User login
Cutting the medicolegal risk of shoulder dystocia
Clip-and-save shoulder dystocia documentation form
Practice recommendations
Among the intrapartum events that constitute bona fide emergencies, shoulder dystocia stands out. This obstetric emergency is the focus of an increasing number of medical liability cases. Most lawsuits involving shoulder dystocia allege negligence as the cause of the brachial plexus injury, fractured clavicle or humerus, or other injury. The defendant physicians named in these suits are often accused of inappropriately managing the prenatal or intrapartum course or the dystocia itself—or of inadequately documenting the steps taken to resolve the emergency.
To glean insights into the litigation process as it involves shoulder dystocia, we retrospectively reviewed all cases closed by the Boston-based ProMutual Group, a major liability insurance carrier, over a 7-year period. We wanted to learn more about the plaintiffs themselves, as well as the clinical and medicolegal factors that led to jury awards or indemnity payments. We also wanted data that could serve as the foundation for guidelines on how to proceed in the event of shoulder dystocia, as well as a documentation tool.
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Nurse and physician document different times
A 31-year-old woman in her 10th week of pregnancy had one prior uncomplicated vaginal delivery of a 9 lb 7 oz infant. Her prenatal course proceeds unremarkably, with a normal glucose tolerance test and total weight gain of 36 lb. At 41 weeks and 2 days, the estimated fetal weight is documented as 4,120 g. Labor is induced with oxytocin. Because of maternal fatigue, vacuum delivery is attempted.
Notes of the physician and the nurse differ regarding the time of the first of 3 vacuum applications.
After delivery of the head, shoulder dystocia is encountered. In a note handwritten immediately after delivery, the physician states that the head was “reconstituted as right occiput anterior with the left shoulder anterior.”
In a note dictated later, however, the same physician states the right shoulder was anterior.
Help is summoned and arrives 20 minutes after the dystocia is first encountered. The time that help was summoned is in question since there is an 18-minute discrepancy between the times the physician and the nurse note that assistance was called.
Despite the use of suprapubic pressure and maneuvers including McRoberts and Wood’s corkscrew, shoulder dystocia persists for 24 minutes. Apgars of the 11 lb 3 oz infant are 0, 1, and 3. The child is resuscitated but dies within 2 days of birth.
Outcome
Settled with a 7-figure indemnity payment.
What the defense experts said
The key issues involve documentation and summoning assistance. Discrepancies in documentation almost always cast doubt upon the credibility of a defendant. Ideally, there should be no discrepancies between nurse and physician notes and, certainly, no discrepancies between 2 notes on the same case by the same physician. If, in this case, the physician realized after writing the first note that the anterior shoulder had been incorrectly identified, a correction should have been written as a separate note.
Use of the shoulder dystocia documentation tool (see) helps create a chronology of events, which may prove vital to a successful defense.
The call for help might not have been delayed if the labor and delivery unit had had a shoulder dystocia protocol including “drills” for all team members. Help should be called as soon as a shoulder dystocia is encountered so that, when needed, it is available. Under no circumstances should it take 20 minutes for assistance to arrive.
Brachial plexus injury not always caused by shoulder dystocia
Between 21% and 42% of shoulder dystocias involve an injury1—usually brachial plexus injury. Plaintiff attorneys have manipulated this fact to attribute many cases of neonatal brachial plexus injury to mismanagement of shoulder dystocia by the obstetrician.
They fault the physician for failing to estimate fetal weight, perform a timely cesarean, use appropriate maneuvers correctly, or have a pediatrician present. They criticize nothing more resoundingly than use of “inappropriate” or “excessive” lateral traction to the fetal head.2
Nontraction injuries. The reality can be strikingly different, however. Some cases of brachial plexus injury involve no traction at all.
- Brachial plexus injuries have been reported in infants who had precipitate vaginal deliveries without any physical intervention by the obstetrician.2
- These injuries also have occurred in infants delivered via cesarean section.1,3,4
- In some cases, brachial plexus injuries have affected the posterior arm of neonates whose anterior arm was involved in shoulder dystocia.1,2,5-7
A retrospective study4 found that, of 39 cases of brachial plexus injury, only 17 were associated with shoulder dystocia. Similar findings have emerged from other studies.2,3,8
Other causes. It is unclear how brachial plexus injuries occur in the absence of shoulder dystocia. Some think they arise in response to infectious agents such as toxoplasmosis, coxsackievirus, mumps, pertussis, or mycoplasma pneumonia.2 Some assume a mechanical cause, such as fetal response to longstanding abnormal intrauterine pressure exerted by maternal conditions such as bicornate uterus and uterine fibroids, especially in the lower segment.1,2
When brachial plexus injuries occur in the absence of shoulder dystocia, they likely originated before labor and delivery.4 Some experts suggest serial electromyelograms within the first 7 days of life to establish a prenatal rather than intrapartum etiology. A positive electromyelogram within 1 week of birth would suggest antepartum causation.2,9
Recognizing risk factors for shoulder dystocia best way to reduce injury
Most brachial plexus injuries or impairments are associated with shoulder dystocia,9 and shoulder dystocia is the most common way brachial plexus injuries are introduced into litigation.
Decreasing the number of brachial plexusrelated liability cases, therefore, depends on decreasing the incidence of shoulder dystocia. Unfortunately, a failsafe method continues to elude both clinicians and researchers.10-12
Retrospective studies have identified certain factors that may—but do not necessarily—increase the risk of shoulder dystocia.
Prenatal risk factors include high maternal or paternal birth weight, maternal obesity, excessive weight gain during pregnancy, advanced age, short stature, multiparity, postdates, prior shoulder dystocia, small pelvis, prior delivery of a macrosomic infant, gestational diabetes in an earlier pregnancy, abnormal blood sugars in the current pregnancy, or fetal macrosomia.13,14-16
Intrapartum risk factors include a rapid or prolonged second stage, failure or arrest of descent, presence of considerable molding, and need for a midpelvic delivery.10,15
Predictability. Prospective studies have not established the predictability of shoulder dystocia. A 2000 study17 showed that 55% of cases with 1 or more risk factors experienced shoulder dystocia. Predictability increases somewhat when both maternal diabetes and fetal macrosomia complicate pregnancy, since the rate of shoulder dystocia in women with diabetes is consistently higher than in nondiabetic gravidas. This becomes a significant issue when the infant weighs more than 4,000 g.
Indications for prophylactic cesarean in women with diabetes
In 1999, Wagner et al9 found that 70% of shoulder dystocias in women with diabetes occurred when the fetal weight exceeded 4,000 g. They concluded that cesarean delivery for infants with an estimated weight over 4,250 g would reduce the rate of shoulder dystocia by 75% and increase the cesarean delivery rate by 1%.
Others are more conservative. Gross and colleagues11 suggested that, for every additional 26 cesarean deliveries, only 1 case of shoulder dystocia would be prevented.
Macrosomia. Most obstetricians and researchers still do not advocate prophylactic cesarean delivery for macrosomia alone because, by some estimates, 98% of macrosomic infants are delivered without difficulty.18 However, they do suggest that obstetricians at least consider the possibility of cesarean delivery for a macrosomic fetus when the woman has diabetes.
In a study completed in 2000, Skolbekken19 suggested a cutoff of 4,250 g for women with diabetes. Dildy20 suggested limits of more than 4,500 g for diabetic women and more than 5,000 g for nondiabetic gravidas. However, Conway and Langer21 assert that a cutoff of 4,500 g is too liberal for women with diabetes and maintain that, at this cutoff, 40% of shoulder dystocias would not be prevented.
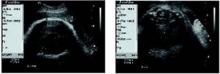
• Ultrasound measurements. Since estimates of fetal weight have a margin of error approaching 40%,9 others have chosen different parameters for determining fetal macrosomia in women with diabetes. In a retrospective study involving 31 women with gestational diabetes, Cohen et al22 found that subtracting the fetal biparietal diameter from the abdominal diameter—with both measurements obtained via ultrasound—yields a predictability score higher than estimated fetal weight. Specifically, if the difference between the 2 measurements is 2.6 cm or more, the rate of shoulder dystocia is high enough to warrant elective cesarean (FIGURE ).
FIGURE Using ultrasound measurements to predict macrosomia
A simple way to predict fetal macrosomia in women with diabetes is to subtract the fetal biparietal diameter (9.3 cm in the scan at left) from the abdominal diameter (average of 12.44 cm in the scan at right). If the difference exceeds 2.6 cm, elective cesarean is warranted. In this case it is 3.14 cm, indicating elective cesarean is warranted.
When dystocia occurs, have a plan and stick to it
Shoulder dystocia immediately places both mother and neonate at risk for temporary or permanent injury. Thus, it is imperative that all obstetricians and other health-care providers who deliver infants have a well developed plan of action for this emergency. They should immediately ask for obstetric assistance and instruct the mother to discontinue any pushing.
Attempts at vigorous downward traction should be avoided, and no fundal pressure should be applied, as these are known to increase the potential for brachial plexus injury. Gentle downward traction is considered the standard of care.17
The obstetrician’s goal is to free the impacted shoulder as quickly as possible, since a fetus can endure only 8 to 10 minutes of asphyxia before permanent neurologic damage occurs.17 The standard of care requires the obstetrician to know and use certain maneuvers to relieve shoulder dystocia. These maneuvers are designed to facilitate vaginal delivery and reduce the risk of permanent brachial plexus injury. The McRoberts maneuver, with flexion and slight rotation of the maternal hips onto the maternal abdomen, is the standard for initial relief of shoulder dystocia.17,23
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Prompt maneuvers, good outcome
A 28-year-old gravida weighing 214 lb has had 2 previous spontaneous deliveries of infants weighing 8 lb 5 oz and 9 lb 3 oz. Except for a weight gain of more than 60 lb, the pregnancy progressed without complications, and a 3-hour glucose tolerance test was normal. At 40 weeks, the obstetrician notes “concern” about an estimated fetal weight of 10 lb. Induction is planned, but spontaneous labor begins before oxytocin can be given. After 5 hours, the head is delivered without difficulty, but shoulder dystocia follows. The obstetrician extends the episiotomy and performs McRoberts and Wood’s corkscrew maneuvers, but the dystocia persists. Upon noting cyanosis, the obstetrician fractures the infant’s clavicle and quickly delivers a 10 lb 9 oz infant with Apgar scores of 8 and 10. Pediatricians examine the child immediately and diagnose Erb’s palsy, which subsequently resolves. X-rays confirm an undisplaced fracture of the right clavicle. Although the child recovers completely, the family sues, alleging a failure to perform cesarean delivery.
Outcome
Case closed with no payment.
What the defense experts said
Key issues are documentation and informed consent. The overriding opinion of 3 experts who reviewed the case for the defense was that cesarean delivery was not indicated and that in fracturing the clavicle, the physician acted responsibly, quickly, and within the standard of care. One defense expert said failure to document exact maneuvers used to relieve shoulder dystocia deviated from the standard of care. Another defense expert said the physician should have obtained informed consent from this at-risk patient, and explained the risks of shoulder dystocia, including neonatal injury, so that the she might have been better able to appreciate the fact that the obstetrician’s fast action may have saved her child from brain damage or death.
Factors that lead to litigation
In a review article, Hickson24 cited factors that prompted families to file medical liability claims following perinatal injury. Some families observed that, in their search for the cause of an injury, they found 1 or more aspects of care to be inappropriate.
The desire for information, perception of being misled, anger with the medical profession, desire to prevent injuries to others, recognition of long-term sequelae, and advice by knowledgeable acquaintances, as well as the need for money, all appeared to contribute to the decision to file medical liability claims.
Convey the risks, and listen carefully. Communication problems between physicians and patients are a contributing factor. Even when physicians provide technically adequate care, families expect answers to their questions and want to feel as though they have been consulted about important medical decisions.24 If these expectations are not met, even patients who have not experienced an adverse outcome may become angry and express dissatisfaction with care.24
The need for communication is critical when shoulder dystocia results in neonatal injury. Empathizing with the family, helping them understand that most brachial plexus injuries are not long-term, and offering to answer their questions both at the moment and later, may help prevent litigation.
This type of communication can be difficult. It helps to realize that an acknowledgment of distress and concern is not an admission of guilt, and an explanation is not an apology.15 However, an absence of communication or an attempt by the physician to place blame may be perceived as an admission of guilt that gives rise to a lawsuit.
Action plan is what counts in court. A review by Gross et al11 concluded that obstetricians should have a shoulder dystocia plan that enables an instant and orderly response. Also recommended is a protocol to help anticipate clinical problems and prevent medicolegal problems.
Gross and colleagues11 found that the Ob/Gyns with the most defensible cases paid close attention to the patient’s history and prenatal course and, upon encountering dystocia, implemented at least 2 maneuvers (if necessary) and thoroughly documented their actions immediately after delivery.
Fetterman15 agreed, asserting that what counts in court is not so much whether the obstetrician employed the McRoberts maneuver before or after the Wood’s corkscrew maneuver but whether he or she had an action plan in mind, implemented that plan properly, and thoroughly documented the actions taken and the reasons underlying them.
Dissecting legal cases for clues to reduced risk
In the review of medicolegal cases for this article, we limited our search to cases closed between Jan 1, 1995, and Dec 31, 2002, using a computer to search for codes specific to shoulder dystocia as well as the phrases “shoulder dystocia,” “Erb’s palsy,” and “brachial plexus injury.” We identified 61 cases involving 117 defendants and created a data sheet to gather information on patient and physician demographics, medical and obstetric history, description of the incident, analysis rendered by defense and plaintiff experts, and legal and financial outcomes.
Of 117 defendants, 76 were obstetricians. There also were 16 hospitals, 15 corporations, 5 certified nurse-midwives, 1 family physician, 1 emergency physician, and 3 persons categorized as “other.” Age, race, and parity of the 61 plaintiffs are given in TABLE 1.
Twenty-six of the 61 cases, involving 74 defendants, closed with no payment. That is, they were either dismissed or closed with a jury verdict for the defense. The remaining 35 cases involved 43 defendants and were closed with an aggregate indemnity payment of $19.2 million. The mean payment was $445,000 per defendant.
These guidelines are recommended to help prevent, predict, and manage shoulder dystocia and brachial plexus injury.
Obtain a prenatal history that includes the birth weights of both parents and any history of prior shoulder dystocia or cesarean delivery performed for “failure to progress.”
Estimate the fetal weight and take into account the risk for shoulder dystocia when the fetus is determined by ultrasound to be macrosomic.
Perform glucose testing on all patients, and follow up on even a single abnormal glucose reading. Discuss the possibility of shoulder dystocia and the accompanying risk of neonatal injury with “at risk” patients.
Consider obtaining informed consent for vaginal delivery of a patient with risk factors for shoulder dystocia.
Consider cesarean delivery for :
- nondiabetic women when the estimated fetal weight (EFW) exceeds 4,500 g,
- diabetic gravidas when the EFW exceeds 4,000 g,
- women with a prior delivery complicated by shoulder dystocia or brachial plexus palsy,
- gravidas with a prolonged second stage and nonprogression of labor, and
- patients who express fear and doubt about vaginal delivery.
Be sparing with the use of oxytocin when the fetus is known or suspected to be macrosomic, taking special care not to be aggressive with induction.
Use forceps or vacuum extraction with caution, and limit the number of attempts with each.
Minimize traction on the fetal head. Traction that is deemed to be “excessive” may be used against the physician in a liability suit. Gentle traction is acceptable.
Do not use or order fundal pressure. It will almost invariably be used against you in a lawsuit.
Be able to define and correctly describe maneuvers generally accepted as the standard for shoulder dystocia and, when necessary, use and document them appropriately. These include the McRoberts, Wood’s corkscrew, Rubin, and Zavanelli maneuvers; extended episiotomy; suprapubic pressure; and fracture of the anterior clavicle.
Be alert to the possibility of brachial plexus injury in the absence of shoulder dystocia. Obstetricians have been erroneously accused of causing brachial plexus injury by plaintiff attorneys who do not understand that this injury is not always the result of dystocia. Thorough contemporaneous documentation is key in these instances.
Request immediate pediatric assessment of a newborn involved in shoulder dystocia. Have the placenta sent for examination, and request cord blood gases.
Communicate openly and honestly with the parents of a child who has suffered a brachial plexus injury. This may be the single greatest tool for reducing the risk of liability litigation.
Consider serial electromyelograms during the first 7 days of life for a neonate with a brachial plexus palsy. These studies can help determine the etiology of the injury.
Use a shoulder dystocia documentation tool such as the one on page 91. Thorough documentation of all relevant prenatal and intrapartum events is critical to a successful defense. A 12-point detailed delivery note as recommended by Fetterman will also prevent or reduce legal risk.15
Schedule shoulder dystocia “drills” in the labor and delivery unit to familiarize obstetric team members with their roles.
Require comanagement of any midwifery patient at increased risk for shoulder dystocia.
Neonatal injury occurred in all 61 cases. Erb’s palsy was the overwhelming pediatric outcome (57 of 61 cases, or 93.4%), with an aggregate indemnity payment of $17.6 million. Fractured humerus was the outcome in 1 case, and 3 cases involved neonatal deaths.
Reasons for indemnity payments included:
- probable liability (18 defendants),
- plaintiff was sympathetic, likely to evoke an emotional response from the jury (8 defendants),
- clear liability (7 defendants),
- defendant would not have made a strong witness in his or her own defense (5 defendants),
- defendant had died or was too ill to stand trial (3 defendants),
- medical record had been altered (2 defendants),
- case was considered too inflammatory to risk a jury award (2 defendants), and
- policy limits were too low to risk a potentially high jury award (1 defendant).
Five defendants were involved in cases with multiple medicolegal issues that argued for settlement.
The effect of birth weight. Notably, 74% of infants involved in these cases were macrosomic (birth weight over 4,000 g). Except for neonatal injury (100%), no single maternal or fetal variable appears with greater frequency.
The mean indemnity in closed cases increased in direct proportion to fetal weight, ranging from $500,000 in cases involving infants whose birth weight was less than 4,000 g to $950,000 when the birth weight was 5,000 g or more.
Maternal factors seen with greatest frequency included obesity, excessive weight gain in pregnancy, and gestational diabetes. Analysis of prepregnant body mass index found that 19 women had a weight within the “obese” category, 18 of whom gave birth to macrosomic infants. Eight of these cases closed without indemnity payment and 10 closed with an aggregate indemnity payment of $6.5 million.
Forty-eight of the 61 plaintiffs (78.8%) exceeded the normal weight gain in pregnancy based on height and prepregnant weight. Twenty-nine of these cases closed with an aggregate indemnity payment of $16.6 million.
Influence of diabetes. Fifty-two of 61 plaintiffs underwent a glucose screening test. Of these, 23 went on to have a glucose tolerance test, with 12 testing positive for gestational diabetes. Further analysis revealed borderline screening glucose values in an additional 16 cases. These women were not considered diabetic by their obstetricians, were not retested for diabetes, and did not receive nutritional counseling. Macrosomic infants were born to 9 of the 12 patients with gestational diabetes and to 13 of the 16 borderline cases.
Of 28 cases with confirmed or suspected gestational diabetes, 14 women delivered infants weighing over 4,250 g; 11 of the 14 weighed more than 4,500 g.
In a comparison of cases involving diabetic and nondiabetic women who delivered infants weighing more than 4,250 g, 82% of the cases involving nondiabetic women closed without payment. Among cases involving diabetes (actual or borderline), the corresponding figure was 27.3%.
Labor and delivery interventions were cited in all cases. Oxytocin was used in 42 of the 61 cases (68.9%), forceps in 9 (14.8%), and vacuum extraction in 7 (11.5%). Suprapubic pressure was used in 37 cases (60.7%), fundal pressure in 9 (14.8%), and traction on the fetal head in 16 cases (26.2%).
In addition, defense experts determined that the McRoberts maneuver was used in 41 cases (67.2%) and the Wood’s corkscrew maneuver in 28 (45.9%).
Seven cases involved a second stage of labor exceeding 2.5 hours. The ratio of wins to losses decreased substantially with the use of oxytocin, forceps, fundal pressure, or a prolonged second stage.
Data were analyzed using selected variables thought to have an association with winning or losing cases and with indemnity (TABLE 2). No statistically significant models emerged. This is likely due to inadequate power (low number of cases) and the large number of interactions between variables relative to the outcomes evaluated.
TABLE 1
Plaintiff demographics
| CHARACTERISTIC | ALL CASES (n = 61) | CASES WITH INDEMNITY (n = 35) |
|---|---|---|
| Mean age, in years (range) | 28 (17–40) | 29 (18–38) |
| Race (%) | ||
| White | 35 (57) | 21 (60) |
| Black | 12 (20) | 7 (20) |
| Hispanic | 11 (18) | 6 (17) |
| Asian | 1 (2) | — |
| Unknown | 2 (3) | 1 (3) |
| Parity (%) | ||
| 0 | 19 (31) | 11 (31) |
| 1 | 26 (43) | 16 (46) |
| 2 | 11 (18) | 7 (20) |
| 3 | 2 (3) | 1 (3) |
| 4 | 2 (3) | — |
| 5 | 1 (2) | — |
TABLE 2
Litigation outcomes for selected prenatal and intrapartum variables
| CHARACTERISTIC | CLOSED WITHOUT INDEMNITY | CLOSED WITHOUT INDEMNITY PAYMENT | MEAN INDEMNITY ($) | TOTAL INDEMNITY ($) |
|---|---|---|---|---|
| Prenatal factors | ||||
| Gestational diabetes | 5 | 7 | 413,300 | 2,893,000 |
| Adjusted diabetes | 10 | 18 | 521,400 | 9,386,000 |
| Obesity | 8 | 13 | 651,300 | 8,466,000 |
| Intrapartum factors | ||||
| Prolonged second stage | 1 | 6 | 707,700 | 4,247,000 |
| Oxytocin induction | 2 | 10 | 406,500 | 4,065,300 |
| Oxytocin augmentation | 15 | 15 | 737,000 | 11,100,000 |
| Forceps delivery | 1 | 8 | 552,100 | 4,416,800 |
| Vacuum extraction | 3 | 4 | 531,300 | 2,125,000 |
| Episiotomy | 20 | 30 | 579,400 | 17,382,100 |
| McRoberts maneuver | 20 | 21 | 542,900 | 11,400,300 |
| Wood’s corkscrew maneuver | 12 | 16 | 652,300 | 10,436,200 |
| Suprapubic pressure | 17 | 20 | 629,000 | 12,579,400 |
| Traction to fetal head | 7 | 9 | 665,500 | 5,989,500 |
| Fundal pressure | 4 | 7 | 660,700 | 4,625,000 |
4 factors raise risk of litigation
After reviewing the literature and analyzing the ProMutual data, we concluded that shoulder dystocia remains largely unpredictable. However, certain clinical factors are clearly associated with an increased risk for litigation:
- prenatal factors,
- labor and delivery interventions,
- maneuvers performed at the time of the dystocia, and
- fetal outcomes.
Prenatal factors. The most significant prenatal factors were maternal obesity, excessive weight gain in pregnancy, and, especially, diabetes and fetal macrosomia.
• Fetal macrosomia. Although most macrosomic infants deliver without complication, fetal macrosomia was involved in more than 72% of the cases reviewed. Because macrosomia represents a risk not only for shoulder dystocia but also for the litigation that may arise from it, it is important to:
- know and document the estimated fetal weight (EFW);
- discuss the risk of dystocia and its sequelae with the mother and her partner; and
- consider cesarean delivery for estimated fetal weights that suggest macrosomia (see the recommendations on page 82 for specifics).
It also is advisable to mobilize a team for the possibility of shoulder dystocia if vaginal delivery is attempted.
Preparing for the increased risk of a macrosomic fetus can be successful only if macrosomia is both diagnosed and anticipated.
Because macrosomia often is accompanied by maternal diabetes, serum glucose testing is recommended for all gravidas, with follow-up of both abnormal and borderline values.
Labor and delivery interventions were cited in all cases, with data indicating a decreasing ratio of wins to losses with the use of oxytocin, forceps, or fundal pressure, and prolonged second stage.
- Use of oxytocin to augment an already established labor carried less risk than oxytocin for labor induction. Ten of the 12 cases (83.3%) in which oxytocin was used for induction closed with an indemnity payment. However, of the 30 cases in which oxytocin was used for labor augmentation, 50% closed with payment.
- • Forceps and a prolonged second stage. Eight of 9 cases (88.9%) involving forceps and 6 of 7 cases (85.7%) involving a prolonged second stage closed with indemnity payment.
Consider cesarean delivery when the second stage is prolonged or labor fails to progress. Be cautious using forceps and vacuum extraction in these circumstances, and limit the number of attempts with either.
Maneuvers performed at the time of dystocia. The most common maneuverswere McRoberts, suprapubic pressure, and Wood’s corkscrew. The ratio of wins to losses decreased with traction of the fetal head and use of fundal pressure.
Part of the risk-management protocol for obstetricians should be appropriate use of McRoberts maneuver, suprapubic pressure, and Wood’s corkscrew, and cutting a large episiotomy. In addition, be careful not to push, pull, rotate the head, or apply fundal pressure.
Fetal outcomes. All cases involved neonatal injury (Erb’s palsy, fractured humerus) or death.
For this reason, we recommend an action plan that includes immediate pediatric or neonatal assessment of neuromuscular function of the infant’s anterior shoulder. Assess the Moro reflex and the possibility of brachial plexus injury and fractures of the clavicle and humerus. Also examine the placenta, send it to pathology, and perform a cord blood gas analysis.
Last words
Shoulder dystocia is the unfortunate complication of a small number of deliveries, but the focus of an increasing number of lawsuits. Because the neonatal injuries that so often accompany shoulder dystocia often lead to litigation, obstetricians should prepare to identify risk and help patients make informed choices. We should be prepared to manage this emergency whenever it occurs and thoroughly document actions.
Dr. Zylstra reports no financial relationships relevant to this article.
1. Gherman RB, Goodwin TM, Ouzounian JG, Miller DA, Paul RH. Brachial plexus palsy associated with cesarean section: an in utero injury? Am J Obstet Gynecol. 1997;177:1162-1164.
2. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93:536-540.
4. Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: an old problem revisited. Am J Obstet Gynecol. 1992;166:1673-1677.
5. Hankins GDV, Clark SL. Brachial plexus palsy involving the posterior shoulder at spontaneous vaginal delivery. Am J Perinatol. 1995;12:44-45.
6. Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: a risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423-427.
7. Dodds SD, Wolfe SW. Perinatal brachial plexus palsy. Curr Opin Pediatrics. 2000;23:40-47.
8. Wade DM. A Comment on the Trial of Brachial Plexus/Erb’s Palsy Medical Malpractice Cases. Evidence Technologies, Inc. 1994.
9. Wagner RK, Nielsen PE, Gonik B. Controversies in labor management: shoulder dystocia. Obstet Gynecol Clin. 1999;26(2):371-382.
10. McFarland M, Hod M, Piper JM, Xenakis EM-J, Langer O. Are labor abnormalities more common in shoulder dystocia? Am J ObstetGynecol. 1995;173:1211-1214.
11. Gross TL, Sokol RJ, Williams T, Thompson K. Shoulder dystocia: A fetal-physician risk. Am J Obstet Gynecol. 1987;156:1408-1418.
12. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
13. Shoulder dystocia and Erb’s palsy. The Keenan Law Firm. 2002. Available at www.shoulderdystociaattorney.com. Accessed July 23, 2004.
14. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71(Part l):390-392.
15. Fetterman HH. Cutting the legal risks of shoulder dystocia. OBG Management. March 1995;41-51.
16. Iffy L, Varadi V, Jakobovits A. Common intrapartum denominators of shoulder dystocia related birth injuries. Zentralbl Gynakol. 1994;116(1):33-37.
17. Calhoun BC, Hume RR, Walters JL. Shoulder Dystocia as a risk for obstetric liability. Legal Medicine. 2000;12-15.
18. Jennett RJ, Tarby TJ. Brachial plexus palsy: an old problem revisited again. Am J Obstet Gynecol. 1997;176:1354-1357.
19. Skolbekken JA. Shoulder dystocia—malpractice or acceptable risk? Acta Obstet Gynecol Scand. 2000;79:750-756.
20. Dildy GA. Fetal macrosomia [slide presentation]. Presented at the 18th Annual Conference on Obstetrics, Gynecology, Perinatal Medicine, and the Law, Boston University School of Medicine and the Center for Human Genetics, January 2002, Kauai, Hawaii.
21. Conway D, Langer O. Elective delivery of infants with macrosomia in diabetic women: reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998;178(5):922-925.
22. Cohen B, Penning S, Major C, Ansley D, Porto M, Garite T. Sonographic prediction of shoulder dystocia in infants of diabetic mothers. Obstet Gynecol. 1998;88:10-13.
23. Gherman RB, Goodwin TM, Souter I, Neumann K, Ouzounian JG, Paul RH. The McRoberts’ maneuver for the alleviation of shoulder dystocia: how successful is it? Am J Obstet Gynecol. 1997;176:656-661.
24. Hickson GB, Clayton DW, Githens PB, Sloan FA. Factors that prompted families to file medical malpractice claims following perinatal injuries. JAMA. 1992;267:1359-1363.
Clip-and-save shoulder dystocia documentation form
Practice recommendations
Among the intrapartum events that constitute bona fide emergencies, shoulder dystocia stands out. This obstetric emergency is the focus of an increasing number of medical liability cases. Most lawsuits involving shoulder dystocia allege negligence as the cause of the brachial plexus injury, fractured clavicle or humerus, or other injury. The defendant physicians named in these suits are often accused of inappropriately managing the prenatal or intrapartum course or the dystocia itself—or of inadequately documenting the steps taken to resolve the emergency.
To glean insights into the litigation process as it involves shoulder dystocia, we retrospectively reviewed all cases closed by the Boston-based ProMutual Group, a major liability insurance carrier, over a 7-year period. We wanted to learn more about the plaintiffs themselves, as well as the clinical and medicolegal factors that led to jury awards or indemnity payments. We also wanted data that could serve as the foundation for guidelines on how to proceed in the event of shoulder dystocia, as well as a documentation tool.
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Nurse and physician document different times
A 31-year-old woman in her 10th week of pregnancy had one prior uncomplicated vaginal delivery of a 9 lb 7 oz infant. Her prenatal course proceeds unremarkably, with a normal glucose tolerance test and total weight gain of 36 lb. At 41 weeks and 2 days, the estimated fetal weight is documented as 4,120 g. Labor is induced with oxytocin. Because of maternal fatigue, vacuum delivery is attempted.
Notes of the physician and the nurse differ regarding the time of the first of 3 vacuum applications.
After delivery of the head, shoulder dystocia is encountered. In a note handwritten immediately after delivery, the physician states that the head was “reconstituted as right occiput anterior with the left shoulder anterior.”
In a note dictated later, however, the same physician states the right shoulder was anterior.
Help is summoned and arrives 20 minutes after the dystocia is first encountered. The time that help was summoned is in question since there is an 18-minute discrepancy between the times the physician and the nurse note that assistance was called.
Despite the use of suprapubic pressure and maneuvers including McRoberts and Wood’s corkscrew, shoulder dystocia persists for 24 minutes. Apgars of the 11 lb 3 oz infant are 0, 1, and 3. The child is resuscitated but dies within 2 days of birth.
Outcome
Settled with a 7-figure indemnity payment.
What the defense experts said
The key issues involve documentation and summoning assistance. Discrepancies in documentation almost always cast doubt upon the credibility of a defendant. Ideally, there should be no discrepancies between nurse and physician notes and, certainly, no discrepancies between 2 notes on the same case by the same physician. If, in this case, the physician realized after writing the first note that the anterior shoulder had been incorrectly identified, a correction should have been written as a separate note.
Use of the shoulder dystocia documentation tool (see) helps create a chronology of events, which may prove vital to a successful defense.
The call for help might not have been delayed if the labor and delivery unit had had a shoulder dystocia protocol including “drills” for all team members. Help should be called as soon as a shoulder dystocia is encountered so that, when needed, it is available. Under no circumstances should it take 20 minutes for assistance to arrive.
Brachial plexus injury not always caused by shoulder dystocia
Between 21% and 42% of shoulder dystocias involve an injury1—usually brachial plexus injury. Plaintiff attorneys have manipulated this fact to attribute many cases of neonatal brachial plexus injury to mismanagement of shoulder dystocia by the obstetrician.
They fault the physician for failing to estimate fetal weight, perform a timely cesarean, use appropriate maneuvers correctly, or have a pediatrician present. They criticize nothing more resoundingly than use of “inappropriate” or “excessive” lateral traction to the fetal head.2
Nontraction injuries. The reality can be strikingly different, however. Some cases of brachial plexus injury involve no traction at all.
- Brachial plexus injuries have been reported in infants who had precipitate vaginal deliveries without any physical intervention by the obstetrician.2
- These injuries also have occurred in infants delivered via cesarean section.1,3,4
- In some cases, brachial plexus injuries have affected the posterior arm of neonates whose anterior arm was involved in shoulder dystocia.1,2,5-7
A retrospective study4 found that, of 39 cases of brachial plexus injury, only 17 were associated with shoulder dystocia. Similar findings have emerged from other studies.2,3,8
Other causes. It is unclear how brachial plexus injuries occur in the absence of shoulder dystocia. Some think they arise in response to infectious agents such as toxoplasmosis, coxsackievirus, mumps, pertussis, or mycoplasma pneumonia.2 Some assume a mechanical cause, such as fetal response to longstanding abnormal intrauterine pressure exerted by maternal conditions such as bicornate uterus and uterine fibroids, especially in the lower segment.1,2
When brachial plexus injuries occur in the absence of shoulder dystocia, they likely originated before labor and delivery.4 Some experts suggest serial electromyelograms within the first 7 days of life to establish a prenatal rather than intrapartum etiology. A positive electromyelogram within 1 week of birth would suggest antepartum causation.2,9
Recognizing risk factors for shoulder dystocia best way to reduce injury
Most brachial plexus injuries or impairments are associated with shoulder dystocia,9 and shoulder dystocia is the most common way brachial plexus injuries are introduced into litigation.
Decreasing the number of brachial plexusrelated liability cases, therefore, depends on decreasing the incidence of shoulder dystocia. Unfortunately, a failsafe method continues to elude both clinicians and researchers.10-12
Retrospective studies have identified certain factors that may—but do not necessarily—increase the risk of shoulder dystocia.
Prenatal risk factors include high maternal or paternal birth weight, maternal obesity, excessive weight gain during pregnancy, advanced age, short stature, multiparity, postdates, prior shoulder dystocia, small pelvis, prior delivery of a macrosomic infant, gestational diabetes in an earlier pregnancy, abnormal blood sugars in the current pregnancy, or fetal macrosomia.13,14-16
Intrapartum risk factors include a rapid or prolonged second stage, failure or arrest of descent, presence of considerable molding, and need for a midpelvic delivery.10,15
Predictability. Prospective studies have not established the predictability of shoulder dystocia. A 2000 study17 showed that 55% of cases with 1 or more risk factors experienced shoulder dystocia. Predictability increases somewhat when both maternal diabetes and fetal macrosomia complicate pregnancy, since the rate of shoulder dystocia in women with diabetes is consistently higher than in nondiabetic gravidas. This becomes a significant issue when the infant weighs more than 4,000 g.
Indications for prophylactic cesarean in women with diabetes
In 1999, Wagner et al9 found that 70% of shoulder dystocias in women with diabetes occurred when the fetal weight exceeded 4,000 g. They concluded that cesarean delivery for infants with an estimated weight over 4,250 g would reduce the rate of shoulder dystocia by 75% and increase the cesarean delivery rate by 1%.
Others are more conservative. Gross and colleagues11 suggested that, for every additional 26 cesarean deliveries, only 1 case of shoulder dystocia would be prevented.
Macrosomia. Most obstetricians and researchers still do not advocate prophylactic cesarean delivery for macrosomia alone because, by some estimates, 98% of macrosomic infants are delivered without difficulty.18 However, they do suggest that obstetricians at least consider the possibility of cesarean delivery for a macrosomic fetus when the woman has diabetes.
In a study completed in 2000, Skolbekken19 suggested a cutoff of 4,250 g for women with diabetes. Dildy20 suggested limits of more than 4,500 g for diabetic women and more than 5,000 g for nondiabetic gravidas. However, Conway and Langer21 assert that a cutoff of 4,500 g is too liberal for women with diabetes and maintain that, at this cutoff, 40% of shoulder dystocias would not be prevented.
• Ultrasound measurements. Since estimates of fetal weight have a margin of error approaching 40%,9 others have chosen different parameters for determining fetal macrosomia in women with diabetes. In a retrospective study involving 31 women with gestational diabetes, Cohen et al22 found that subtracting the fetal biparietal diameter from the abdominal diameter—with both measurements obtained via ultrasound—yields a predictability score higher than estimated fetal weight. Specifically, if the difference between the 2 measurements is 2.6 cm or more, the rate of shoulder dystocia is high enough to warrant elective cesarean (FIGURE ).
FIGURE Using ultrasound measurements to predict macrosomia
A simple way to predict fetal macrosomia in women with diabetes is to subtract the fetal biparietal diameter (9.3 cm in the scan at left) from the abdominal diameter (average of 12.44 cm in the scan at right). If the difference exceeds 2.6 cm, elective cesarean is warranted. In this case it is 3.14 cm, indicating elective cesarean is warranted.
When dystocia occurs, have a plan and stick to it
Shoulder dystocia immediately places both mother and neonate at risk for temporary or permanent injury. Thus, it is imperative that all obstetricians and other health-care providers who deliver infants have a well developed plan of action for this emergency. They should immediately ask for obstetric assistance and instruct the mother to discontinue any pushing.
Attempts at vigorous downward traction should be avoided, and no fundal pressure should be applied, as these are known to increase the potential for brachial plexus injury. Gentle downward traction is considered the standard of care.17
The obstetrician’s goal is to free the impacted shoulder as quickly as possible, since a fetus can endure only 8 to 10 minutes of asphyxia before permanent neurologic damage occurs.17 The standard of care requires the obstetrician to know and use certain maneuvers to relieve shoulder dystocia. These maneuvers are designed to facilitate vaginal delivery and reduce the risk of permanent brachial plexus injury. The McRoberts maneuver, with flexion and slight rotation of the maternal hips onto the maternal abdomen, is the standard for initial relief of shoulder dystocia.17,23
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Prompt maneuvers, good outcome
A 28-year-old gravida weighing 214 lb has had 2 previous spontaneous deliveries of infants weighing 8 lb 5 oz and 9 lb 3 oz. Except for a weight gain of more than 60 lb, the pregnancy progressed without complications, and a 3-hour glucose tolerance test was normal. At 40 weeks, the obstetrician notes “concern” about an estimated fetal weight of 10 lb. Induction is planned, but spontaneous labor begins before oxytocin can be given. After 5 hours, the head is delivered without difficulty, but shoulder dystocia follows. The obstetrician extends the episiotomy and performs McRoberts and Wood’s corkscrew maneuvers, but the dystocia persists. Upon noting cyanosis, the obstetrician fractures the infant’s clavicle and quickly delivers a 10 lb 9 oz infant with Apgar scores of 8 and 10. Pediatricians examine the child immediately and diagnose Erb’s palsy, which subsequently resolves. X-rays confirm an undisplaced fracture of the right clavicle. Although the child recovers completely, the family sues, alleging a failure to perform cesarean delivery.
Outcome
Case closed with no payment.
What the defense experts said
Key issues are documentation and informed consent. The overriding opinion of 3 experts who reviewed the case for the defense was that cesarean delivery was not indicated and that in fracturing the clavicle, the physician acted responsibly, quickly, and within the standard of care. One defense expert said failure to document exact maneuvers used to relieve shoulder dystocia deviated from the standard of care. Another defense expert said the physician should have obtained informed consent from this at-risk patient, and explained the risks of shoulder dystocia, including neonatal injury, so that the she might have been better able to appreciate the fact that the obstetrician’s fast action may have saved her child from brain damage or death.
Factors that lead to litigation
In a review article, Hickson24 cited factors that prompted families to file medical liability claims following perinatal injury. Some families observed that, in their search for the cause of an injury, they found 1 or more aspects of care to be inappropriate.
The desire for information, perception of being misled, anger with the medical profession, desire to prevent injuries to others, recognition of long-term sequelae, and advice by knowledgeable acquaintances, as well as the need for money, all appeared to contribute to the decision to file medical liability claims.
Convey the risks, and listen carefully. Communication problems between physicians and patients are a contributing factor. Even when physicians provide technically adequate care, families expect answers to their questions and want to feel as though they have been consulted about important medical decisions.24 If these expectations are not met, even patients who have not experienced an adverse outcome may become angry and express dissatisfaction with care.24
The need for communication is critical when shoulder dystocia results in neonatal injury. Empathizing with the family, helping them understand that most brachial plexus injuries are not long-term, and offering to answer their questions both at the moment and later, may help prevent litigation.
This type of communication can be difficult. It helps to realize that an acknowledgment of distress and concern is not an admission of guilt, and an explanation is not an apology.15 However, an absence of communication or an attempt by the physician to place blame may be perceived as an admission of guilt that gives rise to a lawsuit.
Action plan is what counts in court. A review by Gross et al11 concluded that obstetricians should have a shoulder dystocia plan that enables an instant and orderly response. Also recommended is a protocol to help anticipate clinical problems and prevent medicolegal problems.
Gross and colleagues11 found that the Ob/Gyns with the most defensible cases paid close attention to the patient’s history and prenatal course and, upon encountering dystocia, implemented at least 2 maneuvers (if necessary) and thoroughly documented their actions immediately after delivery.
Fetterman15 agreed, asserting that what counts in court is not so much whether the obstetrician employed the McRoberts maneuver before or after the Wood’s corkscrew maneuver but whether he or she had an action plan in mind, implemented that plan properly, and thoroughly documented the actions taken and the reasons underlying them.
Dissecting legal cases for clues to reduced risk
In the review of medicolegal cases for this article, we limited our search to cases closed between Jan 1, 1995, and Dec 31, 2002, using a computer to search for codes specific to shoulder dystocia as well as the phrases “shoulder dystocia,” “Erb’s palsy,” and “brachial plexus injury.” We identified 61 cases involving 117 defendants and created a data sheet to gather information on patient and physician demographics, medical and obstetric history, description of the incident, analysis rendered by defense and plaintiff experts, and legal and financial outcomes.
Of 117 defendants, 76 were obstetricians. There also were 16 hospitals, 15 corporations, 5 certified nurse-midwives, 1 family physician, 1 emergency physician, and 3 persons categorized as “other.” Age, race, and parity of the 61 plaintiffs are given in TABLE 1.
Twenty-six of the 61 cases, involving 74 defendants, closed with no payment. That is, they were either dismissed or closed with a jury verdict for the defense. The remaining 35 cases involved 43 defendants and were closed with an aggregate indemnity payment of $19.2 million. The mean payment was $445,000 per defendant.
These guidelines are recommended to help prevent, predict, and manage shoulder dystocia and brachial plexus injury.
Obtain a prenatal history that includes the birth weights of both parents and any history of prior shoulder dystocia or cesarean delivery performed for “failure to progress.”
Estimate the fetal weight and take into account the risk for shoulder dystocia when the fetus is determined by ultrasound to be macrosomic.
Perform glucose testing on all patients, and follow up on even a single abnormal glucose reading. Discuss the possibility of shoulder dystocia and the accompanying risk of neonatal injury with “at risk” patients.
Consider obtaining informed consent for vaginal delivery of a patient with risk factors for shoulder dystocia.
Consider cesarean delivery for :
- nondiabetic women when the estimated fetal weight (EFW) exceeds 4,500 g,
- diabetic gravidas when the EFW exceeds 4,000 g,
- women with a prior delivery complicated by shoulder dystocia or brachial plexus palsy,
- gravidas with a prolonged second stage and nonprogression of labor, and
- patients who express fear and doubt about vaginal delivery.
Be sparing with the use of oxytocin when the fetus is known or suspected to be macrosomic, taking special care not to be aggressive with induction.
Use forceps or vacuum extraction with caution, and limit the number of attempts with each.
Minimize traction on the fetal head. Traction that is deemed to be “excessive” may be used against the physician in a liability suit. Gentle traction is acceptable.
Do not use or order fundal pressure. It will almost invariably be used against you in a lawsuit.
Be able to define and correctly describe maneuvers generally accepted as the standard for shoulder dystocia and, when necessary, use and document them appropriately. These include the McRoberts, Wood’s corkscrew, Rubin, and Zavanelli maneuvers; extended episiotomy; suprapubic pressure; and fracture of the anterior clavicle.
Be alert to the possibility of brachial plexus injury in the absence of shoulder dystocia. Obstetricians have been erroneously accused of causing brachial plexus injury by plaintiff attorneys who do not understand that this injury is not always the result of dystocia. Thorough contemporaneous documentation is key in these instances.
Request immediate pediatric assessment of a newborn involved in shoulder dystocia. Have the placenta sent for examination, and request cord blood gases.
Communicate openly and honestly with the parents of a child who has suffered a brachial plexus injury. This may be the single greatest tool for reducing the risk of liability litigation.
Consider serial electromyelograms during the first 7 days of life for a neonate with a brachial plexus palsy. These studies can help determine the etiology of the injury.
Use a shoulder dystocia documentation tool such as the one on page 91. Thorough documentation of all relevant prenatal and intrapartum events is critical to a successful defense. A 12-point detailed delivery note as recommended by Fetterman will also prevent or reduce legal risk.15
Schedule shoulder dystocia “drills” in the labor and delivery unit to familiarize obstetric team members with their roles.
Require comanagement of any midwifery patient at increased risk for shoulder dystocia.
Neonatal injury occurred in all 61 cases. Erb’s palsy was the overwhelming pediatric outcome (57 of 61 cases, or 93.4%), with an aggregate indemnity payment of $17.6 million. Fractured humerus was the outcome in 1 case, and 3 cases involved neonatal deaths.
Reasons for indemnity payments included:
- probable liability (18 defendants),
- plaintiff was sympathetic, likely to evoke an emotional response from the jury (8 defendants),
- clear liability (7 defendants),
- defendant would not have made a strong witness in his or her own defense (5 defendants),
- defendant had died or was too ill to stand trial (3 defendants),
- medical record had been altered (2 defendants),
- case was considered too inflammatory to risk a jury award (2 defendants), and
- policy limits were too low to risk a potentially high jury award (1 defendant).
Five defendants were involved in cases with multiple medicolegal issues that argued for settlement.
The effect of birth weight. Notably, 74% of infants involved in these cases were macrosomic (birth weight over 4,000 g). Except for neonatal injury (100%), no single maternal or fetal variable appears with greater frequency.
The mean indemnity in closed cases increased in direct proportion to fetal weight, ranging from $500,000 in cases involving infants whose birth weight was less than 4,000 g to $950,000 when the birth weight was 5,000 g or more.
Maternal factors seen with greatest frequency included obesity, excessive weight gain in pregnancy, and gestational diabetes. Analysis of prepregnant body mass index found that 19 women had a weight within the “obese” category, 18 of whom gave birth to macrosomic infants. Eight of these cases closed without indemnity payment and 10 closed with an aggregate indemnity payment of $6.5 million.
Forty-eight of the 61 plaintiffs (78.8%) exceeded the normal weight gain in pregnancy based on height and prepregnant weight. Twenty-nine of these cases closed with an aggregate indemnity payment of $16.6 million.
Influence of diabetes. Fifty-two of 61 plaintiffs underwent a glucose screening test. Of these, 23 went on to have a glucose tolerance test, with 12 testing positive for gestational diabetes. Further analysis revealed borderline screening glucose values in an additional 16 cases. These women were not considered diabetic by their obstetricians, were not retested for diabetes, and did not receive nutritional counseling. Macrosomic infants were born to 9 of the 12 patients with gestational diabetes and to 13 of the 16 borderline cases.
Of 28 cases with confirmed or suspected gestational diabetes, 14 women delivered infants weighing over 4,250 g; 11 of the 14 weighed more than 4,500 g.
In a comparison of cases involving diabetic and nondiabetic women who delivered infants weighing more than 4,250 g, 82% of the cases involving nondiabetic women closed without payment. Among cases involving diabetes (actual or borderline), the corresponding figure was 27.3%.
Labor and delivery interventions were cited in all cases. Oxytocin was used in 42 of the 61 cases (68.9%), forceps in 9 (14.8%), and vacuum extraction in 7 (11.5%). Suprapubic pressure was used in 37 cases (60.7%), fundal pressure in 9 (14.8%), and traction on the fetal head in 16 cases (26.2%).
In addition, defense experts determined that the McRoberts maneuver was used in 41 cases (67.2%) and the Wood’s corkscrew maneuver in 28 (45.9%).
Seven cases involved a second stage of labor exceeding 2.5 hours. The ratio of wins to losses decreased substantially with the use of oxytocin, forceps, fundal pressure, or a prolonged second stage.
Data were analyzed using selected variables thought to have an association with winning or losing cases and with indemnity (TABLE 2). No statistically significant models emerged. This is likely due to inadequate power (low number of cases) and the large number of interactions between variables relative to the outcomes evaluated.
TABLE 1
Plaintiff demographics
| CHARACTERISTIC | ALL CASES (n = 61) | CASES WITH INDEMNITY (n = 35) |
|---|---|---|
| Mean age, in years (range) | 28 (17–40) | 29 (18–38) |
| Race (%) | ||
| White | 35 (57) | 21 (60) |
| Black | 12 (20) | 7 (20) |
| Hispanic | 11 (18) | 6 (17) |
| Asian | 1 (2) | — |
| Unknown | 2 (3) | 1 (3) |
| Parity (%) | ||
| 0 | 19 (31) | 11 (31) |
| 1 | 26 (43) | 16 (46) |
| 2 | 11 (18) | 7 (20) |
| 3 | 2 (3) | 1 (3) |
| 4 | 2 (3) | — |
| 5 | 1 (2) | — |
TABLE 2
Litigation outcomes for selected prenatal and intrapartum variables
| CHARACTERISTIC | CLOSED WITHOUT INDEMNITY | CLOSED WITHOUT INDEMNITY PAYMENT | MEAN INDEMNITY ($) | TOTAL INDEMNITY ($) |
|---|---|---|---|---|
| Prenatal factors | ||||
| Gestational diabetes | 5 | 7 | 413,300 | 2,893,000 |
| Adjusted diabetes | 10 | 18 | 521,400 | 9,386,000 |
| Obesity | 8 | 13 | 651,300 | 8,466,000 |
| Intrapartum factors | ||||
| Prolonged second stage | 1 | 6 | 707,700 | 4,247,000 |
| Oxytocin induction | 2 | 10 | 406,500 | 4,065,300 |
| Oxytocin augmentation | 15 | 15 | 737,000 | 11,100,000 |
| Forceps delivery | 1 | 8 | 552,100 | 4,416,800 |
| Vacuum extraction | 3 | 4 | 531,300 | 2,125,000 |
| Episiotomy | 20 | 30 | 579,400 | 17,382,100 |
| McRoberts maneuver | 20 | 21 | 542,900 | 11,400,300 |
| Wood’s corkscrew maneuver | 12 | 16 | 652,300 | 10,436,200 |
| Suprapubic pressure | 17 | 20 | 629,000 | 12,579,400 |
| Traction to fetal head | 7 | 9 | 665,500 | 5,989,500 |
| Fundal pressure | 4 | 7 | 660,700 | 4,625,000 |
4 factors raise risk of litigation
After reviewing the literature and analyzing the ProMutual data, we concluded that shoulder dystocia remains largely unpredictable. However, certain clinical factors are clearly associated with an increased risk for litigation:
- prenatal factors,
- labor and delivery interventions,
- maneuvers performed at the time of the dystocia, and
- fetal outcomes.
Prenatal factors. The most significant prenatal factors were maternal obesity, excessive weight gain in pregnancy, and, especially, diabetes and fetal macrosomia.
• Fetal macrosomia. Although most macrosomic infants deliver without complication, fetal macrosomia was involved in more than 72% of the cases reviewed. Because macrosomia represents a risk not only for shoulder dystocia but also for the litigation that may arise from it, it is important to:
- know and document the estimated fetal weight (EFW);
- discuss the risk of dystocia and its sequelae with the mother and her partner; and
- consider cesarean delivery for estimated fetal weights that suggest macrosomia (see the recommendations on page 82 for specifics).
It also is advisable to mobilize a team for the possibility of shoulder dystocia if vaginal delivery is attempted.
Preparing for the increased risk of a macrosomic fetus can be successful only if macrosomia is both diagnosed and anticipated.
Because macrosomia often is accompanied by maternal diabetes, serum glucose testing is recommended for all gravidas, with follow-up of both abnormal and borderline values.
Labor and delivery interventions were cited in all cases, with data indicating a decreasing ratio of wins to losses with the use of oxytocin, forceps, or fundal pressure, and prolonged second stage.
- Use of oxytocin to augment an already established labor carried less risk than oxytocin for labor induction. Ten of the 12 cases (83.3%) in which oxytocin was used for induction closed with an indemnity payment. However, of the 30 cases in which oxytocin was used for labor augmentation, 50% closed with payment.
- • Forceps and a prolonged second stage. Eight of 9 cases (88.9%) involving forceps and 6 of 7 cases (85.7%) involving a prolonged second stage closed with indemnity payment.
Consider cesarean delivery when the second stage is prolonged or labor fails to progress. Be cautious using forceps and vacuum extraction in these circumstances, and limit the number of attempts with either.
Maneuvers performed at the time of dystocia. The most common maneuverswere McRoberts, suprapubic pressure, and Wood’s corkscrew. The ratio of wins to losses decreased with traction of the fetal head and use of fundal pressure.
Part of the risk-management protocol for obstetricians should be appropriate use of McRoberts maneuver, suprapubic pressure, and Wood’s corkscrew, and cutting a large episiotomy. In addition, be careful not to push, pull, rotate the head, or apply fundal pressure.
Fetal outcomes. All cases involved neonatal injury (Erb’s palsy, fractured humerus) or death.
For this reason, we recommend an action plan that includes immediate pediatric or neonatal assessment of neuromuscular function of the infant’s anterior shoulder. Assess the Moro reflex and the possibility of brachial plexus injury and fractures of the clavicle and humerus. Also examine the placenta, send it to pathology, and perform a cord blood gas analysis.
Last words
Shoulder dystocia is the unfortunate complication of a small number of deliveries, but the focus of an increasing number of lawsuits. Because the neonatal injuries that so often accompany shoulder dystocia often lead to litigation, obstetricians should prepare to identify risk and help patients make informed choices. We should be prepared to manage this emergency whenever it occurs and thoroughly document actions.
Dr. Zylstra reports no financial relationships relevant to this article.
Clip-and-save shoulder dystocia documentation form
Practice recommendations
Among the intrapartum events that constitute bona fide emergencies, shoulder dystocia stands out. This obstetric emergency is the focus of an increasing number of medical liability cases. Most lawsuits involving shoulder dystocia allege negligence as the cause of the brachial plexus injury, fractured clavicle or humerus, or other injury. The defendant physicians named in these suits are often accused of inappropriately managing the prenatal or intrapartum course or the dystocia itself—or of inadequately documenting the steps taken to resolve the emergency.
To glean insights into the litigation process as it involves shoulder dystocia, we retrospectively reviewed all cases closed by the Boston-based ProMutual Group, a major liability insurance carrier, over a 7-year period. We wanted to learn more about the plaintiffs themselves, as well as the clinical and medicolegal factors that led to jury awards or indemnity payments. We also wanted data that could serve as the foundation for guidelines on how to proceed in the event of shoulder dystocia, as well as a documentation tool.
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Nurse and physician document different times
A 31-year-old woman in her 10th week of pregnancy had one prior uncomplicated vaginal delivery of a 9 lb 7 oz infant. Her prenatal course proceeds unremarkably, with a normal glucose tolerance test and total weight gain of 36 lb. At 41 weeks and 2 days, the estimated fetal weight is documented as 4,120 g. Labor is induced with oxytocin. Because of maternal fatigue, vacuum delivery is attempted.
Notes of the physician and the nurse differ regarding the time of the first of 3 vacuum applications.
After delivery of the head, shoulder dystocia is encountered. In a note handwritten immediately after delivery, the physician states that the head was “reconstituted as right occiput anterior with the left shoulder anterior.”
In a note dictated later, however, the same physician states the right shoulder was anterior.
Help is summoned and arrives 20 minutes after the dystocia is first encountered. The time that help was summoned is in question since there is an 18-minute discrepancy between the times the physician and the nurse note that assistance was called.
Despite the use of suprapubic pressure and maneuvers including McRoberts and Wood’s corkscrew, shoulder dystocia persists for 24 minutes. Apgars of the 11 lb 3 oz infant are 0, 1, and 3. The child is resuscitated but dies within 2 days of birth.
Outcome
Settled with a 7-figure indemnity payment.
What the defense experts said
The key issues involve documentation and summoning assistance. Discrepancies in documentation almost always cast doubt upon the credibility of a defendant. Ideally, there should be no discrepancies between nurse and physician notes and, certainly, no discrepancies between 2 notes on the same case by the same physician. If, in this case, the physician realized after writing the first note that the anterior shoulder had been incorrectly identified, a correction should have been written as a separate note.
Use of the shoulder dystocia documentation tool (see) helps create a chronology of events, which may prove vital to a successful defense.
The call for help might not have been delayed if the labor and delivery unit had had a shoulder dystocia protocol including “drills” for all team members. Help should be called as soon as a shoulder dystocia is encountered so that, when needed, it is available. Under no circumstances should it take 20 minutes for assistance to arrive.
Brachial plexus injury not always caused by shoulder dystocia
Between 21% and 42% of shoulder dystocias involve an injury1—usually brachial plexus injury. Plaintiff attorneys have manipulated this fact to attribute many cases of neonatal brachial plexus injury to mismanagement of shoulder dystocia by the obstetrician.
They fault the physician for failing to estimate fetal weight, perform a timely cesarean, use appropriate maneuvers correctly, or have a pediatrician present. They criticize nothing more resoundingly than use of “inappropriate” or “excessive” lateral traction to the fetal head.2
Nontraction injuries. The reality can be strikingly different, however. Some cases of brachial plexus injury involve no traction at all.
- Brachial plexus injuries have been reported in infants who had precipitate vaginal deliveries without any physical intervention by the obstetrician.2
- These injuries also have occurred in infants delivered via cesarean section.1,3,4
- In some cases, brachial plexus injuries have affected the posterior arm of neonates whose anterior arm was involved in shoulder dystocia.1,2,5-7
A retrospective study4 found that, of 39 cases of brachial plexus injury, only 17 were associated with shoulder dystocia. Similar findings have emerged from other studies.2,3,8
Other causes. It is unclear how brachial plexus injuries occur in the absence of shoulder dystocia. Some think they arise in response to infectious agents such as toxoplasmosis, coxsackievirus, mumps, pertussis, or mycoplasma pneumonia.2 Some assume a mechanical cause, such as fetal response to longstanding abnormal intrauterine pressure exerted by maternal conditions such as bicornate uterus and uterine fibroids, especially in the lower segment.1,2
When brachial plexus injuries occur in the absence of shoulder dystocia, they likely originated before labor and delivery.4 Some experts suggest serial electromyelograms within the first 7 days of life to establish a prenatal rather than intrapartum etiology. A positive electromyelogram within 1 week of birth would suggest antepartum causation.2,9
Recognizing risk factors for shoulder dystocia best way to reduce injury
Most brachial plexus injuries or impairments are associated with shoulder dystocia,9 and shoulder dystocia is the most common way brachial plexus injuries are introduced into litigation.
Decreasing the number of brachial plexusrelated liability cases, therefore, depends on decreasing the incidence of shoulder dystocia. Unfortunately, a failsafe method continues to elude both clinicians and researchers.10-12
Retrospective studies have identified certain factors that may—but do not necessarily—increase the risk of shoulder dystocia.
Prenatal risk factors include high maternal or paternal birth weight, maternal obesity, excessive weight gain during pregnancy, advanced age, short stature, multiparity, postdates, prior shoulder dystocia, small pelvis, prior delivery of a macrosomic infant, gestational diabetes in an earlier pregnancy, abnormal blood sugars in the current pregnancy, or fetal macrosomia.13,14-16
Intrapartum risk factors include a rapid or prolonged second stage, failure or arrest of descent, presence of considerable molding, and need for a midpelvic delivery.10,15
Predictability. Prospective studies have not established the predictability of shoulder dystocia. A 2000 study17 showed that 55% of cases with 1 or more risk factors experienced shoulder dystocia. Predictability increases somewhat when both maternal diabetes and fetal macrosomia complicate pregnancy, since the rate of shoulder dystocia in women with diabetes is consistently higher than in nondiabetic gravidas. This becomes a significant issue when the infant weighs more than 4,000 g.
Indications for prophylactic cesarean in women with diabetes
In 1999, Wagner et al9 found that 70% of shoulder dystocias in women with diabetes occurred when the fetal weight exceeded 4,000 g. They concluded that cesarean delivery for infants with an estimated weight over 4,250 g would reduce the rate of shoulder dystocia by 75% and increase the cesarean delivery rate by 1%.
Others are more conservative. Gross and colleagues11 suggested that, for every additional 26 cesarean deliveries, only 1 case of shoulder dystocia would be prevented.
Macrosomia. Most obstetricians and researchers still do not advocate prophylactic cesarean delivery for macrosomia alone because, by some estimates, 98% of macrosomic infants are delivered without difficulty.18 However, they do suggest that obstetricians at least consider the possibility of cesarean delivery for a macrosomic fetus when the woman has diabetes.
In a study completed in 2000, Skolbekken19 suggested a cutoff of 4,250 g for women with diabetes. Dildy20 suggested limits of more than 4,500 g for diabetic women and more than 5,000 g for nondiabetic gravidas. However, Conway and Langer21 assert that a cutoff of 4,500 g is too liberal for women with diabetes and maintain that, at this cutoff, 40% of shoulder dystocias would not be prevented.
• Ultrasound measurements. Since estimates of fetal weight have a margin of error approaching 40%,9 others have chosen different parameters for determining fetal macrosomia in women with diabetes. In a retrospective study involving 31 women with gestational diabetes, Cohen et al22 found that subtracting the fetal biparietal diameter from the abdominal diameter—with both measurements obtained via ultrasound—yields a predictability score higher than estimated fetal weight. Specifically, if the difference between the 2 measurements is 2.6 cm or more, the rate of shoulder dystocia is high enough to warrant elective cesarean (FIGURE ).
FIGURE Using ultrasound measurements to predict macrosomia
A simple way to predict fetal macrosomia in women with diabetes is to subtract the fetal biparietal diameter (9.3 cm in the scan at left) from the abdominal diameter (average of 12.44 cm in the scan at right). If the difference exceeds 2.6 cm, elective cesarean is warranted. In this case it is 3.14 cm, indicating elective cesarean is warranted.
When dystocia occurs, have a plan and stick to it
Shoulder dystocia immediately places both mother and neonate at risk for temporary or permanent injury. Thus, it is imperative that all obstetricians and other health-care providers who deliver infants have a well developed plan of action for this emergency. They should immediately ask for obstetric assistance and instruct the mother to discontinue any pushing.
Attempts at vigorous downward traction should be avoided, and no fundal pressure should be applied, as these are known to increase the potential for brachial plexus injury. Gentle downward traction is considered the standard of care.17
The obstetrician’s goal is to free the impacted shoulder as quickly as possible, since a fetus can endure only 8 to 10 minutes of asphyxia before permanent neurologic damage occurs.17 The standard of care requires the obstetrician to know and use certain maneuvers to relieve shoulder dystocia. These maneuvers are designed to facilitate vaginal delivery and reduce the risk of permanent brachial plexus injury. The McRoberts maneuver, with flexion and slight rotation of the maternal hips onto the maternal abdomen, is the standard for initial relief of shoulder dystocia.17,23
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Prompt maneuvers, good outcome
A 28-year-old gravida weighing 214 lb has had 2 previous spontaneous deliveries of infants weighing 8 lb 5 oz and 9 lb 3 oz. Except for a weight gain of more than 60 lb, the pregnancy progressed without complications, and a 3-hour glucose tolerance test was normal. At 40 weeks, the obstetrician notes “concern” about an estimated fetal weight of 10 lb. Induction is planned, but spontaneous labor begins before oxytocin can be given. After 5 hours, the head is delivered without difficulty, but shoulder dystocia follows. The obstetrician extends the episiotomy and performs McRoberts and Wood’s corkscrew maneuvers, but the dystocia persists. Upon noting cyanosis, the obstetrician fractures the infant’s clavicle and quickly delivers a 10 lb 9 oz infant with Apgar scores of 8 and 10. Pediatricians examine the child immediately and diagnose Erb’s palsy, which subsequently resolves. X-rays confirm an undisplaced fracture of the right clavicle. Although the child recovers completely, the family sues, alleging a failure to perform cesarean delivery.
Outcome
Case closed with no payment.
What the defense experts said
Key issues are documentation and informed consent. The overriding opinion of 3 experts who reviewed the case for the defense was that cesarean delivery was not indicated and that in fracturing the clavicle, the physician acted responsibly, quickly, and within the standard of care. One defense expert said failure to document exact maneuvers used to relieve shoulder dystocia deviated from the standard of care. Another defense expert said the physician should have obtained informed consent from this at-risk patient, and explained the risks of shoulder dystocia, including neonatal injury, so that the she might have been better able to appreciate the fact that the obstetrician’s fast action may have saved her child from brain damage or death.
Factors that lead to litigation
In a review article, Hickson24 cited factors that prompted families to file medical liability claims following perinatal injury. Some families observed that, in their search for the cause of an injury, they found 1 or more aspects of care to be inappropriate.
The desire for information, perception of being misled, anger with the medical profession, desire to prevent injuries to others, recognition of long-term sequelae, and advice by knowledgeable acquaintances, as well as the need for money, all appeared to contribute to the decision to file medical liability claims.
Convey the risks, and listen carefully. Communication problems between physicians and patients are a contributing factor. Even when physicians provide technically adequate care, families expect answers to their questions and want to feel as though they have been consulted about important medical decisions.24 If these expectations are not met, even patients who have not experienced an adverse outcome may become angry and express dissatisfaction with care.24
The need for communication is critical when shoulder dystocia results in neonatal injury. Empathizing with the family, helping them understand that most brachial plexus injuries are not long-term, and offering to answer their questions both at the moment and later, may help prevent litigation.
This type of communication can be difficult. It helps to realize that an acknowledgment of distress and concern is not an admission of guilt, and an explanation is not an apology.15 However, an absence of communication or an attempt by the physician to place blame may be perceived as an admission of guilt that gives rise to a lawsuit.
Action plan is what counts in court. A review by Gross et al11 concluded that obstetricians should have a shoulder dystocia plan that enables an instant and orderly response. Also recommended is a protocol to help anticipate clinical problems and prevent medicolegal problems.
Gross and colleagues11 found that the Ob/Gyns with the most defensible cases paid close attention to the patient’s history and prenatal course and, upon encountering dystocia, implemented at least 2 maneuvers (if necessary) and thoroughly documented their actions immediately after delivery.
Fetterman15 agreed, asserting that what counts in court is not so much whether the obstetrician employed the McRoberts maneuver before or after the Wood’s corkscrew maneuver but whether he or she had an action plan in mind, implemented that plan properly, and thoroughly documented the actions taken and the reasons underlying them.
Dissecting legal cases for clues to reduced risk
In the review of medicolegal cases for this article, we limited our search to cases closed between Jan 1, 1995, and Dec 31, 2002, using a computer to search for codes specific to shoulder dystocia as well as the phrases “shoulder dystocia,” “Erb’s palsy,” and “brachial plexus injury.” We identified 61 cases involving 117 defendants and created a data sheet to gather information on patient and physician demographics, medical and obstetric history, description of the incident, analysis rendered by defense and plaintiff experts, and legal and financial outcomes.
Of 117 defendants, 76 were obstetricians. There also were 16 hospitals, 15 corporations, 5 certified nurse-midwives, 1 family physician, 1 emergency physician, and 3 persons categorized as “other.” Age, race, and parity of the 61 plaintiffs are given in TABLE 1.
Twenty-six of the 61 cases, involving 74 defendants, closed with no payment. That is, they were either dismissed or closed with a jury verdict for the defense. The remaining 35 cases involved 43 defendants and were closed with an aggregate indemnity payment of $19.2 million. The mean payment was $445,000 per defendant.
These guidelines are recommended to help prevent, predict, and manage shoulder dystocia and brachial plexus injury.
Obtain a prenatal history that includes the birth weights of both parents and any history of prior shoulder dystocia or cesarean delivery performed for “failure to progress.”
Estimate the fetal weight and take into account the risk for shoulder dystocia when the fetus is determined by ultrasound to be macrosomic.
Perform glucose testing on all patients, and follow up on even a single abnormal glucose reading. Discuss the possibility of shoulder dystocia and the accompanying risk of neonatal injury with “at risk” patients.
Consider obtaining informed consent for vaginal delivery of a patient with risk factors for shoulder dystocia.
Consider cesarean delivery for :
- nondiabetic women when the estimated fetal weight (EFW) exceeds 4,500 g,
- diabetic gravidas when the EFW exceeds 4,000 g,
- women with a prior delivery complicated by shoulder dystocia or brachial plexus palsy,
- gravidas with a prolonged second stage and nonprogression of labor, and
- patients who express fear and doubt about vaginal delivery.
Be sparing with the use of oxytocin when the fetus is known or suspected to be macrosomic, taking special care not to be aggressive with induction.
Use forceps or vacuum extraction with caution, and limit the number of attempts with each.
Minimize traction on the fetal head. Traction that is deemed to be “excessive” may be used against the physician in a liability suit. Gentle traction is acceptable.
Do not use or order fundal pressure. It will almost invariably be used against you in a lawsuit.
Be able to define and correctly describe maneuvers generally accepted as the standard for shoulder dystocia and, when necessary, use and document them appropriately. These include the McRoberts, Wood’s corkscrew, Rubin, and Zavanelli maneuvers; extended episiotomy; suprapubic pressure; and fracture of the anterior clavicle.
Be alert to the possibility of brachial plexus injury in the absence of shoulder dystocia. Obstetricians have been erroneously accused of causing brachial plexus injury by plaintiff attorneys who do not understand that this injury is not always the result of dystocia. Thorough contemporaneous documentation is key in these instances.
Request immediate pediatric assessment of a newborn involved in shoulder dystocia. Have the placenta sent for examination, and request cord blood gases.
Communicate openly and honestly with the parents of a child who has suffered a brachial plexus injury. This may be the single greatest tool for reducing the risk of liability litigation.
Consider serial electromyelograms during the first 7 days of life for a neonate with a brachial plexus palsy. These studies can help determine the etiology of the injury.
Use a shoulder dystocia documentation tool such as the one on page 91. Thorough documentation of all relevant prenatal and intrapartum events is critical to a successful defense. A 12-point detailed delivery note as recommended by Fetterman will also prevent or reduce legal risk.15
Schedule shoulder dystocia “drills” in the labor and delivery unit to familiarize obstetric team members with their roles.
Require comanagement of any midwifery patient at increased risk for shoulder dystocia.
Neonatal injury occurred in all 61 cases. Erb’s palsy was the overwhelming pediatric outcome (57 of 61 cases, or 93.4%), with an aggregate indemnity payment of $17.6 million. Fractured humerus was the outcome in 1 case, and 3 cases involved neonatal deaths.
Reasons for indemnity payments included:
- probable liability (18 defendants),
- plaintiff was sympathetic, likely to evoke an emotional response from the jury (8 defendants),
- clear liability (7 defendants),
- defendant would not have made a strong witness in his or her own defense (5 defendants),
- defendant had died or was too ill to stand trial (3 defendants),
- medical record had been altered (2 defendants),
- case was considered too inflammatory to risk a jury award (2 defendants), and
- policy limits were too low to risk a potentially high jury award (1 defendant).
Five defendants were involved in cases with multiple medicolegal issues that argued for settlement.
The effect of birth weight. Notably, 74% of infants involved in these cases were macrosomic (birth weight over 4,000 g). Except for neonatal injury (100%), no single maternal or fetal variable appears with greater frequency.
The mean indemnity in closed cases increased in direct proportion to fetal weight, ranging from $500,000 in cases involving infants whose birth weight was less than 4,000 g to $950,000 when the birth weight was 5,000 g or more.
Maternal factors seen with greatest frequency included obesity, excessive weight gain in pregnancy, and gestational diabetes. Analysis of prepregnant body mass index found that 19 women had a weight within the “obese” category, 18 of whom gave birth to macrosomic infants. Eight of these cases closed without indemnity payment and 10 closed with an aggregate indemnity payment of $6.5 million.
Forty-eight of the 61 plaintiffs (78.8%) exceeded the normal weight gain in pregnancy based on height and prepregnant weight. Twenty-nine of these cases closed with an aggregate indemnity payment of $16.6 million.
Influence of diabetes. Fifty-two of 61 plaintiffs underwent a glucose screening test. Of these, 23 went on to have a glucose tolerance test, with 12 testing positive for gestational diabetes. Further analysis revealed borderline screening glucose values in an additional 16 cases. These women were not considered diabetic by their obstetricians, were not retested for diabetes, and did not receive nutritional counseling. Macrosomic infants were born to 9 of the 12 patients with gestational diabetes and to 13 of the 16 borderline cases.
Of 28 cases with confirmed or suspected gestational diabetes, 14 women delivered infants weighing over 4,250 g; 11 of the 14 weighed more than 4,500 g.
In a comparison of cases involving diabetic and nondiabetic women who delivered infants weighing more than 4,250 g, 82% of the cases involving nondiabetic women closed without payment. Among cases involving diabetes (actual or borderline), the corresponding figure was 27.3%.
Labor and delivery interventions were cited in all cases. Oxytocin was used in 42 of the 61 cases (68.9%), forceps in 9 (14.8%), and vacuum extraction in 7 (11.5%). Suprapubic pressure was used in 37 cases (60.7%), fundal pressure in 9 (14.8%), and traction on the fetal head in 16 cases (26.2%).
In addition, defense experts determined that the McRoberts maneuver was used in 41 cases (67.2%) and the Wood’s corkscrew maneuver in 28 (45.9%).
Seven cases involved a second stage of labor exceeding 2.5 hours. The ratio of wins to losses decreased substantially with the use of oxytocin, forceps, fundal pressure, or a prolonged second stage.
Data were analyzed using selected variables thought to have an association with winning or losing cases and with indemnity (TABLE 2). No statistically significant models emerged. This is likely due to inadequate power (low number of cases) and the large number of interactions between variables relative to the outcomes evaluated.
TABLE 1
Plaintiff demographics
| CHARACTERISTIC | ALL CASES (n = 61) | CASES WITH INDEMNITY (n = 35) |
|---|---|---|
| Mean age, in years (range) | 28 (17–40) | 29 (18–38) |
| Race (%) | ||
| White | 35 (57) | 21 (60) |
| Black | 12 (20) | 7 (20) |
| Hispanic | 11 (18) | 6 (17) |
| Asian | 1 (2) | — |
| Unknown | 2 (3) | 1 (3) |
| Parity (%) | ||
| 0 | 19 (31) | 11 (31) |
| 1 | 26 (43) | 16 (46) |
| 2 | 11 (18) | 7 (20) |
| 3 | 2 (3) | 1 (3) |
| 4 | 2 (3) | — |
| 5 | 1 (2) | — |
TABLE 2
Litigation outcomes for selected prenatal and intrapartum variables
| CHARACTERISTIC | CLOSED WITHOUT INDEMNITY | CLOSED WITHOUT INDEMNITY PAYMENT | MEAN INDEMNITY ($) | TOTAL INDEMNITY ($) |
|---|---|---|---|---|
| Prenatal factors | ||||
| Gestational diabetes | 5 | 7 | 413,300 | 2,893,000 |
| Adjusted diabetes | 10 | 18 | 521,400 | 9,386,000 |
| Obesity | 8 | 13 | 651,300 | 8,466,000 |
| Intrapartum factors | ||||
| Prolonged second stage | 1 | 6 | 707,700 | 4,247,000 |
| Oxytocin induction | 2 | 10 | 406,500 | 4,065,300 |
| Oxytocin augmentation | 15 | 15 | 737,000 | 11,100,000 |
| Forceps delivery | 1 | 8 | 552,100 | 4,416,800 |
| Vacuum extraction | 3 | 4 | 531,300 | 2,125,000 |
| Episiotomy | 20 | 30 | 579,400 | 17,382,100 |
| McRoberts maneuver | 20 | 21 | 542,900 | 11,400,300 |
| Wood’s corkscrew maneuver | 12 | 16 | 652,300 | 10,436,200 |
| Suprapubic pressure | 17 | 20 | 629,000 | 12,579,400 |
| Traction to fetal head | 7 | 9 | 665,500 | 5,989,500 |
| Fundal pressure | 4 | 7 | 660,700 | 4,625,000 |
4 factors raise risk of litigation
After reviewing the literature and analyzing the ProMutual data, we concluded that shoulder dystocia remains largely unpredictable. However, certain clinical factors are clearly associated with an increased risk for litigation:
- prenatal factors,
- labor and delivery interventions,
- maneuvers performed at the time of the dystocia, and
- fetal outcomes.
Prenatal factors. The most significant prenatal factors were maternal obesity, excessive weight gain in pregnancy, and, especially, diabetes and fetal macrosomia.
• Fetal macrosomia. Although most macrosomic infants deliver without complication, fetal macrosomia was involved in more than 72% of the cases reviewed. Because macrosomia represents a risk not only for shoulder dystocia but also for the litigation that may arise from it, it is important to:
- know and document the estimated fetal weight (EFW);
- discuss the risk of dystocia and its sequelae with the mother and her partner; and
- consider cesarean delivery for estimated fetal weights that suggest macrosomia (see the recommendations on page 82 for specifics).
It also is advisable to mobilize a team for the possibility of shoulder dystocia if vaginal delivery is attempted.
Preparing for the increased risk of a macrosomic fetus can be successful only if macrosomia is both diagnosed and anticipated.
Because macrosomia often is accompanied by maternal diabetes, serum glucose testing is recommended for all gravidas, with follow-up of both abnormal and borderline values.
Labor and delivery interventions were cited in all cases, with data indicating a decreasing ratio of wins to losses with the use of oxytocin, forceps, or fundal pressure, and prolonged second stage.
- Use of oxytocin to augment an already established labor carried less risk than oxytocin for labor induction. Ten of the 12 cases (83.3%) in which oxytocin was used for induction closed with an indemnity payment. However, of the 30 cases in which oxytocin was used for labor augmentation, 50% closed with payment.
- • Forceps and a prolonged second stage. Eight of 9 cases (88.9%) involving forceps and 6 of 7 cases (85.7%) involving a prolonged second stage closed with indemnity payment.
Consider cesarean delivery when the second stage is prolonged or labor fails to progress. Be cautious using forceps and vacuum extraction in these circumstances, and limit the number of attempts with either.
Maneuvers performed at the time of dystocia. The most common maneuverswere McRoberts, suprapubic pressure, and Wood’s corkscrew. The ratio of wins to losses decreased with traction of the fetal head and use of fundal pressure.
Part of the risk-management protocol for obstetricians should be appropriate use of McRoberts maneuver, suprapubic pressure, and Wood’s corkscrew, and cutting a large episiotomy. In addition, be careful not to push, pull, rotate the head, or apply fundal pressure.
Fetal outcomes. All cases involved neonatal injury (Erb’s palsy, fractured humerus) or death.
For this reason, we recommend an action plan that includes immediate pediatric or neonatal assessment of neuromuscular function of the infant’s anterior shoulder. Assess the Moro reflex and the possibility of brachial plexus injury and fractures of the clavicle and humerus. Also examine the placenta, send it to pathology, and perform a cord blood gas analysis.
Last words
Shoulder dystocia is the unfortunate complication of a small number of deliveries, but the focus of an increasing number of lawsuits. Because the neonatal injuries that so often accompany shoulder dystocia often lead to litigation, obstetricians should prepare to identify risk and help patients make informed choices. We should be prepared to manage this emergency whenever it occurs and thoroughly document actions.
Dr. Zylstra reports no financial relationships relevant to this article.
1. Gherman RB, Goodwin TM, Ouzounian JG, Miller DA, Paul RH. Brachial plexus palsy associated with cesarean section: an in utero injury? Am J Obstet Gynecol. 1997;177:1162-1164.
2. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93:536-540.
4. Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: an old problem revisited. Am J Obstet Gynecol. 1992;166:1673-1677.
5. Hankins GDV, Clark SL. Brachial plexus palsy involving the posterior shoulder at spontaneous vaginal delivery. Am J Perinatol. 1995;12:44-45.
6. Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: a risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423-427.
7. Dodds SD, Wolfe SW. Perinatal brachial plexus palsy. Curr Opin Pediatrics. 2000;23:40-47.
8. Wade DM. A Comment on the Trial of Brachial Plexus/Erb’s Palsy Medical Malpractice Cases. Evidence Technologies, Inc. 1994.
9. Wagner RK, Nielsen PE, Gonik B. Controversies in labor management: shoulder dystocia. Obstet Gynecol Clin. 1999;26(2):371-382.
10. McFarland M, Hod M, Piper JM, Xenakis EM-J, Langer O. Are labor abnormalities more common in shoulder dystocia? Am J ObstetGynecol. 1995;173:1211-1214.
11. Gross TL, Sokol RJ, Williams T, Thompson K. Shoulder dystocia: A fetal-physician risk. Am J Obstet Gynecol. 1987;156:1408-1418.
12. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
13. Shoulder dystocia and Erb’s palsy. The Keenan Law Firm. 2002. Available at www.shoulderdystociaattorney.com. Accessed July 23, 2004.
14. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71(Part l):390-392.
15. Fetterman HH. Cutting the legal risks of shoulder dystocia. OBG Management. March 1995;41-51.
16. Iffy L, Varadi V, Jakobovits A. Common intrapartum denominators of shoulder dystocia related birth injuries. Zentralbl Gynakol. 1994;116(1):33-37.
17. Calhoun BC, Hume RR, Walters JL. Shoulder Dystocia as a risk for obstetric liability. Legal Medicine. 2000;12-15.
18. Jennett RJ, Tarby TJ. Brachial plexus palsy: an old problem revisited again. Am J Obstet Gynecol. 1997;176:1354-1357.
19. Skolbekken JA. Shoulder dystocia—malpractice or acceptable risk? Acta Obstet Gynecol Scand. 2000;79:750-756.
20. Dildy GA. Fetal macrosomia [slide presentation]. Presented at the 18th Annual Conference on Obstetrics, Gynecology, Perinatal Medicine, and the Law, Boston University School of Medicine and the Center for Human Genetics, January 2002, Kauai, Hawaii.
21. Conway D, Langer O. Elective delivery of infants with macrosomia in diabetic women: reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998;178(5):922-925.
22. Cohen B, Penning S, Major C, Ansley D, Porto M, Garite T. Sonographic prediction of shoulder dystocia in infants of diabetic mothers. Obstet Gynecol. 1998;88:10-13.
23. Gherman RB, Goodwin TM, Souter I, Neumann K, Ouzounian JG, Paul RH. The McRoberts’ maneuver for the alleviation of shoulder dystocia: how successful is it? Am J Obstet Gynecol. 1997;176:656-661.
24. Hickson GB, Clayton DW, Githens PB, Sloan FA. Factors that prompted families to file medical malpractice claims following perinatal injuries. JAMA. 1992;267:1359-1363.
1. Gherman RB, Goodwin TM, Ouzounian JG, Miller DA, Paul RH. Brachial plexus palsy associated with cesarean section: an in utero injury? Am J Obstet Gynecol. 1997;177:1162-1164.
2. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93:536-540.
4. Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: an old problem revisited. Am J Obstet Gynecol. 1992;166:1673-1677.
5. Hankins GDV, Clark SL. Brachial plexus palsy involving the posterior shoulder at spontaneous vaginal delivery. Am J Perinatol. 1995;12:44-45.
6. Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: a risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423-427.
7. Dodds SD, Wolfe SW. Perinatal brachial plexus palsy. Curr Opin Pediatrics. 2000;23:40-47.
8. Wade DM. A Comment on the Trial of Brachial Plexus/Erb’s Palsy Medical Malpractice Cases. Evidence Technologies, Inc. 1994.
9. Wagner RK, Nielsen PE, Gonik B. Controversies in labor management: shoulder dystocia. Obstet Gynecol Clin. 1999;26(2):371-382.
10. McFarland M, Hod M, Piper JM, Xenakis EM-J, Langer O. Are labor abnormalities more common in shoulder dystocia? Am J ObstetGynecol. 1995;173:1211-1214.
11. Gross TL, Sokol RJ, Williams T, Thompson K. Shoulder dystocia: A fetal-physician risk. Am J Obstet Gynecol. 1987;156:1408-1418.
12. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
13. Shoulder dystocia and Erb’s palsy. The Keenan Law Firm. 2002. Available at www.shoulderdystociaattorney.com. Accessed July 23, 2004.
14. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71(Part l):390-392.
15. Fetterman HH. Cutting the legal risks of shoulder dystocia. OBG Management. March 1995;41-51.
16. Iffy L, Varadi V, Jakobovits A. Common intrapartum denominators of shoulder dystocia related birth injuries. Zentralbl Gynakol. 1994;116(1):33-37.
17. Calhoun BC, Hume RR, Walters JL. Shoulder Dystocia as a risk for obstetric liability. Legal Medicine. 2000;12-15.
18. Jennett RJ, Tarby TJ. Brachial plexus palsy: an old problem revisited again. Am J Obstet Gynecol. 1997;176:1354-1357.
19. Skolbekken JA. Shoulder dystocia—malpractice or acceptable risk? Acta Obstet Gynecol Scand. 2000;79:750-756.
20. Dildy GA. Fetal macrosomia [slide presentation]. Presented at the 18th Annual Conference on Obstetrics, Gynecology, Perinatal Medicine, and the Law, Boston University School of Medicine and the Center for Human Genetics, January 2002, Kauai, Hawaii.
21. Conway D, Langer O. Elective delivery of infants with macrosomia in diabetic women: reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998;178(5):922-925.
22. Cohen B, Penning S, Major C, Ansley D, Porto M, Garite T. Sonographic prediction of shoulder dystocia in infants of diabetic mothers. Obstet Gynecol. 1998;88:10-13.
23. Gherman RB, Goodwin TM, Souter I, Neumann K, Ouzounian JG, Paul RH. The McRoberts’ maneuver for the alleviation of shoulder dystocia: how successful is it? Am J Obstet Gynecol. 1997;176:656-661.
24. Hickson GB, Clayton DW, Githens PB, Sloan FA. Factors that prompted families to file medical malpractice claims following perinatal injuries. JAMA. 1992;267:1359-1363.
Primary Care Panels in the VA [published correction appears in: Fed Pract. 2004;21(9):99.]
Carbon Monoxide Monitoring
Atypical Presentations of Clostridium difficile
• New routes, new regimens • Array of options for emergency contraception clip-and-save chart • The IUD makes a comeback
It’s surprising to realize that the birth control pill, which launched a revolution in women’s sexuality and health, has been around for less than 50 years—especially considering the myriad of methods and products on the market today. New types of contraceptives have become available, more are on the way, and noncontraceptive benefits continue to accrue. This article reviews noteworthy changes and upcoming products.
Continuous contraception: New routes, new regimens
When it was introduced in 1960, the oral contraceptive (OC) consisted of 20 or 21 active pills followed by a pill-free interval of 7 or 8 days. No medical reason justified the pill-free interval; it was devised simply to trigger menses and reassure the woman she was not pregnant.
That pill-free interval shrank as we gained awareness of the benefits of continuous OC regimens, particularly in the treatment of endometriosis. Advantages—well documented by Sulak1—include marked reduction or elimination of menses-related symptoms such as menorrhagia, dysmenorrhea, and menstrual migraines.
Continuous OCs became “official” in 2003 with the introduction of Seasonale (ethinyl estradiol, 0.03 mg/levonorgestrel, 0.15 mg), a continuous regimen taken for 84 days, followed by a 7-day pill-free interval.2
This regimen reduces the number of periods to 4 each year.
Breakthrough bleeding diminishes over time. Although breakthrough bleeding is a common side effect of continuous oral contraception—as it is with conventional regimens—it decreases with each successive cycle of Seasonale. By the fourth cycle, it is comparable to the level reported by women on a conventional regimen. Prolonged and heavy bleeding also can be managed by discontinuing pills for 7 days.
Other methods also can be used continuously. Continuous contraception is not limited to oral contraception.
The contraceptive ring (Nuvaring; ethinyl estradiol/etonogestrel) can be left in place for 4 weeks instead of 3 without decreasing efficacy. Both hormone levels are sufficient to prevent pregnancy throughout these weeks.3
Nor is there any reason to doubt the efficacy of continuous use of the contraceptive patch (Evra; ethinyl estradiol/norelgestromin).
New regimens. A regimen of 24 active pills and a 4-day pill-free interval is available in Europe. The shorter pill-free interval allows further reduction of the active components ethinyl estradiol and gestadene. This agent contains 15 μg estradiol per pill.4
A version of Yasmin that contains only 20 μg of ethinyl estradiol, should be released in the United States later this year. It will consist of 24 active pills and a 4-day pill-free interval.
New implantable rod. Implanon, a new single-rod contraceptive implant containing etonogestrel, is available outside the United States and may become available here in the near future.5,6 It can be inserted in 1.6 minutes, removed in 2.6 minutes, and lasts 3 years. No pregnancies were reported in clinical trials.
Lower dose of Depo-Provera effective for 3 months. A new dose and route of administration for Depo-Provera (depotmedroxyprogesterone acetate) is just as effective as the 150-mg intramuscular dose.
The new dose is 104 mg administered subcutaneously every 3 months. When it becomes available in the near future, this preparation should allow women to administer the drug at home.7
Emergency contraception: A variety of methods, but still need a prescription
The different methods of emergency contraception are listed in a clip-and-save chart on page 46, which can be photocopied for convenient reference.
Trussell and colleagues’8 excellent review prefers the term emergency contraception to refer to contraceptive use after intercourse has occurred. The term “morning-after pill” is confusing because the method can be used any time after intercourse for up to 72 hours. It can even be used after 72 hours, albeit at reduced efficacy.
The Yuzpe regimen is the oldest and probably most popular form of contraception. It involves taking 2 Ovral tablets (ethinyl estradiol/levonorgestrel) followed by 2 more tablets 12 hours later. Each dose consists of 100 μg of estradiol and 0.5 mg of norgestrel.
Unfortunately, Ovral is not readily available in pharmacies. An alternative is taking enough OCs to equal the 100-μg dose of ethinyl estradiol and the 0.5 mg dose of norgestrel. For example, 5 tablets of Alesse or Levlite can be substituted for the 2 Ovral.
Efficacy is 75%. That is, if 100 women have unprotected intercourse once during the second or third week of their cycle, about 8 will become pregnant without treatment, and only 2 will become pregnant after treatment—a 75% reduction.
OCs that contain different progestins have not been studied extensively. They appear to work with lower efficacy than pills containing levonorgestrel.
- Common side effects include nausea and vomiting. Co-administration of an anti-nausea agent may be necessary. About 50% of women experience nausea and 20% vomit within 2 hours of taking a dose; some clinicians recommend repeating that dose to assure efficacy. One option is giving two 25-mg tablets of the over-the-counter drug meclizine 1 hour before combined emergency contraceptive pills; this reduces the risk of nausea by 27% and vomiting by 64%, but the risk of drowsiness doubles. Taking the medicine with a meal does not lower the rate of nausea.
- Contraindications. The only contraindication is an established pregnancy, since the drugs are taken so briefly.
Plan B Consists of 0.75 mg levonorgestrel taken within 72 hours of intercourse, followed by a second dose 12 hours later. This regimen has fewer side effects than the Yuzpe plan and may be slightly more effective. Unfortunately, the US Food and Drug Administration failed to approve over-the-counter status for this drug, so a prescription is still necessary.
IUDs. Insertion of a copper IUD (Paraguard)—but not a levonorgestrel-containing device (Mirena)—within 72 hours after intercourse is almost completely effective in preventing pregnancy. It also provides continuing contraception. Its mechanism of action is preventing implantation of a fertilized egg. Mirena has no efficacy in this regard.
“Every Woman, Every Visit,” the American College of Obstetricians and Gynecologists’ public education campaign, urges Ob/Gyns to provide advance prescriptions for emergency contraception at every office visit.
After many years of declining choices in the realm of intrauterine devices (IUDs), the Mirena levonorgestrel-containing system was released in the US market in 2000. Like the Paraguard copper IUD, the Mirena prevents pregnancy at a rate equivalent to tubal ligation. These devices last for 5 and 10 years, respectively.9,10
In the United States, interest in IUDs declined after they were associated with salpingitis and tubo-ovarian abscess. More recent epidemiological evidence indicates that IUDs do not increase the risk of infection over the general population, but the rate is higher than with other forms of contraception, which offer some protection against salpingitis. Antibiotic prophylaxis is not necessary.
The removal rate for pelvic inflammatory disease is much lower for Mirena than for the copper IUD and may be related to the low levels of levonorgestrel, which thicken cervical mucus and prevent sperm transport.9
Ectopic pregnancy rates with the Mirena are about 1/8 to 1/10 those observed in the general population. Once the Mirena device is removed, fertility returns rapidly9-11
Recommended for the chronically ill. According to the World Health Organization, IUDs are the safest form of contraception for medically complicated patients.12 Certainly, they are underutilized in this circumstance.
Noncontraceptive benefits. Slow release of low doses of levonorgestrel by Mirena reduces endometrial thickness and menstrual blood loss.13 In fact, several studies have found the Mirena to be equivalent to endometrial ablation.14 In a randomized study, two thirds of the women scheduled for hysterectomy for abnormal uterine bleeding cancelled surgery due to satisfaction with Mirena’s bleeding profile.15
Ovarian cancer also is reduced.16
Does obesity limit contraceptive efficacy?
Decreased efficacy of the contraceptive patch, observed in overweight women,17 especially those heavier than 198 lb, prompted reevaluation of other forms. In a retrospective analysis, Holt and colleagues18 found a higher pregnancy rate in women heavier than 155 lb as the estrogen dose decreased. In contrast, no pregnancies occurred in women weighing more than 198 lb in a randomized trial2 of 30 μg ethinyl estradiol/150 μg levonorgestrel (Seasonale). It is unclear why Holt failed to analyze progestin content, since it is the progestin that inhibits ovulation and prevents pregnancy.
Unfortunately, few clinical trials involve obese women. For example, the contraceptive ring was not evaluated in obese women.
Despite this shortcoming, I have not changed my prescribing of contraceptives in obese women, but await better, more convincing data. Until then, it seems wise to include a broad range of body weights in future trials.
The author serves on the Speakers Bureau for Barr, Berlex, and Wyeth-Ayerst.
1. Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol. 2002;186:1142-1149.
2. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
3. Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinyl estradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinetics. 2000;39:233-242.
4. Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21 day and 24 day oral contraceptive regimens containing gestodene (60 μg) and ethinyl estradiol (15 μg) on ovarian activity. Fertil Steril. 1999;72:115-120.
5. Croxatto HB, Urbancsek J, Massai R, Coelingh BH, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999;14:976-981.
6. Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
7. Jain J, Dutton C, Nicosia A, Wajszczuk C, Dode FR, Mishell DR. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception. 2004;70:11-18.
8. Trussell J, Ellertson C, Stewart F, Raymond EG, Shochet T. The role of emergency contraception. Am J Obstet Gynecol. 2004;190:S30-S38.
9. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
10. Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization [see comment]. Am J Obstet Gynecol. 1996;174:1161-1168.
11. Andersson K, Batar I, Rybo G. Return to fertility after removal of a levonorgestrelreleasing intrauterine device and Nova-T. Contraception. 1992;46:575-584.
12. World Health Organization. Improving Access to Quality Care in Family Planning: Medical Eligibility Criteria for Contraceptive Use. Geneva: WHO; 2000.
13. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879-883.
14. Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257-263.
15. Lahteenmaki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy [see comment]. BMJ. 1998;316:1122-1126.
16. Ness RB, Grisso JA, Vergona R, et al. Oral contraceptives, other methods of contraception, and risk reduction for ovarian cancer [see comment]. Epidemiology. 2001;12:307-312.
17. Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77:S13-S18.
18. Holt VL, Cushing-Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstet Gynecol. 2002;99:820-827.
It’s surprising to realize that the birth control pill, which launched a revolution in women’s sexuality and health, has been around for less than 50 years—especially considering the myriad of methods and products on the market today. New types of contraceptives have become available, more are on the way, and noncontraceptive benefits continue to accrue. This article reviews noteworthy changes and upcoming products.
Continuous contraception: New routes, new regimens
When it was introduced in 1960, the oral contraceptive (OC) consisted of 20 or 21 active pills followed by a pill-free interval of 7 or 8 days. No medical reason justified the pill-free interval; it was devised simply to trigger menses and reassure the woman she was not pregnant.
That pill-free interval shrank as we gained awareness of the benefits of continuous OC regimens, particularly in the treatment of endometriosis. Advantages—well documented by Sulak1—include marked reduction or elimination of menses-related symptoms such as menorrhagia, dysmenorrhea, and menstrual migraines.
Continuous OCs became “official” in 2003 with the introduction of Seasonale (ethinyl estradiol, 0.03 mg/levonorgestrel, 0.15 mg), a continuous regimen taken for 84 days, followed by a 7-day pill-free interval.2
This regimen reduces the number of periods to 4 each year.
Breakthrough bleeding diminishes over time. Although breakthrough bleeding is a common side effect of continuous oral contraception—as it is with conventional regimens—it decreases with each successive cycle of Seasonale. By the fourth cycle, it is comparable to the level reported by women on a conventional regimen. Prolonged and heavy bleeding also can be managed by discontinuing pills for 7 days.
Other methods also can be used continuously. Continuous contraception is not limited to oral contraception.
The contraceptive ring (Nuvaring; ethinyl estradiol/etonogestrel) can be left in place for 4 weeks instead of 3 without decreasing efficacy. Both hormone levels are sufficient to prevent pregnancy throughout these weeks.3
Nor is there any reason to doubt the efficacy of continuous use of the contraceptive patch (Evra; ethinyl estradiol/norelgestromin).
New regimens. A regimen of 24 active pills and a 4-day pill-free interval is available in Europe. The shorter pill-free interval allows further reduction of the active components ethinyl estradiol and gestadene. This agent contains 15 μg estradiol per pill.4
A version of Yasmin that contains only 20 μg of ethinyl estradiol, should be released in the United States later this year. It will consist of 24 active pills and a 4-day pill-free interval.
New implantable rod. Implanon, a new single-rod contraceptive implant containing etonogestrel, is available outside the United States and may become available here in the near future.5,6 It can be inserted in 1.6 minutes, removed in 2.6 minutes, and lasts 3 years. No pregnancies were reported in clinical trials.
Lower dose of Depo-Provera effective for 3 months. A new dose and route of administration for Depo-Provera (depotmedroxyprogesterone acetate) is just as effective as the 150-mg intramuscular dose.
The new dose is 104 mg administered subcutaneously every 3 months. When it becomes available in the near future, this preparation should allow women to administer the drug at home.7
Emergency contraception: A variety of methods, but still need a prescription
The different methods of emergency contraception are listed in a clip-and-save chart on page 46, which can be photocopied for convenient reference.
Trussell and colleagues’8 excellent review prefers the term emergency contraception to refer to contraceptive use after intercourse has occurred. The term “morning-after pill” is confusing because the method can be used any time after intercourse for up to 72 hours. It can even be used after 72 hours, albeit at reduced efficacy.
The Yuzpe regimen is the oldest and probably most popular form of contraception. It involves taking 2 Ovral tablets (ethinyl estradiol/levonorgestrel) followed by 2 more tablets 12 hours later. Each dose consists of 100 μg of estradiol and 0.5 mg of norgestrel.
Unfortunately, Ovral is not readily available in pharmacies. An alternative is taking enough OCs to equal the 100-μg dose of ethinyl estradiol and the 0.5 mg dose of norgestrel. For example, 5 tablets of Alesse or Levlite can be substituted for the 2 Ovral.
Efficacy is 75%. That is, if 100 women have unprotected intercourse once during the second or third week of their cycle, about 8 will become pregnant without treatment, and only 2 will become pregnant after treatment—a 75% reduction.
OCs that contain different progestins have not been studied extensively. They appear to work with lower efficacy than pills containing levonorgestrel.
- Common side effects include nausea and vomiting. Co-administration of an anti-nausea agent may be necessary. About 50% of women experience nausea and 20% vomit within 2 hours of taking a dose; some clinicians recommend repeating that dose to assure efficacy. One option is giving two 25-mg tablets of the over-the-counter drug meclizine 1 hour before combined emergency contraceptive pills; this reduces the risk of nausea by 27% and vomiting by 64%, but the risk of drowsiness doubles. Taking the medicine with a meal does not lower the rate of nausea.
- Contraindications. The only contraindication is an established pregnancy, since the drugs are taken so briefly.
Plan B Consists of 0.75 mg levonorgestrel taken within 72 hours of intercourse, followed by a second dose 12 hours later. This regimen has fewer side effects than the Yuzpe plan and may be slightly more effective. Unfortunately, the US Food and Drug Administration failed to approve over-the-counter status for this drug, so a prescription is still necessary.
IUDs. Insertion of a copper IUD (Paraguard)—but not a levonorgestrel-containing device (Mirena)—within 72 hours after intercourse is almost completely effective in preventing pregnancy. It also provides continuing contraception. Its mechanism of action is preventing implantation of a fertilized egg. Mirena has no efficacy in this regard.
“Every Woman, Every Visit,” the American College of Obstetricians and Gynecologists’ public education campaign, urges Ob/Gyns to provide advance prescriptions for emergency contraception at every office visit.
After many years of declining choices in the realm of intrauterine devices (IUDs), the Mirena levonorgestrel-containing system was released in the US market in 2000. Like the Paraguard copper IUD, the Mirena prevents pregnancy at a rate equivalent to tubal ligation. These devices last for 5 and 10 years, respectively.9,10
In the United States, interest in IUDs declined after they were associated with salpingitis and tubo-ovarian abscess. More recent epidemiological evidence indicates that IUDs do not increase the risk of infection over the general population, but the rate is higher than with other forms of contraception, which offer some protection against salpingitis. Antibiotic prophylaxis is not necessary.
The removal rate for pelvic inflammatory disease is much lower for Mirena than for the copper IUD and may be related to the low levels of levonorgestrel, which thicken cervical mucus and prevent sperm transport.9
Ectopic pregnancy rates with the Mirena are about 1/8 to 1/10 those observed in the general population. Once the Mirena device is removed, fertility returns rapidly9-11
Recommended for the chronically ill. According to the World Health Organization, IUDs are the safest form of contraception for medically complicated patients.12 Certainly, they are underutilized in this circumstance.
Noncontraceptive benefits. Slow release of low doses of levonorgestrel by Mirena reduces endometrial thickness and menstrual blood loss.13 In fact, several studies have found the Mirena to be equivalent to endometrial ablation.14 In a randomized study, two thirds of the women scheduled for hysterectomy for abnormal uterine bleeding cancelled surgery due to satisfaction with Mirena’s bleeding profile.15
Ovarian cancer also is reduced.16
Does obesity limit contraceptive efficacy?
Decreased efficacy of the contraceptive patch, observed in overweight women,17 especially those heavier than 198 lb, prompted reevaluation of other forms. In a retrospective analysis, Holt and colleagues18 found a higher pregnancy rate in women heavier than 155 lb as the estrogen dose decreased. In contrast, no pregnancies occurred in women weighing more than 198 lb in a randomized trial2 of 30 μg ethinyl estradiol/150 μg levonorgestrel (Seasonale). It is unclear why Holt failed to analyze progestin content, since it is the progestin that inhibits ovulation and prevents pregnancy.
Unfortunately, few clinical trials involve obese women. For example, the contraceptive ring was not evaluated in obese women.
Despite this shortcoming, I have not changed my prescribing of contraceptives in obese women, but await better, more convincing data. Until then, it seems wise to include a broad range of body weights in future trials.
The author serves on the Speakers Bureau for Barr, Berlex, and Wyeth-Ayerst.
It’s surprising to realize that the birth control pill, which launched a revolution in women’s sexuality and health, has been around for less than 50 years—especially considering the myriad of methods and products on the market today. New types of contraceptives have become available, more are on the way, and noncontraceptive benefits continue to accrue. This article reviews noteworthy changes and upcoming products.
Continuous contraception: New routes, new regimens
When it was introduced in 1960, the oral contraceptive (OC) consisted of 20 or 21 active pills followed by a pill-free interval of 7 or 8 days. No medical reason justified the pill-free interval; it was devised simply to trigger menses and reassure the woman she was not pregnant.
That pill-free interval shrank as we gained awareness of the benefits of continuous OC regimens, particularly in the treatment of endometriosis. Advantages—well documented by Sulak1—include marked reduction or elimination of menses-related symptoms such as menorrhagia, dysmenorrhea, and menstrual migraines.
Continuous OCs became “official” in 2003 with the introduction of Seasonale (ethinyl estradiol, 0.03 mg/levonorgestrel, 0.15 mg), a continuous regimen taken for 84 days, followed by a 7-day pill-free interval.2
This regimen reduces the number of periods to 4 each year.
Breakthrough bleeding diminishes over time. Although breakthrough bleeding is a common side effect of continuous oral contraception—as it is with conventional regimens—it decreases with each successive cycle of Seasonale. By the fourth cycle, it is comparable to the level reported by women on a conventional regimen. Prolonged and heavy bleeding also can be managed by discontinuing pills for 7 days.
Other methods also can be used continuously. Continuous contraception is not limited to oral contraception.
The contraceptive ring (Nuvaring; ethinyl estradiol/etonogestrel) can be left in place for 4 weeks instead of 3 without decreasing efficacy. Both hormone levels are sufficient to prevent pregnancy throughout these weeks.3
Nor is there any reason to doubt the efficacy of continuous use of the contraceptive patch (Evra; ethinyl estradiol/norelgestromin).
New regimens. A regimen of 24 active pills and a 4-day pill-free interval is available in Europe. The shorter pill-free interval allows further reduction of the active components ethinyl estradiol and gestadene. This agent contains 15 μg estradiol per pill.4
A version of Yasmin that contains only 20 μg of ethinyl estradiol, should be released in the United States later this year. It will consist of 24 active pills and a 4-day pill-free interval.
New implantable rod. Implanon, a new single-rod contraceptive implant containing etonogestrel, is available outside the United States and may become available here in the near future.5,6 It can be inserted in 1.6 minutes, removed in 2.6 minutes, and lasts 3 years. No pregnancies were reported in clinical trials.
Lower dose of Depo-Provera effective for 3 months. A new dose and route of administration for Depo-Provera (depotmedroxyprogesterone acetate) is just as effective as the 150-mg intramuscular dose.
The new dose is 104 mg administered subcutaneously every 3 months. When it becomes available in the near future, this preparation should allow women to administer the drug at home.7
Emergency contraception: A variety of methods, but still need a prescription
The different methods of emergency contraception are listed in a clip-and-save chart on page 46, which can be photocopied for convenient reference.
Trussell and colleagues’8 excellent review prefers the term emergency contraception to refer to contraceptive use after intercourse has occurred. The term “morning-after pill” is confusing because the method can be used any time after intercourse for up to 72 hours. It can even be used after 72 hours, albeit at reduced efficacy.
The Yuzpe regimen is the oldest and probably most popular form of contraception. It involves taking 2 Ovral tablets (ethinyl estradiol/levonorgestrel) followed by 2 more tablets 12 hours later. Each dose consists of 100 μg of estradiol and 0.5 mg of norgestrel.
Unfortunately, Ovral is not readily available in pharmacies. An alternative is taking enough OCs to equal the 100-μg dose of ethinyl estradiol and the 0.5 mg dose of norgestrel. For example, 5 tablets of Alesse or Levlite can be substituted for the 2 Ovral.
Efficacy is 75%. That is, if 100 women have unprotected intercourse once during the second or third week of their cycle, about 8 will become pregnant without treatment, and only 2 will become pregnant after treatment—a 75% reduction.
OCs that contain different progestins have not been studied extensively. They appear to work with lower efficacy than pills containing levonorgestrel.
- Common side effects include nausea and vomiting. Co-administration of an anti-nausea agent may be necessary. About 50% of women experience nausea and 20% vomit within 2 hours of taking a dose; some clinicians recommend repeating that dose to assure efficacy. One option is giving two 25-mg tablets of the over-the-counter drug meclizine 1 hour before combined emergency contraceptive pills; this reduces the risk of nausea by 27% and vomiting by 64%, but the risk of drowsiness doubles. Taking the medicine with a meal does not lower the rate of nausea.
- Contraindications. The only contraindication is an established pregnancy, since the drugs are taken so briefly.
Plan B Consists of 0.75 mg levonorgestrel taken within 72 hours of intercourse, followed by a second dose 12 hours later. This regimen has fewer side effects than the Yuzpe plan and may be slightly more effective. Unfortunately, the US Food and Drug Administration failed to approve over-the-counter status for this drug, so a prescription is still necessary.
IUDs. Insertion of a copper IUD (Paraguard)—but not a levonorgestrel-containing device (Mirena)—within 72 hours after intercourse is almost completely effective in preventing pregnancy. It also provides continuing contraception. Its mechanism of action is preventing implantation of a fertilized egg. Mirena has no efficacy in this regard.
“Every Woman, Every Visit,” the American College of Obstetricians and Gynecologists’ public education campaign, urges Ob/Gyns to provide advance prescriptions for emergency contraception at every office visit.
After many years of declining choices in the realm of intrauterine devices (IUDs), the Mirena levonorgestrel-containing system was released in the US market in 2000. Like the Paraguard copper IUD, the Mirena prevents pregnancy at a rate equivalent to tubal ligation. These devices last for 5 and 10 years, respectively.9,10
In the United States, interest in IUDs declined after they were associated with salpingitis and tubo-ovarian abscess. More recent epidemiological evidence indicates that IUDs do not increase the risk of infection over the general population, but the rate is higher than with other forms of contraception, which offer some protection against salpingitis. Antibiotic prophylaxis is not necessary.
The removal rate for pelvic inflammatory disease is much lower for Mirena than for the copper IUD and may be related to the low levels of levonorgestrel, which thicken cervical mucus and prevent sperm transport.9
Ectopic pregnancy rates with the Mirena are about 1/8 to 1/10 those observed in the general population. Once the Mirena device is removed, fertility returns rapidly9-11
Recommended for the chronically ill. According to the World Health Organization, IUDs are the safest form of contraception for medically complicated patients.12 Certainly, they are underutilized in this circumstance.
Noncontraceptive benefits. Slow release of low doses of levonorgestrel by Mirena reduces endometrial thickness and menstrual blood loss.13 In fact, several studies have found the Mirena to be equivalent to endometrial ablation.14 In a randomized study, two thirds of the women scheduled for hysterectomy for abnormal uterine bleeding cancelled surgery due to satisfaction with Mirena’s bleeding profile.15
Ovarian cancer also is reduced.16
Does obesity limit contraceptive efficacy?
Decreased efficacy of the contraceptive patch, observed in overweight women,17 especially those heavier than 198 lb, prompted reevaluation of other forms. In a retrospective analysis, Holt and colleagues18 found a higher pregnancy rate in women heavier than 155 lb as the estrogen dose decreased. In contrast, no pregnancies occurred in women weighing more than 198 lb in a randomized trial2 of 30 μg ethinyl estradiol/150 μg levonorgestrel (Seasonale). It is unclear why Holt failed to analyze progestin content, since it is the progestin that inhibits ovulation and prevents pregnancy.
Unfortunately, few clinical trials involve obese women. For example, the contraceptive ring was not evaluated in obese women.
Despite this shortcoming, I have not changed my prescribing of contraceptives in obese women, but await better, more convincing data. Until then, it seems wise to include a broad range of body weights in future trials.
The author serves on the Speakers Bureau for Barr, Berlex, and Wyeth-Ayerst.
1. Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol. 2002;186:1142-1149.
2. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
3. Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinyl estradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinetics. 2000;39:233-242.
4. Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21 day and 24 day oral contraceptive regimens containing gestodene (60 μg) and ethinyl estradiol (15 μg) on ovarian activity. Fertil Steril. 1999;72:115-120.
5. Croxatto HB, Urbancsek J, Massai R, Coelingh BH, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999;14:976-981.
6. Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
7. Jain J, Dutton C, Nicosia A, Wajszczuk C, Dode FR, Mishell DR. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception. 2004;70:11-18.
8. Trussell J, Ellertson C, Stewart F, Raymond EG, Shochet T. The role of emergency contraception. Am J Obstet Gynecol. 2004;190:S30-S38.
9. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
10. Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization [see comment]. Am J Obstet Gynecol. 1996;174:1161-1168.
11. Andersson K, Batar I, Rybo G. Return to fertility after removal of a levonorgestrelreleasing intrauterine device and Nova-T. Contraception. 1992;46:575-584.
12. World Health Organization. Improving Access to Quality Care in Family Planning: Medical Eligibility Criteria for Contraceptive Use. Geneva: WHO; 2000.
13. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879-883.
14. Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257-263.
15. Lahteenmaki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy [see comment]. BMJ. 1998;316:1122-1126.
16. Ness RB, Grisso JA, Vergona R, et al. Oral contraceptives, other methods of contraception, and risk reduction for ovarian cancer [see comment]. Epidemiology. 2001;12:307-312.
17. Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77:S13-S18.
18. Holt VL, Cushing-Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstet Gynecol. 2002;99:820-827.
1. Sulak PJ, Kuehl TJ, Ortiz M, Shull BL. Acceptance of altering the standard 21-day/7-day oral contraceptive regimen to delay menses and reduce hormone withdrawal symptoms. Am J Obstet Gynecol. 2002;186:1142-1149.
2. Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89-96.
3. Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinyl estradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinetics. 2000;39:233-242.
4. Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21 day and 24 day oral contraceptive regimens containing gestodene (60 μg) and ethinyl estradiol (15 μg) on ovarian activity. Fertil Steril. 1999;72:115-120.
5. Croxatto HB, Urbancsek J, Massai R, Coelingh BH, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999;14:976-981.
6. Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
7. Jain J, Dutton C, Nicosia A, Wajszczuk C, Dode FR, Mishell DR. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception. 2004;70:11-18.
8. Trussell J, Ellertson C, Stewart F, Raymond EG, Shochet T. The role of emergency contraception. Am J Obstet Gynecol. 2004;190:S30-S38.
9. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
10. Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of pregnancy after tubal sterilization: findings from the U.S. Collaborative Review of Sterilization [see comment]. Am J Obstet Gynecol. 1996;174:1161-1168.
11. Andersson K, Batar I, Rybo G. Return to fertility after removal of a levonorgestrelreleasing intrauterine device and Nova-T. Contraception. 1992;46:575-584.
12. World Health Organization. Improving Access to Quality Care in Family Planning: Medical Eligibility Criteria for Contraceptive Use. Geneva: WHO; 2000.
13. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879-883.
14. Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol. 1997;90:257-263.
15. Lahteenmaki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy [see comment]. BMJ. 1998;316:1122-1126.
16. Ness RB, Grisso JA, Vergona R, et al. Oral contraceptives, other methods of contraception, and risk reduction for ovarian cancer [see comment]. Epidemiology. 2001;12:307-312.
17. Zieman M, Guillebaud J, Weisberg E, Shangold GA, Fisher AC, Creasy GW. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: the analysis of pooled data. Fertil Steril. 2002;77:S13-S18.
18. Holt VL, Cushing-Haugen KL, Daling JR. Body weight and risk of oral contraceptive failure. Obstet Gynecol. 2002;99:820-827.
Managing menopause-related depression and low libido
- Depression is more likely when perimenopause exceeds 27 months and hot flashes are moderate to severe.
- All serotonin and norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors have sexual side effects, including anorgasmia and loss of libido. Gabapentin is the only psychotropic that improves hot flashes and mood without interfering with sexual function.
- If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.
- Women with androgen deficiency symptoms and low testosterone should at least be considered for testosterone replacement.
Among psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
ANNE’S CASE
Mood improves, but still no libido
You and Anne decide to try the SNRI venlafaxine, 75 mg daily, to treat her hot flashes and depression. Four weeks later, she is having only half as many hot flashes and her mood has improved (Beck Depression Inventory score of 10). She feels much better and wishes to continue the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
Treating low interest in sex
Being angry with one’s partner is the number one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.16 Other agents the FDA is reviewing for erectile dysfunction may help.
Consideration of how hormones affect female sexual desire may suggest what advice to give Anne and how to coordinate her care with a psychiatrist, if necessary. For example, the psychiatrist might treat her sexual complaints and relationship problems while the Ob/Gyn manages gynecologic symptoms.
How androgens affect sexual desire
Testosterone is the hormone of sexual desire in men and women. Other female androgens are androstenedione, androstenediol, 5α-dihydrotestosterone, dehydroepiandrosterone (DHEA), and its sulfate (DHEA-S).
Premenopausal women produce testosterone in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%); DHEA and DHEA-S are produced in the adrenal glands (95%).
Get the cardiologist’s clearance before giving testosterone to a woman with heart disease or an HDL below 45 mg/dL.
Average daily serum testosterone concentrations decline in women between ages 20 and 50. When values in women aged 20 to 29 were compared with those in women 40 to 49, they were 195.6 g/dL and 140.4 g/dL, respectively, for DHEA-S; 51.5 ng/dL and 33.7 ng/dL, respectively, for serum testosterone; and 1.51 pg/mL and 1.03 pg/mL, respectively, for free testosterone.17
Lower levels also are seen with estrogen replacement therapy, oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Total hysterectomy with bilateral oophorectomy induces a sudden 50% loss of testosterone and an 80% decline in estradiol.18
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—compared with what they had when in their 30s.19
The percentage of women reporting low libido increases with age until menopause: 30% at age 30 to 50% at age 50. The rate declines to 27% in women aged 50 to 59.20
Female androgen deficiency syndrome. After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire. Oral estrogen reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in menopausal women.21 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.22
- Symptoms. Diagnosis of female androgen deficiency syndrome23 requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
Value of testosterone replacement
Replacing testosterone can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.25 Muscle and bone stimulation and decreased hot flashes also are reported.26
Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Potential disadvantages. Patients should be informed that testosterone may lower levels of beneficial high-density lipoprotein (HDL) cholesterol. Get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level below 45 mg/dL.
A meta-analysis of 8 clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg daily of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone doses.27
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Monitoring progress. Depending on the patient’s progress through menopause, after 12 to 24 months, it may be possible to reduce the testosterone dose or to give it only 2 to 4 times per week. As estrogen levels drop off through menopause, free testosterone may rise and the increased LH drive to the ovary may increase production of ovarian testosterone.
Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone is tricky.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle third of the cycle—on cycle days 10 to 16, counting the first day of menstrual bleeding as day 1.28
- Oral contraceptives decrease androgen production by the ovary and can result in low libido in some women.29
New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.30
Restoring bioactive testosterone to the normal free androgen index range may improve low libido.
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEA-S. Measuring SHBG helps determine the free, biologically active testosterone level and helps in calculating the free androgen index in women (TABLE 2).31
TABLE 2
Free androgen index values in women, by age
| TO CALCULATE THE FREE ANDROGEN INDEX: | |
|---|---|
| Total testosterone in nmol/L (total testosterone in ng/mL x 0.0347 x 100), divided by sex hormone-binding globulin in nmol/L | |
| AGE | NORMAL RANGE |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Data from Guay et al31 | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 nmol/L (normal range, 1.78 to 2.86).
You and Anne decide to start testosterone replacement therapy. You prescribe Androgel, starting at one seventh of a 2.5-mg foil packet (about 0.35 mg daily of testosterone) and instruct her to rate her sexual energy daily, using a Sexual Energy Scale (see).
The Beck Depression Inventory-II
The questionnaire assesses level of depression by having the patient rate 21 psychological attributes. She chooses 1 of 4 graded statements for each attribute, which are assigned 0 to 3 points. The points are tallied at the end of the test for an overall view of the patient’s depression—or lack thereof.
For details, see: http://marketplace.psychcorp.com/PsychCorp.com/Cultures/en-US/default.htm
The Sexual Energy Scale
This 1-10 scale is designed to identify and follow-up patients with sexual dysfunction due to a general medical condition or substance-induced sexual dysfunction. It can be given at every visit, including at baseline, to evaluate a patient’s sexual energy level and response to therapy. In this assessment, the term “sexual energy” includes ease of arousal, sexual pleasure, orgasms, interest in sex, sexual fantasies, and sexual fulfillment.
Instructions to the patient are: “On a scale of 1 to 10, with 1 being the lowest sexual energy level you have experienced in your adult life, and 10 being the highest sexual energy level you have experienced in your adult life, rate your current energy level.”
Testosterone choices for women
Restoring a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options that I use clinically include:
Methyl testosterone sublingual pills, 0.5 mg daily, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels unless you order the very expensive test that is specific for methyl testosterone.
Two percent vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption.
Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg daily or one seventh of the 2.5-mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations, including a testosterone patch for women and a gel in female-sized doses.
Research is warranted to evaluate the benefits and safety of longer-term interventions with these therapies in women because of the large numbers of women experiencing diminished sexual interest and declining general wellbeing during their late reproductive years.32
Using the Sexual Energy Scale. At every visit, monitor therapeutic response with the Sexual Energy Scale—a scale numbered 1 to 10.33,34 Instruct her to define “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Conversely, “1” would be when she felt the worst sexually and had the least desire.
Supplemental estrogen, progestin. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS (0.625 mg esterified estrogens and 1.25 mg of methyl testosterone). Add a progestin if the patient is postmenopausal with an intact uterus.
A vaginal lubricant is not enough to defeat age-related vaginal atrophy, which can make intercourse difficult or impossible. Women with vaginal dryness need estrogen that can be applied vaginally.
ANNE’S CASE
Libido improves somewhat
Anne returns in 4 weeks with gradually improving sex drive (Sexual Energy Scale score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low.
You increase the Androgel dosage to one fifth of a 2.5-mg packet (0.5 mg daily) and continue to monitor her Sexual Energy Scale ratings at monthly visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Dr. Brizendine is a speaker for Pfizer, Lilly, and Wyeth.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric. 2003;6(Suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med. 2003;348:645-650.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392-398.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279-294.
6. Seppa N. Hormone therapy falls out of favor. Science News. 2002;162:61.-
7. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
8. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
9. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798-811.
10. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839-1854.
11. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum. 2002;29:33-40.
12. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827-2834.
13. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry. 2003;64:473-479.
14. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578-1583.
15. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337-345.
16. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623-628.
17. Guay AR, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):112-120.
18. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5:357-365.
19. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol. 2003;85(2–5):363-366.
20. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
21. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
22. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol. 1997;90:995-998.
23. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
24. Guay AJ, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B. Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):121-129.
25. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab. 2003;17:165-175.
26. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol. 2002;20:106-110.
27. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause. 1999;6:216-224.
28. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums. March 2003;8:3.-
29. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception. 1995;52:363-369.
30. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77(Suppl 4):S83-S88.
31. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther. 2002;28(Suppl 1):129-142.
32. Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
33. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull. 1997;33:761-765.
34. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction. Poster presented at: North American Society for Psychosocial Obstetrics and Gynecology; 2001; Waikoloa, Hawaii.
- Depression is more likely when perimenopause exceeds 27 months and hot flashes are moderate to severe.
- All serotonin and norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors have sexual side effects, including anorgasmia and loss of libido. Gabapentin is the only psychotropic that improves hot flashes and mood without interfering with sexual function.
- If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.
- Women with androgen deficiency symptoms and low testosterone should at least be considered for testosterone replacement.
Among psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
ANNE’S CASE
Mood improves, but still no libido
You and Anne decide to try the SNRI venlafaxine, 75 mg daily, to treat her hot flashes and depression. Four weeks later, she is having only half as many hot flashes and her mood has improved (Beck Depression Inventory score of 10). She feels much better and wishes to continue the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
Treating low interest in sex
Being angry with one’s partner is the number one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.16 Other agents the FDA is reviewing for erectile dysfunction may help.
Consideration of how hormones affect female sexual desire may suggest what advice to give Anne and how to coordinate her care with a psychiatrist, if necessary. For example, the psychiatrist might treat her sexual complaints and relationship problems while the Ob/Gyn manages gynecologic symptoms.
How androgens affect sexual desire
Testosterone is the hormone of sexual desire in men and women. Other female androgens are androstenedione, androstenediol, 5α-dihydrotestosterone, dehydroepiandrosterone (DHEA), and its sulfate (DHEA-S).
Premenopausal women produce testosterone in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%); DHEA and DHEA-S are produced in the adrenal glands (95%).
Get the cardiologist’s clearance before giving testosterone to a woman with heart disease or an HDL below 45 mg/dL.
Average daily serum testosterone concentrations decline in women between ages 20 and 50. When values in women aged 20 to 29 were compared with those in women 40 to 49, they were 195.6 g/dL and 140.4 g/dL, respectively, for DHEA-S; 51.5 ng/dL and 33.7 ng/dL, respectively, for serum testosterone; and 1.51 pg/mL and 1.03 pg/mL, respectively, for free testosterone.17
Lower levels also are seen with estrogen replacement therapy, oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Total hysterectomy with bilateral oophorectomy induces a sudden 50% loss of testosterone and an 80% decline in estradiol.18
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—compared with what they had when in their 30s.19
The percentage of women reporting low libido increases with age until menopause: 30% at age 30 to 50% at age 50. The rate declines to 27% in women aged 50 to 59.20
Female androgen deficiency syndrome. After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire. Oral estrogen reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in menopausal women.21 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.22
- Symptoms. Diagnosis of female androgen deficiency syndrome23 requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
Value of testosterone replacement
Replacing testosterone can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.25 Muscle and bone stimulation and decreased hot flashes also are reported.26
Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Potential disadvantages. Patients should be informed that testosterone may lower levels of beneficial high-density lipoprotein (HDL) cholesterol. Get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level below 45 mg/dL.
A meta-analysis of 8 clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg daily of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone doses.27
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Monitoring progress. Depending on the patient’s progress through menopause, after 12 to 24 months, it may be possible to reduce the testosterone dose or to give it only 2 to 4 times per week. As estrogen levels drop off through menopause, free testosterone may rise and the increased LH drive to the ovary may increase production of ovarian testosterone.
Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone is tricky.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle third of the cycle—on cycle days 10 to 16, counting the first day of menstrual bleeding as day 1.28
- Oral contraceptives decrease androgen production by the ovary and can result in low libido in some women.29
New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.30
Restoring bioactive testosterone to the normal free androgen index range may improve low libido.
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEA-S. Measuring SHBG helps determine the free, biologically active testosterone level and helps in calculating the free androgen index in women (TABLE 2).31
TABLE 2
Free androgen index values in women, by age
| TO CALCULATE THE FREE ANDROGEN INDEX: | |
|---|---|
| Total testosterone in nmol/L (total testosterone in ng/mL x 0.0347 x 100), divided by sex hormone-binding globulin in nmol/L | |
| AGE | NORMAL RANGE |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Data from Guay et al31 | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 nmol/L (normal range, 1.78 to 2.86).
You and Anne decide to start testosterone replacement therapy. You prescribe Androgel, starting at one seventh of a 2.5-mg foil packet (about 0.35 mg daily of testosterone) and instruct her to rate her sexual energy daily, using a Sexual Energy Scale (see).
The Beck Depression Inventory-II
The questionnaire assesses level of depression by having the patient rate 21 psychological attributes. She chooses 1 of 4 graded statements for each attribute, which are assigned 0 to 3 points. The points are tallied at the end of the test for an overall view of the patient’s depression—or lack thereof.
For details, see: http://marketplace.psychcorp.com/PsychCorp.com/Cultures/en-US/default.htm
The Sexual Energy Scale
This 1-10 scale is designed to identify and follow-up patients with sexual dysfunction due to a general medical condition or substance-induced sexual dysfunction. It can be given at every visit, including at baseline, to evaluate a patient’s sexual energy level and response to therapy. In this assessment, the term “sexual energy” includes ease of arousal, sexual pleasure, orgasms, interest in sex, sexual fantasies, and sexual fulfillment.
Instructions to the patient are: “On a scale of 1 to 10, with 1 being the lowest sexual energy level you have experienced in your adult life, and 10 being the highest sexual energy level you have experienced in your adult life, rate your current energy level.”
Testosterone choices for women
Restoring a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options that I use clinically include:
Methyl testosterone sublingual pills, 0.5 mg daily, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels unless you order the very expensive test that is specific for methyl testosterone.
Two percent vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption.
Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg daily or one seventh of the 2.5-mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations, including a testosterone patch for women and a gel in female-sized doses.
Research is warranted to evaluate the benefits and safety of longer-term interventions with these therapies in women because of the large numbers of women experiencing diminished sexual interest and declining general wellbeing during their late reproductive years.32
Using the Sexual Energy Scale. At every visit, monitor therapeutic response with the Sexual Energy Scale—a scale numbered 1 to 10.33,34 Instruct her to define “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Conversely, “1” would be when she felt the worst sexually and had the least desire.
Supplemental estrogen, progestin. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS (0.625 mg esterified estrogens and 1.25 mg of methyl testosterone). Add a progestin if the patient is postmenopausal with an intact uterus.
A vaginal lubricant is not enough to defeat age-related vaginal atrophy, which can make intercourse difficult or impossible. Women with vaginal dryness need estrogen that can be applied vaginally.
ANNE’S CASE
Libido improves somewhat
Anne returns in 4 weeks with gradually improving sex drive (Sexual Energy Scale score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low.
You increase the Androgel dosage to one fifth of a 2.5-mg packet (0.5 mg daily) and continue to monitor her Sexual Energy Scale ratings at monthly visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Dr. Brizendine is a speaker for Pfizer, Lilly, and Wyeth.
- Depression is more likely when perimenopause exceeds 27 months and hot flashes are moderate to severe.
- All serotonin and norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors have sexual side effects, including anorgasmia and loss of libido. Gabapentin is the only psychotropic that improves hot flashes and mood without interfering with sexual function.
- If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.
- Women with androgen deficiency symptoms and low testosterone should at least be considered for testosterone replacement.
Among psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
ANNE’S CASE
Mood improves, but still no libido
You and Anne decide to try the SNRI venlafaxine, 75 mg daily, to treat her hot flashes and depression. Four weeks later, she is having only half as many hot flashes and her mood has improved (Beck Depression Inventory score of 10). She feels much better and wishes to continue the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
Treating low interest in sex
Being angry with one’s partner is the number one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.16 Other agents the FDA is reviewing for erectile dysfunction may help.
Consideration of how hormones affect female sexual desire may suggest what advice to give Anne and how to coordinate her care with a psychiatrist, if necessary. For example, the psychiatrist might treat her sexual complaints and relationship problems while the Ob/Gyn manages gynecologic symptoms.
How androgens affect sexual desire
Testosterone is the hormone of sexual desire in men and women. Other female androgens are androstenedione, androstenediol, 5α-dihydrotestosterone, dehydroepiandrosterone (DHEA), and its sulfate (DHEA-S).
Premenopausal women produce testosterone in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%); DHEA and DHEA-S are produced in the adrenal glands (95%).
Get the cardiologist’s clearance before giving testosterone to a woman with heart disease or an HDL below 45 mg/dL.
Average daily serum testosterone concentrations decline in women between ages 20 and 50. When values in women aged 20 to 29 were compared with those in women 40 to 49, they were 195.6 g/dL and 140.4 g/dL, respectively, for DHEA-S; 51.5 ng/dL and 33.7 ng/dL, respectively, for serum testosterone; and 1.51 pg/mL and 1.03 pg/mL, respectively, for free testosterone.17
Lower levels also are seen with estrogen replacement therapy, oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Total hysterectomy with bilateral oophorectomy induces a sudden 50% loss of testosterone and an 80% decline in estradiol.18
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—compared with what they had when in their 30s.19
The percentage of women reporting low libido increases with age until menopause: 30% at age 30 to 50% at age 50. The rate declines to 27% in women aged 50 to 59.20
Female androgen deficiency syndrome. After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire. Oral estrogen reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in menopausal women.21 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.22
- Symptoms. Diagnosis of female androgen deficiency syndrome23 requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
Value of testosterone replacement
Replacing testosterone can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.25 Muscle and bone stimulation and decreased hot flashes also are reported.26
Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Potential disadvantages. Patients should be informed that testosterone may lower levels of beneficial high-density lipoprotein (HDL) cholesterol. Get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level below 45 mg/dL.
A meta-analysis of 8 clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg daily of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone doses.27
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Monitoring progress. Depending on the patient’s progress through menopause, after 12 to 24 months, it may be possible to reduce the testosterone dose or to give it only 2 to 4 times per week. As estrogen levels drop off through menopause, free testosterone may rise and the increased LH drive to the ovary may increase production of ovarian testosterone.
Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone is tricky.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle third of the cycle—on cycle days 10 to 16, counting the first day of menstrual bleeding as day 1.28
- Oral contraceptives decrease androgen production by the ovary and can result in low libido in some women.29
New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.30
Restoring bioactive testosterone to the normal free androgen index range may improve low libido.
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEA-S. Measuring SHBG helps determine the free, biologically active testosterone level and helps in calculating the free androgen index in women (TABLE 2).31
TABLE 2
Free androgen index values in women, by age
| TO CALCULATE THE FREE ANDROGEN INDEX: | |
|---|---|
| Total testosterone in nmol/L (total testosterone in ng/mL x 0.0347 x 100), divided by sex hormone-binding globulin in nmol/L | |
| AGE | NORMAL RANGE |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Data from Guay et al31 | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 nmol/L (normal range, 1.78 to 2.86).
You and Anne decide to start testosterone replacement therapy. You prescribe Androgel, starting at one seventh of a 2.5-mg foil packet (about 0.35 mg daily of testosterone) and instruct her to rate her sexual energy daily, using a Sexual Energy Scale (see).
The Beck Depression Inventory-II
The questionnaire assesses level of depression by having the patient rate 21 psychological attributes. She chooses 1 of 4 graded statements for each attribute, which are assigned 0 to 3 points. The points are tallied at the end of the test for an overall view of the patient’s depression—or lack thereof.
For details, see: http://marketplace.psychcorp.com/PsychCorp.com/Cultures/en-US/default.htm
The Sexual Energy Scale
This 1-10 scale is designed to identify and follow-up patients with sexual dysfunction due to a general medical condition or substance-induced sexual dysfunction. It can be given at every visit, including at baseline, to evaluate a patient’s sexual energy level and response to therapy. In this assessment, the term “sexual energy” includes ease of arousal, sexual pleasure, orgasms, interest in sex, sexual fantasies, and sexual fulfillment.
Instructions to the patient are: “On a scale of 1 to 10, with 1 being the lowest sexual energy level you have experienced in your adult life, and 10 being the highest sexual energy level you have experienced in your adult life, rate your current energy level.”
Testosterone choices for women
Restoring a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options that I use clinically include:
Methyl testosterone sublingual pills, 0.5 mg daily, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels unless you order the very expensive test that is specific for methyl testosterone.
Two percent vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption.
Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg daily or one seventh of the 2.5-mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations, including a testosterone patch for women and a gel in female-sized doses.
Research is warranted to evaluate the benefits and safety of longer-term interventions with these therapies in women because of the large numbers of women experiencing diminished sexual interest and declining general wellbeing during their late reproductive years.32
Using the Sexual Energy Scale. At every visit, monitor therapeutic response with the Sexual Energy Scale—a scale numbered 1 to 10.33,34 Instruct her to define “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Conversely, “1” would be when she felt the worst sexually and had the least desire.
Supplemental estrogen, progestin. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS (0.625 mg esterified estrogens and 1.25 mg of methyl testosterone). Add a progestin if the patient is postmenopausal with an intact uterus.
A vaginal lubricant is not enough to defeat age-related vaginal atrophy, which can make intercourse difficult or impossible. Women with vaginal dryness need estrogen that can be applied vaginally.
ANNE’S CASE
Libido improves somewhat
Anne returns in 4 weeks with gradually improving sex drive (Sexual Energy Scale score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low.
You increase the Androgel dosage to one fifth of a 2.5-mg packet (0.5 mg daily) and continue to monitor her Sexual Energy Scale ratings at monthly visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Dr. Brizendine is a speaker for Pfizer, Lilly, and Wyeth.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric. 2003;6(Suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med. 2003;348:645-650.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392-398.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279-294.
6. Seppa N. Hormone therapy falls out of favor. Science News. 2002;162:61.-
7. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
8. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
9. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798-811.
10. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839-1854.
11. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum. 2002;29:33-40.
12. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827-2834.
13. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry. 2003;64:473-479.
14. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578-1583.
15. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337-345.
16. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623-628.
17. Guay AR, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):112-120.
18. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5:357-365.
19. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol. 2003;85(2–5):363-366.
20. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
21. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
22. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol. 1997;90:995-998.
23. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
24. Guay AJ, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B. Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):121-129.
25. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab. 2003;17:165-175.
26. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol. 2002;20:106-110.
27. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause. 1999;6:216-224.
28. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums. March 2003;8:3.-
29. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception. 1995;52:363-369.
30. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77(Suppl 4):S83-S88.
31. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther. 2002;28(Suppl 1):129-142.
32. Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
33. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull. 1997;33:761-765.
34. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction. Poster presented at: North American Society for Psychosocial Obstetrics and Gynecology; 2001; Waikoloa, Hawaii.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric. 2003;6(Suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med. 2003;348:645-650.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9:392-398.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279-294.
6. Seppa N. Hormone therapy falls out of favor. Science News. 2002;162:61.-
7. Treatment of menopause-associated vasomotor symptoms: position statement of the North American Menopause Society. Menopause. 2004;11:11-33.
8. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am. 2003;32:325-336.
9. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798-811.
10. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839-1854.
11. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum. 2002;29:33-40.
12. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003;289:2827-2834.
13. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry. 2003;64:473-479.
14. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578-1583.
15. Guttuso T Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337-345.
16. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG. 2001;108:623-628.
17. Guay AR, Munarriz R, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part A. Serum androgen levels in women aged 20-49 years with no complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):112-120.
18. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric. 2002;5:357-365.
19. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol. 2003;85(2–5):363-366.
20. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537-544.
21. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril. 2003;79:1341-1352.
22. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol. 1997;90:995-998.
23. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660-665.
24. Guay AJ, Jacobson J, et al. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B. Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 2004;16(2):121-129.
25. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab. 2003;17:165-175.
26. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol. 2002;20:106-110.
27. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause. 1999;6:216-224.
28. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums. March 2003;8:3.-
29. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception. 1995;52:363-369.
30. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 2002;77(Suppl 4):S83-S88.
31. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther. 2002;28(Suppl 1):129-142.
32. Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10:390-398.
33. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull. 1997;33:761-765.
34. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction. Poster presented at: North American Society for Psychosocial Obstetrics and Gynecology; 2001; Waikoloa, Hawaii.
Nausea and vomiting of pregnancy: Q&A with T. Murphy Goodwin
- About 35% of gravidas have nausea and vomiting severe enough to disrupt their daily routine. As many as 50% of women with severe NVP are not offered antiemetic therapy, studies show.
- At any level of severity, nausea and vomiting cause psychosocial morbidity.
- Multivitamin use at the time of conception reduces the severity of nausea and vomiting.
- When drug therapy is necessary, start with 10 to 25 mg pyridoxine (vitamin B6) 3 or 4 times daily.
- If nausea and vomiting continue, add 12.5 mg doxylamine (by halving the over-the-counter sleep aid Unisom) to each dose of pyridoxine. The pyridoxine-doxylamine combination is the same formulation as the highly effective drug Bendectin, which is no longer available in the US.
Dr. Goodwin is professor of obstetrics and gynecology at the Keck School of Medicine, University of Southern California, Los Angeles.
Many patients assume they must endure nausea, vomiting
- Women do not always mention nausea and vomiting, believing they must “live with it.” Prompt intervention can stave off more severe NVP, so it makes sense for Ob/Gyns to raise the subject early.
GOODWIN: Rather than “new information,” I would call it a change in emphasis. Previously, the focus was almost exclusively on life-threatening hyperemesis gravidarum, while lesser degrees of nausea and vomiting of pregnancy went untreated or undertreated. In reality, approximately 35% of gravidas have NVP severe enough to interfere with their daily routine. Fortunately, effective therapy is available.
OBG MANAGEMENT: Some authorities believe NVP is a continuum between mild upset and hyperemesis gravidarum. How would such a continuum affect patient care?
GOODWIN: Epidemiologic studies suggest that women with mild NVP and those with hyperemesis are significantly similar, strongly supporting the notion of a continuum.2 This fact underscores the importance of treating NVP in its early stages, before it advances to hyperemesis.
My own sense is that a small group of women with hyperemesis are predisposed to it because of rare genetic disorders such as mitochondrial mutations.
OBG MANAGEMENT: Since so many women experience NVP, should obstetricians routinely ask about it, including its psychosocial effects?
GOODWIN: Yes. This is important because of the continuum we mentioned—and because many women consider NVP “normal” and may not mention it unless they are asked.
OBG MANAGEMENT: Do you think some physicians share this mindset?
GOODWIN: Undoubtedly. Studies have shown that as many as 50% of women with severe NVP are not offered antiemetic therapy.3,4 But the days of letting the condition “run its course” are past. Now the best strategy is asking about it—and intervening early.
What causes NVP?
- The factors at play in each patient help determine the best treatment. NVP is more likely with high levels of hCG and estrogen.
How would this concept affect management of NVP?
GOODWIN: Women who are susceptible to NVP because of a vestibular mechanism might respond to a medical regimen that works at that site. Still other women may have “background” gastrointestinal motility dysfunction that makes them more susceptible to NVP; in that case, therapies to treat motility abnormalities would be appropriate. Others may have a strong behavioral overlay and would be expected to respond to the kinds of treatment used for anticipatory nausea and vomiting associated with chemotherapy.
These are theoretical scenarios at present, but a new paradigm will allow new ways of thinking about therapy.
OBG MANAGEMENT: In the early 1990s, you and your colleagues6 reported your study on the link between human chorionic gonadotropin (hCG) and NVP. What is the association?
GOODWIN: A temporal association has attracted the attention of obstetricians for years. In addition, biochemical hyperthyroidism and NVP are strongly associated. Fifty percent to 70% of women with hyperemesis gravidarum have transient hyperthyroidism, with no goiter.7 (If a goiter is present, suspect primary thyroid disorder.) Because hCG causes the biochemical hyperthyroidism related to pregnancy, and because hyperthyroidism by itself rarely causes vomiting, hCG is implicated. However, it remains unclear how hCG would cause NVP—perhaps by stimulating the ovary to produce more estrogen, which causes emesis, or through some other, unknown step.
Interestingly, a number of “high-hCG” conditions are associated with increased NVP: molar pregnancy, multiple gestation, and Down’s syndrome. Conversely, trisomy 18 is a “low-hCG” condition anecdotally associated with reduced NVP.
OBG MANAGEMENT: What is estrogen’s role?
GOODWIN: Estradiol and hCG are the only 2 hormones ever found to differ when women with NVP are compared to controls. Women who get sick with estrogen exposure are much more likely to develop NVP. Some studies have found increased estradiol levels in women with NVP, compared with controls.6,8 Conversely, low estradiol levels may reduce the risk of NVP. Take smoking, for example. It is associated with lower hCG and estradiol levels, and smokers are less likely than non-smokers to have NVP.
Studies also have focused on a possible link between cytokines and hyperemesis, and between hyperemesis and tumor necrosis factor alpha.
OBG MANAGEMENT: Do these findings alter clinical management?
GOODWIN: No. The findings about etiology do not affect management at present.
NVP can be protective or perilous
- Some degree of nausea and vomiting affects the vast majority of pregnancies. Unless it is severe, however, NVP appears to have a protective effect on the fetus.
GOODWIN: About 70% to 85% of gravidas experience it.9 In the spectrum of NVP, about 50% of women experience both nausea and vomiting, 25% experience nausea alone, and 25% are unaffected.10
Hyperemesis gravidarum affects roughly 3 to 20 of every 1,000 pregnancies.
It generally is categorized according to the level of intervention required:
- Mild NVP does not affect daily life.
- Moderate NVP interferes with daily life.
- Severe NVP, or hyperemesis, requires fluid and/or nutritional support. Hyperemesis is further defined as persistent vomiting, weight loss exceeding 5% of prepregnancy weight, and significant ketonuria, with or without electrolyte disturbances. Hospitalization usually is required.
GOODWIN: NVP almost always appears before 10 weeks’ gestation. If it begins any later, it is likely the patient has a different condition associated with nausea and vomiting.
One study found that most women develop NVP at about 4 to 7 weeks’ gestation, and that it resolves at less than 10 weeks in about 30% of women, at 10 to 12 weeks in another 30%, and at 12 to 16 weeks in another 30%.10
As for diurnal changes, symptoms tend to occur with greater frequency early in the day.
OBG MANAGEMENT: Is NVP ever harmful for the fetus?
GOODWIN: It is associated with improved fetal outcomes unless hyperemesis supervenes. Then it is associated with mild fetal growth delays and rare cases of fetal death.
We lack studies of the long-term effects of severe NVP on the fetus, but starvation and weight loss in women during famine have been shown to cause many diverse problems in offspring later in adult life.
OBG MANAGEMENT: What do you make of evidence that suggests NVP plays a protective role in pregnancy?
GOODWIN: If true, it likely represents a vestigial response that is no longer beneficial—similar to thrombophilias in pregnancy, which played an important role protecting women against bleeding at childbirth but now are more of a clinical problem because of the associated thrombosis.
Prevalence. 70% to 85%.1
Presentation. About 50% of women have both nausea and vomiting, 25% have nausea only, and 25% are not affected.10
Hyperemesis gravidarum. 3 to 20 of every 1,000 pregnancies. Marked by persistent vomiting, weight loss exceeding 5% of prepregnancy weight, and significant ketonuria.
Clinical course. If NVP is going to occur, it is present before 10 weeks’ gestation. It resolves at less than 10 weeks’ gestation in about 30% of women, at 10 to 12 weeks in another 30%, and at 12 to 16 weeks in another 30%.10
Predisposing factors. History of illness with estrogen exposure, motion sickness, migraine, or hyperemesis; mother or sister with hyperemesis; female fetus; mitochondrial disorders; multiple gestation; gestational trophoblastic disease; and fetal anomalies such as Down’s syndrome and triploidy.
Undertreatment. As many as 50% of women with severe NVP are not offered therapy.3,4
Physical discomfort. Intensity and character similar to nausea and vomiting induced by cancer chemotherapy, even at milder levels of severity.11
Social and psychological impact. Reduced job efficiency, lost work time, negative impact on family relationships and mental health, decision to terminate an otherwise desired pregnancy. Women with hyperemesis are more likely to have anxiety, depression, somatization, psychoticism, and obsessive-compulsive symptoms, but return to normal after delivery.21
Fetal effects. Improved fetal outcomes unless hyperemesis supervenes. Hyperemesis with maternal weight loss is associated with mild fetal growth delays and rare cases of fetal death.
Maternal effects. Not linked to adverse effects except for hyperemesis gravidarum, in which Wernicke’s encephalopathy, Mallory-Weiss tears, splenic avulsion, esophageal rupture, pneumothorax, acute tubular necrosis, or peripheral neuropathy (due to vitamins B6 and B12 deficiencies) can result.
Effects on the mother and predisposing factors
- Even at milder levels, physical discomfort is considerable. Predisposing factors include a female fetus, history of hyperemesis, migraine headaches, and a tendency to develop motion sickness.
GOODWIN: Serious sequelae are limited to hyperemesis, in which case Mallory-Weiss tears, splenic avulsion, esophageal rupture, pneumothorax, acute tubular necrosis, peripheral neuropathy (due to deficiencies of vitamins B6and B12), or Wernicke’s encephalopathy can result. This last condition is the most serious potential complication, and it can be difficult to recognize. Look for signs of confusion, memory loss, and blunted affect.
Even at milder levels of NVP, however, physical discomfort can be considerable. One prospective study using the McGill Nausea Questionnaire found the nausea experienced by pregnant women to be similar in character and intensity to that experienced by cancer patients on chemotherapy.11
OBG MANAGEMENT: Are there predisposing factors?
GOODWIN: Yes. They include a history of illness upon exposure to estrogen (for example, oral contraceptives), as well as a history of motion sickness and migraine.
Hyperemesis is more likely when the fetus is female. Women who had hyperemesis in a previous pregnancy are likely to experience it again in their next gestation, and their daughters and sisters also are more likely to develop it.
The most widely prescribed drug for nausea and vomiting of pregnancy (NVP), Bendectin, was voluntarily withdrawn from the US market in 1982, after numerous, unsuccessful lawsuits alleged it had caused birth defects.
After its withdrawal, hospitalization rates for NVP doubled while solid evidence of Bendectin’s safety continued to accumulate. In the years since, researchers have found no difference in major malformations between infants in the general population and those born to women who took Bendectin. Nor has there been any decrease in specific malformations. As the New England Journal of Medicine pointed out, a decrease in the number of malformations “would be expected for a truly teratogenic drug estimated to have been used by up to 40% of pregnant women at one time.”18
The drug is still available in Canada, where it is marketed as Diclectin. In the US, its ingredients—pyridoxine (vitamin B6) and doxylamine—can be compounded by a pharmacy, or the patient can be instructed to take 10 to 25 mg of pyridoxine and 12.5 mg of Unisom (half a tablet) 3 or 4 times daily. (Unisom is an over-the-counter sleep aid containing doxylamine succinate.)
Predisposing factors also include pregnancy-related conditions: multiple gestation; gestational trophoblastic disease; and fetal anomalies such as triploidy, Down’s syndrome, and hydrops fetalis. Overall, the chance of fetal defects associated with hyperemesis is extremely small.
The work-up: key signs and tests
- In severe cases, look for Wernicke’s encephalopathy, dehydration, weight loss, other causes of nausea and vomiting, and abnormal lab values.
GOODWIN: Physical assessment is necessary only in severe cases. If nausea and vomiting last more than 3 weeks, signs of Wernicke’s encephalopathy should be sought (this condition is never reported as early as 8 weeks).
Other important signs to look for are dehydration and evidence of other diseases that can cause nausea and vomiting (TABLE). If the patient has experienced vomiting throughout her pregnancy, but it suddenly becomes acute, another condition may be responsible.
It also is important to be aware that abdominal pain, fever, and headache do not represent NVP, but usually reflect other conditions associated with nausea and vomiting.
I ask about the duration and severity of NVP, and whether the woman has lost weight as a result. Weight loss is very important: Women who can’t sustain their weight need nutritional therapy.
OBG MANAGEMENT: Do you order lab tests?
GOODWIN: Yes. In severe cases, I get a liver panel and check amylase, lipase, and electrolytes. A number of abnormalities have been documented when hyperemesis is present. They include elevated liver enzymes, serum bilirubin, and serum amylase or lipase measurements, as well as increased free thyroxine and suppressed thyroid-stimulating hormone.
Serum hCG measurements are generally not useful, however, in exploring whether the patient’s vomiting is caused by hyperemesis gravidarum.
Imaging is necessary only to check for predisposing causes such as twins or molar gestation.
TABLE
Differential diagnosis of nausea and vomiting of pregnancy
| Gastrointestinal disorders |
| Appendicitis |
| Biliary tract disease |
| Esophagitis |
| Gastroenteritis |
| Gastroparesis |
| Hepatitis |
| Intestinal obstruction |
| Pancreatitis |
| Peptic ulcer disease |
| Genitourinary disorders |
| Acute renal failure |
| Degenerating fibroid |
| Nephrolithiasis |
| Pyelonephritis |
| Torsion |
| Metabolic disorders |
| Addison’s disease |
| Diabetic ketoacidosis |
| Hyperparathyroidism |
| Hyperthyroidism |
| Porphyria |
| Pregnancy-related |
| Acute fatty liver of pregnancy |
| Preeclampsia |
| Pregnancy-induced hypertension |
| Miscellaneous |
| Central nervous system lesions |
| Drug toxicity/side effects |
| Eating disorder |
| Migraine |
| Pseudotumor cerebri |
| Vestibular lesions |
| Adapted from Goodwin TM21 |
Do vitamins, rest, diet, ginger, or acupuncture help?
- Vitamins may help prevent NVP. For mild NVP, simple lifestyle changes or alternative remedies may suffice.
GOODWIN: The first step in this case should have been prevention. Two studies have found that multivitamins given at conception help reduce the severity of NVP.13,14
As for lifestyle changes, I would recommend rest and instruct the patient to avoid foods, activities, and other stimuli that exacerbate symptoms. Small, frequent meals are usually suggested to take the place of 3 large daily meals.
Scientifically, however, almost nothing is known about these common recommendations, though 1 study showed protein liquid meals reduced nausea and gastric-motility abnormalities more than carbohydrate or fatty meals with the same caloric content.15
OBG MANAGEMENT: Do you ever recommend alternative remedies such as ginger powder or stimulation of the P6 acupuncture point (eg, via acupuncture, Sea-Band, or ReliefBand)?
GOODWIN: As the ACOG guidelines point out, they are worth a try. When the patient wants to take ginger, I recommend 250 mg by mouth 3 to 4 times daily.
When to start drug therapy
- Pyridoxine (vitamin B6) is the first choice, followed by a combination of pyridoxine and doxylamine, which together form the drug Bendectin (no longer available in the US).
GOODWIN: When symptoms interfere with daily life and the remedies already mentioned fail or patients choose not to use them. In such cases, pyridoxine (vitamin B6) is my first choice. I recommend 10 to 25 mg 3 or 4 times daily. In 1 randomized, controlled trial,16 25 mg of pyridoxine every 8 hours led to a significant reduction in severe vomiting. In another, 10 mg of pyridoxine every 8 hours decreased both nausea and vomiting compared to placebo.17
When pyridoxine alone fails to ease NVP, I add doxylamine (see “Stepwise drug treatment of nausea and vomiting of pregnancy”). When it was commercially available, Bendectin contained the pyridoxine-doxylamine combination (10 mg of each) in 1 pill and was the most commonly prescribed agent, but it is no longer offered in the US. In Canada, it is sold as Diclectin.
The teratogenicity of Bendectin has never been proven despite extensive study, and a 1998 review described the agent’s safety.
Although the manufacturer voluntarily removed the drug in the early 1980s because of lawsuits alleging birth defects, teratogenicity has never been proven despite extensive study. A 1998 review described Diclectin’s safety.18
Fortunately, the combination of pyridoxine and doxylamine is still available—though not in a single pill. Some pharmacies will compound it, or the patient can be given pyridoxine in combination with the over-the-counter sleep aid Unisom, which contains 25 mg doxylamine succinate per tablet.
If pyridoxine alone is ineffective, I generally add 12.5 mg of doxylamine (half a Unisom tablet) to each dose. The patient should be instructed to buy Unisom in tablet form, rather than gel caps, as the active ingredient in the latter is not doxylamine.
Before starting drug therapy, rule out other causes of nausea and vomiting.
STEP 1
Try monotherapy. Start with pyridoxine (vitamin B6), 10 to 25 mg, 3 or 4 times daily.
STEP 2
Add doxylamine (Unisom tablet), 12.5 mg, 3 or 4 times daily, and adjust dosage as necessary according to severity of symptoms. (Note: Half of a 25-mg Unisom tablet = 12.5 mg.)
STEP 3
Add promethazine (Phenergan), 12.5 to 25 mg every 4 hours, orally or rectally, or dimenhydrinate (Dramamine), 50 to 100 mg every 4 to 6 hours, orally or rectally. Not to exceed 400 mg/day; if the patient is taking doxylamine, limit to 200 mg/day.
STEP 4
If the patient is sufficiently hydrated, add any of the following (listed alphabetically):
metoclopramide (Reglan), 5 to 10 mg every 8 hours, intramuscularly or orally, or promethazine, 12.5 to 25 mg every 4 hours, intramuscularly, orally, or rectally, or trimethobenzamide (Tigan), 200 mg every 6 to 8 hours, rectally.
If the patient is dehydrated, give intravenous fluids. For women who require intravenous hydration and have been vomiting for 3 or more weeks, intravenous thiamine, 100 mg daily for 2 to 3 days, followed by intravenous multivitamins, is recommended. (No study has compared different fluid replacements for NVP.) and add any of the following intravenous agents (listed alphabetically): dimenhydrinate, 50 mg (in 50 mL saline over 20 minutes) every 4 to 6 hours, or metoclopramide, 5 to 10 mg every 8 hours, or promethazine, 12.5 to 25 mg every 4 hours.
STEP 5
In refractory cases: Add methylprednisolone (Medrol), 16 mg every 8 hours, orally or intravenously, for 3 days. Taper over 2 weeks to the lowest effective dose. Limit total therapy to 6 weeks. (If given in the first 10 weeks of gestation, corticosteroids may increase risk for oral clefts.) or add ondansetron (Zofran), 8 mg over 15 minutes, every 12 hours, intravenously. Safety, particularly in first trimester, not determined. (Used mainly for emesis; less effect on nausea.)
AT ANY STEP
- Consider parenteral nutrition for dehydration or persistent weight loss. Stop nutrition once the patient achieves relief.
- Consider alternative therapies such as ginger capsules (250 mg 3 times daily) and stimulation of the P6 acupuncture point (via wrist bands or acustimulation).
Adapted from Goodwin TM and American College of Obstetricians and Gynecologists1
Adding other drugs
- When pyridoxine is insufficient (alone or in combination with doxylamine), add promethazine, dimenhydrinate, or another agent.
GOODWIN: The safety of antihistamines for use in pregnancy is well established. There are fewer data for phenothiazines, but they also appear to be quite safe.
As for 5-HT3-receptor antagonists, no teratogenicity has been found for ondansetron, which is used to treat nausea and vomiting associated with chemotherapy. However, in the only randomized trial, it did not appear to offer benefit over promethazine (a drug with characteristics of both antihistamines and phenothiazines and a good safety profile). Another drug, granisetron, has not been studied in regard to NVP.
OBG MANAGEMENT: What drugs do you prescribe besides pyridoxine and doxylamine?
GOODWIN: If the combination of pyridoxine and doxylamine fails to provide relief, I add 12.5 to 25 mg of promethazine every 4 hours (orally or rectally) or 50 to 100 mg of dimenhydrinate every 4 to 6 hours (orally or rectally, but not exceeding 200 mg daily when the patient is also taking doxylamine).
If this regimen fails to ease the patient’s symptoms, other combinations can be suggested.
OBG MANAGEMENT: Any caveats?
GOODWIN: I never give droperidol, a butyrophenone, because the US Food and Drug Administration has warned of an association with cardiac arrhythmias. This linkage may be overstated, but since there are safe alternatives, I avoid this agent.
Last resort: corticosteroids
- Corticosteroids may help resolve refractory hyperemesis gravidarum.
GOODWIN: They do not appear to be teratogenic unless they are given during the first 10 weeks of gestation; then they are associated with a slightly increased risk of oral clefts.19 Even so, they should be a last resort—and then only in refractory cases involving weight loss and the need for enteral or parenteral nutrition. The literature is mixed on the efficacy, but I find that a subset of patients have a dramatic response to corticosteroids.
The usual regimen is 16 mg methylprednisolone 3 times daily for 3 days, followed by tapering over 2 weeks, declining in 4-mg increments. If vomiting occurs during the taper, increase the dose by 4 mg for 1 week, then continue tapering. If vomiting recurs after tapering, restart the drug at 8 to 12 mg/day. All told, therapy should be limited to 4 to 6 weeks.
If there is no improvement during the initial 3 days of therapy, stop the drug altogether.
The steroid can be given orally or intravenously. It also is important to give 1,200 mg daily of calcium throughout the regimen.
Hydration and nutrition for hyperemesis
- Give fluids and nutritional support to maintain weight.
The most extreme psychosocial effect of NVP is the decision to terminate an otherwise desired pregnancy.
GOODWIN: Yes. If she is dehydrated, I replace fluids, taking care to give intravenous thiamine before dextrose if she has been vomiting longer than 3 weeks. Otherwise, Wernicke’s encephalopathy may develop.
I prefer enteral nutrition, provided the patient can tolerate it and a good nutrition team is available to support both physician and patient.
Generally, hospitalization is warranted for women who cannot maintain hydration or nutrition or when the patient and her family cannot cope with the condition. However, depending on community resources, outpatient hydration/nutrition may be a feasible option.
When she is able to tolerate oral hydration and maintain weight—with or without nutritional support—she can be discharged.
Crippling psychosocial effects
- Acknowledging NVP’s disruptive effect—at all levels of severity—is critical. Let her know you aren’t minimizing its impact, and that a range of options are at her disposal.
GOODWIN: Yes. In some cases, it can be crippling. Unfortunately, this dimension is rarely addressed by the physician. Effects can include reduced job efficiency, lost work time (in 1 study, a mean of 62 hours10), and a negative impact on family relationships and mental health. The most extreme effect, of course, is the decision to terminate an otherwise desired pregnancy.
These effects are not limited to hyperemesis; NVP involves psychosocial morbidity at all levels of severity.3 One study concluded that the severity of NVP fails to adequately reflect the distress it causes.3
The patient’s sense of her condition is critical. It is important to find out what effects NVP is having on her daily routine and let her know you aren’t minimizing its impact—also that a range of options are at her disposal.
OBG MANAGEMENT: In the past, haven’t a number of psychodynamic causes been proposed for NVP?
GOODWIN: Yes. These included speculation that the woman was subconsciously rejecting the pregnancy, “hysterical,” maternally dependent or too independent, or denying her femininity. Fortunately, these ideas have been discredited.
Severe NVP does have psychological effects, however. Simpson et al20 found that women with hyperemesis gravidarum were more likely to experience anxiety, depression, somatization, psychoticism, and symptoms of obsessive-compulsive disorder. After delivery, however, they returned to normal and were no more likely than other nonpregnant women to experience these conditions.
NVP is not more likely to occur during an unwanted pregnancy. The rates are about the same as for desired gestations.
OBG MANAGEMENT: Is NVP ever the result of a psychologic condition?
GOODWIN: Very rarely, although a behavioral component may be involved—eg, vomiting as a conditioned response. However, this does not mean that the patient has a disease or is responsible for her own condition.
That kind of thinking is one reason women hesitate to raise the subject.
Dr. Goodwin reports no financial relationships relevant to this article.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Nausea and Vomiting of Pregnancy. Clinical management guidelines for obstetrician-gynecologists. No. 52. Washington, DC: ACOG: April 2004.
2. Klebanoff M, Koslone P, Kaslow R, Rhoads G. Epidemiology of vomiting in early pregnancy. Obstet Gynecol. 1985;66:612-616.
3. Mazzotta P, Stewart D, Atanackovic G, Koren G, Magee LA. Psychosocial morbidity among women with nausea and vomiting of pregnancy: prevalence and association with antiemetic therapy. J Psychosom Obstet Gynaecol. 2000;21:129-136.
4. Mazzota P, Magee L, Koren G. Therapeutic abortions due to severe morning sickness. Unacceptable combination. Can Fam Physician. 1997;43:1055-1057.
5. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol. 2002;186:S184-S189.
6. Goodwin TM, Montoro M, Mestman JH, Pekary AE, Hershman JM. The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab. 1992;75:1333-1337.
7. Goodwin TM, Hershman JM. Hyperthyroidism due to inappropriate production of human chorionic gonadotropin. Clin Obstet Gynecol. 1997;40:32-44.
8. Depue RH, Bernstein L, Ross RK, et al. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and other maternal factors: a seroepidemiologic study. Am J Obstet Gynecol. 1987;156:1137-1141.
9. Vellacott ID, Cooke EJ, James CE. Nausea and vomiting in early pregnancy. Int J Gynaecol Obstet. 1988;27:57-62.
10. Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. Br J Gen Prac. 1993;43:245-248.
11. Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182:931-937.
12. Boles RG, Williams JC. Mitochondrial disease and cyclic vomiting syndrome. Dig Dis Sci. 1999;44:103S-107S.
13. Czeizel AE, Dudas I, Fritz G, Tecsoi A, Hanck A, Kunovits G. The effect of periconceptional multivitamin-mineral supplementation on vertigo, nausea and vomiting in the first trimester of pregnancy. Arch Gynecol Obstet. 1992;251:181-185.
14. Emelianova S, Mazzotta P, Einarson A, Koren G. Prevalence and severity of nausea and vomiting of pregnancy and effect of vitamin supplementation. Clin Invest Med. 1999;22(3):106-110.
15. Jednak MA, Shadigian EM, Kim MS, et al. Protein meals reduce nausea and gastric slow wave dysrhythmic activity in first trimester pregnancy. Am J Physiol. 1999;277:G855-G861.
16. Sahakian V, Rouse D, Sipes S, Rose N, Niebyl J. Vitamin B6 is effective therapy for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled study. Obstet Gynecol. 1991;78:33-36.
17. Vutyavanich T, Wongtrangan S, Ruangsri R. Pyridoxine for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1995;173:881-884.
18. Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128-1137.
19. Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
20. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med. 2001;10:471-477.
21. Goodwin TM. Hyperemesis gravidarum. Clin Obstet Gynecol. 1998;41:597-605.
- About 35% of gravidas have nausea and vomiting severe enough to disrupt their daily routine. As many as 50% of women with severe NVP are not offered antiemetic therapy, studies show.
- At any level of severity, nausea and vomiting cause psychosocial morbidity.
- Multivitamin use at the time of conception reduces the severity of nausea and vomiting.
- When drug therapy is necessary, start with 10 to 25 mg pyridoxine (vitamin B6) 3 or 4 times daily.
- If nausea and vomiting continue, add 12.5 mg doxylamine (by halving the over-the-counter sleep aid Unisom) to each dose of pyridoxine. The pyridoxine-doxylamine combination is the same formulation as the highly effective drug Bendectin, which is no longer available in the US.
Dr. Goodwin is professor of obstetrics and gynecology at the Keck School of Medicine, University of Southern California, Los Angeles.
Many patients assume they must endure nausea, vomiting
- Women do not always mention nausea and vomiting, believing they must “live with it.” Prompt intervention can stave off more severe NVP, so it makes sense for Ob/Gyns to raise the subject early.
GOODWIN: Rather than “new information,” I would call it a change in emphasis. Previously, the focus was almost exclusively on life-threatening hyperemesis gravidarum, while lesser degrees of nausea and vomiting of pregnancy went untreated or undertreated. In reality, approximately 35% of gravidas have NVP severe enough to interfere with their daily routine. Fortunately, effective therapy is available.
OBG MANAGEMENT: Some authorities believe NVP is a continuum between mild upset and hyperemesis gravidarum. How would such a continuum affect patient care?
GOODWIN: Epidemiologic studies suggest that women with mild NVP and those with hyperemesis are significantly similar, strongly supporting the notion of a continuum.2 This fact underscores the importance of treating NVP in its early stages, before it advances to hyperemesis.
My own sense is that a small group of women with hyperemesis are predisposed to it because of rare genetic disorders such as mitochondrial mutations.
OBG MANAGEMENT: Since so many women experience NVP, should obstetricians routinely ask about it, including its psychosocial effects?
GOODWIN: Yes. This is important because of the continuum we mentioned—and because many women consider NVP “normal” and may not mention it unless they are asked.
OBG MANAGEMENT: Do you think some physicians share this mindset?
GOODWIN: Undoubtedly. Studies have shown that as many as 50% of women with severe NVP are not offered antiemetic therapy.3,4 But the days of letting the condition “run its course” are past. Now the best strategy is asking about it—and intervening early.
What causes NVP?
- The factors at play in each patient help determine the best treatment. NVP is more likely with high levels of hCG and estrogen.
How would this concept affect management of NVP?
GOODWIN: Women who are susceptible to NVP because of a vestibular mechanism might respond to a medical regimen that works at that site. Still other women may have “background” gastrointestinal motility dysfunction that makes them more susceptible to NVP; in that case, therapies to treat motility abnormalities would be appropriate. Others may have a strong behavioral overlay and would be expected to respond to the kinds of treatment used for anticipatory nausea and vomiting associated with chemotherapy.
These are theoretical scenarios at present, but a new paradigm will allow new ways of thinking about therapy.
OBG MANAGEMENT: In the early 1990s, you and your colleagues6 reported your study on the link between human chorionic gonadotropin (hCG) and NVP. What is the association?
GOODWIN: A temporal association has attracted the attention of obstetricians for years. In addition, biochemical hyperthyroidism and NVP are strongly associated. Fifty percent to 70% of women with hyperemesis gravidarum have transient hyperthyroidism, with no goiter.7 (If a goiter is present, suspect primary thyroid disorder.) Because hCG causes the biochemical hyperthyroidism related to pregnancy, and because hyperthyroidism by itself rarely causes vomiting, hCG is implicated. However, it remains unclear how hCG would cause NVP—perhaps by stimulating the ovary to produce more estrogen, which causes emesis, or through some other, unknown step.
Interestingly, a number of “high-hCG” conditions are associated with increased NVP: molar pregnancy, multiple gestation, and Down’s syndrome. Conversely, trisomy 18 is a “low-hCG” condition anecdotally associated with reduced NVP.
OBG MANAGEMENT: What is estrogen’s role?
GOODWIN: Estradiol and hCG are the only 2 hormones ever found to differ when women with NVP are compared to controls. Women who get sick with estrogen exposure are much more likely to develop NVP. Some studies have found increased estradiol levels in women with NVP, compared with controls.6,8 Conversely, low estradiol levels may reduce the risk of NVP. Take smoking, for example. It is associated with lower hCG and estradiol levels, and smokers are less likely than non-smokers to have NVP.
Studies also have focused on a possible link between cytokines and hyperemesis, and between hyperemesis and tumor necrosis factor alpha.
OBG MANAGEMENT: Do these findings alter clinical management?
GOODWIN: No. The findings about etiology do not affect management at present.
NVP can be protective or perilous
- Some degree of nausea and vomiting affects the vast majority of pregnancies. Unless it is severe, however, NVP appears to have a protective effect on the fetus.
GOODWIN: About 70% to 85% of gravidas experience it.9 In the spectrum of NVP, about 50% of women experience both nausea and vomiting, 25% experience nausea alone, and 25% are unaffected.10
Hyperemesis gravidarum affects roughly 3 to 20 of every 1,000 pregnancies.
It generally is categorized according to the level of intervention required:
- Mild NVP does not affect daily life.
- Moderate NVP interferes with daily life.
- Severe NVP, or hyperemesis, requires fluid and/or nutritional support. Hyperemesis is further defined as persistent vomiting, weight loss exceeding 5% of prepregnancy weight, and significant ketonuria, with or without electrolyte disturbances. Hospitalization usually is required.
GOODWIN: NVP almost always appears before 10 weeks’ gestation. If it begins any later, it is likely the patient has a different condition associated with nausea and vomiting.
One study found that most women develop NVP at about 4 to 7 weeks’ gestation, and that it resolves at less than 10 weeks in about 30% of women, at 10 to 12 weeks in another 30%, and at 12 to 16 weeks in another 30%.10
As for diurnal changes, symptoms tend to occur with greater frequency early in the day.
OBG MANAGEMENT: Is NVP ever harmful for the fetus?
GOODWIN: It is associated with improved fetal outcomes unless hyperemesis supervenes. Then it is associated with mild fetal growth delays and rare cases of fetal death.
We lack studies of the long-term effects of severe NVP on the fetus, but starvation and weight loss in women during famine have been shown to cause many diverse problems in offspring later in adult life.
OBG MANAGEMENT: What do you make of evidence that suggests NVP plays a protective role in pregnancy?
GOODWIN: If true, it likely represents a vestigial response that is no longer beneficial—similar to thrombophilias in pregnancy, which played an important role protecting women against bleeding at childbirth but now are more of a clinical problem because of the associated thrombosis.
Prevalence. 70% to 85%.1
Presentation. About 50% of women have both nausea and vomiting, 25% have nausea only, and 25% are not affected.10
Hyperemesis gravidarum. 3 to 20 of every 1,000 pregnancies. Marked by persistent vomiting, weight loss exceeding 5% of prepregnancy weight, and significant ketonuria.
Clinical course. If NVP is going to occur, it is present before 10 weeks’ gestation. It resolves at less than 10 weeks’ gestation in about 30% of women, at 10 to 12 weeks in another 30%, and at 12 to 16 weeks in another 30%.10
Predisposing factors. History of illness with estrogen exposure, motion sickness, migraine, or hyperemesis; mother or sister with hyperemesis; female fetus; mitochondrial disorders; multiple gestation; gestational trophoblastic disease; and fetal anomalies such as Down’s syndrome and triploidy.
Undertreatment. As many as 50% of women with severe NVP are not offered therapy.3,4
Physical discomfort. Intensity and character similar to nausea and vomiting induced by cancer chemotherapy, even at milder levels of severity.11
Social and psychological impact. Reduced job efficiency, lost work time, negative impact on family relationships and mental health, decision to terminate an otherwise desired pregnancy. Women with hyperemesis are more likely to have anxiety, depression, somatization, psychoticism, and obsessive-compulsive symptoms, but return to normal after delivery.21
Fetal effects. Improved fetal outcomes unless hyperemesis supervenes. Hyperemesis with maternal weight loss is associated with mild fetal growth delays and rare cases of fetal death.
Maternal effects. Not linked to adverse effects except for hyperemesis gravidarum, in which Wernicke’s encephalopathy, Mallory-Weiss tears, splenic avulsion, esophageal rupture, pneumothorax, acute tubular necrosis, or peripheral neuropathy (due to vitamins B6 and B12 deficiencies) can result.
Effects on the mother and predisposing factors
- Even at milder levels, physical discomfort is considerable. Predisposing factors include a female fetus, history of hyperemesis, migraine headaches, and a tendency to develop motion sickness.
GOODWIN: Serious sequelae are limited to hyperemesis, in which case Mallory-Weiss tears, splenic avulsion, esophageal rupture, pneumothorax, acute tubular necrosis, peripheral neuropathy (due to deficiencies of vitamins B6and B12), or Wernicke’s encephalopathy can result. This last condition is the most serious potential complication, and it can be difficult to recognize. Look for signs of confusion, memory loss, and blunted affect.
Even at milder levels of NVP, however, physical discomfort can be considerable. One prospective study using the McGill Nausea Questionnaire found the nausea experienced by pregnant women to be similar in character and intensity to that experienced by cancer patients on chemotherapy.11
OBG MANAGEMENT: Are there predisposing factors?
GOODWIN: Yes. They include a history of illness upon exposure to estrogen (for example, oral contraceptives), as well as a history of motion sickness and migraine.
Hyperemesis is more likely when the fetus is female. Women who had hyperemesis in a previous pregnancy are likely to experience it again in their next gestation, and their daughters and sisters also are more likely to develop it.
The most widely prescribed drug for nausea and vomiting of pregnancy (NVP), Bendectin, was voluntarily withdrawn from the US market in 1982, after numerous, unsuccessful lawsuits alleged it had caused birth defects.
After its withdrawal, hospitalization rates for NVP doubled while solid evidence of Bendectin’s safety continued to accumulate. In the years since, researchers have found no difference in major malformations between infants in the general population and those born to women who took Bendectin. Nor has there been any decrease in specific malformations. As the New England Journal of Medicine pointed out, a decrease in the number of malformations “would be expected for a truly teratogenic drug estimated to have been used by up to 40% of pregnant women at one time.”18
The drug is still available in Canada, where it is marketed as Diclectin. In the US, its ingredients—pyridoxine (vitamin B6) and doxylamine—can be compounded by a pharmacy, or the patient can be instructed to take 10 to 25 mg of pyridoxine and 12.5 mg of Unisom (half a tablet) 3 or 4 times daily. (Unisom is an over-the-counter sleep aid containing doxylamine succinate.)
Predisposing factors also include pregnancy-related conditions: multiple gestation; gestational trophoblastic disease; and fetal anomalies such as triploidy, Down’s syndrome, and hydrops fetalis. Overall, the chance of fetal defects associated with hyperemesis is extremely small.
The work-up: key signs and tests
- In severe cases, look for Wernicke’s encephalopathy, dehydration, weight loss, other causes of nausea and vomiting, and abnormal lab values.
GOODWIN: Physical assessment is necessary only in severe cases. If nausea and vomiting last more than 3 weeks, signs of Wernicke’s encephalopathy should be sought (this condition is never reported as early as 8 weeks).
Other important signs to look for are dehydration and evidence of other diseases that can cause nausea and vomiting (TABLE). If the patient has experienced vomiting throughout her pregnancy, but it suddenly becomes acute, another condition may be responsible.
It also is important to be aware that abdominal pain, fever, and headache do not represent NVP, but usually reflect other conditions associated with nausea and vomiting.
I ask about the duration and severity of NVP, and whether the woman has lost weight as a result. Weight loss is very important: Women who can’t sustain their weight need nutritional therapy.
OBG MANAGEMENT: Do you order lab tests?
GOODWIN: Yes. In severe cases, I get a liver panel and check amylase, lipase, and electrolytes. A number of abnormalities have been documented when hyperemesis is present. They include elevated liver enzymes, serum bilirubin, and serum amylase or lipase measurements, as well as increased free thyroxine and suppressed thyroid-stimulating hormone.
Serum hCG measurements are generally not useful, however, in exploring whether the patient’s vomiting is caused by hyperemesis gravidarum.
Imaging is necessary only to check for predisposing causes such as twins or molar gestation.
TABLE
Differential diagnosis of nausea and vomiting of pregnancy
| Gastrointestinal disorders |
| Appendicitis |
| Biliary tract disease |
| Esophagitis |
| Gastroenteritis |
| Gastroparesis |
| Hepatitis |
| Intestinal obstruction |
| Pancreatitis |
| Peptic ulcer disease |
| Genitourinary disorders |
| Acute renal failure |
| Degenerating fibroid |
| Nephrolithiasis |
| Pyelonephritis |
| Torsion |
| Metabolic disorders |
| Addison’s disease |
| Diabetic ketoacidosis |
| Hyperparathyroidism |
| Hyperthyroidism |
| Porphyria |
| Pregnancy-related |
| Acute fatty liver of pregnancy |
| Preeclampsia |
| Pregnancy-induced hypertension |
| Miscellaneous |
| Central nervous system lesions |
| Drug toxicity/side effects |
| Eating disorder |
| Migraine |
| Pseudotumor cerebri |
| Vestibular lesions |
| Adapted from Goodwin TM21 |
Do vitamins, rest, diet, ginger, or acupuncture help?
- Vitamins may help prevent NVP. For mild NVP, simple lifestyle changes or alternative remedies may suffice.
GOODWIN: The first step in this case should have been prevention. Two studies have found that multivitamins given at conception help reduce the severity of NVP.13,14
As for lifestyle changes, I would recommend rest and instruct the patient to avoid foods, activities, and other stimuli that exacerbate symptoms. Small, frequent meals are usually suggested to take the place of 3 large daily meals.
Scientifically, however, almost nothing is known about these common recommendations, though 1 study showed protein liquid meals reduced nausea and gastric-motility abnormalities more than carbohydrate or fatty meals with the same caloric content.15
OBG MANAGEMENT: Do you ever recommend alternative remedies such as ginger powder or stimulation of the P6 acupuncture point (eg, via acupuncture, Sea-Band, or ReliefBand)?
GOODWIN: As the ACOG guidelines point out, they are worth a try. When the patient wants to take ginger, I recommend 250 mg by mouth 3 to 4 times daily.
When to start drug therapy
- Pyridoxine (vitamin B6) is the first choice, followed by a combination of pyridoxine and doxylamine, which together form the drug Bendectin (no longer available in the US).
GOODWIN: When symptoms interfere with daily life and the remedies already mentioned fail or patients choose not to use them. In such cases, pyridoxine (vitamin B6) is my first choice. I recommend 10 to 25 mg 3 or 4 times daily. In 1 randomized, controlled trial,16 25 mg of pyridoxine every 8 hours led to a significant reduction in severe vomiting. In another, 10 mg of pyridoxine every 8 hours decreased both nausea and vomiting compared to placebo.17
When pyridoxine alone fails to ease NVP, I add doxylamine (see “Stepwise drug treatment of nausea and vomiting of pregnancy”). When it was commercially available, Bendectin contained the pyridoxine-doxylamine combination (10 mg of each) in 1 pill and was the most commonly prescribed agent, but it is no longer offered in the US. In Canada, it is sold as Diclectin.
The teratogenicity of Bendectin has never been proven despite extensive study, and a 1998 review described the agent’s safety.
Although the manufacturer voluntarily removed the drug in the early 1980s because of lawsuits alleging birth defects, teratogenicity has never been proven despite extensive study. A 1998 review described Diclectin’s safety.18
Fortunately, the combination of pyridoxine and doxylamine is still available—though not in a single pill. Some pharmacies will compound it, or the patient can be given pyridoxine in combination with the over-the-counter sleep aid Unisom, which contains 25 mg doxylamine succinate per tablet.
If pyridoxine alone is ineffective, I generally add 12.5 mg of doxylamine (half a Unisom tablet) to each dose. The patient should be instructed to buy Unisom in tablet form, rather than gel caps, as the active ingredient in the latter is not doxylamine.
Before starting drug therapy, rule out other causes of nausea and vomiting.
STEP 1
Try monotherapy. Start with pyridoxine (vitamin B6), 10 to 25 mg, 3 or 4 times daily.
STEP 2
Add doxylamine (Unisom tablet), 12.5 mg, 3 or 4 times daily, and adjust dosage as necessary according to severity of symptoms. (Note: Half of a 25-mg Unisom tablet = 12.5 mg.)
STEP 3
Add promethazine (Phenergan), 12.5 to 25 mg every 4 hours, orally or rectally, or dimenhydrinate (Dramamine), 50 to 100 mg every 4 to 6 hours, orally or rectally. Not to exceed 400 mg/day; if the patient is taking doxylamine, limit to 200 mg/day.
STEP 4
If the patient is sufficiently hydrated, add any of the following (listed alphabetically):
metoclopramide (Reglan), 5 to 10 mg every 8 hours, intramuscularly or orally, or promethazine, 12.5 to 25 mg every 4 hours, intramuscularly, orally, or rectally, or trimethobenzamide (Tigan), 200 mg every 6 to 8 hours, rectally.
If the patient is dehydrated, give intravenous fluids. For women who require intravenous hydration and have been vomiting for 3 or more weeks, intravenous thiamine, 100 mg daily for 2 to 3 days, followed by intravenous multivitamins, is recommended. (No study has compared different fluid replacements for NVP.) and add any of the following intravenous agents (listed alphabetically): dimenhydrinate, 50 mg (in 50 mL saline over 20 minutes) every 4 to 6 hours, or metoclopramide, 5 to 10 mg every 8 hours, or promethazine, 12.5 to 25 mg every 4 hours.
STEP 5
In refractory cases: Add methylprednisolone (Medrol), 16 mg every 8 hours, orally or intravenously, for 3 days. Taper over 2 weeks to the lowest effective dose. Limit total therapy to 6 weeks. (If given in the first 10 weeks of gestation, corticosteroids may increase risk for oral clefts.) or add ondansetron (Zofran), 8 mg over 15 minutes, every 12 hours, intravenously. Safety, particularly in first trimester, not determined. (Used mainly for emesis; less effect on nausea.)
AT ANY STEP
- Consider parenteral nutrition for dehydration or persistent weight loss. Stop nutrition once the patient achieves relief.
- Consider alternative therapies such as ginger capsules (250 mg 3 times daily) and stimulation of the P6 acupuncture point (via wrist bands or acustimulation).
Adapted from Goodwin TM and American College of Obstetricians and Gynecologists1
Adding other drugs
- When pyridoxine is insufficient (alone or in combination with doxylamine), add promethazine, dimenhydrinate, or another agent.
GOODWIN: The safety of antihistamines for use in pregnancy is well established. There are fewer data for phenothiazines, but they also appear to be quite safe.
As for 5-HT3-receptor antagonists, no teratogenicity has been found for ondansetron, which is used to treat nausea and vomiting associated with chemotherapy. However, in the only randomized trial, it did not appear to offer benefit over promethazine (a drug with characteristics of both antihistamines and phenothiazines and a good safety profile). Another drug, granisetron, has not been studied in regard to NVP.
OBG MANAGEMENT: What drugs do you prescribe besides pyridoxine and doxylamine?
GOODWIN: If the combination of pyridoxine and doxylamine fails to provide relief, I add 12.5 to 25 mg of promethazine every 4 hours (orally or rectally) or 50 to 100 mg of dimenhydrinate every 4 to 6 hours (orally or rectally, but not exceeding 200 mg daily when the patient is also taking doxylamine).
If this regimen fails to ease the patient’s symptoms, other combinations can be suggested.
OBG MANAGEMENT: Any caveats?
GOODWIN: I never give droperidol, a butyrophenone, because the US Food and Drug Administration has warned of an association with cardiac arrhythmias. This linkage may be overstated, but since there are safe alternatives, I avoid this agent.
Last resort: corticosteroids
- Corticosteroids may help resolve refractory hyperemesis gravidarum.
GOODWIN: They do not appear to be teratogenic unless they are given during the first 10 weeks of gestation; then they are associated with a slightly increased risk of oral clefts.19 Even so, they should be a last resort—and then only in refractory cases involving weight loss and the need for enteral or parenteral nutrition. The literature is mixed on the efficacy, but I find that a subset of patients have a dramatic response to corticosteroids.
The usual regimen is 16 mg methylprednisolone 3 times daily for 3 days, followed by tapering over 2 weeks, declining in 4-mg increments. If vomiting occurs during the taper, increase the dose by 4 mg for 1 week, then continue tapering. If vomiting recurs after tapering, restart the drug at 8 to 12 mg/day. All told, therapy should be limited to 4 to 6 weeks.
If there is no improvement during the initial 3 days of therapy, stop the drug altogether.
The steroid can be given orally or intravenously. It also is important to give 1,200 mg daily of calcium throughout the regimen.
Hydration and nutrition for hyperemesis
- Give fluids and nutritional support to maintain weight.
The most extreme psychosocial effect of NVP is the decision to terminate an otherwise desired pregnancy.
GOODWIN: Yes. If she is dehydrated, I replace fluids, taking care to give intravenous thiamine before dextrose if she has been vomiting longer than 3 weeks. Otherwise, Wernicke’s encephalopathy may develop.
I prefer enteral nutrition, provided the patient can tolerate it and a good nutrition team is available to support both physician and patient.
Generally, hospitalization is warranted for women who cannot maintain hydration or nutrition or when the patient and her family cannot cope with the condition. However, depending on community resources, outpatient hydration/nutrition may be a feasible option.
When she is able to tolerate oral hydration and maintain weight—with or without nutritional support—she can be discharged.
Crippling psychosocial effects
- Acknowledging NVP’s disruptive effect—at all levels of severity—is critical. Let her know you aren’t minimizing its impact, and that a range of options are at her disposal.
GOODWIN: Yes. In some cases, it can be crippling. Unfortunately, this dimension is rarely addressed by the physician. Effects can include reduced job efficiency, lost work time (in 1 study, a mean of 62 hours10), and a negative impact on family relationships and mental health. The most extreme effect, of course, is the decision to terminate an otherwise desired pregnancy.
These effects are not limited to hyperemesis; NVP involves psychosocial morbidity at all levels of severity.3 One study concluded that the severity of NVP fails to adequately reflect the distress it causes.3
The patient’s sense of her condition is critical. It is important to find out what effects NVP is having on her daily routine and let her know you aren’t minimizing its impact—also that a range of options are at her disposal.
OBG MANAGEMENT: In the past, haven’t a number of psychodynamic causes been proposed for NVP?
GOODWIN: Yes. These included speculation that the woman was subconsciously rejecting the pregnancy, “hysterical,” maternally dependent or too independent, or denying her femininity. Fortunately, these ideas have been discredited.
Severe NVP does have psychological effects, however. Simpson et al20 found that women with hyperemesis gravidarum were more likely to experience anxiety, depression, somatization, psychoticism, and symptoms of obsessive-compulsive disorder. After delivery, however, they returned to normal and were no more likely than other nonpregnant women to experience these conditions.
NVP is not more likely to occur during an unwanted pregnancy. The rates are about the same as for desired gestations.
OBG MANAGEMENT: Is NVP ever the result of a psychologic condition?
GOODWIN: Very rarely, although a behavioral component may be involved—eg, vomiting as a conditioned response. However, this does not mean that the patient has a disease or is responsible for her own condition.
That kind of thinking is one reason women hesitate to raise the subject.
Dr. Goodwin reports no financial relationships relevant to this article.
- About 35% of gravidas have nausea and vomiting severe enough to disrupt their daily routine. As many as 50% of women with severe NVP are not offered antiemetic therapy, studies show.
- At any level of severity, nausea and vomiting cause psychosocial morbidity.
- Multivitamin use at the time of conception reduces the severity of nausea and vomiting.
- When drug therapy is necessary, start with 10 to 25 mg pyridoxine (vitamin B6) 3 or 4 times daily.
- If nausea and vomiting continue, add 12.5 mg doxylamine (by halving the over-the-counter sleep aid Unisom) to each dose of pyridoxine. The pyridoxine-doxylamine combination is the same formulation as the highly effective drug Bendectin, which is no longer available in the US.
Dr. Goodwin is professor of obstetrics and gynecology at the Keck School of Medicine, University of Southern California, Los Angeles.
Many patients assume they must endure nausea, vomiting
- Women do not always mention nausea and vomiting, believing they must “live with it.” Prompt intervention can stave off more severe NVP, so it makes sense for Ob/Gyns to raise the subject early.
GOODWIN: Rather than “new information,” I would call it a change in emphasis. Previously, the focus was almost exclusively on life-threatening hyperemesis gravidarum, while lesser degrees of nausea and vomiting of pregnancy went untreated or undertreated. In reality, approximately 35% of gravidas have NVP severe enough to interfere with their daily routine. Fortunately, effective therapy is available.
OBG MANAGEMENT: Some authorities believe NVP is a continuum between mild upset and hyperemesis gravidarum. How would such a continuum affect patient care?
GOODWIN: Epidemiologic studies suggest that women with mild NVP and those with hyperemesis are significantly similar, strongly supporting the notion of a continuum.2 This fact underscores the importance of treating NVP in its early stages, before it advances to hyperemesis.
My own sense is that a small group of women with hyperemesis are predisposed to it because of rare genetic disorders such as mitochondrial mutations.
OBG MANAGEMENT: Since so many women experience NVP, should obstetricians routinely ask about it, including its psychosocial effects?
GOODWIN: Yes. This is important because of the continuum we mentioned—and because many women consider NVP “normal” and may not mention it unless they are asked.
OBG MANAGEMENT: Do you think some physicians share this mindset?
GOODWIN: Undoubtedly. Studies have shown that as many as 50% of women with severe NVP are not offered antiemetic therapy.3,4 But the days of letting the condition “run its course” are past. Now the best strategy is asking about it—and intervening early.
What causes NVP?
- The factors at play in each patient help determine the best treatment. NVP is more likely with high levels of hCG and estrogen.
How would this concept affect management of NVP?
GOODWIN: Women who are susceptible to NVP because of a vestibular mechanism might respond to a medical regimen that works at that site. Still other women may have “background” gastrointestinal motility dysfunction that makes them more susceptible to NVP; in that case, therapies to treat motility abnormalities would be appropriate. Others may have a strong behavioral overlay and would be expected to respond to the kinds of treatment used for anticipatory nausea and vomiting associated with chemotherapy.
These are theoretical scenarios at present, but a new paradigm will allow new ways of thinking about therapy.
OBG MANAGEMENT: In the early 1990s, you and your colleagues6 reported your study on the link between human chorionic gonadotropin (hCG) and NVP. What is the association?
GOODWIN: A temporal association has attracted the attention of obstetricians for years. In addition, biochemical hyperthyroidism and NVP are strongly associated. Fifty percent to 70% of women with hyperemesis gravidarum have transient hyperthyroidism, with no goiter.7 (If a goiter is present, suspect primary thyroid disorder.) Because hCG causes the biochemical hyperthyroidism related to pregnancy, and because hyperthyroidism by itself rarely causes vomiting, hCG is implicated. However, it remains unclear how hCG would cause NVP—perhaps by stimulating the ovary to produce more estrogen, which causes emesis, or through some other, unknown step.
Interestingly, a number of “high-hCG” conditions are associated with increased NVP: molar pregnancy, multiple gestation, and Down’s syndrome. Conversely, trisomy 18 is a “low-hCG” condition anecdotally associated with reduced NVP.
OBG MANAGEMENT: What is estrogen’s role?
GOODWIN: Estradiol and hCG are the only 2 hormones ever found to differ when women with NVP are compared to controls. Women who get sick with estrogen exposure are much more likely to develop NVP. Some studies have found increased estradiol levels in women with NVP, compared with controls.6,8 Conversely, low estradiol levels may reduce the risk of NVP. Take smoking, for example. It is associated with lower hCG and estradiol levels, and smokers are less likely than non-smokers to have NVP.
Studies also have focused on a possible link between cytokines and hyperemesis, and between hyperemesis and tumor necrosis factor alpha.
OBG MANAGEMENT: Do these findings alter clinical management?
GOODWIN: No. The findings about etiology do not affect management at present.
NVP can be protective or perilous
- Some degree of nausea and vomiting affects the vast majority of pregnancies. Unless it is severe, however, NVP appears to have a protective effect on the fetus.
GOODWIN: About 70% to 85% of gravidas experience it.9 In the spectrum of NVP, about 50% of women experience both nausea and vomiting, 25% experience nausea alone, and 25% are unaffected.10
Hyperemesis gravidarum affects roughly 3 to 20 of every 1,000 pregnancies.
It generally is categorized according to the level of intervention required:
- Mild NVP does not affect daily life.
- Moderate NVP interferes with daily life.
- Severe NVP, or hyperemesis, requires fluid and/or nutritional support. Hyperemesis is further defined as persistent vomiting, weight loss exceeding 5% of prepregnancy weight, and significant ketonuria, with or without electrolyte disturbances. Hospitalization usually is required.
GOODWIN: NVP almost always appears before 10 weeks’ gestation. If it begins any later, it is likely the patient has a different condition associated with nausea and vomiting.
One study found that most women develop NVP at about 4 to 7 weeks’ gestation, and that it resolves at less than 10 weeks in about 30% of women, at 10 to 12 weeks in another 30%, and at 12 to 16 weeks in another 30%.10
As for diurnal changes, symptoms tend to occur with greater frequency early in the day.
OBG MANAGEMENT: Is NVP ever harmful for the fetus?
GOODWIN: It is associated with improved fetal outcomes unless hyperemesis supervenes. Then it is associated with mild fetal growth delays and rare cases of fetal death.
We lack studies of the long-term effects of severe NVP on the fetus, but starvation and weight loss in women during famine have been shown to cause many diverse problems in offspring later in adult life.
OBG MANAGEMENT: What do you make of evidence that suggests NVP plays a protective role in pregnancy?
GOODWIN: If true, it likely represents a vestigial response that is no longer beneficial—similar to thrombophilias in pregnancy, which played an important role protecting women against bleeding at childbirth but now are more of a clinical problem because of the associated thrombosis.
Prevalence. 70% to 85%.1
Presentation. About 50% of women have both nausea and vomiting, 25% have nausea only, and 25% are not affected.10
Hyperemesis gravidarum. 3 to 20 of every 1,000 pregnancies. Marked by persistent vomiting, weight loss exceeding 5% of prepregnancy weight, and significant ketonuria.
Clinical course. If NVP is going to occur, it is present before 10 weeks’ gestation. It resolves at less than 10 weeks’ gestation in about 30% of women, at 10 to 12 weeks in another 30%, and at 12 to 16 weeks in another 30%.10
Predisposing factors. History of illness with estrogen exposure, motion sickness, migraine, or hyperemesis; mother or sister with hyperemesis; female fetus; mitochondrial disorders; multiple gestation; gestational trophoblastic disease; and fetal anomalies such as Down’s syndrome and triploidy.
Undertreatment. As many as 50% of women with severe NVP are not offered therapy.3,4
Physical discomfort. Intensity and character similar to nausea and vomiting induced by cancer chemotherapy, even at milder levels of severity.11
Social and psychological impact. Reduced job efficiency, lost work time, negative impact on family relationships and mental health, decision to terminate an otherwise desired pregnancy. Women with hyperemesis are more likely to have anxiety, depression, somatization, psychoticism, and obsessive-compulsive symptoms, but return to normal after delivery.21
Fetal effects. Improved fetal outcomes unless hyperemesis supervenes. Hyperemesis with maternal weight loss is associated with mild fetal growth delays and rare cases of fetal death.
Maternal effects. Not linked to adverse effects except for hyperemesis gravidarum, in which Wernicke’s encephalopathy, Mallory-Weiss tears, splenic avulsion, esophageal rupture, pneumothorax, acute tubular necrosis, or peripheral neuropathy (due to vitamins B6 and B12 deficiencies) can result.
Effects on the mother and predisposing factors
- Even at milder levels, physical discomfort is considerable. Predisposing factors include a female fetus, history of hyperemesis, migraine headaches, and a tendency to develop motion sickness.
GOODWIN: Serious sequelae are limited to hyperemesis, in which case Mallory-Weiss tears, splenic avulsion, esophageal rupture, pneumothorax, acute tubular necrosis, peripheral neuropathy (due to deficiencies of vitamins B6and B12), or Wernicke’s encephalopathy can result. This last condition is the most serious potential complication, and it can be difficult to recognize. Look for signs of confusion, memory loss, and blunted affect.
Even at milder levels of NVP, however, physical discomfort can be considerable. One prospective study using the McGill Nausea Questionnaire found the nausea experienced by pregnant women to be similar in character and intensity to that experienced by cancer patients on chemotherapy.11
OBG MANAGEMENT: Are there predisposing factors?
GOODWIN: Yes. They include a history of illness upon exposure to estrogen (for example, oral contraceptives), as well as a history of motion sickness and migraine.
Hyperemesis is more likely when the fetus is female. Women who had hyperemesis in a previous pregnancy are likely to experience it again in their next gestation, and their daughters and sisters also are more likely to develop it.
The most widely prescribed drug for nausea and vomiting of pregnancy (NVP), Bendectin, was voluntarily withdrawn from the US market in 1982, after numerous, unsuccessful lawsuits alleged it had caused birth defects.
After its withdrawal, hospitalization rates for NVP doubled while solid evidence of Bendectin’s safety continued to accumulate. In the years since, researchers have found no difference in major malformations between infants in the general population and those born to women who took Bendectin. Nor has there been any decrease in specific malformations. As the New England Journal of Medicine pointed out, a decrease in the number of malformations “would be expected for a truly teratogenic drug estimated to have been used by up to 40% of pregnant women at one time.”18
The drug is still available in Canada, where it is marketed as Diclectin. In the US, its ingredients—pyridoxine (vitamin B6) and doxylamine—can be compounded by a pharmacy, or the patient can be instructed to take 10 to 25 mg of pyridoxine and 12.5 mg of Unisom (half a tablet) 3 or 4 times daily. (Unisom is an over-the-counter sleep aid containing doxylamine succinate.)
Predisposing factors also include pregnancy-related conditions: multiple gestation; gestational trophoblastic disease; and fetal anomalies such as triploidy, Down’s syndrome, and hydrops fetalis. Overall, the chance of fetal defects associated with hyperemesis is extremely small.
The work-up: key signs and tests
- In severe cases, look for Wernicke’s encephalopathy, dehydration, weight loss, other causes of nausea and vomiting, and abnormal lab values.
GOODWIN: Physical assessment is necessary only in severe cases. If nausea and vomiting last more than 3 weeks, signs of Wernicke’s encephalopathy should be sought (this condition is never reported as early as 8 weeks).
Other important signs to look for are dehydration and evidence of other diseases that can cause nausea and vomiting (TABLE). If the patient has experienced vomiting throughout her pregnancy, but it suddenly becomes acute, another condition may be responsible.
It also is important to be aware that abdominal pain, fever, and headache do not represent NVP, but usually reflect other conditions associated with nausea and vomiting.
I ask about the duration and severity of NVP, and whether the woman has lost weight as a result. Weight loss is very important: Women who can’t sustain their weight need nutritional therapy.
OBG MANAGEMENT: Do you order lab tests?
GOODWIN: Yes. In severe cases, I get a liver panel and check amylase, lipase, and electrolytes. A number of abnormalities have been documented when hyperemesis is present. They include elevated liver enzymes, serum bilirubin, and serum amylase or lipase measurements, as well as increased free thyroxine and suppressed thyroid-stimulating hormone.
Serum hCG measurements are generally not useful, however, in exploring whether the patient’s vomiting is caused by hyperemesis gravidarum.
Imaging is necessary only to check for predisposing causes such as twins or molar gestation.
TABLE
Differential diagnosis of nausea and vomiting of pregnancy
| Gastrointestinal disorders |
| Appendicitis |
| Biliary tract disease |
| Esophagitis |
| Gastroenteritis |
| Gastroparesis |
| Hepatitis |
| Intestinal obstruction |
| Pancreatitis |
| Peptic ulcer disease |
| Genitourinary disorders |
| Acute renal failure |
| Degenerating fibroid |
| Nephrolithiasis |
| Pyelonephritis |
| Torsion |
| Metabolic disorders |
| Addison’s disease |
| Diabetic ketoacidosis |
| Hyperparathyroidism |
| Hyperthyroidism |
| Porphyria |
| Pregnancy-related |
| Acute fatty liver of pregnancy |
| Preeclampsia |
| Pregnancy-induced hypertension |
| Miscellaneous |
| Central nervous system lesions |
| Drug toxicity/side effects |
| Eating disorder |
| Migraine |
| Pseudotumor cerebri |
| Vestibular lesions |
| Adapted from Goodwin TM21 |
Do vitamins, rest, diet, ginger, or acupuncture help?
- Vitamins may help prevent NVP. For mild NVP, simple lifestyle changes or alternative remedies may suffice.
GOODWIN: The first step in this case should have been prevention. Two studies have found that multivitamins given at conception help reduce the severity of NVP.13,14
As for lifestyle changes, I would recommend rest and instruct the patient to avoid foods, activities, and other stimuli that exacerbate symptoms. Small, frequent meals are usually suggested to take the place of 3 large daily meals.
Scientifically, however, almost nothing is known about these common recommendations, though 1 study showed protein liquid meals reduced nausea and gastric-motility abnormalities more than carbohydrate or fatty meals with the same caloric content.15
OBG MANAGEMENT: Do you ever recommend alternative remedies such as ginger powder or stimulation of the P6 acupuncture point (eg, via acupuncture, Sea-Band, or ReliefBand)?
GOODWIN: As the ACOG guidelines point out, they are worth a try. When the patient wants to take ginger, I recommend 250 mg by mouth 3 to 4 times daily.
When to start drug therapy
- Pyridoxine (vitamin B6) is the first choice, followed by a combination of pyridoxine and doxylamine, which together form the drug Bendectin (no longer available in the US).
GOODWIN: When symptoms interfere with daily life and the remedies already mentioned fail or patients choose not to use them. In such cases, pyridoxine (vitamin B6) is my first choice. I recommend 10 to 25 mg 3 or 4 times daily. In 1 randomized, controlled trial,16 25 mg of pyridoxine every 8 hours led to a significant reduction in severe vomiting. In another, 10 mg of pyridoxine every 8 hours decreased both nausea and vomiting compared to placebo.17
When pyridoxine alone fails to ease NVP, I add doxylamine (see “Stepwise drug treatment of nausea and vomiting of pregnancy”). When it was commercially available, Bendectin contained the pyridoxine-doxylamine combination (10 mg of each) in 1 pill and was the most commonly prescribed agent, but it is no longer offered in the US. In Canada, it is sold as Diclectin.
The teratogenicity of Bendectin has never been proven despite extensive study, and a 1998 review described the agent’s safety.
Although the manufacturer voluntarily removed the drug in the early 1980s because of lawsuits alleging birth defects, teratogenicity has never been proven despite extensive study. A 1998 review described Diclectin’s safety.18
Fortunately, the combination of pyridoxine and doxylamine is still available—though not in a single pill. Some pharmacies will compound it, or the patient can be given pyridoxine in combination with the over-the-counter sleep aid Unisom, which contains 25 mg doxylamine succinate per tablet.
If pyridoxine alone is ineffective, I generally add 12.5 mg of doxylamine (half a Unisom tablet) to each dose. The patient should be instructed to buy Unisom in tablet form, rather than gel caps, as the active ingredient in the latter is not doxylamine.
Before starting drug therapy, rule out other causes of nausea and vomiting.
STEP 1
Try monotherapy. Start with pyridoxine (vitamin B6), 10 to 25 mg, 3 or 4 times daily.
STEP 2
Add doxylamine (Unisom tablet), 12.5 mg, 3 or 4 times daily, and adjust dosage as necessary according to severity of symptoms. (Note: Half of a 25-mg Unisom tablet = 12.5 mg.)
STEP 3
Add promethazine (Phenergan), 12.5 to 25 mg every 4 hours, orally or rectally, or dimenhydrinate (Dramamine), 50 to 100 mg every 4 to 6 hours, orally or rectally. Not to exceed 400 mg/day; if the patient is taking doxylamine, limit to 200 mg/day.
STEP 4
If the patient is sufficiently hydrated, add any of the following (listed alphabetically):
metoclopramide (Reglan), 5 to 10 mg every 8 hours, intramuscularly or orally, or promethazine, 12.5 to 25 mg every 4 hours, intramuscularly, orally, or rectally, or trimethobenzamide (Tigan), 200 mg every 6 to 8 hours, rectally.
If the patient is dehydrated, give intravenous fluids. For women who require intravenous hydration and have been vomiting for 3 or more weeks, intravenous thiamine, 100 mg daily for 2 to 3 days, followed by intravenous multivitamins, is recommended. (No study has compared different fluid replacements for NVP.) and add any of the following intravenous agents (listed alphabetically): dimenhydrinate, 50 mg (in 50 mL saline over 20 minutes) every 4 to 6 hours, or metoclopramide, 5 to 10 mg every 8 hours, or promethazine, 12.5 to 25 mg every 4 hours.
STEP 5
In refractory cases: Add methylprednisolone (Medrol), 16 mg every 8 hours, orally or intravenously, for 3 days. Taper over 2 weeks to the lowest effective dose. Limit total therapy to 6 weeks. (If given in the first 10 weeks of gestation, corticosteroids may increase risk for oral clefts.) or add ondansetron (Zofran), 8 mg over 15 minutes, every 12 hours, intravenously. Safety, particularly in first trimester, not determined. (Used mainly for emesis; less effect on nausea.)
AT ANY STEP
- Consider parenteral nutrition for dehydration or persistent weight loss. Stop nutrition once the patient achieves relief.
- Consider alternative therapies such as ginger capsules (250 mg 3 times daily) and stimulation of the P6 acupuncture point (via wrist bands or acustimulation).
Adapted from Goodwin TM and American College of Obstetricians and Gynecologists1
Adding other drugs
- When pyridoxine is insufficient (alone or in combination with doxylamine), add promethazine, dimenhydrinate, or another agent.
GOODWIN: The safety of antihistamines for use in pregnancy is well established. There are fewer data for phenothiazines, but they also appear to be quite safe.
As for 5-HT3-receptor antagonists, no teratogenicity has been found for ondansetron, which is used to treat nausea and vomiting associated with chemotherapy. However, in the only randomized trial, it did not appear to offer benefit over promethazine (a drug with characteristics of both antihistamines and phenothiazines and a good safety profile). Another drug, granisetron, has not been studied in regard to NVP.
OBG MANAGEMENT: What drugs do you prescribe besides pyridoxine and doxylamine?
GOODWIN: If the combination of pyridoxine and doxylamine fails to provide relief, I add 12.5 to 25 mg of promethazine every 4 hours (orally or rectally) or 50 to 100 mg of dimenhydrinate every 4 to 6 hours (orally or rectally, but not exceeding 200 mg daily when the patient is also taking doxylamine).
If this regimen fails to ease the patient’s symptoms, other combinations can be suggested.
OBG MANAGEMENT: Any caveats?
GOODWIN: I never give droperidol, a butyrophenone, because the US Food and Drug Administration has warned of an association with cardiac arrhythmias. This linkage may be overstated, but since there are safe alternatives, I avoid this agent.
Last resort: corticosteroids
- Corticosteroids may help resolve refractory hyperemesis gravidarum.
GOODWIN: They do not appear to be teratogenic unless they are given during the first 10 weeks of gestation; then they are associated with a slightly increased risk of oral clefts.19 Even so, they should be a last resort—and then only in refractory cases involving weight loss and the need for enteral or parenteral nutrition. The literature is mixed on the efficacy, but I find that a subset of patients have a dramatic response to corticosteroids.
The usual regimen is 16 mg methylprednisolone 3 times daily for 3 days, followed by tapering over 2 weeks, declining in 4-mg increments. If vomiting occurs during the taper, increase the dose by 4 mg for 1 week, then continue tapering. If vomiting recurs after tapering, restart the drug at 8 to 12 mg/day. All told, therapy should be limited to 4 to 6 weeks.
If there is no improvement during the initial 3 days of therapy, stop the drug altogether.
The steroid can be given orally or intravenously. It also is important to give 1,200 mg daily of calcium throughout the regimen.
Hydration and nutrition for hyperemesis
- Give fluids and nutritional support to maintain weight.
The most extreme psychosocial effect of NVP is the decision to terminate an otherwise desired pregnancy.
GOODWIN: Yes. If she is dehydrated, I replace fluids, taking care to give intravenous thiamine before dextrose if she has been vomiting longer than 3 weeks. Otherwise, Wernicke’s encephalopathy may develop.
I prefer enteral nutrition, provided the patient can tolerate it and a good nutrition team is available to support both physician and patient.
Generally, hospitalization is warranted for women who cannot maintain hydration or nutrition or when the patient and her family cannot cope with the condition. However, depending on community resources, outpatient hydration/nutrition may be a feasible option.
When she is able to tolerate oral hydration and maintain weight—with or without nutritional support—she can be discharged.
Crippling psychosocial effects
- Acknowledging NVP’s disruptive effect—at all levels of severity—is critical. Let her know you aren’t minimizing its impact, and that a range of options are at her disposal.
GOODWIN: Yes. In some cases, it can be crippling. Unfortunately, this dimension is rarely addressed by the physician. Effects can include reduced job efficiency, lost work time (in 1 study, a mean of 62 hours10), and a negative impact on family relationships and mental health. The most extreme effect, of course, is the decision to terminate an otherwise desired pregnancy.
These effects are not limited to hyperemesis; NVP involves psychosocial morbidity at all levels of severity.3 One study concluded that the severity of NVP fails to adequately reflect the distress it causes.3
The patient’s sense of her condition is critical. It is important to find out what effects NVP is having on her daily routine and let her know you aren’t minimizing its impact—also that a range of options are at her disposal.
OBG MANAGEMENT: In the past, haven’t a number of psychodynamic causes been proposed for NVP?
GOODWIN: Yes. These included speculation that the woman was subconsciously rejecting the pregnancy, “hysterical,” maternally dependent or too independent, or denying her femininity. Fortunately, these ideas have been discredited.
Severe NVP does have psychological effects, however. Simpson et al20 found that women with hyperemesis gravidarum were more likely to experience anxiety, depression, somatization, psychoticism, and symptoms of obsessive-compulsive disorder. After delivery, however, they returned to normal and were no more likely than other nonpregnant women to experience these conditions.
NVP is not more likely to occur during an unwanted pregnancy. The rates are about the same as for desired gestations.
OBG MANAGEMENT: Is NVP ever the result of a psychologic condition?
GOODWIN: Very rarely, although a behavioral component may be involved—eg, vomiting as a conditioned response. However, this does not mean that the patient has a disease or is responsible for her own condition.
That kind of thinking is one reason women hesitate to raise the subject.
Dr. Goodwin reports no financial relationships relevant to this article.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Nausea and Vomiting of Pregnancy. Clinical management guidelines for obstetrician-gynecologists. No. 52. Washington, DC: ACOG: April 2004.
2. Klebanoff M, Koslone P, Kaslow R, Rhoads G. Epidemiology of vomiting in early pregnancy. Obstet Gynecol. 1985;66:612-616.
3. Mazzotta P, Stewart D, Atanackovic G, Koren G, Magee LA. Psychosocial morbidity among women with nausea and vomiting of pregnancy: prevalence and association with antiemetic therapy. J Psychosom Obstet Gynaecol. 2000;21:129-136.
4. Mazzota P, Magee L, Koren G. Therapeutic abortions due to severe morning sickness. Unacceptable combination. Can Fam Physician. 1997;43:1055-1057.
5. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol. 2002;186:S184-S189.
6. Goodwin TM, Montoro M, Mestman JH, Pekary AE, Hershman JM. The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab. 1992;75:1333-1337.
7. Goodwin TM, Hershman JM. Hyperthyroidism due to inappropriate production of human chorionic gonadotropin. Clin Obstet Gynecol. 1997;40:32-44.
8. Depue RH, Bernstein L, Ross RK, et al. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and other maternal factors: a seroepidemiologic study. Am J Obstet Gynecol. 1987;156:1137-1141.
9. Vellacott ID, Cooke EJ, James CE. Nausea and vomiting in early pregnancy. Int J Gynaecol Obstet. 1988;27:57-62.
10. Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. Br J Gen Prac. 1993;43:245-248.
11. Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182:931-937.
12. Boles RG, Williams JC. Mitochondrial disease and cyclic vomiting syndrome. Dig Dis Sci. 1999;44:103S-107S.
13. Czeizel AE, Dudas I, Fritz G, Tecsoi A, Hanck A, Kunovits G. The effect of periconceptional multivitamin-mineral supplementation on vertigo, nausea and vomiting in the first trimester of pregnancy. Arch Gynecol Obstet. 1992;251:181-185.
14. Emelianova S, Mazzotta P, Einarson A, Koren G. Prevalence and severity of nausea and vomiting of pregnancy and effect of vitamin supplementation. Clin Invest Med. 1999;22(3):106-110.
15. Jednak MA, Shadigian EM, Kim MS, et al. Protein meals reduce nausea and gastric slow wave dysrhythmic activity in first trimester pregnancy. Am J Physiol. 1999;277:G855-G861.
16. Sahakian V, Rouse D, Sipes S, Rose N, Niebyl J. Vitamin B6 is effective therapy for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled study. Obstet Gynecol. 1991;78:33-36.
17. Vutyavanich T, Wongtrangan S, Ruangsri R. Pyridoxine for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1995;173:881-884.
18. Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128-1137.
19. Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
20. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med. 2001;10:471-477.
21. Goodwin TM. Hyperemesis gravidarum. Clin Obstet Gynecol. 1998;41:597-605.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin: Nausea and Vomiting of Pregnancy. Clinical management guidelines for obstetrician-gynecologists. No. 52. Washington, DC: ACOG: April 2004.
2. Klebanoff M, Koslone P, Kaslow R, Rhoads G. Epidemiology of vomiting in early pregnancy. Obstet Gynecol. 1985;66:612-616.
3. Mazzotta P, Stewart D, Atanackovic G, Koren G, Magee LA. Psychosocial morbidity among women with nausea and vomiting of pregnancy: prevalence and association with antiemetic therapy. J Psychosom Obstet Gynaecol. 2000;21:129-136.
4. Mazzota P, Magee L, Koren G. Therapeutic abortions due to severe morning sickness. Unacceptable combination. Can Fam Physician. 1997;43:1055-1057.
5. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol. 2002;186:S184-S189.
6. Goodwin TM, Montoro M, Mestman JH, Pekary AE, Hershman JM. The role of chorionic gonadotropin in transient hyperthyroidism of hyperemesis gravidarum. J Clin Endocrinol Metab. 1992;75:1333-1337.
7. Goodwin TM, Hershman JM. Hyperthyroidism due to inappropriate production of human chorionic gonadotropin. Clin Obstet Gynecol. 1997;40:32-44.
8. Depue RH, Bernstein L, Ross RK, et al. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and other maternal factors: a seroepidemiologic study. Am J Obstet Gynecol. 1987;156:1137-1141.
9. Vellacott ID, Cooke EJ, James CE. Nausea and vomiting in early pregnancy. Int J Gynaecol Obstet. 1988;27:57-62.
10. Gadsby R, Barnie-Adshead AM, Jagger C. A prospective study of nausea and vomiting during pregnancy. Br J Gen Prac. 1993;43:245-248.
11. Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182:931-937.
12. Boles RG, Williams JC. Mitochondrial disease and cyclic vomiting syndrome. Dig Dis Sci. 1999;44:103S-107S.
13. Czeizel AE, Dudas I, Fritz G, Tecsoi A, Hanck A, Kunovits G. The effect of periconceptional multivitamin-mineral supplementation on vertigo, nausea and vomiting in the first trimester of pregnancy. Arch Gynecol Obstet. 1992;251:181-185.
14. Emelianova S, Mazzotta P, Einarson A, Koren G. Prevalence and severity of nausea and vomiting of pregnancy and effect of vitamin supplementation. Clin Invest Med. 1999;22(3):106-110.
15. Jednak MA, Shadigian EM, Kim MS, et al. Protein meals reduce nausea and gastric slow wave dysrhythmic activity in first trimester pregnancy. Am J Physiol. 1999;277:G855-G861.
16. Sahakian V, Rouse D, Sipes S, Rose N, Niebyl J. Vitamin B6 is effective therapy for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled study. Obstet Gynecol. 1991;78:33-36.
17. Vutyavanich T, Wongtrangan S, Ruangsri R. Pyridoxine for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1995;173:881-884.
18. Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128-1137.
19. Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
20. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med. 2001;10:471-477.
21. Goodwin TM. Hyperemesis gravidarum. Clin Obstet Gynecol. 1998;41:597-605.