User login
Is the incidence of depressive disorders increased following cerebral concussion?
EVIDENCE SUMMARY
Higher odds of depression in youth and adolescents with concussion
A 2019 prospective cohort study used data from the 2017 Nevada Youth Risk Behavior Surveillance Survey (YRBSS) to evaluate the relationship between concussion and depression in high school students.1 Included students were physically active for at least 60 minutes on 5 or more days per week or played on at least 1 sports team (N = 3427; 9th-12th grade students from 98 schools). When compared to the total population of included students and controlled for covariates, those who self-reported a concussion within the past 12 months (N = 664) had a higher adjusted odds ratio (aOR) of depressive symptoms (aOR = 1.5; 95% confidence interval [CI], 1.1-1.9). Depressive symptoms were reported in 38.1% of patients with a history of concussion, compared to 29.2% of patients who did not report a concussion in the past 12 months.
A 2014 retrospective cohort study examined data from the 2007-2008 National Survey of Children’s Health and evaluated the association between previous concussion and current depression diagnosis in youth ages 12 to 17 years without a current concussion (N = 36,060).2 Parents were contacted by random-digit dialing, prompted with a description of depression, and asked if their child currently had a clinical diagnosis of depression and whether a concussion had ever been diagnosed. A prior diagnosis of concussion was associated with greater risk for current depression compared to youth with no concussion history (aOR = 3.3; 95% CI, 2-5.5). Current depression was reported in 10.1% of patients with a history of concussion compared to 3.4% of patients with no history of concussion.
Findings vary among college athletes
A 2015 case-control study examined the prevalence of depressive symptoms in college athletes diagnosed with concussion compared to an athletic control group.3 The intervention group (N = 84; 77% male; average age, 18.4 years) received a concussion diagnosis from the team physician or certified athletic trainer. The athletic control group (N = 42; 55% male; average age, 18.9 years) reported no concussions in the past year.
The Beck Depression Inventory–Fast Screen (BDI-FS) was administered to the concussion group at baseline and postconcussion, and to the control group at 2 time points, with an average interval of 6.8 weeks. A score of ≥ 4 on the BDI-FS (scoring range, 0-21; higher score suggestive of more severe depression) indicated athletes at risk for depression. Concussed athletes exhibited a statistically significant increase in depression symptoms compared to control participants (20% vs 5%; x21 = 5.2; P = .02).
A 2018 cross-sectional study examined the association between concussion and adverse health outcomes in former college football players who played at least 1 year in college (1999-2001) but had no professional football experience.4 The cohort (N = 204; average age, 35) self-reported (15 years after their college career ended) the number of concussions sustained during high school and college sports performance. Reports were then stratified into 3 categories: no concussions, 1 or 2 concussions, and ≥ 3 concussions. The Patient Health Questionnaire (PHQ-9) was used to screen for depression, with scores categorized to no or mild depression (< 10) and moderate-to-severe depression (≥ 10).
Controlling for body mass index, athletes reporting ≥ 3 concussions had a higher prevalence of depression compared to those reporting no concussions (prevalence ratio [PR] = 4.2; 95% CI, 1.0-16.3) or 1 to 2 concussions (PR = 2.8; 95% CI, 1.3-6.0). No statistically significant association between concussion and depression was observed with athletes reporting 1 to 2 concussions compared to 0 concussions.
A 2015 prospective longitudinal cohort study examined postinjury depressive symptoms in 3 groups of Division 1 male and female college student athletes (N = 21; ages 18-22).5 Physician-diagnosed concussed (N = 7) and injured but nonconcussed (N = 7) athletes completed the Center for Epidemiological Studies Depression Scale (CES-D) at baseline and at 1 week, 1 month, and 3 months postinjury. Sport-matched healthy athletes (N = 7) completed it only at baseline. A CES-D score of ≥ 16 (range, 0-60) indicated a risk for clinical depression. Participants with a history of depression or other injury resulting in ≥ 1 day of time lost within the past 3 months were excluded.
Continue to: While both groups...
While both groups showed a significant increase from baseline CES-D scores, there were no significant differences in depressive symptoms between concussed (mean CES-D score ± standard deviation [SD]: baseline, 6.7 ± 3.9; 1 week, 11 ± 5.3; 1 month, 8.3 ± 5; 3 months, 6.4 ± 5.4) and injured but nonconcussed participants (mean CES-D score ± SD: baseline, 5.7 ± 2.8; 1 week, 9.1 ± 4; 1 month, 8.9 ± 4.6; 3 months, 6.9 ± 2.8) at any of the postinjury time points.
Findings among semipro and pro athletes appear to vary by sport
A 2016 prospective cohort study assessed the impact of concussive events on incidence of depression in active semiprofessional and professional football players who had previously sustained ≥ 1 concussions.6 Participants (N = 27) answered an anonymous online survey that included the revised version of the CES-D (CESD-R) to determine level of depression (a score of ≥ 16 defined clinical depression). Players with a CESD-R score ≥ 16 (N = 16) sustained a significantly greater average number of concussions compared to those who scored < 16 (N = 11; 3.8 vs. 1.6, P = .0004). Players who sustained ≥ 3 concussions scored significantly higher on the CESD-R than players with ≤ 2 concussions (average score, 24 vs 15.6; P = .03).
A 2017 case-control study examined the long-term health outcomes of retired Scottish male rugby players (N = 52; mean age, 54 years) with a history of mild concussion compared to males of similar age with no previous history of concussion (N = 29; mean age, 55).7 The Hospital Anxiety and Depression Scale (HADS) was used to assess depression on a 21-point scale (normal = 0-7; borderline, 8-10; abnormal, 11-21). There was no significant difference observed in mean HADS scores between the rugby players and controls, respectively (2.8 ± 2.1 vs 2.6 ± 2 .8; P = .941).
A 2013 case-control study of 30 retired NFL players with 29 controls matched for age, estimated IQ, and education examined the relationship between a remote history of concussion and current symptoms of depression.8 Concussion history was self-reported by the retired players. Controls with a history of concussion were excluded from the study. The Beck Depression Inventory-II (BDI-II) was used to measure depression symptoms, with a score of 1 to 9 designating minimal depression and ≥ 10 mild-to-moderate depression. Retired players scored significantly higher on the BDI-II compared to the controls (8.8 vs 2.8; P = .001).
Editor’s takeaway
Concussions include cognitive compromise. An astute clinician’s concern for depression as a sequela makes sense. This evidence contributes to that conjecture. However, the authors of this Clinical Inquiry correctly outline the limitations, inconsistencies, and biases of the evidence. The exact relationship—degree and context—between concussion and depression remains vague.
1. Yang MN, Clements-Nolle K, Parrish B, et al. Adolescent concussion and mental health outcomes: a population-based study. Am J Health Behav. 2019;43:258-265.
2. Chrisman SPD, Richardson LP. Prevalence of diagnosed depression in adolescents with history of concussion. J Adolesc Health. 2014;54:582-586.
3. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50:250-255.
4. Kerr ZY, Thomas LC, Simon JE, et al. Association between history of multiple concussions and health outcomes among former college football players. Am J Sports Med. 2018;46:1733-1741.
5. Roiger T, Weidauer L, Kern B. A longitudinal pilot study of depressive symptoms in concussed and injured/nonconcussed National Collegiate Athletic Association Division I student-athletes. J Athl Train. 2015;50:256-261.
6. Pryor J, Larson A, DeBeliso M. The prevalence of depression and concussions in a sample of active North American semi-professional and professional football players. J Lifestyle Med. 2016;6:7-15.
7. McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry. 2017;88:505-511.
8. Didehbani N, Munro Cullum C, Mansinghani S, et al. Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. 2013;28:418-424.
EVIDENCE SUMMARY
Higher odds of depression in youth and adolescents with concussion
A 2019 prospective cohort study used data from the 2017 Nevada Youth Risk Behavior Surveillance Survey (YRBSS) to evaluate the relationship between concussion and depression in high school students.1 Included students were physically active for at least 60 minutes on 5 or more days per week or played on at least 1 sports team (N = 3427; 9th-12th grade students from 98 schools). When compared to the total population of included students and controlled for covariates, those who self-reported a concussion within the past 12 months (N = 664) had a higher adjusted odds ratio (aOR) of depressive symptoms (aOR = 1.5; 95% confidence interval [CI], 1.1-1.9). Depressive symptoms were reported in 38.1% of patients with a history of concussion, compared to 29.2% of patients who did not report a concussion in the past 12 months.
A 2014 retrospective cohort study examined data from the 2007-2008 National Survey of Children’s Health and evaluated the association between previous concussion and current depression diagnosis in youth ages 12 to 17 years without a current concussion (N = 36,060).2 Parents were contacted by random-digit dialing, prompted with a description of depression, and asked if their child currently had a clinical diagnosis of depression and whether a concussion had ever been diagnosed. A prior diagnosis of concussion was associated with greater risk for current depression compared to youth with no concussion history (aOR = 3.3; 95% CI, 2-5.5). Current depression was reported in 10.1% of patients with a history of concussion compared to 3.4% of patients with no history of concussion.
Findings vary among college athletes
A 2015 case-control study examined the prevalence of depressive symptoms in college athletes diagnosed with concussion compared to an athletic control group.3 The intervention group (N = 84; 77% male; average age, 18.4 years) received a concussion diagnosis from the team physician or certified athletic trainer. The athletic control group (N = 42; 55% male; average age, 18.9 years) reported no concussions in the past year.
The Beck Depression Inventory–Fast Screen (BDI-FS) was administered to the concussion group at baseline and postconcussion, and to the control group at 2 time points, with an average interval of 6.8 weeks. A score of ≥ 4 on the BDI-FS (scoring range, 0-21; higher score suggestive of more severe depression) indicated athletes at risk for depression. Concussed athletes exhibited a statistically significant increase in depression symptoms compared to control participants (20% vs 5%; x21 = 5.2; P = .02).
A 2018 cross-sectional study examined the association between concussion and adverse health outcomes in former college football players who played at least 1 year in college (1999-2001) but had no professional football experience.4 The cohort (N = 204; average age, 35) self-reported (15 years after their college career ended) the number of concussions sustained during high school and college sports performance. Reports were then stratified into 3 categories: no concussions, 1 or 2 concussions, and ≥ 3 concussions. The Patient Health Questionnaire (PHQ-9) was used to screen for depression, with scores categorized to no or mild depression (< 10) and moderate-to-severe depression (≥ 10).
Controlling for body mass index, athletes reporting ≥ 3 concussions had a higher prevalence of depression compared to those reporting no concussions (prevalence ratio [PR] = 4.2; 95% CI, 1.0-16.3) or 1 to 2 concussions (PR = 2.8; 95% CI, 1.3-6.0). No statistically significant association between concussion and depression was observed with athletes reporting 1 to 2 concussions compared to 0 concussions.
A 2015 prospective longitudinal cohort study examined postinjury depressive symptoms in 3 groups of Division 1 male and female college student athletes (N = 21; ages 18-22).5 Physician-diagnosed concussed (N = 7) and injured but nonconcussed (N = 7) athletes completed the Center for Epidemiological Studies Depression Scale (CES-D) at baseline and at 1 week, 1 month, and 3 months postinjury. Sport-matched healthy athletes (N = 7) completed it only at baseline. A CES-D score of ≥ 16 (range, 0-60) indicated a risk for clinical depression. Participants with a history of depression or other injury resulting in ≥ 1 day of time lost within the past 3 months were excluded.
Continue to: While both groups...
While both groups showed a significant increase from baseline CES-D scores, there were no significant differences in depressive symptoms between concussed (mean CES-D score ± standard deviation [SD]: baseline, 6.7 ± 3.9; 1 week, 11 ± 5.3; 1 month, 8.3 ± 5; 3 months, 6.4 ± 5.4) and injured but nonconcussed participants (mean CES-D score ± SD: baseline, 5.7 ± 2.8; 1 week, 9.1 ± 4; 1 month, 8.9 ± 4.6; 3 months, 6.9 ± 2.8) at any of the postinjury time points.
Findings among semipro and pro athletes appear to vary by sport
A 2016 prospective cohort study assessed the impact of concussive events on incidence of depression in active semiprofessional and professional football players who had previously sustained ≥ 1 concussions.6 Participants (N = 27) answered an anonymous online survey that included the revised version of the CES-D (CESD-R) to determine level of depression (a score of ≥ 16 defined clinical depression). Players with a CESD-R score ≥ 16 (N = 16) sustained a significantly greater average number of concussions compared to those who scored < 16 (N = 11; 3.8 vs. 1.6, P = .0004). Players who sustained ≥ 3 concussions scored significantly higher on the CESD-R than players with ≤ 2 concussions (average score, 24 vs 15.6; P = .03).
A 2017 case-control study examined the long-term health outcomes of retired Scottish male rugby players (N = 52; mean age, 54 years) with a history of mild concussion compared to males of similar age with no previous history of concussion (N = 29; mean age, 55).7 The Hospital Anxiety and Depression Scale (HADS) was used to assess depression on a 21-point scale (normal = 0-7; borderline, 8-10; abnormal, 11-21). There was no significant difference observed in mean HADS scores between the rugby players and controls, respectively (2.8 ± 2.1 vs 2.6 ± 2 .8; P = .941).
A 2013 case-control study of 30 retired NFL players with 29 controls matched for age, estimated IQ, and education examined the relationship between a remote history of concussion and current symptoms of depression.8 Concussion history was self-reported by the retired players. Controls with a history of concussion were excluded from the study. The Beck Depression Inventory-II (BDI-II) was used to measure depression symptoms, with a score of 1 to 9 designating minimal depression and ≥ 10 mild-to-moderate depression. Retired players scored significantly higher on the BDI-II compared to the controls (8.8 vs 2.8; P = .001).
Editor’s takeaway
Concussions include cognitive compromise. An astute clinician’s concern for depression as a sequela makes sense. This evidence contributes to that conjecture. However, the authors of this Clinical Inquiry correctly outline the limitations, inconsistencies, and biases of the evidence. The exact relationship—degree and context—between concussion and depression remains vague.
EVIDENCE SUMMARY
Higher odds of depression in youth and adolescents with concussion
A 2019 prospective cohort study used data from the 2017 Nevada Youth Risk Behavior Surveillance Survey (YRBSS) to evaluate the relationship between concussion and depression in high school students.1 Included students were physically active for at least 60 minutes on 5 or more days per week or played on at least 1 sports team (N = 3427; 9th-12th grade students from 98 schools). When compared to the total population of included students and controlled for covariates, those who self-reported a concussion within the past 12 months (N = 664) had a higher adjusted odds ratio (aOR) of depressive symptoms (aOR = 1.5; 95% confidence interval [CI], 1.1-1.9). Depressive symptoms were reported in 38.1% of patients with a history of concussion, compared to 29.2% of patients who did not report a concussion in the past 12 months.
A 2014 retrospective cohort study examined data from the 2007-2008 National Survey of Children’s Health and evaluated the association between previous concussion and current depression diagnosis in youth ages 12 to 17 years without a current concussion (N = 36,060).2 Parents were contacted by random-digit dialing, prompted with a description of depression, and asked if their child currently had a clinical diagnosis of depression and whether a concussion had ever been diagnosed. A prior diagnosis of concussion was associated with greater risk for current depression compared to youth with no concussion history (aOR = 3.3; 95% CI, 2-5.5). Current depression was reported in 10.1% of patients with a history of concussion compared to 3.4% of patients with no history of concussion.
Findings vary among college athletes
A 2015 case-control study examined the prevalence of depressive symptoms in college athletes diagnosed with concussion compared to an athletic control group.3 The intervention group (N = 84; 77% male; average age, 18.4 years) received a concussion diagnosis from the team physician or certified athletic trainer. The athletic control group (N = 42; 55% male; average age, 18.9 years) reported no concussions in the past year.
The Beck Depression Inventory–Fast Screen (BDI-FS) was administered to the concussion group at baseline and postconcussion, and to the control group at 2 time points, with an average interval of 6.8 weeks. A score of ≥ 4 on the BDI-FS (scoring range, 0-21; higher score suggestive of more severe depression) indicated athletes at risk for depression. Concussed athletes exhibited a statistically significant increase in depression symptoms compared to control participants (20% vs 5%; x21 = 5.2; P = .02).
A 2018 cross-sectional study examined the association between concussion and adverse health outcomes in former college football players who played at least 1 year in college (1999-2001) but had no professional football experience.4 The cohort (N = 204; average age, 35) self-reported (15 years after their college career ended) the number of concussions sustained during high school and college sports performance. Reports were then stratified into 3 categories: no concussions, 1 or 2 concussions, and ≥ 3 concussions. The Patient Health Questionnaire (PHQ-9) was used to screen for depression, with scores categorized to no or mild depression (< 10) and moderate-to-severe depression (≥ 10).
Controlling for body mass index, athletes reporting ≥ 3 concussions had a higher prevalence of depression compared to those reporting no concussions (prevalence ratio [PR] = 4.2; 95% CI, 1.0-16.3) or 1 to 2 concussions (PR = 2.8; 95% CI, 1.3-6.0). No statistically significant association between concussion and depression was observed with athletes reporting 1 to 2 concussions compared to 0 concussions.
A 2015 prospective longitudinal cohort study examined postinjury depressive symptoms in 3 groups of Division 1 male and female college student athletes (N = 21; ages 18-22).5 Physician-diagnosed concussed (N = 7) and injured but nonconcussed (N = 7) athletes completed the Center for Epidemiological Studies Depression Scale (CES-D) at baseline and at 1 week, 1 month, and 3 months postinjury. Sport-matched healthy athletes (N = 7) completed it only at baseline. A CES-D score of ≥ 16 (range, 0-60) indicated a risk for clinical depression. Participants with a history of depression or other injury resulting in ≥ 1 day of time lost within the past 3 months were excluded.
Continue to: While both groups...
While both groups showed a significant increase from baseline CES-D scores, there were no significant differences in depressive symptoms between concussed (mean CES-D score ± standard deviation [SD]: baseline, 6.7 ± 3.9; 1 week, 11 ± 5.3; 1 month, 8.3 ± 5; 3 months, 6.4 ± 5.4) and injured but nonconcussed participants (mean CES-D score ± SD: baseline, 5.7 ± 2.8; 1 week, 9.1 ± 4; 1 month, 8.9 ± 4.6; 3 months, 6.9 ± 2.8) at any of the postinjury time points.
Findings among semipro and pro athletes appear to vary by sport
A 2016 prospective cohort study assessed the impact of concussive events on incidence of depression in active semiprofessional and professional football players who had previously sustained ≥ 1 concussions.6 Participants (N = 27) answered an anonymous online survey that included the revised version of the CES-D (CESD-R) to determine level of depression (a score of ≥ 16 defined clinical depression). Players with a CESD-R score ≥ 16 (N = 16) sustained a significantly greater average number of concussions compared to those who scored < 16 (N = 11; 3.8 vs. 1.6, P = .0004). Players who sustained ≥ 3 concussions scored significantly higher on the CESD-R than players with ≤ 2 concussions (average score, 24 vs 15.6; P = .03).
A 2017 case-control study examined the long-term health outcomes of retired Scottish male rugby players (N = 52; mean age, 54 years) with a history of mild concussion compared to males of similar age with no previous history of concussion (N = 29; mean age, 55).7 The Hospital Anxiety and Depression Scale (HADS) was used to assess depression on a 21-point scale (normal = 0-7; borderline, 8-10; abnormal, 11-21). There was no significant difference observed in mean HADS scores between the rugby players and controls, respectively (2.8 ± 2.1 vs 2.6 ± 2 .8; P = .941).
A 2013 case-control study of 30 retired NFL players with 29 controls matched for age, estimated IQ, and education examined the relationship between a remote history of concussion and current symptoms of depression.8 Concussion history was self-reported by the retired players. Controls with a history of concussion were excluded from the study. The Beck Depression Inventory-II (BDI-II) was used to measure depression symptoms, with a score of 1 to 9 designating minimal depression and ≥ 10 mild-to-moderate depression. Retired players scored significantly higher on the BDI-II compared to the controls (8.8 vs 2.8; P = .001).
Editor’s takeaway
Concussions include cognitive compromise. An astute clinician’s concern for depression as a sequela makes sense. This evidence contributes to that conjecture. However, the authors of this Clinical Inquiry correctly outline the limitations, inconsistencies, and biases of the evidence. The exact relationship—degree and context—between concussion and depression remains vague.
1. Yang MN, Clements-Nolle K, Parrish B, et al. Adolescent concussion and mental health outcomes: a population-based study. Am J Health Behav. 2019;43:258-265.
2. Chrisman SPD, Richardson LP. Prevalence of diagnosed depression in adolescents with history of concussion. J Adolesc Health. 2014;54:582-586.
3. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50:250-255.
4. Kerr ZY, Thomas LC, Simon JE, et al. Association between history of multiple concussions and health outcomes among former college football players. Am J Sports Med. 2018;46:1733-1741.
5. Roiger T, Weidauer L, Kern B. A longitudinal pilot study of depressive symptoms in concussed and injured/nonconcussed National Collegiate Athletic Association Division I student-athletes. J Athl Train. 2015;50:256-261.
6. Pryor J, Larson A, DeBeliso M. The prevalence of depression and concussions in a sample of active North American semi-professional and professional football players. J Lifestyle Med. 2016;6:7-15.
7. McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry. 2017;88:505-511.
8. Didehbani N, Munro Cullum C, Mansinghani S, et al. Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. 2013;28:418-424.
1. Yang MN, Clements-Nolle K, Parrish B, et al. Adolescent concussion and mental health outcomes: a population-based study. Am J Health Behav. 2019;43:258-265.
2. Chrisman SPD, Richardson LP. Prevalence of diagnosed depression in adolescents with history of concussion. J Adolesc Health. 2014;54:582-586.
3. Vargas G, Rabinowitz A, Meyer J, et al. Predictors and prevalence of postconcussion depression symptoms in collegiate athletes. J Athl Train. 2015;50:250-255.
4. Kerr ZY, Thomas LC, Simon JE, et al. Association between history of multiple concussions and health outcomes among former college football players. Am J Sports Med. 2018;46:1733-1741.
5. Roiger T, Weidauer L, Kern B. A longitudinal pilot study of depressive symptoms in concussed and injured/nonconcussed National Collegiate Athletic Association Division I student-athletes. J Athl Train. 2015;50:256-261.
6. Pryor J, Larson A, DeBeliso M. The prevalence of depression and concussions in a sample of active North American semi-professional and professional football players. J Lifestyle Med. 2016;6:7-15.
7. McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry. 2017;88:505-511.
8. Didehbani N, Munro Cullum C, Mansinghani S, et al. Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol. 2013;28:418-424.
EVIDENCE-BASED ANSWER
Yes, in some populations. Youth and adolescents with self-reported history of concussion had increased risk of depressive disorders (strength of recommendation [SOR]: B, based on a prospective cohort study and a retrospective cohort study). Evidence was inconsistent for college athletes. Athletes with ≥ 3 concussions exhibited more depressive disorders, but no association was observed for those with 1 or 2 concussions compared to nonconcussion injuries (SOR: B, based on a cross-sectional study, a small prospective cohort study, and a case-control study).
In semiprofessional and professional athletes, evidence was variable and may be sport related. Retired rugby players with a history of concussion showed no increase in depression compared to controls with no concussion history (SOR: B, based on a case-control study). Retired football players with previous concussions displayed increased incidence of depression, especially after ≥ 3 concussions (SOR: B, based on a prospective cohort study and a small case-control study).
There is a significant risk of bias in these studies because of their reliance on self-reported concussions, differing definitions of depression, and possible unmeasured confounders in the study designs, making a causative relationship between concussion and depression unclear.
Does XR injectable naltrexone prevent relapse as effectively as daily sublingual buprenorphine-naloxone?
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE-BASED ANSWER:
Yes. Monthly extended-release injectable naltrexone (XR-NTX) treats opioid use disorder as effectively as daily sublingual buprenorphine-naloxone (BUP-NX) without causing any increase in serious adverse events or fatal overdoses. (strength of recommendation: A, 2 good-quality RCTs).
Do electronic reminder systems help patients with T2DM to lose weight?
EVIDENCE SUMMARY
A meta-analysis of 6 RCTs studied the effect of smartphone self-care applications on A1C, weight, blood pressure, and lipids in adult patients with T2DM. All the interventions comprised 4 components: electronic self-management prompts and reminders, personal measuring devices, patient-driven data upload, and remote analysis of the data with feedback. The review excluded studies that used phone calls or lasted fewer than 3 months.
Some improvement in A1C found, but no effect on weight
Telehealth interventions improved A1C more than usual care (6 trials, 884 patients; mean difference = –0.40%; 95% CI, –0.69% to –0.11%).1 A subset of 4 studies with 560 patients evaluated changes in weight. Patients had a mean age of 61 years and average weight of 84 kg (in 3 of 4 studies reporting baseline weight). Aggregate weight loss was insignificant after 3 to 12 months (mean difference = –0.84 kg; 95% CI, –2.04 kg to 0.36 kg, P = .17). Investigators reported no harms. Limitations of the analysis included high heterogeneity in the main outcome of A1C (I2 = 70%) but low heterogeneity within the 4 studies assessing weight (I2 = 30%).
Other, small studies found no change in A1C
Two subsequent small RCTs came to different conclusions than the meta-analysis. One compared the impact of individualized physical activity–based text messages in response to pedometer readings with pedometer use alone.2 It included 126 adult patients (mean age, 50.5 years) with T2DM who had an A1C > 7% and access to an Internet-connected computer. Researchers excluded patients who were unable to perform moderate physical activity or who had cognitive deficits.
At enrollment, researchers supplied all patients with a pedometer and an appointment with a counselor to set goals for physical activity. They sent 2 text messages daily to the intervention group (and none to the control group) based on uploaded pedometer data. One message detailed physical activity progress and the second encouraged increased physical activity. The primary outcome was mean step counts per month; secondary outcomes included A1C and weight measured at 6 months.
The groups showed no significant difference in A1C (mean difference = 0.07%; 95% CI, –0.47% to 0.34%, P = .75) or weight loss (mean difference = 3.1 lb; 95% CI, –24.5 lb to 18.3 lb, P = .77). Many patients (43%) reported difficulty uploading step counts, receiving texts, and responding to texts. The dropout rate was 24%.
A second RCT with 150 patients, using a less elaborate protocol, assessed the effectiveness of tailored text-message reminders compared with nontailored text messages to improve A1C and body mass index (BMI).3 Patients were adult Iranians (mean age, 52.5 years) with T2DM who owned a cell phone and could receive and read text messages.
Patients filled out a diabetic self-care assessment to identify barriers to improving care and were randomized into 3 groups. The first group received tailored text messages (75% addressing the patient’s top 2 barriers to self-care and 25% general messages). The second group received nontailored text messages of encouragement. The control group received no text messages.
Continue to: After 3 months...
After 3 months, BMI was reduced in both messaging groups but not the control group (tailored text = –0.6 kg/m2, nontailored text = –0.5 kg/m2, controls = 0.7 kg/m2; P < .05). A1C levels didn’t change significantly. One limitation of the study was that 30% to 35% of the patients in the intervention group had a university-level education, compared with 12% in the control group.
Recommendations
The Department of Veterans Affairs issued guidelines in 2017 regarding management of patients with T2DM in primary care.4 The guidelines state that all patients should receive individualized self-management education using “modalities tailored to their preferences” (strong recommendation). They further recommend “offering one or more bidirectional telehealth interventions” in coordination with patients’ health care providers (weak recommendation).
The 2017 diabetes self-management recommendations endorsed by the American Diabetes Association state that “strong evidence” shows that incorporating text messaging into diabetes care improves outcomes, enhances feedback loops, and empowers patients.5
Editor’s takeaway
Telehealth offers mechanisms for patients and physicians to enhance communication about health behaviors and health status. But does it alter outcomes? The cited literature suggests that benefits aren’t a forgone conclusion and that acceptability, ease of use, cost, and individualization are critical issues in telehealth design.
1. Cui M, Wu X, Mao J, et al. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0166718.
2. Agboola S, Jethwani K, Lopez L, et al. Text to Move: A randomized controlled trial of a text-messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus. J Med Internet Res. 2016;18:e307.
3. Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016;10:251-258.
4. Guideline summary: VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.innovations.ahrq.gov/qualitytools/department-veterans-affairsdepartment-defense-vadod-clinical-practice-guideline-4. Accessed October 26, 2020.
5. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management, Education and Support. Diabetes Care. 2017;40:1409-1419.
EVIDENCE SUMMARY
A meta-analysis of 6 RCTs studied the effect of smartphone self-care applications on A1C, weight, blood pressure, and lipids in adult patients with T2DM. All the interventions comprised 4 components: electronic self-management prompts and reminders, personal measuring devices, patient-driven data upload, and remote analysis of the data with feedback. The review excluded studies that used phone calls or lasted fewer than 3 months.
Some improvement in A1C found, but no effect on weight
Telehealth interventions improved A1C more than usual care (6 trials, 884 patients; mean difference = –0.40%; 95% CI, –0.69% to –0.11%).1 A subset of 4 studies with 560 patients evaluated changes in weight. Patients had a mean age of 61 years and average weight of 84 kg (in 3 of 4 studies reporting baseline weight). Aggregate weight loss was insignificant after 3 to 12 months (mean difference = –0.84 kg; 95% CI, –2.04 kg to 0.36 kg, P = .17). Investigators reported no harms. Limitations of the analysis included high heterogeneity in the main outcome of A1C (I2 = 70%) but low heterogeneity within the 4 studies assessing weight (I2 = 30%).
Other, small studies found no change in A1C
Two subsequent small RCTs came to different conclusions than the meta-analysis. One compared the impact of individualized physical activity–based text messages in response to pedometer readings with pedometer use alone.2 It included 126 adult patients (mean age, 50.5 years) with T2DM who had an A1C > 7% and access to an Internet-connected computer. Researchers excluded patients who were unable to perform moderate physical activity or who had cognitive deficits.
At enrollment, researchers supplied all patients with a pedometer and an appointment with a counselor to set goals for physical activity. They sent 2 text messages daily to the intervention group (and none to the control group) based on uploaded pedometer data. One message detailed physical activity progress and the second encouraged increased physical activity. The primary outcome was mean step counts per month; secondary outcomes included A1C and weight measured at 6 months.
The groups showed no significant difference in A1C (mean difference = 0.07%; 95% CI, –0.47% to 0.34%, P = .75) or weight loss (mean difference = 3.1 lb; 95% CI, –24.5 lb to 18.3 lb, P = .77). Many patients (43%) reported difficulty uploading step counts, receiving texts, and responding to texts. The dropout rate was 24%.
A second RCT with 150 patients, using a less elaborate protocol, assessed the effectiveness of tailored text-message reminders compared with nontailored text messages to improve A1C and body mass index (BMI).3 Patients were adult Iranians (mean age, 52.5 years) with T2DM who owned a cell phone and could receive and read text messages.
Patients filled out a diabetic self-care assessment to identify barriers to improving care and were randomized into 3 groups. The first group received tailored text messages (75% addressing the patient’s top 2 barriers to self-care and 25% general messages). The second group received nontailored text messages of encouragement. The control group received no text messages.
Continue to: After 3 months...
After 3 months, BMI was reduced in both messaging groups but not the control group (tailored text = –0.6 kg/m2, nontailored text = –0.5 kg/m2, controls = 0.7 kg/m2; P < .05). A1C levels didn’t change significantly. One limitation of the study was that 30% to 35% of the patients in the intervention group had a university-level education, compared with 12% in the control group.
Recommendations
The Department of Veterans Affairs issued guidelines in 2017 regarding management of patients with T2DM in primary care.4 The guidelines state that all patients should receive individualized self-management education using “modalities tailored to their preferences” (strong recommendation). They further recommend “offering one or more bidirectional telehealth interventions” in coordination with patients’ health care providers (weak recommendation).
The 2017 diabetes self-management recommendations endorsed by the American Diabetes Association state that “strong evidence” shows that incorporating text messaging into diabetes care improves outcomes, enhances feedback loops, and empowers patients.5
Editor’s takeaway
Telehealth offers mechanisms for patients and physicians to enhance communication about health behaviors and health status. But does it alter outcomes? The cited literature suggests that benefits aren’t a forgone conclusion and that acceptability, ease of use, cost, and individualization are critical issues in telehealth design.
EVIDENCE SUMMARY
A meta-analysis of 6 RCTs studied the effect of smartphone self-care applications on A1C, weight, blood pressure, and lipids in adult patients with T2DM. All the interventions comprised 4 components: electronic self-management prompts and reminders, personal measuring devices, patient-driven data upload, and remote analysis of the data with feedback. The review excluded studies that used phone calls or lasted fewer than 3 months.
Some improvement in A1C found, but no effect on weight
Telehealth interventions improved A1C more than usual care (6 trials, 884 patients; mean difference = –0.40%; 95% CI, –0.69% to –0.11%).1 A subset of 4 studies with 560 patients evaluated changes in weight. Patients had a mean age of 61 years and average weight of 84 kg (in 3 of 4 studies reporting baseline weight). Aggregate weight loss was insignificant after 3 to 12 months (mean difference = –0.84 kg; 95% CI, –2.04 kg to 0.36 kg, P = .17). Investigators reported no harms. Limitations of the analysis included high heterogeneity in the main outcome of A1C (I2 = 70%) but low heterogeneity within the 4 studies assessing weight (I2 = 30%).
Other, small studies found no change in A1C
Two subsequent small RCTs came to different conclusions than the meta-analysis. One compared the impact of individualized physical activity–based text messages in response to pedometer readings with pedometer use alone.2 It included 126 adult patients (mean age, 50.5 years) with T2DM who had an A1C > 7% and access to an Internet-connected computer. Researchers excluded patients who were unable to perform moderate physical activity or who had cognitive deficits.
At enrollment, researchers supplied all patients with a pedometer and an appointment with a counselor to set goals for physical activity. They sent 2 text messages daily to the intervention group (and none to the control group) based on uploaded pedometer data. One message detailed physical activity progress and the second encouraged increased physical activity. The primary outcome was mean step counts per month; secondary outcomes included A1C and weight measured at 6 months.
The groups showed no significant difference in A1C (mean difference = 0.07%; 95% CI, –0.47% to 0.34%, P = .75) or weight loss (mean difference = 3.1 lb; 95% CI, –24.5 lb to 18.3 lb, P = .77). Many patients (43%) reported difficulty uploading step counts, receiving texts, and responding to texts. The dropout rate was 24%.
A second RCT with 150 patients, using a less elaborate protocol, assessed the effectiveness of tailored text-message reminders compared with nontailored text messages to improve A1C and body mass index (BMI).3 Patients were adult Iranians (mean age, 52.5 years) with T2DM who owned a cell phone and could receive and read text messages.
Patients filled out a diabetic self-care assessment to identify barriers to improving care and were randomized into 3 groups. The first group received tailored text messages (75% addressing the patient’s top 2 barriers to self-care and 25% general messages). The second group received nontailored text messages of encouragement. The control group received no text messages.
Continue to: After 3 months...
After 3 months, BMI was reduced in both messaging groups but not the control group (tailored text = –0.6 kg/m2, nontailored text = –0.5 kg/m2, controls = 0.7 kg/m2; P < .05). A1C levels didn’t change significantly. One limitation of the study was that 30% to 35% of the patients in the intervention group had a university-level education, compared with 12% in the control group.
Recommendations
The Department of Veterans Affairs issued guidelines in 2017 regarding management of patients with T2DM in primary care.4 The guidelines state that all patients should receive individualized self-management education using “modalities tailored to their preferences” (strong recommendation). They further recommend “offering one or more bidirectional telehealth interventions” in coordination with patients’ health care providers (weak recommendation).
The 2017 diabetes self-management recommendations endorsed by the American Diabetes Association state that “strong evidence” shows that incorporating text messaging into diabetes care improves outcomes, enhances feedback loops, and empowers patients.5
Editor’s takeaway
Telehealth offers mechanisms for patients and physicians to enhance communication about health behaviors and health status. But does it alter outcomes? The cited literature suggests that benefits aren’t a forgone conclusion and that acceptability, ease of use, cost, and individualization are critical issues in telehealth design.
1. Cui M, Wu X, Mao J, et al. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0166718.
2. Agboola S, Jethwani K, Lopez L, et al. Text to Move: A randomized controlled trial of a text-messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus. J Med Internet Res. 2016;18:e307.
3. Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016;10:251-258.
4. Guideline summary: VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.innovations.ahrq.gov/qualitytools/department-veterans-affairsdepartment-defense-vadod-clinical-practice-guideline-4. Accessed October 26, 2020.
5. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management, Education and Support. Diabetes Care. 2017;40:1409-1419.
1. Cui M, Wu X, Mao J, et al. T2DM self-management via smartphone applications: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0166718.
2. Agboola S, Jethwani K, Lopez L, et al. Text to Move: A randomized controlled trial of a text-messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus. J Med Internet Res. 2016;18:e307.
3. Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016;10:251-258.
4. Guideline summary: VA/DoD clinical practice guideline for the management of type 2 diabetes mellitus in primary care. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.innovations.ahrq.gov/qualitytools/department-veterans-affairsdepartment-defense-vadod-clinical-practice-guideline-4. Accessed October 26, 2020.
5. Beck J, Greenwood DA, Blanton L, et al. 2017 National Standards for Diabetes Self-Management, Education and Support. Diabetes Care. 2017;40:1409-1419.
EVIDENCE-BASED ANSWER:
PROBABLY NOT—but they may augment self-management. Four-component telehealth systems—including electronic reminders, measuring devices, patient-driven data upload, and remote data analysis—likely don’t result in significant weight reductions in adults with type 2 diabetes (T2DM). However, their use may be associated with a decrease in hemoglobin A1C of about 0.4% (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] and conflicting smaller subsequent RCTs).
Telehealth is considered a reasonable option for augmenting diabetes self-management in patients who are facile with the technology (SOR: C, expert opinion).
Does early introduction of peanuts to an infant’s diet reduce the risk for peanut allergy?
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
EVIDENCE SUMMARY
A 2016 systematic review identified 2 RCTs that examined whether early introduction of peanuts affects subsequent allergies.1 The first RCT recruited 1303 3-month-old infants from the general population in the United Kingdom.2 All patients had either a negative skin prick test (SPT) to peanuts or a negative oral peanut challenge (if an initial SPT was positive). The control group breastfed exclusively until age 6 months, at which time allergenic foods could be introduced at parental discretion.
Timing doesn’t affect peanut allergy in nonallergic patients
The intervention group received 6 common allergenic foods (peanuts, eggs, cow’s milk, wheat, sesame, and whitefish) twice weekly between ages 3 and 6 months. Researchers then performed double-blinded, placebo-controlled oral food challenges at ages 12 and 36 months.
More patients in the late-introduction group demonstrated peanut allergies by age 36 months than in the early-introduction group, but the difference wasn’t significant (2.5% vs 1.2%; P = 0.11).A key weakness of the study was combining peanuts with other common food allergens.2
Children with eczema, egg allergy benefit from earlier peanut introduction
The second RCT divided 640 infants with severe eczema, egg allergy, or both into 2 groups according to their response to an SPT to peanuts: patients with no wheal and patients with a positive wheal measuring 1 to 4 mm.3 Researchers then randomized patients to either early exposure (peanut products given from ages 4 to 11 months) or avoidance (no peanuts until age 60 months). The primary endpoint was a positive clinical response to oral peanut allergen at age 60 months.
In the negative SPT group (atopic children expected to have a lower risk for allergy), patients introduced to peanuts later had a higher rate of subsequent allergy than children exposed earlier (14% vs 2%; absolute risk reduction [ARR] = 12%; 95% confidence interval [CI], 3%-20%; number needed to treat [NNT] = 9).3
In the positive SPT group (atopic children expected to have a higher risk for allergy), later peanut introduction likewise increased risk compared to earlier introduction (35% vs 11%; ARR = 24%; 95% CI, 5%-43%; NNT = 5). Children in the early-exposure group, however, had more URIs, viral exanthems, gastroenteritis, urticaria, and conjunctivitis (4527 events in the early-exposure group vs 4287 in the avoidance group, P = 0.02; about 1 more event per patient over the course of the study).3
The authors of the systematic review performed a meta-analysis of the 2 RCTs (1793 patients). They concluded that early introduction of peanuts to an infant’s diet (between ages 3 and 11 months) decreased the risk for eventual peanut allergy (relative risk [RR] = 0.29; 95% CI, 0.11-0.74), compared with introduction at or after age 1 year.1 A key weakness, however, was the researchers’ choice to combine trials with very different inclusion criteria (infants with severe eczema and a general population).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
A 2017 National Institute of Allergy and Infectious Diseases guideline recommends a 3-tiered approach to peanut introduction: 4
- For children with severe eczema or egg allergy who aren’t currently allergic to peanuts (per SPT or immunoglobulin E [IgE] test), the guideline advises adding peanuts to the diet between ages 4 and 6 months. (Patients with positive SPT or IgE should be referred to an allergy specialist.)
- Children with mild or moderate eczema can be introduced to peanuts around age 6 months “in accordance with family preferences and cultural practices.”
- Children with no evidence of allergy or eczema can be “freely introduced” to peanut-containing foods with no specific guidance on age.
Editor’s takeaway
Good-quality evidence supports family physicians encouraging introduction of foods containing peanuts at age 4 to 6 months for children at increased risk because of atopy, allergies, or eczema.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
1. Ierodiakonou D, Garcia-Larsen V, Logan A, et al. Timing of allergenic food introduction to the infant diet and risk of allergic or autoimmune disease: a systematic review and meta-analysis. JAMA. 2016;316:1181-1192.
2. Perkin MR, Logan K, Tseng A, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733-1743.
3. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
4. Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139:29-44.
EVIDENCE-BASED ANSWER:
Probably not, unless the child has severe eczema or egg allergy. In a general pediatric population, introducing peanuts early (at age 3 to 6 months) doesn’t appear to alter rates of subsequent peanut allergy compared with introduction after age 6 months (strength of recommendation [SOR]: B, randomized clinical trial [RCT] using multiple potential food allergens).
In children with severe eczema, egg allergy, or both, however, the risk for a peanut allergy is 12% to 24% lower when peanut-containing foods are introduced at age 4 to 11 months than after age 1 year. Early introduction of peanuts is associated with about 1 additional mild virus-associated syndrome (upper respiratory infection [URI], exanthem, conjunctivitis, or gastroenteritis) per patient (SOR: B, RCT).
Introducing peanuts before age 1 year is recommended for atopic children without evidence of pre-existing peanut allergy; an earlier start, at age 4 to 6 months, is advised for infants with severe eczema or egg allergy (SOR: C, expert opinion).
Does evidence support the use of supplements to aid in BP control?
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).
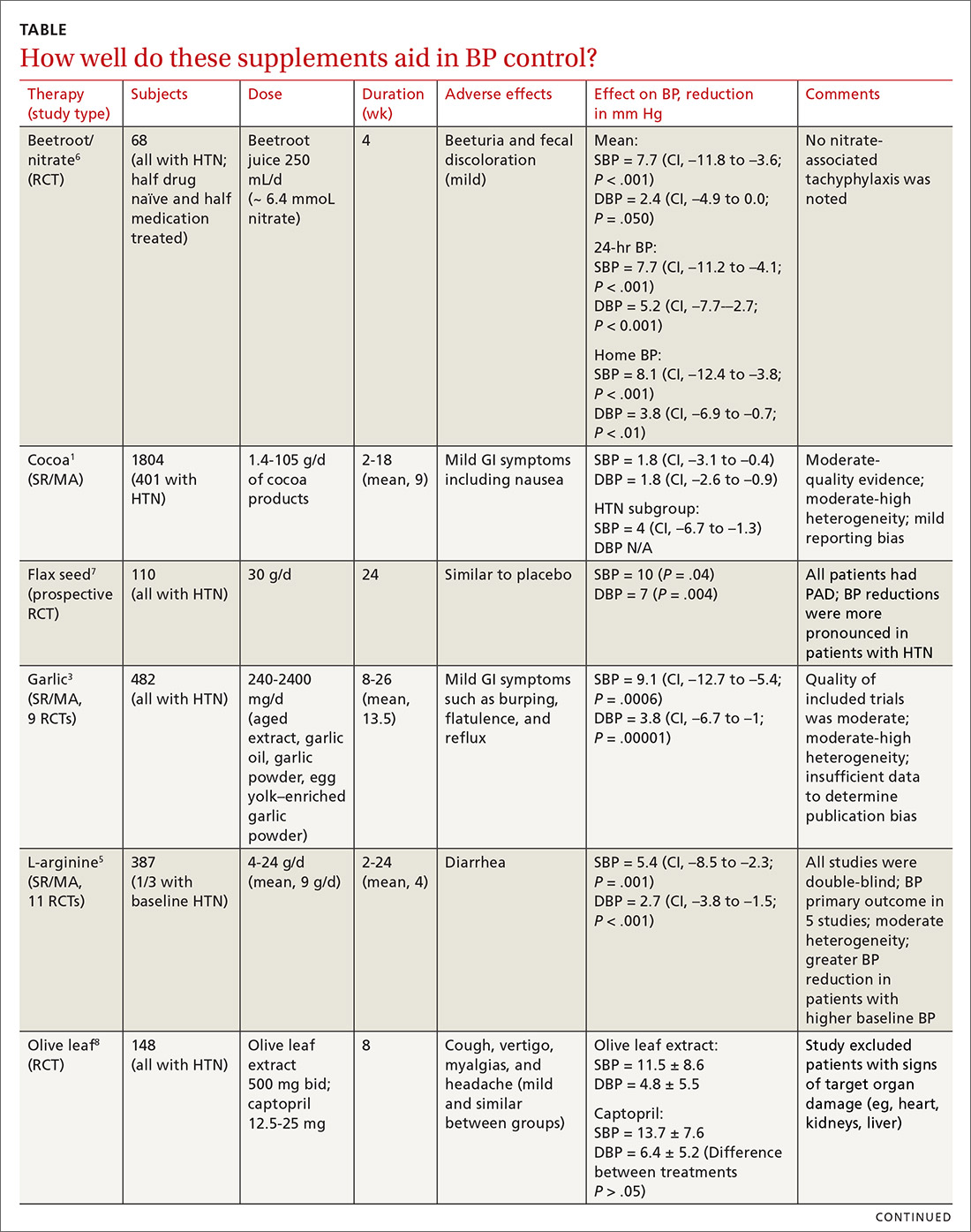
Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.
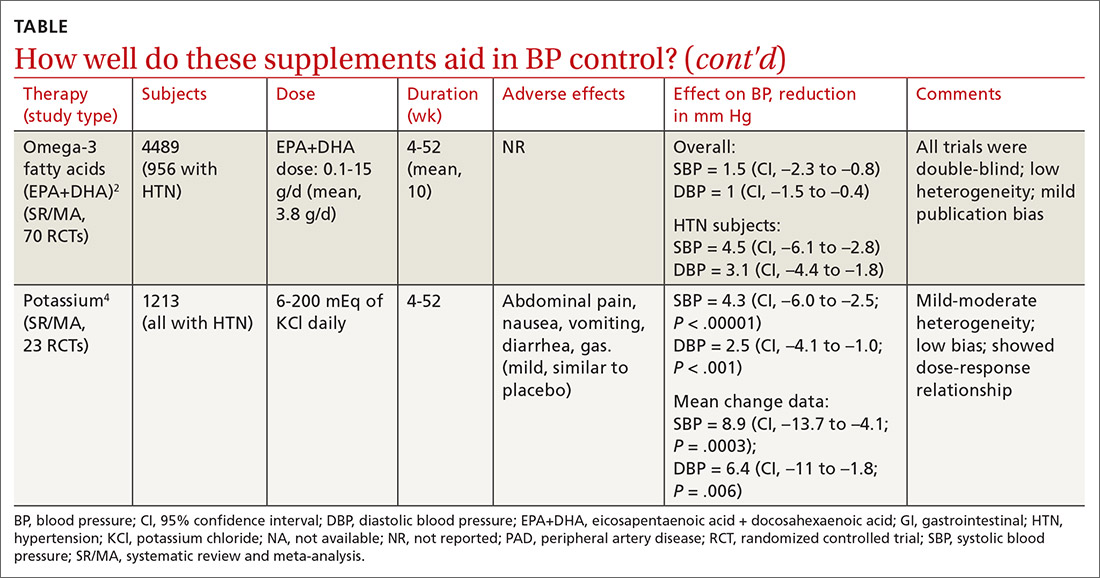
Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).

Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.

Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).

Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.

Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE-BASED ANSWER:
Yes. A number of well-tolerated natural therapies have been shown to reduce systolic and diastolic blood pressure (BP). (See Table1-8 for summary.) However, the studies don’t provide direct evidence of whether the decrease in BP is linked to patient-oriented outcomes. Nor do they allow definitive conclusions concerning the lasting nature of the reductions, because most studies were fewer than 6 months in duration (strength of recommendation: C, disease-oriented evidence).