drugs or a prolonged course of antibiotics. Trichorrhexis invaginata, also referred to as bamboo hair or ball-and-socket defect, is the pathognomonic hair shaft abnormality seen in NS.7 Other hair shaft abnormalities in this syndrome include trichorrhexis nodosa and pili torti.8 Our patient had hair shaft abnormalities of trichorrhexis invaginata and pili torti, which are rare findings. The third component of this syndrome is atopy, which generally manifests as angioedema, urticaria, allergic rhinitis, peripheral eosinophilia, atopic dermatitis–like skin lesions, asthma, and elevated IgE levels.9
Treatment with emollients, topical steroids, tacrolimus, and psoralen plus UVA does not elicit a satisfactory response. The Table highlights the clinical features and management of NS.
Generally, systemic retinoid therapy is helpful in cases of erythrodermic ichthyosis, but a unique feature of NS is that erythroderma may worsen with systemic retinoid therapy, as retinoids aggravate atopic dermatitis by worsening existing xerosis.4 Our case highlights the rare association of trichorrhexis invaginata with pili torti as well as acitretin treatment worsening our patient’s condition. This paradoxical effect of retinoid therapy further confirmed the diagnosis of NS.
- Suhaila O, Muzhirah A. Netherton syndrome: a case report. Malaysian J Pediatr Child Health. 2010;16:26.
- Emre S, Metin A, Demirseren D, et al. Two siblings with Netherton syndrome. Turk J Med Sci. 2010;40:819-823.
- Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141-142.
- Judge MR, Mclean WH, Munro CS. Disorders of keratinization. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 8th ed. Singapore: Wiley-Blackwell; 2010:19.1-19.122.
- Greene SL, Muller SA. Netherton’s syndrome. report of a case and review of the literature. J Am Acad Dermatol. 1985;13:329-337.
- Khan I-U, Chaudhary R. Netherton’s syndrome, an uncommon genodermatosis. J Pakistan Assoc Dermatol. 2006;16.
- Boskabadi H, Maamouri G, Mafinejad S. Netherton syndrome, a case report and review of literature. Iran J Pediatr. 2013;23:611-612.
- Hurwitz S. Hereditary skin disorders: the genodermatoses. In: Hurwitz, ed. Clinical Pediatric Dermatology. Philadelphia, PA: WB Saunders; 1993:173.
- Judge MR, McLean WH, Munro CS. Disorders of keratinization. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 7th ed. Vol 2. Oxford, England: Blackwell Science; 2004:34.35.
- Suhaila O, Muzhirah A. Netherton syndrome: a case report. Malaysian J Pediatr Child Health. 2010;16:26.
- Emre S, Metin A, Demirseren D, et al. Two siblings with Netherton syndrome. Turk J Med Sci. 2010;40:819-823.
- Chavanas S, Bodemer C, Rochat A, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nat Genet. 2000;25:141-142.
- Judge MR, Mclean WH, Munro CS. Disorders of keratinization. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 8th ed. Singapore: Wiley-Blackwell; 2010:19.1-19.122.
- Greene SL, Muller SA. Netherton’s syndrome. report of a case and review of the literature. J Am Acad Dermatol. 1985;13:329-337.
- Khan I-U, Chaudhary R. Netherton’s syndrome, an uncommon genodermatosis. J Pakistan Assoc Dermatol. 2006;16.
- Boskabadi H, Maamouri G, Mafinejad S. Netherton syndrome, a case report and review of literature. Iran J Pediatr. 2013;23:611-612.
- Hurwitz S. Hereditary skin disorders: the genodermatoses. In: Hurwitz, ed. Clinical Pediatric Dermatology. Philadelphia, PA: WB Saunders; 1993:173.
- Judge MR, McLean WH, Munro CS. Disorders of keratinization. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 7th ed. Vol 2. Oxford, England: Blackwell Science; 2004:34.35.
Practice Points
- Netherton syndrome is characterized by generalized erythroderma and scaling, hair shaft abnormalities, and dysregulation of the immune system.
- Treatment is largely symptomatic and includes fragrance-free emollients, keratolytics, tretinoin, and corticosteroids, either alone or in combination.
Adult-Onset Asymmetrical Lipomatosis
To the Editor:
An 85-year-old woman presented with extra growth of subcutaneous fat at the left anterior infradiaphragm that expanded circumferentially to the left back over the last 4 years. Two years prior to the current presentation, the left thigh became visibly thicker than the right. Diffuse subtle lipomatosis affecting the ipsilateral face, neck, arms, calf, and foot was noted at that time. Additionally, the patient had hyperlipidemia, gastroesophageal reflux disease, osteoporosis, and scoliosis, all beginning in her late 60s. She reported no alcohol or tobacco use and was taking rosuvastatin, esomeprazole, calcium, vitamin D, and glucosamine. There was no reported family history of asymmetric growth or bony deformities, and her children were healthy.
On physical examination, the lipomatosis affected the entire left side, most prominently around the abdomen, back, and thighs. The affected side was nontender and nonpruritic; there was no atrophy of the unaffected side (Figure). Maximum thigh circumference was 55.1 cm on the affected side and 52.6 cm on the unaffected side. There were no differences in power, reflex, or sensation between the 2 sides, and no hyperhidrosis or vascular malformations were present. Laboratory investigations, including complete blood cell count, complete metabolic panel, lipids, and thyroid-stimulating and sex hormone panels all were within reference range.
Enzi et al1 reported 2 women who developed asymmetrical lipomatosis between the ages of 13 and 20 years. Acquired asymmetrical lipomatosis should be differentiated from the asymmetrical overgrowth diagnosed in neonates and infants.
Proteus syndrome (PS) is a progressive disease involving a combination of overgrowth in a mosaic distribution, connective tissue and epidermal nevi, ovarian cysts, parotid gland tumor, dysregulated adipose tissue, lymphovascular malformation, and certain facial phenotypes.2,3 The average age of onset is 6 to 18 months, and half of cases present at birth.3,4 Hemihyperplasia-multiple lipomatosis syndrome (HHML) describes a mild and nonprogressive variant that does not satisfy the diagnostic criteria of PS; it typically is diagnosed at birth.5 One case of mild and delayed-onset PS was described in a woman who started developing signs at 15 years of age.6 In comparison, asymmetrical lipomatosis and scoliosis were the only abnormal clinical signs present in our patient, and the lipomatosis developed diffusely, as opposed to the typical mosaic distribution found in PS and HHML. Scoliosis can be found in PS and HHML secondary to hemihypertrophy of vertebra or infiltrative intraspinal lipomatosis.7,8 Our patient’s scoliosis was diagnosed more than 10 years prior to the onset of lipomatosis, likely representing degenerative joint disease.9
Prior reported cases of asymmetrical lipomatosis did not describe treatment.1 Ultrasound-guided or conventional liposuction and lipectomy are mainstream therapies for multiple symmetrical lipomatosis, an acquired lipomatosis typically affecting alcoholics in the fourth decade of life. However, recurrence rates are high for surgical treatment of unencapsulated lipomatosis, likely due to incomplete removal of the adipose tissue.10 Alternative treatments found in case reports, including oral salbutamol, mesotherapy using phosphatidylcholine, and fenofibrate (200 mg/d), require further study.11-13 Our patient was not aesthetically bothered by her lipomatosis; therefore, imaging and treatment options were not pursued. In conclusion, we report a patient with acquired asymmetrical lipomatosis with onset in late adulthood, unique from the existing syndromes of asymmetrical hemihyperplasia.1,14
- Enzi G, Digito M, Enzi GB, et al. Asymmetrical lipomatosis: report of two cases. Postgrad Med J. 1985;61:797-800.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Cohen MM Jr. Proteus syndrome: an update. Am J Med Genet C Semin Med Genet. 2005;137C:38-52.
- Biesecker LG, Peters KF, Darling TN, et al. Clinical differentiation between Proteus syndrome and hemihyperplasia: description of a distinct form of hemihyperplasia. Am J Med Genet. 1998;79:311-318.
- Luo S, Feng Y, Zheng Y, et al. Mild and delayed-onset Proteus syndrome. Eur J Dermatol. 2007;17:172-173.
- Takebayashi T, Yamashita T, Yokogushi K, et al. Scoliosis in Proteus syndrome: case report. Spine. 2001;26:E395-E398.
- Schulte TL, Liljenqvist U, Görgens H, et al. Hemihyperplasia-multiple lipomatosis syndrome (HHML): a challenge in spinal care. Acta Orthop Belg. 2008;74:714-719.
- Robin GC, Span Y, Steinberg R, et al. Scoliosis in the elderly: a follow-up study. Spine. 1982;7:355-359.
- Brea-García B, Cameselle-Teijeiro J, Couto-González I, et al. Madelung’s disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthet Plast Surg. 2013;37:409-416.
- Hasegawa T, Matsukura T, Ikeda S. Mesotherapy for benign symmetric lipomatosis. Aesthet Plast Surg. 2010;34:153-156.
- Zeitler H, Ulrich-Merzenich G, Richter DF, et al. Multiple benign symmetric lipomatosis—a differential diagnosis of obesity. is there a rationale for fibrate treatment? Obes Surg. 2008;18:1354-1356.
- Leung N, Gaer J, Beggs D, et al. Multiple symmetric lipomatosis (Launois‐Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol. 1987;27:601-606.
- Craiglow BG, Ko CJ, Antaya RJ. Two cases of hemihyperplasia-multiple lipomatosis syndrome and review of asymmetric hemihyperplasia syndromes. Pediatr Dermatol. 2014;31:507-510.
To the Editor:
An 85-year-old woman presented with extra growth of subcutaneous fat at the left anterior infradiaphragm that expanded circumferentially to the left back over the last 4 years. Two years prior to the current presentation, the left thigh became visibly thicker than the right. Diffuse subtle lipomatosis affecting the ipsilateral face, neck, arms, calf, and foot was noted at that time. Additionally, the patient had hyperlipidemia, gastroesophageal reflux disease, osteoporosis, and scoliosis, all beginning in her late 60s. She reported no alcohol or tobacco use and was taking rosuvastatin, esomeprazole, calcium, vitamin D, and glucosamine. There was no reported family history of asymmetric growth or bony deformities, and her children were healthy.
On physical examination, the lipomatosis affected the entire left side, most prominently around the abdomen, back, and thighs. The affected side was nontender and nonpruritic; there was no atrophy of the unaffected side (Figure). Maximum thigh circumference was 55.1 cm on the affected side and 52.6 cm on the unaffected side. There were no differences in power, reflex, or sensation between the 2 sides, and no hyperhidrosis or vascular malformations were present. Laboratory investigations, including complete blood cell count, complete metabolic panel, lipids, and thyroid-stimulating and sex hormone panels all were within reference range.
Enzi et al1 reported 2 women who developed asymmetrical lipomatosis between the ages of 13 and 20 years. Acquired asymmetrical lipomatosis should be differentiated from the asymmetrical overgrowth diagnosed in neonates and infants.
Proteus syndrome (PS) is a progressive disease involving a combination of overgrowth in a mosaic distribution, connective tissue and epidermal nevi, ovarian cysts, parotid gland tumor, dysregulated adipose tissue, lymphovascular malformation, and certain facial phenotypes.2,3 The average age of onset is 6 to 18 months, and half of cases present at birth.3,4 Hemihyperplasia-multiple lipomatosis syndrome (HHML) describes a mild and nonprogressive variant that does not satisfy the diagnostic criteria of PS; it typically is diagnosed at birth.5 One case of mild and delayed-onset PS was described in a woman who started developing signs at 15 years of age.6 In comparison, asymmetrical lipomatosis and scoliosis were the only abnormal clinical signs present in our patient, and the lipomatosis developed diffusely, as opposed to the typical mosaic distribution found in PS and HHML. Scoliosis can be found in PS and HHML secondary to hemihypertrophy of vertebra or infiltrative intraspinal lipomatosis.7,8 Our patient’s scoliosis was diagnosed more than 10 years prior to the onset of lipomatosis, likely representing degenerative joint disease.9
Prior reported cases of asymmetrical lipomatosis did not describe treatment.1 Ultrasound-guided or conventional liposuction and lipectomy are mainstream therapies for multiple symmetrical lipomatosis, an acquired lipomatosis typically affecting alcoholics in the fourth decade of life. However, recurrence rates are high for surgical treatment of unencapsulated lipomatosis, likely due to incomplete removal of the adipose tissue.10 Alternative treatments found in case reports, including oral salbutamol, mesotherapy using phosphatidylcholine, and fenofibrate (200 mg/d), require further study.11-13 Our patient was not aesthetically bothered by her lipomatosis; therefore, imaging and treatment options were not pursued. In conclusion, we report a patient with acquired asymmetrical lipomatosis with onset in late adulthood, unique from the existing syndromes of asymmetrical hemihyperplasia.1,14
To the Editor:
An 85-year-old woman presented with extra growth of subcutaneous fat at the left anterior infradiaphragm that expanded circumferentially to the left back over the last 4 years. Two years prior to the current presentation, the left thigh became visibly thicker than the right. Diffuse subtle lipomatosis affecting the ipsilateral face, neck, arms, calf, and foot was noted at that time. Additionally, the patient had hyperlipidemia, gastroesophageal reflux disease, osteoporosis, and scoliosis, all beginning in her late 60s. She reported no alcohol or tobacco use and was taking rosuvastatin, esomeprazole, calcium, vitamin D, and glucosamine. There was no reported family history of asymmetric growth or bony deformities, and her children were healthy.
On physical examination, the lipomatosis affected the entire left side, most prominently around the abdomen, back, and thighs. The affected side was nontender and nonpruritic; there was no atrophy of the unaffected side (Figure). Maximum thigh circumference was 55.1 cm on the affected side and 52.6 cm on the unaffected side. There were no differences in power, reflex, or sensation between the 2 sides, and no hyperhidrosis or vascular malformations were present. Laboratory investigations, including complete blood cell count, complete metabolic panel, lipids, and thyroid-stimulating and sex hormone panels all were within reference range.
Enzi et al1 reported 2 women who developed asymmetrical lipomatosis between the ages of 13 and 20 years. Acquired asymmetrical lipomatosis should be differentiated from the asymmetrical overgrowth diagnosed in neonates and infants.
Proteus syndrome (PS) is a progressive disease involving a combination of overgrowth in a mosaic distribution, connective tissue and epidermal nevi, ovarian cysts, parotid gland tumor, dysregulated adipose tissue, lymphovascular malformation, and certain facial phenotypes.2,3 The average age of onset is 6 to 18 months, and half of cases present at birth.3,4 Hemihyperplasia-multiple lipomatosis syndrome (HHML) describes a mild and nonprogressive variant that does not satisfy the diagnostic criteria of PS; it typically is diagnosed at birth.5 One case of mild and delayed-onset PS was described in a woman who started developing signs at 15 years of age.6 In comparison, asymmetrical lipomatosis and scoliosis were the only abnormal clinical signs present in our patient, and the lipomatosis developed diffusely, as opposed to the typical mosaic distribution found in PS and HHML. Scoliosis can be found in PS and HHML secondary to hemihypertrophy of vertebra or infiltrative intraspinal lipomatosis.7,8 Our patient’s scoliosis was diagnosed more than 10 years prior to the onset of lipomatosis, likely representing degenerative joint disease.9
Prior reported cases of asymmetrical lipomatosis did not describe treatment.1 Ultrasound-guided or conventional liposuction and lipectomy are mainstream therapies for multiple symmetrical lipomatosis, an acquired lipomatosis typically affecting alcoholics in the fourth decade of life. However, recurrence rates are high for surgical treatment of unencapsulated lipomatosis, likely due to incomplete removal of the adipose tissue.10 Alternative treatments found in case reports, including oral salbutamol, mesotherapy using phosphatidylcholine, and fenofibrate (200 mg/d), require further study.11-13 Our patient was not aesthetically bothered by her lipomatosis; therefore, imaging and treatment options were not pursued. In conclusion, we report a patient with acquired asymmetrical lipomatosis with onset in late adulthood, unique from the existing syndromes of asymmetrical hemihyperplasia.1,14
- Enzi G, Digito M, Enzi GB, et al. Asymmetrical lipomatosis: report of two cases. Postgrad Med J. 1985;61:797-800.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Cohen MM Jr. Proteus syndrome: an update. Am J Med Genet C Semin Med Genet. 2005;137C:38-52.
- Biesecker LG, Peters KF, Darling TN, et al. Clinical differentiation between Proteus syndrome and hemihyperplasia: description of a distinct form of hemihyperplasia. Am J Med Genet. 1998;79:311-318.
- Luo S, Feng Y, Zheng Y, et al. Mild and delayed-onset Proteus syndrome. Eur J Dermatol. 2007;17:172-173.
- Takebayashi T, Yamashita T, Yokogushi K, et al. Scoliosis in Proteus syndrome: case report. Spine. 2001;26:E395-E398.
- Schulte TL, Liljenqvist U, Görgens H, et al. Hemihyperplasia-multiple lipomatosis syndrome (HHML): a challenge in spinal care. Acta Orthop Belg. 2008;74:714-719.
- Robin GC, Span Y, Steinberg R, et al. Scoliosis in the elderly: a follow-up study. Spine. 1982;7:355-359.
- Brea-García B, Cameselle-Teijeiro J, Couto-González I, et al. Madelung’s disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthet Plast Surg. 2013;37:409-416.
- Hasegawa T, Matsukura T, Ikeda S. Mesotherapy for benign symmetric lipomatosis. Aesthet Plast Surg. 2010;34:153-156.
- Zeitler H, Ulrich-Merzenich G, Richter DF, et al. Multiple benign symmetric lipomatosis—a differential diagnosis of obesity. is there a rationale for fibrate treatment? Obes Surg. 2008;18:1354-1356.
- Leung N, Gaer J, Beggs D, et al. Multiple symmetric lipomatosis (Launois‐Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol. 1987;27:601-606.
- Craiglow BG, Ko CJ, Antaya RJ. Two cases of hemihyperplasia-multiple lipomatosis syndrome and review of asymmetric hemihyperplasia syndromes. Pediatr Dermatol. 2014;31:507-510.
- Enzi G, Digito M, Enzi GB, et al. Asymmetrical lipomatosis: report of two cases. Postgrad Med J. 1985;61:797-800.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151-1157.
- Cohen MM Jr. Proteus syndrome: an update. Am J Med Genet C Semin Med Genet. 2005;137C:38-52.
- Biesecker LG, Peters KF, Darling TN, et al. Clinical differentiation between Proteus syndrome and hemihyperplasia: description of a distinct form of hemihyperplasia. Am J Med Genet. 1998;79:311-318.
- Luo S, Feng Y, Zheng Y, et al. Mild and delayed-onset Proteus syndrome. Eur J Dermatol. 2007;17:172-173.
- Takebayashi T, Yamashita T, Yokogushi K, et al. Scoliosis in Proteus syndrome: case report. Spine. 2001;26:E395-E398.
- Schulte TL, Liljenqvist U, Görgens H, et al. Hemihyperplasia-multiple lipomatosis syndrome (HHML): a challenge in spinal care. Acta Orthop Belg. 2008;74:714-719.
- Robin GC, Span Y, Steinberg R, et al. Scoliosis in the elderly: a follow-up study. Spine. 1982;7:355-359.
- Brea-García B, Cameselle-Teijeiro J, Couto-González I, et al. Madelung’s disease: comorbidities, fatty mass distribution, and response to treatment of 22 patients. Aesthet Plast Surg. 2013;37:409-416.
- Hasegawa T, Matsukura T, Ikeda S. Mesotherapy for benign symmetric lipomatosis. Aesthet Plast Surg. 2010;34:153-156.
- Zeitler H, Ulrich-Merzenich G, Richter DF, et al. Multiple benign symmetric lipomatosis—a differential diagnosis of obesity. is there a rationale for fibrate treatment? Obes Surg. 2008;18:1354-1356.
- Leung N, Gaer J, Beggs D, et al. Multiple symmetric lipomatosis (Launois‐Bensaude syndrome): effect of oral salbutamol. Clin Endocrinol. 1987;27:601-606.
- Craiglow BG, Ko CJ, Antaya RJ. Two cases of hemihyperplasia-multiple lipomatosis syndrome and review of asymmetric hemihyperplasia syndromes. Pediatr Dermatol. 2014;31:507-510.
Practice Points
- Acquired asymmetrical lipomatosis is a rare condition that can develop at any age; it should be differentiated from existing syndromes of asymmetrical hemihyperplasia.
- Acquired asymmetrical lipomatosis is a clinical diagnosis with no laboratory changes. If the patient is clinically stable and asymptomatic, no further investigation or management is required.
Asboe-Hansen Sign in Toxic Epidermal Necrolysis
To the Editor:
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.
applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
To the Editor:
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.
applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
To the Editor:
A 25-year-old woman with no notable medical history was admitted to the hospital for suspected Stevens-Johnson syndrome (SJS). The patient was started on amoxicillin 7 days prior to the skin eruption for prophylaxis before removal of an intrauterine device. On the day of admission, she reported ocular discomfort, dysphagia, and dysuria. She developed erythema of the conjunctivae, face, chest, and proximal upper extremities, as well as erosions of the vermilion lips. She presented to the local emergency department and was transferred to our institution for urgent dermatologic consultation. On physical examination by the dermatology service, the patient had erythematous macules coalescing into patches with overlying flaccid bullae, some denuded, involving the face, chest, abdomen, back (Figure 1), bilateral upper extremities, bilateral thighs, and labia majora and minora. Additionally, she had conjunctivitis, superficial erosions of the vermilion lips, and tense bullae of the palms and soles. On palpation of the flaccid bullae, the Asboe-Hansen sign was elicited (Figure 2 and video). A shave biopsy of the newly elicited bullae was performed. Pathology showed a subepidermal bulla with confluent necrosis of the epidermis and minimal inflammatory infiltrate. An additional shave biopsy of perilesional skin was obtained for direct immunofluorescence, which was negative for IgG, C3, IgM, and IgA. Based on the clinical presentation involving more than 30% of the patient’s body surface area (BSA) and the pathology findings, a diagnosis of toxic epidermal necrolysis (TEN) was made. The patient remained in the intensive care unit with a multidisciplinary team consisting of dermatology, ophthalmology, gynecology, gastroenterology, and the general surgery burn group. Following treatment with intravenous immunoglobulin, systemic corticosteroids, and aggressive wound care, the patient made a full recovery.
applied to an intact bulla.

Toxic epidermal necrolysis is a rare, acute, life-threatening mucocutaneous disease within a spectrum of adverse cutaneous drug reactions. The estimated worldwide incidence of TEN is 0.4 to 1.9 per million individuals annually.1 Toxic epidermal necrolysis is clinically characterized by diffuse exfoliation of the skin and mucosae with flaccid bullae. These clinical features are a consequence of extensive keratinocyte death, leading to dermoepidermal junction dissociation. Commonly, there is a prodrome of fever, pharyngitis, and painful skin preceding the diffuse erythema and sloughing of skin and mucous membranes. Lesions typically first appear on the trunk and then follow a centrifugal spread, often sparing the distal aspects of the arms and legs.
Toxic epidermal necrolysis is part of a continuous spectrum with SJS. Less than 10% BSA involvement is considered SJS, 10% to 30% BSA involvement is SJS/TEN overlap, and more than 30% BSA detachment is TEN. Stevens-Johnson syndrome can progress to TEN. In TEN, the distribution of cutaneous lesions is more confluent, and mucosal involvement is more severe.2 The differential diagnosis may include staphylococcal scalded skin syndrome, drug-induced linear IgA bullous dermatosis, severe acute graft-vs-host disease, drug reaction with eosinophilia and systemic symptoms, and invasive fungal dermatitis. An accurate diagnosis of TEN is imperative, as the management and morbidity of these diseases are vastly different. Toxic epidermal necrolysis has an estimated mortality rate of 25% to 30%, with sepsis leading to multiorgan failure being the most common cause of death.3
Although the pathophysiology of TEN has yet to be fully elucidated, it is thought to be a T cell–mediated process with CD8+ cells acting as the primary means of keratinocyte death. An estimated 80% to 95% of cases are due to drug reactions.3 The medications that are most commonly associated with TEN include allopurinol, antibiotics, nonsteroidal anti-inflammatory drugs, and anticonvulsants. Symptoms typically begin 7 to 21 days after starting the drug. Less commonly, Mycoplasma pneumoniae, dengue virus, cytomegalovirus, and contrast medium have been reported as inciting factors for TEN.2
The diagnosis of TEN is established by correlating clinical features with a histopathologic examination obtained from a lesional skin biopsy. The classic cutaneous features of TEN begin as erythematous, flesh-colored, dusky to violaceous macules and/or morbilliform or targetoid lesions. These early lesions have the tendency to coalesce. The cutaneous findings will eventually progress into flaccid bullae, diffuse epidermal sloughing, and full-thickness skin necrosis.2,3 The evolution of skin lesions may be rapid or may take several days to develop. On palpation, the Nikolsky (lateral shearing of epidermis with minimal pressure) and Asboe-Hansen sign will be positive in patients with SJS/TEN, demonstrating that the associated blisters are flaccid and may be displaced peripherally.4 For an accurate diagnosis, the biopsy must contain full-thickness epidermis. It is imperative to choose a biopsy site from an acute blister, as old lesions of other diseases, such as erythema multiforme, will eventually become necrotic and mimic the histopathologic appearance of SJS/TEN, potentially leading to an incorrect diagnosis.4 Full-thickness epidermal necrosis has a high sensitivity but low specificity for TEN.3 The histologic features of TEN vary depending on the stage of the disease. Classic histologic findings include satellite necrosis of keratinocytes followed by full-thickness necrosis of keratinocytes and perivascular lymphoid infiltrates. The stratum corneum retains its original structure.4
The Asboe-Hansen sign, also known as the bulla spread sign, was originally described in 1960 as a diagnostic sign for pemphigus vulgaris.5 A positive Asboe-Hansen sign demonstrates the ability to enlarge a bulla in the lateral direction by applying perpendicular mechanical pressure to the roof of an intact bulla. The bulla is extended to adjacent nonblistered skin.6 A positive sign demonstrates decreased adhesion between keratinocytes or between the basal epidermal cells and the dermal connective tissue.5 In addition to pemphigus vulgaris, the Asboe-Hansen sign may be positive in TEN and SJS, as well as other diseases affecting the dermoepidermal junction including pemphigus foliaceus, pemphigus vegetans, and bullous pemphigoid. Asboe-Hansen5 made the argument that a fresh bulla should be biopsied if histopathologic diagnosis is necessary, as older bullae may exhibit epithelial cell regeneration and disturb an accurate diagnosis.
Accurate and early diagnosis of TEN is imperative, as prognosis is strongly correlated with the speed at which the offending drug is discontinued and appropriate medical treatment is initiated. Prompt withdrawal of the offending drug has been reported to reduce the risk for morbidity by 30% per day.7 Although classically associated with the pemphigus group of diseases, the Asboe-Hansen sign is of diagnostic value to the pathologist in diagnosing TEN by reproducing the same microscopic appearance of a fresh spontaneous blister. Due to the notable morbidity and mortality in SJS and TEN, the Asboe-Hansen sign should be attempted for the site of a lesional biopsy, as an accurate diagnosis relies on clinicopathologic correlation.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part I. introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1-173.e13.
- Frech LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. New York, NY: Elsevier; 2012:332-347.
- Schwartz RA, McDonough PH, Lee BW, et al. Toxic epidermal necrolysis: part II. prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69:187.e1–187.e16.
- Elston D, Stratman E, Miller S. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
- Asboe-Hansen G. Blister-spread induced by finger-pressure, a diagnostic sign in pemphigus. J Invest Dermatol. 1960;34:5-9.
- Ganapati S. Eponymous dermatological signs in bullous dermatoses. Indian J Dermatol. 2014;59:21-23.
- Garcia-Doval I, Lecleach L, Bocquet H, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323-327.
Practice Points
- Asboe-Hansen sign is a useful clinical tool for diagnosing toxic epidermal necrolysis (TEN).
- Asboe-Hansen sign can be employed to generate a fresh bulla for lesional skin biopsy in the evaluation of TEN.
Symmetric Lichen Amyloidosis: An Atypical Location on the Bilateral Extensor Surfaces of the Arms
To the Editor:
Lichen amyloidosis (LA) classically presents as a pruritic, hyperkeratotic, papular eruption localized to the pretibial surface of the legs.1 Nonpruritic and generalized variants have been reported but are rare.2 Although it is the most common subtype of primary localized cutaneous amyloidosis, LA is a benign condition but is difficult to eradicate.1 The precise pathophysiology is poorly understood, but chronic frictional irritation is closely associated with the eruption. We present a nongeneralized case of LA in an atypical location.
A healthy 30-year-old woman presented with an intermittent itchy rash on the elbows and knees of 2 years’ duration. The patient was first diagnosed with lichen simplex chronicus (LSC) and initially responded well to treatment with fluocinonide ointment 0.05%. Nearly 2 years after the initial presentation, she developed recurrent symptoms and sought further treatment. She reported frequent scratching in association with episodes of anxiety. Examination revealed numerous 1- to 3-mm, flesh-colored to light brown, monomorphic, dome-shaped papules over the extensor surfaces of the bilateral arms and left pretibial surface (Figure 1).
papules (1–3 mm) over the extensor surfaces of the bilateral arms.
Although in an atypical location, LA was clinically suspected due to the morphology, and a biopsy was performed given the evolving nature of the lesions. The differential diagnosis included LSC, hypertrophic lichen planus, papular mucinosis, prurigo nodularis, and pretibial myxedema. Pathology revealed small eosinophilic globules in the papillary dermis (Figure 2), and cytokeratin 5/6 immunostaining showed amorphous papillary dermal deposits consistent with keratin-derived amyloid deposition (Figure 3). The deposits stained positive for Congo red and displayed apple green birefringence under polarized light. Thus, the diagnosis of LA was confirmed. After limited success with triamcinolone ointment 0.1%, the patient was transitioned to clobetasol cream 0.05% with notable physical and symptomatic improvement.
Amyloidosis is histopathologically characterized by extracellular deposits of amyloid, a polypeptide that polymerizes to form cross-β sheets.3 It is believed that the deposits seen in localized amyloidosis result from local production of amyloid, as opposed to the deposition of circulating light chains that is characteristic of systemic amyloidosis.3 Lichen amyloidosis is the most common subtype of primary localized cutaneous amyloidosis.1 The amyloid in this condition has been found to react immunohistochemically with antikeratin antibody, leading to the conclusion that the amyloid is formed by degeneration of keratinocytes locally due to chronic rubbing and scratching.
4-6
The possibility remains that this patient first presented with LSC 2 years prior and secondarily developed LA due to chronic trauma. Indeed, LA has been proposed as a variant of LSC. In both conditions, scratching seems to be the most important factor in the development of lesions. It has been proposed that treatment should primarily focus on the amelioration of pruritus.5
Five percent to 10% of cases of LA have been found to have some form of upper extremity involvement.7 However, these cases typically are associated with a generalized presentation involving the trunk and arms.2,7 Our patient had no evidence of disease elsewhere. When evaluating a localized, pruritic, monomorphic, papular eruption on the extensor surfaces of the arms, LA may be an important consideration.
- Tay CH, Dacosta JL. Lichen amyloidosis. clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Kandhari R, Ramesh V, Singh A. A generalized, non-pruritic variant of lichen amyloidosis: a case report and a brief review. Indian J Dermatol. 2013;58:328.
- Biewend ML, Menke DM, Calamia KT. The spectrum of localized amyloidosis: a case series of 20 patients and review of the literature. Amyloid. 2006;13:135-142.
- Jambrosic J, From L, Hanna W. Lichen amyloidosus. ultrastructure and pathogenesis. Am J Dermatopathol. 1984;6:151-158.
- Weyers W, Weyers I, Bonczkowitz M, et al. Lichen amyloidosis: a consequence of scratching. J Am Acad Dermatol. 1997;37:923-928.
- Kumakiri M, Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J Invest Dermatol. 1979;73:150-162.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosus: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
To the Editor:
Lichen amyloidosis (LA) classically presents as a pruritic, hyperkeratotic, papular eruption localized to the pretibial surface of the legs.1 Nonpruritic and generalized variants have been reported but are rare.2 Although it is the most common subtype of primary localized cutaneous amyloidosis, LA is a benign condition but is difficult to eradicate.1 The precise pathophysiology is poorly understood, but chronic frictional irritation is closely associated with the eruption. We present a nongeneralized case of LA in an atypical location.
A healthy 30-year-old woman presented with an intermittent itchy rash on the elbows and knees of 2 years’ duration. The patient was first diagnosed with lichen simplex chronicus (LSC) and initially responded well to treatment with fluocinonide ointment 0.05%. Nearly 2 years after the initial presentation, she developed recurrent symptoms and sought further treatment. She reported frequent scratching in association with episodes of anxiety. Examination revealed numerous 1- to 3-mm, flesh-colored to light brown, monomorphic, dome-shaped papules over the extensor surfaces of the bilateral arms and left pretibial surface (Figure 1).
papules (1–3 mm) over the extensor surfaces of the bilateral arms.
Although in an atypical location, LA was clinically suspected due to the morphology, and a biopsy was performed given the evolving nature of the lesions. The differential diagnosis included LSC, hypertrophic lichen planus, papular mucinosis, prurigo nodularis, and pretibial myxedema. Pathology revealed small eosinophilic globules in the papillary dermis (Figure 2), and cytokeratin 5/6 immunostaining showed amorphous papillary dermal deposits consistent with keratin-derived amyloid deposition (Figure 3). The deposits stained positive for Congo red and displayed apple green birefringence under polarized light. Thus, the diagnosis of LA was confirmed. After limited success with triamcinolone ointment 0.1%, the patient was transitioned to clobetasol cream 0.05% with notable physical and symptomatic improvement.
Amyloidosis is histopathologically characterized by extracellular deposits of amyloid, a polypeptide that polymerizes to form cross-β sheets.3 It is believed that the deposits seen in localized amyloidosis result from local production of amyloid, as opposed to the deposition of circulating light chains that is characteristic of systemic amyloidosis.3 Lichen amyloidosis is the most common subtype of primary localized cutaneous amyloidosis.1 The amyloid in this condition has been found to react immunohistochemically with antikeratin antibody, leading to the conclusion that the amyloid is formed by degeneration of keratinocytes locally due to chronic rubbing and scratching.
4-6
The possibility remains that this patient first presented with LSC 2 years prior and secondarily developed LA due to chronic trauma. Indeed, LA has been proposed as a variant of LSC. In both conditions, scratching seems to be the most important factor in the development of lesions. It has been proposed that treatment should primarily focus on the amelioration of pruritus.5
Five percent to 10% of cases of LA have been found to have some form of upper extremity involvement.7 However, these cases typically are associated with a generalized presentation involving the trunk and arms.2,7 Our patient had no evidence of disease elsewhere. When evaluating a localized, pruritic, monomorphic, papular eruption on the extensor surfaces of the arms, LA may be an important consideration.
To the Editor:
Lichen amyloidosis (LA) classically presents as a pruritic, hyperkeratotic, papular eruption localized to the pretibial surface of the legs.1 Nonpruritic and generalized variants have been reported but are rare.2 Although it is the most common subtype of primary localized cutaneous amyloidosis, LA is a benign condition but is difficult to eradicate.1 The precise pathophysiology is poorly understood, but chronic frictional irritation is closely associated with the eruption. We present a nongeneralized case of LA in an atypical location.
A healthy 30-year-old woman presented with an intermittent itchy rash on the elbows and knees of 2 years’ duration. The patient was first diagnosed with lichen simplex chronicus (LSC) and initially responded well to treatment with fluocinonide ointment 0.05%. Nearly 2 years after the initial presentation, she developed recurrent symptoms and sought further treatment. She reported frequent scratching in association with episodes of anxiety. Examination revealed numerous 1- to 3-mm, flesh-colored to light brown, monomorphic, dome-shaped papules over the extensor surfaces of the bilateral arms and left pretibial surface (Figure 1).
papules (1–3 mm) over the extensor surfaces of the bilateral arms.
Although in an atypical location, LA was clinically suspected due to the morphology, and a biopsy was performed given the evolving nature of the lesions. The differential diagnosis included LSC, hypertrophic lichen planus, papular mucinosis, prurigo nodularis, and pretibial myxedema. Pathology revealed small eosinophilic globules in the papillary dermis (Figure 2), and cytokeratin 5/6 immunostaining showed amorphous papillary dermal deposits consistent with keratin-derived amyloid deposition (Figure 3). The deposits stained positive for Congo red and displayed apple green birefringence under polarized light. Thus, the diagnosis of LA was confirmed. After limited success with triamcinolone ointment 0.1%, the patient was transitioned to clobetasol cream 0.05% with notable physical and symptomatic improvement.
Amyloidosis is histopathologically characterized by extracellular deposits of amyloid, a polypeptide that polymerizes to form cross-β sheets.3 It is believed that the deposits seen in localized amyloidosis result from local production of amyloid, as opposed to the deposition of circulating light chains that is characteristic of systemic amyloidosis.3 Lichen amyloidosis is the most common subtype of primary localized cutaneous amyloidosis.1 The amyloid in this condition has been found to react immunohistochemically with antikeratin antibody, leading to the conclusion that the amyloid is formed by degeneration of keratinocytes locally due to chronic rubbing and scratching.
4-6
The possibility remains that this patient first presented with LSC 2 years prior and secondarily developed LA due to chronic trauma. Indeed, LA has been proposed as a variant of LSC. In both conditions, scratching seems to be the most important factor in the development of lesions. It has been proposed that treatment should primarily focus on the amelioration of pruritus.5
Five percent to 10% of cases of LA have been found to have some form of upper extremity involvement.7 However, these cases typically are associated with a generalized presentation involving the trunk and arms.2,7 Our patient had no evidence of disease elsewhere. When evaluating a localized, pruritic, monomorphic, papular eruption on the extensor surfaces of the arms, LA may be an important consideration.
- Tay CH, Dacosta JL. Lichen amyloidosis. clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Kandhari R, Ramesh V, Singh A. A generalized, non-pruritic variant of lichen amyloidosis: a case report and a brief review. Indian J Dermatol. 2013;58:328.
- Biewend ML, Menke DM, Calamia KT. The spectrum of localized amyloidosis: a case series of 20 patients and review of the literature. Amyloid. 2006;13:135-142.
- Jambrosic J, From L, Hanna W. Lichen amyloidosus. ultrastructure and pathogenesis. Am J Dermatopathol. 1984;6:151-158.
- Weyers W, Weyers I, Bonczkowitz M, et al. Lichen amyloidosis: a consequence of scratching. J Am Acad Dermatol. 1997;37:923-928.
- Kumakiri M, Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J Invest Dermatol. 1979;73:150-162.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosus: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
- Tay CH, Dacosta JL. Lichen amyloidosis. clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Kandhari R, Ramesh V, Singh A. A generalized, non-pruritic variant of lichen amyloidosis: a case report and a brief review. Indian J Dermatol. 2013;58:328.
- Biewend ML, Menke DM, Calamia KT. The spectrum of localized amyloidosis: a case series of 20 patients and review of the literature. Amyloid. 2006;13:135-142.
- Jambrosic J, From L, Hanna W. Lichen amyloidosus. ultrastructure and pathogenesis. Am J Dermatopathol. 1984;6:151-158.
- Weyers W, Weyers I, Bonczkowitz M, et al. Lichen amyloidosis: a consequence of scratching. J Am Acad Dermatol. 1997;37:923-928.
- Kumakiri M, Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J Invest Dermatol. 1979;73:150-162.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosus: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
Practice Points
- Lichen amyloidosis (LA) classically presents as a pruritic and papular eruption localized to the pretibial surface of the legs.
- Nonpruritic and generalized variants are rare.
- This case represents a pruritic and nongeneralized
case located on the arms; LA should be considered
for any localized and pruritic eruption on the arms.