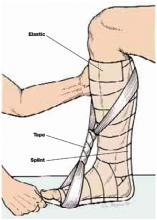
User login
What regimens eradicate Heliobacter pylori?
Fourteen-day triple therapy with a proton pump inhibitor (PPI) plus clarithromycin and either amoxicillin or metronidazole is superior to 7-day therapy in eradicating Heliobacter pylori (strength of recommendation [SOR]: A, high-quality meta-analysis).
Seven-day triple therapy with a PPI or ranitidine bismuth citrate plus clarithromycin and either amoxicillin or metronidazole is also effective (SOR: A, high-quality systematic review).
Three-day quadruple therapy with a combination of PPI, clarithromycin, bismuth subcitrate, and metronidazole or a combination of PPI, clarithromycin, amoxicillin, and metronidazole also appears to be effective (SOR: B, unblinded randomized controlled trial).
Evidence summary
The ideal H pylori eradication regimen should reach an intention-to-treat cure rate of 80% (Table).1 Effective regimens are:
Fourteen-day triple therapy of PPI + clarithromycin + metronidazole or amoxicillin. A meta-analysis of 13 studies found the eradication rate for 14-day therapy was 81% (95% confidence interval [CI], 77%–85%), compared with 72% (95% CI, 68%–76%) for 7-day therapy. The eradication rate for 10-day therapy (83%; 95% CI, 75%–89%), however, was not significantly better than that for 7-day therapy (80%; 95% CI, 71%–86%).2 Side effects were more frequent in the longer therapies, but did not lead to discontinuation of therapy.
Seven-day triple therapy of PPI + clarithromycin + metronidazole or amoxicillin. A high-quality systematic review of 82 studies using 7-day triple therapy found clarithromycin 500 twice daily yielded a higher eradication rate than clarithromycin 250 mg twice daily when combined with a PPI and amoxicillin (87% vs 81%; P<.0001). When clarithromycin was combined with a PPI and metronidazole, the higher dose of clarithromycin did not yield significantly higher eradication rates (88% vs 89%, P=.259).3
Seven-day triple therapy of ranitidine bismuth citrate + clarithromycin + metronidazole or amoxicillin. For these therapies, a high-quality systematic review of 8 studies reported eradication rates of 81% (95% CI, 77%–84%) with amoxicillin and 88% (95% CI, 85%–90%) with metronidazole.4,5 Side effects were not reported in a uniform manner for the 7-day therapies, but were noted to be mild and did not lead to significant discontinuation of therapy. Pooled dropout rates were similar among all regimens.4
Three-day quadruple therapy of PPI + bismuth + clarithromycin + metronidazole or PPI+ clarithromycin + amoxicillin + metronidazole. An otherwise high-quality but unblinded randomized clinical trial of 234 patients demonstrated that 2 days of pretreatment with lansoprazole followed by 3 days of lansoprazole with clarithromycin, amoxicillin, and metronidazole yielded eradication rates comparable with 5-day treatment (81% vs. 89%; P<.05).6
Another randomized clinical trial of 118 patients, blinded to investigators but not patients, showed that quadruple 3-day therapy with lansoprazole + bismuth + clarithromycin + metronidazole was as effective as 7 days of lansoprazole + clarithromycin + metronidazole (87% vs 86%; P=.94), and had significantly shorter duration of side effects (2.6 vs 6.2 days; P<.001). Eradication rates were similar in isolates that were resistant or sensitive to either metronidazole or clarithromycin.7
The problems of emerging clarithromycin and metronidazole resistance have not been
extensively studied. In 1 review, metronida-zole-containing regimens eradicated metronidazole-sensitive strains more effectively than metronidazole-resistant strains (weighted difference, 15%; 95% CI, 8%–20%).4 When an infection is resistant to metronidazole, amoxicillin should be used instead.4 In areas of high clarithromycin and metronidazole resistance, a quadruple regimen might be more effective.7
TABLE
Effective therapies for Heliobacter pylorieradication
| Regimen | Dosage | Duration (days) | Cost ($)b | SOR |
|---|---|---|---|---|
| PPIa | 14 | 210 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 500 mg twice daily or | |||
| amoxicillin | 1000 mg twice daily | |||
| PPI | 7 | 105 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| PPI | 7 | 105 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 500 mg twice daily | |||
| Ranitidine bismuth citrate | 400 mg twice daily | 7 | 85 | A |
| Clarithromycin | 500 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| Ranitidine bismuth citrate | 400 mg twice daily | 7 | 82 | A |
| Clarithromycin | 250 mg twice daily | |||
| Metronidazole | 500 mg twice daily | |||
| PPI | 3 | 46 | B | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 400 mg twice daily | |||
| Bismuth subcitrate | 240 mg twice daily | |||
| PPI (5 days) | 3 | 60 | B | |
| Clarithromycin | 250 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| Metronidazole | 400 mg twice daily | |||
| a. PPI: standard twice-daily dosing—eg, lansoprazole 30 mg or omeprazole 20 mg | ||||
| b. Approximate cost of entire course of therapy from www.drugstore.com, August 2003. | ||||
| PPI, proton pump inhibitor; SOR, strength of recommendation (for an explanation of evidence ratings, see page 779) | ||||
Recommendations from others
The Maastricht Consensus of the European Heliobacter Study Group1 recommends a 7-day triple regimen of PPI + clarithromycin + either metronidazole or amoxicillin or (if clarithromycin resistance is prevalent) PPI + amoxicillin 500 mg 3 times daily + metronidazole 500 mg 3 times daily.
The American College of Gastroenterology recommends 14-day therapy of one of the following options:8
- PPI + clarithromycin + (metronidazole or amoxicillin), or ranitidine bismuth citrate + clarithromycin + (metronidazole or amoxicillin). Tetracycline 500 mg twice a day can be substituted for amoxicillin or metronidazole
- PPI + bismuth subsalicylate 525 mg + metronidazole 500 mg 3 times daily + tetra-cycline 500 mg 4 times daily
- Bismuth subsalicylate 525 mg 4 times daily + metronidazole 250 mg 4 times daily + tetra-cycline 500 mg 4 times daily + H2 receptor antagonist in standard acid-suppression dose (eg, famotidine 20 mg twice a day for 4 weeks).
The Institute for Clinical Systems Improvement recommends as first-choice treatment a 7-day PPI/clarithromycin/amoxicillin combination, and as second choice a 7-day regimen of PPI, tetracycline 250 mg 4 times daily, metronidazole 500 mg twice daily, and bismuth subsalicylate 525 mg 4 times daily.9
Patients beginning complex regimens require counseling
Laura B. Hansen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver, Colorado
The most effective regimens (>80% eradication) for H pylori include a 10- to 14-day course of at least 2 antibiotics and an antisecretory agent. However, even optimal treatment regimens can fail in approximately 10% of patients. Poor compliance is among the most common reasons for treatment failure. Medication side effects can affect up to 50% of patients taking triple-agent regimens.
Treatment regimens with multiple medications administered several times daily can be difficult to follow. Convenient packaging containing all daily medications are available to optimize adherence.
Counseling points for patients should include how to take the medicine correctly, expected side effects, the importance of completing the entire therapy regimen, and warnings of specific interactions (eg, alcohol and metronidazole). Lastly, the patient should be made aware of the cost of the entire regimen, which ranges from $50 to $250.
1. Current European concepts in the management of Heliobacter pylori infection. The Maastricht Consensus Report. European Heliobacter Pylori Study Group. Gut 1997;41:8-13.
2. Calvet X, Garcia N, Lopez T, Gisbert JP, Gene E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxicillin for treating Heliobacter pylori infection. Aliment Pharmacol Ther 2000;14:603-609.
3. Huang J, Hunt RH. The importance of clarithromycin dose in the management of Heliobacter pylori infection: a meta-analysis of triple therapies with a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. Aliment Pharmacol Ther 1999;13:719-729.
4. Janssen MJ, Van Oijen AH, Verbeek AL, Jansen JB, De Boer WA. A systematic comparison of triple therapies for treatment of Heliobacter pylori infection with proton pump inhibitor/ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment Pharmacol Ther 2001;15:613-624.
5. Delaney B, Moayyedi P, Forman D. Heliobacter pylori. Clin Evid [online], Issue 8. London: BMJ Publishing Group, Last updated 2003 March. Available at www.ovid.com. Accessed on March 4, 2003.
6. Treiber G, Wittig J, Ammon S, Walker S, van Doorn LJ, Klotz U. Clinical outcome and influencing factors for a new short-term quadruple therapy for Heliobacter pylori eradication: a randomized controlled trial (MACLOR study). Arch Intern Med. 2002;162:153-160.
7. Wong BC, Wang WH, Wong WM, et al. Three-day lansoprazole quadruple therapy for Heliobacter pylori-positive duodenal ulcers: a randomized controlled study. Aliment PharmacTher 2001;15:843-849.
8. Howden CW, Hunt RH. Guidelines for the management of Heliobacter pylori infection. Ad Hoc Committee on the Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 1998;93:2330-2338.
9. Institute for Clinical Systems Improvement (ICSI). Dyspepsia. Bloomington, Minn: ICSI; last updated January 2003. Available at: http://www.icsi.org/ knowledge/detail.asp?catID=29&itemID=171. Accessed on September 8, 2003.
Fourteen-day triple therapy with a proton pump inhibitor (PPI) plus clarithromycin and either amoxicillin or metronidazole is superior to 7-day therapy in eradicating Heliobacter pylori (strength of recommendation [SOR]: A, high-quality meta-analysis).
Seven-day triple therapy with a PPI or ranitidine bismuth citrate plus clarithromycin and either amoxicillin or metronidazole is also effective (SOR: A, high-quality systematic review).
Three-day quadruple therapy with a combination of PPI, clarithromycin, bismuth subcitrate, and metronidazole or a combination of PPI, clarithromycin, amoxicillin, and metronidazole also appears to be effective (SOR: B, unblinded randomized controlled trial).
Evidence summary
The ideal H pylori eradication regimen should reach an intention-to-treat cure rate of 80% (Table).1 Effective regimens are:
Fourteen-day triple therapy of PPI + clarithromycin + metronidazole or amoxicillin. A meta-analysis of 13 studies found the eradication rate for 14-day therapy was 81% (95% confidence interval [CI], 77%–85%), compared with 72% (95% CI, 68%–76%) for 7-day therapy. The eradication rate for 10-day therapy (83%; 95% CI, 75%–89%), however, was not significantly better than that for 7-day therapy (80%; 95% CI, 71%–86%).2 Side effects were more frequent in the longer therapies, but did not lead to discontinuation of therapy.
Seven-day triple therapy of PPI + clarithromycin + metronidazole or amoxicillin. A high-quality systematic review of 82 studies using 7-day triple therapy found clarithromycin 500 twice daily yielded a higher eradication rate than clarithromycin 250 mg twice daily when combined with a PPI and amoxicillin (87% vs 81%; P<.0001). When clarithromycin was combined with a PPI and metronidazole, the higher dose of clarithromycin did not yield significantly higher eradication rates (88% vs 89%, P=.259).3
Seven-day triple therapy of ranitidine bismuth citrate + clarithromycin + metronidazole or amoxicillin. For these therapies, a high-quality systematic review of 8 studies reported eradication rates of 81% (95% CI, 77%–84%) with amoxicillin and 88% (95% CI, 85%–90%) with metronidazole.4,5 Side effects were not reported in a uniform manner for the 7-day therapies, but were noted to be mild and did not lead to significant discontinuation of therapy. Pooled dropout rates were similar among all regimens.4
Three-day quadruple therapy of PPI + bismuth + clarithromycin + metronidazole or PPI+ clarithromycin + amoxicillin + metronidazole. An otherwise high-quality but unblinded randomized clinical trial of 234 patients demonstrated that 2 days of pretreatment with lansoprazole followed by 3 days of lansoprazole with clarithromycin, amoxicillin, and metronidazole yielded eradication rates comparable with 5-day treatment (81% vs. 89%; P<.05).6
Another randomized clinical trial of 118 patients, blinded to investigators but not patients, showed that quadruple 3-day therapy with lansoprazole + bismuth + clarithromycin + metronidazole was as effective as 7 days of lansoprazole + clarithromycin + metronidazole (87% vs 86%; P=.94), and had significantly shorter duration of side effects (2.6 vs 6.2 days; P<.001). Eradication rates were similar in isolates that were resistant or sensitive to either metronidazole or clarithromycin.7
The problems of emerging clarithromycin and metronidazole resistance have not been
extensively studied. In 1 review, metronida-zole-containing regimens eradicated metronidazole-sensitive strains more effectively than metronidazole-resistant strains (weighted difference, 15%; 95% CI, 8%–20%).4 When an infection is resistant to metronidazole, amoxicillin should be used instead.4 In areas of high clarithromycin and metronidazole resistance, a quadruple regimen might be more effective.7
TABLE
Effective therapies for Heliobacter pylorieradication
| Regimen | Dosage | Duration (days) | Cost ($)b | SOR |
|---|---|---|---|---|
| PPIa | 14 | 210 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 500 mg twice daily or | |||
| amoxicillin | 1000 mg twice daily | |||
| PPI | 7 | 105 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| PPI | 7 | 105 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 500 mg twice daily | |||
| Ranitidine bismuth citrate | 400 mg twice daily | 7 | 85 | A |
| Clarithromycin | 500 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| Ranitidine bismuth citrate | 400 mg twice daily | 7 | 82 | A |
| Clarithromycin | 250 mg twice daily | |||
| Metronidazole | 500 mg twice daily | |||
| PPI | 3 | 46 | B | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 400 mg twice daily | |||
| Bismuth subcitrate | 240 mg twice daily | |||
| PPI (5 days) | 3 | 60 | B | |
| Clarithromycin | 250 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| Metronidazole | 400 mg twice daily | |||
| a. PPI: standard twice-daily dosing—eg, lansoprazole 30 mg or omeprazole 20 mg | ||||
| b. Approximate cost of entire course of therapy from www.drugstore.com, August 2003. | ||||
| PPI, proton pump inhibitor; SOR, strength of recommendation (for an explanation of evidence ratings, see page 779) | ||||
Recommendations from others
The Maastricht Consensus of the European Heliobacter Study Group1 recommends a 7-day triple regimen of PPI + clarithromycin + either metronidazole or amoxicillin or (if clarithromycin resistance is prevalent) PPI + amoxicillin 500 mg 3 times daily + metronidazole 500 mg 3 times daily.
The American College of Gastroenterology recommends 14-day therapy of one of the following options:8
- PPI + clarithromycin + (metronidazole or amoxicillin), or ranitidine bismuth citrate + clarithromycin + (metronidazole or amoxicillin). Tetracycline 500 mg twice a day can be substituted for amoxicillin or metronidazole
- PPI + bismuth subsalicylate 525 mg + metronidazole 500 mg 3 times daily + tetra-cycline 500 mg 4 times daily
- Bismuth subsalicylate 525 mg 4 times daily + metronidazole 250 mg 4 times daily + tetra-cycline 500 mg 4 times daily + H2 receptor antagonist in standard acid-suppression dose (eg, famotidine 20 mg twice a day for 4 weeks).
The Institute for Clinical Systems Improvement recommends as first-choice treatment a 7-day PPI/clarithromycin/amoxicillin combination, and as second choice a 7-day regimen of PPI, tetracycline 250 mg 4 times daily, metronidazole 500 mg twice daily, and bismuth subsalicylate 525 mg 4 times daily.9
Patients beginning complex regimens require counseling
Laura B. Hansen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver, Colorado
The most effective regimens (>80% eradication) for H pylori include a 10- to 14-day course of at least 2 antibiotics and an antisecretory agent. However, even optimal treatment regimens can fail in approximately 10% of patients. Poor compliance is among the most common reasons for treatment failure. Medication side effects can affect up to 50% of patients taking triple-agent regimens.
Treatment regimens with multiple medications administered several times daily can be difficult to follow. Convenient packaging containing all daily medications are available to optimize adherence.
Counseling points for patients should include how to take the medicine correctly, expected side effects, the importance of completing the entire therapy regimen, and warnings of specific interactions (eg, alcohol and metronidazole). Lastly, the patient should be made aware of the cost of the entire regimen, which ranges from $50 to $250.
Fourteen-day triple therapy with a proton pump inhibitor (PPI) plus clarithromycin and either amoxicillin or metronidazole is superior to 7-day therapy in eradicating Heliobacter pylori (strength of recommendation [SOR]: A, high-quality meta-analysis).
Seven-day triple therapy with a PPI or ranitidine bismuth citrate plus clarithromycin and either amoxicillin or metronidazole is also effective (SOR: A, high-quality systematic review).
Three-day quadruple therapy with a combination of PPI, clarithromycin, bismuth subcitrate, and metronidazole or a combination of PPI, clarithromycin, amoxicillin, and metronidazole also appears to be effective (SOR: B, unblinded randomized controlled trial).
Evidence summary
The ideal H pylori eradication regimen should reach an intention-to-treat cure rate of 80% (Table).1 Effective regimens are:
Fourteen-day triple therapy of PPI + clarithromycin + metronidazole or amoxicillin. A meta-analysis of 13 studies found the eradication rate for 14-day therapy was 81% (95% confidence interval [CI], 77%–85%), compared with 72% (95% CI, 68%–76%) for 7-day therapy. The eradication rate for 10-day therapy (83%; 95% CI, 75%–89%), however, was not significantly better than that for 7-day therapy (80%; 95% CI, 71%–86%).2 Side effects were more frequent in the longer therapies, but did not lead to discontinuation of therapy.
Seven-day triple therapy of PPI + clarithromycin + metronidazole or amoxicillin. A high-quality systematic review of 82 studies using 7-day triple therapy found clarithromycin 500 twice daily yielded a higher eradication rate than clarithromycin 250 mg twice daily when combined with a PPI and amoxicillin (87% vs 81%; P<.0001). When clarithromycin was combined with a PPI and metronidazole, the higher dose of clarithromycin did not yield significantly higher eradication rates (88% vs 89%, P=.259).3
Seven-day triple therapy of ranitidine bismuth citrate + clarithromycin + metronidazole or amoxicillin. For these therapies, a high-quality systematic review of 8 studies reported eradication rates of 81% (95% CI, 77%–84%) with amoxicillin and 88% (95% CI, 85%–90%) with metronidazole.4,5 Side effects were not reported in a uniform manner for the 7-day therapies, but were noted to be mild and did not lead to significant discontinuation of therapy. Pooled dropout rates were similar among all regimens.4
Three-day quadruple therapy of PPI + bismuth + clarithromycin + metronidazole or PPI+ clarithromycin + amoxicillin + metronidazole. An otherwise high-quality but unblinded randomized clinical trial of 234 patients demonstrated that 2 days of pretreatment with lansoprazole followed by 3 days of lansoprazole with clarithromycin, amoxicillin, and metronidazole yielded eradication rates comparable with 5-day treatment (81% vs. 89%; P<.05).6
Another randomized clinical trial of 118 patients, blinded to investigators but not patients, showed that quadruple 3-day therapy with lansoprazole + bismuth + clarithromycin + metronidazole was as effective as 7 days of lansoprazole + clarithromycin + metronidazole (87% vs 86%; P=.94), and had significantly shorter duration of side effects (2.6 vs 6.2 days; P<.001). Eradication rates were similar in isolates that were resistant or sensitive to either metronidazole or clarithromycin.7
The problems of emerging clarithromycin and metronidazole resistance have not been
extensively studied. In 1 review, metronida-zole-containing regimens eradicated metronidazole-sensitive strains more effectively than metronidazole-resistant strains (weighted difference, 15%; 95% CI, 8%–20%).4 When an infection is resistant to metronidazole, amoxicillin should be used instead.4 In areas of high clarithromycin and metronidazole resistance, a quadruple regimen might be more effective.7
TABLE
Effective therapies for Heliobacter pylorieradication
| Regimen | Dosage | Duration (days) | Cost ($)b | SOR |
|---|---|---|---|---|
| PPIa | 14 | 210 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 500 mg twice daily or | |||
| amoxicillin | 1000 mg twice daily | |||
| PPI | 7 | 105 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| PPI | 7 | 105 | A | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 500 mg twice daily | |||
| Ranitidine bismuth citrate | 400 mg twice daily | 7 | 85 | A |
| Clarithromycin | 500 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| Ranitidine bismuth citrate | 400 mg twice daily | 7 | 82 | A |
| Clarithromycin | 250 mg twice daily | |||
| Metronidazole | 500 mg twice daily | |||
| PPI | 3 | 46 | B | |
| Clarithromycin | 500 mg twice daily | |||
| Metronidazole | 400 mg twice daily | |||
| Bismuth subcitrate | 240 mg twice daily | |||
| PPI (5 days) | 3 | 60 | B | |
| Clarithromycin | 250 mg twice daily | |||
| Amoxicillin | 1000 mg twice daily | |||
| Metronidazole | 400 mg twice daily | |||
| a. PPI: standard twice-daily dosing—eg, lansoprazole 30 mg or omeprazole 20 mg | ||||
| b. Approximate cost of entire course of therapy from www.drugstore.com, August 2003. | ||||
| PPI, proton pump inhibitor; SOR, strength of recommendation (for an explanation of evidence ratings, see page 779) | ||||
Recommendations from others
The Maastricht Consensus of the European Heliobacter Study Group1 recommends a 7-day triple regimen of PPI + clarithromycin + either metronidazole or amoxicillin or (if clarithromycin resistance is prevalent) PPI + amoxicillin 500 mg 3 times daily + metronidazole 500 mg 3 times daily.
The American College of Gastroenterology recommends 14-day therapy of one of the following options:8
- PPI + clarithromycin + (metronidazole or amoxicillin), or ranitidine bismuth citrate + clarithromycin + (metronidazole or amoxicillin). Tetracycline 500 mg twice a day can be substituted for amoxicillin or metronidazole
- PPI + bismuth subsalicylate 525 mg + metronidazole 500 mg 3 times daily + tetra-cycline 500 mg 4 times daily
- Bismuth subsalicylate 525 mg 4 times daily + metronidazole 250 mg 4 times daily + tetra-cycline 500 mg 4 times daily + H2 receptor antagonist in standard acid-suppression dose (eg, famotidine 20 mg twice a day for 4 weeks).
The Institute for Clinical Systems Improvement recommends as first-choice treatment a 7-day PPI/clarithromycin/amoxicillin combination, and as second choice a 7-day regimen of PPI, tetracycline 250 mg 4 times daily, metronidazole 500 mg twice daily, and bismuth subsalicylate 525 mg 4 times daily.9
Patients beginning complex regimens require counseling
Laura B. Hansen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver, Colorado
The most effective regimens (>80% eradication) for H pylori include a 10- to 14-day course of at least 2 antibiotics and an antisecretory agent. However, even optimal treatment regimens can fail in approximately 10% of patients. Poor compliance is among the most common reasons for treatment failure. Medication side effects can affect up to 50% of patients taking triple-agent regimens.
Treatment regimens with multiple medications administered several times daily can be difficult to follow. Convenient packaging containing all daily medications are available to optimize adherence.
Counseling points for patients should include how to take the medicine correctly, expected side effects, the importance of completing the entire therapy regimen, and warnings of specific interactions (eg, alcohol and metronidazole). Lastly, the patient should be made aware of the cost of the entire regimen, which ranges from $50 to $250.
1. Current European concepts in the management of Heliobacter pylori infection. The Maastricht Consensus Report. European Heliobacter Pylori Study Group. Gut 1997;41:8-13.
2. Calvet X, Garcia N, Lopez T, Gisbert JP, Gene E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxicillin for treating Heliobacter pylori infection. Aliment Pharmacol Ther 2000;14:603-609.
3. Huang J, Hunt RH. The importance of clarithromycin dose in the management of Heliobacter pylori infection: a meta-analysis of triple therapies with a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. Aliment Pharmacol Ther 1999;13:719-729.
4. Janssen MJ, Van Oijen AH, Verbeek AL, Jansen JB, De Boer WA. A systematic comparison of triple therapies for treatment of Heliobacter pylori infection with proton pump inhibitor/ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment Pharmacol Ther 2001;15:613-624.
5. Delaney B, Moayyedi P, Forman D. Heliobacter pylori. Clin Evid [online], Issue 8. London: BMJ Publishing Group, Last updated 2003 March. Available at www.ovid.com. Accessed on March 4, 2003.
6. Treiber G, Wittig J, Ammon S, Walker S, van Doorn LJ, Klotz U. Clinical outcome and influencing factors for a new short-term quadruple therapy for Heliobacter pylori eradication: a randomized controlled trial (MACLOR study). Arch Intern Med. 2002;162:153-160.
7. Wong BC, Wang WH, Wong WM, et al. Three-day lansoprazole quadruple therapy for Heliobacter pylori-positive duodenal ulcers: a randomized controlled study. Aliment PharmacTher 2001;15:843-849.
8. Howden CW, Hunt RH. Guidelines for the management of Heliobacter pylori infection. Ad Hoc Committee on the Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 1998;93:2330-2338.
9. Institute for Clinical Systems Improvement (ICSI). Dyspepsia. Bloomington, Minn: ICSI; last updated January 2003. Available at: http://www.icsi.org/ knowledge/detail.asp?catID=29&itemID=171. Accessed on September 8, 2003.
1. Current European concepts in the management of Heliobacter pylori infection. The Maastricht Consensus Report. European Heliobacter Pylori Study Group. Gut 1997;41:8-13.
2. Calvet X, Garcia N, Lopez T, Gisbert JP, Gene E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxicillin for treating Heliobacter pylori infection. Aliment Pharmacol Ther 2000;14:603-609.
3. Huang J, Hunt RH. The importance of clarithromycin dose in the management of Heliobacter pylori infection: a meta-analysis of triple therapies with a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. Aliment Pharmacol Ther 1999;13:719-729.
4. Janssen MJ, Van Oijen AH, Verbeek AL, Jansen JB, De Boer WA. A systematic comparison of triple therapies for treatment of Heliobacter pylori infection with proton pump inhibitor/ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment Pharmacol Ther 2001;15:613-624.
5. Delaney B, Moayyedi P, Forman D. Heliobacter pylori. Clin Evid [online], Issue 8. London: BMJ Publishing Group, Last updated 2003 March. Available at www.ovid.com. Accessed on March 4, 2003.
6. Treiber G, Wittig J, Ammon S, Walker S, van Doorn LJ, Klotz U. Clinical outcome and influencing factors for a new short-term quadruple therapy for Heliobacter pylori eradication: a randomized controlled trial (MACLOR study). Arch Intern Med. 2002;162:153-160.
7. Wong BC, Wang WH, Wong WM, et al. Three-day lansoprazole quadruple therapy for Heliobacter pylori-positive duodenal ulcers: a randomized controlled study. Aliment PharmacTher 2001;15:843-849.
8. Howden CW, Hunt RH. Guidelines for the management of Heliobacter pylori infection. Ad Hoc Committee on the Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 1998;93:2330-2338.
9. Institute for Clinical Systems Improvement (ICSI). Dyspepsia. Bloomington, Minn: ICSI; last updated January 2003. Available at: http://www.icsi.org/ knowledge/detail.asp?catID=29&itemID=171. Accessed on September 8, 2003.
Evidence-based answers from the Family Physicians Inquiries Network
Is screening for lead poisoning justified?
Evidence is insufficient to recommend for or against universal screening of young children for lead poisoning in high-prevalence communities (strength of recommendation [SOR]: C). In low-prevalence communities, evidence is insufficient to recommend for or against a targeted screening approach, employing locale-specific demographic risk factors and personal risk questionnaires to inform screening decisions (SOR: C).
Although evidence does not suggest that treatment of individuals with elevated blood lead levels improves individual outcomes, public health strategies aimed at decreasing lead in the environment appear to have resulted in a significant decline in the number of children with elevated blood lead levels in recent decades. One could thus argue that screening may identify communities with high rates of lead poisoning, where environmental strategies could be targeted.
Because the epidemiology of lead poisoning continues to change, local and state health authorities must continuously update information on which to base decisions about screening.
Evidence summary
The prevalence of elevated blood lead levels varies widely among different demographic groups and geographic regions, and it has decreased dramatically in the last several decades. Racial and ethnic minorities and children of families with low incomes, who live in the Northeast or Midwest, or who live in older houses continue to be at increased risk.1 Children with blood lead levels 10 μg/dL have been shown to have poorer cognitive and behavioral functioning.2
No studies have demonstrated that screening for lead poisoning improves outcomes. To justify screening, one must therefore extrapolate from indirect evidence, demonstrating that screening tests are accurate and that treatment of children detected by screening is effective. Capillary blood samples are comparable with venous samples for detecting elevated blood lead levels. The sensitivity of capillary samples ranges from 86% to 96% compared with venous samples.3
In low-prevalence areas, questionnaires may inform screening decisions. A questionnaire inquiring about age of housing, presence of peeling paint, ongoing renovations, siblings or playmates with elevated blood lead levels, adults in the home with occupational exposures to lead, and proximity to industrial sources of lead has a sensitivity for detecting blood lead levels 10 μg/dL ranging from 32% to 87%. Sensitivity varies depending on the population and geographic location in which the questionnaire is tested. Accuracy is improved by tailoring the questionnaire based on locally important risk factors.4
Proposed treatments for elevated blood lead levels include chelation therapy, education about hygiene and nutrition, household dust control measures, and soil lead abatement. No good-quality trials have demonstrated that lowering slightly to moderately elevated blood lead levels (10–55 μg/dL) improves patient-oriented outcomes such as cognitive and behavioral functioning. Although 1 observational study of chelation therapy linked lowering blood lead levels with improved cognitive function,3 a randomized controlled trial showed that chelation had no effect on cognitive or behavioral outcomes.5
All other trials evaluating treatment for lead poisoning looked at the intermediate outcome of blood lead levels. A systematic review of randomized controlled trials showed that home dust control interventions reduced the proportion of children with elevated blood lead levels (15 μg/dL) from 14% to 6%.6 A randomized controlled trial of high-efficiency particulate air (HEPA) filtration vacuuming showed no effect.7 More intensive interventions such as soil lead abatement and paint remediation have not proven effective in good-quality randomized controlled trials.
Increasing dietary calcium and iron and decreasing dietary fat are also commonly recommended for children with elevated blood lead levels, based on animal models and cross-sectional studies. The only randomized controlled trial that investigated calcium supplementation showed no effect on blood lead levels.8 Our search revealed no good-quality studies on the effect of iron or fat intake on lead poisoning.
In summary, because the prevalence of lead poisoning varies between communities and continues to change, standard recommendations are not possible. Clinicians must rely on local epidemiologic data to make screening decisions. Although questionnaires are accurate in predicting elevated blood lead levels in some settings, no specific set of questions can be recommended for all populations.
No treatment options for those with mild to moderate elevations in blood lead levels have been shown to improve clinically important outcomes, although some interventions may decrease blood lead levels.
Recommendations from others
The Centers for Disease Control and Prevention (CDC) recommends
- that individual states develop screening plans based on local data
- universal screening at 12 and 24 months of age:
Otherwise screening should be targeted based on a questionnaire on age of housing, recent or ongoing remodeling, and having a sibling or playmate diagnosed with lead poisoning, in addition to questions on locally important risk factors.9
The American Academy of Pediatrics endorses the CDC recommendations.2 The US Preventive Services Task Force, the American Academy of Family Physicians, and the American College of Preventive Medicine all recommend screening for lead poisoning at 12 months of age in children with demographic or geographic risk factors.3,10,11
Lead screening: Think locally
Julia Fashner, MD
St. Joseph Regional Medical Center, South Bend, Ind
The local health department can provide information about lead screening in your community, whether based on blood levels or the housing conditions. If your patients need screening, you may want to add a reminder on a flow sheet in the chart to do a questionnaire or a blood draw. Finding and treating severely elevated lead levels can change outcomes, but for less elevated levels, the evidence shows no benefit. You should work with the health department when considering therapy for children with elevated blood lead levels.
1. Kaufmann RB, Clouse TL, Olson DR, Matte TD. Elevated blood lead levels and blood lead screening among US children aged one to five years: 1988–1994. Pediatrics 2000;106:E79.-
2. Screening for elevated blood lead levels. American Academy of Pediatrics Committee on Environmental Health. Pediatrics 1998;101:1072-1078.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore, Md: Lippincott Williams & Wilkins; 1996.
4. Binns HJ, LeBailly SA, Fingar AR, Saunders S. Evaluation of risk assessment questions used to target blood lead screening in Illinois. Pediatrics 1999;103:100-106.
5. Rogan WJ, Dietrich KN, Ware JH, et al. The effect of chela-tion therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med 2001;344:1421-1426.
6. Haynes E, Lanphear BP, Tohn E, Farr N, Rhoads GG. The effect of interior lead hazard controls on children’s blood lead concentrations: a systematic evaluation. Environ Health Perspect 2002;110:103-107.
7. Hilts SR, Hertzman C, Marion SA. A controlled trial of the effect of HEPA vacuuming on childhood lead exposure. Can J Public Health 1995;86:345-350.
8. Ballew C, Bowman B. Recommending calcium to reduce lead toxicity in children: a critical review. Nutr Rev 2001;59(3 Pt 1):71-79.
9. Centers for Disease Control and Prevention. Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials. Atlanta, Ga: CDC, 1997.
10. AAFP Policy Recommendations for Periodic Health Examinations 2002. Available at http://www.aafp.org/ exam.xml. Accessed on February 9, 2003.
11. Lane WG, Kemper AR. American College of Preventive Medicine Practice Policy Statement. Screening for elevated blood lead levels in children. Am J Prev Med 2001;20:78-82.
Evidence is insufficient to recommend for or against universal screening of young children for lead poisoning in high-prevalence communities (strength of recommendation [SOR]: C). In low-prevalence communities, evidence is insufficient to recommend for or against a targeted screening approach, employing locale-specific demographic risk factors and personal risk questionnaires to inform screening decisions (SOR: C).
Although evidence does not suggest that treatment of individuals with elevated blood lead levels improves individual outcomes, public health strategies aimed at decreasing lead in the environment appear to have resulted in a significant decline in the number of children with elevated blood lead levels in recent decades. One could thus argue that screening may identify communities with high rates of lead poisoning, where environmental strategies could be targeted.
Because the epidemiology of lead poisoning continues to change, local and state health authorities must continuously update information on which to base decisions about screening.
Evidence summary
The prevalence of elevated blood lead levels varies widely among different demographic groups and geographic regions, and it has decreased dramatically in the last several decades. Racial and ethnic minorities and children of families with low incomes, who live in the Northeast or Midwest, or who live in older houses continue to be at increased risk.1 Children with blood lead levels 10 μg/dL have been shown to have poorer cognitive and behavioral functioning.2
No studies have demonstrated that screening for lead poisoning improves outcomes. To justify screening, one must therefore extrapolate from indirect evidence, demonstrating that screening tests are accurate and that treatment of children detected by screening is effective. Capillary blood samples are comparable with venous samples for detecting elevated blood lead levels. The sensitivity of capillary samples ranges from 86% to 96% compared with venous samples.3
In low-prevalence areas, questionnaires may inform screening decisions. A questionnaire inquiring about age of housing, presence of peeling paint, ongoing renovations, siblings or playmates with elevated blood lead levels, adults in the home with occupational exposures to lead, and proximity to industrial sources of lead has a sensitivity for detecting blood lead levels 10 μg/dL ranging from 32% to 87%. Sensitivity varies depending on the population and geographic location in which the questionnaire is tested. Accuracy is improved by tailoring the questionnaire based on locally important risk factors.4
Proposed treatments for elevated blood lead levels include chelation therapy, education about hygiene and nutrition, household dust control measures, and soil lead abatement. No good-quality trials have demonstrated that lowering slightly to moderately elevated blood lead levels (10–55 μg/dL) improves patient-oriented outcomes such as cognitive and behavioral functioning. Although 1 observational study of chelation therapy linked lowering blood lead levels with improved cognitive function,3 a randomized controlled trial showed that chelation had no effect on cognitive or behavioral outcomes.5
All other trials evaluating treatment for lead poisoning looked at the intermediate outcome of blood lead levels. A systematic review of randomized controlled trials showed that home dust control interventions reduced the proportion of children with elevated blood lead levels (15 μg/dL) from 14% to 6%.6 A randomized controlled trial of high-efficiency particulate air (HEPA) filtration vacuuming showed no effect.7 More intensive interventions such as soil lead abatement and paint remediation have not proven effective in good-quality randomized controlled trials.
Increasing dietary calcium and iron and decreasing dietary fat are also commonly recommended for children with elevated blood lead levels, based on animal models and cross-sectional studies. The only randomized controlled trial that investigated calcium supplementation showed no effect on blood lead levels.8 Our search revealed no good-quality studies on the effect of iron or fat intake on lead poisoning.
In summary, because the prevalence of lead poisoning varies between communities and continues to change, standard recommendations are not possible. Clinicians must rely on local epidemiologic data to make screening decisions. Although questionnaires are accurate in predicting elevated blood lead levels in some settings, no specific set of questions can be recommended for all populations.
No treatment options for those with mild to moderate elevations in blood lead levels have been shown to improve clinically important outcomes, although some interventions may decrease blood lead levels.
Recommendations from others
The Centers for Disease Control and Prevention (CDC) recommends
- that individual states develop screening plans based on local data
- universal screening at 12 and 24 months of age:
Otherwise screening should be targeted based on a questionnaire on age of housing, recent or ongoing remodeling, and having a sibling or playmate diagnosed with lead poisoning, in addition to questions on locally important risk factors.9
The American Academy of Pediatrics endorses the CDC recommendations.2 The US Preventive Services Task Force, the American Academy of Family Physicians, and the American College of Preventive Medicine all recommend screening for lead poisoning at 12 months of age in children with demographic or geographic risk factors.3,10,11
Lead screening: Think locally
Julia Fashner, MD
St. Joseph Regional Medical Center, South Bend, Ind
The local health department can provide information about lead screening in your community, whether based on blood levels or the housing conditions. If your patients need screening, you may want to add a reminder on a flow sheet in the chart to do a questionnaire or a blood draw. Finding and treating severely elevated lead levels can change outcomes, but for less elevated levels, the evidence shows no benefit. You should work with the health department when considering therapy for children with elevated blood lead levels.
Evidence is insufficient to recommend for or against universal screening of young children for lead poisoning in high-prevalence communities (strength of recommendation [SOR]: C). In low-prevalence communities, evidence is insufficient to recommend for or against a targeted screening approach, employing locale-specific demographic risk factors and personal risk questionnaires to inform screening decisions (SOR: C).
Although evidence does not suggest that treatment of individuals with elevated blood lead levels improves individual outcomes, public health strategies aimed at decreasing lead in the environment appear to have resulted in a significant decline in the number of children with elevated blood lead levels in recent decades. One could thus argue that screening may identify communities with high rates of lead poisoning, where environmental strategies could be targeted.
Because the epidemiology of lead poisoning continues to change, local and state health authorities must continuously update information on which to base decisions about screening.
Evidence summary
The prevalence of elevated blood lead levels varies widely among different demographic groups and geographic regions, and it has decreased dramatically in the last several decades. Racial and ethnic minorities and children of families with low incomes, who live in the Northeast or Midwest, or who live in older houses continue to be at increased risk.1 Children with blood lead levels 10 μg/dL have been shown to have poorer cognitive and behavioral functioning.2
No studies have demonstrated that screening for lead poisoning improves outcomes. To justify screening, one must therefore extrapolate from indirect evidence, demonstrating that screening tests are accurate and that treatment of children detected by screening is effective. Capillary blood samples are comparable with venous samples for detecting elevated blood lead levels. The sensitivity of capillary samples ranges from 86% to 96% compared with venous samples.3
In low-prevalence areas, questionnaires may inform screening decisions. A questionnaire inquiring about age of housing, presence of peeling paint, ongoing renovations, siblings or playmates with elevated blood lead levels, adults in the home with occupational exposures to lead, and proximity to industrial sources of lead has a sensitivity for detecting blood lead levels 10 μg/dL ranging from 32% to 87%. Sensitivity varies depending on the population and geographic location in which the questionnaire is tested. Accuracy is improved by tailoring the questionnaire based on locally important risk factors.4
Proposed treatments for elevated blood lead levels include chelation therapy, education about hygiene and nutrition, household dust control measures, and soil lead abatement. No good-quality trials have demonstrated that lowering slightly to moderately elevated blood lead levels (10–55 μg/dL) improves patient-oriented outcomes such as cognitive and behavioral functioning. Although 1 observational study of chelation therapy linked lowering blood lead levels with improved cognitive function,3 a randomized controlled trial showed that chelation had no effect on cognitive or behavioral outcomes.5
All other trials evaluating treatment for lead poisoning looked at the intermediate outcome of blood lead levels. A systematic review of randomized controlled trials showed that home dust control interventions reduced the proportion of children with elevated blood lead levels (15 μg/dL) from 14% to 6%.6 A randomized controlled trial of high-efficiency particulate air (HEPA) filtration vacuuming showed no effect.7 More intensive interventions such as soil lead abatement and paint remediation have not proven effective in good-quality randomized controlled trials.
Increasing dietary calcium and iron and decreasing dietary fat are also commonly recommended for children with elevated blood lead levels, based on animal models and cross-sectional studies. The only randomized controlled trial that investigated calcium supplementation showed no effect on blood lead levels.8 Our search revealed no good-quality studies on the effect of iron or fat intake on lead poisoning.
In summary, because the prevalence of lead poisoning varies between communities and continues to change, standard recommendations are not possible. Clinicians must rely on local epidemiologic data to make screening decisions. Although questionnaires are accurate in predicting elevated blood lead levels in some settings, no specific set of questions can be recommended for all populations.
No treatment options for those with mild to moderate elevations in blood lead levels have been shown to improve clinically important outcomes, although some interventions may decrease blood lead levels.
Recommendations from others
The Centers for Disease Control and Prevention (CDC) recommends
- that individual states develop screening plans based on local data
- universal screening at 12 and 24 months of age:
Otherwise screening should be targeted based on a questionnaire on age of housing, recent or ongoing remodeling, and having a sibling or playmate diagnosed with lead poisoning, in addition to questions on locally important risk factors.9
The American Academy of Pediatrics endorses the CDC recommendations.2 The US Preventive Services Task Force, the American Academy of Family Physicians, and the American College of Preventive Medicine all recommend screening for lead poisoning at 12 months of age in children with demographic or geographic risk factors.3,10,11
Lead screening: Think locally
Julia Fashner, MD
St. Joseph Regional Medical Center, South Bend, Ind
The local health department can provide information about lead screening in your community, whether based on blood levels or the housing conditions. If your patients need screening, you may want to add a reminder on a flow sheet in the chart to do a questionnaire or a blood draw. Finding and treating severely elevated lead levels can change outcomes, but for less elevated levels, the evidence shows no benefit. You should work with the health department when considering therapy for children with elevated blood lead levels.
1. Kaufmann RB, Clouse TL, Olson DR, Matte TD. Elevated blood lead levels and blood lead screening among US children aged one to five years: 1988–1994. Pediatrics 2000;106:E79.-
2. Screening for elevated blood lead levels. American Academy of Pediatrics Committee on Environmental Health. Pediatrics 1998;101:1072-1078.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore, Md: Lippincott Williams & Wilkins; 1996.
4. Binns HJ, LeBailly SA, Fingar AR, Saunders S. Evaluation of risk assessment questions used to target blood lead screening in Illinois. Pediatrics 1999;103:100-106.
5. Rogan WJ, Dietrich KN, Ware JH, et al. The effect of chela-tion therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med 2001;344:1421-1426.
6. Haynes E, Lanphear BP, Tohn E, Farr N, Rhoads GG. The effect of interior lead hazard controls on children’s blood lead concentrations: a systematic evaluation. Environ Health Perspect 2002;110:103-107.
7. Hilts SR, Hertzman C, Marion SA. A controlled trial of the effect of HEPA vacuuming on childhood lead exposure. Can J Public Health 1995;86:345-350.
8. Ballew C, Bowman B. Recommending calcium to reduce lead toxicity in children: a critical review. Nutr Rev 2001;59(3 Pt 1):71-79.
9. Centers for Disease Control and Prevention. Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials. Atlanta, Ga: CDC, 1997.
10. AAFP Policy Recommendations for Periodic Health Examinations 2002. Available at http://www.aafp.org/ exam.xml. Accessed on February 9, 2003.
11. Lane WG, Kemper AR. American College of Preventive Medicine Practice Policy Statement. Screening for elevated blood lead levels in children. Am J Prev Med 2001;20:78-82.
1. Kaufmann RB, Clouse TL, Olson DR, Matte TD. Elevated blood lead levels and blood lead screening among US children aged one to five years: 1988–1994. Pediatrics 2000;106:E79.-
2. Screening for elevated blood lead levels. American Academy of Pediatrics Committee on Environmental Health. Pediatrics 1998;101:1072-1078.
3. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Baltimore, Md: Lippincott Williams & Wilkins; 1996.
4. Binns HJ, LeBailly SA, Fingar AR, Saunders S. Evaluation of risk assessment questions used to target blood lead screening in Illinois. Pediatrics 1999;103:100-106.
5. Rogan WJ, Dietrich KN, Ware JH, et al. The effect of chela-tion therapy with succimer on neuropsychological development in children exposed to lead. N Engl J Med 2001;344:1421-1426.
6. Haynes E, Lanphear BP, Tohn E, Farr N, Rhoads GG. The effect of interior lead hazard controls on children’s blood lead concentrations: a systematic evaluation. Environ Health Perspect 2002;110:103-107.
7. Hilts SR, Hertzman C, Marion SA. A controlled trial of the effect of HEPA vacuuming on childhood lead exposure. Can J Public Health 1995;86:345-350.
8. Ballew C, Bowman B. Recommending calcium to reduce lead toxicity in children: a critical review. Nutr Rev 2001;59(3 Pt 1):71-79.
9. Centers for Disease Control and Prevention. Screening Young Children for Lead Poisoning: Guidance for State and Local Public Health Officials. Atlanta, Ga: CDC, 1997.
10. AAFP Policy Recommendations for Periodic Health Examinations 2002. Available at http://www.aafp.org/ exam.xml. Accessed on February 9, 2003.
11. Lane WG, Kemper AR. American College of Preventive Medicine Practice Policy Statement. Screening for elevated blood lead levels in children. Am J Prev Med 2001;20:78-82.
Evidence-based answers from the Family Physicians Inquiries Network
Do nasal decongestants relieve symptoms?
Oral and topical nasal decongestants result in a statistically significant improvement in subjective symptoms of nasal congestion and objective nasal airway resistance in adults’ common colds (strength of recommendation [SOR]: A, based on randomized controlled trials). Evidence is lacking to support the use of decongestants in acute sinusitis.
Evidence summary
Nasal congestion is the most common symptom of the common cold, and hundreds of millions of dollars are spent annually on decongestants. A Cochrane review of 4 randomized controlled trials compared single doses of oxymetazoline, pseudoephedrine, and phenylpropanolamine.1 Included studies involved from 30 to 106 participants, were double-blinded and placebo-controlled, used either topical or oral decongestants for symptoms of less than 5 days’ duration, and measured either subjective or objective relief or adverse events. All 4 studies used nasal airway resistance as an objective measure of nasal congestion, and a combined symptom score as a subjective measure of relief. One study also administered a side-effect questionnaire.
In all studies, topical and oral decongestants were equally efficacious, producing a 13% reduction in subjective symptoms and a significant decrease in nasal airway resistance after 1 dose of decongestant. Only 1 study investigated repeated doses of decongestants and found no significant additional improvement from repeated doses over a 5-day period.
More studies are needed to evaluate efficacy of multiple doses. Clinical interpretation of these results must take into consideration that quality-of-life measures were not evaluated and that none of the studies included children under 12.
Limited data are available on decongestants in sinusitis. Most studies focused on the use of nasal corticosteroids. One placebo-controlled, randomized controlled trial evaluated the effect on mucociliary clearance from adding nasal saline, nasal steroids, or oxymetazoline to antibiotics in acute bacterial sinusitis.2 The group using oxymetazoline increased mucociliary clearance immediately (within 20 minutes). However, at 3 weeks, the improvement in mucociliary clearance in the oxymetazoline group was not significantly different than in the other groups.
An additional prospective, placebo-controlled study evaluated improvement in x-ray findings as well as subjective symptoms in acute sinusitis using phenoxymethyl-penicillin (penicillin V) in combination with oxymetazoline or placebo administered via a variety of nasal delivery systems.3 Oxymetazoline was not significantly different from placebo. Controlled prospective studies are lacking to support the use of decongestants in acute sinusitis.
Recommendations from others
Expert opinion from Current Clinical Topics in Infectious Diseases does not recommend the use of decongestants in sinusitis or the common cold in the absence of concurrent allergic rhinosinusitis.4 This recommendation is based on the lack of evidence regarding efficacy and the known rebound congestion associated with topical decongestants. If a decongestant is prescribed, the oral route is preferred, with the understanding of potential significant side effects of nervousness, insomnia, tachycardia, and hypertension.
Decongestants can do more harm than good
Russell W. Roberts, MD
Louisiana State University Health Sciences Center, Shreveport
Never one to have been impressed with most of the current symptomatic treatments available for the common cold, I have for years been amazed at how quick the public is to purchase and repeatedly use these products.
While a judicious course of decongestants can ease the congestion, when misused they often cause significant harm and discomfort that is difficult to resolve. Patients whom I have assisted through successful discontinuance of topical nasal decongestants are among the most appreciative in my practice.
1. Taverner D, Bickford L, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2000;(2):CD001953. Updated quarterly.
2. Inanli S, Ozturk O, Korkmaz M, Tutkun A, Batman C. The effects of topical agents of fluticasone propionate, oxymetazoline, and 3% and 0.9% sodium chloride solutions on mucociliary clearance in the therapy of acute bacterial rhinosinusitis in vivo. Laryngoscope 2002;112:320-325.
3. Wiklund L, Stierna P, Berglund R, Westrin KM, Tonnesson M. The efficacy of oxymetazoline administered with a nasal bellows container and combined with oral phenoxymethyl-penicillin in the treatment of acute maxillary sinusitis. Acta Otolaryngol Suppl 1994;515:57-64.
4. Chow AW. Acute sinusitis: current status of etiologies, diagnosis, and treatment. Curr Clin Top Infect Dis 2001;21:31-63.
Oral and topical nasal decongestants result in a statistically significant improvement in subjective symptoms of nasal congestion and objective nasal airway resistance in adults’ common colds (strength of recommendation [SOR]: A, based on randomized controlled trials). Evidence is lacking to support the use of decongestants in acute sinusitis.
Evidence summary
Nasal congestion is the most common symptom of the common cold, and hundreds of millions of dollars are spent annually on decongestants. A Cochrane review of 4 randomized controlled trials compared single doses of oxymetazoline, pseudoephedrine, and phenylpropanolamine.1 Included studies involved from 30 to 106 participants, were double-blinded and placebo-controlled, used either topical or oral decongestants for symptoms of less than 5 days’ duration, and measured either subjective or objective relief or adverse events. All 4 studies used nasal airway resistance as an objective measure of nasal congestion, and a combined symptom score as a subjective measure of relief. One study also administered a side-effect questionnaire.
In all studies, topical and oral decongestants were equally efficacious, producing a 13% reduction in subjective symptoms and a significant decrease in nasal airway resistance after 1 dose of decongestant. Only 1 study investigated repeated doses of decongestants and found no significant additional improvement from repeated doses over a 5-day period.
More studies are needed to evaluate efficacy of multiple doses. Clinical interpretation of these results must take into consideration that quality-of-life measures were not evaluated and that none of the studies included children under 12.
Limited data are available on decongestants in sinusitis. Most studies focused on the use of nasal corticosteroids. One placebo-controlled, randomized controlled trial evaluated the effect on mucociliary clearance from adding nasal saline, nasal steroids, or oxymetazoline to antibiotics in acute bacterial sinusitis.2 The group using oxymetazoline increased mucociliary clearance immediately (within 20 minutes). However, at 3 weeks, the improvement in mucociliary clearance in the oxymetazoline group was not significantly different than in the other groups.
An additional prospective, placebo-controlled study evaluated improvement in x-ray findings as well as subjective symptoms in acute sinusitis using phenoxymethyl-penicillin (penicillin V) in combination with oxymetazoline or placebo administered via a variety of nasal delivery systems.3 Oxymetazoline was not significantly different from placebo. Controlled prospective studies are lacking to support the use of decongestants in acute sinusitis.
Recommendations from others
Expert opinion from Current Clinical Topics in Infectious Diseases does not recommend the use of decongestants in sinusitis or the common cold in the absence of concurrent allergic rhinosinusitis.4 This recommendation is based on the lack of evidence regarding efficacy and the known rebound congestion associated with topical decongestants. If a decongestant is prescribed, the oral route is preferred, with the understanding of potential significant side effects of nervousness, insomnia, tachycardia, and hypertension.
Decongestants can do more harm than good
Russell W. Roberts, MD
Louisiana State University Health Sciences Center, Shreveport
Never one to have been impressed with most of the current symptomatic treatments available for the common cold, I have for years been amazed at how quick the public is to purchase and repeatedly use these products.
While a judicious course of decongestants can ease the congestion, when misused they often cause significant harm and discomfort that is difficult to resolve. Patients whom I have assisted through successful discontinuance of topical nasal decongestants are among the most appreciative in my practice.
Oral and topical nasal decongestants result in a statistically significant improvement in subjective symptoms of nasal congestion and objective nasal airway resistance in adults’ common colds (strength of recommendation [SOR]: A, based on randomized controlled trials). Evidence is lacking to support the use of decongestants in acute sinusitis.
Evidence summary
Nasal congestion is the most common symptom of the common cold, and hundreds of millions of dollars are spent annually on decongestants. A Cochrane review of 4 randomized controlled trials compared single doses of oxymetazoline, pseudoephedrine, and phenylpropanolamine.1 Included studies involved from 30 to 106 participants, were double-blinded and placebo-controlled, used either topical or oral decongestants for symptoms of less than 5 days’ duration, and measured either subjective or objective relief or adverse events. All 4 studies used nasal airway resistance as an objective measure of nasal congestion, and a combined symptom score as a subjective measure of relief. One study also administered a side-effect questionnaire.
In all studies, topical and oral decongestants were equally efficacious, producing a 13% reduction in subjective symptoms and a significant decrease in nasal airway resistance after 1 dose of decongestant. Only 1 study investigated repeated doses of decongestants and found no significant additional improvement from repeated doses over a 5-day period.
More studies are needed to evaluate efficacy of multiple doses. Clinical interpretation of these results must take into consideration that quality-of-life measures were not evaluated and that none of the studies included children under 12.
Limited data are available on decongestants in sinusitis. Most studies focused on the use of nasal corticosteroids. One placebo-controlled, randomized controlled trial evaluated the effect on mucociliary clearance from adding nasal saline, nasal steroids, or oxymetazoline to antibiotics in acute bacterial sinusitis.2 The group using oxymetazoline increased mucociliary clearance immediately (within 20 minutes). However, at 3 weeks, the improvement in mucociliary clearance in the oxymetazoline group was not significantly different than in the other groups.
An additional prospective, placebo-controlled study evaluated improvement in x-ray findings as well as subjective symptoms in acute sinusitis using phenoxymethyl-penicillin (penicillin V) in combination with oxymetazoline or placebo administered via a variety of nasal delivery systems.3 Oxymetazoline was not significantly different from placebo. Controlled prospective studies are lacking to support the use of decongestants in acute sinusitis.
Recommendations from others
Expert opinion from Current Clinical Topics in Infectious Diseases does not recommend the use of decongestants in sinusitis or the common cold in the absence of concurrent allergic rhinosinusitis.4 This recommendation is based on the lack of evidence regarding efficacy and the known rebound congestion associated with topical decongestants. If a decongestant is prescribed, the oral route is preferred, with the understanding of potential significant side effects of nervousness, insomnia, tachycardia, and hypertension.
Decongestants can do more harm than good
Russell W. Roberts, MD
Louisiana State University Health Sciences Center, Shreveport
Never one to have been impressed with most of the current symptomatic treatments available for the common cold, I have for years been amazed at how quick the public is to purchase and repeatedly use these products.
While a judicious course of decongestants can ease the congestion, when misused they often cause significant harm and discomfort that is difficult to resolve. Patients whom I have assisted through successful discontinuance of topical nasal decongestants are among the most appreciative in my practice.
1. Taverner D, Bickford L, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2000;(2):CD001953. Updated quarterly.
2. Inanli S, Ozturk O, Korkmaz M, Tutkun A, Batman C. The effects of topical agents of fluticasone propionate, oxymetazoline, and 3% and 0.9% sodium chloride solutions on mucociliary clearance in the therapy of acute bacterial rhinosinusitis in vivo. Laryngoscope 2002;112:320-325.
3. Wiklund L, Stierna P, Berglund R, Westrin KM, Tonnesson M. The efficacy of oxymetazoline administered with a nasal bellows container and combined with oral phenoxymethyl-penicillin in the treatment of acute maxillary sinusitis. Acta Otolaryngol Suppl 1994;515:57-64.
4. Chow AW. Acute sinusitis: current status of etiologies, diagnosis, and treatment. Curr Clin Top Infect Dis 2001;21:31-63.
1. Taverner D, Bickford L, Draper M. Nasal decongestants for the common cold. Cochrane Database Syst Rev 2000;(2):CD001953. Updated quarterly.
2. Inanli S, Ozturk O, Korkmaz M, Tutkun A, Batman C. The effects of topical agents of fluticasone propionate, oxymetazoline, and 3% and 0.9% sodium chloride solutions on mucociliary clearance in the therapy of acute bacterial rhinosinusitis in vivo. Laryngoscope 2002;112:320-325.
3. Wiklund L, Stierna P, Berglund R, Westrin KM, Tonnesson M. The efficacy of oxymetazoline administered with a nasal bellows container and combined with oral phenoxymethyl-penicillin in the treatment of acute maxillary sinusitis. Acta Otolaryngol Suppl 1994;515:57-64.
4. Chow AW. Acute sinusitis: current status of etiologies, diagnosis, and treatment. Curr Clin Top Infect Dis 2001;21:31-63.
Evidence-based answers from the Family Physicians Inquiries Network
Do antiarrhythmics prevent sudden death in patients with heart failure?
Beta-blockers (class II antiarrhythmics) reduce sudden death and total mortality in patients with heart failure (strength of recommendation [SOR]: A, based on systematic reviews of randomized controlled trials). Amiodarone (class III) may reduce sudden death in heart failure (SOR: B, extrapolation from randomized controlled trials), but evidence is weak that it reduces total mortality, and it has significant side effects. Class I and other class III antiarrhythmic agents appear cause an increase in mortality due to sudden death in heart failure (SOR: B, extrapolations from randomized controlled trials).
Evidence summary
Antiarrhythmic agents have been studied in patients with heart failure because these persons have a high incidence of sudden death, presumably from ventricular arrhythmias. Although the implantable defibrillator is an alternative antiarrhythmic device that may be preferred for some patients, we restricted our review to pharmacologic antiarrhythmics.
The beta-blockers bisoprolol, carvedilol, and metoprolol1-3 were studied in large randomized controlled trials. The relative risk reduction (RRR) for sudden death ranged from 10% to 52% in the larger trials and 30% to 39% in meta-analyses.1-4 The absolute risk reduction (ARR) was about 2% to 3% per year for sudden death and 3% to 5% for total mortality (number needed to treat=20–33 per year).
These beta-blockers were well-tolerated, even in class IV New York Heart Association patients, and improved other endpoints. Although we cannot say whether the benefits are a class effect, they were seen with both beta-1 selective and nonselective agents.
Amiodarone was studied in 2 large randomized controlled trials enrolling patients with heart failure, in trials that included patients with or without heart failure at high risk for sudden death (usually post-myocardial infarction or with complex ventricular arrhythmias), and in meta-analyses.5-8 The largest randomized controlled trial in heart failure showed a significant ARR of 2.9% for sudden death,5 but was unblinded. The largest placebo-controlled trial in heart failure failed to detect a significant decrease in sudden death.6
Meta-analyses, weakened by heterogeneity and the inclusion of patients without heart failure, detected a significant 21% to 25% RRR for sudden death,7,8 and an ARR of 2% to 3% per year. The pooled data from the placebo-controlled heart failure trials showed nonsignificant trends: 1.6% per year ARR for sudden death, 0.6% per year for total mortality.
These possible benefits must be balanced against the risk of harm from amiodarone, including excess rates of pulmonary infiltrate (1.1% per year), thyroid dysfunction (6.8% per year), liver enzyme abnormalities (0.6% per year), neuropathy (0.3% per year), and bradycardia (1.6% per year), as well as a discontinuation rate of 41% compared with 27% for placebo.7 No evidence suggested that use of amiodarone in patients with heart failure increased mortality.
Class I antiarrhythmics and other class III agents have not been studied in heart failure trials, but were associated with increased mortality in studies of patients at high risk for ventricular arrhythmia,9,10 including patients with left ventricular dysfunction. Because this increase in mortality is thought to be due to proarrhythmic properties of the drugs, further trials in heart failure patients are unlikely to occur.
Recommendations from others
American College of Cardiology/American Heart Association (ACC/AHA),11 European Society of Cardiology (ESC),12 and Heart Failure Society of America (HFSA) guidelines13 address heart failure. ACC/AHA and ESC reports specifically mention that beta-blockers reduce sudden death. Both strongly support the use of beta-blockers in patients with heart failure.
ACC/AHA finds “conflicting evidence and/or a divergence of opinion about the usefulness/ efficacy” of amiodarone to prevent sudden death and advises: “routine use of amiodarone to prevent sudden death is not recommended.” The ESC and HFSA also recommend against routine use of amiodarone.
All 3 guidelines, however, state that for the control of symptomatic arrhythmias in heart failure, amiodarone is the antiarrhythmic agent of choice. All 3 also recommend not using class I or other class III agents in heart failure.
Beta-blockers reduce mortality in patients with heart failure
Joseph Saseen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Numerous well-controlled clinical trials have conclusively demonstrated that beta-blockers reduce morbidity and mortality (including sudden death) in patients with systolic heart failure. They are considered disease-modifying agents and their use is strongly encouraged. Beta-blocker therapy must be initiated using low doses and only when patients are hemo-dynamically stable, with gradual dose titrations to prevent acute decompensation.
Evidence for amiodarone shows some reduction in sudden death, but these data are less compelling. Moreover, adverse effects and drug interactions complicate long-term amiodarone use. Use of class I (eg, flecainide, procainamide, propafenone) and other class III (sotalol) anti-arrhythmics to reduce sudden death is discouraged.
1. Effect of metoprolol CR/XL in chronic heart failure. Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
2. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Lancet 1999;353:9-13.
3. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-1355.
4. Lee S, Spencer A. Beta-blockers to reduce mortality in patients with systolic dysfunction: a meta-analysis. J Fam Pract 2001;50:499-504.
5. Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344:493-498.
6. Singh SN, Fletcher RD, Fisher SG, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med 1995;333:77-82.
7. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Amiodarone Trials Meta-Analysis Investigators. Lancet 1997;350:1417-1424.
8. Piepoli M, Villani GO, Ponikowski P, Wright A, Flather MD, Coats AJ. Overview and meta-analysis of randomised trials of amiodarone in chronic heart failure. Int J Cardiol 1998;66:1-10.
9. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781-788.
10. Connolly SJ. Meta-analysis of anti-arrhythmic drug trials. Am J Cardiol 1999;84:90R-93R.
11. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. Bethesda, Md: American College of Cardiology; 2001.
12. Priori SG, Aliot E, Blomstrom-Lundqvist C, et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J 2001;22:1374-1450.
13. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction— pharmacological approaches. Heart Failure Society of merica. Pharmacotherapy 2000;20:495-522.
Beta-blockers (class II antiarrhythmics) reduce sudden death and total mortality in patients with heart failure (strength of recommendation [SOR]: A, based on systematic reviews of randomized controlled trials). Amiodarone (class III) may reduce sudden death in heart failure (SOR: B, extrapolation from randomized controlled trials), but evidence is weak that it reduces total mortality, and it has significant side effects. Class I and other class III antiarrhythmic agents appear cause an increase in mortality due to sudden death in heart failure (SOR: B, extrapolations from randomized controlled trials).
Evidence summary
Antiarrhythmic agents have been studied in patients with heart failure because these persons have a high incidence of sudden death, presumably from ventricular arrhythmias. Although the implantable defibrillator is an alternative antiarrhythmic device that may be preferred for some patients, we restricted our review to pharmacologic antiarrhythmics.
The beta-blockers bisoprolol, carvedilol, and metoprolol1-3 were studied in large randomized controlled trials. The relative risk reduction (RRR) for sudden death ranged from 10% to 52% in the larger trials and 30% to 39% in meta-analyses.1-4 The absolute risk reduction (ARR) was about 2% to 3% per year for sudden death and 3% to 5% for total mortality (number needed to treat=20–33 per year).
These beta-blockers were well-tolerated, even in class IV New York Heart Association patients, and improved other endpoints. Although we cannot say whether the benefits are a class effect, they were seen with both beta-1 selective and nonselective agents.
Amiodarone was studied in 2 large randomized controlled trials enrolling patients with heart failure, in trials that included patients with or without heart failure at high risk for sudden death (usually post-myocardial infarction or with complex ventricular arrhythmias), and in meta-analyses.5-8 The largest randomized controlled trial in heart failure showed a significant ARR of 2.9% for sudden death,5 but was unblinded. The largest placebo-controlled trial in heart failure failed to detect a significant decrease in sudden death.6
Meta-analyses, weakened by heterogeneity and the inclusion of patients without heart failure, detected a significant 21% to 25% RRR for sudden death,7,8 and an ARR of 2% to 3% per year. The pooled data from the placebo-controlled heart failure trials showed nonsignificant trends: 1.6% per year ARR for sudden death, 0.6% per year for total mortality.
These possible benefits must be balanced against the risk of harm from amiodarone, including excess rates of pulmonary infiltrate (1.1% per year), thyroid dysfunction (6.8% per year), liver enzyme abnormalities (0.6% per year), neuropathy (0.3% per year), and bradycardia (1.6% per year), as well as a discontinuation rate of 41% compared with 27% for placebo.7 No evidence suggested that use of amiodarone in patients with heart failure increased mortality.
Class I antiarrhythmics and other class III agents have not been studied in heart failure trials, but were associated with increased mortality in studies of patients at high risk for ventricular arrhythmia,9,10 including patients with left ventricular dysfunction. Because this increase in mortality is thought to be due to proarrhythmic properties of the drugs, further trials in heart failure patients are unlikely to occur.
Recommendations from others
American College of Cardiology/American Heart Association (ACC/AHA),11 European Society of Cardiology (ESC),12 and Heart Failure Society of America (HFSA) guidelines13 address heart failure. ACC/AHA and ESC reports specifically mention that beta-blockers reduce sudden death. Both strongly support the use of beta-blockers in patients with heart failure.
ACC/AHA finds “conflicting evidence and/or a divergence of opinion about the usefulness/ efficacy” of amiodarone to prevent sudden death and advises: “routine use of amiodarone to prevent sudden death is not recommended.” The ESC and HFSA also recommend against routine use of amiodarone.
All 3 guidelines, however, state that for the control of symptomatic arrhythmias in heart failure, amiodarone is the antiarrhythmic agent of choice. All 3 also recommend not using class I or other class III agents in heart failure.
Beta-blockers reduce mortality in patients with heart failure
Joseph Saseen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Numerous well-controlled clinical trials have conclusively demonstrated that beta-blockers reduce morbidity and mortality (including sudden death) in patients with systolic heart failure. They are considered disease-modifying agents and their use is strongly encouraged. Beta-blocker therapy must be initiated using low doses and only when patients are hemo-dynamically stable, with gradual dose titrations to prevent acute decompensation.
Evidence for amiodarone shows some reduction in sudden death, but these data are less compelling. Moreover, adverse effects and drug interactions complicate long-term amiodarone use. Use of class I (eg, flecainide, procainamide, propafenone) and other class III (sotalol) anti-arrhythmics to reduce sudden death is discouraged.
Beta-blockers (class II antiarrhythmics) reduce sudden death and total mortality in patients with heart failure (strength of recommendation [SOR]: A, based on systematic reviews of randomized controlled trials). Amiodarone (class III) may reduce sudden death in heart failure (SOR: B, extrapolation from randomized controlled trials), but evidence is weak that it reduces total mortality, and it has significant side effects. Class I and other class III antiarrhythmic agents appear cause an increase in mortality due to sudden death in heart failure (SOR: B, extrapolations from randomized controlled trials).
Evidence summary
Antiarrhythmic agents have been studied in patients with heart failure because these persons have a high incidence of sudden death, presumably from ventricular arrhythmias. Although the implantable defibrillator is an alternative antiarrhythmic device that may be preferred for some patients, we restricted our review to pharmacologic antiarrhythmics.
The beta-blockers bisoprolol, carvedilol, and metoprolol1-3 were studied in large randomized controlled trials. The relative risk reduction (RRR) for sudden death ranged from 10% to 52% in the larger trials and 30% to 39% in meta-analyses.1-4 The absolute risk reduction (ARR) was about 2% to 3% per year for sudden death and 3% to 5% for total mortality (number needed to treat=20–33 per year).
These beta-blockers were well-tolerated, even in class IV New York Heart Association patients, and improved other endpoints. Although we cannot say whether the benefits are a class effect, they were seen with both beta-1 selective and nonselective agents.
Amiodarone was studied in 2 large randomized controlled trials enrolling patients with heart failure, in trials that included patients with or without heart failure at high risk for sudden death (usually post-myocardial infarction or with complex ventricular arrhythmias), and in meta-analyses.5-8 The largest randomized controlled trial in heart failure showed a significant ARR of 2.9% for sudden death,5 but was unblinded. The largest placebo-controlled trial in heart failure failed to detect a significant decrease in sudden death.6
Meta-analyses, weakened by heterogeneity and the inclusion of patients without heart failure, detected a significant 21% to 25% RRR for sudden death,7,8 and an ARR of 2% to 3% per year. The pooled data from the placebo-controlled heart failure trials showed nonsignificant trends: 1.6% per year ARR for sudden death, 0.6% per year for total mortality.
These possible benefits must be balanced against the risk of harm from amiodarone, including excess rates of pulmonary infiltrate (1.1% per year), thyroid dysfunction (6.8% per year), liver enzyme abnormalities (0.6% per year), neuropathy (0.3% per year), and bradycardia (1.6% per year), as well as a discontinuation rate of 41% compared with 27% for placebo.7 No evidence suggested that use of amiodarone in patients with heart failure increased mortality.
Class I antiarrhythmics and other class III agents have not been studied in heart failure trials, but were associated with increased mortality in studies of patients at high risk for ventricular arrhythmia,9,10 including patients with left ventricular dysfunction. Because this increase in mortality is thought to be due to proarrhythmic properties of the drugs, further trials in heart failure patients are unlikely to occur.
Recommendations from others
American College of Cardiology/American Heart Association (ACC/AHA),11 European Society of Cardiology (ESC),12 and Heart Failure Society of America (HFSA) guidelines13 address heart failure. ACC/AHA and ESC reports specifically mention that beta-blockers reduce sudden death. Both strongly support the use of beta-blockers in patients with heart failure.
ACC/AHA finds “conflicting evidence and/or a divergence of opinion about the usefulness/ efficacy” of amiodarone to prevent sudden death and advises: “routine use of amiodarone to prevent sudden death is not recommended.” The ESC and HFSA also recommend against routine use of amiodarone.
All 3 guidelines, however, state that for the control of symptomatic arrhythmias in heart failure, amiodarone is the antiarrhythmic agent of choice. All 3 also recommend not using class I or other class III agents in heart failure.
Beta-blockers reduce mortality in patients with heart failure
Joseph Saseen, PharmD, BCPS
University of Colorado Health Sciences Center, Denver
Numerous well-controlled clinical trials have conclusively demonstrated that beta-blockers reduce morbidity and mortality (including sudden death) in patients with systolic heart failure. They are considered disease-modifying agents and their use is strongly encouraged. Beta-blocker therapy must be initiated using low doses and only when patients are hemo-dynamically stable, with gradual dose titrations to prevent acute decompensation.
Evidence for amiodarone shows some reduction in sudden death, but these data are less compelling. Moreover, adverse effects and drug interactions complicate long-term amiodarone use. Use of class I (eg, flecainide, procainamide, propafenone) and other class III (sotalol) anti-arrhythmics to reduce sudden death is discouraged.
1. Effect of metoprolol CR/XL in chronic heart failure. Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
2. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Lancet 1999;353:9-13.
3. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-1355.
4. Lee S, Spencer A. Beta-blockers to reduce mortality in patients with systolic dysfunction: a meta-analysis. J Fam Pract 2001;50:499-504.
5. Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344:493-498.
6. Singh SN, Fletcher RD, Fisher SG, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med 1995;333:77-82.
7. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Amiodarone Trials Meta-Analysis Investigators. Lancet 1997;350:1417-1424.
8. Piepoli M, Villani GO, Ponikowski P, Wright A, Flather MD, Coats AJ. Overview and meta-analysis of randomised trials of amiodarone in chronic heart failure. Int J Cardiol 1998;66:1-10.
9. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781-788.
10. Connolly SJ. Meta-analysis of anti-arrhythmic drug trials. Am J Cardiol 1999;84:90R-93R.
11. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. Bethesda, Md: American College of Cardiology; 2001.
12. Priori SG, Aliot E, Blomstrom-Lundqvist C, et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J 2001;22:1374-1450.
13. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction— pharmacological approaches. Heart Failure Society of merica. Pharmacotherapy 2000;20:495-522.
1. Effect of metoprolol CR/XL in chronic heart failure. Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-2007.
2. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial Lancet 1999;353:9-13.
3. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-1355.
4. Lee S, Spencer A. Beta-blockers to reduce mortality in patients with systolic dysfunction: a meta-analysis. J Fam Pract 2001;50:499-504.
5. Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet. 1994;344:493-498.
6. Singh SN, Fletcher RD, Fisher SG, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med 1995;333:77-82.
7. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Amiodarone Trials Meta-Analysis Investigators. Lancet 1997;350:1417-1424.
8. Piepoli M, Villani GO, Ponikowski P, Wright A, Flather MD, Coats AJ. Overview and meta-analysis of randomised trials of amiodarone in chronic heart failure. Int J Cardiol 1998;66:1-10.
9. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781-788.
10. Connolly SJ. Meta-analysis of anti-arrhythmic drug trials. Am J Cardiol 1999;84:90R-93R.
11. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult. Bethesda, Md: American College of Cardiology; 2001.
12. Priori SG, Aliot E, Blomstrom-Lundqvist C, et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur Heart J 2001;22:1374-1450.
13. HFSA guidelines for management of patients with heart failure caused by left ventricular systolic dysfunction— pharmacological approaches. Heart Failure Society of merica. Pharmacotherapy 2000;20:495-522.
Evidence-based answers from the Family Physicians Inquiries Network
Does physical therapy improve symptoms of fibromyalgia?
Physical therapy is minimally effective in the treatment of fibromyalgia, with immediate post-treatment improvement in pain and tender points, and both short- and longer-term improved self-efficacy (confidence in performing tasks) (strength of recommendation [SOR]: B, 1 small, high-quality randomized controlled trial, 4 additional small randomized controlled trials).
Multidisciplinary rehabilitation is probably not effective for this disorder but warrants future research, as trial quality is poor (SOR: B, systematic review of 4 small or low-quality and 3 additional randomized controlled trials on widespread pain conditions).
Evidence summary
The goal of physical therapy is to maximize function and reduce impairment to limit disability in patients with musculoskeletal conditions.1 Based on a British study, physical therapists most commonly use exercise, education about correct posture and functional activity, relaxation, and energy conservation and fatigue management.2 For this review, physical therapy is defined as a treatment program that includes patient education and supervised exercise.
In the highest-quality trial, Buckelew and colleagues3 randomized 119 subjects to 1 of 4 groups: biofeedback and relaxation training, exercise training, combination treatment, and an education and attention control program. Individuals were evaluated on measures of pain, function, disease impact, and self-efficacy. Evaluators were blinded to treatment group. Patients were followed for 2 years, and follow-up information was available on 85% of patients.
At immediate postintervention follow-up, all treatment groups were significantly improved on tender-point index score compared with the control group, but this was due to a modest deterioration for the control group rather than improvements in the treatment groups. In addition, all groups showed improvements in self-efficacy for function compared with the control group but not for other self-efficacy measures. While within-group improvements in the treatment groups were seen, no significant differences were seen from the control group.
Another trial randomized 99 patients to 3 groups: education and cognitive behavioral therapy; education, cognitive behavioral therapy and exercise; or a wait-list control group.4 At the 6-month follow-up, the education group scored significantly higher than the others—but only on self-reported measures of daily functioning and self-efficacy.
In another study, 45 patients with fibromyalgia were randomly assigned to a 6-week program combining exercise and multidisciplinary education or to a control group.5 The treatment group had significant improvements in walking distance and for 2 measures on the Fibromyalgia Impact Questionnaire (feeling bad and morning fatigue). Keel and colleagues6 found no immediate treatment benefit following 15 weeks of education, cognitive behavioral therapy, and exercise vs relaxation training in their small randomized controlled trial.6
In contrast, another study reported significant and immediate improvements in 2 groups— exercise and education; exercise, education, and cognitive behavioral therapy—when compared with control patients on self-reported symptoms and knowledge.# The exercise and education group was also better than the control patients in self-reported daily functioning.
We identified 2 additional trials examining different types of physical therapy for fibromyalgia that did not include control groups. In a trial of muscle strengthening vs flexibility training, investigators found no difference between groups on measures including tender points and disease and symptom severity.8 They did find benefits in symptoms and self-efficacy over baseline, but it is not known whether these were sustained.
In a trial comparing 2 physical therapies— body awareness therapy and the Mensendieck system—Kendall and colleagues9 found greater improvements at 18-month follow-up in the Mensendieck group.9 Benefits were seen on the Fibromyalgia Impact Questionnaire, self-efficacy measures, and pain at worst site. The Mensendieck system uses individual interview, analysis of movement patterns, a discussion of possible corrections followed by practice, and relaxation exercises.
Multidisciplinary rehabilitation, often including physical therapy, has also been studied in a limited way. In a systematic review of 7 studies fulfilling inclusion criteria (a total of 1050 patients), Karjalainen and colleagues10 concluded that although education combined with physical training seemed to have some positive results at long-term follow-up, the level of scientific evidence required for recommending these programs for fibromyalgia was lacking.10
Because exercise is believed to be an essential component of physical therapy, we examined the results of a systematic review of exercise for treating fibromyalgia. The authors found 7 high-quality studies, 4 of aerobic training, and concluded that supervised aerobic exercise training had beneficial effects on physical capacity, tender-point threshold, and pain.11 Other investigators have questioned the usefulness of aerobic exercise because long-term benefit remains unclear and compliance is poor.
Recommendations from others
We were unable to find any guidelines for the treatment of fibromyalgia. Patient information sheets from both the American College of Rheumatology (www.rheumatology.org) and American Academy of Orthopaedic Surgeons (orthoinfo.aaos.org) recommend physical modalities such as heat application, massage, and exercise, including fitness training.
Authors of chapters on fibromyalgia in both Kelly’s Textbook of Rheumatology and Harrison’s Principles of Internal Medicine suggest that patients may benefit from regular low-impact aerobic exercise.12,13
Exercise, physical therapy ease pain, “helplessness”
Wail Malaty, MD
Mountain Area Health Education Center, Rural Track Family Practice Residency, Hendersonville, NC, Department of Family Medicine, University of North Carolina, Chapel Hill
Fibromyalgia is a disease of chronic pain. It engenders feelings of helplessness, depression, and loss of control in many patients. In my experience, both physical therapy and exercise can help alleviate these feelings. Physical therapy helps motivated patients perform body movements that they believe may be painful. In this sense, it demonstrates to them the possibility of exercising without excruciating pain. As the evidence suggests, patients who exercise have less pain and feel better in general. Thus, physical therapy can teach patients to actively participate in the management of their disease.
1. Guccione AA. Physical therapy for musculoskeletal syndromes. Rheum Dis Clin North Am 1996;22:551-562.
2. Adams N, Sim J. An overview of fibromyalgia syndrome: mechanisms, differential diagnosis and treatment approaches. Physiotherapy 1998;84:304-318.
3. Buckelew SP, Conway R, Parker J, et al. Biofeedback/relaxation training and exercise interventions for fibromyalgia: a prospective trial. Arthritis Care Res 1998;11:196-209.
4. Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. J Rheumatol 1994;21:714-720.
5. Gowans SE, deHueck A, Voss S, Richardson M. A randomized, controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care Res 1999;12:120-128.
6. Keel PJ, Bodoky C, Gerhard U, Muller W. Comparison of integrated group therapy and group relaxation training for fibromyalgia. Clin J Pain 1998;14:232-238.
7. Vlaeyen JW, Teeken-Gruben NJ, Goossens ME, et al. Cognitive-educational treatment of fibromyalgia: a randomized clinical trial. I. Clinical effects. J Rheumatol 1996;23:1237-1245.
8. Jones KD, Burckhardt CS, Clark SR, Bennett RM, Potempa KM. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J Rheumatol 2002;29:1041-1048.
9. Kendall SA, Ekselius L, Gerdle B, Soren B, Bengtsson A. Feldenkrais intervention in fibromyalgia patients: A pilot study. J Musculoskeletal Pain 2001;9:25-35.
10. Karjalainen K, Malmivaara A, van Tulder M, et al. Multidisciplinary rehabilitation for fibromyalgia and musculoskeletal pain in working age adults. Cochrane Database Syst Rev2000; (2):CD001984. Updated quarterly.
11. Busch A, Schachter CL, Peloso PM, Bombardier C. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev2002; (3):CD003786. Updated quarterly.
12. Claus DJ. Fibromyalgia. In: Ruddy S, Harris ED Jr., Sledge CB, eds. Kelley’s Textbook of Rheumatology. 6th ed. Philadelphia, Pa: W.B. Saunders; 2001;417-427.
13. Gilliland BC. Relapsing polychondritis and other arthritides. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill; 2001;2005-2016.
Physical therapy is minimally effective in the treatment of fibromyalgia, with immediate post-treatment improvement in pain and tender points, and both short- and longer-term improved self-efficacy (confidence in performing tasks) (strength of recommendation [SOR]: B, 1 small, high-quality randomized controlled trial, 4 additional small randomized controlled trials).
Multidisciplinary rehabilitation is probably not effective for this disorder but warrants future research, as trial quality is poor (SOR: B, systematic review of 4 small or low-quality and 3 additional randomized controlled trials on widespread pain conditions).
Evidence summary
The goal of physical therapy is to maximize function and reduce impairment to limit disability in patients with musculoskeletal conditions.1 Based on a British study, physical therapists most commonly use exercise, education about correct posture and functional activity, relaxation, and energy conservation and fatigue management.2 For this review, physical therapy is defined as a treatment program that includes patient education and supervised exercise.
In the highest-quality trial, Buckelew and colleagues3 randomized 119 subjects to 1 of 4 groups: biofeedback and relaxation training, exercise training, combination treatment, and an education and attention control program. Individuals were evaluated on measures of pain, function, disease impact, and self-efficacy. Evaluators were blinded to treatment group. Patients were followed for 2 years, and follow-up information was available on 85% of patients.
At immediate postintervention follow-up, all treatment groups were significantly improved on tender-point index score compared with the control group, but this was due to a modest deterioration for the control group rather than improvements in the treatment groups. In addition, all groups showed improvements in self-efficacy for function compared with the control group but not for other self-efficacy measures. While within-group improvements in the treatment groups were seen, no significant differences were seen from the control group.
Another trial randomized 99 patients to 3 groups: education and cognitive behavioral therapy; education, cognitive behavioral therapy and exercise; or a wait-list control group.4 At the 6-month follow-up, the education group scored significantly higher than the others—but only on self-reported measures of daily functioning and self-efficacy.
In another study, 45 patients with fibromyalgia were randomly assigned to a 6-week program combining exercise and multidisciplinary education or to a control group.5 The treatment group had significant improvements in walking distance and for 2 measures on the Fibromyalgia Impact Questionnaire (feeling bad and morning fatigue). Keel and colleagues6 found no immediate treatment benefit following 15 weeks of education, cognitive behavioral therapy, and exercise vs relaxation training in their small randomized controlled trial.6
In contrast, another study reported significant and immediate improvements in 2 groups— exercise and education; exercise, education, and cognitive behavioral therapy—when compared with control patients on self-reported symptoms and knowledge.# The exercise and education group was also better than the control patients in self-reported daily functioning.
We identified 2 additional trials examining different types of physical therapy for fibromyalgia that did not include control groups. In a trial of muscle strengthening vs flexibility training, investigators found no difference between groups on measures including tender points and disease and symptom severity.8 They did find benefits in symptoms and self-efficacy over baseline, but it is not known whether these were sustained.
In a trial comparing 2 physical therapies— body awareness therapy and the Mensendieck system—Kendall and colleagues9 found greater improvements at 18-month follow-up in the Mensendieck group.9 Benefits were seen on the Fibromyalgia Impact Questionnaire, self-efficacy measures, and pain at worst site. The Mensendieck system uses individual interview, analysis of movement patterns, a discussion of possible corrections followed by practice, and relaxation exercises.
Multidisciplinary rehabilitation, often including physical therapy, has also been studied in a limited way. In a systematic review of 7 studies fulfilling inclusion criteria (a total of 1050 patients), Karjalainen and colleagues10 concluded that although education combined with physical training seemed to have some positive results at long-term follow-up, the level of scientific evidence required for recommending these programs for fibromyalgia was lacking.10
Because exercise is believed to be an essential component of physical therapy, we examined the results of a systematic review of exercise for treating fibromyalgia. The authors found 7 high-quality studies, 4 of aerobic training, and concluded that supervised aerobic exercise training had beneficial effects on physical capacity, tender-point threshold, and pain.11 Other investigators have questioned the usefulness of aerobic exercise because long-term benefit remains unclear and compliance is poor.
Recommendations from others
We were unable to find any guidelines for the treatment of fibromyalgia. Patient information sheets from both the American College of Rheumatology (www.rheumatology.org) and American Academy of Orthopaedic Surgeons (orthoinfo.aaos.org) recommend physical modalities such as heat application, massage, and exercise, including fitness training.
Authors of chapters on fibromyalgia in both Kelly’s Textbook of Rheumatology and Harrison’s Principles of Internal Medicine suggest that patients may benefit from regular low-impact aerobic exercise.12,13
Exercise, physical therapy ease pain, “helplessness”
Wail Malaty, MD
Mountain Area Health Education Center, Rural Track Family Practice Residency, Hendersonville, NC, Department of Family Medicine, University of North Carolina, Chapel Hill
Fibromyalgia is a disease of chronic pain. It engenders feelings of helplessness, depression, and loss of control in many patients. In my experience, both physical therapy and exercise can help alleviate these feelings. Physical therapy helps motivated patients perform body movements that they believe may be painful. In this sense, it demonstrates to them the possibility of exercising without excruciating pain. As the evidence suggests, patients who exercise have less pain and feel better in general. Thus, physical therapy can teach patients to actively participate in the management of their disease.
Physical therapy is minimally effective in the treatment of fibromyalgia, with immediate post-treatment improvement in pain and tender points, and both short- and longer-term improved self-efficacy (confidence in performing tasks) (strength of recommendation [SOR]: B, 1 small, high-quality randomized controlled trial, 4 additional small randomized controlled trials).
Multidisciplinary rehabilitation is probably not effective for this disorder but warrants future research, as trial quality is poor (SOR: B, systematic review of 4 small or low-quality and 3 additional randomized controlled trials on widespread pain conditions).
Evidence summary
The goal of physical therapy is to maximize function and reduce impairment to limit disability in patients with musculoskeletal conditions.1 Based on a British study, physical therapists most commonly use exercise, education about correct posture and functional activity, relaxation, and energy conservation and fatigue management.2 For this review, physical therapy is defined as a treatment program that includes patient education and supervised exercise.
In the highest-quality trial, Buckelew and colleagues3 randomized 119 subjects to 1 of 4 groups: biofeedback and relaxation training, exercise training, combination treatment, and an education and attention control program. Individuals were evaluated on measures of pain, function, disease impact, and self-efficacy. Evaluators were blinded to treatment group. Patients were followed for 2 years, and follow-up information was available on 85% of patients.
At immediate postintervention follow-up, all treatment groups were significantly improved on tender-point index score compared with the control group, but this was due to a modest deterioration for the control group rather than improvements in the treatment groups. In addition, all groups showed improvements in self-efficacy for function compared with the control group but not for other self-efficacy measures. While within-group improvements in the treatment groups were seen, no significant differences were seen from the control group.
Another trial randomized 99 patients to 3 groups: education and cognitive behavioral therapy; education, cognitive behavioral therapy and exercise; or a wait-list control group.4 At the 6-month follow-up, the education group scored significantly higher than the others—but only on self-reported measures of daily functioning and self-efficacy.
In another study, 45 patients with fibromyalgia were randomly assigned to a 6-week program combining exercise and multidisciplinary education or to a control group.5 The treatment group had significant improvements in walking distance and for 2 measures on the Fibromyalgia Impact Questionnaire (feeling bad and morning fatigue). Keel and colleagues6 found no immediate treatment benefit following 15 weeks of education, cognitive behavioral therapy, and exercise vs relaxation training in their small randomized controlled trial.6
In contrast, another study reported significant and immediate improvements in 2 groups— exercise and education; exercise, education, and cognitive behavioral therapy—when compared with control patients on self-reported symptoms and knowledge.# The exercise and education group was also better than the control patients in self-reported daily functioning.
We identified 2 additional trials examining different types of physical therapy for fibromyalgia that did not include control groups. In a trial of muscle strengthening vs flexibility training, investigators found no difference between groups on measures including tender points and disease and symptom severity.8 They did find benefits in symptoms and self-efficacy over baseline, but it is not known whether these were sustained.
In a trial comparing 2 physical therapies— body awareness therapy and the Mensendieck system—Kendall and colleagues9 found greater improvements at 18-month follow-up in the Mensendieck group.9 Benefits were seen on the Fibromyalgia Impact Questionnaire, self-efficacy measures, and pain at worst site. The Mensendieck system uses individual interview, analysis of movement patterns, a discussion of possible corrections followed by practice, and relaxation exercises.
Multidisciplinary rehabilitation, often including physical therapy, has also been studied in a limited way. In a systematic review of 7 studies fulfilling inclusion criteria (a total of 1050 patients), Karjalainen and colleagues10 concluded that although education combined with physical training seemed to have some positive results at long-term follow-up, the level of scientific evidence required for recommending these programs for fibromyalgia was lacking.10
Because exercise is believed to be an essential component of physical therapy, we examined the results of a systematic review of exercise for treating fibromyalgia. The authors found 7 high-quality studies, 4 of aerobic training, and concluded that supervised aerobic exercise training had beneficial effects on physical capacity, tender-point threshold, and pain.11 Other investigators have questioned the usefulness of aerobic exercise because long-term benefit remains unclear and compliance is poor.
Recommendations from others
We were unable to find any guidelines for the treatment of fibromyalgia. Patient information sheets from both the American College of Rheumatology (www.rheumatology.org) and American Academy of Orthopaedic Surgeons (orthoinfo.aaos.org) recommend physical modalities such as heat application, massage, and exercise, including fitness training.
Authors of chapters on fibromyalgia in both Kelly’s Textbook of Rheumatology and Harrison’s Principles of Internal Medicine suggest that patients may benefit from regular low-impact aerobic exercise.12,13
Exercise, physical therapy ease pain, “helplessness”
Wail Malaty, MD
Mountain Area Health Education Center, Rural Track Family Practice Residency, Hendersonville, NC, Department of Family Medicine, University of North Carolina, Chapel Hill
Fibromyalgia is a disease of chronic pain. It engenders feelings of helplessness, depression, and loss of control in many patients. In my experience, both physical therapy and exercise can help alleviate these feelings. Physical therapy helps motivated patients perform body movements that they believe may be painful. In this sense, it demonstrates to them the possibility of exercising without excruciating pain. As the evidence suggests, patients who exercise have less pain and feel better in general. Thus, physical therapy can teach patients to actively participate in the management of their disease.
1. Guccione AA. Physical therapy for musculoskeletal syndromes. Rheum Dis Clin North Am 1996;22:551-562.
2. Adams N, Sim J. An overview of fibromyalgia syndrome: mechanisms, differential diagnosis and treatment approaches. Physiotherapy 1998;84:304-318.
3. Buckelew SP, Conway R, Parker J, et al. Biofeedback/relaxation training and exercise interventions for fibromyalgia: a prospective trial. Arthritis Care Res 1998;11:196-209.
4. Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. J Rheumatol 1994;21:714-720.
5. Gowans SE, deHueck A, Voss S, Richardson M. A randomized, controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care Res 1999;12:120-128.
6. Keel PJ, Bodoky C, Gerhard U, Muller W. Comparison of integrated group therapy and group relaxation training for fibromyalgia. Clin J Pain 1998;14:232-238.
7. Vlaeyen JW, Teeken-Gruben NJ, Goossens ME, et al. Cognitive-educational treatment of fibromyalgia: a randomized clinical trial. I. Clinical effects. J Rheumatol 1996;23:1237-1245.
8. Jones KD, Burckhardt CS, Clark SR, Bennett RM, Potempa KM. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J Rheumatol 2002;29:1041-1048.
9. Kendall SA, Ekselius L, Gerdle B, Soren B, Bengtsson A. Feldenkrais intervention in fibromyalgia patients: A pilot study. J Musculoskeletal Pain 2001;9:25-35.
10. Karjalainen K, Malmivaara A, van Tulder M, et al. Multidisciplinary rehabilitation for fibromyalgia and musculoskeletal pain in working age adults. Cochrane Database Syst Rev2000; (2):CD001984. Updated quarterly.
11. Busch A, Schachter CL, Peloso PM, Bombardier C. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev2002; (3):CD003786. Updated quarterly.
12. Claus DJ. Fibromyalgia. In: Ruddy S, Harris ED Jr., Sledge CB, eds. Kelley’s Textbook of Rheumatology. 6th ed. Philadelphia, Pa: W.B. Saunders; 2001;417-427.
13. Gilliland BC. Relapsing polychondritis and other arthritides. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill; 2001;2005-2016.
1. Guccione AA. Physical therapy for musculoskeletal syndromes. Rheum Dis Clin North Am 1996;22:551-562.
2. Adams N, Sim J. An overview of fibromyalgia syndrome: mechanisms, differential diagnosis and treatment approaches. Physiotherapy 1998;84:304-318.
3. Buckelew SP, Conway R, Parker J, et al. Biofeedback/relaxation training and exercise interventions for fibromyalgia: a prospective trial. Arthritis Care Res 1998;11:196-209.
4. Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. J Rheumatol 1994;21:714-720.
5. Gowans SE, deHueck A, Voss S, Richardson M. A randomized, controlled trial of exercise and education for individuals with fibromyalgia. Arthritis Care Res 1999;12:120-128.
6. Keel PJ, Bodoky C, Gerhard U, Muller W. Comparison of integrated group therapy and group relaxation training for fibromyalgia. Clin J Pain 1998;14:232-238.
7. Vlaeyen JW, Teeken-Gruben NJ, Goossens ME, et al. Cognitive-educational treatment of fibromyalgia: a randomized clinical trial. I. Clinical effects. J Rheumatol 1996;23:1237-1245.
8. Jones KD, Burckhardt CS, Clark SR, Bennett RM, Potempa KM. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J Rheumatol 2002;29:1041-1048.
9. Kendall SA, Ekselius L, Gerdle B, Soren B, Bengtsson A. Feldenkrais intervention in fibromyalgia patients: A pilot study. J Musculoskeletal Pain 2001;9:25-35.
10. Karjalainen K, Malmivaara A, van Tulder M, et al. Multidisciplinary rehabilitation for fibromyalgia and musculoskeletal pain in working age adults. Cochrane Database Syst Rev2000; (2):CD001984. Updated quarterly.
11. Busch A, Schachter CL, Peloso PM, Bombardier C. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev2002; (3):CD003786. Updated quarterly.
12. Claus DJ. Fibromyalgia. In: Ruddy S, Harris ED Jr., Sledge CB, eds. Kelley’s Textbook of Rheumatology. 6th ed. Philadelphia, Pa: W.B. Saunders; 2001;417-427.
13. Gilliland BC. Relapsing polychondritis and other arthritides. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 15th ed. New York, NY: McGraw-Hill; 2001;2005-2016.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for plantar fasciitis?
Mechanical therapies—such as taping, tension night splinting, and rigid arch support—are the most effective treatment for plantar fasciitis (strength of recommendation: A, based on randomized controlled trials). If limited or no improvement is observed after 6 months of mechanical therapy, extracorporeal shock wave therapy (Orthotripsy) is the next treatment of choice (strength of recommendation [SOR]: A, based on meta-analysis of randomized controlled trials). When mechanical therapies and extracorporeal shock wave therapy have failed for more than 1 year, surgical treatment may be considered (SOR: C, based on a case-series study).
Evidence summary
In a prospective, observer-blinded study, 103 subjects were randomized to 1 of 3 treatment categories: anti-inflammatory (etodolac plus corticosteroid injections); accommodative (viscoelastic heel cup); or mechanical (low-dye tapping for 1 month followed by rigid custom orthosis for 2 months).1 After 3 months of treatment, 70% of patients in the mechanical treatment group rated their functional outcome as excellent, compared with only 33% of the anti-inflammatory group and 30% of the accommodative group (P=.005). Additionally, the mechanically treated group was less likely to terminate treatment early because of treatment failure (P<.001).
Several of the same researchers then went a step further to find out which specific mechanical treatment is best. They found no statistically significant difference among treatment with tension night splinting ( Figure 1 ), custom rigid orthosis, and over-the-counter arch supports.2 A retrospective study of 237 subjects also concluded that mechanical treatment is better than anti-inflammatory or accommodative treatments.3
Another prospective, observer-blinded study randomized 116 patients to 1 of 2 groups for 3 months.4 The first group of patients were treated with a nonsteroidal anti-inflammatory drug (piroxicam) and Achilles tendon stretching 3 times a day. The second group received the same treatment but also wore plastic tension night splints in 5° of dorsiflexion. After 3 months, in an intention-to-treat analysis, no statistically significant difference was detected in subjective pain between the 2 groups. In this study, patient compliance with the tension night splinting was poor, and this likely affected the outcome.
From 1993–1995 an observer-blinded randomized controlled trial of 112 patients compared standard with sham extracorporeal shock wave therapy.5 The main outcome measure was patient satisfaction on a 4-step score at 6 months and 5 years. At 6 months, the treatment group had a significantly better 4-step score than the placebo group (P<.0001). In fact, 51% of treatment-group patients were pain-free, while none of the 48 placebo-group patients were painfree. After 5 years, the 4-step score only demonstrated a trend in favor of the treatment group (P<.071) because of a high rate of good results from subsequent surgery in the placebo group. Thirteen percent of the treatment-group patients had undergone a heel operation, compared with 58% of placebo-group patients.
A controlled and observer-blinded study of 302 patients with plantar fasciitis compared standard extracorporeal shock wave therapy with sham treatment.6 The treated patients had significantly lower pain scores (as measured on a visual analog scale) than the placebo group (1.9 vs 4.7). Three months post-treatment, half as many treated patients were taking pain medication when compared with placebo patients. After 1 year of follow-up, 94% of the treatment group patients were still pain-free, with a pain score of <2.
One randomized controlled study of 166 patients found no evidence to support a beneficial effect on pain, function, and quality of life of extracorporeal shock wave therapy over a sham treatment.7 Of note, this study enrolled patients who had a minimum of 6 weeks of symptoms. All recommendations in the US are to reserve extracorporeal shock wave therapy for patients with more than 6 months of symptoms.
A meta-analysis of 8 published studies involving 840 patients whose condition was not improved after conservative therapy for at least 6 months showed that up to 88% of patients experienced good to excellent outcomes with extracorporeal shock wave therapy and were satisfied with the result. 6
As for surgical treatment, in a prospective study of 43 patients with 47 painful heels followed for an average of 31 months, only 49% of the patients were satisfied with their outcome.8 Patient expectations should be considered in preoperative counseling. In contrast to surgery, either open or endoscopic, extracorporeal shock wave therapy does not require the patient avoid weightbearing or a prolonged time for return to work.
FIGURE 1
Tension night splinting
With the knee flexed 90°, secure the splint to the leg with elastic. Remove the splint and moisten, then reapply with the ankle at maximum dorsiflexion. Apply tape in a figure-8 until the fiberglass hardens.
Recommendations from others
Figure 2 has been modified from a clinical practice guideline on the treatment of plantar fasciitis published by the American College of Foot and Ankle Surgeons.9
FIGURE 2
Treatment of plantar fasciitis
Keys to treatment: Avoid overuse, stabilize, be patient
Mark B. Stephens, MD, MS
Uniformed Services University of the Health Sciences, Bethesda, Md
Plantar fasciitis (heel pain syndrome) is one of the most common disorders of the foot and ankle and is notoriously difficult to treat. Patients are commonly symptomatic for months, leading to frustration, poor compliance, and general dissatisfaction.
From a pathophysiologic perspective, plantar fasciitis is a form of overuse syndrome. When approached in this manner, it makes intuitive (and now scientific) sense that stabilization of the proximal fascial enthesis at the point of its insertion to the calcaneus is the key to clinical resolution of symptoms. Activity modification, mechanical therapy, and patience are the essential elements for treating plantar fasciitis.
1. Lynch DM, Goforth WP, Martin JE, Odom RD, Preece CK, Kotter MW. Conservative treatment of plantar fasciitis. A prospective study. J Am Podiatr Med Assoc 1998;88:375-380.
2. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int 1998;19:803-811.
3. Probe RA, Baca M, Adams R, Preece C. Night splint treatment for plantar fasciitis. A prospective randomized study. Clin Orthop 1999;368:190-195.
4. Martin JE, Hosch JC, Goforth WP, Murff RT, Lynch DM, Odom RD. Mechanical treatment of plantar fasciitis: A prospective study. J Am Podiatr Med Assoc 2001;91:55-62.
5. Rompe JD, Schoellner C, Nafe B. Evaluation of low-energy extracorporeal shock-wave application for treatment of chronic plantar fasciitis. J Bone Joint Surg Am 2002;84-A:335-341.
6. Ogden JA, Alvarez R, Levitt R, Cross GL, Marlow M. Shock wave therapy for chronic proximal plantar fasciitis. Clin Orthop 2001;387:47-59.
7. Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA 2002;288:1364-1372.
8. Davies MS, Weiss GA, Saxby TS. Plantar fasciitis: how successful is surgical intervention? Foot Ankle Int 1999;20:803-807.
9. The diagnosis and treatment of heel pain. J Foot Ankle Surg 2001;40:329-340.
Mechanical therapies—such as taping, tension night splinting, and rigid arch support—are the most effective treatment for plantar fasciitis (strength of recommendation: A, based on randomized controlled trials). If limited or no improvement is observed after 6 months of mechanical therapy, extracorporeal shock wave therapy (Orthotripsy) is the next treatment of choice (strength of recommendation [SOR]: A, based on meta-analysis of randomized controlled trials). When mechanical therapies and extracorporeal shock wave therapy have failed for more than 1 year, surgical treatment may be considered (SOR: C, based on a case-series study).
Evidence summary
In a prospective, observer-blinded study, 103 subjects were randomized to 1 of 3 treatment categories: anti-inflammatory (etodolac plus corticosteroid injections); accommodative (viscoelastic heel cup); or mechanical (low-dye tapping for 1 month followed by rigid custom orthosis for 2 months).1 After 3 months of treatment, 70% of patients in the mechanical treatment group rated their functional outcome as excellent, compared with only 33% of the anti-inflammatory group and 30% of the accommodative group (P=.005). Additionally, the mechanically treated group was less likely to terminate treatment early because of treatment failure (P<.001).
Several of the same researchers then went a step further to find out which specific mechanical treatment is best. They found no statistically significant difference among treatment with tension night splinting ( Figure 1 ), custom rigid orthosis, and over-the-counter arch supports.2 A retrospective study of 237 subjects also concluded that mechanical treatment is better than anti-inflammatory or accommodative treatments.3
Another prospective, observer-blinded study randomized 116 patients to 1 of 2 groups for 3 months.4 The first group of patients were treated with a nonsteroidal anti-inflammatory drug (piroxicam) and Achilles tendon stretching 3 times a day. The second group received the same treatment but also wore plastic tension night splints in 5° of dorsiflexion. After 3 months, in an intention-to-treat analysis, no statistically significant difference was detected in subjective pain between the 2 groups. In this study, patient compliance with the tension night splinting was poor, and this likely affected the outcome.
From 1993–1995 an observer-blinded randomized controlled trial of 112 patients compared standard with sham extracorporeal shock wave therapy.5 The main outcome measure was patient satisfaction on a 4-step score at 6 months and 5 years. At 6 months, the treatment group had a significantly better 4-step score than the placebo group (P<.0001). In fact, 51% of treatment-group patients were pain-free, while none of the 48 placebo-group patients were painfree. After 5 years, the 4-step score only demonstrated a trend in favor of the treatment group (P<.071) because of a high rate of good results from subsequent surgery in the placebo group. Thirteen percent of the treatment-group patients had undergone a heel operation, compared with 58% of placebo-group patients.
A controlled and observer-blinded study of 302 patients with plantar fasciitis compared standard extracorporeal shock wave therapy with sham treatment.6 The treated patients had significantly lower pain scores (as measured on a visual analog scale) than the placebo group (1.9 vs 4.7). Three months post-treatment, half as many treated patients were taking pain medication when compared with placebo patients. After 1 year of follow-up, 94% of the treatment group patients were still pain-free, with a pain score of <2.
One randomized controlled study of 166 patients found no evidence to support a beneficial effect on pain, function, and quality of life of extracorporeal shock wave therapy over a sham treatment.7 Of note, this study enrolled patients who had a minimum of 6 weeks of symptoms. All recommendations in the US are to reserve extracorporeal shock wave therapy for patients with more than 6 months of symptoms.
A meta-analysis of 8 published studies involving 840 patients whose condition was not improved after conservative therapy for at least 6 months showed that up to 88% of patients experienced good to excellent outcomes with extracorporeal shock wave therapy and were satisfied with the result. 6
As for surgical treatment, in a prospective study of 43 patients with 47 painful heels followed for an average of 31 months, only 49% of the patients were satisfied with their outcome.8 Patient expectations should be considered in preoperative counseling. In contrast to surgery, either open or endoscopic, extracorporeal shock wave therapy does not require the patient avoid weightbearing or a prolonged time for return to work.
FIGURE 1
Tension night splinting
With the knee flexed 90°, secure the splint to the leg with elastic. Remove the splint and moisten, then reapply with the ankle at maximum dorsiflexion. Apply tape in a figure-8 until the fiberglass hardens.
Recommendations from others
Figure 2 has been modified from a clinical practice guideline on the treatment of plantar fasciitis published by the American College of Foot and Ankle Surgeons.9
FIGURE 2
Treatment of plantar fasciitis
Keys to treatment: Avoid overuse, stabilize, be patient
Mark B. Stephens, MD, MS
Uniformed Services University of the Health Sciences, Bethesda, Md
Plantar fasciitis (heel pain syndrome) is one of the most common disorders of the foot and ankle and is notoriously difficult to treat. Patients are commonly symptomatic for months, leading to frustration, poor compliance, and general dissatisfaction.
From a pathophysiologic perspective, plantar fasciitis is a form of overuse syndrome. When approached in this manner, it makes intuitive (and now scientific) sense that stabilization of the proximal fascial enthesis at the point of its insertion to the calcaneus is the key to clinical resolution of symptoms. Activity modification, mechanical therapy, and patience are the essential elements for treating plantar fasciitis.
Mechanical therapies—such as taping, tension night splinting, and rigid arch support—are the most effective treatment for plantar fasciitis (strength of recommendation: A, based on randomized controlled trials). If limited or no improvement is observed after 6 months of mechanical therapy, extracorporeal shock wave therapy (Orthotripsy) is the next treatment of choice (strength of recommendation [SOR]: A, based on meta-analysis of randomized controlled trials). When mechanical therapies and extracorporeal shock wave therapy have failed for more than 1 year, surgical treatment may be considered (SOR: C, based on a case-series study).
Evidence summary
In a prospective, observer-blinded study, 103 subjects were randomized to 1 of 3 treatment categories: anti-inflammatory (etodolac plus corticosteroid injections); accommodative (viscoelastic heel cup); or mechanical (low-dye tapping for 1 month followed by rigid custom orthosis for 2 months).1 After 3 months of treatment, 70% of patients in the mechanical treatment group rated their functional outcome as excellent, compared with only 33% of the anti-inflammatory group and 30% of the accommodative group (P=.005). Additionally, the mechanically treated group was less likely to terminate treatment early because of treatment failure (P<.001).
Several of the same researchers then went a step further to find out which specific mechanical treatment is best. They found no statistically significant difference among treatment with tension night splinting ( Figure 1 ), custom rigid orthosis, and over-the-counter arch supports.2 A retrospective study of 237 subjects also concluded that mechanical treatment is better than anti-inflammatory or accommodative treatments.3
Another prospective, observer-blinded study randomized 116 patients to 1 of 2 groups for 3 months.4 The first group of patients were treated with a nonsteroidal anti-inflammatory drug (piroxicam) and Achilles tendon stretching 3 times a day. The second group received the same treatment but also wore plastic tension night splints in 5° of dorsiflexion. After 3 months, in an intention-to-treat analysis, no statistically significant difference was detected in subjective pain between the 2 groups. In this study, patient compliance with the tension night splinting was poor, and this likely affected the outcome.
From 1993–1995 an observer-blinded randomized controlled trial of 112 patients compared standard with sham extracorporeal shock wave therapy.5 The main outcome measure was patient satisfaction on a 4-step score at 6 months and 5 years. At 6 months, the treatment group had a significantly better 4-step score than the placebo group (P<.0001). In fact, 51% of treatment-group patients were pain-free, while none of the 48 placebo-group patients were painfree. After 5 years, the 4-step score only demonstrated a trend in favor of the treatment group (P<.071) because of a high rate of good results from subsequent surgery in the placebo group. Thirteen percent of the treatment-group patients had undergone a heel operation, compared with 58% of placebo-group patients.
A controlled and observer-blinded study of 302 patients with plantar fasciitis compared standard extracorporeal shock wave therapy with sham treatment.6 The treated patients had significantly lower pain scores (as measured on a visual analog scale) than the placebo group (1.9 vs 4.7). Three months post-treatment, half as many treated patients were taking pain medication when compared with placebo patients. After 1 year of follow-up, 94% of the treatment group patients were still pain-free, with a pain score of <2.
One randomized controlled study of 166 patients found no evidence to support a beneficial effect on pain, function, and quality of life of extracorporeal shock wave therapy over a sham treatment.7 Of note, this study enrolled patients who had a minimum of 6 weeks of symptoms. All recommendations in the US are to reserve extracorporeal shock wave therapy for patients with more than 6 months of symptoms.
A meta-analysis of 8 published studies involving 840 patients whose condition was not improved after conservative therapy for at least 6 months showed that up to 88% of patients experienced good to excellent outcomes with extracorporeal shock wave therapy and were satisfied with the result. 6
As for surgical treatment, in a prospective study of 43 patients with 47 painful heels followed for an average of 31 months, only 49% of the patients were satisfied with their outcome.8 Patient expectations should be considered in preoperative counseling. In contrast to surgery, either open or endoscopic, extracorporeal shock wave therapy does not require the patient avoid weightbearing or a prolonged time for return to work.
FIGURE 1
Tension night splinting
With the knee flexed 90°, secure the splint to the leg with elastic. Remove the splint and moisten, then reapply with the ankle at maximum dorsiflexion. Apply tape in a figure-8 until the fiberglass hardens.
Recommendations from others
Figure 2 has been modified from a clinical practice guideline on the treatment of plantar fasciitis published by the American College of Foot and Ankle Surgeons.9
FIGURE 2
Treatment of plantar fasciitis
Keys to treatment: Avoid overuse, stabilize, be patient
Mark B. Stephens, MD, MS
Uniformed Services University of the Health Sciences, Bethesda, Md
Plantar fasciitis (heel pain syndrome) is one of the most common disorders of the foot and ankle and is notoriously difficult to treat. Patients are commonly symptomatic for months, leading to frustration, poor compliance, and general dissatisfaction.
From a pathophysiologic perspective, plantar fasciitis is a form of overuse syndrome. When approached in this manner, it makes intuitive (and now scientific) sense that stabilization of the proximal fascial enthesis at the point of its insertion to the calcaneus is the key to clinical resolution of symptoms. Activity modification, mechanical therapy, and patience are the essential elements for treating plantar fasciitis.
1. Lynch DM, Goforth WP, Martin JE, Odom RD, Preece CK, Kotter MW. Conservative treatment of plantar fasciitis. A prospective study. J Am Podiatr Med Assoc 1998;88:375-380.
2. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int 1998;19:803-811.
3. Probe RA, Baca M, Adams R, Preece C. Night splint treatment for plantar fasciitis. A prospective randomized study. Clin Orthop 1999;368:190-195.
4. Martin JE, Hosch JC, Goforth WP, Murff RT, Lynch DM, Odom RD. Mechanical treatment of plantar fasciitis: A prospective study. J Am Podiatr Med Assoc 2001;91:55-62.
5. Rompe JD, Schoellner C, Nafe B. Evaluation of low-energy extracorporeal shock-wave application for treatment of chronic plantar fasciitis. J Bone Joint Surg Am 2002;84-A:335-341.
6. Ogden JA, Alvarez R, Levitt R, Cross GL, Marlow M. Shock wave therapy for chronic proximal plantar fasciitis. Clin Orthop 2001;387:47-59.
7. Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA 2002;288:1364-1372.
8. Davies MS, Weiss GA, Saxby TS. Plantar fasciitis: how successful is surgical intervention? Foot Ankle Int 1999;20:803-807.
9. The diagnosis and treatment of heel pain. J Foot Ankle Surg 2001;40:329-340.
1. Lynch DM, Goforth WP, Martin JE, Odom RD, Preece CK, Kotter MW. Conservative treatment of plantar fasciitis. A prospective study. J Am Podiatr Med Assoc 1998;88:375-380.
2. Martin RL, Irrgang JJ, Conti SF. Outcome study of subjects with insertional plantar fasciitis. Foot Ankle Int 1998;19:803-811.
3. Probe RA, Baca M, Adams R, Preece C. Night splint treatment for plantar fasciitis. A prospective randomized study. Clin Orthop 1999;368:190-195.
4. Martin JE, Hosch JC, Goforth WP, Murff RT, Lynch DM, Odom RD. Mechanical treatment of plantar fasciitis: A prospective study. J Am Podiatr Med Assoc 2001;91:55-62.
5. Rompe JD, Schoellner C, Nafe B. Evaluation of low-energy extracorporeal shock-wave application for treatment of chronic plantar fasciitis. J Bone Joint Surg Am 2002;84-A:335-341.
6. Ogden JA, Alvarez R, Levitt R, Cross GL, Marlow M. Shock wave therapy for chronic proximal plantar fasciitis. Clin Orthop 2001;387:47-59.
7. Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA 2002;288:1364-1372.
8. Davies MS, Weiss GA, Saxby TS. Plantar fasciitis: how successful is surgical intervention? Foot Ankle Int 1999;20:803-807.
9. The diagnosis and treatment of heel pain. J Foot Ankle Surg 2001;40:329-340.
Evidence-based answers from the Family Physicians Inquiries Network
Are nasal steroid sprays effective for otitis media with effusion?
Treatment of otitis media with effusion (OME) with nasal steroids is not recommended (strength of recommendation [SOR]=A, based on systematic review).
Limited evidence exists that shows nasal steroids may increase the rate of resolution of OME in the short term, alone or in combination with antibiotics (SOR: A, based on randomized controlled trials). However, within 3 to 12 weeks, resolution of OME with nasal steroids is no better than placebo. No evidence exists that treatment with nasal steroids has any effect on decreasing potential complications of OME, such as hearing loss and delayed language development.
Evidence summary
OME is diagnosed by visualization of an effusion on otoscopy, by limited tympanic membrane movement on insufflation, or by abnormal tympanometry, all in the absence of acute inflammation. OME is defined as chronic when the effusion has been present for at least 3 months.
The natural course of OME was observed in a longitudinal cohort study of 1439 children aged 2 years in the Netherlands. Single or recurrent flat screening tympanograms were noted in 20% and remitted spontaneously at a rate of 50% every 3 months.1 This prevalence and spontaneous resolution rate is consistent with other studies.
Three randomized controlled trials published in English tested intranasal steroids for OME (Table).
The Lilholdt study enrolled children through a private ear, nose, and throat clinic over autumn, winter, and spring with a primary or new bout of OME.
The Shapiro study enrolled children who had documented allergic rhinitis and OME with failure to respond to 4 weeks of oral antihistamine and decongestant therapy at time of entry. This was the only study with short-term follow-up comparing intranasal steroids with control. The odds ratio for OME persisting after 3 weeks was 2.12 (95% confidence interval [CI], 0.65-6.90).3
The Tracy study enrolled children with chronic OME referred to a chronic ear clinic from October to June. Inclusion criteria included 3 episodes of acute otitis media in the prior 6 months or 4 episodes in the prior 12 months. This was a randomized comparison study with 3 treatment arms: an active nasal spray group and 2 control groups. The odds ratio for OME persisting after short-term follow-up was 0.79 (95% CI, 0.20-3.19); after intermediate follow-up the odds ratio was 0.72 (95% CI, 0.21-2.44).
This study, which included a symptom score after 3 months, favored treatment, with a weighted mean difference of -4.5, but with wide 95% CI of -10.28 to 1.28. An effect was demonstrated on clearing effusions in the short term, but the advantage appeared to vanish for the most part by 3 months. The study did not evaluate improvements in hearing.4
No adverse effects of intranasal steroid treatment were seen except for transient drops in cortisol levels in the Shapiro study, which tested dexamethasone. Approximately 8 randomized controlled trials using oral steroids with and without antibiotics for OME and chronic OME mirror a trend for short-term benefit of treatment, spontaneous resolution, and frequent recurrence.
In summary, limited evidence exists for short-term improvement of OME with intranasal steroids plus antibiotics, and no evidence exists for lasting beneficial effect on effusion or OME associated hearing loss.
TABLE
Clinical trials: Intranasal steroids for otitis media with effusion
| Study | Subjects | Groups | Duration | Outcome |
|---|---|---|---|---|
| Lilholdt 1982 | n=70 (aged 4-14 yrs with OME) | Beclomethasone vs placebo | 2 mo | No benefit at end of treatment month or after second month with no treatment by otoscopy, tympanometry, or audiometry. |
| Spontaneous improvement in 25% and resolution in 25%.1 | ||||
| Shapiro 1982 | n=45 | Dexamethasone vs placebo | 3 wk (aged 2-12 yrs with OME >1 mo) | Normalization of ear pressure and middle ear gradient at 1 and 2 weeks of treatment group over placebo (P<.05). |
| No significant differences by third week.2 | ||||
| Tracy 1998 | n=61 (aged 3-11 yrs with chronic OME) | Beclomethasone + amoxicillin vs placebo + amoxicillin vs amoxicillin alone | 12 wk | Beclomethasone group showed a significantly greater frequency of resolution of chronic effusion at 4 and 8 weeks (P<.05) but not at 12 weeks, with improved middle ear pressures: left (P=.004) and right (P=.010) over the 12 weeks.3 |
| OME, otitis media with effusion | ||||
Recommendations from others
The Canadian Task Force on Preventative Health Care found insufficient evidence to recommend screening for OME to prevent delayed language development.5
The Cochrane Ear, Nose and Throat Disorders Group concludes that both oral and topical intranasal steroids alone or in combination with an antibiotic lead to a quicker resolution of OME in the short term, but no long-term benefit is seen from treating OME effusions or associated hearing loss with topical intranasal steroids.6 They separately reviewed antibiotic treatment for OME, noting the short-term benefit above, but cited several drawbacks including cost and increased antibacterial resistance.7
The American Academy of Family Physicians Clinical Recommendation on Otitis Media with Effusion in Young Children does not recommend steroid medications for treatment of OME in a child of any age.8
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado, Denver
Management of OME can be challenging and expensive—annual costs are estimated at $5 billion. Antibiotics are often inappropriately prescribed for OME, which may promote bacterial resistance. Commonly, clinicians augment OME treatment with antihistamines, decongestants, and steroids. Yet studies such as those cited above confirm that these treatments offer limited or no benefit. We must avoid the kitchen-sink treatment of OME. Furthermore, randomized controlled trials have shown that 80% to 90% of cases of acute otitis media and OME resolve without any therapy.
However, children with chronic OME, especially those with bilateral disease or possible hearing loss, may benefit from tympanostomy tube placement and adenoidectomy. If the OME doesn’t clear within 3 months, refer to an ear, nose, and throat specialist.
Prevention efforts are valuable. Immunization of infants with pneumococcal conjugate vaccine reduced tympanostomy tube placement by 20% to 39%.9,10 Since increased incidence of OME and recurrent acute otitis media are associated with secondhand smoke exposure, motivating parents to quit smoking may further reduce chronic OME.
ACKNOWLEDGMENTS
Thanks to Marianne Broers, MD for her translation of reference 1 from Dutch.
1. Zielhuis GA, Schilder A, van den Broek P. The spontaneous course of otitis media with effusion in young children [in Dutch]. Ned Tijdschr Geneeskd 1991;135:1754-1757.
2. Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle-ear effusion—a double-blind study. Int J Pediatr Otorhinolaryngol 1982;4:133-137.
3. Shapiro GG, Bierman CW, Furukawa CT, et al. Treatment of persistent eustachian tube dysfunction in children with aerosolized nasal dexamethasone phosphate versus placebo. Ann Allergy 1982;49:81-85.
4. Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann Allergy Asthma Immunol 1998;80:198-206.
5. Butler CC, MacMillan HL. Early detection of OME in the first four years of life to prevent delayed language development. Systematic Review & Recommendations. CTF-PHC Technical Report #01-3. September 2000. London, Ont: Canadian Task Force; 2000. Available at: http://www.ctfphc.org, or by request from the task force office [email protected]. Accessed on July 10, 2003.
6. Butler CC, Van Der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2002;(4):CD001935.-
7. Van Balen FAM, Canekin II LJ, Williamson IG. Antibiotic treatment for otitis media with effusion in children aged 6 months-12 years (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
8. American Academy of Family Physicians. Otitis Media with Effusion in Young Children. AAFP Clinical Recommendations, Part II—Clinical Policies. Leawood, Kansas: American Academy of Family Physicians; 1994 (reaffirmed 2000, 2001). Available at: http://www.aafp.org/x1596.xml. Accessed on June 30, 2003.
9. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-195.
10. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-409.
Treatment of otitis media with effusion (OME) with nasal steroids is not recommended (strength of recommendation [SOR]=A, based on systematic review).
Limited evidence exists that shows nasal steroids may increase the rate of resolution of OME in the short term, alone or in combination with antibiotics (SOR: A, based on randomized controlled trials). However, within 3 to 12 weeks, resolution of OME with nasal steroids is no better than placebo. No evidence exists that treatment with nasal steroids has any effect on decreasing potential complications of OME, such as hearing loss and delayed language development.
Evidence summary
OME is diagnosed by visualization of an effusion on otoscopy, by limited tympanic membrane movement on insufflation, or by abnormal tympanometry, all in the absence of acute inflammation. OME is defined as chronic when the effusion has been present for at least 3 months.
The natural course of OME was observed in a longitudinal cohort study of 1439 children aged 2 years in the Netherlands. Single or recurrent flat screening tympanograms were noted in 20% and remitted spontaneously at a rate of 50% every 3 months.1 This prevalence and spontaneous resolution rate is consistent with other studies.
Three randomized controlled trials published in English tested intranasal steroids for OME (Table).
The Lilholdt study enrolled children through a private ear, nose, and throat clinic over autumn, winter, and spring with a primary or new bout of OME.
The Shapiro study enrolled children who had documented allergic rhinitis and OME with failure to respond to 4 weeks of oral antihistamine and decongestant therapy at time of entry. This was the only study with short-term follow-up comparing intranasal steroids with control. The odds ratio for OME persisting after 3 weeks was 2.12 (95% confidence interval [CI], 0.65-6.90).3
The Tracy study enrolled children with chronic OME referred to a chronic ear clinic from October to June. Inclusion criteria included 3 episodes of acute otitis media in the prior 6 months or 4 episodes in the prior 12 months. This was a randomized comparison study with 3 treatment arms: an active nasal spray group and 2 control groups. The odds ratio for OME persisting after short-term follow-up was 0.79 (95% CI, 0.20-3.19); after intermediate follow-up the odds ratio was 0.72 (95% CI, 0.21-2.44).
This study, which included a symptom score after 3 months, favored treatment, with a weighted mean difference of -4.5, but with wide 95% CI of -10.28 to 1.28. An effect was demonstrated on clearing effusions in the short term, but the advantage appeared to vanish for the most part by 3 months. The study did not evaluate improvements in hearing.4
No adverse effects of intranasal steroid treatment were seen except for transient drops in cortisol levels in the Shapiro study, which tested dexamethasone. Approximately 8 randomized controlled trials using oral steroids with and without antibiotics for OME and chronic OME mirror a trend for short-term benefit of treatment, spontaneous resolution, and frequent recurrence.
In summary, limited evidence exists for short-term improvement of OME with intranasal steroids plus antibiotics, and no evidence exists for lasting beneficial effect on effusion or OME associated hearing loss.
TABLE
Clinical trials: Intranasal steroids for otitis media with effusion
| Study | Subjects | Groups | Duration | Outcome |
|---|---|---|---|---|
| Lilholdt 1982 | n=70 (aged 4-14 yrs with OME) | Beclomethasone vs placebo | 2 mo | No benefit at end of treatment month or after second month with no treatment by otoscopy, tympanometry, or audiometry. |
| Spontaneous improvement in 25% and resolution in 25%.1 | ||||
| Shapiro 1982 | n=45 | Dexamethasone vs placebo | 3 wk (aged 2-12 yrs with OME >1 mo) | Normalization of ear pressure and middle ear gradient at 1 and 2 weeks of treatment group over placebo (P<.05). |
| No significant differences by third week.2 | ||||
| Tracy 1998 | n=61 (aged 3-11 yrs with chronic OME) | Beclomethasone + amoxicillin vs placebo + amoxicillin vs amoxicillin alone | 12 wk | Beclomethasone group showed a significantly greater frequency of resolution of chronic effusion at 4 and 8 weeks (P<.05) but not at 12 weeks, with improved middle ear pressures: left (P=.004) and right (P=.010) over the 12 weeks.3 |
| OME, otitis media with effusion | ||||
Recommendations from others
The Canadian Task Force on Preventative Health Care found insufficient evidence to recommend screening for OME to prevent delayed language development.5
The Cochrane Ear, Nose and Throat Disorders Group concludes that both oral and topical intranasal steroids alone or in combination with an antibiotic lead to a quicker resolution of OME in the short term, but no long-term benefit is seen from treating OME effusions or associated hearing loss with topical intranasal steroids.6 They separately reviewed antibiotic treatment for OME, noting the short-term benefit above, but cited several drawbacks including cost and increased antibacterial resistance.7
The American Academy of Family Physicians Clinical Recommendation on Otitis Media with Effusion in Young Children does not recommend steroid medications for treatment of OME in a child of any age.8
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado, Denver
Management of OME can be challenging and expensive—annual costs are estimated at $5 billion. Antibiotics are often inappropriately prescribed for OME, which may promote bacterial resistance. Commonly, clinicians augment OME treatment with antihistamines, decongestants, and steroids. Yet studies such as those cited above confirm that these treatments offer limited or no benefit. We must avoid the kitchen-sink treatment of OME. Furthermore, randomized controlled trials have shown that 80% to 90% of cases of acute otitis media and OME resolve without any therapy.
However, children with chronic OME, especially those with bilateral disease or possible hearing loss, may benefit from tympanostomy tube placement and adenoidectomy. If the OME doesn’t clear within 3 months, refer to an ear, nose, and throat specialist.
Prevention efforts are valuable. Immunization of infants with pneumococcal conjugate vaccine reduced tympanostomy tube placement by 20% to 39%.9,10 Since increased incidence of OME and recurrent acute otitis media are associated with secondhand smoke exposure, motivating parents to quit smoking may further reduce chronic OME.
ACKNOWLEDGMENTS
Thanks to Marianne Broers, MD for her translation of reference 1 from Dutch.
Treatment of otitis media with effusion (OME) with nasal steroids is not recommended (strength of recommendation [SOR]=A, based on systematic review).
Limited evidence exists that shows nasal steroids may increase the rate of resolution of OME in the short term, alone or in combination with antibiotics (SOR: A, based on randomized controlled trials). However, within 3 to 12 weeks, resolution of OME with nasal steroids is no better than placebo. No evidence exists that treatment with nasal steroids has any effect on decreasing potential complications of OME, such as hearing loss and delayed language development.
Evidence summary
OME is diagnosed by visualization of an effusion on otoscopy, by limited tympanic membrane movement on insufflation, or by abnormal tympanometry, all in the absence of acute inflammation. OME is defined as chronic when the effusion has been present for at least 3 months.
The natural course of OME was observed in a longitudinal cohort study of 1439 children aged 2 years in the Netherlands. Single or recurrent flat screening tympanograms were noted in 20% and remitted spontaneously at a rate of 50% every 3 months.1 This prevalence and spontaneous resolution rate is consistent with other studies.
Three randomized controlled trials published in English tested intranasal steroids for OME (Table).
The Lilholdt study enrolled children through a private ear, nose, and throat clinic over autumn, winter, and spring with a primary or new bout of OME.
The Shapiro study enrolled children who had documented allergic rhinitis and OME with failure to respond to 4 weeks of oral antihistamine and decongestant therapy at time of entry. This was the only study with short-term follow-up comparing intranasal steroids with control. The odds ratio for OME persisting after 3 weeks was 2.12 (95% confidence interval [CI], 0.65-6.90).3
The Tracy study enrolled children with chronic OME referred to a chronic ear clinic from October to June. Inclusion criteria included 3 episodes of acute otitis media in the prior 6 months or 4 episodes in the prior 12 months. This was a randomized comparison study with 3 treatment arms: an active nasal spray group and 2 control groups. The odds ratio for OME persisting after short-term follow-up was 0.79 (95% CI, 0.20-3.19); after intermediate follow-up the odds ratio was 0.72 (95% CI, 0.21-2.44).
This study, which included a symptom score after 3 months, favored treatment, with a weighted mean difference of -4.5, but with wide 95% CI of -10.28 to 1.28. An effect was demonstrated on clearing effusions in the short term, but the advantage appeared to vanish for the most part by 3 months. The study did not evaluate improvements in hearing.4
No adverse effects of intranasal steroid treatment were seen except for transient drops in cortisol levels in the Shapiro study, which tested dexamethasone. Approximately 8 randomized controlled trials using oral steroids with and without antibiotics for OME and chronic OME mirror a trend for short-term benefit of treatment, spontaneous resolution, and frequent recurrence.
In summary, limited evidence exists for short-term improvement of OME with intranasal steroids plus antibiotics, and no evidence exists for lasting beneficial effect on effusion or OME associated hearing loss.
TABLE
Clinical trials: Intranasal steroids for otitis media with effusion
| Study | Subjects | Groups | Duration | Outcome |
|---|---|---|---|---|
| Lilholdt 1982 | n=70 (aged 4-14 yrs with OME) | Beclomethasone vs placebo | 2 mo | No benefit at end of treatment month or after second month with no treatment by otoscopy, tympanometry, or audiometry. |
| Spontaneous improvement in 25% and resolution in 25%.1 | ||||
| Shapiro 1982 | n=45 | Dexamethasone vs placebo | 3 wk (aged 2-12 yrs with OME >1 mo) | Normalization of ear pressure and middle ear gradient at 1 and 2 weeks of treatment group over placebo (P<.05). |
| No significant differences by third week.2 | ||||
| Tracy 1998 | n=61 (aged 3-11 yrs with chronic OME) | Beclomethasone + amoxicillin vs placebo + amoxicillin vs amoxicillin alone | 12 wk | Beclomethasone group showed a significantly greater frequency of resolution of chronic effusion at 4 and 8 weeks (P<.05) but not at 12 weeks, with improved middle ear pressures: left (P=.004) and right (P=.010) over the 12 weeks.3 |
| OME, otitis media with effusion | ||||
Recommendations from others
The Canadian Task Force on Preventative Health Care found insufficient evidence to recommend screening for OME to prevent delayed language development.5
The Cochrane Ear, Nose and Throat Disorders Group concludes that both oral and topical intranasal steroids alone or in combination with an antibiotic lead to a quicker resolution of OME in the short term, but no long-term benefit is seen from treating OME effusions or associated hearing loss with topical intranasal steroids.6 They separately reviewed antibiotic treatment for OME, noting the short-term benefit above, but cited several drawbacks including cost and increased antibacterial resistance.7
The American Academy of Family Physicians Clinical Recommendation on Otitis Media with Effusion in Young Children does not recommend steroid medications for treatment of OME in a child of any age.8
Fred Grover, Jr, MD
Department of Family Medicine, University of Colorado, Denver
Management of OME can be challenging and expensive—annual costs are estimated at $5 billion. Antibiotics are often inappropriately prescribed for OME, which may promote bacterial resistance. Commonly, clinicians augment OME treatment with antihistamines, decongestants, and steroids. Yet studies such as those cited above confirm that these treatments offer limited or no benefit. We must avoid the kitchen-sink treatment of OME. Furthermore, randomized controlled trials have shown that 80% to 90% of cases of acute otitis media and OME resolve without any therapy.
However, children with chronic OME, especially those with bilateral disease or possible hearing loss, may benefit from tympanostomy tube placement and adenoidectomy. If the OME doesn’t clear within 3 months, refer to an ear, nose, and throat specialist.
Prevention efforts are valuable. Immunization of infants with pneumococcal conjugate vaccine reduced tympanostomy tube placement by 20% to 39%.9,10 Since increased incidence of OME and recurrent acute otitis media are associated with secondhand smoke exposure, motivating parents to quit smoking may further reduce chronic OME.
ACKNOWLEDGMENTS
Thanks to Marianne Broers, MD for her translation of reference 1 from Dutch.
1. Zielhuis GA, Schilder A, van den Broek P. The spontaneous course of otitis media with effusion in young children [in Dutch]. Ned Tijdschr Geneeskd 1991;135:1754-1757.
2. Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle-ear effusion—a double-blind study. Int J Pediatr Otorhinolaryngol 1982;4:133-137.
3. Shapiro GG, Bierman CW, Furukawa CT, et al. Treatment of persistent eustachian tube dysfunction in children with aerosolized nasal dexamethasone phosphate versus placebo. Ann Allergy 1982;49:81-85.
4. Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann Allergy Asthma Immunol 1998;80:198-206.
5. Butler CC, MacMillan HL. Early detection of OME in the first four years of life to prevent delayed language development. Systematic Review & Recommendations. CTF-PHC Technical Report #01-3. September 2000. London, Ont: Canadian Task Force; 2000. Available at: http://www.ctfphc.org, or by request from the task force office [email protected]. Accessed on July 10, 2003.
6. Butler CC, Van Der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2002;(4):CD001935.-
7. Van Balen FAM, Canekin II LJ, Williamson IG. Antibiotic treatment for otitis media with effusion in children aged 6 months-12 years (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
8. American Academy of Family Physicians. Otitis Media with Effusion in Young Children. AAFP Clinical Recommendations, Part II—Clinical Policies. Leawood, Kansas: American Academy of Family Physicians; 1994 (reaffirmed 2000, 2001). Available at: http://www.aafp.org/x1596.xml. Accessed on June 30, 2003.
9. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-195.
10. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-409.
1. Zielhuis GA, Schilder A, van den Broek P. The spontaneous course of otitis media with effusion in young children [in Dutch]. Ned Tijdschr Geneeskd 1991;135:1754-1757.
2. Lildholdt T, Kortholm B. Beclomethasone nasal spray in the treatment of middle-ear effusion—a double-blind study. Int J Pediatr Otorhinolaryngol 1982;4:133-137.
3. Shapiro GG, Bierman CW, Furukawa CT, et al. Treatment of persistent eustachian tube dysfunction in children with aerosolized nasal dexamethasone phosphate versus placebo. Ann Allergy 1982;49:81-85.
4. Tracy JM, Demain JG, Hoffman KM, Goetz DW. Intranasal beclomethasone as an adjunct to treatment of chronic middle ear effusion. Ann Allergy Asthma Immunol 1998;80:198-206.
5. Butler CC, MacMillan HL. Early detection of OME in the first four years of life to prevent delayed language development. Systematic Review & Recommendations. CTF-PHC Technical Report #01-3. September 2000. London, Ont: Canadian Task Force; 2000. Available at: http://www.ctfphc.org, or by request from the task force office [email protected]. Accessed on July 10, 2003.
6. Butler CC, Van Der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2002;(4):CD001935.-
7. Van Balen FAM, Canekin II LJ, Williamson IG. Antibiotic treatment for otitis media with effusion in children aged 6 months-12 years (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
8. American Academy of Family Physicians. Otitis Media with Effusion in Young Children. AAFP Clinical Recommendations, Part II—Clinical Policies. Leawood, Kansas: American Academy of Family Physicians; 1994 (reaffirmed 2000, 2001). Available at: http://www.aafp.org/x1596.xml. Accessed on June 30, 2003.
9. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-195.
10. Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001;344:403-409.
Evidence-based answers from the Family Physicians Inquiries Network
Does glucosamine relieve arthritis joint pain?
Glucosamine may provide some pain relief. Studies have shown varied results, ranging from glucosamine being superior or equivalent to other agents, to no difference between glucosamine and placebo. However, most of these studies have small sample sizes, short duration, and often other significant flaws. Meta-analyses of available studies suggest a trend toward benefit from glucosamine (strength of recommendation: B).
Glucosamine may help osteoarthritis pain, but it is premature to recommend it universally until better studies are done. Even if glucosamine is effective, this sector of the market is currently unregulated, and products may not contain the amount or kind of glucosamine material advertised on their labels.
Evidence summary
Multiple methodological flaws have characterized studies trying to answer this question over the past 30 to 35 years. The companies manufacturing glucosamine have funded most studies. The overwhelming proportion of positive but marginal results raises the possibility of a publication bias (the tendency to publish only positive or supportive results), and the funding sources for the positive studies make that bias plausible.
Identified flaws in the studies include small sample size, inconsistent diagnostic criteria, variable disease sites, differing routes of administration, inconsistent doses, compositions and forms of glucosamine, the brief durations of studies, and poorly defined endpoints.1 Those problems account for the relatively low quality scores of the studies used in meta-analyses, particularly in earlier ones. Quality scores range from 12% to 52% of optimal and make any definitive conclusions suspect.2
The magnitude of the treatment effect is variable. Meta-analyses demonstrate aggregate treatment effects ranging from 0.36 to 1.02— where a small effect is 0.2, a moderate effect is 0.5, and a large effect is 0.8.2
When more recent, higher-quality studies are analyzed, trends toward benefit and the effect sizes for glucosamine diminish but remain at aggregate values ranging from 0.26 to 0.44.2-4 Statistically significant differences exist in some subgroup analyses and secondary endpoints.5 Typical trends suggest that glucosamine is superior to placebo for pain relief, and less effective but safer than nonsteroidal anti-inflammatory agents.6
Statements about safety are speculative given the brief duration of available trials, most of which lasted <10 weeks.6 Reported adverse effects are few. Mild gastrointestinal, skin, and constitutional symptoms predominate, but seldom at rates much higher than placebo.3-4 Pain relief may require as much as 4 to 6 weeks of therapy, and short studies may not demonstrate these benefits. The possibility of site-specific benefits or a difference in effect from a different dose or form is impossible to determine based on the current literature.
Recommendations from others
The American College of Rheumatology Subcommittee on Osteoarthritis believes that it is too early to issue recommendations for use of glucosamine sulfate or chondroitin sulfate for treatment of osteoarthritis.7
The National Institutes of Health Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT) began recruiting in May 2002. The design of this study is specifically directed at addressing the flaws of previous studies. This study will enroll 1588 patients at 13 study sites, and will use standardized products and doses with a single route of administration in a double-blinded, placebo-controlled fashion.
The GAIT study will measure change in joint space width (baseline to 2 years) and consists of 4 arms: glucosamine vs placebo, chondroitin vs placebo, glucosamine and chondroitin vs placebo, and celecoxib vs placebo. It is likely that the National Institute of Arthritis, Musculoskeletal and Skin Diseases, in collaboration with the National Center for Complementary and Alternative Medicine, will issue recommendations regarding the efficacy of glucosamine when the study is complete in 2005 or 2006. Updates are available at http://nccam.nih.gov/clinicaltrials/ glucosamine.htm.
Russell W. Roberts, MD
Louisiana State University Health Sciences Center, Shreveport, La
Patients frequently ask me if glucosamine, in combination with chondroitin or methylsulfonylmethane (MSM), reduces or prevents arthritis pain. It appears that glucosamine is safe and offers some promise.
I think a 6-week trial in patients with osteoarthritis is reasonable, preferably using glucosamine—a type that complies with the United States Pharmacopia/National Formulary standards—500 mg orally 3 times daily, once it becomes widely available. In my experience, very few patients who give glucosamine an enthusiastic and adequate trial of therapy continue the course for more than a few months. Those who use it longer often acknowledge only modest relief but continue with the hope of preventing further joint degeneration and increased pain, another currently unsubstantiated expectation.
1. Deal CL, Moskowitz RW. Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate and collagen hydrolysate. Rheum Dis Clin North Am 1999;25:379-395.
2. McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA 2000;283:1469-1475.
3. Barclay TS, Tsourounis C, McCart GM. Glucosamine. Ann Pharmacother 1998;32:574-579.
4. Heyneman CA, Rhodes RS. Glucosamine for osteoarthritis: cure or conundrum? Ann Pharmacother 1998;32:602-603.
5. Houpt JB, McMillan R, Wein C, Paget-Dellio SD. Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. J Rheumatol 1999;26:2423-2430.
6. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;(1):CD002946.-
7. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
Glucosamine may provide some pain relief. Studies have shown varied results, ranging from glucosamine being superior or equivalent to other agents, to no difference between glucosamine and placebo. However, most of these studies have small sample sizes, short duration, and often other significant flaws. Meta-analyses of available studies suggest a trend toward benefit from glucosamine (strength of recommendation: B).
Glucosamine may help osteoarthritis pain, but it is premature to recommend it universally until better studies are done. Even if glucosamine is effective, this sector of the market is currently unregulated, and products may not contain the amount or kind of glucosamine material advertised on their labels.
Evidence summary
Multiple methodological flaws have characterized studies trying to answer this question over the past 30 to 35 years. The companies manufacturing glucosamine have funded most studies. The overwhelming proportion of positive but marginal results raises the possibility of a publication bias (the tendency to publish only positive or supportive results), and the funding sources for the positive studies make that bias plausible.
Identified flaws in the studies include small sample size, inconsistent diagnostic criteria, variable disease sites, differing routes of administration, inconsistent doses, compositions and forms of glucosamine, the brief durations of studies, and poorly defined endpoints.1 Those problems account for the relatively low quality scores of the studies used in meta-analyses, particularly in earlier ones. Quality scores range from 12% to 52% of optimal and make any definitive conclusions suspect.2
The magnitude of the treatment effect is variable. Meta-analyses demonstrate aggregate treatment effects ranging from 0.36 to 1.02— where a small effect is 0.2, a moderate effect is 0.5, and a large effect is 0.8.2
When more recent, higher-quality studies are analyzed, trends toward benefit and the effect sizes for glucosamine diminish but remain at aggregate values ranging from 0.26 to 0.44.2-4 Statistically significant differences exist in some subgroup analyses and secondary endpoints.5 Typical trends suggest that glucosamine is superior to placebo for pain relief, and less effective but safer than nonsteroidal anti-inflammatory agents.6
Statements about safety are speculative given the brief duration of available trials, most of which lasted <10 weeks.6 Reported adverse effects are few. Mild gastrointestinal, skin, and constitutional symptoms predominate, but seldom at rates much higher than placebo.3-4 Pain relief may require as much as 4 to 6 weeks of therapy, and short studies may not demonstrate these benefits. The possibility of site-specific benefits or a difference in effect from a different dose or form is impossible to determine based on the current literature.
Recommendations from others
The American College of Rheumatology Subcommittee on Osteoarthritis believes that it is too early to issue recommendations for use of glucosamine sulfate or chondroitin sulfate for treatment of osteoarthritis.7
The National Institutes of Health Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT) began recruiting in May 2002. The design of this study is specifically directed at addressing the flaws of previous studies. This study will enroll 1588 patients at 13 study sites, and will use standardized products and doses with a single route of administration in a double-blinded, placebo-controlled fashion.
The GAIT study will measure change in joint space width (baseline to 2 years) and consists of 4 arms: glucosamine vs placebo, chondroitin vs placebo, glucosamine and chondroitin vs placebo, and celecoxib vs placebo. It is likely that the National Institute of Arthritis, Musculoskeletal and Skin Diseases, in collaboration with the National Center for Complementary and Alternative Medicine, will issue recommendations regarding the efficacy of glucosamine when the study is complete in 2005 or 2006. Updates are available at http://nccam.nih.gov/clinicaltrials/ glucosamine.htm.
Russell W. Roberts, MD
Louisiana State University Health Sciences Center, Shreveport, La
Patients frequently ask me if glucosamine, in combination with chondroitin or methylsulfonylmethane (MSM), reduces or prevents arthritis pain. It appears that glucosamine is safe and offers some promise.
I think a 6-week trial in patients with osteoarthritis is reasonable, preferably using glucosamine—a type that complies with the United States Pharmacopia/National Formulary standards—500 mg orally 3 times daily, once it becomes widely available. In my experience, very few patients who give glucosamine an enthusiastic and adequate trial of therapy continue the course for more than a few months. Those who use it longer often acknowledge only modest relief but continue with the hope of preventing further joint degeneration and increased pain, another currently unsubstantiated expectation.
Glucosamine may provide some pain relief. Studies have shown varied results, ranging from glucosamine being superior or equivalent to other agents, to no difference between glucosamine and placebo. However, most of these studies have small sample sizes, short duration, and often other significant flaws. Meta-analyses of available studies suggest a trend toward benefit from glucosamine (strength of recommendation: B).
Glucosamine may help osteoarthritis pain, but it is premature to recommend it universally until better studies are done. Even if glucosamine is effective, this sector of the market is currently unregulated, and products may not contain the amount or kind of glucosamine material advertised on their labels.
Evidence summary
Multiple methodological flaws have characterized studies trying to answer this question over the past 30 to 35 years. The companies manufacturing glucosamine have funded most studies. The overwhelming proportion of positive but marginal results raises the possibility of a publication bias (the tendency to publish only positive or supportive results), and the funding sources for the positive studies make that bias plausible.
Identified flaws in the studies include small sample size, inconsistent diagnostic criteria, variable disease sites, differing routes of administration, inconsistent doses, compositions and forms of glucosamine, the brief durations of studies, and poorly defined endpoints.1 Those problems account for the relatively low quality scores of the studies used in meta-analyses, particularly in earlier ones. Quality scores range from 12% to 52% of optimal and make any definitive conclusions suspect.2
The magnitude of the treatment effect is variable. Meta-analyses demonstrate aggregate treatment effects ranging from 0.36 to 1.02— where a small effect is 0.2, a moderate effect is 0.5, and a large effect is 0.8.2
When more recent, higher-quality studies are analyzed, trends toward benefit and the effect sizes for glucosamine diminish but remain at aggregate values ranging from 0.26 to 0.44.2-4 Statistically significant differences exist in some subgroup analyses and secondary endpoints.5 Typical trends suggest that glucosamine is superior to placebo for pain relief, and less effective but safer than nonsteroidal anti-inflammatory agents.6
Statements about safety are speculative given the brief duration of available trials, most of which lasted <10 weeks.6 Reported adverse effects are few. Mild gastrointestinal, skin, and constitutional symptoms predominate, but seldom at rates much higher than placebo.3-4 Pain relief may require as much as 4 to 6 weeks of therapy, and short studies may not demonstrate these benefits. The possibility of site-specific benefits or a difference in effect from a different dose or form is impossible to determine based on the current literature.
Recommendations from others
The American College of Rheumatology Subcommittee on Osteoarthritis believes that it is too early to issue recommendations for use of glucosamine sulfate or chondroitin sulfate for treatment of osteoarthritis.7
The National Institutes of Health Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT) began recruiting in May 2002. The design of this study is specifically directed at addressing the flaws of previous studies. This study will enroll 1588 patients at 13 study sites, and will use standardized products and doses with a single route of administration in a double-blinded, placebo-controlled fashion.
The GAIT study will measure change in joint space width (baseline to 2 years) and consists of 4 arms: glucosamine vs placebo, chondroitin vs placebo, glucosamine and chondroitin vs placebo, and celecoxib vs placebo. It is likely that the National Institute of Arthritis, Musculoskeletal and Skin Diseases, in collaboration with the National Center for Complementary and Alternative Medicine, will issue recommendations regarding the efficacy of glucosamine when the study is complete in 2005 or 2006. Updates are available at http://nccam.nih.gov/clinicaltrials/ glucosamine.htm.
Russell W. Roberts, MD
Louisiana State University Health Sciences Center, Shreveport, La
Patients frequently ask me if glucosamine, in combination with chondroitin or methylsulfonylmethane (MSM), reduces or prevents arthritis pain. It appears that glucosamine is safe and offers some promise.
I think a 6-week trial in patients with osteoarthritis is reasonable, preferably using glucosamine—a type that complies with the United States Pharmacopia/National Formulary standards—500 mg orally 3 times daily, once it becomes widely available. In my experience, very few patients who give glucosamine an enthusiastic and adequate trial of therapy continue the course for more than a few months. Those who use it longer often acknowledge only modest relief but continue with the hope of preventing further joint degeneration and increased pain, another currently unsubstantiated expectation.
1. Deal CL, Moskowitz RW. Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate and collagen hydrolysate. Rheum Dis Clin North Am 1999;25:379-395.
2. McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA 2000;283:1469-1475.
3. Barclay TS, Tsourounis C, McCart GM. Glucosamine. Ann Pharmacother 1998;32:574-579.
4. Heyneman CA, Rhodes RS. Glucosamine for osteoarthritis: cure or conundrum? Ann Pharmacother 1998;32:602-603.
5. Houpt JB, McMillan R, Wein C, Paget-Dellio SD. Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. J Rheumatol 1999;26:2423-2430.
6. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;(1):CD002946.-
7. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
1. Deal CL, Moskowitz RW. Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate and collagen hydrolysate. Rheum Dis Clin North Am 1999;25:379-395.
2. McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. JAMA 2000;283:1469-1475.
3. Barclay TS, Tsourounis C, McCart GM. Glucosamine. Ann Pharmacother 1998;32:574-579.
4. Heyneman CA, Rhodes RS. Glucosamine for osteoarthritis: cure or conundrum? Ann Pharmacother 1998;32:602-603.
5. Houpt JB, McMillan R, Wein C, Paget-Dellio SD. Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. J Rheumatol 1999;26:2423-2430.
6. Towheed TE, Anastassiades TP, Shea B, Houpt J, Welch V, Hochberg MC. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev 2001;(1):CD002946.-
7. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum 2000;43:1905-1915.
Evidence-based answers from the Family Physicians Inquiries Network
How should patients with mitral regurgitation be followed?
Patients with mild to moderate mitral regurgitation should be assessed periodically for a worsening condition; those with severe mitral regurgitation should be monitored for development of congestive heart failure, atrial fibrillation, and decline in left ventricular ejection fraction or increase in left ventricular end-diastolic diameter (strength of recommendation [SOR]=B).1-3
Cardiologists and general internists perform equally well in identifying severe mitral regurgitation among patients with known mitral regurgitation.4 Grade I or II murmurs indicate mild or moderate mitral regurgitation; grade IV or greater murmurs indicate severe mitral regurgitation, and grade III murmurs are indeterminate (SOR=B).4
The optimal frequency of evaluation is uncertain. Patients with severe regurgitation should be followed more frequently, with a combination of physical examination and echocardiography (SOR=B).
Evidence summary
A well-done, prospective cohort study enrolled 229 patients (mean age, 66; 70% male) diagnosed with severe mitral regurgitation. Overall 10-year mortality was 43%. Older patients, those with New York Heart Association (NYHA) class III or IV heart failure, or those with left ventricular ejection fraction <60% had higher mortality. Eighty-two percent of patients had surgery within 10 years. Mortality among patients undergoing surgery was equivalent to that of the age-matched US population and significantly less than patients managed without surgery.1
A second report from the same cohort compared the outcomes of patients undergoing early surgery (within 1 month of diagnosis) with those initially treated medically. Eight patients were excluded from this study because they were unsuitable candidates for surgery. The remaining 221 patients were followed based on their original group assignment of early surgery (63 patients) or medical management (158 patients).
Patients undergoing early surgery were more likely to have symptoms at enrollment than those managed medically. Patients in the early surgery group had better 5-year (89% vs 78%) and 10-year (78% vs 65%; P<.05 for both comparisons) survival and were less likely to develop congestive heart failure or atrial fibrillation. These differences remained significant after multivariate adjustment for potential confounders.2
Another cohort study of patients undergoing surgery for severe mitral regurgitation compared the outcomes of 199 patients with NYHA class I/II symptoms with those of 279 patients with NYHA class III/IV symptoms. Patients with NYHA class I/II had better operative outcomes (0.5% vs 5.4%) and better 5-year (90% vs 73%) and 10-year (76% vs 48%) survival than patients with more severe symptoms. In multivariate analysis, NYHA functional class remained inversely associated with survival.3
In a prospective study testing the ability of physical examination to identify severe mitral regurgitation, 170 consecutive patients with mitral regurgitation assessed by echocardiography underwent a clinical examination by internists or cardiologists blinded to the echocardiogram findings. The negative predictive value for absence of severe mitral regurgitation with a murmur less than grade III ranged from 88% to 100%. Murmurs greater than grade III had a predictive value of 91% for severe mitral regurgitation. Grade III murmurs were not predictive of severity.4
This study found no difference in the performance of internists and cardiologists. A systematic review found that cardiologists were able to accurately determine the presence or absence of mitral regurgitation by physical exam, but that trainees (internal medicine house staff and students) were much less accurate in their assessment.5
Recommendations from others
The American College of Cardiology and the American Heart Association recommend that patients with murmurs consistent with mitral regurgitation (holosytolic or late systolic murmurs) undergo echocardiography. Severity of regurgitation determined echocardiographically should dictate subsequent follow-up.
Patients with mild mitral regurgitation should undergo annual physical examination. Patients with moderate mitral regurgitation should undergo annual clinical evaluation and echocardiographic examination. Asymptomatic patients with severe mitral regurgitation should have a clinical and echocardiographic evaluation every 6 to 12 months. Patients with symptoms of heart failure or with mild left ventricular dysfunction (ejection fraction 50%-60% or end-diastolic dimension 45-50 mm) should be referred for surgery. Surgery should be considered in patients with severe mitral regurgitation and atrial fibrillation (SOR=D).6
Stephen Elgert, MD
New Hampshire Dartmouth-Concord Family Practice Residency, Concord
This question is best answered with the following assumptions:
- The mitral regurgitation is not acute (eg, following acute ischemia or frank myocardial infarction) and does not require immediate intervention
- If no other associated valve disease is found, care should be individualized
- Mitral regurgitation is clearly differentiated from mitral valve prolapse (although in reality they may lie on a continuum).
Given these assumptions, stratifying patients into mild, moderate, and severe categories makes the most sense. These recommendations accurately reflect a literature that has few randomized controlled trials to guide us.
As echocardiography and other technology for assessing the cardiovascular system have become readily available, physicians’ ability to accurately auscultate the heart has diminished. Given this, echocardiograms are an increasingly important way to identify and follow patients with all stages of mitral regurgitation.
1. Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996;335:1417-1423.
2. Ling LH, Enriquez-Sarano M, Seward JB, et al. Early surgery in patients with mitral regurgitation due to flail leaflets: a long-term outcome study. Circulation 1997;96:1819-1825.
3. Tribouilloy CM, Enriquez-Sarano M, Schaff HV, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation 1999;99:400-405.
4. Desjardins VA, Enriquez-Sarano M, Tajik AJ, Bailey KR, Seward JB. Intensity of murmurs correlates with severity of valvular regurgitation. Am J Med 1996;100:149-156.
5. Etchells E, Bell C, Robb K. Does this patient have an abnormal systolic murmur? JAMA 1997;277:564-571.
6. Bonow RO, Carabello B, de Leon AJ, Jr, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 1998;98:1949-1984.
Patients with mild to moderate mitral regurgitation should be assessed periodically for a worsening condition; those with severe mitral regurgitation should be monitored for development of congestive heart failure, atrial fibrillation, and decline in left ventricular ejection fraction or increase in left ventricular end-diastolic diameter (strength of recommendation [SOR]=B).1-3
Cardiologists and general internists perform equally well in identifying severe mitral regurgitation among patients with known mitral regurgitation.4 Grade I or II murmurs indicate mild or moderate mitral regurgitation; grade IV or greater murmurs indicate severe mitral regurgitation, and grade III murmurs are indeterminate (SOR=B).4
The optimal frequency of evaluation is uncertain. Patients with severe regurgitation should be followed more frequently, with a combination of physical examination and echocardiography (SOR=B).
Evidence summary
A well-done, prospective cohort study enrolled 229 patients (mean age, 66; 70% male) diagnosed with severe mitral regurgitation. Overall 10-year mortality was 43%. Older patients, those with New York Heart Association (NYHA) class III or IV heart failure, or those with left ventricular ejection fraction <60% had higher mortality. Eighty-two percent of patients had surgery within 10 years. Mortality among patients undergoing surgery was equivalent to that of the age-matched US population and significantly less than patients managed without surgery.1
A second report from the same cohort compared the outcomes of patients undergoing early surgery (within 1 month of diagnosis) with those initially treated medically. Eight patients were excluded from this study because they were unsuitable candidates for surgery. The remaining 221 patients were followed based on their original group assignment of early surgery (63 patients) or medical management (158 patients).
Patients undergoing early surgery were more likely to have symptoms at enrollment than those managed medically. Patients in the early surgery group had better 5-year (89% vs 78%) and 10-year (78% vs 65%; P<.05 for both comparisons) survival and were less likely to develop congestive heart failure or atrial fibrillation. These differences remained significant after multivariate adjustment for potential confounders.2
Another cohort study of patients undergoing surgery for severe mitral regurgitation compared the outcomes of 199 patients with NYHA class I/II symptoms with those of 279 patients with NYHA class III/IV symptoms. Patients with NYHA class I/II had better operative outcomes (0.5% vs 5.4%) and better 5-year (90% vs 73%) and 10-year (76% vs 48%) survival than patients with more severe symptoms. In multivariate analysis, NYHA functional class remained inversely associated with survival.3
In a prospective study testing the ability of physical examination to identify severe mitral regurgitation, 170 consecutive patients with mitral regurgitation assessed by echocardiography underwent a clinical examination by internists or cardiologists blinded to the echocardiogram findings. The negative predictive value for absence of severe mitral regurgitation with a murmur less than grade III ranged from 88% to 100%. Murmurs greater than grade III had a predictive value of 91% for severe mitral regurgitation. Grade III murmurs were not predictive of severity.4
This study found no difference in the performance of internists and cardiologists. A systematic review found that cardiologists were able to accurately determine the presence or absence of mitral regurgitation by physical exam, but that trainees (internal medicine house staff and students) were much less accurate in their assessment.5
Recommendations from others
The American College of Cardiology and the American Heart Association recommend that patients with murmurs consistent with mitral regurgitation (holosytolic or late systolic murmurs) undergo echocardiography. Severity of regurgitation determined echocardiographically should dictate subsequent follow-up.
Patients with mild mitral regurgitation should undergo annual physical examination. Patients with moderate mitral regurgitation should undergo annual clinical evaluation and echocardiographic examination. Asymptomatic patients with severe mitral regurgitation should have a clinical and echocardiographic evaluation every 6 to 12 months. Patients with symptoms of heart failure or with mild left ventricular dysfunction (ejection fraction 50%-60% or end-diastolic dimension 45-50 mm) should be referred for surgery. Surgery should be considered in patients with severe mitral regurgitation and atrial fibrillation (SOR=D).6
Stephen Elgert, MD
New Hampshire Dartmouth-Concord Family Practice Residency, Concord
This question is best answered with the following assumptions:
- The mitral regurgitation is not acute (eg, following acute ischemia or frank myocardial infarction) and does not require immediate intervention
- If no other associated valve disease is found, care should be individualized
- Mitral regurgitation is clearly differentiated from mitral valve prolapse (although in reality they may lie on a continuum).
Given these assumptions, stratifying patients into mild, moderate, and severe categories makes the most sense. These recommendations accurately reflect a literature that has few randomized controlled trials to guide us.
As echocardiography and other technology for assessing the cardiovascular system have become readily available, physicians’ ability to accurately auscultate the heart has diminished. Given this, echocardiograms are an increasingly important way to identify and follow patients with all stages of mitral regurgitation.
Patients with mild to moderate mitral regurgitation should be assessed periodically for a worsening condition; those with severe mitral regurgitation should be monitored for development of congestive heart failure, atrial fibrillation, and decline in left ventricular ejection fraction or increase in left ventricular end-diastolic diameter (strength of recommendation [SOR]=B).1-3
Cardiologists and general internists perform equally well in identifying severe mitral regurgitation among patients with known mitral regurgitation.4 Grade I or II murmurs indicate mild or moderate mitral regurgitation; grade IV or greater murmurs indicate severe mitral regurgitation, and grade III murmurs are indeterminate (SOR=B).4
The optimal frequency of evaluation is uncertain. Patients with severe regurgitation should be followed more frequently, with a combination of physical examination and echocardiography (SOR=B).
Evidence summary
A well-done, prospective cohort study enrolled 229 patients (mean age, 66; 70% male) diagnosed with severe mitral regurgitation. Overall 10-year mortality was 43%. Older patients, those with New York Heart Association (NYHA) class III or IV heart failure, or those with left ventricular ejection fraction <60% had higher mortality. Eighty-two percent of patients had surgery within 10 years. Mortality among patients undergoing surgery was equivalent to that of the age-matched US population and significantly less than patients managed without surgery.1
A second report from the same cohort compared the outcomes of patients undergoing early surgery (within 1 month of diagnosis) with those initially treated medically. Eight patients were excluded from this study because they were unsuitable candidates for surgery. The remaining 221 patients were followed based on their original group assignment of early surgery (63 patients) or medical management (158 patients).
Patients undergoing early surgery were more likely to have symptoms at enrollment than those managed medically. Patients in the early surgery group had better 5-year (89% vs 78%) and 10-year (78% vs 65%; P<.05 for both comparisons) survival and were less likely to develop congestive heart failure or atrial fibrillation. These differences remained significant after multivariate adjustment for potential confounders.2
Another cohort study of patients undergoing surgery for severe mitral regurgitation compared the outcomes of 199 patients with NYHA class I/II symptoms with those of 279 patients with NYHA class III/IV symptoms. Patients with NYHA class I/II had better operative outcomes (0.5% vs 5.4%) and better 5-year (90% vs 73%) and 10-year (76% vs 48%) survival than patients with more severe symptoms. In multivariate analysis, NYHA functional class remained inversely associated with survival.3
In a prospective study testing the ability of physical examination to identify severe mitral regurgitation, 170 consecutive patients with mitral regurgitation assessed by echocardiography underwent a clinical examination by internists or cardiologists blinded to the echocardiogram findings. The negative predictive value for absence of severe mitral regurgitation with a murmur less than grade III ranged from 88% to 100%. Murmurs greater than grade III had a predictive value of 91% for severe mitral regurgitation. Grade III murmurs were not predictive of severity.4
This study found no difference in the performance of internists and cardiologists. A systematic review found that cardiologists were able to accurately determine the presence or absence of mitral regurgitation by physical exam, but that trainees (internal medicine house staff and students) were much less accurate in their assessment.5
Recommendations from others
The American College of Cardiology and the American Heart Association recommend that patients with murmurs consistent with mitral regurgitation (holosytolic or late systolic murmurs) undergo echocardiography. Severity of regurgitation determined echocardiographically should dictate subsequent follow-up.
Patients with mild mitral regurgitation should undergo annual physical examination. Patients with moderate mitral regurgitation should undergo annual clinical evaluation and echocardiographic examination. Asymptomatic patients with severe mitral regurgitation should have a clinical and echocardiographic evaluation every 6 to 12 months. Patients with symptoms of heart failure or with mild left ventricular dysfunction (ejection fraction 50%-60% or end-diastolic dimension 45-50 mm) should be referred for surgery. Surgery should be considered in patients with severe mitral regurgitation and atrial fibrillation (SOR=D).6
Stephen Elgert, MD
New Hampshire Dartmouth-Concord Family Practice Residency, Concord
This question is best answered with the following assumptions:
- The mitral regurgitation is not acute (eg, following acute ischemia or frank myocardial infarction) and does not require immediate intervention
- If no other associated valve disease is found, care should be individualized
- Mitral regurgitation is clearly differentiated from mitral valve prolapse (although in reality they may lie on a continuum).
Given these assumptions, stratifying patients into mild, moderate, and severe categories makes the most sense. These recommendations accurately reflect a literature that has few randomized controlled trials to guide us.
As echocardiography and other technology for assessing the cardiovascular system have become readily available, physicians’ ability to accurately auscultate the heart has diminished. Given this, echocardiograms are an increasingly important way to identify and follow patients with all stages of mitral regurgitation.
1. Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996;335:1417-1423.
2. Ling LH, Enriquez-Sarano M, Seward JB, et al. Early surgery in patients with mitral regurgitation due to flail leaflets: a long-term outcome study. Circulation 1997;96:1819-1825.
3. Tribouilloy CM, Enriquez-Sarano M, Schaff HV, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation 1999;99:400-405.
4. Desjardins VA, Enriquez-Sarano M, Tajik AJ, Bailey KR, Seward JB. Intensity of murmurs correlates with severity of valvular regurgitation. Am J Med 1996;100:149-156.
5. Etchells E, Bell C, Robb K. Does this patient have an abnormal systolic murmur? JAMA 1997;277:564-571.
6. Bonow RO, Carabello B, de Leon AJ, Jr, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 1998;98:1949-1984.
1. Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996;335:1417-1423.
2. Ling LH, Enriquez-Sarano M, Seward JB, et al. Early surgery in patients with mitral regurgitation due to flail leaflets: a long-term outcome study. Circulation 1997;96:1819-1825.
3. Tribouilloy CM, Enriquez-Sarano M, Schaff HV, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation 1999;99:400-405.
4. Desjardins VA, Enriquez-Sarano M, Tajik AJ, Bailey KR, Seward JB. Intensity of murmurs correlates with severity of valvular regurgitation. Am J Med 1996;100:149-156.
5. Etchells E, Bell C, Robb K. Does this patient have an abnormal systolic murmur? JAMA 1997;277:564-571.
6. Bonow RO, Carabello B, de Leon AJ, Jr, et al. Guidelines for the management of patients with valvular heart disease: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation 1998;98:1949-1984.
Evidence-based answers from the Family Physicians Inquiries Network
Heat or ice for acute ankle sprain?
For grade 3 and 4 ankle sprains, ice works better than heat to speed recovery (return to play) (strength of recommendation [SOR]: B, based on a single retrospective cohort study). No studies support faster return to play with the application of heat at any time after injury (SOR: B, based on head-to-head randomized trials). Ice therapy also reduces edema, but the clinical significance of this finding is unclear.
Evidence summary
Studies of ankle sprain use variable diagnostic criteria for sprain and definition of recovery (return to play). They often report indirect outcomes such as edema. The effect of decreased edema on recovery time is not addressed.
Only 1 study has directly compared heat vs ice therapy and recovery time for ankle sprains. A retrospective cohort study of 32 patients in a sports medicine clinic demonstrated that early cryotherapy (within 36 hours of injury) for grades 3 and 4 ankle sprains, when compared with early heat therapy, resulted in earlier return to activity, as defined by ability to walk, climb stairs, run, and jump without pain.1 Grade 3 sprains treated with ice recovered in 11.0 days vs 14.8 days with heat. Grade 4 sprains treated with ice recovered in 13.2 days vs 30.4 days with heat. This study also showed that early application of ice (within 36 hours) decreased time to recovery compared with late application of ice.
However, evidence is heterogeneous about the effect of ice on return to play. In 2 of 3 randomized controlled trials, early application of ice vs placebo did not significantly speed return to play.
One randomized controlled trial compared ice therapy (in the form of a cooling anklet applied upon presentation) with placebo in 143 patients presenting within 24 hours of injury to a university emergency department in England.2 All patients received high-dose nonsteroidal anti-inflammatory agents. Though a trend was found in favor of ice therapy, no statistically significant difference was found in recovery time, as defined by pain relief and ability to bear weight. The grade of sprain was not specifically accounted for in this study.
Another randomized controlled trial compared ice with placebo in 30 patients with grade 3 and 4 sprains referred to a physiotherapy department within 2 days of ankle injury. No statistical difference was found in recovery time, defined as ability to bear weight with only mild to moderate pain.3
However, a randomized controlled trial of 60 patients with acute ankle sprains of all grades presenting to an emergency department compared cryogel plus bandaging with bandaging alone (cooling vs no cooling). This study found the mean time to recovery—defined as decreased pain—was reduced from 14.8 days to 9.7 days with constant cooling for the first 48 hours.4
The application of ice—but not heat—within 24 to 48 hours of acute ankle sprain also reduced edema. Several studies looked at reduction of edema with cooling. One study measured edema in 30 patients with grade 1 and 2 sprains treated with cold, heat, or contrast baths during the third, fourth, and fifth days.5 Only ice therapy alone significantly reduced edema.
Recommendations from others
The American Academy of Orthopaedic Surgeons recommends initial treatment of stable ankle sprains with rest, ice, gentle compression, and elevation (RICE).6 These guidelines are echoed by the American Academy of Family Physicians. In addition, the Institute for Clinical Systems Improvement and the National Guidelines Clearinghouse recommend PRICE, where protecting the ankle is explicitly added to RICE therapy.7
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes; Department of Family Medicine, University of Colorado
Ice should be the first choice for all acute ankle sprains. The immediate goals of treating an ankle sprain are reducing edema, stabilizing the ankle, and enabling early weight-bearing. Applying heat may increase swelling and subsequently slow recovery. Once the initial phase of recovery is achieved through cryotherapy, compression, and elevation, the injured patient may initiate work to increase strength, flexibility, and range of motion of the injured ankle. As a result, icing an ankle sprain facilitates an earlier return to full activity and sports participation by speeding the first phase of recovery.
1. Sloan JP, Hain R, Pownall R. Clinical benefits of early cold therapy in accident and emergency following ankle sprain. Arch Emerg Med 1989;6:1-6.
2. Laba E, Roestenburg M. Clinical evaluation of ice therapy for acute ankle sprain injuries. N Z J Physiother 1989;17:7-9.
3. Basur RL, Shephard E, Mouzas GL. A cooling method in the treatment of ankle sprains. Practitioner 1976;216:708-711.
4. Hocutt JE, Jr, Jaffee R, Rylander CR, Beebe JK. Cryotherapy in ankle sprains. Am J Sports Med 1982;10:316-319.
5. Cote DJ, Prentice WE, Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther 1988;68:1072-1076.
6. American Academy of Orthopaedic Surgeons. Clinical Guideline on Ankle Injury. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 1997:7.
7. Institute for Clinical Systems Improvement (ICSI). Ankle Sprain. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002:24.
For grade 3 and 4 ankle sprains, ice works better than heat to speed recovery (return to play) (strength of recommendation [SOR]: B, based on a single retrospective cohort study). No studies support faster return to play with the application of heat at any time after injury (SOR: B, based on head-to-head randomized trials). Ice therapy also reduces edema, but the clinical significance of this finding is unclear.
Evidence summary
Studies of ankle sprain use variable diagnostic criteria for sprain and definition of recovery (return to play). They often report indirect outcomes such as edema. The effect of decreased edema on recovery time is not addressed.
Only 1 study has directly compared heat vs ice therapy and recovery time for ankle sprains. A retrospective cohort study of 32 patients in a sports medicine clinic demonstrated that early cryotherapy (within 36 hours of injury) for grades 3 and 4 ankle sprains, when compared with early heat therapy, resulted in earlier return to activity, as defined by ability to walk, climb stairs, run, and jump without pain.1 Grade 3 sprains treated with ice recovered in 11.0 days vs 14.8 days with heat. Grade 4 sprains treated with ice recovered in 13.2 days vs 30.4 days with heat. This study also showed that early application of ice (within 36 hours) decreased time to recovery compared with late application of ice.
However, evidence is heterogeneous about the effect of ice on return to play. In 2 of 3 randomized controlled trials, early application of ice vs placebo did not significantly speed return to play.
One randomized controlled trial compared ice therapy (in the form of a cooling anklet applied upon presentation) with placebo in 143 patients presenting within 24 hours of injury to a university emergency department in England.2 All patients received high-dose nonsteroidal anti-inflammatory agents. Though a trend was found in favor of ice therapy, no statistically significant difference was found in recovery time, as defined by pain relief and ability to bear weight. The grade of sprain was not specifically accounted for in this study.
Another randomized controlled trial compared ice with placebo in 30 patients with grade 3 and 4 sprains referred to a physiotherapy department within 2 days of ankle injury. No statistical difference was found in recovery time, defined as ability to bear weight with only mild to moderate pain.3
However, a randomized controlled trial of 60 patients with acute ankle sprains of all grades presenting to an emergency department compared cryogel plus bandaging with bandaging alone (cooling vs no cooling). This study found the mean time to recovery—defined as decreased pain—was reduced from 14.8 days to 9.7 days with constant cooling for the first 48 hours.4
The application of ice—but not heat—within 24 to 48 hours of acute ankle sprain also reduced edema. Several studies looked at reduction of edema with cooling. One study measured edema in 30 patients with grade 1 and 2 sprains treated with cold, heat, or contrast baths during the third, fourth, and fifth days.5 Only ice therapy alone significantly reduced edema.
Recommendations from others
The American Academy of Orthopaedic Surgeons recommends initial treatment of stable ankle sprains with rest, ice, gentle compression, and elevation (RICE).6 These guidelines are echoed by the American Academy of Family Physicians. In addition, the Institute for Clinical Systems Improvement and the National Guidelines Clearinghouse recommend PRICE, where protecting the ankle is explicitly added to RICE therapy.7
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes; Department of Family Medicine, University of Colorado
Ice should be the first choice for all acute ankle sprains. The immediate goals of treating an ankle sprain are reducing edema, stabilizing the ankle, and enabling early weight-bearing. Applying heat may increase swelling and subsequently slow recovery. Once the initial phase of recovery is achieved through cryotherapy, compression, and elevation, the injured patient may initiate work to increase strength, flexibility, and range of motion of the injured ankle. As a result, icing an ankle sprain facilitates an earlier return to full activity and sports participation by speeding the first phase of recovery.
For grade 3 and 4 ankle sprains, ice works better than heat to speed recovery (return to play) (strength of recommendation [SOR]: B, based on a single retrospective cohort study). No studies support faster return to play with the application of heat at any time after injury (SOR: B, based on head-to-head randomized trials). Ice therapy also reduces edema, but the clinical significance of this finding is unclear.
Evidence summary
Studies of ankle sprain use variable diagnostic criteria for sprain and definition of recovery (return to play). They often report indirect outcomes such as edema. The effect of decreased edema on recovery time is not addressed.
Only 1 study has directly compared heat vs ice therapy and recovery time for ankle sprains. A retrospective cohort study of 32 patients in a sports medicine clinic demonstrated that early cryotherapy (within 36 hours of injury) for grades 3 and 4 ankle sprains, when compared with early heat therapy, resulted in earlier return to activity, as defined by ability to walk, climb stairs, run, and jump without pain.1 Grade 3 sprains treated with ice recovered in 11.0 days vs 14.8 days with heat. Grade 4 sprains treated with ice recovered in 13.2 days vs 30.4 days with heat. This study also showed that early application of ice (within 36 hours) decreased time to recovery compared with late application of ice.
However, evidence is heterogeneous about the effect of ice on return to play. In 2 of 3 randomized controlled trials, early application of ice vs placebo did not significantly speed return to play.
One randomized controlled trial compared ice therapy (in the form of a cooling anklet applied upon presentation) with placebo in 143 patients presenting within 24 hours of injury to a university emergency department in England.2 All patients received high-dose nonsteroidal anti-inflammatory agents. Though a trend was found in favor of ice therapy, no statistically significant difference was found in recovery time, as defined by pain relief and ability to bear weight. The grade of sprain was not specifically accounted for in this study.
Another randomized controlled trial compared ice with placebo in 30 patients with grade 3 and 4 sprains referred to a physiotherapy department within 2 days of ankle injury. No statistical difference was found in recovery time, defined as ability to bear weight with only mild to moderate pain.3
However, a randomized controlled trial of 60 patients with acute ankle sprains of all grades presenting to an emergency department compared cryogel plus bandaging with bandaging alone (cooling vs no cooling). This study found the mean time to recovery—defined as decreased pain—was reduced from 14.8 days to 9.7 days with constant cooling for the first 48 hours.4
The application of ice—but not heat—within 24 to 48 hours of acute ankle sprain also reduced edema. Several studies looked at reduction of edema with cooling. One study measured edema in 30 patients with grade 1 and 2 sprains treated with cold, heat, or contrast baths during the third, fourth, and fifth days.5 Only ice therapy alone significantly reduced edema.
Recommendations from others
The American Academy of Orthopaedic Surgeons recommends initial treatment of stable ankle sprains with rest, ice, gentle compression, and elevation (RICE).6 These guidelines are echoed by the American Academy of Family Physicians. In addition, the Institute for Clinical Systems Improvement and the National Guidelines Clearinghouse recommend PRICE, where protecting the ankle is explicitly added to RICE therapy.7
Sourav Poddar, MD
Team Physician, University of Colorado Buffaloes; Department of Family Medicine, University of Colorado
Ice should be the first choice for all acute ankle sprains. The immediate goals of treating an ankle sprain are reducing edema, stabilizing the ankle, and enabling early weight-bearing. Applying heat may increase swelling and subsequently slow recovery. Once the initial phase of recovery is achieved through cryotherapy, compression, and elevation, the injured patient may initiate work to increase strength, flexibility, and range of motion of the injured ankle. As a result, icing an ankle sprain facilitates an earlier return to full activity and sports participation by speeding the first phase of recovery.
1. Sloan JP, Hain R, Pownall R. Clinical benefits of early cold therapy in accident and emergency following ankle sprain. Arch Emerg Med 1989;6:1-6.
2. Laba E, Roestenburg M. Clinical evaluation of ice therapy for acute ankle sprain injuries. N Z J Physiother 1989;17:7-9.
3. Basur RL, Shephard E, Mouzas GL. A cooling method in the treatment of ankle sprains. Practitioner 1976;216:708-711.
4. Hocutt JE, Jr, Jaffee R, Rylander CR, Beebe JK. Cryotherapy in ankle sprains. Am J Sports Med 1982;10:316-319.
5. Cote DJ, Prentice WE, Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther 1988;68:1072-1076.
6. American Academy of Orthopaedic Surgeons. Clinical Guideline on Ankle Injury. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 1997:7.
7. Institute for Clinical Systems Improvement (ICSI). Ankle Sprain. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002:24.
1. Sloan JP, Hain R, Pownall R. Clinical benefits of early cold therapy in accident and emergency following ankle sprain. Arch Emerg Med 1989;6:1-6.
2. Laba E, Roestenburg M. Clinical evaluation of ice therapy for acute ankle sprain injuries. N Z J Physiother 1989;17:7-9.
3. Basur RL, Shephard E, Mouzas GL. A cooling method in the treatment of ankle sprains. Practitioner 1976;216:708-711.
4. Hocutt JE, Jr, Jaffee R, Rylander CR, Beebe JK. Cryotherapy in ankle sprains. Am J Sports Med 1982;10:316-319.
5. Cote DJ, Prentice WE, Jr, Hooker DN, Shields EW. Comparison of three treatment procedures for minimizing ankle sprain swelling. Phys Ther 1988;68:1072-1076.
6. American Academy of Orthopaedic Surgeons. Clinical Guideline on Ankle Injury. Rosemont, Ill: American Academy of Orthopaedic Surgeons; 1997:7.
7. Institute for Clinical Systems Improvement (ICSI). Ankle Sprain. Bloomington, Minn: Institute for Clinical Systems Improvement (ICSI); 2002:24.
Evidence-based answers from the Family Physicians Inquiries Network