User login
Is an outpatient workup safe for patients with a transient ischemic attack?
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
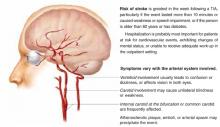
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
There is no compelling evidence that outpatient diagnostic workup of patients with transient ischemic attack (TIA) is less safe than inpatient workup, or that hospitalization prevents stroke or improves stroke outcomes after TIA (strength of recommendation [SOR]: C, based on case series studies). Because the risk of stroke is substantial in the week following a TIA (SOR: A, based on a prospective cohort study), evaluation and treatment for reversible stroke risk factors should be initiated urgently and completed within a week of initial presentation (SOR: C, based on expert consensus opinion).
Risk factors for patients at highest risk for stroke or other cardiovascular events after TIA include age >60 years, diabetes, TIA lasting longer than 10 minutes, and a TIA associated with weakness or speech impairment (SOR: B, based on retrospective cohort study). Hospitalization may be prudent for patients at high risk for cardiovascular events or for those with mental status changes, an inadequate home situation, or the physician’s inability to obtain expedient evaluation (SOR: C, based on case series studies).
Evidence summary
Transient ischemic attack (Figure) is a temporary, focal brain or retinal deficit caused by vascular disease that clears completely in less than 24 hours.1 A large prospective cohort study recently estimated the risk of stroke after a TIA or minor stroke to be 8% to 12% at 7 days and 11% to 15% at 1 month.2
In a large retrospective cohort study, 5% of TIA patients returned to the emergency department with a stroke within the first 2 days after TIA.3 Another 6% returned with a stroke within 90 days. Five independent risk factors were identified: age >60 years, diabetes mellitus, duration of TIA longer than 10 minutes, signs or symptoms of weakness, and speech impairment. Thirty-four percent of patients with all 5 risk factors, and none of the patients without any risk factors, had a stroke within 90 days. Of note, 13% of the TIA patients had an arrhythmia, congestive heart failure, unstable angina, myocardial infarction, stroke, or recurrent TIA within 4 days of initial presenting with a TIA. Twenty-five percent of the patients experienced 1 of these cardiovascular events during the 3 months of follow-up.
In a retrospective case review of TIA and stroke patients, the hospital admissions of 4 of 21 TIA patients were retrospectively categorized as medically justified.4 Admission was categorized as medically justified if the patient had 1 or more of the following criteria: another diagnosis that warranted admission, inadequate home situation, altered mental status, an adverse event during hospitalization including worsening of the deficit, and if the patient underwent some hospital-based treatment that could not be provided on an out-patient basis. Ease and rapidity of evaluation was not considered medically justifiable and outcome improvement (stroke prevention) was not studied.
Two retrospective chart reviews of TIA found considerable practice variability in the evaluation of TIA patient. In 1 study of TIA patients presenting to an emergency department, 81% had a computed tomography scan, 75% had electrocardiogram, and 74% had a complete blood count.5 Carotid Doppler imaging was performed in the emergency department in 16%, and 26% were referred for outpatient Doppler studies. One percent had an ECG in the emergency department, and 16% were given ECGs as outpatients. Seventy-five percent of patients were discharged home. Those hospitalized had a median length of stay of 1 day. In the second study, 31% of the TIA patients had no diagnostic studies performed during the first month after presenting to their primary care physician.6
FIGURE
Expeditious evaluation of TIA is imperative
Recommendations from others
The American Heart Association (AHA) recommends that physicians use a stepwise approach to TIA evaluation as outlined in the Table. The AHA also recommends that the diagnostic evaluation of patients seen within 7 days of a TIA should be completed within 1 week or less. The AHA leaves the decision whether to hospitalize a patient up to the physician based on a patient’s circumstances. The goals of diagnostic testing are to identify or exclude causes of TIA requiring specific therapy, to assess modifiable risk factors, and to determine prognosis.7
The National Stroke Association recommends that patients with known high-grade stenosis in a vascular territory appropriate to the symptoms, and patients with recurrent symptoms, undergo urgent evaluation. Evaluation includes imaging and ruling out other causes of TIA. Patients should be admitted to the hospital if imaging is not immediately available. If indicated, carotid endarterectomy should be performed without delay.8
TABLE
Stepwise diagnostic evaluation for patients with transient ischemic attack
Initial Evaluation
|
Second step (to resolve persistent diagnostic uncertainty as appropriate)
|
| Adapted from Feinberg et al 1994.7 |
Make the patient aware of the risks of TIA and quickly complete the work-up
Jon O. Neher, MD
Valley Medical Center, Renton, Wash
It is important to remember that a diagnosis of TIA can only be made retrospectively. All patients with ongoing focal neurologic signs must be evaluated immediately and (if the symptom duration is less than 3 hours) considered potential candidates for emergent thrombolytic therapy.
The vast majority of TIA patients are asymptomatic during their evaluation. Because they feel well and may have a considerable element of denial, it can be hard to get them to rapidly complete their evaluation in either the inpatient or outpatient setting. It is therefore critical that the patient be made aware that the highest risk period is soon after the TIA and that failure to quickly complete the work-up could have serious negative consequences.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
1. Levy DE. How transient are transient ischemic attacks? Neurology 1988;38:674-677.
2. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326.-
3. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-2906.
4. Henneman PL, Lewis RJ. Is admission medically justified for all patients with acute stroke or transient ischemic attack? Ann Emerg Med 1995;25:458-463.
5. Chang E, Holroyd BR, Kochanski P, Kelly KD, Shuaib A, Rowe BH. Adherence to practice guidelines for transient ischemic attacks in an emergency department. Can J Neurol Sci 2002;29:358-363.
6. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-2946.
7. Feinberg WM, Albers GW, Barnett HJ, et al. Guidelines for the management of transient ischemic attacks. From the Ad Hoc Committee of Guidelines for the Management of Transient Ischemic Attacks of the Stroke Council of the American Heart Association. Circulation 1994;89:2950-2965.
8. Brott TG, Clark WM, Fagan SC, et al. Stroke: The First Hours: Guidelines for Acute Treatment. Englewood, Colo: National Stroke Association; 2000.
Evidence-based answers from the Family Physicians Inquiries Network
Do acetaminophen and an NSAID combined relieve osteoarthritis pain better than either alone?
Combining nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen for short courses provides more relief of pain in osteoarthritis with-out an increase in side effects (strength of recommendation [SOR]=B). Combining acetaminophen at 4 g/d with an NSAID can also decrease the daily dose of NSAID required for pain relief, thus reducing the potential risk from higher-dose NSAID therapy (SOR=B).
Over the long term, however, this combination may increase the risk of upper gastrointestinal (GI) bleeding more than that conferred by the NSAID alone (SOR=B). If combination therapy is necessary, limiting the dose of acetaminophen to 2 g/d minimizes gastrointestinal toxicity. Acetaminophen alone at the lowest dose to provide pain relief is the safest pharmacologic choice for patients with osteoarthritis.
Evidence summary
Clinical guidelines for osteoarthritis recommend acetaminophen as first-line therapy followed by an NSAID or cyclooxygenase-2 (COX-2) inhibitor, and many patients are treated with combination therapy.
Several small randomized controlled trials have compared the individual efficacy of NSAIDs and acetaminophen in osteoarthritis and have found that both provide more pain relief than placebo.1-3There is a trend toward improved pain relief with NSAIDs compared with acetaminophen in the initial treatment period; however, few long-term studies of efficacy have been reported. One randomized controlled trial comparing 750 mg/d naproxen (Aleve, Naprosyn) with 2600 mg/d acetaminophen for 2 years found similar pain relief for both medications and a dropout rate of 65% in both groups.2 Similar numbers of persons taking acetaminophen or naproxen dropped out because of adverse effects (20%) or lack of efficacy (19%), and no difference was seen in functional improvement between the 2 groups.
A 6-week randomized double-blind crossover trial of 227 patients comparing 75 mg diclofenac and 200 mg misoprostol (Arthrotec) with acetaminophen 4 g/d found the diclofenac-misoprostol combination provided more pain control than acetaminophen alone. Adverse events were slightly more common in the diclofenac group (54% vs 46%; P=.046).4
The COX-2 inhibitors rofecoxib (Vioxx) and celecoxib (Celebrex) have been shown to provide equal pain relief compared with naproxen for patients with osteoarthritis.5 One industry-sponsored randomized trial found rofecoxib superior to celecoxib, and both superior to acetaminophen in treatment of osteoarthritis pain.6 There was no difference in the incidence of side effects among the 3 medications. Thirty percent of patients taking 4 g/d acetaminophen discontinued the study because of lack of efficacy, compared with 20% of those taking either celecoxib or rofecoxib.6
Few studies have evaluated the safety or efficacy of the combination of NSAIDs and acetaminophen in osteoarthritis. One double-blind, double-dummy crossover trial of 18 patients with osteoarthritis of the hip compared naproxen at doses of 500 mg and 1000 mg, with and without 4 g/d of acetaminophen, and 1500 mg/d of naproxen alone over 5 one-week trial periods.7Adding acetaminophen improved patient-reported pain scores compared with naproxen alone. Higher doses of naproxen alone provided less pain relief than a lower dose of naproxen combined with acetaminophen. GI side effects increased with the increase in naproxen dose, but were unaffected by the addition of acetaminophen. Functional ability was not affected during this short study. A similar study by the same researchers of patients with rheumatoid arthritis found similar results.7
One randomized, double-blind, crossover trial compared single doses of tolmetin (Tolectin, 100, 150, 200 mg) and acetaminophen (400 mg) alone and in combination with placebo in the control of experimentally induced pain (thermal and electrical stimulation). Acetaminophen alone did not differ from placebo in pain control; however, the combinations of acetaminophen with tolmetin provided similar pain relief to higher doses of tolmetin alone.8 No studies have evaluated the efficacy or safety of acetaminophen combined with rofecoxib or celecoxib.
Regarding the risks of combining acetaminophen with NSAIDs, 1 nested case-control study based on the entire enrollment panel of the British National Health Service characterized the risk of upper GI side effects among persons taking NSAIDs or acetaminophen alone or in combination. The study evaluated medications in use at the time of an upper GI bleed, controlling for age, sex, and concomitant medications (corticosteroids, H2 receptor antagonists, omeprazole, anticoagulants, and others) and excluding patients with varices, alcohol-related disorders, liver disease, and cancer; no attempt was made to control other comorbidities. The relative risk of upper GI perforation or bleeding for patients taking 2g/d acetaminophen or high-dose NSAIDs was 2.4 (95% confidence interval [CI], 1.7–3.5) and 3.6 (95% CI, 2.9–4.3), respectively. Concomitant use of an NSAID with 2 g/d of acetaminophen showed a relative risk of upper GI perforation or bleed of 16.6 (95% CI, 11.0–24.9). Acetaminophen doses <2 g/d conferred no additional risk for serious upper GI side effects.9
A systematic review of selective COX-2 inhibitors vs naproxen found fewer endoscopically detected ulcers in patients taking celecoxib but no difference in serious gastrointestinal bleeds.5 A meta-analysis of randomized controlled trials found a higher incidence of serious thrombotic cardiovascular events among patients taking COX-2 inhibitors compared with naprosyn.10 The safety profile of rofecoxib and celecoxib in the long-term treatment of pain is not fully understood at this time.
Recommendations from others
The American College of Rheumatology (ACR) recommends acetaminophen up to 4 g/d as a first-line pharmacologic treatment for osteoarthritis of the hip and knee, and advises NSAIDs be used at the lowest effective dose if they are necessary for pain control.11 The ACR does not specifically comment on combining NSAID and acetaminophen use. The American Academy of Orthopaedic Surgeons recommends initial use of an NSAID or acetaminophen, but does not comment on the combination of NSAIDs and acetaminophen.12
Adding acetaminophen may be more desirable than switching NSAIDs
Joseph Saseen, PharmD, FCCP, BCPS
University of Colorado Health Sciences Center, Denver
Compared with NSAIDs, acetaminophen has a complementary analgesic mechanism of action and can be safely used in many patients. Additive effects of acetaminophen have not been well described with all NSAIDs (eg, COX-2 inhibitors); however, this combination is inexpensive and overall appears to effectively augment analgesia when combined with NSAIDs. Although observational data demonstrate an increased risk of upper GI bleeding with this combination, selection bias (higher-risk patients being on combination therapy) could reasonably explain this association. Adding acetaminophen may be more desirable than switching NSAIDs for patients with osteoarthritis that have a partial response to their current NSAID therapy.
- Amoxicillin • Amoxil, Biomox, Polymox, Trimox, Wymox
- Cephalexin • Biocef, Keflex
- Celecoxib • Celebrex
- Diclofenac/Misoprostol • Arthrotec
- Ipratropium • Atrovent
- Labetalol • Trandate
- Methyldopa • Aldomet
- Naproxen • Aleve, Anaprox, Naprosyn
- Nitrofurantoin • Furadantin, Macrobid, Macrodantin
- Rofecoxib • Vioxx
- Tiotropium • Spiriva
- Tolmetin • Tolectin
- Triamcinalone • Aristocort, Atolone, Kenacort
- Sulfamethoxazole/Trimethoprim • Bactrim,Cotrim,
- Septra, Sulfatrim
- Sulfisoxazole • Gantrisin
1. Amadio P Jr, Cummings DM. Evaluation of acetaminophen in the management of osteoarthritis of the knee. Curr Ther Res 1983;34:59-66.
2. Williams HJ, Ward JR, Egger MJ, et al. Comparison of naproxen and acetaminophen in a two-year study of treatment of osteoarthritis of the knee. Arthritis Rheum 1993;36:1196-1206.
3. Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Treatment of knee osteoarthritis: relationship of clinical features of joint inflammation to the response to a nonsteroidal antiinflammatory drug or pure analgesic. J Rheumatol 1992;19:1950-1954.
4. Pincus T, Koch GG, Sokka T, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen for patients with osteoarthritis of the hip or knee. Arthritis Rheum 2001;44:1587-1598.
5. Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002;325:619.-
6. Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ. Vioxx. Acetaminophen Celecoxib Trial (VACT) Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA 2002;287:64-71.
7. Seideman P, Samuelson P, Neander G. Naproxen and paracetamol compared with naproxen only in coxarthrosis. Increased effect of the combination in 18 patients. Acta Orthop Scand 1993;64:285-288.
8. Stacher G, Bauer P, Ehn I, Schreiber E. Effects of tolmetin, paracetamol, and of two combinations of tolmetin and paracetamol as compared to placebo on experimentally induced pain. A double blind study. Int J Clin Pharmacol Biopharm 1979;17:250-255.
9. Garcia Rodriguez LA, Hernandez-Diaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res 2001;3:98-101.
10. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-959.
11. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
12. AAOS Clinical Guideline on Osteoarthritis of the Knee. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2003. Available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_oakn.pdf. Accessed on May 11, 2004.
Combining nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen for short courses provides more relief of pain in osteoarthritis with-out an increase in side effects (strength of recommendation [SOR]=B). Combining acetaminophen at 4 g/d with an NSAID can also decrease the daily dose of NSAID required for pain relief, thus reducing the potential risk from higher-dose NSAID therapy (SOR=B).
Over the long term, however, this combination may increase the risk of upper gastrointestinal (GI) bleeding more than that conferred by the NSAID alone (SOR=B). If combination therapy is necessary, limiting the dose of acetaminophen to 2 g/d minimizes gastrointestinal toxicity. Acetaminophen alone at the lowest dose to provide pain relief is the safest pharmacologic choice for patients with osteoarthritis.
Evidence summary
Clinical guidelines for osteoarthritis recommend acetaminophen as first-line therapy followed by an NSAID or cyclooxygenase-2 (COX-2) inhibitor, and many patients are treated with combination therapy.
Several small randomized controlled trials have compared the individual efficacy of NSAIDs and acetaminophen in osteoarthritis and have found that both provide more pain relief than placebo.1-3There is a trend toward improved pain relief with NSAIDs compared with acetaminophen in the initial treatment period; however, few long-term studies of efficacy have been reported. One randomized controlled trial comparing 750 mg/d naproxen (Aleve, Naprosyn) with 2600 mg/d acetaminophen for 2 years found similar pain relief for both medications and a dropout rate of 65% in both groups.2 Similar numbers of persons taking acetaminophen or naproxen dropped out because of adverse effects (20%) or lack of efficacy (19%), and no difference was seen in functional improvement between the 2 groups.
A 6-week randomized double-blind crossover trial of 227 patients comparing 75 mg diclofenac and 200 mg misoprostol (Arthrotec) with acetaminophen 4 g/d found the diclofenac-misoprostol combination provided more pain control than acetaminophen alone. Adverse events were slightly more common in the diclofenac group (54% vs 46%; P=.046).4
The COX-2 inhibitors rofecoxib (Vioxx) and celecoxib (Celebrex) have been shown to provide equal pain relief compared with naproxen for patients with osteoarthritis.5 One industry-sponsored randomized trial found rofecoxib superior to celecoxib, and both superior to acetaminophen in treatment of osteoarthritis pain.6 There was no difference in the incidence of side effects among the 3 medications. Thirty percent of patients taking 4 g/d acetaminophen discontinued the study because of lack of efficacy, compared with 20% of those taking either celecoxib or rofecoxib.6
Few studies have evaluated the safety or efficacy of the combination of NSAIDs and acetaminophen in osteoarthritis. One double-blind, double-dummy crossover trial of 18 patients with osteoarthritis of the hip compared naproxen at doses of 500 mg and 1000 mg, with and without 4 g/d of acetaminophen, and 1500 mg/d of naproxen alone over 5 one-week trial periods.7Adding acetaminophen improved patient-reported pain scores compared with naproxen alone. Higher doses of naproxen alone provided less pain relief than a lower dose of naproxen combined with acetaminophen. GI side effects increased with the increase in naproxen dose, but were unaffected by the addition of acetaminophen. Functional ability was not affected during this short study. A similar study by the same researchers of patients with rheumatoid arthritis found similar results.7
One randomized, double-blind, crossover trial compared single doses of tolmetin (Tolectin, 100, 150, 200 mg) and acetaminophen (400 mg) alone and in combination with placebo in the control of experimentally induced pain (thermal and electrical stimulation). Acetaminophen alone did not differ from placebo in pain control; however, the combinations of acetaminophen with tolmetin provided similar pain relief to higher doses of tolmetin alone.8 No studies have evaluated the efficacy or safety of acetaminophen combined with rofecoxib or celecoxib.
Regarding the risks of combining acetaminophen with NSAIDs, 1 nested case-control study based on the entire enrollment panel of the British National Health Service characterized the risk of upper GI side effects among persons taking NSAIDs or acetaminophen alone or in combination. The study evaluated medications in use at the time of an upper GI bleed, controlling for age, sex, and concomitant medications (corticosteroids, H2 receptor antagonists, omeprazole, anticoagulants, and others) and excluding patients with varices, alcohol-related disorders, liver disease, and cancer; no attempt was made to control other comorbidities. The relative risk of upper GI perforation or bleeding for patients taking 2g/d acetaminophen or high-dose NSAIDs was 2.4 (95% confidence interval [CI], 1.7–3.5) and 3.6 (95% CI, 2.9–4.3), respectively. Concomitant use of an NSAID with 2 g/d of acetaminophen showed a relative risk of upper GI perforation or bleed of 16.6 (95% CI, 11.0–24.9). Acetaminophen doses <2 g/d conferred no additional risk for serious upper GI side effects.9
A systematic review of selective COX-2 inhibitors vs naproxen found fewer endoscopically detected ulcers in patients taking celecoxib but no difference in serious gastrointestinal bleeds.5 A meta-analysis of randomized controlled trials found a higher incidence of serious thrombotic cardiovascular events among patients taking COX-2 inhibitors compared with naprosyn.10 The safety profile of rofecoxib and celecoxib in the long-term treatment of pain is not fully understood at this time.
Recommendations from others
The American College of Rheumatology (ACR) recommends acetaminophen up to 4 g/d as a first-line pharmacologic treatment for osteoarthritis of the hip and knee, and advises NSAIDs be used at the lowest effective dose if they are necessary for pain control.11 The ACR does not specifically comment on combining NSAID and acetaminophen use. The American Academy of Orthopaedic Surgeons recommends initial use of an NSAID or acetaminophen, but does not comment on the combination of NSAIDs and acetaminophen.12
Adding acetaminophen may be more desirable than switching NSAIDs
Joseph Saseen, PharmD, FCCP, BCPS
University of Colorado Health Sciences Center, Denver
Compared with NSAIDs, acetaminophen has a complementary analgesic mechanism of action and can be safely used in many patients. Additive effects of acetaminophen have not been well described with all NSAIDs (eg, COX-2 inhibitors); however, this combination is inexpensive and overall appears to effectively augment analgesia when combined with NSAIDs. Although observational data demonstrate an increased risk of upper GI bleeding with this combination, selection bias (higher-risk patients being on combination therapy) could reasonably explain this association. Adding acetaminophen may be more desirable than switching NSAIDs for patients with osteoarthritis that have a partial response to their current NSAID therapy.
- Amoxicillin • Amoxil, Biomox, Polymox, Trimox, Wymox
- Cephalexin • Biocef, Keflex
- Celecoxib • Celebrex
- Diclofenac/Misoprostol • Arthrotec
- Ipratropium • Atrovent
- Labetalol • Trandate
- Methyldopa • Aldomet
- Naproxen • Aleve, Anaprox, Naprosyn
- Nitrofurantoin • Furadantin, Macrobid, Macrodantin
- Rofecoxib • Vioxx
- Tiotropium • Spiriva
- Tolmetin • Tolectin
- Triamcinalone • Aristocort, Atolone, Kenacort
- Sulfamethoxazole/Trimethoprim • Bactrim,Cotrim,
- Septra, Sulfatrim
- Sulfisoxazole • Gantrisin
Combining nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen for short courses provides more relief of pain in osteoarthritis with-out an increase in side effects (strength of recommendation [SOR]=B). Combining acetaminophen at 4 g/d with an NSAID can also decrease the daily dose of NSAID required for pain relief, thus reducing the potential risk from higher-dose NSAID therapy (SOR=B).
Over the long term, however, this combination may increase the risk of upper gastrointestinal (GI) bleeding more than that conferred by the NSAID alone (SOR=B). If combination therapy is necessary, limiting the dose of acetaminophen to 2 g/d minimizes gastrointestinal toxicity. Acetaminophen alone at the lowest dose to provide pain relief is the safest pharmacologic choice for patients with osteoarthritis.
Evidence summary
Clinical guidelines for osteoarthritis recommend acetaminophen as first-line therapy followed by an NSAID or cyclooxygenase-2 (COX-2) inhibitor, and many patients are treated with combination therapy.
Several small randomized controlled trials have compared the individual efficacy of NSAIDs and acetaminophen in osteoarthritis and have found that both provide more pain relief than placebo.1-3There is a trend toward improved pain relief with NSAIDs compared with acetaminophen in the initial treatment period; however, few long-term studies of efficacy have been reported. One randomized controlled trial comparing 750 mg/d naproxen (Aleve, Naprosyn) with 2600 mg/d acetaminophen for 2 years found similar pain relief for both medications and a dropout rate of 65% in both groups.2 Similar numbers of persons taking acetaminophen or naproxen dropped out because of adverse effects (20%) or lack of efficacy (19%), and no difference was seen in functional improvement between the 2 groups.
A 6-week randomized double-blind crossover trial of 227 patients comparing 75 mg diclofenac and 200 mg misoprostol (Arthrotec) with acetaminophen 4 g/d found the diclofenac-misoprostol combination provided more pain control than acetaminophen alone. Adverse events were slightly more common in the diclofenac group (54% vs 46%; P=.046).4
The COX-2 inhibitors rofecoxib (Vioxx) and celecoxib (Celebrex) have been shown to provide equal pain relief compared with naproxen for patients with osteoarthritis.5 One industry-sponsored randomized trial found rofecoxib superior to celecoxib, and both superior to acetaminophen in treatment of osteoarthritis pain.6 There was no difference in the incidence of side effects among the 3 medications. Thirty percent of patients taking 4 g/d acetaminophen discontinued the study because of lack of efficacy, compared with 20% of those taking either celecoxib or rofecoxib.6
Few studies have evaluated the safety or efficacy of the combination of NSAIDs and acetaminophen in osteoarthritis. One double-blind, double-dummy crossover trial of 18 patients with osteoarthritis of the hip compared naproxen at doses of 500 mg and 1000 mg, with and without 4 g/d of acetaminophen, and 1500 mg/d of naproxen alone over 5 one-week trial periods.7Adding acetaminophen improved patient-reported pain scores compared with naproxen alone. Higher doses of naproxen alone provided less pain relief than a lower dose of naproxen combined with acetaminophen. GI side effects increased with the increase in naproxen dose, but were unaffected by the addition of acetaminophen. Functional ability was not affected during this short study. A similar study by the same researchers of patients with rheumatoid arthritis found similar results.7
One randomized, double-blind, crossover trial compared single doses of tolmetin (Tolectin, 100, 150, 200 mg) and acetaminophen (400 mg) alone and in combination with placebo in the control of experimentally induced pain (thermal and electrical stimulation). Acetaminophen alone did not differ from placebo in pain control; however, the combinations of acetaminophen with tolmetin provided similar pain relief to higher doses of tolmetin alone.8 No studies have evaluated the efficacy or safety of acetaminophen combined with rofecoxib or celecoxib.
Regarding the risks of combining acetaminophen with NSAIDs, 1 nested case-control study based on the entire enrollment panel of the British National Health Service characterized the risk of upper GI side effects among persons taking NSAIDs or acetaminophen alone or in combination. The study evaluated medications in use at the time of an upper GI bleed, controlling for age, sex, and concomitant medications (corticosteroids, H2 receptor antagonists, omeprazole, anticoagulants, and others) and excluding patients with varices, alcohol-related disorders, liver disease, and cancer; no attempt was made to control other comorbidities. The relative risk of upper GI perforation or bleeding for patients taking 2g/d acetaminophen or high-dose NSAIDs was 2.4 (95% confidence interval [CI], 1.7–3.5) and 3.6 (95% CI, 2.9–4.3), respectively. Concomitant use of an NSAID with 2 g/d of acetaminophen showed a relative risk of upper GI perforation or bleed of 16.6 (95% CI, 11.0–24.9). Acetaminophen doses <2 g/d conferred no additional risk for serious upper GI side effects.9
A systematic review of selective COX-2 inhibitors vs naproxen found fewer endoscopically detected ulcers in patients taking celecoxib but no difference in serious gastrointestinal bleeds.5 A meta-analysis of randomized controlled trials found a higher incidence of serious thrombotic cardiovascular events among patients taking COX-2 inhibitors compared with naprosyn.10 The safety profile of rofecoxib and celecoxib in the long-term treatment of pain is not fully understood at this time.
Recommendations from others
The American College of Rheumatology (ACR) recommends acetaminophen up to 4 g/d as a first-line pharmacologic treatment for osteoarthritis of the hip and knee, and advises NSAIDs be used at the lowest effective dose if they are necessary for pain control.11 The ACR does not specifically comment on combining NSAID and acetaminophen use. The American Academy of Orthopaedic Surgeons recommends initial use of an NSAID or acetaminophen, but does not comment on the combination of NSAIDs and acetaminophen.12
Adding acetaminophen may be more desirable than switching NSAIDs
Joseph Saseen, PharmD, FCCP, BCPS
University of Colorado Health Sciences Center, Denver
Compared with NSAIDs, acetaminophen has a complementary analgesic mechanism of action and can be safely used in many patients. Additive effects of acetaminophen have not been well described with all NSAIDs (eg, COX-2 inhibitors); however, this combination is inexpensive and overall appears to effectively augment analgesia when combined with NSAIDs. Although observational data demonstrate an increased risk of upper GI bleeding with this combination, selection bias (higher-risk patients being on combination therapy) could reasonably explain this association. Adding acetaminophen may be more desirable than switching NSAIDs for patients with osteoarthritis that have a partial response to their current NSAID therapy.
- Amoxicillin • Amoxil, Biomox, Polymox, Trimox, Wymox
- Cephalexin • Biocef, Keflex
- Celecoxib • Celebrex
- Diclofenac/Misoprostol • Arthrotec
- Ipratropium • Atrovent
- Labetalol • Trandate
- Methyldopa • Aldomet
- Naproxen • Aleve, Anaprox, Naprosyn
- Nitrofurantoin • Furadantin, Macrobid, Macrodantin
- Rofecoxib • Vioxx
- Tiotropium • Spiriva
- Tolmetin • Tolectin
- Triamcinalone • Aristocort, Atolone, Kenacort
- Sulfamethoxazole/Trimethoprim • Bactrim,Cotrim,
- Septra, Sulfatrim
- Sulfisoxazole • Gantrisin
1. Amadio P Jr, Cummings DM. Evaluation of acetaminophen in the management of osteoarthritis of the knee. Curr Ther Res 1983;34:59-66.
2. Williams HJ, Ward JR, Egger MJ, et al. Comparison of naproxen and acetaminophen in a two-year study of treatment of osteoarthritis of the knee. Arthritis Rheum 1993;36:1196-1206.
3. Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Treatment of knee osteoarthritis: relationship of clinical features of joint inflammation to the response to a nonsteroidal antiinflammatory drug or pure analgesic. J Rheumatol 1992;19:1950-1954.
4. Pincus T, Koch GG, Sokka T, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen for patients with osteoarthritis of the hip or knee. Arthritis Rheum 2001;44:1587-1598.
5. Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002;325:619.-
6. Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ. Vioxx. Acetaminophen Celecoxib Trial (VACT) Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA 2002;287:64-71.
7. Seideman P, Samuelson P, Neander G. Naproxen and paracetamol compared with naproxen only in coxarthrosis. Increased effect of the combination in 18 patients. Acta Orthop Scand 1993;64:285-288.
8. Stacher G, Bauer P, Ehn I, Schreiber E. Effects of tolmetin, paracetamol, and of two combinations of tolmetin and paracetamol as compared to placebo on experimentally induced pain. A double blind study. Int J Clin Pharmacol Biopharm 1979;17:250-255.
9. Garcia Rodriguez LA, Hernandez-Diaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res 2001;3:98-101.
10. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-959.
11. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
12. AAOS Clinical Guideline on Osteoarthritis of the Knee. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2003. Available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_oakn.pdf. Accessed on May 11, 2004.
1. Amadio P Jr, Cummings DM. Evaluation of acetaminophen in the management of osteoarthritis of the knee. Curr Ther Res 1983;34:59-66.
2. Williams HJ, Ward JR, Egger MJ, et al. Comparison of naproxen and acetaminophen in a two-year study of treatment of osteoarthritis of the knee. Arthritis Rheum 1993;36:1196-1206.
3. Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Treatment of knee osteoarthritis: relationship of clinical features of joint inflammation to the response to a nonsteroidal antiinflammatory drug or pure analgesic. J Rheumatol 1992;19:1950-1954.
4. Pincus T, Koch GG, Sokka T, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen for patients with osteoarthritis of the hip or knee. Arthritis Rheum 2001;44:1587-1598.
5. Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002;325:619.-
6. Geba GP, Weaver AL, Polis AB, Dixon ME, Schnitzer TJ. Vioxx. Acetaminophen Celecoxib Trial (VACT) Group. Efficacy of rofecoxib, celecoxib, and acetaminophen in osteoarthritis of the knee: a randomized trial. JAMA 2002;287:64-71.
7. Seideman P, Samuelson P, Neander G. Naproxen and paracetamol compared with naproxen only in coxarthrosis. Increased effect of the combination in 18 patients. Acta Orthop Scand 1993;64:285-288.
8. Stacher G, Bauer P, Ehn I, Schreiber E. Effects of tolmetin, paracetamol, and of two combinations of tolmetin and paracetamol as compared to placebo on experimentally induced pain. A double blind study. Int J Clin Pharmacol Biopharm 1979;17:250-255.
9. Garcia Rodriguez LA, Hernandez-Diaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res 2001;3:98-101.
10. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-959.
11. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
12. AAOS Clinical Guideline on Osteoarthritis of the Knee. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2003. Available at: www.aaos.org/wordhtml/pdfs_r/guidelin/suprt_oakn.pdf. Accessed on May 11, 2004.
Evidence-based answers from the Family Physicians Inquiries Network
Do antibiotics prevent recurrent UTI in children with anatomic abnormalities?
Evidence is insufficient to recommend for or against antibiotic prophylaxis to prevent recurrent urinary tract infections (UTI) in children with anatomic abnormalities. Guidelines acknowledge this lack of evidence, but still recommend using prophylactic antibiotics in children with vesiculoureteral reflux (strength of recommendation: B, based on poor-quality or inconclusive cohort and randomized controlled studies).1-3 No controlled, prospective studies have examined the effectiveness of prophylactic antibiotics to prevent UTI recurrence or renal scarring.
Evidence summary
Recommendations about antibiotic prophylaxis are based on several premises. Reflux predisposes children to acute pyelonephritis; reflux plus infection leads to reflux nephropathy and ultimately to renal scarring. In theory, if antibiotics could be initiated at the appropriate time and be maintained until reflux resolves, we could successfully prevent infection and scarring.4
A recent systematic review evaluated the use of antibiotics to prevent UTI in children.5 This review of 5 randomized controlled trials included a total of 463 children between the ages of 2 months to 16 years. Three out of 5 trials evaluated the effectiveness of antibiotic treatment for 2 to 6 months to prevent subsequent off-treatment recurrence. The 2 smaller trials (n=71) evaluated the use of low-dose long-term antibiotics to prevent UTI.
There was a clinically, but not statistically, significant trend towards reduced risk of UTI during long-term antibiotic treatment (risk reduction [RR]=0.31; 95% confidence interval [CI]=0.10–1.00); however, no sustained benefit was seen once antibiotics were stopped (RR=0.79; 95% CI, 0.61–1.02). There were many problems with the methodological quality of these trials, including significant heterogeneity. The researchers concluded that well-designed randomized controlled trails are still needed to evaluate this commonly used intervention in the pediatric population.4 Benefits for long-term prophylaxis are even less clear in children with low-grade reflux (I–II).5 Furthermore, no randomized controlled trials assess whether prophylaxis prevents the development of new renal scars in children.6
In addition, a recent systematic review of studies done in children with normal urinary tracts, as well in children with neurogenic bladders, found that the available evidence is of low quality. Only 6 out of 31 potential studies fulfilled the inclusion criteria. These were small (mean sample size was 28), and the quality scores of all 6 trials were low, indicating that the evidence may be unreliable.7
Two of 3 studies done in children with normal urinary tracts demonstrated statistically significant higher rates of UTI recurrence in control groups compared with treatment groups receiving 6 to 10 months of either nitrofurantoin or cotrimoxazole (RR=24–31). The third study showed no difference between groups.
One of 2 trials in children with neurogenic bladder demonstrated higher recurrence rates of 2.9 per 10 patient years for patients receiving antibiotics compared with 1.5 in the untreated group. The other study showed lower recurrence rates of 17.1 for patients receiving antibiotics, compared with 33 in the untreated group.7Neither of these findings were statistically significant.
A different meta-analysis of 15 controlled clinical trials in children with neurogenic bladder due to spinal cord dysfunction. This analysis showed that antibiotic prophylaxis was associated with a reduction in asymptomatic bacteruria among children with acute spinal cord injury (P<.05), but there was no significant reduction in symptomatic infections. Prophylaxis resulted in an approximately twofold increase in antimicrobial-resistant bacteria. The researchers concluded that although a clinically important effect has not been excluded, the regular use of antimicrobial prophylaxis for most patients who have neurogenic bladder caused by spinal cord dysfunction is not supported at this time.8
Poor compliance may be an issue with long-term prophylaxis and may represent patient or parent practice.9One study found that in children taking low-dose trimethoprim, 97% of the parents reported giving antibiotics on daily basis, but in 31% of subjects, trimethoprim was not detectable in the urine.6Risk of prophylaxis includes nausea, vomiting, and rash in 8% to 10% of patients; development of resistant organisms; and change in indigenous microflora.6 One study of resistance found that children who received antibiotics for more than 4 weeks in the previous 6 months were more likely to have resistant Escherichia coli isolates than children who had not received prolonged antibiotic treatment (odds ratio [OR]=13.9; 95% CI, 8.2–23.5). Children with abnormalities of the genitourinary tract were approximately 4 times more likely to have resistant isolates of E coli than children without abnormalities of the genitourinary tract (OR=3.9; 95% CI, 2.7–5.7).11
Recommendations from others
The American Academy of Pediatrics, American Urological Association, and the Swedish Medical Research Council guidelines recommend prophylaxis for children with reflux ( Table ), but they all acknowledge that the recommendations are not supported by well-designed randomized controlled trials.1-3 No guidelines are available for children with neurogenic bladder and recurrent urinary tract infections.7
TABLE
Oral antibiotics for prophylaxis of urinary tract infections in children
| Antimicrobial | Prophylaxis dosage |
|---|---|
| Trimethoprim/sulfamethoxazole (TMP/SMX) (Bactrim, Septra) | 2 mg of TMP, 10 mg of SMX per kg as single bedtime or 5 mg of TMP, 25 mg of SMX per kg twice per week |
| Nitrofurantoin (Macrodantin) | 1–2 mg/kg as single daily dose |
| Cephalexin (Keflex) | 10 mg/kg as single daily dose |
| Amoxicillin | 10 mg/kg as single daily dose |
| Sulfisoxazole (Gantrisin Pedatric) | 10–20 mg/kg divided every 12h |
| Modified with permission from AAP 1999;3Allen et al1999.10 | |
UTI prevention most successful when the child exhibits efficiency of voiding
William R Strand MD
Division of Pediatric Urology, University of Texas Southwestern Medical Center, Dallas
The relative benefit of antibiotic prophylaxis in prevention of UTI in children with anatomic abnormalities like vesicoureteral reflux could best be determined if all other risk factors for UTI were controlled. Unfortunately, these other factors are often more significant in promoting UTI than is reflux, and they are also more difficult to quantify. Voiding dysfunction and constipation can both increase bladder storage pressures and postvoid residual urine volumes, and as such greatly predispose children for UTI. Furthermore, a distended colon provides an abundant reservoir of pathogens with an array of uropathogenic virulence factors.
Published reports have failed to detect significant benefit for antibiotic prophylaxis in part because the children studied possess varying risks for UTI. Prevention of UTI is most successful when the child exhibits efficiency of voiding and elimination. Clinical practice in pediatric urology advocates use of antibiotic prophylaxis in children with vesicoureteral reflux. Reflux should be suspected in children with hydroureter, multicystic renal dysplasia, ureteral duplication, and ureterocele.
1. Jodal U, Lindberg U. Guidelines for management of children with urinary tract infection and vesico-ureteric reflux. Recommendations from a Swedish state-of-the-art conference. Swedish Medical Research Council. Acta Paediatr Suppl 1999;88:87-89.
2. Elder JS, Peters CA, Arant BS Jr, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol 1997;157:1846-1851.
3. Practice parameter: the diagnosis treatment and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Pediatrics 1999;103:843-852.
4. Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 2003;348:195-202.
5. Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children. A systematic review of randomized controlled trials. J Pediatr 2001;138:868-874.
6. Bollgren I. Antibacterial prophylaxis in children with urinary tract infection. Acta Paediatr Suppl 1999;88:48-52.
7. Le Saux N, Pham B, Moher D. Evaluating the benefits of antimicrobial prophylaxis to prevent urinary tract infections in children: a systematic review. CMAJ 2000;163:523-529.
8. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002;83:129-138.
9. Ghiro L, Cracco AT, Sartor M, Comacchio S, Zacchello G, Dall’Amico R. Veneto Urinary Tract Infection Study Group. Retrospective study of children with acute pyelonephritis. Evaluation of bacterial etiology, antimicrobial susceptibility, drug management and imaging studies. Nephron 2002;90:8-15.
10. Evidence based clinical guideline for children with first UTI. Health Policy and Clinical Effectiveness Program. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 1999. Available at: www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/uti.htm. Accessed on May 5, 2004.
11. Allen UD, MacDonald N, Fiute L, Chan F, Stephen D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ 1999;160:1436-1440.
Evidence is insufficient to recommend for or against antibiotic prophylaxis to prevent recurrent urinary tract infections (UTI) in children with anatomic abnormalities. Guidelines acknowledge this lack of evidence, but still recommend using prophylactic antibiotics in children with vesiculoureteral reflux (strength of recommendation: B, based on poor-quality or inconclusive cohort and randomized controlled studies).1-3 No controlled, prospective studies have examined the effectiveness of prophylactic antibiotics to prevent UTI recurrence or renal scarring.
Evidence summary
Recommendations about antibiotic prophylaxis are based on several premises. Reflux predisposes children to acute pyelonephritis; reflux plus infection leads to reflux nephropathy and ultimately to renal scarring. In theory, if antibiotics could be initiated at the appropriate time and be maintained until reflux resolves, we could successfully prevent infection and scarring.4
A recent systematic review evaluated the use of antibiotics to prevent UTI in children.5 This review of 5 randomized controlled trials included a total of 463 children between the ages of 2 months to 16 years. Three out of 5 trials evaluated the effectiveness of antibiotic treatment for 2 to 6 months to prevent subsequent off-treatment recurrence. The 2 smaller trials (n=71) evaluated the use of low-dose long-term antibiotics to prevent UTI.
There was a clinically, but not statistically, significant trend towards reduced risk of UTI during long-term antibiotic treatment (risk reduction [RR]=0.31; 95% confidence interval [CI]=0.10–1.00); however, no sustained benefit was seen once antibiotics were stopped (RR=0.79; 95% CI, 0.61–1.02). There were many problems with the methodological quality of these trials, including significant heterogeneity. The researchers concluded that well-designed randomized controlled trails are still needed to evaluate this commonly used intervention in the pediatric population.4 Benefits for long-term prophylaxis are even less clear in children with low-grade reflux (I–II).5 Furthermore, no randomized controlled trials assess whether prophylaxis prevents the development of new renal scars in children.6
In addition, a recent systematic review of studies done in children with normal urinary tracts, as well in children with neurogenic bladders, found that the available evidence is of low quality. Only 6 out of 31 potential studies fulfilled the inclusion criteria. These were small (mean sample size was 28), and the quality scores of all 6 trials were low, indicating that the evidence may be unreliable.7
Two of 3 studies done in children with normal urinary tracts demonstrated statistically significant higher rates of UTI recurrence in control groups compared with treatment groups receiving 6 to 10 months of either nitrofurantoin or cotrimoxazole (RR=24–31). The third study showed no difference between groups.
One of 2 trials in children with neurogenic bladder demonstrated higher recurrence rates of 2.9 per 10 patient years for patients receiving antibiotics compared with 1.5 in the untreated group. The other study showed lower recurrence rates of 17.1 for patients receiving antibiotics, compared with 33 in the untreated group.7Neither of these findings were statistically significant.
A different meta-analysis of 15 controlled clinical trials in children with neurogenic bladder due to spinal cord dysfunction. This analysis showed that antibiotic prophylaxis was associated with a reduction in asymptomatic bacteruria among children with acute spinal cord injury (P<.05), but there was no significant reduction in symptomatic infections. Prophylaxis resulted in an approximately twofold increase in antimicrobial-resistant bacteria. The researchers concluded that although a clinically important effect has not been excluded, the regular use of antimicrobial prophylaxis for most patients who have neurogenic bladder caused by spinal cord dysfunction is not supported at this time.8
Poor compliance may be an issue with long-term prophylaxis and may represent patient or parent practice.9One study found that in children taking low-dose trimethoprim, 97% of the parents reported giving antibiotics on daily basis, but in 31% of subjects, trimethoprim was not detectable in the urine.6Risk of prophylaxis includes nausea, vomiting, and rash in 8% to 10% of patients; development of resistant organisms; and change in indigenous microflora.6 One study of resistance found that children who received antibiotics for more than 4 weeks in the previous 6 months were more likely to have resistant Escherichia coli isolates than children who had not received prolonged antibiotic treatment (odds ratio [OR]=13.9; 95% CI, 8.2–23.5). Children with abnormalities of the genitourinary tract were approximately 4 times more likely to have resistant isolates of E coli than children without abnormalities of the genitourinary tract (OR=3.9; 95% CI, 2.7–5.7).11
Recommendations from others
The American Academy of Pediatrics, American Urological Association, and the Swedish Medical Research Council guidelines recommend prophylaxis for children with reflux ( Table ), but they all acknowledge that the recommendations are not supported by well-designed randomized controlled trials.1-3 No guidelines are available for children with neurogenic bladder and recurrent urinary tract infections.7
TABLE
Oral antibiotics for prophylaxis of urinary tract infections in children
| Antimicrobial | Prophylaxis dosage |
|---|---|
| Trimethoprim/sulfamethoxazole (TMP/SMX) (Bactrim, Septra) | 2 mg of TMP, 10 mg of SMX per kg as single bedtime or 5 mg of TMP, 25 mg of SMX per kg twice per week |
| Nitrofurantoin (Macrodantin) | 1–2 mg/kg as single daily dose |
| Cephalexin (Keflex) | 10 mg/kg as single daily dose |
| Amoxicillin | 10 mg/kg as single daily dose |
| Sulfisoxazole (Gantrisin Pedatric) | 10–20 mg/kg divided every 12h |
| Modified with permission from AAP 1999;3Allen et al1999.10 | |
UTI prevention most successful when the child exhibits efficiency of voiding
William R Strand MD
Division of Pediatric Urology, University of Texas Southwestern Medical Center, Dallas
The relative benefit of antibiotic prophylaxis in prevention of UTI in children with anatomic abnormalities like vesicoureteral reflux could best be determined if all other risk factors for UTI were controlled. Unfortunately, these other factors are often more significant in promoting UTI than is reflux, and they are also more difficult to quantify. Voiding dysfunction and constipation can both increase bladder storage pressures and postvoid residual urine volumes, and as such greatly predispose children for UTI. Furthermore, a distended colon provides an abundant reservoir of pathogens with an array of uropathogenic virulence factors.
Published reports have failed to detect significant benefit for antibiotic prophylaxis in part because the children studied possess varying risks for UTI. Prevention of UTI is most successful when the child exhibits efficiency of voiding and elimination. Clinical practice in pediatric urology advocates use of antibiotic prophylaxis in children with vesicoureteral reflux. Reflux should be suspected in children with hydroureter, multicystic renal dysplasia, ureteral duplication, and ureterocele.
Evidence is insufficient to recommend for or against antibiotic prophylaxis to prevent recurrent urinary tract infections (UTI) in children with anatomic abnormalities. Guidelines acknowledge this lack of evidence, but still recommend using prophylactic antibiotics in children with vesiculoureteral reflux (strength of recommendation: B, based on poor-quality or inconclusive cohort and randomized controlled studies).1-3 No controlled, prospective studies have examined the effectiveness of prophylactic antibiotics to prevent UTI recurrence or renal scarring.
Evidence summary
Recommendations about antibiotic prophylaxis are based on several premises. Reflux predisposes children to acute pyelonephritis; reflux plus infection leads to reflux nephropathy and ultimately to renal scarring. In theory, if antibiotics could be initiated at the appropriate time and be maintained until reflux resolves, we could successfully prevent infection and scarring.4
A recent systematic review evaluated the use of antibiotics to prevent UTI in children.5 This review of 5 randomized controlled trials included a total of 463 children between the ages of 2 months to 16 years. Three out of 5 trials evaluated the effectiveness of antibiotic treatment for 2 to 6 months to prevent subsequent off-treatment recurrence. The 2 smaller trials (n=71) evaluated the use of low-dose long-term antibiotics to prevent UTI.
There was a clinically, but not statistically, significant trend towards reduced risk of UTI during long-term antibiotic treatment (risk reduction [RR]=0.31; 95% confidence interval [CI]=0.10–1.00); however, no sustained benefit was seen once antibiotics were stopped (RR=0.79; 95% CI, 0.61–1.02). There were many problems with the methodological quality of these trials, including significant heterogeneity. The researchers concluded that well-designed randomized controlled trails are still needed to evaluate this commonly used intervention in the pediatric population.4 Benefits for long-term prophylaxis are even less clear in children with low-grade reflux (I–II).5 Furthermore, no randomized controlled trials assess whether prophylaxis prevents the development of new renal scars in children.6
In addition, a recent systematic review of studies done in children with normal urinary tracts, as well in children with neurogenic bladders, found that the available evidence is of low quality. Only 6 out of 31 potential studies fulfilled the inclusion criteria. These were small (mean sample size was 28), and the quality scores of all 6 trials were low, indicating that the evidence may be unreliable.7
Two of 3 studies done in children with normal urinary tracts demonstrated statistically significant higher rates of UTI recurrence in control groups compared with treatment groups receiving 6 to 10 months of either nitrofurantoin or cotrimoxazole (RR=24–31). The third study showed no difference between groups.
One of 2 trials in children with neurogenic bladder demonstrated higher recurrence rates of 2.9 per 10 patient years for patients receiving antibiotics compared with 1.5 in the untreated group. The other study showed lower recurrence rates of 17.1 for patients receiving antibiotics, compared with 33 in the untreated group.7Neither of these findings were statistically significant.
A different meta-analysis of 15 controlled clinical trials in children with neurogenic bladder due to spinal cord dysfunction. This analysis showed that antibiotic prophylaxis was associated with a reduction in asymptomatic bacteruria among children with acute spinal cord injury (P<.05), but there was no significant reduction in symptomatic infections. Prophylaxis resulted in an approximately twofold increase in antimicrobial-resistant bacteria. The researchers concluded that although a clinically important effect has not been excluded, the regular use of antimicrobial prophylaxis for most patients who have neurogenic bladder caused by spinal cord dysfunction is not supported at this time.8
Poor compliance may be an issue with long-term prophylaxis and may represent patient or parent practice.9One study found that in children taking low-dose trimethoprim, 97% of the parents reported giving antibiotics on daily basis, but in 31% of subjects, trimethoprim was not detectable in the urine.6Risk of prophylaxis includes nausea, vomiting, and rash in 8% to 10% of patients; development of resistant organisms; and change in indigenous microflora.6 One study of resistance found that children who received antibiotics for more than 4 weeks in the previous 6 months were more likely to have resistant Escherichia coli isolates than children who had not received prolonged antibiotic treatment (odds ratio [OR]=13.9; 95% CI, 8.2–23.5). Children with abnormalities of the genitourinary tract were approximately 4 times more likely to have resistant isolates of E coli than children without abnormalities of the genitourinary tract (OR=3.9; 95% CI, 2.7–5.7).11
Recommendations from others
The American Academy of Pediatrics, American Urological Association, and the Swedish Medical Research Council guidelines recommend prophylaxis for children with reflux ( Table ), but they all acknowledge that the recommendations are not supported by well-designed randomized controlled trials.1-3 No guidelines are available for children with neurogenic bladder and recurrent urinary tract infections.7
TABLE
Oral antibiotics for prophylaxis of urinary tract infections in children
| Antimicrobial | Prophylaxis dosage |
|---|---|
| Trimethoprim/sulfamethoxazole (TMP/SMX) (Bactrim, Septra) | 2 mg of TMP, 10 mg of SMX per kg as single bedtime or 5 mg of TMP, 25 mg of SMX per kg twice per week |
| Nitrofurantoin (Macrodantin) | 1–2 mg/kg as single daily dose |
| Cephalexin (Keflex) | 10 mg/kg as single daily dose |
| Amoxicillin | 10 mg/kg as single daily dose |
| Sulfisoxazole (Gantrisin Pedatric) | 10–20 mg/kg divided every 12h |
| Modified with permission from AAP 1999;3Allen et al1999.10 | |
UTI prevention most successful when the child exhibits efficiency of voiding
William R Strand MD
Division of Pediatric Urology, University of Texas Southwestern Medical Center, Dallas
The relative benefit of antibiotic prophylaxis in prevention of UTI in children with anatomic abnormalities like vesicoureteral reflux could best be determined if all other risk factors for UTI were controlled. Unfortunately, these other factors are often more significant in promoting UTI than is reflux, and they are also more difficult to quantify. Voiding dysfunction and constipation can both increase bladder storage pressures and postvoid residual urine volumes, and as such greatly predispose children for UTI. Furthermore, a distended colon provides an abundant reservoir of pathogens with an array of uropathogenic virulence factors.
Published reports have failed to detect significant benefit for antibiotic prophylaxis in part because the children studied possess varying risks for UTI. Prevention of UTI is most successful when the child exhibits efficiency of voiding and elimination. Clinical practice in pediatric urology advocates use of antibiotic prophylaxis in children with vesicoureteral reflux. Reflux should be suspected in children with hydroureter, multicystic renal dysplasia, ureteral duplication, and ureterocele.
1. Jodal U, Lindberg U. Guidelines for management of children with urinary tract infection and vesico-ureteric reflux. Recommendations from a Swedish state-of-the-art conference. Swedish Medical Research Council. Acta Paediatr Suppl 1999;88:87-89.
2. Elder JS, Peters CA, Arant BS Jr, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol 1997;157:1846-1851.
3. Practice parameter: the diagnosis treatment and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Pediatrics 1999;103:843-852.
4. Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 2003;348:195-202.
5. Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children. A systematic review of randomized controlled trials. J Pediatr 2001;138:868-874.
6. Bollgren I. Antibacterial prophylaxis in children with urinary tract infection. Acta Paediatr Suppl 1999;88:48-52.
7. Le Saux N, Pham B, Moher D. Evaluating the benefits of antimicrobial prophylaxis to prevent urinary tract infections in children: a systematic review. CMAJ 2000;163:523-529.
8. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002;83:129-138.
9. Ghiro L, Cracco AT, Sartor M, Comacchio S, Zacchello G, Dall’Amico R. Veneto Urinary Tract Infection Study Group. Retrospective study of children with acute pyelonephritis. Evaluation of bacterial etiology, antimicrobial susceptibility, drug management and imaging studies. Nephron 2002;90:8-15.
10. Evidence based clinical guideline for children with first UTI. Health Policy and Clinical Effectiveness Program. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 1999. Available at: www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/uti.htm. Accessed on May 5, 2004.
11. Allen UD, MacDonald N, Fiute L, Chan F, Stephen D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ 1999;160:1436-1440.
1. Jodal U, Lindberg U. Guidelines for management of children with urinary tract infection and vesico-ureteric reflux. Recommendations from a Swedish state-of-the-art conference. Swedish Medical Research Council. Acta Paediatr Suppl 1999;88:87-89.
2. Elder JS, Peters CA, Arant BS Jr, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol 1997;157:1846-1851.
3. Practice parameter: the diagnosis treatment and evaluation of the initial urinary tract infection in febrile infants and young children. American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection. Pediatrics 1999;103:843-852.
4. Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 2003;348:195-202.
5. Williams G, Lee A, Craig J. Antibiotics for the prevention of urinary tract infection in children. A systematic review of randomized controlled trials. J Pediatr 2001;138:868-874.
6. Bollgren I. Antibacterial prophylaxis in children with urinary tract infection. Acta Paediatr Suppl 1999;88:48-52.
7. Le Saux N, Pham B, Moher D. Evaluating the benefits of antimicrobial prophylaxis to prevent urinary tract infections in children: a systematic review. CMAJ 2000;163:523-529.
8. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002;83:129-138.
9. Ghiro L, Cracco AT, Sartor M, Comacchio S, Zacchello G, Dall’Amico R. Veneto Urinary Tract Infection Study Group. Retrospective study of children with acute pyelonephritis. Evaluation of bacterial etiology, antimicrobial susceptibility, drug management and imaging studies. Nephron 2002;90:8-15.
10. Evidence based clinical guideline for children with first UTI. Health Policy and Clinical Effectiveness Program. Cincinnati, Ohio: Cincinnati Children’s Hospital Medical Center; 1999. Available at: www.cincinnatichildrens.org/svc/dept-div/health-policy/ev-based/uti.htm. Accessed on May 5, 2004.
11. Allen UD, MacDonald N, Fiute L, Chan F, Stephen D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ 1999;160:1436-1440.
Evidence-based answers from the Family Physicians Inquiries Network
Should the varicella vaccine be given to all children to prevent chickenpox?
EVIDENCE-BASED ANSWER
Healthy, unimmunized children who have not had varicella infection should be vaccinated (strength of recommendation: A, based on randomized controlled trials). Use of the vaccine in immunocompromised children is still being studied and has not been approved by the Food and Drug Administration (FDA).
Evidence summary
Before the introduction of the varicella vaccine, almost 4 million cases of chickenpox occurred each year in the United States, resulting in 11,000 hospitalizations and 100 deaths.1 Varicella is the leading cause of vaccine-preventable death in children.2
In a search of the literature from 1966 to 2000, a systematic review identified 24 randomized controlled trials and 18 cohort studies of varicella vaccination.3 In children aged 10 months to 14 years, 1 randomized controlled trial found protective efficacy of 100% over 9 months and 98% over 7 years.4 A second trial showed efficacy of 72% over 29 months.5Cohort studies of children report that the vaccine is 84% to 86% effective in preventing varicella and 100% effective in preventing moderate to severe infections.3
Cumulative results of all studies show the number needed to vaccinate to prevent 1 case of varicella ranges from 5.5 to 11.8, and the number needed to prevent 1 complicated case ranges from 550 to 1180.
No direct evidence supports or refutes a reduction in varicella mortality or rates of hospitalization due to vaccination. Randomized controlled trials show no increase in rates of fever or rash among those receiving vaccine; however, cohort studies report fever (0%–36%), local injection site reactions (7%–30%), and rash (5%).3 No clinical trials have shown transmission of vaccine-related varicella zoster virus in immunocompetent patients, and only 3 proven cases of transmission of vaccine virus to susceptible contacts have been documented.6 Some evidence suggests the incidence of herpes zoster is reduced in immunocompromised vaccine recipients, but long-term observation is needed to assess the effect on healthy recipients.7
One concern about the vaccine is that waning immunity over time could result in increased incidence of varicella infection during adulthood. While existing studies document persistence of antibodies for up to 20 years following immunization,3 long-term effectiveness should continue to be monitored.
The FDA has not approved this live-virus vaccine for use in pregnant women and immunocom-promised persons, including transplant recipients and persons receiving corticosteroid therapy. However, the vaccine has been very well-studied in children with leukemia. A review of these studies found that optimal seroconversion requires 2 sequential vaccine doses (86% efficacy). A rash of varying severity was the predominant adverse event in 20% to 50% of vacinees.7 Study of vaccine use in other immunocompromised children has been limited. Early results from a trial in HIV-infected children who were not severely immunocompromised suggests similar tolerance and efficacy compared with children without HIV.8 A systemic review of cost-effectiveness of varicella vaccine is based predominantly on mathematical models.9These models show societal savings due to decrease in unproductive days for parents, but fail to demonstrate actual healthcare savings.
Recommendations from others
The American Academy of Pediatrics (AAP), Advisory Committee on Immunization Practices (ACIP), and American Academy of Family Medicine all recommend vaccinating unimmunized children aged 12 months and older who have not had varicella infection, and not vaccinating children with cellular immunodeficiencies.2,10,11 The AAP suggests the vaccine could be considered for children with acute lymphocytic leukemia and for HIV-infected children with mild or no signs or symptoms. The ACIP guidelines are similar, with the addition that children with impaired humoral immunity may now be vaccinated.
Encourage varicella vaccination, except for the immunocompromised
Kristen Rundell, MD
University of Colorado Health Sciences Center, Denver
For many parents, vaccination decisions are made based on school district requirements. Varicella zoster vaccine is an exception to that rule. Parents can choose to immunize their child at 12 months or wait and let nature take its course—hopefully before the child starts kindergarten. The major concern with the vaccine has been its long-term efficacy. Although no one knows for sure how long immunity is sustained, studies show that detectable antibodies are present for up to 20 years.
As a parent and physician, my decision to vaccinate my daughter was made after I witnessed an 8-year-old boy in the emergency room with respiratory distress secondary to complications from chickenpox. This experience reinforced for me that chickenpox is a life-threatening disease. The effects of chickenpox include scarring as well as time away from work for parents. I therefore encourage varicella vaccination for my patients, with the only exception being those who are immunocompromised, for whom we have no data.
To the question of whether we should we vaccinate children to prevent chickenpox, I give a resounding “yes.”
1. Arvin AM. Varicella vaccine—the first six years. N Engl J Med 2001;344:1007-1009.
2. Centers for Disease Control and Prevention. Prevention of varicella. Update recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999;48(RR-6):1-5.
3. Skull SA, Wang EE. Varicella vaccination—a critical review of the evidence. Arch Dis Child 2001;85:83-90.
4. Weibel RE, Neff BJ, Kuter BJ, et al. Live attenuated varicella vaccine. Efficacy trial in healthy children. N Eng J Med 1984;310:1409-1415.
5. Varis T, Vesikari T. Efficacy of high-titer live attenuated varicella vaccine in healthy young children. J Infect Dis 1996;174(suppl 3):S330-S334.
6. Wise RP, Salive ME, Braun MM, et al. Postlicensure safety surveillance for varicella vaccine. JAMA 2000;284:1271-1279.
7. Gershon AA, LaRussa P, Steinberg S. The varicella vaccine. Clinical trials in immunocompromised individuals. Infect Dis Clin North Am 1996;10:583-594.
8. Levin MJ, Gershon AA, Weinberg A, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr 2001;139:305-310.
9. Rothberg M, Bennish ML, Kao JS, Wong JB. Do the benefits of varicella vaccination outweigh the risks? A decision-analytical model for policymakers and pediatricians. Clin Infect Dis 2002;34:885-894.
10. American. Academy of Family Practice. Periodic Health Examinations. Revision 5.3. Leawood, Kansas: AAFP; 2002.
11. American Academy of Pediatrics. Committee on Infectious Diseases. Varicella vaccine update. Pediatrics 2000;105:136-141.
EVIDENCE-BASED ANSWER
Healthy, unimmunized children who have not had varicella infection should be vaccinated (strength of recommendation: A, based on randomized controlled trials). Use of the vaccine in immunocompromised children is still being studied and has not been approved by the Food and Drug Administration (FDA).
Evidence summary
Before the introduction of the varicella vaccine, almost 4 million cases of chickenpox occurred each year in the United States, resulting in 11,000 hospitalizations and 100 deaths.1 Varicella is the leading cause of vaccine-preventable death in children.2
In a search of the literature from 1966 to 2000, a systematic review identified 24 randomized controlled trials and 18 cohort studies of varicella vaccination.3 In children aged 10 months to 14 years, 1 randomized controlled trial found protective efficacy of 100% over 9 months and 98% over 7 years.4 A second trial showed efficacy of 72% over 29 months.5Cohort studies of children report that the vaccine is 84% to 86% effective in preventing varicella and 100% effective in preventing moderate to severe infections.3
Cumulative results of all studies show the number needed to vaccinate to prevent 1 case of varicella ranges from 5.5 to 11.8, and the number needed to prevent 1 complicated case ranges from 550 to 1180.
No direct evidence supports or refutes a reduction in varicella mortality or rates of hospitalization due to vaccination. Randomized controlled trials show no increase in rates of fever or rash among those receiving vaccine; however, cohort studies report fever (0%–36%), local injection site reactions (7%–30%), and rash (5%).3 No clinical trials have shown transmission of vaccine-related varicella zoster virus in immunocompetent patients, and only 3 proven cases of transmission of vaccine virus to susceptible contacts have been documented.6 Some evidence suggests the incidence of herpes zoster is reduced in immunocompromised vaccine recipients, but long-term observation is needed to assess the effect on healthy recipients.7
One concern about the vaccine is that waning immunity over time could result in increased incidence of varicella infection during adulthood. While existing studies document persistence of antibodies for up to 20 years following immunization,3 long-term effectiveness should continue to be monitored.
The FDA has not approved this live-virus vaccine for use in pregnant women and immunocom-promised persons, including transplant recipients and persons receiving corticosteroid therapy. However, the vaccine has been very well-studied in children with leukemia. A review of these studies found that optimal seroconversion requires 2 sequential vaccine doses (86% efficacy). A rash of varying severity was the predominant adverse event in 20% to 50% of vacinees.7 Study of vaccine use in other immunocompromised children has been limited. Early results from a trial in HIV-infected children who were not severely immunocompromised suggests similar tolerance and efficacy compared with children without HIV.8 A systemic review of cost-effectiveness of varicella vaccine is based predominantly on mathematical models.9These models show societal savings due to decrease in unproductive days for parents, but fail to demonstrate actual healthcare savings.
Recommendations from others
The American Academy of Pediatrics (AAP), Advisory Committee on Immunization Practices (ACIP), and American Academy of Family Medicine all recommend vaccinating unimmunized children aged 12 months and older who have not had varicella infection, and not vaccinating children with cellular immunodeficiencies.2,10,11 The AAP suggests the vaccine could be considered for children with acute lymphocytic leukemia and for HIV-infected children with mild or no signs or symptoms. The ACIP guidelines are similar, with the addition that children with impaired humoral immunity may now be vaccinated.
Encourage varicella vaccination, except for the immunocompromised
Kristen Rundell, MD
University of Colorado Health Sciences Center, Denver
For many parents, vaccination decisions are made based on school district requirements. Varicella zoster vaccine is an exception to that rule. Parents can choose to immunize their child at 12 months or wait and let nature take its course—hopefully before the child starts kindergarten. The major concern with the vaccine has been its long-term efficacy. Although no one knows for sure how long immunity is sustained, studies show that detectable antibodies are present for up to 20 years.
As a parent and physician, my decision to vaccinate my daughter was made after I witnessed an 8-year-old boy in the emergency room with respiratory distress secondary to complications from chickenpox. This experience reinforced for me that chickenpox is a life-threatening disease. The effects of chickenpox include scarring as well as time away from work for parents. I therefore encourage varicella vaccination for my patients, with the only exception being those who are immunocompromised, for whom we have no data.
To the question of whether we should we vaccinate children to prevent chickenpox, I give a resounding “yes.”
EVIDENCE-BASED ANSWER
Healthy, unimmunized children who have not had varicella infection should be vaccinated (strength of recommendation: A, based on randomized controlled trials). Use of the vaccine in immunocompromised children is still being studied and has not been approved by the Food and Drug Administration (FDA).
Evidence summary
Before the introduction of the varicella vaccine, almost 4 million cases of chickenpox occurred each year in the United States, resulting in 11,000 hospitalizations and 100 deaths.1 Varicella is the leading cause of vaccine-preventable death in children.2
In a search of the literature from 1966 to 2000, a systematic review identified 24 randomized controlled trials and 18 cohort studies of varicella vaccination.3 In children aged 10 months to 14 years, 1 randomized controlled trial found protective efficacy of 100% over 9 months and 98% over 7 years.4 A second trial showed efficacy of 72% over 29 months.5Cohort studies of children report that the vaccine is 84% to 86% effective in preventing varicella and 100% effective in preventing moderate to severe infections.3
Cumulative results of all studies show the number needed to vaccinate to prevent 1 case of varicella ranges from 5.5 to 11.8, and the number needed to prevent 1 complicated case ranges from 550 to 1180.
No direct evidence supports or refutes a reduction in varicella mortality or rates of hospitalization due to vaccination. Randomized controlled trials show no increase in rates of fever or rash among those receiving vaccine; however, cohort studies report fever (0%–36%), local injection site reactions (7%–30%), and rash (5%).3 No clinical trials have shown transmission of vaccine-related varicella zoster virus in immunocompetent patients, and only 3 proven cases of transmission of vaccine virus to susceptible contacts have been documented.6 Some evidence suggests the incidence of herpes zoster is reduced in immunocompromised vaccine recipients, but long-term observation is needed to assess the effect on healthy recipients.7
One concern about the vaccine is that waning immunity over time could result in increased incidence of varicella infection during adulthood. While existing studies document persistence of antibodies for up to 20 years following immunization,3 long-term effectiveness should continue to be monitored.
The FDA has not approved this live-virus vaccine for use in pregnant women and immunocom-promised persons, including transplant recipients and persons receiving corticosteroid therapy. However, the vaccine has been very well-studied in children with leukemia. A review of these studies found that optimal seroconversion requires 2 sequential vaccine doses (86% efficacy). A rash of varying severity was the predominant adverse event in 20% to 50% of vacinees.7 Study of vaccine use in other immunocompromised children has been limited. Early results from a trial in HIV-infected children who were not severely immunocompromised suggests similar tolerance and efficacy compared with children without HIV.8 A systemic review of cost-effectiveness of varicella vaccine is based predominantly on mathematical models.9These models show societal savings due to decrease in unproductive days for parents, but fail to demonstrate actual healthcare savings.
Recommendations from others
The American Academy of Pediatrics (AAP), Advisory Committee on Immunization Practices (ACIP), and American Academy of Family Medicine all recommend vaccinating unimmunized children aged 12 months and older who have not had varicella infection, and not vaccinating children with cellular immunodeficiencies.2,10,11 The AAP suggests the vaccine could be considered for children with acute lymphocytic leukemia and for HIV-infected children with mild or no signs or symptoms. The ACIP guidelines are similar, with the addition that children with impaired humoral immunity may now be vaccinated.
Encourage varicella vaccination, except for the immunocompromised
Kristen Rundell, MD
University of Colorado Health Sciences Center, Denver
For many parents, vaccination decisions are made based on school district requirements. Varicella zoster vaccine is an exception to that rule. Parents can choose to immunize their child at 12 months or wait and let nature take its course—hopefully before the child starts kindergarten. The major concern with the vaccine has been its long-term efficacy. Although no one knows for sure how long immunity is sustained, studies show that detectable antibodies are present for up to 20 years.
As a parent and physician, my decision to vaccinate my daughter was made after I witnessed an 8-year-old boy in the emergency room with respiratory distress secondary to complications from chickenpox. This experience reinforced for me that chickenpox is a life-threatening disease. The effects of chickenpox include scarring as well as time away from work for parents. I therefore encourage varicella vaccination for my patients, with the only exception being those who are immunocompromised, for whom we have no data.
To the question of whether we should we vaccinate children to prevent chickenpox, I give a resounding “yes.”
1. Arvin AM. Varicella vaccine—the first six years. N Engl J Med 2001;344:1007-1009.
2. Centers for Disease Control and Prevention. Prevention of varicella. Update recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999;48(RR-6):1-5.
3. Skull SA, Wang EE. Varicella vaccination—a critical review of the evidence. Arch Dis Child 2001;85:83-90.
4. Weibel RE, Neff BJ, Kuter BJ, et al. Live attenuated varicella vaccine. Efficacy trial in healthy children. N Eng J Med 1984;310:1409-1415.
5. Varis T, Vesikari T. Efficacy of high-titer live attenuated varicella vaccine in healthy young children. J Infect Dis 1996;174(suppl 3):S330-S334.
6. Wise RP, Salive ME, Braun MM, et al. Postlicensure safety surveillance for varicella vaccine. JAMA 2000;284:1271-1279.
7. Gershon AA, LaRussa P, Steinberg S. The varicella vaccine. Clinical trials in immunocompromised individuals. Infect Dis Clin North Am 1996;10:583-594.
8. Levin MJ, Gershon AA, Weinberg A, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr 2001;139:305-310.
9. Rothberg M, Bennish ML, Kao JS, Wong JB. Do the benefits of varicella vaccination outweigh the risks? A decision-analytical model for policymakers and pediatricians. Clin Infect Dis 2002;34:885-894.
10. American. Academy of Family Practice. Periodic Health Examinations. Revision 5.3. Leawood, Kansas: AAFP; 2002.
11. American Academy of Pediatrics. Committee on Infectious Diseases. Varicella vaccine update. Pediatrics 2000;105:136-141.
1. Arvin AM. Varicella vaccine—the first six years. N Engl J Med 2001;344:1007-1009.
2. Centers for Disease Control and Prevention. Prevention of varicella. Update recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999;48(RR-6):1-5.
3. Skull SA, Wang EE. Varicella vaccination—a critical review of the evidence. Arch Dis Child 2001;85:83-90.
4. Weibel RE, Neff BJ, Kuter BJ, et al. Live attenuated varicella vaccine. Efficacy trial in healthy children. N Eng J Med 1984;310:1409-1415.
5. Varis T, Vesikari T. Efficacy of high-titer live attenuated varicella vaccine in healthy young children. J Infect Dis 1996;174(suppl 3):S330-S334.
6. Wise RP, Salive ME, Braun MM, et al. Postlicensure safety surveillance for varicella vaccine. JAMA 2000;284:1271-1279.
7. Gershon AA, LaRussa P, Steinberg S. The varicella vaccine. Clinical trials in immunocompromised individuals. Infect Dis Clin North Am 1996;10:583-594.
8. Levin MJ, Gershon AA, Weinberg A, et al. Immunization of HIV-infected children with varicella vaccine. J Pediatr 2001;139:305-310.
9. Rothberg M, Bennish ML, Kao JS, Wong JB. Do the benefits of varicella vaccination outweigh the risks? A decision-analytical model for policymakers and pediatricians. Clin Infect Dis 2002;34:885-894.
10. American. Academy of Family Practice. Periodic Health Examinations. Revision 5.3. Leawood, Kansas: AAFP; 2002.
11. American Academy of Pediatrics. Committee on Infectious Diseases. Varicella vaccine update. Pediatrics 2000;105:136-141.
Evidence-based answers from the Family Physicians Inquiries Network
Are beta-2-agonists or anticholinergics more effective for treating COPD?
Both β2-agonists and anticholinergics appear to improve symptoms for patients with chronic obstructive pulmonary disease (COPD). Recent research indicates that adding a long-acting anti-cholinergic to a β2-agonist may improve quality of life for patients with stable COPD more than the use of β2-agonists alone.
Both drug classes increase exercise capacity and alleviate symptoms of COPD, although neither alters disease progression (strength of recommendation [SOR]: A). Combination therapy can lead to greater improvements in forced expiratory volume in 1 second (FEV1) than either drug alone (SOR: A). However, until recently there were no convincing direct head-to-head comparisons of the 2 classes, and it is unclear whether this difference is clinically significant.
Evidence summary
A review of 33 double-blind randomized placebo-controlled studies showed a significant effect of bronchodilator therapy on exercise capacity in COPD patients in about one half of studies. Anticholinergic agents had significant beneficial effects in the majority, and these effects tended to be somewhat dose-dependent. Short-acting β2-agonists improved exercise capacity in more than two thirds of the studies, but long-acting agents led to mixed outcomes. The researchers identified no superior agent between the 2 classes, citing a lack of adequate studies making a direct comparison.1
A recent Cochrane Review comparing the short-term effects of ipratropium to β2-agonists in changes in FEV1and arterial oxygen pressure (PaO2) concluded there was no evidence that the degree of bronchodilation from ipratropium was greater than that from short-acting β2-agonists.2Subjective endpoints such as dyspnea and quality of life were not assessed, and neither of the above reviews included studies focusing on long-term outcomes.
A 12-week double-blind, double-placebo-controlled parallel group study published in 2000 followed 144 patients (age 64 ± 7 years with a FEV1of 44 ±11% predicted) randomized to receive salmeterol 50 μg twice daily alone, salmeterol 50 μg twice daily plus ipratropium 40 μg 4 times daily, or placebo. Patients were assessed for changes in FEV1, daytime symptom scores, specific airway conductance, and the need for rescue medication. The study demonstrated a significant benefit from the addition of ipratropium to salmeterol in terms of reduction of airway obstruction, but not in symptom control or need for rescue medication.3 However, no patients were randomized to receive ipratropium alone, so comparison of the relative contribution of the 2 classes is limited.
A 6-month, randomized double-blind placebo-controlled study evaluating the efficacy of salmeterol 50 μg twice daily vs tiotropium (a new long-acting inhaled anticholinergic) 18 μg once daily was published in 2002. Endpoints in 623 patients were assessed using 12-hour spirometric performance, transition dyspnea index (TDI), and the St. George Respiratory Questionnaire (SGRQ). (SGRQ is a validated disease-specific instrument designed to measure impact on overall health, daily life, and perceived well-being. It measures activity limitations, symptoms, and psychosocial impact.) Tiotropium showed superiority over salmeterol in all endpoints assessed (0.14 L increase in morning FEV1vs 0.09 L, 1.02 U improvement in TDI score vs 0.24, and –5.14 U improvement of SGRQ total score from baseline vs –3.54). However, it should be noted that a difference of 1 on the TDI score was necessary to suggest a clinical benefit. While the overall difference in SGQR between tiotropium and salmeterol did not reach statistical significance, the proportion of patients in the tiotropium group that reached the clinically significant threshold of 4 units improvement in SGRQ score was significantly higher than in the salmeterol group (51% vs 40%; P<.05).4
In a similar study in 2003, 1207 patients were randomized to receive the above doses of salmeterol, tiotropium, or placebo. Over the course of 6 months, tiotropium was associated with a significant delay in onset of the first exacerbation compared with placebo, and overall it led to the fewest exacerbations per patient-year. Fewer hospital admissions were also demonstrated in the tiotropium group per patient-year, and the number of days that patients were unable to perform usual activities was lowest for the tiotropium group. Again, improvement in TDI and SGRQ scores was significantly greater with tiotropium than placebo. In almost all outcomes, the salmeterol results were intermediate between those of tiotropium and placebo, and were not statistically different from placebo.5
Recommendations from others
The GOLD (Global Strategy for the Diagnosis, Management, and Prevention of COPD) Report states that the choice between β2-agonist, anti-cholinergics, or combination therapy depends on the availability and the response of a given patient in terms of symptom relief and side effects. The 2003 GOLD Workshop Report update further recommends the use of regular treatment with long-acting bronchodilators, including tiotropium, rather than short-acting bronchodilators for moderate-to-severe COPD.6
A separate report for the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians—American Society of Internal Medicine states that both are beneficial for management of acute exacerbations, but that anticholinergics should be considered first because they are associated with fewer and more benign side effects.7
Patient response and tolerance of side effects determine which drug class to use
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Although recent national guidelines for the management of COPD, such as the GOLD report, give more cohesiveness to treatment strategies for patients with COPD, there is still room for tailoring a treatment approach. I find that when choosing between beta-agonists and anticholinergics, patient response and tolerability of side effects determine what I use.
This Clinical Inquiry supports my clinical impression that neither class of drug is significantly superior to the other in regards to COPD outcome measures. In my experience, when neither drug offers a clear advantage, factors affecting compliance and tolerability tend to determine how effective it is for my patients. Therefore, a trial of either class seems reasonable at first and follow-up determines what is used in the long run.
1. Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koeter GH, Postma DS, Kerstjens HA. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002;121:597-608.
2. McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. In: The Cochrane Library, Issue 1,2004. Chichester, UK: John Wiley, Ltd; 2003.
3. van Noord JA, de Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000;15:878-885.
4. Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47-55.
5. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399-404.
6. Fabbri LM, Hurd SS. GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2.
7. Snow V, Lascher S, Mottur-Pilson C. Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
Both β2-agonists and anticholinergics appear to improve symptoms for patients with chronic obstructive pulmonary disease (COPD). Recent research indicates that adding a long-acting anti-cholinergic to a β2-agonist may improve quality of life for patients with stable COPD more than the use of β2-agonists alone.
Both drug classes increase exercise capacity and alleviate symptoms of COPD, although neither alters disease progression (strength of recommendation [SOR]: A). Combination therapy can lead to greater improvements in forced expiratory volume in 1 second (FEV1) than either drug alone (SOR: A). However, until recently there were no convincing direct head-to-head comparisons of the 2 classes, and it is unclear whether this difference is clinically significant.
Evidence summary
A review of 33 double-blind randomized placebo-controlled studies showed a significant effect of bronchodilator therapy on exercise capacity in COPD patients in about one half of studies. Anticholinergic agents had significant beneficial effects in the majority, and these effects tended to be somewhat dose-dependent. Short-acting β2-agonists improved exercise capacity in more than two thirds of the studies, but long-acting agents led to mixed outcomes. The researchers identified no superior agent between the 2 classes, citing a lack of adequate studies making a direct comparison.1
A recent Cochrane Review comparing the short-term effects of ipratropium to β2-agonists in changes in FEV1and arterial oxygen pressure (PaO2) concluded there was no evidence that the degree of bronchodilation from ipratropium was greater than that from short-acting β2-agonists.2Subjective endpoints such as dyspnea and quality of life were not assessed, and neither of the above reviews included studies focusing on long-term outcomes.
A 12-week double-blind, double-placebo-controlled parallel group study published in 2000 followed 144 patients (age 64 ± 7 years with a FEV1of 44 ±11% predicted) randomized to receive salmeterol 50 μg twice daily alone, salmeterol 50 μg twice daily plus ipratropium 40 μg 4 times daily, or placebo. Patients were assessed for changes in FEV1, daytime symptom scores, specific airway conductance, and the need for rescue medication. The study demonstrated a significant benefit from the addition of ipratropium to salmeterol in terms of reduction of airway obstruction, but not in symptom control or need for rescue medication.3 However, no patients were randomized to receive ipratropium alone, so comparison of the relative contribution of the 2 classes is limited.
A 6-month, randomized double-blind placebo-controlled study evaluating the efficacy of salmeterol 50 μg twice daily vs tiotropium (a new long-acting inhaled anticholinergic) 18 μg once daily was published in 2002. Endpoints in 623 patients were assessed using 12-hour spirometric performance, transition dyspnea index (TDI), and the St. George Respiratory Questionnaire (SGRQ). (SGRQ is a validated disease-specific instrument designed to measure impact on overall health, daily life, and perceived well-being. It measures activity limitations, symptoms, and psychosocial impact.) Tiotropium showed superiority over salmeterol in all endpoints assessed (0.14 L increase in morning FEV1vs 0.09 L, 1.02 U improvement in TDI score vs 0.24, and –5.14 U improvement of SGRQ total score from baseline vs –3.54). However, it should be noted that a difference of 1 on the TDI score was necessary to suggest a clinical benefit. While the overall difference in SGQR between tiotropium and salmeterol did not reach statistical significance, the proportion of patients in the tiotropium group that reached the clinically significant threshold of 4 units improvement in SGRQ score was significantly higher than in the salmeterol group (51% vs 40%; P<.05).4
In a similar study in 2003, 1207 patients were randomized to receive the above doses of salmeterol, tiotropium, or placebo. Over the course of 6 months, tiotropium was associated with a significant delay in onset of the first exacerbation compared with placebo, and overall it led to the fewest exacerbations per patient-year. Fewer hospital admissions were also demonstrated in the tiotropium group per patient-year, and the number of days that patients were unable to perform usual activities was lowest for the tiotropium group. Again, improvement in TDI and SGRQ scores was significantly greater with tiotropium than placebo. In almost all outcomes, the salmeterol results were intermediate between those of tiotropium and placebo, and were not statistically different from placebo.5
Recommendations from others
The GOLD (Global Strategy for the Diagnosis, Management, and Prevention of COPD) Report states that the choice between β2-agonist, anti-cholinergics, or combination therapy depends on the availability and the response of a given patient in terms of symptom relief and side effects. The 2003 GOLD Workshop Report update further recommends the use of regular treatment with long-acting bronchodilators, including tiotropium, rather than short-acting bronchodilators for moderate-to-severe COPD.6
A separate report for the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians—American Society of Internal Medicine states that both are beneficial for management of acute exacerbations, but that anticholinergics should be considered first because they are associated with fewer and more benign side effects.7
Patient response and tolerance of side effects determine which drug class to use
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Although recent national guidelines for the management of COPD, such as the GOLD report, give more cohesiveness to treatment strategies for patients with COPD, there is still room for tailoring a treatment approach. I find that when choosing between beta-agonists and anticholinergics, patient response and tolerability of side effects determine what I use.
This Clinical Inquiry supports my clinical impression that neither class of drug is significantly superior to the other in regards to COPD outcome measures. In my experience, when neither drug offers a clear advantage, factors affecting compliance and tolerability tend to determine how effective it is for my patients. Therefore, a trial of either class seems reasonable at first and follow-up determines what is used in the long run.
Both β2-agonists and anticholinergics appear to improve symptoms for patients with chronic obstructive pulmonary disease (COPD). Recent research indicates that adding a long-acting anti-cholinergic to a β2-agonist may improve quality of life for patients with stable COPD more than the use of β2-agonists alone.
Both drug classes increase exercise capacity and alleviate symptoms of COPD, although neither alters disease progression (strength of recommendation [SOR]: A). Combination therapy can lead to greater improvements in forced expiratory volume in 1 second (FEV1) than either drug alone (SOR: A). However, until recently there were no convincing direct head-to-head comparisons of the 2 classes, and it is unclear whether this difference is clinically significant.
Evidence summary
A review of 33 double-blind randomized placebo-controlled studies showed a significant effect of bronchodilator therapy on exercise capacity in COPD patients in about one half of studies. Anticholinergic agents had significant beneficial effects in the majority, and these effects tended to be somewhat dose-dependent. Short-acting β2-agonists improved exercise capacity in more than two thirds of the studies, but long-acting agents led to mixed outcomes. The researchers identified no superior agent between the 2 classes, citing a lack of adequate studies making a direct comparison.1
A recent Cochrane Review comparing the short-term effects of ipratropium to β2-agonists in changes in FEV1and arterial oxygen pressure (PaO2) concluded there was no evidence that the degree of bronchodilation from ipratropium was greater than that from short-acting β2-agonists.2Subjective endpoints such as dyspnea and quality of life were not assessed, and neither of the above reviews included studies focusing on long-term outcomes.
A 12-week double-blind, double-placebo-controlled parallel group study published in 2000 followed 144 patients (age 64 ± 7 years with a FEV1of 44 ±11% predicted) randomized to receive salmeterol 50 μg twice daily alone, salmeterol 50 μg twice daily plus ipratropium 40 μg 4 times daily, or placebo. Patients were assessed for changes in FEV1, daytime symptom scores, specific airway conductance, and the need for rescue medication. The study demonstrated a significant benefit from the addition of ipratropium to salmeterol in terms of reduction of airway obstruction, but not in symptom control or need for rescue medication.3 However, no patients were randomized to receive ipratropium alone, so comparison of the relative contribution of the 2 classes is limited.
A 6-month, randomized double-blind placebo-controlled study evaluating the efficacy of salmeterol 50 μg twice daily vs tiotropium (a new long-acting inhaled anticholinergic) 18 μg once daily was published in 2002. Endpoints in 623 patients were assessed using 12-hour spirometric performance, transition dyspnea index (TDI), and the St. George Respiratory Questionnaire (SGRQ). (SGRQ is a validated disease-specific instrument designed to measure impact on overall health, daily life, and perceived well-being. It measures activity limitations, symptoms, and psychosocial impact.) Tiotropium showed superiority over salmeterol in all endpoints assessed (0.14 L increase in morning FEV1vs 0.09 L, 1.02 U improvement in TDI score vs 0.24, and –5.14 U improvement of SGRQ total score from baseline vs –3.54). However, it should be noted that a difference of 1 on the TDI score was necessary to suggest a clinical benefit. While the overall difference in SGQR between tiotropium and salmeterol did not reach statistical significance, the proportion of patients in the tiotropium group that reached the clinically significant threshold of 4 units improvement in SGRQ score was significantly higher than in the salmeterol group (51% vs 40%; P<.05).4
In a similar study in 2003, 1207 patients were randomized to receive the above doses of salmeterol, tiotropium, or placebo. Over the course of 6 months, tiotropium was associated with a significant delay in onset of the first exacerbation compared with placebo, and overall it led to the fewest exacerbations per patient-year. Fewer hospital admissions were also demonstrated in the tiotropium group per patient-year, and the number of days that patients were unable to perform usual activities was lowest for the tiotropium group. Again, improvement in TDI and SGRQ scores was significantly greater with tiotropium than placebo. In almost all outcomes, the salmeterol results were intermediate between those of tiotropium and placebo, and were not statistically different from placebo.5
Recommendations from others
The GOLD (Global Strategy for the Diagnosis, Management, and Prevention of COPD) Report states that the choice between β2-agonist, anti-cholinergics, or combination therapy depends on the availability and the response of a given patient in terms of symptom relief and side effects. The 2003 GOLD Workshop Report update further recommends the use of regular treatment with long-acting bronchodilators, including tiotropium, rather than short-acting bronchodilators for moderate-to-severe COPD.6
A separate report for the Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians—American Society of Internal Medicine states that both are beneficial for management of acute exacerbations, but that anticholinergics should be considered first because they are associated with fewer and more benign side effects.7
Patient response and tolerance of side effects determine which drug class to use
Grant Hoekzema, MD
Mercy Family Medicine Residency, St. Louis, Mo
Although recent national guidelines for the management of COPD, such as the GOLD report, give more cohesiveness to treatment strategies for patients with COPD, there is still room for tailoring a treatment approach. I find that when choosing between beta-agonists and anticholinergics, patient response and tolerability of side effects determine what I use.
This Clinical Inquiry supports my clinical impression that neither class of drug is significantly superior to the other in regards to COPD outcome measures. In my experience, when neither drug offers a clear advantage, factors affecting compliance and tolerability tend to determine how effective it is for my patients. Therefore, a trial of either class seems reasonable at first and follow-up determines what is used in the long run.
1. Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koeter GH, Postma DS, Kerstjens HA. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002;121:597-608.
2. McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. In: The Cochrane Library, Issue 1,2004. Chichester, UK: John Wiley, Ltd; 2003.
3. van Noord JA, de Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000;15:878-885.
4. Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47-55.
5. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399-404.
6. Fabbri LM, Hurd SS. GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2.
7. Snow V, Lascher S, Mottur-Pilson C. Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
1. Liesker JJ, Wijkstra PJ, Ten Hacken NH, Koeter GH, Postma DS, Kerstjens HA. A systematic review of the effects of bronchodilators on exercise capacity in patients with COPD. Chest 2002;121:597-608.
2. McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. In: The Cochrane Library, Issue 1,2004. Chichester, UK: John Wiley, Ltd; 2003.
3. van Noord JA, de Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000;15:878-885.
4. Donohue JF, van Noord JA, Bateman ED, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122:47-55.
5. Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399-404.
6. Fabbri LM, Hurd SS. GOLD Scientific Committee. Global Strategy for the Diagnosis, Management, and Prevention of COPD: 2003 update. Eur Respir J 2003;22:1-2.
7. Snow V, Lascher S, Mottur-Pilson C. Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians-American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 2001;134:595-599.
Evidence-based answers from the Family Physicians Inquiries Network
What treatments are safe and effective for mild to moderate hypertension in pregnancy?
There is considerable debate concerning the treatment of mild to moderate essential hypertension during pregnancy. Evidence suggests that because of the potential risk of fetal intrauterine growth restriction, treatment of hypertension should be delayed until maternal blood pressure reaches 150–160 mm Hg systolic or 100–110 mm Hg diastolic, as long as the mother has no preexisting end organ damage.
Methyldopa has been the drug of choice for oral treatment, as it is the only medication to have any extended follow-up study. However, a recent meta-analysis raised the possibility of increased fetal mortality (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials).
Labetalol is an effective alternative, but concerns remain that treatment with any beta-blocker increases the risk that infants will be small for gestational age (SGA) (SOR: B, based on small randomized controlled trials with inconsistent results).
There is limited evidence that calcium channel blockers and diuretics are safe alternatives, although evidence is insufficient to prove a clear benefit (SOR: B, based on limited randomized controlled trials). Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), due to similar mechanisms of action, are contraindicated in pregnancy (SOR: B, based on multiple case studies). No other class of anti-hypertensive medications is proven to be harmful in pregnancy.
Evidence summary
Treatment of maternal hypertension during pregnancy is based on maternal and fetal outcomes. Multiple meta-analyses of randomized controlled trials show that the major maternal outcomes improved by treating mild to moderate hypertension are decreased progression to severe hypertension (number needed to treat [NNT]=12; 95% confidence interval [CI], 9–17) and decreased need for additional antihypertensive therapy.1,2 The relative risk (RR) for preventing preeclampsia was 0.99 (95% CI, 0.84–1.18). The risk of preterm delivery was 1.00 (95% CI, 0.87–1.15).
The data for fetal outcomes are important, as the maternal benefits of treatment remain small.3 Much of the debate centers on decreasing uteroplacental perfusion, which may lead to decreased fetal growth. One meta-analysis reviewed 45 trials to evaluate the potential increase in SGA infants caused by any antihypertensive treatment, through quantifying the fall in mean arterial pressure. The analysis found an average decrease in birthweight of 145 g for a 10 mm Hg fall in mean arterial pressurewith no increased perinatal morbidity.4 The clinical significance of this is unclear.
In comparing one agent with another, methyldopa was the most commonly tested agent, with 14 randomized controlled trials of more than 1010 subjects demonstrating its efficacy at reducing blood pressure. Other antihypertensive agents appear better than methyldopa in terms of reducing the risk of infant mortality (RR=0.49; 95% CI, 0.24–0.99),1 but the studies were small and used weak methods, and this finding may be due to bias.5 Meta-analyses of beta-blocker trials show a borderline increase in SGA infants, with no related increase in perinatal mortality, as well as a decrease in the incidence of respiratory distress syndrome.6
Diuretics are effective antihypertensives, especially when combined with other agents, but they are known to decrease the circulating plasma volume, potentially decreasing uteroplacental perfusion. They are generally viewed as safe, as long as the mother is not already at increased risk for perfusion abnormalities (eg, preeclamptic states).7 Calcium channel blockers, though generally regarded as safe and effective, have mostly been evaluated for use late in pregnancy, so their benefit-to-risk ratio remains uncertain.8ACE inhibitors and, by extension, ARBs, due to their similar mechanisms of action, are contraindicated in pregnancy, having been linked to miscarriage, fetal death, fetal renal failure, and malformation.5,9-11
Recommendations from others
The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin states there is no evidence that antihypertensive treatment for mild to moderate hypertension improves maternal or fetal outcomes, even for women who are already receiving hypertension treatment at the time of pregnancy. ACOG suggests treatment may be stopped during pregnancy, or not initiated until blood pressures reach 150–160 mm Hg systolic or 100–110 mm Hg diastolic, unless the mother has underlying renal or cardiovascular disease.9
The National High Blood Pressure Education Program recommends the same guidelines as ACOG,10 whereas the Canadian Hypertension Society consensus panel has chosen 140/90 mm Hg as the level at which treatment should be initiated.11
The British Medical Journal Clinical Evidence Guidelines reiterate that the evidence does not support the benefit of treating mild to moderate hypertension, except in reducing the progression to severe hypertension.5 Methyldopa is consistently the drug of choice in all those making a specific recommendation,9-11 although it should be noted these recommendations were published before the 2003 Cochrane Review.1
Benefits from treatment do not outweigh risks unless maternal BP moderately high
James Holt, MD
East Tennessee State University, Johnson City
I have always felt uneasy with treatment of mild to moderate hypertension in pregnancy, as chronic hypertension must be differentiated from preeclampsia; and the treatments seem counterintuitive. I often see new obstetric patients well into the third trimester, and how I should initially treat an elevated blood pressure has been unclear. Adding the welfare of the unborn baby raises the stakes further.
This Clinical Inquiry helps my decision about initiating treatment, as the benefits from treatment do not outweigh the risks to mother and fetus unless the maternal blood pressure is moderately high, and the recommended thresholds for treatment are rather high for women with no end organ damage. If I must treat her, it appears the best (but not perfect) option is methyldopa.
1. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2003;(1):CD002252.-
2. Magee LA, Duley L. Oral beta blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003;(1):CD002863.-
3. Ferrer RL, Sibai BM, Mulrow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol 2000;96:849-860.
4. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet 2000;355:87-92.
5. Duley L. Childbirth and pregnancy—pre-eclampsia and hypertension. Clin Evid [online], Issue 9, August 2003. London: BMJ Publishing Group; 2003. Available at: clinicalevidence.com. Accessed on August 29, 2003.
6. Magee LA, Elran E, Bull SB, Logan A, Koren G. Risks and benefits of beta-receptor blockers for pregnancy hypertension: overview of the randomized trials. Eur J Obstet Gynecol Reprod Biol 2000;88:15-26.
7. Management of Chronic Hypertension During Pregnancy. Evidence Report/Technology Assessment: Number 14. AHRQ Publication No. 00-E010, August 2000. Rockville, Md: Agency for Healthcare Research and Quality; 2000. Available at: www.ahrq.gov/clinic/epcsums/pregsum.htm. Accessed on May 5, 2004.
8. Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ 1999;318:1332-1336.
9. Gilstrap LC, Ramin SM. ACOG Practice Bulletin No. 29: Chronic hypertension in pregnancy. Obstet Gynecol 2001;98:177-185.
10. National. Heart Lung and Blood Institute. National High Blood Pressure Education Program: Working Group Report on High Blood Pressure in Pregnancy. Bethesda, Md: NHLBI; 2000. Available at: www.nhlbi.nih.gov/health/prof/heart/hbp/hbp_preg.pdf. Accessed on May 5, 2004.
11. Rey E, LeLorier J, Burgess E, Lange IR, Leduc L. Report of Canadian Hypertension Society Consensus Conference: 3. Pharmacologic treatment of hypertensive disorders in pregnancy. CMAJ 1997;157:1245-1254.
There is considerable debate concerning the treatment of mild to moderate essential hypertension during pregnancy. Evidence suggests that because of the potential risk of fetal intrauterine growth restriction, treatment of hypertension should be delayed until maternal blood pressure reaches 150–160 mm Hg systolic or 100–110 mm Hg diastolic, as long as the mother has no preexisting end organ damage.
Methyldopa has been the drug of choice for oral treatment, as it is the only medication to have any extended follow-up study. However, a recent meta-analysis raised the possibility of increased fetal mortality (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials).
Labetalol is an effective alternative, but concerns remain that treatment with any beta-blocker increases the risk that infants will be small for gestational age (SGA) (SOR: B, based on small randomized controlled trials with inconsistent results).
There is limited evidence that calcium channel blockers and diuretics are safe alternatives, although evidence is insufficient to prove a clear benefit (SOR: B, based on limited randomized controlled trials). Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), due to similar mechanisms of action, are contraindicated in pregnancy (SOR: B, based on multiple case studies). No other class of anti-hypertensive medications is proven to be harmful in pregnancy.
Evidence summary
Treatment of maternal hypertension during pregnancy is based on maternal and fetal outcomes. Multiple meta-analyses of randomized controlled trials show that the major maternal outcomes improved by treating mild to moderate hypertension are decreased progression to severe hypertension (number needed to treat [NNT]=12; 95% confidence interval [CI], 9–17) and decreased need for additional antihypertensive therapy.1,2 The relative risk (RR) for preventing preeclampsia was 0.99 (95% CI, 0.84–1.18). The risk of preterm delivery was 1.00 (95% CI, 0.87–1.15).
The data for fetal outcomes are important, as the maternal benefits of treatment remain small.3 Much of the debate centers on decreasing uteroplacental perfusion, which may lead to decreased fetal growth. One meta-analysis reviewed 45 trials to evaluate the potential increase in SGA infants caused by any antihypertensive treatment, through quantifying the fall in mean arterial pressure. The analysis found an average decrease in birthweight of 145 g for a 10 mm Hg fall in mean arterial pressurewith no increased perinatal morbidity.4 The clinical significance of this is unclear.
In comparing one agent with another, methyldopa was the most commonly tested agent, with 14 randomized controlled trials of more than 1010 subjects demonstrating its efficacy at reducing blood pressure. Other antihypertensive agents appear better than methyldopa in terms of reducing the risk of infant mortality (RR=0.49; 95% CI, 0.24–0.99),1 but the studies were small and used weak methods, and this finding may be due to bias.5 Meta-analyses of beta-blocker trials show a borderline increase in SGA infants, with no related increase in perinatal mortality, as well as a decrease in the incidence of respiratory distress syndrome.6
Diuretics are effective antihypertensives, especially when combined with other agents, but they are known to decrease the circulating plasma volume, potentially decreasing uteroplacental perfusion. They are generally viewed as safe, as long as the mother is not already at increased risk for perfusion abnormalities (eg, preeclamptic states).7 Calcium channel blockers, though generally regarded as safe and effective, have mostly been evaluated for use late in pregnancy, so their benefit-to-risk ratio remains uncertain.8ACE inhibitors and, by extension, ARBs, due to their similar mechanisms of action, are contraindicated in pregnancy, having been linked to miscarriage, fetal death, fetal renal failure, and malformation.5,9-11
Recommendations from others
The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin states there is no evidence that antihypertensive treatment for mild to moderate hypertension improves maternal or fetal outcomes, even for women who are already receiving hypertension treatment at the time of pregnancy. ACOG suggests treatment may be stopped during pregnancy, or not initiated until blood pressures reach 150–160 mm Hg systolic or 100–110 mm Hg diastolic, unless the mother has underlying renal or cardiovascular disease.9
The National High Blood Pressure Education Program recommends the same guidelines as ACOG,10 whereas the Canadian Hypertension Society consensus panel has chosen 140/90 mm Hg as the level at which treatment should be initiated.11
The British Medical Journal Clinical Evidence Guidelines reiterate that the evidence does not support the benefit of treating mild to moderate hypertension, except in reducing the progression to severe hypertension.5 Methyldopa is consistently the drug of choice in all those making a specific recommendation,9-11 although it should be noted these recommendations were published before the 2003 Cochrane Review.1
Benefits from treatment do not outweigh risks unless maternal BP moderately high
James Holt, MD
East Tennessee State University, Johnson City
I have always felt uneasy with treatment of mild to moderate hypertension in pregnancy, as chronic hypertension must be differentiated from preeclampsia; and the treatments seem counterintuitive. I often see new obstetric patients well into the third trimester, and how I should initially treat an elevated blood pressure has been unclear. Adding the welfare of the unborn baby raises the stakes further.
This Clinical Inquiry helps my decision about initiating treatment, as the benefits from treatment do not outweigh the risks to mother and fetus unless the maternal blood pressure is moderately high, and the recommended thresholds for treatment are rather high for women with no end organ damage. If I must treat her, it appears the best (but not perfect) option is methyldopa.
There is considerable debate concerning the treatment of mild to moderate essential hypertension during pregnancy. Evidence suggests that because of the potential risk of fetal intrauterine growth restriction, treatment of hypertension should be delayed until maternal blood pressure reaches 150–160 mm Hg systolic or 100–110 mm Hg diastolic, as long as the mother has no preexisting end organ damage.
Methyldopa has been the drug of choice for oral treatment, as it is the only medication to have any extended follow-up study. However, a recent meta-analysis raised the possibility of increased fetal mortality (strength of recommendation [SOR]: A, based on systematic review of randomized controlled trials).
Labetalol is an effective alternative, but concerns remain that treatment with any beta-blocker increases the risk that infants will be small for gestational age (SGA) (SOR: B, based on small randomized controlled trials with inconsistent results).
There is limited evidence that calcium channel blockers and diuretics are safe alternatives, although evidence is insufficient to prove a clear benefit (SOR: B, based on limited randomized controlled trials). Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), due to similar mechanisms of action, are contraindicated in pregnancy (SOR: B, based on multiple case studies). No other class of anti-hypertensive medications is proven to be harmful in pregnancy.
Evidence summary
Treatment of maternal hypertension during pregnancy is based on maternal and fetal outcomes. Multiple meta-analyses of randomized controlled trials show that the major maternal outcomes improved by treating mild to moderate hypertension are decreased progression to severe hypertension (number needed to treat [NNT]=12; 95% confidence interval [CI], 9–17) and decreased need for additional antihypertensive therapy.1,2 The relative risk (RR) for preventing preeclampsia was 0.99 (95% CI, 0.84–1.18). The risk of preterm delivery was 1.00 (95% CI, 0.87–1.15).
The data for fetal outcomes are important, as the maternal benefits of treatment remain small.3 Much of the debate centers on decreasing uteroplacental perfusion, which may lead to decreased fetal growth. One meta-analysis reviewed 45 trials to evaluate the potential increase in SGA infants caused by any antihypertensive treatment, through quantifying the fall in mean arterial pressure. The analysis found an average decrease in birthweight of 145 g for a 10 mm Hg fall in mean arterial pressurewith no increased perinatal morbidity.4 The clinical significance of this is unclear.
In comparing one agent with another, methyldopa was the most commonly tested agent, with 14 randomized controlled trials of more than 1010 subjects demonstrating its efficacy at reducing blood pressure. Other antihypertensive agents appear better than methyldopa in terms of reducing the risk of infant mortality (RR=0.49; 95% CI, 0.24–0.99),1 but the studies were small and used weak methods, and this finding may be due to bias.5 Meta-analyses of beta-blocker trials show a borderline increase in SGA infants, with no related increase in perinatal mortality, as well as a decrease in the incidence of respiratory distress syndrome.6
Diuretics are effective antihypertensives, especially when combined with other agents, but they are known to decrease the circulating plasma volume, potentially decreasing uteroplacental perfusion. They are generally viewed as safe, as long as the mother is not already at increased risk for perfusion abnormalities (eg, preeclamptic states).7 Calcium channel blockers, though generally regarded as safe and effective, have mostly been evaluated for use late in pregnancy, so their benefit-to-risk ratio remains uncertain.8ACE inhibitors and, by extension, ARBs, due to their similar mechanisms of action, are contraindicated in pregnancy, having been linked to miscarriage, fetal death, fetal renal failure, and malformation.5,9-11
Recommendations from others
The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin states there is no evidence that antihypertensive treatment for mild to moderate hypertension improves maternal or fetal outcomes, even for women who are already receiving hypertension treatment at the time of pregnancy. ACOG suggests treatment may be stopped during pregnancy, or not initiated until blood pressures reach 150–160 mm Hg systolic or 100–110 mm Hg diastolic, unless the mother has underlying renal or cardiovascular disease.9
The National High Blood Pressure Education Program recommends the same guidelines as ACOG,10 whereas the Canadian Hypertension Society consensus panel has chosen 140/90 mm Hg as the level at which treatment should be initiated.11
The British Medical Journal Clinical Evidence Guidelines reiterate that the evidence does not support the benefit of treating mild to moderate hypertension, except in reducing the progression to severe hypertension.5 Methyldopa is consistently the drug of choice in all those making a specific recommendation,9-11 although it should be noted these recommendations were published before the 2003 Cochrane Review.1
Benefits from treatment do not outweigh risks unless maternal BP moderately high
James Holt, MD
East Tennessee State University, Johnson City
I have always felt uneasy with treatment of mild to moderate hypertension in pregnancy, as chronic hypertension must be differentiated from preeclampsia; and the treatments seem counterintuitive. I often see new obstetric patients well into the third trimester, and how I should initially treat an elevated blood pressure has been unclear. Adding the welfare of the unborn baby raises the stakes further.
This Clinical Inquiry helps my decision about initiating treatment, as the benefits from treatment do not outweigh the risks to mother and fetus unless the maternal blood pressure is moderately high, and the recommended thresholds for treatment are rather high for women with no end organ damage. If I must treat her, it appears the best (but not perfect) option is methyldopa.
1. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2003;(1):CD002252.-
2. Magee LA, Duley L. Oral beta blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003;(1):CD002863.-
3. Ferrer RL, Sibai BM, Mulrow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol 2000;96:849-860.
4. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet 2000;355:87-92.
5. Duley L. Childbirth and pregnancy—pre-eclampsia and hypertension. Clin Evid [online], Issue 9, August 2003. London: BMJ Publishing Group; 2003. Available at: clinicalevidence.com. Accessed on August 29, 2003.
6. Magee LA, Elran E, Bull SB, Logan A, Koren G. Risks and benefits of beta-receptor blockers for pregnancy hypertension: overview of the randomized trials. Eur J Obstet Gynecol Reprod Biol 2000;88:15-26.
7. Management of Chronic Hypertension During Pregnancy. Evidence Report/Technology Assessment: Number 14. AHRQ Publication No. 00-E010, August 2000. Rockville, Md: Agency for Healthcare Research and Quality; 2000. Available at: www.ahrq.gov/clinic/epcsums/pregsum.htm. Accessed on May 5, 2004.
8. Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ 1999;318:1332-1336.
9. Gilstrap LC, Ramin SM. ACOG Practice Bulletin No. 29: Chronic hypertension in pregnancy. Obstet Gynecol 2001;98:177-185.
10. National. Heart Lung and Blood Institute. National High Blood Pressure Education Program: Working Group Report on High Blood Pressure in Pregnancy. Bethesda, Md: NHLBI; 2000. Available at: www.nhlbi.nih.gov/health/prof/heart/hbp/hbp_preg.pdf. Accessed on May 5, 2004.
11. Rey E, LeLorier J, Burgess E, Lange IR, Leduc L. Report of Canadian Hypertension Society Consensus Conference: 3. Pharmacologic treatment of hypertensive disorders in pregnancy. CMAJ 1997;157:1245-1254.
1. Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2003;(1):CD002252.-
2. Magee LA, Duley L. Oral beta blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003;(1):CD002863.-
3. Ferrer RL, Sibai BM, Mulrow CD, Chiquette E, Stevens KR, Cornell J. Management of mild chronic hypertension during pregnancy: a review. Obstet Gynecol 2000;96:849-860.
4. von Dadelszen P, Ornstein MP, Bull SB, Logan AG, Koren G, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: a meta-analysis. Lancet 2000;355:87-92.
5. Duley L. Childbirth and pregnancy—pre-eclampsia and hypertension. Clin Evid [online], Issue 9, August 2003. London: BMJ Publishing Group; 2003. Available at: clinicalevidence.com. Accessed on August 29, 2003.
6. Magee LA, Elran E, Bull SB, Logan A, Koren G. Risks and benefits of beta-receptor blockers for pregnancy hypertension: overview of the randomized trials. Eur J Obstet Gynecol Reprod Biol 2000;88:15-26.
7. Management of Chronic Hypertension During Pregnancy. Evidence Report/Technology Assessment: Number 14. AHRQ Publication No. 00-E010, August 2000. Rockville, Md: Agency for Healthcare Research and Quality; 2000. Available at: www.ahrq.gov/clinic/epcsums/pregsum.htm. Accessed on May 5, 2004.
8. Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ 1999;318:1332-1336.
9. Gilstrap LC, Ramin SM. ACOG Practice Bulletin No. 29: Chronic hypertension in pregnancy. Obstet Gynecol 2001;98:177-185.
10. National. Heart Lung and Blood Institute. National High Blood Pressure Education Program: Working Group Report on High Blood Pressure in Pregnancy. Bethesda, Md: NHLBI; 2000. Available at: www.nhlbi.nih.gov/health/prof/heart/hbp/hbp_preg.pdf. Accessed on May 5, 2004.
11. Rey E, LeLorier J, Burgess E, Lange IR, Leduc L. Report of Canadian Hypertension Society Consensus Conference: 3. Pharmacologic treatment of hypertensive disorders in pregnancy. CMAJ 1997;157:1245-1254.
Evidence-based answers from the Family Physicians Inquiries Network
Does injection of steroids and lidocaine in the shoulder relieve bursitis?
Subacromial steroid injection may provide a small, short-term benefit compared with placebo. The short-term effectiveness of steroid injection compared with nonsteroidal anti-inflammatory agents (NSAIDs) remains unclear.
Steroid injections are better than physiotherapy alone in the short term. However, injection does not appear to provide any meaningful long-term benefit compared with other therapies (strength of recommendation: B). Data are insufficient to make recommendations regarding the proper timing of injection in the sequence of other treatments. Side effects of steroid injection, such as steroid flare and infection, are rare.
Evidence summary
A Cochrane Review of corticosteroid injections for shoulder pain found 7 randomized controlled trials comparing subacromial steroid injections with placebo.1 The placebos were either injectable anesthetics alone or injectable anesthetics combined with oral placebo tablets. Six of the 7 studies used the anterolateral approach to inject under the acromion.
All studies used a clinical exam for diagnosis that showed pain with range of motion (especially abduction) or pain that was consistent with impingement syndrome. Most of the follow-up times were short, typically 4 to 12 weeks, and the longest study was 33 weeks. Meta-analyses often report the effect size using standard mean difference (SMD). A rule of thumb for interpretation of SMD is a value of 0.2 indicates a small effect, a value of 0.5 indicates a medium effect, and a value of 0.8 or larger indicates a large effect. If the 95% confidence interval [CI] does not include zero, then the SMD is statistically significant at the 5% level (P<.05).2
Two of the studies comparing steroid injection with placebo were methodologically suitable for meta-analysis; these studies showed thatsteroids provided a mild, short-term (4-week)benefit with respect to pain (SMD=0.83; 95% CI,0.39–1.26), function (SMD=0.63; 95% CI,0.20–1.06), and abductive range of motion(SMD=0.82; 95% CI, 0.39–1.25).3,4
Results of the remaining, less rigorous trialswere conflicting and inconclusive. The reviewersalso found 3 randomized controlled trials comparing subacromial steroid injection with oralNSAIDs. The pooled results of these trials,encompassing 120 patients, found no differences in these 3 outcomes at 4 or 6 weeks. The review of an additional trial of 50 patients comparing subacromial steroid injection plus simultaneous oral NSAIDs with oral NSAIDs alone found no differences at 4 weeks. All 11 studies had small sample sizes, and suffered from variable methodological quality and heterogeneous results.
The reviewers concluded that steroids are probably better than placebo but provide little or no benefit in addition to NSAIDs, and that evidence is insufficient to guide treatment. Likewise, a Cochrane Review of multiple interventions for shoulder pain also found “little evidence to support or refute the efficacy of common interventions” and highlighted the need for new, well-designed trials.5
Another Cochrane Review examined 4 randomized controlled trials comparing physiotherapy interventions for shoulder pain.6 They found that steroid injections may be superior to physiotherapy for rotator cuff disease, but the type of physiotherapy and injection sites were not consistent across the studies, making creation of summary estimates inappropriate. The individual studies showed significant short-term benefits (3–7 weeks) of steroid injection over physiotherapy; however, long-term (6–52 weeks) benefits ranged from some benefit to no difference. These studies were consistent regarding age (mean age=53–55 years, SD ± 13–14 years) and complications reported, with the only side effect being postinjection soreness.
Hay et al7 conducted a multicenter, primary care–based randomized controlled trial with more than 200 patients, which was published too recently for inclusion in the Cochrane Review. They found no statistical difference in improvement between steroid injection without physiotherapy and physiotherapy alone at 6 weeks.
In 1996, van der Heijden et al8 systematically reviewed randomized clinical trials of steroid injections for shoulder disorders, including rota-tor cuff disease, adhesive capsulitis, rheumatoid conditions, and periarthritis. They screened more than 200 articles from searches in Medline (1966–1995) and EMBASE (1984–1995) and found 16 articles that met qualifying conditions for further review. Of these, 3 were methodologically adequate for final review. None of these 3 studies provided evidence showing the efficacy of steroid injections. The results of the major trials reviewed can be found in the Table .
TABLE
Major placebo-controlled trials of injectable steroids for shoulder pain
| Steroid (n) | Comparison | Follow-up arms (n) | Reported results | Conclusions |
|---|---|---|---|---|
| Methylprednisolone 1% lignocaine (28) | 1% lignocaine (28) | 12wks | 2 wks:insignificant improvement in steroid arm 2, 4, 6, 12 wks:no difference in pain, range of motion;all P>.05 | No significant advantage of subacromial methyl prednisolone over lignocaine10 |
| Triamcinolone, 0.5% lignocaine, placebo tabs (20) | C1:diclofenac, lignocaine (20) C2:placebo tabs, lignocaine (20) | 4 wks | 4 wks:steroid and C1 showed significant benefit over C2 for pain and range of motion (P<.05) Steroid vs C1:no difference (P=.0268) | Triamcinolone and diclofenac are equivalent, and superior to placebo3 |
| S1:triamcinolone, 1% lidocaine, naproxen (25) S2:triamcinolone, 1% lidocaine, placebo (25) | C1:1% lidocaine, naproxen (25) C2:1% lidocaine, placebo (25) | 4 wks | S1 superior to S2, C1, C2 S2 superior to C1, C2 For pain and clinical index at 2 and 4 wks, P<.05 | Triamcinolone and naproxen superior to placebo.More severe cases see most benefit4 |
| Triamcinolone, placebo tabs (15); reinjection at 3 wks if not better | Saline injection, indomethacin (15); reinjection at 3 wks if not | 6 wks | Pain and global scores improved in both groups (P<0.05), but no difference between them (P>.05) | No difference between indomethacin andtriamcinolone better injection11 |
| S1:methylprednisolone, lidocaine, placebo tabs (12) S2:methylprednisolone, NSAID (12) | C1:acupuncture (12) C2:ultrasound (12) C3:placebo tab, placebo U/S (12) | 4 wks | All patients improved. No differences in pain scores or abduction measurements at 2 or 4 wks (P=n/a) | Painful stiff shoulder may be self-limiting condition and bene- ficial effect may be natural recovery12 |
| Methylprednisolone, 1% lidocaine (104) | Physiotherapy (103) | 6 mos, option of other therapies given at 6 weeks | No differences in disability scores 6 wks:mean difference= –.05 (95% CI, –.02 to 3.0) 6 mos:mean difference= 1.4 (95% CI, –0.2 to 3.0) (7) episodes of unilateral | Physiotherapy and steroid injection were of similar short- and long-term effectiveness for treating new shoulder pain |
| Triamcinolone, 1% lidocaine (19) | 1% lidocaine (21) | Mean:33 wk; range:12–52 wk | Steroid:significant improvements of pain (P<.005) and range of motion (P<.005) vs control.No difference in activities of daily living seen (13) | Subacromial injection of steroids is effective for short-term therapy of impingement syndrome |
Recommendations from others
The American Academy of Orthopaedic Surgeons’ clinical guideline for shoulder pain9 recommends the following for rotator cuff disease: avoidance of irritating activity; anti-inflammatory medications if tolerated; exercises to recover and maintain passive range of motion; exercises to strengthen the rotator cuff once acute symptoms abated. If these are unsuccessful over several weeks, they recommend considering subacromial injection of local anesthetic and a short-acting corticosteroid. They gave their recommendation a “B” rating (some evidence exists to suggest benefit).
Consider injection with anesthetic and steroid for rotator cuff impingement
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes, University of Colorado Health Sciences Center, Denver
Subacromial injection is an integral component of the treatment armamentarium for certain types of shoulder pathology. Diagnostically, injection of a local anesthetic such as lidocaine can help differentiate true weakness caused by a full-thickness rotator cuff tear from inhibition due to inflammation and impingement pain. Strongly consider subacromial injection with both a local anesthetic and corticosteroid for patients with true rotator cuff impingement as diagnosed by positive Neer and Hawkins signs on examination.
If injection is appropriately administered, the patient should have near-immediate and significant reduction of impingement symptoms. They may regain motion sooner and advance quicker through their initial therapy program.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
2. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988.
3. Adebajo AO, Nash P, Hazleman BL. A prospective double blind dummy placebo controlled study comparing triamcinolone hexacetonide injection with oral diclofenac 50 mg TDS in patients with rotator cuff tendinitis. J Rheumatol 1990;17:1207-1210.
4. Petri M, Dobrow R, Neiman R, Whiting-O’Keefe O, Seaman WE. Randomized, double-blind, placebo-controlled study of the treatment of the painful shoulder. Arthritis Rheum 1987;30:1040-1045.
5. Green S, Buchbinder R, Glazier R, Forbes A. Interventions for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
6. Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
7. Hay EM, Thomas E, Paterson SM, Dziedzic K, Croft PR. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Ann Rheum Dis 2003;62:394-399.
8. van der Heijden GJ, van der Windt DA, Kleijnen J, Koes BW, Bouter LM. Steroid injections for shoulder disorders: a systematic review of randomized clinical trials. Br J Gen Pract 1996;46:309-316.
9. American. Academy of Orthopedic Surgeons. AAOS clinical guideline on shoulder pain: support document. Rosemont, IL: AAOS, 2001. Available at: www.guideline.gov/summary/summary.aspx?doc_id=2998. Accessed on May 5, 2004.
10. Vecchio PC, Hazleman BL, King RH. A double-blind trial comparing subacromial methylprednisolone and ligno-caine in acute rotator cuff tendinitis. Br J Rheumatol 1993;32:743-745.
11. White RH, Paull DM, Fleming KW. Rotator cuff tendinitis: comparison of subacromial injection of a long acting corticosteroid versus indomethacin therapy. J Rheumatol 1986;13:608-613.
12. Berry H, Fernandes L, Bloom B, Clarke R, Hamilton EB. Clinical study comparing acupuncture, physiotherapy, injection and oral anti-inflammatory therapy in shoulder cuff lesions. Curr Med Res Opin 1980;7:121-126.
13. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am 1996;78:1685-1689.
Subacromial steroid injection may provide a small, short-term benefit compared with placebo. The short-term effectiveness of steroid injection compared with nonsteroidal anti-inflammatory agents (NSAIDs) remains unclear.
Steroid injections are better than physiotherapy alone in the short term. However, injection does not appear to provide any meaningful long-term benefit compared with other therapies (strength of recommendation: B). Data are insufficient to make recommendations regarding the proper timing of injection in the sequence of other treatments. Side effects of steroid injection, such as steroid flare and infection, are rare.
Evidence summary
A Cochrane Review of corticosteroid injections for shoulder pain found 7 randomized controlled trials comparing subacromial steroid injections with placebo.1 The placebos were either injectable anesthetics alone or injectable anesthetics combined with oral placebo tablets. Six of the 7 studies used the anterolateral approach to inject under the acromion.
All studies used a clinical exam for diagnosis that showed pain with range of motion (especially abduction) or pain that was consistent with impingement syndrome. Most of the follow-up times were short, typically 4 to 12 weeks, and the longest study was 33 weeks. Meta-analyses often report the effect size using standard mean difference (SMD). A rule of thumb for interpretation of SMD is a value of 0.2 indicates a small effect, a value of 0.5 indicates a medium effect, and a value of 0.8 or larger indicates a large effect. If the 95% confidence interval [CI] does not include zero, then the SMD is statistically significant at the 5% level (P<.05).2
Two of the studies comparing steroid injection with placebo were methodologically suitable for meta-analysis; these studies showed thatsteroids provided a mild, short-term (4-week)benefit with respect to pain (SMD=0.83; 95% CI,0.39–1.26), function (SMD=0.63; 95% CI,0.20–1.06), and abductive range of motion(SMD=0.82; 95% CI, 0.39–1.25).3,4
Results of the remaining, less rigorous trialswere conflicting and inconclusive. The reviewersalso found 3 randomized controlled trials comparing subacromial steroid injection with oralNSAIDs. The pooled results of these trials,encompassing 120 patients, found no differences in these 3 outcomes at 4 or 6 weeks. The review of an additional trial of 50 patients comparing subacromial steroid injection plus simultaneous oral NSAIDs with oral NSAIDs alone found no differences at 4 weeks. All 11 studies had small sample sizes, and suffered from variable methodological quality and heterogeneous results.
The reviewers concluded that steroids are probably better than placebo but provide little or no benefit in addition to NSAIDs, and that evidence is insufficient to guide treatment. Likewise, a Cochrane Review of multiple interventions for shoulder pain also found “little evidence to support or refute the efficacy of common interventions” and highlighted the need for new, well-designed trials.5
Another Cochrane Review examined 4 randomized controlled trials comparing physiotherapy interventions for shoulder pain.6 They found that steroid injections may be superior to physiotherapy for rotator cuff disease, but the type of physiotherapy and injection sites were not consistent across the studies, making creation of summary estimates inappropriate. The individual studies showed significant short-term benefits (3–7 weeks) of steroid injection over physiotherapy; however, long-term (6–52 weeks) benefits ranged from some benefit to no difference. These studies were consistent regarding age (mean age=53–55 years, SD ± 13–14 years) and complications reported, with the only side effect being postinjection soreness.
Hay et al7 conducted a multicenter, primary care–based randomized controlled trial with more than 200 patients, which was published too recently for inclusion in the Cochrane Review. They found no statistical difference in improvement between steroid injection without physiotherapy and physiotherapy alone at 6 weeks.
In 1996, van der Heijden et al8 systematically reviewed randomized clinical trials of steroid injections for shoulder disorders, including rota-tor cuff disease, adhesive capsulitis, rheumatoid conditions, and periarthritis. They screened more than 200 articles from searches in Medline (1966–1995) and EMBASE (1984–1995) and found 16 articles that met qualifying conditions for further review. Of these, 3 were methodologically adequate for final review. None of these 3 studies provided evidence showing the efficacy of steroid injections. The results of the major trials reviewed can be found in the Table .
TABLE
Major placebo-controlled trials of injectable steroids for shoulder pain
| Steroid (n) | Comparison | Follow-up arms (n) | Reported results | Conclusions |
|---|---|---|---|---|
| Methylprednisolone 1% lignocaine (28) | 1% lignocaine (28) | 12wks | 2 wks:insignificant improvement in steroid arm 2, 4, 6, 12 wks:no difference in pain, range of motion;all P>.05 | No significant advantage of subacromial methyl prednisolone over lignocaine10 |
| Triamcinolone, 0.5% lignocaine, placebo tabs (20) | C1:diclofenac, lignocaine (20) C2:placebo tabs, lignocaine (20) | 4 wks | 4 wks:steroid and C1 showed significant benefit over C2 for pain and range of motion (P<.05) Steroid vs C1:no difference (P=.0268) | Triamcinolone and diclofenac are equivalent, and superior to placebo3 |
| S1:triamcinolone, 1% lidocaine, naproxen (25) S2:triamcinolone, 1% lidocaine, placebo (25) | C1:1% lidocaine, naproxen (25) C2:1% lidocaine, placebo (25) | 4 wks | S1 superior to S2, C1, C2 S2 superior to C1, C2 For pain and clinical index at 2 and 4 wks, P<.05 | Triamcinolone and naproxen superior to placebo.More severe cases see most benefit4 |
| Triamcinolone, placebo tabs (15); reinjection at 3 wks if not better | Saline injection, indomethacin (15); reinjection at 3 wks if not | 6 wks | Pain and global scores improved in both groups (P<0.05), but no difference between them (P>.05) | No difference between indomethacin andtriamcinolone better injection11 |
| S1:methylprednisolone, lidocaine, placebo tabs (12) S2:methylprednisolone, NSAID (12) | C1:acupuncture (12) C2:ultrasound (12) C3:placebo tab, placebo U/S (12) | 4 wks | All patients improved. No differences in pain scores or abduction measurements at 2 or 4 wks (P=n/a) | Painful stiff shoulder may be self-limiting condition and bene- ficial effect may be natural recovery12 |
| Methylprednisolone, 1% lidocaine (104) | Physiotherapy (103) | 6 mos, option of other therapies given at 6 weeks | No differences in disability scores 6 wks:mean difference= –.05 (95% CI, –.02 to 3.0) 6 mos:mean difference= 1.4 (95% CI, –0.2 to 3.0) (7) episodes of unilateral | Physiotherapy and steroid injection were of similar short- and long-term effectiveness for treating new shoulder pain |
| Triamcinolone, 1% lidocaine (19) | 1% lidocaine (21) | Mean:33 wk; range:12–52 wk | Steroid:significant improvements of pain (P<.005) and range of motion (P<.005) vs control.No difference in activities of daily living seen (13) | Subacromial injection of steroids is effective for short-term therapy of impingement syndrome |
Recommendations from others
The American Academy of Orthopaedic Surgeons’ clinical guideline for shoulder pain9 recommends the following for rotator cuff disease: avoidance of irritating activity; anti-inflammatory medications if tolerated; exercises to recover and maintain passive range of motion; exercises to strengthen the rotator cuff once acute symptoms abated. If these are unsuccessful over several weeks, they recommend considering subacromial injection of local anesthetic and a short-acting corticosteroid. They gave their recommendation a “B” rating (some evidence exists to suggest benefit).
Consider injection with anesthetic and steroid for rotator cuff impingement
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes, University of Colorado Health Sciences Center, Denver
Subacromial injection is an integral component of the treatment armamentarium for certain types of shoulder pathology. Diagnostically, injection of a local anesthetic such as lidocaine can help differentiate true weakness caused by a full-thickness rotator cuff tear from inhibition due to inflammation and impingement pain. Strongly consider subacromial injection with both a local anesthetic and corticosteroid for patients with true rotator cuff impingement as diagnosed by positive Neer and Hawkins signs on examination.
If injection is appropriately administered, the patient should have near-immediate and significant reduction of impingement symptoms. They may regain motion sooner and advance quicker through their initial therapy program.
Subacromial steroid injection may provide a small, short-term benefit compared with placebo. The short-term effectiveness of steroid injection compared with nonsteroidal anti-inflammatory agents (NSAIDs) remains unclear.
Steroid injections are better than physiotherapy alone in the short term. However, injection does not appear to provide any meaningful long-term benefit compared with other therapies (strength of recommendation: B). Data are insufficient to make recommendations regarding the proper timing of injection in the sequence of other treatments. Side effects of steroid injection, such as steroid flare and infection, are rare.
Evidence summary
A Cochrane Review of corticosteroid injections for shoulder pain found 7 randomized controlled trials comparing subacromial steroid injections with placebo.1 The placebos were either injectable anesthetics alone or injectable anesthetics combined with oral placebo tablets. Six of the 7 studies used the anterolateral approach to inject under the acromion.
All studies used a clinical exam for diagnosis that showed pain with range of motion (especially abduction) or pain that was consistent with impingement syndrome. Most of the follow-up times were short, typically 4 to 12 weeks, and the longest study was 33 weeks. Meta-analyses often report the effect size using standard mean difference (SMD). A rule of thumb for interpretation of SMD is a value of 0.2 indicates a small effect, a value of 0.5 indicates a medium effect, and a value of 0.8 or larger indicates a large effect. If the 95% confidence interval [CI] does not include zero, then the SMD is statistically significant at the 5% level (P<.05).2
Two of the studies comparing steroid injection with placebo were methodologically suitable for meta-analysis; these studies showed thatsteroids provided a mild, short-term (4-week)benefit with respect to pain (SMD=0.83; 95% CI,0.39–1.26), function (SMD=0.63; 95% CI,0.20–1.06), and abductive range of motion(SMD=0.82; 95% CI, 0.39–1.25).3,4
Results of the remaining, less rigorous trialswere conflicting and inconclusive. The reviewersalso found 3 randomized controlled trials comparing subacromial steroid injection with oralNSAIDs. The pooled results of these trials,encompassing 120 patients, found no differences in these 3 outcomes at 4 or 6 weeks. The review of an additional trial of 50 patients comparing subacromial steroid injection plus simultaneous oral NSAIDs with oral NSAIDs alone found no differences at 4 weeks. All 11 studies had small sample sizes, and suffered from variable methodological quality and heterogeneous results.
The reviewers concluded that steroids are probably better than placebo but provide little or no benefit in addition to NSAIDs, and that evidence is insufficient to guide treatment. Likewise, a Cochrane Review of multiple interventions for shoulder pain also found “little evidence to support or refute the efficacy of common interventions” and highlighted the need for new, well-designed trials.5
Another Cochrane Review examined 4 randomized controlled trials comparing physiotherapy interventions for shoulder pain.6 They found that steroid injections may be superior to physiotherapy for rotator cuff disease, but the type of physiotherapy and injection sites were not consistent across the studies, making creation of summary estimates inappropriate. The individual studies showed significant short-term benefits (3–7 weeks) of steroid injection over physiotherapy; however, long-term (6–52 weeks) benefits ranged from some benefit to no difference. These studies were consistent regarding age (mean age=53–55 years, SD ± 13–14 years) and complications reported, with the only side effect being postinjection soreness.
Hay et al7 conducted a multicenter, primary care–based randomized controlled trial with more than 200 patients, which was published too recently for inclusion in the Cochrane Review. They found no statistical difference in improvement between steroid injection without physiotherapy and physiotherapy alone at 6 weeks.
In 1996, van der Heijden et al8 systematically reviewed randomized clinical trials of steroid injections for shoulder disorders, including rota-tor cuff disease, adhesive capsulitis, rheumatoid conditions, and periarthritis. They screened more than 200 articles from searches in Medline (1966–1995) and EMBASE (1984–1995) and found 16 articles that met qualifying conditions for further review. Of these, 3 were methodologically adequate for final review. None of these 3 studies provided evidence showing the efficacy of steroid injections. The results of the major trials reviewed can be found in the Table .
TABLE
Major placebo-controlled trials of injectable steroids for shoulder pain
| Steroid (n) | Comparison | Follow-up arms (n) | Reported results | Conclusions |
|---|---|---|---|---|
| Methylprednisolone 1% lignocaine (28) | 1% lignocaine (28) | 12wks | 2 wks:insignificant improvement in steroid arm 2, 4, 6, 12 wks:no difference in pain, range of motion;all P>.05 | No significant advantage of subacromial methyl prednisolone over lignocaine10 |
| Triamcinolone, 0.5% lignocaine, placebo tabs (20) | C1:diclofenac, lignocaine (20) C2:placebo tabs, lignocaine (20) | 4 wks | 4 wks:steroid and C1 showed significant benefit over C2 for pain and range of motion (P<.05) Steroid vs C1:no difference (P=.0268) | Triamcinolone and diclofenac are equivalent, and superior to placebo3 |
| S1:triamcinolone, 1% lidocaine, naproxen (25) S2:triamcinolone, 1% lidocaine, placebo (25) | C1:1% lidocaine, naproxen (25) C2:1% lidocaine, placebo (25) | 4 wks | S1 superior to S2, C1, C2 S2 superior to C1, C2 For pain and clinical index at 2 and 4 wks, P<.05 | Triamcinolone and naproxen superior to placebo.More severe cases see most benefit4 |
| Triamcinolone, placebo tabs (15); reinjection at 3 wks if not better | Saline injection, indomethacin (15); reinjection at 3 wks if not | 6 wks | Pain and global scores improved in both groups (P<0.05), but no difference between them (P>.05) | No difference between indomethacin andtriamcinolone better injection11 |
| S1:methylprednisolone, lidocaine, placebo tabs (12) S2:methylprednisolone, NSAID (12) | C1:acupuncture (12) C2:ultrasound (12) C3:placebo tab, placebo U/S (12) | 4 wks | All patients improved. No differences in pain scores or abduction measurements at 2 or 4 wks (P=n/a) | Painful stiff shoulder may be self-limiting condition and bene- ficial effect may be natural recovery12 |
| Methylprednisolone, 1% lidocaine (104) | Physiotherapy (103) | 6 mos, option of other therapies given at 6 weeks | No differences in disability scores 6 wks:mean difference= –.05 (95% CI, –.02 to 3.0) 6 mos:mean difference= 1.4 (95% CI, –0.2 to 3.0) (7) episodes of unilateral | Physiotherapy and steroid injection were of similar short- and long-term effectiveness for treating new shoulder pain |
| Triamcinolone, 1% lidocaine (19) | 1% lidocaine (21) | Mean:33 wk; range:12–52 wk | Steroid:significant improvements of pain (P<.005) and range of motion (P<.005) vs control.No difference in activities of daily living seen (13) | Subacromial injection of steroids is effective for short-term therapy of impingement syndrome |
Recommendations from others
The American Academy of Orthopaedic Surgeons’ clinical guideline for shoulder pain9 recommends the following for rotator cuff disease: avoidance of irritating activity; anti-inflammatory medications if tolerated; exercises to recover and maintain passive range of motion; exercises to strengthen the rotator cuff once acute symptoms abated. If these are unsuccessful over several weeks, they recommend considering subacromial injection of local anesthetic and a short-acting corticosteroid. They gave their recommendation a “B” rating (some evidence exists to suggest benefit).
Consider injection with anesthetic and steroid for rotator cuff impingement
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes, University of Colorado Health Sciences Center, Denver
Subacromial injection is an integral component of the treatment armamentarium for certain types of shoulder pathology. Diagnostically, injection of a local anesthetic such as lidocaine can help differentiate true weakness caused by a full-thickness rotator cuff tear from inhibition due to inflammation and impingement pain. Strongly consider subacromial injection with both a local anesthetic and corticosteroid for patients with true rotator cuff impingement as diagnosed by positive Neer and Hawkins signs on examination.
If injection is appropriately administered, the patient should have near-immediate and significant reduction of impingement symptoms. They may regain motion sooner and advance quicker through their initial therapy program.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
2. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988.
3. Adebajo AO, Nash P, Hazleman BL. A prospective double blind dummy placebo controlled study comparing triamcinolone hexacetonide injection with oral diclofenac 50 mg TDS in patients with rotator cuff tendinitis. J Rheumatol 1990;17:1207-1210.
4. Petri M, Dobrow R, Neiman R, Whiting-O’Keefe O, Seaman WE. Randomized, double-blind, placebo-controlled study of the treatment of the painful shoulder. Arthritis Rheum 1987;30:1040-1045.
5. Green S, Buchbinder R, Glazier R, Forbes A. Interventions for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
6. Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
7. Hay EM, Thomas E, Paterson SM, Dziedzic K, Croft PR. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Ann Rheum Dis 2003;62:394-399.
8. van der Heijden GJ, van der Windt DA, Kleijnen J, Koes BW, Bouter LM. Steroid injections for shoulder disorders: a systematic review of randomized clinical trials. Br J Gen Pract 1996;46:309-316.
9. American. Academy of Orthopedic Surgeons. AAOS clinical guideline on shoulder pain: support document. Rosemont, IL: AAOS, 2001. Available at: www.guideline.gov/summary/summary.aspx?doc_id=2998. Accessed on May 5, 2004.
10. Vecchio PC, Hazleman BL, King RH. A double-blind trial comparing subacromial methylprednisolone and ligno-caine in acute rotator cuff tendinitis. Br J Rheumatol 1993;32:743-745.
11. White RH, Paull DM, Fleming KW. Rotator cuff tendinitis: comparison of subacromial injection of a long acting corticosteroid versus indomethacin therapy. J Rheumatol 1986;13:608-613.
12. Berry H, Fernandes L, Bloom B, Clarke R, Hamilton EB. Clinical study comparing acupuncture, physiotherapy, injection and oral anti-inflammatory therapy in shoulder cuff lesions. Curr Med Res Opin 1980;7:121-126.
13. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am 1996;78:1685-1689.
1. Buchbinder R, Green S, Youd JM. Corticosteroid injections for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
2. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988.
3. Adebajo AO, Nash P, Hazleman BL. A prospective double blind dummy placebo controlled study comparing triamcinolone hexacetonide injection with oral diclofenac 50 mg TDS in patients with rotator cuff tendinitis. J Rheumatol 1990;17:1207-1210.
4. Petri M, Dobrow R, Neiman R, Whiting-O’Keefe O, Seaman WE. Randomized, double-blind, placebo-controlled study of the treatment of the painful shoulder. Arthritis Rheum 1987;30:1040-1045.
5. Green S, Buchbinder R, Glazier R, Forbes A. Interventions for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
6. Green S, Buchbinder R, Hetrick S. Physiotherapy interventions for shoulder pain (Cochrane Review). In: The Cochrane Library,Issue 2, 2004. Chichester, UK: John Wiley & Sons.
7. Hay EM, Thomas E, Paterson SM, Dziedzic K, Croft PR. A pragmatic randomised controlled trial of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Ann Rheum Dis 2003;62:394-399.
8. van der Heijden GJ, van der Windt DA, Kleijnen J, Koes BW, Bouter LM. Steroid injections for shoulder disorders: a systematic review of randomized clinical trials. Br J Gen Pract 1996;46:309-316.
9. American. Academy of Orthopedic Surgeons. AAOS clinical guideline on shoulder pain: support document. Rosemont, IL: AAOS, 2001. Available at: www.guideline.gov/summary/summary.aspx?doc_id=2998. Accessed on May 5, 2004.
10. Vecchio PC, Hazleman BL, King RH. A double-blind trial comparing subacromial methylprednisolone and ligno-caine in acute rotator cuff tendinitis. Br J Rheumatol 1993;32:743-745.
11. White RH, Paull DM, Fleming KW. Rotator cuff tendinitis: comparison of subacromial injection of a long acting corticosteroid versus indomethacin therapy. J Rheumatol 1986;13:608-613.
12. Berry H, Fernandes L, Bloom B, Clarke R, Hamilton EB. Clinical study comparing acupuncture, physiotherapy, injection and oral anti-inflammatory therapy in shoulder cuff lesions. Curr Med Res Opin 1980;7:121-126.
13. Blair B, Rokito AS, Cuomo F, Jarolem K, Zuckerman JD. Efficacy of injections of corticosteroids for subacromial impingement syndrome. J Bone Joint Surg Am 1996;78:1685-1689.
Evidence-based answers from the Family Physicians Inquiries Network
Should home apnea monitoring be recommended to prevent SIDS?
While home apnea monitoring may find an increased incidence of apnea and bradycardia in preterm infants compared with term infants, no association links these events with sudden infant death syndrome (SIDS). Apnea of prematurity is not a proven risk factor for SIDS. Since apnea of prematurity has not been shown to be a precursor to SIDS, home apnea monitoring for the purpose of preventing SIDS cannot be recommended (strength of recommendation [SOR]: B, based on a single prospective cohort study and multiple case-control studies). Neonates with significant neurologic or pulmonary disease may benefit from apnea monitoring (SOR: C, expert opinion).
Evidence summary
Multiple case-control studies have identified risk factors for SIDS, which are presented along (with odds ratios) in Table 1.1-6 None of these case-control studies found apnea of prematurity to be a risk factor for SIDS.
A prospective cohort study of 1079 infants monitored for cardiorespiratory events, the Collaborative Home Infant Monitor Evaluation (CHIME) study, demonstrated that prior to 43 weeks postconceptional age, preterm infants had a statistically significant greater risk of extreme events (apnea or bradycardia longer than 30 seconds) compared with healthy term infants (Table 2). After 43 weeks postconceptional age, there were no differences in incidence of apnea or bradycardia, comparing preterm and term infants. Neither preterm infants nor infants with apnea, bradycardia, or apparent life-threatening events had increased incidences of SIDS.7
Significant financial costs are associated with home monitoring. The average monthly cost is $300 to $400, not including physician fees. This would lead to an estimated annual cost of $24 million dollars if every infant <1500 grams in the United States were monitored.8
The psychological costs of home apnea monitoring have also been studied. One hundred and four parents of monitored and unmonitored infants were enrolled in a questionnaire study to determine emotional distress and family functioning. As is common among families in the postpartum period, all experienced increased stress. But parents of monitored infants, compared with parents of unmonitored infants, had an increased incidence of subjective depression (number needed to harm [NNH]=7) and hostility (NNH=12) at 2 weeks postpartum. Interestingly, at 1-year follow-up interviews, 83% of parents who had consistently used the monitor reported feeling more secure for having used it and 69% believed that monitor use had been helpful.9
Recommendations from others
The American Academy of Pediatrics (AAP) acknowledges that no established predictive or precursor relationship exists between prolonged apnea and SIDS, stating that the “prevention of SIDS is not an acceptable indication for home cardiorespiratory monitoring.” They issue a weak recommendation that home cardiorespiratory monitoring may be necessary for recurrent apnea, recurrent bradycardia, hypoxemia, chronic lung disease, and technology-dependent infants. Finally, they state that monitoring should be discontinued at 43 weeks postconceptional age or after cessation of extreme cardiorespiratory events, whichever occurs last. The AAP recommends proven practices such as supine sleeping position, a safe sleeping environment, and elimination of prenatal and postnatal exposure to tobacco smoke to decrease the risk of SIDS.8
TABLE 1
Risk factors for SIDS
| Risk factor | Odds ratio (95% CI) |
|---|---|
| Maternal factors | |
| Transport problems for prenatal care1 | 11.8 (2.7–52.7) |
| Education 12 years1 | 4.2 (1.1–15.5) |
| Prenatal smoke exposure3 | 3.7 (2.9–4.6) |
| <7 prenatal visits1 | 3.3 (1.1–9.8) |
| Unmarried3 | 2.0 (1.6–2.5) |
| Paternal factors | |
| Education≤ 12 years1 | 8.8 (1.1–70.8) |
| Parental factors | |
| Parental smoking4 | 5.19 (2.26–11.91) |
| Passive smoke | |
| exposure—all sources5 | 3.50 (1.81–6.75) |
| Maternal consumption of alcohol | |
| First trimester1 | 6.7 (2.2–20.1) |
| Any trimester1 | 3.4 (1.4–10.9) |
| Binge drinking—first trimester1 | 6.3 (1.8–22.8) |
| Binge drinking—any trimester1 | 3.9 (1.4–10.9) |
| Infant care | |
| <3 well-child visits1 | 13.8 (1.7–109.9) |
| Sleeping prone4 | 6.96 (1.51–31.97) |
| ≥2 layers of clothing1 | 3.9 (1.4–10.9) |
| Routine use of reused mattress2 | 3.1 (1.5–6.2) |
| Drug treatment in previous week4 | 2.33 (1.10–4.54) |
| Infant demographics | |
| Low birth weight (2500 g)3 | 3.6 (2.4–5.2) |
| Black3 | 2.5 (1.6–3.9) |
| Male gender6 | 1.47 (1.26–1.70) |
| Table adapted from multiple case-control studies. | |
Apnea monitors are not the answer
Matthew Gannons, MD
Department of Family and Community Medicine and Orthopaedic Surgery, Medical College of Wisconsin
An episode of SIDS is devastating to parents and leaves physicians questioning what more could have been done to prevent the tragedy. Apnea monitors, however, are not the answer. There are clearly downsides to apnea monitors and the added stress they place on parents. I do not think anyone would argue this would be a small price to pay if they helped to prevent SIDS; unfortunately, this is not the case.
I find it interesting that although apnea monitors add stress to parents, most would use them again and many felt they were helpful. This highlights the importance of education and clear communication with parents about SIDS and its prevention. Anecdotally, I have yet to have parents who did not stop using apnea monitor early because of the constant false alarms.
1. Iyasu S, Randall LL, Welty TK, et al. Risk factors for sudden infant death syndrome among northern plains Indians. JAMA 2002;288:2717-2723.
2. Tappin D, Brooke H, Ecob R, Gibson A. Used infant mat-tresses and sudden infant death syndrome in Scotland: case-control study. BMJ 2002;325:1007.-
3. Paris CA, Remler R, Daling JR. Risk factors for sudden infant death syndrome: changes associated with sleep position recommendations. J Pediatr 2001;139:771-777.
4. Brooke H, Gibson A, Tappin D, Brown H. Case-control study of sudden infant death syndrome in Scotland, 1992–1995. BMJ 1997;314:1516-1520.
5. Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, et al. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA 1995;273:795-798.
6. Millar WJ, Hill GB. Prevalence of and risk factors for sudden infant death syndrome in Canada. CMAJ 1993;149:629-635.
7. Ramanathan, R, Corwin MJ, Hunt CE, et al. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA 2001;285:2199-2207.
8. Committee on Fetus and Newborn. American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003;111:914-917.
9. Abendroth D, Moser DK, Dracup K, Doering LV. Do apnea monitors decrease emotional distress in parents of infants at high risk for cardiopulmonary arrest? J Pediatr Health Care 1999;13:50-57.
While home apnea monitoring may find an increased incidence of apnea and bradycardia in preterm infants compared with term infants, no association links these events with sudden infant death syndrome (SIDS). Apnea of prematurity is not a proven risk factor for SIDS. Since apnea of prematurity has not been shown to be a precursor to SIDS, home apnea monitoring for the purpose of preventing SIDS cannot be recommended (strength of recommendation [SOR]: B, based on a single prospective cohort study and multiple case-control studies). Neonates with significant neurologic or pulmonary disease may benefit from apnea monitoring (SOR: C, expert opinion).
Evidence summary
Multiple case-control studies have identified risk factors for SIDS, which are presented along (with odds ratios) in Table 1.1-6 None of these case-control studies found apnea of prematurity to be a risk factor for SIDS.
A prospective cohort study of 1079 infants monitored for cardiorespiratory events, the Collaborative Home Infant Monitor Evaluation (CHIME) study, demonstrated that prior to 43 weeks postconceptional age, preterm infants had a statistically significant greater risk of extreme events (apnea or bradycardia longer than 30 seconds) compared with healthy term infants (Table 2). After 43 weeks postconceptional age, there were no differences in incidence of apnea or bradycardia, comparing preterm and term infants. Neither preterm infants nor infants with apnea, bradycardia, or apparent life-threatening events had increased incidences of SIDS.7
Significant financial costs are associated with home monitoring. The average monthly cost is $300 to $400, not including physician fees. This would lead to an estimated annual cost of $24 million dollars if every infant <1500 grams in the United States were monitored.8
The psychological costs of home apnea monitoring have also been studied. One hundred and four parents of monitored and unmonitored infants were enrolled in a questionnaire study to determine emotional distress and family functioning. As is common among families in the postpartum period, all experienced increased stress. But parents of monitored infants, compared with parents of unmonitored infants, had an increased incidence of subjective depression (number needed to harm [NNH]=7) and hostility (NNH=12) at 2 weeks postpartum. Interestingly, at 1-year follow-up interviews, 83% of parents who had consistently used the monitor reported feeling more secure for having used it and 69% believed that monitor use had been helpful.9
Recommendations from others
The American Academy of Pediatrics (AAP) acknowledges that no established predictive or precursor relationship exists between prolonged apnea and SIDS, stating that the “prevention of SIDS is not an acceptable indication for home cardiorespiratory monitoring.” They issue a weak recommendation that home cardiorespiratory monitoring may be necessary for recurrent apnea, recurrent bradycardia, hypoxemia, chronic lung disease, and technology-dependent infants. Finally, they state that monitoring should be discontinued at 43 weeks postconceptional age or after cessation of extreme cardiorespiratory events, whichever occurs last. The AAP recommends proven practices such as supine sleeping position, a safe sleeping environment, and elimination of prenatal and postnatal exposure to tobacco smoke to decrease the risk of SIDS.8
TABLE 1
Risk factors for SIDS
| Risk factor | Odds ratio (95% CI) |
|---|---|
| Maternal factors | |
| Transport problems for prenatal care1 | 11.8 (2.7–52.7) |
| Education 12 years1 | 4.2 (1.1–15.5) |
| Prenatal smoke exposure3 | 3.7 (2.9–4.6) |
| <7 prenatal visits1 | 3.3 (1.1–9.8) |
| Unmarried3 | 2.0 (1.6–2.5) |
| Paternal factors | |
| Education≤ 12 years1 | 8.8 (1.1–70.8) |
| Parental factors | |
| Parental smoking4 | 5.19 (2.26–11.91) |
| Passive smoke | |
| exposure—all sources5 | 3.50 (1.81–6.75) |
| Maternal consumption of alcohol | |
| First trimester1 | 6.7 (2.2–20.1) |
| Any trimester1 | 3.4 (1.4–10.9) |
| Binge drinking—first trimester1 | 6.3 (1.8–22.8) |
| Binge drinking—any trimester1 | 3.9 (1.4–10.9) |
| Infant care | |
| <3 well-child visits1 | 13.8 (1.7–109.9) |
| Sleeping prone4 | 6.96 (1.51–31.97) |
| ≥2 layers of clothing1 | 3.9 (1.4–10.9) |
| Routine use of reused mattress2 | 3.1 (1.5–6.2) |
| Drug treatment in previous week4 | 2.33 (1.10–4.54) |
| Infant demographics | |
| Low birth weight (2500 g)3 | 3.6 (2.4–5.2) |
| Black3 | 2.5 (1.6–3.9) |
| Male gender6 | 1.47 (1.26–1.70) |
| Table adapted from multiple case-control studies. | |
Apnea monitors are not the answer
Matthew Gannons, MD
Department of Family and Community Medicine and Orthopaedic Surgery, Medical College of Wisconsin
An episode of SIDS is devastating to parents and leaves physicians questioning what more could have been done to prevent the tragedy. Apnea monitors, however, are not the answer. There are clearly downsides to apnea monitors and the added stress they place on parents. I do not think anyone would argue this would be a small price to pay if they helped to prevent SIDS; unfortunately, this is not the case.
I find it interesting that although apnea monitors add stress to parents, most would use them again and many felt they were helpful. This highlights the importance of education and clear communication with parents about SIDS and its prevention. Anecdotally, I have yet to have parents who did not stop using apnea monitor early because of the constant false alarms.
While home apnea monitoring may find an increased incidence of apnea and bradycardia in preterm infants compared with term infants, no association links these events with sudden infant death syndrome (SIDS). Apnea of prematurity is not a proven risk factor for SIDS. Since apnea of prematurity has not been shown to be a precursor to SIDS, home apnea monitoring for the purpose of preventing SIDS cannot be recommended (strength of recommendation [SOR]: B, based on a single prospective cohort study and multiple case-control studies). Neonates with significant neurologic or pulmonary disease may benefit from apnea monitoring (SOR: C, expert opinion).
Evidence summary
Multiple case-control studies have identified risk factors for SIDS, which are presented along (with odds ratios) in Table 1.1-6 None of these case-control studies found apnea of prematurity to be a risk factor for SIDS.
A prospective cohort study of 1079 infants monitored for cardiorespiratory events, the Collaborative Home Infant Monitor Evaluation (CHIME) study, demonstrated that prior to 43 weeks postconceptional age, preterm infants had a statistically significant greater risk of extreme events (apnea or bradycardia longer than 30 seconds) compared with healthy term infants (Table 2). After 43 weeks postconceptional age, there were no differences in incidence of apnea or bradycardia, comparing preterm and term infants. Neither preterm infants nor infants with apnea, bradycardia, or apparent life-threatening events had increased incidences of SIDS.7
Significant financial costs are associated with home monitoring. The average monthly cost is $300 to $400, not including physician fees. This would lead to an estimated annual cost of $24 million dollars if every infant <1500 grams in the United States were monitored.8
The psychological costs of home apnea monitoring have also been studied. One hundred and four parents of monitored and unmonitored infants were enrolled in a questionnaire study to determine emotional distress and family functioning. As is common among families in the postpartum period, all experienced increased stress. But parents of monitored infants, compared with parents of unmonitored infants, had an increased incidence of subjective depression (number needed to harm [NNH]=7) and hostility (NNH=12) at 2 weeks postpartum. Interestingly, at 1-year follow-up interviews, 83% of parents who had consistently used the monitor reported feeling more secure for having used it and 69% believed that monitor use had been helpful.9
Recommendations from others
The American Academy of Pediatrics (AAP) acknowledges that no established predictive or precursor relationship exists between prolonged apnea and SIDS, stating that the “prevention of SIDS is not an acceptable indication for home cardiorespiratory monitoring.” They issue a weak recommendation that home cardiorespiratory monitoring may be necessary for recurrent apnea, recurrent bradycardia, hypoxemia, chronic lung disease, and technology-dependent infants. Finally, they state that monitoring should be discontinued at 43 weeks postconceptional age or after cessation of extreme cardiorespiratory events, whichever occurs last. The AAP recommends proven practices such as supine sleeping position, a safe sleeping environment, and elimination of prenatal and postnatal exposure to tobacco smoke to decrease the risk of SIDS.8
TABLE 1
Risk factors for SIDS
| Risk factor | Odds ratio (95% CI) |
|---|---|
| Maternal factors | |
| Transport problems for prenatal care1 | 11.8 (2.7–52.7) |
| Education 12 years1 | 4.2 (1.1–15.5) |
| Prenatal smoke exposure3 | 3.7 (2.9–4.6) |
| <7 prenatal visits1 | 3.3 (1.1–9.8) |
| Unmarried3 | 2.0 (1.6–2.5) |
| Paternal factors | |
| Education≤ 12 years1 | 8.8 (1.1–70.8) |
| Parental factors | |
| Parental smoking4 | 5.19 (2.26–11.91) |
| Passive smoke | |
| exposure—all sources5 | 3.50 (1.81–6.75) |
| Maternal consumption of alcohol | |
| First trimester1 | 6.7 (2.2–20.1) |
| Any trimester1 | 3.4 (1.4–10.9) |
| Binge drinking—first trimester1 | 6.3 (1.8–22.8) |
| Binge drinking—any trimester1 | 3.9 (1.4–10.9) |
| Infant care | |
| <3 well-child visits1 | 13.8 (1.7–109.9) |
| Sleeping prone4 | 6.96 (1.51–31.97) |
| ≥2 layers of clothing1 | 3.9 (1.4–10.9) |
| Routine use of reused mattress2 | 3.1 (1.5–6.2) |
| Drug treatment in previous week4 | 2.33 (1.10–4.54) |
| Infant demographics | |
| Low birth weight (2500 g)3 | 3.6 (2.4–5.2) |
| Black3 | 2.5 (1.6–3.9) |
| Male gender6 | 1.47 (1.26–1.70) |
| Table adapted from multiple case-control studies. | |
Apnea monitors are not the answer
Matthew Gannons, MD
Department of Family and Community Medicine and Orthopaedic Surgery, Medical College of Wisconsin
An episode of SIDS is devastating to parents and leaves physicians questioning what more could have been done to prevent the tragedy. Apnea monitors, however, are not the answer. There are clearly downsides to apnea monitors and the added stress they place on parents. I do not think anyone would argue this would be a small price to pay if they helped to prevent SIDS; unfortunately, this is not the case.
I find it interesting that although apnea monitors add stress to parents, most would use them again and many felt they were helpful. This highlights the importance of education and clear communication with parents about SIDS and its prevention. Anecdotally, I have yet to have parents who did not stop using apnea monitor early because of the constant false alarms.
1. Iyasu S, Randall LL, Welty TK, et al. Risk factors for sudden infant death syndrome among northern plains Indians. JAMA 2002;288:2717-2723.
2. Tappin D, Brooke H, Ecob R, Gibson A. Used infant mat-tresses and sudden infant death syndrome in Scotland: case-control study. BMJ 2002;325:1007.-
3. Paris CA, Remler R, Daling JR. Risk factors for sudden infant death syndrome: changes associated with sleep position recommendations. J Pediatr 2001;139:771-777.
4. Brooke H, Gibson A, Tappin D, Brown H. Case-control study of sudden infant death syndrome in Scotland, 1992–1995. BMJ 1997;314:1516-1520.
5. Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, et al. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA 1995;273:795-798.
6. Millar WJ, Hill GB. Prevalence of and risk factors for sudden infant death syndrome in Canada. CMAJ 1993;149:629-635.
7. Ramanathan, R, Corwin MJ, Hunt CE, et al. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA 2001;285:2199-2207.
8. Committee on Fetus and Newborn. American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003;111:914-917.
9. Abendroth D, Moser DK, Dracup K, Doering LV. Do apnea monitors decrease emotional distress in parents of infants at high risk for cardiopulmonary arrest? J Pediatr Health Care 1999;13:50-57.
1. Iyasu S, Randall LL, Welty TK, et al. Risk factors for sudden infant death syndrome among northern plains Indians. JAMA 2002;288:2717-2723.
2. Tappin D, Brooke H, Ecob R, Gibson A. Used infant mat-tresses and sudden infant death syndrome in Scotland: case-control study. BMJ 2002;325:1007.-
3. Paris CA, Remler R, Daling JR. Risk factors for sudden infant death syndrome: changes associated with sleep position recommendations. J Pediatr 2001;139:771-777.
4. Brooke H, Gibson A, Tappin D, Brown H. Case-control study of sudden infant death syndrome in Scotland, 1992–1995. BMJ 1997;314:1516-1520.
5. Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, et al. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. JAMA 1995;273:795-798.
6. Millar WJ, Hill GB. Prevalence of and risk factors for sudden infant death syndrome in Canada. CMAJ 1993;149:629-635.
7. Ramanathan, R, Corwin MJ, Hunt CE, et al. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA 2001;285:2199-2207.
8. Committee on Fetus and Newborn. American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003;111:914-917.
9. Abendroth D, Moser DK, Dracup K, Doering LV. Do apnea monitors decrease emotional distress in parents of infants at high risk for cardiopulmonary arrest? J Pediatr Health Care 1999;13:50-57.
Evidence-based answers from the Family Physicians Inquiries Network
Does a short symptom checklist accurately diagnose ADHD?
Several abbreviated checklists perform well in distinguishing children with attention deficit/hyperactivity disorder (ADHD) from those without ADHD under ideal conditions and in research settings. While many guidelines and experts recommend using these checklists as an efficient method to collect data from multiple sources (strength of recommendation: B, based on extrapolation from cohort studies to define test characteristics and consensus opinion), experts point out the subjective nature of responses on behavior rating scales, and the limitations in using checklists as the sole source of information.
The Swanson, Nolan, and Pelham (SNAP) checklist from the Diagnostic and Statistical Manual of Mental Disorders, revised 3rd edition (DSM-III-R) has been shown to have a sensitivity and specificity in excess of 94% to distinguish hyperactive, inattentive, and impulsive children with ADHD from those without ADHD. This was based on criteria in the DSM-III-R. The DSM-IV SNAP checklist (available at www.adhd.net/snap-iv-form.pdf; scoring at www.adhd.net/snap-iv-instructions.pdf), based on the newer diagnostic criteria, has not been adequately evaluated. The ADHD Rating Scale-IV (in DuPaul et al, ADHD Rating Scale IV—Checklists, Norms, and Clinical Interpretations, available from Guilford Press) and the ADD-H Comprehensive Teacher/Parent Rating Scale (ACTeRS; available from MetriTech, Inc at www.metritech.com) are useful for their brevity, but they do not perform as well in differentiating children with ADHD from those without ADHD.
Evidence summary
A variety of brief ADHD-specific rating scales are used for both parent and teacher assessment of child behavior. Rating scales are generally evaluated to establish mean scores for affected and unaffected children. Many scales publish such normative data in commercially available manuals. Some scales have been evaluated by 1 or more independent studies to compare children with and without ADHD. Rating scales have not been evaluated as a sole tool for the diagnosis of ADHD.
The test characteristics of a particular scale depend on the cut points for a positive or negative test. The usefulness of psychological tests in discriminating normal from abnormal behavior is often reported as “effect size.” The effect size is the difference in mean scores between 2 populations divided by an estimate of the individual standard deviation.1 An effect size of 4.0 means that abnormal subjects and normal controls are separated 4 standard deviations and thus almost completely separated. An effect size of 1.0 shows significant overlap between the 2 populations. An effect size of 4.0 is roughly equivalent to a sensitivity and specificity of 97%. An effect size of 1.0 is roughly equal to a sensitivity and specificity of 71%.
Table 1 outlines the characteristics and effect size of several available brief ADHD-specific checklists.2-4,6,11-13 Typically, the gold standard was a clinical diagnostic interview, usually conducted by a clinical psychologist, as well as supporting data from schools and parents.
TABLE
Descriptive characteristics of abbreviated symptom checklists for ADHD
| Effect size | ||||||
|---|---|---|---|---|---|---|
| Scale | Minutes | #Items | Age | Hyperactivity | Inattention | Impulsivity |
| ACTeRS Parent Version | 5–10 | 25 | 5–12 | 1.5 | 2.0 | NA |
| ACTeRS Teacher Version | 5–10 | 24 | 5–12 | NA | NA | NA |
| DSM-IV SNAP | 5–10 | 40 | 6–12 | NA | NA | NA |
| DSM-III–R SNAP | 5–10 | 38 | 6–12 | 3.1–5.1 | 3.5–4.2 | 4.0–5.5 |
| ADHD Rating Scale-IV | 5 | 18 | 5–18 | 1.1 | 1.2 | 1.1 |
| Conners RatingScale,Revised (1997, Short Version)11,12,13 | 5–10 | 27 | 3–17 | NA | NA | NA |
| Numbers reported in ranges indicate multiple studies. | ||||||
| ACTeRS, ADD-H Comprehensive Teacher Rating Scales; DSM, Diagnostic and Statistical Manual of Mental Disorders; SNAP, | ||||||
| Swanson, Nolan, and Pelham; ADHD, attention deficit/hyperactivity disorder; NA, not available. | ||||||
Recommendations from others
The American Academy of Pediatrics states that the use of ADHD-specific checklists is a clinical option when evaluating children for ADHD. They caution that the ADHD scales may function less well in clinicians’ offices than suggested by reported effect size and, in addition, rating scales are subject to bias and may convey a false sense of validity. They also state that it is not known if these scales provide additional information beyond a careful clinical assessment.7
The Institute for Clinical Systems Improvement recommends use of at least 1 ADHD-specific rating scale to be administered to parents and teachers. This information should be used as part of the overall historical database for the child and should not be used as the sole criteria for diagnosis of ADHD.8
Many sources agree that ADHD-specific rating scales allow a rapid and consistent collection of information from multiple sources. However, the information they provide is necessary, but not sufficient, to make a definitive diagnosis of ADHD. In addition to assisting in diagnosis, checklists can be helpful in monitoring treatment changes once a diagnosis has been established.
Gather data from multiple sources
John Hill, MD
Rose Family Medicine Residency/University of Colorado Health Sciences Center, Denver
Sorting out children with ADHD, bipolar disorder, or learning disabilities from lively or distractible children is not a simple matter. Often the objective rating scales miss the more passive, less disruptive, inattentive ADHD children while overdiagnosing high-energy children as having ADHD. Perhaps the new DSM-IV SNAP will provide the objective sensitivity and specificity we desire as clinicians. However, this checklist requires further evaluation.
Information from ACTeRS scales has helped me treat these children, but I prefer to have both parents, if possible, independently complete the form. Obtaining scales from a Special Education teacher or psychologist, when available, in addition to the primary classroom teacher, is invaluable. Still, it often comes down to how a child responds to medication. Proceed with caution if there is a family history of bipolar disorder, as these children often do worse on stimulants and are better treated by our colleagues in child psychiatry.
1. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Orlando, Fla: Academic Press; 1985.
2. Diagnosis of Attention-Deficit/Hyperactivity Disorder. Technical Review #3. Rockville, Md: Agency for Health Care Policy and Research; 1999 August. Available at: www.ahrq.gov/clinic/epcsums/adhdsutr.htm. Accessed on March 31, 2004.
3. DuPaul GJ. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press, 1998.
4. Atkins MS, Pelham WE, Licht MH. A comparison of objective classroom measures and teacher ratings of Attention Deficit Disorder. J Abnorm Child Psychol 1985;13:155-167.
5. Tarnowski KJ, Prinz RJ, Nay SM. Comparative analysis of attentional deficits in hyperactive and learning-disabled children. J Abnorm Psychol 1986;95:341-345.
6. Ullmann RK, Sleator EK, Sprague RL, MetriTech Staff. ACTeRS Teacher and Parent Forms Manual. Champaign, Ill: MetriTech; 1997.
7. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder Pediatrics 2000;105:1158-1170.
8. Diagnosis and Management of Attention Deficit Hyperactivity Disorder in Primary Care for School Age Children and Adolescents. Bloomington, Minn: Institute for Clinical Systems Improvement; 2003. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=163. Accessed on March 31, 2004.
9. Dulcan M. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 1997;36(10 Suppl):85S-121S.
10. Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs. American Medical Association. JAMA 1998;279:1100-1107.
11. Conners CK, Parker JD, Sitarenios G, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:257-268.
12. Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:279-291.
13. Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for the assessment of adolescent psychopathology: factor structure, reliability, validity and diagnostic sensitivity. J Abnorm Child Psychol 1997;25:487-497.
Several abbreviated checklists perform well in distinguishing children with attention deficit/hyperactivity disorder (ADHD) from those without ADHD under ideal conditions and in research settings. While many guidelines and experts recommend using these checklists as an efficient method to collect data from multiple sources (strength of recommendation: B, based on extrapolation from cohort studies to define test characteristics and consensus opinion), experts point out the subjective nature of responses on behavior rating scales, and the limitations in using checklists as the sole source of information.
The Swanson, Nolan, and Pelham (SNAP) checklist from the Diagnostic and Statistical Manual of Mental Disorders, revised 3rd edition (DSM-III-R) has been shown to have a sensitivity and specificity in excess of 94% to distinguish hyperactive, inattentive, and impulsive children with ADHD from those without ADHD. This was based on criteria in the DSM-III-R. The DSM-IV SNAP checklist (available at www.adhd.net/snap-iv-form.pdf; scoring at www.adhd.net/snap-iv-instructions.pdf), based on the newer diagnostic criteria, has not been adequately evaluated. The ADHD Rating Scale-IV (in DuPaul et al, ADHD Rating Scale IV—Checklists, Norms, and Clinical Interpretations, available from Guilford Press) and the ADD-H Comprehensive Teacher/Parent Rating Scale (ACTeRS; available from MetriTech, Inc at www.metritech.com) are useful for their brevity, but they do not perform as well in differentiating children with ADHD from those without ADHD.
Evidence summary
A variety of brief ADHD-specific rating scales are used for both parent and teacher assessment of child behavior. Rating scales are generally evaluated to establish mean scores for affected and unaffected children. Many scales publish such normative data in commercially available manuals. Some scales have been evaluated by 1 or more independent studies to compare children with and without ADHD. Rating scales have not been evaluated as a sole tool for the diagnosis of ADHD.
The test characteristics of a particular scale depend on the cut points for a positive or negative test. The usefulness of psychological tests in discriminating normal from abnormal behavior is often reported as “effect size.” The effect size is the difference in mean scores between 2 populations divided by an estimate of the individual standard deviation.1 An effect size of 4.0 means that abnormal subjects and normal controls are separated 4 standard deviations and thus almost completely separated. An effect size of 1.0 shows significant overlap between the 2 populations. An effect size of 4.0 is roughly equivalent to a sensitivity and specificity of 97%. An effect size of 1.0 is roughly equal to a sensitivity and specificity of 71%.
Table 1 outlines the characteristics and effect size of several available brief ADHD-specific checklists.2-4,6,11-13 Typically, the gold standard was a clinical diagnostic interview, usually conducted by a clinical psychologist, as well as supporting data from schools and parents.
TABLE
Descriptive characteristics of abbreviated symptom checklists for ADHD
| Effect size | ||||||
|---|---|---|---|---|---|---|
| Scale | Minutes | #Items | Age | Hyperactivity | Inattention | Impulsivity |
| ACTeRS Parent Version | 5–10 | 25 | 5–12 | 1.5 | 2.0 | NA |
| ACTeRS Teacher Version | 5–10 | 24 | 5–12 | NA | NA | NA |
| DSM-IV SNAP | 5–10 | 40 | 6–12 | NA | NA | NA |
| DSM-III–R SNAP | 5–10 | 38 | 6–12 | 3.1–5.1 | 3.5–4.2 | 4.0–5.5 |
| ADHD Rating Scale-IV | 5 | 18 | 5–18 | 1.1 | 1.2 | 1.1 |
| Conners RatingScale,Revised (1997, Short Version)11,12,13 | 5–10 | 27 | 3–17 | NA | NA | NA |
| Numbers reported in ranges indicate multiple studies. | ||||||
| ACTeRS, ADD-H Comprehensive Teacher Rating Scales; DSM, Diagnostic and Statistical Manual of Mental Disorders; SNAP, | ||||||
| Swanson, Nolan, and Pelham; ADHD, attention deficit/hyperactivity disorder; NA, not available. | ||||||
Recommendations from others
The American Academy of Pediatrics states that the use of ADHD-specific checklists is a clinical option when evaluating children for ADHD. They caution that the ADHD scales may function less well in clinicians’ offices than suggested by reported effect size and, in addition, rating scales are subject to bias and may convey a false sense of validity. They also state that it is not known if these scales provide additional information beyond a careful clinical assessment.7
The Institute for Clinical Systems Improvement recommends use of at least 1 ADHD-specific rating scale to be administered to parents and teachers. This information should be used as part of the overall historical database for the child and should not be used as the sole criteria for diagnosis of ADHD.8
Many sources agree that ADHD-specific rating scales allow a rapid and consistent collection of information from multiple sources. However, the information they provide is necessary, but not sufficient, to make a definitive diagnosis of ADHD. In addition to assisting in diagnosis, checklists can be helpful in monitoring treatment changes once a diagnosis has been established.
Gather data from multiple sources
John Hill, MD
Rose Family Medicine Residency/University of Colorado Health Sciences Center, Denver
Sorting out children with ADHD, bipolar disorder, or learning disabilities from lively or distractible children is not a simple matter. Often the objective rating scales miss the more passive, less disruptive, inattentive ADHD children while overdiagnosing high-energy children as having ADHD. Perhaps the new DSM-IV SNAP will provide the objective sensitivity and specificity we desire as clinicians. However, this checklist requires further evaluation.
Information from ACTeRS scales has helped me treat these children, but I prefer to have both parents, if possible, independently complete the form. Obtaining scales from a Special Education teacher or psychologist, when available, in addition to the primary classroom teacher, is invaluable. Still, it often comes down to how a child responds to medication. Proceed with caution if there is a family history of bipolar disorder, as these children often do worse on stimulants and are better treated by our colleagues in child psychiatry.
Several abbreviated checklists perform well in distinguishing children with attention deficit/hyperactivity disorder (ADHD) from those without ADHD under ideal conditions and in research settings. While many guidelines and experts recommend using these checklists as an efficient method to collect data from multiple sources (strength of recommendation: B, based on extrapolation from cohort studies to define test characteristics and consensus opinion), experts point out the subjective nature of responses on behavior rating scales, and the limitations in using checklists as the sole source of information.
The Swanson, Nolan, and Pelham (SNAP) checklist from the Diagnostic and Statistical Manual of Mental Disorders, revised 3rd edition (DSM-III-R) has been shown to have a sensitivity and specificity in excess of 94% to distinguish hyperactive, inattentive, and impulsive children with ADHD from those without ADHD. This was based on criteria in the DSM-III-R. The DSM-IV SNAP checklist (available at www.adhd.net/snap-iv-form.pdf; scoring at www.adhd.net/snap-iv-instructions.pdf), based on the newer diagnostic criteria, has not been adequately evaluated. The ADHD Rating Scale-IV (in DuPaul et al, ADHD Rating Scale IV—Checklists, Norms, and Clinical Interpretations, available from Guilford Press) and the ADD-H Comprehensive Teacher/Parent Rating Scale (ACTeRS; available from MetriTech, Inc at www.metritech.com) are useful for their brevity, but they do not perform as well in differentiating children with ADHD from those without ADHD.
Evidence summary
A variety of brief ADHD-specific rating scales are used for both parent and teacher assessment of child behavior. Rating scales are generally evaluated to establish mean scores for affected and unaffected children. Many scales publish such normative data in commercially available manuals. Some scales have been evaluated by 1 or more independent studies to compare children with and without ADHD. Rating scales have not been evaluated as a sole tool for the diagnosis of ADHD.
The test characteristics of a particular scale depend on the cut points for a positive or negative test. The usefulness of psychological tests in discriminating normal from abnormal behavior is often reported as “effect size.” The effect size is the difference in mean scores between 2 populations divided by an estimate of the individual standard deviation.1 An effect size of 4.0 means that abnormal subjects and normal controls are separated 4 standard deviations and thus almost completely separated. An effect size of 1.0 shows significant overlap between the 2 populations. An effect size of 4.0 is roughly equivalent to a sensitivity and specificity of 97%. An effect size of 1.0 is roughly equal to a sensitivity and specificity of 71%.
Table 1 outlines the characteristics and effect size of several available brief ADHD-specific checklists.2-4,6,11-13 Typically, the gold standard was a clinical diagnostic interview, usually conducted by a clinical psychologist, as well as supporting data from schools and parents.
TABLE
Descriptive characteristics of abbreviated symptom checklists for ADHD
| Effect size | ||||||
|---|---|---|---|---|---|---|
| Scale | Minutes | #Items | Age | Hyperactivity | Inattention | Impulsivity |
| ACTeRS Parent Version | 5–10 | 25 | 5–12 | 1.5 | 2.0 | NA |
| ACTeRS Teacher Version | 5–10 | 24 | 5–12 | NA | NA | NA |
| DSM-IV SNAP | 5–10 | 40 | 6–12 | NA | NA | NA |
| DSM-III–R SNAP | 5–10 | 38 | 6–12 | 3.1–5.1 | 3.5–4.2 | 4.0–5.5 |
| ADHD Rating Scale-IV | 5 | 18 | 5–18 | 1.1 | 1.2 | 1.1 |
| Conners RatingScale,Revised (1997, Short Version)11,12,13 | 5–10 | 27 | 3–17 | NA | NA | NA |
| Numbers reported in ranges indicate multiple studies. | ||||||
| ACTeRS, ADD-H Comprehensive Teacher Rating Scales; DSM, Diagnostic and Statistical Manual of Mental Disorders; SNAP, | ||||||
| Swanson, Nolan, and Pelham; ADHD, attention deficit/hyperactivity disorder; NA, not available. | ||||||
Recommendations from others
The American Academy of Pediatrics states that the use of ADHD-specific checklists is a clinical option when evaluating children for ADHD. They caution that the ADHD scales may function less well in clinicians’ offices than suggested by reported effect size and, in addition, rating scales are subject to bias and may convey a false sense of validity. They also state that it is not known if these scales provide additional information beyond a careful clinical assessment.7
The Institute for Clinical Systems Improvement recommends use of at least 1 ADHD-specific rating scale to be administered to parents and teachers. This information should be used as part of the overall historical database for the child and should not be used as the sole criteria for diagnosis of ADHD.8
Many sources agree that ADHD-specific rating scales allow a rapid and consistent collection of information from multiple sources. However, the information they provide is necessary, but not sufficient, to make a definitive diagnosis of ADHD. In addition to assisting in diagnosis, checklists can be helpful in monitoring treatment changes once a diagnosis has been established.
Gather data from multiple sources
John Hill, MD
Rose Family Medicine Residency/University of Colorado Health Sciences Center, Denver
Sorting out children with ADHD, bipolar disorder, or learning disabilities from lively or distractible children is not a simple matter. Often the objective rating scales miss the more passive, less disruptive, inattentive ADHD children while overdiagnosing high-energy children as having ADHD. Perhaps the new DSM-IV SNAP will provide the objective sensitivity and specificity we desire as clinicians. However, this checklist requires further evaluation.
Information from ACTeRS scales has helped me treat these children, but I prefer to have both parents, if possible, independently complete the form. Obtaining scales from a Special Education teacher or psychologist, when available, in addition to the primary classroom teacher, is invaluable. Still, it often comes down to how a child responds to medication. Proceed with caution if there is a family history of bipolar disorder, as these children often do worse on stimulants and are better treated by our colleagues in child psychiatry.
1. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Orlando, Fla: Academic Press; 1985.
2. Diagnosis of Attention-Deficit/Hyperactivity Disorder. Technical Review #3. Rockville, Md: Agency for Health Care Policy and Research; 1999 August. Available at: www.ahrq.gov/clinic/epcsums/adhdsutr.htm. Accessed on March 31, 2004.
3. DuPaul GJ. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press, 1998.
4. Atkins MS, Pelham WE, Licht MH. A comparison of objective classroom measures and teacher ratings of Attention Deficit Disorder. J Abnorm Child Psychol 1985;13:155-167.
5. Tarnowski KJ, Prinz RJ, Nay SM. Comparative analysis of attentional deficits in hyperactive and learning-disabled children. J Abnorm Psychol 1986;95:341-345.
6. Ullmann RK, Sleator EK, Sprague RL, MetriTech Staff. ACTeRS Teacher and Parent Forms Manual. Champaign, Ill: MetriTech; 1997.
7. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder Pediatrics 2000;105:1158-1170.
8. Diagnosis and Management of Attention Deficit Hyperactivity Disorder in Primary Care for School Age Children and Adolescents. Bloomington, Minn: Institute for Clinical Systems Improvement; 2003. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=163. Accessed on March 31, 2004.
9. Dulcan M. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 1997;36(10 Suppl):85S-121S.
10. Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs. American Medical Association. JAMA 1998;279:1100-1107.
11. Conners CK, Parker JD, Sitarenios G, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:257-268.
12. Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:279-291.
13. Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for the assessment of adolescent psychopathology: factor structure, reliability, validity and diagnostic sensitivity. J Abnorm Child Psychol 1997;25:487-497.
1. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Orlando, Fla: Academic Press; 1985.
2. Diagnosis of Attention-Deficit/Hyperactivity Disorder. Technical Review #3. Rockville, Md: Agency for Health Care Policy and Research; 1999 August. Available at: www.ahrq.gov/clinic/epcsums/adhdsutr.htm. Accessed on March 31, 2004.
3. DuPaul GJ. ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York, NY: Guilford Press, 1998.
4. Atkins MS, Pelham WE, Licht MH. A comparison of objective classroom measures and teacher ratings of Attention Deficit Disorder. J Abnorm Child Psychol 1985;13:155-167.
5. Tarnowski KJ, Prinz RJ, Nay SM. Comparative analysis of attentional deficits in hyperactive and learning-disabled children. J Abnorm Psychol 1986;95:341-345.
6. Ullmann RK, Sleator EK, Sprague RL, MetriTech Staff. ACTeRS Teacher and Parent Forms Manual. Champaign, Ill: MetriTech; 1997.
7. Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder Pediatrics 2000;105:1158-1170.
8. Diagnosis and Management of Attention Deficit Hyperactivity Disorder in Primary Care for School Age Children and Adolescents. Bloomington, Minn: Institute for Clinical Systems Improvement; 2003. Available at: www.icsi.org/knowledge/detail.asp?catID=29&itemID=163. Accessed on March 31, 2004.
9. Dulcan M. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 1997;36(10 Suppl):85S-121S.
10. Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs. American Medical Association. JAMA 1998;279:1100-1107.
11. Conners CK, Parker JD, Sitarenios G, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:257-268.
12. Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998;26:279-291.
13. Conners CK, Wells KC, Parker JD, Sitarenios G, Diamond JM, Powell JW. A new self-report scale for the assessment of adolescent psychopathology: factor structure, reliability, validity and diagnostic sensitivity. J Abnorm Child Psychol 1997;25:487-497.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best way to treat patients with white-coat hypertension?
Evidence is conflicting regarding the risk of cardiovascular complications from white-coat hypertension. Some but not all studies show lower cardiovascular event rates for patients with white-coat hypertension compared with those with sustained hypertension (strength of recommendation [SOR]: B, cohort studies with conflicting results and methodological problems).
Little information is available about the use of antihypertensive medication for white-coat hypertension. In 1 small randomized trial, the difference in stroke incidence and cardiovascular complications between active treatment and placebo did not reach statistical significance (SOR: B, based on an underpowered randomized controlled trial). Some experts recommend that patients with white-coat hypertension should be evaluated for evidence of target organ injury and monitored for the development of sustained hypertension (SOR: C, expert opinion).
Evidence summary
A prospective cohort study compared cardiovascular events among patients with white-coat hypertension vs those with sustained hypertension. The study evaluated 479 patients with persistently elevated clinic systolic blood pressures of 140 to 180 mm Hg. Using 24-hour intraarterial ambulatory blood pressure monitoring (ABPM), they found that 126 patients had ambulatory blood pressures below 140/90 mm Hg (white-coat hypertension) while 353 patients maintained pressures above 140/90 mm Hg (sustained hypertension). On average, white-coat hypertension patients were younger than sustained hypertension patients (44 vs 52 years) but were otherwise similar. Over the next 9 years, patients with white-coat hypertension had significantly fewer cardiovascular events than patients with sustained hypertension (Table).1
Another prospective cohort study compared fatal and nonfatal cardiovascular event rates among patients who had white-coat hypertension, sustained hypertension, or were normotensive. Investigators performed 24-hour ABPM on 1187 patients who had clinic blood pressures over 140/90 on three visits. They found that 228 patients had white-coat hypertension, defined as mean ambulatory blood pressures below the 90th percentile of a normotensive population, and 959 patients had sustained hypertension. They followed these patients, along with 205 normotensive controls, for a mean of 3.2 years. Cardiovascular event rates did not differ significantly between normotensive and white-coat hypertension patients (P=.83; see Table), but the difference in event-free survival between the sustained hypertension group and both the white-coat hypertension and normotensive groups was highly significant (P=.002).2
In contrast, a recent 10-year longitudinal study of 146 normotensive people, 76 people with white-coat hypertension, and 344 with sustained hypertension showed that cardiovascular event rates were similar for patients with white-coat and sustained hypertension, and were significantly higher than in the normotensive group (P=.03 overall, P=.03 between white-coat hypertension and normotension and P=.01 between sustained hypertension and normotension).3
One randomized trial evaluated outcomes of antihypertensive therapy for white-coat hypertension for patients aged >60 years. Ninety-nine patients with white-coat hypertension were identified on the basis of systolic blood pressure greater than 160 mm Hg in clinic and normal 24-hour ABPM and were randomized to either place-bo or drug therapy. Active treatment did not significantly lower ambulatory blood pressure in white-coat hypertension, but it did reduce blood pressure measured in clinic. After a year, medication produced an absolute reduction in cardiovascular events of 8.6%, and in stroke of 4.2%. Neither result was statistically significant due to the small sample size.4
TABLE
Cohort studies of patients with white-coat hypertension
| Total number of events | |||||
|---|---|---|---|---|---|
| Patients | Outcome | NT | WCH | SH | P value |
| 479 patients, mean age of 641 | Cardiovascular events | N/A | 15 (11.9%) | 83 (23.5%) | P<.001 |
| 1392 patients, mean age of 512 | Cardiovascular events | 4 (1.9%) | 3 (1.3%) | 37 (5.3%) | WCH: NT vs P=.83 |
| WCH vs SH: P<.0001 | |||||
| 566 patients, mean age of 483 | Cardiovascular events | 10 (6.8%) | 14 (18.4%) | 56 (16.3%) | Overall P=.03 |
| NT vs WCH: P=.03 | |||||
| NT vs SH: P=.01 | |||||
| NT, normotensive; WCH, white-coat hypertension; SH, sustained hypertension | |||||
Recommendations from others
The American College of Cardiology and American Academy of Family Physicians have made no specific recommendations about white-coat hypertension. The Blood Pressure Monitoring Task Force V concluded that a significant number of white-coat hypertension patients become truly hypertensive over years of follow-up.5
Experts agree that patients with white-coat hypertension should be indefinitely monitored for the development of sustained hypertension.6 Treatment is not needed unless the patient has sustained hypertension, evidence of cardiovascular disease, or signs of target organ injury.7,8 Typically, expert opinion recommends confirming the diagnosis of white-coat hypertension with home blood pressure records or ambulatory blood pressure monitoring.
White-coat hypertension represents one point along the continuum of hypertension
Mark B. Stephens, MD, MS
Uniformed Services University, Bethesda, Md
Unfortunately, the best available clinical evidence provides an unfulfilling answer to the question posed by this Clinical Inquiry. It requires inductive reasoning and logic to derive a treatment plan from the evidence presented. Perhaps it is because the diagnosis of white-coat hypertension remains poorly defined and clinically elusive.
Nevertheless, application of the simple principle of “where there’s smoke, there’s fire” fits best here. Clinicians should be aware that white-coat hypertension represents one point along the continuum of hypertensive disease. When diagnosed, patients with white-coat hypertension should at a minimum be followed for associated morbidities and treated when systemic hypertension is identified.
1. Khattar R, Senior R, Lahiri A. Cardiovascular outcomes in white coat versus sustained mild hypertension: a tenyear follow-up study. Circulation 1998;98:1892-1897.
2. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure, an independent predictor of prognosis in essential hypertension. Hypertension 1994;24:793-801.
3. Gustavsen PH, Hoegholm A, Bang L, Kristensen KS. White coat hypertension is a cardiovascular risk factor: a 10-year follow-up study. J Hum Hypertens 2003;17:811-817.
4. Fagard R, Staessen J, Thijs L, et al. Response to anti-hypertensive therapy in older patients with sustained and nonsustained systolic hypertension. Circulation 2000;102:1139-1144.
5. Pickering T, Coats A, Mallion JM, Mancia G, Verecchia P. Blood Pressure Monitoring Task force. Task Force V: White-coat hypertension. Blood Press Monit 1999;4:333-341.
6. Marchiando R, Elston M. Automated ambulatory blood pressure monitoring: clinical utility in the family practice setting. Am Fam Physician 2003;67:2343-2350.
7. Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000;35:844-851.
8. Ernst M, Bergus G. Ambulatory blood pressure monitoring: technology with purpose. Am Fam Physician 2003;67:2262-2270.
Evidence is conflicting regarding the risk of cardiovascular complications from white-coat hypertension. Some but not all studies show lower cardiovascular event rates for patients with white-coat hypertension compared with those with sustained hypertension (strength of recommendation [SOR]: B, cohort studies with conflicting results and methodological problems).
Little information is available about the use of antihypertensive medication for white-coat hypertension. In 1 small randomized trial, the difference in stroke incidence and cardiovascular complications between active treatment and placebo did not reach statistical significance (SOR: B, based on an underpowered randomized controlled trial). Some experts recommend that patients with white-coat hypertension should be evaluated for evidence of target organ injury and monitored for the development of sustained hypertension (SOR: C, expert opinion).
Evidence summary
A prospective cohort study compared cardiovascular events among patients with white-coat hypertension vs those with sustained hypertension. The study evaluated 479 patients with persistently elevated clinic systolic blood pressures of 140 to 180 mm Hg. Using 24-hour intraarterial ambulatory blood pressure monitoring (ABPM), they found that 126 patients had ambulatory blood pressures below 140/90 mm Hg (white-coat hypertension) while 353 patients maintained pressures above 140/90 mm Hg (sustained hypertension). On average, white-coat hypertension patients were younger than sustained hypertension patients (44 vs 52 years) but were otherwise similar. Over the next 9 years, patients with white-coat hypertension had significantly fewer cardiovascular events than patients with sustained hypertension (Table).1
Another prospective cohort study compared fatal and nonfatal cardiovascular event rates among patients who had white-coat hypertension, sustained hypertension, or were normotensive. Investigators performed 24-hour ABPM on 1187 patients who had clinic blood pressures over 140/90 on three visits. They found that 228 patients had white-coat hypertension, defined as mean ambulatory blood pressures below the 90th percentile of a normotensive population, and 959 patients had sustained hypertension. They followed these patients, along with 205 normotensive controls, for a mean of 3.2 years. Cardiovascular event rates did not differ significantly between normotensive and white-coat hypertension patients (P=.83; see Table), but the difference in event-free survival between the sustained hypertension group and both the white-coat hypertension and normotensive groups was highly significant (P=.002).2
In contrast, a recent 10-year longitudinal study of 146 normotensive people, 76 people with white-coat hypertension, and 344 with sustained hypertension showed that cardiovascular event rates were similar for patients with white-coat and sustained hypertension, and were significantly higher than in the normotensive group (P=.03 overall, P=.03 between white-coat hypertension and normotension and P=.01 between sustained hypertension and normotension).3
One randomized trial evaluated outcomes of antihypertensive therapy for white-coat hypertension for patients aged >60 years. Ninety-nine patients with white-coat hypertension were identified on the basis of systolic blood pressure greater than 160 mm Hg in clinic and normal 24-hour ABPM and were randomized to either place-bo or drug therapy. Active treatment did not significantly lower ambulatory blood pressure in white-coat hypertension, but it did reduce blood pressure measured in clinic. After a year, medication produced an absolute reduction in cardiovascular events of 8.6%, and in stroke of 4.2%. Neither result was statistically significant due to the small sample size.4
TABLE
Cohort studies of patients with white-coat hypertension
| Total number of events | |||||
|---|---|---|---|---|---|
| Patients | Outcome | NT | WCH | SH | P value |
| 479 patients, mean age of 641 | Cardiovascular events | N/A | 15 (11.9%) | 83 (23.5%) | P<.001 |
| 1392 patients, mean age of 512 | Cardiovascular events | 4 (1.9%) | 3 (1.3%) | 37 (5.3%) | WCH: NT vs P=.83 |
| WCH vs SH: P<.0001 | |||||
| 566 patients, mean age of 483 | Cardiovascular events | 10 (6.8%) | 14 (18.4%) | 56 (16.3%) | Overall P=.03 |
| NT vs WCH: P=.03 | |||||
| NT vs SH: P=.01 | |||||
| NT, normotensive; WCH, white-coat hypertension; SH, sustained hypertension | |||||
Recommendations from others
The American College of Cardiology and American Academy of Family Physicians have made no specific recommendations about white-coat hypertension. The Blood Pressure Monitoring Task Force V concluded that a significant number of white-coat hypertension patients become truly hypertensive over years of follow-up.5
Experts agree that patients with white-coat hypertension should be indefinitely monitored for the development of sustained hypertension.6 Treatment is not needed unless the patient has sustained hypertension, evidence of cardiovascular disease, or signs of target organ injury.7,8 Typically, expert opinion recommends confirming the diagnosis of white-coat hypertension with home blood pressure records or ambulatory blood pressure monitoring.
White-coat hypertension represents one point along the continuum of hypertension
Mark B. Stephens, MD, MS
Uniformed Services University, Bethesda, Md
Unfortunately, the best available clinical evidence provides an unfulfilling answer to the question posed by this Clinical Inquiry. It requires inductive reasoning and logic to derive a treatment plan from the evidence presented. Perhaps it is because the diagnosis of white-coat hypertension remains poorly defined and clinically elusive.
Nevertheless, application of the simple principle of “where there’s smoke, there’s fire” fits best here. Clinicians should be aware that white-coat hypertension represents one point along the continuum of hypertensive disease. When diagnosed, patients with white-coat hypertension should at a minimum be followed for associated morbidities and treated when systemic hypertension is identified.
Evidence is conflicting regarding the risk of cardiovascular complications from white-coat hypertension. Some but not all studies show lower cardiovascular event rates for patients with white-coat hypertension compared with those with sustained hypertension (strength of recommendation [SOR]: B, cohort studies with conflicting results and methodological problems).
Little information is available about the use of antihypertensive medication for white-coat hypertension. In 1 small randomized trial, the difference in stroke incidence and cardiovascular complications between active treatment and placebo did not reach statistical significance (SOR: B, based on an underpowered randomized controlled trial). Some experts recommend that patients with white-coat hypertension should be evaluated for evidence of target organ injury and monitored for the development of sustained hypertension (SOR: C, expert opinion).
Evidence summary
A prospective cohort study compared cardiovascular events among patients with white-coat hypertension vs those with sustained hypertension. The study evaluated 479 patients with persistently elevated clinic systolic blood pressures of 140 to 180 mm Hg. Using 24-hour intraarterial ambulatory blood pressure monitoring (ABPM), they found that 126 patients had ambulatory blood pressures below 140/90 mm Hg (white-coat hypertension) while 353 patients maintained pressures above 140/90 mm Hg (sustained hypertension). On average, white-coat hypertension patients were younger than sustained hypertension patients (44 vs 52 years) but were otherwise similar. Over the next 9 years, patients with white-coat hypertension had significantly fewer cardiovascular events than patients with sustained hypertension (Table).1
Another prospective cohort study compared fatal and nonfatal cardiovascular event rates among patients who had white-coat hypertension, sustained hypertension, or were normotensive. Investigators performed 24-hour ABPM on 1187 patients who had clinic blood pressures over 140/90 on three visits. They found that 228 patients had white-coat hypertension, defined as mean ambulatory blood pressures below the 90th percentile of a normotensive population, and 959 patients had sustained hypertension. They followed these patients, along with 205 normotensive controls, for a mean of 3.2 years. Cardiovascular event rates did not differ significantly between normotensive and white-coat hypertension patients (P=.83; see Table), but the difference in event-free survival between the sustained hypertension group and both the white-coat hypertension and normotensive groups was highly significant (P=.002).2
In contrast, a recent 10-year longitudinal study of 146 normotensive people, 76 people with white-coat hypertension, and 344 with sustained hypertension showed that cardiovascular event rates were similar for patients with white-coat and sustained hypertension, and were significantly higher than in the normotensive group (P=.03 overall, P=.03 between white-coat hypertension and normotension and P=.01 between sustained hypertension and normotension).3
One randomized trial evaluated outcomes of antihypertensive therapy for white-coat hypertension for patients aged >60 years. Ninety-nine patients with white-coat hypertension were identified on the basis of systolic blood pressure greater than 160 mm Hg in clinic and normal 24-hour ABPM and were randomized to either place-bo or drug therapy. Active treatment did not significantly lower ambulatory blood pressure in white-coat hypertension, but it did reduce blood pressure measured in clinic. After a year, medication produced an absolute reduction in cardiovascular events of 8.6%, and in stroke of 4.2%. Neither result was statistically significant due to the small sample size.4
TABLE
Cohort studies of patients with white-coat hypertension
| Total number of events | |||||
|---|---|---|---|---|---|
| Patients | Outcome | NT | WCH | SH | P value |
| 479 patients, mean age of 641 | Cardiovascular events | N/A | 15 (11.9%) | 83 (23.5%) | P<.001 |
| 1392 patients, mean age of 512 | Cardiovascular events | 4 (1.9%) | 3 (1.3%) | 37 (5.3%) | WCH: NT vs P=.83 |
| WCH vs SH: P<.0001 | |||||
| 566 patients, mean age of 483 | Cardiovascular events | 10 (6.8%) | 14 (18.4%) | 56 (16.3%) | Overall P=.03 |
| NT vs WCH: P=.03 | |||||
| NT vs SH: P=.01 | |||||
| NT, normotensive; WCH, white-coat hypertension; SH, sustained hypertension | |||||
Recommendations from others
The American College of Cardiology and American Academy of Family Physicians have made no specific recommendations about white-coat hypertension. The Blood Pressure Monitoring Task Force V concluded that a significant number of white-coat hypertension patients become truly hypertensive over years of follow-up.5
Experts agree that patients with white-coat hypertension should be indefinitely monitored for the development of sustained hypertension.6 Treatment is not needed unless the patient has sustained hypertension, evidence of cardiovascular disease, or signs of target organ injury.7,8 Typically, expert opinion recommends confirming the diagnosis of white-coat hypertension with home blood pressure records or ambulatory blood pressure monitoring.
White-coat hypertension represents one point along the continuum of hypertension
Mark B. Stephens, MD, MS
Uniformed Services University, Bethesda, Md
Unfortunately, the best available clinical evidence provides an unfulfilling answer to the question posed by this Clinical Inquiry. It requires inductive reasoning and logic to derive a treatment plan from the evidence presented. Perhaps it is because the diagnosis of white-coat hypertension remains poorly defined and clinically elusive.
Nevertheless, application of the simple principle of “where there’s smoke, there’s fire” fits best here. Clinicians should be aware that white-coat hypertension represents one point along the continuum of hypertensive disease. When diagnosed, patients with white-coat hypertension should at a minimum be followed for associated morbidities and treated when systemic hypertension is identified.
1. Khattar R, Senior R, Lahiri A. Cardiovascular outcomes in white coat versus sustained mild hypertension: a tenyear follow-up study. Circulation 1998;98:1892-1897.
2. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure, an independent predictor of prognosis in essential hypertension. Hypertension 1994;24:793-801.
3. Gustavsen PH, Hoegholm A, Bang L, Kristensen KS. White coat hypertension is a cardiovascular risk factor: a 10-year follow-up study. J Hum Hypertens 2003;17:811-817.
4. Fagard R, Staessen J, Thijs L, et al. Response to anti-hypertensive therapy in older patients with sustained and nonsustained systolic hypertension. Circulation 2000;102:1139-1144.
5. Pickering T, Coats A, Mallion JM, Mancia G, Verecchia P. Blood Pressure Monitoring Task force. Task Force V: White-coat hypertension. Blood Press Monit 1999;4:333-341.
6. Marchiando R, Elston M. Automated ambulatory blood pressure monitoring: clinical utility in the family practice setting. Am Fam Physician 2003;67:2343-2350.
7. Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000;35:844-851.
8. Ernst M, Bergus G. Ambulatory blood pressure monitoring: technology with purpose. Am Fam Physician 2003;67:2262-2270.
1. Khattar R, Senior R, Lahiri A. Cardiovascular outcomes in white coat versus sustained mild hypertension: a tenyear follow-up study. Circulation 1998;98:1892-1897.
2. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure, an independent predictor of prognosis in essential hypertension. Hypertension 1994;24:793-801.
3. Gustavsen PH, Hoegholm A, Bang L, Kristensen KS. White coat hypertension is a cardiovascular risk factor: a 10-year follow-up study. J Hum Hypertens 2003;17:811-817.
4. Fagard R, Staessen J, Thijs L, et al. Response to anti-hypertensive therapy in older patients with sustained and nonsustained systolic hypertension. Circulation 2000;102:1139-1144.
5. Pickering T, Coats A, Mallion JM, Mancia G, Verecchia P. Blood Pressure Monitoring Task force. Task Force V: White-coat hypertension. Blood Press Monit 1999;4:333-341.
6. Marchiando R, Elston M. Automated ambulatory blood pressure monitoring: clinical utility in the family practice setting. Am Fam Physician 2003;67:2343-2350.
7. Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000;35:844-851.
8. Ernst M, Bergus G. Ambulatory blood pressure monitoring: technology with purpose. Am Fam Physician 2003;67:2262-2270.
Evidence-based answers from the Family Physicians Inquiries Network