User login
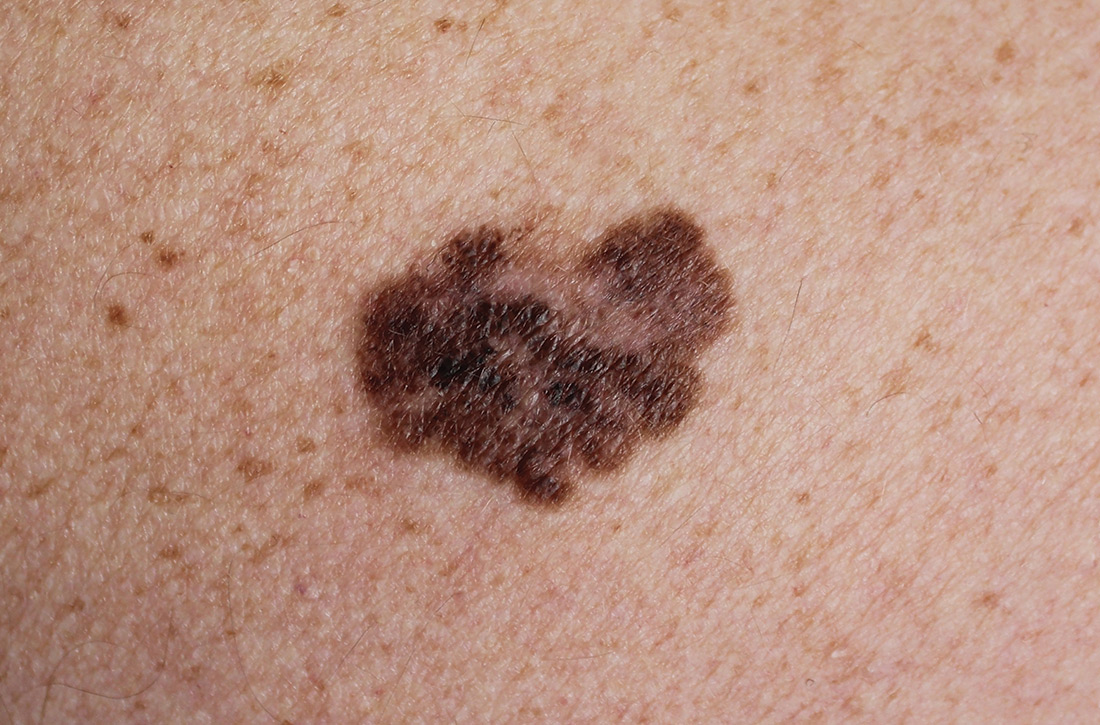
Does screening by primary care providers effectively detect melanoma and other skin cancers?
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE-BASED ANSWER:
Possibly. No trials have directly assessed detection of melanoma and other skin cancers by primary care providers.
Training a group comprised largely of primary care physicians to perform skin cancer screening was associated with a 41% increase in skin cancer diagnoses but no change in melanoma mortality.
Visual screening for melanoma by primary care physicians is 40% sensitive and 86% specific (compared with 49% and 98%, respectively, for dermatologists and plastic surgeons).
Melanomas found by visual screening are 38% more likely to be thin (≤ 0.75 mm) than melanomas discovered without screening, which correlates with improved outcomes.
Visual skin cancer screening overall is associated with false-positive rates as follows: 28 biopsies for each melanoma detected, 9 to 10 biopsies for each basal cell carcinoma, and 28 to 56 biopsies for squamous cell carcinoma. False-positive rates are higher for women—as much as double the rate for men—and younger patients—as much as 20-fold the rate for older patients (strength of recommendations for all foregoing statements: B, cohort studies).
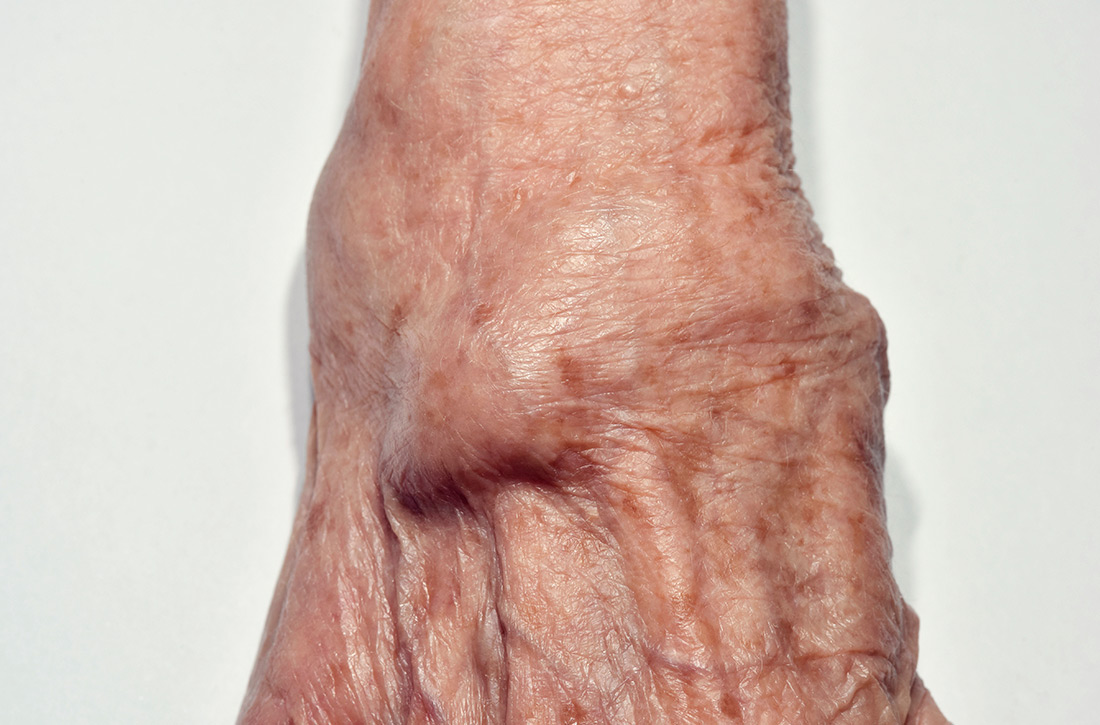
What is the best treatment for wrist ganglion cysts?
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
EVIDENCE SUMMARY
A 2015 meta-analysis of 35 studies (7 RCTs, 6 cohort studies, 22 case series) of 2239 wrist ganglion cysts examined the recurrence rate of cysts after common treatments.1 Two RCTs and 4 cohort studies compared open surgical excision with aspiration with or without corticosteroid injection.
The RCTs found significantly lower recurrence rates following open surgical excision compared with aspiration (2 trials; 60 cysts; risk ratio [RR] = 0.24; 95% confidence interval [CI], 0.08-0.71; number needed to treat [NNT] = 3). The cohort studies likewise found markedly less recurrence of cysts after open surgical excision than aspiration (4 studies; 461 cysts; RR = 0.42; 95% CI, 0.21-0.85; NNT = 4). Recurrence rates didn’t differ between aspiration and observation (2 cohort studies; 209 cysts; RR = 0.99; 95% CI, 0.77-1.28).
Overall, the RCT evidence was of moderate quality because of a lack of significant heterogeneity, and the cohort evidence was graded as very low quality because of heterogeneity.
More evidence of lower recurrence with surgical excision
A 2014 prospective RCT, not included in the foregoing meta-analysis because it was published after the search date, compared ganglion cyst recurrence at 6 months for 2 groups: one group received aspiration accompanied by corticosteroid injection and the other had surgical treatment.2 The trial included 173 patients ages 16 to 47 years with 187 ganglia of the wrist, ankle, or knee (143 wrist ganglia). Patients were excluded if they had a history of recurrent ganglia, prior treatment of ganglia, nearby joint injury, bleeding disorders, pregnancy, compound palmar ganglion, ganglion near arteries, infected ganglion, ganglion associated with arthritic disease, or ganglion measuring < 5 mm in size.
Patients were allowed to choose aspiration with corticosteroid injection or surgical excision. The aspiration group (143 ganglia: 106 wrist, 21 ankle, 16 knee) underwent aspiration using a 19-gauge needle and 10-mL syringe followed by injection of 0.25 to 1.0 mL of triamcinolone acetonide. Aspiration and injection were repeated if indicated at either 6 weeks or 3 months. The surgical excision group comprised 44 ganglia: 37 wrist and 7 ankle.
The success rate at 6 months following aspiration with corticosteroid injection was 81% compared with 93% after surgical excision (NNT = 8). Surgical treatment was associated with significantly less recurrence than aspiration and injection (7% vs 19%; P < .028).
Patients report symptomatic improvement after aspiration
A 2015 retrospective case series assessed the long-term outcomes of 21 patients following aspiration of wrist ganglia.3 The patients, who were 41 to 49 years of age, each had a single wrist ganglion that was treated with aspiration between 2001 and 2011 by a single surgeon. Mean time to follow-up was 6.3 years. Outcomes reviewed included recurrence, satisfaction, and improvement in symptoms—pain, function, range of motion, and appearance—using a 1 to 5 Likert scale (1 = significantly worse; 5 = significantly improved).
Continue to: Overall, 52.4% of patients...
Overall, 52.4% of patients experienced recurrence of their ganglia. However, 95% expressed satisfaction with treatment independent of recurrence. Mean symptom scores improved from baseline for pain (4.1 points), function (3.9 points), range of motion (3.8 points), and appearance (4.1 points). Improvements in all symptoms were independent of recurrence.
Aspiration plus steroids results in 43% recurrence rate
A 2015 prospective study examined the recurrence rate at 1 year after therapy in 30 patients, ages 15 to 55 years, with a wrist ganglion treated by aspiration and steroid injection.4 Patients chose aspiration and steroid injection with 40 mg/mL methyl-prednisolone acetate over reassurance or surgical intervention. The recurrence rate at 1-year follow-up was 43.3% (13 patients).
Editor’s takeaway
Surgical excision of ganglion cysts results in fewer recurrences than aspiration. However, moderately high-quality evidence shows that both methods help most patients.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
1. Head L, Gencarelli JR, Allen M, et al. Wrist ganglion treatment: systematic review and meta-analysis. J Hand Surg Am. 2015;40:546-553.e8.
2. Latif A, Ansar A, Butt MQ. Treatment of ganglions; a five year experience. J Pak Med Assoc. 2014;64:1278-1281.
3. Head L, Allen M, Boyd KU. Long-term outcomes and patient satisfaction following wrist ganglion aspiration. Plast Surg (Oakv). 2015;23:51-53.
4. Hussain S, Akhtar S, Aslam V, et al. Efficacy of aspiration and steroid injection in treatment of ganglion cyst. PJMHS. 2015;9:1403-1405.
EVIDENCE-BASED ANSWER:
Open surgical excision of wrist ganglion cysts is associated with a lower recurrence rate than aspiration with or without corticosteroid injection (strength of recommendation [SOR]: B, systematic review of randomized clinical trials [RCTs] and observational trials and RCT).
Even though the recurrence rate with aspiration is about 50%, most patients are satisfied with aspiration and report a decrease in symptoms involving pain, function, and range of motion (SOR: B, individual cohort and case series).
Do group visits improve HbA1c more than individual visits in patients with T2DM?
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
EVIDENCE-BASED ANSWER:
Yes. In patients with type 2 diabetes mellitus (T2DM), group visits led by health professionals or teams improved glycosylated hemoglobin (HbA1c) by 0.3% to 0.9% over usual care (strength of recommendation [SOR]: B, meta-analyses of randomized clinical trials [RCTs] with moderate to high risk of bias).
Patients taking oral antidiabetic agents alone appear to benefit more than patients on insulin. Peer-led group visits likely have no effect (SOR: B, subgroup analysis within a meta-analysis).
Treatment durations as long as 3 years are associated with larger decreases in HbA1c (by 0.25% per year) than treatment lasting less than a year (SOR: B, meta-analysis of RCTs involving patents with type 1 diabetes and T2DM).
Patients with T2DM should be offered group visits for diabetes education when available (SOR: C, expert opinion).
Does using e-cigarettes increase cigarette smoking in adolescents?
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
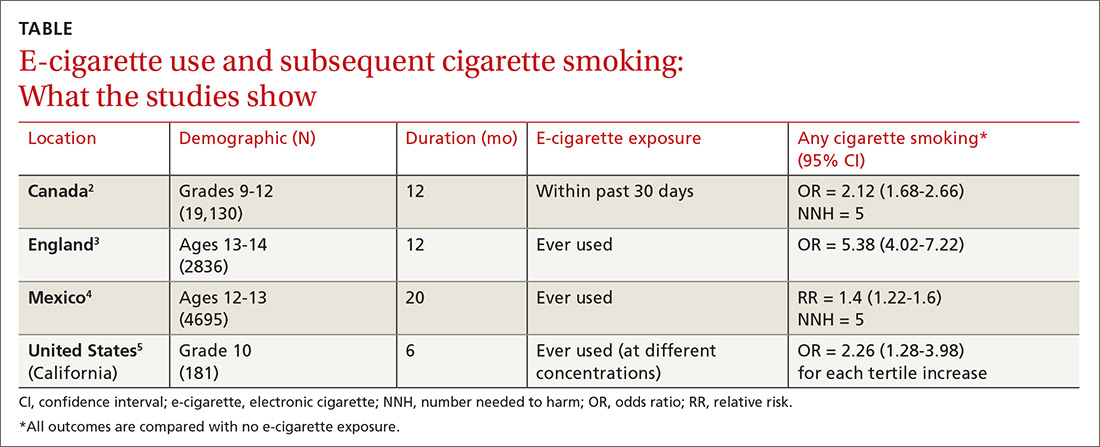
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5

RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5

RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE-BASED ANSWER:
Probably. Electronic cigarette (e-cigarette) use by adolescents is associated with a 2- to 4-fold increase in cigarette smoking over the next year (strength of recommendation: A, meta-analysis and subsequent prospective cohort studies).
Does tranexamic acid reduce mortality in women with postpartum hemorrhage?
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
EVIDENCE SUMMARY
A 2017 double-blind RCT that included 20,060 women with PPH from 21 countries (the WOMAN trial) found that the risk of maternal mortality was significantly lower among women who received tranexamic acid as part of their PPH treatment compared with placebo (1.5% [N = 155] vs 1.9% [N = 191]; P = .045; relative risk [RR] = 0.81; 95% confidence interval [CI], 0.65-1; number needed to treat [NNT] = 250).1
Inclusion criteria were age 16 years or older, postpartum course complicated by hemorrhage of known or unknown etiology, and a case in which the clinician considered using tranexamic acid in addition to the standard of care. PPH was defined as > 500 mL blood loss after vaginal delivery, > 1000 mL blood loss after cesarean section, or blood loss sufficient to produce hemodynamic compromise.
Researchers randomized 10,051 women to the tranexamic acid group and 10,009 to the placebo group. Women in the experimental group received a 1-g IV injection of tranexamic acid over 10 to 20 minutes. A second dose was given if bleeding restarted after 30 minutes and within 24 hours of the first dose.
To reduce mortality give tranexamic acid promptly
Tranexamic acid reduced mortality most effectively compared with placebo when given within 3 hours of delivery (1.2% [N = 89] vs 1.7% [N = 127]; P = .008; RR = 0.69; 95% CI 0.52-0.91; NNT = 200). After 3 hours, no significant decrease in mortality occurred. No significant difference in effect was noted between vaginal and cesarean deliveries nor between uterine atony as the primary cause of hemorrhage and other causes.
Administering tranexamic acid didn’t reduce the composite primary endpoint of hysterectomy or death from all causes. Nor did it reduce the secondary endpoints of intrauterine tamponade, embolization, manual placental extraction, arterial ligation, blood transfusions, or number of units of packed red blood cells. The tranexamic acid group showed a significant decrease in cases of laparotomy for PPH (0.8% vs 1.3%; P = .002; RR = 0.64; 95% CI, 0.49-0.85; NNT = 200).
Women who received tranexamic acid vs placebo showed no significant difference in mortality from pulmonary embolism (0.1% [N = 10] vs 0.1% [N = 11]; P = .82; RR = .9; 95% CI, 0.38-2.13), organ failure ure (0.3% [N = 25] vs 0.2% [N = 18]; P = .29; RR = 1.38; 95% CI, 0.75-2.53), sepsis (0.2% [N = 15] vs 0.1% [N = 8]; P = .15; RR = 1.87; 95% CI, 0.79-4.4), eclampsia (0.02% [N = 2] vs 0.1% [N = 8]; P = .057; RR = .25; 95% CI, 0.05-1.17), or other causes (0.2% [N = 20] vs 0.2% [N = 20]; P = .99; RR = 0.99; 95% CI, 0.54-1.85).
Tranexamic acid doesn’t increase the risk of thromboembolism
A 2018 Cochrane review sought more broadly to determine the general effectiveness and safety of antifibrinolytic drugs in treating primary PPH.2 Of 15 RCTs identified, only 3 met the inclusion criteria for the review, 1 of which was the WOMAN trial (which contributed most of the data in the review). The other trials were a study conducted in France that recruited 152 women and a study of 200 women in Iran that contributed only 1 primary outcome—estimated blood loss—to the review. The former study didn’t report any maternal deaths, and the latter study didn’t look at maternal deaths. The Cochrane review concluded, based on data from the WOMAN trial, that IV tranexamic acid, if given as early as possible, reduced mortality from bleeding in women with primary PPH after both vaginal and cesarean delivery and didn’t increase the risk of thromboembolic events.2
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The newest practice guidelines on the management of postpartum hemorrhage published by the American College of Obstetricians and Gynecologists recommends considering tranexamic acid as an additional agent in managing PPH when initial standard-of-care treatments fail.3
Editor’s takeaway
The large international double-blind, randomized placebo-controlled trial provides convincing evidence that tranexamic acid should be administered readily in cases of PPH.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
1. WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116.
2. Shakur H, Beaumont D, Pavord S, et al. Antifibrinolytic drugs for treating primary postpartum haemorrhage. Cochrane Database Syst Rev. 2018;2:CD012964.
3. Committee on Practice Bulletins-Obstetrics (American College of Obstetricians and Gynecologists). Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet Gynecol. 2017;130:e168-e186.
EVIDENCE-BASED ANSWER:
Yes. When used in conjunction with the standard of care, 1 g intravenous (IV) tranexamic acid given 1 to 3 hours after delivery is associated with a significant reduction in maternal mortality from postpartum hemorrhage (PPH) (strength of recommendation: A, randomized controlled trial [RCT] and Cochrane review).
No known significant risks are associated with the use of tranexamic acid to treat PPH.
Time to conception after miscarriage: How long to wait?
EVIDENCE SUMMARY
To evaluate the longstanding belief that a short IPI after miscarriage is associated with adverse outcomes in subsequent pregnancies, a 2017 systematic review and meta-analysis of 16 studies (3 randomized controlled trials [RCTs] and 13 retrospective cohort studies) with a total of more than 1 million patients compared IPIs shorter and longer than 6 months (miscarriage was defined as any pregnancy loss before 24 weeks).1 The meta-analysis included 10 of the studies (2 RCTs and 8 cohort studies), with a total of 977,972 women and excluded 6 studies because of insufficient data. The outcomes investigated were recurrent miscarriage, preterm birth, stillbirth, pre-eclampsia, and low birthweight in the pregnancy following miscarriage.
Only 1 study reported the specific gestational age of the index miscarriage at 8.6 ± 2.8 weeks.2 All studies adjusted data for age, and some considered other confounders, such as race, smoking status, and body mass index (BMI).
Women included in the meta-analysis were from Asia, Europe, South America, and the United States and had a history of at least 1 miscarriage.1 A study of 257,908 subjects (Conde-Agudelo) also included women with a history of induced abortion from Latin American countries, where abortion is illegal, and made no distinction between spontaneous and induced abortions in those data sets.3 Women with a history of illegal abortion could be at greater risk of subsequent miscarriage than women who underwent a legally performed abortion.
IPI shorter than 6 months carries fewer risks
Excluding the Conde-Agudelo study, women with an IPI < 6 months, compared with > 6 months, had lower risks of subsequent miscarriage (7 studies, 46,313 women; risk ratio [RR] = 0.82; 95% confidence interval [CI], 0.78-0.86) and preterm delivery (7 studies, 60,772 women; RR = 0.79; 95% CI, 0.75-0.83); a higher rate of live births (4 studies, 44,586 women; RR = 1.06; 95% CI, 1.01-1.11); and no increase in stillbirths (4 studies, 44,586 women; RR = 0.88; 95% CI, 0.76-1.02), low birthweight (4 studies, 284,222 women; RR = 1.05; 95% CI, 0.48-2.29) or pre-eclampsia (5 studies, 284,899 women; RR = 0.95; 95% CI, 0.88-1.02) in the subsequent pregnancy.
Including the Conde-Agudelo study, the risk of preterm delivery was the same in women with an IPI < 6 months and > 6 months (8 studies, 318,880 women; RR = 0.93; 95% CI, 0.58-1.48).1 Four of the 10 studies evaluated the risk of miscarriage with an IPI < 3 months compared with > 3 months and found either no difference or a lower risk of subsequent miscarriage.2,4-6
IPI shorter than 3 months has lowest risk of all
A 2017 prospective cohort study examined the association between IPI length and risk of recurrent miscarriage in 514 women who had experienced recent miscarriage (defined as spontaneous pregnancy loss before 20 weeks of gestation).7 Average gestational age at the time of initial miscarriage wasn’t reported. Study participants were 30 years of age on average and predominantly white (76.8%); 12.3% were black.
The authors compared IPIs of < 3 months, 3 to 6 months, and > 18 months with IPIs of 6 to 18 months, which correlates with the IPIs recommended by the World Health Organization (WHO).8 They adjusted for maternal age, race, parity, BMI, and education. An IPI < 3 months was associated with the lowest risk of subsequent miscarriage (7.3% compared with 22.1%; adjusted hazard ratio = 0.33; 95% CI, 0.16-0.71). Women with IPIs of 3 to 6 months and > 18 months didn’t experience statistically significant differences in subsequent miscarriage rates compared with IPIs of 6 to 18 months.7
Continue to: But a short IPI after second-trimester loss increases risk of miscarriage
But a short IPI after second-trimester loss increases risk of miscarriage
By including all miscarriages, the meta-analysis effectively examined IPI after first-trimester loss because first-trimester loss occurs far more frequently than does second-trimester loss.1 A retrospective cohort study of Australian women, not included in the meta-analysis, assessed 4290 patients with a second-trimester pregnancy loss to specifically examine the association between IPI and risk of recurrent pregnancy loss.9
After a pregnancy loss at 14 to 19 weeks, women with an IPI < 3 months, compared with an IPI of 9 to 12 months, had an increased risk of recurrent pregnancy loss (21.9 vs 11.3%; P < .001). Women with an IPI > 9 to 12 months had rates of pregnancy loss similar to an IPI of 3 to 6 months (RR = 1.24; 95% CI, 0.89-1.7) and 6 to 9 months (RR = 1.02; 95% CI, 0.7-1.5). Women who experienced an initial loss at 20 to 23 weeks, for unclear reasons, showed no evidence that the IPI affected the risk of subsequent loss.
Short IPI may be linked to anxiety in first trimester of next pregnancy
A large cohort study of 20,308 pregnant Chinese women, including 1495 with a previous miscarriage, explored the mental health impact of IPI after miscarriage compared with no miscarriage.10 Investigators used the Self-Rating Anxiety Scale to evaluate anxiety and the Center for Epidemiologic Studies Depression Scale to evaluate depression.
Women with an IPI of < 7 months after miscarriage were more likely to experience anxiety symptoms in the subsequent pregnancy than were women with no previous miscarriage (adjusted odds ratio [AOR] = 2.76; 95% CI, 1.4-5.5), whereas women with a history of miscarriage and IPI > 6 months weren’t. Women with IPIs < 7 months and 7 to 12 months, compared with women who had no miscarriage, had an increased risk of depression (AOR = 2.5; 95% CI, 1.4-4.5, and AOR = 2.6; 95% CI, 1.3-5.2, respectively). Women with an IPI > 12 months had no increased risk of depression compared with women with no history of miscarriage.
The odds ratios were adjusted for age, education, BMI, income, and place of residence. The higher rates of depression and anxiety didn’t persist beyond the first trimester of the subsequent pregnancy.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists’ Practice Bulletin on Early Pregnancy Loss states that no quality data exist to support delaying conception after early pregnancy loss (defined as loss of an intrauterine pregnancy in the first trimester) to prevent subsequent pregnancy loss or other pregnancy complications.11
WHO recommends a minimum IPI of at least 6 months after a spontaneous or elective abortion. This recommendation is based on a single multi-center cohort study in Latin America that included women with both spontaneous and induced abortions.8
Editor’s takeaway
High-quality evidence now shows that shorter IPIs after first-trimester miscarriages result in safe subsequent pregnancies. However, some concern remains about second-trimester miscarriages and maternal mental health following a shorter IPI, based on lower-quality evidence.
1. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23:221-231.
2. Wong LF, Schliep KC, Silver RM, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol. 2015;212:375.e1-375.e11.
3. Conde-Agudelo A, Belizan JM, Breman R, et al. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet. 2005;89(suppl 1):S34-S40.
4. Bentolila Y, Ratzon R, Shoham-Vardi I, et al. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2013;26:1459-1464.
5. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591.
6. Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med. 1994;22:235-241.
7. Sundermann AC, Hartmann KE, Jones SH, et al. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol. 2017;130:1312-1318.
8. World Health Organization. Department of Reproductive Health and Research, Department of Making Pregnancy Safer. Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13-15 June 2005. Geneva: World Health Organization, 2007.
9. Roberts CL, Algert CS, Ford JB, et al. Association between interpregnancy interval and the risk of recurrent loss after a midtrimester loss. Hum Reprod. 2016;31:2834-2840.
10. Gong X, Hao J, Tao F, et al. Pregnancy loss and anxiety and depression during subsequent pregnancies: data from the C-ABC study. Eur J Obstet Gynecol Reprod Biol. 2013;166:30-36.
11. American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Gynecology. The American College of Obstetricians and Gynecologists Practice Bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
EVIDENCE SUMMARY
To evaluate the longstanding belief that a short IPI after miscarriage is associated with adverse outcomes in subsequent pregnancies, a 2017 systematic review and meta-analysis of 16 studies (3 randomized controlled trials [RCTs] and 13 retrospective cohort studies) with a total of more than 1 million patients compared IPIs shorter and longer than 6 months (miscarriage was defined as any pregnancy loss before 24 weeks).1 The meta-analysis included 10 of the studies (2 RCTs and 8 cohort studies), with a total of 977,972 women and excluded 6 studies because of insufficient data. The outcomes investigated were recurrent miscarriage, preterm birth, stillbirth, pre-eclampsia, and low birthweight in the pregnancy following miscarriage.
Only 1 study reported the specific gestational age of the index miscarriage at 8.6 ± 2.8 weeks.2 All studies adjusted data for age, and some considered other confounders, such as race, smoking status, and body mass index (BMI).
Women included in the meta-analysis were from Asia, Europe, South America, and the United States and had a history of at least 1 miscarriage.1 A study of 257,908 subjects (Conde-Agudelo) also included women with a history of induced abortion from Latin American countries, where abortion is illegal, and made no distinction between spontaneous and induced abortions in those data sets.3 Women with a history of illegal abortion could be at greater risk of subsequent miscarriage than women who underwent a legally performed abortion.
IPI shorter than 6 months carries fewer risks
Excluding the Conde-Agudelo study, women with an IPI < 6 months, compared with > 6 months, had lower risks of subsequent miscarriage (7 studies, 46,313 women; risk ratio [RR] = 0.82; 95% confidence interval [CI], 0.78-0.86) and preterm delivery (7 studies, 60,772 women; RR = 0.79; 95% CI, 0.75-0.83); a higher rate of live births (4 studies, 44,586 women; RR = 1.06; 95% CI, 1.01-1.11); and no increase in stillbirths (4 studies, 44,586 women; RR = 0.88; 95% CI, 0.76-1.02), low birthweight (4 studies, 284,222 women; RR = 1.05; 95% CI, 0.48-2.29) or pre-eclampsia (5 studies, 284,899 women; RR = 0.95; 95% CI, 0.88-1.02) in the subsequent pregnancy.
Including the Conde-Agudelo study, the risk of preterm delivery was the same in women with an IPI < 6 months and > 6 months (8 studies, 318,880 women; RR = 0.93; 95% CI, 0.58-1.48).1 Four of the 10 studies evaluated the risk of miscarriage with an IPI < 3 months compared with > 3 months and found either no difference or a lower risk of subsequent miscarriage.2,4-6
IPI shorter than 3 months has lowest risk of all
A 2017 prospective cohort study examined the association between IPI length and risk of recurrent miscarriage in 514 women who had experienced recent miscarriage (defined as spontaneous pregnancy loss before 20 weeks of gestation).7 Average gestational age at the time of initial miscarriage wasn’t reported. Study participants were 30 years of age on average and predominantly white (76.8%); 12.3% were black.
The authors compared IPIs of < 3 months, 3 to 6 months, and > 18 months with IPIs of 6 to 18 months, which correlates with the IPIs recommended by the World Health Organization (WHO).8 They adjusted for maternal age, race, parity, BMI, and education. An IPI < 3 months was associated with the lowest risk of subsequent miscarriage (7.3% compared with 22.1%; adjusted hazard ratio = 0.33; 95% CI, 0.16-0.71). Women with IPIs of 3 to 6 months and > 18 months didn’t experience statistically significant differences in subsequent miscarriage rates compared with IPIs of 6 to 18 months.7
Continue to: But a short IPI after second-trimester loss increases risk of miscarriage
But a short IPI after second-trimester loss increases risk of miscarriage
By including all miscarriages, the meta-analysis effectively examined IPI after first-trimester loss because first-trimester loss occurs far more frequently than does second-trimester loss.1 A retrospective cohort study of Australian women, not included in the meta-analysis, assessed 4290 patients with a second-trimester pregnancy loss to specifically examine the association between IPI and risk of recurrent pregnancy loss.9
After a pregnancy loss at 14 to 19 weeks, women with an IPI < 3 months, compared with an IPI of 9 to 12 months, had an increased risk of recurrent pregnancy loss (21.9 vs 11.3%; P < .001). Women with an IPI > 9 to 12 months had rates of pregnancy loss similar to an IPI of 3 to 6 months (RR = 1.24; 95% CI, 0.89-1.7) and 6 to 9 months (RR = 1.02; 95% CI, 0.7-1.5). Women who experienced an initial loss at 20 to 23 weeks, for unclear reasons, showed no evidence that the IPI affected the risk of subsequent loss.
Short IPI may be linked to anxiety in first trimester of next pregnancy
A large cohort study of 20,308 pregnant Chinese women, including 1495 with a previous miscarriage, explored the mental health impact of IPI after miscarriage compared with no miscarriage.10 Investigators used the Self-Rating Anxiety Scale to evaluate anxiety and the Center for Epidemiologic Studies Depression Scale to evaluate depression.
Women with an IPI of < 7 months after miscarriage were more likely to experience anxiety symptoms in the subsequent pregnancy than were women with no previous miscarriage (adjusted odds ratio [AOR] = 2.76; 95% CI, 1.4-5.5), whereas women with a history of miscarriage and IPI > 6 months weren’t. Women with IPIs < 7 months and 7 to 12 months, compared with women who had no miscarriage, had an increased risk of depression (AOR = 2.5; 95% CI, 1.4-4.5, and AOR = 2.6; 95% CI, 1.3-5.2, respectively). Women with an IPI > 12 months had no increased risk of depression compared with women with no history of miscarriage.
The odds ratios were adjusted for age, education, BMI, income, and place of residence. The higher rates of depression and anxiety didn’t persist beyond the first trimester of the subsequent pregnancy.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists’ Practice Bulletin on Early Pregnancy Loss states that no quality data exist to support delaying conception after early pregnancy loss (defined as loss of an intrauterine pregnancy in the first trimester) to prevent subsequent pregnancy loss or other pregnancy complications.11
WHO recommends a minimum IPI of at least 6 months after a spontaneous or elective abortion. This recommendation is based on a single multi-center cohort study in Latin America that included women with both spontaneous and induced abortions.8
Editor’s takeaway
High-quality evidence now shows that shorter IPIs after first-trimester miscarriages result in safe subsequent pregnancies. However, some concern remains about second-trimester miscarriages and maternal mental health following a shorter IPI, based on lower-quality evidence.
EVIDENCE SUMMARY
To evaluate the longstanding belief that a short IPI after miscarriage is associated with adverse outcomes in subsequent pregnancies, a 2017 systematic review and meta-analysis of 16 studies (3 randomized controlled trials [RCTs] and 13 retrospective cohort studies) with a total of more than 1 million patients compared IPIs shorter and longer than 6 months (miscarriage was defined as any pregnancy loss before 24 weeks).1 The meta-analysis included 10 of the studies (2 RCTs and 8 cohort studies), with a total of 977,972 women and excluded 6 studies because of insufficient data. The outcomes investigated were recurrent miscarriage, preterm birth, stillbirth, pre-eclampsia, and low birthweight in the pregnancy following miscarriage.
Only 1 study reported the specific gestational age of the index miscarriage at 8.6 ± 2.8 weeks.2 All studies adjusted data for age, and some considered other confounders, such as race, smoking status, and body mass index (BMI).
Women included in the meta-analysis were from Asia, Europe, South America, and the United States and had a history of at least 1 miscarriage.1 A study of 257,908 subjects (Conde-Agudelo) also included women with a history of induced abortion from Latin American countries, where abortion is illegal, and made no distinction between spontaneous and induced abortions in those data sets.3 Women with a history of illegal abortion could be at greater risk of subsequent miscarriage than women who underwent a legally performed abortion.
IPI shorter than 6 months carries fewer risks
Excluding the Conde-Agudelo study, women with an IPI < 6 months, compared with > 6 months, had lower risks of subsequent miscarriage (7 studies, 46,313 women; risk ratio [RR] = 0.82; 95% confidence interval [CI], 0.78-0.86) and preterm delivery (7 studies, 60,772 women; RR = 0.79; 95% CI, 0.75-0.83); a higher rate of live births (4 studies, 44,586 women; RR = 1.06; 95% CI, 1.01-1.11); and no increase in stillbirths (4 studies, 44,586 women; RR = 0.88; 95% CI, 0.76-1.02), low birthweight (4 studies, 284,222 women; RR = 1.05; 95% CI, 0.48-2.29) or pre-eclampsia (5 studies, 284,899 women; RR = 0.95; 95% CI, 0.88-1.02) in the subsequent pregnancy.
Including the Conde-Agudelo study, the risk of preterm delivery was the same in women with an IPI < 6 months and > 6 months (8 studies, 318,880 women; RR = 0.93; 95% CI, 0.58-1.48).1 Four of the 10 studies evaluated the risk of miscarriage with an IPI < 3 months compared with > 3 months and found either no difference or a lower risk of subsequent miscarriage.2,4-6
IPI shorter than 3 months has lowest risk of all
A 2017 prospective cohort study examined the association between IPI length and risk of recurrent miscarriage in 514 women who had experienced recent miscarriage (defined as spontaneous pregnancy loss before 20 weeks of gestation).7 Average gestational age at the time of initial miscarriage wasn’t reported. Study participants were 30 years of age on average and predominantly white (76.8%); 12.3% were black.
The authors compared IPIs of < 3 months, 3 to 6 months, and > 18 months with IPIs of 6 to 18 months, which correlates with the IPIs recommended by the World Health Organization (WHO).8 They adjusted for maternal age, race, parity, BMI, and education. An IPI < 3 months was associated with the lowest risk of subsequent miscarriage (7.3% compared with 22.1%; adjusted hazard ratio = 0.33; 95% CI, 0.16-0.71). Women with IPIs of 3 to 6 months and > 18 months didn’t experience statistically significant differences in subsequent miscarriage rates compared with IPIs of 6 to 18 months.7
Continue to: But a short IPI after second-trimester loss increases risk of miscarriage
But a short IPI after second-trimester loss increases risk of miscarriage
By including all miscarriages, the meta-analysis effectively examined IPI after first-trimester loss because first-trimester loss occurs far more frequently than does second-trimester loss.1 A retrospective cohort study of Australian women, not included in the meta-analysis, assessed 4290 patients with a second-trimester pregnancy loss to specifically examine the association between IPI and risk of recurrent pregnancy loss.9
After a pregnancy loss at 14 to 19 weeks, women with an IPI < 3 months, compared with an IPI of 9 to 12 months, had an increased risk of recurrent pregnancy loss (21.9 vs 11.3%; P < .001). Women with an IPI > 9 to 12 months had rates of pregnancy loss similar to an IPI of 3 to 6 months (RR = 1.24; 95% CI, 0.89-1.7) and 6 to 9 months (RR = 1.02; 95% CI, 0.7-1.5). Women who experienced an initial loss at 20 to 23 weeks, for unclear reasons, showed no evidence that the IPI affected the risk of subsequent loss.
Short IPI may be linked to anxiety in first trimester of next pregnancy
A large cohort study of 20,308 pregnant Chinese women, including 1495 with a previous miscarriage, explored the mental health impact of IPI after miscarriage compared with no miscarriage.10 Investigators used the Self-Rating Anxiety Scale to evaluate anxiety and the Center for Epidemiologic Studies Depression Scale to evaluate depression.
Women with an IPI of < 7 months after miscarriage were more likely to experience anxiety symptoms in the subsequent pregnancy than were women with no previous miscarriage (adjusted odds ratio [AOR] = 2.76; 95% CI, 1.4-5.5), whereas women with a history of miscarriage and IPI > 6 months weren’t. Women with IPIs < 7 months and 7 to 12 months, compared with women who had no miscarriage, had an increased risk of depression (AOR = 2.5; 95% CI, 1.4-4.5, and AOR = 2.6; 95% CI, 1.3-5.2, respectively). Women with an IPI > 12 months had no increased risk of depression compared with women with no history of miscarriage.
The odds ratios were adjusted for age, education, BMI, income, and place of residence. The higher rates of depression and anxiety didn’t persist beyond the first trimester of the subsequent pregnancy.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists’ Practice Bulletin on Early Pregnancy Loss states that no quality data exist to support delaying conception after early pregnancy loss (defined as loss of an intrauterine pregnancy in the first trimester) to prevent subsequent pregnancy loss or other pregnancy complications.11
WHO recommends a minimum IPI of at least 6 months after a spontaneous or elective abortion. This recommendation is based on a single multi-center cohort study in Latin America that included women with both spontaneous and induced abortions.8
Editor’s takeaway
High-quality evidence now shows that shorter IPIs after first-trimester miscarriages result in safe subsequent pregnancies. However, some concern remains about second-trimester miscarriages and maternal mental health following a shorter IPI, based on lower-quality evidence.
1. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23:221-231.
2. Wong LF, Schliep KC, Silver RM, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol. 2015;212:375.e1-375.e11.
3. Conde-Agudelo A, Belizan JM, Breman R, et al. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet. 2005;89(suppl 1):S34-S40.
4. Bentolila Y, Ratzon R, Shoham-Vardi I, et al. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2013;26:1459-1464.
5. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591.
6. Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med. 1994;22:235-241.
7. Sundermann AC, Hartmann KE, Jones SH, et al. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol. 2017;130:1312-1318.
8. World Health Organization. Department of Reproductive Health and Research, Department of Making Pregnancy Safer. Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13-15 June 2005. Geneva: World Health Organization, 2007.
9. Roberts CL, Algert CS, Ford JB, et al. Association between interpregnancy interval and the risk of recurrent loss after a midtrimester loss. Hum Reprod. 2016;31:2834-2840.
10. Gong X, Hao J, Tao F, et al. Pregnancy loss and anxiety and depression during subsequent pregnancies: data from the C-ABC study. Eur J Obstet Gynecol Reprod Biol. 2013;166:30-36.
11. American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Gynecology. The American College of Obstetricians and Gynecologists Practice Bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
1. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta-analysis. Hum Reprod Update. 2017;23:221-231.
2. Wong LF, Schliep KC, Silver RM, et al. The effect of a very short interpregnancy interval and pregnancy outcomes following a previous pregnancy loss. Am J Obstet Gynecol. 2015;212:375.e1-375.e11.
3. Conde-Agudelo A, Belizan JM, Breman R, et al. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynaecol Obstet. 2005;89(suppl 1):S34-S40.
4. Bentolila Y, Ratzon R, Shoham-Vardi I, et al. Effect of interpregnancy interval on outcomes of pregnancy after recurrent pregnancy loss. J Matern Fetal Neonatal Med. 2013;26:1459-1464.
5. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591.
6. Wyss P, Biedermann K, Huch A. Relevance of the miscarriage-new pregnancy interval. J Perinat Med. 1994;22:235-241.
7. Sundermann AC, Hartmann KE, Jones SH, et al. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol. 2017;130:1312-1318.
8. World Health Organization. Department of Reproductive Health and Research, Department of Making Pregnancy Safer. Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13-15 June 2005. Geneva: World Health Organization, 2007.
9. Roberts CL, Algert CS, Ford JB, et al. Association between interpregnancy interval and the risk of recurrent loss after a midtrimester loss. Hum Reprod. 2016;31:2834-2840.
10. Gong X, Hao J, Tao F, et al. Pregnancy loss and anxiety and depression during subsequent pregnancies: data from the C-ABC study. Eur J Obstet Gynecol Reprod Biol. 2013;166:30-36.
11. American College of Obstetricians and Gynecologists. Committee on Practice Bulletins-Gynecology. The American College of Obstetricians and Gynecologists Practice Bulletin no. 150. Early pregnancy loss. Obstet Gynecol. 2015;125:1258-1267.
EVIDENCE-BASED ANSWER:
An interpregnancy interval (IPI) of < 6 months following miscarriage is associated with an increased live birth rate in subsequent pregnancy, lower risks of preterm birth and subsequent miscarriage, and no difference in rates of stillbirth, pre-eclampsia, and low birth weight infants (strength of recommendation [SOR]: A, well-done meta-analysis). (IPI is defined as the time between the end of one pregnancy and the last menstrual period of a subsequent one.)
A very short IPI (< 3 months), when compared with an IPI of 6 to 18 months, is associated with the lowest rate of subsequent miscarriage (SOR: B, cohort study). However, for women who experience a pregnancy loss at 14 to 19 weeks’ gestation, an IPI < 3 months is associated with an increased risk of miscarriage or birth before 24 weeks’ gestation (SOR: B, cohort study).
Women with a short IPI following miscarriage may be at increased risk for anxiety and depression in the first trimester of the subsequent pregnancy (SOR: B, cohort study).