User login
Care of post–acute COVID-19 patients requires multidisciplinary collaboration
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
In the wake of the COVID-19 pandemic, a population of patients has arisen with a range of symptoms and complications after surviving the acute phase of illness, according to Mezgebe Berhe, MD, of Baylor University Medical Center, Dallas.
Different terms have been used to describe this condition, including post COVID, long COVID, chronic COVID, and long-haulers, Dr. Berhe said in a presentation at SHM Converge, the annual conference of the Society of Hospital Medicine. However, the current medical consensus for a definition is post–acute COVID-19 syndrome.
Acute COVID-19 generally lasts for about 4 weeks after the onset of symptoms, and post–acute COVID-19 is generally defined as “persistent symptoms and/or delayed or long-term complications beyond 4 weeks from the onset of symptoms,” he said. The postacute period may be broken into a subacute phase with symptoms and abnormalities present from 4-12 weeks beyond the acute phase, and then a chronic or post–acute COVID-19 syndrome, with symptoms and abnormalities present beyond 12 weeks after the onset of acute COVID-19.
Patients in the subacute or post–COVID-19 phase of illness are polymerase chain reaction negative and may have multiorgan symptomatology, said Dr. Berhe. Physical symptoms include fatigue, decline in quality of life, joint pain, and muscle weakness; reported mental symptoms include anxiety and depression; sleep disturbance; PTSD; cognitive disturbance (described by patients as “brain fog”); and headaches.
Pulmonary symptoms in post–acute COVID-19 patients include dyspnea, cough, and persistent oxygen requirements; patients also have reported palpitations and chest pain. Thromboembolism, chronic kidney disease, and hair loss also have been reported in COVID-19 patients in the postacute period.
What studies show
Early reports on postacute consequences of COVID-19 have been reported in published studies from the United States, Europe, and China, and the current treatment recommendations are based on findings from these studies, Dr. Berhe said.
In an observational cohort study from 38 hospitals in Michigan, researchers assessed 60-day outcomes for 1,250 COVID-19 patients who were discharged alive from the hospital. The researchers used medical record abstraction and telephone surveys to assess long-term symptoms. Overall, 6.7% of the patients died and 15.1% required hospital readmission. A total of 488 patients completed the telephone survey. Of these, 32.6% reported persistent symptoms, 18.9% reported new or worsening symptoms, 22.9% reported dyspnea while walking up stairs, 15.4% reported a cough, and 13.1% reported a persistent loss of taste or smell.
Data from multiple countries in Europe have shown similar prevalence of post–acute COVID-19 syndrome, but Dr. Berhe highlighted an Italian study in which 87% of 143 patients discharged from hospitals after acute COVID-19 reported at least one symptom at 60 day. “A decline in quality of life, as measured by the EuroQol visual analog scale, was reported by 44.1% of patients” in the Italian study, Dr. Berhe noted.
In a prospective cohort study conducted in Wuhan, China, researchers conducted a comprehensive in-person evaluation of symptoms in 1,733 COVID-19 patients at 6 months from symptom onset, and found that 76% reported at least one symptom, said Dr. Berhe. “Similar to other studies, muscle weakness and fatigue were the most common symptoms, followed by sleep problems and anxiety/depression.
Dr. Berhe also cited a literature review published in Clinical Infectious Diseases that addressed COVID-19 in children; in one study of postacute COVID-19, approximately 12% of children had 5 weeks’ prevalence of persistent symptoms, compared with 22% of adults. This finding should remind clinicians that “Children can have devastating persistent symptoms following acute COVID-19 disease,” Dr. Berhe said.
In the post–acute COVID clinic
“Multidisciplinary collaboration is essential to provide integrated outpatient care to survivors of acute COVID-19,” Dr. Berhe said. Such collaboration includes pulmonary and cardiovascular symptom assessment through virtual or in-person follow-up at 4-6 weeks and at 12 weeks after hospital discharge. For those with dyspnea and persistent oxygen requirements at 12 weeks, consider the 6-minute walk test, pulmonary function test, chest x-ray, pulmonary embolism work-up, echocardiogram, and high-resolution CT of the chest as indicated.
With regard to neuropsychiatry, patients should be screened for anxiety, depression, PTSD, sleep disturbance, and cognitive impairment, said Dr. Berhe.
For hematology, “consider extended thromboprophylaxis for high-risk survivors based on shared decision-making,” he said. The incidence of thrombotic events post COVID is less than 5% so you have to be very selective and they should be in the highest-risk category.
COVID-19 patients with acute kidney infections should have a follow-up with a nephrologist soon after hospital discharge, he added.
From a primary care standpoint, early rehabilitation and patient education are important for managing symptoms; also consider recommending patient enrollment in research studies, Dr. Berhe said.
Dr. Berhe has been involved in multiple clinical trials of treating acute COVID-19 patients, but had no financial conflicts to disclose.
FROM SHM CONVERGE 2021
Some things pediatric hospitalists do for no reason
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
Converge 2021 session
High Value Care in Pediatrics – Things We Do for No Reason
Presenter
Ricardo Quinonez, MD, FAAP, FHM
Session summary
Dr. Ricardo Quinonez, associate professor of pediatrics at Baylor College of Medicine and chief of pediatric hospital medicine at Texas Children’s Hospital, both in Houston, presented key topics in pediatric hospital medicine with low-value care management practices which are not supported by recent literature. This session was a continuation of the popular lecture series first presented at the Society of Hospital Medicine annual conference and the “Choosing Wisely: Things We Do for No Reason” article series in the Journal of Hospital Medicine.
Dr. Quinonez began by discussing high flow nasal cannula (HFNC) in bronchiolitis. At first, early observational studies showed a decrease in intubation rate for children placed on HFNC, which resulted in its high utilization. Randomized, controlled trials (RCTs) later showed that early initiation of HFNC did not affect rates of transfer to the ICU, duration of oxygen need, or length of stay.
He then discussed the treatment of symptomatic spontaneous pneumothorax in children, which is often managed by hospital admission, needle aspiration and chest tube placement, and serial chest x-rays. Instead, recent literature supports an ambulatory approach by placing a device with an 8 French catheter with one way Heimlich valve. After placement, a chest x-ray is performed and if the pneumothorax is stable, the patient is discharged with plans for serial chest x-rays as an outpatient. The device is removed after re-expansion of the lung.
Dr. Quinonez then discussed the frequent pediatric complaint of constipation. He stated that abdominal x-rays for evaluation of “stool burden” are not reliable, and x-rays are recommended against in both U.S. and British guidelines. Furthermore, a high-fiber diet is often recommended as a treatment for constipation. However, after review of recent RCTs and cohort studies, no relationship between a low-fiber diet and constipation was seen. Instead, genetics likely plays a large part in causing constipation.
Lastly, Dr. Quinonez discussed electrolyte testing in children with acute gastroenteritis. Electrolyte testing is commonly performed, yet testing patterns vary greatly across children’s hospitals. One quality improvement project found that after decreasing electrolyte testing by more than a third during hospitalizations, no change in readmission rate or renal replacement therapy was reported.
Key takeaways
- Early use of high flow nasal cannula in bronchiolitis does not affect rates of transfer to the ICU or length of stay.
- Abdominal x-rays to assess for constipation are not recommended and are not reliable in measuring stool burden.
- A low-fiber diet does not cause constipation.
- Quality improvement projects can help physicians “choose wisely” and decrease things we do for no reason.
Dr. Tantoco is an academic med-peds hospitalist at Northwestern Memorial Hospital and Ann & Robert H. Lurie Children’s Hospital of Chicago. She is an instructor of medicine (hospital medicine) and pediatrics at Northwestern University, Chicago.
FROM SHM CONVERGE 2021
Hospital outcomes for children with MIS-C unaffected by initial presentation site
Length of hospital stay and the need for intensive care for pediatric COVID-19 patients with multisystem inflammatory syndrome in children was not significantly different for those who presented first as outpatients or emergency patients, based on data from 34 children.
Multisystem inflammatory syndrome in children (MIS-C) can be challenging to diagnose, as the key characteristics of fever, elevated inflammatory markers, and involvement of at least two organ systems often overlap with other illnesses, said Erin B. Treemarcki, DO, of the University of Utah, Salt Lake City, and colleagues.
“Primary care and urgent care providers are often the first point of health care for children with symptoms of MIS-C,” the researchers wrote. In a study (Poster 142) presented at the annual meeting of the Pediatric Academic Societies, held virtually, the researchers conducted a retrospective review of 34 patients younger than 21 years who were hospitalized with MIS-C at a single center between April 2020 and December 2020. The average age of the patients was 7.9 years, 68% were male, 82% were White, and 53% first presented to an outpatient clinic.
Sixteen patients presented to an emergency department and 18 presented to an ambulatory setting. The length of hospitalization ranged from 3 to 16 days with a median of 6 days, and the PICU stay ranged from 1 to 10 days with a median of 2 days.
Overall, the length of hospital stay and rate of PICU admission were not significantly different between the emergency presentation and outpatient presentation groups. Twenty-four patients entered the PICU, 13 at admission and 11 as transfers. However, the median number of days of symptoms prior to admission was significantly higher for outpatient cases (6 days vs. 4 days, P = .03).
One patient was readmitted to the hospital within 30 days for aseptic meningitis, and none of the patients died.
Initial symptoms were not significantly different for outpatient vs. emergency department patients. The most common initial manifestations of MIS-C included fever (100%), gastrointestinal symptoms (85%), and mucocutaneous symptoms (88%). Mucocutaneous symptoms included rash, oral mucosal changes, conjunctivitis, and hand/foot edema. In addition, 65% of the patients met at least 3 criteria for Kawasaki disease, the researchers noted.
The most common elevated labs at presentation regardless of setting were D-dimer (100%), C-reactive protein (97%), ferritin (97%), procalcitonin (97%), and serum IL-6 (94%).
The study findings were limited by the small sample size and focus on data from a single center. However, the results emphasize the varied presentations of MIS-C and the importance that both primary care and urgent care providers know the signs, as they are often the first point of health care for children with MIS-C, the researchers noted.
Keep looking for factors that put children at risk
“MIS-C is probably the most serious complication of COVID in children, so we as pediatricians on the front line need to know what it looks like,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was surprised by the study finding that children’s length of hospital stay was not affected by presentation setting.
“I would have thought the kids presenting in an outpatient setting would take longer to diagnose, and therefore have a longer hospital stay,” she noted. Instead, the take-home message is that whether the MIS-C diagnosis occurs in the outpatient or emergency setting, the length of stay is the same, and that the most common symptoms are fever, gastrointestinal, mucocutaneous, and cardiac symptoms regardless of initial presentation setting, she said.
More research is needed, and future studies should examine “any potential underlying factors making these particular kids susceptible to MIS-C,” Dr. Kinsella added.
The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts, but serves on the Pediatric News Editorial Advisory Board.
Length of hospital stay and the need for intensive care for pediatric COVID-19 patients with multisystem inflammatory syndrome in children was not significantly different for those who presented first as outpatients or emergency patients, based on data from 34 children.
Multisystem inflammatory syndrome in children (MIS-C) can be challenging to diagnose, as the key characteristics of fever, elevated inflammatory markers, and involvement of at least two organ systems often overlap with other illnesses, said Erin B. Treemarcki, DO, of the University of Utah, Salt Lake City, and colleagues.
“Primary care and urgent care providers are often the first point of health care for children with symptoms of MIS-C,” the researchers wrote. In a study (Poster 142) presented at the annual meeting of the Pediatric Academic Societies, held virtually, the researchers conducted a retrospective review of 34 patients younger than 21 years who were hospitalized with MIS-C at a single center between April 2020 and December 2020. The average age of the patients was 7.9 years, 68% were male, 82% were White, and 53% first presented to an outpatient clinic.
Sixteen patients presented to an emergency department and 18 presented to an ambulatory setting. The length of hospitalization ranged from 3 to 16 days with a median of 6 days, and the PICU stay ranged from 1 to 10 days with a median of 2 days.
Overall, the length of hospital stay and rate of PICU admission were not significantly different between the emergency presentation and outpatient presentation groups. Twenty-four patients entered the PICU, 13 at admission and 11 as transfers. However, the median number of days of symptoms prior to admission was significantly higher for outpatient cases (6 days vs. 4 days, P = .03).
One patient was readmitted to the hospital within 30 days for aseptic meningitis, and none of the patients died.
Initial symptoms were not significantly different for outpatient vs. emergency department patients. The most common initial manifestations of MIS-C included fever (100%), gastrointestinal symptoms (85%), and mucocutaneous symptoms (88%). Mucocutaneous symptoms included rash, oral mucosal changes, conjunctivitis, and hand/foot edema. In addition, 65% of the patients met at least 3 criteria for Kawasaki disease, the researchers noted.
The most common elevated labs at presentation regardless of setting were D-dimer (100%), C-reactive protein (97%), ferritin (97%), procalcitonin (97%), and serum IL-6 (94%).
The study findings were limited by the small sample size and focus on data from a single center. However, the results emphasize the varied presentations of MIS-C and the importance that both primary care and urgent care providers know the signs, as they are often the first point of health care for children with MIS-C, the researchers noted.
Keep looking for factors that put children at risk
“MIS-C is probably the most serious complication of COVID in children, so we as pediatricians on the front line need to know what it looks like,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was surprised by the study finding that children’s length of hospital stay was not affected by presentation setting.
“I would have thought the kids presenting in an outpatient setting would take longer to diagnose, and therefore have a longer hospital stay,” she noted. Instead, the take-home message is that whether the MIS-C diagnosis occurs in the outpatient or emergency setting, the length of stay is the same, and that the most common symptoms are fever, gastrointestinal, mucocutaneous, and cardiac symptoms regardless of initial presentation setting, she said.
More research is needed, and future studies should examine “any potential underlying factors making these particular kids susceptible to MIS-C,” Dr. Kinsella added.
The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts, but serves on the Pediatric News Editorial Advisory Board.
Length of hospital stay and the need for intensive care for pediatric COVID-19 patients with multisystem inflammatory syndrome in children was not significantly different for those who presented first as outpatients or emergency patients, based on data from 34 children.
Multisystem inflammatory syndrome in children (MIS-C) can be challenging to diagnose, as the key characteristics of fever, elevated inflammatory markers, and involvement of at least two organ systems often overlap with other illnesses, said Erin B. Treemarcki, DO, of the University of Utah, Salt Lake City, and colleagues.
“Primary care and urgent care providers are often the first point of health care for children with symptoms of MIS-C,” the researchers wrote. In a study (Poster 142) presented at the annual meeting of the Pediatric Academic Societies, held virtually, the researchers conducted a retrospective review of 34 patients younger than 21 years who were hospitalized with MIS-C at a single center between April 2020 and December 2020. The average age of the patients was 7.9 years, 68% were male, 82% were White, and 53% first presented to an outpatient clinic.
Sixteen patients presented to an emergency department and 18 presented to an ambulatory setting. The length of hospitalization ranged from 3 to 16 days with a median of 6 days, and the PICU stay ranged from 1 to 10 days with a median of 2 days.
Overall, the length of hospital stay and rate of PICU admission were not significantly different between the emergency presentation and outpatient presentation groups. Twenty-four patients entered the PICU, 13 at admission and 11 as transfers. However, the median number of days of symptoms prior to admission was significantly higher for outpatient cases (6 days vs. 4 days, P = .03).
One patient was readmitted to the hospital within 30 days for aseptic meningitis, and none of the patients died.
Initial symptoms were not significantly different for outpatient vs. emergency department patients. The most common initial manifestations of MIS-C included fever (100%), gastrointestinal symptoms (85%), and mucocutaneous symptoms (88%). Mucocutaneous symptoms included rash, oral mucosal changes, conjunctivitis, and hand/foot edema. In addition, 65% of the patients met at least 3 criteria for Kawasaki disease, the researchers noted.
The most common elevated labs at presentation regardless of setting were D-dimer (100%), C-reactive protein (97%), ferritin (97%), procalcitonin (97%), and serum IL-6 (94%).
The study findings were limited by the small sample size and focus on data from a single center. However, the results emphasize the varied presentations of MIS-C and the importance that both primary care and urgent care providers know the signs, as they are often the first point of health care for children with MIS-C, the researchers noted.
Keep looking for factors that put children at risk
“MIS-C is probably the most serious complication of COVID in children, so we as pediatricians on the front line need to know what it looks like,” Karalyn Kinsella, MD, a pediatrician in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was surprised by the study finding that children’s length of hospital stay was not affected by presentation setting.
“I would have thought the kids presenting in an outpatient setting would take longer to diagnose, and therefore have a longer hospital stay,” she noted. Instead, the take-home message is that whether the MIS-C diagnosis occurs in the outpatient or emergency setting, the length of stay is the same, and that the most common symptoms are fever, gastrointestinal, mucocutaneous, and cardiac symptoms regardless of initial presentation setting, she said.
More research is needed, and future studies should examine “any potential underlying factors making these particular kids susceptible to MIS-C,” Dr. Kinsella added.
The researchers had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts, but serves on the Pediatric News Editorial Advisory Board.
FROM PAS 2021
Worse outcomes for patients with COPD and COVID-19
A study of COVID-19 outcomes across the United States bolsters reports from China and Europe that indicate that patients with chronic obstructive pulmonary disease (COPD) and SARS-CoV-2 infection have worse outcomes than those of patients with COVID-19 who do not have COPD.
Investigators at the University of Texas Medical Branch at Galveston, Texas, combed through electronic health records from four geographic regions of the United States and identified a cohort of 6,056 patients with COPD among 150,775 patients whose records indicate either a diagnostic code or a positive laboratory test result for COVID-19.
Their findings indicate that patients with both COPD and COVID-19 “have worse outcomes compared to non-COPD COVID-19 patients, including 14-day hospitalization, length of stay, ICU admission, 30-day mortality, and use of mechanical ventilation,” Daniel Puebla Neira, MD, and colleagues from the University of Texas Medical Branch reported in a thematic poster presented during the American Thoracic Society (ATS) 2021 virtual international conference.
A critical care specialist who was not involved in the study said that the results are concerning but not surprising.
“If you already have a lung disease and you develop an additional lung disease on top of that, you don’t have as much reserve and you’re not going to tolerate the acute COVID infection,” said ATS expert Marc Moss, MD, Roger S. Mitchell Professor of Medicine in the division of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The evidence shows that “patients with COPD should be even more cautious, because if they get sick and develop, they could do worse,” he said in an interview.
Retrospective analysis
Dr. Neira and colleagues assessed the characteristics and outcomes of patients with COPD who were treated for COVID-19 in the United States from March through August 2020.
Baseline demographics of the patients with and those without COPD were similar except that the mean age was higher among patients with COPD (68.62 vs. 47.08 years).
In addition, a significantly higher proportion of patients with COPD had comorbidities compared with those without COPD. Comorbidities included diabetes, hypertension, asthma, chronic kidney disease, end-stage renal disease, stroke, heart failure, cancer, coronary artery disease, and liver disease (P < .0001 for all comparisons).
Among patients with COPD, percentages were higher with respect to the following parameters: 14-day hospitalization for any cause (28.7% vs. 10.4%), COVID-19-related 14-day hospitalization (28.1% vs. 9.9%), ICU use (26.3% vs. 17.9%), mechanical ventilation use (26.3% vs. 16.1%), and 30-day mortality (13.6% vs. 7.2%; P < .0001 for all comparisons).
‘Mechanisms unclear’
“It is unclear what mechanisms drive the association between COPD and mortality in hospitalized patients with COVID-19,” the investigators wrote. “Several biological factors have been proposed, including chronic lung inflammation, oxidative stress, protease-antiprotease imbalance, and increased airway mediators.”
They recommend use of multivariable logistic regression to tease out the effects of covariates among patients with COPD and COVID-19 and call for research into long-term outcomes for these patients, “as survivors of critical illness are increasingly recognized to have cognitive, psychological, and physical consequences.”
Dr. Moss said that in general, the management of patients with COPD and COVID-19 is similar to that for patients with COVID-19 who do not have COPD, although there may be “subtle” differences, such as ventilator settings for patients with COPD.
No source of funding for the study has been disclosed. The investigators and Dr. Moss have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A study of COVID-19 outcomes across the United States bolsters reports from China and Europe that indicate that patients with chronic obstructive pulmonary disease (COPD) and SARS-CoV-2 infection have worse outcomes than those of patients with COVID-19 who do not have COPD.
Investigators at the University of Texas Medical Branch at Galveston, Texas, combed through electronic health records from four geographic regions of the United States and identified a cohort of 6,056 patients with COPD among 150,775 patients whose records indicate either a diagnostic code or a positive laboratory test result for COVID-19.
Their findings indicate that patients with both COPD and COVID-19 “have worse outcomes compared to non-COPD COVID-19 patients, including 14-day hospitalization, length of stay, ICU admission, 30-day mortality, and use of mechanical ventilation,” Daniel Puebla Neira, MD, and colleagues from the University of Texas Medical Branch reported in a thematic poster presented during the American Thoracic Society (ATS) 2021 virtual international conference.
A critical care specialist who was not involved in the study said that the results are concerning but not surprising.
“If you already have a lung disease and you develop an additional lung disease on top of that, you don’t have as much reserve and you’re not going to tolerate the acute COVID infection,” said ATS expert Marc Moss, MD, Roger S. Mitchell Professor of Medicine in the division of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The evidence shows that “patients with COPD should be even more cautious, because if they get sick and develop, they could do worse,” he said in an interview.
Retrospective analysis
Dr. Neira and colleagues assessed the characteristics and outcomes of patients with COPD who were treated for COVID-19 in the United States from March through August 2020.
Baseline demographics of the patients with and those without COPD were similar except that the mean age was higher among patients with COPD (68.62 vs. 47.08 years).
In addition, a significantly higher proportion of patients with COPD had comorbidities compared with those without COPD. Comorbidities included diabetes, hypertension, asthma, chronic kidney disease, end-stage renal disease, stroke, heart failure, cancer, coronary artery disease, and liver disease (P < .0001 for all comparisons).
Among patients with COPD, percentages were higher with respect to the following parameters: 14-day hospitalization for any cause (28.7% vs. 10.4%), COVID-19-related 14-day hospitalization (28.1% vs. 9.9%), ICU use (26.3% vs. 17.9%), mechanical ventilation use (26.3% vs. 16.1%), and 30-day mortality (13.6% vs. 7.2%; P < .0001 for all comparisons).
‘Mechanisms unclear’
“It is unclear what mechanisms drive the association between COPD and mortality in hospitalized patients with COVID-19,” the investigators wrote. “Several biological factors have been proposed, including chronic lung inflammation, oxidative stress, protease-antiprotease imbalance, and increased airway mediators.”
They recommend use of multivariable logistic regression to tease out the effects of covariates among patients with COPD and COVID-19 and call for research into long-term outcomes for these patients, “as survivors of critical illness are increasingly recognized to have cognitive, psychological, and physical consequences.”
Dr. Moss said that in general, the management of patients with COPD and COVID-19 is similar to that for patients with COVID-19 who do not have COPD, although there may be “subtle” differences, such as ventilator settings for patients with COPD.
No source of funding for the study has been disclosed. The investigators and Dr. Moss have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A study of COVID-19 outcomes across the United States bolsters reports from China and Europe that indicate that patients with chronic obstructive pulmonary disease (COPD) and SARS-CoV-2 infection have worse outcomes than those of patients with COVID-19 who do not have COPD.
Investigators at the University of Texas Medical Branch at Galveston, Texas, combed through electronic health records from four geographic regions of the United States and identified a cohort of 6,056 patients with COPD among 150,775 patients whose records indicate either a diagnostic code or a positive laboratory test result for COVID-19.
Their findings indicate that patients with both COPD and COVID-19 “have worse outcomes compared to non-COPD COVID-19 patients, including 14-day hospitalization, length of stay, ICU admission, 30-day mortality, and use of mechanical ventilation,” Daniel Puebla Neira, MD, and colleagues from the University of Texas Medical Branch reported in a thematic poster presented during the American Thoracic Society (ATS) 2021 virtual international conference.
A critical care specialist who was not involved in the study said that the results are concerning but not surprising.
“If you already have a lung disease and you develop an additional lung disease on top of that, you don’t have as much reserve and you’re not going to tolerate the acute COVID infection,” said ATS expert Marc Moss, MD, Roger S. Mitchell Professor of Medicine in the division of pulmonary sciences and critical care medicine at the University of Colorado, Aurora.
The evidence shows that “patients with COPD should be even more cautious, because if they get sick and develop, they could do worse,” he said in an interview.
Retrospective analysis
Dr. Neira and colleagues assessed the characteristics and outcomes of patients with COPD who were treated for COVID-19 in the United States from March through August 2020.
Baseline demographics of the patients with and those without COPD were similar except that the mean age was higher among patients with COPD (68.62 vs. 47.08 years).
In addition, a significantly higher proportion of patients with COPD had comorbidities compared with those without COPD. Comorbidities included diabetes, hypertension, asthma, chronic kidney disease, end-stage renal disease, stroke, heart failure, cancer, coronary artery disease, and liver disease (P < .0001 for all comparisons).
Among patients with COPD, percentages were higher with respect to the following parameters: 14-day hospitalization for any cause (28.7% vs. 10.4%), COVID-19-related 14-day hospitalization (28.1% vs. 9.9%), ICU use (26.3% vs. 17.9%), mechanical ventilation use (26.3% vs. 16.1%), and 30-day mortality (13.6% vs. 7.2%; P < .0001 for all comparisons).
‘Mechanisms unclear’
“It is unclear what mechanisms drive the association between COPD and mortality in hospitalized patients with COVID-19,” the investigators wrote. “Several biological factors have been proposed, including chronic lung inflammation, oxidative stress, protease-antiprotease imbalance, and increased airway mediators.”
They recommend use of multivariable logistic regression to tease out the effects of covariates among patients with COPD and COVID-19 and call for research into long-term outcomes for these patients, “as survivors of critical illness are increasingly recognized to have cognitive, psychological, and physical consequences.”
Dr. Moss said that in general, the management of patients with COPD and COVID-19 is similar to that for patients with COVID-19 who do not have COPD, although there may be “subtle” differences, such as ventilator settings for patients with COPD.
No source of funding for the study has been disclosed. The investigators and Dr. Moss have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Atorvastatin: A potential treatment in COVID-19?
For patients with COVID-19 admitted to intensive care, giving atorvastatin 20 mg/d did not result in a significant reduction in risk for venous or arterial thrombosis, for treatment with extracorporeal membrane oxygenation (ECMO), or for all-cause mortality, compared with placebo in the INSPIRATION-S study.
However, there was a suggestion of benefit in the subgroup of patients who were treated within 7 days of COVID-19 symptom onset.
The study was presented by Behnood Bikdeli, MD, Brigham and Women’s Hospital, Boston, on May 16 at the annual scientific sessions of the American College of Cardiology.
He explained that COVID-19 is characterized by an exuberant immune response and that there is a potential for thrombotic events because of enhanced endothelial activation and a prothrombotic state.
“In this context, it is interesting to think about statins as potential agents to be studied in COVID-19, because as well as having lipid-lowering actions, they are also thought to have anti-inflammatory and antithrombotic effects,” he said.
In the HARP-2 trial of simvastatin in acute respiratory distress syndrome (ARDS), published a few years ago, the main results were neutral, but in the subgroup of patients with hyperinflammatory ARDS, there was a reduction in mortality with simvastatin in comparison with placebo, Dr. Bikdeli noted.
Moreover, in a series of observational studies of patients with COVID-19, use of statins was associated with a reduction in mortality among hospitalized patients. However, there are limited high-quality data to guide clinical practice, he said.
The INSPIRATION study, conducted in 11 hospitals in Iran, had a two-by-two factorial design to investigate different anticoagulant strategies and the use of atorvastatin for COVID-19 patients in the ICU.
In the anticoagulation part of the trial, which was published in JAMA in March 2020, there was no difference in the primary endpoint of an intermediate dose and standard dose of enoxaparin.
For the statin part of the trial (INSPIRATION-S), 605 patients were randomly assigned to receive atorvastatin 20 mg daily or placebo. Patients who had been taking statins beforehand were excluded. Baseline characteristics were similar for the two groups, with around a quarter of patients taking aspirin and more than 90% taking steroids.
Results showed that atorvastatin was not associated with a significant reduction in the primary outcome – a composite of adjudicated venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days – which occurred in 32.7% of the statin group versus 36.3% of the placebo group (odds ratio, 0.84; P = .35).
Atorvastatin was not associated with any significant differences in any of the individual components of the primary composite endpoint. There was also no significant difference in any of the safety endpoints, which included major bleeding and elevations in liver enzyme levels.
Subgroup analyses were mostly consistent with the main findings, with one exception.
In the subgroup of patients who presented within the first 7 days of COVID-19 symptom onset, there was a hint of a potential protective effect with atorvastatin.
In this group of 171 patients, the primary endpoint occurred in 30.9% of those taking atorvastatin versus 40.3% of those taking placebo (OR, 0.60; P = .055).
“This is an interesting observation, and it is plausible, as these patients may be in a different phase of COVID-19 disease. But we need to be cognizant of the multiplicity of comparisons, and this needs to be further investigated in subsequent studies,” Dr. Bikdeli said.
Higher dose in less sick patients a better strategy?
Discussing the study at the ACC presentation, Binita Shah, MD, said the importance of enrolling COVID-19 patients into clinical trials was paramount but that these patients in the ICU may not have been the right population in which to test a statin.
“Maybe for these very sick patients, it is just too late. Trying to rein in the inflammatory cytokine storm and the interaction with thrombosis at this point is very difficult,” Dr. Shah commented.
She suggested that it might be appropriate to try statins in an earlier phase of the disease in order to prevent the inflammatory process, rather than trying to stop it after it had already started.
Dr. Shah also questioned the use of such a low dose of atorvastatin for these patients. “In the cardiovascular literature – at least in ACS [acute coronary syndrome] – high statin doses are used to see short-term benefits. In this very inflammatory milieu, I wonder whether a high-intensity regimen would be more beneficial,” she speculated.
Dr. Bikdeli replied that a low dose of atorvastatin was chosen because early on, several antiviral agents, such as ritonavir, were being used for COVID-19 patients, and these drugs were associated with increases in liver enzyme levels.
“We didn’t want to exacerbate that with high doses of statins,” he said. “But we have now established the safety profile of atorvastatin in these patients, and in retrospect, yes, a higher dose might have been better.”
The INSPIRATION study was funded by the Rajaie Cardiovascular Medical and Research Center, Tehran, Iran. Dr. Bikdeli has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with COVID-19 admitted to intensive care, giving atorvastatin 20 mg/d did not result in a significant reduction in risk for venous or arterial thrombosis, for treatment with extracorporeal membrane oxygenation (ECMO), or for all-cause mortality, compared with placebo in the INSPIRATION-S study.
However, there was a suggestion of benefit in the subgroup of patients who were treated within 7 days of COVID-19 symptom onset.
The study was presented by Behnood Bikdeli, MD, Brigham and Women’s Hospital, Boston, on May 16 at the annual scientific sessions of the American College of Cardiology.
He explained that COVID-19 is characterized by an exuberant immune response and that there is a potential for thrombotic events because of enhanced endothelial activation and a prothrombotic state.
“In this context, it is interesting to think about statins as potential agents to be studied in COVID-19, because as well as having lipid-lowering actions, they are also thought to have anti-inflammatory and antithrombotic effects,” he said.
In the HARP-2 trial of simvastatin in acute respiratory distress syndrome (ARDS), published a few years ago, the main results were neutral, but in the subgroup of patients with hyperinflammatory ARDS, there was a reduction in mortality with simvastatin in comparison with placebo, Dr. Bikdeli noted.
Moreover, in a series of observational studies of patients with COVID-19, use of statins was associated with a reduction in mortality among hospitalized patients. However, there are limited high-quality data to guide clinical practice, he said.
The INSPIRATION study, conducted in 11 hospitals in Iran, had a two-by-two factorial design to investigate different anticoagulant strategies and the use of atorvastatin for COVID-19 patients in the ICU.
In the anticoagulation part of the trial, which was published in JAMA in March 2020, there was no difference in the primary endpoint of an intermediate dose and standard dose of enoxaparin.
For the statin part of the trial (INSPIRATION-S), 605 patients were randomly assigned to receive atorvastatin 20 mg daily or placebo. Patients who had been taking statins beforehand were excluded. Baseline characteristics were similar for the two groups, with around a quarter of patients taking aspirin and more than 90% taking steroids.
Results showed that atorvastatin was not associated with a significant reduction in the primary outcome – a composite of adjudicated venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days – which occurred in 32.7% of the statin group versus 36.3% of the placebo group (odds ratio, 0.84; P = .35).
Atorvastatin was not associated with any significant differences in any of the individual components of the primary composite endpoint. There was also no significant difference in any of the safety endpoints, which included major bleeding and elevations in liver enzyme levels.
Subgroup analyses were mostly consistent with the main findings, with one exception.
In the subgroup of patients who presented within the first 7 days of COVID-19 symptom onset, there was a hint of a potential protective effect with atorvastatin.
In this group of 171 patients, the primary endpoint occurred in 30.9% of those taking atorvastatin versus 40.3% of those taking placebo (OR, 0.60; P = .055).
“This is an interesting observation, and it is plausible, as these patients may be in a different phase of COVID-19 disease. But we need to be cognizant of the multiplicity of comparisons, and this needs to be further investigated in subsequent studies,” Dr. Bikdeli said.
Higher dose in less sick patients a better strategy?
Discussing the study at the ACC presentation, Binita Shah, MD, said the importance of enrolling COVID-19 patients into clinical trials was paramount but that these patients in the ICU may not have been the right population in which to test a statin.
“Maybe for these very sick patients, it is just too late. Trying to rein in the inflammatory cytokine storm and the interaction with thrombosis at this point is very difficult,” Dr. Shah commented.
She suggested that it might be appropriate to try statins in an earlier phase of the disease in order to prevent the inflammatory process, rather than trying to stop it after it had already started.
Dr. Shah also questioned the use of such a low dose of atorvastatin for these patients. “In the cardiovascular literature – at least in ACS [acute coronary syndrome] – high statin doses are used to see short-term benefits. In this very inflammatory milieu, I wonder whether a high-intensity regimen would be more beneficial,” she speculated.
Dr. Bikdeli replied that a low dose of atorvastatin was chosen because early on, several antiviral agents, such as ritonavir, were being used for COVID-19 patients, and these drugs were associated with increases in liver enzyme levels.
“We didn’t want to exacerbate that with high doses of statins,” he said. “But we have now established the safety profile of atorvastatin in these patients, and in retrospect, yes, a higher dose might have been better.”
The INSPIRATION study was funded by the Rajaie Cardiovascular Medical and Research Center, Tehran, Iran. Dr. Bikdeli has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with COVID-19 admitted to intensive care, giving atorvastatin 20 mg/d did not result in a significant reduction in risk for venous or arterial thrombosis, for treatment with extracorporeal membrane oxygenation (ECMO), or for all-cause mortality, compared with placebo in the INSPIRATION-S study.
However, there was a suggestion of benefit in the subgroup of patients who were treated within 7 days of COVID-19 symptom onset.
The study was presented by Behnood Bikdeli, MD, Brigham and Women’s Hospital, Boston, on May 16 at the annual scientific sessions of the American College of Cardiology.
He explained that COVID-19 is characterized by an exuberant immune response and that there is a potential for thrombotic events because of enhanced endothelial activation and a prothrombotic state.
“In this context, it is interesting to think about statins as potential agents to be studied in COVID-19, because as well as having lipid-lowering actions, they are also thought to have anti-inflammatory and antithrombotic effects,” he said.
In the HARP-2 trial of simvastatin in acute respiratory distress syndrome (ARDS), published a few years ago, the main results were neutral, but in the subgroup of patients with hyperinflammatory ARDS, there was a reduction in mortality with simvastatin in comparison with placebo, Dr. Bikdeli noted.
Moreover, in a series of observational studies of patients with COVID-19, use of statins was associated with a reduction in mortality among hospitalized patients. However, there are limited high-quality data to guide clinical practice, he said.
The INSPIRATION study, conducted in 11 hospitals in Iran, had a two-by-two factorial design to investigate different anticoagulant strategies and the use of atorvastatin for COVID-19 patients in the ICU.
In the anticoagulation part of the trial, which was published in JAMA in March 2020, there was no difference in the primary endpoint of an intermediate dose and standard dose of enoxaparin.
For the statin part of the trial (INSPIRATION-S), 605 patients were randomly assigned to receive atorvastatin 20 mg daily or placebo. Patients who had been taking statins beforehand were excluded. Baseline characteristics were similar for the two groups, with around a quarter of patients taking aspirin and more than 90% taking steroids.
Results showed that atorvastatin was not associated with a significant reduction in the primary outcome – a composite of adjudicated venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days – which occurred in 32.7% of the statin group versus 36.3% of the placebo group (odds ratio, 0.84; P = .35).
Atorvastatin was not associated with any significant differences in any of the individual components of the primary composite endpoint. There was also no significant difference in any of the safety endpoints, which included major bleeding and elevations in liver enzyme levels.
Subgroup analyses were mostly consistent with the main findings, with one exception.
In the subgroup of patients who presented within the first 7 days of COVID-19 symptom onset, there was a hint of a potential protective effect with atorvastatin.
In this group of 171 patients, the primary endpoint occurred in 30.9% of those taking atorvastatin versus 40.3% of those taking placebo (OR, 0.60; P = .055).
“This is an interesting observation, and it is plausible, as these patients may be in a different phase of COVID-19 disease. But we need to be cognizant of the multiplicity of comparisons, and this needs to be further investigated in subsequent studies,” Dr. Bikdeli said.
Higher dose in less sick patients a better strategy?
Discussing the study at the ACC presentation, Binita Shah, MD, said the importance of enrolling COVID-19 patients into clinical trials was paramount but that these patients in the ICU may not have been the right population in which to test a statin.
“Maybe for these very sick patients, it is just too late. Trying to rein in the inflammatory cytokine storm and the interaction with thrombosis at this point is very difficult,” Dr. Shah commented.
She suggested that it might be appropriate to try statins in an earlier phase of the disease in order to prevent the inflammatory process, rather than trying to stop it after it had already started.
Dr. Shah also questioned the use of such a low dose of atorvastatin for these patients. “In the cardiovascular literature – at least in ACS [acute coronary syndrome] – high statin doses are used to see short-term benefits. In this very inflammatory milieu, I wonder whether a high-intensity regimen would be more beneficial,” she speculated.
Dr. Bikdeli replied that a low dose of atorvastatin was chosen because early on, several antiviral agents, such as ritonavir, were being used for COVID-19 patients, and these drugs were associated with increases in liver enzyme levels.
“We didn’t want to exacerbate that with high doses of statins,” he said. “But we have now established the safety profile of atorvastatin in these patients, and in retrospect, yes, a higher dose might have been better.”
The INSPIRATION study was funded by the Rajaie Cardiovascular Medical and Research Center, Tehran, Iran. Dr. Bikdeli has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Procalcitonin-guided antibiotic stewardship for lower respiratory tract infection
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 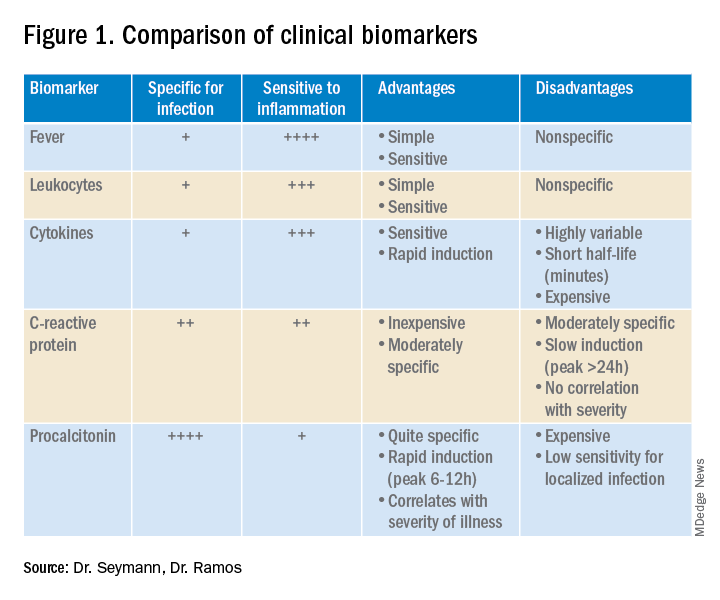
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)
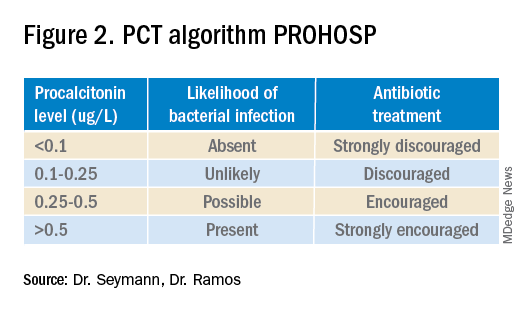
For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.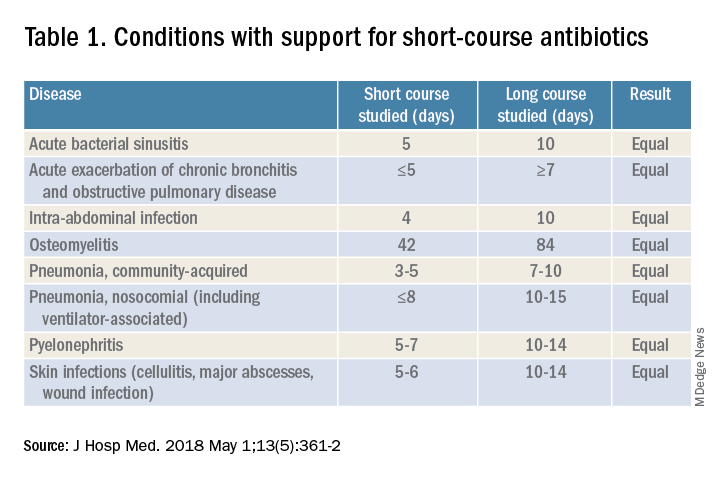
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.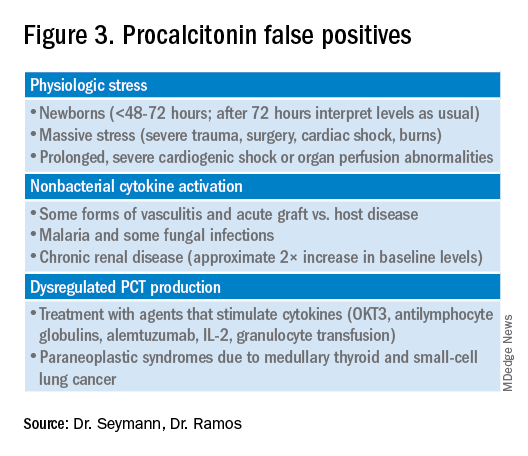
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Dynamics of the assay must be considered
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
COVID-19 in children: Weekly cases drop to 6-month low
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Dr. Fauci: Extraordinary challenges, scientific triumphs with COVID-19
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
FIDELIO-DKD: Finerenone cuts new-onset AFib in patients with type 2 diabetes and CKD
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
FROM ACC 2021
Dapagliflozin misses as treatment for COVID-19 but leaves intriguing signal for benefit
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
FROM ACC 2021













