User login
3 cases of hormone therapy optimized to match the patient problem
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
There are dozens of medications containing combinations of estrogen and progestin. I am often confused by the bewildering proliferation of generic brand names used to describe the same estrogen-progestin (E-P) regimen. For example, the combination medication containing ethinyl estradiol 20 µg plus norethindrone acetate (NEA) 1 mg is available under at least 5 different names: Lo Estrin 1/20 (Warner Chilcot), Junel 1/20 (Teva Pharmaceuticals), Microgestin Fe 1/20 (Mayne Pharma), Gildess 1/20 (Qualitest Pharmaceuticals), and Larin 1/20 (Novast Laboratories). To reduce the confusion, it is often useful to select a single preferred estrogen and progestin and use the dose combinations that are available to treat a wide range of gynecology problems (TABLE). In this editorial I focus on using various dose combinations of ethinyl estradiol and NEA to treat 3 common gynecologic problems.

CASE 1 Polycystic ovary syndrome
A 19-year-old woman reports 4 spontaneous menses in the past year and bothersome facial hair and acne. Her total testosterone concentration is at the upper limit of normal (0.46 ng/mL) and her sex hormone binding globulin (SHBG) concentration is at the lower limit of normal (35 nM). For treatment of the patient’s menstrual disorder, what is an optimal E-P combination?
Prioritize the use of an estrogen-dominant medication
Based on the Rotterdam criteria this woman has polycystic ovary syndrome (PCOS).1 In women with PCOS, luteinizing hormone (LH) secretion is increased, stimulating excessive ovarian production of testosterone.2 In addition, many women with PCOS have decreased hepatic secretion of SHBG, a binding protein that prevents testosterone from entering cells, resulting in excessive bioavailable testosterone.3 The Endocrine Society recommends that women with PCOS who have menstrual dysfunction or hirsutism be treated initially with a combination E-P hormone medication.1 Combination E-P medications suppress pituitary secretion of LH, thereby reducing ovarian production of testosterone, and ethinyl estradiol increases hepatic secretion of SHBG, reducing bioavailable testosterone. These two goals are best accomplished with an oral E-P hormone medication containing ethinyl estradiol doses of 20 µg to 30 µg per pill. An E-P hormone medication containing pills with an ethinyl estradiol dose ≤ 10 µg-daily may stimulate less hepatic production of SHBG than a pill with an ethinyl estradiol dose of 20 µg or 30 µg daily.4,5 In addition, E-P pills containing levonorgestrel suppress SHBG hormone secretion compared with E-P pills with other progestins.6 Therefore, levonorgestrel-containing E-P pills should not be prioritized for use in women with PCOS because the estrogen-induced increase in SHBG will be blunted by levonorgestrel.
CASE 2 Moderate to severe pelvic pain caused by endometriosis
A 25-year-old woman (G0) with severe dysmenorrhea had a laparoscopy showing endometriosis lesions in the cul-de-sac and a peritoneal window near the left uterosacral ligament. Biopsy showed endometriosis. Postoperatively, the patient was treated with an E-P pill containing 30 µg ethinyl estradiol and 0.15 mg desogestrel per pill using a continuous-dosing protocol. During the year following the laparoscopy, her pelvic pain symptoms gradually increased until they became severe, preventing her from performing daily activities on multiple days per month. She was prescribed elagolix but her insurance did not approve the treatment. What alternative treatment would you prescribe?
Continue to: Use progestin-dominant pills to treat pelvic pain...
Use progestin-dominant pills to treat pelvic pain
Cellular activity in endometriosis lesions is stimulated by estradiol and inhibited by a high concentration of androgenic progestins or androgens. This simplified endocrine paradigm explains the effectiveness of hormonal treatments that suppress ovarian estradiol production, including leuprolide, elagolix, medroxyprogesterone acetate, and NEA. For the woman in the above case, I would advocate for elagolix treatment but, following the insurance denial of the prescription, an alternative treatment for moderate or severe pelvic pain caused by endometriosis would be a progestin-dominant hormone medication (for example, NEA 5 mg daily). Norethindrone acetate 5 mg daily may be associated with bothersome adverse effects including weight gain (16% of patients; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%).7
I sometimes see women with moderate to severe pelvic pain caused by endometriosis being treated with norethindrone 0.35 mg daily. This dose of norethindrone is suboptimal for pain treatment because it does not reliably suppress ovarian production of estradiol. In addition, the cells in endometriosis lesions are often resistant to the effects of progesterone, requiring higher dosages to produce secretory or decidual changes. In most situations, I recommend against the use of norethindrone 0.35 mg daily for the treatment of pelvic pain caused by endometriosis.
Patients commonly ask if NEA 5 mg daily has contraceptive efficacy. Although it is not approved at this dosage by the US Food and Drug Administration as a contraceptive,8 norethindrone 0.35 mg daily is approved as a progestin-only contraceptive.9 Norethindrone acetate is rapidly and completely deacetylated to norethindrone and the disposition of oral NEA is indistinguishable from that of norethindrone (which is the FDA-approved dosage mentioned above). Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg daily has contraceptive efficacy, especially if there is good adherence with the daily medication.
CASE 3 Perimenopausal AUB
A 45-year-old woman reports varying menstrual cycle lengths from 24 to 60 days with very heavy menses in some cycles. Pelvic ultrasonography shows no abnormality. Endometrial biopsy shows a proliferative endometrium. Her serum progesterone level, obtained 1 week before the onset of menses, is < 3 ng/mL. She has no past history of heavy menses, easy bruising, excessive bleeding with procedures, or a family history of bleeding problems. She also reports occasional hot flashes that wake her from sleep.
Use an estrogen step-down regimen to manage postmenopause transition
This patient is likely in the perimenopause transition, and the abnormal uterine bleeding (AUB) is caused, in part, by oligo- or anovulation. Perimenopausal women with AUB may have cycles characterized by above normal ovarian estradiol production and below normal progesterone production, or frank anovulation.10 Elevated ovarian estrogen and low progesterone production sets the stage for heavy bleeding in the perimenopause, regardless of the presence of uterine pathology such as fibroids.
For perimenopausal women, one option for treatment of AUB due to anovulation is to prescribe an estrogen step-down regimen. For the 45-year-old woman in this case, initiating treatment with an E-P pill containing ethinyl estradiol 10 µg and NEA 1 mg will likely control the AUB and her occasional hot flash.11 As the woman ages, the ethinyl estradiol dose can be decreased to pills containing 5 µg and then 2.5 µg, covering the transition into postmenopause. Once the woman is in the postmenopause, treatment with transdermal estradiol and oral micronized progesterone is an option to treat menopausal vasomotor symptoms.
Optimize estrogen and progestin treatment for your patients
Many gynecologic problems are effectively treated by estrogen and/or progestin steroids. The dose of estrogen and progestin should be tailored to the specific problem. For PCOS, the estrogen dose selected should be sufficient to safely stimulate hepatic SHBG production. For endometriosis, if a GnRH antagonist is not available to the patient, a high-dose progestin, such as NEA 5 mg, may be an effective treatment. During the perimenopause transition in a woman with AUB, a treatment plan using a sequential E-P step-down program might control symptoms and help smoothly glide the patient into the postmenopause. ●
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592. doi: 10.1210/jc.2013-2350.
- Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467-520. doi: 10.1210/er.2015-1104.
- Zhu JL, Chen Z, Feng WJ, et al. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. doi: 10.1016/j.cca.2019.09.010.
- Oner G, Muderris II. A prospective randomized trial comparing low-dose ethinyl estradiol and drospirenone 24/4 combined oral contraceptive vs. ethinyl estradiol and drospirenone 21/7 combined oral contraceptive in the treatment of hirsutism. Contraception. 2011;84:508-511. doi: 10.1016/j.contraception.2011.03.002.
- Boyd RA, Zegarac EA, Posvar EL, et al. Minimal androgenic activity of a new oral contraceptive containing norethindrone acetate and graduated doses of ethinyl estradiol. Contraception. 2001;63:71-76. doi: 10.1016/s0010-7824(01)00179-2.
- Thorneycroft IH, Stanczyk FZ, Bradshaw KD, et al. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60:255-262. doi: 10.1016/s0010-7824(99)00093-1.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108. doi: 10.1016/j.jpag.2011.09.013.
- Aygestin [package insert]. Pomona, NY: Duramed Pharmaceuticals; 2007.
- Camila [package insert]. Greenville, NC; Mayne Pharma; 2018.
- Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495-1501. doi: 10.1210/jcem.81.4.8636357.
- Speroff L, Symons J, Kempfert N, et al; FemHrt Study Investigators. The effect of varying low-dose combinations of norethindrone acetate and ethinyl estradiol (Femhrt) on the frequency and intensity of vasomotor symptoms. Menopause. 2000;7:383-390. doi: 10.1097/00042192-200011000-00003.
From ideology to articles of faith: The ‘religification’ of political beliefs
Man is a political animal.
— Aristotle, Politics , Book 1, Section 1253a
Religion is the opium of the people.
— Karl Marx, A contribution to the critique of Hegel’s philosophy of right , introduction
Beliefs are at the core of psychiatric practice. Our patients are often shackled by their anomalous beliefs, which are not reality-based. These beliefs are often the primary targets of psychiatric treatment. Consider a day at the office of a psychiatrist who may see several patients impaired by false beliefs, such as:
- My neighbor is reading my mind remotely and is plotting to kill me
- If I ride on a plane, it will crash and I will die
- I am a failure, a worthless person, and a burden on my family
- I am hopeless and helpless; life is too painful and not worth living anymore
- I am a prophet with supernatural gifts, and I can predict the future
- Whenever I take this substance, I feel I can jump out of a window and fly
- If I do not shower 5 times in a row every night before going to bed, something terrible will happen to my family.
Patients with false beliefs obviously need psychiatric care. However, a large number of religious individuals harbor “unusual” beliefs involving angels and devils and hell and paradise after death. Those people of faith are not considered to have a DSM-5 psychiatric disorder. Billions of people around the world belong to one of the approximately 4,300 religions, which they celebrate using one of the more than 6,800 living languages. Psychiatrists encourage patients to have a faith because it can be quite comforting to its adherents, enhancing their social relations and providing them with hope and resilience during the darkest days of life. Regular attendance at a house of worship is a measure of the strong roots of one’s faith.
So why have there been so many religious wars over centuries of recorded history? Why have millions of people died during conflicts among religions? Why does one religious group adamantly believe that theirs is the real God, while the god of other religions is fake? And why have people who withdrew from or refused to adopt a certain religious belief been persecuted; labeled as “heretic,” “infidel,” “heathen,” or “apostate”; and burned at the stake or beheaded? Perhaps religion is not always a kinder, gentler belief system.
Continue to: Recent statistics...
Recent statistics show a precipitous decline in religious observance in the United States.1 So what happens to a society that gradually abandons its previously entrenched religious beliefs and becomes secular? This trend is spreading widely in Europe and North America. But widely held beliefs with powerful personal meaning don’t just fizzle away: they re-emerge in another form. The substantial energy of religious faith must be invested elsewhere and manifested in an alternative form with similar dynamics.
Enter politics!
It seems that humans’ need to uphold a strong belief is so powerful that they either incorporate political doctrines side-by-side with their religious beliefs (if the 2 are compatible) or adopt a strong political belief if they abandon their religion and become secular. This does not have to be an intellectually wrenching change because there are many similarities between hyper-religiosity and fanatic political beliefs (Table).

The toxic hyperpartisanship that has dominated the United States over the past several years may be the culmination of an intensified “religification” of politics. The incendiary mix of religious zealotry and political fanaticism is conducive to intensified loathing, hostility, and animus to those with an opposing political ideology.
So it all boils down to the human imperative of harboring a strong personal belief. What is the origin of beliefs, religious, political, or otherwise? Why does the human species have the overwhelming need to uphold a belief? Research suggests that it is the result of evolution and the phylogenetic enlargement of the brain, including the parietal and medial frontal cortex in humans.2 And according to many studies, abnormal and delusional beliefs encountered in psychiatric practice appear to be caused by altered perception and/or misattribution of aversive meaning.3 Lesions in the right hemisphere have been reported to play an important role in generating delusional beliefs.4 A healthy right hemisphere plays an important role in:
- pragmatic communications
- perceptual integration
- attentional surveillance and anomaly novelty detection
- belief updating.4
Right hemispheric pathology disrupts those functions and can lead to false beliefs such as delusions, or, on a milder scale, strongly held superstitions.
One wonders how the structure and function of the right hemisphere generates and perpetuates a belief in a religion or political ideology that ultimately shapes one’s life. Religiosity and politics are an inherent part of human nature, and they can replace each other or merge together. If one is to believe what Durkheim5 proposed more than a century ago, the existence of belief systems is essential for societal stability. He posited that the absence of stable belief systems can lead to what he labeled “anomie,” leading to a surge of suicide and crime. If that is true, then the coexistence of religious and political beliefs may have a significant upside, but also with a palpable downside when either or both of those belief systems become excessively antagonistic or extreme. Three cheers for religious and political moderation that allows them to peacefully coexist.
1. Jones JM. U.S. church membership falls below majority for first time. Gallup. March 29, 2021. Accessed June 7, 2021. https://news.gallup.com/poll/341963/church-membership-falls-below-majority-first-time.aspx
2. Seitz RJ, Angel HF. Belief formation—a driving force for brain evolution. Brain Cogn. 2020;140:105548. doi: 10.1016/j.bandc.2020.105548
3. Seitz RJ. Beliefs: a challenge in neuropsychological disorders. J Neuropsychol. 2021. doi: 10.1111/jnp.12249
4. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235. doi: 10.1176/appi.neuropsych.16060118
5. Durkheim E. Suicide: a study in sociology. The Free Press; 1951.
Man is a political animal.
— Aristotle, Politics , Book 1, Section 1253a
Religion is the opium of the people.
— Karl Marx, A contribution to the critique of Hegel’s philosophy of right , introduction
Beliefs are at the core of psychiatric practice. Our patients are often shackled by their anomalous beliefs, which are not reality-based. These beliefs are often the primary targets of psychiatric treatment. Consider a day at the office of a psychiatrist who may see several patients impaired by false beliefs, such as:
- My neighbor is reading my mind remotely and is plotting to kill me
- If I ride on a plane, it will crash and I will die
- I am a failure, a worthless person, and a burden on my family
- I am hopeless and helpless; life is too painful and not worth living anymore
- I am a prophet with supernatural gifts, and I can predict the future
- Whenever I take this substance, I feel I can jump out of a window and fly
- If I do not shower 5 times in a row every night before going to bed, something terrible will happen to my family.
Patients with false beliefs obviously need psychiatric care. However, a large number of religious individuals harbor “unusual” beliefs involving angels and devils and hell and paradise after death. Those people of faith are not considered to have a DSM-5 psychiatric disorder. Billions of people around the world belong to one of the approximately 4,300 religions, which they celebrate using one of the more than 6,800 living languages. Psychiatrists encourage patients to have a faith because it can be quite comforting to its adherents, enhancing their social relations and providing them with hope and resilience during the darkest days of life. Regular attendance at a house of worship is a measure of the strong roots of one’s faith.
So why have there been so many religious wars over centuries of recorded history? Why have millions of people died during conflicts among religions? Why does one religious group adamantly believe that theirs is the real God, while the god of other religions is fake? And why have people who withdrew from or refused to adopt a certain religious belief been persecuted; labeled as “heretic,” “infidel,” “heathen,” or “apostate”; and burned at the stake or beheaded? Perhaps religion is not always a kinder, gentler belief system.
Continue to: Recent statistics...
Recent statistics show a precipitous decline in religious observance in the United States.1 So what happens to a society that gradually abandons its previously entrenched religious beliefs and becomes secular? This trend is spreading widely in Europe and North America. But widely held beliefs with powerful personal meaning don’t just fizzle away: they re-emerge in another form. The substantial energy of religious faith must be invested elsewhere and manifested in an alternative form with similar dynamics.
Enter politics!
It seems that humans’ need to uphold a strong belief is so powerful that they either incorporate political doctrines side-by-side with their religious beliefs (if the 2 are compatible) or adopt a strong political belief if they abandon their religion and become secular. This does not have to be an intellectually wrenching change because there are many similarities between hyper-religiosity and fanatic political beliefs (Table).

The toxic hyperpartisanship that has dominated the United States over the past several years may be the culmination of an intensified “religification” of politics. The incendiary mix of religious zealotry and political fanaticism is conducive to intensified loathing, hostility, and animus to those with an opposing political ideology.
So it all boils down to the human imperative of harboring a strong personal belief. What is the origin of beliefs, religious, political, or otherwise? Why does the human species have the overwhelming need to uphold a belief? Research suggests that it is the result of evolution and the phylogenetic enlargement of the brain, including the parietal and medial frontal cortex in humans.2 And according to many studies, abnormal and delusional beliefs encountered in psychiatric practice appear to be caused by altered perception and/or misattribution of aversive meaning.3 Lesions in the right hemisphere have been reported to play an important role in generating delusional beliefs.4 A healthy right hemisphere plays an important role in:
- pragmatic communications
- perceptual integration
- attentional surveillance and anomaly novelty detection
- belief updating.4
Right hemispheric pathology disrupts those functions and can lead to false beliefs such as delusions, or, on a milder scale, strongly held superstitions.
One wonders how the structure and function of the right hemisphere generates and perpetuates a belief in a religion or political ideology that ultimately shapes one’s life. Religiosity and politics are an inherent part of human nature, and they can replace each other or merge together. If one is to believe what Durkheim5 proposed more than a century ago, the existence of belief systems is essential for societal stability. He posited that the absence of stable belief systems can lead to what he labeled “anomie,” leading to a surge of suicide and crime. If that is true, then the coexistence of religious and political beliefs may have a significant upside, but also with a palpable downside when either or both of those belief systems become excessively antagonistic or extreme. Three cheers for religious and political moderation that allows them to peacefully coexist.
Man is a political animal.
— Aristotle, Politics , Book 1, Section 1253a
Religion is the opium of the people.
— Karl Marx, A contribution to the critique of Hegel’s philosophy of right , introduction
Beliefs are at the core of psychiatric practice. Our patients are often shackled by their anomalous beliefs, which are not reality-based. These beliefs are often the primary targets of psychiatric treatment. Consider a day at the office of a psychiatrist who may see several patients impaired by false beliefs, such as:
- My neighbor is reading my mind remotely and is plotting to kill me
- If I ride on a plane, it will crash and I will die
- I am a failure, a worthless person, and a burden on my family
- I am hopeless and helpless; life is too painful and not worth living anymore
- I am a prophet with supernatural gifts, and I can predict the future
- Whenever I take this substance, I feel I can jump out of a window and fly
- If I do not shower 5 times in a row every night before going to bed, something terrible will happen to my family.
Patients with false beliefs obviously need psychiatric care. However, a large number of religious individuals harbor “unusual” beliefs involving angels and devils and hell and paradise after death. Those people of faith are not considered to have a DSM-5 psychiatric disorder. Billions of people around the world belong to one of the approximately 4,300 religions, which they celebrate using one of the more than 6,800 living languages. Psychiatrists encourage patients to have a faith because it can be quite comforting to its adherents, enhancing their social relations and providing them with hope and resilience during the darkest days of life. Regular attendance at a house of worship is a measure of the strong roots of one’s faith.
So why have there been so many religious wars over centuries of recorded history? Why have millions of people died during conflicts among religions? Why does one religious group adamantly believe that theirs is the real God, while the god of other religions is fake? And why have people who withdrew from or refused to adopt a certain religious belief been persecuted; labeled as “heretic,” “infidel,” “heathen,” or “apostate”; and burned at the stake or beheaded? Perhaps religion is not always a kinder, gentler belief system.
Continue to: Recent statistics...
Recent statistics show a precipitous decline in religious observance in the United States.1 So what happens to a society that gradually abandons its previously entrenched religious beliefs and becomes secular? This trend is spreading widely in Europe and North America. But widely held beliefs with powerful personal meaning don’t just fizzle away: they re-emerge in another form. The substantial energy of religious faith must be invested elsewhere and manifested in an alternative form with similar dynamics.
Enter politics!
It seems that humans’ need to uphold a strong belief is so powerful that they either incorporate political doctrines side-by-side with their religious beliefs (if the 2 are compatible) or adopt a strong political belief if they abandon their religion and become secular. This does not have to be an intellectually wrenching change because there are many similarities between hyper-religiosity and fanatic political beliefs (Table).

The toxic hyperpartisanship that has dominated the United States over the past several years may be the culmination of an intensified “religification” of politics. The incendiary mix of religious zealotry and political fanaticism is conducive to intensified loathing, hostility, and animus to those with an opposing political ideology.
So it all boils down to the human imperative of harboring a strong personal belief. What is the origin of beliefs, religious, political, or otherwise? Why does the human species have the overwhelming need to uphold a belief? Research suggests that it is the result of evolution and the phylogenetic enlargement of the brain, including the parietal and medial frontal cortex in humans.2 And according to many studies, abnormal and delusional beliefs encountered in psychiatric practice appear to be caused by altered perception and/or misattribution of aversive meaning.3 Lesions in the right hemisphere have been reported to play an important role in generating delusional beliefs.4 A healthy right hemisphere plays an important role in:
- pragmatic communications
- perceptual integration
- attentional surveillance and anomaly novelty detection
- belief updating.4
Right hemispheric pathology disrupts those functions and can lead to false beliefs such as delusions, or, on a milder scale, strongly held superstitions.
One wonders how the structure and function of the right hemisphere generates and perpetuates a belief in a religion or political ideology that ultimately shapes one’s life. Religiosity and politics are an inherent part of human nature, and they can replace each other or merge together. If one is to believe what Durkheim5 proposed more than a century ago, the existence of belief systems is essential for societal stability. He posited that the absence of stable belief systems can lead to what he labeled “anomie,” leading to a surge of suicide and crime. If that is true, then the coexistence of religious and political beliefs may have a significant upside, but also with a palpable downside when either or both of those belief systems become excessively antagonistic or extreme. Three cheers for religious and political moderation that allows them to peacefully coexist.
1. Jones JM. U.S. church membership falls below majority for first time. Gallup. March 29, 2021. Accessed June 7, 2021. https://news.gallup.com/poll/341963/church-membership-falls-below-majority-first-time.aspx
2. Seitz RJ, Angel HF. Belief formation—a driving force for brain evolution. Brain Cogn. 2020;140:105548. doi: 10.1016/j.bandc.2020.105548
3. Seitz RJ. Beliefs: a challenge in neuropsychological disorders. J Neuropsychol. 2021. doi: 10.1111/jnp.12249
4. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235. doi: 10.1176/appi.neuropsych.16060118
5. Durkheim E. Suicide: a study in sociology. The Free Press; 1951.
1. Jones JM. U.S. church membership falls below majority for first time. Gallup. March 29, 2021. Accessed June 7, 2021. https://news.gallup.com/poll/341963/church-membership-falls-below-majority-first-time.aspx
2. Seitz RJ, Angel HF. Belief formation—a driving force for brain evolution. Brain Cogn. 2020;140:105548. doi: 10.1016/j.bandc.2020.105548
3. Seitz RJ. Beliefs: a challenge in neuropsychological disorders. J Neuropsychol. 2021. doi: 10.1111/jnp.12249
4. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235. doi: 10.1176/appi.neuropsych.16060118
5. Durkheim E. Suicide: a study in sociology. The Free Press; 1951.
Adverse pregnancy outcomes and later cardiovascular disease
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.
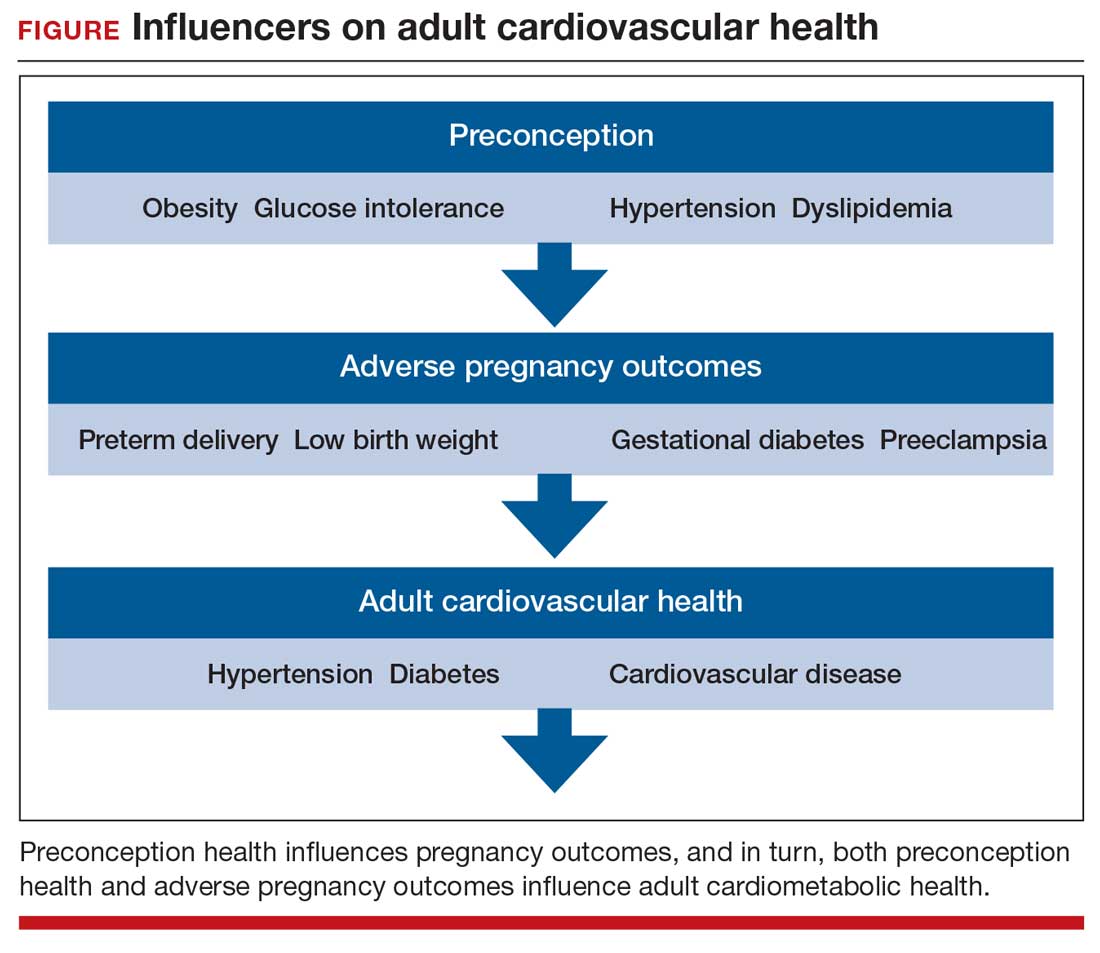
Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
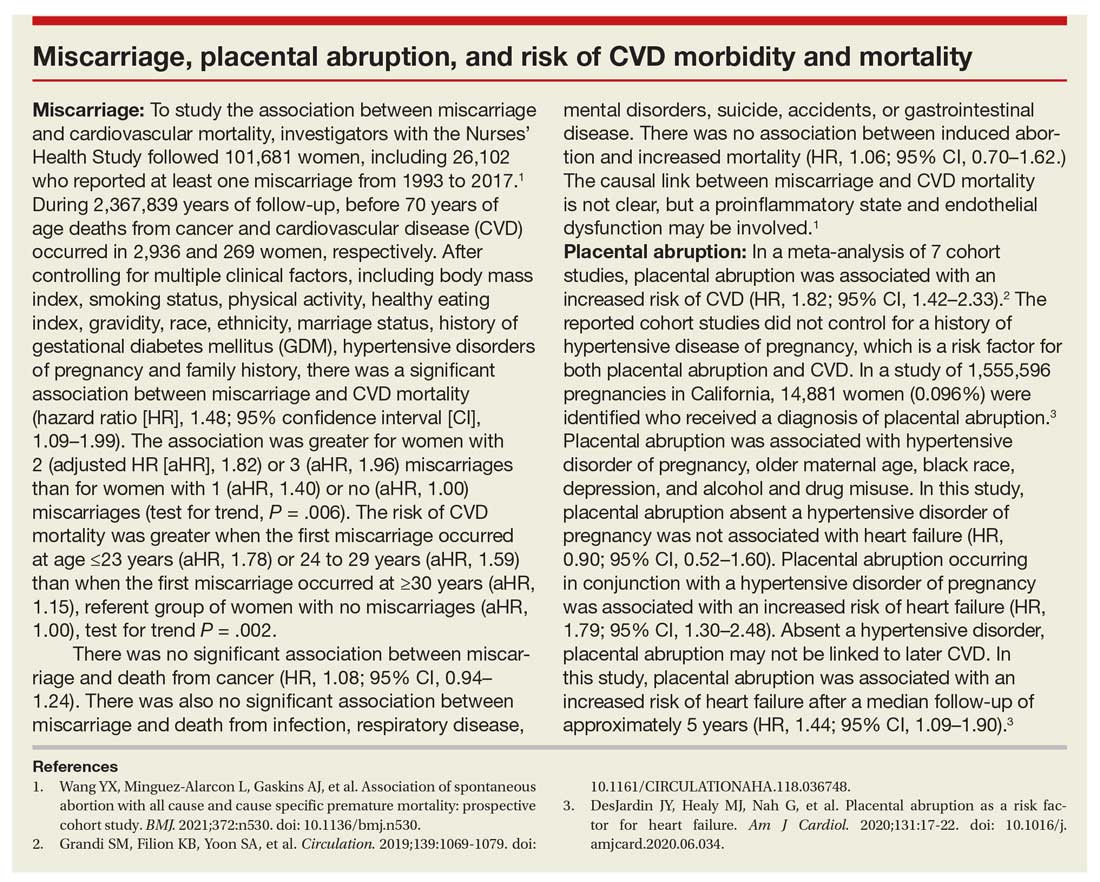
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
Psychiatry is Neurology: White matter pathology permeates psychiatric disorders
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.
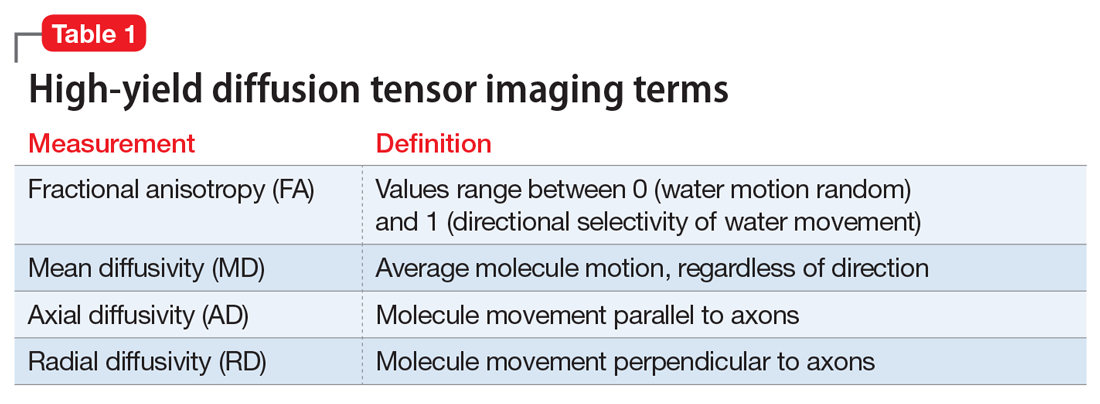
During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28
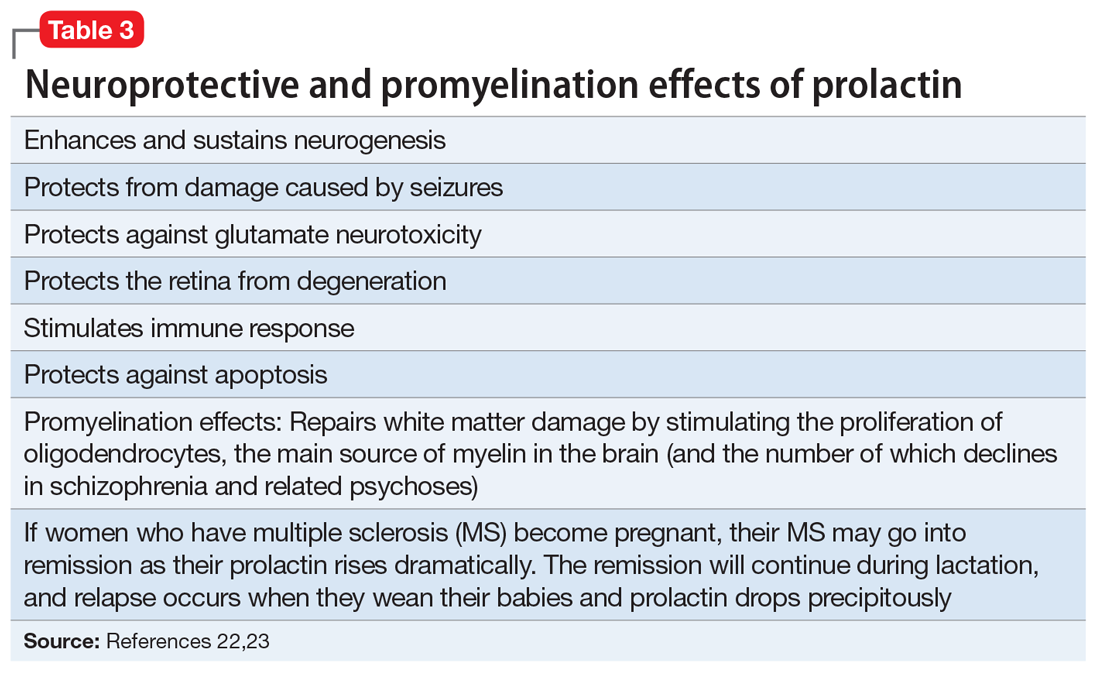
Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.

During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28

Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
Ask neurologists or psychiatrists to name a white matter (WM) brain disease and they are very likely to say multiple sclerosis (MS), a demyelinating brain disorder caused by immune-mediated destruction of oligodendrocytes, the glial cells that manufacture myelin without which brain communications would come to a standstill.
MS is often associated with mood or psychotic disorders, yet it is regarded as a neurologic illness, not a psychiatric disorder.
Many neurologists and psychiatrists may not be aware that during the past few years, multiple diffusion tensor imaging (DTI) studies have revealed that many psychiatric disorders are associated with WM pathology.1
Most people think that the brain is composed mostly of neurons, but in fact the bulk of brain volume (60%) is comprised of WM and only 40% is gray matter, which includes both neurons and glial cells (astroglia, microglia, and oligodendroglia). WM includes >137,000 km of myelinated fibers, an extensive network that connects all brain regions and integrates its complex, multifaceted functions, culminating in a unified sense of self and agency.
The role of the corpus callosum
Early in my research career, I became interested in the corpus callosum, the largest interhemispheric WM commissure connecting homologous areas across the 2 cerebral hemispheres. It is comprised of 200 million fibers of various diameters. Reasons for my fascination with the corpus callosum were:
The studies of Roger Sperry, the 1981 Nobel Laureate who led the team that was awarded the prize for split-brain research, which involved patients whose corpus callosum was cut to prevent the transfer of intractable epilepsy from 1 hemisphere to the other. Using a tachistoscope that he designed, Sperry discovered that the right and left hemispheres are 2 independent spheres of consciousness (ie, 2 individuals) with different skills.2 Cerebral dominance (laterality) fully integrates the 2 hemispheres via the corpus callosum, with a verbal hemisphere (the left, in 90% of people) dominating the other hemisphere and serving as the “spokesman self.” Thus, we all have 2 persons in our brain completely integrated into 1 “self.”2 This led me to wonder about the effects of an impaired corpus callosum on the “unified self.”
Postmortem and MRI studies conducted by our research group showed a significant difference in the thickness of the corpus callosum in a group of patients with schizophrenia vs healthy controls, which implied abnormal connectivity across the left and right hemispheres.3
Continue to: I then conducted a clinical study
I then conducted a clinical study examining patients with tumors impinging on the corpus callosum, which revealed that they developed psychotic symptoms (delusions and hallucinations).4 This study suggested that disrupting the integrity of the callosal inter-hemispheric fibers can trigger fixed false beliefs and perceptual anomalies.4
A ‘dysconnection’ between hemispheres
I translated those observations about the corpus callosum into a published hypothesis5 in which I proposed that Schneider’s First-Rank Symptoms of schizophrenia of thought insertion, thought withdrawal, and thought broadcasting—as well as delusional experiences of “external control”—may be due to a neurobiologic abnormality in the corpus callosum that disrupts the flow of ongoing bits of information transmitted from the left to the right hemisphere, and vice versa. I proposed in my model that this disruption leads to the verbal left hemisphere of a psychotic patient to describe having thoughts inserted into it from an alien source, failing to recognize that the thoughts it is receiving are being transmitted from the disconnected right hemisphere, which is no longer part of the “self.” Similarly, impulses from the right hemispheric consciousness are now perceived by the patient’s verbal left hemisphere (which talks to the examining physician) as “external control.” Thus, I postulated that an abnormal corpus callosum structure would lead to a “dysconnection” (not “disconnection”) between the 2 hemispheres, and that anomalous dysconnectivity may generate both delusions and hallucinations. 6
Two decades later, my assumptions were vindicated when DTI was invented, enabling the measurement of WM integrity, including the corpus callosum, the largest body of WM in the brain. Table 1 defines the main parameters of WM integrity, anisotropy and diffusivity, which measure water flow inside WM fibers.

During the past 15 years, many studies have confirmed the presence of significant abnormalities in the myelinated fibers of the corpus callosum in schizophrenia, which can be considered a validation of my hypothesis that the corpus callosum becomes a dysfunctional channel of communications between the right and left hemisphere. Subsequently, DTI studies have reported a spectrum of WM pathologies in various other cerebral bundles and not only in schizophrenia, but also in other major psychiatric disorders (Table 27-19).
The pathophysiology of WM pathology in many psychiatric disorders may include neurodevelopmental aberrations (genetic, environmental, or both, which may alter WM structure and/or myelination), neuroinflammation, or oxidative stress (free radicals), which can cause disintegration of the vital myelin sheaths, leading to disruption of brain connectivity.6,7 Researchers now consider the brain’s WM network dysconnectivity as generating a variety of psychiatric symptoms, including psychosis, depression, mania, anxiety, autism, aggression, impulsivity, psychopathy, and cognitive impairments.
It is not surprising that WM repair has become a therapeutic target in psychiatry and neurology. Among the strategies being investigated are inhibiting the Nogo-A signaling pathways20 or modulating the Lingo-1 signaling.21 However, the most well-established myelin repair pathway is prolactin, a neuroprotective hormone with several beneficial effects on the brain (Table 322,23), including the proliferation of oligodendroglia, the main source of myelin (and the number of which declines in schizophrenia). Antipsychotics that increase prolactin have been shown to increase WM volume.24,25 It has even been proposed that a decline in oligodendrocytes and low myelin synthesis may be one of the neurobiologic pathologies in schizophrenia.26 One of the 24 neuroprotective properties of the second-generation antipsychotics (SGAs) is the restoration of WM integrity.27 It’s worth noting that WM pathology has been found to be present at the onset of schizophrenia before treatment, and that SGAs have been reported to correct it.28

Continue to: In conclusion...
In conclusion, psychiatric disorders, usually referred to as “mental illnesses,” are unquestionably neurologic disorders. Similarly, all neurologic disorders are associated with psychiatric manifestations. WM pathology is only 1 of numerous structural brain abnormalities that have been documented across psychiatric disorders, which proves that psychiatry is a clinical neuroscience, just like neurology. I strongly advocate that psychiatry and neurology reunite into a single medical specialty. Both focus on disorders of brain structure and/or function, and these disorders also share much more than WM pathology.29
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
1. Sagarwala R and Nasrallah HA. White matter pathology is shared across multiple psychiatric brain disorders: Is abnormal diffusivity a transdiagnostic biomarker for psychopathology? Biomarkers in Neuropsychiatry. 2020;2:00010. https://doi.org/10.1016/j.bionps.2019.100010
2. Pearce JMS; FRCP. The “split brain” and Roger Wolcott Sperry (1913-1994). Rev Neurol (Paris). 2019;175(4):217-220.
3. Nasrallah HA, Andreasen NC, Coffman JA, et al. A controlled magnetic resonance imaging study of corpus callosum thickness in schizophrenia. Biol Psychiatry. 1986;21(3):274-282.
4. Nasrallah HA, McChesney CM. Psychopathology of corpus callosum tumors. Biol Psychiatry. 1981;16(7):663-669.
5. Nasrallah HA. The unintegrated right cerebral hemispheric consciousness as alien intruder: a possible mechanism for Schneiderian delusions in schizophrenia. Compr Psychiatry. 1985;26(3):273-282.
6. Friston K, Brown HR, Siemerkus J, et al. The dysconnection hypothesis (2016). Schizophr Res. 2016;176(2-3):83-94.
7. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
8. Benedetti F, Bollettini I. Recent findings on the role of white matter pathology in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):338-341.
9. Zheng H, Bergamino M, Ford BN, et al; Tulsa 1000 Investigators. Replicable association between human cytomegalovirus infection and reduced white matter fractional anisotropy in major depressive disorder. Neuropsychopharmacology. 2021;46(5):928-938.
10. Sagarwala R, Nasrallah HA. A systematic review of diffusion tensor imaging studies in drug-naïve OCD patients before and after pharmacotherapy. Ann Clin Psychiatry. 2020;32(1):42-47.
11. Lee KS, Lee SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. 2020;1191:61-70.
12. Swanson MR, Hazlett HC. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J Neurodev Disord. 2019;11(1):33.
13. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288-298.
14. Waller R, Dotterer HL, Murray L, et al. White-matter tract abnormalities and antisocial behavior: a systematic review of diffusion tensor imaging studies across development. Neuroimage Clin. 2017;14:201-215.
15. Wolf RC, Pujara MS, Motzkin JC, et al. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum Brain Mapp. 2015;36(10):4202-4209.
16. Puzzo I, Seunarine K, Sully K, et al. Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. J Abnorm Child Psychol. 2018;46(7):1451-1466.
17. Finger EC, Marsh A, Blair KS, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202(3):239-244.
18. Li C, Dong M, Womer FY, et al. Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Hum Brain Mapp. 2021;42(4):1182-1196.
19. Khanbabaei M, Hughes E, Ellegood J, et al. Precocious myelination in a mouse model of autism. Transl Psychiatry. 2019;9(1):251.
20. Petratos S, Theotokis P, Kim MJ, et al. That’s a wrap! Molecular drivers governing neuronal nogo receptor-dependent myelin plasticity and integrity. Front Cell Neurosci. 2020;14:227
21. Fernandez-Enright F, Andrews JL, Newell KA, et al. Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry. 2014;4(1):e348. doi: 10.1038/tp.2013.121
22. Bartzokis G, Lu PH, Stewart SB, et al. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr Res. 2009;113(2-3):322-331.
23. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011 Oct;132(1):35-41
24. Tishler TA, Bartzokis G, Lu PH, et al. Abnormal trajectory of intracortical myelination in schizophrenia implicates white matter in disease pathophysiology and the therapeutic mechanism of action of antipsychotics. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(5):454-462.
25. Ren Y, Wang H, Xiao L. Improving myelin/oligodendrocyte-related dysfunction: a new mechanism of antipsychotics in the treatment of schizophrenia? Int J Neuropsychopharmacol. 2013;16(3):691-700.
26. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7(3):272-281.
27. Chen AT, Nasrallah HA. Neuroprotective effects of the second generation antipsychotics. Schizophr Res. 2019;208:1-7
28. Sagarwala R, Nasrallah HA. (In press.) The effect of antipsychotic medications on white matter integrity in first-episode drug naïve patients with psychosis. Asian Journal of Psychiatry.
29. Nasrallah HA. Let’s tear down the silos and reunify psychiatry and neurology. Current Psychiatry. 2013;12(8):9-10.
2021 Update on cervical disease
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.
Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.
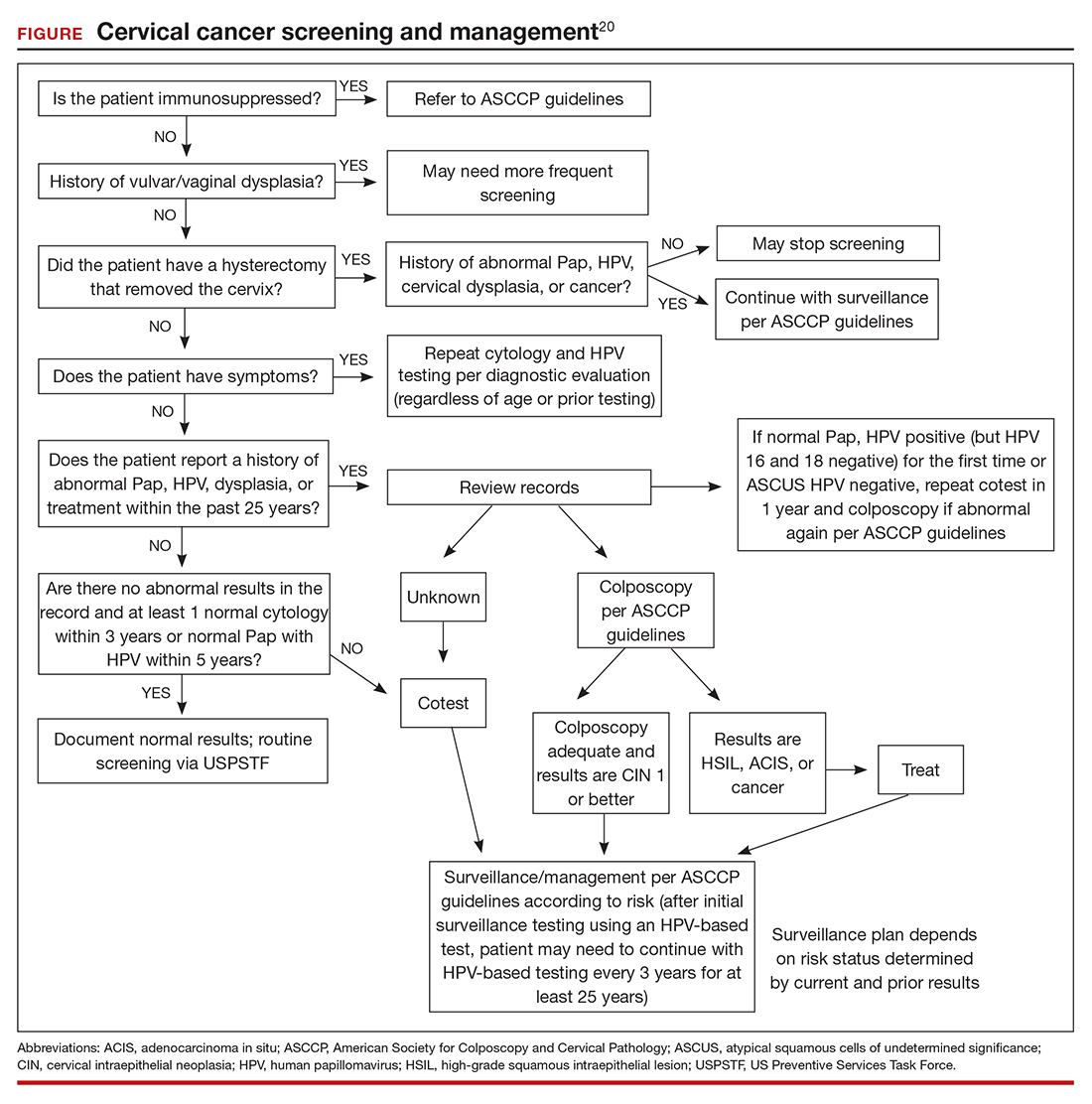
Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.

Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.

Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.

Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.

Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
Obstetric anal sphincter injury: Prevention and repair
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
The rate of obstetric anal sphincter injury (OASIS) is approximately 4.4% of vaginal deliveries, with 3.3% 3rd-degree tears and 1.1% 4th-degree tears.1 In the United States in 2019 there were 3,745,540 births—a 31.7% rate of cesarean delivery (CD) and a 68.3% rate of vaginal delivery—resulting in approximately 112,600 births with OASIS.2 A meta-analysis reported that, among 716,031 vaginal births, the risk factors for OASIS included: forceps delivery (relative risk [RR], 3.15), midline episiotomy (RR, 2.88), occiput posterior fetal position (RR, 2.73), vacuum delivery (RR, 2.60), Asian race (RR, 1.87), primiparity (RR, 1.59), mediolateral episiotomy (RR, 1.55), augmentation of labor (RR, 1.46), and epidural anesthesia (RR, 1.21).3 OASIS is associated with an increased risk for developing postpartum perineal pain, anal incontinence, dyspareunia, and wound breakdown.4 Complications following OASIS repair can trigger many follow-up appointments to assess wound healing and provide physical therapy.
This editorial review focuses on evolving recommendations for preventing and repairing OASIS.
The optimal cutting angle for a mediolateral episiotomy is 60 degrees from the midline
For spontaneous vaginal delivery, a policy of restricted episiotomy reduces the risk of OASIS by approximately 30%.5 With an operative vaginal delivery, especially forceps delivery of a large fetus in the occiput posterior position, a mediolateral episiotomy may help to reduce the risk of OASIS, although there are minimal data from clinical trials to support this practice. In one clinical trial, 407 women were randomly assigned to either a mediolateral or midline episiotomy.6 Approximately 25% of the births in both groups were operative deliveries. The mediolateral episiotomy began in the posterior midline of the vaginal introitus and was carried to the right side of the anal sphincter for 3 cm to 4 cm. The midline episiotomy began in the posterior midline of the vagina and was carried 2 cm to 3 cm into the midline perineal tissue. In the women having a midline or mediolateral episiotomy, a 4th-degree tear occurred in 5.5% and 0.4% of births, respectively. For the midline or mediolateral episiotomy, a third-degree tear occurred in 18.4% and 8.6%, respectively. In a prospective cohort study of 1,302 women with an episiotomy and vaginal birth, the rate of OASIS associated with midline or mediolateral episiotomy was 14.8% and 7%, respectively (P<.05).7 In this study, the operative vaginal delivery rate was 11.6% and 15.2% for the women in the midline and mediolateral groups, respectively.
The angle of the mediolateral episiotomy may influence the rate of OASIS and persistent postpartum perineal pain. In one study, 330 nulliparous women who were assessed to need a mediolateral episiotomy at delivery were randomized to an incision with a 40- or 60-degree angle from the midline.8 Prior to incision, a line was drawn on the skin to mark the course of the incision and then infiltrated with 10 mL of lignocaine. The fetal head was delivered with a Ritgen maneuver. The length of the episiotomy averaged 4 cm in both groups. After delivery, the angle of the episiotomy incision was reassessed. The episiotomy incision cut 60 degrees from the midline was measured on average to be 44 degrees from the midline after delivery of the newborn. Similarly, the incision cut at a 40-degree angle was measured to be 24 degrees from the midline after delivery. The rates of OASIS in the women who had a 40- and 60-degree angle incision were 5.5% and 2.4%, respectively (P = .16).
Continue to: Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair...
Use a prophylactic antibiotic with extended coverage for anaerobes prior to or during your anal sphincter repair
Many experts recommend one dose of a prophylactic antibiotic prior to, or during, OASIS repair in order to reduce the risk of wound complications. In a trial 147 women with OASIS were randomly assigned to receive one dose of a second-generation cephalosporin (cefotetan or cefoxitin) with extended anaerobic coverage or a placebo just before repair of the laceration.9 At 2 weeks postpartum, perineal wound complications were significantly lower in women receiving one dose of prophylactic antibiotic with extended anaerobe coverage compared with placebo—8.2% and 24.1%, respectively (P = .037). Additionally, at 2 weeks postpartum, purulent wound discharge was significantly lower in women receiving antibiotic versus placebo, 4% and 17%, respectively (P = .036). Experts writing for the Society of Obstetricians and Gynaecologists of Canada also recommend one dose of cefotetan or cefoxitin.10 Extended anaerobic coverage also can be achieved by administering a single dose of BOTH cefazolin 2 g by intravenous (IV) infusion PLUS metronidazole 500 mg by IV infusion or oral medication.11 For women with severe penicillin allergy, a recommended regimen is gentamicin 5 mg/kg plus clindamycin 900 mg by IV infusion.11 There is evidence that for colorectal or hysterectomy surgery, expanding prophylactic antibiotic coverage of anaerobes with cefazolin PLUS metronidazole significantly reduces postoperative surgical site infection.12,13 Following an OASIS repair, wound breakdown is a catastrophic problem that may take many months to resolve. Administration of a prophylactic antibiotic with extended coverage of anaerobes may help to prevent wound breakdown.
Prioritize identifying and separately repairing the internal anal sphincter
The internal anal sphincter is a smooth muscle that runs along the outside of the rectal wall and thickens into a sphincter toward the anal canal. The internal anal sphincter is thin and grey-white in appearance, like a veil. By contrast, the external anal sphincter is a thick band of red striated muscle tissue. In one study of 3,333 primiparous women with OASIS, an internal anal sphincter injury was detected in 33% of cases.14 In this large cohort, the rate of internal anal sphincter injury with a 3A tear, a 3B tear, a complete tear of the external sphincter and a 4th-degree perineal tear was 22%, 23%, 42%, and 71%, respectively. The internal anal sphincter is important for maintaining rectal continence and is estimated to contribute 50% to 85% of resting anal tone.15 If injury to the internal anal sphincter is detected at a birth with an OASIS, it is important to separately repair the internal anal sphincter to reduce the risk of postpartum rectal incontinence.16
Polyglactin 910 vs Polydioxanone (PDS) Suture—Is PDS the winner?
Polyglactin 910 (Vicryl) is a braided suture that is absorbed within 56 to 70 days. Polydioxanone suture is a long-lasting monofilament suture that is absorbed within 200 days. Many colorectal surgeons and urogynecologists prefer PDS suture for the repair of both the internal and external anal sphincters.16 Authors of one randomized trial of OASIS repair with Vicryl or PDS suture did not report significant differences in most clinical outcomes.17 However, in this study, anal endosonographic imaging of the internal and external anal sphincter demonstrated more internal sphincter defects but not external sphincter defects when the repair was performed with Vicryl rather than PDS. The investigators concluded that comprehensive training of the surgeon, not choice of suture, is probably the most important factor in achieving a good OASIS repair. However, because many subspecialists favor PDS suture for sphincter repair, specialists in obstetrics and gynecology should consider this option.
Continue to: Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
Can your patient access early secondary repair if they develop a perineal laceration wound breakdown?
The breakdown of an OASIS repair is an obstetric catastrophe with complications that can last many months and sometimes stretch into years. The best approach to a perineal laceration wound breakdown remains controversial. It is optimal if all patients with a wound breakdown can be offered an early secondary repair or healing by secondary intention, permitting the patient to select the best approach for their specific situation.
As noted by the pioneers of early repair of episiotomy dehiscence, Drs. Hankins, Haugh, Gilstrap, Ramin, and others,18-20 conventional doctrine is that an episiotomy repair dehiscence should be managed expectantly, allowing healing by secondary intention and delaying repair of the sphincters for a minimum of 3 to 4 months.21 However, many small case-series report that early secondary repair of a perineal laceration wound breakdown is possible following multiple days of wound preparation prior to the repair, good surgical technique and diligent postoperative follow-up care. One large case series reported on 72 women with complete perineal wound dehiscence who had early secondary repair.22 The median time to complete wound healing following early repair was 28 days. About 36% of the patients had one or more complications, including skin dehiscence, granuloma formation, perineal pain, and sinus formation. A pilot randomized trial reported that, compared with expectant management of a wound breakdown, early repair resulted in a shorter time to wound healing.23
Early repair of perineal wound dehiscence often involves a course of care that extends over multiple weeks. As an example, following a vaginal birth with OASIS and immediate repair, the patient is often discharged from the hospital to home on postpartum day 3. The wound breakdown often is detected between postpartum days 6 to 10. If early secondary repair is selected as the best treatment, 1 to 6 days of daily debridement of the wound is needed to prepare the wound for early secondary repair. The daily debridement required to prepare the wound for early repair is often performed in the hospital, potentially disrupting early mother-newborn bonding. Following the repair, the patient is observed in the hospital for 1 to 3 days and then discharged home with daily wound care and multiple follow-up visits to monitor wound healing. Pelvic floor physical therapy may be initiated when the wound is healed. The prolonged process required for early secondary repair may be best undertaken by a subspecialty practice.24
The surgical repair and postpartum care of OASIS continues to evolve. In your practice you should consider:
- performing a mediolateral episiotomy at a 60-degree angle to reduce the risk of OASIS in situations where there is a high risk of anal sphincter injury, such as in forceps delivery
- using one dose of a prophylactic antibiotic with extended anaerobic coverage, such as cefotetan or cefoxitin
- focus on identifying and separately repairing an internal anal sphincter injury
- using a long-lasting absorbable suture, such as PDS, to repair the internal and external anal sphincters
- ensuring that the patient with a dehiscence following an episiotomy or anal sphincter injury has access to early secondary repair. Standardizing your approach to the prevention and repair of anal sphincter injury will benefit the approximately 112,600 US women who experience OASIS each year. ●
A Cochrane Database Systematic Review reported that moderate-quality evidence showed a decrease in OASIS with the use of intrapartum warm compresses to the perineum and perineal massage.1 Compared with control, intrapartum warm compresses to the perineum did not result in a reduction in first- or second-degree tears, suturing of perineal tears, or use of episiotomy. However, compared with control, intrapartum warm compresses to the perineum was associated with a reduction in OASIS (relative risk [RR], 0.46; 95% confidence interval [CI], 0.27–0.79; 1,799 women; 4 studies; moderate quality evidence; substantial heterogeneity among studies). In addition to a possible reduction in OASIS, warm compresses also may provide the laboring woman, especially those having a natural childbirth, a positive sensory experience and reinforce her perception of the thoughtfulness and caring of her clinicians.
Compared with control, perineal massage was associated with an increase in the rate of an intact perineum (RR, 1.74; 95% CI, 1.11–2.73; 6 studies; 2,618 women; low-quality evidence; substantial heterogeneity among studies) and a decrease in OASIS (RR, 0.49; 95% CI, 0.25–0.94; 5 studies; 2,477 women; moderate quality evidence). Compared with control, perineal massage did not significantly reduce first- or second-degree tears, perineal tears requiring suturing, or the use of episiotomy (very low-quality evidence). Although perineal massage may have benefit, excessive perineal massage likely can contribute to tissue edema and epithelial trauma.
Reference
1. Aasheim V, Nilsen ABC, Reinar LM, et al. Perineal techniques during the second stage of labour for reducing perineal trauma. Cochrane Database Syst Rev. 2017;CD006672.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
- Friedman AM, Ananth CV, Prendergast E, et al. Evaluation of third-degree and fourth-degree laceration rates as quality indicators. Obstet Gynecol. 2015;125:927-937.
- Hamilton BE, Martin JA, Osterman MK. Births: Provisional data for 2019. Vital Statistics Rapid Release; No. 8. Hyattsville MD: National Center for Health Statistics; May 2020. https://www.cdc.gov/nchs/data/vsrr/vsrr-8-508.pdf
- Pergialitotis V, Bellos I, Fanaki M, et al. Risk factors for severe perineal trauma during childbirth: an updated meta-analysis. European J Obstet Gynecol Repro Biol. 2020;247:94-100.
- Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and anal sphincter trauma. 1st ed. London, England: Springer-Verlag; 2009:33-51.
- Jiang H, Qian X, Carroli G, et al. Selective versus routine use of episiotomy for vaginal birth. Cochrane Database Syst Rev. 2017;CD000081.
- Coats PM, Chan KK, Wilkins M, et al. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
- Sooklim R, Thinkhamrop J, Lumbiganon P, et al. The outcomes of midline versus medio-lateral episiotomy. Reprod Health. 2007;4:10.
- El-Din AS, Kamal MM, Amin MA. Comparison between two incision angles of mediolateral episiotomy in primiparous women: a randomized controlled trial. J Obstet Gynaecol Res. 2014;40:1877-1882.
- Duggal N, Mercado C, Daniels K, et al. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111:1268-1273.
- Harvey MA, Pierce M. Obstetrical anal sphincter injuries (OASIS): prevention, recognition and repair. J Obstet Gynecol Can. 2015;37:1131-1148.
- Cox CK, Bugosh MD, Fenner DE, et al. Antibiotic use during repair of obstetrical anal sphincter injury: a qualitative improvement initiative. Int J Gynaecol Obstet. 2021; Epub January 28.
- Deierhoi RJ, Dawes LG, Vick C, et al. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217:763-769.
- Till Sr, Morgan DM, Bazzi AA, et al. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am J Obstet Gynecol. 2017;217:187.e1-e11.
- Pihl S, Blomberg M, Uustal E. Internal anal sphincter injury in the immediate postpartum period: prevalence, risk factors and diagnostic methods in the Swedish perineal laceration registry. European J Obst Gynecol Repro Biol. 2020;245:1-6.
- Fornell EU, Matthiesen L, Sjodahl R, et al. Obstetric anal sphincter injury ten years after: subjective and objective long-term effects. BJOG. 2005;112:312-316.
- Sultan AH, Monga AK, Kumar D, et al. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
- Williams A, Adams EJ, Tincello DG, et al. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
- Hauth JC, Gilstrap LC, Ward SC, et al. Early repair of an external sphincter ani muscle and rectal mucosal dehiscence. Obstet Gynecol. 1986;67:806-809.
- Hankins GD, Hauth JC, Gilstrap LC, et al. Early repair of episiotomy dehiscence. Obstet Gynecol. 1990;75:48-51.
- Ramin SR, Ramus RM, Little BB, et al. Early repair of episiotomy dehiscence associated with infection. Am J Obstet Gynecol. 1992;167:1104-1107.
- Pritchard JA, MacDonald PC, Gant NF. Williams Obstetrics, 17th ed. Norwalk Connecticut: Appleton-Century-Crofts; 1985:349-350.
- Okeahialam NA, Thakar R, Kleprlikova H, et al. Early re-suturing of dehisced obstetric perineal woulds: a 13-year experience. Eur J Obstet Gynecol Repro Biol. 2020;254:69-73.
- Dudley L, Kettle C, Thomas PW, et al. Perineal resuturing versus expectant management following vaginal delivery complicated by a dehisced wound (PREVIEW): a pilot and feasibility randomised controlled trial. BMJ Open. 2017;7:e012766.
- Lewicky-Gaupp C, Leader-Cramer A, Johnson LL, et al. Wound complications after obstetrical anal sphincter injuries. Obstet Gynecol. 2015;125:1088-1093.
10 devastating consequences of psychotic relapses
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
It breaks my heart every time young patients with functional disability and a history of several psychotic episodes are referred to me. It makes me wonder why they weren’t protected from a lifetime of disability with the use of one of the FDA-approved long-acting injectable (LAI) antipsychotics right after discharge from their initial hospitalization for first-episode psychosis (FEP).
Two decades ago, psychiatric research discovered that psychotic episodes are neurotoxic and neurodegenerative, with grave consequences for the brain if they recur. Although many clinicians are aware of the high rate of nonadherence in patients with schizophrenia—which inevitably leads to a psychotic relapse—the vast majority (>99%, in my estimate) never prescribe an LAI after the FEP to guarantee full adherence and protect the patient’s brain from further atrophy due to relapses. The overall rate of LAI antipsychotic use is astonishingly low (approximately 10%), despite the neurologic malignancy of psychotic episodes. Further, LAIs are most often used after a patient has experienced multiple psychotic episodes, at which point the patient has already lost a significant amount of brain tissue and has already descended into a life of permanent disability.
Oral antipsychotics have the same efficacy as their LAI counterparts, and certainly should be used initially in the hospital during FEP to ascertain the absence of an allergic reaction after initial exposure, and to establish tolerability. Inpatient nurses are experts at making sure a reluctant patient actually swallows the pills and does not cheek them to spit them out later. So patients who have had FEP do improve with oral medications in the hospital, but all bets are off that those patients will regularly ingest tablets every day after discharge. Studies show patients have a high rate of nonadherence within days or weeks after leaving the hospital for FEP.1 This leads to repetitive psychotic relapses and rehospitalizations, with dire consequences for young patients with schizophrenia—a very serious brain disorder that had been labeled “the worst disease of mankind”2 in the era before studies showed LAI second-generation antipsychotics for FEP had remarkable rates of relapse prevention and recovery.3,4
Psychiatrists should approach FEP the same way oncologists approach cancer when it is diagnosed as Stage 1. Oncologists immediately take action to prevent the recurrence of the patient’s cancer with chemotherapy and/or radiation therapy, and do not wait for the cancer to advance to Stage 4, with widespread metastasis, before administering these potentially life-saving therapies (despite their toxic adverse effects). In schizophrenia, functional disability is the equivalent of Stage 4 cancer and should be aggressively prevented by using LAIs at the time of initial diagnosis, which is Stage 1 schizophrenia. Knowing the grave consequences of psychotic relapses, there is no logical reason whatsoever not to switch patients who have had FEP to an LAI before they are discharged from the hospital. A well-known study by a UCLA research group that compared patients who had FEP and were assigned to oral vs LAI antipsychotics at the time of discharge reported a stunning difference at the end of 1 year: a 650% higher relapse rate among the oral medication group compared with the LAI group!5 In light of such a massive difference, wouldn’t psychiatrists want to treat their sons or daughters with an LAI antipsychotic right after FEP? I certainly would, and I have always believed in treating every patient like a family member.
Catastrophic consequences
This lack of early intervention with LAI antipsychotics following FEP is the main reason schizophrenia is associated with poor clinical and functional outcomes. Patients are prescribed pills that they often take erratically or not at all, and end up relapsing repeatedly, with multiple catastrophic consequences, such as:
1. Brain tissue loss. Until recently, psychiatry did not know that psychosis destroys gray and white matter in the brain and causes progressive brain atrophy with every psychotic relapse.6,7 The neurotoxicity of psychosis is attributed to 2 destructive processes: neuroinflammation8,9 and free radicals.10 Approximately 11 cc of brain tissue is lost during FEP and with every subsequent relapse.6 Simple math shows that after 3 to 5 relapses, patients’ brains will shrink by 35 cc to 60 cc. No wonder recurrent psychoses lead to a life of permanent disability. As I have said in a past editorial,11 just as cardiologists do everything they can to prevent a second myocardial infarction (“heart attack”), psychiatrists must do the same to prevent a second psychotic episode (“brain attack”).
2. Treatment resistance. With each psychotic episode, the low antipsychotic dose that worked well in FEP is no longer enough and must be increased. The neurodegenerative effects of psychosis implies that the brain structure changes with each episode. Higher and higher doses become necessary with every psychotic recurrence, and studies show that approximately 1 in 8 patients may stop responding altogether after a psychotic relapse.12
Continue to: Disability
3. Disability. Functional disability, both vocational and social, usually begins after the second psychotic episode, which is why it is so important to prevent the second episode.13 Patients usually must drop out of high school or college or quit the job they held before FEP. Most patients with multiple psychotic episodes will never be able to work, get married, have children, live independently, or develop a circle of friends. Disability in schizophrenia is essentially a functional death sentence.14
4. Incarceration and criminalization. So many of our patients with schizophrenia get arrested when they become psychotic and behave erratically due to delusions, hallucinations, or both. They typically are taken to jail instead of a hospital because almost all the state hospitals around the country have been closed. It is outrageous that a medical condition of the brain leads to criminalization of patients with schizophrenia.15 The only solution for this ongoing crisis of incarceration of our patients with schizophrenia is to prevent them from relapsing into psychosis. The so-called deinstitutionalization movement has mutated into trans-institutionalization, moving patients who are medically ill from state hospitals to more restrictive state prisons. Patients with schizophrenia should be surrounded by a mental health team, not by armed prison guards. The rate of recidivism among these individuals is extremely high because patients who are released often stop taking their medications and get re-arrested when their behavior deteriorates.
5. Suicide. The rate of suicide in the first year after FEP is astronomical. A recent study reported an unimaginably high suicide rate: 17,000% higher than that of the general population.16 Many patients with FEP commit suicide after they stop taking their antipsychotic medication, and often no antipsychotic medication is detected in their postmortem blood samples.
6. Homelessness. A disproportionate number of patients with schizophrenia become homeless.17 It started in the 1980s, when the shuttering of state hospitals began and patients with chronic illnesses were released into the community to fend for themselves. Many perished. Others became homeless, living on the streets of urban areas.
7. Early mortality. Schizophrenia has repeatedly been shown to be associated with early mortality, with a loss of approximately 25 potential years of life.17 This is attributed to lifestyle risk factors (eg, sedentary living, poor diet) and multiple medical comorbidities (eg, obesity, diabetes, hypertension). To make things worse, patients with schizophrenia do not receive basic medical care to protect them from cardiovascular morbidity, an appalling disparity of care.18 Interestingly, a recent 7-year follow-up study of patients with schizophrenia found that the lowest rate of mortality from all causes was among patients receiving a second-generation LAI.19 Relapse prevention with LAIs can reduce mortality! According to that study, the worst mortality rate was observed in patients with schizophrenia who were not receiving any antipsychotic medication.
Continue to: Posttraumatic stress disorder
8. Posttraumatic stress disorder (PTSD). Many studies report that psychosis triggers PTSD symptoms20 because delusions and hallucinations can represent a life-threatening experience. The symptoms of PTSD get embedded within the positive and negative symptoms of schizophrenia, and every psychotic relapse serves as a “booster shot” for PTSD, leading to depression, anxiety, personality changes, aggressive behavior, and suicide.
9. Hopelessness, depression, and demoralization. The stigma of a severe psychiatric brain disorder such as schizophrenia, with multiple episodes, disability, incarceration, and homelessness, extends to the patients themselves, who become hopeless and demoralized by a chronic illness that marginalizes them into desperately ill individuals.21 The more psychotic episodes, the more intense the demoralization, hopelessness, and depression.
10. Family burden. The repercussions of psychotic relapses after FEP leads to significant financial and emotional stress on patients’ families.22 The heavy burden of caregiving among family members can be highly distressing, leading to depression and medical illness due to compromised immune functions.
Preventing relapse: It is not rocket science
It is obvious that the single most important therapeutic action for patients with schizophrenia is to prevent psychotic relapses. Even partial nonadherence must be prevented, because a drop of 25% in a patient’s serum antipsychotic level has been reported to lead to a psychotic relapse.23 Preventing relapse after FEP is not rocket science: Switch the patient to an LAI before discharge from the hospital,24 and provide the clinically necessary psychosocial treatments at every monthly follow-up visit (supportive psychotherapy, social skill training, vocational rehabilitation, and cognitive remediation). I have witnessed firsthand how stable and functional a patient who has had FEP can become when started on a second-generation LAI very soon after the onset of the illness.
I will finish with a simple question to my clinician readers: given the many devastating consequences of psychotic relapses, what would you do for your young patient with FEP? I hope you will treat them like a family member, and protect them from brain atrophy, disability, incarceration, homelessness, and suicide by starting them on an LAI antipsychotic before they leave the hospital. We must do no less for this highly vulnerable, young patient population.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.
1. Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468.
2. Where next with psychiatric illness? Nature. 1988;336(6195):95-96.
3. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
4. Kishimoto T, Hagi K, Kurokawa S, et al. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021:S2215-0366(21)00039-0. doi: 10.1016/S2215-0366(21)00039-0
5. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829.
6. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
7. Lei W, Kirkpatrick B, Wang Q, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. 2019;288:12-20.
8. Monji A, Kato TA, Mizoguchi Y, et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115-121.
9. Köhler-Forsberg O, Müller N, Lennox BR. Editorial: The role of inflammation in the etiology and treatment of schizophrenia. Front Psychiatry. 2020;11:603296. doi: 10.3389/fpsyt.2020.603296
10. Noto C, Ota VK, Gadelha A, et al. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216.
11. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
12. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
13. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
14. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: a register-based cohort study in Denmark. Lancet Psychiatry. 2021;8(4):310-319.
15. Kirchebner J, Günther MP, Lau S. Identifying influential factors distinguishing recidivists among offender patients with a diagnosis of schizophrenia via machine learning algorithms. Forensic Sci Int. 2020;315:110435. doi: 10.1016/j.forsciint.2020.110435
16. Zaheer J, Olfson M, Mallia E, et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr Res. 2020;222:382-388.
17. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
18. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
19. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
20. Seedat S, Stein MB, Oosthuizen PP, et al. Linking posttraumatic stress disorder and psychosis: a look at epidemiology, phenomenology, and treatment. J Nerv Ment Dis. 2003;191(10):675-681.
21. Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: A review of the literature. Medicina (Kaunas). 2019;55(5):200.
22. Khalil SA, Elbatrawy AN, Saleh NM, et al. The burden of care and burn out syndrome in caregivers of an Egyptian sample of schizophrenia patients. Int J Soc Psychiatry. 2021;10. doi: 10.1177/0020764021993155
23. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292.
24. Garner KN, Nasrallah HA. Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia. Current Psychiatry. 2015;14(7):33-45.