User login
Alopecia in Association With Sexually Transmitted Disease: A Review
Hair loss has various etiologies. Correct diagnosis of hair disorders is complex and requires the evaluation of clinical presentation, history, physical examination, and laboratory test results. In the patient with a sexually transmitted disease (STD), alopecia may be an important associated finding and can provide clues to diagnosis. This review focuses on the relationship between hair loss and STDs. Specifically, we review alopecia in association with syphilis and human immunodeficiency virus (HIV) infection and the medications used to treat these infections. In addition, we review the literature regarding the putative association between alopecia areata and cytomegalovirus (CMV). There are multiple mechanisms involved in hair loss in these diseases, including the diseases themselves, systemic sequelae of these infections, autoimmune phenomena, and side effects of medications.
Syphilis
When considering the STDs associated with hair loss, syphilis is usually the first STD described because of the large incidence of the disease and its many reported cases of associated hair loss. This is especially important due to the increasing number of current cases of syphilis. Hair loss does not occur in primary syphilis except when associated with a primary chancre of scalp. Hair loss in secondary syphilis, also known as latent syphilis, occurs infrequently; various series report an incidence of 2.9% to 7%.1,2 There are 2 types of secondary syphilitic alopecia. The first is an uncommon symptomatic type found in association with an actual secondary lesion (usually papulosquamous) on the scalp. The second is termed essential syphilitic alopecia, which designates hair loss in the absence of visible syphilitic scalp lesions. Essential syphilitic alopecia has been divided into 3 types: the classic patchy "moth-eaten" alopecia (Figure), a generalized thinning of the hair, and the moth-eaten type in combination with general thinning of the hair. Of these, patchy moth-eaten alopecia occurs most frequently. The diffuse hair loss of essential syphilitic alopecia as the only manifestation of syphilis is uncommon. Cuozzo et al3 described 2 patients in whom the first sign of disease was alopecia.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Moth-eaten alopecia of syphilis is a characteristic manifestation of secondary syphilis that usually affects the scalp and occasionally other areas such as the eyebrows, beard, and pubic area.4 This form of alopecia may be confused with trichotillomania, traction alopecia, and alopecia areata.5 Pareek4 described a case of an unusual location of patchy moth-eaten alopecia that presented on the anterior side of the lower legs of a 30-year-old man in conjunction with patchy alopecia on the scalp and thinning of the eyebrows. With penicillin administration, hair of the legs, scalp, and eyebrows started to grow; the hair was fully regrown within 6 months, which suggests good prognosis with treatment instigation for syphilitic alopecia of all areas.
Jordaan and Louw5 systematically documented the histopathologic features of 12 patients with moth-eaten alopecia. Characteristic features included follicular plugging; a sparse, perivascular and perifollicular lymphocytic infiltrate; telogenization; and follicle-oriented melanin clumping.5 van der Willigen et al6 conducted a study of hair roots in 11 and 8 patients with primary and secondary syphilis, respectively. A decreased number of anagen hair roots; an increased number of catagen hair roots, dysplastic/dystrophic hair roots, and anagen hair roots with sheaths; and more than 20% angulation were observed in both groups.6 In addition, Lee and Hsu7 noted the histopathologic similarity between alopecia syphilitica and alopecia areata. They reported the histopathologic findings of alopecia syphilitica from 9 patients with secondary syphilis and acute hair loss. The alopecia was moth-eaten in 4 patients and was diffuse but slightly moth-eaten in 5. Microscopically, the dermoepidermal interface was not involved. The number of hair follicles was diminished, with increased numbers of catagens and telogens. Lymphocytic infiltration was present around the hair bulbs and fibrous tracts in 8 patients, and plasma cells were present in 4 biopsy specimens. Except for the follicular changes, the findings resembled those of macular/maculopapular syphilides outside the scalp. With the follicular changes, the overall patterns closely resembled alopecia areata. Results of the modified Steiner stain did not reveal spirochetes in any of the patients and failed to differentiate between alopecia syphilitica and alopecia areata. Comparing the alopecia syphilitica patients with 13 patients with alopecia areata, the authors found only a few differentiating features. They concluded that the presence of peribulbar eosinophils strongly suggests alopecia areata.7 Without peribulbar eosinophils, the presence of plasma cells, abundant lymphocytes in the isthmus, or peribulbar lymphoid aggregates suggests alopecia syphilitica. Elston et al8 observed several cases of syphilis with numerous eosinophils in the peribulbar infiltrate and noted that it can be indistinguishable from alopecia areata.
When an associated skin rash or lymphadenopathy is present, the diagnosis of syphilis may be suggested and confirmed by positive serology test results. If such findings are not present, a biopsy specimen to differentiate from other forms of alopecia should be obtained. Because moth-eaten alopecia and alopecia areata have similar resemblance microscopically, syphilis serologic tests are needed.
The treatment of syphilis also has been shown to be a cause of alopecia. Pareek9 described the association of syphilitic alopecia and Herxheimer reaction. A 25-year-old man presented with syphilis with widespread thinning of the scalp hair, eyebrows, and pubic area; the scalp showed patchy moth-eaten alopecia. He was treated with 1 to 2 megaunits of procaine penicillin daily for 10 days. Six hours after the first injection, the patient's temperature rose to 103°F; in addition to malaise, headache, flush, and sore throat, he had a transient skin rash and marked loss of hair. All the symptoms disappeared by the next day. Two to 3 weeks later, the lymphadenopathy had disappeared, and the patient's eyebrows and pubic hair started to regrow. The scalp hair was fully regrown 10 weeks from the onset of treatment. The author concluded that diffuse and extensive hair loss after the first injection of penicillin was part of the Herxheimer reaction.9
HIV
Hair loss is common in patients with HIV; in black patients, this loss may be associated with hair straightening.10 Possible causes of hair loss frequently are present in patients with HIV, including chronic HIV infection itself, acute and chronic systemic infections, local infections, nutritional deficits, immune and endocrine dysregulation, and exposure to multiple drugs.10 Alopecia areata and alopecia universalis also have been reported in patients with HIV.11-14
Smith et al10 studied and reviewed the clinical and histopathologic features of hair loss in 10 patients with HIV. They noted that the most characteristic change in the hair of patients with HIV was hair loss with straightening, sometimes associated with fine hair texture and an increased tendency for broken hairs. These changes are seen in late-stage disease, most commonly in black patients. Each patient had telogen effluvium, and it was observed that any chronic or acute infection (including HIV) can lead to this condition. Nutritional deficits, often prominent in HIV patients, may lead to or potentiate telogen effluvium. Secondary infections and changes in bowel mucosa may lead to specific nutritional deficiencies even before evidence of clinical wasting is seen. In addition to caloric and protein malnutrition that may affect hair growth, minerals such as copper, zinc, and selenium are decreased in patients with HIV. Elevated levels of interleukin 6 and tumor necrosis factor α, which increase epidermal proliferation, may predispose patients to abnormal keratinization by increasing the proliferative rate and nutritional requirements.10
Endocrine regulation is another important factor in hair growth. In late-stage HIV disease, androgen levels decrease while estradiol levels increase. Although thyroid hormone levels are normal in advanced HIV, thyroid functions are elevated to more than expected for the amount of wasting and may contribute to the change of hair texture,10 autoimmune mechanism, associated diseases, and HIV medication side effects.
In the Smith et al10 study, scanning electron microscopy was performed on plucked and pulled hairs of 10 patients with late-stage HIV-1 infection. In addition, scalp biopsy specimens were examined in both vertical and transverse sections. All patients had telogen effluvium. Numerous apoptotic or necrotic keratinocytes were seen in the upper external root sheath follicular epithelium; a mild to moderate perifollicular mononuclear cell infiltrate, often containing eosinophils, also was seen. Additionally, the mononuclear infiltrate was seen surrounding and within the basaloid cells of the follicles in telogen phase; the midfollicular area had the most marked inflammatory infiltrate. Variable dystrophy of the hair shafts also was a consistent feature. Although telogen effluvium is a common response to a wide spectrum of biologic stresses, the presence of apoptotic or necrotic keratinocytes within the upper end of the external root sheath epithelium, as well as dystrophy of hairs, may be markers of hair loss in patients with HIV-1 infection.10
Autoimmune alopecia, including alopecia areata and alopecia universalis, can be seen in association with HIV.11-15 Ostlere et al11 first reported a case of alopecia universalis that developed in a patient 2 years after HIV antibody was detected. The patient showed loss of all scalp hair, eyelashes, eyebrows, and body hair. Two possible mechanisms for the development of alopecia were suggested. The first was that HIV induced nonspecific polyclonal B-cell activation with production of autoantibody either directly or via activated T cells; this supports a humoral theory of alopecia areata pathogenesis. Alternatively, the authors postulated that HIV induced a change in the balance between helper and suppressor cells, which resulted in aberrant cell-mediated immune effect at the hair follicles.11 Werninghaus and Kaminer12 described a similar patient with alopecia universalis; a biopsy specimen revealed perifollicular fibrosis without inflammation.
Stewart and Smoller13 described an HIV-positive patient with altered T-lymphocyte subsets in whom alopecia universalis developed. Results of a skin biopsy of the patient's scalp demonstrated a classic perifollicular lymphocytic infiltrate; results of immunophenotyping of the same specimen revealed that most cells were CD4+ lymphocytes. During the active loss of hair, the patient's ratio of CD4/CD8 cells was decreased; however, the ratio normalized during the period of hair regrowth. Their data suggested that systemic immune dysfunction, as seen in HIV infection, may be more important in mediating alopecia areata than localized immune responses. Because of the proposed mechanism of alopecia areata developing in this patient (ie, influx of CD4+ lymphocytes to the perifollicular regions of skin when the CD4/CD8 cells ratio is low), the authors were surprised that alopecia areata is not more common in patients with HIV infection.13
Cho et al14 described the association of vitiligo and alopecia areata in patients with HIV. They noted that the development of autoimmune diseases, though not life threatening, is an interesting phenomenon that may result from immune dysfunction or from B-cell infection by HIV, Epstein-Barr virus, or other unknown viruses. They described a 47-year-old man who had vitiligo and alopecia areata approximately 2 years after testing positive for HIV antibodies.14 Grossman et al15 described an HIV-seropositive man with acquired eyelash trichomegaly and alopecia areata. They noted that this combination of clinical manifestations is intriguing because the new onset of elongated eyelashes in patients with acquired immunodeficiency syndrome usually has been associated with severe immunosuppression, and alopecia areata has a presumed autoimmune etiology that requires T-cell activation. They concluded that the occurrence of these dichotomous conditions illustrates the potential selective pathogenesis of progressive HIV infection.15
Medications used in the treatment of HIV can play a role in hair loss. Geletko et al16 reported a 33-year-old HIV-infected man who developed alopecia areata after beginning therapy with zidovudine, a nucleoside analogue reverse transcriptase inhibitor. The alopecia reversed after the drug was discontinued. The authors proposed that patients with lower CD4+ counts may be more predisposed to zidovudine-induced alopecia than those in the earlier stages of HIV with higher CD4+ counts.16
Indinavir-related alopecia was described by d'Arminio Monforte et al.17 Of 337 patients given indinavir in combination with nucleoside analogues, 5 patients with HIV developed severe alopecia, which was evident clinically after a mean of 50 days of treatment. All patients were receiving triple therapy that included indinavir. Three patients had diffuse shedding of hair involving the entire scalp, and 2 had circumscribed circular areas of alopecia resulting in complete severe hair loss.17 Bouscarat et al18 reported 10 more cases of hair loss associated with indinavir therapy in patients receiving triple antiviral treatment that included indinavir. Hair loss developed during the first 6 months of indinavir therapy and initially involved the lower limbs. Progressive hair regrowth occurred within 4 months after indinavir was replaced by other treatments.18
Ginarte et al19 described significant alopecia induced by indinavir plus ritonavir therapy in 3 patients a few weeks after beginning treatment. The authors noted that patients receiving indinavir often experience retinoidlike effects such as alopecia, xerosis, and cheilitis. Nonscarring alopecia can develop in patients receiving indinavir, with or without retinoid effects.19 Hair loss also has been noted with the use of crixivan.20
CMV
CMV is a prevalent viral pathogen.21 Most people with acute CMV experience an inapparent infection. The virus usually is spread through close personal contact, including sexual transmission. There has been debate over the link of alopecia areata with CMV. In 1995, Skinner et al22 described using polymerase chain reaction (PCR) techniques to find evidence of CMV DNA in paraffin block sections of lesions of alopecia areata. Of 21 patient biopsy specimens, 10 had alopecia areata and 11 had other hair loss conditions. Of the 10 alopecia areata samples, 9 were positive for CMV; no other hair loss samples were positive for CMV.22 Skinner et al23 theorized that CMV may achieve latency in the hair root. Reactivation of CMV was thought to be one of the pathogenic mechanisms in alopecia areata; the authors argued that a lymphocytic surveillance of not-quite-latent CMV would explain much of the behavior of alopecia areata, which has a tendency for intermittent relapses and remissions.23
The association between alopecia areata and CMV was refuted by Garcia-Hernandez et al,24 who used 3 different PCR assays to detect CMV DNA in skin punch biopsy specimens of 3 patient groups: 40 patients with alopecia areata, 3 patients with HIV and alopecia areata, and 12 patients with other types of alopecia. PCR assays are known to be the most sensitive assay for CMV detection; this study used different PCR assays to achieve maximum sensitivity for CMV. No CMV DNA amplification was found in any of the specimens.24
Offidani et al25 further contradicted this association. The purpose of their study was to clarify the role of CMV infection and to demonstrate the absence of replication of other autoimmune disease–related herpesviruses (eg, Epstein-Barr virus) in the pathogenesis of alopecia areata. After extraction of mRNA from tissue samples of 4 patients with active patchy alopecia areata, reverse transcriptase PCR was carried out using primers specific for some viral members of the β Herpesviridae subfamily of the Herpesviridae family (eg, CMV, Epstein-Barr virus, herpes simplex virus). The authors could not detect any replication of the CMV or other β Herpesviridae in the samples collected, which supports the hypothesis that CMV is not the triggering factor in alopecia areata, neither as a reactivator of the immune response nor as a trigger of the autoimmunity.25
Conclusion
Although many etiologies exist for hair loss, STDs should not be overlooked in a sexually active patient presenting with an otherwise unexplainable cause of this condition. A full workup, including clinical history, physical examination, and laboratory tests, should include STDs in the differential diagnosis (Table).
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Cuozzo DW, Benson PM, Sperling LC, et al. Essential syphilitic alopecia revisited. J Am Acad Dermatol. 1995;32:840-844.
- Pareek SS. Unusual location of syphilitic alopecia: a case report. Sex Transm Dis. 1982;9:43-44.
- Jordaan HF, Louw M. The moth-eaten alopecia of secondary syphilis. a histopathological study of 12 patients. Am J Dermatopathol. 1995;17:158-162.
- van der Willigen AH, Peereboom-Wynia JD, van der Hoek JC, et al. Hair root studies in patients suffering from primary and secondary syphilis. Acta Derm Venereol. 1987;67:250-254.
- Lee JY, Hsu ML. Alopecia syphilitica, a simulator of alopecia areata: histopathology and differential diagnosis. J Cutan Pathol. 1991;18:87-92.
- Elston DM, McCollough ML, Bergfeld WF, et al. Eosinophils in fibrous tracts and near hair bulbs: a helpful diagnostic feature of alopecia areata. J Am Acad Dermatol. 1997;37:101-106.
- Pareek SS. Syphilitic alopecia and Jarisch-Herxheimer reaction. Br J Vener Dis. 1977;53:389-390.
- Smith KJ, Skelton HG, DeRusso D, et al. Clinical and histopathologic features of hair loss in patients with HIV-1 infection. J Am Acad Dermatol. 1996;34:63-68.
- Ostlere LS, Langtry JA, Staughton RC, et al. Alopecia universalis in a patient seropositive for the human immunodeficiency virus. J Am Acad Dermatol. 1992;27:630-631.
- Werninghaus K, Kaminer MS. HIV and alopecia universalis [letter]. J Am Acad Dermatol. 1993;29:667.
- Stewart MI, Smoller BR. Alopecia universalis in an HIV-positive patient: possible insight into pathogenesis. J Cutan Pathol. 1993;20:180-183.
- Cho M, Cohen PR, Duvic M. Vitiligo and alopecia areata in patients with human immunodeficiency virus infection. South Med J. 1995;88:489-491.
- Grossman MC, Cohen PR, Grossman ME. Acquired eyelash trichomegaly and alopecia areata in a human immunodeficiency virus–infected patient. Dermatology. 1996;193:52-53.
- Geletko SM, Segarra M, Mikolich DJ. Alopecia associated with zidovudine therapy. Pharmacotherapy. 1996;16:79-81.
- d'Arminio Monforte A, Testa L, Gianotto M, et al. Indinavir-related alopecia [letter]. AIDS. 1998;12:328.
- Bouscarat F, Prevot MH, Matheron S. Alopecia associated with indinavir therapy [letter]. N Engl J Med. 1999;341:618.
- Ginarte M, Losada E, Prieto A, et al. Generalized hair loss induced by indinavir plus ritonavir therapy [letter]. AIDS. 2002;16:1695-1696.
- Fornataro K, Jefferys R. Crixivan side effect update—hair loss and ingrown toenails. Body Posit. 1999;12:12.
- Taylor GH. Cytomegalovirus. Am Fam Physician. 2003;67:519-524.
- Skinner RB, Light WH, Bale GF, et al. Alopecia areata and
presence of cytomegalovirus DNA [letter]. JAMA.
1995;273:1419-1420. - Skinner RB, Light WH, Leonardi C, et al. A molecular
approach to alopecia areata. J Invest Dermatol.
1995;104(suppl 5):3S-4S. - Garcia-Hernandez MJ, Torres MJ, Palomares JC, et al.
No evidence of cytomegalovirus DNA in alopecia areata
[letter]. J Invest Dermatol. 1998;110:185. - Offidani A, Amerio P, Bernardini ML, et al. Role of
cytomegalovirus replication in alopecia areata pathogenesis.
J Cutan Med Surg. 2000;4:63-65.
Hair loss has various etiologies. Correct diagnosis of hair disorders is complex and requires the evaluation of clinical presentation, history, physical examination, and laboratory test results. In the patient with a sexually transmitted disease (STD), alopecia may be an important associated finding and can provide clues to diagnosis. This review focuses on the relationship between hair loss and STDs. Specifically, we review alopecia in association with syphilis and human immunodeficiency virus (HIV) infection and the medications used to treat these infections. In addition, we review the literature regarding the putative association between alopecia areata and cytomegalovirus (CMV). There are multiple mechanisms involved in hair loss in these diseases, including the diseases themselves, systemic sequelae of these infections, autoimmune phenomena, and side effects of medications.
Syphilis
When considering the STDs associated with hair loss, syphilis is usually the first STD described because of the large incidence of the disease and its many reported cases of associated hair loss. This is especially important due to the increasing number of current cases of syphilis. Hair loss does not occur in primary syphilis except when associated with a primary chancre of scalp. Hair loss in secondary syphilis, also known as latent syphilis, occurs infrequently; various series report an incidence of 2.9% to 7%.1,2 There are 2 types of secondary syphilitic alopecia. The first is an uncommon symptomatic type found in association with an actual secondary lesion (usually papulosquamous) on the scalp. The second is termed essential syphilitic alopecia, which designates hair loss in the absence of visible syphilitic scalp lesions. Essential syphilitic alopecia has been divided into 3 types: the classic patchy "moth-eaten" alopecia (Figure), a generalized thinning of the hair, and the moth-eaten type in combination with general thinning of the hair. Of these, patchy moth-eaten alopecia occurs most frequently. The diffuse hair loss of essential syphilitic alopecia as the only manifestation of syphilis is uncommon. Cuozzo et al3 described 2 patients in whom the first sign of disease was alopecia.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Moth-eaten alopecia of syphilis is a characteristic manifestation of secondary syphilis that usually affects the scalp and occasionally other areas such as the eyebrows, beard, and pubic area.4 This form of alopecia may be confused with trichotillomania, traction alopecia, and alopecia areata.5 Pareek4 described a case of an unusual location of patchy moth-eaten alopecia that presented on the anterior side of the lower legs of a 30-year-old man in conjunction with patchy alopecia on the scalp and thinning of the eyebrows. With penicillin administration, hair of the legs, scalp, and eyebrows started to grow; the hair was fully regrown within 6 months, which suggests good prognosis with treatment instigation for syphilitic alopecia of all areas.
Jordaan and Louw5 systematically documented the histopathologic features of 12 patients with moth-eaten alopecia. Characteristic features included follicular plugging; a sparse, perivascular and perifollicular lymphocytic infiltrate; telogenization; and follicle-oriented melanin clumping.5 van der Willigen et al6 conducted a study of hair roots in 11 and 8 patients with primary and secondary syphilis, respectively. A decreased number of anagen hair roots; an increased number of catagen hair roots, dysplastic/dystrophic hair roots, and anagen hair roots with sheaths; and more than 20% angulation were observed in both groups.6 In addition, Lee and Hsu7 noted the histopathologic similarity between alopecia syphilitica and alopecia areata. They reported the histopathologic findings of alopecia syphilitica from 9 patients with secondary syphilis and acute hair loss. The alopecia was moth-eaten in 4 patients and was diffuse but slightly moth-eaten in 5. Microscopically, the dermoepidermal interface was not involved. The number of hair follicles was diminished, with increased numbers of catagens and telogens. Lymphocytic infiltration was present around the hair bulbs and fibrous tracts in 8 patients, and plasma cells were present in 4 biopsy specimens. Except for the follicular changes, the findings resembled those of macular/maculopapular syphilides outside the scalp. With the follicular changes, the overall patterns closely resembled alopecia areata. Results of the modified Steiner stain did not reveal spirochetes in any of the patients and failed to differentiate between alopecia syphilitica and alopecia areata. Comparing the alopecia syphilitica patients with 13 patients with alopecia areata, the authors found only a few differentiating features. They concluded that the presence of peribulbar eosinophils strongly suggests alopecia areata.7 Without peribulbar eosinophils, the presence of plasma cells, abundant lymphocytes in the isthmus, or peribulbar lymphoid aggregates suggests alopecia syphilitica. Elston et al8 observed several cases of syphilis with numerous eosinophils in the peribulbar infiltrate and noted that it can be indistinguishable from alopecia areata.
When an associated skin rash or lymphadenopathy is present, the diagnosis of syphilis may be suggested and confirmed by positive serology test results. If such findings are not present, a biopsy specimen to differentiate from other forms of alopecia should be obtained. Because moth-eaten alopecia and alopecia areata have similar resemblance microscopically, syphilis serologic tests are needed.
The treatment of syphilis also has been shown to be a cause of alopecia. Pareek9 described the association of syphilitic alopecia and Herxheimer reaction. A 25-year-old man presented with syphilis with widespread thinning of the scalp hair, eyebrows, and pubic area; the scalp showed patchy moth-eaten alopecia. He was treated with 1 to 2 megaunits of procaine penicillin daily for 10 days. Six hours after the first injection, the patient's temperature rose to 103°F; in addition to malaise, headache, flush, and sore throat, he had a transient skin rash and marked loss of hair. All the symptoms disappeared by the next day. Two to 3 weeks later, the lymphadenopathy had disappeared, and the patient's eyebrows and pubic hair started to regrow. The scalp hair was fully regrown 10 weeks from the onset of treatment. The author concluded that diffuse and extensive hair loss after the first injection of penicillin was part of the Herxheimer reaction.9
HIV
Hair loss is common in patients with HIV; in black patients, this loss may be associated with hair straightening.10 Possible causes of hair loss frequently are present in patients with HIV, including chronic HIV infection itself, acute and chronic systemic infections, local infections, nutritional deficits, immune and endocrine dysregulation, and exposure to multiple drugs.10 Alopecia areata and alopecia universalis also have been reported in patients with HIV.11-14
Smith et al10 studied and reviewed the clinical and histopathologic features of hair loss in 10 patients with HIV. They noted that the most characteristic change in the hair of patients with HIV was hair loss with straightening, sometimes associated with fine hair texture and an increased tendency for broken hairs. These changes are seen in late-stage disease, most commonly in black patients. Each patient had telogen effluvium, and it was observed that any chronic or acute infection (including HIV) can lead to this condition. Nutritional deficits, often prominent in HIV patients, may lead to or potentiate telogen effluvium. Secondary infections and changes in bowel mucosa may lead to specific nutritional deficiencies even before evidence of clinical wasting is seen. In addition to caloric and protein malnutrition that may affect hair growth, minerals such as copper, zinc, and selenium are decreased in patients with HIV. Elevated levels of interleukin 6 and tumor necrosis factor α, which increase epidermal proliferation, may predispose patients to abnormal keratinization by increasing the proliferative rate and nutritional requirements.10
Endocrine regulation is another important factor in hair growth. In late-stage HIV disease, androgen levels decrease while estradiol levels increase. Although thyroid hormone levels are normal in advanced HIV, thyroid functions are elevated to more than expected for the amount of wasting and may contribute to the change of hair texture,10 autoimmune mechanism, associated diseases, and HIV medication side effects.
In the Smith et al10 study, scanning electron microscopy was performed on plucked and pulled hairs of 10 patients with late-stage HIV-1 infection. In addition, scalp biopsy specimens were examined in both vertical and transverse sections. All patients had telogen effluvium. Numerous apoptotic or necrotic keratinocytes were seen in the upper external root sheath follicular epithelium; a mild to moderate perifollicular mononuclear cell infiltrate, often containing eosinophils, also was seen. Additionally, the mononuclear infiltrate was seen surrounding and within the basaloid cells of the follicles in telogen phase; the midfollicular area had the most marked inflammatory infiltrate. Variable dystrophy of the hair shafts also was a consistent feature. Although telogen effluvium is a common response to a wide spectrum of biologic stresses, the presence of apoptotic or necrotic keratinocytes within the upper end of the external root sheath epithelium, as well as dystrophy of hairs, may be markers of hair loss in patients with HIV-1 infection.10
Autoimmune alopecia, including alopecia areata and alopecia universalis, can be seen in association with HIV.11-15 Ostlere et al11 first reported a case of alopecia universalis that developed in a patient 2 years after HIV antibody was detected. The patient showed loss of all scalp hair, eyelashes, eyebrows, and body hair. Two possible mechanisms for the development of alopecia were suggested. The first was that HIV induced nonspecific polyclonal B-cell activation with production of autoantibody either directly or via activated T cells; this supports a humoral theory of alopecia areata pathogenesis. Alternatively, the authors postulated that HIV induced a change in the balance between helper and suppressor cells, which resulted in aberrant cell-mediated immune effect at the hair follicles.11 Werninghaus and Kaminer12 described a similar patient with alopecia universalis; a biopsy specimen revealed perifollicular fibrosis without inflammation.
Stewart and Smoller13 described an HIV-positive patient with altered T-lymphocyte subsets in whom alopecia universalis developed. Results of a skin biopsy of the patient's scalp demonstrated a classic perifollicular lymphocytic infiltrate; results of immunophenotyping of the same specimen revealed that most cells were CD4+ lymphocytes. During the active loss of hair, the patient's ratio of CD4/CD8 cells was decreased; however, the ratio normalized during the period of hair regrowth. Their data suggested that systemic immune dysfunction, as seen in HIV infection, may be more important in mediating alopecia areata than localized immune responses. Because of the proposed mechanism of alopecia areata developing in this patient (ie, influx of CD4+ lymphocytes to the perifollicular regions of skin when the CD4/CD8 cells ratio is low), the authors were surprised that alopecia areata is not more common in patients with HIV infection.13
Cho et al14 described the association of vitiligo and alopecia areata in patients with HIV. They noted that the development of autoimmune diseases, though not life threatening, is an interesting phenomenon that may result from immune dysfunction or from B-cell infection by HIV, Epstein-Barr virus, or other unknown viruses. They described a 47-year-old man who had vitiligo and alopecia areata approximately 2 years after testing positive for HIV antibodies.14 Grossman et al15 described an HIV-seropositive man with acquired eyelash trichomegaly and alopecia areata. They noted that this combination of clinical manifestations is intriguing because the new onset of elongated eyelashes in patients with acquired immunodeficiency syndrome usually has been associated with severe immunosuppression, and alopecia areata has a presumed autoimmune etiology that requires T-cell activation. They concluded that the occurrence of these dichotomous conditions illustrates the potential selective pathogenesis of progressive HIV infection.15
Medications used in the treatment of HIV can play a role in hair loss. Geletko et al16 reported a 33-year-old HIV-infected man who developed alopecia areata after beginning therapy with zidovudine, a nucleoside analogue reverse transcriptase inhibitor. The alopecia reversed after the drug was discontinued. The authors proposed that patients with lower CD4+ counts may be more predisposed to zidovudine-induced alopecia than those in the earlier stages of HIV with higher CD4+ counts.16
Indinavir-related alopecia was described by d'Arminio Monforte et al.17 Of 337 patients given indinavir in combination with nucleoside analogues, 5 patients with HIV developed severe alopecia, which was evident clinically after a mean of 50 days of treatment. All patients were receiving triple therapy that included indinavir. Three patients had diffuse shedding of hair involving the entire scalp, and 2 had circumscribed circular areas of alopecia resulting in complete severe hair loss.17 Bouscarat et al18 reported 10 more cases of hair loss associated with indinavir therapy in patients receiving triple antiviral treatment that included indinavir. Hair loss developed during the first 6 months of indinavir therapy and initially involved the lower limbs. Progressive hair regrowth occurred within 4 months after indinavir was replaced by other treatments.18
Ginarte et al19 described significant alopecia induced by indinavir plus ritonavir therapy in 3 patients a few weeks after beginning treatment. The authors noted that patients receiving indinavir often experience retinoidlike effects such as alopecia, xerosis, and cheilitis. Nonscarring alopecia can develop in patients receiving indinavir, with or without retinoid effects.19 Hair loss also has been noted with the use of crixivan.20
CMV
CMV is a prevalent viral pathogen.21 Most people with acute CMV experience an inapparent infection. The virus usually is spread through close personal contact, including sexual transmission. There has been debate over the link of alopecia areata with CMV. In 1995, Skinner et al22 described using polymerase chain reaction (PCR) techniques to find evidence of CMV DNA in paraffin block sections of lesions of alopecia areata. Of 21 patient biopsy specimens, 10 had alopecia areata and 11 had other hair loss conditions. Of the 10 alopecia areata samples, 9 were positive for CMV; no other hair loss samples were positive for CMV.22 Skinner et al23 theorized that CMV may achieve latency in the hair root. Reactivation of CMV was thought to be one of the pathogenic mechanisms in alopecia areata; the authors argued that a lymphocytic surveillance of not-quite-latent CMV would explain much of the behavior of alopecia areata, which has a tendency for intermittent relapses and remissions.23
The association between alopecia areata and CMV was refuted by Garcia-Hernandez et al,24 who used 3 different PCR assays to detect CMV DNA in skin punch biopsy specimens of 3 patient groups: 40 patients with alopecia areata, 3 patients with HIV and alopecia areata, and 12 patients with other types of alopecia. PCR assays are known to be the most sensitive assay for CMV detection; this study used different PCR assays to achieve maximum sensitivity for CMV. No CMV DNA amplification was found in any of the specimens.24
Offidani et al25 further contradicted this association. The purpose of their study was to clarify the role of CMV infection and to demonstrate the absence of replication of other autoimmune disease–related herpesviruses (eg, Epstein-Barr virus) in the pathogenesis of alopecia areata. After extraction of mRNA from tissue samples of 4 patients with active patchy alopecia areata, reverse transcriptase PCR was carried out using primers specific for some viral members of the β Herpesviridae subfamily of the Herpesviridae family (eg, CMV, Epstein-Barr virus, herpes simplex virus). The authors could not detect any replication of the CMV or other β Herpesviridae in the samples collected, which supports the hypothesis that CMV is not the triggering factor in alopecia areata, neither as a reactivator of the immune response nor as a trigger of the autoimmunity.25
Conclusion
Although many etiologies exist for hair loss, STDs should not be overlooked in a sexually active patient presenting with an otherwise unexplainable cause of this condition. A full workup, including clinical history, physical examination, and laboratory tests, should include STDs in the differential diagnosis (Table).
Hair loss has various etiologies. Correct diagnosis of hair disorders is complex and requires the evaluation of clinical presentation, history, physical examination, and laboratory test results. In the patient with a sexually transmitted disease (STD), alopecia may be an important associated finding and can provide clues to diagnosis. This review focuses on the relationship between hair loss and STDs. Specifically, we review alopecia in association with syphilis and human immunodeficiency virus (HIV) infection and the medications used to treat these infections. In addition, we review the literature regarding the putative association between alopecia areata and cytomegalovirus (CMV). There are multiple mechanisms involved in hair loss in these diseases, including the diseases themselves, systemic sequelae of these infections, autoimmune phenomena, and side effects of medications.
Syphilis
When considering the STDs associated with hair loss, syphilis is usually the first STD described because of the large incidence of the disease and its many reported cases of associated hair loss. This is especially important due to the increasing number of current cases of syphilis. Hair loss does not occur in primary syphilis except when associated with a primary chancre of scalp. Hair loss in secondary syphilis, also known as latent syphilis, occurs infrequently; various series report an incidence of 2.9% to 7%.1,2 There are 2 types of secondary syphilitic alopecia. The first is an uncommon symptomatic type found in association with an actual secondary lesion (usually papulosquamous) on the scalp. The second is termed essential syphilitic alopecia, which designates hair loss in the absence of visible syphilitic scalp lesions. Essential syphilitic alopecia has been divided into 3 types: the classic patchy "moth-eaten" alopecia (Figure), a generalized thinning of the hair, and the moth-eaten type in combination with general thinning of the hair. Of these, patchy moth-eaten alopecia occurs most frequently. The diffuse hair loss of essential syphilitic alopecia as the only manifestation of syphilis is uncommon. Cuozzo et al3 described 2 patients in whom the first sign of disease was alopecia.
PLEASE REFER TO THE PDF TO VIEW THE FIGURE
Moth-eaten alopecia of syphilis is a characteristic manifestation of secondary syphilis that usually affects the scalp and occasionally other areas such as the eyebrows, beard, and pubic area.4 This form of alopecia may be confused with trichotillomania, traction alopecia, and alopecia areata.5 Pareek4 described a case of an unusual location of patchy moth-eaten alopecia that presented on the anterior side of the lower legs of a 30-year-old man in conjunction with patchy alopecia on the scalp and thinning of the eyebrows. With penicillin administration, hair of the legs, scalp, and eyebrows started to grow; the hair was fully regrown within 6 months, which suggests good prognosis with treatment instigation for syphilitic alopecia of all areas.
Jordaan and Louw5 systematically documented the histopathologic features of 12 patients with moth-eaten alopecia. Characteristic features included follicular plugging; a sparse, perivascular and perifollicular lymphocytic infiltrate; telogenization; and follicle-oriented melanin clumping.5 van der Willigen et al6 conducted a study of hair roots in 11 and 8 patients with primary and secondary syphilis, respectively. A decreased number of anagen hair roots; an increased number of catagen hair roots, dysplastic/dystrophic hair roots, and anagen hair roots with sheaths; and more than 20% angulation were observed in both groups.6 In addition, Lee and Hsu7 noted the histopathologic similarity between alopecia syphilitica and alopecia areata. They reported the histopathologic findings of alopecia syphilitica from 9 patients with secondary syphilis and acute hair loss. The alopecia was moth-eaten in 4 patients and was diffuse but slightly moth-eaten in 5. Microscopically, the dermoepidermal interface was not involved. The number of hair follicles was diminished, with increased numbers of catagens and telogens. Lymphocytic infiltration was present around the hair bulbs and fibrous tracts in 8 patients, and plasma cells were present in 4 biopsy specimens. Except for the follicular changes, the findings resembled those of macular/maculopapular syphilides outside the scalp. With the follicular changes, the overall patterns closely resembled alopecia areata. Results of the modified Steiner stain did not reveal spirochetes in any of the patients and failed to differentiate between alopecia syphilitica and alopecia areata. Comparing the alopecia syphilitica patients with 13 patients with alopecia areata, the authors found only a few differentiating features. They concluded that the presence of peribulbar eosinophils strongly suggests alopecia areata.7 Without peribulbar eosinophils, the presence of plasma cells, abundant lymphocytes in the isthmus, or peribulbar lymphoid aggregates suggests alopecia syphilitica. Elston et al8 observed several cases of syphilis with numerous eosinophils in the peribulbar infiltrate and noted that it can be indistinguishable from alopecia areata.
When an associated skin rash or lymphadenopathy is present, the diagnosis of syphilis may be suggested and confirmed by positive serology test results. If such findings are not present, a biopsy specimen to differentiate from other forms of alopecia should be obtained. Because moth-eaten alopecia and alopecia areata have similar resemblance microscopically, syphilis serologic tests are needed.
The treatment of syphilis also has been shown to be a cause of alopecia. Pareek9 described the association of syphilitic alopecia and Herxheimer reaction. A 25-year-old man presented with syphilis with widespread thinning of the scalp hair, eyebrows, and pubic area; the scalp showed patchy moth-eaten alopecia. He was treated with 1 to 2 megaunits of procaine penicillin daily for 10 days. Six hours after the first injection, the patient's temperature rose to 103°F; in addition to malaise, headache, flush, and sore throat, he had a transient skin rash and marked loss of hair. All the symptoms disappeared by the next day. Two to 3 weeks later, the lymphadenopathy had disappeared, and the patient's eyebrows and pubic hair started to regrow. The scalp hair was fully regrown 10 weeks from the onset of treatment. The author concluded that diffuse and extensive hair loss after the first injection of penicillin was part of the Herxheimer reaction.9
HIV
Hair loss is common in patients with HIV; in black patients, this loss may be associated with hair straightening.10 Possible causes of hair loss frequently are present in patients with HIV, including chronic HIV infection itself, acute and chronic systemic infections, local infections, nutritional deficits, immune and endocrine dysregulation, and exposure to multiple drugs.10 Alopecia areata and alopecia universalis also have been reported in patients with HIV.11-14
Smith et al10 studied and reviewed the clinical and histopathologic features of hair loss in 10 patients with HIV. They noted that the most characteristic change in the hair of patients with HIV was hair loss with straightening, sometimes associated with fine hair texture and an increased tendency for broken hairs. These changes are seen in late-stage disease, most commonly in black patients. Each patient had telogen effluvium, and it was observed that any chronic or acute infection (including HIV) can lead to this condition. Nutritional deficits, often prominent in HIV patients, may lead to or potentiate telogen effluvium. Secondary infections and changes in bowel mucosa may lead to specific nutritional deficiencies even before evidence of clinical wasting is seen. In addition to caloric and protein malnutrition that may affect hair growth, minerals such as copper, zinc, and selenium are decreased in patients with HIV. Elevated levels of interleukin 6 and tumor necrosis factor α, which increase epidermal proliferation, may predispose patients to abnormal keratinization by increasing the proliferative rate and nutritional requirements.10
Endocrine regulation is another important factor in hair growth. In late-stage HIV disease, androgen levels decrease while estradiol levels increase. Although thyroid hormone levels are normal in advanced HIV, thyroid functions are elevated to more than expected for the amount of wasting and may contribute to the change of hair texture,10 autoimmune mechanism, associated diseases, and HIV medication side effects.
In the Smith et al10 study, scanning electron microscopy was performed on plucked and pulled hairs of 10 patients with late-stage HIV-1 infection. In addition, scalp biopsy specimens were examined in both vertical and transverse sections. All patients had telogen effluvium. Numerous apoptotic or necrotic keratinocytes were seen in the upper external root sheath follicular epithelium; a mild to moderate perifollicular mononuclear cell infiltrate, often containing eosinophils, also was seen. Additionally, the mononuclear infiltrate was seen surrounding and within the basaloid cells of the follicles in telogen phase; the midfollicular area had the most marked inflammatory infiltrate. Variable dystrophy of the hair shafts also was a consistent feature. Although telogen effluvium is a common response to a wide spectrum of biologic stresses, the presence of apoptotic or necrotic keratinocytes within the upper end of the external root sheath epithelium, as well as dystrophy of hairs, may be markers of hair loss in patients with HIV-1 infection.10
Autoimmune alopecia, including alopecia areata and alopecia universalis, can be seen in association with HIV.11-15 Ostlere et al11 first reported a case of alopecia universalis that developed in a patient 2 years after HIV antibody was detected. The patient showed loss of all scalp hair, eyelashes, eyebrows, and body hair. Two possible mechanisms for the development of alopecia were suggested. The first was that HIV induced nonspecific polyclonal B-cell activation with production of autoantibody either directly or via activated T cells; this supports a humoral theory of alopecia areata pathogenesis. Alternatively, the authors postulated that HIV induced a change in the balance between helper and suppressor cells, which resulted in aberrant cell-mediated immune effect at the hair follicles.11 Werninghaus and Kaminer12 described a similar patient with alopecia universalis; a biopsy specimen revealed perifollicular fibrosis without inflammation.
Stewart and Smoller13 described an HIV-positive patient with altered T-lymphocyte subsets in whom alopecia universalis developed. Results of a skin biopsy of the patient's scalp demonstrated a classic perifollicular lymphocytic infiltrate; results of immunophenotyping of the same specimen revealed that most cells were CD4+ lymphocytes. During the active loss of hair, the patient's ratio of CD4/CD8 cells was decreased; however, the ratio normalized during the period of hair regrowth. Their data suggested that systemic immune dysfunction, as seen in HIV infection, may be more important in mediating alopecia areata than localized immune responses. Because of the proposed mechanism of alopecia areata developing in this patient (ie, influx of CD4+ lymphocytes to the perifollicular regions of skin when the CD4/CD8 cells ratio is low), the authors were surprised that alopecia areata is not more common in patients with HIV infection.13
Cho et al14 described the association of vitiligo and alopecia areata in patients with HIV. They noted that the development of autoimmune diseases, though not life threatening, is an interesting phenomenon that may result from immune dysfunction or from B-cell infection by HIV, Epstein-Barr virus, or other unknown viruses. They described a 47-year-old man who had vitiligo and alopecia areata approximately 2 years after testing positive for HIV antibodies.14 Grossman et al15 described an HIV-seropositive man with acquired eyelash trichomegaly and alopecia areata. They noted that this combination of clinical manifestations is intriguing because the new onset of elongated eyelashes in patients with acquired immunodeficiency syndrome usually has been associated with severe immunosuppression, and alopecia areata has a presumed autoimmune etiology that requires T-cell activation. They concluded that the occurrence of these dichotomous conditions illustrates the potential selective pathogenesis of progressive HIV infection.15
Medications used in the treatment of HIV can play a role in hair loss. Geletko et al16 reported a 33-year-old HIV-infected man who developed alopecia areata after beginning therapy with zidovudine, a nucleoside analogue reverse transcriptase inhibitor. The alopecia reversed after the drug was discontinued. The authors proposed that patients with lower CD4+ counts may be more predisposed to zidovudine-induced alopecia than those in the earlier stages of HIV with higher CD4+ counts.16
Indinavir-related alopecia was described by d'Arminio Monforte et al.17 Of 337 patients given indinavir in combination with nucleoside analogues, 5 patients with HIV developed severe alopecia, which was evident clinically after a mean of 50 days of treatment. All patients were receiving triple therapy that included indinavir. Three patients had diffuse shedding of hair involving the entire scalp, and 2 had circumscribed circular areas of alopecia resulting in complete severe hair loss.17 Bouscarat et al18 reported 10 more cases of hair loss associated with indinavir therapy in patients receiving triple antiviral treatment that included indinavir. Hair loss developed during the first 6 months of indinavir therapy and initially involved the lower limbs. Progressive hair regrowth occurred within 4 months after indinavir was replaced by other treatments.18
Ginarte et al19 described significant alopecia induced by indinavir plus ritonavir therapy in 3 patients a few weeks after beginning treatment. The authors noted that patients receiving indinavir often experience retinoidlike effects such as alopecia, xerosis, and cheilitis. Nonscarring alopecia can develop in patients receiving indinavir, with or without retinoid effects.19 Hair loss also has been noted with the use of crixivan.20
CMV
CMV is a prevalent viral pathogen.21 Most people with acute CMV experience an inapparent infection. The virus usually is spread through close personal contact, including sexual transmission. There has been debate over the link of alopecia areata with CMV. In 1995, Skinner et al22 described using polymerase chain reaction (PCR) techniques to find evidence of CMV DNA in paraffin block sections of lesions of alopecia areata. Of 21 patient biopsy specimens, 10 had alopecia areata and 11 had other hair loss conditions. Of the 10 alopecia areata samples, 9 were positive for CMV; no other hair loss samples were positive for CMV.22 Skinner et al23 theorized that CMV may achieve latency in the hair root. Reactivation of CMV was thought to be one of the pathogenic mechanisms in alopecia areata; the authors argued that a lymphocytic surveillance of not-quite-latent CMV would explain much of the behavior of alopecia areata, which has a tendency for intermittent relapses and remissions.23
The association between alopecia areata and CMV was refuted by Garcia-Hernandez et al,24 who used 3 different PCR assays to detect CMV DNA in skin punch biopsy specimens of 3 patient groups: 40 patients with alopecia areata, 3 patients with HIV and alopecia areata, and 12 patients with other types of alopecia. PCR assays are known to be the most sensitive assay for CMV detection; this study used different PCR assays to achieve maximum sensitivity for CMV. No CMV DNA amplification was found in any of the specimens.24
Offidani et al25 further contradicted this association. The purpose of their study was to clarify the role of CMV infection and to demonstrate the absence of replication of other autoimmune disease–related herpesviruses (eg, Epstein-Barr virus) in the pathogenesis of alopecia areata. After extraction of mRNA from tissue samples of 4 patients with active patchy alopecia areata, reverse transcriptase PCR was carried out using primers specific for some viral members of the β Herpesviridae subfamily of the Herpesviridae family (eg, CMV, Epstein-Barr virus, herpes simplex virus). The authors could not detect any replication of the CMV or other β Herpesviridae in the samples collected, which supports the hypothesis that CMV is not the triggering factor in alopecia areata, neither as a reactivator of the immune response nor as a trigger of the autoimmunity.25
Conclusion
Although many etiologies exist for hair loss, STDs should not be overlooked in a sexually active patient presenting with an otherwise unexplainable cause of this condition. A full workup, including clinical history, physical examination, and laboratory tests, should include STDs in the differential diagnosis (Table).
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Cuozzo DW, Benson PM, Sperling LC, et al. Essential syphilitic alopecia revisited. J Am Acad Dermatol. 1995;32:840-844.
- Pareek SS. Unusual location of syphilitic alopecia: a case report. Sex Transm Dis. 1982;9:43-44.
- Jordaan HF, Louw M. The moth-eaten alopecia of secondary syphilis. a histopathological study of 12 patients. Am J Dermatopathol. 1995;17:158-162.
- van der Willigen AH, Peereboom-Wynia JD, van der Hoek JC, et al. Hair root studies in patients suffering from primary and secondary syphilis. Acta Derm Venereol. 1987;67:250-254.
- Lee JY, Hsu ML. Alopecia syphilitica, a simulator of alopecia areata: histopathology and differential diagnosis. J Cutan Pathol. 1991;18:87-92.
- Elston DM, McCollough ML, Bergfeld WF, et al. Eosinophils in fibrous tracts and near hair bulbs: a helpful diagnostic feature of alopecia areata. J Am Acad Dermatol. 1997;37:101-106.
- Pareek SS. Syphilitic alopecia and Jarisch-Herxheimer reaction. Br J Vener Dis. 1977;53:389-390.
- Smith KJ, Skelton HG, DeRusso D, et al. Clinical and histopathologic features of hair loss in patients with HIV-1 infection. J Am Acad Dermatol. 1996;34:63-68.
- Ostlere LS, Langtry JA, Staughton RC, et al. Alopecia universalis in a patient seropositive for the human immunodeficiency virus. J Am Acad Dermatol. 1992;27:630-631.
- Werninghaus K, Kaminer MS. HIV and alopecia universalis [letter]. J Am Acad Dermatol. 1993;29:667.
- Stewart MI, Smoller BR. Alopecia universalis in an HIV-positive patient: possible insight into pathogenesis. J Cutan Pathol. 1993;20:180-183.
- Cho M, Cohen PR, Duvic M. Vitiligo and alopecia areata in patients with human immunodeficiency virus infection. South Med J. 1995;88:489-491.
- Grossman MC, Cohen PR, Grossman ME. Acquired eyelash trichomegaly and alopecia areata in a human immunodeficiency virus–infected patient. Dermatology. 1996;193:52-53.
- Geletko SM, Segarra M, Mikolich DJ. Alopecia associated with zidovudine therapy. Pharmacotherapy. 1996;16:79-81.
- d'Arminio Monforte A, Testa L, Gianotto M, et al. Indinavir-related alopecia [letter]. AIDS. 1998;12:328.
- Bouscarat F, Prevot MH, Matheron S. Alopecia associated with indinavir therapy [letter]. N Engl J Med. 1999;341:618.
- Ginarte M, Losada E, Prieto A, et al. Generalized hair loss induced by indinavir plus ritonavir therapy [letter]. AIDS. 2002;16:1695-1696.
- Fornataro K, Jefferys R. Crixivan side effect update—hair loss and ingrown toenails. Body Posit. 1999;12:12.
- Taylor GH. Cytomegalovirus. Am Fam Physician. 2003;67:519-524.
- Skinner RB, Light WH, Bale GF, et al. Alopecia areata and
presence of cytomegalovirus DNA [letter]. JAMA.
1995;273:1419-1420. - Skinner RB, Light WH, Leonardi C, et al. A molecular
approach to alopecia areata. J Invest Dermatol.
1995;104(suppl 5):3S-4S. - Garcia-Hernandez MJ, Torres MJ, Palomares JC, et al.
No evidence of cytomegalovirus DNA in alopecia areata
[letter]. J Invest Dermatol. 1998;110:185. - Offidani A, Amerio P, Bernardini ML, et al. Role of
cytomegalovirus replication in alopecia areata pathogenesis.
J Cutan Med Surg. 2000;4:63-65.
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Cuozzo DW, Benson PM, Sperling LC, et al. Essential syphilitic alopecia revisited. J Am Acad Dermatol. 1995;32:840-844.
- Pareek SS. Unusual location of syphilitic alopecia: a case report. Sex Transm Dis. 1982;9:43-44.
- Jordaan HF, Louw M. The moth-eaten alopecia of secondary syphilis. a histopathological study of 12 patients. Am J Dermatopathol. 1995;17:158-162.
- van der Willigen AH, Peereboom-Wynia JD, van der Hoek JC, et al. Hair root studies in patients suffering from primary and secondary syphilis. Acta Derm Venereol. 1987;67:250-254.
- Lee JY, Hsu ML. Alopecia syphilitica, a simulator of alopecia areata: histopathology and differential diagnosis. J Cutan Pathol. 1991;18:87-92.
- Elston DM, McCollough ML, Bergfeld WF, et al. Eosinophils in fibrous tracts and near hair bulbs: a helpful diagnostic feature of alopecia areata. J Am Acad Dermatol. 1997;37:101-106.
- Pareek SS. Syphilitic alopecia and Jarisch-Herxheimer reaction. Br J Vener Dis. 1977;53:389-390.
- Smith KJ, Skelton HG, DeRusso D, et al. Clinical and histopathologic features of hair loss in patients with HIV-1 infection. J Am Acad Dermatol. 1996;34:63-68.
- Ostlere LS, Langtry JA, Staughton RC, et al. Alopecia universalis in a patient seropositive for the human immunodeficiency virus. J Am Acad Dermatol. 1992;27:630-631.
- Werninghaus K, Kaminer MS. HIV and alopecia universalis [letter]. J Am Acad Dermatol. 1993;29:667.
- Stewart MI, Smoller BR. Alopecia universalis in an HIV-positive patient: possible insight into pathogenesis. J Cutan Pathol. 1993;20:180-183.
- Cho M, Cohen PR, Duvic M. Vitiligo and alopecia areata in patients with human immunodeficiency virus infection. South Med J. 1995;88:489-491.
- Grossman MC, Cohen PR, Grossman ME. Acquired eyelash trichomegaly and alopecia areata in a human immunodeficiency virus–infected patient. Dermatology. 1996;193:52-53.
- Geletko SM, Segarra M, Mikolich DJ. Alopecia associated with zidovudine therapy. Pharmacotherapy. 1996;16:79-81.
- d'Arminio Monforte A, Testa L, Gianotto M, et al. Indinavir-related alopecia [letter]. AIDS. 1998;12:328.
- Bouscarat F, Prevot MH, Matheron S. Alopecia associated with indinavir therapy [letter]. N Engl J Med. 1999;341:618.
- Ginarte M, Losada E, Prieto A, et al. Generalized hair loss induced by indinavir plus ritonavir therapy [letter]. AIDS. 2002;16:1695-1696.
- Fornataro K, Jefferys R. Crixivan side effect update—hair loss and ingrown toenails. Body Posit. 1999;12:12.
- Taylor GH. Cytomegalovirus. Am Fam Physician. 2003;67:519-524.
- Skinner RB, Light WH, Bale GF, et al. Alopecia areata and
presence of cytomegalovirus DNA [letter]. JAMA.
1995;273:1419-1420. - Skinner RB, Light WH, Leonardi C, et al. A molecular
approach to alopecia areata. J Invest Dermatol.
1995;104(suppl 5):3S-4S. - Garcia-Hernandez MJ, Torres MJ, Palomares JC, et al.
No evidence of cytomegalovirus DNA in alopecia areata
[letter]. J Invest Dermatol. 1998;110:185. - Offidani A, Amerio P, Bernardini ML, et al. Role of
cytomegalovirus replication in alopecia areata pathogenesis.
J Cutan Med Surg. 2000;4:63-65.
URINARY INCONTINENCE
Although urinary incontinence is one of the most common chronic diseases in women, we still don’t understand its pathophysiology, and treatments have been, of necessity, empiric rather than directed at a specific cause. Fortunately, this bleak scenario may be changing, and I think that is the most exciting news about urinary incontinence in 2005.
Urethral deficiency by any name is still a deficiency
Ironically, the most basic description of urinary incontinence may be the most revealing: Incontinence occurs when the urethra cannot stay closed and fails to hold urine in the bladder, where it should be stored until the “right” time and place for emptying. This description applies equally well to women with stress or urge symptoms, but let’s focus on stress incontinence for now.
By that line of thinking, the urethra is deficient in all women with stress incontinence. I believe this to be true, despite the arbitrary label—intrinsic sphincter deficiency, or ISD—that we apply to women with only the most severe symptoms of stress incontinence.
Surgery does not end the quest
Because surgery focuses on eliminating symptoms, it should come as no surprise that incontinence procedures continue to proliferate while we search for the Holy Grail: the perfect surgery that will effectively and durably “fix” the problem without complications or side effects. However, unless we find and correct the underlying problem that gave rise to the incontinence in the first place, we are doomed to fail in our search.
Doom? Failure? Where is the exciting news I promised?
CARE trial underscores efficacy of Burch procedure
Brubaker L, for the Pelvic Floor Disorders Network. Burch colposuspension at the time of sacrocolpopexy in stress continent women reduces bothersome stress urinary symptoms: The CARE randomized trial. Abstract presented at the American Urogynecologic Society Meeting, September 15–17, 2005, Atlanta.
If you have an exceptional memory, you will recall that, in this Update on Urinary Incontinence last year, results from the CARE trial were promised in 2006. Good news! Early results are available now, at least a year before expected.
Superior results with Burch changed the course of the CARE study
The CARE trial (Colpopexy And Urinary Reduction Efforts) was designed to determine the effect of Burch versus no Burch in women without stress incontinence symptoms but with advanced prolapse who were undergoing abdominal sacrocolpopexy. The trial was sponsored by the National Institute of Child Health and Human Development (NICHD) and performed by the Pelvic Floor Disorders Network of investigators from 7 clinical sites and a central coordinating center.
The original sample size was set at 480 women, to be randomized equally to Burch or no Burch, with the primary stress outcome at 3 months after surgery. However, at the first interim analysis, when about half the sample (232 women) had reached the primary outcome, the results showed such a striking benefit in the Burch group that the Data and Safety Monitoring Board for the Pelvic Floor Disorders Network recommended that enrollment be halted while all women continued to receive scheduled follow-up. Therefore, in February 2005, enrollment in the trial was closed, with 322 women randomized to 1 of the 2 arms.
The following results were presented at the annual meeting of the American Urogynecologic Society in September:
- Stress incontinence symptoms were reduced by about half in women after abdominal sacrocolpopexy (from 44% in the no-Burch group to 24% in the Burch group).
- Stress symptom severity improved with Burch. More women (62%) in the no-Burch stress incontinence group were bothered by their symptoms, compared to 32% of women in the Burch group.
- Urge symptoms were no different with Burch. More surprising, women in both groups had similar levels of symptoms measured as the urge endpoint, which included urge incontinence, urgency, frequency, nocturia, or enuresis; or treatment for any of those 5 symptoms. Almost 33% of women in the Burch group met the urge endpoint, compared with 38% in the no-Burch group (difference not statistically significant).
- Serious adverse events were not significantly different between the 2 groups.
Many more questions will be addressed with further analysis of CARE trial data, such as results of urodynamic testing with and without prolapse reduction, and the potential for predicting which subgroup benefits most when Burch is performed. Long-term follow-up data will address durability of results related to incontinence and prolapse. Follow-up is scheduled for 2 years in the CARE trial, and for up to 10 years in the Extended-CARE trial.
Efficacy of anticholinergics
Trospium chloride (Sanctura): another anticholinergic for overactive bladder. The Medical Letter. 2004;46(1188):63–64.
Solifenacin and darifenacin for overactive bladder. The Medical Letter. 2005;47(1204):23–24.
Three more drugs for overactive bladder won FDA approval in 2004 and 2005:
- trospium chloride (Sanctura)
- solifenacin succinate Vesicare)
- darifenacin hydrobromide (Enablex)
According to The Medical Letter, none appears to offer an advantage over long-acting anticholinergics for overactive bladder. Despite the proliferation of anticholinergic drugs for overactive bladder symptoms—or perhaps because of it—one suspects that these medications are not achieving substantial, long-lasting relief of symptoms. One study reported that two thirds of women discontinued therapy within 4 months.1 A comprehensive review of placebo-controlled trials of anticholinergic drugs for overactive bladder estimated that, as a class, even long-acting agents have a very limited effect on symptoms, with approximately 1 fewer incontinent episode and 1 fewer voiding episode per 48 hours.2
REFERENCES
1. Salvatore S, Khullar V, Cardozo L, Milani R, Athanasiou S, Kelleher C. Long-term prospective randomized study comparing two different regimens of oxybutynin as a treatment for detrusor overactivity. Eur J Obstet Gynaecol Reprod Biol. 2005;119:237-241.
2. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
Do anticholinergics and dementia drugs mix?
Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165:808–813.
Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13:324–328.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173:493–498.
Treatment recommendations
As ObGyns become more active in evaluating and treating women with urinary incontinence, we must stay alert for potential adverse drug interactions.
Ideally, behavioral treatment (scheduled voiding, fluid management, bedside commode) and pelvic muscle training should be first-line therapies in elderly women with overactive bladder.
Anticholinergic drugs should be used with caution, if at all, in women taking cholinesterase-inhibiting drugs for dementia.
Elderly patients with overactive bladder are at high risk for drug interactions, especially involving cholinesterase-inhibiting drugs for the treatment of dementia, such as donepezil hydrochloride (Aricept). However, a newer Alzheimer drug, memantine hydrochloride (Namenda), works by a different mechanism and may be less likely to interact directly with anticholinergic drugs for incontinence.
3 studies involving the elderly
Cognitive impairment
Observing a population of older adults with dementia, about half treated with cholinesterase inhibitors for their Alzheimer symptoms, Gill et al found that the patients on cholinesterase inhibitors were more likely to start treatment with an anticholinergic drug for incontinence within a year. They theorized that the cholinesterase-inhibiting drugs possibly contribute to new-onset or worsening urinary incontinence, which in turn leads to treatment with anticholinergic agents.
Jewart and colleagues found better performance in patients with Alzheimer disease who were not taking anticholinergic medication for incontinence.
No or mild cognitive impairment
Lipton et al tested cognitive function with darifenacin for 2 weeks and found no difference between immediate- or controlled-release forms of the drug and placebo. However, the study population consisted of volunteers 65 and older with no or mild cognitive impairment and no use of cholinesterase-inhibiting drugs.
IN THE PIPELINEUrethral injection of muscle-derived cells may restore function
Usiene I, Kim YT, Pruchnic R, et al. Human muscle-derived cells injection increases leak point pressure in a nude rat model of stress urinary incontinence. Abstract presented at the annual meeting of the International Continence Society, August 28–September 2, 2005, Montreal, Quebec. Abstract #2.
Some exciting news: Dr. Michael Chancellor and colleagues at the University of Pittsburgh and at Cook MyoSite in Pittsburgh are working to bring stem cell research to the clinician’s office, with their studies of muscle-derived cells that can be injected into the urethra. (This technique is well-established and currently used for injection of synthetic or biologic material such as bovine collagen.)
What is remarkable about this type of injection is that the muscle cells not only stay put in the urethra, they appear to integrate into the muscle of the urethral sphincter and differentiate into cells that produce new muscle fibers. Newly functioning muscle improves urethral function and, ideally, will be able to restore continence in women with incontinence.
Technique’s success in rats
Could the same be accomplished with muscle-derived stem cells from humans? At the 2005 meeting of the International Continence Society, Chancellor and colleagues described how they injected human muscle-derived stem cells into the urethras of a nude rat model of stress incontinence (via nerve transection). In the injected rats, leak-point pressure measurements were restored to levels similar to those in a control group of rats.
In addition, immunohistochemistry and histology showed persistence of the human muscle-derived stem cells in the injected rats, versus periurethral muscle atrophy in the rats that had nerve transection but no injection.
Clinical testing underway
Clinical trials of this technology in women are now being performed in Toronto. It will be necessary to show safety and efficacy before the stem cell therapy is made available clinically, but there are a couple of factors in its favor:
- In terms of safety, the risks associated with the current crop of injectable materials should not be applicable, at least in theory. Because the injected stem cells are isolated and grown from the patient’s own biopsy, the chance of an adverse immunologic reaction should be zero, absent the rare mistake in labeling or transfer.
- The cell-injection technique is well-established and already used by many clinicians who perform transurethral or periurethral injection of bulking agents for women with incontinence. It will not be necessary to learn a new technique—just injectable material.
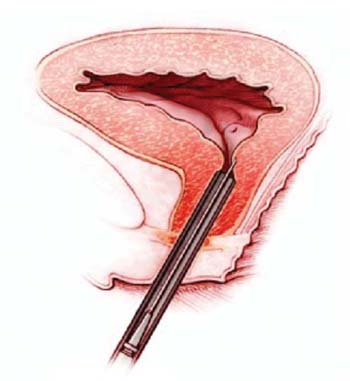
Tissue-engineered sling also in the works
Given that incontinence (and, presumably, the level of urethral damage resulting in some women may have urethral damage so severe as to preclude benefit from the injection of relatively small numbers of researchers is already developing a tissue-engineered sling in the hope that it can be used as a substitute for currently available synthetic or biologic sling materials.
The sling is being developed with the same muscle-derived stem cells, which are seeded onto a scaffold for as little as 2 weeks in a rat model. After the sling was surgically implanted using standard technique, urethral function improved to the level seen among controls.
It may be that the promise of stem cell therapy will come early to the treatment of urinary incontinence. Time will tell.
The author reports no financial relationships relevant to this article.
Although urinary incontinence is one of the most common chronic diseases in women, we still don’t understand its pathophysiology, and treatments have been, of necessity, empiric rather than directed at a specific cause. Fortunately, this bleak scenario may be changing, and I think that is the most exciting news about urinary incontinence in 2005.
Urethral deficiency by any name is still a deficiency
Ironically, the most basic description of urinary incontinence may be the most revealing: Incontinence occurs when the urethra cannot stay closed and fails to hold urine in the bladder, where it should be stored until the “right” time and place for emptying. This description applies equally well to women with stress or urge symptoms, but let’s focus on stress incontinence for now.
By that line of thinking, the urethra is deficient in all women with stress incontinence. I believe this to be true, despite the arbitrary label—intrinsic sphincter deficiency, or ISD—that we apply to women with only the most severe symptoms of stress incontinence.
Surgery does not end the quest
Because surgery focuses on eliminating symptoms, it should come as no surprise that incontinence procedures continue to proliferate while we search for the Holy Grail: the perfect surgery that will effectively and durably “fix” the problem without complications or side effects. However, unless we find and correct the underlying problem that gave rise to the incontinence in the first place, we are doomed to fail in our search.
Doom? Failure? Where is the exciting news I promised?
CARE trial underscores efficacy of Burch procedure
Brubaker L, for the Pelvic Floor Disorders Network. Burch colposuspension at the time of sacrocolpopexy in stress continent women reduces bothersome stress urinary symptoms: The CARE randomized trial. Abstract presented at the American Urogynecologic Society Meeting, September 15–17, 2005, Atlanta.
If you have an exceptional memory, you will recall that, in this Update on Urinary Incontinence last year, results from the CARE trial were promised in 2006. Good news! Early results are available now, at least a year before expected.
Superior results with Burch changed the course of the CARE study
The CARE trial (Colpopexy And Urinary Reduction Efforts) was designed to determine the effect of Burch versus no Burch in women without stress incontinence symptoms but with advanced prolapse who were undergoing abdominal sacrocolpopexy. The trial was sponsored by the National Institute of Child Health and Human Development (NICHD) and performed by the Pelvic Floor Disorders Network of investigators from 7 clinical sites and a central coordinating center.
The original sample size was set at 480 women, to be randomized equally to Burch or no Burch, with the primary stress outcome at 3 months after surgery. However, at the first interim analysis, when about half the sample (232 women) had reached the primary outcome, the results showed such a striking benefit in the Burch group that the Data and Safety Monitoring Board for the Pelvic Floor Disorders Network recommended that enrollment be halted while all women continued to receive scheduled follow-up. Therefore, in February 2005, enrollment in the trial was closed, with 322 women randomized to 1 of the 2 arms.
The following results were presented at the annual meeting of the American Urogynecologic Society in September:
- Stress incontinence symptoms were reduced by about half in women after abdominal sacrocolpopexy (from 44% in the no-Burch group to 24% in the Burch group).
- Stress symptom severity improved with Burch. More women (62%) in the no-Burch stress incontinence group were bothered by their symptoms, compared to 32% of women in the Burch group.
- Urge symptoms were no different with Burch. More surprising, women in both groups had similar levels of symptoms measured as the urge endpoint, which included urge incontinence, urgency, frequency, nocturia, or enuresis; or treatment for any of those 5 symptoms. Almost 33% of women in the Burch group met the urge endpoint, compared with 38% in the no-Burch group (difference not statistically significant).
- Serious adverse events were not significantly different between the 2 groups.
Many more questions will be addressed with further analysis of CARE trial data, such as results of urodynamic testing with and without prolapse reduction, and the potential for predicting which subgroup benefits most when Burch is performed. Long-term follow-up data will address durability of results related to incontinence and prolapse. Follow-up is scheduled for 2 years in the CARE trial, and for up to 10 years in the Extended-CARE trial.
Efficacy of anticholinergics
Trospium chloride (Sanctura): another anticholinergic for overactive bladder. The Medical Letter. 2004;46(1188):63–64.
Solifenacin and darifenacin for overactive bladder. The Medical Letter. 2005;47(1204):23–24.
Three more drugs for overactive bladder won FDA approval in 2004 and 2005:
- trospium chloride (Sanctura)
- solifenacin succinate Vesicare)
- darifenacin hydrobromide (Enablex)
According to The Medical Letter, none appears to offer an advantage over long-acting anticholinergics for overactive bladder. Despite the proliferation of anticholinergic drugs for overactive bladder symptoms—or perhaps because of it—one suspects that these medications are not achieving substantial, long-lasting relief of symptoms. One study reported that two thirds of women discontinued therapy within 4 months.1 A comprehensive review of placebo-controlled trials of anticholinergic drugs for overactive bladder estimated that, as a class, even long-acting agents have a very limited effect on symptoms, with approximately 1 fewer incontinent episode and 1 fewer voiding episode per 48 hours.2
REFERENCES
1. Salvatore S, Khullar V, Cardozo L, Milani R, Athanasiou S, Kelleher C. Long-term prospective randomized study comparing two different regimens of oxybutynin as a treatment for detrusor overactivity. Eur J Obstet Gynaecol Reprod Biol. 2005;119:237-241.
2. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
Do anticholinergics and dementia drugs mix?
Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165:808–813.
Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13:324–328.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173:493–498.
Treatment recommendations
As ObGyns become more active in evaluating and treating women with urinary incontinence, we must stay alert for potential adverse drug interactions.
Ideally, behavioral treatment (scheduled voiding, fluid management, bedside commode) and pelvic muscle training should be first-line therapies in elderly women with overactive bladder.
Anticholinergic drugs should be used with caution, if at all, in women taking cholinesterase-inhibiting drugs for dementia.
Elderly patients with overactive bladder are at high risk for drug interactions, especially involving cholinesterase-inhibiting drugs for the treatment of dementia, such as donepezil hydrochloride (Aricept). However, a newer Alzheimer drug, memantine hydrochloride (Namenda), works by a different mechanism and may be less likely to interact directly with anticholinergic drugs for incontinence.
3 studies involving the elderly
Cognitive impairment
Observing a population of older adults with dementia, about half treated with cholinesterase inhibitors for their Alzheimer symptoms, Gill et al found that the patients on cholinesterase inhibitors were more likely to start treatment with an anticholinergic drug for incontinence within a year. They theorized that the cholinesterase-inhibiting drugs possibly contribute to new-onset or worsening urinary incontinence, which in turn leads to treatment with anticholinergic agents.
Jewart and colleagues found better performance in patients with Alzheimer disease who were not taking anticholinergic medication for incontinence.
No or mild cognitive impairment
Lipton et al tested cognitive function with darifenacin for 2 weeks and found no difference between immediate- or controlled-release forms of the drug and placebo. However, the study population consisted of volunteers 65 and older with no or mild cognitive impairment and no use of cholinesterase-inhibiting drugs.
IN THE PIPELINEUrethral injection of muscle-derived cells may restore function
Usiene I, Kim YT, Pruchnic R, et al. Human muscle-derived cells injection increases leak point pressure in a nude rat model of stress urinary incontinence. Abstract presented at the annual meeting of the International Continence Society, August 28–September 2, 2005, Montreal, Quebec. Abstract #2.
Some exciting news: Dr. Michael Chancellor and colleagues at the University of Pittsburgh and at Cook MyoSite in Pittsburgh are working to bring stem cell research to the clinician’s office, with their studies of muscle-derived cells that can be injected into the urethra. (This technique is well-established and currently used for injection of synthetic or biologic material such as bovine collagen.)
What is remarkable about this type of injection is that the muscle cells not only stay put in the urethra, they appear to integrate into the muscle of the urethral sphincter and differentiate into cells that produce new muscle fibers. Newly functioning muscle improves urethral function and, ideally, will be able to restore continence in women with incontinence.
Technique’s success in rats
Could the same be accomplished with muscle-derived stem cells from humans? At the 2005 meeting of the International Continence Society, Chancellor and colleagues described how they injected human muscle-derived stem cells into the urethras of a nude rat model of stress incontinence (via nerve transection). In the injected rats, leak-point pressure measurements were restored to levels similar to those in a control group of rats.
In addition, immunohistochemistry and histology showed persistence of the human muscle-derived stem cells in the injected rats, versus periurethral muscle atrophy in the rats that had nerve transection but no injection.
Clinical testing underway
Clinical trials of this technology in women are now being performed in Toronto. It will be necessary to show safety and efficacy before the stem cell therapy is made available clinically, but there are a couple of factors in its favor:
- In terms of safety, the risks associated with the current crop of injectable materials should not be applicable, at least in theory. Because the injected stem cells are isolated and grown from the patient’s own biopsy, the chance of an adverse immunologic reaction should be zero, absent the rare mistake in labeling or transfer.
- The cell-injection technique is well-established and already used by many clinicians who perform transurethral or periurethral injection of bulking agents for women with incontinence. It will not be necessary to learn a new technique—just injectable material.

Tissue-engineered sling also in the works
Given that incontinence (and, presumably, the level of urethral damage resulting in some women may have urethral damage so severe as to preclude benefit from the injection of relatively small numbers of researchers is already developing a tissue-engineered sling in the hope that it can be used as a substitute for currently available synthetic or biologic sling materials.
The sling is being developed with the same muscle-derived stem cells, which are seeded onto a scaffold for as little as 2 weeks in a rat model. After the sling was surgically implanted using standard technique, urethral function improved to the level seen among controls.
It may be that the promise of stem cell therapy will come early to the treatment of urinary incontinence. Time will tell.
The author reports no financial relationships relevant to this article.
Although urinary incontinence is one of the most common chronic diseases in women, we still don’t understand its pathophysiology, and treatments have been, of necessity, empiric rather than directed at a specific cause. Fortunately, this bleak scenario may be changing, and I think that is the most exciting news about urinary incontinence in 2005.
Urethral deficiency by any name is still a deficiency
Ironically, the most basic description of urinary incontinence may be the most revealing: Incontinence occurs when the urethra cannot stay closed and fails to hold urine in the bladder, where it should be stored until the “right” time and place for emptying. This description applies equally well to women with stress or urge symptoms, but let’s focus on stress incontinence for now.
By that line of thinking, the urethra is deficient in all women with stress incontinence. I believe this to be true, despite the arbitrary label—intrinsic sphincter deficiency, or ISD—that we apply to women with only the most severe symptoms of stress incontinence.
Surgery does not end the quest
Because surgery focuses on eliminating symptoms, it should come as no surprise that incontinence procedures continue to proliferate while we search for the Holy Grail: the perfect surgery that will effectively and durably “fix” the problem without complications or side effects. However, unless we find and correct the underlying problem that gave rise to the incontinence in the first place, we are doomed to fail in our search.
Doom? Failure? Where is the exciting news I promised?
CARE trial underscores efficacy of Burch procedure
Brubaker L, for the Pelvic Floor Disorders Network. Burch colposuspension at the time of sacrocolpopexy in stress continent women reduces bothersome stress urinary symptoms: The CARE randomized trial. Abstract presented at the American Urogynecologic Society Meeting, September 15–17, 2005, Atlanta.
If you have an exceptional memory, you will recall that, in this Update on Urinary Incontinence last year, results from the CARE trial were promised in 2006. Good news! Early results are available now, at least a year before expected.
Superior results with Burch changed the course of the CARE study
The CARE trial (Colpopexy And Urinary Reduction Efforts) was designed to determine the effect of Burch versus no Burch in women without stress incontinence symptoms but with advanced prolapse who were undergoing abdominal sacrocolpopexy. The trial was sponsored by the National Institute of Child Health and Human Development (NICHD) and performed by the Pelvic Floor Disorders Network of investigators from 7 clinical sites and a central coordinating center.
The original sample size was set at 480 women, to be randomized equally to Burch or no Burch, with the primary stress outcome at 3 months after surgery. However, at the first interim analysis, when about half the sample (232 women) had reached the primary outcome, the results showed such a striking benefit in the Burch group that the Data and Safety Monitoring Board for the Pelvic Floor Disorders Network recommended that enrollment be halted while all women continued to receive scheduled follow-up. Therefore, in February 2005, enrollment in the trial was closed, with 322 women randomized to 1 of the 2 arms.
The following results were presented at the annual meeting of the American Urogynecologic Society in September:
- Stress incontinence symptoms were reduced by about half in women after abdominal sacrocolpopexy (from 44% in the no-Burch group to 24% in the Burch group).
- Stress symptom severity improved with Burch. More women (62%) in the no-Burch stress incontinence group were bothered by their symptoms, compared to 32% of women in the Burch group.
- Urge symptoms were no different with Burch. More surprising, women in both groups had similar levels of symptoms measured as the urge endpoint, which included urge incontinence, urgency, frequency, nocturia, or enuresis; or treatment for any of those 5 symptoms. Almost 33% of women in the Burch group met the urge endpoint, compared with 38% in the no-Burch group (difference not statistically significant).
- Serious adverse events were not significantly different between the 2 groups.
Many more questions will be addressed with further analysis of CARE trial data, such as results of urodynamic testing with and without prolapse reduction, and the potential for predicting which subgroup benefits most when Burch is performed. Long-term follow-up data will address durability of results related to incontinence and prolapse. Follow-up is scheduled for 2 years in the CARE trial, and for up to 10 years in the Extended-CARE trial.
Efficacy of anticholinergics
Trospium chloride (Sanctura): another anticholinergic for overactive bladder. The Medical Letter. 2004;46(1188):63–64.
Solifenacin and darifenacin for overactive bladder. The Medical Letter. 2005;47(1204):23–24.
Three more drugs for overactive bladder won FDA approval in 2004 and 2005:
- trospium chloride (Sanctura)
- solifenacin succinate Vesicare)
- darifenacin hydrobromide (Enablex)
According to The Medical Letter, none appears to offer an advantage over long-acting anticholinergics for overactive bladder. Despite the proliferation of anticholinergic drugs for overactive bladder symptoms—or perhaps because of it—one suspects that these medications are not achieving substantial, long-lasting relief of symptoms. One study reported that two thirds of women discontinued therapy within 4 months.1 A comprehensive review of placebo-controlled trials of anticholinergic drugs for overactive bladder estimated that, as a class, even long-acting agents have a very limited effect on symptoms, with approximately 1 fewer incontinent episode and 1 fewer voiding episode per 48 hours.2
REFERENCES
1. Salvatore S, Khullar V, Cardozo L, Milani R, Athanasiou S, Kelleher C. Long-term prospective randomized study comparing two different regimens of oxybutynin as a treatment for detrusor overactivity. Eur J Obstet Gynaecol Reprod Biol. 2005;119:237-241.
2. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of anticholinergic drugs compared with placebo in the treatment of overactive bladder: systematic review. BMJ. 2003;326:841-844.
Do anticholinergics and dementia drugs mix?
Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165:808–813.
Jewart RD, Green J, Lu CJ, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13:324–328.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173:493–498.
Treatment recommendations
As ObGyns become more active in evaluating and treating women with urinary incontinence, we must stay alert for potential adverse drug interactions.
Ideally, behavioral treatment (scheduled voiding, fluid management, bedside commode) and pelvic muscle training should be first-line therapies in elderly women with overactive bladder.
Anticholinergic drugs should be used with caution, if at all, in women taking cholinesterase-inhibiting drugs for dementia.
Elderly patients with overactive bladder are at high risk for drug interactions, especially involving cholinesterase-inhibiting drugs for the treatment of dementia, such as donepezil hydrochloride (Aricept). However, a newer Alzheimer drug, memantine hydrochloride (Namenda), works by a different mechanism and may be less likely to interact directly with anticholinergic drugs for incontinence.
3 studies involving the elderly
Cognitive impairment
Observing a population of older adults with dementia, about half treated with cholinesterase inhibitors for their Alzheimer symptoms, Gill et al found that the patients on cholinesterase inhibitors were more likely to start treatment with an anticholinergic drug for incontinence within a year. They theorized that the cholinesterase-inhibiting drugs possibly contribute to new-onset or worsening urinary incontinence, which in turn leads to treatment with anticholinergic agents.
Jewart and colleagues found better performance in patients with Alzheimer disease who were not taking anticholinergic medication for incontinence.
No or mild cognitive impairment
Lipton et al tested cognitive function with darifenacin for 2 weeks and found no difference between immediate- or controlled-release forms of the drug and placebo. However, the study population consisted of volunteers 65 and older with no or mild cognitive impairment and no use of cholinesterase-inhibiting drugs.
IN THE PIPELINEUrethral injection of muscle-derived cells may restore function
Usiene I, Kim YT, Pruchnic R, et al. Human muscle-derived cells injection increases leak point pressure in a nude rat model of stress urinary incontinence. Abstract presented at the annual meeting of the International Continence Society, August 28–September 2, 2005, Montreal, Quebec. Abstract #2.
Some exciting news: Dr. Michael Chancellor and colleagues at the University of Pittsburgh and at Cook MyoSite in Pittsburgh are working to bring stem cell research to the clinician’s office, with their studies of muscle-derived cells that can be injected into the urethra. (This technique is well-established and currently used for injection of synthetic or biologic material such as bovine collagen.)
What is remarkable about this type of injection is that the muscle cells not only stay put in the urethra, they appear to integrate into the muscle of the urethral sphincter and differentiate into cells that produce new muscle fibers. Newly functioning muscle improves urethral function and, ideally, will be able to restore continence in women with incontinence.
Technique’s success in rats
Could the same be accomplished with muscle-derived stem cells from humans? At the 2005 meeting of the International Continence Society, Chancellor and colleagues described how they injected human muscle-derived stem cells into the urethras of a nude rat model of stress incontinence (via nerve transection). In the injected rats, leak-point pressure measurements were restored to levels similar to those in a control group of rats.
In addition, immunohistochemistry and histology showed persistence of the human muscle-derived stem cells in the injected rats, versus periurethral muscle atrophy in the rats that had nerve transection but no injection.
Clinical testing underway
Clinical trials of this technology in women are now being performed in Toronto. It will be necessary to show safety and efficacy before the stem cell therapy is made available clinically, but there are a couple of factors in its favor:
- In terms of safety, the risks associated with the current crop of injectable materials should not be applicable, at least in theory. Because the injected stem cells are isolated and grown from the patient’s own biopsy, the chance of an adverse immunologic reaction should be zero, absent the rare mistake in labeling or transfer.
- The cell-injection technique is well-established and already used by many clinicians who perform transurethral or periurethral injection of bulking agents for women with incontinence. It will not be necessary to learn a new technique—just injectable material.

Tissue-engineered sling also in the works
Given that incontinence (and, presumably, the level of urethral damage resulting in some women may have urethral damage so severe as to preclude benefit from the injection of relatively small numbers of researchers is already developing a tissue-engineered sling in the hope that it can be used as a substitute for currently available synthetic or biologic sling materials.
The sling is being developed with the same muscle-derived stem cells, which are seeded onto a scaffold for as little as 2 weeks in a rat model. After the sling was surgically implanted using standard technique, urethral function improved to the level seen among controls.
It may be that the promise of stem cell therapy will come early to the treatment of urinary incontinence. Time will tell.
The author reports no financial relationships relevant to this article.
The 10 principles of practice efficiency
As reimbursement plummets and expenses rise, the options to improve your practice’s profits may seem more limited than ever—either you see more patients or spend less time with the ones you have. There is another option, which is often overlooked: Reduce the constraints on your time by improving your efficiency.
This article outlines 10 practical steps you can take to streamline your practice.
1. Manage the psychology of waiting
The link between patient wait times and practice efficiency may not be immediately apparent, but it does exist. When a patient feels you have kept her waiting unnecessarily, she may conclude that you have no respect for her or her time.
This perception can lead to situations in which you must spend time fielding complaints and making apologies and explanations in the exam room. Although such “service recovery” may consume only a minute of your time, it takes precious minutes away from your efficiency.
Keep patients informed about extended waits
Instruct your staff to inform patients of longer-than-usual waiting times when they first arrive at the office for their appointment. Maister and colleagues found that patients perceive waiting to be longer than it actually is when they are uninformed, uncomfortable, and unoccupied.
Update patients about delays every 15 minutes. Consider asking a member of your clinical team to address the issue if the wait exceeds 30 minutes. Explain lengthy delays, and give patients the choice of waiting or rescheduling. This attention to communication makes patients feel you value their time, too.
Waiting room comforts, personal touches, entertainment
Pay attention to comfort issues such as chairs, room temperature, and back-ground music. Review pre- and post-exam dressing protocols, and make sure to stock extra-large gowns for patients who may need them.
Make your waiting room a delight
Some creative but tasteful ideas to brighten the reception area include hanging artwork from your personal collection or from local artists, many of whom are happy to display their work in exchange for the exposure (place their business cards discreetly next to the work). Other ideas:
- Baby pictures of you and your staff
- Pictures of you at your hobby or with your family
- Interesting memorabilia
- Jigsaw or crossword puzzles, word searches, and other lap games (you can create your own using Web sites such as www.teach-nology.com)
- Personal computers with Internet access
- Free long-distance on available phones (restricted to domestic calls only)
- A diverse magazine collection for patients and their partners
- Pagers to allow patients to walk to nearby shops if delays are lengthy
- Notepads and envelopes for patients to write a letter or 2 (consider offering to stamp and mail completed letters)
- Brochures about services you offer
In short, comfort and entertain your patients, and you can turn the potentially negative situation of waiting into a delight.
2. Review charts in advance
A failure to review charts prior to seeing patients can limit your efficiency, add to your frustration, and give patients the impression that your practice is disorganized.
Tell your staff that chart preparation is a high priority. Require that all charts be previewed 1 or 2 days before patients are seen. Let staff know you expect them to include in the chart the results of tests you ordered at the last exam, communications from physicians to whom you referred the patient, and relevant operative reports.
Don’t overlook billing issues
In addition to the clinical review, an administrative review of a chart in advance of the visit can ensure that your efficiency and good care will be remunerated.
Assign a biller to review your charts for insurance verification and benefits eligibility. He or she also can note any accounts with outstanding balances, and evaluate and resolve any outstanding referral or authorization problems.
Check samples and supplies
As a corollary to chart preparation, assign a staff member to ensure that the pharmaceutical sample closet has adequate supplies for the following day, and review equipment and supplies to see what needs to be ordered.
Red-sticker the low inventory items. To make it easier for staff to spot low inventory, provide small red adhesive flags for them to stick on shelves (or remaining stock) when supplies run low. Appoint a staff member to make the rounds of exam rooms and closets to identify the flags, restock, and order any necessary supplies. Items should not be allowed to run out.
3. Ready the room
A well-prepared exam room means you can care for patients without unnecessary delays. Rooms should be cleaned between—not in front of—patients, and staff should ensure that supplies and equipment are readily available.
A quick and easy tip for equipment preparation is to draw an outline of the equipment in the base of the drawer, or hang a hook on the wall for each piece. That makes it easy to see at a glance whether something is missing—and you’ll always know where to look for the equipment.
If physicians change rooms (eg, at lunch), make sure that the staff knows to restock personalized items such as notepads and prescription pads.
4. Create a start-of-the-day checklist
Provide a written description of what you expect your staff to have accomplished by the start of the clinic. The list may instruct them to:
- Ensure cleanliness of the clinic
- Turn on computers, including those in exam rooms
- Contact the hospital for overnight admissions and cross-match with your schedule
- Review all schedules to anticipate problems
- Evaluate inventory of supplies and equipment in the clinic and each exam room
- Gather and organize incoming results from email and fax machines
- Ready equipment
Commit 5 minutes every morning to huddle with your staff, using your schedule as the agenda. At a minimum, ask your clinical assistant and scheduler to be present.
This huddle is not a meeting. It’s about setting the game plan for the day. Consider the analogy of a football team: The quarterback huddles with his teammates before every play. The team members have practiced the play and are prepared to execute it, but the opposing team creates some dynamics they need to plan for. Your day is similar: You and your team are prepared, but every patient poses a challenge to the routine plan. Predict and prepare for that challenge and you’re more apt to be efficient at managing it. Start huddles a few minutes before the clinic begins.
The huddle is your opportunity to anticipate problems—and solve them before they happen. Don’t let the day control you; predict problems and manage them proactively.
Let’s consider a couple of examples that are a daily occurrence in ObGyn offices, yet wreak havoc in practice efficiency:
The situation: Too few slots for acute needs
Several patients call at 8:15 AM with acute needs that must be handled today in the office. The scheduler has to guess where to direct them. You delivered Mrs. Smith’s baby at 2 AM this morning, yet she is still on your partner’s schedule for 9:30 AM today.
The game plan: The huddle reveals that Mrs. Smith won’t be presenting for her 40-week antepartum visit; 2 patients with acute needs can be scheduled during her now-open slot.
The situation: 3 patients with depression are scheduled for the same slot
Three patients, all in their 50s and all with a history of depression, were scheduled for annual well-woman visits at 10 AM. At 10:05, you’re looking for someone to blame for the scheduling mistake that will cost you—and the rest of your patients—dearly that day.
The game plan: Holding the huddle in advance of the clinic reveals that 3 patients were mistakenly scheduled at the same hour. Decisions are made about where and how to accommodate the patients elsewhere in the schedule. A decision is made to contact 1 or 2 of the patients immediately and ask them to reschedule.
6. Establish intake protocols
An efficient ObGyn knows that, when he or she walks into an exam room, the patient is ready to be seen. Intake protocols tend to vary with the style of practice, but it is wise to set minimum expectations for the following intake activities based on the patient’s chief complaint:
- Documentation of chief complaint and symptoms
- Position of patient; for example, seated or on the exam table
- Dress of patient
- Vital signs
- Date of last menstrual period
- Urine sample
- Standing orders for laboratory or other tests
- Current medications and refills needed
- History
Train staff to anticipate needs
Teach your clinical assistant to anticipate needs for each patient. For example, if a 50-year-old is presenting for her well-woman exam, your assistant should anticipate completing the administrative portions of the mammogram requisition form in advance of the exam.
The power of a simple introduction
Although it might seem the intuitive thing to do, make sure your clinical assistant introduces herself to the patient, provides an overview of her role, and briefly describes the course of the encounter. Thus informed, the patient will be less anxious about the few minutes she may spend waiting for you after your clinical assistant leaves the room.
7. Ask the patient about other concerns—first
Your first step with a patient should be introducing yourself (if she is new) or greeting her warmly (if she is an established patient). A handshake often is appropriate, or a gentle touch of the shoulder.
You might also consider including a place in your charts to record personal notes about the patient, such as a hobby or pet. Exhibit care and concern for your patients, and they will be relaxed and ready to begin the encounter.
To start the exam efficiently, and avoid having the patient withhold important questions until the end of the visit, direct the conversation as follows: “Ms. Jones, I see that you’re here because of painful cramps. Is there anything else that you would like for us to address today?”
If the patient raises an issue that can’t be managed in the time allotted for the visit, tell her the issue is so important you’ll need to schedule another visit in order to address it adequately.
Using the patient’s chief complaint and any other issues that have been raised as a starting point, commence the exam. Make eye contact with the patient whenever possible and keep her informed about your actions during the physical exam.
8. Do the documentation immediately
When the exam is completed, document the encounter. Consider dictating in front of your patient. This strategy can be advantageous because the patient will hear your advice repeated and will be able to provide any clarification needed. You can also document that the note was “dictated in the presence of the patient” this notation is a good way to reduce medicolegal risk. Finally, completing the record before you move on to the next patient means you won’t end up spending extra time at the end of the day recalling that encounter along with all the others.
9. Multitask between patients
Ask your clinical assistant to bring you any outstanding messages and test results so you can review them between patients. Processing work on a real-time basis means less work will be waiting for you at the end of the day. It will save staff time from constantly sorting and re-prioritizing an ever-higher number of messages and tasks accumulating as the day wears on.
The problem with batching
If you batch work until the end of the day, your staff is forced to constantly reorganize the workflow throughout the day, as well as manage all incoming communication from patients. If patients could be counted on to call only once and patiently wait for your response, batching would be more palatable. But when their messages remain unaddressed until the end of the day, chances are that some number of anxious patients will call back.
Batching these communications also increases the odds that your staff’s return calls will be missed. If it seems that your staff is in the phone room most of the day instead of helping you in the clinic, batched communications may be the culprit.
Performing tasks as time permits helps you avoid confronting a big stack of work at the end of the day. This stack is often left to the next day, which means that your team begins every day working in the past. They will try to handle at least some of those messages in the morning hours, which makes it very likely that today’s clinic will fall behind schedule before it even starts.
Avoid batching work by conscientiously reviewing the day’s work as it develops. Tell staff members what they can do to help manage the work, such as completing the administrative portions of Family and Medical Leave Act forms before passing them along to you. Order inked stamps that bear the information you find yourself writing repeatedly. Consider taking a speed-reading course to help you review documents efficiently and effectively.
10. Count your steps
Put a pedometer on your belt during your next clinic, and you’ll be amazed to discover how much walking you do. Although the exercise is wonderful, unnecessary steps reduce your efficiency.
Pay attention to where you walk in the exam room and why. If you have to walk to reach the trash can, put it where you can dispose of garbage without walking. If you have to walk to reach equipment, place those tools nearby. If you have to walk to your office to dictate, invest in a portable machine or a shelf in the hallway that can be used as a workstation.
Watch your steps; saving even 3 or 4 steps per patient will, over the course of the day, improve your efficiency.
Why efficiency matters
The operations of an ObGyn practice are undoubtedly complex; don’t let this complexity overwhelm your efforts to improve efficiency. Even small improvements can quickly add up to a major savings of time.
Efficiency means you can spend more time with each patient, see more patients, or just get home and enjoy some personal time.
The author reports no financial relationships relevant to this article.
Suggested Reading
Delio S. The Efficient Physician: 7 Guiding Principles for a Tech-Savvy Practice. Englewood, Colo: Medical Group Management Association; 2004.
Fitzsimmons JA, Fitzsimmons MJ. Service Management: Operations, Strategy, and Information Technology. New York: McGraw-Hill; 1998.
Goldratt EM, Cox J. The Goal. Great Barrington, Mass: North River Press; 1985.
Heskett JL, Sasser WE Jr, Schlesinger LA. The Service Profit Chain. New York: Free Press; 1997.
Maister DH. The psychology of waiting lines. In: Czepiel JA, Solomon MR, Suprenant CF, eds. The Service Encounter: Managing Employee-Customer Interaction in Service Businesses. Lanham, Md: Lexington Books; 1985. www.davidmaister.com.
Mozena JP, Black SC, Emerick CE. Stop Managing Costs. Milwaukee: American Society for Quality; 1999.
Nolan TW. Reducing Delays and Waiting Times Throughout the Healthcare System. Cambridge, Mass: Institute for Healthcare Improvement; 1996.
Womack JP, Jones DT. Lean Thinking. New York: Simon & Schuster; 1996.
Woodcock EW. Mastering Patient Flow: More Ideas to Increase Efficiency and Earnings. 2nd ed. Englewood, Colo: Medical Group Management Association; 2004.
Zaslove MO. The Successful Physician: A Productivity Handbook for Practitioners. New York: Aspen Publishers; April 1998.
As reimbursement plummets and expenses rise, the options to improve your practice’s profits may seem more limited than ever—either you see more patients or spend less time with the ones you have. There is another option, which is often overlooked: Reduce the constraints on your time by improving your efficiency.
This article outlines 10 practical steps you can take to streamline your practice.
1. Manage the psychology of waiting
The link between patient wait times and practice efficiency may not be immediately apparent, but it does exist. When a patient feels you have kept her waiting unnecessarily, she may conclude that you have no respect for her or her time.
This perception can lead to situations in which you must spend time fielding complaints and making apologies and explanations in the exam room. Although such “service recovery” may consume only a minute of your time, it takes precious minutes away from your efficiency.
Keep patients informed about extended waits
Instruct your staff to inform patients of longer-than-usual waiting times when they first arrive at the office for their appointment. Maister and colleagues found that patients perceive waiting to be longer than it actually is when they are uninformed, uncomfortable, and unoccupied.
Update patients about delays every 15 minutes. Consider asking a member of your clinical team to address the issue if the wait exceeds 30 minutes. Explain lengthy delays, and give patients the choice of waiting or rescheduling. This attention to communication makes patients feel you value their time, too.
Waiting room comforts, personal touches, entertainment
Pay attention to comfort issues such as chairs, room temperature, and back-ground music. Review pre- and post-exam dressing protocols, and make sure to stock extra-large gowns for patients who may need them.
Make your waiting room a delight
Some creative but tasteful ideas to brighten the reception area include hanging artwork from your personal collection or from local artists, many of whom are happy to display their work in exchange for the exposure (place their business cards discreetly next to the work). Other ideas:
- Baby pictures of you and your staff
- Pictures of you at your hobby or with your family
- Interesting memorabilia
- Jigsaw or crossword puzzles, word searches, and other lap games (you can create your own using Web sites such as www.teach-nology.com)
- Personal computers with Internet access
- Free long-distance on available phones (restricted to domestic calls only)
- A diverse magazine collection for patients and their partners
- Pagers to allow patients to walk to nearby shops if delays are lengthy
- Notepads and envelopes for patients to write a letter or 2 (consider offering to stamp and mail completed letters)
- Brochures about services you offer
In short, comfort and entertain your patients, and you can turn the potentially negative situation of waiting into a delight.
2. Review charts in advance
A failure to review charts prior to seeing patients can limit your efficiency, add to your frustration, and give patients the impression that your practice is disorganized.
Tell your staff that chart preparation is a high priority. Require that all charts be previewed 1 or 2 days before patients are seen. Let staff know you expect them to include in the chart the results of tests you ordered at the last exam, communications from physicians to whom you referred the patient, and relevant operative reports.
Don’t overlook billing issues
In addition to the clinical review, an administrative review of a chart in advance of the visit can ensure that your efficiency and good care will be remunerated.
Assign a biller to review your charts for insurance verification and benefits eligibility. He or she also can note any accounts with outstanding balances, and evaluate and resolve any outstanding referral or authorization problems.
Check samples and supplies
As a corollary to chart preparation, assign a staff member to ensure that the pharmaceutical sample closet has adequate supplies for the following day, and review equipment and supplies to see what needs to be ordered.
Red-sticker the low inventory items. To make it easier for staff to spot low inventory, provide small red adhesive flags for them to stick on shelves (or remaining stock) when supplies run low. Appoint a staff member to make the rounds of exam rooms and closets to identify the flags, restock, and order any necessary supplies. Items should not be allowed to run out.
3. Ready the room
A well-prepared exam room means you can care for patients without unnecessary delays. Rooms should be cleaned between—not in front of—patients, and staff should ensure that supplies and equipment are readily available.
A quick and easy tip for equipment preparation is to draw an outline of the equipment in the base of the drawer, or hang a hook on the wall for each piece. That makes it easy to see at a glance whether something is missing—and you’ll always know where to look for the equipment.
If physicians change rooms (eg, at lunch), make sure that the staff knows to restock personalized items such as notepads and prescription pads.
4. Create a start-of-the-day checklist
Provide a written description of what you expect your staff to have accomplished by the start of the clinic. The list may instruct them to:
- Ensure cleanliness of the clinic
- Turn on computers, including those in exam rooms
- Contact the hospital for overnight admissions and cross-match with your schedule
- Review all schedules to anticipate problems
- Evaluate inventory of supplies and equipment in the clinic and each exam room
- Gather and organize incoming results from email and fax machines
- Ready equipment
Commit 5 minutes every morning to huddle with your staff, using your schedule as the agenda. At a minimum, ask your clinical assistant and scheduler to be present.
This huddle is not a meeting. It’s about setting the game plan for the day. Consider the analogy of a football team: The quarterback huddles with his teammates before every play. The team members have practiced the play and are prepared to execute it, but the opposing team creates some dynamics they need to plan for. Your day is similar: You and your team are prepared, but every patient poses a challenge to the routine plan. Predict and prepare for that challenge and you’re more apt to be efficient at managing it. Start huddles a few minutes before the clinic begins.
The huddle is your opportunity to anticipate problems—and solve them before they happen. Don’t let the day control you; predict problems and manage them proactively.
Let’s consider a couple of examples that are a daily occurrence in ObGyn offices, yet wreak havoc in practice efficiency:
The situation: Too few slots for acute needs
Several patients call at 8:15 AM with acute needs that must be handled today in the office. The scheduler has to guess where to direct them. You delivered Mrs. Smith’s baby at 2 AM this morning, yet she is still on your partner’s schedule for 9:30 AM today.
The game plan: The huddle reveals that Mrs. Smith won’t be presenting for her 40-week antepartum visit; 2 patients with acute needs can be scheduled during her now-open slot.
The situation: 3 patients with depression are scheduled for the same slot
Three patients, all in their 50s and all with a history of depression, were scheduled for annual well-woman visits at 10 AM. At 10:05, you’re looking for someone to blame for the scheduling mistake that will cost you—and the rest of your patients—dearly that day.
The game plan: Holding the huddle in advance of the clinic reveals that 3 patients were mistakenly scheduled at the same hour. Decisions are made about where and how to accommodate the patients elsewhere in the schedule. A decision is made to contact 1 or 2 of the patients immediately and ask them to reschedule.
6. Establish intake protocols
An efficient ObGyn knows that, when he or she walks into an exam room, the patient is ready to be seen. Intake protocols tend to vary with the style of practice, but it is wise to set minimum expectations for the following intake activities based on the patient’s chief complaint:
- Documentation of chief complaint and symptoms
- Position of patient; for example, seated or on the exam table
- Dress of patient
- Vital signs
- Date of last menstrual period
- Urine sample
- Standing orders for laboratory or other tests
- Current medications and refills needed
- History
Train staff to anticipate needs
Teach your clinical assistant to anticipate needs for each patient. For example, if a 50-year-old is presenting for her well-woman exam, your assistant should anticipate completing the administrative portions of the mammogram requisition form in advance of the exam.
The power of a simple introduction
Although it might seem the intuitive thing to do, make sure your clinical assistant introduces herself to the patient, provides an overview of her role, and briefly describes the course of the encounter. Thus informed, the patient will be less anxious about the few minutes she may spend waiting for you after your clinical assistant leaves the room.
7. Ask the patient about other concerns—first
Your first step with a patient should be introducing yourself (if she is new) or greeting her warmly (if she is an established patient). A handshake often is appropriate, or a gentle touch of the shoulder.
You might also consider including a place in your charts to record personal notes about the patient, such as a hobby or pet. Exhibit care and concern for your patients, and they will be relaxed and ready to begin the encounter.
To start the exam efficiently, and avoid having the patient withhold important questions until the end of the visit, direct the conversation as follows: “Ms. Jones, I see that you’re here because of painful cramps. Is there anything else that you would like for us to address today?”
If the patient raises an issue that can’t be managed in the time allotted for the visit, tell her the issue is so important you’ll need to schedule another visit in order to address it adequately.
Using the patient’s chief complaint and any other issues that have been raised as a starting point, commence the exam. Make eye contact with the patient whenever possible and keep her informed about your actions during the physical exam.
8. Do the documentation immediately
When the exam is completed, document the encounter. Consider dictating in front of your patient. This strategy can be advantageous because the patient will hear your advice repeated and will be able to provide any clarification needed. You can also document that the note was “dictated in the presence of the patient” this notation is a good way to reduce medicolegal risk. Finally, completing the record before you move on to the next patient means you won’t end up spending extra time at the end of the day recalling that encounter along with all the others.
9. Multitask between patients
Ask your clinical assistant to bring you any outstanding messages and test results so you can review them between patients. Processing work on a real-time basis means less work will be waiting for you at the end of the day. It will save staff time from constantly sorting and re-prioritizing an ever-higher number of messages and tasks accumulating as the day wears on.
The problem with batching
If you batch work until the end of the day, your staff is forced to constantly reorganize the workflow throughout the day, as well as manage all incoming communication from patients. If patients could be counted on to call only once and patiently wait for your response, batching would be more palatable. But when their messages remain unaddressed until the end of the day, chances are that some number of anxious patients will call back.
Batching these communications also increases the odds that your staff’s return calls will be missed. If it seems that your staff is in the phone room most of the day instead of helping you in the clinic, batched communications may be the culprit.
Performing tasks as time permits helps you avoid confronting a big stack of work at the end of the day. This stack is often left to the next day, which means that your team begins every day working in the past. They will try to handle at least some of those messages in the morning hours, which makes it very likely that today’s clinic will fall behind schedule before it even starts.
Avoid batching work by conscientiously reviewing the day’s work as it develops. Tell staff members what they can do to help manage the work, such as completing the administrative portions of Family and Medical Leave Act forms before passing them along to you. Order inked stamps that bear the information you find yourself writing repeatedly. Consider taking a speed-reading course to help you review documents efficiently and effectively.
10. Count your steps
Put a pedometer on your belt during your next clinic, and you’ll be amazed to discover how much walking you do. Although the exercise is wonderful, unnecessary steps reduce your efficiency.
Pay attention to where you walk in the exam room and why. If you have to walk to reach the trash can, put it where you can dispose of garbage without walking. If you have to walk to reach equipment, place those tools nearby. If you have to walk to your office to dictate, invest in a portable machine or a shelf in the hallway that can be used as a workstation.
Watch your steps; saving even 3 or 4 steps per patient will, over the course of the day, improve your efficiency.
Why efficiency matters
The operations of an ObGyn practice are undoubtedly complex; don’t let this complexity overwhelm your efforts to improve efficiency. Even small improvements can quickly add up to a major savings of time.
Efficiency means you can spend more time with each patient, see more patients, or just get home and enjoy some personal time.
The author reports no financial relationships relevant to this article.
Suggested Reading
Delio S. The Efficient Physician: 7 Guiding Principles for a Tech-Savvy Practice. Englewood, Colo: Medical Group Management Association; 2004.
Fitzsimmons JA, Fitzsimmons MJ. Service Management: Operations, Strategy, and Information Technology. New York: McGraw-Hill; 1998.
Goldratt EM, Cox J. The Goal. Great Barrington, Mass: North River Press; 1985.
Heskett JL, Sasser WE Jr, Schlesinger LA. The Service Profit Chain. New York: Free Press; 1997.
Maister DH. The psychology of waiting lines. In: Czepiel JA, Solomon MR, Suprenant CF, eds. The Service Encounter: Managing Employee-Customer Interaction in Service Businesses. Lanham, Md: Lexington Books; 1985. www.davidmaister.com.
Mozena JP, Black SC, Emerick CE. Stop Managing Costs. Milwaukee: American Society for Quality; 1999.
Nolan TW. Reducing Delays and Waiting Times Throughout the Healthcare System. Cambridge, Mass: Institute for Healthcare Improvement; 1996.
Womack JP, Jones DT. Lean Thinking. New York: Simon & Schuster; 1996.
Woodcock EW. Mastering Patient Flow: More Ideas to Increase Efficiency and Earnings. 2nd ed. Englewood, Colo: Medical Group Management Association; 2004.
Zaslove MO. The Successful Physician: A Productivity Handbook for Practitioners. New York: Aspen Publishers; April 1998.
As reimbursement plummets and expenses rise, the options to improve your practice’s profits may seem more limited than ever—either you see more patients or spend less time with the ones you have. There is another option, which is often overlooked: Reduce the constraints on your time by improving your efficiency.
This article outlines 10 practical steps you can take to streamline your practice.
1. Manage the psychology of waiting
The link between patient wait times and practice efficiency may not be immediately apparent, but it does exist. When a patient feels you have kept her waiting unnecessarily, she may conclude that you have no respect for her or her time.
This perception can lead to situations in which you must spend time fielding complaints and making apologies and explanations in the exam room. Although such “service recovery” may consume only a minute of your time, it takes precious minutes away from your efficiency.
Keep patients informed about extended waits
Instruct your staff to inform patients of longer-than-usual waiting times when they first arrive at the office for their appointment. Maister and colleagues found that patients perceive waiting to be longer than it actually is when they are uninformed, uncomfortable, and unoccupied.
Update patients about delays every 15 minutes. Consider asking a member of your clinical team to address the issue if the wait exceeds 30 minutes. Explain lengthy delays, and give patients the choice of waiting or rescheduling. This attention to communication makes patients feel you value their time, too.
Waiting room comforts, personal touches, entertainment
Pay attention to comfort issues such as chairs, room temperature, and back-ground music. Review pre- and post-exam dressing protocols, and make sure to stock extra-large gowns for patients who may need them.
Make your waiting room a delight
Some creative but tasteful ideas to brighten the reception area include hanging artwork from your personal collection or from local artists, many of whom are happy to display their work in exchange for the exposure (place their business cards discreetly next to the work). Other ideas:
- Baby pictures of you and your staff
- Pictures of you at your hobby or with your family
- Interesting memorabilia
- Jigsaw or crossword puzzles, word searches, and other lap games (you can create your own using Web sites such as www.teach-nology.com)
- Personal computers with Internet access
- Free long-distance on available phones (restricted to domestic calls only)
- A diverse magazine collection for patients and their partners
- Pagers to allow patients to walk to nearby shops if delays are lengthy
- Notepads and envelopes for patients to write a letter or 2 (consider offering to stamp and mail completed letters)
- Brochures about services you offer
In short, comfort and entertain your patients, and you can turn the potentially negative situation of waiting into a delight.
2. Review charts in advance
A failure to review charts prior to seeing patients can limit your efficiency, add to your frustration, and give patients the impression that your practice is disorganized.
Tell your staff that chart preparation is a high priority. Require that all charts be previewed 1 or 2 days before patients are seen. Let staff know you expect them to include in the chart the results of tests you ordered at the last exam, communications from physicians to whom you referred the patient, and relevant operative reports.
Don’t overlook billing issues
In addition to the clinical review, an administrative review of a chart in advance of the visit can ensure that your efficiency and good care will be remunerated.
Assign a biller to review your charts for insurance verification and benefits eligibility. He or she also can note any accounts with outstanding balances, and evaluate and resolve any outstanding referral or authorization problems.
Check samples and supplies
As a corollary to chart preparation, assign a staff member to ensure that the pharmaceutical sample closet has adequate supplies for the following day, and review equipment and supplies to see what needs to be ordered.
Red-sticker the low inventory items. To make it easier for staff to spot low inventory, provide small red adhesive flags for them to stick on shelves (or remaining stock) when supplies run low. Appoint a staff member to make the rounds of exam rooms and closets to identify the flags, restock, and order any necessary supplies. Items should not be allowed to run out.
3. Ready the room
A well-prepared exam room means you can care for patients without unnecessary delays. Rooms should be cleaned between—not in front of—patients, and staff should ensure that supplies and equipment are readily available.
A quick and easy tip for equipment preparation is to draw an outline of the equipment in the base of the drawer, or hang a hook on the wall for each piece. That makes it easy to see at a glance whether something is missing—and you’ll always know where to look for the equipment.
If physicians change rooms (eg, at lunch), make sure that the staff knows to restock personalized items such as notepads and prescription pads.
4. Create a start-of-the-day checklist
Provide a written description of what you expect your staff to have accomplished by the start of the clinic. The list may instruct them to:
- Ensure cleanliness of the clinic
- Turn on computers, including those in exam rooms
- Contact the hospital for overnight admissions and cross-match with your schedule
- Review all schedules to anticipate problems
- Evaluate inventory of supplies and equipment in the clinic and each exam room
- Gather and organize incoming results from email and fax machines
- Ready equipment
Commit 5 minutes every morning to huddle with your staff, using your schedule as the agenda. At a minimum, ask your clinical assistant and scheduler to be present.
This huddle is not a meeting. It’s about setting the game plan for the day. Consider the analogy of a football team: The quarterback huddles with his teammates before every play. The team members have practiced the play and are prepared to execute it, but the opposing team creates some dynamics they need to plan for. Your day is similar: You and your team are prepared, but every patient poses a challenge to the routine plan. Predict and prepare for that challenge and you’re more apt to be efficient at managing it. Start huddles a few minutes before the clinic begins.
The huddle is your opportunity to anticipate problems—and solve them before they happen. Don’t let the day control you; predict problems and manage them proactively.
Let’s consider a couple of examples that are a daily occurrence in ObGyn offices, yet wreak havoc in practice efficiency:
The situation: Too few slots for acute needs
Several patients call at 8:15 AM with acute needs that must be handled today in the office. The scheduler has to guess where to direct them. You delivered Mrs. Smith’s baby at 2 AM this morning, yet she is still on your partner’s schedule for 9:30 AM today.
The game plan: The huddle reveals that Mrs. Smith won’t be presenting for her 40-week antepartum visit; 2 patients with acute needs can be scheduled during her now-open slot.
The situation: 3 patients with depression are scheduled for the same slot
Three patients, all in their 50s and all with a history of depression, were scheduled for annual well-woman visits at 10 AM. At 10:05, you’re looking for someone to blame for the scheduling mistake that will cost you—and the rest of your patients—dearly that day.
The game plan: Holding the huddle in advance of the clinic reveals that 3 patients were mistakenly scheduled at the same hour. Decisions are made about where and how to accommodate the patients elsewhere in the schedule. A decision is made to contact 1 or 2 of the patients immediately and ask them to reschedule.
6. Establish intake protocols
An efficient ObGyn knows that, when he or she walks into an exam room, the patient is ready to be seen. Intake protocols tend to vary with the style of practice, but it is wise to set minimum expectations for the following intake activities based on the patient’s chief complaint:
- Documentation of chief complaint and symptoms
- Position of patient; for example, seated or on the exam table
- Dress of patient
- Vital signs
- Date of last menstrual period
- Urine sample
- Standing orders for laboratory or other tests
- Current medications and refills needed
- History
Train staff to anticipate needs
Teach your clinical assistant to anticipate needs for each patient. For example, if a 50-year-old is presenting for her well-woman exam, your assistant should anticipate completing the administrative portions of the mammogram requisition form in advance of the exam.
The power of a simple introduction
Although it might seem the intuitive thing to do, make sure your clinical assistant introduces herself to the patient, provides an overview of her role, and briefly describes the course of the encounter. Thus informed, the patient will be less anxious about the few minutes she may spend waiting for you after your clinical assistant leaves the room.
7. Ask the patient about other concerns—first
Your first step with a patient should be introducing yourself (if she is new) or greeting her warmly (if she is an established patient). A handshake often is appropriate, or a gentle touch of the shoulder.
You might also consider including a place in your charts to record personal notes about the patient, such as a hobby or pet. Exhibit care and concern for your patients, and they will be relaxed and ready to begin the encounter.
To start the exam efficiently, and avoid having the patient withhold important questions until the end of the visit, direct the conversation as follows: “Ms. Jones, I see that you’re here because of painful cramps. Is there anything else that you would like for us to address today?”
If the patient raises an issue that can’t be managed in the time allotted for the visit, tell her the issue is so important you’ll need to schedule another visit in order to address it adequately.
Using the patient’s chief complaint and any other issues that have been raised as a starting point, commence the exam. Make eye contact with the patient whenever possible and keep her informed about your actions during the physical exam.
8. Do the documentation immediately
When the exam is completed, document the encounter. Consider dictating in front of your patient. This strategy can be advantageous because the patient will hear your advice repeated and will be able to provide any clarification needed. You can also document that the note was “dictated in the presence of the patient” this notation is a good way to reduce medicolegal risk. Finally, completing the record before you move on to the next patient means you won’t end up spending extra time at the end of the day recalling that encounter along with all the others.
9. Multitask between patients
Ask your clinical assistant to bring you any outstanding messages and test results so you can review them between patients. Processing work on a real-time basis means less work will be waiting for you at the end of the day. It will save staff time from constantly sorting and re-prioritizing an ever-higher number of messages and tasks accumulating as the day wears on.
The problem with batching
If you batch work until the end of the day, your staff is forced to constantly reorganize the workflow throughout the day, as well as manage all incoming communication from patients. If patients could be counted on to call only once and patiently wait for your response, batching would be more palatable. But when their messages remain unaddressed until the end of the day, chances are that some number of anxious patients will call back.
Batching these communications also increases the odds that your staff’s return calls will be missed. If it seems that your staff is in the phone room most of the day instead of helping you in the clinic, batched communications may be the culprit.
Performing tasks as time permits helps you avoid confronting a big stack of work at the end of the day. This stack is often left to the next day, which means that your team begins every day working in the past. They will try to handle at least some of those messages in the morning hours, which makes it very likely that today’s clinic will fall behind schedule before it even starts.
Avoid batching work by conscientiously reviewing the day’s work as it develops. Tell staff members what they can do to help manage the work, such as completing the administrative portions of Family and Medical Leave Act forms before passing them along to you. Order inked stamps that bear the information you find yourself writing repeatedly. Consider taking a speed-reading course to help you review documents efficiently and effectively.
10. Count your steps
Put a pedometer on your belt during your next clinic, and you’ll be amazed to discover how much walking you do. Although the exercise is wonderful, unnecessary steps reduce your efficiency.
Pay attention to where you walk in the exam room and why. If you have to walk to reach the trash can, put it where you can dispose of garbage without walking. If you have to walk to reach equipment, place those tools nearby. If you have to walk to your office to dictate, invest in a portable machine or a shelf in the hallway that can be used as a workstation.
Watch your steps; saving even 3 or 4 steps per patient will, over the course of the day, improve your efficiency.
Why efficiency matters
The operations of an ObGyn practice are undoubtedly complex; don’t let this complexity overwhelm your efforts to improve efficiency. Even small improvements can quickly add up to a major savings of time.
Efficiency means you can spend more time with each patient, see more patients, or just get home and enjoy some personal time.
The author reports no financial relationships relevant to this article.
Suggested Reading
Delio S. The Efficient Physician: 7 Guiding Principles for a Tech-Savvy Practice. Englewood, Colo: Medical Group Management Association; 2004.
Fitzsimmons JA, Fitzsimmons MJ. Service Management: Operations, Strategy, and Information Technology. New York: McGraw-Hill; 1998.
Goldratt EM, Cox J. The Goal. Great Barrington, Mass: North River Press; 1985.
Heskett JL, Sasser WE Jr, Schlesinger LA. The Service Profit Chain. New York: Free Press; 1997.
Maister DH. The psychology of waiting lines. In: Czepiel JA, Solomon MR, Suprenant CF, eds. The Service Encounter: Managing Employee-Customer Interaction in Service Businesses. Lanham, Md: Lexington Books; 1985. www.davidmaister.com.
Mozena JP, Black SC, Emerick CE. Stop Managing Costs. Milwaukee: American Society for Quality; 1999.
Nolan TW. Reducing Delays and Waiting Times Throughout the Healthcare System. Cambridge, Mass: Institute for Healthcare Improvement; 1996.
Womack JP, Jones DT. Lean Thinking. New York: Simon & Schuster; 1996.
Woodcock EW. Mastering Patient Flow: More Ideas to Increase Efficiency and Earnings. 2nd ed. Englewood, Colo: Medical Group Management Association; 2004.
Zaslove MO. The Successful Physician: A Productivity Handbook for Practitioners. New York: Aspen Publishers; April 1998.
New choices in prenatal screening for Down syndrome
CASE-BASED LEARNING
Her risk is high, but how high? Is invasive testing the only answer?
Mrs. S, a 37-year-old primigravida, has an age-related risk of having a baby with Down syndrome of 1 in 250. Before deciding whether to undergo an invasive diagnostic procedure based on her age alone, she wants to learn more about her risk by having a prenatal screening test. The 2 safest and most informative options: first-trimester combined screening and fully integrated screening. About 5% of women who have the first test are found to be high-risk, and the test identifies about 85% of all cases of Down syndrome. As for fully integrated screening, it identifies about 85% of all Down syndrome cases at a 1% screen-positive rate. Since it offers a faster result, Mrs. S opts for first-trimester screening.
At 11 weeks, 0 days (according to crown-rump length), she undergoes nuchal translucency imaging and has a blood sample drawn to measure pregnancy-associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG). Her test results are reported in multiples of the median (MoM):
| Nuchal translucency | 2.3 mm | 1.62 MoM |
| PAPP-A | 0.5 mIU/mL | 0.79 MoM |
| hCG | 45 IU/mL | 1.25 MoM |
These values suggest that Mrs. S has a risk of having a pregnancy affected by Down syndrome of 1 in 170. In other words, her results are screen-positive.
Can her risk be more precisely quantified without invasive testing?
Had Mrs. S chosen fully integrated screening, the answer to that question would be yes, but it would have meant waiting until the second trimester for the result.
This article describes the 2 screening approaches—first-trimester combined screening and fully integrated screening—as well as the serum-only variant of the integrated test and the established quad marker test. Also discussed are the findings of recent studies, including 2 key trials:
- the First and Second Trimester Evaluation of Risk (FASTER) of aneuploidy trial, published in November1
- the Serum, Urine, and Ultrasound Screening Study (SURUSS), published in 2003 in the United Kingdom2
These 2 trials are the only ones to compare screening markers at different times during pregnancy in the same women—the only way to fairly assess the quality of various marker combinations within and across gestational weeks.
How good is current practice?
In 1995, about 2.5 million of the approximately 4 million gravidas in the United States had maternal serum screening for Down syndrome and open neural tube defects.3 Today, this practice usually involves a serum sample drawn early during the second trimester (15–20 weeks), measurement of 3 or 4 serum markers (the triple or quad test), and calculation and reporting of risk.
Second-trimester serum screening is a relatively easy procedure involving a single blood sample and established risk-calculation methods. Further, the follow-up when a woman is screen-positive—ie, at increased risk—is clear: amniocentesis in the case of Down syndrome risk and targeted ultrasound in the case of open neural tube defects (with amniocentesis backup). For the triple test, we can expect a detection rate of about 70%, and for the quad test, 80%, by identifying 5% of screened women with the highest calculated risk (the effective screen-positive rate).1,2,4
Why the newer options are better
Optimally, prenatal screening should minimize the number of women identified as screen-positive (ie, women at sufficient risk to be offered amniocentesis or comparable procedures) while maximizing the overall detection rate. This point is important because screen-positive status leads to follow-up diagnostic procedures that are necessarily invasive and risky.
First-trimester screening slightly better than the quad test
The option Mrs. S selected entails measuring 3 markers during the late first trimester (11–13 gestational weeks): nuchal translucency, PAPP-A, and hCG or its free βsubunit. These markers constitute a screening test that performs as well as, or slightly better than, the second-trimester quad test. The best estimate of first-trimester screening is an 85% detection rate at a 5% screen-positive rate (compared with about 80% detection rate at a 5% screen-positive rate for the quad test).1,2,5,6
Serum markers or ultrasound alone not enough in first trimester
Two serum markers together, without nuchal translucency, or nuchal translucency alone, without the serum markers, do not constitute a sufficient first-trimester screening test, since they each detect about 60% to 65% of Down syndrome cases with a 5% false-positive rate. This is clearly inferior to the best we can do during the second trimester (about 80% detection rate for a 5% false-positive rate). Only when nuchal translucency and serum markers are used together is first-trimester screening a viable option.
Timing is important in integrated screening
For the integrated screening option, instead of requiring that screening be offered in the late first or early second trimester, each marker is measured when it is most informative. The optimal time for nuchal translucency and PAPP-A measurement is at 10 to 11 weeks, while the optimal time for the measurement of hCG (or its free β subunit), inhibin A, alpha fetoprotein (AFP), and unconjugated estriol (uE3) is at 15 to 20 weeks.
Therefore, the integrated test is accomplished in 2 steps. At about 11 weeks, a woman undergoes nuchal translucency ultrasound imaging and has a blood sample drawn for PAPP-A measurement. At about 15 weeks (the earlier in the second-trimester window the better), she has a second sample drawn for measurement of the quad markers. A risk report then is generated, using all 6 markers to calculate the woman’s new risk. Such a test has to be superior to any test that uses fewer markers or the same markers at less than the optimal time.
The integrated test can also be carried out without nuchal translucency, by measuring PAPP-A during the first trimester and the quad serum markers during the second trimester, for an estimated detection rate of 85% with a 5% false-positive rate.
Integrated option has 1% screen-positive rate
Integrated screening reduces the screen-positive rate by as much as fourfold—to 1% or less. That is, only 1 in 100 women undergoing screening will be called screen-positive, and, in that 1%, approximately 85% of all Down syndrome pregnancies will be found.1,2,7
First-trimester serum markers
The most informative serum marker during the first trimester is PAPP-A, a large glycoprotein complex made by the placenta. In pregnancies affected by Down syndrome, PAPP-A levels tend to be low: about 0.4 MoM on average, or about 2.5 times lower than in unaffected pregnancies.
The second most commonly used serum marker is the free βsubunit of hCG, which is, on average, 1.8 MoM in pregnancies affected by Down syndrome, or almost twice as high as in normal pregnancies.4,8 Studies indicate that hCG and inhibin A are also effective serum markers during the late first trimester, providing screening performance equivalent to that of the free β-hCG when combined with nuchal translucency and PAPP-A.4
Nuchal translucency: A powerful marker
Both the fully integrated and first-trimester screening approaches necessitate ultrasound measurement of nuchal translucency, which is always measured along with fetal crown-rump length. The nuchal translucency value—initially measured in tenths of millimeters—then is normalized for gestational age based on crown-rump length, and reported in multiples of the median, the same unit used to normalize serum screening markers.
FIGURE 1 shows how nuchal translucency values (in millimeters) measured in a general population increase with gestation. The most commonly accepted period of gestation to measure nuchal translucency is between 10 and 13 completed weeks.
Why nuchal translucency is more informative
Nuchal translucency values tend to be increased in Down syndrome pregnancies, as are certain serum markers such as hCG or its free β subunit and inhibin A. However, nuchal translucency is more informative than these markers because there is less overlap between Down syndrome and unaffected case values. This is not because nuchal translucency values tend to be higher in affected pregnancies. In fact, all 3 markers are, on average, about twice as high in cases of Down syndrome as in controls. However, because the distribution of nuchal translucency values in unaffected fetuses is much narrower (or tighter) than is true for hCG (or its free β subunit) or inhibin A, very few unaffected fetuses have increased nuchal translucency values. Therefore, when nuchal translucency is elevated, it is more likely to be associated with an affected pregnancy than is either of the other 2 markers.
FIGURE 2 shows the overlapping distributions in cases and controls for both hCG and nuchal translucency. In unaffected pregnancies, the distribution of values centers around 1 MoM, while in Down syndrome pregnancies, the values center around 2 MoM. About 8% of hCG values in unaffected pregnancies exceed 2 MoM, but only about 1.5% of nuchal translucency values do. Thus, for a detection rate of 50%, the false-positive rate using nuchal translucency is 1.5%, much smaller than the false-positive rate of 8% using hCG.
Imaging expertise is key
While it is beyond the scope of this article to detail the methodology of nuchal translucency measurement, specialized training and ongoing quality assurance are necessary to get the measurement right. Both the Society for Maternal-Fetal Medicine in the United States and the Fetal Medicine Foundation in the United Kingdom provide training and credentialing in nuchal translucency sonography. If performed correctly, it is an excellent screening marker. However, if attempted with no hands-on training, this imaging method yields unreliable results.
FIGURE 1 Nuchal translucency values increase with gestational age
Nuchal translucency values, in millimeters, in 561 pregnancies between 10 and 13 completed weeks of gestation, as estimated by crown-rump length. Reprinted with permission from Drs. Wald and Schucter; data from Schuchter K, et al.9
FIGURE 2 Very few unaffected fetuses have elevated nuchal translucency values
Distribution of values of second-trimester human chorionic gonadotropin (hCG) and first-trimester nuchal translucency among unaffected and Down syndrome pregnancies, both given in multiples of the median (MoM). Note that the unaffected nuchal translucency distribution is much narrower and taller than the unaffected hCG distribution. The scale is a log progression of increasing MoM values because both markers are log-normally distributed in unaffected and Down syndrome gestations. DR=detection rate; FPR=false-positive rate; NT=nuchal translucency.
2 important studies
The SURUSS trial, conducted mainly in the United Kingdom, was an observational study in which all women underwent first-trimester ultrasound measurement of nuchal translucency, as well as first- and second-trimester blood and urine sampling, with all samples stored. After all outcomes were chronicled, case-control sets of first- and second-trimester samples were constructed and assayed for a wide variety of known and potential screening markers. Nuchal translucency data also were analyzed. More than 48,000 women were enrolled, and 101 Down syndrome pregnancies were identified and studied. 2,10
The FASTER trial, completed more recently, was an observational study conducted at 15 enrollment centers in the US and involving more than 38,000 women, among whom 117 Down syndrome cases were identified.1 All women in the trial underwent first-trimester ultrasound examination and blood sampling between 10 weeks 3 days, and 13 weeks 6 days, and were asked to return for a second-trimester blood draw between 15 and 18 completed weeks, after which a report was issued detailing the separate results. In addition, combinations of markers across the trimesters were modeled and compared.
Perhaps the most interesting and surprising finding from the 2 studies is the remarkable similarity of results (TABLE). For 2 large populations on separate continents with a different ethnic mix, the primary findings were almost identical:
- 86% to 87% detection rate at a 5% false-positive rate for first-trimester combined screening (nuchal translucency, PAPP-A, and free β-hCG) and 80% to 83% detection rate at a 5% false-positive rate for second-trimester quad screening (AFP, uE3 , hCG, and inhibin A). In both trials, first-trimester screening was incrementally better than second-trimester screening, but the difference was not statistically significant.
- For integrated screening, a detection rate of 86% to 88% at a false-positive rate of 1%. Nuchal translucency and PAPP-A were measured during the first trimester, and quad markers during the second trimester. At a 5% false-positive rate, the detection rate in both trials was about 95%. With a serum-only integrated test, detection rates were 87% to 88% with a 5% false-positive rate.
- Nuchal translucency was more informative than any of the serum markers tested. This corroborates the rich literature on nuchal translucency published over the past decade. Both trials demonstrated that nuchal translucency measurement is effective when training is adequate, and that ongoing monitoring of quality is essential. However, in both studies, a satisfactory nuchal translucency measurement was not attained in about 7% of all women scanned between 10 and 13 weeks, so a small but significant number of women will not have nuchal translucency included in their risk assessment.
Other notable FASTER findings
Other findings from the FASTER trial that merit special attention:
- An ultrasound finding of cystic hygroma warrants an immediate prenatal diagnostic workup and was associated with an aneuploidy rate of 50% (one third of which was Down syndrome) and adverse outcomes in the great majority of cases.11 Cystic hygroma is uncommon, occurring in about 1 in 300 pregnancies in the first trimester.
- The value of the fetal nasal bone as a first-trimester ultrasound marker is unclear. In the FASTER trial, the nasal bone was studied in about 6,000 of the 38,000 ultrasound examinations, with no detected benefit. Thus, in a nonselected pregnant population, the nasal bone may not be a reasonable marker.12
- Various first- and second-trimester markers are modestly informative about adverse pregnancy outcomes other than aneuploidy (eg, fetal growth restriction, early delivery, and preeclampsia). Only a small proportion of affected pregnancies will be identified by these markers, alone or in combination.13,14
TABLE
5 screening approaches: What the SURUSS and FASTER trials reveal
| TEST | NUCHAL TRANSLUCENCY MEASURED? | METHOD OF PRENATAL DIAGNOSIS | DETECTION RATE (%) AT 1% FALSE-POSITIVE RATE | DETECTION RATE (%) AT 5% FALSE-POSITIVE RATE | FALSE-POSITIVE RATE* (%) TO ACHIEVE: | |||
|---|---|---|---|---|---|---|---|---|
| SURUSS | FASTER | SURUSS | FASTER | 85% DETECTION | 95% DETECTION | |||
| 2nd-trimester triple marker | No | 2nd-trimester amniocentesis | 56 | 45 | 77 | 70 | 14 | 32 |
| 2nd-trimester quad marker | No | 2nd-trimester amniocentesis | 64 | 60 | 83 | 80 | 7.3 | 22 |
| 1st-trimester combined | Yes | 1st-trimester chorionic villus sampling | 72 | 73 | 86 | 87 | 3.8 | 18 |
| Serum integrated test | No | 2nd-trimester amniocentesis | 73 | 73 | 87 | 88 | 3.6 | 15 |
| Full integrated test | Yes | 2nd-trimester amniocentesis | 86 | 88 | 94 | 96 | 0.6 | 4 |
| FASTER=First- and Second-Trimester Evaluation of Risk[1]; SURUSS=Serum, Urine, and Ultrasound Screening Study.2 | ||||||||
| *Based on data from FASTER trial only. | ||||||||
Clinical considerations
Integrated screening
A number of considerations are important:
Tell the patient screening for neural tube defects is included in the integrated test, since it spans the first and second trimesters and includes AFP as one of the markers measured. Thus, women having the integrated test will also be screened for open spina bifida and anencephaly, in addition to Down syndrome.
Hold individual measurements until all results are in. First-trimester nuchal translucency and PAPP-A results are the first to become available. After these tests are performed, a waiting period of about 2 to 5 weeks is required to allow for second-trimester testing, tabulation, and integration into a single risk estimate.
In the case of ultrasound imaging, it is important for the sonographer to explain what is being measured without conveying special import regarding the nuchal translucency measurement—whether it is large or small. If asked, the sonographer should explain that nuchal translucency is only 1 of 6 measures that will determine the patient’s risk.
Advise the patient that definitive diagnosis will not occur until the second trimester. Because the integrated test is reported after the second-trimester serum sample is drawn and assayed, any follow-up diagnostic testing will not be available any sooner than is typical for second-trimester screening (ie, 16–18 gestational weeks).
A very large nuchal translucency measurement may be cause for concern and points to the need for early diagnosis. If a woman having the integrated test is found to have a nuchal translucency measurement of 3 to 4 mm or more (or any cystic hygroma), a clinically reasonable strategy is to offer immediate prenatal diagnosis by CVS rather than continue with the screening test.
Nuchal translucency values of 3 or 4 mm or more are seen in fewer than 1% of women scanned, and are associated with a very high risk of fetal aneuploidy and adverse pregnancy outcomes.
If nuchal translucency ultrasound is not available in your region, the integrated test using serum markers will provide better screening performance than any other serum-only test.
First-trimester combined screening
Offer it as early as possible. The benefit of first-trimester screening is the prospect of early prenatal diagnosis. Therefore, the earlier the test is offered within the accepted time frame of 11 to 13 completed weeks, the more apparent the benefit.
Early screening requires nuchal translucency measurement. First-trimester screening involves the measurement of serum analytes and ultrasound measurement of nuchal translucency. Serum markers without nuchal translucency or nuchal translucency without serum markers provide insufficient screening. If nuchal translucency is unavailable, offer the serum-only form of the integrated test or the second-trimester quad marker test.
Early diagnosis is requisite. Patients who are screen-positive in the first trimester should have CVS as an option for the earliest possible diagnosis. If amniocentesis at 15 weeks or beyond is the only invasive diagnostic procedure available, first-trimester combined screening is not appropriate.
An additional screen for neural tube defects is needed. First-trimester combined screening does not test for risk of open neural tube defects. Most commonly, a second-trimester serum AFP measurement is recommended for all women who have had first-trimester screening. Alternatively, a second-trimester ultrasound scan for fetal anomalies is highly indicative of neural tube defects if it includes the cranial lemon and cerebellar banana signs.
Even newer choices
Within the past year, 2 new screening methods have been proposed: sequential testing and contingent testing.15,16 They are essentially hybrids of the first-trimester and integrated tests. Both identify a very small, very high-risk group based on first-trimester nuchal translucency and serum markers. This group (eg, women having a risk of 1 in 25 and higher) would account for less than 1% of the total screened population and would ultimately be found to have more than 50% of Down syndrome cases.
In sequential testing, all women whose risk is less than the high-risk cutoff (eg, a possible cutoff of less than 1 in 25 or higher) would go on to have the full integrated test. In contingent testing, a second group would be identified as very low-risk based on first-trimester markers (eg, a possible risk cutoff of 1 in 2,000 or lower). Such low-risk women would have a small chance of having their results become high-risk based on the completed integrated test; therefore, they would be identified as screen-negative early and would not have to go on to integrated testing. In contingent testing, only the intermediate group (eg, those between, say, 1 in 25 and 1 in 2,000) would complete the integrated test.
The terms screen-negative and screen-positive are applied to test results to convey the most accurate balance between detection and false-positive rates. However, these terms indicate only risk categories; within these categories, the specific risk assigned is of the greatest value in counseling the patient. For example, a woman with a screen-positive result may have a risk of 1 in 200 or a risk of 1 in 10.
These patient-specific risks are extremely accurate. Thus, a woman with the lower risk (ie, 1 in 200) may choose to forego invasive testing, whereas a woman with the much higher risk (1 in 10) may want definitive diagnosis.
Similarly, women with screen-negative results can have very different risks—as low as 1 in 50,000 or less and as high as being almost screen-positive. Again, it is helpful to counsel each woman using the specific risk calculated for her.
Clinical guidelines
Clinicians have an obligation to help pregnant women choose the best and safest options in diagnosis and treatment.It is not enough to simply offer a menu of therapies or tests and let the patient choose. A clinician would never let the patient decide which medications are safest and most effective, and the same should hold true for screening tests.
The following guidelines may help the clinician and patient make the most informed decision:
- If nuchal translucency ultrasound is available and the gravida presents by 11 to 13 gestational weeks, the integrated test is the safest, most effective screening method to assess Down syndrome risk.
- If a woman wants the earliest prenatal diagnosis, first-trimester combined screening, using nuchal translucency and serum markers, is appropriate.
- If nuchal translucency is unavailable, the serumonly version of the integrated test is the best screening method.
- If a woman presents after 13 weeks’ gestation, the second-trimester quad marker test is best.
Each hybrid test makes sense in theory. However, no one knows yet whether they will work as anticipated once they are implemented clinically, and the appropriate risk cutoffs have not yet been determined. It also is unclear whether women whose risks fall on the edge of the various groupings would be interested in waiting for the integrated test to be completed.
These tests remain investigational.
Dr. Canick is a consultant to and receives grant/research support from Diagnostic Systems. He holds patents on use of estriol in prenatal screening (nos. 5506150 and 5605843).
1. Malone FD, Canick JA, Ball RH, et al. A comparison of first trimester screening, second trimester screening, and the combination of both for evaluation of risk of Down syndrome. N Engl J Med. 2005;353:2001-2011.
2. Wald NJ, Rodeck C, et al. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003;10:56-104.
3. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM. Maternal serum screening for Down syndrome in the United States: a 1995 survey. Am J Obstet Gynecol. 1997;176:1046-1051.
4. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome [published corrections appear in J Med Screen. 1998;5:110; J Med Screen. 1998;5:166]. J Med Screen. 1997;4:181-246.
5. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down’s syndrome. Prenat Diagn. 1997;17:821-829.
6. Malone FD, D’Alton ME. Society for Maternal-Fetal Medicine. First-trimester sonographic screening for Down syndrome. Obstet Gynecol. 2003;102:1066-1079.
7. Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341:461-467.
8. Canick JA, Kellner LH. First trimester screening for aneuploidy: serum biochemical markers. Semin Perinatol. 1999;23:359-368.
9. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10–13 weeks of pregnancy. Prenat Diagn. 1998;18:281-286.
10. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Br J Obstet Gynaecol. 2004;111:521-531.
11. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Trial Research Consortium. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005;106:288-294.
12. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Research Consortium. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004;104:1222-1228.
13. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446-1451.
14. Dugoff L, Hobbins JC, Malone FD, et al. for the FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260-267.
15. Maymon R, Betser M, Dreazen E, et al. A model for disclosing the first trimester part of an integrated Down’s syndrome screening test. Clin Genet. 2004;65:113-119.
16. Wright D, Bradbury I, Benn P, Cuckle H, Ritchie K. Contingent screening for Down syndrome is an efficient alternative to non-disclosure sequential screening. Prenat Diagn. 2004;24:762-766.
CASE-BASED LEARNING
Her risk is high, but how high? Is invasive testing the only answer?
Mrs. S, a 37-year-old primigravida, has an age-related risk of having a baby with Down syndrome of 1 in 250. Before deciding whether to undergo an invasive diagnostic procedure based on her age alone, she wants to learn more about her risk by having a prenatal screening test. The 2 safest and most informative options: first-trimester combined screening and fully integrated screening. About 5% of women who have the first test are found to be high-risk, and the test identifies about 85% of all cases of Down syndrome. As for fully integrated screening, it identifies about 85% of all Down syndrome cases at a 1% screen-positive rate. Since it offers a faster result, Mrs. S opts for first-trimester screening.
At 11 weeks, 0 days (according to crown-rump length), she undergoes nuchal translucency imaging and has a blood sample drawn to measure pregnancy-associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG). Her test results are reported in multiples of the median (MoM):
| Nuchal translucency | 2.3 mm | 1.62 MoM |
| PAPP-A | 0.5 mIU/mL | 0.79 MoM |
| hCG | 45 IU/mL | 1.25 MoM |
These values suggest that Mrs. S has a risk of having a pregnancy affected by Down syndrome of 1 in 170. In other words, her results are screen-positive.
Can her risk be more precisely quantified without invasive testing?
Had Mrs. S chosen fully integrated screening, the answer to that question would be yes, but it would have meant waiting until the second trimester for the result.
This article describes the 2 screening approaches—first-trimester combined screening and fully integrated screening—as well as the serum-only variant of the integrated test and the established quad marker test. Also discussed are the findings of recent studies, including 2 key trials:
- the First and Second Trimester Evaluation of Risk (FASTER) of aneuploidy trial, published in November1
- the Serum, Urine, and Ultrasound Screening Study (SURUSS), published in 2003 in the United Kingdom2
These 2 trials are the only ones to compare screening markers at different times during pregnancy in the same women—the only way to fairly assess the quality of various marker combinations within and across gestational weeks.
How good is current practice?
In 1995, about 2.5 million of the approximately 4 million gravidas in the United States had maternal serum screening for Down syndrome and open neural tube defects.3 Today, this practice usually involves a serum sample drawn early during the second trimester (15–20 weeks), measurement of 3 or 4 serum markers (the triple or quad test), and calculation and reporting of risk.
Second-trimester serum screening is a relatively easy procedure involving a single blood sample and established risk-calculation methods. Further, the follow-up when a woman is screen-positive—ie, at increased risk—is clear: amniocentesis in the case of Down syndrome risk and targeted ultrasound in the case of open neural tube defects (with amniocentesis backup). For the triple test, we can expect a detection rate of about 70%, and for the quad test, 80%, by identifying 5% of screened women with the highest calculated risk (the effective screen-positive rate).1,2,4
Why the newer options are better
Optimally, prenatal screening should minimize the number of women identified as screen-positive (ie, women at sufficient risk to be offered amniocentesis or comparable procedures) while maximizing the overall detection rate. This point is important because screen-positive status leads to follow-up diagnostic procedures that are necessarily invasive and risky.
First-trimester screening slightly better than the quad test
The option Mrs. S selected entails measuring 3 markers during the late first trimester (11–13 gestational weeks): nuchal translucency, PAPP-A, and hCG or its free βsubunit. These markers constitute a screening test that performs as well as, or slightly better than, the second-trimester quad test. The best estimate of first-trimester screening is an 85% detection rate at a 5% screen-positive rate (compared with about 80% detection rate at a 5% screen-positive rate for the quad test).1,2,5,6
Serum markers or ultrasound alone not enough in first trimester
Two serum markers together, without nuchal translucency, or nuchal translucency alone, without the serum markers, do not constitute a sufficient first-trimester screening test, since they each detect about 60% to 65% of Down syndrome cases with a 5% false-positive rate. This is clearly inferior to the best we can do during the second trimester (about 80% detection rate for a 5% false-positive rate). Only when nuchal translucency and serum markers are used together is first-trimester screening a viable option.
Timing is important in integrated screening
For the integrated screening option, instead of requiring that screening be offered in the late first or early second trimester, each marker is measured when it is most informative. The optimal time for nuchal translucency and PAPP-A measurement is at 10 to 11 weeks, while the optimal time for the measurement of hCG (or its free β subunit), inhibin A, alpha fetoprotein (AFP), and unconjugated estriol (uE3) is at 15 to 20 weeks.
Therefore, the integrated test is accomplished in 2 steps. At about 11 weeks, a woman undergoes nuchal translucency ultrasound imaging and has a blood sample drawn for PAPP-A measurement. At about 15 weeks (the earlier in the second-trimester window the better), she has a second sample drawn for measurement of the quad markers. A risk report then is generated, using all 6 markers to calculate the woman’s new risk. Such a test has to be superior to any test that uses fewer markers or the same markers at less than the optimal time.
The integrated test can also be carried out without nuchal translucency, by measuring PAPP-A during the first trimester and the quad serum markers during the second trimester, for an estimated detection rate of 85% with a 5% false-positive rate.
Integrated option has 1% screen-positive rate
Integrated screening reduces the screen-positive rate by as much as fourfold—to 1% or less. That is, only 1 in 100 women undergoing screening will be called screen-positive, and, in that 1%, approximately 85% of all Down syndrome pregnancies will be found.1,2,7
First-trimester serum markers
The most informative serum marker during the first trimester is PAPP-A, a large glycoprotein complex made by the placenta. In pregnancies affected by Down syndrome, PAPP-A levels tend to be low: about 0.4 MoM on average, or about 2.5 times lower than in unaffected pregnancies.
The second most commonly used serum marker is the free βsubunit of hCG, which is, on average, 1.8 MoM in pregnancies affected by Down syndrome, or almost twice as high as in normal pregnancies.4,8 Studies indicate that hCG and inhibin A are also effective serum markers during the late first trimester, providing screening performance equivalent to that of the free β-hCG when combined with nuchal translucency and PAPP-A.4
Nuchal translucency: A powerful marker
Both the fully integrated and first-trimester screening approaches necessitate ultrasound measurement of nuchal translucency, which is always measured along with fetal crown-rump length. The nuchal translucency value—initially measured in tenths of millimeters—then is normalized for gestational age based on crown-rump length, and reported in multiples of the median, the same unit used to normalize serum screening markers.
FIGURE 1 shows how nuchal translucency values (in millimeters) measured in a general population increase with gestation. The most commonly accepted period of gestation to measure nuchal translucency is between 10 and 13 completed weeks.
Why nuchal translucency is more informative
Nuchal translucency values tend to be increased in Down syndrome pregnancies, as are certain serum markers such as hCG or its free β subunit and inhibin A. However, nuchal translucency is more informative than these markers because there is less overlap between Down syndrome and unaffected case values. This is not because nuchal translucency values tend to be higher in affected pregnancies. In fact, all 3 markers are, on average, about twice as high in cases of Down syndrome as in controls. However, because the distribution of nuchal translucency values in unaffected fetuses is much narrower (or tighter) than is true for hCG (or its free β subunit) or inhibin A, very few unaffected fetuses have increased nuchal translucency values. Therefore, when nuchal translucency is elevated, it is more likely to be associated with an affected pregnancy than is either of the other 2 markers.
FIGURE 2 shows the overlapping distributions in cases and controls for both hCG and nuchal translucency. In unaffected pregnancies, the distribution of values centers around 1 MoM, while in Down syndrome pregnancies, the values center around 2 MoM. About 8% of hCG values in unaffected pregnancies exceed 2 MoM, but only about 1.5% of nuchal translucency values do. Thus, for a detection rate of 50%, the false-positive rate using nuchal translucency is 1.5%, much smaller than the false-positive rate of 8% using hCG.
Imaging expertise is key
While it is beyond the scope of this article to detail the methodology of nuchal translucency measurement, specialized training and ongoing quality assurance are necessary to get the measurement right. Both the Society for Maternal-Fetal Medicine in the United States and the Fetal Medicine Foundation in the United Kingdom provide training and credentialing in nuchal translucency sonography. If performed correctly, it is an excellent screening marker. However, if attempted with no hands-on training, this imaging method yields unreliable results.
FIGURE 1 Nuchal translucency values increase with gestational age
Nuchal translucency values, in millimeters, in 561 pregnancies between 10 and 13 completed weeks of gestation, as estimated by crown-rump length. Reprinted with permission from Drs. Wald and Schucter; data from Schuchter K, et al.9
FIGURE 2 Very few unaffected fetuses have elevated nuchal translucency values
Distribution of values of second-trimester human chorionic gonadotropin (hCG) and first-trimester nuchal translucency among unaffected and Down syndrome pregnancies, both given in multiples of the median (MoM). Note that the unaffected nuchal translucency distribution is much narrower and taller than the unaffected hCG distribution. The scale is a log progression of increasing MoM values because both markers are log-normally distributed in unaffected and Down syndrome gestations. DR=detection rate; FPR=false-positive rate; NT=nuchal translucency.
2 important studies
The SURUSS trial, conducted mainly in the United Kingdom, was an observational study in which all women underwent first-trimester ultrasound measurement of nuchal translucency, as well as first- and second-trimester blood and urine sampling, with all samples stored. After all outcomes were chronicled, case-control sets of first- and second-trimester samples were constructed and assayed for a wide variety of known and potential screening markers. Nuchal translucency data also were analyzed. More than 48,000 women were enrolled, and 101 Down syndrome pregnancies were identified and studied. 2,10
The FASTER trial, completed more recently, was an observational study conducted at 15 enrollment centers in the US and involving more than 38,000 women, among whom 117 Down syndrome cases were identified.1 All women in the trial underwent first-trimester ultrasound examination and blood sampling between 10 weeks 3 days, and 13 weeks 6 days, and were asked to return for a second-trimester blood draw between 15 and 18 completed weeks, after which a report was issued detailing the separate results. In addition, combinations of markers across the trimesters were modeled and compared.
Perhaps the most interesting and surprising finding from the 2 studies is the remarkable similarity of results (TABLE). For 2 large populations on separate continents with a different ethnic mix, the primary findings were almost identical:
- 86% to 87% detection rate at a 5% false-positive rate for first-trimester combined screening (nuchal translucency, PAPP-A, and free β-hCG) and 80% to 83% detection rate at a 5% false-positive rate for second-trimester quad screening (AFP, uE3 , hCG, and inhibin A). In both trials, first-trimester screening was incrementally better than second-trimester screening, but the difference was not statistically significant.
- For integrated screening, a detection rate of 86% to 88% at a false-positive rate of 1%. Nuchal translucency and PAPP-A were measured during the first trimester, and quad markers during the second trimester. At a 5% false-positive rate, the detection rate in both trials was about 95%. With a serum-only integrated test, detection rates were 87% to 88% with a 5% false-positive rate.
- Nuchal translucency was more informative than any of the serum markers tested. This corroborates the rich literature on nuchal translucency published over the past decade. Both trials demonstrated that nuchal translucency measurement is effective when training is adequate, and that ongoing monitoring of quality is essential. However, in both studies, a satisfactory nuchal translucency measurement was not attained in about 7% of all women scanned between 10 and 13 weeks, so a small but significant number of women will not have nuchal translucency included in their risk assessment.
Other notable FASTER findings
Other findings from the FASTER trial that merit special attention:
- An ultrasound finding of cystic hygroma warrants an immediate prenatal diagnostic workup and was associated with an aneuploidy rate of 50% (one third of which was Down syndrome) and adverse outcomes in the great majority of cases.11 Cystic hygroma is uncommon, occurring in about 1 in 300 pregnancies in the first trimester.
- The value of the fetal nasal bone as a first-trimester ultrasound marker is unclear. In the FASTER trial, the nasal bone was studied in about 6,000 of the 38,000 ultrasound examinations, with no detected benefit. Thus, in a nonselected pregnant population, the nasal bone may not be a reasonable marker.12
- Various first- and second-trimester markers are modestly informative about adverse pregnancy outcomes other than aneuploidy (eg, fetal growth restriction, early delivery, and preeclampsia). Only a small proportion of affected pregnancies will be identified by these markers, alone or in combination.13,14
TABLE
5 screening approaches: What the SURUSS and FASTER trials reveal
| TEST | NUCHAL TRANSLUCENCY MEASURED? | METHOD OF PRENATAL DIAGNOSIS | DETECTION RATE (%) AT 1% FALSE-POSITIVE RATE | DETECTION RATE (%) AT 5% FALSE-POSITIVE RATE | FALSE-POSITIVE RATE* (%) TO ACHIEVE: | |||
|---|---|---|---|---|---|---|---|---|
| SURUSS | FASTER | SURUSS | FASTER | 85% DETECTION | 95% DETECTION | |||
| 2nd-trimester triple marker | No | 2nd-trimester amniocentesis | 56 | 45 | 77 | 70 | 14 | 32 |
| 2nd-trimester quad marker | No | 2nd-trimester amniocentesis | 64 | 60 | 83 | 80 | 7.3 | 22 |
| 1st-trimester combined | Yes | 1st-trimester chorionic villus sampling | 72 | 73 | 86 | 87 | 3.8 | 18 |
| Serum integrated test | No | 2nd-trimester amniocentesis | 73 | 73 | 87 | 88 | 3.6 | 15 |
| Full integrated test | Yes | 2nd-trimester amniocentesis | 86 | 88 | 94 | 96 | 0.6 | 4 |
| FASTER=First- and Second-Trimester Evaluation of Risk[1]; SURUSS=Serum, Urine, and Ultrasound Screening Study.2 | ||||||||
| *Based on data from FASTER trial only. | ||||||||
Clinical considerations
Integrated screening
A number of considerations are important:
Tell the patient screening for neural tube defects is included in the integrated test, since it spans the first and second trimesters and includes AFP as one of the markers measured. Thus, women having the integrated test will also be screened for open spina bifida and anencephaly, in addition to Down syndrome.
Hold individual measurements until all results are in. First-trimester nuchal translucency and PAPP-A results are the first to become available. After these tests are performed, a waiting period of about 2 to 5 weeks is required to allow for second-trimester testing, tabulation, and integration into a single risk estimate.
In the case of ultrasound imaging, it is important for the sonographer to explain what is being measured without conveying special import regarding the nuchal translucency measurement—whether it is large or small. If asked, the sonographer should explain that nuchal translucency is only 1 of 6 measures that will determine the patient’s risk.
Advise the patient that definitive diagnosis will not occur until the second trimester. Because the integrated test is reported after the second-trimester serum sample is drawn and assayed, any follow-up diagnostic testing will not be available any sooner than is typical for second-trimester screening (ie, 16–18 gestational weeks).
A very large nuchal translucency measurement may be cause for concern and points to the need for early diagnosis. If a woman having the integrated test is found to have a nuchal translucency measurement of 3 to 4 mm or more (or any cystic hygroma), a clinically reasonable strategy is to offer immediate prenatal diagnosis by CVS rather than continue with the screening test.
Nuchal translucency values of 3 or 4 mm or more are seen in fewer than 1% of women scanned, and are associated with a very high risk of fetal aneuploidy and adverse pregnancy outcomes.
If nuchal translucency ultrasound is not available in your region, the integrated test using serum markers will provide better screening performance than any other serum-only test.
First-trimester combined screening
Offer it as early as possible. The benefit of first-trimester screening is the prospect of early prenatal diagnosis. Therefore, the earlier the test is offered within the accepted time frame of 11 to 13 completed weeks, the more apparent the benefit.
Early screening requires nuchal translucency measurement. First-trimester screening involves the measurement of serum analytes and ultrasound measurement of nuchal translucency. Serum markers without nuchal translucency or nuchal translucency without serum markers provide insufficient screening. If nuchal translucency is unavailable, offer the serum-only form of the integrated test or the second-trimester quad marker test.
Early diagnosis is requisite. Patients who are screen-positive in the first trimester should have CVS as an option for the earliest possible diagnosis. If amniocentesis at 15 weeks or beyond is the only invasive diagnostic procedure available, first-trimester combined screening is not appropriate.
An additional screen for neural tube defects is needed. First-trimester combined screening does not test for risk of open neural tube defects. Most commonly, a second-trimester serum AFP measurement is recommended for all women who have had first-trimester screening. Alternatively, a second-trimester ultrasound scan for fetal anomalies is highly indicative of neural tube defects if it includes the cranial lemon and cerebellar banana signs.
Even newer choices
Within the past year, 2 new screening methods have been proposed: sequential testing and contingent testing.15,16 They are essentially hybrids of the first-trimester and integrated tests. Both identify a very small, very high-risk group based on first-trimester nuchal translucency and serum markers. This group (eg, women having a risk of 1 in 25 and higher) would account for less than 1% of the total screened population and would ultimately be found to have more than 50% of Down syndrome cases.
In sequential testing, all women whose risk is less than the high-risk cutoff (eg, a possible cutoff of less than 1 in 25 or higher) would go on to have the full integrated test. In contingent testing, a second group would be identified as very low-risk based on first-trimester markers (eg, a possible risk cutoff of 1 in 2,000 or lower). Such low-risk women would have a small chance of having their results become high-risk based on the completed integrated test; therefore, they would be identified as screen-negative early and would not have to go on to integrated testing. In contingent testing, only the intermediate group (eg, those between, say, 1 in 25 and 1 in 2,000) would complete the integrated test.
The terms screen-negative and screen-positive are applied to test results to convey the most accurate balance between detection and false-positive rates. However, these terms indicate only risk categories; within these categories, the specific risk assigned is of the greatest value in counseling the patient. For example, a woman with a screen-positive result may have a risk of 1 in 200 or a risk of 1 in 10.
These patient-specific risks are extremely accurate. Thus, a woman with the lower risk (ie, 1 in 200) may choose to forego invasive testing, whereas a woman with the much higher risk (1 in 10) may want definitive diagnosis.
Similarly, women with screen-negative results can have very different risks—as low as 1 in 50,000 or less and as high as being almost screen-positive. Again, it is helpful to counsel each woman using the specific risk calculated for her.
Clinical guidelines
Clinicians have an obligation to help pregnant women choose the best and safest options in diagnosis and treatment.It is not enough to simply offer a menu of therapies or tests and let the patient choose. A clinician would never let the patient decide which medications are safest and most effective, and the same should hold true for screening tests.
The following guidelines may help the clinician and patient make the most informed decision:
- If nuchal translucency ultrasound is available and the gravida presents by 11 to 13 gestational weeks, the integrated test is the safest, most effective screening method to assess Down syndrome risk.
- If a woman wants the earliest prenatal diagnosis, first-trimester combined screening, using nuchal translucency and serum markers, is appropriate.
- If nuchal translucency is unavailable, the serumonly version of the integrated test is the best screening method.
- If a woman presents after 13 weeks’ gestation, the second-trimester quad marker test is best.
Each hybrid test makes sense in theory. However, no one knows yet whether they will work as anticipated once they are implemented clinically, and the appropriate risk cutoffs have not yet been determined. It also is unclear whether women whose risks fall on the edge of the various groupings would be interested in waiting for the integrated test to be completed.
These tests remain investigational.
Dr. Canick is a consultant to and receives grant/research support from Diagnostic Systems. He holds patents on use of estriol in prenatal screening (nos. 5506150 and 5605843).
CASE-BASED LEARNING
Her risk is high, but how high? Is invasive testing the only answer?
Mrs. S, a 37-year-old primigravida, has an age-related risk of having a baby with Down syndrome of 1 in 250. Before deciding whether to undergo an invasive diagnostic procedure based on her age alone, she wants to learn more about her risk by having a prenatal screening test. The 2 safest and most informative options: first-trimester combined screening and fully integrated screening. About 5% of women who have the first test are found to be high-risk, and the test identifies about 85% of all cases of Down syndrome. As for fully integrated screening, it identifies about 85% of all Down syndrome cases at a 1% screen-positive rate. Since it offers a faster result, Mrs. S opts for first-trimester screening.
At 11 weeks, 0 days (according to crown-rump length), she undergoes nuchal translucency imaging and has a blood sample drawn to measure pregnancy-associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG). Her test results are reported in multiples of the median (MoM):
| Nuchal translucency | 2.3 mm | 1.62 MoM |
| PAPP-A | 0.5 mIU/mL | 0.79 MoM |
| hCG | 45 IU/mL | 1.25 MoM |
These values suggest that Mrs. S has a risk of having a pregnancy affected by Down syndrome of 1 in 170. In other words, her results are screen-positive.
Can her risk be more precisely quantified without invasive testing?
Had Mrs. S chosen fully integrated screening, the answer to that question would be yes, but it would have meant waiting until the second trimester for the result.
This article describes the 2 screening approaches—first-trimester combined screening and fully integrated screening—as well as the serum-only variant of the integrated test and the established quad marker test. Also discussed are the findings of recent studies, including 2 key trials:
- the First and Second Trimester Evaluation of Risk (FASTER) of aneuploidy trial, published in November1
- the Serum, Urine, and Ultrasound Screening Study (SURUSS), published in 2003 in the United Kingdom2
These 2 trials are the only ones to compare screening markers at different times during pregnancy in the same women—the only way to fairly assess the quality of various marker combinations within and across gestational weeks.
How good is current practice?
In 1995, about 2.5 million of the approximately 4 million gravidas in the United States had maternal serum screening for Down syndrome and open neural tube defects.3 Today, this practice usually involves a serum sample drawn early during the second trimester (15–20 weeks), measurement of 3 or 4 serum markers (the triple or quad test), and calculation and reporting of risk.
Second-trimester serum screening is a relatively easy procedure involving a single blood sample and established risk-calculation methods. Further, the follow-up when a woman is screen-positive—ie, at increased risk—is clear: amniocentesis in the case of Down syndrome risk and targeted ultrasound in the case of open neural tube defects (with amniocentesis backup). For the triple test, we can expect a detection rate of about 70%, and for the quad test, 80%, by identifying 5% of screened women with the highest calculated risk (the effective screen-positive rate).1,2,4
Why the newer options are better
Optimally, prenatal screening should minimize the number of women identified as screen-positive (ie, women at sufficient risk to be offered amniocentesis or comparable procedures) while maximizing the overall detection rate. This point is important because screen-positive status leads to follow-up diagnostic procedures that are necessarily invasive and risky.
First-trimester screening slightly better than the quad test
The option Mrs. S selected entails measuring 3 markers during the late first trimester (11–13 gestational weeks): nuchal translucency, PAPP-A, and hCG or its free βsubunit. These markers constitute a screening test that performs as well as, or slightly better than, the second-trimester quad test. The best estimate of first-trimester screening is an 85% detection rate at a 5% screen-positive rate (compared with about 80% detection rate at a 5% screen-positive rate for the quad test).1,2,5,6
Serum markers or ultrasound alone not enough in first trimester
Two serum markers together, without nuchal translucency, or nuchal translucency alone, without the serum markers, do not constitute a sufficient first-trimester screening test, since they each detect about 60% to 65% of Down syndrome cases with a 5% false-positive rate. This is clearly inferior to the best we can do during the second trimester (about 80% detection rate for a 5% false-positive rate). Only when nuchal translucency and serum markers are used together is first-trimester screening a viable option.
Timing is important in integrated screening
For the integrated screening option, instead of requiring that screening be offered in the late first or early second trimester, each marker is measured when it is most informative. The optimal time for nuchal translucency and PAPP-A measurement is at 10 to 11 weeks, while the optimal time for the measurement of hCG (or its free β subunit), inhibin A, alpha fetoprotein (AFP), and unconjugated estriol (uE3) is at 15 to 20 weeks.
Therefore, the integrated test is accomplished in 2 steps. At about 11 weeks, a woman undergoes nuchal translucency ultrasound imaging and has a blood sample drawn for PAPP-A measurement. At about 15 weeks (the earlier in the second-trimester window the better), she has a second sample drawn for measurement of the quad markers. A risk report then is generated, using all 6 markers to calculate the woman’s new risk. Such a test has to be superior to any test that uses fewer markers or the same markers at less than the optimal time.
The integrated test can also be carried out without nuchal translucency, by measuring PAPP-A during the first trimester and the quad serum markers during the second trimester, for an estimated detection rate of 85% with a 5% false-positive rate.
Integrated option has 1% screen-positive rate
Integrated screening reduces the screen-positive rate by as much as fourfold—to 1% or less. That is, only 1 in 100 women undergoing screening will be called screen-positive, and, in that 1%, approximately 85% of all Down syndrome pregnancies will be found.1,2,7
First-trimester serum markers
The most informative serum marker during the first trimester is PAPP-A, a large glycoprotein complex made by the placenta. In pregnancies affected by Down syndrome, PAPP-A levels tend to be low: about 0.4 MoM on average, or about 2.5 times lower than in unaffected pregnancies.
The second most commonly used serum marker is the free βsubunit of hCG, which is, on average, 1.8 MoM in pregnancies affected by Down syndrome, or almost twice as high as in normal pregnancies.4,8 Studies indicate that hCG and inhibin A are also effective serum markers during the late first trimester, providing screening performance equivalent to that of the free β-hCG when combined with nuchal translucency and PAPP-A.4
Nuchal translucency: A powerful marker
Both the fully integrated and first-trimester screening approaches necessitate ultrasound measurement of nuchal translucency, which is always measured along with fetal crown-rump length. The nuchal translucency value—initially measured in tenths of millimeters—then is normalized for gestational age based on crown-rump length, and reported in multiples of the median, the same unit used to normalize serum screening markers.
FIGURE 1 shows how nuchal translucency values (in millimeters) measured in a general population increase with gestation. The most commonly accepted period of gestation to measure nuchal translucency is between 10 and 13 completed weeks.
Why nuchal translucency is more informative
Nuchal translucency values tend to be increased in Down syndrome pregnancies, as are certain serum markers such as hCG or its free β subunit and inhibin A. However, nuchal translucency is more informative than these markers because there is less overlap between Down syndrome and unaffected case values. This is not because nuchal translucency values tend to be higher in affected pregnancies. In fact, all 3 markers are, on average, about twice as high in cases of Down syndrome as in controls. However, because the distribution of nuchal translucency values in unaffected fetuses is much narrower (or tighter) than is true for hCG (or its free β subunit) or inhibin A, very few unaffected fetuses have increased nuchal translucency values. Therefore, when nuchal translucency is elevated, it is more likely to be associated with an affected pregnancy than is either of the other 2 markers.
FIGURE 2 shows the overlapping distributions in cases and controls for both hCG and nuchal translucency. In unaffected pregnancies, the distribution of values centers around 1 MoM, while in Down syndrome pregnancies, the values center around 2 MoM. About 8% of hCG values in unaffected pregnancies exceed 2 MoM, but only about 1.5% of nuchal translucency values do. Thus, for a detection rate of 50%, the false-positive rate using nuchal translucency is 1.5%, much smaller than the false-positive rate of 8% using hCG.
Imaging expertise is key
While it is beyond the scope of this article to detail the methodology of nuchal translucency measurement, specialized training and ongoing quality assurance are necessary to get the measurement right. Both the Society for Maternal-Fetal Medicine in the United States and the Fetal Medicine Foundation in the United Kingdom provide training and credentialing in nuchal translucency sonography. If performed correctly, it is an excellent screening marker. However, if attempted with no hands-on training, this imaging method yields unreliable results.
FIGURE 1 Nuchal translucency values increase with gestational age
Nuchal translucency values, in millimeters, in 561 pregnancies between 10 and 13 completed weeks of gestation, as estimated by crown-rump length. Reprinted with permission from Drs. Wald and Schucter; data from Schuchter K, et al.9
FIGURE 2 Very few unaffected fetuses have elevated nuchal translucency values
Distribution of values of second-trimester human chorionic gonadotropin (hCG) and first-trimester nuchal translucency among unaffected and Down syndrome pregnancies, both given in multiples of the median (MoM). Note that the unaffected nuchal translucency distribution is much narrower and taller than the unaffected hCG distribution. The scale is a log progression of increasing MoM values because both markers are log-normally distributed in unaffected and Down syndrome gestations. DR=detection rate; FPR=false-positive rate; NT=nuchal translucency.
2 important studies
The SURUSS trial, conducted mainly in the United Kingdom, was an observational study in which all women underwent first-trimester ultrasound measurement of nuchal translucency, as well as first- and second-trimester blood and urine sampling, with all samples stored. After all outcomes were chronicled, case-control sets of first- and second-trimester samples were constructed and assayed for a wide variety of known and potential screening markers. Nuchal translucency data also were analyzed. More than 48,000 women were enrolled, and 101 Down syndrome pregnancies were identified and studied. 2,10
The FASTER trial, completed more recently, was an observational study conducted at 15 enrollment centers in the US and involving more than 38,000 women, among whom 117 Down syndrome cases were identified.1 All women in the trial underwent first-trimester ultrasound examination and blood sampling between 10 weeks 3 days, and 13 weeks 6 days, and were asked to return for a second-trimester blood draw between 15 and 18 completed weeks, after which a report was issued detailing the separate results. In addition, combinations of markers across the trimesters were modeled and compared.
Perhaps the most interesting and surprising finding from the 2 studies is the remarkable similarity of results (TABLE). For 2 large populations on separate continents with a different ethnic mix, the primary findings were almost identical:
- 86% to 87% detection rate at a 5% false-positive rate for first-trimester combined screening (nuchal translucency, PAPP-A, and free β-hCG) and 80% to 83% detection rate at a 5% false-positive rate for second-trimester quad screening (AFP, uE3 , hCG, and inhibin A). In both trials, first-trimester screening was incrementally better than second-trimester screening, but the difference was not statistically significant.
- For integrated screening, a detection rate of 86% to 88% at a false-positive rate of 1%. Nuchal translucency and PAPP-A were measured during the first trimester, and quad markers during the second trimester. At a 5% false-positive rate, the detection rate in both trials was about 95%. With a serum-only integrated test, detection rates were 87% to 88% with a 5% false-positive rate.
- Nuchal translucency was more informative than any of the serum markers tested. This corroborates the rich literature on nuchal translucency published over the past decade. Both trials demonstrated that nuchal translucency measurement is effective when training is adequate, and that ongoing monitoring of quality is essential. However, in both studies, a satisfactory nuchal translucency measurement was not attained in about 7% of all women scanned between 10 and 13 weeks, so a small but significant number of women will not have nuchal translucency included in their risk assessment.
Other notable FASTER findings
Other findings from the FASTER trial that merit special attention:
- An ultrasound finding of cystic hygroma warrants an immediate prenatal diagnostic workup and was associated with an aneuploidy rate of 50% (one third of which was Down syndrome) and adverse outcomes in the great majority of cases.11 Cystic hygroma is uncommon, occurring in about 1 in 300 pregnancies in the first trimester.
- The value of the fetal nasal bone as a first-trimester ultrasound marker is unclear. In the FASTER trial, the nasal bone was studied in about 6,000 of the 38,000 ultrasound examinations, with no detected benefit. Thus, in a nonselected pregnant population, the nasal bone may not be a reasonable marker.12
- Various first- and second-trimester markers are modestly informative about adverse pregnancy outcomes other than aneuploidy (eg, fetal growth restriction, early delivery, and preeclampsia). Only a small proportion of affected pregnancies will be identified by these markers, alone or in combination.13,14
TABLE
5 screening approaches: What the SURUSS and FASTER trials reveal
| TEST | NUCHAL TRANSLUCENCY MEASURED? | METHOD OF PRENATAL DIAGNOSIS | DETECTION RATE (%) AT 1% FALSE-POSITIVE RATE | DETECTION RATE (%) AT 5% FALSE-POSITIVE RATE | FALSE-POSITIVE RATE* (%) TO ACHIEVE: | |||
|---|---|---|---|---|---|---|---|---|
| SURUSS | FASTER | SURUSS | FASTER | 85% DETECTION | 95% DETECTION | |||
| 2nd-trimester triple marker | No | 2nd-trimester amniocentesis | 56 | 45 | 77 | 70 | 14 | 32 |
| 2nd-trimester quad marker | No | 2nd-trimester amniocentesis | 64 | 60 | 83 | 80 | 7.3 | 22 |
| 1st-trimester combined | Yes | 1st-trimester chorionic villus sampling | 72 | 73 | 86 | 87 | 3.8 | 18 |
| Serum integrated test | No | 2nd-trimester amniocentesis | 73 | 73 | 87 | 88 | 3.6 | 15 |
| Full integrated test | Yes | 2nd-trimester amniocentesis | 86 | 88 | 94 | 96 | 0.6 | 4 |
| FASTER=First- and Second-Trimester Evaluation of Risk[1]; SURUSS=Serum, Urine, and Ultrasound Screening Study.2 | ||||||||
| *Based on data from FASTER trial only. | ||||||||
Clinical considerations
Integrated screening
A number of considerations are important:
Tell the patient screening for neural tube defects is included in the integrated test, since it spans the first and second trimesters and includes AFP as one of the markers measured. Thus, women having the integrated test will also be screened for open spina bifida and anencephaly, in addition to Down syndrome.
Hold individual measurements until all results are in. First-trimester nuchal translucency and PAPP-A results are the first to become available. After these tests are performed, a waiting period of about 2 to 5 weeks is required to allow for second-trimester testing, tabulation, and integration into a single risk estimate.
In the case of ultrasound imaging, it is important for the sonographer to explain what is being measured without conveying special import regarding the nuchal translucency measurement—whether it is large or small. If asked, the sonographer should explain that nuchal translucency is only 1 of 6 measures that will determine the patient’s risk.
Advise the patient that definitive diagnosis will not occur until the second trimester. Because the integrated test is reported after the second-trimester serum sample is drawn and assayed, any follow-up diagnostic testing will not be available any sooner than is typical for second-trimester screening (ie, 16–18 gestational weeks).
A very large nuchal translucency measurement may be cause for concern and points to the need for early diagnosis. If a woman having the integrated test is found to have a nuchal translucency measurement of 3 to 4 mm or more (or any cystic hygroma), a clinically reasonable strategy is to offer immediate prenatal diagnosis by CVS rather than continue with the screening test.
Nuchal translucency values of 3 or 4 mm or more are seen in fewer than 1% of women scanned, and are associated with a very high risk of fetal aneuploidy and adverse pregnancy outcomes.
If nuchal translucency ultrasound is not available in your region, the integrated test using serum markers will provide better screening performance than any other serum-only test.
First-trimester combined screening
Offer it as early as possible. The benefit of first-trimester screening is the prospect of early prenatal diagnosis. Therefore, the earlier the test is offered within the accepted time frame of 11 to 13 completed weeks, the more apparent the benefit.
Early screening requires nuchal translucency measurement. First-trimester screening involves the measurement of serum analytes and ultrasound measurement of nuchal translucency. Serum markers without nuchal translucency or nuchal translucency without serum markers provide insufficient screening. If nuchal translucency is unavailable, offer the serum-only form of the integrated test or the second-trimester quad marker test.
Early diagnosis is requisite. Patients who are screen-positive in the first trimester should have CVS as an option for the earliest possible diagnosis. If amniocentesis at 15 weeks or beyond is the only invasive diagnostic procedure available, first-trimester combined screening is not appropriate.
An additional screen for neural tube defects is needed. First-trimester combined screening does not test for risk of open neural tube defects. Most commonly, a second-trimester serum AFP measurement is recommended for all women who have had first-trimester screening. Alternatively, a second-trimester ultrasound scan for fetal anomalies is highly indicative of neural tube defects if it includes the cranial lemon and cerebellar banana signs.
Even newer choices
Within the past year, 2 new screening methods have been proposed: sequential testing and contingent testing.15,16 They are essentially hybrids of the first-trimester and integrated tests. Both identify a very small, very high-risk group based on first-trimester nuchal translucency and serum markers. This group (eg, women having a risk of 1 in 25 and higher) would account for less than 1% of the total screened population and would ultimately be found to have more than 50% of Down syndrome cases.
In sequential testing, all women whose risk is less than the high-risk cutoff (eg, a possible cutoff of less than 1 in 25 or higher) would go on to have the full integrated test. In contingent testing, a second group would be identified as very low-risk based on first-trimester markers (eg, a possible risk cutoff of 1 in 2,000 or lower). Such low-risk women would have a small chance of having their results become high-risk based on the completed integrated test; therefore, they would be identified as screen-negative early and would not have to go on to integrated testing. In contingent testing, only the intermediate group (eg, those between, say, 1 in 25 and 1 in 2,000) would complete the integrated test.
The terms screen-negative and screen-positive are applied to test results to convey the most accurate balance between detection and false-positive rates. However, these terms indicate only risk categories; within these categories, the specific risk assigned is of the greatest value in counseling the patient. For example, a woman with a screen-positive result may have a risk of 1 in 200 or a risk of 1 in 10.
These patient-specific risks are extremely accurate. Thus, a woman with the lower risk (ie, 1 in 200) may choose to forego invasive testing, whereas a woman with the much higher risk (1 in 10) may want definitive diagnosis.
Similarly, women with screen-negative results can have very different risks—as low as 1 in 50,000 or less and as high as being almost screen-positive. Again, it is helpful to counsel each woman using the specific risk calculated for her.
Clinical guidelines
Clinicians have an obligation to help pregnant women choose the best and safest options in diagnosis and treatment.It is not enough to simply offer a menu of therapies or tests and let the patient choose. A clinician would never let the patient decide which medications are safest and most effective, and the same should hold true for screening tests.
The following guidelines may help the clinician and patient make the most informed decision:
- If nuchal translucency ultrasound is available and the gravida presents by 11 to 13 gestational weeks, the integrated test is the safest, most effective screening method to assess Down syndrome risk.
- If a woman wants the earliest prenatal diagnosis, first-trimester combined screening, using nuchal translucency and serum markers, is appropriate.
- If nuchal translucency is unavailable, the serumonly version of the integrated test is the best screening method.
- If a woman presents after 13 weeks’ gestation, the second-trimester quad marker test is best.
Each hybrid test makes sense in theory. However, no one knows yet whether they will work as anticipated once they are implemented clinically, and the appropriate risk cutoffs have not yet been determined. It also is unclear whether women whose risks fall on the edge of the various groupings would be interested in waiting for the integrated test to be completed.
These tests remain investigational.
Dr. Canick is a consultant to and receives grant/research support from Diagnostic Systems. He holds patents on use of estriol in prenatal screening (nos. 5506150 and 5605843).
1. Malone FD, Canick JA, Ball RH, et al. A comparison of first trimester screening, second trimester screening, and the combination of both for evaluation of risk of Down syndrome. N Engl J Med. 2005;353:2001-2011.
2. Wald NJ, Rodeck C, et al. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003;10:56-104.
3. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM. Maternal serum screening for Down syndrome in the United States: a 1995 survey. Am J Obstet Gynecol. 1997;176:1046-1051.
4. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome [published corrections appear in J Med Screen. 1998;5:110; J Med Screen. 1998;5:166]. J Med Screen. 1997;4:181-246.
5. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down’s syndrome. Prenat Diagn. 1997;17:821-829.
6. Malone FD, D’Alton ME. Society for Maternal-Fetal Medicine. First-trimester sonographic screening for Down syndrome. Obstet Gynecol. 2003;102:1066-1079.
7. Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341:461-467.
8. Canick JA, Kellner LH. First trimester screening for aneuploidy: serum biochemical markers. Semin Perinatol. 1999;23:359-368.
9. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10–13 weeks of pregnancy. Prenat Diagn. 1998;18:281-286.
10. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Br J Obstet Gynaecol. 2004;111:521-531.
11. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Trial Research Consortium. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005;106:288-294.
12. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Research Consortium. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004;104:1222-1228.
13. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446-1451.
14. Dugoff L, Hobbins JC, Malone FD, et al. for the FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260-267.
15. Maymon R, Betser M, Dreazen E, et al. A model for disclosing the first trimester part of an integrated Down’s syndrome screening test. Clin Genet. 2004;65:113-119.
16. Wright D, Bradbury I, Benn P, Cuckle H, Ritchie K. Contingent screening for Down syndrome is an efficient alternative to non-disclosure sequential screening. Prenat Diagn. 2004;24:762-766.
1. Malone FD, Canick JA, Ball RH, et al. A comparison of first trimester screening, second trimester screening, and the combination of both for evaluation of risk of Down syndrome. N Engl J Med. 2005;353:2001-2011.
2. Wald NJ, Rodeck C, et al. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003;10:56-104.
3. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM. Maternal serum screening for Down syndrome in the United States: a 1995 survey. Am J Obstet Gynecol. 1997;176:1046-1051.
4. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome [published corrections appear in J Med Screen. 1998;5:110; J Med Screen. 1998;5:166]. J Med Screen. 1997;4:181-246.
5. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down’s syndrome. Prenat Diagn. 1997;17:821-829.
6. Malone FD, D’Alton ME. Society for Maternal-Fetal Medicine. First-trimester sonographic screening for Down syndrome. Obstet Gynecol. 2003;102:1066-1079.
7. Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341:461-467.
8. Canick JA, Kellner LH. First trimester screening for aneuploidy: serum biochemical markers. Semin Perinatol. 1999;23:359-368.
9. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10–13 weeks of pregnancy. Prenat Diagn. 1998;18:281-286.
10. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Br J Obstet Gynaecol. 2004;111:521-531.
11. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Trial Research Consortium. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005;106:288-294.
12. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Research Consortium. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004;104:1222-1228.
13. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446-1451.
14. Dugoff L, Hobbins JC, Malone FD, et al. for the FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260-267.
15. Maymon R, Betser M, Dreazen E, et al. A model for disclosing the first trimester part of an integrated Down’s syndrome screening test. Clin Genet. 2004;65:113-119.
16. Wright D, Bradbury I, Benn P, Cuckle H, Ritchie K. Contingent screening for Down syndrome is an efficient alternative to non-disclosure sequential screening. Prenat Diagn. 2004;24:762-766.
Assessing Clinicians' Knowledge of Herbal Medicine
Managing Chronic, Nonmalignant Pain in Patients with a Substance Use Disorder
Reducing the legal risks of labor induction and augmentation
- 18 common allegations in oxytocin-related litigation
- 6 risk-reducing strategies
Martin L. Gimovsky, MD
WHAT’S YOUR VERDICT?
Does this patient have grounds for a lawsuit?
At 41 weeks’ estimated gestation, Elena, a 32-year-old primipara with an uneventful antepartum course, is scheduled for induction of labor for postdates. On admission she is 1 cm dilated and 70% effaced, with the fetal vertex at –3 station. Fetal heart rate monitoring shows a normal baseline, moderate variability, and accelerations. No decelerations are observed.
After the membranes are ruptured artificially, labor progresses slowly, and chorioamnionitis is suspected.
Fetal tachycardia with minimal variability and variable decelerations develops. Oxytocin is titrated to achieve uterine contractions every 2 minutes. Elena eventually becomes completely dilated and pushes for 95 minutes. During this time, the fetal variable decelerations increase in duration, with loss of variability and continued tachycardia.
Because of these findings, delivery is expedited with a vacuum extractor. The newborn is depressed, admitted to the neonatal intensive care unit for respiratory support to “rule out sepsis,” and is later found to have neurologic injury.
In your opinion, does Elena have grounds for a lawsuit?
If such a case spurs a lawsuit, as it often will, the plaintiff’s attorney is likely to declare any or all of these allegations:
- failure to discontinue oxytocin in light of nonreassuring fetal heart rate
- failure to identify and respond to uterine hyperstimulation
- failure to identify and respond to fetal distress
- failure to react in a timely manner to fetal distress
- inappropriate delivery method
- failure to use a fetal scalp electrode
- failure to recognize and act upon arrest of dilatation in a timely manner
These allegations are only the most probable ones in circumstances such as Elena’s. When unanticipated morbidity or death occurs after oxytocin is used, physicians and nurses may find themselves facing any of the 18 allegations listed in the TABLE—or even others.
In court, these allegations will be based on the opinions of independent physicians, certified nurse-midwives, and registered nurses with the education, experience, and credentials to qualify as “experts.” Courts usually allow experts when the substance of the allegations is beyond the public’s general knowledge.
Although allegations often include inaccuracies, erroneous assumptions, and conclusions based on “information and belief” rather than scientific evidence, they remain part of the claim until disproved over the course of the legal proceedings.
TABLE
18 common allegations in oxytocin-related litigation
| 1. Unnecessary induction due to lack of medical indication |
| 2. Failure to establish fetal well-being prior to initiating oxytocin |
| 3. Failure to adequately monitor fetal heart rate during oxytocin infusion |
| 4. Failure to adequately monitor uterine contractions |
| 5. Failure to place a spiral electrode and/or intrauterine pressure catheter |
| 6. Failure to discontinue oxytocin in light of nonreassuring fetal heart rate |
| 7. Failure to identify and respond to fetal distress |
| 8. Delay in identifying and responding to nonreassuring fetal heart rate |
| 9. Failure to notify provider of nonreassuring fetal heart rate |
| 10. Failure to identify and respond to uterine hyperstimulation and/or elevated resting tone |
| 11. Inappropriate titration of oxytocin not based on accepted protocols |
| 12. Administration of oxytocin without a physician’s order |
| 13. Failure to follow physician’s order |
| 14. Failure to order a cesarean section when fetal heart rate became nonreassuring |
| 15. Delay in cesarean section after being ordered by the physician |
| 16. Failure to follow hospital policies and procedures |
| 17. Inadequate policies and procedures governing oxytocin administration |
| 18. Failure to initiate chain of command |
Elective inductions can spell trouble
Although the rate of induction has more than doubled since 1989, to 20.6% of births or more than 840,000 pregnancies in 2003,1 still no consensus exists for patient selection. In some centers, inductions are reserved for women with medical indications only, whereas in others, more than half are elective.2
Because of this divergence, when there is a negative outcome after an elective induction, the obstetrician can anticipate an allegation of unnecessary induction due to lack of a medical indication.
Fetal monitoring
Proven or not, it’s the norm
Although we lack overwhelming proof of its superiority to intermittent auscultation,3 electronic fetal monitoring (EFM) is used in most labor and delivery settings during oxytocin administration for induction or augmentation of labor—and fetal heart rate and uterine activity typically guide initiation and titration of oxytocin.
Nevertheless, because EFM is the unofficial standard, an obstetrician who chooses to use intermittent auscultation of fetal heart rate during oxytocin infusion can anticipate strong criticism if the delivery results in a compromised neonate.
A 2005 Cochrane review4 of 18,561 births compared EFM with intermittent auscultation in labor and delivery and found fewer neonatal seizures in the EFM group but no differences in Apgar scores of less than 4 and 7, NICU admissions, perinatal deaths, or cerebral palsy.
“Default” intervals
Not only is the type of fetal monitoring important, but also how closely and how often the strip is evaluated. However, no studies have determined the optimal frequency of EFM interpretation during normal labors, let alone those induced or augmented with oxytocin. Furthermore, no single best methodology has been identified. Rather, the “default” timing of EFM interpretation has been loosely based on the historical practice of evaluating and documenting intermittent auscultation at 30-minute intervals during active labor and 15-minute intervals during the second stage for low-risk patients. For high-risk patients, the intervals have been every 15 minutes during the active phase and every 5 minutes during the second stage.
What will expert witnesses look for?
After adverse outcomes, the EFM tracing will be examined closely by “experts” looking for evidence that it contained abnormalities demonstrating fetal compromise or predicting the infant’s injury or death.
These experts also scrutinize the actions of physicians and nurses for appropriateness, timeliness, and effectiveness; the timing of the decision for expedited delivery; and the events occurring between that decision and the time of delivery or abdominal skin incision.
The monitor’s shortcomings
Many courts now require experts to base their opinions on reliable scientific studies; however, in malpractice claims involving EFM, expert interpretation often is based on the expert’s own personal or institutional experience or common practices rather than scientific evidence.
One of the most pervasive public misconceptions is that fetal monitoring can reliably detect when a fetus lacks sufficient oxygen, is experiencing a physiologically stressful labor that is depleting oxygen reserves, or is becoming asphyxiated. In reality, the positive predictive value (ability of the technology to identify the compromised fetus without including healthy fetuses) is very low: 0.14%. Thus, of 1,000 fetuses with nonreassuring tracings, only 1 or 2 are actually compromised.5 This may explain why providers and nurses are reluctant to deem all nonreassuring recordings as accurate.
The only thing EFM reliably identifies with a high degree of specificity is the oxygenated fetus that is not experiencing metabolic acidemia. Recordings with “nonreassuring” features are statistically unlikely to imply a diagnosis of fetal metabolic acidosis, hypoxemia, or stress or distress.
Should EFM precede oxytocin?
No minimal duration of monitoring prior to oxytocin administration has been consistently determined. Researchers do not even agree that initial monitoring of the fetus scheduled for induction has benefit.
This does not mean that oxytocin can be started without knowledge of the maternal and fetal condition—only that the best timing and methods of assessment prior to induction of labor are unknown.
What is “nonreassuring”?
Starting oxytocin in a woman with a “non-reassuring” tracing opens the OB to criticism. This is the most contentious aspect of medical and nursing management because we lack standardized definitions of “reassuring” and “nonreassuring.”
Nurses typically label a tracing nonreassuring based solely on decelerations or other variant patterns such as tachycardia. However, while a tracing’s individual characteristics may reflect a variety of etiologies (one of which is decreased uteroplacental perfusion), variability and/or accelerations signify an overall reassuring status, or fetal tolerance of labor.
Physicians generally examine the tracing in light of other clinical factors, such as labor progress, historical data, or parity—and also in light of any specific actions that have been taken and the expected time of their peak effect.
When to notify the OB
Another contentious issue in labor induction is exactly when nurses should notify the physician of a nonreassuring fetal heart rate. Unfortunately, there is no consensus about this question, either; again, most EFM tracings requiring nursing intervention exhibit an overall reassuring status.
Because evaluation of nonreassuring findings may take several minutes, nurses usually notify the physician when their assessment is complete. If the worrisome tracing resolves after intervention, a nurse may appropriately postpone notification until the next opportunity for communication with the physician.
Uterine monitoring
Can monitoring predict rupture?
In cases involving uterine rupture and/or placental abruption, experts may allege that the event could have been predicted with an intrauterine pressure catheter. However, in a study of “controlled” uterine rupture (recording of intrauterine pressure before and during uterine incision at the time of cesarean section), Devoe et al6 found no real differences in contraction frequency or duration, peak contraction pressures, or uterine resting tone prior to and after uterine “rupture” (incision).
We also lack prospective studies demonstrating that intrauterine pressure catheterization can predict placental abruption. Placement of the device purely for this reason is not indicated.
Titration of oxytocin
No consensus on frequency or intensity of contractions
Criticism of the method of oxytocin titration is common in malpractice claims because no data satisfactorily define adequate frequency or intensity of contractions.
Nor do we have widely accepted terminology to describe uterine activity. For example, hyperstimulation is sometimes defined as increased frequency of contractions with an abnormal fetal heart rate tracing, and sometimes as increased frequency of contractions without a nonreassuring fetal heart rate. The same inconsistencies hold true for the terms “hypertonus,” “tetany,” “tachysystole,” and others.
“Adequate labor pattern” has been defined as 3 to 5 contractions in 10 minutes or 7 contractions in 15 minutes,7 even though these criteria are based on limited data. Although clinically adequate labor is defined by cervical dilatation and effacement with fetal descent, this definition frequently leaves us titrating oxytocin by “trial and error.” Fortunately, the half-life of oxytocin is short, and we can use fetal and uterine response to guide titration.
No definitive predictors of rupture, abruption, asphyxiation
When uterine rupture, placental abruption, and/or variant fetal heart patterns occur with hyperstimulation or elevated resting tone, the possibility of a cause-and-effect will be explored in legal claims. Although uterine rupture has been attributed to oxytocin in older, nonprospective, uncontrolled studies, more recent investigations8 failed to confirm this link.
The effect of uterine hyperstimulation on fetal oxygenation is even less well established. Contractions increase placental vascular resistance, which in turn decreases uteroplacental blood flow. This phenomenon has been demonstrated in studies utilizing Doppler velocimetry,9 radioangiography,10 and fetal pulse oximetry.11 However, none have been able to quantify, in millimeters of mercury, the intensity of uterine contractions or baseline tonus required to compromise fetal oxygenation.
Risk-reducing tactics
These strategies12 do not represent the standard of care, but may help reduce liability:
- Routinely assess fetal heart rate during examination of the laboring patient.
- Document EFM interpretation comprehensively. Include baseline, variability, accelerations, decelerations, and uterine activity, as well as overall impression.
- Date and time every entry.
- When notified of a finding, detail the notification, as well as the orders and plan of care communicated to the nurse.
- Develop a mechanism for documentation when you are located outside the hospital (eg, progress notes that are later posted in the chart).
- Use digital storage and retrieval with central monitoring of displays to allow physicians to observe EFM tracings via remote access.
- Use handheld PDA-type displays.
- Go to the bedside to evaluate a patient when nurses ask you to do so. Document date and time, and the fetal heart rate interpretation.
- Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion (FIGURE 1).
- Avoid further increases in oxytocin once adequate labor (progressive cervical change) is established.
- Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes (FIGURE 2).
- Use National Institute of Child Health and Human Development terminology in verbal communications with nurses and physicians (see the Web version of this article for a downloadable PDF file of this terminology).
Martin L. Gimovsky, MD
Program Director, Department of Obstetrics and Gynecology, Newark Beth Israel Medical Center, Newark, NJ
Clinical Professor of Obstetrics and Gynecology, Mount Sinai School of Medicine, New York City
As obstetricians, we are fortunate to participate in the most basic aspect of the human condition: the need to reproduce. Sometimes it is easy to overlook this fact, given the routine nature of many of our practices.
A case in point: oxytocin administration to induce or augment labor, an everyday occurrence in virtually all labor and delivery suites. Oxytocin is so ubiquitous, it can be easy to use it less than meticulously. Although the risks associated with its use are largely recognized, and the appropriate responses well known, a few points bear repeating.
Twin challenges: Protect and document
Safe and judicious use of oxytocin involves 2 challenges: minimizing medical risks to mother and fetus, and creating a supportive medical record. As in all aspects of medical care, we are required to know how to handle the clinical situation, and to document our skill, knowledge, and experience. Nowhere is this of greater concern than in the management of labor and delivery.
Here are 6 additional strategies for reducing legal risks of oxytocin use in labor.
1. Start with a written note
I suggest entering a written note into the record prior to administering oxytocin, outlining the reasoning behind the decision to proceed. Taking this pretreatment pause or “time out”—as the Joint Commission on Accreditation of Healthcare Organizations calls it—provides an opportunity to consider the risks, benefits, and alternatives of oxytocin use. This note should include the medical indication.
2. Conduct a comprehensive consent process
A passive signature on a general consent form is a minimalist way of demonstrating patient consent. By beginning the charting at the time of the consent discussion, you can demonstrate your consideration of the patient’s understanding and desires, not to mention your adherence to the highest standards of care.
Was an alternative approach possible? The patient should have the benefit of your opinion as well as a discussion of other reasonable strategies. Involving her in an active discussion is a fundamental component of informed consent—especially since improper consent is a frequent allegation in malpractice actions.
3. Describe both uterine and fetal responses
Because oxytocin directly affects uterine activity and indirectly affects placental perfusion, any chart notation needs to include references to both. For example, the comment that “contractions are every 2 minutes” requires the additional observation that the fetal heart rate tracing “is reassuring,”…“unchanged from earlier,”…or “demonstrates changes that are being evaluated.”
Whether a notation is made at the time of a routine labor check or when the physician is called to the bedside, comments on both uterine activity and fetal response are needed.
4. Discontinue oxytocin when the uterus overreacts
On occasion, excessive uterine activity may occur when oxytocin is first administered. Excessive uterine activity on a continuing basis can lead to fetal asphyxia. Although reducing the oxytocin dose will ultimately diminish uterine activity, I teach residents to discontinue oxytocin completely as soon as excessive uterine activity occurs.
Because this is a clinically important intervention, the medical record should be notated.
5. Adjust oxytocin to reflect changes in labor patterns
It makes good sense to avoid further oxytocin increases once the patient is in active labor (ie, progressive cervical change) and to decrease doses when contractions occur more frequently than every 2 minutes, even in the face of a reassuring fetal heart rate. This is not a situation in which, “if a little is good, a lot is better.”
6. Consider including a labor curve
Adding a labor curve or partograph to the chart can be a further safeguard, as it makes it easy to identify prolonged labors and potential complications in a timely manner.
All 6 strategies help demonstrate and preserve your hard work and concern for the patient. As always, adherence to principles of sound care and communication is the bedrock of successful obstetrics. There is no substitute.
The author reports no financial relationships relevant to this article.
FIGURE 1 Tachysystole with decelerations signifies uterine hyperstimulation
Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion. This tracing shows uterine hyperstimulation (tachysystole with decelerations).
FIGURE 2 Titrate oxytocin to “normalize” contractions
Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes. This tracing shows 6 uterine contractions in 10 minutes. The fetal heart rate channel demonstrates moderate variability and, therefore, fetal tolerance of a frequent contraction pattern.
Why policies and procedures are a double-edged sword
Although policies and procedures are intended to help guide health care assessments and interventions, they are routinely subpoenaed and entered as evidence in an attempt to define the standard of care. Failure to follow these policies and procedures may be viewed by expert witnesses as a breach in that standard.
Use of oxytocin requires a medical or nursing professional to make judgments based on training, experience, and knowledge. Although policies and procedures cannot address every possible scenario or replace informed judgment, physicians and nurses are routinely criticized for failing to administer oxytocin or otherwise proceed exactly as outlined.
Some reasons policies and procedures should not be viewed as standard of care:
- They are typically written by a person in an administrative position who does not actually provide the care outlined.
- They are usually not routinely updated as new literature is published.
- Since they do not provide guidelines for unanticipated or unusual situations, deviation from policy is reasonable and even necessary in many scenarios.
- They are rarely written to reflect “reasonable” care; instead, they suggest an “ideal” level of care.
Reasonable protocols. Every physician and health care provider should be familiar with the hospital’s policies and procedures and help hospital personnel revise those that appear to limit the physician’s ability to easily adjust care or exercise judgment. Among the suggestions:
- Make all recommendations practical. This means they can be followed most of the time in most situations.
- Avoid terms such as “mandatory,” “always,” “never,” “should,” or “must.”
- Limit recommendations that can be considered “endpoints” for increasing oxytocin, such as: “Increase oxytocin until contractions are 2 to 3 minutes apart and 60 seconds in duration.” Recommendations written in this fashion are difficult to follow clinically; although the criteria may be met, labor may not progress, warranting an increase in oxytocin beyond the endpoints in the guidelines. Guidelines that discuss considerations for decreasing or discontinuing the drug would be better.
It also is important to foster understanding among medical and nursing staff that policies and procedures are guidelines and that medical and nursing judgment supersedes policy recommendations.
The authors report no financial relationships relevant to this article.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Birth: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1-116.
2. Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstet Gynecol. 2002;100:164-167.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
4. Thacker SB, et al. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2005;(3):ISSN 1464-780X.
5. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 62, May 2005: Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;105:1161-1169.
6. Devoe LD, Croom CS, et al. The prediction of “controlled” uterine rupture by the use of intrauterine pressure catheters. Obstet Gynecol. 1992;80:626-629.
7. Norwitz ER, Robinson JN, Repke JT. Labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics. Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone; 2002;355.-
8. Phelan JP, Korst LM, Settles DK. Uterine activity patterns in uterine rupture: a case-control study. Obstet Gynecol. 1998;92:394-397.
9. Bower S, Campbell S, Vyas S, McGirr C. Braxton-Hicks contractions can alter uteroplacental perfusion. Ultrasound Obstet Gynecol. 1991;1:46-49.
10. Borell U, Fernstroem I, Ohlson L, Wiqvist N. Influence of uterine contractions on the uteroplacental blood flow at term. Am J Obstet Gynecol. 1965;93:44-57.
11. Johnson N, van Oudgaarden E, Montague I, McNamara H. The effect of oxytocin-induced hyperstimulation on fetal oxygen. Br J Obstet Gynaecol. 1994;101:805-807.
12. Lucidi RS, Chez RA, et al. The clinical use of intrauterine pressure catheters. J Matern Fetal Med. 2001;10:420-422.
- 18 common allegations in oxytocin-related litigation
- 6 risk-reducing strategies
Martin L. Gimovsky, MD
WHAT’S YOUR VERDICT?
Does this patient have grounds for a lawsuit?
At 41 weeks’ estimated gestation, Elena, a 32-year-old primipara with an uneventful antepartum course, is scheduled for induction of labor for postdates. On admission she is 1 cm dilated and 70% effaced, with the fetal vertex at –3 station. Fetal heart rate monitoring shows a normal baseline, moderate variability, and accelerations. No decelerations are observed.
After the membranes are ruptured artificially, labor progresses slowly, and chorioamnionitis is suspected.
Fetal tachycardia with minimal variability and variable decelerations develops. Oxytocin is titrated to achieve uterine contractions every 2 minutes. Elena eventually becomes completely dilated and pushes for 95 minutes. During this time, the fetal variable decelerations increase in duration, with loss of variability and continued tachycardia.
Because of these findings, delivery is expedited with a vacuum extractor. The newborn is depressed, admitted to the neonatal intensive care unit for respiratory support to “rule out sepsis,” and is later found to have neurologic injury.
In your opinion, does Elena have grounds for a lawsuit?
If such a case spurs a lawsuit, as it often will, the plaintiff’s attorney is likely to declare any or all of these allegations:
- failure to discontinue oxytocin in light of nonreassuring fetal heart rate
- failure to identify and respond to uterine hyperstimulation
- failure to identify and respond to fetal distress
- failure to react in a timely manner to fetal distress
- inappropriate delivery method
- failure to use a fetal scalp electrode
- failure to recognize and act upon arrest of dilatation in a timely manner
These allegations are only the most probable ones in circumstances such as Elena’s. When unanticipated morbidity or death occurs after oxytocin is used, physicians and nurses may find themselves facing any of the 18 allegations listed in the TABLE—or even others.
In court, these allegations will be based on the opinions of independent physicians, certified nurse-midwives, and registered nurses with the education, experience, and credentials to qualify as “experts.” Courts usually allow experts when the substance of the allegations is beyond the public’s general knowledge.
Although allegations often include inaccuracies, erroneous assumptions, and conclusions based on “information and belief” rather than scientific evidence, they remain part of the claim until disproved over the course of the legal proceedings.
TABLE
18 common allegations in oxytocin-related litigation
| 1. Unnecessary induction due to lack of medical indication |
| 2. Failure to establish fetal well-being prior to initiating oxytocin |
| 3. Failure to adequately monitor fetal heart rate during oxytocin infusion |
| 4. Failure to adequately monitor uterine contractions |
| 5. Failure to place a spiral electrode and/or intrauterine pressure catheter |
| 6. Failure to discontinue oxytocin in light of nonreassuring fetal heart rate |
| 7. Failure to identify and respond to fetal distress |
| 8. Delay in identifying and responding to nonreassuring fetal heart rate |
| 9. Failure to notify provider of nonreassuring fetal heart rate |
| 10. Failure to identify and respond to uterine hyperstimulation and/or elevated resting tone |
| 11. Inappropriate titration of oxytocin not based on accepted protocols |
| 12. Administration of oxytocin without a physician’s order |
| 13. Failure to follow physician’s order |
| 14. Failure to order a cesarean section when fetal heart rate became nonreassuring |
| 15. Delay in cesarean section after being ordered by the physician |
| 16. Failure to follow hospital policies and procedures |
| 17. Inadequate policies and procedures governing oxytocin administration |
| 18. Failure to initiate chain of command |
Elective inductions can spell trouble
Although the rate of induction has more than doubled since 1989, to 20.6% of births or more than 840,000 pregnancies in 2003,1 still no consensus exists for patient selection. In some centers, inductions are reserved for women with medical indications only, whereas in others, more than half are elective.2
Because of this divergence, when there is a negative outcome after an elective induction, the obstetrician can anticipate an allegation of unnecessary induction due to lack of a medical indication.
Fetal monitoring
Proven or not, it’s the norm
Although we lack overwhelming proof of its superiority to intermittent auscultation,3 electronic fetal monitoring (EFM) is used in most labor and delivery settings during oxytocin administration for induction or augmentation of labor—and fetal heart rate and uterine activity typically guide initiation and titration of oxytocin.
Nevertheless, because EFM is the unofficial standard, an obstetrician who chooses to use intermittent auscultation of fetal heart rate during oxytocin infusion can anticipate strong criticism if the delivery results in a compromised neonate.
A 2005 Cochrane review4 of 18,561 births compared EFM with intermittent auscultation in labor and delivery and found fewer neonatal seizures in the EFM group but no differences in Apgar scores of less than 4 and 7, NICU admissions, perinatal deaths, or cerebral palsy.
“Default” intervals
Not only is the type of fetal monitoring important, but also how closely and how often the strip is evaluated. However, no studies have determined the optimal frequency of EFM interpretation during normal labors, let alone those induced or augmented with oxytocin. Furthermore, no single best methodology has been identified. Rather, the “default” timing of EFM interpretation has been loosely based on the historical practice of evaluating and documenting intermittent auscultation at 30-minute intervals during active labor and 15-minute intervals during the second stage for low-risk patients. For high-risk patients, the intervals have been every 15 minutes during the active phase and every 5 minutes during the second stage.
What will expert witnesses look for?
After adverse outcomes, the EFM tracing will be examined closely by “experts” looking for evidence that it contained abnormalities demonstrating fetal compromise or predicting the infant’s injury or death.
These experts also scrutinize the actions of physicians and nurses for appropriateness, timeliness, and effectiveness; the timing of the decision for expedited delivery; and the events occurring between that decision and the time of delivery or abdominal skin incision.
The monitor’s shortcomings
Many courts now require experts to base their opinions on reliable scientific studies; however, in malpractice claims involving EFM, expert interpretation often is based on the expert’s own personal or institutional experience or common practices rather than scientific evidence.
One of the most pervasive public misconceptions is that fetal monitoring can reliably detect when a fetus lacks sufficient oxygen, is experiencing a physiologically stressful labor that is depleting oxygen reserves, or is becoming asphyxiated. In reality, the positive predictive value (ability of the technology to identify the compromised fetus without including healthy fetuses) is very low: 0.14%. Thus, of 1,000 fetuses with nonreassuring tracings, only 1 or 2 are actually compromised.5 This may explain why providers and nurses are reluctant to deem all nonreassuring recordings as accurate.
The only thing EFM reliably identifies with a high degree of specificity is the oxygenated fetus that is not experiencing metabolic acidemia. Recordings with “nonreassuring” features are statistically unlikely to imply a diagnosis of fetal metabolic acidosis, hypoxemia, or stress or distress.
Should EFM precede oxytocin?
No minimal duration of monitoring prior to oxytocin administration has been consistently determined. Researchers do not even agree that initial monitoring of the fetus scheduled for induction has benefit.
This does not mean that oxytocin can be started without knowledge of the maternal and fetal condition—only that the best timing and methods of assessment prior to induction of labor are unknown.
What is “nonreassuring”?
Starting oxytocin in a woman with a “non-reassuring” tracing opens the OB to criticism. This is the most contentious aspect of medical and nursing management because we lack standardized definitions of “reassuring” and “nonreassuring.”
Nurses typically label a tracing nonreassuring based solely on decelerations or other variant patterns such as tachycardia. However, while a tracing’s individual characteristics may reflect a variety of etiologies (one of which is decreased uteroplacental perfusion), variability and/or accelerations signify an overall reassuring status, or fetal tolerance of labor.
Physicians generally examine the tracing in light of other clinical factors, such as labor progress, historical data, or parity—and also in light of any specific actions that have been taken and the expected time of their peak effect.
When to notify the OB
Another contentious issue in labor induction is exactly when nurses should notify the physician of a nonreassuring fetal heart rate. Unfortunately, there is no consensus about this question, either; again, most EFM tracings requiring nursing intervention exhibit an overall reassuring status.
Because evaluation of nonreassuring findings may take several minutes, nurses usually notify the physician when their assessment is complete. If the worrisome tracing resolves after intervention, a nurse may appropriately postpone notification until the next opportunity for communication with the physician.
Uterine monitoring
Can monitoring predict rupture?
In cases involving uterine rupture and/or placental abruption, experts may allege that the event could have been predicted with an intrauterine pressure catheter. However, in a study of “controlled” uterine rupture (recording of intrauterine pressure before and during uterine incision at the time of cesarean section), Devoe et al6 found no real differences in contraction frequency or duration, peak contraction pressures, or uterine resting tone prior to and after uterine “rupture” (incision).
We also lack prospective studies demonstrating that intrauterine pressure catheterization can predict placental abruption. Placement of the device purely for this reason is not indicated.
Titration of oxytocin
No consensus on frequency or intensity of contractions
Criticism of the method of oxytocin titration is common in malpractice claims because no data satisfactorily define adequate frequency or intensity of contractions.
Nor do we have widely accepted terminology to describe uterine activity. For example, hyperstimulation is sometimes defined as increased frequency of contractions with an abnormal fetal heart rate tracing, and sometimes as increased frequency of contractions without a nonreassuring fetal heart rate. The same inconsistencies hold true for the terms “hypertonus,” “tetany,” “tachysystole,” and others.
“Adequate labor pattern” has been defined as 3 to 5 contractions in 10 minutes or 7 contractions in 15 minutes,7 even though these criteria are based on limited data. Although clinically adequate labor is defined by cervical dilatation and effacement with fetal descent, this definition frequently leaves us titrating oxytocin by “trial and error.” Fortunately, the half-life of oxytocin is short, and we can use fetal and uterine response to guide titration.
No definitive predictors of rupture, abruption, asphyxiation
When uterine rupture, placental abruption, and/or variant fetal heart patterns occur with hyperstimulation or elevated resting tone, the possibility of a cause-and-effect will be explored in legal claims. Although uterine rupture has been attributed to oxytocin in older, nonprospective, uncontrolled studies, more recent investigations8 failed to confirm this link.
The effect of uterine hyperstimulation on fetal oxygenation is even less well established. Contractions increase placental vascular resistance, which in turn decreases uteroplacental blood flow. This phenomenon has been demonstrated in studies utilizing Doppler velocimetry,9 radioangiography,10 and fetal pulse oximetry.11 However, none have been able to quantify, in millimeters of mercury, the intensity of uterine contractions or baseline tonus required to compromise fetal oxygenation.
Risk-reducing tactics
These strategies12 do not represent the standard of care, but may help reduce liability:
- Routinely assess fetal heart rate during examination of the laboring patient.
- Document EFM interpretation comprehensively. Include baseline, variability, accelerations, decelerations, and uterine activity, as well as overall impression.
- Date and time every entry.
- When notified of a finding, detail the notification, as well as the orders and plan of care communicated to the nurse.
- Develop a mechanism for documentation when you are located outside the hospital (eg, progress notes that are later posted in the chart).
- Use digital storage and retrieval with central monitoring of displays to allow physicians to observe EFM tracings via remote access.
- Use handheld PDA-type displays.
- Go to the bedside to evaluate a patient when nurses ask you to do so. Document date and time, and the fetal heart rate interpretation.
- Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion (FIGURE 1).
- Avoid further increases in oxytocin once adequate labor (progressive cervical change) is established.
- Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes (FIGURE 2).
- Use National Institute of Child Health and Human Development terminology in verbal communications with nurses and physicians (see the Web version of this article for a downloadable PDF file of this terminology).
Martin L. Gimovsky, MD
Program Director, Department of Obstetrics and Gynecology, Newark Beth Israel Medical Center, Newark, NJ
Clinical Professor of Obstetrics and Gynecology, Mount Sinai School of Medicine, New York City
As obstetricians, we are fortunate to participate in the most basic aspect of the human condition: the need to reproduce. Sometimes it is easy to overlook this fact, given the routine nature of many of our practices.
A case in point: oxytocin administration to induce or augment labor, an everyday occurrence in virtually all labor and delivery suites. Oxytocin is so ubiquitous, it can be easy to use it less than meticulously. Although the risks associated with its use are largely recognized, and the appropriate responses well known, a few points bear repeating.
Twin challenges: Protect and document
Safe and judicious use of oxytocin involves 2 challenges: minimizing medical risks to mother and fetus, and creating a supportive medical record. As in all aspects of medical care, we are required to know how to handle the clinical situation, and to document our skill, knowledge, and experience. Nowhere is this of greater concern than in the management of labor and delivery.
Here are 6 additional strategies for reducing legal risks of oxytocin use in labor.
1. Start with a written note
I suggest entering a written note into the record prior to administering oxytocin, outlining the reasoning behind the decision to proceed. Taking this pretreatment pause or “time out”—as the Joint Commission on Accreditation of Healthcare Organizations calls it—provides an opportunity to consider the risks, benefits, and alternatives of oxytocin use. This note should include the medical indication.
2. Conduct a comprehensive consent process
A passive signature on a general consent form is a minimalist way of demonstrating patient consent. By beginning the charting at the time of the consent discussion, you can demonstrate your consideration of the patient’s understanding and desires, not to mention your adherence to the highest standards of care.
Was an alternative approach possible? The patient should have the benefit of your opinion as well as a discussion of other reasonable strategies. Involving her in an active discussion is a fundamental component of informed consent—especially since improper consent is a frequent allegation in malpractice actions.
3. Describe both uterine and fetal responses
Because oxytocin directly affects uterine activity and indirectly affects placental perfusion, any chart notation needs to include references to both. For example, the comment that “contractions are every 2 minutes” requires the additional observation that the fetal heart rate tracing “is reassuring,”…“unchanged from earlier,”…or “demonstrates changes that are being evaluated.”
Whether a notation is made at the time of a routine labor check or when the physician is called to the bedside, comments on both uterine activity and fetal response are needed.
4. Discontinue oxytocin when the uterus overreacts
On occasion, excessive uterine activity may occur when oxytocin is first administered. Excessive uterine activity on a continuing basis can lead to fetal asphyxia. Although reducing the oxytocin dose will ultimately diminish uterine activity, I teach residents to discontinue oxytocin completely as soon as excessive uterine activity occurs.
Because this is a clinically important intervention, the medical record should be notated.
5. Adjust oxytocin to reflect changes in labor patterns
It makes good sense to avoid further oxytocin increases once the patient is in active labor (ie, progressive cervical change) and to decrease doses when contractions occur more frequently than every 2 minutes, even in the face of a reassuring fetal heart rate. This is not a situation in which, “if a little is good, a lot is better.”
6. Consider including a labor curve
Adding a labor curve or partograph to the chart can be a further safeguard, as it makes it easy to identify prolonged labors and potential complications in a timely manner.
All 6 strategies help demonstrate and preserve your hard work and concern for the patient. As always, adherence to principles of sound care and communication is the bedrock of successful obstetrics. There is no substitute.
The author reports no financial relationships relevant to this article.
FIGURE 1 Tachysystole with decelerations signifies uterine hyperstimulation
Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion. This tracing shows uterine hyperstimulation (tachysystole with decelerations).
FIGURE 2 Titrate oxytocin to “normalize” contractions
Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes. This tracing shows 6 uterine contractions in 10 minutes. The fetal heart rate channel demonstrates moderate variability and, therefore, fetal tolerance of a frequent contraction pattern.
Why policies and procedures are a double-edged sword
Although policies and procedures are intended to help guide health care assessments and interventions, they are routinely subpoenaed and entered as evidence in an attempt to define the standard of care. Failure to follow these policies and procedures may be viewed by expert witnesses as a breach in that standard.
Use of oxytocin requires a medical or nursing professional to make judgments based on training, experience, and knowledge. Although policies and procedures cannot address every possible scenario or replace informed judgment, physicians and nurses are routinely criticized for failing to administer oxytocin or otherwise proceed exactly as outlined.
Some reasons policies and procedures should not be viewed as standard of care:
- They are typically written by a person in an administrative position who does not actually provide the care outlined.
- They are usually not routinely updated as new literature is published.
- Since they do not provide guidelines for unanticipated or unusual situations, deviation from policy is reasonable and even necessary in many scenarios.
- They are rarely written to reflect “reasonable” care; instead, they suggest an “ideal” level of care.
Reasonable protocols. Every physician and health care provider should be familiar with the hospital’s policies and procedures and help hospital personnel revise those that appear to limit the physician’s ability to easily adjust care or exercise judgment. Among the suggestions:
- Make all recommendations practical. This means they can be followed most of the time in most situations.
- Avoid terms such as “mandatory,” “always,” “never,” “should,” or “must.”
- Limit recommendations that can be considered “endpoints” for increasing oxytocin, such as: “Increase oxytocin until contractions are 2 to 3 minutes apart and 60 seconds in duration.” Recommendations written in this fashion are difficult to follow clinically; although the criteria may be met, labor may not progress, warranting an increase in oxytocin beyond the endpoints in the guidelines. Guidelines that discuss considerations for decreasing or discontinuing the drug would be better.
It also is important to foster understanding among medical and nursing staff that policies and procedures are guidelines and that medical and nursing judgment supersedes policy recommendations.
The authors report no financial relationships relevant to this article.
- 18 common allegations in oxytocin-related litigation
- 6 risk-reducing strategies
Martin L. Gimovsky, MD
WHAT’S YOUR VERDICT?
Does this patient have grounds for a lawsuit?
At 41 weeks’ estimated gestation, Elena, a 32-year-old primipara with an uneventful antepartum course, is scheduled for induction of labor for postdates. On admission she is 1 cm dilated and 70% effaced, with the fetal vertex at –3 station. Fetal heart rate monitoring shows a normal baseline, moderate variability, and accelerations. No decelerations are observed.
After the membranes are ruptured artificially, labor progresses slowly, and chorioamnionitis is suspected.
Fetal tachycardia with minimal variability and variable decelerations develops. Oxytocin is titrated to achieve uterine contractions every 2 minutes. Elena eventually becomes completely dilated and pushes for 95 minutes. During this time, the fetal variable decelerations increase in duration, with loss of variability and continued tachycardia.
Because of these findings, delivery is expedited with a vacuum extractor. The newborn is depressed, admitted to the neonatal intensive care unit for respiratory support to “rule out sepsis,” and is later found to have neurologic injury.
In your opinion, does Elena have grounds for a lawsuit?
If such a case spurs a lawsuit, as it often will, the plaintiff’s attorney is likely to declare any or all of these allegations:
- failure to discontinue oxytocin in light of nonreassuring fetal heart rate
- failure to identify and respond to uterine hyperstimulation
- failure to identify and respond to fetal distress
- failure to react in a timely manner to fetal distress
- inappropriate delivery method
- failure to use a fetal scalp electrode
- failure to recognize and act upon arrest of dilatation in a timely manner
These allegations are only the most probable ones in circumstances such as Elena’s. When unanticipated morbidity or death occurs after oxytocin is used, physicians and nurses may find themselves facing any of the 18 allegations listed in the TABLE—or even others.
In court, these allegations will be based on the opinions of independent physicians, certified nurse-midwives, and registered nurses with the education, experience, and credentials to qualify as “experts.” Courts usually allow experts when the substance of the allegations is beyond the public’s general knowledge.
Although allegations often include inaccuracies, erroneous assumptions, and conclusions based on “information and belief” rather than scientific evidence, they remain part of the claim until disproved over the course of the legal proceedings.
TABLE
18 common allegations in oxytocin-related litigation
| 1. Unnecessary induction due to lack of medical indication |
| 2. Failure to establish fetal well-being prior to initiating oxytocin |
| 3. Failure to adequately monitor fetal heart rate during oxytocin infusion |
| 4. Failure to adequately monitor uterine contractions |
| 5. Failure to place a spiral electrode and/or intrauterine pressure catheter |
| 6. Failure to discontinue oxytocin in light of nonreassuring fetal heart rate |
| 7. Failure to identify and respond to fetal distress |
| 8. Delay in identifying and responding to nonreassuring fetal heart rate |
| 9. Failure to notify provider of nonreassuring fetal heart rate |
| 10. Failure to identify and respond to uterine hyperstimulation and/or elevated resting tone |
| 11. Inappropriate titration of oxytocin not based on accepted protocols |
| 12. Administration of oxytocin without a physician’s order |
| 13. Failure to follow physician’s order |
| 14. Failure to order a cesarean section when fetal heart rate became nonreassuring |
| 15. Delay in cesarean section after being ordered by the physician |
| 16. Failure to follow hospital policies and procedures |
| 17. Inadequate policies and procedures governing oxytocin administration |
| 18. Failure to initiate chain of command |
Elective inductions can spell trouble
Although the rate of induction has more than doubled since 1989, to 20.6% of births or more than 840,000 pregnancies in 2003,1 still no consensus exists for patient selection. In some centers, inductions are reserved for women with medical indications only, whereas in others, more than half are elective.2
Because of this divergence, when there is a negative outcome after an elective induction, the obstetrician can anticipate an allegation of unnecessary induction due to lack of a medical indication.
Fetal monitoring
Proven or not, it’s the norm
Although we lack overwhelming proof of its superiority to intermittent auscultation,3 electronic fetal monitoring (EFM) is used in most labor and delivery settings during oxytocin administration for induction or augmentation of labor—and fetal heart rate and uterine activity typically guide initiation and titration of oxytocin.
Nevertheless, because EFM is the unofficial standard, an obstetrician who chooses to use intermittent auscultation of fetal heart rate during oxytocin infusion can anticipate strong criticism if the delivery results in a compromised neonate.
A 2005 Cochrane review4 of 18,561 births compared EFM with intermittent auscultation in labor and delivery and found fewer neonatal seizures in the EFM group but no differences in Apgar scores of less than 4 and 7, NICU admissions, perinatal deaths, or cerebral palsy.
“Default” intervals
Not only is the type of fetal monitoring important, but also how closely and how often the strip is evaluated. However, no studies have determined the optimal frequency of EFM interpretation during normal labors, let alone those induced or augmented with oxytocin. Furthermore, no single best methodology has been identified. Rather, the “default” timing of EFM interpretation has been loosely based on the historical practice of evaluating and documenting intermittent auscultation at 30-minute intervals during active labor and 15-minute intervals during the second stage for low-risk patients. For high-risk patients, the intervals have been every 15 minutes during the active phase and every 5 minutes during the second stage.
What will expert witnesses look for?
After adverse outcomes, the EFM tracing will be examined closely by “experts” looking for evidence that it contained abnormalities demonstrating fetal compromise or predicting the infant’s injury or death.
These experts also scrutinize the actions of physicians and nurses for appropriateness, timeliness, and effectiveness; the timing of the decision for expedited delivery; and the events occurring between that decision and the time of delivery or abdominal skin incision.
The monitor’s shortcomings
Many courts now require experts to base their opinions on reliable scientific studies; however, in malpractice claims involving EFM, expert interpretation often is based on the expert’s own personal or institutional experience or common practices rather than scientific evidence.
One of the most pervasive public misconceptions is that fetal monitoring can reliably detect when a fetus lacks sufficient oxygen, is experiencing a physiologically stressful labor that is depleting oxygen reserves, or is becoming asphyxiated. In reality, the positive predictive value (ability of the technology to identify the compromised fetus without including healthy fetuses) is very low: 0.14%. Thus, of 1,000 fetuses with nonreassuring tracings, only 1 or 2 are actually compromised.5 This may explain why providers and nurses are reluctant to deem all nonreassuring recordings as accurate.
The only thing EFM reliably identifies with a high degree of specificity is the oxygenated fetus that is not experiencing metabolic acidemia. Recordings with “nonreassuring” features are statistically unlikely to imply a diagnosis of fetal metabolic acidosis, hypoxemia, or stress or distress.
Should EFM precede oxytocin?
No minimal duration of monitoring prior to oxytocin administration has been consistently determined. Researchers do not even agree that initial monitoring of the fetus scheduled for induction has benefit.
This does not mean that oxytocin can be started without knowledge of the maternal and fetal condition—only that the best timing and methods of assessment prior to induction of labor are unknown.
What is “nonreassuring”?
Starting oxytocin in a woman with a “non-reassuring” tracing opens the OB to criticism. This is the most contentious aspect of medical and nursing management because we lack standardized definitions of “reassuring” and “nonreassuring.”
Nurses typically label a tracing nonreassuring based solely on decelerations or other variant patterns such as tachycardia. However, while a tracing’s individual characteristics may reflect a variety of etiologies (one of which is decreased uteroplacental perfusion), variability and/or accelerations signify an overall reassuring status, or fetal tolerance of labor.
Physicians generally examine the tracing in light of other clinical factors, such as labor progress, historical data, or parity—and also in light of any specific actions that have been taken and the expected time of their peak effect.
When to notify the OB
Another contentious issue in labor induction is exactly when nurses should notify the physician of a nonreassuring fetal heart rate. Unfortunately, there is no consensus about this question, either; again, most EFM tracings requiring nursing intervention exhibit an overall reassuring status.
Because evaluation of nonreassuring findings may take several minutes, nurses usually notify the physician when their assessment is complete. If the worrisome tracing resolves after intervention, a nurse may appropriately postpone notification until the next opportunity for communication with the physician.
Uterine monitoring
Can monitoring predict rupture?
In cases involving uterine rupture and/or placental abruption, experts may allege that the event could have been predicted with an intrauterine pressure catheter. However, in a study of “controlled” uterine rupture (recording of intrauterine pressure before and during uterine incision at the time of cesarean section), Devoe et al6 found no real differences in contraction frequency or duration, peak contraction pressures, or uterine resting tone prior to and after uterine “rupture” (incision).
We also lack prospective studies demonstrating that intrauterine pressure catheterization can predict placental abruption. Placement of the device purely for this reason is not indicated.
Titration of oxytocin
No consensus on frequency or intensity of contractions
Criticism of the method of oxytocin titration is common in malpractice claims because no data satisfactorily define adequate frequency or intensity of contractions.
Nor do we have widely accepted terminology to describe uterine activity. For example, hyperstimulation is sometimes defined as increased frequency of contractions with an abnormal fetal heart rate tracing, and sometimes as increased frequency of contractions without a nonreassuring fetal heart rate. The same inconsistencies hold true for the terms “hypertonus,” “tetany,” “tachysystole,” and others.
“Adequate labor pattern” has been defined as 3 to 5 contractions in 10 minutes or 7 contractions in 15 minutes,7 even though these criteria are based on limited data. Although clinically adequate labor is defined by cervical dilatation and effacement with fetal descent, this definition frequently leaves us titrating oxytocin by “trial and error.” Fortunately, the half-life of oxytocin is short, and we can use fetal and uterine response to guide titration.
No definitive predictors of rupture, abruption, asphyxiation
When uterine rupture, placental abruption, and/or variant fetal heart patterns occur with hyperstimulation or elevated resting tone, the possibility of a cause-and-effect will be explored in legal claims. Although uterine rupture has been attributed to oxytocin in older, nonprospective, uncontrolled studies, more recent investigations8 failed to confirm this link.
The effect of uterine hyperstimulation on fetal oxygenation is even less well established. Contractions increase placental vascular resistance, which in turn decreases uteroplacental blood flow. This phenomenon has been demonstrated in studies utilizing Doppler velocimetry,9 radioangiography,10 and fetal pulse oximetry.11 However, none have been able to quantify, in millimeters of mercury, the intensity of uterine contractions or baseline tonus required to compromise fetal oxygenation.
Risk-reducing tactics
These strategies12 do not represent the standard of care, but may help reduce liability:
- Routinely assess fetal heart rate during examination of the laboring patient.
- Document EFM interpretation comprehensively. Include baseline, variability, accelerations, decelerations, and uterine activity, as well as overall impression.
- Date and time every entry.
- When notified of a finding, detail the notification, as well as the orders and plan of care communicated to the nurse.
- Develop a mechanism for documentation when you are located outside the hospital (eg, progress notes that are later posted in the chart).
- Use digital storage and retrieval with central monitoring of displays to allow physicians to observe EFM tracings via remote access.
- Use handheld PDA-type displays.
- Go to the bedside to evaluate a patient when nurses ask you to do so. Document date and time, and the fetal heart rate interpretation.
- Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion (FIGURE 1).
- Avoid further increases in oxytocin once adequate labor (progressive cervical change) is established.
- Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes (FIGURE 2).
- Use National Institute of Child Health and Human Development terminology in verbal communications with nurses and physicians (see the Web version of this article for a downloadable PDF file of this terminology).
Martin L. Gimovsky, MD
Program Director, Department of Obstetrics and Gynecology, Newark Beth Israel Medical Center, Newark, NJ
Clinical Professor of Obstetrics and Gynecology, Mount Sinai School of Medicine, New York City
As obstetricians, we are fortunate to participate in the most basic aspect of the human condition: the need to reproduce. Sometimes it is easy to overlook this fact, given the routine nature of many of our practices.
A case in point: oxytocin administration to induce or augment labor, an everyday occurrence in virtually all labor and delivery suites. Oxytocin is so ubiquitous, it can be easy to use it less than meticulously. Although the risks associated with its use are largely recognized, and the appropriate responses well known, a few points bear repeating.
Twin challenges: Protect and document
Safe and judicious use of oxytocin involves 2 challenges: minimizing medical risks to mother and fetus, and creating a supportive medical record. As in all aspects of medical care, we are required to know how to handle the clinical situation, and to document our skill, knowledge, and experience. Nowhere is this of greater concern than in the management of labor and delivery.
Here are 6 additional strategies for reducing legal risks of oxytocin use in labor.
1. Start with a written note
I suggest entering a written note into the record prior to administering oxytocin, outlining the reasoning behind the decision to proceed. Taking this pretreatment pause or “time out”—as the Joint Commission on Accreditation of Healthcare Organizations calls it—provides an opportunity to consider the risks, benefits, and alternatives of oxytocin use. This note should include the medical indication.
2. Conduct a comprehensive consent process
A passive signature on a general consent form is a minimalist way of demonstrating patient consent. By beginning the charting at the time of the consent discussion, you can demonstrate your consideration of the patient’s understanding and desires, not to mention your adherence to the highest standards of care.
Was an alternative approach possible? The patient should have the benefit of your opinion as well as a discussion of other reasonable strategies. Involving her in an active discussion is a fundamental component of informed consent—especially since improper consent is a frequent allegation in malpractice actions.
3. Describe both uterine and fetal responses
Because oxytocin directly affects uterine activity and indirectly affects placental perfusion, any chart notation needs to include references to both. For example, the comment that “contractions are every 2 minutes” requires the additional observation that the fetal heart rate tracing “is reassuring,”…“unchanged from earlier,”…or “demonstrates changes that are being evaluated.”
Whether a notation is made at the time of a routine labor check or when the physician is called to the bedside, comments on both uterine activity and fetal response are needed.
4. Discontinue oxytocin when the uterus overreacts
On occasion, excessive uterine activity may occur when oxytocin is first administered. Excessive uterine activity on a continuing basis can lead to fetal asphyxia. Although reducing the oxytocin dose will ultimately diminish uterine activity, I teach residents to discontinue oxytocin completely as soon as excessive uterine activity occurs.
Because this is a clinically important intervention, the medical record should be notated.
5. Adjust oxytocin to reflect changes in labor patterns
It makes good sense to avoid further oxytocin increases once the patient is in active labor (ie, progressive cervical change) and to decrease doses when contractions occur more frequently than every 2 minutes, even in the face of a reassuring fetal heart rate. This is not a situation in which, “if a little is good, a lot is better.”
6. Consider including a labor curve
Adding a labor curve or partograph to the chart can be a further safeguard, as it makes it easy to identify prolonged labors and potential complications in a timely manner.
All 6 strategies help demonstrate and preserve your hard work and concern for the patient. As always, adherence to principles of sound care and communication is the bedrock of successful obstetrics. There is no substitute.
The author reports no financial relationships relevant to this article.
FIGURE 1 Tachysystole with decelerations signifies uterine hyperstimulation
Decrease or discontinue oxytocin when variant fetal heart rate patterns suggest decreased uteroplacental perfusion. This tracing shows uterine hyperstimulation (tachysystole with decelerations).
FIGURE 2 Titrate oxytocin to “normalize” contractions
Consider decreasing oxytocin—or avoid further increases—when uterine contractions are more frequent than 5 in 10 minutes or 7 in 15 minutes. This tracing shows 6 uterine contractions in 10 minutes. The fetal heart rate channel demonstrates moderate variability and, therefore, fetal tolerance of a frequent contraction pattern.
Why policies and procedures are a double-edged sword
Although policies and procedures are intended to help guide health care assessments and interventions, they are routinely subpoenaed and entered as evidence in an attempt to define the standard of care. Failure to follow these policies and procedures may be viewed by expert witnesses as a breach in that standard.
Use of oxytocin requires a medical or nursing professional to make judgments based on training, experience, and knowledge. Although policies and procedures cannot address every possible scenario or replace informed judgment, physicians and nurses are routinely criticized for failing to administer oxytocin or otherwise proceed exactly as outlined.
Some reasons policies and procedures should not be viewed as standard of care:
- They are typically written by a person in an administrative position who does not actually provide the care outlined.
- They are usually not routinely updated as new literature is published.
- Since they do not provide guidelines for unanticipated or unusual situations, deviation from policy is reasonable and even necessary in many scenarios.
- They are rarely written to reflect “reasonable” care; instead, they suggest an “ideal” level of care.
Reasonable protocols. Every physician and health care provider should be familiar with the hospital’s policies and procedures and help hospital personnel revise those that appear to limit the physician’s ability to easily adjust care or exercise judgment. Among the suggestions:
- Make all recommendations practical. This means they can be followed most of the time in most situations.
- Avoid terms such as “mandatory,” “always,” “never,” “should,” or “must.”
- Limit recommendations that can be considered “endpoints” for increasing oxytocin, such as: “Increase oxytocin until contractions are 2 to 3 minutes apart and 60 seconds in duration.” Recommendations written in this fashion are difficult to follow clinically; although the criteria may be met, labor may not progress, warranting an increase in oxytocin beyond the endpoints in the guidelines. Guidelines that discuss considerations for decreasing or discontinuing the drug would be better.
It also is important to foster understanding among medical and nursing staff that policies and procedures are guidelines and that medical and nursing judgment supersedes policy recommendations.
The authors report no financial relationships relevant to this article.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Birth: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1-116.
2. Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstet Gynecol. 2002;100:164-167.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
4. Thacker SB, et al. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2005;(3):ISSN 1464-780X.
5. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 62, May 2005: Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;105:1161-1169.
6. Devoe LD, Croom CS, et al. The prediction of “controlled” uterine rupture by the use of intrauterine pressure catheters. Obstet Gynecol. 1992;80:626-629.
7. Norwitz ER, Robinson JN, Repke JT. Labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics. Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone; 2002;355.-
8. Phelan JP, Korst LM, Settles DK. Uterine activity patterns in uterine rupture: a case-control study. Obstet Gynecol. 1998;92:394-397.
9. Bower S, Campbell S, Vyas S, McGirr C. Braxton-Hicks contractions can alter uteroplacental perfusion. Ultrasound Obstet Gynecol. 1991;1:46-49.
10. Borell U, Fernstroem I, Ohlson L, Wiqvist N. Influence of uterine contractions on the uteroplacental blood flow at term. Am J Obstet Gynecol. 1965;93:44-57.
11. Johnson N, van Oudgaarden E, Montague I, McNamara H. The effect of oxytocin-induced hyperstimulation on fetal oxygen. Br J Obstet Gynaecol. 1994;101:805-807.
12. Lucidi RS, Chez RA, et al. The clinical use of intrauterine pressure catheters. J Matern Fetal Med. 2001;10:420-422.
1. Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Birth: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1-116.
2. Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstet Gynecol. 2002;100:164-167.
3. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445-1454.
4. Thacker SB, et al. Continuous electronic heart rate monitoring for fetal assessment during labor. Cochrane Database Syst Rev. 2005;(3):ISSN 1464-780X.
5. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin Number 62, May 2005: Intrapartum fetal heart rate monitoring. Obstet Gynecol. 2005;105:1161-1169.
6. Devoe LD, Croom CS, et al. The prediction of “controlled” uterine rupture by the use of intrauterine pressure catheters. Obstet Gynecol. 1992;80:626-629.
7. Norwitz ER, Robinson JN, Repke JT. Labor and delivery. In: Gabbe SG, Niebyl JR, Simpson JL, eds. Obstetrics. Normal and Problem Pregnancies. 4th ed. New York: Churchill Livingstone; 2002;355.-
8. Phelan JP, Korst LM, Settles DK. Uterine activity patterns in uterine rupture: a case-control study. Obstet Gynecol. 1998;92:394-397.
9. Bower S, Campbell S, Vyas S, McGirr C. Braxton-Hicks contractions can alter uteroplacental perfusion. Ultrasound Obstet Gynecol. 1991;1:46-49.
10. Borell U, Fernstroem I, Ohlson L, Wiqvist N. Influence of uterine contractions on the uteroplacental blood flow at term. Am J Obstet Gynecol. 1965;93:44-57.
11. Johnson N, van Oudgaarden E, Montague I, McNamara H. The effect of oxytocin-induced hyperstimulation on fetal oxygen. Br J Obstet Gynaecol. 1994;101:805-807.
12. Lucidi RS, Chez RA, et al. The clinical use of intrauterine pressure catheters. J Matern Fetal Med. 2001;10:420-422.
Preventing fragility fractures: Effective drugs and doses
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P<.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P<.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P<.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.