User login
Brittle Diabetes: Drilling Down to the Cause
How to Evaluate Vaginal Bleeding and Discharge
Four Steps to Diagnosing Drug Overdose
Misconceptions About Opioid Dosing
Cardiac Assessment in Acute Stroke
UPDATE: MINIMALLY INVASIVE SURGERY
Cervical stenosis and difficult uterine and vaginal anatomy pose a challenge for the gynecologist who needs access to the cervix and uterus to evaluate pathology. Overcoming this hurdle requires a careful, considered approach to avoid the complications of dilation, such as laceration, creation of a false passage, uterine perforation, and failed procedures. Care and consideration also ensure a successful and comfortable procedure; save the patient a great deal of time and the higher expense of the operating room (OR); and avert the need for general anesthesia.
In this first Update on Minimally Invasive Surgery, I will:
- describe the continuing shift from the OR to office for many gynecologic procedures
- review recent data on cervical softening
- outline the components of mechanical dilation
- offer tips on pain relief.
Need for cervical access should not prohibit office-based procedures
Cervical access is critical to increase the percentage of procedures performed in the office setting. The office has long been the ideal environment for minor procedures such as endometrial biopsy, dilation and curettage, diagnostic hysteroscopy, hysterosonography, and insertion of an intrauterine device—but difficulty traversing the cervix has relegated many of these procedures to the OR.
Minor procedures such as tubal sterilization and endometrial ablation have begun to move from the outpatient environment into the office as well, upping the number of office procedures that require safe access to the endometrial cavity.
For example, hysteroscopic tubal occlusion (Essure) is performed transcervically, thereby eliminating all incisions and the need for general anesthesia. Approximately 50% of all Essure sterilization procedures performed in the United States today are done in an office, and that percentage is expected to rise to 60% in 2009.1
The smallest operative hysteroscopes that allow for placement of Essure coils have an outer-sheath diameter between 5 and 6 mm. Even with such small diameters, cervical dilation is sometimes needed.
Endometrial ablation offers women who have menorrhagia a minimally invasive option for treatment. Several FDA-approved devices are used safely in the office.2-5 Cervical dilation requirements for these devices range from 5 to 7.8 mm, making cervical access paramount (TABLE).
A number of measures, such as cervical softening and mechanical dilation, can ease dilation in an office setting so that a stenotic cervix no longer requires an OR for the procedure to be completed. Successful in-office cervical dilation also greatly reduces cost.
TABLE
Size of the instrument varies across endometrial ablation systems
| Instrument | Diameter | Instrument | Diameter |
|---|---|---|---|
| ThermaChoice (uterine balloon therapy) | 5 mm | NovaSure | 7.2 mm |
| Her Option (cryoablation therapy) | 5 mm | Hydro ThermAblator | 7.8 mm |
New data back efficacy of vaginal misoprostol for cervical softening
da Costa AR, Pinto-Neto AM, Amorim M, Paiva LH, Scavuzzi A, Schettini J. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Minim Invasive Gynecol. 2008;15:67–73.
Waddell G, Desindes S, Takser L, Bequchemin M, Bessett P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Minim Invasive Gynecol. 2008;15:739–744.
Uckuyu A, Ozcimen E, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. J Minim Invasive Gynecol. 2008;15:472–475.
Valente EP, Amorim MM, da Costa AR, de Miranda VD. Vaginal misoprostol prior to diagnostic hysteroscopy in patients of reproductive age: a randomized clinical trial. J Minim Invasive Gynecol. 2008;15:452–458.
A synthetic analog of prostaglandin E1, misoprostol is thought to act on the extracellular matrix of the cervix, leading to water absorption, neutrophil collagenase release, and cervical softening. Smooth muscle is activated by the drug, especially in the uterus.
Pharmacokinetic studies suggest that the oral route of misoprostol has the shortest interval to peak serum concentration (within 30 minutes of ingestion), but that concentration declines within 1 hour. The vaginal route, on the other hand, has fewer side effects, with longer duration and approximately three times the bioavailability.6,7 Peak values are equal to those of orally administered misoprostol. They are attained at 60 minutes, then decline slowly, reaching 50% of peak values by 240 minutes. Serum concentration remains elevated, improving efficacy.
I recommend an interval of 4 to 12 hours between vaginal placement and the start of the procedure.
Vaginal route is clearly effective in premenopausal women
In premenopausal women, several recent randomized clinical trials show that vaginal misoprostol, administered before hysteroscopy, not only decreases pain and the force and amount of dilation needed, but also reduces complications of cervical dilation.8 As Uckuyu and colleagues observe, these findings are consistent in nulliparous women who have a history of cesarean delivery and who receive 400 μg of vaginal misoprostol 6 to 12 hours before hysteroscopy.
Several studies found no improvement in ease of dilation or operative time in menopausal women who received misoprostol before hysteroscopy.9,10 However, in their randomized, placebo-controlled trial, da Costa and colleagues found that women who received 200 μg of vaginal misoprostol 8 hours before hysteroscopy had a significant decrease in intraprocedural pain associated with cervical dilation.
Recommended protocol
For women at significant risk of cervical stenosis, give 400 μg of intravaginal misoprostol approximately 12 hours before the scheduled procedure. The patient should begin round-the-clock use of a nonsteroidal anti-inflammatory drug (NSAID) an hour before insertion of the misoprostol tablets.
Side effects of vaginal and oral misoprostol include occasional diarrhea, abdominal cramping, uterine bleeding, and pyrexia. These effects are usually mild and limited.8 Concomitant administration of NSAIDs reduces or eliminates these side effects.
Although you may sometimes find an incompletely dissolved tablet within the vagina, active medication usually has been absorbed, leaving the less soluble vehicle behind.
Frequent causes of stenosis include:
- Loop electrosurgical excision procedure, conization, and laser vaporization—In one series, 43% of cases of cervical stenosis resulted from one of these procedures, with a recurrence rate of 14%.11
- Scarring of the external os—Common in the parous cervix. Usually, only minimal dilation is needed; the remainder of the cervix is traversed easily.
- Narrow or closed external os—Common in menopausal women and increasingly common in nulligravid and nulliparous women as the rate of elective cesarean delivery rises. In these cases, both the endocervical canal and internal os are narrow, necessitating dilation through the entire length of the cervix.
- Genital atrophy—In postmenopausal women, cervical stenosis as a result of genital atrophy is associated with pain upon cervical dilation. The situation generally necessitates local anesthesia.12
When mechanical dilation is necessary, a few prerequisites can make a difference
By assessing each patient carefully, the gynecologist can customize the intervention. Accordingly, it is wise to have multiple types of dilators accessible to accommodate varying clinical needs and anatomic scenarios.
Begin by stabilizing the cervix. Use of a single-toothed tenaculum, placed on the anterior lip of the cervix, has several advantages. Countertraction against the dilating instrument can facilitate more controlled placement of the dilator, preventing perforation of the uterus. This maneuver is especially useful when the cervical canal and internal os are tight or resistant.
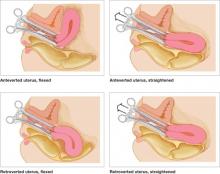
Use of a tenaculum when placing a hysteroscope produces a similar result and can add an element of safety to uterine access, especially when the uterus is significantly flexed (FIGURE 1).
FIGURE 1 Straighten a flexed uterus before dilating the cervix
When the uterus is flexed, creating acute angulation at the cervicouterine junction, it can be difficult to move the dilator through the internal os. By placing a tenaculum at the 12 o’clock position on the cervix of an anteverted uterus, or 6 o’clock on a retroverted uterus, and applying outward traction, you can straighten the distorted canal.
A local anesthetic can help
It is helpful to administer a local anesthetic before placing a penetrating instrument such as the tenaculum. Patients are usually grateful for the extra few minutes taken to ensure their comfort.
If the cervix is resistant, or the patient is uncomfortable, after initial attempts to dilate the cervix, consider placing a paracervical or intracervical stromal local anesthetic. Lidocaine 1% has a rapid onset of action, reaching peak effectiveness in just a few minutes, with a duration of approximately 60 minutes. Bupivacaine 0.25% has a slightly slower onset of action (8–10 minutes), but offers long duration—about 240 minutes.
Place 5 to 10 cc of local anesthetic paracervically at the 4 and 8 o’clock positions. The choice of anesthetic depends on the procedure. For example, a nulligravid patient may benefit from bupivacaine because cervical dilation can cause significant and prolonged cramping. Bupivacaine is more potent than lidocaine and, therefore, potentially more cardiotoxic, so caution is advised.
Used correctly, local anesthetic facilitates cervical dilation.
Types of dilators
Many instruments are available. Be familiar with the benefits and shortcomings of each to achieve successful cervical dilation in the office.
Lacrimal-duct dilators. These instruments have long been used by gynecologists for cervical access when the closed external os is no more than a tiny dimple. These ophthalmologic instruments come in diameters smaller than 1 mm and allow the closed menopausal cervix to be dilated enough to allow placement of more traditional instruments (FIGURE 2).
Traditional dilators. Tapered metal or plastic dilators facilitate access to a tightly stenotic external os (FIGURE 3). These instruments have a more symmetrical segment proximal to the tip to allow gentle, gradual dilation of the canal. However, when dilation is needed for the entire length of the cervix, as it frequently is in nulliparous women, the tapered end will not dilate the internal os unless it is passed far enough into the canal—but passing the instrument too far increases the risk of uterine perforation. In this scenario, a symmetric dilator may be a better option to achieve uniform diameter of the endocervical canal from external to internal os. Be cautious: These symmetric dilators sometimes require additional force.
When the entire length of the cervix needs to be dilated, it may be wise to use a symmetric dilator that is the same size as the tapered dilator before increasing the diameter, to reduce the risk of perforation.
FIGURE 2 A borrowed tool to unlock the external os
From the world of ophthalmology, a lacrimal-duct dilator facilitates dilation of the extremely stenotic cervix.
FIGURE 3 Tapered instruments allow gradual dilation
Tapered cervical dilators, like this disposable set, have a more symmetrical segment proximal to the tip.
When every dilator is too large
Occasionally, the external os is so completely closed that even a lacrimal-duct dilator cannot be placed. In this situation, administer 2 cc of local anesthetic in the region of the external os, then gently penetrate the os using the tip of a #11 scalpel blade. This technique generally allows quick and easy access to the endocervical canal. (Typically, the entire canal will not be stenotic and, once the external opening is created, easy dilation can be accomplished.)
Navigating a crooked cervical canal
When the cervical canal is distorted, sometimes even the most carefully selected dilator will fail.
Why?
Because the diameter of the cervix is not the obstacle.
When a straight—or even partially curved—dilator cannot traverse the tortuous path of a distorted canal, the small flexible hysteroscope may offer a solution, allowing identification of the anatomic obstruction and visualization of the course of the cervical canal. Prior visualization of the canal enhances proprioception with the dilating instrument and improves the likelihood of safe uterine access.
The use of concomitant ultrasonography may also be helpful.11
From the standpoint of a payer, obtaining surgical access to the site of a procedure is included in the procedure payment. This is generally the rule for surgical access on the day of the procedure. Some payers reimburse separately, however, when access (specifically, dilation of the cervix) is performed a day, or few days, before the procedure because of an anatomic problem—such as the cervical stenosis discussed in the main “Update” article.
What are your options?
You have several coding options available for dilation of the cervix, depending on the approach you take:
- When you’ve given oral misoprostol, bill the visit at which you prescribed the drug.
- When you’ve inserted misoprostol vaginally, report code 59200 [insertion of cervical dilator (e.g., laminaria, prostaglandin) (separate procedure)].
The fact that 59200 is found in the “Maternity Care and Delivery” chapter of the CPT does not limit its use to obstetric cases. Because this code has a zero-day global period, you are not considered to be in the postoperative period of the first procedure when the surgical procedure is performed the next day (or even longer afterward), and the two procedures will not be bundled.
Another method of cervical dilation is represented by code 57800 [Dilation of cervical canal, instrumental (separate procedure)], which also has a zero-day global period.
The diagnosis code must support the service
That’s true whichever technique you choose. In this case, the diagnosis code will be either:
- 622.4 (Stricture and stenosis of cervix) or
- 752.49 (Other anomalies of cervix, vagina, and external female genitalia)—when the stenosis is the result of a congenital condition.—
MELANIE WITT, RN, CPC, COBGC, MA
1. Essure Earnings Report Fourth Quarter 2008. Mountain View, Calif: Conceptus Inc.
2. Farrugia M, Hussain S. Hysteroscopic endometrial ablation using the Hydro ThermAblator in an outpatient hysteroscopy clinic: feasibility and acceptability. J Minim Invasive Gynecol. 2006;13:178-182.
3. Fernandez H, Capella S, Audiebert F. Uterine thermal balloon therapy under local anesthesia for the treatment of menorrhagia: a pilot study. Hum Reprod. 1997;12:2511-2514.
4. Bertrand J. Use of NovaSure endometrial ablation system in an office setting environment. J Minim Invasive Gynecol. 2005;12(5 Suppl):14.-
5. Roy K, Whiteside D, Manjon J, et al. Assessment of procedure tolerability during Her Option endometrial cryoablation in an outpatient setting. J Minim Invasive Gynecol. 2005;12(5 Suppl):87.-
6. Khan RU, El-Rafeay H, Sharma S, Sooranna D, Stafford M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103:866-869.
7. Zieman M, Fong S, Benowitz N, Bankster D, Darney P. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
8. Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstet Gynecol. 1999;94:427-430.
9. Fung TM, Lam MW, Wong SF, Ho LC. A randomized placebo-controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal women. BJOG. 2002;119:561-565.
10. Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in postmenopausal women. Hum Reprod. 2001;16:1486-1488.
11. Valle RF, Sankpal R, Marlow J, Cohen L. Cervical stenosis: a challenging clinical entity. J Gynecol Surg. 2002;18:129-143.
12. Perez-Medina T, Bajo MJ, Martinez-Cortes L, Castellanos P, Perez de Avila I. Six thousand office diagnostic-operative hysteroscopies. Int J Obstet Gynecol. 2000;71:33-38.
Cervical stenosis and difficult uterine and vaginal anatomy pose a challenge for the gynecologist who needs access to the cervix and uterus to evaluate pathology. Overcoming this hurdle requires a careful, considered approach to avoid the complications of dilation, such as laceration, creation of a false passage, uterine perforation, and failed procedures. Care and consideration also ensure a successful and comfortable procedure; save the patient a great deal of time and the higher expense of the operating room (OR); and avert the need for general anesthesia.
In this first Update on Minimally Invasive Surgery, I will:
- describe the continuing shift from the OR to office for many gynecologic procedures
- review recent data on cervical softening
- outline the components of mechanical dilation
- offer tips on pain relief.
Need for cervical access should not prohibit office-based procedures
Cervical access is critical to increase the percentage of procedures performed in the office setting. The office has long been the ideal environment for minor procedures such as endometrial biopsy, dilation and curettage, diagnostic hysteroscopy, hysterosonography, and insertion of an intrauterine device—but difficulty traversing the cervix has relegated many of these procedures to the OR.
Minor procedures such as tubal sterilization and endometrial ablation have begun to move from the outpatient environment into the office as well, upping the number of office procedures that require safe access to the endometrial cavity.
For example, hysteroscopic tubal occlusion (Essure) is performed transcervically, thereby eliminating all incisions and the need for general anesthesia. Approximately 50% of all Essure sterilization procedures performed in the United States today are done in an office, and that percentage is expected to rise to 60% in 2009.1
The smallest operative hysteroscopes that allow for placement of Essure coils have an outer-sheath diameter between 5 and 6 mm. Even with such small diameters, cervical dilation is sometimes needed.
Endometrial ablation offers women who have menorrhagia a minimally invasive option for treatment. Several FDA-approved devices are used safely in the office.2-5 Cervical dilation requirements for these devices range from 5 to 7.8 mm, making cervical access paramount (TABLE).
A number of measures, such as cervical softening and mechanical dilation, can ease dilation in an office setting so that a stenotic cervix no longer requires an OR for the procedure to be completed. Successful in-office cervical dilation also greatly reduces cost.
TABLE
Size of the instrument varies across endometrial ablation systems
| Instrument | Diameter | Instrument | Diameter |
|---|---|---|---|
| ThermaChoice (uterine balloon therapy) | 5 mm | NovaSure | 7.2 mm |
| Her Option (cryoablation therapy) | 5 mm | Hydro ThermAblator | 7.8 mm |
New data back efficacy of vaginal misoprostol for cervical softening
da Costa AR, Pinto-Neto AM, Amorim M, Paiva LH, Scavuzzi A, Schettini J. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Minim Invasive Gynecol. 2008;15:67–73.
Waddell G, Desindes S, Takser L, Bequchemin M, Bessett P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Minim Invasive Gynecol. 2008;15:739–744.
Uckuyu A, Ozcimen E, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. J Minim Invasive Gynecol. 2008;15:472–475.
Valente EP, Amorim MM, da Costa AR, de Miranda VD. Vaginal misoprostol prior to diagnostic hysteroscopy in patients of reproductive age: a randomized clinical trial. J Minim Invasive Gynecol. 2008;15:452–458.
A synthetic analog of prostaglandin E1, misoprostol is thought to act on the extracellular matrix of the cervix, leading to water absorption, neutrophil collagenase release, and cervical softening. Smooth muscle is activated by the drug, especially in the uterus.
Pharmacokinetic studies suggest that the oral route of misoprostol has the shortest interval to peak serum concentration (within 30 minutes of ingestion), but that concentration declines within 1 hour. The vaginal route, on the other hand, has fewer side effects, with longer duration and approximately three times the bioavailability.6,7 Peak values are equal to those of orally administered misoprostol. They are attained at 60 minutes, then decline slowly, reaching 50% of peak values by 240 minutes. Serum concentration remains elevated, improving efficacy.
I recommend an interval of 4 to 12 hours between vaginal placement and the start of the procedure.
Vaginal route is clearly effective in premenopausal women
In premenopausal women, several recent randomized clinical trials show that vaginal misoprostol, administered before hysteroscopy, not only decreases pain and the force and amount of dilation needed, but also reduces complications of cervical dilation.8 As Uckuyu and colleagues observe, these findings are consistent in nulliparous women who have a history of cesarean delivery and who receive 400 μg of vaginal misoprostol 6 to 12 hours before hysteroscopy.
Several studies found no improvement in ease of dilation or operative time in menopausal women who received misoprostol before hysteroscopy.9,10 However, in their randomized, placebo-controlled trial, da Costa and colleagues found that women who received 200 μg of vaginal misoprostol 8 hours before hysteroscopy had a significant decrease in intraprocedural pain associated with cervical dilation.
Recommended protocol
For women at significant risk of cervical stenosis, give 400 μg of intravaginal misoprostol approximately 12 hours before the scheduled procedure. The patient should begin round-the-clock use of a nonsteroidal anti-inflammatory drug (NSAID) an hour before insertion of the misoprostol tablets.
Side effects of vaginal and oral misoprostol include occasional diarrhea, abdominal cramping, uterine bleeding, and pyrexia. These effects are usually mild and limited.8 Concomitant administration of NSAIDs reduces or eliminates these side effects.
Although you may sometimes find an incompletely dissolved tablet within the vagina, active medication usually has been absorbed, leaving the less soluble vehicle behind.
Frequent causes of stenosis include:
- Loop electrosurgical excision procedure, conization, and laser vaporization—In one series, 43% of cases of cervical stenosis resulted from one of these procedures, with a recurrence rate of 14%.11
- Scarring of the external os—Common in the parous cervix. Usually, only minimal dilation is needed; the remainder of the cervix is traversed easily.
- Narrow or closed external os—Common in menopausal women and increasingly common in nulligravid and nulliparous women as the rate of elective cesarean delivery rises. In these cases, both the endocervical canal and internal os are narrow, necessitating dilation through the entire length of the cervix.
- Genital atrophy—In postmenopausal women, cervical stenosis as a result of genital atrophy is associated with pain upon cervical dilation. The situation generally necessitates local anesthesia.12
When mechanical dilation is necessary, a few prerequisites can make a difference
By assessing each patient carefully, the gynecologist can customize the intervention. Accordingly, it is wise to have multiple types of dilators accessible to accommodate varying clinical needs and anatomic scenarios.
Begin by stabilizing the cervix. Use of a single-toothed tenaculum, placed on the anterior lip of the cervix, has several advantages. Countertraction against the dilating instrument can facilitate more controlled placement of the dilator, preventing perforation of the uterus. This maneuver is especially useful when the cervical canal and internal os are tight or resistant.
Use of a tenaculum when placing a hysteroscope produces a similar result and can add an element of safety to uterine access, especially when the uterus is significantly flexed (FIGURE 1).
FIGURE 1 Straighten a flexed uterus before dilating the cervix
When the uterus is flexed, creating acute angulation at the cervicouterine junction, it can be difficult to move the dilator through the internal os. By placing a tenaculum at the 12 o’clock position on the cervix of an anteverted uterus, or 6 o’clock on a retroverted uterus, and applying outward traction, you can straighten the distorted canal.
A local anesthetic can help
It is helpful to administer a local anesthetic before placing a penetrating instrument such as the tenaculum. Patients are usually grateful for the extra few minutes taken to ensure their comfort.
If the cervix is resistant, or the patient is uncomfortable, after initial attempts to dilate the cervix, consider placing a paracervical or intracervical stromal local anesthetic. Lidocaine 1% has a rapid onset of action, reaching peak effectiveness in just a few minutes, with a duration of approximately 60 minutes. Bupivacaine 0.25% has a slightly slower onset of action (8–10 minutes), but offers long duration—about 240 minutes.
Place 5 to 10 cc of local anesthetic paracervically at the 4 and 8 o’clock positions. The choice of anesthetic depends on the procedure. For example, a nulligravid patient may benefit from bupivacaine because cervical dilation can cause significant and prolonged cramping. Bupivacaine is more potent than lidocaine and, therefore, potentially more cardiotoxic, so caution is advised.
Used correctly, local anesthetic facilitates cervical dilation.
Types of dilators
Many instruments are available. Be familiar with the benefits and shortcomings of each to achieve successful cervical dilation in the office.
Lacrimal-duct dilators. These instruments have long been used by gynecologists for cervical access when the closed external os is no more than a tiny dimple. These ophthalmologic instruments come in diameters smaller than 1 mm and allow the closed menopausal cervix to be dilated enough to allow placement of more traditional instruments (FIGURE 2).
Traditional dilators. Tapered metal or plastic dilators facilitate access to a tightly stenotic external os (FIGURE 3). These instruments have a more symmetrical segment proximal to the tip to allow gentle, gradual dilation of the canal. However, when dilation is needed for the entire length of the cervix, as it frequently is in nulliparous women, the tapered end will not dilate the internal os unless it is passed far enough into the canal—but passing the instrument too far increases the risk of uterine perforation. In this scenario, a symmetric dilator may be a better option to achieve uniform diameter of the endocervical canal from external to internal os. Be cautious: These symmetric dilators sometimes require additional force.
When the entire length of the cervix needs to be dilated, it may be wise to use a symmetric dilator that is the same size as the tapered dilator before increasing the diameter, to reduce the risk of perforation.
FIGURE 2 A borrowed tool to unlock the external os
From the world of ophthalmology, a lacrimal-duct dilator facilitates dilation of the extremely stenotic cervix.
FIGURE 3 Tapered instruments allow gradual dilation
Tapered cervical dilators, like this disposable set, have a more symmetrical segment proximal to the tip.
When every dilator is too large
Occasionally, the external os is so completely closed that even a lacrimal-duct dilator cannot be placed. In this situation, administer 2 cc of local anesthetic in the region of the external os, then gently penetrate the os using the tip of a #11 scalpel blade. This technique generally allows quick and easy access to the endocervical canal. (Typically, the entire canal will not be stenotic and, once the external opening is created, easy dilation can be accomplished.)
Navigating a crooked cervical canal
When the cervical canal is distorted, sometimes even the most carefully selected dilator will fail.
Why?
Because the diameter of the cervix is not the obstacle.
When a straight—or even partially curved—dilator cannot traverse the tortuous path of a distorted canal, the small flexible hysteroscope may offer a solution, allowing identification of the anatomic obstruction and visualization of the course of the cervical canal. Prior visualization of the canal enhances proprioception with the dilating instrument and improves the likelihood of safe uterine access.
The use of concomitant ultrasonography may also be helpful.11
From the standpoint of a payer, obtaining surgical access to the site of a procedure is included in the procedure payment. This is generally the rule for surgical access on the day of the procedure. Some payers reimburse separately, however, when access (specifically, dilation of the cervix) is performed a day, or few days, before the procedure because of an anatomic problem—such as the cervical stenosis discussed in the main “Update” article.
What are your options?
You have several coding options available for dilation of the cervix, depending on the approach you take:
- When you’ve given oral misoprostol, bill the visit at which you prescribed the drug.
- When you’ve inserted misoprostol vaginally, report code 59200 [insertion of cervical dilator (e.g., laminaria, prostaglandin) (separate procedure)].
The fact that 59200 is found in the “Maternity Care and Delivery” chapter of the CPT does not limit its use to obstetric cases. Because this code has a zero-day global period, you are not considered to be in the postoperative period of the first procedure when the surgical procedure is performed the next day (or even longer afterward), and the two procedures will not be bundled.
Another method of cervical dilation is represented by code 57800 [Dilation of cervical canal, instrumental (separate procedure)], which also has a zero-day global period.
The diagnosis code must support the service
That’s true whichever technique you choose. In this case, the diagnosis code will be either:
- 622.4 (Stricture and stenosis of cervix) or
- 752.49 (Other anomalies of cervix, vagina, and external female genitalia)—when the stenosis is the result of a congenital condition.—
MELANIE WITT, RN, CPC, COBGC, MA
Cervical stenosis and difficult uterine and vaginal anatomy pose a challenge for the gynecologist who needs access to the cervix and uterus to evaluate pathology. Overcoming this hurdle requires a careful, considered approach to avoid the complications of dilation, such as laceration, creation of a false passage, uterine perforation, and failed procedures. Care and consideration also ensure a successful and comfortable procedure; save the patient a great deal of time and the higher expense of the operating room (OR); and avert the need for general anesthesia.
In this first Update on Minimally Invasive Surgery, I will:
- describe the continuing shift from the OR to office for many gynecologic procedures
- review recent data on cervical softening
- outline the components of mechanical dilation
- offer tips on pain relief.
Need for cervical access should not prohibit office-based procedures
Cervical access is critical to increase the percentage of procedures performed in the office setting. The office has long been the ideal environment for minor procedures such as endometrial biopsy, dilation and curettage, diagnostic hysteroscopy, hysterosonography, and insertion of an intrauterine device—but difficulty traversing the cervix has relegated many of these procedures to the OR.
Minor procedures such as tubal sterilization and endometrial ablation have begun to move from the outpatient environment into the office as well, upping the number of office procedures that require safe access to the endometrial cavity.
For example, hysteroscopic tubal occlusion (Essure) is performed transcervically, thereby eliminating all incisions and the need for general anesthesia. Approximately 50% of all Essure sterilization procedures performed in the United States today are done in an office, and that percentage is expected to rise to 60% in 2009.1
The smallest operative hysteroscopes that allow for placement of Essure coils have an outer-sheath diameter between 5 and 6 mm. Even with such small diameters, cervical dilation is sometimes needed.
Endometrial ablation offers women who have menorrhagia a minimally invasive option for treatment. Several FDA-approved devices are used safely in the office.2-5 Cervical dilation requirements for these devices range from 5 to 7.8 mm, making cervical access paramount (TABLE).
A number of measures, such as cervical softening and mechanical dilation, can ease dilation in an office setting so that a stenotic cervix no longer requires an OR for the procedure to be completed. Successful in-office cervical dilation also greatly reduces cost.
TABLE
Size of the instrument varies across endometrial ablation systems
| Instrument | Diameter | Instrument | Diameter |
|---|---|---|---|
| ThermaChoice (uterine balloon therapy) | 5 mm | NovaSure | 7.2 mm |
| Her Option (cryoablation therapy) | 5 mm | Hydro ThermAblator | 7.8 mm |
New data back efficacy of vaginal misoprostol for cervical softening
da Costa AR, Pinto-Neto AM, Amorim M, Paiva LH, Scavuzzi A, Schettini J. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Minim Invasive Gynecol. 2008;15:67–73.
Waddell G, Desindes S, Takser L, Bequchemin M, Bessett P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Minim Invasive Gynecol. 2008;15:739–744.
Uckuyu A, Ozcimen E, Sevinc FC, Zeyneloglu HB. Efficacy of vaginal misoprostol before hysteroscopy for cervical priming in patients who have undergone cesarean section and no vaginal deliveries. J Minim Invasive Gynecol. 2008;15:472–475.
Valente EP, Amorim MM, da Costa AR, de Miranda VD. Vaginal misoprostol prior to diagnostic hysteroscopy in patients of reproductive age: a randomized clinical trial. J Minim Invasive Gynecol. 2008;15:452–458.
A synthetic analog of prostaglandin E1, misoprostol is thought to act on the extracellular matrix of the cervix, leading to water absorption, neutrophil collagenase release, and cervical softening. Smooth muscle is activated by the drug, especially in the uterus.
Pharmacokinetic studies suggest that the oral route of misoprostol has the shortest interval to peak serum concentration (within 30 minutes of ingestion), but that concentration declines within 1 hour. The vaginal route, on the other hand, has fewer side effects, with longer duration and approximately three times the bioavailability.6,7 Peak values are equal to those of orally administered misoprostol. They are attained at 60 minutes, then decline slowly, reaching 50% of peak values by 240 minutes. Serum concentration remains elevated, improving efficacy.
I recommend an interval of 4 to 12 hours between vaginal placement and the start of the procedure.
Vaginal route is clearly effective in premenopausal women
In premenopausal women, several recent randomized clinical trials show that vaginal misoprostol, administered before hysteroscopy, not only decreases pain and the force and amount of dilation needed, but also reduces complications of cervical dilation.8 As Uckuyu and colleagues observe, these findings are consistent in nulliparous women who have a history of cesarean delivery and who receive 400 μg of vaginal misoprostol 6 to 12 hours before hysteroscopy.
Several studies found no improvement in ease of dilation or operative time in menopausal women who received misoprostol before hysteroscopy.9,10 However, in their randomized, placebo-controlled trial, da Costa and colleagues found that women who received 200 μg of vaginal misoprostol 8 hours before hysteroscopy had a significant decrease in intraprocedural pain associated with cervical dilation.
Recommended protocol
For women at significant risk of cervical stenosis, give 400 μg of intravaginal misoprostol approximately 12 hours before the scheduled procedure. The patient should begin round-the-clock use of a nonsteroidal anti-inflammatory drug (NSAID) an hour before insertion of the misoprostol tablets.
Side effects of vaginal and oral misoprostol include occasional diarrhea, abdominal cramping, uterine bleeding, and pyrexia. These effects are usually mild and limited.8 Concomitant administration of NSAIDs reduces or eliminates these side effects.
Although you may sometimes find an incompletely dissolved tablet within the vagina, active medication usually has been absorbed, leaving the less soluble vehicle behind.
Frequent causes of stenosis include:
- Loop electrosurgical excision procedure, conization, and laser vaporization—In one series, 43% of cases of cervical stenosis resulted from one of these procedures, with a recurrence rate of 14%.11
- Scarring of the external os—Common in the parous cervix. Usually, only minimal dilation is needed; the remainder of the cervix is traversed easily.
- Narrow or closed external os—Common in menopausal women and increasingly common in nulligravid and nulliparous women as the rate of elective cesarean delivery rises. In these cases, both the endocervical canal and internal os are narrow, necessitating dilation through the entire length of the cervix.
- Genital atrophy—In postmenopausal women, cervical stenosis as a result of genital atrophy is associated with pain upon cervical dilation. The situation generally necessitates local anesthesia.12
When mechanical dilation is necessary, a few prerequisites can make a difference
By assessing each patient carefully, the gynecologist can customize the intervention. Accordingly, it is wise to have multiple types of dilators accessible to accommodate varying clinical needs and anatomic scenarios.
Begin by stabilizing the cervix. Use of a single-toothed tenaculum, placed on the anterior lip of the cervix, has several advantages. Countertraction against the dilating instrument can facilitate more controlled placement of the dilator, preventing perforation of the uterus. This maneuver is especially useful when the cervical canal and internal os are tight or resistant.
Use of a tenaculum when placing a hysteroscope produces a similar result and can add an element of safety to uterine access, especially when the uterus is significantly flexed (FIGURE 1).
FIGURE 1 Straighten a flexed uterus before dilating the cervix
When the uterus is flexed, creating acute angulation at the cervicouterine junction, it can be difficult to move the dilator through the internal os. By placing a tenaculum at the 12 o’clock position on the cervix of an anteverted uterus, or 6 o’clock on a retroverted uterus, and applying outward traction, you can straighten the distorted canal.
A local anesthetic can help
It is helpful to administer a local anesthetic before placing a penetrating instrument such as the tenaculum. Patients are usually grateful for the extra few minutes taken to ensure their comfort.
If the cervix is resistant, or the patient is uncomfortable, after initial attempts to dilate the cervix, consider placing a paracervical or intracervical stromal local anesthetic. Lidocaine 1% has a rapid onset of action, reaching peak effectiveness in just a few minutes, with a duration of approximately 60 minutes. Bupivacaine 0.25% has a slightly slower onset of action (8–10 minutes), but offers long duration—about 240 minutes.
Place 5 to 10 cc of local anesthetic paracervically at the 4 and 8 o’clock positions. The choice of anesthetic depends on the procedure. For example, a nulligravid patient may benefit from bupivacaine because cervical dilation can cause significant and prolonged cramping. Bupivacaine is more potent than lidocaine and, therefore, potentially more cardiotoxic, so caution is advised.
Used correctly, local anesthetic facilitates cervical dilation.
Types of dilators
Many instruments are available. Be familiar with the benefits and shortcomings of each to achieve successful cervical dilation in the office.
Lacrimal-duct dilators. These instruments have long been used by gynecologists for cervical access when the closed external os is no more than a tiny dimple. These ophthalmologic instruments come in diameters smaller than 1 mm and allow the closed menopausal cervix to be dilated enough to allow placement of more traditional instruments (FIGURE 2).
Traditional dilators. Tapered metal or plastic dilators facilitate access to a tightly stenotic external os (FIGURE 3). These instruments have a more symmetrical segment proximal to the tip to allow gentle, gradual dilation of the canal. However, when dilation is needed for the entire length of the cervix, as it frequently is in nulliparous women, the tapered end will not dilate the internal os unless it is passed far enough into the canal—but passing the instrument too far increases the risk of uterine perforation. In this scenario, a symmetric dilator may be a better option to achieve uniform diameter of the endocervical canal from external to internal os. Be cautious: These symmetric dilators sometimes require additional force.
When the entire length of the cervix needs to be dilated, it may be wise to use a symmetric dilator that is the same size as the tapered dilator before increasing the diameter, to reduce the risk of perforation.
FIGURE 2 A borrowed tool to unlock the external os
From the world of ophthalmology, a lacrimal-duct dilator facilitates dilation of the extremely stenotic cervix.
FIGURE 3 Tapered instruments allow gradual dilation
Tapered cervical dilators, like this disposable set, have a more symmetrical segment proximal to the tip.
When every dilator is too large
Occasionally, the external os is so completely closed that even a lacrimal-duct dilator cannot be placed. In this situation, administer 2 cc of local anesthetic in the region of the external os, then gently penetrate the os using the tip of a #11 scalpel blade. This technique generally allows quick and easy access to the endocervical canal. (Typically, the entire canal will not be stenotic and, once the external opening is created, easy dilation can be accomplished.)
Navigating a crooked cervical canal
When the cervical canal is distorted, sometimes even the most carefully selected dilator will fail.
Why?
Because the diameter of the cervix is not the obstacle.
When a straight—or even partially curved—dilator cannot traverse the tortuous path of a distorted canal, the small flexible hysteroscope may offer a solution, allowing identification of the anatomic obstruction and visualization of the course of the cervical canal. Prior visualization of the canal enhances proprioception with the dilating instrument and improves the likelihood of safe uterine access.
The use of concomitant ultrasonography may also be helpful.11
From the standpoint of a payer, obtaining surgical access to the site of a procedure is included in the procedure payment. This is generally the rule for surgical access on the day of the procedure. Some payers reimburse separately, however, when access (specifically, dilation of the cervix) is performed a day, or few days, before the procedure because of an anatomic problem—such as the cervical stenosis discussed in the main “Update” article.
What are your options?
You have several coding options available for dilation of the cervix, depending on the approach you take:
- When you’ve given oral misoprostol, bill the visit at which you prescribed the drug.
- When you’ve inserted misoprostol vaginally, report code 59200 [insertion of cervical dilator (e.g., laminaria, prostaglandin) (separate procedure)].
The fact that 59200 is found in the “Maternity Care and Delivery” chapter of the CPT does not limit its use to obstetric cases. Because this code has a zero-day global period, you are not considered to be in the postoperative period of the first procedure when the surgical procedure is performed the next day (or even longer afterward), and the two procedures will not be bundled.
Another method of cervical dilation is represented by code 57800 [Dilation of cervical canal, instrumental (separate procedure)], which also has a zero-day global period.
The diagnosis code must support the service
That’s true whichever technique you choose. In this case, the diagnosis code will be either:
- 622.4 (Stricture and stenosis of cervix) or
- 752.49 (Other anomalies of cervix, vagina, and external female genitalia)—when the stenosis is the result of a congenital condition.—
MELANIE WITT, RN, CPC, COBGC, MA
1. Essure Earnings Report Fourth Quarter 2008. Mountain View, Calif: Conceptus Inc.
2. Farrugia M, Hussain S. Hysteroscopic endometrial ablation using the Hydro ThermAblator in an outpatient hysteroscopy clinic: feasibility and acceptability. J Minim Invasive Gynecol. 2006;13:178-182.
3. Fernandez H, Capella S, Audiebert F. Uterine thermal balloon therapy under local anesthesia for the treatment of menorrhagia: a pilot study. Hum Reprod. 1997;12:2511-2514.
4. Bertrand J. Use of NovaSure endometrial ablation system in an office setting environment. J Minim Invasive Gynecol. 2005;12(5 Suppl):14.-
5. Roy K, Whiteside D, Manjon J, et al. Assessment of procedure tolerability during Her Option endometrial cryoablation in an outpatient setting. J Minim Invasive Gynecol. 2005;12(5 Suppl):87.-
6. Khan RU, El-Rafeay H, Sharma S, Sooranna D, Stafford M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103:866-869.
7. Zieman M, Fong S, Benowitz N, Bankster D, Darney P. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
8. Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstet Gynecol. 1999;94:427-430.
9. Fung TM, Lam MW, Wong SF, Ho LC. A randomized placebo-controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal women. BJOG. 2002;119:561-565.
10. Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in postmenopausal women. Hum Reprod. 2001;16:1486-1488.
11. Valle RF, Sankpal R, Marlow J, Cohen L. Cervical stenosis: a challenging clinical entity. J Gynecol Surg. 2002;18:129-143.
12. Perez-Medina T, Bajo MJ, Martinez-Cortes L, Castellanos P, Perez de Avila I. Six thousand office diagnostic-operative hysteroscopies. Int J Obstet Gynecol. 2000;71:33-38.
1. Essure Earnings Report Fourth Quarter 2008. Mountain View, Calif: Conceptus Inc.
2. Farrugia M, Hussain S. Hysteroscopic endometrial ablation using the Hydro ThermAblator in an outpatient hysteroscopy clinic: feasibility and acceptability. J Minim Invasive Gynecol. 2006;13:178-182.
3. Fernandez H, Capella S, Audiebert F. Uterine thermal balloon therapy under local anesthesia for the treatment of menorrhagia: a pilot study. Hum Reprod. 1997;12:2511-2514.
4. Bertrand J. Use of NovaSure endometrial ablation system in an office setting environment. J Minim Invasive Gynecol. 2005;12(5 Suppl):14.-
5. Roy K, Whiteside D, Manjon J, et al. Assessment of procedure tolerability during Her Option endometrial cryoablation in an outpatient setting. J Minim Invasive Gynecol. 2005;12(5 Suppl):87.-
6. Khan RU, El-Rafeay H, Sharma S, Sooranna D, Stafford M. Oral, rectal, and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103:866-869.
7. Zieman M, Fong S, Benowitz N, Bankster D, Darney P. Absorption kinetics of misoprostol with oral or vaginal administration. Obstet Gynecol. 1997;90:88-92.
8. Preutthipan S, Herabutya Y. A randomized controlled trial of vaginal misoprostol for cervical priming before hysteroscopy. Obstet Gynecol. 1999;94:427-430.
9. Fung TM, Lam MW, Wong SF, Ho LC. A randomized placebo-controlled trial of vaginal misoprostol for cervical priming before hysteroscopy in postmenopausal women. BJOG. 2002;119:561-565.
10. Ngai SW, Chan YM, Ho PC. The use of misoprostol prior to hysteroscopy in postmenopausal women. Hum Reprod. 2001;16:1486-1488.
11. Valle RF, Sankpal R, Marlow J, Cohen L. Cervical stenosis: a challenging clinical entity. J Gynecol Surg. 2002;18:129-143.
12. Perez-Medina T, Bajo MJ, Martinez-Cortes L, Castellanos P, Perez de Avila I. Six thousand office diagnostic-operative hysteroscopies. Int J Obstet Gynecol. 2000;71:33-38.
Best practices for call—to make for a sustainable career
The authors report no financial relationships relevant to this article.
Call is a fact of life for most obstetricians; there’s no alternative to having obstetric care available 24 hours a day, 7 days a week. Although we recognize call as part of the job we’ve accepted, many of us have a love–hate relationship with the call schedule.
One of the most fulfilling experiences in our career is following a patient through her pregnancy and then safely placing a baby in her arms. And call is the time during which many of us earn a significant part of our income. But it is also a time when we can never fully relax—particularly as we become more aware of the potential safety issues and medicolegal concerns inherent in traditional call practices.
We studied the matter with the goal of making call more palatable
In 2004 and 2005, we surveyed 66 obstetricians, attempting to talk to one person from every large or medium-sized group practice in the state of Wisconsin.
Our aim? To identify patterns in call practice that might be beneficial to our groups and other obstetricians.
Some of our findings were published in the American Journal of Obstetrics and Gynecology.1 We have since formulated suggestions for groups to consider when they design or modify their call practices.
Those suggestions form the bulk of this article. Please read on—you may find that they apply to your work.
A shortage of physicians?
Residencies in obstetrics and gynecology are increasingly hard to fill. The medical malpractice climate is often cited as a major reason, but studies demonstrate that “lifestyle” is as much or more of a concern for medical students who are deciding on a specialty.2-4
At the other end of the career trajectory, obstetricians are retiring from the specialty earlier than in the past, and research shows that obstetric call is one of the most important variables driving retirement.5 The combined effect of these two realities will likely challenge our ability to maintain sufficient numbers of obstetricians.
Although the restriction of resident work hours has drawn attention of late, the work-life demands of practicing obstetricians have been largely ignored. (For an exception, see “The unbearable unhappiness of the ObGyn: A crisis looms,” by Louis Weinstein, MD, in the December 2008 issue of OBG Management at www.obgmanagement.com.)
We found significant differences between residents’ call and the typical private-practice call (TABLE).
TABLE
Residency versus private practice: Which call pattern is more onerous?
| Residency | Private practice |
|---|---|
| More intense | Less intense |
| Focused (often on only one area) | Multiple responsibilities and sometimes multiple hospitals |
| More likely to go without sleep | Less likely to go without sleep |
| Shorter duration | Longer duration |
Dangers of call
Although 56% of respondents to our survey indicated that they go without sleep for 24 hours most or some of the time, only 13% reported being concerned that fatigue limits their ability to safely deliver care.1 This finding runs contrary to many studies that demonstrate that prolonged periods of wakefulness are associated with a high risk of error and potential compromise of patient safety.
The need to be in several places at once
“A bigger concern than fatigue is the risk inherent in handling multiple simultaneous responsibilities”Perhaps a bigger concern than fatigue—and largely unexplored in scientific study—is the risk inherent in handling multiple simultaneous responsibilities. It is not uncommon for a doctor to be seeing one patient in the clinic while another patient is being prepped in the operating room and a third patient is in labor.
“I can be two places at the same time on a good day with a tailwind, but never three,” one OB joked.
Even when the OB’s activity is limited to the labor and delivery unit, it is not unusual for two patients to be delivering at once, sometimes in different hospitals.
In our study, 26% of obstetricians delivered in more than one hospital, with the maximum being five hospitals.1 One OB proudly described having five patients in five different hospitals and being fortunate enough to deliver them all.
Is it possible, or wise, to attempt to please every patient?
It can sometimes be difficult to balance patient satisfaction and patient safety. Most women in labor prefer to have their own doctor provide their care. At one time, they seemed to have accepted the fact that the physician might be late for an office visit because of a simultaneous delivery.6 Now, however, they seem less accepting of even this inconvenience.
There is no “standard” call pattern
Overall, we found no standard pattern of call. Each system seems to have evolved, or been designed, to meet the needs required to provide care.
Our perception is that call arrangements must balance two main concerns: safety and sustainability. Someone must be available and able to function, but the call pattern cannot be so onerous that the doctors sharing it find it unlivable. Each group of obstetricians who provide care needs to identify rules that ensure safety—but also care that can be delivered over years of a career.
Best practices
We have several suggestions for best practices, though we recognize that some of them may not be practical for every practice. However, we believe that these generalizations may be useful to a broad range of obstetric call groups.
Deliver in one hospital only
The obstetricians we surveyed who were delivering at multiple hospitals indicated that the decision to do so was patient-driven; many physicians were dissatisfied with this practice.
Groups that had restricted themselves to one hospital felt that this decision had made their call easier and more sustainable.
Develop a formal backup policy
Many survey respondents indicated that, even without a formal policy, they can call a partner or other obstetrician in the community when the volume of work becomes too much to handle. We found that there is a true brotherhood and sisterhood of obstetricians who will drop everything to help when called upon.
Certainly, the volume of deliveries and other responsibilities will determine how frequently you need to call your backup. Unless a formal backup system is in place, however, there is no certainty that you will be able to reach another obstetrician and that he or she will be able to help. When you need assistance, it’s a terrible distraction to spend 30 minutes going down the list of your partners, trying to figure out who is in town and who isn’t. What if you call one of your partners late Saturday night and find him or her to be in no condition to perform?
If your call is busy enough, make sure a designated backup is carrying a beeper and understands his or her call responsibilities.
Restrict your responsibilities while on call
In a large practice, where it is not unusual for at least one or two patients to be in labor at any given time, consider assigning the call person solely to labor and delivery to ensure adequate availability for emergencies.
The American College of Obstetricians and Gynecologists recommends that a provider be “immediately available” when a patient is attempting vaginal birth after cesarean or when oxytocin is being utilized.7 Although the definition of “immediately available” is not codified, it probably means that the obstetrician should not be doing a major surgical procedure or seeing a full schedule of patients in an office 10 miles from the hospital.
Leaders in one large hospital chain have defined being immediately available as being available within 5 minutes. 8
Restrict responsibilities after being on call
This recommendation, too, is volume-driven. If it is likely that you will get little or no sleep during call, the next day’s activities should not include a difficult hysterectomy for severe endometriosis or endometrial cancer. If you must schedule these cases, do so with the patient’s understanding that last-minute rescheduling may be needed.
Even seeing a full slate of clinic patients may be challenging and could have a negative impact on patient satisfaction if you do not sleep the night before. Keep your next day short, and concentrate on activities that require limited mental and physical attention.
Align reimbursement systems
It became apparent, during our discussions with obstetricians in our survey, that financial incentives were aligned in ways that could potentially cause the physicians to overextend themselves. Although none of our respondents expressed concern about this fact from a safety standpoint, it was clear that people may sometimes work when they shouldn’t because of their desire to capture the charges for the care given.
A Canadian study reported a significant drop in elective inductions, as well as increased mean duration of labor, after implementing an income-pooling remuneration system. 9
Take call intelligently
Don’t begin call with a sleep deficit. Make sure you get a good night’s rest the night before. If possible, learn how to take “combat naps.” Even 20-minute naps can be helpful.
In our survey, all respondents indicated that their hospital had dedicated call quarters. Some institutions even provided meals and exercise facilities. See “How to combat fatigue (and win) during call”
Here are more ways to improve call
Depending on the size of your call pool and volume of deliveries, you might consider the following options to improve your call system.
A few simple measures can boost mental and physical alertness during extended duty.
Physical activity—This is the best strategy to counter fatigue. Stretch often, and walk around. Bright lights help.
Talk—Active participation in a conversation helps keep you focused; passive listening does not.
Drink caffeinated beverages—This calls for moderation, of course. Caffeine isn’t, and shouldn’t be, a cure-all.
Eat well and keep hydrated—A healthy diet and lots of uncaffeinated fluids keep your body running smoothly.
Take short naps—Even 20 minutes can help.
Support your colleagues—Cover another physician long enough for him or her to take a nap, and then take your turn.
Call for help—Call in backup if you are faced with a difficult situation and sense symptoms of serious fatigue in yourself. Also, watch for those symptoms in your colleagues.
Take a shower—A change of clothes helps, too.
Source: Adapted from “Fatigue countermeasures: alertness management in flight operations.” Available at http://humanfactors.arc.nasa.gov/zteam. Accessed March 12, 2009.
If you increase the number of obstetricians in your call pool, the number of calls you take may diminish. However, the volume of activity will probably increase as a result, so that you have a greater chance of being busy while on call. Hiring a nurse-midwife may decrease the number of uncomplicated vaginal deliveries you perform, but you still need to be prepared to provide backup.
Shorten the call duration
The most common duration of call in our study was 24 hours. However, some call pools take call for a weekend or week at a time.1 This gives the physician a longer interval between calls, but the unpredictability of the patient load may make this a horrendously long period of time.
Another potential disadvantage of a shortened call, especially when it is abbreviated to less than 24 hours, is that it increases the number of handoffs in patient care and, therefore, enhances the risk that the circumstances of any given patient will be incompletely understood at this time.
In a busy OB practice, handoffs usually involve a meeting of obstetricians in labor and delivery to “run the board.” When participants attempt to make these handoffs as complete as possible, patient safety is significantly improved.
One way to ensure completeness of patient handoffs is to borrow training and skills from the world of airline pilots. There, crew resource management has introduced the concept of SBAR [Situation-Background-Assessment-Recommendation] as a specific tool to decrease risk inherent in handoffs.
Another helpful idea is attending nurses’ report sessions. These reports can provide you with useful information that you may not have recognized otherwise. By giving them your attention, you may also strengthen relationships with the nurses, your first line of defense.
Develop in-house call
Dildy and colleagues estimated that a call volume of approximately 2,400 deliveries a year would justify a hospital developing 24-hour in-house obstetric coverage.10 In our study, almost all of the hospitals that required in-house call did so because they had residencies, and in-house staff call was required.
Although Clark and colleagues found 24-hour in-house call to be safer than regular call in their review of closed perinatal claims at one large hospital chain, there has been a paucity of studies that confirm or extend our knowledge in this area.11
Hire laborists
Weinstein introduced the word “laborist” into our lexicon in a 2003 paper.12 Barbieri suggested the addition of “nocturnalist” or “weekendist” as possible terms to describe specialists assigned to work certain shifts that prove particularly onerous to practicing obstetricians.13 A number of hospitals and groups are examining and developing this call model.14 However, little research has been conducted on its effects on patient safety and obstetric practice.
For more information, visit www.oblaborist.org and http://obgynhospitalist.com.
Balance—that is the goal
For some of us, perfect balance between safety and patient satisfaction, and between work and home life, may be impossible. Nevertheless, we all need to explore ways to make call a safe and sustainable practice. For many of us, the growing recognition that we have more beautiful, sunny Sunday afternoons behind us than in front of us may be the signal to shift our focus to less demanding, and time-depleting, call schedules.
1. Schauberger CW, Gribble RK, Rooney BL. On call: a survey of Wisconsin obstetric groups. Am J Obstet Gynecol. 2007;196:39.e1-39.e4.
2. Dorsey ER, Jarjoura D, Rutecki GW. Influence of controllable lifestyle on recent trends in specialty choice by US medical students. JAMA. 2003;290:1173-1178.
3. Gariti DL, Zollinger TW, Look KY. Factors detracting students from applying for an obstetrics and gynecology residency. Am J Obstet Gynecol. 2005;193:289-293.
4. Deutsch A, McCarthy J, Murray K, Sayer R. Why are fewer medical students in Florida choosing obstetrics and gynecology? South Med J. 2007;100:1095-1098.
5. Bettes BA, Chalas E, Coleman VH, Schulkin J. Heavier workload, less personal control: impact of delivery on obstetrician/gynecologists’ career satisfaction. Am J Obstet Gynecol. 2004;190:851-857.
6. Pradhan A, Raman A, Lim C, Yoon E, Ananth C. The patient perspective. Is the hospitalist model a viable option for obstetric care [abstract]. Obstet Gynecol. 2008;111:40S-41S.
7. American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 6th ed. Elk Grove, Ill: American Academy of Pediatrics; 2007:157.
8. Clark SL, Belfort MA, Byrum SL, Meyers JA, Perlin JB. Improved outcomes, fewer cesarean deliveries, and reduced litigation: results of a new paradigm in patient safety. Am J Obstet Gynecol. 2008;199:105.e1-105.e7.
9. Bland ES, Oppenheimer LW, Holmes P, Wen SW. The effect of income pooling within a call group on rates of obstetric intervention. CMAJ. 2001;164:337-339.
10. Dildy GA, Clark SL, Belfort MA, Herbst MA, Thompson J. Twenty-four hour, in-house obstetric coverage in a community-based tertiary care hospital [abstract]. Obstet Gynecol. 2007;109:43S.-
11. Clark SL, Belfort MA, Dildy GA. Reducing obstetric litigation through alterations in practice patterns—experience with 189 closed claims. Am J Obstet Gynecol. 2006;195:S118.-
12. Weinstein L. The laborist: a new focus of practice for the obstetrician. Am J Obstet Gynecol. 2003;188:310-312.
13. Barbieri RL. Will the “ists” preserve the rewards of OB practice? OBG Management. 2007;199(9):8-12.
14. Yates J. The laborists are here, but can they thrive in US hospitals? OBG Management. 2008;20(8):26-34.
The authors report no financial relationships relevant to this article.
Call is a fact of life for most obstetricians; there’s no alternative to having obstetric care available 24 hours a day, 7 days a week. Although we recognize call as part of the job we’ve accepted, many of us have a love–hate relationship with the call schedule.
One of the most fulfilling experiences in our career is following a patient through her pregnancy and then safely placing a baby in her arms. And call is the time during which many of us earn a significant part of our income. But it is also a time when we can never fully relax—particularly as we become more aware of the potential safety issues and medicolegal concerns inherent in traditional call practices.
We studied the matter with the goal of making call more palatable
In 2004 and 2005, we surveyed 66 obstetricians, attempting to talk to one person from every large or medium-sized group practice in the state of Wisconsin.
Our aim? To identify patterns in call practice that might be beneficial to our groups and other obstetricians.
Some of our findings were published in the American Journal of Obstetrics and Gynecology.1 We have since formulated suggestions for groups to consider when they design or modify their call practices.
Those suggestions form the bulk of this article. Please read on—you may find that they apply to your work.
A shortage of physicians?
Residencies in obstetrics and gynecology are increasingly hard to fill. The medical malpractice climate is often cited as a major reason, but studies demonstrate that “lifestyle” is as much or more of a concern for medical students who are deciding on a specialty.2-4
At the other end of the career trajectory, obstetricians are retiring from the specialty earlier than in the past, and research shows that obstetric call is one of the most important variables driving retirement.5 The combined effect of these two realities will likely challenge our ability to maintain sufficient numbers of obstetricians.
Although the restriction of resident work hours has drawn attention of late, the work-life demands of practicing obstetricians have been largely ignored. (For an exception, see “The unbearable unhappiness of the ObGyn: A crisis looms,” by Louis Weinstein, MD, in the December 2008 issue of OBG Management at www.obgmanagement.com.)
We found significant differences between residents’ call and the typical private-practice call (TABLE).
TABLE
Residency versus private practice: Which call pattern is more onerous?
| Residency | Private practice |
|---|---|
| More intense | Less intense |
| Focused (often on only one area) | Multiple responsibilities and sometimes multiple hospitals |
| More likely to go without sleep | Less likely to go without sleep |
| Shorter duration | Longer duration |
Dangers of call
Although 56% of respondents to our survey indicated that they go without sleep for 24 hours most or some of the time, only 13% reported being concerned that fatigue limits their ability to safely deliver care.1 This finding runs contrary to many studies that demonstrate that prolonged periods of wakefulness are associated with a high risk of error and potential compromise of patient safety.
The need to be in several places at once
“A bigger concern than fatigue is the risk inherent in handling multiple simultaneous responsibilities”Perhaps a bigger concern than fatigue—and largely unexplored in scientific study—is the risk inherent in handling multiple simultaneous responsibilities. It is not uncommon for a doctor to be seeing one patient in the clinic while another patient is being prepped in the operating room and a third patient is in labor.
“I can be two places at the same time on a good day with a tailwind, but never three,” one OB joked.
Even when the OB’s activity is limited to the labor and delivery unit, it is not unusual for two patients to be delivering at once, sometimes in different hospitals.
In our study, 26% of obstetricians delivered in more than one hospital, with the maximum being five hospitals.1 One OB proudly described having five patients in five different hospitals and being fortunate enough to deliver them all.
Is it possible, or wise, to attempt to please every patient?
It can sometimes be difficult to balance patient satisfaction and patient safety. Most women in labor prefer to have their own doctor provide their care. At one time, they seemed to have accepted the fact that the physician might be late for an office visit because of a simultaneous delivery.6 Now, however, they seem less accepting of even this inconvenience.
There is no “standard” call pattern
Overall, we found no standard pattern of call. Each system seems to have evolved, or been designed, to meet the needs required to provide care.
Our perception is that call arrangements must balance two main concerns: safety and sustainability. Someone must be available and able to function, but the call pattern cannot be so onerous that the doctors sharing it find it unlivable. Each group of obstetricians who provide care needs to identify rules that ensure safety—but also care that can be delivered over years of a career.
Best practices
We have several suggestions for best practices, though we recognize that some of them may not be practical for every practice. However, we believe that these generalizations may be useful to a broad range of obstetric call groups.
Deliver in one hospital only
The obstetricians we surveyed who were delivering at multiple hospitals indicated that the decision to do so was patient-driven; many physicians were dissatisfied with this practice.
Groups that had restricted themselves to one hospital felt that this decision had made their call easier and more sustainable.
Develop a formal backup policy
Many survey respondents indicated that, even without a formal policy, they can call a partner or other obstetrician in the community when the volume of work becomes too much to handle. We found that there is a true brotherhood and sisterhood of obstetricians who will drop everything to help when called upon.
Certainly, the volume of deliveries and other responsibilities will determine how frequently you need to call your backup. Unless a formal backup system is in place, however, there is no certainty that you will be able to reach another obstetrician and that he or she will be able to help. When you need assistance, it’s a terrible distraction to spend 30 minutes going down the list of your partners, trying to figure out who is in town and who isn’t. What if you call one of your partners late Saturday night and find him or her to be in no condition to perform?
If your call is busy enough, make sure a designated backup is carrying a beeper and understands his or her call responsibilities.
Restrict your responsibilities while on call
In a large practice, where it is not unusual for at least one or two patients to be in labor at any given time, consider assigning the call person solely to labor and delivery to ensure adequate availability for emergencies.
The American College of Obstetricians and Gynecologists recommends that a provider be “immediately available” when a patient is attempting vaginal birth after cesarean or when oxytocin is being utilized.7 Although the definition of “immediately available” is not codified, it probably means that the obstetrician should not be doing a major surgical procedure or seeing a full schedule of patients in an office 10 miles from the hospital.
Leaders in one large hospital chain have defined being immediately available as being available within 5 minutes. 8
Restrict responsibilities after being on call
This recommendation, too, is volume-driven. If it is likely that you will get little or no sleep during call, the next day’s activities should not include a difficult hysterectomy for severe endometriosis or endometrial cancer. If you must schedule these cases, do so with the patient’s understanding that last-minute rescheduling may be needed.
Even seeing a full slate of clinic patients may be challenging and could have a negative impact on patient satisfaction if you do not sleep the night before. Keep your next day short, and concentrate on activities that require limited mental and physical attention.
Align reimbursement systems
It became apparent, during our discussions with obstetricians in our survey, that financial incentives were aligned in ways that could potentially cause the physicians to overextend themselves. Although none of our respondents expressed concern about this fact from a safety standpoint, it was clear that people may sometimes work when they shouldn’t because of their desire to capture the charges for the care given.
A Canadian study reported a significant drop in elective inductions, as well as increased mean duration of labor, after implementing an income-pooling remuneration system. 9
Take call intelligently
Don’t begin call with a sleep deficit. Make sure you get a good night’s rest the night before. If possible, learn how to take “combat naps.” Even 20-minute naps can be helpful.
In our survey, all respondents indicated that their hospital had dedicated call quarters. Some institutions even provided meals and exercise facilities. See “How to combat fatigue (and win) during call”
Here are more ways to improve call
Depending on the size of your call pool and volume of deliveries, you might consider the following options to improve your call system.
A few simple measures can boost mental and physical alertness during extended duty.
Physical activity—This is the best strategy to counter fatigue. Stretch often, and walk around. Bright lights help.
Talk—Active participation in a conversation helps keep you focused; passive listening does not.
Drink caffeinated beverages—This calls for moderation, of course. Caffeine isn’t, and shouldn’t be, a cure-all.
Eat well and keep hydrated—A healthy diet and lots of uncaffeinated fluids keep your body running smoothly.
Take short naps—Even 20 minutes can help.
Support your colleagues—Cover another physician long enough for him or her to take a nap, and then take your turn.
Call for help—Call in backup if you are faced with a difficult situation and sense symptoms of serious fatigue in yourself. Also, watch for those symptoms in your colleagues.
Take a shower—A change of clothes helps, too.
Source: Adapted from “Fatigue countermeasures: alertness management in flight operations.” Available at http://humanfactors.arc.nasa.gov/zteam. Accessed March 12, 2009.
If you increase the number of obstetricians in your call pool, the number of calls you take may diminish. However, the volume of activity will probably increase as a result, so that you have a greater chance of being busy while on call. Hiring a nurse-midwife may decrease the number of uncomplicated vaginal deliveries you perform, but you still need to be prepared to provide backup.
Shorten the call duration
The most common duration of call in our study was 24 hours. However, some call pools take call for a weekend or week at a time.1 This gives the physician a longer interval between calls, but the unpredictability of the patient load may make this a horrendously long period of time.
Another potential disadvantage of a shortened call, especially when it is abbreviated to less than 24 hours, is that it increases the number of handoffs in patient care and, therefore, enhances the risk that the circumstances of any given patient will be incompletely understood at this time.
In a busy OB practice, handoffs usually involve a meeting of obstetricians in labor and delivery to “run the board.” When participants attempt to make these handoffs as complete as possible, patient safety is significantly improved.
One way to ensure completeness of patient handoffs is to borrow training and skills from the world of airline pilots. There, crew resource management has introduced the concept of SBAR [Situation-Background-Assessment-Recommendation] as a specific tool to decrease risk inherent in handoffs.
Another helpful idea is attending nurses’ report sessions. These reports can provide you with useful information that you may not have recognized otherwise. By giving them your attention, you may also strengthen relationships with the nurses, your first line of defense.
Develop in-house call
Dildy and colleagues estimated that a call volume of approximately 2,400 deliveries a year would justify a hospital developing 24-hour in-house obstetric coverage.10 In our study, almost all of the hospitals that required in-house call did so because they had residencies, and in-house staff call was required.
Although Clark and colleagues found 24-hour in-house call to be safer than regular call in their review of closed perinatal claims at one large hospital chain, there has been a paucity of studies that confirm or extend our knowledge in this area.11
Hire laborists
Weinstein introduced the word “laborist” into our lexicon in a 2003 paper.12 Barbieri suggested the addition of “nocturnalist” or “weekendist” as possible terms to describe specialists assigned to work certain shifts that prove particularly onerous to practicing obstetricians.13 A number of hospitals and groups are examining and developing this call model.14 However, little research has been conducted on its effects on patient safety and obstetric practice.
For more information, visit www.oblaborist.org and http://obgynhospitalist.com.
Balance—that is the goal
For some of us, perfect balance between safety and patient satisfaction, and between work and home life, may be impossible. Nevertheless, we all need to explore ways to make call a safe and sustainable practice. For many of us, the growing recognition that we have more beautiful, sunny Sunday afternoons behind us than in front of us may be the signal to shift our focus to less demanding, and time-depleting, call schedules.
The authors report no financial relationships relevant to this article.
Call is a fact of life for most obstetricians; there’s no alternative to having obstetric care available 24 hours a day, 7 days a week. Although we recognize call as part of the job we’ve accepted, many of us have a love–hate relationship with the call schedule.
One of the most fulfilling experiences in our career is following a patient through her pregnancy and then safely placing a baby in her arms. And call is the time during which many of us earn a significant part of our income. But it is also a time when we can never fully relax—particularly as we become more aware of the potential safety issues and medicolegal concerns inherent in traditional call practices.
We studied the matter with the goal of making call more palatable
In 2004 and 2005, we surveyed 66 obstetricians, attempting to talk to one person from every large or medium-sized group practice in the state of Wisconsin.
Our aim? To identify patterns in call practice that might be beneficial to our groups and other obstetricians.
Some of our findings were published in the American Journal of Obstetrics and Gynecology.1 We have since formulated suggestions for groups to consider when they design or modify their call practices.
Those suggestions form the bulk of this article. Please read on—you may find that they apply to your work.
A shortage of physicians?
Residencies in obstetrics and gynecology are increasingly hard to fill. The medical malpractice climate is often cited as a major reason, but studies demonstrate that “lifestyle” is as much or more of a concern for medical students who are deciding on a specialty.2-4
At the other end of the career trajectory, obstetricians are retiring from the specialty earlier than in the past, and research shows that obstetric call is one of the most important variables driving retirement.5 The combined effect of these two realities will likely challenge our ability to maintain sufficient numbers of obstetricians.
Although the restriction of resident work hours has drawn attention of late, the work-life demands of practicing obstetricians have been largely ignored. (For an exception, see “The unbearable unhappiness of the ObGyn: A crisis looms,” by Louis Weinstein, MD, in the December 2008 issue of OBG Management at www.obgmanagement.com.)
We found significant differences between residents’ call and the typical private-practice call (TABLE).
TABLE
Residency versus private practice: Which call pattern is more onerous?
| Residency | Private practice |
|---|---|
| More intense | Less intense |
| Focused (often on only one area) | Multiple responsibilities and sometimes multiple hospitals |
| More likely to go without sleep | Less likely to go without sleep |
| Shorter duration | Longer duration |
Dangers of call
Although 56% of respondents to our survey indicated that they go without sleep for 24 hours most or some of the time, only 13% reported being concerned that fatigue limits their ability to safely deliver care.1 This finding runs contrary to many studies that demonstrate that prolonged periods of wakefulness are associated with a high risk of error and potential compromise of patient safety.
The need to be in several places at once
“A bigger concern than fatigue is the risk inherent in handling multiple simultaneous responsibilities”Perhaps a bigger concern than fatigue—and largely unexplored in scientific study—is the risk inherent in handling multiple simultaneous responsibilities. It is not uncommon for a doctor to be seeing one patient in the clinic while another patient is being prepped in the operating room and a third patient is in labor.
“I can be two places at the same time on a good day with a tailwind, but never three,” one OB joked.
Even when the OB’s activity is limited to the labor and delivery unit, it is not unusual for two patients to be delivering at once, sometimes in different hospitals.
In our study, 26% of obstetricians delivered in more than one hospital, with the maximum being five hospitals.1 One OB proudly described having five patients in five different hospitals and being fortunate enough to deliver them all.
Is it possible, or wise, to attempt to please every patient?
It can sometimes be difficult to balance patient satisfaction and patient safety. Most women in labor prefer to have their own doctor provide their care. At one time, they seemed to have accepted the fact that the physician might be late for an office visit because of a simultaneous delivery.6 Now, however, they seem less accepting of even this inconvenience.
There is no “standard” call pattern
Overall, we found no standard pattern of call. Each system seems to have evolved, or been designed, to meet the needs required to provide care.
Our perception is that call arrangements must balance two main concerns: safety and sustainability. Someone must be available and able to function, but the call pattern cannot be so onerous that the doctors sharing it find it unlivable. Each group of obstetricians who provide care needs to identify rules that ensure safety—but also care that can be delivered over years of a career.
Best practices
We have several suggestions for best practices, though we recognize that some of them may not be practical for every practice. However, we believe that these generalizations may be useful to a broad range of obstetric call groups.
Deliver in one hospital only
The obstetricians we surveyed who were delivering at multiple hospitals indicated that the decision to do so was patient-driven; many physicians were dissatisfied with this practice.
Groups that had restricted themselves to one hospital felt that this decision had made their call easier and more sustainable.
Develop a formal backup policy
Many survey respondents indicated that, even without a formal policy, they can call a partner or other obstetrician in the community when the volume of work becomes too much to handle. We found that there is a true brotherhood and sisterhood of obstetricians who will drop everything to help when called upon.
Certainly, the volume of deliveries and other responsibilities will determine how frequently you need to call your backup. Unless a formal backup system is in place, however, there is no certainty that you will be able to reach another obstetrician and that he or she will be able to help. When you need assistance, it’s a terrible distraction to spend 30 minutes going down the list of your partners, trying to figure out who is in town and who isn’t. What if you call one of your partners late Saturday night and find him or her to be in no condition to perform?
If your call is busy enough, make sure a designated backup is carrying a beeper and understands his or her call responsibilities.
Restrict your responsibilities while on call
In a large practice, where it is not unusual for at least one or two patients to be in labor at any given time, consider assigning the call person solely to labor and delivery to ensure adequate availability for emergencies.
The American College of Obstetricians and Gynecologists recommends that a provider be “immediately available” when a patient is attempting vaginal birth after cesarean or when oxytocin is being utilized.7 Although the definition of “immediately available” is not codified, it probably means that the obstetrician should not be doing a major surgical procedure or seeing a full schedule of patients in an office 10 miles from the hospital.
Leaders in one large hospital chain have defined being immediately available as being available within 5 minutes. 8
Restrict responsibilities after being on call
This recommendation, too, is volume-driven. If it is likely that you will get little or no sleep during call, the next day’s activities should not include a difficult hysterectomy for severe endometriosis or endometrial cancer. If you must schedule these cases, do so with the patient’s understanding that last-minute rescheduling may be needed.
Even seeing a full slate of clinic patients may be challenging and could have a negative impact on patient satisfaction if you do not sleep the night before. Keep your next day short, and concentrate on activities that require limited mental and physical attention.
Align reimbursement systems
It became apparent, during our discussions with obstetricians in our survey, that financial incentives were aligned in ways that could potentially cause the physicians to overextend themselves. Although none of our respondents expressed concern about this fact from a safety standpoint, it was clear that people may sometimes work when they shouldn’t because of their desire to capture the charges for the care given.
A Canadian study reported a significant drop in elective inductions, as well as increased mean duration of labor, after implementing an income-pooling remuneration system. 9
Take call intelligently
Don’t begin call with a sleep deficit. Make sure you get a good night’s rest the night before. If possible, learn how to take “combat naps.” Even 20-minute naps can be helpful.
In our survey, all respondents indicated that their hospital had dedicated call quarters. Some institutions even provided meals and exercise facilities. See “How to combat fatigue (and win) during call”
Here are more ways to improve call
Depending on the size of your call pool and volume of deliveries, you might consider the following options to improve your call system.
A few simple measures can boost mental and physical alertness during extended duty.
Physical activity—This is the best strategy to counter fatigue. Stretch often, and walk around. Bright lights help.
Talk—Active participation in a conversation helps keep you focused; passive listening does not.
Drink caffeinated beverages—This calls for moderation, of course. Caffeine isn’t, and shouldn’t be, a cure-all.
Eat well and keep hydrated—A healthy diet and lots of uncaffeinated fluids keep your body running smoothly.
Take short naps—Even 20 minutes can help.
Support your colleagues—Cover another physician long enough for him or her to take a nap, and then take your turn.
Call for help—Call in backup if you are faced with a difficult situation and sense symptoms of serious fatigue in yourself. Also, watch for those symptoms in your colleagues.
Take a shower—A change of clothes helps, too.
Source: Adapted from “Fatigue countermeasures: alertness management in flight operations.” Available at http://humanfactors.arc.nasa.gov/zteam. Accessed March 12, 2009.
If you increase the number of obstetricians in your call pool, the number of calls you take may diminish. However, the volume of activity will probably increase as a result, so that you have a greater chance of being busy while on call. Hiring a nurse-midwife may decrease the number of uncomplicated vaginal deliveries you perform, but you still need to be prepared to provide backup.
Shorten the call duration
The most common duration of call in our study was 24 hours. However, some call pools take call for a weekend or week at a time.1 This gives the physician a longer interval between calls, but the unpredictability of the patient load may make this a horrendously long period of time.
Another potential disadvantage of a shortened call, especially when it is abbreviated to less than 24 hours, is that it increases the number of handoffs in patient care and, therefore, enhances the risk that the circumstances of any given patient will be incompletely understood at this time.
In a busy OB practice, handoffs usually involve a meeting of obstetricians in labor and delivery to “run the board.” When participants attempt to make these handoffs as complete as possible, patient safety is significantly improved.
One way to ensure completeness of patient handoffs is to borrow training and skills from the world of airline pilots. There, crew resource management has introduced the concept of SBAR [Situation-Background-Assessment-Recommendation] as a specific tool to decrease risk inherent in handoffs.
Another helpful idea is attending nurses’ report sessions. These reports can provide you with useful information that you may not have recognized otherwise. By giving them your attention, you may also strengthen relationships with the nurses, your first line of defense.
Develop in-house call
Dildy and colleagues estimated that a call volume of approximately 2,400 deliveries a year would justify a hospital developing 24-hour in-house obstetric coverage.10 In our study, almost all of the hospitals that required in-house call did so because they had residencies, and in-house staff call was required.
Although Clark and colleagues found 24-hour in-house call to be safer than regular call in their review of closed perinatal claims at one large hospital chain, there has been a paucity of studies that confirm or extend our knowledge in this area.11
Hire laborists
Weinstein introduced the word “laborist” into our lexicon in a 2003 paper.12 Barbieri suggested the addition of “nocturnalist” or “weekendist” as possible terms to describe specialists assigned to work certain shifts that prove particularly onerous to practicing obstetricians.13 A number of hospitals and groups are examining and developing this call model.14 However, little research has been conducted on its effects on patient safety and obstetric practice.
For more information, visit www.oblaborist.org and http://obgynhospitalist.com.
Balance—that is the goal
For some of us, perfect balance between safety and patient satisfaction, and between work and home life, may be impossible. Nevertheless, we all need to explore ways to make call a safe and sustainable practice. For many of us, the growing recognition that we have more beautiful, sunny Sunday afternoons behind us than in front of us may be the signal to shift our focus to less demanding, and time-depleting, call schedules.
1. Schauberger CW, Gribble RK, Rooney BL. On call: a survey of Wisconsin obstetric groups. Am J Obstet Gynecol. 2007;196:39.e1-39.e4.
2. Dorsey ER, Jarjoura D, Rutecki GW. Influence of controllable lifestyle on recent trends in specialty choice by US medical students. JAMA. 2003;290:1173-1178.
3. Gariti DL, Zollinger TW, Look KY. Factors detracting students from applying for an obstetrics and gynecology residency. Am J Obstet Gynecol. 2005;193:289-293.
4. Deutsch A, McCarthy J, Murray K, Sayer R. Why are fewer medical students in Florida choosing obstetrics and gynecology? South Med J. 2007;100:1095-1098.
5. Bettes BA, Chalas E, Coleman VH, Schulkin J. Heavier workload, less personal control: impact of delivery on obstetrician/gynecologists’ career satisfaction. Am J Obstet Gynecol. 2004;190:851-857.
6. Pradhan A, Raman A, Lim C, Yoon E, Ananth C. The patient perspective. Is the hospitalist model a viable option for obstetric care [abstract]. Obstet Gynecol. 2008;111:40S-41S.
7. American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 6th ed. Elk Grove, Ill: American Academy of Pediatrics; 2007:157.
8. Clark SL, Belfort MA, Byrum SL, Meyers JA, Perlin JB. Improved outcomes, fewer cesarean deliveries, and reduced litigation: results of a new paradigm in patient safety. Am J Obstet Gynecol. 2008;199:105.e1-105.e7.
9. Bland ES, Oppenheimer LW, Holmes P, Wen SW. The effect of income pooling within a call group on rates of obstetric intervention. CMAJ. 2001;164:337-339.
10. Dildy GA, Clark SL, Belfort MA, Herbst MA, Thompson J. Twenty-four hour, in-house obstetric coverage in a community-based tertiary care hospital [abstract]. Obstet Gynecol. 2007;109:43S.-
11. Clark SL, Belfort MA, Dildy GA. Reducing obstetric litigation through alterations in practice patterns—experience with 189 closed claims. Am J Obstet Gynecol. 2006;195:S118.-
12. Weinstein L. The laborist: a new focus of practice for the obstetrician. Am J Obstet Gynecol. 2003;188:310-312.
13. Barbieri RL. Will the “ists” preserve the rewards of OB practice? OBG Management. 2007;199(9):8-12.
14. Yates J. The laborists are here, but can they thrive in US hospitals? OBG Management. 2008;20(8):26-34.
1. Schauberger CW, Gribble RK, Rooney BL. On call: a survey of Wisconsin obstetric groups. Am J Obstet Gynecol. 2007;196:39.e1-39.e4.
2. Dorsey ER, Jarjoura D, Rutecki GW. Influence of controllable lifestyle on recent trends in specialty choice by US medical students. JAMA. 2003;290:1173-1178.
3. Gariti DL, Zollinger TW, Look KY. Factors detracting students from applying for an obstetrics and gynecology residency. Am J Obstet Gynecol. 2005;193:289-293.
4. Deutsch A, McCarthy J, Murray K, Sayer R. Why are fewer medical students in Florida choosing obstetrics and gynecology? South Med J. 2007;100:1095-1098.
5. Bettes BA, Chalas E, Coleman VH, Schulkin J. Heavier workload, less personal control: impact of delivery on obstetrician/gynecologists’ career satisfaction. Am J Obstet Gynecol. 2004;190:851-857.
6. Pradhan A, Raman A, Lim C, Yoon E, Ananth C. The patient perspective. Is the hospitalist model a viable option for obstetric care [abstract]. Obstet Gynecol. 2008;111:40S-41S.
7. American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Guidelines for Perinatal Care. 6th ed. Elk Grove, Ill: American Academy of Pediatrics; 2007:157.
8. Clark SL, Belfort MA, Byrum SL, Meyers JA, Perlin JB. Improved outcomes, fewer cesarean deliveries, and reduced litigation: results of a new paradigm in patient safety. Am J Obstet Gynecol. 2008;199:105.e1-105.e7.
9. Bland ES, Oppenheimer LW, Holmes P, Wen SW. The effect of income pooling within a call group on rates of obstetric intervention. CMAJ. 2001;164:337-339.
10. Dildy GA, Clark SL, Belfort MA, Herbst MA, Thompson J. Twenty-four hour, in-house obstetric coverage in a community-based tertiary care hospital [abstract]. Obstet Gynecol. 2007;109:43S.-
11. Clark SL, Belfort MA, Dildy GA. Reducing obstetric litigation through alterations in practice patterns—experience with 189 closed claims. Am J Obstet Gynecol. 2006;195:S118.-
12. Weinstein L. The laborist: a new focus of practice for the obstetrician. Am J Obstet Gynecol. 2003;188:310-312.
13. Barbieri RL. Will the “ists” preserve the rewards of OB practice? OBG Management. 2007;199(9):8-12.
14. Yates J. The laborists are here, but can they thrive in US hospitals? OBG Management. 2008;20(8):26-34.
Preeclampsia and eclampsia: 7 management challenges (and zero shortcuts)
CASE: At risk, or just very pregnant?
At her first prenatal visit, a 31-year-old gravida has blood pressure (BP) of 100/60 mm Hg, no proteinuria, and normal weight for her gestational age. As she enters the third trimester, however, her BP rises to 138/86 mm Hg, she now has proteinuria of 1+, and she has gained 10 lb in the past 2 weeks.
Does she have preeclampsia, or do these findings reflect normal development in the last trimester?
These findings, in and of themselves, may not indicate preeclampsia—but they do suggest a serious risk of developing the disease.
Preeclampsia complicates approximately 3% to 7% of nulliparous pregnancies in the United States, and about 0.8% to 5% of multiparous pregnancies.
Although severe preeclampsia represents only a fraction of those amounts, and eclampsia an even lower percentage, they are potentially catastrophic complications of pregnancy and one of the leading causes of maternal death. They also are responsible for a large percentage of infants born prematurely as a result of a worsening maternal or fetal condition.
Preeclampsia and eclampsia are obstetric diseases, and obstetricians are the group best equipped to diagnose, evaluate, and manage them. In this article, we highlight seven challenges that obstetricians face when managing preeclampsia and eclampsia, and offer useful strategies to help minimize morbidity and mortality in both mother and infant.
CHALLENGE NO. 1: Making the diagnosis
Good prenatal care is a prerequisite
We can’t overemphasize the importance of early and adequate prenatal care! Although the diagnostic criteria for preeclampsia have been widely established—persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period—the condition does not always play by the rules. With close monitoring of weight, urine protein, and BP, the clinician can identify and follow potentially worrisome trends.
Earlier diagnostic criteria—which included a rise in systolic BP of 30 mm Hg or a rise in diastolic BP of 15 mm Hg above initial baseline BP, as well as the presence of pathologic edema—may have been revised, but it remains important for clinicians to put all pieces of clinical information together at each visit. For example, given her rising BP, proteinuria, and weight gain, the patient in the opening case must be considered at risk for preeclampsia. Suspicion also is justified if the patient has any of the risk factors for preeclampsia in TABLE 1.
TABLE 1
Risk factors for preeclampsia
|
|
|
Early detection is critical
Early identification of preeclampsia may allow for interventions, including delivery, that will lessen the risk of progression to severe preeclampsia and eclampsia and reduce fetal and maternal morbidity and mortality. It is, therefore, essential for the clinician to ask specifically about signs and symptoms of preeclampsia and to listen carefully to the answers.
Signs and symptoms may sometimes be typical:
- weight gain
- increasing edema
- persistent headache
- blurred vision.
- malaise
- nausea
- epigastric discomfort
- right upper-quadrant discomfort.
Diagnostic criteria
The diagnosis of preeclampsia is based on persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period.1 Other criteria have been applied, such as a rise in systolic or diastolic BP above baseline and urine dipstick criteria for proteinuria, but BP above 140/90 mm Hg and proteinuria above 300 mg are most frequently used in medical centers in the United States.2
Gestational hypertension and chronic hypertension do sometimes coexist with superimposed preeclampsia, but should not be confused with preeclampsia or lead to management decisions that should apply only to patients with preeclampsia.3
Before severe preeclampsia can be diagnosed, the initial criteria for preeclampsia should have been fulfilled, along with one or more of the findings listed in TABLE 2.
Attempts to predict preeclampsia have met with poor results. Measurement of the ratio of uterine artery systolic to diastolic flow has not been informative in the general healthy population of pregnant women. Nor has uric acid determination been useful; it generally has very poor predictive value and should be interpreted with caution.
TABLE 2
13 criteria for establishing severe preeclampsia
|
|
|
Hospitalization is essential for severe disease
Mild preeclampsia can be managed expectantly until fetal maturity or 37 weeks’ gestation. Severe preeclampsia can be managed expectantly in the mid trimester or early third trimester if both mother and fetus are stable, but hospitalization is necessary in a tertiary care facility that has critical-care OB expertise, an ICU facility, and a NICU facility and personnel on site.
Distinguish an existing condition from superimposed preeclampsia
One of the most difficult management challenges is the diagnosis of superimposed preeclampsia. Patients who have chronic hypertension often have underlying renal disease as well; in these patients, it may be difficult, if not impossible, to distinguish a worsening underlying medical condition from superimposed preeclampsia.
Our advice is not to agonize about this difference too much in the patient at or near term, as delivery may be indicated and the patient’s postpartum course may help resolve the question, with rapid resolution tending to favor a diagnosis of superimposed preeclampsia.
It also is important to note whether these patients are receiving antihypertensive therapy. If they are, hospitalization is recommended until delivery once the diagnosis of superimposed preeclampsia is made.
Given that the use of antihypertensive agents removes one of the major indicators of disease progression (i.e., rising BP), it is our practice to deliver these patients according to our severe preeclampsia management protocol and not to carry such pregnancies beyond 34 weeks. In carefully selected cases, the pregnancy can be continued to 37 weeks, but the decision to do so should be weighed carefully—ideally, with input from a maternal–fetal medicine specialist.
CHALLENGE NO. 2: Forgoing shortcuts
Evaluation and management of preeclampsia are relatively straightforward, but there are no shortcuts. Many patients who feel well initially may push for outpatient evaluation, but once a diagnosis of preeclampsia is established, in-hospital evaluation is preferable, at least until the degree of illness can be determined, fetal well-being can be established, and the patient’s candidacy for subsequent outpatient management can be more fully determined.
In-hospital management may be particularly useful for patients who have any of the risk factors for preeclampsia listed in TABLE 1.
Initial evaluation consists of:
- fetal nonstress testing
- amniotic fluid index
- serial BP determination
- 24-hour urine collection
- initial laboratory evaluation comprising a complete blood count with platelets and aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine levels.
If fetal and maternal evaluations are reassuring, and if the patient has remained stable, then outpatient management may be considered. In general, if proteinuria exceeds 1 g in 24 hours, in-hospital management is recommended, regardless of other parameters.
If outpatient management is considered, the level of care and surveillance must mirror what could be provided in the hospital. Hospitalization alone will not prevent all cases from progressing to severe preeclampsia or eclampsia, but daily and diligent observation and evaluation may minimize the risk.
CHALLENGE NO. 3: Treating the disease
Appropriate treatment of preeclampsia requires not only that the patient show up for prenatal care, but also that we:
- are certain of the diagnosis
- recognize the potential seriousness of the disease
- are thorough (remember, no shortcuts!).
Many experts in the field of preeclampsia have stated, on numerous occasions, that preeclampsia is more than simple hypertension. It is almost never advisable to initiate antihypertensive therapy for a patient in the third trimester when she was previously normotensive, because one runs the risk of masking a key clinical parameter used to assess disease progression.
In our institutions, any patient who is taking antihypertensive medication and in whom we are entertaining a diagnosis of preeclampsia is recommended for hospitalization for the duration of her pregnancy or until a diagnosis of preeclampsia can be ruled out with reasonable certainty.
Expectant management beyond 37 weeks does not benefit mother or fetus
Because preeclampsia is a multisystem disease, it has maternal, placental, and fetal consequences. The cure for preeclampsia remains delivery of the placenta. Expectant management offers no maternal benefit, but does offer some potential neonatal benefits if prematurity is a concern. Once concerns about prematurity have been largely eliminated, generally by achieving a gestational age of 37 weeks, further expectant management is not indicated, offers little or no additional benefit to the fetus, and leaves both mother and fetus at risk.
Therefore, once the pregnancy reaches 37 weeks, delivery is recommended.
When preeclampsia is severe, and when it is superimposed in a patient who is taking antihypertensive medication, we generally do not continue the pregnancy beyond 34 weeks.4 In our institutions, most patients who are being expectantly managed for severe preeclampsia remote from term—and who have remained stable—are delivered between 32 and 34 weeks’ gestation, depending on the specific clinical circumstances.
CHALLENGE NO. 4: Controlling blood pressure
Cerebrovascular accident (stroke) is the leading cause of maternal mortality from preeclampsia in the United States. Not all cases can be prevented, but one suggested preventive strategy is adequate BP control. Some cases of stroke in the setting of preeclampsia will occur despite systemic BP readings that are not considered to be in a dangerous range. One reason may be an override of normal cerebral blood flow autoregulatory mechanisms, resulting in increased cerebral blood flow, rising cerebral perfusion pressures, and vessel rupture. Such occurrences may sometimes, but not always, be related to coagulopathy.
When a patient has elevated BP, generally defined as persistent systolic pressures above 160 to 170 mm Hg and persistent diastolic pressures above 105 to 110 mm Hg, antihypertensive therapy is indicated and should be administered in a timely fashion.
Labetalol, nifedipine, and hydralazine have all been used effectively in such acute settings, when administered parenterally (except nifedipine, which may be given orally) and when given in proper dosages (TABLE 3).
Avoid oral use of labetalol or hydralazine to treat acute hypertensive emergencies.
TABLE 3
Pharmacotherapy of acute hypertension
| Drug | Dosage | Directions |
|---|---|---|
| Hydralazine* | 5 mg IV | Repeat in 10 min, then give 10 mg IV every 20 min until BP stabilizes (140–150/90–100 mm Hg) |
| Labetalol* | 10–20 mg IV push | Repeat every 10-20 min, doubling the dosage each time until a maximum total cumulative dosage of 300 mg has been given |
| Nifedipine* | 10 mg | Repeat in 20 min for four doses (maximum 40 mg); then give 10–20 mg orally every 4–6 h to achieve a stable BP of 140–150/90–100 mm Hg |
| * If target blood pressure is not reached after the maximum dosage of an agent is given, then additional or alternative pharmacotherapy must be utilized. | ||
Goals for treatment
In the antepartum patient, the goal is to maintain systolic BP at 140 to 150 mm Hg and diastolic pressure at 90 to 100 mm Hg to keep from inadvertently inducing uteroplacental insufficiency secondary to reduced uterine blood flow.
In the delivered patient, the risk of mild hypotension is not quite as great, although an attempt to rapidly return the patient to her previous normal BP profile may cause symptomatic hypotension.
If a patient develops a true hypertensive crisis with hypertensive encephalopathy (which generally occurs at BPs exceeding 240/140 mm Hg), then emergent intervention with a rapidly acting agent such as sodium nitroprusside is necessary and should be managed by someone skilled in critical care and the use of such drugs.
CHALLENGE NO. 5: Preventing seizures
Magnesium sulfate is the drug of choice to prevent both initial and recurrent eclamptic seizures.5 Two large clinical trials ended any doubts about its efficacy, demonstrating its superiority over both phenytoin and diazepam in the settings of preeclampsia and eclampsia.
Magnesium sulfate is best administered intravenously (IV) via continuous infusion pump. An initial bolus of 4 to 6 g is given over 15 to 30 minutes; this amount does not need to be adjusted to the patient’s level of renal function. A continuous infusion of magnesium sulfate is usually initiated at a rate of 2 g/hour. It is this infusion dosage that may need to be altered, based on the patient’s urine output and renal function.
Evidence of magnesium toxicity includes:
- loss of deep-tendon reflexes
- respiratory depression
- blurred vision
- cardiotoxicity.
There is no debate about the utility of magnesium sulfate in severe preeclampsia, but when it comes to intrapartum management of mild preeclampsia or cases in which preeclampsia first manifests in the postpartum period, data are not so clear. This debate will not be resolved to anyone’s satisfaction in the course of this article. Historically, the practice has been to use magnesium in these circumstances, but the pendulum has begun to shift based on a few arguments:
- Eclampsia is a rare event (about 1 case for every 300 to 1,000 deliveries).
- Most cases occur outside of the hospital.
- Some women experience seizures before preeclampsia has been diagnosed.
- Some patients experience seizures while taking magnesium sulfate.
Regardless of one’s position on this debate, there is broad consensus that regular careful clinical assessment of the patient who has preeclampsia is essential to minimize morbidity and mortality. This disease can progress from mild to severe rapidly. Only through regular careful assessment can a physician observe this change soon enough to alter management as necessary.
Treatment of magnesium toxicity
Most often, an ampule of 10% calcium gluconate (1 g) is administered IV to reverse the effects of suspected magnesium toxicity.
In addition, because magnesium freely crosses the placenta, we recommend that a newborn resuscitation team be present at all deliveries during which the mother was receiving magnesium sulfate because neonatal respiratory and cardiac depression have been reported in this setting.
CHALLENGE NO. 6: Delivering the patient
Preeclampsia, severe preeclampsia, and eclampsia present a dilemma for the managing clinician: subject her to the rigors of labor, or to the heightened risk of cesarean delivery? Overall, a properly managed vaginal delivery is less hemodynamically stressful than cesarean delivery for the mother. To accomplish vaginal delivery, it is necessary to provide optimal anesthesia and analgesia.
Risks of regional anesthesia
Women who have preeclampsia are volume-depleted. As such, they are prone to hypotension after administration of regional anesthesia if the block sets up too rapidly. For this reason, epidural anesthesia or some of the newer combined techniques offer optimal analgesia by allowing for slower implementation of the regional block.
Women who have preeclampsia, especially severe preeclampsia, are usually candidates for regional analgesia and anesthesia. Some requisites for regional anesthesia under these conditions include the following:
- The patient can tolerate preblock hydration.
- She has adequate IV access.
- There is a reproducible means of determining BP.
- The patient has a normal coagulation profile. (A normal platelet count with normal transaminase should be sufficient to confirm this; women who have preeclampsia are not at increased risk of having altered prothrombin time, partial thromboplastin time, or fibrinogen levels, provided there are no other mitigating clinical circumstances.)
- The anesthesiology team is skilled in the administration of regional anesthesia.
If eclampsia occurs
Do not proceed to emergent cesarean section. Rather, stabilize the mother, protect her from injury during the seizure, protect her airway, and allow the seizure to take its course.
Begin magnesium at once. If it was being infused before the seizure, consider giving an additional 2-g bolus over several minutes. As the mother stabilizes, the fetal heart rate will recover and she can be reassessed to determine optimal timing and route of delivery.
Continue magnesium after delivery?
Yes, but how long remains unclear. Most authorities have recommended 24 hours, based on the observation that most eclamptic seizures that occur in the first 48 hours postpartum actually occur in the first 24 hours.
Clinical assessment can guide management to some degree. The most reliable sign of disease resolution is spontaneous, brisk diuresis, so some clinicians use this finding as an indication to discontinue magnesium.
Regardless of clinical preference, if magnesium sulfate is being used postpartum, continue it until there is evidence of disease resolution, such as the diuresis noted above.
When HELLP syndrome arises
If HELLP [Hemolysis, Elevated Liver en zymes, Low Platelets] syndrome is present, continue magnesium sulfate until there is laboratory evidence of improvement in the platelet count and transaminase. Because a return to normal levels can take several days, it is not required before discontinuation of magnesium in cases of HELLP syndrome. However, at the time of discontinuation, it should be clear that there is no longer evidence of a worsening laboratory or clinical trend.
CHALLENGE NO. 7: Managing HELLP, atypical eclampsia
These two diagnoses pose daunting clinical challenges too numerous to cover in detail in this article, but a few key points merit consideration. When HELLP syndrome is diagnosed (using established criteria, TABLE 4), follow guidelines for severe preeclampsia. Use of dexamethasone remains somewhat controversial, as randomized clinical trials so far do not support it.6
Atypical eclampsia has been defined as eclampsia that occurs before 20 weeks’ gestation or from 48 hours to 14 days after delivery. Its management is similar to the management of eclampsia, with BP control and magnesium sulfate being the mainstays of therapy.
Because of the relative rarity of atypical eclampsia, we recommend neurologic consultation in these cases to evaluate for other possible causes of seizure.
TABLE 4
HELLP syndrome—Sibai criteria
|
CASE RESOLVED
After initial hospitalization, the patient is monitored as an outpatient until 35 weeks’ gestation, when more labile BP and increased proteinuria necessitate hospitalization. However, her preeclampsia remains mild by definition and, after continued reassuring fetal testing, she undergoes labor induction at 37 weeks.
1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-167.
3. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
4. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514-519.
5. Altman D, Carroli G, Duley L, et al:. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
6. Fonseca JE, Méndez F, Cataño C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005;193:1591-1598.
CASE: At risk, or just very pregnant?
At her first prenatal visit, a 31-year-old gravida has blood pressure (BP) of 100/60 mm Hg, no proteinuria, and normal weight for her gestational age. As she enters the third trimester, however, her BP rises to 138/86 mm Hg, she now has proteinuria of 1+, and she has gained 10 lb in the past 2 weeks.
Does she have preeclampsia, or do these findings reflect normal development in the last trimester?
These findings, in and of themselves, may not indicate preeclampsia—but they do suggest a serious risk of developing the disease.
Preeclampsia complicates approximately 3% to 7% of nulliparous pregnancies in the United States, and about 0.8% to 5% of multiparous pregnancies.
Although severe preeclampsia represents only a fraction of those amounts, and eclampsia an even lower percentage, they are potentially catastrophic complications of pregnancy and one of the leading causes of maternal death. They also are responsible for a large percentage of infants born prematurely as a result of a worsening maternal or fetal condition.
Preeclampsia and eclampsia are obstetric diseases, and obstetricians are the group best equipped to diagnose, evaluate, and manage them. In this article, we highlight seven challenges that obstetricians face when managing preeclampsia and eclampsia, and offer useful strategies to help minimize morbidity and mortality in both mother and infant.
CHALLENGE NO. 1: Making the diagnosis
Good prenatal care is a prerequisite
We can’t overemphasize the importance of early and adequate prenatal care! Although the diagnostic criteria for preeclampsia have been widely established—persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period—the condition does not always play by the rules. With close monitoring of weight, urine protein, and BP, the clinician can identify and follow potentially worrisome trends.
Earlier diagnostic criteria—which included a rise in systolic BP of 30 mm Hg or a rise in diastolic BP of 15 mm Hg above initial baseline BP, as well as the presence of pathologic edema—may have been revised, but it remains important for clinicians to put all pieces of clinical information together at each visit. For example, given her rising BP, proteinuria, and weight gain, the patient in the opening case must be considered at risk for preeclampsia. Suspicion also is justified if the patient has any of the risk factors for preeclampsia in TABLE 1.
TABLE 1
Risk factors for preeclampsia
|
|
|
Early detection is critical
Early identification of preeclampsia may allow for interventions, including delivery, that will lessen the risk of progression to severe preeclampsia and eclampsia and reduce fetal and maternal morbidity and mortality. It is, therefore, essential for the clinician to ask specifically about signs and symptoms of preeclampsia and to listen carefully to the answers.
Signs and symptoms may sometimes be typical:
- weight gain
- increasing edema
- persistent headache
- blurred vision.
- malaise
- nausea
- epigastric discomfort
- right upper-quadrant discomfort.
Diagnostic criteria
The diagnosis of preeclampsia is based on persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period.1 Other criteria have been applied, such as a rise in systolic or diastolic BP above baseline and urine dipstick criteria for proteinuria, but BP above 140/90 mm Hg and proteinuria above 300 mg are most frequently used in medical centers in the United States.2
Gestational hypertension and chronic hypertension do sometimes coexist with superimposed preeclampsia, but should not be confused with preeclampsia or lead to management decisions that should apply only to patients with preeclampsia.3
Before severe preeclampsia can be diagnosed, the initial criteria for preeclampsia should have been fulfilled, along with one or more of the findings listed in TABLE 2.
Attempts to predict preeclampsia have met with poor results. Measurement of the ratio of uterine artery systolic to diastolic flow has not been informative in the general healthy population of pregnant women. Nor has uric acid determination been useful; it generally has very poor predictive value and should be interpreted with caution.
TABLE 2
13 criteria for establishing severe preeclampsia
|
|
|
Hospitalization is essential for severe disease
Mild preeclampsia can be managed expectantly until fetal maturity or 37 weeks’ gestation. Severe preeclampsia can be managed expectantly in the mid trimester or early third trimester if both mother and fetus are stable, but hospitalization is necessary in a tertiary care facility that has critical-care OB expertise, an ICU facility, and a NICU facility and personnel on site.
Distinguish an existing condition from superimposed preeclampsia
One of the most difficult management challenges is the diagnosis of superimposed preeclampsia. Patients who have chronic hypertension often have underlying renal disease as well; in these patients, it may be difficult, if not impossible, to distinguish a worsening underlying medical condition from superimposed preeclampsia.
Our advice is not to agonize about this difference too much in the patient at or near term, as delivery may be indicated and the patient’s postpartum course may help resolve the question, with rapid resolution tending to favor a diagnosis of superimposed preeclampsia.
It also is important to note whether these patients are receiving antihypertensive therapy. If they are, hospitalization is recommended until delivery once the diagnosis of superimposed preeclampsia is made.
Given that the use of antihypertensive agents removes one of the major indicators of disease progression (i.e., rising BP), it is our practice to deliver these patients according to our severe preeclampsia management protocol and not to carry such pregnancies beyond 34 weeks. In carefully selected cases, the pregnancy can be continued to 37 weeks, but the decision to do so should be weighed carefully—ideally, with input from a maternal–fetal medicine specialist.
CHALLENGE NO. 2: Forgoing shortcuts
Evaluation and management of preeclampsia are relatively straightforward, but there are no shortcuts. Many patients who feel well initially may push for outpatient evaluation, but once a diagnosis of preeclampsia is established, in-hospital evaluation is preferable, at least until the degree of illness can be determined, fetal well-being can be established, and the patient’s candidacy for subsequent outpatient management can be more fully determined.
In-hospital management may be particularly useful for patients who have any of the risk factors for preeclampsia listed in TABLE 1.
Initial evaluation consists of:
- fetal nonstress testing
- amniotic fluid index
- serial BP determination
- 24-hour urine collection
- initial laboratory evaluation comprising a complete blood count with platelets and aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine levels.
If fetal and maternal evaluations are reassuring, and if the patient has remained stable, then outpatient management may be considered. In general, if proteinuria exceeds 1 g in 24 hours, in-hospital management is recommended, regardless of other parameters.
If outpatient management is considered, the level of care and surveillance must mirror what could be provided in the hospital. Hospitalization alone will not prevent all cases from progressing to severe preeclampsia or eclampsia, but daily and diligent observation and evaluation may minimize the risk.
CHALLENGE NO. 3: Treating the disease
Appropriate treatment of preeclampsia requires not only that the patient show up for prenatal care, but also that we:
- are certain of the diagnosis
- recognize the potential seriousness of the disease
- are thorough (remember, no shortcuts!).
Many experts in the field of preeclampsia have stated, on numerous occasions, that preeclampsia is more than simple hypertension. It is almost never advisable to initiate antihypertensive therapy for a patient in the third trimester when she was previously normotensive, because one runs the risk of masking a key clinical parameter used to assess disease progression.
In our institutions, any patient who is taking antihypertensive medication and in whom we are entertaining a diagnosis of preeclampsia is recommended for hospitalization for the duration of her pregnancy or until a diagnosis of preeclampsia can be ruled out with reasonable certainty.
Expectant management beyond 37 weeks does not benefit mother or fetus
Because preeclampsia is a multisystem disease, it has maternal, placental, and fetal consequences. The cure for preeclampsia remains delivery of the placenta. Expectant management offers no maternal benefit, but does offer some potential neonatal benefits if prematurity is a concern. Once concerns about prematurity have been largely eliminated, generally by achieving a gestational age of 37 weeks, further expectant management is not indicated, offers little or no additional benefit to the fetus, and leaves both mother and fetus at risk.
Therefore, once the pregnancy reaches 37 weeks, delivery is recommended.
When preeclampsia is severe, and when it is superimposed in a patient who is taking antihypertensive medication, we generally do not continue the pregnancy beyond 34 weeks.4 In our institutions, most patients who are being expectantly managed for severe preeclampsia remote from term—and who have remained stable—are delivered between 32 and 34 weeks’ gestation, depending on the specific clinical circumstances.
CHALLENGE NO. 4: Controlling blood pressure
Cerebrovascular accident (stroke) is the leading cause of maternal mortality from preeclampsia in the United States. Not all cases can be prevented, but one suggested preventive strategy is adequate BP control. Some cases of stroke in the setting of preeclampsia will occur despite systemic BP readings that are not considered to be in a dangerous range. One reason may be an override of normal cerebral blood flow autoregulatory mechanisms, resulting in increased cerebral blood flow, rising cerebral perfusion pressures, and vessel rupture. Such occurrences may sometimes, but not always, be related to coagulopathy.
When a patient has elevated BP, generally defined as persistent systolic pressures above 160 to 170 mm Hg and persistent diastolic pressures above 105 to 110 mm Hg, antihypertensive therapy is indicated and should be administered in a timely fashion.
Labetalol, nifedipine, and hydralazine have all been used effectively in such acute settings, when administered parenterally (except nifedipine, which may be given orally) and when given in proper dosages (TABLE 3).
Avoid oral use of labetalol or hydralazine to treat acute hypertensive emergencies.
TABLE 3
Pharmacotherapy of acute hypertension
| Drug | Dosage | Directions |
|---|---|---|
| Hydralazine* | 5 mg IV | Repeat in 10 min, then give 10 mg IV every 20 min until BP stabilizes (140–150/90–100 mm Hg) |
| Labetalol* | 10–20 mg IV push | Repeat every 10-20 min, doubling the dosage each time until a maximum total cumulative dosage of 300 mg has been given |
| Nifedipine* | 10 mg | Repeat in 20 min for four doses (maximum 40 mg); then give 10–20 mg orally every 4–6 h to achieve a stable BP of 140–150/90–100 mm Hg |
| * If target blood pressure is not reached after the maximum dosage of an agent is given, then additional or alternative pharmacotherapy must be utilized. | ||
Goals for treatment
In the antepartum patient, the goal is to maintain systolic BP at 140 to 150 mm Hg and diastolic pressure at 90 to 100 mm Hg to keep from inadvertently inducing uteroplacental insufficiency secondary to reduced uterine blood flow.
In the delivered patient, the risk of mild hypotension is not quite as great, although an attempt to rapidly return the patient to her previous normal BP profile may cause symptomatic hypotension.
If a patient develops a true hypertensive crisis with hypertensive encephalopathy (which generally occurs at BPs exceeding 240/140 mm Hg), then emergent intervention with a rapidly acting agent such as sodium nitroprusside is necessary and should be managed by someone skilled in critical care and the use of such drugs.
CHALLENGE NO. 5: Preventing seizures
Magnesium sulfate is the drug of choice to prevent both initial and recurrent eclamptic seizures.5 Two large clinical trials ended any doubts about its efficacy, demonstrating its superiority over both phenytoin and diazepam in the settings of preeclampsia and eclampsia.
Magnesium sulfate is best administered intravenously (IV) via continuous infusion pump. An initial bolus of 4 to 6 g is given over 15 to 30 minutes; this amount does not need to be adjusted to the patient’s level of renal function. A continuous infusion of magnesium sulfate is usually initiated at a rate of 2 g/hour. It is this infusion dosage that may need to be altered, based on the patient’s urine output and renal function.
Evidence of magnesium toxicity includes:
- loss of deep-tendon reflexes
- respiratory depression
- blurred vision
- cardiotoxicity.
There is no debate about the utility of magnesium sulfate in severe preeclampsia, but when it comes to intrapartum management of mild preeclampsia or cases in which preeclampsia first manifests in the postpartum period, data are not so clear. This debate will not be resolved to anyone’s satisfaction in the course of this article. Historically, the practice has been to use magnesium in these circumstances, but the pendulum has begun to shift based on a few arguments:
- Eclampsia is a rare event (about 1 case for every 300 to 1,000 deliveries).
- Most cases occur outside of the hospital.
- Some women experience seizures before preeclampsia has been diagnosed.
- Some patients experience seizures while taking magnesium sulfate.
Regardless of one’s position on this debate, there is broad consensus that regular careful clinical assessment of the patient who has preeclampsia is essential to minimize morbidity and mortality. This disease can progress from mild to severe rapidly. Only through regular careful assessment can a physician observe this change soon enough to alter management as necessary.
Treatment of magnesium toxicity
Most often, an ampule of 10% calcium gluconate (1 g) is administered IV to reverse the effects of suspected magnesium toxicity.
In addition, because magnesium freely crosses the placenta, we recommend that a newborn resuscitation team be present at all deliveries during which the mother was receiving magnesium sulfate because neonatal respiratory and cardiac depression have been reported in this setting.
CHALLENGE NO. 6: Delivering the patient
Preeclampsia, severe preeclampsia, and eclampsia present a dilemma for the managing clinician: subject her to the rigors of labor, or to the heightened risk of cesarean delivery? Overall, a properly managed vaginal delivery is less hemodynamically stressful than cesarean delivery for the mother. To accomplish vaginal delivery, it is necessary to provide optimal anesthesia and analgesia.
Risks of regional anesthesia
Women who have preeclampsia are volume-depleted. As such, they are prone to hypotension after administration of regional anesthesia if the block sets up too rapidly. For this reason, epidural anesthesia or some of the newer combined techniques offer optimal analgesia by allowing for slower implementation of the regional block.
Women who have preeclampsia, especially severe preeclampsia, are usually candidates for regional analgesia and anesthesia. Some requisites for regional anesthesia under these conditions include the following:
- The patient can tolerate preblock hydration.
- She has adequate IV access.
- There is a reproducible means of determining BP.
- The patient has a normal coagulation profile. (A normal platelet count with normal transaminase should be sufficient to confirm this; women who have preeclampsia are not at increased risk of having altered prothrombin time, partial thromboplastin time, or fibrinogen levels, provided there are no other mitigating clinical circumstances.)
- The anesthesiology team is skilled in the administration of regional anesthesia.
If eclampsia occurs
Do not proceed to emergent cesarean section. Rather, stabilize the mother, protect her from injury during the seizure, protect her airway, and allow the seizure to take its course.
Begin magnesium at once. If it was being infused before the seizure, consider giving an additional 2-g bolus over several minutes. As the mother stabilizes, the fetal heart rate will recover and she can be reassessed to determine optimal timing and route of delivery.
Continue magnesium after delivery?
Yes, but how long remains unclear. Most authorities have recommended 24 hours, based on the observation that most eclamptic seizures that occur in the first 48 hours postpartum actually occur in the first 24 hours.
Clinical assessment can guide management to some degree. The most reliable sign of disease resolution is spontaneous, brisk diuresis, so some clinicians use this finding as an indication to discontinue magnesium.
Regardless of clinical preference, if magnesium sulfate is being used postpartum, continue it until there is evidence of disease resolution, such as the diuresis noted above.
When HELLP syndrome arises
If HELLP [Hemolysis, Elevated Liver en zymes, Low Platelets] syndrome is present, continue magnesium sulfate until there is laboratory evidence of improvement in the platelet count and transaminase. Because a return to normal levels can take several days, it is not required before discontinuation of magnesium in cases of HELLP syndrome. However, at the time of discontinuation, it should be clear that there is no longer evidence of a worsening laboratory or clinical trend.
CHALLENGE NO. 7: Managing HELLP, atypical eclampsia
These two diagnoses pose daunting clinical challenges too numerous to cover in detail in this article, but a few key points merit consideration. When HELLP syndrome is diagnosed (using established criteria, TABLE 4), follow guidelines for severe preeclampsia. Use of dexamethasone remains somewhat controversial, as randomized clinical trials so far do not support it.6
Atypical eclampsia has been defined as eclampsia that occurs before 20 weeks’ gestation or from 48 hours to 14 days after delivery. Its management is similar to the management of eclampsia, with BP control and magnesium sulfate being the mainstays of therapy.
Because of the relative rarity of atypical eclampsia, we recommend neurologic consultation in these cases to evaluate for other possible causes of seizure.
TABLE 4
HELLP syndrome—Sibai criteria
|
CASE RESOLVED
After initial hospitalization, the patient is monitored as an outpatient until 35 weeks’ gestation, when more labile BP and increased proteinuria necessitate hospitalization. However, her preeclampsia remains mild by definition and, after continued reassuring fetal testing, she undergoes labor induction at 37 weeks.
CASE: At risk, or just very pregnant?
At her first prenatal visit, a 31-year-old gravida has blood pressure (BP) of 100/60 mm Hg, no proteinuria, and normal weight for her gestational age. As she enters the third trimester, however, her BP rises to 138/86 mm Hg, she now has proteinuria of 1+, and she has gained 10 lb in the past 2 weeks.
Does she have preeclampsia, or do these findings reflect normal development in the last trimester?
These findings, in and of themselves, may not indicate preeclampsia—but they do suggest a serious risk of developing the disease.
Preeclampsia complicates approximately 3% to 7% of nulliparous pregnancies in the United States, and about 0.8% to 5% of multiparous pregnancies.
Although severe preeclampsia represents only a fraction of those amounts, and eclampsia an even lower percentage, they are potentially catastrophic complications of pregnancy and one of the leading causes of maternal death. They also are responsible for a large percentage of infants born prematurely as a result of a worsening maternal or fetal condition.
Preeclampsia and eclampsia are obstetric diseases, and obstetricians are the group best equipped to diagnose, evaluate, and manage them. In this article, we highlight seven challenges that obstetricians face when managing preeclampsia and eclampsia, and offer useful strategies to help minimize morbidity and mortality in both mother and infant.
CHALLENGE NO. 1: Making the diagnosis
Good prenatal care is a prerequisite
We can’t overemphasize the importance of early and adequate prenatal care! Although the diagnostic criteria for preeclampsia have been widely established—persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period—the condition does not always play by the rules. With close monitoring of weight, urine protein, and BP, the clinician can identify and follow potentially worrisome trends.
Earlier diagnostic criteria—which included a rise in systolic BP of 30 mm Hg or a rise in diastolic BP of 15 mm Hg above initial baseline BP, as well as the presence of pathologic edema—may have been revised, but it remains important for clinicians to put all pieces of clinical information together at each visit. For example, given her rising BP, proteinuria, and weight gain, the patient in the opening case must be considered at risk for preeclampsia. Suspicion also is justified if the patient has any of the risk factors for preeclampsia in TABLE 1.
TABLE 1
Risk factors for preeclampsia
|
|
|
Early detection is critical
Early identification of preeclampsia may allow for interventions, including delivery, that will lessen the risk of progression to severe preeclampsia and eclampsia and reduce fetal and maternal morbidity and mortality. It is, therefore, essential for the clinician to ask specifically about signs and symptoms of preeclampsia and to listen carefully to the answers.
Signs and symptoms may sometimes be typical:
- weight gain
- increasing edema
- persistent headache
- blurred vision.
- malaise
- nausea
- epigastric discomfort
- right upper-quadrant discomfort.
Diagnostic criteria
The diagnosis of preeclampsia is based on persistent BP elevation above 140/90 mm Hg and proteinuria exceeding 300 mg over a 24-hour collection period.1 Other criteria have been applied, such as a rise in systolic or diastolic BP above baseline and urine dipstick criteria for proteinuria, but BP above 140/90 mm Hg and proteinuria above 300 mg are most frequently used in medical centers in the United States.2
Gestational hypertension and chronic hypertension do sometimes coexist with superimposed preeclampsia, but should not be confused with preeclampsia or lead to management decisions that should apply only to patients with preeclampsia.3
Before severe preeclampsia can be diagnosed, the initial criteria for preeclampsia should have been fulfilled, along with one or more of the findings listed in TABLE 2.
Attempts to predict preeclampsia have met with poor results. Measurement of the ratio of uterine artery systolic to diastolic flow has not been informative in the general healthy population of pregnant women. Nor has uric acid determination been useful; it generally has very poor predictive value and should be interpreted with caution.
TABLE 2
13 criteria for establishing severe preeclampsia
|
|
|
Hospitalization is essential for severe disease
Mild preeclampsia can be managed expectantly until fetal maturity or 37 weeks’ gestation. Severe preeclampsia can be managed expectantly in the mid trimester or early third trimester if both mother and fetus are stable, but hospitalization is necessary in a tertiary care facility that has critical-care OB expertise, an ICU facility, and a NICU facility and personnel on site.
Distinguish an existing condition from superimposed preeclampsia
One of the most difficult management challenges is the diagnosis of superimposed preeclampsia. Patients who have chronic hypertension often have underlying renal disease as well; in these patients, it may be difficult, if not impossible, to distinguish a worsening underlying medical condition from superimposed preeclampsia.
Our advice is not to agonize about this difference too much in the patient at or near term, as delivery may be indicated and the patient’s postpartum course may help resolve the question, with rapid resolution tending to favor a diagnosis of superimposed preeclampsia.
It also is important to note whether these patients are receiving antihypertensive therapy. If they are, hospitalization is recommended until delivery once the diagnosis of superimposed preeclampsia is made.
Given that the use of antihypertensive agents removes one of the major indicators of disease progression (i.e., rising BP), it is our practice to deliver these patients according to our severe preeclampsia management protocol and not to carry such pregnancies beyond 34 weeks. In carefully selected cases, the pregnancy can be continued to 37 weeks, but the decision to do so should be weighed carefully—ideally, with input from a maternal–fetal medicine specialist.
CHALLENGE NO. 2: Forgoing shortcuts
Evaluation and management of preeclampsia are relatively straightforward, but there are no shortcuts. Many patients who feel well initially may push for outpatient evaluation, but once a diagnosis of preeclampsia is established, in-hospital evaluation is preferable, at least until the degree of illness can be determined, fetal well-being can be established, and the patient’s candidacy for subsequent outpatient management can be more fully determined.
In-hospital management may be particularly useful for patients who have any of the risk factors for preeclampsia listed in TABLE 1.
Initial evaluation consists of:
- fetal nonstress testing
- amniotic fluid index
- serial BP determination
- 24-hour urine collection
- initial laboratory evaluation comprising a complete blood count with platelets and aspartate aminotransferase (AST), alanine aminotransferase (ALT), and creatinine levels.
If fetal and maternal evaluations are reassuring, and if the patient has remained stable, then outpatient management may be considered. In general, if proteinuria exceeds 1 g in 24 hours, in-hospital management is recommended, regardless of other parameters.
If outpatient management is considered, the level of care and surveillance must mirror what could be provided in the hospital. Hospitalization alone will not prevent all cases from progressing to severe preeclampsia or eclampsia, but daily and diligent observation and evaluation may minimize the risk.
CHALLENGE NO. 3: Treating the disease
Appropriate treatment of preeclampsia requires not only that the patient show up for prenatal care, but also that we:
- are certain of the diagnosis
- recognize the potential seriousness of the disease
- are thorough (remember, no shortcuts!).
Many experts in the field of preeclampsia have stated, on numerous occasions, that preeclampsia is more than simple hypertension. It is almost never advisable to initiate antihypertensive therapy for a patient in the third trimester when she was previously normotensive, because one runs the risk of masking a key clinical parameter used to assess disease progression.
In our institutions, any patient who is taking antihypertensive medication and in whom we are entertaining a diagnosis of preeclampsia is recommended for hospitalization for the duration of her pregnancy or until a diagnosis of preeclampsia can be ruled out with reasonable certainty.
Expectant management beyond 37 weeks does not benefit mother or fetus
Because preeclampsia is a multisystem disease, it has maternal, placental, and fetal consequences. The cure for preeclampsia remains delivery of the placenta. Expectant management offers no maternal benefit, but does offer some potential neonatal benefits if prematurity is a concern. Once concerns about prematurity have been largely eliminated, generally by achieving a gestational age of 37 weeks, further expectant management is not indicated, offers little or no additional benefit to the fetus, and leaves both mother and fetus at risk.
Therefore, once the pregnancy reaches 37 weeks, delivery is recommended.
When preeclampsia is severe, and when it is superimposed in a patient who is taking antihypertensive medication, we generally do not continue the pregnancy beyond 34 weeks.4 In our institutions, most patients who are being expectantly managed for severe preeclampsia remote from term—and who have remained stable—are delivered between 32 and 34 weeks’ gestation, depending on the specific clinical circumstances.
CHALLENGE NO. 4: Controlling blood pressure
Cerebrovascular accident (stroke) is the leading cause of maternal mortality from preeclampsia in the United States. Not all cases can be prevented, but one suggested preventive strategy is adequate BP control. Some cases of stroke in the setting of preeclampsia will occur despite systemic BP readings that are not considered to be in a dangerous range. One reason may be an override of normal cerebral blood flow autoregulatory mechanisms, resulting in increased cerebral blood flow, rising cerebral perfusion pressures, and vessel rupture. Such occurrences may sometimes, but not always, be related to coagulopathy.
When a patient has elevated BP, generally defined as persistent systolic pressures above 160 to 170 mm Hg and persistent diastolic pressures above 105 to 110 mm Hg, antihypertensive therapy is indicated and should be administered in a timely fashion.
Labetalol, nifedipine, and hydralazine have all been used effectively in such acute settings, when administered parenterally (except nifedipine, which may be given orally) and when given in proper dosages (TABLE 3).
Avoid oral use of labetalol or hydralazine to treat acute hypertensive emergencies.
TABLE 3
Pharmacotherapy of acute hypertension
| Drug | Dosage | Directions |
|---|---|---|
| Hydralazine* | 5 mg IV | Repeat in 10 min, then give 10 mg IV every 20 min until BP stabilizes (140–150/90–100 mm Hg) |
| Labetalol* | 10–20 mg IV push | Repeat every 10-20 min, doubling the dosage each time until a maximum total cumulative dosage of 300 mg has been given |
| Nifedipine* | 10 mg | Repeat in 20 min for four doses (maximum 40 mg); then give 10–20 mg orally every 4–6 h to achieve a stable BP of 140–150/90–100 mm Hg |
| * If target blood pressure is not reached after the maximum dosage of an agent is given, then additional or alternative pharmacotherapy must be utilized. | ||
Goals for treatment
In the antepartum patient, the goal is to maintain systolic BP at 140 to 150 mm Hg and diastolic pressure at 90 to 100 mm Hg to keep from inadvertently inducing uteroplacental insufficiency secondary to reduced uterine blood flow.
In the delivered patient, the risk of mild hypotension is not quite as great, although an attempt to rapidly return the patient to her previous normal BP profile may cause symptomatic hypotension.
If a patient develops a true hypertensive crisis with hypertensive encephalopathy (which generally occurs at BPs exceeding 240/140 mm Hg), then emergent intervention with a rapidly acting agent such as sodium nitroprusside is necessary and should be managed by someone skilled in critical care and the use of such drugs.
CHALLENGE NO. 5: Preventing seizures
Magnesium sulfate is the drug of choice to prevent both initial and recurrent eclamptic seizures.5 Two large clinical trials ended any doubts about its efficacy, demonstrating its superiority over both phenytoin and diazepam in the settings of preeclampsia and eclampsia.
Magnesium sulfate is best administered intravenously (IV) via continuous infusion pump. An initial bolus of 4 to 6 g is given over 15 to 30 minutes; this amount does not need to be adjusted to the patient’s level of renal function. A continuous infusion of magnesium sulfate is usually initiated at a rate of 2 g/hour. It is this infusion dosage that may need to be altered, based on the patient’s urine output and renal function.
Evidence of magnesium toxicity includes:
- loss of deep-tendon reflexes
- respiratory depression
- blurred vision
- cardiotoxicity.
There is no debate about the utility of magnesium sulfate in severe preeclampsia, but when it comes to intrapartum management of mild preeclampsia or cases in which preeclampsia first manifests in the postpartum period, data are not so clear. This debate will not be resolved to anyone’s satisfaction in the course of this article. Historically, the practice has been to use magnesium in these circumstances, but the pendulum has begun to shift based on a few arguments:
- Eclampsia is a rare event (about 1 case for every 300 to 1,000 deliveries).
- Most cases occur outside of the hospital.
- Some women experience seizures before preeclampsia has been diagnosed.
- Some patients experience seizures while taking magnesium sulfate.
Regardless of one’s position on this debate, there is broad consensus that regular careful clinical assessment of the patient who has preeclampsia is essential to minimize morbidity and mortality. This disease can progress from mild to severe rapidly. Only through regular careful assessment can a physician observe this change soon enough to alter management as necessary.
Treatment of magnesium toxicity
Most often, an ampule of 10% calcium gluconate (1 g) is administered IV to reverse the effects of suspected magnesium toxicity.
In addition, because magnesium freely crosses the placenta, we recommend that a newborn resuscitation team be present at all deliveries during which the mother was receiving magnesium sulfate because neonatal respiratory and cardiac depression have been reported in this setting.
CHALLENGE NO. 6: Delivering the patient
Preeclampsia, severe preeclampsia, and eclampsia present a dilemma for the managing clinician: subject her to the rigors of labor, or to the heightened risk of cesarean delivery? Overall, a properly managed vaginal delivery is less hemodynamically stressful than cesarean delivery for the mother. To accomplish vaginal delivery, it is necessary to provide optimal anesthesia and analgesia.
Risks of regional anesthesia
Women who have preeclampsia are volume-depleted. As such, they are prone to hypotension after administration of regional anesthesia if the block sets up too rapidly. For this reason, epidural anesthesia or some of the newer combined techniques offer optimal analgesia by allowing for slower implementation of the regional block.
Women who have preeclampsia, especially severe preeclampsia, are usually candidates for regional analgesia and anesthesia. Some requisites for regional anesthesia under these conditions include the following:
- The patient can tolerate preblock hydration.
- She has adequate IV access.
- There is a reproducible means of determining BP.
- The patient has a normal coagulation profile. (A normal platelet count with normal transaminase should be sufficient to confirm this; women who have preeclampsia are not at increased risk of having altered prothrombin time, partial thromboplastin time, or fibrinogen levels, provided there are no other mitigating clinical circumstances.)
- The anesthesiology team is skilled in the administration of regional anesthesia.
If eclampsia occurs
Do not proceed to emergent cesarean section. Rather, stabilize the mother, protect her from injury during the seizure, protect her airway, and allow the seizure to take its course.
Begin magnesium at once. If it was being infused before the seizure, consider giving an additional 2-g bolus over several minutes. As the mother stabilizes, the fetal heart rate will recover and she can be reassessed to determine optimal timing and route of delivery.
Continue magnesium after delivery?
Yes, but how long remains unclear. Most authorities have recommended 24 hours, based on the observation that most eclamptic seizures that occur in the first 48 hours postpartum actually occur in the first 24 hours.
Clinical assessment can guide management to some degree. The most reliable sign of disease resolution is spontaneous, brisk diuresis, so some clinicians use this finding as an indication to discontinue magnesium.
Regardless of clinical preference, if magnesium sulfate is being used postpartum, continue it until there is evidence of disease resolution, such as the diuresis noted above.
When HELLP syndrome arises
If HELLP [Hemolysis, Elevated Liver en zymes, Low Platelets] syndrome is present, continue magnesium sulfate until there is laboratory evidence of improvement in the platelet count and transaminase. Because a return to normal levels can take several days, it is not required before discontinuation of magnesium in cases of HELLP syndrome. However, at the time of discontinuation, it should be clear that there is no longer evidence of a worsening laboratory or clinical trend.
CHALLENGE NO. 7: Managing HELLP, atypical eclampsia
These two diagnoses pose daunting clinical challenges too numerous to cover in detail in this article, but a few key points merit consideration. When HELLP syndrome is diagnosed (using established criteria, TABLE 4), follow guidelines for severe preeclampsia. Use of dexamethasone remains somewhat controversial, as randomized clinical trials so far do not support it.6
Atypical eclampsia has been defined as eclampsia that occurs before 20 weeks’ gestation or from 48 hours to 14 days after delivery. Its management is similar to the management of eclampsia, with BP control and magnesium sulfate being the mainstays of therapy.
Because of the relative rarity of atypical eclampsia, we recommend neurologic consultation in these cases to evaluate for other possible causes of seizure.
TABLE 4
HELLP syndrome—Sibai criteria
|
CASE RESOLVED
After initial hospitalization, the patient is monitored as an outpatient until 35 weeks’ gestation, when more labile BP and increased proteinuria necessitate hospitalization. However, her preeclampsia remains mild by definition and, after continued reassuring fetal testing, she undergoes labor induction at 37 weeks.
1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-167.
3. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
4. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514-519.
5. Altman D, Carroli G, Duley L, et al:. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
6. Fonseca JE, Méndez F, Cataño C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005;193:1591-1598.
1. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1-S22.
2. ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159-167.
3. Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402-410.
4. Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514-519.
5. Altman D, Carroli G, Duley L, et al:. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
6. Fonseca JE, Méndez F, Cataño C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obstet Gynecol. 2005;193:1591-1598.