User login
Which medications work best for menorrhagia?
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE-BASED ANSWER:
Four medications have been shown to reduce menstrual blood loss (MBL) significantly in placebo-controlled randomized controlled trials (RCTs): the levonorgestrel-releasing intrauterine system (LNG-IUS), tranexamic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and danazol, a synthetic steroid (strength of recommendation: A, meta-analyses of RCTs).
A single trial showed that the LNG-IUS reduced MBL by about 100 mL, compared with placebo. In a meta-analysis of 4 placebo-controlled RCTs, tranexamic acid reduced MBL by about 53 mL, roughly a 40% to 50% decrease. The 8 NSAID trials (5 mefenamic acid, 2 naproxen, 1 ibuprofen) demonstrated effectiveness, but the effect size is difficult to quantify. The single danazol RCT used a subjective scoring system without reporting MBL.
No studies compared all effective medical therapies against one another. In head-to-head comparisons, women were more likely to experience improvement with the LNG-IUS than with tranexamic acid (number needed to treat [NNT] = 2 to 6). Both treatments are superior to NSAIDs. Danazol is also more efficacious than NSAIDs, but its use is limited by its adverse effects, including teratogenicity.
No placebo-controlled trials have studied oral contraceptive pills (OCPs) or oral progesterone to treat menorrhagia. However, multiple comparative RCTs have demonstrated that these commonly prescribed medications significantly decrease MBL. Trials have shown the reduction to be inferior to LNG-IUS and danazol and equivalent to NSAIDs.
Does concurrent use of clopidogrel and PPIs increase CV risk in patients with ACS?
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
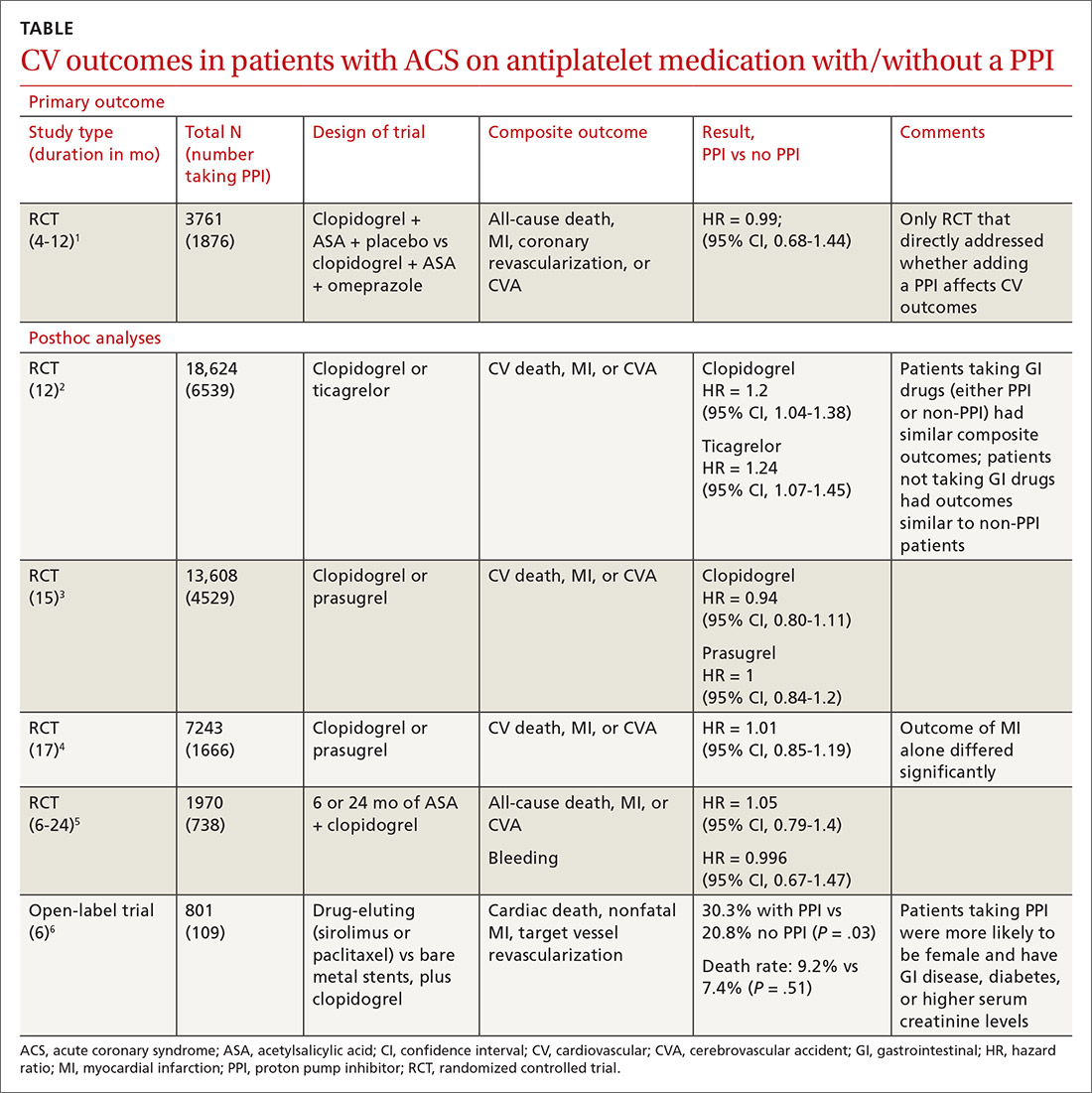
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.

Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.

Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE-BASED ANSWER:
No. Adding a proton pump inhibitor (PPI) in patients taking antiplatelet medications such as clopidogrel for acute coronary syndrome (ACS) doesn’t increase the composite risk of cardiovascular (CV) events: CV death, myocardial infarction (MI), and cerebrovascular accident (CVA) (strength of recommendation: B, randomized, controlled trial [RCT] and prepon-derance of posthoc analyses of large RCTs).
Do cinnamon supplements improve glycemic control in adults with T2DM?
EVIDENCE SUMMARY
A 2013 systematic review of 10 randomized controlled trials (RCTs) with a total of 543 patients with type 2 diabetes evaluated the effect of cinnamon (120 mg/d to 6 g/d) on measures of glycemic control.1 Study duration ranged from 4 to 18 weeks. Fasting glucose levels demonstrated small but statistically significant reductions (−24.6 mg/dL; 95% confidence interval [CI], −40.5 to −8.7 mg/dL), whereas hemoglobin A1C levels didn’t differ between treatment and control groups (−0.16%; 95% CI, −0.39% to 0.02%). Study limitations included heterogeneity of cinnamon dosing and formulation and concurrent use of oral hypoglycemic agents.
Studies of glycemic control produce mixed results
A 2012 systematic review of 10 RCTs comprising 577 patients with type 1 (72 patients) or type 2 (505 patients) diabetes evaluated the effects of cinnamon supplements (mean dose, 1.9 g/d) on glycemic control compared with placebo, active control, or no treatment.2 Study duration ranged from 4.3 to 16 weeks (mean, 10.8 weeks). Studies evaluating hemoglobin A1C lasted at least 12 weeks.
Fasting glucose as measured in 8 studies (338 patients) and hemoglobin A1C as measured in 6 studies (405 patients) didn’t differ between treatment groups (mean fasting glucose difference = −0.91 mmol/L; 95% CI, −1.93 to 0.11; mean hemoglobin A1C difference = −0.06; 95% CI, −0.29 to 0.18). The risk for bias was assessed as high or unclear in 8 studies and moderate in 2 studies.
A 2012 systematic review and meta-analysis of 6 RCTs including 435 patients with type 2 diabetes evaluated the impact of cinnamon supplements (1 to 6 g/d) on glycemic control.3 Participants consumed cinnamon for 40 to 160 days. Hemoglobin A1C decreased by 0.09% (95% CI, 0.04% to 0.14%) in 5 trials (375 patients), and fasting glucose decreased by 0.84 mmol/L (CI, 0.66 to 1.02) in 5 trials (326 patients). Study limitations included heterogeneity of cinnamon dosing and study population.
RECOMMENDATIONS
The American Diabetes Association finds insufficient evidence to support the use of herbs or spices, including cinnamon, in treating diabetes.4
Editor’s Takeaway
Meta-analyses of multiple small, lower-quality studies yield uncertain conclusions. If cinnamon does improve glycemic control, the benefit is minimal—but so is therisk.
1. Allen RW, Schwartzman E, Baker WL, et al. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452-459.
2. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
3. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: systematic review and meta-analysis. Clin Nutr. 2012;31:609-615.
4. American Diabetes Association. Standards of medical care in diabetes—2017. 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33-S43.
EVIDENCE SUMMARY
A 2013 systematic review of 10 randomized controlled trials (RCTs) with a total of 543 patients with type 2 diabetes evaluated the effect of cinnamon (120 mg/d to 6 g/d) on measures of glycemic control.1 Study duration ranged from 4 to 18 weeks. Fasting glucose levels demonstrated small but statistically significant reductions (−24.6 mg/dL; 95% confidence interval [CI], −40.5 to −8.7 mg/dL), whereas hemoglobin A1C levels didn’t differ between treatment and control groups (−0.16%; 95% CI, −0.39% to 0.02%). Study limitations included heterogeneity of cinnamon dosing and formulation and concurrent use of oral hypoglycemic agents.
Studies of glycemic control produce mixed results
A 2012 systematic review of 10 RCTs comprising 577 patients with type 1 (72 patients) or type 2 (505 patients) diabetes evaluated the effects of cinnamon supplements (mean dose, 1.9 g/d) on glycemic control compared with placebo, active control, or no treatment.2 Study duration ranged from 4.3 to 16 weeks (mean, 10.8 weeks). Studies evaluating hemoglobin A1C lasted at least 12 weeks.
Fasting glucose as measured in 8 studies (338 patients) and hemoglobin A1C as measured in 6 studies (405 patients) didn’t differ between treatment groups (mean fasting glucose difference = −0.91 mmol/L; 95% CI, −1.93 to 0.11; mean hemoglobin A1C difference = −0.06; 95% CI, −0.29 to 0.18). The risk for bias was assessed as high or unclear in 8 studies and moderate in 2 studies.
A 2012 systematic review and meta-analysis of 6 RCTs including 435 patients with type 2 diabetes evaluated the impact of cinnamon supplements (1 to 6 g/d) on glycemic control.3 Participants consumed cinnamon for 40 to 160 days. Hemoglobin A1C decreased by 0.09% (95% CI, 0.04% to 0.14%) in 5 trials (375 patients), and fasting glucose decreased by 0.84 mmol/L (CI, 0.66 to 1.02) in 5 trials (326 patients). Study limitations included heterogeneity of cinnamon dosing and study population.
RECOMMENDATIONS
The American Diabetes Association finds insufficient evidence to support the use of herbs or spices, including cinnamon, in treating diabetes.4
Editor’s Takeaway
Meta-analyses of multiple small, lower-quality studies yield uncertain conclusions. If cinnamon does improve glycemic control, the benefit is minimal—but so is therisk.
EVIDENCE SUMMARY
A 2013 systematic review of 10 randomized controlled trials (RCTs) with a total of 543 patients with type 2 diabetes evaluated the effect of cinnamon (120 mg/d to 6 g/d) on measures of glycemic control.1 Study duration ranged from 4 to 18 weeks. Fasting glucose levels demonstrated small but statistically significant reductions (−24.6 mg/dL; 95% confidence interval [CI], −40.5 to −8.7 mg/dL), whereas hemoglobin A1C levels didn’t differ between treatment and control groups (−0.16%; 95% CI, −0.39% to 0.02%). Study limitations included heterogeneity of cinnamon dosing and formulation and concurrent use of oral hypoglycemic agents.
Studies of glycemic control produce mixed results
A 2012 systematic review of 10 RCTs comprising 577 patients with type 1 (72 patients) or type 2 (505 patients) diabetes evaluated the effects of cinnamon supplements (mean dose, 1.9 g/d) on glycemic control compared with placebo, active control, or no treatment.2 Study duration ranged from 4.3 to 16 weeks (mean, 10.8 weeks). Studies evaluating hemoglobin A1C lasted at least 12 weeks.
Fasting glucose as measured in 8 studies (338 patients) and hemoglobin A1C as measured in 6 studies (405 patients) didn’t differ between treatment groups (mean fasting glucose difference = −0.91 mmol/L; 95% CI, −1.93 to 0.11; mean hemoglobin A1C difference = −0.06; 95% CI, −0.29 to 0.18). The risk for bias was assessed as high or unclear in 8 studies and moderate in 2 studies.
A 2012 systematic review and meta-analysis of 6 RCTs including 435 patients with type 2 diabetes evaluated the impact of cinnamon supplements (1 to 6 g/d) on glycemic control.3 Participants consumed cinnamon for 40 to 160 days. Hemoglobin A1C decreased by 0.09% (95% CI, 0.04% to 0.14%) in 5 trials (375 patients), and fasting glucose decreased by 0.84 mmol/L (CI, 0.66 to 1.02) in 5 trials (326 patients). Study limitations included heterogeneity of cinnamon dosing and study population.
RECOMMENDATIONS
The American Diabetes Association finds insufficient evidence to support the use of herbs or spices, including cinnamon, in treating diabetes.4
Editor’s Takeaway
Meta-analyses of multiple small, lower-quality studies yield uncertain conclusions. If cinnamon does improve glycemic control, the benefit is minimal—but so is therisk.
1. Allen RW, Schwartzman E, Baker WL, et al. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452-459.
2. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
3. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: systematic review and meta-analysis. Clin Nutr. 2012;31:609-615.
4. American Diabetes Association. Standards of medical care in diabetes—2017. 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33-S43.
1. Allen RW, Schwartzman E, Baker WL, et al. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11:452-459.
2. Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;(9):CD007170.
3. Akilen R, Tsiami A, Devendra D, et al. Cinnamon in glycaemic control: systematic review and meta-analysis. Clin Nutr. 2012;31:609-615.
4. American Diabetes Association. Standards of medical care in diabetes—2017. 4. Lifestyle management. Diabetes Care. 2017;40(suppl 1):S33-S43.
EVIDENCE-BASED ANSWER:
The answer isn’t clear. Cinnamon supplements for adults with type 2 diabetes haven’t been shown to decrease hemoglobin A1C (strength of recommendation [SOR]: C, multiple systematic reviews of disease-oriented outcomes).
Cinnamon supplements have shown inconsistent effects on fasting glucose levels (SOR: C, multiple systematic reviews and a single meta-analysis of disease-oriented outcomes). Supplements decreased fasting glucose levels in some studies, but the evidence isn’t consistent and hasn’t been correlated with clinically significant improvements in glycemic control.
Does vitamin D supplementation reduce asthma exacerbations?
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
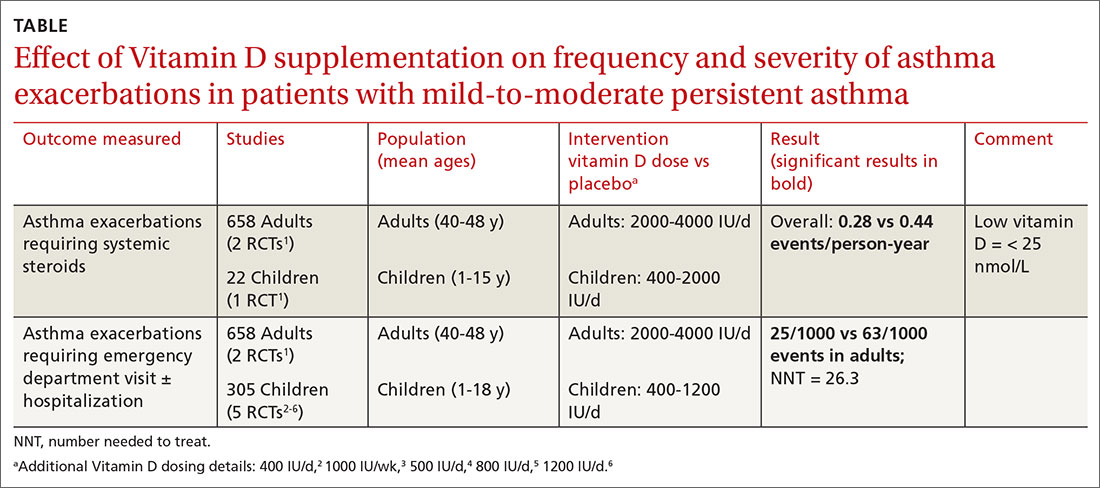
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).

Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).

Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE-BASED ANSWER:
Yes, to some extent it does, and primarily in patients with low vitamin D levels. Supplementation reduces asthma exacerbations requiring systemic steroids by 30% overall in adults and children with mild-to-moderate asthma (number needed to treat [NNT] = 7.7). The outcome is driven by the effect in patients with vitamin D levels < 25 nmol/L (NNT = 4.3), however; supplementation doesn’t decrease exacerbations in patients with higher levels. Supplementation also reduces, by a smaller amount (NNT = 26.3), the odds of exacerbations requiring emergency department care or hospitalization (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
In children, vitamin D supplementation may also reduce exacerbations and improve symptom scores (SOR: C, low-quality RCTs).
Vitamin D doesn’t improve forced expiratory volume in 1 second (FEV1) or standardized asthma control test scores. Also, it isn’t associated with serious adverse effects (SOR: A, meta-analysis of RCTs).
Do prophylactic antipyretics reduce vaccination-associated symptoms in children?
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE-BASED ANSWER:
Yes for acetaminophen, not so much for ibuprofen. Prophylactic acetaminophen reduces the odds of febrile reactions in the first 48 hours after vaccination by 40% to 65% and pain of all grades by 36% to 43%. In contrast, prophylactic ibuprofen reduces pain of all grades by 34% only after primary vaccination and doesn’t alter pain after boosters. Nor does it alter early febrile reactions (strength of recommendation [SOR]: B, meta-analysis of randomized clinical trials [RCTs] with moderate-to-high risk of bias).
Prophylactic administration of acetaminophen or ibuprofen is associated with a reduction in antibody response to the primary vaccine series and to influenza vaccine, but antibody responses still achieve seroprotective levels (SOR: C, bench research).