User login
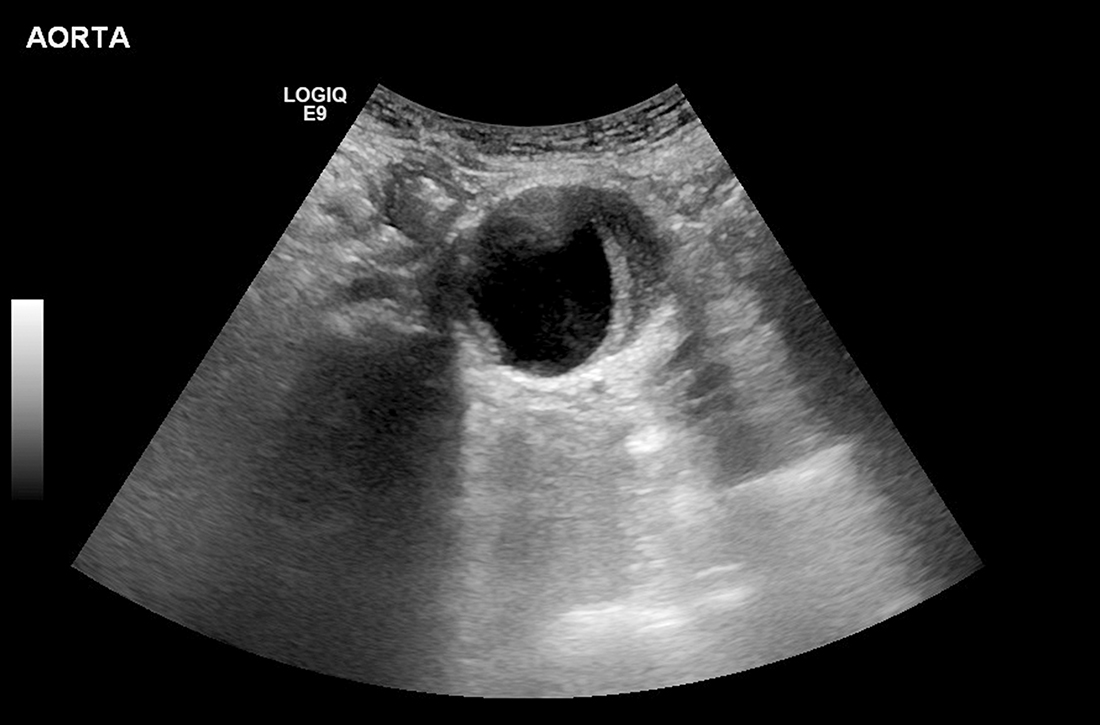
Can family physicians accurately screen for AAA with point-of-care ultrasound?
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
EVIDENCE SUMMARY
Meta-analysis demonstrates accuracy of nonradiologist providers with POCUS
A systematic review and meta-analysis (11 studies; 946 exams) compared nonradiologist-performed AAA screening with POCUS vs radiologist-performed aortic imaging as a gold standard. Eight trials involved emergency medicine physicians (718 exams); 1 trial, surgical residents (104 exams); 1 trial, primary care internal medicine physicians (79 exams); and 1 trial, rural family physicians (45 exams). The majority of studies were conducted in Ireland, the United Kingdom, Australia, and Canada, with 4 trials performed in the United States.1
Researchers compared all POCUS exam findings with radiologist-performed imaging (using ultrasound, computed tomography, magnetic resonance imaging, or angiography) and with operative findings or pathology where available. There were 193 true positives, 8 false-positives, 740 true negatives, and 5 false-negatives. Primary care physicians identified 6 patients with AAA, with no false-positives or false-negatives. Overall, POCUS demonstrated a sensitivity of 0.975 (95% CI, 0.942 to 0.992) and a specificity of 0.989 (95% CI, 0.979 to 0.995).1
Nonradiologist providers received POCUS training as follows: emergency medicine residents, 5 hours to 3 days; emergency medicine physicians, 4 to 24 hours of didactics, 50 AAA scans, or American College of Emergency Medicine certification; and primary care physicians, 2.3 hours or 50 AAA scans. Information on training for surgical residents was not supplied. The authors rated the studies for quality (10-14 points on the 14-point QUADA quality score) and heterogeneity (I2 = 0 for sensitivity and I2 = .38 for specificity).1
European studies support FPs’ ability to diagnose AAA with POCUS
Two subsequent prospective diagnostic accuracy studies both found that POCUS performed by family physicians had 100% concordance with radiologist overread. The first study (in Spain) included 106 men (ages 50 and older; mean, 69 years) with chronic hypertension or a history of tobacco use. One family physician underwent training (duration not reported) by a radiologist, including experience measuring standard cross-sections of the aorta. Radiologists reviewed all POCUS images, which identified 6 patients with AAA (confirmed by CT scan). The concordance between the family physician and the radiologists was absolute (kappa = 1.0; sensitivity and specificity, 100%; positive and negative predictive values, both 1.0).2
The second study (in Denmark) compared 29 POCUS screenings for AAA performed by 5 family physicians vs a gold standard of a radiologist-performed abdominal ultrasound blinded to previous ultrasound findings. Four of the family physicians were board certified and 1 was a final-year resident in training. They all underwent a 3-day ultrasonography course that included initial e-learning followed by 2 days of hands-on training; all passed a final certification exam. The family physicians identified 1 patient with AAA. Radiologists overread all the scans and found 100% agreement with the 1 positive AAA and the 28 negative scans.3
Recommendations from others
In 2019, the US Preventive Services Task Force (USPSTF) offered a Grade “B” (moderate net benefit) recommendation for screening with ultrasonography for AAA in men ages 65 to 75 years who have ever smoked, and a Grade “C” recommendation (small net benefit) for screening men ages 65 to 75 years who have never smoked.4 In 2017, the Canadian Task Force on Preventive Health Care recommended screening all men ages 65 to 80 years with 1 ultrasound exam for AAA (weak recommendation; moderate-quality evidence). The Canadian Task Force also noted that, with adequate training, AAA screening could be performed in a family practice setting.5
Editor’s takeaway
While these studies evaluating POCUS performed by nonradiologists included a small number of family physicians, their finding that all participants (attending physicians and residents) demonstrate high sensitivity and specificity for AAA detection with relatively limited training bodes well for more widespread use of the technology. Offering POCUS to detect AAAs in family physician offices has the potential to dramatically improve access to USPSTF-recommended screening.
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
1. Concannon E, McHugh S, Healy DA, et al. Diagnostic accuracy of non-radiologist performed ultrasound for abdominal aortic aneurysm: systematic review and meta-analysis. Int J Clin Pract. 2014;9:1122-1129. doi: 10.1111/ijcp.12453
2. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis [article in Spanish]. Med Clin (Barc). 2013;141:417-422. doi: 10.1016/j.medcli.2013.02.038
3. Lindgaard K, Riisgaard L. ‘Validation of ultrasound examinations performed by general practitioners’. Scand J Prim Health Care. 2017;3:256-261. doi: 10.1080/02813432.2017.1358437
4. US Preventive Task Force. Screening for abdominal aortic aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:2211-2218. doi:10.1001/jama.2019.18928
5. Canadian Task Force on Preventive Health Care. Recommendations on screening for abdominal aortic aneurysm in primary care. CMAJ. 2017;189:E1137-E1145. doi: 10.1503/cmaj.170118
EVIDENCE-BASED ANSWER:
Likely yes. Point-of-care ultrasound (POCUS) screening for abdominal aortic aneurysm (AAA) by nonradiologist physicians is 98% sensitive and 99% specific, compared with imaging performed by radiologists (strength of recommendation [SOR]: B, meta-analysis of diagnostic accuracy studies mostly involving emergency medicine physicians). European family physicians demonstrated 100% concordance with radiologist readings (SOR: C, very small subsequent diagnostic accuracy studies).
Is event-driven PrEP dosing for HIV as effective as daily dosing?
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE-BASED ANSWER:
Probably, although there are no head-to-head trials comparing the 2 dosing regimens. Event-driven pre-exposure prophylaxis (PrEP) dosing reduces HIV conversion by 86% compared to placebo (strength of recommendation [SOR]: B, large randomized controlled trial [RCT]). Daily PrEP reduces HIV conversion by 44% to 86% (SOR: B, based on open-label RCTs).
Event-driven PrEP regimens may be associated with lower adherence when compared with daily PrEP regimens (average of 70% for event-driven PrEP vs average of 92% for daily PrEP) (SOR: B, based on open-label and cohort trials). Event-driven PrEP regimens have lower medication costs, and they are associated with no difference in the rate of sexually transmitted infections (STIs) (SOR: B, based on prospective cohort studies). Patients may prefer them to daily regimens (75% choose event-driven PrEP vs 25% choose daily PrEP) (SOR: B, based on the preponderance of prospective cohort studies with conflicting results).
Is NPH associated with fewer adverse events than analog basal insulin for adults with T2D?
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
Evidence summary
No difference in overall hypoglycemia risk between glargine and NPH
A 2015 systematic review and meta-analysis of 28 RCTs compared efficacy and safety outcomes for insulin glargine, NPH insulin, premixed insulin preparations, and insulin detemir in 12,669 adults with type 2 diabetes (T2D) who were also taking an oral antidiabetic drug (OAD).1 In the comparison of glargine to NPH, there was no difference in risk for hypoglycemia (5 trials; N not provided; risk ratio [RR] = 0.92; 0.84-1.001).
Symptomatic hypoglycemia (6 RCTs; RR = 0.89; 0.83-0.96) and nocturnal hypoglycemia (6 RCTs; RR = 0.63; 0.51-0.77) occurred significantly less frequently in those treated with glargine and an OAD compared to NPH and an OAD. The risk for severe hypoglycemia was not different between regimens (5 RCTs; RR = 0.76; 0.47-1.23). Weight gain was also similar (6 RCTs; weighted mean difference [WMD] = 0.36 kg [–0.12 to 0.84]). This review was limited by the fact that many of the trials were of moderate quality, the majority were funded by pharmaceutical companies, fasting glucose goals varied between trials, and some trials had a short duration (6 months).
There may be some advantages of glargine over NPH
A 2008 meta-analysis of 12 RCTs (5 of which were not included in the 2015 review) with 4385 patients with T2D compared fasting plasma glucose (FPG), A1C, hypoglycemia, and body weight for patients treated with NPH vs with glargine.2 Researchers found a significant difference in patient-reported hypoglycemia (10 trials; N not provided; 59% vs 53%; P < .001), symptomatic hypoglycemia (6 trials; 51% vs 43%; P < .0001), and nocturnal hypoglycemia (8 trials; 33% vs 19%; P < .001), favoring glargine over NPH. However, there was no difference between these 2 groups in confirmed hypoglycemia (2 trials; 10% vs 6.3%; P = .11) or severe hypoglycemia (7 trials; 2.4% vs 1.4%; P = .07). Of note, there was no difference between groups in FPG or A1C and a smaller weight gain in the NPH group (6 trials; WMD = 0.33 kg; 95% CI, –0.61 to –0.06). This review did not assess potential biases in the included trials.
Other results indicate a significant benefit from glargine
A 2014 RCT (published after the systematic review search date) compared hypoglycemia risk between NPH and glargine in 1017 adults ages 30 to 70 years who’d had T2D for at least 1 year.3 Patients were randomized to receive an OAD paired with either once-daily glargine or twice-daily NPH. Insulin doses were titrated over the first 3 years of the study to achieve standard glycemic control (described as FPG < 120 mg/dL; this goal was changed to < 100 mg/dL after the first year).
Over 5 years, once-daily glargine resulted in a significantly lower risk for all symptomatic hypoglycemia (odds ratio [OR] = 0.71; 95% CI, 0.52-0.98) and for any severe event (OR = 0.62; 95% CI, 0.41-0.95) compared to NPH. Using a logistic regression model, the authors predicted that if 25 patients were treated with NPH instead of glargine, 1 additional patient would experience at least 1 severe hypoglycemic event. This trial was funded by a pharmaceutical company.
Hypoglycemia requiring hospital care was similar for basal insulin and NPH
A 2018 retrospective observational study (N = 25,489) analyzed the association between the initiation of basal insulin analogs vs NPH with hypoglycemia-related ED visits or hospital admissions.4 Adults older than 19 years with clinically recognized diabetes were identified using electronic medical records; those included in the analysis had newly initiated basal insulin therapy during the prior 12 months. Data was gathered via chart review.
The difference in ED visits or hospital admissions was not different between groups (mean difference = 3.1 events per 100 person-years; 95% CI, –1.5 to 7.7). Among 4428 patients matched by propensity score, there was again no difference for hypoglycemia-related ED visits or hospital admissions with insulin analog use (adjusted hazard ratio = 1.16; 95% CI, 0.71-1.78).
Editor’s takeaway
Meta-analysis of large RCTs shows the glargine insulin adverse effects profile, specifically nonsevere hypoglycemia, to be inconsistently better than NPH. These small differences, plus once-daily dosing, may encourage prescribing of analog basal insulin, but price and the need for more than once-daily dosing remain worthy considerations.
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
1. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649-662. doi:10.1007/s00592-014-0698-4
2. Bazzano LA, Lee LJ, Shi L, et al. Safety and efficacy of glargine compared with NPH insulin for the treatment of type 2 diabetes: a meta-analysis of randomized controlled trials. Diabet Med. 2008;25:924-932. doi:10.1111/j.1464-5491.2008.02517.x
3. Rosenstock J, Fonseca V, Schinzel S, et al. Reduced risk of hypoglycemia with once-daily glargine versus twice-daily NPH and number needed to harm with NPH to demonstrate the risk of one additional hypoglycemic event in type 2 diabetes: evidence from a long-term controlled trial. J Diabetes Complications. 2014;28:742-749. doi:10.1016/j.jdiacomp.2014.04.003
4. Lipska KJ, Parker MM, Moffet HH, et al. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA. 2018;320:53-62. doi:10.1001/jama.2018.7993
EVIDENCE-BASED ANSWER:
NO. Insulin glargine may lead to less patient-reported, symptomatic, and nocturnal hypoglycemia, although overall, there may not be a difference in the risk for severe hypoglycemia or hypoglycemia-related emergency department (ED) visits and hospitalizations (strength of recommendation [SOR]: B, systematic review of randomized controlled trials [RCTs], individual RCTs, and observational study).
Is ketamine effective and safe for treatment-resistant depression?
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
Evidence Summary
Single-dose IV ketamine elicits a short-term response
A meta-analysis of RCTs evaluating a single dose of IV ketamine vs placebo for severe depression found that it increased the chance of a treatment response for up to 1 week afterward. Studies included patients with severe (N = 30), treatment-resistant (N = 40), and psychotic depression (N = 10), based on Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition criteria.1
The primary outcome was treatment response: either an improvement of > 50% on a standardized depression scale or a Clinical Global Impression–Improvement scale score of 1 or 2 (“very much” and “much” improved, respectively, as assessed by a clinician). Ketamine increased the likelihood of short-term response or improvement at 24 hours (3 RCTs; N = 56; odds ratio [OR] = 11; 95% CI, 2-58); at 72 hours (3 RCTs; N = 56; OR = 13; 95% CI, 2-66); and at 7 days (4 RCTs; N = 88; OR = 2.6; 95% CI, 1.1-6.2).1 Response rates equaled placebo at 2 weeks. The authors rated the RCTs as low quality.
Another systematic review of single-dose IV ketamine vs placebo for major depression and bipolar disorder included 3 additional small, low-quality RCTs, 2 of which showed short-term response to ketamine. The authors used Hedge’s g statistic to standardize effect size (a score of magnitude 0.2 indicates a small effect; 0.6, moderate; 1.2, large; and 2, very large). One RCT (n = 26) found a very large 1-day response (effect size: –2; 95% CI, –2.8 to –1.3), and 2 RCTs found conflicting responses at 12 days (RCT with N = 18: effect size: –0.2; 95% CI, –0.4 to 0.02 [no significant response] vs RCT with N = 8: effect size: –1.5; 95% CI, –2.5 to –0.5).2
More frequent dosing of IV ketamine improves symptoms
An RCT (N = 67) evaluating twice- or thrice-weekly IV ketamine vs placebo in patients with recurrent depression (with at least 1 treatment failure) found that ketamine significantly improved standardized depression scores and response rates at 15 days. Patients with clinically significant suicidality were excluded.3
Researchers randomized patients to IV ketamine (0.05 mg/kg) twice or thrice weekly or to saline control and used the 60-point Montgomery-Asberg Depression Rating Scale (MADRS). A response was defined as a reduction of the MADRS score by 50%.
Both ketamine arms produced greater symptom improvement at 15 days, compared to placebo (twice weekly: −18.4 vs −5.7; P < 0.001; thrice weekly: −17.7 vs −3.1; P < 0.001) in addition to higher response rates (twice weekly: 69% vs 15%; P = .005; number needed to treat [NNT] = 2; and thrice-weekly: 54% vs 6%; P = .004; NNT = 2).3 There was no significant difference between twice- or thrice-weekly dosing. The study was flawed by dropouts (N = 57 at 15 days and N = 25 at 28 days), primarily attributed to ketamine adverse effects, that prevented assessment beyond 2 weeks.
Oral ketamine has a moderate effecton depression
A systematic review included 2 low-quality RCTs evaluating oral ketamine vs placebo as adjunctive treatment with sertraline, and oral ketamine vs diclofenac, and found improvement in patients with moderate depression.4 In the first RCT (n = 45), researchers found that oral ketamine (25 mg bid) plus sertraline (25 mg titrated up to 150 mg/d) produced more treatment responses (> 50% reduction on a standardized depression rating scale) than placebo plus sertraline (2 weeks: 85.4% vs 42.5%; P < .001; 6 weeks: 85.4% vs 57.5%; P = .005).4
In the second RCT (n = 23), researchers randomized patients with mild-to-moderate depression and comorbid chronic headaches to take oral ketamine (50 mg tid) or oral diclofenac (50 mg tid) and measured effect size on standardized depression scores at 3 weeks (no difference) and 6 weeks (Cohen d effect size = 0.79 [rated as a moderate effect]; P = .017).4
Nasal esketamine + oral antidepressants boosts treatment response rates
A meta-analysis with 4 RCTs (N = 708) evaluating intranasal esketamine vs placebo as an adjunct to oral antidepressants for patients with predominantly treatment-resistant major depression found that it boosted response rates by about 40%. Researchers randomized patients to intranasal esketamine (mostly 28-84 mg twice weekly for 28 days) or placebo spray as an adjunct to oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine).
The primary outcomes were treatment response (≥ 50% reduction in depression scores) or remission (a MADRS score < 12). Adjunctive intranasal esketamine produced greater rates of treatment response compared to placebo at 24 hours (21% vs 7%; relative risk [RR] = 8.4; 95% CI, 1.4 to 21.2; P < .02; NNT = 7) and at 28 days (59% vs 43%; RR = 1.4; 95% CI, 1.2 to 1.60; P < .0001; NNT = 6).5 Adjunctive intranasal esketamine also produced greater rates of remission at the end of the study (mostly at 28 days), compared with placebo (41% vs 25%; RR = 1.4; 95% CI, 1.2 to 1.7; P = .0004; NNT = 7).5 The authors rated study quality as moderate to high.
Adverse effects are common, may cause Tx discontinuation
Ketamine-produced adverse effects (AEs) included confusion (2 trials; N = 76; OR = 3.7; 95% CI, 1.1-12) and emotional blunting (1 trial; N = 30; OR = 23; 95% CI, 1.1-489).1
A 2018 systematic review assessed the safety of ketamine in depression after single and repeated dose in 60 studies (N = 899; 20 RCTs, 17 open-label-trials, 20 case series, and 3 retrospective studies). The most common AEs reported were headache (35% of studies), dizziness (33%), dissociation (28%), elevated blood pressure (28%), and blurred vision (23%), with the majority reported to resolve shortly after administration. The most common psychiatric AE was anxiety (15%).6 Included studies varied greatly in design and dosage form, and no meta-analysis could be performed.
Nasal esketamine produced more AEs causing discontinuation than did placebo (5.8% vs 1.5%; RR = 3.5; 95% CI, 1.34-8.9; number needed to harm [NNH] = 23), including blurred vision, dizziness, sedation, nausea, and dysphoria.5A review (5 RCTs and 1 open-label trial; N = 1708) analyzing the cardiac safety profile of intranasal esketamine adjuvant therapy found that it produced transient and asymptomatic blood pressure elevations (OR = 3.2; 95% CI, 1.9-5.8; NNH = 13).7
Recommendations from others
A clinical practice guideline from the US Veterans Administration lists IV ketamine as 1 of the therapeutic options for patients with treatment-resistant depression and suicidal ideation.8 However, a Department of Veterans Affairs Panel restricted its use to a pre-approved case-by-case basis.8
Editor’s takeaway
Physicians with patients facing the all-too-common problem of treatment-resistant major depression will be wondering if ketamine, either by itself or as an augmentation therapy, can help. Unfortunately, the outcomes we report here are too short term to answer that question, and we must await the results of further studies. Augmentation with intranasal esketamine, at a cost of $370/month, might offer some promise.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
1. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;(9):CD011612.
2. Coyle CM, Laws KR. The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol. 2015;30:152‐163.
3. Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816‐826.
4. Rosenblat JD, Carvalho AF, Li M, et al. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80:18r12475.
5. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord. 2020;265:63‐70.
6. Short B, Fong J, Galvez V, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78.
7. Doherty T, Wajs E, Melkote R, et al. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the Clinical Development Program. CNS Drugs. 2020;34:299‐310.
8. Sall J, Brenner L, Millikan Bell AM, et al. Assessment and management of patients at risk for suicide: synopsis of the 2019 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guidelines. Ann Intern Med. 2019;171:343-353.
EVIDENCE-BASED ANSWER:
MAYBE, but it’s too soon to tell. There is limited evidence that ketamine by itself is effective in the very short term. Single-dose intravenous (IV) ketamine is more likely than placebo (odds ratio = 11-13) to produce improvement (> 50%) in standardized depression scores in 1 to 3 days, lasting up to a week. Twice- or thrice-weekly IV ketamine improves symptom scores by 20%-25% over 2 weeks (strength of recommendation [SOR]: B, meta-analysis of small, low-quality, randomized controlled trials [RCTs] and a single small RCT).
Augmentation of sertraline with daily oral ketamine moderately improves symptom scores for 6 weeks in patients with moderate depression (SOR: B, small, low-quality RCTs).
Augmentation of oral antidepressants (duloxetine, escitalopram, sertraline, venlafaxine) with intranasal esketamine spray improves response and remission rates at 4 weeks (16% for both outcomes) in patients with predominantly treatment-resistant major depression (SOR: A, meta-analysis of RCTs).
Ketamine therapy is associated with confusion, emotional blunting, headache, dizziness, and blurred vision (SOR: A, meta-analyses).
Nasal esketamine spray produces the adverse effects of dizziness, vertigo, and blurred vision severe enough to cause discontinuation in 4% of patients; it also can produce transient elevation of blood pressure (SOR: A, meta-analyses).
Is metformin effective for reducing weight in obese or overweight adolescents?
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
EVIDENCE SUMMARY
Metformin has modest effects on body weight
A large systematic review and meta-analysis (38 RCTs; n = 2199) published in 2020 evaluated metformin therapy in children and adolescents (including those with metabolic disease, growth problems, and psychological disorders in addition to obesity and overweight).1 Over an average of 6 months, metformin use modestly reduced BMI (weighted mean difference [WMD] = –1.07 kg/m2; 95% CI, –1.43 to –0.72 kg/m2) and body weight (WMD = –2.51 kg; 95% CI, –3.14 to –1.89 kg) for all participants.1
However, the authors also performed a meta-analysis of trials involving obese or overweight youth without other comorbidities. Participants in these trials ranged in age from 7 to 17 years (mean not supplied; most trials, 12-15 years), had a BMI greater than the 95th percentile for age, and took doses of metformin ranging from 1500 to 3000 mg (most trials, 1500-2000 mg/d for 24 weeks).1 In this analysis, metformin reduced body weight (8 trials; n = 616; WMD = –2.06 kg; 95% CI, –3.47 to –0.65 kg) and body fat mass (–1.9%; 95% CI, –3.25% to –0.56%). But it did not reduce BMI (12 trials; n = 826; WMD = –0.76 kg/m2; 95 % CI, –1.61 to 0.08 kg/m2) or improve lean body mass (2 trials; N = 98; WMD = –0.74 kg; 95% CI, –2.4 to 0.91 kg).1
The authors of this meta-analysis did not include an evaluation of the quality of the individual RCTs.
Metformin has benefits but also adverse effects
A 2016 Cochrane systematic review and meta-analysis assessed 8 trials (total n = 543) evaluating metformin vs placebo in adolescents prescribed exercise and lifestyle support.2 This meta-analysis included 4 trials (n = 294) with obese or overweight adolescents that were also included in the newer meta-analysis,1 as well as 4 trials involving obese adolescents with insulin resistance. The authors did not assess the effects of metformin on obese or overweight adolescents separately.
Over 6 months, metformin use reduced BMI (WMD = –1.35 kg/m2; 95% CI –2 to –0.69 kg/m2).2 Metformin commonly produced gastrointestinal symptoms: diarrhea, flatulence (rates not given), and nausea in 15% to 42% compared with 3% to 21% with placebo (no comparison statistic supplied), however rarely to the point of discontinuation (< 5%).2 Nine participants withdrew due to adverse effects: 5 in the metformin group and 4 in the placebo group. The authors rated the quality of the included trials as low to moderate.
An evidence report and systematic review (42 RCTs; total n = 6956) compared the efficacy of several approaches for weight loss in adolescents, including metformin (6 of the 8 RCTs included in the 2020 meta-analysis1) and lifestyle interventions.3 Interventions comprising exercise and diet counseling for > 26 hours over 6 to 12 months produced decreases in BMI (–0.86 kg/m2; 95% CI –1.44 to –0.29 kg/m2) but not weight (–2 kg; 95% CI –3.2 to 1.2 kg).3
Recommendations from others
The US Preventive Services Task Force states that metformin treatment in adolescents who are overweight or obese produces a small reduction in BMI when compared to placebo, but the clinical significance of this reduction is unclear.3
Editor’s takeaway
The idea of using medications for weight loss remains seductive, given how hard it can be for patients to achieve significant, lasting weight loss through lifestyle modification. Evidence suggests that metformin can help in this regard but not enough to recommend it. In addition, metformin therapy is associated with gastrointestinal adverse effects.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
1. Sadeghi A, Mousavi SM, Mokhtari T, et al. Metformin therapy reduces obesity indices in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Child Obes. 2020;16:174-191.
2. Mead E, Atkinson G, Richter B, et al. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev. 2016;11:CD012436.
3. O’Connor EA, Evans CV, Burda BU, et al. Screening for obesity and intervention for weight management in children and adolescents: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:2427-2444.
EVIDENCE-BASED ANSWER:
Yes, to some degree—but it is of uncertain clinical significance. Over a period of 6 months, metformin modestly reduced weight (–2.1 kg) and body fat mass (–1.9%), but not body mass index (BMI) or lean body mass, in adolescents who were overweight or obese. This is comparable to lifestyle interventions (diet and exercise) supported with > 26 hours of counseling, which modestly improved BMI but not weight. (Strength of recommendation [SOR]: A, based on a large meta-analysis of randomized controlled trials [RCTs] of variable quality).
Which detoxification regimens are effective for alcohol withdrawal syndrome?
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
EVIDENCE SUMMARY
Benzodiazepines work—but how do they compare?
A 2010 Cochrane meta-analysis of 64 RCTs and controlled clinical trials (CCTs; N = 4309) evaluated the use of benzodiazepines for treatment of AWS in adults.1 This systematic review compared benzodiazepines
- vs placebo (10 studies)
- vs other drugs, including phenobarbital, carbamazepine, topiramate, lamotrigine, gabapentin, haloperidol, clonidine, hydroxyzine, propranolol, and baclofen (42 studies)
- to other benzodiazepines, including chlordiazepoxide, alprazolam, diazepam, and lorazepam (18 studies)
- in combination with other drugs vs other drugs alone (3 studies)
- administered on a fixed schedule vs symptom-triggered administration (3 studies).
Primary outcomes included efficacy (alcohol withdrawal seizures, alcohol withdrawal delirium, alcohol withdrawal symptoms, global improvement), safety (adverse events and severe, life-threatening adverse events), and acceptability (dropouts and dropouts due to adverse events).
Benzodiazepines performed better than placebo for seizures in 3 studies (N = 324), with a relative risk (RR) of 0.16 (95% confidence interval [CI], 0.04-0.69). Studies assessing the described outcomes between benzodiazepines and other drugs were often of small sample size and heterogeneous in interventions and outcomes, limiting the ability to draw clear conclusions regarding benzodiazepine superiority. Comparisons of different benzodiazepines with each other and comparisons of benzodiazepines combined with other drugs vs other drugs alone did not reach statistical significance. Data on harms of benzodiazepines were lacking.
Anticonvulsants are not better than placebo for AWS
Another 2010 Cochrane meta-analysis of 56 RCTs and CCTs (N = 4076) evaluated the use of anticonvulsants for AWS.2 This systematic review compared anticonvulsants
- vs placebo (17 studies)
- vs other drugs, such as bromocriptine, piracetam, gamma-hydroxybutyric acid, trifluoperazine, clonidine, and various benzodiazepines (32 studies)
- to other anticonvulsants (10 studies)
- in combination with other drugs vs other drugs alone (6 studies)
- in combination with other drugs vs different anticonvulsants (1 study).
Primary outcomes included reductions in alcohol withdrawal seizures, adverse events, and acceptability of medication as indicated by participant dropouts.
Anticonvulsants were not superior to placebo for any outcome. Three studies (N = 260) favored carbamazepine over benzodiazepine (oxazepam or lorazepam) for 1 secondary outcome: a reduction of Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA-Ar) score (maximum score of 7; mean difference [MD] = –1 [95% CI, –1.9 to –0.2]).
Continue to: Gabapentin is effective; less sedating than chlordiazepoxide
Gabapentin is effective; less sedating than chlordiazepoxide
A 2013 RCT of US veterans with AWS (N = 26; 25 men; average age, 53.5 years) compared gabapentin and chlordiazepoxide.3 Endpoints were ratings on the Epworth Sleepiness Scale (ESS; maximum score = 24), Penn Alcohol Craving Scale (PACS; maximum score, 30), and CIWA-Ar.
In the early treatment period (Days 1-4), ESS and PACS scores did not differ significantly between groups. At end of treatment (Days 5-7), ESS and PACS scores were lower in gabapentin-treated patients (ESS: MD = –3.7; 95% CI, –7.2 to –0.19; P = .04; PACS: MD = –6.05; 95% CI –12.82 to 0.72; P = .08). CIWA-Ar did not differ between treatment groups.
Recommendations from others
In January 2020, the American Society of Addiction Medicine (ASAM) published a clinical practice guideline for alcohol withdrawal management. Protocols for diagnosis, assessment, level of care determination, and management are delineated.4
Benzodiazepines are the first-line treatment for moderate-to-severe AWS, or when there is risk for severe AWS. In the ambulatory setting, when AWS is mild and there is no risk for worsening, AWS can be managed with supportive care or with either benzodiazepines, gabapentin, or carbamazepine as monotherapy. ASAM recommends long-acting benzodiazepines (eg, chlordiazepoxide or diazepam) over short-acting benzodiazepines (eg, alprazolam or lorazepam), except in the elderly and those with liver or lung disease.5
Editor’s takeaway
Dozens of small trials and meta-analyses confirm the benefits (sometimes marginal) of sedation to treat alcohol withdrawal. Given that the evidence fails to point to the superiority of 1 agent over another, it seems reasonable to make treatment decisions based on physician and perhaps patient preference. This review does not support a change in clinical practice.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
1. Amato L, Minozzi S, Vecchi S, et al. Benzodiazepines for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005063.
2. Minozzi S, Amato L, Vecchi S, et al. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064.
3. Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47:961-969.
4. American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management 2020. Accessed March 2, 2021. www.asam.org/docs/default-source/quality-science/the_asam_clinical_practice_guideline_on_alcohol-1.pdf
5. Ries RK, Fiellin DA, Miller SC, et al. The ASAM Principles of Addiction Medicine. 4th ed. Lippincott Williams & Wilkins; 2014.
EVIDENCE-BASED ANSWER:
Benzodiazepines remain the first-line regimen for alcohol withdrawal syndrome (AWS) and are the only class more effective than placebo for reducing seizure (strength of recommendation [SOR]: B, based on 3 medium-quality randomized controlled trials [RCTs]). Anticonvulsants are no more effective than placebo at reducing seizures (SOR: B, based on 10 moderate-quality RCTs). Gabapentin reduces withdrawal symptoms and is less sedating than benzodiazepines (SOR: B, based on 1 medium-quality RCT). Carbamazepine also reduces withdrawal symptoms (SOR: B, based on 3 RCTs). Evidence of benzodiazepine superiority to other drugs with respect to safety is lacking (SOR: A, based on a meta-analysis).