User login
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
THE DIAGNOSIS: Cutaneous Cryptococcosis
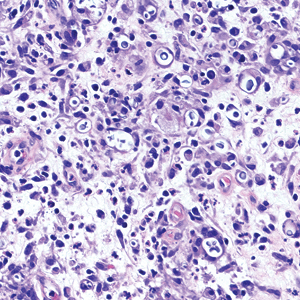
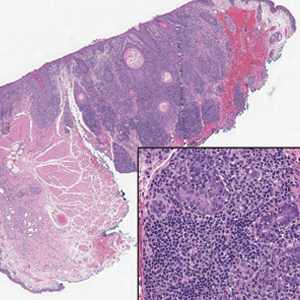
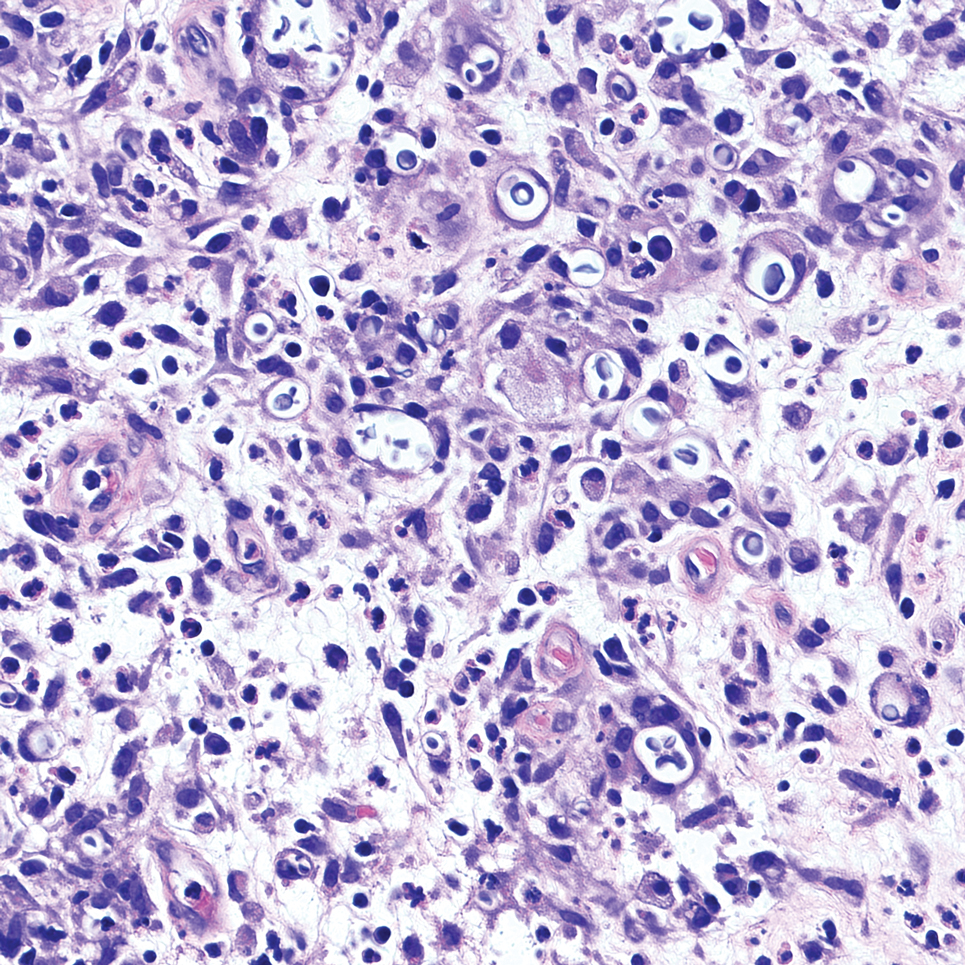
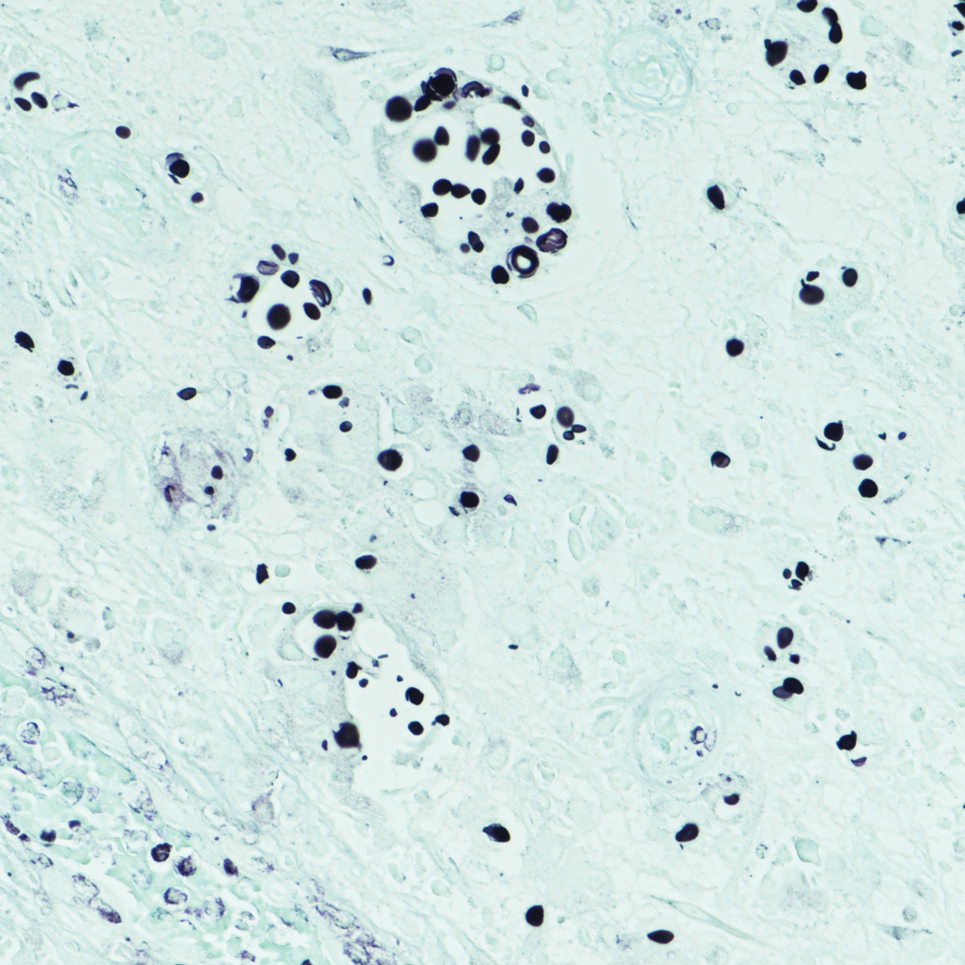
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
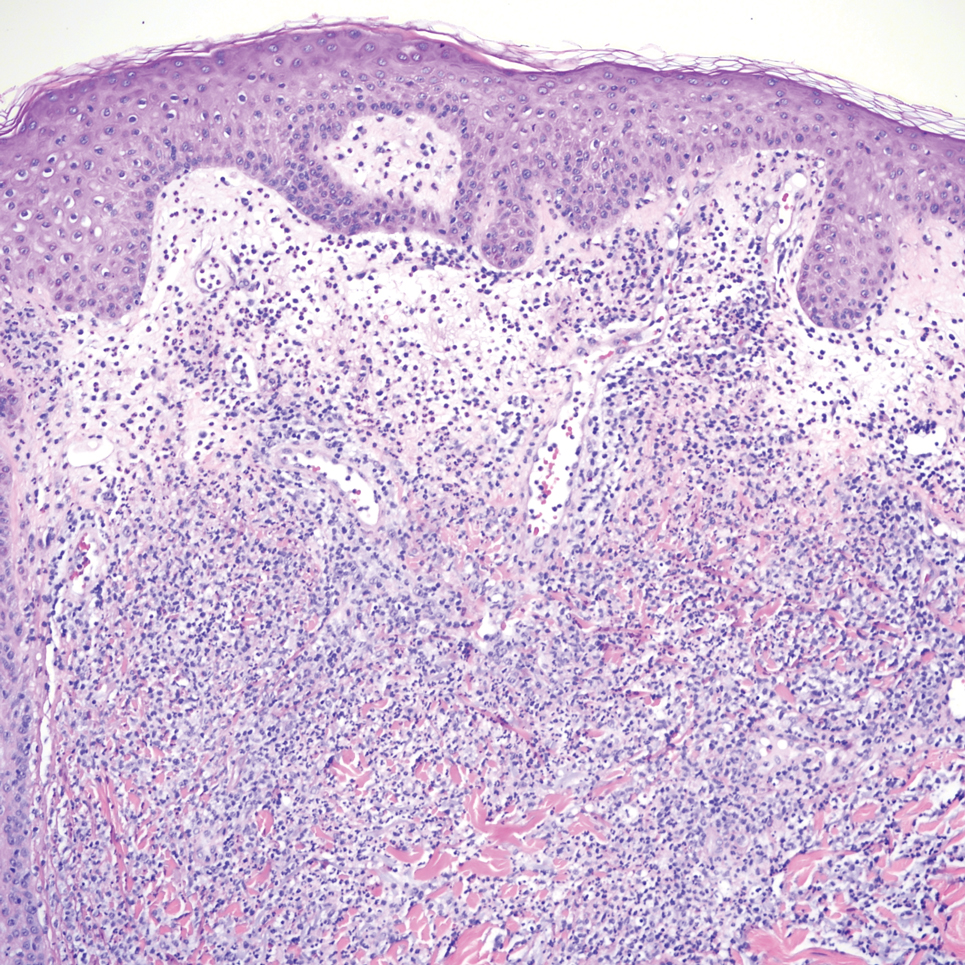
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
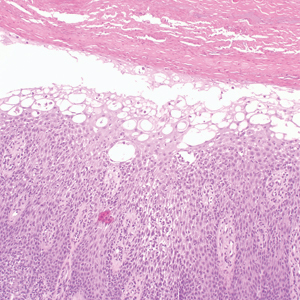
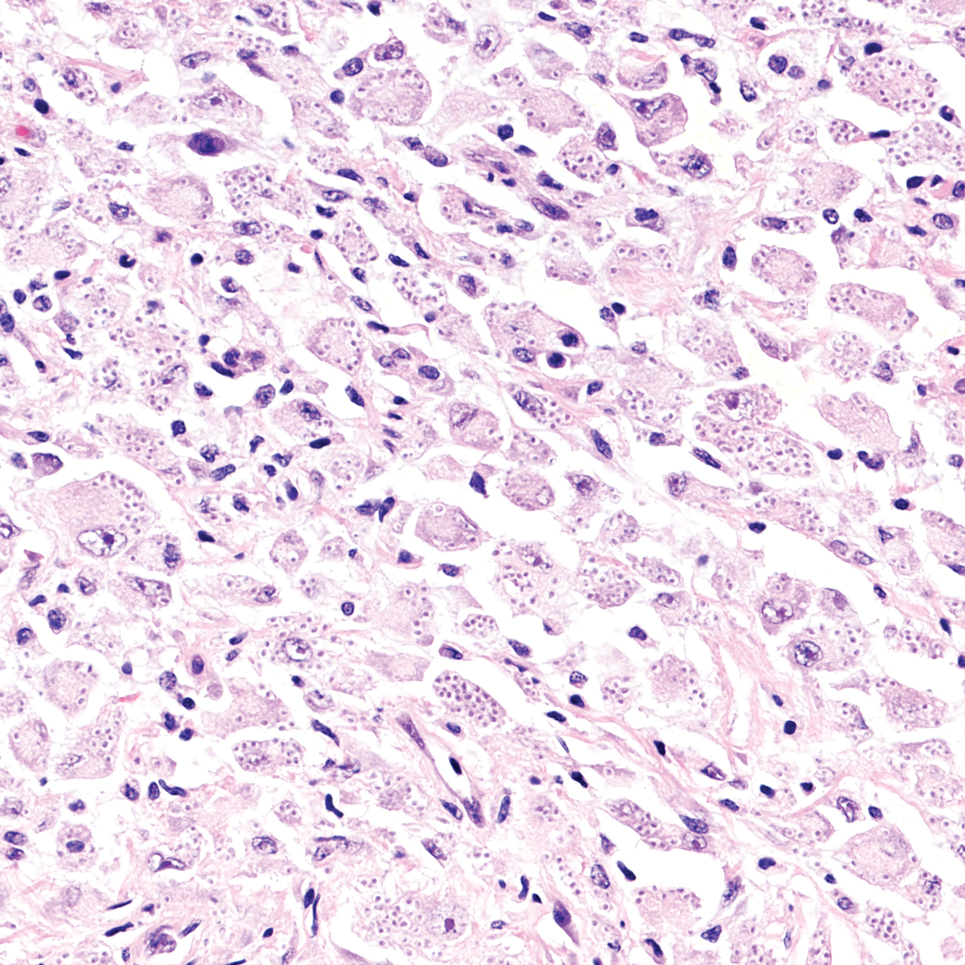
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
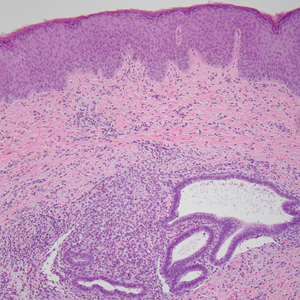
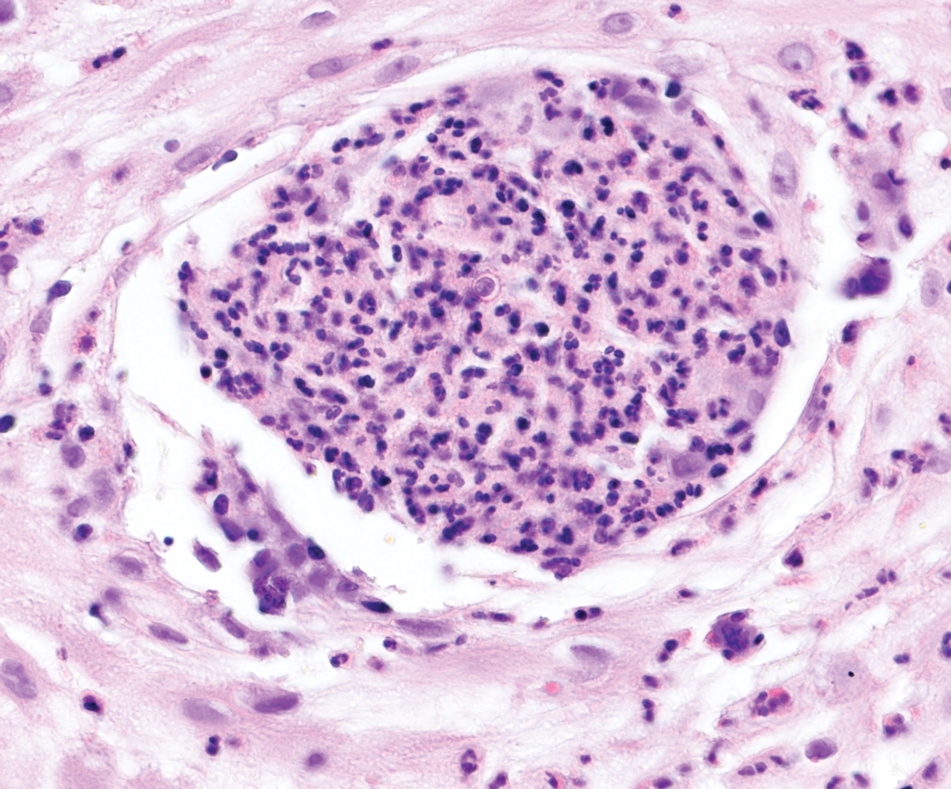
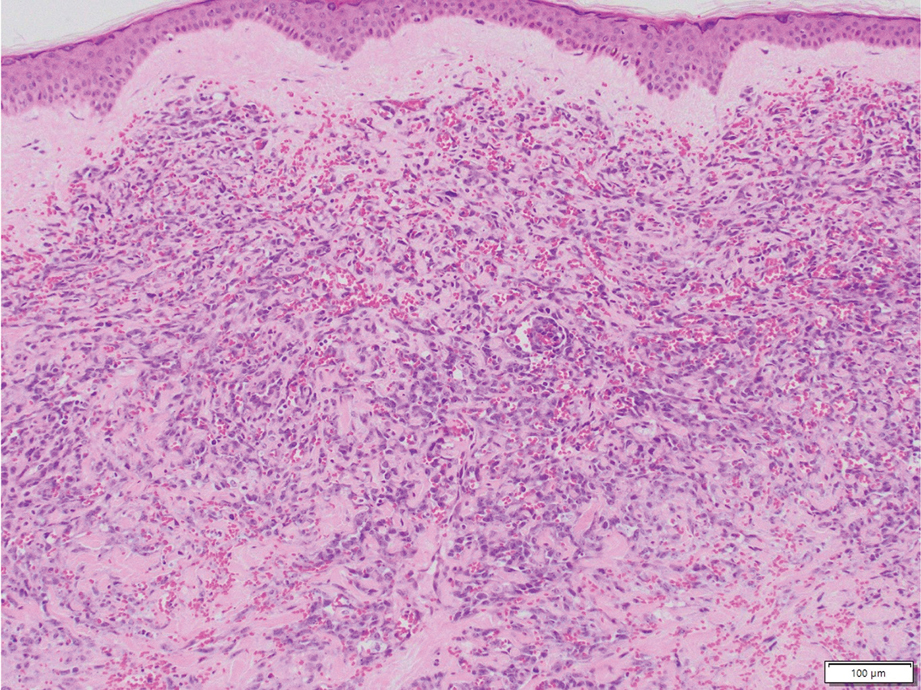
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13
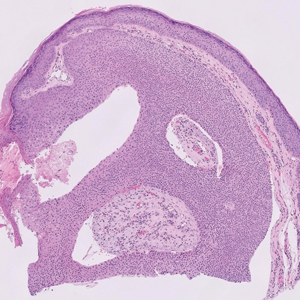
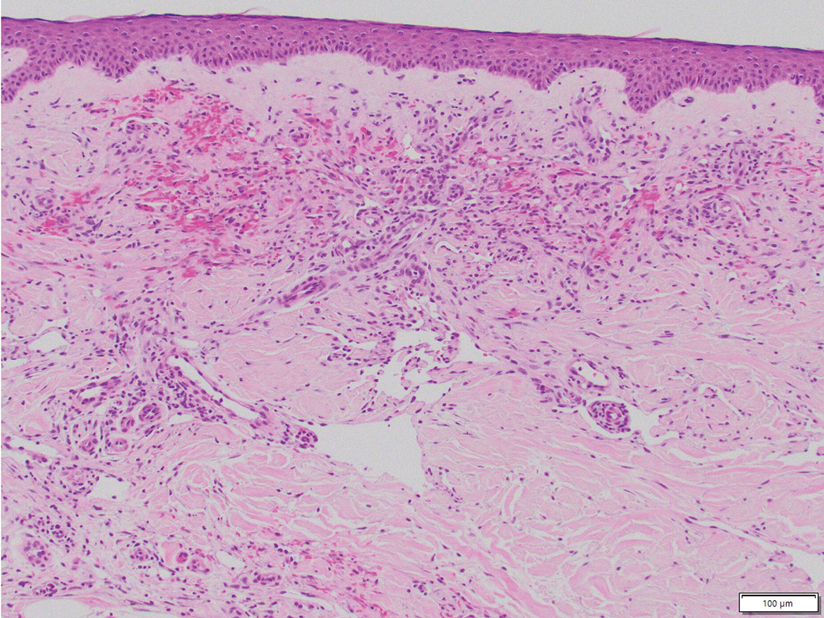
Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10
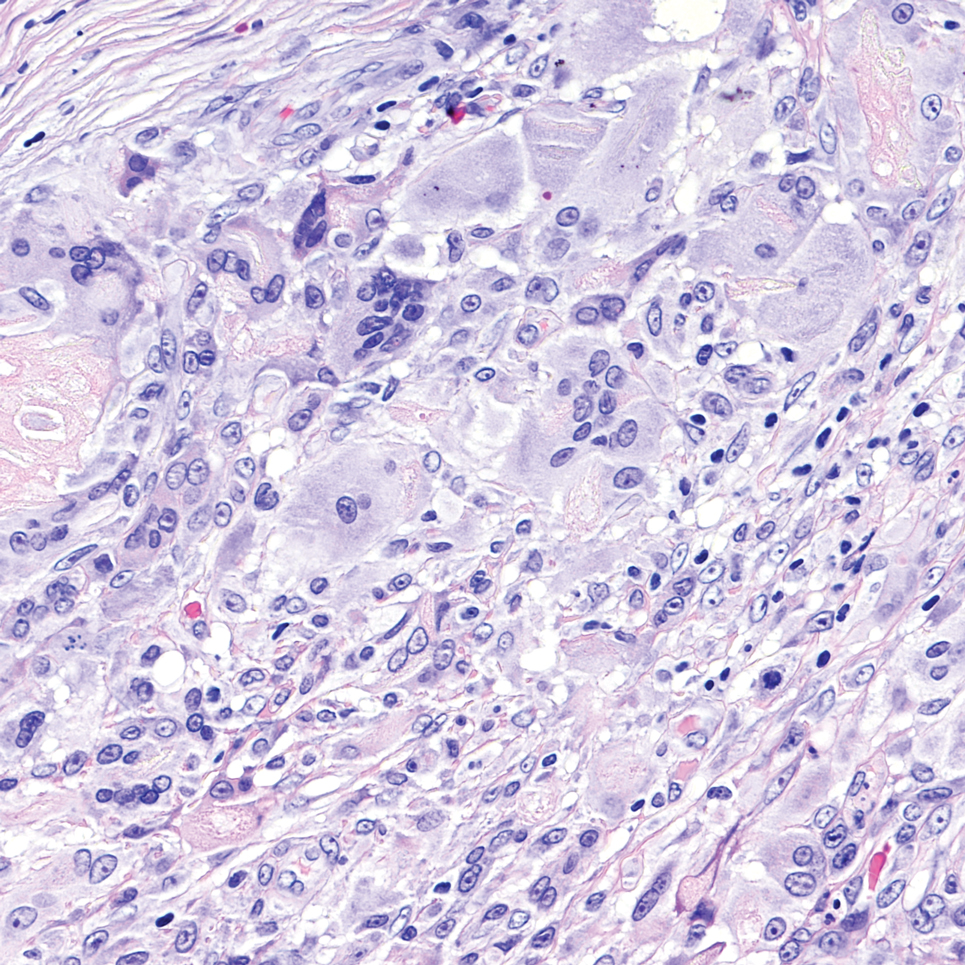
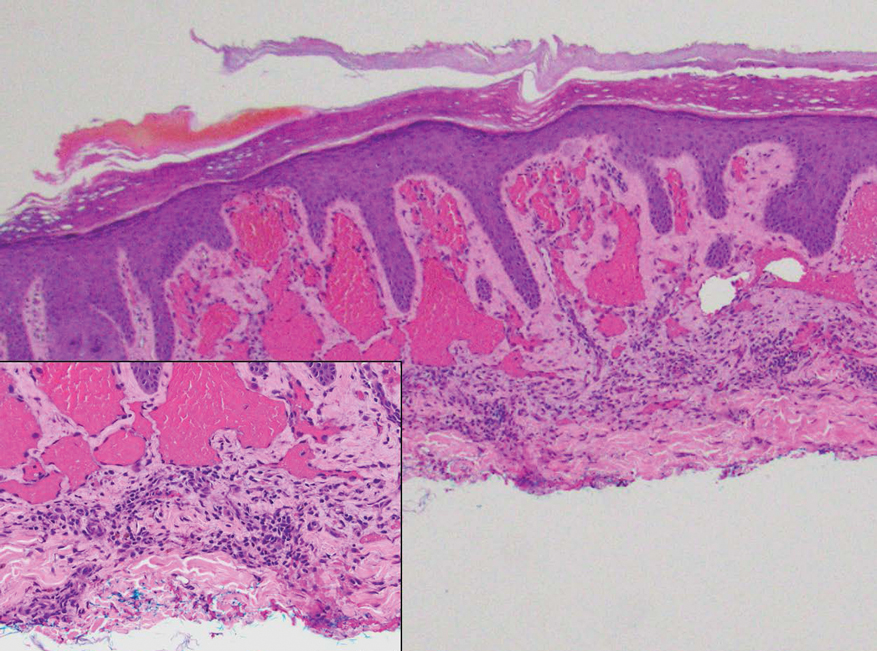
Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

THE DIAGNOSIS: Cutaneous Cryptococcosis
Biopsy of the ulcerated nodule showed numerous yeastlike organisms within clear mucinous capsules and with some surrounding inflammation. On Grocott methenamine silver staining, the organisms stained black. Workup for disseminated cryptococcus was negative, leading to a diagnosis of primary cutaneous cryptococcosis in the setting of immunosuppression. Notably, cryptococcosis infection has been reported in patients taking fingolimod (a sphingosine-1-phosphate receptor) for multiple sclerosis, which was the case for our patient.1
The genus Cryptococcus comprises more than 30 species of encapsulated basidiomycetous fungi distributed ubiquitously in nature. Currently, only 2 species are known to cause infectious disease in humans: Cryptococcus neoformans, which affects both immunocompromised and immunocompetent patients and frequently is isolated from pigeon droppings, as well as Cryptococcus gatti, which primarily affects immunocompetent patients and is more commonly isolated from soil and decaying wood.2
Primary cutaneous cryptococcosis (PCC), characterized by direct inoculation of C neoformans or C gatti via skin injury, is rare and typically is seen in patients with decreased cell-mediated immunity, such as those on chronic corticosteroid therapy, solid-organ transplant recipients, and those with HIV.3 Primary cutaneous cryptococcosis typically manifests as a solitary or confined lesion on exposed areas of the skin and often is accompanied by regional lymphadenopathy.4,5 The most common cutaneous findings associated with PCC include ulceration, cellulitis, and whitlow.5 In immunocompetent hosts, frequently affected sites include the arms, fingers, and face, while the trunk and lower extremities are more commonly affected in immunocompromised hosts.3 Secondary cutaneous cryptococcosis occurs through hematologic spread in patients with disseminated cryptococcosis after inhalation of Cryptococcosis spores and differs from PCC in that it typically manifests as multiple lesions scattered on both exposed and covered areas of the skin. Patients also may have signs and symptoms of disseminated cryptococcosis such as pneumonia and/or meningitis at presentation.5
Despite the difference between PCC and secondary cutaneous cryptococcosis, almost every type of skin lesion has been observed in cryptococcosis, including pustules, nodules, vesicles, acneform lesions, purpura, ulcers, abscesses, molluscumlike lesions, granulomas, draining sinuses, and cellulitis.6,7
Cutaneous cryptococcosis generally is associated with 2 types of histologic reactions: gelatinous and granulomatous. The gelatinous reaction shows numerous yeastlike organisms ranging from 4 μm to 12 μm in diameter with large mucinous polysaccharide capsules and scant inflammation. Organisms may be seen in mucoid sheets.8 The granulomatous type shows a more pronounced reaction with fewer organisms ranging from 2 μm to 4 μm in diameter found within giant cells, histiocytes, and lymphocytes.6,9 Areas of necrosis occasionally can be observed.8
It is important to consider infection with Blastomyces dermatitidis and Histoplasma capsulatum in the differential Both entities can manifest as necrotizing granulomas on histology (Figures 1 and 2).10 Microscopic morphology can help differentiate these pathogenic fungi from Cryptococcus diagnosis of cryptococcosis. species which show pleomorphic, narrow-based budding yeast with wide capsules. In contrast, H capsulatum is characterized by small, intracellular, yeastlike cells with microconidia and macroconidia, while B dermatitidis is distinguished by spherical, thick-walled cells with broad-based budding.11 Capsular material also can help distinguish Cryptococcus from other pathogenic fungi. Special stains highlighting the polysaccharide capsule of Cryptococcus can best identify the yeast. The capsule stains red with periodic acid–Schiff, blue with Alcian blue, and black with Grocott methenamine silver. Mucicarmine is especially useful as it can stain the mucinous capsule pinkish red and typically does not stain other pathogenic fungi.12 Capsule-deficient organisms can lead to considerable difficulties in diagnosis given the organisms can vary in size and may mimic H capsulatum or B dermatitidis. The Fontana-Masson stain is a valuable tool in identifying capsule-deficient organisms, as melanin is found in Cryptococcus cell walls; thus, positive staining excludes H capsulatum and B dermatitidis.13


Cutaneous foreign body granuloma, which refers to a granulomatous inflammatory reaction to a foreign body in the skin, is another differential diagnosis that is important to distinguish from cutaneous cryptococcosis. On histology, a collection of histiocytes surround the inert material, forming giant cells without an immune response (Figure 3).10 In contrast, granulomas caused by infectious etiologies (eg, Cryptococcus species) have an associated adaptive immune response and can be further classified as necrotizing or non-necrotizing. Necrotizing granulomas have a distinct central necrosis with a surrounding lymphohistiocytic reaction with peripheral chronic inflammation.10

Sweet syndrome is another mimicker of cutaneous cryptococcosis. A histologic variant of Sweet syndrome has been reported that has characteristic cutaneous lesions clinically but shows basophilic bodies with a surrounding halo on pathology that can be mistaken for Cryptococcus yeast. Classic histopathology of Sweet syndrome features papillary dermal edema with neutrophil or histiocytelike inflammatory infiltrate (Figure 4). Identification of Sweet syndrome can be aided by positive myeloperoxidase staining and negative periodic acid–Schiff staining.14,15

- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
- Lehmann NM, Kammeyer JA. Cerebral venous thrombosis due to Cryptococcus in a multiple sclerosis patient on fingolimod. Case Rep Neurol. 2022; 14:286-290. doi:10.1159/000524359
- Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. doi:10.1016/j.idc.2015.10.006.
- Christianson JC, Engber W, Andes D. Primary cutaneous cryptococcosis in immunocompetent and immunocompromised hosts. Med Mycol. 2003;41:177-188. doi:10.1080/1369378031000137224
- Tilak R, Prakash P, Nigam C, et al. Cryptococcal meningitis with an antecedent cutaneous Cryptococcal lesion. Dermatol Online J. 2009;15:12.
- Neuville S, Dromer F, Morin O, et al. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36:337-347. doi:10.1086/345956
- Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32:844-850.
- Anderson DJ, Schmidt C, Goodman J, Pomeroy C. Cryptococcal disease presenting as cellulitis. Clin Infect Dis. 1992;14:666-672. doi:10.1093/clinids/14.3.666
- Moore M. Cryptococcosis with cutaneous manifestations: four cases with a review of published reports. J Invest Dermatol. 1957;28(2):159-182. doi: 10.1038/jid.1957.17
- Phan NQ, Tirado M, Moeckel SMC, et al. Cutaneous and pulmonary cryptococcosis in an immunocompetent patient. J Dtsch Dermatol Ges. 2019;17:1283-1286. doi:10.1111/ddg.13997.
- Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. J Clin Tuberc Other Mycobact Dis. 2017;7:1-12. doi: 10.1016/j.jctube.2017.02.001
- Fridlington E, Colome-Grimmer M, Kelly E, et al. Tzanck smear as a rapid diagnostic tool for disseminated cryptococcal infection. Arch Dermatol. 2006;142:25-27. doi: 10.1001/archderm.142.1.25
- Hernandez AD. Cutaneous Cryptococcosis. Dermatol Clin. 1989; 7:269-274.
- Ro JY, Lee SS, Ayala AG. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987;111:53-57.
- Jordan AA, Graciaa DS, Gopalsamy SN, et al. Sweet syndrome imitating cutaneous cryptococcal disease. Open Forum Infect Dis. 2022;9:ofac608. doi: 10.1093/ofid/ofac608
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi: 10.1111/cup.12019
Pink Ulcerated Nodule on the Forearm
Pink Ulcerated Nodule on the Forearm
A 51-year-old man with a history of multiple sclerosis treated with fingolimod presented to the dermatology department with an ulcerated lesion on the left forearm of 2 to 3 months’ duration. The patient reported that he recently presented to the emergency department for drainage of the lesion, which was unsuccessful. Shortly after, he traumatized the lesion at his construction job. At the current presentation, physical examination revealed a 1-cm, flesh-colored to faintly pink, ulcerated nodule on the left forearm. A biopsy was performed.
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
THE DIAGNOSIS: Poroma
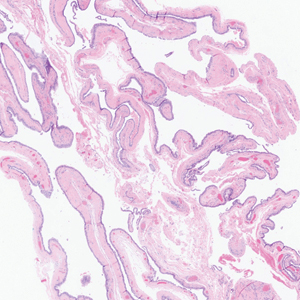
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
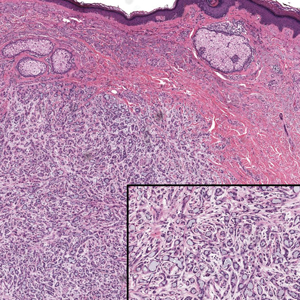
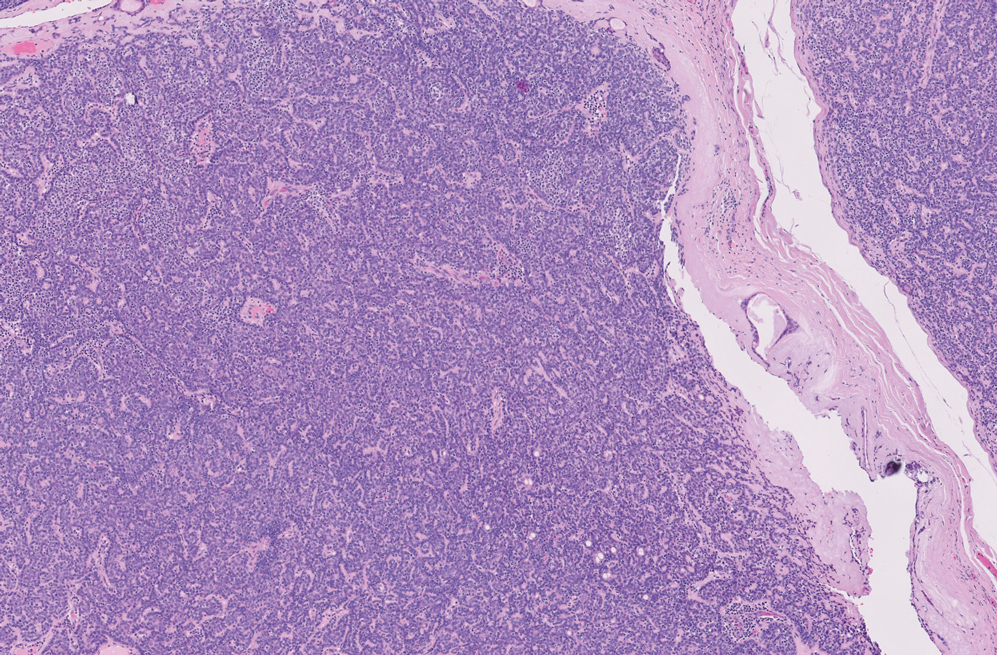
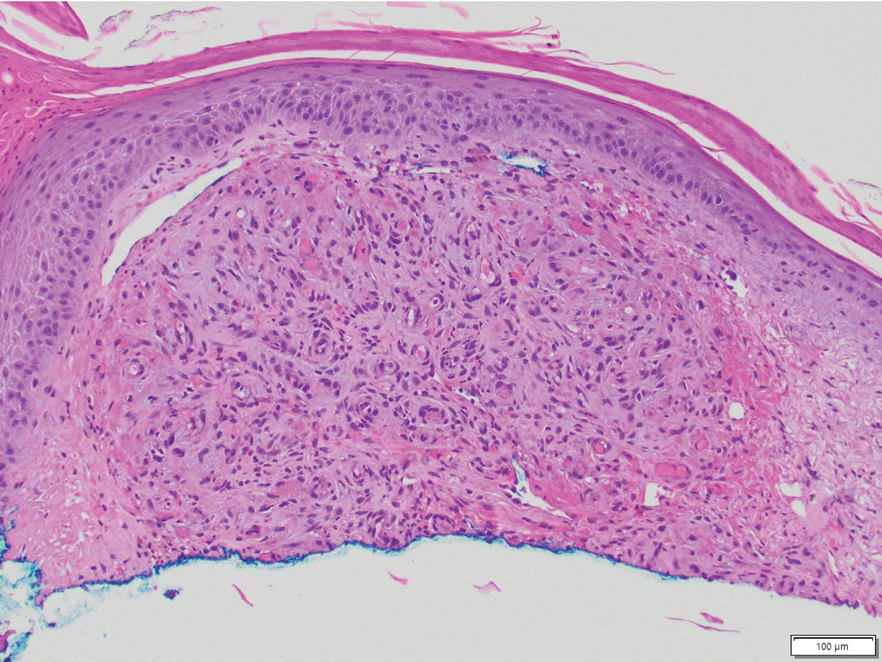
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11
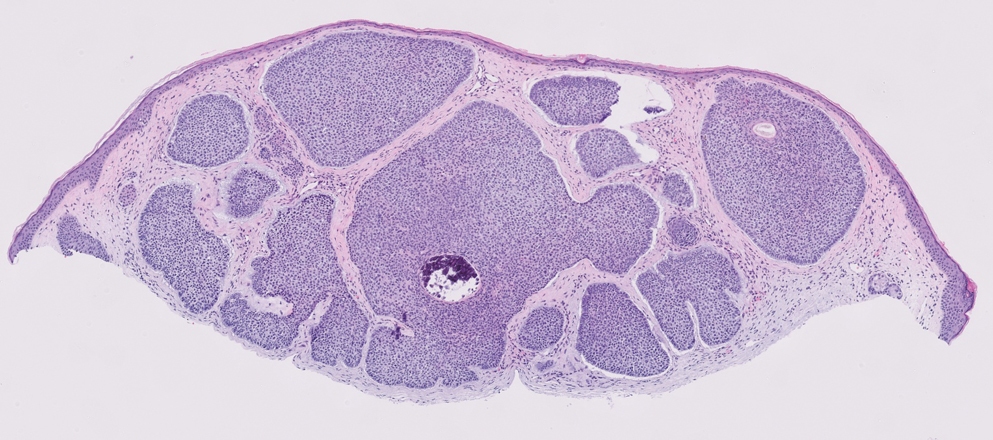
Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5
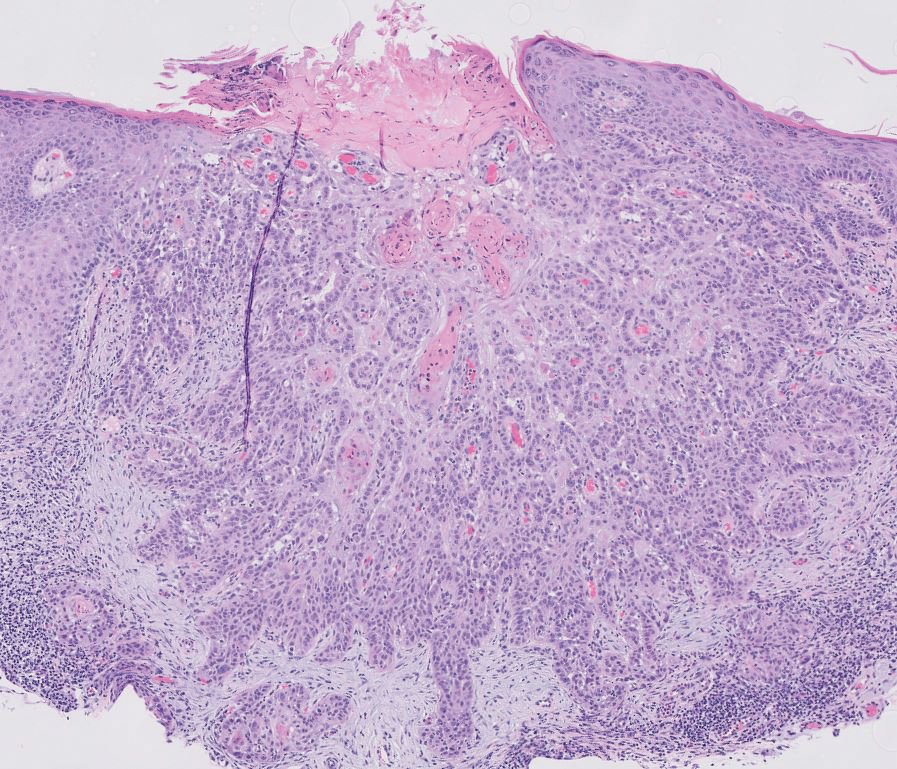
Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13
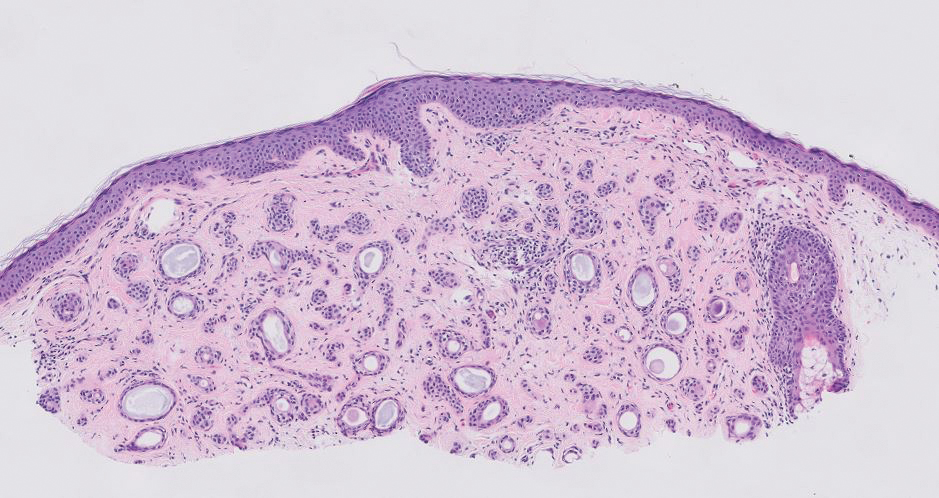
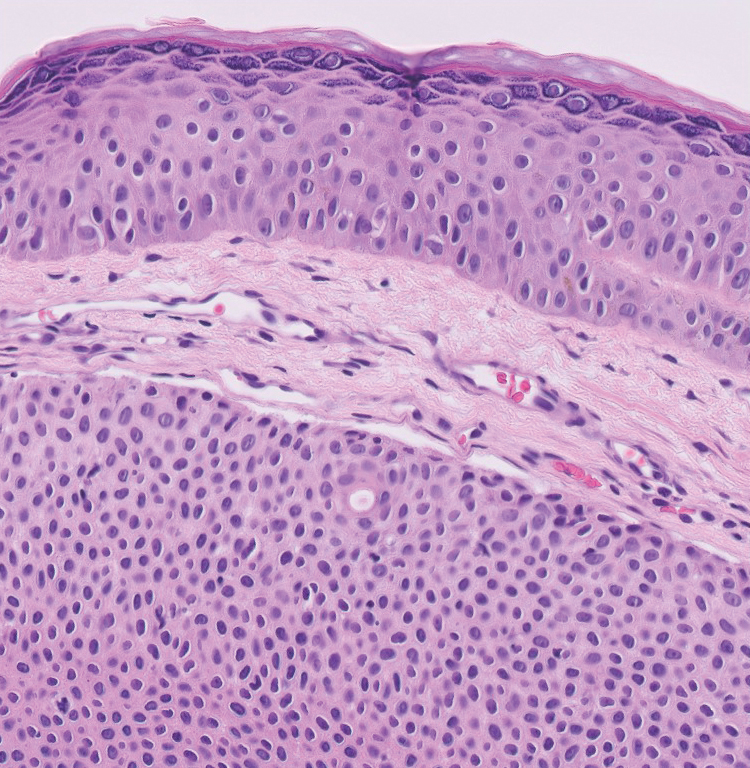
Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4
Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
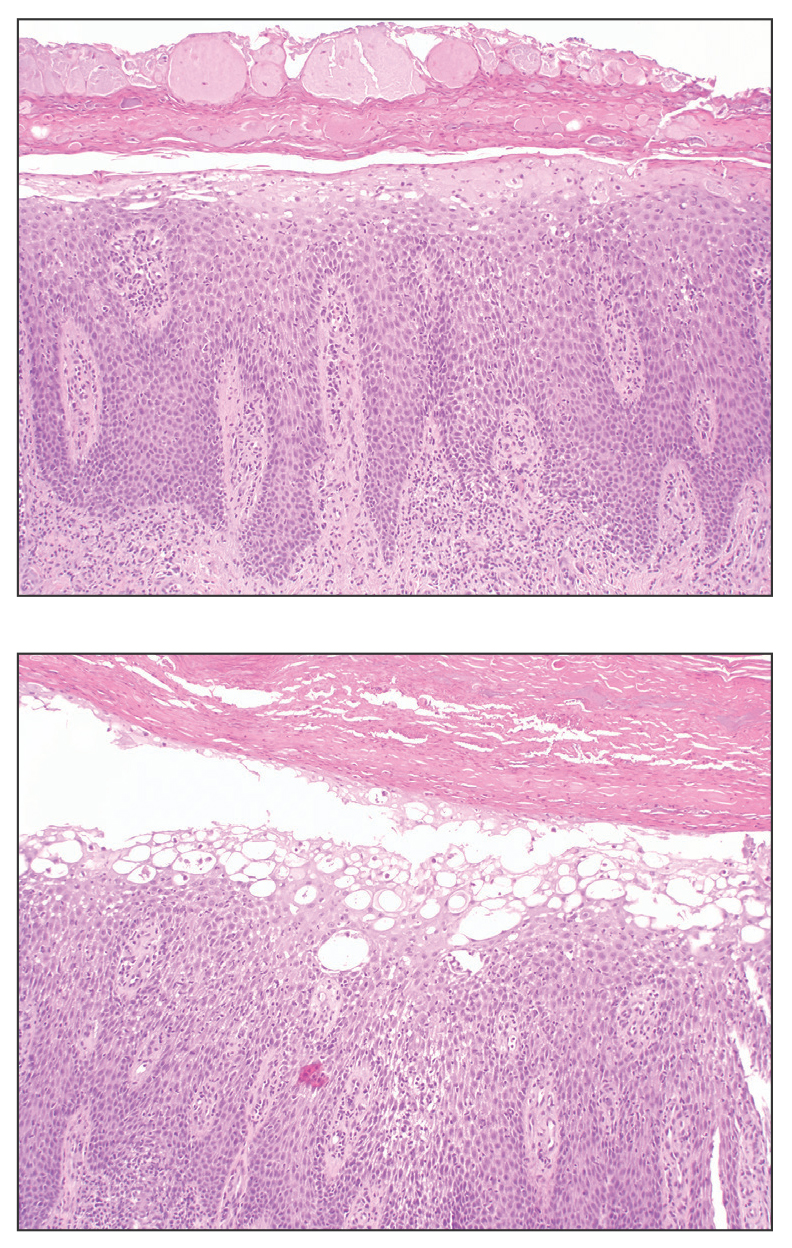
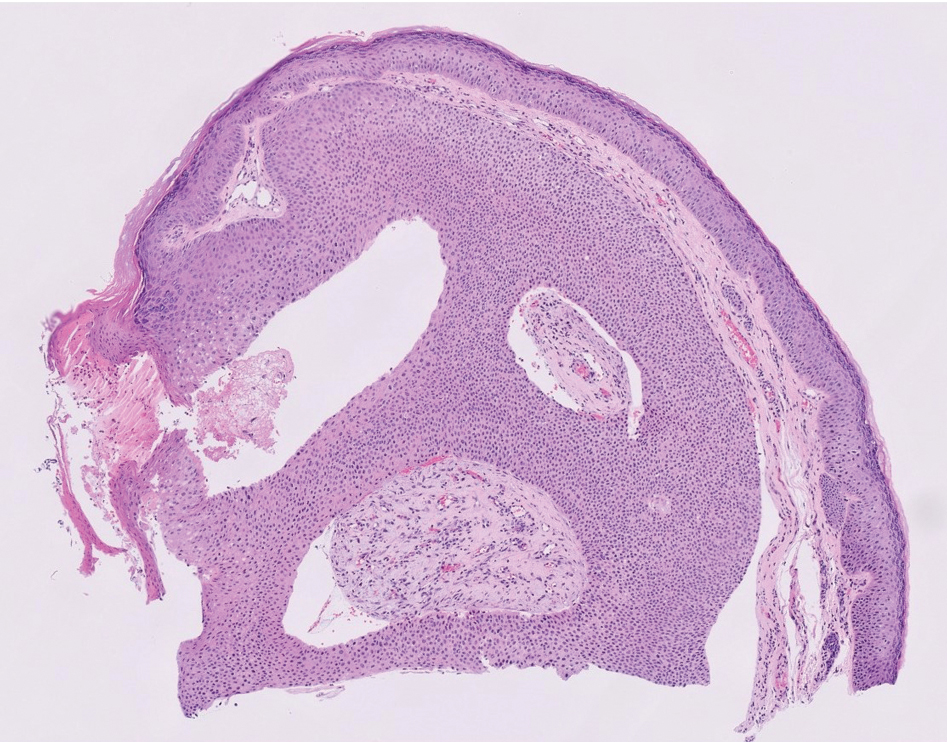
A 57-year-old man with no notable medical history presented to the dermatology clinic for evaluation of an asymptomatic papule on the left lower eyelid. The patient reported that the lesion seemed to wax and wane in size over time. Physical examination revealed a small, pink, verrucous papule on the left lower eyelid. A shave biopsy of the lesion revealed a well-circumscribed collection of small, monomorphic, cuboidal cells with basophilic round nuclei, inconspicuous nucleoli, and compact eosinophilic cytoplasm (top) with focal areas of duct formation (bottom) that was sharply demarcated from normal keratinocytes.
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
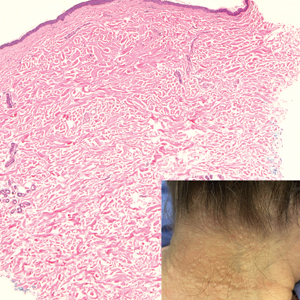
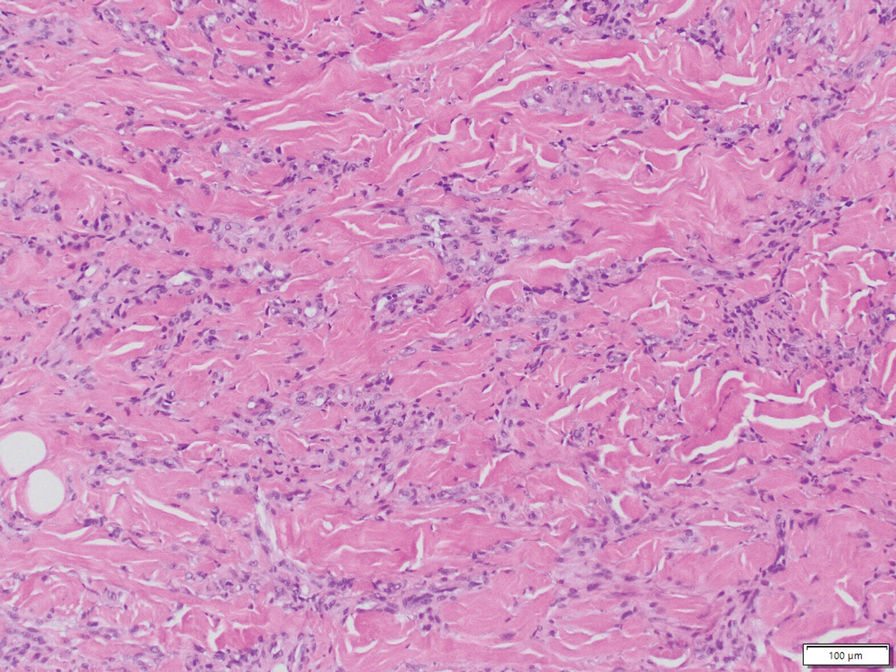
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
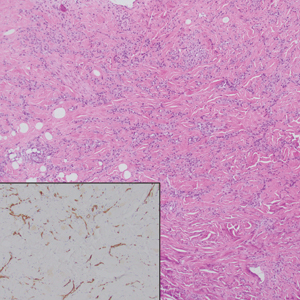
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.
The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6
Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9
Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
A 46-year-old woman with a history of systemic lupus erythematosus and end-stage renal disease presented to the dermatology department with painful ulcers on the extensor surfaces of the elbows, knees, and ankles of 2 months’ duration. Physical examination revealed angulated ulcers with surrounding pink erythema. A 4-mm punch biopsy and CD31 immunostaining of the left knee revealed dystrophic elastic fibers and purplish calciumlike depositions on connective tissue fibers in the mid to deep dermis.
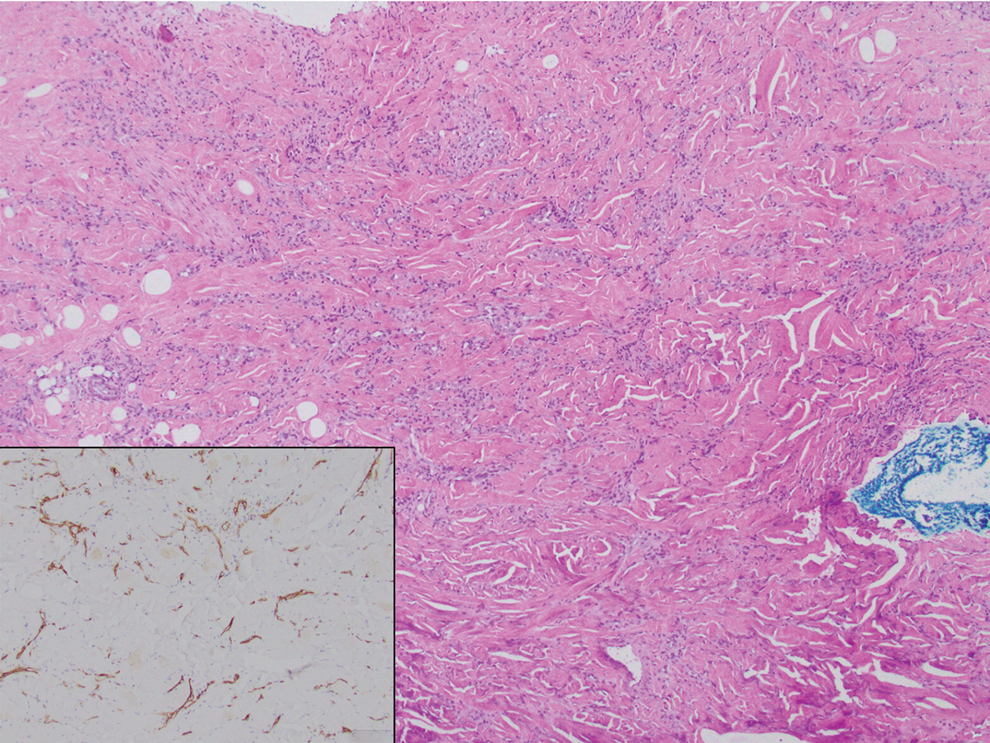
Solitary Lesion on the Umbilicus
Solitary Lesion on the Umbilicus
THE DIAGNOSIS: Cutaneous Endometriosis
Endometriosis is the ectopic presence of endometrial tissue and occurs in approximately 13% of women of childbearing age.1 This non-neoplastic lesion can manifest on the skin in less than 5.5% of endometriosis cases worldwide. Historically, secondary cutaneous endometriosis (CE) most frequently has been associated with prior gynecologic surgery (often cesarean section)2; however, an increased incidence of primary CE in patients without prior surgical history recently has been documented in the literature.3 While secondary CE usually manifests at the site of a surgical scar, primary CE has a predilection for the umbilicus (Villar nodule). In both primary and secondary CE, patients present clinically with a solitary nodule and abdominal pain that may be exacerbated during menstruation. Bleeding without associated pain may be more common in primary CE, while bleeding with pain may be more common in secondary CE. Cutaneous endometriosis often is overlooked given its low incidence, leading to delayed diagnosis. Primary CE often is misdiagnosed clinically as a pyogenic granuloma, Sister Mary Joseph nodule, or keloid, while secondary CE may be mistaken for a fibroma, incisional hernia, or granuloma.2
Primary and secondary CE have identical histopathologic features. Glands of variable size consisting of a single epithelial layer of columnar cells are present in the reticular dermis or subcutis (quiz image).4 The accompanying periglandular stroma often is uniform, consisting of spindle-shaped basophilic cells with abundant vascular structures. The stroma may contain moderate numbers of mitotic figures, a chronic inflammatory infiltrate, and extravasated red blood cells. The ectopic tissue may be inactive or display morphologic changes resembling those of the endometrium in the normal menstrual cycle.4 As the ectopic tissue progresses through the stages of menstruation, the glandular morphology also transforms. The proliferative stage demonstrates increased epithelial mitotic figures, the secretory stage exhibits intraluminal secretion, and during menstruation there are degenerative epithelial cells and evidence of vascular congestion. A mixture of glandular stages may be seen in biopsy results. Robust immunohistochemical expression of CD10 in the endometrial stroma can aid in diagnosis (Figure 1). Estrogen and progesterone receptor immunostaining also shows strong nuclear positivity, except in decidualized tissue.4 Unlike intestinal glands, endometrial glands do not express CDX2 or CK20.5 Complete surgical excision of CE usually is curative; however, recurrence has been documented in 10% (3/30) of cases.2
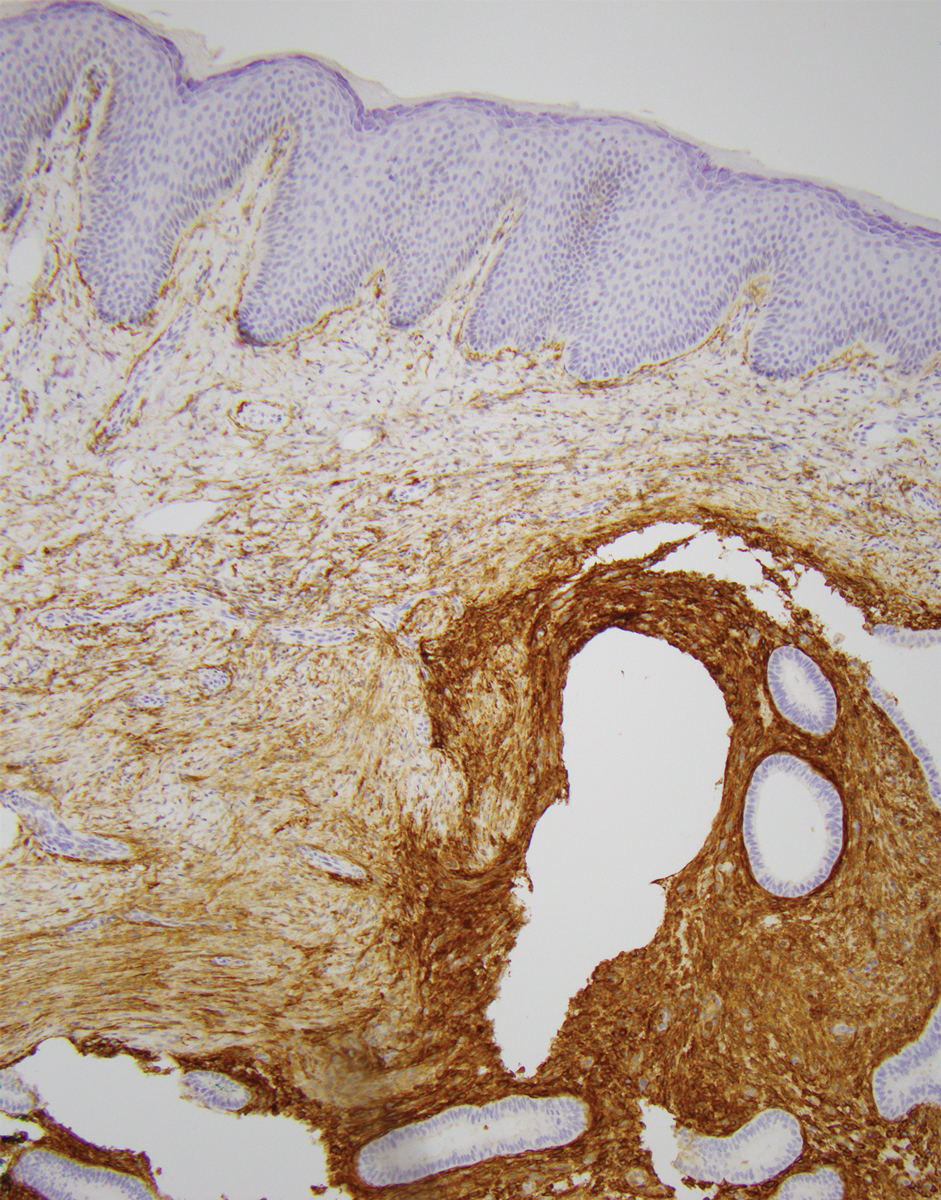
Breast carcinoma is the most common internal malignancy associated with cutaneous metastasis and may develop prior to visceral diagnosis. It is possible that tumor cells travel through the communicating networks of the cutaneous lymphatic ducts and the mammary lymphatic plexus; however, cutaneous manifestation often is located on the ipsilateral breast, and therefore tumor expansion rather than true metastasis cannot always be ruled out. On histopathology, findings of breast adenocarcinoma include tumor cells that tend to show either interstitial, nodular, mixed, or intravascular growth patterns (Figure 2). Tumor cells may invade the stroma in clusters or as individual cells. Sites of distant metastasis may show an increased likelihood of vascular and lymphatic invasion.6
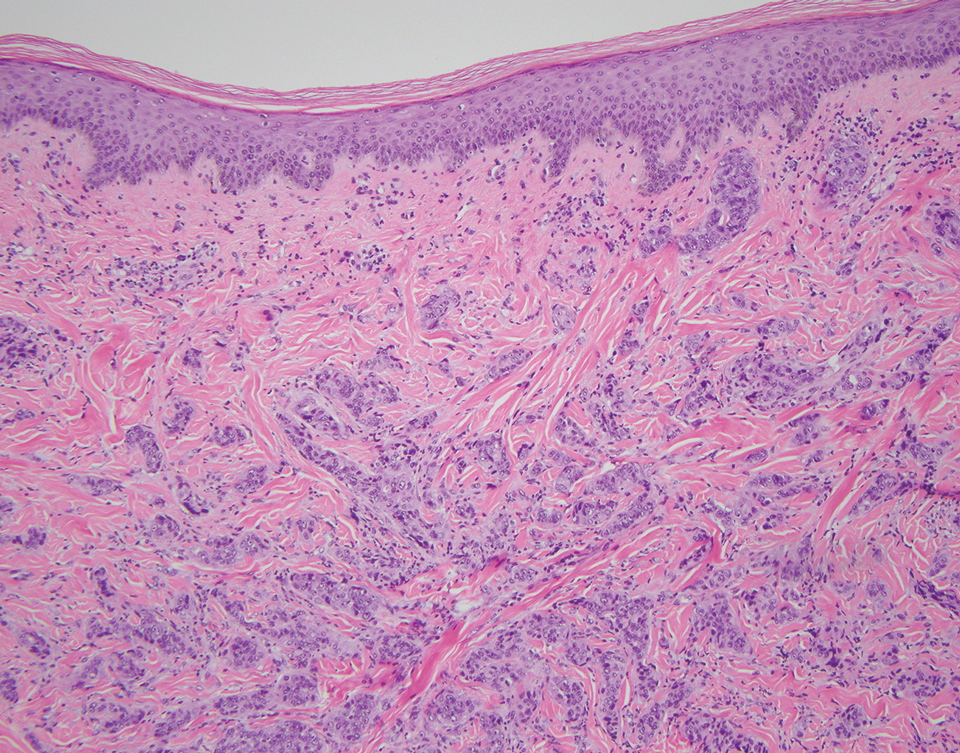
Nodular hidradenoma often manifests as a solitary nodule in the head or neck region, predominantly in women.7 Pathology shows well-demarcated intradermal aggregates of tumor cells within a hyalinized stroma; connection to the epidermis is not a feature of nodular hidradenoma. The epithelial component consists of polygonal cells with eosinophilic to amphophilic cytoplasm as well as large glycogenated cells with pale to clear cytoplasm (leading to the alternative term clear cell hidradenoma)(Figure 3). The cystic portion represents deterioration of tumor cells. Surgical excision usually is curative, although lesions may recur. Malignant transformation is rare.7
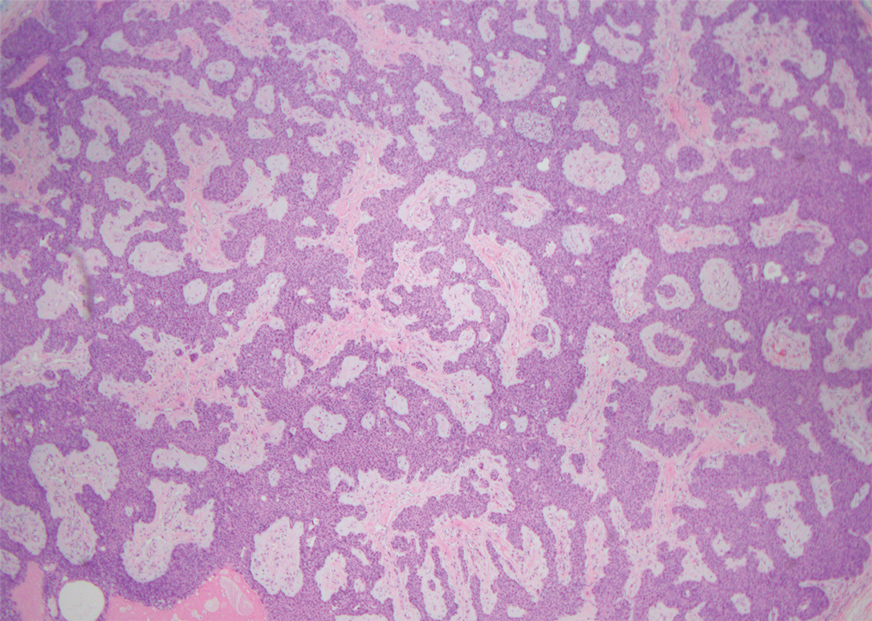
Sister Mary Joseph nodule is a cutaneous involvement of the umbilicus by a metastatic malignancy, often from an intra-abdominal primary malignancy (most commonly ovarian carcinoma in women and colonic carcinoma in men). Clinically, patients present with a solitary firm nodule or plaque within the umbilicus.8,9 Histopathology recapitulates the primary tumor (Figure 4).9 Sister Mary Joseph nodule portends a poor prognosis, with a survival rate of less than 8 months from the time of diagnosis.10
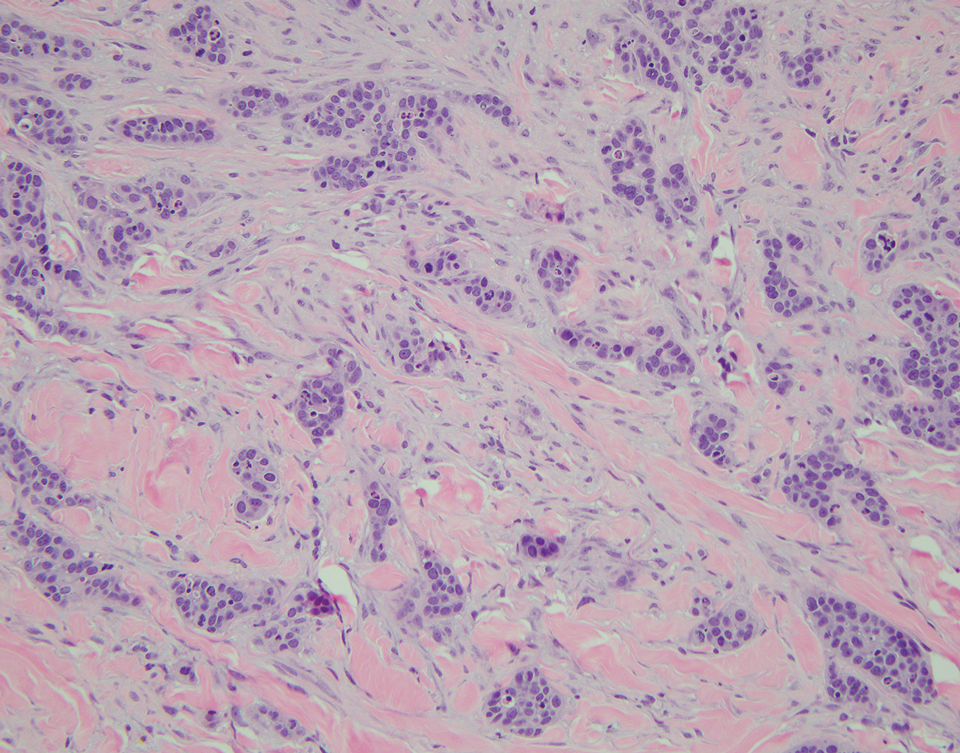
Urachal duct cyst develops from a remnant of the urachus that closed appropriately at the umbilicus and bladder but did not completely regress. It may manifest as an extraperitoneal mass at the umbilicus. Clinically, urachal duct cysts may be asymptomatic until an inciting event (eg, inflammation, deposition of calculus, or malignancy) occurs.11 Histopathology shows cystically dilated structures lined with a transitional epithelium (Figure 5).12 Urachal duct cysts usually are diagnosed in children or young adults and subsequently are excised.11
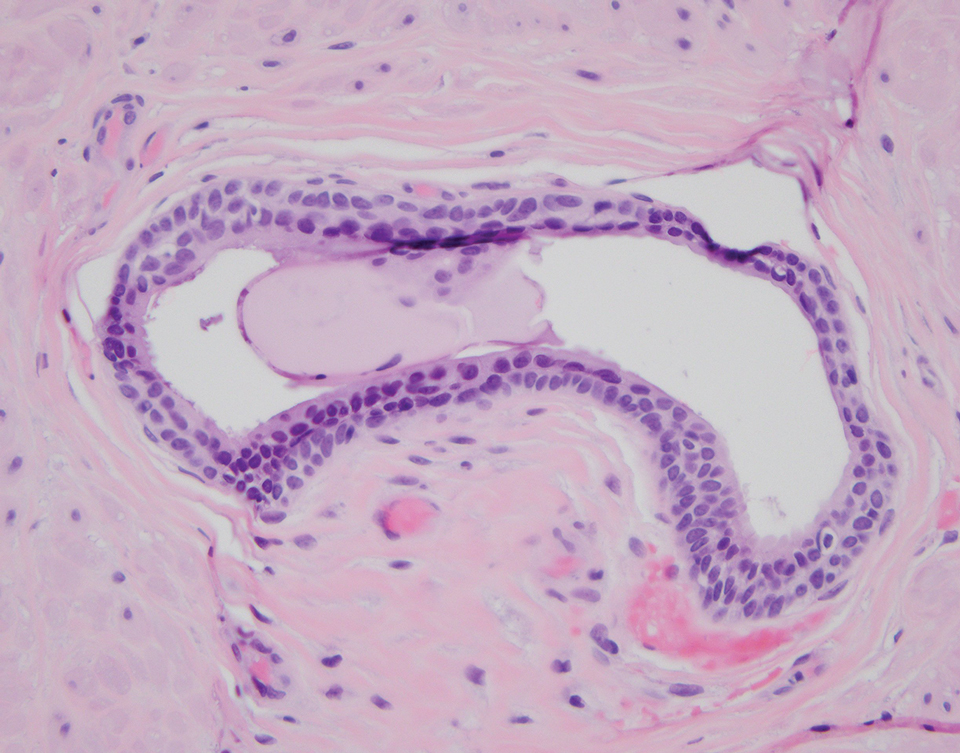
- Harder C, Velho RV, Brandes I, et al. Assessing the true prevalence of endometriosis: a narrative review of literature data. Int J Gynaecol Obstet. 2024;167:883-900. doi:10.1002/ijgo.15756
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, et al. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. Feb 2018;221:58-63. doi:10.1016 /j.ejogrb.2017.11.024
- Dridi D, Chiaffarino F, Parazzini F, et al. Umbilical endometriosis: a systematic literature review and pathogenic theory proposal. J Clin Med. 2022;11:995. doi:10.3390/jcm11040995
- Farooq U, Laureano AC, Miteva M, Elgart GW. Cutaneous endometriosis: diagnostic immunohistochemistry and clinicopathologic correlation. J Cutan Pathol. 2011;38:525-528. doi:10.1111/j.1600-0560.2011.01681.x
- Gadducci A, Zannoni GF. Endometriosis-associated extraovarian malignancies: a challenging question for the clinician and the pathologist. Anticancer Res. 2020;40:2429-2438. doi:10.21873/anticanres.14212
- Ronen S, Suster D, Chen WS, et al. Histologic patterns of cutaneous metastases of breast carcinoma: a clinicopathologic study of 232 cases. Am J Dermatopathol. 2021;43:401-411. doi:10.1097 /DAD.0000000000001841
- Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470. doi:10.1097/DAD.0b013e31821a4d33
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273. doi:10.1097/MAJ.0b013e3181954187
- Powell FC, Cooper AJ, Massa MC, et al. Sister Mary Joseph’s nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610-615. doi:10.1016/s0190-9622(84)80265-0
- Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: real-world data shows abysmal outcome. Int J Cancer. 2021;149: 1266-1273. doi:10.1002/ijc.33684
- Al-Salem A. An Illustrated Guide to Pediatric Urology. 1st ed. Springer Cham; 2016.
- Schubert GE, Pavkovic MB, Bethke-Bedürftig BA. Tubular urachal remnants in adult bladders. J Urol. 1982;127:40-42. doi:10.1016/s0022- 5347(17)53595-8
THE DIAGNOSIS: Cutaneous Endometriosis
Endometriosis is the ectopic presence of endometrial tissue and occurs in approximately 13% of women of childbearing age.1 This non-neoplastic lesion can manifest on the skin in less than 5.5% of endometriosis cases worldwide. Historically, secondary cutaneous endometriosis (CE) most frequently has been associated with prior gynecologic surgery (often cesarean section)2; however, an increased incidence of primary CE in patients without prior surgical history recently has been documented in the literature.3 While secondary CE usually manifests at the site of a surgical scar, primary CE has a predilection for the umbilicus (Villar nodule). In both primary and secondary CE, patients present clinically with a solitary nodule and abdominal pain that may be exacerbated during menstruation. Bleeding without associated pain may be more common in primary CE, while bleeding with pain may be more common in secondary CE. Cutaneous endometriosis often is overlooked given its low incidence, leading to delayed diagnosis. Primary CE often is misdiagnosed clinically as a pyogenic granuloma, Sister Mary Joseph nodule, or keloid, while secondary CE may be mistaken for a fibroma, incisional hernia, or granuloma.2
Primary and secondary CE have identical histopathologic features. Glands of variable size consisting of a single epithelial layer of columnar cells are present in the reticular dermis or subcutis (quiz image).4 The accompanying periglandular stroma often is uniform, consisting of spindle-shaped basophilic cells with abundant vascular structures. The stroma may contain moderate numbers of mitotic figures, a chronic inflammatory infiltrate, and extravasated red blood cells. The ectopic tissue may be inactive or display morphologic changes resembling those of the endometrium in the normal menstrual cycle.4 As the ectopic tissue progresses through the stages of menstruation, the glandular morphology also transforms. The proliferative stage demonstrates increased epithelial mitotic figures, the secretory stage exhibits intraluminal secretion, and during menstruation there are degenerative epithelial cells and evidence of vascular congestion. A mixture of glandular stages may be seen in biopsy results. Robust immunohistochemical expression of CD10 in the endometrial stroma can aid in diagnosis (Figure 1). Estrogen and progesterone receptor immunostaining also shows strong nuclear positivity, except in decidualized tissue.4 Unlike intestinal glands, endometrial glands do not express CDX2 or CK20.5 Complete surgical excision of CE usually is curative; however, recurrence has been documented in 10% (3/30) of cases.2

Breast carcinoma is the most common internal malignancy associated with cutaneous metastasis and may develop prior to visceral diagnosis. It is possible that tumor cells travel through the communicating networks of the cutaneous lymphatic ducts and the mammary lymphatic plexus; however, cutaneous manifestation often is located on the ipsilateral breast, and therefore tumor expansion rather than true metastasis cannot always be ruled out. On histopathology, findings of breast adenocarcinoma include tumor cells that tend to show either interstitial, nodular, mixed, or intravascular growth patterns (Figure 2). Tumor cells may invade the stroma in clusters or as individual cells. Sites of distant metastasis may show an increased likelihood of vascular and lymphatic invasion.6

Nodular hidradenoma often manifests as a solitary nodule in the head or neck region, predominantly in women.7 Pathology shows well-demarcated intradermal aggregates of tumor cells within a hyalinized stroma; connection to the epidermis is not a feature of nodular hidradenoma. The epithelial component consists of polygonal cells with eosinophilic to amphophilic cytoplasm as well as large glycogenated cells with pale to clear cytoplasm (leading to the alternative term clear cell hidradenoma)(Figure 3). The cystic portion represents deterioration of tumor cells. Surgical excision usually is curative, although lesions may recur. Malignant transformation is rare.7

Sister Mary Joseph nodule is a cutaneous involvement of the umbilicus by a metastatic malignancy, often from an intra-abdominal primary malignancy (most commonly ovarian carcinoma in women and colonic carcinoma in men). Clinically, patients present with a solitary firm nodule or plaque within the umbilicus.8,9 Histopathology recapitulates the primary tumor (Figure 4).9 Sister Mary Joseph nodule portends a poor prognosis, with a survival rate of less than 8 months from the time of diagnosis.10

Urachal duct cyst develops from a remnant of the urachus that closed appropriately at the umbilicus and bladder but did not completely regress. It may manifest as an extraperitoneal mass at the umbilicus. Clinically, urachal duct cysts may be asymptomatic until an inciting event (eg, inflammation, deposition of calculus, or malignancy) occurs.11 Histopathology shows cystically dilated structures lined with a transitional epithelium (Figure 5).12 Urachal duct cysts usually are diagnosed in children or young adults and subsequently are excised.11

THE DIAGNOSIS: Cutaneous Endometriosis
Endometriosis is the ectopic presence of endometrial tissue and occurs in approximately 13% of women of childbearing age.1 This non-neoplastic lesion can manifest on the skin in less than 5.5% of endometriosis cases worldwide. Historically, secondary cutaneous endometriosis (CE) most frequently has been associated with prior gynecologic surgery (often cesarean section)2; however, an increased incidence of primary CE in patients without prior surgical history recently has been documented in the literature.3 While secondary CE usually manifests at the site of a surgical scar, primary CE has a predilection for the umbilicus (Villar nodule). In both primary and secondary CE, patients present clinically with a solitary nodule and abdominal pain that may be exacerbated during menstruation. Bleeding without associated pain may be more common in primary CE, while bleeding with pain may be more common in secondary CE. Cutaneous endometriosis often is overlooked given its low incidence, leading to delayed diagnosis. Primary CE often is misdiagnosed clinically as a pyogenic granuloma, Sister Mary Joseph nodule, or keloid, while secondary CE may be mistaken for a fibroma, incisional hernia, or granuloma.2
Primary and secondary CE have identical histopathologic features. Glands of variable size consisting of a single epithelial layer of columnar cells are present in the reticular dermis or subcutis (quiz image).4 The accompanying periglandular stroma often is uniform, consisting of spindle-shaped basophilic cells with abundant vascular structures. The stroma may contain moderate numbers of mitotic figures, a chronic inflammatory infiltrate, and extravasated red blood cells. The ectopic tissue may be inactive or display morphologic changes resembling those of the endometrium in the normal menstrual cycle.4 As the ectopic tissue progresses through the stages of menstruation, the glandular morphology also transforms. The proliferative stage demonstrates increased epithelial mitotic figures, the secretory stage exhibits intraluminal secretion, and during menstruation there are degenerative epithelial cells and evidence of vascular congestion. A mixture of glandular stages may be seen in biopsy results. Robust immunohistochemical expression of CD10 in the endometrial stroma can aid in diagnosis (Figure 1). Estrogen and progesterone receptor immunostaining also shows strong nuclear positivity, except in decidualized tissue.4 Unlike intestinal glands, endometrial glands do not express CDX2 or CK20.5 Complete surgical excision of CE usually is curative; however, recurrence has been documented in 10% (3/30) of cases.2

Breast carcinoma is the most common internal malignancy associated with cutaneous metastasis and may develop prior to visceral diagnosis. It is possible that tumor cells travel through the communicating networks of the cutaneous lymphatic ducts and the mammary lymphatic plexus; however, cutaneous manifestation often is located on the ipsilateral breast, and therefore tumor expansion rather than true metastasis cannot always be ruled out. On histopathology, findings of breast adenocarcinoma include tumor cells that tend to show either interstitial, nodular, mixed, or intravascular growth patterns (Figure 2). Tumor cells may invade the stroma in clusters or as individual cells. Sites of distant metastasis may show an increased likelihood of vascular and lymphatic invasion.6

Nodular hidradenoma often manifests as a solitary nodule in the head or neck region, predominantly in women.7 Pathology shows well-demarcated intradermal aggregates of tumor cells within a hyalinized stroma; connection to the epidermis is not a feature of nodular hidradenoma. The epithelial component consists of polygonal cells with eosinophilic to amphophilic cytoplasm as well as large glycogenated cells with pale to clear cytoplasm (leading to the alternative term clear cell hidradenoma)(Figure 3). The cystic portion represents deterioration of tumor cells. Surgical excision usually is curative, although lesions may recur. Malignant transformation is rare.7

Sister Mary Joseph nodule is a cutaneous involvement of the umbilicus by a metastatic malignancy, often from an intra-abdominal primary malignancy (most commonly ovarian carcinoma in women and colonic carcinoma in men). Clinically, patients present with a solitary firm nodule or plaque within the umbilicus.8,9 Histopathology recapitulates the primary tumor (Figure 4).9 Sister Mary Joseph nodule portends a poor prognosis, with a survival rate of less than 8 months from the time of diagnosis.10

Urachal duct cyst develops from a remnant of the urachus that closed appropriately at the umbilicus and bladder but did not completely regress. It may manifest as an extraperitoneal mass at the umbilicus. Clinically, urachal duct cysts may be asymptomatic until an inciting event (eg, inflammation, deposition of calculus, or malignancy) occurs.11 Histopathology shows cystically dilated structures lined with a transitional epithelium (Figure 5).12 Urachal duct cysts usually are diagnosed in children or young adults and subsequently are excised.11

- Harder C, Velho RV, Brandes I, et al. Assessing the true prevalence of endometriosis: a narrative review of literature data. Int J Gynaecol Obstet. 2024;167:883-900. doi:10.1002/ijgo.15756
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, et al. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. Feb 2018;221:58-63. doi:10.1016 /j.ejogrb.2017.11.024
- Dridi D, Chiaffarino F, Parazzini F, et al. Umbilical endometriosis: a systematic literature review and pathogenic theory proposal. J Clin Med. 2022;11:995. doi:10.3390/jcm11040995
- Farooq U, Laureano AC, Miteva M, Elgart GW. Cutaneous endometriosis: diagnostic immunohistochemistry and clinicopathologic correlation. J Cutan Pathol. 2011;38:525-528. doi:10.1111/j.1600-0560.2011.01681.x
- Gadducci A, Zannoni GF. Endometriosis-associated extraovarian malignancies: a challenging question for the clinician and the pathologist. Anticancer Res. 2020;40:2429-2438. doi:10.21873/anticanres.14212
- Ronen S, Suster D, Chen WS, et al. Histologic patterns of cutaneous metastases of breast carcinoma: a clinicopathologic study of 232 cases. Am J Dermatopathol. 2021;43:401-411. doi:10.1097 /DAD.0000000000001841
- Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470. doi:10.1097/DAD.0b013e31821a4d33
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273. doi:10.1097/MAJ.0b013e3181954187
- Powell FC, Cooper AJ, Massa MC, et al. Sister Mary Joseph’s nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610-615. doi:10.1016/s0190-9622(84)80265-0
- Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: real-world data shows abysmal outcome. Int J Cancer. 2021;149: 1266-1273. doi:10.1002/ijc.33684
- Al-Salem A. An Illustrated Guide to Pediatric Urology. 1st ed. Springer Cham; 2016.
- Schubert GE, Pavkovic MB, Bethke-Bedürftig BA. Tubular urachal remnants in adult bladders. J Urol. 1982;127:40-42. doi:10.1016/s0022- 5347(17)53595-8
- Harder C, Velho RV, Brandes I, et al. Assessing the true prevalence of endometriosis: a narrative review of literature data. Int J Gynaecol Obstet. 2024;167:883-900. doi:10.1002/ijgo.15756
- Lopez-Soto A, Sanchez-Zapata MI, Martinez-Cendan JP, et al. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. Feb 2018;221:58-63. doi:10.1016 /j.ejogrb.2017.11.024
- Dridi D, Chiaffarino F, Parazzini F, et al. Umbilical endometriosis: a systematic literature review and pathogenic theory proposal. J Clin Med. 2022;11:995. doi:10.3390/jcm11040995
- Farooq U, Laureano AC, Miteva M, Elgart GW. Cutaneous endometriosis: diagnostic immunohistochemistry and clinicopathologic correlation. J Cutan Pathol. 2011;38:525-528. doi:10.1111/j.1600-0560.2011.01681.x
- Gadducci A, Zannoni GF. Endometriosis-associated extraovarian malignancies: a challenging question for the clinician and the pathologist. Anticancer Res. 2020;40:2429-2438. doi:10.21873/anticanres.14212
- Ronen S, Suster D, Chen WS, et al. Histologic patterns of cutaneous metastases of breast carcinoma: a clinicopathologic study of 232 cases. Am J Dermatopathol. 2021;43:401-411. doi:10.1097 /DAD.0000000000001841
- Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470. doi:10.1097/DAD.0b013e31821a4d33
- Abu-Hilal M, Newman JS. Sister Mary Joseph and her nodule: historical and clinical perspective. Am J Med Sci. 2009;337:271-273. doi:10.1097/MAJ.0b013e3181954187
- Powell FC, Cooper AJ, Massa MC, et al. Sister Mary Joseph’s nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610-615. doi:10.1016/s0190-9622(84)80265-0
- Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: real-world data shows abysmal outcome. Int J Cancer. 2021;149: 1266-1273. doi:10.1002/ijc.33684
- Al-Salem A. An Illustrated Guide to Pediatric Urology. 1st ed. Springer Cham; 2016.
- Schubert GE, Pavkovic MB, Bethke-Bedürftig BA. Tubular urachal remnants in adult bladders. J Urol. 1982;127:40-42. doi:10.1016/s0022- 5347(17)53595-8
Solitary Lesion on the Umbilicus
Solitary Lesion on the Umbilicus
A 33-year-old woman with no notable medical or surgical history presented to our clinic with a solitary indurated nodule on the umbilicus that had been progressively enlarging for 1 year. The patient reported that she had undergone piercing of the umbilicus more than 5 years prior. She noted that the lesion was uncomfortable and pruritic and occasionally bled spontaneously. Physical examination revealed no other mucosal or cutaneous findings. A shave biopsy of the nodule was performed.
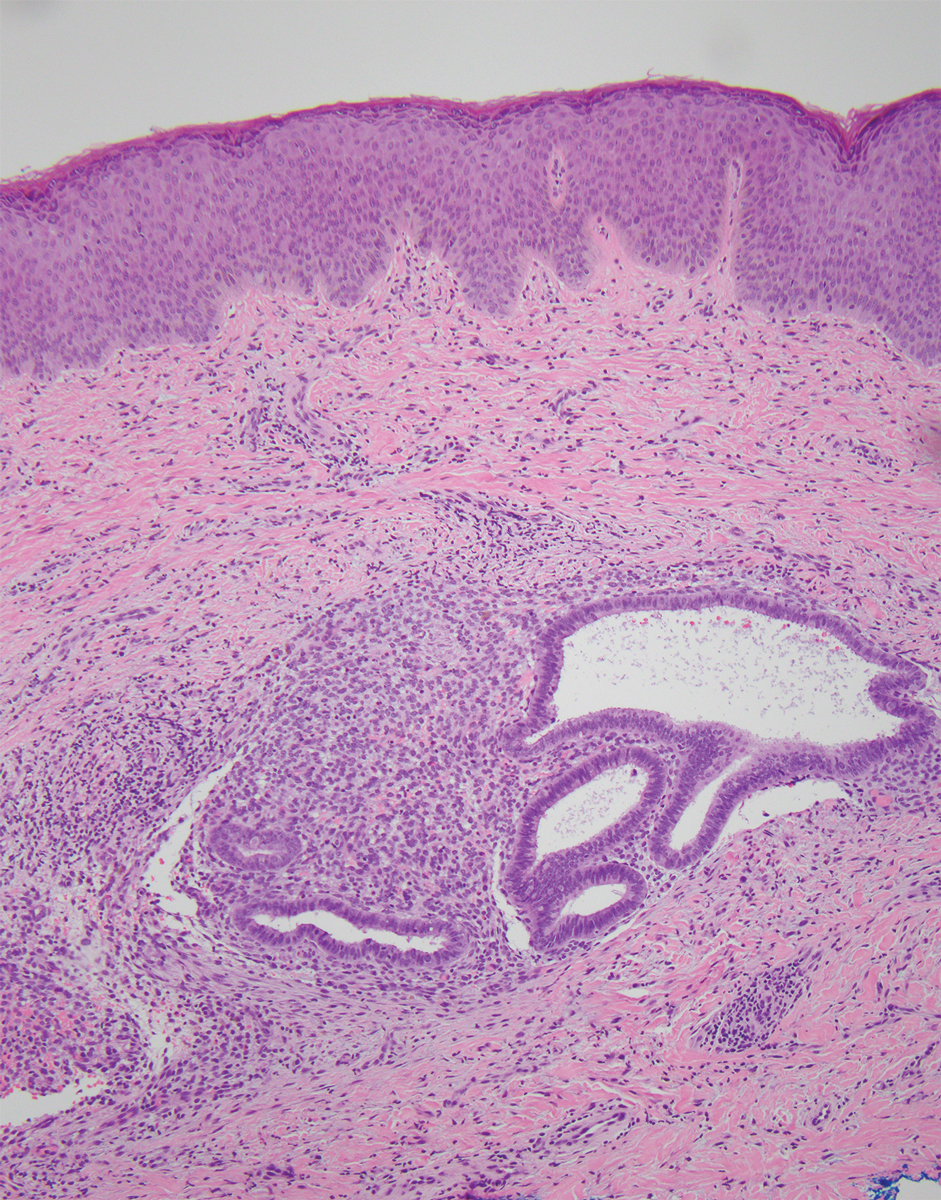
Scalp Nodule With Copious Fluid
Scalp Nodule With Copious Fluid
The Diagnosis: Apocrine Hidrocystoma
Histopathology of the excised nodule revealed a partially collapsed, multiloculated dermal cyst lined with apocrine cells, which was consistent with a diagnosis of apocrine hidrocystoma. Apocrine hidrocystomas are cysts that range from flesh-colored to blue-black and most commonly manifest as solitary lesions on the face, particularly near the eyelids.1,2 Apocrine hidrocystomas typically range from 1 to 10 mm in diameter and contain fluid that can be colorless, yellow-brown, or blue-black.1,2 Apocrine hidrocystomas usually are reported between the ages of 30 and 70 years and have no sex predilection.3
Apocrine hidrocystomas are thought to develop from adenomatous growth of apocrine sweat gland coils.4 The term apocrine hidrocystoma has been used interchangeably with apocrine cystadenoma, though some investigators have recommended using the latter term only for lesions with true papillary projections.5 Definitive diagnosis is obtained through histopathology, which typically shows unilocular or multilocular cystic spaces in the dermis lined by an apocrine secretory epithelium. These secretory cells often demonstrate decapitation secretion and apical snouting. The cyst wall may send pseudopapillary projections into the cystic cavity.1,2 While apocrine and eccrine hidrocystomas previously were recognized as separate entities, it has been suggested that so-called eccrine hidrocystomas are truly apocrine in nature, with a cyst wall that is compressed by the cyst contents.4
Apocrine hidrocystomas are benign and do not require treatment; however, they may be removed for cosmetic purposes, most commonly via surgical excision. Lesions treated with needle puncture as monotherapy frequently recur. Other successful methods for removal include cyst puncture followed by hypertonic glucose sclerotherapy, trichloroacetic acid injection, botulinum toxin A injection, or CO2 laser treatment.3,6
Several clinical and histopathologic findings can distinguish between apocrine hidrocystomas and other diagnoses in the differential. Lipomas are common benign tumors composed of mature fat that typically manifest as solitary, painless, soft nodules with a normal overlying epidermis. They frequently are distributed on the neck, arms, legs, and buttocks. While the differential for our patient initially included lipoma, these lesions do not contain or release fluid, which was present in our patient. On histopathology, lipoma shows a uniform population of mature fat cells with small, uniform, and eccentric nuclei (Figure 1).7
Epidermal inclusion cysts are derived from the follicular infundibulum and commonly are found on the face and upper trunk. They manifest as flesh-colored dermal nodules and may have a visible punctum. As opposed to the cystic cavities lined with apocrine cells seen in apocrine hidrocystomas, epidermal inclusion cysts are lined with a stratified squamous epithelium, are filled with laminated keratin, and have a visible granular layer (Figure 2).8
Pilar cysts, also known as trichilemmal cysts, clinically resemble epidermal inclusion cysts but are derived from the outer root sheath of hair follicles, manifesting as flesh-colored dermal nodules almost always found on the scalp. On histopathology, pilar cysts are lined with stratified squamous epithelial cells without a visible granular layer and are filled with compact eosinophilic keratin (Figure 3).8
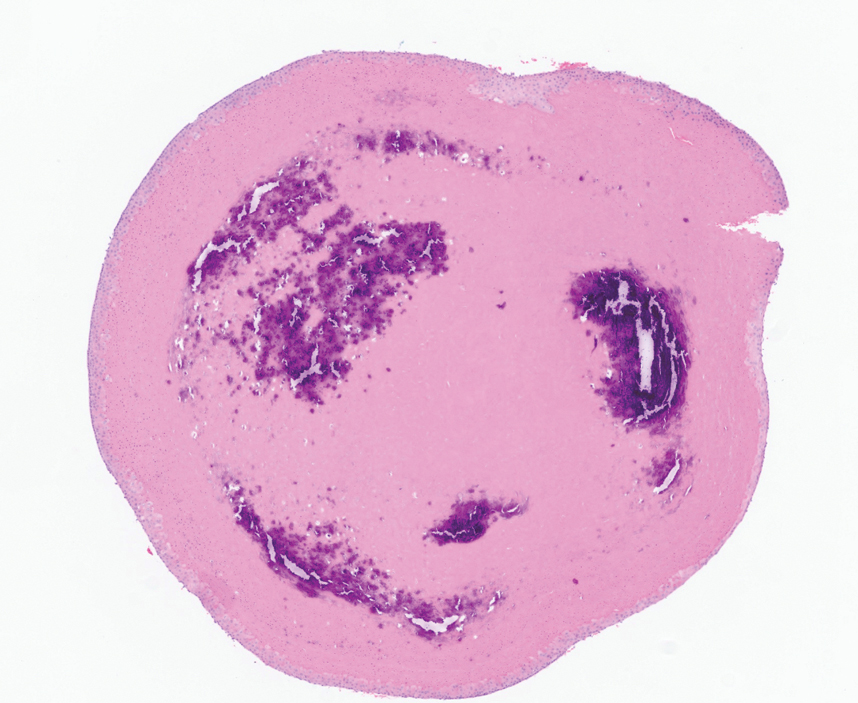
Tubular apocrine adenomas are benign neoplasms of the apocrine glands that manifest as smooth nodules. They are within the same spectrum as papillary eccrine adenomas, appearing more frequently on the legs and less frequently on the face and scalp.9 Histopathology generally demonstrates well-circumscribed lobules of tubular structures in the dermis. Similar to apocrine hidrocystomas, tubular apocrine adenomas will demonstrate an inner layer of columnar apocrine cells with decapitation secretion, but the tubular architecture helps differentiate it from other adnexal tumors (Figure 4).10
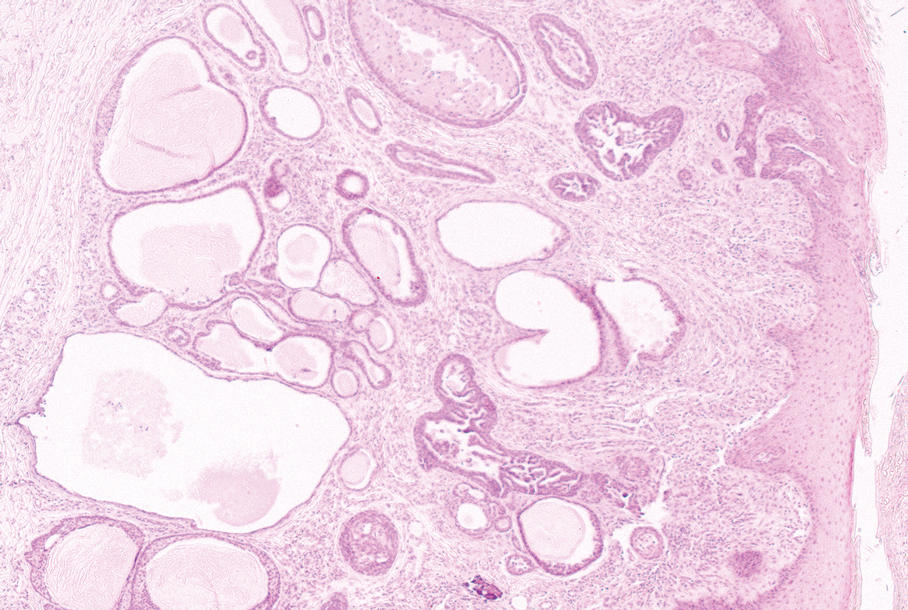
The clinical manifestation of the apocrine hidrocystoma in our patient was unusual due to its size and location. Apocrine hidrocystomas rarely are found on the scalp, with few other cases found in the literature. To our knowledge, this is the largest apocrine hidrocystoma found on the scalp to date, although there is at least 1 other published case of an apocrine hidrocystoma on the scalp measuring at least 3 cm in diameter.11 Our case highlights the importance of recognizing atypical manifestations of apocrine hidrocystomas, as a lesion on the midline scalp that discharges a thin fluid might raise initial concern for an intracranial connection. Awareness of atypical manifestations of common lesions can expand dermatologists’ differential diagnoses and help them to reassure patients.
- Smith JD. Apocrine hidrocystoma (cystadenoma). Arch Dermatol. 1974;109:700. doi:10.1001/archderm.1974.01630050046010
- Mehregan AH. Apocrine cystadenoma: a clinicopathologic study with special reference to the pigmented variety. Arch Dermatol. 1964;90:274. doi:10.1001/archderm.1964.01600030024005
- Hafsi W, Badri T, Shah F. Apocrine hidrocystoma. StatPearls [Internet]. Updated April 13, 2024. Accessed November 6, 2024. http://www.ncbi.nlm.nih.gov/books/NBK448109/
- de Viragh PA, Szeimies RM, Eckert F. Apocrine cystadenoma, apocrine hidrocystoma, and eccrine hidrocystoma: three distinct tumors defined by expression of keratins and human milk fat globulin 1. J Cutan Pathol. 1997;24:249-255. doi:10.1111/j.1600-0560.1997.tb01590.x
- Sugiyama A, Sugiura M, Piris A, et al. Apocrine cystadenoma and apocrine hidrocystoma: examination of 21 cases with emphasis on nomenclature according to proliferative features. J Cutan Pathol. 2007;34:912-917. doi:10.1111/j.1600-0560.2007.00757.x
- Bickley LK, Goldberg DJ, Imaeda S, et al. Treatment of multiple apocrine hidrocystomas with the carbon dioxide (CO2) laser. J Dermatol Surg Oncol. 1989;15:599-602. doi:10.1111/j.1524-4725.1989.tb03597.x
- Kaddu S. Smooth muscle, adipose and cartilage neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2086-2101.
- Stone MS. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Requena L, Sangüeza O. Tubular adenoma. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:127-136. doi:10.1007/978-3-319-45704-8_12
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
The Diagnosis: Apocrine Hidrocystoma
Histopathology of the excised nodule revealed a partially collapsed, multiloculated dermal cyst lined with apocrine cells, which was consistent with a diagnosis of apocrine hidrocystoma. Apocrine hidrocystomas are cysts that range from flesh-colored to blue-black and most commonly manifest as solitary lesions on the face, particularly near the eyelids.1,2 Apocrine hidrocystomas typically range from 1 to 10 mm in diameter and contain fluid that can be colorless, yellow-brown, or blue-black.1,2 Apocrine hidrocystomas usually are reported between the ages of 30 and 70 years and have no sex predilection.3
Apocrine hidrocystomas are thought to develop from adenomatous growth of apocrine sweat gland coils.4 The term apocrine hidrocystoma has been used interchangeably with apocrine cystadenoma, though some investigators have recommended using the latter term only for lesions with true papillary projections.5 Definitive diagnosis is obtained through histopathology, which typically shows unilocular or multilocular cystic spaces in the dermis lined by an apocrine secretory epithelium. These secretory cells often demonstrate decapitation secretion and apical snouting. The cyst wall may send pseudopapillary projections into the cystic cavity.1,2 While apocrine and eccrine hidrocystomas previously were recognized as separate entities, it has been suggested that so-called eccrine hidrocystomas are truly apocrine in nature, with a cyst wall that is compressed by the cyst contents.4
Apocrine hidrocystomas are benign and do not require treatment; however, they may be removed for cosmetic purposes, most commonly via surgical excision. Lesions treated with needle puncture as monotherapy frequently recur. Other successful methods for removal include cyst puncture followed by hypertonic glucose sclerotherapy, trichloroacetic acid injection, botulinum toxin A injection, or CO2 laser treatment.3,6
Several clinical and histopathologic findings can distinguish between apocrine hidrocystomas and other diagnoses in the differential. Lipomas are common benign tumors composed of mature fat that typically manifest as solitary, painless, soft nodules with a normal overlying epidermis. They frequently are distributed on the neck, arms, legs, and buttocks. While the differential for our patient initially included lipoma, these lesions do not contain or release fluid, which was present in our patient. On histopathology, lipoma shows a uniform population of mature fat cells with small, uniform, and eccentric nuclei (Figure 1).7

Epidermal inclusion cysts are derived from the follicular infundibulum and commonly are found on the face and upper trunk. They manifest as flesh-colored dermal nodules and may have a visible punctum. As opposed to the cystic cavities lined with apocrine cells seen in apocrine hidrocystomas, epidermal inclusion cysts are lined with a stratified squamous epithelium, are filled with laminated keratin, and have a visible granular layer (Figure 2).8

Pilar cysts, also known as trichilemmal cysts, clinically resemble epidermal inclusion cysts but are derived from the outer root sheath of hair follicles, manifesting as flesh-colored dermal nodules almost always found on the scalp. On histopathology, pilar cysts are lined with stratified squamous epithelial cells without a visible granular layer and are filled with compact eosinophilic keratin (Figure 3).8

Tubular apocrine adenomas are benign neoplasms of the apocrine glands that manifest as smooth nodules. They are within the same spectrum as papillary eccrine adenomas, appearing more frequently on the legs and less frequently on the face and scalp.9 Histopathology generally demonstrates well-circumscribed lobules of tubular structures in the dermis. Similar to apocrine hidrocystomas, tubular apocrine adenomas will demonstrate an inner layer of columnar apocrine cells with decapitation secretion, but the tubular architecture helps differentiate it from other adnexal tumors (Figure 4).10

The clinical manifestation of the apocrine hidrocystoma in our patient was unusual due to its size and location. Apocrine hidrocystomas rarely are found on the scalp, with few other cases found in the literature. To our knowledge, this is the largest apocrine hidrocystoma found on the scalp to date, although there is at least 1 other published case of an apocrine hidrocystoma on the scalp measuring at least 3 cm in diameter.11 Our case highlights the importance of recognizing atypical manifestations of apocrine hidrocystomas, as a lesion on the midline scalp that discharges a thin fluid might raise initial concern for an intracranial connection. Awareness of atypical manifestations of common lesions can expand dermatologists’ differential diagnoses and help them to reassure patients.
The Diagnosis: Apocrine Hidrocystoma
Histopathology of the excised nodule revealed a partially collapsed, multiloculated dermal cyst lined with apocrine cells, which was consistent with a diagnosis of apocrine hidrocystoma. Apocrine hidrocystomas are cysts that range from flesh-colored to blue-black and most commonly manifest as solitary lesions on the face, particularly near the eyelids.1,2 Apocrine hidrocystomas typically range from 1 to 10 mm in diameter and contain fluid that can be colorless, yellow-brown, or blue-black.1,2 Apocrine hidrocystomas usually are reported between the ages of 30 and 70 years and have no sex predilection.3
Apocrine hidrocystomas are thought to develop from adenomatous growth of apocrine sweat gland coils.4 The term apocrine hidrocystoma has been used interchangeably with apocrine cystadenoma, though some investigators have recommended using the latter term only for lesions with true papillary projections.5 Definitive diagnosis is obtained through histopathology, which typically shows unilocular or multilocular cystic spaces in the dermis lined by an apocrine secretory epithelium. These secretory cells often demonstrate decapitation secretion and apical snouting. The cyst wall may send pseudopapillary projections into the cystic cavity.1,2 While apocrine and eccrine hidrocystomas previously were recognized as separate entities, it has been suggested that so-called eccrine hidrocystomas are truly apocrine in nature, with a cyst wall that is compressed by the cyst contents.4
Apocrine hidrocystomas are benign and do not require treatment; however, they may be removed for cosmetic purposes, most commonly via surgical excision. Lesions treated with needle puncture as monotherapy frequently recur. Other successful methods for removal include cyst puncture followed by hypertonic glucose sclerotherapy, trichloroacetic acid injection, botulinum toxin A injection, or CO2 laser treatment.3,6
Several clinical and histopathologic findings can distinguish between apocrine hidrocystomas and other diagnoses in the differential. Lipomas are common benign tumors composed of mature fat that typically manifest as solitary, painless, soft nodules with a normal overlying epidermis. They frequently are distributed on the neck, arms, legs, and buttocks. While the differential for our patient initially included lipoma, these lesions do not contain or release fluid, which was present in our patient. On histopathology, lipoma shows a uniform population of mature fat cells with small, uniform, and eccentric nuclei (Figure 1).7

Epidermal inclusion cysts are derived from the follicular infundibulum and commonly are found on the face and upper trunk. They manifest as flesh-colored dermal nodules and may have a visible punctum. As opposed to the cystic cavities lined with apocrine cells seen in apocrine hidrocystomas, epidermal inclusion cysts are lined with a stratified squamous epithelium, are filled with laminated keratin, and have a visible granular layer (Figure 2).8

Pilar cysts, also known as trichilemmal cysts, clinically resemble epidermal inclusion cysts but are derived from the outer root sheath of hair follicles, manifesting as flesh-colored dermal nodules almost always found on the scalp. On histopathology, pilar cysts are lined with stratified squamous epithelial cells without a visible granular layer and are filled with compact eosinophilic keratin (Figure 3).8

Tubular apocrine adenomas are benign neoplasms of the apocrine glands that manifest as smooth nodules. They are within the same spectrum as papillary eccrine adenomas, appearing more frequently on the legs and less frequently on the face and scalp.9 Histopathology generally demonstrates well-circumscribed lobules of tubular structures in the dermis. Similar to apocrine hidrocystomas, tubular apocrine adenomas will demonstrate an inner layer of columnar apocrine cells with decapitation secretion, but the tubular architecture helps differentiate it from other adnexal tumors (Figure 4).10

The clinical manifestation of the apocrine hidrocystoma in our patient was unusual due to its size and location. Apocrine hidrocystomas rarely are found on the scalp, with few other cases found in the literature. To our knowledge, this is the largest apocrine hidrocystoma found on the scalp to date, although there is at least 1 other published case of an apocrine hidrocystoma on the scalp measuring at least 3 cm in diameter.11 Our case highlights the importance of recognizing atypical manifestations of apocrine hidrocystomas, as a lesion on the midline scalp that discharges a thin fluid might raise initial concern for an intracranial connection. Awareness of atypical manifestations of common lesions can expand dermatologists’ differential diagnoses and help them to reassure patients.
- Smith JD. Apocrine hidrocystoma (cystadenoma). Arch Dermatol. 1974;109:700. doi:10.1001/archderm.1974.01630050046010
- Mehregan AH. Apocrine cystadenoma: a clinicopathologic study with special reference to the pigmented variety. Arch Dermatol. 1964;90:274. doi:10.1001/archderm.1964.01600030024005
- Hafsi W, Badri T, Shah F. Apocrine hidrocystoma. StatPearls [Internet]. Updated April 13, 2024. Accessed November 6, 2024. http://www.ncbi.nlm.nih.gov/books/NBK448109/
- de Viragh PA, Szeimies RM, Eckert F. Apocrine cystadenoma, apocrine hidrocystoma, and eccrine hidrocystoma: three distinct tumors defined by expression of keratins and human milk fat globulin 1. J Cutan Pathol. 1997;24:249-255. doi:10.1111/j.1600-0560.1997.tb01590.x
- Sugiyama A, Sugiura M, Piris A, et al. Apocrine cystadenoma and apocrine hidrocystoma: examination of 21 cases with emphasis on nomenclature according to proliferative features. J Cutan Pathol. 2007;34:912-917. doi:10.1111/j.1600-0560.2007.00757.x
- Bickley LK, Goldberg DJ, Imaeda S, et al. Treatment of multiple apocrine hidrocystomas with the carbon dioxide (CO2) laser. J Dermatol Surg Oncol. 1989;15:599-602. doi:10.1111/j.1524-4725.1989.tb03597.x
- Kaddu S. Smooth muscle, adipose and cartilage neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2086-2101.
- Stone MS. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Requena L, Sangüeza O. Tubular adenoma. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:127-136. doi:10.1007/978-3-319-45704-8_12
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Smith JD. Apocrine hidrocystoma (cystadenoma). Arch Dermatol. 1974;109:700. doi:10.1001/archderm.1974.01630050046010
- Mehregan AH. Apocrine cystadenoma: a clinicopathologic study with special reference to the pigmented variety. Arch Dermatol. 1964;90:274. doi:10.1001/archderm.1964.01600030024005
- Hafsi W, Badri T, Shah F. Apocrine hidrocystoma. StatPearls [Internet]. Updated April 13, 2024. Accessed November 6, 2024. http://www.ncbi.nlm.nih.gov/books/NBK448109/
- de Viragh PA, Szeimies RM, Eckert F. Apocrine cystadenoma, apocrine hidrocystoma, and eccrine hidrocystoma: three distinct tumors defined by expression of keratins and human milk fat globulin 1. J Cutan Pathol. 1997;24:249-255. doi:10.1111/j.1600-0560.1997.tb01590.x
- Sugiyama A, Sugiura M, Piris A, et al. Apocrine cystadenoma and apocrine hidrocystoma: examination of 21 cases with emphasis on nomenclature according to proliferative features. J Cutan Pathol. 2007;34:912-917. doi:10.1111/j.1600-0560.2007.00757.x
- Bickley LK, Goldberg DJ, Imaeda S, et al. Treatment of multiple apocrine hidrocystomas with the carbon dioxide (CO2) laser. J Dermatol Surg Oncol. 1989;15:599-602. doi:10.1111/j.1524-4725.1989.tb03597.x
- Kaddu S. Smooth muscle, adipose and cartilage neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:2086-2101.
- Stone MS. Cysts. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Requena L, Sangüeza O. Tubular adenoma. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:127-136. doi:10.1007/978-3-319-45704-8_12
- McCalmont TH, Pincus LB. Adnexal neoplasms. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1057-1074.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
Scalp Nodule With Copious Fluid
Scalp Nodule With Copious Fluid
A 48-year-old woman presented to the dermatology clinic with a suspected cyst on the occipital scalp. The patient noted that the lesion had been present for years and denied any pain, pruritus, or drainage from the site. Physical examination revealed a soft, flesh-colored, subcutaneous nodule measuring 4.2×3.2 cm on the midline occipital scalp. During excision, the lesion drained a copious amount of thin yellowish fluid.
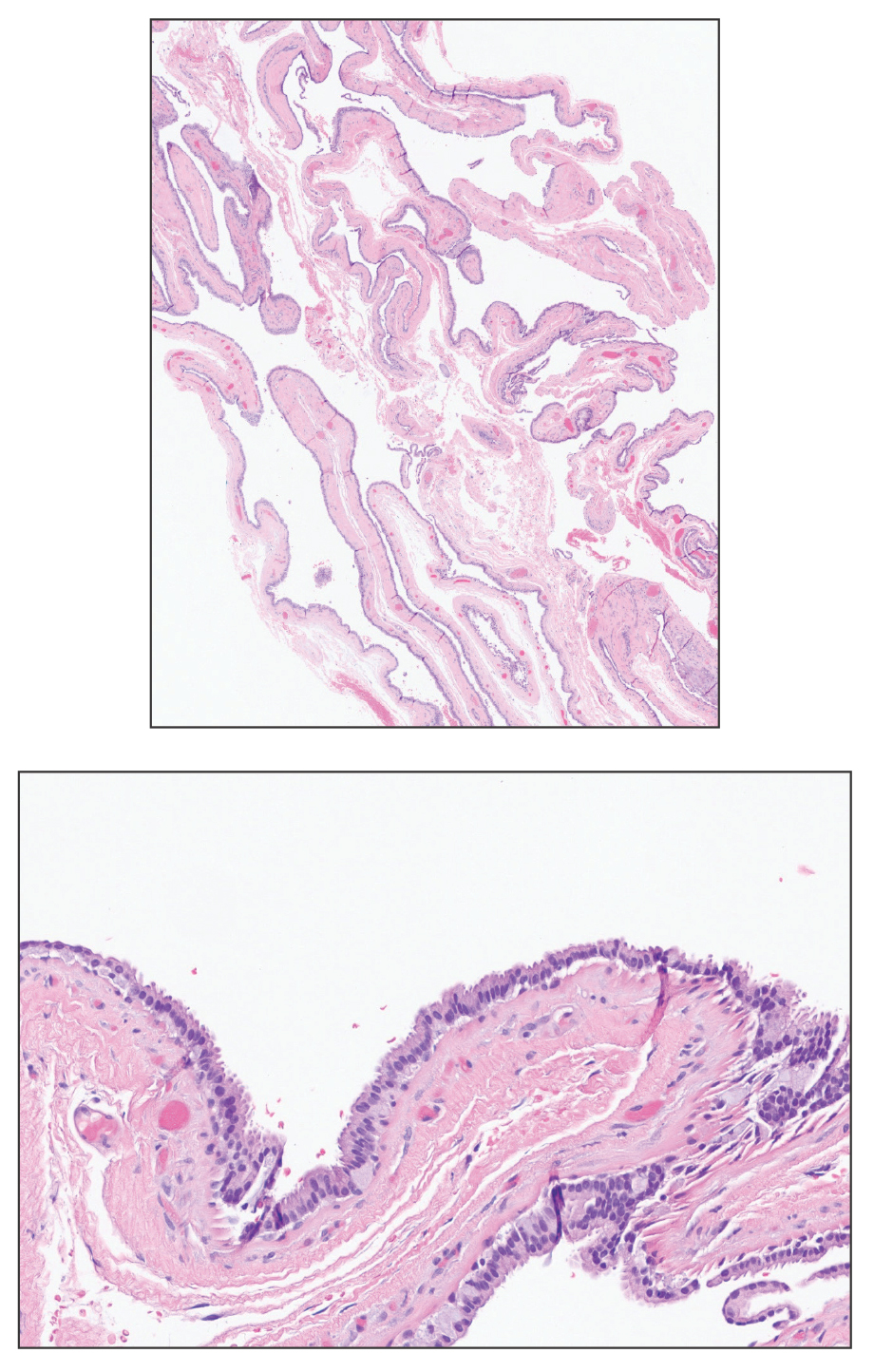
Asymptomatic Papules on the Neck
THE DIAGNOSIS: White Fibrous Papulosis
Given the histopathology findings, location on a sun-exposed site, lack of any additional systemic signs or symptoms, and no family history of similar lesions to suggest an underlying genetic condition, a diagnosis of white fibrous papulosis (WFP) was made. White fibrous papulosis is a relatively rare cutaneous disorder that was first reported by Shimizu et al1 in 1985. It is characterized by numerous grouped, 2- to 3-mm, white to flesh-colored papules that in most cases are confined to the neck in middle-aged to elderly individuals; however, cases involving the upper trunk and axillae also have been reported.1-3 The etiology of this condition is unclear but is thought to be related to aging and chronic exposure to UV light. Although treatment is not required, various modalities including tretinoin, excision, and laser therapy have been trialed with varying success.2,4 Our patient elected not to proceed with treatment.
Histologically, WFP may manifest similarly to connective tissue nevi; the overall architecture is nonspecific with focally thickened collagen and often elastic fibers that may be normal to reduced and/or fragmented, as well as an overall decrease in superficial dermal elastic tissue.3,5 Therefore, the differential diagnosis may include connective tissue nevi and require clinical correlation to make a correct diagnosis.
Pseudoxanthoma elasticum (PXE) is an autosomalrecessive disorder most commonly related to mutations in the ATP binding cassette subfamily C member 6 (ABCC6) gene that tends to manifest clinically on the neck and flexural extremities.6 This disease affects elastic fibers, which may become calcified over time. Pseudoxanthoma elasticum is associated with ocular complications relating to the Bruch membrane of the retina and angioid streaks; choroidal neovascularization involving the damaged Bruch membrane and episodes of acute retinopathy may result in vision loss in later stages of the disease.7 Involvement of the elastic laminae of arteries can be associated with cardiovascular and cerebrovascular complications such as stroke, coronary artery disease, claudication, and aneurysms. Involvement of the gastrointestinal or genitourinary tracts also may occur and most commonly manifests with bleeding. Pathologic alterations in the elastic fibers of the lungs also have been reported in patients with PXE.8 Histologically, PXE exhibits increased abnormally clumped and fragmented elastic fibers in the superficial dermis, often with calcification (Figure 1). Pseudo-PXE related to D-penicillamine use often lacks calcification and has a bramble bush appearance.9

Fibrofolliculomas may manifest alone or in association with an underlying condition such as Birt-Hogg-Dubé syndrome, in which lesions are most frequently seen scattered on the scalp, face, ears, neck, or upper trunk.10 This condition is related to a folliculin (FLCN) gene germline mutation. Birt-Hogg-Dubé syndrome also may be associated with acrochordons, trichodiscomas, renal cancer, and lung cysts with or without spontaneous pneumothorax. Less frequently noted findings include oral papules, epidermal cysts, angiofibromas, lipomas/angiolipomas, parotid gland tumors, and thyroid neoplasms. Connective tissue nevi/collagenomas can appear clinically similar to fibrofolliculomas; true connective tissue nevi are reported less commonly in Birt-Hogg-Dubé syndrome.11 Histologically, a fibrofolliculoma manifests with epidermal strands originating from a hair follicle associated with prominent surrounding connective tissue (Figure 2).
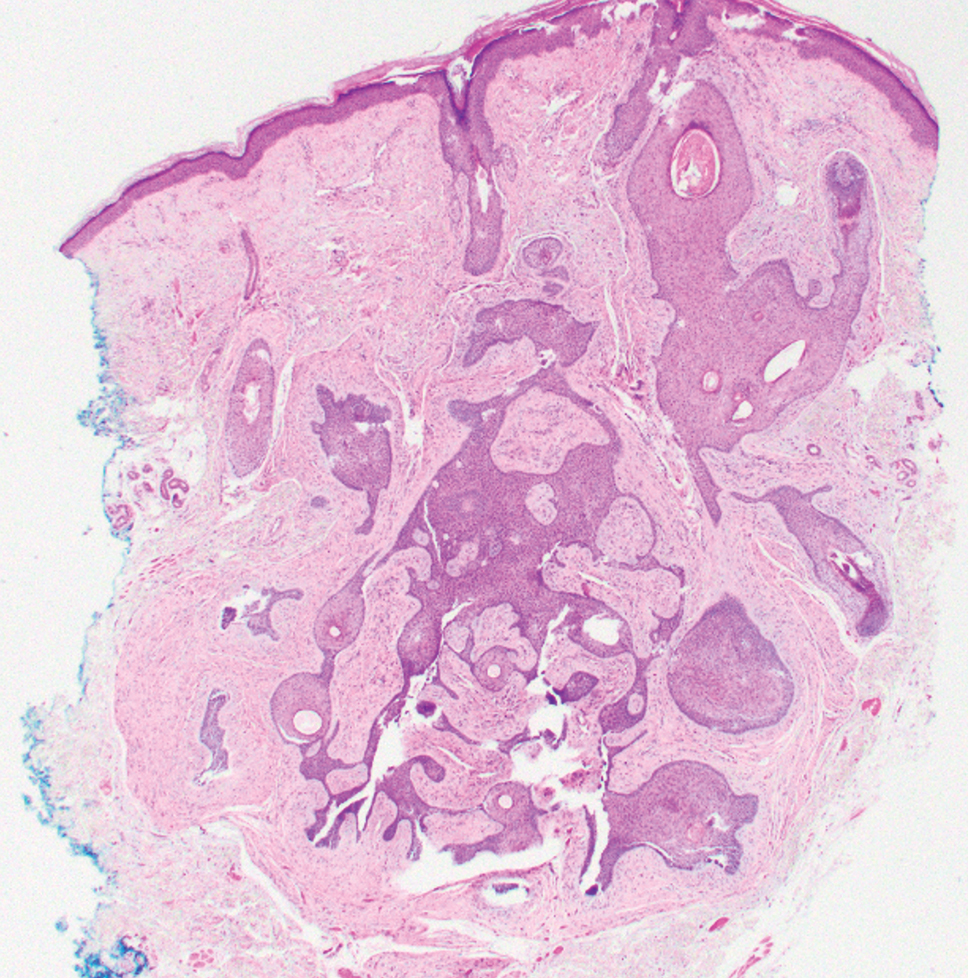
Elastofibroma dorsi is a benign tumor of connective tissue that most commonly manifests clinically as a solitary subcutaneous mass on the back near the inferior angle of the scapula; it typically develops below the rhomboid major and latissimus dorsi muscles.12 The pathogenesis is uncertain, but some patients have reported a family history of the condition or a history of repetitive shoulder movement/trauma prior to onset; the mass may be asymptomatic or associated with pain and/or swelling. Those affected tend to be older than 50 years.13 Histologically, thickened and rounded to beaded elastic fibers are seen admixed with collagen (Figure 3).
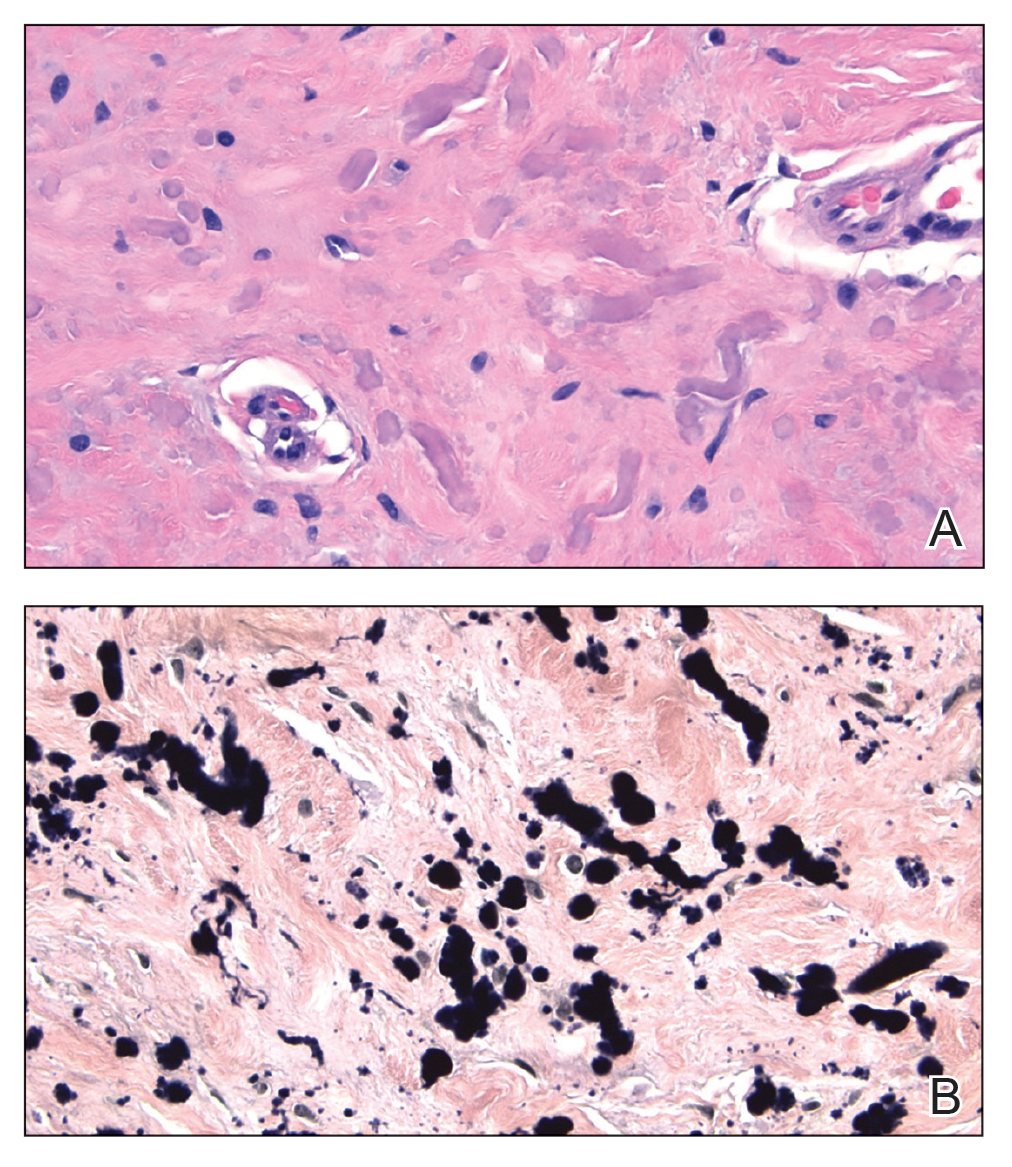
Actinic (solar) elastosis frequently is encountered in many skin biopsies and is caused by chronic photodamage. More hypertrophic variants, such as papular or nodular solar elastosis, may clinically manifest similarly to WFP.14 Histologically, actinic elastosis manifests as a considerable increase in elastic tissue in the papillary and superficial reticular dermis (Figure 4).
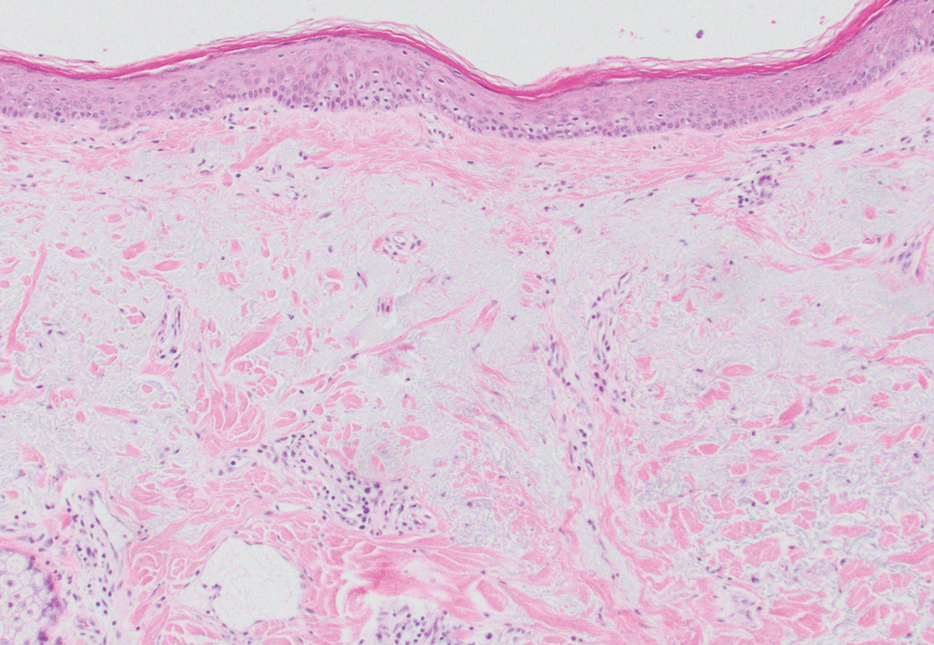
- Shimizu H, Nishikawa T, Kimura S. White fibrous papulosis of the neck: review of our 16 cases. Nihon Hifuka Gakkai Zasshi. 1985;95:1077-1084.
- Teo W, Pang S. White fibrous papulosis of the chest and back. J Am Acad Dermatol. 2012;66:AB33.
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635.
- Lueangarun S, Panchaprateep R. White fibrous papulosis of the neck treated with fractionated 1550-nm erbium glass laser: a case report. J Lasers Med Sci. 2016;7:256-258.
- Rios-Gomez M, Ramos-Garibay JA, Perez-Santana ME, et al. White fibrous papulosis of the neck: a case report. Cureus. 2022;14:E25661.
- Váradi A, Szabó Z, Pomozi V, et al. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671-682.
- Gliem M, Birtel J, Müller PL, et al. Acute retinopathy in pseudoxanthoma elasticum. JAMA Ophthalmol. 2019;137:1165-1173.
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12:85. doi:10.1186/s13023-017-0639-8
- Chisti MA, Binamer Y, Alfadley A, et al. D-penicillamine-induced pseudo-pseudoxanthoma elasticum and extensive elastosis perforans serpiginosa with excellent response to acitretin. Ann Saudi Med. 2019;39:56-60.
- Criscito MC, Mu EW, Meehan SA, et al. Dermoscopic features of a solitary fibrofolliculoma on the left cheek. J Am Acad Dermatol. 2017;76(2 suppl 1):S8-S9.
- Sattler EC, Steinlein OK. Birt-Hogg-Dubé syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Updated January 30, 2020. Accessed February 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1522
- Patnayak R, Jena A, Settipalli S, et al. Elastofibroma: an uncommon tumor revisited. J Cutan Aesthet Surg. 2016;9:34-37. doi:10.4103/0974- 2077.178543
- Chandrasekar CR, Grimer RJ, Carter SR, et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi:10.1155/2008/756565
- Kwittken J. Papular elastosis. Cutis. 2000;66:81-83.
THE DIAGNOSIS: White Fibrous Papulosis
Given the histopathology findings, location on a sun-exposed site, lack of any additional systemic signs or symptoms, and no family history of similar lesions to suggest an underlying genetic condition, a diagnosis of white fibrous papulosis (WFP) was made. White fibrous papulosis is a relatively rare cutaneous disorder that was first reported by Shimizu et al1 in 1985. It is characterized by numerous grouped, 2- to 3-mm, white to flesh-colored papules that in most cases are confined to the neck in middle-aged to elderly individuals; however, cases involving the upper trunk and axillae also have been reported.1-3 The etiology of this condition is unclear but is thought to be related to aging and chronic exposure to UV light. Although treatment is not required, various modalities including tretinoin, excision, and laser therapy have been trialed with varying success.2,4 Our patient elected not to proceed with treatment.
Histologically, WFP may manifest similarly to connective tissue nevi; the overall architecture is nonspecific with focally thickened collagen and often elastic fibers that may be normal to reduced and/or fragmented, as well as an overall decrease in superficial dermal elastic tissue.3,5 Therefore, the differential diagnosis may include connective tissue nevi and require clinical correlation to make a correct diagnosis.
Pseudoxanthoma elasticum (PXE) is an autosomalrecessive disorder most commonly related to mutations in the ATP binding cassette subfamily C member 6 (ABCC6) gene that tends to manifest clinically on the neck and flexural extremities.6 This disease affects elastic fibers, which may become calcified over time. Pseudoxanthoma elasticum is associated with ocular complications relating to the Bruch membrane of the retina and angioid streaks; choroidal neovascularization involving the damaged Bruch membrane and episodes of acute retinopathy may result in vision loss in later stages of the disease.7 Involvement of the elastic laminae of arteries can be associated with cardiovascular and cerebrovascular complications such as stroke, coronary artery disease, claudication, and aneurysms. Involvement of the gastrointestinal or genitourinary tracts also may occur and most commonly manifests with bleeding. Pathologic alterations in the elastic fibers of the lungs also have been reported in patients with PXE.8 Histologically, PXE exhibits increased abnormally clumped and fragmented elastic fibers in the superficial dermis, often with calcification (Figure 1). Pseudo-PXE related to D-penicillamine use often lacks calcification and has a bramble bush appearance.9

Fibrofolliculomas may manifest alone or in association with an underlying condition such as Birt-Hogg-Dubé syndrome, in which lesions are most frequently seen scattered on the scalp, face, ears, neck, or upper trunk.10 This condition is related to a folliculin (FLCN) gene germline mutation. Birt-Hogg-Dubé syndrome also may be associated with acrochordons, trichodiscomas, renal cancer, and lung cysts with or without spontaneous pneumothorax. Less frequently noted findings include oral papules, epidermal cysts, angiofibromas, lipomas/angiolipomas, parotid gland tumors, and thyroid neoplasms. Connective tissue nevi/collagenomas can appear clinically similar to fibrofolliculomas; true connective tissue nevi are reported less commonly in Birt-Hogg-Dubé syndrome.11 Histologically, a fibrofolliculoma manifests with epidermal strands originating from a hair follicle associated with prominent surrounding connective tissue (Figure 2).

Elastofibroma dorsi is a benign tumor of connective tissue that most commonly manifests clinically as a solitary subcutaneous mass on the back near the inferior angle of the scapula; it typically develops below the rhomboid major and latissimus dorsi muscles.12 The pathogenesis is uncertain, but some patients have reported a family history of the condition or a history of repetitive shoulder movement/trauma prior to onset; the mass may be asymptomatic or associated with pain and/or swelling. Those affected tend to be older than 50 years.13 Histologically, thickened and rounded to beaded elastic fibers are seen admixed with collagen (Figure 3).

Actinic (solar) elastosis frequently is encountered in many skin biopsies and is caused by chronic photodamage. More hypertrophic variants, such as papular or nodular solar elastosis, may clinically manifest similarly to WFP.14 Histologically, actinic elastosis manifests as a considerable increase in elastic tissue in the papillary and superficial reticular dermis (Figure 4).

THE DIAGNOSIS: White Fibrous Papulosis
Given the histopathology findings, location on a sun-exposed site, lack of any additional systemic signs or symptoms, and no family history of similar lesions to suggest an underlying genetic condition, a diagnosis of white fibrous papulosis (WFP) was made. White fibrous papulosis is a relatively rare cutaneous disorder that was first reported by Shimizu et al1 in 1985. It is characterized by numerous grouped, 2- to 3-mm, white to flesh-colored papules that in most cases are confined to the neck in middle-aged to elderly individuals; however, cases involving the upper trunk and axillae also have been reported.1-3 The etiology of this condition is unclear but is thought to be related to aging and chronic exposure to UV light. Although treatment is not required, various modalities including tretinoin, excision, and laser therapy have been trialed with varying success.2,4 Our patient elected not to proceed with treatment.
Histologically, WFP may manifest similarly to connective tissue nevi; the overall architecture is nonspecific with focally thickened collagen and often elastic fibers that may be normal to reduced and/or fragmented, as well as an overall decrease in superficial dermal elastic tissue.3,5 Therefore, the differential diagnosis may include connective tissue nevi and require clinical correlation to make a correct diagnosis.
Pseudoxanthoma elasticum (PXE) is an autosomalrecessive disorder most commonly related to mutations in the ATP binding cassette subfamily C member 6 (ABCC6) gene that tends to manifest clinically on the neck and flexural extremities.6 This disease affects elastic fibers, which may become calcified over time. Pseudoxanthoma elasticum is associated with ocular complications relating to the Bruch membrane of the retina and angioid streaks; choroidal neovascularization involving the damaged Bruch membrane and episodes of acute retinopathy may result in vision loss in later stages of the disease.7 Involvement of the elastic laminae of arteries can be associated with cardiovascular and cerebrovascular complications such as stroke, coronary artery disease, claudication, and aneurysms. Involvement of the gastrointestinal or genitourinary tracts also may occur and most commonly manifests with bleeding. Pathologic alterations in the elastic fibers of the lungs also have been reported in patients with PXE.8 Histologically, PXE exhibits increased abnormally clumped and fragmented elastic fibers in the superficial dermis, often with calcification (Figure 1). Pseudo-PXE related to D-penicillamine use often lacks calcification and has a bramble bush appearance.9

Fibrofolliculomas may manifest alone or in association with an underlying condition such as Birt-Hogg-Dubé syndrome, in which lesions are most frequently seen scattered on the scalp, face, ears, neck, or upper trunk.10 This condition is related to a folliculin (FLCN) gene germline mutation. Birt-Hogg-Dubé syndrome also may be associated with acrochordons, trichodiscomas, renal cancer, and lung cysts with or without spontaneous pneumothorax. Less frequently noted findings include oral papules, epidermal cysts, angiofibromas, lipomas/angiolipomas, parotid gland tumors, and thyroid neoplasms. Connective tissue nevi/collagenomas can appear clinically similar to fibrofolliculomas; true connective tissue nevi are reported less commonly in Birt-Hogg-Dubé syndrome.11 Histologically, a fibrofolliculoma manifests with epidermal strands originating from a hair follicle associated with prominent surrounding connective tissue (Figure 2).

Elastofibroma dorsi is a benign tumor of connective tissue that most commonly manifests clinically as a solitary subcutaneous mass on the back near the inferior angle of the scapula; it typically develops below the rhomboid major and latissimus dorsi muscles.12 The pathogenesis is uncertain, but some patients have reported a family history of the condition or a history of repetitive shoulder movement/trauma prior to onset; the mass may be asymptomatic or associated with pain and/or swelling. Those affected tend to be older than 50 years.13 Histologically, thickened and rounded to beaded elastic fibers are seen admixed with collagen (Figure 3).

Actinic (solar) elastosis frequently is encountered in many skin biopsies and is caused by chronic photodamage. More hypertrophic variants, such as papular or nodular solar elastosis, may clinically manifest similarly to WFP.14 Histologically, actinic elastosis manifests as a considerable increase in elastic tissue in the papillary and superficial reticular dermis (Figure 4).

- Shimizu H, Nishikawa T, Kimura S. White fibrous papulosis of the neck: review of our 16 cases. Nihon Hifuka Gakkai Zasshi. 1985;95:1077-1084.
- Teo W, Pang S. White fibrous papulosis of the chest and back. J Am Acad Dermatol. 2012;66:AB33.
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635.
- Lueangarun S, Panchaprateep R. White fibrous papulosis of the neck treated with fractionated 1550-nm erbium glass laser: a case report. J Lasers Med Sci. 2016;7:256-258.
- Rios-Gomez M, Ramos-Garibay JA, Perez-Santana ME, et al. White fibrous papulosis of the neck: a case report. Cureus. 2022;14:E25661.
- Váradi A, Szabó Z, Pomozi V, et al. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671-682.
- Gliem M, Birtel J, Müller PL, et al. Acute retinopathy in pseudoxanthoma elasticum. JAMA Ophthalmol. 2019;137:1165-1173.
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12:85. doi:10.1186/s13023-017-0639-8
- Chisti MA, Binamer Y, Alfadley A, et al. D-penicillamine-induced pseudo-pseudoxanthoma elasticum and extensive elastosis perforans serpiginosa with excellent response to acitretin. Ann Saudi Med. 2019;39:56-60.
- Criscito MC, Mu EW, Meehan SA, et al. Dermoscopic features of a solitary fibrofolliculoma on the left cheek. J Am Acad Dermatol. 2017;76(2 suppl 1):S8-S9.
- Sattler EC, Steinlein OK. Birt-Hogg-Dubé syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Updated January 30, 2020. Accessed February 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1522
- Patnayak R, Jena A, Settipalli S, et al. Elastofibroma: an uncommon tumor revisited. J Cutan Aesthet Surg. 2016;9:34-37. doi:10.4103/0974- 2077.178543
- Chandrasekar CR, Grimer RJ, Carter SR, et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi:10.1155/2008/756565
- Kwittken J. Papular elastosis. Cutis. 2000;66:81-83.
- Shimizu H, Nishikawa T, Kimura S. White fibrous papulosis of the neck: review of our 16 cases. Nihon Hifuka Gakkai Zasshi. 1985;95:1077-1084.
- Teo W, Pang S. White fibrous papulosis of the chest and back. J Am Acad Dermatol. 2012;66:AB33.
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635.
- Lueangarun S, Panchaprateep R. White fibrous papulosis of the neck treated with fractionated 1550-nm erbium glass laser: a case report. J Lasers Med Sci. 2016;7:256-258.
- Rios-Gomez M, Ramos-Garibay JA, Perez-Santana ME, et al. White fibrous papulosis of the neck: a case report. Cureus. 2022;14:E25661.
- Váradi A, Szabó Z, Pomozi V, et al. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets. 2011;12:671-682.
- Gliem M, Birtel J, Müller PL, et al. Acute retinopathy in pseudoxanthoma elasticum. JAMA Ophthalmol. 2019;137:1165-1173.
- Germain DP. Pseudoxanthoma elasticum. Orphanet J Rare Dis. 2017;12:85. doi:10.1186/s13023-017-0639-8
- Chisti MA, Binamer Y, Alfadley A, et al. D-penicillamine-induced pseudo-pseudoxanthoma elasticum and extensive elastosis perforans serpiginosa with excellent response to acitretin. Ann Saudi Med. 2019;39:56-60.
- Criscito MC, Mu EW, Meehan SA, et al. Dermoscopic features of a solitary fibrofolliculoma on the left cheek. J Am Acad Dermatol. 2017;76(2 suppl 1):S8-S9.
- Sattler EC, Steinlein OK. Birt-Hogg-Dubé syndrome. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Updated January 30, 2020. Accessed February 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1522
- Patnayak R, Jena A, Settipalli S, et al. Elastofibroma: an uncommon tumor revisited. J Cutan Aesthet Surg. 2016;9:34-37. doi:10.4103/0974- 2077.178543
- Chandrasekar CR, Grimer RJ, Carter SR, et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi:10.1155/2008/756565
- Kwittken J. Papular elastosis. Cutis. 2000;66:81-83.
A 70-year-old woman with a history of osteoporosis and breast cancer presented for evaluation of asymptomatic, 2- to 3-mm, white to flesh-colored papules concentrated on the inferior occipital scalp and posterior neck (inset) for at least several months. She had no additional systemic signs or symptoms, and there was no family history of similar skin findings. A punch biopsy was performed.
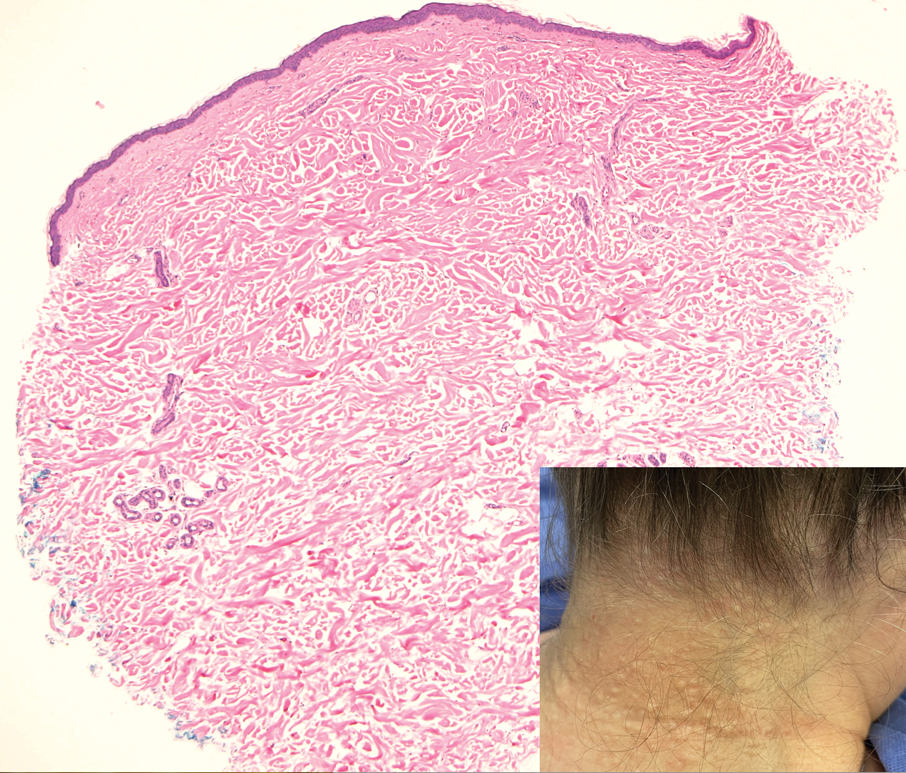
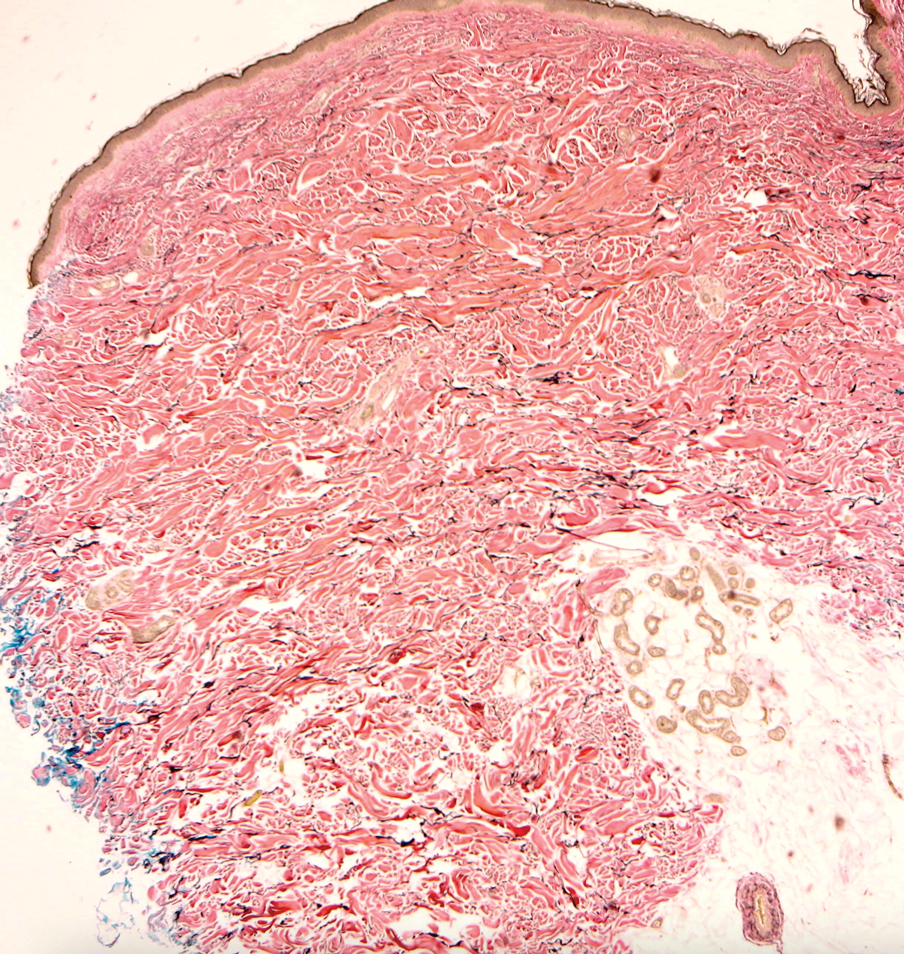
Acute Tender Papules on the Arms and Legs
The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).
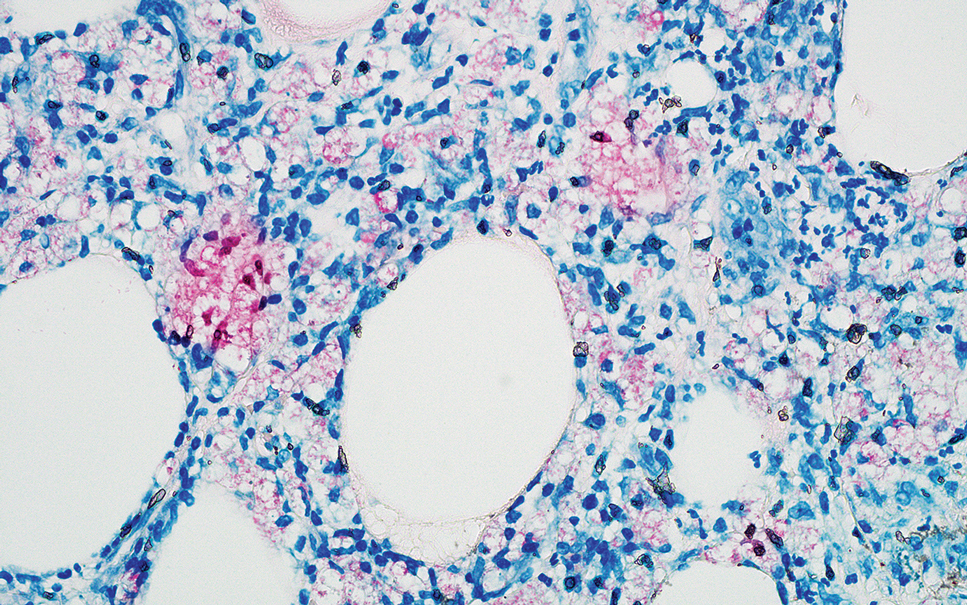
Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.
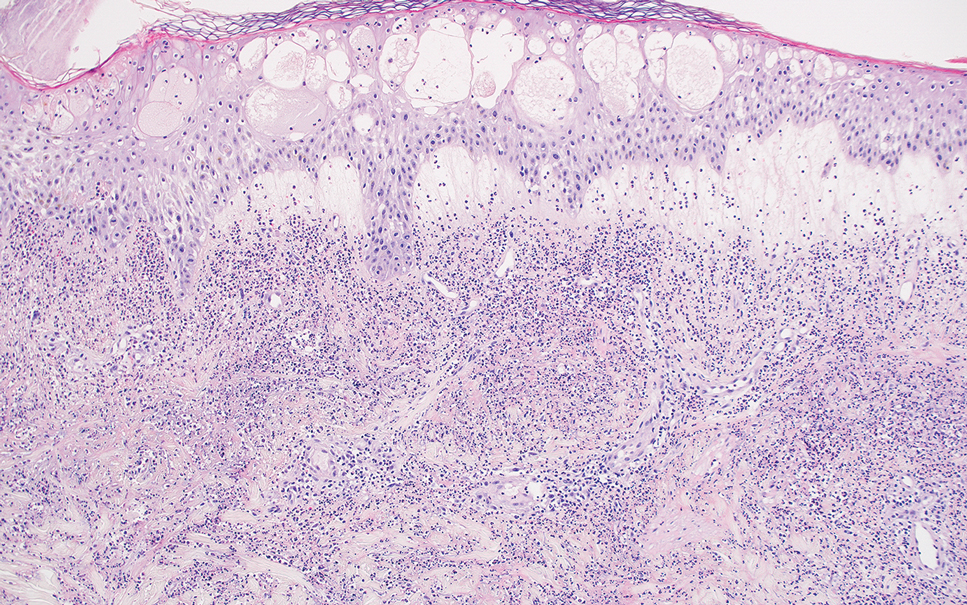
Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.
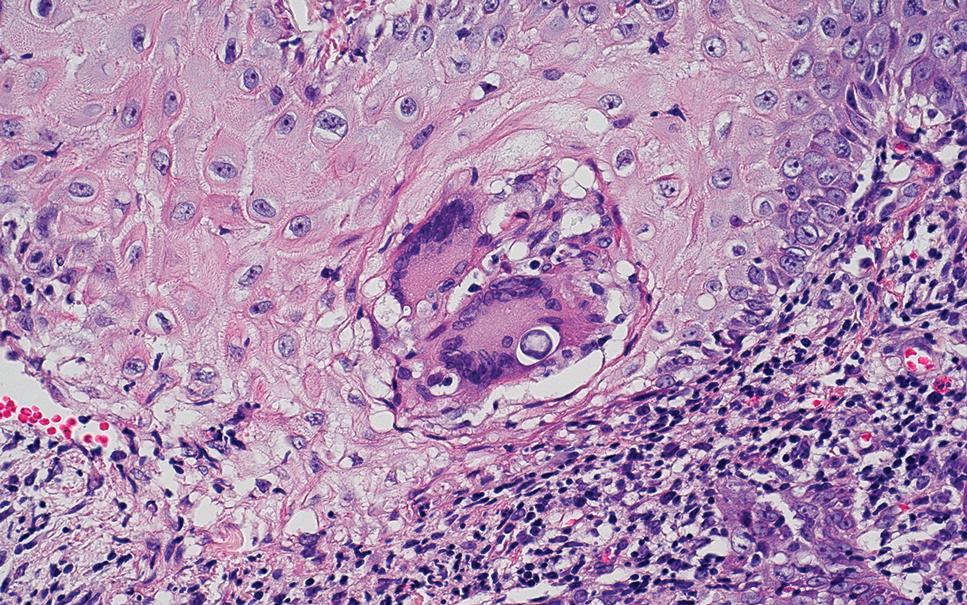
Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9
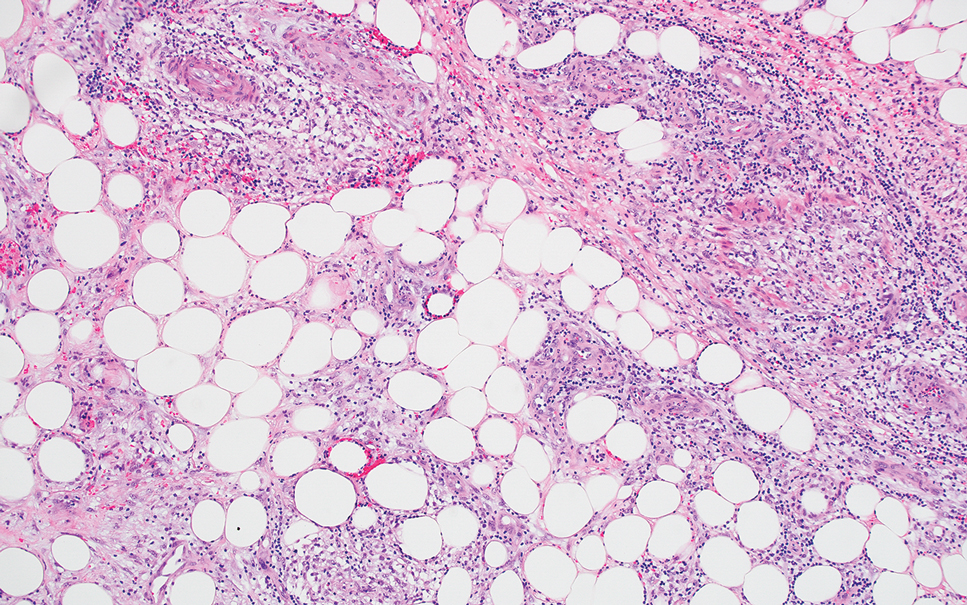
Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).
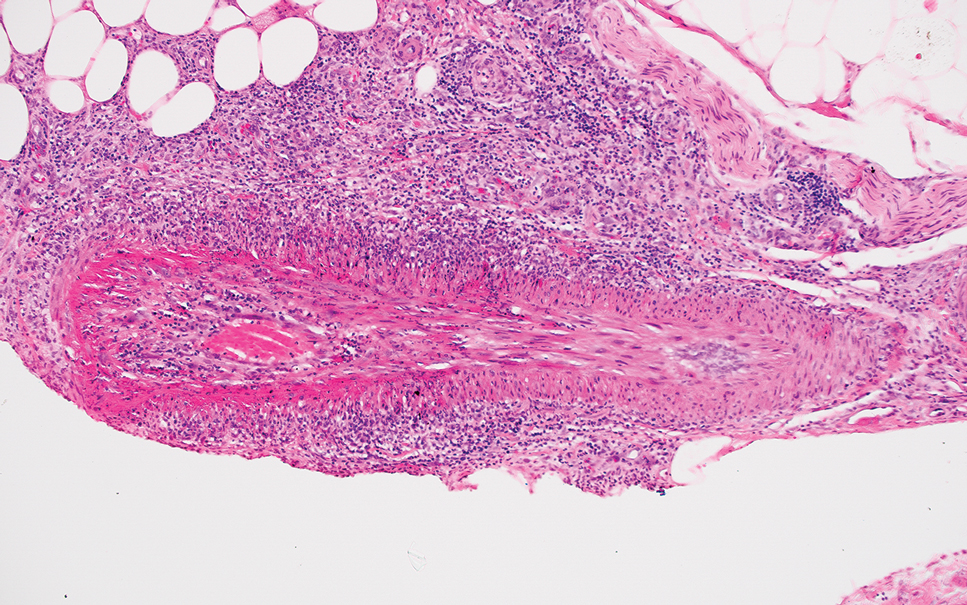
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
A 66-year-old man presented with new tender erythematous papules scattered over the arms and legs. A biopsy of a lesion on the left thigh was performed.
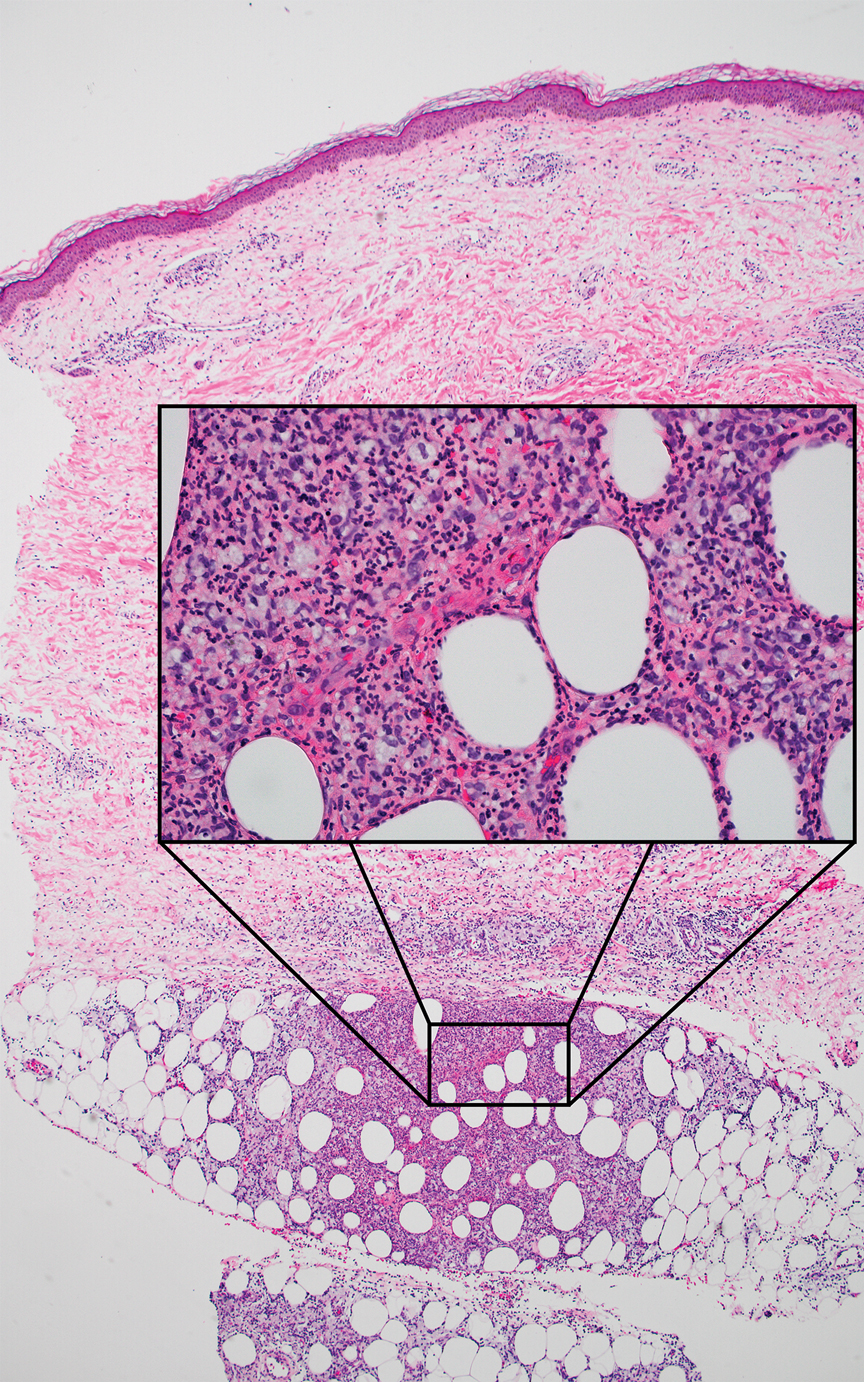
Slowly Enlarging Nodule on the Neck
The Diagnosis: Microsecretory Adenocarcinoma
Microscopically, the tumor was relatively well circumscribed but had irregular borders. It consisted of microcysts and tubules lined by flattened to plump eosinophilic cells with mildly enlarged nuclei and intraluminal basophilic secretions. Peripheral lymphocytic aggregates also were seen in the mid and deep reticular dermis. Tumor necrosis, lymphovascular invasion, and notable mitotic activity were absent. Immunohistochemistry was diffusely positive for cytokeratin (CK) 7 and CK5/6. Occasional tumor cells showed variable expression of alpha smooth muscle actin, S-100 protein, and p40 and p63 antibodies. Immunohistochemistry was negative for CK20; GATA binding protein 3; MYB proto-oncogene, transcription factor; and insulinoma-associated protein 1. A dual-color, break-apart fluorescence in situ hybridization probe identified a rearrangement of the SS18 (SYT) gene locus on chromosome 18. The nodule was excised with clear surgical margins, and the patient had no evidence of recurrent disease or metastasis at 2-year follow-up.
In recent years, there has been a growing recognition of the pivotal role played by gene fusions in driving oncogenesis, encompassing a diverse range of benign and malignant cutaneous neoplasms. These investigations have shed light on previously unknown mechanisms and pathways contributing to the pathogenesis of these neoplastic conditions, offering invaluable insights into their underlying biology. As a result, our ability to classify and diagnose these cutaneous tumors has improved. A notable example of how our current understanding has evolved is the discovery of the new cutaneous adnexal tumor microsecretory adenocarcinoma (MSA). Initially described by Bishop et al1 in 2019 as predominantly occurring in the intraoral minor salivary glands, rare instances of primary cutaneous MSA involving the head and neck regions also have been reported.2 Microsecretory adenocarcinoma represents an important addition to the group of fusion-driven tumors with both salivary gland and cutaneous adnexal analogues, characterized by a MEF2C::SS18 gene fusion. This entity is now recognized as a group of cutaneous adnexal tumors with distinct gene fusions, including both relatively recently discovered entities (eg, secretory carcinoma with NTRK fusions) and previously known entities with newly identified gene fusions (eg, poroid neoplasms with NUTM1, YAP1, or WWTR1 fusions; hidradenomatous neoplasms with CRTC1::MAML2 fusions; and adenoid cystic carcinoma with MYB, MYBL1, and/or NFIB rearrangements).3
Microsecretory adenocarcinoma exhibits a high degree of morphologic consistency, characterized by a microcystic-predominant growth pattern, uniform intercalated ductlike tumor cells with attenuated eosinophilic to clear cytoplasm, monotonous oval hyperchromatic nuclei with indistinct nucleoli, abundant basophilic luminal secretions, and a variably cellular fibromyxoid stroma. It also shows rounded borders with subtle infiltrative growth. Occasionally, pseudoepitheliomatous hyperplasia, tumor-associated lymphoid proliferation, or metaplastic bone formation may accompany MSA. Perineural invasion is rare, necrosis is absent, and mitotic rates generally are low, contributing to its distinctive histopathologic features that aid in accurate diagnosis and differentiation from other entities. Immunohistochemistry reveals diffuse positivity for CK7 and patchy to diffuse expression of S-100 in tumor cells as well as variable expression of p40 and p63. Highly specific SS18 gene translocations at chromosome 18q are useful for diagnosing MSA when found alongside its characteristic appearance, and SS18 break-apart fluorescence in situ hybridization can serve reliably as an accurate diagnostic method (Figure 1).4 Our case illustrates how molecular analysis assists in distinguishing MSA from other cutaneous adnexal tumors, exemplifying the power of our evolving understanding in refining diagnostic accuracy and guiding targeted therapies in clinical practice.
The differential diagnosis of MSA includes tubular adenoma, secretory carcinoma, cribriform tumor (previously carcinoma), and metastatic adenocarcinoma. Tubular adenoma is a rare benign neoplasm that predominantly affects females and can manifest at any age in adulthood. It typically manifests as a slow-growing, occasionally pedunculated nodule, often measuring less than 2 cm. Although it most commonly manifests on the scalp, tubular adenoma also may arise in diverse sites such as the face, axillae, lower extremities, or genitalia.
Notably, scalp lesions often are associated with nevus sebaceus of Jadassohn or syringocystadenoma papilliferum. Microscopically, tubular adenoma is well circumscribed within the dermis and may extend into the subcutis in some cases. Its distinctive appearance consists of variably sized tubules lined by a double or multilayered cuboidal to columnar epithelium, frequently displaying apocrine decapitation secretion (Figure 2). Cystic changes and intraluminal papillae devoid of true fibrovascular cores frequently are observed. Immunohistochemically, luminal epithelial cells express epithelial membrane antigen and carcinoembryonic antigen, while the myoepithelial layer expresses smooth muscle markers, p40, and S-100 protein. BRAF V600E mutation can be detected using immunohistochemistry, with excellent sensitivity and specificity using the anti-BRAF V600E antibody (clone VE1).5 Distinguishing tubular adenoma from MSA is achievable by observing its larger, more variable tubules, along with the consistent presence of a peripheral myoepithelial layer.
Secretory carcinoma is recognized as a low-grade gene fusion–driven carcinoma that primarily arises in salivary glands (both major and minor), with occasional occurrences in the breast and extremely rare instances in other locations such as the skin, thyroid gland, and lung.6 Although the axilla is the most common cutaneous site, diverse locations such as the neck, eyelids, extremities, and nipples also have been documented. Secretory carcinoma affects individuals across a wide age range (13–71 years).6 The hallmark tumors exhibit densely packed, sievelike microcystic glands and tubular spaces filled with abundant eosinophilic intraluminal secretions (Figure 3). Additionally, morphologic variants, such as predominantly papillary, papillary-cystic, macrocystic, solid, partially mucinous, and mixed-pattern neoplasms, have been described. Secretory carcinoma shares certain features with MSA; however, it is distinguished by the presence of pronounced eosinophilic secretions, plump and vacuolated cytoplasm, and a less conspicuous fibromyxoid stroma. Immunohistochemistry reveals tumor cells that are positive for CK7, SOX-10, S-100, mammaglobin, MUC4, and variably GATA-3. Genetically, secretory carcinoma exhibits distinct characteristics, commonly showing the ETV6::NTRK3 fusion, detectable through molecular techniques or pan-TRK immunohistochemistry, while RET fusions and other rare variants are less frequent.7
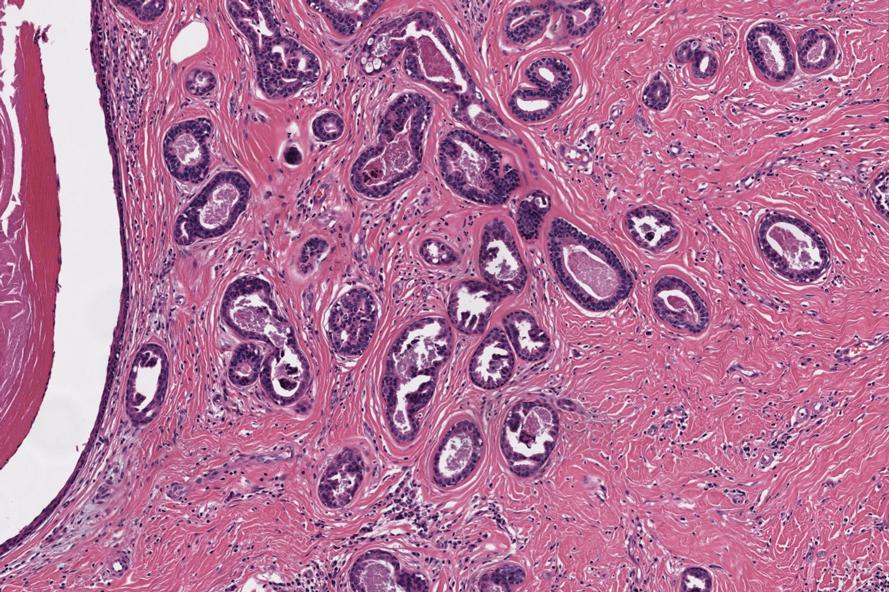
In 1998, Requena et al8 introduced the concept of primary cutaneous cribriform carcinoma. Despite initially being classified as a carcinoma, the malignant potential of this tumor remains uncertain. Consequently, the term cribriform tumor now has become the preferred terminology for denoting this rare entity.9 Primary cutaneous cribriform tumors are observed more commonly in women and typically affect individuals aged 20 to 55 years (mean, 44 years). Predominant locations include the upper and lower extremities, especially the thighs, knees, and legs, with additional cases occurring on the head and trunk. Microscopically, cribriform tumor is characterized by a partially circumscribed, unencapsulated dermal nodule composed of round or oval nuclei displaying hyperchromatism and mild pleomorphism. The defining aspect of its morphology revolves around interspersed small round cavities that give rise to the hallmark cribriform pattern (Figure 4). Although MSA occasionally may exhibit a cribriform architectural pattern, it typically lacks the distinctive feature of thin, threadlike, intraluminal bridging strands observed in cribriform tumors. Similarly, luminal cells within the cribriform tumor express CK7 and exhibit variable S-100 expression. It is recognized as an indolent neoplasm with uncertain malignant potential.
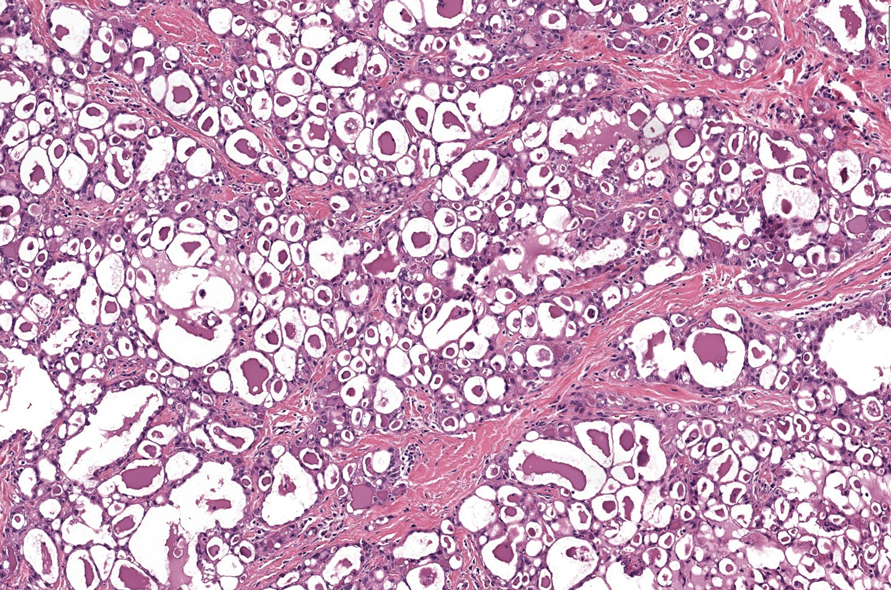
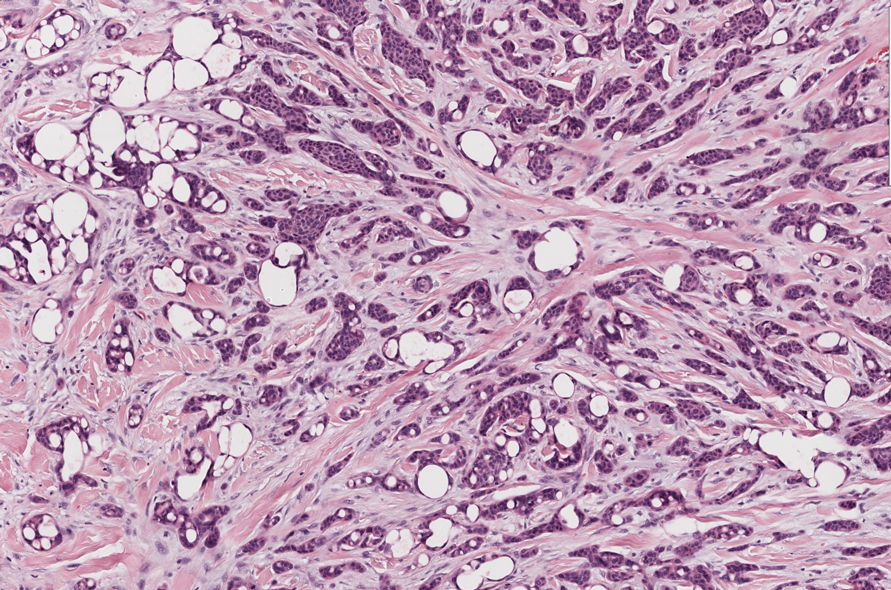
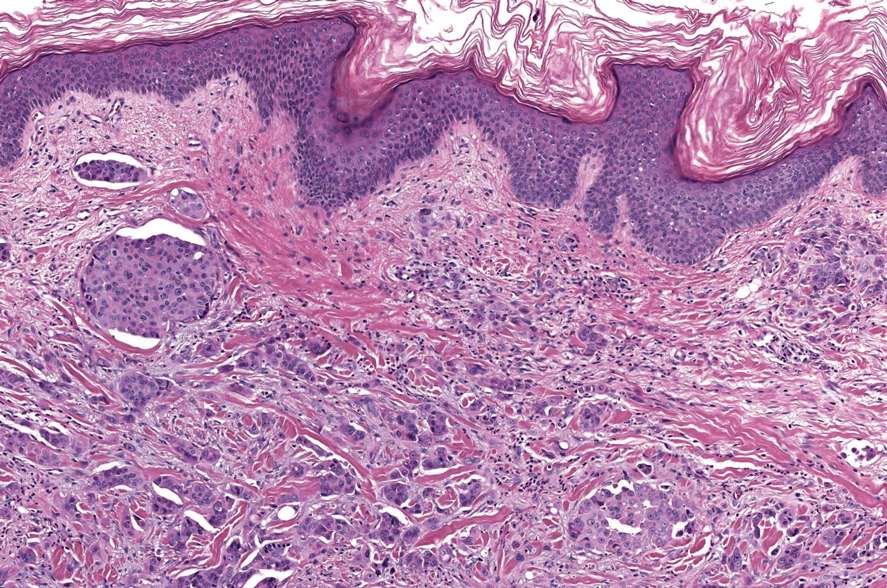
The histopathologic features of metastatic carcinomas can overlap with those of primary cutaneous tumors, particularly adnexal neoplasms.10 However, several key features can aid in the differentiation of cutaneous metastases, including a dermal-based growth pattern with or without subcutaneous involvement, the presence of multiple lesions, and the occurrence of lymphovascular invasion (Figure 5). Conversely, features that suggest a primary cutaneous adnexal neoplasm include the presence of superimposed in situ disease, carcinoma developing within a benign adnexal neoplasm, and notable stromal and/or vascular hyalinization within benign-appearing areas. In some cases, it can be difficult to determine the primary site of origin of a metastatic carcinoma to the skin based on morphologic features alone. In these cases, immunohistochemistry can be helpful. The most cost-effective and time-efficient approach to accurate diagnosis is to obtain a comprehensive clinical history. If there is a known history of cancer, a small panel of organ-specific immunohistochemical studies can be performed to confirm the diagnosis. If there is no known history, an algorithmic approach can be used to identify the primary site of origin. In all circumstances, it cannot be stressed enough that acquiring a thorough clinical history before conducting any diagnostic examinations is paramount.
- Bishop JA, Weinreb I, Swanson D, et al. Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol. 2019;43:1023-1032.
- Bishop JA, Williams EA, McLean AC, et al. Microsecretory adenocarcinoma of the skin harboring recurrent SS18 fusions: a cutaneous analog to a newly described salivary gland tumor. J Cutan Pathol. 2023;50:134-139.
- Macagno N, Sohier Pierre, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers (Basel). 2022;14:476.
- Bishop JA, Koduru P, Veremis BM, et al. SS18 break-apart fluorescence in situ hybridization is a practical and effective method for diagnosing microsecretory adenocarcinoma of salivary glands. Head Neck Pathol. 2021;15:723-726.
- Liau JY, Tsai JH, Huang WC, et al. BRAF and KRAS mutations in tubular apocrine adenoma and papillary eccrine adenoma of the skin. Hum Pathol. 2018;73:59-65.
- Chang MD, Arthur AK, Garcia JJ, et al. ETV6 rearrangement in a case of mammary analogue secretory carcinoma of the skin. J Cutan Pathol. 2016;43:1045-1049.
- Skalova A, Baneckova M, Thompson LDR, et al. Expanding the molecular spectrum of secretory carcinoma of salivary glands with a novel VIM-RET fusion. Am J Surg Pathol. 2020;44:1295-1307.
- Requena L, Kiryu H, Ackerman AB. Neoplasms With Apocrine Differentiation. Lippencott-Raven; 1998.
- Kazakov DV, Llamas-Velasco M, Fernandez-Flores A, et al. Cribriform tumour (previously carcinoma). In: WHO Classification of Tumours: Skin Tumours. 5th ed. International Agency for Research on Cancer; 2024.
- Habaermehl G, Ko J. Cutaneous metastases: a review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019;143:943-957.
The Diagnosis: Microsecretory Adenocarcinoma
Microscopically, the tumor was relatively well circumscribed but had irregular borders. It consisted of microcysts and tubules lined by flattened to plump eosinophilic cells with mildly enlarged nuclei and intraluminal basophilic secretions. Peripheral lymphocytic aggregates also were seen in the mid and deep reticular dermis. Tumor necrosis, lymphovascular invasion, and notable mitotic activity were absent. Immunohistochemistry was diffusely positive for cytokeratin (CK) 7 and CK5/6. Occasional tumor cells showed variable expression of alpha smooth muscle actin, S-100 protein, and p40 and p63 antibodies. Immunohistochemistry was negative for CK20; GATA binding protein 3; MYB proto-oncogene, transcription factor; and insulinoma-associated protein 1. A dual-color, break-apart fluorescence in situ hybridization probe identified a rearrangement of the SS18 (SYT) gene locus on chromosome 18. The nodule was excised with clear surgical margins, and the patient had no evidence of recurrent disease or metastasis at 2-year follow-up.
In recent years, there has been a growing recognition of the pivotal role played by gene fusions in driving oncogenesis, encompassing a diverse range of benign and malignant cutaneous neoplasms. These investigations have shed light on previously unknown mechanisms and pathways contributing to the pathogenesis of these neoplastic conditions, offering invaluable insights into their underlying biology. As a result, our ability to classify and diagnose these cutaneous tumors has improved. A notable example of how our current understanding has evolved is the discovery of the new cutaneous adnexal tumor microsecretory adenocarcinoma (MSA). Initially described by Bishop et al1 in 2019 as predominantly occurring in the intraoral minor salivary glands, rare instances of primary cutaneous MSA involving the head and neck regions also have been reported.2 Microsecretory adenocarcinoma represents an important addition to the group of fusion-driven tumors with both salivary gland and cutaneous adnexal analogues, characterized by a MEF2C::SS18 gene fusion. This entity is now recognized as a group of cutaneous adnexal tumors with distinct gene fusions, including both relatively recently discovered entities (eg, secretory carcinoma with NTRK fusions) and previously known entities with newly identified gene fusions (eg, poroid neoplasms with NUTM1, YAP1, or WWTR1 fusions; hidradenomatous neoplasms with CRTC1::MAML2 fusions; and adenoid cystic carcinoma with MYB, MYBL1, and/or NFIB rearrangements).3
Microsecretory adenocarcinoma exhibits a high degree of morphologic consistency, characterized by a microcystic-predominant growth pattern, uniform intercalated ductlike tumor cells with attenuated eosinophilic to clear cytoplasm, monotonous oval hyperchromatic nuclei with indistinct nucleoli, abundant basophilic luminal secretions, and a variably cellular fibromyxoid stroma. It also shows rounded borders with subtle infiltrative growth. Occasionally, pseudoepitheliomatous hyperplasia, tumor-associated lymphoid proliferation, or metaplastic bone formation may accompany MSA. Perineural invasion is rare, necrosis is absent, and mitotic rates generally are low, contributing to its distinctive histopathologic features that aid in accurate diagnosis and differentiation from other entities. Immunohistochemistry reveals diffuse positivity for CK7 and patchy to diffuse expression of S-100 in tumor cells as well as variable expression of p40 and p63. Highly specific SS18 gene translocations at chromosome 18q are useful for diagnosing MSA when found alongside its characteristic appearance, and SS18 break-apart fluorescence in situ hybridization can serve reliably as an accurate diagnostic method (Figure 1).4 Our case illustrates how molecular analysis assists in distinguishing MSA from other cutaneous adnexal tumors, exemplifying the power of our evolving understanding in refining diagnostic accuracy and guiding targeted therapies in clinical practice.
The differential diagnosis of MSA includes tubular adenoma, secretory carcinoma, cribriform tumor (previously carcinoma), and metastatic adenocarcinoma. Tubular adenoma is a rare benign neoplasm that predominantly affects females and can manifest at any age in adulthood. It typically manifests as a slow-growing, occasionally pedunculated nodule, often measuring less than 2 cm. Although it most commonly manifests on the scalp, tubular adenoma also may arise in diverse sites such as the face, axillae, lower extremities, or genitalia.

Notably, scalp lesions often are associated with nevus sebaceus of Jadassohn or syringocystadenoma papilliferum. Microscopically, tubular adenoma is well circumscribed within the dermis and may extend into the subcutis in some cases. Its distinctive appearance consists of variably sized tubules lined by a double or multilayered cuboidal to columnar epithelium, frequently displaying apocrine decapitation secretion (Figure 2). Cystic changes and intraluminal papillae devoid of true fibrovascular cores frequently are observed. Immunohistochemically, luminal epithelial cells express epithelial membrane antigen and carcinoembryonic antigen, while the myoepithelial layer expresses smooth muscle markers, p40, and S-100 protein. BRAF V600E mutation can be detected using immunohistochemistry, with excellent sensitivity and specificity using the anti-BRAF V600E antibody (clone VE1).5 Distinguishing tubular adenoma from MSA is achievable by observing its larger, more variable tubules, along with the consistent presence of a peripheral myoepithelial layer.
Secretory carcinoma is recognized as a low-grade gene fusion–driven carcinoma that primarily arises in salivary glands (both major and minor), with occasional occurrences in the breast and extremely rare instances in other locations such as the skin, thyroid gland, and lung.6 Although the axilla is the most common cutaneous site, diverse locations such as the neck, eyelids, extremities, and nipples also have been documented. Secretory carcinoma affects individuals across a wide age range (13–71 years).6 The hallmark tumors exhibit densely packed, sievelike microcystic glands and tubular spaces filled with abundant eosinophilic intraluminal secretions (Figure 3). Additionally, morphologic variants, such as predominantly papillary, papillary-cystic, macrocystic, solid, partially mucinous, and mixed-pattern neoplasms, have been described. Secretory carcinoma shares certain features with MSA; however, it is distinguished by the presence of pronounced eosinophilic secretions, plump and vacuolated cytoplasm, and a less conspicuous fibromyxoid stroma. Immunohistochemistry reveals tumor cells that are positive for CK7, SOX-10, S-100, mammaglobin, MUC4, and variably GATA-3. Genetically, secretory carcinoma exhibits distinct characteristics, commonly showing the ETV6::NTRK3 fusion, detectable through molecular techniques or pan-TRK immunohistochemistry, while RET fusions and other rare variants are less frequent.7

In 1998, Requena et al8 introduced the concept of primary cutaneous cribriform carcinoma. Despite initially being classified as a carcinoma, the malignant potential of this tumor remains uncertain. Consequently, the term cribriform tumor now has become the preferred terminology for denoting this rare entity.9 Primary cutaneous cribriform tumors are observed more commonly in women and typically affect individuals aged 20 to 55 years (mean, 44 years). Predominant locations include the upper and lower extremities, especially the thighs, knees, and legs, with additional cases occurring on the head and trunk. Microscopically, cribriform tumor is characterized by a partially circumscribed, unencapsulated dermal nodule composed of round or oval nuclei displaying hyperchromatism and mild pleomorphism. The defining aspect of its morphology revolves around interspersed small round cavities that give rise to the hallmark cribriform pattern (Figure 4). Although MSA occasionally may exhibit a cribriform architectural pattern, it typically lacks the distinctive feature of thin, threadlike, intraluminal bridging strands observed in cribriform tumors. Similarly, luminal cells within the cribriform tumor express CK7 and exhibit variable S-100 expression. It is recognized as an indolent neoplasm with uncertain malignant potential.



The histopathologic features of metastatic carcinomas can overlap with those of primary cutaneous tumors, particularly adnexal neoplasms.10 However, several key features can aid in the differentiation of cutaneous metastases, including a dermal-based growth pattern with or without subcutaneous involvement, the presence of multiple lesions, and the occurrence of lymphovascular invasion (Figure 5). Conversely, features that suggest a primary cutaneous adnexal neoplasm include the presence of superimposed in situ disease, carcinoma developing within a benign adnexal neoplasm, and notable stromal and/or vascular hyalinization within benign-appearing areas. In some cases, it can be difficult to determine the primary site of origin of a metastatic carcinoma to the skin based on morphologic features alone. In these cases, immunohistochemistry can be helpful. The most cost-effective and time-efficient approach to accurate diagnosis is to obtain a comprehensive clinical history. If there is a known history of cancer, a small panel of organ-specific immunohistochemical studies can be performed to confirm the diagnosis. If there is no known history, an algorithmic approach can be used to identify the primary site of origin. In all circumstances, it cannot be stressed enough that acquiring a thorough clinical history before conducting any diagnostic examinations is paramount.
The Diagnosis: Microsecretory Adenocarcinoma
Microscopically, the tumor was relatively well circumscribed but had irregular borders. It consisted of microcysts and tubules lined by flattened to plump eosinophilic cells with mildly enlarged nuclei and intraluminal basophilic secretions. Peripheral lymphocytic aggregates also were seen in the mid and deep reticular dermis. Tumor necrosis, lymphovascular invasion, and notable mitotic activity were absent. Immunohistochemistry was diffusely positive for cytokeratin (CK) 7 and CK5/6. Occasional tumor cells showed variable expression of alpha smooth muscle actin, S-100 protein, and p40 and p63 antibodies. Immunohistochemistry was negative for CK20; GATA binding protein 3; MYB proto-oncogene, transcription factor; and insulinoma-associated protein 1. A dual-color, break-apart fluorescence in situ hybridization probe identified a rearrangement of the SS18 (SYT) gene locus on chromosome 18. The nodule was excised with clear surgical margins, and the patient had no evidence of recurrent disease or metastasis at 2-year follow-up.
In recent years, there has been a growing recognition of the pivotal role played by gene fusions in driving oncogenesis, encompassing a diverse range of benign and malignant cutaneous neoplasms. These investigations have shed light on previously unknown mechanisms and pathways contributing to the pathogenesis of these neoplastic conditions, offering invaluable insights into their underlying biology. As a result, our ability to classify and diagnose these cutaneous tumors has improved. A notable example of how our current understanding has evolved is the discovery of the new cutaneous adnexal tumor microsecretory adenocarcinoma (MSA). Initially described by Bishop et al1 in 2019 as predominantly occurring in the intraoral minor salivary glands, rare instances of primary cutaneous MSA involving the head and neck regions also have been reported.2 Microsecretory adenocarcinoma represents an important addition to the group of fusion-driven tumors with both salivary gland and cutaneous adnexal analogues, characterized by a MEF2C::SS18 gene fusion. This entity is now recognized as a group of cutaneous adnexal tumors with distinct gene fusions, including both relatively recently discovered entities (eg, secretory carcinoma with NTRK fusions) and previously known entities with newly identified gene fusions (eg, poroid neoplasms with NUTM1, YAP1, or WWTR1 fusions; hidradenomatous neoplasms with CRTC1::MAML2 fusions; and adenoid cystic carcinoma with MYB, MYBL1, and/or NFIB rearrangements).3
Microsecretory adenocarcinoma exhibits a high degree of morphologic consistency, characterized by a microcystic-predominant growth pattern, uniform intercalated ductlike tumor cells with attenuated eosinophilic to clear cytoplasm, monotonous oval hyperchromatic nuclei with indistinct nucleoli, abundant basophilic luminal secretions, and a variably cellular fibromyxoid stroma. It also shows rounded borders with subtle infiltrative growth. Occasionally, pseudoepitheliomatous hyperplasia, tumor-associated lymphoid proliferation, or metaplastic bone formation may accompany MSA. Perineural invasion is rare, necrosis is absent, and mitotic rates generally are low, contributing to its distinctive histopathologic features that aid in accurate diagnosis and differentiation from other entities. Immunohistochemistry reveals diffuse positivity for CK7 and patchy to diffuse expression of S-100 in tumor cells as well as variable expression of p40 and p63. Highly specific SS18 gene translocations at chromosome 18q are useful for diagnosing MSA when found alongside its characteristic appearance, and SS18 break-apart fluorescence in situ hybridization can serve reliably as an accurate diagnostic method (Figure 1).4 Our case illustrates how molecular analysis assists in distinguishing MSA from other cutaneous adnexal tumors, exemplifying the power of our evolving understanding in refining diagnostic accuracy and guiding targeted therapies in clinical practice.
The differential diagnosis of MSA includes tubular adenoma, secretory carcinoma, cribriform tumor (previously carcinoma), and metastatic adenocarcinoma. Tubular adenoma is a rare benign neoplasm that predominantly affects females and can manifest at any age in adulthood. It typically manifests as a slow-growing, occasionally pedunculated nodule, often measuring less than 2 cm. Although it most commonly manifests on the scalp, tubular adenoma also may arise in diverse sites such as the face, axillae, lower extremities, or genitalia.

Notably, scalp lesions often are associated with nevus sebaceus of Jadassohn or syringocystadenoma papilliferum. Microscopically, tubular adenoma is well circumscribed within the dermis and may extend into the subcutis in some cases. Its distinctive appearance consists of variably sized tubules lined by a double or multilayered cuboidal to columnar epithelium, frequently displaying apocrine decapitation secretion (Figure 2). Cystic changes and intraluminal papillae devoid of true fibrovascular cores frequently are observed. Immunohistochemically, luminal epithelial cells express epithelial membrane antigen and carcinoembryonic antigen, while the myoepithelial layer expresses smooth muscle markers, p40, and S-100 protein. BRAF V600E mutation can be detected using immunohistochemistry, with excellent sensitivity and specificity using the anti-BRAF V600E antibody (clone VE1).5 Distinguishing tubular adenoma from MSA is achievable by observing its larger, more variable tubules, along with the consistent presence of a peripheral myoepithelial layer.
Secretory carcinoma is recognized as a low-grade gene fusion–driven carcinoma that primarily arises in salivary glands (both major and minor), with occasional occurrences in the breast and extremely rare instances in other locations such as the skin, thyroid gland, and lung.6 Although the axilla is the most common cutaneous site, diverse locations such as the neck, eyelids, extremities, and nipples also have been documented. Secretory carcinoma affects individuals across a wide age range (13–71 years).6 The hallmark tumors exhibit densely packed, sievelike microcystic glands and tubular spaces filled with abundant eosinophilic intraluminal secretions (Figure 3). Additionally, morphologic variants, such as predominantly papillary, papillary-cystic, macrocystic, solid, partially mucinous, and mixed-pattern neoplasms, have been described. Secretory carcinoma shares certain features with MSA; however, it is distinguished by the presence of pronounced eosinophilic secretions, plump and vacuolated cytoplasm, and a less conspicuous fibromyxoid stroma. Immunohistochemistry reveals tumor cells that are positive for CK7, SOX-10, S-100, mammaglobin, MUC4, and variably GATA-3. Genetically, secretory carcinoma exhibits distinct characteristics, commonly showing the ETV6::NTRK3 fusion, detectable through molecular techniques or pan-TRK immunohistochemistry, while RET fusions and other rare variants are less frequent.7

In 1998, Requena et al8 introduced the concept of primary cutaneous cribriform carcinoma. Despite initially being classified as a carcinoma, the malignant potential of this tumor remains uncertain. Consequently, the term cribriform tumor now has become the preferred terminology for denoting this rare entity.9 Primary cutaneous cribriform tumors are observed more commonly in women and typically affect individuals aged 20 to 55 years (mean, 44 years). Predominant locations include the upper and lower extremities, especially the thighs, knees, and legs, with additional cases occurring on the head and trunk. Microscopically, cribriform tumor is characterized by a partially circumscribed, unencapsulated dermal nodule composed of round or oval nuclei displaying hyperchromatism and mild pleomorphism. The defining aspect of its morphology revolves around interspersed small round cavities that give rise to the hallmark cribriform pattern (Figure 4). Although MSA occasionally may exhibit a cribriform architectural pattern, it typically lacks the distinctive feature of thin, threadlike, intraluminal bridging strands observed in cribriform tumors. Similarly, luminal cells within the cribriform tumor express CK7 and exhibit variable S-100 expression. It is recognized as an indolent neoplasm with uncertain malignant potential.



The histopathologic features of metastatic carcinomas can overlap with those of primary cutaneous tumors, particularly adnexal neoplasms.10 However, several key features can aid in the differentiation of cutaneous metastases, including a dermal-based growth pattern with or without subcutaneous involvement, the presence of multiple lesions, and the occurrence of lymphovascular invasion (Figure 5). Conversely, features that suggest a primary cutaneous adnexal neoplasm include the presence of superimposed in situ disease, carcinoma developing within a benign adnexal neoplasm, and notable stromal and/or vascular hyalinization within benign-appearing areas. In some cases, it can be difficult to determine the primary site of origin of a metastatic carcinoma to the skin based on morphologic features alone. In these cases, immunohistochemistry can be helpful. The most cost-effective and time-efficient approach to accurate diagnosis is to obtain a comprehensive clinical history. If there is a known history of cancer, a small panel of organ-specific immunohistochemical studies can be performed to confirm the diagnosis. If there is no known history, an algorithmic approach can be used to identify the primary site of origin. In all circumstances, it cannot be stressed enough that acquiring a thorough clinical history before conducting any diagnostic examinations is paramount.
- Bishop JA, Weinreb I, Swanson D, et al. Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol. 2019;43:1023-1032.
- Bishop JA, Williams EA, McLean AC, et al. Microsecretory adenocarcinoma of the skin harboring recurrent SS18 fusions: a cutaneous analog to a newly described salivary gland tumor. J Cutan Pathol. 2023;50:134-139.
- Macagno N, Sohier Pierre, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers (Basel). 2022;14:476.
- Bishop JA, Koduru P, Veremis BM, et al. SS18 break-apart fluorescence in situ hybridization is a practical and effective method for diagnosing microsecretory adenocarcinoma of salivary glands. Head Neck Pathol. 2021;15:723-726.
- Liau JY, Tsai JH, Huang WC, et al. BRAF and KRAS mutations in tubular apocrine adenoma and papillary eccrine adenoma of the skin. Hum Pathol. 2018;73:59-65.
- Chang MD, Arthur AK, Garcia JJ, et al. ETV6 rearrangement in a case of mammary analogue secretory carcinoma of the skin. J Cutan Pathol. 2016;43:1045-1049.
- Skalova A, Baneckova M, Thompson LDR, et al. Expanding the molecular spectrum of secretory carcinoma of salivary glands with a novel VIM-RET fusion. Am J Surg Pathol. 2020;44:1295-1307.
- Requena L, Kiryu H, Ackerman AB. Neoplasms With Apocrine Differentiation. Lippencott-Raven; 1998.
- Kazakov DV, Llamas-Velasco M, Fernandez-Flores A, et al. Cribriform tumour (previously carcinoma). In: WHO Classification of Tumours: Skin Tumours. 5th ed. International Agency for Research on Cancer; 2024.
- Habaermehl G, Ko J. Cutaneous metastases: a review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019;143:943-957.
- Bishop JA, Weinreb I, Swanson D, et al. Microsecretory adenocarcinoma: a novel salivary gland tumor characterized by a recurrent MEF2C-SS18 fusion. Am J Surg Pathol. 2019;43:1023-1032.
- Bishop JA, Williams EA, McLean AC, et al. Microsecretory adenocarcinoma of the skin harboring recurrent SS18 fusions: a cutaneous analog to a newly described salivary gland tumor. J Cutan Pathol. 2023;50:134-139.
- Macagno N, Sohier Pierre, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers (Basel). 2022;14:476.
- Bishop JA, Koduru P, Veremis BM, et al. SS18 break-apart fluorescence in situ hybridization is a practical and effective method for diagnosing microsecretory adenocarcinoma of salivary glands. Head Neck Pathol. 2021;15:723-726.
- Liau JY, Tsai JH, Huang WC, et al. BRAF and KRAS mutations in tubular apocrine adenoma and papillary eccrine adenoma of the skin. Hum Pathol. 2018;73:59-65.
- Chang MD, Arthur AK, Garcia JJ, et al. ETV6 rearrangement in a case of mammary analogue secretory carcinoma of the skin. J Cutan Pathol. 2016;43:1045-1049.
- Skalova A, Baneckova M, Thompson LDR, et al. Expanding the molecular spectrum of secretory carcinoma of salivary glands with a novel VIM-RET fusion. Am J Surg Pathol. 2020;44:1295-1307.
- Requena L, Kiryu H, Ackerman AB. Neoplasms With Apocrine Differentiation. Lippencott-Raven; 1998.
- Kazakov DV, Llamas-Velasco M, Fernandez-Flores A, et al. Cribriform tumour (previously carcinoma). In: WHO Classification of Tumours: Skin Tumours. 5th ed. International Agency for Research on Cancer; 2024.
- Habaermehl G, Ko J. Cutaneous metastases: a review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019;143:943-957.
A 74-year-old man presented with an asymptomatic nodule on the left neck measuring approximately 2 cm. An excisional biopsy was obtained for histopathologic evaluation.
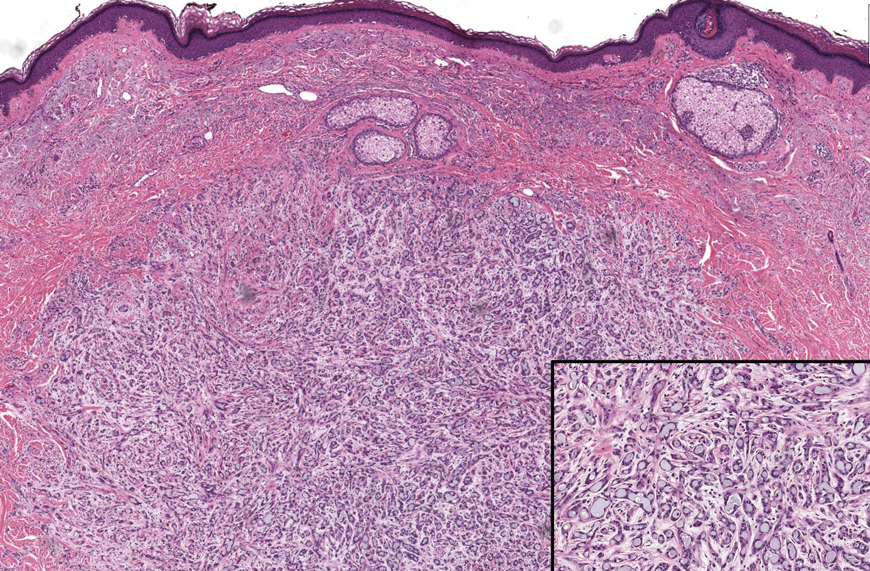
Progressive Eyelash Loss and Scale of the Right Eyelid
The Diagnosis: Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) characterized by folliculotropism and follicular-based lesions. The clinical manifestation of FMF can vary and includes patches, plaques, or tumors resembling nonfolliculotropic MF; acneform lesions including comedones and pustules; or areas of alopecia. Lesions commonly involve the head and neck but also can be seen on the trunk or extremities. Folliculotropic mycosis fungoides can be accompanied by pruritus or superimposed secondary infection.
Histologic features of FMF include follicular (perifollicular or intrafollicular) infiltration by atypical T cells showing cerebriform nuclei.1 In early lesions, there may be only mild superficial perivascular inflammation without notable lymphocyte atypia, making diagnosis challenging. 2,3 Mucinous degeneration of the follicles—termed follicular mucinosis—is a common histologic finding in FMF.1,2 Follicular mucinosis is not exclusive to FMF; it can be primary/idiopathic or secondary to underlying inflammatory or neoplastic disorders such as FMF. On immunohistochemistry, FMF most commonly demonstrates a helper T cell phenotype that is positive for CD3 and CD4 and negative for CD8, with aberrant loss of CD7 and variably CD5, which is similar to classic MF. Occasionally, larger CD30+ cells also can be present in the dermis. T-cell gene rearrangement studies will demonstrate T-cell receptor clonality in most cases.2
Many large retrospective cohort studies have suggested that patients with FMF have a worse prognosis than classic MF, with a 5-year survival rate of 62% to 87% for early-stage FMF vs more than 90% for classic patchand plaque-stage MF.4-7 However, a 2016 study suggested histologic evaluation may be able to further differentiate clinically identical cases into indolent and aggressive forms of FMF with considerably different outcomes based on the density of the perifollicular infiltrate.5 The presence of follicular mucinosis has no impact on prognosis compared to cases without follicular mucinosis.1,2
Alopecia mucinosa is characterized by infiltrating, erythematous, scaling plaques localized to the head and neck.8 It is diagnosed clinically, and histopathology shows follicular mucinosis. The terms alopecia mucinosa and follicular mucinosis often are used interchangeably. Over the past few decades, 3 variants have been categorized: primary acute, primary chronic, and secondary. The primary acute form manifests in children and young adults as solitary lesions, which often resolve spontaneously. In contrast, the primary chronic form manifests in older adults as multiple disseminated lesions with a chronic relapsing course.8,9 The secondary form can occur in the setting of other disorders, including lupus erythematosus, hypertrophic lichen planus, alopecia areata, and neoplasms such as MF or Hodgkin lymphoma.9 The histopathologic findings are similar for all types of alopecia mucinosa, with cystic pools of mucin deposition in the sebaceous glands and external root sheath of the follicles as well as associated inflammation composed of lymphocytes and eosinophils (Figure 1).9,10 The inflammatory infiltrate rarely extends into the epidermis or upper portion of the hair follicle. Although histopathology alone cannot reliably distinguish between primary and secondary forms of alopecia mucinosa, MF (including follicular MF) or another underlying cutaneous T-cell lymphoma should be considered if inflammation extends into the upper dermis, epidermis, or follicles or is in a dense bandlike distribution.11 On immunohistochemistry, lymphocytes should show positivity for CD3, CD4, and CD8. The CD4:CD8 ratio often is 1:1 in alopecia mucinosa, while in FMF it is approximately 3:1.10 CD7 commonly is negative but can be present in a small percentage of cases.12 T-cell receptor gene rearrangement studies have detected clonality in both primary and secondary alopecia mucinosa and thus cannot be used alone to distinguish between the two.10 Given the overlap in histopathologic and immunohistochemical features of primary and secondary alopecia mucinosa, definitive diagnosis cannot be made with any single modality and should be based on correlating clinical presentation, histopathology, immunohistochemistry, and molecular analyses.
Inflammatory dermatoses including seborrheic dermatitis also are in the differential diagnosis for FMF. Seborrheic dermatitis is a common chronic inflammatory skin disorder affecting 1% to 3% of the general population. 13 Patients usually present with scaly and greasy plaques and papules localized to areas with increased sebaceous glands and high sebum production such as the face, scalp, and intertriginous regions. The distribution often is symmetrical, and the severity of disease can vary substantially.13 Sebopsoriasis is an entity with overlapping features of seborrheic dermatitis and psoriasis, including thicker, more erythematous plaques that are more elevated. Histopathology of seborrheic dermatitis reveals spongiotic inflammation in the epidermis characterized by rounding of the keratinocytes, widening of the intercellular spaces, and accumulation of intracellular edema, causing the formation of clear spaces in the epidermis (Figure 2). Focal parakeratosis, usually in the follicular ostia, and mounds of scaly crust often are present. 14 A periodic acid–Schiff stain should be performed to rule out infectious dermatophytes, which can show similar clinical and histologic features. More chronic cases of seborrheic dermatitis often can take on histologic features of psoriasis, namely epidermal hyperplasia with thinning over dermal papillae, though the hyperplasia in psoriasis is more regular.
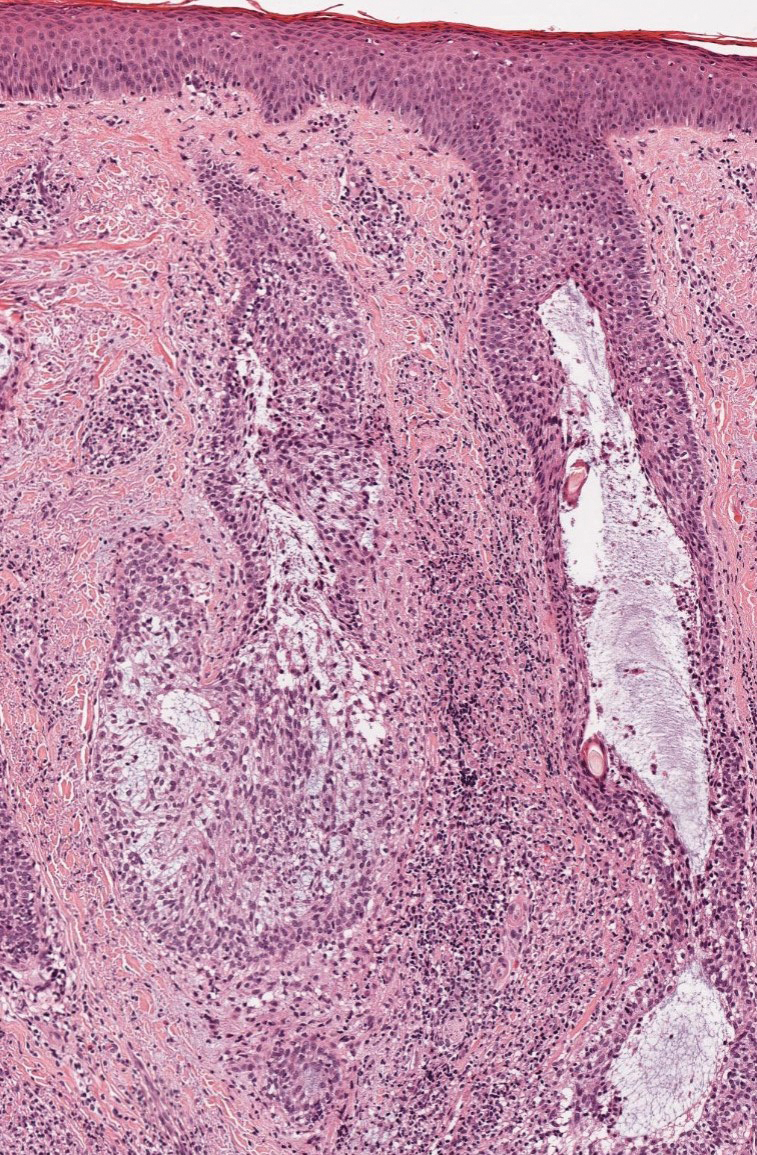
Alopecia areata is an immune-mediated disorder characterized by nonscarring hair loss; it affects approximately 0.1% to 0.2% of the general population.15 The pathogenesis involves the premature transition of hair follicles in the anagen (growth) phase to the catagen ( nonproliferative/involution) and telogen (resting) phases, resulting in sudden hair shedding and decreased regrowth. Clinically, it is characterized by asymptomatic hair loss that occurs most frequently on the scalp and other areas of the head, including eyelashes, eyebrows, and facial hair, but also can occur on the extremities. There are several variants; the most common is patchy alopecia, which features smooth circular areas of hair loss that progress over several weeks. Some patients can progress to loss of all scalp hairs (alopecia totalis) or all hairs throughout the body (alopecia universalis). 15 Patients typically will have spontaneous regrowth of hair, with up to 50% of those with limited hair loss recovering within a year.16 The disease has a chronic/ relapsing course, and patients often will have multiple episodes of hair loss. Histopathologic features can vary depending on the stage of disease. In acute cases, a peribulbar lymphocytic infiltrate preferentially involving anagen-stage hair follicles is seen, with associated necrosis, edema, and pigment incontinence (Figure 3).16 In chronic alopecia areata, the inflammation may be less brisk, and follicular miniaturization often is seen. Additionally, increased proportions of catagen- or telogen-stage follicles are present.16,17 On immunohistochemistry, lymphocytes express both CD4 and CD8, with a slightly increased CD4:CD8 ratio in active disease.18
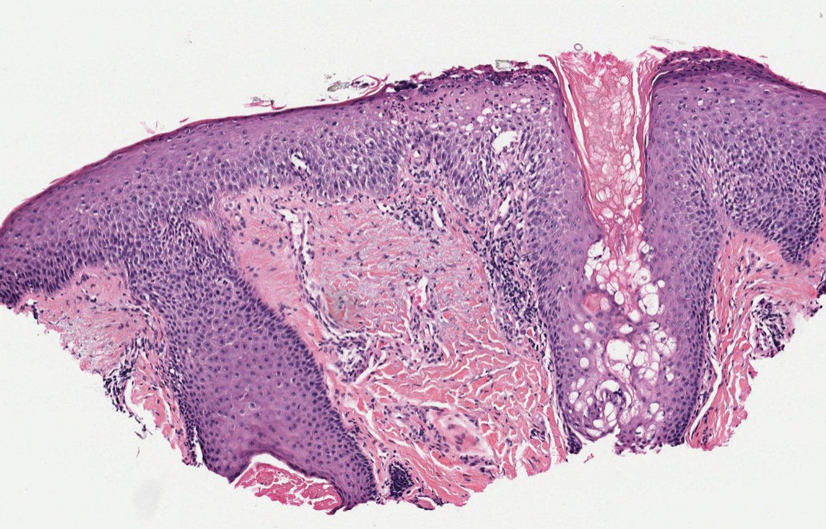
Psoriatic alopecia describes hair loss that occurs in patients with psoriasis. Patients present with scaly, erythematous, psoriasiform plaques or patches, as well as decreased hair density, finer hairs, and increased dystrophic hair bulbs within the psoriatic plaques.19 It often is nonscarring and resolves with therapy, though scarring may occur with secondary infection. Psoriatic alopecia may occur in the setting of classic psoriasis and also may occur in psoriasiform drug eruptions, including those caused by tumor necrosis factor inhibitors.20,21 Histologic features include atrophy of sebaceous glands, epidermal changes with hypogranulosis and psoriasiform hyperplasia, decreased hair follicle density, and neutrophils in the stratum spinosum (Figure 4). There often is associated perifollicular lymphocytic inflammation with small lymphocytes that do not have notable morphologic abnormalities.
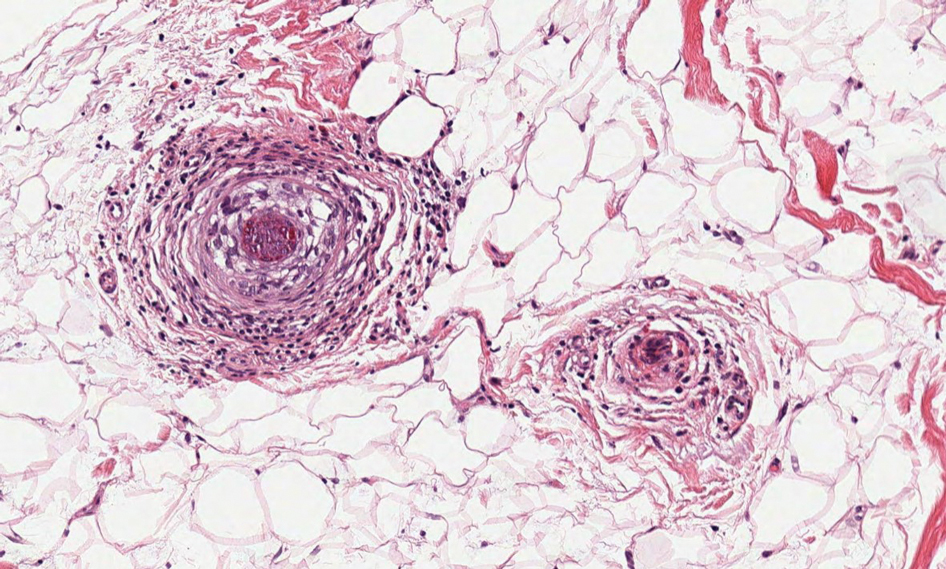
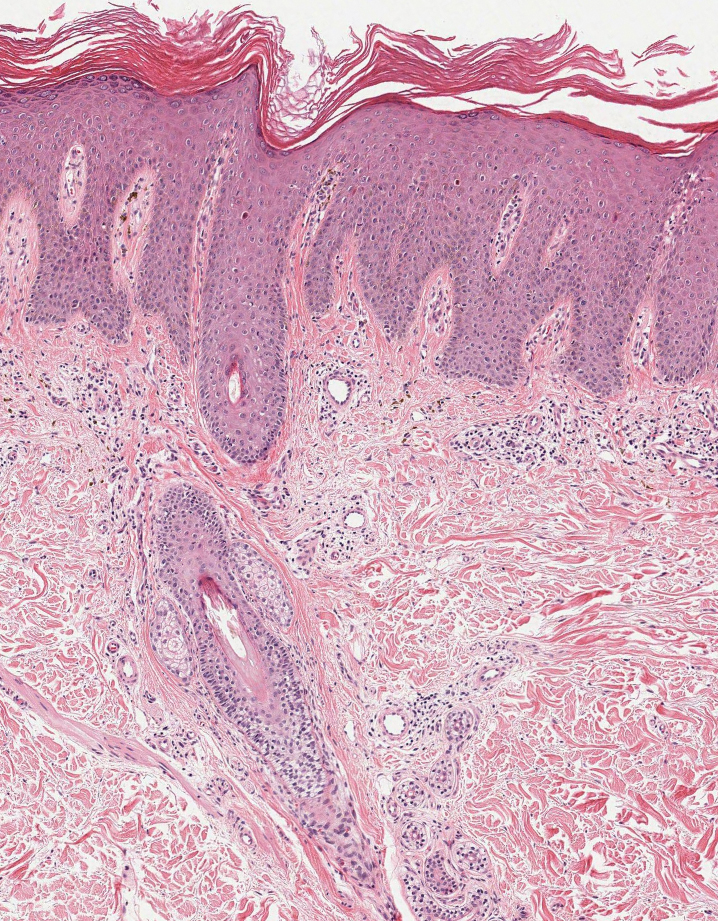
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714. doi:10.1182/blood-2018-11-881268
- Malveira MIB, Pascoal G, Gamonal SBL, et al. Folliculotropic mycosis fungoides: challenging clinical, histopathological and immunohistochemical diagnosis. An Bras Dermatol. 2017;92(5 suppl 1):73-75. doi:10.1590/abd1806-4841.20175634
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525- 530. doi:10.1034/j.1600-0560.2001.281006.x
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides: a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198. doi:10.1001/archderm.138.2.191
- van Santen S, Roach REJ, van Doorn R, et al. Clinical staging and prognostic factors in folliculotropic mycosis fungoides. JAMA Dermatol. 2016;152:992-1000. doi:10.1001/jamadermatol.2016.1597
- Lehman JS, Cook-Norris RH, Weed BR, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010;146:607-613. doi:10.1001/archdermatol.2010.101
- Gerami P, Rosen S, Kuzel T, et al. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008;144:738-746. doi:10.1001/archderm.144.6.738
- Büchner SA, Meier M, Rufli TH. Follicular mucinosis associated with mycosis fungoides. Dermatology. 1991;183:66-67. doi:10.1159/000247639
- Akinsanya AO, Tschen JA. Follicular mucinosis: a case report. Cureus. 2019;11:E4746. doi:10.7759/cureus.4746
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19. doi:10.1111/j.1600-0560.2009.01338.x
- Khalil J, Kurban M, Abbas O. Follicular mucinosis: a review. Int J Dermatol. 2021;60:159-165. doi:10.1111/ijd.15165
- Zvulunov A, Shkalim V, Ben-Amitai D, et al. Clinical and histopathologic spectrum of alopecia mucinosa/follicular mucinosis and its natural history in children. J Am Acad Dermatol. 2012;67:1174-1181. doi:10.1016/j.jaad.2012.04.015
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31:343-351. doi:10.1016/j.clindermatol.2013.01.001
- Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26; quiz 19-20. doi:10.1111/j .1468-3083.2004.00693.x
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12. doi:10.1016/j .jaad.2017.04.1141
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-88, quiz 189-90. doi:10.1016/j.jaad.2009.10.032
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139:1555-1559. doi:10.1001/archderm .139.12.1555
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11(2 pt 1):216-223. doi:10.1016 /s0190-9622(84)70152-6
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721. doi:10.1111/ced.12715
- Afaasiev OK, Zhang CZ, Ruhoy SM. TNF-inhibitor associated psoriatic alopecia: diagnostic utility of sebaceous lobule atrophy. J Cutan Pathol. 2017;44:563-539. doi:10.1111/cup.12932
- Silva CY, Brown KL, Kurban AK, et al. Psoriatic alopecia—fact or fiction? A clinicohistologic reappraisal. Indian J Dermatol Venereol Leprol. 2012;78:611-619. doi:10.4103/0378-6323.100574
The Diagnosis: Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) characterized by folliculotropism and follicular-based lesions. The clinical manifestation of FMF can vary and includes patches, plaques, or tumors resembling nonfolliculotropic MF; acneform lesions including comedones and pustules; or areas of alopecia. Lesions commonly involve the head and neck but also can be seen on the trunk or extremities. Folliculotropic mycosis fungoides can be accompanied by pruritus or superimposed secondary infection.
Histologic features of FMF include follicular (perifollicular or intrafollicular) infiltration by atypical T cells showing cerebriform nuclei.1 In early lesions, there may be only mild superficial perivascular inflammation without notable lymphocyte atypia, making diagnosis challenging. 2,3 Mucinous degeneration of the follicles—termed follicular mucinosis—is a common histologic finding in FMF.1,2 Follicular mucinosis is not exclusive to FMF; it can be primary/idiopathic or secondary to underlying inflammatory or neoplastic disorders such as FMF. On immunohistochemistry, FMF most commonly demonstrates a helper T cell phenotype that is positive for CD3 and CD4 and negative for CD8, with aberrant loss of CD7 and variably CD5, which is similar to classic MF. Occasionally, larger CD30+ cells also can be present in the dermis. T-cell gene rearrangement studies will demonstrate T-cell receptor clonality in most cases.2
Many large retrospective cohort studies have suggested that patients with FMF have a worse prognosis than classic MF, with a 5-year survival rate of 62% to 87% for early-stage FMF vs more than 90% for classic patchand plaque-stage MF.4-7 However, a 2016 study suggested histologic evaluation may be able to further differentiate clinically identical cases into indolent and aggressive forms of FMF with considerably different outcomes based on the density of the perifollicular infiltrate.5 The presence of follicular mucinosis has no impact on prognosis compared to cases without follicular mucinosis.1,2
Alopecia mucinosa is characterized by infiltrating, erythematous, scaling plaques localized to the head and neck.8 It is diagnosed clinically, and histopathology shows follicular mucinosis. The terms alopecia mucinosa and follicular mucinosis often are used interchangeably. Over the past few decades, 3 variants have been categorized: primary acute, primary chronic, and secondary. The primary acute form manifests in children and young adults as solitary lesions, which often resolve spontaneously. In contrast, the primary chronic form manifests in older adults as multiple disseminated lesions with a chronic relapsing course.8,9 The secondary form can occur in the setting of other disorders, including lupus erythematosus, hypertrophic lichen planus, alopecia areata, and neoplasms such as MF or Hodgkin lymphoma.9 The histopathologic findings are similar for all types of alopecia mucinosa, with cystic pools of mucin deposition in the sebaceous glands and external root sheath of the follicles as well as associated inflammation composed of lymphocytes and eosinophils (Figure 1).9,10 The inflammatory infiltrate rarely extends into the epidermis or upper portion of the hair follicle. Although histopathology alone cannot reliably distinguish between primary and secondary forms of alopecia mucinosa, MF (including follicular MF) or another underlying cutaneous T-cell lymphoma should be considered if inflammation extends into the upper dermis, epidermis, or follicles or is in a dense bandlike distribution.11 On immunohistochemistry, lymphocytes should show positivity for CD3, CD4, and CD8. The CD4:CD8 ratio often is 1:1 in alopecia mucinosa, while in FMF it is approximately 3:1.10 CD7 commonly is negative but can be present in a small percentage of cases.12 T-cell receptor gene rearrangement studies have detected clonality in both primary and secondary alopecia mucinosa and thus cannot be used alone to distinguish between the two.10 Given the overlap in histopathologic and immunohistochemical features of primary and secondary alopecia mucinosa, definitive diagnosis cannot be made with any single modality and should be based on correlating clinical presentation, histopathology, immunohistochemistry, and molecular analyses.
Inflammatory dermatoses including seborrheic dermatitis also are in the differential diagnosis for FMF. Seborrheic dermatitis is a common chronic inflammatory skin disorder affecting 1% to 3% of the general population. 13 Patients usually present with scaly and greasy plaques and papules localized to areas with increased sebaceous glands and high sebum production such as the face, scalp, and intertriginous regions. The distribution often is symmetrical, and the severity of disease can vary substantially.13 Sebopsoriasis is an entity with overlapping features of seborrheic dermatitis and psoriasis, including thicker, more erythematous plaques that are more elevated. Histopathology of seborrheic dermatitis reveals spongiotic inflammation in the epidermis characterized by rounding of the keratinocytes, widening of the intercellular spaces, and accumulation of intracellular edema, causing the formation of clear spaces in the epidermis (Figure 2). Focal parakeratosis, usually in the follicular ostia, and mounds of scaly crust often are present. 14 A periodic acid–Schiff stain should be performed to rule out infectious dermatophytes, which can show similar clinical and histologic features. More chronic cases of seborrheic dermatitis often can take on histologic features of psoriasis, namely epidermal hyperplasia with thinning over dermal papillae, though the hyperplasia in psoriasis is more regular.

Alopecia areata is an immune-mediated disorder characterized by nonscarring hair loss; it affects approximately 0.1% to 0.2% of the general population.15 The pathogenesis involves the premature transition of hair follicles in the anagen (growth) phase to the catagen ( nonproliferative/involution) and telogen (resting) phases, resulting in sudden hair shedding and decreased regrowth. Clinically, it is characterized by asymptomatic hair loss that occurs most frequently on the scalp and other areas of the head, including eyelashes, eyebrows, and facial hair, but also can occur on the extremities. There are several variants; the most common is patchy alopecia, which features smooth circular areas of hair loss that progress over several weeks. Some patients can progress to loss of all scalp hairs (alopecia totalis) or all hairs throughout the body (alopecia universalis). 15 Patients typically will have spontaneous regrowth of hair, with up to 50% of those with limited hair loss recovering within a year.16 The disease has a chronic/ relapsing course, and patients often will have multiple episodes of hair loss. Histopathologic features can vary depending on the stage of disease. In acute cases, a peribulbar lymphocytic infiltrate preferentially involving anagen-stage hair follicles is seen, with associated necrosis, edema, and pigment incontinence (Figure 3).16 In chronic alopecia areata, the inflammation may be less brisk, and follicular miniaturization often is seen. Additionally, increased proportions of catagen- or telogen-stage follicles are present.16,17 On immunohistochemistry, lymphocytes express both CD4 and CD8, with a slightly increased CD4:CD8 ratio in active disease.18

Psoriatic alopecia describes hair loss that occurs in patients with psoriasis. Patients present with scaly, erythematous, psoriasiform plaques or patches, as well as decreased hair density, finer hairs, and increased dystrophic hair bulbs within the psoriatic plaques.19 It often is nonscarring and resolves with therapy, though scarring may occur with secondary infection. Psoriatic alopecia may occur in the setting of classic psoriasis and also may occur in psoriasiform drug eruptions, including those caused by tumor necrosis factor inhibitors.20,21 Histologic features include atrophy of sebaceous glands, epidermal changes with hypogranulosis and psoriasiform hyperplasia, decreased hair follicle density, and neutrophils in the stratum spinosum (Figure 4). There often is associated perifollicular lymphocytic inflammation with small lymphocytes that do not have notable morphologic abnormalities.


The Diagnosis: Folliculotropic Mycosis Fungoides
Folliculotropic mycosis fungoides (FMF) is a variant of mycosis fungoides (MF) characterized by folliculotropism and follicular-based lesions. The clinical manifestation of FMF can vary and includes patches, plaques, or tumors resembling nonfolliculotropic MF; acneform lesions including comedones and pustules; or areas of alopecia. Lesions commonly involve the head and neck but also can be seen on the trunk or extremities. Folliculotropic mycosis fungoides can be accompanied by pruritus or superimposed secondary infection.
Histologic features of FMF include follicular (perifollicular or intrafollicular) infiltration by atypical T cells showing cerebriform nuclei.1 In early lesions, there may be only mild superficial perivascular inflammation without notable lymphocyte atypia, making diagnosis challenging. 2,3 Mucinous degeneration of the follicles—termed follicular mucinosis—is a common histologic finding in FMF.1,2 Follicular mucinosis is not exclusive to FMF; it can be primary/idiopathic or secondary to underlying inflammatory or neoplastic disorders such as FMF. On immunohistochemistry, FMF most commonly demonstrates a helper T cell phenotype that is positive for CD3 and CD4 and negative for CD8, with aberrant loss of CD7 and variably CD5, which is similar to classic MF. Occasionally, larger CD30+ cells also can be present in the dermis. T-cell gene rearrangement studies will demonstrate T-cell receptor clonality in most cases.2
Many large retrospective cohort studies have suggested that patients with FMF have a worse prognosis than classic MF, with a 5-year survival rate of 62% to 87% for early-stage FMF vs more than 90% for classic patchand plaque-stage MF.4-7 However, a 2016 study suggested histologic evaluation may be able to further differentiate clinically identical cases into indolent and aggressive forms of FMF with considerably different outcomes based on the density of the perifollicular infiltrate.5 The presence of follicular mucinosis has no impact on prognosis compared to cases without follicular mucinosis.1,2
Alopecia mucinosa is characterized by infiltrating, erythematous, scaling plaques localized to the head and neck.8 It is diagnosed clinically, and histopathology shows follicular mucinosis. The terms alopecia mucinosa and follicular mucinosis often are used interchangeably. Over the past few decades, 3 variants have been categorized: primary acute, primary chronic, and secondary. The primary acute form manifests in children and young adults as solitary lesions, which often resolve spontaneously. In contrast, the primary chronic form manifests in older adults as multiple disseminated lesions with a chronic relapsing course.8,9 The secondary form can occur in the setting of other disorders, including lupus erythematosus, hypertrophic lichen planus, alopecia areata, and neoplasms such as MF or Hodgkin lymphoma.9 The histopathologic findings are similar for all types of alopecia mucinosa, with cystic pools of mucin deposition in the sebaceous glands and external root sheath of the follicles as well as associated inflammation composed of lymphocytes and eosinophils (Figure 1).9,10 The inflammatory infiltrate rarely extends into the epidermis or upper portion of the hair follicle. Although histopathology alone cannot reliably distinguish between primary and secondary forms of alopecia mucinosa, MF (including follicular MF) or another underlying cutaneous T-cell lymphoma should be considered if inflammation extends into the upper dermis, epidermis, or follicles or is in a dense bandlike distribution.11 On immunohistochemistry, lymphocytes should show positivity for CD3, CD4, and CD8. The CD4:CD8 ratio often is 1:1 in alopecia mucinosa, while in FMF it is approximately 3:1.10 CD7 commonly is negative but can be present in a small percentage of cases.12 T-cell receptor gene rearrangement studies have detected clonality in both primary and secondary alopecia mucinosa and thus cannot be used alone to distinguish between the two.10 Given the overlap in histopathologic and immunohistochemical features of primary and secondary alopecia mucinosa, definitive diagnosis cannot be made with any single modality and should be based on correlating clinical presentation, histopathology, immunohistochemistry, and molecular analyses.
Inflammatory dermatoses including seborrheic dermatitis also are in the differential diagnosis for FMF. Seborrheic dermatitis is a common chronic inflammatory skin disorder affecting 1% to 3% of the general population. 13 Patients usually present with scaly and greasy plaques and papules localized to areas with increased sebaceous glands and high sebum production such as the face, scalp, and intertriginous regions. The distribution often is symmetrical, and the severity of disease can vary substantially.13 Sebopsoriasis is an entity with overlapping features of seborrheic dermatitis and psoriasis, including thicker, more erythematous plaques that are more elevated. Histopathology of seborrheic dermatitis reveals spongiotic inflammation in the epidermis characterized by rounding of the keratinocytes, widening of the intercellular spaces, and accumulation of intracellular edema, causing the formation of clear spaces in the epidermis (Figure 2). Focal parakeratosis, usually in the follicular ostia, and mounds of scaly crust often are present. 14 A periodic acid–Schiff stain should be performed to rule out infectious dermatophytes, which can show similar clinical and histologic features. More chronic cases of seborrheic dermatitis often can take on histologic features of psoriasis, namely epidermal hyperplasia with thinning over dermal papillae, though the hyperplasia in psoriasis is more regular.

Alopecia areata is an immune-mediated disorder characterized by nonscarring hair loss; it affects approximately 0.1% to 0.2% of the general population.15 The pathogenesis involves the premature transition of hair follicles in the anagen (growth) phase to the catagen ( nonproliferative/involution) and telogen (resting) phases, resulting in sudden hair shedding and decreased regrowth. Clinically, it is characterized by asymptomatic hair loss that occurs most frequently on the scalp and other areas of the head, including eyelashes, eyebrows, and facial hair, but also can occur on the extremities. There are several variants; the most common is patchy alopecia, which features smooth circular areas of hair loss that progress over several weeks. Some patients can progress to loss of all scalp hairs (alopecia totalis) or all hairs throughout the body (alopecia universalis). 15 Patients typically will have spontaneous regrowth of hair, with up to 50% of those with limited hair loss recovering within a year.16 The disease has a chronic/ relapsing course, and patients often will have multiple episodes of hair loss. Histopathologic features can vary depending on the stage of disease. In acute cases, a peribulbar lymphocytic infiltrate preferentially involving anagen-stage hair follicles is seen, with associated necrosis, edema, and pigment incontinence (Figure 3).16 In chronic alopecia areata, the inflammation may be less brisk, and follicular miniaturization often is seen. Additionally, increased proportions of catagen- or telogen-stage follicles are present.16,17 On immunohistochemistry, lymphocytes express both CD4 and CD8, with a slightly increased CD4:CD8 ratio in active disease.18

Psoriatic alopecia describes hair loss that occurs in patients with psoriasis. Patients present with scaly, erythematous, psoriasiform plaques or patches, as well as decreased hair density, finer hairs, and increased dystrophic hair bulbs within the psoriatic plaques.19 It often is nonscarring and resolves with therapy, though scarring may occur with secondary infection. Psoriatic alopecia may occur in the setting of classic psoriasis and also may occur in psoriasiform drug eruptions, including those caused by tumor necrosis factor inhibitors.20,21 Histologic features include atrophy of sebaceous glands, epidermal changes with hypogranulosis and psoriasiform hyperplasia, decreased hair follicle density, and neutrophils in the stratum spinosum (Figure 4). There often is associated perifollicular lymphocytic inflammation with small lymphocytes that do not have notable morphologic abnormalities.


- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714. doi:10.1182/blood-2018-11-881268
- Malveira MIB, Pascoal G, Gamonal SBL, et al. Folliculotropic mycosis fungoides: challenging clinical, histopathological and immunohistochemical diagnosis. An Bras Dermatol. 2017;92(5 suppl 1):73-75. doi:10.1590/abd1806-4841.20175634
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525- 530. doi:10.1034/j.1600-0560.2001.281006.x
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides: a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198. doi:10.1001/archderm.138.2.191
- van Santen S, Roach REJ, van Doorn R, et al. Clinical staging and prognostic factors in folliculotropic mycosis fungoides. JAMA Dermatol. 2016;152:992-1000. doi:10.1001/jamadermatol.2016.1597
- Lehman JS, Cook-Norris RH, Weed BR, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010;146:607-613. doi:10.1001/archdermatol.2010.101
- Gerami P, Rosen S, Kuzel T, et al. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008;144:738-746. doi:10.1001/archderm.144.6.738
- Büchner SA, Meier M, Rufli TH. Follicular mucinosis associated with mycosis fungoides. Dermatology. 1991;183:66-67. doi:10.1159/000247639
- Akinsanya AO, Tschen JA. Follicular mucinosis: a case report. Cureus. 2019;11:E4746. doi:10.7759/cureus.4746
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19. doi:10.1111/j.1600-0560.2009.01338.x
- Khalil J, Kurban M, Abbas O. Follicular mucinosis: a review. Int J Dermatol. 2021;60:159-165. doi:10.1111/ijd.15165
- Zvulunov A, Shkalim V, Ben-Amitai D, et al. Clinical and histopathologic spectrum of alopecia mucinosa/follicular mucinosis and its natural history in children. J Am Acad Dermatol. 2012;67:1174-1181. doi:10.1016/j.jaad.2012.04.015
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31:343-351. doi:10.1016/j.clindermatol.2013.01.001
- Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26; quiz 19-20. doi:10.1111/j .1468-3083.2004.00693.x
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12. doi:10.1016/j .jaad.2017.04.1141
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-88, quiz 189-90. doi:10.1016/j.jaad.2009.10.032
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139:1555-1559. doi:10.1001/archderm .139.12.1555
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11(2 pt 1):216-223. doi:10.1016 /s0190-9622(84)70152-6
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721. doi:10.1111/ced.12715
- Afaasiev OK, Zhang CZ, Ruhoy SM. TNF-inhibitor associated psoriatic alopecia: diagnostic utility of sebaceous lobule atrophy. J Cutan Pathol. 2017;44:563-539. doi:10.1111/cup.12932
- Silva CY, Brown KL, Kurban AK, et al. Psoriatic alopecia—fact or fiction? A clinicohistologic reappraisal. Indian J Dermatol Venereol Leprol. 2012;78:611-619. doi:10.4103/0378-6323.100574
- Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703-1714. doi:10.1182/blood-2018-11-881268
- Malveira MIB, Pascoal G, Gamonal SBL, et al. Folliculotropic mycosis fungoides: challenging clinical, histopathological and immunohistochemical diagnosis. An Bras Dermatol. 2017;92(5 suppl 1):73-75. doi:10.1590/abd1806-4841.20175634
- Flaig MJ, Cerroni L, Schuhmann K, et al. Follicular mycosis fungoides: a histopathologic analysis of nine cases. J Cutan Pathol. 2001;28:525- 530. doi:10.1034/j.1600-0560.2001.281006.x
- van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides: a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol. 2002;138:191-198. doi:10.1001/archderm.138.2.191
- van Santen S, Roach REJ, van Doorn R, et al. Clinical staging and prognostic factors in folliculotropic mycosis fungoides. JAMA Dermatol. 2016;152:992-1000. doi:10.1001/jamadermatol.2016.1597
- Lehman JS, Cook-Norris RH, Weed BR, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010;146:607-613. doi:10.1001/archdermatol.2010.101
- Gerami P, Rosen S, Kuzel T, et al. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol. 2008;144:738-746. doi:10.1001/archderm.144.6.738
- Büchner SA, Meier M, Rufli TH. Follicular mucinosis associated with mycosis fungoides. Dermatology. 1991;183:66-67. doi:10.1159/000247639
- Akinsanya AO, Tschen JA. Follicular mucinosis: a case report. Cureus. 2019;11:E4746. doi:10.7759/cureus.4746
- Rongioletti F, De Lucchi S, Meyes D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37:15-19. doi:10.1111/j.1600-0560.2009.01338.x
- Khalil J, Kurban M, Abbas O. Follicular mucinosis: a review. Int J Dermatol. 2021;60:159-165. doi:10.1111/ijd.15165
- Zvulunov A, Shkalim V, Ben-Amitai D, et al. Clinical and histopathologic spectrum of alopecia mucinosa/follicular mucinosis and its natural history in children. J Am Acad Dermatol. 2012;67:1174-1181. doi:10.1016/j.jaad.2012.04.015
- Dessinioti C, Katsambas A. Seborrheic dermatitis: etiology, risk factors, and treatments: facts and controversies. Clin Dermatol. 2013;31:343-351. doi:10.1016/j.clindermatol.2013.01.001
- Gupta AK, Bluhm R. Seborrheic dermatitis. J Eur Acad Dermatol Venereol. 2004;18:13-26; quiz 19-20. doi:10.1111/j .1468-3083.2004.00693.x
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78:1-12. doi:10.1016/j .jaad.2017.04.1141
- Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: part I. clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177-88, quiz 189-90. doi:10.1016/j.jaad.2009.10.032
- Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139:1555-1559. doi:10.1001/archderm .139.12.1555
- Todes-Taylor N, Turner R, Wood GS, et al. T cell subpopulations in alopecia areata. J Am Acad Dermatol. 1984;11(2 pt 1):216-223. doi:10.1016 /s0190-9622(84)70152-6
- George SM, Taylor MR, Farrant PB. Psoriatic alopecia. Clin Exp Dermatol. 2015;40:717-721. doi:10.1111/ced.12715
- Afaasiev OK, Zhang CZ, Ruhoy SM. TNF-inhibitor associated psoriatic alopecia: diagnostic utility of sebaceous lobule atrophy. J Cutan Pathol. 2017;44:563-539. doi:10.1111/cup.12932
- Silva CY, Brown KL, Kurban AK, et al. Psoriatic alopecia—fact or fiction? A clinicohistologic reappraisal. Indian J Dermatol Venereol Leprol. 2012;78:611-619. doi:10.4103/0378-6323.100574
An 88-year-old man presented with progressive eyelash loss and scale involving the right eyelids (top). Dermatopathologic examination was performed (bottom).
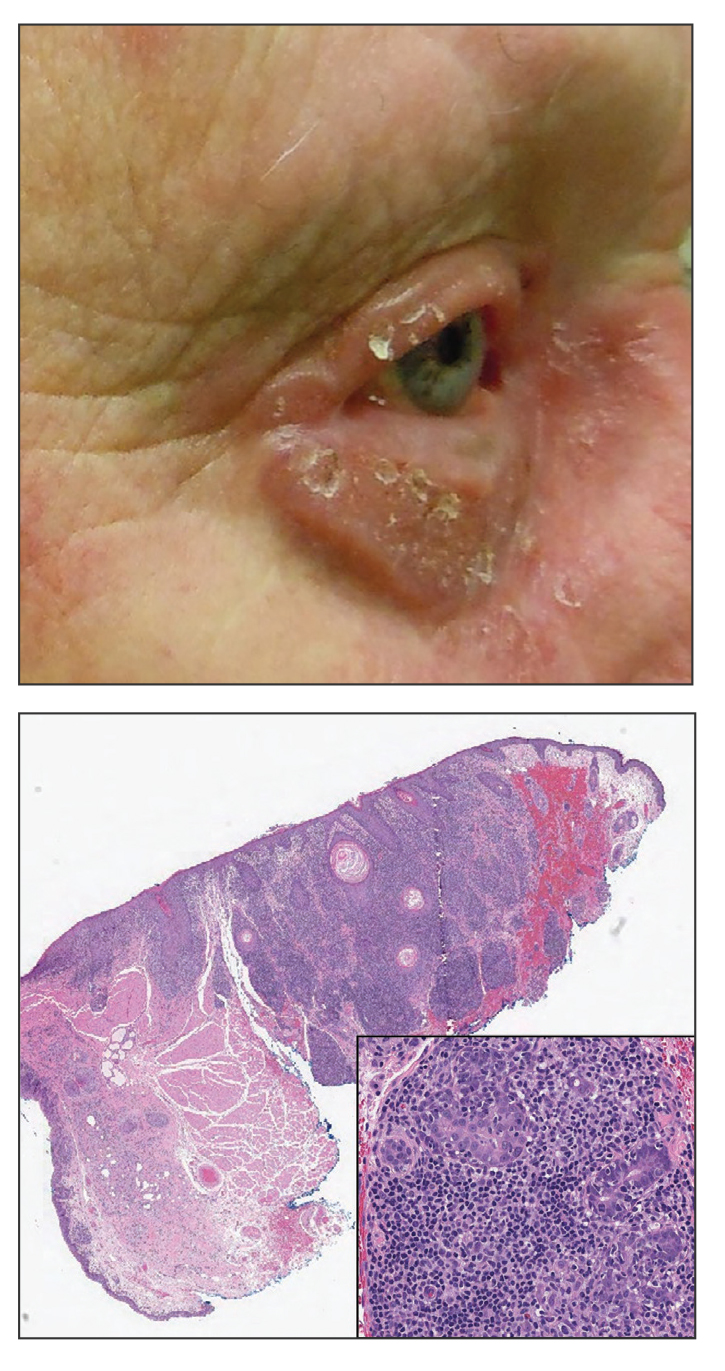
Erythematous Flaky Rash on the Toe
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13
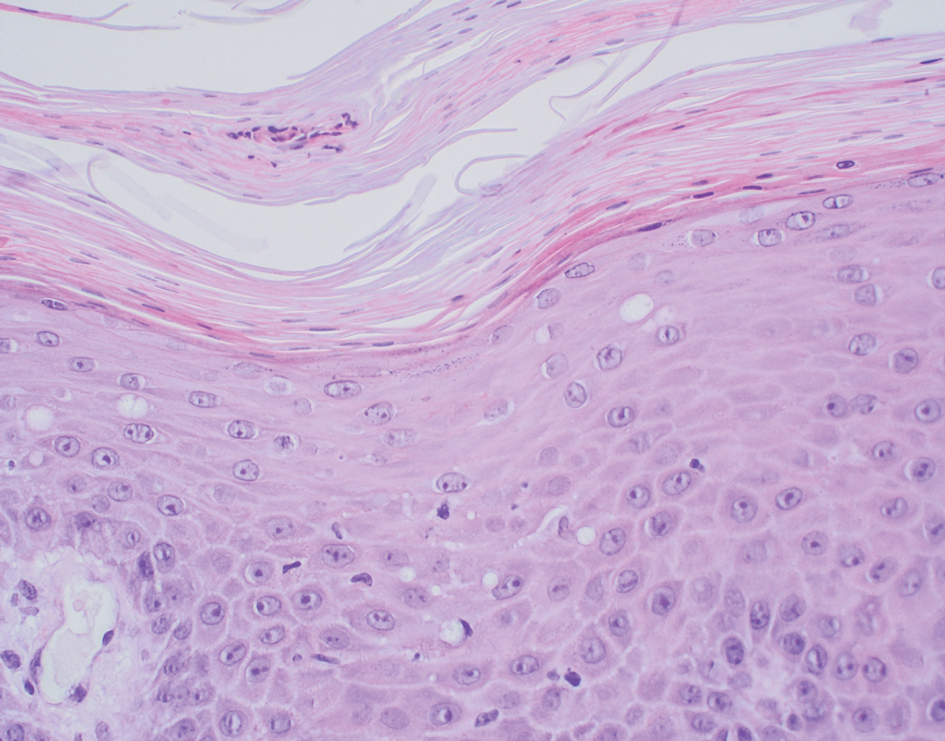
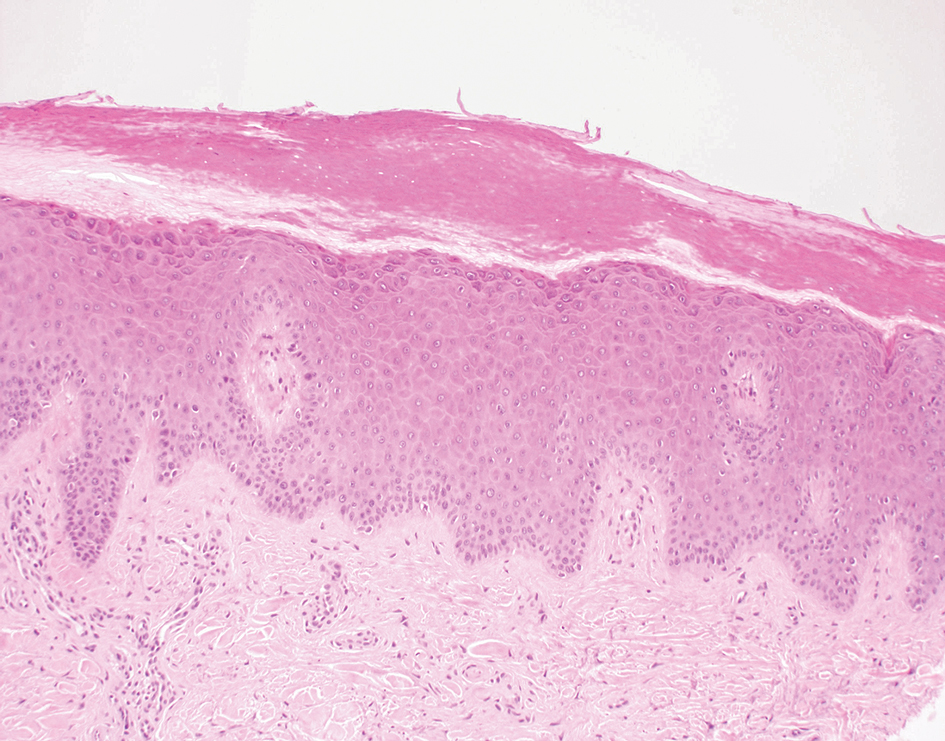
The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13
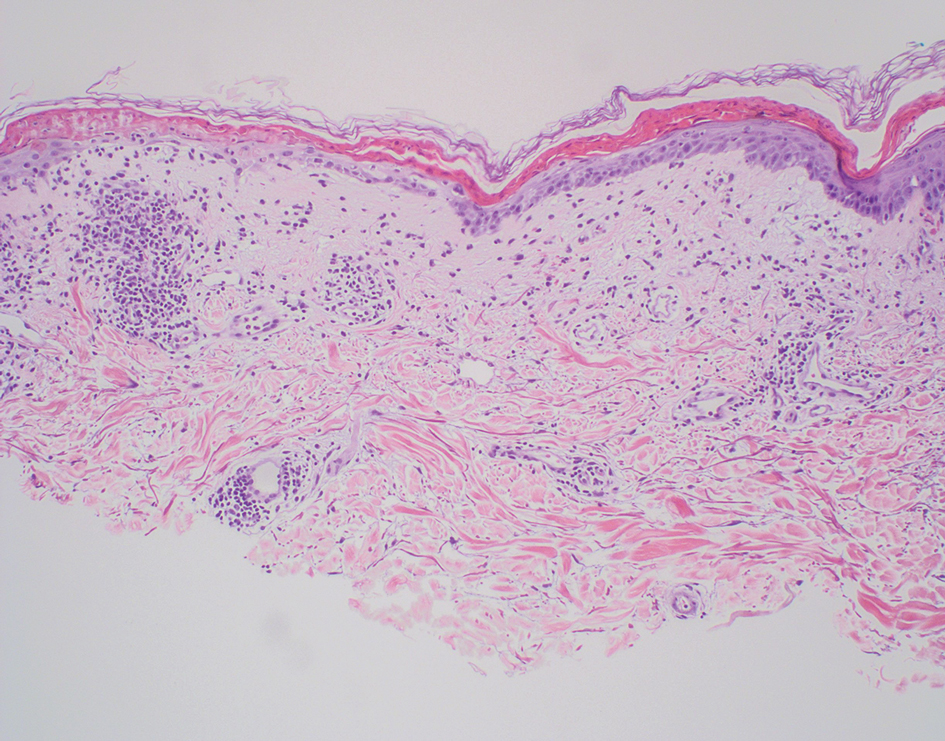
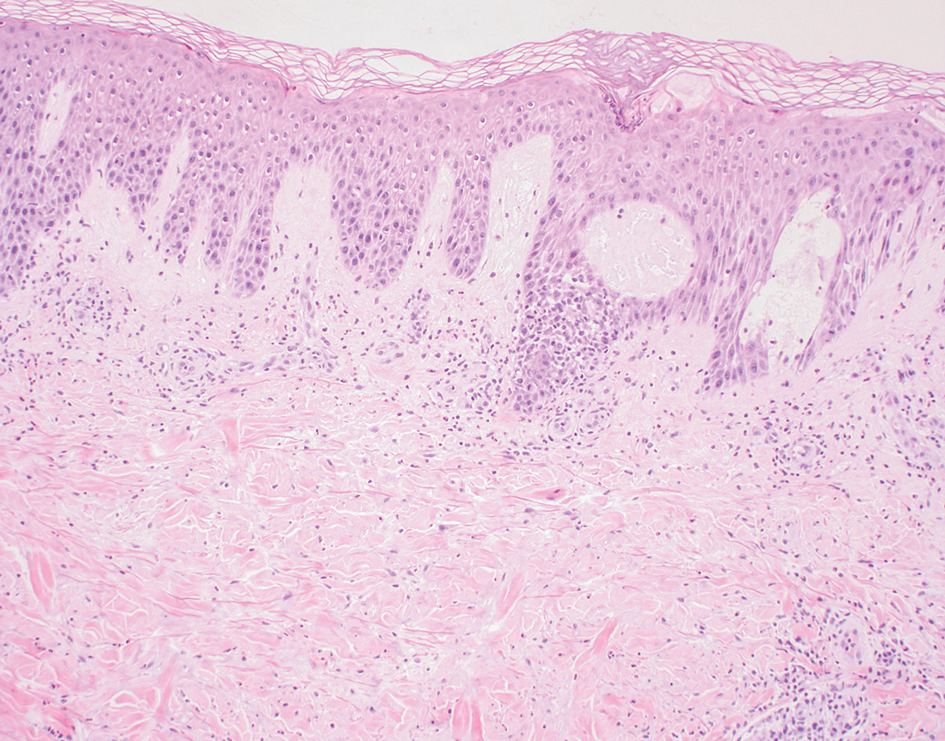
The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13


The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13


The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13


The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13


The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
A 62-year-old man presented with an erythematous flaky rash associated with burning pain on the right medial second toe that persisted for several months. Prior treatment with econazole, ciclopirox, and oral amoxicillin had failed. A shave biopsy was performed.