User login
Managing Chronic, Nonmalignant Pain in Patients with a Substance Use Disorder
Reducing the legal risks of labor induction and augmentation
- 18 common allegations in oxytocin-related litigation
- 6 risk-reducing strategies
Martin L. Gimovsky, MD
WHAT’S YOUR VERDICT?
Does this patient have grounds for a lawsuit?
At 41 weeks’ estimated gestation, Elena, a 32-year-old primipara with an uneventful antepartum course, is scheduled for induction of labor for postdates. On admission she is 1 cm dilated and 70% effaced, with the fetal vertex at –3 station. Fetal heart rate monitoring shows a normal baseline, moderate variability, and accelerations. No decelerations are observed.
After the membranes are ruptured artificially, labor progresses slowly, and chorioamnionitis is suspected.
Fetal tachycardia with minimal variability and variable decelerations develops. Oxytocin is titrated to achieve uterine contractions every 2 minutes. Elena eventually becomes completely dilated and pushes for 95 minutes. During this time, the fetal variable decelerations increase in duration, with loss of variability and continued tachycardia.
Because of these findings, delivery is expedited with a vacuum extractor. The newborn is depressed, admitted to the neonatal intensive care unit for respiratory support to “rule out sepsis,” and is later found to have neurologic injury.
In your opinion, does Elena have grounds for a lawsuit?
If such a case spurs a lawsuit, as it often will, the plaintiff’s attorney is likely to declare any or all of these allegations:
- failure to discontinue oxytocin in light of nonreassuring fetal heart rate
- failure to identify and respond to uterine hyperstimulation
- failure to identify and respond to fetal distress
- failure to react in a timely manner to fetal distress
- inappropriate delivery method
- failure to use a fetal scalp electrode
- failure to recognize and act upon arrest of dilatation in a timely manner
These allegations are only the most probable ones in circumstances such as Elena’s. When unanticipated morbidity or death occurs after oxytocin is used, physicians and nurses may find themselves facing any of the 18 allegations listed in the TABLE—or even others.
In court, these allegations will be based on the opinions of independent physicians, certified nurse-midwives, and registered nurses with the education, experience, and credentials to qualify as “experts.” Courts usually allow experts when the substance of the allegations is beyond the public’s general knowledge.
Although allegations often include inaccuracies, erroneous assumptions, and conclusions based on “information and belief” rather than scientific evidence, they remain part of the claim until disproved over the course of the legal proceedings.
TABLE
18 common allegations in oxytocin-related litigation
| 1. Unnecessary induction due to lack of medical indication |
| 2. Failure to establish fetal well-being prior to initiating oxytocin |
| 3. Failure to adequately monitor fetal heart rate during oxytocin infusion |
| 4. Failure to adequately monitor uterine contractions |
| 5. Failure to place a spiral electrode and/or intrauterine pressure catheter |
| 6. Failure to discontinue oxytocin in light of nonreassuring fetal heart rate |
| 7. Failure to identify and respond to fetal distress |
| 8. Delay in identifying and responding to nonreassuring fetal heart rate |
| 9. Failure to notify provider of nonreassuring fetal heart rate |
| 10. Failure to identify and respond to uterine hyperstimulation and/or elevated resting tone |
| 11. Inappropriate titration of oxytocin not based on accepted protocols |
| 12. Administration of oxytocin without a physician’s order |
| 13. Failure to follow physician’s order |
| 14. Failure to order a cesarean section when fetal heart rate became nonreassuring |
| 15. Delay in cesarean section after being ordered by the physician |
| 16. Failure to follow hospital policies and procedures |
| 17. Inadequate policies and procedures governing oxytocin administration |
| 18. Failure to initiate chain of command |
Elective inductions can spell trouble
Although the rate of induction has more than doubled since 1989, to 20.6% of births or more than 840,000 pregnancies in 2003,1 still no consensus exists for patient selection. In some centers, inductions are reserved for women with medical indications only, whereas in others, more than half are elective.2
Because of this divergence, when there is a negative outcome after an elective induction, the obstetrician can anticipate an allegation of unnecessary induction due to lack of a medical indication.
Fetal monitoring
Proven or not, it’s the norm
Although we lack overwhelming proof of its superiority to intermittent auscultation,3 electronic fetal monitoring (EFM) is used in most labor and delivery settings during oxytocin administration for induction or augmentation of labor—and fetal heart rate and uterine activity typically guide initiation and titration of oxytocin.
Nevertheless, because EFM is the unofficial standard, an obstetrician who chooses to use intermittent auscultation of fetal heart rate during oxytocin infusion can anticipate strong criticism if the delivery results in a compromised neonate.
A 2005 Cochrane review4 of 18,561 births compared EFM with intermittent auscultation in labor and delivery and found fewer neonatal seizures in the EFM group but no differences in Apgar scores of less than 4 and 7, NICU admissions, perinatal deaths, or cerebral palsy.
“Default” intervals
Not only is the type of fetal monitoring important, but also how closely and how often the strip is evaluated. However, no studies have determined the optimal frequency of EFM interpretation during normal labors, let alone those induced or augmented with oxytocin. Furthermore, no single best methodology has been identified. Rather, the “default” timing of EFM interpretation has been loosely based on the historical practice of evaluating and documenting intermittent auscultation at 30-minute intervals during active labor and 15-minute intervals during the second stage for low-risk patients. For high-risk patients, the intervals have been every 15 minutes during the active phase and every 5 minutes during the second stage.
What will expert witnesses look for?
After adverse outcomes, the EFM tracing will be examined closely by “experts” looking for evidence that it contained abnormalities demonstrating fetal compromise or predicting the infant’s injury or death.
These experts also scrutinize the actions of physicians and nurses for appropriateness, timeliness, and effectiveness; the timing of the decision for expedited delivery; and the events occurring between that decision and the time of delivery or abdominal skin incision.
The monitor’s shortcomings
Many courts now require experts to base their opinions on reliable scientific studies; however, in malpractice claims involving EFM, expert interpretation often is based on the expert’s own personal or institutional experience or common practices rather than scientific evidence.
One of the most pervasive public misconceptions is that fetal monitoring can reliably detect when a fetus lacks sufficient oxygen, is experiencing a physiologically stressful labor that is depleting oxygen reserves, or is becoming asphyxiated. In reality, the positive predictive value (ability of the technology to identify the compromised fetus without including healthy fetuses) is very low: 0.14%. Thus, of 1,000 fetuses with nonreassuring tracings, only 1 or 2 are actually compromised.5 This may explain why providers and nurses are reluctant to deem all nonreassuring recordings as accurate.
The only thing EFM reliably identifies with a high degree of specificity is the oxygenated fetus that is not experiencing metabolic acidemia. Recordings with “nonreassuring” features are statistically unlikely to imply a diagnosis of fetal metabolic acidosis, hypoxemia, or stress or distress.
Should EFM precede oxytocin?
No minimal duration of monitoring prior to oxytocin administration has been consistently determined. Researchers do not even agree that initial monitoring of the fetus scheduled for induction has benefit.
This does not mean that oxytocin can be started without knowledge of the maternal and fetal condition—only that the best timing and methods of assessment prior to induction of labor are unknown.
What is “nonreassuring”?
Starting oxytocin in a woman with a “non-reassuring” tracing opens the OB to criticism. This is the most contentious aspect of medical and nursing management because we lack standardized definitions of “reassuring” and “nonreassuring.”
Nurses typically label a tracing nonreassuring based solely on decelerations or other variant patterns such as tachycardia. However, while a tracing’s individual characteristics may reflect a variety of etiologies (one of which is decreased uteroplacental perfusion), variability and/or accelerations signify an overall reassuring status, or fetal tolerance of labor.
Physicians generally examine the tracing in light of other clinical factors, such as labor progress, historical data, or parity—and also in light of any specific actions that have been taken and the expected time of their peak effect.
When to notify the OB
Another contentious issue in labor induction is exactly when nurses should notify the physician of a nonreassuring fetal heart rate. Unfortunately, there is no consensus about this question, either; again, most EFM tracings requiring nursing intervention exhibit an overall reassuring status.
Because evaluation of nonreassuring findings may take several minutes, nurses usually notify the physician when their assessment is complete. If the worrisome tracing resolves after intervention, a nurse may appropriately postpone notification until the next opportunity for communication with the physician.
Uterine monitoring
Can monitoring predict rupture?
In cases involving uterine rupture and/or placental abruption, experts may allege that the event could have been predicted with an intrauterine pressure catheter. However, in a study of “controlled” uterine rupture (recording of intrauterine pressure before and during uterine incision at the time of cesarean section), Devoe et al6 found no real differences in contraction frequency or duration, peak contraction pressures, or uterine resting tone prior to and after uterine “rupture” (incision).
We also lack prospective studies demonstrating that intrauterine pressure catheterization can predict placental abruption. Placement of the device purely for this reason is not indicated.
Titration of oxytocin
No consensus on frequency or intensity of contractions
Criticism of the method of oxytocin titration is common in malpractice claims because no data satisfactorily define adequate frequency or intensity of contractions.
Nor do we have widely accepted terminology to describe uterine activity. For example, hyperstimulation is sometimes defined as increased frequency of contractions with an abnormal fetal heart rate tracing, and sometimes as increased frequency of contractions without a nonreassuring fetal heart rate. The same inconsistencies hold true for the terms “hypertonus,” “tetany,” “tachysystole,” and others.
“Adequate labor pattern” has been defined as 3 to 5 contractions in 10 minutes or 7 contractions in 15 minutes,7 even though these criteria are based on limited data. Although clinically adequate labor is defined by cervical dilatation and effacement with fetal descent, this definition frequently leaves us titrating oxytocin by “trial and error.” Fortunately, the half-life of oxytocin is short, and we can use fetal and uterine response to guide titration.
No definitive predictors of rupture, abruption, asphyxiation
When uterine rupture, placental abruption, and/or variant fetal heart patterns occur with hyperstimulation or elevated resting tone, the possibility of a cause-and-effect will be explored in legal claims. Although uterine rupture has been attributed to oxytocin in older, nonprospective, uncontrolled studies, more recent investigations8 failed to confirm this link.
The effect of uterine hyperstimulation on fetal oxygenation is even less well established. Contractions increase placental vascular resistance, which in turn decreases uteroplacental blood flow. This phenomenon has been demonstrated in studies utilizing Doppler velocimetry,9 radioangiography,10 and fetal pulse oximetry.11 However, none have been able to quantify, in millimeters of mercury, the intensity of uterine contractions or baseline tonus required to compromise fetal oxygenation.
Risk-reducing tactics
These strategies12 do not represent the standard of care, but may help reduce liability:
- Routinely assess fetal heart rate during examination of the laboring patient.
- Document EFM interpretation comprehensively. Include baseline, variability, accelerations, decelerations, and uterine activity, as well as overall impression.
- Date and time every entry.
- When notified of a finding, detail the notification, as well as the orders and plan of care communicated to the nurse.
- Develop a mechanism for documentation when you are located outside the hospital (eg, progress notes that are later posted in the chart).
- Use digital storage and retrieval with central monitoring of displays to allow physicians to observe EFM tracings via remote access.
- Use handheld PDA-type displays.
- Go to the bedside to evaluate a patient when nurses ask you to do so. Document date and time, and the fetal heart rate interpretation.
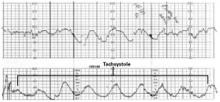
- Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion (FIGURE 1).
- Avoid further increases in oxytocin once adequate labor (progressive cervical change) is established.
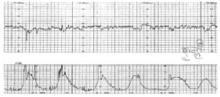
- Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes (FIGURE 2).
- Use National Institute of Child Health and Human Development terminology in verbal communications with nurses and physicians (see the Web version of this article for a downloadable PDF file of this terminology).
Martin L. Gimovsky, MD
Program Director, Department of Obstetrics and Gynecology, Newark Beth Israel Medical Center, Newark, NJ
Clinical Professor of Obstetrics and Gynecology, Mount Sinai School of Medicine, New York City
As obstetricians, we are fortunate to participate in the most basic aspect of the human condition: the need to reproduce. Sometimes it is easy to overlook this fact, given the routine nature of many of our practices.
A case in point: oxytocin administration to induce or augment labor, an everyday occurrence in virtually all labor and delivery suites. Oxytocin is so ubiquitous, it can be easy to use it less than meticulously. Although the risks associated with its use are largely recognized, and the appropriate responses well known, a few points bear repeating.
Twin challenges: Protect and document
Safe and judicious use of oxytocin involves 2 challenges: minimizing medical risks to mother and fetus, and creating a supportive medical record. As in all aspects of medical care, we are required to know how to handle the clinical situation, and to document our skill, knowledge, and experience. Nowhere is this of greater concern than in the management of labor and delivery.
Here are 6 additional strategies for reducing legal risks of oxytocin use in labor.
1. Start with a written note
I suggest entering a written note into the record prior to administering oxytocin, outlining the reasoning behind the decision to proceed. Taking this pretreatment pause or “time out”—as the Joint Commission on Accreditation of Healthcare Organizations calls it—provides an opportunity to consider the risks, benefits, and alternatives of oxytocin use. This note should include the medical indication.
2. Conduct a comprehensive consent process
A passive signature on a general consent form is a minimalist way of demonstrating patient consent. By beginning the charting at the time of the consent discussion, you can demonstrate your consideration of the patient’s understanding and desires, not to mention your adherence to the highest standards of care.
Was an alternative approach possible? The patient should have the benefit of your opinion as well as a discussion of other reasonable strategies. Involving her in an active discussion is a fundamental component of informed consent—especially since improper consent is a frequent allegation in malpractice actions.
3. Describe both uterine and fetal responses
Because oxytocin directly affects uterine activity and indirectly affects placental perfusion, any chart notation needs to include references to both. For example, the comment that “contractions are every 2 minutes” requires the additional observation that the fetal heart rate tracing “is reassuring,”…“unchanged from earlier,”…or “demonstrates changes that are being evaluated.”
Whether a notation is made at the time of a routine labor check or when the physician is called to the bedside, comments on both uterine activity and fetal response are needed.
4. Discontinue oxytocin when the uterus overreacts
On occasion, excessive uterine activity may occur when oxytocin is first administered. Excessive uterine activity on a continuing basis can lead to fetal asphyxia. Although reducing the oxytocin dose will ultimately diminish uterine activity, I teach residents to discontinue oxytocin completely as soon as excessive uterine activity occurs.
Because this is a clinically important intervention, the medical record should be notated.
5. Adjust oxytocin to reflect changes in labor patterns
It makes good sense to avoid further oxytocin increases once the patient is in active labor (ie, progressive cervical change) and to decrease doses when contractions occur more frequently than every 2 minutes, even in the face of a reassuring fetal heart rate. This is not a situation in which, “if a little is good, a lot is better.”
6. Consider including a labor curve
Adding a labor curve or partograph to the chart can be a further safeguard, as it makes it easy to identify prolonged labors and potential complications in a timely manner.
All 6 strategies help demonstrate and preserve your hard work and concern for the patient. As always, adherence to principles of sound care and communication is the bedrock of successful obstetrics. There is no substitute.
The author reports no financial relationships relevant to this article.
FIGURE 1 Tachysystole with decelerations signifies uterine hyperstimulation
Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion. This tracing shows uterine hyperstimulation (tachysystole with decelerations).
FIGURE 2 Titrate oxytocin to “normalize” contractions
Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes. This tracing shows 6 uterine contractions in 10 minutes. The fetal heart rate channel demonstrates moderate variability and, therefore, fetal tolerance of a frequent contraction pattern.
Why policies and procedures are a double-edged sword
Although policies and procedures are intended to help guide health care assessments and interventions, they are routinely subpoenaed and entered as evidence in an attempt to define the standard of care. Failure to follow these policies and procedures may be viewed by expert witnesses as a breach in that standard.
Use of oxytocin requires a medical or nursing professional to make judgments based on training, experience, and knowledge. Although policies and procedures cannot address every possible scenario or replace informed judgment, physicians and nurses are routinely criticized for failing to administer oxytocin or otherwise proceed exactly as outlined.
Some reasons policies and procedures should not be viewed as standard of care:
- They are typically written by a person in an administrative position who does not actually provide the care outlined.
- They are usually not routinely updated as new literature is published.
- Since they do not provide guidelines for unanticipated or unusual situations, deviation from policy is reasonable and even necessary in many scenarios.
- They are rarely written to reflect “reasonable” care; instead, they suggest an “ideal” level of care.
Reasonable protocols. Every physician and health care provider should be familiar with the hospital’s policies and procedures and help hospital personnel revise those that appear to limit the physician’s ability to easily adjust care or exercise judgment. Among the suggestions:
- Make all recommendations practical. This means they can be followed most of the time in most situations.
- Avoid terms such as “mandatory,” “always,” “never,” “should,” or “must.”
- Limit recommendations that can be considered “endpoints” for increasing oxytocin, such as: “Increase oxytocin until contractions are 2 to 3 minutes apart and 60 seconds in duration.” Recommendations written in this fashion are difficult to follow clinically; although the criteria may be met, labor may not progress, warranting an increase in oxytocin beyond the endpoints in the guidelines. Guidelines that discuss considerations for decreasing or discontinuing the drug would be better.
It also is important to foster understanding among medical and nursing staff that policies and procedures are guidelines and that medical and nursing judgment supersedes policy recommendations.
The authors report no financial relationships relevant to this article.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Birth: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1-116.
2. Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstet Gynecol. 2002;100:164-167.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
4. Thacker SB, et al. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2005;(3):ISSN 1464-780X.
5. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 62, May 2005: Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;105:1161-1169.
6. Devoe LD, Croom CS, et al. The prediction of “controlled” uterine rupture by the use of intrauterine pressure catheters. Obstet Gynecol. 1992;80:626-629.
7. Norwitz ER, Robinson JN, Repke JT. Labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics. Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone; 2002;355.-
8. Phelan JP, Korst LM, Settles DK. Uterine activity patterns in uterine rupture: a case-control study. Obstet Gynecol. 1998;92:394-397.
9. Bower S, Campbell S, Vyas S, McGirr C. Braxton-Hicks contractions can alter uteroplacental perfusion. Ultrasound Obstet Gynecol. 1991;1:46-49.
10. Borell U, Fernstroem I, Ohlson L, Wiqvist N. Influence of uterine contractions on the uteroplacental blood flow at term. Am J Obstet Gynecol. 1965;93:44-57.
11. Johnson N, van Oudgaarden E, Montague I, McNamara H. The effect of oxytocin-induced hyperstimulation on fetal oxygen. Br J Obstet Gynaecol. 1994;101:805-807.
12. Lucidi RS, Chez RA, et al. The clinical use of intrauterine pressure catheters. J Matern Fetal Med. 2001;10:420-422.
- 18 common allegations in oxytocin-related litigation
- 6 risk-reducing strategies
Martin L. Gimovsky, MD
WHAT’S YOUR VERDICT?
Does this patient have grounds for a lawsuit?
At 41 weeks’ estimated gestation, Elena, a 32-year-old primipara with an uneventful antepartum course, is scheduled for induction of labor for postdates. On admission she is 1 cm dilated and 70% effaced, with the fetal vertex at –3 station. Fetal heart rate monitoring shows a normal baseline, moderate variability, and accelerations. No decelerations are observed.
After the membranes are ruptured artificially, labor progresses slowly, and chorioamnionitis is suspected.
Fetal tachycardia with minimal variability and variable decelerations develops. Oxytocin is titrated to achieve uterine contractions every 2 minutes. Elena eventually becomes completely dilated and pushes for 95 minutes. During this time, the fetal variable decelerations increase in duration, with loss of variability and continued tachycardia.
Because of these findings, delivery is expedited with a vacuum extractor. The newborn is depressed, admitted to the neonatal intensive care unit for respiratory support to “rule out sepsis,” and is later found to have neurologic injury.
In your opinion, does Elena have grounds for a lawsuit?
If such a case spurs a lawsuit, as it often will, the plaintiff’s attorney is likely to declare any or all of these allegations:
- failure to discontinue oxytocin in light of nonreassuring fetal heart rate
- failure to identify and respond to uterine hyperstimulation
- failure to identify and respond to fetal distress
- failure to react in a timely manner to fetal distress
- inappropriate delivery method
- failure to use a fetal scalp electrode
- failure to recognize and act upon arrest of dilatation in a timely manner
These allegations are only the most probable ones in circumstances such as Elena’s. When unanticipated morbidity or death occurs after oxytocin is used, physicians and nurses may find themselves facing any of the 18 allegations listed in the TABLE—or even others.
In court, these allegations will be based on the opinions of independent physicians, certified nurse-midwives, and registered nurses with the education, experience, and credentials to qualify as “experts.” Courts usually allow experts when the substance of the allegations is beyond the public’s general knowledge.
Although allegations often include inaccuracies, erroneous assumptions, and conclusions based on “information and belief” rather than scientific evidence, they remain part of the claim until disproved over the course of the legal proceedings.
TABLE
18 common allegations in oxytocin-related litigation
| 1. Unnecessary induction due to lack of medical indication |
| 2. Failure to establish fetal well-being prior to initiating oxytocin |
| 3. Failure to adequately monitor fetal heart rate during oxytocin infusion |
| 4. Failure to adequately monitor uterine contractions |
| 5. Failure to place a spiral electrode and/or intrauterine pressure catheter |
| 6. Failure to discontinue oxytocin in light of nonreassuring fetal heart rate |
| 7. Failure to identify and respond to fetal distress |
| 8. Delay in identifying and responding to nonreassuring fetal heart rate |
| 9. Failure to notify provider of nonreassuring fetal heart rate |
| 10. Failure to identify and respond to uterine hyperstimulation and/or elevated resting tone |
| 11. Inappropriate titration of oxytocin not based on accepted protocols |
| 12. Administration of oxytocin without a physician’s order |
| 13. Failure to follow physician’s order |
| 14. Failure to order a cesarean section when fetal heart rate became nonreassuring |
| 15. Delay in cesarean section after being ordered by the physician |
| 16. Failure to follow hospital policies and procedures |
| 17. Inadequate policies and procedures governing oxytocin administration |
| 18. Failure to initiate chain of command |
Elective inductions can spell trouble
Although the rate of induction has more than doubled since 1989, to 20.6% of births or more than 840,000 pregnancies in 2003,1 still no consensus exists for patient selection. In some centers, inductions are reserved for women with medical indications only, whereas in others, more than half are elective.2
Because of this divergence, when there is a negative outcome after an elective induction, the obstetrician can anticipate an allegation of unnecessary induction due to lack of a medical indication.
Fetal monitoring
Proven or not, it’s the norm
Although we lack overwhelming proof of its superiority to intermittent auscultation,3 electronic fetal monitoring (EFM) is used in most labor and delivery settings during oxytocin administration for induction or augmentation of labor—and fetal heart rate and uterine activity typically guide initiation and titration of oxytocin.
Nevertheless, because EFM is the unofficial standard, an obstetrician who chooses to use intermittent auscultation of fetal heart rate during oxytocin infusion can anticipate strong criticism if the delivery results in a compromised neonate.
A 2005 Cochrane review4 of 18,561 births compared EFM with intermittent auscultation in labor and delivery and found fewer neonatal seizures in the EFM group but no differences in Apgar scores of less than 4 and 7, NICU admissions, perinatal deaths, or cerebral palsy.
“Default” intervals
Not only is the type of fetal monitoring important, but also how closely and how often the strip is evaluated. However, no studies have determined the optimal frequency of EFM interpretation during normal labors, let alone those induced or augmented with oxytocin. Furthermore, no single best methodology has been identified. Rather, the “default” timing of EFM interpretation has been loosely based on the historical practice of evaluating and documenting intermittent auscultation at 30-minute intervals during active labor and 15-minute intervals during the second stage for low-risk patients. For high-risk patients, the intervals have been every 15 minutes during the active phase and every 5 minutes during the second stage.
What will expert witnesses look for?
After adverse outcomes, the EFM tracing will be examined closely by “experts” looking for evidence that it contained abnormalities demonstrating fetal compromise or predicting the infant’s injury or death.
These experts also scrutinize the actions of physicians and nurses for appropriateness, timeliness, and effectiveness; the timing of the decision for expedited delivery; and the events occurring between that decision and the time of delivery or abdominal skin incision.
The monitor’s shortcomings
Many courts now require experts to base their opinions on reliable scientific studies; however, in malpractice claims involving EFM, expert interpretation often is based on the expert’s own personal or institutional experience or common practices rather than scientific evidence.
One of the most pervasive public misconceptions is that fetal monitoring can reliably detect when a fetus lacks sufficient oxygen, is experiencing a physiologically stressful labor that is depleting oxygen reserves, or is becoming asphyxiated. In reality, the positive predictive value (ability of the technology to identify the compromised fetus without including healthy fetuses) is very low: 0.14%. Thus, of 1,000 fetuses with nonreassuring tracings, only 1 or 2 are actually compromised.5 This may explain why providers and nurses are reluctant to deem all nonreassuring recordings as accurate.
The only thing EFM reliably identifies with a high degree of specificity is the oxygenated fetus that is not experiencing metabolic acidemia. Recordings with “nonreassuring” features are statistically unlikely to imply a diagnosis of fetal metabolic acidosis, hypoxemia, or stress or distress.
Should EFM precede oxytocin?
No minimal duration of monitoring prior to oxytocin administration has been consistently determined. Researchers do not even agree that initial monitoring of the fetus scheduled for induction has benefit.
This does not mean that oxytocin can be started without knowledge of the maternal and fetal condition—only that the best timing and methods of assessment prior to induction of labor are unknown.
What is “nonreassuring”?
Starting oxytocin in a woman with a “non-reassuring” tracing opens the OB to criticism. This is the most contentious aspect of medical and nursing management because we lack standardized definitions of “reassuring” and “nonreassuring.”
Nurses typically label a tracing nonreassuring based solely on decelerations or other variant patterns such as tachycardia. However, while a tracing’s individual characteristics may reflect a variety of etiologies (one of which is decreased uteroplacental perfusion), variability and/or accelerations signify an overall reassuring status, or fetal tolerance of labor.
Physicians generally examine the tracing in light of other clinical factors, such as labor progress, historical data, or parity—and also in light of any specific actions that have been taken and the expected time of their peak effect.
When to notify the OB
Another contentious issue in labor induction is exactly when nurses should notify the physician of a nonreassuring fetal heart rate. Unfortunately, there is no consensus about this question, either; again, most EFM tracings requiring nursing intervention exhibit an overall reassuring status.
Because evaluation of nonreassuring findings may take several minutes, nurses usually notify the physician when their assessment is complete. If the worrisome tracing resolves after intervention, a nurse may appropriately postpone notification until the next opportunity for communication with the physician.
Uterine monitoring
Can monitoring predict rupture?
In cases involving uterine rupture and/or placental abruption, experts may allege that the event could have been predicted with an intrauterine pressure catheter. However, in a study of “controlled” uterine rupture (recording of intrauterine pressure before and during uterine incision at the time of cesarean section), Devoe et al6 found no real differences in contraction frequency or duration, peak contraction pressures, or uterine resting tone prior to and after uterine “rupture” (incision).
We also lack prospective studies demonstrating that intrauterine pressure catheterization can predict placental abruption. Placement of the device purely for this reason is not indicated.
Titration of oxytocin
No consensus on frequency or intensity of contractions
Criticism of the method of oxytocin titration is common in malpractice claims because no data satisfactorily define adequate frequency or intensity of contractions.
Nor do we have widely accepted terminology to describe uterine activity. For example, hyperstimulation is sometimes defined as increased frequency of contractions with an abnormal fetal heart rate tracing, and sometimes as increased frequency of contractions without a nonreassuring fetal heart rate. The same inconsistencies hold true for the terms “hypertonus,” “tetany,” “tachysystole,” and others.
“Adequate labor pattern” has been defined as 3 to 5 contractions in 10 minutes or 7 contractions in 15 minutes,7 even though these criteria are based on limited data. Although clinically adequate labor is defined by cervical dilatation and effacement with fetal descent, this definition frequently leaves us titrating oxytocin by “trial and error.” Fortunately, the half-life of oxytocin is short, and we can use fetal and uterine response to guide titration.
No definitive predictors of rupture, abruption, asphyxiation
When uterine rupture, placental abruption, and/or variant fetal heart patterns occur with hyperstimulation or elevated resting tone, the possibility of a cause-and-effect will be explored in legal claims. Although uterine rupture has been attributed to oxytocin in older, nonprospective, uncontrolled studies, more recent investigations8 failed to confirm this link.
The effect of uterine hyperstimulation on fetal oxygenation is even less well established. Contractions increase placental vascular resistance, which in turn decreases uteroplacental blood flow. This phenomenon has been demonstrated in studies utilizing Doppler velocimetry,9 radioangiography,10 and fetal pulse oximetry.11 However, none have been able to quantify, in millimeters of mercury, the intensity of uterine contractions or baseline tonus required to compromise fetal oxygenation.
Risk-reducing tactics
These strategies12 do not represent the standard of care, but may help reduce liability:
- Routinely assess fetal heart rate during examination of the laboring patient.
- Document EFM interpretation comprehensively. Include baseline, variability, accelerations, decelerations, and uterine activity, as well as overall impression.
- Date and time every entry.
- When notified of a finding, detail the notification, as well as the orders and plan of care communicated to the nurse.
- Develop a mechanism for documentation when you are located outside the hospital (eg, progress notes that are later posted in the chart).
- Use digital storage and retrieval with central monitoring of displays to allow physicians to observe EFM tracings via remote access.
- Use handheld PDA-type displays.
- Go to the bedside to evaluate a patient when nurses ask you to do so. Document date and time, and the fetal heart rate interpretation.
- Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion (FIGURE 1).
- Avoid further increases in oxytocin once adequate labor (progressive cervical change) is established.
- Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes (FIGURE 2).
- Use National Institute of Child Health and Human Development terminology in verbal communications with nurses and physicians (see the Web version of this article for a downloadable PDF file of this terminology).
Martin L. Gimovsky, MD
Program Director, Department of Obstetrics and Gynecology, Newark Beth Israel Medical Center, Newark, NJ
Clinical Professor of Obstetrics and Gynecology, Mount Sinai School of Medicine, New York City
As obstetricians, we are fortunate to participate in the most basic aspect of the human condition: the need to reproduce. Sometimes it is easy to overlook this fact, given the routine nature of many of our practices.
A case in point: oxytocin administration to induce or augment labor, an everyday occurrence in virtually all labor and delivery suites. Oxytocin is so ubiquitous, it can be easy to use it less than meticulously. Although the risks associated with its use are largely recognized, and the appropriate responses well known, a few points bear repeating.
Twin challenges: Protect and document
Safe and judicious use of oxytocin involves 2 challenges: minimizing medical risks to mother and fetus, and creating a supportive medical record. As in all aspects of medical care, we are required to know how to handle the clinical situation, and to document our skill, knowledge, and experience. Nowhere is this of greater concern than in the management of labor and delivery.
Here are 6 additional strategies for reducing legal risks of oxytocin use in labor.
1. Start with a written note
I suggest entering a written note into the record prior to administering oxytocin, outlining the reasoning behind the decision to proceed. Taking this pretreatment pause or “time out”—as the Joint Commission on Accreditation of Healthcare Organizations calls it—provides an opportunity to consider the risks, benefits, and alternatives of oxytocin use. This note should include the medical indication.
2. Conduct a comprehensive consent process
A passive signature on a general consent form is a minimalist way of demonstrating patient consent. By beginning the charting at the time of the consent discussion, you can demonstrate your consideration of the patient’s understanding and desires, not to mention your adherence to the highest standards of care.
Was an alternative approach possible? The patient should have the benefit of your opinion as well as a discussion of other reasonable strategies. Involving her in an active discussion is a fundamental component of informed consent—especially since improper consent is a frequent allegation in malpractice actions.
3. Describe both uterine and fetal responses
Because oxytocin directly affects uterine activity and indirectly affects placental perfusion, any chart notation needs to include references to both. For example, the comment that “contractions are every 2 minutes” requires the additional observation that the fetal heart rate tracing “is reassuring,”…“unchanged from earlier,”…or “demonstrates changes that are being evaluated.”
Whether a notation is made at the time of a routine labor check or when the physician is called to the bedside, comments on both uterine activity and fetal response are needed.
4. Discontinue oxytocin when the uterus overreacts
On occasion, excessive uterine activity may occur when oxytocin is first administered. Excessive uterine activity on a continuing basis can lead to fetal asphyxia. Although reducing the oxytocin dose will ultimately diminish uterine activity, I teach residents to discontinue oxytocin completely as soon as excessive uterine activity occurs.
Because this is a clinically important intervention, the medical record should be notated.
5. Adjust oxytocin to reflect changes in labor patterns
It makes good sense to avoid further oxytocin increases once the patient is in active labor (ie, progressive cervical change) and to decrease doses when contractions occur more frequently than every 2 minutes, even in the face of a reassuring fetal heart rate. This is not a situation in which, “if a little is good, a lot is better.”
6. Consider including a labor curve
Adding a labor curve or partograph to the chart can be a further safeguard, as it makes it easy to identify prolonged labors and potential complications in a timely manner.
All 6 strategies help demonstrate and preserve your hard work and concern for the patient. As always, adherence to principles of sound care and communication is the bedrock of successful obstetrics. There is no substitute.
The author reports no financial relationships relevant to this article.
FIGURE 1 Tachysystole with decelerations signifies uterine hyperstimulation
Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion. This tracing shows uterine hyperstimulation (tachysystole with decelerations).
FIGURE 2 Titrate oxytocin to “normalize” contractions
Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes. This tracing shows 6 uterine contractions in 10 minutes. The fetal heart rate channel demonstrates moderate variability and, therefore, fetal tolerance of a frequent contraction pattern.
Why policies and procedures are a double-edged sword
Although policies and procedures are intended to help guide health care assessments and interventions, they are routinely subpoenaed and entered as evidence in an attempt to define the standard of care. Failure to follow these policies and procedures may be viewed by expert witnesses as a breach in that standard.
Use of oxytocin requires a medical or nursing professional to make judgments based on training, experience, and knowledge. Although policies and procedures cannot address every possible scenario or replace informed judgment, physicians and nurses are routinely criticized for failing to administer oxytocin or otherwise proceed exactly as outlined.
Some reasons policies and procedures should not be viewed as standard of care:
- They are typically written by a person in an administrative position who does not actually provide the care outlined.
- They are usually not routinely updated as new literature is published.
- Since they do not provide guidelines for unanticipated or unusual situations, deviation from policy is reasonable and even necessary in many scenarios.
- They are rarely written to reflect “reasonable” care; instead, they suggest an “ideal” level of care.
Reasonable protocols. Every physician and health care provider should be familiar with the hospital’s policies and procedures and help hospital personnel revise those that appear to limit the physician’s ability to easily adjust care or exercise judgment. Among the suggestions:
- Make all recommendations practical. This means they can be followed most of the time in most situations.
- Avoid terms such as “mandatory,” “always,” “never,” “should,” or “must.”
- Limit recommendations that can be considered “endpoints” for increasing oxytocin, such as: “Increase oxytocin until contractions are 2 to 3 minutes apart and 60 seconds in duration.” Recommendations written in this fashion are difficult to follow clinically; although the criteria may be met, labor may not progress, warranting an increase in oxytocin beyond the endpoints in the guidelines. Guidelines that discuss considerations for decreasing or discontinuing the drug would be better.
It also is important to foster understanding among medical and nursing staff that policies and procedures are guidelines and that medical and nursing judgment supersedes policy recommendations.
The authors report no financial relationships relevant to this article.
- 18 common allegations in oxytocin-related litigation
- 6 risk-reducing strategies
Martin L. Gimovsky, MD
WHAT’S YOUR VERDICT?
Does this patient have grounds for a lawsuit?
At 41 weeks’ estimated gestation, Elena, a 32-year-old primipara with an uneventful antepartum course, is scheduled for induction of labor for postdates. On admission she is 1 cm dilated and 70% effaced, with the fetal vertex at –3 station. Fetal heart rate monitoring shows a normal baseline, moderate variability, and accelerations. No decelerations are observed.
After the membranes are ruptured artificially, labor progresses slowly, and chorioamnionitis is suspected.
Fetal tachycardia with minimal variability and variable decelerations develops. Oxytocin is titrated to achieve uterine contractions every 2 minutes. Elena eventually becomes completely dilated and pushes for 95 minutes. During this time, the fetal variable decelerations increase in duration, with loss of variability and continued tachycardia.
Because of these findings, delivery is expedited with a vacuum extractor. The newborn is depressed, admitted to the neonatal intensive care unit for respiratory support to “rule out sepsis,” and is later found to have neurologic injury.
In your opinion, does Elena have grounds for a lawsuit?
If such a case spurs a lawsuit, as it often will, the plaintiff’s attorney is likely to declare any or all of these allegations:
- failure to discontinue oxytocin in light of nonreassuring fetal heart rate
- failure to identify and respond to uterine hyperstimulation
- failure to identify and respond to fetal distress
- failure to react in a timely manner to fetal distress
- inappropriate delivery method
- failure to use a fetal scalp electrode
- failure to recognize and act upon arrest of dilatation in a timely manner
These allegations are only the most probable ones in circumstances such as Elena’s. When unanticipated morbidity or death occurs after oxytocin is used, physicians and nurses may find themselves facing any of the 18 allegations listed in the TABLE—or even others.
In court, these allegations will be based on the opinions of independent physicians, certified nurse-midwives, and registered nurses with the education, experience, and credentials to qualify as “experts.” Courts usually allow experts when the substance of the allegations is beyond the public’s general knowledge.
Although allegations often include inaccuracies, erroneous assumptions, and conclusions based on “information and belief” rather than scientific evidence, they remain part of the claim until disproved over the course of the legal proceedings.
TABLE
18 common allegations in oxytocin-related litigation
| 1. Unnecessary induction due to lack of medical indication |
| 2. Failure to establish fetal well-being prior to initiating oxytocin |
| 3. Failure to adequately monitor fetal heart rate during oxytocin infusion |
| 4. Failure to adequately monitor uterine contractions |
| 5. Failure to place a spiral electrode and/or intrauterine pressure catheter |
| 6. Failure to discontinue oxytocin in light of nonreassuring fetal heart rate |
| 7. Failure to identify and respond to fetal distress |
| 8. Delay in identifying and responding to nonreassuring fetal heart rate |
| 9. Failure to notify provider of nonreassuring fetal heart rate |
| 10. Failure to identify and respond to uterine hyperstimulation and/or elevated resting tone |
| 11. Inappropriate titration of oxytocin not based on accepted protocols |
| 12. Administration of oxytocin without a physician’s order |
| 13. Failure to follow physician’s order |
| 14. Failure to order a cesarean section when fetal heart rate became nonreassuring |
| 15. Delay in cesarean section after being ordered by the physician |
| 16. Failure to follow hospital policies and procedures |
| 17. Inadequate policies and procedures governing oxytocin administration |
| 18. Failure to initiate chain of command |
Elective inductions can spell trouble
Although the rate of induction has more than doubled since 1989, to 20.6% of births or more than 840,000 pregnancies in 2003,1 still no consensus exists for patient selection. In some centers, inductions are reserved for women with medical indications only, whereas in others, more than half are elective.2
Because of this divergence, when there is a negative outcome after an elective induction, the obstetrician can anticipate an allegation of unnecessary induction due to lack of a medical indication.
Fetal monitoring
Proven or not, it’s the norm
Although we lack overwhelming proof of its superiority to intermittent auscultation,3 electronic fetal monitoring (EFM) is used in most labor and delivery settings during oxytocin administration for induction or augmentation of labor—and fetal heart rate and uterine activity typically guide initiation and titration of oxytocin.
Nevertheless, because EFM is the unofficial standard, an obstetrician who chooses to use intermittent auscultation of fetal heart rate during oxytocin infusion can anticipate strong criticism if the delivery results in a compromised neonate.
A 2005 Cochrane review4 of 18,561 births compared EFM with intermittent auscultation in labor and delivery and found fewer neonatal seizures in the EFM group but no differences in Apgar scores of less than 4 and 7, NICU admissions, perinatal deaths, or cerebral palsy.
“Default” intervals
Not only is the type of fetal monitoring important, but also how closely and how often the strip is evaluated. However, no studies have determined the optimal frequency of EFM interpretation during normal labors, let alone those induced or augmented with oxytocin. Furthermore, no single best methodology has been identified. Rather, the “default” timing of EFM interpretation has been loosely based on the historical practice of evaluating and documenting intermittent auscultation at 30-minute intervals during active labor and 15-minute intervals during the second stage for low-risk patients. For high-risk patients, the intervals have been every 15 minutes during the active phase and every 5 minutes during the second stage.
What will expert witnesses look for?
After adverse outcomes, the EFM tracing will be examined closely by “experts” looking for evidence that it contained abnormalities demonstrating fetal compromise or predicting the infant’s injury or death.
These experts also scrutinize the actions of physicians and nurses for appropriateness, timeliness, and effectiveness; the timing of the decision for expedited delivery; and the events occurring between that decision and the time of delivery or abdominal skin incision.
The monitor’s shortcomings
Many courts now require experts to base their opinions on reliable scientific studies; however, in malpractice claims involving EFM, expert interpretation often is based on the expert’s own personal or institutional experience or common practices rather than scientific evidence.
One of the most pervasive public misconceptions is that fetal monitoring can reliably detect when a fetus lacks sufficient oxygen, is experiencing a physiologically stressful labor that is depleting oxygen reserves, or is becoming asphyxiated. In reality, the positive predictive value (ability of the technology to identify the compromised fetus without including healthy fetuses) is very low: 0.14%. Thus, of 1,000 fetuses with nonreassuring tracings, only 1 or 2 are actually compromised.5 This may explain why providers and nurses are reluctant to deem all nonreassuring recordings as accurate.
The only thing EFM reliably identifies with a high degree of specificity is the oxygenated fetus that is not experiencing metabolic acidemia. Recordings with “nonreassuring” features are statistically unlikely to imply a diagnosis of fetal metabolic acidosis, hypoxemia, or stress or distress.
Should EFM precede oxytocin?
No minimal duration of monitoring prior to oxytocin administration has been consistently determined. Researchers do not even agree that initial monitoring of the fetus scheduled for induction has benefit.
This does not mean that oxytocin can be started without knowledge of the maternal and fetal condition—only that the best timing and methods of assessment prior to induction of labor are unknown.
What is “nonreassuring”?
Starting oxytocin in a woman with a “non-reassuring” tracing opens the OB to criticism. This is the most contentious aspect of medical and nursing management because we lack standardized definitions of “reassuring” and “nonreassuring.”
Nurses typically label a tracing nonreassuring based solely on decelerations or other variant patterns such as tachycardia. However, while a tracing’s individual characteristics may reflect a variety of etiologies (one of which is decreased uteroplacental perfusion), variability and/or accelerations signify an overall reassuring status, or fetal tolerance of labor.
Physicians generally examine the tracing in light of other clinical factors, such as labor progress, historical data, or parity—and also in light of any specific actions that have been taken and the expected time of their peak effect.
When to notify the OB
Another contentious issue in labor induction is exactly when nurses should notify the physician of a nonreassuring fetal heart rate. Unfortunately, there is no consensus about this question, either; again, most EFM tracings requiring nursing intervention exhibit an overall reassuring status.
Because evaluation of nonreassuring findings may take several minutes, nurses usually notify the physician when their assessment is complete. If the worrisome tracing resolves after intervention, a nurse may appropriately postpone notification until the next opportunity for communication with the physician.
Uterine monitoring
Can monitoring predict rupture?
In cases involving uterine rupture and/or placental abruption, experts may allege that the event could have been predicted with an intrauterine pressure catheter. However, in a study of “controlled” uterine rupture (recording of intrauterine pressure before and during uterine incision at the time of cesarean section), Devoe et al6 found no real differences in contraction frequency or duration, peak contraction pressures, or uterine resting tone prior to and after uterine “rupture” (incision).
We also lack prospective studies demonstrating that intrauterine pressure catheterization can predict placental abruption. Placement of the device purely for this reason is not indicated.
Titration of oxytocin
No consensus on frequency or intensity of contractions
Criticism of the method of oxytocin titration is common in malpractice claims because no data satisfactorily define adequate frequency or intensity of contractions.
Nor do we have widely accepted terminology to describe uterine activity. For example, hyperstimulation is sometimes defined as increased frequency of contractions with an abnormal fetal heart rate tracing, and sometimes as increased frequency of contractions without a nonreassuring fetal heart rate. The same inconsistencies hold true for the terms “hypertonus,” “tetany,” “tachysystole,” and others.
“Adequate labor pattern” has been defined as 3 to 5 contractions in 10 minutes or 7 contractions in 15 minutes,7 even though these criteria are based on limited data. Although clinically adequate labor is defined by cervical dilatation and effacement with fetal descent, this definition frequently leaves us titrating oxytocin by “trial and error.” Fortunately, the half-life of oxytocin is short, and we can use fetal and uterine response to guide titration.
No definitive predictors of rupture, abruption, asphyxiation
When uterine rupture, placental abruption, and/or variant fetal heart patterns occur with hyperstimulation or elevated resting tone, the possibility of a cause-and-effect will be explored in legal claims. Although uterine rupture has been attributed to oxytocin in older, nonprospective, uncontrolled studies, more recent investigations8 failed to confirm this link.
The effect of uterine hyperstimulation on fetal oxygenation is even less well established. Contractions increase placental vascular resistance, which in turn decreases uteroplacental blood flow. This phenomenon has been demonstrated in studies utilizing Doppler velocimetry,9 radioangiography,10 and fetal pulse oximetry.11 However, none have been able to quantify, in millimeters of mercury, the intensity of uterine contractions or baseline tonus required to compromise fetal oxygenation.
Risk-reducing tactics
These strategies12 do not represent the standard of care, but may help reduce liability:
- Routinely assess fetal heart rate during examination of the laboring patient.
- Document EFM interpretation comprehensively. Include baseline, variability, accelerations, decelerations, and uterine activity, as well as overall impression.
- Date and time every entry.
- When notified of a finding, detail the notification, as well as the orders and plan of care communicated to the nurse.
- Develop a mechanism for documentation when you are located outside the hospital (eg, progress notes that are later posted in the chart).
- Use digital storage and retrieval with central monitoring of displays to allow physicians to observe EFM tracings via remote access.
- Use handheld PDA-type displays.
- Go to the bedside to evaluate a patient when nurses ask you to do so. Document date and time, and the fetal heart rate interpretation.
- Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion (FIGURE 1).
- Avoid further increases in oxytocin once adequate labor (progressive cervical change) is established.
- Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes (FIGURE 2).
- Use National Institute of Child Health and Human Development terminology in verbal communications with nurses and physicians (see the Web version of this article for a downloadable PDF file of this terminology).
Martin L. Gimovsky, MD
Program Director, Department of Obstetrics and Gynecology, Newark Beth Israel Medical Center, Newark, NJ
Clinical Professor of Obstetrics and Gynecology, Mount Sinai School of Medicine, New York City
As obstetricians, we are fortunate to participate in the most basic aspect of the human condition: the need to reproduce. Sometimes it is easy to overlook this fact, given the routine nature of many of our practices.
A case in point: oxytocin administration to induce or augment labor, an everyday occurrence in virtually all labor and delivery suites. Oxytocin is so ubiquitous, it can be easy to use it less than meticulously. Although the risks associated with its use are largely recognized, and the appropriate responses well known, a few points bear repeating.
Twin challenges: Protect and document
Safe and judicious use of oxytocin involves 2 challenges: minimizing medical risks to mother and fetus, and creating a supportive medical record. As in all aspects of medical care, we are required to know how to handle the clinical situation, and to document our skill, knowledge, and experience. Nowhere is this of greater concern than in the management of labor and delivery.
Here are 6 additional strategies for reducing legal risks of oxytocin use in labor.
1. Start with a written note
I suggest entering a written note into the record prior to administering oxytocin, outlining the reasoning behind the decision to proceed. Taking this pretreatment pause or “time out”—as the Joint Commission on Accreditation of Healthcare Organizations calls it—provides an opportunity to consider the risks, benefits, and alternatives of oxytocin use. This note should include the medical indication.
2. Conduct a comprehensive consent process
A passive signature on a general consent form is a minimalist way of demonstrating patient consent. By beginning the charting at the time of the consent discussion, you can demonstrate your consideration of the patient’s understanding and desires, not to mention your adherence to the highest standards of care.
Was an alternative approach possible? The patient should have the benefit of your opinion as well as a discussion of other reasonable strategies. Involving her in an active discussion is a fundamental component of informed consent—especially since improper consent is a frequent allegation in malpractice actions.
3. Describe both uterine and fetal responses
Because oxytocin directly affects uterine activity and indirectly affects placental perfusion, any chart notation needs to include references to both. For example, the comment that “contractions are every 2 minutes” requires the additional observation that the fetal heart rate tracing “is reassuring,”…“unchanged from earlier,”…or “demonstrates changes that are being evaluated.”
Whether a notation is made at the time of a routine labor check or when the physician is called to the bedside, comments on both uterine activity and fetal response are needed.
4. Discontinue oxytocin when the uterus overreacts
On occasion, excessive uterine activity may occur when oxytocin is first administered. Excessive uterine activity on a continuing basis can lead to fetal asphyxia. Although reducing the oxytocin dose will ultimately diminish uterine activity, I teach residents to discontinue oxytocin completely as soon as excessive uterine activity occurs.
Because this is a clinically important intervention, the medical record should be notated.
5. Adjust oxytocin to reflect changes in labor patterns
It makes good sense to avoid further oxytocin increases once the patient is in active labor (ie, progressive cervical change) and to decrease doses when contractions occur more frequently than every 2 minutes, even in the face of a reassuring fetal heart rate. This is not a situation in which, “if a little is good, a lot is better.”
6. Consider including a labor curve
Adding a labor curve or partograph to the chart can be a further safeguard, as it makes it easy to identify prolonged labors and potential complications in a timely manner.
All 6 strategies help demonstrate and preserve your hard work and concern for the patient. As always, adherence to principles of sound care and communication is the bedrock of successful obstetrics. There is no substitute.
The author reports no financial relationships relevant to this article.
FIGURE 1 Tachysystole with decelerations signifies uterine hyperstimulation
Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion. This tracing shows uterine hyperstimulation (tachysystole with decelerations).
FIGURE 2 Titrate oxytocin to “normalize” contractions
Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes. This tracing shows 6 uterine contractions in 10 minutes. The fetal heart rate channel demonstrates moderate variability and, therefore, fetal tolerance of a frequent contraction pattern.
Why policies and procedures are a double-edged sword
Although policies and procedures are intended to help guide health care assessments and interventions, they are routinely subpoenaed and entered as evidence in an attempt to define the standard of care. Failure to follow these policies and procedures may be viewed by expert witnesses as a breach in that standard.
Use of oxytocin requires a medical or nursing professional to make judgments based on training, experience, and knowledge. Although policies and procedures cannot address every possible scenario or replace informed judgment, physicians and nurses are routinely criticized for failing to administer oxytocin or otherwise proceed exactly as outlined.
Some reasons policies and procedures should not be viewed as standard of care:
- They are typically written by a person in an administrative position who does not actually provide the care outlined.
- They are usually not routinely updated as new literature is published.
- Since they do not provide guidelines for unanticipated or unusual situations, deviation from policy is reasonable and even necessary in many scenarios.
- They are rarely written to reflect “reasonable” care; instead, they suggest an “ideal” level of care.
Reasonable protocols. Every physician and health care provider should be familiar with the hospital’s policies and procedures and help hospital personnel revise those that appear to limit the physician’s ability to easily adjust care or exercise judgment. Among the suggestions:
- Make all recommendations practical. This means they can be followed most of the time in most situations.
- Avoid terms such as “mandatory,” “always,” “never,” “should,” or “must.”
- Limit recommendations that can be considered “endpoints” for increasing oxytocin, such as: “Increase oxytocin until contractions are 2 to 3 minutes apart and 60 seconds in duration.” Recommendations written in this fashion are difficult to follow clinically; although the criteria may be met, labor may not progress, warranting an increase in oxytocin beyond the endpoints in the guidelines. Guidelines that discuss considerations for decreasing or discontinuing the drug would be better.
It also is important to foster understanding among medical and nursing staff that policies and procedures are guidelines and that medical and nursing judgment supersedes policy recommendations.
The authors report no financial relationships relevant to this article.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Birth: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1-116.
2. Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstet Gynecol. 2002;100:164-167.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
4. Thacker SB, et al. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2005;(3):ISSN 1464-780X.
5. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 62, May 2005: Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;105:1161-1169.
6. Devoe LD, Croom CS, et al. The prediction of “controlled” uterine rupture by the use of intrauterine pressure catheters. Obstet Gynecol. 1992;80:626-629.
7. Norwitz ER, Robinson JN, Repke JT. Labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics. Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone; 2002;355.-
8. Phelan JP, Korst LM, Settles DK. Uterine activity patterns in uterine rupture: a case-control study. Obstet Gynecol. 1998;92:394-397.
9. Bower S, Campbell S, Vyas S, McGirr C. Braxton-Hicks contractions can alter uteroplacental perfusion. Ultrasound Obstet Gynecol. 1991;1:46-49.
10. Borell U, Fernstroem I, Ohlson L, Wiqvist N. Influence of uterine contractions on the uteroplacental blood flow at term. Am J Obstet Gynecol. 1965;93:44-57.
11. Johnson N, van Oudgaarden E, Montague I, McNamara H. The effect of oxytocin-induced hyperstimulation on fetal oxygen. Br J Obstet Gynaecol. 1994;101:805-807.
12. Lucidi RS, Chez RA, et al. The clinical use of intrauterine pressure catheters. J Matern Fetal Med. 2001;10:420-422.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Birth: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1-116.
2. Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstet Gynecol. 2002;100:164-167.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
4. Thacker SB, et al. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2005;(3):ISSN 1464-780X.
5. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 62, May 2005: Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;105:1161-1169.
6. Devoe LD, Croom CS, et al. The prediction of “controlled” uterine rupture by the use of intrauterine pressure catheters. Obstet Gynecol. 1992;80:626-629.
7. Norwitz ER, Robinson JN, Repke JT. Labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics. Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone; 2002;355.-
8. Phelan JP, Korst LM, Settles DK. Uterine activity patterns in uterine rupture: a case-control study. Obstet Gynecol. 1998;92:394-397.
9. Bower S, Campbell S, Vyas S, McGirr C. Braxton-Hicks contractions can alter uteroplacental perfusion. Ultrasound Obstet Gynecol. 1991;1:46-49.
10. Borell U, Fernstroem I, Ohlson L, Wiqvist N. Influence of uterine contractions on the uteroplacental blood flow at term. Am J Obstet Gynecol. 1965;93:44-57.
11. Johnson N, van Oudgaarden E, Montague I, McNamara H. The effect of oxytocin-induced hyperstimulation on fetal oxygen. Br J Obstet Gynaecol. 1994;101:805-807.
12. Lucidi RS, Chez RA, et al. The clinical use of intrauterine pressure catheters. J Matern Fetal Med. 2001;10:420-422.
Preventing fragility fractures: Effective drugs and doses
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
Primary Care Evaluation of Dizziness
Vascular Malformations of the Colon
Tradition is yielding to new technology’s advantages, time-tested though they are not—yet
Even as we scramble to gather definitive evidence on the immediate and long-term benefits of new technologies, they are supplanting tradition in the surgical treatment of incontinence and prolapse. Surgeons have been swift to adopt synthetic mesh and the new generation of needle suspension procedures, which offer the double advantage of a shorter operative time and shorter postoperative recovery. Yet, we lack well-designed randomized prospective clinical studies on whether outcomes and complication rates are better than traditional therapies such as vaginal colporrhaphy and paravaginal repair.
There hasn’t been time.
These innovations came onto the market in rapid succession, accompanied by aggressive corporate promotion, physician interest, and, in turn, pressure from patients. Improved reimbursement for quicker, easier procedures also entices many physicians to become “early adopters.” (Recent addition of the CPT code for mesh/graft use in prolapse surgery [CPT 57267], increases reimbursement over traditional procedures.)
It is important to keep a cautious but open mind. Given the blind needle techniques and use of biomaterial grafts and synthetic meshes, these procedures may not be for every surgeon or every patient. As always, astute clinical judgment and critical analysis of the data and anecdotal experience are recommended.
Transobturator sling
The needle-guided synthetic mesh midurethral sling was rapidly adopted as the treatment of choice for stress urinary incontinence due to urethral hypermobility and intrinsic sphincter deficiency, soon after it was described in 1995.1
With the transvaginal tape (TVT) procedure, the learning curve was shorter and so were hospital stays and recovery, compared with abdominal Burch colposuspension and traditional bladder neck slings. Furthermore, cost efficiency improved,2 and the persistent cure rate was 85% from 2 to 8 years.3
However, needle passage through the retropubic space can cause vascular, bowel, or bladder injury, even in the hands of experienced surgeons. An August 2005 French survey4 of 92 surgeons who performed 12,280 TVT procedures reported these complications: perioperative bladder injuries, 901 (7.34%); cases of complete postoperative urinary retention requiring catheterization, 809 (6.59%); vaginal mesh exposure, 26 (0.21%); retropubic or vulvovaginal hematoma, 39 (0.32%); and major organ injuries, 10 (0.08%).
The transobturator (TOT) approach, introduced in 2003,5 is simpler, with fewer complications. The sling is placed in a similar manner in the midurethral position, but the insertion points overlie the obturator space in the genitofemoral crease lateral to the vagina. A needle passing through the obturator membrane exits the vaginal incision without entering the retropubic space, theoretically averting risk of bowel, bladder, and major blood vessel injury.
Although the TOT is thought to be safer in this regard, complications including urinary retention, obturator hematoma and nerve injury, and urethral injury/erosion have been reported.6
A variety of TOT sling kits are available, none with proven superiority.
In a recent randomized, prospective trial in which 61 women had TVT or TOT, there were no bladder injuries in the TOT group, and 9.7% (n=3) in the TVT group (P>.05). The postoperative urinary retention rate was 25.8% (n=8) in the TVT group and 13.3% (n=4) in the TOT group (P>.05). Cure rates (83.9% vs 90%), improvement (9.7% vs 3.3%), and failure (6.5% vs 6.7%) were similar.7
The transobturator suburethral sling is encouraging, although it is unclear whether it is effective in patients with intrinsic sphincter deficiency, especially with a fixed or lead-pipe urethra. We need studies to determine how to match the right procedure to the right patient.
Which sling for which patients?
My indications for TOT vs. TVT, which are based on personal experience and available data, may change as data accumulate (TABLE). Indications are often surgeon-specific, depending on clinical experience.
In our review of 210 TOT slings over a 16-month period at 2 centers, we found a cure rate of 88% and an improvement rate of 1.9%. The complication rate was 24%; intraoperative and postoperative complications were all minor and mostly self-limited8: 1 cystotomy, 1 urethral injury, 2 hematomas, 1 erosion, 16 complaints of transient groin pain, 5 cases of urinary retention requiring reoperation, and 23 cases of de novo urge incontinence.
TABLE
Transvaginal vs transobturator sling
| INDICATIONS | ADVANTAGES |
|---|---|
| Transvaginal (retropubic) | |
| Physically active patient | Avoids groin discomfort with activity |
| Thin, young patient | Long term data available |
| Limited urethral hypermobility/internal sphincter dysfunction | Data supports use/dynamic backboard |
| Transobturator | |
| Elderly patient | Less postop voiding dysfunction |
| Significant overactive bladder/urge incontinence | Less urethral obstruction |
| Previous retropubic surgery | Less risk of retropubic complication |
| Obesity | Less risk of needle-passage complication |
| Inexperience with TVT | Less risk of periop complications |
Adjustable suburethral sling
One of the challenges in placing a suburethral sling is adjustment for efficacy without overcorrection and resultant bladder neck obstruction, urinary retention, or persistent and refractory overactive bladder symptoms. An adjustable transvaginal midurethral synthetic sling procedure was recently introduced in the United States: the Remeex Tensionfree Readjustable Tape, (Neomedic International, Spain). A retropubic minimally invasive midurethral sling is attached to sutures that are taken through a tensioning device placed above the fascia in the suprapubic region. The tensioning device has a small adjustment kit similar to a screwdriver, which is left in place at the time of surgery. The sling is intentionally left loose for postoperative adjustment. Following surgery, a filling cystometrogram confirms stress incontinence. The sling is then progressively tightened until the leaking ceases. This technology is designed to prevent or correct overtightening, and avert bladder outlet obstruction. The sling can be adjusted via a small suprapubic incision, even years later; adjustment has been reported up to 7 years later.
In a recent study of 62 patients with stress urinary incontinence, 58 patients (94%) were completely dry and cured, and 4 patients (6%) reported occasional slight urine leakage. Operative time was 20 to 40 minutes (only stress urinary incontinence and cystocele). Six patients required long-term readjustment (5 to increase tension and 1 to reduce tension). No major intra-operative complications occurred. Late complications included suprapubic wound pain (12 transitional and relieved with analgesics), 3 urinary tract infections, 2 wound seromas, 1 case requiring prosthesis removal due to infection, and 3 cases of hyperactivity de novo, which required anticholinergic treatment.9
Although postoperative urinary retention or postoperative failure is relatively uncommon in transvaginal or transobturator suburethral sling procedures performed by experienced surgeons, the adjustable sling may be especially useful in patients with increased risk of postoperative voiding dysfunction, as well as limited urethral hypermobility/fixed urethra, because the sling can be adjusted long after the operation. Risks include infection due to foreign body (indwelling placement of the tensioning device) as well as palpation and incisional discomfort in very thin patients. Further clinical experience is needed, but the concept of a sling that can be adjusted immediately or even years later is appealing.
Graft/mesh augmentation for prolapse repair
Augmentation of pelvic prolapse repair using mesh and graft materials is used increasingly in an effort to improve long-term outcomes, although we lack randomized prospective data and long-term outcome studies. Synthetic materials offer ready availability, consistent tissue properties, cost effectiveness, and permanent placement, although there are risks: infection, dyspareunia, and erosion or exposure. Success and complications may depend on surgical technique, choice of material, patient selection, postoperative management, or other factors.
The overall success rate was 94% at a mean of 17 months after operation, in a study of 63 women in whom polypropylene mesh was used for augmentation of cystocele and rectocele. However, the authors recommended abandonment of the procedure due to an unacceptably high rate of complications.10 In the 32 women undergoing anterior repair, sexual activity rate did not alter, but dyspareunia increased in 20%. Urge and stress incontinence did not change, but urgency improved in 10%; 13% had vaginal erosion of the mesh. Of the 31 patients undergoing posterior repair, sexual activity decreased by 12% and dyspareunia increased in 63%. Constipation improved in 15% and anal incontinence in 4%; 6.5% had vaginal erosion of mesh and 1 required mesh removal for abscess.
In another study, results were improved and complications were fewer. After 2 years, 24 of 26 women who had posterior repair with polypropylene mesh were cured (92.3%) and 1 had asymptomatic stage 2 rectocele. All but 1 reported improved symptoms and quality of life. No postop infection or rectovaginal fistula was reported; there were 3 vaginal erosions (12%), and 2 patients had de novo dyspareunia (7.7%).11
To make graft/mesh augmentation easier and faster, needle-suspension techniques were recently introduced. Needles are inserted either through the transobturator space (anterior mesh placement) or ischiorectal fascia (posterior placement) and exit through the pelvic sidewall in proximity to the ischial spine. A multi-arm mesh is then attached to the needles, which are withdrawn. Tension secures the mesh and provides “tension-free” anterior or posterior wall support. Colporrhaphy can be performed prior to mesh placement at the surgeon’s discretion.
Because we have few data on patient selection or long-term safety and efficacy (most of it presented at recent meetings12,13 ), these techniques call for caution. Blind needle passage can be associated with complications such as rectal injury and rectovaginal fistula.14 But complication rates may reflect early evolution and may improve with time and experience.
The author receives grant/research support and/or serves as a consultant for American Medical Systems, Boston Scientific, CR Bard, Mentor, Novartis, and Pfizer.
1. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
2. Kilonzo M, Vale L, Stearns SC, et al. Cost effectiveness of tension-free vaginal tape for the surgical management of female stress incontinence. Int J Technol Assess Health Care. 2004;20:455-463.
3. Holmgren C, Nilsson S, Lanner L, Hellberg D. Long-term results with tension-free vaginal tape on mixed and stress urinary incontinence. Obstet Gynecol. 2005;106:38-43.
4. Agostini A, Bretelle F, Franchi F, Roger V, Cravello L, Blanc B. Immediate complications of tension-free vaginal tape (TVT): results of a French survey. Eur J Obstet Gynecol Reprod Biol. 2005 Aug 8; [Epub ahead of print].
5. Delorme E, Droupy S, de Tayrac R, Delmas V. Transobturator tape (Uratape). A new minimally invasive method in the treatment of urinary incontinence in women. Prog Urol. 2003;13:656-659.
6. Game X, et al. Obturator infected hematoma and urethral erosion following transobturator tape implantation. J Urol. 2004;171:1629.-
7. deTayrac R, Deffieux X, Droupy S, et al. A prospective randomized trial comparing tension-free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence [retracted in: Am J Obstet Gynecol. 2005;192:339]. Am J Obstet Gynecol. 2004;190:602-608.
8. Rajan S, Diwadkar G, Hurwitz S, Kohli N, Roberts L, Moore R, Miklos J. Transobturator (TOT) suburethral sling: early US data on safety and efficacy. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark. Poster 52, abstract ID 982.
9. Cabrera Pérez J, Bravo Fernández I, Pérez G, González Enguita C, Vela Navarrete R. Analysis of the suburethral sling TRT (tension free readjustable tape) results in female SUI treatment. Abstract presented at: LXX Congreso Nacional de Urología; June 4–7, 2005; San Sebastián, Spain.
10. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with Prolene mesh. BJOG. 2005;112:107-111.
11. de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2005 May 21; [Epub ahead of print].
12. Rane AM, Naidu AK, Barry CL, Nyok LY, Corstiaans AC. A novel transobturator system for the repair of anterior vaginal wall prolapse: a pilot study. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
13. Cosson M, Caquent F, Collinet P, Rosenthal C, Clave H, Debodinance P, Garbin O, Berrocal J, Villet R, Jacquetin B. Prolift mesh for pelvic organ prolapse surgical treatment using the TVM group technique: a retrospective study of 687 patients. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
14. Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2005 Jul 29; [Epub ahead of print].
Even as we scramble to gather definitive evidence on the immediate and long-term benefits of new technologies, they are supplanting tradition in the surgical treatment of incontinence and prolapse. Surgeons have been swift to adopt synthetic mesh and the new generation of needle suspension procedures, which offer the double advantage of a shorter operative time and shorter postoperative recovery. Yet, we lack well-designed randomized prospective clinical studies on whether outcomes and complication rates are better than traditional therapies such as vaginal colporrhaphy and paravaginal repair.
There hasn’t been time.
These innovations came onto the market in rapid succession, accompanied by aggressive corporate promotion, physician interest, and, in turn, pressure from patients. Improved reimbursement for quicker, easier procedures also entices many physicians to become “early adopters.” (Recent addition of the CPT code for mesh/graft use in prolapse surgery [CPT 57267], increases reimbursement over traditional procedures.)
It is important to keep a cautious but open mind. Given the blind needle techniques and use of biomaterial grafts and synthetic meshes, these procedures may not be for every surgeon or every patient. As always, astute clinical judgment and critical analysis of the data and anecdotal experience are recommended.
Transobturator sling
The needle-guided synthetic mesh midurethral sling was rapidly adopted as the treatment of choice for stress urinary incontinence due to urethral hypermobility and intrinsic sphincter deficiency, soon after it was described in 1995.1
With the transvaginal tape (TVT) procedure, the learning curve was shorter and so were hospital stays and recovery, compared with abdominal Burch colposuspension and traditional bladder neck slings. Furthermore, cost efficiency improved,2 and the persistent cure rate was 85% from 2 to 8 years.3
However, needle passage through the retropubic space can cause vascular, bowel, or bladder injury, even in the hands of experienced surgeons. An August 2005 French survey4 of 92 surgeons who performed 12,280 TVT procedures reported these complications: perioperative bladder injuries, 901 (7.34%); cases of complete postoperative urinary retention requiring catheterization, 809 (6.59%); vaginal mesh exposure, 26 (0.21%); retropubic or vulvovaginal hematoma, 39 (0.32%); and major organ injuries, 10 (0.08%).
The transobturator (TOT) approach, introduced in 2003,5 is simpler, with fewer complications. The sling is placed in a similar manner in the midurethral position, but the insertion points overlie the obturator space in the genitofemoral crease lateral to the vagina. A needle passing through the obturator membrane exits the vaginal incision without entering the retropubic space, theoretically averting risk of bowel, bladder, and major blood vessel injury.
Although the TOT is thought to be safer in this regard, complications including urinary retention, obturator hematoma and nerve injury, and urethral injury/erosion have been reported.6
A variety of TOT sling kits are available, none with proven superiority.
In a recent randomized, prospective trial in which 61 women had TVT or TOT, there were no bladder injuries in the TOT group, and 9.7% (n=3) in the TVT group (P>.05). The postoperative urinary retention rate was 25.8% (n=8) in the TVT group and 13.3% (n=4) in the TOT group (P>.05). Cure rates (83.9% vs 90%), improvement (9.7% vs 3.3%), and failure (6.5% vs 6.7%) were similar.7
The transobturator suburethral sling is encouraging, although it is unclear whether it is effective in patients with intrinsic sphincter deficiency, especially with a fixed or lead-pipe urethra. We need studies to determine how to match the right procedure to the right patient.
Which sling for which patients?
My indications for TOT vs. TVT, which are based on personal experience and available data, may change as data accumulate (TABLE). Indications are often surgeon-specific, depending on clinical experience.
In our review of 210 TOT slings over a 16-month period at 2 centers, we found a cure rate of 88% and an improvement rate of 1.9%. The complication rate was 24%; intraoperative and postoperative complications were all minor and mostly self-limited8: 1 cystotomy, 1 urethral injury, 2 hematomas, 1 erosion, 16 complaints of transient groin pain, 5 cases of urinary retention requiring reoperation, and 23 cases of de novo urge incontinence.
TABLE
Transvaginal vs transobturator sling
| INDICATIONS | ADVANTAGES |
|---|---|
| Transvaginal (retropubic) | |
| Physically active patient | Avoids groin discomfort with activity |
| Thin, young patient | Long term data available |
| Limited urethral hypermobility/internal sphincter dysfunction | Data supports use/dynamic backboard |
| Transobturator | |
| Elderly patient | Less postop voiding dysfunction |
| Significant overactive bladder/urge incontinence | Less urethral obstruction |
| Previous retropubic surgery | Less risk of retropubic complication |
| Obesity | Less risk of needle-passage complication |
| Inexperience with TVT | Less risk of periop complications |
Adjustable suburethral sling
One of the challenges in placing a suburethral sling is adjustment for efficacy without overcorrection and resultant bladder neck obstruction, urinary retention, or persistent and refractory overactive bladder symptoms. An adjustable transvaginal midurethral synthetic sling procedure was recently introduced in the United States: the Remeex Tensionfree Readjustable Tape, (Neomedic International, Spain). A retropubic minimally invasive midurethral sling is attached to sutures that are taken through a tensioning device placed above the fascia in the suprapubic region. The tensioning device has a small adjustment kit similar to a screwdriver, which is left in place at the time of surgery. The sling is intentionally left loose for postoperative adjustment. Following surgery, a filling cystometrogram confirms stress incontinence. The sling is then progressively tightened until the leaking ceases. This technology is designed to prevent or correct overtightening, and avert bladder outlet obstruction. The sling can be adjusted via a small suprapubic incision, even years later; adjustment has been reported up to 7 years later.
In a recent study of 62 patients with stress urinary incontinence, 58 patients (94%) were completely dry and cured, and 4 patients (6%) reported occasional slight urine leakage. Operative time was 20 to 40 minutes (only stress urinary incontinence and cystocele). Six patients required long-term readjustment (5 to increase tension and 1 to reduce tension). No major intra-operative complications occurred. Late complications included suprapubic wound pain (12 transitional and relieved with analgesics), 3 urinary tract infections, 2 wound seromas, 1 case requiring prosthesis removal due to infection, and 3 cases of hyperactivity de novo, which required anticholinergic treatment.9
Although postoperative urinary retention or postoperative failure is relatively uncommon in transvaginal or transobturator suburethral sling procedures performed by experienced surgeons, the adjustable sling may be especially useful in patients with increased risk of postoperative voiding dysfunction, as well as limited urethral hypermobility/fixed urethra, because the sling can be adjusted long after the operation. Risks include infection due to foreign body (indwelling placement of the tensioning device) as well as palpation and incisional discomfort in very thin patients. Further clinical experience is needed, but the concept of a sling that can be adjusted immediately or even years later is appealing.
Graft/mesh augmentation for prolapse repair
Augmentation of pelvic prolapse repair using mesh and graft materials is used increasingly in an effort to improve long-term outcomes, although we lack randomized prospective data and long-term outcome studies. Synthetic materials offer ready availability, consistent tissue properties, cost effectiveness, and permanent placement, although there are risks: infection, dyspareunia, and erosion or exposure. Success and complications may depend on surgical technique, choice of material, patient selection, postoperative management, or other factors.
The overall success rate was 94% at a mean of 17 months after operation, in a study of 63 women in whom polypropylene mesh was used for augmentation of cystocele and rectocele. However, the authors recommended abandonment of the procedure due to an unacceptably high rate of complications.10 In the 32 women undergoing anterior repair, sexual activity rate did not alter, but dyspareunia increased in 20%. Urge and stress incontinence did not change, but urgency improved in 10%; 13% had vaginal erosion of the mesh. Of the 31 patients undergoing posterior repair, sexual activity decreased by 12% and dyspareunia increased in 63%. Constipation improved in 15% and anal incontinence in 4%; 6.5% had vaginal erosion of mesh and 1 required mesh removal for abscess.
In another study, results were improved and complications were fewer. After 2 years, 24 of 26 women who had posterior repair with polypropylene mesh were cured (92.3%) and 1 had asymptomatic stage 2 rectocele. All but 1 reported improved symptoms and quality of life. No postop infection or rectovaginal fistula was reported; there were 3 vaginal erosions (12%), and 2 patients had de novo dyspareunia (7.7%).11
To make graft/mesh augmentation easier and faster, needle-suspension techniques were recently introduced. Needles are inserted either through the transobturator space (anterior mesh placement) or ischiorectal fascia (posterior placement) and exit through the pelvic sidewall in proximity to the ischial spine. A multi-arm mesh is then attached to the needles, which are withdrawn. Tension secures the mesh and provides “tension-free” anterior or posterior wall support. Colporrhaphy can be performed prior to mesh placement at the surgeon’s discretion.
Because we have few data on patient selection or long-term safety and efficacy (most of it presented at recent meetings12,13 ), these techniques call for caution. Blind needle passage can be associated with complications such as rectal injury and rectovaginal fistula.14 But complication rates may reflect early evolution and may improve with time and experience.
The author receives grant/research support and/or serves as a consultant for American Medical Systems, Boston Scientific, CR Bard, Mentor, Novartis, and Pfizer.
Even as we scramble to gather definitive evidence on the immediate and long-term benefits of new technologies, they are supplanting tradition in the surgical treatment of incontinence and prolapse. Surgeons have been swift to adopt synthetic mesh and the new generation of needle suspension procedures, which offer the double advantage of a shorter operative time and shorter postoperative recovery. Yet, we lack well-designed randomized prospective clinical studies on whether outcomes and complication rates are better than traditional therapies such as vaginal colporrhaphy and paravaginal repair.
There hasn’t been time.
These innovations came onto the market in rapid succession, accompanied by aggressive corporate promotion, physician interest, and, in turn, pressure from patients. Improved reimbursement for quicker, easier procedures also entices many physicians to become “early adopters.” (Recent addition of the CPT code for mesh/graft use in prolapse surgery [CPT 57267], increases reimbursement over traditional procedures.)
It is important to keep a cautious but open mind. Given the blind needle techniques and use of biomaterial grafts and synthetic meshes, these procedures may not be for every surgeon or every patient. As always, astute clinical judgment and critical analysis of the data and anecdotal experience are recommended.
Transobturator sling
The needle-guided synthetic mesh midurethral sling was rapidly adopted as the treatment of choice for stress urinary incontinence due to urethral hypermobility and intrinsic sphincter deficiency, soon after it was described in 1995.1
With the transvaginal tape (TVT) procedure, the learning curve was shorter and so were hospital stays and recovery, compared with abdominal Burch colposuspension and traditional bladder neck slings. Furthermore, cost efficiency improved,2 and the persistent cure rate was 85% from 2 to 8 years.3
However, needle passage through the retropubic space can cause vascular, bowel, or bladder injury, even in the hands of experienced surgeons. An August 2005 French survey4 of 92 surgeons who performed 12,280 TVT procedures reported these complications: perioperative bladder injuries, 901 (7.34%); cases of complete postoperative urinary retention requiring catheterization, 809 (6.59%); vaginal mesh exposure, 26 (0.21%); retropubic or vulvovaginal hematoma, 39 (0.32%); and major organ injuries, 10 (0.08%).
The transobturator (TOT) approach, introduced in 2003,5 is simpler, with fewer complications. The sling is placed in a similar manner in the midurethral position, but the insertion points overlie the obturator space in the genitofemoral crease lateral to the vagina. A needle passing through the obturator membrane exits the vaginal incision without entering the retropubic space, theoretically averting risk of bowel, bladder, and major blood vessel injury.
Although the TOT is thought to be safer in this regard, complications including urinary retention, obturator hematoma and nerve injury, and urethral injury/erosion have been reported.6
A variety of TOT sling kits are available, none with proven superiority.
In a recent randomized, prospective trial in which 61 women had TVT or TOT, there were no bladder injuries in the TOT group, and 9.7% (n=3) in the TVT group (P>.05). The postoperative urinary retention rate was 25.8% (n=8) in the TVT group and 13.3% (n=4) in the TOT group (P>.05). Cure rates (83.9% vs 90%), improvement (9.7% vs 3.3%), and failure (6.5% vs 6.7%) were similar.7
The transobturator suburethral sling is encouraging, although it is unclear whether it is effective in patients with intrinsic sphincter deficiency, especially with a fixed or lead-pipe urethra. We need studies to determine how to match the right procedure to the right patient.
Which sling for which patients?
My indications for TOT vs. TVT, which are based on personal experience and available data, may change as data accumulate (TABLE). Indications are often surgeon-specific, depending on clinical experience.
In our review of 210 TOT slings over a 16-month period at 2 centers, we found a cure rate of 88% and an improvement rate of 1.9%. The complication rate was 24%; intraoperative and postoperative complications were all minor and mostly self-limited8: 1 cystotomy, 1 urethral injury, 2 hematomas, 1 erosion, 16 complaints of transient groin pain, 5 cases of urinary retention requiring reoperation, and 23 cases of de novo urge incontinence.
TABLE
Transvaginal vs transobturator sling
| INDICATIONS | ADVANTAGES |
|---|---|
| Transvaginal (retropubic) | |
| Physically active patient | Avoids groin discomfort with activity |
| Thin, young patient | Long term data available |
| Limited urethral hypermobility/internal sphincter dysfunction | Data supports use/dynamic backboard |
| Transobturator | |
| Elderly patient | Less postop voiding dysfunction |
| Significant overactive bladder/urge incontinence | Less urethral obstruction |
| Previous retropubic surgery | Less risk of retropubic complication |
| Obesity | Less risk of needle-passage complication |
| Inexperience with TVT | Less risk of periop complications |
Adjustable suburethral sling
One of the challenges in placing a suburethral sling is adjustment for efficacy without overcorrection and resultant bladder neck obstruction, urinary retention, or persistent and refractory overactive bladder symptoms. An adjustable transvaginal midurethral synthetic sling procedure was recently introduced in the United States: the Remeex Tensionfree Readjustable Tape, (Neomedic International, Spain). A retropubic minimally invasive midurethral sling is attached to sutures that are taken through a tensioning device placed above the fascia in the suprapubic region. The tensioning device has a small adjustment kit similar to a screwdriver, which is left in place at the time of surgery. The sling is intentionally left loose for postoperative adjustment. Following surgery, a filling cystometrogram confirms stress incontinence. The sling is then progressively tightened until the leaking ceases. This technology is designed to prevent or correct overtightening, and avert bladder outlet obstruction. The sling can be adjusted via a small suprapubic incision, even years later; adjustment has been reported up to 7 years later.
In a recent study of 62 patients with stress urinary incontinence, 58 patients (94%) were completely dry and cured, and 4 patients (6%) reported occasional slight urine leakage. Operative time was 20 to 40 minutes (only stress urinary incontinence and cystocele). Six patients required long-term readjustment (5 to increase tension and 1 to reduce tension). No major intra-operative complications occurred. Late complications included suprapubic wound pain (12 transitional and relieved with analgesics), 3 urinary tract infections, 2 wound seromas, 1 case requiring prosthesis removal due to infection, and 3 cases of hyperactivity de novo, which required anticholinergic treatment.9
Although postoperative urinary retention or postoperative failure is relatively uncommon in transvaginal or transobturator suburethral sling procedures performed by experienced surgeons, the adjustable sling may be especially useful in patients with increased risk of postoperative voiding dysfunction, as well as limited urethral hypermobility/fixed urethra, because the sling can be adjusted long after the operation. Risks include infection due to foreign body (indwelling placement of the tensioning device) as well as palpation and incisional discomfort in very thin patients. Further clinical experience is needed, but the concept of a sling that can be adjusted immediately or even years later is appealing.
Graft/mesh augmentation for prolapse repair
Augmentation of pelvic prolapse repair using mesh and graft materials is used increasingly in an effort to improve long-term outcomes, although we lack randomized prospective data and long-term outcome studies. Synthetic materials offer ready availability, consistent tissue properties, cost effectiveness, and permanent placement, although there are risks: infection, dyspareunia, and erosion or exposure. Success and complications may depend on surgical technique, choice of material, patient selection, postoperative management, or other factors.
The overall success rate was 94% at a mean of 17 months after operation, in a study of 63 women in whom polypropylene mesh was used for augmentation of cystocele and rectocele. However, the authors recommended abandonment of the procedure due to an unacceptably high rate of complications.10 In the 32 women undergoing anterior repair, sexual activity rate did not alter, but dyspareunia increased in 20%. Urge and stress incontinence did not change, but urgency improved in 10%; 13% had vaginal erosion of the mesh. Of the 31 patients undergoing posterior repair, sexual activity decreased by 12% and dyspareunia increased in 63%. Constipation improved in 15% and anal incontinence in 4%; 6.5% had vaginal erosion of mesh and 1 required mesh removal for abscess.
In another study, results were improved and complications were fewer. After 2 years, 24 of 26 women who had posterior repair with polypropylene mesh were cured (92.3%) and 1 had asymptomatic stage 2 rectocele. All but 1 reported improved symptoms and quality of life. No postop infection or rectovaginal fistula was reported; there were 3 vaginal erosions (12%), and 2 patients had de novo dyspareunia (7.7%).11
To make graft/mesh augmentation easier and faster, needle-suspension techniques were recently introduced. Needles are inserted either through the transobturator space (anterior mesh placement) or ischiorectal fascia (posterior placement) and exit through the pelvic sidewall in proximity to the ischial spine. A multi-arm mesh is then attached to the needles, which are withdrawn. Tension secures the mesh and provides “tension-free” anterior or posterior wall support. Colporrhaphy can be performed prior to mesh placement at the surgeon’s discretion.
Because we have few data on patient selection or long-term safety and efficacy (most of it presented at recent meetings12,13 ), these techniques call for caution. Blind needle passage can be associated with complications such as rectal injury and rectovaginal fistula.14 But complication rates may reflect early evolution and may improve with time and experience.
The author receives grant/research support and/or serves as a consultant for American Medical Systems, Boston Scientific, CR Bard, Mentor, Novartis, and Pfizer.
1. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
2. Kilonzo M, Vale L, Stearns SC, et al. Cost effectiveness of tension-free vaginal tape for the surgical management of female stress incontinence. Int J Technol Assess Health Care. 2004;20:455-463.
3. Holmgren C, Nilsson S, Lanner L, Hellberg D. Long-term results with tension-free vaginal tape on mixed and stress urinary incontinence. Obstet Gynecol. 2005;106:38-43.
4. Agostini A, Bretelle F, Franchi F, Roger V, Cravello L, Blanc B. Immediate complications of tension-free vaginal tape (TVT): results of a French survey. Eur J Obstet Gynecol Reprod Biol. 2005 Aug 8; [Epub ahead of print].
5. Delorme E, Droupy S, de Tayrac R, Delmas V. Transobturator tape (Uratape). A new minimally invasive method in the treatment of urinary incontinence in women. Prog Urol. 2003;13:656-659.
6. Game X, et al. Obturator infected hematoma and urethral erosion following transobturator tape implantation. J Urol. 2004;171:1629.-
7. deTayrac R, Deffieux X, Droupy S, et al. A prospective randomized trial comparing tension-free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence [retracted in: Am J Obstet Gynecol. 2005;192:339]. Am J Obstet Gynecol. 2004;190:602-608.
8. Rajan S, Diwadkar G, Hurwitz S, Kohli N, Roberts L, Moore R, Miklos J. Transobturator (TOT) suburethral sling: early US data on safety and efficacy. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark. Poster 52, abstract ID 982.
9. Cabrera Pérez J, Bravo Fernández I, Pérez G, González Enguita C, Vela Navarrete R. Analysis of the suburethral sling TRT (tension free readjustable tape) results in female SUI treatment. Abstract presented at: LXX Congreso Nacional de Urología; June 4–7, 2005; San Sebastián, Spain.
10. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with Prolene mesh. BJOG. 2005;112:107-111.
11. de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2005 May 21; [Epub ahead of print].
12. Rane AM, Naidu AK, Barry CL, Nyok LY, Corstiaans AC. A novel transobturator system for the repair of anterior vaginal wall prolapse: a pilot study. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
13. Cosson M, Caquent F, Collinet P, Rosenthal C, Clave H, Debodinance P, Garbin O, Berrocal J, Villet R, Jacquetin B. Prolift mesh for pelvic organ prolapse surgical treatment using the TVM group technique: a retrospective study of 687 patients. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
14. Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2005 Jul 29; [Epub ahead of print].
1. Ulmsten U, Petros P. Intravaginal slingplasty (IVS): an ambulatory surgical procedure for treatment of female urinary incontinence. Scand J Urol Nephrol. 1995;29:75-82.
2. Kilonzo M, Vale L, Stearns SC, et al. Cost effectiveness of tension-free vaginal tape for the surgical management of female stress incontinence. Int J Technol Assess Health Care. 2004;20:455-463.
3. Holmgren C, Nilsson S, Lanner L, Hellberg D. Long-term results with tension-free vaginal tape on mixed and stress urinary incontinence. Obstet Gynecol. 2005;106:38-43.
4. Agostini A, Bretelle F, Franchi F, Roger V, Cravello L, Blanc B. Immediate complications of tension-free vaginal tape (TVT): results of a French survey. Eur J Obstet Gynecol Reprod Biol. 2005 Aug 8; [Epub ahead of print].
5. Delorme E, Droupy S, de Tayrac R, Delmas V. Transobturator tape (Uratape). A new minimally invasive method in the treatment of urinary incontinence in women. Prog Urol. 2003;13:656-659.
6. Game X, et al. Obturator infected hematoma and urethral erosion following transobturator tape implantation. J Urol. 2004;171:1629.-
7. deTayrac R, Deffieux X, Droupy S, et al. A prospective randomized trial comparing tension-free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence [retracted in: Am J Obstet Gynecol. 2005;192:339]. Am J Obstet Gynecol. 2004;190:602-608.
8. Rajan S, Diwadkar G, Hurwitz S, Kohli N, Roberts L, Moore R, Miklos J. Transobturator (TOT) suburethral sling: early US data on safety and efficacy. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark. Poster 52, abstract ID 982.
9. Cabrera Pérez J, Bravo Fernández I, Pérez G, González Enguita C, Vela Navarrete R. Analysis of the suburethral sling TRT (tension free readjustable tape) results in female SUI treatment. Abstract presented at: LXX Congreso Nacional de Urología; June 4–7, 2005; San Sebastián, Spain.
10. Milani R, Salvatore S, Soligo M, Pifarotti P, Meschia M, Cortese M. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with Prolene mesh. BJOG. 2005;112:107-111.
11. de Tayrac R, Picone O, Chauveaud-Lambling A, Fernandez H. A 2-year anatomical and functional assessment of transvaginal rectocele repair using a polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2005 May 21; [Epub ahead of print].
12. Rane AM, Naidu AK, Barry CL, Nyok LY, Corstiaans AC. A novel transobturator system for the repair of anterior vaginal wall prolapse: a pilot study. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
13. Cosson M, Caquent F, Collinet P, Rosenthal C, Clave H, Debodinance P, Garbin O, Berrocal J, Villet R, Jacquetin B. Prolift mesh for pelvic organ prolapse surgical treatment using the TVM group technique: a retrospective study of 687 patients. Abstract presented at: 2005 Meeting of the International Urogynecology Association; August 9–12, 2005; Copenhagen, Denmark.
14. Hilger WS, Cornella JL. Rectovaginal fistula after posterior intravaginal slingplasty and polypropylene mesh augmented rectocele repair. Int Urogynecol J Pelvic Floor Dysfunct. 2005 Jul 29; [Epub ahead of print].
Defusing the angry patient
The cases below show why patients get angry, what clues signal anger, what to say, and what not to say. But the focus throughout is how to prepare yourself and your staff to defuse the angry patient, from the first encounter through the essential follow-up: the office visit that you set up specifically to address her anger and reduce the risk of legal action.
Tracing anger’s fuse
KIM’S CASE
Kim is a 21-year-old G1P0000 with type 1 diabetes who did not comply well with her insulin and diet regimens. At 39 weeks’ gestation, with an estimated fetal weight of 4,000 g, she demands a vaginal delivery. At delivery, severe shoulder dystocia occurs, resulting in what the family later recalls as chaotic activity in the delivery room and severe nerve damage to the newborn’s left arm. When you meet with the couple and the wife’s sister later that day, all 3 express intense anger at you.
How should you respond?
Anger can occur when there is an unexpected adverse outcome, or when a patient feels responsible for a poor outcome. Either way, you may be a “safer” target than the actual cause.
In Kim’s case, each family member feels angry for a different reason, and you should try to draw their reasons out in conversation so they can be addressed. Don’t assume all are angry for the same reason.
- The new mother feels terrible because her noncompliance contributed to the outcome. She expresses her anger at you to overcome feelings of failure.
- The husband is angry with his wife because she did not follow her diet and insulin instructions carefully, but he is afraid to confront her after nearly losing their newborn son. He therefore directs his anger at you.
- The sister is angry with you out of a sense of helplessness and a desire to “make someone pay.”
In conversation, describe each step of the delivery process, to clarify misconceptions, and discuss the controversy over how best to manage a 4,000-g infant when the mother has diabetes. Also realize that the “chaotic activity” the family witnessed during the delivery may have contributed to their anger. An explanation of what was actually taking place may allay some of their concern.
In this case, knowing the patient was diabetic, the physician should have discussed the risks of various delivery methods well before the actual birth.
When is anger a healthy response and when is it pathologic?
It is beneficial when we use it to inform our actions. For example, if a physician feels angry about the proposed changes to Medicaid and writes a letter to her congressman, she is using anger constructively.
Anger is a problem when it changes to aggression, is buried, or is used to manipulate others. Research has linked chronic anger-management problems with interpersonal strife, difficulties at work, poor decision-making, increased risk-taking, substance abuse, coronary heart disease, stroke, chronic pain, disruption of motor activities (such as driving), and susceptibility to depression, guilt, and shame.2
Greenberg3 identified 4 ways of classifying expressed emotions such as anger, which the following 4 cases illustrate:
CASE 1. When anger sparks a change for the better A patient expresses anger at having to wait more than an hour to see you. By bringing it to your attention, she makes it possible for you to address the issue with office staff.
When anger is a primary adaptive response, it enables one to organize for action and to hold another responsible for injuring her. This is a healthy expression of anger; it lets a person act in congruence with both cognition and emotion.
CASE 2. When anger gets stuck Upon arrival, a patient informs your staff that she has no intention of being kept waiting. This woman considers even the slightest delay a personal affront.
When anger is a primary maladaptive response, it indicates a stuck pattern of behavior and emotional expression in which anger becomes a reflexive reaction, rather than an action. When anger takes this form, it can cause an individual to overestimate the threat to herself and damage relationships through aversive behavior.
CASE 3. Anger as a cover A woman with a newly discovered breast lump is afraid she has cancer. Rather than express this fear, she accuses you of withholding information.
In this instance, anger conceals an underlying emotion, and is therefore the secondary emotion. Sometimes the person is aware of the underlying feeling, sometimes not.
CASE 4. Anger as manipulation A patient wants cab fare provided for her and her children to get to their appointments; the norm is to provide bus fare. The patient threatens action and negative word-of-mouth, complains to the insurance company, and ultimately gets the cab fare.
Anger can be used as an instrument to manipulate others, to bring about a desired outcome.
How anger rears its head
“In your face”
Although a woman may express—or contain—her anger in any number of ways, a few styles tend to predominate. Probably the most fearsome patient is the one with an “in your face” style, who yells, swears, or threatens people. If a nurse doesn’t jump to her demands, she asks for the supervisor and threatens to file a report.
Although it can be intimidating, there is one advantage to this style of anger: At least you know where you stand.
“I don’t do anger”
At the other end of the continuum is the woman who denies her anger. We once had a patient who proclaimed, “I don’t do anger,” but the midwife who referred her described her as one of the angriest patients she had ever seen. In our encounter, she was visibly tense and responded in short, clipped sentences, but we could not address her anger directly because she refused to admit its existence.
Noncompliance as power struggle
Indirect anger falls somewhere between the above 2 extremes. Women tend to be socialized to withhold their anger to preserve relationships, so they often feel safer expressing it indirectly. Passive-aggressive behavior is one example. The patient may arrive late or forget her insulin logs, or she may say everything is fine but call later in the day with an important concern. This conduct may seem like noncompliance, but noncompliance can be rooted in anger. Communication can become a power struggle in which the patient demonstrates her anger by refusing to do as you ask. Dropping out is the ultimate expression of indirect anger; the patient merely quits.
Somatization
Another way indirect anger manifests is through somatization, an unconscious process in which the patient does not articulate her emotions but experiences them physically. Treatment often has little effect.
The quiet woman
Also be aware that women often stifle their emotions until they feel overwhelmed and resentful, at which point they may explode.
PHYSIOLOGIC
- Shortness of breath
- Rapid breathing
- Pressured speech: louder and faster
- Clenched teeth, fists
- Muscle tension
- Rapid heart rate
- Shakiness, trembling
- Tight jaw
- Indigestion, nausea, diarrhea
- Headache
- Flushing, sweating
- Fatigue
BEHAVIORAL
- Pointing a finger
- Getting in another person’s “space”
- Leaning toward the other person
- Rolling eyes
- Raising the voice
- Profanity
- Harsh or hostile tone
- Strong or extreme language
- Sarcasm
- Making accusations
- Slamming doors or phones
- Aggression toward a person or object
COGNITIVE
- Dichotomous thinking: all or nothing, black or white
- Exaggeration and generalization: always, never
- Distorted thinking
- Rigid ideation: “It must be this way or else,” “I will not stand for this,” etc
The slippery slope of how to respond
The correct response to anger is empathy, which should be heartfelt, if at all possible.
Unfortunately, personality and personal issues sometimes impede our ability to empathize openly. It is important to avoid paternalism, evasiveness, and self-blame.
Paternalism in many ways is built into the medical hierarchy. Our specialized knowledge is the reason we’re consulted in the first place, and an intellectual or condescending remark on our part may be a natural defense to a patient’s angry attack, but such a reaction only fuels the fire. Nor should we ever use our role as an authority to dismiss the patient’s anger.
Evasiveness is another frequent response to anger, but can lead to detachment and feed the patient’s perception that you are unfeeling.
Acquiescing to her demands in hopes of avoiding further confrontation or a lawsuit may decrease her anger, but increase your own resentment.
Worst of all is self-blame, in which the physician assumes and internalizes responsibility for failing the patient or lacking perfect knowledge. Though this approach may quickly quell the patient’s anger, it can harm the physician-patient relationship and your emotional health.
Loss of control. Some diseases or conditions have uncontrollable outcomes. For example, a woman with ovarian cancer may feel angry when she realizes she cannot necessarily get better by following a particular plan of action.
“Why me?” Feelings of perceived injustice arise when circumstances seem particularly unfair, as when a woman experiences fetal death in utero despite responsible self-care, and sees less responsible women deliver healthy babies.
Not listening, inattention. Poor communication often leaves the patient feeling as though you failed to listen to her concerns. For example, when you give her less than full attention, an obese woman with gestational diabetes may feel blamed for her own problems.
History of sexual abuse. In women with a history of sexual trauma, obstetric or gynecologic procedures can sometimes remind them of abuse, and themes of powerlessness and lack of control may be reenacted. While it may have been unsafe—or even fatal—for these women to express anger at the original perpetrator, they may feel safer directing it at you.
When a patient pushes your buttons
ERIN’S CASE
At 36 weeks’ gestation, Erin calls your office at the end of the day on a Friday with an emergency: She couldn’t sleep the day before. You feel angry; why? Is it because you had plans to go out to dinner and now will be late? Is it because Erin has been demanding and needy throughout her pregnancy? Is it because Erin doesn’t follow the protocol for nonemergencies and acts as though everything is an emergency? Is it because no matter what you do, Erin hasn’t been satisfied with her care?
These questions can help you decide how to proceed. In any tense situation, the first step is to identify the angry party. This is not always as straightforward as it sounds, since anger is frequently displaced. It may be the patient or her family who shows signs of anger. It may be you. Or it may be both.
Dealing with an angry patient is easier if you keep sight of your own triggers and sore spots. This self-awareness allows you to assert control and contain your emotional reaction.
Coach yourself through an angry encounter. When you recognize your own limits, it is easier to manage difficult situations using “self-talk” to coach yourself through them. For example, when your patient becomes angry, you might remind yourself, “Take a deep breath.” As she complains, you might tell yourself, “This is not personal, even though it feels that way. What is she trying to tell me?”
Formal role-playing and informal discussions of real or hypothetical cases are another strategy.
How to conduct an anger-defusing visit
Set an appointment to talk it out
When a patient expresses anger that does not abate with empathetic listening, consider scheduling a special visit to address her concerns. And prepare for it:
Don’t sit behind a desk. Determine where the conversation will be held, including the seating arrangement. It is inappropriate to sit behind a desk for the discussion, as this arrangement can exacerbate the division between you and the patient. If your office is not conducive to a “relaxed” talk, consider a more neutral site.
Check the exits. Always consider the possibility that the patient or a family member may feel outraged to the point of physical abuse. Make sure security personnel are readily available; if necessary, agree upon a prearranged signal for them to intervene. Also consider whether you will have easy access to the door. During the conversation, do not accept or tolerate threats.
Make it soon. When emotions run high, it is probably best to talk within a week. Avoid a delay of several weeks in hopes that anger will dissipate. What usually happens during such a long interval is that the family becomes angrier and seeks outside advice, much of which is negative. The patient also may change doctors because of the anger and abandonment she feels.
Schedule plenty of time. When enough time is devoted to the discussion, anger generally begins to dissipate. Therefore, do not limit conversation if the case is especially contentious and the risk of a lawsuit very real. On the contrary, it may take 2 hours or longer for the anger to soften.
Rehearse. A role-playing scenario with a colleague can help you experience the full impact of how anger alters a discussion.
Here’s what to say
JULIA’S CASE
A 48-year-old patient reported a breast lump at her last annual visit. At that time, upon examination, you palpated a small mass. Since Julia also has fibrocystic breast disease, you explained that the lump was probably benign, but you referred her for a mammogram and encouraged her to get it as soon as possible. When she finally did—8 months later—invasive carcinoma was diagnosed, necessitating a lumpectomy and chemotherapy.
At her next annual visit, Julia is confrontational, accusing you of downplaying the risk of breast cancer and causing her delay in seeking a mammogram. When she remains outraged despite your calm reminder that you recommended an expeditious mammogram, you schedule an appointment to discuss the matter.
What are your next steps?
Though the logistics are straightforward, success depends on the specific patient and any family members involved.
In general, you should strive to:
- Establish an agenda and review it with the patient.
- Describe the facts of the case in lay terms so that the patient, her family, and any others receive the same information. This helps eliminate erroneous assumptions and misinformation. For example, Julia’s family may not be aware that you encouraged her to obtain a mammogram as soon as possible.
- Express empathy that she has to go through this serious time in her life, and acknowledge that a cancer diagnosis and treatment are tremendously stressful.
- Search for the reasons behind the anger. Ask how the patient and her family have arrived at their conclusions.
- Control anger and prevent it from escalating. Keep your voice steady and maintain an open body language. Avoid signs of your own anger.
- Ask for her perception of what has been said, at the conclusion. If family members are present, ask for any other issues that need clarification.
- Close the discussion respectfully.
- Document the conversation by noting the time, persons present, what was discussed, attitudes of the family, and any plans for follow-up. How thorough should this medical note be? If you were to read your own documentation 5 years from now (the length of time it often takes a disputed malpractice claim to come to trial), would the written facts of the conversation enable you to reconstruct the complete interaction with the family? If the answer is yes, the documentation is complete.
In short, directly address the causes of the anger after laying out the facts and expressing your empathy or condolence.
When anger flares
Ask the question. The first step is intervening to interrupt the momentum. One way is to ask, “What would you have wanted us to do?” This usually changes the pace of the conversation by giving the patient a chance to talk at some length.
Acknowledge. Another tactic is saying, “I would be very angry, too, if I were in your shoes, and I would want explanations as well.” By making such a statement, you co-opt the patient’s anger momentarily, which can be enough to defuse the situation.
From that point on, making sure the anger doesn’t get out of control is the challenge.
Uphold principles. During such a conversation, basic principles should prevail:
- Express respect
- Be honest
- Remain calm
- Articulate empathy for the patient’s loss or injury.
Practices that prevent suits
Address absence of facts
In the early aftermath of an event, key information may remain unknown. It takes time to complete and evaluate interviews, equipment analysis, and test results.
When the patient asks, “Why did this happen?” it is appropriate to respond: “We do not know and will not know until all the necessary information is collected and reviewed. I think you would agree that it would not be helpful for me to speculate or conjecture. But as soon as we have all the facts we can present them to you.”
Get key information from patients
Not only is it important for you to share information with the patient, but she should provide key details about the event in question, too. This step is especially important when there is a concern about patient noncompliance.
If such noncompliance comes to light, share it with the people who must complete an incident evaluation. It will help put the outcome in context and neutralize lingering hostility.
Identify 1 spokesperson
Angry, dissatisfied patients and their families often seek out answers from other health professionals, such as nurses who participated in treatment. Instruct staff members and colleagues to decline to comment directly to the patient.
Train staff in verbal control
Staff should be taught how to respond when confronted by an angry patient. Unfortunately, shouts and threats to go to the press or to sue are not unusual.
Books on negotiation or conflict management are available in most libraries, and many colleges or other institutions offer classes on anger management. Unfortunately, such training remains only a peripheral part of medical education.
Dr. Woods and Dr. Bennett report no financial relationships relevant to this article. Ms. Rozovsky is President of Quality Medical Communications LLC, a company that produces video and written material for care provider education.
1. Woods JR, Rozovsky FA. What Do I Say? Communicating Intended or Unanticipated Outcomes in Obstetrics. San Francisco: Jossey-Bass; 2003.
2. Kassinove H, Tafrate RC. Anger Management: The Complete Treatment Guidebook for Practitioners. Atascadero, Calif: Impact Publishers; 2002.
3. Greenberg LS. Emotion-Focused Therapy: Coaching Clients to Work Through Their Feelings. Washington, DC: American Psychological Association; 2002.
The cases below show why patients get angry, what clues signal anger, what to say, and what not to say. But the focus throughout is how to prepare yourself and your staff to defuse the angry patient, from the first encounter through the essential follow-up: the office visit that you set up specifically to address her anger and reduce the risk of legal action.
Tracing anger’s fuse
KIM’S CASE
Kim is a 21-year-old G1P0000 with type 1 diabetes who did not comply well with her insulin and diet regimens. At 39 weeks’ gestation, with an estimated fetal weight of 4,000 g, she demands a vaginal delivery. At delivery, severe shoulder dystocia occurs, resulting in what the family later recalls as chaotic activity in the delivery room and severe nerve damage to the newborn’s left arm. When you meet with the couple and the wife’s sister later that day, all 3 express intense anger at you.
How should you respond?
Anger can occur when there is an unexpected adverse outcome, or when a patient feels responsible for a poor outcome. Either way, you may be a “safer” target than the actual cause.
In Kim’s case, each family member feels angry for a different reason, and you should try to draw their reasons out in conversation so they can be addressed. Don’t assume all are angry for the same reason.
- The new mother feels terrible because her noncompliance contributed to the outcome. She expresses her anger at you to overcome feelings of failure.
- The husband is angry with his wife because she did not follow her diet and insulin instructions carefully, but he is afraid to confront her after nearly losing their newborn son. He therefore directs his anger at you.
- The sister is angry with you out of a sense of helplessness and a desire to “make someone pay.”
In conversation, describe each step of the delivery process, to clarify misconceptions, and discuss the controversy over how best to manage a 4,000-g infant when the mother has diabetes. Also realize that the “chaotic activity” the family witnessed during the delivery may have contributed to their anger. An explanation of what was actually taking place may allay some of their concern.
In this case, knowing the patient was diabetic, the physician should have discussed the risks of various delivery methods well before the actual birth.
When is anger a healthy response and when is it pathologic?
It is beneficial when we use it to inform our actions. For example, if a physician feels angry about the proposed changes to Medicaid and writes a letter to her congressman, she is using anger constructively.
Anger is a problem when it changes to aggression, is buried, or is used to manipulate others. Research has linked chronic anger-management problems with interpersonal strife, difficulties at work, poor decision-making, increased risk-taking, substance abuse, coronary heart disease, stroke, chronic pain, disruption of motor activities (such as driving), and susceptibility to depression, guilt, and shame.2
Greenberg3 identified 4 ways of classifying expressed emotions such as anger, which the following 4 cases illustrate:
CASE 1. When anger sparks a change for the better A patient expresses anger at having to wait more than an hour to see you. By bringing it to your attention, she makes it possible for you to address the issue with office staff.
When anger is a primary adaptive response, it enables one to organize for action and to hold another responsible for injuring her. This is a healthy expression of anger; it lets a person act in congruence with both cognition and emotion.
CASE 2. When anger gets stuck Upon arrival, a patient informs your staff that she has no intention of being kept waiting. This woman considers even the slightest delay a personal affront.
When anger is a primary maladaptive response, it indicates a stuck pattern of behavior and emotional expression in which anger becomes a reflexive reaction, rather than an action. When anger takes this form, it can cause an individual to overestimate the threat to herself and damage relationships through aversive behavior.
CASE 3. Anger as a cover A woman with a newly discovered breast lump is afraid she has cancer. Rather than express this fear, she accuses you of withholding information.
In this instance, anger conceals an underlying emotion, and is therefore the secondary emotion. Sometimes the person is aware of the underlying feeling, sometimes not.
CASE 4. Anger as manipulation A patient wants cab fare provided for her and her children to get to their appointments; the norm is to provide bus fare. The patient threatens action and negative word-of-mouth, complains to the insurance company, and ultimately gets the cab fare.
Anger can be used as an instrument to manipulate others, to bring about a desired outcome.
How anger rears its head
“In your face”
Although a woman may express—or contain—her anger in any number of ways, a few styles tend to predominate. Probably the most fearsome patient is the one with an “in your face” style, who yells, swears, or threatens people. If a nurse doesn’t jump to her demands, she asks for the supervisor and threatens to file a report.
Although it can be intimidating, there is one advantage to this style of anger: At least you know where you stand.
“I don’t do anger”
At the other end of the continuum is the woman who denies her anger. We once had a patient who proclaimed, “I don’t do anger,” but the midwife who referred her described her as one of the angriest patients she had ever seen. In our encounter, she was visibly tense and responded in short, clipped sentences, but we could not address her anger directly because she refused to admit its existence.
Noncompliance as power struggle
Indirect anger falls somewhere between the above 2 extremes. Women tend to be socialized to withhold their anger to preserve relationships, so they often feel safer expressing it indirectly. Passive-aggressive behavior is one example. The patient may arrive late or forget her insulin logs, or she may say everything is fine but call later in the day with an important concern. This conduct may seem like noncompliance, but noncompliance can be rooted in anger. Communication can become a power struggle in which the patient demonstrates her anger by refusing to do as you ask. Dropping out is the ultimate expression of indirect anger; the patient merely quits.
Somatization
Another way indirect anger manifests is through somatization, an unconscious process in which the patient does not articulate her emotions but experiences them physically. Treatment often has little effect.
The quiet woman
Also be aware that women often stifle their emotions until they feel overwhelmed and resentful, at which point they may explode.
PHYSIOLOGIC
- Shortness of breath
- Rapid breathing
- Pressured speech: louder and faster
- Clenched teeth, fists
- Muscle tension
- Rapid heart rate
- Shakiness, trembling
- Tight jaw
- Indigestion, nausea, diarrhea
- Headache
- Flushing, sweating
- Fatigue
BEHAVIORAL
- Pointing a finger
- Getting in another person’s “space”
- Leaning toward the other person
- Rolling eyes
- Raising the voice
- Profanity
- Harsh or hostile tone
- Strong or extreme language
- Sarcasm
- Making accusations
- Slamming doors or phones
- Aggression toward a person or object
COGNITIVE
- Dichotomous thinking: all or nothing, black or white
- Exaggeration and generalization: always, never
- Distorted thinking
- Rigid ideation: “It must be this way or else,” “I will not stand for this,” etc
The slippery slope of how to respond
The correct response to anger is empathy, which should be heartfelt, if at all possible.
Unfortunately, personality and personal issues sometimes impede our ability to empathize openly. It is important to avoid paternalism, evasiveness, and self-blame.
Paternalism in many ways is built into the medical hierarchy. Our specialized knowledge is the reason we’re consulted in the first place, and an intellectual or condescending remark on our part may be a natural defense to a patient’s angry attack, but such a reaction only fuels the fire. Nor should we ever use our role as an authority to dismiss the patient’s anger.
Evasiveness is another frequent response to anger, but can lead to detachment and feed the patient’s perception that you are unfeeling.
Acquiescing to her demands in hopes of avoiding further confrontation or a lawsuit may decrease her anger, but increase your own resentment.
Worst of all is self-blame, in which the physician assumes and internalizes responsibility for failing the patient or lacking perfect knowledge. Though this approach may quickly quell the patient’s anger, it can harm the physician-patient relationship and your emotional health.
Loss of control. Some diseases or conditions have uncontrollable outcomes. For example, a woman with ovarian cancer may feel angry when she realizes she cannot necessarily get better by following a particular plan of action.
“Why me?” Feelings of perceived injustice arise when circumstances seem particularly unfair, as when a woman experiences fetal death in utero despite responsible self-care, and sees less responsible women deliver healthy babies.
Not listening, inattention. Poor communication often leaves the patient feeling as though you failed to listen to her concerns. For example, when you give her less than full attention, an obese woman with gestational diabetes may feel blamed for her own problems.
History of sexual abuse. In women with a history of sexual trauma, obstetric or gynecologic procedures can sometimes remind them of abuse, and themes of powerlessness and lack of control may be reenacted. While it may have been unsafe—or even fatal—for these women to express anger at the original perpetrator, they may feel safer directing it at you.
When a patient pushes your buttons
ERIN’S CASE
At 36 weeks’ gestation, Erin calls your office at the end of the day on a Friday with an emergency: She couldn’t sleep the day before. You feel angry; why? Is it because you had plans to go out to dinner and now will be late? Is it because Erin has been demanding and needy throughout her pregnancy? Is it because Erin doesn’t follow the protocol for nonemergencies and acts as though everything is an emergency? Is it because no matter what you do, Erin hasn’t been satisfied with her care?
These questions can help you decide how to proceed. In any tense situation, the first step is to identify the angry party. This is not always as straightforward as it sounds, since anger is frequently displaced. It may be the patient or her family who shows signs of anger. It may be you. Or it may be both.
Dealing with an angry patient is easier if you keep sight of your own triggers and sore spots. This self-awareness allows you to assert control and contain your emotional reaction.
Coach yourself through an angry encounter. When you recognize your own limits, it is easier to manage difficult situations using “self-talk” to coach yourself through them. For example, when your patient becomes angry, you might remind yourself, “Take a deep breath.” As she complains, you might tell yourself, “This is not personal, even though it feels that way. What is she trying to tell me?”
Formal role-playing and informal discussions of real or hypothetical cases are another strategy.
How to conduct an anger-defusing visit
Set an appointment to talk it out
When a patient expresses anger that does not abate with empathetic listening, consider scheduling a special visit to address her concerns. And prepare for it:
Don’t sit behind a desk. Determine where the conversation will be held, including the seating arrangement. It is inappropriate to sit behind a desk for the discussion, as this arrangement can exacerbate the division between you and the patient. If your office is not conducive to a “relaxed” talk, consider a more neutral site.
Check the exits. Always consider the possibility that the patient or a family member may feel outraged to the point of physical abuse. Make sure security personnel are readily available; if necessary, agree upon a prearranged signal for them to intervene. Also consider whether you will have easy access to the door. During the conversation, do not accept or tolerate threats.
Make it soon. When emotions run high, it is probably best to talk within a week. Avoid a delay of several weeks in hopes that anger will dissipate. What usually happens during such a long interval is that the family becomes angrier and seeks outside advice, much of which is negative. The patient also may change doctors because of the anger and abandonment she feels.
Schedule plenty of time. When enough time is devoted to the discussion, anger generally begins to dissipate. Therefore, do not limit conversation if the case is especially contentious and the risk of a lawsuit very real. On the contrary, it may take 2 hours or longer for the anger to soften.
Rehearse. A role-playing scenario with a colleague can help you experience the full impact of how anger alters a discussion.
Here’s what to say
JULIA’S CASE
A 48-year-old patient reported a breast lump at her last annual visit. At that time, upon examination, you palpated a small mass. Since Julia also has fibrocystic breast disease, you explained that the lump was probably benign, but you referred her for a mammogram and encouraged her to get it as soon as possible. When she finally did—8 months later—invasive carcinoma was diagnosed, necessitating a lumpectomy and chemotherapy.
At her next annual visit, Julia is confrontational, accusing you of downplaying the risk of breast cancer and causing her delay in seeking a mammogram. When she remains outraged despite your calm reminder that you recommended an expeditious mammogram, you schedule an appointment to discuss the matter.
What are your next steps?
Though the logistics are straightforward, success depends on the specific patient and any family members involved.
In general, you should strive to:
- Establish an agenda and review it with the patient.
- Describe the facts of the case in lay terms so that the patient, her family, and any others receive the same information. This helps eliminate erroneous assumptions and misinformation. For example, Julia’s family may not be aware that you encouraged her to obtain a mammogram as soon as possible.
- Express empathy that she has to go through this serious time in her life, and acknowledge that a cancer diagnosis and treatment are tremendously stressful.
- Search for the reasons behind the anger. Ask how the patient and her family have arrived at their conclusions.
- Control anger and prevent it from escalating. Keep your voice steady and maintain an open body language. Avoid signs of your own anger.
- Ask for her perception of what has been said, at the conclusion. If family members are present, ask for any other issues that need clarification.
- Close the discussion respectfully.
- Document the conversation by noting the time, persons present, what was discussed, attitudes of the family, and any plans for follow-up. How thorough should this medical note be? If you were to read your own documentation 5 years from now (the length of time it often takes a disputed malpractice claim to come to trial), would the written facts of the conversation enable you to reconstruct the complete interaction with the family? If the answer is yes, the documentation is complete.
In short, directly address the causes of the anger after laying out the facts and expressing your empathy or condolence.
When anger flares
Ask the question. The first step is intervening to interrupt the momentum. One way is to ask, “What would you have wanted us to do?” This usually changes the pace of the conversation by giving the patient a chance to talk at some length.
Acknowledge. Another tactic is saying, “I would be very angry, too, if I were in your shoes, and I would want explanations as well.” By making such a statement, you co-opt the patient’s anger momentarily, which can be enough to defuse the situation.
From that point on, making sure the anger doesn’t get out of control is the challenge.
Uphold principles. During such a conversation, basic principles should prevail:
- Express respect
- Be honest
- Remain calm
- Articulate empathy for the patient’s loss or injury.
Practices that prevent suits
Address absence of facts
In the early aftermath of an event, key information may remain unknown. It takes time to complete and evaluate interviews, equipment analysis, and test results.
When the patient asks, “Why did this happen?” it is appropriate to respond: “We do not know and will not know until all the necessary information is collected and reviewed. I think you would agree that it would not be helpful for me to speculate or conjecture. But as soon as we have all the facts we can present them to you.”
Get key information from patients
Not only is it important for you to share information with the patient, but she should provide key details about the event in question, too. This step is especially important when there is a concern about patient noncompliance.
If such noncompliance comes to light, share it with the people who must complete an incident evaluation. It will help put the outcome in context and neutralize lingering hostility.
Identify 1 spokesperson
Angry, dissatisfied patients and their families often seek out answers from other health professionals, such as nurses who participated in treatment. Instruct staff members and colleagues to decline to comment directly to the patient.
Train staff in verbal control
Staff should be taught how to respond when confronted by an angry patient. Unfortunately, shouts and threats to go to the press or to sue are not unusual.
Books on negotiation or conflict management are available in most libraries, and many colleges or other institutions offer classes on anger management. Unfortunately, such training remains only a peripheral part of medical education.
Dr. Woods and Dr. Bennett report no financial relationships relevant to this article. Ms. Rozovsky is President of Quality Medical Communications LLC, a company that produces video and written material for care provider education.
The cases below show why patients get angry, what clues signal anger, what to say, and what not to say. But the focus throughout is how to prepare yourself and your staff to defuse the angry patient, from the first encounter through the essential follow-up: the office visit that you set up specifically to address her anger and reduce the risk of legal action.
Tracing anger’s fuse
KIM’S CASE
Kim is a 21-year-old G1P0000 with type 1 diabetes who did not comply well with her insulin and diet regimens. At 39 weeks’ gestation, with an estimated fetal weight of 4,000 g, she demands a vaginal delivery. At delivery, severe shoulder dystocia occurs, resulting in what the family later recalls as chaotic activity in the delivery room and severe nerve damage to the newborn’s left arm. When you meet with the couple and the wife’s sister later that day, all 3 express intense anger at you.
How should you respond?
Anger can occur when there is an unexpected adverse outcome, or when a patient feels responsible for a poor outcome. Either way, you may be a “safer” target than the actual cause.
In Kim’s case, each family member feels angry for a different reason, and you should try to draw their reasons out in conversation so they can be addressed. Don’t assume all are angry for the same reason.
- The new mother feels terrible because her noncompliance contributed to the outcome. She expresses her anger at you to overcome feelings of failure.
- The husband is angry with his wife because she did not follow her diet and insulin instructions carefully, but he is afraid to confront her after nearly losing their newborn son. He therefore directs his anger at you.
- The sister is angry with you out of a sense of helplessness and a desire to “make someone pay.”
In conversation, describe each step of the delivery process, to clarify misconceptions, and discuss the controversy over how best to manage a 4,000-g infant when the mother has diabetes. Also realize that the “chaotic activity” the family witnessed during the delivery may have contributed to their anger. An explanation of what was actually taking place may allay some of their concern.
In this case, knowing the patient was diabetic, the physician should have discussed the risks of various delivery methods well before the actual birth.
When is anger a healthy response and when is it pathologic?
It is beneficial when we use it to inform our actions. For example, if a physician feels angry about the proposed changes to Medicaid and writes a letter to her congressman, she is using anger constructively.
Anger is a problem when it changes to aggression, is buried, or is used to manipulate others. Research has linked chronic anger-management problems with interpersonal strife, difficulties at work, poor decision-making, increased risk-taking, substance abuse, coronary heart disease, stroke, chronic pain, disruption of motor activities (such as driving), and susceptibility to depression, guilt, and shame.2
Greenberg3 identified 4 ways of classifying expressed emotions such as anger, which the following 4 cases illustrate:
CASE 1. When anger sparks a change for the better A patient expresses anger at having to wait more than an hour to see you. By bringing it to your attention, she makes it possible for you to address the issue with office staff.
When anger is a primary adaptive response, it enables one to organize for action and to hold another responsible for injuring her. This is a healthy expression of anger; it lets a person act in congruence with both cognition and emotion.
CASE 2. When anger gets stuck Upon arrival, a patient informs your staff that she has no intention of being kept waiting. This woman considers even the slightest delay a personal affront.
When anger is a primary maladaptive response, it indicates a stuck pattern of behavior and emotional expression in which anger becomes a reflexive reaction, rather than an action. When anger takes this form, it can cause an individual to overestimate the threat to herself and damage relationships through aversive behavior.
CASE 3. Anger as a cover A woman with a newly discovered breast lump is afraid she has cancer. Rather than express this fear, she accuses you of withholding information.
In this instance, anger conceals an underlying emotion, and is therefore the secondary emotion. Sometimes the person is aware of the underlying feeling, sometimes not.
CASE 4. Anger as manipulation A patient wants cab fare provided for her and her children to get to their appointments; the norm is to provide bus fare. The patient threatens action and negative word-of-mouth, complains to the insurance company, and ultimately gets the cab fare.
Anger can be used as an instrument to manipulate others, to bring about a desired outcome.
How anger rears its head
“In your face”
Although a woman may express—or contain—her anger in any number of ways, a few styles tend to predominate. Probably the most fearsome patient is the one with an “in your face” style, who yells, swears, or threatens people. If a nurse doesn’t jump to her demands, she asks for the supervisor and threatens to file a report.
Although it can be intimidating, there is one advantage to this style of anger: At least you know where you stand.
“I don’t do anger”
At the other end of the continuum is the woman who denies her anger. We once had a patient who proclaimed, “I don’t do anger,” but the midwife who referred her described her as one of the angriest patients she had ever seen. In our encounter, she was visibly tense and responded in short, clipped sentences, but we could not address her anger directly because she refused to admit its existence.
Noncompliance as power struggle
Indirect anger falls somewhere between the above 2 extremes. Women tend to be socialized to withhold their anger to preserve relationships, so they often feel safer expressing it indirectly. Passive-aggressive behavior is one example. The patient may arrive late or forget her insulin logs, or she may say everything is fine but call later in the day with an important concern. This conduct may seem like noncompliance, but noncompliance can be rooted in anger. Communication can become a power struggle in which the patient demonstrates her anger by refusing to do as you ask. Dropping out is the ultimate expression of indirect anger; the patient merely quits.
Somatization
Another way indirect anger manifests is through somatization, an unconscious process in which the patient does not articulate her emotions but experiences them physically. Treatment often has little effect.
The quiet woman
Also be aware that women often stifle their emotions until they feel overwhelmed and resentful, at which point they may explode.
PHYSIOLOGIC
- Shortness of breath
- Rapid breathing
- Pressured speech: louder and faster
- Clenched teeth, fists
- Muscle tension
- Rapid heart rate
- Shakiness, trembling
- Tight jaw
- Indigestion, nausea, diarrhea
- Headache
- Flushing, sweating
- Fatigue
BEHAVIORAL
- Pointing a finger
- Getting in another person’s “space”
- Leaning toward the other person
- Rolling eyes
- Raising the voice
- Profanity
- Harsh or hostile tone
- Strong or extreme language
- Sarcasm
- Making accusations
- Slamming doors or phones
- Aggression toward a person or object
COGNITIVE
- Dichotomous thinking: all or nothing, black or white
- Exaggeration and generalization: always, never
- Distorted thinking
- Rigid ideation: “It must be this way or else,” “I will not stand for this,” etc
The slippery slope of how to respond
The correct response to anger is empathy, which should be heartfelt, if at all possible.
Unfortunately, personality and personal issues sometimes impede our ability to empathize openly. It is important to avoid paternalism, evasiveness, and self-blame.
Paternalism in many ways is built into the medical hierarchy. Our specialized knowledge is the reason we’re consulted in the first place, and an intellectual or condescending remark on our part may be a natural defense to a patient’s angry attack, but such a reaction only fuels the fire. Nor should we ever use our role as an authority to dismiss the patient’s anger.
Evasiveness is another frequent response to anger, but can lead to detachment and feed the patient’s perception that you are unfeeling.
Acquiescing to her demands in hopes of avoiding further confrontation or a lawsuit may decrease her anger, but increase your own resentment.
Worst of all is self-blame, in which the physician assumes and internalizes responsibility for failing the patient or lacking perfect knowledge. Though this approach may quickly quell the patient’s anger, it can harm the physician-patient relationship and your emotional health.
Loss of control. Some diseases or conditions have uncontrollable outcomes. For example, a woman with ovarian cancer may feel angry when she realizes she cannot necessarily get better by following a particular plan of action.
“Why me?” Feelings of perceived injustice arise when circumstances seem particularly unfair, as when a woman experiences fetal death in utero despite responsible self-care, and sees less responsible women deliver healthy babies.
Not listening, inattention. Poor communication often leaves the patient feeling as though you failed to listen to her concerns. For example, when you give her less than full attention, an obese woman with gestational diabetes may feel blamed for her own problems.
History of sexual abuse. In women with a history of sexual trauma, obstetric or gynecologic procedures can sometimes remind them of abuse, and themes of powerlessness and lack of control may be reenacted. While it may have been unsafe—or even fatal—for these women to express anger at the original perpetrator, they may feel safer directing it at you.
When a patient pushes your buttons
ERIN’S CASE
At 36 weeks’ gestation, Erin calls your office at the end of the day on a Friday with an emergency: She couldn’t sleep the day before. You feel angry; why? Is it because you had plans to go out to dinner and now will be late? Is it because Erin has been demanding and needy throughout her pregnancy? Is it because Erin doesn’t follow the protocol for nonemergencies and acts as though everything is an emergency? Is it because no matter what you do, Erin hasn’t been satisfied with her care?
These questions can help you decide how to proceed. In any tense situation, the first step is to identify the angry party. This is not always as straightforward as it sounds, since anger is frequently displaced. It may be the patient or her family who shows signs of anger. It may be you. Or it may be both.
Dealing with an angry patient is easier if you keep sight of your own triggers and sore spots. This self-awareness allows you to assert control and contain your emotional reaction.
Coach yourself through an angry encounter. When you recognize your own limits, it is easier to manage difficult situations using “self-talk” to coach yourself through them. For example, when your patient becomes angry, you might remind yourself, “Take a deep breath.” As she complains, you might tell yourself, “This is not personal, even though it feels that way. What is she trying to tell me?”
Formal role-playing and informal discussions of real or hypothetical cases are another strategy.
How to conduct an anger-defusing visit
Set an appointment to talk it out
When a patient expresses anger that does not abate with empathetic listening, consider scheduling a special visit to address her concerns. And prepare for it:
Don’t sit behind a desk. Determine where the conversation will be held, including the seating arrangement. It is inappropriate to sit behind a desk for the discussion, as this arrangement can exacerbate the division between you and the patient. If your office is not conducive to a “relaxed” talk, consider a more neutral site.
Check the exits. Always consider the possibility that the patient or a family member may feel outraged to the point of physical abuse. Make sure security personnel are readily available; if necessary, agree upon a prearranged signal for them to intervene. Also consider whether you will have easy access to the door. During the conversation, do not accept or tolerate threats.
Make it soon. When emotions run high, it is probably best to talk within a week. Avoid a delay of several weeks in hopes that anger will dissipate. What usually happens during such a long interval is that the family becomes angrier and seeks outside advice, much of which is negative. The patient also may change doctors because of the anger and abandonment she feels.
Schedule plenty of time. When enough time is devoted to the discussion, anger generally begins to dissipate. Therefore, do not limit conversation if the case is especially contentious and the risk of a lawsuit very real. On the contrary, it may take 2 hours or longer for the anger to soften.
Rehearse. A role-playing scenario with a colleague can help you experience the full impact of how anger alters a discussion.
Here’s what to say
JULIA’S CASE
A 48-year-old patient reported a breast lump at her last annual visit. At that time, upon examination, you palpated a small mass. Since Julia also has fibrocystic breast disease, you explained that the lump was probably benign, but you referred her for a mammogram and encouraged her to get it as soon as possible. When she finally did—8 months later—invasive carcinoma was diagnosed, necessitating a lumpectomy and chemotherapy.
At her next annual visit, Julia is confrontational, accusing you of downplaying the risk of breast cancer and causing her delay in seeking a mammogram. When she remains outraged despite your calm reminder that you recommended an expeditious mammogram, you schedule an appointment to discuss the matter.
What are your next steps?
Though the logistics are straightforward, success depends on the specific patient and any family members involved.
In general, you should strive to:
- Establish an agenda and review it with the patient.
- Describe the facts of the case in lay terms so that the patient, her family, and any others receive the same information. This helps eliminate erroneous assumptions and misinformation. For example, Julia’s family may not be aware that you encouraged her to obtain a mammogram as soon as possible.
- Express empathy that she has to go through this serious time in her life, and acknowledge that a cancer diagnosis and treatment are tremendously stressful.
- Search for the reasons behind the anger. Ask how the patient and her family have arrived at their conclusions.
- Control anger and prevent it from escalating. Keep your voice steady and maintain an open body language. Avoid signs of your own anger.
- Ask for her perception of what has been said, at the conclusion. If family members are present, ask for any other issues that need clarification.
- Close the discussion respectfully.
- Document the conversation by noting the time, persons present, what was discussed, attitudes of the family, and any plans for follow-up. How thorough should this medical note be? If you were to read your own documentation 5 years from now (the length of time it often takes a disputed malpractice claim to come to trial), would the written facts of the conversation enable you to reconstruct the complete interaction with the family? If the answer is yes, the documentation is complete.
In short, directly address the causes of the anger after laying out the facts and expressing your empathy or condolence.
When anger flares
Ask the question. The first step is intervening to interrupt the momentum. One way is to ask, “What would you have wanted us to do?” This usually changes the pace of the conversation by giving the patient a chance to talk at some length.
Acknowledge. Another tactic is saying, “I would be very angry, too, if I were in your shoes, and I would want explanations as well.” By making such a statement, you co-opt the patient’s anger momentarily, which can be enough to defuse the situation.
From that point on, making sure the anger doesn’t get out of control is the challenge.
Uphold principles. During such a conversation, basic principles should prevail:
- Express respect
- Be honest
- Remain calm
- Articulate empathy for the patient’s loss or injury.
Practices that prevent suits
Address absence of facts
In the early aftermath of an event, key information may remain unknown. It takes time to complete and evaluate interviews, equipment analysis, and test results.
When the patient asks, “Why did this happen?” it is appropriate to respond: “We do not know and will not know until all the necessary information is collected and reviewed. I think you would agree that it would not be helpful for me to speculate or conjecture. But as soon as we have all the facts we can present them to you.”
Get key information from patients
Not only is it important for you to share information with the patient, but she should provide key details about the event in question, too. This step is especially important when there is a concern about patient noncompliance.
If such noncompliance comes to light, share it with the people who must complete an incident evaluation. It will help put the outcome in context and neutralize lingering hostility.
Identify 1 spokesperson
Angry, dissatisfied patients and their families often seek out answers from other health professionals, such as nurses who participated in treatment. Instruct staff members and colleagues to decline to comment directly to the patient.
Train staff in verbal control
Staff should be taught how to respond when confronted by an angry patient. Unfortunately, shouts and threats to go to the press or to sue are not unusual.
Books on negotiation or conflict management are available in most libraries, and many colleges or other institutions offer classes on anger management. Unfortunately, such training remains only a peripheral part of medical education.
Dr. Woods and Dr. Bennett report no financial relationships relevant to this article. Ms. Rozovsky is President of Quality Medical Communications LLC, a company that produces video and written material for care provider education.
1. Woods JR, Rozovsky FA. What Do I Say? Communicating Intended or Unanticipated Outcomes in Obstetrics. San Francisco: Jossey-Bass; 2003.
2. Kassinove H, Tafrate RC. Anger Management: The Complete Treatment Guidebook for Practitioners. Atascadero, Calif: Impact Publishers; 2002.
3. Greenberg LS. Emotion-Focused Therapy: Coaching Clients to Work Through Their Feelings. Washington, DC: American Psychological Association; 2002.
1. Woods JR, Rozovsky FA. What Do I Say? Communicating Intended or Unanticipated Outcomes in Obstetrics. San Francisco: Jossey-Bass; 2003.
2. Kassinove H, Tafrate RC. Anger Management: The Complete Treatment Guidebook for Practitioners. Atascadero, Calif: Impact Publishers; 2002.
3. Greenberg LS. Emotion-Focused Therapy: Coaching Clients to Work Through Their Feelings. Washington, DC: American Psychological Association; 2002.
Current management of diabetic pregnancy
This article describes the rationale for intensive treatment with these agents and other interventions to prevent both hypoglycemia and hyperglycemia.
Intensive therapy requires:
- memory-based self-monitoring of blood glucose, which empowers patients to take charge of glycemic control and provides feedback on the timing and dose of insulin administration,
- dietary regulation,
- strict criteria for initiation of pharmacologic therapy,
- multiple injections of insulin or its equivalent when diet alone is insufficient, and
- an interdisciplinary management team.
Neither race nor ethnicity predicts treatment duration or success.5-7
Blood glucose goals
Regardless of the treatment, the primary goal is always to achieve glycemic control, because it reduces the incidence of hypoglycemia, hyperglycemia, and ketosis. For type 1 and type 2 diabetes, glycemic control is important to prevent further deterioration of complications such as vasculopathy and nephropathy.
Goals of treatment are achieving the following blood glucose concentrations (in milligrams per deciliter):
- mean: 90 to 105
- fasting: 60 to 90
- preprandial: 80 to 95
- postprandial: less than 120
In the process, the clinician needs to anticipate how pregnancy will affect preexisting disease, and how diabetes will affect pregnancy outcomes, in patients with any of the 3 types of diabetes.
2 diet protocols
For all types of diabetes, the foundation is diet—specifically, using nutritional therapy to achieve and maintain a maternal blood glucose profile comparable to that of a nondiabetic woman.
Two approaches are recommended:
- reducing carbohydrate intake to 40% to 50% of total calories or
- limiting carbohydrate consumption to foods with a low glycemic index for approximately 60% of calories.
Calculating calories: Same for all
The daily caloric intake is based on the prepregnancy body mass index (BMI) and uses the same formula for all 3 types of diabetes10,11:
- For a BMI less than 20 (underweight), daily caloric intake should be 35 to 38 kcal/kg.
- For a BMI of 20 to 25 (normal weight), the patient should consume 30 kcal/kg.
- For a BMI of 26 and higher (overweight, obese, morbidly obese), caloric intake should be 20 to 25 kcal/kg.
In addition, the daily allotment of calories is divided into 3 main meals and 3 to 4 snacks, with adjustments for the patient’s time constraints, work schedule, and other individual factors.
To encourage compliance, the diet also should reflect the patient’s cultural preferences.
How do you know when diet fails?
Women with pregestational diabetes are usually already taking insulin or other pharmacologic agents by the time they conceive. There is no consensus or hard data on how long a woman who develops gestational diabetes mellitus should remain on a diet before starting drug treatment.
In a study evaluating the time required to achieve glycemic control with diet alone during a 4-week period, 70% of patients with fasting plasma below 95 mg/dL achieved established levels of glycemic control within 2 weeks with no substantial improvement thereafter.8,9 In contrast, in patients with fasting plasma glucose of more than 95 mg/dL, most patients failed to achieve the desired level of glycemic control throughout the 4-week period.
Hypoglycemia after exercise can be a positive marker
I recommend 20 to 30 minutes of exercise 3 to 4 times weekly for gravidas with diabetes, provided they are willing and able to perform it, because it can improve post-prandial blood glucose levels and insulin sensitivity.12
Blood glucose should be measured after exercise, especially in women with type 1 diabetes.
Hypoglycemic reactions during and after exercise may be positive markers of improved insulin sensitivity. Low blood glucose necessitates adjustment of the insulin dose and carbohydrate intake. Extra monitoring is warranted after evening exercise, as glucose uptake increases for several hours after exercise and can cause nocturnal hypoglycemia.
Intensive therapy: Why, when, how
The healthy body secretes insulin over 24 hours independent of nutrient intake. Basal insulin secretion maintains metabolic homeostasis by preventing excessive hepatic glucose production and the mobilization of free fatty acids from adipose tissue stores. This also helps maintain protein balance. Insulin secretion increases several times in response to the ingestion of food.
Treatment or consequences
Langer O, Yogev Y, Most O, et al. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol. 2005;192;989-997.
When diabetic women receive adequate preconception care and counseling and achieve glycemic control, the rate of congenital anomalies declines to levels seen in the general population.69-72
On the other hand, maternal hyperglycemia and resultant fetal hyperinsulinemia are central to the pathophysiology of diabetic complications:
- type 1 and type 2 diabetes—congenital malformations
- all pregnancies compromised by diabetes—increased rates of deviant fetal growth (macrosomia and intrauterine growth restriction), neonatal metabolic, hematological and respiratory complications, birth trauma, stillbirth, cesarean delivery and intensive care admissions.
I tell patients, “Some improvement is better than none” I explain to my patients how pregnancy itself imposes risk, and why it is crucial to follow protocols and achieve glycemic control. I explain the maternal and fetal complications associated with various glucose thresholds, and the added risks of maternal age, body composition, disease severity, and so on.
However, I also stress that even some improvement in glucose control is better than no improvement.
Insulin dosage requires frequent adjustment
To determine the insulin dose needed to achieve glycemic control in pregnant gravidas, multiple blood glucose measurements are needed because insulin requirements steadily increase throughout pregnancy in women with pregestational diabetes.13-16 Jovanovic and Peterson13 quantified these increases as 0.7, 0.8, 0.9, and 1.0 U/kg per day in the first trimester and at weeks 18, 26, and 36, respectively.
Using memory-based reflectance meters to monitor blood glucose, my colleagues and I observed that insulin requirements during pregnancy in women with pregestational diabetes are triphasic (TABLE) and require frequent assessment with individualized adjustment of the insulin dose in each trimester.16 Women with type 2 diabetes require significantly higher doses of insulin each trimester, compared with women with type 1 diabetes.
In women with gestational diabetes, we observed a biphasic increase in insulin requirements17:
- Insulin requirements increased up to the 30th week of gestation, necessitating frequent dose adjustments.
- After 30 weeks, insulin requirements stabilized, requiring minimal or no dose adjustments. Insulin requirements for obese subjects were 0.9 U/kg per day, compared with 0.8 U/kg per day for nonobese women.
TABLE
Insulin requirements during pregnancy for women with pregestational diabetes
| INSULIN REQUIREMENT (UNITS/KG/DAY) | ||
|---|---|---|
| TRIMESTER | TYPE 1 DIABETES | TYPE 2 DIABETES |
| 1 | 0.86 | 0.86 |
| 2 | 0.95 | 1.18 |
| 3 | 1.19 | 1.62 |
| Insulin requirements vary with gestational diabetes | ||
When to start drugs
Most women with pregestational diabetes are treated with insulin prior to pregnancy. Thus, the main task during pregnancy is maintaining or improving glycemic control. In gestational diabetes, pharmacologic therapy (insulin or glyburide) is initiated only when regulation of the diet fails to achieve the desired level of glycemic control or when the disease is severe enough to mandate therapy.
Authorities disagree on the threshold of severity that necessitates pharmacologic intervention (glyburide or insulin). Some suggest a threshold of fasting plasma glucose of at least 95 mg/dL,18-20 which will decrease the rate of macrosomic and large-for-gestational-age infants,19,21 while others suggest at least 105 mg/dL.19,22
All authorities agree that drug therapy should be started when postprandial glucose levels are 120 mg/dL or higher at 2 hours or 140 mg/dL or higher at 1 hour.
Using these standards, 30% to 50% of women with gestational diabetes require pharmacologic therapy when diet alone fails to reduce glucose levels.
Determining insulin requirements
The insulin algorithm for women with gestational diabetes is based on prepregnancy BMI:
- For women with a BMI of 25 and less, the insulin dose is 0.8 U/kg.
- For women with a BMI of more than 25 (overweight and obese), it is 1.0 U/kg.
Once the total insulin dose is calculated, it is divided so that two thirds is administered in the morning and one third in the afternoon or evening. The morning dose is further divided in a ratio of 2 to 1 (intermediate and rapid-acting) and the evening dose into a ratio of 1-to-1 (rapid-acting and intermediate). The rapid-acting dose is administered with the evening meal, while the intermediate dose is given just before bedtime.
If the patient with gestational diabetes has not achieved the desired level of glycemic control after 3 to 7 days, increase the total dose by 10% to 20% and thereafter adjust it when needed.
Fine points of insulin therapy
The actual total insulin dose in women with gestational diabetes is 40% higher than the calculated dose16; this provides a margin of safety and avoids severe hypoglycemic episodes. As a rule of thumb, self-monitoring of blood glucose is necessary before every administration of insulin.
The failure to introduce insulin therapy in a timely fashion may lead to fetal hyperinsulinemia and associated complications. Conversely, premature initiation of insulin in women who could have achieved glycemic control with diet alone leads to unnecessary drug treatment.
When gestational diabetes is diagnosed after 30 to 33 weeks’ gestation and there is little time left to gain the desired level of control, pharmacologic intervention is recommended. There is greater flexibility when gestational diabetes is diagnosed early in the third trimester.
Which form of insulin is best?
Human insulin is recommended when insulin is prescribed during pregnancy, and the same type of insulin is used for pregestational and gestational diabetes. The main differences:
- use of the insulin pump in type 1 diabetes and
- the insulin dose, which is based on insulin requirements for each type of diabetes.
Regular insulin and insulin lispro are the 2 most common rapid-acting forms of insulin in use.
Pros and cons of insulin lispro
Mounting evidence of the benefits of insulin lispro for type 1 and type 2 diabetes in nonpregnant individuals includes:
- fewer episodes of severe hypoglycemia,
- limited postprandial glucose excursions, and
- a possible decrease in glycosylated hemoglobin when the drug is administered by continuous subcutaneous infusion.23
Neither the American Diabetes Association22 nor the American College of Obstetricians and Gynecologists19 endorses the use of insulin analogs. The reason: these drugs have not been adequately tested in pregnancy, although insulin lispro is categorized as a class B drug.
Data on insulin lispro are limited and abstracted from studies with relatively small sample sizes (only 244 gravidas reported thus far in the literature). Most case reports describe improved glycemic control, increased patient satisfaction, and fewer hypoglycemic episodes, but lack sufficient data on maternal and neonatal outcomes. Even so, many obstetricians have administered the drug with no adverse outcome.
In my opinion, insulin lispro can and should be used in pregnancy because of its ability to produce more physiologic insulin patterns and because the data against it are anecdotal. In contrast, insulin aspart and glargine should be avoided in pregnancy because data on their effects are limited.24-40
Individualizing the insulin regimen
A relatively high dose of insulin (about 50–90 U) is needed to achieve glycemic control in gestational diabetes. In contrast, in type 1 diabetes, a lower dose is necessary (50–60 U). Because of the different glycemic profile of women with type 1 diabetes, individualizing the insulin regimen is accepted practice.
The carbohydrate algorithm. For every 15 g of carbohydrates ingested at mealtime, 1 U of rapid-acting insulin analog (insulin lispro or insulin aspart) is required.
If postprandial glucose is continuously elevated (>120 mg/dL) at 2 hours, an increase in rapid-acting insulin is required. Thus, the carbohydrate algorithm may change to 1 U of insulin for every 12 g of carbohydrates until the appropriate ratio is achieved.
If hypoglycemia or relative hypoglycemia occurs, the amount of carbohydrates should increase for each unit of insulin. For example, the adjusted dose would be 1 U of insulin for every 18 g of carbohydrates.
The range of these algorithms is influenced by prepregnancy BMI, disease severity, type of diabetes, and type of carbohydrate (ie, complex versus simple).41
Recognizing patterns of severity. The second algorithm involves identifying the glucose severity pattern (ie, hyperglycemia, hypoglycemia). For example, the total dose of insulin required (0.8–1.0 U/kg) is divided into a ratio of 60% for intermediate or long-acting insulin (basal dose) and 40% for the premeal dose. If the glucose level falls above the targeted level, a single unit of insulin lispro or insulin aspart is added for every 30 mg/dL, but not exceeding 3 U at one time. If glucose levels remain high, redistribute the calculated insulin dose to obtain an improved actual dose (ie, reconfigure the new calculated dose throughout the day based on patient need).
Retinopathy
Poor glucose control may contribute to or worsen diabetic retinopathy—the leading cause of blindness in diabetic patients 24 to 64 years of age—by increasing intracellular accumulation of glucose and its metabolic products. This damages the tiny blood vessels inside the retina, beginning with the formation of microaneurysms and progressing to blockage and, potentially, proliferation of fragile, abnormal, new blood vessels. If vessels leak blood, vision can be severely impaired or obliterated.
Patients at risk should achieve glycemic control gradually. Rapid initiation of stringent glycemic control can cause short-term progression of retinopathy, especially in hypertensive patients, although there are no apparent long-term effects.
Diabetic nephropathy
This complication increases the risk of preeclampsia, chronic hypertension, and fetal growth restriction, and is the most common cause of end-stage renal disease. Proteinuria often increases during pregnancy in diabetic women, but renal function generally remains stable. Nevertheless, advanced diabetic nephropathy (serum creatinine >1.5 mg/dL or creatinine clearance of ≤90 mL/min) can cause further deterioration.
The stacking effect explains why most women with gestational diabetes need no regular or rapid-acting insulin at lunchtime yet are still able to maintain the desired level of glucose control.
Special needs in type 1 disease
Because glucose levels in women with type 1 diabetes typically vary widely on a daily or even hourly basis, the insulin dose should be flexible. For example, the patient may need 1 U of rapid-acting insulin for every 25 mg/dL of blood glucose above 125 mg/dL, or 1 U for every 20 mg/dL above 120 mg/dL, and so on. I encourage patients to titrate based on half-unit increments, which can be measured in an insulin syringe.
Insulin pumps. Insulin lispro and insulin aspart are approved for administration as a continuous subcutaneous infusion. However, use of the pump in pregnancy has been limited—as has its research.
Improved metabolic control is a potential advantage of the pump. When the patient is motivated and alert, use of the pump can reduce nocturnal hypoglycemia and morning hyperglycemia caused by the “dawn phenomenon” (an abrupt rise in glucose level in the early morning).
Disadvantages of the pump include cost, diabetic ketoacidosis, and hypoglycemia (caused by malfunction or infection at the infusion site). Maternal and fetal outcomes are comparable whether the insulin pump or intensive therapy is used. However, improvements in lifestyle and metabolic control may justify use of the pump in women who have trouble achieving glycemic control.43
The many advantages of glyburide
Oral agents can be a pragmatic alternative to insulin in pregnancy because they are easy to administer and noninvasive. Many experts and authoritative bodies in the US recommend glyburide (sulfonylurea) as an alternative pharmacologic therapy during pregnancy.20,44-48 Others recommend further evaluation.19,22,49,50
Although some oral agents cross the placenta, they do not necessarily cause a toxic or teratogenic effect on the fetus. Glyburide, a class B drug, does not cross the placenta.51-53 It increases insulin secretion and diminishes insulin resistance by lowering glucose toxicity. Its onset of action is about 4 hours, and the duration of action is about 10 hours. Thus, after achieving the targeted therapeutic level, glyburide covers the basal requirement as well as postprandial glucose excursions.
The starting dose is 2.5 mg orally in the morning. If the targeted level of glycemia is not attained, add 2.5 mg to the morning dose. If indicated (after 3 to 7 days), add 5 mg in the evening. Thereafter, increase the dose in 5-mg increments, up to a total of 20 mg per day. If the patient does not achieve acceptable glycemic control, add long-acting insulin.
Evidence on oral agents. Several retrospective and randomized studies evaluated oral agents in pregnancy. Most demonstrated that these agents are comparable to insulin in glycemic control and pregnancy outcome.54-61
In a randomized study, my colleagues and I found comparable pregnancy outcomes for glyburide and insulin.56 Recently we reconfirmed our original observation62 that hypoglycemic episodes are more common in insulin-treated patients than in those taking glyburide. In this study, we used continuous glucose monitoring and found hypoglycemic episodes in 63% of the insulin-treated women with gestational diabetes, but only in 28% of those taking glyburide.
We further analyzed the association between glyburide dose, gestational diabetes severity, and selected maternal and neonatal factors.63 Not surprisingly, we found that the glyburide dose increased with the severity of gestational diabetes. The success rate (ie, achievement of glycemic control) decreased as disease severity increased. However, there was no difference between glyburide- and insulin-treated patients at each level of severity. Thus, achieving glycemic control—not any particular mode of pharmacologic therapy—is the key to improving pregnancy outcome in gestational diabetes.
When costs of insulin therapy and glyburide treatment are compared, the latter is considerably less expensive.64
Ensuring fetal health and a safe delivery
Three principles form the basis of obstetric care for women with diabetes:
- fetal testing to prevent stillbirth and compromised fetal states at delivery,
- lung-maturity testing to prevent hyaline membrane disease, and
- determining the best time and method of delivery to prevent fetal compromise, macrosomia, and shoulder dystocia.
Fetal testing
At our institution, we begin fetal testing at 32 weeks’ gestation in all women regardless of diabetes type—even earlier in women with vascular/hypertensive disorders. This includes assessing fetal movements 3 times daily and nonstress testing weekly. This approach has led to a stillbirth rate of 2.5 per 1,000, compared with 4 per 1,000 in the general population.4
Is amniocentesis warranted to determine lung maturity?
A major goal of fetal surveillance in gestational diabetes is preventing lung disease. Inadequately controlled gestational diabetes can increase the risk of respiratory distress syndrome or delay lung maturity. Thus, assessing fetal pulmonary status by confirming gestational age or fetal size can be misleading.65,66
The delay in lung maturity among infants of diabetic mothers is 1 to 2 weeks.67 This delay was associated with poorly controlled diabetes in several studies. Thus, this subgroup of patients stands to benefit most from amniocentesis.
At our institution, the common practice is to test for lung maturity before any elective delivery at less than 38 weeks’ gestation. However, when the clinician determines that delivery would be beneficial, as in cases of poorly controlled diabetes, noncompliance, or other obstetric indications, we deliver the infant regardless of lung maturity. These fetuses experience minimal lung morbidity after 37 weeks’ gestation.
The bottom line: Compromised lung maturity in a live infant is preferable to a deceased infant with healthy lungs.
Timing of delivery
Most experts agree that women with diabetes should be delivered at term—though the definition of “term” ranges from 38 to 42 weeks’ gestation.
At our institution, in addition to the established routine obstetric indications for delivery, 4 additional indications mandate elective delivery for women with diabetes:
- Fetal macrosomia (weight >4,000 g). For large-for-gestational-age fetuses (>90th percentile), induction of labor may be appropriate when fetal weight ranges from 3,800 to 4,000 g and the gestational age is at least 38 weeks. Delivering these fetuses reduces the risk for shoulder dystocia, an ominous complication of diabetes in pregnancy.
- History of previous stillbirth—often the result of poorly controlled diabetes—also warrants induction of labor.
- Poor compliance or glycemic control. This includes the failure to test blood glucose enough to determine glycemic control; inability or unwillingness to adhere to the diabetic protocol, such as fetal testing; and missed appointments.
- Presence of vasculopathy-related hypertension.
The road ahead
More pharmacologic alternatives are on the horizon and may include metformin and other oral antidiabetic drugs, insulin glargine and oral insulin, and a technologically improved insulin pump that can interact directly with blood glucose levels.
The author reports no financial relationships relevant to this article.
1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
2. American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2000;23(suppl 2):S27-S31.
3. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33). Lancet. 1998;352:837-853.
4. Langer O, Rodriguez DA, Xenakis EMJ, et al. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1994;170:1036-1047.
5. Langer N, Langer O. Comparison of pregnancy mood profiles in gestational diabetes and preexisting diabetes. Diabetes Educ. 2000;26:667-672.
6. Langer O, Langer N, Piper JM, Elliott B, Anyaegbunam A. Cultural diversity as a factor in self-monitoring blood glucose in gestational diabetes. J Assoc Acad Minor Phys. 1995;6:73-77.
7. Langer N, Langer O. Emotional adjustment to diagnosis and intensified treatment of gestational diabetes. Obstet Gynecol. 1994;84:329-334.
8. McFarland MB, Langer O, et al. Dietary therapy for gestational diabetes: how long is long enough? Obstet Gynecol. 1999;93:978-982.
9. Langer O. Management of gestational diabetes. Clin Obstet Gynecol. 1999;93:978-982.
10. Dornhorst A, Frost G. Nutritional management in diabetic pregnancy: a time for reason not dogma. In: Hod M, Jovanovic L, DiRenzo GC, deLevia A, Langer O, eds. Diabetes and Pregnancy. United Kingdom: Taylor & Francis; 2003;340-358.
11. Luke B. Dietary management. In: Reece EA, Coustan DR, Gabbe SG, eds. Diabetes in Women. Philadelphia: Lippincott Williams & Wilkins; 2004;273-281.
12. Artal R. Exercise: the alternative therapeutic intervention for gestational diabetes. Clin Obstet Gynecol. 2003;46:479-487.
13. Jovanovic L, Peterson CM. Optimal insulin delivery for the pregnant diabetic patient. Diabetes Care. 1982;5:24-31.
14. Rayburn W. Changes in insulin therapy during pregnancy. Am J Perinatol. 1985;2:271-277.
15. Weiss P, Hofmann H. Intensified conventional insulin therapy for the pregnant diabetic patient. Obstet Gynecol. 1984;64:629-633.
16. Langer O, et al. Pregestational diabetes: insulin requirements throughout pregnancy. Am J Obstet Gynecol. 1988;159:616-620.
17. Langer O, Anyaegbunam A, et al. Gestational diabetes: insulin requirements in pregnancy. Am J Obstet Gynecol. 1987;157:669-675.
18. Metzger BE, Coustan DR. Organizing committee. Summary and Recommendations of the Fourth International Workshop-Conference on Gestational Diabetes. Diabetes Care. 1998;21(suppl 2):B161-B167.
19. American College of Obstetricians and Gynecologists. Clinical management guidelines for obstetrician-gynecologists. No. 30. Gestational diabetes. Washington, DC: ACOG; 2001.
20. Reece EA, Homko C, Miodovnik M, et al. A consensus report of the diabetes in pregnancy study group of North America Conference. J Matern Fetal Neonatal Med. 2002;12:362-364.
21. Langer O. Maternal glycemic criteria for insulin therapy in gestational diabetes mellitus. Diabetes Care. 1998;21(suppl 2):B91-B98.
22. American Diabetes Association. Position statement on gestational diabetes mellitus. Diabetes Care. 2004;27(suppl 1):S88-S90.
23. Holleman F, Hoekstra JBL. Insulin lispro. N Engl J Med. 1997;337:176-183.
24. Diamond T, Kormas N. Possible adverse fetal effect of insulin lispro [letter] [with discussion]. N Engl J Med. 1997;337:1009-1010.
25. Jovanovic L, Ilic S, Pettitt, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care. 1999;22:1422-1427.
26. Kitzmiller JL, Main E, Ward B, et al. Insulin lispro and the development of proliferative diabetic retinopathy during pregnancy [letter]. Diabetes Care. 1999;22:874-876.
27. Bhattacharyya A, Vice P. Insulin lispro, pregnancy, and retinopathy. Diabetes Care. 1999;22:2101-2102.
28. Buchbinder A, Miodovnik M, McElvy S, et al. Is insulin lispro associated with the development or progression of diabetic retinopathy during pregnancy? Am J Obstet Gynecol. 2000;183:1162-1165.
29. Bhattacharyya A, Brown S, Hughes S, et al. Insulin lispro and regular insulin in pregnancy. QJ Med. 2001;94:255-260.
30. Persson B, Swahn ML, Hjertberg R, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes mellitus. Diabetes Res Clin Pract. 2002;58:115-121.
31. Loukovaara S, Immonen I, Teramo KA, et al. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care. 2003;26:1193-1198.
32. Durand-Gonzalez KN, Guillausseau N, Anciaux ML, et al. Allergy to insulin in a woman with gestational diabetes mellitus: transient efficiency of continuous subcutaneous insulin lispro infusion. Diabetes Metab. 2003;29:432-434.
33. Garg S, Frias JP, Anil S, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes: glycemic control and maternal and fetal outcomes. Endocr Pract. 2003;9:187-193.
34. Mecacci F, Carignani L, Cioni R, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non-diabetic pregnant women. Eur J Obstet Gynecol Reprod Biol. 2003;111:19-24.
35. Carr KJE, Idama T, et al. A randomized controlled trial of insulin lispro given before or after meals in pregnant women with type 1 diabetesthe effect on glycaemic excursion. J Obstet Gynaecol. 2004;24:382-386.
36. Idama TO, Lindow SW, French M, et al. Preliminary experience with the use of insulin lispro in pregnant diabetic women. J Obstet Gynaecol. 2001;21:350-351.
37. Cypryk K, Sobczak M, Pertynska-Marczewska M, et al. Pregnancy complications and perinatal outcome in diabetic women treated with Humalog (insulin lispro) or regular human insulin during pregnancy. Med Sci Monit. 2004;10:PI29-PI32.
38. Masson EA, et al. Pregnancy outcome in type 1 diabetes mellitus treated with insulin lispro (Humalog). Diabetes Med. 2003;20:46-50.
39. Pettitt DJ, Opsina P, Kolaczynski JW, et al. Comparison of an insulin analogue, insulin aspart, and regular human insulin with no insulin in gestational diabetes mellitus. Diabetes Care. 2003;26:183-186.
40. Devlin JT, Hothersall L, Wilkins JL. Use of insulin glargine during pregnancy in a type 1 diabetic women. Diabetes Care. 2002;25:1095-1096.
41. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus. JAMA. 2003;289:2254-2264.
42. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
43. Gabbe SG, Graves CR. Management of diabetes mellitus complicating pregnancy. Obstet Gynecol. 2003;102:857-868.
44. Greene MF. Oral hypoglycemic drugs for gestational diabetes [editorial]. N Engl J Med. 2000;343:1178-1179.
45. Cefalo RC. A comparison of glyburide and insulin in women with gestational diabetes mellitus. Obstet Gynecol Surv. 2001;56:126-127.
46. Koren G. The use of glyburide in gestational diabetes: an ideal example of “bench to bedside.” Pediatr Res. 2001;49:734.-
47. Ryan EA. Glyburide was as safe and effective as insulin in gestational diabetes. Evid Based Med. 2001;6:79.-Available at: http://ebm.bmjjournals.com/cgi/content/full/6/3/79. Accessed September 14, 2005.
48. Saade G. Gestational diabetes mellitus: a pill or a shot? Obstet Gynecol. 2005;105:456-457.
49. Coustan DR. Oral hypoglycemic agents for the ob/gyn. Contemp OB/GYN. 2001;45-63.
50. Jovanovic L. The use of oral agents during pregnancy to treat gestational diabetes. Curr Diab Rep. 2001;1:69-70.
51. Elliot B, Langer O, et al. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
52. Elliot B, Schenker S, Langer O, et al. Comparative placental transport of oral hypoglycemic agents: a model of human placental drug transfer. Am J Obstet Gynecol. 1994;171:653-660.
53. Elliot B, Langer O, Schussling F. A model of human placental drug transfer. Am J Obstet Gynecol. 1997;176:527-530.
54. Towner D, Kjos SL, Leung B, et al. Congenital malformations in pregnancies complicated by NIDDM. Diabetes Care. 1995;18:1446-1451.
55. Gutzin S, Kozer E, Magee L, et al. The safety of oral hypoglycemic agents in the first trimester of pregnancy: a meta-analysis. Can J Clin Pharmacol. 2003;10:179-183.
56. Langer O, Conway DL, Berkus MD, et al. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134-1138.
57. Lim JM, Tayob Y, O’Brien PM, Shaw RW. A comparison between the pregnancy outcome of women with gestation diabetes treated with glibenclamide and those treated with insulin. Med J Malaysia. 1997;52:377-381.
58. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. J Matern Fetal Neonatal Med. 2004;15:51-55.
59. Kremer CJ, Duff P. Glyburide for the treatment of gestational diabetes. Am J Obstet Gynecol. 2004;190:1438-1439.
60. Coetzee EJ, Jackson WP. Oral hypoglycaemics in the first trimester and fetal outcome. S Afr Med J. 1984;65:635-637.
61. Coetzee EJ, Jackson WP. The management of non-insulin-dependent diabetes during pregnancy. Diabetes Res Clin Pract. 1985:86;1:281-287.
62. Yogev Y, Ben-Haroush A, Chen R, et al. Undiagnosed asymptomatic hypoglycemia: diet, insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104:88-93.
63. Langer O, Yogev Y, Xenakis EMJ, et al. Insulin and glyburide therapy: dosage, severity level of gestational diabetes and pregnancy outcome. Am J Obstet Gynecol. 2005;192:134-139.
64. Goetzel L, Wilkins I. Glyburide compared to insulin for the treatment of gestational diabetes mellitus: a cost analysis. J Perinatol. 2002;22:403-406.
65. Gluck L, Kulovich MB. Lecithin/sphingomyelin ratios in amniotic fluid in normal and abnormal pregnancy. Am J Obstet Gynecol. 1973;115:539-546.
66. Kulovich MV, Gluck L. The lung profile: complicated pregnancy. Am J Obstet Gynecol. 1979;135:64-70.
67. Langer O. The controversy surrounding fetal lung maturity in diabetes in pregnancy: a re-evaluation. J Matern Fetal Neonatal Med. 2002;12:428-432.
68. Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519-1522.
69. Fuhrmann K, Reiher H, Semmler K, et al. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983;6:219-223.
70. Rosenn B, Miodovnik M, Combs CA, et al. Glycemic thresholds for spontaneous abortion and congenital malformations in insulindependent diabetes mellitus. Obstet Gynecol. 1994;84:515-520.
71. Kitzmiller JL, Gavin LA, Gin GD, et al. Preconception care of diabetes: glycemic control prevents congenital anomalies. JAMA. 1991;265:731-736.
72. Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol. 1996;174:1343-1353.
This article describes the rationale for intensive treatment with these agents and other interventions to prevent both hypoglycemia and hyperglycemia.
Intensive therapy requires:
- memory-based self-monitoring of blood glucose, which empowers patients to take charge of glycemic control and provides feedback on the timing and dose of insulin administration,
- dietary regulation,
- strict criteria for initiation of pharmacologic therapy,
- multiple injections of insulin or its equivalent when diet alone is insufficient, and
- an interdisciplinary management team.
Neither race nor ethnicity predicts treatment duration or success.5-7
Blood glucose goals
Regardless of the treatment, the primary goal is always to achieve glycemic control, because it reduces the incidence of hypoglycemia, hyperglycemia, and ketosis. For type 1 and type 2 diabetes, glycemic control is important to prevent further deterioration of complications such as vasculopathy and nephropathy.
Goals of treatment are achieving the following blood glucose concentrations (in milligrams per deciliter):
- mean: 90 to 105
- fasting: 60 to 90
- preprandial: 80 to 95
- postprandial: less than 120
In the process, the clinician needs to anticipate how pregnancy will affect preexisting disease, and how diabetes will affect pregnancy outcomes, in patients with any of the 3 types of diabetes.
2 diet protocols
For all types of diabetes, the foundation is diet—specifically, using nutritional therapy to achieve and maintain a maternal blood glucose profile comparable to that of a nondiabetic woman.
Two approaches are recommended:
- reducing carbohydrate intake to 40% to 50% of total calories or
- limiting carbohydrate consumption to foods with a low glycemic index for approximately 60% of calories.
Calculating calories: Same for all
The daily caloric intake is based on the prepregnancy body mass index (BMI) and uses the same formula for all 3 types of diabetes10,11:
- For a BMI less than 20 (underweight), daily caloric intake should be 35 to 38 kcal/kg.
- For a BMI of 20 to 25 (normal weight), the patient should consume 30 kcal/kg.
- For a BMI of 26 and higher (overweight, obese, morbidly obese), caloric intake should be 20 to 25 kcal/kg.
In addition, the daily allotment of calories is divided into 3 main meals and 3 to 4 snacks, with adjustments for the patient’s time constraints, work schedule, and other individual factors.
To encourage compliance, the diet also should reflect the patient’s cultural preferences.
How do you know when diet fails?
Women with pregestational diabetes are usually already taking insulin or other pharmacologic agents by the time they conceive. There is no consensus or hard data on how long a woman who develops gestational diabetes mellitus should remain on a diet before starting drug treatment.
In a study evaluating the time required to achieve glycemic control with diet alone during a 4-week period, 70% of patients with fasting plasma below 95 mg/dL achieved established levels of glycemic control within 2 weeks with no substantial improvement thereafter.8,9 In contrast, in patients with fasting plasma glucose of more than 95 mg/dL, most patients failed to achieve the desired level of glycemic control throughout the 4-week period.
Hypoglycemia after exercise can be a positive marker
I recommend 20 to 30 minutes of exercise 3 to 4 times weekly for gravidas with diabetes, provided they are willing and able to perform it, because it can improve post-prandial blood glucose levels and insulin sensitivity.12
Blood glucose should be measured after exercise, especially in women with type 1 diabetes.
Hypoglycemic reactions during and after exercise may be positive markers of improved insulin sensitivity. Low blood glucose necessitates adjustment of the insulin dose and carbohydrate intake. Extra monitoring is warranted after evening exercise, as glucose uptake increases for several hours after exercise and can cause nocturnal hypoglycemia.
Intensive therapy: Why, when, how
The healthy body secretes insulin over 24 hours independent of nutrient intake. Basal insulin secretion maintains metabolic homeostasis by preventing excessive hepatic glucose production and the mobilization of free fatty acids from adipose tissue stores. This also helps maintain protein balance. Insulin secretion increases several times in response to the ingestion of food.
Treatment or consequences
Langer O, Yogev Y, Most O, et al. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol. 2005;192;989-997.
When diabetic women receive adequate preconception care and counseling and achieve glycemic control, the rate of congenital anomalies declines to levels seen in the general population.69-72
On the other hand, maternal hyperglycemia and resultant fetal hyperinsulinemia are central to the pathophysiology of diabetic complications:
- type 1 and type 2 diabetes—congenital malformations
- all pregnancies compromised by diabetes—increased rates of deviant fetal growth (macrosomia and intrauterine growth restriction), neonatal metabolic, hematological and respiratory complications, birth trauma, stillbirth, cesarean delivery and intensive care admissions.
I tell patients, “Some improvement is better than none” I explain to my patients how pregnancy itself imposes risk, and why it is crucial to follow protocols and achieve glycemic control. I explain the maternal and fetal complications associated with various glucose thresholds, and the added risks of maternal age, body composition, disease severity, and so on.
However, I also stress that even some improvement in glucose control is better than no improvement.
Insulin dosage requires frequent adjustment
To determine the insulin dose needed to achieve glycemic control in pregnant gravidas, multiple blood glucose measurements are needed because insulin requirements steadily increase throughout pregnancy in women with pregestational diabetes.13-16 Jovanovic and Peterson13 quantified these increases as 0.7, 0.8, 0.9, and 1.0 U/kg per day in the first trimester and at weeks 18, 26, and 36, respectively.
Using memory-based reflectance meters to monitor blood glucose, my colleagues and I observed that insulin requirements during pregnancy in women with pregestational diabetes are triphasic (TABLE) and require frequent assessment with individualized adjustment of the insulin dose in each trimester.16 Women with type 2 diabetes require significantly higher doses of insulin each trimester, compared with women with type 1 diabetes.
In women with gestational diabetes, we observed a biphasic increase in insulin requirements17:
- Insulin requirements increased up to the 30th week of gestation, necessitating frequent dose adjustments.
- After 30 weeks, insulin requirements stabilized, requiring minimal or no dose adjustments. Insulin requirements for obese subjects were 0.9 U/kg per day, compared with 0.8 U/kg per day for nonobese women.
TABLE
Insulin requirements during pregnancy for women with pregestational diabetes
| INSULIN REQUIREMENT (UNITS/KG/DAY) | ||
|---|---|---|
| TRIMESTER | TYPE 1 DIABETES | TYPE 2 DIABETES |
| 1 | 0.86 | 0.86 |
| 2 | 0.95 | 1.18 |
| 3 | 1.19 | 1.62 |
| Insulin requirements vary with gestational diabetes | ||
When to start drugs
Most women with pregestational diabetes are treated with insulin prior to pregnancy. Thus, the main task during pregnancy is maintaining or improving glycemic control. In gestational diabetes, pharmacologic therapy (insulin or glyburide) is initiated only when regulation of the diet fails to achieve the desired level of glycemic control or when the disease is severe enough to mandate therapy.
Authorities disagree on the threshold of severity that necessitates pharmacologic intervention (glyburide or insulin). Some suggest a threshold of fasting plasma glucose of at least 95 mg/dL,18-20 which will decrease the rate of macrosomic and large-for-gestational-age infants,19,21 while others suggest at least 105 mg/dL.19,22
All authorities agree that drug therapy should be started when postprandial glucose levels are 120 mg/dL or higher at 2 hours or 140 mg/dL or higher at 1 hour.
Using these standards, 30% to 50% of women with gestational diabetes require pharmacologic therapy when diet alone fails to reduce glucose levels.
Determining insulin requirements
The insulin algorithm for women with gestational diabetes is based on prepregnancy BMI:
- For women with a BMI of 25 and less, the insulin dose is 0.8 U/kg.
- For women with a BMI of more than 25 (overweight and obese), it is 1.0 U/kg.
Once the total insulin dose is calculated, it is divided so that two thirds is administered in the morning and one third in the afternoon or evening. The morning dose is further divided in a ratio of 2 to 1 (intermediate and rapid-acting) and the evening dose into a ratio of 1-to-1 (rapid-acting and intermediate). The rapid-acting dose is administered with the evening meal, while the intermediate dose is given just before bedtime.
If the patient with gestational diabetes has not achieved the desired level of glycemic control after 3 to 7 days, increase the total dose by 10% to 20% and thereafter adjust it when needed.
Fine points of insulin therapy
The actual total insulin dose in women with gestational diabetes is 40% higher than the calculated dose16; this provides a margin of safety and avoids severe hypoglycemic episodes. As a rule of thumb, self-monitoring of blood glucose is necessary before every administration of insulin.
The failure to introduce insulin therapy in a timely fashion may lead to fetal hyperinsulinemia and associated complications. Conversely, premature initiation of insulin in women who could have achieved glycemic control with diet alone leads to unnecessary drug treatment.
When gestational diabetes is diagnosed after 30 to 33 weeks’ gestation and there is little time left to gain the desired level of control, pharmacologic intervention is recommended. There is greater flexibility when gestational diabetes is diagnosed early in the third trimester.
Which form of insulin is best?
Human insulin is recommended when insulin is prescribed during pregnancy, and the same type of insulin is used for pregestational and gestational diabetes. The main differences:
- use of the insulin pump in type 1 diabetes and
- the insulin dose, which is based on insulin requirements for each type of diabetes.
Regular insulin and insulin lispro are the 2 most common rapid-acting forms of insulin in use.
Pros and cons of insulin lispro
Mounting evidence of the benefits of insulin lispro for type 1 and type 2 diabetes in nonpregnant individuals includes:
- fewer episodes of severe hypoglycemia,
- limited postprandial glucose excursions, and
- a possible decrease in glycosylated hemoglobin when the drug is administered by continuous subcutaneous infusion.23
Neither the American Diabetes Association22 nor the American College of Obstetricians and Gynecologists19 endorses the use of insulin analogs. The reason: these drugs have not been adequately tested in pregnancy, although insulin lispro is categorized as a class B drug.
Data on insulin lispro are limited and abstracted from studies with relatively small sample sizes (only 244 gravidas reported thus far in the literature). Most case reports describe improved glycemic control, increased patient satisfaction, and fewer hypoglycemic episodes, but lack sufficient data on maternal and neonatal outcomes. Even so, many obstetricians have administered the drug with no adverse outcome.
In my opinion, insulin lispro can and should be used in pregnancy because of its ability to produce more physiologic insulin patterns and because the data against it are anecdotal. In contrast, insulin aspart and glargine should be avoided in pregnancy because data on their effects are limited.24-40
Individualizing the insulin regimen
A relatively high dose of insulin (about 50–90 U) is needed to achieve glycemic control in gestational diabetes. In contrast, in type 1 diabetes, a lower dose is necessary (50–60 U). Because of the different glycemic profile of women with type 1 diabetes, individualizing the insulin regimen is accepted practice.
The carbohydrate algorithm. For every 15 g of carbohydrates ingested at mealtime, 1 U of rapid-acting insulin analog (insulin lispro or insulin aspart) is required.
If postprandial glucose is continuously elevated (>120 mg/dL) at 2 hours, an increase in rapid-acting insulin is required. Thus, the carbohydrate algorithm may change to 1 U of insulin for every 12 g of carbohydrates until the appropriate ratio is achieved.
If hypoglycemia or relative hypoglycemia occurs, the amount of carbohydrates should increase for each unit of insulin. For example, the adjusted dose would be 1 U of insulin for every 18 g of carbohydrates.
The range of these algorithms is influenced by prepregnancy BMI, disease severity, type of diabetes, and type of carbohydrate (ie, complex versus simple).41
Recognizing patterns of severity. The second algorithm involves identifying the glucose severity pattern (ie, hyperglycemia, hypoglycemia). For example, the total dose of insulin required (0.8–1.0 U/kg) is divided into a ratio of 60% for intermediate or long-acting insulin (basal dose) and 40% for the premeal dose. If the glucose level falls above the targeted level, a single unit of insulin lispro or insulin aspart is added for every 30 mg/dL, but not exceeding 3 U at one time. If glucose levels remain high, redistribute the calculated insulin dose to obtain an improved actual dose (ie, reconfigure the new calculated dose throughout the day based on patient need).
Retinopathy
Poor glucose control may contribute to or worsen diabetic retinopathy—the leading cause of blindness in diabetic patients 24 to 64 years of age—by increasing intracellular accumulation of glucose and its metabolic products. This damages the tiny blood vessels inside the retina, beginning with the formation of microaneurysms and progressing to blockage and, potentially, proliferation of fragile, abnormal, new blood vessels. If vessels leak blood, vision can be severely impaired or obliterated.
Patients at risk should achieve glycemic control gradually. Rapid initiation of stringent glycemic control can cause short-term progression of retinopathy, especially in hypertensive patients, although there are no apparent long-term effects.
Diabetic nephropathy
This complication increases the risk of preeclampsia, chronic hypertension, and fetal growth restriction, and is the most common cause of end-stage renal disease. Proteinuria often increases during pregnancy in diabetic women, but renal function generally remains stable. Nevertheless, advanced diabetic nephropathy (serum creatinine >1.5 mg/dL or creatinine clearance of ≤90 mL/min) can cause further deterioration.
The stacking effect explains why most women with gestational diabetes need no regular or rapid-acting insulin at lunchtime yet are still able to maintain the desired level of glucose control.
Special needs in type 1 disease
Because glucose levels in women with type 1 diabetes typically vary widely on a daily or even hourly basis, the insulin dose should be flexible. For example, the patient may need 1 U of rapid-acting insulin for every 25 mg/dL of blood glucose above 125 mg/dL, or 1 U for every 20 mg/dL above 120 mg/dL, and so on. I encourage patients to titrate based on half-unit increments, which can be measured in an insulin syringe.
Insulin pumps. Insulin lispro and insulin aspart are approved for administration as a continuous subcutaneous infusion. However, use of the pump in pregnancy has been limited—as has its research.
Improved metabolic control is a potential advantage of the pump. When the patient is motivated and alert, use of the pump can reduce nocturnal hypoglycemia and morning hyperglycemia caused by the “dawn phenomenon” (an abrupt rise in glucose level in the early morning).
Disadvantages of the pump include cost, diabetic ketoacidosis, and hypoglycemia (caused by malfunction or infection at the infusion site). Maternal and fetal outcomes are comparable whether the insulin pump or intensive therapy is used. However, improvements in lifestyle and metabolic control may justify use of the pump in women who have trouble achieving glycemic control.43
The many advantages of glyburide
Oral agents can be a pragmatic alternative to insulin in pregnancy because they are easy to administer and noninvasive. Many experts and authoritative bodies in the US recommend glyburide (sulfonylurea) as an alternative pharmacologic therapy during pregnancy.20,44-48 Others recommend further evaluation.19,22,49,50
Although some oral agents cross the placenta, they do not necessarily cause a toxic or teratogenic effect on the fetus. Glyburide, a class B drug, does not cross the placenta.51-53 It increases insulin secretion and diminishes insulin resistance by lowering glucose toxicity. Its onset of action is about 4 hours, and the duration of action is about 10 hours. Thus, after achieving the targeted therapeutic level, glyburide covers the basal requirement as well as postprandial glucose excursions.
The starting dose is 2.5 mg orally in the morning. If the targeted level of glycemia is not attained, add 2.5 mg to the morning dose. If indicated (after 3 to 7 days), add 5 mg in the evening. Thereafter, increase the dose in 5-mg increments, up to a total of 20 mg per day. If the patient does not achieve acceptable glycemic control, add long-acting insulin.
Evidence on oral agents. Several retrospective and randomized studies evaluated oral agents in pregnancy. Most demonstrated that these agents are comparable to insulin in glycemic control and pregnancy outcome.54-61
In a randomized study, my colleagues and I found comparable pregnancy outcomes for glyburide and insulin.56 Recently we reconfirmed our original observation62 that hypoglycemic episodes are more common in insulin-treated patients than in those taking glyburide. In this study, we used continuous glucose monitoring and found hypoglycemic episodes in 63% of the insulin-treated women with gestational diabetes, but only in 28% of those taking glyburide.
We further analyzed the association between glyburide dose, gestational diabetes severity, and selected maternal and neonatal factors.63 Not surprisingly, we found that the glyburide dose increased with the severity of gestational diabetes. The success rate (ie, achievement of glycemic control) decreased as disease severity increased. However, there was no difference between glyburide- and insulin-treated patients at each level of severity. Thus, achieving glycemic control—not any particular mode of pharmacologic therapy—is the key to improving pregnancy outcome in gestational diabetes.
When costs of insulin therapy and glyburide treatment are compared, the latter is considerably less expensive.64
Ensuring fetal health and a safe delivery
Three principles form the basis of obstetric care for women with diabetes:
- fetal testing to prevent stillbirth and compromised fetal states at delivery,
- lung-maturity testing to prevent hyaline membrane disease, and
- determining the best time and method of delivery to prevent fetal compromise, macrosomia, and shoulder dystocia.
Fetal testing
At our institution, we begin fetal testing at 32 weeks’ gestation in all women regardless of diabetes type—even earlier in women with vascular/hypertensive disorders. This includes assessing fetal movements 3 times daily and nonstress testing weekly. This approach has led to a stillbirth rate of 2.5 per 1,000, compared with 4 per 1,000 in the general population.4
Is amniocentesis warranted to determine lung maturity?
A major goal of fetal surveillance in gestational diabetes is preventing lung disease. Inadequately controlled gestational diabetes can increase the risk of respiratory distress syndrome or delay lung maturity. Thus, assessing fetal pulmonary status by confirming gestational age or fetal size can be misleading.65,66
The delay in lung maturity among infants of diabetic mothers is 1 to 2 weeks.67 This delay was associated with poorly controlled diabetes in several studies. Thus, this subgroup of patients stands to benefit most from amniocentesis.
At our institution, the common practice is to test for lung maturity before any elective delivery at less than 38 weeks’ gestation. However, when the clinician determines that delivery would be beneficial, as in cases of poorly controlled diabetes, noncompliance, or other obstetric indications, we deliver the infant regardless of lung maturity. These fetuses experience minimal lung morbidity after 37 weeks’ gestation.
The bottom line: Compromised lung maturity in a live infant is preferable to a deceased infant with healthy lungs.
Timing of delivery
Most experts agree that women with diabetes should be delivered at term—though the definition of “term” ranges from 38 to 42 weeks’ gestation.
At our institution, in addition to the established routine obstetric indications for delivery, 4 additional indications mandate elective delivery for women with diabetes:
- Fetal macrosomia (weight >4,000 g). For large-for-gestational-age fetuses (>90th percentile), induction of labor may be appropriate when fetal weight ranges from 3,800 to 4,000 g and the gestational age is at least 38 weeks. Delivering these fetuses reduces the risk for shoulder dystocia, an ominous complication of diabetes in pregnancy.
- History of previous stillbirth—often the result of poorly controlled diabetes—also warrants induction of labor.
- Poor compliance or glycemic control. This includes the failure to test blood glucose enough to determine glycemic control; inability or unwillingness to adhere to the diabetic protocol, such as fetal testing; and missed appointments.
- Presence of vasculopathy-related hypertension.
The road ahead
More pharmacologic alternatives are on the horizon and may include metformin and other oral antidiabetic drugs, insulin glargine and oral insulin, and a technologically improved insulin pump that can interact directly with blood glucose levels.
The author reports no financial relationships relevant to this article.
This article describes the rationale for intensive treatment with these agents and other interventions to prevent both hypoglycemia and hyperglycemia.
Intensive therapy requires:
- memory-based self-monitoring of blood glucose, which empowers patients to take charge of glycemic control and provides feedback on the timing and dose of insulin administration,
- dietary regulation,
- strict criteria for initiation of pharmacologic therapy,
- multiple injections of insulin or its equivalent when diet alone is insufficient, and
- an interdisciplinary management team.
Neither race nor ethnicity predicts treatment duration or success.5-7
Blood glucose goals
Regardless of the treatment, the primary goal is always to achieve glycemic control, because it reduces the incidence of hypoglycemia, hyperglycemia, and ketosis. For type 1 and type 2 diabetes, glycemic control is important to prevent further deterioration of complications such as vasculopathy and nephropathy.
Goals of treatment are achieving the following blood glucose concentrations (in milligrams per deciliter):
- mean: 90 to 105
- fasting: 60 to 90
- preprandial: 80 to 95
- postprandial: less than 120
In the process, the clinician needs to anticipate how pregnancy will affect preexisting disease, and how diabetes will affect pregnancy outcomes, in patients with any of the 3 types of diabetes.
2 diet protocols
For all types of diabetes, the foundation is diet—specifically, using nutritional therapy to achieve and maintain a maternal blood glucose profile comparable to that of a nondiabetic woman.
Two approaches are recommended:
- reducing carbohydrate intake to 40% to 50% of total calories or
- limiting carbohydrate consumption to foods with a low glycemic index for approximately 60% of calories.
Calculating calories: Same for all
The daily caloric intake is based on the prepregnancy body mass index (BMI) and uses the same formula for all 3 types of diabetes10,11:
- For a BMI less than 20 (underweight), daily caloric intake should be 35 to 38 kcal/kg.
- For a BMI of 20 to 25 (normal weight), the patient should consume 30 kcal/kg.
- For a BMI of 26 and higher (overweight, obese, morbidly obese), caloric intake should be 20 to 25 kcal/kg.
In addition, the daily allotment of calories is divided into 3 main meals and 3 to 4 snacks, with adjustments for the patient’s time constraints, work schedule, and other individual factors.
To encourage compliance, the diet also should reflect the patient’s cultural preferences.
How do you know when diet fails?
Women with pregestational diabetes are usually already taking insulin or other pharmacologic agents by the time they conceive. There is no consensus or hard data on how long a woman who develops gestational diabetes mellitus should remain on a diet before starting drug treatment.
In a study evaluating the time required to achieve glycemic control with diet alone during a 4-week period, 70% of patients with fasting plasma below 95 mg/dL achieved established levels of glycemic control within 2 weeks with no substantial improvement thereafter.8,9 In contrast, in patients with fasting plasma glucose of more than 95 mg/dL, most patients failed to achieve the desired level of glycemic control throughout the 4-week period.
Hypoglycemia after exercise can be a positive marker
I recommend 20 to 30 minutes of exercise 3 to 4 times weekly for gravidas with diabetes, provided they are willing and able to perform it, because it can improve post-prandial blood glucose levels and insulin sensitivity.12
Blood glucose should be measured after exercise, especially in women with type 1 diabetes.
Hypoglycemic reactions during and after exercise may be positive markers of improved insulin sensitivity. Low blood glucose necessitates adjustment of the insulin dose and carbohydrate intake. Extra monitoring is warranted after evening exercise, as glucose uptake increases for several hours after exercise and can cause nocturnal hypoglycemia.
Intensive therapy: Why, when, how
The healthy body secretes insulin over 24 hours independent of nutrient intake. Basal insulin secretion maintains metabolic homeostasis by preventing excessive hepatic glucose production and the mobilization of free fatty acids from adipose tissue stores. This also helps maintain protein balance. Insulin secretion increases several times in response to the ingestion of food.
Treatment or consequences
Langer O, Yogev Y, Most O, et al. Gestational diabetes: The consequences of not treating. Am J Obstet Gynecol. 2005;192;989-997.
When diabetic women receive adequate preconception care and counseling and achieve glycemic control, the rate of congenital anomalies declines to levels seen in the general population.69-72
On the other hand, maternal hyperglycemia and resultant fetal hyperinsulinemia are central to the pathophysiology of diabetic complications:
- type 1 and type 2 diabetes—congenital malformations
- all pregnancies compromised by diabetes—increased rates of deviant fetal growth (macrosomia and intrauterine growth restriction), neonatal metabolic, hematological and respiratory complications, birth trauma, stillbirth, cesarean delivery and intensive care admissions.
I tell patients, “Some improvement is better than none” I explain to my patients how pregnancy itself imposes risk, and why it is crucial to follow protocols and achieve glycemic control. I explain the maternal and fetal complications associated with various glucose thresholds, and the added risks of maternal age, body composition, disease severity, and so on.
However, I also stress that even some improvement in glucose control is better than no improvement.
Insulin dosage requires frequent adjustment
To determine the insulin dose needed to achieve glycemic control in pregnant gravidas, multiple blood glucose measurements are needed because insulin requirements steadily increase throughout pregnancy in women with pregestational diabetes.13-16 Jovanovic and Peterson13 quantified these increases as 0.7, 0.8, 0.9, and 1.0 U/kg per day in the first trimester and at weeks 18, 26, and 36, respectively.
Using memory-based reflectance meters to monitor blood glucose, my colleagues and I observed that insulin requirements during pregnancy in women with pregestational diabetes are triphasic (TABLE) and require frequent assessment with individualized adjustment of the insulin dose in each trimester.16 Women with type 2 diabetes require significantly higher doses of insulin each trimester, compared with women with type 1 diabetes.
In women with gestational diabetes, we observed a biphasic increase in insulin requirements17:
- Insulin requirements increased up to the 30th week of gestation, necessitating frequent dose adjustments.
- After 30 weeks, insulin requirements stabilized, requiring minimal or no dose adjustments. Insulin requirements for obese subjects were 0.9 U/kg per day, compared with 0.8 U/kg per day for nonobese women.
TABLE
Insulin requirements during pregnancy for women with pregestational diabetes
| INSULIN REQUIREMENT (UNITS/KG/DAY) | ||
|---|---|---|
| TRIMESTER | TYPE 1 DIABETES | TYPE 2 DIABETES |
| 1 | 0.86 | 0.86 |
| 2 | 0.95 | 1.18 |
| 3 | 1.19 | 1.62 |
| Insulin requirements vary with gestational diabetes | ||
When to start drugs
Most women with pregestational diabetes are treated with insulin prior to pregnancy. Thus, the main task during pregnancy is maintaining or improving glycemic control. In gestational diabetes, pharmacologic therapy (insulin or glyburide) is initiated only when regulation of the diet fails to achieve the desired level of glycemic control or when the disease is severe enough to mandate therapy.
Authorities disagree on the threshold of severity that necessitates pharmacologic intervention (glyburide or insulin). Some suggest a threshold of fasting plasma glucose of at least 95 mg/dL,18-20 which will decrease the rate of macrosomic and large-for-gestational-age infants,19,21 while others suggest at least 105 mg/dL.19,22
All authorities agree that drug therapy should be started when postprandial glucose levels are 120 mg/dL or higher at 2 hours or 140 mg/dL or higher at 1 hour.
Using these standards, 30% to 50% of women with gestational diabetes require pharmacologic therapy when diet alone fails to reduce glucose levels.
Determining insulin requirements
The insulin algorithm for women with gestational diabetes is based on prepregnancy BMI:
- For women with a BMI of 25 and less, the insulin dose is 0.8 U/kg.
- For women with a BMI of more than 25 (overweight and obese), it is 1.0 U/kg.
Once the total insulin dose is calculated, it is divided so that two thirds is administered in the morning and one third in the afternoon or evening. The morning dose is further divided in a ratio of 2 to 1 (intermediate and rapid-acting) and the evening dose into a ratio of 1-to-1 (rapid-acting and intermediate). The rapid-acting dose is administered with the evening meal, while the intermediate dose is given just before bedtime.
If the patient with gestational diabetes has not achieved the desired level of glycemic control after 3 to 7 days, increase the total dose by 10% to 20% and thereafter adjust it when needed.
Fine points of insulin therapy
The actual total insulin dose in women with gestational diabetes is 40% higher than the calculated dose16; this provides a margin of safety and avoids severe hypoglycemic episodes. As a rule of thumb, self-monitoring of blood glucose is necessary before every administration of insulin.
The failure to introduce insulin therapy in a timely fashion may lead to fetal hyperinsulinemia and associated complications. Conversely, premature initiation of insulin in women who could have achieved glycemic control with diet alone leads to unnecessary drug treatment.
When gestational diabetes is diagnosed after 30 to 33 weeks’ gestation and there is little time left to gain the desired level of control, pharmacologic intervention is recommended. There is greater flexibility when gestational diabetes is diagnosed early in the third trimester.
Which form of insulin is best?
Human insulin is recommended when insulin is prescribed during pregnancy, and the same type of insulin is used for pregestational and gestational diabetes. The main differences:
- use of the insulin pump in type 1 diabetes and
- the insulin dose, which is based on insulin requirements for each type of diabetes.
Regular insulin and insulin lispro are the 2 most common rapid-acting forms of insulin in use.
Pros and cons of insulin lispro
Mounting evidence of the benefits of insulin lispro for type 1 and type 2 diabetes in nonpregnant individuals includes:
- fewer episodes of severe hypoglycemia,
- limited postprandial glucose excursions, and
- a possible decrease in glycosylated hemoglobin when the drug is administered by continuous subcutaneous infusion.23
Neither the American Diabetes Association22 nor the American College of Obstetricians and Gynecologists19 endorses the use of insulin analogs. The reason: these drugs have not been adequately tested in pregnancy, although insulin lispro is categorized as a class B drug.
Data on insulin lispro are limited and abstracted from studies with relatively small sample sizes (only 244 gravidas reported thus far in the literature). Most case reports describe improved glycemic control, increased patient satisfaction, and fewer hypoglycemic episodes, but lack sufficient data on maternal and neonatal outcomes. Even so, many obstetricians have administered the drug with no adverse outcome.
In my opinion, insulin lispro can and should be used in pregnancy because of its ability to produce more physiologic insulin patterns and because the data against it are anecdotal. In contrast, insulin aspart and glargine should be avoided in pregnancy because data on their effects are limited.24-40
Individualizing the insulin regimen
A relatively high dose of insulin (about 50–90 U) is needed to achieve glycemic control in gestational diabetes. In contrast, in type 1 diabetes, a lower dose is necessary (50–60 U). Because of the different glycemic profile of women with type 1 diabetes, individualizing the insulin regimen is accepted practice.
The carbohydrate algorithm. For every 15 g of carbohydrates ingested at mealtime, 1 U of rapid-acting insulin analog (insulin lispro or insulin aspart) is required.
If postprandial glucose is continuously elevated (>120 mg/dL) at 2 hours, an increase in rapid-acting insulin is required. Thus, the carbohydrate algorithm may change to 1 U of insulin for every 12 g of carbohydrates until the appropriate ratio is achieved.
If hypoglycemia or relative hypoglycemia occurs, the amount of carbohydrates should increase for each unit of insulin. For example, the adjusted dose would be 1 U of insulin for every 18 g of carbohydrates.
The range of these algorithms is influenced by prepregnancy BMI, disease severity, type of diabetes, and type of carbohydrate (ie, complex versus simple).41
Recognizing patterns of severity. The second algorithm involves identifying the glucose severity pattern (ie, hyperglycemia, hypoglycemia). For example, the total dose of insulin required (0.8–1.0 U/kg) is divided into a ratio of 60% for intermediate or long-acting insulin (basal dose) and 40% for the premeal dose. If the glucose level falls above the targeted level, a single unit of insulin lispro or insulin aspart is added for every 30 mg/dL, but not exceeding 3 U at one time. If glucose levels remain high, redistribute the calculated insulin dose to obtain an improved actual dose (ie, reconfigure the new calculated dose throughout the day based on patient need).
Retinopathy
Poor glucose control may contribute to or worsen diabetic retinopathy—the leading cause of blindness in diabetic patients 24 to 64 years of age—by increasing intracellular accumulation of glucose and its metabolic products. This damages the tiny blood vessels inside the retina, beginning with the formation of microaneurysms and progressing to blockage and, potentially, proliferation of fragile, abnormal, new blood vessels. If vessels leak blood, vision can be severely impaired or obliterated.
Patients at risk should achieve glycemic control gradually. Rapid initiation of stringent glycemic control can cause short-term progression of retinopathy, especially in hypertensive patients, although there are no apparent long-term effects.
Diabetic nephropathy
This complication increases the risk of preeclampsia, chronic hypertension, and fetal growth restriction, and is the most common cause of end-stage renal disease. Proteinuria often increases during pregnancy in diabetic women, but renal function generally remains stable. Nevertheless, advanced diabetic nephropathy (serum creatinine >1.5 mg/dL or creatinine clearance of ≤90 mL/min) can cause further deterioration.
The stacking effect explains why most women with gestational diabetes need no regular or rapid-acting insulin at lunchtime yet are still able to maintain the desired level of glucose control.
Special needs in type 1 disease
Because glucose levels in women with type 1 diabetes typically vary widely on a daily or even hourly basis, the insulin dose should be flexible. For example, the patient may need 1 U of rapid-acting insulin for every 25 mg/dL of blood glucose above 125 mg/dL, or 1 U for every 20 mg/dL above 120 mg/dL, and so on. I encourage patients to titrate based on half-unit increments, which can be measured in an insulin syringe.
Insulin pumps. Insulin lispro and insulin aspart are approved for administration as a continuous subcutaneous infusion. However, use of the pump in pregnancy has been limited—as has its research.
Improved metabolic control is a potential advantage of the pump. When the patient is motivated and alert, use of the pump can reduce nocturnal hypoglycemia and morning hyperglycemia caused by the “dawn phenomenon” (an abrupt rise in glucose level in the early morning).
Disadvantages of the pump include cost, diabetic ketoacidosis, and hypoglycemia (caused by malfunction or infection at the infusion site). Maternal and fetal outcomes are comparable whether the insulin pump or intensive therapy is used. However, improvements in lifestyle and metabolic control may justify use of the pump in women who have trouble achieving glycemic control.43
The many advantages of glyburide
Oral agents can be a pragmatic alternative to insulin in pregnancy because they are easy to administer and noninvasive. Many experts and authoritative bodies in the US recommend glyburide (sulfonylurea) as an alternative pharmacologic therapy during pregnancy.20,44-48 Others recommend further evaluation.19,22,49,50
Although some oral agents cross the placenta, they do not necessarily cause a toxic or teratogenic effect on the fetus. Glyburide, a class B drug, does not cross the placenta.51-53 It increases insulin secretion and diminishes insulin resistance by lowering glucose toxicity. Its onset of action is about 4 hours, and the duration of action is about 10 hours. Thus, after achieving the targeted therapeutic level, glyburide covers the basal requirement as well as postprandial glucose excursions.
The starting dose is 2.5 mg orally in the morning. If the targeted level of glycemia is not attained, add 2.5 mg to the morning dose. If indicated (after 3 to 7 days), add 5 mg in the evening. Thereafter, increase the dose in 5-mg increments, up to a total of 20 mg per day. If the patient does not achieve acceptable glycemic control, add long-acting insulin.
Evidence on oral agents. Several retrospective and randomized studies evaluated oral agents in pregnancy. Most demonstrated that these agents are comparable to insulin in glycemic control and pregnancy outcome.54-61
In a randomized study, my colleagues and I found comparable pregnancy outcomes for glyburide and insulin.56 Recently we reconfirmed our original observation62 that hypoglycemic episodes are more common in insulin-treated patients than in those taking glyburide. In this study, we used continuous glucose monitoring and found hypoglycemic episodes in 63% of the insulin-treated women with gestational diabetes, but only in 28% of those taking glyburide.
We further analyzed the association between glyburide dose, gestational diabetes severity, and selected maternal and neonatal factors.63 Not surprisingly, we found that the glyburide dose increased with the severity of gestational diabetes. The success rate (ie, achievement of glycemic control) decreased as disease severity increased. However, there was no difference between glyburide- and insulin-treated patients at each level of severity. Thus, achieving glycemic control—not any particular mode of pharmacologic therapy—is the key to improving pregnancy outcome in gestational diabetes.
When costs of insulin therapy and glyburide treatment are compared, the latter is considerably less expensive.64
Ensuring fetal health and a safe delivery
Three principles form the basis of obstetric care for women with diabetes:
- fetal testing to prevent stillbirth and compromised fetal states at delivery,
- lung-maturity testing to prevent hyaline membrane disease, and
- determining the best time and method of delivery to prevent fetal compromise, macrosomia, and shoulder dystocia.
Fetal testing
At our institution, we begin fetal testing at 32 weeks’ gestation in all women regardless of diabetes type—even earlier in women with vascular/hypertensive disorders. This includes assessing fetal movements 3 times daily and nonstress testing weekly. This approach has led to a stillbirth rate of 2.5 per 1,000, compared with 4 per 1,000 in the general population.4
Is amniocentesis warranted to determine lung maturity?
A major goal of fetal surveillance in gestational diabetes is preventing lung disease. Inadequately controlled gestational diabetes can increase the risk of respiratory distress syndrome or delay lung maturity. Thus, assessing fetal pulmonary status by confirming gestational age or fetal size can be misleading.65,66
The delay in lung maturity among infants of diabetic mothers is 1 to 2 weeks.67 This delay was associated with poorly controlled diabetes in several studies. Thus, this subgroup of patients stands to benefit most from amniocentesis.
At our institution, the common practice is to test for lung maturity before any elective delivery at less than 38 weeks’ gestation. However, when the clinician determines that delivery would be beneficial, as in cases of poorly controlled diabetes, noncompliance, or other obstetric indications, we deliver the infant regardless of lung maturity. These fetuses experience minimal lung morbidity after 37 weeks’ gestation.
The bottom line: Compromised lung maturity in a live infant is preferable to a deceased infant with healthy lungs.
Timing of delivery
Most experts agree that women with diabetes should be delivered at term—though the definition of “term” ranges from 38 to 42 weeks’ gestation.
At our institution, in addition to the established routine obstetric indications for delivery, 4 additional indications mandate elective delivery for women with diabetes:
- Fetal macrosomia (weight >4,000 g). For large-for-gestational-age fetuses (>90th percentile), induction of labor may be appropriate when fetal weight ranges from 3,800 to 4,000 g and the gestational age is at least 38 weeks. Delivering these fetuses reduces the risk for shoulder dystocia, an ominous complication of diabetes in pregnancy.
- History of previous stillbirth—often the result of poorly controlled diabetes—also warrants induction of labor.
- Poor compliance or glycemic control. This includes the failure to test blood glucose enough to determine glycemic control; inability or unwillingness to adhere to the diabetic protocol, such as fetal testing; and missed appointments.
- Presence of vasculopathy-related hypertension.
The road ahead
More pharmacologic alternatives are on the horizon and may include metformin and other oral antidiabetic drugs, insulin glargine and oral insulin, and a technologically improved insulin pump that can interact directly with blood glucose levels.
The author reports no financial relationships relevant to this article.
1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
2. American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2000;23(suppl 2):S27-S31.
3. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33). Lancet. 1998;352:837-853.
4. Langer O, Rodriguez DA, Xenakis EMJ, et al. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1994;170:1036-1047.
5. Langer N, Langer O. Comparison of pregnancy mood profiles in gestational diabetes and preexisting diabetes. Diabetes Educ. 2000;26:667-672.
6. Langer O, Langer N, Piper JM, Elliott B, Anyaegbunam A. Cultural diversity as a factor in self-monitoring blood glucose in gestational diabetes. J Assoc Acad Minor Phys. 1995;6:73-77.
7. Langer N, Langer O. Emotional adjustment to diagnosis and intensified treatment of gestational diabetes. Obstet Gynecol. 1994;84:329-334.
8. McFarland MB, Langer O, et al. Dietary therapy for gestational diabetes: how long is long enough? Obstet Gynecol. 1999;93:978-982.
9. Langer O. Management of gestational diabetes. Clin Obstet Gynecol. 1999;93:978-982.
10. Dornhorst A, Frost G. Nutritional management in diabetic pregnancy: a time for reason not dogma. In: Hod M, Jovanovic L, DiRenzo GC, deLevia A, Langer O, eds. Diabetes and Pregnancy. United Kingdom: Taylor & Francis; 2003;340-358.
11. Luke B. Dietary management. In: Reece EA, Coustan DR, Gabbe SG, eds. Diabetes in Women. Philadelphia: Lippincott Williams & Wilkins; 2004;273-281.
12. Artal R. Exercise: the alternative therapeutic intervention for gestational diabetes. Clin Obstet Gynecol. 2003;46:479-487.
13. Jovanovic L, Peterson CM. Optimal insulin delivery for the pregnant diabetic patient. Diabetes Care. 1982;5:24-31.
14. Rayburn W. Changes in insulin therapy during pregnancy. Am J Perinatol. 1985;2:271-277.
15. Weiss P, Hofmann H. Intensified conventional insulin therapy for the pregnant diabetic patient. Obstet Gynecol. 1984;64:629-633.
16. Langer O, et al. Pregestational diabetes: insulin requirements throughout pregnancy. Am J Obstet Gynecol. 1988;159:616-620.
17. Langer O, Anyaegbunam A, et al. Gestational diabetes: insulin requirements in pregnancy. Am J Obstet Gynecol. 1987;157:669-675.
18. Metzger BE, Coustan DR. Organizing committee. Summary and Recommendations of the Fourth International Workshop-Conference on Gestational Diabetes. Diabetes Care. 1998;21(suppl 2):B161-B167.
19. American College of Obstetricians and Gynecologists. Clinical management guidelines for obstetrician-gynecologists. No. 30. Gestational diabetes. Washington, DC: ACOG; 2001.
20. Reece EA, Homko C, Miodovnik M, et al. A consensus report of the diabetes in pregnancy study group of North America Conference. J Matern Fetal Neonatal Med. 2002;12:362-364.
21. Langer O. Maternal glycemic criteria for insulin therapy in gestational diabetes mellitus. Diabetes Care. 1998;21(suppl 2):B91-B98.
22. American Diabetes Association. Position statement on gestational diabetes mellitus. Diabetes Care. 2004;27(suppl 1):S88-S90.
23. Holleman F, Hoekstra JBL. Insulin lispro. N Engl J Med. 1997;337:176-183.
24. Diamond T, Kormas N. Possible adverse fetal effect of insulin lispro [letter] [with discussion]. N Engl J Med. 1997;337:1009-1010.
25. Jovanovic L, Ilic S, Pettitt, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care. 1999;22:1422-1427.
26. Kitzmiller JL, Main E, Ward B, et al. Insulin lispro and the development of proliferative diabetic retinopathy during pregnancy [letter]. Diabetes Care. 1999;22:874-876.
27. Bhattacharyya A, Vice P. Insulin lispro, pregnancy, and retinopathy. Diabetes Care. 1999;22:2101-2102.
28. Buchbinder A, Miodovnik M, McElvy S, et al. Is insulin lispro associated with the development or progression of diabetic retinopathy during pregnancy? Am J Obstet Gynecol. 2000;183:1162-1165.
29. Bhattacharyya A, Brown S, Hughes S, et al. Insulin lispro and regular insulin in pregnancy. QJ Med. 2001;94:255-260.
30. Persson B, Swahn ML, Hjertberg R, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes mellitus. Diabetes Res Clin Pract. 2002;58:115-121.
31. Loukovaara S, Immonen I, Teramo KA, et al. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care. 2003;26:1193-1198.
32. Durand-Gonzalez KN, Guillausseau N, Anciaux ML, et al. Allergy to insulin in a woman with gestational diabetes mellitus: transient efficiency of continuous subcutaneous insulin lispro infusion. Diabetes Metab. 2003;29:432-434.
33. Garg S, Frias JP, Anil S, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes: glycemic control and maternal and fetal outcomes. Endocr Pract. 2003;9:187-193.
34. Mecacci F, Carignani L, Cioni R, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non-diabetic pregnant women. Eur J Obstet Gynecol Reprod Biol. 2003;111:19-24.
35. Carr KJE, Idama T, et al. A randomized controlled trial of insulin lispro given before or after meals in pregnant women with type 1 diabetesthe effect on glycaemic excursion. J Obstet Gynaecol. 2004;24:382-386.
36. Idama TO, Lindow SW, French M, et al. Preliminary experience with the use of insulin lispro in pregnant diabetic women. J Obstet Gynaecol. 2001;21:350-351.
37. Cypryk K, Sobczak M, Pertynska-Marczewska M, et al. Pregnancy complications and perinatal outcome in diabetic women treated with Humalog (insulin lispro) or regular human insulin during pregnancy. Med Sci Monit. 2004;10:PI29-PI32.
38. Masson EA, et al. Pregnancy outcome in type 1 diabetes mellitus treated with insulin lispro (Humalog). Diabetes Med. 2003;20:46-50.
39. Pettitt DJ, Opsina P, Kolaczynski JW, et al. Comparison of an insulin analogue, insulin aspart, and regular human insulin with no insulin in gestational diabetes mellitus. Diabetes Care. 2003;26:183-186.
40. Devlin JT, Hothersall L, Wilkins JL. Use of insulin glargine during pregnancy in a type 1 diabetic women. Diabetes Care. 2002;25:1095-1096.
41. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus. JAMA. 2003;289:2254-2264.
42. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
43. Gabbe SG, Graves CR. Management of diabetes mellitus complicating pregnancy. Obstet Gynecol. 2003;102:857-868.
44. Greene MF. Oral hypoglycemic drugs for gestational diabetes [editorial]. N Engl J Med. 2000;343:1178-1179.
45. Cefalo RC. A comparison of glyburide and insulin in women with gestational diabetes mellitus. Obstet Gynecol Surv. 2001;56:126-127.
46. Koren G. The use of glyburide in gestational diabetes: an ideal example of “bench to bedside.” Pediatr Res. 2001;49:734.-
47. Ryan EA. Glyburide was as safe and effective as insulin in gestational diabetes. Evid Based Med. 2001;6:79.-Available at: http://ebm.bmjjournals.com/cgi/content/full/6/3/79. Accessed September 14, 2005.
48. Saade G. Gestational diabetes mellitus: a pill or a shot? Obstet Gynecol. 2005;105:456-457.
49. Coustan DR. Oral hypoglycemic agents for the ob/gyn. Contemp OB/GYN. 2001;45-63.
50. Jovanovic L. The use of oral agents during pregnancy to treat gestational diabetes. Curr Diab Rep. 2001;1:69-70.
51. Elliot B, Langer O, et al. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
52. Elliot B, Schenker S, Langer O, et al. Comparative placental transport of oral hypoglycemic agents: a model of human placental drug transfer. Am J Obstet Gynecol. 1994;171:653-660.
53. Elliot B, Langer O, Schussling F. A model of human placental drug transfer. Am J Obstet Gynecol. 1997;176:527-530.
54. Towner D, Kjos SL, Leung B, et al. Congenital malformations in pregnancies complicated by NIDDM. Diabetes Care. 1995;18:1446-1451.
55. Gutzin S, Kozer E, Magee L, et al. The safety of oral hypoglycemic agents in the first trimester of pregnancy: a meta-analysis. Can J Clin Pharmacol. 2003;10:179-183.
56. Langer O, Conway DL, Berkus MD, et al. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134-1138.
57. Lim JM, Tayob Y, O’Brien PM, Shaw RW. A comparison between the pregnancy outcome of women with gestation diabetes treated with glibenclamide and those treated with insulin. Med J Malaysia. 1997;52:377-381.
58. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. J Matern Fetal Neonatal Med. 2004;15:51-55.
59. Kremer CJ, Duff P. Glyburide for the treatment of gestational diabetes. Am J Obstet Gynecol. 2004;190:1438-1439.
60. Coetzee EJ, Jackson WP. Oral hypoglycaemics in the first trimester and fetal outcome. S Afr Med J. 1984;65:635-637.
61. Coetzee EJ, Jackson WP. The management of non-insulin-dependent diabetes during pregnancy. Diabetes Res Clin Pract. 1985:86;1:281-287.
62. Yogev Y, Ben-Haroush A, Chen R, et al. Undiagnosed asymptomatic hypoglycemia: diet, insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104:88-93.
63. Langer O, Yogev Y, Xenakis EMJ, et al. Insulin and glyburide therapy: dosage, severity level of gestational diabetes and pregnancy outcome. Am J Obstet Gynecol. 2005;192:134-139.
64. Goetzel L, Wilkins I. Glyburide compared to insulin for the treatment of gestational diabetes mellitus: a cost analysis. J Perinatol. 2002;22:403-406.
65. Gluck L, Kulovich MB. Lecithin/sphingomyelin ratios in amniotic fluid in normal and abnormal pregnancy. Am J Obstet Gynecol. 1973;115:539-546.
66. Kulovich MV, Gluck L. The lung profile: complicated pregnancy. Am J Obstet Gynecol. 1979;135:64-70.
67. Langer O. The controversy surrounding fetal lung maturity in diabetes in pregnancy: a re-evaluation. J Matern Fetal Neonatal Med. 2002;12:428-432.
68. Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519-1522.
69. Fuhrmann K, Reiher H, Semmler K, et al. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983;6:219-223.
70. Rosenn B, Miodovnik M, Combs CA, et al. Glycemic thresholds for spontaneous abortion and congenital malformations in insulindependent diabetes mellitus. Obstet Gynecol. 1994;84:515-520.
71. Kitzmiller JL, Gavin LA, Gin GD, et al. Preconception care of diabetes: glycemic control prevents congenital anomalies. JAMA. 1991;265:731-736.
72. Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol. 1996;174:1343-1353.
1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
2. American Diabetes Association. Implications of the United Kingdom Prospective Diabetes Study. Diabetes Care. 2000;23(suppl 2):S27-S31.
3. Intensive blood-glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33). Lancet. 1998;352:837-853.
4. Langer O, Rodriguez DA, Xenakis EMJ, et al. Intensified versus conventional management of gestational diabetes. Am J Obstet Gynecol. 1994;170:1036-1047.
5. Langer N, Langer O. Comparison of pregnancy mood profiles in gestational diabetes and preexisting diabetes. Diabetes Educ. 2000;26:667-672.
6. Langer O, Langer N, Piper JM, Elliott B, Anyaegbunam A. Cultural diversity as a factor in self-monitoring blood glucose in gestational diabetes. J Assoc Acad Minor Phys. 1995;6:73-77.
7. Langer N, Langer O. Emotional adjustment to diagnosis and intensified treatment of gestational diabetes. Obstet Gynecol. 1994;84:329-334.
8. McFarland MB, Langer O, et al. Dietary therapy for gestational diabetes: how long is long enough? Obstet Gynecol. 1999;93:978-982.
9. Langer O. Management of gestational diabetes. Clin Obstet Gynecol. 1999;93:978-982.
10. Dornhorst A, Frost G. Nutritional management in diabetic pregnancy: a time for reason not dogma. In: Hod M, Jovanovic L, DiRenzo GC, deLevia A, Langer O, eds. Diabetes and Pregnancy. United Kingdom: Taylor & Francis; 2003;340-358.
11. Luke B. Dietary management. In: Reece EA, Coustan DR, Gabbe SG, eds. Diabetes in Women. Philadelphia: Lippincott Williams & Wilkins; 2004;273-281.
12. Artal R. Exercise: the alternative therapeutic intervention for gestational diabetes. Clin Obstet Gynecol. 2003;46:479-487.
13. Jovanovic L, Peterson CM. Optimal insulin delivery for the pregnant diabetic patient. Diabetes Care. 1982;5:24-31.
14. Rayburn W. Changes in insulin therapy during pregnancy. Am J Perinatol. 1985;2:271-277.
15. Weiss P, Hofmann H. Intensified conventional insulin therapy for the pregnant diabetic patient. Obstet Gynecol. 1984;64:629-633.
16. Langer O, et al. Pregestational diabetes: insulin requirements throughout pregnancy. Am J Obstet Gynecol. 1988;159:616-620.
17. Langer O, Anyaegbunam A, et al. Gestational diabetes: insulin requirements in pregnancy. Am J Obstet Gynecol. 1987;157:669-675.
18. Metzger BE, Coustan DR. Organizing committee. Summary and Recommendations of the Fourth International Workshop-Conference on Gestational Diabetes. Diabetes Care. 1998;21(suppl 2):B161-B167.
19. American College of Obstetricians and Gynecologists. Clinical management guidelines for obstetrician-gynecologists. No. 30. Gestational diabetes. Washington, DC: ACOG; 2001.
20. Reece EA, Homko C, Miodovnik M, et al. A consensus report of the diabetes in pregnancy study group of North America Conference. J Matern Fetal Neonatal Med. 2002;12:362-364.
21. Langer O. Maternal glycemic criteria for insulin therapy in gestational diabetes mellitus. Diabetes Care. 1998;21(suppl 2):B91-B98.
22. American Diabetes Association. Position statement on gestational diabetes mellitus. Diabetes Care. 2004;27(suppl 1):S88-S90.
23. Holleman F, Hoekstra JBL. Insulin lispro. N Engl J Med. 1997;337:176-183.
24. Diamond T, Kormas N. Possible adverse fetal effect of insulin lispro [letter] [with discussion]. N Engl J Med. 1997;337:1009-1010.
25. Jovanovic L, Ilic S, Pettitt, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care. 1999;22:1422-1427.
26. Kitzmiller JL, Main E, Ward B, et al. Insulin lispro and the development of proliferative diabetic retinopathy during pregnancy [letter]. Diabetes Care. 1999;22:874-876.
27. Bhattacharyya A, Vice P. Insulin lispro, pregnancy, and retinopathy. Diabetes Care. 1999;22:2101-2102.
28. Buchbinder A, Miodovnik M, McElvy S, et al. Is insulin lispro associated with the development or progression of diabetic retinopathy during pregnancy? Am J Obstet Gynecol. 2000;183:1162-1165.
29. Bhattacharyya A, Brown S, Hughes S, et al. Insulin lispro and regular insulin in pregnancy. QJ Med. 2001;94:255-260.
30. Persson B, Swahn ML, Hjertberg R, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes mellitus. Diabetes Res Clin Pract. 2002;58:115-121.
31. Loukovaara S, Immonen I, Teramo KA, et al. Progression of retinopathy during pregnancy in type 1 diabetic women treated with insulin lispro. Diabetes Care. 2003;26:1193-1198.
32. Durand-Gonzalez KN, Guillausseau N, Anciaux ML, et al. Allergy to insulin in a woman with gestational diabetes mellitus: transient efficiency of continuous subcutaneous insulin lispro infusion. Diabetes Metab. 2003;29:432-434.
33. Garg S, Frias JP, Anil S, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes: glycemic control and maternal and fetal outcomes. Endocr Pract. 2003;9:187-193.
34. Mecacci F, Carignani L, Cioni R, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non-diabetic pregnant women. Eur J Obstet Gynecol Reprod Biol. 2003;111:19-24.
35. Carr KJE, Idama T, et al. A randomized controlled trial of insulin lispro given before or after meals in pregnant women with type 1 diabetesthe effect on glycaemic excursion. J Obstet Gynaecol. 2004;24:382-386.
36. Idama TO, Lindow SW, French M, et al. Preliminary experience with the use of insulin lispro in pregnant diabetic women. J Obstet Gynaecol. 2001;21:350-351.
37. Cypryk K, Sobczak M, Pertynska-Marczewska M, et al. Pregnancy complications and perinatal outcome in diabetic women treated with Humalog (insulin lispro) or regular human insulin during pregnancy. Med Sci Monit. 2004;10:PI29-PI32.
38. Masson EA, et al. Pregnancy outcome in type 1 diabetes mellitus treated with insulin lispro (Humalog). Diabetes Med. 2003;20:46-50.
39. Pettitt DJ, Opsina P, Kolaczynski JW, et al. Comparison of an insulin analogue, insulin aspart, and regular human insulin with no insulin in gestational diabetes mellitus. Diabetes Care. 2003;26:183-186.
40. Devlin JT, Hothersall L, Wilkins JL. Use of insulin glargine during pregnancy in a type 1 diabetic women. Diabetes Care. 2002;25:1095-1096.
41. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus. JAMA. 2003;289:2254-2264.
42. Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183.
43. Gabbe SG, Graves CR. Management of diabetes mellitus complicating pregnancy. Obstet Gynecol. 2003;102:857-868.
44. Greene MF. Oral hypoglycemic drugs for gestational diabetes [editorial]. N Engl J Med. 2000;343:1178-1179.
45. Cefalo RC. A comparison of glyburide and insulin in women with gestational diabetes mellitus. Obstet Gynecol Surv. 2001;56:126-127.
46. Koren G. The use of glyburide in gestational diabetes: an ideal example of “bench to bedside.” Pediatr Res. 2001;49:734.-
47. Ryan EA. Glyburide was as safe and effective as insulin in gestational diabetes. Evid Based Med. 2001;6:79.-Available at: http://ebm.bmjjournals.com/cgi/content/full/6/3/79. Accessed September 14, 2005.
48. Saade G. Gestational diabetes mellitus: a pill or a shot? Obstet Gynecol. 2005;105:456-457.
49. Coustan DR. Oral hypoglycemic agents for the ob/gyn. Contemp OB/GYN. 2001;45-63.
50. Jovanovic L. The use of oral agents during pregnancy to treat gestational diabetes. Curr Diab Rep. 2001;1:69-70.
51. Elliot B, Langer O, et al. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
52. Elliot B, Schenker S, Langer O, et al. Comparative placental transport of oral hypoglycemic agents: a model of human placental drug transfer. Am J Obstet Gynecol. 1994;171:653-660.
53. Elliot B, Langer O, Schussling F. A model of human placental drug transfer. Am J Obstet Gynecol. 1997;176:527-530.
54. Towner D, Kjos SL, Leung B, et al. Congenital malformations in pregnancies complicated by NIDDM. Diabetes Care. 1995;18:1446-1451.
55. Gutzin S, Kozer E, Magee L, et al. The safety of oral hypoglycemic agents in the first trimester of pregnancy: a meta-analysis. Can J Clin Pharmacol. 2003;10:179-183.
56. Langer O, Conway DL, Berkus MD, et al. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134-1138.
57. Lim JM, Tayob Y, O’Brien PM, Shaw RW. A comparison between the pregnancy outcome of women with gestation diabetes treated with glibenclamide and those treated with insulin. Med J Malaysia. 1997;52:377-381.
58. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. J Matern Fetal Neonatal Med. 2004;15:51-55.
59. Kremer CJ, Duff P. Glyburide for the treatment of gestational diabetes. Am J Obstet Gynecol. 2004;190:1438-1439.
60. Coetzee EJ, Jackson WP. Oral hypoglycaemics in the first trimester and fetal outcome. S Afr Med J. 1984;65:635-637.
61. Coetzee EJ, Jackson WP. The management of non-insulin-dependent diabetes during pregnancy. Diabetes Res Clin Pract. 1985:86;1:281-287.
62. Yogev Y, Ben-Haroush A, Chen R, et al. Undiagnosed asymptomatic hypoglycemia: diet, insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104:88-93.
63. Langer O, Yogev Y, Xenakis EMJ, et al. Insulin and glyburide therapy: dosage, severity level of gestational diabetes and pregnancy outcome. Am J Obstet Gynecol. 2005;192:134-139.
64. Goetzel L, Wilkins I. Glyburide compared to insulin for the treatment of gestational diabetes mellitus: a cost analysis. J Perinatol. 2002;22:403-406.
65. Gluck L, Kulovich MB. Lecithin/sphingomyelin ratios in amniotic fluid in normal and abnormal pregnancy. Am J Obstet Gynecol. 1973;115:539-546.
66. Kulovich MV, Gluck L. The lung profile: complicated pregnancy. Am J Obstet Gynecol. 1979;135:64-70.
67. Langer O. The controversy surrounding fetal lung maturity in diabetes in pregnancy: a re-evaluation. J Matern Fetal Neonatal Med. 2002;12:428-432.
68. Mokdad AH, Serdula MK, Dietz WH, et al. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519-1522.
69. Fuhrmann K, Reiher H, Semmler K, et al. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983;6:219-223.
70. Rosenn B, Miodovnik M, Combs CA, et al. Glycemic thresholds for spontaneous abortion and congenital malformations in insulindependent diabetes mellitus. Obstet Gynecol. 1994;84:515-520.
71. Kitzmiller JL, Gavin LA, Gin GD, et al. Preconception care of diabetes: glycemic control prevents congenital anomalies. JAMA. 1991;265:731-736.
72. Pregnancy outcomes in the Diabetes Control and Complications Trial. Am J Obstet Gynecol. 1996;174:1343-1353.
New screening tests: HSV, CMV, HBV, HCV, parvovirus, and HIV
Technology makes many things possible, but not without imposing new responsibilities. When it comes to viral infections, diagnosis through serology testing, antigen assays, or amplification techniques such as polymerase chain reaction (PCR) now is possible for a number of diseases, including:
- herpes simplex virus (HSV),
- cytomegalovirus (CMV),
- hepatitis B and C viruses (HBV, HCV),
- parvovirus B19 (B19), and
- human immunodeficiency virus (HIV).
The responsibilities that come along with this ability: keeping up to date and selecting the most sensitive and specific test possible. This article reviews the latest tests and offers advice on their use in detecting 6 viruses.
How to pick the right test
When a patient’s presentation suggests viral infection, when something in her history raises a red flag, or when she reports possible exposure to a virus, the right test is critical to the diagnosis. The right test also helps prevent false positives and avoid confusion—but which test is best?
Which immunoglobulins matter?
Many of us have been taught that immunoglobulin M (IgM) correlates with acute infection, but that is not necessarily the case. Because of its high molecular weight, IgM is found most commonly in the intravascular compartment and is not transported to the fetus. IgM usually becomes apparent early during the course of infection. It has a half-life of 10 days and usually—but not always—regresses to undetectable levels over a few months.
The misconception that IgM is found only in acute infection and disappears within 3 months causes many clinicians to test for it and to misinterpret the results. In many cases, IgM fails to develop after acute infection. In others, it may persist for as long as 2 years after primary infection. It also can be detected with recurrent or reactivated infections.1
Immunoglobulin G (IgG) has a longer half-life (21 days) and is the most common immunoglobulin in humans. It is found in tissues and serum and readily crosses the placenta. It can be detected shortly after acute infection, exhibiting a steep rise and fall over several weeks after primary infection. IgG also is a sign of past infection.1
Telltale sign of acute infection
It now seems clear that an IgG antibody produced within the first months after primary infection binds to its antigen poorly. After this initial period, the IgG binds with greater intensity (ie, higher avidity) to that specific antigen (virus). Assays that measure this binding intensity are called avidity assays and are expressed as a percentage of IgG bound to the antigen after treatment with denaturing agents.
Avidity assays have been developed and studied for a variety of viruses.2 The detection of low-avidity IgG can be considered a more reliable sign of acute infection than IgM.
Herpes simplex virus
SERENA’S CASE
Monthly irritation and a vulvar lesion
Serena, 22, complains of irritation and pruritus that precede her period each month. She has tried treating herself with over-the-counter and prescription antifungal medication, without much relief. She presents to your office as an add-on patient and reports that the irritation started 1 day ago and usually lasts 7 days. Physical examination reveals a small area on the left vulva that is inflamed, with 2 small fissures. You obtain a vaginal pH, but it is normal, and there are negative findings on the wet prep.
In view of Serena’s history of recurrent symptoms and atypical lesions, recurrent herpes is a likely diagnosis, so you culture the lesion and order IgG type-specific serology for HSV 1 and 2. The culture is negative, but serology is positive for the HSV-2 antibody. Thus, serology confirms genital herpes.
Although Serena’s culture was negative, false-negative cultures are common with HSV, and serology testing usually is necessary to make the diagnosis of recurrent genital herpes.
HSV-2 is widespread: About 1 in 4 adults is infected. Of these, fewer than 1 in 10 is aware he or she has the virus. Thus, it makes sense to test for HSV-2 when physical findings suggest that it may be present.
Thanks to type-specific serology tests, HSV 1 and/or 2 infection can be diagnosed with confidence.3 Type-specific tests determine the IgG antibody response to an envelope glycoprotein (gG) of HSV. In the diagnosis of primary infection, the test detects seroconversion from a negative to a positive titer. The earliest time for antibody production is 3 weeks after infection—but 8 to 12 weeks should elapse prior to testing for seroconversion.4
The US Food and Drug Administration (FDA) has approved an office test for HSV-2 antibodies. The biokitHSV-2 rapid test (biokit USA, Lexington, Mass) requires blood or serum—though statistical analysis indicates that serum demonstrates higher sensitivity with this assay than does capillary whole blood—and can be performed in any outpatient setting, with excellent sensitivity and specificity.
Flaws of IgM testing and viral cultures
IgM testing for HSV is not advised, as the presence of IgM does not correlate with acute disease. As for cultures, laboratories have been slow to change from HSV cultures to PCR testing from suspected HSV lesions. Because viral cultures for HSV have extremely low sensitivity, only positive results are clinically useful.
Primary infection yields a higher positive culture rate because of increased viral shedding. In contrast, recurrent disease, with its low levels of viral shedding, yields significantly lower positive culture rates.5
Cytomegalovirus
CATHY’S CASE
Flu-like symptoms and plans to conceive
When Cathy, 28, comes in for her annual visit, she reports that she has been experiencing exhaustion, chills, and body aches for several weeks and wonders whether she might have mononucleosis. She also mentions that she and her husband are trying to have a child. She recently started a new job in a day care center, working directly with young children. You decide to test her for the Epstein-Barr virus. Since she is planning to conceive, you also test her for CMV, and the test is positive.
How do you counsel Cathy?
CMV infection usually is diagnosed after clinical findings in the individual or fetus (by ultrasound examination) suggest this infection, or when the clinician suspects it for other reasons.
In young adults, primary CMV infection can cause flu- or mononucleosis-like symptoms such as extreme fatigue, fever, chills, and/or body aches, though they generally resolve within several weeks without further morbidity. However, CMV can cause grave illness—even death—in immunocompromised patients, who may continue to experience the disease intermittently after the first outbreak. CMV also can seriously impair development in infants who are infected in utero. The vast majority of these infants appear normal at birth and only later exhibit problems.
Cathy should be counseled to postpone conception until the virus clears, to avoid jeopardizing her infant’s health.
Testing for CMV during pregnancy
Diagnosing primary infection in pregnancy can be difficult. In the United States, we do not screen for CMV prior to pregnancy, except for special circumstances such as Cathy’s, because the virus is widespread and causes few problems in healthy, nonpregnant individuals. This lack of screening renders the best diagnostic method (ie, seroconversion, or finding CMV IgG antibody in a previously seronegative woman) impossible in most cases. Rather, the clinician usually orders IgG and IgM antibody tests, hoping they will help determine whether a primary infection is present.
In these situations, detection of the IgM antibody during pregnancy is vital to diagnose primary infection.
IgG avidity test clarifies IgM findings
The enzyme-linked immunosorbent assay (ELISA) and the capture ELISA assay are used to detect CMV IgG and IgM antibodies. However, the ELISA for IgM can yield false-positive results due to the presence of:
- IgG (especially at high titers),
- rheumatoid factor of the IgM class (IgM-RF),
- reaction between IgM antibodies and cellular antigens, and
- primary Epstein-Barr viral infection, which can stimulate production of CMV IgM antibody in CMV-immune individuals.
Thus, a positive IgM finding in the serum of a pregnant woman can be caused by acute primary CMV infection, the convalescent phase of a primary CMV infection, or simply persistent IgM antibodies.6,7
That’s where the IgG avidity test comes in. It can help confirm and clarify the clinical significance of finding the IgM antibody and distinguish between primary and recurrent CMV infection. Using the IgM antibody test together with the IgG avidity test increases the likelihood of a correct diagnosis.
A positive IgM with a low avidity index is highly suggestive of a recent (less than 3 months) primary CMV infection.
Standardization of the IgG and IgM assays and the IgG avidity assay is urgently needed to reduce the likelihood of incorrect diagnosis and unwanted intervention in otherwise normal pregnancies.
Further testing can confirm fetal transmission
If primary CMV infection is detected in a pregnant woman, accurate prenatal diagnosis is possible to determine whether the infection has been transmitted to the fetus.
Sophisticated tests such as PCR make in utero diagnosis possible. In fact, PCR remains the most accurate means of diagnosing CMV, with sensitivities ranging from 80% to 100% in prospective studies of gravidas infected with cytomegalovirus.8
Hepatitis B
LIAN’S CASE
Pregnant, with no history of vaccination
Lian, 25, was born in Taiwan and has been in the United States 6 years. She presents for her first prenatal visit at 12 weeks’ gestation and reports no medical history, including no vaccination against hepatitis. This is her first pregnancy. Because testing for hepatitis B is routine in pregnancy, she undergoes a hepatitis B surface antigen test (HBsAg), which is positive.
What is your next step?
Lian’s positive test suggests she is a carrier for HBV. Additional testing can clarify her status and direct treatment and prevention.
Unlike some of the other viruses covered in this article, HBV has been well studied, and the identifying proteins have been described. This information has made it possible to produce sensitive and specific tests that can determine whether a patient is infected, a carrier, or immune to HBV.
Screening for carrier status is universal among gravidas in the United States. Standard ELISA assays have been developed for several HBV proteins as well as the antibody.
Signs of a carrier
The carrier state for HBV is determined by measuring HBsAg, the envelope protein of the virus, which circulates freely in the serum after acute infection (1–10 weeks) and in carrier patients. A woman is a carrier if HBsAg persists 6 months after primary infection.
The nucleocapsid core protein of the hepatitis B core antigen (HBcAg) and its secretory product e antigen (HBeAg) are produced after viral multiplication. There is no assay for HBcAg, but the hepatitis B surface antibody (HBsAb) develops after acute HBV infection. In the carrier state, no HBsAb is detected. However, HBcAb is detected in virtually all infected individuals, usually 4 to 8 weeks after infection.
HBeAg production is a sign of active production of the virus in the liver and is associated with more severe disease. It is usually cleared by 16 weeks post-infection. The antibody to the e protein (HBeAb) occurs more than 16 weeks after infection, and is a finding that suggests less severe disease.
The only finding in the serum of patients successfully vaccinated against HBV is HBsAb.9,10
How to qualify infection status
In Lian’s case, further testing should include liver function and other markers of HBV infection. The presence of hepatitis B e antibody (HBeAb) and e antigen (HBeAg) can define infection and the risk of transmitting the virus to the newborn. For example, when HBeAg is present, the patient has a higher risk of transmitting the virus to other people, including her fetus. When HBeAb is present, the patient has a lower risk of transmitting the virus to other people, including her fetus.
In addition, the viral load can be measured via PCR; if the viral titers are elevated, appropriate antiviral therapy can be given during and after pregnancy.
HBV DNA testing is more sensitive than HBeAg to detect viruses in the blood. When it is performed, DNA testing is generally in addition to regular serologic tests. It also is used to monitor therapy in individuals with chronic HBV.
Lian should also be referred for gastrointestinal (GI) consultation for management during her pregnancy, as well as long-term follow-up after delivery. Vaccination of the partner may be required to prevent his infection.
Hepatitis C
JESSICA’S CASE
Partner had hepatitis
Jessica is a 20-year-old college student home for the summer. She schedules an appointment to discuss contraception, but during her visit she also asks to be screened for sexually transmitted diseases (STDs) and wants to know if there is any way to determine whether she has had a hepatitis B vaccination. In further discussion, she tells you that she was sexually involved with a male who had hepatitis.
You order testing for STDs, including antibody testing for HBV and HCV. The results suggest that Jessica was vaccinated for HBV but is HCV-positive.
How should you proceed?
HCV infection in women is diagnosed by ELISA assays sensitive enough to detect HCV antibody. However, the US Centers for Disease Control and Prevention recommend that a person be diagnosed with HCV only after a positive test for HCV antibodies is confirmed by a more specific test, such as a nucleic amplification test or recombinant immunoblot assay.11,1 2 Thus, Jessica should undergo further testing, including liver function tests and measurement of her viral load. Supplemental testing also is recommended for patients with a borderline result on their initial ELISA.
Referral for GI consultation is indicated for Jessica’s management. The finding of HCV virus also influences which contraceptives she should be offered: Avoid any hormonal contraceptive metabolized by the liver.
Parvovirus B19
GRETCHEN’S CASE
Pregnant and occupationally exposed
Gretchen is a 31-year-old elementary school teacher who is 20 weeks pregnant with her second child. She schedules an appointment with you because she is concerned about cases of B19, or Fifth disease, reported at her school. She has been teaching in the school for 7 years and has never been tested for B19 infection. She reports no symptoms and observes that there have been no cases of B19 among the students in her classroom.
You order IgG antibody testing, which reveals that Gretchen has immunity.
In light of this finding, how do you counsel her?
Gretchen can be reassured that, though she was obviously infected in the past, B19 poses no risk to this pregnancy. Roughly 50% of women are, like Gretchen, already immune to B19. Even if a woman is exposed to the virus during pregnancy, both she and the fetus are usually only mildly affected. However, B19 infection can cause severe anemia in the fetus and trigger spontaneous abortion—although this occurs in less than 5% of pregnancies infected with B19 and is more likely to occur during the first half of a pregnancy. Fetal exposure to B19 appears to cause no birth defects or mental retardation.
In general, testing for B19 in pregnancy is warranted after exposure to the virus to assess immunity or susceptibility. The best way to do so is by testing for IgM and IgG antibodies using an ELISA technique. The IgM antibody is produced within a few days of primary infection and persists for 2 to 3 months. The IgG antibody can be found 1 week after acute infection and persists perhaps for life.
The finding of the IgG antibody in an immunocompetent patient with no IgM antibody demonstrates immunity.13,14 In some cases, additional testing using an IgG avidity assay and/or B19 DNA PCR may be necessary.15
TABLE
Sensitivity and specificity of viral screening tests
| TESTS | SENSITIVITY (%) | SPECIFICITY (%) | COMMENT |
|---|---|---|---|
| Genital herpes | |||
| IgG (ELISA) | Must be type-specific based on gG protein | ||
| HSV-1 | 91–96 | 96–97 | |
| HSV-2 | 96–100 | 92–95 | |
| Point-of-care rapid test (biokitHSV-2) | 99–100 | 96–97 | Only for HSV-2 |
| IgM | DO NOT USE | ||
| Culture of lesion in mother | 50 | 90 | Positive result clinically useful |
| PCR | >90 | >90 | Usually reserved for testing cerebrospinal fluid for encephalitis (in newborns, children, and adults) |
| Cytomegalovirus | |||
| IgG | >90 | 80–92 | |
| IgM | 65–78 | 65–82 | |
| Culture of amniotic fluid | 70 (newborns with CMV) | 100 | Culture of the amniotic fluid may be negative even with infection |
| PCR of amniotic fluid | 77 | 100 | |
| Hepatitis B | |||
| HBcAb | 95 | 95 | Marker of past infection |
| HBsAb | 95 | 95 | Only marker post-vaccination |
| HBsAg | 95 | 95 | Carrier |
| HBeAb | Marker of past infection | ||
| HBeAg | Only in carrier state. Carrier who also demonstrates HBeAg has a high risk of transmission to the newborn | ||
| Hepatitis C | |||
| HCVAb (ELISA) | 99 | 99 | 3rd-generation ELISA |
| HCV RNA-PCR | >95 | >95 | Correlates with infectivity |
| Parvovirus B19 | |||
| IgG (ELISA) | 97 | 94 | |
| IgM (antibody capture enzyme immunoassay) | 89 | 99 | Must use a reliable lab for determination of IgM because of the high incidence of false positives |
| PCR in fetus | 92 | 94 | Currently, reference lab can perform this test. Use on amniotic fluid to confirm in utero infection after primary infection in the mother is diagnosed |
| Human immunodeficiency virus | |||
| ELISA | 99 | 99 | Screening test, repeated if positive |
| Western blot | 99 | 99 | Confirmatory test |
| p24 antigen | May be positive early in acute infection before antibody response | ||
| HIV-1 RNA by PCR | May be positive early in acute infection before antibody response | ||
| ELISA=Enzyme-linked immunosorbent assay; HBcAb=hepatitis B core antibody; HBeAb=hepatitis B early antibody; HBeAg=hepatitis B early antigen; HBsAb=hepatitis B surface antibody; HBsAg=hepatitis B surface antigen; HCV=hepatitis C virus; HSV=herpes simplex virus; Ig=immunoglobulin; PCR=polymerase chain reaction. | |||
ANGELA’S CASE
Recurrent vaginitis and numerous sexual partners
After 2 years of recurrent yeast infections, 41-year-old Angela comes to your office seeking more definitive therapy. She says she is tired of frequent infections that respond to oral and topical therapy but recur less than a week after therapy ends. She reports that she was divorced 8 years ago and has had numerous sexual partners since then. One in particular had a history of drug abuse. In response to your questions, Angela reports no history of diabetes or drug abuse herself.
Physical examination reveals severe external vulvar erythema and edema, and severe vaginal overgrowth of a “cottage-cheese” discharge consistent with Candida vaginitis. Given Angela’s history and physical findings, you order an HIV test, obtaining written permission for it. The test is reported as positive.
Does Angela have HIV?
It is impossible to tell without a repeat ELISA test and confirmation by Western blot, because false-positive results do occur.
Testing for HIV is now common in ObGyn offices. All women who are sexually active, diagnosed with another STD, or are pregnant are encouraged to undergo HIV screening. Testing may need to be repeated within 6 months in consideration of the incubation period of the virus. If the woman has a new relationship or additional history of high-risk sexual practices, testing should also be repeated.
Highly sensitive and specific ELISA assays have been developed for both HIV-1 and HIV-2. Both types of HIV have the same mode of transmission and incur opportunistic infections and other conditions. However, HIV-2 develops more slowly, is generally milder, and appears to be less transmissible than HIV-1.
HIV-2 occurs primarily in West Africa, although cases have been reported in Western Europe and India.
Several generations of assays have been approved for HIV-1 and HIV-2 over the past 2 decades. Today, third-generation assays using enzyme-coupled HIV antigens are standard. These tests are able to detect HIV antibody within 22 days of acute infection.16
Rapid tests (<30 minutes) and saliva tests are available for HIV screening. The IgG antibody is found as a transudate in oral secretions. Therefore, a technique that absorbs and concentrates IgG antibody from the oral cavity can be used to assay for HIV antibodies. An oral assay has been approved, but only for HIV-1 testing.17
Beware of false-positive and false-negative HIV results
Because of the enormous impact of an HIV diagnosis, it is critical to counsel patients that HIV screening assays can be falsely positive or falsely negative. However, compared with tests for the other viral infections covered in this article, HIV testing is perhaps the most sensitive and specific of FDA-approved tests.
False-positive rates range from 1 in 1,000 to 1 in 2,000 tests.
False negatives are less common and usually occur because of the time it takes for a patient to seroconvert after acute infection, which can be up to 1 year after infection.16
Confirm positive results via Western blot
The Western blot test is the gold standard for confirming ELISA-positive HIV results. For this highly sensitive (>99%) test, specific HIV proteins are obtained, separated by electrophoresis, and incubated with the serum along with control specimens. The CDC recognizes 3 results: positive, negative, or indeterminate.
A positive Western blot reacts to 2 bands (from p24, gp41, and gp120/160). A negative result reacts to no bands, and an indeterminate test reacts to only 1 band. Several chronic autoimmune diseases such as systemic lupus erythematosus and Hashimoto’s thyroiditis are associated with p24 reactivity.18
Other HIV tests
In addition to antibody tests, antigen and RNA tests can diagnose and help monitor the course of the disease and response to therapy. The most widely used are the p24 antigen detection test and the PCR assay for HIV-1 RNA.
Finding p24 antigen in the serum precedes the antibody response and may diagnose HIV earlier than ELISA. PCR testing for HIV viral RNA may be positive earlier than the p24 and ELISA antigen tests.19
Dr. Baker reports that he receives grant/research support from GlaxoSmithKline and Merck, is a consultant to GlaxoSmithKline, and is on the speakers bureaus of 3M, GlaxoSmithKline, and Pfizer.
1. Heyman B. Regulation of antibody responses via antibodies, complement and Fc receptors. Annu Rev Immunol. 2000;18:709-737.
2. Ashley RL. Sorting out the new HSV type specific antibody tests. Sex Transm Infect. 2001;77:232-237.
3. Bodeus M, Van Ranst M, Bernard P, Hubinont C, Goubau P. Anticytomegalovirus IgG avidity in pregnancy: a 2-year prospective study. Fetal Diagn Ther. 2002;17:362-366.
4. Ashley-Morrow R, Krantz E, Wald A. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex Transm Dis. 2003;30:310-314.
5. Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
6. Eggers M, Metzger C, Enders G. Differentiation between acute primary and recurrent human cytomegalovirus infection in pregnancy, using a microneutralization assay. J Med Virol. 1998;56:351-358.
7. Maine GT, Lazzarotto T, Landini MP. New developments in the diagnosis of maternal and congenital CMV infection. Expert Rev Mol Diagn. 2001;1:19-29.
8. Bodeus M, Hubinont C, Bernard P, Bouckaert A, Thomas K, Goubau P. Prenatal diagnosis of human cytomegalovirus by culture and polymerase chain reaction: 98 pregnancies leading to congenital infection. Prenat Diagn. 1999;19:314-317.
9. Dinsmoor MJ. Hepatitis in the obstetric patient. Infect Dis Clin North Am. 1997;11:77-91.
10. Duff P. Hepatitis in pregnancy. Semin Perinatol. 1998;22:277-283.
11. Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36(suppl 1):S65-S73.
12. Dienstag JL. Sexual and perinatal transmission of hepatitis C. Hepatology. 1997;26(suppl 1):S66-S70.
13. Jordan JA. Diagnosing human parvovirus B19 infection: guidelines for test selection. Mol Diagn. 2001;6:307-312.
14. Brown KE. Parvovirus B19. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier; 2005.
15. Katta R. Parvovirus B19: a review. Dermatol Clin. 2002;20:333-342.
16. Beelaert G, Vercauteren G, Fransen K, et al. Comparative evaluation of eight commercial enzyme linked immunosorbent assays and 14 simple assays for detection of antibodies to HIV. J Virol Methods. 2002;105:197-206.
17. Schramm W, Angulo GB, Torres PC, Burgess-Cassler A. A simple saliva-based test for detecting antibodies to human immunodeficiency virus. Clin Diagn Lab Immunol. 1999;6:577-580.
18. Makuwa M, Souquiere S, Niangui MT, et al. Reliability of rapid diagnostic tests for HIV variant infection. J Virol Methods. 2002;103:183-190.
19. Dodd RY, Notari EP, 4th, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975-979.
Technology makes many things possible, but not without imposing new responsibilities. When it comes to viral infections, diagnosis through serology testing, antigen assays, or amplification techniques such as polymerase chain reaction (PCR) now is possible for a number of diseases, including:
- herpes simplex virus (HSV),
- cytomegalovirus (CMV),
- hepatitis B and C viruses (HBV, HCV),
- parvovirus B19 (B19), and
- human immunodeficiency virus (HIV).
The responsibilities that come along with this ability: keeping up to date and selecting the most sensitive and specific test possible. This article reviews the latest tests and offers advice on their use in detecting 6 viruses.
How to pick the right test
When a patient’s presentation suggests viral infection, when something in her history raises a red flag, or when she reports possible exposure to a virus, the right test is critical to the diagnosis. The right test also helps prevent false positives and avoid confusion—but which test is best?
Which immunoglobulins matter?
Many of us have been taught that immunoglobulin M (IgM) correlates with acute infection, but that is not necessarily the case. Because of its high molecular weight, IgM is found most commonly in the intravascular compartment and is not transported to the fetus. IgM usually becomes apparent early during the course of infection. It has a half-life of 10 days and usually—but not always—regresses to undetectable levels over a few months.
The misconception that IgM is found only in acute infection and disappears within 3 months causes many clinicians to test for it and to misinterpret the results. In many cases, IgM fails to develop after acute infection. In others, it may persist for as long as 2 years after primary infection. It also can be detected with recurrent or reactivated infections.1
Immunoglobulin G (IgG) has a longer half-life (21 days) and is the most common immunoglobulin in humans. It is found in tissues and serum and readily crosses the placenta. It can be detected shortly after acute infection, exhibiting a steep rise and fall over several weeks after primary infection. IgG also is a sign of past infection.1
Telltale sign of acute infection
It now seems clear that an IgG antibody produced within the first months after primary infection binds to its antigen poorly. After this initial period, the IgG binds with greater intensity (ie, higher avidity) to that specific antigen (virus). Assays that measure this binding intensity are called avidity assays and are expressed as a percentage of IgG bound to the antigen after treatment with denaturing agents.
Avidity assays have been developed and studied for a variety of viruses.2 The detection of low-avidity IgG can be considered a more reliable sign of acute infection than IgM.
Herpes simplex virus
SERENA’S CASE
Monthly irritation and a vulvar lesion
Serena, 22, complains of irritation and pruritus that precede her period each month. She has tried treating herself with over-the-counter and prescription antifungal medication, without much relief. She presents to your office as an add-on patient and reports that the irritation started 1 day ago and usually lasts 7 days. Physical examination reveals a small area on the left vulva that is inflamed, with 2 small fissures. You obtain a vaginal pH, but it is normal, and there are negative findings on the wet prep.
In view of Serena’s history of recurrent symptoms and atypical lesions, recurrent herpes is a likely diagnosis, so you culture the lesion and order IgG type-specific serology for HSV 1 and 2. The culture is negative, but serology is positive for the HSV-2 antibody. Thus, serology confirms genital herpes.
Although Serena’s culture was negative, false-negative cultures are common with HSV, and serology testing usually is necessary to make the diagnosis of recurrent genital herpes.
HSV-2 is widespread: About 1 in 4 adults is infected. Of these, fewer than 1 in 10 is aware he or she has the virus. Thus, it makes sense to test for HSV-2 when physical findings suggest that it may be present.
Thanks to type-specific serology tests, HSV 1 and/or 2 infection can be diagnosed with confidence.3 Type-specific tests determine the IgG antibody response to an envelope glycoprotein (gG) of HSV. In the diagnosis of primary infection, the test detects seroconversion from a negative to a positive titer. The earliest time for antibody production is 3 weeks after infection—but 8 to 12 weeks should elapse prior to testing for seroconversion.4
The US Food and Drug Administration (FDA) has approved an office test for HSV-2 antibodies. The biokitHSV-2 rapid test (biokit USA, Lexington, Mass) requires blood or serum—though statistical analysis indicates that serum demonstrates higher sensitivity with this assay than does capillary whole blood—and can be performed in any outpatient setting, with excellent sensitivity and specificity.
Flaws of IgM testing and viral cultures
IgM testing for HSV is not advised, as the presence of IgM does not correlate with acute disease. As for cultures, laboratories have been slow to change from HSV cultures to PCR testing from suspected HSV lesions. Because viral cultures for HSV have extremely low sensitivity, only positive results are clinically useful.
Primary infection yields a higher positive culture rate because of increased viral shedding. In contrast, recurrent disease, with its low levels of viral shedding, yields significantly lower positive culture rates.5
Cytomegalovirus
CATHY’S CASE
Flu-like symptoms and plans to conceive
When Cathy, 28, comes in for her annual visit, she reports that she has been experiencing exhaustion, chills, and body aches for several weeks and wonders whether she might have mononucleosis. She also mentions that she and her husband are trying to have a child. She recently started a new job in a day care center, working directly with young children. You decide to test her for the Epstein-Barr virus. Since she is planning to conceive, you also test her for CMV, and the test is positive.
How do you counsel Cathy?
CMV infection usually is diagnosed after clinical findings in the individual or fetus (by ultrasound examination) suggest this infection, or when the clinician suspects it for other reasons.
In young adults, primary CMV infection can cause flu- or mononucleosis-like symptoms such as extreme fatigue, fever, chills, and/or body aches, though they generally resolve within several weeks without further morbidity. However, CMV can cause grave illness—even death—in immunocompromised patients, who may continue to experience the disease intermittently after the first outbreak. CMV also can seriously impair development in infants who are infected in utero. The vast majority of these infants appear normal at birth and only later exhibit problems.
Cathy should be counseled to postpone conception until the virus clears, to avoid jeopardizing her infant’s health.
Testing for CMV during pregnancy
Diagnosing primary infection in pregnancy can be difficult. In the United States, we do not screen for CMV prior to pregnancy, except for special circumstances such as Cathy’s, because the virus is widespread and causes few problems in healthy, nonpregnant individuals. This lack of screening renders the best diagnostic method (ie, seroconversion, or finding CMV IgG antibody in a previously seronegative woman) impossible in most cases. Rather, the clinician usually orders IgG and IgM antibody tests, hoping they will help determine whether a primary infection is present.
In these situations, detection of the IgM antibody during pregnancy is vital to diagnose primary infection.
IgG avidity test clarifies IgM findings
The enzyme-linked immunosorbent assay (ELISA) and the capture ELISA assay are used to detect CMV IgG and IgM antibodies. However, the ELISA for IgM can yield false-positive results due to the presence of:
- IgG (especially at high titers),
- rheumatoid factor of the IgM class (IgM-RF),
- reaction between IgM antibodies and cellular antigens, and
- primary Epstein-Barr viral infection, which can stimulate production of CMV IgM antibody in CMV-immune individuals.
Thus, a positive IgM finding in the serum of a pregnant woman can be caused by acute primary CMV infection, the convalescent phase of a primary CMV infection, or simply persistent IgM antibodies.6,7
That’s where the IgG avidity test comes in. It can help confirm and clarify the clinical significance of finding the IgM antibody and distinguish between primary and recurrent CMV infection. Using the IgM antibody test together with the IgG avidity test increases the likelihood of a correct diagnosis.
A positive IgM with a low avidity index is highly suggestive of a recent (less than 3 months) primary CMV infection.
Standardization of the IgG and IgM assays and the IgG avidity assay is urgently needed to reduce the likelihood of incorrect diagnosis and unwanted intervention in otherwise normal pregnancies.
Further testing can confirm fetal transmission
If primary CMV infection is detected in a pregnant woman, accurate prenatal diagnosis is possible to determine whether the infection has been transmitted to the fetus.
Sophisticated tests such as PCR make in utero diagnosis possible. In fact, PCR remains the most accurate means of diagnosing CMV, with sensitivities ranging from 80% to 100% in prospective studies of gravidas infected with cytomegalovirus.8
Hepatitis B
LIAN’S CASE
Pregnant, with no history of vaccination
Lian, 25, was born in Taiwan and has been in the United States 6 years. She presents for her first prenatal visit at 12 weeks’ gestation and reports no medical history, including no vaccination against hepatitis. This is her first pregnancy. Because testing for hepatitis B is routine in pregnancy, she undergoes a hepatitis B surface antigen test (HBsAg), which is positive.
What is your next step?
Lian’s positive test suggests she is a carrier for HBV. Additional testing can clarify her status and direct treatment and prevention.
Unlike some of the other viruses covered in this article, HBV has been well studied, and the identifying proteins have been described. This information has made it possible to produce sensitive and specific tests that can determine whether a patient is infected, a carrier, or immune to HBV.
Screening for carrier status is universal among gravidas in the United States. Standard ELISA assays have been developed for several HBV proteins as well as the antibody.
Signs of a carrier
The carrier state for HBV is determined by measuring HBsAg, the envelope protein of the virus, which circulates freely in the serum after acute infection (1–10 weeks) and in carrier patients. A woman is a carrier if HBsAg persists 6 months after primary infection.
The nucleocapsid core protein of the hepatitis B core antigen (HBcAg) and its secretory product e antigen (HBeAg) are produced after viral multiplication. There is no assay for HBcAg, but the hepatitis B surface antibody (HBsAb) develops after acute HBV infection. In the carrier state, no HBsAb is detected. However, HBcAb is detected in virtually all infected individuals, usually 4 to 8 weeks after infection.
HBeAg production is a sign of active production of the virus in the liver and is associated with more severe disease. It is usually cleared by 16 weeks post-infection. The antibody to the e protein (HBeAb) occurs more than 16 weeks after infection, and is a finding that suggests less severe disease.
The only finding in the serum of patients successfully vaccinated against HBV is HBsAb.9,10
How to qualify infection status
In Lian’s case, further testing should include liver function and other markers of HBV infection. The presence of hepatitis B e antibody (HBeAb) and e antigen (HBeAg) can define infection and the risk of transmitting the virus to the newborn. For example, when HBeAg is present, the patient has a higher risk of transmitting the virus to other people, including her fetus. When HBeAb is present, the patient has a lower risk of transmitting the virus to other people, including her fetus.
In addition, the viral load can be measured via PCR; if the viral titers are elevated, appropriate antiviral therapy can be given during and after pregnancy.
HBV DNA testing is more sensitive than HBeAg to detect viruses in the blood. When it is performed, DNA testing is generally in addition to regular serologic tests. It also is used to monitor therapy in individuals with chronic HBV.
Lian should also be referred for gastrointestinal (GI) consultation for management during her pregnancy, as well as long-term follow-up after delivery. Vaccination of the partner may be required to prevent his infection.
Hepatitis C
JESSICA’S CASE
Partner had hepatitis
Jessica is a 20-year-old college student home for the summer. She schedules an appointment to discuss contraception, but during her visit she also asks to be screened for sexually transmitted diseases (STDs) and wants to know if there is any way to determine whether she has had a hepatitis B vaccination. In further discussion, she tells you that she was sexually involved with a male who had hepatitis.
You order testing for STDs, including antibody testing for HBV and HCV. The results suggest that Jessica was vaccinated for HBV but is HCV-positive.
How should you proceed?
HCV infection in women is diagnosed by ELISA assays sensitive enough to detect HCV antibody. However, the US Centers for Disease Control and Prevention recommend that a person be diagnosed with HCV only after a positive test for HCV antibodies is confirmed by a more specific test, such as a nucleic amplification test or recombinant immunoblot assay.11,1 2 Thus, Jessica should undergo further testing, including liver function tests and measurement of her viral load. Supplemental testing also is recommended for patients with a borderline result on their initial ELISA.
Referral for GI consultation is indicated for Jessica’s management. The finding of HCV virus also influences which contraceptives she should be offered: Avoid any hormonal contraceptive metabolized by the liver.
Parvovirus B19
GRETCHEN’S CASE
Pregnant and occupationally exposed
Gretchen is a 31-year-old elementary school teacher who is 20 weeks pregnant with her second child. She schedules an appointment with you because she is concerned about cases of B19, or Fifth disease, reported at her school. She has been teaching in the school for 7 years and has never been tested for B19 infection. She reports no symptoms and observes that there have been no cases of B19 among the students in her classroom.
You order IgG antibody testing, which reveals that Gretchen has immunity.
In light of this finding, how do you counsel her?
Gretchen can be reassured that, though she was obviously infected in the past, B19 poses no risk to this pregnancy. Roughly 50% of women are, like Gretchen, already immune to B19. Even if a woman is exposed to the virus during pregnancy, both she and the fetus are usually only mildly affected. However, B19 infection can cause severe anemia in the fetus and trigger spontaneous abortion—although this occurs in less than 5% of pregnancies infected with B19 and is more likely to occur during the first half of a pregnancy. Fetal exposure to B19 appears to cause no birth defects or mental retardation.
In general, testing for B19 in pregnancy is warranted after exposure to the virus to assess immunity or susceptibility. The best way to do so is by testing for IgM and IgG antibodies using an ELISA technique. The IgM antibody is produced within a few days of primary infection and persists for 2 to 3 months. The IgG antibody can be found 1 week after acute infection and persists perhaps for life.
The finding of the IgG antibody in an immunocompetent patient with no IgM antibody demonstrates immunity.13,14 In some cases, additional testing using an IgG avidity assay and/or B19 DNA PCR may be necessary.15
TABLE
Sensitivity and specificity of viral screening tests
| TESTS | SENSITIVITY (%) | SPECIFICITY (%) | COMMENT |
|---|---|---|---|
| Genital herpes | |||
| IgG (ELISA) | Must be type-specific based on gG protein | ||
| HSV-1 | 91–96 | 96–97 | |
| HSV-2 | 96–100 | 92–95 | |
| Point-of-care rapid test (biokitHSV-2) | 99–100 | 96–97 | Only for HSV-2 |
| IgM | DO NOT USE | ||
| Culture of lesion in mother | 50 | 90 | Positive result clinically useful |
| PCR | >90 | >90 | Usually reserved for testing cerebrospinal fluid for encephalitis (in newborns, children, and adults) |
| Cytomegalovirus | |||
| IgG | >90 | 80–92 | |
| IgM | 65–78 | 65–82 | |
| Culture of amniotic fluid | 70 (newborns with CMV) | 100 | Culture of the amniotic fluid may be negative even with infection |
| PCR of amniotic fluid | 77 | 100 | |
| Hepatitis B | |||
| HBcAb | 95 | 95 | Marker of past infection |
| HBsAb | 95 | 95 | Only marker post-vaccination |
| HBsAg | 95 | 95 | Carrier |
| HBeAb | Marker of past infection | ||
| HBeAg | Only in carrier state. Carrier who also demonstrates HBeAg has a high risk of transmission to the newborn | ||
| Hepatitis C | |||
| HCVAb (ELISA) | 99 | 99 | 3rd-generation ELISA |
| HCV RNA-PCR | >95 | >95 | Correlates with infectivity |
| Parvovirus B19 | |||
| IgG (ELISA) | 97 | 94 | |
| IgM (antibody capture enzyme immunoassay) | 89 | 99 | Must use a reliable lab for determination of IgM because of the high incidence of false positives |
| PCR in fetus | 92 | 94 | Currently, reference lab can perform this test. Use on amniotic fluid to confirm in utero infection after primary infection in the mother is diagnosed |
| Human immunodeficiency virus | |||
| ELISA | 99 | 99 | Screening test, repeated if positive |
| Western blot | 99 | 99 | Confirmatory test |
| p24 antigen | May be positive early in acute infection before antibody response | ||
| HIV-1 RNA by PCR | May be positive early in acute infection before antibody response | ||
| ELISA=Enzyme-linked immunosorbent assay; HBcAb=hepatitis B core antibody; HBeAb=hepatitis B early antibody; HBeAg=hepatitis B early antigen; HBsAb=hepatitis B surface antibody; HBsAg=hepatitis B surface antigen; HCV=hepatitis C virus; HSV=herpes simplex virus; Ig=immunoglobulin; PCR=polymerase chain reaction. | |||
ANGELA’S CASE
Recurrent vaginitis and numerous sexual partners
After 2 years of recurrent yeast infections, 41-year-old Angela comes to your office seeking more definitive therapy. She says she is tired of frequent infections that respond to oral and topical therapy but recur less than a week after therapy ends. She reports that she was divorced 8 years ago and has had numerous sexual partners since then. One in particular had a history of drug abuse. In response to your questions, Angela reports no history of diabetes or drug abuse herself.
Physical examination reveals severe external vulvar erythema and edema, and severe vaginal overgrowth of a “cottage-cheese” discharge consistent with Candida vaginitis. Given Angela’s history and physical findings, you order an HIV test, obtaining written permission for it. The test is reported as positive.
Does Angela have HIV?
It is impossible to tell without a repeat ELISA test and confirmation by Western blot, because false-positive results do occur.
Testing for HIV is now common in ObGyn offices. All women who are sexually active, diagnosed with another STD, or are pregnant are encouraged to undergo HIV screening. Testing may need to be repeated within 6 months in consideration of the incubation period of the virus. If the woman has a new relationship or additional history of high-risk sexual practices, testing should also be repeated.
Highly sensitive and specific ELISA assays have been developed for both HIV-1 and HIV-2. Both types of HIV have the same mode of transmission and incur opportunistic infections and other conditions. However, HIV-2 develops more slowly, is generally milder, and appears to be less transmissible than HIV-1.
HIV-2 occurs primarily in West Africa, although cases have been reported in Western Europe and India.
Several generations of assays have been approved for HIV-1 and HIV-2 over the past 2 decades. Today, third-generation assays using enzyme-coupled HIV antigens are standard. These tests are able to detect HIV antibody within 22 days of acute infection.16
Rapid tests (<30 minutes) and saliva tests are available for HIV screening. The IgG antibody is found as a transudate in oral secretions. Therefore, a technique that absorbs and concentrates IgG antibody from the oral cavity can be used to assay for HIV antibodies. An oral assay has been approved, but only for HIV-1 testing.17
Beware of false-positive and false-negative HIV results
Because of the enormous impact of an HIV diagnosis, it is critical to counsel patients that HIV screening assays can be falsely positive or falsely negative. However, compared with tests for the other viral infections covered in this article, HIV testing is perhaps the most sensitive and specific of FDA-approved tests.
False-positive rates range from 1 in 1,000 to 1 in 2,000 tests.
False negatives are less common and usually occur because of the time it takes for a patient to seroconvert after acute infection, which can be up to 1 year after infection.16
Confirm positive results via Western blot
The Western blot test is the gold standard for confirming ELISA-positive HIV results. For this highly sensitive (>99%) test, specific HIV proteins are obtained, separated by electrophoresis, and incubated with the serum along with control specimens. The CDC recognizes 3 results: positive, negative, or indeterminate.
A positive Western blot reacts to 2 bands (from p24, gp41, and gp120/160). A negative result reacts to no bands, and an indeterminate test reacts to only 1 band. Several chronic autoimmune diseases such as systemic lupus erythematosus and Hashimoto’s thyroiditis are associated with p24 reactivity.18
Other HIV tests
In addition to antibody tests, antigen and RNA tests can diagnose and help monitor the course of the disease and response to therapy. The most widely used are the p24 antigen detection test and the PCR assay for HIV-1 RNA.
Finding p24 antigen in the serum precedes the antibody response and may diagnose HIV earlier than ELISA. PCR testing for HIV viral RNA may be positive earlier than the p24 and ELISA antigen tests.19
Dr. Baker reports that he receives grant/research support from GlaxoSmithKline and Merck, is a consultant to GlaxoSmithKline, and is on the speakers bureaus of 3M, GlaxoSmithKline, and Pfizer.
Technology makes many things possible, but not without imposing new responsibilities. When it comes to viral infections, diagnosis through serology testing, antigen assays, or amplification techniques such as polymerase chain reaction (PCR) now is possible for a number of diseases, including:
- herpes simplex virus (HSV),
- cytomegalovirus (CMV),
- hepatitis B and C viruses (HBV, HCV),
- parvovirus B19 (B19), and
- human immunodeficiency virus (HIV).
The responsibilities that come along with this ability: keeping up to date and selecting the most sensitive and specific test possible. This article reviews the latest tests and offers advice on their use in detecting 6 viruses.
How to pick the right test
When a patient’s presentation suggests viral infection, when something in her history raises a red flag, or when she reports possible exposure to a virus, the right test is critical to the diagnosis. The right test also helps prevent false positives and avoid confusion—but which test is best?
Which immunoglobulins matter?
Many of us have been taught that immunoglobulin M (IgM) correlates with acute infection, but that is not necessarily the case. Because of its high molecular weight, IgM is found most commonly in the intravascular compartment and is not transported to the fetus. IgM usually becomes apparent early during the course of infection. It has a half-life of 10 days and usually—but not always—regresses to undetectable levels over a few months.
The misconception that IgM is found only in acute infection and disappears within 3 months causes many clinicians to test for it and to misinterpret the results. In many cases, IgM fails to develop after acute infection. In others, it may persist for as long as 2 years after primary infection. It also can be detected with recurrent or reactivated infections.1
Immunoglobulin G (IgG) has a longer half-life (21 days) and is the most common immunoglobulin in humans. It is found in tissues and serum and readily crosses the placenta. It can be detected shortly after acute infection, exhibiting a steep rise and fall over several weeks after primary infection. IgG also is a sign of past infection.1
Telltale sign of acute infection
It now seems clear that an IgG antibody produced within the first months after primary infection binds to its antigen poorly. After this initial period, the IgG binds with greater intensity (ie, higher avidity) to that specific antigen (virus). Assays that measure this binding intensity are called avidity assays and are expressed as a percentage of IgG bound to the antigen after treatment with denaturing agents.
Avidity assays have been developed and studied for a variety of viruses.2 The detection of low-avidity IgG can be considered a more reliable sign of acute infection than IgM.
Herpes simplex virus
SERENA’S CASE
Monthly irritation and a vulvar lesion
Serena, 22, complains of irritation and pruritus that precede her period each month. She has tried treating herself with over-the-counter and prescription antifungal medication, without much relief. She presents to your office as an add-on patient and reports that the irritation started 1 day ago and usually lasts 7 days. Physical examination reveals a small area on the left vulva that is inflamed, with 2 small fissures. You obtain a vaginal pH, but it is normal, and there are negative findings on the wet prep.
In view of Serena’s history of recurrent symptoms and atypical lesions, recurrent herpes is a likely diagnosis, so you culture the lesion and order IgG type-specific serology for HSV 1 and 2. The culture is negative, but serology is positive for the HSV-2 antibody. Thus, serology confirms genital herpes.
Although Serena’s culture was negative, false-negative cultures are common with HSV, and serology testing usually is necessary to make the diagnosis of recurrent genital herpes.
HSV-2 is widespread: About 1 in 4 adults is infected. Of these, fewer than 1 in 10 is aware he or she has the virus. Thus, it makes sense to test for HSV-2 when physical findings suggest that it may be present.
Thanks to type-specific serology tests, HSV 1 and/or 2 infection can be diagnosed with confidence.3 Type-specific tests determine the IgG antibody response to an envelope glycoprotein (gG) of HSV. In the diagnosis of primary infection, the test detects seroconversion from a negative to a positive titer. The earliest time for antibody production is 3 weeks after infection—but 8 to 12 weeks should elapse prior to testing for seroconversion.4
The US Food and Drug Administration (FDA) has approved an office test for HSV-2 antibodies. The biokitHSV-2 rapid test (biokit USA, Lexington, Mass) requires blood or serum—though statistical analysis indicates that serum demonstrates higher sensitivity with this assay than does capillary whole blood—and can be performed in any outpatient setting, with excellent sensitivity and specificity.
Flaws of IgM testing and viral cultures
IgM testing for HSV is not advised, as the presence of IgM does not correlate with acute disease. As for cultures, laboratories have been slow to change from HSV cultures to PCR testing from suspected HSV lesions. Because viral cultures for HSV have extremely low sensitivity, only positive results are clinically useful.
Primary infection yields a higher positive culture rate because of increased viral shedding. In contrast, recurrent disease, with its low levels of viral shedding, yields significantly lower positive culture rates.5
Cytomegalovirus
CATHY’S CASE
Flu-like symptoms and plans to conceive
When Cathy, 28, comes in for her annual visit, she reports that she has been experiencing exhaustion, chills, and body aches for several weeks and wonders whether she might have mononucleosis. She also mentions that she and her husband are trying to have a child. She recently started a new job in a day care center, working directly with young children. You decide to test her for the Epstein-Barr virus. Since she is planning to conceive, you also test her for CMV, and the test is positive.
How do you counsel Cathy?
CMV infection usually is diagnosed after clinical findings in the individual or fetus (by ultrasound examination) suggest this infection, or when the clinician suspects it for other reasons.
In young adults, primary CMV infection can cause flu- or mononucleosis-like symptoms such as extreme fatigue, fever, chills, and/or body aches, though they generally resolve within several weeks without further morbidity. However, CMV can cause grave illness—even death—in immunocompromised patients, who may continue to experience the disease intermittently after the first outbreak. CMV also can seriously impair development in infants who are infected in utero. The vast majority of these infants appear normal at birth and only later exhibit problems.
Cathy should be counseled to postpone conception until the virus clears, to avoid jeopardizing her infant’s health.
Testing for CMV during pregnancy
Diagnosing primary infection in pregnancy can be difficult. In the United States, we do not screen for CMV prior to pregnancy, except for special circumstances such as Cathy’s, because the virus is widespread and causes few problems in healthy, nonpregnant individuals. This lack of screening renders the best diagnostic method (ie, seroconversion, or finding CMV IgG antibody in a previously seronegative woman) impossible in most cases. Rather, the clinician usually orders IgG and IgM antibody tests, hoping they will help determine whether a primary infection is present.
In these situations, detection of the IgM antibody during pregnancy is vital to diagnose primary infection.
IgG avidity test clarifies IgM findings
The enzyme-linked immunosorbent assay (ELISA) and the capture ELISA assay are used to detect CMV IgG and IgM antibodies. However, the ELISA for IgM can yield false-positive results due to the presence of:
- IgG (especially at high titers),
- rheumatoid factor of the IgM class (IgM-RF),
- reaction between IgM antibodies and cellular antigens, and
- primary Epstein-Barr viral infection, which can stimulate production of CMV IgM antibody in CMV-immune individuals.
Thus, a positive IgM finding in the serum of a pregnant woman can be caused by acute primary CMV infection, the convalescent phase of a primary CMV infection, or simply persistent IgM antibodies.6,7
That’s where the IgG avidity test comes in. It can help confirm and clarify the clinical significance of finding the IgM antibody and distinguish between primary and recurrent CMV infection. Using the IgM antibody test together with the IgG avidity test increases the likelihood of a correct diagnosis.
A positive IgM with a low avidity index is highly suggestive of a recent (less than 3 months) primary CMV infection.
Standardization of the IgG and IgM assays and the IgG avidity assay is urgently needed to reduce the likelihood of incorrect diagnosis and unwanted intervention in otherwise normal pregnancies.
Further testing can confirm fetal transmission
If primary CMV infection is detected in a pregnant woman, accurate prenatal diagnosis is possible to determine whether the infection has been transmitted to the fetus.
Sophisticated tests such as PCR make in utero diagnosis possible. In fact, PCR remains the most accurate means of diagnosing CMV, with sensitivities ranging from 80% to 100% in prospective studies of gravidas infected with cytomegalovirus.8
Hepatitis B
LIAN’S CASE
Pregnant, with no history of vaccination
Lian, 25, was born in Taiwan and has been in the United States 6 years. She presents for her first prenatal visit at 12 weeks’ gestation and reports no medical history, including no vaccination against hepatitis. This is her first pregnancy. Because testing for hepatitis B is routine in pregnancy, she undergoes a hepatitis B surface antigen test (HBsAg), which is positive.
What is your next step?
Lian’s positive test suggests she is a carrier for HBV. Additional testing can clarify her status and direct treatment and prevention.
Unlike some of the other viruses covered in this article, HBV has been well studied, and the identifying proteins have been described. This information has made it possible to produce sensitive and specific tests that can determine whether a patient is infected, a carrier, or immune to HBV.
Screening for carrier status is universal among gravidas in the United States. Standard ELISA assays have been developed for several HBV proteins as well as the antibody.
Signs of a carrier
The carrier state for HBV is determined by measuring HBsAg, the envelope protein of the virus, which circulates freely in the serum after acute infection (1–10 weeks) and in carrier patients. A woman is a carrier if HBsAg persists 6 months after primary infection.
The nucleocapsid core protein of the hepatitis B core antigen (HBcAg) and its secretory product e antigen (HBeAg) are produced after viral multiplication. There is no assay for HBcAg, but the hepatitis B surface antibody (HBsAb) develops after acute HBV infection. In the carrier state, no HBsAb is detected. However, HBcAb is detected in virtually all infected individuals, usually 4 to 8 weeks after infection.
HBeAg production is a sign of active production of the virus in the liver and is associated with more severe disease. It is usually cleared by 16 weeks post-infection. The antibody to the e protein (HBeAb) occurs more than 16 weeks after infection, and is a finding that suggests less severe disease.
The only finding in the serum of patients successfully vaccinated against HBV is HBsAb.9,10
How to qualify infection status
In Lian’s case, further testing should include liver function and other markers of HBV infection. The presence of hepatitis B e antibody (HBeAb) and e antigen (HBeAg) can define infection and the risk of transmitting the virus to the newborn. For example, when HBeAg is present, the patient has a higher risk of transmitting the virus to other people, including her fetus. When HBeAb is present, the patient has a lower risk of transmitting the virus to other people, including her fetus.
In addition, the viral load can be measured via PCR; if the viral titers are elevated, appropriate antiviral therapy can be given during and after pregnancy.
HBV DNA testing is more sensitive than HBeAg to detect viruses in the blood. When it is performed, DNA testing is generally in addition to regular serologic tests. It also is used to monitor therapy in individuals with chronic HBV.
Lian should also be referred for gastrointestinal (GI) consultation for management during her pregnancy, as well as long-term follow-up after delivery. Vaccination of the partner may be required to prevent his infection.
Hepatitis C
JESSICA’S CASE
Partner had hepatitis
Jessica is a 20-year-old college student home for the summer. She schedules an appointment to discuss contraception, but during her visit she also asks to be screened for sexually transmitted diseases (STDs) and wants to know if there is any way to determine whether she has had a hepatitis B vaccination. In further discussion, she tells you that she was sexually involved with a male who had hepatitis.
You order testing for STDs, including antibody testing for HBV and HCV. The results suggest that Jessica was vaccinated for HBV but is HCV-positive.
How should you proceed?
HCV infection in women is diagnosed by ELISA assays sensitive enough to detect HCV antibody. However, the US Centers for Disease Control and Prevention recommend that a person be diagnosed with HCV only after a positive test for HCV antibodies is confirmed by a more specific test, such as a nucleic amplification test or recombinant immunoblot assay.11,1 2 Thus, Jessica should undergo further testing, including liver function tests and measurement of her viral load. Supplemental testing also is recommended for patients with a borderline result on their initial ELISA.
Referral for GI consultation is indicated for Jessica’s management. The finding of HCV virus also influences which contraceptives she should be offered: Avoid any hormonal contraceptive metabolized by the liver.
Parvovirus B19
GRETCHEN’S CASE
Pregnant and occupationally exposed
Gretchen is a 31-year-old elementary school teacher who is 20 weeks pregnant with her second child. She schedules an appointment with you because she is concerned about cases of B19, or Fifth disease, reported at her school. She has been teaching in the school for 7 years and has never been tested for B19 infection. She reports no symptoms and observes that there have been no cases of B19 among the students in her classroom.
You order IgG antibody testing, which reveals that Gretchen has immunity.
In light of this finding, how do you counsel her?
Gretchen can be reassured that, though she was obviously infected in the past, B19 poses no risk to this pregnancy. Roughly 50% of women are, like Gretchen, already immune to B19. Even if a woman is exposed to the virus during pregnancy, both she and the fetus are usually only mildly affected. However, B19 infection can cause severe anemia in the fetus and trigger spontaneous abortion—although this occurs in less than 5% of pregnancies infected with B19 and is more likely to occur during the first half of a pregnancy. Fetal exposure to B19 appears to cause no birth defects or mental retardation.
In general, testing for B19 in pregnancy is warranted after exposure to the virus to assess immunity or susceptibility. The best way to do so is by testing for IgM and IgG antibodies using an ELISA technique. The IgM antibody is produced within a few days of primary infection and persists for 2 to 3 months. The IgG antibody can be found 1 week after acute infection and persists perhaps for life.
The finding of the IgG antibody in an immunocompetent patient with no IgM antibody demonstrates immunity.13,14 In some cases, additional testing using an IgG avidity assay and/or B19 DNA PCR may be necessary.15
TABLE
Sensitivity and specificity of viral screening tests
| TESTS | SENSITIVITY (%) | SPECIFICITY (%) | COMMENT |
|---|---|---|---|
| Genital herpes | |||
| IgG (ELISA) | Must be type-specific based on gG protein | ||
| HSV-1 | 91–96 | 96–97 | |
| HSV-2 | 96–100 | 92–95 | |
| Point-of-care rapid test (biokitHSV-2) | 99–100 | 96–97 | Only for HSV-2 |
| IgM | DO NOT USE | ||
| Culture of lesion in mother | 50 | 90 | Positive result clinically useful |
| PCR | >90 | >90 | Usually reserved for testing cerebrospinal fluid for encephalitis (in newborns, children, and adults) |
| Cytomegalovirus | |||
| IgG | >90 | 80–92 | |
| IgM | 65–78 | 65–82 | |
| Culture of amniotic fluid | 70 (newborns with CMV) | 100 | Culture of the amniotic fluid may be negative even with infection |
| PCR of amniotic fluid | 77 | 100 | |
| Hepatitis B | |||
| HBcAb | 95 | 95 | Marker of past infection |
| HBsAb | 95 | 95 | Only marker post-vaccination |
| HBsAg | 95 | 95 | Carrier |
| HBeAb | Marker of past infection | ||
| HBeAg | Only in carrier state. Carrier who also demonstrates HBeAg has a high risk of transmission to the newborn | ||
| Hepatitis C | |||
| HCVAb (ELISA) | 99 | 99 | 3rd-generation ELISA |
| HCV RNA-PCR | >95 | >95 | Correlates with infectivity |
| Parvovirus B19 | |||
| IgG (ELISA) | 97 | 94 | |
| IgM (antibody capture enzyme immunoassay) | 89 | 99 | Must use a reliable lab for determination of IgM because of the high incidence of false positives |
| PCR in fetus | 92 | 94 | Currently, reference lab can perform this test. Use on amniotic fluid to confirm in utero infection after primary infection in the mother is diagnosed |
| Human immunodeficiency virus | |||
| ELISA | 99 | 99 | Screening test, repeated if positive |
| Western blot | 99 | 99 | Confirmatory test |
| p24 antigen | May be positive early in acute infection before antibody response | ||
| HIV-1 RNA by PCR | May be positive early in acute infection before antibody response | ||
| ELISA=Enzyme-linked immunosorbent assay; HBcAb=hepatitis B core antibody; HBeAb=hepatitis B early antibody; HBeAg=hepatitis B early antigen; HBsAb=hepatitis B surface antibody; HBsAg=hepatitis B surface antigen; HCV=hepatitis C virus; HSV=herpes simplex virus; Ig=immunoglobulin; PCR=polymerase chain reaction. | |||
ANGELA’S CASE
Recurrent vaginitis and numerous sexual partners
After 2 years of recurrent yeast infections, 41-year-old Angela comes to your office seeking more definitive therapy. She says she is tired of frequent infections that respond to oral and topical therapy but recur less than a week after therapy ends. She reports that she was divorced 8 years ago and has had numerous sexual partners since then. One in particular had a history of drug abuse. In response to your questions, Angela reports no history of diabetes or drug abuse herself.
Physical examination reveals severe external vulvar erythema and edema, and severe vaginal overgrowth of a “cottage-cheese” discharge consistent with Candida vaginitis. Given Angela’s history and physical findings, you order an HIV test, obtaining written permission for it. The test is reported as positive.
Does Angela have HIV?
It is impossible to tell without a repeat ELISA test and confirmation by Western blot, because false-positive results do occur.
Testing for HIV is now common in ObGyn offices. All women who are sexually active, diagnosed with another STD, or are pregnant are encouraged to undergo HIV screening. Testing may need to be repeated within 6 months in consideration of the incubation period of the virus. If the woman has a new relationship or additional history of high-risk sexual practices, testing should also be repeated.
Highly sensitive and specific ELISA assays have been developed for both HIV-1 and HIV-2. Both types of HIV have the same mode of transmission and incur opportunistic infections and other conditions. However, HIV-2 develops more slowly, is generally milder, and appears to be less transmissible than HIV-1.
HIV-2 occurs primarily in West Africa, although cases have been reported in Western Europe and India.
Several generations of assays have been approved for HIV-1 and HIV-2 over the past 2 decades. Today, third-generation assays using enzyme-coupled HIV antigens are standard. These tests are able to detect HIV antibody within 22 days of acute infection.16
Rapid tests (<30 minutes) and saliva tests are available for HIV screening. The IgG antibody is found as a transudate in oral secretions. Therefore, a technique that absorbs and concentrates IgG antibody from the oral cavity can be used to assay for HIV antibodies. An oral assay has been approved, but only for HIV-1 testing.17
Beware of false-positive and false-negative HIV results
Because of the enormous impact of an HIV diagnosis, it is critical to counsel patients that HIV screening assays can be falsely positive or falsely negative. However, compared with tests for the other viral infections covered in this article, HIV testing is perhaps the most sensitive and specific of FDA-approved tests.
False-positive rates range from 1 in 1,000 to 1 in 2,000 tests.
False negatives are less common and usually occur because of the time it takes for a patient to seroconvert after acute infection, which can be up to 1 year after infection.16
Confirm positive results via Western blot
The Western blot test is the gold standard for confirming ELISA-positive HIV results. For this highly sensitive (>99%) test, specific HIV proteins are obtained, separated by electrophoresis, and incubated with the serum along with control specimens. The CDC recognizes 3 results: positive, negative, or indeterminate.
A positive Western blot reacts to 2 bands (from p24, gp41, and gp120/160). A negative result reacts to no bands, and an indeterminate test reacts to only 1 band. Several chronic autoimmune diseases such as systemic lupus erythematosus and Hashimoto’s thyroiditis are associated with p24 reactivity.18
Other HIV tests
In addition to antibody tests, antigen and RNA tests can diagnose and help monitor the course of the disease and response to therapy. The most widely used are the p24 antigen detection test and the PCR assay for HIV-1 RNA.
Finding p24 antigen in the serum precedes the antibody response and may diagnose HIV earlier than ELISA. PCR testing for HIV viral RNA may be positive earlier than the p24 and ELISA antigen tests.19
Dr. Baker reports that he receives grant/research support from GlaxoSmithKline and Merck, is a consultant to GlaxoSmithKline, and is on the speakers bureaus of 3M, GlaxoSmithKline, and Pfizer.
1. Heyman B. Regulation of antibody responses via antibodies, complement and Fc receptors. Annu Rev Immunol. 2000;18:709-737.
2. Ashley RL. Sorting out the new HSV type specific antibody tests. Sex Transm Infect. 2001;77:232-237.
3. Bodeus M, Van Ranst M, Bernard P, Hubinont C, Goubau P. Anticytomegalovirus IgG avidity in pregnancy: a 2-year prospective study. Fetal Diagn Ther. 2002;17:362-366.
4. Ashley-Morrow R, Krantz E, Wald A. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex Transm Dis. 2003;30:310-314.
5. Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
6. Eggers M, Metzger C, Enders G. Differentiation between acute primary and recurrent human cytomegalovirus infection in pregnancy, using a microneutralization assay. J Med Virol. 1998;56:351-358.
7. Maine GT, Lazzarotto T, Landini MP. New developments in the diagnosis of maternal and congenital CMV infection. Expert Rev Mol Diagn. 2001;1:19-29.
8. Bodeus M, Hubinont C, Bernard P, Bouckaert A, Thomas K, Goubau P. Prenatal diagnosis of human cytomegalovirus by culture and polymerase chain reaction: 98 pregnancies leading to congenital infection. Prenat Diagn. 1999;19:314-317.
9. Dinsmoor MJ. Hepatitis in the obstetric patient. Infect Dis Clin North Am. 1997;11:77-91.
10. Duff P. Hepatitis in pregnancy. Semin Perinatol. 1998;22:277-283.
11. Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36(suppl 1):S65-S73.
12. Dienstag JL. Sexual and perinatal transmission of hepatitis C. Hepatology. 1997;26(suppl 1):S66-S70.
13. Jordan JA. Diagnosing human parvovirus B19 infection: guidelines for test selection. Mol Diagn. 2001;6:307-312.
14. Brown KE. Parvovirus B19. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier; 2005.
15. Katta R. Parvovirus B19: a review. Dermatol Clin. 2002;20:333-342.
16. Beelaert G, Vercauteren G, Fransen K, et al. Comparative evaluation of eight commercial enzyme linked immunosorbent assays and 14 simple assays for detection of antibodies to HIV. J Virol Methods. 2002;105:197-206.
17. Schramm W, Angulo GB, Torres PC, Burgess-Cassler A. A simple saliva-based test for detecting antibodies to human immunodeficiency virus. Clin Diagn Lab Immunol. 1999;6:577-580.
18. Makuwa M, Souquiere S, Niangui MT, et al. Reliability of rapid diagnostic tests for HIV variant infection. J Virol Methods. 2002;103:183-190.
19. Dodd RY, Notari EP, 4th, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975-979.
1. Heyman B. Regulation of antibody responses via antibodies, complement and Fc receptors. Annu Rev Immunol. 2000;18:709-737.
2. Ashley RL. Sorting out the new HSV type specific antibody tests. Sex Transm Infect. 2001;77:232-237.
3. Bodeus M, Van Ranst M, Bernard P, Hubinont C, Goubau P. Anticytomegalovirus IgG avidity in pregnancy: a 2-year prospective study. Fetal Diagn Ther. 2002;17:362-366.
4. Ashley-Morrow R, Krantz E, Wald A. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex Transm Dis. 2003;30:310-314.
5. Wald A, Huang ML, Carrell D, Selke S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345-1351.
6. Eggers M, Metzger C, Enders G. Differentiation between acute primary and recurrent human cytomegalovirus infection in pregnancy, using a microneutralization assay. J Med Virol. 1998;56:351-358.
7. Maine GT, Lazzarotto T, Landini MP. New developments in the diagnosis of maternal and congenital CMV infection. Expert Rev Mol Diagn. 2001;1:19-29.
8. Bodeus M, Hubinont C, Bernard P, Bouckaert A, Thomas K, Goubau P. Prenatal diagnosis of human cytomegalovirus by culture and polymerase chain reaction: 98 pregnancies leading to congenital infection. Prenat Diagn. 1999;19:314-317.
9. Dinsmoor MJ. Hepatitis in the obstetric patient. Infect Dis Clin North Am. 1997;11:77-91.
10. Duff P. Hepatitis in pregnancy. Semin Perinatol. 1998;22:277-283.
11. Pawlotsky JM. Use and interpretation of virological tests for hepatitis C. Hepatology. 2002;36(suppl 1):S65-S73.
12. Dienstag JL. Sexual and perinatal transmission of hepatitis C. Hepatology. 1997;26(suppl 1):S66-S70.
13. Jordan JA. Diagnosing human parvovirus B19 infection: guidelines for test selection. Mol Diagn. 2001;6:307-312.
14. Brown KE. Parvovirus B19. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier; 2005.
15. Katta R. Parvovirus B19: a review. Dermatol Clin. 2002;20:333-342.
16. Beelaert G, Vercauteren G, Fransen K, et al. Comparative evaluation of eight commercial enzyme linked immunosorbent assays and 14 simple assays for detection of antibodies to HIV. J Virol Methods. 2002;105:197-206.
17. Schramm W, Angulo GB, Torres PC, Burgess-Cassler A. A simple saliva-based test for detecting antibodies to human immunodeficiency virus. Clin Diagn Lab Immunol. 1999;6:577-580.
18. Makuwa M, Souquiere S, Niangui MT, et al. Reliability of rapid diagnostic tests for HIV variant infection. J Virol Methods. 2002;103:183-190.
19. Dodd RY, Notari EP, 4th, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975-979.