User login
How can you help athletes prevent and treat shin splints?
Encourage patients who are concerned about shin splints to decrease the intensity of their running; suggest rest and ice and foot orthoses, to treat the condition. Reducing running intensity probably reduces lower extremity soft tissue injuries (strength of recommendation [SOR]: B, low-quality randomized controlled trials [RCTs]), although doing stretching exercises doesn’t. Rest and ice alone promote faster recovery than rest and ice combined with nonsteroidal anti-inflammatory drugs (NSAIDs), a walking cast, or heel-cord stretching. (SOR: B, low-quality RCT). Although foot orthoses or insoles don’t prevent lower limb injuries (SOR: A, systematic review), they do significantly relieve symptoms and promote return to running (SOR: C, poor-quality cohort studies). Lower limb fasciotomy can reduce symptoms caused by shin splints in athletes, but the rate of return to previous level of sports activity is modest at best (SOR: C, case series).
Review, rest, follow-up
Doug Aukerman, MD
Pennsylvania State University, State College
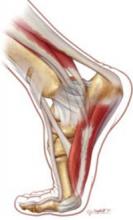
Shin splints are an early indicator of abnormal stresses that cause periostitis along the tibia. Medial tibial stress syndrome (MTSS) is best thought of as part of a continuum, with shin splints at the mild end and tibial stress fracture at the severe extreme. I find that reviewing biomechanical forces and training schedules often helps prevent and treat injury. Aging footwear and training errors, including a rapid increase in training volume, are common culprits when biomechanics are normal. Adequate rest to allow the body to respond to the stresses of training can help prevent stress-related injuries.
My treatment regimen for shin splints includes a period of relative rest that allows the runner to remain active but reduces the stresses placed on the tibia and musculature of the lower leg. Relative rest may comprise either reducing running distance and intensity or changing the mode of exercise to deep water running or cycling. Consider follow-up examination to ensure that the tibial pain doesn’t become more localized, indicating a possible stress fracture.
Evidence summary
MTSS, or shin splints, is the most common cause of exertional leg pain in athletes. MTSS is an overuse injury characterized by pain along the posteromedial aspect of the distal two-thirds of the tibia.
Reduced running time, frequency, and distance may prevent injury
A systematic review of 12 trials of 8806 mostly young male military recruits, evaluated nonsurgical interventions to prevent lower limb soft tissue running injuries, including MTSS.1 Five trials investigating stretching and 4 trials of shoe insoles showed no benefit. Three studies showed that reducing the duration of running (to between 15 and 30 minutes), frequency (to 1 or 3 days a week), or distance (to 16.5 fewer kilometers over 12 weeks) reduced the risk of all injuries. Methods and outcome measures of the studies were heterogeneous, limiting the generalizability of these results.
A prospective RCT of custom semi-rigid shoe orthoses in the boots of Danish army recruits found a significantly lower prevalence of MTSS at 3-month follow-up in the intervention group (13% compared with 24%, P=.005; NNT=5).2 However, a systematic review of methods to prevent MTSS in sports found no high-quality studies in nonmilitary populations.3 No difference in the incidence of MTSS was noted with shoe inserts, different combat boots, or stretching routines.
To treat shin splints, consider orthotics, rest, and ice
Two studies retrospectively surveyed runners about their response to orthotics to treat MTSS.4,5 In one study, 16 of 41 collegiate cross-country runners were prescribed orthotics; 14 (88%) reported relief or improvement in their symptoms and return to running within 4 weeks.4 The other survey, of long-distance runners, found that 70% who had used or were using orthotics for a presumed diagnosis of MTSS reported complete relief or great improvement.5
A study of 97 US naval midshipmen who developed MTSS during summer training programs compared randomly assigned treatments of rest and ice to rest and ice plus anti-inflammatory medication (aspirin or phenylbutazone), rest and ice plus heel-cord stretching, or use of a short walking cast for 1 week.6 Subjects assigned to a treatment program of rest and ice alone lost significantly fewer days from running than the other treatment groups (P<.03).
A recent RCT of soldiers with MTSS compared treatment with a leg orthosis with no orthotic use; all subjects underwent icing and activity modification.7 No significant differences in outcome measures (days to completion of a 0.5-mile run, global rating of change, or number of treatment sessions) were noted between the treatment groups. Small sample size, high dropout rate, and small effect size limit the power of this study.
Fasciotomy can relieve symptoms but may not improve postop activity
Three case series reported on deep posterior compartment fasciotomy to treat MTSS in athletes.8-10 Surgery significantly reduced pain levels (P<.001) by an average of 72% on the visual analog pain scale for 46 patients who had failed conservative therapy for at least 12 months.8 However, only 41% returned to their pre-symptom level of sports activity. Another case series of mostly running athletes reported that 21 (78%) of 27 patients exhibited excellent or good healing after surgery.9 In a series of 9 patients with an average of 39 months of symptoms, 5 reported complete relief at follow-up (42±6 months), and 7 trained more than they had preoperatively.10
One case series of superficial posterior compartment fasciotomy for MTSS in 35 athletes reported that, among the 32 athletes available for follow-up, 72% (23) reported improved symptoms, but 69% (22) had a lower level of activity postoperatively.11
Recommendations
The American College of Sports Medicine (ACSM) recommends at least 7 to 10 days of rest from painful activities to treat MTSS.12 Running in a pool and cycling to maintain aerobic fitness during the rest period are considered safe activities. Stretching and strengthening exercises are not recommended while symptoms persist. Patients should return to running gradually. ACSM also suggests that orthotics are useful for preventing injury and treating some patients.
1. Yeung EW, Yeung SS. Interventions for preventing lower limb soft-tissue injuries in runners. Cochrane Database Syst Rev. 2005;(4):CD001256.-
2. Larsen K, Weidich F, Leboeuf-Yde C. Can custom-made biomechanic shoe orthoses prevent problems in the back and lower extremities? A randomized, controlled intervention trial of 146 military conscripts. J Manipulative Physiol Ther. 2002;25:326-331.
3. Thacker SB, Gilchrist J, Stroup DF, et al. The prevention of shin splints in sports: a systematic review of literature. Med Sci Sports Exerc. 2002;34:32-40.
4. Eickhoff CA, Hossain SA, Slawski DP. From the field. Effects of prescribed foot orthoses on medial tibial stress syndrome in collegiate cross-country runners. Clin Kinesiol. 2000;54:76-80.
5. Gross ML, Davlin LB, Evanski PM. Effectiveness of orthotic shoe inserts in the long-distance runner. Am J Sports Med. 1991;19:409-412.
6. Andrish JT, Bergfeld JA, Walheim J. A prospective study on the management of shin splints. J Bone Joint Surg Am. 1974;56:1697-1700.
7. Johnston E, Flynn T, Bean M, et al. A randomized controlled trial of a leg orthosis versus traditional treatment for soldiers with shin splints: a pilot study. Mil Med. 2006;171:40-44.
8. Yates B, Allen MJ, Barnes MR. Outcome of surgical treatment of medial tibial stress syndrome. J Bone Joint Surg Am. 2003;85-A:1974-1980.
9. Järvinnen M, Aho H, Niittymäki S. Results of the surgical treatment of the medial tibial syndrome in athletes Int J Sports Med. 1989;10:55-57
10. Wallenstein R. Results of fasciotomy in patients with medial tibial syndrome or chronic anterior-compartment syndrome. .J Bone Joint Surg Am. 1983;65-A:1252-1255.
11. Holen KJ, Engebretsen L, Grontvedt T, et al. Surgical treatment of medial tibial stress syndrome (shin splint) by fasciotomy of the superficial posterior compartment of the leg. Scand J Med Sci Sports. 1995;5:40-43.
12. Beck BR. Exercise-induced leg pain. Current Comment. March 2002. American College of Sports Medicine. Available at: www.acsm.org/AM/Template.cfm?Section=Search§ion=20026&template=/CM/ContentDisplaycfm&ContentFileID=299. Accessed December 14, 2006.
Encourage patients who are concerned about shin splints to decrease the intensity of their running; suggest rest and ice and foot orthoses, to treat the condition. Reducing running intensity probably reduces lower extremity soft tissue injuries (strength of recommendation [SOR]: B, low-quality randomized controlled trials [RCTs]), although doing stretching exercises doesn’t. Rest and ice alone promote faster recovery than rest and ice combined with nonsteroidal anti-inflammatory drugs (NSAIDs), a walking cast, or heel-cord stretching. (SOR: B, low-quality RCT). Although foot orthoses or insoles don’t prevent lower limb injuries (SOR: A, systematic review), they do significantly relieve symptoms and promote return to running (SOR: C, poor-quality cohort studies). Lower limb fasciotomy can reduce symptoms caused by shin splints in athletes, but the rate of return to previous level of sports activity is modest at best (SOR: C, case series).
Review, rest, follow-up
Doug Aukerman, MD
Pennsylvania State University, State College
Shin splints are an early indicator of abnormal stresses that cause periostitis along the tibia. Medial tibial stress syndrome (MTSS) is best thought of as part of a continuum, with shin splints at the mild end and tibial stress fracture at the severe extreme. I find that reviewing biomechanical forces and training schedules often helps prevent and treat injury. Aging footwear and training errors, including a rapid increase in training volume, are common culprits when biomechanics are normal. Adequate rest to allow the body to respond to the stresses of training can help prevent stress-related injuries.
My treatment regimen for shin splints includes a period of relative rest that allows the runner to remain active but reduces the stresses placed on the tibia and musculature of the lower leg. Relative rest may comprise either reducing running distance and intensity or changing the mode of exercise to deep water running or cycling. Consider follow-up examination to ensure that the tibial pain doesn’t become more localized, indicating a possible stress fracture.
Evidence summary
MTSS, or shin splints, is the most common cause of exertional leg pain in athletes. MTSS is an overuse injury characterized by pain along the posteromedial aspect of the distal two-thirds of the tibia.
Reduced running time, frequency, and distance may prevent injury
A systematic review of 12 trials of 8806 mostly young male military recruits, evaluated nonsurgical interventions to prevent lower limb soft tissue running injuries, including MTSS.1 Five trials investigating stretching and 4 trials of shoe insoles showed no benefit. Three studies showed that reducing the duration of running (to between 15 and 30 minutes), frequency (to 1 or 3 days a week), or distance (to 16.5 fewer kilometers over 12 weeks) reduced the risk of all injuries. Methods and outcome measures of the studies were heterogeneous, limiting the generalizability of these results.
A prospective RCT of custom semi-rigid shoe orthoses in the boots of Danish army recruits found a significantly lower prevalence of MTSS at 3-month follow-up in the intervention group (13% compared with 24%, P=.005; NNT=5).2 However, a systematic review of methods to prevent MTSS in sports found no high-quality studies in nonmilitary populations.3 No difference in the incidence of MTSS was noted with shoe inserts, different combat boots, or stretching routines.
To treat shin splints, consider orthotics, rest, and ice
Two studies retrospectively surveyed runners about their response to orthotics to treat MTSS.4,5 In one study, 16 of 41 collegiate cross-country runners were prescribed orthotics; 14 (88%) reported relief or improvement in their symptoms and return to running within 4 weeks.4 The other survey, of long-distance runners, found that 70% who had used or were using orthotics for a presumed diagnosis of MTSS reported complete relief or great improvement.5
A study of 97 US naval midshipmen who developed MTSS during summer training programs compared randomly assigned treatments of rest and ice to rest and ice plus anti-inflammatory medication (aspirin or phenylbutazone), rest and ice plus heel-cord stretching, or use of a short walking cast for 1 week.6 Subjects assigned to a treatment program of rest and ice alone lost significantly fewer days from running than the other treatment groups (P<.03).
A recent RCT of soldiers with MTSS compared treatment with a leg orthosis with no orthotic use; all subjects underwent icing and activity modification.7 No significant differences in outcome measures (days to completion of a 0.5-mile run, global rating of change, or number of treatment sessions) were noted between the treatment groups. Small sample size, high dropout rate, and small effect size limit the power of this study.
Fasciotomy can relieve symptoms but may not improve postop activity
Three case series reported on deep posterior compartment fasciotomy to treat MTSS in athletes.8-10 Surgery significantly reduced pain levels (P<.001) by an average of 72% on the visual analog pain scale for 46 patients who had failed conservative therapy for at least 12 months.8 However, only 41% returned to their pre-symptom level of sports activity. Another case series of mostly running athletes reported that 21 (78%) of 27 patients exhibited excellent or good healing after surgery.9 In a series of 9 patients with an average of 39 months of symptoms, 5 reported complete relief at follow-up (42±6 months), and 7 trained more than they had preoperatively.10
One case series of superficial posterior compartment fasciotomy for MTSS in 35 athletes reported that, among the 32 athletes available for follow-up, 72% (23) reported improved symptoms, but 69% (22) had a lower level of activity postoperatively.11
Recommendations
The American College of Sports Medicine (ACSM) recommends at least 7 to 10 days of rest from painful activities to treat MTSS.12 Running in a pool and cycling to maintain aerobic fitness during the rest period are considered safe activities. Stretching and strengthening exercises are not recommended while symptoms persist. Patients should return to running gradually. ACSM also suggests that orthotics are useful for preventing injury and treating some patients.
Encourage patients who are concerned about shin splints to decrease the intensity of their running; suggest rest and ice and foot orthoses, to treat the condition. Reducing running intensity probably reduces lower extremity soft tissue injuries (strength of recommendation [SOR]: B, low-quality randomized controlled trials [RCTs]), although doing stretching exercises doesn’t. Rest and ice alone promote faster recovery than rest and ice combined with nonsteroidal anti-inflammatory drugs (NSAIDs), a walking cast, or heel-cord stretching. (SOR: B, low-quality RCT). Although foot orthoses or insoles don’t prevent lower limb injuries (SOR: A, systematic review), they do significantly relieve symptoms and promote return to running (SOR: C, poor-quality cohort studies). Lower limb fasciotomy can reduce symptoms caused by shin splints in athletes, but the rate of return to previous level of sports activity is modest at best (SOR: C, case series).
Review, rest, follow-up
Doug Aukerman, MD
Pennsylvania State University, State College
Shin splints are an early indicator of abnormal stresses that cause periostitis along the tibia. Medial tibial stress syndrome (MTSS) is best thought of as part of a continuum, with shin splints at the mild end and tibial stress fracture at the severe extreme. I find that reviewing biomechanical forces and training schedules often helps prevent and treat injury. Aging footwear and training errors, including a rapid increase in training volume, are common culprits when biomechanics are normal. Adequate rest to allow the body to respond to the stresses of training can help prevent stress-related injuries.
My treatment regimen for shin splints includes a period of relative rest that allows the runner to remain active but reduces the stresses placed on the tibia and musculature of the lower leg. Relative rest may comprise either reducing running distance and intensity or changing the mode of exercise to deep water running or cycling. Consider follow-up examination to ensure that the tibial pain doesn’t become more localized, indicating a possible stress fracture.
Evidence summary
MTSS, or shin splints, is the most common cause of exertional leg pain in athletes. MTSS is an overuse injury characterized by pain along the posteromedial aspect of the distal two-thirds of the tibia.
Reduced running time, frequency, and distance may prevent injury
A systematic review of 12 trials of 8806 mostly young male military recruits, evaluated nonsurgical interventions to prevent lower limb soft tissue running injuries, including MTSS.1 Five trials investigating stretching and 4 trials of shoe insoles showed no benefit. Three studies showed that reducing the duration of running (to between 15 and 30 minutes), frequency (to 1 or 3 days a week), or distance (to 16.5 fewer kilometers over 12 weeks) reduced the risk of all injuries. Methods and outcome measures of the studies were heterogeneous, limiting the generalizability of these results.
A prospective RCT of custom semi-rigid shoe orthoses in the boots of Danish army recruits found a significantly lower prevalence of MTSS at 3-month follow-up in the intervention group (13% compared with 24%, P=.005; NNT=5).2 However, a systematic review of methods to prevent MTSS in sports found no high-quality studies in nonmilitary populations.3 No difference in the incidence of MTSS was noted with shoe inserts, different combat boots, or stretching routines.
To treat shin splints, consider orthotics, rest, and ice
Two studies retrospectively surveyed runners about their response to orthotics to treat MTSS.4,5 In one study, 16 of 41 collegiate cross-country runners were prescribed orthotics; 14 (88%) reported relief or improvement in their symptoms and return to running within 4 weeks.4 The other survey, of long-distance runners, found that 70% who had used or were using orthotics for a presumed diagnosis of MTSS reported complete relief or great improvement.5
A study of 97 US naval midshipmen who developed MTSS during summer training programs compared randomly assigned treatments of rest and ice to rest and ice plus anti-inflammatory medication (aspirin or phenylbutazone), rest and ice plus heel-cord stretching, or use of a short walking cast for 1 week.6 Subjects assigned to a treatment program of rest and ice alone lost significantly fewer days from running than the other treatment groups (P<.03).
A recent RCT of soldiers with MTSS compared treatment with a leg orthosis with no orthotic use; all subjects underwent icing and activity modification.7 No significant differences in outcome measures (days to completion of a 0.5-mile run, global rating of change, or number of treatment sessions) were noted between the treatment groups. Small sample size, high dropout rate, and small effect size limit the power of this study.
Fasciotomy can relieve symptoms but may not improve postop activity
Three case series reported on deep posterior compartment fasciotomy to treat MTSS in athletes.8-10 Surgery significantly reduced pain levels (P<.001) by an average of 72% on the visual analog pain scale for 46 patients who had failed conservative therapy for at least 12 months.8 However, only 41% returned to their pre-symptom level of sports activity. Another case series of mostly running athletes reported that 21 (78%) of 27 patients exhibited excellent or good healing after surgery.9 In a series of 9 patients with an average of 39 months of symptoms, 5 reported complete relief at follow-up (42±6 months), and 7 trained more than they had preoperatively.10
One case series of superficial posterior compartment fasciotomy for MTSS in 35 athletes reported that, among the 32 athletes available for follow-up, 72% (23) reported improved symptoms, but 69% (22) had a lower level of activity postoperatively.11
Recommendations
The American College of Sports Medicine (ACSM) recommends at least 7 to 10 days of rest from painful activities to treat MTSS.12 Running in a pool and cycling to maintain aerobic fitness during the rest period are considered safe activities. Stretching and strengthening exercises are not recommended while symptoms persist. Patients should return to running gradually. ACSM also suggests that orthotics are useful for preventing injury and treating some patients.
1. Yeung EW, Yeung SS. Interventions for preventing lower limb soft-tissue injuries in runners. Cochrane Database Syst Rev. 2005;(4):CD001256.-
2. Larsen K, Weidich F, Leboeuf-Yde C. Can custom-made biomechanic shoe orthoses prevent problems in the back and lower extremities? A randomized, controlled intervention trial of 146 military conscripts. J Manipulative Physiol Ther. 2002;25:326-331.
3. Thacker SB, Gilchrist J, Stroup DF, et al. The prevention of shin splints in sports: a systematic review of literature. Med Sci Sports Exerc. 2002;34:32-40.
4. Eickhoff CA, Hossain SA, Slawski DP. From the field. Effects of prescribed foot orthoses on medial tibial stress syndrome in collegiate cross-country runners. Clin Kinesiol. 2000;54:76-80.
5. Gross ML, Davlin LB, Evanski PM. Effectiveness of orthotic shoe inserts in the long-distance runner. Am J Sports Med. 1991;19:409-412.
6. Andrish JT, Bergfeld JA, Walheim J. A prospective study on the management of shin splints. J Bone Joint Surg Am. 1974;56:1697-1700.
7. Johnston E, Flynn T, Bean M, et al. A randomized controlled trial of a leg orthosis versus traditional treatment for soldiers with shin splints: a pilot study. Mil Med. 2006;171:40-44.
8. Yates B, Allen MJ, Barnes MR. Outcome of surgical treatment of medial tibial stress syndrome. J Bone Joint Surg Am. 2003;85-A:1974-1980.
9. Järvinnen M, Aho H, Niittymäki S. Results of the surgical treatment of the medial tibial syndrome in athletes Int J Sports Med. 1989;10:55-57
10. Wallenstein R. Results of fasciotomy in patients with medial tibial syndrome or chronic anterior-compartment syndrome. .J Bone Joint Surg Am. 1983;65-A:1252-1255.
11. Holen KJ, Engebretsen L, Grontvedt T, et al. Surgical treatment of medial tibial stress syndrome (shin splint) by fasciotomy of the superficial posterior compartment of the leg. Scand J Med Sci Sports. 1995;5:40-43.
12. Beck BR. Exercise-induced leg pain. Current Comment. March 2002. American College of Sports Medicine. Available at: www.acsm.org/AM/Template.cfm?Section=Search§ion=20026&template=/CM/ContentDisplaycfm&ContentFileID=299. Accessed December 14, 2006.
1. Yeung EW, Yeung SS. Interventions for preventing lower limb soft-tissue injuries in runners. Cochrane Database Syst Rev. 2005;(4):CD001256.-
2. Larsen K, Weidich F, Leboeuf-Yde C. Can custom-made biomechanic shoe orthoses prevent problems in the back and lower extremities? A randomized, controlled intervention trial of 146 military conscripts. J Manipulative Physiol Ther. 2002;25:326-331.
3. Thacker SB, Gilchrist J, Stroup DF, et al. The prevention of shin splints in sports: a systematic review of literature. Med Sci Sports Exerc. 2002;34:32-40.
4. Eickhoff CA, Hossain SA, Slawski DP. From the field. Effects of prescribed foot orthoses on medial tibial stress syndrome in collegiate cross-country runners. Clin Kinesiol. 2000;54:76-80.
5. Gross ML, Davlin LB, Evanski PM. Effectiveness of orthotic shoe inserts in the long-distance runner. Am J Sports Med. 1991;19:409-412.
6. Andrish JT, Bergfeld JA, Walheim J. A prospective study on the management of shin splints. J Bone Joint Surg Am. 1974;56:1697-1700.
7. Johnston E, Flynn T, Bean M, et al. A randomized controlled trial of a leg orthosis versus traditional treatment for soldiers with shin splints: a pilot study. Mil Med. 2006;171:40-44.
8. Yates B, Allen MJ, Barnes MR. Outcome of surgical treatment of medial tibial stress syndrome. J Bone Joint Surg Am. 2003;85-A:1974-1980.
9. Järvinnen M, Aho H, Niittymäki S. Results of the surgical treatment of the medial tibial syndrome in athletes Int J Sports Med. 1989;10:55-57
10. Wallenstein R. Results of fasciotomy in patients with medial tibial syndrome or chronic anterior-compartment syndrome. .J Bone Joint Surg Am. 1983;65-A:1252-1255.
11. Holen KJ, Engebretsen L, Grontvedt T, et al. Surgical treatment of medial tibial stress syndrome (shin splint) by fasciotomy of the superficial posterior compartment of the leg. Scand J Med Sci Sports. 1995;5:40-43.
12. Beck BR. Exercise-induced leg pain. Current Comment. March 2002. American College of Sports Medicine. Available at: www.acsm.org/AM/Template.cfm?Section=Search§ion=20026&template=/CM/ContentDisplaycfm&ContentFileID=299. Accessed December 14, 2006.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best test for peripheral vascular disease?
An ankle-brachial index is best for evaluating patients with symptoms of claudication (strength of recommendation [SOR]: B, multiple cohort studies). That said, duplex ultrasonography or magnetic resonance angiography may be a preferable first step if immediate revascularization appears necessary (SOR: C, expert consensus and case reports). In addition, a toe-brachial index may be superior to an ankle-brachial index for evaluating elderly and diabetic patients (SOR: C, expert consensus and case reports).
After considering the accuracy, cost, and risk of available tests, an appropriate stepwise approach begins with a complete history and targeted physical examination (palpation of pulses) (SOR: B, consistent cohort studies); then obtain an ankle-brachial index to confirm the diagnosis.
Ankle-brachial index: An underused test
Vincent Lo, MD
San Joaquin Family Medicine Residency, French Camp, Calif
In my experience, the ankle-brachial index is often underused because of a lack of time, equipment, and proper training. Nonetheless, as the evidence makes clear, this test is the best approach for patients with symptoms of claudication.
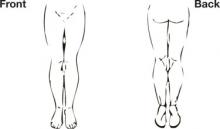
Another option you may want to consider is the Edinburgh Claudication Questionnaire (FIGURE). This tool has a strong positive predictive value and positive likelihood ratio and may allow for a presumptive diagnosis, especially among patients with significant risk factors such as diabetes, hypertension, hyperlipidemia, coronary artery disease, and tobacco use. It has limitations, though, which are detailed in this Clinical Inquiry.
For initial management of peripheral vascular disease (PVD), you can recommend lifestyle modification, antiplatelet agents, and aggressive control of blood glucose, blood pressure, and lipids without further testing. Reserve Doppler ultrasound and magnetic resonance angiography for patients who require revascularization or don’t respond to medical therapy.
Evidence summary
PVD is a progressive atherosclerotic narrowing of arteries in the extremities. The prevalence increases with age—it is less than 1% in people between 40 and 49 years of age and 15% in people 70 years and older.1,2 Risk factors are the same as for coronary artery disease.1
Weighing your options
Methods for evaluating patients for PVD include medical history, physical examination (inspection of the extremities and palpation of peripheral pulses), and ancillary testing (ankle-brachial index, duplex ultrasound, and magnetic resonance imaging with contrast, and angiography). The toe-brachial index may be useful in the elderly and patients with advanced diabetes because noncompressible vasculature in these patients may render the ankle-brachial index unreliable.3
1. Do you get a pain or discomfort in your leg(s) when you walk?
□ Yes □ No □ I am unable to walk
If you answered “Yes” to question (1), please answer the following questions. Otherwise, you need not continue.
2. Does this pain ever begin when you are standing still or sitting?
□ Yes □ No
3. Do you get it if you walk uphill or hurry?
□ Yes □ No
4. Do you get it when you walk at an ordinary pace on the level?
□ Yes □ No
5. What happens to it if you stand still?
□ Usually continues more than 10 minutes.
□ Usually disappears in 10 minutes or less.
6. Where do you get this pain or discomfort?
Mark the place(s) with an “x” on the diagram below.
Definition of positive classification requires all of the following responses: “Yes” to (1), “No” to (2), “Yes” to (3), and “usually disappears in 10 minutes or less” to (5); grade 1=“No” to (4) and grade 2=“Yes” to (4). If these criteria are fulfilled, a definite claudicant is one who indicates pain in the calf, regardless of whether pain is also marked in other sites; a diagnosis of atypical claudication is made if pain is indicated in the thigh or buttock, in the absence of any calf pain. Patients should not be considered to have claudication if pain is indicated in the hamstrings, feet, shins, joints, or appears to radiate, in the absence of any pain in the calf.
Source: Leng GC, Fowkes FG.4 Adapted with permission.
The TABLE lists the sensitivities, specificities, positive predictive values, and likelihood ratios for commonly used tests.3-8 The calculations assume peripheral vascular disease to have a prevalence of 14% among people older than 60 years, based on pooled results from several epidemiologic studies.1
A questionnaire-based history of claudication (the Edinburgh Claudication Questionnaire) has been shown to have a high positive predictive value and likelihood ratio for PVD; physical examination techniques appear to be less predictive.4-7 However, the gold standard in the questionnaire study was “clinician determination,” which carries a risk of subjectivity and lack of testing independence that may make the test appear more accurate than it is in typical application.
A stepwise approach
After balancing the accuracy, cost, and risk of available tests, an appropriate stepwise approach to evaluation for PVD is:
First, do a complete history and targeted physical examination (palpation of pulses).
Next, obtain an ankle-brachial index to confirm the diagnosis.
Then, proceed directly to either duplex ultrasonography or magnetic resonance angiography when revascularization is clearly needed.9
TABLE
Commonly used tests for peripheral vascular disease
| TEST | COMPARISON/STANDARD | SENSITIVITY % (95% CI, IF REPORTED) | SENSITIVITY % (95% CI, IF REPORTED) | SPECIFICITY % (95% CI, IF REPORTED) | PPV* | LR- | LR+ | REIMBURSEMENT ESTIMATE† |
|---|---|---|---|---|---|---|---|---|
| MEDICAL HISTORY | CPT CODE | ESTIMATED COST | ||||||
| Edinburgh Claudication Questionnaire4 | Claudication by clinician assessment | 91.0 (88.1-94.5) | 99.0 (98.9-100.0) | 0.955 | 0.09 | 91.0 | 99203 | $87.29 |
| PHYSICAL EXAMINATION | ||||||||
| Color abnormality of extremity skin (pale, red, or blue)5 | ABI<0.9 | 35.0 | 87.0 | 0.305 | 0.75 | 2.7 | 99203 | $87.29 |
| Cool skin unilaterally5 | ABI<0.9 | 10.0 | 98.0 | 0.449 | 0.92 | 5.0 | 99203 | $87.29 |
| Any abnormal pulse by palpation6 | Multiple criteria‡ | 76.9 | 86.4 | 0.479 | 0.27 | 5.7 | 99203 | $87.29 |
| Presence of femoral bruit6 | Multiple criteria‡ | 20.0 | 95.7 | 0.431 | 0.84 | 4.7 | 99203 | $87.29 |
| Absent pedal pulses (dorsalis pedis and posterior tibial)7 | ABI<0.9 | 63.0 | 99.0 | 0.912 | 0.37 | 63.9 | 99203 | $87.29 |
| LABORATORY INVESTIGATIONS | ||||||||
| ABI <0.93 | Conventional angiography | 79.0 | 96.0 | 0.763 | 0.22 | 19.8 | 93923 | $165.18 |
| Duplex ultrasound8 | Conventional angiography | 87.6 (84.4-90.8) | 94.7 (93.2-96.2) | 0.729 | 0.13 | 16.5 | 93923 | $165.18 |
| Gadolinium-enhanced magnetic resonance angiography8 | Conventional angiographyy | 97.5 (95.7-99.3) | 96.2 (94.4-97.9) | 0.807 | 0.03 | 25.7 | 73725 | $504.00 |
| ABI, ankle-brachial index; CI, confidence interval; LR, likelihood ratio; PPV, positive predictive value. | ||||||||
| *Based on a prevalence of peripheral vascular disease of 14% (Pasternak RC et al1). | ||||||||
| † Based on estimated Medicare-approved CPT reimbursement rates, https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp. Accessed December 2, 2007. History and physical items based on a new-patient visit of moderate complexity, CPT Code 99203. Cost estimate for conventional invasive angiography (angiography, extremity, unilateral, radiological supervision and interpretation, CPT Code 75710) $426.14 (Downstate Illinois estimates). | ||||||||
| ‡ Multiple criteria = segmental blood pressure, flow velocity by Doppler, postocclusive reactive hyperemia, pulse reappearance half-time, small or large vessel peripheral arterial disease, and surgery. | ||||||||
The major advantages of the ankle-brachial index include low cost and non-invasiveness (low potential for harm). However, it doesn’t detect proximal aneurysms or PVD distal to the ankle, and it may be difficult to perform on patients with noncompressible distal vasculature. Adequately evaluating such patients may require invasive testing.
Recommendations
The US Preventive Services Task Force recommends against (D recommendation) any screening tests for PVD in patients without symptoms.10 The American College of Cardiology gives a class I recommendation (tests for which there is evidence or general agreement that a procedure is useful, beneficial, or effective) to the ankle-brachial index as the baseline diagnostic tool for establishing peripheral vascular disease, except in elderly patients or those with advanced diabetes, for whom the test is unreliable.11
1. Pasternak RC, Criqui MH, Benjamin EJ, et al. Atherosclerotic Vascular Disease Conference: Writing Group I: Epidemiology. Circulation. 2004;109:2605-2612.
2. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738-743.
3. American College of Cardiology, American Heart Association. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report. Circulation. 2006;113:e463-654.
4. Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101-1109.
5. Stoffers HE, Kester AD, Kaiser V, et al. Diagnostic value of signs and symptoms associated with peripheral arterial occlusive disease seen in general practice: a multivariable approach. Med Decis Making. 1997;17:61-70.
6. Criqui MH, Fronek A, Klauber MR, et al. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985;71:516-522.
7. McGee SR, Boyko EJ. Physical examination and chronic lower-extremity ischemia: a critical review. Arch Intern Med. 1998;158:1357-1364.
8. Visser K, Myriam Hunink MG. Peripheral arterial disease: gadolinium-enhanced MR angiography versus color-guided duplex US-A meta-analysis. Radiology. 2000;216:67-77.
9. Sondtheimer DL. Peripheral vascular disease: diagnosis and treatment. Am Fam Physician. 2006;73:1971-1976.
10. US Preventive Services Task Force. Screening for Peripheral Vascular Disease: Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; August, 2005.
11. American College of Cardiology, American Heart Association. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation. 2006;113:1474-1547.
An ankle-brachial index is best for evaluating patients with symptoms of claudication (strength of recommendation [SOR]: B, multiple cohort studies). That said, duplex ultrasonography or magnetic resonance angiography may be a preferable first step if immediate revascularization appears necessary (SOR: C, expert consensus and case reports). In addition, a toe-brachial index may be superior to an ankle-brachial index for evaluating elderly and diabetic patients (SOR: C, expert consensus and case reports).
After considering the accuracy, cost, and risk of available tests, an appropriate stepwise approach begins with a complete history and targeted physical examination (palpation of pulses) (SOR: B, consistent cohort studies); then obtain an ankle-brachial index to confirm the diagnosis.
Ankle-brachial index: An underused test
Vincent Lo, MD
San Joaquin Family Medicine Residency, French Camp, Calif
In my experience, the ankle-brachial index is often underused because of a lack of time, equipment, and proper training. Nonetheless, as the evidence makes clear, this test is the best approach for patients with symptoms of claudication.
Another option you may want to consider is the Edinburgh Claudication Questionnaire (FIGURE). This tool has a strong positive predictive value and positive likelihood ratio and may allow for a presumptive diagnosis, especially among patients with significant risk factors such as diabetes, hypertension, hyperlipidemia, coronary artery disease, and tobacco use. It has limitations, though, which are detailed in this Clinical Inquiry.
For initial management of peripheral vascular disease (PVD), you can recommend lifestyle modification, antiplatelet agents, and aggressive control of blood glucose, blood pressure, and lipids without further testing. Reserve Doppler ultrasound and magnetic resonance angiography for patients who require revascularization or don’t respond to medical therapy.
Evidence summary
PVD is a progressive atherosclerotic narrowing of arteries in the extremities. The prevalence increases with age—it is less than 1% in people between 40 and 49 years of age and 15% in people 70 years and older.1,2 Risk factors are the same as for coronary artery disease.1
Weighing your options
Methods for evaluating patients for PVD include medical history, physical examination (inspection of the extremities and palpation of peripheral pulses), and ancillary testing (ankle-brachial index, duplex ultrasound, and magnetic resonance imaging with contrast, and angiography). The toe-brachial index may be useful in the elderly and patients with advanced diabetes because noncompressible vasculature in these patients may render the ankle-brachial index unreliable.3
1. Do you get a pain or discomfort in your leg(s) when you walk?
□ Yes □ No □ I am unable to walk
If you answered “Yes” to question (1), please answer the following questions. Otherwise, you need not continue.
2. Does this pain ever begin when you are standing still or sitting?
□ Yes □ No
3. Do you get it if you walk uphill or hurry?
□ Yes □ No
4. Do you get it when you walk at an ordinary pace on the level?
□ Yes □ No
5. What happens to it if you stand still?
□ Usually continues more than 10 minutes.
□ Usually disappears in 10 minutes or less.
6. Where do you get this pain or discomfort?
Mark the place(s) with an “x” on the diagram below.
Definition of positive classification requires all of the following responses: “Yes” to (1), “No” to (2), “Yes” to (3), and “usually disappears in 10 minutes or less” to (5); grade 1=“No” to (4) and grade 2=“Yes” to (4). If these criteria are fulfilled, a definite claudicant is one who indicates pain in the calf, regardless of whether pain is also marked in other sites; a diagnosis of atypical claudication is made if pain is indicated in the thigh or buttock, in the absence of any calf pain. Patients should not be considered to have claudication if pain is indicated in the hamstrings, feet, shins, joints, or appears to radiate, in the absence of any pain in the calf.
Source: Leng GC, Fowkes FG.4 Adapted with permission.
The TABLE lists the sensitivities, specificities, positive predictive values, and likelihood ratios for commonly used tests.3-8 The calculations assume peripheral vascular disease to have a prevalence of 14% among people older than 60 years, based on pooled results from several epidemiologic studies.1
A questionnaire-based history of claudication (the Edinburgh Claudication Questionnaire) has been shown to have a high positive predictive value and likelihood ratio for PVD; physical examination techniques appear to be less predictive.4-7 However, the gold standard in the questionnaire study was “clinician determination,” which carries a risk of subjectivity and lack of testing independence that may make the test appear more accurate than it is in typical application.
A stepwise approach
After balancing the accuracy, cost, and risk of available tests, an appropriate stepwise approach to evaluation for PVD is:
First, do a complete history and targeted physical examination (palpation of pulses).
Next, obtain an ankle-brachial index to confirm the diagnosis.
Then, proceed directly to either duplex ultrasonography or magnetic resonance angiography when revascularization is clearly needed.9
TABLE
Commonly used tests for peripheral vascular disease
| TEST | COMPARISON/STANDARD | SENSITIVITY % (95% CI, IF REPORTED) | SENSITIVITY % (95% CI, IF REPORTED) | SPECIFICITY % (95% CI, IF REPORTED) | PPV* | LR- | LR+ | REIMBURSEMENT ESTIMATE† |
|---|---|---|---|---|---|---|---|---|
| MEDICAL HISTORY | CPT CODE | ESTIMATED COST | ||||||
| Edinburgh Claudication Questionnaire4 | Claudication by clinician assessment | 91.0 (88.1-94.5) | 99.0 (98.9-100.0) | 0.955 | 0.09 | 91.0 | 99203 | $87.29 |
| PHYSICAL EXAMINATION | ||||||||
| Color abnormality of extremity skin (pale, red, or blue)5 | ABI<0.9 | 35.0 | 87.0 | 0.305 | 0.75 | 2.7 | 99203 | $87.29 |
| Cool skin unilaterally5 | ABI<0.9 | 10.0 | 98.0 | 0.449 | 0.92 | 5.0 | 99203 | $87.29 |
| Any abnormal pulse by palpation6 | Multiple criteria‡ | 76.9 | 86.4 | 0.479 | 0.27 | 5.7 | 99203 | $87.29 |
| Presence of femoral bruit6 | Multiple criteria‡ | 20.0 | 95.7 | 0.431 | 0.84 | 4.7 | 99203 | $87.29 |
| Absent pedal pulses (dorsalis pedis and posterior tibial)7 | ABI<0.9 | 63.0 | 99.0 | 0.912 | 0.37 | 63.9 | 99203 | $87.29 |
| LABORATORY INVESTIGATIONS | ||||||||
| ABI <0.93 | Conventional angiography | 79.0 | 96.0 | 0.763 | 0.22 | 19.8 | 93923 | $165.18 |
| Duplex ultrasound8 | Conventional angiography | 87.6 (84.4-90.8) | 94.7 (93.2-96.2) | 0.729 | 0.13 | 16.5 | 93923 | $165.18 |
| Gadolinium-enhanced magnetic resonance angiography8 | Conventional angiographyy | 97.5 (95.7-99.3) | 96.2 (94.4-97.9) | 0.807 | 0.03 | 25.7 | 73725 | $504.00 |
| ABI, ankle-brachial index; CI, confidence interval; LR, likelihood ratio; PPV, positive predictive value. | ||||||||
| *Based on a prevalence of peripheral vascular disease of 14% (Pasternak RC et al1). | ||||||||
| † Based on estimated Medicare-approved CPT reimbursement rates, https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp. Accessed December 2, 2007. History and physical items based on a new-patient visit of moderate complexity, CPT Code 99203. Cost estimate for conventional invasive angiography (angiography, extremity, unilateral, radiological supervision and interpretation, CPT Code 75710) $426.14 (Downstate Illinois estimates). | ||||||||
| ‡ Multiple criteria = segmental blood pressure, flow velocity by Doppler, postocclusive reactive hyperemia, pulse reappearance half-time, small or large vessel peripheral arterial disease, and surgery. | ||||||||
The major advantages of the ankle-brachial index include low cost and non-invasiveness (low potential for harm). However, it doesn’t detect proximal aneurysms or PVD distal to the ankle, and it may be difficult to perform on patients with noncompressible distal vasculature. Adequately evaluating such patients may require invasive testing.
Recommendations
The US Preventive Services Task Force recommends against (D recommendation) any screening tests for PVD in patients without symptoms.10 The American College of Cardiology gives a class I recommendation (tests for which there is evidence or general agreement that a procedure is useful, beneficial, or effective) to the ankle-brachial index as the baseline diagnostic tool for establishing peripheral vascular disease, except in elderly patients or those with advanced diabetes, for whom the test is unreliable.11
An ankle-brachial index is best for evaluating patients with symptoms of claudication (strength of recommendation [SOR]: B, multiple cohort studies). That said, duplex ultrasonography or magnetic resonance angiography may be a preferable first step if immediate revascularization appears necessary (SOR: C, expert consensus and case reports). In addition, a toe-brachial index may be superior to an ankle-brachial index for evaluating elderly and diabetic patients (SOR: C, expert consensus and case reports).
After considering the accuracy, cost, and risk of available tests, an appropriate stepwise approach begins with a complete history and targeted physical examination (palpation of pulses) (SOR: B, consistent cohort studies); then obtain an ankle-brachial index to confirm the diagnosis.
Ankle-brachial index: An underused test
Vincent Lo, MD
San Joaquin Family Medicine Residency, French Camp, Calif
In my experience, the ankle-brachial index is often underused because of a lack of time, equipment, and proper training. Nonetheless, as the evidence makes clear, this test is the best approach for patients with symptoms of claudication.
Another option you may want to consider is the Edinburgh Claudication Questionnaire (FIGURE). This tool has a strong positive predictive value and positive likelihood ratio and may allow for a presumptive diagnosis, especially among patients with significant risk factors such as diabetes, hypertension, hyperlipidemia, coronary artery disease, and tobacco use. It has limitations, though, which are detailed in this Clinical Inquiry.
For initial management of peripheral vascular disease (PVD), you can recommend lifestyle modification, antiplatelet agents, and aggressive control of blood glucose, blood pressure, and lipids without further testing. Reserve Doppler ultrasound and magnetic resonance angiography for patients who require revascularization or don’t respond to medical therapy.
Evidence summary
PVD is a progressive atherosclerotic narrowing of arteries in the extremities. The prevalence increases with age—it is less than 1% in people between 40 and 49 years of age and 15% in people 70 years and older.1,2 Risk factors are the same as for coronary artery disease.1
Weighing your options
Methods for evaluating patients for PVD include medical history, physical examination (inspection of the extremities and palpation of peripheral pulses), and ancillary testing (ankle-brachial index, duplex ultrasound, and magnetic resonance imaging with contrast, and angiography). The toe-brachial index may be useful in the elderly and patients with advanced diabetes because noncompressible vasculature in these patients may render the ankle-brachial index unreliable.3
1. Do you get a pain or discomfort in your leg(s) when you walk?
□ Yes □ No □ I am unable to walk
If you answered “Yes” to question (1), please answer the following questions. Otherwise, you need not continue.
2. Does this pain ever begin when you are standing still or sitting?
□ Yes □ No
3. Do you get it if you walk uphill or hurry?
□ Yes □ No
4. Do you get it when you walk at an ordinary pace on the level?
□ Yes □ No
5. What happens to it if you stand still?
□ Usually continues more than 10 minutes.
□ Usually disappears in 10 minutes or less.
6. Where do you get this pain or discomfort?
Mark the place(s) with an “x” on the diagram below.
Definition of positive classification requires all of the following responses: “Yes” to (1), “No” to (2), “Yes” to (3), and “usually disappears in 10 minutes or less” to (5); grade 1=“No” to (4) and grade 2=“Yes” to (4). If these criteria are fulfilled, a definite claudicant is one who indicates pain in the calf, regardless of whether pain is also marked in other sites; a diagnosis of atypical claudication is made if pain is indicated in the thigh or buttock, in the absence of any calf pain. Patients should not be considered to have claudication if pain is indicated in the hamstrings, feet, shins, joints, or appears to radiate, in the absence of any pain in the calf.
Source: Leng GC, Fowkes FG.4 Adapted with permission.
The TABLE lists the sensitivities, specificities, positive predictive values, and likelihood ratios for commonly used tests.3-8 The calculations assume peripheral vascular disease to have a prevalence of 14% among people older than 60 years, based on pooled results from several epidemiologic studies.1
A questionnaire-based history of claudication (the Edinburgh Claudication Questionnaire) has been shown to have a high positive predictive value and likelihood ratio for PVD; physical examination techniques appear to be less predictive.4-7 However, the gold standard in the questionnaire study was “clinician determination,” which carries a risk of subjectivity and lack of testing independence that may make the test appear more accurate than it is in typical application.
A stepwise approach
After balancing the accuracy, cost, and risk of available tests, an appropriate stepwise approach to evaluation for PVD is:
First, do a complete history and targeted physical examination (palpation of pulses).
Next, obtain an ankle-brachial index to confirm the diagnosis.
Then, proceed directly to either duplex ultrasonography or magnetic resonance angiography when revascularization is clearly needed.9
TABLE
Commonly used tests for peripheral vascular disease
| TEST | COMPARISON/STANDARD | SENSITIVITY % (95% CI, IF REPORTED) | SENSITIVITY % (95% CI, IF REPORTED) | SPECIFICITY % (95% CI, IF REPORTED) | PPV* | LR- | LR+ | REIMBURSEMENT ESTIMATE† |
|---|---|---|---|---|---|---|---|---|
| MEDICAL HISTORY | CPT CODE | ESTIMATED COST | ||||||
| Edinburgh Claudication Questionnaire4 | Claudication by clinician assessment | 91.0 (88.1-94.5) | 99.0 (98.9-100.0) | 0.955 | 0.09 | 91.0 | 99203 | $87.29 |
| PHYSICAL EXAMINATION | ||||||||
| Color abnormality of extremity skin (pale, red, or blue)5 | ABI<0.9 | 35.0 | 87.0 | 0.305 | 0.75 | 2.7 | 99203 | $87.29 |
| Cool skin unilaterally5 | ABI<0.9 | 10.0 | 98.0 | 0.449 | 0.92 | 5.0 | 99203 | $87.29 |
| Any abnormal pulse by palpation6 | Multiple criteria‡ | 76.9 | 86.4 | 0.479 | 0.27 | 5.7 | 99203 | $87.29 |
| Presence of femoral bruit6 | Multiple criteria‡ | 20.0 | 95.7 | 0.431 | 0.84 | 4.7 | 99203 | $87.29 |
| Absent pedal pulses (dorsalis pedis and posterior tibial)7 | ABI<0.9 | 63.0 | 99.0 | 0.912 | 0.37 | 63.9 | 99203 | $87.29 |
| LABORATORY INVESTIGATIONS | ||||||||
| ABI <0.93 | Conventional angiography | 79.0 | 96.0 | 0.763 | 0.22 | 19.8 | 93923 | $165.18 |
| Duplex ultrasound8 | Conventional angiography | 87.6 (84.4-90.8) | 94.7 (93.2-96.2) | 0.729 | 0.13 | 16.5 | 93923 | $165.18 |
| Gadolinium-enhanced magnetic resonance angiography8 | Conventional angiographyy | 97.5 (95.7-99.3) | 96.2 (94.4-97.9) | 0.807 | 0.03 | 25.7 | 73725 | $504.00 |
| ABI, ankle-brachial index; CI, confidence interval; LR, likelihood ratio; PPV, positive predictive value. | ||||||||
| *Based on a prevalence of peripheral vascular disease of 14% (Pasternak RC et al1). | ||||||||
| † Based on estimated Medicare-approved CPT reimbursement rates, https://catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp. Accessed December 2, 2007. History and physical items based on a new-patient visit of moderate complexity, CPT Code 99203. Cost estimate for conventional invasive angiography (angiography, extremity, unilateral, radiological supervision and interpretation, CPT Code 75710) $426.14 (Downstate Illinois estimates). | ||||||||
| ‡ Multiple criteria = segmental blood pressure, flow velocity by Doppler, postocclusive reactive hyperemia, pulse reappearance half-time, small or large vessel peripheral arterial disease, and surgery. | ||||||||
The major advantages of the ankle-brachial index include low cost and non-invasiveness (low potential for harm). However, it doesn’t detect proximal aneurysms or PVD distal to the ankle, and it may be difficult to perform on patients with noncompressible distal vasculature. Adequately evaluating such patients may require invasive testing.
Recommendations
The US Preventive Services Task Force recommends against (D recommendation) any screening tests for PVD in patients without symptoms.10 The American College of Cardiology gives a class I recommendation (tests for which there is evidence or general agreement that a procedure is useful, beneficial, or effective) to the ankle-brachial index as the baseline diagnostic tool for establishing peripheral vascular disease, except in elderly patients or those with advanced diabetes, for whom the test is unreliable.11
1. Pasternak RC, Criqui MH, Benjamin EJ, et al. Atherosclerotic Vascular Disease Conference: Writing Group I: Epidemiology. Circulation. 2004;109:2605-2612.
2. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738-743.
3. American College of Cardiology, American Heart Association. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report. Circulation. 2006;113:e463-654.
4. Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101-1109.
5. Stoffers HE, Kester AD, Kaiser V, et al. Diagnostic value of signs and symptoms associated with peripheral arterial occlusive disease seen in general practice: a multivariable approach. Med Decis Making. 1997;17:61-70.
6. Criqui MH, Fronek A, Klauber MR, et al. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985;71:516-522.
7. McGee SR, Boyko EJ. Physical examination and chronic lower-extremity ischemia: a critical review. Arch Intern Med. 1998;158:1357-1364.
8. Visser K, Myriam Hunink MG. Peripheral arterial disease: gadolinium-enhanced MR angiography versus color-guided duplex US-A meta-analysis. Radiology. 2000;216:67-77.
9. Sondtheimer DL. Peripheral vascular disease: diagnosis and treatment. Am Fam Physician. 2006;73:1971-1976.
10. US Preventive Services Task Force. Screening for Peripheral Vascular Disease: Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; August, 2005.
11. American College of Cardiology, American Heart Association. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation. 2006;113:1474-1547.
1. Pasternak RC, Criqui MH, Benjamin EJ, et al. Atherosclerotic Vascular Disease Conference: Writing Group I: Epidemiology. Circulation. 2004;109:2605-2612.
2. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738-743.
3. American College of Cardiology, American Heart Association. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report. Circulation. 2006;113:e463-654.
4. Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. J Clin Epidemiol. 1992;45:1101-1109.
5. Stoffers HE, Kester AD, Kaiser V, et al. Diagnostic value of signs and symptoms associated with peripheral arterial occlusive disease seen in general practice: a multivariable approach. Med Decis Making. 1997;17:61-70.
6. Criqui MH, Fronek A, Klauber MR, et al. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985;71:516-522.
7. McGee SR, Boyko EJ. Physical examination and chronic lower-extremity ischemia: a critical review. Arch Intern Med. 1998;158:1357-1364.
8. Visser K, Myriam Hunink MG. Peripheral arterial disease: gadolinium-enhanced MR angiography versus color-guided duplex US-A meta-analysis. Radiology. 2000;216:67-77.
9. Sondtheimer DL. Peripheral vascular disease: diagnosis and treatment. Am Fam Physician. 2006;73:1971-1976.
10. US Preventive Services Task Force. Screening for Peripheral Vascular Disease: Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; August, 2005.
11. American College of Cardiology, American Heart Association. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation. 2006;113:1474-1547.
Evidence-based answers from the Family Physicians Inquiries Network
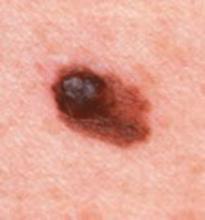
Is training patients in self-examination an effective way to screen for melanoma?
No, it’s not. No studies demonstrate that training patients to examine their skin decreases mortality from melanoma in the general population. Nor is there any evidence to suggest that teaching patients to monitor their skin for suspicious lesions results in earlier detection of melanoma, better prognosis at diagnosis, or better clinical outcomes. However, patients who have had melanoma and perform self-examination have a lower risk of death from subsequent occurrences than those who do not (strength of recommendation [SOR]: B, based on a case-control study). Among patients who find their own melanomas, those who are more knowledgeable about melanoma and aware of their skin are less likely to delay seeking treatment or to have thick lesions upon presentation (SOR: B, based on a retrospective cohort study). Patients who detect melanomas themselves know more about the characteristic features of melanoma and are more likely to perform regular skin self-examinations than patients whose lesions are found by a physician (SOR: C, based on a case series).
Tailor recommendations to the patient
Daniel J. Van Durme, MD
Department of Family Medicine and Rural Health, Florida State University College of Medicine, Tallahassee
Self-examination of the skin is like breast and testicular self-exams—often recommended but not proven to decrease mortality from cancer. Certainly, patients who have already had one melanoma should check the skin regularly. The approach to patients without a history of melanoma is less clear. For some, regular self-examination decreases anxiety and increases their sense of control. Other patients, however, find self-examination anxiety provoking because it reminds them that they may have a cancer. They may worry that they aren’t examining themselves as often, or as well, as they should.
When caring for anxious patients, it may be best to educate them about the features of melanoma and then tell them, “Don’t bother with skin self-exams, just let me know if something new shows up.” Encourage them to schedule routine office visits, avoid sun exposure, and engage in proven healthy behaviors, such as exercise.
Evidence summary
Effects of self-examination on patients with melanoma
Two studies of a group of Connecticut residents examined the relationship between skin self-examination (SSE) and melanoma. The first, a case-control study compared SSE among 650 Caucasian patients newly diagnosed with melanoma and 549 age- and sex-matched controls.1 Fifteen percent of all patients practiced SSE. Investigators monitored a statewide registry system for 5.4 years to identify 110 participants with melanoma who had major adverse outcomes (distant metastases or death).
Patients with a history of melanoma who practiced SSE had lower mortality (odds ratio [OR]=0.42; 95% confidence interval [CI], 0.21-0.85) than those who didn’t. One potential weakness of the study was possible lead-time bias, which could overestimate the risk reduction from SSE. Another was the 5.4-year follow-up, because malignant melanoma can recur as long as 10 years later.
In the second study, involving a cohort of 255 patients from the first study who discovered their own melanomas, researchers questioned patients about their knowledge of melanoma signs and symptoms, awareness of the appearance of their own skin, and whether they delayed seeking medical attention for >3 months after detecting the initial lesion.2
More knowledgeable patients were less likely to have a thick (≥0.75 mm) tumor and a delayed diagnosis (OR=0.34; 95% CI, 0.13-0.88). Similarly, patients with greater awareness of their skin were less likely to have a thick tumor and a delayed diagnosis (OR=0.50; 95% CI, 0.28-0.89 and OR=0.30; 95% CI, 0.12-0.71, respectively). Investigators found no significant difference in mortality based on knowledge or skin awareness.
Which patients are more likely to detect melanoma?
A retrospective series of 816 consecutive cases of newly diagnosed melanoma investigated the frequency of self-detection in a Mediterranean population at intermediate risk.3 Subjects were statistically more likely to find melanoma themselves if they had a lesion on the lower limbs, were of younger age (49.8 vs 52.9 years of age), had fewer atypical nevi, had >8 years education, were knowledgeable about the characteristic features of melanoma, and performed regular SSE (P<.01 for all comparisons).
Recommendations
The US Preventive Services Task Force finds insufficient evidence to recommend for or against routine counseling by primary care clinicians to prevent skin cancer.4
The Cancer Care Ontario Program in Evidence-based Care, on the other hand, advises health care providers to perform annual total-body skin examinations of high-risk patients and teach the patients to examine themselves.5
The American Cancer Society (ACS) and the American Academy of Dermatology (AAD) both provide information about recognizing melanoma on their Web sites and recommend that people at high risk perform monthly self-examinations. They further advise such people to periodically see a health care professional qualified to diagnose skin cancer (ACS), or a dermatologist (AAD), for a complete skin examination.6,7
1. Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23
2. Oliveria SA, Christos PJ, Halpern AC, et al. Patient knowledge, awareness, and delay in seeking medical attention for malignant melanoma. J Clin Epidemiol 1999;52:1111-1116
3. Carli P, De Giorgi V, Palli D, et al. Self-detected cutaneous melanomas in Italian patients. Clin Exp Dermatol. 2004;29:593-596
4. Counseling to prevent skin cancer: recommendations and rationale of the U.S. Preventive Services Task Force. MMWR Recomm Rep. 2003;52(RR-15):13-17
5. From L, Marrett L, Rosen C, et al. Screening for skin cancer: a clinical practice guideline. Toronto, Canada: Program in Evidence-Based Care, Cancer Care Ontario; 2007. Available at: www.cancercare.on.ca/pdf/pebc15-1s.pdf. Accessed April 14, 2008.
6. American Academy of Dermatology. alignant melanoma. Available at: www.aad.org/public/Publications/pamphlets/MalignantMelanoma.htm. Revised 2005. Accessed March 7, 2008.
7. American Cancer Society online Detailed guide: skin cancer-melanoma. Can melanoma be found early? Available at: www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_melanoma_be_found_early_50.asp. Accessed March 7, 2008.
No, it’s not. No studies demonstrate that training patients to examine their skin decreases mortality from melanoma in the general population. Nor is there any evidence to suggest that teaching patients to monitor their skin for suspicious lesions results in earlier detection of melanoma, better prognosis at diagnosis, or better clinical outcomes. However, patients who have had melanoma and perform self-examination have a lower risk of death from subsequent occurrences than those who do not (strength of recommendation [SOR]: B, based on a case-control study). Among patients who find their own melanomas, those who are more knowledgeable about melanoma and aware of their skin are less likely to delay seeking treatment or to have thick lesions upon presentation (SOR: B, based on a retrospective cohort study). Patients who detect melanomas themselves know more about the characteristic features of melanoma and are more likely to perform regular skin self-examinations than patients whose lesions are found by a physician (SOR: C, based on a case series).
Tailor recommendations to the patient
Daniel J. Van Durme, MD
Department of Family Medicine and Rural Health, Florida State University College of Medicine, Tallahassee
Self-examination of the skin is like breast and testicular self-exams—often recommended but not proven to decrease mortality from cancer. Certainly, patients who have already had one melanoma should check the skin regularly. The approach to patients without a history of melanoma is less clear. For some, regular self-examination decreases anxiety and increases their sense of control. Other patients, however, find self-examination anxiety provoking because it reminds them that they may have a cancer. They may worry that they aren’t examining themselves as often, or as well, as they should.
When caring for anxious patients, it may be best to educate them about the features of melanoma and then tell them, “Don’t bother with skin self-exams, just let me know if something new shows up.” Encourage them to schedule routine office visits, avoid sun exposure, and engage in proven healthy behaviors, such as exercise.
Evidence summary
Effects of self-examination on patients with melanoma
Two studies of a group of Connecticut residents examined the relationship between skin self-examination (SSE) and melanoma. The first, a case-control study compared SSE among 650 Caucasian patients newly diagnosed with melanoma and 549 age- and sex-matched controls.1 Fifteen percent of all patients practiced SSE. Investigators monitored a statewide registry system for 5.4 years to identify 110 participants with melanoma who had major adverse outcomes (distant metastases or death).
Patients with a history of melanoma who practiced SSE had lower mortality (odds ratio [OR]=0.42; 95% confidence interval [CI], 0.21-0.85) than those who didn’t. One potential weakness of the study was possible lead-time bias, which could overestimate the risk reduction from SSE. Another was the 5.4-year follow-up, because malignant melanoma can recur as long as 10 years later.
In the second study, involving a cohort of 255 patients from the first study who discovered their own melanomas, researchers questioned patients about their knowledge of melanoma signs and symptoms, awareness of the appearance of their own skin, and whether they delayed seeking medical attention for >3 months after detecting the initial lesion.2
More knowledgeable patients were less likely to have a thick (≥0.75 mm) tumor and a delayed diagnosis (OR=0.34; 95% CI, 0.13-0.88). Similarly, patients with greater awareness of their skin were less likely to have a thick tumor and a delayed diagnosis (OR=0.50; 95% CI, 0.28-0.89 and OR=0.30; 95% CI, 0.12-0.71, respectively). Investigators found no significant difference in mortality based on knowledge or skin awareness.
Which patients are more likely to detect melanoma?
A retrospective series of 816 consecutive cases of newly diagnosed melanoma investigated the frequency of self-detection in a Mediterranean population at intermediate risk.3 Subjects were statistically more likely to find melanoma themselves if they had a lesion on the lower limbs, were of younger age (49.8 vs 52.9 years of age), had fewer atypical nevi, had >8 years education, were knowledgeable about the characteristic features of melanoma, and performed regular SSE (P<.01 for all comparisons).
Recommendations
The US Preventive Services Task Force finds insufficient evidence to recommend for or against routine counseling by primary care clinicians to prevent skin cancer.4
The Cancer Care Ontario Program in Evidence-based Care, on the other hand, advises health care providers to perform annual total-body skin examinations of high-risk patients and teach the patients to examine themselves.5
The American Cancer Society (ACS) and the American Academy of Dermatology (AAD) both provide information about recognizing melanoma on their Web sites and recommend that people at high risk perform monthly self-examinations. They further advise such people to periodically see a health care professional qualified to diagnose skin cancer (ACS), or a dermatologist (AAD), for a complete skin examination.6,7
No, it’s not. No studies demonstrate that training patients to examine their skin decreases mortality from melanoma in the general population. Nor is there any evidence to suggest that teaching patients to monitor their skin for suspicious lesions results in earlier detection of melanoma, better prognosis at diagnosis, or better clinical outcomes. However, patients who have had melanoma and perform self-examination have a lower risk of death from subsequent occurrences than those who do not (strength of recommendation [SOR]: B, based on a case-control study). Among patients who find their own melanomas, those who are more knowledgeable about melanoma and aware of their skin are less likely to delay seeking treatment or to have thick lesions upon presentation (SOR: B, based on a retrospective cohort study). Patients who detect melanomas themselves know more about the characteristic features of melanoma and are more likely to perform regular skin self-examinations than patients whose lesions are found by a physician (SOR: C, based on a case series).
Tailor recommendations to the patient
Daniel J. Van Durme, MD
Department of Family Medicine and Rural Health, Florida State University College of Medicine, Tallahassee
Self-examination of the skin is like breast and testicular self-exams—often recommended but not proven to decrease mortality from cancer. Certainly, patients who have already had one melanoma should check the skin regularly. The approach to patients without a history of melanoma is less clear. For some, regular self-examination decreases anxiety and increases their sense of control. Other patients, however, find self-examination anxiety provoking because it reminds them that they may have a cancer. They may worry that they aren’t examining themselves as often, or as well, as they should.
When caring for anxious patients, it may be best to educate them about the features of melanoma and then tell them, “Don’t bother with skin self-exams, just let me know if something new shows up.” Encourage them to schedule routine office visits, avoid sun exposure, and engage in proven healthy behaviors, such as exercise.
Evidence summary
Effects of self-examination on patients with melanoma
Two studies of a group of Connecticut residents examined the relationship between skin self-examination (SSE) and melanoma. The first, a case-control study compared SSE among 650 Caucasian patients newly diagnosed with melanoma and 549 age- and sex-matched controls.1 Fifteen percent of all patients practiced SSE. Investigators monitored a statewide registry system for 5.4 years to identify 110 participants with melanoma who had major adverse outcomes (distant metastases or death).
Patients with a history of melanoma who practiced SSE had lower mortality (odds ratio [OR]=0.42; 95% confidence interval [CI], 0.21-0.85) than those who didn’t. One potential weakness of the study was possible lead-time bias, which could overestimate the risk reduction from SSE. Another was the 5.4-year follow-up, because malignant melanoma can recur as long as 10 years later.
In the second study, involving a cohort of 255 patients from the first study who discovered their own melanomas, researchers questioned patients about their knowledge of melanoma signs and symptoms, awareness of the appearance of their own skin, and whether they delayed seeking medical attention for >3 months after detecting the initial lesion.2
More knowledgeable patients were less likely to have a thick (≥0.75 mm) tumor and a delayed diagnosis (OR=0.34; 95% CI, 0.13-0.88). Similarly, patients with greater awareness of their skin were less likely to have a thick tumor and a delayed diagnosis (OR=0.50; 95% CI, 0.28-0.89 and OR=0.30; 95% CI, 0.12-0.71, respectively). Investigators found no significant difference in mortality based on knowledge or skin awareness.
Which patients are more likely to detect melanoma?
A retrospective series of 816 consecutive cases of newly diagnosed melanoma investigated the frequency of self-detection in a Mediterranean population at intermediate risk.3 Subjects were statistically more likely to find melanoma themselves if they had a lesion on the lower limbs, were of younger age (49.8 vs 52.9 years of age), had fewer atypical nevi, had >8 years education, were knowledgeable about the characteristic features of melanoma, and performed regular SSE (P<.01 for all comparisons).
Recommendations
The US Preventive Services Task Force finds insufficient evidence to recommend for or against routine counseling by primary care clinicians to prevent skin cancer.4
The Cancer Care Ontario Program in Evidence-based Care, on the other hand, advises health care providers to perform annual total-body skin examinations of high-risk patients and teach the patients to examine themselves.5
The American Cancer Society (ACS) and the American Academy of Dermatology (AAD) both provide information about recognizing melanoma on their Web sites and recommend that people at high risk perform monthly self-examinations. They further advise such people to periodically see a health care professional qualified to diagnose skin cancer (ACS), or a dermatologist (AAD), for a complete skin examination.6,7
1. Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23
2. Oliveria SA, Christos PJ, Halpern AC, et al. Patient knowledge, awareness, and delay in seeking medical attention for malignant melanoma. J Clin Epidemiol 1999;52:1111-1116
3. Carli P, De Giorgi V, Palli D, et al. Self-detected cutaneous melanomas in Italian patients. Clin Exp Dermatol. 2004;29:593-596
4. Counseling to prevent skin cancer: recommendations and rationale of the U.S. Preventive Services Task Force. MMWR Recomm Rep. 2003;52(RR-15):13-17
5. From L, Marrett L, Rosen C, et al. Screening for skin cancer: a clinical practice guideline. Toronto, Canada: Program in Evidence-Based Care, Cancer Care Ontario; 2007. Available at: www.cancercare.on.ca/pdf/pebc15-1s.pdf. Accessed April 14, 2008.
6. American Academy of Dermatology. alignant melanoma. Available at: www.aad.org/public/Publications/pamphlets/MalignantMelanoma.htm. Revised 2005. Accessed March 7, 2008.
7. American Cancer Society online Detailed guide: skin cancer-melanoma. Can melanoma be found early? Available at: www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_melanoma_be_found_early_50.asp. Accessed March 7, 2008.
1. Berwick M, Begg CB, Fine JA, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23
2. Oliveria SA, Christos PJ, Halpern AC, et al. Patient knowledge, awareness, and delay in seeking medical attention for malignant melanoma. J Clin Epidemiol 1999;52:1111-1116
3. Carli P, De Giorgi V, Palli D, et al. Self-detected cutaneous melanomas in Italian patients. Clin Exp Dermatol. 2004;29:593-596
4. Counseling to prevent skin cancer: recommendations and rationale of the U.S. Preventive Services Task Force. MMWR Recomm Rep. 2003;52(RR-15):13-17
5. From L, Marrett L, Rosen C, et al. Screening for skin cancer: a clinical practice guideline. Toronto, Canada: Program in Evidence-Based Care, Cancer Care Ontario; 2007. Available at: www.cancercare.on.ca/pdf/pebc15-1s.pdf. Accessed April 14, 2008.
6. American Academy of Dermatology. alignant melanoma. Available at: www.aad.org/public/Publications/pamphlets/MalignantMelanoma.htm. Revised 2005. Accessed March 7, 2008.
7. American Cancer Society online Detailed guide: skin cancer-melanoma. Can melanoma be found early? Available at: www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_melanoma_be_found_early_50.asp. Accessed March 7, 2008.
Evidence-based answers from the Family Physicians Inquiries Network
What could be behind your elderly patient’s subjective memory complaints?
Depression, anxiety, and dementia, as well as older age, female gender, lower education level, and decreased physical activity, have all been associated with memory loss reported by patients or family members (strength of recommendation [SOR]: B, cross-sectional studies). Memory complaints in patients with no cognitive impairment on short cognitive screening tests, such as the mini-mental status exam, may predict dementia (SOR: B, longitudinal studies). No consistent evidence supports pharmacologic treatment of reported memory loss that is not corroborated by objective findings (SOR: B, nonrandomized, poor-quality studies).
Is depression or polypharmacy at work?
Rajasree Nair, MD
Baylor College of Medicine, Houston, Tex
As the population ages, primary care physicians encounter a significant number of patients with memory loss and dementia. In clinical practice, patients with subjective memory complaints but normal cognitive testing present a diagnostic dilemma. Close attention to comorbid psychiatric conditions such as depression, anxiety, and substance use disorders, as well as polypharmacy, is essential.
While the US Preventive Services Task Force indicates that there is insufficient evidence to screen, it notes that recognizing cognitive impairment early not only facilitates diagnostic and treatment decisions, but also allows clinicians to anticipate problems the patient may have in understanding and adhering to recommended therapy. Even though evidence of early or minimal dementia may be difficult to detect, identifying it promptly enables physicians to counsel patients and caregivers on the course of disease progression, warning signs, medication adherence, finances, and safety.
Evidence Summary
Several cross-sectional studies indicate that patients with subjective memory loss are more likely to be older, female, less physically active, in poorer health, less educated, and more depressed or anxious than unaffected patients.1-4 These studies concentrate mostly on elderly people living in the community.
A study of 1883 patients with normal baseline short-cognitive test results found that those with subjective memory complaints had a higher incidence of dementia.5 At 5-year follow-up, 15% of patients with baseline subjective memory complaints had developed dementia compared to only 6% of those without such complaints (odds ratio=2.7; 95% confidence interval [CI], 1.45-4.98).
A prospective cohort study that followed 158 patients with no evidence of dementia showed a significant correlation between informant-reported memory problems and development of dementia at 5 years.6 Forty-five percent of patients with informant-reported memory problems developed dementia after 5 years compared with 25% of patients who had only self-reported memory problems (P =.02). This result suggests that subjective memory problems reported by observers (family or caregivers) may be more predictive of dementia than self-reported memory complaints.
Donepezil, ginkgo biloba may not help these patients
Most trials of interventions to preserve memory have not enrolled patients with subjective memory complaints. However, data from trials that enrolled either asymptomatic elderly patients or patients with mild cognitive impairment don’t support the use of donepezil, ginkgo biloba, NSAIDs, COX-2 inhibitors, vitamin E, vitamin B6, vitamin B12, statins, hormone replacement therapy, or omega-3 fatty acids to delay progression to dementia.7-16
Could mental exercise help?
One systematic review of 22 longitudinal cohort studies, which included more than 29,000 patients, evaluated complex patterns of mental activity in early, mid-, and late-life in relation to the incidence of dementia. Dementia was diagnosed at a significantly lower rate in patients with a higher level of cognitive exercise, such as memory-based leisure activities and social interactions, than those with less rigorous daily cognitive challenges (relative risk=0.54; 95% CI, 0.49-0.59).17
This raises the possibility that mental exercise has neuroprotective effects. No randomized trials exist to support this hypothesis, however.
Recommendations
There is no consensus regarding the nomenclature applied to reported memory loss and mild cognitive impairment. The Clinical Manual of Geriatric Psychiatry provides definitions that can be used in the clinical setting (TABLE).18
The US Preventive Services Task Force concludes that evidence is insufficient to recommend for or against routine screening for dementia in older adults (I recommendation).19 However, the Task Force notes that clinicians should assess cognitive function whenever they suspect impairment or deterioration based on direct observation, patient report, or concerns raised by family members, friends, or care-takers.
The American Geriatrics Society20 and American Academy of Neurology (AAN)21 acknowledge the subtle difference between age-associated memory impairment and mild cognitive impairment, and the difficulty of differentiating normal changes of aging from abnormal changes. The AAN’s guidelines for early detection of dementia emphasize the importance of diagnosing mild cognitive impairment or dementia early. However, the guidelines specifically exclude patients with subjective memory loss unaccompanied by objective cognitive deficits and offer no further discussion about these patients.
TABLE
Features of age-associated memory impairment vs mild cognitive impairment
| FEATURE | AGE-ASSOCIATED MEMORY IMPAIRMENT | MILD COGNITIVE IMPAIRMENT |
|---|---|---|
| Clinical presentation |
|
|
| Memory test results |
|
|
| Clinical course |
|
|
| ADLs, activities of daily living; IADLs, instrumental activities of daily living; SD, standard deviation. | ||
| Source: Spar JE, La Rue A. Clinical Manual of Geriatric Psychiatry.18 | ||
1. St John P, Montgomery P. Is subjective memory loss correlated with MMSE scores or dementia? J Geriatr Psychiatry Neurol. 2003;16:80-83.
2. Lautenschlager NT, Flicker L, Vasikaran S, et al. Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. Am J Geriatr Psychiatry. 2005;13:731-734.
3. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983-991.
4. Mol ME, van Boxtel MP, Willems D, et al. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht aging study. Int J Geriatr Psychiatry. 2006;21:432-441.
5. Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045-2051.
6. Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual’s complaints of memory impairment in early dementia. Neurology. 2000;55:1724-1726.
7. Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;(3):CD006104.-
8. Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and demetia. Cochrane Database Syst Rev. 2007;(2):CD003120.-
9. Etminan M, Gill S, Samii A. Effect of nonsteroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. Br Med J. 2003;327:128-131.
10. Aisen P, Schafer K, Grundman M. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819-2826.
11. Isaac M, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2000;(4):CD002854.-
12. Malouf R, Grimley Evans J. Vitamin B6 for cognition. Cochrane Database Syst Rev. 2003;(4):CD004393.-
13. Malouf M, Grimley Evans J, Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev. 2003;(4):004514.-
14. Scott HD, Laake K. Statins for the prevention of Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(3):003160.-
15. US Preventive Services Task Force. Postmenopausal hormone replacement therapy for the primary prevention of chronic conditions: recommendations and rationale. Am Fam Physician. 2003;67:358-364.
16. Lim WS, Gammack JK, Van Niekerk JK, et al. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst Rev. 2006;(1):CD005379.-
17. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441-454.
18. Spar JE, La Rue A. Clinical Manual of Geriatric Psychiatry. Illustrated ed. Arlington, Va: American Psychiatric Publishing, Inc; 2006.
19. US Preventive Services Task Force. Screening for dementia: recommendation and rationale summary for patients. Ann Intern Med. 2003;138:925-926.
20. Durso SC, Gwyther L, Roos B, et al. Clinical Practice Guidelines. Abstracted from the American Academy of Neurology’s dementia guidelines for early detection, diagnosis and management of dementia. New York: American Geriatrics Society; 2006. Available at: www.americangeriatrics.org/products/positionpapers/aan_dementia.shtml. Accessed April 9, 2007.
21. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
Depression, anxiety, and dementia, as well as older age, female gender, lower education level, and decreased physical activity, have all been associated with memory loss reported by patients or family members (strength of recommendation [SOR]: B, cross-sectional studies). Memory complaints in patients with no cognitive impairment on short cognitive screening tests, such as the mini-mental status exam, may predict dementia (SOR: B, longitudinal studies). No consistent evidence supports pharmacologic treatment of reported memory loss that is not corroborated by objective findings (SOR: B, nonrandomized, poor-quality studies).
Is depression or polypharmacy at work?
Rajasree Nair, MD
Baylor College of Medicine, Houston, Tex
As the population ages, primary care physicians encounter a significant number of patients with memory loss and dementia. In clinical practice, patients with subjective memory complaints but normal cognitive testing present a diagnostic dilemma. Close attention to comorbid psychiatric conditions such as depression, anxiety, and substance use disorders, as well as polypharmacy, is essential.
While the US Preventive Services Task Force indicates that there is insufficient evidence to screen, it notes that recognizing cognitive impairment early not only facilitates diagnostic and treatment decisions, but also allows clinicians to anticipate problems the patient may have in understanding and adhering to recommended therapy. Even though evidence of early or minimal dementia may be difficult to detect, identifying it promptly enables physicians to counsel patients and caregivers on the course of disease progression, warning signs, medication adherence, finances, and safety.
Evidence Summary
Several cross-sectional studies indicate that patients with subjective memory loss are more likely to be older, female, less physically active, in poorer health, less educated, and more depressed or anxious than unaffected patients.1-4 These studies concentrate mostly on elderly people living in the community.
A study of 1883 patients with normal baseline short-cognitive test results found that those with subjective memory complaints had a higher incidence of dementia.5 At 5-year follow-up, 15% of patients with baseline subjective memory complaints had developed dementia compared to only 6% of those without such complaints (odds ratio=2.7; 95% confidence interval [CI], 1.45-4.98).
A prospective cohort study that followed 158 patients with no evidence of dementia showed a significant correlation between informant-reported memory problems and development of dementia at 5 years.6 Forty-five percent of patients with informant-reported memory problems developed dementia after 5 years compared with 25% of patients who had only self-reported memory problems (P =.02). This result suggests that subjective memory problems reported by observers (family or caregivers) may be more predictive of dementia than self-reported memory complaints.
Donepezil, ginkgo biloba may not help these patients
Most trials of interventions to preserve memory have not enrolled patients with subjective memory complaints. However, data from trials that enrolled either asymptomatic elderly patients or patients with mild cognitive impairment don’t support the use of donepezil, ginkgo biloba, NSAIDs, COX-2 inhibitors, vitamin E, vitamin B6, vitamin B12, statins, hormone replacement therapy, or omega-3 fatty acids to delay progression to dementia.7-16
Could mental exercise help?
One systematic review of 22 longitudinal cohort studies, which included more than 29,000 patients, evaluated complex patterns of mental activity in early, mid-, and late-life in relation to the incidence of dementia. Dementia was diagnosed at a significantly lower rate in patients with a higher level of cognitive exercise, such as memory-based leisure activities and social interactions, than those with less rigorous daily cognitive challenges (relative risk=0.54; 95% CI, 0.49-0.59).17
This raises the possibility that mental exercise has neuroprotective effects. No randomized trials exist to support this hypothesis, however.
Recommendations
There is no consensus regarding the nomenclature applied to reported memory loss and mild cognitive impairment. The Clinical Manual of Geriatric Psychiatry provides definitions that can be used in the clinical setting (TABLE).18
The US Preventive Services Task Force concludes that evidence is insufficient to recommend for or against routine screening for dementia in older adults (I recommendation).19 However, the Task Force notes that clinicians should assess cognitive function whenever they suspect impairment or deterioration based on direct observation, patient report, or concerns raised by family members, friends, or care-takers.
The American Geriatrics Society20 and American Academy of Neurology (AAN)21 acknowledge the subtle difference between age-associated memory impairment and mild cognitive impairment, and the difficulty of differentiating normal changes of aging from abnormal changes. The AAN’s guidelines for early detection of dementia emphasize the importance of diagnosing mild cognitive impairment or dementia early. However, the guidelines specifically exclude patients with subjective memory loss unaccompanied by objective cognitive deficits and offer no further discussion about these patients.
TABLE
Features of age-associated memory impairment vs mild cognitive impairment
| FEATURE | AGE-ASSOCIATED MEMORY IMPAIRMENT | MILD COGNITIVE IMPAIRMENT |
|---|---|---|
| Clinical presentation |
|
|
| Memory test results |
|
|
| Clinical course |
|
|
| ADLs, activities of daily living; IADLs, instrumental activities of daily living; SD, standard deviation. | ||
| Source: Spar JE, La Rue A. Clinical Manual of Geriatric Psychiatry.18 | ||
Depression, anxiety, and dementia, as well as older age, female gender, lower education level, and decreased physical activity, have all been associated with memory loss reported by patients or family members (strength of recommendation [SOR]: B, cross-sectional studies). Memory complaints in patients with no cognitive impairment on short cognitive screening tests, such as the mini-mental status exam, may predict dementia (SOR: B, longitudinal studies). No consistent evidence supports pharmacologic treatment of reported memory loss that is not corroborated by objective findings (SOR: B, nonrandomized, poor-quality studies).
Is depression or polypharmacy at work?
Rajasree Nair, MD
Baylor College of Medicine, Houston, Tex
As the population ages, primary care physicians encounter a significant number of patients with memory loss and dementia. In clinical practice, patients with subjective memory complaints but normal cognitive testing present a diagnostic dilemma. Close attention to comorbid psychiatric conditions such as depression, anxiety, and substance use disorders, as well as polypharmacy, is essential.
While the US Preventive Services Task Force indicates that there is insufficient evidence to screen, it notes that recognizing cognitive impairment early not only facilitates diagnostic and treatment decisions, but also allows clinicians to anticipate problems the patient may have in understanding and adhering to recommended therapy. Even though evidence of early or minimal dementia may be difficult to detect, identifying it promptly enables physicians to counsel patients and caregivers on the course of disease progression, warning signs, medication adherence, finances, and safety.
Evidence Summary
Several cross-sectional studies indicate that patients with subjective memory loss are more likely to be older, female, less physically active, in poorer health, less educated, and more depressed or anxious than unaffected patients.1-4 These studies concentrate mostly on elderly people living in the community.
A study of 1883 patients with normal baseline short-cognitive test results found that those with subjective memory complaints had a higher incidence of dementia.5 At 5-year follow-up, 15% of patients with baseline subjective memory complaints had developed dementia compared to only 6% of those without such complaints (odds ratio=2.7; 95% confidence interval [CI], 1.45-4.98).
A prospective cohort study that followed 158 patients with no evidence of dementia showed a significant correlation between informant-reported memory problems and development of dementia at 5 years.6 Forty-five percent of patients with informant-reported memory problems developed dementia after 5 years compared with 25% of patients who had only self-reported memory problems (P =.02). This result suggests that subjective memory problems reported by observers (family or caregivers) may be more predictive of dementia than self-reported memory complaints.
Donepezil, ginkgo biloba may not help these patients
Most trials of interventions to preserve memory have not enrolled patients with subjective memory complaints. However, data from trials that enrolled either asymptomatic elderly patients or patients with mild cognitive impairment don’t support the use of donepezil, ginkgo biloba, NSAIDs, COX-2 inhibitors, vitamin E, vitamin B6, vitamin B12, statins, hormone replacement therapy, or omega-3 fatty acids to delay progression to dementia.7-16
Could mental exercise help?
One systematic review of 22 longitudinal cohort studies, which included more than 29,000 patients, evaluated complex patterns of mental activity in early, mid-, and late-life in relation to the incidence of dementia. Dementia was diagnosed at a significantly lower rate in patients with a higher level of cognitive exercise, such as memory-based leisure activities and social interactions, than those with less rigorous daily cognitive challenges (relative risk=0.54; 95% CI, 0.49-0.59).17
This raises the possibility that mental exercise has neuroprotective effects. No randomized trials exist to support this hypothesis, however.
Recommendations
There is no consensus regarding the nomenclature applied to reported memory loss and mild cognitive impairment. The Clinical Manual of Geriatric Psychiatry provides definitions that can be used in the clinical setting (TABLE).18
The US Preventive Services Task Force concludes that evidence is insufficient to recommend for or against routine screening for dementia in older adults (I recommendation).19 However, the Task Force notes that clinicians should assess cognitive function whenever they suspect impairment or deterioration based on direct observation, patient report, or concerns raised by family members, friends, or care-takers.
The American Geriatrics Society20 and American Academy of Neurology (AAN)21 acknowledge the subtle difference between age-associated memory impairment and mild cognitive impairment, and the difficulty of differentiating normal changes of aging from abnormal changes. The AAN’s guidelines for early detection of dementia emphasize the importance of diagnosing mild cognitive impairment or dementia early. However, the guidelines specifically exclude patients with subjective memory loss unaccompanied by objective cognitive deficits and offer no further discussion about these patients.
TABLE
Features of age-associated memory impairment vs mild cognitive impairment
| FEATURE | AGE-ASSOCIATED MEMORY IMPAIRMENT | MILD COGNITIVE IMPAIRMENT |
|---|---|---|
| Clinical presentation |
|
|
| Memory test results |
|
|
| Clinical course |
|
|
| ADLs, activities of daily living; IADLs, instrumental activities of daily living; SD, standard deviation. | ||
| Source: Spar JE, La Rue A. Clinical Manual of Geriatric Psychiatry.18 | ||
1. St John P, Montgomery P. Is subjective memory loss correlated with MMSE scores or dementia? J Geriatr Psychiatry Neurol. 2003;16:80-83.
2. Lautenschlager NT, Flicker L, Vasikaran S, et al. Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. Am J Geriatr Psychiatry. 2005;13:731-734.
3. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983-991.
4. Mol ME, van Boxtel MP, Willems D, et al. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht aging study. Int J Geriatr Psychiatry. 2006;21:432-441.
5. Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045-2051.
6. Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual’s complaints of memory impairment in early dementia. Neurology. 2000;55:1724-1726.
7. Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;(3):CD006104.-
8. Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and demetia. Cochrane Database Syst Rev. 2007;(2):CD003120.-
9. Etminan M, Gill S, Samii A. Effect of nonsteroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. Br Med J. 2003;327:128-131.
10. Aisen P, Schafer K, Grundman M. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819-2826.
11. Isaac M, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2000;(4):CD002854.-
12. Malouf R, Grimley Evans J. Vitamin B6 for cognition. Cochrane Database Syst Rev. 2003;(4):CD004393.-
13. Malouf M, Grimley Evans J, Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev. 2003;(4):004514.-
14. Scott HD, Laake K. Statins for the prevention of Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(3):003160.-
15. US Preventive Services Task Force. Postmenopausal hormone replacement therapy for the primary prevention of chronic conditions: recommendations and rationale. Am Fam Physician. 2003;67:358-364.
16. Lim WS, Gammack JK, Van Niekerk JK, et al. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst Rev. 2006;(1):CD005379.-
17. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441-454.
18. Spar JE, La Rue A. Clinical Manual of Geriatric Psychiatry. Illustrated ed. Arlington, Va: American Psychiatric Publishing, Inc; 2006.
19. US Preventive Services Task Force. Screening for dementia: recommendation and rationale summary for patients. Ann Intern Med. 2003;138:925-926.
20. Durso SC, Gwyther L, Roos B, et al. Clinical Practice Guidelines. Abstracted from the American Academy of Neurology’s dementia guidelines for early detection, diagnosis and management of dementia. New York: American Geriatrics Society; 2006. Available at: www.americangeriatrics.org/products/positionpapers/aan_dementia.shtml. Accessed April 9, 2007.
21. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
1. St John P, Montgomery P. Is subjective memory loss correlated with MMSE scores or dementia? J Geriatr Psychiatry Neurol. 2003;16:80-83.
2. Lautenschlager NT, Flicker L, Vasikaran S, et al. Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. Am J Geriatr Psychiatry. 2005;13:731-734.
3. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983-991.
4. Mol ME, van Boxtel MP, Willems D, et al. Do subjective memory complaints predict cognitive dysfunction over time? A six-year follow-up of the Maastricht aging study. Int J Geriatr Psychiatry. 2006;21:432-441.
5. Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045-2051.
6. Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual’s complaints of memory impairment in early dementia. Neurology. 2000;55:1724-1726.
7. Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;(3):CD006104.-
8. Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and demetia. Cochrane Database Syst Rev. 2007;(2):CD003120.-
9. Etminan M, Gill S, Samii A. Effect of nonsteroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. Br Med J. 2003;327:128-131.
10. Aisen P, Schafer K, Grundman M. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819-2826.
11. Isaac M, Quinn R, Tabet N. Vitamin E for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst Rev. 2000;(4):CD002854.-
12. Malouf R, Grimley Evans J. Vitamin B6 for cognition. Cochrane Database Syst Rev. 2003;(4):CD004393.-
13. Malouf M, Grimley Evans J, Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev. 2003;(4):004514.-
14. Scott HD, Laake K. Statins for the prevention of Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(3):003160.-
15. US Preventive Services Task Force. Postmenopausal hormone replacement therapy for the primary prevention of chronic conditions: recommendations and rationale. Am Fam Physician. 2003;67:358-364.
16. Lim WS, Gammack JK, Van Niekerk JK, et al. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst Rev. 2006;(1):CD005379.-
17. Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441-454.
18. Spar JE, La Rue A. Clinical Manual of Geriatric Psychiatry. Illustrated ed. Arlington, Va: American Psychiatric Publishing, Inc; 2006.
19. US Preventive Services Task Force. Screening for dementia: recommendation and rationale summary for patients. Ann Intern Med. 2003;138:925-926.
20. Durso SC, Gwyther L, Roos B, et al. Clinical Practice Guidelines. Abstracted from the American Academy of Neurology’s dementia guidelines for early detection, diagnosis and management of dementia. New York: American Geriatrics Society; 2006. Available at: www.americangeriatrics.org/products/positionpapers/aan_dementia.shtml. Accessed April 9, 2007.
21. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
Evidence-based answers from the Family Physicians Inquiries Network
Which drugs are safest for moderate to severe depression in adolescents?
Selective serotonin-reuptake inhibitors (SSRIs) appear to be the safest, given current data.
Major safety concerns—prompting a US Food and Drug administration (FDA) black box label warning—have been raised about increased risk of suicidality (ideation, behavior, and attempts) among adolescents receiving antidepressant therapy. Studies indicate that SSRIs and venlafaxine increase the absolute risk of suicidality by 1% to 2% compared with placebo. However, no suicides occurred during any study. On detailed subanalysis, each SSRI was as safe as placebo, and only venlafaxine demonstrated a statistically significant increase in risk of suicidality (strength of recommendation [SOR]: A, meta-analysis).
Information about the safety of tricyclic antidepressants in young people is limited because adverse effects have not been systematically reported in trials (SOR: A, meta-analysis).
(For information on the efficacy of antidepressants in adolescents, see the Clinical Inquiry on page 330.)
Treat with vigilance
Jason Jason Crawford, MD, MPH
University of Nevada School of Medicine, Reno
Based on the available evidence, I’m confident in my decision to use SSRIs to treat depressed adolescents. But the FDA black box warning often leaves me with a sense of apprehension as I write the prescription. I consider this a healthy reminder that depression in adolescents is no small matter, and its treatment shouldn’t be taken lightly.
In my practice, patient and family education always accompany the use of SSRIs, as does weekly follow-up in the beginning. Of the many patient education resources available on the Internet, my favorite Web sites include:
- www.familydoctor.org,
- www.aacap.org (Facts for Families),
- www.kidshealth.org (features pages for both parents and teenagers), and
- www.medlineplus.gov (handouts in English and Spanish and interactive tutorials).
Finally, no adolescent leaves my office without suicide precautions and phone numbers of local and national suicide prevention hotlines (www.suicidehotlines.com).
Evidence summary
In 2003, the United Kingdom’s Expert Working Group of the Committee on Safety of Medicines (CSM) and the FDA conducted a review and meta-analysis, respectively, of the newer antidepressants used in children and adolescents.1,2 Based on these studies, the FDA mandated that all antidepressant labels carry a black box warning about the increased risk of suicidal thinking and behavior (suicidality) among adolescents taking the medications.
No suicides, but a two-fold increase in suicidality
A recent study that incorporated the FDA meta-analysis analyzed data from 4582 patients. Although no completed suicides were reported in any trial, the drug-treated groups had a two-fold in-crease in suicidality compared with placebo groups (4% vs 2%; number needed to harm [NNH]=50).3 A recent Cochrane review confirmed the increase in absolute risk (1.8; 95% confidence interval [CI], 1.19-2.72).4
Two subsequent analyses estimated the difference in risk to be 1.6-fold with a 95% CI of 1.0-2.7 (3% vs 2%; NNH=112).5,6 The lower estimated risk results largely from a statistical reframing rather than any major difference in data analyzed. The analyses used a random-effects model instead of a fixed-effects model to calculate suicidality, assuming heterogeneity in the drugs used, trial de-signs, and outcome measures.3,6 All of the analyzed trials excluded patients at high risk for suicide, defined uniquely in the exclusion criteria for each trial.
The TABLE summarizes the increased risk of suicidality for each drug. Notably, venlafaxine had the greatest—and only statistically significant—increased risk, mostly because of suicidal ideation (7 of 9 events in 182 treated patients vs none in 179 placebo patients).6 (For a de-tailed look at the risk of suicidal ideation, suicidal behavior, or suicide attempt, see TABLE W1, available online at www. jfponline.com.)
Data on tricyclics in adolescents are scarce
Evidence concerning the safety of tri-cyclic antidepressants in adolescents is limited because adverse effects have not been systematically reported. A 2002 Cochrane meta-analysis found a statistically significant increase in rates of vertigo, orthostatic hypotension, tremor, and dry mouth among children and adolescents taking tricyclic antidepressants compared with placebo. The drugs are modestly effective in treating depression in adolescents; concerns about side effects and safety, however, have limited their use.7
Recommendations
The FDA encourages patients taking antidepressants and their families to be alert for signs of impulsive behavior or suicidal tendencies and to have a safety plan. The FDA, American Academy of Child and Adolescent Psychiatry, and the Society for Adolescent Medicine endorse close follow-up with periodic objective assessment.8-10
The Society for Adolescent Medicine explicitly directs clinicians to consider the FDA black box warning in the con-text of the need to treat major depressive disorder in adolescents and endorses pharmacotherapy for appropriately selected patients.10 Similarly, the American College of Neuropsychopharmacology argues that the risk-to-benefit ratio favors drug treatment for moderate to severe adolescent depression.11
TABLE
Risk of suicidality among young people taking antidepressants—Pooled results of 2 meta-analyses1,4
| DRUG | META-ANALYSIS | OR* (95% CI) |
|---|---|---|
| Citalopram, escitalopram | Hammad3 Dubicka6 | 1.37 (0.53-3.50) 1.21 (0.60-2.45) |
| Fluoxetine | Hammad Dubicka | 1.53 (0.74-3.16) 1.36 (0.65-2.88) |
| Paroxetine | Hammad Dubicka | 2.15 (0.71-6.52) 1.53 (0.61-3.84) |
| Sertraline | Hammad Dubicka | 2.16 (0.48-9.62) 2.47 (0.47-12.9) |
| Venlafaxine | Hammad Dubicka | 8.84 (1.12-69.51) 14.83 (1.93-114.0) |
| *Includes suicidal ideation, behavior, and attempts. CI, confidence interval; OR, odds ratio. | ||
1. US Food and Drug Administration Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. Available at: www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Accessed October 29, 2007.
2. Medicines and Healthcare products Regulatory Agency, Committee on Safety of Medicines. Use of selective serotonin reuptake inhibitors (SSRIs) in children and adolescents with major depressive disorder (MDD). Available at: www.mhra.gov.uk/. Accessed October 29, 2007.
3. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332-339.
4. Hetrick S, Merry S, McKenzie J, et al. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2007;(3):CD004851.-
5. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696(and Web-only content http://jama.ama-assn.org/cgi/content/full/297/15/1683; accessed June 13, 2007).
6. Dubicka B, Hadley S, Roberts C. Suicidal behaviour in youths with depression treated with new-generation antidepressants. Br J Psychiatry. 2006;189:393-398.
7. Hazell P, O’Connell D, Heathcote D, et al. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev. 2002;(2):CD002317.-
8. US Food and Drug Administration Medication guide about using antidepressants in children and Teenagers.Revised January 26, 2005. Available at: www.fda.gov/cder/drug/antidepressants/MG_template.pdf. Accessed June 2, 2007.
9. Birmaher B, Brent DA, Benson RS, et al. Summary of the practice parameters for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 1998;37:1234-1238.
10. Lock J, Walker LR, Rickert VI, et al. Suicidality in adolescents being treated with antidepressant medications and the black box label: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2005;36:92-93.
11. Mann JJ, Graham E, Baldessarini RJ, et al. ACNP Task Force report on SSRIs and suicidal behavior in youth. Neuropsychopharmacology. 2006;31:473-492.
Selective serotonin-reuptake inhibitors (SSRIs) appear to be the safest, given current data.
Major safety concerns—prompting a US Food and Drug administration (FDA) black box label warning—have been raised about increased risk of suicidality (ideation, behavior, and attempts) among adolescents receiving antidepressant therapy. Studies indicate that SSRIs and venlafaxine increase the absolute risk of suicidality by 1% to 2% compared with placebo. However, no suicides occurred during any study. On detailed subanalysis, each SSRI was as safe as placebo, and only venlafaxine demonstrated a statistically significant increase in risk of suicidality (strength of recommendation [SOR]: A, meta-analysis).
Information about the safety of tricyclic antidepressants in young people is limited because adverse effects have not been systematically reported in trials (SOR: A, meta-analysis).
(For information on the efficacy of antidepressants in adolescents, see the Clinical Inquiry on page 330.)
Treat with vigilance
Jason Jason Crawford, MD, MPH
University of Nevada School of Medicine, Reno
Based on the available evidence, I’m confident in my decision to use SSRIs to treat depressed adolescents. But the FDA black box warning often leaves me with a sense of apprehension as I write the prescription. I consider this a healthy reminder that depression in adolescents is no small matter, and its treatment shouldn’t be taken lightly.
In my practice, patient and family education always accompany the use of SSRIs, as does weekly follow-up in the beginning. Of the many patient education resources available on the Internet, my favorite Web sites include:
- www.familydoctor.org,
- www.aacap.org (Facts for Families),
- www.kidshealth.org (features pages for both parents and teenagers), and
- www.medlineplus.gov (handouts in English and Spanish and interactive tutorials).
Finally, no adolescent leaves my office without suicide precautions and phone numbers of local and national suicide prevention hotlines (www.suicidehotlines.com).
Evidence summary
In 2003, the United Kingdom’s Expert Working Group of the Committee on Safety of Medicines (CSM) and the FDA conducted a review and meta-analysis, respectively, of the newer antidepressants used in children and adolescents.1,2 Based on these studies, the FDA mandated that all antidepressant labels carry a black box warning about the increased risk of suicidal thinking and behavior (suicidality) among adolescents taking the medications.
No suicides, but a two-fold increase in suicidality
A recent study that incorporated the FDA meta-analysis analyzed data from 4582 patients. Although no completed suicides were reported in any trial, the drug-treated groups had a two-fold in-crease in suicidality compared with placebo groups (4% vs 2%; number needed to harm [NNH]=50).3 A recent Cochrane review confirmed the increase in absolute risk (1.8; 95% confidence interval [CI], 1.19-2.72).4
Two subsequent analyses estimated the difference in risk to be 1.6-fold with a 95% CI of 1.0-2.7 (3% vs 2%; NNH=112).5,6 The lower estimated risk results largely from a statistical reframing rather than any major difference in data analyzed. The analyses used a random-effects model instead of a fixed-effects model to calculate suicidality, assuming heterogeneity in the drugs used, trial de-signs, and outcome measures.3,6 All of the analyzed trials excluded patients at high risk for suicide, defined uniquely in the exclusion criteria for each trial.
The TABLE summarizes the increased risk of suicidality for each drug. Notably, venlafaxine had the greatest—and only statistically significant—increased risk, mostly because of suicidal ideation (7 of 9 events in 182 treated patients vs none in 179 placebo patients).6 (For a de-tailed look at the risk of suicidal ideation, suicidal behavior, or suicide attempt, see TABLE W1, available online at www. jfponline.com.)
Data on tricyclics in adolescents are scarce
Evidence concerning the safety of tri-cyclic antidepressants in adolescents is limited because adverse effects have not been systematically reported. A 2002 Cochrane meta-analysis found a statistically significant increase in rates of vertigo, orthostatic hypotension, tremor, and dry mouth among children and adolescents taking tricyclic antidepressants compared with placebo. The drugs are modestly effective in treating depression in adolescents; concerns about side effects and safety, however, have limited their use.7
Recommendations
The FDA encourages patients taking antidepressants and their families to be alert for signs of impulsive behavior or suicidal tendencies and to have a safety plan. The FDA, American Academy of Child and Adolescent Psychiatry, and the Society for Adolescent Medicine endorse close follow-up with periodic objective assessment.8-10
The Society for Adolescent Medicine explicitly directs clinicians to consider the FDA black box warning in the con-text of the need to treat major depressive disorder in adolescents and endorses pharmacotherapy for appropriately selected patients.10 Similarly, the American College of Neuropsychopharmacology argues that the risk-to-benefit ratio favors drug treatment for moderate to severe adolescent depression.11
TABLE
Risk of suicidality among young people taking antidepressants—Pooled results of 2 meta-analyses1,4
| DRUG | META-ANALYSIS | OR* (95% CI) |
|---|---|---|
| Citalopram, escitalopram | Hammad3 Dubicka6 | 1.37 (0.53-3.50) 1.21 (0.60-2.45) |
| Fluoxetine | Hammad Dubicka | 1.53 (0.74-3.16) 1.36 (0.65-2.88) |
| Paroxetine | Hammad Dubicka | 2.15 (0.71-6.52) 1.53 (0.61-3.84) |
| Sertraline | Hammad Dubicka | 2.16 (0.48-9.62) 2.47 (0.47-12.9) |
| Venlafaxine | Hammad Dubicka | 8.84 (1.12-69.51) 14.83 (1.93-114.0) |
| *Includes suicidal ideation, behavior, and attempts. CI, confidence interval; OR, odds ratio. | ||
Selective serotonin-reuptake inhibitors (SSRIs) appear to be the safest, given current data.
Major safety concerns—prompting a US Food and Drug administration (FDA) black box label warning—have been raised about increased risk of suicidality (ideation, behavior, and attempts) among adolescents receiving antidepressant therapy. Studies indicate that SSRIs and venlafaxine increase the absolute risk of suicidality by 1% to 2% compared with placebo. However, no suicides occurred during any study. On detailed subanalysis, each SSRI was as safe as placebo, and only venlafaxine demonstrated a statistically significant increase in risk of suicidality (strength of recommendation [SOR]: A, meta-analysis).
Information about the safety of tricyclic antidepressants in young people is limited because adverse effects have not been systematically reported in trials (SOR: A, meta-analysis).
(For information on the efficacy of antidepressants in adolescents, see the Clinical Inquiry on page 330.)
Treat with vigilance
Jason Jason Crawford, MD, MPH
University of Nevada School of Medicine, Reno
Based on the available evidence, I’m confident in my decision to use SSRIs to treat depressed adolescents. But the FDA black box warning often leaves me with a sense of apprehension as I write the prescription. I consider this a healthy reminder that depression in adolescents is no small matter, and its treatment shouldn’t be taken lightly.
In my practice, patient and family education always accompany the use of SSRIs, as does weekly follow-up in the beginning. Of the many patient education resources available on the Internet, my favorite Web sites include:
- www.familydoctor.org,
- www.aacap.org (Facts for Families),
- www.kidshealth.org (features pages for both parents and teenagers), and
- www.medlineplus.gov (handouts in English and Spanish and interactive tutorials).
Finally, no adolescent leaves my office without suicide precautions and phone numbers of local and national suicide prevention hotlines (www.suicidehotlines.com).
Evidence summary
In 2003, the United Kingdom’s Expert Working Group of the Committee on Safety of Medicines (CSM) and the FDA conducted a review and meta-analysis, respectively, of the newer antidepressants used in children and adolescents.1,2 Based on these studies, the FDA mandated that all antidepressant labels carry a black box warning about the increased risk of suicidal thinking and behavior (suicidality) among adolescents taking the medications.
No suicides, but a two-fold increase in suicidality
A recent study that incorporated the FDA meta-analysis analyzed data from 4582 patients. Although no completed suicides were reported in any trial, the drug-treated groups had a two-fold in-crease in suicidality compared with placebo groups (4% vs 2%; number needed to harm [NNH]=50).3 A recent Cochrane review confirmed the increase in absolute risk (1.8; 95% confidence interval [CI], 1.19-2.72).4
Two subsequent analyses estimated the difference in risk to be 1.6-fold with a 95% CI of 1.0-2.7 (3% vs 2%; NNH=112).5,6 The lower estimated risk results largely from a statistical reframing rather than any major difference in data analyzed. The analyses used a random-effects model instead of a fixed-effects model to calculate suicidality, assuming heterogeneity in the drugs used, trial de-signs, and outcome measures.3,6 All of the analyzed trials excluded patients at high risk for suicide, defined uniquely in the exclusion criteria for each trial.
The TABLE summarizes the increased risk of suicidality for each drug. Notably, venlafaxine had the greatest—and only statistically significant—increased risk, mostly because of suicidal ideation (7 of 9 events in 182 treated patients vs none in 179 placebo patients).6 (For a de-tailed look at the risk of suicidal ideation, suicidal behavior, or suicide attempt, see TABLE W1, available online at www. jfponline.com.)
Data on tricyclics in adolescents are scarce
Evidence concerning the safety of tri-cyclic antidepressants in adolescents is limited because adverse effects have not been systematically reported. A 2002 Cochrane meta-analysis found a statistically significant increase in rates of vertigo, orthostatic hypotension, tremor, and dry mouth among children and adolescents taking tricyclic antidepressants compared with placebo. The drugs are modestly effective in treating depression in adolescents; concerns about side effects and safety, however, have limited their use.7
Recommendations
The FDA encourages patients taking antidepressants and their families to be alert for signs of impulsive behavior or suicidal tendencies and to have a safety plan. The FDA, American Academy of Child and Adolescent Psychiatry, and the Society for Adolescent Medicine endorse close follow-up with periodic objective assessment.8-10
The Society for Adolescent Medicine explicitly directs clinicians to consider the FDA black box warning in the con-text of the need to treat major depressive disorder in adolescents and endorses pharmacotherapy for appropriately selected patients.10 Similarly, the American College of Neuropsychopharmacology argues that the risk-to-benefit ratio favors drug treatment for moderate to severe adolescent depression.11
TABLE
Risk of suicidality among young people taking antidepressants—Pooled results of 2 meta-analyses1,4
| DRUG | META-ANALYSIS | OR* (95% CI) |
|---|---|---|
| Citalopram, escitalopram | Hammad3 Dubicka6 | 1.37 (0.53-3.50) 1.21 (0.60-2.45) |
| Fluoxetine | Hammad Dubicka | 1.53 (0.74-3.16) 1.36 (0.65-2.88) |
| Paroxetine | Hammad Dubicka | 2.15 (0.71-6.52) 1.53 (0.61-3.84) |
| Sertraline | Hammad Dubicka | 2.16 (0.48-9.62) 2.47 (0.47-12.9) |
| Venlafaxine | Hammad Dubicka | 8.84 (1.12-69.51) 14.83 (1.93-114.0) |
| *Includes suicidal ideation, behavior, and attempts. CI, confidence interval; OR, odds ratio. | ||
1. US Food and Drug Administration Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. Available at: www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Accessed October 29, 2007.
2. Medicines and Healthcare products Regulatory Agency, Committee on Safety of Medicines. Use of selective serotonin reuptake inhibitors (SSRIs) in children and adolescents with major depressive disorder (MDD). Available at: www.mhra.gov.uk/. Accessed October 29, 2007.
3. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332-339.
4. Hetrick S, Merry S, McKenzie J, et al. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2007;(3):CD004851.-
5. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696(and Web-only content http://jama.ama-assn.org/cgi/content/full/297/15/1683; accessed June 13, 2007).
6. Dubicka B, Hadley S, Roberts C. Suicidal behaviour in youths with depression treated with new-generation antidepressants. Br J Psychiatry. 2006;189:393-398.
7. Hazell P, O’Connell D, Heathcote D, et al. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev. 2002;(2):CD002317.-
8. US Food and Drug Administration Medication guide about using antidepressants in children and Teenagers.Revised January 26, 2005. Available at: www.fda.gov/cder/drug/antidepressants/MG_template.pdf. Accessed June 2, 2007.
9. Birmaher B, Brent DA, Benson RS, et al. Summary of the practice parameters for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 1998;37:1234-1238.
10. Lock J, Walker LR, Rickert VI, et al. Suicidality in adolescents being treated with antidepressant medications and the black box label: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2005;36:92-93.
11. Mann JJ, Graham E, Baldessarini RJ, et al. ACNP Task Force report on SSRIs and suicidal behavior in youth. Neuropsychopharmacology. 2006;31:473-492.
1. US Food and Drug Administration Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. Available at: www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. Accessed October 29, 2007.
2. Medicines and Healthcare products Regulatory Agency, Committee on Safety of Medicines. Use of selective serotonin reuptake inhibitors (SSRIs) in children and adolescents with major depressive disorder (MDD). Available at: www.mhra.gov.uk/. Accessed October 29, 2007.
3. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332-339.
4. Hetrick S, Merry S, McKenzie J, et al. Selective serotonin reuptake inhibitors (SSRIs) for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2007;(3):CD004851.-
5. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683-1696(and Web-only content http://jama.ama-assn.org/cgi/content/full/297/15/1683; accessed June 13, 2007).
6. Dubicka B, Hadley S, Roberts C. Suicidal behaviour in youths with depression treated with new-generation antidepressants. Br J Psychiatry. 2006;189:393-398.
7. Hazell P, O’Connell D, Heathcote D, et al. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev. 2002;(2):CD002317.-
8. US Food and Drug Administration Medication guide about using antidepressants in children and Teenagers.Revised January 26, 2005. Available at: www.fda.gov/cder/drug/antidepressants/MG_template.pdf. Accessed June 2, 2007.
9. Birmaher B, Brent DA, Benson RS, et al. Summary of the practice parameters for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 1998;37:1234-1238.
10. Lock J, Walker LR, Rickert VI, et al. Suicidality in adolescents being treated with antidepressant medications and the black box label: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2005;36:92-93.
11. Mann JJ, Graham E, Baldessarini RJ, et al. ACNP Task Force report on SSRIs and suicidal behavior in youth. Neuropsychopharmacology. 2006;31:473-492.
Evidence-based answers from the Family Physicians Inquiries Network
Should you evaluate for CAD in seniors with premature ventricular contractions?
Yes. Current guidelines suggest evaluating patients with premature ventricular contractions (PVCs) and associated risk factors for underlying coronary artery disease (strength of recommendation [SOR]: C, expert opinion).
Frequent PVCs are associated with acute myocardial infarction and sudden death in patients without known coronary artery disease (CAD). They are linked to increased mortality from all causes in elderly patients with a history of CAD, left ventricular dysfunction, hypertension, or valvular heart disease. Frequent PVCs during recovery from exercise stress testing are also associated with increased mortality.
There is strong evidence against suppressing PVCs with antiarrhythmics (SOR: A, randomized controlled trials [RCTs]).
Stress preventive measures
Jennifer Lochner, MD
Oregon Health and Sciences University, Portland
I find myself discussing PVCs most often with young women who don’t have known heart disease—rather than the elderly. I often discover PVCs on physical examination in the office or see them on a Holter monitor ordered to rule out other more worrisome arrhythmias.
This reminds me that I need to not only consider the issue of treatment aimed at suppressing PVCs (not helpful except when the patient has significant symptoms), but also to consider whether the patient has risk factors for CAD.
In future discussions with patients about PVCs, I plan to shift the focus to measures to prevent CAD—specifically tobacco cessation, weight management, daily physical activity, and a healthy diet.
Evidence summary
A consistent definition of frequent PVCs doesn’t exist in the literature. Some studies have found a significant risk of death or acute myocardial infarction associated with >30 PVCs per hour.1,2 The 2006 American College of Cardiology/American Heart Association/European Society of Cardiology guideline defines frequent PVCs as >10 per hour.3
Despite the association between frequent PVCs and increased risk of death and cardiac events, our review didn’t find studies that indicate the utility of evaluation strategies for higher-risk patients.
Frequent PVCs predict increased mortality
The Framingham study looked at the prognostic implications of frequent PVCs (>30 per hour) in a cohort of symptomatic patients examined over a 6-year period.1 Men, but not women, had a significant increase in all-cause mortality (relative risk [RR]=2.36; 95% confidence interval [CI], 1.65-3.2) and myocardial infarction or sudden death (RR=2.12; 95% CI, 1.33-3.38). The Copenhagen Holter study of a cohort of healthy patients demonstrated an increased risk of myocardial infarction or cardiovascular death in patients with >30 PVCs per hour (hazard ratio [HR]=2.85, 95% CI, 1.16-7.0).2
Frequent PVCs occurring during recovery from stress testing are also associated with increased mortality. A large prospective cohort study followed more than 29,000 patients with varying degrees of risk for 5 years. After adjusting for confounding variables, frequent PVCs (≥7 per minute or more complex ventricular ectopy) during recovery predicted an increased risk of death (HR=1.5; 95% CI, 1.1-1.9). Frequent PVCs arising during exercise stress testing were not associated with increased risk.4
Suppressing PVCs is a bad idea
Studies have evaluated whether suppressing PVCs with antiarrhythmic agents improves prognosis. Both Cardiac Arrhythmia Suppression Trials (CAST I: encainide and flecainide; CAST II: moricizine) showed that suppressing frequent PVCs significantly increased mortality in the treatment groups.5,6
Recommendations
In 2006, the American College of Cardiology, American Heart Association, and European Society of Cardiology published their Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death.3
The TABLE summarizes characteristics of patients with PVCs who were at higher risk of underlying cardiac disease and death. All patients with PVCs should have a history and physical examination, electrocardiogram, and electrolyte studies. Higher-risk patients should be considered for further evaluation, including stress testing, echocardiography, and ambulatory electrocardiogram (SOR: C, opinion).
TABLE
Characteristics of patients with PVCs who are at higher risk of cardiac disease/death
| PATIENT CHARACTERISTICS | LOWER RISK | HIGHER RISK |
|---|---|---|
| Morphology | Unifocal PVCs<10 PVCs per hour | Complex multifocal PVCs Ventricular tachycardia Ventricular fibrillation >10 PVCs per hour |
| Symptoms | Asymptomatic | Palpitations Presyncope Syncope |
| Preexisting conditions | None | Known history of CAD Structural heart disease Valvular heart disease Cardiomyopathy |
| CAD, coronary artery disease, PVCs, premature ventricular contractions. | ||
| Source: American College of Cardiology et al.3 | ||
1. Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham heart study. Ann Intern Med. 1992;117:990-996.
2. Sajadieh A, Nielsen OW, Rasmussen V, et al. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age 55 or older. Am J Cardiol. 2006;97:1351-1357.
3. American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2006;48:e247-e346.
4. Frolkis JP, Pothier CE, Blackstone EH, et al. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781-790.
5. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406-412.
6. The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227-233.
Yes. Current guidelines suggest evaluating patients with premature ventricular contractions (PVCs) and associated risk factors for underlying coronary artery disease (strength of recommendation [SOR]: C, expert opinion).
Frequent PVCs are associated with acute myocardial infarction and sudden death in patients without known coronary artery disease (CAD). They are linked to increased mortality from all causes in elderly patients with a history of CAD, left ventricular dysfunction, hypertension, or valvular heart disease. Frequent PVCs during recovery from exercise stress testing are also associated with increased mortality.
There is strong evidence against suppressing PVCs with antiarrhythmics (SOR: A, randomized controlled trials [RCTs]).
Stress preventive measures
Jennifer Lochner, MD
Oregon Health and Sciences University, Portland
I find myself discussing PVCs most often with young women who don’t have known heart disease—rather than the elderly. I often discover PVCs on physical examination in the office or see them on a Holter monitor ordered to rule out other more worrisome arrhythmias.
This reminds me that I need to not only consider the issue of treatment aimed at suppressing PVCs (not helpful except when the patient has significant symptoms), but also to consider whether the patient has risk factors for CAD.
In future discussions with patients about PVCs, I plan to shift the focus to measures to prevent CAD—specifically tobacco cessation, weight management, daily physical activity, and a healthy diet.
Evidence summary
A consistent definition of frequent PVCs doesn’t exist in the literature. Some studies have found a significant risk of death or acute myocardial infarction associated with >30 PVCs per hour.1,2 The 2006 American College of Cardiology/American Heart Association/European Society of Cardiology guideline defines frequent PVCs as >10 per hour.3
Despite the association between frequent PVCs and increased risk of death and cardiac events, our review didn’t find studies that indicate the utility of evaluation strategies for higher-risk patients.
Frequent PVCs predict increased mortality
The Framingham study looked at the prognostic implications of frequent PVCs (>30 per hour) in a cohort of symptomatic patients examined over a 6-year period.1 Men, but not women, had a significant increase in all-cause mortality (relative risk [RR]=2.36; 95% confidence interval [CI], 1.65-3.2) and myocardial infarction or sudden death (RR=2.12; 95% CI, 1.33-3.38). The Copenhagen Holter study of a cohort of healthy patients demonstrated an increased risk of myocardial infarction or cardiovascular death in patients with >30 PVCs per hour (hazard ratio [HR]=2.85, 95% CI, 1.16-7.0).2
Frequent PVCs occurring during recovery from stress testing are also associated with increased mortality. A large prospective cohort study followed more than 29,000 patients with varying degrees of risk for 5 years. After adjusting for confounding variables, frequent PVCs (≥7 per minute or more complex ventricular ectopy) during recovery predicted an increased risk of death (HR=1.5; 95% CI, 1.1-1.9). Frequent PVCs arising during exercise stress testing were not associated with increased risk.4
Suppressing PVCs is a bad idea
Studies have evaluated whether suppressing PVCs with antiarrhythmic agents improves prognosis. Both Cardiac Arrhythmia Suppression Trials (CAST I: encainide and flecainide; CAST II: moricizine) showed that suppressing frequent PVCs significantly increased mortality in the treatment groups.5,6
Recommendations
In 2006, the American College of Cardiology, American Heart Association, and European Society of Cardiology published their Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death.3
The TABLE summarizes characteristics of patients with PVCs who were at higher risk of underlying cardiac disease and death. All patients with PVCs should have a history and physical examination, electrocardiogram, and electrolyte studies. Higher-risk patients should be considered for further evaluation, including stress testing, echocardiography, and ambulatory electrocardiogram (SOR: C, opinion).
TABLE
Characteristics of patients with PVCs who are at higher risk of cardiac disease/death
| PATIENT CHARACTERISTICS | LOWER RISK | HIGHER RISK |
|---|---|---|
| Morphology | Unifocal PVCs<10 PVCs per hour | Complex multifocal PVCs Ventricular tachycardia Ventricular fibrillation >10 PVCs per hour |
| Symptoms | Asymptomatic | Palpitations Presyncope Syncope |
| Preexisting conditions | None | Known history of CAD Structural heart disease Valvular heart disease Cardiomyopathy |
| CAD, coronary artery disease, PVCs, premature ventricular contractions. | ||
| Source: American College of Cardiology et al.3 | ||
Yes. Current guidelines suggest evaluating patients with premature ventricular contractions (PVCs) and associated risk factors for underlying coronary artery disease (strength of recommendation [SOR]: C, expert opinion).
Frequent PVCs are associated with acute myocardial infarction and sudden death in patients without known coronary artery disease (CAD). They are linked to increased mortality from all causes in elderly patients with a history of CAD, left ventricular dysfunction, hypertension, or valvular heart disease. Frequent PVCs during recovery from exercise stress testing are also associated with increased mortality.
There is strong evidence against suppressing PVCs with antiarrhythmics (SOR: A, randomized controlled trials [RCTs]).
Stress preventive measures
Jennifer Lochner, MD
Oregon Health and Sciences University, Portland
I find myself discussing PVCs most often with young women who don’t have known heart disease—rather than the elderly. I often discover PVCs on physical examination in the office or see them on a Holter monitor ordered to rule out other more worrisome arrhythmias.
This reminds me that I need to not only consider the issue of treatment aimed at suppressing PVCs (not helpful except when the patient has significant symptoms), but also to consider whether the patient has risk factors for CAD.
In future discussions with patients about PVCs, I plan to shift the focus to measures to prevent CAD—specifically tobacco cessation, weight management, daily physical activity, and a healthy diet.
Evidence summary
A consistent definition of frequent PVCs doesn’t exist in the literature. Some studies have found a significant risk of death or acute myocardial infarction associated with >30 PVCs per hour.1,2 The 2006 American College of Cardiology/American Heart Association/European Society of Cardiology guideline defines frequent PVCs as >10 per hour.3
Despite the association between frequent PVCs and increased risk of death and cardiac events, our review didn’t find studies that indicate the utility of evaluation strategies for higher-risk patients.
Frequent PVCs predict increased mortality
The Framingham study looked at the prognostic implications of frequent PVCs (>30 per hour) in a cohort of symptomatic patients examined over a 6-year period.1 Men, but not women, had a significant increase in all-cause mortality (relative risk [RR]=2.36; 95% confidence interval [CI], 1.65-3.2) and myocardial infarction or sudden death (RR=2.12; 95% CI, 1.33-3.38). The Copenhagen Holter study of a cohort of healthy patients demonstrated an increased risk of myocardial infarction or cardiovascular death in patients with >30 PVCs per hour (hazard ratio [HR]=2.85, 95% CI, 1.16-7.0).2
Frequent PVCs occurring during recovery from stress testing are also associated with increased mortality. A large prospective cohort study followed more than 29,000 patients with varying degrees of risk for 5 years. After adjusting for confounding variables, frequent PVCs (≥7 per minute or more complex ventricular ectopy) during recovery predicted an increased risk of death (HR=1.5; 95% CI, 1.1-1.9). Frequent PVCs arising during exercise stress testing were not associated with increased risk.4
Suppressing PVCs is a bad idea
Studies have evaluated whether suppressing PVCs with antiarrhythmic agents improves prognosis. Both Cardiac Arrhythmia Suppression Trials (CAST I: encainide and flecainide; CAST II: moricizine) showed that suppressing frequent PVCs significantly increased mortality in the treatment groups.5,6
Recommendations
In 2006, the American College of Cardiology, American Heart Association, and European Society of Cardiology published their Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death.3
The TABLE summarizes characteristics of patients with PVCs who were at higher risk of underlying cardiac disease and death. All patients with PVCs should have a history and physical examination, electrocardiogram, and electrolyte studies. Higher-risk patients should be considered for further evaluation, including stress testing, echocardiography, and ambulatory electrocardiogram (SOR: C, opinion).
TABLE
Characteristics of patients with PVCs who are at higher risk of cardiac disease/death
| PATIENT CHARACTERISTICS | LOWER RISK | HIGHER RISK |
|---|---|---|
| Morphology | Unifocal PVCs<10 PVCs per hour | Complex multifocal PVCs Ventricular tachycardia Ventricular fibrillation >10 PVCs per hour |
| Symptoms | Asymptomatic | Palpitations Presyncope Syncope |
| Preexisting conditions | None | Known history of CAD Structural heart disease Valvular heart disease Cardiomyopathy |
| CAD, coronary artery disease, PVCs, premature ventricular contractions. | ||
| Source: American College of Cardiology et al.3 | ||
1. Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham heart study. Ann Intern Med. 1992;117:990-996.
2. Sajadieh A, Nielsen OW, Rasmussen V, et al. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age 55 or older. Am J Cardiol. 2006;97:1351-1357.
3. American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2006;48:e247-e346.
4. Frolkis JP, Pothier CE, Blackstone EH, et al. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781-790.
5. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406-412.
6. The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227-233.
1. Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham heart study. Ann Intern Med. 1992;117:990-996.
2. Sajadieh A, Nielsen OW, Rasmussen V, et al. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age 55 or older. Am J Cardiol. 2006;97:1351-1357.
3. American College of Cardiology, American Heart Association, European Society of Cardiology. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2006;48:e247-e346.
4. Frolkis JP, Pothier CE, Blackstone EH, et al. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781-790.
5. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406-412.
6. The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227-233.
Evidence-based answers from the Family Physicians Inquiries Network
How should you evaluate elevated calcium in an asymptomatic patient?
First, establish that true hypercalcemia exists by repeating the serum calcium and measuring or calculating the physiologically active serum calcium when abnormalities in blood pH or albumin are found (SOR: C, expert opinion). Patients with unexplained asymptomatic true hypercalcemia should be screened for primary hyperparathyroidism (PHPT) and malignancy using an intact parathyroid hormone (PTH) level by immunoradioassay (SOR: C, expert opinion). Other recommended tests that can distinguish PHPT from malignancy and familial hypocalciuric hypercalcemia, as well as help manage patients with PHPT include urinary 24-hour calcium and creatinine levels, parathyroid hormone related peptide (PTHrP), alkaline phosphatase, calcitriol, and bone densitometry (SOR: C, expert opinion).
Choose tests carefully to reduce false positives
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Including serum calcium measurements in the chemistry panels that physicians use to manage common conditions such as hypertension has resulted in an epidemic of incidental hypercalcemia. Tempting as it may be to ignore these unexpected numbers, they point to a significant underlying condition in some patients. This puts the family physician in a familiar clinical position—having to worry a patient just enough to convince him to consent to a careful, stepwise evaluation while somehow reassuring him that usually no problem is found. The best solution is to order each test for a reason, which would reduce the number of false positives that we spend so much time chasing.
Evidence summary
Make sure it’s true hypercalcemia
Measuring calcium levels in asymptomatic patients often leads to false-positive elevations caused by random error or changes in the level of physiologically active calcium because of alterations in blood pH or serum albumin. Serum calcium levels between 10.0 and 12.0 mg/dL indicate mild hypercalcemia; levels >14.0 mg/dL are severe. Because changes in pH and serum albumin levels alter levels of physiologically active calcium, authoritative sources recommend measuring or calculating physiologically active calcium if blood pH or albumin is abnormal.1,2 To determine the level, use the equation [4.0 – (plasma albumin)] × 0.8 + (serum calcium) or measure serum ionized calcium.2 Normal levels of serum ionized calcium for adults older than 19 years are 1.13 to 1.32 mmol/L, although the exact range can vary from laboratory to laboratory. Elevated physiologically active calcium indicates true hypercalcemia.
Assess for the most common causes, PHPT and malignancy
Evaluation of the patient with true hypercalcemia should include a detailed history, physical examination, and assessment of risk factors for all causes of hypercalcemia.1,2 PHPT and malignancy are the two most common causes of asymptomatic true hypercalcemia (TABLE).2
Laboratory evaluation targeting these causes, beginning with an intact PTH level, is a logical first step.1,2 Persistent hypercalcemia in the presence of elevated or inappropriately normal PTH concentrations confirms the diagnosis of PHPT.3 When serum calcium rises, PTH is normally suppressed. Normal intact PTH and low 24-hour urinary calcium excretion distinguishes patients with PHPT from those with less common familial hypocalciuric hypercalcemia.1,2
Most patients with PHPT are asymptomatic, although some eventually develop bone loss, nephrolithiasis, and renal colic.4,5 A 10-year prospective cohort study of patients with PHPT found that 21% of asymptomatic patients developed decreased bone density at one or more sites.6 None acquired kidney stones, but hypercalcemia and hypercalciuria did worsen in 10 of 52 patients. A guideline and a review on PHPT recommend measuring creatinine clearance and obtaining a bone densitometry study of the distal third of the radius, hip, and lumbar spine to assess for end-organ changes related to the condition; declining renal function and osteoporosis may be indications for surgery.3,5
Malignancy is the most common cause of low intact PTH and true hypercalcemia, especially when the calcium level is >14 mg/dL.1 A PTHrP >1.0 pmol/L is highly specific for malignancy because this level does not occur in healthy people.1 In a prospective case series of patients with hypercalcemia and malignancy, 54% had elevated PTHrP levels.7 The authors found that an elevated PTHrP in patients younger than 65 years of age doubles the risk of death from malignancy compared to patients the same age with normal PTHrP (hazard ratio=1.9; 95% CI, 1.1-3.4).
TABLE
Causes of hypercalcemia
| Primary hyperparathyroidism |
Malignancy
|
| Chronic renal failure |
| Endocrine disorders (hyperthyroidism, pheochromocytoma, Addison’s disease) |
| Familial hypocalciuric hypercalcemia |
| Immobilization |
| Laboratory artifact resulting from altered albumin concentration or pH |
| Medications (vitamin A toxicity [dietary fads, isotretinoin overdose], estrogens, antiestrogens, thiazides, lithium) |
| Milk alkali syndrome |
| Vitamin D toxicity (granulomatous disease [sarcoidosis, tuberculosis], vitamin D supplementation) |
| Based on Hutton E,1 and Carroll MF et al.2 |
Identify less common causes
Serum calcitriol in association with a low intact PTH level and elevated calcium lower than 14 mg/dL helps differentiate the less common causes of hypercalcemia. Calcitriol is high in granulomatous diseases such as sarcoidosis, tuberculosis, and histoplasmosis, and normal in hyperthyroidism and Addison’s disease.1
Immobilization as a cause of hypercalcemia can be distinguished from PHPT by history and normal PTH levels and from malignancy by a normal alkaline phosphatase level.1
Recommendations
In addition to the recommendations discussed previously, Williams Textbook of Endocrinology advises repeating the initial calcium level twice and measuring serum BUN, creatinine, electrolytes, albumin, globulin, and phosphate.8 The authors recommend a generalized work-up for malignancy, including mammography, chest radiography with or without CT, abdominal CT, serum and urine immunoelectrophoresis, and temporary discontinuation of lithium for patients taking the drug. They also recommend using PTHrP only when PTH is suppressed but an underlying malignancy can’t be found.
1. Hutton E. Evaluation and management of hypercalcemia. JAAPA. 2005;18(6):30-35.
2. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
3. Belezikian JP, Potts JT, Fuleihan GE, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353-5361.
4. AACE/AAES task force on primary hyperparathyroidism. The American Association of Clinical endocrinologists and the American Association of endocrine surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11(1):49-54.
5. Silverberg SJ, Bilezikian JP. The diagnosis and management of asympyomatic primary hyperparathyroidism. Nat Clin Pract Endocrinol Metab. 2006;2:494-503.
6. Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249-1255.
7. Truong NU, deB Edwards MD, Papavacillicu V, et al. Parathyroid hormone related peptide and survival of patients with cancer and hypercalcemia. Am J Med. 2003;115:115-121.
8. Bringhurst FR, Dernay MB, Kronenberg HM. Approach to the hypercalcemic patient. In: Williams RH, Larsen PR. Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders;2003:1336-1339.
First, establish that true hypercalcemia exists by repeating the serum calcium and measuring or calculating the physiologically active serum calcium when abnormalities in blood pH or albumin are found (SOR: C, expert opinion). Patients with unexplained asymptomatic true hypercalcemia should be screened for primary hyperparathyroidism (PHPT) and malignancy using an intact parathyroid hormone (PTH) level by immunoradioassay (SOR: C, expert opinion). Other recommended tests that can distinguish PHPT from malignancy and familial hypocalciuric hypercalcemia, as well as help manage patients with PHPT include urinary 24-hour calcium and creatinine levels, parathyroid hormone related peptide (PTHrP), alkaline phosphatase, calcitriol, and bone densitometry (SOR: C, expert opinion).
Choose tests carefully to reduce false positives
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Including serum calcium measurements in the chemistry panels that physicians use to manage common conditions such as hypertension has resulted in an epidemic of incidental hypercalcemia. Tempting as it may be to ignore these unexpected numbers, they point to a significant underlying condition in some patients. This puts the family physician in a familiar clinical position—having to worry a patient just enough to convince him to consent to a careful, stepwise evaluation while somehow reassuring him that usually no problem is found. The best solution is to order each test for a reason, which would reduce the number of false positives that we spend so much time chasing.
Evidence summary
Make sure it’s true hypercalcemia
Measuring calcium levels in asymptomatic patients often leads to false-positive elevations caused by random error or changes in the level of physiologically active calcium because of alterations in blood pH or serum albumin. Serum calcium levels between 10.0 and 12.0 mg/dL indicate mild hypercalcemia; levels >14.0 mg/dL are severe. Because changes in pH and serum albumin levels alter levels of physiologically active calcium, authoritative sources recommend measuring or calculating physiologically active calcium if blood pH or albumin is abnormal.1,2 To determine the level, use the equation [4.0 – (plasma albumin)] × 0.8 + (serum calcium) or measure serum ionized calcium.2 Normal levels of serum ionized calcium for adults older than 19 years are 1.13 to 1.32 mmol/L, although the exact range can vary from laboratory to laboratory. Elevated physiologically active calcium indicates true hypercalcemia.
Assess for the most common causes, PHPT and malignancy
Evaluation of the patient with true hypercalcemia should include a detailed history, physical examination, and assessment of risk factors for all causes of hypercalcemia.1,2 PHPT and malignancy are the two most common causes of asymptomatic true hypercalcemia (TABLE).2
Laboratory evaluation targeting these causes, beginning with an intact PTH level, is a logical first step.1,2 Persistent hypercalcemia in the presence of elevated or inappropriately normal PTH concentrations confirms the diagnosis of PHPT.3 When serum calcium rises, PTH is normally suppressed. Normal intact PTH and low 24-hour urinary calcium excretion distinguishes patients with PHPT from those with less common familial hypocalciuric hypercalcemia.1,2
Most patients with PHPT are asymptomatic, although some eventually develop bone loss, nephrolithiasis, and renal colic.4,5 A 10-year prospective cohort study of patients with PHPT found that 21% of asymptomatic patients developed decreased bone density at one or more sites.6 None acquired kidney stones, but hypercalcemia and hypercalciuria did worsen in 10 of 52 patients. A guideline and a review on PHPT recommend measuring creatinine clearance and obtaining a bone densitometry study of the distal third of the radius, hip, and lumbar spine to assess for end-organ changes related to the condition; declining renal function and osteoporosis may be indications for surgery.3,5
Malignancy is the most common cause of low intact PTH and true hypercalcemia, especially when the calcium level is >14 mg/dL.1 A PTHrP >1.0 pmol/L is highly specific for malignancy because this level does not occur in healthy people.1 In a prospective case series of patients with hypercalcemia and malignancy, 54% had elevated PTHrP levels.7 The authors found that an elevated PTHrP in patients younger than 65 years of age doubles the risk of death from malignancy compared to patients the same age with normal PTHrP (hazard ratio=1.9; 95% CI, 1.1-3.4).
TABLE
Causes of hypercalcemia
| Primary hyperparathyroidism |
Malignancy
|
| Chronic renal failure |
| Endocrine disorders (hyperthyroidism, pheochromocytoma, Addison’s disease) |
| Familial hypocalciuric hypercalcemia |
| Immobilization |
| Laboratory artifact resulting from altered albumin concentration or pH |
| Medications (vitamin A toxicity [dietary fads, isotretinoin overdose], estrogens, antiestrogens, thiazides, lithium) |
| Milk alkali syndrome |
| Vitamin D toxicity (granulomatous disease [sarcoidosis, tuberculosis], vitamin D supplementation) |
| Based on Hutton E,1 and Carroll MF et al.2 |
Identify less common causes
Serum calcitriol in association with a low intact PTH level and elevated calcium lower than 14 mg/dL helps differentiate the less common causes of hypercalcemia. Calcitriol is high in granulomatous diseases such as sarcoidosis, tuberculosis, and histoplasmosis, and normal in hyperthyroidism and Addison’s disease.1
Immobilization as a cause of hypercalcemia can be distinguished from PHPT by history and normal PTH levels and from malignancy by a normal alkaline phosphatase level.1
Recommendations
In addition to the recommendations discussed previously, Williams Textbook of Endocrinology advises repeating the initial calcium level twice and measuring serum BUN, creatinine, electrolytes, albumin, globulin, and phosphate.8 The authors recommend a generalized work-up for malignancy, including mammography, chest radiography with or without CT, abdominal CT, serum and urine immunoelectrophoresis, and temporary discontinuation of lithium for patients taking the drug. They also recommend using PTHrP only when PTH is suppressed but an underlying malignancy can’t be found.
First, establish that true hypercalcemia exists by repeating the serum calcium and measuring or calculating the physiologically active serum calcium when abnormalities in blood pH or albumin are found (SOR: C, expert opinion). Patients with unexplained asymptomatic true hypercalcemia should be screened for primary hyperparathyroidism (PHPT) and malignancy using an intact parathyroid hormone (PTH) level by immunoradioassay (SOR: C, expert opinion). Other recommended tests that can distinguish PHPT from malignancy and familial hypocalciuric hypercalcemia, as well as help manage patients with PHPT include urinary 24-hour calcium and creatinine levels, parathyroid hormone related peptide (PTHrP), alkaline phosphatase, calcitriol, and bone densitometry (SOR: C, expert opinion).
Choose tests carefully to reduce false positives
Jon O. Neher, MD
Valley Family Medicine, Renton, Wash
Including serum calcium measurements in the chemistry panels that physicians use to manage common conditions such as hypertension has resulted in an epidemic of incidental hypercalcemia. Tempting as it may be to ignore these unexpected numbers, they point to a significant underlying condition in some patients. This puts the family physician in a familiar clinical position—having to worry a patient just enough to convince him to consent to a careful, stepwise evaluation while somehow reassuring him that usually no problem is found. The best solution is to order each test for a reason, which would reduce the number of false positives that we spend so much time chasing.
Evidence summary
Make sure it’s true hypercalcemia
Measuring calcium levels in asymptomatic patients often leads to false-positive elevations caused by random error or changes in the level of physiologically active calcium because of alterations in blood pH or serum albumin. Serum calcium levels between 10.0 and 12.0 mg/dL indicate mild hypercalcemia; levels >14.0 mg/dL are severe. Because changes in pH and serum albumin levels alter levels of physiologically active calcium, authoritative sources recommend measuring or calculating physiologically active calcium if blood pH or albumin is abnormal.1,2 To determine the level, use the equation [4.0 – (plasma albumin)] × 0.8 + (serum calcium) or measure serum ionized calcium.2 Normal levels of serum ionized calcium for adults older than 19 years are 1.13 to 1.32 mmol/L, although the exact range can vary from laboratory to laboratory. Elevated physiologically active calcium indicates true hypercalcemia.
Assess for the most common causes, PHPT and malignancy
Evaluation of the patient with true hypercalcemia should include a detailed history, physical examination, and assessment of risk factors for all causes of hypercalcemia.1,2 PHPT and malignancy are the two most common causes of asymptomatic true hypercalcemia (TABLE).2
Laboratory evaluation targeting these causes, beginning with an intact PTH level, is a logical first step.1,2 Persistent hypercalcemia in the presence of elevated or inappropriately normal PTH concentrations confirms the diagnosis of PHPT.3 When serum calcium rises, PTH is normally suppressed. Normal intact PTH and low 24-hour urinary calcium excretion distinguishes patients with PHPT from those with less common familial hypocalciuric hypercalcemia.1,2
Most patients with PHPT are asymptomatic, although some eventually develop bone loss, nephrolithiasis, and renal colic.4,5 A 10-year prospective cohort study of patients with PHPT found that 21% of asymptomatic patients developed decreased bone density at one or more sites.6 None acquired kidney stones, but hypercalcemia and hypercalciuria did worsen in 10 of 52 patients. A guideline and a review on PHPT recommend measuring creatinine clearance and obtaining a bone densitometry study of the distal third of the radius, hip, and lumbar spine to assess for end-organ changes related to the condition; declining renal function and osteoporosis may be indications for surgery.3,5
Malignancy is the most common cause of low intact PTH and true hypercalcemia, especially when the calcium level is >14 mg/dL.1 A PTHrP >1.0 pmol/L is highly specific for malignancy because this level does not occur in healthy people.1 In a prospective case series of patients with hypercalcemia and malignancy, 54% had elevated PTHrP levels.7 The authors found that an elevated PTHrP in patients younger than 65 years of age doubles the risk of death from malignancy compared to patients the same age with normal PTHrP (hazard ratio=1.9; 95% CI, 1.1-3.4).
TABLE
Causes of hypercalcemia
| Primary hyperparathyroidism |
Malignancy
|
| Chronic renal failure |
| Endocrine disorders (hyperthyroidism, pheochromocytoma, Addison’s disease) |
| Familial hypocalciuric hypercalcemia |
| Immobilization |
| Laboratory artifact resulting from altered albumin concentration or pH |
| Medications (vitamin A toxicity [dietary fads, isotretinoin overdose], estrogens, antiestrogens, thiazides, lithium) |
| Milk alkali syndrome |
| Vitamin D toxicity (granulomatous disease [sarcoidosis, tuberculosis], vitamin D supplementation) |
| Based on Hutton E,1 and Carroll MF et al.2 |
Identify less common causes
Serum calcitriol in association with a low intact PTH level and elevated calcium lower than 14 mg/dL helps differentiate the less common causes of hypercalcemia. Calcitriol is high in granulomatous diseases such as sarcoidosis, tuberculosis, and histoplasmosis, and normal in hyperthyroidism and Addison’s disease.1
Immobilization as a cause of hypercalcemia can be distinguished from PHPT by history and normal PTH levels and from malignancy by a normal alkaline phosphatase level.1
Recommendations
In addition to the recommendations discussed previously, Williams Textbook of Endocrinology advises repeating the initial calcium level twice and measuring serum BUN, creatinine, electrolytes, albumin, globulin, and phosphate.8 The authors recommend a generalized work-up for malignancy, including mammography, chest radiography with or without CT, abdominal CT, serum and urine immunoelectrophoresis, and temporary discontinuation of lithium for patients taking the drug. They also recommend using PTHrP only when PTH is suppressed but an underlying malignancy can’t be found.
1. Hutton E. Evaluation and management of hypercalcemia. JAAPA. 2005;18(6):30-35.
2. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
3. Belezikian JP, Potts JT, Fuleihan GE, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353-5361.
4. AACE/AAES task force on primary hyperparathyroidism. The American Association of Clinical endocrinologists and the American Association of endocrine surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11(1):49-54.
5. Silverberg SJ, Bilezikian JP. The diagnosis and management of asympyomatic primary hyperparathyroidism. Nat Clin Pract Endocrinol Metab. 2006;2:494-503.
6. Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249-1255.
7. Truong NU, deB Edwards MD, Papavacillicu V, et al. Parathyroid hormone related peptide and survival of patients with cancer and hypercalcemia. Am J Med. 2003;115:115-121.
8. Bringhurst FR, Dernay MB, Kronenberg HM. Approach to the hypercalcemic patient. In: Williams RH, Larsen PR. Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders;2003:1336-1339.
1. Hutton E. Evaluation and management of hypercalcemia. JAAPA. 2005;18(6):30-35.
2. Carroll MF, Schade DS. A practical approach to hypercalcemia. Am Fam Physician. 2003;67:1959-1966.
3. Belezikian JP, Potts JT, Fuleihan GE, et al. Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab. 2002;87:5353-5361.
4. AACE/AAES task force on primary hyperparathyroidism. The American Association of Clinical endocrinologists and the American Association of endocrine surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11(1):49-54.
5. Silverberg SJ, Bilezikian JP. The diagnosis and management of asympyomatic primary hyperparathyroidism. Nat Clin Pract Endocrinol Metab. 2006;2:494-503.
6. Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249-1255.
7. Truong NU, deB Edwards MD, Papavacillicu V, et al. Parathyroid hormone related peptide and survival of patients with cancer and hypercalcemia. Am J Med. 2003;115:115-121.
8. Bringhurst FR, Dernay MB, Kronenberg HM. Approach to the hypercalcemic patient. In: Williams RH, Larsen PR. Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders;2003:1336-1339.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the best way to treat Achilles tendonopathy?
Rest and ice are considered first-line therapy for acute Achilles tendonopathy (strength of recommendation [SOR]: C, expert opinion), as is nonsteroidal anti-inflammatory drugs (NSAIDs) (SOR: B, systematic review).
Chronic noninsertional Achilles tendonopathy should be treated with eccentric calf-muscle training (ECMT) (SOR: B, 3 randomized controlled trials [RCTs]). Continuing an activity such as running, while monitoring pain, is as effective as resting while enrolled in an eccentric strengthening program (SOR: B, 1 RCT). If conservative management fails, surgery may help (SOR: C, case series).
Treat tendonopathy one step at a time
Charles W. Webb, DO
Oregon Health and Science University, Portland, Ore
I’ve found stepwise treatment of Achilles tendonopathy to be the most useful approach for my patients. Initially, I recommend NSAIDs, stretching, and decreasing the offending activity. If the patient hasn’t improved in 2 weeks, I refer him for physical therapy with a focus on flexibility and eccentric strengthening. I recommend applying ice for 20 minutes after each therapy or exercise session and 2 or 3 more times a day.
If the patient doesn’t improve in another 2 to 3 weeks, I have him (or her) wear a cam walking boot (fracture boot) constantly, except when bathing, for 2 to 6 weeks. The boot maintains the ankle in a neutral position, facilitating the rest required for healing. I’ve found that most patients can avoid surgery by wearing the boot until they are free of pain for a week (but no longer than 6 weeks) and then undergoing aggressive physical therapy to strengthen the ankle.
Evidence summary
While patients may refer to this condition as “Achilles tendonitis,” the phrase is actually a misnomer. That’s because in tendonitis, the inflammation is limited to the surrounding structures, but doesn’t involve the tendon itself.
Acute symptoms respond to NSAIDs
A Cochrane review of treatments for acute Achilles tendonopathy included 9 randomized trials with a total of 697 patients. Two RCTs showed evidence that NSAIDs alleviate acute symptoms. In 1 trial of 212 patients who received an oral NSAID or placebo for 14 days, significantly fewer patients in the placebo group achieved a good or excellent symptom relief (relative risk [RR]=0.42; 95% confidence interval [CI], 0.21-0.86). A second RCT of 243 patients compared treatment with a topical NSAID to placebo for 21 days; significantly fewer participants who took placebo returned to their pre-injury level of activity (RR=0.78; 95% CI, 0.63-0.95). Low-dose heparin injection, heel pads, topical laser therapy, and peritendinous steroid injection produced no significant decrease in pain compared with placebo.1
Continue to exercise?
Patients with chronic noninsertional Achilles tendonopathy may be able to continue activity during eccentric calf-muscle training without exacerbating the tendonopathy, according to an RCT of 38 patients that compared rest with eccentric training and continued activity with eccentric training.
SHOWN HERE: MEDIAL FOOT AND ANKLE WITHOUT INJURY.
Eccentric calf-muscle training helps chronic tendonopathy
ECMT is an effective intervention for chronic noninsertional Achilles tendonopathy. An observational study of ECMT in 78 patients (101 tendons total) with chronic noninsertional Achilles tendonopathy found that 89% were back to their preinjury activity level after a 12-week regimen of ECMT, as indicated by significantly lower scores on a 100-point visual analog scale (67 at baseline vs 10.2 at 12 weeks; P<0.001).2
An RCT compared ECMT or repetitive shock wave therapy to a “wait-and-see” control group (25 patients in each group). After 4 months, both the ECMT and shock wave therapy groups reported a significant decrease in pain compared with controls (P< 0.001; mean difference in 10-point pain score, 2.4; 95% CI, 1.3-3.5). Sixty percent of participants in the ECMT group reported complete recovery or significant improvement (P<0.001; number needed to treat [NNT]=3; mean difference in 6-point Likert scale=–1.6; 95% CI, –2.4 to 0.8).3
During an ECMT program, patients may be able to continue activity without exacerbating Achilles tendonopathy. An RCT of 38 patients compared rest with eccentric training and continued activity and eccentric training for 12 weeks to 6 months using a pain-monitored model. Both groups showed similar improvement in pain and function when measured with the Swedish version of the Achilles assessment questionnaire (VISA-A-S 100-point scale: 0=worst function, 100=completely recovered). The baseline mean VISA-A-S scores were 57 for both groups; the mean scores after 12 months were 85 for the exercise group and 91 for the rest group.4
Another option: The AirHeel brace
An AirHeel brace may be a viable alternative to ECMT, especially if the patient can’t tolerate eccentric muscle training because of pain. The brace was designed for Achilles tendonopathy and posterior heel pain. It applies intermittent compression to minimize swelling and promote circulation.
An RCT evaluated 100 patients with chronic noninsertional Achilles tendonopathy who were treated with ECMT, an AirHeel Brace, or a combination of the 2 for 12 weeks. At 1-year follow-up, all 3 groups showed a significant reduction in pain measured by a 10-point visual analog scale (ECMT 30%, brace 27%, combination 53%; P<0.0001). All the groups also showed significantly improved function as measured by an American Orthopaedic Foot and Ankle Society Score (10%, 10%, and 30%, respectively; P<0.001). No significant differences in outcomes were found across the treatment groups, and 90% of participants reached their preinjury activity level after 1 year.4
Shock wave therapy and topical glyceryl trinitrate
The role of repetitive shock wave therapy and topical glyceryl trinitrate in the treatment of chronic noninsertional Achilles tendonopathy is less clear. In a double-blinded RCT of 49 patients, shock wave therapy did not produce better outcomes than placebo.5
However, a prospective cohort study of 68 patients found that patients who received shock wave therapy had significantly lower mean visual analog scale scores for pain compared with controls after 1 month (P<0.001), 3 months (P<0.001), and 12 months (P<0.001) of treatment.6 In a double-blinded RCT of 65 patients, more patients treated with topical glyceryl trinitrate therapy were asymptomatic during activities of daily living at 6 months compared with placebo (P<0.001).7
Both shock wave therapy and topical glyceryl trinitrate may have a significant role to play in treating chronic noninsertional Achilles tendonopathy, but more studies are needed to support their use.
Surgery appears to work better for athletes
Surgery is an option for patients with chronic noninsertional Achilles tendonopathy who have failed conservative measures and a 3- to 6-month rehabilitation program. In a systematic review of 26 studies of patients managed surgically, the mean success rate (full return to preinjury activity level) was 77%. However, a negative correlation was observed between the reported success rate and the overall methods score, a rating of the quality of studies (r=0.53; P<0.01). Only 5 of the studies reviewed were prospective cohort studies; the remaining 21 were retrospective cohort studies and case studies.8
Athletes responded significantly better to surgery than nonathletic patients, returning to full activity in 4.5 months compared with 7.1 months for nonathletes (P<0.03). Fewer athletes had surgical complications (9% compared with 19% of nonathletes).9 More studies are needed to clarify the role of surgery in managing Achilles tendonopathy.
Recommendations
The American College of Foot and Ankle Surgeons recommends initial management by reducing pressure around the affected area combined with heel lifts, orthotics, NSAID therapy, and physical therapy. Immobilization may be used on a case-by-case basis. Local steroid injections aren’t recommended. Patients with resistant tendonopathy should be referred to a foot and ankle surgeon.10
1. McLaughlan G, Handoll H. Interventions for treating acute and chronic Achilles tendonitis. Cochrane Database Syst Rev. 2001;(2):CD000232.-
2. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
3. Rompe J, Nafe B, Furia J, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis. Am J Sports Med. 2007;35:374-383.
4. Silbernagel K, Thomee R, Eriksson B, et al. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy. Am J Sports Med. 2007;35:897-906.
5. Costa M, Shepstone L, Donnell S, et al. Shock wave therapy for chronic Achilles tendon pain. Clin Orthop. 2005;440:199-204.
6. Furia J. High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 2006;34:733-740.
7. Paoloni J, Appleyard R, Nelson J, et al. Topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. J Bone Joint Surg Am. 2004;86:916-922.
8. Tallon C, Coleman B, Khan K, et al. Outcome of surgery for chronic Achilles tendinopathy. Am J Sports Med. 2001;29:315-320.
9. Maffuli N, Testa V, Capasso G, et al. Surgery for chronic Achilles tendinopathy yields worse results in nonathletic patients. Clin J Sport Med. 2006;16:123-128.
10. Thomas J, Christensen J, Kravitz S, et al. The diagnosis and treatment of heel pain. J Foot Ankle Surg. 2001;40:329-337.
Rest and ice are considered first-line therapy for acute Achilles tendonopathy (strength of recommendation [SOR]: C, expert opinion), as is nonsteroidal anti-inflammatory drugs (NSAIDs) (SOR: B, systematic review).
Chronic noninsertional Achilles tendonopathy should be treated with eccentric calf-muscle training (ECMT) (SOR: B, 3 randomized controlled trials [RCTs]). Continuing an activity such as running, while monitoring pain, is as effective as resting while enrolled in an eccentric strengthening program (SOR: B, 1 RCT). If conservative management fails, surgery may help (SOR: C, case series).
Treat tendonopathy one step at a time
Charles W. Webb, DO
Oregon Health and Science University, Portland, Ore
I’ve found stepwise treatment of Achilles tendonopathy to be the most useful approach for my patients. Initially, I recommend NSAIDs, stretching, and decreasing the offending activity. If the patient hasn’t improved in 2 weeks, I refer him for physical therapy with a focus on flexibility and eccentric strengthening. I recommend applying ice for 20 minutes after each therapy or exercise session and 2 or 3 more times a day.
If the patient doesn’t improve in another 2 to 3 weeks, I have him (or her) wear a cam walking boot (fracture boot) constantly, except when bathing, for 2 to 6 weeks. The boot maintains the ankle in a neutral position, facilitating the rest required for healing. I’ve found that most patients can avoid surgery by wearing the boot until they are free of pain for a week (but no longer than 6 weeks) and then undergoing aggressive physical therapy to strengthen the ankle.
Evidence summary
While patients may refer to this condition as “Achilles tendonitis,” the phrase is actually a misnomer. That’s because in tendonitis, the inflammation is limited to the surrounding structures, but doesn’t involve the tendon itself.
Acute symptoms respond to NSAIDs
A Cochrane review of treatments for acute Achilles tendonopathy included 9 randomized trials with a total of 697 patients. Two RCTs showed evidence that NSAIDs alleviate acute symptoms. In 1 trial of 212 patients who received an oral NSAID or placebo for 14 days, significantly fewer patients in the placebo group achieved a good or excellent symptom relief (relative risk [RR]=0.42; 95% confidence interval [CI], 0.21-0.86). A second RCT of 243 patients compared treatment with a topical NSAID to placebo for 21 days; significantly fewer participants who took placebo returned to their pre-injury level of activity (RR=0.78; 95% CI, 0.63-0.95). Low-dose heparin injection, heel pads, topical laser therapy, and peritendinous steroid injection produced no significant decrease in pain compared with placebo.1
Continue to exercise?
Patients with chronic noninsertional Achilles tendonopathy may be able to continue activity during eccentric calf-muscle training without exacerbating the tendonopathy, according to an RCT of 38 patients that compared rest with eccentric training and continued activity with eccentric training.
SHOWN HERE: MEDIAL FOOT AND ANKLE WITHOUT INJURY.
Eccentric calf-muscle training helps chronic tendonopathy
ECMT is an effective intervention for chronic noninsertional Achilles tendonopathy. An observational study of ECMT in 78 patients (101 tendons total) with chronic noninsertional Achilles tendonopathy found that 89% were back to their preinjury activity level after a 12-week regimen of ECMT, as indicated by significantly lower scores on a 100-point visual analog scale (67 at baseline vs 10.2 at 12 weeks; P<0.001).2
An RCT compared ECMT or repetitive shock wave therapy to a “wait-and-see” control group (25 patients in each group). After 4 months, both the ECMT and shock wave therapy groups reported a significant decrease in pain compared with controls (P< 0.001; mean difference in 10-point pain score, 2.4; 95% CI, 1.3-3.5). Sixty percent of participants in the ECMT group reported complete recovery or significant improvement (P<0.001; number needed to treat [NNT]=3; mean difference in 6-point Likert scale=–1.6; 95% CI, –2.4 to 0.8).3
During an ECMT program, patients may be able to continue activity without exacerbating Achilles tendonopathy. An RCT of 38 patients compared rest with eccentric training and continued activity and eccentric training for 12 weeks to 6 months using a pain-monitored model. Both groups showed similar improvement in pain and function when measured with the Swedish version of the Achilles assessment questionnaire (VISA-A-S 100-point scale: 0=worst function, 100=completely recovered). The baseline mean VISA-A-S scores were 57 for both groups; the mean scores after 12 months were 85 for the exercise group and 91 for the rest group.4
Another option: The AirHeel brace
An AirHeel brace may be a viable alternative to ECMT, especially if the patient can’t tolerate eccentric muscle training because of pain. The brace was designed for Achilles tendonopathy and posterior heel pain. It applies intermittent compression to minimize swelling and promote circulation.
An RCT evaluated 100 patients with chronic noninsertional Achilles tendonopathy who were treated with ECMT, an AirHeel Brace, or a combination of the 2 for 12 weeks. At 1-year follow-up, all 3 groups showed a significant reduction in pain measured by a 10-point visual analog scale (ECMT 30%, brace 27%, combination 53%; P<0.0001). All the groups also showed significantly improved function as measured by an American Orthopaedic Foot and Ankle Society Score (10%, 10%, and 30%, respectively; P<0.001). No significant differences in outcomes were found across the treatment groups, and 90% of participants reached their preinjury activity level after 1 year.4
Shock wave therapy and topical glyceryl trinitrate
The role of repetitive shock wave therapy and topical glyceryl trinitrate in the treatment of chronic noninsertional Achilles tendonopathy is less clear. In a double-blinded RCT of 49 patients, shock wave therapy did not produce better outcomes than placebo.5
However, a prospective cohort study of 68 patients found that patients who received shock wave therapy had significantly lower mean visual analog scale scores for pain compared with controls after 1 month (P<0.001), 3 months (P<0.001), and 12 months (P<0.001) of treatment.6 In a double-blinded RCT of 65 patients, more patients treated with topical glyceryl trinitrate therapy were asymptomatic during activities of daily living at 6 months compared with placebo (P<0.001).7
Both shock wave therapy and topical glyceryl trinitrate may have a significant role to play in treating chronic noninsertional Achilles tendonopathy, but more studies are needed to support their use.
Surgery appears to work better for athletes
Surgery is an option for patients with chronic noninsertional Achilles tendonopathy who have failed conservative measures and a 3- to 6-month rehabilitation program. In a systematic review of 26 studies of patients managed surgically, the mean success rate (full return to preinjury activity level) was 77%. However, a negative correlation was observed between the reported success rate and the overall methods score, a rating of the quality of studies (r=0.53; P<0.01). Only 5 of the studies reviewed were prospective cohort studies; the remaining 21 were retrospective cohort studies and case studies.8
Athletes responded significantly better to surgery than nonathletic patients, returning to full activity in 4.5 months compared with 7.1 months for nonathletes (P<0.03). Fewer athletes had surgical complications (9% compared with 19% of nonathletes).9 More studies are needed to clarify the role of surgery in managing Achilles tendonopathy.
Recommendations
The American College of Foot and Ankle Surgeons recommends initial management by reducing pressure around the affected area combined with heel lifts, orthotics, NSAID therapy, and physical therapy. Immobilization may be used on a case-by-case basis. Local steroid injections aren’t recommended. Patients with resistant tendonopathy should be referred to a foot and ankle surgeon.10
Rest and ice are considered first-line therapy for acute Achilles tendonopathy (strength of recommendation [SOR]: C, expert opinion), as is nonsteroidal anti-inflammatory drugs (NSAIDs) (SOR: B, systematic review).
Chronic noninsertional Achilles tendonopathy should be treated with eccentric calf-muscle training (ECMT) (SOR: B, 3 randomized controlled trials [RCTs]). Continuing an activity such as running, while monitoring pain, is as effective as resting while enrolled in an eccentric strengthening program (SOR: B, 1 RCT). If conservative management fails, surgery may help (SOR: C, case series).
Treat tendonopathy one step at a time
Charles W. Webb, DO
Oregon Health and Science University, Portland, Ore
I’ve found stepwise treatment of Achilles tendonopathy to be the most useful approach for my patients. Initially, I recommend NSAIDs, stretching, and decreasing the offending activity. If the patient hasn’t improved in 2 weeks, I refer him for physical therapy with a focus on flexibility and eccentric strengthening. I recommend applying ice for 20 minutes after each therapy or exercise session and 2 or 3 more times a day.
If the patient doesn’t improve in another 2 to 3 weeks, I have him (or her) wear a cam walking boot (fracture boot) constantly, except when bathing, for 2 to 6 weeks. The boot maintains the ankle in a neutral position, facilitating the rest required for healing. I’ve found that most patients can avoid surgery by wearing the boot until they are free of pain for a week (but no longer than 6 weeks) and then undergoing aggressive physical therapy to strengthen the ankle.
Evidence summary
While patients may refer to this condition as “Achilles tendonitis,” the phrase is actually a misnomer. That’s because in tendonitis, the inflammation is limited to the surrounding structures, but doesn’t involve the tendon itself.
Acute symptoms respond to NSAIDs
A Cochrane review of treatments for acute Achilles tendonopathy included 9 randomized trials with a total of 697 patients. Two RCTs showed evidence that NSAIDs alleviate acute symptoms. In 1 trial of 212 patients who received an oral NSAID or placebo for 14 days, significantly fewer patients in the placebo group achieved a good or excellent symptom relief (relative risk [RR]=0.42; 95% confidence interval [CI], 0.21-0.86). A second RCT of 243 patients compared treatment with a topical NSAID to placebo for 21 days; significantly fewer participants who took placebo returned to their pre-injury level of activity (RR=0.78; 95% CI, 0.63-0.95). Low-dose heparin injection, heel pads, topical laser therapy, and peritendinous steroid injection produced no significant decrease in pain compared with placebo.1
Continue to exercise?
Patients with chronic noninsertional Achilles tendonopathy may be able to continue activity during eccentric calf-muscle training without exacerbating the tendonopathy, according to an RCT of 38 patients that compared rest with eccentric training and continued activity with eccentric training.
SHOWN HERE: MEDIAL FOOT AND ANKLE WITHOUT INJURY.
Eccentric calf-muscle training helps chronic tendonopathy
ECMT is an effective intervention for chronic noninsertional Achilles tendonopathy. An observational study of ECMT in 78 patients (101 tendons total) with chronic noninsertional Achilles tendonopathy found that 89% were back to their preinjury activity level after a 12-week regimen of ECMT, as indicated by significantly lower scores on a 100-point visual analog scale (67 at baseline vs 10.2 at 12 weeks; P<0.001).2
An RCT compared ECMT or repetitive shock wave therapy to a “wait-and-see” control group (25 patients in each group). After 4 months, both the ECMT and shock wave therapy groups reported a significant decrease in pain compared with controls (P< 0.001; mean difference in 10-point pain score, 2.4; 95% CI, 1.3-3.5). Sixty percent of participants in the ECMT group reported complete recovery or significant improvement (P<0.001; number needed to treat [NNT]=3; mean difference in 6-point Likert scale=–1.6; 95% CI, –2.4 to 0.8).3
During an ECMT program, patients may be able to continue activity without exacerbating Achilles tendonopathy. An RCT of 38 patients compared rest with eccentric training and continued activity and eccentric training for 12 weeks to 6 months using a pain-monitored model. Both groups showed similar improvement in pain and function when measured with the Swedish version of the Achilles assessment questionnaire (VISA-A-S 100-point scale: 0=worst function, 100=completely recovered). The baseline mean VISA-A-S scores were 57 for both groups; the mean scores after 12 months were 85 for the exercise group and 91 for the rest group.4
Another option: The AirHeel brace
An AirHeel brace may be a viable alternative to ECMT, especially if the patient can’t tolerate eccentric muscle training because of pain. The brace was designed for Achilles tendonopathy and posterior heel pain. It applies intermittent compression to minimize swelling and promote circulation.
An RCT evaluated 100 patients with chronic noninsertional Achilles tendonopathy who were treated with ECMT, an AirHeel Brace, or a combination of the 2 for 12 weeks. At 1-year follow-up, all 3 groups showed a significant reduction in pain measured by a 10-point visual analog scale (ECMT 30%, brace 27%, combination 53%; P<0.0001). All the groups also showed significantly improved function as measured by an American Orthopaedic Foot and Ankle Society Score (10%, 10%, and 30%, respectively; P<0.001). No significant differences in outcomes were found across the treatment groups, and 90% of participants reached their preinjury activity level after 1 year.4
Shock wave therapy and topical glyceryl trinitrate
The role of repetitive shock wave therapy and topical glyceryl trinitrate in the treatment of chronic noninsertional Achilles tendonopathy is less clear. In a double-blinded RCT of 49 patients, shock wave therapy did not produce better outcomes than placebo.5
However, a prospective cohort study of 68 patients found that patients who received shock wave therapy had significantly lower mean visual analog scale scores for pain compared with controls after 1 month (P<0.001), 3 months (P<0.001), and 12 months (P<0.001) of treatment.6 In a double-blinded RCT of 65 patients, more patients treated with topical glyceryl trinitrate therapy were asymptomatic during activities of daily living at 6 months compared with placebo (P<0.001).7
Both shock wave therapy and topical glyceryl trinitrate may have a significant role to play in treating chronic noninsertional Achilles tendonopathy, but more studies are needed to support their use.
Surgery appears to work better for athletes
Surgery is an option for patients with chronic noninsertional Achilles tendonopathy who have failed conservative measures and a 3- to 6-month rehabilitation program. In a systematic review of 26 studies of patients managed surgically, the mean success rate (full return to preinjury activity level) was 77%. However, a negative correlation was observed between the reported success rate and the overall methods score, a rating of the quality of studies (r=0.53; P<0.01). Only 5 of the studies reviewed were prospective cohort studies; the remaining 21 were retrospective cohort studies and case studies.8
Athletes responded significantly better to surgery than nonathletic patients, returning to full activity in 4.5 months compared with 7.1 months for nonathletes (P<0.03). Fewer athletes had surgical complications (9% compared with 19% of nonathletes).9 More studies are needed to clarify the role of surgery in managing Achilles tendonopathy.
Recommendations
The American College of Foot and Ankle Surgeons recommends initial management by reducing pressure around the affected area combined with heel lifts, orthotics, NSAID therapy, and physical therapy. Immobilization may be used on a case-by-case basis. Local steroid injections aren’t recommended. Patients with resistant tendonopathy should be referred to a foot and ankle surgeon.10
1. McLaughlan G, Handoll H. Interventions for treating acute and chronic Achilles tendonitis. Cochrane Database Syst Rev. 2001;(2):CD000232.-
2. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
3. Rompe J, Nafe B, Furia J, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis. Am J Sports Med. 2007;35:374-383.
4. Silbernagel K, Thomee R, Eriksson B, et al. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy. Am J Sports Med. 2007;35:897-906.
5. Costa M, Shepstone L, Donnell S, et al. Shock wave therapy for chronic Achilles tendon pain. Clin Orthop. 2005;440:199-204.
6. Furia J. High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 2006;34:733-740.
7. Paoloni J, Appleyard R, Nelson J, et al. Topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. J Bone Joint Surg Am. 2004;86:916-922.
8. Tallon C, Coleman B, Khan K, et al. Outcome of surgery for chronic Achilles tendinopathy. Am J Sports Med. 2001;29:315-320.
9. Maffuli N, Testa V, Capasso G, et al. Surgery for chronic Achilles tendinopathy yields worse results in nonathletic patients. Clin J Sport Med. 2006;16:123-128.
10. Thomas J, Christensen J, Kravitz S, et al. The diagnosis and treatment of heel pain. J Foot Ankle Surg. 2001;40:329-337.
1. McLaughlan G, Handoll H. Interventions for treating acute and chronic Achilles tendonitis. Cochrane Database Syst Rev. 2001;(2):CD000232.-
2. Fahlstrom M, Jonsson P, Lorentzon R, et al. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surg Sports Traumatol Arthrosc. 2003;11:327-333.
3. Rompe J, Nafe B, Furia J, et al. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis. Am J Sports Med. 2007;35:374-383.
4. Silbernagel K, Thomee R, Eriksson B, et al. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with Achilles tendinopathy. Am J Sports Med. 2007;35:897-906.
5. Costa M, Shepstone L, Donnell S, et al. Shock wave therapy for chronic Achilles tendon pain. Clin Orthop. 2005;440:199-204.
6. Furia J. High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 2006;34:733-740.
7. Paoloni J, Appleyard R, Nelson J, et al. Topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. J Bone Joint Surg Am. 2004;86:916-922.
8. Tallon C, Coleman B, Khan K, et al. Outcome of surgery for chronic Achilles tendinopathy. Am J Sports Med. 2001;29:315-320.
9. Maffuli N, Testa V, Capasso G, et al. Surgery for chronic Achilles tendinopathy yields worse results in nonathletic patients. Clin J Sport Med. 2006;16:123-128.
10. Thomas J, Christensen J, Kravitz S, et al. The diagnosis and treatment of heel pain. J Foot Ankle Surg. 2001;40:329-337.
Evidence-based answers from the Family Physicians Inquiries Network
What’s the most effective treatment for giardiasis?
A single 2-g dose of tinidazole is the best treatment (strength of recommendation [SOR]: A, based on meta-analysis). Other drugs, such as nitazoxanide, metronidazole, mebendazole, and albendazole, can also be used (SOR: A, based on randomized controlled trial [RCT] of patient-oriented outcomes), but tinidazole has a higher clinical cure rate than these drugs. It also has a comparable side-effect profile and requires only 1 dose.
The real challenge is diagnosis
Cynthia Brown, MD
University of Nevada, Reno
As this review points out, all the available treatments for giardiasis are effective. Additional prescribing considerations include cost (500 mg metronidazole costs about 30 cents, for example, while 2 mg tinidazole costs $18) and insurance coverage. Tinidazole and metronidazole, unlike the other medications, require that the patient abstain from alcohol for 72 hours after dosing.
In my experience, the biggest challenge in treating giardiasis is deciding when to consider it in the differential and when to test for it. Presentations vary from vague symptoms such as bloating to severe diarrhea. Often the patient has not been exposed to well or stream water. You can test stool samples for ova and parasites, or serum for fluorescent antibody or enzyme-linked immunosorbent assay (ELISA).
Evidence summary
Giardia lamblia is a protozoan parasite found worldwide. Infection typically results from ingesting cysts in contaminated food or water. Patients with giardiasis may be asymptomatic or have mild to severe gastrointestinal symptoms, including explosive diarrhea, abdominal pain, steatorrhea, flatulence, bloating, nausea, and vomiting. Treatment varies widely based on geographic location, physician preference, and availability and cost of medication (TABLE).1
TABLE 1
Drugs commonly used to treat giardiasis
| DRUG | ADULT DOSE | SCHEDULE | COMMENT |
|---|---|---|---|
| Tinidazole | 2 g | 1 time | Can be given to children 3 years of age and older Pregnancy drug class C |
| Metronidazole | 250, 500,or 750 mg | 1 time or 3 times daily for 5 days. (Usually 250 mg, 3 times a day, for 5 days) | Contraindicated in first trimester of pregnancy |
| Mebendazole | 100 mg | Twice daily for 5 days | Contraindicated in first trimester of pregnancy Pregnancy drug class B |
| Nitazoxanide | 500 mg | Twice daily for 3 days | Can be given to children 1 year of age and older Available in liquid form Pregnancy drug class B |
| Albendazole | 200-400 mg | Twice daily for 5 days | Pregnancy drug class C |
| Sources: Beach M,1 and Gilbert DM et al.8 | |||
Tinidazole is the treatment of choice
A 2006 Cochrane Review compared 34 trials of many drug therapies for giardiasis.2 The review, which is being updated to include additional publications, evaluated both head-to-head and placebo-controlled studies, looking at dosage as well as length of drug therapy.
The review found that a single dose of tinidazole had a higher clinical cure rate than other therapies such as metronidazole (odds ratio [OR]=5.33; 95% confidence interval [CI], 2.66-10.67)2 along with a comparable side-effect profile. These findings favor tinidazole as the treatment of choice for symptomatic giardiasis.
How effective are other drugs?
The 2006 Cochrane Review found no difference in clinical cure rate between short-term treatment (3 days) with metronidazole and longer therapy with metronidazole or other drugs. Subsequently, a single dose of metronidazole was found to be as effective as treatment for 5 days or longer (OR=0.33, 95% CI 0.08-1.34).
Since publication of the Cochrane review, several studies have further evaluated mebendazole.
- An RCT in Cuban children 5 to 15 years of age found no difference in clinical cure rate between a 5-day course of mebendazole and more traditional therapy with quinacrine.3
- Another RCT comparing 5 days of mebendazole with 7 days of metronidazole in 7- to 12-year-old Iranian children showed no statistical difference in microbiologic cure between the 2 regimens.4
- Single-dose tinidazole was superior to 3 doses of mebendazole in a single day in an RCT of 122 Cuban children that measured microbiologic cure (NNT=5.5 patients with tinidazole vs mebendazole).5
Two RCTs found nitazoxanide to be effective (number needed to treat [NNT]=1.82) compared to placebo in adolescents and adults.6 A 3-day course of nitazoxanide was as effective as 5 days of metronidazole (80% vs 85%, P=0.61) in resolving clinical giardiasis.7
An RCT of albendazole, 400 mg for 5 days, in 28 adults found it to be as effective as 500 mg metronidazole given 3 times a day for 5 days (80% vs 83%) but less likely than metronidazole (2% vs 18%) to cause anorexia (number needed to harm [NNH]=6.25).
Recommendations
The Centers for Disease Control and Prevention recommends tinidazole, metronidazole, quinacrine, albendazole, or nitazoxanide to treat giardiasis; however, it doesn’t indicate a preference for 1 medicine over another.1 The Infectious Diseases Society of America has no guideline. The Sanford Guide to Antimicrobial Therapy recommends either a single 2-g dose of tinidazole or 500 mg of nitazoxanide PO bid for 3 days as primary treatment.8
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force Medical Service, nor the US Air Force.
1. Beach M. Prevention of specific infectious diseases—giardiasis. In: Arguin PM, Kozarsky PE, Navin AW eds. Centers for Disease Control and Prevention. Health Information for International Travel 2005-2006. Atlanta: US Department of Health and Human Services, Public Health Service; 2005. Available at: www2.ncid.cdc.gov/travel/yb/utils/ybGet.asp?section=dis&obj=giardiasis.htm. Accessed March 7, 2008.
2. Zaat JO, Mank T, Assendelft WJ. Drugs for treating giardiasis. Cochrane Database Syst Rev. 2005:CD000217.-
3. Canete R, Escobedo A, Gonzalez M, et al. Randomized clinical study of five days’ therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol. 2006;12:6366-6370.
4. Sadjjadi SM, Alborzi AW, Mostovfi H. Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr. 2001;47:176-178.
5. Canete R, Escobedo A, Gonzalez M, et al. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin. 2006;22:2131-2136.
6. Rossignol JF, Ayoub A, Ayers MS, et al. Treatment of diarrhea caused by Giardia intestinalis and Entameba histolytica or E dispar: A Randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381-384.
7. Ortiz JJ, Ayoub A, Gargala G, et al. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment Pharmacol Ther. 2001;15:1409-1415.
8. Gilbert DM, Eliopoulos GM, Moellering RC, et al. The Sanford Guide to Antimicrobial Therapy 2006. 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006:95.
A single 2-g dose of tinidazole is the best treatment (strength of recommendation [SOR]: A, based on meta-analysis). Other drugs, such as nitazoxanide, metronidazole, mebendazole, and albendazole, can also be used (SOR: A, based on randomized controlled trial [RCT] of patient-oriented outcomes), but tinidazole has a higher clinical cure rate than these drugs. It also has a comparable side-effect profile and requires only 1 dose.
The real challenge is diagnosis
Cynthia Brown, MD
University of Nevada, Reno
As this review points out, all the available treatments for giardiasis are effective. Additional prescribing considerations include cost (500 mg metronidazole costs about 30 cents, for example, while 2 mg tinidazole costs $18) and insurance coverage. Tinidazole and metronidazole, unlike the other medications, require that the patient abstain from alcohol for 72 hours after dosing.
In my experience, the biggest challenge in treating giardiasis is deciding when to consider it in the differential and when to test for it. Presentations vary from vague symptoms such as bloating to severe diarrhea. Often the patient has not been exposed to well or stream water. You can test stool samples for ova and parasites, or serum for fluorescent antibody or enzyme-linked immunosorbent assay (ELISA).
Evidence summary
Giardia lamblia is a protozoan parasite found worldwide. Infection typically results from ingesting cysts in contaminated food or water. Patients with giardiasis may be asymptomatic or have mild to severe gastrointestinal symptoms, including explosive diarrhea, abdominal pain, steatorrhea, flatulence, bloating, nausea, and vomiting. Treatment varies widely based on geographic location, physician preference, and availability and cost of medication (TABLE).1
TABLE 1
Drugs commonly used to treat giardiasis
| DRUG | ADULT DOSE | SCHEDULE | COMMENT |
|---|---|---|---|
| Tinidazole | 2 g | 1 time | Can be given to children 3 years of age and older Pregnancy drug class C |
| Metronidazole | 250, 500,or 750 mg | 1 time or 3 times daily for 5 days. (Usually 250 mg, 3 times a day, for 5 days) | Contraindicated in first trimester of pregnancy |
| Mebendazole | 100 mg | Twice daily for 5 days | Contraindicated in first trimester of pregnancy Pregnancy drug class B |
| Nitazoxanide | 500 mg | Twice daily for 3 days | Can be given to children 1 year of age and older Available in liquid form Pregnancy drug class B |
| Albendazole | 200-400 mg | Twice daily for 5 days | Pregnancy drug class C |
| Sources: Beach M,1 and Gilbert DM et al.8 | |||
Tinidazole is the treatment of choice
A 2006 Cochrane Review compared 34 trials of many drug therapies for giardiasis.2 The review, which is being updated to include additional publications, evaluated both head-to-head and placebo-controlled studies, looking at dosage as well as length of drug therapy.
The review found that a single dose of tinidazole had a higher clinical cure rate than other therapies such as metronidazole (odds ratio [OR]=5.33; 95% confidence interval [CI], 2.66-10.67)2 along with a comparable side-effect profile. These findings favor tinidazole as the treatment of choice for symptomatic giardiasis.
How effective are other drugs?
The 2006 Cochrane Review found no difference in clinical cure rate between short-term treatment (3 days) with metronidazole and longer therapy with metronidazole or other drugs. Subsequently, a single dose of metronidazole was found to be as effective as treatment for 5 days or longer (OR=0.33, 95% CI 0.08-1.34).
Since publication of the Cochrane review, several studies have further evaluated mebendazole.
- An RCT in Cuban children 5 to 15 years of age found no difference in clinical cure rate between a 5-day course of mebendazole and more traditional therapy with quinacrine.3
- Another RCT comparing 5 days of mebendazole with 7 days of metronidazole in 7- to 12-year-old Iranian children showed no statistical difference in microbiologic cure between the 2 regimens.4
- Single-dose tinidazole was superior to 3 doses of mebendazole in a single day in an RCT of 122 Cuban children that measured microbiologic cure (NNT=5.5 patients with tinidazole vs mebendazole).5
Two RCTs found nitazoxanide to be effective (number needed to treat [NNT]=1.82) compared to placebo in adolescents and adults.6 A 3-day course of nitazoxanide was as effective as 5 days of metronidazole (80% vs 85%, P=0.61) in resolving clinical giardiasis.7
An RCT of albendazole, 400 mg for 5 days, in 28 adults found it to be as effective as 500 mg metronidazole given 3 times a day for 5 days (80% vs 83%) but less likely than metronidazole (2% vs 18%) to cause anorexia (number needed to harm [NNH]=6.25).
Recommendations
The Centers for Disease Control and Prevention recommends tinidazole, metronidazole, quinacrine, albendazole, or nitazoxanide to treat giardiasis; however, it doesn’t indicate a preference for 1 medicine over another.1 The Infectious Diseases Society of America has no guideline. The Sanford Guide to Antimicrobial Therapy recommends either a single 2-g dose of tinidazole or 500 mg of nitazoxanide PO bid for 3 days as primary treatment.8
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force Medical Service, nor the US Air Force.
A single 2-g dose of tinidazole is the best treatment (strength of recommendation [SOR]: A, based on meta-analysis). Other drugs, such as nitazoxanide, metronidazole, mebendazole, and albendazole, can also be used (SOR: A, based on randomized controlled trial [RCT] of patient-oriented outcomes), but tinidazole has a higher clinical cure rate than these drugs. It also has a comparable side-effect profile and requires only 1 dose.
The real challenge is diagnosis
Cynthia Brown, MD
University of Nevada, Reno
As this review points out, all the available treatments for giardiasis are effective. Additional prescribing considerations include cost (500 mg metronidazole costs about 30 cents, for example, while 2 mg tinidazole costs $18) and insurance coverage. Tinidazole and metronidazole, unlike the other medications, require that the patient abstain from alcohol for 72 hours after dosing.
In my experience, the biggest challenge in treating giardiasis is deciding when to consider it in the differential and when to test for it. Presentations vary from vague symptoms such as bloating to severe diarrhea. Often the patient has not been exposed to well or stream water. You can test stool samples for ova and parasites, or serum for fluorescent antibody or enzyme-linked immunosorbent assay (ELISA).
Evidence summary
Giardia lamblia is a protozoan parasite found worldwide. Infection typically results from ingesting cysts in contaminated food or water. Patients with giardiasis may be asymptomatic or have mild to severe gastrointestinal symptoms, including explosive diarrhea, abdominal pain, steatorrhea, flatulence, bloating, nausea, and vomiting. Treatment varies widely based on geographic location, physician preference, and availability and cost of medication (TABLE).1
TABLE 1
Drugs commonly used to treat giardiasis
| DRUG | ADULT DOSE | SCHEDULE | COMMENT |
|---|---|---|---|
| Tinidazole | 2 g | 1 time | Can be given to children 3 years of age and older Pregnancy drug class C |
| Metronidazole | 250, 500,or 750 mg | 1 time or 3 times daily for 5 days. (Usually 250 mg, 3 times a day, for 5 days) | Contraindicated in first trimester of pregnancy |
| Mebendazole | 100 mg | Twice daily for 5 days | Contraindicated in first trimester of pregnancy Pregnancy drug class B |
| Nitazoxanide | 500 mg | Twice daily for 3 days | Can be given to children 1 year of age and older Available in liquid form Pregnancy drug class B |
| Albendazole | 200-400 mg | Twice daily for 5 days | Pregnancy drug class C |
| Sources: Beach M,1 and Gilbert DM et al.8 | |||
Tinidazole is the treatment of choice
A 2006 Cochrane Review compared 34 trials of many drug therapies for giardiasis.2 The review, which is being updated to include additional publications, evaluated both head-to-head and placebo-controlled studies, looking at dosage as well as length of drug therapy.
The review found that a single dose of tinidazole had a higher clinical cure rate than other therapies such as metronidazole (odds ratio [OR]=5.33; 95% confidence interval [CI], 2.66-10.67)2 along with a comparable side-effect profile. These findings favor tinidazole as the treatment of choice for symptomatic giardiasis.
How effective are other drugs?
The 2006 Cochrane Review found no difference in clinical cure rate between short-term treatment (3 days) with metronidazole and longer therapy with metronidazole or other drugs. Subsequently, a single dose of metronidazole was found to be as effective as treatment for 5 days or longer (OR=0.33, 95% CI 0.08-1.34).
Since publication of the Cochrane review, several studies have further evaluated mebendazole.
- An RCT in Cuban children 5 to 15 years of age found no difference in clinical cure rate between a 5-day course of mebendazole and more traditional therapy with quinacrine.3
- Another RCT comparing 5 days of mebendazole with 7 days of metronidazole in 7- to 12-year-old Iranian children showed no statistical difference in microbiologic cure between the 2 regimens.4
- Single-dose tinidazole was superior to 3 doses of mebendazole in a single day in an RCT of 122 Cuban children that measured microbiologic cure (NNT=5.5 patients with tinidazole vs mebendazole).5
Two RCTs found nitazoxanide to be effective (number needed to treat [NNT]=1.82) compared to placebo in adolescents and adults.6 A 3-day course of nitazoxanide was as effective as 5 days of metronidazole (80% vs 85%, P=0.61) in resolving clinical giardiasis.7
An RCT of albendazole, 400 mg for 5 days, in 28 adults found it to be as effective as 500 mg metronidazole given 3 times a day for 5 days (80% vs 83%) but less likely than metronidazole (2% vs 18%) to cause anorexia (number needed to harm [NNH]=6.25).
Recommendations
The Centers for Disease Control and Prevention recommends tinidazole, metronidazole, quinacrine, albendazole, or nitazoxanide to treat giardiasis; however, it doesn’t indicate a preference for 1 medicine over another.1 The Infectious Diseases Society of America has no guideline. The Sanford Guide to Antimicrobial Therapy recommends either a single 2-g dose of tinidazole or 500 mg of nitazoxanide PO bid for 3 days as primary treatment.8
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force Medical Service, nor the US Air Force.
1. Beach M. Prevention of specific infectious diseases—giardiasis. In: Arguin PM, Kozarsky PE, Navin AW eds. Centers for Disease Control and Prevention. Health Information for International Travel 2005-2006. Atlanta: US Department of Health and Human Services, Public Health Service; 2005. Available at: www2.ncid.cdc.gov/travel/yb/utils/ybGet.asp?section=dis&obj=giardiasis.htm. Accessed March 7, 2008.
2. Zaat JO, Mank T, Assendelft WJ. Drugs for treating giardiasis. Cochrane Database Syst Rev. 2005:CD000217.-
3. Canete R, Escobedo A, Gonzalez M, et al. Randomized clinical study of five days’ therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol. 2006;12:6366-6370.
4. Sadjjadi SM, Alborzi AW, Mostovfi H. Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr. 2001;47:176-178.
5. Canete R, Escobedo A, Gonzalez M, et al. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin. 2006;22:2131-2136.
6. Rossignol JF, Ayoub A, Ayers MS, et al. Treatment of diarrhea caused by Giardia intestinalis and Entameba histolytica or E dispar: A Randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381-384.
7. Ortiz JJ, Ayoub A, Gargala G, et al. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment Pharmacol Ther. 2001;15:1409-1415.
8. Gilbert DM, Eliopoulos GM, Moellering RC, et al. The Sanford Guide to Antimicrobial Therapy 2006. 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006:95.
1. Beach M. Prevention of specific infectious diseases—giardiasis. In: Arguin PM, Kozarsky PE, Navin AW eds. Centers for Disease Control and Prevention. Health Information for International Travel 2005-2006. Atlanta: US Department of Health and Human Services, Public Health Service; 2005. Available at: www2.ncid.cdc.gov/travel/yb/utils/ybGet.asp?section=dis&obj=giardiasis.htm. Accessed March 7, 2008.
2. Zaat JO, Mank T, Assendelft WJ. Drugs for treating giardiasis. Cochrane Database Syst Rev. 2005:CD000217.-
3. Canete R, Escobedo A, Gonzalez M, et al. Randomized clinical study of five days’ therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children. World J Gastroenterol. 2006;12:6366-6370.
4. Sadjjadi SM, Alborzi AW, Mostovfi H. Comparative clinical trial of mebendazole and metronidazole in giardiasis of children. J Trop Pediatr. 2001;47:176-178.
5. Canete R, Escobedo A, Gonzalez M, et al. A randomized, controlled, open-label trial of a single day of mebendazole versus a single dose of tinidazole in the treatment of giardiasis in children. Curr Med Res Opin. 2006;22:2131-2136.
6. Rossignol JF, Ayoub A, Ayers MS, et al. Treatment of diarrhea caused by Giardia intestinalis and Entameba histolytica or E dispar: A Randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis. 2001;184:381-384.
7. Ortiz JJ, Ayoub A, Gargala G, et al. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from northern Peru. Aliment Pharmacol Ther. 2001;15:1409-1415.
8. Gilbert DM, Eliopoulos GM, Moellering RC, et al. The Sanford Guide to Antimicrobial Therapy 2006. 36th ed. Sperryville, Va: Antimicrobial Therapy; 2006:95.
Evidence-based answers from the Family Physicians Inquiries Network
What is the clinical workup for failure to thrive?
The clinical evaluation of failure to thrive (FTT) includes a thorough history and physical examination; observation of parent–child interactions; observation and documentation of the child’s feeding patterns; and a home visit by an appropriately trained health care professional (Strength of Recommendation [SOR]: C). Further diagnostic testing should be performed as indicated by positive findings from the history and physical exam or if the child’s weight has not improved at follow-up (SOR: C).
A complex problem that requires a team approach
Robert Gauer, MD
Family Medicine Residency Clinic Faculty, Womack Army Medical Center, Fort Bragg, NC
We admit several infants with FTT to the hospital each month from a large population of young families at Fort Bragg, NC, and manage many more in our outpatient practice. Our experience confirms that FTT is a complex problem with many potential causes.
Laboratory and other evaluation beyond history, physical examination, and observation rarely help establish the diagnosis or prognosis. Incidental abnormalities occasionally change management, but more often result in false positives.
Close follow-up and a multidisciplinary team approach generally uncover the cause and lead to successful treatment. Children who don’t respond to treatment or have a suspected “organic” cause of FTT always warrant further laboratory investigation to identify the 1% of cases that result from a diagnosable disease. FTT can also be the sole indication of neglect or nonaccidental trauma, with devastating consequences.
Early identification of an infant or child approaching the diagnostic criteria for FTT is critical. Diagnosis and intervention may be delayed by inaccurate growth curve points, loss of a growth chart in a busy practice, or lack of well-child visits. Our experience with early detection and a multidisciplinary team treatment approach has been highly successful.
Evidence summary
FTT is a generic term used to describe a child whose current weight (or trajectory of weight gain) does not equal that of other children of similar age, gender, and ethnicity. No single accepted anthropometric measure can be used to diagnose the condition.1
FTT has been variously defined in children who:
- drop more than 2 standard percentile lines on standardized growth charts,2
- are below the third percentile for weight,
- have weight-for-length <80% of ideal weight,3
- have height-for-weight less than the third percentile,4
- have weight-for-height less than the 10th percentile, or have weight-for-age less than 2 standard deviations below the mean for age.
Recent updates of standardized growth charts for children are available from the Centers for Disease Control and Prevention at www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm.
A complex diagnosis
FTT occurs when nutritive intake is insufficient to meet demands for growth (TABLE). It is usually manifested by failure to gain weight. In more severe cases, height and head circumference are affected. FTT is also associated with lower developmental testing scores,5 persistent poor growth, increased susceptibility to infections, and an increased prevalence of behavioral disorders and neurologic disability.4 As many as 10% of children seen in primary-care settings show signs of FTT.2
Children with FTT are most often identified when parents raise concerns about the child’s feeding or growth patterns or when a physician notes a decrease in the child’s growth on physical examination. The terms “organic” and “inorganic” or “nonorganic” FTT, often used to guide diagnostic thinking, are outdated because most cases of FTT are influenced by many variables.6 FTT represents the final common pathway of disruptions in the complex system of biological, psychosocial, and environmental factors contributing to a child’s growth and development.
TABLE
Failure to thrive: Causes and physical findings
| GENERIC CAUSE | ASSOCIATED CONDITIONS | PHYSICAL FINDINGS* | DIAGNOSTIC EVALUATION |
|---|---|---|---|
| Inadequate caloric intake | Poor food intake Chronic illness Inappropriate type/volume of feeding Food not available Parental withholding Neglect Poverty | Signs of neglect or abuse Minimal subcutaneous fat Protuberant abdomen | Complete dietary history and psychosocial evaluation Complete blood count (CBC) Basic metabolic profile Lead screening |
| Inadequate caloric absorption | Gastrointestinal causes Malabsorption Chronic vomiting Pancreatic insufficiency Celiac disease Chronic reflux Inflammatory bowel disease Chronic renal disease Cystic fibrosis | Dysmorphism suggestive of chronic disease Organomegaly Skin/mucosal changes | Stool pathogens Stool fat Cystic fibrosis screening CBC/erythrocyte sedimentation rate (ESR) Basic metabolic profile Urinalysis (U/A) |
| Excessive caloric expenditure | Hyperthyroidism Chronic disease (cardiac, renal, endocrine, hepatic) Malignancy | Dysmporphism Skin dysmorphology Cardiac findings | TSH CBC/ESR Basic metabolic profile Liver function tests |
| *Abnormal weight is observed in all cases. | |||
| Modified from Skuse DH et al., .8 Bergman P et al., .13 Krugman SD et al.14 | |||
FTT has 3 basic causes
1. Inadequate caloric intake. More than 80% of children with poor growth do not have an underlying medical disorder.7 The initial workup, therefore, should include a thorough dietary and psychosocial history. Find out exactly what the child eats, how often he eats, and what behaviors he exhibits at mealtimes.
A detailed prenatal history (including birth weight and pregnancy complications) and medical history for both the child and parents can identify underlying metabolic, endocrine, or familial disorders. It is always important to look for signs of child abuse, because children with FTT are more likely to be victims of abuse than normal-weight peers.3 That said, other factors are responsible for poor nutritional intake in as many as 80% of cases.8
2. Inadequate caloric absorption (mal-absorption). This usually results from persistent emesis or malabsorption. Emesis can be caused by reflux, obstruction, medication, food sensitivities, or underlying metabolic disease. Malabsorption most often arises from chronic diarrhea, celiac disease, protein-losing enteropathy, food sensitivities, or excessive juice intake.
3. Excessive caloric expenditure. Such expenditure is associated with underlying medical conditions such as congenital heart disease, chronic hypoxia (pulmonary disease), hyperthyroidism, metabolic disease (diabetes, renal tubular acidosis), chronic immunodeficiency, recurrent infection, or malignancy.
FTT accounts for between 1% and 5% of all pediatric hospitalizations.9 Children who continue to exhibit poor growth despite adequate outpatient evaluation should be admitted to the hospital. Admission is also indicated if abuse is suspected.
Recommendations
The American Academy of Pediatrics recommends that physicians consider child neglect as a cause of FTT, particularly in cases that do not resolve with appropriate medical intervention.3
The American Gastroenterological Association10 and World Gastroenterology Organization11 recommend that physicians consider celiac sprue in children presenting with FTT. Interestingly, the Cochrane Database of Systematic Reviews suggests that there is little systematic evidence to support routine growth monitoring in children.12
1. Olsen EM. Failure to thrive: still a problem of definition. Clin Pediatr. 2006;45(1):1-6.
2. Wright CM. Identification and management of failure to thrive: a community perspective. Arch Dis Child. 2000;82(1):5-9.
3. Block RW, Krebs NF. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116:1234-1237.
4. Perrin EC, Cole CH, Frank DA, et al. Criteria for determining disability in infants and children: failure to thrive. Evid Rep Technol Assess (Summ). 2003;(72):1-5.
5. Rudolf MC, Logan S. What is the long-term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90:925-931.
6. Gahagan S, Holmes R. A stepwise approach to evaluation of undernutrition and failure to thrive. Pediatr Clin North Am. 1998;45:169-187.
7. Gahagan S. Failure to thrive: a consequence of undernutrition. Pediatr Rev. 2006;27:e1-11.
8. Skuse DH, Gill D, Reilly S, Wolke D, et al. Failure to thrive and the risk of child abuse: a prospective population survey. J Med Screen. 1995;2:145-149.
9. Shah MD. Failure to thrive in children. J Clin Gastroenterol. 2002;35:371-374.
10. American Gastroenterological Association. American Gastroenterological Association medical position statement: celiac sprue. Gastroenterology. 2001;120:1522-1525.
11. WGO-OMGE practice guideline: celiac disease 2005. Available at: www.worldgastroenterology.org/globalguidelines/guide13/guideline13.htm. Accessed September 30, 2007.
12. Panpanich R, Garner P. Growth monitoring in children. Cochrane Database Syst Rev. 1999;(4):CD001443.-
13. Bergman P, Graham J. An approach to “failure to thrive.” Aust Fam Physician. 2005;34:725-729.
14. Krugman SD, Dubowitz H. Failure to thrive. Am Fam Physician. 2003;68:879-884.
The clinical evaluation of failure to thrive (FTT) includes a thorough history and physical examination; observation of parent–child interactions; observation and documentation of the child’s feeding patterns; and a home visit by an appropriately trained health care professional (Strength of Recommendation [SOR]: C). Further diagnostic testing should be performed as indicated by positive findings from the history and physical exam or if the child’s weight has not improved at follow-up (SOR: C).
A complex problem that requires a team approach
Robert Gauer, MD
Family Medicine Residency Clinic Faculty, Womack Army Medical Center, Fort Bragg, NC
We admit several infants with FTT to the hospital each month from a large population of young families at Fort Bragg, NC, and manage many more in our outpatient practice. Our experience confirms that FTT is a complex problem with many potential causes.
Laboratory and other evaluation beyond history, physical examination, and observation rarely help establish the diagnosis or prognosis. Incidental abnormalities occasionally change management, but more often result in false positives.
Close follow-up and a multidisciplinary team approach generally uncover the cause and lead to successful treatment. Children who don’t respond to treatment or have a suspected “organic” cause of FTT always warrant further laboratory investigation to identify the 1% of cases that result from a diagnosable disease. FTT can also be the sole indication of neglect or nonaccidental trauma, with devastating consequences.
Early identification of an infant or child approaching the diagnostic criteria for FTT is critical. Diagnosis and intervention may be delayed by inaccurate growth curve points, loss of a growth chart in a busy practice, or lack of well-child visits. Our experience with early detection and a multidisciplinary team treatment approach has been highly successful.
Evidence summary
FTT is a generic term used to describe a child whose current weight (or trajectory of weight gain) does not equal that of other children of similar age, gender, and ethnicity. No single accepted anthropometric measure can be used to diagnose the condition.1
FTT has been variously defined in children who:
- drop more than 2 standard percentile lines on standardized growth charts,2
- are below the third percentile for weight,
- have weight-for-length <80% of ideal weight,3
- have height-for-weight less than the third percentile,4
- have weight-for-height less than the 10th percentile, or have weight-for-age less than 2 standard deviations below the mean for age.
Recent updates of standardized growth charts for children are available from the Centers for Disease Control and Prevention at www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm.
A complex diagnosis
FTT occurs when nutritive intake is insufficient to meet demands for growth (TABLE). It is usually manifested by failure to gain weight. In more severe cases, height and head circumference are affected. FTT is also associated with lower developmental testing scores,5 persistent poor growth, increased susceptibility to infections, and an increased prevalence of behavioral disorders and neurologic disability.4 As many as 10% of children seen in primary-care settings show signs of FTT.2
Children with FTT are most often identified when parents raise concerns about the child’s feeding or growth patterns or when a physician notes a decrease in the child’s growth on physical examination. The terms “organic” and “inorganic” or “nonorganic” FTT, often used to guide diagnostic thinking, are outdated because most cases of FTT are influenced by many variables.6 FTT represents the final common pathway of disruptions in the complex system of biological, psychosocial, and environmental factors contributing to a child’s growth and development.
TABLE
Failure to thrive: Causes and physical findings
| GENERIC CAUSE | ASSOCIATED CONDITIONS | PHYSICAL FINDINGS* | DIAGNOSTIC EVALUATION |
|---|---|---|---|
| Inadequate caloric intake | Poor food intake Chronic illness Inappropriate type/volume of feeding Food not available Parental withholding Neglect Poverty | Signs of neglect or abuse Minimal subcutaneous fat Protuberant abdomen | Complete dietary history and psychosocial evaluation Complete blood count (CBC) Basic metabolic profile Lead screening |
| Inadequate caloric absorption | Gastrointestinal causes Malabsorption Chronic vomiting Pancreatic insufficiency Celiac disease Chronic reflux Inflammatory bowel disease Chronic renal disease Cystic fibrosis | Dysmorphism suggestive of chronic disease Organomegaly Skin/mucosal changes | Stool pathogens Stool fat Cystic fibrosis screening CBC/erythrocyte sedimentation rate (ESR) Basic metabolic profile Urinalysis (U/A) |
| Excessive caloric expenditure | Hyperthyroidism Chronic disease (cardiac, renal, endocrine, hepatic) Malignancy | Dysmporphism Skin dysmorphology Cardiac findings | TSH CBC/ESR Basic metabolic profile Liver function tests |
| *Abnormal weight is observed in all cases. | |||
| Modified from Skuse DH et al., .8 Bergman P et al., .13 Krugman SD et al.14 | |||
FTT has 3 basic causes
1. Inadequate caloric intake. More than 80% of children with poor growth do not have an underlying medical disorder.7 The initial workup, therefore, should include a thorough dietary and psychosocial history. Find out exactly what the child eats, how often he eats, and what behaviors he exhibits at mealtimes.
A detailed prenatal history (including birth weight and pregnancy complications) and medical history for both the child and parents can identify underlying metabolic, endocrine, or familial disorders. It is always important to look for signs of child abuse, because children with FTT are more likely to be victims of abuse than normal-weight peers.3 That said, other factors are responsible for poor nutritional intake in as many as 80% of cases.8
2. Inadequate caloric absorption (mal-absorption). This usually results from persistent emesis or malabsorption. Emesis can be caused by reflux, obstruction, medication, food sensitivities, or underlying metabolic disease. Malabsorption most often arises from chronic diarrhea, celiac disease, protein-losing enteropathy, food sensitivities, or excessive juice intake.
3. Excessive caloric expenditure. Such expenditure is associated with underlying medical conditions such as congenital heart disease, chronic hypoxia (pulmonary disease), hyperthyroidism, metabolic disease (diabetes, renal tubular acidosis), chronic immunodeficiency, recurrent infection, or malignancy.
FTT accounts for between 1% and 5% of all pediatric hospitalizations.9 Children who continue to exhibit poor growth despite adequate outpatient evaluation should be admitted to the hospital. Admission is also indicated if abuse is suspected.
Recommendations
The American Academy of Pediatrics recommends that physicians consider child neglect as a cause of FTT, particularly in cases that do not resolve with appropriate medical intervention.3
The American Gastroenterological Association10 and World Gastroenterology Organization11 recommend that physicians consider celiac sprue in children presenting with FTT. Interestingly, the Cochrane Database of Systematic Reviews suggests that there is little systematic evidence to support routine growth monitoring in children.12
The clinical evaluation of failure to thrive (FTT) includes a thorough history and physical examination; observation of parent–child interactions; observation and documentation of the child’s feeding patterns; and a home visit by an appropriately trained health care professional (Strength of Recommendation [SOR]: C). Further diagnostic testing should be performed as indicated by positive findings from the history and physical exam or if the child’s weight has not improved at follow-up (SOR: C).
A complex problem that requires a team approach
Robert Gauer, MD
Family Medicine Residency Clinic Faculty, Womack Army Medical Center, Fort Bragg, NC
We admit several infants with FTT to the hospital each month from a large population of young families at Fort Bragg, NC, and manage many more in our outpatient practice. Our experience confirms that FTT is a complex problem with many potential causes.
Laboratory and other evaluation beyond history, physical examination, and observation rarely help establish the diagnosis or prognosis. Incidental abnormalities occasionally change management, but more often result in false positives.
Close follow-up and a multidisciplinary team approach generally uncover the cause and lead to successful treatment. Children who don’t respond to treatment or have a suspected “organic” cause of FTT always warrant further laboratory investigation to identify the 1% of cases that result from a diagnosable disease. FTT can also be the sole indication of neglect or nonaccidental trauma, with devastating consequences.
Early identification of an infant or child approaching the diagnostic criteria for FTT is critical. Diagnosis and intervention may be delayed by inaccurate growth curve points, loss of a growth chart in a busy practice, or lack of well-child visits. Our experience with early detection and a multidisciplinary team treatment approach has been highly successful.
Evidence summary
FTT is a generic term used to describe a child whose current weight (or trajectory of weight gain) does not equal that of other children of similar age, gender, and ethnicity. No single accepted anthropometric measure can be used to diagnose the condition.1
FTT has been variously defined in children who:
- drop more than 2 standard percentile lines on standardized growth charts,2
- are below the third percentile for weight,
- have weight-for-length <80% of ideal weight,3
- have height-for-weight less than the third percentile,4
- have weight-for-height less than the 10th percentile, or have weight-for-age less than 2 standard deviations below the mean for age.
Recent updates of standardized growth charts for children are available from the Centers for Disease Control and Prevention at www.cdc.gov/nchs/about/major/nhanes/growthcharts/clinical_charts.htm.
A complex diagnosis
FTT occurs when nutritive intake is insufficient to meet demands for growth (TABLE). It is usually manifested by failure to gain weight. In more severe cases, height and head circumference are affected. FTT is also associated with lower developmental testing scores,5 persistent poor growth, increased susceptibility to infections, and an increased prevalence of behavioral disorders and neurologic disability.4 As many as 10% of children seen in primary-care settings show signs of FTT.2
Children with FTT are most often identified when parents raise concerns about the child’s feeding or growth patterns or when a physician notes a decrease in the child’s growth on physical examination. The terms “organic” and “inorganic” or “nonorganic” FTT, often used to guide diagnostic thinking, are outdated because most cases of FTT are influenced by many variables.6 FTT represents the final common pathway of disruptions in the complex system of biological, psychosocial, and environmental factors contributing to a child’s growth and development.
TABLE
Failure to thrive: Causes and physical findings
| GENERIC CAUSE | ASSOCIATED CONDITIONS | PHYSICAL FINDINGS* | DIAGNOSTIC EVALUATION |
|---|---|---|---|
| Inadequate caloric intake | Poor food intake Chronic illness Inappropriate type/volume of feeding Food not available Parental withholding Neglect Poverty | Signs of neglect or abuse Minimal subcutaneous fat Protuberant abdomen | Complete dietary history and psychosocial evaluation Complete blood count (CBC) Basic metabolic profile Lead screening |
| Inadequate caloric absorption | Gastrointestinal causes Malabsorption Chronic vomiting Pancreatic insufficiency Celiac disease Chronic reflux Inflammatory bowel disease Chronic renal disease Cystic fibrosis | Dysmorphism suggestive of chronic disease Organomegaly Skin/mucosal changes | Stool pathogens Stool fat Cystic fibrosis screening CBC/erythrocyte sedimentation rate (ESR) Basic metabolic profile Urinalysis (U/A) |
| Excessive caloric expenditure | Hyperthyroidism Chronic disease (cardiac, renal, endocrine, hepatic) Malignancy | Dysmporphism Skin dysmorphology Cardiac findings | TSH CBC/ESR Basic metabolic profile Liver function tests |
| *Abnormal weight is observed in all cases. | |||
| Modified from Skuse DH et al., .8 Bergman P et al., .13 Krugman SD et al.14 | |||
FTT has 3 basic causes
1. Inadequate caloric intake. More than 80% of children with poor growth do not have an underlying medical disorder.7 The initial workup, therefore, should include a thorough dietary and psychosocial history. Find out exactly what the child eats, how often he eats, and what behaviors he exhibits at mealtimes.
A detailed prenatal history (including birth weight and pregnancy complications) and medical history for both the child and parents can identify underlying metabolic, endocrine, or familial disorders. It is always important to look for signs of child abuse, because children with FTT are more likely to be victims of abuse than normal-weight peers.3 That said, other factors are responsible for poor nutritional intake in as many as 80% of cases.8
2. Inadequate caloric absorption (mal-absorption). This usually results from persistent emesis or malabsorption. Emesis can be caused by reflux, obstruction, medication, food sensitivities, or underlying metabolic disease. Malabsorption most often arises from chronic diarrhea, celiac disease, protein-losing enteropathy, food sensitivities, or excessive juice intake.
3. Excessive caloric expenditure. Such expenditure is associated with underlying medical conditions such as congenital heart disease, chronic hypoxia (pulmonary disease), hyperthyroidism, metabolic disease (diabetes, renal tubular acidosis), chronic immunodeficiency, recurrent infection, or malignancy.
FTT accounts for between 1% and 5% of all pediatric hospitalizations.9 Children who continue to exhibit poor growth despite adequate outpatient evaluation should be admitted to the hospital. Admission is also indicated if abuse is suspected.
Recommendations
The American Academy of Pediatrics recommends that physicians consider child neglect as a cause of FTT, particularly in cases that do not resolve with appropriate medical intervention.3
The American Gastroenterological Association10 and World Gastroenterology Organization11 recommend that physicians consider celiac sprue in children presenting with FTT. Interestingly, the Cochrane Database of Systematic Reviews suggests that there is little systematic evidence to support routine growth monitoring in children.12
1. Olsen EM. Failure to thrive: still a problem of definition. Clin Pediatr. 2006;45(1):1-6.
2. Wright CM. Identification and management of failure to thrive: a community perspective. Arch Dis Child. 2000;82(1):5-9.
3. Block RW, Krebs NF. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116:1234-1237.
4. Perrin EC, Cole CH, Frank DA, et al. Criteria for determining disability in infants and children: failure to thrive. Evid Rep Technol Assess (Summ). 2003;(72):1-5.
5. Rudolf MC, Logan S. What is the long-term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90:925-931.
6. Gahagan S, Holmes R. A stepwise approach to evaluation of undernutrition and failure to thrive. Pediatr Clin North Am. 1998;45:169-187.
7. Gahagan S. Failure to thrive: a consequence of undernutrition. Pediatr Rev. 2006;27:e1-11.
8. Skuse DH, Gill D, Reilly S, Wolke D, et al. Failure to thrive and the risk of child abuse: a prospective population survey. J Med Screen. 1995;2:145-149.
9. Shah MD. Failure to thrive in children. J Clin Gastroenterol. 2002;35:371-374.
10. American Gastroenterological Association. American Gastroenterological Association medical position statement: celiac sprue. Gastroenterology. 2001;120:1522-1525.
11. WGO-OMGE practice guideline: celiac disease 2005. Available at: www.worldgastroenterology.org/globalguidelines/guide13/guideline13.htm. Accessed September 30, 2007.
12. Panpanich R, Garner P. Growth monitoring in children. Cochrane Database Syst Rev. 1999;(4):CD001443.-
13. Bergman P, Graham J. An approach to “failure to thrive.” Aust Fam Physician. 2005;34:725-729.
14. Krugman SD, Dubowitz H. Failure to thrive. Am Fam Physician. 2003;68:879-884.
1. Olsen EM. Failure to thrive: still a problem of definition. Clin Pediatr. 2006;45(1):1-6.
2. Wright CM. Identification and management of failure to thrive: a community perspective. Arch Dis Child. 2000;82(1):5-9.
3. Block RW, Krebs NF. Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116:1234-1237.
4. Perrin EC, Cole CH, Frank DA, et al. Criteria for determining disability in infants and children: failure to thrive. Evid Rep Technol Assess (Summ). 2003;(72):1-5.
5. Rudolf MC, Logan S. What is the long-term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90:925-931.
6. Gahagan S, Holmes R. A stepwise approach to evaluation of undernutrition and failure to thrive. Pediatr Clin North Am. 1998;45:169-187.
7. Gahagan S. Failure to thrive: a consequence of undernutrition. Pediatr Rev. 2006;27:e1-11.
8. Skuse DH, Gill D, Reilly S, Wolke D, et al. Failure to thrive and the risk of child abuse: a prospective population survey. J Med Screen. 1995;2:145-149.
9. Shah MD. Failure to thrive in children. J Clin Gastroenterol. 2002;35:371-374.
10. American Gastroenterological Association. American Gastroenterological Association medical position statement: celiac sprue. Gastroenterology. 2001;120:1522-1525.
11. WGO-OMGE practice guideline: celiac disease 2005. Available at: www.worldgastroenterology.org/globalguidelines/guide13/guideline13.htm. Accessed September 30, 2007.
12. Panpanich R, Garner P. Growth monitoring in children. Cochrane Database Syst Rev. 1999;(4):CD001443.-
13. Bergman P, Graham J. An approach to “failure to thrive.” Aust Fam Physician. 2005;34:725-729.
14. Krugman SD, Dubowitz H. Failure to thrive. Am Fam Physician. 2003;68:879-884.
Evidence-based answers from the Family Physicians Inquiries Network