User login
Relugolix combination therapy: A novel hormonal treatment for AUB associated with uterine fibroids
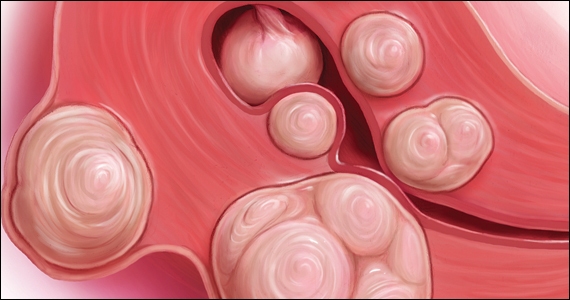
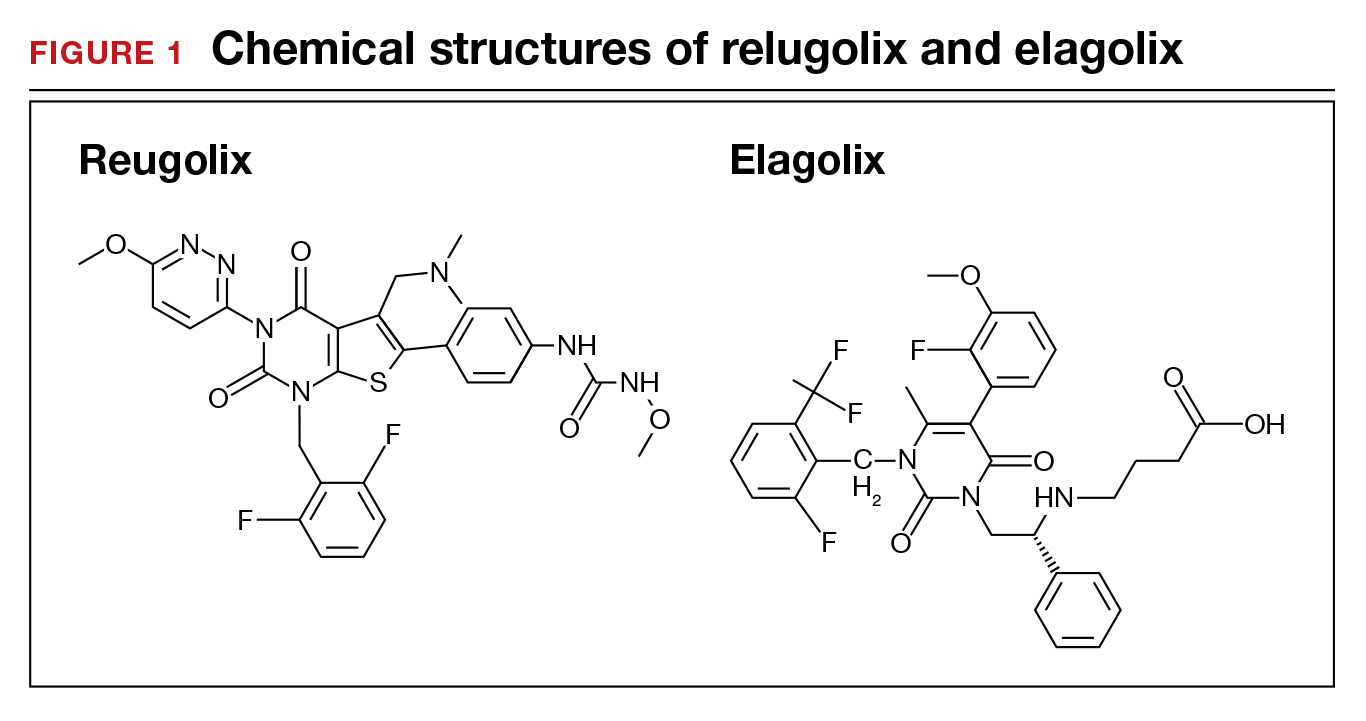
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.
Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.
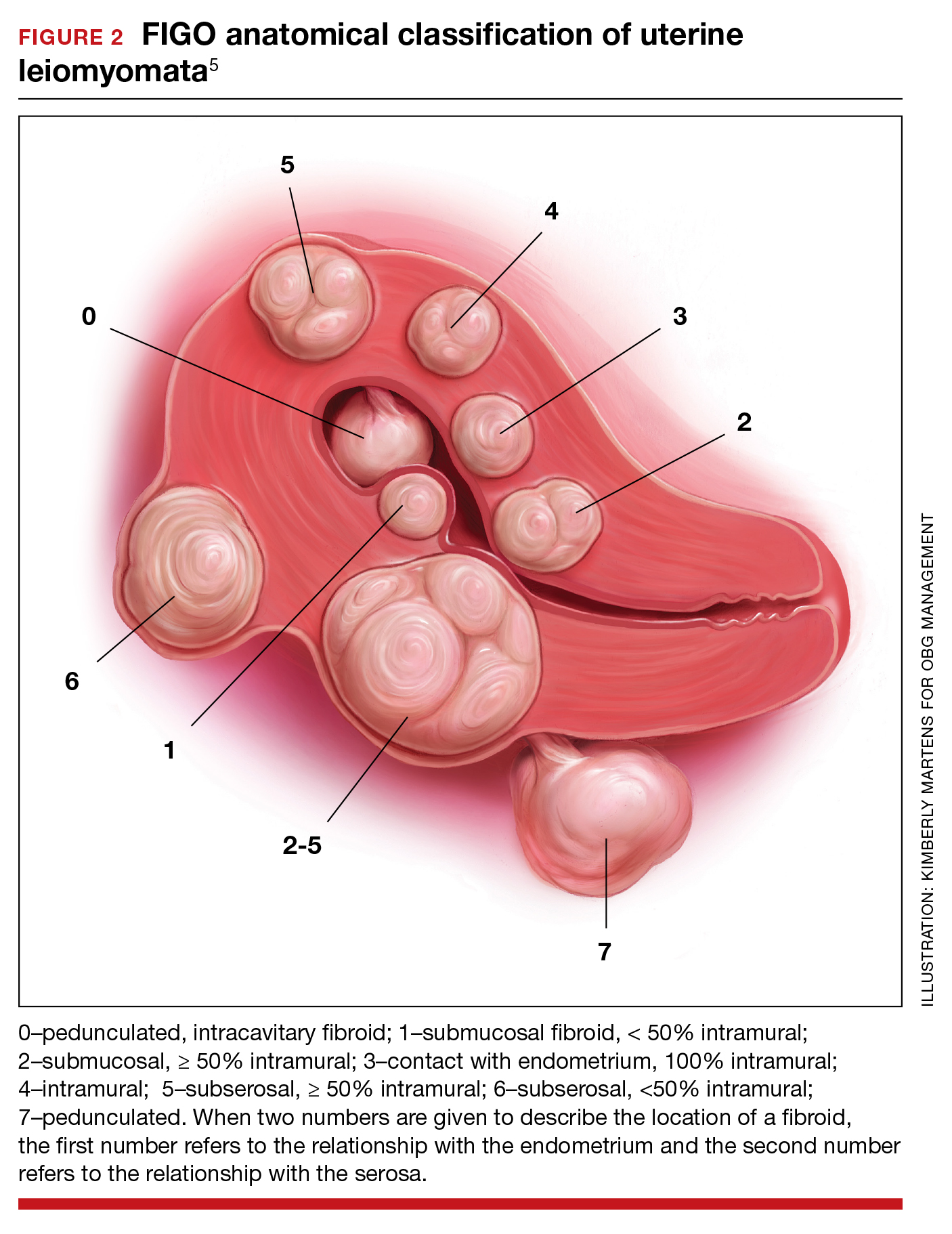
The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
Beyond DSM symptoms: Behavioral clues to diagnosing bipolar II disorder
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
Colorectal cancer screening, 2021: An update
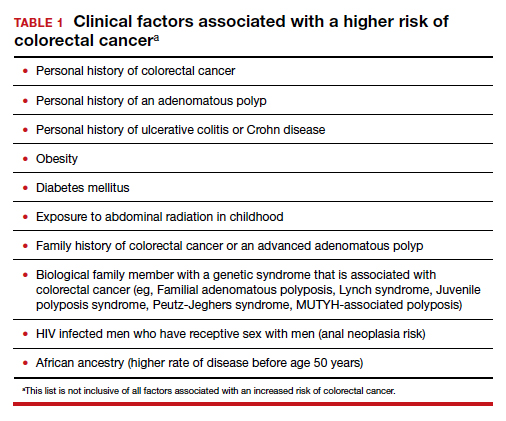
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.
Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).
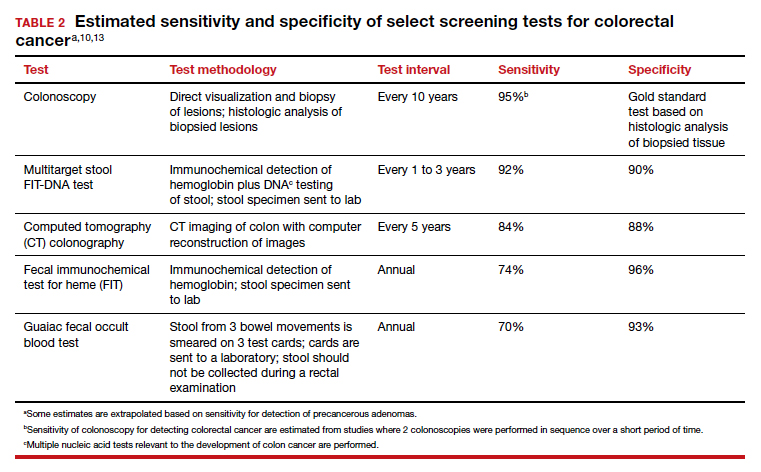
In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
Colorectal cancer is a common disease that has a very lengthy natural history of progression from small (<8 mm) to large (≥8 mm) polyps, then to dysplasia, and eventually to invasive cancer. It is estimated that this progression takes 10 years.1 The long natural history from preneoplasia to cancer makes colorectal cancer an ideal target for screening. Screening for colorectal cancer is divided into two clinical pathways, screening for people at average risk and for those at high risk. Clinical factors that increase the risk of colorectal cancer are listed in TABLE 1. This editorial is focused on the clinical approach to screening for people at average risk for colorectal cancer.

Colorectal cancer is the second most common cause of cancer death
The top 6 causes of cancer death in the United States are2:
- lung cancer (23% of cancer deaths)
- colon and rectum (9%)
- pancreas (8%)
- female breast (7%)
- prostate (5%)
- liver/bile ducts (5%).
In 2020 it is estimated that 147,950 people were diagnosed with colorectal cancer, including 17,930 people less than 50 years of age.3 In 2020, it is also estimated that 53,200 people in the United States died of colorectal cancer, including 3,640 people younger than age 50.3 By contrast, the American Cancer Society estimates that, in 2021, cervical cancer will be diagnosed in 14,480 women and 4,290 women with the disease will die.4
According to a Centers for Disease Control and Prevention (CDC) study, among people 50 to 64 years of age, 63% report being up to date with colorectal cancer screening—leaving a full one-third not up to date with their screening.5 Among people aged 65 to 75, 79% report being up to date with colorectal cancer screening. Among those aged 50 to 64, those with health insurance were more likely to be up to date with screening than people without insurance—67% versus 33%, respectively. People with a household income greater than $75,000 and less than $35,000 reported up-to-date screening rates of 71% and 55%, respectively. Among people aged 50 to 64, non-Hispanic White and Black people reported similar rates of being up to date with colorectal screening (66% and 65%, respectively). Hispanic people, however, reported a significantly lower rate of being up to date with colorectal cancer screening (51%).5
A weakness of this CDC study is that the response rate from the surveyed population was less than 50%, raising questions about validity and generalizability of the reported results. Of note, other studies report that Black men may have lower rates of colorectal cancer screening than non-Black men.6 These data show that focused interventions to improve colorectal cancer screening are required for people 50 to 64 years of age, particularly among underinsured and some minority populations.
Continue to: Inequitable health outcomes for colorectal cancer...
Inequitable health outcomes for colorectal cancer
The purpose of screening for cancer is to reduce the morbidity and mortality associated with the disease. Based on the Surveillance, Epidemiology and End Results (SEER) national reporting system, from 2014 to 2018 colorectal death rates per 100,000 adults were 18 for Black adults; 15.1 for American Indian/Alaska native adults; 13.6 for White non-Hispanic adults; 10.9 for White, Hispanic adults; and 9.4 for Asian/Pacific Islander adults.7 Lack of access to and a lower utilization rate of high-quality colon cancer screening modalities, for example colonoscopy, and a lower rate of optimal colon cancer treatment may account for the higher colorectal death rate among Black adults.8,9
Colorectal cancer screening should begin at age 45
In 2015 the Agency for Health Research and Quality (AHRQ) published data showing that the benefit of initiating screening for colorectal cancer at 45 years of age outweighed the additional cost.10 In 2018, the American Cancer Society recommended that screening for colorectal cancer should begin at age 45.11 In 2021, after resisting the change for many years, the US Preventive Services Task Force (USPSTF) also recommended that screening for colorectal cancer should begin at 45.7 The new recommendation is based on statistical models that showed a significant increase in life-years gained at a small incremental cost. The USPSTF also recommended that clinicians and patients could consider discontinuing colorectal cancer screening at 75 years of age because the net benefit of continuing screening after age 75 is minimal.
Prior to 2021 the USPSTF recommended that screening be initiated at age 50. However, from 2010 to 2020 there was a significant increase in the percentage of new cases of colorectal cancer detected in people younger than 50. In 2010, colon and rectal cancer among people under 50 years of age accounted for 5% and 9% of all cases, respectively.12 In 2020, colon and rectal cancer in people younger than age 50 accounted for 11% and 15% of all cases, respectively.3
Options for colon cancer screening
There are many options for colorectal cancer screening (TABLE 2).10,13 Experts conclude that the best colorectal cancer screening test is the test that the patient will complete. Among options for screening, colonoscopy and the multitarget stool FIT-DNA test (Cologuard) have greater sensitivity for detecting colorectal precancer and cancer lesions compared with fecal immunochemical testing (FIT), computed tomography colonography imaging (CTC), and stool guaiac testing (see TABLE 1).

In my practice, I suggest patients use either colonoscopy (every 10 years) or the multitarget stool FIT-DNA test (every 1 to 3 years) for screening. Most of my patients select colonoscopy, but some prefer the multitarget stool FIT-DNA test because they fear the pre-colonoscopy bowel preparation and the risk of bowel perforation with colonoscopy. Most colonoscopy procedures are performed with sedation, requiring an adult to take responsibility for transporting the patient to their residence, adding complexity to the performance of colonoscopy. These two tests are discussed in more detail below.
Colonoscopy
Colonoscopy occupies a unique position among the options for colorectal cancer screening because it is both a screening test and the gold standard for diagnosis, based on histologic analysis of the polypoid tissue biopsied at the time of colonoscopy. For all other screening tests, if the test yields an abnormal result, it is necessary to perform a colonoscopy. Colonoscopy screening offers the advantage of “one and done for 10 years.” In my practice it is much easier to manage a test that is performed every 10 years than a test that should be performed annually.
Colonoscopy also accounts for most of the harms of colorectal screening because of serious procedure complications, including bowel perforation (1 in 2,000 cases) and major bleeding (1 in 500 cases).7
Continue to: Multitarget stool FIT-DNA test (Cologuard)...
Multitarget stool FIT-DNA test (Cologuard)
The multitarget stool FIT-DNA test is a remarkable innovation in cancer screening combining 3 independent biomarkers associated with precancerous lesions and colorectal cancer.14 The 3 test components include14:
- a fecal immunochemical test (FIT) for hemoglobin (which uses antibodies to detect hemoglobin)
- a test for epigenetic changes in the methylation pattern of promoter DNA, including the promoter regions on the N-Myc Downstream-Regulated Gene 4 (NDRG4) and Bone Morphogenetic Protein 3 (BMP3) genes
- a test for 7 gene mutations in the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS).
In addition, the amount of the beta-actin DNA present in the stool specimen is assessed and used as a quantitative control for the total amount of DNA in the specimen.
In one large clinical study, 9,989 people at average risk for colorectal cancer were screened with both a multitarget stool FIT-DNA test and a stool FIT test.15 Positive test results triggered a referral for colonoscopy. Among this cohort, 1% of participants were diagnosed with colorectal cancer and 7.6% with a precancerous lesion. The sensitivity of the multitarget stool FIT-DNA test and the FIT test for detecting colorectal cancer was 92.3% and 73.8%, respectively. The sensitivities of the multitarget stool FIT-DNA test and the FIT test for detecting precancerous lesions were 42.4% and 23.8%, respectively. The specificity of the FIT-DNA and FIT tests for detecting any cancer or precancerous lesion was 90% and 96.4%, respectively.15 The FIT test is less expensive than the multitarget stool FIT-DNA test. Eligible patients can order the FIT test through a Quest website.16 In June 2021 the published cost was $89 for the test plus a $6 physician fee. Most insurers will reimburse the expense of the test for eligible patients.
The multitarget stool FIT-DNA test should be performed every 1 to 3 years. Unlike colonoscopy or CT colonography, the stool is collected at home and sent to a testing laboratory, saving the patient time and travel costs. A disadvantage of the test is that it is more expensive than FIT or guaiac testing. Eligible patients can request a test kit by completing a telemedicine visit through the Cologuard website.17 One website lists the cost of a Cologuard test at $599.18 This test is eligible for reimbursement by most insurers.
Ensure patients are informed of needed screening
Most obstetrician-gynecologists have many women in their practice who are aged 45 to 64, a key target group for colorectal cancer screening. The American Cancer Society and the USPSTF strongly recommend that people in this age range be screened for colorectal cancer. Given that one-third of people these ages have not been screened, obstetrician-gynecologists can play an important role in reducing the health burden of the second most common cause of cancer death by ensuring that their patients are up to date with colorectal screening. ●
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening, clinical guidelines and rationale. Gastroenterology. 1997;112:594. doi: 10.1053/gast.1997.v112.agast970594.
- Centers for Disease Control and Prevention website. An update on cancer deaths in the United States. Accessed July 14, 2021.
- Siegel RL, Miller KD, Goding SA, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. doi: 10.3322/caac.21601.
- American Cancer Society website. Key statistics for cervical cancer. https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed July 14, 2021.
- Joseph DA, King JB, Dowling NF, et al. Vital signs: colorectal cancer screening test use, United States. Morb Mortal Wkly Rep. 2020;69:253-259.
- Rogers CR, Matthews P, Xu L, et al. Interventions for increasing colorectal cancer screening uptake among African-American men: a systematic review and meta-analysis. PLoS One. 2020;15:e0238354. doi: 10.1371/journal.pone.0238354.
- US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977. doi: 10.1001/jama.2021.6238.
- Carethers JM, Doubeni CA. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354-367. doi: 10.1053/j.gastro.2019.10.029.
- Rutter CM, Knudsen AB, Lin JS, et al. Black and White differences in colorectal cancer screening and screening outcomes: a narrative review. Cancer Epidemiol Biomarkers Prev. 2021;30:3-12. doi: 10.1158/1055-9965.EPI-19-1537.
- Zauber A, Knudsen A, Rutter CM, et al; Writing Committee of the Cancer Intervention and Surveillance Modeling Network (CISNET) Colorectal Cancer Working Group. Evaluating the benefits and harms of colorectal cancer screening strategies: a collaborative modeling approach. AHRQ Publication No. 14-05203-EF-2. Rockville, MD: Agency for Healthcare Research and Quality; October 2015. file:///C:/Users/loconnor/Downloads/cisnet-draft-modeling-report.pdf. Accessed July 15, 2021.
- American Cancer Society website. Cancer screening guidelines by age. . Accessed July 15, 2021.
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. doi: 10.1001/jamasurg.2014.1756.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595. doi: 10.1001/jama.2016.6828.
- FDA summary of safety and effectiveness data. https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130017B.pdf. Accessed July 15, 2021.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Mulitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. doi: 10.1056/NEJMoa1311194.
- FIT colorectal cancer screening. Quest Diagnostics website. https://questdirect.questdiagnostics.com/products/fit-colorectal-cancer-screening/d41c67cb-a16d-4ad6-82b9-1a77d32daf41?utm_source=google&utm_medium=cpc&utm_campaign=71700000081635378&utm_content=58700006943838348&utm_term=p62498361603&gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYAiAAEgKHqfD_BwE. Accessed July 15, 2021.
- Request Cologuard without leaving your home. Cologuard website. https://www.cologuard.com/how-to-get-cologuard?gclsrc=aw.ds&gclid=EAIaIQobChMIgZLq9NOI8QIVufvjBx0slQWPEAAYASAAEgKHIfD_BwE. Accessed July 15, 2021.
- Cologuard. Colonoscopy Assist website. https: //colonoscopyassist.com/Cologuard.html. Accessed July 15, 2021.
Needed: More studies of CSF molecular biomarkers in psychiatric disorders
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.
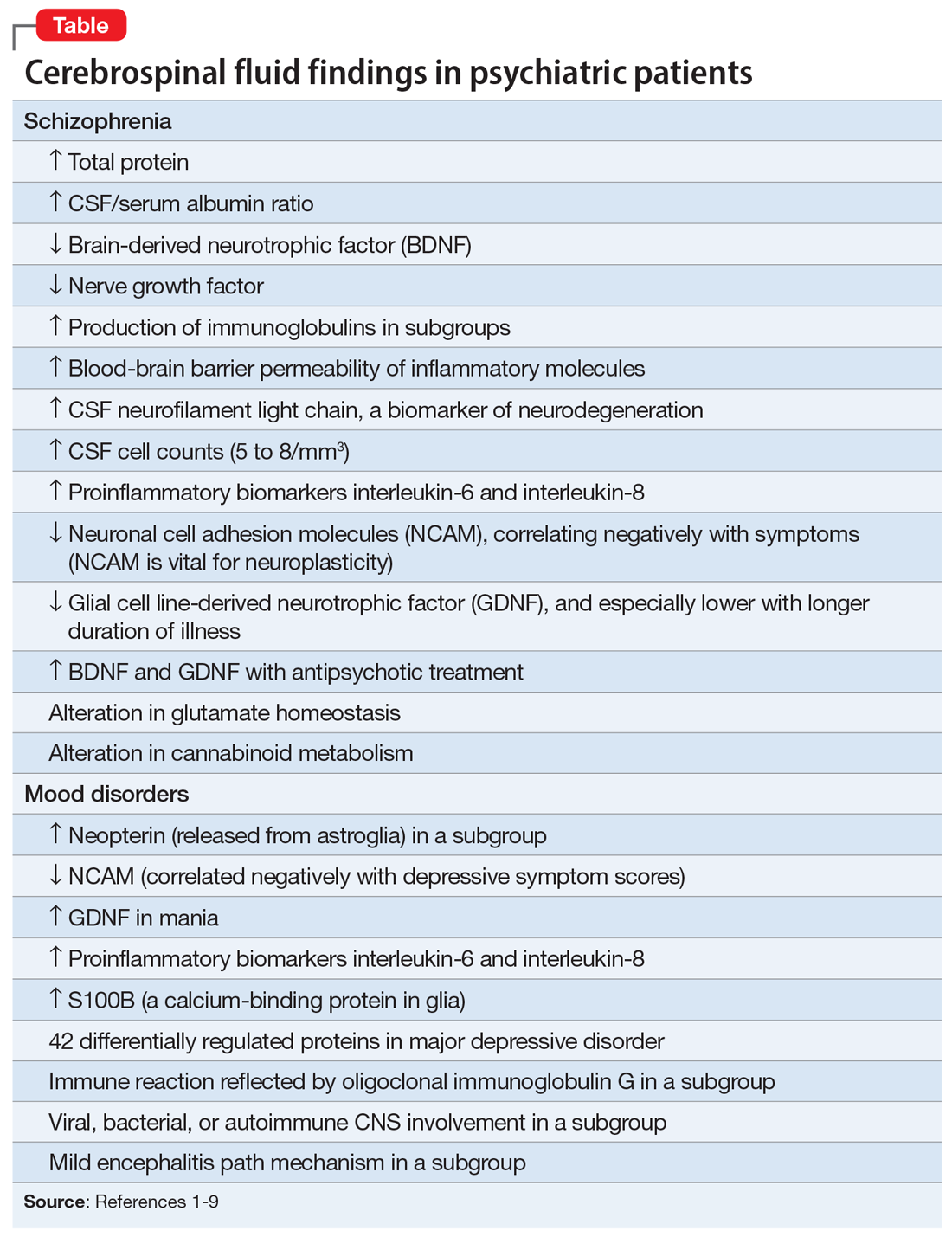
If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.

If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
Psychiatry and neurology are the brain’s twin medical disciplines. Unlike neurologic brain disorders, where localizing the “lesion” is a primary objective, psychiatric brain disorders are much more subtle, with no “gross” lesions but numerous cellular and molecular pathologies within neural circuits.
Measuring the molecular components of the cerebrospinal fluid (CSF), the glorious “sewage system” of the brain, may help reveal granular clues to the neurobiology of psychiatric disorders.
Mental illnesses involve the disruption of brain structures and functions in a diffuse manner across the cortex. Abnormal neuroplasticity has been implicated in several major psychiatric disorders. Examples include hypoplasia of the hippocampus in major depressive disorder and cortical thinning/dysplasia in schizophrenia. Reductions of neurotropic factors such as nerve growth factor or brain-derived neurotropic factor have been reported in mood and psychotic disorders, and appear to correlate with neuroplasticity changes.
Recent advances in psychiatric neuroscience have provided many clues to the pathophysiology of psychopathological conditions, including neuroinflammation, oxidative stress, apoptosis, impaired energy metabolism, abnormal metabolomics and lipidomics, and hypo- and hyperfunction of various neurotransmitters systems (especially glutamate N-methyl-
Thus, psychiatric research should focus on exploring and detecting molecular signatures (ie, biomarkers) of psychiatric disorders, including biomarkers of axonal and synaptic damage, glial activation, and oxidative stress. This is especially critical given the extensive heterogeneity of schizophrenia and mood and anxiety disorders. The CSF is a vastly unexploited substrate for discovering molecular biomarkers that will pave the way to precision psychiatry, and possibly open the door for completely new therapeutic strategies to tackle the most challenging neuropsychiatric disorders.
A role for CSF analysis
It’s quite puzzling why acute psychiatric episodes of schizophrenia, bipolar disorder, major depressive disorder, or panic attacks are not routinely assessed with a spinal tap, in conjunction with other brain measures such as neuroimaging (morphology, spectroscopy, cerebral blood flow, and diffusion tensor imaging) as well as a comprehensive neurocognitive examination and neurophysiological tests such as pre-pulse inhibition, mismatch negativity, and P-50, N-10, and P-300 evoked potentials. Combining CSF analysis with all those measures may help us stratify the spectra of psychosis, depression, and anxiety, as well as posttraumatic stress disorder and obsessive-compulsive disorder, into unique biotypes with overlapping clinical phenotypes and specific treatment approaches.
There are relatively few published CSF studies in psychiatric patients (mostly schizophrenia and bipolar and depressive disorders). The Table1-9 shows some of those findings. More than 365 biomarkers have been reported in schizophrenia, most of them in serum and tissue.10 However, none of them can be used for diagnostic purposes because schizophrenia is a syndrome comprised of several hundred different diseases (biotypes) that have similar clinical symptoms. Many of the serum and tissue biomarkers have not been studied in CSF, and they must if advances in the neurobiology and treatment of the psychotic and mood spectra are to be achieved. And adapting the CSF biomarkers described in neurologic disorders such as multiple sclerosis11 to schizophrenia and bipolar disorder (which also have well-established myelin pathologies) may yield a trove of neurobiologic findings.

If CSF studies eventually prove to be very useful for identifying subtypes for diagnosis and treatment, psychiatrists do not have to do the lumbar puncture themselves, but may refer patients to a “spinal tap” laboratory, just as they refer patients to a phlebotomy laboratory for routine blood tests. The adoption of CSF assessment in psychiatry will solidify its status as a clinical neuroscience, like its sister, neurology.
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133
1. Vasic N, Connemann BJ, Wolf RC, et al. Cerebrospinal fluid biomarker candidates of schizophrenia: where do we stand? Eur Arch Psychiatry Clin Neurosci. 2012;262(5):375-391.
2. Pollak TA, Drndarski S, Stone JM, et al. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5(1):79-92.
3. Katisko K, Cajanus A, Jääskeläinen O, et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J Neurol. 2020;267(1):162-167.
4. Bechter K, Reiber H, Herzog S, et al. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44(5):321-330.
5. Hidese S, Hattori K, Sasayama D, et al. Cerebrospinal fluid neural cell adhesion molecule levels and their correlation with clinical variables in patients with schizophrenia, bipolar disorder, and major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:12-18.
6. Tunca Z, Kıvırcık Akdede B, Özerdem A, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia: a comparative study in four major psychiatric disorders. Eur Psychiatry. 2015;30(2):198-204.
7. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics. 2017;14(6):499-514.
8. Kroksmark H, Vinberg M. Does S100B have a potential role in affective disorders? A literature review. Nord J Psychiatry. 2018;72(7):462-470.
9. Orlovska-Waast S, Köhler-Forsberg O, Brix SW, et al. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(6):869-887.
10. Nasrallah HA. Lab tests for psychiatric disorders: few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
11. Porter L, Shoushtarizadeh A, Jelinek GA, et al. Metabolomic biomarkers of multiple sclerosis: a systematic review. Front Mol Biosci. 2020;7:574133. doi: 10.3389/fmolb.2020.574133