User login
Cover
Opioid-Induced Hearing Loss:
A Trend to Keep Listening For?
When and how to place an autologous rectus fascia pubovaginal sling
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
The authors report no financial relationships relevant to this article.
Developed in partnership with International Academy of Pelvic Surgery.
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
The authors report no financial relationships relevant to this article.
Developed in partnership with International Academy of Pelvic Surgery.
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
The authors report no financial relationships relevant to this article.
Developed in partnership with International Academy of Pelvic Surgery.
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
UPDATE ON OSTEOPOROSIS
What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis?
Steven R. Goldstein, MD (Examining the Evidence, August 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate?
Andrew M. Kaunitz, MD; David A. Grimes, MD (Guest Editorial, August 2011)
Osteoporosis is a significant health issue—and it is likely to remain so as more and more women live longer and longer. In fact, increasing age is the single biggest risk factor for osteoporotic fragility fracture.
Over the past year, important research has improved our understanding in diverse areas of bone health. In this Update, I highlight studies that:
- seek to elucidate the optimal frequency of dual-energy x-ray absorptiometry (DXA) imaging to assess bone mineral density
- review secondary causes of osteoporosis besides menopause-related estrogen deficiency
- explore the use of quantitative ultrasound (QUS) to predict the risk of fracture
- report on a new class of pharmaceutical agents that inhibit the bone-resorption enzyme Cathepsin K.
All of these issues are clinically relevant to the ObGyn specialty because, when it comes to our patients’ bone health, we often function as the primary care physician.
When is DXA indicated—and how often should it be repeated?
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233.
Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739–742.
American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone density testing interval and transition to osteoporosis in older women” [press release]. http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed October 15, 2012.
Recommendations from professional societies, such as the National Osteoporosis Foundation, the International Society of Clinical Densitometry, and the American College of Obstetricians and Gynecologists say virtually the same thing about DXA imaging: Screening is appropriate for women 65 years and older and for postmenopausal women younger than age 65 who have risk factors for fracture. Risk factors include:
- history of a fragility fracture
- body weight less than 127 lb
- medical causes of bone loss, such as medication or disease
- parental history of hip fracture
- current smoker
- alcoholism
- rheumatoid arthritis.
The measurement of bone mineral density (BMD) has been the cornerstone of the diagnosis of osteopenia and osteoporosis since these classifications were introduced by the World Health Organization (WHO) in 1994. Although we are now able to evaluate a woman’s fracture risk using the FRAX tool, which does not require BMD assessment, DXA scanning has become entrenched in routine clinical practice in the United States. In addition, patients who use drugs to reduce their fracture risk often demand periodic testing to see how they are doing. Even women who do not take medications often want periodic assessment to confirm that they are not “losing bone.” Medicare allows for testing every 23 months.
23-month screening interval does not fit all women
Gourlay and colleagues prospectively followed 4,957 women aged 67 years or older who had no history of hip or vertebral fracture and who were not being treated for osteoporosis. After follow-up for as long as 15 years, investigators found that the better a woman’s initial bone density, the longer it took for her to develop osteoporosis. For example, among women over 67 years of age who had a T-score of –1.0 or better, it would take 16.8 years for 10% of this population to develop osteoporosis. In contrast, among women over 67 years of age who had a T-score of –2.0, it would take only 1.1 years for 10% of this population to develop osteoporosis.
This finding certainly calls into question the notion that all patients should be screened every 23 months. It may be better to think of screening as a way of triaging patients for decisions relative to subsequent follow-up.
Media distorted take-home message
This study was the focus of considerable attention from the media, which implied that too much DXA screening is being performed. In reality, only 13% of women over the age of 65 undergo a baseline DXA scan. However, routine follow-up of all patients at 23-month intervals is clearly not appropriate.
Because this study primarily involved white women older than age 67, extrapolation of its findings to other groups may not be appropriate. Nevertheless, the study helps to underscore the fact that reliance on BMD measurement alone should not be used to determine the need for therapeutic intervention. The FRAX tool can be used on an annual basis to assess a woman’s risk of fracture and does not require follow-up DXA imaging at any arbitrary interval.
In healthy older women, an interval of 23 months for repeat BMD assessment makes little sense. For women who have excellent initial T-scores, clinicians can lengthen this interval significantly.
However, strict reliance on the T-score isn’t the best way to predict a woman’s fracture risk or determine when pharmacologic intervention is warranted. Rather, yearly assessment using a tool such as FRAX should become the standard of care.
Some secondary causes of osteoporosis are overlooked or underappreciated
Miller PD. Unrecognized and unappreciated secondary causes of osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):613–628.
The fractures traditionally associated with osteoporosis involve the hip and vertebrae, although low-trauma fractures of the humerus, forearm, femur shaft, tibia, and fibula are also associated with a high risk of future fracture in untreated women.
Once a clinician is confident that a patient has osteoporosis, the question is whether the diagnosis is postmenopausal osteoporosis—or some other form of the disease. Although estrogen deficiency is the most common cause of osteoporosis in postmenopausal women, many other conditions may accompany estrogen deficiency and contribute to impaired bone strength in this population.
Among the culprits are some conditions that are not often encountered in the average gynecologic practice: monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, celiac disease, Crohn’s disease, and other inflammatory bowel diseases. In addition, bariatric surgery, eating disorders, primary hyperparathyroidism, and a number of medications have been implicated in BMD loss or increased risk of fracture, or both. Among the problematic drugs of particular interest to us as gynecologists are aromatase inhibitors, depot medroxyprogesterone acetate, proton pump inhibitors, and gonadotropin-releasing hormone (GnRH) agonists.
Other medications that can affect BMD are glucocorticoids, unfractionated heparin, selective serotonin reuptake inhibitors, excessive amounts of thyroid replacement agents, and some antiseizure medications.
If you suspect a secondary cause of osteoporosis, be prepared to perform a basic workup that includes:
- a careful history and physical examination
- complete blood count
- a chemistry profile, including serum calcium, phosphorous, electrolytes, alkaline phosphatase, and creatinine.
In addition, measurement of 25-hydroxy vitamin D and thyroid-stimulating hormone (TSH) may be helpful, as may serum protein electrophoresis.
Patients who have clinical or laboratory abnormalities suggestive of a secondary cause of osteoporosis are usually referred to a metabolic bone specialist (endocrinology or rheumatology).
When a patient has any clinical history that suggests a secondary cause of bone loss other than menopause-related estrogen deficiency, simple laboratory tests are appropriate and may uncover a condition that necessitates referral to a metabolic bone expert.
Quantitative ultrasound assessment of bone can help predict a woman’s risk of fracture
Guglielmi G, Rossini M, Nicolosi MG, Tagno A, Lentini G, de Terlizzi F. Three-year prospective study on fracture risk in postmenopausal women by quantitative ultrasound at the phalanges [published online ahead of print August 15, 2012]. J Clin Densitom. doi:10.1016 /j.jocd.2012.07.006.
Chan MY, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Quantitative ultrasound and fracture risk prediction in non-osteoporotic men and women as defined by WHO criteria [published online ahead of print August 10, 2012]. Osteoporos Int. doi:10.1007/s00198-012 -2001-2.
I became interested in bone health through my longstanding interest in ultrasound, when a manufacturer asked me to evaluate equipment designed to assess bone density of the heel through quantitative ultrasound (QUS). This modality is not the diagnostic imaging we are familiar with in obstetrics and gynecology. In QUS, the homogeneity of healthy bone promotes sound transmission, whereas the voids and discontinuity of osteoporotic bone impede it. Therefore, normal bone has a faster speed of sound than less healthy bone. The other important quantitative measure is broadband ultrasound attenuation (BUA). Healthy bone is dense and absorbs and scatters sound to a greater extent than osteoporotic bone does.
Two trials of QUS
In 2010, Guglielmi and colleagues contacted 2,210 Italian women who had undergone QUS of the phalanges in 2006–2007. These women had an average age of 60.9 years, entered menopause at an average age of 49.3 years, and had a mean body mass index (BMI) of 26.5 kg/m2. By 2010, this group had experienced 108 new major osteoporotic fractures, including 23 hip fractures and 56 vertebral fractures. Investigators found a statistically significant correlation between QUS findings and fracture risk.
Chan and colleagues focused on 312 women 62 to 92 years of age who had femoral neck BMD, as measured by DXA, of –2.5 or better. QUS was measured as BUA at the calcaneus. The incidence of any fragility fracture was ascertained by radiographic reports during the follow-up period from 1994 to 2011. Eighty women (26%) experienced at least one fragility fracture during follow-up. After adjustment for covariates, women were significantly more likely to experience any fracture if BUA was decreased (hazard ratio [HR], 1.50; 95% confidence interval [CI], 1.13–1.99).
When the models that included BUA were compared with those that used femoral neck BMD, they had a greater area under the curve (0.71, 0.85, 0.71 for any fracture, hip fracture, and vertebral fracture, respectively) and yielded a net reclassification improvement of 16.4% (P=.009) when combined with femoral neck BMD. These findings suggest that calcaneal BUA is an independent predictor of fracture risk in women who have nonosteoporotic BMD.
In an era of increasing pressure to reduce costs, QUS assessment of bone is a promising modality that may be useful as a screening tool. Although it measures different variables than DXA imaging (more microarchitecture, less true density), it seems to predict the risk of fracture at less cost without ionizing radiation.
In the pipeline: A drug that curbs bone resorption without diminishing bone formation
Williams SC. Potential first-in-class osteoporosis drug speeds through trials. Nat Med. 2012;18(8):1158.
Ng KW. Potential role of odanacatib in the treatment of osteoporosis. Clin Interv Aging. 2012;7:235–247.
Alendronate was the first of the oral bisphosphonates to be approved by the US Food and Drug Administration (FDA). Once it was approved in 1999, the drug quickly became the most widely used bone agent in clinical practice and was soon joined by other oral and intravenous bisphosphonates. Regrettably, highly publicized adverse effects have caused many patients to shy away from this class of drugs. Two years ago, the FDA approved denosumab, a subcutaneous injectable agent that is a RANK ligand inhibitor.
The bisphosphonates and denosumab increase bone mass by shutting down the osteoclasts responsible for bone resorption, but they also inhibit creation of new bone. A new category of drug that inhibits the bone-resorption enzyme Cathepsin K appears to inhibit bone resorption without diminishing bone formation. Trials of two previous agents in this class were halted because of adverse effects—particularly effects to the skin, where the enzyme is expressed in addition to bone. However, Phase 2 trials in which odanacatib was compared with alendronate found that the new drug increased BMD almost twice as much as alendronate did, with less reduction in serum markers of bone formation.
Phase 3 trials of odanacatib in 16,000 women older than age 65 recently were halted so that the manufacturer could pursue regulatory approval ahead of the previous schedule. Although Phase 3 data have not been published yet, odanacatib may prove to be an exciting alternative to existing therapies.
Odanacatib is not yet available. However, by discussing therapies that may be “around the corner” with our patients, we demonstrate that we are staying ahead of the curve of scientific development.
We want to hear from you! Tell us what you think.
What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis?
Steven R. Goldstein, MD (Examining the Evidence, August 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate?
Andrew M. Kaunitz, MD; David A. Grimes, MD (Guest Editorial, August 2011)
Osteoporosis is a significant health issue—and it is likely to remain so as more and more women live longer and longer. In fact, increasing age is the single biggest risk factor for osteoporotic fragility fracture.
Over the past year, important research has improved our understanding in diverse areas of bone health. In this Update, I highlight studies that:
- seek to elucidate the optimal frequency of dual-energy x-ray absorptiometry (DXA) imaging to assess bone mineral density
- review secondary causes of osteoporosis besides menopause-related estrogen deficiency
- explore the use of quantitative ultrasound (QUS) to predict the risk of fracture
- report on a new class of pharmaceutical agents that inhibit the bone-resorption enzyme Cathepsin K.
All of these issues are clinically relevant to the ObGyn specialty because, when it comes to our patients’ bone health, we often function as the primary care physician.
When is DXA indicated—and how often should it be repeated?
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233.
Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739–742.
American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone density testing interval and transition to osteoporosis in older women” [press release]. http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed October 15, 2012.
Recommendations from professional societies, such as the National Osteoporosis Foundation, the International Society of Clinical Densitometry, and the American College of Obstetricians and Gynecologists say virtually the same thing about DXA imaging: Screening is appropriate for women 65 years and older and for postmenopausal women younger than age 65 who have risk factors for fracture. Risk factors include:
- history of a fragility fracture
- body weight less than 127 lb
- medical causes of bone loss, such as medication or disease
- parental history of hip fracture
- current smoker
- alcoholism
- rheumatoid arthritis.
The measurement of bone mineral density (BMD) has been the cornerstone of the diagnosis of osteopenia and osteoporosis since these classifications were introduced by the World Health Organization (WHO) in 1994. Although we are now able to evaluate a woman’s fracture risk using the FRAX tool, which does not require BMD assessment, DXA scanning has become entrenched in routine clinical practice in the United States. In addition, patients who use drugs to reduce their fracture risk often demand periodic testing to see how they are doing. Even women who do not take medications often want periodic assessment to confirm that they are not “losing bone.” Medicare allows for testing every 23 months.
23-month screening interval does not fit all women
Gourlay and colleagues prospectively followed 4,957 women aged 67 years or older who had no history of hip or vertebral fracture and who were not being treated for osteoporosis. After follow-up for as long as 15 years, investigators found that the better a woman’s initial bone density, the longer it took for her to develop osteoporosis. For example, among women over 67 years of age who had a T-score of –1.0 or better, it would take 16.8 years for 10% of this population to develop osteoporosis. In contrast, among women over 67 years of age who had a T-score of –2.0, it would take only 1.1 years for 10% of this population to develop osteoporosis.
This finding certainly calls into question the notion that all patients should be screened every 23 months. It may be better to think of screening as a way of triaging patients for decisions relative to subsequent follow-up.
Media distorted take-home message
This study was the focus of considerable attention from the media, which implied that too much DXA screening is being performed. In reality, only 13% of women over the age of 65 undergo a baseline DXA scan. However, routine follow-up of all patients at 23-month intervals is clearly not appropriate.
Because this study primarily involved white women older than age 67, extrapolation of its findings to other groups may not be appropriate. Nevertheless, the study helps to underscore the fact that reliance on BMD measurement alone should not be used to determine the need for therapeutic intervention. The FRAX tool can be used on an annual basis to assess a woman’s risk of fracture and does not require follow-up DXA imaging at any arbitrary interval.
In healthy older women, an interval of 23 months for repeat BMD assessment makes little sense. For women who have excellent initial T-scores, clinicians can lengthen this interval significantly.
However, strict reliance on the T-score isn’t the best way to predict a woman’s fracture risk or determine when pharmacologic intervention is warranted. Rather, yearly assessment using a tool such as FRAX should become the standard of care.
Some secondary causes of osteoporosis are overlooked or underappreciated
Miller PD. Unrecognized and unappreciated secondary causes of osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):613–628.
The fractures traditionally associated with osteoporosis involve the hip and vertebrae, although low-trauma fractures of the humerus, forearm, femur shaft, tibia, and fibula are also associated with a high risk of future fracture in untreated women.
Once a clinician is confident that a patient has osteoporosis, the question is whether the diagnosis is postmenopausal osteoporosis—or some other form of the disease. Although estrogen deficiency is the most common cause of osteoporosis in postmenopausal women, many other conditions may accompany estrogen deficiency and contribute to impaired bone strength in this population.
Among the culprits are some conditions that are not often encountered in the average gynecologic practice: monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, celiac disease, Crohn’s disease, and other inflammatory bowel diseases. In addition, bariatric surgery, eating disorders, primary hyperparathyroidism, and a number of medications have been implicated in BMD loss or increased risk of fracture, or both. Among the problematic drugs of particular interest to us as gynecologists are aromatase inhibitors, depot medroxyprogesterone acetate, proton pump inhibitors, and gonadotropin-releasing hormone (GnRH) agonists.
Other medications that can affect BMD are glucocorticoids, unfractionated heparin, selective serotonin reuptake inhibitors, excessive amounts of thyroid replacement agents, and some antiseizure medications.
If you suspect a secondary cause of osteoporosis, be prepared to perform a basic workup that includes:
- a careful history and physical examination
- complete blood count
- a chemistry profile, including serum calcium, phosphorous, electrolytes, alkaline phosphatase, and creatinine.
In addition, measurement of 25-hydroxy vitamin D and thyroid-stimulating hormone (TSH) may be helpful, as may serum protein electrophoresis.
Patients who have clinical or laboratory abnormalities suggestive of a secondary cause of osteoporosis are usually referred to a metabolic bone specialist (endocrinology or rheumatology).
When a patient has any clinical history that suggests a secondary cause of bone loss other than menopause-related estrogen deficiency, simple laboratory tests are appropriate and may uncover a condition that necessitates referral to a metabolic bone expert.
Quantitative ultrasound assessment of bone can help predict a woman’s risk of fracture
Guglielmi G, Rossini M, Nicolosi MG, Tagno A, Lentini G, de Terlizzi F. Three-year prospective study on fracture risk in postmenopausal women by quantitative ultrasound at the phalanges [published online ahead of print August 15, 2012]. J Clin Densitom. doi:10.1016 /j.jocd.2012.07.006.
Chan MY, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Quantitative ultrasound and fracture risk prediction in non-osteoporotic men and women as defined by WHO criteria [published online ahead of print August 10, 2012]. Osteoporos Int. doi:10.1007/s00198-012 -2001-2.
I became interested in bone health through my longstanding interest in ultrasound, when a manufacturer asked me to evaluate equipment designed to assess bone density of the heel through quantitative ultrasound (QUS). This modality is not the diagnostic imaging we are familiar with in obstetrics and gynecology. In QUS, the homogeneity of healthy bone promotes sound transmission, whereas the voids and discontinuity of osteoporotic bone impede it. Therefore, normal bone has a faster speed of sound than less healthy bone. The other important quantitative measure is broadband ultrasound attenuation (BUA). Healthy bone is dense and absorbs and scatters sound to a greater extent than osteoporotic bone does.
Two trials of QUS
In 2010, Guglielmi and colleagues contacted 2,210 Italian women who had undergone QUS of the phalanges in 2006–2007. These women had an average age of 60.9 years, entered menopause at an average age of 49.3 years, and had a mean body mass index (BMI) of 26.5 kg/m2. By 2010, this group had experienced 108 new major osteoporotic fractures, including 23 hip fractures and 56 vertebral fractures. Investigators found a statistically significant correlation between QUS findings and fracture risk.
Chan and colleagues focused on 312 women 62 to 92 years of age who had femoral neck BMD, as measured by DXA, of –2.5 or better. QUS was measured as BUA at the calcaneus. The incidence of any fragility fracture was ascertained by radiographic reports during the follow-up period from 1994 to 2011. Eighty women (26%) experienced at least one fragility fracture during follow-up. After adjustment for covariates, women were significantly more likely to experience any fracture if BUA was decreased (hazard ratio [HR], 1.50; 95% confidence interval [CI], 1.13–1.99).
When the models that included BUA were compared with those that used femoral neck BMD, they had a greater area under the curve (0.71, 0.85, 0.71 for any fracture, hip fracture, and vertebral fracture, respectively) and yielded a net reclassification improvement of 16.4% (P=.009) when combined with femoral neck BMD. These findings suggest that calcaneal BUA is an independent predictor of fracture risk in women who have nonosteoporotic BMD.
In an era of increasing pressure to reduce costs, QUS assessment of bone is a promising modality that may be useful as a screening tool. Although it measures different variables than DXA imaging (more microarchitecture, less true density), it seems to predict the risk of fracture at less cost without ionizing radiation.
In the pipeline: A drug that curbs bone resorption without diminishing bone formation
Williams SC. Potential first-in-class osteoporosis drug speeds through trials. Nat Med. 2012;18(8):1158.
Ng KW. Potential role of odanacatib in the treatment of osteoporosis. Clin Interv Aging. 2012;7:235–247.
Alendronate was the first of the oral bisphosphonates to be approved by the US Food and Drug Administration (FDA). Once it was approved in 1999, the drug quickly became the most widely used bone agent in clinical practice and was soon joined by other oral and intravenous bisphosphonates. Regrettably, highly publicized adverse effects have caused many patients to shy away from this class of drugs. Two years ago, the FDA approved denosumab, a subcutaneous injectable agent that is a RANK ligand inhibitor.
The bisphosphonates and denosumab increase bone mass by shutting down the osteoclasts responsible for bone resorption, but they also inhibit creation of new bone. A new category of drug that inhibits the bone-resorption enzyme Cathepsin K appears to inhibit bone resorption without diminishing bone formation. Trials of two previous agents in this class were halted because of adverse effects—particularly effects to the skin, where the enzyme is expressed in addition to bone. However, Phase 2 trials in which odanacatib was compared with alendronate found that the new drug increased BMD almost twice as much as alendronate did, with less reduction in serum markers of bone formation.
Phase 3 trials of odanacatib in 16,000 women older than age 65 recently were halted so that the manufacturer could pursue regulatory approval ahead of the previous schedule. Although Phase 3 data have not been published yet, odanacatib may prove to be an exciting alternative to existing therapies.
Odanacatib is not yet available. However, by discussing therapies that may be “around the corner” with our patients, we demonstrate that we are staying ahead of the curve of scientific development.
We want to hear from you! Tell us what you think.
What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis?
Steven R. Goldstein, MD (Examining the Evidence, August 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate?
Andrew M. Kaunitz, MD; David A. Grimes, MD (Guest Editorial, August 2011)
Osteoporosis is a significant health issue—and it is likely to remain so as more and more women live longer and longer. In fact, increasing age is the single biggest risk factor for osteoporotic fragility fracture.
Over the past year, important research has improved our understanding in diverse areas of bone health. In this Update, I highlight studies that:
- seek to elucidate the optimal frequency of dual-energy x-ray absorptiometry (DXA) imaging to assess bone mineral density
- review secondary causes of osteoporosis besides menopause-related estrogen deficiency
- explore the use of quantitative ultrasound (QUS) to predict the risk of fracture
- report on a new class of pharmaceutical agents that inhibit the bone-resorption enzyme Cathepsin K.
All of these issues are clinically relevant to the ObGyn specialty because, when it comes to our patients’ bone health, we often function as the primary care physician.
When is DXA indicated—and how often should it be repeated?
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233.
Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739–742.
American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone density testing interval and transition to osteoporosis in older women” [press release]. http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed October 15, 2012.
Recommendations from professional societies, such as the National Osteoporosis Foundation, the International Society of Clinical Densitometry, and the American College of Obstetricians and Gynecologists say virtually the same thing about DXA imaging: Screening is appropriate for women 65 years and older and for postmenopausal women younger than age 65 who have risk factors for fracture. Risk factors include:
- history of a fragility fracture
- body weight less than 127 lb
- medical causes of bone loss, such as medication or disease
- parental history of hip fracture
- current smoker
- alcoholism
- rheumatoid arthritis.
The measurement of bone mineral density (BMD) has been the cornerstone of the diagnosis of osteopenia and osteoporosis since these classifications were introduced by the World Health Organization (WHO) in 1994. Although we are now able to evaluate a woman’s fracture risk using the FRAX tool, which does not require BMD assessment, DXA scanning has become entrenched in routine clinical practice in the United States. In addition, patients who use drugs to reduce their fracture risk often demand periodic testing to see how they are doing. Even women who do not take medications often want periodic assessment to confirm that they are not “losing bone.” Medicare allows for testing every 23 months.
23-month screening interval does not fit all women
Gourlay and colleagues prospectively followed 4,957 women aged 67 years or older who had no history of hip or vertebral fracture and who were not being treated for osteoporosis. After follow-up for as long as 15 years, investigators found that the better a woman’s initial bone density, the longer it took for her to develop osteoporosis. For example, among women over 67 years of age who had a T-score of –1.0 or better, it would take 16.8 years for 10% of this population to develop osteoporosis. In contrast, among women over 67 years of age who had a T-score of –2.0, it would take only 1.1 years for 10% of this population to develop osteoporosis.
This finding certainly calls into question the notion that all patients should be screened every 23 months. It may be better to think of screening as a way of triaging patients for decisions relative to subsequent follow-up.
Media distorted take-home message
This study was the focus of considerable attention from the media, which implied that too much DXA screening is being performed. In reality, only 13% of women over the age of 65 undergo a baseline DXA scan. However, routine follow-up of all patients at 23-month intervals is clearly not appropriate.
Because this study primarily involved white women older than age 67, extrapolation of its findings to other groups may not be appropriate. Nevertheless, the study helps to underscore the fact that reliance on BMD measurement alone should not be used to determine the need for therapeutic intervention. The FRAX tool can be used on an annual basis to assess a woman’s risk of fracture and does not require follow-up DXA imaging at any arbitrary interval.
In healthy older women, an interval of 23 months for repeat BMD assessment makes little sense. For women who have excellent initial T-scores, clinicians can lengthen this interval significantly.
However, strict reliance on the T-score isn’t the best way to predict a woman’s fracture risk or determine when pharmacologic intervention is warranted. Rather, yearly assessment using a tool such as FRAX should become the standard of care.
Some secondary causes of osteoporosis are overlooked or underappreciated
Miller PD. Unrecognized and unappreciated secondary causes of osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):613–628.
The fractures traditionally associated with osteoporosis involve the hip and vertebrae, although low-trauma fractures of the humerus, forearm, femur shaft, tibia, and fibula are also associated with a high risk of future fracture in untreated women.
Once a clinician is confident that a patient has osteoporosis, the question is whether the diagnosis is postmenopausal osteoporosis—or some other form of the disease. Although estrogen deficiency is the most common cause of osteoporosis in postmenopausal women, many other conditions may accompany estrogen deficiency and contribute to impaired bone strength in this population.
Among the culprits are some conditions that are not often encountered in the average gynecologic practice: monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, celiac disease, Crohn’s disease, and other inflammatory bowel diseases. In addition, bariatric surgery, eating disorders, primary hyperparathyroidism, and a number of medications have been implicated in BMD loss or increased risk of fracture, or both. Among the problematic drugs of particular interest to us as gynecologists are aromatase inhibitors, depot medroxyprogesterone acetate, proton pump inhibitors, and gonadotropin-releasing hormone (GnRH) agonists.
Other medications that can affect BMD are glucocorticoids, unfractionated heparin, selective serotonin reuptake inhibitors, excessive amounts of thyroid replacement agents, and some antiseizure medications.
If you suspect a secondary cause of osteoporosis, be prepared to perform a basic workup that includes:
- a careful history and physical examination
- complete blood count
- a chemistry profile, including serum calcium, phosphorous, electrolytes, alkaline phosphatase, and creatinine.
In addition, measurement of 25-hydroxy vitamin D and thyroid-stimulating hormone (TSH) may be helpful, as may serum protein electrophoresis.
Patients who have clinical or laboratory abnormalities suggestive of a secondary cause of osteoporosis are usually referred to a metabolic bone specialist (endocrinology or rheumatology).
When a patient has any clinical history that suggests a secondary cause of bone loss other than menopause-related estrogen deficiency, simple laboratory tests are appropriate and may uncover a condition that necessitates referral to a metabolic bone expert.
Quantitative ultrasound assessment of bone can help predict a woman’s risk of fracture
Guglielmi G, Rossini M, Nicolosi MG, Tagno A, Lentini G, de Terlizzi F. Three-year prospective study on fracture risk in postmenopausal women by quantitative ultrasound at the phalanges [published online ahead of print August 15, 2012]. J Clin Densitom. doi:10.1016 /j.jocd.2012.07.006.
Chan MY, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Quantitative ultrasound and fracture risk prediction in non-osteoporotic men and women as defined by WHO criteria [published online ahead of print August 10, 2012]. Osteoporos Int. doi:10.1007/s00198-012 -2001-2.
I became interested in bone health through my longstanding interest in ultrasound, when a manufacturer asked me to evaluate equipment designed to assess bone density of the heel through quantitative ultrasound (QUS). This modality is not the diagnostic imaging we are familiar with in obstetrics and gynecology. In QUS, the homogeneity of healthy bone promotes sound transmission, whereas the voids and discontinuity of osteoporotic bone impede it. Therefore, normal bone has a faster speed of sound than less healthy bone. The other important quantitative measure is broadband ultrasound attenuation (BUA). Healthy bone is dense and absorbs and scatters sound to a greater extent than osteoporotic bone does.
Two trials of QUS
In 2010, Guglielmi and colleagues contacted 2,210 Italian women who had undergone QUS of the phalanges in 2006–2007. These women had an average age of 60.9 years, entered menopause at an average age of 49.3 years, and had a mean body mass index (BMI) of 26.5 kg/m2. By 2010, this group had experienced 108 new major osteoporotic fractures, including 23 hip fractures and 56 vertebral fractures. Investigators found a statistically significant correlation between QUS findings and fracture risk.
Chan and colleagues focused on 312 women 62 to 92 years of age who had femoral neck BMD, as measured by DXA, of –2.5 or better. QUS was measured as BUA at the calcaneus. The incidence of any fragility fracture was ascertained by radiographic reports during the follow-up period from 1994 to 2011. Eighty women (26%) experienced at least one fragility fracture during follow-up. After adjustment for covariates, women were significantly more likely to experience any fracture if BUA was decreased (hazard ratio [HR], 1.50; 95% confidence interval [CI], 1.13–1.99).
When the models that included BUA were compared with those that used femoral neck BMD, they had a greater area under the curve (0.71, 0.85, 0.71 for any fracture, hip fracture, and vertebral fracture, respectively) and yielded a net reclassification improvement of 16.4% (P=.009) when combined with femoral neck BMD. These findings suggest that calcaneal BUA is an independent predictor of fracture risk in women who have nonosteoporotic BMD.
In an era of increasing pressure to reduce costs, QUS assessment of bone is a promising modality that may be useful as a screening tool. Although it measures different variables than DXA imaging (more microarchitecture, less true density), it seems to predict the risk of fracture at less cost without ionizing radiation.
In the pipeline: A drug that curbs bone resorption without diminishing bone formation
Williams SC. Potential first-in-class osteoporosis drug speeds through trials. Nat Med. 2012;18(8):1158.
Ng KW. Potential role of odanacatib in the treatment of osteoporosis. Clin Interv Aging. 2012;7:235–247.
Alendronate was the first of the oral bisphosphonates to be approved by the US Food and Drug Administration (FDA). Once it was approved in 1999, the drug quickly became the most widely used bone agent in clinical practice and was soon joined by other oral and intravenous bisphosphonates. Regrettably, highly publicized adverse effects have caused many patients to shy away from this class of drugs. Two years ago, the FDA approved denosumab, a subcutaneous injectable agent that is a RANK ligand inhibitor.
The bisphosphonates and denosumab increase bone mass by shutting down the osteoclasts responsible for bone resorption, but they also inhibit creation of new bone. A new category of drug that inhibits the bone-resorption enzyme Cathepsin K appears to inhibit bone resorption without diminishing bone formation. Trials of two previous agents in this class were halted because of adverse effects—particularly effects to the skin, where the enzyme is expressed in addition to bone. However, Phase 2 trials in which odanacatib was compared with alendronate found that the new drug increased BMD almost twice as much as alendronate did, with less reduction in serum markers of bone formation.
Phase 3 trials of odanacatib in 16,000 women older than age 65 recently were halted so that the manufacturer could pursue regulatory approval ahead of the previous schedule. Although Phase 3 data have not been published yet, odanacatib may prove to be an exciting alternative to existing therapies.
Odanacatib is not yet available. However, by discussing therapies that may be “around the corner” with our patients, we demonstrate that we are staying ahead of the curve of scientific development.
We want to hear from you! Tell us what you think.
Polycystic ovary syndrome: The long-term metabolic risks
Part 1. Where we stand with diagnosis and treatment—and
where we're going
Women with polycystic ovary syndrome (PCOS), compared with women without the condition, have a greater chance of developing metabolic syndrome (also known as syndrome X). Recent data have drawn attention to these long-term metabolic risks of PCOS. What is metabolic syndrome, and how can its first-line treatment, metformin, affect my patient’s symptoms of PCOS, including hyperandrogenism, anovulation, infertility, weight loss, and early pregnancy loss?
We address these questions in part 3 of this four-part series, which will continue to be posted on the OBG Management Web site. [Editor’s note: For readers’ ease of access, all installments of this series will, as they are published, be collected on a single Web page of links.]
Metabolic syndrome
![]()
Metabolic syndrome is a cluster of risk factors for cardiovascular disease that, together, increase a woman’s likelihood of a heart attack or stroke (by fourfold compared with those free of the condition) and increase the chance of her developing diabetes mellitus (DM). According to the American Heart Association, between 20% and 25% of the US adult population (between 58 and 73 million men and women) has metabolic syndrome.1
The diagnostic criteria for metabolic syndrome are presence of at least three of the following:
- abdominal obesity (excessive fat tissue in and around the abdomen)
- atherogenic dyslipidemia (blood fat disorders—including elevated triglyceride level, low high-density lipoprotein cholesterol [HDL-C] level, and elevated low-density cholesterol [LDL-C] level—that foster arterial plaque buildup)
- elevated blood pressure
- insulin resistance or glucose intolerance
- prothrombotic state (which is a high level of fibrinogen or plasminogen activator inhibitor–1 in the blood)
- proinflammatory state (which is elevated plasma C-reactive protein level).
Metformin
![]()
Metformin, a biguanide antidiabetic drug, was first described in the scientific literature in 1957.2 It was first marketed in France in 1979, but it did not receive approval by the US Food and Drug Administration (FDA) for DM until 1994. In contrast to sulfonylurea medications, which work rapidly to control elevated blood glucose levels by increasing pancreatic insulin production, metformin is an insulin-sensitizing agent—it improves peripheral insulin sensitivity and suppresses hepatic gluconeogenesis. Metformin is preferred for initial DM treatment because it does not induce hypoglycemia. Although metformin is not FDA-approved to treat PCOS, it is increasingly being used to treat the syndrome in patients with impaired glucose tolerance and those with no impaired glucose tolerance. More recent research has focused on metformin’s effect on other associated maladies of PCOS, including hirsutism, acne, weight loss, anovulation, pregnancy, and pregnancy loss.
Metformin and hirsutism
![]()
PCOS and its associated hyperinsulinemic state causes excess ovarian androgen production and reduces hepatic sex hormone binding globulin (SHBG) production. As treatment with metformin results in lower circulating insulin, the net affect is reduced ovarian androgen production and less free testosterone. Thus, it is reasonable to think metformin would be effective in the treatment of hirsutism. However, conflicting results have been reported with respect to this issue.
While some study results suggest an improvement in patients’ hirsutism symptoms with metformin treatment, results of a recent meta-analysis of randomized controlled trials involving treatment with metformin for at least 6 months for hirsutism suggest that insulin sensitizers provide limited or no important benefit for women with hirsutism. Of 348 studies, 16 trials (22 comparisons) that were eligible for inclusion in the meta-analysis showed a small decrease in Ferriman-Gallwey scores in women treated with insulin sensitizers compared with women treated with placebo. There was no significant difference in hirsutism between women treated with insulin sensitizers and women treated with oral contraceptives; metformin was inferior to both spironolactone and flutamide. Further study into metformin’s role in treatment for hirsutism is warranted.3
Metformin and acne
![]()
The use of insulin-sensitizing agents, such as metformin, to treat acne also requires more research. The same mechanism of action that infers metformin’s use in hirsutism also applies to its use in acne treatment. In a Cochrane Review4 of randomized controlled trials comparing insulin-sensitizing agents to OCs (alone or in combination) for treating acne, limited data demonstrated no evidence of difference in effect between metformin and the OC. This analysis included six trials, four of which compared metformin with an OC (104 participants) and two of which compared an OC combined with metformin with an OC alone (70 participants).
Metformin and weight loss
![]()
Weight loss leads to greater improvements in overall health, increased fecundity, and improved pregnancy outcome. In spite of the advantages, most patients with PCOS have difficulty losing weight and often regain lost weight over time. Many investigators have raised the question as to whether treatment with insulin-sensitizing drugs contributes to weight loss, compared with diet or a lifestyle modification program.
A systematic search of the literature for randomized controlled trials in women of reproductive age that assessed the effect of insulin-sensitizing drugs on weight loss compared with placebo and diet and/or a lifestyle modification program, revealed 14 trials in the literature, including two in women with PCOS.5 Treatment with metformin showed a statistically significant decrease in body mass index compared with placebo, with some indication of greater effect with high-dose metformin (>1,500 mg/day) and longer duration of therapy (>8 weeks).
Clearly, a structured lifestyle modification program to achieve weight loss should still be the first-line treatment in obese women with or without PCOS. Further adequately powered studies are necessary to confirm such findings.2 As new weight loss drugs become available, they should also be considered for treatment of obesity in women with PCOS.
Metformin and anovulation
![]()
While metformin may offer limited assistance with weight loss, especially when combined with diet and lifestyle therapy, and weight loss generally improves ovulation in overweight women with PCOS, there is no evidence that metformin is a powerful ovulatory drug.6 However, results of a meta-analysis that included 17 studies totaling more than 1,600 women with PCOS, showed that metformin did improve ovulation, especially in non-clomiphene–resistant women.7 Metformin alone did not increase the odds of pregnancy, but in combination with clomiphene, pregnancy was increased. The combination of metformin and clomiphene was especially beneficial in clomiphene-resistant women.
It is important to note that, in women with PCOS, treatment with metformin alone, and in combination with clomiphene, helps to reduce the number of multiple pregnancies, compared with treatment with clomiphine alone.9
Metformin and early pregnancy loss
![]()
While there is mixed evidence as to metformin’s effect on early pregnancy loss, the strongest evidence to date does not indicate a beneficial effect. In a large, randomized, prospective study of 626 infertile women with PCOS, the rate of pregnancy loss was similar between the clomiphene only and clomiphene plus metformin groups, and there was a slight trend for an increase in pregnancy loss in the metformin alone group.9
In a comprehensive review of the literature, Mathur and colleagues concludes that, while some studies have found improvements or no difference in the rates of early pregnancy loss with metformin (alone or in combination with clomiphene), there are “no conclusive data to support a beneficial effect of metformin on pregnancy loss.”6
Metformin and pregnancy outcomes
![]()
In a meta-analysis of eight studies of women with PCOS or DM exposed to metformin during the first trimester of pregnancy with major fetal malformations as the primary outcome, the authors concluded there was no evidence of an increased risk with metformin.8
While it is logical to say that metformin could even be beneficial during pregnancy, given its effect of reducing the risk of developing gestational diabetes, there is inadequate evidence to support the use of metformin during pregnancy at this time.6,8
In the next installment: The authors address several questions about current opinion and future considerations:
|
We want to hear from you! Tell us what you think.
1. Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928-934.
2. Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755-772.
3. Cosma M, Swiglo BA, Flynn DN, et al. Insulin sensitizers for the treatment of hirsutism: a systematic review and meta-analyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1135-1142.
4. Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsuitism acne, and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.-
5. Nieuwenhuis-Ruifrok AE, Kuchenbecker WK, Hoek A, Middleton P, Norman RJ. Insulin sensitizing drugs for weight loss in women of reproductive age who are overweight or obese: systematic review and meta-analysis. Hum Reprod Update. 2009;15(1):57-58.
6. Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199(6):596-609.
7. Creanga AA, Bradley HM, McCormick C, Witkop CT. Use of metformin in polycystic ovary syndrome: a meta-analysis. Obstet Gynecol. 2008;111(4):959-968.
8. Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril. 2006;86(3):658-663.
9. Legro RS, Barnhart HX, Schlaff WD, et al. Cooperative Multicenter Reproductive Medicine Network. Clomiphene metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551-566.
Part 1. Where we stand with diagnosis and treatment—and
where we're going
Women with polycystic ovary syndrome (PCOS), compared with women without the condition, have a greater chance of developing metabolic syndrome (also known as syndrome X). Recent data have drawn attention to these long-term metabolic risks of PCOS. What is metabolic syndrome, and how can its first-line treatment, metformin, affect my patient’s symptoms of PCOS, including hyperandrogenism, anovulation, infertility, weight loss, and early pregnancy loss?
We address these questions in part 3 of this four-part series, which will continue to be posted on the OBG Management Web site. [Editor’s note: For readers’ ease of access, all installments of this series will, as they are published, be collected on a single Web page of links.]
Metabolic syndrome
![]()
Metabolic syndrome is a cluster of risk factors for cardiovascular disease that, together, increase a woman’s likelihood of a heart attack or stroke (by fourfold compared with those free of the condition) and increase the chance of her developing diabetes mellitus (DM). According to the American Heart Association, between 20% and 25% of the US adult population (between 58 and 73 million men and women) has metabolic syndrome.1
The diagnostic criteria for metabolic syndrome are presence of at least three of the following:
- abdominal obesity (excessive fat tissue in and around the abdomen)
- atherogenic dyslipidemia (blood fat disorders—including elevated triglyceride level, low high-density lipoprotein cholesterol [HDL-C] level, and elevated low-density cholesterol [LDL-C] level—that foster arterial plaque buildup)
- elevated blood pressure
- insulin resistance or glucose intolerance
- prothrombotic state (which is a high level of fibrinogen or plasminogen activator inhibitor–1 in the blood)
- proinflammatory state (which is elevated plasma C-reactive protein level).
Metformin
![]()
Metformin, a biguanide antidiabetic drug, was first described in the scientific literature in 1957.2 It was first marketed in France in 1979, but it did not receive approval by the US Food and Drug Administration (FDA) for DM until 1994. In contrast to sulfonylurea medications, which work rapidly to control elevated blood glucose levels by increasing pancreatic insulin production, metformin is an insulin-sensitizing agent—it improves peripheral insulin sensitivity and suppresses hepatic gluconeogenesis. Metformin is preferred for initial DM treatment because it does not induce hypoglycemia. Although metformin is not FDA-approved to treat PCOS, it is increasingly being used to treat the syndrome in patients with impaired glucose tolerance and those with no impaired glucose tolerance. More recent research has focused on metformin’s effect on other associated maladies of PCOS, including hirsutism, acne, weight loss, anovulation, pregnancy, and pregnancy loss.
Metformin and hirsutism
![]()
PCOS and its associated hyperinsulinemic state causes excess ovarian androgen production and reduces hepatic sex hormone binding globulin (SHBG) production. As treatment with metformin results in lower circulating insulin, the net affect is reduced ovarian androgen production and less free testosterone. Thus, it is reasonable to think metformin would be effective in the treatment of hirsutism. However, conflicting results have been reported with respect to this issue.
While some study results suggest an improvement in patients’ hirsutism symptoms with metformin treatment, results of a recent meta-analysis of randomized controlled trials involving treatment with metformin for at least 6 months for hirsutism suggest that insulin sensitizers provide limited or no important benefit for women with hirsutism. Of 348 studies, 16 trials (22 comparisons) that were eligible for inclusion in the meta-analysis showed a small decrease in Ferriman-Gallwey scores in women treated with insulin sensitizers compared with women treated with placebo. There was no significant difference in hirsutism between women treated with insulin sensitizers and women treated with oral contraceptives; metformin was inferior to both spironolactone and flutamide. Further study into metformin’s role in treatment for hirsutism is warranted.3
Metformin and acne
![]()
The use of insulin-sensitizing agents, such as metformin, to treat acne also requires more research. The same mechanism of action that infers metformin’s use in hirsutism also applies to its use in acne treatment. In a Cochrane Review4 of randomized controlled trials comparing insulin-sensitizing agents to OCs (alone or in combination) for treating acne, limited data demonstrated no evidence of difference in effect between metformin and the OC. This analysis included six trials, four of which compared metformin with an OC (104 participants) and two of which compared an OC combined with metformin with an OC alone (70 participants).
Metformin and weight loss
![]()
Weight loss leads to greater improvements in overall health, increased fecundity, and improved pregnancy outcome. In spite of the advantages, most patients with PCOS have difficulty losing weight and often regain lost weight over time. Many investigators have raised the question as to whether treatment with insulin-sensitizing drugs contributes to weight loss, compared with diet or a lifestyle modification program.
A systematic search of the literature for randomized controlled trials in women of reproductive age that assessed the effect of insulin-sensitizing drugs on weight loss compared with placebo and diet and/or a lifestyle modification program, revealed 14 trials in the literature, including two in women with PCOS.5 Treatment with metformin showed a statistically significant decrease in body mass index compared with placebo, with some indication of greater effect with high-dose metformin (>1,500 mg/day) and longer duration of therapy (>8 weeks).
Clearly, a structured lifestyle modification program to achieve weight loss should still be the first-line treatment in obese women with or without PCOS. Further adequately powered studies are necessary to confirm such findings.2 As new weight loss drugs become available, they should also be considered for treatment of obesity in women with PCOS.
Metformin and anovulation
![]()
While metformin may offer limited assistance with weight loss, especially when combined with diet and lifestyle therapy, and weight loss generally improves ovulation in overweight women with PCOS, there is no evidence that metformin is a powerful ovulatory drug.6 However, results of a meta-analysis that included 17 studies totaling more than 1,600 women with PCOS, showed that metformin did improve ovulation, especially in non-clomiphene–resistant women.7 Metformin alone did not increase the odds of pregnancy, but in combination with clomiphene, pregnancy was increased. The combination of metformin and clomiphene was especially beneficial in clomiphene-resistant women.
It is important to note that, in women with PCOS, treatment with metformin alone, and in combination with clomiphene, helps to reduce the number of multiple pregnancies, compared with treatment with clomiphine alone.9
Metformin and early pregnancy loss
![]()
While there is mixed evidence as to metformin’s effect on early pregnancy loss, the strongest evidence to date does not indicate a beneficial effect. In a large, randomized, prospective study of 626 infertile women with PCOS, the rate of pregnancy loss was similar between the clomiphene only and clomiphene plus metformin groups, and there was a slight trend for an increase in pregnancy loss in the metformin alone group.9
In a comprehensive review of the literature, Mathur and colleagues concludes that, while some studies have found improvements or no difference in the rates of early pregnancy loss with metformin (alone or in combination with clomiphene), there are “no conclusive data to support a beneficial effect of metformin on pregnancy loss.”6
Metformin and pregnancy outcomes
![]()
In a meta-analysis of eight studies of women with PCOS or DM exposed to metformin during the first trimester of pregnancy with major fetal malformations as the primary outcome, the authors concluded there was no evidence of an increased risk with metformin.8
While it is logical to say that metformin could even be beneficial during pregnancy, given its effect of reducing the risk of developing gestational diabetes, there is inadequate evidence to support the use of metformin during pregnancy at this time.6,8
In the next installment: The authors address several questions about current opinion and future considerations:
|
We want to hear from you! Tell us what you think.
Part 1. Where we stand with diagnosis and treatment—and
where we're going
Women with polycystic ovary syndrome (PCOS), compared with women without the condition, have a greater chance of developing metabolic syndrome (also known as syndrome X). Recent data have drawn attention to these long-term metabolic risks of PCOS. What is metabolic syndrome, and how can its first-line treatment, metformin, affect my patient’s symptoms of PCOS, including hyperandrogenism, anovulation, infertility, weight loss, and early pregnancy loss?
We address these questions in part 3 of this four-part series, which will continue to be posted on the OBG Management Web site. [Editor’s note: For readers’ ease of access, all installments of this series will, as they are published, be collected on a single Web page of links.]
Metabolic syndrome
![]()
Metabolic syndrome is a cluster of risk factors for cardiovascular disease that, together, increase a woman’s likelihood of a heart attack or stroke (by fourfold compared with those free of the condition) and increase the chance of her developing diabetes mellitus (DM). According to the American Heart Association, between 20% and 25% of the US adult population (between 58 and 73 million men and women) has metabolic syndrome.1
The diagnostic criteria for metabolic syndrome are presence of at least three of the following:
- abdominal obesity (excessive fat tissue in and around the abdomen)
- atherogenic dyslipidemia (blood fat disorders—including elevated triglyceride level, low high-density lipoprotein cholesterol [HDL-C] level, and elevated low-density cholesterol [LDL-C] level—that foster arterial plaque buildup)
- elevated blood pressure
- insulin resistance or glucose intolerance
- prothrombotic state (which is a high level of fibrinogen or plasminogen activator inhibitor–1 in the blood)
- proinflammatory state (which is elevated plasma C-reactive protein level).
Metformin
![]()
Metformin, a biguanide antidiabetic drug, was first described in the scientific literature in 1957.2 It was first marketed in France in 1979, but it did not receive approval by the US Food and Drug Administration (FDA) for DM until 1994. In contrast to sulfonylurea medications, which work rapidly to control elevated blood glucose levels by increasing pancreatic insulin production, metformin is an insulin-sensitizing agent—it improves peripheral insulin sensitivity and suppresses hepatic gluconeogenesis. Metformin is preferred for initial DM treatment because it does not induce hypoglycemia. Although metformin is not FDA-approved to treat PCOS, it is increasingly being used to treat the syndrome in patients with impaired glucose tolerance and those with no impaired glucose tolerance. More recent research has focused on metformin’s effect on other associated maladies of PCOS, including hirsutism, acne, weight loss, anovulation, pregnancy, and pregnancy loss.
Metformin and hirsutism
![]()
PCOS and its associated hyperinsulinemic state causes excess ovarian androgen production and reduces hepatic sex hormone binding globulin (SHBG) production. As treatment with metformin results in lower circulating insulin, the net affect is reduced ovarian androgen production and less free testosterone. Thus, it is reasonable to think metformin would be effective in the treatment of hirsutism. However, conflicting results have been reported with respect to this issue.
While some study results suggest an improvement in patients’ hirsutism symptoms with metformin treatment, results of a recent meta-analysis of randomized controlled trials involving treatment with metformin for at least 6 months for hirsutism suggest that insulin sensitizers provide limited or no important benefit for women with hirsutism. Of 348 studies, 16 trials (22 comparisons) that were eligible for inclusion in the meta-analysis showed a small decrease in Ferriman-Gallwey scores in women treated with insulin sensitizers compared with women treated with placebo. There was no significant difference in hirsutism between women treated with insulin sensitizers and women treated with oral contraceptives; metformin was inferior to both spironolactone and flutamide. Further study into metformin’s role in treatment for hirsutism is warranted.3
Metformin and acne
![]()
The use of insulin-sensitizing agents, such as metformin, to treat acne also requires more research. The same mechanism of action that infers metformin’s use in hirsutism also applies to its use in acne treatment. In a Cochrane Review4 of randomized controlled trials comparing insulin-sensitizing agents to OCs (alone or in combination) for treating acne, limited data demonstrated no evidence of difference in effect between metformin and the OC. This analysis included six trials, four of which compared metformin with an OC (104 participants) and two of which compared an OC combined with metformin with an OC alone (70 participants).
Metformin and weight loss
![]()
Weight loss leads to greater improvements in overall health, increased fecundity, and improved pregnancy outcome. In spite of the advantages, most patients with PCOS have difficulty losing weight and often regain lost weight over time. Many investigators have raised the question as to whether treatment with insulin-sensitizing drugs contributes to weight loss, compared with diet or a lifestyle modification program.
A systematic search of the literature for randomized controlled trials in women of reproductive age that assessed the effect of insulin-sensitizing drugs on weight loss compared with placebo and diet and/or a lifestyle modification program, revealed 14 trials in the literature, including two in women with PCOS.5 Treatment with metformin showed a statistically significant decrease in body mass index compared with placebo, with some indication of greater effect with high-dose metformin (>1,500 mg/day) and longer duration of therapy (>8 weeks).
Clearly, a structured lifestyle modification program to achieve weight loss should still be the first-line treatment in obese women with or without PCOS. Further adequately powered studies are necessary to confirm such findings.2 As new weight loss drugs become available, they should also be considered for treatment of obesity in women with PCOS.
Metformin and anovulation
![]()
While metformin may offer limited assistance with weight loss, especially when combined with diet and lifestyle therapy, and weight loss generally improves ovulation in overweight women with PCOS, there is no evidence that metformin is a powerful ovulatory drug.6 However, results of a meta-analysis that included 17 studies totaling more than 1,600 women with PCOS, showed that metformin did improve ovulation, especially in non-clomiphene–resistant women.7 Metformin alone did not increase the odds of pregnancy, but in combination with clomiphene, pregnancy was increased. The combination of metformin and clomiphene was especially beneficial in clomiphene-resistant women.
It is important to note that, in women with PCOS, treatment with metformin alone, and in combination with clomiphene, helps to reduce the number of multiple pregnancies, compared with treatment with clomiphine alone.9
Metformin and early pregnancy loss
![]()
While there is mixed evidence as to metformin’s effect on early pregnancy loss, the strongest evidence to date does not indicate a beneficial effect. In a large, randomized, prospective study of 626 infertile women with PCOS, the rate of pregnancy loss was similar between the clomiphene only and clomiphene plus metformin groups, and there was a slight trend for an increase in pregnancy loss in the metformin alone group.9
In a comprehensive review of the literature, Mathur and colleagues concludes that, while some studies have found improvements or no difference in the rates of early pregnancy loss with metformin (alone or in combination with clomiphene), there are “no conclusive data to support a beneficial effect of metformin on pregnancy loss.”6
Metformin and pregnancy outcomes
![]()
In a meta-analysis of eight studies of women with PCOS or DM exposed to metformin during the first trimester of pregnancy with major fetal malformations as the primary outcome, the authors concluded there was no evidence of an increased risk with metformin.8
While it is logical to say that metformin could even be beneficial during pregnancy, given its effect of reducing the risk of developing gestational diabetes, there is inadequate evidence to support the use of metformin during pregnancy at this time.6,8
In the next installment: The authors address several questions about current opinion and future considerations:
|
We want to hear from you! Tell us what you think.
1. Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928-934.
2. Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755-772.
3. Cosma M, Swiglo BA, Flynn DN, et al. Insulin sensitizers for the treatment of hirsutism: a systematic review and meta-analyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1135-1142.
4. Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsuitism acne, and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.-
5. Nieuwenhuis-Ruifrok AE, Kuchenbecker WK, Hoek A, Middleton P, Norman RJ. Insulin sensitizing drugs for weight loss in women of reproductive age who are overweight or obese: systematic review and meta-analysis. Hum Reprod Update. 2009;15(1):57-58.
6. Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199(6):596-609.
7. Creanga AA, Bradley HM, McCormick C, Witkop CT. Use of metformin in polycystic ovary syndrome: a meta-analysis. Obstet Gynecol. 2008;111(4):959-968.
8. Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril. 2006;86(3):658-663.
9. Legro RS, Barnhart HX, Schlaff WD, et al. Cooperative Multicenter Reproductive Medicine Network. Clomiphene metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551-566.
1. Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928-934.
2. Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755-772.
3. Cosma M, Swiglo BA, Flynn DN, et al. Insulin sensitizers for the treatment of hirsutism: a systematic review and meta-analyses of randomized controlled trials. J Clin Endocrinol Metab. 2008;93(4):1135-1142.
4. Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsuitism acne, and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.-
5. Nieuwenhuis-Ruifrok AE, Kuchenbecker WK, Hoek A, Middleton P, Norman RJ. Insulin sensitizing drugs for weight loss in women of reproductive age who are overweight or obese: systematic review and meta-analysis. Hum Reprod Update. 2009;15(1):57-58.
6. Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199(6):596-609.
7. Creanga AA, Bradley HM, McCormick C, Witkop CT. Use of metformin in polycystic ovary syndrome: a meta-analysis. Obstet Gynecol. 2008;111(4):959-968.
8. Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril. 2006;86(3):658-663.
9. Legro RS, Barnhart HX, Schlaff WD, et al. Cooperative Multicenter Reproductive Medicine Network. Clomiphene metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551-566.
Management of Hypertensive Urgency and Emergency
An estimated 1% to 2% of patients with chronic hypertension will at some time develop hypertensive urgency or emergency.1 According to recent data from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2010,2 the prevalence of hypertension has remained stable at 30.5% among men and 28.5% among women in the United States; however, 74% of the hypertensive population is unaware of having this condition. Furthermore, 71.6% of hypertensive patients are managed for the condition, and in only 46.5% is blood pressure well controlled.2
In 2006, essential hypertension was estimated to account for more than 44 million emergency department visits in the US. The direct and indirect costs of hypertension totaled $73 billion in 2009.3,4
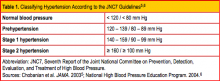
NEW TERMINOLOGY AND CLASSIFICATION
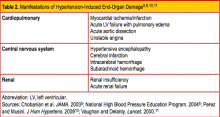
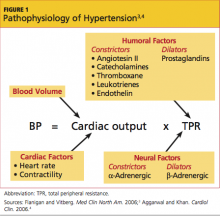
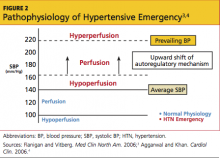
The terms malignant hypertension, hypertensive crisis, and accelerated hypertension have been replaced by hypertensive urgency or hypertensive emergency. Hypertensive urgency and emergency are differentiated by the absence or presence of acute end-organ damage, respectively.
Given the inconsistent terminology used, database searches can be challenging. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7),5,6 published in 2003, is considered the gold standard for categorizing hypertension in the outpatient setting. The JNC7 authorsclassify normal blood pressure as < 120/< 80 mm Hg. The document further classifies blood pressure into the stages shown in Table 1.5,6 Blood pressure higher than 180 mm Hg systolic and/or 120 mm Hg diastolic is generally considered severe hypertension— a designation that includes hypertensive urgency and hypertensive emergency.6
What Defines Hypertensive Urgency/Emergency?
Hypertensive urgency is defined as a diastolic blood pressure of 110 mm Hg or greater without the acute signs of end-organ damage.7 Some sources suggest that a patient must also have certain risk factors (eg, heart disease, renal disease) to be given this diagnosis.8 The presence of acute and rapidly evolving end-organ damage with an elevated diastolic blood pressure, usually greater than 120 mm Hg, establishes a diagnosis of hypertensive emergency.6,8,9
No specific blood pressure measurement indicates a hypertensive emergency, however; rather, the defining feature of this diagnosis is the presence of progressive target end-organ damage.7 This is most commonly manifested in cardiopulmonary, central nervous system, and/or renal findings; for the specific forms of end-organ damage, see Table 2.5,6,10,11 Preeclampsia and eclampsia are also considered manifestations of hypertensive end-organ damage but are beyond the scope of this article.5,11
The most common form of organ damage associated with hypertension is ischemic heart disease, in the form of either heart failure or acute coronary syndrome.12
PATHOPHYSIOLOGY
Blood pressure is calculated by cardiac output (ie, stroke volume multiplied by heart rate) multiplied by total peripheral resistance. Total peripheral resistance is influenced by a variety of humoral and neural factors, also known as vasoactive substances (see Figure 13,4). During an episode of acute hypertension, a failure of autoregulatory function occurs, precipitated by one or more of a host of potential causes. This failure of autoregulation then leads to increased systemic vascular resistance. In the setting of end-organ damage, release of inflammatory markers ensues, which ultimately causes endovascular injury and fibrin necrosis of arterioles.4,10,11
The renin-angiotensin-aldosterone system also plays a significant role in the cascade of hypertension, stimulating decreased renal perfusion and lowering tubular sodium concentration. This in turn stimulates aldosterone to increase blood pressure by maintaining excess volume through sodium retention and potassium excretion, further potentiating the cycle of uncontrolled blood pressure.4,13,14
Patients with chronically elevated blood pressures have a compensatory response, lying in the threshold mechanism, that protects against end-organ damage. Acute changes in blood pressure are better tolerated in these patients because of their decreased propensity for hypoperfusion.4 In contrast, normotensive patients who experience precipitous changes in blood pressure are at increased risk for organ hypoperfusion. The main concern regarding organ hypoperfusion is that it can lead to ischemia4 (see Figure 23,4).
PATIENT HISTORY
Acute hypertensive urgency or emergency can be triggered by many factors. Systemic etiologies (including kidney disease) caused by immunologic mediators or renal artery stenosis can cause or exacerbate hypertension. The patient should be asked about his or her normal blood pressure range, as this may offer clues to medication compliance. Rebound hypertension can be seen in patients who abruptly discontinue medications such as clonidine or β-blockers, as this causes an increase in sympathetic outflow.9,15
All patients should be queried regarding their use of OTC medications and other drug use, including cocaine, methamphetamines, phencyclidine, and alcohol.1,4,11,16 Patients taking monoamine oxidase inhibitors (MAOIs) are at increased risk for serious medication interactions; concomitant administration of MAOIs with other antidepressants can lead to a hypertensive reaction, but also to serotonin syndrome.1,17 Because MAOIs inhibit the breakdown of tyramine, patients taking them should avoid tyramine-containing foods and herbal supplements (including, but not limited to, St. John's wort, ginseng, and yohimbine).1,15,18
Acute hypertensive episodes can also occur as a result of preeclampsia or eclampsia in pregnant women, pheochromocytoma, primary aldosteronism, glucocorticoid excess (Cushing syndrome), or central nervous system disorders (eg, cerebrovascular accident, head trauma, brain tumors).9,11,19
PHYSICAL EXAMINATION
The purpose of the physical examination is to determine whether end-organ damage is present.1,11 The fundoscopic exam may reveal papilledema, a sign of increased intracranial pressure (ICP). Flame hemorrhages, cotton wool spots or arteriovenous nicking suggest a long-standing history of uncontrolled hypertension or diabetes.7,9 The neck should be assessed for jugular venous distention, which may be elevated in decompensated heart failure or pulmonary edema.11
The cardiac exam may reveal an irregular rate and rhythm, displaced apical pulse, gallop, or murmur. On pulmonary exam, rales may be auscultated, suggestive of pulmonary edema.9,15
The abdominal exam should include listening for a renal artery bruit.1 The neurologic exam may demonstrate altered mental status (possibly indicating hypertensive encephalopathy) or focal findings, if the patient has had an underlying ischemic or hemorrhagic event.9
LABORATORY STUDIES AND IMAGING
In most cases, a serum chemistry panel is warranted to identify any renal dysfunction. Urinalysis may reveal proteinuria, possibly indicating renal damage.4,9,15
Any patient complaining of chest pain should have an ECG to look for ischemic changes or presence of a left bundle branch block, and serial cardiac enzymes to rule out acute coronary syndrome.15 Access to previous ECGs is helpful in differentiating between new and old conductive abnormalities.
A chest x-ray should be performed in patients who complain of shortness of breath and/or chest pain. A widened mediastinum can represent aortic dissection.4,15 Evidence of pulmonary edema should prompt the clinician to assess for left ventricular dysfunction or valvular insufficiencies by echocardiogram. Chest CT should be pursued in patients with clinical suspicion for dissection.1,15,20
Patients presenting with a headache or focal neurologic abnormalities warrant a head CT to rule out stroke.15 Urine drug screening is appropriate if the patient history suggests illicit drug use.12
"FIRST, DO NO HARM"
Treatment of hypertensive emergency and urgency varies from traditional treatment for hypertension. Aggressive blood pressure control in patients presenting with acute ischemic stroke has been associated with poorer patient outcomes.21,22 Thus, treating the patient and not the numbers is the first general recommendation for treatment of hypertensive emergency and urgency. It is important for the clinician to remember the Hippocratic oath, "First do no harm," when treating these patients.
Other general recommendations are derived from theory, physiology, and smaller clinical trials; their application must be individualized according to the patient's needs. These recommendations include aiming for a reduction in mean arterial blood pressure of no more than 10% to 25% within the first hour, a goal blood pressure of 160/90 mm Hg within the first 8 hours, and normalization of blood pressure over 8 to 24 hours.12
While the use of pharmacologic agents may be warranted, it is important to consider that elevated blood pressure may be a reaction to pain or stress and may be best treated alternatively. Recommendations for permissive hypertension in acute ischemic stroke will be discussed below.
TREATMENT: HYPERTENSIVE URGENCY
The treatment of hypertensive urgency is usually immediate and warrants close follow-up. Although elevated blood pressures can be alarming to the patient, hypertensive urgency usually develops over days to weeks.8 In this setting, it is not necessary to lower blood pressure acutely.12 A rapid decrease in blood pressure can actually cause symptomatic hypotension, resulting in hypoperfusion to the brain.5,6,8
After ruling out end-organ damage, the next step is to treat according to the guidelines for hypertensive urgency.5,6 These recommendations include the use of rapid-onset oral antihypertensive agents, such as clonidine, labetalol, or captopril.23 Use of these agents is only suggested for gradual, short-term reduction of blood pressure (ie, over 24 to 48 hours) while the patient is being monitored for potential hypertension-related organ damage, either in the emergency department or in an observational hospital setting.5,6,23
Once the short-acting agents have adequately reduced blood pressure, long-term agents can be chosen to prevent rebound hypertension.16 Patients are typically monitored for 24 hours in the hospital during this transition. Upon discharge, the patient should be scheduled for follow-up within one to two days.11 Patient education, including a discussion of medication adherence, weight loss, and reduced dietary salt, is key to prevent recurrences and optimize overall treatment compliance.
TREATMENT: HYPERTENSIVE EMERGENCY
Treatment of hypertensive emergency always warrants hospitalization, usually in the ICU.5,6 IV antihypertensive medications (eg, nicardipine, fenoldopam, labetalol, esmolol, phentolamine) are preferred. Their use often necessitates continuous blood pressure monitoring via arterial line, allowing the clinician to perform ongoing medication titration. In hypertensive emergencies, the purpose of treatment is to preserve brain, kidney, and heart function.4
Goal-directed therapy is initiated even before the patient evaluation has been fully completed. Patient assessment continues after treatment is begun to avoid overly aggressive blood pressure reduction, which can increase the risk for patient demise or morbidity.4
Exceptions in the treatment of hypertensive emergencies (particularly of specific disease states) will be discussed below, along with other treatment considerations. Patient comorbidities, for example, must be considered in the choice of antihypertensive agents.
Focused Treatment for Specific Hypertensive Emergencies
Hypertensive encephalopathy. This condition, associated with severe hypertension, is indicated by an abrupt change in mental status. During this acute end-organ damage event, a failure of cerebral autoregulation occurs, with increased pressure in the vascular endothelium leading to arteriole dilation that in turn can result in hyperperfusion of the brain, cerebral edema, and microhemorrhages.23
Because presentation of hypertensive encephalopathy may be similar to that in patients with acute stroke, hemorrhage, or brain lesions, these and other potential causes must be ruled out. While blood pressure treatment goals correspond with general recommendations,5,6 caution must be taken not to reduce blood pressure too swiftly; thus, continuous monitoring is warranted. If the patient's neurologic function worsens, treatment should be suspended and blood pressure allowed to rise slowly.4
Preferred antihypertensives for patients with hypertensive encephalopathy include labetalol, nicardipine, and fenoldopam23 (see Table 34-6,21,23). Centrally acting antihypertensives, such as clonidine, methyldopa, or reserpine,24 should not be used, as they can cause central nervous system depression and may cloud the patient's sensorium further.
Myocardial ischemia/infarction. During an acute hypertensive event, the workload on the heart and activation of the renin-angiotensin-aldosterone system can lead to acute coronary ischemia or infarction.23 Treatment is aimed at increasing blood flow to the myocardium and reducing the workload on the heart. Antihypertensives are combined with reperfusion (eg, angioplasty) and/or thrombolytics to preserve myocardial structure and function. Standard agents to reduce blood pressure include IV nitroglycerin and β-blockers. Systolic blood pressure is reduced until symptoms subside or diastolic blood pressure is reduced to 100 mm Hg or lower. Adjuncts such as morphine and oxygen are used to reduce patient discomfort and improve oxygen delivery to the myocardium.4
Acute left ventricular failure. In this potential manifestation of hypertensive emergency, the left ventricle initially attempts to compensate for rising blood pressure and becomes hypertrophic. Once the myocardium can no longer meet the demand, left ventricular function decompensates, causing a flow backup that leads to acute pulmonary edema.23
Blood pressure goals mirror those in the general treatment recommendations but focus specifically on reducing preload and afterload, improving myocardial contractility and decreasing peripheral vascular resistance. The preferred agents in this setting are IV nitroglycerin and ACE inhibitors, along with loop diuretics, morphine, and oxygen.4 Medications that increase workload on the heart (eg, hydralazine, clonidine) should be avoided.23
Aortic dissection. This is a true medical emergency that can result in significant morbidity and mortality. Type A dissection occurs proximally, at the ascending aorta, whereas type B dissection occurs at the level of the descending aorta. Typically, type B dissection is managed medically, as surgical treatment carries a significant risk for paralysis.4 Both types of aortic dissection are strongly associated with uncontrolled hypertension and in some patients may be precipitated by an acute hypertensive event. In such cases, the goal for blood pressure reduction is to decrease the shearing forces associated with the dissection. This is accomplished by lowering both blood pressure and pulse rate.12
While cases of type A dissection are usually managed surgically, all affected patients will require some component of medical management and tight blood pressure control. The current recommendation for blood pressure in aortic dissection is swift downward titration to a goal systolic blood pressure of 100 to 110 mm Hg.4 A β-blocker in combination with a vasodilator, administered intravenously, should be used for swift blood pressure reduction.4,25
IV nitroprusside, a potent vasodilator, is the preferred agent, but its use requires intra-arterial blood pressure monitoring.23 Because nitroprusside is metabolized to cyanide, its use can lead to lethal toxicity, especially in patients with hepatic or renal impairment.11 In this patient population, IV labetalol or esmolol may be used instead.4,25
Acute renal failure. In the setting of an acute hypertensive episode, it is often difficult to determine whether acute renal failure is the cause or the effect. Regardless, rapid reduction in blood pressure is warranted to preserve renal function and to stop the cycle of microvascular kidney destruction. Blood pressure goals are aligned with the general treatment recommendations. The preferred antihypertensive agent is IV fenoldopam, a dopamine receptor agonist that directly dilates renal arterioles, improving renal perfusion and promoting diuresis.4,26 Nicardipine, a calcium channel blocker, may be considered as an alternative.23,27
Treatment Considerations in Stroke
Elevated blood pressure is common in the early stages of stroke. Numerous studies have analyzed overall outcomes in patients presenting with ischemic stroke and uncontrolled hypertension. In this setting, evidence suggests a poorer prognosis in patients treated aggressively with antihypertensive agents.4,21,22
The association between dramatic reduction in blood pressure and poor prognoses lies in the theory of the ischemic penumbra. This is an area around the core of ischemic tissue that receives enough blood flow to maintain neuronal activity for a few hours after initial injury, but this tissue is susceptible to further infarction. Precipitous drops in blood pressure can reduce blood flow to collateral vessels, resulting in hypoperfusion of the penumbra and leading to further neurologic damage.
Details and current treatment recommendations for each of the various types of stroke follow.
Acute intracerebral hemorrhage. Uncontrolled hypertension is often associated with intracerebral hemorrhage (ICH), either as a risk factor or a factor that contributes to the event. Once a patient has experienced an acute brain insult, blood pressure can become even more uncontrolled. Extension of the hematoma and a worsening outcome are the main concerns in treating the patient with concomitant blood pressure elevation and ICH. Also of concern is maintaining adequate perfusion to the penumbra. Additionally, transient hypoperfusion can develop when the ICP is elevated and the mean arterial pressure (MAP) is acutely lowered, thus reducing the cerebral perfusion pressure (CPP; CPP = MAP - ICP).
Researchers have acknowledged there is insufficient evidence to offer management guidelines for blood pressure reduction in patients with ICH.28 The 2007 recommendations from the American Heart Association/ American Stroke Association (AHA/ASA) for blood pressure management in patients with acute ICH29 are as follows: In the setting of ICH in patients with uncontrolled blood pressure, treatment should be aggressive if systolic blood pressure exceeds 200 mm Hg or MAP exceeds 150 mm Hg. A treatment goal to consider is reducing systolic blood pres sure to 160 mm Hg or less (or MAP to below 130 mm Hg).29 Patients with elevated ICP should undergo placement of a ventriculostomy to maintain a CPP between 60 and 80 mm Hg, although the risk for infection or intracerebral hemorrhage must be weighed against the potential benefits.29,30
When blood pressure reduction is required, the MAP should not be lowered more than 20% in a 24-hour period. Recommended agents include IV nicardipine, labetalol, enalapril, hydralazine, or esmolol.31
Acute ischemic stroke. Long-term control of blood pressure in patients who have experienced stroke remains undisputed, as it improves outcomes. However, in the setting of acute ischemic stroke (AIS), initiating blood pressure control is more liberal. Optimal control of blood pressure during management of AIS is imperative to reduce morbidity and mortality.32 Areas affected by edematous brain tissue are at increased risk for bleeding (ie, hemorrhagic expansion).
Patients who present with AIS require careful history taking to elicit their average blood pressure range; this will help the clinician determine goal pressures during management of the acute stroke phase.33 The primary rationale for treating blood pressure in this acute setting is to prevent hemorrhagic expansion at sites with potential for bleeding.34
According to the 2007 AHA/ ASA recommendations for management of blood pressure in AIS,35 patients who are eligible for thrombolysis should have a systolic blood pressure goal below 180 mm Hg and diastolic blood pressure below 105 mm Hg. Patients who will not receive thrombolytics should have blood pressure lowered only if systolic blood pressure exceeds 220 mm Hg or diastolic blood pressure exceeds 110 mm Hg.35,36 Appropriately refraining from reducing blood pressure is known as permissive hypertension.
Given the fragility of the cerebral brain tissue after AIS, permissive hypertension is intended to protect the penumbra and preserve cerebral blood flow. In patients who require blood pressure reduction because of other medical conditions (eg, decompensated heart failure), blood pressure should not be lowered more than 10% to 15% in a 24-hour period.9,31,36 No specific antihypertensives are preferred in patients with AIS: IV enalapril, esmolol, labetalol, or nicardipine can be used.31
Subarachnoid hemorrhage. The two complications of a subarachnoid hemorrhage (SAH) that most contribute to morbidity and mortality are rebleeding and vasospasms; elevated blood pressure can contribute to both. Thus, blood pressure control in patients with SAH is imperative.
Patients with acute SAH often require blood pressure monitoring via arterial line, as well as ICP monitoring. Blood pressure goals are similar to those in patients with ICH. The preferred agent for blood pressure control is nimodipine, which offers the secondary benefit of vasospasm prevention.37
CONCLUSION
Patients presenting with urgent or emergent hypertension need expeditious evaluation to avoid the significant morbidity and mortality associated with acute end-organ damage. Hypertensive urgency is defined as a diastolic blood pressure of greater than 120 mm Hg without evidence of end-organ damage.
In cases of hypertensive emergency, in which acute end-organ damage is present, lowering blood pressure should be directed by the type of end-organ damage and/or underlying comorbidities. In general, blood pressure should not be lowered more than 10% to 25% within the first hour, with normalization achieved over the next 8 to 24 hours.
In cases of acute ischemic stroke, permissive hypertension is recommended. Above all, treat patients, not numbers, bearing in mind the Hippocratic oath: Primum non nocere, or "First, do no harm."
REFERENCES
- Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6): 1949-1962.
- Guo F, He D, Zhang W, Walton RG. Trends in Prevalence, Awareness, Management, and Control of Hypertension Among United States Adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599-606.
- Flanigan JS, Vitberg D. Hypertensive emergency and severe hypertension: what to treat, who to treat, and how to treat. Med Clin North Am. 2006;90(3):439-451.
- Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
- National High Blood Pressure Education Program Coordinating Committee, National Heart Lung and Blood Institute, NIH. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 04-5230. August 2004. www .nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed September 20, 2012.
- Houston M. Hypertensive emergencies and urgencies: pathophysiology and clinical aspects. Am Heart J. 1986;111(1):205-210.
- Kessler CS, Joudeh Y. Evaluation and treatment of severe asymptomatic hypertension. Am Fam Physician. 2010;81(4):470-476.
- Vaidya CK, Ouellette JR. Hypertensive urgency and emergency. Hosp Physician. Mar 2007:43-50. www.turner-white.com/memberfile.php?Pub Code=hp_mar07_hypertensive.pdf. Accessed September 20, 2012.
- Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies: a Cochrane systematic review. J Hum Hypertens. 2008;22(9):596-607.
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411-417.
- Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006; 33(3):613-623.
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 suppl B):9-20.
- Flack JM. Epidemiology and unmet needs in hypertension. J Manag Care Pharm. 2007;13(e suppl B):2-8.
- Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19(6):502-512.
- Hebert CJ, Vidt DG. Hypertensive crises. Prim Care. 2008;35(3):475-487.
- Shulman KI, Fischer HD, Herrmann N, et al. Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry. 2009;70(12):1681-1696.
- Musso NR, Vergassola C, Pende A, Lotti G. Yohimbine effects on blood pressure and plasma catecholamines in human hypertension. Am J Hypertens. 1995;8(6):565-571.
- Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6(6): 493-499.
- Society of Critical Care Medicine. Fundamental Critical Care Support Course. www.sccm.org/ fccs_and_training_courses/fccs/pages/default .aspx. Accessed September 20, 2012.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
- Bernardini GL, Yavagal DR. Management of ischemic stroke: current concepts and treatment options. Hosp Physician. Sep 2006:13-23. www.turner-white.com/memberfile.php?PubCode=hp_sep06_is chemic.pdf. Accessed September 20, 2012.
- Varon J. Treatment of acute severe hypertension: current and newer agents. Drugs. 2008;68(3): 283-297.
- Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27 suppl 3:S49-S54.
- Gupta PK, Gupta H, Khoynezhad A. Hypertensive emergency in aortic dissection and thoracic aortic aneurysm: a review of management. Pharmaceuticals. 2009;2(3):66-76.
- Post JB 4th, Frishman WH. Fenoldopam: a new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998;38(1):2-13.
- Suzuki S, Ohtsuka S, Ishikawa K, Yamaguchi I. Effects of nicardipine on coronary, vertebral and renal arterial flows in patients with essential hypertension. Hypertens Res. 2003;26(3):193-199.
- Anderson CS, Huang Y, Arima H, et al; INTERACT Investigators. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41(2):307-312.
- Broderick J, Connolly S, Feldmann E, et al. Quality of Care and Outcomes in Research Interdisciplinary Working Group Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391-413.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Brott T, Lu M, Kothari R, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29(8):1504-1509.
- Aiyagari V, Badruddin A. Management of hypertension in acute stroke. Expert Rev Cardiovasc Ther. 2009;7(6):637-646.
- Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520-526.
- Bonita R, Beaglehole R. The enigma of the decline in stroke deaths in the United States the search for an explanation. Stroke. 1996;27(3): 370-372.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655-1711.
- Heitsch L, Jauch EC. Management of hypertension in the setting of acute ischemic stroke. Curr Hypertens Rep. 2007;9(6):506-511.
- Barker FG II, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414.
An estimated 1% to 2% of patients with chronic hypertension will at some time develop hypertensive urgency or emergency.1 According to recent data from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2010,2 the prevalence of hypertension has remained stable at 30.5% among men and 28.5% among women in the United States; however, 74% of the hypertensive population is unaware of having this condition. Furthermore, 71.6% of hypertensive patients are managed for the condition, and in only 46.5% is blood pressure well controlled.2
In 2006, essential hypertension was estimated to account for more than 44 million emergency department visits in the US. The direct and indirect costs of hypertension totaled $73 billion in 2009.3,4
NEW TERMINOLOGY AND CLASSIFICATION
The terms malignant hypertension, hypertensive crisis, and accelerated hypertension have been replaced by hypertensive urgency or hypertensive emergency. Hypertensive urgency and emergency are differentiated by the absence or presence of acute end-organ damage, respectively.
Given the inconsistent terminology used, database searches can be challenging. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7),5,6 published in 2003, is considered the gold standard for categorizing hypertension in the outpatient setting. The JNC7 authorsclassify normal blood pressure as < 120/< 80 mm Hg. The document further classifies blood pressure into the stages shown in Table 1.5,6 Blood pressure higher than 180 mm Hg systolic and/or 120 mm Hg diastolic is generally considered severe hypertension— a designation that includes hypertensive urgency and hypertensive emergency.6
What Defines Hypertensive Urgency/Emergency?
Hypertensive urgency is defined as a diastolic blood pressure of 110 mm Hg or greater without the acute signs of end-organ damage.7 Some sources suggest that a patient must also have certain risk factors (eg, heart disease, renal disease) to be given this diagnosis.8 The presence of acute and rapidly evolving end-organ damage with an elevated diastolic blood pressure, usually greater than 120 mm Hg, establishes a diagnosis of hypertensive emergency.6,8,9
No specific blood pressure measurement indicates a hypertensive emergency, however; rather, the defining feature of this diagnosis is the presence of progressive target end-organ damage.7 This is most commonly manifested in cardiopulmonary, central nervous system, and/or renal findings; for the specific forms of end-organ damage, see Table 2.5,6,10,11 Preeclampsia and eclampsia are also considered manifestations of hypertensive end-organ damage but are beyond the scope of this article.5,11
The most common form of organ damage associated with hypertension is ischemic heart disease, in the form of either heart failure or acute coronary syndrome.12
PATHOPHYSIOLOGY
Blood pressure is calculated by cardiac output (ie, stroke volume multiplied by heart rate) multiplied by total peripheral resistance. Total peripheral resistance is influenced by a variety of humoral and neural factors, also known as vasoactive substances (see Figure 13,4). During an episode of acute hypertension, a failure of autoregulatory function occurs, precipitated by one or more of a host of potential causes. This failure of autoregulation then leads to increased systemic vascular resistance. In the setting of end-organ damage, release of inflammatory markers ensues, which ultimately causes endovascular injury and fibrin necrosis of arterioles.4,10,11
The renin-angiotensin-aldosterone system also plays a significant role in the cascade of hypertension, stimulating decreased renal perfusion and lowering tubular sodium concentration. This in turn stimulates aldosterone to increase blood pressure by maintaining excess volume through sodium retention and potassium excretion, further potentiating the cycle of uncontrolled blood pressure.4,13,14
Patients with chronically elevated blood pressures have a compensatory response, lying in the threshold mechanism, that protects against end-organ damage. Acute changes in blood pressure are better tolerated in these patients because of their decreased propensity for hypoperfusion.4 In contrast, normotensive patients who experience precipitous changes in blood pressure are at increased risk for organ hypoperfusion. The main concern regarding organ hypoperfusion is that it can lead to ischemia4 (see Figure 23,4).
PATIENT HISTORY
Acute hypertensive urgency or emergency can be triggered by many factors. Systemic etiologies (including kidney disease) caused by immunologic mediators or renal artery stenosis can cause or exacerbate hypertension. The patient should be asked about his or her normal blood pressure range, as this may offer clues to medication compliance. Rebound hypertension can be seen in patients who abruptly discontinue medications such as clonidine or β-blockers, as this causes an increase in sympathetic outflow.9,15
All patients should be queried regarding their use of OTC medications and other drug use, including cocaine, methamphetamines, phencyclidine, and alcohol.1,4,11,16 Patients taking monoamine oxidase inhibitors (MAOIs) are at increased risk for serious medication interactions; concomitant administration of MAOIs with other antidepressants can lead to a hypertensive reaction, but also to serotonin syndrome.1,17 Because MAOIs inhibit the breakdown of tyramine, patients taking them should avoid tyramine-containing foods and herbal supplements (including, but not limited to, St. John's wort, ginseng, and yohimbine).1,15,18
Acute hypertensive episodes can also occur as a result of preeclampsia or eclampsia in pregnant women, pheochromocytoma, primary aldosteronism, glucocorticoid excess (Cushing syndrome), or central nervous system disorders (eg, cerebrovascular accident, head trauma, brain tumors).9,11,19
PHYSICAL EXAMINATION
The purpose of the physical examination is to determine whether end-organ damage is present.1,11 The fundoscopic exam may reveal papilledema, a sign of increased intracranial pressure (ICP). Flame hemorrhages, cotton wool spots or arteriovenous nicking suggest a long-standing history of uncontrolled hypertension or diabetes.7,9 The neck should be assessed for jugular venous distention, which may be elevated in decompensated heart failure or pulmonary edema.11
The cardiac exam may reveal an irregular rate and rhythm, displaced apical pulse, gallop, or murmur. On pulmonary exam, rales may be auscultated, suggestive of pulmonary edema.9,15
The abdominal exam should include listening for a renal artery bruit.1 The neurologic exam may demonstrate altered mental status (possibly indicating hypertensive encephalopathy) or focal findings, if the patient has had an underlying ischemic or hemorrhagic event.9
LABORATORY STUDIES AND IMAGING
In most cases, a serum chemistry panel is warranted to identify any renal dysfunction. Urinalysis may reveal proteinuria, possibly indicating renal damage.4,9,15
Any patient complaining of chest pain should have an ECG to look for ischemic changes or presence of a left bundle branch block, and serial cardiac enzymes to rule out acute coronary syndrome.15 Access to previous ECGs is helpful in differentiating between new and old conductive abnormalities.
A chest x-ray should be performed in patients who complain of shortness of breath and/or chest pain. A widened mediastinum can represent aortic dissection.4,15 Evidence of pulmonary edema should prompt the clinician to assess for left ventricular dysfunction or valvular insufficiencies by echocardiogram. Chest CT should be pursued in patients with clinical suspicion for dissection.1,15,20
Patients presenting with a headache or focal neurologic abnormalities warrant a head CT to rule out stroke.15 Urine drug screening is appropriate if the patient history suggests illicit drug use.12
"FIRST, DO NO HARM"
Treatment of hypertensive emergency and urgency varies from traditional treatment for hypertension. Aggressive blood pressure control in patients presenting with acute ischemic stroke has been associated with poorer patient outcomes.21,22 Thus, treating the patient and not the numbers is the first general recommendation for treatment of hypertensive emergency and urgency. It is important for the clinician to remember the Hippocratic oath, "First do no harm," when treating these patients.
Other general recommendations are derived from theory, physiology, and smaller clinical trials; their application must be individualized according to the patient's needs. These recommendations include aiming for a reduction in mean arterial blood pressure of no more than 10% to 25% within the first hour, a goal blood pressure of 160/90 mm Hg within the first 8 hours, and normalization of blood pressure over 8 to 24 hours.12
While the use of pharmacologic agents may be warranted, it is important to consider that elevated blood pressure may be a reaction to pain or stress and may be best treated alternatively. Recommendations for permissive hypertension in acute ischemic stroke will be discussed below.
TREATMENT: HYPERTENSIVE URGENCY
The treatment of hypertensive urgency is usually immediate and warrants close follow-up. Although elevated blood pressures can be alarming to the patient, hypertensive urgency usually develops over days to weeks.8 In this setting, it is not necessary to lower blood pressure acutely.12 A rapid decrease in blood pressure can actually cause symptomatic hypotension, resulting in hypoperfusion to the brain.5,6,8
After ruling out end-organ damage, the next step is to treat according to the guidelines for hypertensive urgency.5,6 These recommendations include the use of rapid-onset oral antihypertensive agents, such as clonidine, labetalol, or captopril.23 Use of these agents is only suggested for gradual, short-term reduction of blood pressure (ie, over 24 to 48 hours) while the patient is being monitored for potential hypertension-related organ damage, either in the emergency department or in an observational hospital setting.5,6,23
Once the short-acting agents have adequately reduced blood pressure, long-term agents can be chosen to prevent rebound hypertension.16 Patients are typically monitored for 24 hours in the hospital during this transition. Upon discharge, the patient should be scheduled for follow-up within one to two days.11 Patient education, including a discussion of medication adherence, weight loss, and reduced dietary salt, is key to prevent recurrences and optimize overall treatment compliance.
TREATMENT: HYPERTENSIVE EMERGENCY
Treatment of hypertensive emergency always warrants hospitalization, usually in the ICU.5,6 IV antihypertensive medications (eg, nicardipine, fenoldopam, labetalol, esmolol, phentolamine) are preferred. Their use often necessitates continuous blood pressure monitoring via arterial line, allowing the clinician to perform ongoing medication titration. In hypertensive emergencies, the purpose of treatment is to preserve brain, kidney, and heart function.4
Goal-directed therapy is initiated even before the patient evaluation has been fully completed. Patient assessment continues after treatment is begun to avoid overly aggressive blood pressure reduction, which can increase the risk for patient demise or morbidity.4
Exceptions in the treatment of hypertensive emergencies (particularly of specific disease states) will be discussed below, along with other treatment considerations. Patient comorbidities, for example, must be considered in the choice of antihypertensive agents.
Focused Treatment for Specific Hypertensive Emergencies
Hypertensive encephalopathy. This condition, associated with severe hypertension, is indicated by an abrupt change in mental status. During this acute end-organ damage event, a failure of cerebral autoregulation occurs, with increased pressure in the vascular endothelium leading to arteriole dilation that in turn can result in hyperperfusion of the brain, cerebral edema, and microhemorrhages.23
Because presentation of hypertensive encephalopathy may be similar to that in patients with acute stroke, hemorrhage, or brain lesions, these and other potential causes must be ruled out. While blood pressure treatment goals correspond with general recommendations,5,6 caution must be taken not to reduce blood pressure too swiftly; thus, continuous monitoring is warranted. If the patient's neurologic function worsens, treatment should be suspended and blood pressure allowed to rise slowly.4
Preferred antihypertensives for patients with hypertensive encephalopathy include labetalol, nicardipine, and fenoldopam23 (see Table 34-6,21,23). Centrally acting antihypertensives, such as clonidine, methyldopa, or reserpine,24 should not be used, as they can cause central nervous system depression and may cloud the patient's sensorium further.
Myocardial ischemia/infarction. During an acute hypertensive event, the workload on the heart and activation of the renin-angiotensin-aldosterone system can lead to acute coronary ischemia or infarction.23 Treatment is aimed at increasing blood flow to the myocardium and reducing the workload on the heart. Antihypertensives are combined with reperfusion (eg, angioplasty) and/or thrombolytics to preserve myocardial structure and function. Standard agents to reduce blood pressure include IV nitroglycerin and β-blockers. Systolic blood pressure is reduced until symptoms subside or diastolic blood pressure is reduced to 100 mm Hg or lower. Adjuncts such as morphine and oxygen are used to reduce patient discomfort and improve oxygen delivery to the myocardium.4
Acute left ventricular failure. In this potential manifestation of hypertensive emergency, the left ventricle initially attempts to compensate for rising blood pressure and becomes hypertrophic. Once the myocardium can no longer meet the demand, left ventricular function decompensates, causing a flow backup that leads to acute pulmonary edema.23
Blood pressure goals mirror those in the general treatment recommendations but focus specifically on reducing preload and afterload, improving myocardial contractility and decreasing peripheral vascular resistance. The preferred agents in this setting are IV nitroglycerin and ACE inhibitors, along with loop diuretics, morphine, and oxygen.4 Medications that increase workload on the heart (eg, hydralazine, clonidine) should be avoided.23
Aortic dissection. This is a true medical emergency that can result in significant morbidity and mortality. Type A dissection occurs proximally, at the ascending aorta, whereas type B dissection occurs at the level of the descending aorta. Typically, type B dissection is managed medically, as surgical treatment carries a significant risk for paralysis.4 Both types of aortic dissection are strongly associated with uncontrolled hypertension and in some patients may be precipitated by an acute hypertensive event. In such cases, the goal for blood pressure reduction is to decrease the shearing forces associated with the dissection. This is accomplished by lowering both blood pressure and pulse rate.12
While cases of type A dissection are usually managed surgically, all affected patients will require some component of medical management and tight blood pressure control. The current recommendation for blood pressure in aortic dissection is swift downward titration to a goal systolic blood pressure of 100 to 110 mm Hg.4 A β-blocker in combination with a vasodilator, administered intravenously, should be used for swift blood pressure reduction.4,25
IV nitroprusside, a potent vasodilator, is the preferred agent, but its use requires intra-arterial blood pressure monitoring.23 Because nitroprusside is metabolized to cyanide, its use can lead to lethal toxicity, especially in patients with hepatic or renal impairment.11 In this patient population, IV labetalol or esmolol may be used instead.4,25
Acute renal failure. In the setting of an acute hypertensive episode, it is often difficult to determine whether acute renal failure is the cause or the effect. Regardless, rapid reduction in blood pressure is warranted to preserve renal function and to stop the cycle of microvascular kidney destruction. Blood pressure goals are aligned with the general treatment recommendations. The preferred antihypertensive agent is IV fenoldopam, a dopamine receptor agonist that directly dilates renal arterioles, improving renal perfusion and promoting diuresis.4,26 Nicardipine, a calcium channel blocker, may be considered as an alternative.23,27
Treatment Considerations in Stroke
Elevated blood pressure is common in the early stages of stroke. Numerous studies have analyzed overall outcomes in patients presenting with ischemic stroke and uncontrolled hypertension. In this setting, evidence suggests a poorer prognosis in patients treated aggressively with antihypertensive agents.4,21,22
The association between dramatic reduction in blood pressure and poor prognoses lies in the theory of the ischemic penumbra. This is an area around the core of ischemic tissue that receives enough blood flow to maintain neuronal activity for a few hours after initial injury, but this tissue is susceptible to further infarction. Precipitous drops in blood pressure can reduce blood flow to collateral vessels, resulting in hypoperfusion of the penumbra and leading to further neurologic damage.
Details and current treatment recommendations for each of the various types of stroke follow.
Acute intracerebral hemorrhage. Uncontrolled hypertension is often associated with intracerebral hemorrhage (ICH), either as a risk factor or a factor that contributes to the event. Once a patient has experienced an acute brain insult, blood pressure can become even more uncontrolled. Extension of the hematoma and a worsening outcome are the main concerns in treating the patient with concomitant blood pressure elevation and ICH. Also of concern is maintaining adequate perfusion to the penumbra. Additionally, transient hypoperfusion can develop when the ICP is elevated and the mean arterial pressure (MAP) is acutely lowered, thus reducing the cerebral perfusion pressure (CPP; CPP = MAP - ICP).
Researchers have acknowledged there is insufficient evidence to offer management guidelines for blood pressure reduction in patients with ICH.28 The 2007 recommendations from the American Heart Association/ American Stroke Association (AHA/ASA) for blood pressure management in patients with acute ICH29 are as follows: In the setting of ICH in patients with uncontrolled blood pressure, treatment should be aggressive if systolic blood pressure exceeds 200 mm Hg or MAP exceeds 150 mm Hg. A treatment goal to consider is reducing systolic blood pres sure to 160 mm Hg or less (or MAP to below 130 mm Hg).29 Patients with elevated ICP should undergo placement of a ventriculostomy to maintain a CPP between 60 and 80 mm Hg, although the risk for infection or intracerebral hemorrhage must be weighed against the potential benefits.29,30
When blood pressure reduction is required, the MAP should not be lowered more than 20% in a 24-hour period. Recommended agents include IV nicardipine, labetalol, enalapril, hydralazine, or esmolol.31
Acute ischemic stroke. Long-term control of blood pressure in patients who have experienced stroke remains undisputed, as it improves outcomes. However, in the setting of acute ischemic stroke (AIS), initiating blood pressure control is more liberal. Optimal control of blood pressure during management of AIS is imperative to reduce morbidity and mortality.32 Areas affected by edematous brain tissue are at increased risk for bleeding (ie, hemorrhagic expansion).
Patients who present with AIS require careful history taking to elicit their average blood pressure range; this will help the clinician determine goal pressures during management of the acute stroke phase.33 The primary rationale for treating blood pressure in this acute setting is to prevent hemorrhagic expansion at sites with potential for bleeding.34
According to the 2007 AHA/ ASA recommendations for management of blood pressure in AIS,35 patients who are eligible for thrombolysis should have a systolic blood pressure goal below 180 mm Hg and diastolic blood pressure below 105 mm Hg. Patients who will not receive thrombolytics should have blood pressure lowered only if systolic blood pressure exceeds 220 mm Hg or diastolic blood pressure exceeds 110 mm Hg.35,36 Appropriately refraining from reducing blood pressure is known as permissive hypertension.
Given the fragility of the cerebral brain tissue after AIS, permissive hypertension is intended to protect the penumbra and preserve cerebral blood flow. In patients who require blood pressure reduction because of other medical conditions (eg, decompensated heart failure), blood pressure should not be lowered more than 10% to 15% in a 24-hour period.9,31,36 No specific antihypertensives are preferred in patients with AIS: IV enalapril, esmolol, labetalol, or nicardipine can be used.31
Subarachnoid hemorrhage. The two complications of a subarachnoid hemorrhage (SAH) that most contribute to morbidity and mortality are rebleeding and vasospasms; elevated blood pressure can contribute to both. Thus, blood pressure control in patients with SAH is imperative.
Patients with acute SAH often require blood pressure monitoring via arterial line, as well as ICP monitoring. Blood pressure goals are similar to those in patients with ICH. The preferred agent for blood pressure control is nimodipine, which offers the secondary benefit of vasospasm prevention.37
CONCLUSION
Patients presenting with urgent or emergent hypertension need expeditious evaluation to avoid the significant morbidity and mortality associated with acute end-organ damage. Hypertensive urgency is defined as a diastolic blood pressure of greater than 120 mm Hg without evidence of end-organ damage.
In cases of hypertensive emergency, in which acute end-organ damage is present, lowering blood pressure should be directed by the type of end-organ damage and/or underlying comorbidities. In general, blood pressure should not be lowered more than 10% to 25% within the first hour, with normalization achieved over the next 8 to 24 hours.
In cases of acute ischemic stroke, permissive hypertension is recommended. Above all, treat patients, not numbers, bearing in mind the Hippocratic oath: Primum non nocere, or "First, do no harm."
REFERENCES
- Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6): 1949-1962.
- Guo F, He D, Zhang W, Walton RG. Trends in Prevalence, Awareness, Management, and Control of Hypertension Among United States Adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599-606.
- Flanigan JS, Vitberg D. Hypertensive emergency and severe hypertension: what to treat, who to treat, and how to treat. Med Clin North Am. 2006;90(3):439-451.
- Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
- National High Blood Pressure Education Program Coordinating Committee, National Heart Lung and Blood Institute, NIH. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 04-5230. August 2004. www .nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed September 20, 2012.
- Houston M. Hypertensive emergencies and urgencies: pathophysiology and clinical aspects. Am Heart J. 1986;111(1):205-210.
- Kessler CS, Joudeh Y. Evaluation and treatment of severe asymptomatic hypertension. Am Fam Physician. 2010;81(4):470-476.
- Vaidya CK, Ouellette JR. Hypertensive urgency and emergency. Hosp Physician. Mar 2007:43-50. www.turner-white.com/memberfile.php?Pub Code=hp_mar07_hypertensive.pdf. Accessed September 20, 2012.
- Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies: a Cochrane systematic review. J Hum Hypertens. 2008;22(9):596-607.
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411-417.
- Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006; 33(3):613-623.
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 suppl B):9-20.
- Flack JM. Epidemiology and unmet needs in hypertension. J Manag Care Pharm. 2007;13(e suppl B):2-8.
- Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19(6):502-512.
- Hebert CJ, Vidt DG. Hypertensive crises. Prim Care. 2008;35(3):475-487.
- Shulman KI, Fischer HD, Herrmann N, et al. Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry. 2009;70(12):1681-1696.
- Musso NR, Vergassola C, Pende A, Lotti G. Yohimbine effects on blood pressure and plasma catecholamines in human hypertension. Am J Hypertens. 1995;8(6):565-571.
- Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6(6): 493-499.
- Society of Critical Care Medicine. Fundamental Critical Care Support Course. www.sccm.org/ fccs_and_training_courses/fccs/pages/default .aspx. Accessed September 20, 2012.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
- Bernardini GL, Yavagal DR. Management of ischemic stroke: current concepts and treatment options. Hosp Physician. Sep 2006:13-23. www.turner-white.com/memberfile.php?PubCode=hp_sep06_is chemic.pdf. Accessed September 20, 2012.
- Varon J. Treatment of acute severe hypertension: current and newer agents. Drugs. 2008;68(3): 283-297.
- Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27 suppl 3:S49-S54.
- Gupta PK, Gupta H, Khoynezhad A. Hypertensive emergency in aortic dissection and thoracic aortic aneurysm: a review of management. Pharmaceuticals. 2009;2(3):66-76.
- Post JB 4th, Frishman WH. Fenoldopam: a new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998;38(1):2-13.
- Suzuki S, Ohtsuka S, Ishikawa K, Yamaguchi I. Effects of nicardipine on coronary, vertebral and renal arterial flows in patients with essential hypertension. Hypertens Res. 2003;26(3):193-199.
- Anderson CS, Huang Y, Arima H, et al; INTERACT Investigators. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41(2):307-312.
- Broderick J, Connolly S, Feldmann E, et al. Quality of Care and Outcomes in Research Interdisciplinary Working Group Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391-413.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Brott T, Lu M, Kothari R, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29(8):1504-1509.
- Aiyagari V, Badruddin A. Management of hypertension in acute stroke. Expert Rev Cardiovasc Ther. 2009;7(6):637-646.
- Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520-526.
- Bonita R, Beaglehole R. The enigma of the decline in stroke deaths in the United States the search for an explanation. Stroke. 1996;27(3): 370-372.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655-1711.
- Heitsch L, Jauch EC. Management of hypertension in the setting of acute ischemic stroke. Curr Hypertens Rep. 2007;9(6):506-511.
- Barker FG II, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414.
An estimated 1% to 2% of patients with chronic hypertension will at some time develop hypertensive urgency or emergency.1 According to recent data from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2010,2 the prevalence of hypertension has remained stable at 30.5% among men and 28.5% among women in the United States; however, 74% of the hypertensive population is unaware of having this condition. Furthermore, 71.6% of hypertensive patients are managed for the condition, and in only 46.5% is blood pressure well controlled.2
In 2006, essential hypertension was estimated to account for more than 44 million emergency department visits in the US. The direct and indirect costs of hypertension totaled $73 billion in 2009.3,4
NEW TERMINOLOGY AND CLASSIFICATION
The terms malignant hypertension, hypertensive crisis, and accelerated hypertension have been replaced by hypertensive urgency or hypertensive emergency. Hypertensive urgency and emergency are differentiated by the absence or presence of acute end-organ damage, respectively.
Given the inconsistent terminology used, database searches can be challenging. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7),5,6 published in 2003, is considered the gold standard for categorizing hypertension in the outpatient setting. The JNC7 authorsclassify normal blood pressure as < 120/< 80 mm Hg. The document further classifies blood pressure into the stages shown in Table 1.5,6 Blood pressure higher than 180 mm Hg systolic and/or 120 mm Hg diastolic is generally considered severe hypertension— a designation that includes hypertensive urgency and hypertensive emergency.6
What Defines Hypertensive Urgency/Emergency?
Hypertensive urgency is defined as a diastolic blood pressure of 110 mm Hg or greater without the acute signs of end-organ damage.7 Some sources suggest that a patient must also have certain risk factors (eg, heart disease, renal disease) to be given this diagnosis.8 The presence of acute and rapidly evolving end-organ damage with an elevated diastolic blood pressure, usually greater than 120 mm Hg, establishes a diagnosis of hypertensive emergency.6,8,9
No specific blood pressure measurement indicates a hypertensive emergency, however; rather, the defining feature of this diagnosis is the presence of progressive target end-organ damage.7 This is most commonly manifested in cardiopulmonary, central nervous system, and/or renal findings; for the specific forms of end-organ damage, see Table 2.5,6,10,11 Preeclampsia and eclampsia are also considered manifestations of hypertensive end-organ damage but are beyond the scope of this article.5,11
The most common form of organ damage associated with hypertension is ischemic heart disease, in the form of either heart failure or acute coronary syndrome.12
PATHOPHYSIOLOGY
Blood pressure is calculated by cardiac output (ie, stroke volume multiplied by heart rate) multiplied by total peripheral resistance. Total peripheral resistance is influenced by a variety of humoral and neural factors, also known as vasoactive substances (see Figure 13,4). During an episode of acute hypertension, a failure of autoregulatory function occurs, precipitated by one or more of a host of potential causes. This failure of autoregulation then leads to increased systemic vascular resistance. In the setting of end-organ damage, release of inflammatory markers ensues, which ultimately causes endovascular injury and fibrin necrosis of arterioles.4,10,11
The renin-angiotensin-aldosterone system also plays a significant role in the cascade of hypertension, stimulating decreased renal perfusion and lowering tubular sodium concentration. This in turn stimulates aldosterone to increase blood pressure by maintaining excess volume through sodium retention and potassium excretion, further potentiating the cycle of uncontrolled blood pressure.4,13,14
Patients with chronically elevated blood pressures have a compensatory response, lying in the threshold mechanism, that protects against end-organ damage. Acute changes in blood pressure are better tolerated in these patients because of their decreased propensity for hypoperfusion.4 In contrast, normotensive patients who experience precipitous changes in blood pressure are at increased risk for organ hypoperfusion. The main concern regarding organ hypoperfusion is that it can lead to ischemia4 (see Figure 23,4).
PATIENT HISTORY
Acute hypertensive urgency or emergency can be triggered by many factors. Systemic etiologies (including kidney disease) caused by immunologic mediators or renal artery stenosis can cause or exacerbate hypertension. The patient should be asked about his or her normal blood pressure range, as this may offer clues to medication compliance. Rebound hypertension can be seen in patients who abruptly discontinue medications such as clonidine or β-blockers, as this causes an increase in sympathetic outflow.9,15
All patients should be queried regarding their use of OTC medications and other drug use, including cocaine, methamphetamines, phencyclidine, and alcohol.1,4,11,16 Patients taking monoamine oxidase inhibitors (MAOIs) are at increased risk for serious medication interactions; concomitant administration of MAOIs with other antidepressants can lead to a hypertensive reaction, but also to serotonin syndrome.1,17 Because MAOIs inhibit the breakdown of tyramine, patients taking them should avoid tyramine-containing foods and herbal supplements (including, but not limited to, St. John's wort, ginseng, and yohimbine).1,15,18
Acute hypertensive episodes can also occur as a result of preeclampsia or eclampsia in pregnant women, pheochromocytoma, primary aldosteronism, glucocorticoid excess (Cushing syndrome), or central nervous system disorders (eg, cerebrovascular accident, head trauma, brain tumors).9,11,19
PHYSICAL EXAMINATION
The purpose of the physical examination is to determine whether end-organ damage is present.1,11 The fundoscopic exam may reveal papilledema, a sign of increased intracranial pressure (ICP). Flame hemorrhages, cotton wool spots or arteriovenous nicking suggest a long-standing history of uncontrolled hypertension or diabetes.7,9 The neck should be assessed for jugular venous distention, which may be elevated in decompensated heart failure or pulmonary edema.11
The cardiac exam may reveal an irregular rate and rhythm, displaced apical pulse, gallop, or murmur. On pulmonary exam, rales may be auscultated, suggestive of pulmonary edema.9,15
The abdominal exam should include listening for a renal artery bruit.1 The neurologic exam may demonstrate altered mental status (possibly indicating hypertensive encephalopathy) or focal findings, if the patient has had an underlying ischemic or hemorrhagic event.9
LABORATORY STUDIES AND IMAGING
In most cases, a serum chemistry panel is warranted to identify any renal dysfunction. Urinalysis may reveal proteinuria, possibly indicating renal damage.4,9,15
Any patient complaining of chest pain should have an ECG to look for ischemic changes or presence of a left bundle branch block, and serial cardiac enzymes to rule out acute coronary syndrome.15 Access to previous ECGs is helpful in differentiating between new and old conductive abnormalities.
A chest x-ray should be performed in patients who complain of shortness of breath and/or chest pain. A widened mediastinum can represent aortic dissection.4,15 Evidence of pulmonary edema should prompt the clinician to assess for left ventricular dysfunction or valvular insufficiencies by echocardiogram. Chest CT should be pursued in patients with clinical suspicion for dissection.1,15,20
Patients presenting with a headache or focal neurologic abnormalities warrant a head CT to rule out stroke.15 Urine drug screening is appropriate if the patient history suggests illicit drug use.12
"FIRST, DO NO HARM"
Treatment of hypertensive emergency and urgency varies from traditional treatment for hypertension. Aggressive blood pressure control in patients presenting with acute ischemic stroke has been associated with poorer patient outcomes.21,22 Thus, treating the patient and not the numbers is the first general recommendation for treatment of hypertensive emergency and urgency. It is important for the clinician to remember the Hippocratic oath, "First do no harm," when treating these patients.
Other general recommendations are derived from theory, physiology, and smaller clinical trials; their application must be individualized according to the patient's needs. These recommendations include aiming for a reduction in mean arterial blood pressure of no more than 10% to 25% within the first hour, a goal blood pressure of 160/90 mm Hg within the first 8 hours, and normalization of blood pressure over 8 to 24 hours.12
While the use of pharmacologic agents may be warranted, it is important to consider that elevated blood pressure may be a reaction to pain or stress and may be best treated alternatively. Recommendations for permissive hypertension in acute ischemic stroke will be discussed below.
TREATMENT: HYPERTENSIVE URGENCY
The treatment of hypertensive urgency is usually immediate and warrants close follow-up. Although elevated blood pressures can be alarming to the patient, hypertensive urgency usually develops over days to weeks.8 In this setting, it is not necessary to lower blood pressure acutely.12 A rapid decrease in blood pressure can actually cause symptomatic hypotension, resulting in hypoperfusion to the brain.5,6,8
After ruling out end-organ damage, the next step is to treat according to the guidelines for hypertensive urgency.5,6 These recommendations include the use of rapid-onset oral antihypertensive agents, such as clonidine, labetalol, or captopril.23 Use of these agents is only suggested for gradual, short-term reduction of blood pressure (ie, over 24 to 48 hours) while the patient is being monitored for potential hypertension-related organ damage, either in the emergency department or in an observational hospital setting.5,6,23
Once the short-acting agents have adequately reduced blood pressure, long-term agents can be chosen to prevent rebound hypertension.16 Patients are typically monitored for 24 hours in the hospital during this transition. Upon discharge, the patient should be scheduled for follow-up within one to two days.11 Patient education, including a discussion of medication adherence, weight loss, and reduced dietary salt, is key to prevent recurrences and optimize overall treatment compliance.
TREATMENT: HYPERTENSIVE EMERGENCY
Treatment of hypertensive emergency always warrants hospitalization, usually in the ICU.5,6 IV antihypertensive medications (eg, nicardipine, fenoldopam, labetalol, esmolol, phentolamine) are preferred. Their use often necessitates continuous blood pressure monitoring via arterial line, allowing the clinician to perform ongoing medication titration. In hypertensive emergencies, the purpose of treatment is to preserve brain, kidney, and heart function.4
Goal-directed therapy is initiated even before the patient evaluation has been fully completed. Patient assessment continues after treatment is begun to avoid overly aggressive blood pressure reduction, which can increase the risk for patient demise or morbidity.4
Exceptions in the treatment of hypertensive emergencies (particularly of specific disease states) will be discussed below, along with other treatment considerations. Patient comorbidities, for example, must be considered in the choice of antihypertensive agents.
Focused Treatment for Specific Hypertensive Emergencies
Hypertensive encephalopathy. This condition, associated with severe hypertension, is indicated by an abrupt change in mental status. During this acute end-organ damage event, a failure of cerebral autoregulation occurs, with increased pressure in the vascular endothelium leading to arteriole dilation that in turn can result in hyperperfusion of the brain, cerebral edema, and microhemorrhages.23
Because presentation of hypertensive encephalopathy may be similar to that in patients with acute stroke, hemorrhage, or brain lesions, these and other potential causes must be ruled out. While blood pressure treatment goals correspond with general recommendations,5,6 caution must be taken not to reduce blood pressure too swiftly; thus, continuous monitoring is warranted. If the patient's neurologic function worsens, treatment should be suspended and blood pressure allowed to rise slowly.4
Preferred antihypertensives for patients with hypertensive encephalopathy include labetalol, nicardipine, and fenoldopam23 (see Table 34-6,21,23). Centrally acting antihypertensives, such as clonidine, methyldopa, or reserpine,24 should not be used, as they can cause central nervous system depression and may cloud the patient's sensorium further.
Myocardial ischemia/infarction. During an acute hypertensive event, the workload on the heart and activation of the renin-angiotensin-aldosterone system can lead to acute coronary ischemia or infarction.23 Treatment is aimed at increasing blood flow to the myocardium and reducing the workload on the heart. Antihypertensives are combined with reperfusion (eg, angioplasty) and/or thrombolytics to preserve myocardial structure and function. Standard agents to reduce blood pressure include IV nitroglycerin and β-blockers. Systolic blood pressure is reduced until symptoms subside or diastolic blood pressure is reduced to 100 mm Hg or lower. Adjuncts such as morphine and oxygen are used to reduce patient discomfort and improve oxygen delivery to the myocardium.4
Acute left ventricular failure. In this potential manifestation of hypertensive emergency, the left ventricle initially attempts to compensate for rising blood pressure and becomes hypertrophic. Once the myocardium can no longer meet the demand, left ventricular function decompensates, causing a flow backup that leads to acute pulmonary edema.23
Blood pressure goals mirror those in the general treatment recommendations but focus specifically on reducing preload and afterload, improving myocardial contractility and decreasing peripheral vascular resistance. The preferred agents in this setting are IV nitroglycerin and ACE inhibitors, along with loop diuretics, morphine, and oxygen.4 Medications that increase workload on the heart (eg, hydralazine, clonidine) should be avoided.23
Aortic dissection. This is a true medical emergency that can result in significant morbidity and mortality. Type A dissection occurs proximally, at the ascending aorta, whereas type B dissection occurs at the level of the descending aorta. Typically, type B dissection is managed medically, as surgical treatment carries a significant risk for paralysis.4 Both types of aortic dissection are strongly associated with uncontrolled hypertension and in some patients may be precipitated by an acute hypertensive event. In such cases, the goal for blood pressure reduction is to decrease the shearing forces associated with the dissection. This is accomplished by lowering both blood pressure and pulse rate.12
While cases of type A dissection are usually managed surgically, all affected patients will require some component of medical management and tight blood pressure control. The current recommendation for blood pressure in aortic dissection is swift downward titration to a goal systolic blood pressure of 100 to 110 mm Hg.4 A β-blocker in combination with a vasodilator, administered intravenously, should be used for swift blood pressure reduction.4,25
IV nitroprusside, a potent vasodilator, is the preferred agent, but its use requires intra-arterial blood pressure monitoring.23 Because nitroprusside is metabolized to cyanide, its use can lead to lethal toxicity, especially in patients with hepatic or renal impairment.11 In this patient population, IV labetalol or esmolol may be used instead.4,25
Acute renal failure. In the setting of an acute hypertensive episode, it is often difficult to determine whether acute renal failure is the cause or the effect. Regardless, rapid reduction in blood pressure is warranted to preserve renal function and to stop the cycle of microvascular kidney destruction. Blood pressure goals are aligned with the general treatment recommendations. The preferred antihypertensive agent is IV fenoldopam, a dopamine receptor agonist that directly dilates renal arterioles, improving renal perfusion and promoting diuresis.4,26 Nicardipine, a calcium channel blocker, may be considered as an alternative.23,27
Treatment Considerations in Stroke
Elevated blood pressure is common in the early stages of stroke. Numerous studies have analyzed overall outcomes in patients presenting with ischemic stroke and uncontrolled hypertension. In this setting, evidence suggests a poorer prognosis in patients treated aggressively with antihypertensive agents.4,21,22
The association between dramatic reduction in blood pressure and poor prognoses lies in the theory of the ischemic penumbra. This is an area around the core of ischemic tissue that receives enough blood flow to maintain neuronal activity for a few hours after initial injury, but this tissue is susceptible to further infarction. Precipitous drops in blood pressure can reduce blood flow to collateral vessels, resulting in hypoperfusion of the penumbra and leading to further neurologic damage.
Details and current treatment recommendations for each of the various types of stroke follow.
Acute intracerebral hemorrhage. Uncontrolled hypertension is often associated with intracerebral hemorrhage (ICH), either as a risk factor or a factor that contributes to the event. Once a patient has experienced an acute brain insult, blood pressure can become even more uncontrolled. Extension of the hematoma and a worsening outcome are the main concerns in treating the patient with concomitant blood pressure elevation and ICH. Also of concern is maintaining adequate perfusion to the penumbra. Additionally, transient hypoperfusion can develop when the ICP is elevated and the mean arterial pressure (MAP) is acutely lowered, thus reducing the cerebral perfusion pressure (CPP; CPP = MAP - ICP).
Researchers have acknowledged there is insufficient evidence to offer management guidelines for blood pressure reduction in patients with ICH.28 The 2007 recommendations from the American Heart Association/ American Stroke Association (AHA/ASA) for blood pressure management in patients with acute ICH29 are as follows: In the setting of ICH in patients with uncontrolled blood pressure, treatment should be aggressive if systolic blood pressure exceeds 200 mm Hg or MAP exceeds 150 mm Hg. A treatment goal to consider is reducing systolic blood pres sure to 160 mm Hg or less (or MAP to below 130 mm Hg).29 Patients with elevated ICP should undergo placement of a ventriculostomy to maintain a CPP between 60 and 80 mm Hg, although the risk for infection or intracerebral hemorrhage must be weighed against the potential benefits.29,30
When blood pressure reduction is required, the MAP should not be lowered more than 20% in a 24-hour period. Recommended agents include IV nicardipine, labetalol, enalapril, hydralazine, or esmolol.31
Acute ischemic stroke. Long-term control of blood pressure in patients who have experienced stroke remains undisputed, as it improves outcomes. However, in the setting of acute ischemic stroke (AIS), initiating blood pressure control is more liberal. Optimal control of blood pressure during management of AIS is imperative to reduce morbidity and mortality.32 Areas affected by edematous brain tissue are at increased risk for bleeding (ie, hemorrhagic expansion).
Patients who present with AIS require careful history taking to elicit their average blood pressure range; this will help the clinician determine goal pressures during management of the acute stroke phase.33 The primary rationale for treating blood pressure in this acute setting is to prevent hemorrhagic expansion at sites with potential for bleeding.34
According to the 2007 AHA/ ASA recommendations for management of blood pressure in AIS,35 patients who are eligible for thrombolysis should have a systolic blood pressure goal below 180 mm Hg and diastolic blood pressure below 105 mm Hg. Patients who will not receive thrombolytics should have blood pressure lowered only if systolic blood pressure exceeds 220 mm Hg or diastolic blood pressure exceeds 110 mm Hg.35,36 Appropriately refraining from reducing blood pressure is known as permissive hypertension.
Given the fragility of the cerebral brain tissue after AIS, permissive hypertension is intended to protect the penumbra and preserve cerebral blood flow. In patients who require blood pressure reduction because of other medical conditions (eg, decompensated heart failure), blood pressure should not be lowered more than 10% to 15% in a 24-hour period.9,31,36 No specific antihypertensives are preferred in patients with AIS: IV enalapril, esmolol, labetalol, or nicardipine can be used.31
Subarachnoid hemorrhage. The two complications of a subarachnoid hemorrhage (SAH) that most contribute to morbidity and mortality are rebleeding and vasospasms; elevated blood pressure can contribute to both. Thus, blood pressure control in patients with SAH is imperative.
Patients with acute SAH often require blood pressure monitoring via arterial line, as well as ICP monitoring. Blood pressure goals are similar to those in patients with ICH. The preferred agent for blood pressure control is nimodipine, which offers the secondary benefit of vasospasm prevention.37
CONCLUSION
Patients presenting with urgent or emergent hypertension need expeditious evaluation to avoid the significant morbidity and mortality associated with acute end-organ damage. Hypertensive urgency is defined as a diastolic blood pressure of greater than 120 mm Hg without evidence of end-organ damage.
In cases of hypertensive emergency, in which acute end-organ damage is present, lowering blood pressure should be directed by the type of end-organ damage and/or underlying comorbidities. In general, blood pressure should not be lowered more than 10% to 25% within the first hour, with normalization achieved over the next 8 to 24 hours.
In cases of acute ischemic stroke, permissive hypertension is recommended. Above all, treat patients, not numbers, bearing in mind the Hippocratic oath: Primum non nocere, or "First, do no harm."
REFERENCES
- Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6): 1949-1962.
- Guo F, He D, Zhang W, Walton RG. Trends in Prevalence, Awareness, Management, and Control of Hypertension Among United States Adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599-606.
- Flanigan JS, Vitberg D. Hypertensive emergency and severe hypertension: what to treat, who to treat, and how to treat. Med Clin North Am. 2006;90(3):439-451.
- Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
- National High Blood Pressure Education Program Coordinating Committee, National Heart Lung and Blood Institute, NIH. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 04-5230. August 2004. www .nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed September 20, 2012.
- Houston M. Hypertensive emergencies and urgencies: pathophysiology and clinical aspects. Am Heart J. 1986;111(1):205-210.
- Kessler CS, Joudeh Y. Evaluation and treatment of severe asymptomatic hypertension. Am Fam Physician. 2010;81(4):470-476.
- Vaidya CK, Ouellette JR. Hypertensive urgency and emergency. Hosp Physician. Mar 2007:43-50. www.turner-white.com/memberfile.php?Pub Code=hp_mar07_hypertensive.pdf. Accessed September 20, 2012.
- Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies: a Cochrane systematic review. J Hum Hypertens. 2008;22(9):596-607.
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411-417.
- Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006; 33(3):613-623.
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 suppl B):9-20.
- Flack JM. Epidemiology and unmet needs in hypertension. J Manag Care Pharm. 2007;13(e suppl B):2-8.
- Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19(6):502-512.
- Hebert CJ, Vidt DG. Hypertensive crises. Prim Care. 2008;35(3):475-487.
- Shulman KI, Fischer HD, Herrmann N, et al. Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry. 2009;70(12):1681-1696.
- Musso NR, Vergassola C, Pende A, Lotti G. Yohimbine effects on blood pressure and plasma catecholamines in human hypertension. Am J Hypertens. 1995;8(6):565-571.
- Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6(6): 493-499.
- Society of Critical Care Medicine. Fundamental Critical Care Support Course. www.sccm.org/ fccs_and_training_courses/fccs/pages/default .aspx. Accessed September 20, 2012.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
- Bernardini GL, Yavagal DR. Management of ischemic stroke: current concepts and treatment options. Hosp Physician. Sep 2006:13-23. www.turner-white.com/memberfile.php?PubCode=hp_sep06_is chemic.pdf. Accessed September 20, 2012.
- Varon J. Treatment of acute severe hypertension: current and newer agents. Drugs. 2008;68(3): 283-297.
- Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27 suppl 3:S49-S54.
- Gupta PK, Gupta H, Khoynezhad A. Hypertensive emergency in aortic dissection and thoracic aortic aneurysm: a review of management. Pharmaceuticals. 2009;2(3):66-76.
- Post JB 4th, Frishman WH. Fenoldopam: a new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998;38(1):2-13.
- Suzuki S, Ohtsuka S, Ishikawa K, Yamaguchi I. Effects of nicardipine on coronary, vertebral and renal arterial flows in patients with essential hypertension. Hypertens Res. 2003;26(3):193-199.
- Anderson CS, Huang Y, Arima H, et al; INTERACT Investigators. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41(2):307-312.
- Broderick J, Connolly S, Feldmann E, et al. Quality of Care and Outcomes in Research Interdisciplinary Working Group Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391-413.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Brott T, Lu M, Kothari R, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29(8):1504-1509.
- Aiyagari V, Badruddin A. Management of hypertension in acute stroke. Expert Rev Cardiovasc Ther. 2009;7(6):637-646.
- Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520-526.
- Bonita R, Beaglehole R. The enigma of the decline in stroke deaths in the United States the search for an explanation. Stroke. 1996;27(3): 370-372.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655-1711.
- Heitsch L, Jauch EC. Management of hypertension in the setting of acute ischemic stroke. Curr Hypertens Rep. 2007;9(6):506-511.
- Barker FG II, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414.