User login
Hyperbaric Oxygen for a U.S. Soldier with Oral Trauma
Group Therapy to Treat Substance Use and Traumatic Symptoms in Female Veterans
Maybe it’s nerves: Common pathway may explain pain
Many gynecologists now recognize that surgery is of little benefit in the initial diagnosis and treatment of the syndrome of chronic pelvic pain, but effective alternatives have not been well established either. Within the last year, however, new research has given us a better understanding of its causes, evaluation, and management. This Update discusses new findings on the following patient care issues:
- How a common nerve pathway may affect chronic pelvic pain patterns
- Transvaginal ultrasound in the evaluation of acute versus chronic pelvic pain
- The placebo effect of surgery
- What we can and cannot expect from endometriosis resection
- The role of adhesions in pain
- Limits of hysterectomy
- Medical therapy
Any nerve plexus injury may lead to pain
Quinn M. Obstetric denervation–gynaecological reinnervation: disruption of the inferior hypogastric plexus in childbirth as a source of gynaecological symptoms. Med Hypoth. 2004;63:390–393.
When we fit together the pieces of the chronic pelvic pain puzzle, a picture emerges that suggests the pelvic organs are connected functionally, not just by anatomical proximity. Recent commercial promotion of drugs for diseases of the bladder and bowel has raised our awareness of interstitial cystitis and irritable bowel syndrome as factors in chronic pelvic pain, and we recognize that bowel and bladder symptoms often accompany gynecologic symptoms, such as dysmenorrhea and vulvodynia. Now, a hypothesis introduced by Martin Quinn suggests disruption of the inferior hypogastric nervous plexus during childbirth may result in reinnervation changes that cause visceral pain years later. He found collateral nervesprouting and a chaotic distribution of nerve fibers when special stains were used on surgical specimens.
According to this hypothesis:
- Cesarean section is not the answer to this childbirth-related injury, because cesarean section injures the nerve plexus.
- Hysterectomy would be effective for chronic pain only if abnormal nerve regeneration is restricted to the uterus.
DIAGNOSISUltrasound is more useful for acute than chronic pain
Clinicians are taught that a good history and physical examination are the most important diagnostic tools in evaluating symptoms, but we often use imaging studies as well, including routine transvaginal ultrasound in the evaluation of pelvic pain. This analysis of published studies identified transvaginal ultrasound as an extension of the bimanual exam, but observed its greatest utility for acute rather than chronic pelvic pain. In chronic pelvic pain, laparoscopic findings, if abnormal, commonly include endometriosis and adhesions—for which transvaginal ultrasound is not very useful unless there is fixation or enlargement of the ovary.
This review describes use of ultrasound for identification of heterogeneous myometrial echotexture, asymmetric uterine enlargement, and subendometrial cysts as features of adenomyosis, and reports a positive predictive value of 68% to 86% in published series.
SURGERYExcision can be effective—so can sham surgery
Some gynecologists still choose surgery as a first-line treatment, although a landmark randomized trial published 14 years ago proved that a nonsurgical approach more effectively resolves chronic pelvic pain symptoms.1 The enthusiasm for surgery is highest when endometriosis is suspected, and some gynecologists still believe that the only adequate treatment is physical removal or destruction of implants.
Pain relief has been attributed to laparoscopic treatment of endometriosis, but cause-and-effect is uncertain, in part because of confounding factors.
For example, in a report on outcomes after ablative therapy for stage 3 or 4 endometriosis with endometriotic cysts, Jones and Sutton2 considered surgery successful because 87.7% of subjects were satisfied 1 year later. This interpretation can be questioned, however, given that patients who did not want to conceive were treated with oral contraceptives or gonadotropin-releasing hormone analog after surgery. The extent to which symptoms responded to the medication rather than the surgery is not known.
Also unknown is the extent to which symptoms respond to the placebo effect of surgery. Sutton and colleagues had previously shown that pain relief 3 months after laser laparoscopy was no greater than after sham surgery,3 but by 6 months, pain relief in the sham surgery group was not sustained, and was lower than in the real surgery group.
The new study by Abbott et al re-addressed the placebo effect of surgery by randomizing 39 women with pain and visible endometriosis implants to either diagnostic laparoscopy or laparoscopic excision of endometriosis. Six months after the surgery, the women had a second laparoscopic procedure during which the extent of endometriosis was reevaluated and visible disease was resected. In other words, all women had resection of endometriosis, although in half of the subjects, the resection was preceded by a sham operation.
Six months after the first operation, 80% of the resection group said they were improved, compared to 32% of the sham surgery group. Six months after the second operation, 83% of those who initially had sham surgery were improved.
This study shows that surgical resection can be effective in reducing pain associated with visible endometriosis, but there are 2 important additional findings:
- The placebo response of 32% is considerable and not to be ignored.
- Despite aggressive excisional surgery with its risks of major organ injury, up to 20% of subjects did not improve.
Is adhesiolysis helpful or not?
Hammoud A, Gago A, Diamond MP. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82:1483–1491.
Adhesions may be blamed for chronic pelvic pain, although randomized trials have shown adhesiolysis no more effective than sham surgery.4,5 Hammoud et al hypothesized that adhesions cause pain when they distort normal anatomy and pull on peritoneum, but stress that this idea has not been validated.
Their study found substantial evidence against the theory that adhesions cause pain, and suggests that pain and adhesions may both be due to an underlying process such as endometriosis.
They also review the evidence on the important complications that may occur with attempted surgical adhesiolysis.
Hysterectomy less helpful with preop depression
Hartmann KE, Ma C, Lamvu GM, Langenberg PW, Steege JF, Kjerulff KH. Quality of life and sexual function after hysterectomy in women with preoperative pain and depression. Obstet Gynecol. 2004;104:701–709.
Some gynecologists use removal of the uterus as the definitive treatment for chronic pain, although no controlled studies have examined the effectiveness of this operation compared to nonsurgical treatments. Hartmann et al evaluated quality of life and sexual function after hysterectomy in women who had pain, depression, or both pain and depression prior to surgery.
Results were compared between these groups and with women who had neither pain nor depression before surgery. Women with both pain and depression were more likely to have impaired quality of life after hysterectomy than were women with pain or depression alone or women with neither pain nor depression.
Two years later, pelvic pain was still troubling 19.4% of women with preoperative depression and pain, and only 9.3% of women with preoperative pain only.
Hysterectomy led to improvement in many quality of life measures and sexual function in women with pain, depression, or both. The authors concluded, “Overall we do not do harm when we perform hysterectomy for these complex patients.”
That conclusion, however, fails to consider surgical complications, time lost from work or other activities, or monetary costs, which were not evaluated.
There was no nonsurgical comparison group, and the authors point out that their study did not address the possibility that nonsurgical treatments may be as effective or more effective than hysterectomy.
REFERENCES
1. Peters AAW, van Dorst E, Jellis B, van Zuuren E, Hermans J, Trimbos JB. A randomized trial to compare 2 different approaches to women with chronic pelvic pain. Obstet Gynecol. 1991;77:740-744.
2. Jones KD, Sutton C. Patient satisfaction and changes in pain scores after ablative laparoscopic surgery for stage III–IV endometriosis and endometriotic cysts. Fertil Steril. 2003;79:1086-1090.
3. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild and moderate endometriosis. Fertil Steril. 1994;62:696-700.
4. Peters AAW, Trimbos-Kemper GCM, Admiral C, Trimbos JB. A randomized clinical trial on the benefit of adhesiolysis in patients with intraperitoneal adhesions and chronic pelvic pain. Br J Obstet Gynaecol. 1992;99:59-62.
5. Swank DJ, Swank-Bordewijk SC, Hop WC, et al. Laparoscopic adhesiolysis in patients with chronic abdominal pain: a blinded randomized controlled multi-centre trial. Lancet. 2003;361:1247-1251.
MEDICAL THERAPYLetrozole for endometriosis?
The discovery that endometriosis implants may contain the aromatase enzyme prompted consideration of aromatase inhibitors as a nonsurgical treatment for endometriosis. These agents, which prevent conversion of androgens to estrogens, are used in the management of breast cancer.
This pilot study evaluated the use of the aromatase inhibitor letrozole in 10 women in whom medical and surgical therapy for endometriosis had failed. Addback therapy with norethindrone acetate was given to prevent the decrease in bone mineral density that might have occurred with letrozole alone. In 9 of the 10 women, pain decreased over the 6 months of the study.
This encouraging result suggests that larger trials with control subjects and longer follow-up will be worthwhile.
Recommendation: Use GnRH agonist
Nasir L, Bope ET. Management of pelvic pain from dysmenorrhea or endometriosis. J Am Board Fam Pract. 2004;17:S43–S47.
Recommendations from the Family Practice Pain Education Project published at the end of 2004 support use of nonsurgical therapies for endometriosis, based in part on the findings of Ling et al,1 which demonstrated the effectiveness of empirical therapy.
ACOG agrees
That recommendation is similar to the nonsurgical approach to chronic pelvic pain recommended in 1999 in an ACOG Practice Bulletin2:
“Therapy with a GnRH agonist is an appropriate approach to the management of women with chronic pelvic pain, even in the absence of surgical confirmation of endometriosis, provided that a detailed initial evaluation fails to demonstrate some other cause of pelvic pain.”
The author is a speaker and consultant for TAP Pharmaceuticals.
1. Ling FW for the Pelvic Pain Study Group. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstet Gynecol. 1999;93:51-58.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #11: Medical Management of Endometriosis. Washington, DC: ACOG; December 1999.
Many gynecologists now recognize that surgery is of little benefit in the initial diagnosis and treatment of the syndrome of chronic pelvic pain, but effective alternatives have not been well established either. Within the last year, however, new research has given us a better understanding of its causes, evaluation, and management. This Update discusses new findings on the following patient care issues:
- How a common nerve pathway may affect chronic pelvic pain patterns
- Transvaginal ultrasound in the evaluation of acute versus chronic pelvic pain
- The placebo effect of surgery
- What we can and cannot expect from endometriosis resection
- The role of adhesions in pain
- Limits of hysterectomy
- Medical therapy
Any nerve plexus injury may lead to pain
Quinn M. Obstetric denervation–gynaecological reinnervation: disruption of the inferior hypogastric plexus in childbirth as a source of gynaecological symptoms. Med Hypoth. 2004;63:390–393.
When we fit together the pieces of the chronic pelvic pain puzzle, a picture emerges that suggests the pelvic organs are connected functionally, not just by anatomical proximity. Recent commercial promotion of drugs for diseases of the bladder and bowel has raised our awareness of interstitial cystitis and irritable bowel syndrome as factors in chronic pelvic pain, and we recognize that bowel and bladder symptoms often accompany gynecologic symptoms, such as dysmenorrhea and vulvodynia. Now, a hypothesis introduced by Martin Quinn suggests disruption of the inferior hypogastric nervous plexus during childbirth may result in reinnervation changes that cause visceral pain years later. He found collateral nervesprouting and a chaotic distribution of nerve fibers when special stains were used on surgical specimens.
According to this hypothesis:
- Cesarean section is not the answer to this childbirth-related injury, because cesarean section injures the nerve plexus.
- Hysterectomy would be effective for chronic pain only if abnormal nerve regeneration is restricted to the uterus.
DIAGNOSISUltrasound is more useful for acute than chronic pain
Clinicians are taught that a good history and physical examination are the most important diagnostic tools in evaluating symptoms, but we often use imaging studies as well, including routine transvaginal ultrasound in the evaluation of pelvic pain. This analysis of published studies identified transvaginal ultrasound as an extension of the bimanual exam, but observed its greatest utility for acute rather than chronic pelvic pain. In chronic pelvic pain, laparoscopic findings, if abnormal, commonly include endometriosis and adhesions—for which transvaginal ultrasound is not very useful unless there is fixation or enlargement of the ovary.
This review describes use of ultrasound for identification of heterogeneous myometrial echotexture, asymmetric uterine enlargement, and subendometrial cysts as features of adenomyosis, and reports a positive predictive value of 68% to 86% in published series.
SURGERYExcision can be effective—so can sham surgery
Some gynecologists still choose surgery as a first-line treatment, although a landmark randomized trial published 14 years ago proved that a nonsurgical approach more effectively resolves chronic pelvic pain symptoms.1 The enthusiasm for surgery is highest when endometriosis is suspected, and some gynecologists still believe that the only adequate treatment is physical removal or destruction of implants.
Pain relief has been attributed to laparoscopic treatment of endometriosis, but cause-and-effect is uncertain, in part because of confounding factors.
For example, in a report on outcomes after ablative therapy for stage 3 or 4 endometriosis with endometriotic cysts, Jones and Sutton2 considered surgery successful because 87.7% of subjects were satisfied 1 year later. This interpretation can be questioned, however, given that patients who did not want to conceive were treated with oral contraceptives or gonadotropin-releasing hormone analog after surgery. The extent to which symptoms responded to the medication rather than the surgery is not known.
Also unknown is the extent to which symptoms respond to the placebo effect of surgery. Sutton and colleagues had previously shown that pain relief 3 months after laser laparoscopy was no greater than after sham surgery,3 but by 6 months, pain relief in the sham surgery group was not sustained, and was lower than in the real surgery group.
The new study by Abbott et al re-addressed the placebo effect of surgery by randomizing 39 women with pain and visible endometriosis implants to either diagnostic laparoscopy or laparoscopic excision of endometriosis. Six months after the surgery, the women had a second laparoscopic procedure during which the extent of endometriosis was reevaluated and visible disease was resected. In other words, all women had resection of endometriosis, although in half of the subjects, the resection was preceded by a sham operation.
Six months after the first operation, 80% of the resection group said they were improved, compared to 32% of the sham surgery group. Six months after the second operation, 83% of those who initially had sham surgery were improved.
This study shows that surgical resection can be effective in reducing pain associated with visible endometriosis, but there are 2 important additional findings:
- The placebo response of 32% is considerable and not to be ignored.
- Despite aggressive excisional surgery with its risks of major organ injury, up to 20% of subjects did not improve.
Is adhesiolysis helpful or not?
Hammoud A, Gago A, Diamond MP. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82:1483–1491.
Adhesions may be blamed for chronic pelvic pain, although randomized trials have shown adhesiolysis no more effective than sham surgery.4,5 Hammoud et al hypothesized that adhesions cause pain when they distort normal anatomy and pull on peritoneum, but stress that this idea has not been validated.
Their study found substantial evidence against the theory that adhesions cause pain, and suggests that pain and adhesions may both be due to an underlying process such as endometriosis.
They also review the evidence on the important complications that may occur with attempted surgical adhesiolysis.
Hysterectomy less helpful with preop depression
Hartmann KE, Ma C, Lamvu GM, Langenberg PW, Steege JF, Kjerulff KH. Quality of life and sexual function after hysterectomy in women with preoperative pain and depression. Obstet Gynecol. 2004;104:701–709.
Some gynecologists use removal of the uterus as the definitive treatment for chronic pain, although no controlled studies have examined the effectiveness of this operation compared to nonsurgical treatments. Hartmann et al evaluated quality of life and sexual function after hysterectomy in women who had pain, depression, or both pain and depression prior to surgery.
Results were compared between these groups and with women who had neither pain nor depression before surgery. Women with both pain and depression were more likely to have impaired quality of life after hysterectomy than were women with pain or depression alone or women with neither pain nor depression.
Two years later, pelvic pain was still troubling 19.4% of women with preoperative depression and pain, and only 9.3% of women with preoperative pain only.
Hysterectomy led to improvement in many quality of life measures and sexual function in women with pain, depression, or both. The authors concluded, “Overall we do not do harm when we perform hysterectomy for these complex patients.”
That conclusion, however, fails to consider surgical complications, time lost from work or other activities, or monetary costs, which were not evaluated.
There was no nonsurgical comparison group, and the authors point out that their study did not address the possibility that nonsurgical treatments may be as effective or more effective than hysterectomy.
REFERENCES
1. Peters AAW, van Dorst E, Jellis B, van Zuuren E, Hermans J, Trimbos JB. A randomized trial to compare 2 different approaches to women with chronic pelvic pain. Obstet Gynecol. 1991;77:740-744.
2. Jones KD, Sutton C. Patient satisfaction and changes in pain scores after ablative laparoscopic surgery for stage III–IV endometriosis and endometriotic cysts. Fertil Steril. 2003;79:1086-1090.
3. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild and moderate endometriosis. Fertil Steril. 1994;62:696-700.
4. Peters AAW, Trimbos-Kemper GCM, Admiral C, Trimbos JB. A randomized clinical trial on the benefit of adhesiolysis in patients with intraperitoneal adhesions and chronic pelvic pain. Br J Obstet Gynaecol. 1992;99:59-62.
5. Swank DJ, Swank-Bordewijk SC, Hop WC, et al. Laparoscopic adhesiolysis in patients with chronic abdominal pain: a blinded randomized controlled multi-centre trial. Lancet. 2003;361:1247-1251.
MEDICAL THERAPYLetrozole for endometriosis?
The discovery that endometriosis implants may contain the aromatase enzyme prompted consideration of aromatase inhibitors as a nonsurgical treatment for endometriosis. These agents, which prevent conversion of androgens to estrogens, are used in the management of breast cancer.
This pilot study evaluated the use of the aromatase inhibitor letrozole in 10 women in whom medical and surgical therapy for endometriosis had failed. Addback therapy with norethindrone acetate was given to prevent the decrease in bone mineral density that might have occurred with letrozole alone. In 9 of the 10 women, pain decreased over the 6 months of the study.
This encouraging result suggests that larger trials with control subjects and longer follow-up will be worthwhile.
Recommendation: Use GnRH agonist
Nasir L, Bope ET. Management of pelvic pain from dysmenorrhea or endometriosis. J Am Board Fam Pract. 2004;17:S43–S47.
Recommendations from the Family Practice Pain Education Project published at the end of 2004 support use of nonsurgical therapies for endometriosis, based in part on the findings of Ling et al,1 which demonstrated the effectiveness of empirical therapy.
ACOG agrees
That recommendation is similar to the nonsurgical approach to chronic pelvic pain recommended in 1999 in an ACOG Practice Bulletin2:
“Therapy with a GnRH agonist is an appropriate approach to the management of women with chronic pelvic pain, even in the absence of surgical confirmation of endometriosis, provided that a detailed initial evaluation fails to demonstrate some other cause of pelvic pain.”
The author is a speaker and consultant for TAP Pharmaceuticals.
Many gynecologists now recognize that surgery is of little benefit in the initial diagnosis and treatment of the syndrome of chronic pelvic pain, but effective alternatives have not been well established either. Within the last year, however, new research has given us a better understanding of its causes, evaluation, and management. This Update discusses new findings on the following patient care issues:
- How a common nerve pathway may affect chronic pelvic pain patterns
- Transvaginal ultrasound in the evaluation of acute versus chronic pelvic pain
- The placebo effect of surgery
- What we can and cannot expect from endometriosis resection
- The role of adhesions in pain
- Limits of hysterectomy
- Medical therapy
Any nerve plexus injury may lead to pain
Quinn M. Obstetric denervation–gynaecological reinnervation: disruption of the inferior hypogastric plexus in childbirth as a source of gynaecological symptoms. Med Hypoth. 2004;63:390–393.
When we fit together the pieces of the chronic pelvic pain puzzle, a picture emerges that suggests the pelvic organs are connected functionally, not just by anatomical proximity. Recent commercial promotion of drugs for diseases of the bladder and bowel has raised our awareness of interstitial cystitis and irritable bowel syndrome as factors in chronic pelvic pain, and we recognize that bowel and bladder symptoms often accompany gynecologic symptoms, such as dysmenorrhea and vulvodynia. Now, a hypothesis introduced by Martin Quinn suggests disruption of the inferior hypogastric nervous plexus during childbirth may result in reinnervation changes that cause visceral pain years later. He found collateral nervesprouting and a chaotic distribution of nerve fibers when special stains were used on surgical specimens.
According to this hypothesis:
- Cesarean section is not the answer to this childbirth-related injury, because cesarean section injures the nerve plexus.
- Hysterectomy would be effective for chronic pain only if abnormal nerve regeneration is restricted to the uterus.
DIAGNOSISUltrasound is more useful for acute than chronic pain
Clinicians are taught that a good history and physical examination are the most important diagnostic tools in evaluating symptoms, but we often use imaging studies as well, including routine transvaginal ultrasound in the evaluation of pelvic pain. This analysis of published studies identified transvaginal ultrasound as an extension of the bimanual exam, but observed its greatest utility for acute rather than chronic pelvic pain. In chronic pelvic pain, laparoscopic findings, if abnormal, commonly include endometriosis and adhesions—for which transvaginal ultrasound is not very useful unless there is fixation or enlargement of the ovary.
This review describes use of ultrasound for identification of heterogeneous myometrial echotexture, asymmetric uterine enlargement, and subendometrial cysts as features of adenomyosis, and reports a positive predictive value of 68% to 86% in published series.
SURGERYExcision can be effective—so can sham surgery
Some gynecologists still choose surgery as a first-line treatment, although a landmark randomized trial published 14 years ago proved that a nonsurgical approach more effectively resolves chronic pelvic pain symptoms.1 The enthusiasm for surgery is highest when endometriosis is suspected, and some gynecologists still believe that the only adequate treatment is physical removal or destruction of implants.
Pain relief has been attributed to laparoscopic treatment of endometriosis, but cause-and-effect is uncertain, in part because of confounding factors.
For example, in a report on outcomes after ablative therapy for stage 3 or 4 endometriosis with endometriotic cysts, Jones and Sutton2 considered surgery successful because 87.7% of subjects were satisfied 1 year later. This interpretation can be questioned, however, given that patients who did not want to conceive were treated with oral contraceptives or gonadotropin-releasing hormone analog after surgery. The extent to which symptoms responded to the medication rather than the surgery is not known.
Also unknown is the extent to which symptoms respond to the placebo effect of surgery. Sutton and colleagues had previously shown that pain relief 3 months after laser laparoscopy was no greater than after sham surgery,3 but by 6 months, pain relief in the sham surgery group was not sustained, and was lower than in the real surgery group.
The new study by Abbott et al re-addressed the placebo effect of surgery by randomizing 39 women with pain and visible endometriosis implants to either diagnostic laparoscopy or laparoscopic excision of endometriosis. Six months after the surgery, the women had a second laparoscopic procedure during which the extent of endometriosis was reevaluated and visible disease was resected. In other words, all women had resection of endometriosis, although in half of the subjects, the resection was preceded by a sham operation.
Six months after the first operation, 80% of the resection group said they were improved, compared to 32% of the sham surgery group. Six months after the second operation, 83% of those who initially had sham surgery were improved.
This study shows that surgical resection can be effective in reducing pain associated with visible endometriosis, but there are 2 important additional findings:
- The placebo response of 32% is considerable and not to be ignored.
- Despite aggressive excisional surgery with its risks of major organ injury, up to 20% of subjects did not improve.
Is adhesiolysis helpful or not?
Hammoud A, Gago A, Diamond MP. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82:1483–1491.
Adhesions may be blamed for chronic pelvic pain, although randomized trials have shown adhesiolysis no more effective than sham surgery.4,5 Hammoud et al hypothesized that adhesions cause pain when they distort normal anatomy and pull on peritoneum, but stress that this idea has not been validated.
Their study found substantial evidence against the theory that adhesions cause pain, and suggests that pain and adhesions may both be due to an underlying process such as endometriosis.
They also review the evidence on the important complications that may occur with attempted surgical adhesiolysis.
Hysterectomy less helpful with preop depression
Hartmann KE, Ma C, Lamvu GM, Langenberg PW, Steege JF, Kjerulff KH. Quality of life and sexual function after hysterectomy in women with preoperative pain and depression. Obstet Gynecol. 2004;104:701–709.
Some gynecologists use removal of the uterus as the definitive treatment for chronic pain, although no controlled studies have examined the effectiveness of this operation compared to nonsurgical treatments. Hartmann et al evaluated quality of life and sexual function after hysterectomy in women who had pain, depression, or both pain and depression prior to surgery.
Results were compared between these groups and with women who had neither pain nor depression before surgery. Women with both pain and depression were more likely to have impaired quality of life after hysterectomy than were women with pain or depression alone or women with neither pain nor depression.
Two years later, pelvic pain was still troubling 19.4% of women with preoperative depression and pain, and only 9.3% of women with preoperative pain only.
Hysterectomy led to improvement in many quality of life measures and sexual function in women with pain, depression, or both. The authors concluded, “Overall we do not do harm when we perform hysterectomy for these complex patients.”
That conclusion, however, fails to consider surgical complications, time lost from work or other activities, or monetary costs, which were not evaluated.
There was no nonsurgical comparison group, and the authors point out that their study did not address the possibility that nonsurgical treatments may be as effective or more effective than hysterectomy.
REFERENCES
1. Peters AAW, van Dorst E, Jellis B, van Zuuren E, Hermans J, Trimbos JB. A randomized trial to compare 2 different approaches to women with chronic pelvic pain. Obstet Gynecol. 1991;77:740-744.
2. Jones KD, Sutton C. Patient satisfaction and changes in pain scores after ablative laparoscopic surgery for stage III–IV endometriosis and endometriotic cysts. Fertil Steril. 2003;79:1086-1090.
3. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild and moderate endometriosis. Fertil Steril. 1994;62:696-700.
4. Peters AAW, Trimbos-Kemper GCM, Admiral C, Trimbos JB. A randomized clinical trial on the benefit of adhesiolysis in patients with intraperitoneal adhesions and chronic pelvic pain. Br J Obstet Gynaecol. 1992;99:59-62.
5. Swank DJ, Swank-Bordewijk SC, Hop WC, et al. Laparoscopic adhesiolysis in patients with chronic abdominal pain: a blinded randomized controlled multi-centre trial. Lancet. 2003;361:1247-1251.
MEDICAL THERAPYLetrozole for endometriosis?
The discovery that endometriosis implants may contain the aromatase enzyme prompted consideration of aromatase inhibitors as a nonsurgical treatment for endometriosis. These agents, which prevent conversion of androgens to estrogens, are used in the management of breast cancer.
This pilot study evaluated the use of the aromatase inhibitor letrozole in 10 women in whom medical and surgical therapy for endometriosis had failed. Addback therapy with norethindrone acetate was given to prevent the decrease in bone mineral density that might have occurred with letrozole alone. In 9 of the 10 women, pain decreased over the 6 months of the study.
This encouraging result suggests that larger trials with control subjects and longer follow-up will be worthwhile.
Recommendation: Use GnRH agonist
Nasir L, Bope ET. Management of pelvic pain from dysmenorrhea or endometriosis. J Am Board Fam Pract. 2004;17:S43–S47.
Recommendations from the Family Practice Pain Education Project published at the end of 2004 support use of nonsurgical therapies for endometriosis, based in part on the findings of Ling et al,1 which demonstrated the effectiveness of empirical therapy.
ACOG agrees
That recommendation is similar to the nonsurgical approach to chronic pelvic pain recommended in 1999 in an ACOG Practice Bulletin2:
“Therapy with a GnRH agonist is an appropriate approach to the management of women with chronic pelvic pain, even in the absence of surgical confirmation of endometriosis, provided that a detailed initial evaluation fails to demonstrate some other cause of pelvic pain.”
The author is a speaker and consultant for TAP Pharmaceuticals.
1. Ling FW for the Pelvic Pain Study Group. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstet Gynecol. 1999;93:51-58.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #11: Medical Management of Endometriosis. Washington, DC: ACOG; December 1999.
1. Ling FW for the Pelvic Pain Study Group. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstet Gynecol. 1999;93:51-58.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #11: Medical Management of Endometriosis. Washington, DC: ACOG; December 1999.
A practical plan to detect and manage HELLP syndrome
Here’s a disturbing fact: If it looks like HELLP syndrome, and impairs the patient like HELLP syndrome, it isn’t necessarily HELLP syndrome. A plethora of diagnostic criteria from different investigators over the years has confused the issue of what constitutes this syndrome—not to mention how to manage it.
A management issue has also attracted recent attention: use of corticosteroids either antepartum to enhance maternal status so that epidural anesthesia can be administered, or postpartum to improve platelets. Such improvements are only transient, however, and we lack definitive data on the benefits.
One thing is certain, however. The combination of hemolysis, liver dysfunction or injury, and platelet consumption in women with preeclampsia makes adverse maternal and perinatal outcomes more likely and leaves no room for expectant management.
HELLP syndrome also has become a major issue in litigation against obstetricians and medical and surgical consultants. Lawsuits usually allege misdiagnosed preeclampsia, delayed delivery, or improper recognition and management of complications.
Pinning HELLP Down
One of the best tools to identify HELLP syndrome is a healthy dose of suspicion, since it can affect any pregnant woman at any time: antepartum, intrapartum, or within 1 week postpartum. Approximately 72% of cases are diagnosed before delivery, and the rest are diagnosed during the first week postpartum.
Weinstein noted that the signs and symptoms of HELLP syndrome can occur without clinical evidence of severe preeclampsia (severe hypertension and/or severe proteinuria). Indeed, he reported that hypertension can be mild or absent in most patients with HELLP, and proteinuria can be mild.
Weinstein coined the term HELLP syndrome in 1982 to describe these abnormalities in women with preeclampsia:
- H = hemolysis
- EL = elevated liver enzymes
- LP = low platelets
Another obstacle to early detection: Patients may have nonspecific signs and symptoms, none of which are diagnostic of classical preeclampsia.
However, HELLP syndrome is most common in women who have already been diagnosed with gestational hypertension and/or preeclampsia.
HELLP is more likely with severe hypertension
Overall, the incidence of HELLP syndrome in women with gestational hypertension/preeclampsia increases with the severity of the condition. HELLP syndrome also is more likely in women with early-onset hypertension/preeclampsia (before 34 weeks’ gestation).
Making The Diagnosis
HELLP syndrome is diagnosed when all 3 of the following are present:
- Hemolysis, defined as the presence of microangiopathic hemolytic anemia. This is the hallmark of the triad.
- Elevated liver enzymes (either aspartate aminotransferase [AST] or alanine aminotransferase [ALT]). This component signifies liver cell ischemia and/or necrosis.
- Low platelet count (<100,000/mm3). TABLE 1 summarizes the laboratory criteria for the diagnosis.
When to begin testing
In women with new-onset hypertension, order a complete blood count with platelets and liver enzyme analysis at the time of diagnosis and serially thereafter. The frequency of these tests depends on the initial test results, severity of disease, and onset of symptoms.
In women without hypertension, I recommend obtaining the same blood tests at the onset of any of the signs and symptoms listed in TABLE 2.
TABLE 2
Conditions that heighten the risk of HELLP
|
Assessing test results
Clinicians should be familiar with the upper limit for liverenzyme tests in their laboratory. I suggest a cutoff more than twice the upper limit for a particular test.
Also keep in mind that these parameters are dynamic; some women will meet only some of the criteria early in the disease process. Moreover, maternal complications are substantially higher when all 3 components are present than when only 1 or 2 are present.
Look for these clinical findings
Hypertension. Most women with HELLP syndrome have hypertension. In 15% to 50% of cases, the hypertension is mild, but it may be absent in 15%.
Proteinuria. Most patients also have proteinuria by dipstick (≥1+). Proteinuria may be absent in approximately 13% of women with HELLP syndrome, although they will likely have many of the symptoms reported by women with severe preeclampsia.
TABLE 3 lists the signs and symptoms to be expected in these patients, along with their frequency.
TABLE 3
Signs and symptoms
| CONDITION | FREQUENCY (%) |
|---|---|
| Hypertension | 85 |
| Proteinuria | 87 |
| Right upper quadrant or epigastric pain | 40–90 |
| Nausea or vomiting | 29–84 |
| Headaches | 33–60 |
| Visual changes | 10–20 |
| Mucosal bleeding | 10 |
| Jaundice | 5 |
The usual times of onset
Antepartum cases. As was previously noted, HELLP syndrome usually develops before delivery, with the most frequent onset being before 37 weeks’ gestation ( TABLE 4).
In the postpartum period, most cases develop within 48 hours after delivery. Of these, approximately 90% occur in women who had antepartum preeclampsia that progressed to HELLP syndrome in the postpartum period. However, approximately 20% of postpartum cases develop more than 48 hours after delivery.
Another important point: HELLP syndrome can develop for the first time postpartum in women who had no evidence of preeclampsia before or during labor. Thus, it is important to educate all postpartum women to report new symptoms (listed in TABLE 3) as soon as possible. When these symptoms develop, evaluate the patient for both preeclampsia and HELLP syndrome.
TABLE 4
Usual times of onset*
| RELATION TO DELIVERY | PERCENTAGE |
| Antepartum | 72 |
| Postpartum | 28 |
| ≤48 hours | 80 |
| >48 hours | 20 |
| GESTATIONAL AGE (WEEKS) | PERCENTAGE |
| 17–20 | 2 |
| 21–27 | 10 |
| 28–36 | 68 |
| >37 | 20 |
| * Based on 700 cases | |
Risk for life-threatening maternal complications
When all components of HELLP syndrome are present in a woman with preeclampsia, the risk of maternal death and serious maternal morbidities increases substantially (TABLE 5). The rate of these complications depends on gestational age at onset, presence of associated obstetric complications (eclampsia, abruptio placentae, peripartum hemorrhage, or fetal demise) or preexisting conditions (lupus, renal disease, chronic hypertension, or type 1 diabetes).
Abruptio placentae increases the risk of disseminated intravascular coagulopathy (DIC), as well as the need for blood transfusions.
Marked ascites (>1 L) leads to higher rates of cardiopulmonary complications.
TABLE 5
Maternal complications
| COMPLICATION | FREQUENCY (%) |
|---|---|
| Death | 1 |
| Adult respiratory distress syndrome | 1 |
| Laryngeal edema | 1–2 |
| Liver failure or hemorrhage | 1–2 |
| Acute renal failure | 5–8 |
| Pulmonary edema | 6–8 |
| Pleural effusions | 6–10 |
| Abruptio placentae | 10–15 |
| Disseminated intravascular coagulopathy | 10–15 |
| Marked ascites | 10–15 |
Differential diagnosis
When diagnosing HELLP syndrome, confirm or exclude the conditions listed in TABLE 6, since the presenting symptoms and clinical and laboratory findings in women with HELLP syndrome overlap those of several microangiopathic disorders that can develop during pregnancy and/or postpartum. In some women, preeclampsia may be superimposed on one of these disorders, further confounding an already difficult differential diagnosis.
Because of the remarkably similar clinical and laboratory findings of these diseases, make every effort to achieve an accurate diagnosis, since management and outcomes may differ among these conditions.
TABLE 6
Differential diagnosis
|
Initial Management
Hospitalize the patient
Because HELLP syndrome usually is characterized by progressive and sometimes sudden deterioration in maternal and fetal conditions, patients should be hospitalized and observed in a labor and delivery unit.
Initially, assume the patient has severe preeclampsia and treat her with intravenous magnesium sulfate to prevent convulsions and antihypertensive medications as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg.
Blood tests should include:
- complete blood count with platelet count,
- peripheral smear evaluation,
- serum AST,
- lactate dehydrogenase,
- creatinine,
- bilirubin, and
- coagulation studies.
These tests help confirm the diagnosis and check for the presence of DIC, massive hemolysis, severe anemia, or renal failure.
The first priority is to assess the patient for the presence of cardiovascular complications, signs of liver hematoma or hemorrhage, and abruptio placentae. If any is present—particularly hypotension, hypovolemia, DIC, or pulmonary edema—make every effort to stabilize the maternal condition.
Can delivery wait 48 hours for corticosteroids?
Evaluate fetal status by heart rate monitoring or biophysical profile, and confirm gestational age. Then decide whether delivery is indicated or can be delayed for 48 hours so that corticosteroids can be given.
No room for expectant management. Do not consider expectant management in women with true HELLP syndrome. Delivery can only be delayed for a maximum of 48 hours—and only when both mother and fetus are stable, at 24 to 34 weeks’ gestation, and awaiting the benefit of corticosteroids.
Corticosteroid dosing. My practice is to give 2 doses of either betamethasone 12 mg intramuscularly every 12 hours or dexamethasone 12 mg intravenously every 12 hours. This is to improve maternal status, at least temporarily.
Initiate delivery within 24 hours after the last steroid dose, with continuous monitoring in the labor and delivery unit.
Although some women may demonstrate transient improvement in their blood tests (eg, increased platelet count or decreased AST levels), delivery is still indicated. Conversely, in some cases, maternal and fetal conditions may deteriorate, mandating delivery before the 2 doses of steroids are completed.
Delivery Considerations
HELLP syndrome does not justify immediate cesarean
Patients with HELLP syndrome in labor or with rupture of membranes can deliver vaginally in the absence of obstetric complications. In addition, induction or augmentation of labor is acceptable with either oxytocin infusion or prostaglandins if the fetal gestational age is 32 weeks or more and the cervical Bishop score exceeds 5.
TABLE 7 lists the indications for elective cesarean delivery and summarizes management during surgery. It is important to stabilize the maternal condition, correct coagulopathy, and have blood or blood products available before initiating surgery.
TABLE 7
Cesarean delivery: Indications and management
| Indications for cesarean |
|
| Management during cesarean |
|
Watch for oozing from surgical sites
In a cesarean section, generalized oozing from the surgical site can occur during the operation or immediately postpartum because of the continued drop in platelet count in some of these patients. Thus, it is advisable to insert a subfascial drain and to leave the skin incision open for at least 48 hours to avoid hematoma formation in these areas (FIGURE 1).
FIGURE 1 Insert subfascial drain at cesarean section
Because generalized oozing from the surgical site can occur intraoperatively or immediately postpartum, insert a subfascial drain and leave the skin incision open for at least 48 hours to avoid hematoma formation.
Small doses of systemic opioids are best
For maternal analgesia during labor, give small, intermittent doses of systemic opioids. For repair of episiotomy or vulvar or vaginal lacerations, use local infiltration anesthesia.
Avoid pudendal block because of the potential for bleeding and hematoma formation in this area. Epidural anesthesia may be used after consultation with the anesthesiologist if the platelet count exceeds 75,000/mm3.
Some authors report rising platelet counts after intravenous dexamethasone and, with the improved platelets, greater use of epidural anesthesia, especially in women who achieved a 24-hour latency period before delivery. However, since the platelet count may drop again, insert the epidural catheter once the desired platelet level (with anesthesiologist approval) is reached.
Suspected Liver Hematoma
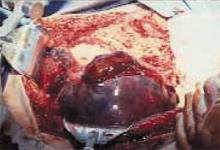
A rare and potentially life-threatening complication of HELLP syndrome is subcapsular liver hematoma (FIGURE 2). Unfortunately, the rarity of this complication sometimes causes it to be overlooked.
FIGURE 2 Rare but life-threatening: Subcapsular liver hematoma
Liver hematomas can develop antepartum, intrapartum, or postpartum. Presenting symptoms may include severe epigastric or retrosternal pain in association with respiratory difficulty (pain on inspiration), with or without shoulder or neck pain.
Early signs and symptoms
Liver hematomas can develop antepartum, during labor, or in the postpartum period. Presenting symptoms may include severe epigastric or retrosternal pain in association with breathing difficulty (pain on inspiration), with or without shoulder or neck pain.
When profound hypovolemic shock occurs in a previously hypertensive patient, suspect rupture of a liver hematoma. Diagnosis can be made by ultrasound or computed tomography (CT) imaging of the liver, both of which can also confirm intraperitoneal bleeding.
In most cases, rupture involves the right lobe of the liver and is preceded by a parenchymal liver hematoma.
Mortality can exceed 50%
Maternal and fetal mortality increase substantially when a subcapsular liver hematoma is present. In fact, mortality may exceed 50% when frank rupture of the capsule involves liver tissue.
Choose conservative management whenever possible
Management of subcapsular liver hematoma depends on maternal hemodynamic status, integrity of the capsule (ruptured or intact), and the fetal condition.
Conservative management is preferable in hemodynamically stable women with an unruptured hematoma. It consists of close monitoring of the patient’s hemodynamic and coagulation status and serial assessment of the hematoma with ultrasound or CT scan.
Avoid exogenous trauma to the liver, such as frequent abdominal palpation, emesis, or convulsions. Any sudden increase in intraabdominal pressure can led to rupture of the hematoma.
When rupture occurs
This surgical emergency requires an acute multidisciplinary team, including an Ob/Gyn, anesthesiologist, highly qualified surgeon, and a representative of the hospital’s blood bank.
Maternal resuscitation should include:
- transfusion of packed red blood cells to maintain blood pressure and tissue perfusion,
- correction of coagulopathy with fresh frozen plasma and platelets, and
- laparotomy, preferably using a cell saver.
Options at laparotomy include:
- packing and drainage (preferred),
- ligation of the hepatic lacerations,
- embolization of the hepatic artery to the affected liver segment, and
- loosely suturing omentum or surgical mesh to the liver surface.
Postpartum Care
In women who develop HELLP prior to delivery, closely monitor postpartum vital signs, intake and output, and symptoms in intensive care or a similar facility for at least 48 hours.
During this time, my practice is to give the patient intravenous magnesium sulfate and antihypertensive medications as needed to keep systolic blood pressure below 155 mm Hg (the standard is 160 mm Hg) and diastolic blood pressure below 105 mm Hg.
The rationale for this treatment is to prevent bleeding in the brain if the woman has thrombocytopenia.
When HELLP appears in the postpartum period
Several maternal complications from HELLP syndrome may not appear until immediately postpartum. Thus, all women with preeclampsia require close monitoring of vital signs, fluid intake and output, laboratory values, and pulse oximetry for at least 48 hours.
Also continue magnesium sulfate in the postpartum period and keep maternal blood pressure below 155 mm Hg systolic and 105 mm Hg diastolic.
Time to recovery
Most patients begin to improve or completely recover within 72 hours, while others deteriorate further or fail to recover for as long as 1 week after delivery. Thus, some women may require intensive monitoring for several days because of the risk of pulmonary edema, renal failure, or adult respiratory distress syndrome.
Keep in mind that, in some of these women, the cause of the postpartum deterioration may be something other than HELLP syndrome(TABLE 6).
Watch for sudden hypotension
A sudden drop in blood pressure to hypotensive levels can be an early sign of severe hemolysis or unrecognized intraperitoneal blood loss (from surgical sites or ruptured liver hematoma), as well as sepsis.
In a woman with severe hemoconcentration (ie, severe vasoconstriction), sudden hypotension also may indicate excessive vasodilation from antihypertensive drugs such as hydralazine or nifedipine, resulting in relative hypovolemia.
Such a case requires volume resuscitation, blood transfusion (if indicated), and evaluation for unrecognized bleeding.
Use of steroids
Some authors recommend giving intravenous dexamethasone (5 to 10 mg every 12 hours) for approximately 48 hours after delivery in women who develop antepartum or postpartum HELLP. They claim this treatment improves maternal blood tests, shortens recovery, and reduces maternal morbidity.
However, at present, no data indicate this approach has clinical benefit—and the risks are unknown. For these reasons, treatment with intravenous dexamethasone after delivery remains empiric.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
1. Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation. Does HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221-225.
2. Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1996;175:460-464.
3. Egerman RS, Sibai BM. Recognizing and managing HELLP syndrome and its imitators. Contemporary Ob/Gyn. 1997;(October):129-149.
4. Magann EF, Perry KG, Jr, Meydrech EF, Harris RL, Chauchan SP, Martin JN, Jr. Postpartum corticosteroids: accelerated recovery from the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171:1154-1158.
5. Martin JN, Jr, Thigsen BD, Rose CH, et al. Maternal benefit of high-dose intravenous corticosteroid therapy for HELLP. Am J Obstet Gynecol. 2003;189:830-834.
6. O’Brien JM, Milligan DA, Barton JR. Impact of high-dose corticosteroid therapy for patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:921-924.
7. O’Brien JM, Shumate SA, Satchwell SL, Milligan DA, Barton JR. Maternal benefit to corticosteroid therapy in patients with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome: impact on the rate of regional anesthesia. Am J Obstet Gynecol. 2002;186:475-479.
8. Rinehart BK, Terrone DA, Magann EF, Martin RW, May WL, Martin JN, Jr. Preeclampsia-associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999;196-202.
9. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000-1006.
10. Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162:311-316.
11. Sibai BM. Diagnosis, controversies, and management of HELLP syndrome. Obstet Gynecol. 2004;103:981-991.
12. Tompkins MJ, Thiagarajah S. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: the benefit of corticosteroids. Am J Obstet Gynecol. 1999;181:304-309.
13. VanPampus MG, Wolf H, et al. Maternal and perinatal outcome after expectant management of the HELLP syndrome compared with preeclampsia without HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 1998;76:31.-
14. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-167.
15. Weinstein L. Preeclampsia/eclampsia with hemolysis, elevated liver enzymes and thrombocytopenia. Obstet Gynecol. 1985;66:657-660.
Here’s a disturbing fact: If it looks like HELLP syndrome, and impairs the patient like HELLP syndrome, it isn’t necessarily HELLP syndrome. A plethora of diagnostic criteria from different investigators over the years has confused the issue of what constitutes this syndrome—not to mention how to manage it.
A management issue has also attracted recent attention: use of corticosteroids either antepartum to enhance maternal status so that epidural anesthesia can be administered, or postpartum to improve platelets. Such improvements are only transient, however, and we lack definitive data on the benefits.
One thing is certain, however. The combination of hemolysis, liver dysfunction or injury, and platelet consumption in women with preeclampsia makes adverse maternal and perinatal outcomes more likely and leaves no room for expectant management.
HELLP syndrome also has become a major issue in litigation against obstetricians and medical and surgical consultants. Lawsuits usually allege misdiagnosed preeclampsia, delayed delivery, or improper recognition and management of complications.
Pinning HELLP Down
One of the best tools to identify HELLP syndrome is a healthy dose of suspicion, since it can affect any pregnant woman at any time: antepartum, intrapartum, or within 1 week postpartum. Approximately 72% of cases are diagnosed before delivery, and the rest are diagnosed during the first week postpartum.
Weinstein noted that the signs and symptoms of HELLP syndrome can occur without clinical evidence of severe preeclampsia (severe hypertension and/or severe proteinuria). Indeed, he reported that hypertension can be mild or absent in most patients with HELLP, and proteinuria can be mild.
Weinstein coined the term HELLP syndrome in 1982 to describe these abnormalities in women with preeclampsia:
- H = hemolysis
- EL = elevated liver enzymes
- LP = low platelets
Another obstacle to early detection: Patients may have nonspecific signs and symptoms, none of which are diagnostic of classical preeclampsia.
However, HELLP syndrome is most common in women who have already been diagnosed with gestational hypertension and/or preeclampsia.
HELLP is more likely with severe hypertension
Overall, the incidence of HELLP syndrome in women with gestational hypertension/preeclampsia increases with the severity of the condition. HELLP syndrome also is more likely in women with early-onset hypertension/preeclampsia (before 34 weeks’ gestation).
Making The Diagnosis
HELLP syndrome is diagnosed when all 3 of the following are present:
- Hemolysis, defined as the presence of microangiopathic hemolytic anemia. This is the hallmark of the triad.
- Elevated liver enzymes (either aspartate aminotransferase [AST] or alanine aminotransferase [ALT]). This component signifies liver cell ischemia and/or necrosis.
- Low platelet count (<100,000/mm3). TABLE 1 summarizes the laboratory criteria for the diagnosis.
When to begin testing
In women with new-onset hypertension, order a complete blood count with platelets and liver enzyme analysis at the time of diagnosis and serially thereafter. The frequency of these tests depends on the initial test results, severity of disease, and onset of symptoms.
In women without hypertension, I recommend obtaining the same blood tests at the onset of any of the signs and symptoms listed in TABLE 2.
TABLE 2
Conditions that heighten the risk of HELLP
|
Assessing test results
Clinicians should be familiar with the upper limit for liverenzyme tests in their laboratory. I suggest a cutoff more than twice the upper limit for a particular test.
Also keep in mind that these parameters are dynamic; some women will meet only some of the criteria early in the disease process. Moreover, maternal complications are substantially higher when all 3 components are present than when only 1 or 2 are present.
Look for these clinical findings
Hypertension. Most women with HELLP syndrome have hypertension. In 15% to 50% of cases, the hypertension is mild, but it may be absent in 15%.
Proteinuria. Most patients also have proteinuria by dipstick (≥1+). Proteinuria may be absent in approximately 13% of women with HELLP syndrome, although they will likely have many of the symptoms reported by women with severe preeclampsia.
TABLE 3 lists the signs and symptoms to be expected in these patients, along with their frequency.
TABLE 3
Signs and symptoms
| CONDITION | FREQUENCY (%) |
|---|---|
| Hypertension | 85 |
| Proteinuria | 87 |
| Right upper quadrant or epigastric pain | 40–90 |
| Nausea or vomiting | 29–84 |
| Headaches | 33–60 |
| Visual changes | 10–20 |
| Mucosal bleeding | 10 |
| Jaundice | 5 |
The usual times of onset
Antepartum cases. As was previously noted, HELLP syndrome usually develops before delivery, with the most frequent onset being before 37 weeks’ gestation ( TABLE 4).
In the postpartum period, most cases develop within 48 hours after delivery. Of these, approximately 90% occur in women who had antepartum preeclampsia that progressed to HELLP syndrome in the postpartum period. However, approximately 20% of postpartum cases develop more than 48 hours after delivery.
Another important point: HELLP syndrome can develop for the first time postpartum in women who had no evidence of preeclampsia before or during labor. Thus, it is important to educate all postpartum women to report new symptoms (listed in TABLE 3) as soon as possible. When these symptoms develop, evaluate the patient for both preeclampsia and HELLP syndrome.
TABLE 4
Usual times of onset*
| RELATION TO DELIVERY | PERCENTAGE |
| Antepartum | 72 |
| Postpartum | 28 |
| ≤48 hours | 80 |
| >48 hours | 20 |
| GESTATIONAL AGE (WEEKS) | PERCENTAGE |
| 17–20 | 2 |
| 21–27 | 10 |
| 28–36 | 68 |
| >37 | 20 |
| * Based on 700 cases | |
Risk for life-threatening maternal complications
When all components of HELLP syndrome are present in a woman with preeclampsia, the risk of maternal death and serious maternal morbidities increases substantially (TABLE 5). The rate of these complications depends on gestational age at onset, presence of associated obstetric complications (eclampsia, abruptio placentae, peripartum hemorrhage, or fetal demise) or preexisting conditions (lupus, renal disease, chronic hypertension, or type 1 diabetes).
Abruptio placentae increases the risk of disseminated intravascular coagulopathy (DIC), as well as the need for blood transfusions.
Marked ascites (>1 L) leads to higher rates of cardiopulmonary complications.
TABLE 5
Maternal complications
| COMPLICATION | FREQUENCY (%) |
|---|---|
| Death | 1 |
| Adult respiratory distress syndrome | 1 |
| Laryngeal edema | 1–2 |
| Liver failure or hemorrhage | 1–2 |
| Acute renal failure | 5–8 |
| Pulmonary edema | 6–8 |
| Pleural effusions | 6–10 |
| Abruptio placentae | 10–15 |
| Disseminated intravascular coagulopathy | 10–15 |
| Marked ascites | 10–15 |
Differential diagnosis
When diagnosing HELLP syndrome, confirm or exclude the conditions listed in TABLE 6, since the presenting symptoms and clinical and laboratory findings in women with HELLP syndrome overlap those of several microangiopathic disorders that can develop during pregnancy and/or postpartum. In some women, preeclampsia may be superimposed on one of these disorders, further confounding an already difficult differential diagnosis.
Because of the remarkably similar clinical and laboratory findings of these diseases, make every effort to achieve an accurate diagnosis, since management and outcomes may differ among these conditions.
TABLE 6
Differential diagnosis
|
Initial Management
Hospitalize the patient
Because HELLP syndrome usually is characterized by progressive and sometimes sudden deterioration in maternal and fetal conditions, patients should be hospitalized and observed in a labor and delivery unit.
Initially, assume the patient has severe preeclampsia and treat her with intravenous magnesium sulfate to prevent convulsions and antihypertensive medications as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg.
Blood tests should include:
- complete blood count with platelet count,
- peripheral smear evaluation,
- serum AST,
- lactate dehydrogenase,
- creatinine,
- bilirubin, and
- coagulation studies.
These tests help confirm the diagnosis and check for the presence of DIC, massive hemolysis, severe anemia, or renal failure.
The first priority is to assess the patient for the presence of cardiovascular complications, signs of liver hematoma or hemorrhage, and abruptio placentae. If any is present—particularly hypotension, hypovolemia, DIC, or pulmonary edema—make every effort to stabilize the maternal condition.
Can delivery wait 48 hours for corticosteroids?
Evaluate fetal status by heart rate monitoring or biophysical profile, and confirm gestational age. Then decide whether delivery is indicated or can be delayed for 48 hours so that corticosteroids can be given.
No room for expectant management. Do not consider expectant management in women with true HELLP syndrome. Delivery can only be delayed for a maximum of 48 hours—and only when both mother and fetus are stable, at 24 to 34 weeks’ gestation, and awaiting the benefit of corticosteroids.
Corticosteroid dosing. My practice is to give 2 doses of either betamethasone 12 mg intramuscularly every 12 hours or dexamethasone 12 mg intravenously every 12 hours. This is to improve maternal status, at least temporarily.
Initiate delivery within 24 hours after the last steroid dose, with continuous monitoring in the labor and delivery unit.
Although some women may demonstrate transient improvement in their blood tests (eg, increased platelet count or decreased AST levels), delivery is still indicated. Conversely, in some cases, maternal and fetal conditions may deteriorate, mandating delivery before the 2 doses of steroids are completed.
Delivery Considerations
HELLP syndrome does not justify immediate cesarean
Patients with HELLP syndrome in labor or with rupture of membranes can deliver vaginally in the absence of obstetric complications. In addition, induction or augmentation of labor is acceptable with either oxytocin infusion or prostaglandins if the fetal gestational age is 32 weeks or more and the cervical Bishop score exceeds 5.
TABLE 7 lists the indications for elective cesarean delivery and summarizes management during surgery. It is important to stabilize the maternal condition, correct coagulopathy, and have blood or blood products available before initiating surgery.
TABLE 7
Cesarean delivery: Indications and management
| Indications for cesarean |
|
| Management during cesarean |
|
Watch for oozing from surgical sites
In a cesarean section, generalized oozing from the surgical site can occur during the operation or immediately postpartum because of the continued drop in platelet count in some of these patients. Thus, it is advisable to insert a subfascial drain and to leave the skin incision open for at least 48 hours to avoid hematoma formation in these areas (FIGURE 1).
FIGURE 1 Insert subfascial drain at cesarean section
Because generalized oozing from the surgical site can occur intraoperatively or immediately postpartum, insert a subfascial drain and leave the skin incision open for at least 48 hours to avoid hematoma formation.
Small doses of systemic opioids are best
For maternal analgesia during labor, give small, intermittent doses of systemic opioids. For repair of episiotomy or vulvar or vaginal lacerations, use local infiltration anesthesia.
Avoid pudendal block because of the potential for bleeding and hematoma formation in this area. Epidural anesthesia may be used after consultation with the anesthesiologist if the platelet count exceeds 75,000/mm3.
Some authors report rising platelet counts after intravenous dexamethasone and, with the improved platelets, greater use of epidural anesthesia, especially in women who achieved a 24-hour latency period before delivery. However, since the platelet count may drop again, insert the epidural catheter once the desired platelet level (with anesthesiologist approval) is reached.
Suspected Liver Hematoma
A rare and potentially life-threatening complication of HELLP syndrome is subcapsular liver hematoma (FIGURE 2). Unfortunately, the rarity of this complication sometimes causes it to be overlooked.
FIGURE 2 Rare but life-threatening: Subcapsular liver hematoma
Liver hematomas can develop antepartum, intrapartum, or postpartum. Presenting symptoms may include severe epigastric or retrosternal pain in association with respiratory difficulty (pain on inspiration), with or without shoulder or neck pain.
Early signs and symptoms
Liver hematomas can develop antepartum, during labor, or in the postpartum period. Presenting symptoms may include severe epigastric or retrosternal pain in association with breathing difficulty (pain on inspiration), with or without shoulder or neck pain.
When profound hypovolemic shock occurs in a previously hypertensive patient, suspect rupture of a liver hematoma. Diagnosis can be made by ultrasound or computed tomography (CT) imaging of the liver, both of which can also confirm intraperitoneal bleeding.
In most cases, rupture involves the right lobe of the liver and is preceded by a parenchymal liver hematoma.
Mortality can exceed 50%
Maternal and fetal mortality increase substantially when a subcapsular liver hematoma is present. In fact, mortality may exceed 50% when frank rupture of the capsule involves liver tissue.
Choose conservative management whenever possible
Management of subcapsular liver hematoma depends on maternal hemodynamic status, integrity of the capsule (ruptured or intact), and the fetal condition.
Conservative management is preferable in hemodynamically stable women with an unruptured hematoma. It consists of close monitoring of the patient’s hemodynamic and coagulation status and serial assessment of the hematoma with ultrasound or CT scan.
Avoid exogenous trauma to the liver, such as frequent abdominal palpation, emesis, or convulsions. Any sudden increase in intraabdominal pressure can led to rupture of the hematoma.
When rupture occurs
This surgical emergency requires an acute multidisciplinary team, including an Ob/Gyn, anesthesiologist, highly qualified surgeon, and a representative of the hospital’s blood bank.
Maternal resuscitation should include:
- transfusion of packed red blood cells to maintain blood pressure and tissue perfusion,
- correction of coagulopathy with fresh frozen plasma and platelets, and
- laparotomy, preferably using a cell saver.
Options at laparotomy include:
- packing and drainage (preferred),
- ligation of the hepatic lacerations,
- embolization of the hepatic artery to the affected liver segment, and
- loosely suturing omentum or surgical mesh to the liver surface.
Postpartum Care
In women who develop HELLP prior to delivery, closely monitor postpartum vital signs, intake and output, and symptoms in intensive care or a similar facility for at least 48 hours.
During this time, my practice is to give the patient intravenous magnesium sulfate and antihypertensive medications as needed to keep systolic blood pressure below 155 mm Hg (the standard is 160 mm Hg) and diastolic blood pressure below 105 mm Hg.
The rationale for this treatment is to prevent bleeding in the brain if the woman has thrombocytopenia.
When HELLP appears in the postpartum period
Several maternal complications from HELLP syndrome may not appear until immediately postpartum. Thus, all women with preeclampsia require close monitoring of vital signs, fluid intake and output, laboratory values, and pulse oximetry for at least 48 hours.
Also continue magnesium sulfate in the postpartum period and keep maternal blood pressure below 155 mm Hg systolic and 105 mm Hg diastolic.
Time to recovery
Most patients begin to improve or completely recover within 72 hours, while others deteriorate further or fail to recover for as long as 1 week after delivery. Thus, some women may require intensive monitoring for several days because of the risk of pulmonary edema, renal failure, or adult respiratory distress syndrome.
Keep in mind that, in some of these women, the cause of the postpartum deterioration may be something other than HELLP syndrome(TABLE 6).
Watch for sudden hypotension
A sudden drop in blood pressure to hypotensive levels can be an early sign of severe hemolysis or unrecognized intraperitoneal blood loss (from surgical sites or ruptured liver hematoma), as well as sepsis.
In a woman with severe hemoconcentration (ie, severe vasoconstriction), sudden hypotension also may indicate excessive vasodilation from antihypertensive drugs such as hydralazine or nifedipine, resulting in relative hypovolemia.
Such a case requires volume resuscitation, blood transfusion (if indicated), and evaluation for unrecognized bleeding.
Use of steroids
Some authors recommend giving intravenous dexamethasone (5 to 10 mg every 12 hours) for approximately 48 hours after delivery in women who develop antepartum or postpartum HELLP. They claim this treatment improves maternal blood tests, shortens recovery, and reduces maternal morbidity.
However, at present, no data indicate this approach has clinical benefit—and the risks are unknown. For these reasons, treatment with intravenous dexamethasone after delivery remains empiric.
The author reports no financial relationships relevant to this article.
Here’s a disturbing fact: If it looks like HELLP syndrome, and impairs the patient like HELLP syndrome, it isn’t necessarily HELLP syndrome. A plethora of diagnostic criteria from different investigators over the years has confused the issue of what constitutes this syndrome—not to mention how to manage it.
A management issue has also attracted recent attention: use of corticosteroids either antepartum to enhance maternal status so that epidural anesthesia can be administered, or postpartum to improve platelets. Such improvements are only transient, however, and we lack definitive data on the benefits.
One thing is certain, however. The combination of hemolysis, liver dysfunction or injury, and platelet consumption in women with preeclampsia makes adverse maternal and perinatal outcomes more likely and leaves no room for expectant management.
HELLP syndrome also has become a major issue in litigation against obstetricians and medical and surgical consultants. Lawsuits usually allege misdiagnosed preeclampsia, delayed delivery, or improper recognition and management of complications.
Pinning HELLP Down
One of the best tools to identify HELLP syndrome is a healthy dose of suspicion, since it can affect any pregnant woman at any time: antepartum, intrapartum, or within 1 week postpartum. Approximately 72% of cases are diagnosed before delivery, and the rest are diagnosed during the first week postpartum.
Weinstein noted that the signs and symptoms of HELLP syndrome can occur without clinical evidence of severe preeclampsia (severe hypertension and/or severe proteinuria). Indeed, he reported that hypertension can be mild or absent in most patients with HELLP, and proteinuria can be mild.
Weinstein coined the term HELLP syndrome in 1982 to describe these abnormalities in women with preeclampsia:
- H = hemolysis
- EL = elevated liver enzymes
- LP = low platelets
Another obstacle to early detection: Patients may have nonspecific signs and symptoms, none of which are diagnostic of classical preeclampsia.
However, HELLP syndrome is most common in women who have already been diagnosed with gestational hypertension and/or preeclampsia.
HELLP is more likely with severe hypertension
Overall, the incidence of HELLP syndrome in women with gestational hypertension/preeclampsia increases with the severity of the condition. HELLP syndrome also is more likely in women with early-onset hypertension/preeclampsia (before 34 weeks’ gestation).
Making The Diagnosis
HELLP syndrome is diagnosed when all 3 of the following are present:
- Hemolysis, defined as the presence of microangiopathic hemolytic anemia. This is the hallmark of the triad.
- Elevated liver enzymes (either aspartate aminotransferase [AST] or alanine aminotransferase [ALT]). This component signifies liver cell ischemia and/or necrosis.
- Low platelet count (<100,000/mm3). TABLE 1 summarizes the laboratory criteria for the diagnosis.
When to begin testing
In women with new-onset hypertension, order a complete blood count with platelets and liver enzyme analysis at the time of diagnosis and serially thereafter. The frequency of these tests depends on the initial test results, severity of disease, and onset of symptoms.
In women without hypertension, I recommend obtaining the same blood tests at the onset of any of the signs and symptoms listed in TABLE 2.
TABLE 2
Conditions that heighten the risk of HELLP
|
Assessing test results
Clinicians should be familiar with the upper limit for liverenzyme tests in their laboratory. I suggest a cutoff more than twice the upper limit for a particular test.
Also keep in mind that these parameters are dynamic; some women will meet only some of the criteria early in the disease process. Moreover, maternal complications are substantially higher when all 3 components are present than when only 1 or 2 are present.
Look for these clinical findings
Hypertension. Most women with HELLP syndrome have hypertension. In 15% to 50% of cases, the hypertension is mild, but it may be absent in 15%.
Proteinuria. Most patients also have proteinuria by dipstick (≥1+). Proteinuria may be absent in approximately 13% of women with HELLP syndrome, although they will likely have many of the symptoms reported by women with severe preeclampsia.
TABLE 3 lists the signs and symptoms to be expected in these patients, along with their frequency.
TABLE 3
Signs and symptoms
| CONDITION | FREQUENCY (%) |
|---|---|
| Hypertension | 85 |
| Proteinuria | 87 |
| Right upper quadrant or epigastric pain | 40–90 |
| Nausea or vomiting | 29–84 |
| Headaches | 33–60 |
| Visual changes | 10–20 |
| Mucosal bleeding | 10 |
| Jaundice | 5 |
The usual times of onset
Antepartum cases. As was previously noted, HELLP syndrome usually develops before delivery, with the most frequent onset being before 37 weeks’ gestation ( TABLE 4).
In the postpartum period, most cases develop within 48 hours after delivery. Of these, approximately 90% occur in women who had antepartum preeclampsia that progressed to HELLP syndrome in the postpartum period. However, approximately 20% of postpartum cases develop more than 48 hours after delivery.
Another important point: HELLP syndrome can develop for the first time postpartum in women who had no evidence of preeclampsia before or during labor. Thus, it is important to educate all postpartum women to report new symptoms (listed in TABLE 3) as soon as possible. When these symptoms develop, evaluate the patient for both preeclampsia and HELLP syndrome.
TABLE 4
Usual times of onset*
| RELATION TO DELIVERY | PERCENTAGE |
| Antepartum | 72 |
| Postpartum | 28 |
| ≤48 hours | 80 |
| >48 hours | 20 |
| GESTATIONAL AGE (WEEKS) | PERCENTAGE |
| 17–20 | 2 |
| 21–27 | 10 |
| 28–36 | 68 |
| >37 | 20 |
| * Based on 700 cases | |
Risk for life-threatening maternal complications
When all components of HELLP syndrome are present in a woman with preeclampsia, the risk of maternal death and serious maternal morbidities increases substantially (TABLE 5). The rate of these complications depends on gestational age at onset, presence of associated obstetric complications (eclampsia, abruptio placentae, peripartum hemorrhage, or fetal demise) or preexisting conditions (lupus, renal disease, chronic hypertension, or type 1 diabetes).
Abruptio placentae increases the risk of disseminated intravascular coagulopathy (DIC), as well as the need for blood transfusions.
Marked ascites (>1 L) leads to higher rates of cardiopulmonary complications.
TABLE 5
Maternal complications
| COMPLICATION | FREQUENCY (%) |
|---|---|
| Death | 1 |
| Adult respiratory distress syndrome | 1 |
| Laryngeal edema | 1–2 |
| Liver failure or hemorrhage | 1–2 |
| Acute renal failure | 5–8 |
| Pulmonary edema | 6–8 |
| Pleural effusions | 6–10 |
| Abruptio placentae | 10–15 |
| Disseminated intravascular coagulopathy | 10–15 |
| Marked ascites | 10–15 |
Differential diagnosis
When diagnosing HELLP syndrome, confirm or exclude the conditions listed in TABLE 6, since the presenting symptoms and clinical and laboratory findings in women with HELLP syndrome overlap those of several microangiopathic disorders that can develop during pregnancy and/or postpartum. In some women, preeclampsia may be superimposed on one of these disorders, further confounding an already difficult differential diagnosis.
Because of the remarkably similar clinical and laboratory findings of these diseases, make every effort to achieve an accurate diagnosis, since management and outcomes may differ among these conditions.
TABLE 6
Differential diagnosis
|
Initial Management
Hospitalize the patient
Because HELLP syndrome usually is characterized by progressive and sometimes sudden deterioration in maternal and fetal conditions, patients should be hospitalized and observed in a labor and delivery unit.
Initially, assume the patient has severe preeclampsia and treat her with intravenous magnesium sulfate to prevent convulsions and antihypertensive medications as needed to keep systolic blood pressure below 160 mm Hg and diastolic blood pressure below 105 mm Hg.
Blood tests should include:
- complete blood count with platelet count,
- peripheral smear evaluation,
- serum AST,
- lactate dehydrogenase,
- creatinine,
- bilirubin, and
- coagulation studies.
These tests help confirm the diagnosis and check for the presence of DIC, massive hemolysis, severe anemia, or renal failure.
The first priority is to assess the patient for the presence of cardiovascular complications, signs of liver hematoma or hemorrhage, and abruptio placentae. If any is present—particularly hypotension, hypovolemia, DIC, or pulmonary edema—make every effort to stabilize the maternal condition.
Can delivery wait 48 hours for corticosteroids?
Evaluate fetal status by heart rate monitoring or biophysical profile, and confirm gestational age. Then decide whether delivery is indicated or can be delayed for 48 hours so that corticosteroids can be given.
No room for expectant management. Do not consider expectant management in women with true HELLP syndrome. Delivery can only be delayed for a maximum of 48 hours—and only when both mother and fetus are stable, at 24 to 34 weeks’ gestation, and awaiting the benefit of corticosteroids.
Corticosteroid dosing. My practice is to give 2 doses of either betamethasone 12 mg intramuscularly every 12 hours or dexamethasone 12 mg intravenously every 12 hours. This is to improve maternal status, at least temporarily.
Initiate delivery within 24 hours after the last steroid dose, with continuous monitoring in the labor and delivery unit.
Although some women may demonstrate transient improvement in their blood tests (eg, increased platelet count or decreased AST levels), delivery is still indicated. Conversely, in some cases, maternal and fetal conditions may deteriorate, mandating delivery before the 2 doses of steroids are completed.
Delivery Considerations
HELLP syndrome does not justify immediate cesarean
Patients with HELLP syndrome in labor or with rupture of membranes can deliver vaginally in the absence of obstetric complications. In addition, induction or augmentation of labor is acceptable with either oxytocin infusion or prostaglandins if the fetal gestational age is 32 weeks or more and the cervical Bishop score exceeds 5.
TABLE 7 lists the indications for elective cesarean delivery and summarizes management during surgery. It is important to stabilize the maternal condition, correct coagulopathy, and have blood or blood products available before initiating surgery.
TABLE 7
Cesarean delivery: Indications and management
| Indications for cesarean |
|
| Management during cesarean |
|
Watch for oozing from surgical sites
In a cesarean section, generalized oozing from the surgical site can occur during the operation or immediately postpartum because of the continued drop in platelet count in some of these patients. Thus, it is advisable to insert a subfascial drain and to leave the skin incision open for at least 48 hours to avoid hematoma formation in these areas (FIGURE 1).
FIGURE 1 Insert subfascial drain at cesarean section
Because generalized oozing from the surgical site can occur intraoperatively or immediately postpartum, insert a subfascial drain and leave the skin incision open for at least 48 hours to avoid hematoma formation.
Small doses of systemic opioids are best
For maternal analgesia during labor, give small, intermittent doses of systemic opioids. For repair of episiotomy or vulvar or vaginal lacerations, use local infiltration anesthesia.
Avoid pudendal block because of the potential for bleeding and hematoma formation in this area. Epidural anesthesia may be used after consultation with the anesthesiologist if the platelet count exceeds 75,000/mm3.
Some authors report rising platelet counts after intravenous dexamethasone and, with the improved platelets, greater use of epidural anesthesia, especially in women who achieved a 24-hour latency period before delivery. However, since the platelet count may drop again, insert the epidural catheter once the desired platelet level (with anesthesiologist approval) is reached.
Suspected Liver Hematoma
A rare and potentially life-threatening complication of HELLP syndrome is subcapsular liver hematoma (FIGURE 2). Unfortunately, the rarity of this complication sometimes causes it to be overlooked.
FIGURE 2 Rare but life-threatening: Subcapsular liver hematoma
Liver hematomas can develop antepartum, intrapartum, or postpartum. Presenting symptoms may include severe epigastric or retrosternal pain in association with respiratory difficulty (pain on inspiration), with or without shoulder or neck pain.
Early signs and symptoms
Liver hematomas can develop antepartum, during labor, or in the postpartum period. Presenting symptoms may include severe epigastric or retrosternal pain in association with breathing difficulty (pain on inspiration), with or without shoulder or neck pain.
When profound hypovolemic shock occurs in a previously hypertensive patient, suspect rupture of a liver hematoma. Diagnosis can be made by ultrasound or computed tomography (CT) imaging of the liver, both of which can also confirm intraperitoneal bleeding.
In most cases, rupture involves the right lobe of the liver and is preceded by a parenchymal liver hematoma.
Mortality can exceed 50%
Maternal and fetal mortality increase substantially when a subcapsular liver hematoma is present. In fact, mortality may exceed 50% when frank rupture of the capsule involves liver tissue.
Choose conservative management whenever possible
Management of subcapsular liver hematoma depends on maternal hemodynamic status, integrity of the capsule (ruptured or intact), and the fetal condition.
Conservative management is preferable in hemodynamically stable women with an unruptured hematoma. It consists of close monitoring of the patient’s hemodynamic and coagulation status and serial assessment of the hematoma with ultrasound or CT scan.
Avoid exogenous trauma to the liver, such as frequent abdominal palpation, emesis, or convulsions. Any sudden increase in intraabdominal pressure can led to rupture of the hematoma.
When rupture occurs
This surgical emergency requires an acute multidisciplinary team, including an Ob/Gyn, anesthesiologist, highly qualified surgeon, and a representative of the hospital’s blood bank.
Maternal resuscitation should include:
- transfusion of packed red blood cells to maintain blood pressure and tissue perfusion,
- correction of coagulopathy with fresh frozen plasma and platelets, and
- laparotomy, preferably using a cell saver.
Options at laparotomy include:
- packing and drainage (preferred),
- ligation of the hepatic lacerations,
- embolization of the hepatic artery to the affected liver segment, and
- loosely suturing omentum or surgical mesh to the liver surface.
Postpartum Care
In women who develop HELLP prior to delivery, closely monitor postpartum vital signs, intake and output, and symptoms in intensive care or a similar facility for at least 48 hours.
During this time, my practice is to give the patient intravenous magnesium sulfate and antihypertensive medications as needed to keep systolic blood pressure below 155 mm Hg (the standard is 160 mm Hg) and diastolic blood pressure below 105 mm Hg.
The rationale for this treatment is to prevent bleeding in the brain if the woman has thrombocytopenia.
When HELLP appears in the postpartum period
Several maternal complications from HELLP syndrome may not appear until immediately postpartum. Thus, all women with preeclampsia require close monitoring of vital signs, fluid intake and output, laboratory values, and pulse oximetry for at least 48 hours.
Also continue magnesium sulfate in the postpartum period and keep maternal blood pressure below 155 mm Hg systolic and 105 mm Hg diastolic.
Time to recovery
Most patients begin to improve or completely recover within 72 hours, while others deteriorate further or fail to recover for as long as 1 week after delivery. Thus, some women may require intensive monitoring for several days because of the risk of pulmonary edema, renal failure, or adult respiratory distress syndrome.
Keep in mind that, in some of these women, the cause of the postpartum deterioration may be something other than HELLP syndrome(TABLE 6).
Watch for sudden hypotension
A sudden drop in blood pressure to hypotensive levels can be an early sign of severe hemolysis or unrecognized intraperitoneal blood loss (from surgical sites or ruptured liver hematoma), as well as sepsis.
In a woman with severe hemoconcentration (ie, severe vasoconstriction), sudden hypotension also may indicate excessive vasodilation from antihypertensive drugs such as hydralazine or nifedipine, resulting in relative hypovolemia.
Such a case requires volume resuscitation, blood transfusion (if indicated), and evaluation for unrecognized bleeding.
Use of steroids
Some authors recommend giving intravenous dexamethasone (5 to 10 mg every 12 hours) for approximately 48 hours after delivery in women who develop antepartum or postpartum HELLP. They claim this treatment improves maternal blood tests, shortens recovery, and reduces maternal morbidity.
However, at present, no data indicate this approach has clinical benefit—and the risks are unknown. For these reasons, treatment with intravenous dexamethasone after delivery remains empiric.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
1. Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation. Does HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221-225.
2. Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1996;175:460-464.
3. Egerman RS, Sibai BM. Recognizing and managing HELLP syndrome and its imitators. Contemporary Ob/Gyn. 1997;(October):129-149.
4. Magann EF, Perry KG, Jr, Meydrech EF, Harris RL, Chauchan SP, Martin JN, Jr. Postpartum corticosteroids: accelerated recovery from the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171:1154-1158.
5. Martin JN, Jr, Thigsen BD, Rose CH, et al. Maternal benefit of high-dose intravenous corticosteroid therapy for HELLP. Am J Obstet Gynecol. 2003;189:830-834.
6. O’Brien JM, Milligan DA, Barton JR. Impact of high-dose corticosteroid therapy for patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:921-924.
7. O’Brien JM, Shumate SA, Satchwell SL, Milligan DA, Barton JR. Maternal benefit to corticosteroid therapy in patients with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome: impact on the rate of regional anesthesia. Am J Obstet Gynecol. 2002;186:475-479.
8. Rinehart BK, Terrone DA, Magann EF, Martin RW, May WL, Martin JN, Jr. Preeclampsia-associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999;196-202.
9. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000-1006.
10. Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162:311-316.
11. Sibai BM. Diagnosis, controversies, and management of HELLP syndrome. Obstet Gynecol. 2004;103:981-991.
12. Tompkins MJ, Thiagarajah S. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: the benefit of corticosteroids. Am J Obstet Gynecol. 1999;181:304-309.
13. VanPampus MG, Wolf H, et al. Maternal and perinatal outcome after expectant management of the HELLP syndrome compared with preeclampsia without HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 1998;76:31.-
14. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-167.
15. Weinstein L. Preeclampsia/eclampsia with hemolysis, elevated liver enzymes and thrombocytopenia. Obstet Gynecol. 1985;66:657-660.
BIBLIOGRAPHY
1. Abramovici D, Friedman SA, Mercer BM, Audibert F, Kao L, Sibai BM. Neonatal outcome in severe preeclampsia at 24 to 36 weeks’ gestation. Does HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome matter? Am J Obstet Gynecol. 1999;180:221-225.
2. Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol. 1996;175:460-464.
3. Egerman RS, Sibai BM. Recognizing and managing HELLP syndrome and its imitators. Contemporary Ob/Gyn. 1997;(October):129-149.
4. Magann EF, Perry KG, Jr, Meydrech EF, Harris RL, Chauchan SP, Martin JN, Jr. Postpartum corticosteroids: accelerated recovery from the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol. 1994;171:1154-1158.
5. Martin JN, Jr, Thigsen BD, Rose CH, et al. Maternal benefit of high-dose intravenous corticosteroid therapy for HELLP. Am J Obstet Gynecol. 2003;189:830-834.
6. O’Brien JM, Milligan DA, Barton JR. Impact of high-dose corticosteroid therapy for patients with HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Am J Obstet Gynecol. 2000;183:921-924.
7. O’Brien JM, Shumate SA, Satchwell SL, Milligan DA, Barton JR. Maternal benefit to corticosteroid therapy in patients with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome: impact on the rate of regional anesthesia. Am J Obstet Gynecol. 2002;186:475-479.
8. Rinehart BK, Terrone DA, Magann EF, Martin RW, May WL, Martin JN, Jr. Preeclampsia-associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999;196-202.
9. Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169:1000-1006.
10. Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing? Am J Obstet Gynecol. 1990;162:311-316.
11. Sibai BM. Diagnosis, controversies, and management of HELLP syndrome. Obstet Gynecol. 2004;103:981-991.
12. Tompkins MJ, Thiagarajah S. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome: the benefit of corticosteroids. Am J Obstet Gynecol. 1999;181:304-309.
13. VanPampus MG, Wolf H, et al. Maternal and perinatal outcome after expectant management of the HELLP syndrome compared with preeclampsia without HELLP syndrome. Eur J Obstet Gynecol Reprod Biol. 1998;76:31.-
14. Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: A severe consequence of hypertension in pregnancy. Am J Obstet Gynecol. 1982;142:159-167.
15. Weinstein L. Preeclampsia/eclampsia with hemolysis, elevated liver enzymes and thrombocytopenia. Obstet Gynecol. 1985;66:657-660.
Sizing up insulin resistance—one treatment doesn’t fit all
Is PCOS a way station on the road to diabetes?
Treatment algorithm based on 4 degrees of insulin resistance
Admittedly, some evidence supports this approach. For example, a recent meta-analysis1 demonstrated that metformin improves ovulation and, in conjunction with clomiphene citrate, boosts pregnancy rates. That may be rationale enough to use the drug routinely for ovulatory-related infertility in women with PCOS.
But for the rest of our PCOS patients, measuring the degree of insulin resistance—by assessing the patient for metabolic syndrome and glucose intolerance—can yield a reasonably accurate view of long-term risk, as well as the optimal intervention for a given patient.
More good news: Effective treatment does exist, including lifestyle modification and pharmacologic therapy. And lest we assume drugs are the strongest medicine, consider this: Intensive lifestyle intervention reduces the risk of diabetes by as much as 58%—about twice the efficacy of medication.2
While many small reports implicate insulin resistance or hyperinsulinemia in a variety of reproductive disorders and even endometrial cancer, the quality and quantity of that evidence pale in comparison with data showing it can cause type 2 diabetes.3 Anything we can do to slow the progression is bound to benefit the patient and, in the long run, help prevent cardiovascular disease as well.
How to identify insulin resistance
The best way is to assess the patient for metabolic syndrome (TABLE) and then measure the 2-hour glucose level after a 75-g oral glucose load. The World Health Organization criterion for impaired glucose tolerance (IGT) after this test is a plasma glucose level of 140–199 mg/dL.4
TABLE
Is it metabolic syndrome or not?
| 3 or more risk factors make the diagnosis | |
|---|---|
| RISK FACTOR | CUTOFF |
| Abdominal obesity (waist circumference) | >88 cm (>35 in) |
| Triglycerides | 150 mg/dL |
| HDL cholesterol | |
| Blood pressure | 130/85 mm Hg |
| Fasting and/or 2-hour glucose from a 75-g oral glucose tolerance test | 110–126 mg/dL* and/or 2-hour glucose level of 140–199 mg/dL† |
| HDL = high-density lipoprotein | |
| *As recommended by the Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults | |
| †As recommended by ACOG and AACE | |
Limitations of other tests
The problem with traditional research tests such as euglycemic clamp studies and intravenous glucose/insulin tolerance tests is that they are invasive, labor intensive, time-consuming, and require a skilled team to perform—all of which translate into a poor clinical test. And among the limitations of the homeostatic models devised to replace them—which use fasting glucose and insulin levels as surrogate measures of these dynamic tests—is poor sensitivity in patients with IGT.
These models also have shifting cutoff levels in different studies in different populations.
Clinical parameters are more practical
Rather than rely on these traditional and homeostatic tests, some experts focus on validated clinical parameters. For example, the Third Report of the National Cholesterol Education Program Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP-III) defined metabolic syndrome using biometric and biochemical measures of centripetal obesity, hypertension, fasting hyperglycemia, and dyslipidemia (TABLE).5
Metabolic syndrome is closely related to insulin resistance syndrome, or syndrome X, which is characterized by dyslipidemia (depressed high-density lipoprotein [HDL] cholesterol and elevated triglycerides), hypertension, and glucose intolerance.6
Other groups such as ACOG7 and the American Association of Clinical Endocrinologists8 recommend adding a modified glucose tolerance test to the fasting blood tests used to identify metabolic syndrome.
Although a 2-hour glucose level after dynamic challenge does not test insulin sensitivity, it is more likely to identify a pathological relationship between insulin sensitivity and compensatory insulin secretion—one that is less detectable using fasting measures of glucose and insulin. One reason: In most women with IGT, fasting glucose levels are in the normal range. Thus, the fasting test alone would provide little discriminatory information.
Why glucose intolerance is important
Impaired glucose tolerance is a strong risk factor for diabetes, and recent studies show it is possible to delay progression to diabetes in women with IGT using lifestyle and, when appropriate, pharmacotherapy.2,9,10 IGT also identifies excess risk for mortality, especially in women.11 This is important because in obese women with PCOS, IGT approaches 40%.12-14 And the incidence of type 2 diabetes among women with PCOS is 11.9%, compared with only 1.4% in healthy controls.15
In type 2 diabetes, as well as polycystic ovary syndrome (PCOS), a main component is peripheral insulin resistance,20 although women with PCOS have somewhat better beta cell function than diabetic women, with initial hypersecretion and compensation. Over time, dysfunction develops, leading to inadequate insulin secretion, beta cell exhaustion, fasting hyperglycemia, and frank type 2 diabetes.
Just what is insulin resistance?
Insulin is the primary anabolic hormone in the body, acting in diverse ways in different tissues. Yet insulin resistance is usually defined as a single action: decreased insulin-mediated glucose uptake by peripheral tissues (largest utilizer: skeletal muscle).
Most research tests, such as euglycemic clamp studies, focus on glucose uptake—or its disappearance from the circulation—during dynamic challenge tests. The higher the glucose uptake, the greater the insulin sensitivity and the lower the eventual risk of developing diabetes.
Signs of insulin resistance in PCOS patients
In women with PCOS, the picture is complicated by selective tissue sensitivity to insulin and/or selective actions within tissues that are either sensitive (adrenal or ovary) or resistant (skeletal muscle) to insulin.21
Women with PCOS are profoundly resistant to insulin at the level of skeletal muscle, where 85% to 90% of insulin is utilized. This is comparable to the resistance in women with type 2 diabetes.22
In the target tissues of women with PCOS, compensatory hyperinsulinemia is thought to create aberrant states, suggesting that these tissues are differentially responsive to insulin’s action. For instance, hyperinsulinemia drives excess ovarian and adrenal androgen production, stimulates the proliferation of the piloseba-ceous unit (worsening acne and hirsutism), and suppresses hepatic sex hormone binding globulin production, thus increasing the bioavailable androgen load.
Insulin resistance is not always a disorder
Determining precisely who is insulin-resistant, and assigning clinical cutoffs, are complicated tasks. We lack standardized assays for insulin, and results vary from lab to lab. Normal insulin sensitivity varies widely and is influenced by age, gender, ethnicity, diet, and obesity. Simply put, not all people with impaired insulin sensitivity are necessarily suffering from a disorder. Pregnancy, a temporary condition of markedly diminished insulin sensitivity, is one example.
Thus, establishing limits for normal degrees of insulin sensitivity is arbitrary; often the bottom 10% to 25% of a population is labeled “insulin-resistant.”
Managing impaired glucose tolerance
Diet and exercise double risk reduction
In a recent trial by the Diabetes Prevention Program Research Group, which offers excellent guidelines for intervention,2 both metformin and lifestyle intervention reduced diabetes risk, although lifestyle was far more effective (58% reduction versus 29%).
That trial randomized 3,234 men and women with IGT to 3 treatments: conventional lifestyle recommendations, intensive and active lifestyle intervention, or insulin sensitization with metformin.
Intensive lifestyle intervention involved a case manager to ensure compliance with the study’s goals: at least a 7% loss in body weight maintained over the life of the study, and at least 150 minutes of exercise weekly. Exercise was key. Individuals who complied with intensive lifestyle intervention exercised an average of 6 hours weekly over the 4 years of the trial. The other groups exercised on average less than 2 hours weekly.
For metabolic abnormalities, start with lifestyle
With this information, it is possible to devise an algorithm for women with PCOS, depending on their degree of metabolic abnormality (FIGURE).
All individuals—even those taking medication—should be counseled about the importance of a healthy lifestyle, including staying physically active and quitting smoking. In addition:
- In women with metabolic syndrome, intensive lifestyle intervention is warranted, preferably supervised (ie, by a registered dietician and exercise trainer). This may involve out-of-pocket expense, but expert advice in these areas requires a professional. Obese, metabolically challenged women should also avoid overstrenuous exercise programs.
- Impaired glucose tolerance arouses further concern; insulin sensitization with metformin may be appropriate.
- If type 2 diabetes is diagnosed, repeat the blood tests to confirm the diagnosis and then evaluate the patient for sequelae and refer her for more intensive management.
If metabolic syndrome progresses, or if individual parameters change for the worse, additional therapy may be warranted, such as altering the dose or the choice of insulin sensitizer.
Guidelines into action
SUZANNE’S CASE
Suzanne is a 32-year-old mother of twins who presents with PCOS at a new-patient appointment, seeking advice about longterm care. She has a history of irregular menses; she conceived her twins on clomiphene 6 years ago, and now is being treated with an oral contraceptive (OC) containing 30 μg ethinyl estradiol, which she has taken for 4 years. She does not desire fertility.
Although Suzanne has a history of hirsutism, and occasionally plucks chest hair, she has been satisfied with her response to the OC. She has no other medical problems and does not smoke. Although she has a strong family history of type 2 diabetes, with both parents now on oral agents, there is no family history of premature heart disease.
She is 5 ft 9 inches tall and weighs 200 lb, with a body mass index of 29.5 kg/m2 (a BMI of 25 to 30 is overweight). Her blood pressure is 110 mm Hg systolic, 70 mm Hg diastolic, and her waist circumference is 90 cm. She has mild hirsutism. Other physical examination findings, including breast and pelvic examinations, are normal.
A fasting lipid profile reveals that Suzanne’s HDL cholesterol is 55 mg/dL, triglycerides are 175 mg/dL, and total and low-density lipoprotein (LDL) cholesterol are normal. A modified oral glucose tolerance test (OGTT) shows a fasting glucose level of 95 mg/dL and a 2-hour glucose level of 180 mg/dL, consistent with IGT.
Diagnostic phase. Polycystic ovary syndrome (PCOS) signs and symptoms are reported as such until you identify PCOS. For example, if the patient has excessive body hair, use hirsutism code ICD-9-CM 704.1; for obesity, code for unspecified obesity (278.00), morbid or severe obesity (278.01), or obesity of endocrine origin (259.9). If she has irregular menstrual periods, use the code for that condition (626.4).
Use the code for polycystic ovaries and PCOS (ICD-9-CM 256.4) once you have a diagnosis, during management, or when additional metabolic studies are done to rule out coexisting problems.
Link tests to suspected condition. A battery of laboratory tests will usually be part of diagnosis. Because many payers do not reimburse for routine screening tests, it is important to indicate that these tests are being performed to diagnose a suspected condition.
When screening women for metabolic syndrome and glucose intolerance, you may consider:
- Lipid panel (CPT 80061), linked to a diagnosis of obesity or a family history of cardiovascular disease (V17.4).
- Diabetes screening (CPT 82947 for fasting glucose plus 82950 for the 2-hour post glucose specimen) linked to a history of gestational diabetes (V13.29, other genital system and obstetric disorders) or family history of diabetes (V18.0), and possibly obesity.
After diagnosis, use E/M codes. Once you confirm PCOS and determine that management of affected systems is required, most follow-up care will be reported using evaluation and management (E/M) codes (99212–99215 for the established patient). Also use the E/M codes for initial physician encounters for diagnosis: consultation codes if another provider sends the patient to you for evaluation, or new/established patient codes if not.
Document counseling time. Some visits may entail counseling, so it is important to document counseling time (as well as total face-to-face time) with the patient. This allows you to select the E/M code based on total time, rather than on 2 of the 3 key components of history, examination, and/or medical decision-making.
The linking diagnosis during the management phase will be PCOS (256.4), along with any supporting diagnosis related to coexisting problems being managed at the time of the visit.
—Melanie Witt, RN, CPC, MA
Consider overall risk, modifiable factors
Besides the IGT, this patient has other risk factors for diabetes, including her BMI, strong family history, and several stigmata of metabolic syndrome, although she does not meet the ATP-III criteria for the syndrome. Nevertheless, the IGT merits attention.
While small case series suggest OCs can worsen glucose tolerance in women with PCOS, the overall evidence is conflicting. The Nurses Health Study16 found no association between type 2 diabetes and OC use.
One treatment option would be to discontinue the OC and see whether glucose tolerance normalizes, but Suzanne expresses a desire to continue the OC, given her overall satisfaction with its contraceptive benefit and control of hirsutism. Another factor to consider is the profound lifetime benefit OCs offer in protection against endometrial cancer.
I recommend a structured lifestyle intervention and would refer her to an exercise physiologist and dietician, because I am concerned about her other risk factors for diabetes, including her weight and strong family history.
When to add metformin
This patient also may benefit from concomitant use of metformin. Although we lack evidence that the combination of intensive lifestyle intervention and metformin is superior to lifestyle intervention alone, there is suggestive evidence from a small pilot clinical trial in women with PCOS.17
In Suzanne’s case I would treat the IGT more aggressively, given her strong family history of diabetes and her overweight status, by recommending that she add metformin to her regimen.
Higher doses may be more effective. Although the Diabetes Prevention Program study recommends a metformin dose of 850 mg twice a day, I prefer 2,000 mg a day in 2 divided doses. A dose-ranging study for type 2 diabetes found this to be the most effective dose for improving glycemic parameters.18 I use a step-up regimen of 500-mg doses over 5 to 7 days until the 2,000-mg dose is attained, and will plateau the patient at a lower dosage if she does not tolerate the higher amount, most commonly due to gastrointestinal side effects.
Monitor at least annually
This patient warrants visits at least yearly to monitor her condition. The visits should include a lipid profile and, every few years, an OGTT. A glycosylated hemoglobin in lieu of the OGTT may be another option.
It also is prudent to monitor renal function at baseline and at least annually, given the renal clearance of metformin.
In addition, I would continue the OC, as there are no known interactions between OCs and metformin, and at least 1 randomized study19 suggests the combination of the 2 is metabolically superior to an OC alone.
Scant evidence suggests that metformin alone improves hirsutism or results in eumenorrhea, and there are no data on endometrial cancer protection.
Dr. Legro has received grant support from Pfizer and served as a consultant for Ortho-McNeil and Abbott Laboratories.
1. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951-953.
2. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19:491-503.
4. Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples. Diabetes. 1989;38:1630-1635.
5. Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486-2497.
6. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
7. American College of Obstetricians and Gynecologists. Practice Bulletin #41: Polycystic Ovary Syndrome. Washington, DC: ACOG; December 2002. Obstet Gynecol. 2002;100:1389-1402.
8. Bloomgarden ZT. American Association of Clinical Endocrinologists (AACE) Consensus Conference on the Insulin Resistance Syndrome: 25-26 August 2002, Washington, DC. Diabetes Care. 2003;26:933-939.
9. Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230-6.
10. Laaksonen DE, Niskanen L, Lakka HM, Lakka TA, Uusitupa M. Epidemiology and treatment of the metabolic syndrome. Ann Med. 2004;36:332-346.
11. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The Decode Study Group. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354:617-621.
12. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165-169.
13. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141-146.
14. Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. 2004;90:66-71.
15. Talbott EO, Zborowski JV, Sutton-Tyrrell K, McHugh-Pemu KP, Guzick DS. Cardiovascular risk in women with polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:111-133.
16. Chasen-Taber L, Willett WC, Stampfer MJ, et al. A prospective study of oral contraceptives and NIDDM among US women. Diabetes Care. 1997;20:330-335.
17. Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82:421-429.
18. Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose–response trial. Am J Med. Dec. 1997;103:491-497.
19. Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17:1729-1737.
20. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663-1672.
21. Poretsky L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev. 1991;12:3-13.
22. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165-1174.
Is PCOS a way station on the road to diabetes?
Treatment algorithm based on 4 degrees of insulin resistance
Admittedly, some evidence supports this approach. For example, a recent meta-analysis1 demonstrated that metformin improves ovulation and, in conjunction with clomiphene citrate, boosts pregnancy rates. That may be rationale enough to use the drug routinely for ovulatory-related infertility in women with PCOS.
But for the rest of our PCOS patients, measuring the degree of insulin resistance—by assessing the patient for metabolic syndrome and glucose intolerance—can yield a reasonably accurate view of long-term risk, as well as the optimal intervention for a given patient.
More good news: Effective treatment does exist, including lifestyle modification and pharmacologic therapy. And lest we assume drugs are the strongest medicine, consider this: Intensive lifestyle intervention reduces the risk of diabetes by as much as 58%—about twice the efficacy of medication.2
While many small reports implicate insulin resistance or hyperinsulinemia in a variety of reproductive disorders and even endometrial cancer, the quality and quantity of that evidence pale in comparison with data showing it can cause type 2 diabetes.3 Anything we can do to slow the progression is bound to benefit the patient and, in the long run, help prevent cardiovascular disease as well.
How to identify insulin resistance
The best way is to assess the patient for metabolic syndrome (TABLE) and then measure the 2-hour glucose level after a 75-g oral glucose load. The World Health Organization criterion for impaired glucose tolerance (IGT) after this test is a plasma glucose level of 140–199 mg/dL.4
TABLE
Is it metabolic syndrome or not?
| 3 or more risk factors make the diagnosis | |
|---|---|
| RISK FACTOR | CUTOFF |
| Abdominal obesity (waist circumference) | >88 cm (>35 in) |
| Triglycerides | 150 mg/dL |
| HDL cholesterol | |
| Blood pressure | 130/85 mm Hg |
| Fasting and/or 2-hour glucose from a 75-g oral glucose tolerance test | 110–126 mg/dL* and/or 2-hour glucose level of 140–199 mg/dL† |
| HDL = high-density lipoprotein | |
| *As recommended by the Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults | |
| †As recommended by ACOG and AACE | |
Limitations of other tests
The problem with traditional research tests such as euglycemic clamp studies and intravenous glucose/insulin tolerance tests is that they are invasive, labor intensive, time-consuming, and require a skilled team to perform—all of which translate into a poor clinical test. And among the limitations of the homeostatic models devised to replace them—which use fasting glucose and insulin levels as surrogate measures of these dynamic tests—is poor sensitivity in patients with IGT.
These models also have shifting cutoff levels in different studies in different populations.
Clinical parameters are more practical
Rather than rely on these traditional and homeostatic tests, some experts focus on validated clinical parameters. For example, the Third Report of the National Cholesterol Education Program Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP-III) defined metabolic syndrome using biometric and biochemical measures of centripetal obesity, hypertension, fasting hyperglycemia, and dyslipidemia (TABLE).5
Metabolic syndrome is closely related to insulin resistance syndrome, or syndrome X, which is characterized by dyslipidemia (depressed high-density lipoprotein [HDL] cholesterol and elevated triglycerides), hypertension, and glucose intolerance.6
Other groups such as ACOG7 and the American Association of Clinical Endocrinologists8 recommend adding a modified glucose tolerance test to the fasting blood tests used to identify metabolic syndrome.
Although a 2-hour glucose level after dynamic challenge does not test insulin sensitivity, it is more likely to identify a pathological relationship between insulin sensitivity and compensatory insulin secretion—one that is less detectable using fasting measures of glucose and insulin. One reason: In most women with IGT, fasting glucose levels are in the normal range. Thus, the fasting test alone would provide little discriminatory information.
Why glucose intolerance is important
Impaired glucose tolerance is a strong risk factor for diabetes, and recent studies show it is possible to delay progression to diabetes in women with IGT using lifestyle and, when appropriate, pharmacotherapy.2,9,10 IGT also identifies excess risk for mortality, especially in women.11 This is important because in obese women with PCOS, IGT approaches 40%.12-14 And the incidence of type 2 diabetes among women with PCOS is 11.9%, compared with only 1.4% in healthy controls.15
In type 2 diabetes, as well as polycystic ovary syndrome (PCOS), a main component is peripheral insulin resistance,20 although women with PCOS have somewhat better beta cell function than diabetic women, with initial hypersecretion and compensation. Over time, dysfunction develops, leading to inadequate insulin secretion, beta cell exhaustion, fasting hyperglycemia, and frank type 2 diabetes.
Just what is insulin resistance?
Insulin is the primary anabolic hormone in the body, acting in diverse ways in different tissues. Yet insulin resistance is usually defined as a single action: decreased insulin-mediated glucose uptake by peripheral tissues (largest utilizer: skeletal muscle).
Most research tests, such as euglycemic clamp studies, focus on glucose uptake—or its disappearance from the circulation—during dynamic challenge tests. The higher the glucose uptake, the greater the insulin sensitivity and the lower the eventual risk of developing diabetes.
Signs of insulin resistance in PCOS patients
In women with PCOS, the picture is complicated by selective tissue sensitivity to insulin and/or selective actions within tissues that are either sensitive (adrenal or ovary) or resistant (skeletal muscle) to insulin.21
Women with PCOS are profoundly resistant to insulin at the level of skeletal muscle, where 85% to 90% of insulin is utilized. This is comparable to the resistance in women with type 2 diabetes.22
In the target tissues of women with PCOS, compensatory hyperinsulinemia is thought to create aberrant states, suggesting that these tissues are differentially responsive to insulin’s action. For instance, hyperinsulinemia drives excess ovarian and adrenal androgen production, stimulates the proliferation of the piloseba-ceous unit (worsening acne and hirsutism), and suppresses hepatic sex hormone binding globulin production, thus increasing the bioavailable androgen load.
Insulin resistance is not always a disorder
Determining precisely who is insulin-resistant, and assigning clinical cutoffs, are complicated tasks. We lack standardized assays for insulin, and results vary from lab to lab. Normal insulin sensitivity varies widely and is influenced by age, gender, ethnicity, diet, and obesity. Simply put, not all people with impaired insulin sensitivity are necessarily suffering from a disorder. Pregnancy, a temporary condition of markedly diminished insulin sensitivity, is one example.
Thus, establishing limits for normal degrees of insulin sensitivity is arbitrary; often the bottom 10% to 25% of a population is labeled “insulin-resistant.”
Managing impaired glucose tolerance
Diet and exercise double risk reduction
In a recent trial by the Diabetes Prevention Program Research Group, which offers excellent guidelines for intervention,2 both metformin and lifestyle intervention reduced diabetes risk, although lifestyle was far more effective (58% reduction versus 29%).
That trial randomized 3,234 men and women with IGT to 3 treatments: conventional lifestyle recommendations, intensive and active lifestyle intervention, or insulin sensitization with metformin.
Intensive lifestyle intervention involved a case manager to ensure compliance with the study’s goals: at least a 7% loss in body weight maintained over the life of the study, and at least 150 minutes of exercise weekly. Exercise was key. Individuals who complied with intensive lifestyle intervention exercised an average of 6 hours weekly over the 4 years of the trial. The other groups exercised on average less than 2 hours weekly.
For metabolic abnormalities, start with lifestyle
With this information, it is possible to devise an algorithm for women with PCOS, depending on their degree of metabolic abnormality (FIGURE).
All individuals—even those taking medication—should be counseled about the importance of a healthy lifestyle, including staying physically active and quitting smoking. In addition:
- In women with metabolic syndrome, intensive lifestyle intervention is warranted, preferably supervised (ie, by a registered dietician and exercise trainer). This may involve out-of-pocket expense, but expert advice in these areas requires a professional. Obese, metabolically challenged women should also avoid overstrenuous exercise programs.
- Impaired glucose tolerance arouses further concern; insulin sensitization with metformin may be appropriate.
- If type 2 diabetes is diagnosed, repeat the blood tests to confirm the diagnosis and then evaluate the patient for sequelae and refer her for more intensive management.
If metabolic syndrome progresses, or if individual parameters change for the worse, additional therapy may be warranted, such as altering the dose or the choice of insulin sensitizer.
Guidelines into action
SUZANNE’S CASE
Suzanne is a 32-year-old mother of twins who presents with PCOS at a new-patient appointment, seeking advice about longterm care. She has a history of irregular menses; she conceived her twins on clomiphene 6 years ago, and now is being treated with an oral contraceptive (OC) containing 30 μg ethinyl estradiol, which she has taken for 4 years. She does not desire fertility.
Although Suzanne has a history of hirsutism, and occasionally plucks chest hair, she has been satisfied with her response to the OC. She has no other medical problems and does not smoke. Although she has a strong family history of type 2 diabetes, with both parents now on oral agents, there is no family history of premature heart disease.
She is 5 ft 9 inches tall and weighs 200 lb, with a body mass index of 29.5 kg/m2 (a BMI of 25 to 30 is overweight). Her blood pressure is 110 mm Hg systolic, 70 mm Hg diastolic, and her waist circumference is 90 cm. She has mild hirsutism. Other physical examination findings, including breast and pelvic examinations, are normal.
A fasting lipid profile reveals that Suzanne’s HDL cholesterol is 55 mg/dL, triglycerides are 175 mg/dL, and total and low-density lipoprotein (LDL) cholesterol are normal. A modified oral glucose tolerance test (OGTT) shows a fasting glucose level of 95 mg/dL and a 2-hour glucose level of 180 mg/dL, consistent with IGT.
Diagnostic phase. Polycystic ovary syndrome (PCOS) signs and symptoms are reported as such until you identify PCOS. For example, if the patient has excessive body hair, use hirsutism code ICD-9-CM 704.1; for obesity, code for unspecified obesity (278.00), morbid or severe obesity (278.01), or obesity of endocrine origin (259.9). If she has irregular menstrual periods, use the code for that condition (626.4).
Use the code for polycystic ovaries and PCOS (ICD-9-CM 256.4) once you have a diagnosis, during management, or when additional metabolic studies are done to rule out coexisting problems.
Link tests to suspected condition. A battery of laboratory tests will usually be part of diagnosis. Because many payers do not reimburse for routine screening tests, it is important to indicate that these tests are being performed to diagnose a suspected condition.
When screening women for metabolic syndrome and glucose intolerance, you may consider:
- Lipid panel (CPT 80061), linked to a diagnosis of obesity or a family history of cardiovascular disease (V17.4).
- Diabetes screening (CPT 82947 for fasting glucose plus 82950 for the 2-hour post glucose specimen) linked to a history of gestational diabetes (V13.29, other genital system and obstetric disorders) or family history of diabetes (V18.0), and possibly obesity.
After diagnosis, use E/M codes. Once you confirm PCOS and determine that management of affected systems is required, most follow-up care will be reported using evaluation and management (E/M) codes (99212–99215 for the established patient). Also use the E/M codes for initial physician encounters for diagnosis: consultation codes if another provider sends the patient to you for evaluation, or new/established patient codes if not.
Document counseling time. Some visits may entail counseling, so it is important to document counseling time (as well as total face-to-face time) with the patient. This allows you to select the E/M code based on total time, rather than on 2 of the 3 key components of history, examination, and/or medical decision-making.
The linking diagnosis during the management phase will be PCOS (256.4), along with any supporting diagnosis related to coexisting problems being managed at the time of the visit.
—Melanie Witt, RN, CPC, MA
Consider overall risk, modifiable factors
Besides the IGT, this patient has other risk factors for diabetes, including her BMI, strong family history, and several stigmata of metabolic syndrome, although she does not meet the ATP-III criteria for the syndrome. Nevertheless, the IGT merits attention.
While small case series suggest OCs can worsen glucose tolerance in women with PCOS, the overall evidence is conflicting. The Nurses Health Study16 found no association between type 2 diabetes and OC use.
One treatment option would be to discontinue the OC and see whether glucose tolerance normalizes, but Suzanne expresses a desire to continue the OC, given her overall satisfaction with its contraceptive benefit and control of hirsutism. Another factor to consider is the profound lifetime benefit OCs offer in protection against endometrial cancer.
I recommend a structured lifestyle intervention and would refer her to an exercise physiologist and dietician, because I am concerned about her other risk factors for diabetes, including her weight and strong family history.
When to add metformin
This patient also may benefit from concomitant use of metformin. Although we lack evidence that the combination of intensive lifestyle intervention and metformin is superior to lifestyle intervention alone, there is suggestive evidence from a small pilot clinical trial in women with PCOS.17
In Suzanne’s case I would treat the IGT more aggressively, given her strong family history of diabetes and her overweight status, by recommending that she add metformin to her regimen.
Higher doses may be more effective. Although the Diabetes Prevention Program study recommends a metformin dose of 850 mg twice a day, I prefer 2,000 mg a day in 2 divided doses. A dose-ranging study for type 2 diabetes found this to be the most effective dose for improving glycemic parameters.18 I use a step-up regimen of 500-mg doses over 5 to 7 days until the 2,000-mg dose is attained, and will plateau the patient at a lower dosage if she does not tolerate the higher amount, most commonly due to gastrointestinal side effects.
Monitor at least annually
This patient warrants visits at least yearly to monitor her condition. The visits should include a lipid profile and, every few years, an OGTT. A glycosylated hemoglobin in lieu of the OGTT may be another option.
It also is prudent to monitor renal function at baseline and at least annually, given the renal clearance of metformin.
In addition, I would continue the OC, as there are no known interactions between OCs and metformin, and at least 1 randomized study19 suggests the combination of the 2 is metabolically superior to an OC alone.
Scant evidence suggests that metformin alone improves hirsutism or results in eumenorrhea, and there are no data on endometrial cancer protection.
Dr. Legro has received grant support from Pfizer and served as a consultant for Ortho-McNeil and Abbott Laboratories.
Is PCOS a way station on the road to diabetes?
Treatment algorithm based on 4 degrees of insulin resistance
Admittedly, some evidence supports this approach. For example, a recent meta-analysis1 demonstrated that metformin improves ovulation and, in conjunction with clomiphene citrate, boosts pregnancy rates. That may be rationale enough to use the drug routinely for ovulatory-related infertility in women with PCOS.
But for the rest of our PCOS patients, measuring the degree of insulin resistance—by assessing the patient for metabolic syndrome and glucose intolerance—can yield a reasonably accurate view of long-term risk, as well as the optimal intervention for a given patient.
More good news: Effective treatment does exist, including lifestyle modification and pharmacologic therapy. And lest we assume drugs are the strongest medicine, consider this: Intensive lifestyle intervention reduces the risk of diabetes by as much as 58%—about twice the efficacy of medication.2
While many small reports implicate insulin resistance or hyperinsulinemia in a variety of reproductive disorders and even endometrial cancer, the quality and quantity of that evidence pale in comparison with data showing it can cause type 2 diabetes.3 Anything we can do to slow the progression is bound to benefit the patient and, in the long run, help prevent cardiovascular disease as well.
How to identify insulin resistance
The best way is to assess the patient for metabolic syndrome (TABLE) and then measure the 2-hour glucose level after a 75-g oral glucose load. The World Health Organization criterion for impaired glucose tolerance (IGT) after this test is a plasma glucose level of 140–199 mg/dL.4
TABLE
Is it metabolic syndrome or not?
| 3 or more risk factors make the diagnosis | |
|---|---|
| RISK FACTOR | CUTOFF |
| Abdominal obesity (waist circumference) | >88 cm (>35 in) |
| Triglycerides | 150 mg/dL |
| HDL cholesterol | |
| Blood pressure | 130/85 mm Hg |
| Fasting and/or 2-hour glucose from a 75-g oral glucose tolerance test | 110–126 mg/dL* and/or 2-hour glucose level of 140–199 mg/dL† |
| HDL = high-density lipoprotein | |
| *As recommended by the Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults | |
| †As recommended by ACOG and AACE | |
Limitations of other tests
The problem with traditional research tests such as euglycemic clamp studies and intravenous glucose/insulin tolerance tests is that they are invasive, labor intensive, time-consuming, and require a skilled team to perform—all of which translate into a poor clinical test. And among the limitations of the homeostatic models devised to replace them—which use fasting glucose and insulin levels as surrogate measures of these dynamic tests—is poor sensitivity in patients with IGT.
These models also have shifting cutoff levels in different studies in different populations.
Clinical parameters are more practical
Rather than rely on these traditional and homeostatic tests, some experts focus on validated clinical parameters. For example, the Third Report of the National Cholesterol Education Program Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III, or ATP-III) defined metabolic syndrome using biometric and biochemical measures of centripetal obesity, hypertension, fasting hyperglycemia, and dyslipidemia (TABLE).5
Metabolic syndrome is closely related to insulin resistance syndrome, or syndrome X, which is characterized by dyslipidemia (depressed high-density lipoprotein [HDL] cholesterol and elevated triglycerides), hypertension, and glucose intolerance.6
Other groups such as ACOG7 and the American Association of Clinical Endocrinologists8 recommend adding a modified glucose tolerance test to the fasting blood tests used to identify metabolic syndrome.
Although a 2-hour glucose level after dynamic challenge does not test insulin sensitivity, it is more likely to identify a pathological relationship between insulin sensitivity and compensatory insulin secretion—one that is less detectable using fasting measures of glucose and insulin. One reason: In most women with IGT, fasting glucose levels are in the normal range. Thus, the fasting test alone would provide little discriminatory information.
Why glucose intolerance is important
Impaired glucose tolerance is a strong risk factor for diabetes, and recent studies show it is possible to delay progression to diabetes in women with IGT using lifestyle and, when appropriate, pharmacotherapy.2,9,10 IGT also identifies excess risk for mortality, especially in women.11 This is important because in obese women with PCOS, IGT approaches 40%.12-14 And the incidence of type 2 diabetes among women with PCOS is 11.9%, compared with only 1.4% in healthy controls.15
In type 2 diabetes, as well as polycystic ovary syndrome (PCOS), a main component is peripheral insulin resistance,20 although women with PCOS have somewhat better beta cell function than diabetic women, with initial hypersecretion and compensation. Over time, dysfunction develops, leading to inadequate insulin secretion, beta cell exhaustion, fasting hyperglycemia, and frank type 2 diabetes.
Just what is insulin resistance?
Insulin is the primary anabolic hormone in the body, acting in diverse ways in different tissues. Yet insulin resistance is usually defined as a single action: decreased insulin-mediated glucose uptake by peripheral tissues (largest utilizer: skeletal muscle).
Most research tests, such as euglycemic clamp studies, focus on glucose uptake—or its disappearance from the circulation—during dynamic challenge tests. The higher the glucose uptake, the greater the insulin sensitivity and the lower the eventual risk of developing diabetes.
Signs of insulin resistance in PCOS patients
In women with PCOS, the picture is complicated by selective tissue sensitivity to insulin and/or selective actions within tissues that are either sensitive (adrenal or ovary) or resistant (skeletal muscle) to insulin.21
Women with PCOS are profoundly resistant to insulin at the level of skeletal muscle, where 85% to 90% of insulin is utilized. This is comparable to the resistance in women with type 2 diabetes.22
In the target tissues of women with PCOS, compensatory hyperinsulinemia is thought to create aberrant states, suggesting that these tissues are differentially responsive to insulin’s action. For instance, hyperinsulinemia drives excess ovarian and adrenal androgen production, stimulates the proliferation of the piloseba-ceous unit (worsening acne and hirsutism), and suppresses hepatic sex hormone binding globulin production, thus increasing the bioavailable androgen load.
Insulin resistance is not always a disorder
Determining precisely who is insulin-resistant, and assigning clinical cutoffs, are complicated tasks. We lack standardized assays for insulin, and results vary from lab to lab. Normal insulin sensitivity varies widely and is influenced by age, gender, ethnicity, diet, and obesity. Simply put, not all people with impaired insulin sensitivity are necessarily suffering from a disorder. Pregnancy, a temporary condition of markedly diminished insulin sensitivity, is one example.
Thus, establishing limits for normal degrees of insulin sensitivity is arbitrary; often the bottom 10% to 25% of a population is labeled “insulin-resistant.”
Managing impaired glucose tolerance
Diet and exercise double risk reduction
In a recent trial by the Diabetes Prevention Program Research Group, which offers excellent guidelines for intervention,2 both metformin and lifestyle intervention reduced diabetes risk, although lifestyle was far more effective (58% reduction versus 29%).
That trial randomized 3,234 men and women with IGT to 3 treatments: conventional lifestyle recommendations, intensive and active lifestyle intervention, or insulin sensitization with metformin.
Intensive lifestyle intervention involved a case manager to ensure compliance with the study’s goals: at least a 7% loss in body weight maintained over the life of the study, and at least 150 minutes of exercise weekly. Exercise was key. Individuals who complied with intensive lifestyle intervention exercised an average of 6 hours weekly over the 4 years of the trial. The other groups exercised on average less than 2 hours weekly.
For metabolic abnormalities, start with lifestyle
With this information, it is possible to devise an algorithm for women with PCOS, depending on their degree of metabolic abnormality (FIGURE).
All individuals—even those taking medication—should be counseled about the importance of a healthy lifestyle, including staying physically active and quitting smoking. In addition:
- In women with metabolic syndrome, intensive lifestyle intervention is warranted, preferably supervised (ie, by a registered dietician and exercise trainer). This may involve out-of-pocket expense, but expert advice in these areas requires a professional. Obese, metabolically challenged women should also avoid overstrenuous exercise programs.
- Impaired glucose tolerance arouses further concern; insulin sensitization with metformin may be appropriate.
- If type 2 diabetes is diagnosed, repeat the blood tests to confirm the diagnosis and then evaluate the patient for sequelae and refer her for more intensive management.
If metabolic syndrome progresses, or if individual parameters change for the worse, additional therapy may be warranted, such as altering the dose or the choice of insulin sensitizer.
Guidelines into action
SUZANNE’S CASE
Suzanne is a 32-year-old mother of twins who presents with PCOS at a new-patient appointment, seeking advice about longterm care. She has a history of irregular menses; she conceived her twins on clomiphene 6 years ago, and now is being treated with an oral contraceptive (OC) containing 30 μg ethinyl estradiol, which she has taken for 4 years. She does not desire fertility.
Although Suzanne has a history of hirsutism, and occasionally plucks chest hair, she has been satisfied with her response to the OC. She has no other medical problems and does not smoke. Although she has a strong family history of type 2 diabetes, with both parents now on oral agents, there is no family history of premature heart disease.
She is 5 ft 9 inches tall and weighs 200 lb, with a body mass index of 29.5 kg/m2 (a BMI of 25 to 30 is overweight). Her blood pressure is 110 mm Hg systolic, 70 mm Hg diastolic, and her waist circumference is 90 cm. She has mild hirsutism. Other physical examination findings, including breast and pelvic examinations, are normal.
A fasting lipid profile reveals that Suzanne’s HDL cholesterol is 55 mg/dL, triglycerides are 175 mg/dL, and total and low-density lipoprotein (LDL) cholesterol are normal. A modified oral glucose tolerance test (OGTT) shows a fasting glucose level of 95 mg/dL and a 2-hour glucose level of 180 mg/dL, consistent with IGT.
Diagnostic phase. Polycystic ovary syndrome (PCOS) signs and symptoms are reported as such until you identify PCOS. For example, if the patient has excessive body hair, use hirsutism code ICD-9-CM 704.1; for obesity, code for unspecified obesity (278.00), morbid or severe obesity (278.01), or obesity of endocrine origin (259.9). If she has irregular menstrual periods, use the code for that condition (626.4).
Use the code for polycystic ovaries and PCOS (ICD-9-CM 256.4) once you have a diagnosis, during management, or when additional metabolic studies are done to rule out coexisting problems.
Link tests to suspected condition. A battery of laboratory tests will usually be part of diagnosis. Because many payers do not reimburse for routine screening tests, it is important to indicate that these tests are being performed to diagnose a suspected condition.
When screening women for metabolic syndrome and glucose intolerance, you may consider:
- Lipid panel (CPT 80061), linked to a diagnosis of obesity or a family history of cardiovascular disease (V17.4).
- Diabetes screening (CPT 82947 for fasting glucose plus 82950 for the 2-hour post glucose specimen) linked to a history of gestational diabetes (V13.29, other genital system and obstetric disorders) or family history of diabetes (V18.0), and possibly obesity.
After diagnosis, use E/M codes. Once you confirm PCOS and determine that management of affected systems is required, most follow-up care will be reported using evaluation and management (E/M) codes (99212–99215 for the established patient). Also use the E/M codes for initial physician encounters for diagnosis: consultation codes if another provider sends the patient to you for evaluation, or new/established patient codes if not.
Document counseling time. Some visits may entail counseling, so it is important to document counseling time (as well as total face-to-face time) with the patient. This allows you to select the E/M code based on total time, rather than on 2 of the 3 key components of history, examination, and/or medical decision-making.
The linking diagnosis during the management phase will be PCOS (256.4), along with any supporting diagnosis related to coexisting problems being managed at the time of the visit.
—Melanie Witt, RN, CPC, MA
Consider overall risk, modifiable factors
Besides the IGT, this patient has other risk factors for diabetes, including her BMI, strong family history, and several stigmata of metabolic syndrome, although she does not meet the ATP-III criteria for the syndrome. Nevertheless, the IGT merits attention.
While small case series suggest OCs can worsen glucose tolerance in women with PCOS, the overall evidence is conflicting. The Nurses Health Study16 found no association between type 2 diabetes and OC use.
One treatment option would be to discontinue the OC and see whether glucose tolerance normalizes, but Suzanne expresses a desire to continue the OC, given her overall satisfaction with its contraceptive benefit and control of hirsutism. Another factor to consider is the profound lifetime benefit OCs offer in protection against endometrial cancer.
I recommend a structured lifestyle intervention and would refer her to an exercise physiologist and dietician, because I am concerned about her other risk factors for diabetes, including her weight and strong family history.
When to add metformin
This patient also may benefit from concomitant use of metformin. Although we lack evidence that the combination of intensive lifestyle intervention and metformin is superior to lifestyle intervention alone, there is suggestive evidence from a small pilot clinical trial in women with PCOS.17
In Suzanne’s case I would treat the IGT more aggressively, given her strong family history of diabetes and her overweight status, by recommending that she add metformin to her regimen.
Higher doses may be more effective. Although the Diabetes Prevention Program study recommends a metformin dose of 850 mg twice a day, I prefer 2,000 mg a day in 2 divided doses. A dose-ranging study for type 2 diabetes found this to be the most effective dose for improving glycemic parameters.18 I use a step-up regimen of 500-mg doses over 5 to 7 days until the 2,000-mg dose is attained, and will plateau the patient at a lower dosage if she does not tolerate the higher amount, most commonly due to gastrointestinal side effects.
Monitor at least annually
This patient warrants visits at least yearly to monitor her condition. The visits should include a lipid profile and, every few years, an OGTT. A glycosylated hemoglobin in lieu of the OGTT may be another option.
It also is prudent to monitor renal function at baseline and at least annually, given the renal clearance of metformin.
In addition, I would continue the OC, as there are no known interactions between OCs and metformin, and at least 1 randomized study19 suggests the combination of the 2 is metabolically superior to an OC alone.
Scant evidence suggests that metformin alone improves hirsutism or results in eumenorrhea, and there are no data on endometrial cancer protection.
Dr. Legro has received grant support from Pfizer and served as a consultant for Ortho-McNeil and Abbott Laboratories.
1. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951-953.
2. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19:491-503.
4. Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples. Diabetes. 1989;38:1630-1635.
5. Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486-2497.
6. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
7. American College of Obstetricians and Gynecologists. Practice Bulletin #41: Polycystic Ovary Syndrome. Washington, DC: ACOG; December 2002. Obstet Gynecol. 2002;100:1389-1402.
8. Bloomgarden ZT. American Association of Clinical Endocrinologists (AACE) Consensus Conference on the Insulin Resistance Syndrome: 25-26 August 2002, Washington, DC. Diabetes Care. 2003;26:933-939.
9. Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230-6.
10. Laaksonen DE, Niskanen L, Lakka HM, Lakka TA, Uusitupa M. Epidemiology and treatment of the metabolic syndrome. Ann Med. 2004;36:332-346.
11. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The Decode Study Group. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354:617-621.
12. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165-169.
13. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141-146.
14. Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. 2004;90:66-71.
15. Talbott EO, Zborowski JV, Sutton-Tyrrell K, McHugh-Pemu KP, Guzick DS. Cardiovascular risk in women with polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:111-133.
16. Chasen-Taber L, Willett WC, Stampfer MJ, et al. A prospective study of oral contraceptives and NIDDM among US women. Diabetes Care. 1997;20:330-335.
17. Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82:421-429.
18. Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose–response trial. Am J Med. Dec. 1997;103:491-497.
19. Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17:1729-1737.
20. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663-1672.
21. Poretsky L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev. 1991;12:3-13.
22. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165-1174.
1. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951-953.
2. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
3. Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19:491-503.
4. Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance. Results from two national samples. Diabetes. 1989;38:1630-1635.
5. Expert Panel on the Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486-2497.
6. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595-1607.
7. American College of Obstetricians and Gynecologists. Practice Bulletin #41: Polycystic Ovary Syndrome. Washington, DC: ACOG; December 2002. Obstet Gynecol. 2002;100:1389-1402.
8. Bloomgarden ZT. American Association of Clinical Endocrinologists (AACE) Consensus Conference on the Insulin Resistance Syndrome: 25-26 August 2002, Washington, DC. Diabetes Care. 2003;26:933-939.
9. Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230-6.
10. Laaksonen DE, Niskanen L, Lakka HM, Lakka TA, Uusitupa M. Epidemiology and treatment of the metabolic syndrome. Ann Med. 2004;36:332-346.
11. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The Decode Study Group. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354:617-621.
12. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165-169.
13. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141-146.
14. Ehrmann DA, Kasza K, Azziz R, Legro RS, Ghazzi MN. Effects of race and family history of type 2 diabetes on metabolic status of women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab. 2004;90:66-71.
15. Talbott EO, Zborowski JV, Sutton-Tyrrell K, McHugh-Pemu KP, Guzick DS. Cardiovascular risk in women with polycystic ovary syndrome. Obstet Gynecol Clin North Am. 2001;28:111-133.
16. Chasen-Taber L, Willett WC, Stampfer MJ, et al. A prospective study of oral contraceptives and NIDDM among US women. Diabetes Care. 1997;20:330-335.
17. Hoeger KM, Kochman L, Wixom N, Craig K, Miller RK, Guzick DS. A randomized, 48-week, placebo-controlled trial of intensive lifestyle modification and/or metformin therapy in overweight women with polycystic ovary syndrome: a pilot study. Fertil Steril. 2004;82:421-429.
18. Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose–response trial. Am J Med. Dec. 1997;103:491-497.
19. Elter K, Imir G, Durmusoglu F. Clinical, endocrine and metabolic effects of metformin added to ethinyl estradiol-cyproterone acetate in non-obese women with polycystic ovarian syndrome: a randomized controlled study. Hum Reprod. 2002;17:1729-1737.
20. Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663-1672.
21. Poretsky L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev. 1991;12:3-13.
22. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165-1174.
Managing Posttraumatic Stress Disorder: A Present-Centered Approach
End-of-Life Care: Comparing Two Approaches
Get ready for a practice makeover
As soon as next year, the human papillomavirus (HPV) vaccine could transform clinical practice more than anything since the Pap smear was introduced 60 years ago.
A second large trial has shown extraordinary efficacy, and now 2 manufacturers, GlaxoSmithKline and Merck, are conducting late-phase clinical trials and working toward registering their vaccines for clinical use in 2006. Only last month, Merck and GSK signed an agreement that resolves their competing intellectual property claims—removing one more barrier to rapid commercialization.
But there are other important new developments that apply to practice now:
- Colposcopy, it appears, is far less reliable for identifying cervical intraepithelial neoplasia (CIN) 2,3 than we thought.
- The long-term risk of preterm delivery with loop electrosurgical excision procedures (LEEP) points to a need to counsel patients and consider all management options for women with CIN 1.
- The high rates of spontaneous regression of low-grade squamous intraepithelial lesion (LSIL) cytologic changes in young women are now better defined, and indicate colposcopy is not always needed.
Colposcopy not as sensitive as we thought
Pretorius R, Zhang W, Bellinson J, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004; 191:430–434.
We need to carefully follow up whenever colposcopy does not identify a CIN 2,3 lesion. This study also reinforces the need for diagnostic excisional procedures in women with an HSIL Pap result, and who are found after colposcopy to have CIN 1 or less (FIGURE 1).
Unfortunately, colposcopy is highly subjective. Accuracy depends on training and experience. Nevertheless, it is the standard of care for identifying CIN 2,3 and invasive cervical cancer in women with abnormal Pap results. Colposcopy was thought to be a sensitive but rather nonspecific method for identifying high-grade neoplasia. A 1998 comprehensive meta-analysis estimated that colposcopy had a weighted mean sensitivity for distinguishing normal tissue from abnormal tissue of 0.96 (95% confidence interval [CI], 0.95-0.97) and a weighted mean specificity of 0.48 (95% CI, 0.47-0.49).1 This means that colposcopy would miss a biopsy-confirmed cervical abnormality in only about 4% of patients. However, more recent follow-up studies have reported much higher false negative rates for colposcopy.
Pretorius and colleagues studied women enrolled in a cervical cancer screening trial conducted in Shanxi, China. The colposcopy in this study was performed by attending gynecologic oncologists who worked closely with a team of US-based gynecologic oncologists. The women in the study had biopsies taken of all areas classified as abnormal by colposcopy. In addition, random 4-quadrant cervical biopsies were obtained from colposcopically normal regions of the cervix.
A total of 364 women with a satisfactory colposcopy and biopsy-confirmed CIN 2 or greater lesions were identified. Even though all 364 women had a satisfactory colposcopic examination, only 57.1% of the women with biopsy-confirmed CIN 2 or worse were detected by the colposcopically-directed biopsy; the remaining 42.9% were detected by the random biopsies of colposcopically normal-appearing tissue. The lesions that were missed by colposcopy tended to be smaller than those identified by colposcopy and were more frequently CIN 2 rather than CIN 3 lesions.
This study also evaluated the role of endocervical curettage, and found that even among women with a satisfactory colposcopic examination, a significant proportion (5.5%) of cases of CIN 2,3 or worse were detected only by using endocervical curettage.
FIGURE 1 Repeat colposcopy and biopsy may reveal high-grade lesion
Low-grade cervical intraepithelial neoplasia (CIN 1) of the cervix. This young woman has a well-defined acetowhite lesion of her cervix that was diagnosed as a CIN 1 on cervical biopsy. In many such cases, repeat colposcopy and biopsy identifies an area of high-grade lesion that was missed at the initial colposcopy.
REFERENCE
1. Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91:626-631.
LEEP raises risk of preterm birth
Sadler L, Saftlas A, Wang W, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100-2106.
We need to counsel women that LEEP will increase their risk for preterm premature rupture of membranes (PPROM) and preterm delivery. We must recognize that it is desirable to follow, rather than treat, biopsy-confirmed CIN 1, and to limit the depth of excision to 1 cm or less whenever possible.
Although Consensus Guidelines state that both ablative and excisional methods are acceptable forms of managing women with satisfactory colposcopy and CIN 2,3, for most clinicians, LEEP has completely replaced laser ablation and cryotherapy for treatment of CIN.1 Because LEEP is so widely utilized, its effects on fertility and preterm delivery, as well as other adverse pregnancy outcomes, are of great concern.
LEEP became widely adopted since its introduction in the early 1990s because it yields a tissue specimen for histological evaluation and is less expensive and easier to perform than laser ablation.
Many consider CIN 2,3 biomarkers the next step away from the Pap smear, toward more accurate molecular testing. One of the more promising biomarkers is p16INK4A, a cyclin-dependent kinase inhibitor involved in control of the cell cycle. Wang et al took tissue blocks from a large population-based screening study and evaluated the performance of p16INK4A on the full diagnostic spectrum of lesions. A very strong correlation was seen between identification of p16INK4A in the lesion and CIN 2,3; 100% of CIN 3 lesions showed diffuse staining with p16INK4A.
Wang S, Trunk M, Schiffman, M et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360.
Unfortunately, most studies of the impact of LEEP on fertility and pregnancy have been limited or inconclusive, and most lacked statistical power to detect a doubling of risk. The New Zealand study conducted by Sadler and colleagues—a large retrospective cohort study—compared delivery outcomes of 426 untreated women with 652 women treated by laser conization, laser ablation, or LEEP. Women who had LEEP or laser cone treatment were at significantly increased risk of rupture of membranes before 37 weeks’ gestation. Notably, in women who had undergone a LEEP, the adjusted relative risk (RR) for PPROM was 1.9 (95% CI, 1.0-3.8) compared to the untreated women. Laser ablation did not increase risk (RR 1.1). This study demonstrate that women who have undergone LEEP have almost twice the risk for PPROM as untreated women, should be of concern to all gynecologists.
Risk of both PPROM and preterm delivery increased as depth of cervical tissue removed increased. Women in whom 1 cm or less of tissue was excised had no increased risk of PPROM or preterm birth; women in whom more than 1.7 cm of tissue was excised had an adjusted relative risk of 3.6 (95% CI, 1.8-7.5).
In a Canadian study published only last month, Samson and colleagues found PPROM was almost 4 times more common among women who had had a LEEP.2
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
2. Samson S, Bentley JR, Fahey T, McKay D, Gill G. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325-332.
LSIL cytology meaningless?
Moscicki A, Shiboski S, Hills N, et al. Regression of low-grade squamous intraepithelial lesions in young women. Lancet. 2004;364:1678–1683.
This study shows just how meaningless LSIL cytology is in young women—and it portends changes in the next Consensus Guidelines. Colposcopy for all adolescents and young women is unwarranted, the authors stated. They recommend monitoring with repeat cytology instead.
For over a decade it has been widely appreciated that many CIN 1 lesions spontaneously regress in the absence of therapy.1 Based on what we recently learned from natural history studies of HPV, we know that the majority of LSIL cytology results and biopsy-confirmed CIN 1 lesions represent nothing more than the morphological manifestation of a productive HPV infection.2 HPV infections, including those with high-risk types of HPV, are typically self-limited (FIGURE 2). In approximately 90% of women, HPV shedding stops spontaneously within 24 months.
However, in the United States, most women with LSIL undergo colposcopy, and many clinicians continue to treat women with biopsy-confirmed CIN 1. These approaches do correspond to the most recent Consensus Guidelines, which recommend colposcopy for women with LSIL, and state that follow-up with treatment, as well as treatment with ablative or excisional methods, are acceptable management options for women with CIN 1.3
Regarding adolescents with LSIL, the guidelines made an exception to performing a colposcopy. For these patients, an acceptable management option is follow-up without initial colposcopy, using a protocol of repeat cytological testing at 6 and 12 months, or HPV testing at 12 months.
To better define the best way to manage young women with LSIL, Moscicki and colleagues followed a cohort of 204 young women (ages 13 to 22 years), who had an LSIL Pap result, for up to 80 months (median 61 months). HSIL cytology (N=6) or biopsy-confirmed CIN 2,3 (N=17) was found in only 11.3% of the women. After 36 months, only 6% had persistent LSIL.
The remainder had had 3 consecutive negative Pap results, and the median time to developing the first of 3 negative Pap results was only 8 months.
FIGURE 2 Even high-risk HPV types usually abate in young women
Liquid-based cytology specimen diagnosed as low-grade squamous intraepithelial lesion (LSIL), with marked koilocytosis with multinucleation, perinuclear halos, and nuclear atypia. These features typify productive HPV infection that usually regresses spontaneously in young women.
REFERENCES
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727-735.
2. Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med 2003;348:489-490.
3. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
Bivalent vaccine vanquishes HPV
Harper D, Franco E, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765.
HPV vaccine may be registered for clinical use next year. Since two-thirds of cervical cancers are caused by only 2 types of high-risk HPV—HPV 16 and HPV 18—a vaccine that prevents infection with HPV 16 and 18 could reduce cervical cancer and high-grade precursor lesions by more than half.
Extraordinary efficacy—100% against persistent infections and 91.6% against incident HPV 16 or 18 infections—was found in this Phase II trial of a bivalent HPV vaccine made by GlaxoSmithKline—the second such trial to show high efficacy for an HPV vaccine. Merck found high efficacy for its monovalent vaccine. Both companies are conducting Phase III registration trials.
Harper and colleagues observed these efficacy rates in women who took all their scheduled vaccinations. They used bivalent HPV 16 and 18 vaccine in a study of 1,113 women randomized to receive 3 doses of vaccine or placebo over a 6-month period. All were followed for up to 27 months.
The vaccine was also highly effective against cytological abnormalities associated with HPV 16 or 18 and was generally safe, well tolerated, and highly immunogenic.
In 2002, a Phase II trial of a monovalent HPV 16 vaccine produced by Merck demonstrated efficacy of 100% over 18 months in preventing persistent HPV 16 infection or CIN associated with HPV 16.1
Both companies’ vaccines consist of viral-like particles that are made by producing recombinant L1 capsid protein of the specific HPV type and then allowing the recombinant L1 capsid proteins to assemble into a structure that appears identical to the native virus, but lacks infectious DNA.
Each year, 470,000 women develop invasive cervical cancer, and 230,000 die, globally. Vaccination is a particularly attractive strategy for preventing cervical cancer in developing countries, where less than 5% of women have ever been screened.
Yet these numbers do not begin to take into account the huge costs and burden of disease due to noninvasive cervical cancer precursors and abnormal screening cytology. In the United States alone, we spend up to $6 billion a year on prevention and treatment of cervical cancer.
The author reports no financial relationships relevant to this article.
REFERENCE
1. Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.
As soon as next year, the human papillomavirus (HPV) vaccine could transform clinical practice more than anything since the Pap smear was introduced 60 years ago.
A second large trial has shown extraordinary efficacy, and now 2 manufacturers, GlaxoSmithKline and Merck, are conducting late-phase clinical trials and working toward registering their vaccines for clinical use in 2006. Only last month, Merck and GSK signed an agreement that resolves their competing intellectual property claims—removing one more barrier to rapid commercialization.
But there are other important new developments that apply to practice now:
- Colposcopy, it appears, is far less reliable for identifying cervical intraepithelial neoplasia (CIN) 2,3 than we thought.
- The long-term risk of preterm delivery with loop electrosurgical excision procedures (LEEP) points to a need to counsel patients and consider all management options for women with CIN 1.
- The high rates of spontaneous regression of low-grade squamous intraepithelial lesion (LSIL) cytologic changes in young women are now better defined, and indicate colposcopy is not always needed.
Colposcopy not as sensitive as we thought
Pretorius R, Zhang W, Bellinson J, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004; 191:430–434.
We need to carefully follow up whenever colposcopy does not identify a CIN 2,3 lesion. This study also reinforces the need for diagnostic excisional procedures in women with an HSIL Pap result, and who are found after colposcopy to have CIN 1 or less (FIGURE 1).
Unfortunately, colposcopy is highly subjective. Accuracy depends on training and experience. Nevertheless, it is the standard of care for identifying CIN 2,3 and invasive cervical cancer in women with abnormal Pap results. Colposcopy was thought to be a sensitive but rather nonspecific method for identifying high-grade neoplasia. A 1998 comprehensive meta-analysis estimated that colposcopy had a weighted mean sensitivity for distinguishing normal tissue from abnormal tissue of 0.96 (95% confidence interval [CI], 0.95-0.97) and a weighted mean specificity of 0.48 (95% CI, 0.47-0.49).1 This means that colposcopy would miss a biopsy-confirmed cervical abnormality in only about 4% of patients. However, more recent follow-up studies have reported much higher false negative rates for colposcopy.
Pretorius and colleagues studied women enrolled in a cervical cancer screening trial conducted in Shanxi, China. The colposcopy in this study was performed by attending gynecologic oncologists who worked closely with a team of US-based gynecologic oncologists. The women in the study had biopsies taken of all areas classified as abnormal by colposcopy. In addition, random 4-quadrant cervical biopsies were obtained from colposcopically normal regions of the cervix.
A total of 364 women with a satisfactory colposcopy and biopsy-confirmed CIN 2 or greater lesions were identified. Even though all 364 women had a satisfactory colposcopic examination, only 57.1% of the women with biopsy-confirmed CIN 2 or worse were detected by the colposcopically-directed biopsy; the remaining 42.9% were detected by the random biopsies of colposcopically normal-appearing tissue. The lesions that were missed by colposcopy tended to be smaller than those identified by colposcopy and were more frequently CIN 2 rather than CIN 3 lesions.
This study also evaluated the role of endocervical curettage, and found that even among women with a satisfactory colposcopic examination, a significant proportion (5.5%) of cases of CIN 2,3 or worse were detected only by using endocervical curettage.
FIGURE 1 Repeat colposcopy and biopsy may reveal high-grade lesion
Low-grade cervical intraepithelial neoplasia (CIN 1) of the cervix. This young woman has a well-defined acetowhite lesion of her cervix that was diagnosed as a CIN 1 on cervical biopsy. In many such cases, repeat colposcopy and biopsy identifies an area of high-grade lesion that was missed at the initial colposcopy.
REFERENCE
1. Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91:626-631.
LEEP raises risk of preterm birth
Sadler L, Saftlas A, Wang W, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100-2106.
We need to counsel women that LEEP will increase their risk for preterm premature rupture of membranes (PPROM) and preterm delivery. We must recognize that it is desirable to follow, rather than treat, biopsy-confirmed CIN 1, and to limit the depth of excision to 1 cm or less whenever possible.
Although Consensus Guidelines state that both ablative and excisional methods are acceptable forms of managing women with satisfactory colposcopy and CIN 2,3, for most clinicians, LEEP has completely replaced laser ablation and cryotherapy for treatment of CIN.1 Because LEEP is so widely utilized, its effects on fertility and preterm delivery, as well as other adverse pregnancy outcomes, are of great concern.
LEEP became widely adopted since its introduction in the early 1990s because it yields a tissue specimen for histological evaluation and is less expensive and easier to perform than laser ablation.
Many consider CIN 2,3 biomarkers the next step away from the Pap smear, toward more accurate molecular testing. One of the more promising biomarkers is p16INK4A, a cyclin-dependent kinase inhibitor involved in control of the cell cycle. Wang et al took tissue blocks from a large population-based screening study and evaluated the performance of p16INK4A on the full diagnostic spectrum of lesions. A very strong correlation was seen between identification of p16INK4A in the lesion and CIN 2,3; 100% of CIN 3 lesions showed diffuse staining with p16INK4A.
Wang S, Trunk M, Schiffman, M et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360.
Unfortunately, most studies of the impact of LEEP on fertility and pregnancy have been limited or inconclusive, and most lacked statistical power to detect a doubling of risk. The New Zealand study conducted by Sadler and colleagues—a large retrospective cohort study—compared delivery outcomes of 426 untreated women with 652 women treated by laser conization, laser ablation, or LEEP. Women who had LEEP or laser cone treatment were at significantly increased risk of rupture of membranes before 37 weeks’ gestation. Notably, in women who had undergone a LEEP, the adjusted relative risk (RR) for PPROM was 1.9 (95% CI, 1.0-3.8) compared to the untreated women. Laser ablation did not increase risk (RR 1.1). This study demonstrate that women who have undergone LEEP have almost twice the risk for PPROM as untreated women, should be of concern to all gynecologists.
Risk of both PPROM and preterm delivery increased as depth of cervical tissue removed increased. Women in whom 1 cm or less of tissue was excised had no increased risk of PPROM or preterm birth; women in whom more than 1.7 cm of tissue was excised had an adjusted relative risk of 3.6 (95% CI, 1.8-7.5).
In a Canadian study published only last month, Samson and colleagues found PPROM was almost 4 times more common among women who had had a LEEP.2
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
2. Samson S, Bentley JR, Fahey T, McKay D, Gill G. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325-332.
LSIL cytology meaningless?
Moscicki A, Shiboski S, Hills N, et al. Regression of low-grade squamous intraepithelial lesions in young women. Lancet. 2004;364:1678–1683.
This study shows just how meaningless LSIL cytology is in young women—and it portends changes in the next Consensus Guidelines. Colposcopy for all adolescents and young women is unwarranted, the authors stated. They recommend monitoring with repeat cytology instead.
For over a decade it has been widely appreciated that many CIN 1 lesions spontaneously regress in the absence of therapy.1 Based on what we recently learned from natural history studies of HPV, we know that the majority of LSIL cytology results and biopsy-confirmed CIN 1 lesions represent nothing more than the morphological manifestation of a productive HPV infection.2 HPV infections, including those with high-risk types of HPV, are typically self-limited (FIGURE 2). In approximately 90% of women, HPV shedding stops spontaneously within 24 months.
However, in the United States, most women with LSIL undergo colposcopy, and many clinicians continue to treat women with biopsy-confirmed CIN 1. These approaches do correspond to the most recent Consensus Guidelines, which recommend colposcopy for women with LSIL, and state that follow-up with treatment, as well as treatment with ablative or excisional methods, are acceptable management options for women with CIN 1.3
Regarding adolescents with LSIL, the guidelines made an exception to performing a colposcopy. For these patients, an acceptable management option is follow-up without initial colposcopy, using a protocol of repeat cytological testing at 6 and 12 months, or HPV testing at 12 months.
To better define the best way to manage young women with LSIL, Moscicki and colleagues followed a cohort of 204 young women (ages 13 to 22 years), who had an LSIL Pap result, for up to 80 months (median 61 months). HSIL cytology (N=6) or biopsy-confirmed CIN 2,3 (N=17) was found in only 11.3% of the women. After 36 months, only 6% had persistent LSIL.
The remainder had had 3 consecutive negative Pap results, and the median time to developing the first of 3 negative Pap results was only 8 months.
FIGURE 2 Even high-risk HPV types usually abate in young women
Liquid-based cytology specimen diagnosed as low-grade squamous intraepithelial lesion (LSIL), with marked koilocytosis with multinucleation, perinuclear halos, and nuclear atypia. These features typify productive HPV infection that usually regresses spontaneously in young women.
REFERENCES
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727-735.
2. Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med 2003;348:489-490.
3. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
Bivalent vaccine vanquishes HPV
Harper D, Franco E, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765.
HPV vaccine may be registered for clinical use next year. Since two-thirds of cervical cancers are caused by only 2 types of high-risk HPV—HPV 16 and HPV 18—a vaccine that prevents infection with HPV 16 and 18 could reduce cervical cancer and high-grade precursor lesions by more than half.
Extraordinary efficacy—100% against persistent infections and 91.6% against incident HPV 16 or 18 infections—was found in this Phase II trial of a bivalent HPV vaccine made by GlaxoSmithKline—the second such trial to show high efficacy for an HPV vaccine. Merck found high efficacy for its monovalent vaccine. Both companies are conducting Phase III registration trials.
Harper and colleagues observed these efficacy rates in women who took all their scheduled vaccinations. They used bivalent HPV 16 and 18 vaccine in a study of 1,113 women randomized to receive 3 doses of vaccine or placebo over a 6-month period. All were followed for up to 27 months.
The vaccine was also highly effective against cytological abnormalities associated with HPV 16 or 18 and was generally safe, well tolerated, and highly immunogenic.
In 2002, a Phase II trial of a monovalent HPV 16 vaccine produced by Merck demonstrated efficacy of 100% over 18 months in preventing persistent HPV 16 infection or CIN associated with HPV 16.1
Both companies’ vaccines consist of viral-like particles that are made by producing recombinant L1 capsid protein of the specific HPV type and then allowing the recombinant L1 capsid proteins to assemble into a structure that appears identical to the native virus, but lacks infectious DNA.
Each year, 470,000 women develop invasive cervical cancer, and 230,000 die, globally. Vaccination is a particularly attractive strategy for preventing cervical cancer in developing countries, where less than 5% of women have ever been screened.
Yet these numbers do not begin to take into account the huge costs and burden of disease due to noninvasive cervical cancer precursors and abnormal screening cytology. In the United States alone, we spend up to $6 billion a year on prevention and treatment of cervical cancer.
The author reports no financial relationships relevant to this article.
REFERENCE
1. Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.
As soon as next year, the human papillomavirus (HPV) vaccine could transform clinical practice more than anything since the Pap smear was introduced 60 years ago.
A second large trial has shown extraordinary efficacy, and now 2 manufacturers, GlaxoSmithKline and Merck, are conducting late-phase clinical trials and working toward registering their vaccines for clinical use in 2006. Only last month, Merck and GSK signed an agreement that resolves their competing intellectual property claims—removing one more barrier to rapid commercialization.
But there are other important new developments that apply to practice now:
- Colposcopy, it appears, is far less reliable for identifying cervical intraepithelial neoplasia (CIN) 2,3 than we thought.
- The long-term risk of preterm delivery with loop electrosurgical excision procedures (LEEP) points to a need to counsel patients and consider all management options for women with CIN 1.
- The high rates of spontaneous regression of low-grade squamous intraepithelial lesion (LSIL) cytologic changes in young women are now better defined, and indicate colposcopy is not always needed.
Colposcopy not as sensitive as we thought
Pretorius R, Zhang W, Bellinson J, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004; 191:430–434.
We need to carefully follow up whenever colposcopy does not identify a CIN 2,3 lesion. This study also reinforces the need for diagnostic excisional procedures in women with an HSIL Pap result, and who are found after colposcopy to have CIN 1 or less (FIGURE 1).
Unfortunately, colposcopy is highly subjective. Accuracy depends on training and experience. Nevertheless, it is the standard of care for identifying CIN 2,3 and invasive cervical cancer in women with abnormal Pap results. Colposcopy was thought to be a sensitive but rather nonspecific method for identifying high-grade neoplasia. A 1998 comprehensive meta-analysis estimated that colposcopy had a weighted mean sensitivity for distinguishing normal tissue from abnormal tissue of 0.96 (95% confidence interval [CI], 0.95-0.97) and a weighted mean specificity of 0.48 (95% CI, 0.47-0.49).1 This means that colposcopy would miss a biopsy-confirmed cervical abnormality in only about 4% of patients. However, more recent follow-up studies have reported much higher false negative rates for colposcopy.
Pretorius and colleagues studied women enrolled in a cervical cancer screening trial conducted in Shanxi, China. The colposcopy in this study was performed by attending gynecologic oncologists who worked closely with a team of US-based gynecologic oncologists. The women in the study had biopsies taken of all areas classified as abnormal by colposcopy. In addition, random 4-quadrant cervical biopsies were obtained from colposcopically normal regions of the cervix.
A total of 364 women with a satisfactory colposcopy and biopsy-confirmed CIN 2 or greater lesions were identified. Even though all 364 women had a satisfactory colposcopic examination, only 57.1% of the women with biopsy-confirmed CIN 2 or worse were detected by the colposcopically-directed biopsy; the remaining 42.9% were detected by the random biopsies of colposcopically normal-appearing tissue. The lesions that were missed by colposcopy tended to be smaller than those identified by colposcopy and were more frequently CIN 2 rather than CIN 3 lesions.
This study also evaluated the role of endocervical curettage, and found that even among women with a satisfactory colposcopic examination, a significant proportion (5.5%) of cases of CIN 2,3 or worse were detected only by using endocervical curettage.
FIGURE 1 Repeat colposcopy and biopsy may reveal high-grade lesion
Low-grade cervical intraepithelial neoplasia (CIN 1) of the cervix. This young woman has a well-defined acetowhite lesion of her cervix that was diagnosed as a CIN 1 on cervical biopsy. In many such cases, repeat colposcopy and biopsy identifies an area of high-grade lesion that was missed at the initial colposcopy.
REFERENCE
1. Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91:626-631.
LEEP raises risk of preterm birth
Sadler L, Saftlas A, Wang W, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100-2106.
We need to counsel women that LEEP will increase their risk for preterm premature rupture of membranes (PPROM) and preterm delivery. We must recognize that it is desirable to follow, rather than treat, biopsy-confirmed CIN 1, and to limit the depth of excision to 1 cm or less whenever possible.
Although Consensus Guidelines state that both ablative and excisional methods are acceptable forms of managing women with satisfactory colposcopy and CIN 2,3, for most clinicians, LEEP has completely replaced laser ablation and cryotherapy for treatment of CIN.1 Because LEEP is so widely utilized, its effects on fertility and preterm delivery, as well as other adverse pregnancy outcomes, are of great concern.
LEEP became widely adopted since its introduction in the early 1990s because it yields a tissue specimen for histological evaluation and is less expensive and easier to perform than laser ablation.
Many consider CIN 2,3 biomarkers the next step away from the Pap smear, toward more accurate molecular testing. One of the more promising biomarkers is p16INK4A, a cyclin-dependent kinase inhibitor involved in control of the cell cycle. Wang et al took tissue blocks from a large population-based screening study and evaluated the performance of p16INK4A on the full diagnostic spectrum of lesions. A very strong correlation was seen between identification of p16INK4A in the lesion and CIN 2,3; 100% of CIN 3 lesions showed diffuse staining with p16INK4A.
Wang S, Trunk M, Schiffman, M et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360.
Unfortunately, most studies of the impact of LEEP on fertility and pregnancy have been limited or inconclusive, and most lacked statistical power to detect a doubling of risk. The New Zealand study conducted by Sadler and colleagues—a large retrospective cohort study—compared delivery outcomes of 426 untreated women with 652 women treated by laser conization, laser ablation, or LEEP. Women who had LEEP or laser cone treatment were at significantly increased risk of rupture of membranes before 37 weeks’ gestation. Notably, in women who had undergone a LEEP, the adjusted relative risk (RR) for PPROM was 1.9 (95% CI, 1.0-3.8) compared to the untreated women. Laser ablation did not increase risk (RR 1.1). This study demonstrate that women who have undergone LEEP have almost twice the risk for PPROM as untreated women, should be of concern to all gynecologists.
Risk of both PPROM and preterm delivery increased as depth of cervical tissue removed increased. Women in whom 1 cm or less of tissue was excised had no increased risk of PPROM or preterm birth; women in whom more than 1.7 cm of tissue was excised had an adjusted relative risk of 3.6 (95% CI, 1.8-7.5).
In a Canadian study published only last month, Samson and colleagues found PPROM was almost 4 times more common among women who had had a LEEP.2
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
2. Samson S, Bentley JR, Fahey T, McKay D, Gill G. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325-332.
LSIL cytology meaningless?
Moscicki A, Shiboski S, Hills N, et al. Regression of low-grade squamous intraepithelial lesions in young women. Lancet. 2004;364:1678–1683.
This study shows just how meaningless LSIL cytology is in young women—and it portends changes in the next Consensus Guidelines. Colposcopy for all adolescents and young women is unwarranted, the authors stated. They recommend monitoring with repeat cytology instead.
For over a decade it has been widely appreciated that many CIN 1 lesions spontaneously regress in the absence of therapy.1 Based on what we recently learned from natural history studies of HPV, we know that the majority of LSIL cytology results and biopsy-confirmed CIN 1 lesions represent nothing more than the morphological manifestation of a productive HPV infection.2 HPV infections, including those with high-risk types of HPV, are typically self-limited (FIGURE 2). In approximately 90% of women, HPV shedding stops spontaneously within 24 months.
However, in the United States, most women with LSIL undergo colposcopy, and many clinicians continue to treat women with biopsy-confirmed CIN 1. These approaches do correspond to the most recent Consensus Guidelines, which recommend colposcopy for women with LSIL, and state that follow-up with treatment, as well as treatment with ablative or excisional methods, are acceptable management options for women with CIN 1.3
Regarding adolescents with LSIL, the guidelines made an exception to performing a colposcopy. For these patients, an acceptable management option is follow-up without initial colposcopy, using a protocol of repeat cytological testing at 6 and 12 months, or HPV testing at 12 months.
To better define the best way to manage young women with LSIL, Moscicki and colleagues followed a cohort of 204 young women (ages 13 to 22 years), who had an LSIL Pap result, for up to 80 months (median 61 months). HSIL cytology (N=6) or biopsy-confirmed CIN 2,3 (N=17) was found in only 11.3% of the women. After 36 months, only 6% had persistent LSIL.
The remainder had had 3 consecutive negative Pap results, and the median time to developing the first of 3 negative Pap results was only 8 months.
FIGURE 2 Even high-risk HPV types usually abate in young women
Liquid-based cytology specimen diagnosed as low-grade squamous intraepithelial lesion (LSIL), with marked koilocytosis with multinucleation, perinuclear halos, and nuclear atypia. These features typify productive HPV infection that usually regresses spontaneously in young women.
REFERENCES
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727-735.
2. Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med 2003;348:489-490.
3. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
Bivalent vaccine vanquishes HPV
Harper D, Franco E, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765.
HPV vaccine may be registered for clinical use next year. Since two-thirds of cervical cancers are caused by only 2 types of high-risk HPV—HPV 16 and HPV 18—a vaccine that prevents infection with HPV 16 and 18 could reduce cervical cancer and high-grade precursor lesions by more than half.
Extraordinary efficacy—100% against persistent infections and 91.6% against incident HPV 16 or 18 infections—was found in this Phase II trial of a bivalent HPV vaccine made by GlaxoSmithKline—the second such trial to show high efficacy for an HPV vaccine. Merck found high efficacy for its monovalent vaccine. Both companies are conducting Phase III registration trials.
Harper and colleagues observed these efficacy rates in women who took all their scheduled vaccinations. They used bivalent HPV 16 and 18 vaccine in a study of 1,113 women randomized to receive 3 doses of vaccine or placebo over a 6-month period. All were followed for up to 27 months.
The vaccine was also highly effective against cytological abnormalities associated with HPV 16 or 18 and was generally safe, well tolerated, and highly immunogenic.
In 2002, a Phase II trial of a monovalent HPV 16 vaccine produced by Merck demonstrated efficacy of 100% over 18 months in preventing persistent HPV 16 infection or CIN associated with HPV 16.1
Both companies’ vaccines consist of viral-like particles that are made by producing recombinant L1 capsid protein of the specific HPV type and then allowing the recombinant L1 capsid proteins to assemble into a structure that appears identical to the native virus, but lacks infectious DNA.
Each year, 470,000 women develop invasive cervical cancer, and 230,000 die, globally. Vaccination is a particularly attractive strategy for preventing cervical cancer in developing countries, where less than 5% of women have ever been screened.
Yet these numbers do not begin to take into account the huge costs and burden of disease due to noninvasive cervical cancer precursors and abnormal screening cytology. In the United States alone, we spend up to $6 billion a year on prevention and treatment of cervical cancer.
The author reports no financial relationships relevant to this article.
REFERENCE
1. Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.
Does menopause always justify bone density testing?
- 4 case studies
- Drug treatment based on T-scores and risk factors Reasonable options if T-score is borderline
- When is a follow-up in 1 year vital? When is a 2- or 3-year interval safe?
- BMD test techniques, sites, and T-scores
This question begs for a simple yes or no, but it is best answered by asking a second question, “Do I need to know my patient’s bone density to give her the best care possible at menopause?” If the answer is yes, then bone density testing is a must, because there is no other way to know what her bone density actually is.
How, then, does this knowledge affect clinical decision-making?
Our concern, of course, is whether we need to intervene pharmacologically to preserve the strength of the skeleton. Even though bone mineral density (BMD) does not completely account for bone strength, it does determine some 60% to 80% of bone strength, and it is still the best predictor of an initial fracture.
Of immediate concern to the physician caring for a woman entering the postmenopausal period is whether she has sufficient bone mass to withstand the bone loss that estrogen deficiency will impose—without developing a dangerously fragile skeletal structure.
Women start losing bone mass years before menopause. While she is still in her mid-40s, a woman’s spinal bone density begins to diminish due to accumulating dietary calcium deficiency, declining physical activity, and declining estradiol levels. (Unless menopause occurs earlier for any reason, however, bone density in the spine is thought to remain relatively stable from the time peak bone mass is attained, before age 30 in most skeletal sites,1 until the mid-40s.) The exact age at which the proximal femur begins to lose bone is more controversial. Cross-sectional studies have suggested that bone loss may in fact begin in a woman’s 20s, almost immediately after reaching peak bone mass. Others have suggested that bone loss does not begin until later, in her 30s.2
A variety of risk factors are modifiable, but one that we cannot modify—genetics— may play the predominant role in determining peak bone mass. Other factors include nutrition, physical activity, intervening illnesses, medications, and lifestyle factors like smoking and alcohol use.
Expect bone loss with any cause of estrogen decline
Postmenopausal bone loss is inexorable in the absence of estrogen replacement, as well as after stopping estrogen replacement therapy (ERT) or hormone replacement therapy (HRT). If your patient stops ERT or HRT, from a skeletal perspective she has just become postmenopausal again. By measuring her bone density, you can ascertain whether bone loss— which will certainly occur—will further deplete bone mass that is already less than ideal. If so, immediate intervention to prevent bone loss is appropriate.
One key longitudinal study,3 for example, found that perimenopausal women lost an average of 2.3% per year from the spine; postmenopausal women, 0.5%. The authors observed these losses in peri- and postmenopausal women, assessed over an average of 27 months. (Women were classified as perimenopausal if they became postmenopausal during the study.)
Calcium intake of 1,000 mg/day or more does not stop bone loss
In a study designed to evaluate the effectiveness of alendronate compared with placebo in preventing bone loss in women within 3 years of menopause, McClung et al4 found a 3% to 4% bone loss at the end of 3 years in the placebo group, despite total calcium intakes of 1,000 mg per day or more.
Stopping HT merits equal concern
Estrogen deficiency precipitated by stopping hormone therapy is due the same concern as that created by menopause itself. Although the exact rates vary in studies, it is clear that bone loss begins when ERT or HRT stops, just as it does with onset of menopause. Hysterectomized postmenopausal women who received ERT for 2 years were found to have a 4.5% decline in posterior-anterior (PA) lumbar spine bone density and a 1.2% decline in total hip bone density only 1 year after estrogen withdrawal.5 This loss occurred despite calcium supplementation.
Trémollieres et al found a 1.64% per year loss of bone density from the spine for the first 2 years after discontinuing HRT, which was similar to that seen in estrogen-deficient women for the first 2 years immediately after menopause.6 In the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, women who stopped HRT after 3 years lost bone density at an annual rate of 1.04% from the spine and 1.01% from the hip during 4 years of follow-up.7
A conservative assessment of the rate of bone loss in the first few years after menopause or cessation of hormone therapy is about 1% per year from the spine and proximal femur. At first glance, 1% per year does not appear worrisome. But within 10 years of menopause, at or about the age of only 60, 10% of the bone mass that was present at menopause is gone. In 15 years, at least 15% is gone because of estrogen deficiency.
Unquestionably, many women have stopped ERT or HRT or are choosing not to begin, due to media attention on negative findings from trials such as the combined-continuous HRT arm of the Wo men’s Health Initiative (WHI) and the Heart and Estrogen Replacement Study (HERS-I). Reviews of the National Prescription Audit database and National Disease and Therapeutic Index database confirmed a subsequent marked drop in prescriptions for ERT or HRT,8 despite WHI findings showing that combined-continuous HRT significantly reduces the risk of spine and hip fracture.9
Anne:Onset of hot flashes is a “teachable moment”
“Anne,” a 53-year-old Caucasian woman, has come to see you because of hot flashes that have begun to trouble her since her menstrual periods stopped 8 months ago.
Although she knows that estrogen replacement would help relieve her hot flashes, she is uncertain whether to use it, having heard negative media reports about WHI findings. She has no family or personal history of breast cancer, but is very frightened at even the slightest possibility of increasing her personal risk for breast cancer. She is 5’5” tall and weighs 120 lb. She broke her right wrist in a fall at age 46.
Don’t miss this opportunity!
Though Anne’s visit was prompted by distress over hot flashes, night sweats, and related symptoms of sleep disruption, daytime fatigue, mental lapses, and irritability, it’s a “teachable moment” to discuss osteoporosis prevention and testing. As is typical, her primary desire is relief from hot flashes, yet bone loss is a more serious threat.
If long-term inter vention starts early, bone loss and osteoporosis are preventable; in that context, onset of hot flashes can be seen as a positive force, since they prompted her to seek medical help.
Beth:Concerned because of her mother’s hip fracture
Occasionally a patient will raise the issue of osteoporosis herself. “Beth” is a 49-year-old woman who reports that her last menstrual period 3 months ago was very light in comparison to what she considers normal. Her periods have become irregular over the last year, initially being about 21 days apart, but now 10 to 12 weeks apart. She says she may have noticed an occasional hot flash, but it was not troublesome. She is concerned about the menstrual irregularity and wonders if she is close to menopause.
While she is not psychologically troubled about cessation of menstrual periods, she is concerned about potential bone loss due to estrogen deficiency. With additional questioning, you discover that her mother had a hip fracture.
Carol:Believes her risk low and refuses BMD test
“Carol,” on the other hand, says she doesn’t need bone density testing, because she is not interested in taking any medication to prevent or treat osteoporosis.
If Carol truly will not consider preventive medications, then bone density testing is certainly not indicated. The few patients who refuse to consider medications or testing tend to think their risk is slight. Careful questioning often elicits this belief. They may exercise, avoid cigarette smoke, and consume more than adequate amounts of calcium supplements or dairy products.
Unfortunately, such admirable habits in no way prevent estrogen-deficient bone loss.
Genetically determined low BMD
And no woman can overcome the effects of a genetically determined lower-than-average peak bone density, which may exist without the patient’s knowledge. Without a bone density test, the patient is making an uninformed decision and it is from this perspective that this situation is best approached. Her decision should always be respected, but it is our responsibility to insure that it is an informed decision.
Drug intervention based on T-score
By measuring the bone density at menopause, we can determine if pharmacologic intervention to prevent bone loss needs to start immediately. According to the National Osteoporosis Foundation (NOF) and the American Association of Clinical Endocrinologists (AACE) guidelines, if a woman’s T-score is below -1.5 and she has even 1 other risk factor, pharmacologic intervention is warranted.12,13
This level of bone density is clearly above the threshold for a diagnosis of osteoporosis based on the WHO criteria. Nevertheless, this patient’s estrogen deficiency will further deplete her already lower-than-normal bone density, and could be rapidly devastating. Knowledge of her T-score gives us potential to prevent fractures, now that we have drugs to prevent such devastation.
Three guidelines (TABLE)12-14 recommend pharmacologic intervention if the T-score is -2.5 or lower, and these guidelines differ only in the intervention threshold that also requires an additional risk factor. Note that all 3 recommend pharmacologic intervention when there is only a single risk factor in addition to a bone density level that would not be considered osteoporotic by WHO criteria.
The Food and Drug Administration (FDA) has approved drugs for prevention or treatment of postmenopausal osteoporosis, or both, based on whether data demonstrate that a drug:
- inhibits or stops bone loss, for the prevention indication, or
- reduces fracture risk, for the treatment indication.
The FDA-approved dosages of nonestrogen agents may vary by indication (TABLE). In clinical practice, however, the distinction between prevention and treatment is often less clear, leaving the dosage to the judgment of the clinician.
The complete list of clinical risk factors to consider in initiating therapy based on the T-score is lengthy; furthermore, an ever-increasing number of medications and diseases are now known to contribute to bone loss. The 5 major risk factors listed in the margin below are some of the most important to consider along with the T-score.
TABLE
Drug intervention is appropriate when there are…
| NO RISK FACTORS AND A T-SCORE: | RISK FACTORS AND A T-SCORE: | |
|---|---|---|
| National Osteoporosis Foundation | Below -2.0 | Below -1.5 |
| American Association of Clinical Endocrinologists | At or below -2.5 | -1.5 or poorer |
| North American Menopause Society | Below -2.5 | -2.0 or poorer |
| FDA-approved agents for prevention and treatment of postmenopausal osteoporosis* | ||
| Alendronate | ||
| Prevention | 5 mg po qd or 35 mg po qw | |
| Treatment | 10 mg po qd or 70 mg po qw | |
| Ibandronate† | 2.5 mg po qd | |
| Risedronate | 5 mg po qd or 35 mg po qw | |
| Raloxifene | 60 mg po qd | |
| *Unless otherwise noted, doses are the same for prevention or treatment | ||
| †Although FDA-approved, ibandronate is not currently marketed in the United States | ||
Follow-up testing intervals
Once your patient begins drug therapy, it is appropriate to follow up periodically with bone densitometry. The skeletal site measured at follow-up and the intervals between are dictated by reimbursement, as well as scientific issues. Many insurers, including Medicare, reimburse only once every 2 years.16 Exceptions are few.
From a scientific standpoint, BMD increases at the PA lumbar spine may be sufficiently great to be detected in only 1 year, with potent agents like the bisphosphonates or teriparatide. Since changes in PA lumbar spine density are generally less with raloxifene or salmon calcitonin, waiting 2 years to remeasure the PA lumbar spine is entirely appropriate here.
The PA lumbar spine is the preferred site for monitoring therapy because its higher percentage of trabecular bone generally results in a greater magnitude of change than at the proximal femur.
However, the slower rate of change at the proximal femur means that it need not be measured more often than 2 or even 3 years.
If your patient’s bone density is above the pharmacologic intervention threshold, it is always appropriate to counsel her on nonpharmacologic measures to preserve her skeleton: adequate dietary or supplemental calcium and vitamin D, regular weight-bearing or resistance exercise, and avoidance of cigarette smoke.
But we cannot assume that these are sufficient to protect her skeleton. Follow-up bone density studies are recommended to identify women who will lose bone despite these measures, and for whom pharmacologic intervention is warranted. AACE and the North American Menopause Society (NAMS) recommend follow-up bone density studies every 3 to 5 years in postmenopausal women in whom pharmacologic intervention is not deemed immediately necessary.13,14
A more specific approach based on the patient’s lowest T-score at either the PA lumbar spine or femoral neck has been suggested.17 If her T-score is greater than 0, a repeat study is suggested in 5 years. If however, the T-score is 0 to -0.5, a repeat study is suggested in 3 years. A repeat study should be done in only 1 year if the T-score is -0.5 to -1.
This approach assumes that you wish to know when the patient’s T-score might fall below the normal range established by the WHO, that is, below a T-score of -1, and assumes a rate of bone loss of approximately 0.5 SD (or 0.5 T-score units) per year. If you know you would not intervene until the T-score reaches -1.5 or -2, you can adjust the interval accordingly.
Precision in bone density testing is integral to accurate drug therapy monitoring. When properly performed, dual energy x-ray absorptiometry (DXA) bone density measurements are highly, but not perfectly, reproducible. To reflect actual biologic changes, a measured change i n BMD must have sufficient magnitude.
In general, for measurements at the PA lumbar spine or total hip, a change of 2.77% is needed for 95% confidence. The bone densitometry testing facility should provide the clinician with the exact magnitude of the change necessary for a given level of statistical confidence.18 It is also important to remember that while increases in BMD are desirable and reassuring, no loss of BMD may also be considered efficacious.
The guidelines
The NOF, AACE, the American College of Obstetricians and Gynecologists (ACOG), NAMS and the United States Preventive Services Task Force (USPSTF) guidelines12-14,19,20 agree that all postmenopausal women age 65 and older should have bone density testing. With the exception of the USPSTF, they also agree that all postmenopausal women under age 65 with risk factors should be tested. (The USPSTF limits this recommendation to women age 60 to 64.)
In reality, all postmenopausal women should have bone density testing because the list of risk factors is so comprehensive that it is unusual to find a woman who does not have at least 1 risk factor for osteoporosis.
Anne:Test again in 1 year
With this information in mind, let’s again consider Anne, the 53-year-old woman who sought help for hot flashes. Her visit was an opportunity to discuss osteoporosis prevention. Of the major risk factors, Anne has 2: weight less than 127 lb and a fracture after age 40. Based on the recommendations from the NOF, AACE, ACOG, and NAMS, bone density testing is appropriate.
A DXA study of both proximal femurs shows bone density data for each femur individually as well as the mean BMD value for each region of interest for both femurs. There are 5 regions of interest in the proximal femur: the total hip (or total femur), the femoral neck, Wa rd’s area, the trochanter, and the shaft. The total hip or femoral neck is preferred for diagnosis. Based on her normal T-scores, Anne does not meet any of the pharmacologic intervention guidelines. She should nevertheless be counseled on nonpharmacologic interventions to prevent bone loss.
She should have another bone density study in 1 year. Anne’s PA lumbar spine DXA study is not shown, but it provided no additional information. The PA lumbar spine would be the preferred site for follow-up in 1 year, however.
The recommendation for follow-up in 1 year would not change even if you elected to begin low-dose combined-continuous HRT for relief of hot flashes. Although HRT would be expected to preserve her skeleton, follow-up testing in 1 year for confirmation is appropriate.21
Beth:Treat now, test in 1 yr
Beth has an important risk factor: her mother’s hip fracture. This raises the possibility that she is genetically predisposed to lowerthan-average peak bone density. She meets NOF, AACE, NAMS, and ACOG guidelines for bone density testing.
At the PA lumbar spine, it is preferable to use either the L1-L4 BMD or the L2-L4 BMD and the corresponding T-score. In either case, Beth’s T-score is disturbingly low at -3.7 and -3.6, respectively. Either T-score meets the diagnosis of osteoporosis based on WHO criteria.
This single bone density study does not reveal whether she has lost bone density from a previously higher level or whether her current bone density represents her peak bone density. It is incumbent on the physician to evaluate her medically to exclude possible causes of bone loss other than estrogen deficiency, which might require a different or additional therapy.
Beth certainly meets NOF, AACE, and NAMS guidelines for drug intervention.
A follow-up PA lumbar spine DXA study is indicated in 1 year. Although she has osteoporosis, she has not yet had a fracture. For now, her diagnosis is nothing more than a test result. An osteoporotic fracture will change that. Immediate intervention with drug therapy can preserve her skeletal mass and her quality of life.
Donna:Borderline T-scores
Treatment decisions are not always as clear as in the cases of Anne, Beth, and Carol. Consider Donna, age 54, who is 2 years postmenopausal and in good health. However, her mother reportedly had a dowager’s hump at the time of her death. Although Donna was never told that her mother had osteoporosis, you suspect that she did because of the kyphosis. Donna is fairly sedentary, thin, and continues to smoke. She is not using HRT and rarely takes nutritional supplements of any kind. L 1 - L 4 PA lumbar spine T-score is -1.4; total hip is -1.3.
The dilemma is that Donna does not meet any guideline for pharmacologic intervention based on T-score, even in the presence of risk factors. Both T-scores are just above the NOF and AACE cutoff points, even in the presence of risk factors.
But the guidelines are not hard and fast rules. T- score cutoff points, with or without other risk factors, were chosen to balance the potential benefit and any potential harm of pharmacologic therapy with the risk of fracture if untreated. So, while it may seem arbitrary to recommend treatment when the T- score is -1.5 with risk factors, yet not if the T-score is -1.4 or -1.3, there is a substantive rationale behind the recommendation. Still, there is no substitute for your judgment.
What is a reasonable course?
She has 6 risk factors for bone loss and osteoporosis: estrogen deficiency, current smoking, probable family history, thinness, sedentary lifestyle, and probable calcium deficiency. Every attempt to modify the risk factors that can be modified is worth the effort—smoking cessation, exercise, and calcium and vitamin D supplementation would benefit her skeleton.
Important: Test again in 1 year. It is extremely important to repeat bone density testing at the lumbar spine in 1 year. If the nonpharmacologic interventions you recommend prove insufficient to radically slow the anticipated bone loss, she will fall below a T-score of -1.5 in the next year.
On the other hand, if she demonstrates that she can maintain her bone density with nonpharmacologic measures, a prescription may not be warranted. It would not be unreasonable to allow her this 1 year, because at her relatively young age of 54, at this bone density, her short-term risk of fracture is actually quite low.
“Yes” to both questions
If bone density is low—particularly if it is low and a woman has risk factors for osteoporosis—pharmacologic intervention can be reasonably expected to prevent the devastating consequences of osteoporosis. The question, “Does this menopausal woman need pharmacologic intervention to prevent or treat osteoporosis now, or might she need it later?” can be answered by measuring bone density. It is a question we would be remiss not to ask. Bone density measurement, preferably at the PA lumbar spine and proximal femur by DXA, is the only way to answer this all-important question. To provide the best care possible for a woman who has just become menopausal, you do need to know her bone density. The simple answer to both original questions then, is yes.
Techniques and sites
Bone densitometry can be performed using any of several techniques: dual energy x-ray absorptiometry (DXA), quantitative computerized tomography (QCT), radiographic absorptiometry (RA), or quantitative ultrasound (QUS).
Similarly, bone densitometry can be performed at a myriad of skeletal sites such as the PA lumbar spine, lateral lumbar spine, proximal femur, forearm, phalanges, calcaneus, and total body.
Guidelines are based on PA lumbar or proximal femur by DXA. It is correct that virtually all sites, measured by any technique, predict an individual’s fracture risk, but guidelines for diagnosis of osteoporosis and pharmacologic intervention to prevent or treat osteoporosis are overwhelmingly based on measurements of the PA lumbar spine or proximal femur by DXA.10-14 This is not because of any inadequacy or inaccuracy of the other technologies at these or other skeletal sites. It is because of the use of the World Health Organization (WHO) criteria for diagnosis of osteoporosis and the reliance upon the T-score in intervention guidelines.
WHO diagnosis based on T-score
| DIAGNOSTIC CATEGORY | T-SCORE CRITERIA |
|---|---|
| Normal | -1 or better |
| Osteopenia (low bone mass) | Between -1 and -2.5 |
| Osteoporosis | -2.5 or poorer |
| Severe osteoporosis | -2.5 or poorer, with a fragility fracture |
In its sentinel 1994 guidelines, the WHO defined osteoporosis as a bone density of 2.5 standard deviations (SD) or more below the average bone density for a young adult.15 This threshold was chosen in an attempt to reconcile the prevalence of the disease created by the threshold and the observed lifetime fracture risks. The data used to reach this conclusion were largely based on single-photon absorptiometry (SPA) data from the mid-radius, dual-photon absorptiometry (DXA’s predecessor) and DXA data from the PA lumbar spine and proximal femur.
The WHO warned that applying these criteria in persons measured by other technologies or at other skeletal sites could result in a different diagnostic category. When physicians did apply the criteria in clinical practice, WHO’s prediction became a reality that was quickly recognized and discussed in the literature.
It became clear that we could not apply the WHO criteria to all technologies and all skeletal sites.
Consequentially, major osteoporosis-related medical organizations issued guidelines calling for restricting the diagnosis of osteoporosis based on the WHO criteria to bone density studies performed at the PA lumbar spine and proximal femur using DXA.
T-score means above or below “average”
The T- score on modern bone density reports, although not a technically correct use of the term, indicates your patient’s number of SDs above or below that of the average value for a young adult. If your patient’s BMD is below the average value for a young adult, a minus sign is placed in front of the T- score. The young-adult average value is always assigned a T- score value of 0. For example, a BMD that is 2.2 SD below the average value for a young adult of the same sex would be assigned a T- score of -2.2. Because the WHO defined osteoporosis based on the number of SDs below the average for a young adult, the WHO criteria readily translate to a T- score.
Dr. Bonnick reports research support from Merck and Roche/GSK; consultant fees from Merck, Roche/GSK, and Wyeth; and speaking fees from Merck, Roche/GSK, and Procter & Gamble.
1. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16:393S-399S.
2. Hui SL, Perkins AJ, Zhous L, et al. Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab. 2002;87:1539-1543.
3. Pouilles JM, Trémollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int. 1993;53:340-343.
4. McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med. 1998;128:253-261.
5. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
6. Trémollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int. 2001;12:385-390.
7. Greendale GA, Espeland M, Slone S, Marcus R, BarrettConnor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
8. Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trend response to recent evidence. JAMA. 2004;291:104-106.
9. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259.
11. Miller PD, Siris ES, Barrett-Connor E, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222-2230.
12. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: NOF; 2003.
13. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001edition, with selected updates for 2003. Endocr Pract. 2003;9:545-564.
14. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
15. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994.
16. Department of Health and Human Services. Health Care Finance Administration. Medicare program; Medicare coverage of and payment for bone mass measurements. 42 CFR Part 410, Federal Register 63:June 24, 1989.
17. Abrahamsen B, Nissen N, Hermann AP, et al. When should densitometry be repeated in healthy peri- and postmenopausal women: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2002;17:2061-2067.
18. Bonnick SL, Johnston CC, Kleerekoper M, et al. The importance of precision in bone density measurements. J Clin Densitom. 2001;4:105-110.
19. American College of Obstetricians and Gynecologists. ACOG releases recommendations for bone density screening for osteoporosis. Washington, DC: ACOG; 2002.
20. US Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
21. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
- 4 case studies
- Drug treatment based on T-scores and risk factors Reasonable options if T-score is borderline
- When is a follow-up in 1 year vital? When is a 2- or 3-year interval safe?
- BMD test techniques, sites, and T-scores
This question begs for a simple yes or no, but it is best answered by asking a second question, “Do I need to know my patient’s bone density to give her the best care possible at menopause?” If the answer is yes, then bone density testing is a must, because there is no other way to know what her bone density actually is.
How, then, does this knowledge affect clinical decision-making?
Our concern, of course, is whether we need to intervene pharmacologically to preserve the strength of the skeleton. Even though bone mineral density (BMD) does not completely account for bone strength, it does determine some 60% to 80% of bone strength, and it is still the best predictor of an initial fracture.
Of immediate concern to the physician caring for a woman entering the postmenopausal period is whether she has sufficient bone mass to withstand the bone loss that estrogen deficiency will impose—without developing a dangerously fragile skeletal structure.
Women start losing bone mass years before menopause. While she is still in her mid-40s, a woman’s spinal bone density begins to diminish due to accumulating dietary calcium deficiency, declining physical activity, and declining estradiol levels. (Unless menopause occurs earlier for any reason, however, bone density in the spine is thought to remain relatively stable from the time peak bone mass is attained, before age 30 in most skeletal sites,1 until the mid-40s.) The exact age at which the proximal femur begins to lose bone is more controversial. Cross-sectional studies have suggested that bone loss may in fact begin in a woman’s 20s, almost immediately after reaching peak bone mass. Others have suggested that bone loss does not begin until later, in her 30s.2
A variety of risk factors are modifiable, but one that we cannot modify—genetics— may play the predominant role in determining peak bone mass. Other factors include nutrition, physical activity, intervening illnesses, medications, and lifestyle factors like smoking and alcohol use.
Expect bone loss with any cause of estrogen decline
Postmenopausal bone loss is inexorable in the absence of estrogen replacement, as well as after stopping estrogen replacement therapy (ERT) or hormone replacement therapy (HRT). If your patient stops ERT or HRT, from a skeletal perspective she has just become postmenopausal again. By measuring her bone density, you can ascertain whether bone loss— which will certainly occur—will further deplete bone mass that is already less than ideal. If so, immediate intervention to prevent bone loss is appropriate.
One key longitudinal study,3 for example, found that perimenopausal women lost an average of 2.3% per year from the spine; postmenopausal women, 0.5%. The authors observed these losses in peri- and postmenopausal women, assessed over an average of 27 months. (Women were classified as perimenopausal if they became postmenopausal during the study.)
Calcium intake of 1,000 mg/day or more does not stop bone loss
In a study designed to evaluate the effectiveness of alendronate compared with placebo in preventing bone loss in women within 3 years of menopause, McClung et al4 found a 3% to 4% bone loss at the end of 3 years in the placebo group, despite total calcium intakes of 1,000 mg per day or more.
Stopping HT merits equal concern
Estrogen deficiency precipitated by stopping hormone therapy is due the same concern as that created by menopause itself. Although the exact rates vary in studies, it is clear that bone loss begins when ERT or HRT stops, just as it does with onset of menopause. Hysterectomized postmenopausal women who received ERT for 2 years were found to have a 4.5% decline in posterior-anterior (PA) lumbar spine bone density and a 1.2% decline in total hip bone density only 1 year after estrogen withdrawal.5 This loss occurred despite calcium supplementation.
Trémollieres et al found a 1.64% per year loss of bone density from the spine for the first 2 years after discontinuing HRT, which was similar to that seen in estrogen-deficient women for the first 2 years immediately after menopause.6 In the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, women who stopped HRT after 3 years lost bone density at an annual rate of 1.04% from the spine and 1.01% from the hip during 4 years of follow-up.7
A conservative assessment of the rate of bone loss in the first few years after menopause or cessation of hormone therapy is about 1% per year from the spine and proximal femur. At first glance, 1% per year does not appear worrisome. But within 10 years of menopause, at or about the age of only 60, 10% of the bone mass that was present at menopause is gone. In 15 years, at least 15% is gone because of estrogen deficiency.
Unquestionably, many women have stopped ERT or HRT or are choosing not to begin, due to media attention on negative findings from trials such as the combined-continuous HRT arm of the Wo men’s Health Initiative (WHI) and the Heart and Estrogen Replacement Study (HERS-I). Reviews of the National Prescription Audit database and National Disease and Therapeutic Index database confirmed a subsequent marked drop in prescriptions for ERT or HRT,8 despite WHI findings showing that combined-continuous HRT significantly reduces the risk of spine and hip fracture.9
Anne:Onset of hot flashes is a “teachable moment”
“Anne,” a 53-year-old Caucasian woman, has come to see you because of hot flashes that have begun to trouble her since her menstrual periods stopped 8 months ago.
Although she knows that estrogen replacement would help relieve her hot flashes, she is uncertain whether to use it, having heard negative media reports about WHI findings. She has no family or personal history of breast cancer, but is very frightened at even the slightest possibility of increasing her personal risk for breast cancer. She is 5’5” tall and weighs 120 lb. She broke her right wrist in a fall at age 46.
Don’t miss this opportunity!
Though Anne’s visit was prompted by distress over hot flashes, night sweats, and related symptoms of sleep disruption, daytime fatigue, mental lapses, and irritability, it’s a “teachable moment” to discuss osteoporosis prevention and testing. As is typical, her primary desire is relief from hot flashes, yet bone loss is a more serious threat.
If long-term inter vention starts early, bone loss and osteoporosis are preventable; in that context, onset of hot flashes can be seen as a positive force, since they prompted her to seek medical help.
Beth:Concerned because of her mother’s hip fracture
Occasionally a patient will raise the issue of osteoporosis herself. “Beth” is a 49-year-old woman who reports that her last menstrual period 3 months ago was very light in comparison to what she considers normal. Her periods have become irregular over the last year, initially being about 21 days apart, but now 10 to 12 weeks apart. She says she may have noticed an occasional hot flash, but it was not troublesome. She is concerned about the menstrual irregularity and wonders if she is close to menopause.
While she is not psychologically troubled about cessation of menstrual periods, she is concerned about potential bone loss due to estrogen deficiency. With additional questioning, you discover that her mother had a hip fracture.
Carol:Believes her risk low and refuses BMD test
“Carol,” on the other hand, says she doesn’t need bone density testing, because she is not interested in taking any medication to prevent or treat osteoporosis.
If Carol truly will not consider preventive medications, then bone density testing is certainly not indicated. The few patients who refuse to consider medications or testing tend to think their risk is slight. Careful questioning often elicits this belief. They may exercise, avoid cigarette smoke, and consume more than adequate amounts of calcium supplements or dairy products.
Unfortunately, such admirable habits in no way prevent estrogen-deficient bone loss.
Genetically determined low BMD
And no woman can overcome the effects of a genetically determined lower-than-average peak bone density, which may exist without the patient’s knowledge. Without a bone density test, the patient is making an uninformed decision and it is from this perspective that this situation is best approached. Her decision should always be respected, but it is our responsibility to insure that it is an informed decision.
Drug intervention based on T-score
By measuring the bone density at menopause, we can determine if pharmacologic intervention to prevent bone loss needs to start immediately. According to the National Osteoporosis Foundation (NOF) and the American Association of Clinical Endocrinologists (AACE) guidelines, if a woman’s T-score is below -1.5 and she has even 1 other risk factor, pharmacologic intervention is warranted.12,13
This level of bone density is clearly above the threshold for a diagnosis of osteoporosis based on the WHO criteria. Nevertheless, this patient’s estrogen deficiency will further deplete her already lower-than-normal bone density, and could be rapidly devastating. Knowledge of her T-score gives us potential to prevent fractures, now that we have drugs to prevent such devastation.
Three guidelines (TABLE)12-14 recommend pharmacologic intervention if the T-score is -2.5 or lower, and these guidelines differ only in the intervention threshold that also requires an additional risk factor. Note that all 3 recommend pharmacologic intervention when there is only a single risk factor in addition to a bone density level that would not be considered osteoporotic by WHO criteria.
The Food and Drug Administration (FDA) has approved drugs for prevention or treatment of postmenopausal osteoporosis, or both, based on whether data demonstrate that a drug:
- inhibits or stops bone loss, for the prevention indication, or
- reduces fracture risk, for the treatment indication.
The FDA-approved dosages of nonestrogen agents may vary by indication (TABLE). In clinical practice, however, the distinction between prevention and treatment is often less clear, leaving the dosage to the judgment of the clinician.
The complete list of clinical risk factors to consider in initiating therapy based on the T-score is lengthy; furthermore, an ever-increasing number of medications and diseases are now known to contribute to bone loss. The 5 major risk factors listed in the margin below are some of the most important to consider along with the T-score.
TABLE
Drug intervention is appropriate when there are…
| NO RISK FACTORS AND A T-SCORE: | RISK FACTORS AND A T-SCORE: | |
|---|---|---|
| National Osteoporosis Foundation | Below -2.0 | Below -1.5 |
| American Association of Clinical Endocrinologists | At or below -2.5 | -1.5 or poorer |
| North American Menopause Society | Below -2.5 | -2.0 or poorer |
| FDA-approved agents for prevention and treatment of postmenopausal osteoporosis* | ||
| Alendronate | ||
| Prevention | 5 mg po qd or 35 mg po qw | |
| Treatment | 10 mg po qd or 70 mg po qw | |
| Ibandronate† | 2.5 mg po qd | |
| Risedronate | 5 mg po qd or 35 mg po qw | |
| Raloxifene | 60 mg po qd | |
| *Unless otherwise noted, doses are the same for prevention or treatment | ||
| †Although FDA-approved, ibandronate is not currently marketed in the United States | ||
Follow-up testing intervals
Once your patient begins drug therapy, it is appropriate to follow up periodically with bone densitometry. The skeletal site measured at follow-up and the intervals between are dictated by reimbursement, as well as scientific issues. Many insurers, including Medicare, reimburse only once every 2 years.16 Exceptions are few.
From a scientific standpoint, BMD increases at the PA lumbar spine may be sufficiently great to be detected in only 1 year, with potent agents like the bisphosphonates or teriparatide. Since changes in PA lumbar spine density are generally less with raloxifene or salmon calcitonin, waiting 2 years to remeasure the PA lumbar spine is entirely appropriate here.
The PA lumbar spine is the preferred site for monitoring therapy because its higher percentage of trabecular bone generally results in a greater magnitude of change than at the proximal femur.
However, the slower rate of change at the proximal femur means that it need not be measured more often than 2 or even 3 years.
If your patient’s bone density is above the pharmacologic intervention threshold, it is always appropriate to counsel her on nonpharmacologic measures to preserve her skeleton: adequate dietary or supplemental calcium and vitamin D, regular weight-bearing or resistance exercise, and avoidance of cigarette smoke.
But we cannot assume that these are sufficient to protect her skeleton. Follow-up bone density studies are recommended to identify women who will lose bone despite these measures, and for whom pharmacologic intervention is warranted. AACE and the North American Menopause Society (NAMS) recommend follow-up bone density studies every 3 to 5 years in postmenopausal women in whom pharmacologic intervention is not deemed immediately necessary.13,14
A more specific approach based on the patient’s lowest T-score at either the PA lumbar spine or femoral neck has been suggested.17 If her T-score is greater than 0, a repeat study is suggested in 5 years. If however, the T-score is 0 to -0.5, a repeat study is suggested in 3 years. A repeat study should be done in only 1 year if the T-score is -0.5 to -1.
This approach assumes that you wish to know when the patient’s T-score might fall below the normal range established by the WHO, that is, below a T-score of -1, and assumes a rate of bone loss of approximately 0.5 SD (or 0.5 T-score units) per year. If you know you would not intervene until the T-score reaches -1.5 or -2, you can adjust the interval accordingly.
Precision in bone density testing is integral to accurate drug therapy monitoring. When properly performed, dual energy x-ray absorptiometry (DXA) bone density measurements are highly, but not perfectly, reproducible. To reflect actual biologic changes, a measured change i n BMD must have sufficient magnitude.
In general, for measurements at the PA lumbar spine or total hip, a change of 2.77% is needed for 95% confidence. The bone densitometry testing facility should provide the clinician with the exact magnitude of the change necessary for a given level of statistical confidence.18 It is also important to remember that while increases in BMD are desirable and reassuring, no loss of BMD may also be considered efficacious.
The guidelines
The NOF, AACE, the American College of Obstetricians and Gynecologists (ACOG), NAMS and the United States Preventive Services Task Force (USPSTF) guidelines12-14,19,20 agree that all postmenopausal women age 65 and older should have bone density testing. With the exception of the USPSTF, they also agree that all postmenopausal women under age 65 with risk factors should be tested. (The USPSTF limits this recommendation to women age 60 to 64.)
In reality, all postmenopausal women should have bone density testing because the list of risk factors is so comprehensive that it is unusual to find a woman who does not have at least 1 risk factor for osteoporosis.
Anne:Test again in 1 year
With this information in mind, let’s again consider Anne, the 53-year-old woman who sought help for hot flashes. Her visit was an opportunity to discuss osteoporosis prevention. Of the major risk factors, Anne has 2: weight less than 127 lb and a fracture after age 40. Based on the recommendations from the NOF, AACE, ACOG, and NAMS, bone density testing is appropriate.
A DXA study of both proximal femurs shows bone density data for each femur individually as well as the mean BMD value for each region of interest for both femurs. There are 5 regions of interest in the proximal femur: the total hip (or total femur), the femoral neck, Wa rd’s area, the trochanter, and the shaft. The total hip or femoral neck is preferred for diagnosis. Based on her normal T-scores, Anne does not meet any of the pharmacologic intervention guidelines. She should nevertheless be counseled on nonpharmacologic interventions to prevent bone loss.
She should have another bone density study in 1 year. Anne’s PA lumbar spine DXA study is not shown, but it provided no additional information. The PA lumbar spine would be the preferred site for follow-up in 1 year, however.
The recommendation for follow-up in 1 year would not change even if you elected to begin low-dose combined-continuous HRT for relief of hot flashes. Although HRT would be expected to preserve her skeleton, follow-up testing in 1 year for confirmation is appropriate.21
Beth:Treat now, test in 1 yr
Beth has an important risk factor: her mother’s hip fracture. This raises the possibility that she is genetically predisposed to lowerthan-average peak bone density. She meets NOF, AACE, NAMS, and ACOG guidelines for bone density testing.
At the PA lumbar spine, it is preferable to use either the L1-L4 BMD or the L2-L4 BMD and the corresponding T-score. In either case, Beth’s T-score is disturbingly low at -3.7 and -3.6, respectively. Either T-score meets the diagnosis of osteoporosis based on WHO criteria.
This single bone density study does not reveal whether she has lost bone density from a previously higher level or whether her current bone density represents her peak bone density. It is incumbent on the physician to evaluate her medically to exclude possible causes of bone loss other than estrogen deficiency, which might require a different or additional therapy.
Beth certainly meets NOF, AACE, and NAMS guidelines for drug intervention.
A follow-up PA lumbar spine DXA study is indicated in 1 year. Although she has osteoporosis, she has not yet had a fracture. For now, her diagnosis is nothing more than a test result. An osteoporotic fracture will change that. Immediate intervention with drug therapy can preserve her skeletal mass and her quality of life.
Donna:Borderline T-scores
Treatment decisions are not always as clear as in the cases of Anne, Beth, and Carol. Consider Donna, age 54, who is 2 years postmenopausal and in good health. However, her mother reportedly had a dowager’s hump at the time of her death. Although Donna was never told that her mother had osteoporosis, you suspect that she did because of the kyphosis. Donna is fairly sedentary, thin, and continues to smoke. She is not using HRT and rarely takes nutritional supplements of any kind. L 1 - L 4 PA lumbar spine T-score is -1.4; total hip is -1.3.
The dilemma is that Donna does not meet any guideline for pharmacologic intervention based on T-score, even in the presence of risk factors. Both T-scores are just above the NOF and AACE cutoff points, even in the presence of risk factors.
But the guidelines are not hard and fast rules. T- score cutoff points, with or without other risk factors, were chosen to balance the potential benefit and any potential harm of pharmacologic therapy with the risk of fracture if untreated. So, while it may seem arbitrary to recommend treatment when the T- score is -1.5 with risk factors, yet not if the T-score is -1.4 or -1.3, there is a substantive rationale behind the recommendation. Still, there is no substitute for your judgment.
What is a reasonable course?
She has 6 risk factors for bone loss and osteoporosis: estrogen deficiency, current smoking, probable family history, thinness, sedentary lifestyle, and probable calcium deficiency. Every attempt to modify the risk factors that can be modified is worth the effort—smoking cessation, exercise, and calcium and vitamin D supplementation would benefit her skeleton.
Important: Test again in 1 year. It is extremely important to repeat bone density testing at the lumbar spine in 1 year. If the nonpharmacologic interventions you recommend prove insufficient to radically slow the anticipated bone loss, she will fall below a T-score of -1.5 in the next year.
On the other hand, if she demonstrates that she can maintain her bone density with nonpharmacologic measures, a prescription may not be warranted. It would not be unreasonable to allow her this 1 year, because at her relatively young age of 54, at this bone density, her short-term risk of fracture is actually quite low.
“Yes” to both questions
If bone density is low—particularly if it is low and a woman has risk factors for osteoporosis—pharmacologic intervention can be reasonably expected to prevent the devastating consequences of osteoporosis. The question, “Does this menopausal woman need pharmacologic intervention to prevent or treat osteoporosis now, or might she need it later?” can be answered by measuring bone density. It is a question we would be remiss not to ask. Bone density measurement, preferably at the PA lumbar spine and proximal femur by DXA, is the only way to answer this all-important question. To provide the best care possible for a woman who has just become menopausal, you do need to know her bone density. The simple answer to both original questions then, is yes.
Techniques and sites
Bone densitometry can be performed using any of several techniques: dual energy x-ray absorptiometry (DXA), quantitative computerized tomography (QCT), radiographic absorptiometry (RA), or quantitative ultrasound (QUS).
Similarly, bone densitometry can be performed at a myriad of skeletal sites such as the PA lumbar spine, lateral lumbar spine, proximal femur, forearm, phalanges, calcaneus, and total body.
Guidelines are based on PA lumbar or proximal femur by DXA. It is correct that virtually all sites, measured by any technique, predict an individual’s fracture risk, but guidelines for diagnosis of osteoporosis and pharmacologic intervention to prevent or treat osteoporosis are overwhelmingly based on measurements of the PA lumbar spine or proximal femur by DXA.10-14 This is not because of any inadequacy or inaccuracy of the other technologies at these or other skeletal sites. It is because of the use of the World Health Organization (WHO) criteria for diagnosis of osteoporosis and the reliance upon the T-score in intervention guidelines.
WHO diagnosis based on T-score
| DIAGNOSTIC CATEGORY | T-SCORE CRITERIA |
|---|---|
| Normal | -1 or better |
| Osteopenia (low bone mass) | Between -1 and -2.5 |
| Osteoporosis | -2.5 or poorer |
| Severe osteoporosis | -2.5 or poorer, with a fragility fracture |
In its sentinel 1994 guidelines, the WHO defined osteoporosis as a bone density of 2.5 standard deviations (SD) or more below the average bone density for a young adult.15 This threshold was chosen in an attempt to reconcile the prevalence of the disease created by the threshold and the observed lifetime fracture risks. The data used to reach this conclusion were largely based on single-photon absorptiometry (SPA) data from the mid-radius, dual-photon absorptiometry (DXA’s predecessor) and DXA data from the PA lumbar spine and proximal femur.
The WHO warned that applying these criteria in persons measured by other technologies or at other skeletal sites could result in a different diagnostic category. When physicians did apply the criteria in clinical practice, WHO’s prediction became a reality that was quickly recognized and discussed in the literature.
It became clear that we could not apply the WHO criteria to all technologies and all skeletal sites.
Consequentially, major osteoporosis-related medical organizations issued guidelines calling for restricting the diagnosis of osteoporosis based on the WHO criteria to bone density studies performed at the PA lumbar spine and proximal femur using DXA.
T-score means above or below “average”
The T- score on modern bone density reports, although not a technically correct use of the term, indicates your patient’s number of SDs above or below that of the average value for a young adult. If your patient’s BMD is below the average value for a young adult, a minus sign is placed in front of the T- score. The young-adult average value is always assigned a T- score value of 0. For example, a BMD that is 2.2 SD below the average value for a young adult of the same sex would be assigned a T- score of -2.2. Because the WHO defined osteoporosis based on the number of SDs below the average for a young adult, the WHO criteria readily translate to a T- score.
Dr. Bonnick reports research support from Merck and Roche/GSK; consultant fees from Merck, Roche/GSK, and Wyeth; and speaking fees from Merck, Roche/GSK, and Procter & Gamble.
- 4 case studies
- Drug treatment based on T-scores and risk factors Reasonable options if T-score is borderline
- When is a follow-up in 1 year vital? When is a 2- or 3-year interval safe?
- BMD test techniques, sites, and T-scores
This question begs for a simple yes or no, but it is best answered by asking a second question, “Do I need to know my patient’s bone density to give her the best care possible at menopause?” If the answer is yes, then bone density testing is a must, because there is no other way to know what her bone density actually is.
How, then, does this knowledge affect clinical decision-making?
Our concern, of course, is whether we need to intervene pharmacologically to preserve the strength of the skeleton. Even though bone mineral density (BMD) does not completely account for bone strength, it does determine some 60% to 80% of bone strength, and it is still the best predictor of an initial fracture.
Of immediate concern to the physician caring for a woman entering the postmenopausal period is whether she has sufficient bone mass to withstand the bone loss that estrogen deficiency will impose—without developing a dangerously fragile skeletal structure.
Women start losing bone mass years before menopause. While she is still in her mid-40s, a woman’s spinal bone density begins to diminish due to accumulating dietary calcium deficiency, declining physical activity, and declining estradiol levels. (Unless menopause occurs earlier for any reason, however, bone density in the spine is thought to remain relatively stable from the time peak bone mass is attained, before age 30 in most skeletal sites,1 until the mid-40s.) The exact age at which the proximal femur begins to lose bone is more controversial. Cross-sectional studies have suggested that bone loss may in fact begin in a woman’s 20s, almost immediately after reaching peak bone mass. Others have suggested that bone loss does not begin until later, in her 30s.2
A variety of risk factors are modifiable, but one that we cannot modify—genetics— may play the predominant role in determining peak bone mass. Other factors include nutrition, physical activity, intervening illnesses, medications, and lifestyle factors like smoking and alcohol use.
Expect bone loss with any cause of estrogen decline
Postmenopausal bone loss is inexorable in the absence of estrogen replacement, as well as after stopping estrogen replacement therapy (ERT) or hormone replacement therapy (HRT). If your patient stops ERT or HRT, from a skeletal perspective she has just become postmenopausal again. By measuring her bone density, you can ascertain whether bone loss— which will certainly occur—will further deplete bone mass that is already less than ideal. If so, immediate intervention to prevent bone loss is appropriate.
One key longitudinal study,3 for example, found that perimenopausal women lost an average of 2.3% per year from the spine; postmenopausal women, 0.5%. The authors observed these losses in peri- and postmenopausal women, assessed over an average of 27 months. (Women were classified as perimenopausal if they became postmenopausal during the study.)
Calcium intake of 1,000 mg/day or more does not stop bone loss
In a study designed to evaluate the effectiveness of alendronate compared with placebo in preventing bone loss in women within 3 years of menopause, McClung et al4 found a 3% to 4% bone loss at the end of 3 years in the placebo group, despite total calcium intakes of 1,000 mg per day or more.
Stopping HT merits equal concern
Estrogen deficiency precipitated by stopping hormone therapy is due the same concern as that created by menopause itself. Although the exact rates vary in studies, it is clear that bone loss begins when ERT or HRT stops, just as it does with onset of menopause. Hysterectomized postmenopausal women who received ERT for 2 years were found to have a 4.5% decline in posterior-anterior (PA) lumbar spine bone density and a 1.2% decline in total hip bone density only 1 year after estrogen withdrawal.5 This loss occurred despite calcium supplementation.
Trémollieres et al found a 1.64% per year loss of bone density from the spine for the first 2 years after discontinuing HRT, which was similar to that seen in estrogen-deficient women for the first 2 years immediately after menopause.6 In the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, women who stopped HRT after 3 years lost bone density at an annual rate of 1.04% from the spine and 1.01% from the hip during 4 years of follow-up.7
A conservative assessment of the rate of bone loss in the first few years after menopause or cessation of hormone therapy is about 1% per year from the spine and proximal femur. At first glance, 1% per year does not appear worrisome. But within 10 years of menopause, at or about the age of only 60, 10% of the bone mass that was present at menopause is gone. In 15 years, at least 15% is gone because of estrogen deficiency.
Unquestionably, many women have stopped ERT or HRT or are choosing not to begin, due to media attention on negative findings from trials such as the combined-continuous HRT arm of the Wo men’s Health Initiative (WHI) and the Heart and Estrogen Replacement Study (HERS-I). Reviews of the National Prescription Audit database and National Disease and Therapeutic Index database confirmed a subsequent marked drop in prescriptions for ERT or HRT,8 despite WHI findings showing that combined-continuous HRT significantly reduces the risk of spine and hip fracture.9
Anne:Onset of hot flashes is a “teachable moment”
“Anne,” a 53-year-old Caucasian woman, has come to see you because of hot flashes that have begun to trouble her since her menstrual periods stopped 8 months ago.
Although she knows that estrogen replacement would help relieve her hot flashes, she is uncertain whether to use it, having heard negative media reports about WHI findings. She has no family or personal history of breast cancer, but is very frightened at even the slightest possibility of increasing her personal risk for breast cancer. She is 5’5” tall and weighs 120 lb. She broke her right wrist in a fall at age 46.
Don’t miss this opportunity!
Though Anne’s visit was prompted by distress over hot flashes, night sweats, and related symptoms of sleep disruption, daytime fatigue, mental lapses, and irritability, it’s a “teachable moment” to discuss osteoporosis prevention and testing. As is typical, her primary desire is relief from hot flashes, yet bone loss is a more serious threat.
If long-term inter vention starts early, bone loss and osteoporosis are preventable; in that context, onset of hot flashes can be seen as a positive force, since they prompted her to seek medical help.
Beth:Concerned because of her mother’s hip fracture
Occasionally a patient will raise the issue of osteoporosis herself. “Beth” is a 49-year-old woman who reports that her last menstrual period 3 months ago was very light in comparison to what she considers normal. Her periods have become irregular over the last year, initially being about 21 days apart, but now 10 to 12 weeks apart. She says she may have noticed an occasional hot flash, but it was not troublesome. She is concerned about the menstrual irregularity and wonders if she is close to menopause.
While she is not psychologically troubled about cessation of menstrual periods, she is concerned about potential bone loss due to estrogen deficiency. With additional questioning, you discover that her mother had a hip fracture.
Carol:Believes her risk low and refuses BMD test
“Carol,” on the other hand, says she doesn’t need bone density testing, because she is not interested in taking any medication to prevent or treat osteoporosis.
If Carol truly will not consider preventive medications, then bone density testing is certainly not indicated. The few patients who refuse to consider medications or testing tend to think their risk is slight. Careful questioning often elicits this belief. They may exercise, avoid cigarette smoke, and consume more than adequate amounts of calcium supplements or dairy products.
Unfortunately, such admirable habits in no way prevent estrogen-deficient bone loss.
Genetically determined low BMD
And no woman can overcome the effects of a genetically determined lower-than-average peak bone density, which may exist without the patient’s knowledge. Without a bone density test, the patient is making an uninformed decision and it is from this perspective that this situation is best approached. Her decision should always be respected, but it is our responsibility to insure that it is an informed decision.
Drug intervention based on T-score
By measuring the bone density at menopause, we can determine if pharmacologic intervention to prevent bone loss needs to start immediately. According to the National Osteoporosis Foundation (NOF) and the American Association of Clinical Endocrinologists (AACE) guidelines, if a woman’s T-score is below -1.5 and she has even 1 other risk factor, pharmacologic intervention is warranted.12,13
This level of bone density is clearly above the threshold for a diagnosis of osteoporosis based on the WHO criteria. Nevertheless, this patient’s estrogen deficiency will further deplete her already lower-than-normal bone density, and could be rapidly devastating. Knowledge of her T-score gives us potential to prevent fractures, now that we have drugs to prevent such devastation.
Three guidelines (TABLE)12-14 recommend pharmacologic intervention if the T-score is -2.5 or lower, and these guidelines differ only in the intervention threshold that also requires an additional risk factor. Note that all 3 recommend pharmacologic intervention when there is only a single risk factor in addition to a bone density level that would not be considered osteoporotic by WHO criteria.
The Food and Drug Administration (FDA) has approved drugs for prevention or treatment of postmenopausal osteoporosis, or both, based on whether data demonstrate that a drug:
- inhibits or stops bone loss, for the prevention indication, or
- reduces fracture risk, for the treatment indication.
The FDA-approved dosages of nonestrogen agents may vary by indication (TABLE). In clinical practice, however, the distinction between prevention and treatment is often less clear, leaving the dosage to the judgment of the clinician.
The complete list of clinical risk factors to consider in initiating therapy based on the T-score is lengthy; furthermore, an ever-increasing number of medications and diseases are now known to contribute to bone loss. The 5 major risk factors listed in the margin below are some of the most important to consider along with the T-score.
TABLE
Drug intervention is appropriate when there are…
| NO RISK FACTORS AND A T-SCORE: | RISK FACTORS AND A T-SCORE: | |
|---|---|---|
| National Osteoporosis Foundation | Below -2.0 | Below -1.5 |
| American Association of Clinical Endocrinologists | At or below -2.5 | -1.5 or poorer |
| North American Menopause Society | Below -2.5 | -2.0 or poorer |
| FDA-approved agents for prevention and treatment of postmenopausal osteoporosis* | ||
| Alendronate | ||
| Prevention | 5 mg po qd or 35 mg po qw | |
| Treatment | 10 mg po qd or 70 mg po qw | |
| Ibandronate† | 2.5 mg po qd | |
| Risedronate | 5 mg po qd or 35 mg po qw | |
| Raloxifene | 60 mg po qd | |
| *Unless otherwise noted, doses are the same for prevention or treatment | ||
| †Although FDA-approved, ibandronate is not currently marketed in the United States | ||
Follow-up testing intervals
Once your patient begins drug therapy, it is appropriate to follow up periodically with bone densitometry. The skeletal site measured at follow-up and the intervals between are dictated by reimbursement, as well as scientific issues. Many insurers, including Medicare, reimburse only once every 2 years.16 Exceptions are few.
From a scientific standpoint, BMD increases at the PA lumbar spine may be sufficiently great to be detected in only 1 year, with potent agents like the bisphosphonates or teriparatide. Since changes in PA lumbar spine density are generally less with raloxifene or salmon calcitonin, waiting 2 years to remeasure the PA lumbar spine is entirely appropriate here.
The PA lumbar spine is the preferred site for monitoring therapy because its higher percentage of trabecular bone generally results in a greater magnitude of change than at the proximal femur.
However, the slower rate of change at the proximal femur means that it need not be measured more often than 2 or even 3 years.
If your patient’s bone density is above the pharmacologic intervention threshold, it is always appropriate to counsel her on nonpharmacologic measures to preserve her skeleton: adequate dietary or supplemental calcium and vitamin D, regular weight-bearing or resistance exercise, and avoidance of cigarette smoke.
But we cannot assume that these are sufficient to protect her skeleton. Follow-up bone density studies are recommended to identify women who will lose bone despite these measures, and for whom pharmacologic intervention is warranted. AACE and the North American Menopause Society (NAMS) recommend follow-up bone density studies every 3 to 5 years in postmenopausal women in whom pharmacologic intervention is not deemed immediately necessary.13,14
A more specific approach based on the patient’s lowest T-score at either the PA lumbar spine or femoral neck has been suggested.17 If her T-score is greater than 0, a repeat study is suggested in 5 years. If however, the T-score is 0 to -0.5, a repeat study is suggested in 3 years. A repeat study should be done in only 1 year if the T-score is -0.5 to -1.
This approach assumes that you wish to know when the patient’s T-score might fall below the normal range established by the WHO, that is, below a T-score of -1, and assumes a rate of bone loss of approximately 0.5 SD (or 0.5 T-score units) per year. If you know you would not intervene until the T-score reaches -1.5 or -2, you can adjust the interval accordingly.
Precision in bone density testing is integral to accurate drug therapy monitoring. When properly performed, dual energy x-ray absorptiometry (DXA) bone density measurements are highly, but not perfectly, reproducible. To reflect actual biologic changes, a measured change i n BMD must have sufficient magnitude.
In general, for measurements at the PA lumbar spine or total hip, a change of 2.77% is needed for 95% confidence. The bone densitometry testing facility should provide the clinician with the exact magnitude of the change necessary for a given level of statistical confidence.18 It is also important to remember that while increases in BMD are desirable and reassuring, no loss of BMD may also be considered efficacious.
The guidelines
The NOF, AACE, the American College of Obstetricians and Gynecologists (ACOG), NAMS and the United States Preventive Services Task Force (USPSTF) guidelines12-14,19,20 agree that all postmenopausal women age 65 and older should have bone density testing. With the exception of the USPSTF, they also agree that all postmenopausal women under age 65 with risk factors should be tested. (The USPSTF limits this recommendation to women age 60 to 64.)
In reality, all postmenopausal women should have bone density testing because the list of risk factors is so comprehensive that it is unusual to find a woman who does not have at least 1 risk factor for osteoporosis.
Anne:Test again in 1 year
With this information in mind, let’s again consider Anne, the 53-year-old woman who sought help for hot flashes. Her visit was an opportunity to discuss osteoporosis prevention. Of the major risk factors, Anne has 2: weight less than 127 lb and a fracture after age 40. Based on the recommendations from the NOF, AACE, ACOG, and NAMS, bone density testing is appropriate.
A DXA study of both proximal femurs shows bone density data for each femur individually as well as the mean BMD value for each region of interest for both femurs. There are 5 regions of interest in the proximal femur: the total hip (or total femur), the femoral neck, Wa rd’s area, the trochanter, and the shaft. The total hip or femoral neck is preferred for diagnosis. Based on her normal T-scores, Anne does not meet any of the pharmacologic intervention guidelines. She should nevertheless be counseled on nonpharmacologic interventions to prevent bone loss.
She should have another bone density study in 1 year. Anne’s PA lumbar spine DXA study is not shown, but it provided no additional information. The PA lumbar spine would be the preferred site for follow-up in 1 year, however.
The recommendation for follow-up in 1 year would not change even if you elected to begin low-dose combined-continuous HRT for relief of hot flashes. Although HRT would be expected to preserve her skeleton, follow-up testing in 1 year for confirmation is appropriate.21
Beth:Treat now, test in 1 yr
Beth has an important risk factor: her mother’s hip fracture. This raises the possibility that she is genetically predisposed to lowerthan-average peak bone density. She meets NOF, AACE, NAMS, and ACOG guidelines for bone density testing.
At the PA lumbar spine, it is preferable to use either the L1-L4 BMD or the L2-L4 BMD and the corresponding T-score. In either case, Beth’s T-score is disturbingly low at -3.7 and -3.6, respectively. Either T-score meets the diagnosis of osteoporosis based on WHO criteria.
This single bone density study does not reveal whether she has lost bone density from a previously higher level or whether her current bone density represents her peak bone density. It is incumbent on the physician to evaluate her medically to exclude possible causes of bone loss other than estrogen deficiency, which might require a different or additional therapy.
Beth certainly meets NOF, AACE, and NAMS guidelines for drug intervention.
A follow-up PA lumbar spine DXA study is indicated in 1 year. Although she has osteoporosis, she has not yet had a fracture. For now, her diagnosis is nothing more than a test result. An osteoporotic fracture will change that. Immediate intervention with drug therapy can preserve her skeletal mass and her quality of life.
Donna:Borderline T-scores
Treatment decisions are not always as clear as in the cases of Anne, Beth, and Carol. Consider Donna, age 54, who is 2 years postmenopausal and in good health. However, her mother reportedly had a dowager’s hump at the time of her death. Although Donna was never told that her mother had osteoporosis, you suspect that she did because of the kyphosis. Donna is fairly sedentary, thin, and continues to smoke. She is not using HRT and rarely takes nutritional supplements of any kind. L 1 - L 4 PA lumbar spine T-score is -1.4; total hip is -1.3.
The dilemma is that Donna does not meet any guideline for pharmacologic intervention based on T-score, even in the presence of risk factors. Both T-scores are just above the NOF and AACE cutoff points, even in the presence of risk factors.
But the guidelines are not hard and fast rules. T- score cutoff points, with or without other risk factors, were chosen to balance the potential benefit and any potential harm of pharmacologic therapy with the risk of fracture if untreated. So, while it may seem arbitrary to recommend treatment when the T- score is -1.5 with risk factors, yet not if the T-score is -1.4 or -1.3, there is a substantive rationale behind the recommendation. Still, there is no substitute for your judgment.
What is a reasonable course?
She has 6 risk factors for bone loss and osteoporosis: estrogen deficiency, current smoking, probable family history, thinness, sedentary lifestyle, and probable calcium deficiency. Every attempt to modify the risk factors that can be modified is worth the effort—smoking cessation, exercise, and calcium and vitamin D supplementation would benefit her skeleton.
Important: Test again in 1 year. It is extremely important to repeat bone density testing at the lumbar spine in 1 year. If the nonpharmacologic interventions you recommend prove insufficient to radically slow the anticipated bone loss, she will fall below a T-score of -1.5 in the next year.
On the other hand, if she demonstrates that she can maintain her bone density with nonpharmacologic measures, a prescription may not be warranted. It would not be unreasonable to allow her this 1 year, because at her relatively young age of 54, at this bone density, her short-term risk of fracture is actually quite low.
“Yes” to both questions
If bone density is low—particularly if it is low and a woman has risk factors for osteoporosis—pharmacologic intervention can be reasonably expected to prevent the devastating consequences of osteoporosis. The question, “Does this menopausal woman need pharmacologic intervention to prevent or treat osteoporosis now, or might she need it later?” can be answered by measuring bone density. It is a question we would be remiss not to ask. Bone density measurement, preferably at the PA lumbar spine and proximal femur by DXA, is the only way to answer this all-important question. To provide the best care possible for a woman who has just become menopausal, you do need to know her bone density. The simple answer to both original questions then, is yes.
Techniques and sites
Bone densitometry can be performed using any of several techniques: dual energy x-ray absorptiometry (DXA), quantitative computerized tomography (QCT), radiographic absorptiometry (RA), or quantitative ultrasound (QUS).
Similarly, bone densitometry can be performed at a myriad of skeletal sites such as the PA lumbar spine, lateral lumbar spine, proximal femur, forearm, phalanges, calcaneus, and total body.
Guidelines are based on PA lumbar or proximal femur by DXA. It is correct that virtually all sites, measured by any technique, predict an individual’s fracture risk, but guidelines for diagnosis of osteoporosis and pharmacologic intervention to prevent or treat osteoporosis are overwhelmingly based on measurements of the PA lumbar spine or proximal femur by DXA.10-14 This is not because of any inadequacy or inaccuracy of the other technologies at these or other skeletal sites. It is because of the use of the World Health Organization (WHO) criteria for diagnosis of osteoporosis and the reliance upon the T-score in intervention guidelines.
WHO diagnosis based on T-score
| DIAGNOSTIC CATEGORY | T-SCORE CRITERIA |
|---|---|
| Normal | -1 or better |
| Osteopenia (low bone mass) | Between -1 and -2.5 |
| Osteoporosis | -2.5 or poorer |
| Severe osteoporosis | -2.5 or poorer, with a fragility fracture |
In its sentinel 1994 guidelines, the WHO defined osteoporosis as a bone density of 2.5 standard deviations (SD) or more below the average bone density for a young adult.15 This threshold was chosen in an attempt to reconcile the prevalence of the disease created by the threshold and the observed lifetime fracture risks. The data used to reach this conclusion were largely based on single-photon absorptiometry (SPA) data from the mid-radius, dual-photon absorptiometry (DXA’s predecessor) and DXA data from the PA lumbar spine and proximal femur.
The WHO warned that applying these criteria in persons measured by other technologies or at other skeletal sites could result in a different diagnostic category. When physicians did apply the criteria in clinical practice, WHO’s prediction became a reality that was quickly recognized and discussed in the literature.
It became clear that we could not apply the WHO criteria to all technologies and all skeletal sites.
Consequentially, major osteoporosis-related medical organizations issued guidelines calling for restricting the diagnosis of osteoporosis based on the WHO criteria to bone density studies performed at the PA lumbar spine and proximal femur using DXA.
T-score means above or below “average”
The T- score on modern bone density reports, although not a technically correct use of the term, indicates your patient’s number of SDs above or below that of the average value for a young adult. If your patient’s BMD is below the average value for a young adult, a minus sign is placed in front of the T- score. The young-adult average value is always assigned a T- score value of 0. For example, a BMD that is 2.2 SD below the average value for a young adult of the same sex would be assigned a T- score of -2.2. Because the WHO defined osteoporosis based on the number of SDs below the average for a young adult, the WHO criteria readily translate to a T- score.
Dr. Bonnick reports research support from Merck and Roche/GSK; consultant fees from Merck, Roche/GSK, and Wyeth; and speaking fees from Merck, Roche/GSK, and Procter & Gamble.
1. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16:393S-399S.
2. Hui SL, Perkins AJ, Zhous L, et al. Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab. 2002;87:1539-1543.
3. Pouilles JM, Trémollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int. 1993;53:340-343.
4. McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med. 1998;128:253-261.
5. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
6. Trémollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int. 2001;12:385-390.
7. Greendale GA, Espeland M, Slone S, Marcus R, BarrettConnor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
8. Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trend response to recent evidence. JAMA. 2004;291:104-106.
9. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259.
11. Miller PD, Siris ES, Barrett-Connor E, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222-2230.
12. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: NOF; 2003.
13. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001edition, with selected updates for 2003. Endocr Pract. 2003;9:545-564.
14. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
15. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994.
16. Department of Health and Human Services. Health Care Finance Administration. Medicare program; Medicare coverage of and payment for bone mass measurements. 42 CFR Part 410, Federal Register 63:June 24, 1989.
17. Abrahamsen B, Nissen N, Hermann AP, et al. When should densitometry be repeated in healthy peri- and postmenopausal women: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2002;17:2061-2067.
18. Bonnick SL, Johnston CC, Kleerekoper M, et al. The importance of precision in bone density measurements. J Clin Densitom. 2001;4:105-110.
19. American College of Obstetricians and Gynecologists. ACOG releases recommendations for bone density screening for osteoporosis. Washington, DC: ACOG; 2002.
20. US Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
21. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
1. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16:393S-399S.
2. Hui SL, Perkins AJ, Zhous L, et al. Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab. 2002;87:1539-1543.
3. Pouilles JM, Trémollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int. 1993;53:340-343.
4. McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med. 1998;128:253-261.
5. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
6. Trémollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int. 2001;12:385-390.
7. Greendale GA, Espeland M, Slone S, Marcus R, BarrettConnor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
8. Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trend response to recent evidence. JAMA. 2004;291:104-106.
9. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259.
11. Miller PD, Siris ES, Barrett-Connor E, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222-2230.
12. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: NOF; 2003.
13. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001edition, with selected updates for 2003. Endocr Pract. 2003;9:545-564.
14. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
15. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994.
16. Department of Health and Human Services. Health Care Finance Administration. Medicare program; Medicare coverage of and payment for bone mass measurements. 42 CFR Part 410, Federal Register 63:June 24, 1989.
17. Abrahamsen B, Nissen N, Hermann AP, et al. When should densitometry be repeated in healthy peri- and postmenopausal women: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2002;17:2061-2067.
18. Bonnick SL, Johnston CC, Kleerekoper M, et al. The importance of precision in bone density measurements. J Clin Densitom. 2001;4:105-110.
19. American College of Obstetricians and Gynecologists. ACOG releases recommendations for bone density screening for osteoporosis. Washington, DC: ACOG; 2002.
20. US Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
21. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
Expectant management of preeclampsia
Once you decide to expectantly manage a patient with preeclampsia, the balancing act begins. That means weighing fetal benefits against maternal risks, since the only justification for expectant management is to prolong pregnancy for fetal gain—there is no advantage to the mother.
The best approach is to classify the woman’s preeclampsia by the degree of severity and gestational age at the time of diagnosis, then follow recommendations tailored to that particular category.
This article offers guidelines for expectant management of mild and severe preeclampsia, preeclampsia superimposed on a preexisting medical condition, and intrapartum and postpartum care.
Mild preeclampsia
The earlier preeclampsia develops, the greater the risk it will become severe. The need for hospitalization depends on gestational age, blood pressure, proteinuria levels, maternal symptoms, and reliability of the patient.
Preeclampsia is mild when systolic blood pressure reaches 140 to 159 mm Hg or diastolic pressure measures 90 to 109 mm Hg on at least 2 occasions more than 6 hours apart after 20 weeks’ gestation in a woman who previously had normal blood pressure. In preeclampsia, this hypertension is accompanied by proteinuria of 0.3 to 4.9 g in a 24-hour urine sample (1+ or 2+ by dipstick on 2 occasions).
At or beyond 37 weeks’ gestation
In general, women diagnosed with preeclampsia at this gestational age have pregnancy outcomes similar to those of normotensive gravidas. Thus, they benefit from induction of labor and delivery.
32 to 36 weeks’ gestation
Close maternal and fetal evaluation is essential. (It is assumed these women have no labor or membrane rupture and normal fetal testing; otherwise, delivery is indicated at 34 weeks or beyond.)
In general, hospitalization is indicated when any of the following circumstances are present (FIGURE 1):
- the patient is unreliable,
- 2 or more systolic blood pressure readings exceed 150 mm Hg,
- 2 or more diastolic blood pressure readings exceed 100 mm Hg,
- proteinuria occurs at a rate exceeding 1 g/24 hours, or
- persistent maternal symptoms are present.
FIGURE 1 Treatment of mild preeclampsia in healthy women
Before 32 weeks’ gestation
These women are at high risk of progressing to severe disease. They also are more likely to have adverse perinatal outcomes such as intrauterine growth restriction (IUGR) (15% to 20%), preterm delivery (50%), and abruptio placentae (1% to 2%), compared with women diagnosed with preeclampsia at 32 to 36 weeks. In addition, they require more antenatal surveillance than women who develop preeclampsia later in pregnancy.
I recommend hospitalization at the time of diagnosis when women develop mild preeclampsia before 32 weeks.
What and when to monitor
Maternal evaluation should include:
- monitoring of blood pressure at least daily (at home or in the hospital),
- daily urine dipstick evaluation to monitor changes in proteinuria,
- twice-weekly platelet count and liver enzymes, and
- documentation of symptoms. (Instruct all women to report the onset of severe headaches, visual changes, altered mental status, epigastric or right upper quadrant pain, and any nausea or vomiting.)
Fetal evaluation should include:
- serial ultrasound every 3 weeks to estimate fetal weight and amniotic fluid status,
- nonstress testing every week, and
- daily fetal movement counts.
If a nonstress test is nonreactive, it should be confirmed by biophysical profile.
All testing should be promptly repeated if the maternal clinical condition deteriorates.
No need for bed rest, diuretics, or antihypertensive medications
Although expectantly managed patients with mild preeclampsia should be advised to restrict daily activity, there is no need for complete bed rest. Nor have diuretics or other antihypertensive drugs been shown to prolong gestation. On the contrary, these medications may mask severe preeclampsia.
Antihypertensive medications reduce the rate of severe hypertension but do not improve perinatal outcome. If these drugs are used to treat mild disease remote from term, hospitalize the patient and manage her as though she has severe preeclampsia.
Hospitalization versus outpatient management
Although she may be hospitalized at the time of diagnosis, a woman with preeclampsia may switch to outpatient management if systolic or diastolic blood pressure declines, proteinuria diminishes to 1 g/24 hours or less, and there are no maternal symptoms or evidence of severe IUGR. Otherwise, these women should remain hospitalized until delivery.
In cases that begin with outpatient management, prompt hospitalization is indicated if there is clinical evidence that the disease is progressing (ie, new symptoms, labor or rupture of membranes, vaginal bleeding, or increased blood pressures or proteinuria) or IUGR and/or oligohydramnios.
Instruct all women to report symptoms and changes in fetal movement.
When to deliver
Whether the gravida is hospitalized or an outpatient, delivery is indicated at 37 weeks. Earlier delivery may be warranted if nonreassuring maternal or fetal conditions develop. (FIGURE 1 summarizes management of mild preeclampsia.)
Severe preeclampsia
Expectant management is safe in properly selected women with severe disease, although maternal and fetal conditions can deteriorate. Hospitalization and daily monitoring are required.
Preeclampsia is severe when any of the following are present:
- systolic blood pressure of 160 mm Hg or higher or diastolic pressure of 110 mm Hg or above on 2 occasions at least 6 hours apart while the patient is on bed rest
- proteinuria of 5 g or more in a 24-hour urine specimen,
- oliguria of less than 500 mL in 24 hours,
- cerebral or visual disturbances,
- pulmonary edema or cyanosis,
- severe epigastric or right upper-quadrant pain, or
- thrombocytopenia.
When gestational hypertension or preeclampsia is severe, hospitalization in the labor and delivery suite is warranted. These women should receive intravenous (IV) magnesium sulfate to reduce the risk of convulsions and antihypertensive drugs to treat severe levels of hypertension, if present. The aim of antihypertensive treatment is to keep diastolic blood pressure between 90 and 105 mm Hg and systolic blood pressure below 160 mm Hg.
During observation, assess maternal and fetal conditions and decide whether delivery is indicated (FIGURE 2).
Expectant management is warranted only for gestations between 23 and 32 weeks’ gestation, provided maternal and fetal conditions are stable (FIGURE 2).
Keep in mind that both maternal and fetal conditions may progressively deteriorate. Thus, these pregnancies involve higher rates of maternal morbidity and significant risk of neonatal morbidity. For this reason, expectant management should proceed only in a tertiary-care center with adequate maternal and neonatal facilities.
Recommended counseling
Advise these patients of the potential risks and benefits of expectant management, which requires daily monitoring of maternal and fetal conditions. Also explain that the decision to continue expectant management will be revisited on a daily basis and that the median number of days pregnancy is prolonged in these cases is 7 (range 2 to 35).
Another important fact to relay: Only 2 randomized trials involving 133 women have compared expectant management to aggressive management in early-onset preeclampsia. However, retrospective and observational studies involving more than 700 women suggest expectant management reduces short-term neonatal morbidity with minimal risk to the mother.
Superimposed preeclampsia
Women who develop preeclampsia on top of chronic hypertension, renal disease, or type 1 diabetes have a markedly higher risk of morbidity, including perinatal morbidity, than women without preexisting conditions.
Women with superimposed preeclampsia may be managed in the hospital, since these pregnancies are associated with higher rates of abruptio placentae (2% to 5%), preterm delivery (56%), IUGR (13% to 15%), and perinatal death (8%). Thus, these women benefit from very close maternal and fetal monitoring.
Superimposed preeclampsia is not classified according to severity.
In general, maternal and perinatal morbidities are substantially higher in women who have preexisting conditions than in healthy women who develop preeclampsia.
Chronic hypertension
Indications for delivery are similar to those described for healthy women with preeclampsia, as is antihypertensive therapy.
If the woman develops preeclampsia while using antihypertensive drugs, delivery should be considered beyond 34 weeks’ gestation.
How preeclampsia affects renal function
Women with renal disease or dysfunction (serum creatinine ≥1.2 mg/dL) prior to or early in pregnancy face an increased risk of adverse neonatal outcomes, regardless of whether preeclampsia also develops. These women also face an increased risk of deteriorating renal function during pregnancy (particularly if preeclampsia or severe hypertension develops) and beyond (more than 6 months postpartum).
Start antihypertensive medications as soon as possible, with the goal of keeping systolic blood pressure below 140 mm Hg and diastolic blood pressure below 90 mm Hg throughout gestation.
Delivery is indicated with the onset of preeclampsia or significant deterioration in renal function.
Diabetes warrants aggressive therapy
Women with type 1 diabetes have a higher risk of preeclampsia, maternal and fetal morbidity, and perinatal mortality. These risks multiply in women who have hypertension and/or diabetic nephropathy. Worsening of retinopathy and nephropathy also is more likely in women who have hypertension. Thus, aggressive management of blood sugars with insulin should be accompanied by aggressive control of blood pressure, with the goal of keeping systolic pressure below 130 mm Hg and diastolic pressure below 85 mm Hg.
Choosing antihypertensive drugs. Calcium-channel blockers are preferred to control blood pressure during pregnancy in women with diabetes. Outside of pregnancy, angiotensin-converting enzyme (ACE) inhibitors are best to avert long-term complications, but avoid these drugs in pregnancy (along with angiotensin-receptor blockers), particularly beyond 16 weeks.
Delivery is indicated in all women with vascular diabetes mellitus beyond 34 weeks when preeclampsia is present.
Intrapartum management
Close fetal heart rate and maternal blood pressure monitoring are mainstays, along with magnesium sulfate and antihypertensive therapy.
All women with preeclampsia should receive continuous monitoring of fetal heart rate and uterine activity, with special vigilance for hyperstimulation and onset of vaginal bleeding during labor. (For a description of potential maternal complications, see TABLE 1; fetal complications are described in FIGURE 3.)
Uterine irritability, recurrent variable or late decelerations, and the development of vaginal bleeding may be the first signs of abruptio placentae.
I recommend recording maternal blood pressure at least hourly to detect progression from mild to severe hypertension and to determine the need for antihypertensive therapy.
TABLE 1
Likelihood of maternal complications
| Disease progresses during labor (from mild to severe) | 10% |
| Eclampsia | |
| • Mild disease | <0.5% |
| • Severe preeclampsia | 1–2% |
| Stroke (encephalopathy or hemorrhage) | <1% |
| Mainly with severe or early onset disease | |
| Pulmonary edema | 1–2% |
| Usually associated with fluid overload or long-standing chronic hypertension | |
Prevent progression to eclampsia
Magnesium sulfate is the drug of choice in women with preeclampsia. Recent reviews indicate that it reduces the rate of convulsions from 2% to 0.6% in women with severe preeclampsia. In women with mild preeclampsia, the benefit of magnesium sulfate remains unclear.
I recommend IV magnesium sulfate during labor and postpartum when a woman has the indications listed in TABLE 2.
The dose of magnesium sulfate is 6 g IV loading over 20 minutes, followed by a maintenance dose of 2 g/hour.
Magnesium sulfate should be started before surgery (elective cesarean delivery) and continued for at least 12 hours postpartum (I prefer 24 hours).
TABLE 2
When to give prophylactic magnesium sulfate
| Use intrapartum and for at least 12 hours postpartum |
|---|
When the patient has:
|
When treating hypertension in labor, avoid “hypotensive overshoot”
The goal of intrapartum treatment is to lower maternal blood pressure without causing precipitous hypotensive overshoot that may lead to reduced maternal organ perfusion, particularly uteroplacental blood flow. Such acute lowering of maternal blood pressure is a common cause of nonreassuring fetal heart rate patterns during labor.
What blood pressure necessitates treatment? There is no doubt that severe levels of hypertension should be treated to avoid potential cerebrovascular and cardiovascular complications in healthy women. However, there is disagreement about what constitutes severe hypertension.
In previously healthy women, I recommend antihypertensive therapy for systolic pressures of 170 mm Hg or above and/or for diastolic pressures of 110 mm Hg or above.
For women with thrombocytopenia, disseminated intravascular coagulation, or pulmonary edema, I recommend treatment for systolic pressures of 160 mm Hg or above and diastolic pressures of 105 mm Hg or above. This latter group should also be given IV furosemide (20 to 40 mg) to promote diuresis. I also recommend treatment at these levels in the postpartum period.
For women with diabetes, renal disease, or left ventricular cardiac disease, antihypertensive medications should be used to keep systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg during labor and postpartum. Further, patients in congestive heart failure or with left ventricular diastolic dysfunction should receive furosemide in addition to antihypertensive drugs.
Choosing a drug. My drugs of choice are IV labetalol and oral nifedipine. These 2 drugs, along with IV hydralazine, are the most commonly recommended medications for severe hypertension in pregnancy (TABLE 3).
Although many authorities prefer hydralazine, recent data indicate that, compared with IV labetalol and oral nifedipine, IV hydralazine is associated with more maternal side effects and worse perinatal outcomes (more fetal distress in labor).
TABLE 3
Drug profiles: Dosing and side effects of antihypertensives used in pregnancy
| MEDICATION | ONSET OF ACTION | DOSE | SIDE EFFECTS |
|---|---|---|---|
| Hydralazine | 10-20 minutes | 5-10 mg intravenously every 20 minutes up to maximum dose of 30 mg | More maternal side effects and worse perinatal outcomes than labetalol or nifedipine. |
| Skin blisters; chest pain; general feeling of discomfort, illness, or weakness; joint or muscle pain; sore throat and fever; swollen lymph glands | |||
| Labetalol* | 10-15 minutes | 10-20 mg intravenously, then 40-80 mg every 10 minutes up to maximum dose of 220 mg/hour or continuous infusion of 1-2 mg/minute | Breathing difficulty and/or wheezing, cold hands and feet, mental depression, shortness of breath, slow heartbeat, swelling of lower extremities, back or joint pain, chest pain, confusion, fever and sore throat, hallucinations, irregular heartbeat, unusual bleeding and bruising, yellow eyes or skin |
| Nifedipine | 5-10 minutes | 10-20 mg orally, repeated in 30 minutes, up to maximum dose of 50 mg/hour | Breathing difficulty, coughing, or wheezing; irregular or fast, poundingheartbeat; skin rash; swelling of lower extremities; chest pain; fainting; painful, swollen joints; vision impairment |
| Sodium nitroprusside† | 0.5-5 minutes | 0.25-5 μg/kg/minute by intravenous infusion | Risk of fetal cyanide poisoning with prolonged treatment. |
| Maternal effects include symptoms of hypothyroidism, headache, abdominal pain, drowsiness, nausea, involuntary muscle movements, perspiration, restlessness, paraesthesia, palpitations, dizziness, retching, tachycardia | |||
| *In women with asthma and congestive heart failure | |||
| †Rarely needed except in hypertensive encephalopathy or cerebral hemorrhage | |||
Postpartum management
Because preeclampsia can worsen, or first appear, in the postpartum period, extra vigilance is important, and pharmacotherapy may be appropriate.
Management of preeclampsia does not end with delivery of the fetus and the placenta. These events do signal the beginning of the curative process, but complications can occur in the postpartum period. Indeed, in some women, the disease process worsens immediately postpartum. Therefore, women with diagnosed preeclampsia or severe gestational hypertension require close monitoring of blood pressure and maternal symptoms and accurate measurement of fluid intake and urine output. Some of these women are at increased risk for pulmonary edema; exacerbation of severe hypertension; eclampsia; and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.
Treating postpartum hypertension
Women who continue to have severe hypertension (systolic pressure at or above 155 mm Hg or diastolic pressure of 105 mm Hg or higher) will benefit from oral nifedipine (10 mg every 6 hours) or long-acting nifedipine (10 to 20 mg twice daily), the drugs of choice because of their favorable effects on renal function.
Women with severe hypertension also may require diuretics for better control of blood pressure, as may women with a history of congestive heart failure or left ventricular dysfunction.
Start women with vascular diabetes mellitus or diabetic nephropathy on ACE inhibitors immediately postpartum.
Patients can be discharged home once blood pressure is stable, provided there are no maternal symptoms of preeclampsia.
Postpartum preeclampsia can develop even in healthy women
Because severe hypertension or preeclampsia may develop for the first time in the postpartum period, it is important to educate all gravidas about the signs and symptoms. All health-care providers should be on the lookout for these symptoms as well.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (England). 2001;(2)pCD002252.-
Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6-14.
Amorim MMR, Santas LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283-1288.
Duley L, Galmezoglu AM, Henderson-Smart DJ. Magnesium sulfate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev (England). 2003;(2)pCD000025.-
Friedman SA, Lubarsky S, Schiff E. Expectant management of severe preeclampsia remote from term. Clin Obstet Gynecol. 1999;42:470-478.
Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190:1590-1597.
Hall DR, Odendaal HJ, Kirten GF, Smith J. Expectant management of early onset, severe preeclampsia, perinatal outcome. BJOG. 2000;107:1258-1264.
Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilia. Hypertension in Pregnancy. 2001;20:35-44.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Magee LA, Cham C, Waterman EJ, Ohlsson A, Von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:1-10.
Magee LA, Ornstein MP, Von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
Magpie Trial Group. Do women with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised, placebo-controlled trial. Lancet. 2002;359:1877-1890.
Report of the National High Blood Pressure Education Program. Working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-22.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Sibai BM, Lindheimer MD, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;229:667-671.
Sibai BM. Magnesium sulfate prophylaxis in preeclampsia. Lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
Vigil-DeGracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe preeclampsia and preeclampsia superimposed on chronic hypertension between 24 and 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2003;107:24-27.
Once you decide to expectantly manage a patient with preeclampsia, the balancing act begins. That means weighing fetal benefits against maternal risks, since the only justification for expectant management is to prolong pregnancy for fetal gain—there is no advantage to the mother.
The best approach is to classify the woman’s preeclampsia by the degree of severity and gestational age at the time of diagnosis, then follow recommendations tailored to that particular category.
This article offers guidelines for expectant management of mild and severe preeclampsia, preeclampsia superimposed on a preexisting medical condition, and intrapartum and postpartum care.
Mild preeclampsia
The earlier preeclampsia develops, the greater the risk it will become severe. The need for hospitalization depends on gestational age, blood pressure, proteinuria levels, maternal symptoms, and reliability of the patient.
Preeclampsia is mild when systolic blood pressure reaches 140 to 159 mm Hg or diastolic pressure measures 90 to 109 mm Hg on at least 2 occasions more than 6 hours apart after 20 weeks’ gestation in a woman who previously had normal blood pressure. In preeclampsia, this hypertension is accompanied by proteinuria of 0.3 to 4.9 g in a 24-hour urine sample (1+ or 2+ by dipstick on 2 occasions).
At or beyond 37 weeks’ gestation
In general, women diagnosed with preeclampsia at this gestational age have pregnancy outcomes similar to those of normotensive gravidas. Thus, they benefit from induction of labor and delivery.
32 to 36 weeks’ gestation
Close maternal and fetal evaluation is essential. (It is assumed these women have no labor or membrane rupture and normal fetal testing; otherwise, delivery is indicated at 34 weeks or beyond.)
In general, hospitalization is indicated when any of the following circumstances are present (FIGURE 1):
- the patient is unreliable,
- 2 or more systolic blood pressure readings exceed 150 mm Hg,
- 2 or more diastolic blood pressure readings exceed 100 mm Hg,
- proteinuria occurs at a rate exceeding 1 g/24 hours, or
- persistent maternal symptoms are present.
FIGURE 1 Treatment of mild preeclampsia in healthy women
Before 32 weeks’ gestation
These women are at high risk of progressing to severe disease. They also are more likely to have adverse perinatal outcomes such as intrauterine growth restriction (IUGR) (15% to 20%), preterm delivery (50%), and abruptio placentae (1% to 2%), compared with women diagnosed with preeclampsia at 32 to 36 weeks. In addition, they require more antenatal surveillance than women who develop preeclampsia later in pregnancy.
I recommend hospitalization at the time of diagnosis when women develop mild preeclampsia before 32 weeks.
What and when to monitor
Maternal evaluation should include:
- monitoring of blood pressure at least daily (at home or in the hospital),
- daily urine dipstick evaluation to monitor changes in proteinuria,
- twice-weekly platelet count and liver enzymes, and
- documentation of symptoms. (Instruct all women to report the onset of severe headaches, visual changes, altered mental status, epigastric or right upper quadrant pain, and any nausea or vomiting.)
Fetal evaluation should include:
- serial ultrasound every 3 weeks to estimate fetal weight and amniotic fluid status,
- nonstress testing every week, and
- daily fetal movement counts.
If a nonstress test is nonreactive, it should be confirmed by biophysical profile.
All testing should be promptly repeated if the maternal clinical condition deteriorates.
No need for bed rest, diuretics, or antihypertensive medications
Although expectantly managed patients with mild preeclampsia should be advised to restrict daily activity, there is no need for complete bed rest. Nor have diuretics or other antihypertensive drugs been shown to prolong gestation. On the contrary, these medications may mask severe preeclampsia.
Antihypertensive medications reduce the rate of severe hypertension but do not improve perinatal outcome. If these drugs are used to treat mild disease remote from term, hospitalize the patient and manage her as though she has severe preeclampsia.
Hospitalization versus outpatient management
Although she may be hospitalized at the time of diagnosis, a woman with preeclampsia may switch to outpatient management if systolic or diastolic blood pressure declines, proteinuria diminishes to 1 g/24 hours or less, and there are no maternal symptoms or evidence of severe IUGR. Otherwise, these women should remain hospitalized until delivery.
In cases that begin with outpatient management, prompt hospitalization is indicated if there is clinical evidence that the disease is progressing (ie, new symptoms, labor or rupture of membranes, vaginal bleeding, or increased blood pressures or proteinuria) or IUGR and/or oligohydramnios.
Instruct all women to report symptoms and changes in fetal movement.
When to deliver
Whether the gravida is hospitalized or an outpatient, delivery is indicated at 37 weeks. Earlier delivery may be warranted if nonreassuring maternal or fetal conditions develop. (FIGURE 1 summarizes management of mild preeclampsia.)
Severe preeclampsia
Expectant management is safe in properly selected women with severe disease, although maternal and fetal conditions can deteriorate. Hospitalization and daily monitoring are required.
Preeclampsia is severe when any of the following are present:
- systolic blood pressure of 160 mm Hg or higher or diastolic pressure of 110 mm Hg or above on 2 occasions at least 6 hours apart while the patient is on bed rest
- proteinuria of 5 g or more in a 24-hour urine specimen,
- oliguria of less than 500 mL in 24 hours,
- cerebral or visual disturbances,
- pulmonary edema or cyanosis,
- severe epigastric or right upper-quadrant pain, or
- thrombocytopenia.
When gestational hypertension or preeclampsia is severe, hospitalization in the labor and delivery suite is warranted. These women should receive intravenous (IV) magnesium sulfate to reduce the risk of convulsions and antihypertensive drugs to treat severe levels of hypertension, if present. The aim of antihypertensive treatment is to keep diastolic blood pressure between 90 and 105 mm Hg and systolic blood pressure below 160 mm Hg.
During observation, assess maternal and fetal conditions and decide whether delivery is indicated (FIGURE 2).
Expectant management is warranted only for gestations between 23 and 32 weeks’ gestation, provided maternal and fetal conditions are stable (FIGURE 2).
Keep in mind that both maternal and fetal conditions may progressively deteriorate. Thus, these pregnancies involve higher rates of maternal morbidity and significant risk of neonatal morbidity. For this reason, expectant management should proceed only in a tertiary-care center with adequate maternal and neonatal facilities.
Recommended counseling
Advise these patients of the potential risks and benefits of expectant management, which requires daily monitoring of maternal and fetal conditions. Also explain that the decision to continue expectant management will be revisited on a daily basis and that the median number of days pregnancy is prolonged in these cases is 7 (range 2 to 35).
Another important fact to relay: Only 2 randomized trials involving 133 women have compared expectant management to aggressive management in early-onset preeclampsia. However, retrospective and observational studies involving more than 700 women suggest expectant management reduces short-term neonatal morbidity with minimal risk to the mother.
Superimposed preeclampsia
Women who develop preeclampsia on top of chronic hypertension, renal disease, or type 1 diabetes have a markedly higher risk of morbidity, including perinatal morbidity, than women without preexisting conditions.
Women with superimposed preeclampsia may be managed in the hospital, since these pregnancies are associated with higher rates of abruptio placentae (2% to 5%), preterm delivery (56%), IUGR (13% to 15%), and perinatal death (8%). Thus, these women benefit from very close maternal and fetal monitoring.
Superimposed preeclampsia is not classified according to severity.
In general, maternal and perinatal morbidities are substantially higher in women who have preexisting conditions than in healthy women who develop preeclampsia.
Chronic hypertension
Indications for delivery are similar to those described for healthy women with preeclampsia, as is antihypertensive therapy.
If the woman develops preeclampsia while using antihypertensive drugs, delivery should be considered beyond 34 weeks’ gestation.
How preeclampsia affects renal function
Women with renal disease or dysfunction (serum creatinine ≥1.2 mg/dL) prior to or early in pregnancy face an increased risk of adverse neonatal outcomes, regardless of whether preeclampsia also develops. These women also face an increased risk of deteriorating renal function during pregnancy (particularly if preeclampsia or severe hypertension develops) and beyond (more than 6 months postpartum).
Start antihypertensive medications as soon as possible, with the goal of keeping systolic blood pressure below 140 mm Hg and diastolic blood pressure below 90 mm Hg throughout gestation.
Delivery is indicated with the onset of preeclampsia or significant deterioration in renal function.
Diabetes warrants aggressive therapy
Women with type 1 diabetes have a higher risk of preeclampsia, maternal and fetal morbidity, and perinatal mortality. These risks multiply in women who have hypertension and/or diabetic nephropathy. Worsening of retinopathy and nephropathy also is more likely in women who have hypertension. Thus, aggressive management of blood sugars with insulin should be accompanied by aggressive control of blood pressure, with the goal of keeping systolic pressure below 130 mm Hg and diastolic pressure below 85 mm Hg.
Choosing antihypertensive drugs. Calcium-channel blockers are preferred to control blood pressure during pregnancy in women with diabetes. Outside of pregnancy, angiotensin-converting enzyme (ACE) inhibitors are best to avert long-term complications, but avoid these drugs in pregnancy (along with angiotensin-receptor blockers), particularly beyond 16 weeks.
Delivery is indicated in all women with vascular diabetes mellitus beyond 34 weeks when preeclampsia is present.
Intrapartum management
Close fetal heart rate and maternal blood pressure monitoring are mainstays, along with magnesium sulfate and antihypertensive therapy.
All women with preeclampsia should receive continuous monitoring of fetal heart rate and uterine activity, with special vigilance for hyperstimulation and onset of vaginal bleeding during labor. (For a description of potential maternal complications, see TABLE 1; fetal complications are described in FIGURE 3.)
Uterine irritability, recurrent variable or late decelerations, and the development of vaginal bleeding may be the first signs of abruptio placentae.
I recommend recording maternal blood pressure at least hourly to detect progression from mild to severe hypertension and to determine the need for antihypertensive therapy.
TABLE 1
Likelihood of maternal complications
| Disease progresses during labor (from mild to severe) | 10% |
| Eclampsia | |
| • Mild disease | <0.5% |
| • Severe preeclampsia | 1–2% |
| Stroke (encephalopathy or hemorrhage) | <1% |
| Mainly with severe or early onset disease | |
| Pulmonary edema | 1–2% |
| Usually associated with fluid overload or long-standing chronic hypertension | |
Prevent progression to eclampsia
Magnesium sulfate is the drug of choice in women with preeclampsia. Recent reviews indicate that it reduces the rate of convulsions from 2% to 0.6% in women with severe preeclampsia. In women with mild preeclampsia, the benefit of magnesium sulfate remains unclear.
I recommend IV magnesium sulfate during labor and postpartum when a woman has the indications listed in TABLE 2.
The dose of magnesium sulfate is 6 g IV loading over 20 minutes, followed by a maintenance dose of 2 g/hour.
Magnesium sulfate should be started before surgery (elective cesarean delivery) and continued for at least 12 hours postpartum (I prefer 24 hours).
TABLE 2
When to give prophylactic magnesium sulfate
| Use intrapartum and for at least 12 hours postpartum |
|---|
When the patient has:
|
When treating hypertension in labor, avoid “hypotensive overshoot”
The goal of intrapartum treatment is to lower maternal blood pressure without causing precipitous hypotensive overshoot that may lead to reduced maternal organ perfusion, particularly uteroplacental blood flow. Such acute lowering of maternal blood pressure is a common cause of nonreassuring fetal heart rate patterns during labor.
What blood pressure necessitates treatment? There is no doubt that severe levels of hypertension should be treated to avoid potential cerebrovascular and cardiovascular complications in healthy women. However, there is disagreement about what constitutes severe hypertension.
In previously healthy women, I recommend antihypertensive therapy for systolic pressures of 170 mm Hg or above and/or for diastolic pressures of 110 mm Hg or above.
For women with thrombocytopenia, disseminated intravascular coagulation, or pulmonary edema, I recommend treatment for systolic pressures of 160 mm Hg or above and diastolic pressures of 105 mm Hg or above. This latter group should also be given IV furosemide (20 to 40 mg) to promote diuresis. I also recommend treatment at these levels in the postpartum period.
For women with diabetes, renal disease, or left ventricular cardiac disease, antihypertensive medications should be used to keep systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg during labor and postpartum. Further, patients in congestive heart failure or with left ventricular diastolic dysfunction should receive furosemide in addition to antihypertensive drugs.
Choosing a drug. My drugs of choice are IV labetalol and oral nifedipine. These 2 drugs, along with IV hydralazine, are the most commonly recommended medications for severe hypertension in pregnancy (TABLE 3).
Although many authorities prefer hydralazine, recent data indicate that, compared with IV labetalol and oral nifedipine, IV hydralazine is associated with more maternal side effects and worse perinatal outcomes (more fetal distress in labor).
TABLE 3
Drug profiles: Dosing and side effects of antihypertensives used in pregnancy
| MEDICATION | ONSET OF ACTION | DOSE | SIDE EFFECTS |
|---|---|---|---|
| Hydralazine | 10-20 minutes | 5-10 mg intravenously every 20 minutes up to maximum dose of 30 mg | More maternal side effects and worse perinatal outcomes than labetalol or nifedipine. |
| Skin blisters; chest pain; general feeling of discomfort, illness, or weakness; joint or muscle pain; sore throat and fever; swollen lymph glands | |||
| Labetalol* | 10-15 minutes | 10-20 mg intravenously, then 40-80 mg every 10 minutes up to maximum dose of 220 mg/hour or continuous infusion of 1-2 mg/minute | Breathing difficulty and/or wheezing, cold hands and feet, mental depression, shortness of breath, slow heartbeat, swelling of lower extremities, back or joint pain, chest pain, confusion, fever and sore throat, hallucinations, irregular heartbeat, unusual bleeding and bruising, yellow eyes or skin |
| Nifedipine | 5-10 minutes | 10-20 mg orally, repeated in 30 minutes, up to maximum dose of 50 mg/hour | Breathing difficulty, coughing, or wheezing; irregular or fast, poundingheartbeat; skin rash; swelling of lower extremities; chest pain; fainting; painful, swollen joints; vision impairment |
| Sodium nitroprusside† | 0.5-5 minutes | 0.25-5 μg/kg/minute by intravenous infusion | Risk of fetal cyanide poisoning with prolonged treatment. |
| Maternal effects include symptoms of hypothyroidism, headache, abdominal pain, drowsiness, nausea, involuntary muscle movements, perspiration, restlessness, paraesthesia, palpitations, dizziness, retching, tachycardia | |||
| *In women with asthma and congestive heart failure | |||
| †Rarely needed except in hypertensive encephalopathy or cerebral hemorrhage | |||
Postpartum management
Because preeclampsia can worsen, or first appear, in the postpartum period, extra vigilance is important, and pharmacotherapy may be appropriate.
Management of preeclampsia does not end with delivery of the fetus and the placenta. These events do signal the beginning of the curative process, but complications can occur in the postpartum period. Indeed, in some women, the disease process worsens immediately postpartum. Therefore, women with diagnosed preeclampsia or severe gestational hypertension require close monitoring of blood pressure and maternal symptoms and accurate measurement of fluid intake and urine output. Some of these women are at increased risk for pulmonary edema; exacerbation of severe hypertension; eclampsia; and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.
Treating postpartum hypertension
Women who continue to have severe hypertension (systolic pressure at or above 155 mm Hg or diastolic pressure of 105 mm Hg or higher) will benefit from oral nifedipine (10 mg every 6 hours) or long-acting nifedipine (10 to 20 mg twice daily), the drugs of choice because of their favorable effects on renal function.
Women with severe hypertension also may require diuretics for better control of blood pressure, as may women with a history of congestive heart failure or left ventricular dysfunction.
Start women with vascular diabetes mellitus or diabetic nephropathy on ACE inhibitors immediately postpartum.
Patients can be discharged home once blood pressure is stable, provided there are no maternal symptoms of preeclampsia.
Postpartum preeclampsia can develop even in healthy women
Because severe hypertension or preeclampsia may develop for the first time in the postpartum period, it is important to educate all gravidas about the signs and symptoms. All health-care providers should be on the lookout for these symptoms as well.
The author reports no financial relationships relevant to this article.
Once you decide to expectantly manage a patient with preeclampsia, the balancing act begins. That means weighing fetal benefits against maternal risks, since the only justification for expectant management is to prolong pregnancy for fetal gain—there is no advantage to the mother.
The best approach is to classify the woman’s preeclampsia by the degree of severity and gestational age at the time of diagnosis, then follow recommendations tailored to that particular category.
This article offers guidelines for expectant management of mild and severe preeclampsia, preeclampsia superimposed on a preexisting medical condition, and intrapartum and postpartum care.
Mild preeclampsia
The earlier preeclampsia develops, the greater the risk it will become severe. The need for hospitalization depends on gestational age, blood pressure, proteinuria levels, maternal symptoms, and reliability of the patient.
Preeclampsia is mild when systolic blood pressure reaches 140 to 159 mm Hg or diastolic pressure measures 90 to 109 mm Hg on at least 2 occasions more than 6 hours apart after 20 weeks’ gestation in a woman who previously had normal blood pressure. In preeclampsia, this hypertension is accompanied by proteinuria of 0.3 to 4.9 g in a 24-hour urine sample (1+ or 2+ by dipstick on 2 occasions).
At or beyond 37 weeks’ gestation
In general, women diagnosed with preeclampsia at this gestational age have pregnancy outcomes similar to those of normotensive gravidas. Thus, they benefit from induction of labor and delivery.
32 to 36 weeks’ gestation
Close maternal and fetal evaluation is essential. (It is assumed these women have no labor or membrane rupture and normal fetal testing; otherwise, delivery is indicated at 34 weeks or beyond.)
In general, hospitalization is indicated when any of the following circumstances are present (FIGURE 1):
- the patient is unreliable,
- 2 or more systolic blood pressure readings exceed 150 mm Hg,
- 2 or more diastolic blood pressure readings exceed 100 mm Hg,
- proteinuria occurs at a rate exceeding 1 g/24 hours, or
- persistent maternal symptoms are present.
FIGURE 1 Treatment of mild preeclampsia in healthy women
Before 32 weeks’ gestation
These women are at high risk of progressing to severe disease. They also are more likely to have adverse perinatal outcomes such as intrauterine growth restriction (IUGR) (15% to 20%), preterm delivery (50%), and abruptio placentae (1% to 2%), compared with women diagnosed with preeclampsia at 32 to 36 weeks. In addition, they require more antenatal surveillance than women who develop preeclampsia later in pregnancy.
I recommend hospitalization at the time of diagnosis when women develop mild preeclampsia before 32 weeks.
What and when to monitor
Maternal evaluation should include:
- monitoring of blood pressure at least daily (at home or in the hospital),
- daily urine dipstick evaluation to monitor changes in proteinuria,
- twice-weekly platelet count and liver enzymes, and
- documentation of symptoms. (Instruct all women to report the onset of severe headaches, visual changes, altered mental status, epigastric or right upper quadrant pain, and any nausea or vomiting.)
Fetal evaluation should include:
- serial ultrasound every 3 weeks to estimate fetal weight and amniotic fluid status,
- nonstress testing every week, and
- daily fetal movement counts.
If a nonstress test is nonreactive, it should be confirmed by biophysical profile.
All testing should be promptly repeated if the maternal clinical condition deteriorates.
No need for bed rest, diuretics, or antihypertensive medications
Although expectantly managed patients with mild preeclampsia should be advised to restrict daily activity, there is no need for complete bed rest. Nor have diuretics or other antihypertensive drugs been shown to prolong gestation. On the contrary, these medications may mask severe preeclampsia.
Antihypertensive medications reduce the rate of severe hypertension but do not improve perinatal outcome. If these drugs are used to treat mild disease remote from term, hospitalize the patient and manage her as though she has severe preeclampsia.
Hospitalization versus outpatient management
Although she may be hospitalized at the time of diagnosis, a woman with preeclampsia may switch to outpatient management if systolic or diastolic blood pressure declines, proteinuria diminishes to 1 g/24 hours or less, and there are no maternal symptoms or evidence of severe IUGR. Otherwise, these women should remain hospitalized until delivery.
In cases that begin with outpatient management, prompt hospitalization is indicated if there is clinical evidence that the disease is progressing (ie, new symptoms, labor or rupture of membranes, vaginal bleeding, or increased blood pressures or proteinuria) or IUGR and/or oligohydramnios.
Instruct all women to report symptoms and changes in fetal movement.
When to deliver
Whether the gravida is hospitalized or an outpatient, delivery is indicated at 37 weeks. Earlier delivery may be warranted if nonreassuring maternal or fetal conditions develop. (FIGURE 1 summarizes management of mild preeclampsia.)
Severe preeclampsia
Expectant management is safe in properly selected women with severe disease, although maternal and fetal conditions can deteriorate. Hospitalization and daily monitoring are required.
Preeclampsia is severe when any of the following are present:
- systolic blood pressure of 160 mm Hg or higher or diastolic pressure of 110 mm Hg or above on 2 occasions at least 6 hours apart while the patient is on bed rest
- proteinuria of 5 g or more in a 24-hour urine specimen,
- oliguria of less than 500 mL in 24 hours,
- cerebral or visual disturbances,
- pulmonary edema or cyanosis,
- severe epigastric or right upper-quadrant pain, or
- thrombocytopenia.
When gestational hypertension or preeclampsia is severe, hospitalization in the labor and delivery suite is warranted. These women should receive intravenous (IV) magnesium sulfate to reduce the risk of convulsions and antihypertensive drugs to treat severe levels of hypertension, if present. The aim of antihypertensive treatment is to keep diastolic blood pressure between 90 and 105 mm Hg and systolic blood pressure below 160 mm Hg.
During observation, assess maternal and fetal conditions and decide whether delivery is indicated (FIGURE 2).
Expectant management is warranted only for gestations between 23 and 32 weeks’ gestation, provided maternal and fetal conditions are stable (FIGURE 2).
Keep in mind that both maternal and fetal conditions may progressively deteriorate. Thus, these pregnancies involve higher rates of maternal morbidity and significant risk of neonatal morbidity. For this reason, expectant management should proceed only in a tertiary-care center with adequate maternal and neonatal facilities.
Recommended counseling
Advise these patients of the potential risks and benefits of expectant management, which requires daily monitoring of maternal and fetal conditions. Also explain that the decision to continue expectant management will be revisited on a daily basis and that the median number of days pregnancy is prolonged in these cases is 7 (range 2 to 35).
Another important fact to relay: Only 2 randomized trials involving 133 women have compared expectant management to aggressive management in early-onset preeclampsia. However, retrospective and observational studies involving more than 700 women suggest expectant management reduces short-term neonatal morbidity with minimal risk to the mother.
Superimposed preeclampsia
Women who develop preeclampsia on top of chronic hypertension, renal disease, or type 1 diabetes have a markedly higher risk of morbidity, including perinatal morbidity, than women without preexisting conditions.
Women with superimposed preeclampsia may be managed in the hospital, since these pregnancies are associated with higher rates of abruptio placentae (2% to 5%), preterm delivery (56%), IUGR (13% to 15%), and perinatal death (8%). Thus, these women benefit from very close maternal and fetal monitoring.
Superimposed preeclampsia is not classified according to severity.
In general, maternal and perinatal morbidities are substantially higher in women who have preexisting conditions than in healthy women who develop preeclampsia.
Chronic hypertension
Indications for delivery are similar to those described for healthy women with preeclampsia, as is antihypertensive therapy.
If the woman develops preeclampsia while using antihypertensive drugs, delivery should be considered beyond 34 weeks’ gestation.
How preeclampsia affects renal function
Women with renal disease or dysfunction (serum creatinine ≥1.2 mg/dL) prior to or early in pregnancy face an increased risk of adverse neonatal outcomes, regardless of whether preeclampsia also develops. These women also face an increased risk of deteriorating renal function during pregnancy (particularly if preeclampsia or severe hypertension develops) and beyond (more than 6 months postpartum).
Start antihypertensive medications as soon as possible, with the goal of keeping systolic blood pressure below 140 mm Hg and diastolic blood pressure below 90 mm Hg throughout gestation.
Delivery is indicated with the onset of preeclampsia or significant deterioration in renal function.
Diabetes warrants aggressive therapy
Women with type 1 diabetes have a higher risk of preeclampsia, maternal and fetal morbidity, and perinatal mortality. These risks multiply in women who have hypertension and/or diabetic nephropathy. Worsening of retinopathy and nephropathy also is more likely in women who have hypertension. Thus, aggressive management of blood sugars with insulin should be accompanied by aggressive control of blood pressure, with the goal of keeping systolic pressure below 130 mm Hg and diastolic pressure below 85 mm Hg.
Choosing antihypertensive drugs. Calcium-channel blockers are preferred to control blood pressure during pregnancy in women with diabetes. Outside of pregnancy, angiotensin-converting enzyme (ACE) inhibitors are best to avert long-term complications, but avoid these drugs in pregnancy (along with angiotensin-receptor blockers), particularly beyond 16 weeks.
Delivery is indicated in all women with vascular diabetes mellitus beyond 34 weeks when preeclampsia is present.
Intrapartum management
Close fetal heart rate and maternal blood pressure monitoring are mainstays, along with magnesium sulfate and antihypertensive therapy.
All women with preeclampsia should receive continuous monitoring of fetal heart rate and uterine activity, with special vigilance for hyperstimulation and onset of vaginal bleeding during labor. (For a description of potential maternal complications, see TABLE 1; fetal complications are described in FIGURE 3.)
Uterine irritability, recurrent variable or late decelerations, and the development of vaginal bleeding may be the first signs of abruptio placentae.
I recommend recording maternal blood pressure at least hourly to detect progression from mild to severe hypertension and to determine the need for antihypertensive therapy.
TABLE 1
Likelihood of maternal complications
| Disease progresses during labor (from mild to severe) | 10% |
| Eclampsia | |
| • Mild disease | <0.5% |
| • Severe preeclampsia | 1–2% |
| Stroke (encephalopathy or hemorrhage) | <1% |
| Mainly with severe or early onset disease | |
| Pulmonary edema | 1–2% |
| Usually associated with fluid overload or long-standing chronic hypertension | |
Prevent progression to eclampsia
Magnesium sulfate is the drug of choice in women with preeclampsia. Recent reviews indicate that it reduces the rate of convulsions from 2% to 0.6% in women with severe preeclampsia. In women with mild preeclampsia, the benefit of magnesium sulfate remains unclear.
I recommend IV magnesium sulfate during labor and postpartum when a woman has the indications listed in TABLE 2.
The dose of magnesium sulfate is 6 g IV loading over 20 minutes, followed by a maintenance dose of 2 g/hour.
Magnesium sulfate should be started before surgery (elective cesarean delivery) and continued for at least 12 hours postpartum (I prefer 24 hours).
TABLE 2
When to give prophylactic magnesium sulfate
| Use intrapartum and for at least 12 hours postpartum |
|---|
When the patient has:
|
When treating hypertension in labor, avoid “hypotensive overshoot”
The goal of intrapartum treatment is to lower maternal blood pressure without causing precipitous hypotensive overshoot that may lead to reduced maternal organ perfusion, particularly uteroplacental blood flow. Such acute lowering of maternal blood pressure is a common cause of nonreassuring fetal heart rate patterns during labor.
What blood pressure necessitates treatment? There is no doubt that severe levels of hypertension should be treated to avoid potential cerebrovascular and cardiovascular complications in healthy women. However, there is disagreement about what constitutes severe hypertension.
In previously healthy women, I recommend antihypertensive therapy for systolic pressures of 170 mm Hg or above and/or for diastolic pressures of 110 mm Hg or above.
For women with thrombocytopenia, disseminated intravascular coagulation, or pulmonary edema, I recommend treatment for systolic pressures of 160 mm Hg or above and diastolic pressures of 105 mm Hg or above. This latter group should also be given IV furosemide (20 to 40 mg) to promote diuresis. I also recommend treatment at these levels in the postpartum period.
For women with diabetes, renal disease, or left ventricular cardiac disease, antihypertensive medications should be used to keep systolic pressure below 140 mm Hg and diastolic pressure below 90 mm Hg during labor and postpartum. Further, patients in congestive heart failure or with left ventricular diastolic dysfunction should receive furosemide in addition to antihypertensive drugs.
Choosing a drug. My drugs of choice are IV labetalol and oral nifedipine. These 2 drugs, along with IV hydralazine, are the most commonly recommended medications for severe hypertension in pregnancy (TABLE 3).
Although many authorities prefer hydralazine, recent data indicate that, compared with IV labetalol and oral nifedipine, IV hydralazine is associated with more maternal side effects and worse perinatal outcomes (more fetal distress in labor).
TABLE 3
Drug profiles: Dosing and side effects of antihypertensives used in pregnancy
| MEDICATION | ONSET OF ACTION | DOSE | SIDE EFFECTS |
|---|---|---|---|
| Hydralazine | 10-20 minutes | 5-10 mg intravenously every 20 minutes up to maximum dose of 30 mg | More maternal side effects and worse perinatal outcomes than labetalol or nifedipine. |
| Skin blisters; chest pain; general feeling of discomfort, illness, or weakness; joint or muscle pain; sore throat and fever; swollen lymph glands | |||
| Labetalol* | 10-15 minutes | 10-20 mg intravenously, then 40-80 mg every 10 minutes up to maximum dose of 220 mg/hour or continuous infusion of 1-2 mg/minute | Breathing difficulty and/or wheezing, cold hands and feet, mental depression, shortness of breath, slow heartbeat, swelling of lower extremities, back or joint pain, chest pain, confusion, fever and sore throat, hallucinations, irregular heartbeat, unusual bleeding and bruising, yellow eyes or skin |
| Nifedipine | 5-10 minutes | 10-20 mg orally, repeated in 30 minutes, up to maximum dose of 50 mg/hour | Breathing difficulty, coughing, or wheezing; irregular or fast, poundingheartbeat; skin rash; swelling of lower extremities; chest pain; fainting; painful, swollen joints; vision impairment |
| Sodium nitroprusside† | 0.5-5 minutes | 0.25-5 μg/kg/minute by intravenous infusion | Risk of fetal cyanide poisoning with prolonged treatment. |
| Maternal effects include symptoms of hypothyroidism, headache, abdominal pain, drowsiness, nausea, involuntary muscle movements, perspiration, restlessness, paraesthesia, palpitations, dizziness, retching, tachycardia | |||
| *In women with asthma and congestive heart failure | |||
| †Rarely needed except in hypertensive encephalopathy or cerebral hemorrhage | |||
Postpartum management
Because preeclampsia can worsen, or first appear, in the postpartum period, extra vigilance is important, and pharmacotherapy may be appropriate.
Management of preeclampsia does not end with delivery of the fetus and the placenta. These events do signal the beginning of the curative process, but complications can occur in the postpartum period. Indeed, in some women, the disease process worsens immediately postpartum. Therefore, women with diagnosed preeclampsia or severe gestational hypertension require close monitoring of blood pressure and maternal symptoms and accurate measurement of fluid intake and urine output. Some of these women are at increased risk for pulmonary edema; exacerbation of severe hypertension; eclampsia; and hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome.
Treating postpartum hypertension
Women who continue to have severe hypertension (systolic pressure at or above 155 mm Hg or diastolic pressure of 105 mm Hg or higher) will benefit from oral nifedipine (10 mg every 6 hours) or long-acting nifedipine (10 to 20 mg twice daily), the drugs of choice because of their favorable effects on renal function.
Women with severe hypertension also may require diuretics for better control of blood pressure, as may women with a history of congestive heart failure or left ventricular dysfunction.
Start women with vascular diabetes mellitus or diabetic nephropathy on ACE inhibitors immediately postpartum.
Patients can be discharged home once blood pressure is stable, provided there are no maternal symptoms of preeclampsia.
Postpartum preeclampsia can develop even in healthy women
Because severe hypertension or preeclampsia may develop for the first time in the postpartum period, it is important to educate all gravidas about the signs and symptoms. All health-care providers should be on the lookout for these symptoms as well.
The author reports no financial relationships relevant to this article.
BIBLIOGRAPHY
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (England). 2001;(2)pCD002252.-
Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6-14.
Amorim MMR, Santas LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283-1288.
Duley L, Galmezoglu AM, Henderson-Smart DJ. Magnesium sulfate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev (England). 2003;(2)pCD000025.-
Friedman SA, Lubarsky S, Schiff E. Expectant management of severe preeclampsia remote from term. Clin Obstet Gynecol. 1999;42:470-478.
Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190:1590-1597.
Hall DR, Odendaal HJ, Kirten GF, Smith J. Expectant management of early onset, severe preeclampsia, perinatal outcome. BJOG. 2000;107:1258-1264.
Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilia. Hypertension in Pregnancy. 2001;20:35-44.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Magee LA, Cham C, Waterman EJ, Ohlsson A, Von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:1-10.
Magee LA, Ornstein MP, Von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
Magpie Trial Group. Do women with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised, placebo-controlled trial. Lancet. 2002;359:1877-1890.
Report of the National High Blood Pressure Education Program. Working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-22.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Sibai BM, Lindheimer MD, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;229:667-671.
Sibai BM. Magnesium sulfate prophylaxis in preeclampsia. Lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
Vigil-DeGracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe preeclampsia and preeclampsia superimposed on chronic hypertension between 24 and 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2003;107:24-27.
BIBLIOGRAPHY
Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (England). 2001;(2)pCD002252.-
Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6-14.
Amorim MMR, Santas LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. Am J Obstet Gynecol. 1999;180:1283-1288.
Duley L, Galmezoglu AM, Henderson-Smart DJ. Magnesium sulfate and other anticonvulsants for women with preeclampsia. Cochrane Database Syst Rev (England). 2003;(2)pCD000025.-
Friedman SA, Lubarsky S, Schiff E. Expectant management of severe preeclampsia remote from term. Clin Obstet Gynecol. 1999;42:470-478.
Haddad B, Deis S, Goffinet F, et al. Maternal and perinatal outcomes during expectant management of 239 severe preeclamptic women between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2004;190:1590-1597.
Hall DR, Odendaal HJ, Kirten GF, Smith J. Expectant management of early onset, severe preeclampsia, perinatal outcome. BJOG. 2000;107:1258-1264.
Kupferminc MJ, Fait G, Many A, et al. Low molecular weight heparin for the prevention of obstetric complications in women with thrombophilia. Hypertension in Pregnancy. 2001;20:35-44.
Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111-166.
Magee LA, Cham C, Waterman EJ, Ohlsson A, Von Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. BMJ. 2003;327:1-10.
Magee LA, Ornstein MP, Von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332-1336.
Magpie Trial Group. Do women with preeclampsia, and their babies, benefit from magnesium sulfate? The Magpie Trial: a randomised, placebo-controlled trial. Lancet. 2002;359:1877-1890.
Report of the National High Blood Pressure Education Program. Working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1-22.
Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369-377.
Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181-192.
Sibai BM, Lindheimer MD, Hauth J, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;229:667-671.
Sibai BM. Magnesium sulfate prophylaxis in preeclampsia. Lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
Vigil-DeGracia P, Montufar-Rueda C, Ruiz J. Expectant management of severe preeclampsia and preeclampsia superimposed on chronic hypertension between 24 and 34 weeks’ gestation. Eur J Obstet Gynecol Reprod Biol. 2003;107:24-27.