User login
Ectopic pregnancy: A 5-step plan for medical management
- In properly selected cases, medical therapy and surgery produce similar outcomes, but medicine is less expensive.
- Surgery is still the first choice for hemorrhage, medical failure, rupture or near-rupture, and when medical therapy is contraindicated.
- Systemic methotrexate and laparoscopic salpingostomy produce similar success rates and long-term fertility.
- Single-dose methotrexate is associated with a higher risk of rupture than multiple doses.
Although ectopic pregnancy remains a leading cause of life-threatening first-trimester morbidity, accounting for about 9% of maternal deaths annually,1 we now are able to diagnose and treat most cases well before rupture occurs—in some cases, as early as 5 weeks’ gestation. As a result, medical therapy with systemic methotrexate has become the first-line treatment, with surgery reserved for hemorrhage, medical failures, neglected cases, and circumstances in which medical therapy is contraindicated.
Early diagnosis not only makes medical therapy possible, it also is cheaper, since it avoids rupture, blood loss, and surgery; preserves fertility; and minimizes lost productivity. This is important because ectopic pregnancy is an expensive condition, with an annual health-care bill exceeding $1 billion.2
Despite this progress, serious challenges remain. Medical management is not for everyone. Success is inversely related to initial serum human chorionic gonadotropin (hCG) levels3 and diminishes substantially when embryonic cardiac activity is observed during ultrasound imaging.4
In addition, because medical therapy has made outpatient treatment the norm in most cases, it has become virtually impossible to chart the prevalence of ectopic pregnancy. In past years, when hospital records were used, ectopic pregnancy rates were increasing relentlessly, from 4.5 per 1,000 pregnancies in 1970 to 16.8 in 1989 and 19.7 (108,000 cases) in 1992.1,5
Reasons for increasing rates
Today the prevalence of ectopic pregnancy is probably still rising, for several reasons:
- a greater incidence of risk factors such as sexually transmitted and tubal disease,6
- improved diagnostic methods, and
- the use of assisted reproductive technology (ART) to treat infertility (roughly 2% of ART pregnancies are ectopic).7
This article describes a 5-step approach to diagnosis and medical management with multiple-dose methotrexate, as well as fine points of treatment and basic surgical technique. It includes a protocol for multiple-dose methotrexate, a table summarizing treatment outcomes, and several case histories.
Likelihood of ectopic pregnancy
If 100 women present with a positive pregnancy test and pain and bleeding, approximately 60 will have a normal pregnancy, 30 are experiencing spontaneous abortion, and 9 have an ectopic pregnancy.8
STEP 1Assess risk factors and symptoms
The first step in early diagnosis is being vigilant for risk factors and symptoms associated with ectopic pregnancy, most of which are well known9:
Tubal disease carries a 3.5-fold common adjusted odds ratio (OR) for ectopic pregnancy. In addition, women with a previous ectopic pregnancy are 6 to 8 times more likely to experience another, while a history of tubal surgery raises that likelihood to 21. A history of pelvic infection, including gonorrhea, serologically confirmed chlamydia, and pelvic inflammatory disease, increases the risk of ectopic pregnancy 2 to 4 times.9
Contraception. Intrauterine devices (IUDs) are associated with an increased OR of 6.4.10 This does not mean that IUDs cause ectopic pregnancy. Rather, when a woman with an IUD becomes pregnant, an ectopic gestation should be high on the list of possibilities. A similar relationship exists between ectopic pregnancy and tubal ligation, which carries an OR of 9.3.9 Oral contraceptives are associated with a reduced risk of ectopic pregnancy unless they are used as emergency contraception (ie, after fertilization), in which case they are associated with an increased risk (TABLE 1).
Diethylstilbestrol exposure in utero alters fallopian tube morphology and can lead to absent or minimal fimbrial tissue, a small tubal os, and decreased length and caliber of the tube.11 Abnormal tubal anatomy caused by this exposure multiplies the risk of ectopic pregnancy by a factor of 5.9
In vitro fertilization. When blocked tubes are treated, the embryos can migrate retrograde into the oviduct, implant, and eventually rupture. The OR for ectopic pregnancy with assisted reproduction is 4.0.8
Although a patient’s ß-hCG levels, symptoms, or imaging may suggest ectopic pregnancy, missed diagnoses abound, especially when the physician omits 1 element of the triad: ß-hCG levels, ultrasound imaging, and curettage.
CASE 1: Pain and bleeding, with a high hCG
Mrs. Jones presents with pain and bleeding and a ß-hCG level of 6,000 mIU/mL. Ultrasound imaging reveals no intrauterine pregnancy. Should you presume the diagnosis is ectopic pregnancy and start methotrexate therapy? Or should you play it safe and perform curettage?
To explore these questions, Barnhart and colleagues22 performed a retrospective cohort analysis involving women with ß-hCG levels above 2,000 mIU/mL and no ultrasound evidence of an intrauterine pregnancy. They found that, when the physician presumed a diagnosis of ectopic pregnancy on the basis of ultrasound and ß-hCG levels alone, the diagnosis was wrong in almost 40% of cases.
Where’s the harm in presumptive treatment?
Some practitioners argue that proceeding with methotrexate therapy under these circumstances causes no harm. However, presumptive treatment unnecessarily exposes women to the side effects of chemotherapy and artificially inflates methotrexate success rates. Presumptive treatment does not decrease overall side effects or save money. It also falsely labels a woman as having an ectopic pregnancy, which directly affects future diagnosis and prognosis.
For these reasons, always perform uterine curettage when ultrasound imaging is inconclusive and ß-hCG levels are below normal.
CASE 2: Pregnant and in pain, with an adnexal mass
A pregnant patient complaining of moderate pain has a 4-cm adnexal mass identified at ultrasound, with no evidence of an intrauterine gestational sac. What is her diagnosis?
It’s impossible to know based on the ultrasound alone—even though the ultrasonographer may diagnose ectopic pregnancy. Unless you interpret these findings in light of her ß-hCG levels, you have no way of knowing whether she is experiencing a normal gestation, spontaneous abortion, or ectopic pregnancy. In this case, the adnexal mass turned out to be a corpus luteum with hydrosalpinx, and the woman had a viable intrauterine pregnancy.
CASE 3: Intrauterine pregnancy and pain
A 28-year-old gravida 1 para 0 at 8 weeks’ gestation has a fetal heart rate of 160 following in vitro fertilization. She has a history of tubal disease and complains of severe left lower quadrant pain of sudden onset. Repeat ultrasound shows multiple bilateral ovarian cysts with a gestational sac and fetal heart rate of 144 in the left adnexa. How do you proceed?
Heterotopic pregnancy sometimes complicates in vitro fertilization and can be a difficult diagnosis when multiple cysts from superovulation obscure visualization of the adnexal implantation.
The best treatment is laparoscopic removal of the ectopic implantation. Methotrexate is contraindicated because of the possibility of injuring the viable intrauterine pregnancy.
Cigarette smoking increases the likelihood of ectopic pregnancy 2.5 times,12 probably by affecting ciliary action within the fallopian tubes.
Salpingitis isthmic nodosa is anatomic thickening of the proximal portion of the fallopian tubes with multiple lumen diverticula. It increases the risk of ectopic pregnancy 1.5 times, compared with age- and race-matched controls.13
Don’t depend solely on risk factors. Many ectopic pregnancies present without them.
Symptoms. Many ectopic pregnancies never produce symptoms; rather, they resolve spontaneously or are timely diagnosed and treated medically. Risk factors should therefore be examined in any woman in early pregnancy and investigated further if ectopic pregnancy is likely.
When symptoms do occur, they usually involve 1 or all of the classic triad: amenorrhea, irregular bleeding, and lower abdominal pain. In addition, syncope, shock, and pain radiating to the patient’s shoulder can result from hemoperitoneum.
TABLE 1
High, moderate, and low levels of risk factors for ectopic pregnancy
| RISK FACTOR | ODDS RATIO* |
|---|---|
| High risk | |
| Tubal surgery | 21.0 |
| Tubal ligation | 9.3 |
| Previous ectopic pregnancy | 8.3 |
| In utero exposure to diethylstilbestrol | 5.6 |
| Use of intrauterine device | 4.2–45.0 |
| Documented tubal pathology | 3.8–21.0 |
| Assisted reproduction | 4.0 |
| Emergency contraception | High |
| Moderate risk | |
| Infertility | 2.5–21.0 |
| Previous genital infections | 2.5–3.7 |
| Multiple sexual partners | 2.1 |
| Salpingitis (isthmic) | 1.5 |
| Slight risk | |
| Previous pelvic, abdominal surgery | 0.9–3.8 |
| Cigarette smoking | 2.3–2.5 |
| Vaginal douching | 1.1–3.1 |
| Early age at first intercourse (<18 years) | 1.6 |
| Reprinted with permission from Elsevier (The Lancet, 1998, vol 351, 1115–1120). | |
| * Single values = common odds ratio from homogeneous studies; point estimates = range of values from heterogeneous studies | |
STEP 2Document the pregnancy and measure ß-hCG
Once you identify the high-risk patient, or a woman comes in complaining of pain and spotting or bleeding, run a pregnancy test to confirm that she is pregnant and, if it is positive, obtain a quantitative ß-hCG.
ß-hCG levels are normally measured using enzyme-linked immunosorbent assays (ELISA), which detect ß-hCG in urine and serum at levels as low as 20 mIU/mL and 10 mIU/mL, respectively.14 ß-hCG is produced by trophoblastic cells in normal pregnancy, and approximately doubles every 2 days when titers are below 10,000 mIU/mL15—although in some normal pregnancies, ß-hCG may increase as slowly as 53% or as rapidly as 230% over 2 days.16 Eighty-five percent of abnormal pregnancies—whether intrauterine or ectopic—have impaired ß-hCG production with prolonged doubling time. Thus, in failing pregnancies, ß-hCG levels will plateau or fail to rise normally.
A single ß-hCG level fails to predict the risk of rupture, since ectopic pregnancies can rupture at ß-hCG levels as low as 10 mIU/mL or far exceeding 10,000 mIU/mL, or at any level in between.
STEP 3Obtain an ultrasound scan
Transvaginal ultrasound reliably detects normal intrauterine gestations when ß-hCG passes somewhere between 1,000 mIU/mL and 2,000 mIU/mL (First International Reference Preparation), depending on the expertise of the ultrasonographer and the particular equipment used.8,17 This is known as the “discriminatory zone.” ß-hCG levels reach this zone as early as 1 week after missed menses.18
The discriminatory zone is not the lowest ß-hCG concentration at which an intrauterine pregnancy can be visualized via ultrasound. Rather, it is the value at which any intrauterine pregnancy will be apparent. At that value, the absence of an intrauterine pregnancy confirms—by negative conclusion—that the patient has a nonviable gestation.
When intrauterine pregnancy is visualized. The diagnosis is definitive and the woman’s symptoms can be explained as “threatened abortion.” No further investigation is necessary aside from routine prenatal care if the pregnancy continues.
When an extrauterine gestation is observed, such as a gestational sac with a detectable fetal heart rate, ectopic pregnancy can be diagnosed with 100% specificity but low sensitivity (15% to 20%). A complex adnexal mass without an intrauterine pregnancy improves sensitivity from 21% to 84% at the expense of lower specificity (93% to 99.5%).19
Even when an adnexal mass is visualized, cardiac activity is not usually present. If cardiac activity is apparent, proceed to surgery, since methotrexate usually will not resolve these gestations.
Be aware that some adnexal masses suspicious for ectopic pregnancy may turn out to be other entities, such as a corpus luteum, hydrosalpinx, ovarian neoplasm, or endometrioma. Unless a fetal heart rate is detected by ultrasound, the diagnosis is uncertain and curettage is needed to establish a definitive diagnosis.
No intrauterine pregnancy, no extrauterine mass. Despite the high resolution of transvaginal ultrasound, many patients with ectopic pregnancy have no apparent adnexal mass,20 particularly when diagnosis is early. In these cases, proceed to curettage (step 4).
Don’t interpret ultrasound findings in a vacuum
This is especially unwise when ß-hCG levels are low—even when the ultrasound report points to intrauterine pregnancy. At ß-hCG levels below 1,500 mIU/mL, the sensitivity of ultrasound in diagnosing intrauterine pregnancy drops from 98% to 33% and predictive value is substantially lower. Interpret ultrasound and ß-hCG levels together for greater accuracy.
How size influences management
Ultrasound can detect ectopic pregnancies as small as 2 cm. In general, an ectopic sac size larger than 4 cm should be treated surgically.
STEP 4Perform uterine curettage
If ultrasound imaging is inconclusive and ß-hCG levels are plateauing or rising subnormally, perform uterine curettage. If ß-hCG levels decrease 15% or more 8 to 12 hours after the procedure, a complete abortion can be strongly suspected.21 If ß-hCG levels plateau or rise, the trophoblasts were not removed by curettage, and ectopic pregnancy is diagnosed.21
Keep in mind these important points:
- Without uterine curettage, roughly 40% of ectopic pregnancy diagnoses are incorrect.22
- Because curettage will result in termination of pregnancy, it is vital that it be limited to cases involving abnormal ß-hCG levels, not normally rising values.
STEP 5Administer methotrexate
Medical management is indicated when the following circumstances are present:
- No viable intrauterine pregnancy is present.
- No rupture has occurred.
- Any adnexal mass is 4 cm in size or smaller.
- ß-hCG levels are below 10,000 mIU/mL.
If there is a positive fetal heart beat, surgery is preferred.
Firm diagnosis of ectopic pregnancy is essential prior to methotrexate administration. If the drug is given to a woman carrying a viable pregnancy, it may result in loss of the pregnancy or methotrexate embryopathy.23
How methotrexate resolves ectopic pregnancy
Systemic methotrexate has been used successfully in the treatment of ectopic pregnancy for more than 20 years. A folic acid antagonist, it inhibits de novo synthesis of purines and pyrimidines. It thus interferes with DNA synthesis and cell multiplication.24 Actively proliferating trophoblasts are particularly vulnerable.25
When methotrexate is administered to normally pregnant women, it blunts the normal ß-hCG increment over the next 7 days. Circulating progesterone and 17-a-hydroxyprogesterone concentrations also decline, and abortion occurs.26
Methotrexate directly impairs trophoblastic production of hCG; the decrement in corpus luteum progesterone is a secondary event.
Side effects include abdominal distress, chills and fever, dizziness, immunosuppression, leukopenia, malaise, nausea, ulcerative stomatitis, photosensitivity, and undue fatigue.
Breastfeeding is an absolute contraindication to methotrexate, while relative contraindications include abnormal liver function tests, blood dyscrasias, excessive alcohol consumption, HIV/AIDS, psoriasis, ongoing radiotherapy, rheumatoid arthritis, and significant pulmonary disease.
Multiple-dose methotrexate is superior
In a recent meta-analysis comparing single-and multiple-dose regimens of methotrexate, Barnhart and colleagues4 found the latter to be more effective, although the single dose was more commonly given. The single-dose regimen was associated with a significantly greater chance of failure in both crude (OR 1.71; 1.04, 2.82) and adjusted (OR 4.74; 1.77, 12.62) analyses. Consequently, we advocate the multiple-dose regimen detailed in TABLE 2: 1 mg of methotrexate per kilogram of body weight on day 1, alternating with 0.1 mg of leucovorin per kilogram on succeeding days. Continue this regimen until ß-hCG levels decline in 2 consecutive daily titers, or 4 doses of methotrexate are given, whichever comes first.
If treatment is unsuccessful after 4 doses, additional methotrexate is unlikely to be effective and will involve significant additional cost and morbidity. If ß-hCG levels plateau or continue to rise, surgery is indicated.
Although multiple-dose therapy is more effective than a single dose, the optimal number of doses probably falls somewhere between 1 and 4.4 A 2-dose protocol currently under investigation may provide an optimal compromise.
Artificially high efficacy rates with “single-dose” therapy. Unusually high success rates in initial studies of the single-dose regimen may have been due to the inclusion of spontaneously aborting intrauterine pregnancies.27 Although 6 subsequent studies—1 cohort and 5 case-control studies involving 304 patients—found an overall success rate (no surgical intervention) of 87.2%, 11.5% of participants required more than 1 dose.9
Still the standard. Despite its lower efficacy rates, single-dose methotrexate remains the “standard” in the United States, as recommended in an American College of Obstetricians and Gynecologists practice bulletin.28 The usual intramuscular dose is 50 mg per square meter of body surface area, with ß-hCG titers on days 4 and 7 and an additional dose if ß-hCG levels fail to resolve.
What the evidence shows
Multidose methotrexate. Twelve studies measured the success of multiple-dose systemic methotrexate (TABLE 3); they included 1 randomized, controlled trial, 1 cohort study, and 10 case series. Between 1982 and 1997, 325 cases were treated with multiple-dose methotrexate. Of these, 93.8% had successful resolution with no subsequent therapy, and 78.9% of the 161 women tested had patent oviducts. In addition, of 95 women hoping to conceive, 57.9% had a subsequent intrauterine pregnancy and 7.4% developed a recurrent ectopic pregnancy. These rates compared favorably with numerous laparoscopic surgery series published during the same years.9
Methotrexate versus laparoscopic salpingostomy. In 1 randomized clinical comparison,29 100 women with laparoscopy-confirmed ectopic pregnancy were randomized to methotrexate or laparoscopic salpingostomy. Of the 51 patients treated medically, 7 (14%) required surgical intervention for active bleeding and/or tubal rupture. An additional course of methotrexate was required in 2 patients (4%) for persistent trophoblasts.
Of the 49 patients in the salpingostomy group, 4 women (8%) failed therapy and required salpingectomies, and 10 patients (20%) were treated with methotrexate for persistent trophoblasts.
Tubal patency was present in 23 of 42 women (55%) in the methotrexate group, compared with 23 of 39 (59%) in the salpingostomy group.
Overall, this randomized study29 and previous meta-analysis demonstrate that systemic multiple-dose methotrexate is comparable in efficacy to laparoscopic salpingostomy.
TABLE 2
Multiple-dose methotrexate protocol
Discontinue treatment when there is a decline in 2 consecutive ß-hCG titers or after 4 doses, whichever comes first.
| DAY | INTERVENTION | DOSE (MG) |
|---|---|---|
| 1 | Baseline studies ß-hCG titer, CBC, and platelets Methotrexate | 1.0 |
| 2 | Leucovori | 0.1 |
| 3 | Methotrexate | 1.0 |
| 4 | Leucovorin ß-hCG titer | 0.1 |
| 5 | Methotrexate ß-hCG titer | 1.0 |
| 6 | Leucovorin ß-hCG titer | 0.1 |
| 7 | Methotrexate ß-hCG titer | 1.0 |
| 8 | Leucovorin ß-hCG titer CBC and platelets Renal and liver function tests | 0.1 |
| Weekly | ß-hCG titer until negative |
TABLE 3
Treatment outcomes for ectopic pregnancy
| METHOD | NUMBER OF STUDIES | NUMBER OF PATIENTS | NUMBER WITH SUCCESSFUL RESOLUTION | TUBAL PATENCY RATE | SUBSEQUENT FERTILITY RATE | |
|---|---|---|---|---|---|---|
| INTRAUTERINE PREGNANCY | ECTOPIC PREGNANCY | |||||
| Conservative laparoscopic surgery | 32 | 1,626 | 1,516 (93%) | 170/223 (76%) | 366/647 (57%) | 87/647 (13%) |
| Variable-dose methotrexate | 12 | 338 | 314 (93%) | 136/182 (75%) | 55/95 (58%) | 7/95 (7%) |
| Single-dose methotrexate | 7 | 393 | 340 (87%) | 61/75 (81%) | 39/64 (61%) | 5/64 (8%) |
| Direct-injection methotrexate | 21 | 660 | 502 (76%) | 130/162 (80%) | 87/152 (57%) | 9/152 (6%) |
| Expectant management | 14 | 628 | 425 (68%) | 60/79 (76%) | 12/14 (86%) | 1/14 (7%) |
| Reprinted with permission from Elsevier (The Lancet, 1998, vol 351, 1115–1120). | ||||||
Fine points of treatment
During methotrexate therapy, examine the patient only once to avoid triggering a rupture, and counsel her to avoid intercourse for the same reason.30 Do not perform repeat vaginal ultrasound examination. Also inform her that transient pain (“separation pain”) from tubal abortion frequently occurs 3 to 7 days after the start of therapy, lasts 4 to 12 hours, and then resolves.31 This is perhaps the most difficult aspect of methotrexate therapy, as it is not always easy to differentiate the pain of tubal abortion from the pain of rupture.
If ß-hCG titers continue to rise rapidly between methotrexate doses, rupture is more likely and surgery should proceed.32
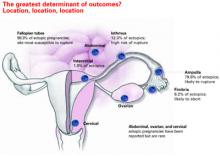
Overall, isthmic ectopic pregnancies (12.3% of ectopics) appear to be at a particularly high risk for rupture and comprise nearly half of methotrexate failures.32 Unfortunately, there is no way to identify isthmic pregnancies without surgery.
Avoid NSAIDs and GI-“unfriendly” foods. Counsel the patient to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) because they may impair natural hemostasis. Gasforming foods such as leeks, corn, and cabbage can cause distension, which may be mistaken for rupture.
Also instruct the patient to avoid folic acid, which impairs the efficacy of methotrexate.
Ultrasound surveillance is unnecessary. If an adnexal mass was identified at initial imaging, there is no need to view it again, since these masses tend to enlarge and form hematomas and can cause undue anxiety in both physician and patient. In properly selected patients, multidose methotrexate with monitoring of ß-hCG levels should suffice.
When surgery is indicated
Surgical intervention is necessary when pain is severe, persists beyond 12 hours, and is associated with orthostatic hypotension, falling hematocrit, or persistently elevated ß-hCG levels after methotrexate therapy.
Laparoscopy is the preferred approach. Advantages include less blood loss and analgesia,33 shortened postoperative recovery, and lower costs.
Technique. For unruptured ampullary ectopic pregnancy, salpingostomy is preferred. Make a linear incision over the bulging antimesenteric border of the fallopian tube using electrocautery, scissors, or laser. Remove the products of conception using forceps or suction, and leave the incision to heal by secondary intention.
For isthmic pregnancies, use segmental excision followed by delayed microsurgical anastomosis.34 The isthmic tubal lumen is narrower and the muscularis thicker than in the ampulla. Thus, the isthmus is predisposed to greater damage after salpingostomy and greater rates of proximal obstruction.33
An increased risk of persistent ectopic pregnancy has been a criticism of salpingostomy. However, when 1 dose of systemic methotrexate is combined with salpingostomy, the risk of persistent pregnancy is virtually eliminated.35
When rupture occurs, salpingectomy is the first choice for treatment, as it arrests hemorrhage and shortens the procedure. Either laparoscopy or laparotomy is appropriate.
The authors report no financial relationships relevant to this article.
1. Centers for Disease Control and Prevention. Ectopic Pregnancy - United States, 1990-1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
2. Washington AE, Katz P. Ectopic pregnancy in the United States: economic consequences and payment source trends. Obstet Gynecol. 1993;81:287-292.
3. Lipscomb GH, McCord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999;341:1974-1978.
4. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am. 2000;27:653-667.
5. Goldner TE, Lawson HW, Xia Z, et al. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42:73-85.
6. Ankum WM, Mol BW, Van der Veen F, et al. Risk factors for ectopic pregnancy: a meta analysis. Fertil Steril. 1996;65:1093-1099.
7. 2001 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinical Reports; December 2003
8. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
9. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351:1115-1120.
10. A multinational case-control study of ectopic pregnancy. The World Health Organization’s Special Programme of Research, Development and Research Training in Human Reproduction: Task Force on Intrauterine Devices for Fertility Regulation. Clin Reprod Fertil. 1985;3:131-143.
11. Russell JB. The etiology of ectopic pregnancy. Clin Obstet Gynecol. 1987;30:181-190.
12. Chow WH, Daling JR, Cates W, Jr, et al. Epidemiology of ectopic pregnancy. Epidemiol Rev. 1987;9:70-94.
13. Majmudar B, Henderson PH, III, Semple E. Salpingitis isthmica nodosa: a high-risk factor for tubal pregnancy. Obstet Gynecol. 1983;62:73-78.
14. Christensen H, Thyssen HH, Schebye O, et al. Three highly sensitive “bedside” serum and urine tests for pregnancy compared. Clin Chem. 1990;36:1686-1688.
15. Kadar N, Romero R. Further observations on serial human chorionic gonadotropin patterns in ectopic pregnancies and spontaneous abortions. Fertil Steril. 1988;50:367-370.
16. Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol. 2004;104:50-55.
17. Bateman BG, Nunley WC, Jr, Kolp LA, et al. Vaginal sonography findings and hCG dynamics of early intrauterine and tubal pregnancies. Obstet Gynecol. 1990;75:421-427.
18. Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol. 1981;58:156-161.
19. Brown DL, Doubilet PM. Transvaginal sonography for diagnosing ectopic pregnancy: positivity criteria and performance characteristics. J Ultrasound Med. 1994;13:259-266.
20. Russell SA, Filly RA, Damato N. Sonographic diagnosis of ectopic pregnancy with endovaginal probes: what really has changed? J Ultrasound Med. 1993;12:145-151.
21. Stovall TG, Ling FW, Carson SA, et al. Serum progesterone and uterine curettage in differential diagnosis of ectopic pregnancy. Fertil Steril. 1992;57:456-457.
22. Barnhart KT, Katz I, Hummel A, Gracia CR. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
23. Adam MP, Manning MA, Beck AE, et al. Methotrexate/misoprostol embryopathy: report of four cases resulting from failed medical abortion. Am J Med Genet. 2003;123A:72-78.
24. DeLoia JA, Stewart-Akers AM, Creinin MD. Effects of methotrexate on trophoblast proliferation and local immune responses. Hum Reprod. 1998;13:1063-1069.
25. Sand PK, Stubblefield PA, Ory SJ. Methotrexate inhibition of normal trophoblasts in vitro. Am J Obstet Gynecol. 1986;155:324-329.
26. Creinin MD, Stewart-Akers AM, DeLoia JA. Methotrexate effects on trophoblast and the corpus luteum in early pregnancy. Am J Obstet Gynecol. 1998;179:604-609.
27. Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754-757.
28. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #3: Medical Management of Tubal Pregnancy. Washington, DC: ACOG; 1998.
29. Hajenius PJ, Engelsbel S, Mol BW, et al. Randomised trial of systemic methotrexate versus laparoscopic salpingostomy in tubal pregnancy. Lancet. 1997;350:774-779.
30. Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754-757.
31. Lipscomb GH, Puckett KJ, Bran D, et al. Management of separation pain after single-dose methotrexate therapy for ectopic pregnancy. Prim Care Update Ob Gyns. 1998;5:175.-
32. Dudley P, et al. Characterizing ectopic pregnancies that rupture despite treatment with methotrexate. Fertil Steril [in press].
33. Murphy AA, Nager CW, Wujek JJ, et al. Operative laparoscopy versus laparotomy for the management of ectopic pregnancy: a prospective trial. Fertil Steril. 1992;57:1180-1185.
34. Balasch J, Barri PN. Treatment of ectopic pregnancy: the new gynaecological dilemma. Hum Reprod. 1994;9:547-558.
35. Gracia CR, Bron HA, Barnhart KT. Prophylactic methotrexate after linear salpingostomy: a decision analysis. Fertil Steril. 2001;76:1191-1195.
36. Breen JL. A 21-year survey of 654 ectopic pregnancies. Am J Obstet Gynecol. 1970;106:1004-1019.
- In properly selected cases, medical therapy and surgery produce similar outcomes, but medicine is less expensive.
- Surgery is still the first choice for hemorrhage, medical failure, rupture or near-rupture, and when medical therapy is contraindicated.
- Systemic methotrexate and laparoscopic salpingostomy produce similar success rates and long-term fertility.
- Single-dose methotrexate is associated with a higher risk of rupture than multiple doses.
Although ectopic pregnancy remains a leading cause of life-threatening first-trimester morbidity, accounting for about 9% of maternal deaths annually,1 we now are able to diagnose and treat most cases well before rupture occurs—in some cases, as early as 5 weeks’ gestation. As a result, medical therapy with systemic methotrexate has become the first-line treatment, with surgery reserved for hemorrhage, medical failures, neglected cases, and circumstances in which medical therapy is contraindicated.
Early diagnosis not only makes medical therapy possible, it also is cheaper, since it avoids rupture, blood loss, and surgery; preserves fertility; and minimizes lost productivity. This is important because ectopic pregnancy is an expensive condition, with an annual health-care bill exceeding $1 billion.2
Despite this progress, serious challenges remain. Medical management is not for everyone. Success is inversely related to initial serum human chorionic gonadotropin (hCG) levels3 and diminishes substantially when embryonic cardiac activity is observed during ultrasound imaging.4
In addition, because medical therapy has made outpatient treatment the norm in most cases, it has become virtually impossible to chart the prevalence of ectopic pregnancy. In past years, when hospital records were used, ectopic pregnancy rates were increasing relentlessly, from 4.5 per 1,000 pregnancies in 1970 to 16.8 in 1989 and 19.7 (108,000 cases) in 1992.1,5
Reasons for increasing rates
Today the prevalence of ectopic pregnancy is probably still rising, for several reasons:
- a greater incidence of risk factors such as sexually transmitted and tubal disease,6
- improved diagnostic methods, and
- the use of assisted reproductive technology (ART) to treat infertility (roughly 2% of ART pregnancies are ectopic).7
This article describes a 5-step approach to diagnosis and medical management with multiple-dose methotrexate, as well as fine points of treatment and basic surgical technique. It includes a protocol for multiple-dose methotrexate, a table summarizing treatment outcomes, and several case histories.
Likelihood of ectopic pregnancy
If 100 women present with a positive pregnancy test and pain and bleeding, approximately 60 will have a normal pregnancy, 30 are experiencing spontaneous abortion, and 9 have an ectopic pregnancy.8
STEP 1Assess risk factors and symptoms
The first step in early diagnosis is being vigilant for risk factors and symptoms associated with ectopic pregnancy, most of which are well known9:
Tubal disease carries a 3.5-fold common adjusted odds ratio (OR) for ectopic pregnancy. In addition, women with a previous ectopic pregnancy are 6 to 8 times more likely to experience another, while a history of tubal surgery raises that likelihood to 21. A history of pelvic infection, including gonorrhea, serologically confirmed chlamydia, and pelvic inflammatory disease, increases the risk of ectopic pregnancy 2 to 4 times.9
Contraception. Intrauterine devices (IUDs) are associated with an increased OR of 6.4.10 This does not mean that IUDs cause ectopic pregnancy. Rather, when a woman with an IUD becomes pregnant, an ectopic gestation should be high on the list of possibilities. A similar relationship exists between ectopic pregnancy and tubal ligation, which carries an OR of 9.3.9 Oral contraceptives are associated with a reduced risk of ectopic pregnancy unless they are used as emergency contraception (ie, after fertilization), in which case they are associated with an increased risk (TABLE 1).
Diethylstilbestrol exposure in utero alters fallopian tube morphology and can lead to absent or minimal fimbrial tissue, a small tubal os, and decreased length and caliber of the tube.11 Abnormal tubal anatomy caused by this exposure multiplies the risk of ectopic pregnancy by a factor of 5.9
In vitro fertilization. When blocked tubes are treated, the embryos can migrate retrograde into the oviduct, implant, and eventually rupture. The OR for ectopic pregnancy with assisted reproduction is 4.0.8
Although a patient’s ß-hCG levels, symptoms, or imaging may suggest ectopic pregnancy, missed diagnoses abound, especially when the physician omits 1 element of the triad: ß-hCG levels, ultrasound imaging, and curettage.
CASE 1: Pain and bleeding, with a high hCG
Mrs. Jones presents with pain and bleeding and a ß-hCG level of 6,000 mIU/mL. Ultrasound imaging reveals no intrauterine pregnancy. Should you presume the diagnosis is ectopic pregnancy and start methotrexate therapy? Or should you play it safe and perform curettage?
To explore these questions, Barnhart and colleagues22 performed a retrospective cohort analysis involving women with ß-hCG levels above 2,000 mIU/mL and no ultrasound evidence of an intrauterine pregnancy. They found that, when the physician presumed a diagnosis of ectopic pregnancy on the basis of ultrasound and ß-hCG levels alone, the diagnosis was wrong in almost 40% of cases.
Where’s the harm in presumptive treatment?
Some practitioners argue that proceeding with methotrexate therapy under these circumstances causes no harm. However, presumptive treatment unnecessarily exposes women to the side effects of chemotherapy and artificially inflates methotrexate success rates. Presumptive treatment does not decrease overall side effects or save money. It also falsely labels a woman as having an ectopic pregnancy, which directly affects future diagnosis and prognosis.
For these reasons, always perform uterine curettage when ultrasound imaging is inconclusive and ß-hCG levels are below normal.
CASE 2: Pregnant and in pain, with an adnexal mass
A pregnant patient complaining of moderate pain has a 4-cm adnexal mass identified at ultrasound, with no evidence of an intrauterine gestational sac. What is her diagnosis?
It’s impossible to know based on the ultrasound alone—even though the ultrasonographer may diagnose ectopic pregnancy. Unless you interpret these findings in light of her ß-hCG levels, you have no way of knowing whether she is experiencing a normal gestation, spontaneous abortion, or ectopic pregnancy. In this case, the adnexal mass turned out to be a corpus luteum with hydrosalpinx, and the woman had a viable intrauterine pregnancy.
CASE 3: Intrauterine pregnancy and pain
A 28-year-old gravida 1 para 0 at 8 weeks’ gestation has a fetal heart rate of 160 following in vitro fertilization. She has a history of tubal disease and complains of severe left lower quadrant pain of sudden onset. Repeat ultrasound shows multiple bilateral ovarian cysts with a gestational sac and fetal heart rate of 144 in the left adnexa. How do you proceed?
Heterotopic pregnancy sometimes complicates in vitro fertilization and can be a difficult diagnosis when multiple cysts from superovulation obscure visualization of the adnexal implantation.
The best treatment is laparoscopic removal of the ectopic implantation. Methotrexate is contraindicated because of the possibility of injuring the viable intrauterine pregnancy.
Cigarette smoking increases the likelihood of ectopic pregnancy 2.5 times,12 probably by affecting ciliary action within the fallopian tubes.
Salpingitis isthmic nodosa is anatomic thickening of the proximal portion of the fallopian tubes with multiple lumen diverticula. It increases the risk of ectopic pregnancy 1.5 times, compared with age- and race-matched controls.13
Don’t depend solely on risk factors. Many ectopic pregnancies present without them.
Symptoms. Many ectopic pregnancies never produce symptoms; rather, they resolve spontaneously or are timely diagnosed and treated medically. Risk factors should therefore be examined in any woman in early pregnancy and investigated further if ectopic pregnancy is likely.
When symptoms do occur, they usually involve 1 or all of the classic triad: amenorrhea, irregular bleeding, and lower abdominal pain. In addition, syncope, shock, and pain radiating to the patient’s shoulder can result from hemoperitoneum.
TABLE 1
High, moderate, and low levels of risk factors for ectopic pregnancy
| RISK FACTOR | ODDS RATIO* |
|---|---|
| High risk | |
| Tubal surgery | 21.0 |
| Tubal ligation | 9.3 |
| Previous ectopic pregnancy | 8.3 |
| In utero exposure to diethylstilbestrol | 5.6 |
| Use of intrauterine device | 4.2–45.0 |
| Documented tubal pathology | 3.8–21.0 |
| Assisted reproduction | 4.0 |
| Emergency contraception | High |
| Moderate risk | |
| Infertility | 2.5–21.0 |
| Previous genital infections | 2.5–3.7 |
| Multiple sexual partners | 2.1 |
| Salpingitis (isthmic) | 1.5 |
| Slight risk | |
| Previous pelvic, abdominal surgery | 0.9–3.8 |
| Cigarette smoking | 2.3–2.5 |
| Vaginal douching | 1.1–3.1 |
| Early age at first intercourse (<18 years) | 1.6 |
| Reprinted with permission from Elsevier (The Lancet, 1998, vol 351, 1115–1120). | |
| * Single values = common odds ratio from homogeneous studies; point estimates = range of values from heterogeneous studies | |
STEP 2Document the pregnancy and measure ß-hCG
Once you identify the high-risk patient, or a woman comes in complaining of pain and spotting or bleeding, run a pregnancy test to confirm that she is pregnant and, if it is positive, obtain a quantitative ß-hCG.
ß-hCG levels are normally measured using enzyme-linked immunosorbent assays (ELISA), which detect ß-hCG in urine and serum at levels as low as 20 mIU/mL and 10 mIU/mL, respectively.14 ß-hCG is produced by trophoblastic cells in normal pregnancy, and approximately doubles every 2 days when titers are below 10,000 mIU/mL15—although in some normal pregnancies, ß-hCG may increase as slowly as 53% or as rapidly as 230% over 2 days.16 Eighty-five percent of abnormal pregnancies—whether intrauterine or ectopic—have impaired ß-hCG production with prolonged doubling time. Thus, in failing pregnancies, ß-hCG levels will plateau or fail to rise normally.
A single ß-hCG level fails to predict the risk of rupture, since ectopic pregnancies can rupture at ß-hCG levels as low as 10 mIU/mL or far exceeding 10,000 mIU/mL, or at any level in between.
STEP 3Obtain an ultrasound scan
Transvaginal ultrasound reliably detects normal intrauterine gestations when ß-hCG passes somewhere between 1,000 mIU/mL and 2,000 mIU/mL (First International Reference Preparation), depending on the expertise of the ultrasonographer and the particular equipment used.8,17 This is known as the “discriminatory zone.” ß-hCG levels reach this zone as early as 1 week after missed menses.18
The discriminatory zone is not the lowest ß-hCG concentration at which an intrauterine pregnancy can be visualized via ultrasound. Rather, it is the value at which any intrauterine pregnancy will be apparent. At that value, the absence of an intrauterine pregnancy confirms—by negative conclusion—that the patient has a nonviable gestation.
When intrauterine pregnancy is visualized. The diagnosis is definitive and the woman’s symptoms can be explained as “threatened abortion.” No further investigation is necessary aside from routine prenatal care if the pregnancy continues.
When an extrauterine gestation is observed, such as a gestational sac with a detectable fetal heart rate, ectopic pregnancy can be diagnosed with 100% specificity but low sensitivity (15% to 20%). A complex adnexal mass without an intrauterine pregnancy improves sensitivity from 21% to 84% at the expense of lower specificity (93% to 99.5%).19
Even when an adnexal mass is visualized, cardiac activity is not usually present. If cardiac activity is apparent, proceed to surgery, since methotrexate usually will not resolve these gestations.
Be aware that some adnexal masses suspicious for ectopic pregnancy may turn out to be other entities, such as a corpus luteum, hydrosalpinx, ovarian neoplasm, or endometrioma. Unless a fetal heart rate is detected by ultrasound, the diagnosis is uncertain and curettage is needed to establish a definitive diagnosis.
No intrauterine pregnancy, no extrauterine mass. Despite the high resolution of transvaginal ultrasound, many patients with ectopic pregnancy have no apparent adnexal mass,20 particularly when diagnosis is early. In these cases, proceed to curettage (step 4).
Don’t interpret ultrasound findings in a vacuum
This is especially unwise when ß-hCG levels are low—even when the ultrasound report points to intrauterine pregnancy. At ß-hCG levels below 1,500 mIU/mL, the sensitivity of ultrasound in diagnosing intrauterine pregnancy drops from 98% to 33% and predictive value is substantially lower. Interpret ultrasound and ß-hCG levels together for greater accuracy.
How size influences management
Ultrasound can detect ectopic pregnancies as small as 2 cm. In general, an ectopic sac size larger than 4 cm should be treated surgically.
STEP 4Perform uterine curettage
If ultrasound imaging is inconclusive and ß-hCG levels are plateauing or rising subnormally, perform uterine curettage. If ß-hCG levels decrease 15% or more 8 to 12 hours after the procedure, a complete abortion can be strongly suspected.21 If ß-hCG levels plateau or rise, the trophoblasts were not removed by curettage, and ectopic pregnancy is diagnosed.21
Keep in mind these important points:
- Without uterine curettage, roughly 40% of ectopic pregnancy diagnoses are incorrect.22
- Because curettage will result in termination of pregnancy, it is vital that it be limited to cases involving abnormal ß-hCG levels, not normally rising values.
STEP 5Administer methotrexate
Medical management is indicated when the following circumstances are present:
- No viable intrauterine pregnancy is present.
- No rupture has occurred.
- Any adnexal mass is 4 cm in size or smaller.
- ß-hCG levels are below 10,000 mIU/mL.
If there is a positive fetal heart beat, surgery is preferred.
Firm diagnosis of ectopic pregnancy is essential prior to methotrexate administration. If the drug is given to a woman carrying a viable pregnancy, it may result in loss of the pregnancy or methotrexate embryopathy.23
How methotrexate resolves ectopic pregnancy
Systemic methotrexate has been used successfully in the treatment of ectopic pregnancy for more than 20 years. A folic acid antagonist, it inhibits de novo synthesis of purines and pyrimidines. It thus interferes with DNA synthesis and cell multiplication.24 Actively proliferating trophoblasts are particularly vulnerable.25
When methotrexate is administered to normally pregnant women, it blunts the normal ß-hCG increment over the next 7 days. Circulating progesterone and 17-a-hydroxyprogesterone concentrations also decline, and abortion occurs.26
Methotrexate directly impairs trophoblastic production of hCG; the decrement in corpus luteum progesterone is a secondary event.
Side effects include abdominal distress, chills and fever, dizziness, immunosuppression, leukopenia, malaise, nausea, ulcerative stomatitis, photosensitivity, and undue fatigue.
Breastfeeding is an absolute contraindication to methotrexate, while relative contraindications include abnormal liver function tests, blood dyscrasias, excessive alcohol consumption, HIV/AIDS, psoriasis, ongoing radiotherapy, rheumatoid arthritis, and significant pulmonary disease.
Multiple-dose methotrexate is superior
In a recent meta-analysis comparing single-and multiple-dose regimens of methotrexate, Barnhart and colleagues4 found the latter to be more effective, although the single dose was more commonly given. The single-dose regimen was associated with a significantly greater chance of failure in both crude (OR 1.71; 1.04, 2.82) and adjusted (OR 4.74; 1.77, 12.62) analyses. Consequently, we advocate the multiple-dose regimen detailed in TABLE 2: 1 mg of methotrexate per kilogram of body weight on day 1, alternating with 0.1 mg of leucovorin per kilogram on succeeding days. Continue this regimen until ß-hCG levels decline in 2 consecutive daily titers, or 4 doses of methotrexate are given, whichever comes first.
If treatment is unsuccessful after 4 doses, additional methotrexate is unlikely to be effective and will involve significant additional cost and morbidity. If ß-hCG levels plateau or continue to rise, surgery is indicated.
Although multiple-dose therapy is more effective than a single dose, the optimal number of doses probably falls somewhere between 1 and 4.4 A 2-dose protocol currently under investigation may provide an optimal compromise.
Artificially high efficacy rates with “single-dose” therapy. Unusually high success rates in initial studies of the single-dose regimen may have been due to the inclusion of spontaneously aborting intrauterine pregnancies.27 Although 6 subsequent studies—1 cohort and 5 case-control studies involving 304 patients—found an overall success rate (no surgical intervention) of 87.2%, 11.5% of participants required more than 1 dose.9
Still the standard. Despite its lower efficacy rates, single-dose methotrexate remains the “standard” in the United States, as recommended in an American College of Obstetricians and Gynecologists practice bulletin.28 The usual intramuscular dose is 50 mg per square meter of body surface area, with ß-hCG titers on days 4 and 7 and an additional dose if ß-hCG levels fail to resolve.
What the evidence shows
Multidose methotrexate. Twelve studies measured the success of multiple-dose systemic methotrexate (TABLE 3); they included 1 randomized, controlled trial, 1 cohort study, and 10 case series. Between 1982 and 1997, 325 cases were treated with multiple-dose methotrexate. Of these, 93.8% had successful resolution with no subsequent therapy, and 78.9% of the 161 women tested had patent oviducts. In addition, of 95 women hoping to conceive, 57.9% had a subsequent intrauterine pregnancy and 7.4% developed a recurrent ectopic pregnancy. These rates compared favorably with numerous laparoscopic surgery series published during the same years.9
Methotrexate versus laparoscopic salpingostomy. In 1 randomized clinical comparison,29 100 women with laparoscopy-confirmed ectopic pregnancy were randomized to methotrexate or laparoscopic salpingostomy. Of the 51 patients treated medically, 7 (14%) required surgical intervention for active bleeding and/or tubal rupture. An additional course of methotrexate was required in 2 patients (4%) for persistent trophoblasts.
Of the 49 patients in the salpingostomy group, 4 women (8%) failed therapy and required salpingectomies, and 10 patients (20%) were treated with methotrexate for persistent trophoblasts.
Tubal patency was present in 23 of 42 women (55%) in the methotrexate group, compared with 23 of 39 (59%) in the salpingostomy group.
Overall, this randomized study29 and previous meta-analysis demonstrate that systemic multiple-dose methotrexate is comparable in efficacy to laparoscopic salpingostomy.
TABLE 2
Multiple-dose methotrexate protocol
Discontinue treatment when there is a decline in 2 consecutive ß-hCG titers or after 4 doses, whichever comes first.
| DAY | INTERVENTION | DOSE (MG) |
|---|---|---|
| 1 | Baseline studies ß-hCG titer, CBC, and platelets Methotrexate | 1.0 |
| 2 | Leucovori | 0.1 |
| 3 | Methotrexate | 1.0 |
| 4 | Leucovorin ß-hCG titer | 0.1 |
| 5 | Methotrexate ß-hCG titer | 1.0 |
| 6 | Leucovorin ß-hCG titer | 0.1 |
| 7 | Methotrexate ß-hCG titer | 1.0 |
| 8 | Leucovorin ß-hCG titer CBC and platelets Renal and liver function tests | 0.1 |
| Weekly | ß-hCG titer until negative |
TABLE 3
Treatment outcomes for ectopic pregnancy
| METHOD | NUMBER OF STUDIES | NUMBER OF PATIENTS | NUMBER WITH SUCCESSFUL RESOLUTION | TUBAL PATENCY RATE | SUBSEQUENT FERTILITY RATE | |
|---|---|---|---|---|---|---|
| INTRAUTERINE PREGNANCY | ECTOPIC PREGNANCY | |||||
| Conservative laparoscopic surgery | 32 | 1,626 | 1,516 (93%) | 170/223 (76%) | 366/647 (57%) | 87/647 (13%) |
| Variable-dose methotrexate | 12 | 338 | 314 (93%) | 136/182 (75%) | 55/95 (58%) | 7/95 (7%) |
| Single-dose methotrexate | 7 | 393 | 340 (87%) | 61/75 (81%) | 39/64 (61%) | 5/64 (8%) |
| Direct-injection methotrexate | 21 | 660 | 502 (76%) | 130/162 (80%) | 87/152 (57%) | 9/152 (6%) |
| Expectant management | 14 | 628 | 425 (68%) | 60/79 (76%) | 12/14 (86%) | 1/14 (7%) |
| Reprinted with permission from Elsevier (The Lancet, 1998, vol 351, 1115–1120). | ||||||
Fine points of treatment
During methotrexate therapy, examine the patient only once to avoid triggering a rupture, and counsel her to avoid intercourse for the same reason.30 Do not perform repeat vaginal ultrasound examination. Also inform her that transient pain (“separation pain”) from tubal abortion frequently occurs 3 to 7 days after the start of therapy, lasts 4 to 12 hours, and then resolves.31 This is perhaps the most difficult aspect of methotrexate therapy, as it is not always easy to differentiate the pain of tubal abortion from the pain of rupture.
If ß-hCG titers continue to rise rapidly between methotrexate doses, rupture is more likely and surgery should proceed.32
Overall, isthmic ectopic pregnancies (12.3% of ectopics) appear to be at a particularly high risk for rupture and comprise nearly half of methotrexate failures.32 Unfortunately, there is no way to identify isthmic pregnancies without surgery.
Avoid NSAIDs and GI-“unfriendly” foods. Counsel the patient to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) because they may impair natural hemostasis. Gasforming foods such as leeks, corn, and cabbage can cause distension, which may be mistaken for rupture.
Also instruct the patient to avoid folic acid, which impairs the efficacy of methotrexate.
Ultrasound surveillance is unnecessary. If an adnexal mass was identified at initial imaging, there is no need to view it again, since these masses tend to enlarge and form hematomas and can cause undue anxiety in both physician and patient. In properly selected patients, multidose methotrexate with monitoring of ß-hCG levels should suffice.
When surgery is indicated
Surgical intervention is necessary when pain is severe, persists beyond 12 hours, and is associated with orthostatic hypotension, falling hematocrit, or persistently elevated ß-hCG levels after methotrexate therapy.
Laparoscopy is the preferred approach. Advantages include less blood loss and analgesia,33 shortened postoperative recovery, and lower costs.
Technique. For unruptured ampullary ectopic pregnancy, salpingostomy is preferred. Make a linear incision over the bulging antimesenteric border of the fallopian tube using electrocautery, scissors, or laser. Remove the products of conception using forceps or suction, and leave the incision to heal by secondary intention.
For isthmic pregnancies, use segmental excision followed by delayed microsurgical anastomosis.34 The isthmic tubal lumen is narrower and the muscularis thicker than in the ampulla. Thus, the isthmus is predisposed to greater damage after salpingostomy and greater rates of proximal obstruction.33
An increased risk of persistent ectopic pregnancy has been a criticism of salpingostomy. However, when 1 dose of systemic methotrexate is combined with salpingostomy, the risk of persistent pregnancy is virtually eliminated.35
When rupture occurs, salpingectomy is the first choice for treatment, as it arrests hemorrhage and shortens the procedure. Either laparoscopy or laparotomy is appropriate.
The authors report no financial relationships relevant to this article.
- In properly selected cases, medical therapy and surgery produce similar outcomes, but medicine is less expensive.
- Surgery is still the first choice for hemorrhage, medical failure, rupture or near-rupture, and when medical therapy is contraindicated.
- Systemic methotrexate and laparoscopic salpingostomy produce similar success rates and long-term fertility.
- Single-dose methotrexate is associated with a higher risk of rupture than multiple doses.
Although ectopic pregnancy remains a leading cause of life-threatening first-trimester morbidity, accounting for about 9% of maternal deaths annually,1 we now are able to diagnose and treat most cases well before rupture occurs—in some cases, as early as 5 weeks’ gestation. As a result, medical therapy with systemic methotrexate has become the first-line treatment, with surgery reserved for hemorrhage, medical failures, neglected cases, and circumstances in which medical therapy is contraindicated.
Early diagnosis not only makes medical therapy possible, it also is cheaper, since it avoids rupture, blood loss, and surgery; preserves fertility; and minimizes lost productivity. This is important because ectopic pregnancy is an expensive condition, with an annual health-care bill exceeding $1 billion.2
Despite this progress, serious challenges remain. Medical management is not for everyone. Success is inversely related to initial serum human chorionic gonadotropin (hCG) levels3 and diminishes substantially when embryonic cardiac activity is observed during ultrasound imaging.4
In addition, because medical therapy has made outpatient treatment the norm in most cases, it has become virtually impossible to chart the prevalence of ectopic pregnancy. In past years, when hospital records were used, ectopic pregnancy rates were increasing relentlessly, from 4.5 per 1,000 pregnancies in 1970 to 16.8 in 1989 and 19.7 (108,000 cases) in 1992.1,5
Reasons for increasing rates
Today the prevalence of ectopic pregnancy is probably still rising, for several reasons:
- a greater incidence of risk factors such as sexually transmitted and tubal disease,6
- improved diagnostic methods, and
- the use of assisted reproductive technology (ART) to treat infertility (roughly 2% of ART pregnancies are ectopic).7
This article describes a 5-step approach to diagnosis and medical management with multiple-dose methotrexate, as well as fine points of treatment and basic surgical technique. It includes a protocol for multiple-dose methotrexate, a table summarizing treatment outcomes, and several case histories.
Likelihood of ectopic pregnancy
If 100 women present with a positive pregnancy test and pain and bleeding, approximately 60 will have a normal pregnancy, 30 are experiencing spontaneous abortion, and 9 have an ectopic pregnancy.8
STEP 1Assess risk factors and symptoms
The first step in early diagnosis is being vigilant for risk factors and symptoms associated with ectopic pregnancy, most of which are well known9:
Tubal disease carries a 3.5-fold common adjusted odds ratio (OR) for ectopic pregnancy. In addition, women with a previous ectopic pregnancy are 6 to 8 times more likely to experience another, while a history of tubal surgery raises that likelihood to 21. A history of pelvic infection, including gonorrhea, serologically confirmed chlamydia, and pelvic inflammatory disease, increases the risk of ectopic pregnancy 2 to 4 times.9
Contraception. Intrauterine devices (IUDs) are associated with an increased OR of 6.4.10 This does not mean that IUDs cause ectopic pregnancy. Rather, when a woman with an IUD becomes pregnant, an ectopic gestation should be high on the list of possibilities. A similar relationship exists between ectopic pregnancy and tubal ligation, which carries an OR of 9.3.9 Oral contraceptives are associated with a reduced risk of ectopic pregnancy unless they are used as emergency contraception (ie, after fertilization), in which case they are associated with an increased risk (TABLE 1).
Diethylstilbestrol exposure in utero alters fallopian tube morphology and can lead to absent or minimal fimbrial tissue, a small tubal os, and decreased length and caliber of the tube.11 Abnormal tubal anatomy caused by this exposure multiplies the risk of ectopic pregnancy by a factor of 5.9
In vitro fertilization. When blocked tubes are treated, the embryos can migrate retrograde into the oviduct, implant, and eventually rupture. The OR for ectopic pregnancy with assisted reproduction is 4.0.8
Although a patient’s ß-hCG levels, symptoms, or imaging may suggest ectopic pregnancy, missed diagnoses abound, especially when the physician omits 1 element of the triad: ß-hCG levels, ultrasound imaging, and curettage.
CASE 1: Pain and bleeding, with a high hCG
Mrs. Jones presents with pain and bleeding and a ß-hCG level of 6,000 mIU/mL. Ultrasound imaging reveals no intrauterine pregnancy. Should you presume the diagnosis is ectopic pregnancy and start methotrexate therapy? Or should you play it safe and perform curettage?
To explore these questions, Barnhart and colleagues22 performed a retrospective cohort analysis involving women with ß-hCG levels above 2,000 mIU/mL and no ultrasound evidence of an intrauterine pregnancy. They found that, when the physician presumed a diagnosis of ectopic pregnancy on the basis of ultrasound and ß-hCG levels alone, the diagnosis was wrong in almost 40% of cases.
Where’s the harm in presumptive treatment?
Some practitioners argue that proceeding with methotrexate therapy under these circumstances causes no harm. However, presumptive treatment unnecessarily exposes women to the side effects of chemotherapy and artificially inflates methotrexate success rates. Presumptive treatment does not decrease overall side effects or save money. It also falsely labels a woman as having an ectopic pregnancy, which directly affects future diagnosis and prognosis.
For these reasons, always perform uterine curettage when ultrasound imaging is inconclusive and ß-hCG levels are below normal.
CASE 2: Pregnant and in pain, with an adnexal mass
A pregnant patient complaining of moderate pain has a 4-cm adnexal mass identified at ultrasound, with no evidence of an intrauterine gestational sac. What is her diagnosis?
It’s impossible to know based on the ultrasound alone—even though the ultrasonographer may diagnose ectopic pregnancy. Unless you interpret these findings in light of her ß-hCG levels, you have no way of knowing whether she is experiencing a normal gestation, spontaneous abortion, or ectopic pregnancy. In this case, the adnexal mass turned out to be a corpus luteum with hydrosalpinx, and the woman had a viable intrauterine pregnancy.
CASE 3: Intrauterine pregnancy and pain
A 28-year-old gravida 1 para 0 at 8 weeks’ gestation has a fetal heart rate of 160 following in vitro fertilization. She has a history of tubal disease and complains of severe left lower quadrant pain of sudden onset. Repeat ultrasound shows multiple bilateral ovarian cysts with a gestational sac and fetal heart rate of 144 in the left adnexa. How do you proceed?
Heterotopic pregnancy sometimes complicates in vitro fertilization and can be a difficult diagnosis when multiple cysts from superovulation obscure visualization of the adnexal implantation.
The best treatment is laparoscopic removal of the ectopic implantation. Methotrexate is contraindicated because of the possibility of injuring the viable intrauterine pregnancy.
Cigarette smoking increases the likelihood of ectopic pregnancy 2.5 times,12 probably by affecting ciliary action within the fallopian tubes.
Salpingitis isthmic nodosa is anatomic thickening of the proximal portion of the fallopian tubes with multiple lumen diverticula. It increases the risk of ectopic pregnancy 1.5 times, compared with age- and race-matched controls.13
Don’t depend solely on risk factors. Many ectopic pregnancies present without them.
Symptoms. Many ectopic pregnancies never produce symptoms; rather, they resolve spontaneously or are timely diagnosed and treated medically. Risk factors should therefore be examined in any woman in early pregnancy and investigated further if ectopic pregnancy is likely.
When symptoms do occur, they usually involve 1 or all of the classic triad: amenorrhea, irregular bleeding, and lower abdominal pain. In addition, syncope, shock, and pain radiating to the patient’s shoulder can result from hemoperitoneum.
TABLE 1
High, moderate, and low levels of risk factors for ectopic pregnancy
| RISK FACTOR | ODDS RATIO* |
|---|---|
| High risk | |
| Tubal surgery | 21.0 |
| Tubal ligation | 9.3 |
| Previous ectopic pregnancy | 8.3 |
| In utero exposure to diethylstilbestrol | 5.6 |
| Use of intrauterine device | 4.2–45.0 |
| Documented tubal pathology | 3.8–21.0 |
| Assisted reproduction | 4.0 |
| Emergency contraception | High |
| Moderate risk | |
| Infertility | 2.5–21.0 |
| Previous genital infections | 2.5–3.7 |
| Multiple sexual partners | 2.1 |
| Salpingitis (isthmic) | 1.5 |
| Slight risk | |
| Previous pelvic, abdominal surgery | 0.9–3.8 |
| Cigarette smoking | 2.3–2.5 |
| Vaginal douching | 1.1–3.1 |
| Early age at first intercourse (<18 years) | 1.6 |
| Reprinted with permission from Elsevier (The Lancet, 1998, vol 351, 1115–1120). | |
| * Single values = common odds ratio from homogeneous studies; point estimates = range of values from heterogeneous studies | |
STEP 2Document the pregnancy and measure ß-hCG
Once you identify the high-risk patient, or a woman comes in complaining of pain and spotting or bleeding, run a pregnancy test to confirm that she is pregnant and, if it is positive, obtain a quantitative ß-hCG.
ß-hCG levels are normally measured using enzyme-linked immunosorbent assays (ELISA), which detect ß-hCG in urine and serum at levels as low as 20 mIU/mL and 10 mIU/mL, respectively.14 ß-hCG is produced by trophoblastic cells in normal pregnancy, and approximately doubles every 2 days when titers are below 10,000 mIU/mL15—although in some normal pregnancies, ß-hCG may increase as slowly as 53% or as rapidly as 230% over 2 days.16 Eighty-five percent of abnormal pregnancies—whether intrauterine or ectopic—have impaired ß-hCG production with prolonged doubling time. Thus, in failing pregnancies, ß-hCG levels will plateau or fail to rise normally.
A single ß-hCG level fails to predict the risk of rupture, since ectopic pregnancies can rupture at ß-hCG levels as low as 10 mIU/mL or far exceeding 10,000 mIU/mL, or at any level in between.
STEP 3Obtain an ultrasound scan
Transvaginal ultrasound reliably detects normal intrauterine gestations when ß-hCG passes somewhere between 1,000 mIU/mL and 2,000 mIU/mL (First International Reference Preparation), depending on the expertise of the ultrasonographer and the particular equipment used.8,17 This is known as the “discriminatory zone.” ß-hCG levels reach this zone as early as 1 week after missed menses.18
The discriminatory zone is not the lowest ß-hCG concentration at which an intrauterine pregnancy can be visualized via ultrasound. Rather, it is the value at which any intrauterine pregnancy will be apparent. At that value, the absence of an intrauterine pregnancy confirms—by negative conclusion—that the patient has a nonviable gestation.
When intrauterine pregnancy is visualized. The diagnosis is definitive and the woman’s symptoms can be explained as “threatened abortion.” No further investigation is necessary aside from routine prenatal care if the pregnancy continues.
When an extrauterine gestation is observed, such as a gestational sac with a detectable fetal heart rate, ectopic pregnancy can be diagnosed with 100% specificity but low sensitivity (15% to 20%). A complex adnexal mass without an intrauterine pregnancy improves sensitivity from 21% to 84% at the expense of lower specificity (93% to 99.5%).19
Even when an adnexal mass is visualized, cardiac activity is not usually present. If cardiac activity is apparent, proceed to surgery, since methotrexate usually will not resolve these gestations.
Be aware that some adnexal masses suspicious for ectopic pregnancy may turn out to be other entities, such as a corpus luteum, hydrosalpinx, ovarian neoplasm, or endometrioma. Unless a fetal heart rate is detected by ultrasound, the diagnosis is uncertain and curettage is needed to establish a definitive diagnosis.
No intrauterine pregnancy, no extrauterine mass. Despite the high resolution of transvaginal ultrasound, many patients with ectopic pregnancy have no apparent adnexal mass,20 particularly when diagnosis is early. In these cases, proceed to curettage (step 4).
Don’t interpret ultrasound findings in a vacuum
This is especially unwise when ß-hCG levels are low—even when the ultrasound report points to intrauterine pregnancy. At ß-hCG levels below 1,500 mIU/mL, the sensitivity of ultrasound in diagnosing intrauterine pregnancy drops from 98% to 33% and predictive value is substantially lower. Interpret ultrasound and ß-hCG levels together for greater accuracy.
How size influences management
Ultrasound can detect ectopic pregnancies as small as 2 cm. In general, an ectopic sac size larger than 4 cm should be treated surgically.
STEP 4Perform uterine curettage
If ultrasound imaging is inconclusive and ß-hCG levels are plateauing or rising subnormally, perform uterine curettage. If ß-hCG levels decrease 15% or more 8 to 12 hours after the procedure, a complete abortion can be strongly suspected.21 If ß-hCG levels plateau or rise, the trophoblasts were not removed by curettage, and ectopic pregnancy is diagnosed.21
Keep in mind these important points:
- Without uterine curettage, roughly 40% of ectopic pregnancy diagnoses are incorrect.22
- Because curettage will result in termination of pregnancy, it is vital that it be limited to cases involving abnormal ß-hCG levels, not normally rising values.
STEP 5Administer methotrexate
Medical management is indicated when the following circumstances are present:
- No viable intrauterine pregnancy is present.
- No rupture has occurred.
- Any adnexal mass is 4 cm in size or smaller.
- ß-hCG levels are below 10,000 mIU/mL.
If there is a positive fetal heart beat, surgery is preferred.
Firm diagnosis of ectopic pregnancy is essential prior to methotrexate administration. If the drug is given to a woman carrying a viable pregnancy, it may result in loss of the pregnancy or methotrexate embryopathy.23
How methotrexate resolves ectopic pregnancy
Systemic methotrexate has been used successfully in the treatment of ectopic pregnancy for more than 20 years. A folic acid antagonist, it inhibits de novo synthesis of purines and pyrimidines. It thus interferes with DNA synthesis and cell multiplication.24 Actively proliferating trophoblasts are particularly vulnerable.25
When methotrexate is administered to normally pregnant women, it blunts the normal ß-hCG increment over the next 7 days. Circulating progesterone and 17-a-hydroxyprogesterone concentrations also decline, and abortion occurs.26
Methotrexate directly impairs trophoblastic production of hCG; the decrement in corpus luteum progesterone is a secondary event.
Side effects include abdominal distress, chills and fever, dizziness, immunosuppression, leukopenia, malaise, nausea, ulcerative stomatitis, photosensitivity, and undue fatigue.
Breastfeeding is an absolute contraindication to methotrexate, while relative contraindications include abnormal liver function tests, blood dyscrasias, excessive alcohol consumption, HIV/AIDS, psoriasis, ongoing radiotherapy, rheumatoid arthritis, and significant pulmonary disease.
Multiple-dose methotrexate is superior
In a recent meta-analysis comparing single-and multiple-dose regimens of methotrexate, Barnhart and colleagues4 found the latter to be more effective, although the single dose was more commonly given. The single-dose regimen was associated with a significantly greater chance of failure in both crude (OR 1.71; 1.04, 2.82) and adjusted (OR 4.74; 1.77, 12.62) analyses. Consequently, we advocate the multiple-dose regimen detailed in TABLE 2: 1 mg of methotrexate per kilogram of body weight on day 1, alternating with 0.1 mg of leucovorin per kilogram on succeeding days. Continue this regimen until ß-hCG levels decline in 2 consecutive daily titers, or 4 doses of methotrexate are given, whichever comes first.
If treatment is unsuccessful after 4 doses, additional methotrexate is unlikely to be effective and will involve significant additional cost and morbidity. If ß-hCG levels plateau or continue to rise, surgery is indicated.
Although multiple-dose therapy is more effective than a single dose, the optimal number of doses probably falls somewhere between 1 and 4.4 A 2-dose protocol currently under investigation may provide an optimal compromise.
Artificially high efficacy rates with “single-dose” therapy. Unusually high success rates in initial studies of the single-dose regimen may have been due to the inclusion of spontaneously aborting intrauterine pregnancies.27 Although 6 subsequent studies—1 cohort and 5 case-control studies involving 304 patients—found an overall success rate (no surgical intervention) of 87.2%, 11.5% of participants required more than 1 dose.9
Still the standard. Despite its lower efficacy rates, single-dose methotrexate remains the “standard” in the United States, as recommended in an American College of Obstetricians and Gynecologists practice bulletin.28 The usual intramuscular dose is 50 mg per square meter of body surface area, with ß-hCG titers on days 4 and 7 and an additional dose if ß-hCG levels fail to resolve.
What the evidence shows
Multidose methotrexate. Twelve studies measured the success of multiple-dose systemic methotrexate (TABLE 3); they included 1 randomized, controlled trial, 1 cohort study, and 10 case series. Between 1982 and 1997, 325 cases were treated with multiple-dose methotrexate. Of these, 93.8% had successful resolution with no subsequent therapy, and 78.9% of the 161 women tested had patent oviducts. In addition, of 95 women hoping to conceive, 57.9% had a subsequent intrauterine pregnancy and 7.4% developed a recurrent ectopic pregnancy. These rates compared favorably with numerous laparoscopic surgery series published during the same years.9
Methotrexate versus laparoscopic salpingostomy. In 1 randomized clinical comparison,29 100 women with laparoscopy-confirmed ectopic pregnancy were randomized to methotrexate or laparoscopic salpingostomy. Of the 51 patients treated medically, 7 (14%) required surgical intervention for active bleeding and/or tubal rupture. An additional course of methotrexate was required in 2 patients (4%) for persistent trophoblasts.
Of the 49 patients in the salpingostomy group, 4 women (8%) failed therapy and required salpingectomies, and 10 patients (20%) were treated with methotrexate for persistent trophoblasts.
Tubal patency was present in 23 of 42 women (55%) in the methotrexate group, compared with 23 of 39 (59%) in the salpingostomy group.
Overall, this randomized study29 and previous meta-analysis demonstrate that systemic multiple-dose methotrexate is comparable in efficacy to laparoscopic salpingostomy.
TABLE 2
Multiple-dose methotrexate protocol
Discontinue treatment when there is a decline in 2 consecutive ß-hCG titers or after 4 doses, whichever comes first.
| DAY | INTERVENTION | DOSE (MG) |
|---|---|---|
| 1 | Baseline studies ß-hCG titer, CBC, and platelets Methotrexate | 1.0 |
| 2 | Leucovori | 0.1 |
| 3 | Methotrexate | 1.0 |
| 4 | Leucovorin ß-hCG titer | 0.1 |
| 5 | Methotrexate ß-hCG titer | 1.0 |
| 6 | Leucovorin ß-hCG titer | 0.1 |
| 7 | Methotrexate ß-hCG titer | 1.0 |
| 8 | Leucovorin ß-hCG titer CBC and platelets Renal and liver function tests | 0.1 |
| Weekly | ß-hCG titer until negative |
TABLE 3
Treatment outcomes for ectopic pregnancy
| METHOD | NUMBER OF STUDIES | NUMBER OF PATIENTS | NUMBER WITH SUCCESSFUL RESOLUTION | TUBAL PATENCY RATE | SUBSEQUENT FERTILITY RATE | |
|---|---|---|---|---|---|---|
| INTRAUTERINE PREGNANCY | ECTOPIC PREGNANCY | |||||
| Conservative laparoscopic surgery | 32 | 1,626 | 1,516 (93%) | 170/223 (76%) | 366/647 (57%) | 87/647 (13%) |
| Variable-dose methotrexate | 12 | 338 | 314 (93%) | 136/182 (75%) | 55/95 (58%) | 7/95 (7%) |
| Single-dose methotrexate | 7 | 393 | 340 (87%) | 61/75 (81%) | 39/64 (61%) | 5/64 (8%) |
| Direct-injection methotrexate | 21 | 660 | 502 (76%) | 130/162 (80%) | 87/152 (57%) | 9/152 (6%) |
| Expectant management | 14 | 628 | 425 (68%) | 60/79 (76%) | 12/14 (86%) | 1/14 (7%) |
| Reprinted with permission from Elsevier (The Lancet, 1998, vol 351, 1115–1120). | ||||||
Fine points of treatment
During methotrexate therapy, examine the patient only once to avoid triggering a rupture, and counsel her to avoid intercourse for the same reason.30 Do not perform repeat vaginal ultrasound examination. Also inform her that transient pain (“separation pain”) from tubal abortion frequently occurs 3 to 7 days after the start of therapy, lasts 4 to 12 hours, and then resolves.31 This is perhaps the most difficult aspect of methotrexate therapy, as it is not always easy to differentiate the pain of tubal abortion from the pain of rupture.
If ß-hCG titers continue to rise rapidly between methotrexate doses, rupture is more likely and surgery should proceed.32
Overall, isthmic ectopic pregnancies (12.3% of ectopics) appear to be at a particularly high risk for rupture and comprise nearly half of methotrexate failures.32 Unfortunately, there is no way to identify isthmic pregnancies without surgery.
Avoid NSAIDs and GI-“unfriendly” foods. Counsel the patient to avoid nonsteroidal anti-inflammatory drugs (NSAIDs) because they may impair natural hemostasis. Gasforming foods such as leeks, corn, and cabbage can cause distension, which may be mistaken for rupture.
Also instruct the patient to avoid folic acid, which impairs the efficacy of methotrexate.
Ultrasound surveillance is unnecessary. If an adnexal mass was identified at initial imaging, there is no need to view it again, since these masses tend to enlarge and form hematomas and can cause undue anxiety in both physician and patient. In properly selected patients, multidose methotrexate with monitoring of ß-hCG levels should suffice.
When surgery is indicated
Surgical intervention is necessary when pain is severe, persists beyond 12 hours, and is associated with orthostatic hypotension, falling hematocrit, or persistently elevated ß-hCG levels after methotrexate therapy.
Laparoscopy is the preferred approach. Advantages include less blood loss and analgesia,33 shortened postoperative recovery, and lower costs.
Technique. For unruptured ampullary ectopic pregnancy, salpingostomy is preferred. Make a linear incision over the bulging antimesenteric border of the fallopian tube using electrocautery, scissors, or laser. Remove the products of conception using forceps or suction, and leave the incision to heal by secondary intention.
For isthmic pregnancies, use segmental excision followed by delayed microsurgical anastomosis.34 The isthmic tubal lumen is narrower and the muscularis thicker than in the ampulla. Thus, the isthmus is predisposed to greater damage after salpingostomy and greater rates of proximal obstruction.33
An increased risk of persistent ectopic pregnancy has been a criticism of salpingostomy. However, when 1 dose of systemic methotrexate is combined with salpingostomy, the risk of persistent pregnancy is virtually eliminated.35
When rupture occurs, salpingectomy is the first choice for treatment, as it arrests hemorrhage and shortens the procedure. Either laparoscopy or laparotomy is appropriate.
The authors report no financial relationships relevant to this article.
1. Centers for Disease Control and Prevention. Ectopic Pregnancy - United States, 1990-1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
2. Washington AE, Katz P. Ectopic pregnancy in the United States: economic consequences and payment source trends. Obstet Gynecol. 1993;81:287-292.
3. Lipscomb GH, McCord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999;341:1974-1978.
4. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am. 2000;27:653-667.
5. Goldner TE, Lawson HW, Xia Z, et al. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42:73-85.
6. Ankum WM, Mol BW, Van der Veen F, et al. Risk factors for ectopic pregnancy: a meta analysis. Fertil Steril. 1996;65:1093-1099.
7. 2001 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinical Reports; December 2003
8. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
9. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351:1115-1120.
10. A multinational case-control study of ectopic pregnancy. The World Health Organization’s Special Programme of Research, Development and Research Training in Human Reproduction: Task Force on Intrauterine Devices for Fertility Regulation. Clin Reprod Fertil. 1985;3:131-143.
11. Russell JB. The etiology of ectopic pregnancy. Clin Obstet Gynecol. 1987;30:181-190.
12. Chow WH, Daling JR, Cates W, Jr, et al. Epidemiology of ectopic pregnancy. Epidemiol Rev. 1987;9:70-94.
13. Majmudar B, Henderson PH, III, Semple E. Salpingitis isthmica nodosa: a high-risk factor for tubal pregnancy. Obstet Gynecol. 1983;62:73-78.
14. Christensen H, Thyssen HH, Schebye O, et al. Three highly sensitive “bedside” serum and urine tests for pregnancy compared. Clin Chem. 1990;36:1686-1688.
15. Kadar N, Romero R. Further observations on serial human chorionic gonadotropin patterns in ectopic pregnancies and spontaneous abortions. Fertil Steril. 1988;50:367-370.
16. Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol. 2004;104:50-55.
17. Bateman BG, Nunley WC, Jr, Kolp LA, et al. Vaginal sonography findings and hCG dynamics of early intrauterine and tubal pregnancies. Obstet Gynecol. 1990;75:421-427.
18. Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol. 1981;58:156-161.
19. Brown DL, Doubilet PM. Transvaginal sonography for diagnosing ectopic pregnancy: positivity criteria and performance characteristics. J Ultrasound Med. 1994;13:259-266.
20. Russell SA, Filly RA, Damato N. Sonographic diagnosis of ectopic pregnancy with endovaginal probes: what really has changed? J Ultrasound Med. 1993;12:145-151.
21. Stovall TG, Ling FW, Carson SA, et al. Serum progesterone and uterine curettage in differential diagnosis of ectopic pregnancy. Fertil Steril. 1992;57:456-457.
22. Barnhart KT, Katz I, Hummel A, Gracia CR. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
23. Adam MP, Manning MA, Beck AE, et al. Methotrexate/misoprostol embryopathy: report of four cases resulting from failed medical abortion. Am J Med Genet. 2003;123A:72-78.
24. DeLoia JA, Stewart-Akers AM, Creinin MD. Effects of methotrexate on trophoblast proliferation and local immune responses. Hum Reprod. 1998;13:1063-1069.
25. Sand PK, Stubblefield PA, Ory SJ. Methotrexate inhibition of normal trophoblasts in vitro. Am J Obstet Gynecol. 1986;155:324-329.
26. Creinin MD, Stewart-Akers AM, DeLoia JA. Methotrexate effects on trophoblast and the corpus luteum in early pregnancy. Am J Obstet Gynecol. 1998;179:604-609.
27. Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754-757.
28. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #3: Medical Management of Tubal Pregnancy. Washington, DC: ACOG; 1998.
29. Hajenius PJ, Engelsbel S, Mol BW, et al. Randomised trial of systemic methotrexate versus laparoscopic salpingostomy in tubal pregnancy. Lancet. 1997;350:774-779.
30. Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754-757.
31. Lipscomb GH, Puckett KJ, Bran D, et al. Management of separation pain after single-dose methotrexate therapy for ectopic pregnancy. Prim Care Update Ob Gyns. 1998;5:175.-
32. Dudley P, et al. Characterizing ectopic pregnancies that rupture despite treatment with methotrexate. Fertil Steril [in press].
33. Murphy AA, Nager CW, Wujek JJ, et al. Operative laparoscopy versus laparotomy for the management of ectopic pregnancy: a prospective trial. Fertil Steril. 1992;57:1180-1185.
34. Balasch J, Barri PN. Treatment of ectopic pregnancy: the new gynaecological dilemma. Hum Reprod. 1994;9:547-558.
35. Gracia CR, Bron HA, Barnhart KT. Prophylactic methotrexate after linear salpingostomy: a decision analysis. Fertil Steril. 2001;76:1191-1195.
36. Breen JL. A 21-year survey of 654 ectopic pregnancies. Am J Obstet Gynecol. 1970;106:1004-1019.
1. Centers for Disease Control and Prevention. Ectopic Pregnancy - United States, 1990-1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
2. Washington AE, Katz P. Ectopic pregnancy in the United States: economic consequences and payment source trends. Obstet Gynecol. 1993;81:287-292.
3. Lipscomb GH, McCord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999;341:1974-1978.
4. Barnhart K, Esposito M, Coutifaris C. An update on the medical treatment of ectopic pregnancy. Obstet Gynecol Clin North Am. 2000;27:653-667.
5. Goldner TE, Lawson HW, Xia Z, et al. Surveillance for ectopic pregnancy—United States, 1970-1989. MMWR CDC Surveill Summ. 1993;42:73-85.
6. Ankum WM, Mol BW, Van der Veen F, et al. Risk factors for ectopic pregnancy: a meta analysis. Fertil Steril. 1996;65:1093-1099.
7. 2001 Assisted Reproductive Technology Success Rates: National Summary and Fertility Clinical Reports; December 2003
8. Barnhart K, Mennuti MT, Benjamin I, et al. Prompt diagnosis of ectopic pregnancy in an emergency department setting. Obstet Gynecol. 1994;84:1010-1015.
9. Pisarska MD, Carson SA, Buster JE. Ectopic pregnancy. Lancet. 1998;351:1115-1120.
10. A multinational case-control study of ectopic pregnancy. The World Health Organization’s Special Programme of Research, Development and Research Training in Human Reproduction: Task Force on Intrauterine Devices for Fertility Regulation. Clin Reprod Fertil. 1985;3:131-143.
11. Russell JB. The etiology of ectopic pregnancy. Clin Obstet Gynecol. 1987;30:181-190.
12. Chow WH, Daling JR, Cates W, Jr, et al. Epidemiology of ectopic pregnancy. Epidemiol Rev. 1987;9:70-94.
13. Majmudar B, Henderson PH, III, Semple E. Salpingitis isthmica nodosa: a high-risk factor for tubal pregnancy. Obstet Gynecol. 1983;62:73-78.
14. Christensen H, Thyssen HH, Schebye O, et al. Three highly sensitive “bedside” serum and urine tests for pregnancy compared. Clin Chem. 1990;36:1686-1688.
15. Kadar N, Romero R. Further observations on serial human chorionic gonadotropin patterns in ectopic pregnancies and spontaneous abortions. Fertil Steril. 1988;50:367-370.
16. Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol. 2004;104:50-55.
17. Bateman BG, Nunley WC, Jr, Kolp LA, et al. Vaginal sonography findings and hCG dynamics of early intrauterine and tubal pregnancies. Obstet Gynecol. 1990;75:421-427.
18. Kadar N, DeVore G, Romero R. Discriminatory hCG zone: its use in the sonographic evaluation for ectopic pregnancy. Obstet Gynecol. 1981;58:156-161.
19. Brown DL, Doubilet PM. Transvaginal sonography for diagnosing ectopic pregnancy: positivity criteria and performance characteristics. J Ultrasound Med. 1994;13:259-266.
20. Russell SA, Filly RA, Damato N. Sonographic diagnosis of ectopic pregnancy with endovaginal probes: what really has changed? J Ultrasound Med. 1993;12:145-151.
21. Stovall TG, Ling FW, Carson SA, et al. Serum progesterone and uterine curettage in differential diagnosis of ectopic pregnancy. Fertil Steril. 1992;57:456-457.
22. Barnhart KT, Katz I, Hummel A, Gracia CR. Presumed diagnosis of ectopic pregnancy. Obstet Gynecol. 2002;100:505-510.
23. Adam MP, Manning MA, Beck AE, et al. Methotrexate/misoprostol embryopathy: report of four cases resulting from failed medical abortion. Am J Med Genet. 2003;123A:72-78.
24. DeLoia JA, Stewart-Akers AM, Creinin MD. Effects of methotrexate on trophoblast proliferation and local immune responses. Hum Reprod. 1998;13:1063-1069.
25. Sand PK, Stubblefield PA, Ory SJ. Methotrexate inhibition of normal trophoblasts in vitro. Am J Obstet Gynecol. 1986;155:324-329.
26. Creinin MD, Stewart-Akers AM, DeLoia JA. Methotrexate effects on trophoblast and the corpus luteum in early pregnancy. Am J Obstet Gynecol. 1998;179:604-609.
27. Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754-757.
28. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #3: Medical Management of Tubal Pregnancy. Washington, DC: ACOG; 1998.
29. Hajenius PJ, Engelsbel S, Mol BW, et al. Randomised trial of systemic methotrexate versus laparoscopic salpingostomy in tubal pregnancy. Lancet. 1997;350:774-779.
30. Stovall TG, Ling FW, Gray LA. Single-dose methotrexate for treatment of ectopic pregnancy. Obstet Gynecol. 1991;77:754-757.
31. Lipscomb GH, Puckett KJ, Bran D, et al. Management of separation pain after single-dose methotrexate therapy for ectopic pregnancy. Prim Care Update Ob Gyns. 1998;5:175.-
32. Dudley P, et al. Characterizing ectopic pregnancies that rupture despite treatment with methotrexate. Fertil Steril [in press].
33. Murphy AA, Nager CW, Wujek JJ, et al. Operative laparoscopy versus laparotomy for the management of ectopic pregnancy: a prospective trial. Fertil Steril. 1992;57:1180-1185.
34. Balasch J, Barri PN. Treatment of ectopic pregnancy: the new gynaecological dilemma. Hum Reprod. 1994;9:547-558.
35. Gracia CR, Bron HA, Barnhart KT. Prophylactic methotrexate after linear salpingostomy: a decision analysis. Fertil Steril. 2001;76:1191-1195.
36. Breen JL. A 21-year survey of 654 ectopic pregnancies. Am J Obstet Gynecol. 1970;106:1004-1019.
Use of Complementary and Alternative Therapies Among Veterans: A Pilot Study
Brief, Focused Provider Consultation
Ovarian cancer: Identifying and managing high-risk patients
- Ask every patient: ”Has anyone in your family had breast cancer under the age of 35, or colorectal, uterine, or ovarian cancer?
- Genetic testing is appropriate only when pre- and post-test counseling is available, the test can be interpreted, and the results will help in medical and surgical management.
- Oral contraceptives may reduce risk by 10% per year for up to 5 to 7 years of use.
- Bilateral salpingo-oophorectomy in BRCA carriers reduces the risk of ovarian cancer by more than 90% and the risk of breast cancer by more than 50%.
A 42-year-old woman of Ashkenazi Jewish ancestry, whose mother had a diagnosis of ovarian cancer a year earlier, is worried that she may also be at risk.
Her family has experienced no other cases of breast, colorectal, or ovarian cancer. She has 2 children and has used oral contraceptives for 9 years.
Primary prevention is an alternative to diagnosis of ovarian cancer at an early stage—a goal that is all too often unattainable.
If we identify women with increased risk based on their family history, environmental exposure, hormone use, or reproductive experiences, we can counsel them about lifestyle changes, chemoprophylaxis, or preventive surgery, depending on their reproductive desires and overall ovarian cancer risk.
Using 2 hypothetical cases, this article describes a quick history to screen for potential high risk, and summarizes what we can advise concerned patients, based on findings to date.
Topics include:
- genetic risk assessment
- when to refer to a genetic counselor
- risk factors
- oral contraceptives for primary prevention
- prophylactic surgery
Despite advances in medical and surgical therapies, ovarian cancer remains the deadliest gynecologic malignancy and the fifth leading cause of cancer deaths among women in the United States. This year the disease will strike an estimated 25,400 women in this country and kill more than 14,000.1
Early detection versus primary prevention
One of the biggest problems is well known: In its early stages, ovarian cancer often is asymptomatic. Even when advanced, symptoms tend to be vague and are often dismissed by patients and their doctors. By the time the diagnosis is suspected, most women have disease beyond the ovary.
Because curability greatly depends on stage at presentation, much research is directed toward early detection. Advances in high-resolution imaging and novel blood or urine tumor markers may one day offer effective screening, but at present these methods are unproven.
3 genetic syndromes
Three genetic syndromes account for the vast majority of familial ovarian cancer and approximately 10% of all ovarian cancers. They are:
- breast-ovarian cancer syndrome,
- site-specific ovarian cancer syndrome, and
- hereditary nonpolyposis colorectal cancer syndrome (HNPCC) (Lynch II).2
HNPCC is caused by mutations in a series of genes responsible for repairing errors in DNA replication. Inactivation of these so-called mismatch repair genes results in a high incidence of right-sided colon cancer, endometrial cancer, and ovarian cancer.3
Gene mutations in different populations
The lifetime risk of developing ovarian cancer in the United States is about 1.4%. However, among women with BRCA1 or BRCA2 mutations, the risk rises to 20% to 60%.4
These genes also impart a significant lifetime risk of breast cancer in women and, in the case of BRCA2, in men as well.
Less than 0.15% of the general population carries BRCA1 or BRCA2 mutations. However, the carrier rate is dependent on ethnic background.5 Founder mutations have been identified among multiple unrelated families in Iceland, the Netherlands, and Sweden, and among Jews of Central or Eastern European descent (Ashkenazi).
The best described founder mutations are the 185delAG and 5382insC mutations in BRCA1 and the 6174delT mutation in BRCA2, occurring in Ashkenazi Jews at a carrier rate of 2%.6
Ovarian cancer in Ashkenazi women more likely genetic than sporadic
Although an Ashkenazi woman is no more likely to develop ovarian cancer than a noncarrier, if she does develop the disease, it is far more likely to be genetic rather than sporadic.
Consequently, if a woman of Ashkenazi Jewish descent develops ovarian cancer, there is a 40% chance she carries a mutation in one of these 2 genes.7 Her first-degree relatives (mother, sisters, daughters) have a 20% risk of being gene carriers (50% in autosomal dominant transmission).
Therefore, an Ashkenazi Jewish woman needs only 1 first-degree relative with ovarian cancer to be considered for further genetic counseling.
The 3-2-1 rule for genetic counseling
Genetic counseling is indicated when the patient meets the “3-2-1” rule, known as the modified Amsterdam criteria:
- 3 affected individuals with either colorectal or ovarian cancer, in
- 2 successive generations, with at least
- 1 who developed cancer under the age of 50 years.
When HNPCC is present
Women with documented HNPCC have a 70% lifetime risk of developing endometrial cancer and a lifetime risk of ovarian cancer of 11% or more. Testing for mutation in mismatch repair genes can be performed on peripheral leukocytes. Alternatively, the primary tumor from affected individuals can be assessed for the presence of microsatellite instability, a consequence of defective mismatch repair.3
How to obtain a family history
Women with a significant family history of breast, colorectal, endometrial, or ovarian cancer may face an elevated risk of ovarian cancer. Basic questions about the occurrence of these malignancies in first-degree (mother, sister, daughter) or second-degree (aunt, grandmother) relatives must be a component of every gynecologic history.
1-question assessment
The following question is a simple but effective way to assess familial cancer risk: “Has anyone in your family had breast cancer under the age of 35, or colorectal, uterine, or ovarian cancer?” If so, ask further questions about the relative’s age at onset and how the affected person is related to the patient.
Although an informal pedigree can be constructed in a few moments, a formal pedigree is a more daunting task. More than half of family histories of ovarian cancer are inaccurate—and the error rate increases if the affected family member is a distant relative.8
Moreover, not all ovarian cancers are associated with genetic syndromes. Serous epithelial ovarian, fallopian tube, and peritoneal cancers dominate in BRCA-associated syndromes, whereas mucinous epithelial cancers are very rare. Germ-cell and stromal neoplasms as well as epithelial tumors of low malignant potential are excluded when assessing genetic risk.
Additional documentation is often necessary to verify the history. Such medical detective work may be beyond what a generalist is willing to perform. In that case, referral to a genetic counselor is the next step.
When to refer
Recommendation. If she is of Ashkenazi Jewish descent, like the patient described at the beginning of this article, only 1 first-degree relative indicates referral for genetic counseling.
If the patient claims at least 2 first-degree or second-degree relatives with breast cancer under the age of 35, or with ovarian cancer, suspect a genetic syndrome.
Family histories of particular concern include breast and ovarian cancer in a single individual, or any case of male breast cancer. Refer these patients to genetic counseling for formal pedigree analysis and possible testing for genetic predisposition. In the case of HNPCC, refer the patient if the modified Amsterdam criteria are met.
What does a genetic counselor do?
One of the most important roles of a genetic counselor is constructing as accurate a pedigree as possible. In some cases, this requires much effort on the part of both patient and counselor. It is not uncommon for the counselor to request outside pathology reports, operative notes, and death certificates to verify cancer cases. Pathology slides also may be necessary, while tissue blocks occasionally are requested to assist in genetic testing.
When testing is warranted
Genetic predisposition testing should be undertaken only after formal consultation with a genetic counselor who has expertise in cancer predisposition syndromes. As a policy statement from the American Society of Clinical Oncology concludes, genetic testing for cancer susceptibility should be performed only when:
Obesity is associated with increased risk of ovarian cancer mortality.
- pre- and post-test counseling is available,
- the test can be interpreted, and
- the results will help in medical and surgical management.
CASE 2
A 65-year-old obese nullipara is currently on year 10 of combined hormone replacement therapy (HRT). Although she has no family history of breast or ovarian cancer, she asks about her risk of developing ovarian cancer.
Other risk factors
Findings on the following potential risk factors for ovarian cancer may help address this patient’s concerns:
Dietary factors
In a recent study, obesity was associated with an increased risk of ovarian cancer mortality.10 Women who eat a diet high in saturated fat and low in vegetable fiber also may face an increased risk.
In 1989, the observation that Swedish women had both a high risk of ovarian cancer and the highest per capita dairy consumption in the world led some investigators to postulate a relationship between lactose consumption and ovarian cancer. The reason: When compared with matched controls, women with ovarian cancer were more likely to have high levels of galactose, a component sugar of the disaccharide lactose and a known oocyte toxin.11
This observation, however, has been inconsistent. Therefore, no specific dietary strategy can be recommended to reduce the risk of ovarian cancer.
Recommendation. I would advise this patient to maintain a normal body mass index. Data are insufficient to support more specific diet recommendations.
Talc exposure
When talc is placed on the perineum, it may enter the vagina and ascend to the upper genital tract. Because talc is structurally similar to asbestos, it may theoretically increase the ovarian cancer risk. The observation that women who undergo tubal sterilization procedures or hysterectomy have a lower risk of ovarian cancer supports the ascending-carcinogen hypothesis.
Multiple case-control studies have shown a small but consistent increased risk with perineal application of talc (odds ratio 1.3, 95% confidence interval [CI], 1.1–1.6).12 The risk appears to be time- and dose-dependent, with greater risk associated with more frequent application of perineal talc over a long duration.
Recommendation. The practice of applying genuine talc to the perineum should be discouraged. Cornstarch-based dusting powders are widely available.
Infertility drugs
One of the most difficult issues to study is the relationship between infertility drugs and ovarian cancer, although we know that unexplained infertility is an independent risk factor for ovarian cancer.
A retrospective study that claimed an association between prolonged clomiphene exposure and ovarian cancer13 was not restricted to invasive epithelial ovarian cancers, but included granulosa cell tumors. These estrogen-secreting neoplasms of stromal origin may directly contribute to infertility by disrupting normal follicular maturation and the menstrual cycle.
A number of studies—including a large collaborative analysis of 12 case-control studies—have reported an association between fertility drugs and invasive epithelial ovarian cancer.14 In addition, many of the theoretical models of epithelial ovarian cancer pathogenesis implicate both incessant ovulation and high gonadotropin levels as important steps in malignant transformation of ovarian epithelium.
Oral contraceptives (OCs), which reduce ovulatory events, and moderate gonadotropin levels are associated with a consistent and significant protective effect.
Recommendation. It seems prudent, in the absence of convincing data, to use fertility medication only when absolutely indicated, at the lowest effective dose, and for the shortest duration possible without compromising treatment success.
However, prior exposure to these agents should not be considered an indication for increased surveillance or prophylactic surgery.
Estrogen replacement therapy
Women on estrogen replacement therapy appear to have an increased risk of ovarian cancer. When compared to nonusers, “ever-users” had a relative risk of ovarian cancer of 2.2 (95% CI, 1.53–3.17), and the risk increased with the duration of use.15 Long-term users, defined as women who used estrogen replacement therapy for at least 20 years, had a relative risk of 3.2 (95% CI, 1.7–5.7).16
Although some studies suggest a protective effect of combination hormone replacement regimens that include both estrogen and progesterone, this observation has not been confirmed. Thus, long-term estrogen users should consider an increased risk of developing ovarian cancer when deciding whether to initiate or continue estrogen replacement therapy.
Recommendation. I would advise the Case 2 patient to stop HRT.
Primary prevention
Oral contraceptives
OCs reduce the risk of ovarian cancer significantly. A number of studies have demonstrated a 10% risk reduction per year for up to 5 to 7 years of use.17 This effect appears to persist for at least 10 years after OCs are discontinued. It also has been observed in patients known to be BRCA1 and BRCA2 carriers and is the basis for recommending OCs as a chemoprophylactic method in known carriers who wish to retain fertility.18
Long-term estrogen users should consider increased risk of ovarian cancer. Ovarian cancer: High-risk patients.
However, use of OCs by BRCA carriers is not without some controversy. An Israeli population-based study19 of ovarian cancer and OC use demonstrated a protective effect of pregnancy but not use of OCs. It is unclear why the Israeli data is inconsistent with prior published reports.
Recommendation. In Case 1, the patient has taken OCs for 9 years. Her risk has been substantially reduced, and I would advise her to continue OC pills when not attempting to get pregnant, until she is near menopause.
Prophylactic surgery
In high-risk women, preventive surgery may substantially reduce but not completely eliminate the risk of ovarian cancer. For example, bilateral salpingo-oophorectomy in BRCA carriers reduces the risk of ovarian cancer by more than 90% and the risk of breast cancer by more than 50%.20,21
The operation should be reserved for women with known mutations in BRCA1 or BRCA2 or who have a family history consistent with one of the genetic syndromes associated with ovarian cancer.
The addition of hysterectomy does not appear to increase the efficacy of the operation and should be performed only for concurrent gynecologic indications or if the patient has HNPCC.
Patients should be informed that prophylactic surgery does not protect against subsequent papillary serous carcinoma of the peritoneum. They also should be warned that about 7% of operations detect occult ovarian or tubal carcinoma, which may not be identified until final pathology reports are issued.22 Pathologists should be instructed to submit the entire specimen for sectioning to reduce risk of missing microscopic occult malignancy.
In addition, the patient should be prepared for surgical menopause.
Effective primary prevention strategies such as chemoprophylaxis and prophylactic surgery, when appropriately applied, may spare many women the devastating consequences of this dreaded disease.
Internet resources
- Ovarian Cancer (PDQ): Prevention of ovarian cancer http://www.cancer.gov/cancerinfo/pdq/prevention/ovarian/healthprofessional
- Myriad Genetics: BRCA1/2 mutation prevalence tables http:///www.myriadtests.com/provider/mutprevo.htm
- National Society of Genetic Counselors: Locate a genetic counselor specializing in cancer risk assessment http://www.nsgc.org/resourcelink.asp
1. American Cancer Society. Cancer Facts and Figures 2003. Atlanta, Ga: American Cancer Society; 2003.
2. Trimble EL, Karlan BY, Lagasse LD, et al. Diagnosing the correct ovarian cancer syndrome. Obstet Gynecol. 1991;78:1023-1026.
3. Vasen HFA, Watson P, Mecklin J-P, Lynch HT. and the International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. New clinical criteria for hereditary non-polyposis colon cancer (HNPCC, Lynch Syndrome): proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453-1456.
4. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265-271.
5. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117-1130.
6. Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401-1408.
7. Lu K, Muto MG, Cramer DW, et al. Prevalence of BRCA mutations among women of Ashkenazi Jewish descent with epithelial ovarian cancer. Obstet Gynecol. 1998;92:596-600.
8. Parent ME, Ghadirian P, Lacroix A, et al. The reliability of recollections of family history: implications for the medical provider. J Cancer Educ. 1997;12:114-120.
9. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397-2406.
10. Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer. A follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95:1244-1248.
11. Cramer DW, Harlow BL, Willett WC, et al. Galactose consumption and metabolism in relation to the risk of ovarian cancer. Lancet. 1989;2:66-71.
12. Gertig DM, Hunter DJ, Cramer DW, et al. Prospective study of talc use and ovarian cancer. J Natl Cancer Inst. 2000;92:249-252.
13. Rossing MA, Daling JR, Weiss NS, et al. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331:771-776.
14. Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk II. Invasive epithelial cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1184-1203.
15. Rodriguez C, Patel AV, Calle EE, et al. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA. 2001;285:1460-1465.
16. Lacey JV, Jr, Mink PJ, Jubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334-341.
17. The reduction in risk of ovarian cancer associated with oral contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med. 1987;316:650-655.
18. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Study Group. N Engl J Med. 1998;339:424-428.
19. Modan B, Hartge P, Hirsh-Yechezkel G, et al. Parity, oral contraceptives and the risk of ovarian cancer among carriers and non-carriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235-240.
20. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
21. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
22. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728.-
- Ask every patient: ”Has anyone in your family had breast cancer under the age of 35, or colorectal, uterine, or ovarian cancer?
- Genetic testing is appropriate only when pre- and post-test counseling is available, the test can be interpreted, and the results will help in medical and surgical management.
- Oral contraceptives may reduce risk by 10% per year for up to 5 to 7 years of use.
- Bilateral salpingo-oophorectomy in BRCA carriers reduces the risk of ovarian cancer by more than 90% and the risk of breast cancer by more than 50%.
A 42-year-old woman of Ashkenazi Jewish ancestry, whose mother had a diagnosis of ovarian cancer a year earlier, is worried that she may also be at risk.
Her family has experienced no other cases of breast, colorectal, or ovarian cancer. She has 2 children and has used oral contraceptives for 9 years.
Primary prevention is an alternative to diagnosis of ovarian cancer at an early stage—a goal that is all too often unattainable.
If we identify women with increased risk based on their family history, environmental exposure, hormone use, or reproductive experiences, we can counsel them about lifestyle changes, chemoprophylaxis, or preventive surgery, depending on their reproductive desires and overall ovarian cancer risk.
Using 2 hypothetical cases, this article describes a quick history to screen for potential high risk, and summarizes what we can advise concerned patients, based on findings to date.
Topics include:
- genetic risk assessment
- when to refer to a genetic counselor
- risk factors
- oral contraceptives for primary prevention
- prophylactic surgery
Despite advances in medical and surgical therapies, ovarian cancer remains the deadliest gynecologic malignancy and the fifth leading cause of cancer deaths among women in the United States. This year the disease will strike an estimated 25,400 women in this country and kill more than 14,000.1
Early detection versus primary prevention
One of the biggest problems is well known: In its early stages, ovarian cancer often is asymptomatic. Even when advanced, symptoms tend to be vague and are often dismissed by patients and their doctors. By the time the diagnosis is suspected, most women have disease beyond the ovary.
Because curability greatly depends on stage at presentation, much research is directed toward early detection. Advances in high-resolution imaging and novel blood or urine tumor markers may one day offer effective screening, but at present these methods are unproven.
3 genetic syndromes
Three genetic syndromes account for the vast majority of familial ovarian cancer and approximately 10% of all ovarian cancers. They are:
- breast-ovarian cancer syndrome,
- site-specific ovarian cancer syndrome, and
- hereditary nonpolyposis colorectal cancer syndrome (HNPCC) (Lynch II).2
HNPCC is caused by mutations in a series of genes responsible for repairing errors in DNA replication. Inactivation of these so-called mismatch repair genes results in a high incidence of right-sided colon cancer, endometrial cancer, and ovarian cancer.3
Gene mutations in different populations
The lifetime risk of developing ovarian cancer in the United States is about 1.4%. However, among women with BRCA1 or BRCA2 mutations, the risk rises to 20% to 60%.4
These genes also impart a significant lifetime risk of breast cancer in women and, in the case of BRCA2, in men as well.
Less than 0.15% of the general population carries BRCA1 or BRCA2 mutations. However, the carrier rate is dependent on ethnic background.5 Founder mutations have been identified among multiple unrelated families in Iceland, the Netherlands, and Sweden, and among Jews of Central or Eastern European descent (Ashkenazi).
The best described founder mutations are the 185delAG and 5382insC mutations in BRCA1 and the 6174delT mutation in BRCA2, occurring in Ashkenazi Jews at a carrier rate of 2%.6
Ovarian cancer in Ashkenazi women more likely genetic than sporadic
Although an Ashkenazi woman is no more likely to develop ovarian cancer than a noncarrier, if she does develop the disease, it is far more likely to be genetic rather than sporadic.
Consequently, if a woman of Ashkenazi Jewish descent develops ovarian cancer, there is a 40% chance she carries a mutation in one of these 2 genes.7 Her first-degree relatives (mother, sisters, daughters) have a 20% risk of being gene carriers (50% in autosomal dominant transmission).
Therefore, an Ashkenazi Jewish woman needs only 1 first-degree relative with ovarian cancer to be considered for further genetic counseling.
The 3-2-1 rule for genetic counseling
Genetic counseling is indicated when the patient meets the “3-2-1” rule, known as the modified Amsterdam criteria:
- 3 affected individuals with either colorectal or ovarian cancer, in
- 2 successive generations, with at least
- 1 who developed cancer under the age of 50 years.
When HNPCC is present
Women with documented HNPCC have a 70% lifetime risk of developing endometrial cancer and a lifetime risk of ovarian cancer of 11% or more. Testing for mutation in mismatch repair genes can be performed on peripheral leukocytes. Alternatively, the primary tumor from affected individuals can be assessed for the presence of microsatellite instability, a consequence of defective mismatch repair.3
How to obtain a family history
Women with a significant family history of breast, colorectal, endometrial, or ovarian cancer may face an elevated risk of ovarian cancer. Basic questions about the occurrence of these malignancies in first-degree (mother, sister, daughter) or second-degree (aunt, grandmother) relatives must be a component of every gynecologic history.
1-question assessment
The following question is a simple but effective way to assess familial cancer risk: “Has anyone in your family had breast cancer under the age of 35, or colorectal, uterine, or ovarian cancer?” If so, ask further questions about the relative’s age at onset and how the affected person is related to the patient.
Although an informal pedigree can be constructed in a few moments, a formal pedigree is a more daunting task. More than half of family histories of ovarian cancer are inaccurate—and the error rate increases if the affected family member is a distant relative.8
Moreover, not all ovarian cancers are associated with genetic syndromes. Serous epithelial ovarian, fallopian tube, and peritoneal cancers dominate in BRCA-associated syndromes, whereas mucinous epithelial cancers are very rare. Germ-cell and stromal neoplasms as well as epithelial tumors of low malignant potential are excluded when assessing genetic risk.
Additional documentation is often necessary to verify the history. Such medical detective work may be beyond what a generalist is willing to perform. In that case, referral to a genetic counselor is the next step.
When to refer
Recommendation. If she is of Ashkenazi Jewish descent, like the patient described at the beginning of this article, only 1 first-degree relative indicates referral for genetic counseling.
If the patient claims at least 2 first-degree or second-degree relatives with breast cancer under the age of 35, or with ovarian cancer, suspect a genetic syndrome.
Family histories of particular concern include breast and ovarian cancer in a single individual, or any case of male breast cancer. Refer these patients to genetic counseling for formal pedigree analysis and possible testing for genetic predisposition. In the case of HNPCC, refer the patient if the modified Amsterdam criteria are met.
What does a genetic counselor do?
One of the most important roles of a genetic counselor is constructing as accurate a pedigree as possible. In some cases, this requires much effort on the part of both patient and counselor. It is not uncommon for the counselor to request outside pathology reports, operative notes, and death certificates to verify cancer cases. Pathology slides also may be necessary, while tissue blocks occasionally are requested to assist in genetic testing.
When testing is warranted
Genetic predisposition testing should be undertaken only after formal consultation with a genetic counselor who has expertise in cancer predisposition syndromes. As a policy statement from the American Society of Clinical Oncology concludes, genetic testing for cancer susceptibility should be performed only when:
Obesity is associated with increased risk of ovarian cancer mortality.
- pre- and post-test counseling is available,
- the test can be interpreted, and
- the results will help in medical and surgical management.
CASE 2
A 65-year-old obese nullipara is currently on year 10 of combined hormone replacement therapy (HRT). Although she has no family history of breast or ovarian cancer, she asks about her risk of developing ovarian cancer.
Other risk factors
Findings on the following potential risk factors for ovarian cancer may help address this patient’s concerns:
Dietary factors
In a recent study, obesity was associated with an increased risk of ovarian cancer mortality.10 Women who eat a diet high in saturated fat and low in vegetable fiber also may face an increased risk.
In 1989, the observation that Swedish women had both a high risk of ovarian cancer and the highest per capita dairy consumption in the world led some investigators to postulate a relationship between lactose consumption and ovarian cancer. The reason: When compared with matched controls, women with ovarian cancer were more likely to have high levels of galactose, a component sugar of the disaccharide lactose and a known oocyte toxin.11
This observation, however, has been inconsistent. Therefore, no specific dietary strategy can be recommended to reduce the risk of ovarian cancer.
Recommendation. I would advise this patient to maintain a normal body mass index. Data are insufficient to support more specific diet recommendations.
Talc exposure
When talc is placed on the perineum, it may enter the vagina and ascend to the upper genital tract. Because talc is structurally similar to asbestos, it may theoretically increase the ovarian cancer risk. The observation that women who undergo tubal sterilization procedures or hysterectomy have a lower risk of ovarian cancer supports the ascending-carcinogen hypothesis.
Multiple case-control studies have shown a small but consistent increased risk with perineal application of talc (odds ratio 1.3, 95% confidence interval [CI], 1.1–1.6).12 The risk appears to be time- and dose-dependent, with greater risk associated with more frequent application of perineal talc over a long duration.
Recommendation. The practice of applying genuine talc to the perineum should be discouraged. Cornstarch-based dusting powders are widely available.
Infertility drugs
One of the most difficult issues to study is the relationship between infertility drugs and ovarian cancer, although we know that unexplained infertility is an independent risk factor for ovarian cancer.
A retrospective study that claimed an association between prolonged clomiphene exposure and ovarian cancer13 was not restricted to invasive epithelial ovarian cancers, but included granulosa cell tumors. These estrogen-secreting neoplasms of stromal origin may directly contribute to infertility by disrupting normal follicular maturation and the menstrual cycle.
A number of studies—including a large collaborative analysis of 12 case-control studies—have reported an association between fertility drugs and invasive epithelial ovarian cancer.14 In addition, many of the theoretical models of epithelial ovarian cancer pathogenesis implicate both incessant ovulation and high gonadotropin levels as important steps in malignant transformation of ovarian epithelium.
Oral contraceptives (OCs), which reduce ovulatory events, and moderate gonadotropin levels are associated with a consistent and significant protective effect.
Recommendation. It seems prudent, in the absence of convincing data, to use fertility medication only when absolutely indicated, at the lowest effective dose, and for the shortest duration possible without compromising treatment success.
However, prior exposure to these agents should not be considered an indication for increased surveillance or prophylactic surgery.
Estrogen replacement therapy
Women on estrogen replacement therapy appear to have an increased risk of ovarian cancer. When compared to nonusers, “ever-users” had a relative risk of ovarian cancer of 2.2 (95% CI, 1.53–3.17), and the risk increased with the duration of use.15 Long-term users, defined as women who used estrogen replacement therapy for at least 20 years, had a relative risk of 3.2 (95% CI, 1.7–5.7).16
Although some studies suggest a protective effect of combination hormone replacement regimens that include both estrogen and progesterone, this observation has not been confirmed. Thus, long-term estrogen users should consider an increased risk of developing ovarian cancer when deciding whether to initiate or continue estrogen replacement therapy.
Recommendation. I would advise the Case 2 patient to stop HRT.
Primary prevention
Oral contraceptives
OCs reduce the risk of ovarian cancer significantly. A number of studies have demonstrated a 10% risk reduction per year for up to 5 to 7 years of use.17 This effect appears to persist for at least 10 years after OCs are discontinued. It also has been observed in patients known to be BRCA1 and BRCA2 carriers and is the basis for recommending OCs as a chemoprophylactic method in known carriers who wish to retain fertility.18
Long-term estrogen users should consider increased risk of ovarian cancer. Ovarian cancer: High-risk patients.
However, use of OCs by BRCA carriers is not without some controversy. An Israeli population-based study19 of ovarian cancer and OC use demonstrated a protective effect of pregnancy but not use of OCs. It is unclear why the Israeli data is inconsistent with prior published reports.
Recommendation. In Case 1, the patient has taken OCs for 9 years. Her risk has been substantially reduced, and I would advise her to continue OC pills when not attempting to get pregnant, until she is near menopause.
Prophylactic surgery
In high-risk women, preventive surgery may substantially reduce but not completely eliminate the risk of ovarian cancer. For example, bilateral salpingo-oophorectomy in BRCA carriers reduces the risk of ovarian cancer by more than 90% and the risk of breast cancer by more than 50%.20,21
The operation should be reserved for women with known mutations in BRCA1 or BRCA2 or who have a family history consistent with one of the genetic syndromes associated with ovarian cancer.
The addition of hysterectomy does not appear to increase the efficacy of the operation and should be performed only for concurrent gynecologic indications or if the patient has HNPCC.
Patients should be informed that prophylactic surgery does not protect against subsequent papillary serous carcinoma of the peritoneum. They also should be warned that about 7% of operations detect occult ovarian or tubal carcinoma, which may not be identified until final pathology reports are issued.22 Pathologists should be instructed to submit the entire specimen for sectioning to reduce risk of missing microscopic occult malignancy.
In addition, the patient should be prepared for surgical menopause.
Effective primary prevention strategies such as chemoprophylaxis and prophylactic surgery, when appropriately applied, may spare many women the devastating consequences of this dreaded disease.
Internet resources
- Ovarian Cancer (PDQ): Prevention of ovarian cancer http://www.cancer.gov/cancerinfo/pdq/prevention/ovarian/healthprofessional
- Myriad Genetics: BRCA1/2 mutation prevalence tables http:///www.myriadtests.com/provider/mutprevo.htm
- National Society of Genetic Counselors: Locate a genetic counselor specializing in cancer risk assessment http://www.nsgc.org/resourcelink.asp
- Ask every patient: ”Has anyone in your family had breast cancer under the age of 35, or colorectal, uterine, or ovarian cancer?
- Genetic testing is appropriate only when pre- and post-test counseling is available, the test can be interpreted, and the results will help in medical and surgical management.
- Oral contraceptives may reduce risk by 10% per year for up to 5 to 7 years of use.
- Bilateral salpingo-oophorectomy in BRCA carriers reduces the risk of ovarian cancer by more than 90% and the risk of breast cancer by more than 50%.
A 42-year-old woman of Ashkenazi Jewish ancestry, whose mother had a diagnosis of ovarian cancer a year earlier, is worried that she may also be at risk.
Her family has experienced no other cases of breast, colorectal, or ovarian cancer. She has 2 children and has used oral contraceptives for 9 years.
Primary prevention is an alternative to diagnosis of ovarian cancer at an early stage—a goal that is all too often unattainable.
If we identify women with increased risk based on their family history, environmental exposure, hormone use, or reproductive experiences, we can counsel them about lifestyle changes, chemoprophylaxis, or preventive surgery, depending on their reproductive desires and overall ovarian cancer risk.
Using 2 hypothetical cases, this article describes a quick history to screen for potential high risk, and summarizes what we can advise concerned patients, based on findings to date.
Topics include:
- genetic risk assessment
- when to refer to a genetic counselor
- risk factors
- oral contraceptives for primary prevention
- prophylactic surgery
Despite advances in medical and surgical therapies, ovarian cancer remains the deadliest gynecologic malignancy and the fifth leading cause of cancer deaths among women in the United States. This year the disease will strike an estimated 25,400 women in this country and kill more than 14,000.1
Early detection versus primary prevention
One of the biggest problems is well known: In its early stages, ovarian cancer often is asymptomatic. Even when advanced, symptoms tend to be vague and are often dismissed by patients and their doctors. By the time the diagnosis is suspected, most women have disease beyond the ovary.
Because curability greatly depends on stage at presentation, much research is directed toward early detection. Advances in high-resolution imaging and novel blood or urine tumor markers may one day offer effective screening, but at present these methods are unproven.
3 genetic syndromes
Three genetic syndromes account for the vast majority of familial ovarian cancer and approximately 10% of all ovarian cancers. They are:
- breast-ovarian cancer syndrome,
- site-specific ovarian cancer syndrome, and
- hereditary nonpolyposis colorectal cancer syndrome (HNPCC) (Lynch II).2
HNPCC is caused by mutations in a series of genes responsible for repairing errors in DNA replication. Inactivation of these so-called mismatch repair genes results in a high incidence of right-sided colon cancer, endometrial cancer, and ovarian cancer.3
Gene mutations in different populations
The lifetime risk of developing ovarian cancer in the United States is about 1.4%. However, among women with BRCA1 or BRCA2 mutations, the risk rises to 20% to 60%.4
These genes also impart a significant lifetime risk of breast cancer in women and, in the case of BRCA2, in men as well.
Less than 0.15% of the general population carries BRCA1 or BRCA2 mutations. However, the carrier rate is dependent on ethnic background.5 Founder mutations have been identified among multiple unrelated families in Iceland, the Netherlands, and Sweden, and among Jews of Central or Eastern European descent (Ashkenazi).
The best described founder mutations are the 185delAG and 5382insC mutations in BRCA1 and the 6174delT mutation in BRCA2, occurring in Ashkenazi Jews at a carrier rate of 2%.6
Ovarian cancer in Ashkenazi women more likely genetic than sporadic
Although an Ashkenazi woman is no more likely to develop ovarian cancer than a noncarrier, if she does develop the disease, it is far more likely to be genetic rather than sporadic.
Consequently, if a woman of Ashkenazi Jewish descent develops ovarian cancer, there is a 40% chance she carries a mutation in one of these 2 genes.7 Her first-degree relatives (mother, sisters, daughters) have a 20% risk of being gene carriers (50% in autosomal dominant transmission).
Therefore, an Ashkenazi Jewish woman needs only 1 first-degree relative with ovarian cancer to be considered for further genetic counseling.
The 3-2-1 rule for genetic counseling
Genetic counseling is indicated when the patient meets the “3-2-1” rule, known as the modified Amsterdam criteria:
- 3 affected individuals with either colorectal or ovarian cancer, in
- 2 successive generations, with at least
- 1 who developed cancer under the age of 50 years.
When HNPCC is present
Women with documented HNPCC have a 70% lifetime risk of developing endometrial cancer and a lifetime risk of ovarian cancer of 11% or more. Testing for mutation in mismatch repair genes can be performed on peripheral leukocytes. Alternatively, the primary tumor from affected individuals can be assessed for the presence of microsatellite instability, a consequence of defective mismatch repair.3
How to obtain a family history
Women with a significant family history of breast, colorectal, endometrial, or ovarian cancer may face an elevated risk of ovarian cancer. Basic questions about the occurrence of these malignancies in first-degree (mother, sister, daughter) or second-degree (aunt, grandmother) relatives must be a component of every gynecologic history.
1-question assessment
The following question is a simple but effective way to assess familial cancer risk: “Has anyone in your family had breast cancer under the age of 35, or colorectal, uterine, or ovarian cancer?” If so, ask further questions about the relative’s age at onset and how the affected person is related to the patient.
Although an informal pedigree can be constructed in a few moments, a formal pedigree is a more daunting task. More than half of family histories of ovarian cancer are inaccurate—and the error rate increases if the affected family member is a distant relative.8
Moreover, not all ovarian cancers are associated with genetic syndromes. Serous epithelial ovarian, fallopian tube, and peritoneal cancers dominate in BRCA-associated syndromes, whereas mucinous epithelial cancers are very rare. Germ-cell and stromal neoplasms as well as epithelial tumors of low malignant potential are excluded when assessing genetic risk.
Additional documentation is often necessary to verify the history. Such medical detective work may be beyond what a generalist is willing to perform. In that case, referral to a genetic counselor is the next step.
When to refer
Recommendation. If she is of Ashkenazi Jewish descent, like the patient described at the beginning of this article, only 1 first-degree relative indicates referral for genetic counseling.
If the patient claims at least 2 first-degree or second-degree relatives with breast cancer under the age of 35, or with ovarian cancer, suspect a genetic syndrome.
Family histories of particular concern include breast and ovarian cancer in a single individual, or any case of male breast cancer. Refer these patients to genetic counseling for formal pedigree analysis and possible testing for genetic predisposition. In the case of HNPCC, refer the patient if the modified Amsterdam criteria are met.
What does a genetic counselor do?
One of the most important roles of a genetic counselor is constructing as accurate a pedigree as possible. In some cases, this requires much effort on the part of both patient and counselor. It is not uncommon for the counselor to request outside pathology reports, operative notes, and death certificates to verify cancer cases. Pathology slides also may be necessary, while tissue blocks occasionally are requested to assist in genetic testing.
When testing is warranted
Genetic predisposition testing should be undertaken only after formal consultation with a genetic counselor who has expertise in cancer predisposition syndromes. As a policy statement from the American Society of Clinical Oncology concludes, genetic testing for cancer susceptibility should be performed only when:
Obesity is associated with increased risk of ovarian cancer mortality.
- pre- and post-test counseling is available,
- the test can be interpreted, and
- the results will help in medical and surgical management.
CASE 2
A 65-year-old obese nullipara is currently on year 10 of combined hormone replacement therapy (HRT). Although she has no family history of breast or ovarian cancer, she asks about her risk of developing ovarian cancer.
Other risk factors
Findings on the following potential risk factors for ovarian cancer may help address this patient’s concerns:
Dietary factors
In a recent study, obesity was associated with an increased risk of ovarian cancer mortality.10 Women who eat a diet high in saturated fat and low in vegetable fiber also may face an increased risk.
In 1989, the observation that Swedish women had both a high risk of ovarian cancer and the highest per capita dairy consumption in the world led some investigators to postulate a relationship between lactose consumption and ovarian cancer. The reason: When compared with matched controls, women with ovarian cancer were more likely to have high levels of galactose, a component sugar of the disaccharide lactose and a known oocyte toxin.11
This observation, however, has been inconsistent. Therefore, no specific dietary strategy can be recommended to reduce the risk of ovarian cancer.
Recommendation. I would advise this patient to maintain a normal body mass index. Data are insufficient to support more specific diet recommendations.
Talc exposure
When talc is placed on the perineum, it may enter the vagina and ascend to the upper genital tract. Because talc is structurally similar to asbestos, it may theoretically increase the ovarian cancer risk. The observation that women who undergo tubal sterilization procedures or hysterectomy have a lower risk of ovarian cancer supports the ascending-carcinogen hypothesis.
Multiple case-control studies have shown a small but consistent increased risk with perineal application of talc (odds ratio 1.3, 95% confidence interval [CI], 1.1–1.6).12 The risk appears to be time- and dose-dependent, with greater risk associated with more frequent application of perineal talc over a long duration.
Recommendation. The practice of applying genuine talc to the perineum should be discouraged. Cornstarch-based dusting powders are widely available.
Infertility drugs
One of the most difficult issues to study is the relationship between infertility drugs and ovarian cancer, although we know that unexplained infertility is an independent risk factor for ovarian cancer.
A retrospective study that claimed an association between prolonged clomiphene exposure and ovarian cancer13 was not restricted to invasive epithelial ovarian cancers, but included granulosa cell tumors. These estrogen-secreting neoplasms of stromal origin may directly contribute to infertility by disrupting normal follicular maturation and the menstrual cycle.
A number of studies—including a large collaborative analysis of 12 case-control studies—have reported an association between fertility drugs and invasive epithelial ovarian cancer.14 In addition, many of the theoretical models of epithelial ovarian cancer pathogenesis implicate both incessant ovulation and high gonadotropin levels as important steps in malignant transformation of ovarian epithelium.
Oral contraceptives (OCs), which reduce ovulatory events, and moderate gonadotropin levels are associated with a consistent and significant protective effect.
Recommendation. It seems prudent, in the absence of convincing data, to use fertility medication only when absolutely indicated, at the lowest effective dose, and for the shortest duration possible without compromising treatment success.
However, prior exposure to these agents should not be considered an indication for increased surveillance or prophylactic surgery.
Estrogen replacement therapy
Women on estrogen replacement therapy appear to have an increased risk of ovarian cancer. When compared to nonusers, “ever-users” had a relative risk of ovarian cancer of 2.2 (95% CI, 1.53–3.17), and the risk increased with the duration of use.15 Long-term users, defined as women who used estrogen replacement therapy for at least 20 years, had a relative risk of 3.2 (95% CI, 1.7–5.7).16
Although some studies suggest a protective effect of combination hormone replacement regimens that include both estrogen and progesterone, this observation has not been confirmed. Thus, long-term estrogen users should consider an increased risk of developing ovarian cancer when deciding whether to initiate or continue estrogen replacement therapy.
Recommendation. I would advise the Case 2 patient to stop HRT.
Primary prevention
Oral contraceptives
OCs reduce the risk of ovarian cancer significantly. A number of studies have demonstrated a 10% risk reduction per year for up to 5 to 7 years of use.17 This effect appears to persist for at least 10 years after OCs are discontinued. It also has been observed in patients known to be BRCA1 and BRCA2 carriers and is the basis for recommending OCs as a chemoprophylactic method in known carriers who wish to retain fertility.18
Long-term estrogen users should consider increased risk of ovarian cancer. Ovarian cancer: High-risk patients.
However, use of OCs by BRCA carriers is not without some controversy. An Israeli population-based study19 of ovarian cancer and OC use demonstrated a protective effect of pregnancy but not use of OCs. It is unclear why the Israeli data is inconsistent with prior published reports.
Recommendation. In Case 1, the patient has taken OCs for 9 years. Her risk has been substantially reduced, and I would advise her to continue OC pills when not attempting to get pregnant, until she is near menopause.
Prophylactic surgery
In high-risk women, preventive surgery may substantially reduce but not completely eliminate the risk of ovarian cancer. For example, bilateral salpingo-oophorectomy in BRCA carriers reduces the risk of ovarian cancer by more than 90% and the risk of breast cancer by more than 50%.20,21
The operation should be reserved for women with known mutations in BRCA1 or BRCA2 or who have a family history consistent with one of the genetic syndromes associated with ovarian cancer.
The addition of hysterectomy does not appear to increase the efficacy of the operation and should be performed only for concurrent gynecologic indications or if the patient has HNPCC.
Patients should be informed that prophylactic surgery does not protect against subsequent papillary serous carcinoma of the peritoneum. They also should be warned that about 7% of operations detect occult ovarian or tubal carcinoma, which may not be identified until final pathology reports are issued.22 Pathologists should be instructed to submit the entire specimen for sectioning to reduce risk of missing microscopic occult malignancy.
In addition, the patient should be prepared for surgical menopause.
Effective primary prevention strategies such as chemoprophylaxis and prophylactic surgery, when appropriately applied, may spare many women the devastating consequences of this dreaded disease.
Internet resources
- Ovarian Cancer (PDQ): Prevention of ovarian cancer http://www.cancer.gov/cancerinfo/pdq/prevention/ovarian/healthprofessional
- Myriad Genetics: BRCA1/2 mutation prevalence tables http:///www.myriadtests.com/provider/mutprevo.htm
- National Society of Genetic Counselors: Locate a genetic counselor specializing in cancer risk assessment http://www.nsgc.org/resourcelink.asp
1. American Cancer Society. Cancer Facts and Figures 2003. Atlanta, Ga: American Cancer Society; 2003.
2. Trimble EL, Karlan BY, Lagasse LD, et al. Diagnosing the correct ovarian cancer syndrome. Obstet Gynecol. 1991;78:1023-1026.
3. Vasen HFA, Watson P, Mecklin J-P, Lynch HT. and the International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. New clinical criteria for hereditary non-polyposis colon cancer (HNPCC, Lynch Syndrome): proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453-1456.
4. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265-271.
5. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117-1130.
6. Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401-1408.
7. Lu K, Muto MG, Cramer DW, et al. Prevalence of BRCA mutations among women of Ashkenazi Jewish descent with epithelial ovarian cancer. Obstet Gynecol. 1998;92:596-600.
8. Parent ME, Ghadirian P, Lacroix A, et al. The reliability of recollections of family history: implications for the medical provider. J Cancer Educ. 1997;12:114-120.
9. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397-2406.
10. Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer. A follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95:1244-1248.
11. Cramer DW, Harlow BL, Willett WC, et al. Galactose consumption and metabolism in relation to the risk of ovarian cancer. Lancet. 1989;2:66-71.
12. Gertig DM, Hunter DJ, Cramer DW, et al. Prospective study of talc use and ovarian cancer. J Natl Cancer Inst. 2000;92:249-252.
13. Rossing MA, Daling JR, Weiss NS, et al. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331:771-776.
14. Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk II. Invasive epithelial cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1184-1203.
15. Rodriguez C, Patel AV, Calle EE, et al. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA. 2001;285:1460-1465.
16. Lacey JV, Jr, Mink PJ, Jubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334-341.
17. The reduction in risk of ovarian cancer associated with oral contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med. 1987;316:650-655.
18. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Study Group. N Engl J Med. 1998;339:424-428.
19. Modan B, Hartge P, Hirsh-Yechezkel G, et al. Parity, oral contraceptives and the risk of ovarian cancer among carriers and non-carriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235-240.
20. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
21. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
22. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728.-
1. American Cancer Society. Cancer Facts and Figures 2003. Atlanta, Ga: American Cancer Society; 2003.
2. Trimble EL, Karlan BY, Lagasse LD, et al. Diagnosing the correct ovarian cancer syndrome. Obstet Gynecol. 1991;78:1023-1026.
3. Vasen HFA, Watson P, Mecklin J-P, Lynch HT. and the International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. New clinical criteria for hereditary non-polyposis colon cancer (HNPCC, Lynch Syndrome): proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453-1456.
4. Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265-271.
5. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117-1130.
6. Struewing JP, Hartge P, Wacholder S, et al. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401-1408.
7. Lu K, Muto MG, Cramer DW, et al. Prevalence of BRCA mutations among women of Ashkenazi Jewish descent with epithelial ovarian cancer. Obstet Gynecol. 1998;92:596-600.
8. Parent ME, Ghadirian P, Lacroix A, et al. The reliability of recollections of family history: implications for the medical provider. J Cancer Educ. 1997;12:114-120.
9. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397-2406.
10. Engeland A, Tretli S, Bjorge T. Height, body mass index, and ovarian cancer. A follow-up of 1.1 million Norwegian women. J Natl Cancer Inst. 2003;95:1244-1248.
11. Cramer DW, Harlow BL, Willett WC, et al. Galactose consumption and metabolism in relation to the risk of ovarian cancer. Lancet. 1989;2:66-71.
12. Gertig DM, Hunter DJ, Cramer DW, et al. Prospective study of talc use and ovarian cancer. J Natl Cancer Inst. 2000;92:249-252.
13. Rossing MA, Daling JR, Weiss NS, et al. Ovarian tumors in a cohort of infertile women. N Engl J Med. 1994;331:771-776.
14. Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk II. Invasive epithelial cancers in white women. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1184-1203.
15. Rodriguez C, Patel AV, Calle EE, et al. Estrogen replacement therapy and ovarian cancer mortality in a large prospective study of US women. JAMA. 2001;285:1460-1465.
16. Lacey JV, Jr, Mink PJ, Jubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334-341.
17. The reduction in risk of ovarian cancer associated with oral contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med. 1987;316:650-655.
18. Narod SA, Risch H, Moslehi R, et al. Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary Ovarian Cancer Study Group. N Engl J Med. 1998;339:424-428.
19. Modan B, Hartge P, Hirsh-Yechezkel G, et al. Parity, oral contraceptives and the risk of ovarian cancer among carriers and non-carriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235-240.
20. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
21. Kauff ND, Satagopan JM, Robson ME, et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609-1615.
22. Lu KH, Garber JE, Cramer DW, et al. Occult ovarian tumors in women with BRCA1 or BRCA2 mutations undergoing prophylactic oophorectomy. J Clin Oncol. 2000;18:2728.-
PPROM: New strategies for expectant management
- A large, prospective, randomized, controlled trial clearly showed that antibiotics decrease neonatal morbidities and prolong the interval between rupture of membranes and delivery.
- Avoid digital cervical examination during testing for rupture of membranes because it may hasten delivery and increase neonatal morbidity.
- Consider giving magnesium sulfate and corticosteroids to patients with preterm premature rupture of membranes at or beyond 23 weeks, provided there is no evidence of infection. After 32 weeks, deliver the infant if either intraamniotic infection or fetal lung maturity is present.
- At delivery, amnioinfusion decreases variable decelerations and improves pH when clinically indicated.
The outlook for preterm premature rupture of membranes (PPROM) has improved considerably since a landmark study showed clear benefits of antibiotics.
Previously, approximately 80% of women with PPROM experienced spontaneous labor within 48 hours with expectant management.
We now know that infection is a major cause of PPROM and that antibiotic therapy decreases neonatal morbidity and increases the interval from rupture of membranes to delivery. These benefits are most evident at early gestational ages.
This article reviews newer studies, as well as that breakthrough 1997 study, and their implications for diagnosis and treatment, including optimal drug regimens. Recommendations for 4 special situations are also discussed:
- presence of cerclage,
- herpes simplex infection,
- bleeding, and
- outpatient management.
Despite progress, research is still needed. For example, because much of our information about clinical interventions for PPROM, such as corticosteroid therapy and use of tocolytics, predates the use of antibiotics, many earlier studies need to be repeated.
A variety of methods confirm the diagnosis
Approximately 90% of patients with PPROM report nonurinary fluid leaking from the vagina.1,2 Nevertheless, a history of leaking fluid should be confirmed by examination.
Nitrazine paper test
The most common way to diagnose rupture of membranes involves exposing nitrazine paper to the leaking fluid. If the fluid is alkaline, the paper will turn bright blue.
However, seminal fluid, urine, and blood can also turn nitrazine paper blue. Therefore, a confirmatory method should be used with nitrazine paper testing.
Fern pattern test
When amniotic fluid proteins are allowed to dry and then examined under a microscope, they will exhibit a “fern” pattern.
The combination of nitrazine paper and the fern test has a specificity of over 90%.
Avoid digital cervical examination
Digital examination during testing may diminish latency (the period from rupture of membranes to delivery) and increase neonatal morbidity.3
Testing at early gestational ages
Occasionally, PPROM occurs early in the middle trimester. At such early gestations, amniotic fluid may not fern or produce the classic blue color when exposed to nitrazine paper.
Other tests may be used, however:
- Ultrasound can help in evaluating the quantity of amniotic fluid.
- Indigo carmine dye can be placed in the amniotic cavity, with a tampon inserted in the vagina. If the tampon turns blue when the patient ambulates, rupture of membranes is confirmed.
- Alpha-fetoprotein and human choriogonadotropin. When alpha-fetoprotein is present and human choriogonadotropin is highly concentrated in amniotic fluid, ruptured membranes are confirmed.4
Fortunately, a large majority of patients can be diagnosed using clinical history, nitrazine paper, and the fern pattern test.
Definition
Preterm premature rupture of membranes (PPROM) is ruptured membranes before the 37th week of gestation.
Incidence
PPROM complicates 3% to 4.5% of all pregnancies and is responsible for 30% to 40% of all preterm births.26 This high incidence makes it a major cause of premature birth in the United States.
Predisposing factors
African-American women appear to have a higher incidence of PPROM than Caucasian women.27
Smoking is strongly correlated with PPROM,28 as it is with most poor perinatal outcomes.
Nutritional deficiencies in hydroxyproline, vitamin C, copper, and zinc also are linked.26
The precise cause is unknown
Ascending infection29 and uterine bleeding1 are both strongly correlated with PPROM. An incompetent cervix is also thought to play a role, as are conditions involving connective tissue abnormalities, such as those found in Ehlers-Danlos syndrome.
Unfortunately, we have yet to identify a factor or factors that could be linked to a specific cause for any given patient. Such knowledge would enable us to abandon empiric treatment in favor of more specific management guidelines.
Infection plays a major role in most cases
Bacteria in the vagina ascend through the intracervical canal and establish subclinical infection in the lower uterine segment.29
This activates macrophages and polymorphoneutrophils as a host defense mechanism. Macrophages release interleukin-6, interleukin-8, and tumor necrosing factors, while lysis of polymorphoneutrophils triggers the release of proteolytic enzymes.
These enzymes not only destroy the invasive microorganisms, but are capable of damaging the chorion and amnion, increasing the likelihood of ruptured membranes.
As we come to understand these mechanisms more fully, we should be able to tailor therapy to specific etiologies, thereby optimizing outcomes.30
Gestational age determines management
The number 1 factor determining management is gestational age.
Before 23 weeks, the pregnancy is considered previable
Counsel these patients about the risk of infection, the prognosis of extreme prematurity, and the generally poor outcome of these pregnancies, which can involve pulmonary hypoplasia secondary to severe oligohydramnios (FIGURE 1).
Induction of labor is an option to terminate the pregnancy. If the patient chooses expectant management, monitor her closely for infection.4 Outpatient management is acceptable for the highly compliant patient. However, once fetal viability is attained, readmit the patient to monitor maternal and fetal well-being.
Between 23 and 32 weeks
Treat these women expectantly in the hospital unless there is evidence of infection or fetal compromise. Management includes intravenous antibiotic therapy and corticosteroids, as recommended by the National Institutes of Health (FIGURE 2).5
Although it is controversial, consider magnesium sulfate tocolytic therapy if the patient is actively contracting with no evidence of infection.6
Some authorities recommend amniocentesis (at any gestational age) to rule out infection prior to expectant management, while others consider the appropriate use of clinical parameters to be adequate.6
After monitoring the patient in the labor suite for 24 to 48 hours, transfer her to the antenatal ward, if she is stable, for frequent fetal surveillance and maternal evaluation. Continue to evaluate her for signs of intraamniotic infection such as fever, uterine tenderness, and foulsmelling discharge.
Many authorities recommend daily nonstress testing or biophysical profiles to evaluate fetal well-being.7
Between 32 and 34 weeks
Management for this gestational age range is the most controversial (FIGURE 3). Mercer et al8 demonstrated that expectant management is harmful to the neonate if fetal lung maturity is present. Most experts contend that some method of fetal lung assessment should be undertaken. The most effective method is sampling amniotic fluid by amniocentesis. Not only can fetal lung maturity be determined, but the fluid can be analyzed for evidence of infection using the Gram stain, glucose level, and white blood cell count.
When an amniocentesis site is unavailable, consider assessing fetal lung maturity via a pooled amniotic fluid sample from the vagina.9 Deliver the infant if either intraamniotic infection or fetal lung maturity is present.
If there is no evidence of fetal lung maturity, expectant management is usually recommended for pregnancies between 32 and 34 weeks.
In a recent survey of maternal-fetal medicine specialists in the United States, 58% recommended delivery at 34 weeks of gestation.10
For patients who are at less than 34 weeks upon presentation—or to be treated expectantly—consider giving antibiotics, corticosteroids, and tocolysis during the first 48 hours.
FIGURE 1 Managing premature rupture of membranes: Less than 23 weeks’ gestation
FIGURE 2 Managing premature rupture of membranes: 23 to <32 weeks’ gestation
The new standard of care: antibiotic therapy
In the 1980s, several studies suggested antibiotics were beneficial for PPROM patients.11 More recently, the Maternal-Fetal Medicine Network published the results of a large, prospective, randomized, double-blind, placebo-controlled trial clearly demonstrating that antibiotics prolong the latency period and decrease neonatal morbidities (see “Antibiotics in PPROM improve outcomes in Maternal-Fetal Medicine Network study”).12
Limited- versus broad-spectrum antibiotics
Although antibiotic therapy is now the standard of care for patients with PPROM, it remains unclear which antibiotic is best. Some researchers have recommended limited-spectrum antibiotics, while others prefer a broader-spectrum approach.13 Several recent studies14 have suggested that erythromycin is effective for eradicating Mycoplasma and Ureaplasma and beneficial for the neonate.
Optimal duration of antibiotic therapy is unclear
Most early studies advocated standard treatment from the time of ruptured membranes until delivery, while others recommended 7 to 10 days of therapy. Several recent trials15 have found 3 days of therapy to be equivalent to 7 days. However, these studies lack sufficient power to warrant a shorter duration of therapy at this time.
Numerous small, prospective, randomized trials comparing antibiotics with placebo have demonstrated prolonged latency (period from rupture of membranes to delivery) in the women with preterm premature rupture of membranes (PPROM) who were given antibiotics.
However, these studies lacked sufficient power to establish a decrease in neonatal morbidity with antibiotics, although a meta-analysis by Mercer and Arheart31 suggested such a decrease existed.
The breakthrough trial
Finally, in 1997, the Maternal-Fetal Medicine Network of the National Institute of Child Health and Human Development published a study large enough to definitively answer the question.12
A significant decrease in morbidity and mortality
Since these findings were published, controversy has shifted to the choice of antibiotics and the length and route of administration.
In this study, 614 women between 24 and 32 weeks of gestation with confirmed PPROM were randomized to intravenous ampicillin (2 g every 6 hours) and erythromycin (250 mg every 6 hours) for 48 hours followed by oral amoxicillin (250 mg every 8 hours) and erythromycin base (333 mg every 8 hours) for 5 days, or placebo. None of the women had received corticosteroids for fetal maturation or antibiotic treatment within 1 week of randomization.
In the study group, the composite primary outcome of interest decreased significantly—pregnancies with at least 1 of the following: fetal or infant mortality, respiratory distress, severe intraventricular hemorrhage, stage 2 or 3 necrotizing enterocolitis, or sepsis within 72 hours of birth.
Practice recommendation
I use ampicillin (2 g IV every 6 hours) and azithromycin (500 mg orally or IV on day 1 followed by 250 mg every day for 4 days) because data from the ORACLE trial14 suggested an increased incidence of necrotizing enterocolitis with amoxicillin and clavulanic acid. I also treat patients for 7 full days, even though preliminary data suggests 3 days may be adequate.15
More trials ahead
Expect to see many more randomized trials seeking to establish the best antibiotic, route of administration, and length of treatment.
Corticosteroids beneficial if infection is treated
In 1998, the National Institutes of Health issued a Consensus Statement5 recommending corticosteroid therapy to accelerate fetal lung maturity and decrease neonatal morbidity in PPROM patients. Many authorities questioned the wisdom of this recommendation, since earlier studies had suggested an increased risk of maternal morbidity when corticosteroids are given as therapy for PPROM.16 However, these early studies did not use antibiotics to treat infection—the primary cause of PPROM—and many patients underwent digital vaginal exams, which probably contributed to unfavorable maternal and neonatal outcomes.
I recently began treating PPROM patients with antibiotics for 12 hours prior to administering corticosteroids and noted a considerable benefit for steroid therapy.17 I therefore recommend that PPROM patients who are treated expectantly receive corticosteroids unless there is evidence of an infection.
Tocolysis: jury still out
The use of tocolytics also is controversial. Many original studies reported no overall benefit and a significant increase in maternal morbidity when tocolytic therapy was given for ruptured membranes.18 However, many patients in these trials underwent digital vaginal exams and were not given antibiotics, and many received corticosteroids.
In my practice, I give magnesium sulfate and corticosteroids for the first 48 hours to women at or beyond 23 weeks’ gestation, provided there is no evidence of infection.19 However, additional data is needed before this management protocol can be considered the standard of care.
Assess fetal well-being frequently
Initial recommendations for antepartum testing were based on the high perinatal morbidity and mortality associated with PPROM. Nonstress testing was the foundation of therapy, and many authorities advocated daily nonstress testing even in the absence of definitive proof of its benefit.4
In the late 1980s, Vintzileos20 and other experts recommended daily biophysical profiles, suggesting that this strategy could identify patients with subclinical infection.
Daily biophysical profiles: No benefit beyond that of daily nonstress test
My colleagues and I7 conducted the only reported prospective, randomized trial comparing daily biophysical profiles to daily nonstress testing with backup biophysical profiles for abnormalities. We concluded that daily biophysical profiles provide no benefit beyond that achieved with daily nonstress testing. Whether a less intense testing protocol would produce the same benefit is unclear.
Important to recognize high risk of poor perinatal outcome
The important point in managing patients with PPROM is recognizing the high risk of poor perinatal outcome. For this reason, frequent evaluation of fetal well-being is an essential element of any management plan.
Determine mode of delivery as usual, but monitor fetus
In PPROM cases, the fetus often is intolerant of labor, primarily because of oligohydramnios and subclinical infection. For this reason, monitor the fetus very closely in the intrapartum period. Amnioinfusion decreases variable decelerations and improves pH in these patients and should be considered when it is clinically indicated.21
Determine the mode of delivery using routine obstetrical indications. Deliver viable breech infants by cesarean section.
Few data exist on the benefit of cesarean for the very premature neonate; use of routine obstetrical indicators is advisable.
Special situations
Four conditions may affect management of the patient with PPROM:
Cerclage in place
Numerous early studies suggested that this foreign body is a focus of infection and recommended removal. However, recent reports have not substantiated these findings.22 A large, multicenter, prospective, randomized trial is underway, which compares expectant management with cerclage removal when membranes rupture. It should provide a definitive answer.
Herpes simplex infection
If the patient with PPROM has a clinical outbreak of herpes simplex virus, weigh the risk of early delivery against the risk of herpes simplex infection. Major et al23 compiled a case series showing that infants did not become infected with herpes simplex virus when women were managed expectantly. Still, consider prophylactic therapy with antiviral agents in expectantly managed patients. At later gestational ages, most experts recommend delivery.
Bleeding
Placental abruptions occur in about 5% of PPROM pregnancies.24 Some authorities contend that the cause of abruption is placental shearing following leakage of amniotic fluid and decreased intrauterine volume. However, bleeding is rarely substantial enough to warrant delivery. Even so, monitor women with active bleeding in the labor suite, remaining vigilant for evidence of fetal compromise.
Outpatient management
Only 1 study has evaluated outpatient management of women with PPROM.25 Until more definitive information is available, the American College of Obstetricians and Gynecologists recommends that outpatient management be limited to approved study protocols.6
The author reports no financial relationships relevant to this article.
1. Iams JD, Stilson R, Johnson FF, et al. Symptoms that precede preterm labor and preterm premature rupture of the membranes. Am J Obstet Gynecol. 1990;162:486.-
2. Friedman ML, McElin TW. Diagnosis of ruptured fetal membranes: Clinical study and review of the literature. Am J Obstet Gynecol. 1969;104:544-550.
3. Lewis DF, Major CA, Towers CV, et al. Effects of digital vaginal examinations on latency period in preterm premature rupture of membranes. Obstet Gynecol. 1992;80:630-634.
4. Garite TJ. Management of premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):837-847.
5. National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: effect of corticosteroids for fetal maturation on perinatal outcomes. Am J Obstet Gynecol. 1995;173:246-252.
6. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #1: Premature Rupture of Membranes. Washington, DC: ACOG; June 1998.
7. Lewis DF, Adair CD, Weeks JW, et al. A randomized clinical trial of nonstress test versus biophysical profile in preterm premature rupture of membranes. Am J Obstet Gynecol. 1999;181:1495-1499.
8. Mercer BM, Crocker LG, Boe NM, et al. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
9. Lewis DF, Towers CV, Major CA, et al. Use of Amniostat-FLM in detecting the presence of phosphatidylglycerol in vaginal pool samples in preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:1139-1143.
10. Healy AJ, Veille J-C, Sciscione A, et al. The timing of elective delivery in preterm rupture of the membranes: a survey of members of the Society of Maternal-Fetal Medicine. Am J Obstet Gynecol. 2004;190(5):1479-1481.
11. Amon E, Lewis SV, Sibai BM, et al. Ampicillin prophylaxis in preterm premature rupture of the membranes: a prospective randomized study. Am J Obstet Gynecol. 1998;159:539.-
12. Mercer B, Miodovnik M, Thurnau G, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes: a randomized controlled trial. JAMA. 1997;278:989-995.
13. Lewis DF, Fontenot MT, Brooks GG, et al. Latency period after preterm premature rupture of membranes: a prospective, randomized, double-blind study comparing ampicillin and ampicillin-sulbactum. Obstet Gynecol. 1995;86:392-395.
14. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Oracle Collaborative Group. Broad spectrum antibiotics for preterm, prelabor rupture of fetal membranes: the ORACLE I randomized trial. Lancet. 2001;357:979-988.
15. Lewis DF, Adair CD, Robichaux AG, et al. Antibiotic therapy in preterm premature rupture of membranes: are seven days necessary? A preliminary, randomized clinical trial. Am J Obstet Gynecol. 2003;188:1413-1416;discussion 1416-1417.
16. Vidaeff AC, Ramin SM, Gilstrap III LC. Antenatal corticosteroids in women with preterm premature rupture of the membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):797-805.
17. Lewis DF, Brody K, Edwards MS, Brouillette RM. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
18. Garite TJ, Keegan KA, Freeman RK, et al. A randomized trial of ritodrine tocolysis versus expectant management in patients with premature rupture of membranes at 25 to 30 weeks of gestation. Am J Obstet Gynecol. 1987;157:388-393.
19. Fontenot T, Lewis DF. Tocolytic therapy with preterm premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):787-796.
20. Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes: an early predictor of fetal infection. Obstet Gynecol. 1985;152:510.-
21. Nageotte MP, Freeman RK, Garite TJ, et al. Prophylactic intrapartum amnioinfusion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1985;153:557.-
22. Lee RM, Major CA. Controversial and special situations in the management of preterm premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):877-884.
23. Major CA, Towers CV, Lewis DF, Garite TJ. Expectant management of preterm premature rupture of membranes complicated by active recurrent genital herpes. Am J Obstet Gynecol. 2003;188:1551-1554.
24. Major CA, de Veciana M, Lewis DF, Morgan MA. Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications? Am J Obstet Gynecol. 1995;172:672-676.
25. Carlan SJ, O’Brien WF, Parsons MT, et al. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
26. Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):721-734.
27. Savitz DA, Blackmore CA, Thorp JM. Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. Am J Obstet Gynecol. 1991;164:467.-
28. Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy: their effects on preterm birth. JAMA. 1986;255:82.-
29. Romero R, Ghidini A, Mazor M, et al. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769.-
30. Asrat T. Intra-amniotic infection in patients with preterm prelabor rupture of membranes: pathophysiology, detection, and management. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):735-751.
31. Mercer B, Arheart K. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995;346:1271-1279.
- A large, prospective, randomized, controlled trial clearly showed that antibiotics decrease neonatal morbidities and prolong the interval between rupture of membranes and delivery.
- Avoid digital cervical examination during testing for rupture of membranes because it may hasten delivery and increase neonatal morbidity.
- Consider giving magnesium sulfate and corticosteroids to patients with preterm premature rupture of membranes at or beyond 23 weeks, provided there is no evidence of infection. After 32 weeks, deliver the infant if either intraamniotic infection or fetal lung maturity is present.
- At delivery, amnioinfusion decreases variable decelerations and improves pH when clinically indicated.
The outlook for preterm premature rupture of membranes (PPROM) has improved considerably since a landmark study showed clear benefits of antibiotics.
Previously, approximately 80% of women with PPROM experienced spontaneous labor within 48 hours with expectant management.
We now know that infection is a major cause of PPROM and that antibiotic therapy decreases neonatal morbidity and increases the interval from rupture of membranes to delivery. These benefits are most evident at early gestational ages.
This article reviews newer studies, as well as that breakthrough 1997 study, and their implications for diagnosis and treatment, including optimal drug regimens. Recommendations for 4 special situations are also discussed:
- presence of cerclage,
- herpes simplex infection,
- bleeding, and
- outpatient management.
Despite progress, research is still needed. For example, because much of our information about clinical interventions for PPROM, such as corticosteroid therapy and use of tocolytics, predates the use of antibiotics, many earlier studies need to be repeated.
A variety of methods confirm the diagnosis
Approximately 90% of patients with PPROM report nonurinary fluid leaking from the vagina.1,2 Nevertheless, a history of leaking fluid should be confirmed by examination.
Nitrazine paper test
The most common way to diagnose rupture of membranes involves exposing nitrazine paper to the leaking fluid. If the fluid is alkaline, the paper will turn bright blue.
However, seminal fluid, urine, and blood can also turn nitrazine paper blue. Therefore, a confirmatory method should be used with nitrazine paper testing.
Fern pattern test
When amniotic fluid proteins are allowed to dry and then examined under a microscope, they will exhibit a “fern” pattern.
The combination of nitrazine paper and the fern test has a specificity of over 90%.
Avoid digital cervical examination
Digital examination during testing may diminish latency (the period from rupture of membranes to delivery) and increase neonatal morbidity.3
Testing at early gestational ages
Occasionally, PPROM occurs early in the middle trimester. At such early gestations, amniotic fluid may not fern or produce the classic blue color when exposed to nitrazine paper.
Other tests may be used, however:
- Ultrasound can help in evaluating the quantity of amniotic fluid.
- Indigo carmine dye can be placed in the amniotic cavity, with a tampon inserted in the vagina. If the tampon turns blue when the patient ambulates, rupture of membranes is confirmed.
- Alpha-fetoprotein and human choriogonadotropin. When alpha-fetoprotein is present and human choriogonadotropin is highly concentrated in amniotic fluid, ruptured membranes are confirmed.4
Fortunately, a large majority of patients can be diagnosed using clinical history, nitrazine paper, and the fern pattern test.
Definition
Preterm premature rupture of membranes (PPROM) is ruptured membranes before the 37th week of gestation.
Incidence
PPROM complicates 3% to 4.5% of all pregnancies and is responsible for 30% to 40% of all preterm births.26 This high incidence makes it a major cause of premature birth in the United States.
Predisposing factors
African-American women appear to have a higher incidence of PPROM than Caucasian women.27
Smoking is strongly correlated with PPROM,28 as it is with most poor perinatal outcomes.
Nutritional deficiencies in hydroxyproline, vitamin C, copper, and zinc also are linked.26
The precise cause is unknown
Ascending infection29 and uterine bleeding1 are both strongly correlated with PPROM. An incompetent cervix is also thought to play a role, as are conditions involving connective tissue abnormalities, such as those found in Ehlers-Danlos syndrome.
Unfortunately, we have yet to identify a factor or factors that could be linked to a specific cause for any given patient. Such knowledge would enable us to abandon empiric treatment in favor of more specific management guidelines.
Infection plays a major role in most cases
Bacteria in the vagina ascend through the intracervical canal and establish subclinical infection in the lower uterine segment.29
This activates macrophages and polymorphoneutrophils as a host defense mechanism. Macrophages release interleukin-6, interleukin-8, and tumor necrosing factors, while lysis of polymorphoneutrophils triggers the release of proteolytic enzymes.
These enzymes not only destroy the invasive microorganisms, but are capable of damaging the chorion and amnion, increasing the likelihood of ruptured membranes.
As we come to understand these mechanisms more fully, we should be able to tailor therapy to specific etiologies, thereby optimizing outcomes.30
Gestational age determines management
The number 1 factor determining management is gestational age.
Before 23 weeks, the pregnancy is considered previable
Counsel these patients about the risk of infection, the prognosis of extreme prematurity, and the generally poor outcome of these pregnancies, which can involve pulmonary hypoplasia secondary to severe oligohydramnios (FIGURE 1).
Induction of labor is an option to terminate the pregnancy. If the patient chooses expectant management, monitor her closely for infection.4 Outpatient management is acceptable for the highly compliant patient. However, once fetal viability is attained, readmit the patient to monitor maternal and fetal well-being.
Between 23 and 32 weeks
Treat these women expectantly in the hospital unless there is evidence of infection or fetal compromise. Management includes intravenous antibiotic therapy and corticosteroids, as recommended by the National Institutes of Health (FIGURE 2).5
Although it is controversial, consider magnesium sulfate tocolytic therapy if the patient is actively contracting with no evidence of infection.6
Some authorities recommend amniocentesis (at any gestational age) to rule out infection prior to expectant management, while others consider the appropriate use of clinical parameters to be adequate.6
After monitoring the patient in the labor suite for 24 to 48 hours, transfer her to the antenatal ward, if she is stable, for frequent fetal surveillance and maternal evaluation. Continue to evaluate her for signs of intraamniotic infection such as fever, uterine tenderness, and foulsmelling discharge.
Many authorities recommend daily nonstress testing or biophysical profiles to evaluate fetal well-being.7
Between 32 and 34 weeks
Management for this gestational age range is the most controversial (FIGURE 3). Mercer et al8 demonstrated that expectant management is harmful to the neonate if fetal lung maturity is present. Most experts contend that some method of fetal lung assessment should be undertaken. The most effective method is sampling amniotic fluid by amniocentesis. Not only can fetal lung maturity be determined, but the fluid can be analyzed for evidence of infection using the Gram stain, glucose level, and white blood cell count.
When an amniocentesis site is unavailable, consider assessing fetal lung maturity via a pooled amniotic fluid sample from the vagina.9 Deliver the infant if either intraamniotic infection or fetal lung maturity is present.
If there is no evidence of fetal lung maturity, expectant management is usually recommended for pregnancies between 32 and 34 weeks.
In a recent survey of maternal-fetal medicine specialists in the United States, 58% recommended delivery at 34 weeks of gestation.10
For patients who are at less than 34 weeks upon presentation—or to be treated expectantly—consider giving antibiotics, corticosteroids, and tocolysis during the first 48 hours.
FIGURE 1 Managing premature rupture of membranes: Less than 23 weeks’ gestation
FIGURE 2 Managing premature rupture of membranes: 23 to <32 weeks’ gestation
The new standard of care: antibiotic therapy
In the 1980s, several studies suggested antibiotics were beneficial for PPROM patients.11 More recently, the Maternal-Fetal Medicine Network published the results of a large, prospective, randomized, double-blind, placebo-controlled trial clearly demonstrating that antibiotics prolong the latency period and decrease neonatal morbidities (see “Antibiotics in PPROM improve outcomes in Maternal-Fetal Medicine Network study”).12
Limited- versus broad-spectrum antibiotics
Although antibiotic therapy is now the standard of care for patients with PPROM, it remains unclear which antibiotic is best. Some researchers have recommended limited-spectrum antibiotics, while others prefer a broader-spectrum approach.13 Several recent studies14 have suggested that erythromycin is effective for eradicating Mycoplasma and Ureaplasma and beneficial for the neonate.
Optimal duration of antibiotic therapy is unclear
Most early studies advocated standard treatment from the time of ruptured membranes until delivery, while others recommended 7 to 10 days of therapy. Several recent trials15 have found 3 days of therapy to be equivalent to 7 days. However, these studies lack sufficient power to warrant a shorter duration of therapy at this time.
Numerous small, prospective, randomized trials comparing antibiotics with placebo have demonstrated prolonged latency (period from rupture of membranes to delivery) in the women with preterm premature rupture of membranes (PPROM) who were given antibiotics.
However, these studies lacked sufficient power to establish a decrease in neonatal morbidity with antibiotics, although a meta-analysis by Mercer and Arheart31 suggested such a decrease existed.
The breakthrough trial
Finally, in 1997, the Maternal-Fetal Medicine Network of the National Institute of Child Health and Human Development published a study large enough to definitively answer the question.12
A significant decrease in morbidity and mortality
Since these findings were published, controversy has shifted to the choice of antibiotics and the length and route of administration.
In this study, 614 women between 24 and 32 weeks of gestation with confirmed PPROM were randomized to intravenous ampicillin (2 g every 6 hours) and erythromycin (250 mg every 6 hours) for 48 hours followed by oral amoxicillin (250 mg every 8 hours) and erythromycin base (333 mg every 8 hours) for 5 days, or placebo. None of the women had received corticosteroids for fetal maturation or antibiotic treatment within 1 week of randomization.
In the study group, the composite primary outcome of interest decreased significantly—pregnancies with at least 1 of the following: fetal or infant mortality, respiratory distress, severe intraventricular hemorrhage, stage 2 or 3 necrotizing enterocolitis, or sepsis within 72 hours of birth.
Practice recommendation
I use ampicillin (2 g IV every 6 hours) and azithromycin (500 mg orally or IV on day 1 followed by 250 mg every day for 4 days) because data from the ORACLE trial14 suggested an increased incidence of necrotizing enterocolitis with amoxicillin and clavulanic acid. I also treat patients for 7 full days, even though preliminary data suggests 3 days may be adequate.15
More trials ahead
Expect to see many more randomized trials seeking to establish the best antibiotic, route of administration, and length of treatment.
Corticosteroids beneficial if infection is treated
In 1998, the National Institutes of Health issued a Consensus Statement5 recommending corticosteroid therapy to accelerate fetal lung maturity and decrease neonatal morbidity in PPROM patients. Many authorities questioned the wisdom of this recommendation, since earlier studies had suggested an increased risk of maternal morbidity when corticosteroids are given as therapy for PPROM.16 However, these early studies did not use antibiotics to treat infection—the primary cause of PPROM—and many patients underwent digital vaginal exams, which probably contributed to unfavorable maternal and neonatal outcomes.
I recently began treating PPROM patients with antibiotics for 12 hours prior to administering corticosteroids and noted a considerable benefit for steroid therapy.17 I therefore recommend that PPROM patients who are treated expectantly receive corticosteroids unless there is evidence of an infection.
Tocolysis: jury still out
The use of tocolytics also is controversial. Many original studies reported no overall benefit and a significant increase in maternal morbidity when tocolytic therapy was given for ruptured membranes.18 However, many patients in these trials underwent digital vaginal exams and were not given antibiotics, and many received corticosteroids.
In my practice, I give magnesium sulfate and corticosteroids for the first 48 hours to women at or beyond 23 weeks’ gestation, provided there is no evidence of infection.19 However, additional data is needed before this management protocol can be considered the standard of care.
Assess fetal well-being frequently
Initial recommendations for antepartum testing were based on the high perinatal morbidity and mortality associated with PPROM. Nonstress testing was the foundation of therapy, and many authorities advocated daily nonstress testing even in the absence of definitive proof of its benefit.4
In the late 1980s, Vintzileos20 and other experts recommended daily biophysical profiles, suggesting that this strategy could identify patients with subclinical infection.
Daily biophysical profiles: No benefit beyond that of daily nonstress test
My colleagues and I7 conducted the only reported prospective, randomized trial comparing daily biophysical profiles to daily nonstress testing with backup biophysical profiles for abnormalities. We concluded that daily biophysical profiles provide no benefit beyond that achieved with daily nonstress testing. Whether a less intense testing protocol would produce the same benefit is unclear.
Important to recognize high risk of poor perinatal outcome
The important point in managing patients with PPROM is recognizing the high risk of poor perinatal outcome. For this reason, frequent evaluation of fetal well-being is an essential element of any management plan.
Determine mode of delivery as usual, but monitor fetus
In PPROM cases, the fetus often is intolerant of labor, primarily because of oligohydramnios and subclinical infection. For this reason, monitor the fetus very closely in the intrapartum period. Amnioinfusion decreases variable decelerations and improves pH in these patients and should be considered when it is clinically indicated.21
Determine the mode of delivery using routine obstetrical indications. Deliver viable breech infants by cesarean section.
Few data exist on the benefit of cesarean for the very premature neonate; use of routine obstetrical indicators is advisable.
Special situations
Four conditions may affect management of the patient with PPROM:
Cerclage in place
Numerous early studies suggested that this foreign body is a focus of infection and recommended removal. However, recent reports have not substantiated these findings.22 A large, multicenter, prospective, randomized trial is underway, which compares expectant management with cerclage removal when membranes rupture. It should provide a definitive answer.
Herpes simplex infection
If the patient with PPROM has a clinical outbreak of herpes simplex virus, weigh the risk of early delivery against the risk of herpes simplex infection. Major et al23 compiled a case series showing that infants did not become infected with herpes simplex virus when women were managed expectantly. Still, consider prophylactic therapy with antiviral agents in expectantly managed patients. At later gestational ages, most experts recommend delivery.
Bleeding
Placental abruptions occur in about 5% of PPROM pregnancies.24 Some authorities contend that the cause of abruption is placental shearing following leakage of amniotic fluid and decreased intrauterine volume. However, bleeding is rarely substantial enough to warrant delivery. Even so, monitor women with active bleeding in the labor suite, remaining vigilant for evidence of fetal compromise.
Outpatient management
Only 1 study has evaluated outpatient management of women with PPROM.25 Until more definitive information is available, the American College of Obstetricians and Gynecologists recommends that outpatient management be limited to approved study protocols.6
The author reports no financial relationships relevant to this article.
- A large, prospective, randomized, controlled trial clearly showed that antibiotics decrease neonatal morbidities and prolong the interval between rupture of membranes and delivery.
- Avoid digital cervical examination during testing for rupture of membranes because it may hasten delivery and increase neonatal morbidity.
- Consider giving magnesium sulfate and corticosteroids to patients with preterm premature rupture of membranes at or beyond 23 weeks, provided there is no evidence of infection. After 32 weeks, deliver the infant if either intraamniotic infection or fetal lung maturity is present.
- At delivery, amnioinfusion decreases variable decelerations and improves pH when clinically indicated.
The outlook for preterm premature rupture of membranes (PPROM) has improved considerably since a landmark study showed clear benefits of antibiotics.
Previously, approximately 80% of women with PPROM experienced spontaneous labor within 48 hours with expectant management.
We now know that infection is a major cause of PPROM and that antibiotic therapy decreases neonatal morbidity and increases the interval from rupture of membranes to delivery. These benefits are most evident at early gestational ages.
This article reviews newer studies, as well as that breakthrough 1997 study, and their implications for diagnosis and treatment, including optimal drug regimens. Recommendations for 4 special situations are also discussed:
- presence of cerclage,
- herpes simplex infection,
- bleeding, and
- outpatient management.
Despite progress, research is still needed. For example, because much of our information about clinical interventions for PPROM, such as corticosteroid therapy and use of tocolytics, predates the use of antibiotics, many earlier studies need to be repeated.
A variety of methods confirm the diagnosis
Approximately 90% of patients with PPROM report nonurinary fluid leaking from the vagina.1,2 Nevertheless, a history of leaking fluid should be confirmed by examination.
Nitrazine paper test
The most common way to diagnose rupture of membranes involves exposing nitrazine paper to the leaking fluid. If the fluid is alkaline, the paper will turn bright blue.
However, seminal fluid, urine, and blood can also turn nitrazine paper blue. Therefore, a confirmatory method should be used with nitrazine paper testing.
Fern pattern test
When amniotic fluid proteins are allowed to dry and then examined under a microscope, they will exhibit a “fern” pattern.
The combination of nitrazine paper and the fern test has a specificity of over 90%.
Avoid digital cervical examination
Digital examination during testing may diminish latency (the period from rupture of membranes to delivery) and increase neonatal morbidity.3
Testing at early gestational ages
Occasionally, PPROM occurs early in the middle trimester. At such early gestations, amniotic fluid may not fern or produce the classic blue color when exposed to nitrazine paper.
Other tests may be used, however:
- Ultrasound can help in evaluating the quantity of amniotic fluid.
- Indigo carmine dye can be placed in the amniotic cavity, with a tampon inserted in the vagina. If the tampon turns blue when the patient ambulates, rupture of membranes is confirmed.
- Alpha-fetoprotein and human choriogonadotropin. When alpha-fetoprotein is present and human choriogonadotropin is highly concentrated in amniotic fluid, ruptured membranes are confirmed.4
Fortunately, a large majority of patients can be diagnosed using clinical history, nitrazine paper, and the fern pattern test.
Definition
Preterm premature rupture of membranes (PPROM) is ruptured membranes before the 37th week of gestation.
Incidence
PPROM complicates 3% to 4.5% of all pregnancies and is responsible for 30% to 40% of all preterm births.26 This high incidence makes it a major cause of premature birth in the United States.
Predisposing factors
African-American women appear to have a higher incidence of PPROM than Caucasian women.27
Smoking is strongly correlated with PPROM,28 as it is with most poor perinatal outcomes.
Nutritional deficiencies in hydroxyproline, vitamin C, copper, and zinc also are linked.26
The precise cause is unknown
Ascending infection29 and uterine bleeding1 are both strongly correlated with PPROM. An incompetent cervix is also thought to play a role, as are conditions involving connective tissue abnormalities, such as those found in Ehlers-Danlos syndrome.
Unfortunately, we have yet to identify a factor or factors that could be linked to a specific cause for any given patient. Such knowledge would enable us to abandon empiric treatment in favor of more specific management guidelines.
Infection plays a major role in most cases
Bacteria in the vagina ascend through the intracervical canal and establish subclinical infection in the lower uterine segment.29
This activates macrophages and polymorphoneutrophils as a host defense mechanism. Macrophages release interleukin-6, interleukin-8, and tumor necrosing factors, while lysis of polymorphoneutrophils triggers the release of proteolytic enzymes.
These enzymes not only destroy the invasive microorganisms, but are capable of damaging the chorion and amnion, increasing the likelihood of ruptured membranes.
As we come to understand these mechanisms more fully, we should be able to tailor therapy to specific etiologies, thereby optimizing outcomes.30
Gestational age determines management
The number 1 factor determining management is gestational age.
Before 23 weeks, the pregnancy is considered previable
Counsel these patients about the risk of infection, the prognosis of extreme prematurity, and the generally poor outcome of these pregnancies, which can involve pulmonary hypoplasia secondary to severe oligohydramnios (FIGURE 1).
Induction of labor is an option to terminate the pregnancy. If the patient chooses expectant management, monitor her closely for infection.4 Outpatient management is acceptable for the highly compliant patient. However, once fetal viability is attained, readmit the patient to monitor maternal and fetal well-being.
Between 23 and 32 weeks
Treat these women expectantly in the hospital unless there is evidence of infection or fetal compromise. Management includes intravenous antibiotic therapy and corticosteroids, as recommended by the National Institutes of Health (FIGURE 2).5
Although it is controversial, consider magnesium sulfate tocolytic therapy if the patient is actively contracting with no evidence of infection.6
Some authorities recommend amniocentesis (at any gestational age) to rule out infection prior to expectant management, while others consider the appropriate use of clinical parameters to be adequate.6
After monitoring the patient in the labor suite for 24 to 48 hours, transfer her to the antenatal ward, if she is stable, for frequent fetal surveillance and maternal evaluation. Continue to evaluate her for signs of intraamniotic infection such as fever, uterine tenderness, and foulsmelling discharge.
Many authorities recommend daily nonstress testing or biophysical profiles to evaluate fetal well-being.7
Between 32 and 34 weeks
Management for this gestational age range is the most controversial (FIGURE 3). Mercer et al8 demonstrated that expectant management is harmful to the neonate if fetal lung maturity is present. Most experts contend that some method of fetal lung assessment should be undertaken. The most effective method is sampling amniotic fluid by amniocentesis. Not only can fetal lung maturity be determined, but the fluid can be analyzed for evidence of infection using the Gram stain, glucose level, and white blood cell count.
When an amniocentesis site is unavailable, consider assessing fetal lung maturity via a pooled amniotic fluid sample from the vagina.9 Deliver the infant if either intraamniotic infection or fetal lung maturity is present.
If there is no evidence of fetal lung maturity, expectant management is usually recommended for pregnancies between 32 and 34 weeks.
In a recent survey of maternal-fetal medicine specialists in the United States, 58% recommended delivery at 34 weeks of gestation.10
For patients who are at less than 34 weeks upon presentation—or to be treated expectantly—consider giving antibiotics, corticosteroids, and tocolysis during the first 48 hours.
FIGURE 1 Managing premature rupture of membranes: Less than 23 weeks’ gestation
FIGURE 2 Managing premature rupture of membranes: 23 to <32 weeks’ gestation
The new standard of care: antibiotic therapy
In the 1980s, several studies suggested antibiotics were beneficial for PPROM patients.11 More recently, the Maternal-Fetal Medicine Network published the results of a large, prospective, randomized, double-blind, placebo-controlled trial clearly demonstrating that antibiotics prolong the latency period and decrease neonatal morbidities (see “Antibiotics in PPROM improve outcomes in Maternal-Fetal Medicine Network study”).12
Limited- versus broad-spectrum antibiotics
Although antibiotic therapy is now the standard of care for patients with PPROM, it remains unclear which antibiotic is best. Some researchers have recommended limited-spectrum antibiotics, while others prefer a broader-spectrum approach.13 Several recent studies14 have suggested that erythromycin is effective for eradicating Mycoplasma and Ureaplasma and beneficial for the neonate.
Optimal duration of antibiotic therapy is unclear
Most early studies advocated standard treatment from the time of ruptured membranes until delivery, while others recommended 7 to 10 days of therapy. Several recent trials15 have found 3 days of therapy to be equivalent to 7 days. However, these studies lack sufficient power to warrant a shorter duration of therapy at this time.
Numerous small, prospective, randomized trials comparing antibiotics with placebo have demonstrated prolonged latency (period from rupture of membranes to delivery) in the women with preterm premature rupture of membranes (PPROM) who were given antibiotics.
However, these studies lacked sufficient power to establish a decrease in neonatal morbidity with antibiotics, although a meta-analysis by Mercer and Arheart31 suggested such a decrease existed.
The breakthrough trial
Finally, in 1997, the Maternal-Fetal Medicine Network of the National Institute of Child Health and Human Development published a study large enough to definitively answer the question.12
A significant decrease in morbidity and mortality
Since these findings were published, controversy has shifted to the choice of antibiotics and the length and route of administration.
In this study, 614 women between 24 and 32 weeks of gestation with confirmed PPROM were randomized to intravenous ampicillin (2 g every 6 hours) and erythromycin (250 mg every 6 hours) for 48 hours followed by oral amoxicillin (250 mg every 8 hours) and erythromycin base (333 mg every 8 hours) for 5 days, or placebo. None of the women had received corticosteroids for fetal maturation or antibiotic treatment within 1 week of randomization.
In the study group, the composite primary outcome of interest decreased significantly—pregnancies with at least 1 of the following: fetal or infant mortality, respiratory distress, severe intraventricular hemorrhage, stage 2 or 3 necrotizing enterocolitis, or sepsis within 72 hours of birth.
Practice recommendation
I use ampicillin (2 g IV every 6 hours) and azithromycin (500 mg orally or IV on day 1 followed by 250 mg every day for 4 days) because data from the ORACLE trial14 suggested an increased incidence of necrotizing enterocolitis with amoxicillin and clavulanic acid. I also treat patients for 7 full days, even though preliminary data suggests 3 days may be adequate.15
More trials ahead
Expect to see many more randomized trials seeking to establish the best antibiotic, route of administration, and length of treatment.
Corticosteroids beneficial if infection is treated
In 1998, the National Institutes of Health issued a Consensus Statement5 recommending corticosteroid therapy to accelerate fetal lung maturity and decrease neonatal morbidity in PPROM patients. Many authorities questioned the wisdom of this recommendation, since earlier studies had suggested an increased risk of maternal morbidity when corticosteroids are given as therapy for PPROM.16 However, these early studies did not use antibiotics to treat infection—the primary cause of PPROM—and many patients underwent digital vaginal exams, which probably contributed to unfavorable maternal and neonatal outcomes.
I recently began treating PPROM patients with antibiotics for 12 hours prior to administering corticosteroids and noted a considerable benefit for steroid therapy.17 I therefore recommend that PPROM patients who are treated expectantly receive corticosteroids unless there is evidence of an infection.
Tocolysis: jury still out
The use of tocolytics also is controversial. Many original studies reported no overall benefit and a significant increase in maternal morbidity when tocolytic therapy was given for ruptured membranes.18 However, many patients in these trials underwent digital vaginal exams and were not given antibiotics, and many received corticosteroids.
In my practice, I give magnesium sulfate and corticosteroids for the first 48 hours to women at or beyond 23 weeks’ gestation, provided there is no evidence of infection.19 However, additional data is needed before this management protocol can be considered the standard of care.
Assess fetal well-being frequently
Initial recommendations for antepartum testing were based on the high perinatal morbidity and mortality associated with PPROM. Nonstress testing was the foundation of therapy, and many authorities advocated daily nonstress testing even in the absence of definitive proof of its benefit.4
In the late 1980s, Vintzileos20 and other experts recommended daily biophysical profiles, suggesting that this strategy could identify patients with subclinical infection.
Daily biophysical profiles: No benefit beyond that of daily nonstress test
My colleagues and I7 conducted the only reported prospective, randomized trial comparing daily biophysical profiles to daily nonstress testing with backup biophysical profiles for abnormalities. We concluded that daily biophysical profiles provide no benefit beyond that achieved with daily nonstress testing. Whether a less intense testing protocol would produce the same benefit is unclear.
Important to recognize high risk of poor perinatal outcome
The important point in managing patients with PPROM is recognizing the high risk of poor perinatal outcome. For this reason, frequent evaluation of fetal well-being is an essential element of any management plan.
Determine mode of delivery as usual, but monitor fetus
In PPROM cases, the fetus often is intolerant of labor, primarily because of oligohydramnios and subclinical infection. For this reason, monitor the fetus very closely in the intrapartum period. Amnioinfusion decreases variable decelerations and improves pH in these patients and should be considered when it is clinically indicated.21
Determine the mode of delivery using routine obstetrical indications. Deliver viable breech infants by cesarean section.
Few data exist on the benefit of cesarean for the very premature neonate; use of routine obstetrical indicators is advisable.
Special situations
Four conditions may affect management of the patient with PPROM:
Cerclage in place
Numerous early studies suggested that this foreign body is a focus of infection and recommended removal. However, recent reports have not substantiated these findings.22 A large, multicenter, prospective, randomized trial is underway, which compares expectant management with cerclage removal when membranes rupture. It should provide a definitive answer.
Herpes simplex infection
If the patient with PPROM has a clinical outbreak of herpes simplex virus, weigh the risk of early delivery against the risk of herpes simplex infection. Major et al23 compiled a case series showing that infants did not become infected with herpes simplex virus when women were managed expectantly. Still, consider prophylactic therapy with antiviral agents in expectantly managed patients. At later gestational ages, most experts recommend delivery.
Bleeding
Placental abruptions occur in about 5% of PPROM pregnancies.24 Some authorities contend that the cause of abruption is placental shearing following leakage of amniotic fluid and decreased intrauterine volume. However, bleeding is rarely substantial enough to warrant delivery. Even so, monitor women with active bleeding in the labor suite, remaining vigilant for evidence of fetal compromise.
Outpatient management
Only 1 study has evaluated outpatient management of women with PPROM.25 Until more definitive information is available, the American College of Obstetricians and Gynecologists recommends that outpatient management be limited to approved study protocols.6
The author reports no financial relationships relevant to this article.
1. Iams JD, Stilson R, Johnson FF, et al. Symptoms that precede preterm labor and preterm premature rupture of the membranes. Am J Obstet Gynecol. 1990;162:486.-
2. Friedman ML, McElin TW. Diagnosis of ruptured fetal membranes: Clinical study and review of the literature. Am J Obstet Gynecol. 1969;104:544-550.
3. Lewis DF, Major CA, Towers CV, et al. Effects of digital vaginal examinations on latency period in preterm premature rupture of membranes. Obstet Gynecol. 1992;80:630-634.
4. Garite TJ. Management of premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):837-847.
5. National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: effect of corticosteroids for fetal maturation on perinatal outcomes. Am J Obstet Gynecol. 1995;173:246-252.
6. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #1: Premature Rupture of Membranes. Washington, DC: ACOG; June 1998.
7. Lewis DF, Adair CD, Weeks JW, et al. A randomized clinical trial of nonstress test versus biophysical profile in preterm premature rupture of membranes. Am J Obstet Gynecol. 1999;181:1495-1499.
8. Mercer BM, Crocker LG, Boe NM, et al. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
9. Lewis DF, Towers CV, Major CA, et al. Use of Amniostat-FLM in detecting the presence of phosphatidylglycerol in vaginal pool samples in preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:1139-1143.
10. Healy AJ, Veille J-C, Sciscione A, et al. The timing of elective delivery in preterm rupture of the membranes: a survey of members of the Society of Maternal-Fetal Medicine. Am J Obstet Gynecol. 2004;190(5):1479-1481.
11. Amon E, Lewis SV, Sibai BM, et al. Ampicillin prophylaxis in preterm premature rupture of the membranes: a prospective randomized study. Am J Obstet Gynecol. 1998;159:539.-
12. Mercer B, Miodovnik M, Thurnau G, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes: a randomized controlled trial. JAMA. 1997;278:989-995.
13. Lewis DF, Fontenot MT, Brooks GG, et al. Latency period after preterm premature rupture of membranes: a prospective, randomized, double-blind study comparing ampicillin and ampicillin-sulbactum. Obstet Gynecol. 1995;86:392-395.
14. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Oracle Collaborative Group. Broad spectrum antibiotics for preterm, prelabor rupture of fetal membranes: the ORACLE I randomized trial. Lancet. 2001;357:979-988.
15. Lewis DF, Adair CD, Robichaux AG, et al. Antibiotic therapy in preterm premature rupture of membranes: are seven days necessary? A preliminary, randomized clinical trial. Am J Obstet Gynecol. 2003;188:1413-1416;discussion 1416-1417.
16. Vidaeff AC, Ramin SM, Gilstrap III LC. Antenatal corticosteroids in women with preterm premature rupture of the membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):797-805.
17. Lewis DF, Brody K, Edwards MS, Brouillette RM. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
18. Garite TJ, Keegan KA, Freeman RK, et al. A randomized trial of ritodrine tocolysis versus expectant management in patients with premature rupture of membranes at 25 to 30 weeks of gestation. Am J Obstet Gynecol. 1987;157:388-393.
19. Fontenot T, Lewis DF. Tocolytic therapy with preterm premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):787-796.
20. Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes: an early predictor of fetal infection. Obstet Gynecol. 1985;152:510.-
21. Nageotte MP, Freeman RK, Garite TJ, et al. Prophylactic intrapartum amnioinfusion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1985;153:557.-
22. Lee RM, Major CA. Controversial and special situations in the management of preterm premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):877-884.
23. Major CA, Towers CV, Lewis DF, Garite TJ. Expectant management of preterm premature rupture of membranes complicated by active recurrent genital herpes. Am J Obstet Gynecol. 2003;188:1551-1554.
24. Major CA, de Veciana M, Lewis DF, Morgan MA. Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications? Am J Obstet Gynecol. 1995;172:672-676.
25. Carlan SJ, O’Brien WF, Parsons MT, et al. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
26. Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):721-734.
27. Savitz DA, Blackmore CA, Thorp JM. Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. Am J Obstet Gynecol. 1991;164:467.-
28. Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy: their effects on preterm birth. JAMA. 1986;255:82.-
29. Romero R, Ghidini A, Mazor M, et al. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769.-
30. Asrat T. Intra-amniotic infection in patients with preterm prelabor rupture of membranes: pathophysiology, detection, and management. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):735-751.
31. Mercer B, Arheart K. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995;346:1271-1279.
1. Iams JD, Stilson R, Johnson FF, et al. Symptoms that precede preterm labor and preterm premature rupture of the membranes. Am J Obstet Gynecol. 1990;162:486.-
2. Friedman ML, McElin TW. Diagnosis of ruptured fetal membranes: Clinical study and review of the literature. Am J Obstet Gynecol. 1969;104:544-550.
3. Lewis DF, Major CA, Towers CV, et al. Effects of digital vaginal examinations on latency period in preterm premature rupture of membranes. Obstet Gynecol. 1992;80:630-634.
4. Garite TJ. Management of premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):837-847.
5. National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: effect of corticosteroids for fetal maturation on perinatal outcomes. Am J Obstet Gynecol. 1995;173:246-252.
6. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #1: Premature Rupture of Membranes. Washington, DC: ACOG; June 1998.
7. Lewis DF, Adair CD, Weeks JW, et al. A randomized clinical trial of nonstress test versus biophysical profile in preterm premature rupture of membranes. Am J Obstet Gynecol. 1999;181:1495-1499.
8. Mercer BM, Crocker LG, Boe NM, et al. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
9. Lewis DF, Towers CV, Major CA, et al. Use of Amniostat-FLM in detecting the presence of phosphatidylglycerol in vaginal pool samples in preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:1139-1143.
10. Healy AJ, Veille J-C, Sciscione A, et al. The timing of elective delivery in preterm rupture of the membranes: a survey of members of the Society of Maternal-Fetal Medicine. Am J Obstet Gynecol. 2004;190(5):1479-1481.
11. Amon E, Lewis SV, Sibai BM, et al. Ampicillin prophylaxis in preterm premature rupture of the membranes: a prospective randomized study. Am J Obstet Gynecol. 1998;159:539.-
12. Mercer B, Miodovnik M, Thurnau G, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes: a randomized controlled trial. JAMA. 1997;278:989-995.
13. Lewis DF, Fontenot MT, Brooks GG, et al. Latency period after preterm premature rupture of membranes: a prospective, randomized, double-blind study comparing ampicillin and ampicillin-sulbactum. Obstet Gynecol. 1995;86:392-395.
14. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Oracle Collaborative Group. Broad spectrum antibiotics for preterm, prelabor rupture of fetal membranes: the ORACLE I randomized trial. Lancet. 2001;357:979-988.
15. Lewis DF, Adair CD, Robichaux AG, et al. Antibiotic therapy in preterm premature rupture of membranes: are seven days necessary? A preliminary, randomized clinical trial. Am J Obstet Gynecol. 2003;188:1413-1416;discussion 1416-1417.
16. Vidaeff AC, Ramin SM, Gilstrap III LC. Antenatal corticosteroids in women with preterm premature rupture of the membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):797-805.
17. Lewis DF, Brody K, Edwards MS, Brouillette RM. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
18. Garite TJ, Keegan KA, Freeman RK, et al. A randomized trial of ritodrine tocolysis versus expectant management in patients with premature rupture of membranes at 25 to 30 weeks of gestation. Am J Obstet Gynecol. 1987;157:388-393.
19. Fontenot T, Lewis DF. Tocolytic therapy with preterm premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):787-796.
20. Vintzileos AM, Campbell WA, Nochimson DJ, et al. The fetal biophysical profile in patients with premature rupture of the membranes: an early predictor of fetal infection. Obstet Gynecol. 1985;152:510.-
21. Nageotte MP, Freeman RK, Garite TJ, et al. Prophylactic intrapartum amnioinfusion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1985;153:557.-
22. Lee RM, Major CA. Controversial and special situations in the management of preterm premature rupture of membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):877-884.
23. Major CA, Towers CV, Lewis DF, Garite TJ. Expectant management of preterm premature rupture of membranes complicated by active recurrent genital herpes. Am J Obstet Gynecol. 2003;188:1551-1554.
24. Major CA, de Veciana M, Lewis DF, Morgan MA. Preterm premature rupture of membranes and abruptio placentae: is there an association between these pregnancy complications? Am J Obstet Gynecol. 1995;172:672-676.
25. Carlan SJ, O’Brien WF, Parsons MT, et al. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
26. Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):721-734.
27. Savitz DA, Blackmore CA, Thorp JM. Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. Am J Obstet Gynecol. 1991;164:467.-
28. Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy: their effects on preterm birth. JAMA. 1986;255:82.-
29. Romero R, Ghidini A, Mazor M, et al. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769.-
30. Asrat T. Intra-amniotic infection in patients with preterm prelabor rupture of membranes: pathophysiology, detection, and management. Clinics in Perinatology: Prelabor Rupture of Membranes. 2001;28(4):735-751.
31. Mercer B, Arheart K. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995;346:1271-1279.
Practice Pearls for Titrating Antiglycemics
Over 100 Unnecessary Psychiatric Admissions
A Model for Mental Health Care
Sexual dysfunction: The challenge of treatment
Case reports of patients helped by therapy,
Say you see 100 adult patients this week. How many have a sexual dysfunction? 10? 20? Would you believe 43? In a National Health and Social Life Survey1 of 1,749 women and 1,410 men aged 18 to 59 years, 43% of women and 31% of men reported sexual dysfunction. Rates that high make sexual dysfunction an important public health concern—one that is highly associated with impaired well-being and negative experiences in sexual relationships.
Similar findings marked a study2 of general practice patients in Britain: 41% of 979 women and 34% of 789 men reported sexual dysfunction. Although 52% wanted professional help, only 10% had received it.
As primary care providers, Ob/Gyns must identify common problems, treat those within our expertise, and refer the others. In all likelihood, we are the first physicians women see about a sexual problem. For these reasons—and because dysfunction is so common—it is appropriate that we screen for and address female sexual dysfunction.
This article presents a medical model of management and tells how to take a sexual history, formulate a differential diagnosis, manage common sexual problems, and identify indications for referral.
The medical model
According to the medical model, a patient presents with a problem, which is investigated by history and physical examination. A differential diagnosis is formulated, and appropriate laboratory tests or other evaluations are conducted. The patient’s problem is compared to a normal physiologic process to determine pathophysiology. Diagnosis and therapy follow.
The medical model for sexual dysfunction is the same as that for any disease, such as asthma, in which normal breathing is interrupted. In the sexual response cycle, physiologic changes can be measured and specifically described, as Masters and Johnson explained in 1966 in Human Sexual Response (FIGURE 1). Any barriers that interrupt this progression “block” the normal response cycle, resulting in sexual dysfunction. We aim to identify and remove or get around any barriers.
FIGURE 1 Use the sexual response cycle in dialogue with the patient
Most patients with sexual dysfunction can identify the phase in the Masters and Johnson sexual response cycle where normal progression is interrupted. Using a sketch of this cycle helps patients understand and articulate their problem.
The sexual response cycle
Desire initiates the cycle, followed by the excitement phase, during which breathing rate and pulse increase, blood flow shifts away from muscles and into the skin and pelvic organs, and there is an associated expansion, lengthening, and lubrication of the vagina. A plateau follows, during which there is no further increase in signs such as increased pulse rate. Orgasm is the first point in the response cycle that is different in men and women. Men have an obligatory resolution phase and return to baseline state; women can return to the plateau state and then have another orgasm. The resolution phase lengthens in duration in both sexes with increasing age.
The sexual response cycle is innate, like any physiologic process. It cannot be taught or learned, and is not under voluntary control. It is critical that patients understand that the sexual response cycle is a natural function that everyone has—just as everyone has innate capacity to breathe. Patients do not learn it, any more than they have to learn respiration.
Sexual behavior, however, is learned, and sexual dysfunctions are exhibited by behavior. Sex therapy is a learning process, the aim of which is to change the behavior. Sexual dysfunctions have both cognitive and physical components; although response is physiologic, it can be altered by emotion, as can respiration.
The relationship is the patient. Sexual dysfunction occurs within a relationship, and the relationship needs and stands to benefit from therapy.
Reassuring anxious, angry patients
Approach is critical, and must reflect an understanding that sexual dysfunction is real. Many patients have been told that the problem is “all in their head” or that they are deliberately acting dysfunctionally.
Confrontation and support are useful communication tools. Confrontation means identifying behaviors and beliefs and how beliefs influence behaviors. Support means conveying to the patient that you understand that she is in distress and that it couldn’t have happened any other way for her.
A “therapeutic mirror” technique can show the patient her beliefs, attitudes, and behaviors. The therapist repeats the the patient’s beliefs and behaviors until the patient states that they are an accurate reflection, or tells the therapist what is inaccurate.
Assure the patient that anxiety is to be expected, and that her probelm is common and treatable. These patients believe they have thought of everything, and nothing has worked. Just reassuring them may help.
Anger must also be acknowledged—and these patients are angry. Their anger may be directed at their partner, a former physician, or you. You may elicit a surprising outburst of pent-up anger when you ask what seems to be a neutral question.
The patient always has an internal theory and everything you say is filtered through this theory. If there is a dichotomy, it must be addressed before you can proceed successfully. Usually, you can simply ask, “What do you think is the cause?” Often she will tell you. If she is uncertain, you might say, “I realize you don’t really know. What do you think it might be? What are you afraid it might be?”
You have to ask
Many patients do not express the fact that their chief complaint is sexual dysfunction. (See How to take a sexual history,).
In many cases, sexual dysfunction may be suggested in the investigation of another complaint such as infertility, pelvic pain with no particular pattern, malaise, or depression. With these complaints, it is important to pursue a sexual history. Determine any associated or causal condition and look for associated dysfunction.
The sexual history is like a history for any disease, in that you begin with the present illness. The differential diagnosis begins with a determination of where in the cycle the dysfunction occurs.
- One way to start is to describe the normal human sexual response. I find it best to sketch out the response cycle (FIGURE 1 ) on a piece of paper and ask, “Is this what happens to you?” Most patients understand and can tell where in the cycle the interruption occurs.
- 3 questions in a review of systems disclose nearly all sexual dysfunction:
- “How often do you have intercourse?” The only “right” answer is “as often as I want to.” If the patient is not having intercourse, is it by choice or involuntary?
- “Do you have pain with intercourse?”
- “How often do you have orgasm?” Most answer “hardly ever” or “most of the time.”
- Allegories like these are useful:
- Elements and example questions
Physical examination
Ageneral physical examination with pelvic examination should always be performed. If the patient complains of pain, the site of pain should be determined. A wet prep is useful to exclude vaginitis. A patient may have dyspareunia from vaginitis even if there are no obvious physical signs.
The most common dysfunctions: Desire phase disorders
Female sexual arousal disorder is either low libido or inhibited sexual desire. In both cases the patient is likely to report that she has no desire. But if you ask what happens when her partner wants to have intercourse, a patient with low libido describes low interest behavior, and a patient with inhibited desire describes aversion behavior.
Patients with low libido sometimes report that their orgasms are less intense, or that they do not become excited. Women on estrogen replacement after oophorectomy may have low libido related to decreased androgen.
Depression is by far the most common cause. Other contributors: chronic disease, hypoestrogenic states, hyperprolactinemia, and breastfeeding.
Inhibited sexual desire, which is a result of pain or other dysfunction, is far more common than low libido. Careful history usually reveals aversion behavior. They avoid going to bed at the same time as their partner, or develop other behaviors that preclude intercourse.
Women with inhibited desire have a conditioned negative response. In studies, these patients exhibit normal objective measures of vaginal vasocongestion in response to sexual stimuli, but self-reported subjective measures of arousal do not correlate.
They report that they simply have no desire, but the cause of their avoidance is negative conditioning. Here’s where the therapist supports, but also confronts their belief system, and attempts to help them understand that they have a normal desire for intercourse. The therapist may ask the patient to consider whether she ever has sexual dreams, reads romance novels, or fantasizes, to show that these phenomena demonstrate normal desire.
The role of testosterone in inhibited sexual desire
Measuring serum testosterone isn’t helpful. It is my belief that inhibited desire is most often related to other factors. Counseling should be the first-line therapy. Drug therapy without counseling is less likely to be effective than therapy that includes counseling.
Normal serum testosterone levels are 20 to 80 ng/dL in reproductive-age women, who produce 0.2 to 0.3 mg/day of testosterone. Levels increase 10% to 20% at midcycle. Testosterone falls 40% to 60% at natural menopause and by similar amounts with use of oral contraceptive pills, and by 80% with surgical menopause.
Testosterone levels have little correlation with sexual desire in cycling women or women on oral contraceptives.3 Most studies have found no correlation between testosterone levels and sexual function in perimenopausal or naturally menopausal women.4,5 Other studies have failed to show a difference in testosterone levels between women with arousal disorders and normal controls.6
Testosterone therapy may be effective in surgically menopausal women with low libido. Shifrin and colleagues7 randomized 75 surgically menopausal women being treated with conjugated equine estrogens to placebo or transdermal testosterone, 150 or 300 μg per day, in a crossover trial. They reported, “Despite an appreciable placebo response, the higher testosterone dose resulted in further increases in scores for frequency of sexual activity and pleasure-orgasm. At the higher dose, the percentages of women who had sexual fantasies, masturbated, or engaged in sexual intercourse at least once a week increased 2 to 3 times from base line.”
Testosterone is not yet approved for female sexual dysfunction, but a transdermal testosterone is being proposed for women with hypoactive sexual desire disorder who have had bilateral oophorectomy.
Excitement phase disorders
These disorders are caused by pain with intercourse. What the patient says about the site of pain and when she began experiencing pain help form the differential diagnosis.
Dyspareunia can be due to physiological causes such as obstetric laceration, endometriosis, pelvic inflammatory disease, vaginitis, vulvar disease, and vestibulitis. Interstitial cystitis and inflammatory bowel disease can cause dyspareunia, as can a simple fungal infection. Ask how long the pain continues after intercourse. If internal pain continues for hours, it is highly suggestive of intra-abdominal pathology.
Any cause of pain can begin a self-perpetuating conditioned response cycle that leads to inhibited desire. Anything that causes pain elicits aversion behavior, even without the stimulus. With conditioned response comes failure of excitement, which is where the sexual response cycle is interrupted.
When pain interrupts the sexual response cycle, the patient may still have intercourse, but without excitement, lubrication, and vaginal expansion—and with consequent further pain. Further attempts at intercourse then cause more pain, leading to the cycle of dyspareunia. Pain may be introital, vaginal, or deep. The pain may be reproduced on exam.
Treatment is twofold. Identify and correct source of pain first. Then the patient must learn that intercourse is not painful but pleasurable. Sensate focus exercises are often useful.
Vaginismus, an involuntary spasm of muscles around the outer third of the vagina, may make penetration impossible. Vaginismus can be caused by pain, severe negative parental attitudes about sex, or extreme religious orthodoxy. These patients may be hyper-feminine. They often have bizarre mental images of their genitals. They usually have a partner who supports the dysfunction.
Treatment involves dilators, but the purpose is not to dilate the vagina. It is to learn to have something in the vagina and to confront fears and feelings rregarding penetration. Treatment must involve the partner.
Plateau and orgasmic dysfunctions
Delayed or absent orgasm is common. It may be primary or secondary, global, situational, or random. Etiologies include performance anxiety, fear of loss of control, and boredom. Patients with orgasmic dysfunction often develop “spectator” behavior. When they reach plateau they begin to wonder if they will have orgasm. They begin to watch to see what is happening rather than participating. As a result, they lose excitement and do not have orgasm. They become increasingly anxious about whether they will achieve orgasm. Anxiety interferes with the sexual response cycle, and orgasm does not occur.
Treatment involves sensate focus. By learning to focus on the sensations that occur, the patient learns not to focus on anxiety or assume the spectator frame of mind.
When the patient reports “no desire” but describes aversion behavior, inhibited desire is typically a conditioned response to the experience of pain during intercourse. Pain—from any cause—during sexual intercourse, can begin a conditioned response that requires recognition, treatment, and, often, counseling to eliminate.
How can the Ob/Gyn help? Therapy model: P LI SS IT
Permission giving. Many sexual problems can be solved by permission-giving. A patient might be bored and wish to try new positions; permission may be all that is required.
LImited information. A patient might have orgasm with masturbation but not penetration. Explaining to the patient that 50% of women do not have orgasm with penetration alone and that it is OK to combine manual stimulation with penetration may be all that is needed.
Specific Suggestions. A patient on decongestants may note decreased lubrication. Use of a lubricant might help. A patient with the “busy mother syndrome” may need specific instruction to make time for a relationship.
Intensive Therapy. Patients with childhood sexual abuse probably require intensive therapy. Vaginismus may require intensive therapy.
- 47 yo woman married to a physician for 23 years. No intercourse for 20 years, and no communication outside the bedroom about their sexual relationship. She is very angry. Her last gynecologist said she should get a good divorce attorney.
- 50 yo woman complains of no desire and lack of lubrication. Married for 20 years. She has insulin-dependent diabetes. Her last gynecologist told her to use KY jelly. She became very angry saying KY jelly was “too clinical.”
- 30 yo woman referred for severe dyspareunia and pelvic pain. Prior workup including IVP, ultrasound, and laparoscopy found no organic cause. She describes severe inhibited sexual desire and had not attempted intercourse in 4 months.
- 34 yo married woman complains of anorgasmia since starting fluoxetine (Prozac) 2 years ago. She had orgasm prior to that. After changing to bupropion (Wellbutrin), her anorgasmia continued.
Summary
Many gynecologists are concerned about lack of time or skills to deal with sexual problems. However, it often takes less time to deal with the problem than to ignore it. If a problem is uncovered during a scheduled brief visit, the patient can be given a follow-up appointment to ensure adequate time.
Dr. Carey is a member of the Female Sexual Dysfunction CME Steering Committee, sponsored by the University of Medicine and Dentistry of New Jersey, through a grant from Proctor and Gamble.
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
2. Dunn KM, Croft PR, Hackett GI. Sexual problems: a study of the prevalence and need for health care in the general population. Fam Pract. 1998;15:519-524.
3. Bancroft J, Sherwin BB, Alexander GM, Davidson DW, Walker A. Oral contraceptives, androgens, and the sexuality of young women: II. The role of androgens. Arch Sex Behav. 1991;20:121-135.
4. Dennerstein L, Dudley EC, Hopper JL, Burger H. Sexuality, hormones and the menopausal transition. Maturitas. 1997;26:83-93.
5. Kirchengast S, Hartmann B, Gruber D, Huber J. Decreased sexual interest and its relationship to body build in postmenopausal women. Maturitas. 1996;23:63-71.
6. Schreiner-Engel P, Schiavi RC, White D, Ghizzani A. Low sexual desire in women: the role of reproductive hormones. Horm Behav. 1989;23:221-234.
7. Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
Case reports of patients helped by therapy,
Say you see 100 adult patients this week. How many have a sexual dysfunction? 10? 20? Would you believe 43? In a National Health and Social Life Survey1 of 1,749 women and 1,410 men aged 18 to 59 years, 43% of women and 31% of men reported sexual dysfunction. Rates that high make sexual dysfunction an important public health concern—one that is highly associated with impaired well-being and negative experiences in sexual relationships.
Similar findings marked a study2 of general practice patients in Britain: 41% of 979 women and 34% of 789 men reported sexual dysfunction. Although 52% wanted professional help, only 10% had received it.
As primary care providers, Ob/Gyns must identify common problems, treat those within our expertise, and refer the others. In all likelihood, we are the first physicians women see about a sexual problem. For these reasons—and because dysfunction is so common—it is appropriate that we screen for and address female sexual dysfunction.
This article presents a medical model of management and tells how to take a sexual history, formulate a differential diagnosis, manage common sexual problems, and identify indications for referral.
The medical model
According to the medical model, a patient presents with a problem, which is investigated by history and physical examination. A differential diagnosis is formulated, and appropriate laboratory tests or other evaluations are conducted. The patient’s problem is compared to a normal physiologic process to determine pathophysiology. Diagnosis and therapy follow.
The medical model for sexual dysfunction is the same as that for any disease, such as asthma, in which normal breathing is interrupted. In the sexual response cycle, physiologic changes can be measured and specifically described, as Masters and Johnson explained in 1966 in Human Sexual Response (FIGURE 1). Any barriers that interrupt this progression “block” the normal response cycle, resulting in sexual dysfunction. We aim to identify and remove or get around any barriers.
FIGURE 1 Use the sexual response cycle in dialogue with the patient
Most patients with sexual dysfunction can identify the phase in the Masters and Johnson sexual response cycle where normal progression is interrupted. Using a sketch of this cycle helps patients understand and articulate their problem.
The sexual response cycle
Desire initiates the cycle, followed by the excitement phase, during which breathing rate and pulse increase, blood flow shifts away from muscles and into the skin and pelvic organs, and there is an associated expansion, lengthening, and lubrication of the vagina. A plateau follows, during which there is no further increase in signs such as increased pulse rate. Orgasm is the first point in the response cycle that is different in men and women. Men have an obligatory resolution phase and return to baseline state; women can return to the plateau state and then have another orgasm. The resolution phase lengthens in duration in both sexes with increasing age.
The sexual response cycle is innate, like any physiologic process. It cannot be taught or learned, and is not under voluntary control. It is critical that patients understand that the sexual response cycle is a natural function that everyone has—just as everyone has innate capacity to breathe. Patients do not learn it, any more than they have to learn respiration.
Sexual behavior, however, is learned, and sexual dysfunctions are exhibited by behavior. Sex therapy is a learning process, the aim of which is to change the behavior. Sexual dysfunctions have both cognitive and physical components; although response is physiologic, it can be altered by emotion, as can respiration.
The relationship is the patient. Sexual dysfunction occurs within a relationship, and the relationship needs and stands to benefit from therapy.
Reassuring anxious, angry patients
Approach is critical, and must reflect an understanding that sexual dysfunction is real. Many patients have been told that the problem is “all in their head” or that they are deliberately acting dysfunctionally.
Confrontation and support are useful communication tools. Confrontation means identifying behaviors and beliefs and how beliefs influence behaviors. Support means conveying to the patient that you understand that she is in distress and that it couldn’t have happened any other way for her.
A “therapeutic mirror” technique can show the patient her beliefs, attitudes, and behaviors. The therapist repeats the the patient’s beliefs and behaviors until the patient states that they are an accurate reflection, or tells the therapist what is inaccurate.
Assure the patient that anxiety is to be expected, and that her probelm is common and treatable. These patients believe they have thought of everything, and nothing has worked. Just reassuring them may help.
Anger must also be acknowledged—and these patients are angry. Their anger may be directed at their partner, a former physician, or you. You may elicit a surprising outburst of pent-up anger when you ask what seems to be a neutral question.
The patient always has an internal theory and everything you say is filtered through this theory. If there is a dichotomy, it must be addressed before you can proceed successfully. Usually, you can simply ask, “What do you think is the cause?” Often she will tell you. If she is uncertain, you might say, “I realize you don’t really know. What do you think it might be? What are you afraid it might be?”
You have to ask
Many patients do not express the fact that their chief complaint is sexual dysfunction. (See How to take a sexual history,).
In many cases, sexual dysfunction may be suggested in the investigation of another complaint such as infertility, pelvic pain with no particular pattern, malaise, or depression. With these complaints, it is important to pursue a sexual history. Determine any associated or causal condition and look for associated dysfunction.
The sexual history is like a history for any disease, in that you begin with the present illness. The differential diagnosis begins with a determination of where in the cycle the dysfunction occurs.
- One way to start is to describe the normal human sexual response. I find it best to sketch out the response cycle (FIGURE 1 ) on a piece of paper and ask, “Is this what happens to you?” Most patients understand and can tell where in the cycle the interruption occurs.
- 3 questions in a review of systems disclose nearly all sexual dysfunction:
- “How often do you have intercourse?” The only “right” answer is “as often as I want to.” If the patient is not having intercourse, is it by choice or involuntary?
- “Do you have pain with intercourse?”
- “How often do you have orgasm?” Most answer “hardly ever” or “most of the time.”
- Allegories like these are useful:
- Elements and example questions
Physical examination
Ageneral physical examination with pelvic examination should always be performed. If the patient complains of pain, the site of pain should be determined. A wet prep is useful to exclude vaginitis. A patient may have dyspareunia from vaginitis even if there are no obvious physical signs.
The most common dysfunctions: Desire phase disorders
Female sexual arousal disorder is either low libido or inhibited sexual desire. In both cases the patient is likely to report that she has no desire. But if you ask what happens when her partner wants to have intercourse, a patient with low libido describes low interest behavior, and a patient with inhibited desire describes aversion behavior.
Patients with low libido sometimes report that their orgasms are less intense, or that they do not become excited. Women on estrogen replacement after oophorectomy may have low libido related to decreased androgen.
Depression is by far the most common cause. Other contributors: chronic disease, hypoestrogenic states, hyperprolactinemia, and breastfeeding.
Inhibited sexual desire, which is a result of pain or other dysfunction, is far more common than low libido. Careful history usually reveals aversion behavior. They avoid going to bed at the same time as their partner, or develop other behaviors that preclude intercourse.
Women with inhibited desire have a conditioned negative response. In studies, these patients exhibit normal objective measures of vaginal vasocongestion in response to sexual stimuli, but self-reported subjective measures of arousal do not correlate.
They report that they simply have no desire, but the cause of their avoidance is negative conditioning. Here’s where the therapist supports, but also confronts their belief system, and attempts to help them understand that they have a normal desire for intercourse. The therapist may ask the patient to consider whether she ever has sexual dreams, reads romance novels, or fantasizes, to show that these phenomena demonstrate normal desire.
The role of testosterone in inhibited sexual desire
Measuring serum testosterone isn’t helpful. It is my belief that inhibited desire is most often related to other factors. Counseling should be the first-line therapy. Drug therapy without counseling is less likely to be effective than therapy that includes counseling.
Normal serum testosterone levels are 20 to 80 ng/dL in reproductive-age women, who produce 0.2 to 0.3 mg/day of testosterone. Levels increase 10% to 20% at midcycle. Testosterone falls 40% to 60% at natural menopause and by similar amounts with use of oral contraceptive pills, and by 80% with surgical menopause.
Testosterone levels have little correlation with sexual desire in cycling women or women on oral contraceptives.3 Most studies have found no correlation between testosterone levels and sexual function in perimenopausal or naturally menopausal women.4,5 Other studies have failed to show a difference in testosterone levels between women with arousal disorders and normal controls.6
Testosterone therapy may be effective in surgically menopausal women with low libido. Shifrin and colleagues7 randomized 75 surgically menopausal women being treated with conjugated equine estrogens to placebo or transdermal testosterone, 150 or 300 μg per day, in a crossover trial. They reported, “Despite an appreciable placebo response, the higher testosterone dose resulted in further increases in scores for frequency of sexual activity and pleasure-orgasm. At the higher dose, the percentages of women who had sexual fantasies, masturbated, or engaged in sexual intercourse at least once a week increased 2 to 3 times from base line.”
Testosterone is not yet approved for female sexual dysfunction, but a transdermal testosterone is being proposed for women with hypoactive sexual desire disorder who have had bilateral oophorectomy.
Excitement phase disorders
These disorders are caused by pain with intercourse. What the patient says about the site of pain and when she began experiencing pain help form the differential diagnosis.
Dyspareunia can be due to physiological causes such as obstetric laceration, endometriosis, pelvic inflammatory disease, vaginitis, vulvar disease, and vestibulitis. Interstitial cystitis and inflammatory bowel disease can cause dyspareunia, as can a simple fungal infection. Ask how long the pain continues after intercourse. If internal pain continues for hours, it is highly suggestive of intra-abdominal pathology.
Any cause of pain can begin a self-perpetuating conditioned response cycle that leads to inhibited desire. Anything that causes pain elicits aversion behavior, even without the stimulus. With conditioned response comes failure of excitement, which is where the sexual response cycle is interrupted.
When pain interrupts the sexual response cycle, the patient may still have intercourse, but without excitement, lubrication, and vaginal expansion—and with consequent further pain. Further attempts at intercourse then cause more pain, leading to the cycle of dyspareunia. Pain may be introital, vaginal, or deep. The pain may be reproduced on exam.
Treatment is twofold. Identify and correct source of pain first. Then the patient must learn that intercourse is not painful but pleasurable. Sensate focus exercises are often useful.
Vaginismus, an involuntary spasm of muscles around the outer third of the vagina, may make penetration impossible. Vaginismus can be caused by pain, severe negative parental attitudes about sex, or extreme religious orthodoxy. These patients may be hyper-feminine. They often have bizarre mental images of their genitals. They usually have a partner who supports the dysfunction.
Treatment involves dilators, but the purpose is not to dilate the vagina. It is to learn to have something in the vagina and to confront fears and feelings rregarding penetration. Treatment must involve the partner.
Plateau and orgasmic dysfunctions
Delayed or absent orgasm is common. It may be primary or secondary, global, situational, or random. Etiologies include performance anxiety, fear of loss of control, and boredom. Patients with orgasmic dysfunction often develop “spectator” behavior. When they reach plateau they begin to wonder if they will have orgasm. They begin to watch to see what is happening rather than participating. As a result, they lose excitement and do not have orgasm. They become increasingly anxious about whether they will achieve orgasm. Anxiety interferes with the sexual response cycle, and orgasm does not occur.
Treatment involves sensate focus. By learning to focus on the sensations that occur, the patient learns not to focus on anxiety or assume the spectator frame of mind.
When the patient reports “no desire” but describes aversion behavior, inhibited desire is typically a conditioned response to the experience of pain during intercourse. Pain—from any cause—during sexual intercourse, can begin a conditioned response that requires recognition, treatment, and, often, counseling to eliminate.
How can the Ob/Gyn help? Therapy model: P LI SS IT
Permission giving. Many sexual problems can be solved by permission-giving. A patient might be bored and wish to try new positions; permission may be all that is required.
LImited information. A patient might have orgasm with masturbation but not penetration. Explaining to the patient that 50% of women do not have orgasm with penetration alone and that it is OK to combine manual stimulation with penetration may be all that is needed.
Specific Suggestions. A patient on decongestants may note decreased lubrication. Use of a lubricant might help. A patient with the “busy mother syndrome” may need specific instruction to make time for a relationship.
Intensive Therapy. Patients with childhood sexual abuse probably require intensive therapy. Vaginismus may require intensive therapy.
- 47 yo woman married to a physician for 23 years. No intercourse for 20 years, and no communication outside the bedroom about their sexual relationship. She is very angry. Her last gynecologist said she should get a good divorce attorney.
- 50 yo woman complains of no desire and lack of lubrication. Married for 20 years. She has insulin-dependent diabetes. Her last gynecologist told her to use KY jelly. She became very angry saying KY jelly was “too clinical.”
- 30 yo woman referred for severe dyspareunia and pelvic pain. Prior workup including IVP, ultrasound, and laparoscopy found no organic cause. She describes severe inhibited sexual desire and had not attempted intercourse in 4 months.
- 34 yo married woman complains of anorgasmia since starting fluoxetine (Prozac) 2 years ago. She had orgasm prior to that. After changing to bupropion (Wellbutrin), her anorgasmia continued.
Summary
Many gynecologists are concerned about lack of time or skills to deal with sexual problems. However, it often takes less time to deal with the problem than to ignore it. If a problem is uncovered during a scheduled brief visit, the patient can be given a follow-up appointment to ensure adequate time.
Dr. Carey is a member of the Female Sexual Dysfunction CME Steering Committee, sponsored by the University of Medicine and Dentistry of New Jersey, through a grant from Proctor and Gamble.
Case reports of patients helped by therapy,
Say you see 100 adult patients this week. How many have a sexual dysfunction? 10? 20? Would you believe 43? In a National Health and Social Life Survey1 of 1,749 women and 1,410 men aged 18 to 59 years, 43% of women and 31% of men reported sexual dysfunction. Rates that high make sexual dysfunction an important public health concern—one that is highly associated with impaired well-being and negative experiences in sexual relationships.
Similar findings marked a study2 of general practice patients in Britain: 41% of 979 women and 34% of 789 men reported sexual dysfunction. Although 52% wanted professional help, only 10% had received it.
As primary care providers, Ob/Gyns must identify common problems, treat those within our expertise, and refer the others. In all likelihood, we are the first physicians women see about a sexual problem. For these reasons—and because dysfunction is so common—it is appropriate that we screen for and address female sexual dysfunction.
This article presents a medical model of management and tells how to take a sexual history, formulate a differential diagnosis, manage common sexual problems, and identify indications for referral.
The medical model
According to the medical model, a patient presents with a problem, which is investigated by history and physical examination. A differential diagnosis is formulated, and appropriate laboratory tests or other evaluations are conducted. The patient’s problem is compared to a normal physiologic process to determine pathophysiology. Diagnosis and therapy follow.
The medical model for sexual dysfunction is the same as that for any disease, such as asthma, in which normal breathing is interrupted. In the sexual response cycle, physiologic changes can be measured and specifically described, as Masters and Johnson explained in 1966 in Human Sexual Response (FIGURE 1). Any barriers that interrupt this progression “block” the normal response cycle, resulting in sexual dysfunction. We aim to identify and remove or get around any barriers.
FIGURE 1 Use the sexual response cycle in dialogue with the patient
Most patients with sexual dysfunction can identify the phase in the Masters and Johnson sexual response cycle where normal progression is interrupted. Using a sketch of this cycle helps patients understand and articulate their problem.
The sexual response cycle
Desire initiates the cycle, followed by the excitement phase, during which breathing rate and pulse increase, blood flow shifts away from muscles and into the skin and pelvic organs, and there is an associated expansion, lengthening, and lubrication of the vagina. A plateau follows, during which there is no further increase in signs such as increased pulse rate. Orgasm is the first point in the response cycle that is different in men and women. Men have an obligatory resolution phase and return to baseline state; women can return to the plateau state and then have another orgasm. The resolution phase lengthens in duration in both sexes with increasing age.
The sexual response cycle is innate, like any physiologic process. It cannot be taught or learned, and is not under voluntary control. It is critical that patients understand that the sexual response cycle is a natural function that everyone has—just as everyone has innate capacity to breathe. Patients do not learn it, any more than they have to learn respiration.
Sexual behavior, however, is learned, and sexual dysfunctions are exhibited by behavior. Sex therapy is a learning process, the aim of which is to change the behavior. Sexual dysfunctions have both cognitive and physical components; although response is physiologic, it can be altered by emotion, as can respiration.
The relationship is the patient. Sexual dysfunction occurs within a relationship, and the relationship needs and stands to benefit from therapy.
Reassuring anxious, angry patients
Approach is critical, and must reflect an understanding that sexual dysfunction is real. Many patients have been told that the problem is “all in their head” or that they are deliberately acting dysfunctionally.
Confrontation and support are useful communication tools. Confrontation means identifying behaviors and beliefs and how beliefs influence behaviors. Support means conveying to the patient that you understand that she is in distress and that it couldn’t have happened any other way for her.
A “therapeutic mirror” technique can show the patient her beliefs, attitudes, and behaviors. The therapist repeats the the patient’s beliefs and behaviors until the patient states that they are an accurate reflection, or tells the therapist what is inaccurate.
Assure the patient that anxiety is to be expected, and that her probelm is common and treatable. These patients believe they have thought of everything, and nothing has worked. Just reassuring them may help.
Anger must also be acknowledged—and these patients are angry. Their anger may be directed at their partner, a former physician, or you. You may elicit a surprising outburst of pent-up anger when you ask what seems to be a neutral question.
The patient always has an internal theory and everything you say is filtered through this theory. If there is a dichotomy, it must be addressed before you can proceed successfully. Usually, you can simply ask, “What do you think is the cause?” Often she will tell you. If she is uncertain, you might say, “I realize you don’t really know. What do you think it might be? What are you afraid it might be?”
You have to ask
Many patients do not express the fact that their chief complaint is sexual dysfunction. (See How to take a sexual history,).
In many cases, sexual dysfunction may be suggested in the investigation of another complaint such as infertility, pelvic pain with no particular pattern, malaise, or depression. With these complaints, it is important to pursue a sexual history. Determine any associated or causal condition and look for associated dysfunction.
The sexual history is like a history for any disease, in that you begin with the present illness. The differential diagnosis begins with a determination of where in the cycle the dysfunction occurs.
- One way to start is to describe the normal human sexual response. I find it best to sketch out the response cycle (FIGURE 1 ) on a piece of paper and ask, “Is this what happens to you?” Most patients understand and can tell where in the cycle the interruption occurs.
- 3 questions in a review of systems disclose nearly all sexual dysfunction:
- “How often do you have intercourse?” The only “right” answer is “as often as I want to.” If the patient is not having intercourse, is it by choice or involuntary?
- “Do you have pain with intercourse?”
- “How often do you have orgasm?” Most answer “hardly ever” or “most of the time.”
- Allegories like these are useful:
- Elements and example questions
Physical examination
Ageneral physical examination with pelvic examination should always be performed. If the patient complains of pain, the site of pain should be determined. A wet prep is useful to exclude vaginitis. A patient may have dyspareunia from vaginitis even if there are no obvious physical signs.
The most common dysfunctions: Desire phase disorders
Female sexual arousal disorder is either low libido or inhibited sexual desire. In both cases the patient is likely to report that she has no desire. But if you ask what happens when her partner wants to have intercourse, a patient with low libido describes low interest behavior, and a patient with inhibited desire describes aversion behavior.
Patients with low libido sometimes report that their orgasms are less intense, or that they do not become excited. Women on estrogen replacement after oophorectomy may have low libido related to decreased androgen.
Depression is by far the most common cause. Other contributors: chronic disease, hypoestrogenic states, hyperprolactinemia, and breastfeeding.
Inhibited sexual desire, which is a result of pain or other dysfunction, is far more common than low libido. Careful history usually reveals aversion behavior. They avoid going to bed at the same time as their partner, or develop other behaviors that preclude intercourse.
Women with inhibited desire have a conditioned negative response. In studies, these patients exhibit normal objective measures of vaginal vasocongestion in response to sexual stimuli, but self-reported subjective measures of arousal do not correlate.
They report that they simply have no desire, but the cause of their avoidance is negative conditioning. Here’s where the therapist supports, but also confronts their belief system, and attempts to help them understand that they have a normal desire for intercourse. The therapist may ask the patient to consider whether she ever has sexual dreams, reads romance novels, or fantasizes, to show that these phenomena demonstrate normal desire.
The role of testosterone in inhibited sexual desire
Measuring serum testosterone isn’t helpful. It is my belief that inhibited desire is most often related to other factors. Counseling should be the first-line therapy. Drug therapy without counseling is less likely to be effective than therapy that includes counseling.
Normal serum testosterone levels are 20 to 80 ng/dL in reproductive-age women, who produce 0.2 to 0.3 mg/day of testosterone. Levels increase 10% to 20% at midcycle. Testosterone falls 40% to 60% at natural menopause and by similar amounts with use of oral contraceptive pills, and by 80% with surgical menopause.
Testosterone levels have little correlation with sexual desire in cycling women or women on oral contraceptives.3 Most studies have found no correlation between testosterone levels and sexual function in perimenopausal or naturally menopausal women.4,5 Other studies have failed to show a difference in testosterone levels between women with arousal disorders and normal controls.6
Testosterone therapy may be effective in surgically menopausal women with low libido. Shifrin and colleagues7 randomized 75 surgically menopausal women being treated with conjugated equine estrogens to placebo or transdermal testosterone, 150 or 300 μg per day, in a crossover trial. They reported, “Despite an appreciable placebo response, the higher testosterone dose resulted in further increases in scores for frequency of sexual activity and pleasure-orgasm. At the higher dose, the percentages of women who had sexual fantasies, masturbated, or engaged in sexual intercourse at least once a week increased 2 to 3 times from base line.”
Testosterone is not yet approved for female sexual dysfunction, but a transdermal testosterone is being proposed for women with hypoactive sexual desire disorder who have had bilateral oophorectomy.
Excitement phase disorders
These disorders are caused by pain with intercourse. What the patient says about the site of pain and when she began experiencing pain help form the differential diagnosis.
Dyspareunia can be due to physiological causes such as obstetric laceration, endometriosis, pelvic inflammatory disease, vaginitis, vulvar disease, and vestibulitis. Interstitial cystitis and inflammatory bowel disease can cause dyspareunia, as can a simple fungal infection. Ask how long the pain continues after intercourse. If internal pain continues for hours, it is highly suggestive of intra-abdominal pathology.
Any cause of pain can begin a self-perpetuating conditioned response cycle that leads to inhibited desire. Anything that causes pain elicits aversion behavior, even without the stimulus. With conditioned response comes failure of excitement, which is where the sexual response cycle is interrupted.
When pain interrupts the sexual response cycle, the patient may still have intercourse, but without excitement, lubrication, and vaginal expansion—and with consequent further pain. Further attempts at intercourse then cause more pain, leading to the cycle of dyspareunia. Pain may be introital, vaginal, or deep. The pain may be reproduced on exam.
Treatment is twofold. Identify and correct source of pain first. Then the patient must learn that intercourse is not painful but pleasurable. Sensate focus exercises are often useful.
Vaginismus, an involuntary spasm of muscles around the outer third of the vagina, may make penetration impossible. Vaginismus can be caused by pain, severe negative parental attitudes about sex, or extreme religious orthodoxy. These patients may be hyper-feminine. They often have bizarre mental images of their genitals. They usually have a partner who supports the dysfunction.
Treatment involves dilators, but the purpose is not to dilate the vagina. It is to learn to have something in the vagina and to confront fears and feelings rregarding penetration. Treatment must involve the partner.
Plateau and orgasmic dysfunctions
Delayed or absent orgasm is common. It may be primary or secondary, global, situational, or random. Etiologies include performance anxiety, fear of loss of control, and boredom. Patients with orgasmic dysfunction often develop “spectator” behavior. When they reach plateau they begin to wonder if they will have orgasm. They begin to watch to see what is happening rather than participating. As a result, they lose excitement and do not have orgasm. They become increasingly anxious about whether they will achieve orgasm. Anxiety interferes with the sexual response cycle, and orgasm does not occur.
Treatment involves sensate focus. By learning to focus on the sensations that occur, the patient learns not to focus on anxiety or assume the spectator frame of mind.
When the patient reports “no desire” but describes aversion behavior, inhibited desire is typically a conditioned response to the experience of pain during intercourse. Pain—from any cause—during sexual intercourse, can begin a conditioned response that requires recognition, treatment, and, often, counseling to eliminate.
How can the Ob/Gyn help? Therapy model: P LI SS IT
Permission giving. Many sexual problems can be solved by permission-giving. A patient might be bored and wish to try new positions; permission may be all that is required.
LImited information. A patient might have orgasm with masturbation but not penetration. Explaining to the patient that 50% of women do not have orgasm with penetration alone and that it is OK to combine manual stimulation with penetration may be all that is needed.
Specific Suggestions. A patient on decongestants may note decreased lubrication. Use of a lubricant might help. A patient with the “busy mother syndrome” may need specific instruction to make time for a relationship.
Intensive Therapy. Patients with childhood sexual abuse probably require intensive therapy. Vaginismus may require intensive therapy.
- 47 yo woman married to a physician for 23 years. No intercourse for 20 years, and no communication outside the bedroom about their sexual relationship. She is very angry. Her last gynecologist said she should get a good divorce attorney.
- 50 yo woman complains of no desire and lack of lubrication. Married for 20 years. She has insulin-dependent diabetes. Her last gynecologist told her to use KY jelly. She became very angry saying KY jelly was “too clinical.”
- 30 yo woman referred for severe dyspareunia and pelvic pain. Prior workup including IVP, ultrasound, and laparoscopy found no organic cause. She describes severe inhibited sexual desire and had not attempted intercourse in 4 months.
- 34 yo married woman complains of anorgasmia since starting fluoxetine (Prozac) 2 years ago. She had orgasm prior to that. After changing to bupropion (Wellbutrin), her anorgasmia continued.
Summary
Many gynecologists are concerned about lack of time or skills to deal with sexual problems. However, it often takes less time to deal with the problem than to ignore it. If a problem is uncovered during a scheduled brief visit, the patient can be given a follow-up appointment to ensure adequate time.
Dr. Carey is a member of the Female Sexual Dysfunction CME Steering Committee, sponsored by the University of Medicine and Dentistry of New Jersey, through a grant from Proctor and Gamble.
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
2. Dunn KM, Croft PR, Hackett GI. Sexual problems: a study of the prevalence and need for health care in the general population. Fam Pract. 1998;15:519-524.
3. Bancroft J, Sherwin BB, Alexander GM, Davidson DW, Walker A. Oral contraceptives, androgens, and the sexuality of young women: II. The role of androgens. Arch Sex Behav. 1991;20:121-135.
4. Dennerstein L, Dudley EC, Hopper JL, Burger H. Sexuality, hormones and the menopausal transition. Maturitas. 1997;26:83-93.
5. Kirchengast S, Hartmann B, Gruber D, Huber J. Decreased sexual interest and its relationship to body build in postmenopausal women. Maturitas. 1996;23:63-71.
6. Schreiner-Engel P, Schiavi RC, White D, Ghizzani A. Low sexual desire in women: the role of reproductive hormones. Horm Behav. 1989;23:221-234.
7. Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
2. Dunn KM, Croft PR, Hackett GI. Sexual problems: a study of the prevalence and need for health care in the general population. Fam Pract. 1998;15:519-524.
3. Bancroft J, Sherwin BB, Alexander GM, Davidson DW, Walker A. Oral contraceptives, androgens, and the sexuality of young women: II. The role of androgens. Arch Sex Behav. 1991;20:121-135.
4. Dennerstein L, Dudley EC, Hopper JL, Burger H. Sexuality, hormones and the menopausal transition. Maturitas. 1997;26:83-93.
5. Kirchengast S, Hartmann B, Gruber D, Huber J. Decreased sexual interest and its relationship to body build in postmenopausal women. Maturitas. 1996;23:63-71.
6. Schreiner-Engel P, Schiavi RC, White D, Ghizzani A. Low sexual desire in women: the role of reproductive hormones. Horm Behav. 1989;23:221-234.
7. Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682-688.
Estradiol gel: A new option in hormone replacement therapy
- Percutaneous estradiol gel can be prescribed at low doses.
- Relief of menopausal symptoms can begin as early as 2 weeks after starting treatment.
- Treatment with estradiol gel maintains or increases bone mineral density.
- No large randomized, controlled trials have explored the effect of estradiol gel on coronary artery disease. It appears to have metabolic effects similar to those of oral estradiol.
- Because percutaneous estradiol stimulates the endometrium, women with an intact uterus should also take a progestogen.
Although the Women’s Health Initiative1 discouraged many menopausal women from using oral estrogen, it failed to address the risks of treatment with other dosages or forms of estrogen and progestin.
Nor has any other trial of similar size and complexity taken up the issue. However, numerous smaller studies have been published.
This article summarizes findings on percutaneous delivery of estradiol gel (EstroGel), the most recently FDA-approved estrogen option for treatment of menopausal symptoms.
It describes the overall safety of estradiol gel, as well as its effectson:
- menopausal symptoms,
- bone,
- metabolism, and
- endometrium.
(In this article, “percutaneous delivery” refers to estradiol gel applied to the skin, and “transdermal estradiol” indicates delivery via a transdermal reservoir or matrix system, otherwise known as “the patch.” I have used an arbitrary definition to distinguish the gel from other methods of delivering estradiol across the skin. For example, Estrasorb is a liposomal formulation that is applied to the skin of the thigh. Although it is a percutaneous estradiol similar to the gel, this article focuses only on the latter option.)
Easy to apply, few skin reactions
Percutaneous estradiol gel formulations have been available for almost 30 years in Europe, where they are utilized by a majority of women on hormone therapy. In the United States, the hydroalcoholic gel is packaged in a pump that delivers 64 standardized 1.25-g doses, which contain 0.75 mg of 17ß-estradiol. Once it is applied, the gel is absorbed into an intradermal reservoir (FIGURE) and dries in 2 to 5 minutes, leaving no residue.
Patient selection. Estradiol gel is well-suited for patients who are concerned about the risks of oral estrogen (as portrayed in the mainstream press following the Women’s Health Initiative) and want to avoid that route of administration, as well as women who dislike or have difficulty swallowing pills. Percutaneous administration also is appropriate for physically active women who may have adhesion problems or skin irritation with the transdermal patch, or those who have had reactions to local adhesives in the past. The patient should be motivated to apply the gel on a daily basis.
Indications are moderate to severe vasomotor symptoms in menopausal women, and moderate to severe symptoms of vulvar and vaginal atrophy, although topical vaginal products should also be considered for the latter indication.
Contraindications are undiagnosed abnormal vaginal bleeding; history of breast cancer, other estrogen-dependent malignancy, stroke, heart attack, or liver disease or dysfunction; active thrombophlebitis or thromboembolic disorders (or a history of these); and known or suspected pregnancy.
Common side effects include headache, breast pain, irregular vaginal bleeding or spotting, stomach cramps or bloating, nausea and vomiting, and hair loss.
- Skin reactions are infrequent, but should be taken into account when discussing per-cutaneous or transdermal delivery of any drug. However, estradiol gel appears to cause fewer skin reactions than the patch. In a study over more than 5 years, 0 of 157 women treated with percutaneous estradiol reported skin irritation.2 Other comparisons found similar outcomes.3,4
Onset of action is rapid, as it is with the transdermal patch.5 Dose variability is minimized when the gel is applied at the same time every day to a large area of skin, preferably the arm, although all application sites appear to produce similar results: abdomen, shoulders, arms, and inner thigh.6 The gel should not be applied to the breast or vagina.
Diminished effect with skin washing. In 1 trial, site washing 1/2 hour after application significantly decreased bioavailability and time to reach peak plasma concentrations.7 For this reason, the gel should be not applied before a bath, shower, or sauna.
Dosing options. The initial dose is 1.25 mg of gel, which is 1 pump of the bottle. The gel is collected in the palm of 1 hand and applied to the skin of the opposite arm from the wrist to the shoulder. The dose can be titrated by adding a second pump of the gel and applying it to the opposite arm. The dose can be lowered by using less than a full depression of the pump.
Stable, physiologic estrogen levels
Estradiol gel produces relatively stable serum estradiol levels, and therapeutic estradiol levels similar to those seen with other formulations, routes of administration, and dosages.
Percutaneous administration produces serum estrone to estradiol ratios close to 1, in contrast to higher ratios (5:1) with oral administration.8-11 The lower ratio approximates levels during the menstrual cycle of premenopausal women. (TABLE 1 gives estradiol and estrone levels from different studies following administration of estradiol as a gel, oral formulation, and transdermal patch.)
The percutaneous route also allows for delivery directly to the systemic circulation, avoiding gastrointestinal and first-pass hepatic metabolism and elimination. In contrast, oral micronized estradiol causes large fluctuations in serum estradiol and estrone levels due to absorption and metabolism.8
More stable serum levels than with the patch. One study12 comparing percutaneous and transdermal estradiol found similar interindividual variability but less stable serum levels in women using the transdermal system. A separate study13 also reported greater fluctuation of serum estradiol levels in women using a transdermal system than in those using the gel.
TABLE 1
Serum estradiol and estrone levels for various routes of estradiol
| AUTHOR | THERAPY | ESTRADIOL LEVEL (PG/ML) | ESTRONE LEVEL (PG/ML) |
|---|---|---|---|
| Scott et al8 | E2gel 3 mg/day | 102.9±39.9 | 120.0±50.5 |
| E2gel 1.5 mg/day | 68.1±27.4 | 90.6±45.7 | |
| Transdermal estradiol 50 μg/day | 41.1±13.5 | 45.0±15.9 | |
| Oral micronized estradiol 2 mg/day | 114.0±65.2 | 575.2±279.9 | |
| Palacios et al10 | E2gel 1.5 mg/day | 75.7±1.0 | 58.5±2.9 |
| Oral conjugated estrogen 0.625 mg/day | 39.6±4.6 | 126.0±7.6 | |
| Basdevant et al11 | E2gel 3 mg/day | 221.0±35.0 | 146.0±19.0 |
| Oral micronized estradiol 2 mg/day | 121.0±27.0 | 811.0±370.0 | |
| Archer15 | E2gel 0.75 mg/day | 33.5* | 49.0* |
| E2gel 1.5 mg/day | 65.0* | 58.0* | |
| Placebo | 5.0* | 23.0* | |
| *Median value | |||
| E2gel = percutaneous estradiol gel | |||
Studies compared relief of menopausal symptoms
Estradiol gel effectively relieved meno-pausal symptoms in randomized, double-blind studies, open label comparisons, and observational trials in postmenopausal women, with and without the addition of various progestins.
Symptom relief in comparison with baseline values is statistically significant as early as 2 weeks after initiating treatment. Relief of up to 2 years’ duration has been reported.3,14
Several studies have compared symptom relief achieved with estradiol gel, oral estrogen, transdermal delivery systems, and placebo3,9,14-18; findings are summarized in TABLE 2.
Same efficacy when progestogen is added. The following studies, and other studies,14 demonstrated that estradiol gel relieves menopausal symptoms whether it is administered alone or in combination with a progestogen, with efficacy similar to other estrogen formulations:
Climacteric symptoms decreased to the same extent when estradiol gel was combined with a levonorgestrel-releasing intrauterine device, oral micronized progesterone, or vaginal micronized progesterone.19
A study20 that added lynestrenol decreased the frequency of hot flushes and night sweats more than in women using estradiol gel alone. However, negative mood symptoms were more pronounced in the progestin-treated group.
Estradiol gel 1 mg/day in combination with monthly or quarterly oral medroxyprog-esterone acetate reduced the severity of hot flushes, sweating, and vaginal dryness, according to a 12-month trial.21
Symptoms decreased the same whether a levonorgestrel-releasing IUD or oral or vaginal progesterone was added.
Bone mineral density maintained or increased
Several randomized, controlled trials have documented the effects of estradiol gel on bone mineral density (BMD) and various markers of bone metabolism. In these studies, BMD remained steady22,23 or increased10,24,25 following treatment, and estradiol gel remained effective for up to 4 years.25 Estradiol gel maintained or increased BMD with or without addition of progestins.22,23,26
These investigations involved measurement of BMD at the lumbar spine, forearms, or hip, as well as biologic markers of bone turnover such as urinary hydroxyproline/creatinine ratio, serum alkaline phosphatase, and serum osteocalcin.
Serum estradiol and skeletal uptake of a bone-seeking agent also were determined. Estradiol gel regimens ranged from 0.75 mg/day to 3 mg/day, and populations included both surgical and natural menopausal subjects in several countries.
Effects comparable to oral estrogen. Compared with oral conjugated estrogen, which increased BMD at the lumbar spine by 4.3% (±3.2%), estradiol gel produced increases of 5.6% (±2.9%) at 24 months and 4.7% (±3.2%) at 36 months.10
The case. A 52-year-old woman with no menses for 8 months presents for management complaining of disabling hot flushes. Although she is moderately obese, with a body mass index of 29, she is normotensive without any other significant medical history except for hysterectomy at age 44 for excessive bleeding.
Counseling. During her 20s, the patient tried to use oral contraceptives on 3 separate occasions, but was unable to continue them because of nausea. Although she is interested in estrogen therapy for her vasomotor symptoms, she is concerned about the possibility of experiencing nausea again. You explain that one of the benefits of percutaneous estradiol is that it avoids the gastrointestinal tract.
Physical findings. Her physical examination is within normal limits, and gynecologic examination confirms no uterus and finds no palpable adnexal masses.
Outcome. After weighing the pros and cons, she elects to use estradiol gel. At her 3-month follow-up, she reports effective relief and good compliance.
Minimum level of protection achieved. Following a comparison of oral and percutaneous estradiol, Reginster et al27 suggested that a minimum estradiol level of 60 pg/mL is necessary to prevent postmenopausal bone loss. Mean serum estradiol levels in women receiving 1.5 mg/day estradiol gel were 75.7 pg/mL and 78.4 pg/mL at 24 and 36 months, respectively, in a study by Palacios et al,10 and 85.8 pg/mL in a study by Devogelaer and colleagues.24 In these trials, BMD increased, and it remained steady in other investigations.28,29
More recent trials suggest that lower serum estradiol levels secondary to smaller estrogen doses have the capacity to maintain BMD.30,31 A 1.9% mean increase of BMD at the lumbar spine was reported in women with a mean serum concentration of 17 pg/mL in a 2-year study30 of a transdermal estradiol delivery system. The percutaneous route does not appear to limit these beneficial effects.
How are metabolic factors affected?
In general, oral estrogens produce beneficial changes in lipid metabolism, particularly higher levels of high-density lipoprotein (HDL) cholesterol. However, they also elevate triglyceride and glucose levels. How this plays out clinically is unclear. Both the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study (HERS) found no cardioprotective effects of oral estrogen despite increases in HDL and decreases in low-density lipoprotein (LDL) levels.1,32
Oral versus percutaneous estradiol. No long-term studies of this magnitude have investigated the effect of percutaneous estradiol on coronary artery disease, although numerous clinical trials have shown that the route of estrogen delivery affects many of the metabolic variables used to estimate the risk of negative cardiac outcomes. It remains to be determined whether percutaneous estradiol affects these metabolic factors in ways that influence morbidity and mortality.
In 1 trial,33 oral conjugated estrogen led to the following significant changes in lipids:
- increase in very low density lipoprotein (VLDL) cholesterol,
- decrease in LDL cholesterol (but not the LDL apoprotein B),
- increase in HDL and apoprotein A1, and
- significant increase in HDL2 cholesterol.
In a separate study,9 oral conjugated estrogen had a 2.5-fold increase in serum angiotensin, and percutaneous estradiol gel, no effect.
Same effects when progestin is added. Beneficial effects were not diminished when oral micronized progesterone was added to percutaneous estradiol.34 Estradiol gel significantly reduced total serum cholesterol and LDL cholesterol in the first year of treatment, compared with placebo.
Coagulation effects. Percutaneous estradiol has fewer negative effects on coagulation factors than its oral counterpart. One study35 investigating combination therapy with micronized progesterone compared the effects of percutaneous estradiol and oral estradiol valerate. The group receiving percutaneous estradiol gel/micronized progesterone had no significant changes in plasminogen activator inhibitor, tissue-type plasminogen concentration, and global fibrinolytic capacity. The other group had a significant decrease in mean tissue-type plasminogen concentration and plasminogen activator inhibitor activity and a significant rise in global fibrinolytic capacity.
The oral estradiol—but not the percutaneous formulation—significantly increased the mean value of prothrombin activation peptide and decreased mean antithrombin activity compared with no treatment.
Poor glycemic control may increase the risk of cardiovascular disease, but oral and percutaneous estradiol appear to have similar glycemic effects. A comparative trial36 of oral estradiol valerate 2 mg/day and percutaneous estradiol 1 mg/day found no differences in glycosylated hemoglobin A1c levels (declined in both groups) or in fasting and 2-hour post-prandial blood glucose levels (constant in both groups) or insulin sensitivity.
Treatment duration may also influence how percutaneous estradiol affects metabolic factors. An open-label longitudinal prospective study37 of 30 women receiving estradiol gel 1.5 mg/day for 6 months found a significant decrease in lipoprotein (a), apoprotein A-I, apoprotein B, HDL cholesterol, and HDL3 cholesterol. At 1 year, however, these changes were not significant.
No association with venous thromboembolism. Transdermal estradiol administered as a patch or percutaneous gel had no effect on the risk of venous thromboembolism in a multicenter case-control investigation.38
In contrast, a recent retrospective study found a risk of venous thromboembolism that was at least 4 times greater with oral estrogen than with transdermal estradiol.38
Use a progestogen to prevent endometrial hyperplasia
Like other forms of estrogen, percutaneous estradiol stimulates the endometrium. For this reason, women who have an intact uterus should use a progestogen in an adequate dose to prevent hyperplastic changes.39 Although numerous regimens appear to be effective, the optimal route, dose, and duration of progestin in women using percutaneous estrogen remain to be determined.
Percutaneous estradiol versus other routes of administration. Endometrial thickness and amenorrhea rates over 6 months were not statistically different among 54 women treated with estradiol gel 1.5 mg/day, transdermal estradiol 50 μg/day, or oral estradiol valerate 2 mg/day—all in combination with nomegestrol acetate 2.5 mg/day.40 The overall rates of no bleeding or spotting over 6 cycles of treatment were 78% in the percutaneous estradiol group, 48% in the transdermal group, and 60% in the oral group.
Although the percutaneous estradiol group had a lower overall incidence of no bleeding or spotting than the other groups, the difference was not significant. Nor were there significant differences in the variation of endometrial thickness from baseline: A mean increase of 1.5 mm (±0.4 mm) was reported in the percutaneous group, compared with 1.5 mm (±0.7 mm) and 1.7 mm (±0.6 mm) in the transdermal and oral estrogen groups, respectively.
Estrogenic and progestogenic effects were similar for transdermal and percutaneous estradiol (with dydrogesterone 10 mg/day for days 1 to 12) after a baseline atropic endometrium was identified.3
Estradiol gel produces stable, physiologic serum estradiol levels and has a serum estrone to estradiol ratio close to 1.
The most effective progestogen dosage and duration are unknown, although many regimens have been studied:
- Percutaneous estradiol 1.5 mg/day for the first 24 days of the month in combination with nomegestrol acetate 5 mg/day for days 11 to 24: Over 6 months, researchers found a secretory pattern in the majority of women and no evidence of hyperplasia.18
- Percutaneous estradiol 3 mg/day for 3 of every 4 weeks in combination with nomegestrol acetate 5 mg/day for 10 days: No reduction in estrogenic endometrial effects.41,42
- Estradiol gel 3 mg/day for 3 of every 4 weeks in combination with 200 mg or 300 mg oral micronized progesterone for the last 10 days of treatment: Dose was too low or treatment too short to produce a complete secretory transformation of the endometrium.43 However, the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial44 found no increased occurrence of hyperplasia in women using micronized progesterone 200 mg for 12 days of each cycle for more than 3 years in combination with oral conjugated estrogen 0.625 mg.
- Percutaneous estradiol 1.5 mg/day combined with micronized progesterone 100 mg daily (orally or vaginally) for the first 25 days of the month: Fully inhibited mitoses and induced amenorrhea in most of the women studied, with amenorrhea rates of 93.3% at 3 months and 91.6% at 6 months.45
- Estradiol gel 1.5 mg/day with 100 mg vaginal micronized progesterone for 21 days per cycle: Stable endometrial thickness and no endometrial hyperplasia over 12 months.46 However, breakthrough bleeding was reported in up to 30% of subjects, principally in the second 3 months of treatment.
- Percutaneous estradiol 1 mg/day for 3 months with oral medroxyprogesterone acetate 20 mg/day for the last 14 days, followed by a 7-day free interval: No reports of endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 2 mg/day for 21 days with 10 mg oral medroxyprogesterone acetate for the last 14 days, followed by a 7-day free interval: No endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 1 mg/day with medroxyprogesterone acetate 10 mg on days 1-12 every month or every 3 months: Endometrial hyperplasia was found in 1 woman (0.3%) in the group receiving the progestogen every 3 months. Endometrial histology did not differ between women taking medroxyprogesterone monthly and those taking it every 3 months.21
- Estradiol gel 3 mg/day with oral micronized progesterone 200 mg for 12 days of each cycle: Regular withdrawal bleeding in 70% of women.34
- Percutaneous estradiol 1.5 mg/day in combination with a levonorgestrel-releasing intrauterine device: 80% of women were amenorrheic at 1 year, with a mean endometrial thickness 3 mm; at 5 years, 100% of women had epithelial atrophy.47
- Estradiol gel 1.5 mg for 21 days with 200 mg oral progesterone for 14 days (126 women); 3 mg percutaneous estradiol for 21 days with 300 mg oral progesterone for 10 days (23 women); 1.5 mg estradiol gel with 300 mg oral progesterone (3 women); or 3 mg estradiol gel for 28 days with 200 mg progesterone for 14 days (5 women): No evidence of hyperplasia after 5 years of treatment.2
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Moyer DL, de Lignieres B, Driguez P, et al. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril. 1993;59:992-997.
3. Hirvonen E, Cacciatore B, Wahlström T, et al. Effects of transdermal oestrogen therapy in postmenopausal women: a comparative study of an oestradiol gel and an oestradiol delivering patch. BJOG. 1997;104(Suppl 16):26-31.
4. Travassos de Figueiredo Alves S, et al. Comparison of gel and patch estradiol replacement in Brazil, a tropical country. Maturitas. 2000;36:69-74.
5. Henzl MR, Loomba PK. Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice. J Reprod Med. 2003;48:525-540.
6. Holst J, et al. Serum estrogen levels after topic application of estradiol-17 beta on two different cutaneous areas. Acta Obstet Gynecol Scand. 1987;662:151-152.
7. Jarvinen A, Granander M, Nykanen S, Laine T, Geurts P, Viitanen A. Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. Br J Obstet Gynaecol. 1997;104(Suppl 16):14-18.
8. Scott RT, Ross B, Anderson C, Archer DF. Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol. Obstet Gynecol. 1991;77:758-764.
9. Dupont A, Dupont P, Cusan M, et al. Comparative endocrinological and clinical effects of percutaneous estradiol and oral conjugated estrogens as replacement therapy in menopausal women. Maturitas. 1991;13:297-311.
10. Palacios S, Menéndez C, Jurado AR, Vargas JC. Effects of percutaneous oestradiol versus oral oestrogens on bone density. Maturitas. 1995;20:209-213.
11. Basdevant A, de Lignieres B, Simon P, et al. Hepatic lipase activity during oral and parenteral 17ß-estradiol replacement therapy: high-density lipoprotein increase may not be antiatherogenic. Fertil Steril. 1991;55:1112-1117.
12. Simon JA, et al. Are there significant differences between patch and gel cutaneous estradiol therapy? In: Genazzani AR, Petraglia F, Volpe A, Facchinetti F, eds. Recent Research on Gynecological Endocrinology. Casterton Hall, Carnforth, Lancashire, UK: Parthenon Publishing, Casterton Hall; 1998;317-324.
13. Paoletti AM, et al. Comparison of pharmacokinetic profiles of 17ß-estradiol gel 0.6mg/g (Gelestra) with a transdermal delivery system (Estraderm TTS 50) in postmenopausal women at steady state. Maturitas. 2001;40:203-209.
14. Jensen PB, Jensen J, Riis BJ, et al. Climacteric symptoms after oral and percutaneous hormone replacement therapy. Maturitas. 1987;9:207-215.
15. Archer DF. for the EstroGel Study Group. Percutaneous 17ß-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
16. Kornafel KL, March CM. Estradiol gel in the treatment of menopausal symptoms: a placebo-controlled double-blind case study of efficacy and safety. South Med J. 1992;85:270-273.
17. Hirvonen E, Lamberg-Allardt C, Lankinen KS, Geurts P, Wilén-Rosenqvist G. Transdermal oestradiol gel in the treatment of the climacterium: a comparison with oral therapy. BJOG. 1997;104(Suppl 16):19-25.
18. Foidart JM, Béliard A, Hedon B, et al. Impact of percutaneous oestradiol gels in postmenopausal hormone replacement therapy on clinical symptoms and endometrium. BJOG. 1997;104:305-310.
19. Suvanto-Luukkonen E, Sundström H, Penttinen J, et al. Percutaneous estradiol gel with an intrauterine levonorgestrel releasing device or natural progesterone in hormone replacement therapy. Maturitas. 1997;26:211-17.
20. Holst J, et al. Progestogen addition during oestrogen replacement therapy—effects on vasomotor symptoms and mood. Maturitas. 1989;11:13-20.
21. Hirvonen E, et al. Effect of an estradiol gel with monthly or quarterly progestogen on menopausal symptoms and bleeding. Climacteric. 2000;3:262-270.
22. Ng HT, Chang SP, Yang TS, Cho MP, Wei TC. Estradiol administered in a percutaneous gel for the prevention of postmenopausal bone loss. Asia-Oceania J Obstet Gynaecol. 1993;19(2):115-119.
23. Riis B, Thomsen K, et al. Does calcium supplementation prevent postmenopausal bone loss? A double blind, controlled clinical study. N Engl J Med. 1987;316:173-177.
24. Devogelaer JP, Lecart C, Dupret P, De Nayer P, Nagant De Deuxchaisnes C. Long-term effects of percutaneous estradiol on bone loss and bone metabolism in post-menopausal hysterectomized women. Maturitas. 1998;28:243-249.
25. Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four year randomized study. Am J Med. 1995;99:36-42.
26. Sun A, Lin S, Yu W, et al. Percutaneous estrogen in prevention of early post-menopausal bone loss in Chinese women. Chin Med J. 2002;115:1790-1795.
27. Reginster JY, Sarlet N, Deroisy R, et al. Minimum levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
28. Cicinelli E, Cantatore FP, Galantino P, et al. Effects of continuous percutaneous estradiol administration on skeletal turnover in postmenopausal women: a 1-year prospective controlled study. Eur J Obstet Gynecol Reprod Biol. 1996;69(2):109-113.
29. Riis B, et al. The effect of percutaneous estradiol and natural progesterone on post-menopausal bone loss. Am J Obstet Gynecol. 1987;156:61-65.
30. Notelvitz M, John VA, Good WR. Effectiveness of Alora estradiol matrix transdermal delivery system in improving lumbar bone mineral density in healthy, post-menopausal women. Menopause. 2002;9:343-353.
31. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17ß-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-1048.
32. Hully S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
33. Moorjani S, Dupont A, Labrie F, et al. Changes in plasma lipoprotein and apolipoprotein composition in relation to oral versus percutaneous administration of estrogen alone or in cyclic association with Utrogestan in menopausal women. J Clin Endocrinol Metab. 1991;73:373-379.
34. Jensen J, Riis BJ, Strøm V, Nilas L, Christiansen C. Long-term effects of percutaneous estrogens and oral progesterone on serum lipoproteins in postmenopausal women. Am J Obstet Gyencol. 1987;156:66-71.
35. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071-3078.
36. Karjalainen A, Paassilta M, Heikkinen J, et al. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrin. 2001;54:165-173.
37. Haines CJ, Chung TKH, Masarei JRL, Tomlinson B, Lau JTF. The effect of per-cutaneous oestrogen replacement therapy on Lp(a) and other lipoproteins. Maturitas. 1995;22:219-225.
38. Scarabin PY, Oger E, Plu-Bureau G. EStrogen and THromboEmbolism Risk (ESTHER) Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
39. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
40. Blanc B, Cravello L, Micheletti MC, et al. Continuous hormone replacement therapy for menopause combining nomegestrol acetate and gel, patch, or oral estrogen: a comparison of amenorrhea rates. Clin Therap. 1998;20(5):901-912.
41. Holst J. Percutaneous estrogen therapy. Endometrial response and metabolic effects. Acta Obstet Gynecol Scand Suppl. 1983;115:1-30
42. Holst J, Cajander S, von Schoultz B. Cellular morphometric analysis of the post-menopausal endometrium during treatment with percutaneous estradiol-17ß with and without oral gestagen. Acta Obstet Gynecol Scand. 1983;62:267-270.
43. Holst J, Cajander S, von Schoultz B. Endometrial response in post-menopausal women during treatment with percutaneous 17ß-oestradiol opposed by oral progesterone. Maturitas. 1986;8:201-207.
44. Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370-375.
45. Gillet JY, Andre G, Faguer B, et al. Induction of amenorrhea during hormone replacement therapy: optimized micronized progesterone dose. A multicenter study. Maturitas. 1994;19:103-115.
46. Vilodre LC, et al. Endometrial response to a cyclic regimen of percutaneous 17ß-estradiol and low-dose vaginal micronized progesterone in women with mild-to-moderate hypertension. Gynecol Endocrinol. 2003;17:323-328.
47. Suvanto-Luukkonen E, Kauppila A. The levonorgestrel intrauterine system in menopausal hormone replacement therapy: five-year experience. Fertil Steril. 1999;72:161-163.
- Percutaneous estradiol gel can be prescribed at low doses.
- Relief of menopausal symptoms can begin as early as 2 weeks after starting treatment.
- Treatment with estradiol gel maintains or increases bone mineral density.
- No large randomized, controlled trials have explored the effect of estradiol gel on coronary artery disease. It appears to have metabolic effects similar to those of oral estradiol.
- Because percutaneous estradiol stimulates the endometrium, women with an intact uterus should also take a progestogen.
Although the Women’s Health Initiative1 discouraged many menopausal women from using oral estrogen, it failed to address the risks of treatment with other dosages or forms of estrogen and progestin.
Nor has any other trial of similar size and complexity taken up the issue. However, numerous smaller studies have been published.
This article summarizes findings on percutaneous delivery of estradiol gel (EstroGel), the most recently FDA-approved estrogen option for treatment of menopausal symptoms.
It describes the overall safety of estradiol gel, as well as its effectson:
- menopausal symptoms,
- bone,
- metabolism, and
- endometrium.
(In this article, “percutaneous delivery” refers to estradiol gel applied to the skin, and “transdermal estradiol” indicates delivery via a transdermal reservoir or matrix system, otherwise known as “the patch.” I have used an arbitrary definition to distinguish the gel from other methods of delivering estradiol across the skin. For example, Estrasorb is a liposomal formulation that is applied to the skin of the thigh. Although it is a percutaneous estradiol similar to the gel, this article focuses only on the latter option.)
Easy to apply, few skin reactions
Percutaneous estradiol gel formulations have been available for almost 30 years in Europe, where they are utilized by a majority of women on hormone therapy. In the United States, the hydroalcoholic gel is packaged in a pump that delivers 64 standardized 1.25-g doses, which contain 0.75 mg of 17ß-estradiol. Once it is applied, the gel is absorbed into an intradermal reservoir (FIGURE) and dries in 2 to 5 minutes, leaving no residue.
Patient selection. Estradiol gel is well-suited for patients who are concerned about the risks of oral estrogen (as portrayed in the mainstream press following the Women’s Health Initiative) and want to avoid that route of administration, as well as women who dislike or have difficulty swallowing pills. Percutaneous administration also is appropriate for physically active women who may have adhesion problems or skin irritation with the transdermal patch, or those who have had reactions to local adhesives in the past. The patient should be motivated to apply the gel on a daily basis.
Indications are moderate to severe vasomotor symptoms in menopausal women, and moderate to severe symptoms of vulvar and vaginal atrophy, although topical vaginal products should also be considered for the latter indication.
Contraindications are undiagnosed abnormal vaginal bleeding; history of breast cancer, other estrogen-dependent malignancy, stroke, heart attack, or liver disease or dysfunction; active thrombophlebitis or thromboembolic disorders (or a history of these); and known or suspected pregnancy.
Common side effects include headache, breast pain, irregular vaginal bleeding or spotting, stomach cramps or bloating, nausea and vomiting, and hair loss.
- Skin reactions are infrequent, but should be taken into account when discussing per-cutaneous or transdermal delivery of any drug. However, estradiol gel appears to cause fewer skin reactions than the patch. In a study over more than 5 years, 0 of 157 women treated with percutaneous estradiol reported skin irritation.2 Other comparisons found similar outcomes.3,4
Onset of action is rapid, as it is with the transdermal patch.5 Dose variability is minimized when the gel is applied at the same time every day to a large area of skin, preferably the arm, although all application sites appear to produce similar results: abdomen, shoulders, arms, and inner thigh.6 The gel should not be applied to the breast or vagina.
Diminished effect with skin washing. In 1 trial, site washing 1/2 hour after application significantly decreased bioavailability and time to reach peak plasma concentrations.7 For this reason, the gel should be not applied before a bath, shower, or sauna.
Dosing options. The initial dose is 1.25 mg of gel, which is 1 pump of the bottle. The gel is collected in the palm of 1 hand and applied to the skin of the opposite arm from the wrist to the shoulder. The dose can be titrated by adding a second pump of the gel and applying it to the opposite arm. The dose can be lowered by using less than a full depression of the pump.
Stable, physiologic estrogen levels
Estradiol gel produces relatively stable serum estradiol levels, and therapeutic estradiol levels similar to those seen with other formulations, routes of administration, and dosages.
Percutaneous administration produces serum estrone to estradiol ratios close to 1, in contrast to higher ratios (5:1) with oral administration.8-11 The lower ratio approximates levels during the menstrual cycle of premenopausal women. (TABLE 1 gives estradiol and estrone levels from different studies following administration of estradiol as a gel, oral formulation, and transdermal patch.)
The percutaneous route also allows for delivery directly to the systemic circulation, avoiding gastrointestinal and first-pass hepatic metabolism and elimination. In contrast, oral micronized estradiol causes large fluctuations in serum estradiol and estrone levels due to absorption and metabolism.8
More stable serum levels than with the patch. One study12 comparing percutaneous and transdermal estradiol found similar interindividual variability but less stable serum levels in women using the transdermal system. A separate study13 also reported greater fluctuation of serum estradiol levels in women using a transdermal system than in those using the gel.
TABLE 1
Serum estradiol and estrone levels for various routes of estradiol
| AUTHOR | THERAPY | ESTRADIOL LEVEL (PG/ML) | ESTRONE LEVEL (PG/ML) |
|---|---|---|---|
| Scott et al8 | E2gel 3 mg/day | 102.9±39.9 | 120.0±50.5 |
| E2gel 1.5 mg/day | 68.1±27.4 | 90.6±45.7 | |
| Transdermal estradiol 50 μg/day | 41.1±13.5 | 45.0±15.9 | |
| Oral micronized estradiol 2 mg/day | 114.0±65.2 | 575.2±279.9 | |
| Palacios et al10 | E2gel 1.5 mg/day | 75.7±1.0 | 58.5±2.9 |
| Oral conjugated estrogen 0.625 mg/day | 39.6±4.6 | 126.0±7.6 | |
| Basdevant et al11 | E2gel 3 mg/day | 221.0±35.0 | 146.0±19.0 |
| Oral micronized estradiol 2 mg/day | 121.0±27.0 | 811.0±370.0 | |
| Archer15 | E2gel 0.75 mg/day | 33.5* | 49.0* |
| E2gel 1.5 mg/day | 65.0* | 58.0* | |
| Placebo | 5.0* | 23.0* | |
| *Median value | |||
| E2gel = percutaneous estradiol gel | |||
Studies compared relief of menopausal symptoms
Estradiol gel effectively relieved meno-pausal symptoms in randomized, double-blind studies, open label comparisons, and observational trials in postmenopausal women, with and without the addition of various progestins.
Symptom relief in comparison with baseline values is statistically significant as early as 2 weeks after initiating treatment. Relief of up to 2 years’ duration has been reported.3,14
Several studies have compared symptom relief achieved with estradiol gel, oral estrogen, transdermal delivery systems, and placebo3,9,14-18; findings are summarized in TABLE 2.
Same efficacy when progestogen is added. The following studies, and other studies,14 demonstrated that estradiol gel relieves menopausal symptoms whether it is administered alone or in combination with a progestogen, with efficacy similar to other estrogen formulations:
Climacteric symptoms decreased to the same extent when estradiol gel was combined with a levonorgestrel-releasing intrauterine device, oral micronized progesterone, or vaginal micronized progesterone.19
A study20 that added lynestrenol decreased the frequency of hot flushes and night sweats more than in women using estradiol gel alone. However, negative mood symptoms were more pronounced in the progestin-treated group.
Estradiol gel 1 mg/day in combination with monthly or quarterly oral medroxyprog-esterone acetate reduced the severity of hot flushes, sweating, and vaginal dryness, according to a 12-month trial.21
Symptoms decreased the same whether a levonorgestrel-releasing IUD or oral or vaginal progesterone was added.
Bone mineral density maintained or increased
Several randomized, controlled trials have documented the effects of estradiol gel on bone mineral density (BMD) and various markers of bone metabolism. In these studies, BMD remained steady22,23 or increased10,24,25 following treatment, and estradiol gel remained effective for up to 4 years.25 Estradiol gel maintained or increased BMD with or without addition of progestins.22,23,26
These investigations involved measurement of BMD at the lumbar spine, forearms, or hip, as well as biologic markers of bone turnover such as urinary hydroxyproline/creatinine ratio, serum alkaline phosphatase, and serum osteocalcin.
Serum estradiol and skeletal uptake of a bone-seeking agent also were determined. Estradiol gel regimens ranged from 0.75 mg/day to 3 mg/day, and populations included both surgical and natural menopausal subjects in several countries.
Effects comparable to oral estrogen. Compared with oral conjugated estrogen, which increased BMD at the lumbar spine by 4.3% (±3.2%), estradiol gel produced increases of 5.6% (±2.9%) at 24 months and 4.7% (±3.2%) at 36 months.10
The case. A 52-year-old woman with no menses for 8 months presents for management complaining of disabling hot flushes. Although she is moderately obese, with a body mass index of 29, she is normotensive without any other significant medical history except for hysterectomy at age 44 for excessive bleeding.
Counseling. During her 20s, the patient tried to use oral contraceptives on 3 separate occasions, but was unable to continue them because of nausea. Although she is interested in estrogen therapy for her vasomotor symptoms, she is concerned about the possibility of experiencing nausea again. You explain that one of the benefits of percutaneous estradiol is that it avoids the gastrointestinal tract.
Physical findings. Her physical examination is within normal limits, and gynecologic examination confirms no uterus and finds no palpable adnexal masses.
Outcome. After weighing the pros and cons, she elects to use estradiol gel. At her 3-month follow-up, she reports effective relief and good compliance.
Minimum level of protection achieved. Following a comparison of oral and percutaneous estradiol, Reginster et al27 suggested that a minimum estradiol level of 60 pg/mL is necessary to prevent postmenopausal bone loss. Mean serum estradiol levels in women receiving 1.5 mg/day estradiol gel were 75.7 pg/mL and 78.4 pg/mL at 24 and 36 months, respectively, in a study by Palacios et al,10 and 85.8 pg/mL in a study by Devogelaer and colleagues.24 In these trials, BMD increased, and it remained steady in other investigations.28,29
More recent trials suggest that lower serum estradiol levels secondary to smaller estrogen doses have the capacity to maintain BMD.30,31 A 1.9% mean increase of BMD at the lumbar spine was reported in women with a mean serum concentration of 17 pg/mL in a 2-year study30 of a transdermal estradiol delivery system. The percutaneous route does not appear to limit these beneficial effects.
How are metabolic factors affected?
In general, oral estrogens produce beneficial changes in lipid metabolism, particularly higher levels of high-density lipoprotein (HDL) cholesterol. However, they also elevate triglyceride and glucose levels. How this plays out clinically is unclear. Both the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study (HERS) found no cardioprotective effects of oral estrogen despite increases in HDL and decreases in low-density lipoprotein (LDL) levels.1,32
Oral versus percutaneous estradiol. No long-term studies of this magnitude have investigated the effect of percutaneous estradiol on coronary artery disease, although numerous clinical trials have shown that the route of estrogen delivery affects many of the metabolic variables used to estimate the risk of negative cardiac outcomes. It remains to be determined whether percutaneous estradiol affects these metabolic factors in ways that influence morbidity and mortality.
In 1 trial,33 oral conjugated estrogen led to the following significant changes in lipids:
- increase in very low density lipoprotein (VLDL) cholesterol,
- decrease in LDL cholesterol (but not the LDL apoprotein B),
- increase in HDL and apoprotein A1, and
- significant increase in HDL2 cholesterol.
In a separate study,9 oral conjugated estrogen had a 2.5-fold increase in serum angiotensin, and percutaneous estradiol gel, no effect.
Same effects when progestin is added. Beneficial effects were not diminished when oral micronized progesterone was added to percutaneous estradiol.34 Estradiol gel significantly reduced total serum cholesterol and LDL cholesterol in the first year of treatment, compared with placebo.
Coagulation effects. Percutaneous estradiol has fewer negative effects on coagulation factors than its oral counterpart. One study35 investigating combination therapy with micronized progesterone compared the effects of percutaneous estradiol and oral estradiol valerate. The group receiving percutaneous estradiol gel/micronized progesterone had no significant changes in plasminogen activator inhibitor, tissue-type plasminogen concentration, and global fibrinolytic capacity. The other group had a significant decrease in mean tissue-type plasminogen concentration and plasminogen activator inhibitor activity and a significant rise in global fibrinolytic capacity.
The oral estradiol—but not the percutaneous formulation—significantly increased the mean value of prothrombin activation peptide and decreased mean antithrombin activity compared with no treatment.
Poor glycemic control may increase the risk of cardiovascular disease, but oral and percutaneous estradiol appear to have similar glycemic effects. A comparative trial36 of oral estradiol valerate 2 mg/day and percutaneous estradiol 1 mg/day found no differences in glycosylated hemoglobin A1c levels (declined in both groups) or in fasting and 2-hour post-prandial blood glucose levels (constant in both groups) or insulin sensitivity.
Treatment duration may also influence how percutaneous estradiol affects metabolic factors. An open-label longitudinal prospective study37 of 30 women receiving estradiol gel 1.5 mg/day for 6 months found a significant decrease in lipoprotein (a), apoprotein A-I, apoprotein B, HDL cholesterol, and HDL3 cholesterol. At 1 year, however, these changes were not significant.
No association with venous thromboembolism. Transdermal estradiol administered as a patch or percutaneous gel had no effect on the risk of venous thromboembolism in a multicenter case-control investigation.38
In contrast, a recent retrospective study found a risk of venous thromboembolism that was at least 4 times greater with oral estrogen than with transdermal estradiol.38
Use a progestogen to prevent endometrial hyperplasia
Like other forms of estrogen, percutaneous estradiol stimulates the endometrium. For this reason, women who have an intact uterus should use a progestogen in an adequate dose to prevent hyperplastic changes.39 Although numerous regimens appear to be effective, the optimal route, dose, and duration of progestin in women using percutaneous estrogen remain to be determined.
Percutaneous estradiol versus other routes of administration. Endometrial thickness and amenorrhea rates over 6 months were not statistically different among 54 women treated with estradiol gel 1.5 mg/day, transdermal estradiol 50 μg/day, or oral estradiol valerate 2 mg/day—all in combination with nomegestrol acetate 2.5 mg/day.40 The overall rates of no bleeding or spotting over 6 cycles of treatment were 78% in the percutaneous estradiol group, 48% in the transdermal group, and 60% in the oral group.
Although the percutaneous estradiol group had a lower overall incidence of no bleeding or spotting than the other groups, the difference was not significant. Nor were there significant differences in the variation of endometrial thickness from baseline: A mean increase of 1.5 mm (±0.4 mm) was reported in the percutaneous group, compared with 1.5 mm (±0.7 mm) and 1.7 mm (±0.6 mm) in the transdermal and oral estrogen groups, respectively.
Estrogenic and progestogenic effects were similar for transdermal and percutaneous estradiol (with dydrogesterone 10 mg/day for days 1 to 12) after a baseline atropic endometrium was identified.3
Estradiol gel produces stable, physiologic serum estradiol levels and has a serum estrone to estradiol ratio close to 1.
The most effective progestogen dosage and duration are unknown, although many regimens have been studied:
- Percutaneous estradiol 1.5 mg/day for the first 24 days of the month in combination with nomegestrol acetate 5 mg/day for days 11 to 24: Over 6 months, researchers found a secretory pattern in the majority of women and no evidence of hyperplasia.18
- Percutaneous estradiol 3 mg/day for 3 of every 4 weeks in combination with nomegestrol acetate 5 mg/day for 10 days: No reduction in estrogenic endometrial effects.41,42
- Estradiol gel 3 mg/day for 3 of every 4 weeks in combination with 200 mg or 300 mg oral micronized progesterone for the last 10 days of treatment: Dose was too low or treatment too short to produce a complete secretory transformation of the endometrium.43 However, the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial44 found no increased occurrence of hyperplasia in women using micronized progesterone 200 mg for 12 days of each cycle for more than 3 years in combination with oral conjugated estrogen 0.625 mg.
- Percutaneous estradiol 1.5 mg/day combined with micronized progesterone 100 mg daily (orally or vaginally) for the first 25 days of the month: Fully inhibited mitoses and induced amenorrhea in most of the women studied, with amenorrhea rates of 93.3% at 3 months and 91.6% at 6 months.45
- Estradiol gel 1.5 mg/day with 100 mg vaginal micronized progesterone for 21 days per cycle: Stable endometrial thickness and no endometrial hyperplasia over 12 months.46 However, breakthrough bleeding was reported in up to 30% of subjects, principally in the second 3 months of treatment.
- Percutaneous estradiol 1 mg/day for 3 months with oral medroxyprogesterone acetate 20 mg/day for the last 14 days, followed by a 7-day free interval: No reports of endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 2 mg/day for 21 days with 10 mg oral medroxyprogesterone acetate for the last 14 days, followed by a 7-day free interval: No endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 1 mg/day with medroxyprogesterone acetate 10 mg on days 1-12 every month or every 3 months: Endometrial hyperplasia was found in 1 woman (0.3%) in the group receiving the progestogen every 3 months. Endometrial histology did not differ between women taking medroxyprogesterone monthly and those taking it every 3 months.21
- Estradiol gel 3 mg/day with oral micronized progesterone 200 mg for 12 days of each cycle: Regular withdrawal bleeding in 70% of women.34
- Percutaneous estradiol 1.5 mg/day in combination with a levonorgestrel-releasing intrauterine device: 80% of women were amenorrheic at 1 year, with a mean endometrial thickness 3 mm; at 5 years, 100% of women had epithelial atrophy.47
- Estradiol gel 1.5 mg for 21 days with 200 mg oral progesterone for 14 days (126 women); 3 mg percutaneous estradiol for 21 days with 300 mg oral progesterone for 10 days (23 women); 1.5 mg estradiol gel with 300 mg oral progesterone (3 women); or 3 mg estradiol gel for 28 days with 200 mg progesterone for 14 days (5 women): No evidence of hyperplasia after 5 years of treatment.2
- Percutaneous estradiol gel can be prescribed at low doses.
- Relief of menopausal symptoms can begin as early as 2 weeks after starting treatment.
- Treatment with estradiol gel maintains or increases bone mineral density.
- No large randomized, controlled trials have explored the effect of estradiol gel on coronary artery disease. It appears to have metabolic effects similar to those of oral estradiol.
- Because percutaneous estradiol stimulates the endometrium, women with an intact uterus should also take a progestogen.
Although the Women’s Health Initiative1 discouraged many menopausal women from using oral estrogen, it failed to address the risks of treatment with other dosages or forms of estrogen and progestin.
Nor has any other trial of similar size and complexity taken up the issue. However, numerous smaller studies have been published.
This article summarizes findings on percutaneous delivery of estradiol gel (EstroGel), the most recently FDA-approved estrogen option for treatment of menopausal symptoms.
It describes the overall safety of estradiol gel, as well as its effectson:
- menopausal symptoms,
- bone,
- metabolism, and
- endometrium.
(In this article, “percutaneous delivery” refers to estradiol gel applied to the skin, and “transdermal estradiol” indicates delivery via a transdermal reservoir or matrix system, otherwise known as “the patch.” I have used an arbitrary definition to distinguish the gel from other methods of delivering estradiol across the skin. For example, Estrasorb is a liposomal formulation that is applied to the skin of the thigh. Although it is a percutaneous estradiol similar to the gel, this article focuses only on the latter option.)
Easy to apply, few skin reactions
Percutaneous estradiol gel formulations have been available for almost 30 years in Europe, where they are utilized by a majority of women on hormone therapy. In the United States, the hydroalcoholic gel is packaged in a pump that delivers 64 standardized 1.25-g doses, which contain 0.75 mg of 17ß-estradiol. Once it is applied, the gel is absorbed into an intradermal reservoir (FIGURE) and dries in 2 to 5 minutes, leaving no residue.
Patient selection. Estradiol gel is well-suited for patients who are concerned about the risks of oral estrogen (as portrayed in the mainstream press following the Women’s Health Initiative) and want to avoid that route of administration, as well as women who dislike or have difficulty swallowing pills. Percutaneous administration also is appropriate for physically active women who may have adhesion problems or skin irritation with the transdermal patch, or those who have had reactions to local adhesives in the past. The patient should be motivated to apply the gel on a daily basis.
Indications are moderate to severe vasomotor symptoms in menopausal women, and moderate to severe symptoms of vulvar and vaginal atrophy, although topical vaginal products should also be considered for the latter indication.
Contraindications are undiagnosed abnormal vaginal bleeding; history of breast cancer, other estrogen-dependent malignancy, stroke, heart attack, or liver disease or dysfunction; active thrombophlebitis or thromboembolic disorders (or a history of these); and known or suspected pregnancy.
Common side effects include headache, breast pain, irregular vaginal bleeding or spotting, stomach cramps or bloating, nausea and vomiting, and hair loss.
- Skin reactions are infrequent, but should be taken into account when discussing per-cutaneous or transdermal delivery of any drug. However, estradiol gel appears to cause fewer skin reactions than the patch. In a study over more than 5 years, 0 of 157 women treated with percutaneous estradiol reported skin irritation.2 Other comparisons found similar outcomes.3,4
Onset of action is rapid, as it is with the transdermal patch.5 Dose variability is minimized when the gel is applied at the same time every day to a large area of skin, preferably the arm, although all application sites appear to produce similar results: abdomen, shoulders, arms, and inner thigh.6 The gel should not be applied to the breast or vagina.
Diminished effect with skin washing. In 1 trial, site washing 1/2 hour after application significantly decreased bioavailability and time to reach peak plasma concentrations.7 For this reason, the gel should be not applied before a bath, shower, or sauna.
Dosing options. The initial dose is 1.25 mg of gel, which is 1 pump of the bottle. The gel is collected in the palm of 1 hand and applied to the skin of the opposite arm from the wrist to the shoulder. The dose can be titrated by adding a second pump of the gel and applying it to the opposite arm. The dose can be lowered by using less than a full depression of the pump.
Stable, physiologic estrogen levels
Estradiol gel produces relatively stable serum estradiol levels, and therapeutic estradiol levels similar to those seen with other formulations, routes of administration, and dosages.
Percutaneous administration produces serum estrone to estradiol ratios close to 1, in contrast to higher ratios (5:1) with oral administration.8-11 The lower ratio approximates levels during the menstrual cycle of premenopausal women. (TABLE 1 gives estradiol and estrone levels from different studies following administration of estradiol as a gel, oral formulation, and transdermal patch.)
The percutaneous route also allows for delivery directly to the systemic circulation, avoiding gastrointestinal and first-pass hepatic metabolism and elimination. In contrast, oral micronized estradiol causes large fluctuations in serum estradiol and estrone levels due to absorption and metabolism.8
More stable serum levels than with the patch. One study12 comparing percutaneous and transdermal estradiol found similar interindividual variability but less stable serum levels in women using the transdermal system. A separate study13 also reported greater fluctuation of serum estradiol levels in women using a transdermal system than in those using the gel.
TABLE 1
Serum estradiol and estrone levels for various routes of estradiol
| AUTHOR | THERAPY | ESTRADIOL LEVEL (PG/ML) | ESTRONE LEVEL (PG/ML) |
|---|---|---|---|
| Scott et al8 | E2gel 3 mg/day | 102.9±39.9 | 120.0±50.5 |
| E2gel 1.5 mg/day | 68.1±27.4 | 90.6±45.7 | |
| Transdermal estradiol 50 μg/day | 41.1±13.5 | 45.0±15.9 | |
| Oral micronized estradiol 2 mg/day | 114.0±65.2 | 575.2±279.9 | |
| Palacios et al10 | E2gel 1.5 mg/day | 75.7±1.0 | 58.5±2.9 |
| Oral conjugated estrogen 0.625 mg/day | 39.6±4.6 | 126.0±7.6 | |
| Basdevant et al11 | E2gel 3 mg/day | 221.0±35.0 | 146.0±19.0 |
| Oral micronized estradiol 2 mg/day | 121.0±27.0 | 811.0±370.0 | |
| Archer15 | E2gel 0.75 mg/day | 33.5* | 49.0* |
| E2gel 1.5 mg/day | 65.0* | 58.0* | |
| Placebo | 5.0* | 23.0* | |
| *Median value | |||
| E2gel = percutaneous estradiol gel | |||
Studies compared relief of menopausal symptoms
Estradiol gel effectively relieved meno-pausal symptoms in randomized, double-blind studies, open label comparisons, and observational trials in postmenopausal women, with and without the addition of various progestins.
Symptom relief in comparison with baseline values is statistically significant as early as 2 weeks after initiating treatment. Relief of up to 2 years’ duration has been reported.3,14
Several studies have compared symptom relief achieved with estradiol gel, oral estrogen, transdermal delivery systems, and placebo3,9,14-18; findings are summarized in TABLE 2.
Same efficacy when progestogen is added. The following studies, and other studies,14 demonstrated that estradiol gel relieves menopausal symptoms whether it is administered alone or in combination with a progestogen, with efficacy similar to other estrogen formulations:
Climacteric symptoms decreased to the same extent when estradiol gel was combined with a levonorgestrel-releasing intrauterine device, oral micronized progesterone, or vaginal micronized progesterone.19
A study20 that added lynestrenol decreased the frequency of hot flushes and night sweats more than in women using estradiol gel alone. However, negative mood symptoms were more pronounced in the progestin-treated group.
Estradiol gel 1 mg/day in combination with monthly or quarterly oral medroxyprog-esterone acetate reduced the severity of hot flushes, sweating, and vaginal dryness, according to a 12-month trial.21
Symptoms decreased the same whether a levonorgestrel-releasing IUD or oral or vaginal progesterone was added.
Bone mineral density maintained or increased
Several randomized, controlled trials have documented the effects of estradiol gel on bone mineral density (BMD) and various markers of bone metabolism. In these studies, BMD remained steady22,23 or increased10,24,25 following treatment, and estradiol gel remained effective for up to 4 years.25 Estradiol gel maintained or increased BMD with or without addition of progestins.22,23,26
These investigations involved measurement of BMD at the lumbar spine, forearms, or hip, as well as biologic markers of bone turnover such as urinary hydroxyproline/creatinine ratio, serum alkaline phosphatase, and serum osteocalcin.
Serum estradiol and skeletal uptake of a bone-seeking agent also were determined. Estradiol gel regimens ranged from 0.75 mg/day to 3 mg/day, and populations included both surgical and natural menopausal subjects in several countries.
Effects comparable to oral estrogen. Compared with oral conjugated estrogen, which increased BMD at the lumbar spine by 4.3% (±3.2%), estradiol gel produced increases of 5.6% (±2.9%) at 24 months and 4.7% (±3.2%) at 36 months.10
The case. A 52-year-old woman with no menses for 8 months presents for management complaining of disabling hot flushes. Although she is moderately obese, with a body mass index of 29, she is normotensive without any other significant medical history except for hysterectomy at age 44 for excessive bleeding.
Counseling. During her 20s, the patient tried to use oral contraceptives on 3 separate occasions, but was unable to continue them because of nausea. Although she is interested in estrogen therapy for her vasomotor symptoms, she is concerned about the possibility of experiencing nausea again. You explain that one of the benefits of percutaneous estradiol is that it avoids the gastrointestinal tract.
Physical findings. Her physical examination is within normal limits, and gynecologic examination confirms no uterus and finds no palpable adnexal masses.
Outcome. After weighing the pros and cons, she elects to use estradiol gel. At her 3-month follow-up, she reports effective relief and good compliance.
Minimum level of protection achieved. Following a comparison of oral and percutaneous estradiol, Reginster et al27 suggested that a minimum estradiol level of 60 pg/mL is necessary to prevent postmenopausal bone loss. Mean serum estradiol levels in women receiving 1.5 mg/day estradiol gel were 75.7 pg/mL and 78.4 pg/mL at 24 and 36 months, respectively, in a study by Palacios et al,10 and 85.8 pg/mL in a study by Devogelaer and colleagues.24 In these trials, BMD increased, and it remained steady in other investigations.28,29
More recent trials suggest that lower serum estradiol levels secondary to smaller estrogen doses have the capacity to maintain BMD.30,31 A 1.9% mean increase of BMD at the lumbar spine was reported in women with a mean serum concentration of 17 pg/mL in a 2-year study30 of a transdermal estradiol delivery system. The percutaneous route does not appear to limit these beneficial effects.
How are metabolic factors affected?
In general, oral estrogens produce beneficial changes in lipid metabolism, particularly higher levels of high-density lipoprotein (HDL) cholesterol. However, they also elevate triglyceride and glucose levels. How this plays out clinically is unclear. Both the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study (HERS) found no cardioprotective effects of oral estrogen despite increases in HDL and decreases in low-density lipoprotein (LDL) levels.1,32
Oral versus percutaneous estradiol. No long-term studies of this magnitude have investigated the effect of percutaneous estradiol on coronary artery disease, although numerous clinical trials have shown that the route of estrogen delivery affects many of the metabolic variables used to estimate the risk of negative cardiac outcomes. It remains to be determined whether percutaneous estradiol affects these metabolic factors in ways that influence morbidity and mortality.
In 1 trial,33 oral conjugated estrogen led to the following significant changes in lipids:
- increase in very low density lipoprotein (VLDL) cholesterol,
- decrease in LDL cholesterol (but not the LDL apoprotein B),
- increase in HDL and apoprotein A1, and
- significant increase in HDL2 cholesterol.
In a separate study,9 oral conjugated estrogen had a 2.5-fold increase in serum angiotensin, and percutaneous estradiol gel, no effect.
Same effects when progestin is added. Beneficial effects were not diminished when oral micronized progesterone was added to percutaneous estradiol.34 Estradiol gel significantly reduced total serum cholesterol and LDL cholesterol in the first year of treatment, compared with placebo.
Coagulation effects. Percutaneous estradiol has fewer negative effects on coagulation factors than its oral counterpart. One study35 investigating combination therapy with micronized progesterone compared the effects of percutaneous estradiol and oral estradiol valerate. The group receiving percutaneous estradiol gel/micronized progesterone had no significant changes in plasminogen activator inhibitor, tissue-type plasminogen concentration, and global fibrinolytic capacity. The other group had a significant decrease in mean tissue-type plasminogen concentration and plasminogen activator inhibitor activity and a significant rise in global fibrinolytic capacity.
The oral estradiol—but not the percutaneous formulation—significantly increased the mean value of prothrombin activation peptide and decreased mean antithrombin activity compared with no treatment.
Poor glycemic control may increase the risk of cardiovascular disease, but oral and percutaneous estradiol appear to have similar glycemic effects. A comparative trial36 of oral estradiol valerate 2 mg/day and percutaneous estradiol 1 mg/day found no differences in glycosylated hemoglobin A1c levels (declined in both groups) or in fasting and 2-hour post-prandial blood glucose levels (constant in both groups) or insulin sensitivity.
Treatment duration may also influence how percutaneous estradiol affects metabolic factors. An open-label longitudinal prospective study37 of 30 women receiving estradiol gel 1.5 mg/day for 6 months found a significant decrease in lipoprotein (a), apoprotein A-I, apoprotein B, HDL cholesterol, and HDL3 cholesterol. At 1 year, however, these changes were not significant.
No association with venous thromboembolism. Transdermal estradiol administered as a patch or percutaneous gel had no effect on the risk of venous thromboembolism in a multicenter case-control investigation.38
In contrast, a recent retrospective study found a risk of venous thromboembolism that was at least 4 times greater with oral estrogen than with transdermal estradiol.38
Use a progestogen to prevent endometrial hyperplasia
Like other forms of estrogen, percutaneous estradiol stimulates the endometrium. For this reason, women who have an intact uterus should use a progestogen in an adequate dose to prevent hyperplastic changes.39 Although numerous regimens appear to be effective, the optimal route, dose, and duration of progestin in women using percutaneous estrogen remain to be determined.
Percutaneous estradiol versus other routes of administration. Endometrial thickness and amenorrhea rates over 6 months were not statistically different among 54 women treated with estradiol gel 1.5 mg/day, transdermal estradiol 50 μg/day, or oral estradiol valerate 2 mg/day—all in combination with nomegestrol acetate 2.5 mg/day.40 The overall rates of no bleeding or spotting over 6 cycles of treatment were 78% in the percutaneous estradiol group, 48% in the transdermal group, and 60% in the oral group.
Although the percutaneous estradiol group had a lower overall incidence of no bleeding or spotting than the other groups, the difference was not significant. Nor were there significant differences in the variation of endometrial thickness from baseline: A mean increase of 1.5 mm (±0.4 mm) was reported in the percutaneous group, compared with 1.5 mm (±0.7 mm) and 1.7 mm (±0.6 mm) in the transdermal and oral estrogen groups, respectively.
Estrogenic and progestogenic effects were similar for transdermal and percutaneous estradiol (with dydrogesterone 10 mg/day for days 1 to 12) after a baseline atropic endometrium was identified.3
Estradiol gel produces stable, physiologic serum estradiol levels and has a serum estrone to estradiol ratio close to 1.
The most effective progestogen dosage and duration are unknown, although many regimens have been studied:
- Percutaneous estradiol 1.5 mg/day for the first 24 days of the month in combination with nomegestrol acetate 5 mg/day for days 11 to 24: Over 6 months, researchers found a secretory pattern in the majority of women and no evidence of hyperplasia.18
- Percutaneous estradiol 3 mg/day for 3 of every 4 weeks in combination with nomegestrol acetate 5 mg/day for 10 days: No reduction in estrogenic endometrial effects.41,42
- Estradiol gel 3 mg/day for 3 of every 4 weeks in combination with 200 mg or 300 mg oral micronized progesterone for the last 10 days of treatment: Dose was too low or treatment too short to produce a complete secretory transformation of the endometrium.43 However, the Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial44 found no increased occurrence of hyperplasia in women using micronized progesterone 200 mg for 12 days of each cycle for more than 3 years in combination with oral conjugated estrogen 0.625 mg.
- Percutaneous estradiol 1.5 mg/day combined with micronized progesterone 100 mg daily (orally or vaginally) for the first 25 days of the month: Fully inhibited mitoses and induced amenorrhea in most of the women studied, with amenorrhea rates of 93.3% at 3 months and 91.6% at 6 months.45
- Estradiol gel 1.5 mg/day with 100 mg vaginal micronized progesterone for 21 days per cycle: Stable endometrial thickness and no endometrial hyperplasia over 12 months.46 However, breakthrough bleeding was reported in up to 30% of subjects, principally in the second 3 months of treatment.
- Percutaneous estradiol 1 mg/day for 3 months with oral medroxyprogesterone acetate 20 mg/day for the last 14 days, followed by a 7-day free interval: No reports of endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 2 mg/day for 21 days with 10 mg oral medroxyprogesterone acetate for the last 14 days, followed by a 7-day free interval: No endometrial hyperplasia or suspect changes at 12 and 24 months.17
- Estradiol gel 1 mg/day with medroxyprogesterone acetate 10 mg on days 1-12 every month or every 3 months: Endometrial hyperplasia was found in 1 woman (0.3%) in the group receiving the progestogen every 3 months. Endometrial histology did not differ between women taking medroxyprogesterone monthly and those taking it every 3 months.21
- Estradiol gel 3 mg/day with oral micronized progesterone 200 mg for 12 days of each cycle: Regular withdrawal bleeding in 70% of women.34
- Percutaneous estradiol 1.5 mg/day in combination with a levonorgestrel-releasing intrauterine device: 80% of women were amenorrheic at 1 year, with a mean endometrial thickness 3 mm; at 5 years, 100% of women had epithelial atrophy.47
- Estradiol gel 1.5 mg for 21 days with 200 mg oral progesterone for 14 days (126 women); 3 mg percutaneous estradiol for 21 days with 300 mg oral progesterone for 10 days (23 women); 1.5 mg estradiol gel with 300 mg oral progesterone (3 women); or 3 mg estradiol gel for 28 days with 200 mg progesterone for 14 days (5 women): No evidence of hyperplasia after 5 years of treatment.2
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Moyer DL, de Lignieres B, Driguez P, et al. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril. 1993;59:992-997.
3. Hirvonen E, Cacciatore B, Wahlström T, et al. Effects of transdermal oestrogen therapy in postmenopausal women: a comparative study of an oestradiol gel and an oestradiol delivering patch. BJOG. 1997;104(Suppl 16):26-31.
4. Travassos de Figueiredo Alves S, et al. Comparison of gel and patch estradiol replacement in Brazil, a tropical country. Maturitas. 2000;36:69-74.
5. Henzl MR, Loomba PK. Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice. J Reprod Med. 2003;48:525-540.
6. Holst J, et al. Serum estrogen levels after topic application of estradiol-17 beta on two different cutaneous areas. Acta Obstet Gynecol Scand. 1987;662:151-152.
7. Jarvinen A, Granander M, Nykanen S, Laine T, Geurts P, Viitanen A. Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. Br J Obstet Gynaecol. 1997;104(Suppl 16):14-18.
8. Scott RT, Ross B, Anderson C, Archer DF. Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol. Obstet Gynecol. 1991;77:758-764.
9. Dupont A, Dupont P, Cusan M, et al. Comparative endocrinological and clinical effects of percutaneous estradiol and oral conjugated estrogens as replacement therapy in menopausal women. Maturitas. 1991;13:297-311.
10. Palacios S, Menéndez C, Jurado AR, Vargas JC. Effects of percutaneous oestradiol versus oral oestrogens on bone density. Maturitas. 1995;20:209-213.
11. Basdevant A, de Lignieres B, Simon P, et al. Hepatic lipase activity during oral and parenteral 17ß-estradiol replacement therapy: high-density lipoprotein increase may not be antiatherogenic. Fertil Steril. 1991;55:1112-1117.
12. Simon JA, et al. Are there significant differences between patch and gel cutaneous estradiol therapy? In: Genazzani AR, Petraglia F, Volpe A, Facchinetti F, eds. Recent Research on Gynecological Endocrinology. Casterton Hall, Carnforth, Lancashire, UK: Parthenon Publishing, Casterton Hall; 1998;317-324.
13. Paoletti AM, et al. Comparison of pharmacokinetic profiles of 17ß-estradiol gel 0.6mg/g (Gelestra) with a transdermal delivery system (Estraderm TTS 50) in postmenopausal women at steady state. Maturitas. 2001;40:203-209.
14. Jensen PB, Jensen J, Riis BJ, et al. Climacteric symptoms after oral and percutaneous hormone replacement therapy. Maturitas. 1987;9:207-215.
15. Archer DF. for the EstroGel Study Group. Percutaneous 17ß-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
16. Kornafel KL, March CM. Estradiol gel in the treatment of menopausal symptoms: a placebo-controlled double-blind case study of efficacy and safety. South Med J. 1992;85:270-273.
17. Hirvonen E, Lamberg-Allardt C, Lankinen KS, Geurts P, Wilén-Rosenqvist G. Transdermal oestradiol gel in the treatment of the climacterium: a comparison with oral therapy. BJOG. 1997;104(Suppl 16):19-25.
18. Foidart JM, Béliard A, Hedon B, et al. Impact of percutaneous oestradiol gels in postmenopausal hormone replacement therapy on clinical symptoms and endometrium. BJOG. 1997;104:305-310.
19. Suvanto-Luukkonen E, Sundström H, Penttinen J, et al. Percutaneous estradiol gel with an intrauterine levonorgestrel releasing device or natural progesterone in hormone replacement therapy. Maturitas. 1997;26:211-17.
20. Holst J, et al. Progestogen addition during oestrogen replacement therapy—effects on vasomotor symptoms and mood. Maturitas. 1989;11:13-20.
21. Hirvonen E, et al. Effect of an estradiol gel with monthly or quarterly progestogen on menopausal symptoms and bleeding. Climacteric. 2000;3:262-270.
22. Ng HT, Chang SP, Yang TS, Cho MP, Wei TC. Estradiol administered in a percutaneous gel for the prevention of postmenopausal bone loss. Asia-Oceania J Obstet Gynaecol. 1993;19(2):115-119.
23. Riis B, Thomsen K, et al. Does calcium supplementation prevent postmenopausal bone loss? A double blind, controlled clinical study. N Engl J Med. 1987;316:173-177.
24. Devogelaer JP, Lecart C, Dupret P, De Nayer P, Nagant De Deuxchaisnes C. Long-term effects of percutaneous estradiol on bone loss and bone metabolism in post-menopausal hysterectomized women. Maturitas. 1998;28:243-249.
25. Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four year randomized study. Am J Med. 1995;99:36-42.
26. Sun A, Lin S, Yu W, et al. Percutaneous estrogen in prevention of early post-menopausal bone loss in Chinese women. Chin Med J. 2002;115:1790-1795.
27. Reginster JY, Sarlet N, Deroisy R, et al. Minimum levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
28. Cicinelli E, Cantatore FP, Galantino P, et al. Effects of continuous percutaneous estradiol administration on skeletal turnover in postmenopausal women: a 1-year prospective controlled study. Eur J Obstet Gynecol Reprod Biol. 1996;69(2):109-113.
29. Riis B, et al. The effect of percutaneous estradiol and natural progesterone on post-menopausal bone loss. Am J Obstet Gynecol. 1987;156:61-65.
30. Notelvitz M, John VA, Good WR. Effectiveness of Alora estradiol matrix transdermal delivery system in improving lumbar bone mineral density in healthy, post-menopausal women. Menopause. 2002;9:343-353.
31. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17ß-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-1048.
32. Hully S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
33. Moorjani S, Dupont A, Labrie F, et al. Changes in plasma lipoprotein and apolipoprotein composition in relation to oral versus percutaneous administration of estrogen alone or in cyclic association with Utrogestan in menopausal women. J Clin Endocrinol Metab. 1991;73:373-379.
34. Jensen J, Riis BJ, Strøm V, Nilas L, Christiansen C. Long-term effects of percutaneous estrogens and oral progesterone on serum lipoproteins in postmenopausal women. Am J Obstet Gyencol. 1987;156:66-71.
35. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071-3078.
36. Karjalainen A, Paassilta M, Heikkinen J, et al. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrin. 2001;54:165-173.
37. Haines CJ, Chung TKH, Masarei JRL, Tomlinson B, Lau JTF. The effect of per-cutaneous oestrogen replacement therapy on Lp(a) and other lipoproteins. Maturitas. 1995;22:219-225.
38. Scarabin PY, Oger E, Plu-Bureau G. EStrogen and THromboEmbolism Risk (ESTHER) Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
39. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
40. Blanc B, Cravello L, Micheletti MC, et al. Continuous hormone replacement therapy for menopause combining nomegestrol acetate and gel, patch, or oral estrogen: a comparison of amenorrhea rates. Clin Therap. 1998;20(5):901-912.
41. Holst J. Percutaneous estrogen therapy. Endometrial response and metabolic effects. Acta Obstet Gynecol Scand Suppl. 1983;115:1-30
42. Holst J, Cajander S, von Schoultz B. Cellular morphometric analysis of the post-menopausal endometrium during treatment with percutaneous estradiol-17ß with and without oral gestagen. Acta Obstet Gynecol Scand. 1983;62:267-270.
43. Holst J, Cajander S, von Schoultz B. Endometrial response in post-menopausal women during treatment with percutaneous 17ß-oestradiol opposed by oral progesterone. Maturitas. 1986;8:201-207.
44. Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370-375.
45. Gillet JY, Andre G, Faguer B, et al. Induction of amenorrhea during hormone replacement therapy: optimized micronized progesterone dose. A multicenter study. Maturitas. 1994;19:103-115.
46. Vilodre LC, et al. Endometrial response to a cyclic regimen of percutaneous 17ß-estradiol and low-dose vaginal micronized progesterone in women with mild-to-moderate hypertension. Gynecol Endocrinol. 2003;17:323-328.
47. Suvanto-Luukkonen E, Kauppila A. The levonorgestrel intrauterine system in menopausal hormone replacement therapy: five-year experience. Fertil Steril. 1999;72:161-163.
1. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
2. Moyer DL, de Lignieres B, Driguez P, et al. Prevention of endometrial hyperplasia by progesterone during long-term estradiol replacement: influence of bleeding pattern and secretory changes. Fertil Steril. 1993;59:992-997.
3. Hirvonen E, Cacciatore B, Wahlström T, et al. Effects of transdermal oestrogen therapy in postmenopausal women: a comparative study of an oestradiol gel and an oestradiol delivering patch. BJOG. 1997;104(Suppl 16):26-31.
4. Travassos de Figueiredo Alves S, et al. Comparison of gel and patch estradiol replacement in Brazil, a tropical country. Maturitas. 2000;36:69-74.
5. Henzl MR, Loomba PK. Transdermal delivery of sex steroids for hormone replacement therapy and contraception. A review of principles and practice. J Reprod Med. 2003;48:525-540.
6. Holst J, et al. Serum estrogen levels after topic application of estradiol-17 beta on two different cutaneous areas. Acta Obstet Gynecol Scand. 1987;662:151-152.
7. Jarvinen A, Granander M, Nykanen S, Laine T, Geurts P, Viitanen A. Steady-state pharmacokinetics of oestradiol gel in post-menopausal women: effects of application area and washing. Br J Obstet Gynaecol. 1997;104(Suppl 16):14-18.
8. Scott RT, Ross B, Anderson C, Archer DF. Pharmacokinetics of percutaneous estradiol: a crossover study using a gel and a transdermal system in comparison with oral micronized estradiol. Obstet Gynecol. 1991;77:758-764.
9. Dupont A, Dupont P, Cusan M, et al. Comparative endocrinological and clinical effects of percutaneous estradiol and oral conjugated estrogens as replacement therapy in menopausal women. Maturitas. 1991;13:297-311.
10. Palacios S, Menéndez C, Jurado AR, Vargas JC. Effects of percutaneous oestradiol versus oral oestrogens on bone density. Maturitas. 1995;20:209-213.
11. Basdevant A, de Lignieres B, Simon P, et al. Hepatic lipase activity during oral and parenteral 17ß-estradiol replacement therapy: high-density lipoprotein increase may not be antiatherogenic. Fertil Steril. 1991;55:1112-1117.
12. Simon JA, et al. Are there significant differences between patch and gel cutaneous estradiol therapy? In: Genazzani AR, Petraglia F, Volpe A, Facchinetti F, eds. Recent Research on Gynecological Endocrinology. Casterton Hall, Carnforth, Lancashire, UK: Parthenon Publishing, Casterton Hall; 1998;317-324.
13. Paoletti AM, et al. Comparison of pharmacokinetic profiles of 17ß-estradiol gel 0.6mg/g (Gelestra) with a transdermal delivery system (Estraderm TTS 50) in postmenopausal women at steady state. Maturitas. 2001;40:203-209.
14. Jensen PB, Jensen J, Riis BJ, et al. Climacteric symptoms after oral and percutaneous hormone replacement therapy. Maturitas. 1987;9:207-215.
15. Archer DF. for the EstroGel Study Group. Percutaneous 17ß-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516-521.
16. Kornafel KL, March CM. Estradiol gel in the treatment of menopausal symptoms: a placebo-controlled double-blind case study of efficacy and safety. South Med J. 1992;85:270-273.
17. Hirvonen E, Lamberg-Allardt C, Lankinen KS, Geurts P, Wilén-Rosenqvist G. Transdermal oestradiol gel in the treatment of the climacterium: a comparison with oral therapy. BJOG. 1997;104(Suppl 16):19-25.
18. Foidart JM, Béliard A, Hedon B, et al. Impact of percutaneous oestradiol gels in postmenopausal hormone replacement therapy on clinical symptoms and endometrium. BJOG. 1997;104:305-310.
19. Suvanto-Luukkonen E, Sundström H, Penttinen J, et al. Percutaneous estradiol gel with an intrauterine levonorgestrel releasing device or natural progesterone in hormone replacement therapy. Maturitas. 1997;26:211-17.
20. Holst J, et al. Progestogen addition during oestrogen replacement therapy—effects on vasomotor symptoms and mood. Maturitas. 1989;11:13-20.
21. Hirvonen E, et al. Effect of an estradiol gel with monthly or quarterly progestogen on menopausal symptoms and bleeding. Climacteric. 2000;3:262-270.
22. Ng HT, Chang SP, Yang TS, Cho MP, Wei TC. Estradiol administered in a percutaneous gel for the prevention of postmenopausal bone loss. Asia-Oceania J Obstet Gynaecol. 1993;19(2):115-119.
23. Riis B, Thomsen K, et al. Does calcium supplementation prevent postmenopausal bone loss? A double blind, controlled clinical study. N Engl J Med. 1987;316:173-177.
24. Devogelaer JP, Lecart C, Dupret P, De Nayer P, Nagant De Deuxchaisnes C. Long-term effects of percutaneous estradiol on bone loss and bone metabolism in post-menopausal hysterectomized women. Maturitas. 1998;28:243-249.
25. Wimalawansa SJ. Combined therapy with estrogen and etidronate has an additive effect on bone mineral density in the hip and vertebrae: four year randomized study. Am J Med. 1995;99:36-42.
26. Sun A, Lin S, Yu W, et al. Percutaneous estrogen in prevention of early post-menopausal bone loss in Chinese women. Chin Med J. 2002;115:1790-1795.
27. Reginster JY, Sarlet N, Deroisy R, et al. Minimum levels of serum estradiol prevent postmenopausal bone loss. Calcif Tissue Int. 1992;51:340-343.
28. Cicinelli E, Cantatore FP, Galantino P, et al. Effects of continuous percutaneous estradiol administration on skeletal turnover in postmenopausal women: a 1-year prospective controlled study. Eur J Obstet Gynecol Reprod Biol. 1996;69(2):109-113.
29. Riis B, et al. The effect of percutaneous estradiol and natural progesterone on post-menopausal bone loss. Am J Obstet Gynecol. 1987;156:61-65.
30. Notelvitz M, John VA, Good WR. Effectiveness of Alora estradiol matrix transdermal delivery system in improving lumbar bone mineral density in healthy, post-menopausal women. Menopause. 2002;9:343-353.
31. Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17ß-estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042-1048.
32. Hully S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
33. Moorjani S, Dupont A, Labrie F, et al. Changes in plasma lipoprotein and apolipoprotein composition in relation to oral versus percutaneous administration of estrogen alone or in cyclic association with Utrogestan in menopausal women. J Clin Endocrinol Metab. 1991;73:373-379.
34. Jensen J, Riis BJ, Strøm V, Nilas L, Christiansen C. Long-term effects of percutaneous estrogens and oral progesterone on serum lipoproteins in postmenopausal women. Am J Obstet Gyencol. 1987;156:66-71.
35. Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071-3078.
36. Karjalainen A, Paassilta M, Heikkinen J, et al. Effects of peroral and transdermal oestrogen replacement therapy on glucose and insulin metabolism. Clin Endocrin. 2001;54:165-173.
37. Haines CJ, Chung TKH, Masarei JRL, Tomlinson B, Lau JTF. The effect of per-cutaneous oestrogen replacement therapy on Lp(a) and other lipoproteins. Maturitas. 1995;22:219-225.
38. Scarabin PY, Oger E, Plu-Bureau G. EStrogen and THromboEmbolism Risk (ESTHER) Study Group. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428-432.
39. Archer DF. The effect of the duration of progestin use on the occurrence of endometrial cancer in postmenopausal women. Menopause. 2001;8:245-251.
40. Blanc B, Cravello L, Micheletti MC, et al. Continuous hormone replacement therapy for menopause combining nomegestrol acetate and gel, patch, or oral estrogen: a comparison of amenorrhea rates. Clin Therap. 1998;20(5):901-912.
41. Holst J. Percutaneous estrogen therapy. Endometrial response and metabolic effects. Acta Obstet Gynecol Scand Suppl. 1983;115:1-30
42. Holst J, Cajander S, von Schoultz B. Cellular morphometric analysis of the post-menopausal endometrium during treatment with percutaneous estradiol-17ß with and without oral gestagen. Acta Obstet Gynecol Scand. 1983;62:267-270.
43. Holst J, Cajander S, von Schoultz B. Endometrial response in post-menopausal women during treatment with percutaneous 17ß-oestradiol opposed by oral progesterone. Maturitas. 1986;8:201-207.
44. Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370-375.
45. Gillet JY, Andre G, Faguer B, et al. Induction of amenorrhea during hormone replacement therapy: optimized micronized progesterone dose. A multicenter study. Maturitas. 1994;19:103-115.
46. Vilodre LC, et al. Endometrial response to a cyclic regimen of percutaneous 17ß-estradiol and low-dose vaginal micronized progesterone in women with mild-to-moderate hypertension. Gynecol Endocrinol. 2003;17:323-328.
47. Suvanto-Luukkonen E, Kauppila A. The levonorgestrel intrauterine system in menopausal hormone replacement therapy: five-year experience. Fertil Steril. 1999;72:161-163.