User login
Informed Consent Substitutes: A Decision Making Flow Chart
Reducing Wait Times for Cardiac Consultation
Milk-Alkali Syndrome [published correction appears in: Fed Pract. 2005;22(3):66.]
Frozen eggs and other marvels take “hi-tech” up a notch or 2
Be prepared for questions when the marketing starts
We can expect younger patients wishing to preserve reproductive capacity to ask our advice on freezing their eggs. (This technology is of limited applicability to the average reproductive-aged woman). The official position of the American Society for Reproductive Medicine is that, until outcome data are available, it is too early to incorporate this practice into general use.
However, a growing number of assisted reproductive technology (ART) centers will be offering—and marketing—the procedure.
Related to the growing interest in preserving female fertility so that women can delay childbearing: a momentous discovery reported in 2004 suggests that our time-honored dogma on growth of new human oocytes may be wrong.
In addition, 2 laboratory studies suggest a future for assisted reproductive technologies, when every embryo is assessed for its likelihood to result in a healthy infant.
These reports demonstrate the excitement of translational research in bringing basic discoveries into the clinical arena. They highlight the incredible development of both stem cell biology and nanotechnology, and suggest how such fields are rapidly becoming relevant in the modern practice of infertility. While it is difficult to predict an accurate timeline, or certainty, of the application of these discoveries to a modern infertility practice, it is safe to say that these and similar discoveries will influence our usual practice in the foreseeable future.
Oocytes unlimited after all?
Johnson J, Canning J, Kaneko T, Pru J, Tilly J. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150.
Along-held belief is that women reach their peak oocyte number at midgestation, and that number continuously declines until oocytes are depleted, sometime after menopause. This study from Jonathan Tilly’s group at Harvard Medical School presents evidence for germline stem cells in the ovaries of mice capable of replenishing oocytes into adult life. Removal of these germline stem cells caused a rapid depletion of primordial follicles, suggesting that the primordial follicle reserve was continuously replenished from germline stem cells.
While it is quite a leap to assume that human ovaries contain similar germline stem cells, the possibility that reproductive age woman may be able to develop new oocytes is truly exciting. If such oogonial stem cells exist in women—and could be harvested and cryopreserved, as mature oocytes are now being harvested and preserved—it opens the possibility that a renewable supply of oocytes may be available for women who wish to preserve the capacity to reproduce.
Practical implications
Whether for personal use following cancer therapy or oophorectomy, or as a source of oocytes for donation to women who have lost ovarian function, the potential holds great promise. However, extensive further work is required, not the least of which is independent verification of Dr. Tilly’s findings and the extension of that discovery to women.
For the practicing obstetrician/gynecologist fielding a question about “growing new eggs” from a savvy patient surfing the Net, know that the concept has a scientific basis but is definitely not ready for prime time.
New standard ahead for embryo assessment
In vivo assessment without injury
Kulkarni RN, Roper MG, Dahlgren G, Shih DQ, Kauri LM, Peters JL, Stoffel M, Kennedy RT. Islet secretory defect in insulin receptor substrate 1 null mice is linked with reduced calcium signaling and expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SECA)-2b and -3. Diabetes. 2004;53:1517–1525.
The assessment of human embryos for their viability and the likelihood of implanting and developing into a healthy baby has been a challenge for in vitro fertilization programs worldwide. Recent interest has focused on removing single cells from embryos and performing genetic studies. However, this is expensive, time consuming, and runs the risk of damaging embryos in the process of removing single blastomeres.
This study by Kennedy and colleagues, while not immediately applicable to human ART programs, demonstrates a technique for monitoring the secretion products from individual pancreatic beta cells. Using microelectrodes placed adjacent to individually cultured beta cells, insulin secretion from each cell was estimated by measuring the number of exocytotic events in normal and IRS-1 knock-out mice. The in vivo measurement of specific secretion products from individual cells without injuring them is an exciting example of the miniaturization of clinical science.
Practical implications
The extension of this technique to the safe assessment of human embryos in vivo while they are in culture is logical and opens up an entire area of both investigation and potential clinical practice. If the metabolic activity of the individual embryos indicates the likelihood of implantation and development, then this could become a new standard in the assessment of every embryo resulting from an ART cycle.
Much work will be required to translate this exciting new technique to human embryos, but it does offer a logical approach to the longstanding problem of embryo assessment.
Real-time assessment
Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reproductive Biomedicine Online. 2004;1(Jul-Aug):75–81.
One of the challenges of assessing individual human embryos in vivo is the dilution effect on any secretion products secreted from an embryo into the relatively vast amount of media in a traditional ART Petri dish. This report describes the first of a series of microfluidic devices designed to separate motile from nonmotile spermatozoa in very small volumes. Such devices can be used to isolate motile sperm from nonmotile sperm and debris for ART procedures when numbers are exceptionally low.
However, similar microfluidic devices are being developed to include tiny chambers that could be used to contain individual human embryos receiving a constant stream of nutrient media with a constant output of spent media and secretory products. In conjunction with the capacity to analyze such tiny amounts of secretory products in vivo from individual cells, this suggests a system for evaluating all human embryos in modern microchambers and continually monitoring for appropriate secretion and subsequent selection for optimal reproductive capacity.
Practical implications
The promise of real-time embryo assessment is certainly upon us, though much work needs to be done to develop these microfluidic incubation chambers before they will be clinically applicable.
For the practicing Ob/Gyn, it is useful to know that the era of preimplantation evaluation of all embryos is not far off. Whether that will translate into fewer fetal/neonatal defects remains to be seen.
The author reports no financial relationships relevant to this article.
Be prepared for questions when the marketing starts
We can expect younger patients wishing to preserve reproductive capacity to ask our advice on freezing their eggs. (This technology is of limited applicability to the average reproductive-aged woman). The official position of the American Society for Reproductive Medicine is that, until outcome data are available, it is too early to incorporate this practice into general use.
However, a growing number of assisted reproductive technology (ART) centers will be offering—and marketing—the procedure.
Related to the growing interest in preserving female fertility so that women can delay childbearing: a momentous discovery reported in 2004 suggests that our time-honored dogma on growth of new human oocytes may be wrong.
In addition, 2 laboratory studies suggest a future for assisted reproductive technologies, when every embryo is assessed for its likelihood to result in a healthy infant.
These reports demonstrate the excitement of translational research in bringing basic discoveries into the clinical arena. They highlight the incredible development of both stem cell biology and nanotechnology, and suggest how such fields are rapidly becoming relevant in the modern practice of infertility. While it is difficult to predict an accurate timeline, or certainty, of the application of these discoveries to a modern infertility practice, it is safe to say that these and similar discoveries will influence our usual practice in the foreseeable future.
Oocytes unlimited after all?
Johnson J, Canning J, Kaneko T, Pru J, Tilly J. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150.
Along-held belief is that women reach their peak oocyte number at midgestation, and that number continuously declines until oocytes are depleted, sometime after menopause. This study from Jonathan Tilly’s group at Harvard Medical School presents evidence for germline stem cells in the ovaries of mice capable of replenishing oocytes into adult life. Removal of these germline stem cells caused a rapid depletion of primordial follicles, suggesting that the primordial follicle reserve was continuously replenished from germline stem cells.
While it is quite a leap to assume that human ovaries contain similar germline stem cells, the possibility that reproductive age woman may be able to develop new oocytes is truly exciting. If such oogonial stem cells exist in women—and could be harvested and cryopreserved, as mature oocytes are now being harvested and preserved—it opens the possibility that a renewable supply of oocytes may be available for women who wish to preserve the capacity to reproduce.
Practical implications
Whether for personal use following cancer therapy or oophorectomy, or as a source of oocytes for donation to women who have lost ovarian function, the potential holds great promise. However, extensive further work is required, not the least of which is independent verification of Dr. Tilly’s findings and the extension of that discovery to women.
For the practicing obstetrician/gynecologist fielding a question about “growing new eggs” from a savvy patient surfing the Net, know that the concept has a scientific basis but is definitely not ready for prime time.
New standard ahead for embryo assessment
In vivo assessment without injury
Kulkarni RN, Roper MG, Dahlgren G, Shih DQ, Kauri LM, Peters JL, Stoffel M, Kennedy RT. Islet secretory defect in insulin receptor substrate 1 null mice is linked with reduced calcium signaling and expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SECA)-2b and -3. Diabetes. 2004;53:1517–1525.
The assessment of human embryos for their viability and the likelihood of implanting and developing into a healthy baby has been a challenge for in vitro fertilization programs worldwide. Recent interest has focused on removing single cells from embryos and performing genetic studies. However, this is expensive, time consuming, and runs the risk of damaging embryos in the process of removing single blastomeres.
This study by Kennedy and colleagues, while not immediately applicable to human ART programs, demonstrates a technique for monitoring the secretion products from individual pancreatic beta cells. Using microelectrodes placed adjacent to individually cultured beta cells, insulin secretion from each cell was estimated by measuring the number of exocytotic events in normal and IRS-1 knock-out mice. The in vivo measurement of specific secretion products from individual cells without injuring them is an exciting example of the miniaturization of clinical science.
Practical implications
The extension of this technique to the safe assessment of human embryos in vivo while they are in culture is logical and opens up an entire area of both investigation and potential clinical practice. If the metabolic activity of the individual embryos indicates the likelihood of implantation and development, then this could become a new standard in the assessment of every embryo resulting from an ART cycle.
Much work will be required to translate this exciting new technique to human embryos, but it does offer a logical approach to the longstanding problem of embryo assessment.
Real-time assessment
Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reproductive Biomedicine Online. 2004;1(Jul-Aug):75–81.
One of the challenges of assessing individual human embryos in vivo is the dilution effect on any secretion products secreted from an embryo into the relatively vast amount of media in a traditional ART Petri dish. This report describes the first of a series of microfluidic devices designed to separate motile from nonmotile spermatozoa in very small volumes. Such devices can be used to isolate motile sperm from nonmotile sperm and debris for ART procedures when numbers are exceptionally low.
However, similar microfluidic devices are being developed to include tiny chambers that could be used to contain individual human embryos receiving a constant stream of nutrient media with a constant output of spent media and secretory products. In conjunction with the capacity to analyze such tiny amounts of secretory products in vivo from individual cells, this suggests a system for evaluating all human embryos in modern microchambers and continually monitoring for appropriate secretion and subsequent selection for optimal reproductive capacity.
Practical implications
The promise of real-time embryo assessment is certainly upon us, though much work needs to be done to develop these microfluidic incubation chambers before they will be clinically applicable.
For the practicing Ob/Gyn, it is useful to know that the era of preimplantation evaluation of all embryos is not far off. Whether that will translate into fewer fetal/neonatal defects remains to be seen.
The author reports no financial relationships relevant to this article.
Be prepared for questions when the marketing starts
We can expect younger patients wishing to preserve reproductive capacity to ask our advice on freezing their eggs. (This technology is of limited applicability to the average reproductive-aged woman). The official position of the American Society for Reproductive Medicine is that, until outcome data are available, it is too early to incorporate this practice into general use.
However, a growing number of assisted reproductive technology (ART) centers will be offering—and marketing—the procedure.
Related to the growing interest in preserving female fertility so that women can delay childbearing: a momentous discovery reported in 2004 suggests that our time-honored dogma on growth of new human oocytes may be wrong.
In addition, 2 laboratory studies suggest a future for assisted reproductive technologies, when every embryo is assessed for its likelihood to result in a healthy infant.
These reports demonstrate the excitement of translational research in bringing basic discoveries into the clinical arena. They highlight the incredible development of both stem cell biology and nanotechnology, and suggest how such fields are rapidly becoming relevant in the modern practice of infertility. While it is difficult to predict an accurate timeline, or certainty, of the application of these discoveries to a modern infertility practice, it is safe to say that these and similar discoveries will influence our usual practice in the foreseeable future.
Oocytes unlimited after all?
Johnson J, Canning J, Kaneko T, Pru J, Tilly J. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150.
Along-held belief is that women reach their peak oocyte number at midgestation, and that number continuously declines until oocytes are depleted, sometime after menopause. This study from Jonathan Tilly’s group at Harvard Medical School presents evidence for germline stem cells in the ovaries of mice capable of replenishing oocytes into adult life. Removal of these germline stem cells caused a rapid depletion of primordial follicles, suggesting that the primordial follicle reserve was continuously replenished from germline stem cells.
While it is quite a leap to assume that human ovaries contain similar germline stem cells, the possibility that reproductive age woman may be able to develop new oocytes is truly exciting. If such oogonial stem cells exist in women—and could be harvested and cryopreserved, as mature oocytes are now being harvested and preserved—it opens the possibility that a renewable supply of oocytes may be available for women who wish to preserve the capacity to reproduce.
Practical implications
Whether for personal use following cancer therapy or oophorectomy, or as a source of oocytes for donation to women who have lost ovarian function, the potential holds great promise. However, extensive further work is required, not the least of which is independent verification of Dr. Tilly’s findings and the extension of that discovery to women.
For the practicing obstetrician/gynecologist fielding a question about “growing new eggs” from a savvy patient surfing the Net, know that the concept has a scientific basis but is definitely not ready for prime time.
New standard ahead for embryo assessment
In vivo assessment without injury
Kulkarni RN, Roper MG, Dahlgren G, Shih DQ, Kauri LM, Peters JL, Stoffel M, Kennedy RT. Islet secretory defect in insulin receptor substrate 1 null mice is linked with reduced calcium signaling and expression of sarco(endo)plasmic reticulum Ca2+-ATPase (SECA)-2b and -3. Diabetes. 2004;53:1517–1525.
The assessment of human embryos for their viability and the likelihood of implanting and developing into a healthy baby has been a challenge for in vitro fertilization programs worldwide. Recent interest has focused on removing single cells from embryos and performing genetic studies. However, this is expensive, time consuming, and runs the risk of damaging embryos in the process of removing single blastomeres.
This study by Kennedy and colleagues, while not immediately applicable to human ART programs, demonstrates a technique for monitoring the secretion products from individual pancreatic beta cells. Using microelectrodes placed adjacent to individually cultured beta cells, insulin secretion from each cell was estimated by measuring the number of exocytotic events in normal and IRS-1 knock-out mice. The in vivo measurement of specific secretion products from individual cells without injuring them is an exciting example of the miniaturization of clinical science.
Practical implications
The extension of this technique to the safe assessment of human embryos in vivo while they are in culture is logical and opens up an entire area of both investigation and potential clinical practice. If the metabolic activity of the individual embryos indicates the likelihood of implantation and development, then this could become a new standard in the assessment of every embryo resulting from an ART cycle.
Much work will be required to translate this exciting new technique to human embryos, but it does offer a logical approach to the longstanding problem of embryo assessment.
Real-time assessment
Schuster TG, Cho B, Keller LM, Takayama S, Smith GD. Isolation of motile spermatozoa from semen samples using microfluidics. Reproductive Biomedicine Online. 2004;1(Jul-Aug):75–81.
One of the challenges of assessing individual human embryos in vivo is the dilution effect on any secretion products secreted from an embryo into the relatively vast amount of media in a traditional ART Petri dish. This report describes the first of a series of microfluidic devices designed to separate motile from nonmotile spermatozoa in very small volumes. Such devices can be used to isolate motile sperm from nonmotile sperm and debris for ART procedures when numbers are exceptionally low.
However, similar microfluidic devices are being developed to include tiny chambers that could be used to contain individual human embryos receiving a constant stream of nutrient media with a constant output of spent media and secretory products. In conjunction with the capacity to analyze such tiny amounts of secretory products in vivo from individual cells, this suggests a system for evaluating all human embryos in modern microchambers and continually monitoring for appropriate secretion and subsequent selection for optimal reproductive capacity.
Practical implications
The promise of real-time embryo assessment is certainly upon us, though much work needs to be done to develop these microfluidic incubation chambers before they will be clinically applicable.
For the practicing Ob/Gyn, it is useful to know that the era of preimplantation evaluation of all embryos is not far off. Whether that will translate into fewer fetal/neonatal defects remains to be seen.
The author reports no financial relationships relevant to this article.
OBG Management ©2005 Dowden Health Media
Preeclampsia: 3 preemptive tactics
We routinely use every means possible to overcome the complications of hypertensive disorders and related preterm births. Yet our best opportunity to reduce morbidity and mortality could be before preeclampsia develops.
Preemptive tactics can be effective in preventing or reducing severity of preeclampsia. The patient’s active cooperation is a must, but the effort to recruit her cooperation can mean a better outcome.
If a diabetic or hypertensive woman doesn’t take her medications properly or if an obese woman postpones weight loss until after preeclampsia develops, it is too late to reduce the level of risk.
At-risk patients can benefit from being informed of any other ways to reduce risk as well; for example, by controlling the number of fetuses transferred via assisted reproductive techniques.
Trends that are driving up the prevalence of risk factors will only increase the number of preconception and obstetric cases with high-risk potential:
- The increased proportion of births among nulliparous women and women older than 35 years.
- The increased proportion of multifetal gestation as a result of assisted reproductive therapy.
- The increased prevalence of obesity in women, which is likely to lead to greater frequency of gestational diabetes, insulin resistance, and chronic hypertension.
Step 1Start risk-reducing tactics as early as possible
Retrospective studies have identified factors that multiply the risk of preeclampsia. Some are identifiable—and modifiable—before conception or beginning at the first prenatal visit (TABLE 1).
- Identify risk factors and recruit the patient’s efforts to reduce risks—before conception whenever possible.
- Set up prenatal care to watch closely for signal findings and make a prompt diagnosis.
- Develop a delivery plan that balances maternal and fetal needs. Identify indications for delivery.
Preconception risk factors
Obesity carries a 10 to 15% risk for preeclampsia. Prevention or effective treatment can greatly reduce risk.
Hypertension.Women with uncontrolled hypertension should have their blood pressure controlled prior to conception and as early as possible in the first trimester. In these women, the risk of preeclampsia may be reduced to below the 10 to 40% rate, depending on severity.
Renal disease. Risk for an adverse pregnancy outcome depends on maternal renal function at time of conception. Women should be encouraged to conceive while serum creatinine is less than 1.2 mg/dl.
Pregestational diabetes mellitus. Risk for preeclampsia and adverse outcomes depends on duration of diabetes, as well as vascular complications and blood sugar control prior to conception and early in pregnancy. Encourage these women to complete childbearing as early as possible and before vascular complications develop, and to aggressively control their diabetes and hypertension (if present) at least a few months prior to conception and throughout pregnancy.
Maternal age older than 35 years increases risk depending on associated medical conditions, nulliparity, and need for assisted reproductive therapy. These women are more likely to be nulliparous, overweight, chronically hypertensive, and to require assisted reproductive therapy. ART may involve multifetal gestation and donor insemination or oocyte donation—both of which increase risk and severity of preeclampsia. Therefore, these patients need to be made aware of their risks and helped to take steps to minimize risks.
TABLE 1
Preconception risk factors for preeclampsia
| 20 to 30% | Previous preeclampsia |
| 50% | Previous preeclampsia at 28 weeks |
| 15 to 25% | Chronic hypertension |
| 40% | Severe hypertension |
| 25% | Renal disease |
| 20% | Pregestational diabetes mellitus |
| 10 to 15% | Class B/C diabetes |
| 35% | Class F/R diabetes |
| 10 to 40% | Thrombophilia |
| 10 to 15% | Obesity/insulin resistance |
| 10 to 20% | Age >35 years |
| 10 to 15% | Family history of preeclampsia |
| 6 to 7% | Nulliparity/primipaternity |
Pregnancy-related risk factors
Many risk factors may be identified for the first time during pregnancy (TABLE 2). It is important to realize that the magnitude of risk depends on number of risk factors.
Nulliparity and primipaternity. Over the past decade, several epidemiologic studies suggested that immune maladaptation plays an important pathogenetic role in development of preeclampsia.
Generally, preeclampsia is considered a disease of first pregnancy. Indeed, a previous miscarriage of a previous normotensive pregnancy with the same partner is has a lowered frequency of preeclampsia. This protective effect is lost, however, with change of partner, suggesting that primipaternity increases the rate of preeclampsia.
A large prospective study on the relation between duration of sperm exposure with a partner and the rate of preeclampsia showed that women who conceive after a cohabitation period of 0 to 4 months have a 10-fold rate of preeclampsia, compared to those who conceive after a cohabitation period of at least 12 months. A similar study confirmed these findings.
The protective effects of long-term sperm exposure could explain the high frequency of preeclampsia in teenage pregnancy. (These women tend to have limited sperm exposure with a partner, or multiple partners). Thus, it is important to teach these women about their risks and the need for regular prenatal care.
Multifetal gestation increases the rate as well as the severity of preeclampsia, and the rate increases with the number of fetuses. Lowering the number of embryos transferred will substantially reduce the risk of preeclampsia and adverse outcomes.
There is no therapy to prevent preeclampsia in these women; however, we should acknowledge the increased risk and develop antenatal-care programs that allow close observation and early detection of preeclampsia in these women.
Hydropic degeneration of placenta. It is well-established that pregnancies complicated by fetal hydrops or hydropic degeneration of the placenta (with or without a coexisting fetus) are at very high risk for preeclampsia. In these cases, preeclampsia usually develops in the second trimester and is usually severe, and therefore causes substantial maternal and perinatal morbidities. Development of preeclampsia in such pregnancies requires immediate hospitalization and consideration for prompt delivery.
Unexplained elevated serum markers in the second trimester. Maternal serum screening with alpha fetoprotein (AFP), human chorionic gonadotropin (HCG) and inhibin A is commonly used to identify those at risk for aneuploidy or neural tube defects.
Unexplained elevations in AFP, HCG or inhibin A have been associated with increased adverse pregnancy outcome such as fetal death, intrauterine growth restriction (IUGR), preterm delivery, and preeclampsia. However, the data on the association between abnormalities in these biomarkers and preeclampsia have been inconsistent. Nevertheless, retrospective studies suggest that elevation in these serum markers during the second trimester increases the risk of preeclampsia by at least twofold. The risk is probably higher in those who have abnormalities in more than 1 of these markers. Since unexplained abnormalities of these serum markers may reflect early placental pathology, it is suggested that these pregnancies may benefit from close obstetric surveillance.
Serum and urinary markers of abnormal angiogenesis and subsequent preeclampsia were strongly associate, in newly published studies reported by Levine and colleagues. For example, circulating soluble fms-like tyrosine kinase (sFLt1) is elevated in pregnant women prior to onset of preeclampsia, whereas urinary placental growth factor is reduced several weeks prior to clinical onset of preeclampsia. Both of these markers appear to hold some promise.
Unexplained proteinuria or hematuria. Generally, proteinuria is considered a late manifestation of preeclampsia. However, recent retrospective studies suggest that some women with preeclampsia, particularly those with HELLP syndrome, might not have hypertension (>140 mm Hg systolic or >90 mm Hg diastolic). In some women, persistent proteinuria (3+ on dipstick) or >300 mg/24 hour may be the first sign of preeclampsia or could be a marker of silent renal disease.
No prospective studies have evaluated the risk of preeclampsia in asymptomatic women with persistent proteinuria. I suggest, however, that women with this finding will benefit from intensified obstetric surveillance (more frequent prenatal visits) and/or biochemical evaluation (platelet count, liver enzymes), particularly if they have headaches, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, or respiratory symptoms (chest pain or shortness of breath)—likewise, for pregnant women with persistent hematuria of unknown origin.
Unexplained fetal growth restriction. Impaired trophoblast invasion is a key features of pregnancies complicated by preeclampsia or unexplained IUGR. Preeclampsia can manifest either as a maternal syndrome (hypertension and proteinuria with or without symptoms) or a fetal abnormal growth syndrome.
In clinical practice, most cases of unexplained IUGR are probably delivered before the maternal syndrome develops. In some cases, unexplained IUGR may be the first manifestation of preeclampsia, particularly those with IUGR before 34 weeks’ gestation. The absolute risk of clinical preeclampsia in such women is unknown because of lack of prospective data. Nevertheless, a woman with idiopathic IUGR prior to 34 weeks’ gestation whose pregnancy is managed expectantly is at increased risk for future preeclampsia. These women should receive intensive maternal surveillance for preeclampsia, and a diagnosis of preeclampsia should be considered in those who develop maternal symptoms or abnormal blood tests.
Abnormal uterine artery Doppler velocimetry at 18 to 24 weeks’ gestation. Several observational studies reported an association between elevated uterine artery resistance as measured by Doppler (with or without presence of a notch) in the second trimester and subsequent preeclampsia and/or IUGR. The reported rates of preeclampsia among women with abnormal Doppler results range from 6% to 40%. The risk varies depending on the site measured, gestational age at time of measurement, normal indices used, abnormality on repeat measurement, and population studied.
A systemic review of 27 studies, which included approximately 13,000 women, revealed that an abnormal uterine artery Doppler waveform increases the risk of preeclampsia by 4- to 6-fold, compared to normal Doppler results. The review concluded that uterine artery Doppler evaluation has a limited value as a screening test to predict preeclampsia.
What should the physician do when faced with an ultrasound report indicating an abnormal uterine artery Doppler finding?
Is low-dose aspirin helpful? Several randomized trials evaluated the potential role of low-dose aspirin in reducing the risk of preeclampsia in women with abnormal uterine artery Doppler indices. A meta-analysis suggested that low-dose aspirin significantly reduced the rate of preeclampsia (16% in placebo versus 10% with aspirin, odds ratio of 0.55). This analysis included a total of 498 subjects.
In contrast, a recent randomized trial in 560 women with abnormal uterine artery Doppler at 23 weeks’, who were assigned to aspirin 150 mg or placebo, found no differences in rates of preeclampsia (18% versus 19%) or in preeclampsia requiring delivery before 34 weeks’ (6% versus 8%). A similar randomized trial using 100 mg aspirin daily in 237 women with abnormal uterine artery Doppler at 22 to 24 weeks revealed no reduction in rate of preeclampsia compared to placebo.
Consequently, low-dose aspirin is not recommended for prevention of preeclamp-sia in these women.
Close surveillance is warranted. Although there is no available proven therapy to reduce the risk of preeclampsia in these women, they should be closely observed because of the increased rate of adverse outcomes, including preeclampsia.
TABLE 2
Pregnancy-related risk factors for preeclampsia
| Magnitude of risk depends on the number of factors | |
|---|---|
| 2-fold normal | Unexplained midtrimester elevations of serum AFP, HCG, inhibin-A |
| 10 to 30% | Abnormal uterine artery Doppler velocimetry |
| 0 to 30% | Hydrops/hydropic degeneration of placenta |
| 10 to 20% | Multifetal gestation (depends on number of fetuses and maternal age) |
| 10% | Partner who fathered preeclampsia in another woman |
| 8 to 10% | Gestational diabetes mellitus |
| 8 to 10% | Limited sperm exposure (teenage pregnancy) |
| 6 to 7% | Nulliparity/primipaternity |
| Limited data | Donor insemination, oocyte donation |
| Limited data | Unexplained persistent proteinuria or hematuria |
| Unknown | Unexplained fetal growth restriction |
Step 2Watch for signal findings, diagnose preeclampsia early
Signs and symptoms may call for close surveillance at any time. Early detection of preeclampsia is the best way to reduce adverse outcomes.
Prenatal care does not prevent preeclampsia, of course. All pregnant women are at risk, some more than others. Still, adequate and proper prenatal care is the best strategy to detect preeclampsia early.
We may need to modify the frequency and type of maternal and fetal surveillance at any time. Thus, patients with multiple risk factors or risk exceeding 10% should have more frequent visits, especially beyond 24 weeks. Maternal blood pressure (both systolic and diastolic), urine protein values, abrupt and excessive weight gain, maternal symptoms, and fetal growth warrant particular attention.
Diagnostic criteria vary with risk
The diagnosis of preeclampsia is different in patients with different risk factors. In healthy nulliparous women, the diagnosis requires persistent hypertension and proteinuria (new onset after 20 weeks’ gestation). However, in some patients the diagnosis should be made based on new onset hypertension and maternal symptoms or abnormal blood tests (low platelets or elevated liver enzymes).
Urine dipstick is a reliable screening test in women who remain normotensive.
24-hour urine measurement is the best test to confirm proteinuria if hypertension develops. Several studies found that urine dipstick values less than (1+) and random urine protein to creatinine ratio measurements are not accurate to predict proteinuria in women with gestational hypertension.
When is it gestational hypertension? The term applies only women with all of these findings:
- mild hypertension <160/<110 mm Hg
- proteinuria <300 mg/24-hour urine
- normal platelet count
- normal liver enzymes
- normal fetal growth
- no maternal symptoms
Once gestational hypertension is diagnosed, obtain blood tests and ultrasound evaluation to document fetal growth and amniotic fluid status.
Women with severe gestational hypertension and those with abnormal tests should be diagnosed as having preeclampsia and managed as such.
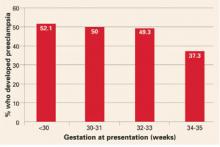
Women with gestational hypertension are at high risk for preeclampsia, and risk of progression depends on gestational age at time of diagnosis. Women who develop gestational hypertension at 24 to 35 weeks have a 46% chance of developing preeclampsia with a high rate of preterm delivery (32% <36 weeks and 12.5% <34 weeks) (FIGURE). These women require very close surveillance. In contrast, maternal and perinatal outcome is usually favorable when only mild gestational hypertension develops at or beyond 36 weeks.
When hypertension, proteinuria occur before 20 weeks
The traditional diagnostic criteria for preeclampsia in healthy women are not reliable in women who have either hypertension or proteinuria prior to 20 weeks’ gestation, particularly in those taking antihypertensive medications and in those who have class F diabetes mellitus. Because of the physiologic changes during pregnancy, women with diabetes and renal disease will have serial increases in blood pressure as well as protein excretion with advanced gestational age, particularly in the third trimester. Diagnostic criteria (TABLE 3) should be individualized based on medical conditions and current therapy. Antihypertensive drugs and preexisting proteinuria make it more difficult to classify preeclampsia as mild or severe.
TABLE 3
Diagnostic criteria
| GESTATIONAL HYPERTENSION IN HEALTHY WOMEN | |
| Blood pressure <160 mm Hg diastolic and <110 mm Hg systolic | |
| Proteinuria <300 mg/24-hour collection | |
| Platelet count >100,000/mm3 | |
| Normal liver enzymes | |
| No maternal symptoms | |
| No intrauterine growth restriction or oligohydraminos by ultrasound | |
| PREECLAMPSIA IN WOMEN WITH PREEXISTING MEDICAL CONDITIONS | |
| Condition | Criteria |
| Hypertension only | Proteinuria >500 mg/24-hours or thrombocytopenia |
| Proteinuria only | New onset hypertension plus symptoms or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | Worsening severe hypertension and/or new onset of symptoms, thrombocytopenia, elevated liver enzymes |
FIGURE Whether preeclampsia will develop depends on when gestational hypertension begins
Adapted from Barton JR, et al. Am J Obstet Gynecol. 2001;184:979-983.
Step 3Consider how to balance risk to mother and fetus
Once a diagnosis is made, promptly evaluate mother and fetus, continue close surveillance, select those who will benefit from hospitalization, and identify indications for delivery (TABLE 4).
Delivery will always reduce the risks for the mother, but in certain situations, it might not be the best option for an extremely premature fetus. Sometimes delivery is best for both mother and fetus.
The best strategy takes into consideration:
- maternal and fetal status at initial evaluation,
- preexisting medical conditions that could affect pregnancy outcome,
- fetal gestational age at time of diagnosis,
- labor or rupture of fetal membranes (both could affect management), and
- maternal choice of available options.
Women who remain undelivered require close maternal and fetal evaluation. In otherwise healthy women, management depends on whether the preeclampsia is mild or severe, and, if there are other medical conditions, on the status of those conditions, as well.
TABLE 4
Indications for delivery
| Consider delivery in gravidas with 1 or more indications |
|---|
| Gestational age ≥38 weeks for mild disease |
| Gestational age ≥34 weeks for severe disease |
| 33-34 weeks with severe disease after steroids |
| Onset of labor and/or membrane rupture ≥34 weeks |
| Eclampsia or pulmonary edema (any gestational age) |
| HELLP syndrome (any gestational age) |
| Severe cerebral symptoms or epigastric pain |
| Acute renal insufficiency (serum creatinine >1.2 mg/dl) |
| Persistent thrombocytopenia (platelet count <100,000) |
| Maternal desire for delivery |
| Severe oligohydraminos or IUGR < 5th percentile |
| Nonreassuring fetal testing |
Chronic hypertension
- Underlies 30% of cases of hypertension during pregnancy.
- Begins before pregnancy or before 20 weeks’ gestation.
Gestational hypertension
- The most common form of hypertension during pregnancy.
- Acute onset beyond 20 weeks’ gestation in a woman known to be normotensive before pregnancy or prior to 20 weeks’ gestation.
Preeclampsia
- Can superimpose upon chronic hypertension, renal disease, or connective tissue disease, or develop in women with gestational hypertension.
- Preeclampsia in healthy nulliparous women: hypertension and proteinuria after 20 weeks’ gestation.
- Preeclampsia in women with preexisting chronic hypertension and absent proteinuria: an exacerbation of hypertension and new onset proteinuria.
Eclampsia
- Development of convulsions in women with hypertensive disorders of pregnancy.
“HELLP syndrome”
- Hemolysis,
- Elevated liver enzymes, and
- Low platelet count
Suspected or confirmed preeclampsia in a woman who has documented evidence of hemolysis (abnormal peripheral smear, or elevated bilirubin, or anemia, or low heptoglobin levels), plus elevated liver enzymes (AST or ALT), and thrombocytopenia (platelet count below 100,000).
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol. 2001;184:979-983.
Boggess KA, Lief S, Martha AP, Moos K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227-231.
Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66-71.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med. 1998;338:701-705.
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219-224.
Chien PE, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An Overview. Br J Obstet Gynaecol. 2000;107:196-208.
Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: A meta-analysis. Obstet Gynecol. 2001;98:861-866.
Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Dekker G, Robillard PY. The birth interval hypothesis - Does it really indicate the end of the primipaternity hypothesis? J Reprod Immunol. 2003;59:245-251.
Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209-215.
Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848-52.
Einarsson JI, Sangi-Haghpeykar H, Gardner NO. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol. 2003;188:1241-1243.
Hauth JC, Ewell MG, Levine RL, Esterlitz JR, Sibai BM, Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Hnat MD, Sibai BM, Caritis S, Hiouth J, Lindheimer MD, MacPherson C. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparous. Am J Obstet Gynecol. 2002;186:422-426.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Levine RG, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77-85.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P. The importance of genetic and environmental effects for preeclampsia and gestational hypertension: a family study. Br J Obstet Gynaecol. 2004;111:200-206.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
Ragip A Al, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol. 2004;104:367-371.
Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol. 2003;157:1108-1114.
Sibai BM, Caritis S, Hauth J, Lilndheimer MD, MacPherson C, Klebanoff M, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938-942.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-33377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Subtil D, Goeusse P, Houfflin-Debarge V, Puech F, Lequien P, Breart G, Uzan S, Quandalle F, Delcourt YM, Malek YM. Essai Regional Aspirine Mere-Enfant (ERASME) Collaborative Group. Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 2). Br J Obstet Gynaecol. 2003;110:485-491.
Wen SW, Demissie K, Yang Q, Walker MC. Maternal morbidity and obstetric complications in triplet pregnancies and quadruplet and higher-order multiple pregnancies. Am J Obstet Gynecol. 2004;191:254-258.
Yu CKH, Papageorghiou AT, Parra M, Dias RP, Nicolaides KH. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;22:233-239.
We routinely use every means possible to overcome the complications of hypertensive disorders and related preterm births. Yet our best opportunity to reduce morbidity and mortality could be before preeclampsia develops.
Preemptive tactics can be effective in preventing or reducing severity of preeclampsia. The patient’s active cooperation is a must, but the effort to recruit her cooperation can mean a better outcome.
If a diabetic or hypertensive woman doesn’t take her medications properly or if an obese woman postpones weight loss until after preeclampsia develops, it is too late to reduce the level of risk.
At-risk patients can benefit from being informed of any other ways to reduce risk as well; for example, by controlling the number of fetuses transferred via assisted reproductive techniques.
Trends that are driving up the prevalence of risk factors will only increase the number of preconception and obstetric cases with high-risk potential:
- The increased proportion of births among nulliparous women and women older than 35 years.
- The increased proportion of multifetal gestation as a result of assisted reproductive therapy.
- The increased prevalence of obesity in women, which is likely to lead to greater frequency of gestational diabetes, insulin resistance, and chronic hypertension.
Step 1Start risk-reducing tactics as early as possible
Retrospective studies have identified factors that multiply the risk of preeclampsia. Some are identifiable—and modifiable—before conception or beginning at the first prenatal visit (TABLE 1).
- Identify risk factors and recruit the patient’s efforts to reduce risks—before conception whenever possible.
- Set up prenatal care to watch closely for signal findings and make a prompt diagnosis.
- Develop a delivery plan that balances maternal and fetal needs. Identify indications for delivery.
Preconception risk factors
Obesity carries a 10 to 15% risk for preeclampsia. Prevention or effective treatment can greatly reduce risk.
Hypertension.Women with uncontrolled hypertension should have their blood pressure controlled prior to conception and as early as possible in the first trimester. In these women, the risk of preeclampsia may be reduced to below the 10 to 40% rate, depending on severity.
Renal disease. Risk for an adverse pregnancy outcome depends on maternal renal function at time of conception. Women should be encouraged to conceive while serum creatinine is less than 1.2 mg/dl.
Pregestational diabetes mellitus. Risk for preeclampsia and adverse outcomes depends on duration of diabetes, as well as vascular complications and blood sugar control prior to conception and early in pregnancy. Encourage these women to complete childbearing as early as possible and before vascular complications develop, and to aggressively control their diabetes and hypertension (if present) at least a few months prior to conception and throughout pregnancy.
Maternal age older than 35 years increases risk depending on associated medical conditions, nulliparity, and need for assisted reproductive therapy. These women are more likely to be nulliparous, overweight, chronically hypertensive, and to require assisted reproductive therapy. ART may involve multifetal gestation and donor insemination or oocyte donation—both of which increase risk and severity of preeclampsia. Therefore, these patients need to be made aware of their risks and helped to take steps to minimize risks.
TABLE 1
Preconception risk factors for preeclampsia
| 20 to 30% | Previous preeclampsia |
| 50% | Previous preeclampsia at 28 weeks |
| 15 to 25% | Chronic hypertension |
| 40% | Severe hypertension |
| 25% | Renal disease |
| 20% | Pregestational diabetes mellitus |
| 10 to 15% | Class B/C diabetes |
| 35% | Class F/R diabetes |
| 10 to 40% | Thrombophilia |
| 10 to 15% | Obesity/insulin resistance |
| 10 to 20% | Age >35 years |
| 10 to 15% | Family history of preeclampsia |
| 6 to 7% | Nulliparity/primipaternity |
Pregnancy-related risk factors
Many risk factors may be identified for the first time during pregnancy (TABLE 2). It is important to realize that the magnitude of risk depends on number of risk factors.
Nulliparity and primipaternity. Over the past decade, several epidemiologic studies suggested that immune maladaptation plays an important pathogenetic role in development of preeclampsia.
Generally, preeclampsia is considered a disease of first pregnancy. Indeed, a previous miscarriage of a previous normotensive pregnancy with the same partner is has a lowered frequency of preeclampsia. This protective effect is lost, however, with change of partner, suggesting that primipaternity increases the rate of preeclampsia.
A large prospective study on the relation between duration of sperm exposure with a partner and the rate of preeclampsia showed that women who conceive after a cohabitation period of 0 to 4 months have a 10-fold rate of preeclampsia, compared to those who conceive after a cohabitation period of at least 12 months. A similar study confirmed these findings.
The protective effects of long-term sperm exposure could explain the high frequency of preeclampsia in teenage pregnancy. (These women tend to have limited sperm exposure with a partner, or multiple partners). Thus, it is important to teach these women about their risks and the need for regular prenatal care.
Multifetal gestation increases the rate as well as the severity of preeclampsia, and the rate increases with the number of fetuses. Lowering the number of embryos transferred will substantially reduce the risk of preeclampsia and adverse outcomes.
There is no therapy to prevent preeclampsia in these women; however, we should acknowledge the increased risk and develop antenatal-care programs that allow close observation and early detection of preeclampsia in these women.
Hydropic degeneration of placenta. It is well-established that pregnancies complicated by fetal hydrops or hydropic degeneration of the placenta (with or without a coexisting fetus) are at very high risk for preeclampsia. In these cases, preeclampsia usually develops in the second trimester and is usually severe, and therefore causes substantial maternal and perinatal morbidities. Development of preeclampsia in such pregnancies requires immediate hospitalization and consideration for prompt delivery.
Unexplained elevated serum markers in the second trimester. Maternal serum screening with alpha fetoprotein (AFP), human chorionic gonadotropin (HCG) and inhibin A is commonly used to identify those at risk for aneuploidy or neural tube defects.
Unexplained elevations in AFP, HCG or inhibin A have been associated with increased adverse pregnancy outcome such as fetal death, intrauterine growth restriction (IUGR), preterm delivery, and preeclampsia. However, the data on the association between abnormalities in these biomarkers and preeclampsia have been inconsistent. Nevertheless, retrospective studies suggest that elevation in these serum markers during the second trimester increases the risk of preeclampsia by at least twofold. The risk is probably higher in those who have abnormalities in more than 1 of these markers. Since unexplained abnormalities of these serum markers may reflect early placental pathology, it is suggested that these pregnancies may benefit from close obstetric surveillance.
Serum and urinary markers of abnormal angiogenesis and subsequent preeclampsia were strongly associate, in newly published studies reported by Levine and colleagues. For example, circulating soluble fms-like tyrosine kinase (sFLt1) is elevated in pregnant women prior to onset of preeclampsia, whereas urinary placental growth factor is reduced several weeks prior to clinical onset of preeclampsia. Both of these markers appear to hold some promise.
Unexplained proteinuria or hematuria. Generally, proteinuria is considered a late manifestation of preeclampsia. However, recent retrospective studies suggest that some women with preeclampsia, particularly those with HELLP syndrome, might not have hypertension (>140 mm Hg systolic or >90 mm Hg diastolic). In some women, persistent proteinuria (3+ on dipstick) or >300 mg/24 hour may be the first sign of preeclampsia or could be a marker of silent renal disease.
No prospective studies have evaluated the risk of preeclampsia in asymptomatic women with persistent proteinuria. I suggest, however, that women with this finding will benefit from intensified obstetric surveillance (more frequent prenatal visits) and/or biochemical evaluation (platelet count, liver enzymes), particularly if they have headaches, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, or respiratory symptoms (chest pain or shortness of breath)—likewise, for pregnant women with persistent hematuria of unknown origin.
Unexplained fetal growth restriction. Impaired trophoblast invasion is a key features of pregnancies complicated by preeclampsia or unexplained IUGR. Preeclampsia can manifest either as a maternal syndrome (hypertension and proteinuria with or without symptoms) or a fetal abnormal growth syndrome.
In clinical practice, most cases of unexplained IUGR are probably delivered before the maternal syndrome develops. In some cases, unexplained IUGR may be the first manifestation of preeclampsia, particularly those with IUGR before 34 weeks’ gestation. The absolute risk of clinical preeclampsia in such women is unknown because of lack of prospective data. Nevertheless, a woman with idiopathic IUGR prior to 34 weeks’ gestation whose pregnancy is managed expectantly is at increased risk for future preeclampsia. These women should receive intensive maternal surveillance for preeclampsia, and a diagnosis of preeclampsia should be considered in those who develop maternal symptoms or abnormal blood tests.
Abnormal uterine artery Doppler velocimetry at 18 to 24 weeks’ gestation. Several observational studies reported an association between elevated uterine artery resistance as measured by Doppler (with or without presence of a notch) in the second trimester and subsequent preeclampsia and/or IUGR. The reported rates of preeclampsia among women with abnormal Doppler results range from 6% to 40%. The risk varies depending on the site measured, gestational age at time of measurement, normal indices used, abnormality on repeat measurement, and population studied.
A systemic review of 27 studies, which included approximately 13,000 women, revealed that an abnormal uterine artery Doppler waveform increases the risk of preeclampsia by 4- to 6-fold, compared to normal Doppler results. The review concluded that uterine artery Doppler evaluation has a limited value as a screening test to predict preeclampsia.
What should the physician do when faced with an ultrasound report indicating an abnormal uterine artery Doppler finding?
Is low-dose aspirin helpful? Several randomized trials evaluated the potential role of low-dose aspirin in reducing the risk of preeclampsia in women with abnormal uterine artery Doppler indices. A meta-analysis suggested that low-dose aspirin significantly reduced the rate of preeclampsia (16% in placebo versus 10% with aspirin, odds ratio of 0.55). This analysis included a total of 498 subjects.
In contrast, a recent randomized trial in 560 women with abnormal uterine artery Doppler at 23 weeks’, who were assigned to aspirin 150 mg or placebo, found no differences in rates of preeclampsia (18% versus 19%) or in preeclampsia requiring delivery before 34 weeks’ (6% versus 8%). A similar randomized trial using 100 mg aspirin daily in 237 women with abnormal uterine artery Doppler at 22 to 24 weeks revealed no reduction in rate of preeclampsia compared to placebo.
Consequently, low-dose aspirin is not recommended for prevention of preeclamp-sia in these women.
Close surveillance is warranted. Although there is no available proven therapy to reduce the risk of preeclampsia in these women, they should be closely observed because of the increased rate of adverse outcomes, including preeclampsia.
TABLE 2
Pregnancy-related risk factors for preeclampsia
| Magnitude of risk depends on the number of factors | |
|---|---|
| 2-fold normal | Unexplained midtrimester elevations of serum AFP, HCG, inhibin-A |
| 10 to 30% | Abnormal uterine artery Doppler velocimetry |
| 0 to 30% | Hydrops/hydropic degeneration of placenta |
| 10 to 20% | Multifetal gestation (depends on number of fetuses and maternal age) |
| 10% | Partner who fathered preeclampsia in another woman |
| 8 to 10% | Gestational diabetes mellitus |
| 8 to 10% | Limited sperm exposure (teenage pregnancy) |
| 6 to 7% | Nulliparity/primipaternity |
| Limited data | Donor insemination, oocyte donation |
| Limited data | Unexplained persistent proteinuria or hematuria |
| Unknown | Unexplained fetal growth restriction |
Step 2Watch for signal findings, diagnose preeclampsia early
Signs and symptoms may call for close surveillance at any time. Early detection of preeclampsia is the best way to reduce adverse outcomes.
Prenatal care does not prevent preeclampsia, of course. All pregnant women are at risk, some more than others. Still, adequate and proper prenatal care is the best strategy to detect preeclampsia early.
We may need to modify the frequency and type of maternal and fetal surveillance at any time. Thus, patients with multiple risk factors or risk exceeding 10% should have more frequent visits, especially beyond 24 weeks. Maternal blood pressure (both systolic and diastolic), urine protein values, abrupt and excessive weight gain, maternal symptoms, and fetal growth warrant particular attention.
Diagnostic criteria vary with risk
The diagnosis of preeclampsia is different in patients with different risk factors. In healthy nulliparous women, the diagnosis requires persistent hypertension and proteinuria (new onset after 20 weeks’ gestation). However, in some patients the diagnosis should be made based on new onset hypertension and maternal symptoms or abnormal blood tests (low platelets or elevated liver enzymes).
Urine dipstick is a reliable screening test in women who remain normotensive.
24-hour urine measurement is the best test to confirm proteinuria if hypertension develops. Several studies found that urine dipstick values less than (1+) and random urine protein to creatinine ratio measurements are not accurate to predict proteinuria in women with gestational hypertension.
When is it gestational hypertension? The term applies only women with all of these findings:
- mild hypertension <160/<110 mm Hg
- proteinuria <300 mg/24-hour urine
- normal platelet count
- normal liver enzymes
- normal fetal growth
- no maternal symptoms
Once gestational hypertension is diagnosed, obtain blood tests and ultrasound evaluation to document fetal growth and amniotic fluid status.
Women with severe gestational hypertension and those with abnormal tests should be diagnosed as having preeclampsia and managed as such.
Women with gestational hypertension are at high risk for preeclampsia, and risk of progression depends on gestational age at time of diagnosis. Women who develop gestational hypertension at 24 to 35 weeks have a 46% chance of developing preeclampsia with a high rate of preterm delivery (32% <36 weeks and 12.5% <34 weeks) (FIGURE). These women require very close surveillance. In contrast, maternal and perinatal outcome is usually favorable when only mild gestational hypertension develops at or beyond 36 weeks.
When hypertension, proteinuria occur before 20 weeks
The traditional diagnostic criteria for preeclampsia in healthy women are not reliable in women who have either hypertension or proteinuria prior to 20 weeks’ gestation, particularly in those taking antihypertensive medications and in those who have class F diabetes mellitus. Because of the physiologic changes during pregnancy, women with diabetes and renal disease will have serial increases in blood pressure as well as protein excretion with advanced gestational age, particularly in the third trimester. Diagnostic criteria (TABLE 3) should be individualized based on medical conditions and current therapy. Antihypertensive drugs and preexisting proteinuria make it more difficult to classify preeclampsia as mild or severe.
TABLE 3
Diagnostic criteria
| GESTATIONAL HYPERTENSION IN HEALTHY WOMEN | |
| Blood pressure <160 mm Hg diastolic and <110 mm Hg systolic | |
| Proteinuria <300 mg/24-hour collection | |
| Platelet count >100,000/mm3 | |
| Normal liver enzymes | |
| No maternal symptoms | |
| No intrauterine growth restriction or oligohydraminos by ultrasound | |
| PREECLAMPSIA IN WOMEN WITH PREEXISTING MEDICAL CONDITIONS | |
| Condition | Criteria |
| Hypertension only | Proteinuria >500 mg/24-hours or thrombocytopenia |
| Proteinuria only | New onset hypertension plus symptoms or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | Worsening severe hypertension and/or new onset of symptoms, thrombocytopenia, elevated liver enzymes |
FIGURE Whether preeclampsia will develop depends on when gestational hypertension begins
Adapted from Barton JR, et al. Am J Obstet Gynecol. 2001;184:979-983.
Step 3Consider how to balance risk to mother and fetus
Once a diagnosis is made, promptly evaluate mother and fetus, continue close surveillance, select those who will benefit from hospitalization, and identify indications for delivery (TABLE 4).
Delivery will always reduce the risks for the mother, but in certain situations, it might not be the best option for an extremely premature fetus. Sometimes delivery is best for both mother and fetus.
The best strategy takes into consideration:
- maternal and fetal status at initial evaluation,
- preexisting medical conditions that could affect pregnancy outcome,
- fetal gestational age at time of diagnosis,
- labor or rupture of fetal membranes (both could affect management), and
- maternal choice of available options.
Women who remain undelivered require close maternal and fetal evaluation. In otherwise healthy women, management depends on whether the preeclampsia is mild or severe, and, if there are other medical conditions, on the status of those conditions, as well.
TABLE 4
Indications for delivery
| Consider delivery in gravidas with 1 or more indications |
|---|
| Gestational age ≥38 weeks for mild disease |
| Gestational age ≥34 weeks for severe disease |
| 33-34 weeks with severe disease after steroids |
| Onset of labor and/or membrane rupture ≥34 weeks |
| Eclampsia or pulmonary edema (any gestational age) |
| HELLP syndrome (any gestational age) |
| Severe cerebral symptoms or epigastric pain |
| Acute renal insufficiency (serum creatinine >1.2 mg/dl) |
| Persistent thrombocytopenia (platelet count <100,000) |
| Maternal desire for delivery |
| Severe oligohydraminos or IUGR < 5th percentile |
| Nonreassuring fetal testing |
Chronic hypertension
- Underlies 30% of cases of hypertension during pregnancy.
- Begins before pregnancy or before 20 weeks’ gestation.
Gestational hypertension
- The most common form of hypertension during pregnancy.
- Acute onset beyond 20 weeks’ gestation in a woman known to be normotensive before pregnancy or prior to 20 weeks’ gestation.
Preeclampsia
- Can superimpose upon chronic hypertension, renal disease, or connective tissue disease, or develop in women with gestational hypertension.
- Preeclampsia in healthy nulliparous women: hypertension and proteinuria after 20 weeks’ gestation.
- Preeclampsia in women with preexisting chronic hypertension and absent proteinuria: an exacerbation of hypertension and new onset proteinuria.
Eclampsia
- Development of convulsions in women with hypertensive disorders of pregnancy.
“HELLP syndrome”
- Hemolysis,
- Elevated liver enzymes, and
- Low platelet count
Suspected or confirmed preeclampsia in a woman who has documented evidence of hemolysis (abnormal peripheral smear, or elevated bilirubin, or anemia, or low heptoglobin levels), plus elevated liver enzymes (AST or ALT), and thrombocytopenia (platelet count below 100,000).
The author reports no financial relationships relevant to this article.
We routinely use every means possible to overcome the complications of hypertensive disorders and related preterm births. Yet our best opportunity to reduce morbidity and mortality could be before preeclampsia develops.
Preemptive tactics can be effective in preventing or reducing severity of preeclampsia. The patient’s active cooperation is a must, but the effort to recruit her cooperation can mean a better outcome.
If a diabetic or hypertensive woman doesn’t take her medications properly or if an obese woman postpones weight loss until after preeclampsia develops, it is too late to reduce the level of risk.
At-risk patients can benefit from being informed of any other ways to reduce risk as well; for example, by controlling the number of fetuses transferred via assisted reproductive techniques.
Trends that are driving up the prevalence of risk factors will only increase the number of preconception and obstetric cases with high-risk potential:
- The increased proportion of births among nulliparous women and women older than 35 years.
- The increased proportion of multifetal gestation as a result of assisted reproductive therapy.
- The increased prevalence of obesity in women, which is likely to lead to greater frequency of gestational diabetes, insulin resistance, and chronic hypertension.
Step 1Start risk-reducing tactics as early as possible
Retrospective studies have identified factors that multiply the risk of preeclampsia. Some are identifiable—and modifiable—before conception or beginning at the first prenatal visit (TABLE 1).
- Identify risk factors and recruit the patient’s efforts to reduce risks—before conception whenever possible.
- Set up prenatal care to watch closely for signal findings and make a prompt diagnosis.
- Develop a delivery plan that balances maternal and fetal needs. Identify indications for delivery.
Preconception risk factors
Obesity carries a 10 to 15% risk for preeclampsia. Prevention or effective treatment can greatly reduce risk.
Hypertension.Women with uncontrolled hypertension should have their blood pressure controlled prior to conception and as early as possible in the first trimester. In these women, the risk of preeclampsia may be reduced to below the 10 to 40% rate, depending on severity.
Renal disease. Risk for an adverse pregnancy outcome depends on maternal renal function at time of conception. Women should be encouraged to conceive while serum creatinine is less than 1.2 mg/dl.
Pregestational diabetes mellitus. Risk for preeclampsia and adverse outcomes depends on duration of diabetes, as well as vascular complications and blood sugar control prior to conception and early in pregnancy. Encourage these women to complete childbearing as early as possible and before vascular complications develop, and to aggressively control their diabetes and hypertension (if present) at least a few months prior to conception and throughout pregnancy.
Maternal age older than 35 years increases risk depending on associated medical conditions, nulliparity, and need for assisted reproductive therapy. These women are more likely to be nulliparous, overweight, chronically hypertensive, and to require assisted reproductive therapy. ART may involve multifetal gestation and donor insemination or oocyte donation—both of which increase risk and severity of preeclampsia. Therefore, these patients need to be made aware of their risks and helped to take steps to minimize risks.
TABLE 1
Preconception risk factors for preeclampsia
| 20 to 30% | Previous preeclampsia |
| 50% | Previous preeclampsia at 28 weeks |
| 15 to 25% | Chronic hypertension |
| 40% | Severe hypertension |
| 25% | Renal disease |
| 20% | Pregestational diabetes mellitus |
| 10 to 15% | Class B/C diabetes |
| 35% | Class F/R diabetes |
| 10 to 40% | Thrombophilia |
| 10 to 15% | Obesity/insulin resistance |
| 10 to 20% | Age >35 years |
| 10 to 15% | Family history of preeclampsia |
| 6 to 7% | Nulliparity/primipaternity |
Pregnancy-related risk factors
Many risk factors may be identified for the first time during pregnancy (TABLE 2). It is important to realize that the magnitude of risk depends on number of risk factors.
Nulliparity and primipaternity. Over the past decade, several epidemiologic studies suggested that immune maladaptation plays an important pathogenetic role in development of preeclampsia.
Generally, preeclampsia is considered a disease of first pregnancy. Indeed, a previous miscarriage of a previous normotensive pregnancy with the same partner is has a lowered frequency of preeclampsia. This protective effect is lost, however, with change of partner, suggesting that primipaternity increases the rate of preeclampsia.
A large prospective study on the relation between duration of sperm exposure with a partner and the rate of preeclampsia showed that women who conceive after a cohabitation period of 0 to 4 months have a 10-fold rate of preeclampsia, compared to those who conceive after a cohabitation period of at least 12 months. A similar study confirmed these findings.
The protective effects of long-term sperm exposure could explain the high frequency of preeclampsia in teenage pregnancy. (These women tend to have limited sperm exposure with a partner, or multiple partners). Thus, it is important to teach these women about their risks and the need for regular prenatal care.
Multifetal gestation increases the rate as well as the severity of preeclampsia, and the rate increases with the number of fetuses. Lowering the number of embryos transferred will substantially reduce the risk of preeclampsia and adverse outcomes.
There is no therapy to prevent preeclampsia in these women; however, we should acknowledge the increased risk and develop antenatal-care programs that allow close observation and early detection of preeclampsia in these women.
Hydropic degeneration of placenta. It is well-established that pregnancies complicated by fetal hydrops or hydropic degeneration of the placenta (with or without a coexisting fetus) are at very high risk for preeclampsia. In these cases, preeclampsia usually develops in the second trimester and is usually severe, and therefore causes substantial maternal and perinatal morbidities. Development of preeclampsia in such pregnancies requires immediate hospitalization and consideration for prompt delivery.
Unexplained elevated serum markers in the second trimester. Maternal serum screening with alpha fetoprotein (AFP), human chorionic gonadotropin (HCG) and inhibin A is commonly used to identify those at risk for aneuploidy or neural tube defects.
Unexplained elevations in AFP, HCG or inhibin A have been associated with increased adverse pregnancy outcome such as fetal death, intrauterine growth restriction (IUGR), preterm delivery, and preeclampsia. However, the data on the association between abnormalities in these biomarkers and preeclampsia have been inconsistent. Nevertheless, retrospective studies suggest that elevation in these serum markers during the second trimester increases the risk of preeclampsia by at least twofold. The risk is probably higher in those who have abnormalities in more than 1 of these markers. Since unexplained abnormalities of these serum markers may reflect early placental pathology, it is suggested that these pregnancies may benefit from close obstetric surveillance.
Serum and urinary markers of abnormal angiogenesis and subsequent preeclampsia were strongly associate, in newly published studies reported by Levine and colleagues. For example, circulating soluble fms-like tyrosine kinase (sFLt1) is elevated in pregnant women prior to onset of preeclampsia, whereas urinary placental growth factor is reduced several weeks prior to clinical onset of preeclampsia. Both of these markers appear to hold some promise.
Unexplained proteinuria or hematuria. Generally, proteinuria is considered a late manifestation of preeclampsia. However, recent retrospective studies suggest that some women with preeclampsia, particularly those with HELLP syndrome, might not have hypertension (>140 mm Hg systolic or >90 mm Hg diastolic). In some women, persistent proteinuria (3+ on dipstick) or >300 mg/24 hour may be the first sign of preeclampsia or could be a marker of silent renal disease.
No prospective studies have evaluated the risk of preeclampsia in asymptomatic women with persistent proteinuria. I suggest, however, that women with this finding will benefit from intensified obstetric surveillance (more frequent prenatal visits) and/or biochemical evaluation (platelet count, liver enzymes), particularly if they have headaches, visual changes, epigastric or right upper quadrant pain, nausea or vomiting, or respiratory symptoms (chest pain or shortness of breath)—likewise, for pregnant women with persistent hematuria of unknown origin.
Unexplained fetal growth restriction. Impaired trophoblast invasion is a key features of pregnancies complicated by preeclampsia or unexplained IUGR. Preeclampsia can manifest either as a maternal syndrome (hypertension and proteinuria with or without symptoms) or a fetal abnormal growth syndrome.
In clinical practice, most cases of unexplained IUGR are probably delivered before the maternal syndrome develops. In some cases, unexplained IUGR may be the first manifestation of preeclampsia, particularly those with IUGR before 34 weeks’ gestation. The absolute risk of clinical preeclampsia in such women is unknown because of lack of prospective data. Nevertheless, a woman with idiopathic IUGR prior to 34 weeks’ gestation whose pregnancy is managed expectantly is at increased risk for future preeclampsia. These women should receive intensive maternal surveillance for preeclampsia, and a diagnosis of preeclampsia should be considered in those who develop maternal symptoms or abnormal blood tests.
Abnormal uterine artery Doppler velocimetry at 18 to 24 weeks’ gestation. Several observational studies reported an association between elevated uterine artery resistance as measured by Doppler (with or without presence of a notch) in the second trimester and subsequent preeclampsia and/or IUGR. The reported rates of preeclampsia among women with abnormal Doppler results range from 6% to 40%. The risk varies depending on the site measured, gestational age at time of measurement, normal indices used, abnormality on repeat measurement, and population studied.
A systemic review of 27 studies, which included approximately 13,000 women, revealed that an abnormal uterine artery Doppler waveform increases the risk of preeclampsia by 4- to 6-fold, compared to normal Doppler results. The review concluded that uterine artery Doppler evaluation has a limited value as a screening test to predict preeclampsia.
What should the physician do when faced with an ultrasound report indicating an abnormal uterine artery Doppler finding?
Is low-dose aspirin helpful? Several randomized trials evaluated the potential role of low-dose aspirin in reducing the risk of preeclampsia in women with abnormal uterine artery Doppler indices. A meta-analysis suggested that low-dose aspirin significantly reduced the rate of preeclampsia (16% in placebo versus 10% with aspirin, odds ratio of 0.55). This analysis included a total of 498 subjects.
In contrast, a recent randomized trial in 560 women with abnormal uterine artery Doppler at 23 weeks’, who were assigned to aspirin 150 mg or placebo, found no differences in rates of preeclampsia (18% versus 19%) or in preeclampsia requiring delivery before 34 weeks’ (6% versus 8%). A similar randomized trial using 100 mg aspirin daily in 237 women with abnormal uterine artery Doppler at 22 to 24 weeks revealed no reduction in rate of preeclampsia compared to placebo.
Consequently, low-dose aspirin is not recommended for prevention of preeclamp-sia in these women.
Close surveillance is warranted. Although there is no available proven therapy to reduce the risk of preeclampsia in these women, they should be closely observed because of the increased rate of adverse outcomes, including preeclampsia.
TABLE 2
Pregnancy-related risk factors for preeclampsia
| Magnitude of risk depends on the number of factors | |
|---|---|
| 2-fold normal | Unexplained midtrimester elevations of serum AFP, HCG, inhibin-A |
| 10 to 30% | Abnormal uterine artery Doppler velocimetry |
| 0 to 30% | Hydrops/hydropic degeneration of placenta |
| 10 to 20% | Multifetal gestation (depends on number of fetuses and maternal age) |
| 10% | Partner who fathered preeclampsia in another woman |
| 8 to 10% | Gestational diabetes mellitus |
| 8 to 10% | Limited sperm exposure (teenage pregnancy) |
| 6 to 7% | Nulliparity/primipaternity |
| Limited data | Donor insemination, oocyte donation |
| Limited data | Unexplained persistent proteinuria or hematuria |
| Unknown | Unexplained fetal growth restriction |
Step 2Watch for signal findings, diagnose preeclampsia early
Signs and symptoms may call for close surveillance at any time. Early detection of preeclampsia is the best way to reduce adverse outcomes.
Prenatal care does not prevent preeclampsia, of course. All pregnant women are at risk, some more than others. Still, adequate and proper prenatal care is the best strategy to detect preeclampsia early.
We may need to modify the frequency and type of maternal and fetal surveillance at any time. Thus, patients with multiple risk factors or risk exceeding 10% should have more frequent visits, especially beyond 24 weeks. Maternal blood pressure (both systolic and diastolic), urine protein values, abrupt and excessive weight gain, maternal symptoms, and fetal growth warrant particular attention.
Diagnostic criteria vary with risk
The diagnosis of preeclampsia is different in patients with different risk factors. In healthy nulliparous women, the diagnosis requires persistent hypertension and proteinuria (new onset after 20 weeks’ gestation). However, in some patients the diagnosis should be made based on new onset hypertension and maternal symptoms or abnormal blood tests (low platelets or elevated liver enzymes).
Urine dipstick is a reliable screening test in women who remain normotensive.
24-hour urine measurement is the best test to confirm proteinuria if hypertension develops. Several studies found that urine dipstick values less than (1+) and random urine protein to creatinine ratio measurements are not accurate to predict proteinuria in women with gestational hypertension.
When is it gestational hypertension? The term applies only women with all of these findings:
- mild hypertension <160/<110 mm Hg
- proteinuria <300 mg/24-hour urine
- normal platelet count
- normal liver enzymes
- normal fetal growth
- no maternal symptoms
Once gestational hypertension is diagnosed, obtain blood tests and ultrasound evaluation to document fetal growth and amniotic fluid status.
Women with severe gestational hypertension and those with abnormal tests should be diagnosed as having preeclampsia and managed as such.
Women with gestational hypertension are at high risk for preeclampsia, and risk of progression depends on gestational age at time of diagnosis. Women who develop gestational hypertension at 24 to 35 weeks have a 46% chance of developing preeclampsia with a high rate of preterm delivery (32% <36 weeks and 12.5% <34 weeks) (FIGURE). These women require very close surveillance. In contrast, maternal and perinatal outcome is usually favorable when only mild gestational hypertension develops at or beyond 36 weeks.
When hypertension, proteinuria occur before 20 weeks
The traditional diagnostic criteria for preeclampsia in healthy women are not reliable in women who have either hypertension or proteinuria prior to 20 weeks’ gestation, particularly in those taking antihypertensive medications and in those who have class F diabetes mellitus. Because of the physiologic changes during pregnancy, women with diabetes and renal disease will have serial increases in blood pressure as well as protein excretion with advanced gestational age, particularly in the third trimester. Diagnostic criteria (TABLE 3) should be individualized based on medical conditions and current therapy. Antihypertensive drugs and preexisting proteinuria make it more difficult to classify preeclampsia as mild or severe.
TABLE 3
Diagnostic criteria
| GESTATIONAL HYPERTENSION IN HEALTHY WOMEN | |
| Blood pressure <160 mm Hg diastolic and <110 mm Hg systolic | |
| Proteinuria <300 mg/24-hour collection | |
| Platelet count >100,000/mm3 | |
| Normal liver enzymes | |
| No maternal symptoms | |
| No intrauterine growth restriction or oligohydraminos by ultrasound | |
| PREECLAMPSIA IN WOMEN WITH PREEXISTING MEDICAL CONDITIONS | |
| Condition | Criteria |
| Hypertension only | Proteinuria >500 mg/24-hours or thrombocytopenia |
| Proteinuria only | New onset hypertension plus symptoms or thrombocytopenia or elevated liver enzymes |
| Hypertension plus proteinuria (renal disease or class F diabetes) | Worsening severe hypertension and/or new onset of symptoms, thrombocytopenia, elevated liver enzymes |
FIGURE Whether preeclampsia will develop depends on when gestational hypertension begins
Adapted from Barton JR, et al. Am J Obstet Gynecol. 2001;184:979-983.
Step 3Consider how to balance risk to mother and fetus
Once a diagnosis is made, promptly evaluate mother and fetus, continue close surveillance, select those who will benefit from hospitalization, and identify indications for delivery (TABLE 4).
Delivery will always reduce the risks for the mother, but in certain situations, it might not be the best option for an extremely premature fetus. Sometimes delivery is best for both mother and fetus.
The best strategy takes into consideration:
- maternal and fetal status at initial evaluation,
- preexisting medical conditions that could affect pregnancy outcome,
- fetal gestational age at time of diagnosis,
- labor or rupture of fetal membranes (both could affect management), and
- maternal choice of available options.
Women who remain undelivered require close maternal and fetal evaluation. In otherwise healthy women, management depends on whether the preeclampsia is mild or severe, and, if there are other medical conditions, on the status of those conditions, as well.
TABLE 4
Indications for delivery
| Consider delivery in gravidas with 1 or more indications |
|---|
| Gestational age ≥38 weeks for mild disease |
| Gestational age ≥34 weeks for severe disease |
| 33-34 weeks with severe disease after steroids |
| Onset of labor and/or membrane rupture ≥34 weeks |
| Eclampsia or pulmonary edema (any gestational age) |
| HELLP syndrome (any gestational age) |
| Severe cerebral symptoms or epigastric pain |
| Acute renal insufficiency (serum creatinine >1.2 mg/dl) |
| Persistent thrombocytopenia (platelet count <100,000) |
| Maternal desire for delivery |
| Severe oligohydraminos or IUGR < 5th percentile |
| Nonreassuring fetal testing |
Chronic hypertension
- Underlies 30% of cases of hypertension during pregnancy.
- Begins before pregnancy or before 20 weeks’ gestation.
Gestational hypertension
- The most common form of hypertension during pregnancy.
- Acute onset beyond 20 weeks’ gestation in a woman known to be normotensive before pregnancy or prior to 20 weeks’ gestation.
Preeclampsia
- Can superimpose upon chronic hypertension, renal disease, or connective tissue disease, or develop in women with gestational hypertension.
- Preeclampsia in healthy nulliparous women: hypertension and proteinuria after 20 weeks’ gestation.
- Preeclampsia in women with preexisting chronic hypertension and absent proteinuria: an exacerbation of hypertension and new onset proteinuria.
Eclampsia
- Development of convulsions in women with hypertensive disorders of pregnancy.
“HELLP syndrome”
- Hemolysis,
- Elevated liver enzymes, and
- Low platelet count
Suspected or confirmed preeclampsia in a woman who has documented evidence of hemolysis (abnormal peripheral smear, or elevated bilirubin, or anemia, or low heptoglobin levels), plus elevated liver enzymes (AST or ALT), and thrombocytopenia (platelet count below 100,000).
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol. 2001;184:979-983.
Boggess KA, Lief S, Martha AP, Moos K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227-231.
Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66-71.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med. 1998;338:701-705.
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219-224.
Chien PE, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An Overview. Br J Obstet Gynaecol. 2000;107:196-208.
Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: A meta-analysis. Obstet Gynecol. 2001;98:861-866.
Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Dekker G, Robillard PY. The birth interval hypothesis - Does it really indicate the end of the primipaternity hypothesis? J Reprod Immunol. 2003;59:245-251.
Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209-215.
Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848-52.
Einarsson JI, Sangi-Haghpeykar H, Gardner NO. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol. 2003;188:1241-1243.
Hauth JC, Ewell MG, Levine RL, Esterlitz JR, Sibai BM, Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Hnat MD, Sibai BM, Caritis S, Hiouth J, Lindheimer MD, MacPherson C. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparous. Am J Obstet Gynecol. 2002;186:422-426.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Levine RG, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77-85.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P. The importance of genetic and environmental effects for preeclampsia and gestational hypertension: a family study. Br J Obstet Gynaecol. 2004;111:200-206.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
Ragip A Al, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol. 2004;104:367-371.
Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol. 2003;157:1108-1114.
Sibai BM, Caritis S, Hauth J, Lilndheimer MD, MacPherson C, Klebanoff M, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938-942.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-33377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Subtil D, Goeusse P, Houfflin-Debarge V, Puech F, Lequien P, Breart G, Uzan S, Quandalle F, Delcourt YM, Malek YM. Essai Regional Aspirine Mere-Enfant (ERASME) Collaborative Group. Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 2). Br J Obstet Gynaecol. 2003;110:485-491.
Wen SW, Demissie K, Yang Q, Walker MC. Maternal morbidity and obstetric complications in triplet pregnancies and quadruplet and higher-order multiple pregnancies. Am J Obstet Gynecol. 2004;191:254-258.
Yu CKH, Papageorghiou AT, Parra M, Dias RP, Nicolaides KH. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;22:233-239.
BIBLIOGRAPHY
Barton JR, O’Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol. 2001;184:979-983.
Boggess KA, Lief S, Martha AP, Moos K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227-231.
Buchbinder A, Sibai BM, Caritis S, MacPherson C, Hauth J, Lindheimer MD. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186:66-71.
Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med. 1998;338:701-705.
Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219-224.
Chien PE, Arnott N, Gordon A, Owen P, Khan KS. How useful is uterine artery Doppler flow velocimetry in the prediction of pre-eclampsia, intrauterine growth retardation and perinatal death? An Overview. Br J Obstet Gynaecol. 2000;107:196-208.
Coomarasamy A, Papaioannou S, Gee H, Khan KS. Aspirin for the prevention of preeclampsia in women with abnormal uterine artery Doppler: A meta-analysis. Obstet Gynecol. 2001;98:861-866.
Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Dekker G, Robillard PY. The birth interval hypothesis - Does it really indicate the end of the primipaternity hypothesis? J Reprod Immunol. 2003;59:245-251.
Dekker G, Sibai B. Primary, secondary, and tertiary prevention of pre-eclampsia. Lancet. 2001;357:209-215.
Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol. 2003;189:848-52.
Einarsson JI, Sangi-Haghpeykar H, Gardner NO. Sperm exposure and development of preeclampsia. Am J Obstet Gynecol. 2003;188:1241-1243.
Hauth JC, Ewell MG, Levine RL, Esterlitz JR, Sibai BM, Curet LB. Pregnancy outcomes in healthy nulliparous women who subsequently developed hypertension. Obstet Gynecol. 2000;95:24-28.
Hnat MD, Sibai BM, Caritis S, Hiouth J, Lindheimer MD, MacPherson C. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparous. Am J Obstet Gynecol. 2002;186:422-426.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Levine RG, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77-85.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683.
Nilsson E, Salonen Ros H, Cnattingius S, Lichtenstein P. The importance of genetic and environmental effects for preeclampsia and gestational hypertension: a family study. Br J Obstet Gynaecol. 2004;111:200-206.
O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14:368-374.
Ragip A Al, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol. 2004;104:367-371.
Saftlas AF, Levine RJ, Klebanoff MA, Martz KL, Ewell MG, Morris CD, Sibai BM. Abortion, changed paternity, and risk of preeclampsia in nulliparous women. Am J Epidemiol. 2003;157:1108-1114.
Sibai BM, Caritis S, Hauth J, Lilndheimer MD, MacPherson C, Klebanoff M, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938-942.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-33377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Subtil D, Goeusse P, Houfflin-Debarge V, Puech F, Lequien P, Breart G, Uzan S, Quandalle F, Delcourt YM, Malek YM. Essai Regional Aspirine Mere-Enfant (ERASME) Collaborative Group. Randomised comparison of uterine artery Doppler and aspirin (100 mg) with placebo in nulliparous women: the Essai Regional Aspirine Mere-Enfant study (Part 2). Br J Obstet Gynaecol. 2003;110:485-491.
Wen SW, Demissie K, Yang Q, Walker MC. Maternal morbidity and obstetric complications in triplet pregnancies and quadruplet and higher-order multiple pregnancies. Am J Obstet Gynecol. 2004;191:254-258.
Yu CKH, Papageorghiou AT, Parra M, Dias RP, Nicolaides KH. Randomized controlled trial using low-dose aspirin in the prevention of pre-eclampsia in women with abnormal uterine artery Doppler at 23 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;22:233-239.
The Challenge of Delivering Mental Health Care in Rural Clinics
The nightmare of litigation: A survivor’s true story
The author reports no relevant financial relationships.
“I was stunned, bewildered, and disoriented. Surely this wasn’t happening to me. I felt cornered like a trapped animal and just had to escape so I spent most of the day wandering around in a daze. It was like living a dream—no, more like a nightmare.”
The victim of an accident, criminal assault, or terrorist attack? No, this was David, an obstetrician describing to me his reaction on being sued for medical malpractice. A day that started off as hectic but routine suddenly turned into a nightmare. Later, colleagues would tell him not to worry, that he’d be OK and that litigation was a “normal” part of medical practice. But it didn’t feel normal to him, as the memories of that day continued to replay in thoughts and dreams.
Malpractice liability may be omnipresent, but that doesn’t mean getting sued is a “normal” everyday hazard that Ob/Gyns should be able to take in stride. Litigation is frequently unfair, abusive, and traumatizing, and can cause acute stress disorder and even posttraumatic stress disorder (PTSD) in both physicians and patients.
David’s story
In this true story, an obstetrician suffering disabling litigation stress reclaims a sense of empowerment and control as he becomes aware of the nature of litigation stress. In the process, he learns how to listen, understand, and support patients, employees, and colleagues in times of stress.
During one-on-one telephone sessions, his trauma was acknowledged and named; his losses were identified and mourned in safety; and his isolation was relieved in a healing supportive relationship.
The initial shock
This was his first. “I was a litigation virgin,” he sardonically commented. “You know, when you’re jumping the waves in the ocean at high tide and then you become confident, you turn your back, and this big one hits you? It felt like that. I had just begun to relax, believing it wouldn’t happen to me. Then the lawsuit hit. It was a patient I’ve known for years. I delivered her other children and regarded her almost as a friend, someone I liked and trusted.
“I’ve made mistakes in the past but this wasn’t one of those times. It’s so unfair—instead of being grateful that I saved her 9.5-pound baby, she hunted down a lawyer on the Internet. The Web is full of them just waiting to pounce.”
The aftershocks
David recounted the journal articles1 he’d looked up, which recommended that he share his feelings with a trusted colleague. Other articles cautioned against a possible “discoverable” confidence.2 Colleagues’ attempts at reassurance did not really comfort him.
Sociable persons who have a thoughtful, active coping style and a strong sense of their ability to control their destiny have more capacity to resist stress.
Ask yourself:
- Am I a loner?
- Do I assign control of my destiny to others?
- Am I a perfectionist?
- Do I tend to beat up on myself when I miss the mark?
- Is my primary identity that of physician?
- Do I lack a community of support?
- Do I lack stress reduction practices?
- Do I suffer from burnout?
- Do I have a history of serious trauma?
If you answer yes to any of these questions, you are probably at greater risk of litigation stress. Begin attending to your personal needs and well-being now.
Expand your resilience. You have invested time and money in your education; now invest in yourself.
David contacted me when it became increasingly difficult for him to see patients. He said that he felt he had to be constantly on guard, watching every word and action as if patients were an enemy waiting to ambush him. He dreaded going to work and wondered if he should quit obstetrics.
No, he did not want to see a psychiatrist or a psychotherapist. He wasn’t crazy, he wasn’t thinking of suicide or anything like that, he said, and the last thing he needed was the credential committee of his local hospital breathing down his neck.
He spoke in a a lifeless monotone, reciting the facts of the case as he had told and retold them many times. He sighed often and used negative expressions such as can’t, but, should, have to, if only. He was articulating a lament—an expression of suffering and loss, which is not uncommon among physicians3,4 and patients.5 Within his narrative ran an unbroken thread of helplessness, grief, despair, and absence of meaning and hope.
Rather than premature reassurance and comfort, what David needed was to have his trauma named and acknowledged. Choosing my words carefully, I summarized his story and asked whether I had heard and understood him correctly. He verified that I had. Going a step further, I reflected back his underlying emotions as I had heard them—his feelings of fear, helplessness, sadness, isolation, betrayal, violation, anger, and injustice. Then I paused to create space for his response. Soon, the silence was interrupted by the sounds of his sobbing. When he regained his composure, David apologized for losing control. This lawsuit had been a huge strain, he explained.
Symptoms of acute stress reaction
I agreed, pointing out that he had probably experienced an acute stress reaction: feelings of intense fear, horror, and helplessness in response to an unusually traumatic event threatening death or serious physical injury to self or others.
This explained his fright and dazed disorientation on the day he learned of the litigation.6 While the lawsuit was not life-threatening, it threatened his identity, career, and survival as a physician.
Symptoms of PTSD
Usually acute stress reaction settles down, but sometimes it progresses beyond a month into posttraumatic stress disorder, a pervasive chronic anxiety disorder characterized by 3 clusters of symptoms:
- Recurrent, intrusive recollection of the events; recurrent flashbacks and dreams.
- Persistent avoidance of stimuli associated with the event; numbness, detachment, avoidance of patients.
- Persistent symptoms of increased arousal; insomnia, hypervigilance, irritability, difficulty with concentration.
“I am a rock” mentality may predispose to PTSD
Litigation, because of its protracted nature, is particularly retraumatizing. David concurred: “This explains why just opening a lawyer’s letter now causes my heart to pound.”
Unlike the military, physicians do not enter a stressful environment organized into teams. Should trauma and acute stress reaction occur, most physicians continue working despite their intense physical responses. There is little community support, so withdrawal and isolation is the norm, and this “norm” may predispose to posttraumatic stress disorder.
As a result, some physicians manifest behavioral problems such as being hyperreactive, aloof, or disruptive, or they abuse alcohol and drugs. Ironically, these behaviors probably lay groundwork for additional lawsuits.7
Counting up the losses
David asked what I meant by “losses.” I explained that the nature of trauma is to create loss.
- Together we listed his loss of:
trust
safety
peace of mind
sense of justice
integrity of personal boundaries
control
self-esteem
self-confidence
passion
idealism
If you notice that you are stunned, bewildered, and feeling overwhelmed, even disoriented, accept that you may not be able to think clearly for a while. Avoid complex tasks and major decisions.
Take care of your physical health. Obstetricians take sleep deprivation, lack of exercise, long hours, and irregular eating habits for granted. This, however, is not the time to neglect your basic needs. If necessary, take time off (though many prefer to keep to a regular, albeit moderated, familiar schedule).
Do not isolate yourself. Share your feelings with those you can trust. Consider seeing an individual, such as a psychotherapist, who is trained to listen therapeutically. Do not use your lawyer for this purpose.
Limit use of substances (such as sedatives, hypnotics, alcohol) and limit activities (burying yourself in work or exercise) aimed at numbing your emotions.
Conserve your energy. You have limited control over legal proceedings. You can, however, apply your energy to improving your well-being.
If you develop symptoms of depression, do not hesitate to seek psychiatric help and certainly do not attempt to self-medicate.
For many reasons, not the least being shame, physicians avoid consulting a mental health professional and repercussions can be serious.16
Remember your life partner, children, and others around you may be affected too. Be gentle with them.
The power to choose how to respond
While he could not stop the lawsuit, he did have the power to choose how to respond to it. It was his choice whether to be demolished by this lawsuit or to use it to grow personally and professionally. If he agreed, I would partner him in transforming his suffering into growth. On the other hand, should his symptoms not recede, he would need to see a psychiatrist.
By now I had:
- validated his trauma, losses, and suffering
- provided him a cognitive framework
- interrupted his lament
- created safety for him to express his emotions
- emphasized he was not helpless, and that he had choices
- offered to partner with him, thereby relieving his isolation
- role-modeled listening
- offered him hope and a sense of some control.
A set-up for litigation stress
Surveys reveal that many medical students are exposed to serious trauma such as sexual abuse or domestic violence prior to entering medical school.8 They then enter medical training, which has been described as a “neglectful abusive family system,”9 and which adds trauma and toxic shame—this continues into a career punctuated with acute episodes of severe trauma such as medical errors, unexpected death of patients, and litigation stress.
Breast cancer, traumatic birth cause acute stress
David read books on trauma10 and suffering,11,12 and began to explore ways to apply his new insight. He read journal articles that described acute stress reaction in patients diagnosed with breast cancer,13 traumatic birth,14 and spontaneous abortion.15 Now he understood why patients sometimes left his office bewildered and disoriented, unable to retain any information, and why patients with chronic trauma experience functional somatic symptoms. He also learned how to respond more effectively.
The outcome: Self-empowerment
Together we studied his written narratives of patient encounters and did role plays of these encounters. He was a good student, and his ability to communicate empathy and support eventually matched his technical proficiency. Increasingly, not only patients, but also employees and colleagues turned to him for listening in times of stress. Their positive feedback enhanced his sense of well-being. His newly acquired empowerment and sense of control was key to his success.
Over the course of 8 months, he traveled full circle from trauma victim to healer.
Litigation stress: Take it seriously
When taken seriously, much can be done to transform litigation stress into physician empowerment. Studies need to be done on stress disorders in physicians, so as to refute the culture of denial that exists around the trauma inflicted by malpractice litigation. Innovative programs need to be developed to minimize the harmful effect of litigation and to support physicians suffering litigation stress.
1. Meier D, Back A, Morrison R. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
2. Physician Insurance Litigation Stress Support Services. http://www.phyins.com/pi/claims/stress.html
3. Daugird A, Spencer D. Physician reactions to the health care revolution: a grief model approach. Arch Fam Med. 1996;5:497-500.
4. Loder D. The saddest day of my life. Berks County Medical Record. 1998(5);89:6.-
5. Bub B. The Lament, Hidden Key to Effective Listening. Medical Humanities. In press.
6. Christensen JF, Levinson W, Dunn PM. The impact of perceived mistakes on physicians. J Gen Intern Med. 1992;7:424-431.
7. Kennedy J. Physicians’ feelings about themselves and their patients. Letter. JAMA. 2002;287:1113.-
8. Ambuel B, Butler D, Hamberger LK, et al. Female and male students’ exposure to violence: impact on well-being and perceived capacity to help battered women. J Comparative Fam Studies. 2003;34:113-135.
9. McKegney C. Medical Education: A neglectful and abusive family system. Fam Med. 1989;452-457.
10. Herman JL. Trauma and Recovery. London: Rivers Oram Press; 1997.
11. Schneider JM. Finding My Way. Healing and Transformation through Loss and Grief. Seasons Press. 1994.
12. Frankl VE. Man’s Search for Meaning. Beacon Press. 1946.
13. McGarvey EL, Canterbury RJ, Cohen RB. Evidence of acute stress disorder after diagnosis of cancer. Southern Med J. 1998;91:864-866.
14. Reynolds JL. Posttraumatic stress disorder after childbirth: the phenomenon of traumatic birth. Can Med Assoc J. 1997;156:831-835.
15. Bowles SV, James LC, Solursh DS, Yancey MK, Epperly TD, Folen RA, Masone M. Acute and posttraumatic stress disorder after spontaneous abortion. Am Fam Phys. 2000;61:1689-1696.
16. Brunk D. Suicide is top cause of early death in physicians–far higher than in general population. http://www.findarticles.com/p/articles/mi_m0CYD/is_5_38/ai_98830125
The author reports no relevant financial relationships.
“I was stunned, bewildered, and disoriented. Surely this wasn’t happening to me. I felt cornered like a trapped animal and just had to escape so I spent most of the day wandering around in a daze. It was like living a dream—no, more like a nightmare.”
The victim of an accident, criminal assault, or terrorist attack? No, this was David, an obstetrician describing to me his reaction on being sued for medical malpractice. A day that started off as hectic but routine suddenly turned into a nightmare. Later, colleagues would tell him not to worry, that he’d be OK and that litigation was a “normal” part of medical practice. But it didn’t feel normal to him, as the memories of that day continued to replay in thoughts and dreams.
Malpractice liability may be omnipresent, but that doesn’t mean getting sued is a “normal” everyday hazard that Ob/Gyns should be able to take in stride. Litigation is frequently unfair, abusive, and traumatizing, and can cause acute stress disorder and even posttraumatic stress disorder (PTSD) in both physicians and patients.
David’s story
In this true story, an obstetrician suffering disabling litigation stress reclaims a sense of empowerment and control as he becomes aware of the nature of litigation stress. In the process, he learns how to listen, understand, and support patients, employees, and colleagues in times of stress.
During one-on-one telephone sessions, his trauma was acknowledged and named; his losses were identified and mourned in safety; and his isolation was relieved in a healing supportive relationship.
The initial shock
This was his first. “I was a litigation virgin,” he sardonically commented. “You know, when you’re jumping the waves in the ocean at high tide and then you become confident, you turn your back, and this big one hits you? It felt like that. I had just begun to relax, believing it wouldn’t happen to me. Then the lawsuit hit. It was a patient I’ve known for years. I delivered her other children and regarded her almost as a friend, someone I liked and trusted.
“I’ve made mistakes in the past but this wasn’t one of those times. It’s so unfair—instead of being grateful that I saved her 9.5-pound baby, she hunted down a lawyer on the Internet. The Web is full of them just waiting to pounce.”
The aftershocks
David recounted the journal articles1 he’d looked up, which recommended that he share his feelings with a trusted colleague. Other articles cautioned against a possible “discoverable” confidence.2 Colleagues’ attempts at reassurance did not really comfort him.
Sociable persons who have a thoughtful, active coping style and a strong sense of their ability to control their destiny have more capacity to resist stress.
Ask yourself:
- Am I a loner?
- Do I assign control of my destiny to others?
- Am I a perfectionist?
- Do I tend to beat up on myself when I miss the mark?
- Is my primary identity that of physician?
- Do I lack a community of support?
- Do I lack stress reduction practices?
- Do I suffer from burnout?
- Do I have a history of serious trauma?
If you answer yes to any of these questions, you are probably at greater risk of litigation stress. Begin attending to your personal needs and well-being now.
Expand your resilience. You have invested time and money in your education; now invest in yourself.
David contacted me when it became increasingly difficult for him to see patients. He said that he felt he had to be constantly on guard, watching every word and action as if patients were an enemy waiting to ambush him. He dreaded going to work and wondered if he should quit obstetrics.
No, he did not want to see a psychiatrist or a psychotherapist. He wasn’t crazy, he wasn’t thinking of suicide or anything like that, he said, and the last thing he needed was the credential committee of his local hospital breathing down his neck.
He spoke in a a lifeless monotone, reciting the facts of the case as he had told and retold them many times. He sighed often and used negative expressions such as can’t, but, should, have to, if only. He was articulating a lament—an expression of suffering and loss, which is not uncommon among physicians3,4 and patients.5 Within his narrative ran an unbroken thread of helplessness, grief, despair, and absence of meaning and hope.
Rather than premature reassurance and comfort, what David needed was to have his trauma named and acknowledged. Choosing my words carefully, I summarized his story and asked whether I had heard and understood him correctly. He verified that I had. Going a step further, I reflected back his underlying emotions as I had heard them—his feelings of fear, helplessness, sadness, isolation, betrayal, violation, anger, and injustice. Then I paused to create space for his response. Soon, the silence was interrupted by the sounds of his sobbing. When he regained his composure, David apologized for losing control. This lawsuit had been a huge strain, he explained.
Symptoms of acute stress reaction
I agreed, pointing out that he had probably experienced an acute stress reaction: feelings of intense fear, horror, and helplessness in response to an unusually traumatic event threatening death or serious physical injury to self or others.
This explained his fright and dazed disorientation on the day he learned of the litigation.6 While the lawsuit was not life-threatening, it threatened his identity, career, and survival as a physician.
Symptoms of PTSD
Usually acute stress reaction settles down, but sometimes it progresses beyond a month into posttraumatic stress disorder, a pervasive chronic anxiety disorder characterized by 3 clusters of symptoms:
- Recurrent, intrusive recollection of the events; recurrent flashbacks and dreams.
- Persistent avoidance of stimuli associated with the event; numbness, detachment, avoidance of patients.
- Persistent symptoms of increased arousal; insomnia, hypervigilance, irritability, difficulty with concentration.
“I am a rock” mentality may predispose to PTSD
Litigation, because of its protracted nature, is particularly retraumatizing. David concurred: “This explains why just opening a lawyer’s letter now causes my heart to pound.”
Unlike the military, physicians do not enter a stressful environment organized into teams. Should trauma and acute stress reaction occur, most physicians continue working despite their intense physical responses. There is little community support, so withdrawal and isolation is the norm, and this “norm” may predispose to posttraumatic stress disorder.
As a result, some physicians manifest behavioral problems such as being hyperreactive, aloof, or disruptive, or they abuse alcohol and drugs. Ironically, these behaviors probably lay groundwork for additional lawsuits.7
Counting up the losses
David asked what I meant by “losses.” I explained that the nature of trauma is to create loss.
- Together we listed his loss of:
trust
safety
peace of mind
sense of justice
integrity of personal boundaries
control
self-esteem
self-confidence
passion
idealism
If you notice that you are stunned, bewildered, and feeling overwhelmed, even disoriented, accept that you may not be able to think clearly for a while. Avoid complex tasks and major decisions.
Take care of your physical health. Obstetricians take sleep deprivation, lack of exercise, long hours, and irregular eating habits for granted. This, however, is not the time to neglect your basic needs. If necessary, take time off (though many prefer to keep to a regular, albeit moderated, familiar schedule).
Do not isolate yourself. Share your feelings with those you can trust. Consider seeing an individual, such as a psychotherapist, who is trained to listen therapeutically. Do not use your lawyer for this purpose.
Limit use of substances (such as sedatives, hypnotics, alcohol) and limit activities (burying yourself in work or exercise) aimed at numbing your emotions.
Conserve your energy. You have limited control over legal proceedings. You can, however, apply your energy to improving your well-being.
If you develop symptoms of depression, do not hesitate to seek psychiatric help and certainly do not attempt to self-medicate.
For many reasons, not the least being shame, physicians avoid consulting a mental health professional and repercussions can be serious.16
Remember your life partner, children, and others around you may be affected too. Be gentle with them.
The power to choose how to respond
While he could not stop the lawsuit, he did have the power to choose how to respond to it. It was his choice whether to be demolished by this lawsuit or to use it to grow personally and professionally. If he agreed, I would partner him in transforming his suffering into growth. On the other hand, should his symptoms not recede, he would need to see a psychiatrist.
By now I had:
- validated his trauma, losses, and suffering
- provided him a cognitive framework
- interrupted his lament
- created safety for him to express his emotions
- emphasized he was not helpless, and that he had choices
- offered to partner with him, thereby relieving his isolation
- role-modeled listening
- offered him hope and a sense of some control.
A set-up for litigation stress
Surveys reveal that many medical students are exposed to serious trauma such as sexual abuse or domestic violence prior to entering medical school.8 They then enter medical training, which has been described as a “neglectful abusive family system,”9 and which adds trauma and toxic shame—this continues into a career punctuated with acute episodes of severe trauma such as medical errors, unexpected death of patients, and litigation stress.
Breast cancer, traumatic birth cause acute stress
David read books on trauma10 and suffering,11,12 and began to explore ways to apply his new insight. He read journal articles that described acute stress reaction in patients diagnosed with breast cancer,13 traumatic birth,14 and spontaneous abortion.15 Now he understood why patients sometimes left his office bewildered and disoriented, unable to retain any information, and why patients with chronic trauma experience functional somatic symptoms. He also learned how to respond more effectively.
The outcome: Self-empowerment
Together we studied his written narratives of patient encounters and did role plays of these encounters. He was a good student, and his ability to communicate empathy and support eventually matched his technical proficiency. Increasingly, not only patients, but also employees and colleagues turned to him for listening in times of stress. Their positive feedback enhanced his sense of well-being. His newly acquired empowerment and sense of control was key to his success.
Over the course of 8 months, he traveled full circle from trauma victim to healer.
Litigation stress: Take it seriously
When taken seriously, much can be done to transform litigation stress into physician empowerment. Studies need to be done on stress disorders in physicians, so as to refute the culture of denial that exists around the trauma inflicted by malpractice litigation. Innovative programs need to be developed to minimize the harmful effect of litigation and to support physicians suffering litigation stress.
The author reports no relevant financial relationships.
“I was stunned, bewildered, and disoriented. Surely this wasn’t happening to me. I felt cornered like a trapped animal and just had to escape so I spent most of the day wandering around in a daze. It was like living a dream—no, more like a nightmare.”
The victim of an accident, criminal assault, or terrorist attack? No, this was David, an obstetrician describing to me his reaction on being sued for medical malpractice. A day that started off as hectic but routine suddenly turned into a nightmare. Later, colleagues would tell him not to worry, that he’d be OK and that litigation was a “normal” part of medical practice. But it didn’t feel normal to him, as the memories of that day continued to replay in thoughts and dreams.
Malpractice liability may be omnipresent, but that doesn’t mean getting sued is a “normal” everyday hazard that Ob/Gyns should be able to take in stride. Litigation is frequently unfair, abusive, and traumatizing, and can cause acute stress disorder and even posttraumatic stress disorder (PTSD) in both physicians and patients.
David’s story
In this true story, an obstetrician suffering disabling litigation stress reclaims a sense of empowerment and control as he becomes aware of the nature of litigation stress. In the process, he learns how to listen, understand, and support patients, employees, and colleagues in times of stress.
During one-on-one telephone sessions, his trauma was acknowledged and named; his losses were identified and mourned in safety; and his isolation was relieved in a healing supportive relationship.
The initial shock
This was his first. “I was a litigation virgin,” he sardonically commented. “You know, when you’re jumping the waves in the ocean at high tide and then you become confident, you turn your back, and this big one hits you? It felt like that. I had just begun to relax, believing it wouldn’t happen to me. Then the lawsuit hit. It was a patient I’ve known for years. I delivered her other children and regarded her almost as a friend, someone I liked and trusted.
“I’ve made mistakes in the past but this wasn’t one of those times. It’s so unfair—instead of being grateful that I saved her 9.5-pound baby, she hunted down a lawyer on the Internet. The Web is full of them just waiting to pounce.”
The aftershocks
David recounted the journal articles1 he’d looked up, which recommended that he share his feelings with a trusted colleague. Other articles cautioned against a possible “discoverable” confidence.2 Colleagues’ attempts at reassurance did not really comfort him.
Sociable persons who have a thoughtful, active coping style and a strong sense of their ability to control their destiny have more capacity to resist stress.
Ask yourself:
- Am I a loner?
- Do I assign control of my destiny to others?
- Am I a perfectionist?
- Do I tend to beat up on myself when I miss the mark?
- Is my primary identity that of physician?
- Do I lack a community of support?
- Do I lack stress reduction practices?
- Do I suffer from burnout?
- Do I have a history of serious trauma?
If you answer yes to any of these questions, you are probably at greater risk of litigation stress. Begin attending to your personal needs and well-being now.
Expand your resilience. You have invested time and money in your education; now invest in yourself.
David contacted me when it became increasingly difficult for him to see patients. He said that he felt he had to be constantly on guard, watching every word and action as if patients were an enemy waiting to ambush him. He dreaded going to work and wondered if he should quit obstetrics.
No, he did not want to see a psychiatrist or a psychotherapist. He wasn’t crazy, he wasn’t thinking of suicide or anything like that, he said, and the last thing he needed was the credential committee of his local hospital breathing down his neck.
He spoke in a a lifeless monotone, reciting the facts of the case as he had told and retold them many times. He sighed often and used negative expressions such as can’t, but, should, have to, if only. He was articulating a lament—an expression of suffering and loss, which is not uncommon among physicians3,4 and patients.5 Within his narrative ran an unbroken thread of helplessness, grief, despair, and absence of meaning and hope.
Rather than premature reassurance and comfort, what David needed was to have his trauma named and acknowledged. Choosing my words carefully, I summarized his story and asked whether I had heard and understood him correctly. He verified that I had. Going a step further, I reflected back his underlying emotions as I had heard them—his feelings of fear, helplessness, sadness, isolation, betrayal, violation, anger, and injustice. Then I paused to create space for his response. Soon, the silence was interrupted by the sounds of his sobbing. When he regained his composure, David apologized for losing control. This lawsuit had been a huge strain, he explained.
Symptoms of acute stress reaction
I agreed, pointing out that he had probably experienced an acute stress reaction: feelings of intense fear, horror, and helplessness in response to an unusually traumatic event threatening death or serious physical injury to self or others.
This explained his fright and dazed disorientation on the day he learned of the litigation.6 While the lawsuit was not life-threatening, it threatened his identity, career, and survival as a physician.
Symptoms of PTSD
Usually acute stress reaction settles down, but sometimes it progresses beyond a month into posttraumatic stress disorder, a pervasive chronic anxiety disorder characterized by 3 clusters of symptoms:
- Recurrent, intrusive recollection of the events; recurrent flashbacks and dreams.
- Persistent avoidance of stimuli associated with the event; numbness, detachment, avoidance of patients.
- Persistent symptoms of increased arousal; insomnia, hypervigilance, irritability, difficulty with concentration.
“I am a rock” mentality may predispose to PTSD
Litigation, because of its protracted nature, is particularly retraumatizing. David concurred: “This explains why just opening a lawyer’s letter now causes my heart to pound.”
Unlike the military, physicians do not enter a stressful environment organized into teams. Should trauma and acute stress reaction occur, most physicians continue working despite their intense physical responses. There is little community support, so withdrawal and isolation is the norm, and this “norm” may predispose to posttraumatic stress disorder.
As a result, some physicians manifest behavioral problems such as being hyperreactive, aloof, or disruptive, or they abuse alcohol and drugs. Ironically, these behaviors probably lay groundwork for additional lawsuits.7
Counting up the losses
David asked what I meant by “losses.” I explained that the nature of trauma is to create loss.
- Together we listed his loss of:
trust
safety
peace of mind
sense of justice
integrity of personal boundaries
control
self-esteem
self-confidence
passion
idealism
If you notice that you are stunned, bewildered, and feeling overwhelmed, even disoriented, accept that you may not be able to think clearly for a while. Avoid complex tasks and major decisions.
Take care of your physical health. Obstetricians take sleep deprivation, lack of exercise, long hours, and irregular eating habits for granted. This, however, is not the time to neglect your basic needs. If necessary, take time off (though many prefer to keep to a regular, albeit moderated, familiar schedule).
Do not isolate yourself. Share your feelings with those you can trust. Consider seeing an individual, such as a psychotherapist, who is trained to listen therapeutically. Do not use your lawyer for this purpose.
Limit use of substances (such as sedatives, hypnotics, alcohol) and limit activities (burying yourself in work or exercise) aimed at numbing your emotions.
Conserve your energy. You have limited control over legal proceedings. You can, however, apply your energy to improving your well-being.
If you develop symptoms of depression, do not hesitate to seek psychiatric help and certainly do not attempt to self-medicate.
For many reasons, not the least being shame, physicians avoid consulting a mental health professional and repercussions can be serious.16
Remember your life partner, children, and others around you may be affected too. Be gentle with them.
The power to choose how to respond
While he could not stop the lawsuit, he did have the power to choose how to respond to it. It was his choice whether to be demolished by this lawsuit or to use it to grow personally and professionally. If he agreed, I would partner him in transforming his suffering into growth. On the other hand, should his symptoms not recede, he would need to see a psychiatrist.
By now I had:
- validated his trauma, losses, and suffering
- provided him a cognitive framework
- interrupted his lament
- created safety for him to express his emotions
- emphasized he was not helpless, and that he had choices
- offered to partner with him, thereby relieving his isolation
- role-modeled listening
- offered him hope and a sense of some control.
A set-up for litigation stress
Surveys reveal that many medical students are exposed to serious trauma such as sexual abuse or domestic violence prior to entering medical school.8 They then enter medical training, which has been described as a “neglectful abusive family system,”9 and which adds trauma and toxic shame—this continues into a career punctuated with acute episodes of severe trauma such as medical errors, unexpected death of patients, and litigation stress.
Breast cancer, traumatic birth cause acute stress
David read books on trauma10 and suffering,11,12 and began to explore ways to apply his new insight. He read journal articles that described acute stress reaction in patients diagnosed with breast cancer,13 traumatic birth,14 and spontaneous abortion.15 Now he understood why patients sometimes left his office bewildered and disoriented, unable to retain any information, and why patients with chronic trauma experience functional somatic symptoms. He also learned how to respond more effectively.
The outcome: Self-empowerment
Together we studied his written narratives of patient encounters and did role plays of these encounters. He was a good student, and his ability to communicate empathy and support eventually matched his technical proficiency. Increasingly, not only patients, but also employees and colleagues turned to him for listening in times of stress. Their positive feedback enhanced his sense of well-being. His newly acquired empowerment and sense of control was key to his success.
Over the course of 8 months, he traveled full circle from trauma victim to healer.
Litigation stress: Take it seriously
When taken seriously, much can be done to transform litigation stress into physician empowerment. Studies need to be done on stress disorders in physicians, so as to refute the culture of denial that exists around the trauma inflicted by malpractice litigation. Innovative programs need to be developed to minimize the harmful effect of litigation and to support physicians suffering litigation stress.
1. Meier D, Back A, Morrison R. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
2. Physician Insurance Litigation Stress Support Services. http://www.phyins.com/pi/claims/stress.html
3. Daugird A, Spencer D. Physician reactions to the health care revolution: a grief model approach. Arch Fam Med. 1996;5:497-500.
4. Loder D. The saddest day of my life. Berks County Medical Record. 1998(5);89:6.-
5. Bub B. The Lament, Hidden Key to Effective Listening. Medical Humanities. In press.
6. Christensen JF, Levinson W, Dunn PM. The impact of perceived mistakes on physicians. J Gen Intern Med. 1992;7:424-431.
7. Kennedy J. Physicians’ feelings about themselves and their patients. Letter. JAMA. 2002;287:1113.-
8. Ambuel B, Butler D, Hamberger LK, et al. Female and male students’ exposure to violence: impact on well-being and perceived capacity to help battered women. J Comparative Fam Studies. 2003;34:113-135.
9. McKegney C. Medical Education: A neglectful and abusive family system. Fam Med. 1989;452-457.
10. Herman JL. Trauma and Recovery. London: Rivers Oram Press; 1997.
11. Schneider JM. Finding My Way. Healing and Transformation through Loss and Grief. Seasons Press. 1994.
12. Frankl VE. Man’s Search for Meaning. Beacon Press. 1946.
13. McGarvey EL, Canterbury RJ, Cohen RB. Evidence of acute stress disorder after diagnosis of cancer. Southern Med J. 1998;91:864-866.
14. Reynolds JL. Posttraumatic stress disorder after childbirth: the phenomenon of traumatic birth. Can Med Assoc J. 1997;156:831-835.
15. Bowles SV, James LC, Solursh DS, Yancey MK, Epperly TD, Folen RA, Masone M. Acute and posttraumatic stress disorder after spontaneous abortion. Am Fam Phys. 2000;61:1689-1696.
16. Brunk D. Suicide is top cause of early death in physicians–far higher than in general population. http://www.findarticles.com/p/articles/mi_m0CYD/is_5_38/ai_98830125
1. Meier D, Back A, Morrison R. The inner life of physicians and care of the seriously ill. JAMA. 2001;286:3007-3014.
2. Physician Insurance Litigation Stress Support Services. http://www.phyins.com/pi/claims/stress.html
3. Daugird A, Spencer D. Physician reactions to the health care revolution: a grief model approach. Arch Fam Med. 1996;5:497-500.
4. Loder D. The saddest day of my life. Berks County Medical Record. 1998(5);89:6.-
5. Bub B. The Lament, Hidden Key to Effective Listening. Medical Humanities. In press.
6. Christensen JF, Levinson W, Dunn PM. The impact of perceived mistakes on physicians. J Gen Intern Med. 1992;7:424-431.
7. Kennedy J. Physicians’ feelings about themselves and their patients. Letter. JAMA. 2002;287:1113.-
8. Ambuel B, Butler D, Hamberger LK, et al. Female and male students’ exposure to violence: impact on well-being and perceived capacity to help battered women. J Comparative Fam Studies. 2003;34:113-135.
9. McKegney C. Medical Education: A neglectful and abusive family system. Fam Med. 1989;452-457.
10. Herman JL. Trauma and Recovery. London: Rivers Oram Press; 1997.
11. Schneider JM. Finding My Way. Healing and Transformation through Loss and Grief. Seasons Press. 1994.
12. Frankl VE. Man’s Search for Meaning. Beacon Press. 1946.
13. McGarvey EL, Canterbury RJ, Cohen RB. Evidence of acute stress disorder after diagnosis of cancer. Southern Med J. 1998;91:864-866.
14. Reynolds JL. Posttraumatic stress disorder after childbirth: the phenomenon of traumatic birth. Can Med Assoc J. 1997;156:831-835.
15. Bowles SV, James LC, Solursh DS, Yancey MK, Epperly TD, Folen RA, Masone M. Acute and posttraumatic stress disorder after spontaneous abortion. Am Fam Phys. 2000;61:1689-1696.
16. Brunk D. Suicide is top cause of early death in physicians–far higher than in general population. http://www.findarticles.com/p/articles/mi_m0CYD/is_5_38/ai_98830125
New screening basics for the generalist
Overall, you must determine the extent to which you will provide and interpret genetic testing and when to refer patients to a specialist. This article aims to simplify that decision by reviewing guidelines and key studies in 3 areas:
- For genetic carrier screening for people of Ashkenazi Jewish heritage, add familial dysautonomia to the list of screened diseases.
- Screening for Down syndrome is now possible in the first trimester.
- Greater genetic risks may be present among children born as a result of assisted reproductive technology (ART), although it’s unclear whether the cause is their parents’ infertility or ART itself.
On the plus side, molecular DNA diagnostics are increasingly sophisticated, readily available, and cost-efficient. The downside: As the list of recommended studies grows, successful testing programs are harder to achieve because of the need to educate patients—and yourself—about each test.
Preconception testing may be especially advisable in women with infertility because it can identify carriers and detect conditions related to infertility or its treatment. With 1% of US births attributable to ART, the possibility of genetic effects continues to raise concern.
Add another disease to genetic carrier screening
ACOG Committee Opinion #298: Prenatal and preconception carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet Gynecol. 2004;104:425–428.
Add familial dysautonomia to carrier screening when patients—or their partners—are of Ashkenazi Jewish heritage. That’s the advice from an American College of Obstetricians and Gynecologists (ACOG) committee opinion. Also conduct previously recommended screening for Tay-Sachs disease, Canavan disease, and cystic fibrosis, and advise patients that testing is available for several other diseases as well (TABLE 1). For Tay-Sachs disease, screening also is urged for patients of French Canadian and Cajun descent.
ACOG emphasizes the importance of assessing these risks prior to pregnancy to allow time for the partner to be tested, if necessary.
Among the Ashkenazi Jewish population, DNA testing detects more than 95% of carriers of autosomal disorders by analyzing the small number of mutations responsible. Tay-Sachs was the first disease for which mutations were identified.
Familial dysautonomia is caused by a single mutation in the gene IKBKAP in more than 99% of affected patients. It has a carrier rate (1/32) similar to that of Tay-Sachs disease (1/30) and involves substantial morbidity of the autonomic and sensory nervous system, with symptoms such as abnormal sweating, pain/temperature insensitivity, and labile blood pressure. Treatment may relieve symptoms, but does not cure the disease.
Refer non-Ashkenazi partners of identified carriers. Although non-Ashkenazi partners are less likely to be carriers, the exact carrier frequency and detection rates for these people are unknown (except for Tay-Sachs disease and cystic fibrosis). In these situations, it may be wise to refer the patient and her partner for genetic counseling to clarify the sensitivity of DNA analysis and the advisability of possible alternative testing by enzyme analysis.
What a generalist should offer. Because the availability of genetic testing will continue to increase, ACOG recommends that generalists provide:
- patient education on the disorders,
- referral sources for additional counseling and prenatal diagnostic testing,
- informed consent when obtaining samples for genetic testing, and
- assurance of confidentiality.
Screen for Down syndrome in the first trimester
Screening for Down syndrome is now available in the first trimester; ACOG recommends using ultrasound and maternal serum screening, with 3 criteria:
- standardized, continuous quality assurance,
- ability to counsel patients about the testing options, and
- access to appropriate diagnostic testing.
TABLE 2).
Integrated versus contingency screening. “Integrated” screening combines information from the first trimester (nuchal lucency and serum screen) with serum screening in the second trimester. This approach yields the lowest screen-positive rate (2.6%) and a high detection rate (90%), but has an important shortcoming: The results are not disclosed until the second trimester.
“Contingency” screening is emerging as an alternative: High first-trimester risks are relayed to the patient, while women with low screen values are excused from further testing. Patients with intermediate risk proceed to second-trimester serum screening.
Disadvantages of this approach include the need to coordinate the various steps and adequately inform the patient of them.
Added value of first-trimester nuchal lucency screening. Increased nuchal translucency alone is an important screen for structural abnormalities and adverse pregnancy outcomes. If a karyotypically normal fetus has an increased first-trimester nuchal lucency, the possibility of a structural anomaly on second-trimester ultrasound increases 2-to 10-fold. Absolute risk rises with increasing nuchal lucency.
Since an average of 10% to 15% of the identified anomalies are cardiac defects, fetal echocardiogram and a comprehensive fetal survey are appropriate in the second trimester.
TABLE 2
Detecting Down syndrome: Which test is best?
| MODALITY | SCREEN-POSITIVE RATE | DETECTION RATE |
|---|---|---|
| Maternal age >35 years | 18% | 30% |
| Triple screen (MSAFP, beta-hCG, estriol) | 5% | 65% |
| Quad screen (triple plus inhibin) | 5% | 75% |
| First-trimester (nuchal lucency, PAPP-A, free beta-hCG) | 5% | 80% |
| Integrated (first-trimester nuchal lucency and serum screen combined with second-trimester serum screen) | 2.5% | 90% |
| MSAFP = maternal serum alpha-fetoprotein, PAPP-A = pregnancy-associated plasma protein-A | ||
REFERENCE
1. Wapner R, Thom E, Simpsoon JL, et al. First-trimester screening for trisomies 21 and 18. N Engl J Med. 2003;349:1405-1413.
Are children conceived with ART at increased risk?
Children born as a result of ART may face a higher risk of inherited disorders and congenital malformations, but it is unclear whether the risks are due to their parents’ infertility or to ART.
For this reason, it may be wise to refer ART patients for additional genetic counseling and fetal structural surveillance by ultrasound.
Schieve and colleagues attempted to clarify the risks by reviewing the theoretical and empiric literature. Two studies provide the bulk of evidence. In Western Australia, the background risk of birth defects doubled in infants conceived with ART: 9% risk in both intracytoplasmic sperm injection and IVF patients, compared with 4% with spontaneous conception.1 This study is notable because ART programs are more highly regulated in Australia and similar methods were used to ascertain congenital anomalies in both groups.
A comparable study2 in Sweden also noted an increased risk, but attributed it to the underlying cause of the parents’ infertility rather than to ART itself. The reason: The increased risk disappeared when the authors adjusted for the period of “involuntary childlessness.” However, they provided very little detail on how involuntary childlessness was defined and “whether and how strongly this measure is correlated with infertility severity in Sweden.”2
Imprinting disorders among ART offspring. Schieve et al also explored imprinting disorders, since diseases such as Beckwith-Wiedemann syndrome are attributed to them. Imprinting is an epigenetic phenomenon in which the allele of only 1 parent is active at a particular gene locus. The inactive—or imprinted—allele is rendered nonfunctional, often through methylation. Gametogenesis and preimplantation are times of increased imprinting. Identified imprinted genes include those that control embryonic growth and differentiation.
Analyses of Beckwith-Wiedemann syndrome registries in the United States, France, and the UK3-5 revealed a 3- to 6-fold increase in ART conception among infants with the syndrome. Case reports of other rare imprinted disorders such as Angelman syndrome and retinoblastoma are also beginning to appear.
Direct treatment effect not established. According to Schieve et al and others, evidence of an increased risk of defects following ART does not indicate whether a direct treatment effect is present. Future studies that address methodological flaws are sure to be time-consuming; they also will require large sample sizes and consistent ascertainment and ART treatment.
Dr. Wilkins-Haug reports no relevant financial relationships.
1. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725-730.
2. Ericson A, Kallen B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001;16:504-509.
3. DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156-160.
4. Maher ER, Brueton LA, Bowdin SC, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) [published erratum appears in J Med Genet. 2003;40:304]. J Med Genet. 2003;40:62-64.
5. Gicquel C, Gaston V, Mandelbaum J, et al. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72:1338-1341.
Overall, you must determine the extent to which you will provide and interpret genetic testing and when to refer patients to a specialist. This article aims to simplify that decision by reviewing guidelines and key studies in 3 areas:
- For genetic carrier screening for people of Ashkenazi Jewish heritage, add familial dysautonomia to the list of screened diseases.
- Screening for Down syndrome is now possible in the first trimester.
- Greater genetic risks may be present among children born as a result of assisted reproductive technology (ART), although it’s unclear whether the cause is their parents’ infertility or ART itself.
On the plus side, molecular DNA diagnostics are increasingly sophisticated, readily available, and cost-efficient. The downside: As the list of recommended studies grows, successful testing programs are harder to achieve because of the need to educate patients—and yourself—about each test.
Preconception testing may be especially advisable in women with infertility because it can identify carriers and detect conditions related to infertility or its treatment. With 1% of US births attributable to ART, the possibility of genetic effects continues to raise concern.
Add another disease to genetic carrier screening
ACOG Committee Opinion #298: Prenatal and preconception carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet Gynecol. 2004;104:425–428.
Add familial dysautonomia to carrier screening when patients—or their partners—are of Ashkenazi Jewish heritage. That’s the advice from an American College of Obstetricians and Gynecologists (ACOG) committee opinion. Also conduct previously recommended screening for Tay-Sachs disease, Canavan disease, and cystic fibrosis, and advise patients that testing is available for several other diseases as well (TABLE 1). For Tay-Sachs disease, screening also is urged for patients of French Canadian and Cajun descent.
ACOG emphasizes the importance of assessing these risks prior to pregnancy to allow time for the partner to be tested, if necessary.
Among the Ashkenazi Jewish population, DNA testing detects more than 95% of carriers of autosomal disorders by analyzing the small number of mutations responsible. Tay-Sachs was the first disease for which mutations were identified.
Familial dysautonomia is caused by a single mutation in the gene IKBKAP in more than 99% of affected patients. It has a carrier rate (1/32) similar to that of Tay-Sachs disease (1/30) and involves substantial morbidity of the autonomic and sensory nervous system, with symptoms such as abnormal sweating, pain/temperature insensitivity, and labile blood pressure. Treatment may relieve symptoms, but does not cure the disease.
Refer non-Ashkenazi partners of identified carriers. Although non-Ashkenazi partners are less likely to be carriers, the exact carrier frequency and detection rates for these people are unknown (except for Tay-Sachs disease and cystic fibrosis). In these situations, it may be wise to refer the patient and her partner for genetic counseling to clarify the sensitivity of DNA analysis and the advisability of possible alternative testing by enzyme analysis.
What a generalist should offer. Because the availability of genetic testing will continue to increase, ACOG recommends that generalists provide:
- patient education on the disorders,
- referral sources for additional counseling and prenatal diagnostic testing,
- informed consent when obtaining samples for genetic testing, and
- assurance of confidentiality.
Screen for Down syndrome in the first trimester
Screening for Down syndrome is now available in the first trimester; ACOG recommends using ultrasound and maternal serum screening, with 3 criteria:
- standardized, continuous quality assurance,
- ability to counsel patients about the testing options, and
- access to appropriate diagnostic testing.
TABLE 2).
Integrated versus contingency screening. “Integrated” screening combines information from the first trimester (nuchal lucency and serum screen) with serum screening in the second trimester. This approach yields the lowest screen-positive rate (2.6%) and a high detection rate (90%), but has an important shortcoming: The results are not disclosed until the second trimester.
“Contingency” screening is emerging as an alternative: High first-trimester risks are relayed to the patient, while women with low screen values are excused from further testing. Patients with intermediate risk proceed to second-trimester serum screening.
Disadvantages of this approach include the need to coordinate the various steps and adequately inform the patient of them.
Added value of first-trimester nuchal lucency screening. Increased nuchal translucency alone is an important screen for structural abnormalities and adverse pregnancy outcomes. If a karyotypically normal fetus has an increased first-trimester nuchal lucency, the possibility of a structural anomaly on second-trimester ultrasound increases 2-to 10-fold. Absolute risk rises with increasing nuchal lucency.
Since an average of 10% to 15% of the identified anomalies are cardiac defects, fetal echocardiogram and a comprehensive fetal survey are appropriate in the second trimester.
TABLE 2
Detecting Down syndrome: Which test is best?
| MODALITY | SCREEN-POSITIVE RATE | DETECTION RATE |
|---|---|---|
| Maternal age >35 years | 18% | 30% |
| Triple screen (MSAFP, beta-hCG, estriol) | 5% | 65% |
| Quad screen (triple plus inhibin) | 5% | 75% |
| First-trimester (nuchal lucency, PAPP-A, free beta-hCG) | 5% | 80% |
| Integrated (first-trimester nuchal lucency and serum screen combined with second-trimester serum screen) | 2.5% | 90% |
| MSAFP = maternal serum alpha-fetoprotein, PAPP-A = pregnancy-associated plasma protein-A | ||
REFERENCE
1. Wapner R, Thom E, Simpsoon JL, et al. First-trimester screening for trisomies 21 and 18. N Engl J Med. 2003;349:1405-1413.
Are children conceived with ART at increased risk?
Children born as a result of ART may face a higher risk of inherited disorders and congenital malformations, but it is unclear whether the risks are due to their parents’ infertility or to ART.
For this reason, it may be wise to refer ART patients for additional genetic counseling and fetal structural surveillance by ultrasound.
Schieve and colleagues attempted to clarify the risks by reviewing the theoretical and empiric literature. Two studies provide the bulk of evidence. In Western Australia, the background risk of birth defects doubled in infants conceived with ART: 9% risk in both intracytoplasmic sperm injection and IVF patients, compared with 4% with spontaneous conception.1 This study is notable because ART programs are more highly regulated in Australia and similar methods were used to ascertain congenital anomalies in both groups.
A comparable study2 in Sweden also noted an increased risk, but attributed it to the underlying cause of the parents’ infertility rather than to ART itself. The reason: The increased risk disappeared when the authors adjusted for the period of “involuntary childlessness.” However, they provided very little detail on how involuntary childlessness was defined and “whether and how strongly this measure is correlated with infertility severity in Sweden.”2
Imprinting disorders among ART offspring. Schieve et al also explored imprinting disorders, since diseases such as Beckwith-Wiedemann syndrome are attributed to them. Imprinting is an epigenetic phenomenon in which the allele of only 1 parent is active at a particular gene locus. The inactive—or imprinted—allele is rendered nonfunctional, often through methylation. Gametogenesis and preimplantation are times of increased imprinting. Identified imprinted genes include those that control embryonic growth and differentiation.
Analyses of Beckwith-Wiedemann syndrome registries in the United States, France, and the UK3-5 revealed a 3- to 6-fold increase in ART conception among infants with the syndrome. Case reports of other rare imprinted disorders such as Angelman syndrome and retinoblastoma are also beginning to appear.
Direct treatment effect not established. According to Schieve et al and others, evidence of an increased risk of defects following ART does not indicate whether a direct treatment effect is present. Future studies that address methodological flaws are sure to be time-consuming; they also will require large sample sizes and consistent ascertainment and ART treatment.
Dr. Wilkins-Haug reports no relevant financial relationships.
Overall, you must determine the extent to which you will provide and interpret genetic testing and when to refer patients to a specialist. This article aims to simplify that decision by reviewing guidelines and key studies in 3 areas:
- For genetic carrier screening for people of Ashkenazi Jewish heritage, add familial dysautonomia to the list of screened diseases.
- Screening for Down syndrome is now possible in the first trimester.
- Greater genetic risks may be present among children born as a result of assisted reproductive technology (ART), although it’s unclear whether the cause is their parents’ infertility or ART itself.
On the plus side, molecular DNA diagnostics are increasingly sophisticated, readily available, and cost-efficient. The downside: As the list of recommended studies grows, successful testing programs are harder to achieve because of the need to educate patients—and yourself—about each test.
Preconception testing may be especially advisable in women with infertility because it can identify carriers and detect conditions related to infertility or its treatment. With 1% of US births attributable to ART, the possibility of genetic effects continues to raise concern.
Add another disease to genetic carrier screening
ACOG Committee Opinion #298: Prenatal and preconception carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet Gynecol. 2004;104:425–428.
Add familial dysautonomia to carrier screening when patients—or their partners—are of Ashkenazi Jewish heritage. That’s the advice from an American College of Obstetricians and Gynecologists (ACOG) committee opinion. Also conduct previously recommended screening for Tay-Sachs disease, Canavan disease, and cystic fibrosis, and advise patients that testing is available for several other diseases as well (TABLE 1). For Tay-Sachs disease, screening also is urged for patients of French Canadian and Cajun descent.
ACOG emphasizes the importance of assessing these risks prior to pregnancy to allow time for the partner to be tested, if necessary.
Among the Ashkenazi Jewish population, DNA testing detects more than 95% of carriers of autosomal disorders by analyzing the small number of mutations responsible. Tay-Sachs was the first disease for which mutations were identified.
Familial dysautonomia is caused by a single mutation in the gene IKBKAP in more than 99% of affected patients. It has a carrier rate (1/32) similar to that of Tay-Sachs disease (1/30) and involves substantial morbidity of the autonomic and sensory nervous system, with symptoms such as abnormal sweating, pain/temperature insensitivity, and labile blood pressure. Treatment may relieve symptoms, but does not cure the disease.
Refer non-Ashkenazi partners of identified carriers. Although non-Ashkenazi partners are less likely to be carriers, the exact carrier frequency and detection rates for these people are unknown (except for Tay-Sachs disease and cystic fibrosis). In these situations, it may be wise to refer the patient and her partner for genetic counseling to clarify the sensitivity of DNA analysis and the advisability of possible alternative testing by enzyme analysis.
What a generalist should offer. Because the availability of genetic testing will continue to increase, ACOG recommends that generalists provide:
- patient education on the disorders,
- referral sources for additional counseling and prenatal diagnostic testing,
- informed consent when obtaining samples for genetic testing, and
- assurance of confidentiality.
Screen for Down syndrome in the first trimester
Screening for Down syndrome is now available in the first trimester; ACOG recommends using ultrasound and maternal serum screening, with 3 criteria:
- standardized, continuous quality assurance,
- ability to counsel patients about the testing options, and
- access to appropriate diagnostic testing.
TABLE 2).
Integrated versus contingency screening. “Integrated” screening combines information from the first trimester (nuchal lucency and serum screen) with serum screening in the second trimester. This approach yields the lowest screen-positive rate (2.6%) and a high detection rate (90%), but has an important shortcoming: The results are not disclosed until the second trimester.
“Contingency” screening is emerging as an alternative: High first-trimester risks are relayed to the patient, while women with low screen values are excused from further testing. Patients with intermediate risk proceed to second-trimester serum screening.
Disadvantages of this approach include the need to coordinate the various steps and adequately inform the patient of them.
Added value of first-trimester nuchal lucency screening. Increased nuchal translucency alone is an important screen for structural abnormalities and adverse pregnancy outcomes. If a karyotypically normal fetus has an increased first-trimester nuchal lucency, the possibility of a structural anomaly on second-trimester ultrasound increases 2-to 10-fold. Absolute risk rises with increasing nuchal lucency.
Since an average of 10% to 15% of the identified anomalies are cardiac defects, fetal echocardiogram and a comprehensive fetal survey are appropriate in the second trimester.
TABLE 2
Detecting Down syndrome: Which test is best?
| MODALITY | SCREEN-POSITIVE RATE | DETECTION RATE |
|---|---|---|
| Maternal age >35 years | 18% | 30% |
| Triple screen (MSAFP, beta-hCG, estriol) | 5% | 65% |
| Quad screen (triple plus inhibin) | 5% | 75% |
| First-trimester (nuchal lucency, PAPP-A, free beta-hCG) | 5% | 80% |
| Integrated (first-trimester nuchal lucency and serum screen combined with second-trimester serum screen) | 2.5% | 90% |
| MSAFP = maternal serum alpha-fetoprotein, PAPP-A = pregnancy-associated plasma protein-A | ||
REFERENCE
1. Wapner R, Thom E, Simpsoon JL, et al. First-trimester screening for trisomies 21 and 18. N Engl J Med. 2003;349:1405-1413.
Are children conceived with ART at increased risk?
Children born as a result of ART may face a higher risk of inherited disorders and congenital malformations, but it is unclear whether the risks are due to their parents’ infertility or to ART.
For this reason, it may be wise to refer ART patients for additional genetic counseling and fetal structural surveillance by ultrasound.
Schieve and colleagues attempted to clarify the risks by reviewing the theoretical and empiric literature. Two studies provide the bulk of evidence. In Western Australia, the background risk of birth defects doubled in infants conceived with ART: 9% risk in both intracytoplasmic sperm injection and IVF patients, compared with 4% with spontaneous conception.1 This study is notable because ART programs are more highly regulated in Australia and similar methods were used to ascertain congenital anomalies in both groups.
A comparable study2 in Sweden also noted an increased risk, but attributed it to the underlying cause of the parents’ infertility rather than to ART itself. The reason: The increased risk disappeared when the authors adjusted for the period of “involuntary childlessness.” However, they provided very little detail on how involuntary childlessness was defined and “whether and how strongly this measure is correlated with infertility severity in Sweden.”2
Imprinting disorders among ART offspring. Schieve et al also explored imprinting disorders, since diseases such as Beckwith-Wiedemann syndrome are attributed to them. Imprinting is an epigenetic phenomenon in which the allele of only 1 parent is active at a particular gene locus. The inactive—or imprinted—allele is rendered nonfunctional, often through methylation. Gametogenesis and preimplantation are times of increased imprinting. Identified imprinted genes include those that control embryonic growth and differentiation.
Analyses of Beckwith-Wiedemann syndrome registries in the United States, France, and the UK3-5 revealed a 3- to 6-fold increase in ART conception among infants with the syndrome. Case reports of other rare imprinted disorders such as Angelman syndrome and retinoblastoma are also beginning to appear.
Direct treatment effect not established. According to Schieve et al and others, evidence of an increased risk of defects following ART does not indicate whether a direct treatment effect is present. Future studies that address methodological flaws are sure to be time-consuming; they also will require large sample sizes and consistent ascertainment and ART treatment.
Dr. Wilkins-Haug reports no relevant financial relationships.
1. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725-730.
2. Ericson A, Kallen B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001;16:504-509.
3. DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156-160.
4. Maher ER, Brueton LA, Bowdin SC, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) [published erratum appears in J Med Genet. 2003;40:304]. J Med Genet. 2003;40:62-64.
5. Gicquel C, Gaston V, Mandelbaum J, et al. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72:1338-1341.
1. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725-730.
2. Ericson A, Kallen B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001;16:504-509.
3. DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156-160.
4. Maher ER, Brueton LA, Bowdin SC, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) [published erratum appears in J Med Genet. 2003;40:304]. J Med Genet. 2003;40:62-64.
5. Gicquel C, Gaston V, Mandelbaum J, et al. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72:1338-1341.
How to judge an herbal remedy
Safety issues surrounding herbal medicine are complex: possible toxicity of herbal constituents, presence of contaminants or adulterants, and potential interactions between herbs and prescription drugs. In addition, the preparations are often poor in quality. One reason: They are inadequately regulated, a problem many experts hope to change. Cost evaluations of herbal medicines are not available.
This article offers guidelines for prescribing herbal medications, as well as advice on when they are unwise.
TABLE 1
10 best-selling herbal medicines
| RANK | HERB | RETAIL SALES ($ MILLIONS)* |
|---|---|---|
| 1 | Ginkgo biloba | $46 |
| 2 | Echinacea | $40 |
| 3 | Garlic | $35 |
| 4 | Ginseng | $31 |
| 5 | Soy | $28 |
| 6 | Saw palmetto | $25 |
| 7 | St John’s wort | $24 |
| 8 | Valerian | $12 |
| 9 | Cranberry | $10 |
| 10 | Black cohosh | $10 |
| * US, 2001 data | ||
Is the herb effective for the patient’s condition?
Although data are incomplete, some treatments have shown promise (TABLE 2), and findings indicate serious adverse effects of certain treatments (TABLE 3).
Besides safety, the critical question is: Does the remedy work for the patient’s condition? Do not prescribe or recommend an herbal remedy if the answer is not a firm yes.
Herbal medicines usually contain a range of pharmacologically active compounds. In some cases, it is unclear which constituents produce the therapeutic effect. Testing for efficacy in this situation is obviously more complex than with synthetic drugs. One approach is to view the entire herbal extract as the active component.
To optimize the reproducibility of efficacy studies, extracts must be sufficiently characterized. This is often achieved by standardizing the amount of a single key constituent (eg, a pharmacologically active ingredient or a marker suitable substance if such an ingredient is unknown).
Once the dilemma of standardization is solved, herbal medicines are scrutinized in much the same way as other drugs. The literature contains several randomized, clinical trials and systematic reviews/meta-analyses of these studies.3,4 The Cochrane database includes about 30 systematic reviews of herbal medicines, and several authoritative books recently were published.3-6
Unfortunately, systematic reviews are often limited by the paucity and varied methodological quality of the primary studies,3,7 and research funds are generally scarce, in part because plants cannot be patented.
Generalizations about the efficacy of herbal medicines are not possible. Each remedy must be judged on its own merits. Some herbal products have demon strated efficacy for certain conditions, while others have not. Overall, few products have been subjected to extensive clinical testing.3
The bottom line? As a review in the New England Journal of Medicine concluded, “Clinicians should not prescribe or recommend herbal remedies without well-established efficacy.”7
Tradition is no guarantee, as in the case of kava
Consumers are attracted to herbal medicines in part because they equate “natural” with “safe.” Yet some herbal medicines pose serious risks.7
First, the active ingredients in herbal preparations can cause both desirable and undesirable effects. TABLE 3 lists examples of commonly used herbal medicines that have been associated with serious adverse effects.3 Traditional use is no guarantee of safety and no acceptable substitute for data.8
A poignant example is kava (Piper methysticum), an herbal remedy that has been used for centuries, apparently without problems. Numerous rigorous clinical trials have shown it to be a powerful anxiolytic agent,9 but it was recently associated with several cases of serious liver damage.10 As a result, it was withdrawn from the markets of several European countries, and the US Food and Drug Administration (FDA) has issued warnings about its hepatotoxic potential.
Second, the active ingredients in herbal medicines can interact with prescription drugs. For instance, extracts of St. John’s wort (Hypericum perforatum) act as an enzyme inducer on the cytochrome P450 system and increase the activity of the P-glycoprotein transmembrane transporter mechanism. Both effects lead to a reduction of the plasma level of several conventional drugs.11 Perhaps the most serious consequence would be insufficiently low cyclosporine levels in patients after organ transplantation, which jeopardize the success of this procedure.12
Third, some herbal medicines (particularly Asian herbal mixtures) are contaminated with heavy metals13; contain misidentified, toxic herbal ingredients14; or are adulterated with prescription drugs.15 Be sure an herbal medication cannot cause harm before prescribing or recommending it.
TABLE 3
7 herbal medicines associated with serious adverse effects*
| COMMON (LATIN) NAME | INDICATION | ADVERSE EFFECTS (EXAMPLES) |
|---|---|---|
| Aloe vera (Aloe barbadensis) | Various | Juice may cause intestinal pain and electrolyte loss |
| Feverfew (Tanacetum parthenium) | Migraine prevention | “Post-fever syndrome” after discontinuation (migraine, anxiety, insomnia, muscle stiffness) |
| Hawthorn (Crataegus) | Congestive heart failure | Additive effects with other cardiac glycosides |
| Kava (Piper methysticum) | Anxiety | Toxic liver damage |
| St. John’s wort (Hypericum perforatum) | Depression | Increased clearance of a range of prescribed drugs |
| Tea tree oil (Malaleuca alternifolia) | Skin problems (external) | Allergic reactions |
| Valerian (Valeriana officinalis) | Insomnia | Morning hangover |
| * This is a sampling only. Also, without positive safety data, herbal medications cannot be considered safe for pregnant or nursing women. | ||
Uneven quality marks herbal medicines
The quality of an herbal preparation contributes to its efficacy and safety. Herbal dietary supplements usually are unregulated as drugs and can vary widely in quality—to the point of being ineffective.7,16
In the United States, herbal preparations must meet the requirements set forth in the Dietary Supplement and Health Education Act (DSHEA) of 1994. Thus, they are marketed without FDA approval of their efficacy and safety. The DSHEA prohibits companies from making medical claims for dietary supplements, but does allow structure or functional claims. If safety concerns arise, the burden of proof lies not with the manufacturer, but with the FDA.
Many experts believe this regulation is insufficient to guarantee consumer safety and argue for it to be changed.16 In Europe, new legislation will soon require efficacy to be based on bibliographic data, and safety will be governed as it is with conventional drugs.17
Not enough data to base decisions on cost
As a general rule, clinicians should try to recommend treatments that save money for patients and the health-care system. Although herbal medications are relatively inexpensive, few proper economic analyses exist.18,19 So far, only 1 cost evaluation20 of an herbal medicine has been published. This study involved treatment of symptomatic chronic venous insufficiency and compared the cost-effectiveness of compression stockings with that of an extract of horse chestnut seeds; the treatments were comparable.
For the prescribing physician, this means decisions cannot be based on conclusive cost-analyses. Until such studies are available, decisions must be informed by our knowledge of the efficacy, safety, and quality of herbal medications.
One of 5 Ayurvedic herbal medicine products may contain potentially toxic levels of lead, mercury, and/or arsenic, according to a study in the December 15 issue of JAMA. The Ayurvedic tradition is a holistic healing system that originated in India. When researchers tested Ayurvedic products produced in South Asia and sold in the Boston area, 14 of 70 contained heavy metals. If taken according to the package directions, the preparations would exceed published standards for the metals, some of them by a huge margin.
Pharmaceuticals in an herbal remedy?
Among other hazards detected in herbal products are undeclared prescription drugs mixed into the ingredients of some Chinese preparations, according to the FDA. And last May, Consumer Reports identified 12 dietary supplements “too dangerous to be on the market,” yet all were readily available in stores or online. They include comfrey, androstenedione, chaparral, and kava.
Pose the question
All the more reason to ask patients what products they may be using. Ask specifically about herbal or natural remedies, since many people do not consider them drugs and fail to disclose them to physicians.
—The editors
1. Eisenberg DM, David RB, Ettner SL, et al. Trends in alternative medicine use in the United States. JAMA. 1998;280:1569-1575.
2. Blumenthal M. Herb sales down in mainstream market, up in natural food stores. Herbal Gram. 2002;55:60.-
3. Ernst E, Pittler MH, Stevinson C, White AR. The Desktop Guide to Complementary and Alternative Medicine. Edinburgh: Mosby; 2001.
4. Fugh-Berman A. The 5-minute herb & dietary supplement consult. Philadelphia: Lippincott Williams & Wilkins; 2003.
5. Capasso F, Gaginella TS, Grandolini G, Izzo AA. Phytotherapy: A Quick Reference to Herbal Medicine. Berlin: Springer-Verlag; 2003.
6. Schulz V, Hänsel R, Tyler VE. Rational Phytotherapy. Berlin: Springer-Verlag; 2001.
7. De Smet PAGM. Herbal remedies. N Engl J Med. 2002;347:2046-2056.
8. Ernst E, De Smet PAGM, Shaw D, Murray V. Traditional remedies and the “test of time.” Eur J Clin Pharmacol. 1998;54:99-100.
9. Pittler MH, Ernst E. Kava extract for treating anxiety. Cochrane Library 2002.
10. Teschke R, Gaus W, Loew D. Kava extracts: safety and risks including rare hepatotoxicity. Phytomed. 2003;10:440-446.
11. Carlo GD, Borrelli F, Ernst E, Izzo AA. St. John’s wort: Prozac from the plant kingdom. TRENDS in Pharmacol Sci. 2001;22:292-297.
12. Ernst E. St John’s wort supplements endanger the success of organ transplantation. Arch Surg. 2002;137:316-319.
13. Ernst E, Thompson Coon J. Heavy metals in traditional Chinese medicines: a systematic review. Clin Pharmacol Ther. 2001;70:497-504.
14. Nortier JL, Martinez MC. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686-1692.
15. Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Int Med. 2002;251:107-113.
16. De Angelis CD, Fontanarosa PB. Drugs alias dietary supplements. JAMA. 2003;290:1519-1520.
17. Silano M, De Vincenzi M, De Vincenzi A, Silano V. The new European legislation on traditional herbal medicines: main features and perspectives. Fitoterapia. 2004;75:107-116.
18. Kernick D, White A. Applying economic evaluation to complementary and alternative medicine. In: Kernick DE, ed. Getting Health Economics into Practice. Oxford: Radcliffe Medical Press; 2002;173-180.
19. De Smet PAGM, Bonsel G, Van der Kuy A, et al. Introduction to the pharmacoeconomics of herbal medicines. Pharmacoeconomics. 2000;18:1-7.
20. Rychlik R, Marshall M, Bachinger A, et al. Ökonomische Aspekte der Therapie der chronisch venösen Insuffizienz. Gesundh ökon Qual Manag. 1997;2:86-91.
Safety issues surrounding herbal medicine are complex: possible toxicity of herbal constituents, presence of contaminants or adulterants, and potential interactions between herbs and prescription drugs. In addition, the preparations are often poor in quality. One reason: They are inadequately regulated, a problem many experts hope to change. Cost evaluations of herbal medicines are not available.
This article offers guidelines for prescribing herbal medications, as well as advice on when they are unwise.
TABLE 1
10 best-selling herbal medicines
| RANK | HERB | RETAIL SALES ($ MILLIONS)* |
|---|---|---|
| 1 | Ginkgo biloba | $46 |
| 2 | Echinacea | $40 |
| 3 | Garlic | $35 |
| 4 | Ginseng | $31 |
| 5 | Soy | $28 |
| 6 | Saw palmetto | $25 |
| 7 | St John’s wort | $24 |
| 8 | Valerian | $12 |
| 9 | Cranberry | $10 |
| 10 | Black cohosh | $10 |
| * US, 2001 data | ||
Is the herb effective for the patient’s condition?
Although data are incomplete, some treatments have shown promise (TABLE 2), and findings indicate serious adverse effects of certain treatments (TABLE 3).
Besides safety, the critical question is: Does the remedy work for the patient’s condition? Do not prescribe or recommend an herbal remedy if the answer is not a firm yes.
Herbal medicines usually contain a range of pharmacologically active compounds. In some cases, it is unclear which constituents produce the therapeutic effect. Testing for efficacy in this situation is obviously more complex than with synthetic drugs. One approach is to view the entire herbal extract as the active component.
To optimize the reproducibility of efficacy studies, extracts must be sufficiently characterized. This is often achieved by standardizing the amount of a single key constituent (eg, a pharmacologically active ingredient or a marker suitable substance if such an ingredient is unknown).
Once the dilemma of standardization is solved, herbal medicines are scrutinized in much the same way as other drugs. The literature contains several randomized, clinical trials and systematic reviews/meta-analyses of these studies.3,4 The Cochrane database includes about 30 systematic reviews of herbal medicines, and several authoritative books recently were published.3-6
Unfortunately, systematic reviews are often limited by the paucity and varied methodological quality of the primary studies,3,7 and research funds are generally scarce, in part because plants cannot be patented.
Generalizations about the efficacy of herbal medicines are not possible. Each remedy must be judged on its own merits. Some herbal products have demon strated efficacy for certain conditions, while others have not. Overall, few products have been subjected to extensive clinical testing.3
The bottom line? As a review in the New England Journal of Medicine concluded, “Clinicians should not prescribe or recommend herbal remedies without well-established efficacy.”7
Tradition is no guarantee, as in the case of kava
Consumers are attracted to herbal medicines in part because they equate “natural” with “safe.” Yet some herbal medicines pose serious risks.7
First, the active ingredients in herbal preparations can cause both desirable and undesirable effects. TABLE 3 lists examples of commonly used herbal medicines that have been associated with serious adverse effects.3 Traditional use is no guarantee of safety and no acceptable substitute for data.8
A poignant example is kava (Piper methysticum), an herbal remedy that has been used for centuries, apparently without problems. Numerous rigorous clinical trials have shown it to be a powerful anxiolytic agent,9 but it was recently associated with several cases of serious liver damage.10 As a result, it was withdrawn from the markets of several European countries, and the US Food and Drug Administration (FDA) has issued warnings about its hepatotoxic potential.
Second, the active ingredients in herbal medicines can interact with prescription drugs. For instance, extracts of St. John’s wort (Hypericum perforatum) act as an enzyme inducer on the cytochrome P450 system and increase the activity of the P-glycoprotein transmembrane transporter mechanism. Both effects lead to a reduction of the plasma level of several conventional drugs.11 Perhaps the most serious consequence would be insufficiently low cyclosporine levels in patients after organ transplantation, which jeopardize the success of this procedure.12
Third, some herbal medicines (particularly Asian herbal mixtures) are contaminated with heavy metals13; contain misidentified, toxic herbal ingredients14; or are adulterated with prescription drugs.15 Be sure an herbal medication cannot cause harm before prescribing or recommending it.
TABLE 3
7 herbal medicines associated with serious adverse effects*
| COMMON (LATIN) NAME | INDICATION | ADVERSE EFFECTS (EXAMPLES) |
|---|---|---|
| Aloe vera (Aloe barbadensis) | Various | Juice may cause intestinal pain and electrolyte loss |
| Feverfew (Tanacetum parthenium) | Migraine prevention | “Post-fever syndrome” after discontinuation (migraine, anxiety, insomnia, muscle stiffness) |
| Hawthorn (Crataegus) | Congestive heart failure | Additive effects with other cardiac glycosides |
| Kava (Piper methysticum) | Anxiety | Toxic liver damage |
| St. John’s wort (Hypericum perforatum) | Depression | Increased clearance of a range of prescribed drugs |
| Tea tree oil (Malaleuca alternifolia) | Skin problems (external) | Allergic reactions |
| Valerian (Valeriana officinalis) | Insomnia | Morning hangover |
| * This is a sampling only. Also, without positive safety data, herbal medications cannot be considered safe for pregnant or nursing women. | ||
Uneven quality marks herbal medicines
The quality of an herbal preparation contributes to its efficacy and safety. Herbal dietary supplements usually are unregulated as drugs and can vary widely in quality—to the point of being ineffective.7,16
In the United States, herbal preparations must meet the requirements set forth in the Dietary Supplement and Health Education Act (DSHEA) of 1994. Thus, they are marketed without FDA approval of their efficacy and safety. The DSHEA prohibits companies from making medical claims for dietary supplements, but does allow structure or functional claims. If safety concerns arise, the burden of proof lies not with the manufacturer, but with the FDA.
Many experts believe this regulation is insufficient to guarantee consumer safety and argue for it to be changed.16 In Europe, new legislation will soon require efficacy to be based on bibliographic data, and safety will be governed as it is with conventional drugs.17
Not enough data to base decisions on cost
As a general rule, clinicians should try to recommend treatments that save money for patients and the health-care system. Although herbal medications are relatively inexpensive, few proper economic analyses exist.18,19 So far, only 1 cost evaluation20 of an herbal medicine has been published. This study involved treatment of symptomatic chronic venous insufficiency and compared the cost-effectiveness of compression stockings with that of an extract of horse chestnut seeds; the treatments were comparable.
For the prescribing physician, this means decisions cannot be based on conclusive cost-analyses. Until such studies are available, decisions must be informed by our knowledge of the efficacy, safety, and quality of herbal medications.
One of 5 Ayurvedic herbal medicine products may contain potentially toxic levels of lead, mercury, and/or arsenic, according to a study in the December 15 issue of JAMA. The Ayurvedic tradition is a holistic healing system that originated in India. When researchers tested Ayurvedic products produced in South Asia and sold in the Boston area, 14 of 70 contained heavy metals. If taken according to the package directions, the preparations would exceed published standards for the metals, some of them by a huge margin.
Pharmaceuticals in an herbal remedy?
Among other hazards detected in herbal products are undeclared prescription drugs mixed into the ingredients of some Chinese preparations, according to the FDA. And last May, Consumer Reports identified 12 dietary supplements “too dangerous to be on the market,” yet all were readily available in stores or online. They include comfrey, androstenedione, chaparral, and kava.
Pose the question
All the more reason to ask patients what products they may be using. Ask specifically about herbal or natural remedies, since many people do not consider them drugs and fail to disclose them to physicians.
—The editors
Safety issues surrounding herbal medicine are complex: possible toxicity of herbal constituents, presence of contaminants or adulterants, and potential interactions between herbs and prescription drugs. In addition, the preparations are often poor in quality. One reason: They are inadequately regulated, a problem many experts hope to change. Cost evaluations of herbal medicines are not available.
This article offers guidelines for prescribing herbal medications, as well as advice on when they are unwise.
TABLE 1
10 best-selling herbal medicines
| RANK | HERB | RETAIL SALES ($ MILLIONS)* |
|---|---|---|
| 1 | Ginkgo biloba | $46 |
| 2 | Echinacea | $40 |
| 3 | Garlic | $35 |
| 4 | Ginseng | $31 |
| 5 | Soy | $28 |
| 6 | Saw palmetto | $25 |
| 7 | St John’s wort | $24 |
| 8 | Valerian | $12 |
| 9 | Cranberry | $10 |
| 10 | Black cohosh | $10 |
| * US, 2001 data | ||
Is the herb effective for the patient’s condition?
Although data are incomplete, some treatments have shown promise (TABLE 2), and findings indicate serious adverse effects of certain treatments (TABLE 3).
Besides safety, the critical question is: Does the remedy work for the patient’s condition? Do not prescribe or recommend an herbal remedy if the answer is not a firm yes.
Herbal medicines usually contain a range of pharmacologically active compounds. In some cases, it is unclear which constituents produce the therapeutic effect. Testing for efficacy in this situation is obviously more complex than with synthetic drugs. One approach is to view the entire herbal extract as the active component.
To optimize the reproducibility of efficacy studies, extracts must be sufficiently characterized. This is often achieved by standardizing the amount of a single key constituent (eg, a pharmacologically active ingredient or a marker suitable substance if such an ingredient is unknown).
Once the dilemma of standardization is solved, herbal medicines are scrutinized in much the same way as other drugs. The literature contains several randomized, clinical trials and systematic reviews/meta-analyses of these studies.3,4 The Cochrane database includes about 30 systematic reviews of herbal medicines, and several authoritative books recently were published.3-6
Unfortunately, systematic reviews are often limited by the paucity and varied methodological quality of the primary studies,3,7 and research funds are generally scarce, in part because plants cannot be patented.
Generalizations about the efficacy of herbal medicines are not possible. Each remedy must be judged on its own merits. Some herbal products have demon strated efficacy for certain conditions, while others have not. Overall, few products have been subjected to extensive clinical testing.3
The bottom line? As a review in the New England Journal of Medicine concluded, “Clinicians should not prescribe or recommend herbal remedies without well-established efficacy.”7
Tradition is no guarantee, as in the case of kava
Consumers are attracted to herbal medicines in part because they equate “natural” with “safe.” Yet some herbal medicines pose serious risks.7
First, the active ingredients in herbal preparations can cause both desirable and undesirable effects. TABLE 3 lists examples of commonly used herbal medicines that have been associated with serious adverse effects.3 Traditional use is no guarantee of safety and no acceptable substitute for data.8
A poignant example is kava (Piper methysticum), an herbal remedy that has been used for centuries, apparently without problems. Numerous rigorous clinical trials have shown it to be a powerful anxiolytic agent,9 but it was recently associated with several cases of serious liver damage.10 As a result, it was withdrawn from the markets of several European countries, and the US Food and Drug Administration (FDA) has issued warnings about its hepatotoxic potential.
Second, the active ingredients in herbal medicines can interact with prescription drugs. For instance, extracts of St. John’s wort (Hypericum perforatum) act as an enzyme inducer on the cytochrome P450 system and increase the activity of the P-glycoprotein transmembrane transporter mechanism. Both effects lead to a reduction of the plasma level of several conventional drugs.11 Perhaps the most serious consequence would be insufficiently low cyclosporine levels in patients after organ transplantation, which jeopardize the success of this procedure.12
Third, some herbal medicines (particularly Asian herbal mixtures) are contaminated with heavy metals13; contain misidentified, toxic herbal ingredients14; or are adulterated with prescription drugs.15 Be sure an herbal medication cannot cause harm before prescribing or recommending it.
TABLE 3
7 herbal medicines associated with serious adverse effects*
| COMMON (LATIN) NAME | INDICATION | ADVERSE EFFECTS (EXAMPLES) |
|---|---|---|
| Aloe vera (Aloe barbadensis) | Various | Juice may cause intestinal pain and electrolyte loss |
| Feverfew (Tanacetum parthenium) | Migraine prevention | “Post-fever syndrome” after discontinuation (migraine, anxiety, insomnia, muscle stiffness) |
| Hawthorn (Crataegus) | Congestive heart failure | Additive effects with other cardiac glycosides |
| Kava (Piper methysticum) | Anxiety | Toxic liver damage |
| St. John’s wort (Hypericum perforatum) | Depression | Increased clearance of a range of prescribed drugs |
| Tea tree oil (Malaleuca alternifolia) | Skin problems (external) | Allergic reactions |
| Valerian (Valeriana officinalis) | Insomnia | Morning hangover |
| * This is a sampling only. Also, without positive safety data, herbal medications cannot be considered safe for pregnant or nursing women. | ||
Uneven quality marks herbal medicines
The quality of an herbal preparation contributes to its efficacy and safety. Herbal dietary supplements usually are unregulated as drugs and can vary widely in quality—to the point of being ineffective.7,16
In the United States, herbal preparations must meet the requirements set forth in the Dietary Supplement and Health Education Act (DSHEA) of 1994. Thus, they are marketed without FDA approval of their efficacy and safety. The DSHEA prohibits companies from making medical claims for dietary supplements, but does allow structure or functional claims. If safety concerns arise, the burden of proof lies not with the manufacturer, but with the FDA.
Many experts believe this regulation is insufficient to guarantee consumer safety and argue for it to be changed.16 In Europe, new legislation will soon require efficacy to be based on bibliographic data, and safety will be governed as it is with conventional drugs.17
Not enough data to base decisions on cost
As a general rule, clinicians should try to recommend treatments that save money for patients and the health-care system. Although herbal medications are relatively inexpensive, few proper economic analyses exist.18,19 So far, only 1 cost evaluation20 of an herbal medicine has been published. This study involved treatment of symptomatic chronic venous insufficiency and compared the cost-effectiveness of compression stockings with that of an extract of horse chestnut seeds; the treatments were comparable.
For the prescribing physician, this means decisions cannot be based on conclusive cost-analyses. Until such studies are available, decisions must be informed by our knowledge of the efficacy, safety, and quality of herbal medications.
One of 5 Ayurvedic herbal medicine products may contain potentially toxic levels of lead, mercury, and/or arsenic, according to a study in the December 15 issue of JAMA. The Ayurvedic tradition is a holistic healing system that originated in India. When researchers tested Ayurvedic products produced in South Asia and sold in the Boston area, 14 of 70 contained heavy metals. If taken according to the package directions, the preparations would exceed published standards for the metals, some of them by a huge margin.
Pharmaceuticals in an herbal remedy?
Among other hazards detected in herbal products are undeclared prescription drugs mixed into the ingredients of some Chinese preparations, according to the FDA. And last May, Consumer Reports identified 12 dietary supplements “too dangerous to be on the market,” yet all were readily available in stores or online. They include comfrey, androstenedione, chaparral, and kava.
Pose the question
All the more reason to ask patients what products they may be using. Ask specifically about herbal or natural remedies, since many people do not consider them drugs and fail to disclose them to physicians.
—The editors
1. Eisenberg DM, David RB, Ettner SL, et al. Trends in alternative medicine use in the United States. JAMA. 1998;280:1569-1575.
2. Blumenthal M. Herb sales down in mainstream market, up in natural food stores. Herbal Gram. 2002;55:60.-
3. Ernst E, Pittler MH, Stevinson C, White AR. The Desktop Guide to Complementary and Alternative Medicine. Edinburgh: Mosby; 2001.
4. Fugh-Berman A. The 5-minute herb & dietary supplement consult. Philadelphia: Lippincott Williams & Wilkins; 2003.
5. Capasso F, Gaginella TS, Grandolini G, Izzo AA. Phytotherapy: A Quick Reference to Herbal Medicine. Berlin: Springer-Verlag; 2003.
6. Schulz V, Hänsel R, Tyler VE. Rational Phytotherapy. Berlin: Springer-Verlag; 2001.
7. De Smet PAGM. Herbal remedies. N Engl J Med. 2002;347:2046-2056.
8. Ernst E, De Smet PAGM, Shaw D, Murray V. Traditional remedies and the “test of time.” Eur J Clin Pharmacol. 1998;54:99-100.
9. Pittler MH, Ernst E. Kava extract for treating anxiety. Cochrane Library 2002.
10. Teschke R, Gaus W, Loew D. Kava extracts: safety and risks including rare hepatotoxicity. Phytomed. 2003;10:440-446.
11. Carlo GD, Borrelli F, Ernst E, Izzo AA. St. John’s wort: Prozac from the plant kingdom. TRENDS in Pharmacol Sci. 2001;22:292-297.
12. Ernst E. St John’s wort supplements endanger the success of organ transplantation. Arch Surg. 2002;137:316-319.
13. Ernst E, Thompson Coon J. Heavy metals in traditional Chinese medicines: a systematic review. Clin Pharmacol Ther. 2001;70:497-504.
14. Nortier JL, Martinez MC. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686-1692.
15. Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Int Med. 2002;251:107-113.
16. De Angelis CD, Fontanarosa PB. Drugs alias dietary supplements. JAMA. 2003;290:1519-1520.
17. Silano M, De Vincenzi M, De Vincenzi A, Silano V. The new European legislation on traditional herbal medicines: main features and perspectives. Fitoterapia. 2004;75:107-116.
18. Kernick D, White A. Applying economic evaluation to complementary and alternative medicine. In: Kernick DE, ed. Getting Health Economics into Practice. Oxford: Radcliffe Medical Press; 2002;173-180.
19. De Smet PAGM, Bonsel G, Van der Kuy A, et al. Introduction to the pharmacoeconomics of herbal medicines. Pharmacoeconomics. 2000;18:1-7.
20. Rychlik R, Marshall M, Bachinger A, et al. Ökonomische Aspekte der Therapie der chronisch venösen Insuffizienz. Gesundh ökon Qual Manag. 1997;2:86-91.
1. Eisenberg DM, David RB, Ettner SL, et al. Trends in alternative medicine use in the United States. JAMA. 1998;280:1569-1575.
2. Blumenthal M. Herb sales down in mainstream market, up in natural food stores. Herbal Gram. 2002;55:60.-
3. Ernst E, Pittler MH, Stevinson C, White AR. The Desktop Guide to Complementary and Alternative Medicine. Edinburgh: Mosby; 2001.
4. Fugh-Berman A. The 5-minute herb & dietary supplement consult. Philadelphia: Lippincott Williams & Wilkins; 2003.
5. Capasso F, Gaginella TS, Grandolini G, Izzo AA. Phytotherapy: A Quick Reference to Herbal Medicine. Berlin: Springer-Verlag; 2003.
6. Schulz V, Hänsel R, Tyler VE. Rational Phytotherapy. Berlin: Springer-Verlag; 2001.
7. De Smet PAGM. Herbal remedies. N Engl J Med. 2002;347:2046-2056.
8. Ernst E, De Smet PAGM, Shaw D, Murray V. Traditional remedies and the “test of time.” Eur J Clin Pharmacol. 1998;54:99-100.
9. Pittler MH, Ernst E. Kava extract for treating anxiety. Cochrane Library 2002.
10. Teschke R, Gaus W, Loew D. Kava extracts: safety and risks including rare hepatotoxicity. Phytomed. 2003;10:440-446.
11. Carlo GD, Borrelli F, Ernst E, Izzo AA. St. John’s wort: Prozac from the plant kingdom. TRENDS in Pharmacol Sci. 2001;22:292-297.
12. Ernst E. St John’s wort supplements endanger the success of organ transplantation. Arch Surg. 2002;137:316-319.
13. Ernst E, Thompson Coon J. Heavy metals in traditional Chinese medicines: a systematic review. Clin Pharmacol Ther. 2001;70:497-504.
14. Nortier JL, Martinez MC. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med. 2000;342:1686-1692.
15. Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Int Med. 2002;251:107-113.
16. De Angelis CD, Fontanarosa PB. Drugs alias dietary supplements. JAMA. 2003;290:1519-1520.
17. Silano M, De Vincenzi M, De Vincenzi A, Silano V. The new European legislation on traditional herbal medicines: main features and perspectives. Fitoterapia. 2004;75:107-116.
18. Kernick D, White A. Applying economic evaluation to complementary and alternative medicine. In: Kernick DE, ed. Getting Health Economics into Practice. Oxford: Radcliffe Medical Press; 2002;173-180.
19. De Smet PAGM, Bonsel G, Van der Kuy A, et al. Introduction to the pharmacoeconomics of herbal medicines. Pharmacoeconomics. 2000;18:1-7.
20. Rychlik R, Marshall M, Bachinger A, et al. Ökonomische Aspekte der Therapie der chronisch venösen Insuffizienz. Gesundh ökon Qual Manag. 1997;2:86-91.
Metabolic syndrome: When and how to intervene
- First-line therapies for both lipid and nonlipid risk factors? Weight loss and regular exercise.
- Reduce low-density lipoprotein (LDL) cholesterol to less than 100 mg/dL when metabolic syndrome is present.
- Lower the total of LDL and very-low-density lipoprotein (VLDL) cholesterol to less than 130 mg/dL, especially in patients with borderline (150 to 199 mg/dL) or high (200 mg/dL or above) triglycerides.
- When drug intervention is needed to lower non-HDL cholesterol, use an LDL-lowering drug or add nicotinic acid or fibrate to reduce VLDL.
This risk are serious. Metabolic syndrome amplifies morbidity and mortality due to diabetes mellitus and cardiovascular disease to such an extent that the National Cholesterol Education Program identifies it as a critical target of risk reduction, second only to reducing low-density lipoprotein (LDL) cholesterol.2
In our primary care capacity, Ob/Gyns are likely to be the first to identify metabolic syndrome and intervene—and intervention makes a difference. An aggressive approach to lipid lowering is critical. However, solid evidence confirms that weight loss and physical activity eliminate some or all of the risk factors in many patients. There’s the challenge. Notably, research reported in theNew England Journal of Medicine found that, with a nutritionist’s guidance, many patients who were counseled about these lifestyle changes reduced their risk of type 2 diabetes by 58% over 3 years.3
This article reviews key studies linking metabolic syndrome to heart disease, diabetes, and death; and describes diagnostic and management fundamentals.
What defines metabolic syndrome?
Women with 3 or more of these factors have metabolic syndrome:
- Abdominal obesity; ie, waist circumference exceeding 35 inches (88 cm).
- Triglyceride level of 150 mg/dL or more.
- High-density lipoprotein (HDL) cholesterol below 50 mg/dL.
- Blood pressure 130/85 mm Hg or above.
- Fasting glucose of 100 mg/dL or above.2
Women being treated for hypertension or diabetes can be presumed to meet the criteria for those components of metabolic syndrome.
Though the syndrome affects men and women equally overall, Hispanic and African-American women have a 26% and 57% higher incidence, respectively, than men of the same ethnic and racial background.1
Obesity and age drive full-blown syndrome
Insulin resistance, dyslipidemia, and other components of metabolic syndrome exist because of intrinsic genetic susceptibility, which occurs to varying degrees throughout the population.
Some conditions cause this genetic susceptibility to blossom into the full-blown syndrome. Obesity is the driving force for much of this expression.
Age is a highly important factor. Prevalence of metabolic syndrome climbs sharply above the age of 40—in both men and women—so much so that the syndrome is close to becoming the common feature for older age groups (FIGURE 1).
Studies find link to diabetes, cardiovascular disease
What evidence do we have that this syndrome is associated with an increased risk of diabetes, heart disease, and death?
In a study of slightly more than 1,000 males with 10 years of follow-up, Lakka et al4 found a 3.5-fold increased risk of cardiovascular disease mortality with metabolic syndrome. This risk is as high as or higher than the risk for cardiovascular disease in men with type 2 diabetes, which has been described in many other studies.
Risk rises with number of components
A more recent study explored the impact of the number of components of metabolic syndrome present.5 After controlling for age, family history of diabetes, alcohol intake, and cigarette smoking, investigators found a multivariate-adjusted relative risk of cardiovascular disease, compared with an absence of components, of 3.18, 3.48, 12.55, and 14.15 (P.001 for the presence of and or more components respectively. corresponding relative risks type diabetes were>P.001>
Another recent study used the coronary artery calcium score as a surrogate for cardiovascular disease.6 This measure is increasingly recognized as a marker of underlying atherosclerosis. In both men and women, the amount of calcium in the coronary arteries increased with the number of metabolic syndrome components.
Dyslipidemia is a critical component
Several studies have identified dyslipidemia as the key component of metabolic syndrome. That is not to say that other components are unimportant—only that lipid abnormalities appear to have the greatest impact.
In a trial from the Third National Health and Nutrition Examination Study (NHANES III),7 the large dataset that has been studied extensively for this disorder, low HDL cholesterol and high blood pressure in the presence of overt diabetes appeared to account for much of the excess risk associated with metabolic syndrome. In fact, blood pressure, HDL cholesterol, and diabetes—but not metabolic syndrome per se—were significant multivariate predictors of prevalent CHD.7
Twice the risk of myocardial infarction and stroke
Another recent study8 found twice the risk of myocardial infarction and stroke when metabolic syndrome was present.
Investigators used logistic regression to estimate the association of the syndrome as a whole and each of its 5 component conditions separately with a history of myocardial infarction (MI), stroke, and either MI or stroke (MI/stroke).
Metabolic syndrome was significantly related in multivariate analysis to MI (odds ratio [OR], 2.01; 95% confidence interval [CI], 1.53 to 2.64), stroke (OR, 2.16; 95% CI, 1.48 to 3.16), and MI/stroke (OR, 2.05; 95% CI, 1.64 to 2.57).
Among the 5 component conditions of metabolic syndrome, the following were independently and significantly related to MI/stroke8:
- insulin resistance (OR, 1.30; 95% CI, 1.03 to 1.66),
- low HDL cholesterol (OR, 1.35; 95% CI, 1.05 to 1.74),
- hypertension (OR, 1.44; 95% CI, 1.00 to 2.08), and
- high triglycerides (OR, 1.66; 95% CI, 1.20 to 2.30).
With nutritionist counseling, glucose-impaired patients lost weight
Can lifestyle adjustments alone prevent type 2 diabetes to any great extent? Can anything be done to get overweight patients with impaired glucose to stick to a diet and exercise regimen?
Yes to both questions, according to researchers who conducted a randomized, controlled trial3 of lifestyle changes among 522 middle-aged, overweight men (n = 172) and women (n = 350) with impaired glucose tolerance and a mean body mass index of 31.
Chief intervention was nutritionist counseling
Nevertheless, getting the study participants to live healthier was a complex undertaking. The intervention group received individualized counseling to encourage them to:
- reduce their weight by 5% or more
- reduce fat consumption to less than 30%
- limit saturated fat intake to less than 10%
- eat 15 g or more of fiber per 1,000 kcal of intake
- exercise moderately for at least 30 minutes daily
- eat whole-grain products, fruits and vegetables, low-fat dairy products and meat, and vegetable oils rich in monounsaturated fatty acids.
Each person in the intervention group met with a nutritionist 7 times during the first year of the study and every 3 months thereafter. Dietary advice was based on 3-day diaries of food intake, completed quarterly.
Endurance exercise was recommended to increase aerobic capacity and improve cardiorespiratory function. In addition, progressive, individually tailored, circuit-type resistance training was offered to improve muscle strength. During the first year of the study, the rate of participation in these resistance training sessions ranged from 50% to 85%.
A very different picture for controls
In contrast to the individualized attention focused on the intervention group, controls received general oral and written information about diet and exercise at the beginning of the trial and at each annual visit, but no detailed counseling. They also completed a 3-day food diary at the beginning of the study and at each annual visit.
Risk of type 2 diabetes 58% lower
The percentage of patients in the intervention group who achieved a particular goal ranged from 25% (fiber consumption) to 86% (exercise). Net weight loss at the end of the second year was 3.5 ± 5.5 kg in the intervention group versus 0.8 ± 4.4 kg in the control group (P.001 for both comparisons>
While this weight loss was not dramatic, the differences between groups was substantial. For example, individuals who lost at least 5% of their baseline weight had an odds ratio for diabetes of 0.3 (95 percent confidence interval, 0.1 to 0.7).
Over the duration of the trial, the cumulative incidence of type 2 diabetes was 58% lower in the intervention group than in the control group (P.001>3 When women were singled out, the incidence of diabetes was 54% lower in the intervention group than among controls.
The failure to make any changes in lifestyle led to an incidence of diabetes very near the 35% estimate for this high-risk population.
Patients willingly stuck to diet, exercise
The dropout rate was low, and the researchers concluded that patients with impaired glucose tolerance are “willing and able to participate in a demanding intervention program if it is made available to them.”13
Unique lipid triad
High triglycerides, small LDL particles, and low HDL form the characteristic lipid profile of women with metabolic syndrome. For classification of the different levels of cholesterol, see TABLE 1.
High triglycerides heighten risk. High triglyceride levels carry an increased, independent risk of cardiovascular disease, particularly in women. As levels exceed 200 mg/dL, that risk rises sharply (FIGURE 2).9 Other studies, including a metaanalysis, have confirmed this finding.
Low HDL cholesterol is another independent risk factor for cardiovascular disease— one that is independent of standard risk markers such as LDL cholesterol. At high total cholesterol levels, the risk of cardiovascular disease increases, but that risk is even higher when HDL is low.10
Small LDL cholesterol particles. The characteristic LDL abnormality in patients with metabolic syndrome is not elevated levels, but a shift in size from larger to smaller LDL particles. In fact, the cardiovascular disease risk associated with small LDL particles is several times higher than the risk associated with the larger particles.
Smaller particles are more atherogenic than larger LDL particles despite their lower cholesterol content. The reasons:
They are cleared more slowly from plasma, taken up more readily by the artery wall, and more actively retained.
They are more rapidly oxidized, an important step in the atherogenic process.
At any level of LDL, there are more particles circulating.
Individuals tend to cluster into 2 groups based on LDL particle size: those with larger LDL particles, who usually have relatively lower triglyceride levels, and those with smaller LDL particles, who tend to have higher triglycerides. At triglyceride levels above 150 mg/dL—the cutoff for metabolic syndrome—individuals are more likely to have smaller LDL particles.
What is the risk associated with smaller particles? A study from 2001 by St. Pierre and colleagues11 showed that, at any level of triglycerides, LDL cholesterol, or apolipoprotein B (another LDL-related risk marker), the risk of coronary heart disease associated with small LDL particles is more than 3 times the risk associated with larger LDL particles.
TABLE 1
ATP III classification of LDL, total, and HDL cholesterol (mg/dL)
| LEVEL | STATUS |
|---|---|
| LDL cholesterol | |
| Optimal | |
| 100–129 | Near or above optimal |
| 130–159 | Borderline high |
| 160 –189 | High |
| ≥190 | Very high |
| Total cholesterol | |
| Desirable | |
| 200–239 | Borderline high |
| ≥240 | High |
| HDL cholesterol | |
| Low | |
| ≥60 | High |
| LDL = low-density lipoprotein | |
| HDL = high-density lipoprotein | |
| Source: NCEP.2 Reprinted with permission | |
C-reactive protein is an important marker
C-reactive protein is an important marker of the inflammation linked to heart disease. Elevated C-reactive protein also is associated with insulin resistance and adiposity. The trigger for the liver’s production of C-reactive protein is a cytokine released in large part by adipose tissue and endothelial cells.
Because a standardized, highly sensitive assay to measure plasma C-reactive protein is now available, there is a movement to include it in the definition of metabolic syndrome. As a recent study shows, the level of C-reactive protein rises with the number of components of metabolic syndrome.12 Levels tend to be higher in women than in men.
In addition, as Ridker et al13 and others have shown, as the levels of C-reactive protein rise from low (3 mg/L), so does the risk of cardiovascular disease.
Moreover, high C-reactive protein levels add to the risk associated with standard cholesterol-based risk factors. Thus, adding plasma C-reactive protein to standard lipid screening may help predict the risk of cardiovascular disease in women with high as well as low cholesterol levels.13 For example, if an individual has both elevated C-reactive protein and the metabolic syndrome, the relative risk of cardiovascular disease is more than twice the risk in women with high C-reactive protein alone.
First-line therapies are weight loss, exercise
According to ATP III2, the aims of managing metabolic syndrome are:
- to reduce causes of the syndrome, such as obesity and inactivity, and
- to treat lipid and nonlipid risk factors.
Weight loss enhances efforts to lower LDL cholesterol and reduces the impact of all risk factors for metabolic syndrome.2
Physical activity can reduce the risk of cardiovascular disease by improving cardiovascular fitness and coronary blood flow. Regular physical activity reduces very-lowdensity lipoprotein (VLDL) cholesterol levels, increases HDL cholesterol, and can lower LDL levels in some individuals. It also may help reduce blood pressure and insulin resistance.
ATP III recommends regular physical activity as a key component of managing high serum cholesterol.2 For more information on these interventions, see “Integrating evidence and experience”.
Why aggressive lipid lowering?
The current goal is reducing LDL cholesterol to less than 100 mg/dL when metabolic syndrome is present. Even lower levels, eg, less than 70 mg/dL, may be advisable when both cardiovascular disease and metabolic syndrome are present.14
A broader measure of atherogenic lipoproteins is total cholesterol minus HDL cholesterol. This measure incorporates some of the triglyceride-rich lipoproteins involved in atherosclerosis. The target is less than 130 mg/dL (TABLE 2).2 All people with borderline (150 to 199 mg/dL) or high (200 mg/dL or above) triglycerides should be managed to achieve this goal. Weight reduction and physical activity are critical, even with drug therapy.
TABLE 2
Comparison of LDL and non-HDL cholesterol goals for 3 risk categories
| RISK CATEGORY | LDL GOAL (MG/DL) | NON-HDL GOAL (MG/DL) |
|---|---|---|
| Coronary heart disease or risk equivalent (10-year risk for coronary heart disease >20%) | ||
| 2 or more risk factors and 10-year risk 20% | ||
| 0–1 risk factor | ||
| LDL = low-density lipoprotein | ||
| HDL = high-density lipoprotein | ||
| Source: NCEP.2 Reprinted with permission | ||
When to use drug therapy
Pharmacologic intervention to lower non-HDL cholesterol may involve use of an LDL-lowering drug or the addition of nicotinic acid or fibrate to reduce VLDL.
When triglyceride levels are extremely high (500 mg/dL or higher), the primary goal of therapy is preventing acute pancreatitis. This may require a combination of low-fat diet, weight loss, regular physical activity, and a triglyceride-lowering drug.2 Once triglyceride levels decline to less than 500 mg/dL, the emphasis can return to reducing cardiovascular risk.
When LDL cholesterol is very high. LDL cholesterol levels of 190 mg/dL or higher, usually signify genetic hypercholesterolemia.2 Early detection—preferably, in young adults—is crucial to prevent coronary heart disease, and a combination of drugs usually is necessary to reduce LDL cholesterol levels. Otherwise, aim for the goals in TABLE 2.
Benefits of statins. In a post hoc analysis of data from the Scandinavian Simvastatin Survival Study, which enrolled patients with elevated LDL cholesterol and coronary heart disease, those with the triad of elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides were more likely than patients with isolated high LDL cholesterol to have other characteristics of the metabolic syndrome. They also had a greater risk of coronary heart disease on placebo and received greater benefit with simvastatin therapy.15
Fibrates and HDL cholesterol. In a subgroup analysis from the Department of Veterans Affairs High-Density Lipoprotein Intervention Trial, investigators explored the efficacy of gemfibrozil in men with coronary heart disease, HDL cholesterol levels of 40 mg/dL or below, and LDL cholesterol of 140 mg/dL or less.16
Participants were given 1,200 mg of gemfibrozil daily and followed for an average of 5.1 years. The drug was most effective in those with diabetes, reducing death from coronary heart disease by 41% (hazard ratio, 0.59; 95% confidence interval, 0.39–0.91; P.02>
Among men without diabetes, gemfibrozil was most effective for those in the highest quartile for fasting plasma insulin (risk reduction 35%; P.04>
Among those who had coronary heart disease and low HDL cholesterol, the drug reduced major cardiovascular events.
Nicotinic acid improves each of the common lipid abnormalities found in metabolic syndrome.17 Early concern that it can precipitate or worsen diabetes has largely been disproved, although some data suggest that it can slightly aggravate insulin resistance and elevate blood glucose.
When it comes to cognitive function, is metabolic syndrome a “brain drain”?
A recent prospective observational study14 found a link between metabolic syndrome and cognitive impairment in the elderly, particularly when inflammation also was present.
Hypertension, diabetes, and other cardiovascular and metabolic risk factors are thought to play a role in the development of Alzheimer’s disease and vascular dementia.
Researchers followed 2,632 elderly men and women over 5 years (mean age: 74), documenting metabolic syndrome in 1,016. Those with metabolic syndrome were more likely to have cognitive impairment (26% versus 21%; multivariate-adjusted relative risk [RR], 1.20; 95% confidence interval [CI], 1.02–1.41) than were those without the syndrome.
Investigators also documented high inflammation in the study population, defining it as higher-than-median serum levels of both interleukin 6 (≥2 pg/mL) and C-reactive protein (≥2 mg/L). They then assessed its relationship to cognitive decline.
Those with both metabolic syndrome and high inflammation had an increased likelihood of cognitive impairment, compared with those without metabolic syndrome (multivariate-adjusted RR, 1.66; 95% CI, 1.19–2.32).
Those with metabolic syndrome and low inflammation had a low likelihood of impairment (multivariate-adjusted RR, 1.08; 95% CI, 0.89–1.30).
These findings held true even after adjusting for demographics, comorbidities, and health habits. It remains to be seen whether attempts to prevent metabolic syndrome or lower inflammation also limit cognitive impairment.
1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Study. JAMA. 2002;287:356-359.
2. Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
3. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
4. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709-2716.
5. Nakanishi N, Takatorige T, Fukuda H, et al. Components of the metabolic syndrome as predictors of cardiovascular disease and type 2 diabetes in middle-aged Japanese men. Diabetes Res Clin Pract. 2004;64:59-70.
6. Reilly MP, Wolfe ML, Rhodes T, et al. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803-809.
7. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210-1214.
8. Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42-46.
9. Castelli WP. Epidemiology of triglycerides: a view from Framingham. Am J Cardiol. 1992;70(19):3H-9H.
10. Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835-2838.
11. St. Pierre, et al. Circulation. 2001;104:2295.-
12. Rutter MK, Meigs JB, Sullivan LM, et al. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380-385.
13. Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813-1818.
14. Yaffe K, Kamaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237-2242.
15. Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046-3051.
16. Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs highdensity lipoprotein intervention trial (VA-HIT). Arch Intern Med. 2002;162:2597-2604.
17. Meyers CD, Kashyap ML. Management of the metabolic syndrome—nicotinic acid. Endocrinol Metab Clin North Am. 2004;33:557-575.
- First-line therapies for both lipid and nonlipid risk factors? Weight loss and regular exercise.
- Reduce low-density lipoprotein (LDL) cholesterol to less than 100 mg/dL when metabolic syndrome is present.
- Lower the total of LDL and very-low-density lipoprotein (VLDL) cholesterol to less than 130 mg/dL, especially in patients with borderline (150 to 199 mg/dL) or high (200 mg/dL or above) triglycerides.
- When drug intervention is needed to lower non-HDL cholesterol, use an LDL-lowering drug or add nicotinic acid or fibrate to reduce VLDL.
This risk are serious. Metabolic syndrome amplifies morbidity and mortality due to diabetes mellitus and cardiovascular disease to such an extent that the National Cholesterol Education Program identifies it as a critical target of risk reduction, second only to reducing low-density lipoprotein (LDL) cholesterol.2
In our primary care capacity, Ob/Gyns are likely to be the first to identify metabolic syndrome and intervene—and intervention makes a difference. An aggressive approach to lipid lowering is critical. However, solid evidence confirms that weight loss and physical activity eliminate some or all of the risk factors in many patients. There’s the challenge. Notably, research reported in theNew England Journal of Medicine found that, with a nutritionist’s guidance, many patients who were counseled about these lifestyle changes reduced their risk of type 2 diabetes by 58% over 3 years.3
This article reviews key studies linking metabolic syndrome to heart disease, diabetes, and death; and describes diagnostic and management fundamentals.
What defines metabolic syndrome?
Women with 3 or more of these factors have metabolic syndrome:
- Abdominal obesity; ie, waist circumference exceeding 35 inches (88 cm).
- Triglyceride level of 150 mg/dL or more.
- High-density lipoprotein (HDL) cholesterol below 50 mg/dL.
- Blood pressure 130/85 mm Hg or above.
- Fasting glucose of 100 mg/dL or above.2
Women being treated for hypertension or diabetes can be presumed to meet the criteria for those components of metabolic syndrome.
Though the syndrome affects men and women equally overall, Hispanic and African-American women have a 26% and 57% higher incidence, respectively, than men of the same ethnic and racial background.1
Obesity and age drive full-blown syndrome
Insulin resistance, dyslipidemia, and other components of metabolic syndrome exist because of intrinsic genetic susceptibility, which occurs to varying degrees throughout the population.
Some conditions cause this genetic susceptibility to blossom into the full-blown syndrome. Obesity is the driving force for much of this expression.
Age is a highly important factor. Prevalence of metabolic syndrome climbs sharply above the age of 40—in both men and women—so much so that the syndrome is close to becoming the common feature for older age groups (FIGURE 1).
Studies find link to diabetes, cardiovascular disease
What evidence do we have that this syndrome is associated with an increased risk of diabetes, heart disease, and death?
In a study of slightly more than 1,000 males with 10 years of follow-up, Lakka et al4 found a 3.5-fold increased risk of cardiovascular disease mortality with metabolic syndrome. This risk is as high as or higher than the risk for cardiovascular disease in men with type 2 diabetes, which has been described in many other studies.
Risk rises with number of components
A more recent study explored the impact of the number of components of metabolic syndrome present.5 After controlling for age, family history of diabetes, alcohol intake, and cigarette smoking, investigators found a multivariate-adjusted relative risk of cardiovascular disease, compared with an absence of components, of 3.18, 3.48, 12.55, and 14.15 (P.001 for the presence of and or more components respectively. corresponding relative risks type diabetes were>P.001>
Another recent study used the coronary artery calcium score as a surrogate for cardiovascular disease.6 This measure is increasingly recognized as a marker of underlying atherosclerosis. In both men and women, the amount of calcium in the coronary arteries increased with the number of metabolic syndrome components.
Dyslipidemia is a critical component
Several studies have identified dyslipidemia as the key component of metabolic syndrome. That is not to say that other components are unimportant—only that lipid abnormalities appear to have the greatest impact.
In a trial from the Third National Health and Nutrition Examination Study (NHANES III),7 the large dataset that has been studied extensively for this disorder, low HDL cholesterol and high blood pressure in the presence of overt diabetes appeared to account for much of the excess risk associated with metabolic syndrome. In fact, blood pressure, HDL cholesterol, and diabetes—but not metabolic syndrome per se—were significant multivariate predictors of prevalent CHD.7
Twice the risk of myocardial infarction and stroke
Another recent study8 found twice the risk of myocardial infarction and stroke when metabolic syndrome was present.
Investigators used logistic regression to estimate the association of the syndrome as a whole and each of its 5 component conditions separately with a history of myocardial infarction (MI), stroke, and either MI or stroke (MI/stroke).
Metabolic syndrome was significantly related in multivariate analysis to MI (odds ratio [OR], 2.01; 95% confidence interval [CI], 1.53 to 2.64), stroke (OR, 2.16; 95% CI, 1.48 to 3.16), and MI/stroke (OR, 2.05; 95% CI, 1.64 to 2.57).
Among the 5 component conditions of metabolic syndrome, the following were independently and significantly related to MI/stroke8:
- insulin resistance (OR, 1.30; 95% CI, 1.03 to 1.66),
- low HDL cholesterol (OR, 1.35; 95% CI, 1.05 to 1.74),
- hypertension (OR, 1.44; 95% CI, 1.00 to 2.08), and
- high triglycerides (OR, 1.66; 95% CI, 1.20 to 2.30).
With nutritionist counseling, glucose-impaired patients lost weight
Can lifestyle adjustments alone prevent type 2 diabetes to any great extent? Can anything be done to get overweight patients with impaired glucose to stick to a diet and exercise regimen?
Yes to both questions, according to researchers who conducted a randomized, controlled trial3 of lifestyle changes among 522 middle-aged, overweight men (n = 172) and women (n = 350) with impaired glucose tolerance and a mean body mass index of 31.
Chief intervention was nutritionist counseling
Nevertheless, getting the study participants to live healthier was a complex undertaking. The intervention group received individualized counseling to encourage them to:
- reduce their weight by 5% or more
- reduce fat consumption to less than 30%
- limit saturated fat intake to less than 10%
- eat 15 g or more of fiber per 1,000 kcal of intake
- exercise moderately for at least 30 minutes daily
- eat whole-grain products, fruits and vegetables, low-fat dairy products and meat, and vegetable oils rich in monounsaturated fatty acids.
Each person in the intervention group met with a nutritionist 7 times during the first year of the study and every 3 months thereafter. Dietary advice was based on 3-day diaries of food intake, completed quarterly.
Endurance exercise was recommended to increase aerobic capacity and improve cardiorespiratory function. In addition, progressive, individually tailored, circuit-type resistance training was offered to improve muscle strength. During the first year of the study, the rate of participation in these resistance training sessions ranged from 50% to 85%.
A very different picture for controls
In contrast to the individualized attention focused on the intervention group, controls received general oral and written information about diet and exercise at the beginning of the trial and at each annual visit, but no detailed counseling. They also completed a 3-day food diary at the beginning of the study and at each annual visit.
Risk of type 2 diabetes 58% lower
The percentage of patients in the intervention group who achieved a particular goal ranged from 25% (fiber consumption) to 86% (exercise). Net weight loss at the end of the second year was 3.5 ± 5.5 kg in the intervention group versus 0.8 ± 4.4 kg in the control group (P.001 for both comparisons>
While this weight loss was not dramatic, the differences between groups was substantial. For example, individuals who lost at least 5% of their baseline weight had an odds ratio for diabetes of 0.3 (95 percent confidence interval, 0.1 to 0.7).
Over the duration of the trial, the cumulative incidence of type 2 diabetes was 58% lower in the intervention group than in the control group (P.001>3 When women were singled out, the incidence of diabetes was 54% lower in the intervention group than among controls.
The failure to make any changes in lifestyle led to an incidence of diabetes very near the 35% estimate for this high-risk population.
Patients willingly stuck to diet, exercise
The dropout rate was low, and the researchers concluded that patients with impaired glucose tolerance are “willing and able to participate in a demanding intervention program if it is made available to them.”13
Unique lipid triad
High triglycerides, small LDL particles, and low HDL form the characteristic lipid profile of women with metabolic syndrome. For classification of the different levels of cholesterol, see TABLE 1.
High triglycerides heighten risk. High triglyceride levels carry an increased, independent risk of cardiovascular disease, particularly in women. As levels exceed 200 mg/dL, that risk rises sharply (FIGURE 2).9 Other studies, including a metaanalysis, have confirmed this finding.
Low HDL cholesterol is another independent risk factor for cardiovascular disease— one that is independent of standard risk markers such as LDL cholesterol. At high total cholesterol levels, the risk of cardiovascular disease increases, but that risk is even higher when HDL is low.10
Small LDL cholesterol particles. The characteristic LDL abnormality in patients with metabolic syndrome is not elevated levels, but a shift in size from larger to smaller LDL particles. In fact, the cardiovascular disease risk associated with small LDL particles is several times higher than the risk associated with the larger particles.
Smaller particles are more atherogenic than larger LDL particles despite their lower cholesterol content. The reasons:
They are cleared more slowly from plasma, taken up more readily by the artery wall, and more actively retained.
They are more rapidly oxidized, an important step in the atherogenic process.
At any level of LDL, there are more particles circulating.
Individuals tend to cluster into 2 groups based on LDL particle size: those with larger LDL particles, who usually have relatively lower triglyceride levels, and those with smaller LDL particles, who tend to have higher triglycerides. At triglyceride levels above 150 mg/dL—the cutoff for metabolic syndrome—individuals are more likely to have smaller LDL particles.
What is the risk associated with smaller particles? A study from 2001 by St. Pierre and colleagues11 showed that, at any level of triglycerides, LDL cholesterol, or apolipoprotein B (another LDL-related risk marker), the risk of coronary heart disease associated with small LDL particles is more than 3 times the risk associated with larger LDL particles.
TABLE 1
ATP III classification of LDL, total, and HDL cholesterol (mg/dL)
| LEVEL | STATUS |
|---|---|
| LDL cholesterol | |
| Optimal | |
| 100–129 | Near or above optimal |
| 130–159 | Borderline high |
| 160 –189 | High |
| ≥190 | Very high |
| Total cholesterol | |
| Desirable | |
| 200–239 | Borderline high |
| ≥240 | High |
| HDL cholesterol | |
| Low | |
| ≥60 | High |
| LDL = low-density lipoprotein | |
| HDL = high-density lipoprotein | |
| Source: NCEP.2 Reprinted with permission | |
C-reactive protein is an important marker
C-reactive protein is an important marker of the inflammation linked to heart disease. Elevated C-reactive protein also is associated with insulin resistance and adiposity. The trigger for the liver’s production of C-reactive protein is a cytokine released in large part by adipose tissue and endothelial cells.
Because a standardized, highly sensitive assay to measure plasma C-reactive protein is now available, there is a movement to include it in the definition of metabolic syndrome. As a recent study shows, the level of C-reactive protein rises with the number of components of metabolic syndrome.12 Levels tend to be higher in women than in men.
In addition, as Ridker et al13 and others have shown, as the levels of C-reactive protein rise from low (3 mg/L), so does the risk of cardiovascular disease.
Moreover, high C-reactive protein levels add to the risk associated with standard cholesterol-based risk factors. Thus, adding plasma C-reactive protein to standard lipid screening may help predict the risk of cardiovascular disease in women with high as well as low cholesterol levels.13 For example, if an individual has both elevated C-reactive protein and the metabolic syndrome, the relative risk of cardiovascular disease is more than twice the risk in women with high C-reactive protein alone.
First-line therapies are weight loss, exercise
According to ATP III2, the aims of managing metabolic syndrome are:
- to reduce causes of the syndrome, such as obesity and inactivity, and
- to treat lipid and nonlipid risk factors.
Weight loss enhances efforts to lower LDL cholesterol and reduces the impact of all risk factors for metabolic syndrome.2
Physical activity can reduce the risk of cardiovascular disease by improving cardiovascular fitness and coronary blood flow. Regular physical activity reduces very-lowdensity lipoprotein (VLDL) cholesterol levels, increases HDL cholesterol, and can lower LDL levels in some individuals. It also may help reduce blood pressure and insulin resistance.
ATP III recommends regular physical activity as a key component of managing high serum cholesterol.2 For more information on these interventions, see “Integrating evidence and experience”.
Why aggressive lipid lowering?
The current goal is reducing LDL cholesterol to less than 100 mg/dL when metabolic syndrome is present. Even lower levels, eg, less than 70 mg/dL, may be advisable when both cardiovascular disease and metabolic syndrome are present.14
A broader measure of atherogenic lipoproteins is total cholesterol minus HDL cholesterol. This measure incorporates some of the triglyceride-rich lipoproteins involved in atherosclerosis. The target is less than 130 mg/dL (TABLE 2).2 All people with borderline (150 to 199 mg/dL) or high (200 mg/dL or above) triglycerides should be managed to achieve this goal. Weight reduction and physical activity are critical, even with drug therapy.
TABLE 2
Comparison of LDL and non-HDL cholesterol goals for 3 risk categories
| RISK CATEGORY | LDL GOAL (MG/DL) | NON-HDL GOAL (MG/DL) |
|---|---|---|
| Coronary heart disease or risk equivalent (10-year risk for coronary heart disease >20%) | ||
| 2 or more risk factors and 10-year risk 20% | ||
| 0–1 risk factor | ||
| LDL = low-density lipoprotein | ||
| HDL = high-density lipoprotein | ||
| Source: NCEP.2 Reprinted with permission | ||
When to use drug therapy
Pharmacologic intervention to lower non-HDL cholesterol may involve use of an LDL-lowering drug or the addition of nicotinic acid or fibrate to reduce VLDL.
When triglyceride levels are extremely high (500 mg/dL or higher), the primary goal of therapy is preventing acute pancreatitis. This may require a combination of low-fat diet, weight loss, regular physical activity, and a triglyceride-lowering drug.2 Once triglyceride levels decline to less than 500 mg/dL, the emphasis can return to reducing cardiovascular risk.
When LDL cholesterol is very high. LDL cholesterol levels of 190 mg/dL or higher, usually signify genetic hypercholesterolemia.2 Early detection—preferably, in young adults—is crucial to prevent coronary heart disease, and a combination of drugs usually is necessary to reduce LDL cholesterol levels. Otherwise, aim for the goals in TABLE 2.
Benefits of statins. In a post hoc analysis of data from the Scandinavian Simvastatin Survival Study, which enrolled patients with elevated LDL cholesterol and coronary heart disease, those with the triad of elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides were more likely than patients with isolated high LDL cholesterol to have other characteristics of the metabolic syndrome. They also had a greater risk of coronary heart disease on placebo and received greater benefit with simvastatin therapy.15
Fibrates and HDL cholesterol. In a subgroup analysis from the Department of Veterans Affairs High-Density Lipoprotein Intervention Trial, investigators explored the efficacy of gemfibrozil in men with coronary heart disease, HDL cholesterol levels of 40 mg/dL or below, and LDL cholesterol of 140 mg/dL or less.16
Participants were given 1,200 mg of gemfibrozil daily and followed for an average of 5.1 years. The drug was most effective in those with diabetes, reducing death from coronary heart disease by 41% (hazard ratio, 0.59; 95% confidence interval, 0.39–0.91; P.02>
Among men without diabetes, gemfibrozil was most effective for those in the highest quartile for fasting plasma insulin (risk reduction 35%; P.04>
Among those who had coronary heart disease and low HDL cholesterol, the drug reduced major cardiovascular events.
Nicotinic acid improves each of the common lipid abnormalities found in metabolic syndrome.17 Early concern that it can precipitate or worsen diabetes has largely been disproved, although some data suggest that it can slightly aggravate insulin resistance and elevate blood glucose.
When it comes to cognitive function, is metabolic syndrome a “brain drain”?
A recent prospective observational study14 found a link between metabolic syndrome and cognitive impairment in the elderly, particularly when inflammation also was present.
Hypertension, diabetes, and other cardiovascular and metabolic risk factors are thought to play a role in the development of Alzheimer’s disease and vascular dementia.
Researchers followed 2,632 elderly men and women over 5 years (mean age: 74), documenting metabolic syndrome in 1,016. Those with metabolic syndrome were more likely to have cognitive impairment (26% versus 21%; multivariate-adjusted relative risk [RR], 1.20; 95% confidence interval [CI], 1.02–1.41) than were those without the syndrome.
Investigators also documented high inflammation in the study population, defining it as higher-than-median serum levels of both interleukin 6 (≥2 pg/mL) and C-reactive protein (≥2 mg/L). They then assessed its relationship to cognitive decline.
Those with both metabolic syndrome and high inflammation had an increased likelihood of cognitive impairment, compared with those without metabolic syndrome (multivariate-adjusted RR, 1.66; 95% CI, 1.19–2.32).
Those with metabolic syndrome and low inflammation had a low likelihood of impairment (multivariate-adjusted RR, 1.08; 95% CI, 0.89–1.30).
These findings held true even after adjusting for demographics, comorbidities, and health habits. It remains to be seen whether attempts to prevent metabolic syndrome or lower inflammation also limit cognitive impairment.
- First-line therapies for both lipid and nonlipid risk factors? Weight loss and regular exercise.
- Reduce low-density lipoprotein (LDL) cholesterol to less than 100 mg/dL when metabolic syndrome is present.
- Lower the total of LDL and very-low-density lipoprotein (VLDL) cholesterol to less than 130 mg/dL, especially in patients with borderline (150 to 199 mg/dL) or high (200 mg/dL or above) triglycerides.
- When drug intervention is needed to lower non-HDL cholesterol, use an LDL-lowering drug or add nicotinic acid or fibrate to reduce VLDL.
This risk are serious. Metabolic syndrome amplifies morbidity and mortality due to diabetes mellitus and cardiovascular disease to such an extent that the National Cholesterol Education Program identifies it as a critical target of risk reduction, second only to reducing low-density lipoprotein (LDL) cholesterol.2
In our primary care capacity, Ob/Gyns are likely to be the first to identify metabolic syndrome and intervene—and intervention makes a difference. An aggressive approach to lipid lowering is critical. However, solid evidence confirms that weight loss and physical activity eliminate some or all of the risk factors in many patients. There’s the challenge. Notably, research reported in theNew England Journal of Medicine found that, with a nutritionist’s guidance, many patients who were counseled about these lifestyle changes reduced their risk of type 2 diabetes by 58% over 3 years.3
This article reviews key studies linking metabolic syndrome to heart disease, diabetes, and death; and describes diagnostic and management fundamentals.
What defines metabolic syndrome?
Women with 3 or more of these factors have metabolic syndrome:
- Abdominal obesity; ie, waist circumference exceeding 35 inches (88 cm).
- Triglyceride level of 150 mg/dL or more.
- High-density lipoprotein (HDL) cholesterol below 50 mg/dL.
- Blood pressure 130/85 mm Hg or above.
- Fasting glucose of 100 mg/dL or above.2
Women being treated for hypertension or diabetes can be presumed to meet the criteria for those components of metabolic syndrome.
Though the syndrome affects men and women equally overall, Hispanic and African-American women have a 26% and 57% higher incidence, respectively, than men of the same ethnic and racial background.1
Obesity and age drive full-blown syndrome
Insulin resistance, dyslipidemia, and other components of metabolic syndrome exist because of intrinsic genetic susceptibility, which occurs to varying degrees throughout the population.
Some conditions cause this genetic susceptibility to blossom into the full-blown syndrome. Obesity is the driving force for much of this expression.
Age is a highly important factor. Prevalence of metabolic syndrome climbs sharply above the age of 40—in both men and women—so much so that the syndrome is close to becoming the common feature for older age groups (FIGURE 1).
Studies find link to diabetes, cardiovascular disease
What evidence do we have that this syndrome is associated with an increased risk of diabetes, heart disease, and death?
In a study of slightly more than 1,000 males with 10 years of follow-up, Lakka et al4 found a 3.5-fold increased risk of cardiovascular disease mortality with metabolic syndrome. This risk is as high as or higher than the risk for cardiovascular disease in men with type 2 diabetes, which has been described in many other studies.
Risk rises with number of components
A more recent study explored the impact of the number of components of metabolic syndrome present.5 After controlling for age, family history of diabetes, alcohol intake, and cigarette smoking, investigators found a multivariate-adjusted relative risk of cardiovascular disease, compared with an absence of components, of 3.18, 3.48, 12.55, and 14.15 (P.001 for the presence of and or more components respectively. corresponding relative risks type diabetes were>P.001>
Another recent study used the coronary artery calcium score as a surrogate for cardiovascular disease.6 This measure is increasingly recognized as a marker of underlying atherosclerosis. In both men and women, the amount of calcium in the coronary arteries increased with the number of metabolic syndrome components.
Dyslipidemia is a critical component
Several studies have identified dyslipidemia as the key component of metabolic syndrome. That is not to say that other components are unimportant—only that lipid abnormalities appear to have the greatest impact.
In a trial from the Third National Health and Nutrition Examination Study (NHANES III),7 the large dataset that has been studied extensively for this disorder, low HDL cholesterol and high blood pressure in the presence of overt diabetes appeared to account for much of the excess risk associated with metabolic syndrome. In fact, blood pressure, HDL cholesterol, and diabetes—but not metabolic syndrome per se—were significant multivariate predictors of prevalent CHD.7
Twice the risk of myocardial infarction and stroke
Another recent study8 found twice the risk of myocardial infarction and stroke when metabolic syndrome was present.
Investigators used logistic regression to estimate the association of the syndrome as a whole and each of its 5 component conditions separately with a history of myocardial infarction (MI), stroke, and either MI or stroke (MI/stroke).
Metabolic syndrome was significantly related in multivariate analysis to MI (odds ratio [OR], 2.01; 95% confidence interval [CI], 1.53 to 2.64), stroke (OR, 2.16; 95% CI, 1.48 to 3.16), and MI/stroke (OR, 2.05; 95% CI, 1.64 to 2.57).
Among the 5 component conditions of metabolic syndrome, the following were independently and significantly related to MI/stroke8:
- insulin resistance (OR, 1.30; 95% CI, 1.03 to 1.66),
- low HDL cholesterol (OR, 1.35; 95% CI, 1.05 to 1.74),
- hypertension (OR, 1.44; 95% CI, 1.00 to 2.08), and
- high triglycerides (OR, 1.66; 95% CI, 1.20 to 2.30).
With nutritionist counseling, glucose-impaired patients lost weight
Can lifestyle adjustments alone prevent type 2 diabetes to any great extent? Can anything be done to get overweight patients with impaired glucose to stick to a diet and exercise regimen?
Yes to both questions, according to researchers who conducted a randomized, controlled trial3 of lifestyle changes among 522 middle-aged, overweight men (n = 172) and women (n = 350) with impaired glucose tolerance and a mean body mass index of 31.
Chief intervention was nutritionist counseling
Nevertheless, getting the study participants to live healthier was a complex undertaking. The intervention group received individualized counseling to encourage them to:
- reduce their weight by 5% or more
- reduce fat consumption to less than 30%
- limit saturated fat intake to less than 10%
- eat 15 g or more of fiber per 1,000 kcal of intake
- exercise moderately for at least 30 minutes daily
- eat whole-grain products, fruits and vegetables, low-fat dairy products and meat, and vegetable oils rich in monounsaturated fatty acids.
Each person in the intervention group met with a nutritionist 7 times during the first year of the study and every 3 months thereafter. Dietary advice was based on 3-day diaries of food intake, completed quarterly.
Endurance exercise was recommended to increase aerobic capacity and improve cardiorespiratory function. In addition, progressive, individually tailored, circuit-type resistance training was offered to improve muscle strength. During the first year of the study, the rate of participation in these resistance training sessions ranged from 50% to 85%.
A very different picture for controls
In contrast to the individualized attention focused on the intervention group, controls received general oral and written information about diet and exercise at the beginning of the trial and at each annual visit, but no detailed counseling. They also completed a 3-day food diary at the beginning of the study and at each annual visit.
Risk of type 2 diabetes 58% lower
The percentage of patients in the intervention group who achieved a particular goal ranged from 25% (fiber consumption) to 86% (exercise). Net weight loss at the end of the second year was 3.5 ± 5.5 kg in the intervention group versus 0.8 ± 4.4 kg in the control group (P.001 for both comparisons>
While this weight loss was not dramatic, the differences between groups was substantial. For example, individuals who lost at least 5% of their baseline weight had an odds ratio for diabetes of 0.3 (95 percent confidence interval, 0.1 to 0.7).
Over the duration of the trial, the cumulative incidence of type 2 diabetes was 58% lower in the intervention group than in the control group (P.001>3 When women were singled out, the incidence of diabetes was 54% lower in the intervention group than among controls.
The failure to make any changes in lifestyle led to an incidence of diabetes very near the 35% estimate for this high-risk population.
Patients willingly stuck to diet, exercise
The dropout rate was low, and the researchers concluded that patients with impaired glucose tolerance are “willing and able to participate in a demanding intervention program if it is made available to them.”13
Unique lipid triad
High triglycerides, small LDL particles, and low HDL form the characteristic lipid profile of women with metabolic syndrome. For classification of the different levels of cholesterol, see TABLE 1.
High triglycerides heighten risk. High triglyceride levels carry an increased, independent risk of cardiovascular disease, particularly in women. As levels exceed 200 mg/dL, that risk rises sharply (FIGURE 2).9 Other studies, including a metaanalysis, have confirmed this finding.
Low HDL cholesterol is another independent risk factor for cardiovascular disease— one that is independent of standard risk markers such as LDL cholesterol. At high total cholesterol levels, the risk of cardiovascular disease increases, but that risk is even higher when HDL is low.10
Small LDL cholesterol particles. The characteristic LDL abnormality in patients with metabolic syndrome is not elevated levels, but a shift in size from larger to smaller LDL particles. In fact, the cardiovascular disease risk associated with small LDL particles is several times higher than the risk associated with the larger particles.
Smaller particles are more atherogenic than larger LDL particles despite their lower cholesterol content. The reasons:
They are cleared more slowly from plasma, taken up more readily by the artery wall, and more actively retained.
They are more rapidly oxidized, an important step in the atherogenic process.
At any level of LDL, there are more particles circulating.
Individuals tend to cluster into 2 groups based on LDL particle size: those with larger LDL particles, who usually have relatively lower triglyceride levels, and those with smaller LDL particles, who tend to have higher triglycerides. At triglyceride levels above 150 mg/dL—the cutoff for metabolic syndrome—individuals are more likely to have smaller LDL particles.
What is the risk associated with smaller particles? A study from 2001 by St. Pierre and colleagues11 showed that, at any level of triglycerides, LDL cholesterol, or apolipoprotein B (another LDL-related risk marker), the risk of coronary heart disease associated with small LDL particles is more than 3 times the risk associated with larger LDL particles.
TABLE 1
ATP III classification of LDL, total, and HDL cholesterol (mg/dL)
| LEVEL | STATUS |
|---|---|
| LDL cholesterol | |
| Optimal | |
| 100–129 | Near or above optimal |
| 130–159 | Borderline high |
| 160 –189 | High |
| ≥190 | Very high |
| Total cholesterol | |
| Desirable | |
| 200–239 | Borderline high |
| ≥240 | High |
| HDL cholesterol | |
| Low | |
| ≥60 | High |
| LDL = low-density lipoprotein | |
| HDL = high-density lipoprotein | |
| Source: NCEP.2 Reprinted with permission | |
C-reactive protein is an important marker
C-reactive protein is an important marker of the inflammation linked to heart disease. Elevated C-reactive protein also is associated with insulin resistance and adiposity. The trigger for the liver’s production of C-reactive protein is a cytokine released in large part by adipose tissue and endothelial cells.
Because a standardized, highly sensitive assay to measure plasma C-reactive protein is now available, there is a movement to include it in the definition of metabolic syndrome. As a recent study shows, the level of C-reactive protein rises with the number of components of metabolic syndrome.12 Levels tend to be higher in women than in men.
In addition, as Ridker et al13 and others have shown, as the levels of C-reactive protein rise from low (3 mg/L), so does the risk of cardiovascular disease.
Moreover, high C-reactive protein levels add to the risk associated with standard cholesterol-based risk factors. Thus, adding plasma C-reactive protein to standard lipid screening may help predict the risk of cardiovascular disease in women with high as well as low cholesterol levels.13 For example, if an individual has both elevated C-reactive protein and the metabolic syndrome, the relative risk of cardiovascular disease is more than twice the risk in women with high C-reactive protein alone.
First-line therapies are weight loss, exercise
According to ATP III2, the aims of managing metabolic syndrome are:
- to reduce causes of the syndrome, such as obesity and inactivity, and
- to treat lipid and nonlipid risk factors.
Weight loss enhances efforts to lower LDL cholesterol and reduces the impact of all risk factors for metabolic syndrome.2
Physical activity can reduce the risk of cardiovascular disease by improving cardiovascular fitness and coronary blood flow. Regular physical activity reduces very-lowdensity lipoprotein (VLDL) cholesterol levels, increases HDL cholesterol, and can lower LDL levels in some individuals. It also may help reduce blood pressure and insulin resistance.
ATP III recommends regular physical activity as a key component of managing high serum cholesterol.2 For more information on these interventions, see “Integrating evidence and experience”.
Why aggressive lipid lowering?
The current goal is reducing LDL cholesterol to less than 100 mg/dL when metabolic syndrome is present. Even lower levels, eg, less than 70 mg/dL, may be advisable when both cardiovascular disease and metabolic syndrome are present.14
A broader measure of atherogenic lipoproteins is total cholesterol minus HDL cholesterol. This measure incorporates some of the triglyceride-rich lipoproteins involved in atherosclerosis. The target is less than 130 mg/dL (TABLE 2).2 All people with borderline (150 to 199 mg/dL) or high (200 mg/dL or above) triglycerides should be managed to achieve this goal. Weight reduction and physical activity are critical, even with drug therapy.
TABLE 2
Comparison of LDL and non-HDL cholesterol goals for 3 risk categories
| RISK CATEGORY | LDL GOAL (MG/DL) | NON-HDL GOAL (MG/DL) |
|---|---|---|
| Coronary heart disease or risk equivalent (10-year risk for coronary heart disease >20%) | ||
| 2 or more risk factors and 10-year risk 20% | ||
| 0–1 risk factor | ||
| LDL = low-density lipoprotein | ||
| HDL = high-density lipoprotein | ||
| Source: NCEP.2 Reprinted with permission | ||
When to use drug therapy
Pharmacologic intervention to lower non-HDL cholesterol may involve use of an LDL-lowering drug or the addition of nicotinic acid or fibrate to reduce VLDL.
When triglyceride levels are extremely high (500 mg/dL or higher), the primary goal of therapy is preventing acute pancreatitis. This may require a combination of low-fat diet, weight loss, regular physical activity, and a triglyceride-lowering drug.2 Once triglyceride levels decline to less than 500 mg/dL, the emphasis can return to reducing cardiovascular risk.
When LDL cholesterol is very high. LDL cholesterol levels of 190 mg/dL or higher, usually signify genetic hypercholesterolemia.2 Early detection—preferably, in young adults—is crucial to prevent coronary heart disease, and a combination of drugs usually is necessary to reduce LDL cholesterol levels. Otherwise, aim for the goals in TABLE 2.
Benefits of statins. In a post hoc analysis of data from the Scandinavian Simvastatin Survival Study, which enrolled patients with elevated LDL cholesterol and coronary heart disease, those with the triad of elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides were more likely than patients with isolated high LDL cholesterol to have other characteristics of the metabolic syndrome. They also had a greater risk of coronary heart disease on placebo and received greater benefit with simvastatin therapy.15
Fibrates and HDL cholesterol. In a subgroup analysis from the Department of Veterans Affairs High-Density Lipoprotein Intervention Trial, investigators explored the efficacy of gemfibrozil in men with coronary heart disease, HDL cholesterol levels of 40 mg/dL or below, and LDL cholesterol of 140 mg/dL or less.16
Participants were given 1,200 mg of gemfibrozil daily and followed for an average of 5.1 years. The drug was most effective in those with diabetes, reducing death from coronary heart disease by 41% (hazard ratio, 0.59; 95% confidence interval, 0.39–0.91; P.02>
Among men without diabetes, gemfibrozil was most effective for those in the highest quartile for fasting plasma insulin (risk reduction 35%; P.04>
Among those who had coronary heart disease and low HDL cholesterol, the drug reduced major cardiovascular events.
Nicotinic acid improves each of the common lipid abnormalities found in metabolic syndrome.17 Early concern that it can precipitate or worsen diabetes has largely been disproved, although some data suggest that it can slightly aggravate insulin resistance and elevate blood glucose.
When it comes to cognitive function, is metabolic syndrome a “brain drain”?
A recent prospective observational study14 found a link between metabolic syndrome and cognitive impairment in the elderly, particularly when inflammation also was present.
Hypertension, diabetes, and other cardiovascular and metabolic risk factors are thought to play a role in the development of Alzheimer’s disease and vascular dementia.
Researchers followed 2,632 elderly men and women over 5 years (mean age: 74), documenting metabolic syndrome in 1,016. Those with metabolic syndrome were more likely to have cognitive impairment (26% versus 21%; multivariate-adjusted relative risk [RR], 1.20; 95% confidence interval [CI], 1.02–1.41) than were those without the syndrome.
Investigators also documented high inflammation in the study population, defining it as higher-than-median serum levels of both interleukin 6 (≥2 pg/mL) and C-reactive protein (≥2 mg/L). They then assessed its relationship to cognitive decline.
Those with both metabolic syndrome and high inflammation had an increased likelihood of cognitive impairment, compared with those without metabolic syndrome (multivariate-adjusted RR, 1.66; 95% CI, 1.19–2.32).
Those with metabolic syndrome and low inflammation had a low likelihood of impairment (multivariate-adjusted RR, 1.08; 95% CI, 0.89–1.30).
These findings held true even after adjusting for demographics, comorbidities, and health habits. It remains to be seen whether attempts to prevent metabolic syndrome or lower inflammation also limit cognitive impairment.
1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Study. JAMA. 2002;287:356-359.
2. Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
3. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
4. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709-2716.
5. Nakanishi N, Takatorige T, Fukuda H, et al. Components of the metabolic syndrome as predictors of cardiovascular disease and type 2 diabetes in middle-aged Japanese men. Diabetes Res Clin Pract. 2004;64:59-70.
6. Reilly MP, Wolfe ML, Rhodes T, et al. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803-809.
7. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210-1214.
8. Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42-46.
9. Castelli WP. Epidemiology of triglycerides: a view from Framingham. Am J Cardiol. 1992;70(19):3H-9H.
10. Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835-2838.
11. St. Pierre, et al. Circulation. 2001;104:2295.-
12. Rutter MK, Meigs JB, Sullivan LM, et al. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380-385.
13. Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813-1818.
14. Yaffe K, Kamaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237-2242.
15. Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046-3051.
16. Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs highdensity lipoprotein intervention trial (VA-HIT). Arch Intern Med. 2002;162:2597-2604.
17. Meyers CD, Kashyap ML. Management of the metabolic syndrome—nicotinic acid. Endocrinol Metab Clin North Am. 2004;33:557-575.
1. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Study. JAMA. 2002;287:356-359.
2. Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
3. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.
4. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709-2716.
5. Nakanishi N, Takatorige T, Fukuda H, et al. Components of the metabolic syndrome as predictors of cardiovascular disease and type 2 diabetes in middle-aged Japanese men. Diabetes Res Clin Pract. 2004;64:59-70.
6. Reilly MP, Wolfe ML, Rhodes T, et al. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803-809.
7. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210-1214.
8. Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42-46.
9. Castelli WP. Epidemiology of triglycerides: a view from Framingham. Am J Cardiol. 1992;70(19):3H-9H.
10. Castelli WP, Garrison RJ, Wilson PW, et al. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256:2835-2838.
11. St. Pierre, et al. Circulation. 2001;104:2295.-
12. Rutter MK, Meigs JB, Sullivan LM, et al. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380-385.
13. Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813-1818.
14. Yaffe K, Kamaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237-2242.
15. Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046-3051.
16. Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs highdensity lipoprotein intervention trial (VA-HIT). Arch Intern Med. 2002;162:2597-2604.
17. Meyers CD, Kashyap ML. Management of the metabolic syndrome—nicotinic acid. Endocrinol Metab Clin North Am. 2004;33:557-575.