User login
When does benign shyness become social anxiety, a treatable disorder?
Since the appearance of social anxiety disorder (SAD) in the DSM-III in 1980, research on its prevalence, characteristics, and treatment have grown (Box 11,2). In addition to the name, the definition of SAD has changed over the years; as a result, its prevalence has increased in recent cohort studies. This has led to debate over whether the experience of shyness is being over-pathologized, or whether SAD has been underdiagnosed in earlier decades. Those who argue that shyness is being over-pathologized note that it is a normal human experience that has evolutionary functions (eg, preventing engagement in harmful social relationships3). Others argue that a high degree of shyness is not beneficial in terms of evolution because it causes the individual to be shunned, so to speak, by society.4
Why worry about ‘over-pathologizing’?
The medicalization of shyness might be a reflection of Western societal values of assertiveness and gregariousness; other societies that value modesty and reticence do not over-pathologize shyness.5 It is important not to assume that someone who is shy necessarily has a “pathologic” level of social anxiety, especially because some people who are shy view that condition as a positive quality, much like sensitivity and conscientiousness.5
The broader issue of what constitutes a mental disorder arises in this debate. A “disorder” is a socially constructed label that describes a set of symptoms occurring together and its associated behaviors, not a real entity with etiological homogeneity.6 Labeling emotional problems “disordered” assumes that happiness is the natural homeostatic state, and distressing emotional states are abnormal and need to be changed.7 A diagnostic label can help improve communication and understand maladaptive behaviors; if that label is reified, however, it can lead to assumptions that the etiology, course, and treatment response are known. Proponents of the diagnostic psychiatric nomenclature have acknowledged the dangers of over-pathologizing normal experiences of living (such as fear) by way of diagnostic labeling.8
Determining when shyness becomes a clinically significant problem—what we call SAD here—demands a delicate distinction that has important implications for treatment. On one hand, if shyness is over-pathologized, persons who neither desire nor need treatment might be subjected to unnecessary and costly intervention. On the other hand, if SAD is underdiagnosed, some persons will not receive treatment that might be beneficial to them.
In this article, we review the similarities and differences between shyness and SAD, and provide recommendations for determining when shyness becomes a more clinically significant problem. We also highlight the importance of this distinction as it pertains to management, and provide suggestions for treatment approaches.
SAD: Definition, prevalence
SAD is defined as a significant fear of embarrassment or humiliation in social or performance-based situations, to a point at which the affected person often avoids these situations or endures them only with a high level of distress9 (Table 1, and Box 2). SAD can be distinguished from other anxiety disorders based on the source and content of the fear (ie, the source being social interaction or performance situations, and the content being a fear that one will show a behavior that will cause embarrassment). SAD also should be distinguished from autism spectrum disorders, in which persons have limited social communication capabilities and inadequate age-appropriate social relationships.
SAD is most highly comorbid with mood and anxiety disorders, with rates of at least 30% in clinical samples.10 The disorder also is highly comorbid with avoidant personality disorder—to a point at which it is argued that they are one and the same disorder.11
As with other psychiatric disorders, anxiety must cause significant impairment or distress. What constitutes significant impairment or distress is subjective, and the arbitrary nature of this criterion can influence estimates of the prevalence of SAD. For example, prevalence ranges as widely as 1.9% to 20.4% when different cut-offs are used for distress ratings and the number of impaired domains.12
The prevalence of SAD varies from 1 epidemiological study to another (ie, the Epidemiological Catchment Area [ECA] Study and the National Comorbidity Survey [NCS])—in part, a consequence of the differing definitions of significant impairment or distress. The ECA study assessed the clinical significance of each symptom in anxiety disorders; the NCS assessed overall clinical significance of the disorder. When the clinical significance criterion was applied at the symptom level to the NCS dataset (as was done in the ECA study), 1-year prevalence decreased by 50% (from 7.4% to 3.7%).13 The manner in which significant impairment or distress is defined (ie, conservatively or liberally) impacts whether social anxiety symptoms are classified as disordered or non-disordered.
Shyness: Definition, prevalence
Shyness often refers to 1) anxiety, inhibition, reticence, or a combination of these findings, in social and interpersonal situations, and 2) a fear of negative evaluation by others.14 It is a normal facet of personality that combines the experience of social anxiety and inhibited behavior,15 and also has been described as a stable temperament.16 Shyness is common; in the NCS study,17 26% of women and 19% of men characterized themselves as “very shy”; in the NCS Adolescent study,18 nearly 50% of adolescents self-identified as shy.
Persons who are shy tend to self-report greater social anxiety and embarrassment in social situations than non-shy persons do; they also might experience greater autonomic reactivity—especially blushing—in social or performance situations.15 Furthermore, shy persons are more likely to have axis I comorbidity and traits of introversion and neuroticism, compared with non-shy persons.14
Research suggests that temperament and behavioral inhibition are risk factors for mood and anxiety disorders, and appear to have a particularly strong relationship with SAD.19 A recent prospective study showed that shyness tends to increase steeply in toddlerhood, then stabilizes in childhood. Shyness in childhood—but not toddlerhood—is predictive of anxiety, depression, and poorer social skills in adolescence.20
A qualitative, or just quantitative, difference?
It is clear that SAD and shyness share several features—including anxiety and embarrassment—in social interactions. This raises a question: Are SAD and shyness distinct qualitatively, or do they represent points along a continuum, with SAD being an extreme form of shyness?
Continuum hypothesis. Support for the continuum hypothesis includes evidence that SAD and shyness share several features, including autonomic arousal, deficits in social skills (eg, aversion of gaze, difficulty initiating and maintaining conversation), avoidance of social situations, and fear of negative evaluation.21,22 In addition, both shyness and SAD are highly heritable,23 and mothers of shy children have a significantly higher rate of SAD than non-shy children do.24 No familial or genetic studies have compared heritability and familial aggregation in shyness and SAD.
According to the continuum hypothesis, if SAD is an extreme form of shyness, all (or nearly all) persons who have a diagnosis of SAD also would be characterized as shy. However, only approximately one-half of such persons report having been shy in childhood.17 Less than one-quarter of shy persons meet criteria for SAD.14,18 Because many persons who are shy do not meet criteria for SAD, and many who have SAD were not considered shy earlier in life, it has been suggested that this supports a qualitative distinction.
Qualitative distinctiveness. Despite having similarities, several features distinguish the experience of SAD from that of shyness. Compared with shyness, a SAD diagnosis is associated with:
- greater comorbidity
- greater severity of avoidance and impairment
- poorer quality of life.18,21,25
Studies that compared SAD, shyness without SAD, and non-shyness have shown that the shyness without SAD group more closely resembles the non-shy group than the SAD group—particularly with regard to impairment, presence of substance use, and other behavioral problems.18,25
Given the evidence, experts have concluded that shyness and a SAD diagnosis are overlapping yet different constructs that encapsulate qualitative and quantitative differences.25 There is a spectrum of shyness that ranges from a normative level to a higher level that overlaps the experience of SAD, but the 2 states represent different constructs.25
Guidance for making an assessment. Because of similarities in anxiety, embarrassment, and other symptoms in social situations, the best way to determine whether shyness crosses the line into a clinically significant problem is to assess the severity of the anxiety and associated degree of impairment and distress. More severe anxiety paired with distress about having anxiety and significant impairment in multiple areas of functioning might indicate more problematic social anxiety—a diagnosis of SAD—not just “normal” shyness.
It is important to take into account the environmental and cultural context of a patient’s distress and impairment because these features might fall within a normal range, given immediate circumstances (such as speaking in front of a large audience when one is not normally called on to do so, to a degree that does not interfere with general social functioning6).
What is considered a normative range depends on the developmental stage:
- Among children, a greater level of shyness might be considered more normative when it manifests during developmental stages in which separation anxiety appears.
- Among adolescents, a greater level of shyness might be considered normative especially during early adolescence (when social relationships become more important), and during times of transition (ie, entering high school).
- In adulthood, a greater level of normative shyness or social anxiety might be present during a major life change (eg, beginning to date again after the loss of a lengthy marriage or romantic relationship).
Assessment tools
Assessment tools can help you differentiate normal shyness from SAD. Several empirically-validated rating scales exist, including clinician-rated and self-report scales.
Liebowitz Social Anxiety Scale26 rates the severity of fear and avoidance in a variety of social interaction and performance-based situations. However, it was developed primarily as a clinician-rated scale and might be more burdensome to complete in practice. In addition, it does not provide cut-offs to indicate when more clinically significant anxiety might be likely.
Clinically Useful Social Anxiety Disorder Outcome Scale (CUSADOS)27 and Mini-Social Phobia Inventory (Mini-SPIN)28 are brief self-report scales that provide cut-offs to suggest further assessment is warranted. A cut-off score of 16 on the CUSADOS suggests the presence of SAD with 73% diagnostic efficiency.
One disadvantage to relying on a rating scale alone is the narrow focus on symptoms. Given that shyness and SAD share similar symptoms, it is necessary to assess the degree of impairment related to these symptoms to determine whether the problem is clinically significant. The overly narrow focus on symptoms utilized by the biomedical approach has been criticized for contributing to the medicalization of normal shyness.5
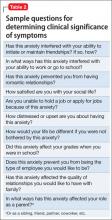
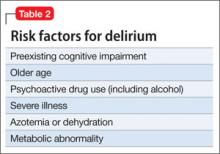
Diagnostic interviews, such as the Structured Clinical Interview for DSM-IV Axis I Disorders29 include sections on SAD that assess avoidance and impairment/distress associated with anxiety. Because these interviews may increase the time burden during an office visit, there are several general questions outside of a structured interview that you can ask, such as: “Has this anxiety interfered with your ability to initiate or maintain friendships? If so, how?” (Table 2). Persons with clinically significant social anxiety, rather than shyness, tend to report greater effects on their relationships and on work or school performance, as well as greater distress about having that anxiety.
Treatment approaches based on distinctions
Exercise care in making the distinction between normal shyness and dysfunctional and impairing levels of anxiety characteristic of SAD, because persons who display normal shyness but who are overdiagnosed might feel stigmatized by a diagnostic label.5 Also, overpathologizing shyness takes what is a social problem out of context, and could promote treatment strategies that might not be helpful or effective.30
Unnecessary diagnosis might lead to unnecessary treatment, such as prescribing an antidepressant or benzodiazepine. Avoiding such a situation is important, because of the side effects associated with medication and the potential for dependence and withdrawal effects with benzodiazepines.
Persons who exhibit normal shyness do not require medical treatment and, often, do not want it. However, some people may be interested in improving their ability to function in social interactions. Self-help approaches or brief psychotherapy (eg, cognitive-behavioral therapy [CBT]) should be the first step—and might be all that is necessary.
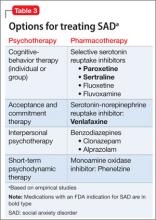
The opposite side of the problem. Under-recognition of clinically significant social anxiety can lead to under-treatment, which is common even in patients with a SAD diagnosis.31 Treatment options include CBT, medication, and CBT combined with medication (Table 3):
- several studies have demonstrated the short- and long-term efficacy of CBT alone for SAD
- medication alone has been efficacious in the short-term, but less efficacious than CBT in the long-term
- combined treatment also has been shown to be more efficacious than CBT or medication alone in the short-term
- there is evidence to suggest that CBT alone is more efficacious in the long-term compared with combined treatment.a
CBT is recommended as an appropriate first-line option, especially for mild and moderate SAD; it is the preferred initial treatment option of the United Kingdom’s National Institute for Health and Care Excellence (NICE). For more severe presentations (such as the presence of comorbidity) or when a patient did not respond to an adequate course of CBT, combined treatment might be an option—the goal being to taper the medication and continue CBT as a longer-term treatment. Research has shown that continuing CBT while discontinuing medication helps prevent relapse.32,33
Appropriate pharmacotherapy options include selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.34 Increasingly, benzodiazepines are considered less desirable; they are not recommended for routine use in SAD in the NICE guidelines. Those guidelines call for continuing pharmacotherapy for 6 months when a patient responds to treatment within 3 months, then discontinuing medication with the aid of CBT.
Bottom Line
The severity of anxiety and associated impairment and distress are the main variables that differentiate normal shyness and clinically significant social anxiety. Taking care not to over-pathologize normal shyness and common social anxiety concerns or underdiagnose severe, impairing social anxiety disorder has important implications for treatment—and for whether a patient needs treatment at all.
Related Resources
National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment, and treatment of social anxiety disorder. http://guidance.nice.org.uk/cg159.
• Hofmann SG, DiBartolo PM, eds. Social anxiety: clinical, developmental, and social perspectives, 2nd ed. London, United Kingdom: Academic Press; 2010.
• The Shyness Institute. www.shyness.com.
Drug Brand Names
Alprazolam • Xanax Clonazepam • Klonopin Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Phenelzine • Nardil
Sertraline • Zoloft Venlafaxine • Effexor
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Kristy L. Dalrymple, PhD, discusses, treating social anxiety disorder. Dr. Dalrymple is Staff Psychologist, Department of Psychiatry, Rhode Island Hospital, and Assistant Professor of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, Rhode Island.
1. Bruce LC, Coles ME, Heimberg RG. Social phobia and social anxiety disorder: effect of disorder name on recommendation for treatment. Am J Psychiatry. 2012;169(5):538.
2. Bögels SM, Alden L, Beidel DC, et al. Social anxiety disorder: questions and answers for the DSM-V. Depress Anxiety. 2010;27:168-189.
3. Wakefield JC, Horwitz AV, Schmitz MF. Are we overpathologizing the socially anxious? Social phobia from a harmful dysfunction perspective. Can J Psychiatry. 2005;50(6):317-319.
4. Campbell-Sills L, Stein MB. Justifying the diagnostic status of social phobia: a reply to Wakefield, Horwitz, and Schmitz. Can J Psychiatry. 2005;50(6):320-323.
5. Scott S. The medicalisation of shyness: from social misfits to social fitness. Sociology of Health and Illness. 2006;28(2):133-153.
6. Wakefield JC. The DSM-5 debate over the bereavement exclusion: psychiatric diagnosis and the future of empirically supported treatment. Clin Psychol Rev. 2013; 33(7):825-845.
7. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. New York, NY: Guilford Press; 2012.
8. Kupfer DJ, First MB, Regier DA, eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Dalrymple KL, Zimmerman M. Does comorbid social anxiety disorder impact the clinical presentation of principal major depressive disorder? J Affect Disord. 2007;100:241-247.
11. Dalrymple KL. Issues and controversies surrounding the diagnosis and treatment of social anxiety disorder. Expert Rev Neurother. 2012;12(8):993-1008.
12. Furmark T, Tillfors M, Everz PO, et al. Social phobia in the general population: prevalence and sociodemographic profile. Soc Psychiatry Psychiatr Epidemiol. 1999;34:416-424.
13. Narrow WE, Rae DS, Robins LN, et al. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115-123.
14. Heiser NA, Turner SM, Beidel DC. Shyness: relationship to social phobia and other psychiatric disorders. Behav Res Ther. 2003;41:209-221.
15. Hofmann SG, Moscovitch DA, Hyo-Jin K. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiology. 2006;61:134-142.
16. Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Hofmann SG, DiBartolo PM, eds. From social anxiety to social phobia: multiple perspectives. Needham Heights, MA: Allyn & Bacon; 2001:216-234.
17. Cox BJ, MacPherson PS, Enns MW. Psychiatric correlates of childhood shyness in a nationally representative sample. Behav Res Ther. 2005;43:1019-1027.
18. Burstein M, Ameli-Grillon L, Merikangas KR. Shyness versus social phobia in US youth. Pediatrics. 2011;128:917-925.
19. Hirshfeld-Becker DR, Micco J, Henin A, et al. Behavioral inhibition. Depress Anxiety. 2008;25:357-367.
20. Karevold E, Ystrom E, Coplan RJ, et al. A prospective longitudinal study of shyness from infancy to adolescence: stability, age-related changes, and prediction of socio-emotional functioning. J Abnorm Child Psychol. 2012; 40:1167-1177.
21. Chavira DA, Stein MB, Malcarne VL. Scrutinizing the relationship between shyness and social phobia. J Anxiety Disord. 2002;16:585-598.
22. Schneier FR, Blanco C, Antia SX, et al. The social anxiety spectrum. Psychiatr Clin N Am. 2002;25:757-774.
23. Stein MB, Chavira DA, Jang KL. Bringing up bashful baby: developmental pathways to social phobia. Psychiatr Clin N Am. 2001;24:797-818.
24. Cooper PJ, Eke M. Childhood shyness and maternal social phobia: a community study. Br J Psychiatry. 1999;174:439-443.
25. Heiser NA, Turner SM, Beidel DC, et al. Differentiating social phobia from shyness. J Anxiety Disord. 2009;23:469-476.
26. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141-173.
27. Dalrymple, KL, Martinez J, Tepe E, et al. A clinically useful social anxiety disorder outcome scale. Compr Psychiatry. 2013;54(7):758-765.
28. Connor KM, Kobak KA, Churchill LE, et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14(2):137-140.
29. First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997.
30. Conrad P. Medicalization and social control. Ann Rev Sociology. 1992;18:209-232.
31. Zimmerman M, Chelminski I. Clinician recognition of anxiety disorders in depressed outpatients. J Psychiatr Res. 2003;37:325-333.
32. Gelernter CS, Uhde TW, Cimbolic P, et al. Cognitive-behavioral and pharmacological treatments of social phobia: a controlled study. Arch Gen Psychiatry. 1991;48:938-945.
33. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(suppl 5):34-41.
34. Blanco C, Bragdon LB, Schneier FR, et al. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol. 2013;16:235-249.
Since the appearance of social anxiety disorder (SAD) in the DSM-III in 1980, research on its prevalence, characteristics, and treatment have grown (Box 11,2). In addition to the name, the definition of SAD has changed over the years; as a result, its prevalence has increased in recent cohort studies. This has led to debate over whether the experience of shyness is being over-pathologized, or whether SAD has been underdiagnosed in earlier decades. Those who argue that shyness is being over-pathologized note that it is a normal human experience that has evolutionary functions (eg, preventing engagement in harmful social relationships3). Others argue that a high degree of shyness is not beneficial in terms of evolution because it causes the individual to be shunned, so to speak, by society.4
Why worry about ‘over-pathologizing’?
The medicalization of shyness might be a reflection of Western societal values of assertiveness and gregariousness; other societies that value modesty and reticence do not over-pathologize shyness.5 It is important not to assume that someone who is shy necessarily has a “pathologic” level of social anxiety, especially because some people who are shy view that condition as a positive quality, much like sensitivity and conscientiousness.5
The broader issue of what constitutes a mental disorder arises in this debate. A “disorder” is a socially constructed label that describes a set of symptoms occurring together and its associated behaviors, not a real entity with etiological homogeneity.6 Labeling emotional problems “disordered” assumes that happiness is the natural homeostatic state, and distressing emotional states are abnormal and need to be changed.7 A diagnostic label can help improve communication and understand maladaptive behaviors; if that label is reified, however, it can lead to assumptions that the etiology, course, and treatment response are known. Proponents of the diagnostic psychiatric nomenclature have acknowledged the dangers of over-pathologizing normal experiences of living (such as fear) by way of diagnostic labeling.8
Determining when shyness becomes a clinically significant problem—what we call SAD here—demands a delicate distinction that has important implications for treatment. On one hand, if shyness is over-pathologized, persons who neither desire nor need treatment might be subjected to unnecessary and costly intervention. On the other hand, if SAD is underdiagnosed, some persons will not receive treatment that might be beneficial to them.
In this article, we review the similarities and differences between shyness and SAD, and provide recommendations for determining when shyness becomes a more clinically significant problem. We also highlight the importance of this distinction as it pertains to management, and provide suggestions for treatment approaches.
SAD: Definition, prevalence
SAD is defined as a significant fear of embarrassment or humiliation in social or performance-based situations, to a point at which the affected person often avoids these situations or endures them only with a high level of distress9 (Table 1, and Box 2). SAD can be distinguished from other anxiety disorders based on the source and content of the fear (ie, the source being social interaction or performance situations, and the content being a fear that one will show a behavior that will cause embarrassment). SAD also should be distinguished from autism spectrum disorders, in which persons have limited social communication capabilities and inadequate age-appropriate social relationships.
SAD is most highly comorbid with mood and anxiety disorders, with rates of at least 30% in clinical samples.10 The disorder also is highly comorbid with avoidant personality disorder—to a point at which it is argued that they are one and the same disorder.11
As with other psychiatric disorders, anxiety must cause significant impairment or distress. What constitutes significant impairment or distress is subjective, and the arbitrary nature of this criterion can influence estimates of the prevalence of SAD. For example, prevalence ranges as widely as 1.9% to 20.4% when different cut-offs are used for distress ratings and the number of impaired domains.12
The prevalence of SAD varies from 1 epidemiological study to another (ie, the Epidemiological Catchment Area [ECA] Study and the National Comorbidity Survey [NCS])—in part, a consequence of the differing definitions of significant impairment or distress. The ECA study assessed the clinical significance of each symptom in anxiety disorders; the NCS assessed overall clinical significance of the disorder. When the clinical significance criterion was applied at the symptom level to the NCS dataset (as was done in the ECA study), 1-year prevalence decreased by 50% (from 7.4% to 3.7%).13 The manner in which significant impairment or distress is defined (ie, conservatively or liberally) impacts whether social anxiety symptoms are classified as disordered or non-disordered.
Shyness: Definition, prevalence
Shyness often refers to 1) anxiety, inhibition, reticence, or a combination of these findings, in social and interpersonal situations, and 2) a fear of negative evaluation by others.14 It is a normal facet of personality that combines the experience of social anxiety and inhibited behavior,15 and also has been described as a stable temperament.16 Shyness is common; in the NCS study,17 26% of women and 19% of men characterized themselves as “very shy”; in the NCS Adolescent study,18 nearly 50% of adolescents self-identified as shy.
Persons who are shy tend to self-report greater social anxiety and embarrassment in social situations than non-shy persons do; they also might experience greater autonomic reactivity—especially blushing—in social or performance situations.15 Furthermore, shy persons are more likely to have axis I comorbidity and traits of introversion and neuroticism, compared with non-shy persons.14
Research suggests that temperament and behavioral inhibition are risk factors for mood and anxiety disorders, and appear to have a particularly strong relationship with SAD.19 A recent prospective study showed that shyness tends to increase steeply in toddlerhood, then stabilizes in childhood. Shyness in childhood—but not toddlerhood—is predictive of anxiety, depression, and poorer social skills in adolescence.20
A qualitative, or just quantitative, difference?
It is clear that SAD and shyness share several features—including anxiety and embarrassment—in social interactions. This raises a question: Are SAD and shyness distinct qualitatively, or do they represent points along a continuum, with SAD being an extreme form of shyness?
Continuum hypothesis. Support for the continuum hypothesis includes evidence that SAD and shyness share several features, including autonomic arousal, deficits in social skills (eg, aversion of gaze, difficulty initiating and maintaining conversation), avoidance of social situations, and fear of negative evaluation.21,22 In addition, both shyness and SAD are highly heritable,23 and mothers of shy children have a significantly higher rate of SAD than non-shy children do.24 No familial or genetic studies have compared heritability and familial aggregation in shyness and SAD.
According to the continuum hypothesis, if SAD is an extreme form of shyness, all (or nearly all) persons who have a diagnosis of SAD also would be characterized as shy. However, only approximately one-half of such persons report having been shy in childhood.17 Less than one-quarter of shy persons meet criteria for SAD.14,18 Because many persons who are shy do not meet criteria for SAD, and many who have SAD were not considered shy earlier in life, it has been suggested that this supports a qualitative distinction.
Qualitative distinctiveness. Despite having similarities, several features distinguish the experience of SAD from that of shyness. Compared with shyness, a SAD diagnosis is associated with:
- greater comorbidity
- greater severity of avoidance and impairment
- poorer quality of life.18,21,25
Studies that compared SAD, shyness without SAD, and non-shyness have shown that the shyness without SAD group more closely resembles the non-shy group than the SAD group—particularly with regard to impairment, presence of substance use, and other behavioral problems.18,25
Given the evidence, experts have concluded that shyness and a SAD diagnosis are overlapping yet different constructs that encapsulate qualitative and quantitative differences.25 There is a spectrum of shyness that ranges from a normative level to a higher level that overlaps the experience of SAD, but the 2 states represent different constructs.25
Guidance for making an assessment. Because of similarities in anxiety, embarrassment, and other symptoms in social situations, the best way to determine whether shyness crosses the line into a clinically significant problem is to assess the severity of the anxiety and associated degree of impairment and distress. More severe anxiety paired with distress about having anxiety and significant impairment in multiple areas of functioning might indicate more problematic social anxiety—a diagnosis of SAD—not just “normal” shyness.
It is important to take into account the environmental and cultural context of a patient’s distress and impairment because these features might fall within a normal range, given immediate circumstances (such as speaking in front of a large audience when one is not normally called on to do so, to a degree that does not interfere with general social functioning6).
What is considered a normative range depends on the developmental stage:
- Among children, a greater level of shyness might be considered more normative when it manifests during developmental stages in which separation anxiety appears.
- Among adolescents, a greater level of shyness might be considered normative especially during early adolescence (when social relationships become more important), and during times of transition (ie, entering high school).
- In adulthood, a greater level of normative shyness or social anxiety might be present during a major life change (eg, beginning to date again after the loss of a lengthy marriage or romantic relationship).
Assessment tools
Assessment tools can help you differentiate normal shyness from SAD. Several empirically-validated rating scales exist, including clinician-rated and self-report scales.
Liebowitz Social Anxiety Scale26 rates the severity of fear and avoidance in a variety of social interaction and performance-based situations. However, it was developed primarily as a clinician-rated scale and might be more burdensome to complete in practice. In addition, it does not provide cut-offs to indicate when more clinically significant anxiety might be likely.
Clinically Useful Social Anxiety Disorder Outcome Scale (CUSADOS)27 and Mini-Social Phobia Inventory (Mini-SPIN)28 are brief self-report scales that provide cut-offs to suggest further assessment is warranted. A cut-off score of 16 on the CUSADOS suggests the presence of SAD with 73% diagnostic efficiency.
One disadvantage to relying on a rating scale alone is the narrow focus on symptoms. Given that shyness and SAD share similar symptoms, it is necessary to assess the degree of impairment related to these symptoms to determine whether the problem is clinically significant. The overly narrow focus on symptoms utilized by the biomedical approach has been criticized for contributing to the medicalization of normal shyness.5
Diagnostic interviews, such as the Structured Clinical Interview for DSM-IV Axis I Disorders29 include sections on SAD that assess avoidance and impairment/distress associated with anxiety. Because these interviews may increase the time burden during an office visit, there are several general questions outside of a structured interview that you can ask, such as: “Has this anxiety interfered with your ability to initiate or maintain friendships? If so, how?” (Table 2). Persons with clinically significant social anxiety, rather than shyness, tend to report greater effects on their relationships and on work or school performance, as well as greater distress about having that anxiety.
Treatment approaches based on distinctions
Exercise care in making the distinction between normal shyness and dysfunctional and impairing levels of anxiety characteristic of SAD, because persons who display normal shyness but who are overdiagnosed might feel stigmatized by a diagnostic label.5 Also, overpathologizing shyness takes what is a social problem out of context, and could promote treatment strategies that might not be helpful or effective.30
Unnecessary diagnosis might lead to unnecessary treatment, such as prescribing an antidepressant or benzodiazepine. Avoiding such a situation is important, because of the side effects associated with medication and the potential for dependence and withdrawal effects with benzodiazepines.
Persons who exhibit normal shyness do not require medical treatment and, often, do not want it. However, some people may be interested in improving their ability to function in social interactions. Self-help approaches or brief psychotherapy (eg, cognitive-behavioral therapy [CBT]) should be the first step—and might be all that is necessary.
The opposite side of the problem. Under-recognition of clinically significant social anxiety can lead to under-treatment, which is common even in patients with a SAD diagnosis.31 Treatment options include CBT, medication, and CBT combined with medication (Table 3):
- several studies have demonstrated the short- and long-term efficacy of CBT alone for SAD
- medication alone has been efficacious in the short-term, but less efficacious than CBT in the long-term
- combined treatment also has been shown to be more efficacious than CBT or medication alone in the short-term
- there is evidence to suggest that CBT alone is more efficacious in the long-term compared with combined treatment.a
CBT is recommended as an appropriate first-line option, especially for mild and moderate SAD; it is the preferred initial treatment option of the United Kingdom’s National Institute for Health and Care Excellence (NICE). For more severe presentations (such as the presence of comorbidity) or when a patient did not respond to an adequate course of CBT, combined treatment might be an option—the goal being to taper the medication and continue CBT as a longer-term treatment. Research has shown that continuing CBT while discontinuing medication helps prevent relapse.32,33
Appropriate pharmacotherapy options include selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.34 Increasingly, benzodiazepines are considered less desirable; they are not recommended for routine use in SAD in the NICE guidelines. Those guidelines call for continuing pharmacotherapy for 6 months when a patient responds to treatment within 3 months, then discontinuing medication with the aid of CBT.
Bottom Line
The severity of anxiety and associated impairment and distress are the main variables that differentiate normal shyness and clinically significant social anxiety. Taking care not to over-pathologize normal shyness and common social anxiety concerns or underdiagnose severe, impairing social anxiety disorder has important implications for treatment—and for whether a patient needs treatment at all.
Related Resources
National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment, and treatment of social anxiety disorder. http://guidance.nice.org.uk/cg159.
• Hofmann SG, DiBartolo PM, eds. Social anxiety: clinical, developmental, and social perspectives, 2nd ed. London, United Kingdom: Academic Press; 2010.
• The Shyness Institute. www.shyness.com.
Drug Brand Names
Alprazolam • Xanax Clonazepam • Klonopin Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Phenelzine • Nardil
Sertraline • Zoloft Venlafaxine • Effexor
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Kristy L. Dalrymple, PhD, discusses, treating social anxiety disorder. Dr. Dalrymple is Staff Psychologist, Department of Psychiatry, Rhode Island Hospital, and Assistant Professor of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, Rhode Island.
Since the appearance of social anxiety disorder (SAD) in the DSM-III in 1980, research on its prevalence, characteristics, and treatment have grown (Box 11,2). In addition to the name, the definition of SAD has changed over the years; as a result, its prevalence has increased in recent cohort studies. This has led to debate over whether the experience of shyness is being over-pathologized, or whether SAD has been underdiagnosed in earlier decades. Those who argue that shyness is being over-pathologized note that it is a normal human experience that has evolutionary functions (eg, preventing engagement in harmful social relationships3). Others argue that a high degree of shyness is not beneficial in terms of evolution because it causes the individual to be shunned, so to speak, by society.4
Why worry about ‘over-pathologizing’?
The medicalization of shyness might be a reflection of Western societal values of assertiveness and gregariousness; other societies that value modesty and reticence do not over-pathologize shyness.5 It is important not to assume that someone who is shy necessarily has a “pathologic” level of social anxiety, especially because some people who are shy view that condition as a positive quality, much like sensitivity and conscientiousness.5
The broader issue of what constitutes a mental disorder arises in this debate. A “disorder” is a socially constructed label that describes a set of symptoms occurring together and its associated behaviors, not a real entity with etiological homogeneity.6 Labeling emotional problems “disordered” assumes that happiness is the natural homeostatic state, and distressing emotional states are abnormal and need to be changed.7 A diagnostic label can help improve communication and understand maladaptive behaviors; if that label is reified, however, it can lead to assumptions that the etiology, course, and treatment response are known. Proponents of the diagnostic psychiatric nomenclature have acknowledged the dangers of over-pathologizing normal experiences of living (such as fear) by way of diagnostic labeling.8
Determining when shyness becomes a clinically significant problem—what we call SAD here—demands a delicate distinction that has important implications for treatment. On one hand, if shyness is over-pathologized, persons who neither desire nor need treatment might be subjected to unnecessary and costly intervention. On the other hand, if SAD is underdiagnosed, some persons will not receive treatment that might be beneficial to them.
In this article, we review the similarities and differences between shyness and SAD, and provide recommendations for determining when shyness becomes a more clinically significant problem. We also highlight the importance of this distinction as it pertains to management, and provide suggestions for treatment approaches.
SAD: Definition, prevalence
SAD is defined as a significant fear of embarrassment or humiliation in social or performance-based situations, to a point at which the affected person often avoids these situations or endures them only with a high level of distress9 (Table 1, and Box 2). SAD can be distinguished from other anxiety disorders based on the source and content of the fear (ie, the source being social interaction or performance situations, and the content being a fear that one will show a behavior that will cause embarrassment). SAD also should be distinguished from autism spectrum disorders, in which persons have limited social communication capabilities and inadequate age-appropriate social relationships.
SAD is most highly comorbid with mood and anxiety disorders, with rates of at least 30% in clinical samples.10 The disorder also is highly comorbid with avoidant personality disorder—to a point at which it is argued that they are one and the same disorder.11
As with other psychiatric disorders, anxiety must cause significant impairment or distress. What constitutes significant impairment or distress is subjective, and the arbitrary nature of this criterion can influence estimates of the prevalence of SAD. For example, prevalence ranges as widely as 1.9% to 20.4% when different cut-offs are used for distress ratings and the number of impaired domains.12
The prevalence of SAD varies from 1 epidemiological study to another (ie, the Epidemiological Catchment Area [ECA] Study and the National Comorbidity Survey [NCS])—in part, a consequence of the differing definitions of significant impairment or distress. The ECA study assessed the clinical significance of each symptom in anxiety disorders; the NCS assessed overall clinical significance of the disorder. When the clinical significance criterion was applied at the symptom level to the NCS dataset (as was done in the ECA study), 1-year prevalence decreased by 50% (from 7.4% to 3.7%).13 The manner in which significant impairment or distress is defined (ie, conservatively or liberally) impacts whether social anxiety symptoms are classified as disordered or non-disordered.
Shyness: Definition, prevalence
Shyness often refers to 1) anxiety, inhibition, reticence, or a combination of these findings, in social and interpersonal situations, and 2) a fear of negative evaluation by others.14 It is a normal facet of personality that combines the experience of social anxiety and inhibited behavior,15 and also has been described as a stable temperament.16 Shyness is common; in the NCS study,17 26% of women and 19% of men characterized themselves as “very shy”; in the NCS Adolescent study,18 nearly 50% of adolescents self-identified as shy.
Persons who are shy tend to self-report greater social anxiety and embarrassment in social situations than non-shy persons do; they also might experience greater autonomic reactivity—especially blushing—in social or performance situations.15 Furthermore, shy persons are more likely to have axis I comorbidity and traits of introversion and neuroticism, compared with non-shy persons.14
Research suggests that temperament and behavioral inhibition are risk factors for mood and anxiety disorders, and appear to have a particularly strong relationship with SAD.19 A recent prospective study showed that shyness tends to increase steeply in toddlerhood, then stabilizes in childhood. Shyness in childhood—but not toddlerhood—is predictive of anxiety, depression, and poorer social skills in adolescence.20
A qualitative, or just quantitative, difference?
It is clear that SAD and shyness share several features—including anxiety and embarrassment—in social interactions. This raises a question: Are SAD and shyness distinct qualitatively, or do they represent points along a continuum, with SAD being an extreme form of shyness?
Continuum hypothesis. Support for the continuum hypothesis includes evidence that SAD and shyness share several features, including autonomic arousal, deficits in social skills (eg, aversion of gaze, difficulty initiating and maintaining conversation), avoidance of social situations, and fear of negative evaluation.21,22 In addition, both shyness and SAD are highly heritable,23 and mothers of shy children have a significantly higher rate of SAD than non-shy children do.24 No familial or genetic studies have compared heritability and familial aggregation in shyness and SAD.
According to the continuum hypothesis, if SAD is an extreme form of shyness, all (or nearly all) persons who have a diagnosis of SAD also would be characterized as shy. However, only approximately one-half of such persons report having been shy in childhood.17 Less than one-quarter of shy persons meet criteria for SAD.14,18 Because many persons who are shy do not meet criteria for SAD, and many who have SAD were not considered shy earlier in life, it has been suggested that this supports a qualitative distinction.
Qualitative distinctiveness. Despite having similarities, several features distinguish the experience of SAD from that of shyness. Compared with shyness, a SAD diagnosis is associated with:
- greater comorbidity
- greater severity of avoidance and impairment
- poorer quality of life.18,21,25
Studies that compared SAD, shyness without SAD, and non-shyness have shown that the shyness without SAD group more closely resembles the non-shy group than the SAD group—particularly with regard to impairment, presence of substance use, and other behavioral problems.18,25
Given the evidence, experts have concluded that shyness and a SAD diagnosis are overlapping yet different constructs that encapsulate qualitative and quantitative differences.25 There is a spectrum of shyness that ranges from a normative level to a higher level that overlaps the experience of SAD, but the 2 states represent different constructs.25
Guidance for making an assessment. Because of similarities in anxiety, embarrassment, and other symptoms in social situations, the best way to determine whether shyness crosses the line into a clinically significant problem is to assess the severity of the anxiety and associated degree of impairment and distress. More severe anxiety paired with distress about having anxiety and significant impairment in multiple areas of functioning might indicate more problematic social anxiety—a diagnosis of SAD—not just “normal” shyness.
It is important to take into account the environmental and cultural context of a patient’s distress and impairment because these features might fall within a normal range, given immediate circumstances (such as speaking in front of a large audience when one is not normally called on to do so, to a degree that does not interfere with general social functioning6).
What is considered a normative range depends on the developmental stage:
- Among children, a greater level of shyness might be considered more normative when it manifests during developmental stages in which separation anxiety appears.
- Among adolescents, a greater level of shyness might be considered normative especially during early adolescence (when social relationships become more important), and during times of transition (ie, entering high school).
- In adulthood, a greater level of normative shyness or social anxiety might be present during a major life change (eg, beginning to date again after the loss of a lengthy marriage or romantic relationship).
Assessment tools
Assessment tools can help you differentiate normal shyness from SAD. Several empirically-validated rating scales exist, including clinician-rated and self-report scales.
Liebowitz Social Anxiety Scale26 rates the severity of fear and avoidance in a variety of social interaction and performance-based situations. However, it was developed primarily as a clinician-rated scale and might be more burdensome to complete in practice. In addition, it does not provide cut-offs to indicate when more clinically significant anxiety might be likely.
Clinically Useful Social Anxiety Disorder Outcome Scale (CUSADOS)27 and Mini-Social Phobia Inventory (Mini-SPIN)28 are brief self-report scales that provide cut-offs to suggest further assessment is warranted. A cut-off score of 16 on the CUSADOS suggests the presence of SAD with 73% diagnostic efficiency.
One disadvantage to relying on a rating scale alone is the narrow focus on symptoms. Given that shyness and SAD share similar symptoms, it is necessary to assess the degree of impairment related to these symptoms to determine whether the problem is clinically significant. The overly narrow focus on symptoms utilized by the biomedical approach has been criticized for contributing to the medicalization of normal shyness.5
Diagnostic interviews, such as the Structured Clinical Interview for DSM-IV Axis I Disorders29 include sections on SAD that assess avoidance and impairment/distress associated with anxiety. Because these interviews may increase the time burden during an office visit, there are several general questions outside of a structured interview that you can ask, such as: “Has this anxiety interfered with your ability to initiate or maintain friendships? If so, how?” (Table 2). Persons with clinically significant social anxiety, rather than shyness, tend to report greater effects on their relationships and on work or school performance, as well as greater distress about having that anxiety.
Treatment approaches based on distinctions
Exercise care in making the distinction between normal shyness and dysfunctional and impairing levels of anxiety characteristic of SAD, because persons who display normal shyness but who are overdiagnosed might feel stigmatized by a diagnostic label.5 Also, overpathologizing shyness takes what is a social problem out of context, and could promote treatment strategies that might not be helpful or effective.30
Unnecessary diagnosis might lead to unnecessary treatment, such as prescribing an antidepressant or benzodiazepine. Avoiding such a situation is important, because of the side effects associated with medication and the potential for dependence and withdrawal effects with benzodiazepines.
Persons who exhibit normal shyness do not require medical treatment and, often, do not want it. However, some people may be interested in improving their ability to function in social interactions. Self-help approaches or brief psychotherapy (eg, cognitive-behavioral therapy [CBT]) should be the first step—and might be all that is necessary.
The opposite side of the problem. Under-recognition of clinically significant social anxiety can lead to under-treatment, which is common even in patients with a SAD diagnosis.31 Treatment options include CBT, medication, and CBT combined with medication (Table 3):
- several studies have demonstrated the short- and long-term efficacy of CBT alone for SAD
- medication alone has been efficacious in the short-term, but less efficacious than CBT in the long-term
- combined treatment also has been shown to be more efficacious than CBT or medication alone in the short-term
- there is evidence to suggest that CBT alone is more efficacious in the long-term compared with combined treatment.a
CBT is recommended as an appropriate first-line option, especially for mild and moderate SAD; it is the preferred initial treatment option of the United Kingdom’s National Institute for Health and Care Excellence (NICE). For more severe presentations (such as the presence of comorbidity) or when a patient did not respond to an adequate course of CBT, combined treatment might be an option—the goal being to taper the medication and continue CBT as a longer-term treatment. Research has shown that continuing CBT while discontinuing medication helps prevent relapse.32,33
Appropriate pharmacotherapy options include selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.34 Increasingly, benzodiazepines are considered less desirable; they are not recommended for routine use in SAD in the NICE guidelines. Those guidelines call for continuing pharmacotherapy for 6 months when a patient responds to treatment within 3 months, then discontinuing medication with the aid of CBT.
Bottom Line
The severity of anxiety and associated impairment and distress are the main variables that differentiate normal shyness and clinically significant social anxiety. Taking care not to over-pathologize normal shyness and common social anxiety concerns or underdiagnose severe, impairing social anxiety disorder has important implications for treatment—and for whether a patient needs treatment at all.
Related Resources
National Institute for Health and Care Excellence. Social anxiety disorder: recognition, assessment, and treatment of social anxiety disorder. http://guidance.nice.org.uk/cg159.
• Hofmann SG, DiBartolo PM, eds. Social anxiety: clinical, developmental, and social perspectives, 2nd ed. London, United Kingdom: Academic Press; 2010.
• The Shyness Institute. www.shyness.com.
Drug Brand Names
Alprazolam • Xanax Clonazepam • Klonopin Fluoxetine • Prozac
Fluvoxamine • Luvox Paroxetine • Paxil Phenelzine • Nardil
Sertraline • Zoloft Venlafaxine • Effexor
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Kristy L. Dalrymple, PhD, discusses, treating social anxiety disorder. Dr. Dalrymple is Staff Psychologist, Department of Psychiatry, Rhode Island Hospital, and Assistant Professor of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, Rhode Island.
1. Bruce LC, Coles ME, Heimberg RG. Social phobia and social anxiety disorder: effect of disorder name on recommendation for treatment. Am J Psychiatry. 2012;169(5):538.
2. Bögels SM, Alden L, Beidel DC, et al. Social anxiety disorder: questions and answers for the DSM-V. Depress Anxiety. 2010;27:168-189.
3. Wakefield JC, Horwitz AV, Schmitz MF. Are we overpathologizing the socially anxious? Social phobia from a harmful dysfunction perspective. Can J Psychiatry. 2005;50(6):317-319.
4. Campbell-Sills L, Stein MB. Justifying the diagnostic status of social phobia: a reply to Wakefield, Horwitz, and Schmitz. Can J Psychiatry. 2005;50(6):320-323.
5. Scott S. The medicalisation of shyness: from social misfits to social fitness. Sociology of Health and Illness. 2006;28(2):133-153.
6. Wakefield JC. The DSM-5 debate over the bereavement exclusion: psychiatric diagnosis and the future of empirically supported treatment. Clin Psychol Rev. 2013; 33(7):825-845.
7. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. New York, NY: Guilford Press; 2012.
8. Kupfer DJ, First MB, Regier DA, eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Dalrymple KL, Zimmerman M. Does comorbid social anxiety disorder impact the clinical presentation of principal major depressive disorder? J Affect Disord. 2007;100:241-247.
11. Dalrymple KL. Issues and controversies surrounding the diagnosis and treatment of social anxiety disorder. Expert Rev Neurother. 2012;12(8):993-1008.
12. Furmark T, Tillfors M, Everz PO, et al. Social phobia in the general population: prevalence and sociodemographic profile. Soc Psychiatry Psychiatr Epidemiol. 1999;34:416-424.
13. Narrow WE, Rae DS, Robins LN, et al. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115-123.
14. Heiser NA, Turner SM, Beidel DC. Shyness: relationship to social phobia and other psychiatric disorders. Behav Res Ther. 2003;41:209-221.
15. Hofmann SG, Moscovitch DA, Hyo-Jin K. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiology. 2006;61:134-142.
16. Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Hofmann SG, DiBartolo PM, eds. From social anxiety to social phobia: multiple perspectives. Needham Heights, MA: Allyn & Bacon; 2001:216-234.
17. Cox BJ, MacPherson PS, Enns MW. Psychiatric correlates of childhood shyness in a nationally representative sample. Behav Res Ther. 2005;43:1019-1027.
18. Burstein M, Ameli-Grillon L, Merikangas KR. Shyness versus social phobia in US youth. Pediatrics. 2011;128:917-925.
19. Hirshfeld-Becker DR, Micco J, Henin A, et al. Behavioral inhibition. Depress Anxiety. 2008;25:357-367.
20. Karevold E, Ystrom E, Coplan RJ, et al. A prospective longitudinal study of shyness from infancy to adolescence: stability, age-related changes, and prediction of socio-emotional functioning. J Abnorm Child Psychol. 2012; 40:1167-1177.
21. Chavira DA, Stein MB, Malcarne VL. Scrutinizing the relationship between shyness and social phobia. J Anxiety Disord. 2002;16:585-598.
22. Schneier FR, Blanco C, Antia SX, et al. The social anxiety spectrum. Psychiatr Clin N Am. 2002;25:757-774.
23. Stein MB, Chavira DA, Jang KL. Bringing up bashful baby: developmental pathways to social phobia. Psychiatr Clin N Am. 2001;24:797-818.
24. Cooper PJ, Eke M. Childhood shyness and maternal social phobia: a community study. Br J Psychiatry. 1999;174:439-443.
25. Heiser NA, Turner SM, Beidel DC, et al. Differentiating social phobia from shyness. J Anxiety Disord. 2009;23:469-476.
26. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141-173.
27. Dalrymple, KL, Martinez J, Tepe E, et al. A clinically useful social anxiety disorder outcome scale. Compr Psychiatry. 2013;54(7):758-765.
28. Connor KM, Kobak KA, Churchill LE, et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14(2):137-140.
29. First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997.
30. Conrad P. Medicalization and social control. Ann Rev Sociology. 1992;18:209-232.
31. Zimmerman M, Chelminski I. Clinician recognition of anxiety disorders in depressed outpatients. J Psychiatr Res. 2003;37:325-333.
32. Gelernter CS, Uhde TW, Cimbolic P, et al. Cognitive-behavioral and pharmacological treatments of social phobia: a controlled study. Arch Gen Psychiatry. 1991;48:938-945.
33. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(suppl 5):34-41.
34. Blanco C, Bragdon LB, Schneier FR, et al. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol. 2013;16:235-249.
1. Bruce LC, Coles ME, Heimberg RG. Social phobia and social anxiety disorder: effect of disorder name on recommendation for treatment. Am J Psychiatry. 2012;169(5):538.
2. Bögels SM, Alden L, Beidel DC, et al. Social anxiety disorder: questions and answers for the DSM-V. Depress Anxiety. 2010;27:168-189.
3. Wakefield JC, Horwitz AV, Schmitz MF. Are we overpathologizing the socially anxious? Social phobia from a harmful dysfunction perspective. Can J Psychiatry. 2005;50(6):317-319.
4. Campbell-Sills L, Stein MB. Justifying the diagnostic status of social phobia: a reply to Wakefield, Horwitz, and Schmitz. Can J Psychiatry. 2005;50(6):320-323.
5. Scott S. The medicalisation of shyness: from social misfits to social fitness. Sociology of Health and Illness. 2006;28(2):133-153.
6. Wakefield JC. The DSM-5 debate over the bereavement exclusion: psychiatric diagnosis and the future of empirically supported treatment. Clin Psychol Rev. 2013; 33(7):825-845.
7. Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: the process and practice of mindful change. New York, NY: Guilford Press; 2012.
8. Kupfer DJ, First MB, Regier DA, eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Dalrymple KL, Zimmerman M. Does comorbid social anxiety disorder impact the clinical presentation of principal major depressive disorder? J Affect Disord. 2007;100:241-247.
11. Dalrymple KL. Issues and controversies surrounding the diagnosis and treatment of social anxiety disorder. Expert Rev Neurother. 2012;12(8):993-1008.
12. Furmark T, Tillfors M, Everz PO, et al. Social phobia in the general population: prevalence and sociodemographic profile. Soc Psychiatry Psychiatr Epidemiol. 1999;34:416-424.
13. Narrow WE, Rae DS, Robins LN, et al. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry. 2002;59:115-123.
14. Heiser NA, Turner SM, Beidel DC. Shyness: relationship to social phobia and other psychiatric disorders. Behav Res Ther. 2003;41:209-221.
15. Hofmann SG, Moscovitch DA, Hyo-Jin K. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. Int J Psychophysiology. 2006;61:134-142.
16. Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Hofmann SG, DiBartolo PM, eds. From social anxiety to social phobia: multiple perspectives. Needham Heights, MA: Allyn & Bacon; 2001:216-234.
17. Cox BJ, MacPherson PS, Enns MW. Psychiatric correlates of childhood shyness in a nationally representative sample. Behav Res Ther. 2005;43:1019-1027.
18. Burstein M, Ameli-Grillon L, Merikangas KR. Shyness versus social phobia in US youth. Pediatrics. 2011;128:917-925.
19. Hirshfeld-Becker DR, Micco J, Henin A, et al. Behavioral inhibition. Depress Anxiety. 2008;25:357-367.
20. Karevold E, Ystrom E, Coplan RJ, et al. A prospective longitudinal study of shyness from infancy to adolescence: stability, age-related changes, and prediction of socio-emotional functioning. J Abnorm Child Psychol. 2012; 40:1167-1177.
21. Chavira DA, Stein MB, Malcarne VL. Scrutinizing the relationship between shyness and social phobia. J Anxiety Disord. 2002;16:585-598.
22. Schneier FR, Blanco C, Antia SX, et al. The social anxiety spectrum. Psychiatr Clin N Am. 2002;25:757-774.
23. Stein MB, Chavira DA, Jang KL. Bringing up bashful baby: developmental pathways to social phobia. Psychiatr Clin N Am. 2001;24:797-818.
24. Cooper PJ, Eke M. Childhood shyness and maternal social phobia: a community study. Br J Psychiatry. 1999;174:439-443.
25. Heiser NA, Turner SM, Beidel DC, et al. Differentiating social phobia from shyness. J Anxiety Disord. 2009;23:469-476.
26. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141-173.
27. Dalrymple, KL, Martinez J, Tepe E, et al. A clinically useful social anxiety disorder outcome scale. Compr Psychiatry. 2013;54(7):758-765.
28. Connor KM, Kobak KA, Churchill LE, et al. Mini-SPIN: a brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14(2):137-140.
29. First MB, Gibbon M, Spitzer RL, et al. Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II). Washington, DC: American Psychiatric Press, Inc; 1997.
30. Conrad P. Medicalization and social control. Ann Rev Sociology. 1992;18:209-232.
31. Zimmerman M, Chelminski I. Clinician recognition of anxiety disorders in depressed outpatients. J Psychiatr Res. 2003;37:325-333.
32. Gelernter CS, Uhde TW, Cimbolic P, et al. Cognitive-behavioral and pharmacological treatments of social phobia: a controlled study. Arch Gen Psychiatry. 1991;48:938-945.
33. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry. 2004;65(suppl 5):34-41.
34. Blanco C, Bragdon LB, Schneier FR, et al. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol. 2013;16:235-249.
Overcoming medication nonadherence in schizophrenia: Strategies that can reduce harm
Medication nonadherence is a common problem when treating patients with schizophrenia that can worsen prognosis and lead to sub-optimal treatment outcomes. In this article, we discuss common reasons for nonadherence and describe evidence-based treatments intended to increase adherence and improve outcomes (Box).1-6
Common reasons for nonadherence
The primary predictor of future nonadherence is a history of nonadherence. It is important to understand patients’ reasons for nonadherence so that practical and evidence-based solutions can be implemented into the treatment plans of individual patients.
The 2009 Expert Consensus Guidelines on Adherence Problems in Patients with Serious and Persistent Mental Illness divided variables related to nonadherence into 3 categories:
- those that lie within the patient (intrinsic)
- those that are related to the patient’s relationship with healthcare providers, family, or caregivers (extrinsic)
- those that are related to the healthcare delivery system (extrinsic).7
Among intrinsic variables, studies have shown a correlation between nonadherence and education level, lower socioeconomic status, homelessness, and male sex.7 (The Expert Consensus Guidelines considered homelessness to be an intrinsic factor because it was used as a demographic variable in the studies.)
Cognitive and negative symptoms associated with schizophrenia are an intrinsic risk factor for nonadherence because patients might not remember when or how to take medication.7 In a study by Freudenreich and co-workers8 of 81 outpatients who had a diagnosis of schizophrenia, the presence of negative symptoms predicted a negative attitude toward psychotropic medications. Poor insight might be the result of cognitive dysfunction associated with schizophrenia, and often is due to a lack of awareness of the importance of taking medications.
Limited insight into the need for treatment can be problematic early in the course of the illness when it may be directly related to positive symptoms. Perkins and colleagues9 demonstrated that patients recovering from a first psychotic episode who had limited insight into their illness and lacked desire to seek treatment were less adherent with medication. In another study, 5% of psychiatrists surveyed thought that many of their patients with schizophrenia were nonadherent because those patients did not believe that medications were effective or useful.10
Comorbid substance abuse disorders can contribute to medication nonadherence. In an analysis of 6,731 patients with schizophrenia, Novick and co-workers reported that alcohol dependence and substance abuse in the previous month predicted medication nonadherence.11 Hunt and colleagues demonstrated that, among 99 nonadherent patients with schizophrenia, time to first readmission was shorter for patients with comorbid substance abuse disorders compared with patients who had a diagnosis of schizophrenia only. Over the 4-year study period, the 28 patients who had a dual diagnosis (schizophrenia and substance abuse) accounted for 57% of all hospital readmissions.12
Several variables that affect medication adherence are related to the patient’s relationship with healthcare providers, family, caregivers, and the service delivery system.7 These include:
- the perceived stigma of being given a diagnosis of a serious mental illness
- adverse effects related to medications
- poor social and family support
- difficulty gaining access to mental health services.7,10
Societal stigma associated with seeking treatment from a mental health professional may contribute to nonadherence in some patients. In 1 study,13 36% of people surveyed would not want to work closely with a person who has a serious mental illness.
Adverse effects contribute significantly to nonadherence
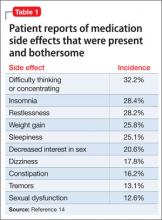
Limited treatment options (which may be expensive) can make it difficult to manage the adverse effects of antipsychotics. In a cross-sectional survey of 876 patients, investigators reported that: 1) <50% of patients were adherent with medication, and 2) 80% experienced ≥1 side effect that was reported to be “somewhat bothersome” in self-ratings (Table 1).14 Extrapyramidal symptoms (EPS) and agitation were most strongly associated with nonadherence; weight gain, akathisia, and sexual dysfunction also were associated with nonadherence.14 This study did not distinguish adverse effects associated with first-generation antipsychotics (FGAs) from those associated with second-generation antipsychotics (SGAs), even though 71.7% of patients studied were taking an SGA.
A meta-analysis by Leucht and co-workers15 compared 15 antipsychotics (the FGAs haloperidol and chlorpromazine and 13 SGAs) for efficacy and tolerability in schizophrenia. Haloperidol had the highest rate of discontinuation for any
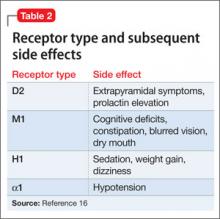
Antipsychotic binding affinities to dopamine 2 (D2), serotonin 2A (5-HT2A), histamine (H1), and other receptors have an impact on a medication’s side-effect profile. Because of individual patient characteristics, you might be faced with choosing a medication that has a lower risk of EPS but a higher risk of weight gain and metabolic complications—or the inverse. Understanding binding affinities, side-effect profiles, and how to minimize or utilize adverse effects (ie, giving a drug that is approved to treat schizophrenia and is associated with weight gain to a patient with schizophrenia who has lost weight) may lead to greater adherence (Table 216 and Table 317).
Adequate support is essential
The therapeutic alliance plays a key role in patients’ attitudes toward taking medication. Magura and colleagues18 found that one-third of psychiatric patients (13% of whom had a diagnosis of schizophrenia) reported that their psychiatrist did not spend enough time with them explaining side effects, and felt “rushed.”
Patients with schizophrenia often require access to social support systems provided by family members, friends, and community agencies that provide case management and attendant care services. Patients who are adherent to medication tend to have greater perceived family involvement in medication treatment, and tend to have been raised in a family that had more of a positive attitude toward medication.19
In our practice, we have observed that recent state and federal budget cuts have resulted in patients having greater difficulty gaining access to case management and attendant care services, which then leads to increased rates of medication nonadherence. Be aware that variables such as limited office hours, financial hardship, and cultural and language barriers can compromise a patient’s ability to seek and continue care.
In the following section, we lay out techniques for improving adherence in patients with schizophrenia.
Employ general and specific strategies to boost adherence
How can you raise medication adherence concerns with patients, keeping in mind that they often overestimate their adherence?
Ask. Some clinicians ask questions such as “Are you taking your medication?”, although a more effective approach might be to ask how the patient is taking his (her) medication. Asking questions such as “When do you take your medication?” and “In the past week, how many doses do you think you missed?” might be more effective ways to inquire about adherence.7
The Expert Consensus Guidelines recommend asking patients about medication adherence monthly for those who are stable, doing well, and believed to be adherent. For those who are new to a practice or who are not doing well, inquire about medication adherence at least weekly.7
In our practice, patients who are unstable but do not require inpatient hospitalization typically are seen more often in the clinic, or are referred to intensive outpatient or partial hospitalization programs. If an unstable patient is unable to come in for more frequent appointments, we arrange phone conferences between her and her provider. If a patient is not doing well and has a case manager, we often ask that case manager to visit the patient, in person, more often than he (she) would otherwise.
Take a nonjudgmental approach when raising these issues with patients. Questions such as “We all forget to take our medication sometimes; do you?” help to normalize nonadherence, and improve the therapeutic alliance, and might result in the patient being more honest with the clinician.7 Because patients may be apprehensive about discussing adverse events, clinicians must be proactive about improving the therapeutic alliance and making patients feel comfortable when discussing sensitive topics. Clinicians should try to convey the idea that, although adherence is a concern, so is quality of life. A clinicians’ willingness to take a flexible approach that is nonpunitive nor authoritarian can aid the therapeutic alliance and improve overall adherence.
Be sensitive to financial, cultural, and language variables that can affect access to care. The Expert Consensus Guidelines recommend asking patients if they can afford their medication. In our practice, we have seen patients with schizophrenia discharged from the hospital only to be readmitted 1 month later because they could not afford to fill their prescriptions.
It is important to have translation services available, in person or by phone, for patients who do not speak English. Furthermore, it is important to understand the limitations that your practice might place on access to care. Ask patients if they have ever had trouble making an appointment when they needed to be seen, or if they called the office with a question and did not receive an answer in a timely fashion; doing so allows you to assess the practice’s ability to meet patients’ needs and helps you build a therapeutic alliance.
Make objective assessments. It is important for practitioners to not base their assessment of medication adherence solely on subjective findings. Asking patients to bring in their medication bottles for pill counts and checking with the patients’ pharmacies for information about refill frequency can provide some objective data. Electronic monitoring systems use microprocessors inserted into bottle caps to record the occurrence and timing of each bottle opening. Studies show that these electronic monitoring systems are the gold standard for determining medication adherence and could be used in cases where it is unclear if the patient is taking his (her) medication.7,20 Such systems have successfully monitored medication adherence in clinical trials, but their use in clinical practice is complicated by ethical and legal considerations and cost issues.
Simplify the regimen. Using medications with once-daily dosing, for example, can help improve adherence. Pfeiffer and co-workers21 found that patients whose medication regimens were changed from once daily to more than once daily experienced a decrease in medication adherence. Conversely, a decrease in dosing frequency was significantly associated with improved adherence. More than once-daily dosing was only weakly associated with poorer adherence among patients already on a stable regimen.
Discussing positive and negative aspects of past medication trials with a patient and inquiring if she prefers a specific medication can be an effective way to build the therapeutic relationship and help with adherence.
Direct patients to psychosocial interventions. These can be broadly classified as:
- educational approaches
- group therapy approaches
- family interventions
- cognitive treatments
- combination approaches.
Psychoeducational approaches have limited effect on improving adherence when delivered to individual patients. However, 1 study showed that psychoeducation was effective at improving adherence when extended to include the patient’s family.22
Motivational interviewing techniques, behavioral approaches, and family interventions are effective at increasing medication adherence. One study looked at the value of training a patient-identified informant to supervise and administer medication. This person, usually a family member or close support, was responsible for obtaining medication from the pharmacy, administering the medication, and recording adherence. After 1 year, 67% of patients who used an informant were adherent, compared with only 45% in the group that did not have informant support.22 Case managers, attendant care workers, home health nurses, and assertive community treatment (ACT) teams also can participate in this manner; it is important, therefore, for you to be aware of the resources available in your community and to understand your role as patient advocate.
Substance abuse is a strong risk factor for nonadherence among patients with schizophrenia,18 which makes it important to assess patients for substance use and encourage those who do abuse to seek treatment. Although 1 study showed no correlation between Alcoholics Anonymous (AA) attendance and medication adherence,12 many AA and Narcotics Anonymous groups do not discuss psychiatric medications during group meetings. Magura and colleagues encouraged the use of “dual focus” groups that involve mental health professionals and addiction treatment specialists discussing mental health and substance abuse issues at the same setting.18
Prescribe long-acting injectable antipsychotics. Typically, long-acting injectable antipsychotics (LAIs) are reserved for patients who have a history of nonadherence. In a small study (N = 97) comparing LAI risperidone and oral risperidone or oral haloperidol, patients treated with an LAI had significantly fewer all-cause discontinuations (26.0%, compared with 70.2%) at 24 months.23 The Adherence to Treatment and Therapeutic Strategies in Schizophrenic Patients study examined 1,848 patients with schizophrenia and reached similar findings regarding LAI antipsychotics.24 (Note: Aripiprazole, fluphenazine, haloperidol, olanzapine, and paliperidone also are available in an LAI formulation.)
Bottom Line
Antipsychotic nonadherence in schizophrenia is a major problem for patients, families, and society. Being able to identify patients at risk for nonadherence, understanding the reasons for their nonadherence, and seeking practical solutions to the problem are all the responsibility of the treating physician. Psychoeducation, addressing substance abuse, modifying dosing, and using long-acting injectable antipsychotics may help improve adherence.
Related Resources
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Clin Psychiatry. 2009;70(suppl 4):1-46.
- National Alliance on Mental Illness. www.nami.org.
- Assertive Community Treatment (ACT) Organization. www.actassociation.org.
Drug Brand Names
Aripiprazole • Abilify Chlorpromazine • Thorazine Clozapine • Clozaril Fluphenazine • Permitil Haloperidol • Haldol Iloperidone • Fanapt Olanzapine • Zyprexa Paliperidone • Invega Perphenazine • Trilafon Quetiapine • Seroquel Risperidone • Risperdal Ziprasidone • Geodon
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Dr. McKnight reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23:2305-2312.
2. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892-909.
3. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
4. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
5. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemial Drug Saf. 2003;12(5):423-424.
6. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891.
7. Velligan D, Weiden P, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness. J Psychiatr Pract. 2010;16(1):34-45.
8. Freudenreich O, Cather C, Evins A, et al. Attitudes of schizophrenia outpatients toward psychiatric medications: relationship to clinical variables and insight. J Clin Psychiatry. 2004;65(10):1372-1376.
9. Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83(1):53-63.
10. Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence. 2013;7:121-132.
11. Novick D, Haro J, Suarez D, et al. Predictors and clinical consequences of nonadherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2-3):109-113.
12. Hunt GE, Bergen J, Bashir M, et al. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival four years after a relapse. Schizophr Res. 2002;54(3):253-264.
13. McGinty EE, Webster DW, Barry CL. Effects of news media messages about mass shootings on attitudes toward persons with serious mental illness and public support for gun control policies. Am J Psychiatry. 2013;170(5):494-501.
14. DiBonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;1382(9896): 951-962.
16. Robinson DS. Antipsychotics: pharmacology and clinical decision making. Primary Psychiatry. 2007;14(10):23-25.
17. Robinson D, Correll CU, Kane JM, et al. Practical dosing strategies in the treatment of schizophrenia. CNS Spectr. 2010;15:4(suppl 6):1-16.
18. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
19. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1-3):176-181.
20. Byerly M, Nakonezny P, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30:437-452.
21. Pfeiffer PN, Ganoczy D, Valenstein M. Dosing frequency and adherence to antipsychotic medications. Psychiatr Serv. 2008;59(10):1207-1210.
22. Farooq S, Nazar Z, Irfan M, et al. Schizophrenia medication adherence in a resource-poor setting: randomized controlled trial of supervised treatment in out-patients for schizophrenia (STOPS). Br J Psychiatry. 2011;199(6):467-472.
23. Emsley R, Oosthuizen P, Koen L, et al. Oral vs injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
24. Gutierrez-Casares JR, Canãs F, Rodriguez-Morales A, et al. Adherence to treatment and therapeutic strategies in schizophrenic patients: the ADHERE study. CNS Spectr. 2010;15(5):327-337.
Medication nonadherence is a common problem when treating patients with schizophrenia that can worsen prognosis and lead to sub-optimal treatment outcomes. In this article, we discuss common reasons for nonadherence and describe evidence-based treatments intended to increase adherence and improve outcomes (Box).1-6
Common reasons for nonadherence
The primary predictor of future nonadherence is a history of nonadherence. It is important to understand patients’ reasons for nonadherence so that practical and evidence-based solutions can be implemented into the treatment plans of individual patients.
The 2009 Expert Consensus Guidelines on Adherence Problems in Patients with Serious and Persistent Mental Illness divided variables related to nonadherence into 3 categories:
- those that lie within the patient (intrinsic)
- those that are related to the patient’s relationship with healthcare providers, family, or caregivers (extrinsic)
- those that are related to the healthcare delivery system (extrinsic).7
Among intrinsic variables, studies have shown a correlation between nonadherence and education level, lower socioeconomic status, homelessness, and male sex.7 (The Expert Consensus Guidelines considered homelessness to be an intrinsic factor because it was used as a demographic variable in the studies.)
Cognitive and negative symptoms associated with schizophrenia are an intrinsic risk factor for nonadherence because patients might not remember when or how to take medication.7 In a study by Freudenreich and co-workers8 of 81 outpatients who had a diagnosis of schizophrenia, the presence of negative symptoms predicted a negative attitude toward psychotropic medications. Poor insight might be the result of cognitive dysfunction associated with schizophrenia, and often is due to a lack of awareness of the importance of taking medications.
Limited insight into the need for treatment can be problematic early in the course of the illness when it may be directly related to positive symptoms. Perkins and colleagues9 demonstrated that patients recovering from a first psychotic episode who had limited insight into their illness and lacked desire to seek treatment were less adherent with medication. In another study, 5% of psychiatrists surveyed thought that many of their patients with schizophrenia were nonadherent because those patients did not believe that medications were effective or useful.10
Comorbid substance abuse disorders can contribute to medication nonadherence. In an analysis of 6,731 patients with schizophrenia, Novick and co-workers reported that alcohol dependence and substance abuse in the previous month predicted medication nonadherence.11 Hunt and colleagues demonstrated that, among 99 nonadherent patients with schizophrenia, time to first readmission was shorter for patients with comorbid substance abuse disorders compared with patients who had a diagnosis of schizophrenia only. Over the 4-year study period, the 28 patients who had a dual diagnosis (schizophrenia and substance abuse) accounted for 57% of all hospital readmissions.12
Several variables that affect medication adherence are related to the patient’s relationship with healthcare providers, family, caregivers, and the service delivery system.7 These include:
- the perceived stigma of being given a diagnosis of a serious mental illness
- adverse effects related to medications
- poor social and family support
- difficulty gaining access to mental health services.7,10
Societal stigma associated with seeking treatment from a mental health professional may contribute to nonadherence in some patients. In 1 study,13 36% of people surveyed would not want to work closely with a person who has a serious mental illness.
Adverse effects contribute significantly to nonadherence
Limited treatment options (which may be expensive) can make it difficult to manage the adverse effects of antipsychotics. In a cross-sectional survey of 876 patients, investigators reported that: 1) <50% of patients were adherent with medication, and 2) 80% experienced ≥1 side effect that was reported to be “somewhat bothersome” in self-ratings (Table 1).14 Extrapyramidal symptoms (EPS) and agitation were most strongly associated with nonadherence; weight gain, akathisia, and sexual dysfunction also were associated with nonadherence.14 This study did not distinguish adverse effects associated with first-generation antipsychotics (FGAs) from those associated with second-generation antipsychotics (SGAs), even though 71.7% of patients studied were taking an SGA.
A meta-analysis by Leucht and co-workers15 compared 15 antipsychotics (the FGAs haloperidol and chlorpromazine and 13 SGAs) for efficacy and tolerability in schizophrenia. Haloperidol had the highest rate of discontinuation for any
Antipsychotic binding affinities to dopamine 2 (D2), serotonin 2A (5-HT2A), histamine (H1), and other receptors have an impact on a medication’s side-effect profile. Because of individual patient characteristics, you might be faced with choosing a medication that has a lower risk of EPS but a higher risk of weight gain and metabolic complications—or the inverse. Understanding binding affinities, side-effect profiles, and how to minimize or utilize adverse effects (ie, giving a drug that is approved to treat schizophrenia and is associated with weight gain to a patient with schizophrenia who has lost weight) may lead to greater adherence (Table 216 and Table 317).
Adequate support is essential
The therapeutic alliance plays a key role in patients’ attitudes toward taking medication. Magura and colleagues18 found that one-third of psychiatric patients (13% of whom had a diagnosis of schizophrenia) reported that their psychiatrist did not spend enough time with them explaining side effects, and felt “rushed.”
Patients with schizophrenia often require access to social support systems provided by family members, friends, and community agencies that provide case management and attendant care services. Patients who are adherent to medication tend to have greater perceived family involvement in medication treatment, and tend to have been raised in a family that had more of a positive attitude toward medication.19
In our practice, we have observed that recent state and federal budget cuts have resulted in patients having greater difficulty gaining access to case management and attendant care services, which then leads to increased rates of medication nonadherence. Be aware that variables such as limited office hours, financial hardship, and cultural and language barriers can compromise a patient’s ability to seek and continue care.
In the following section, we lay out techniques for improving adherence in patients with schizophrenia.
Employ general and specific strategies to boost adherence
How can you raise medication adherence concerns with patients, keeping in mind that they often overestimate their adherence?
Ask. Some clinicians ask questions such as “Are you taking your medication?”, although a more effective approach might be to ask how the patient is taking his (her) medication. Asking questions such as “When do you take your medication?” and “In the past week, how many doses do you think you missed?” might be more effective ways to inquire about adherence.7
The Expert Consensus Guidelines recommend asking patients about medication adherence monthly for those who are stable, doing well, and believed to be adherent. For those who are new to a practice or who are not doing well, inquire about medication adherence at least weekly.7
In our practice, patients who are unstable but do not require inpatient hospitalization typically are seen more often in the clinic, or are referred to intensive outpatient or partial hospitalization programs. If an unstable patient is unable to come in for more frequent appointments, we arrange phone conferences between her and her provider. If a patient is not doing well and has a case manager, we often ask that case manager to visit the patient, in person, more often than he (she) would otherwise.
Take a nonjudgmental approach when raising these issues with patients. Questions such as “We all forget to take our medication sometimes; do you?” help to normalize nonadherence, and improve the therapeutic alliance, and might result in the patient being more honest with the clinician.7 Because patients may be apprehensive about discussing adverse events, clinicians must be proactive about improving the therapeutic alliance and making patients feel comfortable when discussing sensitive topics. Clinicians should try to convey the idea that, although adherence is a concern, so is quality of life. A clinicians’ willingness to take a flexible approach that is nonpunitive nor authoritarian can aid the therapeutic alliance and improve overall adherence.
Be sensitive to financial, cultural, and language variables that can affect access to care. The Expert Consensus Guidelines recommend asking patients if they can afford their medication. In our practice, we have seen patients with schizophrenia discharged from the hospital only to be readmitted 1 month later because they could not afford to fill their prescriptions.
It is important to have translation services available, in person or by phone, for patients who do not speak English. Furthermore, it is important to understand the limitations that your practice might place on access to care. Ask patients if they have ever had trouble making an appointment when they needed to be seen, or if they called the office with a question and did not receive an answer in a timely fashion; doing so allows you to assess the practice’s ability to meet patients’ needs and helps you build a therapeutic alliance.
Make objective assessments. It is important for practitioners to not base their assessment of medication adherence solely on subjective findings. Asking patients to bring in their medication bottles for pill counts and checking with the patients’ pharmacies for information about refill frequency can provide some objective data. Electronic monitoring systems use microprocessors inserted into bottle caps to record the occurrence and timing of each bottle opening. Studies show that these electronic monitoring systems are the gold standard for determining medication adherence and could be used in cases where it is unclear if the patient is taking his (her) medication.7,20 Such systems have successfully monitored medication adherence in clinical trials, but their use in clinical practice is complicated by ethical and legal considerations and cost issues.
Simplify the regimen. Using medications with once-daily dosing, for example, can help improve adherence. Pfeiffer and co-workers21 found that patients whose medication regimens were changed from once daily to more than once daily experienced a decrease in medication adherence. Conversely, a decrease in dosing frequency was significantly associated with improved adherence. More than once-daily dosing was only weakly associated with poorer adherence among patients already on a stable regimen.
Discussing positive and negative aspects of past medication trials with a patient and inquiring if she prefers a specific medication can be an effective way to build the therapeutic relationship and help with adherence.
Direct patients to psychosocial interventions. These can be broadly classified as:
- educational approaches
- group therapy approaches
- family interventions
- cognitive treatments
- combination approaches.
Psychoeducational approaches have limited effect on improving adherence when delivered to individual patients. However, 1 study showed that psychoeducation was effective at improving adherence when extended to include the patient’s family.22
Motivational interviewing techniques, behavioral approaches, and family interventions are effective at increasing medication adherence. One study looked at the value of training a patient-identified informant to supervise and administer medication. This person, usually a family member or close support, was responsible for obtaining medication from the pharmacy, administering the medication, and recording adherence. After 1 year, 67% of patients who used an informant were adherent, compared with only 45% in the group that did not have informant support.22 Case managers, attendant care workers, home health nurses, and assertive community treatment (ACT) teams also can participate in this manner; it is important, therefore, for you to be aware of the resources available in your community and to understand your role as patient advocate.
Substance abuse is a strong risk factor for nonadherence among patients with schizophrenia,18 which makes it important to assess patients for substance use and encourage those who do abuse to seek treatment. Although 1 study showed no correlation between Alcoholics Anonymous (AA) attendance and medication adherence,12 many AA and Narcotics Anonymous groups do not discuss psychiatric medications during group meetings. Magura and colleagues encouraged the use of “dual focus” groups that involve mental health professionals and addiction treatment specialists discussing mental health and substance abuse issues at the same setting.18
Prescribe long-acting injectable antipsychotics. Typically, long-acting injectable antipsychotics (LAIs) are reserved for patients who have a history of nonadherence. In a small study (N = 97) comparing LAI risperidone and oral risperidone or oral haloperidol, patients treated with an LAI had significantly fewer all-cause discontinuations (26.0%, compared with 70.2%) at 24 months.23 The Adherence to Treatment and Therapeutic Strategies in Schizophrenic Patients study examined 1,848 patients with schizophrenia and reached similar findings regarding LAI antipsychotics.24 (Note: Aripiprazole, fluphenazine, haloperidol, olanzapine, and paliperidone also are available in an LAI formulation.)
Bottom Line
Antipsychotic nonadherence in schizophrenia is a major problem for patients, families, and society. Being able to identify patients at risk for nonadherence, understanding the reasons for their nonadherence, and seeking practical solutions to the problem are all the responsibility of the treating physician. Psychoeducation, addressing substance abuse, modifying dosing, and using long-acting injectable antipsychotics may help improve adherence.
Related Resources
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Clin Psychiatry. 2009;70(suppl 4):1-46.
- National Alliance on Mental Illness. www.nami.org.
- Assertive Community Treatment (ACT) Organization. www.actassociation.org.
Drug Brand Names
Aripiprazole • Abilify Chlorpromazine • Thorazine Clozapine • Clozaril Fluphenazine • Permitil Haloperidol • Haldol Iloperidone • Fanapt Olanzapine • Zyprexa Paliperidone • Invega Perphenazine • Trilafon Quetiapine • Seroquel Risperidone • Risperdal Ziprasidone • Geodon
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Dr. McKnight reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Medication nonadherence is a common problem when treating patients with schizophrenia that can worsen prognosis and lead to sub-optimal treatment outcomes. In this article, we discuss common reasons for nonadherence and describe evidence-based treatments intended to increase adherence and improve outcomes (Box).1-6
Common reasons for nonadherence
The primary predictor of future nonadherence is a history of nonadherence. It is important to understand patients’ reasons for nonadherence so that practical and evidence-based solutions can be implemented into the treatment plans of individual patients.
The 2009 Expert Consensus Guidelines on Adherence Problems in Patients with Serious and Persistent Mental Illness divided variables related to nonadherence into 3 categories:
- those that lie within the patient (intrinsic)
- those that are related to the patient’s relationship with healthcare providers, family, or caregivers (extrinsic)
- those that are related to the healthcare delivery system (extrinsic).7
Among intrinsic variables, studies have shown a correlation between nonadherence and education level, lower socioeconomic status, homelessness, and male sex.7 (The Expert Consensus Guidelines considered homelessness to be an intrinsic factor because it was used as a demographic variable in the studies.)
Cognitive and negative symptoms associated with schizophrenia are an intrinsic risk factor for nonadherence because patients might not remember when or how to take medication.7 In a study by Freudenreich and co-workers8 of 81 outpatients who had a diagnosis of schizophrenia, the presence of negative symptoms predicted a negative attitude toward psychotropic medications. Poor insight might be the result of cognitive dysfunction associated with schizophrenia, and often is due to a lack of awareness of the importance of taking medications.
Limited insight into the need for treatment can be problematic early in the course of the illness when it may be directly related to positive symptoms. Perkins and colleagues9 demonstrated that patients recovering from a first psychotic episode who had limited insight into their illness and lacked desire to seek treatment were less adherent with medication. In another study, 5% of psychiatrists surveyed thought that many of their patients with schizophrenia were nonadherent because those patients did not believe that medications were effective or useful.10
Comorbid substance abuse disorders can contribute to medication nonadherence. In an analysis of 6,731 patients with schizophrenia, Novick and co-workers reported that alcohol dependence and substance abuse in the previous month predicted medication nonadherence.11 Hunt and colleagues demonstrated that, among 99 nonadherent patients with schizophrenia, time to first readmission was shorter for patients with comorbid substance abuse disorders compared with patients who had a diagnosis of schizophrenia only. Over the 4-year study period, the 28 patients who had a dual diagnosis (schizophrenia and substance abuse) accounted for 57% of all hospital readmissions.12
Several variables that affect medication adherence are related to the patient’s relationship with healthcare providers, family, caregivers, and the service delivery system.7 These include:
- the perceived stigma of being given a diagnosis of a serious mental illness
- adverse effects related to medications
- poor social and family support
- difficulty gaining access to mental health services.7,10
Societal stigma associated with seeking treatment from a mental health professional may contribute to nonadherence in some patients. In 1 study,13 36% of people surveyed would not want to work closely with a person who has a serious mental illness.
Adverse effects contribute significantly to nonadherence
Limited treatment options (which may be expensive) can make it difficult to manage the adverse effects of antipsychotics. In a cross-sectional survey of 876 patients, investigators reported that: 1) <50% of patients were adherent with medication, and 2) 80% experienced ≥1 side effect that was reported to be “somewhat bothersome” in self-ratings (Table 1).14 Extrapyramidal symptoms (EPS) and agitation were most strongly associated with nonadherence; weight gain, akathisia, and sexual dysfunction also were associated with nonadherence.14 This study did not distinguish adverse effects associated with first-generation antipsychotics (FGAs) from those associated with second-generation antipsychotics (SGAs), even though 71.7% of patients studied were taking an SGA.
A meta-analysis by Leucht and co-workers15 compared 15 antipsychotics (the FGAs haloperidol and chlorpromazine and 13 SGAs) for efficacy and tolerability in schizophrenia. Haloperidol had the highest rate of discontinuation for any
Antipsychotic binding affinities to dopamine 2 (D2), serotonin 2A (5-HT2A), histamine (H1), and other receptors have an impact on a medication’s side-effect profile. Because of individual patient characteristics, you might be faced with choosing a medication that has a lower risk of EPS but a higher risk of weight gain and metabolic complications—or the inverse. Understanding binding affinities, side-effect profiles, and how to minimize or utilize adverse effects (ie, giving a drug that is approved to treat schizophrenia and is associated with weight gain to a patient with schizophrenia who has lost weight) may lead to greater adherence (Table 216 and Table 317).
Adequate support is essential
The therapeutic alliance plays a key role in patients’ attitudes toward taking medication. Magura and colleagues18 found that one-third of psychiatric patients (13% of whom had a diagnosis of schizophrenia) reported that their psychiatrist did not spend enough time with them explaining side effects, and felt “rushed.”
Patients with schizophrenia often require access to social support systems provided by family members, friends, and community agencies that provide case management and attendant care services. Patients who are adherent to medication tend to have greater perceived family involvement in medication treatment, and tend to have been raised in a family that had more of a positive attitude toward medication.19
In our practice, we have observed that recent state and federal budget cuts have resulted in patients having greater difficulty gaining access to case management and attendant care services, which then leads to increased rates of medication nonadherence. Be aware that variables such as limited office hours, financial hardship, and cultural and language barriers can compromise a patient’s ability to seek and continue care.
In the following section, we lay out techniques for improving adherence in patients with schizophrenia.
Employ general and specific strategies to boost adherence
How can you raise medication adherence concerns with patients, keeping in mind that they often overestimate their adherence?
Ask. Some clinicians ask questions such as “Are you taking your medication?”, although a more effective approach might be to ask how the patient is taking his (her) medication. Asking questions such as “When do you take your medication?” and “In the past week, how many doses do you think you missed?” might be more effective ways to inquire about adherence.7
The Expert Consensus Guidelines recommend asking patients about medication adherence monthly for those who are stable, doing well, and believed to be adherent. For those who are new to a practice or who are not doing well, inquire about medication adherence at least weekly.7
In our practice, patients who are unstable but do not require inpatient hospitalization typically are seen more often in the clinic, or are referred to intensive outpatient or partial hospitalization programs. If an unstable patient is unable to come in for more frequent appointments, we arrange phone conferences between her and her provider. If a patient is not doing well and has a case manager, we often ask that case manager to visit the patient, in person, more often than he (she) would otherwise.
Take a nonjudgmental approach when raising these issues with patients. Questions such as “We all forget to take our medication sometimes; do you?” help to normalize nonadherence, and improve the therapeutic alliance, and might result in the patient being more honest with the clinician.7 Because patients may be apprehensive about discussing adverse events, clinicians must be proactive about improving the therapeutic alliance and making patients feel comfortable when discussing sensitive topics. Clinicians should try to convey the idea that, although adherence is a concern, so is quality of life. A clinicians’ willingness to take a flexible approach that is nonpunitive nor authoritarian can aid the therapeutic alliance and improve overall adherence.
Be sensitive to financial, cultural, and language variables that can affect access to care. The Expert Consensus Guidelines recommend asking patients if they can afford their medication. In our practice, we have seen patients with schizophrenia discharged from the hospital only to be readmitted 1 month later because they could not afford to fill their prescriptions.
It is important to have translation services available, in person or by phone, for patients who do not speak English. Furthermore, it is important to understand the limitations that your practice might place on access to care. Ask patients if they have ever had trouble making an appointment when they needed to be seen, or if they called the office with a question and did not receive an answer in a timely fashion; doing so allows you to assess the practice’s ability to meet patients’ needs and helps you build a therapeutic alliance.
Make objective assessments. It is important for practitioners to not base their assessment of medication adherence solely on subjective findings. Asking patients to bring in their medication bottles for pill counts and checking with the patients’ pharmacies for information about refill frequency can provide some objective data. Electronic monitoring systems use microprocessors inserted into bottle caps to record the occurrence and timing of each bottle opening. Studies show that these electronic monitoring systems are the gold standard for determining medication adherence and could be used in cases where it is unclear if the patient is taking his (her) medication.7,20 Such systems have successfully monitored medication adherence in clinical trials, but their use in clinical practice is complicated by ethical and legal considerations and cost issues.
Simplify the regimen. Using medications with once-daily dosing, for example, can help improve adherence. Pfeiffer and co-workers21 found that patients whose medication regimens were changed from once daily to more than once daily experienced a decrease in medication adherence. Conversely, a decrease in dosing frequency was significantly associated with improved adherence. More than once-daily dosing was only weakly associated with poorer adherence among patients already on a stable regimen.
Discussing positive and negative aspects of past medication trials with a patient and inquiring if she prefers a specific medication can be an effective way to build the therapeutic relationship and help with adherence.
Direct patients to psychosocial interventions. These can be broadly classified as:
- educational approaches
- group therapy approaches
- family interventions
- cognitive treatments
- combination approaches.
Psychoeducational approaches have limited effect on improving adherence when delivered to individual patients. However, 1 study showed that psychoeducation was effective at improving adherence when extended to include the patient’s family.22
Motivational interviewing techniques, behavioral approaches, and family interventions are effective at increasing medication adherence. One study looked at the value of training a patient-identified informant to supervise and administer medication. This person, usually a family member or close support, was responsible for obtaining medication from the pharmacy, administering the medication, and recording adherence. After 1 year, 67% of patients who used an informant were adherent, compared with only 45% in the group that did not have informant support.22 Case managers, attendant care workers, home health nurses, and assertive community treatment (ACT) teams also can participate in this manner; it is important, therefore, for you to be aware of the resources available in your community and to understand your role as patient advocate.
Substance abuse is a strong risk factor for nonadherence among patients with schizophrenia,18 which makes it important to assess patients for substance use and encourage those who do abuse to seek treatment. Although 1 study showed no correlation between Alcoholics Anonymous (AA) attendance and medication adherence,12 many AA and Narcotics Anonymous groups do not discuss psychiatric medications during group meetings. Magura and colleagues encouraged the use of “dual focus” groups that involve mental health professionals and addiction treatment specialists discussing mental health and substance abuse issues at the same setting.18
Prescribe long-acting injectable antipsychotics. Typically, long-acting injectable antipsychotics (LAIs) are reserved for patients who have a history of nonadherence. In a small study (N = 97) comparing LAI risperidone and oral risperidone or oral haloperidol, patients treated with an LAI had significantly fewer all-cause discontinuations (26.0%, compared with 70.2%) at 24 months.23 The Adherence to Treatment and Therapeutic Strategies in Schizophrenic Patients study examined 1,848 patients with schizophrenia and reached similar findings regarding LAI antipsychotics.24 (Note: Aripiprazole, fluphenazine, haloperidol, olanzapine, and paliperidone also are available in an LAI formulation.)
Bottom Line
Antipsychotic nonadherence in schizophrenia is a major problem for patients, families, and society. Being able to identify patients at risk for nonadherence, understanding the reasons for their nonadherence, and seeking practical solutions to the problem are all the responsibility of the treating physician. Psychoeducation, addressing substance abuse, modifying dosing, and using long-acting injectable antipsychotics may help improve adherence.
Related Resources
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Clin Psychiatry. 2009;70(suppl 4):1-46.
- National Alliance on Mental Illness. www.nami.org.
- Assertive Community Treatment (ACT) Organization. www.actassociation.org.
Drug Brand Names
Aripiprazole • Abilify Chlorpromazine • Thorazine Clozapine • Clozaril Fluphenazine • Permitil Haloperidol • Haldol Iloperidone • Fanapt Olanzapine • Zyprexa Paliperidone • Invega Perphenazine • Trilafon Quetiapine • Seroquel Risperidone • Risperdal Ziprasidone • Geodon
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Dr. McKnight reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23:2305-2312.
2. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892-909.
3. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
4. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
5. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemial Drug Saf. 2003;12(5):423-424.
6. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891.
7. Velligan D, Weiden P, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness. J Psychiatr Pract. 2010;16(1):34-45.
8. Freudenreich O, Cather C, Evins A, et al. Attitudes of schizophrenia outpatients toward psychiatric medications: relationship to clinical variables and insight. J Clin Psychiatry. 2004;65(10):1372-1376.
9. Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83(1):53-63.
10. Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence. 2013;7:121-132.
11. Novick D, Haro J, Suarez D, et al. Predictors and clinical consequences of nonadherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2-3):109-113.
12. Hunt GE, Bergen J, Bashir M, et al. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival four years after a relapse. Schizophr Res. 2002;54(3):253-264.
13. McGinty EE, Webster DW, Barry CL. Effects of news media messages about mass shootings on attitudes toward persons with serious mental illness and public support for gun control policies. Am J Psychiatry. 2013;170(5):494-501.
14. DiBonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;1382(9896): 951-962.
16. Robinson DS. Antipsychotics: pharmacology and clinical decision making. Primary Psychiatry. 2007;14(10):23-25.
17. Robinson D, Correll CU, Kane JM, et al. Practical dosing strategies in the treatment of schizophrenia. CNS Spectr. 2010;15:4(suppl 6):1-16.
18. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
19. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1-3):176-181.
20. Byerly M, Nakonezny P, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30:437-452.
21. Pfeiffer PN, Ganoczy D, Valenstein M. Dosing frequency and adherence to antipsychotic medications. Psychiatr Serv. 2008;59(10):1207-1210.
22. Farooq S, Nazar Z, Irfan M, et al. Schizophrenia medication adherence in a resource-poor setting: randomized controlled trial of supervised treatment in out-patients for schizophrenia (STOPS). Br J Psychiatry. 2011;199(6):467-472.
23. Emsley R, Oosthuizen P, Koen L, et al. Oral vs injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
24. Gutierrez-Casares JR, Canãs F, Rodriguez-Morales A, et al. Adherence to treatment and therapeutic strategies in schizophrenic patients: the ADHERE study. CNS Spectr. 2010;15(5):327-337.
1. Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23:2305-2312.
2. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892-909.
3. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637-651.
4. Olfson M, Mechanic D, Hansell S, et al. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216-222.
5. Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemial Drug Saf. 2003;12(5):423-424.
6. Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886-891.
7. Velligan D, Weiden P, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness. J Psychiatr Pract. 2010;16(1):34-45.
8. Freudenreich O, Cather C, Evins A, et al. Attitudes of schizophrenia outpatients toward psychiatric medications: relationship to clinical variables and insight. J Clin Psychiatry. 2004;65(10):1372-1376.
9. Perkins DO, Johnson JL, Hamer RM, et al. Predictors of antipsychotic medication adherence in patients recovering from a first psychotic episode. Schizophr Res. 2006;83(1):53-63.
10. Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence. 2013;7:121-132.
11. Novick D, Haro J, Suarez D, et al. Predictors and clinical consequences of nonadherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2-3):109-113.
12. Hunt GE, Bergen J, Bashir M, et al. Medication compliance and comorbid substance abuse in schizophrenia: impact on community survival four years after a relapse. Schizophr Res. 2002;54(3):253-264.
13. McGinty EE, Webster DW, Barry CL. Effects of news media messages about mass shootings on attitudes toward persons with serious mental illness and public support for gun control policies. Am J Psychiatry. 2013;170(5):494-501.
14. DiBonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20.
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;1382(9896): 951-962.
16. Robinson DS. Antipsychotics: pharmacology and clinical decision making. Primary Psychiatry. 2007;14(10):23-25.
17. Robinson D, Correll CU, Kane JM, et al. Practical dosing strategies in the treatment of schizophrenia. CNS Spectr. 2010;15:4(suppl 6):1-16.
18. Magura S, Rosenblum A, Fong C. Factors associated with medication adherence among psychiatric outpatients at substance abuse risk. Open Addict J. 2011;4:58-64.
19. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1-3):176-181.
20. Byerly M, Nakonezny P, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30:437-452.
21. Pfeiffer PN, Ganoczy D, Valenstein M. Dosing frequency and adherence to antipsychotic medications. Psychiatr Serv. 2008;59(10):1207-1210.
22. Farooq S, Nazar Z, Irfan M, et al. Schizophrenia medication adherence in a resource-poor setting: randomized controlled trial of supervised treatment in out-patients for schizophrenia (STOPS). Br J Psychiatry. 2011;199(6):467-472.
23. Emsley R, Oosthuizen P, Koen L, et al. Oral vs injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
24. Gutierrez-Casares JR, Canãs F, Rodriguez-Morales A, et al. Adherence to treatment and therapeutic strategies in schizophrenic patients: the ADHERE study. CNS Spectr. 2010;15(5):327-337.
Violent behavior in autism spectrum disorder: Is it a fact, or fiction?
When Kanner first described autism,1 the disorder was believed to be an uncommon condition, occurring in 4 of every 10,000 children. Over the past few years, however, the rate of autism has increased substantially. Autism is now regarded as a childhood-onset spectrum disordera characterized by persistent deficits in social communication, with a restricted pattern of interests and activities, occurring in approximately 1% of children.3
In DSM-IV-TR, Asperger’s disorder (AD), first described as “autistic psychopathy,”4 is categorized as a subtype of ASD in which the patient, without a history of language delay or mental retardation, has autistic social deficits that do not meet full criteria for autism.
DSM-5 eliminated AD as an independent category, including it instead as part of ASD.5 The label “high-functioning autism” is sometimes used to refer to persons with autism who have normal intelligence (usually defined as full-scale IQ >70), whereas those who have severe intellectual and communication disability are referred to as “low-functioning.” I use “high-functioning autism” and “Asperger’s disorder” interchangeably.
Violent crime and ASD/AD
Reports in the past 2 decades have described violent behavior in persons with ASD/AD. Because of the sensational and unusual nature of these criminal incidents, there is a perception by the public that persons with these disorders, especially those with AD, are predisposed to violent behavior. (Incidents allegedly committed by persons with ASD include the 2007 Virginia Tech campus shooting and the 2012 Newtown, Connecticut, school massacre.6)
Yet neither the original descriptions by Kanner (of autism) and Asperger, nor follow-up studies based on the initial samples studied, showed an increased prevalence of violent crime among persons with ASD/AD.7
In this article, I examine the evidence behind the claim that people who have ASD/AD are predisposed to criminal violence. At the conclusion, you should, as a physician without special training in autism, have a better understanding of when to suspect ASD/AD in an adult who is involved in criminal behavior.
When should you suspect ASD/AD in an adult?
Although autism is a childhood-onset disorder, its symptoms persist across the life
span. If the diagnosis is missed in childhood, which is likely to happen if the person has normal intelligence and relatively good verbal skills, he (she) might come to medical attention for the first time as an adult.
Because most psychiatrists who treat adults do not receive adequate training in the assessment of childhood psychiatric disorders, ASD/AD might be misdiagnosed as schizophrenia or another psychotic disorder. What clues help identify underlying ASD/AD when a patient is referred to you for psychiatric evaluation after allegedly committing a violent crime?
Clue #1. He makes no attempt to deny or conceal the act. The behavior appears to be part of ritualistic behavior or excessive interest (Table).
Often, the alleged crime occurs when the patient’s excessive interests “get out of control,” perhaps because of an external event. For example, a teenager with AD who is fixated on video games might stumble upon pornographic web sites and begin making obscene telephone calls. Particular attention should be paid to a history of rigid, restricted interests beginning in early childhood.
These restricted interests change over time and correlate with intelligence level: The higher the level of intelligence, the more sophisticated the level of fixation. Examples of fixations include computers, technology, and scientific experiments and pursuits. Repeated acts of arson have been reported to be part of an autistic person’s fixation with starting fires.8
Clue #2. He appears to lack sound and prudent judgment despite normal intelligence.
Although most patients with ASD score in the intellectually disabled or mentally retarded range, at least one-third have an IQ in the normal range.9 Examine school records and reports from other agencies when evaluating a patient. Pay attention to a history of difficulty relating to peers at an early age, combined with evidence of rigid, restricted fixations and interests.
It is important to obtain a reliable history going back to early childhood, and not rely just on the patient’s mental status; presenting symptoms might mask underlying traits of ASD, especially in higher-functioning adults. (I once cared for a young man with ASD who had been fired a few days after landing his first job selling used cars because he was “sexually harassing” his colleagues. When questioned, he said that he was only trying to be “friendly” and “practicing his social skills.”)
Clue #3. He has been given a diagnosis of schizophrenia without a clear history of hallucinations or delusions.
Differentiating chronic schizophrenia and autism in adults is not always easy, especially in those who have an intellectual disability. In patients whose cognitive and verbal skills are relatively well preserved (such as AD), the presence of intense, focused interests, a pedantic manner of speaking, and abnormalities of nonverbal communication can help clarify the diagnosis. In particular, a recorded history of “childhood schizophrenia” or “obsessive-compulsive behavior” going back to preschool years should alert you to possible ASD.
Scales and screens. Apart from obtaining an accurate developmental history from a variety of sources, you can use rating scales and screening instruments, such as the Social and Communication Questionnaire10—although their utility is limited in adults. It is important not to risk overdiagnosis on the basis of these instruments alone: The gold standard of diagnosis remains clinical. The critical point is that the combination of core symptoms of social communication deficits and restricted interests is more important than the presence of a single symptom. A touch of oddity does not mean that one has ASD/AD.
Is the prevalence of violent crime increased in ASD/AD?
It is important to distinguish violent crime from aggressive behavior. The latter, which can be verbal or nonverbal, is not always intentional or malevolent. In some persons who have an intellectual disability, a desire to communicate might lead to inappropriate touching or pushing. This distinction is particularly relevant to psychiatrists because many people who have ASD have an intellectual disability.
Violent crime is more deliberate, serious, and planned. It involves force or threat of force. According to the Federal Bureau of Investigation Uniform Crime Reporting Program, violent crime comprises four offenses: murder and non-negligent manslaughter, forcible rape, robbery, and aggravated assault.11
Earlier descriptions of ASD/AD did not mention criminal violence as an important feature of these disorders. However, reports began to emerge about two decades ago suggesting that people who have ASD—particularly AD—are prone to violent crime. Some of the patients described in Wing’s original series12 of AD showed violent tendencies, ranging from sudden outbursts of violence to injury to others because of fixation on hobbies such as chemistry experimentation.
Reports such as these were based on isolated case reports or select samples, such as residents of maximum-security hospitals. Scragg and Shah, for example, surveyed the male population of Broadmoor Hospital, a high-security facility in the United Kingdom, and found that the prevalence of AD was higher than expected in the general population.13
Recent reports have not been able to confirm that violent crime is increased in persons with ASD, however:
- In a clinical sample of 313 Danish adults with ASD (age 25 to 59) drawn from the Danish Register of Criminality, Mouridsen and colleagues found that persons with ASD had a lower rate of criminal conviction than matched controls (9%, compared with 18%).14
- In a small community study, Woodbury-Smith and colleagues examined the prevalence rates and types of offending behavior in persons with ASD. Based on official records, only two (18%) had a history of criminal conviction.15
The role of psychiatric comorbidity
Psychiatric disorders are common in persons who have ASD. In one study, 70% of a sample of 114 children with ASD (age 10 to 14) had a psychiatric disorder, based on a parent interview.16 Although people with mental illness are not inherently criminal or violent, having an additional psychiatric disorder independently increases the risk of offending behavior.17 For example, the association of attention-deficit/hyperactivity disorder with criminality is well established.16 Some patients with severe depression and psychotic disorders, including schizophrenia, also are at increased risk of committing a violent act.
To examine the contribution of mental health factors to the commission of crime by persons with ASD, Newman and Ghaziuddin18 used online databases to identify relevant articles, which were then cross-referenced with keyword searches for “violence,” “crime,” “murder,” “assault,” “rape,” and “sex offenses.” Thirty-seven cases were identified in the 17 publications that met inclusion criteria. Out of these, 30% had a definite psychiatric disorder and 54% had a probable psychiatric disorder at the time they committed the crime.18
Any patient with ASD/AD who is evaluated for criminal behavior should be screened for a comorbid psychiatric disorder. In adolescents, stressors such as bullying in school and problems surrounding dating might contribute to offending behavior.
What are management options in the face of violence?
Managing ASD/AD when an offending behavior has occurred first requires a correct diagnosis.19 Professionals working in the criminal justice system have little awareness of the variants of ASD; a defendant with an intellectual disability and a characteristic facial appearance (for example, someone with Down syndrome) can be easily identified, but a high-functioning person who has mild autistic features often is missed. This is more likely to occur in adults because the symptoms of ASD, including the type and severity of isolated interests, change over time.
Here is how I recommend that you proceed:
Step #1. Confirm the ASD diagnosis based on developmental history and the presence of persistent social and communication deficits plus restricted interests.
Step #2. Screen for comorbid psychiatric and medical disorders, including depression, psychosis, and seizure disorder.
Step #3. Treat any disorders you identify with a combination of medication and behavioral intervention.
Step #4. Carefully examine the circumstances surrounding the offending behavior. Involve forensic services on a case-by-case basis, depending on the type and seriousness of the offending behavior (see Related Resources for information on the role of forensic services). When the crime does not involve serious violence, lengthy incarceration might be unnecessary. Because psychopathy and ASD/AD are not mutually exclusive, persons who commit a heinous crime, such as rape or murder, should be dealt with in accordance with the law.
Need for greater awareness of the complexion of ASD
Patients who have ASD/AD form a heterogeneous group in which the levels of cognitive and communication skills are variable. Those who are low-functioning and who have severe behavioral and adaptive deficits occasionally commit aggressive acts against their caregivers.
Most patients with ASD/AD are neither violent nor criminal. Those who are at the higher end of the spectrum, with relatively preserved communication and intellectual skills, occasionally indulge in criminal behavior—behavior that is nonviolent and results from their inability to read social cues or excessive preoccupations.
Most reports that link criminal violence with ASD are based on isolated case reports or on biased samples that use unreliable diagnostic criteria. In higher-functioning persons with ASD, violent crime is almost always precipitated by a comorbid psychiatric disorder, such as severe depression and psychosis.
In short: There is a need to increase our awareness of the special challenges faced by persons with ASD/AD in the criminal justice system.
aGiven the term pervasive developmental disorders (PDD) in the DSM-IV-TR, the spectrum includes autistic disorder, Asperger’s disorder, and pervasive developmental disorder not otherwise specified.2
Bottom Line
Most people who have an autism spectrum disorder (ASD) do not commit violent crime. When violent crime occurs at the hands of a person with ASD, it is almost always precipitated by a comorbid psychiatric disorder, such as severe depression or psychosis. Treating a person with ASD who has committed a violent crime is multimodal, including forensic services when necessary.
Related Resources
- Autism Speaks. No link between autism and violence. www.autismspeaks.org/science/science-news/no-link-between-autism-and-violence.
- Haskins BG, Silva JA. Asperger’s disorder and criminal behavior: Forensic-psychiatric considerations. J Am Acad Psychiatry Law. 2006;34(3):374-384.
- Newman SS, Ghaziuddin M. Violent crime and Asperger syndrome: the role of psychiatric comorbidity. J Autism Dev Disord. 2008;38:1848-1852.
- Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11(1):115-129.
Disclosure
Dr. Ghaziuddin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217-250.
2. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 2000.
3. Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1-19.
4. Asperger H. Die autistichen psychopathen im kindesalter. Arch Psychiatr Nervenkr. 1944;117:76-136.
5. Happe F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child and Adolesc Psychiatry. 2011;50:540-542.
6. Walkup JT, Rubin DH. Social withdrawal and violence. N Engl J Med. 2013;368:399-401.
7. Hippler K, Vidding E, Klicpera C, et al. Brief report: no increase in criminal convictions in Asperger’s original cohort. J Autism Dev Disord. 2010;40:774-780.
8. Siponmaa L, Kristiansson M, Jonson C, et al. Juvenile and young adult mentally disordered offenders: the role of child neuropsychiatric disorders. J Am Acad Psychiatry Law. 2001;29(4):420-426.
9. Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009;30(6):1107-1114.
10. Rutter M, Bailey A, Lord C. Social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003.
11. US Department of Justice. Violent crime. http://www2.fbi.gov/ucr/cius2009/offenses/violent_crime. Published September, 2010. Accessed April 26, 2013.
12. Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11(1):115-129.
13. Scragg P, Shah A. The prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165:67-72.
14. Mouridsen SE, Rich B, Isager T, et al. Pervasive developmental disorders and criminal behaviour: a case control study. Int J Offender Ther Comp Criminol. 2008; 52(2):196-205.
15. Woodbury-Smith MR, Clare ICH, Holland AJ, et al. High functioning autistic spectrum disorders, offending and other law-breaking: findings from a community sample. J Forens Psychiatry Psychol. 2006;17(1):108-120.
16. Simonoff E, Pickles A, Charman T, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):
921-929.
17. Ghaziuddin M. Mental health aspects of autism and Asperger syndrome. London, United Kingdom: Jessica Kingsley Press; 2005.
18. Newman SS, Ghaziuddin M. Violent crime and Asperger syndrome: the role of psychiatric comorbidity. J Autism Dev Disord. 2008;38:1848-1852.
19. Wing L. Asperger’s syndrome: management requires diagnosis. The Journal of Forensic Psychiatry. 1997;8(2):253-257.
When Kanner first described autism,1 the disorder was believed to be an uncommon condition, occurring in 4 of every 10,000 children. Over the past few years, however, the rate of autism has increased substantially. Autism is now regarded as a childhood-onset spectrum disordera characterized by persistent deficits in social communication, with a restricted pattern of interests and activities, occurring in approximately 1% of children.3
In DSM-IV-TR, Asperger’s disorder (AD), first described as “autistic psychopathy,”4 is categorized as a subtype of ASD in which the patient, without a history of language delay or mental retardation, has autistic social deficits that do not meet full criteria for autism.
DSM-5 eliminated AD as an independent category, including it instead as part of ASD.5 The label “high-functioning autism” is sometimes used to refer to persons with autism who have normal intelligence (usually defined as full-scale IQ >70), whereas those who have severe intellectual and communication disability are referred to as “low-functioning.” I use “high-functioning autism” and “Asperger’s disorder” interchangeably.
Violent crime and ASD/AD
Reports in the past 2 decades have described violent behavior in persons with ASD/AD. Because of the sensational and unusual nature of these criminal incidents, there is a perception by the public that persons with these disorders, especially those with AD, are predisposed to violent behavior. (Incidents allegedly committed by persons with ASD include the 2007 Virginia Tech campus shooting and the 2012 Newtown, Connecticut, school massacre.6)
Yet neither the original descriptions by Kanner (of autism) and Asperger, nor follow-up studies based on the initial samples studied, showed an increased prevalence of violent crime among persons with ASD/AD.7
In this article, I examine the evidence behind the claim that people who have ASD/AD are predisposed to criminal violence. At the conclusion, you should, as a physician without special training in autism, have a better understanding of when to suspect ASD/AD in an adult who is involved in criminal behavior.
When should you suspect ASD/AD in an adult?
Although autism is a childhood-onset disorder, its symptoms persist across the life
span. If the diagnosis is missed in childhood, which is likely to happen if the person has normal intelligence and relatively good verbal skills, he (she) might come to medical attention for the first time as an adult.
Because most psychiatrists who treat adults do not receive adequate training in the assessment of childhood psychiatric disorders, ASD/AD might be misdiagnosed as schizophrenia or another psychotic disorder. What clues help identify underlying ASD/AD when a patient is referred to you for psychiatric evaluation after allegedly committing a violent crime?
Clue #1. He makes no attempt to deny or conceal the act. The behavior appears to be part of ritualistic behavior or excessive interest (Table).
Often, the alleged crime occurs when the patient’s excessive interests “get out of control,” perhaps because of an external event. For example, a teenager with AD who is fixated on video games might stumble upon pornographic web sites and begin making obscene telephone calls. Particular attention should be paid to a history of rigid, restricted interests beginning in early childhood.
These restricted interests change over time and correlate with intelligence level: The higher the level of intelligence, the more sophisticated the level of fixation. Examples of fixations include computers, technology, and scientific experiments and pursuits. Repeated acts of arson have been reported to be part of an autistic person’s fixation with starting fires.8
Clue #2. He appears to lack sound and prudent judgment despite normal intelligence.
Although most patients with ASD score in the intellectually disabled or mentally retarded range, at least one-third have an IQ in the normal range.9 Examine school records and reports from other agencies when evaluating a patient. Pay attention to a history of difficulty relating to peers at an early age, combined with evidence of rigid, restricted fixations and interests.
It is important to obtain a reliable history going back to early childhood, and not rely just on the patient’s mental status; presenting symptoms might mask underlying traits of ASD, especially in higher-functioning adults. (I once cared for a young man with ASD who had been fired a few days after landing his first job selling used cars because he was “sexually harassing” his colleagues. When questioned, he said that he was only trying to be “friendly” and “practicing his social skills.”)
Clue #3. He has been given a diagnosis of schizophrenia without a clear history of hallucinations or delusions.
Differentiating chronic schizophrenia and autism in adults is not always easy, especially in those who have an intellectual disability. In patients whose cognitive and verbal skills are relatively well preserved (such as AD), the presence of intense, focused interests, a pedantic manner of speaking, and abnormalities of nonverbal communication can help clarify the diagnosis. In particular, a recorded history of “childhood schizophrenia” or “obsessive-compulsive behavior” going back to preschool years should alert you to possible ASD.
Scales and screens. Apart from obtaining an accurate developmental history from a variety of sources, you can use rating scales and screening instruments, such as the Social and Communication Questionnaire10—although their utility is limited in adults. It is important not to risk overdiagnosis on the basis of these instruments alone: The gold standard of diagnosis remains clinical. The critical point is that the combination of core symptoms of social communication deficits and restricted interests is more important than the presence of a single symptom. A touch of oddity does not mean that one has ASD/AD.
Is the prevalence of violent crime increased in ASD/AD?
It is important to distinguish violent crime from aggressive behavior. The latter, which can be verbal or nonverbal, is not always intentional or malevolent. In some persons who have an intellectual disability, a desire to communicate might lead to inappropriate touching or pushing. This distinction is particularly relevant to psychiatrists because many people who have ASD have an intellectual disability.
Violent crime is more deliberate, serious, and planned. It involves force or threat of force. According to the Federal Bureau of Investigation Uniform Crime Reporting Program, violent crime comprises four offenses: murder and non-negligent manslaughter, forcible rape, robbery, and aggravated assault.11
Earlier descriptions of ASD/AD did not mention criminal violence as an important feature of these disorders. However, reports began to emerge about two decades ago suggesting that people who have ASD—particularly AD—are prone to violent crime. Some of the patients described in Wing’s original series12 of AD showed violent tendencies, ranging from sudden outbursts of violence to injury to others because of fixation on hobbies such as chemistry experimentation.
Reports such as these were based on isolated case reports or select samples, such as residents of maximum-security hospitals. Scragg and Shah, for example, surveyed the male population of Broadmoor Hospital, a high-security facility in the United Kingdom, and found that the prevalence of AD was higher than expected in the general population.13
Recent reports have not been able to confirm that violent crime is increased in persons with ASD, however:
- In a clinical sample of 313 Danish adults with ASD (age 25 to 59) drawn from the Danish Register of Criminality, Mouridsen and colleagues found that persons with ASD had a lower rate of criminal conviction than matched controls (9%, compared with 18%).14
- In a small community study, Woodbury-Smith and colleagues examined the prevalence rates and types of offending behavior in persons with ASD. Based on official records, only two (18%) had a history of criminal conviction.15
The role of psychiatric comorbidity
Psychiatric disorders are common in persons who have ASD. In one study, 70% of a sample of 114 children with ASD (age 10 to 14) had a psychiatric disorder, based on a parent interview.16 Although people with mental illness are not inherently criminal or violent, having an additional psychiatric disorder independently increases the risk of offending behavior.17 For example, the association of attention-deficit/hyperactivity disorder with criminality is well established.16 Some patients with severe depression and psychotic disorders, including schizophrenia, also are at increased risk of committing a violent act.
To examine the contribution of mental health factors to the commission of crime by persons with ASD, Newman and Ghaziuddin18 used online databases to identify relevant articles, which were then cross-referenced with keyword searches for “violence,” “crime,” “murder,” “assault,” “rape,” and “sex offenses.” Thirty-seven cases were identified in the 17 publications that met inclusion criteria. Out of these, 30% had a definite psychiatric disorder and 54% had a probable psychiatric disorder at the time they committed the crime.18
Any patient with ASD/AD who is evaluated for criminal behavior should be screened for a comorbid psychiatric disorder. In adolescents, stressors such as bullying in school and problems surrounding dating might contribute to offending behavior.
What are management options in the face of violence?
Managing ASD/AD when an offending behavior has occurred first requires a correct diagnosis.19 Professionals working in the criminal justice system have little awareness of the variants of ASD; a defendant with an intellectual disability and a characteristic facial appearance (for example, someone with Down syndrome) can be easily identified, but a high-functioning person who has mild autistic features often is missed. This is more likely to occur in adults because the symptoms of ASD, including the type and severity of isolated interests, change over time.
Here is how I recommend that you proceed:
Step #1. Confirm the ASD diagnosis based on developmental history and the presence of persistent social and communication deficits plus restricted interests.
Step #2. Screen for comorbid psychiatric and medical disorders, including depression, psychosis, and seizure disorder.
Step #3. Treat any disorders you identify with a combination of medication and behavioral intervention.
Step #4. Carefully examine the circumstances surrounding the offending behavior. Involve forensic services on a case-by-case basis, depending on the type and seriousness of the offending behavior (see Related Resources for information on the role of forensic services). When the crime does not involve serious violence, lengthy incarceration might be unnecessary. Because psychopathy and ASD/AD are not mutually exclusive, persons who commit a heinous crime, such as rape or murder, should be dealt with in accordance with the law.
Need for greater awareness of the complexion of ASD
Patients who have ASD/AD form a heterogeneous group in which the levels of cognitive and communication skills are variable. Those who are low-functioning and who have severe behavioral and adaptive deficits occasionally commit aggressive acts against their caregivers.
Most patients with ASD/AD are neither violent nor criminal. Those who are at the higher end of the spectrum, with relatively preserved communication and intellectual skills, occasionally indulge in criminal behavior—behavior that is nonviolent and results from their inability to read social cues or excessive preoccupations.
Most reports that link criminal violence with ASD are based on isolated case reports or on biased samples that use unreliable diagnostic criteria. In higher-functioning persons with ASD, violent crime is almost always precipitated by a comorbid psychiatric disorder, such as severe depression and psychosis.
In short: There is a need to increase our awareness of the special challenges faced by persons with ASD/AD in the criminal justice system.
aGiven the term pervasive developmental disorders (PDD) in the DSM-IV-TR, the spectrum includes autistic disorder, Asperger’s disorder, and pervasive developmental disorder not otherwise specified.2
Bottom Line
Most people who have an autism spectrum disorder (ASD) do not commit violent crime. When violent crime occurs at the hands of a person with ASD, it is almost always precipitated by a comorbid psychiatric disorder, such as severe depression or psychosis. Treating a person with ASD who has committed a violent crime is multimodal, including forensic services when necessary.
Related Resources
- Autism Speaks. No link between autism and violence. www.autismspeaks.org/science/science-news/no-link-between-autism-and-violence.
- Haskins BG, Silva JA. Asperger’s disorder and criminal behavior: Forensic-psychiatric considerations. J Am Acad Psychiatry Law. 2006;34(3):374-384.
- Newman SS, Ghaziuddin M. Violent crime and Asperger syndrome: the role of psychiatric comorbidity. J Autism Dev Disord. 2008;38:1848-1852.
- Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11(1):115-129.
Disclosure
Dr. Ghaziuddin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When Kanner first described autism,1 the disorder was believed to be an uncommon condition, occurring in 4 of every 10,000 children. Over the past few years, however, the rate of autism has increased substantially. Autism is now regarded as a childhood-onset spectrum disordera characterized by persistent deficits in social communication, with a restricted pattern of interests and activities, occurring in approximately 1% of children.3
In DSM-IV-TR, Asperger’s disorder (AD), first described as “autistic psychopathy,”4 is categorized as a subtype of ASD in which the patient, without a history of language delay or mental retardation, has autistic social deficits that do not meet full criteria for autism.
DSM-5 eliminated AD as an independent category, including it instead as part of ASD.5 The label “high-functioning autism” is sometimes used to refer to persons with autism who have normal intelligence (usually defined as full-scale IQ >70), whereas those who have severe intellectual and communication disability are referred to as “low-functioning.” I use “high-functioning autism” and “Asperger’s disorder” interchangeably.
Violent crime and ASD/AD
Reports in the past 2 decades have described violent behavior in persons with ASD/AD. Because of the sensational and unusual nature of these criminal incidents, there is a perception by the public that persons with these disorders, especially those with AD, are predisposed to violent behavior. (Incidents allegedly committed by persons with ASD include the 2007 Virginia Tech campus shooting and the 2012 Newtown, Connecticut, school massacre.6)
Yet neither the original descriptions by Kanner (of autism) and Asperger, nor follow-up studies based on the initial samples studied, showed an increased prevalence of violent crime among persons with ASD/AD.7
In this article, I examine the evidence behind the claim that people who have ASD/AD are predisposed to criminal violence. At the conclusion, you should, as a physician without special training in autism, have a better understanding of when to suspect ASD/AD in an adult who is involved in criminal behavior.
When should you suspect ASD/AD in an adult?
Although autism is a childhood-onset disorder, its symptoms persist across the life
span. If the diagnosis is missed in childhood, which is likely to happen if the person has normal intelligence and relatively good verbal skills, he (she) might come to medical attention for the first time as an adult.
Because most psychiatrists who treat adults do not receive adequate training in the assessment of childhood psychiatric disorders, ASD/AD might be misdiagnosed as schizophrenia or another psychotic disorder. What clues help identify underlying ASD/AD when a patient is referred to you for psychiatric evaluation after allegedly committing a violent crime?
Clue #1. He makes no attempt to deny or conceal the act. The behavior appears to be part of ritualistic behavior or excessive interest (Table).
Often, the alleged crime occurs when the patient’s excessive interests “get out of control,” perhaps because of an external event. For example, a teenager with AD who is fixated on video games might stumble upon pornographic web sites and begin making obscene telephone calls. Particular attention should be paid to a history of rigid, restricted interests beginning in early childhood.
These restricted interests change over time and correlate with intelligence level: The higher the level of intelligence, the more sophisticated the level of fixation. Examples of fixations include computers, technology, and scientific experiments and pursuits. Repeated acts of arson have been reported to be part of an autistic person’s fixation with starting fires.8
Clue #2. He appears to lack sound and prudent judgment despite normal intelligence.
Although most patients with ASD score in the intellectually disabled or mentally retarded range, at least one-third have an IQ in the normal range.9 Examine school records and reports from other agencies when evaluating a patient. Pay attention to a history of difficulty relating to peers at an early age, combined with evidence of rigid, restricted fixations and interests.
It is important to obtain a reliable history going back to early childhood, and not rely just on the patient’s mental status; presenting symptoms might mask underlying traits of ASD, especially in higher-functioning adults. (I once cared for a young man with ASD who had been fired a few days after landing his first job selling used cars because he was “sexually harassing” his colleagues. When questioned, he said that he was only trying to be “friendly” and “practicing his social skills.”)
Clue #3. He has been given a diagnosis of schizophrenia without a clear history of hallucinations or delusions.
Differentiating chronic schizophrenia and autism in adults is not always easy, especially in those who have an intellectual disability. In patients whose cognitive and verbal skills are relatively well preserved (such as AD), the presence of intense, focused interests, a pedantic manner of speaking, and abnormalities of nonverbal communication can help clarify the diagnosis. In particular, a recorded history of “childhood schizophrenia” or “obsessive-compulsive behavior” going back to preschool years should alert you to possible ASD.
Scales and screens. Apart from obtaining an accurate developmental history from a variety of sources, you can use rating scales and screening instruments, such as the Social and Communication Questionnaire10—although their utility is limited in adults. It is important not to risk overdiagnosis on the basis of these instruments alone: The gold standard of diagnosis remains clinical. The critical point is that the combination of core symptoms of social communication deficits and restricted interests is more important than the presence of a single symptom. A touch of oddity does not mean that one has ASD/AD.
Is the prevalence of violent crime increased in ASD/AD?
It is important to distinguish violent crime from aggressive behavior. The latter, which can be verbal or nonverbal, is not always intentional or malevolent. In some persons who have an intellectual disability, a desire to communicate might lead to inappropriate touching or pushing. This distinction is particularly relevant to psychiatrists because many people who have ASD have an intellectual disability.
Violent crime is more deliberate, serious, and planned. It involves force or threat of force. According to the Federal Bureau of Investigation Uniform Crime Reporting Program, violent crime comprises four offenses: murder and non-negligent manslaughter, forcible rape, robbery, and aggravated assault.11
Earlier descriptions of ASD/AD did not mention criminal violence as an important feature of these disorders. However, reports began to emerge about two decades ago suggesting that people who have ASD—particularly AD—are prone to violent crime. Some of the patients described in Wing’s original series12 of AD showed violent tendencies, ranging from sudden outbursts of violence to injury to others because of fixation on hobbies such as chemistry experimentation.
Reports such as these were based on isolated case reports or select samples, such as residents of maximum-security hospitals. Scragg and Shah, for example, surveyed the male population of Broadmoor Hospital, a high-security facility in the United Kingdom, and found that the prevalence of AD was higher than expected in the general population.13
Recent reports have not been able to confirm that violent crime is increased in persons with ASD, however:
- In a clinical sample of 313 Danish adults with ASD (age 25 to 59) drawn from the Danish Register of Criminality, Mouridsen and colleagues found that persons with ASD had a lower rate of criminal conviction than matched controls (9%, compared with 18%).14
- In a small community study, Woodbury-Smith and colleagues examined the prevalence rates and types of offending behavior in persons with ASD. Based on official records, only two (18%) had a history of criminal conviction.15
The role of psychiatric comorbidity
Psychiatric disorders are common in persons who have ASD. In one study, 70% of a sample of 114 children with ASD (age 10 to 14) had a psychiatric disorder, based on a parent interview.16 Although people with mental illness are not inherently criminal or violent, having an additional psychiatric disorder independently increases the risk of offending behavior.17 For example, the association of attention-deficit/hyperactivity disorder with criminality is well established.16 Some patients with severe depression and psychotic disorders, including schizophrenia, also are at increased risk of committing a violent act.
To examine the contribution of mental health factors to the commission of crime by persons with ASD, Newman and Ghaziuddin18 used online databases to identify relevant articles, which were then cross-referenced with keyword searches for “violence,” “crime,” “murder,” “assault,” “rape,” and “sex offenses.” Thirty-seven cases were identified in the 17 publications that met inclusion criteria. Out of these, 30% had a definite psychiatric disorder and 54% had a probable psychiatric disorder at the time they committed the crime.18
Any patient with ASD/AD who is evaluated for criminal behavior should be screened for a comorbid psychiatric disorder. In adolescents, stressors such as bullying in school and problems surrounding dating might contribute to offending behavior.
What are management options in the face of violence?
Managing ASD/AD when an offending behavior has occurred first requires a correct diagnosis.19 Professionals working in the criminal justice system have little awareness of the variants of ASD; a defendant with an intellectual disability and a characteristic facial appearance (for example, someone with Down syndrome) can be easily identified, but a high-functioning person who has mild autistic features often is missed. This is more likely to occur in adults because the symptoms of ASD, including the type and severity of isolated interests, change over time.
Here is how I recommend that you proceed:
Step #1. Confirm the ASD diagnosis based on developmental history and the presence of persistent social and communication deficits plus restricted interests.
Step #2. Screen for comorbid psychiatric and medical disorders, including depression, psychosis, and seizure disorder.
Step #3. Treat any disorders you identify with a combination of medication and behavioral intervention.
Step #4. Carefully examine the circumstances surrounding the offending behavior. Involve forensic services on a case-by-case basis, depending on the type and seriousness of the offending behavior (see Related Resources for information on the role of forensic services). When the crime does not involve serious violence, lengthy incarceration might be unnecessary. Because psychopathy and ASD/AD are not mutually exclusive, persons who commit a heinous crime, such as rape or murder, should be dealt with in accordance with the law.
Need for greater awareness of the complexion of ASD
Patients who have ASD/AD form a heterogeneous group in which the levels of cognitive and communication skills are variable. Those who are low-functioning and who have severe behavioral and adaptive deficits occasionally commit aggressive acts against their caregivers.
Most patients with ASD/AD are neither violent nor criminal. Those who are at the higher end of the spectrum, with relatively preserved communication and intellectual skills, occasionally indulge in criminal behavior—behavior that is nonviolent and results from their inability to read social cues or excessive preoccupations.
Most reports that link criminal violence with ASD are based on isolated case reports or on biased samples that use unreliable diagnostic criteria. In higher-functioning persons with ASD, violent crime is almost always precipitated by a comorbid psychiatric disorder, such as severe depression and psychosis.
In short: There is a need to increase our awareness of the special challenges faced by persons with ASD/AD in the criminal justice system.
aGiven the term pervasive developmental disorders (PDD) in the DSM-IV-TR, the spectrum includes autistic disorder, Asperger’s disorder, and pervasive developmental disorder not otherwise specified.2
Bottom Line
Most people who have an autism spectrum disorder (ASD) do not commit violent crime. When violent crime occurs at the hands of a person with ASD, it is almost always precipitated by a comorbid psychiatric disorder, such as severe depression or psychosis. Treating a person with ASD who has committed a violent crime is multimodal, including forensic services when necessary.
Related Resources
- Autism Speaks. No link between autism and violence. www.autismspeaks.org/science/science-news/no-link-between-autism-and-violence.
- Haskins BG, Silva JA. Asperger’s disorder and criminal behavior: Forensic-psychiatric considerations. J Am Acad Psychiatry Law. 2006;34(3):374-384.
- Newman SS, Ghaziuddin M. Violent crime and Asperger syndrome: the role of psychiatric comorbidity. J Autism Dev Disord. 2008;38:1848-1852.
- Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11(1):115-129.
Disclosure
Dr. Ghaziuddin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217-250.
2. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 2000.
3. Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1-19.
4. Asperger H. Die autistichen psychopathen im kindesalter. Arch Psychiatr Nervenkr. 1944;117:76-136.
5. Happe F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child and Adolesc Psychiatry. 2011;50:540-542.
6. Walkup JT, Rubin DH. Social withdrawal and violence. N Engl J Med. 2013;368:399-401.
7. Hippler K, Vidding E, Klicpera C, et al. Brief report: no increase in criminal convictions in Asperger’s original cohort. J Autism Dev Disord. 2010;40:774-780.
8. Siponmaa L, Kristiansson M, Jonson C, et al. Juvenile and young adult mentally disordered offenders: the role of child neuropsychiatric disorders. J Am Acad Psychiatry Law. 2001;29(4):420-426.
9. Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009;30(6):1107-1114.
10. Rutter M, Bailey A, Lord C. Social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003.
11. US Department of Justice. Violent crime. http://www2.fbi.gov/ucr/cius2009/offenses/violent_crime. Published September, 2010. Accessed April 26, 2013.
12. Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11(1):115-129.
13. Scragg P, Shah A. The prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165:67-72.
14. Mouridsen SE, Rich B, Isager T, et al. Pervasive developmental disorders and criminal behaviour: a case control study. Int J Offender Ther Comp Criminol. 2008; 52(2):196-205.
15. Woodbury-Smith MR, Clare ICH, Holland AJ, et al. High functioning autistic spectrum disorders, offending and other law-breaking: findings from a community sample. J Forens Psychiatry Psychol. 2006;17(1):108-120.
16. Simonoff E, Pickles A, Charman T, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):
921-929.
17. Ghaziuddin M. Mental health aspects of autism and Asperger syndrome. London, United Kingdom: Jessica Kingsley Press; 2005.
18. Newman SS, Ghaziuddin M. Violent crime and Asperger syndrome: the role of psychiatric comorbidity. J Autism Dev Disord. 2008;38:1848-1852.
19. Wing L. Asperger’s syndrome: management requires diagnosis. The Journal of Forensic Psychiatry. 1997;8(2):253-257.
1. Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217-250.
2. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 2000.
3. Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders--Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1-19.
4. Asperger H. Die autistichen psychopathen im kindesalter. Arch Psychiatr Nervenkr. 1944;117:76-136.
5. Happe F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child and Adolesc Psychiatry. 2011;50:540-542.
6. Walkup JT, Rubin DH. Social withdrawal and violence. N Engl J Med. 2013;368:399-401.
7. Hippler K, Vidding E, Klicpera C, et al. Brief report: no increase in criminal convictions in Asperger’s original cohort. J Autism Dev Disord. 2010;40:774-780.
8. Siponmaa L, Kristiansson M, Jonson C, et al. Juvenile and young adult mentally disordered offenders: the role of child neuropsychiatric disorders. J Am Acad Psychiatry Law. 2001;29(4):420-426.
9. Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009;30(6):1107-1114.
10. Rutter M, Bailey A, Lord C. Social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003.
11. US Department of Justice. Violent crime. http://www2.fbi.gov/ucr/cius2009/offenses/violent_crime. Published September, 2010. Accessed April 26, 2013.
12. Wing L. Asperger’s syndrome: a clinical account. Psychol Med. 1981;11(1):115-129.
13. Scragg P, Shah A. The prevalence of Asperger’s syndrome in a secure hospital. Br J Psychiatry. 1994;165:67-72.
14. Mouridsen SE, Rich B, Isager T, et al. Pervasive developmental disorders and criminal behaviour: a case control study. Int J Offender Ther Comp Criminol. 2008; 52(2):196-205.
15. Woodbury-Smith MR, Clare ICH, Holland AJ, et al. High functioning autistic spectrum disorders, offending and other law-breaking: findings from a community sample. J Forens Psychiatry Psychol. 2006;17(1):108-120.
16. Simonoff E, Pickles A, Charman T, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47(8):
921-929.
17. Ghaziuddin M. Mental health aspects of autism and Asperger syndrome. London, United Kingdom: Jessica Kingsley Press; 2005.
18. Newman SS, Ghaziuddin M. Violent crime and Asperger syndrome: the role of psychiatric comorbidity. J Autism Dev Disord. 2008;38:1848-1852.
19. Wing L. Asperger’s syndrome: management requires diagnosis. The Journal of Forensic Psychiatry. 1997;8(2):253-257.
“I just saw Big Bird. He was 100 feet tall!” Malingering in the emergency room
The economic downturn in the United States has prompted numerous state and county budget cuts, in turn forcing many patients to receive their mental health care in the emergency room (ER). Most patients evaluated in the ER for mental health-related reasons have a legitimate psychiatric crisis—but that isn’t always the case. And as the number of people seeking care in the ER has increased, it appears that so too has the number of those who feign symptoms for secondary gain—that is, who are malingering.
This article highlights several red flags for malingered behavior; emphasizes typical (compared with atypical) symptoms of psychosis; and provides an overview of four instruments that you can use to help assess for malingering in the ED.
A difficult diagnosis
No single factor is indicative of malingering, and no objective tests exist to diagnose malingering definitively. Rather, the tests we discuss provide additional information that can help formulate a clinical impression.
According to DSM-5, malingering is “…the intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives…”1 Despite a relatively straightforward definition, the diagnosis is difficult to make because it is a diagnosis of exclusion.
Even with sufficient evidence, many clinicians are reluctant to diagnose malingering because they fear retaliation and diagnostic uncertainty. Psychiatrists also might be reluctant to diagnose malingering because the negative connotation that the label carries risks stigmatizing a patient who might, in fact, be suffering. This is true especially when there is suspicion of partial malingering, the conscious exaggeration of existing symptoms.
Despite physicians’ reluctance to diagnose malingering, it is a real problem, especially in the ER. Research suggests that as many as 13% of patients in the ER feign illness, and that their secondary gain most often includes food, shelter, prescription drugs, financial gain, and avoidance of jail, work, or family responsibilities.2
CASE REPORT ‘The voices are telling me to kill myself’
Mr. K, a 36-year-old white man, walks into the ER on a late December day. He tells the triage nurse that he suicidal; she escorts him to the psychiatric pod of the ER. Nursing staff provide line-of-sight care, monitor his vital signs, and draw blood for testing.
Within hours, Mr. K is deemed “medically cleared” and ready for assessment by the psychiatric social worker.
Interview and assessment. During the interview with the social worker, Mr. K reports that he has been depressed, adamantly maintaining that he is suicidal, with a plan to “walk in traffic” or “eat the end of a gun.” The social worker places him on a 72-hour involuntary psychiatric hold. ER physicians order psychiatric consultation.
Mr. K is well-known to the psychiatrist on call, from prior ER visits and psychiatric hospital admissions. In fact, two days earlier, he put a psychiatric nurse in a headlock while being escorted from the psychiatric inpatient unit under protest.
On assessment by the psychiatrist, Mr. K continues to endorse feeling suicidal; he adds: “If I don’t get some help, I’m gonna kill somebody else!”
Without prompting, the patient states that “the voices are telling me to kill myself.” He says that those voices have been relentless since he left the hospital two days earlier. According to Mr. K, nothing he did helped quiet the voices, although previous prescriptions for quetiapine have been helpful.
Mr. K says that he is unable to recall the clinic or name of his prior psychiatrist. He claims that he was hospitalized four months ago, (despite the psychiatrist’s knowledge that he had been discharged two days ago) and estimates that his psychotic symptoms began one year ago. He explains that he is homeless and does not have social support. He is unable to provide a telephone number or a name to contact family for collateral information.
Mental status exam. The mental status examination reveals a tall, thin, disheveled man who has poor dentition. He is now calm and cooperative despite his reported level of distress. His speech is unremarkable and his eye contact is appropriate. His thought process is linear, organized, and coherent.
Mr. K does not endorse additional symptoms, but is quick to agree with the psychiatrist’s follow-up questions about hallucinations: “Yeah! I’ve been seeing all kinds of crazy stuff.” When prompted for details, he says, “I just saw Big Bird… He was 100 feet tall!”
Lab testing. Mr. K’s blood work is remarkable for positive urine toxicology for amphetamines.
Nursing notes indicate that Mr. K slept overnight and ate 100% of the food on his dinner and breakfast trays.
Red flags flying
Mr. K’s case highlights several red flags that should raise suspicion of malingering (Table 1)3,4:
- A conditional statement by which a patient threatens to harm himself or others, contingent upon a demand—for example, “If I don’t get A, I’ll do B.”
- An overly dramatic presentation, in which the patient is quick to endorse
distressing symptoms. Consider Mr. K: He was quick to report that he saw Big Bird, and that this Sesame Street character “was 100 feet tall.” Patients who have been experiencing true psychotic symptoms might be reluctant to speak of their distressing symptoms, especially if they have not experienced such symptoms in the past (the first psychotic break). Mr. K, however, volunteered and called attention to particularly dramatic psychotic symptoms. - A subjective report of distress that is inconsistent with the objective presentation. Mr. K’s report of depression—a diagnosis that typically includes insomnia and poor appetite—was inconsistent with his behavior: He slept and he ate all of his meals.
Atypical (vs typical) psychosis
Malingering can occur in various arenas and take many different forms. In forensic settings, such as prison, malingered conditions more often present as posttraumatic stress disorder or cognitive impairment.5 In non-forensic settings, such as the ER, the most commonly malingered conditions include suicidality and psychosis.
To detect malingered psychosis, one must first understand how true psychotic symptoms manifest. The following discussion describes and compares typical and atypical symptoms of psychosis; examples are given in Table 2.6,7No single atypical psychotic symptom is indicative of malingering. Rather, a collection of atypical symptoms, when considered in clinical context, should raise suspicion of malingering and prompt you to seek additional collateral information or perform appropriate testing for malingering.
Hallucinations
Typically, hallucinations take three forms: auditory, visual, and tactile. In primary psychiatric conditions, auditory hallucinations are the most common of those three.
Tactile hallucinations can be present during episodes of substance intoxication or withdrawal (eg, so-called coke bugs).
Auditory hallucinations. Patients who malinger psychosis are often unaware of the nuances of hallucinations. For example, they might report the atypical symptom of continuous voices; in fact, most patients who have schizophrenia hear voices intermittently. Keep in mind, too, that 75% of patients who have schizophrenia hear male and female voices, and that 70% have some type of coping strategy to minimize their internal stimuli (eg, listening to music).6,7
Visual hallucinations are most often associated with neurologic disease, but also occur often in primary psychotic disorders, such as schizophrenia.
Patients who malinger psychotic symptoms often are open to suggestion, and are quick to endorse visual hallucinations. When asked to describe their hallucinations, however, they often respond without details (“I don’t know”). Other times, they overcompensate with wild exaggeration of atypical visions—recall Mr. K’s description of a towering Big Bird. Asked if the visions are in black and white, they might eagerly agree. Research suggests, however, that patients who have schizophrenia more often experience life-sized hallucinations of vivid scenes with family members, religious figures, or animals.8 Furthermore, genuine visual hallucinations typically are in color.
Putting malingering in the differential
Regardless of the number of atypical symptoms a patient exhibits, malingering will be missed if you do not include it in the differential diagnosis. This fact was made evident in a 1973 study.9
In that study, Rosenhan and seven of his colleagues—a psychology graduate student, three psychologists, a pediatrician, a psychiatrist, a painter, and a housewife—presented to various ERs and intake units, and, as they had been instructed, endorsed vague auditory hallucinations of “empty,” “hollow,” or “thud” sounds—but nothing more. All were admitted to psychiatric hospitals. Once admitted, they refrained (again, as instructed) from endorsing or exhibiting any psychotic symptoms.
Despite the vague nature of the reported auditory hallucinations and how rapidly symptoms resolved on admission, seven of these pseudo-patients were given a diagnosis of schizophrenia, and one was given a diagnosis of manic-depressive psychosis. Duration of admission ranged from 7 to 52 days (average, 19 days). None of the study participants were suspected of feigning symptoms.
It’s fortunate that, since then, mental health professionals have developed more structured techniques of assessment to detect malingering in inpatient and triage settings.
Testing to identify and assess malingering
The ER is a fast-paced environment, in which treatment teams are challenged to make rapid clinical assessments. With the overwhelming number of patients seeking mental health care in the ER, however, overall wait times are increasing; in some regions, it is common to write, then to rewrite, involuntary psychiatric holds for patients awaiting transfer to a psychiatric hospital. This extended duration presents an opportunity to serially evaluate patients suspected of malingering.
Even in environments that allow for a more comprehensive evaluation (eg, jail or inpatient psychiatric wards), few psychometric tests have been validated to detect malingering. The most validated tests include the Structured Interview of Reported Symptoms (SIRS), distributed now as the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2), and the Minnesota Multiphasic Personality Inventory Revised (MMPI-2). These tests typically require ≥30 minutes to administer and generally are not feasible in the fast-paced ER.
Despite the high prevalence of malingered behaviors in the ER, no single test has been validated in such a setting. Furthermore, there is no test designed to specifically assess for malingered suicidality or homicidality. The results of one test do not, in isolation, represent a comprehensive neuropsychological examination; rather, those results provide additional data to formulate a clinical impression. The instruments discussed below are administered and scored in a defined, objective manner.
When evaluating a patient whom you suspect of malingering, gathering collateral information—from family members, friends, nurses, social workers, emergency medicine physicians, and others—becomes important. You might discover pertinent information in ambulance and police reports and a review of the patient’s prior ER visits.
During the initial interview, ask open-ended questions; do not lead the patient by listing clusters of symptoms associated with a particular diagnosis. Because it is often difficult for a patient to malinger symptoms for a prolonged period, serial observations of a patient’s behavior and interview responses over time can provide additional information to make a clinical diagnosis of malingering.4
What testing is feasible in the ER?
Miller Forensic Assessment of Symptoms Test. The M-FAST measures rare symptom combinations, excessive reporting, and atypical symptoms of psychosis, using the same principles as the SIRS-2.
The 25-item screen begins by advising the examinee that he (she) will be asked questions about his psychological symptoms and that the questions that follow might or might not apply to his specific symptoms.
After that brief introduction, the examinee is asked if he hears ringing in his ears. Based on his response, the examiner reads one of two responses—both of which suggest the false notion that patients with true mental illness will suffer from ringing in their ears.
The examinee is then asked a series of Yes or No questions. Some pertain to legitimate symptoms a person with a psychotic illness might suffer (such as, “Do voices tell you to do things? Yes or No?”). Conversely, other questions screen for improbable symptoms that are atypical of patients who have a true psychotic disorder (such as “On many days I feel so bad that I can’t even remember my full name: Yes or No?”).
The exam concludes with a question about a ringing in the examinee’s ear. Affirmative responses are tallied; a score of ≥6 in a clinical setting is 83% specific and 93% sensitive for malingering.10
Visual Memory Test. Rey’s 15-Item Visual Memory Test capitalizes on the false belief that intellectual deficits, in addition to psychotic symptoms, make a claim of mental illness more believable.
In this simple test, the provider tells the examinee, “I am going to show you a card with 15 things on it that I want you to remember. When I take the card away, I want you to write down as many of the 15 things as you can remember.”3 The examinee is shown 15 common symbols (eg, 1, 2, 3; A, B, C; I, II, III, a, b, c; and the geometrics ●, ■, ▲).
At 5 seconds, the examinee is prompted, “Be sure to remember all of them.” After 10 seconds, the stimulus is removed, and the examinee is asked to recreate the figure.
Normative data indicate that even a patient who has a severe traumatic brain injury is able to recreate at least eight of the symbols. Although controversial, research indicates that a score of <9 symbols is predictive of malingering with 40% sensitivity and 100% specificity.11
Critics argued that confounding variables (IQ, memory disorder, age) might skew the quantitative score. For that reason, the same group developed the Rey’s II Test, which includes a supplementary qualitative scoring system that emphasizes embellishment errors (eg, the wrong symbol) and ordering errors (eg, wrong row). The Rey’s II Test proved to be more sensitive (accurate classification of malingers): A cut-off score of ≥2 qualitative errors is predictive of malingering with 86% sensitivity and 100% specificity.12
Coin-in-the-Hand Test. Perhaps the simplest test to administer is the Coin-in-the-Hand, designed to seem—superficially—to be a challenging memory test.
The patient must guess in which hand the examiner is holding a coin. The patient is shown the coin for two seconds, and then asked to close his eyes and count back from 10. The patient then points to one of the two clenched hands.
This task is repeated 10 times; each time, the provider gives verbal feedback about the accuracy or inaccuracy of that attempt. Studies indicate that a patient who has a severe traumatic brain injury is able to score 85% correct. A score <85%, however, suggests feigning of symptoms (sensitivity, 92.5%; specificity 87.5%).13 Hanley and co-workers demonstrated that people who are simulating cognitive impairment had a mean accurate response of 4.1, whereas people who had true amnesia had a mean accurate response of 9.65.14
Persons who feign psychosis or mood symptoms often inaccurately believe that people with mental illness also have cognitive impairment. Both Rey’s test and the Coin-in-the-Hand Test capitalize on this misconception.
Mini-Mental State Examination. Research also has shown that the Folstein Mini-Mental State Examination (MMSE) can screen for malingered cognitive impairment. Powell compared 40 mental health clinicians who were instructed to feign psychosis and 40 patients with schizophrenia. Using the MMSE, the researchers found that the malingers more often gave approximate answers.15 Moreover, Myers argued that, when compared with Rey’s Test, the MMSE is superior for assessing malingered cognitive impairment because it has a higher positive predictive value (67%, compared with 43% for Rey’s Test) and a higher negative predictive value (93% and 89%).16
What can you do for these patients after diagnosis?
Malingering is not considered a psychiatric diagnosis; there are no indicated therapies with which to manage it—only guidelines. When you suspect a patient of malingering, you should avoid accusing him (her) of faking symptoms. Rather, when feasible, gently confront the person and provide the opportunity for him to explain his current behaviors. For example, you might say: “I’ve treated many patients with the symptoms that you’re reporting, but the details you provide are different, and don’t ring completely true. Is there anything else that could explain this?”17
Regardless of a patient’s challenging behaviors, it is important to remember that people who feign illness—whether partial malingering or pure malingering—often do need help. The assistance they require, however, might be best obtained from a housing agency, a chemical dependency program, or another social service—not from the ER. Identifying malingered behaviors saves time and money and shifts limited resources to people who have a legitimate mental health condition.
Last, despite an empathetic approach, some malingering patients continue to feign symptoms—as Mr. K did.
CASE CONTINUED
Although the psychiatrist on call considered forsaking the police to escort Mr. K out of the ER, he eventually agreed to leave the hospital on his own, stating, “My death is going to be on your hands.”
Eight days later, Mr. K visited the ER at a different hospital, endorsing chronic pain and demanding narcotics.
Bottom Line
As the number of people seeking care in the emergency room (ER) has increased, so has the number of those who feign symptoms for secondary gain. No single factor is indicative of malingering, and no objective tests exist to diagnose it definitively. Furthermore, there are no indicated therapies with which to manage malingering—only guidelines. Keep in mind that people who feign illness, whether partial or pure malingering, often do need help—although not the services of an ER.
Related Resources
- Miller Forensic Assessment of Symptoms Test (M-FAST). Psychological Assessment Resources, Inc. www4.parinc.com (enter “M-FAST” in search field).
- Duffy S. Malingering psychological symptoms: An empirical review. Illinois State University, Department of Psychology. http://psychology.illinoisstate.edu/cc/Comps/Duffy%20-%20Malingering.pdf. Accessed September 10, 2013.
Drug Brand Names
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
M. Cait Brady, MD, shares strategies for assessing malingering. Dr. Brady is a Third-Year Resident in General Psychiatry, University of California, Davis Medical Center - Sacramento, Sacramento, California.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Yates BD, Nordquist CR, Schultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
3. Reccoppa L. Mentally ill or malingering? 3 clues cast doubt. Current Psychiatry. 2009;8(12):110.
4. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
5. Gunn J, Taylor P. Forensic psychiatry: clinical, legal and ethical issues. Oxford, United Kingdom: Butterworth-Heinemann; 1998.
6. Farhall J, Greenwood K, Jackson H. Coping with hallucinated voices in schizophrenia: a review of self-initiated and therapeutic interventions. Clin Psychol Rev. 2007;27(4):476-493.
7. Goodwin DW, Anderson P, Rosenthal R. Clinical significance of hallucinations in psychiatric disorders: a study of 116 hallucinatory patients. Arch Gen Psychiatry. 1971;24:76-80.
8. Small IJ, Small JG, Andersen JM. Clinical characteristics of hallucinations of schizophrenia. Dis Nerv Syst. 1966;27(5):349-353.
9. Rosenhan DL. On being sane in insane places. Science. 1973;179(70):250-258.
10. Miller HA. M-FAST interview booklet. Lutz, FL: Psychological Assessment Resources; 2001.
11. Hom J, Denney RL. Detection of response bias in forensic neuropsychology. Binghamton, NY: Haworth Medical Press; 2002.
12. Whitney KA, Hook JN, Steiner AR, et al. Is the Rey 15-Item Memory Test II (Rey II) a valid symptom validity test?: comparison with the TOMM. Appl Neuropsychol. 2008;15(4):287-292.
13. Kelly PJ, Baker GA, van den Broek MD, et al. The detection of malingering in memory performance: the sensitivity and specificity of four measures in a UK population. Br J Clin Psychol. 2005;44(3):333-341.
14. Hanley JR, Backer G, Ledson S. Detecting the faking of amnesia: a comparison of the effectiveness of three different techniques for distinguishing simulators from patients with amnesia. J Clin Exp Neuropsychol. 1999;21(1):59-69.
15. Rogers R. Clinical assessment of malingering and deception, 3rd ed. New York, NY: The Gilford Press; 2008:54.
16. Myers W, Hall R, Tolou-Shams M. Prevalence and assessment of malingering in homicide defendants using the mini-mental state examination and the Rey 15-Item Memory Test. Homicide Stud. 2013;17(3):314-328.
17. Resnick PJ. In session with Phillip J. Resnick, MD: malingering of psychiatric symptoms. Prim Psychiatry. 2006;13(6):35-38.
The economic downturn in the United States has prompted numerous state and county budget cuts, in turn forcing many patients to receive their mental health care in the emergency room (ER). Most patients evaluated in the ER for mental health-related reasons have a legitimate psychiatric crisis—but that isn’t always the case. And as the number of people seeking care in the ER has increased, it appears that so too has the number of those who feign symptoms for secondary gain—that is, who are malingering.
This article highlights several red flags for malingered behavior; emphasizes typical (compared with atypical) symptoms of psychosis; and provides an overview of four instruments that you can use to help assess for malingering in the ED.
A difficult diagnosis
No single factor is indicative of malingering, and no objective tests exist to diagnose malingering definitively. Rather, the tests we discuss provide additional information that can help formulate a clinical impression.
According to DSM-5, malingering is “…the intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives…”1 Despite a relatively straightforward definition, the diagnosis is difficult to make because it is a diagnosis of exclusion.
Even with sufficient evidence, many clinicians are reluctant to diagnose malingering because they fear retaliation and diagnostic uncertainty. Psychiatrists also might be reluctant to diagnose malingering because the negative connotation that the label carries risks stigmatizing a patient who might, in fact, be suffering. This is true especially when there is suspicion of partial malingering, the conscious exaggeration of existing symptoms.
Despite physicians’ reluctance to diagnose malingering, it is a real problem, especially in the ER. Research suggests that as many as 13% of patients in the ER feign illness, and that their secondary gain most often includes food, shelter, prescription drugs, financial gain, and avoidance of jail, work, or family responsibilities.2
CASE REPORT ‘The voices are telling me to kill myself’
Mr. K, a 36-year-old white man, walks into the ER on a late December day. He tells the triage nurse that he suicidal; she escorts him to the psychiatric pod of the ER. Nursing staff provide line-of-sight care, monitor his vital signs, and draw blood for testing.
Within hours, Mr. K is deemed “medically cleared” and ready for assessment by the psychiatric social worker.
Interview and assessment. During the interview with the social worker, Mr. K reports that he has been depressed, adamantly maintaining that he is suicidal, with a plan to “walk in traffic” or “eat the end of a gun.” The social worker places him on a 72-hour involuntary psychiatric hold. ER physicians order psychiatric consultation.
Mr. K is well-known to the psychiatrist on call, from prior ER visits and psychiatric hospital admissions. In fact, two days earlier, he put a psychiatric nurse in a headlock while being escorted from the psychiatric inpatient unit under protest.
On assessment by the psychiatrist, Mr. K continues to endorse feeling suicidal; he adds: “If I don’t get some help, I’m gonna kill somebody else!”
Without prompting, the patient states that “the voices are telling me to kill myself.” He says that those voices have been relentless since he left the hospital two days earlier. According to Mr. K, nothing he did helped quiet the voices, although previous prescriptions for quetiapine have been helpful.
Mr. K says that he is unable to recall the clinic or name of his prior psychiatrist. He claims that he was hospitalized four months ago, (despite the psychiatrist’s knowledge that he had been discharged two days ago) and estimates that his psychotic symptoms began one year ago. He explains that he is homeless and does not have social support. He is unable to provide a telephone number or a name to contact family for collateral information.
Mental status exam. The mental status examination reveals a tall, thin, disheveled man who has poor dentition. He is now calm and cooperative despite his reported level of distress. His speech is unremarkable and his eye contact is appropriate. His thought process is linear, organized, and coherent.
Mr. K does not endorse additional symptoms, but is quick to agree with the psychiatrist’s follow-up questions about hallucinations: “Yeah! I’ve been seeing all kinds of crazy stuff.” When prompted for details, he says, “I just saw Big Bird… He was 100 feet tall!”
Lab testing. Mr. K’s blood work is remarkable for positive urine toxicology for amphetamines.
Nursing notes indicate that Mr. K slept overnight and ate 100% of the food on his dinner and breakfast trays.
Red flags flying
Mr. K’s case highlights several red flags that should raise suspicion of malingering (Table 1)3,4:
- A conditional statement by which a patient threatens to harm himself or others, contingent upon a demand—for example, “If I don’t get A, I’ll do B.”
- An overly dramatic presentation, in which the patient is quick to endorse
distressing symptoms. Consider Mr. K: He was quick to report that he saw Big Bird, and that this Sesame Street character “was 100 feet tall.” Patients who have been experiencing true psychotic symptoms might be reluctant to speak of their distressing symptoms, especially if they have not experienced such symptoms in the past (the first psychotic break). Mr. K, however, volunteered and called attention to particularly dramatic psychotic symptoms. - A subjective report of distress that is inconsistent with the objective presentation. Mr. K’s report of depression—a diagnosis that typically includes insomnia and poor appetite—was inconsistent with his behavior: He slept and he ate all of his meals.
Atypical (vs typical) psychosis
Malingering can occur in various arenas and take many different forms. In forensic settings, such as prison, malingered conditions more often present as posttraumatic stress disorder or cognitive impairment.5 In non-forensic settings, such as the ER, the most commonly malingered conditions include suicidality and psychosis.
To detect malingered psychosis, one must first understand how true psychotic symptoms manifest. The following discussion describes and compares typical and atypical symptoms of psychosis; examples are given in Table 2.6,7No single atypical psychotic symptom is indicative of malingering. Rather, a collection of atypical symptoms, when considered in clinical context, should raise suspicion of malingering and prompt you to seek additional collateral information or perform appropriate testing for malingering.
Hallucinations
Typically, hallucinations take three forms: auditory, visual, and tactile. In primary psychiatric conditions, auditory hallucinations are the most common of those three.
Tactile hallucinations can be present during episodes of substance intoxication or withdrawal (eg, so-called coke bugs).
Auditory hallucinations. Patients who malinger psychosis are often unaware of the nuances of hallucinations. For example, they might report the atypical symptom of continuous voices; in fact, most patients who have schizophrenia hear voices intermittently. Keep in mind, too, that 75% of patients who have schizophrenia hear male and female voices, and that 70% have some type of coping strategy to minimize their internal stimuli (eg, listening to music).6,7
Visual hallucinations are most often associated with neurologic disease, but also occur often in primary psychotic disorders, such as schizophrenia.
Patients who malinger psychotic symptoms often are open to suggestion, and are quick to endorse visual hallucinations. When asked to describe their hallucinations, however, they often respond without details (“I don’t know”). Other times, they overcompensate with wild exaggeration of atypical visions—recall Mr. K’s description of a towering Big Bird. Asked if the visions are in black and white, they might eagerly agree. Research suggests, however, that patients who have schizophrenia more often experience life-sized hallucinations of vivid scenes with family members, religious figures, or animals.8 Furthermore, genuine visual hallucinations typically are in color.
Putting malingering in the differential
Regardless of the number of atypical symptoms a patient exhibits, malingering will be missed if you do not include it in the differential diagnosis. This fact was made evident in a 1973 study.9
In that study, Rosenhan and seven of his colleagues—a psychology graduate student, three psychologists, a pediatrician, a psychiatrist, a painter, and a housewife—presented to various ERs and intake units, and, as they had been instructed, endorsed vague auditory hallucinations of “empty,” “hollow,” or “thud” sounds—but nothing more. All were admitted to psychiatric hospitals. Once admitted, they refrained (again, as instructed) from endorsing or exhibiting any psychotic symptoms.
Despite the vague nature of the reported auditory hallucinations and how rapidly symptoms resolved on admission, seven of these pseudo-patients were given a diagnosis of schizophrenia, and one was given a diagnosis of manic-depressive psychosis. Duration of admission ranged from 7 to 52 days (average, 19 days). None of the study participants were suspected of feigning symptoms.
It’s fortunate that, since then, mental health professionals have developed more structured techniques of assessment to detect malingering in inpatient and triage settings.
Testing to identify and assess malingering
The ER is a fast-paced environment, in which treatment teams are challenged to make rapid clinical assessments. With the overwhelming number of patients seeking mental health care in the ER, however, overall wait times are increasing; in some regions, it is common to write, then to rewrite, involuntary psychiatric holds for patients awaiting transfer to a psychiatric hospital. This extended duration presents an opportunity to serially evaluate patients suspected of malingering.
Even in environments that allow for a more comprehensive evaluation (eg, jail or inpatient psychiatric wards), few psychometric tests have been validated to detect malingering. The most validated tests include the Structured Interview of Reported Symptoms (SIRS), distributed now as the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2), and the Minnesota Multiphasic Personality Inventory Revised (MMPI-2). These tests typically require ≥30 minutes to administer and generally are not feasible in the fast-paced ER.
Despite the high prevalence of malingered behaviors in the ER, no single test has been validated in such a setting. Furthermore, there is no test designed to specifically assess for malingered suicidality or homicidality. The results of one test do not, in isolation, represent a comprehensive neuropsychological examination; rather, those results provide additional data to formulate a clinical impression. The instruments discussed below are administered and scored in a defined, objective manner.
When evaluating a patient whom you suspect of malingering, gathering collateral information—from family members, friends, nurses, social workers, emergency medicine physicians, and others—becomes important. You might discover pertinent information in ambulance and police reports and a review of the patient’s prior ER visits.
During the initial interview, ask open-ended questions; do not lead the patient by listing clusters of symptoms associated with a particular diagnosis. Because it is often difficult for a patient to malinger symptoms for a prolonged period, serial observations of a patient’s behavior and interview responses over time can provide additional information to make a clinical diagnosis of malingering.4
What testing is feasible in the ER?
Miller Forensic Assessment of Symptoms Test. The M-FAST measures rare symptom combinations, excessive reporting, and atypical symptoms of psychosis, using the same principles as the SIRS-2.
The 25-item screen begins by advising the examinee that he (she) will be asked questions about his psychological symptoms and that the questions that follow might or might not apply to his specific symptoms.
After that brief introduction, the examinee is asked if he hears ringing in his ears. Based on his response, the examiner reads one of two responses—both of which suggest the false notion that patients with true mental illness will suffer from ringing in their ears.
The examinee is then asked a series of Yes or No questions. Some pertain to legitimate symptoms a person with a psychotic illness might suffer (such as, “Do voices tell you to do things? Yes or No?”). Conversely, other questions screen for improbable symptoms that are atypical of patients who have a true psychotic disorder (such as “On many days I feel so bad that I can’t even remember my full name: Yes or No?”).
The exam concludes with a question about a ringing in the examinee’s ear. Affirmative responses are tallied; a score of ≥6 in a clinical setting is 83% specific and 93% sensitive for malingering.10
Visual Memory Test. Rey’s 15-Item Visual Memory Test capitalizes on the false belief that intellectual deficits, in addition to psychotic symptoms, make a claim of mental illness more believable.
In this simple test, the provider tells the examinee, “I am going to show you a card with 15 things on it that I want you to remember. When I take the card away, I want you to write down as many of the 15 things as you can remember.”3 The examinee is shown 15 common symbols (eg, 1, 2, 3; A, B, C; I, II, III, a, b, c; and the geometrics ●, ■, ▲).
At 5 seconds, the examinee is prompted, “Be sure to remember all of them.” After 10 seconds, the stimulus is removed, and the examinee is asked to recreate the figure.
Normative data indicate that even a patient who has a severe traumatic brain injury is able to recreate at least eight of the symbols. Although controversial, research indicates that a score of <9 symbols is predictive of malingering with 40% sensitivity and 100% specificity.11
Critics argued that confounding variables (IQ, memory disorder, age) might skew the quantitative score. For that reason, the same group developed the Rey’s II Test, which includes a supplementary qualitative scoring system that emphasizes embellishment errors (eg, the wrong symbol) and ordering errors (eg, wrong row). The Rey’s II Test proved to be more sensitive (accurate classification of malingers): A cut-off score of ≥2 qualitative errors is predictive of malingering with 86% sensitivity and 100% specificity.12
Coin-in-the-Hand Test. Perhaps the simplest test to administer is the Coin-in-the-Hand, designed to seem—superficially—to be a challenging memory test.
The patient must guess in which hand the examiner is holding a coin. The patient is shown the coin for two seconds, and then asked to close his eyes and count back from 10. The patient then points to one of the two clenched hands.
This task is repeated 10 times; each time, the provider gives verbal feedback about the accuracy or inaccuracy of that attempt. Studies indicate that a patient who has a severe traumatic brain injury is able to score 85% correct. A score <85%, however, suggests feigning of symptoms (sensitivity, 92.5%; specificity 87.5%).13 Hanley and co-workers demonstrated that people who are simulating cognitive impairment had a mean accurate response of 4.1, whereas people who had true amnesia had a mean accurate response of 9.65.14
Persons who feign psychosis or mood symptoms often inaccurately believe that people with mental illness also have cognitive impairment. Both Rey’s test and the Coin-in-the-Hand Test capitalize on this misconception.
Mini-Mental State Examination. Research also has shown that the Folstein Mini-Mental State Examination (MMSE) can screen for malingered cognitive impairment. Powell compared 40 mental health clinicians who were instructed to feign psychosis and 40 patients with schizophrenia. Using the MMSE, the researchers found that the malingers more often gave approximate answers.15 Moreover, Myers argued that, when compared with Rey’s Test, the MMSE is superior for assessing malingered cognitive impairment because it has a higher positive predictive value (67%, compared with 43% for Rey’s Test) and a higher negative predictive value (93% and 89%).16
What can you do for these patients after diagnosis?
Malingering is not considered a psychiatric diagnosis; there are no indicated therapies with which to manage it—only guidelines. When you suspect a patient of malingering, you should avoid accusing him (her) of faking symptoms. Rather, when feasible, gently confront the person and provide the opportunity for him to explain his current behaviors. For example, you might say: “I’ve treated many patients with the symptoms that you’re reporting, but the details you provide are different, and don’t ring completely true. Is there anything else that could explain this?”17
Regardless of a patient’s challenging behaviors, it is important to remember that people who feign illness—whether partial malingering or pure malingering—often do need help. The assistance they require, however, might be best obtained from a housing agency, a chemical dependency program, or another social service—not from the ER. Identifying malingered behaviors saves time and money and shifts limited resources to people who have a legitimate mental health condition.
Last, despite an empathetic approach, some malingering patients continue to feign symptoms—as Mr. K did.
CASE CONTINUED
Although the psychiatrist on call considered forsaking the police to escort Mr. K out of the ER, he eventually agreed to leave the hospital on his own, stating, “My death is going to be on your hands.”
Eight days later, Mr. K visited the ER at a different hospital, endorsing chronic pain and demanding narcotics.
Bottom Line
As the number of people seeking care in the emergency room (ER) has increased, so has the number of those who feign symptoms for secondary gain. No single factor is indicative of malingering, and no objective tests exist to diagnose it definitively. Furthermore, there are no indicated therapies with which to manage malingering—only guidelines. Keep in mind that people who feign illness, whether partial or pure malingering, often do need help—although not the services of an ER.
Related Resources
- Miller Forensic Assessment of Symptoms Test (M-FAST). Psychological Assessment Resources, Inc. www4.parinc.com (enter “M-FAST” in search field).
- Duffy S. Malingering psychological symptoms: An empirical review. Illinois State University, Department of Psychology. http://psychology.illinoisstate.edu/cc/Comps/Duffy%20-%20Malingering.pdf. Accessed September 10, 2013.
Drug Brand Names
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
M. Cait Brady, MD, shares strategies for assessing malingering. Dr. Brady is a Third-Year Resident in General Psychiatry, University of California, Davis Medical Center - Sacramento, Sacramento, California.
The economic downturn in the United States has prompted numerous state and county budget cuts, in turn forcing many patients to receive their mental health care in the emergency room (ER). Most patients evaluated in the ER for mental health-related reasons have a legitimate psychiatric crisis—but that isn’t always the case. And as the number of people seeking care in the ER has increased, it appears that so too has the number of those who feign symptoms for secondary gain—that is, who are malingering.
This article highlights several red flags for malingered behavior; emphasizes typical (compared with atypical) symptoms of psychosis; and provides an overview of four instruments that you can use to help assess for malingering in the ED.
A difficult diagnosis
No single factor is indicative of malingering, and no objective tests exist to diagnose malingering definitively. Rather, the tests we discuss provide additional information that can help formulate a clinical impression.
According to DSM-5, malingering is “…the intentional production of false or grossly exaggerated physical or psychological symptoms, motivated by external incentives…”1 Despite a relatively straightforward definition, the diagnosis is difficult to make because it is a diagnosis of exclusion.
Even with sufficient evidence, many clinicians are reluctant to diagnose malingering because they fear retaliation and diagnostic uncertainty. Psychiatrists also might be reluctant to diagnose malingering because the negative connotation that the label carries risks stigmatizing a patient who might, in fact, be suffering. This is true especially when there is suspicion of partial malingering, the conscious exaggeration of existing symptoms.
Despite physicians’ reluctance to diagnose malingering, it is a real problem, especially in the ER. Research suggests that as many as 13% of patients in the ER feign illness, and that their secondary gain most often includes food, shelter, prescription drugs, financial gain, and avoidance of jail, work, or family responsibilities.2
CASE REPORT ‘The voices are telling me to kill myself’
Mr. K, a 36-year-old white man, walks into the ER on a late December day. He tells the triage nurse that he suicidal; she escorts him to the psychiatric pod of the ER. Nursing staff provide line-of-sight care, monitor his vital signs, and draw blood for testing.
Within hours, Mr. K is deemed “medically cleared” and ready for assessment by the psychiatric social worker.
Interview and assessment. During the interview with the social worker, Mr. K reports that he has been depressed, adamantly maintaining that he is suicidal, with a plan to “walk in traffic” or “eat the end of a gun.” The social worker places him on a 72-hour involuntary psychiatric hold. ER physicians order psychiatric consultation.
Mr. K is well-known to the psychiatrist on call, from prior ER visits and psychiatric hospital admissions. In fact, two days earlier, he put a psychiatric nurse in a headlock while being escorted from the psychiatric inpatient unit under protest.
On assessment by the psychiatrist, Mr. K continues to endorse feeling suicidal; he adds: “If I don’t get some help, I’m gonna kill somebody else!”
Without prompting, the patient states that “the voices are telling me to kill myself.” He says that those voices have been relentless since he left the hospital two days earlier. According to Mr. K, nothing he did helped quiet the voices, although previous prescriptions for quetiapine have been helpful.
Mr. K says that he is unable to recall the clinic or name of his prior psychiatrist. He claims that he was hospitalized four months ago, (despite the psychiatrist’s knowledge that he had been discharged two days ago) and estimates that his psychotic symptoms began one year ago. He explains that he is homeless and does not have social support. He is unable to provide a telephone number or a name to contact family for collateral information.
Mental status exam. The mental status examination reveals a tall, thin, disheveled man who has poor dentition. He is now calm and cooperative despite his reported level of distress. His speech is unremarkable and his eye contact is appropriate. His thought process is linear, organized, and coherent.
Mr. K does not endorse additional symptoms, but is quick to agree with the psychiatrist’s follow-up questions about hallucinations: “Yeah! I’ve been seeing all kinds of crazy stuff.” When prompted for details, he says, “I just saw Big Bird… He was 100 feet tall!”
Lab testing. Mr. K’s blood work is remarkable for positive urine toxicology for amphetamines.
Nursing notes indicate that Mr. K slept overnight and ate 100% of the food on his dinner and breakfast trays.
Red flags flying
Mr. K’s case highlights several red flags that should raise suspicion of malingering (Table 1)3,4:
- A conditional statement by which a patient threatens to harm himself or others, contingent upon a demand—for example, “If I don’t get A, I’ll do B.”
- An overly dramatic presentation, in which the patient is quick to endorse
distressing symptoms. Consider Mr. K: He was quick to report that he saw Big Bird, and that this Sesame Street character “was 100 feet tall.” Patients who have been experiencing true psychotic symptoms might be reluctant to speak of their distressing symptoms, especially if they have not experienced such symptoms in the past (the first psychotic break). Mr. K, however, volunteered and called attention to particularly dramatic psychotic symptoms. - A subjective report of distress that is inconsistent with the objective presentation. Mr. K’s report of depression—a diagnosis that typically includes insomnia and poor appetite—was inconsistent with his behavior: He slept and he ate all of his meals.
Atypical (vs typical) psychosis
Malingering can occur in various arenas and take many different forms. In forensic settings, such as prison, malingered conditions more often present as posttraumatic stress disorder or cognitive impairment.5 In non-forensic settings, such as the ER, the most commonly malingered conditions include suicidality and psychosis.
To detect malingered psychosis, one must first understand how true psychotic symptoms manifest. The following discussion describes and compares typical and atypical symptoms of psychosis; examples are given in Table 2.6,7No single atypical psychotic symptom is indicative of malingering. Rather, a collection of atypical symptoms, when considered in clinical context, should raise suspicion of malingering and prompt you to seek additional collateral information or perform appropriate testing for malingering.
Hallucinations
Typically, hallucinations take three forms: auditory, visual, and tactile. In primary psychiatric conditions, auditory hallucinations are the most common of those three.
Tactile hallucinations can be present during episodes of substance intoxication or withdrawal (eg, so-called coke bugs).
Auditory hallucinations. Patients who malinger psychosis are often unaware of the nuances of hallucinations. For example, they might report the atypical symptom of continuous voices; in fact, most patients who have schizophrenia hear voices intermittently. Keep in mind, too, that 75% of patients who have schizophrenia hear male and female voices, and that 70% have some type of coping strategy to minimize their internal stimuli (eg, listening to music).6,7
Visual hallucinations are most often associated with neurologic disease, but also occur often in primary psychotic disorders, such as schizophrenia.
Patients who malinger psychotic symptoms often are open to suggestion, and are quick to endorse visual hallucinations. When asked to describe their hallucinations, however, they often respond without details (“I don’t know”). Other times, they overcompensate with wild exaggeration of atypical visions—recall Mr. K’s description of a towering Big Bird. Asked if the visions are in black and white, they might eagerly agree. Research suggests, however, that patients who have schizophrenia more often experience life-sized hallucinations of vivid scenes with family members, religious figures, or animals.8 Furthermore, genuine visual hallucinations typically are in color.
Putting malingering in the differential
Regardless of the number of atypical symptoms a patient exhibits, malingering will be missed if you do not include it in the differential diagnosis. This fact was made evident in a 1973 study.9
In that study, Rosenhan and seven of his colleagues—a psychology graduate student, three psychologists, a pediatrician, a psychiatrist, a painter, and a housewife—presented to various ERs and intake units, and, as they had been instructed, endorsed vague auditory hallucinations of “empty,” “hollow,” or “thud” sounds—but nothing more. All were admitted to psychiatric hospitals. Once admitted, they refrained (again, as instructed) from endorsing or exhibiting any psychotic symptoms.
Despite the vague nature of the reported auditory hallucinations and how rapidly symptoms resolved on admission, seven of these pseudo-patients were given a diagnosis of schizophrenia, and one was given a diagnosis of manic-depressive psychosis. Duration of admission ranged from 7 to 52 days (average, 19 days). None of the study participants were suspected of feigning symptoms.
It’s fortunate that, since then, mental health professionals have developed more structured techniques of assessment to detect malingering in inpatient and triage settings.
Testing to identify and assess malingering
The ER is a fast-paced environment, in which treatment teams are challenged to make rapid clinical assessments. With the overwhelming number of patients seeking mental health care in the ER, however, overall wait times are increasing; in some regions, it is common to write, then to rewrite, involuntary psychiatric holds for patients awaiting transfer to a psychiatric hospital. This extended duration presents an opportunity to serially evaluate patients suspected of malingering.
Even in environments that allow for a more comprehensive evaluation (eg, jail or inpatient psychiatric wards), few psychometric tests have been validated to detect malingering. The most validated tests include the Structured Interview of Reported Symptoms (SIRS), distributed now as the Structured Interview of Reported Symptoms, 2nd edition (SIRS-2), and the Minnesota Multiphasic Personality Inventory Revised (MMPI-2). These tests typically require ≥30 minutes to administer and generally are not feasible in the fast-paced ER.
Despite the high prevalence of malingered behaviors in the ER, no single test has been validated in such a setting. Furthermore, there is no test designed to specifically assess for malingered suicidality or homicidality. The results of one test do not, in isolation, represent a comprehensive neuropsychological examination; rather, those results provide additional data to formulate a clinical impression. The instruments discussed below are administered and scored in a defined, objective manner.
When evaluating a patient whom you suspect of malingering, gathering collateral information—from family members, friends, nurses, social workers, emergency medicine physicians, and others—becomes important. You might discover pertinent information in ambulance and police reports and a review of the patient’s prior ER visits.
During the initial interview, ask open-ended questions; do not lead the patient by listing clusters of symptoms associated with a particular diagnosis. Because it is often difficult for a patient to malinger symptoms for a prolonged period, serial observations of a patient’s behavior and interview responses over time can provide additional information to make a clinical diagnosis of malingering.4
What testing is feasible in the ER?
Miller Forensic Assessment of Symptoms Test. The M-FAST measures rare symptom combinations, excessive reporting, and atypical symptoms of psychosis, using the same principles as the SIRS-2.
The 25-item screen begins by advising the examinee that he (she) will be asked questions about his psychological symptoms and that the questions that follow might or might not apply to his specific symptoms.
After that brief introduction, the examinee is asked if he hears ringing in his ears. Based on his response, the examiner reads one of two responses—both of which suggest the false notion that patients with true mental illness will suffer from ringing in their ears.
The examinee is then asked a series of Yes or No questions. Some pertain to legitimate symptoms a person with a psychotic illness might suffer (such as, “Do voices tell you to do things? Yes or No?”). Conversely, other questions screen for improbable symptoms that are atypical of patients who have a true psychotic disorder (such as “On many days I feel so bad that I can’t even remember my full name: Yes or No?”).
The exam concludes with a question about a ringing in the examinee’s ear. Affirmative responses are tallied; a score of ≥6 in a clinical setting is 83% specific and 93% sensitive for malingering.10
Visual Memory Test. Rey’s 15-Item Visual Memory Test capitalizes on the false belief that intellectual deficits, in addition to psychotic symptoms, make a claim of mental illness more believable.
In this simple test, the provider tells the examinee, “I am going to show you a card with 15 things on it that I want you to remember. When I take the card away, I want you to write down as many of the 15 things as you can remember.”3 The examinee is shown 15 common symbols (eg, 1, 2, 3; A, B, C; I, II, III, a, b, c; and the geometrics ●, ■, ▲).
At 5 seconds, the examinee is prompted, “Be sure to remember all of them.” After 10 seconds, the stimulus is removed, and the examinee is asked to recreate the figure.
Normative data indicate that even a patient who has a severe traumatic brain injury is able to recreate at least eight of the symbols. Although controversial, research indicates that a score of <9 symbols is predictive of malingering with 40% sensitivity and 100% specificity.11
Critics argued that confounding variables (IQ, memory disorder, age) might skew the quantitative score. For that reason, the same group developed the Rey’s II Test, which includes a supplementary qualitative scoring system that emphasizes embellishment errors (eg, the wrong symbol) and ordering errors (eg, wrong row). The Rey’s II Test proved to be more sensitive (accurate classification of malingers): A cut-off score of ≥2 qualitative errors is predictive of malingering with 86% sensitivity and 100% specificity.12
Coin-in-the-Hand Test. Perhaps the simplest test to administer is the Coin-in-the-Hand, designed to seem—superficially—to be a challenging memory test.
The patient must guess in which hand the examiner is holding a coin. The patient is shown the coin for two seconds, and then asked to close his eyes and count back from 10. The patient then points to one of the two clenched hands.
This task is repeated 10 times; each time, the provider gives verbal feedback about the accuracy or inaccuracy of that attempt. Studies indicate that a patient who has a severe traumatic brain injury is able to score 85% correct. A score <85%, however, suggests feigning of symptoms (sensitivity, 92.5%; specificity 87.5%).13 Hanley and co-workers demonstrated that people who are simulating cognitive impairment had a mean accurate response of 4.1, whereas people who had true amnesia had a mean accurate response of 9.65.14
Persons who feign psychosis or mood symptoms often inaccurately believe that people with mental illness also have cognitive impairment. Both Rey’s test and the Coin-in-the-Hand Test capitalize on this misconception.
Mini-Mental State Examination. Research also has shown that the Folstein Mini-Mental State Examination (MMSE) can screen for malingered cognitive impairment. Powell compared 40 mental health clinicians who were instructed to feign psychosis and 40 patients with schizophrenia. Using the MMSE, the researchers found that the malingers more often gave approximate answers.15 Moreover, Myers argued that, when compared with Rey’s Test, the MMSE is superior for assessing malingered cognitive impairment because it has a higher positive predictive value (67%, compared with 43% for Rey’s Test) and a higher negative predictive value (93% and 89%).16
What can you do for these patients after diagnosis?
Malingering is not considered a psychiatric diagnosis; there are no indicated therapies with which to manage it—only guidelines. When you suspect a patient of malingering, you should avoid accusing him (her) of faking symptoms. Rather, when feasible, gently confront the person and provide the opportunity for him to explain his current behaviors. For example, you might say: “I’ve treated many patients with the symptoms that you’re reporting, but the details you provide are different, and don’t ring completely true. Is there anything else that could explain this?”17
Regardless of a patient’s challenging behaviors, it is important to remember that people who feign illness—whether partial malingering or pure malingering—often do need help. The assistance they require, however, might be best obtained from a housing agency, a chemical dependency program, or another social service—not from the ER. Identifying malingered behaviors saves time and money and shifts limited resources to people who have a legitimate mental health condition.
Last, despite an empathetic approach, some malingering patients continue to feign symptoms—as Mr. K did.
CASE CONTINUED
Although the psychiatrist on call considered forsaking the police to escort Mr. K out of the ER, he eventually agreed to leave the hospital on his own, stating, “My death is going to be on your hands.”
Eight days later, Mr. K visited the ER at a different hospital, endorsing chronic pain and demanding narcotics.
Bottom Line
As the number of people seeking care in the emergency room (ER) has increased, so has the number of those who feign symptoms for secondary gain. No single factor is indicative of malingering, and no objective tests exist to diagnose it definitively. Furthermore, there are no indicated therapies with which to manage malingering—only guidelines. Keep in mind that people who feign illness, whether partial or pure malingering, often do need help—although not the services of an ER.
Related Resources
- Miller Forensic Assessment of Symptoms Test (M-FAST). Psychological Assessment Resources, Inc. www4.parinc.com (enter “M-FAST” in search field).
- Duffy S. Malingering psychological symptoms: An empirical review. Illinois State University, Department of Psychology. http://psychology.illinoisstate.edu/cc/Comps/Duffy%20-%20Malingering.pdf. Accessed September 10, 2013.
Drug Brand Names
Quetiapine • Seroquel
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
M. Cait Brady, MD, shares strategies for assessing malingering. Dr. Brady is a Third-Year Resident in General Psychiatry, University of California, Davis Medical Center - Sacramento, Sacramento, California.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Yates BD, Nordquist CR, Schultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
3. Reccoppa L. Mentally ill or malingering? 3 clues cast doubt. Current Psychiatry. 2009;8(12):110.
4. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
5. Gunn J, Taylor P. Forensic psychiatry: clinical, legal and ethical issues. Oxford, United Kingdom: Butterworth-Heinemann; 1998.
6. Farhall J, Greenwood K, Jackson H. Coping with hallucinated voices in schizophrenia: a review of self-initiated and therapeutic interventions. Clin Psychol Rev. 2007;27(4):476-493.
7. Goodwin DW, Anderson P, Rosenthal R. Clinical significance of hallucinations in psychiatric disorders: a study of 116 hallucinatory patients. Arch Gen Psychiatry. 1971;24:76-80.
8. Small IJ, Small JG, Andersen JM. Clinical characteristics of hallucinations of schizophrenia. Dis Nerv Syst. 1966;27(5):349-353.
9. Rosenhan DL. On being sane in insane places. Science. 1973;179(70):250-258.
10. Miller HA. M-FAST interview booklet. Lutz, FL: Psychological Assessment Resources; 2001.
11. Hom J, Denney RL. Detection of response bias in forensic neuropsychology. Binghamton, NY: Haworth Medical Press; 2002.
12. Whitney KA, Hook JN, Steiner AR, et al. Is the Rey 15-Item Memory Test II (Rey II) a valid symptom validity test?: comparison with the TOMM. Appl Neuropsychol. 2008;15(4):287-292.
13. Kelly PJ, Baker GA, van den Broek MD, et al. The detection of malingering in memory performance: the sensitivity and specificity of four measures in a UK population. Br J Clin Psychol. 2005;44(3):333-341.
14. Hanley JR, Backer G, Ledson S. Detecting the faking of amnesia: a comparison of the effectiveness of three different techniques for distinguishing simulators from patients with amnesia. J Clin Exp Neuropsychol. 1999;21(1):59-69.
15. Rogers R. Clinical assessment of malingering and deception, 3rd ed. New York, NY: The Gilford Press; 2008:54.
16. Myers W, Hall R, Tolou-Shams M. Prevalence and assessment of malingering in homicide defendants using the mini-mental state examination and the Rey 15-Item Memory Test. Homicide Stud. 2013;17(3):314-328.
17. Resnick PJ. In session with Phillip J. Resnick, MD: malingering of psychiatric symptoms. Prim Psychiatry. 2006;13(6):35-38.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Yates BD, Nordquist CR, Schultz-Ross RA. Feigned psychiatric symptoms in the emergency room. Psychiatr Serv. 1996;47(9):998-1000.
3. Reccoppa L. Mentally ill or malingering? 3 clues cast doubt. Current Psychiatry. 2009;8(12):110.
4. Resnick PJ, Knoll J. Faking it: how to detect malingered psychosis. Current Psychiatry. 2005;4(11):12-25.
5. Gunn J, Taylor P. Forensic psychiatry: clinical, legal and ethical issues. Oxford, United Kingdom: Butterworth-Heinemann; 1998.
6. Farhall J, Greenwood K, Jackson H. Coping with hallucinated voices in schizophrenia: a review of self-initiated and therapeutic interventions. Clin Psychol Rev. 2007;27(4):476-493.
7. Goodwin DW, Anderson P, Rosenthal R. Clinical significance of hallucinations in psychiatric disorders: a study of 116 hallucinatory patients. Arch Gen Psychiatry. 1971;24:76-80.
8. Small IJ, Small JG, Andersen JM. Clinical characteristics of hallucinations of schizophrenia. Dis Nerv Syst. 1966;27(5):349-353.
9. Rosenhan DL. On being sane in insane places. Science. 1973;179(70):250-258.
10. Miller HA. M-FAST interview booklet. Lutz, FL: Psychological Assessment Resources; 2001.
11. Hom J, Denney RL. Detection of response bias in forensic neuropsychology. Binghamton, NY: Haworth Medical Press; 2002.
12. Whitney KA, Hook JN, Steiner AR, et al. Is the Rey 15-Item Memory Test II (Rey II) a valid symptom validity test?: comparison with the TOMM. Appl Neuropsychol. 2008;15(4):287-292.
13. Kelly PJ, Baker GA, van den Broek MD, et al. The detection of malingering in memory performance: the sensitivity and specificity of four measures in a UK population. Br J Clin Psychol. 2005;44(3):333-341.
14. Hanley JR, Backer G, Ledson S. Detecting the faking of amnesia: a comparison of the effectiveness of three different techniques for distinguishing simulators from patients with amnesia. J Clin Exp Neuropsychol. 1999;21(1):59-69.
15. Rogers R. Clinical assessment of malingering and deception, 3rd ed. New York, NY: The Gilford Press; 2008:54.
16. Myers W, Hall R, Tolou-Shams M. Prevalence and assessment of malingering in homicide defendants using the mini-mental state examination and the Rey 15-Item Memory Test. Homicide Stud. 2013;17(3):314-328.
17. Resnick PJ. In session with Phillip J. Resnick, MD: malingering of psychiatric symptoms. Prim Psychiatry. 2006;13(6):35-38.
Investigational treatments for cognitive impairment in schizophrenia
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Available treatments for schizophrenia (eg, antipsychotics) are primarily effective on positive symptoms (hallucinations, delusions, etc.). It is, however, increasingly clear that schizophrenia also is a severe neuropsychiatric illness associated with deficits in cognitive function. These deficits represent a core feature of the disorder, and are a major determinant of long-term disability.1 Cognitive dysfunction is among the earliest signs of illness that, typically, presents in the prodromal phase.
Since the formulation of the dopaminergic model of schizophrenia, cognitive studies of the disease primarily have examined dysfunction in dopaminergic-rich regions of the brain, such as the prefrontal cortex, and, therefore, have focused largely on executive functioning. But neurocognitive deficits in schizophrenia are not limited to executive functioning; comparable deficits have been observed across multiple areas of cognition.2
More recent formulations of cognitive dysfunction in schizophrenia divide deficits into multiple domains. These include verbal, visual, and working memory; attention and vigilance; speed of processing, reasoning, and problem solving; and social cognition (Table). Neurocognitive impairments often are closely associated with deficits in early sensory processing and basic neurophysiology.3
The prevalence of cognitive dysfunction also can be estimated using baseline data from the large-scale Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial.4 Although cognitive dysfunction was not one of the inclusion criteria in CATIE, most patients who were enrolled had profound cognitive deficits.5 Furthermore, meta-analyses6 suggest that composite neurocognitive measures can explain as much as 60% of the variance of overall functioning in schizophrenia.
Antipsychotics aren’t the answer
The cognitive-enhancing benefits of antipsychotic medications are minimal.7 As evidence of a direct relationship between cognitive dysfunction and long-term functional outcome in schizophrenia becomes established, the need for safe and effective treatment for these symptoms becomes more urgent. Given the mechanistic complexity of the potential cause of poor cognitive performance, the search for an effective treatment is ongoing—but that search has not been successful.
Despite mixed results for recent novel mechanism trials (http://newsroom.lilly.com/releasedetail.cfm?releaseid=703018) and a number of companies ceasing drug development, the work to develop safe and effective treatments for cognitive dysfunction in schizophrenia continues, as exemplified by National Institute of Mental Health-initiated programs to spur development of drugs that work by a novel mechanism. Rather than simply assessing novel compounds with paper-and-pencil cognitive scales, such programs seek to assess the ability of the compound to engage with the intended receptor (target),9 using imaging or electrophysiological tools. Without utilization of a target engagement biomarker, there is no way to know whether 1) the drug simply does not get into the brain in sufficient concentration to be effective in humans or 2) the overall mechanism is wrong.
In this article, we review several promising targets and techniques that are the subject of active research on the treatment of cognitive disorders in schizophrenia. This list isn’t exhaustive; our aim is to highlight a few of the promising treatments now being studied in clinical trials.
Acetylcholine receptors
Acetylcholine receptors comprise two major families, nicotinic and muscarinic receptors; evidence implicates deficits of both families in schizophrenia.10 Following up on epidemiological studies11 of the high percentage of schizophrenia patients who smoke tobacco (60% to 90%), the role of alpha-7 nicotinic acetylcholine receptors (á7 nAchR) has been explored. Nicotine itself might normalize some disrupted auditory processes, as measured by electroencephalography.12
Several clinical trials of partial á7 nAchR agonists have been conducted, with EVP-6124 and TC-5619 furthest along in development.
EVP-6124. Information is unavailable publicly on EVP-6124, except for an abstract presented in 2011 at the 51st Annual Meeting of the American College of Neuropsychopharmacology.13 In that study, 319 patients with schizophrenia were randomized to EVP-6124 (0.3 mg/d or 1 mg/d [n = 213]) or placebo (n = 106) adjunctive to at least 4 weeks of non-clozapine antipsychotics. Efficacy was shown up to 1 mg, in a dose-responsive manner. Modest, but significant, improvements in cognition, clinical function, and negative symptoms were seen. The most commonly reported side effects were headache (3.8%), nausea (3.2%), and nasopharyngitis (2.5%). Phase III studies are underway.
TC-5619. This partial á7 nAchR also showed positive results recently in a Phase II trial. Significant (P < .05) improvement was demonstrated in executive function in the Groton Maze Learning Task of the CogState Schizophrenia Battery and the Scale for Assessment of Negative Symptoms.14
Strong anatomic links also exist between muscarinic acetylcholine receptors and the brain dopaminergic system, especially muscarinic type-1 and type-4 (M1 and M4) receptors. The potential utility of an M1, M4, or combined M/M4 agonist is also supported by studies of M1 and M4 knockout mice, with particular evidence of cognitive enhancement with the use of M1 agonists.15
GSK1034702. Administration of the M1 allosteric agonist GSK1034702 to healthy human smokers, using the nicotine abstinence model of cognitive dysfunction, resulted in improvements in immediate recall.16
Xanomeline. In a small pilot study of 20 schizophrenia patients, xanomeline, a mixed M1/M4 agonist, demonstrated significant improvements in verbal learning, short-term memory, and overall symptoms.17
Dopamine receptors
All marketed antipsychotics block the dopamine type-2 (D2) receptor18; they are primarily effective on positive symptoms.4 In contrast, a role for the dopamine type-1 (D1) receptor in cognition is suggested by studies that demonstrate reduced D1 and N-methyl-d-aspartate (NMDA) glutamate receptor function in the prefrontal cortex.19-22
In a model of cognitive impairment in non-human primates, low-dose intermittent dosing of D1-receptor agonists produced improvements in cognitive function.23 This strategy aims to sensitize, rather than induce tolerance, to the effects of the D1-receptor agonist. Benefits were primarily seen in working memory. Phase II trials of a potent D1-receptor agonist, DAR-100A, the active enantiomer of dihydrexidine24 are ongoing (www.clinicaltrials.gov/ct2/show/NCT01519557).
Glutamatergic receptors
Intoxication with NMDA antagonists (such as phencyclidine and ketamine) yields a phenotype with similarity to schizophrenia.25 More than 20 years of research has provided evidence for the role of glutamatergic NMDA receptors in the pathophysiology of schizophrenia.26,27
NMDA receptors are distributed widely in the brain, but specific glutamatergic processes are localized to areas that are associated with cognition. This relative distribution provides a convenient framework from which to view the pattern of cognitive dysfunction associated with schizophrenia:
• NMDA receptors in the hippocampus are involved in learning and memory acquisition
• NMDA receptors in the visual cortex and auditory cortex are fundamental for auditory and visual sensory memory.
Previous reviews of ketamine administration have described cognitive deficits in healthy control subjects, comparable to what is seen in schizophrenia.28 The deficits are noted primarily in measures of executive functioning, attention/vigilance, verbal fluency, and visual and verbal working memory.
Most treatment studies of glutamatergic-based drugs have focused on positive and negative symptoms. Two recent comprehensive meta-analyses29,30 of NMDA-based treatments support small-to-moderate effect size improvement in total symptoms and in negative symptoms, in patients with chronic schizophrenia, when the drugs are used in combination with non-clozapine antipsychotics.
Bitopertin. A novel glycine-transport inhibitor, bitopertin, showed significant improvement in negative symptoms as an adjunctive treatment in a large Phase II trial.31,32 In the “per protocol” population (ie, patients who completed 8 weeks of treatment without any major protocol violations [n = 231]), negative symptoms diminished to a significantly (P < .05) greater degree from baseline in the 10 mg/d and 30 mg/d dosage groups, compared with placebo. Phase III studies of bitopertin are ongoing (www.clinicaltrials.gov/ct2/show/NCT01192906).
Direct evidence of a cognitive benefit of glutamatergic-based drugs is limited. In a recent large, multicenter study, low dosage D-serine (~30 mg/kg/d) did not separate from placebo,33 but an open-label study suggests increased efficacy with dosages >30 mg/kg/d.34 In addition to symptomatic improvements, a highly significant, large effect-size improvement was seen for overall cognition for dosages ≥60 mg/kg/d, leading to a significant dose-by-time interaction (P < .01).
Combination approaches. The value of combining glutamatergic medication and a cognitive training program is supported by the role of NMDA receptors in learning. For example, D-cycloserine, a glycine-site partial agonist, has been shown in several studies to enhance learning and behavioral therapies in anxiety disorders.35 Although an initial study in schizophrenia was negative for the effectiveness of D-serine (a glycine-site full agonist) and combined cognitive training,36 further research is ongoing to evaluate a role for such combined therapy.37,38
Brain stimulation
Two nonpharmacotherapeutic brain stimulation techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have been applied in the study of schizophrenia symptoms, particularly for enhancing cognition.39 Both techniques use electric stimulation to influence activity of underlying brain regions: rTMS utilizes a magnetic coil and electromagnetic induction; tDCS, in contrast, utilizes constant low (<2 mA) direct current to specific regions of the scalp.
Cortical neuronal excitability is increased by anodal tDCS and high-frequency rTMS and reduced by cathodal tDCS and low-frequency rTMS. Both tDCS and rTMS appear to be NMDA receptor-dependent. tDCS is relatively inexpensive and requires less expertise to administer than rTMS does.
Both techniques might be efficacious for treating resistant auditory hallucinations.40,41 Applying rTMS over the left dorsolateral prefrontal cortex has led to improvement in verbal learning and visuomotor tracking in patients with schizophrenia.39 Stimulation of both sides of the prefrontal cortex with rTMS has brought improvement in visual memory, executive function, spatial working memory, and attention. Few papers have been published so far regarding enhancement of cognition with tDCS in schizophrenia,42 but beneficial effects of this technique have been seen across several disorders.43
Cognitive remediation techniques
A fundamental starting point for cognitive remediation is the idea that there is plasticity in the brain and that repetitive practice can lead to cognitive improvement. Cognitive remediation therapy often adopts computerized programs and exercises that attempt to improve psychosocial function by targeting structures of the brain that are involved in cognitive function, such as attention, working memory, executive functioning, planning, and cognitive flexibility.
In schizophrenia, cognitive remediation studies have traditionally targeted higher-order processes, such as attention and higher level processes, that might lead to improvement in overall cognition and function.44 Cognitive remediation typically is utilized complementary to pharmacotherapy, with some studies supporting the use of combined use of cognition-enhancing drugs and remediation programs.
A 2007 meta-analysis showed a medium-size but significant improvement in cognition through the use of cognitive remediation therapy45—especially when it is combined with psychiatric rehabilitation. More recent studies utilizing techniques that focus on bottom-up (auditory and visual processing) techniques has shown significant improvements.46-48 Several multicenter studies utilizing Posit Science programs combined with antipsychotic medication are ongoing (www.clinicaltrials.gov/ct2/show/NCT01173874 and www.clinicaltrials.gov/ct2/show/NCT01422902).
Bottom Line
Although cognitive dysfunction is a leading cause of disability in schizophrenia, no treatments are approved for this condition. Numerous novel-mechanism and nonpharmaceutical modalities are actively being studied for this difficult-to-treat problem, however—offering hope to patients.
Related Resources
Javitt DC, Zukin SR, Heresco-Levy U, et al. Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Has an angel shown the way? Schizophr Bull. 2012; 38(5):958-966.
Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;(213):11-37.
Millan MJ, Agid Y, Brune M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012; 11(2):141-168.
Drug Brand Names
D-cycloserine • Seromycin Ketamine • Ketalar
Xanomeline • Lumeron, Memcor
Disclosures
Dr. Kantrowitz receives grant or research support from EnVivo, the National Institute of Mental Health, Novartis, Pfizer, Roche-Genentech, the Stanley Foundation, and Sunovion; is a consultant to Health Advances, LLC, the Healthcare Advisory Board, Otsuka Pharmaceuticals, Strategic Edge Communications, and Vindico Medical Education; and owns a small number of shares of common stock in GlaxoSmithKline. Ms. Levy and Dr. Ballon report no financial relationships with manufacturers of any products mentioned in this article or with manufacturers of competing products.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
1. Bowie CR, Reichenberg A, Patterson TL, et al. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163(3):418-425.
2. Kern RS, Gold JM, Dickinson D, et al. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr Res. 2011;126(1-3):124-131.
3. Javitt DC, Spencer KM, Thaker GK, et al. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7(1):68-83.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209-1253.
5. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033-2046.
6. Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119-136.
7. Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633-647.
8. Yan J. NIMH tries to jumpstart drug innovations. Psychiatric News. 2013;48(1):8-10.
9. Javitt DC, Schoepp D, Kalivas PW, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2.
10. Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413-420.
11. D’Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62(3):1564-1573.
12. Adler LE, Olincy A, Waldo M, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull. 1998; 24(2):189-202.
13. Meltzer HY, Gawryl M, Ward S, et al. EVP-6124, an alpha-7 nicotinic partial agonist, reduces positive effects on cognition, clinical function, and negative symptoms in patients with chronic schizophrenia on stable antipsychotic therapy. Neuropsychopharmacology. 2011;36:S170-S171.
14. Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968-975.
15. Digby GJ, Noetzel MJ, Bubser M, et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci. 2012;32(25):8532-8544.
16. Nathan PJ, Watson J, Lund J, et al. The potent M1 receptor allosteric agonist GSK1034702 improves episodic memory in humans in the nicotine abstinence model of cognitive dysfunction. Int J Neuropsychopharmacol. 2013;16(4):721-731.
17. Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033-1039.
18. Di Forti M, Lappin LM, Murray RM. Risk factors for schizophrenia—all roads lead to dopamine. Eur Neuropsychopharmacol. 2007;17(suppl 2):S101-S107.
19. Krystal JH, D’Souza DC, Mathalon D, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 2003;169(3-4): 215-233.
20. Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9(5):404-416.
21. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708-3719.
22. Martinez A, Ramanathan DS, Foxe JJ, et al. The role of spatial attention in the selection of real and illusory objects. J Neurosci. 2007;27(30):7963-7973.
23. Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287(5460):2020-2022.
24. Slifstein M, Suckow RF, Javitch JA, et al. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31(1):293-304.
25. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301-1308.
26. Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010; 83(3-4):108-121.
27. Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4(3):189-200.
28. Kantrowitz JT, Javitt DC. Glutamatergic approaches to the conceptualization and treatment of schizophrenia. In: Javitt DC, Kantrowitz JT, eds. Handbook of neurochemistry and molecular neurobiology. New York, NY: Springer; 2009:3-36.
29. Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16(5):522-537.
30. Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25(10):859-868.
31. Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320-S321.
32. Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-(( S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53(12):4603-4614.
33. Weiser M, Heresco-Levy U, Davidson M, et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J Clin Psychiatry. 2012;73(6):e728-e734.
34. Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121(1-3):125-130.
35. Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118-1126.
36. D’Souza DC, Radhakrishnan R, Perry E, et al. Feasibility, safety, and efficacy of the combination of D-serine and computerized cognitive retraining in schizophrenia: an international collaborative pilot study. Neuropsychopharmacology. 2013;38(3):492-503.
37. Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69-74.
38. Kantrowitz J, Sehatpour P, Oakman E, et al. D-Serine and NMDA based sensory modulation. Poster presented at: 3rd Biennial Schizophrenia International Research Conference; April 14-18, 2012; Florence, Italy.
39. Demirtas-Tatlidede, A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566-578.
40. Brunelin J, Mondino M, Gassab L, et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012;169(7):719-724.
41. Matheson SL, Green MJ, Loo C, et al. Quality assessment and comparison of evidence for electroconvulsive therapy and repetitive transcranial magnetic stimulation for schizophrenia: a systematic meta-review. Schizophr Res. 2012;118(1-3):201-210.
42. Vercammen A, Rushby JA, Loo C, et al. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res. 2011;131(1-3):198-205.
43. Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011; 29(6):463-492.
44. Keefe RS, Vinogradov S, Medalia A, et al. Report from the working group conference on multisite trial design for cognitive remediation in schizophrenia. Schizophr Bull. 2011;37(5):1057-1065.
45. McGurk SR, Twamley EW, Sitzer DI, et al. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791-1802.
46. Fisher M, Holland C, Merzenich MM, et al. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805-811.
47. Norton DJ, McBain RK, Ongür D, et al. Perceptual training strongly improves visual motion perception in schizophrenia. Brain Cogn. 2011;77(2):248-256.
48. Kantrowitz JT, Revheim N, Pasternak R, et al. It’s all in the cards: effect of stimulus manipulation on Wisconsin Card Sorting Test performance in schizophrenia. Psychiatry Res. 2009;168(3):198-204.
How best to engage patients in their psychiatric care
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
Providing patients and their families with information and education about their psychiatric illness is a central tenet of mental health care. Discussions about diagnostic impressions, treatment options, and the risks and benefits of interventions are customary. Additionally, patients and families often receive written material or referral to other information sources, including self-help books and a growing number of online resources. Although patient education remains a useful and expected element of good care, there is evidence that, alone, it is insufficient to change health behaviors.1
A growing body of literature and clinical experience suggests that self-management strategies complement patient education and improve treatment outcomes for patients with chronic illnesses, including psychiatric conditions.2,3 The Cochrane Collaboration describes patient education as “teaching or training of patients concerning their own health needs,” and self-management as “the individual’s ability to manage the symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a long-term disorder.”4
In this article, we review:
• characteristics of long-term care models
• literature supporting the benefits of self-management programs
• clinical initiatives illustrating important elements of self-management support
• opportunities and challenges faced by clinicians, patients, families, clinics, and healthcare systems implementing self-management programs.
Principles of self-management
Chronic care models must account for conditions in which the clinical course can be variable, that are not amenable to a cure, and that demand long-term treatment. Optimal health outcomes rely on patients accurately monitoring, reporting, and responding to their symptoms, while engaging in critical health-related behaviors. In addition, clinicians must teach, partner with, and motivate patients to engage in crucial disease-management activities. Although typically not considered in this light, we believe most psychiatric disorders are best approached through a long-term care model, and benefit from self-management principles.
Basics of self-management include:
• patients must formulate goals and learn skills relevant to their disease
• problems are patient-selected and targeted with individualized, flexible treatment plans.
Corbin and Strauss believe effective “self-managers” achieve competency in three areas:
• role management, which entails healthy adjustments to changes in role
responsibilities, expectations, and self-identity
• emotional management, which often is particularly challenging in psychiatric conditions because of the emotional disruption inherent in living with a psychiatric illness.6
Proficiency in these three “self-manager” domains is enhanced by mastering five key self-management skills outlined in Table 1.7
Successful intervention programs vary widely with regard to individual vs group formats, communication interface, and involved health professionals. However, evidence indicates that problem solving, decision making, and action planning are key components.7 Successful planning includes:
• detailed descriptions of what, how, when, and where the activity will be accomplished
• assessment of patient confidence and adjustment of plans if confidence is limited
• continuous monitoring and self-tailoring of plans through collaborative discussions with providers, fostering a spirit of partnership and ownership.
Table 2 illustrates elements of successful action plans in our Action Planning Worksheet. Adapted from the work of Scharzer,8 Prochaska,9 and Clark,10 we developed this self-management tool for individual and group treatment settings. It serves as a vehicle for collaborative patient-provider discussion and planning. The nuts-and-bolts nature of the discussions inevitably leads to learning new, important, and often unexpected information about our patients’ daily lives and the challenges they face as they share their dominant priorities, fears, and insecurities.
Patients provide consistently positive feedback about action planning, and clinicians often find that the process reveals fruitful areas for further psychotherapeutic intervention. Examples include identifying a range of negative automatic thoughts or catastrophic thinking impeding initiation of important activation, and exposure activities for depressed or anxious patients.
Knowing what to do is different than actually doing it. Changing behavior is difficult in the best circumstances, let alone with the strain of a chronic illness. It is critical to recognize that the presence of depressive symptoms significantly reduces the likelihood that patients will employ self-management practices.11 When combined with anxiety and the impairment of motivation and executive functioning that is common in psychiatric conditions, it is not surprising that patients with a mental health condition struggle to embrace ownership of their illness and engage in critical health behaviors—which may include adhering to medication regimens; maintaining a healthy sleep cycle, nutrition, and exercise routines; vigilant symptom surveillance; and carrying out an agreed-upon action plan.
Interventions
Professionally-guided “light-touch” interventions and technology-assisted self-management interventions also can improve patient engagement in activities through individual encounters, group forums, and technology-mediated exchanges, including telephone, email, text message, tele-health, and web-based interventions.13 The DE-STRESS (Delivery of Self Training and Education for Stressful Situations) model illustrates these principles. This 8-week program combines elements of face-to-face, email, telephone, and web-based assignments and exchanges, and demonstrates a decline in posttraumatic stress disorder, depression, and anxiety scores.14 Our Michigan Depression Outreach and Collaborative Care program is another example of a self-management intervention (Box 1).
• modeling and social persuasion through having patients observe and engage others as they struggle to overcome similar obstacles
• re-interpretation of symptoms aimed at fostering the belief that symptoms generally are multi-determined with several potential explanations, and vary with daily routines.
Helping patients understand when common symptoms such as impaired concentration or dizziness should be “watched”—rather than responded to aggressively—is crucial for effective long-term management.
Challenges
Health care delivery and medical educational models have been slow to embrace this change to long-term care models because doing so involves what might be uncomfortable shifts in roles and responsibilities. Effective care for long-term illness necessitates that the patient become an expert on his (her) illness, and be an active participant and partner in their treatment. Preparing health professionals for this new role as teacher, mentor, and collaborator presents a challenge to health care systems and educational programs across disciplines.
When trying to facilitate effective patient-provider partnerships, it is important to recognize the variability in patient preference for what and how information is shared, how decisions are made, and the role patients are asked to play in their care. Patients differ in their desire for an active or collaborative shared-decision model; some prefer more directive provider communication and a passive role.18 Preferences are influenced by variables such as age, sex, race, anxiety level, and education.19 Open discussion of these matters between caregivers and patients is important; studies have shown that failure to address these issues of “fit” can
impede communication, healthy behavior, and positive outcomes.
Bottom Line
Emerging care models demand that health care providers become teachers and motivators to help patients develop and implement patterns of health surveillance and intervention that will optimize their well-being and functionality. As active collaborators in their care, patients form a partnership with their care teams, allowing for regular, reciprocal exchange of information and shared decision-making. This shift to a partnership creates new, exciting roles and responsibilities for all parties.
Related Resources
- Improving Chronic Illness Care. www.improvingchroniccare.org.
- Chronic disease self-management program (Better Choices, Better Health workshop). Stanford School of Medicine. http://patienteducation.stanford.edu/programs/cdsmp.html.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Thomas E. Fluent, MD, talks about addressing patient resistance to a self-management model of care. Dr. Fluent is Clinical Assistant Professor, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
1. Bodenheimer T, Lorig K, Holman H, et al. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469-2475.
2. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909-1914.
3. Druss BG, Zhao L, von Esenwein SA, et al. The Health and Recovery Peer (HARP) Program: a peer-led intervention to improve medical self-management for persons with serious mental illness. Schizophr Res. 2010;118(1-3):264-270.
4. Tomkins S, Collins A. Promoting optimal self-care: consultation techniques that improve quality of life for patients and clinicians. London, United Kingdom: National Health Service; 2005.
5. Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511-544.
6. Corbin JM, Strauss AL. Unending work and care : managing chronic illness at home. 1st ed. San Francisco, CA: Jossey-Bass Publishers; 1988.
7. Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1-7.
8. Schwarzer R. Social-cognitive factors in changing health-related behaviors. Current Directions in Psychological Science. 2001;10(2):47-51.
9. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. Am Psycholt. 1992;47(9):1102-1114.
10. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav. 2001; 28(6):769-782.
11. Hibbard JH, Mahoney ER, Stock R, et al. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42(4):1443-1463.
12. Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65(11):
1232-1245.
13. Tumur I, Kaltenthaler E, Ferriter M, et al. Computerised cognitive behaviour therapy for obsessive-compulsive disorder: a systematic review. Psychother Psychosom. 2007; 76(4):196-202.
14. Litz BT, Engel CC, Bryant RA, et al. A randomized, controlled proof-of-concept trial of an Internet-based, therapist-assisted self-management treatment for posttraumatic stress disorder. Am J Psychiatry. 2007;164(11):1676-1683.
15. Holman H, Lorig K. Patient self-management: a key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243.
16. Williams A, Hagerty BM, Brasington SJ, et al. Stress Gym: feasibility of deploying a web-enhanced behavioral self-management program for stress in a military setting. Mil Med. 2010;175(7):487-493.
17. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry. 2012;169(8):790-804.
18. Davison BJ, Breckon E. Factors influencing treatment decision making and information p of prostate cancer patients on active surveillance. Patient Educ Couns. 2012;87(3):369-374.
19. Chewning B, Bylund CL, Shah B, et al. Patient p for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9-18.
Hospitalized, elderly, and delirious: What should you do for these patients?
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
Delirium is a common condition in hospitalized older patients. Often, a report of a “change in mental status” is the reason geriatric patients are sent to the emergency room for evaluation, although delirium also can develop after admission.
Delirium is a marker of underlying medical illness that needs careful workup and treatment. The condition can be iatrogenic, resulting from prescribed medication or a surgical procedure; most often, it is the consequence of multiple factors. Delirium can be expensive, because it increases hospital length of stay and overall costs—particularly if the patient is discharged to a nursing facility, not to home. Patients with delirium are at higher risk of death.
Delirium often goes unrecognized by physicians and nursing staff, and is not documented in medical records. Educating the medical staff on the identification and management of delirium is a key role for consulting psychiatrists.
CASE: Confused and agitated
Ms. T, a 93-year-old resident of an assisted living facility with a history of three
cerebral vascular accidents, atrial fibrillation, hypertension, multiple deep venous thromboses, blindness in her right eye, and deafness in her right ear without a hearing aid, is brought to the hospital after a syncopal episode lasting 10 minutes that was followed by slurred speech, confusion, and transient hypotension. Her dentist recently started her on azithromycin.
In the emergency room, Ms. T’s elevated blood pressure is managed with hydralazine and diltiazem. A CT scan of the head rules out hemorrhagic stroke. Complete blood count and tests of electrolytes, vitamin B12, and thyroid-stimulating hormone are within normal limits; urinalysis is negative for urinary tract infection.
Ms. T is noted to be in and out of sleep, with some confusion. She is maintained without oral food or fluids because of concerns about her ability to swallow. After 5 or 6 hours in the ER, Ms. T is transferred to a medical unit, where she becomes agitated and paranoid, with the delusion that her daughter is an impostor. She yells, is combative, and refuses medication.
Her confusion and behaviors become worse at night: She pulls out her IV line and telemetry leads. Blood pressure remains elevated, for which she receives additional doses of hydralazine.
For behavioral management, the medical team orders a one-time IM dose of haloperidol and starts her on risperidone, 0.5 mg every 4 hours as needed, which Ms. T refuses to take. She is incontinent and has foul-smelling urine.
Ms. T’s family is shocked at her condition; nursing staff is frustrated. With her worsening paranoia, delusions, and combative behaviors towards the nursing staff, psychiatry is consulted.
How to recognize and diagnose
The Box lists DSM-5 criteria for delirium.1 The key feature is a disturbance in attention—what was referred to in DSM-IV-TR as “disturbance in consciousness.” That finding contrasts with what is seen in dementia, with its hallmark memory impairment and chronic deterioration.
In a hospital setting, the question is often asked: Does this patient have dementia or delirium? In many cases, the answer is both, because preexisting cognitive impairment is an important risk factor for delirium.
In addition to the standard clinical interview, several screening instruments or delirium rating scales have been developed. The most commonly used (Table 1) is the Confusion Assessment Method developed by Inouye and colleagues.2
Subtypes of delirium have been described, largely based on motor activity. Patients can present as hyperactive, hypoactive, mixed, or neither.3 Psychiatrists are more likely to be consulted regarding patients with hyperactive delirium, because they are the ones who scream, pull out their IV line, hallucinate, and are delusional, insisting they “have to go home”—such as the patient described in the case above.
Patients with hypoactive delirium often, on the other hand, are difficult to recognize; they present with lethargy, drowsiness, apathy, and confusion. They become withdrawn and answer slowly4; often, psychiatry is consulted to assess them for depression.
Delirium can be difficult to diagnose in patients with underlying dementia, who are not able to provide information. In such cases, obtaining collateral information from a family member or primary caretaker is crucial. Knowing the patient’s baseline helps to determine whether there has been an acute change in mental status.
CASE CONTINUED: Acute mental status changes
Ms. T’s daughter reports that her mother has not been in this condition before. At baseline, Ms. T has had memory problems but no paranoia, delusions, or agitated behaviors. Her daughter also reports that Ms. T has visual and hearing impairments and is not wearing her hearing aid.
The acute change in mental status and the perceptual disturbances indicate that Ms. T has delirium, not dementia.
Who is likely to develop delirium?
Risk factors for delirium (Table 2) include preexisting cognitive impairment, older age, vision and hearing impairment, use of psychoactive drugs, severe illness, azotemia and dehydration, a metabolic abnormality, and infection. Male sex also seems to be a risk factor, perhaps because men are more likely to abuse alcohol before admission.
Many patients become delirious after starting a new medication. An experienced geriatrician teaches that the main causes of delirium are “drugs, drugs, drugs, infections, and everything else” (Kenneth Rockwood, MD, personal communication, 2012). At admission, urinary tract infection and pneumonia are common causes of delirium, especially in geriatric patients.
What is the clinical course?
The clinical course varies widely. Delirium often is the reason that a patient is brought to the hospital, presenting with the condition at admission or early in hospitalization. The highest incidence among surgical patients appears to be on the third postoperative day—in some cases because of alcohol or drug withdrawal.
As noted in the DSM-5 criteria, delirium often comes on acutely, over hours or days. Symptoms can persist for weeks after initial onset of episodes of delirium.5 Symptoms fluctuate over the course of the day; at times, they can be missed if a provider sees the patient only while she (he) is clearer and doesn’t review nursing notes from other shifts.
How does delirium affect outcome?
Delirium has been shown to be associated with prolonged hospital stay (21 days, compared with 11 days in the absence of delirium), functional decline during hospitalization, and increased admission to long-term care (36% compared with 13%).6 In a study by O’Keefe and Lavan,6 delirious patients were more likely to sustain falls and to develop urinary incontinence, pressure sores, and other complications during hospitalization.
Older patients with delirium superimposed on dementia had a more than twofold increased risk of mortality compared with patients with dementia alone or with neither dementia nor delirium.7 Rockwood found that an episode of delirium was associated with a much higher rate of subsequent dementia.8
Think of an acute medical illness as a “stress test” for the brain, such that, if the patient develops delirium, it suggests an underlying brain disease that was not evident before the acute episode. After hip fracture, for example, delirium was independently associated with poor functional recovery at 1 month9 and at 2 years.10
Older patients admitted to a skilled nursing facility with delirium are more likely to experience one or more complications (73% compared with 41%).11 In the study by Marcantonio and colleagues, patients with delirium were more than twice as likely to be hospitalized again within 30 days (30% and 13%), and less than half as likely to be discharged to the community (30% and 73%). Table 3 summarizes the impact of delirium on outcomes.
Appropriate management steps
Identifying and treating underlying medical illness is the definitive treatment for delirium; in a geriatric patient with multiple medical comorbidities the pathogenesis often is multifactorial or a definitive precipitant cannot always be identified.12
Managing a patient with delirium includes both non-pharmacotherapeutic interventions, which should be considered first-line, and pharmacotherapeutic interventions. Non-pharmacotherapeutic interventions include, but are not limited to:
• support and close observation by nursing staff
• placing a clock or calendar in the room
• frequent reorientation and reminders
• placing familiar possessions in the room
• putting the patient in an isolated room with a window
• regulating the sleep-wake cycle.4
Pharmacotherapeutic intervention in delirium should be used for behavioral symptoms, but only for the minimum duration necessary4 and preferably oral or IV. No drugs are FDA-approved for delirium, which means that use of any agent is off-label.13
Antipsychotics are the mainstay of pharmacotherapy for delirium in most settings. The use of antipsychotics relates to the dopamine excess-acetylcholine deficiency hypothesis of delirium pathophysiology.12 Haloperidol remains the first-line agent because it is available in multiple dosages and can be given by various routes. IV haloperidol appears to carry less risk of extrapyramidal symptoms than oral haloperidol does but, as with all antipsychotics, its use warrants monitoring for QTc prolongation.12
Studies have not shown that atypical antipsychotics are superior to typical antipsychotics for delirium. Multiple studies have shown that atypicals are as efficacious as haloperidol.
Benzodiazepines are the treatment of choice for delirium caused by alcohol withdrawal. A Cochrane review found no evidence that benzodiazepines were helpful in treating delirium unrelated to alcohol withdrawal.14 In some studies, benzodiazepines were associated with an increased risk of delirium, especially in patients in the intensive care unit.15
More recently, cholinesterase inhibitors have been used to treat delirium. The reasoning behind their use is the hypothesis of a central cholinergic deficiency in delirium.12 Regrettably, there have been few well-conducted studies of these agents in delirium, and a Cochrane review found no significant benefit for cholinesterase inhibitors.16 With the same hypothesis in mind, anticholinergic medications in patients with delirium should be avoided because these agents could exacerbate delirium by further decreasing the acetylcholine level.
Because delirium is common in the hospitalized population (especially older patients), a number of studies have examined strategies to prevent or reduce its development. Inouye and colleagues conducted a controlled clinical trial, in which they intervened to reduce six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual and hearing impairment, and dehydration in hospitalized geriatric patients. The number and duration of events of delirium were significantly lower in the intervention group.17
Brummel et al reported that reducing modifiable risk factors in intensive care unit patients—including sedation management, minimizing deliriogenic medications (anticholinergics, antihistamines), minimizing sleep disruption, and encouraging early mobility—could prevent or reduce the incidence of delirium.15
CASE CONCLUDED: Return to baseline
Ms. T’s medications are minimized or discontinued, including azithromycin, based on case reports in the literature. She is stabilized hemodynamically.
Clinicians educate Ms. T’s family about delirium. To address Ms. T’s aggressive and paranoid behaviors, clinicians request that a family member is present to reassure Ms. T. She is continued on low-dose haloperidol. The family also is asked to bring Ms. T’s hearing aid and eyeglasses.
MRI is performed after Ms. T’s behavior is under control. The scan is negative for a new stroke.
Repeat blood tests the following day show an elevated white blood cell count; urinalysis is positive for a urinary tract infection. Ms. T is started on antibiotics. Subsequent urine culture shows no bacterial growth; the antibiotics are stopped after 3 days.
Ms. T slowly improves. According to her family, she is back at baseline in 3 or 4 days.
This case illustrates the complexity of trying to identify the precise cause of delirium among the many that could be involved. Often, no single cause can be found.18
Bottom Line
Delirium is a common and potentially life-threatening condition in hospitalized geriatric patients. General hospital psychiatrists should know how to recognize and treat the condition in collaboration with their medical colleagues.
Related Resources
- Treating delirium: a quick reference guide. Arlington, VA: American Psychiatric Association. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1662986.
- Cook IA. Guideline watch: practice guidelines for the treatment of patients with delirium. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1681952.
- Fearing MA, Inouye SK. Delirium. In: Blazer DG, Steffens D, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:229-241.
- Ghandour A, Saab R, Mehr D. Detecting and treating delirium—key interventions you may be missing. J Fam Pract. 2011;60(12):726-734.
- Leentjens AF, Rundell J, Rummans T, et al. Delirium: an evidence-based medicine (EBM) monograph for psychosomatic medicine practice. J Psychosom Res. 2012;73:149-152.
- Liptzin B, Jacobson SA. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Comprehensive textbook of psychiatry. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009:4066-4073.
Drug Brand Names
Azithromycin • Zithromax Hydralazine • Apresoline
Diltiazem • Cardizem Risperidone • Risperdal
Haloperidol • Haldo
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Featured Audio
Benjamin Liptzin, MD, describes the distinction between dementia and delirium. Dr. Liptzin is Chair of Psychiatry, Baystate Medical Center, Springfield, Massachusetts, and Professor and Deputy Chair, Department of Psychiatry, Tufts University School of Medicine, Boston, Massachusetts.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013.
2. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948.
3. Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843-845.
4. Martins S, Fernandes L. Delirium in elderly people: a review. Front Neurol. 2012;3:101.
5. Levkoff SE, Liptzin B, Evans D, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230-238.
6. O’Keefe S, Lavan J. The prognostic significance of delirium in older hospitalized patients. J Am Geriatr Soc. 1997;45:247-248.
7. Tsai MC, Weng HH, Chou SY, et al. One-year mortality of elderly inpatients with delirium, dementia or depression seen by a consultation-liaison service. Psychosomatics. 2012;53:433-438.
8. Rockwood K, Cosway S, Carver D, et al. The risk of dementia and death after delirium. Age Ageing. 1999;28:551-556.
9. Marcantonio E, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618-624.
10. Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M27-M34.
11. Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of elders admitted to post-acute facilities with delirium. J Am Geriatr Soc. 2005;53:963-969.
12. Bledowski J, Trutia A. A review of pharmacologic management and prevention strategies of delirium in the intensive care unit. Psychosomatics. 2012;53:203-211.
13. Breitbart W, Alici-Evcimen Y. Why off-label antipsychotics remain first-choice drugs for delirium. Current Psychiatry. 2007;6(9):49-63.
14. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. doi: 10.1002/14651858.CD006379.pub3.
15. Brummel NE, Girard TD. Preventing delirium in the ICU. Crit Care Clin. 2013;(29):51-65.
16. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008(1):CD005317. doi: 10.1002/14651858.CD005317.
17. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669-676.
18. Francis J, Martin D, Kapoor WN. A prospective study of delirium in hospitalized elderly. JAMA. 1990;263:1097-1101.
No laughing matter: Laughter is good psychiatric medicine
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
Participatory pharmacotherapy: 10 strategies for enhancing adherence
Psychiatric patients stand to benefit greatly from adhering to prescribed pharmacotherapy, but many patients typically do not follow their medication regimens.1,2 Three months after pharmacotherapy is initiated, approximately 50% of patients with major depressive disorder (MDD) do not take their prescribed antidepressants.3 Adherence rates in patients with schizophrenia range from 50% to 60%, and patients with bipolar disorder have adherence rates as low as 35%.4-6 One possible explanation for “treatment-resistant” depression, schizophrenia, and bipolar disorder may simply be nonadherence to prescribed pharmacotherapy.
Several strategies have been used to address this vexing problem (Table 1).7,8 They include individual and family psychoeducation,9,10 cognitive-behavioral therapy,11 interpersonal and social rhythm therapy, and family-focused therapy. This article describes an additional strategy I call “participatory pharmacotherapy.” In this model, the patient becomes a partner in the process of treatment choices and decision-making. This encourages patients to provide their own opinions and points of view regarding medication use. The prescribing clinician makes the patient feel that he or she has been listened to and understood. This and other techniques emphasize forming a therapeutic alliance with the patient before initiating pharmacotherapy. The patient provides information on his or her family history, medical and psychiatric history, and experience with previous medications, with a specific focus on which medications worked best for the patient and family members diagnosed with a similar condition.
Getting patients to participate
One of the fundamental tasks is to encourage patients to accept a participatory role, determine their underlying diagnosis, and co-create a treatment plan that will be most compatible with their illness and their personality. There are 10 components of establishing and practicing participatory pharmacotherapy.
1. Encourage patients to share their opinion of what a desirable treatment outcome should be. Some patients have unrealistic expectations about what medications can achieve. Clarify with patients what would be a realistic expectation of pharmacotherapy, and modify the patient’s beliefs to be compatible with a more probable outcome. For example, Ms. D, a 46-year-old mother of 2, is diagnosed with MDD, recurrent type without psychotic features. She states she expects pharmacotherapy will alleviate all symptoms and allow her to achieve a new healthy, happy state in which she will be able to laugh, socialize, and have fun every day for the rest of her life. Although achieving remission is a realistic and desirable treatment goal, Ms. D’s expectations are idealistic. Helping Ms. D accept and agree to realistic and achievable outcomes will improve her adherence to prescribed medications.
2. Encourage patients to share their ideas of how a desirable outcome can be accomplished. Similar to their expectations of outcomes, some patients have an unrealistic understanding of how treatment is conducted. Some patients expect treatment to be limited to prescribed medications or a one-time injection of a curative drug. Others prefer to use herbs and supplements and want to avoid prescribed medications. Understanding the patient’s expectations of how treatment is carried out will allow clinicians to provide patients with a rational view of treatment and establish a partnership based on realistic expectations.
3. Engage patients in choosing the best medication for them. Many patients have preconceived ideas about medications and which medicine would be best for them. They get this information from various sources, including family members and friends who benefitted from a specific drug, personal experience with medications, and exposure to drug advertising.
Understanding the patient’s preference for a specific medication and why he or she made such a choice is critical because doing so can take advantage of the patient’s self-fulfilling prophecies and improve the chances of obtaining a better outcome. For example, Mr. O, a 52-year-old father of 3, has been experiencing recurrent episodes of severe panic attacks. His clinician asked him to describe medications that in his opinion were most helpful in the past. He said he preferred clonazepam because it had helped him control the panic attacks and had minimal side effects, but he discontinued it after a previous psychotherapist told him he would become addicted to it. Obtaining this information was valuable because the clinician was able to clarify guidelines for clonazepam use without the risk of dependence. Mr. O is prescribed clonazepam, which he takes consistently and responds to excellently.
4. Involve patients in setting treatment goals and targeting symptoms to be relieved. Actively listen when patients describe their symptoms, discomforts, and past experiences with treatments. I invite patients to speak uninterrupted for 5 to 10 minutes, even if they talk about issues that seem irrelevant. I then summarize the patient’s major points and ask, “And what else?” After he or she says, “That’s it,” I ask the patient to assign a priority to alleviating each symptom.
For example, Ms. J, a 38-year-old married mother of 2, was diagnosed with bipolar II disorder. She listed her highest priority as controlling her impulsive shopping rather than alleviating depression, insomnia, or overeating. She had been forced to declare bankruptcy twice, and she was determined to never do so again. She also wanted to regain her husband’s trust and her ability to manage her finances. Ensuring that Ms. J felt understood regarding this issue increased the chances of establishing a solid treatment partnership. Providing Ms. J with a menu of treatment choices and asking her to describe her previous experiences with medications helped her and the clinician choose a medication that is compatible with her desire to control her impulsive shopping.
5. Engage patients in choosing the best delivery system for the prescribed medication. For many medications, clinicians can choose from a variety of delivery systems, including pills, transdermal patches, rectal or vaginal suppositories, creams, ointments, orally disintegrating tablets, liquids, and intramuscular injections. Patients have varying beliefs about the efficacy of particular delivery systems, based on personal experiences or what they have learned from the media, their family and friends, or the Internet. For example, Ms. S, age 28, experienced recurrent, disabling anxiety attacks. When asked about the best way of providing medication to relieve her symptoms, she chose gluteal injections because, as a child, her pediatrician had treated her for an unspecified illness by injecting medication in her buttock, which rapidly relieved her symptoms. This left her with the impression that injectable medications were the best therapeutic delivery system. After discussing the practicalities and availability of fast-acting medications to control panic attacks, we agreed to use orally disintegrating clonazepam, which is absorbed swiftly and provides fast symptom relief. Ms. S reported favorable results and was pleased with the process of developing this strategy with her clinician.
6. Involve patients in choosing the times and frequency of medication administration. The timing and frequency of medication administration can be used to enhance desirable therapeutic effects. For example, an antidepressant that causes sedation and somnolence could be taken at bedtime to help alleviate insomnia. Some studies have shown that taking a medication once a day improves adherence compared with taking the same medication in divided doses.13 Other patients may wish to take a medication several times a day so they can keep the medication in their purse or briefcase and feel confident that if they need a medication for immediate symptom relief, it will be readily available.
7. Teach patients to self-monitor changes and improvements in target symptoms. Engaging patients in a system of self-monitoring improves their chances of achieving successful treatment outcomes.14 Instruct patients to create a list of symptoms and monitor the intensity of each symptom using a rating scale of 1 to 5, where 1 represents the lowest intensity and 5 represents the highest. As for frequency, patients can rate each symptom from “not present” to “present most of the time.”
Self-monitoring allows patients to observe which daily behaviors and lifestyle choices make symptoms better and which make them worse. For example, Mrs. P, a 38-year-old married mother of 2, had anxiety and panic attacks associated with low self-esteem and chronic depression. Her clinician instructed her to use a 1-page form to monitor the frequency and intensity of her anxiety and panic symptoms by focusing on the physical manifestations, such as rapid heartbeat, shortness of breath, nausea, tremors, dry mouth, frequent urination, and diarrhea to see if there was any correlation between her behaviors and her symptoms.
8. Instruct patients to call you to report any changes, including minor successes. Early in my career, toward the end of each appointment after I’d prescribed medications I’d tell patients, “Please call me if you have a problem.” Frequently, patients would call with a list of problems and side effects that they believed were caused by the newly prescribed medication. Later, I realized that I may have inadvertently encouraged patients to develop problems so they would have a reason to call me. To achieve a more favorable outcome I changed the way I communicate. I now say, “Please call me next week, even if you begin to feel better with this new medication.” The phone call is now associated with the idea that they will “get better,” and internalizing such a suggestion allows patients to talk with the clinician and report favorable treatment results.
9. Tell patients to monitor their successes by relabeling and reframing their symptoms. Mr. B, age 28, has MDD and reports irritability, insomnia, short temper, and restlessness. After reviewing his desired treatment outcome, we discuss the benefits of pharmacotherapy. I tell him the new medication will improve the quality and length of his sleep, which will allow his body and mind to recharge his “internal batteries” and restore health and energy. When we discuss side effects, I tell him to expect a dry mouth, which will be his signal that the medication is working. This discussion helps patients reframe side effects and improves their ability to tolerate side effects and adhere to pharmacotherapy.
10. Harness the placebo effect and the power of suggestion to increase chances of achieving the best treatment outcomes. In a previous article,12 I reviewed the principles of recognizing and enhancing the placebo effect and the power of suggestion to improve the chances of achieving better pharmacotherapy outcomes. When practicing participatory pharmacotherapy, clinicians are consciously aware of the power embedded in their words and are careful to use language that enhances the placebo effect and the power of suggestion when prescribing medications. Use the patient’s own language as a way of pacing yourself to the patient’s description of his or her distress. For example, Ms. R, a 42-year-old mother of 3, describes her experiences seeking help for her anxiety and depression, stating that she has not yet found the right combination of medications that provide benefits with tolerable side effects. Her clinician responds by focusing on the word “yet” (pacing) stating, “even though you have not yet found the right combination of medications to provide the most desirable benefit of beginning healing and restoring your hope, I promise to work with you and together we will try to achieve an improvement in your overall health and well-being.” This response includes several positive words and suggestions of future success, which are referred to as leading.
Not all patients will respond to participatory pharmacotherapy. Some factors will make patients good candidates for this approach, and others should be considered exclusionary qualities (Table 2).
Bottom Line
“Participatory pharmacotherapy” involves identifying patients as partners in the process of treatment choice and decision-making, encouraging them to provide their opinions regarding medication use, and making patients feel they have been heard and understood. This technique emphasizes forming a therapeutic alliance with the patient to improve patients’ adherence to pharmacotherapy and optimize treatment outcomes.
Related Resources
- Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;16(2);CD000011.
- Mahone IH. Shared decision making and serious mental illness. Arch Psychiatr Nurs. 2008;22(6):334-343.
- Russel CL, Ruppar TM, Metteson M. Improving medication adherence: moving from intention and motivation to a personal systems approach. Nurs Clin North Am. 2011;46(3):271-281.
- Tibaldi G, Salvador-Carulla L, Garcia-Gutierrez JC. From treatment adherence to advanced shared decision making: New professional strategies and attitudes in mental health care. Curr Clin Pharmacol. 2011;6(2):91-99.
Drug Brand Name
Clonazepam • Klonopin
Disclosure
Dr. Torem reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159:1653-1664.
2. Nosé M, Barbui C, Gray R, et al. Clinical interventions for treatment non-adherence in psychosis: meta-analysis. Br J Psychiatry. 2003;183:197-206.
3. Vergouwen AC, van Hout HP, Bakker A. Methods to improve patient compliance in the use of antidepressants. Ned Tijdschr Geneeskd. 2002;146:204-207.
4. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature.
J Clin Psychiatry. 2002;63:892-909.
5. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry. 2002;63:1121-1128.
6. Colom F, Vieta E, Martinez-Aran A, et al. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry. 2000;61:549-555.
7. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
8. Osterberg LG, Rudd R. Medication adherence for antihypertensive therapy. In: Oparil S, Weber MA, eds. Hypertension: a companion to Brenner and Rector’s the kidney. 2nd ed. Philadelphia. PA: Elsevier Saunders; 2005:848.
9. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from expert consensus guidelines. J Psychiatr Pract. 2010;16:306-324.
10. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008; 165:1408-1419.
11. Szentagotai A, David D. The efficacy of cognitive-behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71:66-72.
12. Torem MS. Words to the wise: 4 secrets of successful pharmacotherapy. Current Psychiatry. 2008;7(12):19-24.
13. Medic G, Higashi K, Littlewood KJ, et al. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2013; 9:119-131.
14. Virdi N, Daskiran M, Nigam S, et al. The association of self-monitoring of blood glucose use with medication adherence and glycemic control in patients with type 2 diabetes initiating non-insulin treatment. Diabetes Technol Ther. 2012;14(9):790-798.
Psychiatric patients stand to benefit greatly from adhering to prescribed pharmacotherapy, but many patients typically do not follow their medication regimens.1,2 Three months after pharmacotherapy is initiated, approximately 50% of patients with major depressive disorder (MDD) do not take their prescribed antidepressants.3 Adherence rates in patients with schizophrenia range from 50% to 60%, and patients with bipolar disorder have adherence rates as low as 35%.4-6 One possible explanation for “treatment-resistant” depression, schizophrenia, and bipolar disorder may simply be nonadherence to prescribed pharmacotherapy.
Several strategies have been used to address this vexing problem (Table 1).7,8 They include individual and family psychoeducation,9,10 cognitive-behavioral therapy,11 interpersonal and social rhythm therapy, and family-focused therapy. This article describes an additional strategy I call “participatory pharmacotherapy.” In this model, the patient becomes a partner in the process of treatment choices and decision-making. This encourages patients to provide their own opinions and points of view regarding medication use. The prescribing clinician makes the patient feel that he or she has been listened to and understood. This and other techniques emphasize forming a therapeutic alliance with the patient before initiating pharmacotherapy. The patient provides information on his or her family history, medical and psychiatric history, and experience with previous medications, with a specific focus on which medications worked best for the patient and family members diagnosed with a similar condition.
Getting patients to participate
One of the fundamental tasks is to encourage patients to accept a participatory role, determine their underlying diagnosis, and co-create a treatment plan that will be most compatible with their illness and their personality. There are 10 components of establishing and practicing participatory pharmacotherapy.
1. Encourage patients to share their opinion of what a desirable treatment outcome should be. Some patients have unrealistic expectations about what medications can achieve. Clarify with patients what would be a realistic expectation of pharmacotherapy, and modify the patient’s beliefs to be compatible with a more probable outcome. For example, Ms. D, a 46-year-old mother of 2, is diagnosed with MDD, recurrent type without psychotic features. She states she expects pharmacotherapy will alleviate all symptoms and allow her to achieve a new healthy, happy state in which she will be able to laugh, socialize, and have fun every day for the rest of her life. Although achieving remission is a realistic and desirable treatment goal, Ms. D’s expectations are idealistic. Helping Ms. D accept and agree to realistic and achievable outcomes will improve her adherence to prescribed medications.
2. Encourage patients to share their ideas of how a desirable outcome can be accomplished. Similar to their expectations of outcomes, some patients have an unrealistic understanding of how treatment is conducted. Some patients expect treatment to be limited to prescribed medications or a one-time injection of a curative drug. Others prefer to use herbs and supplements and want to avoid prescribed medications. Understanding the patient’s expectations of how treatment is carried out will allow clinicians to provide patients with a rational view of treatment and establish a partnership based on realistic expectations.
3. Engage patients in choosing the best medication for them. Many patients have preconceived ideas about medications and which medicine would be best for them. They get this information from various sources, including family members and friends who benefitted from a specific drug, personal experience with medications, and exposure to drug advertising.
Understanding the patient’s preference for a specific medication and why he or she made such a choice is critical because doing so can take advantage of the patient’s self-fulfilling prophecies and improve the chances of obtaining a better outcome. For example, Mr. O, a 52-year-old father of 3, has been experiencing recurrent episodes of severe panic attacks. His clinician asked him to describe medications that in his opinion were most helpful in the past. He said he preferred clonazepam because it had helped him control the panic attacks and had minimal side effects, but he discontinued it after a previous psychotherapist told him he would become addicted to it. Obtaining this information was valuable because the clinician was able to clarify guidelines for clonazepam use without the risk of dependence. Mr. O is prescribed clonazepam, which he takes consistently and responds to excellently.
4. Involve patients in setting treatment goals and targeting symptoms to be relieved. Actively listen when patients describe their symptoms, discomforts, and past experiences with treatments. I invite patients to speak uninterrupted for 5 to 10 minutes, even if they talk about issues that seem irrelevant. I then summarize the patient’s major points and ask, “And what else?” After he or she says, “That’s it,” I ask the patient to assign a priority to alleviating each symptom.
For example, Ms. J, a 38-year-old married mother of 2, was diagnosed with bipolar II disorder. She listed her highest priority as controlling her impulsive shopping rather than alleviating depression, insomnia, or overeating. She had been forced to declare bankruptcy twice, and she was determined to never do so again. She also wanted to regain her husband’s trust and her ability to manage her finances. Ensuring that Ms. J felt understood regarding this issue increased the chances of establishing a solid treatment partnership. Providing Ms. J with a menu of treatment choices and asking her to describe her previous experiences with medications helped her and the clinician choose a medication that is compatible with her desire to control her impulsive shopping.
5. Engage patients in choosing the best delivery system for the prescribed medication. For many medications, clinicians can choose from a variety of delivery systems, including pills, transdermal patches, rectal or vaginal suppositories, creams, ointments, orally disintegrating tablets, liquids, and intramuscular injections. Patients have varying beliefs about the efficacy of particular delivery systems, based on personal experiences or what they have learned from the media, their family and friends, or the Internet. For example, Ms. S, age 28, experienced recurrent, disabling anxiety attacks. When asked about the best way of providing medication to relieve her symptoms, she chose gluteal injections because, as a child, her pediatrician had treated her for an unspecified illness by injecting medication in her buttock, which rapidly relieved her symptoms. This left her with the impression that injectable medications were the best therapeutic delivery system. After discussing the practicalities and availability of fast-acting medications to control panic attacks, we agreed to use orally disintegrating clonazepam, which is absorbed swiftly and provides fast symptom relief. Ms. S reported favorable results and was pleased with the process of developing this strategy with her clinician.
6. Involve patients in choosing the times and frequency of medication administration. The timing and frequency of medication administration can be used to enhance desirable therapeutic effects. For example, an antidepressant that causes sedation and somnolence could be taken at bedtime to help alleviate insomnia. Some studies have shown that taking a medication once a day improves adherence compared with taking the same medication in divided doses.13 Other patients may wish to take a medication several times a day so they can keep the medication in their purse or briefcase and feel confident that if they need a medication for immediate symptom relief, it will be readily available.
7. Teach patients to self-monitor changes and improvements in target symptoms. Engaging patients in a system of self-monitoring improves their chances of achieving successful treatment outcomes.14 Instruct patients to create a list of symptoms and monitor the intensity of each symptom using a rating scale of 1 to 5, where 1 represents the lowest intensity and 5 represents the highest. As for frequency, patients can rate each symptom from “not present” to “present most of the time.”
Self-monitoring allows patients to observe which daily behaviors and lifestyle choices make symptoms better and which make them worse. For example, Mrs. P, a 38-year-old married mother of 2, had anxiety and panic attacks associated with low self-esteem and chronic depression. Her clinician instructed her to use a 1-page form to monitor the frequency and intensity of her anxiety and panic symptoms by focusing on the physical manifestations, such as rapid heartbeat, shortness of breath, nausea, tremors, dry mouth, frequent urination, and diarrhea to see if there was any correlation between her behaviors and her symptoms.
8. Instruct patients to call you to report any changes, including minor successes. Early in my career, toward the end of each appointment after I’d prescribed medications I’d tell patients, “Please call me if you have a problem.” Frequently, patients would call with a list of problems and side effects that they believed were caused by the newly prescribed medication. Later, I realized that I may have inadvertently encouraged patients to develop problems so they would have a reason to call me. To achieve a more favorable outcome I changed the way I communicate. I now say, “Please call me next week, even if you begin to feel better with this new medication.” The phone call is now associated with the idea that they will “get better,” and internalizing such a suggestion allows patients to talk with the clinician and report favorable treatment results.
9. Tell patients to monitor their successes by relabeling and reframing their symptoms. Mr. B, age 28, has MDD and reports irritability, insomnia, short temper, and restlessness. After reviewing his desired treatment outcome, we discuss the benefits of pharmacotherapy. I tell him the new medication will improve the quality and length of his sleep, which will allow his body and mind to recharge his “internal batteries” and restore health and energy. When we discuss side effects, I tell him to expect a dry mouth, which will be his signal that the medication is working. This discussion helps patients reframe side effects and improves their ability to tolerate side effects and adhere to pharmacotherapy.
10. Harness the placebo effect and the power of suggestion to increase chances of achieving the best treatment outcomes. In a previous article,12 I reviewed the principles of recognizing and enhancing the placebo effect and the power of suggestion to improve the chances of achieving better pharmacotherapy outcomes. When practicing participatory pharmacotherapy, clinicians are consciously aware of the power embedded in their words and are careful to use language that enhances the placebo effect and the power of suggestion when prescribing medications. Use the patient’s own language as a way of pacing yourself to the patient’s description of his or her distress. For example, Ms. R, a 42-year-old mother of 3, describes her experiences seeking help for her anxiety and depression, stating that she has not yet found the right combination of medications that provide benefits with tolerable side effects. Her clinician responds by focusing on the word “yet” (pacing) stating, “even though you have not yet found the right combination of medications to provide the most desirable benefit of beginning healing and restoring your hope, I promise to work with you and together we will try to achieve an improvement in your overall health and well-being.” This response includes several positive words and suggestions of future success, which are referred to as leading.
Not all patients will respond to participatory pharmacotherapy. Some factors will make patients good candidates for this approach, and others should be considered exclusionary qualities (Table 2).
Bottom Line
“Participatory pharmacotherapy” involves identifying patients as partners in the process of treatment choice and decision-making, encouraging them to provide their opinions regarding medication use, and making patients feel they have been heard and understood. This technique emphasizes forming a therapeutic alliance with the patient to improve patients’ adherence to pharmacotherapy and optimize treatment outcomes.
Related Resources
- Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;16(2);CD000011.
- Mahone IH. Shared decision making and serious mental illness. Arch Psychiatr Nurs. 2008;22(6):334-343.
- Russel CL, Ruppar TM, Metteson M. Improving medication adherence: moving from intention and motivation to a personal systems approach. Nurs Clin North Am. 2011;46(3):271-281.
- Tibaldi G, Salvador-Carulla L, Garcia-Gutierrez JC. From treatment adherence to advanced shared decision making: New professional strategies and attitudes in mental health care. Curr Clin Pharmacol. 2011;6(2):91-99.
Drug Brand Name
Clonazepam • Klonopin
Disclosure
Dr. Torem reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatric patients stand to benefit greatly from adhering to prescribed pharmacotherapy, but many patients typically do not follow their medication regimens.1,2 Three months after pharmacotherapy is initiated, approximately 50% of patients with major depressive disorder (MDD) do not take their prescribed antidepressants.3 Adherence rates in patients with schizophrenia range from 50% to 60%, and patients with bipolar disorder have adherence rates as low as 35%.4-6 One possible explanation for “treatment-resistant” depression, schizophrenia, and bipolar disorder may simply be nonadherence to prescribed pharmacotherapy.
Several strategies have been used to address this vexing problem (Table 1).7,8 They include individual and family psychoeducation,9,10 cognitive-behavioral therapy,11 interpersonal and social rhythm therapy, and family-focused therapy. This article describes an additional strategy I call “participatory pharmacotherapy.” In this model, the patient becomes a partner in the process of treatment choices and decision-making. This encourages patients to provide their own opinions and points of view regarding medication use. The prescribing clinician makes the patient feel that he or she has been listened to and understood. This and other techniques emphasize forming a therapeutic alliance with the patient before initiating pharmacotherapy. The patient provides information on his or her family history, medical and psychiatric history, and experience with previous medications, with a specific focus on which medications worked best for the patient and family members diagnosed with a similar condition.
Getting patients to participate
One of the fundamental tasks is to encourage patients to accept a participatory role, determine their underlying diagnosis, and co-create a treatment plan that will be most compatible with their illness and their personality. There are 10 components of establishing and practicing participatory pharmacotherapy.
1. Encourage patients to share their opinion of what a desirable treatment outcome should be. Some patients have unrealistic expectations about what medications can achieve. Clarify with patients what would be a realistic expectation of pharmacotherapy, and modify the patient’s beliefs to be compatible with a more probable outcome. For example, Ms. D, a 46-year-old mother of 2, is diagnosed with MDD, recurrent type without psychotic features. She states she expects pharmacotherapy will alleviate all symptoms and allow her to achieve a new healthy, happy state in which she will be able to laugh, socialize, and have fun every day for the rest of her life. Although achieving remission is a realistic and desirable treatment goal, Ms. D’s expectations are idealistic. Helping Ms. D accept and agree to realistic and achievable outcomes will improve her adherence to prescribed medications.
2. Encourage patients to share their ideas of how a desirable outcome can be accomplished. Similar to their expectations of outcomes, some patients have an unrealistic understanding of how treatment is conducted. Some patients expect treatment to be limited to prescribed medications or a one-time injection of a curative drug. Others prefer to use herbs and supplements and want to avoid prescribed medications. Understanding the patient’s expectations of how treatment is carried out will allow clinicians to provide patients with a rational view of treatment and establish a partnership based on realistic expectations.
3. Engage patients in choosing the best medication for them. Many patients have preconceived ideas about medications and which medicine would be best for them. They get this information from various sources, including family members and friends who benefitted from a specific drug, personal experience with medications, and exposure to drug advertising.
Understanding the patient’s preference for a specific medication and why he or she made such a choice is critical because doing so can take advantage of the patient’s self-fulfilling prophecies and improve the chances of obtaining a better outcome. For example, Mr. O, a 52-year-old father of 3, has been experiencing recurrent episodes of severe panic attacks. His clinician asked him to describe medications that in his opinion were most helpful in the past. He said he preferred clonazepam because it had helped him control the panic attacks and had minimal side effects, but he discontinued it after a previous psychotherapist told him he would become addicted to it. Obtaining this information was valuable because the clinician was able to clarify guidelines for clonazepam use without the risk of dependence. Mr. O is prescribed clonazepam, which he takes consistently and responds to excellently.
4. Involve patients in setting treatment goals and targeting symptoms to be relieved. Actively listen when patients describe their symptoms, discomforts, and past experiences with treatments. I invite patients to speak uninterrupted for 5 to 10 minutes, even if they talk about issues that seem irrelevant. I then summarize the patient’s major points and ask, “And what else?” After he or she says, “That’s it,” I ask the patient to assign a priority to alleviating each symptom.
For example, Ms. J, a 38-year-old married mother of 2, was diagnosed with bipolar II disorder. She listed her highest priority as controlling her impulsive shopping rather than alleviating depression, insomnia, or overeating. She had been forced to declare bankruptcy twice, and she was determined to never do so again. She also wanted to regain her husband’s trust and her ability to manage her finances. Ensuring that Ms. J felt understood regarding this issue increased the chances of establishing a solid treatment partnership. Providing Ms. J with a menu of treatment choices and asking her to describe her previous experiences with medications helped her and the clinician choose a medication that is compatible with her desire to control her impulsive shopping.
5. Engage patients in choosing the best delivery system for the prescribed medication. For many medications, clinicians can choose from a variety of delivery systems, including pills, transdermal patches, rectal or vaginal suppositories, creams, ointments, orally disintegrating tablets, liquids, and intramuscular injections. Patients have varying beliefs about the efficacy of particular delivery systems, based on personal experiences or what they have learned from the media, their family and friends, or the Internet. For example, Ms. S, age 28, experienced recurrent, disabling anxiety attacks. When asked about the best way of providing medication to relieve her symptoms, she chose gluteal injections because, as a child, her pediatrician had treated her for an unspecified illness by injecting medication in her buttock, which rapidly relieved her symptoms. This left her with the impression that injectable medications were the best therapeutic delivery system. After discussing the practicalities and availability of fast-acting medications to control panic attacks, we agreed to use orally disintegrating clonazepam, which is absorbed swiftly and provides fast symptom relief. Ms. S reported favorable results and was pleased with the process of developing this strategy with her clinician.
6. Involve patients in choosing the times and frequency of medication administration. The timing and frequency of medication administration can be used to enhance desirable therapeutic effects. For example, an antidepressant that causes sedation and somnolence could be taken at bedtime to help alleviate insomnia. Some studies have shown that taking a medication once a day improves adherence compared with taking the same medication in divided doses.13 Other patients may wish to take a medication several times a day so they can keep the medication in their purse or briefcase and feel confident that if they need a medication for immediate symptom relief, it will be readily available.
7. Teach patients to self-monitor changes and improvements in target symptoms. Engaging patients in a system of self-monitoring improves their chances of achieving successful treatment outcomes.14 Instruct patients to create a list of symptoms and monitor the intensity of each symptom using a rating scale of 1 to 5, where 1 represents the lowest intensity and 5 represents the highest. As for frequency, patients can rate each symptom from “not present” to “present most of the time.”
Self-monitoring allows patients to observe which daily behaviors and lifestyle choices make symptoms better and which make them worse. For example, Mrs. P, a 38-year-old married mother of 2, had anxiety and panic attacks associated with low self-esteem and chronic depression. Her clinician instructed her to use a 1-page form to monitor the frequency and intensity of her anxiety and panic symptoms by focusing on the physical manifestations, such as rapid heartbeat, shortness of breath, nausea, tremors, dry mouth, frequent urination, and diarrhea to see if there was any correlation between her behaviors and her symptoms.
8. Instruct patients to call you to report any changes, including minor successes. Early in my career, toward the end of each appointment after I’d prescribed medications I’d tell patients, “Please call me if you have a problem.” Frequently, patients would call with a list of problems and side effects that they believed were caused by the newly prescribed medication. Later, I realized that I may have inadvertently encouraged patients to develop problems so they would have a reason to call me. To achieve a more favorable outcome I changed the way I communicate. I now say, “Please call me next week, even if you begin to feel better with this new medication.” The phone call is now associated with the idea that they will “get better,” and internalizing such a suggestion allows patients to talk with the clinician and report favorable treatment results.
9. Tell patients to monitor their successes by relabeling and reframing their symptoms. Mr. B, age 28, has MDD and reports irritability, insomnia, short temper, and restlessness. After reviewing his desired treatment outcome, we discuss the benefits of pharmacotherapy. I tell him the new medication will improve the quality and length of his sleep, which will allow his body and mind to recharge his “internal batteries” and restore health and energy. When we discuss side effects, I tell him to expect a dry mouth, which will be his signal that the medication is working. This discussion helps patients reframe side effects and improves their ability to tolerate side effects and adhere to pharmacotherapy.
10. Harness the placebo effect and the power of suggestion to increase chances of achieving the best treatment outcomes. In a previous article,12 I reviewed the principles of recognizing and enhancing the placebo effect and the power of suggestion to improve the chances of achieving better pharmacotherapy outcomes. When practicing participatory pharmacotherapy, clinicians are consciously aware of the power embedded in their words and are careful to use language that enhances the placebo effect and the power of suggestion when prescribing medications. Use the patient’s own language as a way of pacing yourself to the patient’s description of his or her distress. For example, Ms. R, a 42-year-old mother of 3, describes her experiences seeking help for her anxiety and depression, stating that she has not yet found the right combination of medications that provide benefits with tolerable side effects. Her clinician responds by focusing on the word “yet” (pacing) stating, “even though you have not yet found the right combination of medications to provide the most desirable benefit of beginning healing and restoring your hope, I promise to work with you and together we will try to achieve an improvement in your overall health and well-being.” This response includes several positive words and suggestions of future success, which are referred to as leading.
Not all patients will respond to participatory pharmacotherapy. Some factors will make patients good candidates for this approach, and others should be considered exclusionary qualities (Table 2).
Bottom Line
“Participatory pharmacotherapy” involves identifying patients as partners in the process of treatment choice and decision-making, encouraging them to provide their opinions regarding medication use, and making patients feel they have been heard and understood. This technique emphasizes forming a therapeutic alliance with the patient to improve patients’ adherence to pharmacotherapy and optimize treatment outcomes.
Related Resources
- Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;16(2);CD000011.
- Mahone IH. Shared decision making and serious mental illness. Arch Psychiatr Nurs. 2008;22(6):334-343.
- Russel CL, Ruppar TM, Metteson M. Improving medication adherence: moving from intention and motivation to a personal systems approach. Nurs Clin North Am. 2011;46(3):271-281.
- Tibaldi G, Salvador-Carulla L, Garcia-Gutierrez JC. From treatment adherence to advanced shared decision making: New professional strategies and attitudes in mental health care. Curr Clin Pharmacol. 2011;6(2):91-99.
Drug Brand Name
Clonazepam • Klonopin
Disclosure
Dr. Torem reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159:1653-1664.
2. Nosé M, Barbui C, Gray R, et al. Clinical interventions for treatment non-adherence in psychosis: meta-analysis. Br J Psychiatry. 2003;183:197-206.
3. Vergouwen AC, van Hout HP, Bakker A. Methods to improve patient compliance in the use of antidepressants. Ned Tijdschr Geneeskd. 2002;146:204-207.
4. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature.
J Clin Psychiatry. 2002;63:892-909.
5. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry. 2002;63:1121-1128.
6. Colom F, Vieta E, Martinez-Aran A, et al. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry. 2000;61:549-555.
7. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
8. Osterberg LG, Rudd R. Medication adherence for antihypertensive therapy. In: Oparil S, Weber MA, eds. Hypertension: a companion to Brenner and Rector’s the kidney. 2nd ed. Philadelphia. PA: Elsevier Saunders; 2005:848.
9. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from expert consensus guidelines. J Psychiatr Pract. 2010;16:306-324.
10. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008; 165:1408-1419.
11. Szentagotai A, David D. The efficacy of cognitive-behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71:66-72.
12. Torem MS. Words to the wise: 4 secrets of successful pharmacotherapy. Current Psychiatry. 2008;7(12):19-24.
13. Medic G, Higashi K, Littlewood KJ, et al. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2013; 9:119-131.
14. Virdi N, Daskiran M, Nigam S, et al. The association of self-monitoring of blood glucose use with medication adherence and glycemic control in patients with type 2 diabetes initiating non-insulin treatment. Diabetes Technol Ther. 2012;14(9):790-798.
1. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159:1653-1664.
2. Nosé M, Barbui C, Gray R, et al. Clinical interventions for treatment non-adherence in psychosis: meta-analysis. Br J Psychiatry. 2003;183:197-206.
3. Vergouwen AC, van Hout HP, Bakker A. Methods to improve patient compliance in the use of antidepressants. Ned Tijdschr Geneeskd. 2002;146:204-207.
4. Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature.
J Clin Psychiatry. 2002;63:892-909.
5. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry. 2002;63:1121-1128.
6. Colom F, Vieta E, Martinez-Aran A, et al. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry. 2000;61:549-555.
7. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497.
8. Osterberg LG, Rudd R. Medication adherence for antihypertensive therapy. In: Oparil S, Weber MA, eds. Hypertension: a companion to Brenner and Rector’s the kidney. 2nd ed. Philadelphia. PA: Elsevier Saunders; 2005:848.
9. Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from expert consensus guidelines. J Psychiatr Pract. 2010;16:306-324.
10. Miklowitz DJ. Adjunctive psychotherapy for bipolar disorder: state of the evidence. Am J Psychiatry. 2008; 165:1408-1419.
11. Szentagotai A, David D. The efficacy of cognitive-behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71:66-72.
12. Torem MS. Words to the wise: 4 secrets of successful pharmacotherapy. Current Psychiatry. 2008;7(12):19-24.
13. Medic G, Higashi K, Littlewood KJ, et al. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2013; 9:119-131.
14. Virdi N, Daskiran M, Nigam S, et al. The association of self-monitoring of blood glucose use with medication adherence and glycemic control in patients with type 2 diabetes initiating non-insulin treatment. Diabetes Technol Ther. 2012;14(9):790-798.
Atypical antipsychotics during pregnancy
Although clinicians and patients generally are cautious when prescribing or using antipsychotics during pregnancy, inadequately controlled psychiatric illness poses risks to both mother and child. Calculating the risks and benefits of antipsychotic use during pregnancy is limited by an incomplete understanding of the true effectiveness and full spectrum of risks of these medications. Ethical principles prohibit the type of rigorous research that would be needed to achieve clarity on this issue. This article reviews studies that might help guide clinicians who are considering prescribing an atypical antipsychotic to manage psychiatric illness in a pregnant woman.
Antipsychotic efficacy in pregnancy
All atypical antipsychotics available in the United States are FDA-approved for treating schizophrenia; some also have been approved for treating bipolar disorder, unipolar depression, or symptoms associated with autism (Table 1). Atypical antipsychotics frequently are used off-label for these and other categories of psychiatric illness, including unipolar depression, generalized anxiety disorder, and obsessive-compulsive disorder.
Studies of pharmacotherapy in pregnant women tend to focus more on safety rather than efficacy. Clinical decisions for an individual patient are best made based on knowledge about which medications have been effective for that patient in the past (Algorithm).
However, safety concerns in pregnancy may require modifying an existing regimen. In other cases, new symptoms arise during pregnancy and necessitate new medications. Additionally, a drug’s effectiveness may be affected by physiologic changes of pregnancy that can alter drug metabolism,1 potentially necessitating dose changes (Box 1).
Risks of treatment vs illness
Complete safety data on the use of any psychotropic medication during pregnancy are not available. To date, studies of atypical antipsychotics do not support any increased risk for congenital malformations large enough to be detected in medium-sized samples,2-4 although it is possible that there are increases in risk that are below the detection limit of these studies. Data regarding delivery outcomes are conflicting and difficult to interpret.
Several studies2-4 have yielded inconsistent results, including:
• risks for increased birth weight and large for gestational age3
• risks for low birth weight and small for gestational age2
• no significant differences from controls.4
Atypical antipsychotics increase the risk of gestational diabetes, whereas typical antipsychotics do not appear to increase this risk.4
Until recently, research has been limited by difficulties in separating the effects of treatment from the effects of psychiatric illness, which include intrauterine growth retardation, prematurity, preterm birth, low Apgar scores, and congenital defects.5 In addition, most studies address early and easily measurable outcomes such as preterm labor, birth weight, and congenital malformations. Researchers are just beginning to investigate more subtle and long-term potential behavioral effects.
Several recent studies have explored outcomes associated with antipsychotic use during pregnancy while attempting to separate the effects of treatment from those of disease (Box 2).
Data on atypicals
Aripiprazole. Case reports of aripiprazole use during pregnancy have reported difficulties including transient unexplained fetal tachycardia that required emergent caesarean section6 and transient respiratory distress.7 Several small case series were not powered to detect risks related to aripiprazole.8,9
Animal data suggest teratogenic potential at dosages 3 and 10 times the maximum recommended human dose.10,11 Two studies7,12 that measured placental transfer of aripiprazole found cord-to-maternal serum concentration ratios ranging from 0.47 to 0.63, which is similar to the ratios for quetiapine and risperidone and lower than those for olanzapine and haloperidol.13
There are insufficient data to identify risks related to aripiprazole compared with other drugs in its class, and fewer reports are available than for other atypical antipsychotics such as quetiapine and olanzapine. Placental transfer appears to be on the lower end of the spectrum for drugs in this class. Aripiprazole would be an acceptable choice for a woman who had a history of response to aripiprazole but likely would not be a first choice for a woman requiring a new medication during pregnancy.
Clozapine. In case reports, adverse effects associated with clozapine exposure during pregnancy include major malformations, gestational metabolic complications, poor pregnancy outcome, and perinatal adverse reactions. In one case, neonatal plasma clozapine concentrations were found to be twice that found in maternal plasma.14 Animal data have shown no evidence of increased teratogenicity at 2 to 4 times the maximum recommended human dosages.15 Boden et al8 found an increased risk for gestational diabetes and macrocephaly with clozapine (11 exposures). Four other series2-4,16 were underpowered to detect concerns related specifically to clozapine.
There are insufficient data to identify risks related specifically to clozapine use during pregnancy. However, the rare but severe adverse effects associated with clozapine in other patient populations—including agranulocytosis and severe constipation17—could be devastating in a pregnant patient, which suggests this medication would not be a first-line treatment.
Olanzapine. In postmarketing surveillance studies and case reports, there have been have anecdotal cases of fetal malformations related to olanzapine use during pregnancy. Several larger studies2-4,8,16 did not find higher rates of congenital malformations or any pattern of malformation types, although none were designed or powered to examine rare events. Animal data show no evidence of teratogenicity.18 A study comparing rates of placental passage of antipsychotics13 found higher rates for olanzapine than for quetiapine and risperidone, as well as higher prevalence of low birth weight and perinatal complications. A neonatal withdrawal syndrome has also been reported.19 Boden et al8 found an increased risk for gestational diabetes and macrocephaly with olanzapine.
Data suggest that olanzapine may be associated with somewhat higher rates of the adverse effects attributable to atypical antipsychotics (gestational diabetes and possibly macrocephaly), which could be related to olanzapine’s relatively higher rate of placental passage. Olanzapine could be a reasonable choice in a woman who had a history of good response to this medication, but would be lower priority than quetiapine when a new drug is indicated during pregnancy.
Quetiapine. In clinical trials, quetiapine had lower rates of placental passage compared with risperidone and olanzapine.13 One case report found only small changes in quetiapine serum levels during pregnancy.20 Prospective studies (90 exposures,8 36 exposures,2 7 exposures,16 4 exposures,3 and 4 exposures4) show no increase in fetal malformations or adverse neonatal health outcomes related to quetiapine, and manufacturer safety data reveal no teratogenic effect, although delays in fetal skeletal ossification were seen in rats and rabbits at doses comparable to the recommended human range.21
Quetiapine is a reasonable first choice when a new atypical antipsychotic is indicated for a pregnant patient.
Risperidone. Rates of placental passage of risperidone are higher compared with quetiapine.13 Postmarketing surveillance data (265 exposures22 and 10 exposures23) and prospective studies (including 72 exposures,8 49 exposures,2 51 exposures,4 16 exposures,16 and 5 exposures3) suggest risperidone has no major teratogenic effect. When malformations were present, they were similar to expected rates and types of malformations, and no specific malformation type was overrepresented. However, in some cases, researchers noted a withdrawal-emergent syndrome that included various combinations of tremors, irritability, poor feeding, and somnolence.22 Animal data are similarly reassuring, although increases in early fetal mortality and (potentially related) changes in maternal behavior have been observed in rats.24,25 A major caveat with risperidone is its propensity to cause hyperprolactinemia, which is detrimental to efforts to conceive and maintain a pregnancy.26,27
Risperidone is not associated with higher rates of adverse events in pregnancy than other atypical antipsychotics. It would not be a first choice for a woman trying to conceive or in the early stages of pregnancy, but would be a reasonable choice for a woman already well into pregnancy.
Ziprasidone. Available reports are few and generally do not report findings on ziprasidone separately.8,28 Manufacturer data includes 5 spontaneous abortions, one malformation, and one stillbirth among 57 exposures,4 and available animal data suggest significant developmental toxicity and impaired fertility.29 In pregnant rats, ziprasidone dosed as low as 0.5 times the maximum human recommended dose resulted in delayed fetal skeletal ossification, increased stillbirths, and decreased fetal weight and postnatal survival, and ziprasidone dosed as low as 0.2 times the maximum recommended human dose resulted in developmental delays and neurobehavioral impairments in offspring. In pregnant rabbits, ziprasidone dosed at 3 times the maximum recommended human dose resulted in cardiac and renal malformations.29
Although available data are too sparse to draw reliable conclusions, the small amount of human data plus animal data suggest that ziprasidone should be less preferred than other atypical antipsychotics during pregnancy.
Lurasidone. No data addressing lurasidone use in humans during pregnancy are available. Material submitted to the FDA includes no evidence of teratogenicity or embryo-fetal toxicity in rat and rabbit studies using 3 and 12 times the maximum recommended human dose (80 mg) based on a body surface area comparison.30
Asenapine. No data specifically addressing asenapine use in humans during pregnancy are available. Studies in rats and rabbits found no increase in teratogenicity, but did find increases in postimplantation loss and decreases in pup survival and weight gain with maternal doses equivalent to less than the maximum recommended human dose.31
Iloperidone. No data specifically addressing iloperidone use in humans during pregnancy are available. Animal studies of iloperidone found multiple developmental toxicities when iloperidone was administered during gestation.32 In one study, pregnant rats were given up to 26 times the maximum recommended human dose of 24 mg/d during the period of organogenesis. The highest dose caused increased early intrauterine deaths, decreased fetal weight and length, decreased fetal skeletal ossification, and increased minor fetal skeletal anomalies and variations. In a similar study using pregnant rabbits, the highest dose caused increased early intrauterine deaths and decreased fetal viability at term.
Paliperidone. In animal studies, there were no increases in fetal abnormalities when pregnant rats and rabbits were treated with up to 8 times the maximum recommended human dose of paliperidone during the period of organogenesis.33
A single case report34 measured levels of risperidone and its 9-hydroxy metabolite, paliperidone, in the breast milk of a mother who had taken risperidone during pregnancy and in the serum of her child. 9-OH-risperidone dose in breast milk was calculated as 4.7% of the weight-adjusted maternal dose, and serum levels in the infant were undetectable. No ill effects on the child were observed.
It is not possible to draw solid conclusions about atypical antipsychotics’ potential effects on human development from animal studies. Because of the lack of human data for the newer atypical antipsychotics—asenapine, iloperidone, lurasidone, paliperidone—in general these agents would not be advisable as first-line medications for treating pregnant women.
A few caveats
All atypical antipsychotics share the propensity to trigger or worsen glucose intolerance, which can have significant negative consequences in a pregnant patient. When deciding to use an atypical antipsychotic during pregnancy, blood glucose should be monitored carefully and regularly.
Because all atypical antipsychotics (except clozapine) are FDA pregnancy class C—indicating that animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks—the decision to use these medications must be based on an individualized assessment of risks and benefits. Patients and their providers together should make a fully informed decision.
There is an urgent need for larger and better-designed investigations that will be sufficiently powered to detect differences in outcomes—particularly major malformations, preterm delivery, adverse events in labor and delivery, metabolic and anthropometric effects on the newborn, and neurodevelopmental and psychiatric outcomes for individuals exposed in utero—between women without mental illness, untreated women with mental illness, and women receiving atypical antipsychotics during pregnancy. Further research into the pharmacokinetics and clinical efficacy of antipsychotics in pregnant women also would be useful. Clinicians can assist with these efforts by submitting their patient data to a pregnancy registry maintained by the Massachusetts General Hospital (see Related Resources).
Bottom Line
Treatment with atypical antipsychotics during pregnancy may increase the risk of adverse birth outcomes, but inadequately controlled mental illness also carries some degree of risk. The decision to use any atypical antipsychotic during pregnancy must be based on an individualized assessment of risks and benefits and made by the pregnant patient and her provider.
Related Resources
- Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36(3):518-544. www.ncbi.nlm.nih.gov/pmc/articles/PMC2879689.
- Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. www.womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Paliperidone • Invega
Clozapine • Clozaril Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Iloperidone • Fanapt Ziprasidone • Geodon
Lurasidone • Latuda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30(3):781-789.
2. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4): 444-449; quiz 546.
3. Newham JJ, Thomas SH, MacRitchie K, et al. Birth weight of infants after maternal exposure to typical and atypical antipsychotics: prospective comparison study. Br J Psychiatry. 2008;192(5):333-337.
4. Reis M, Kallen B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28(3):279-288.
5. Matevosyan NR. Pregnancy and postpartum specifics in women with schizophrenia: a meta-study. Arch Gynecol Obstet. 2011;283(2):141-147.
6. Mendhekar DN, Sunder KR, Andrade C. Aripiprazole use in a pregnant schizoaffective woman. Bipolar Disord. 2006;8(3):299-300.
7. Watanabe N, Kasahara M, Sugibayashi R, et al. Perinatal use of aripiprazole: a case report. J Clin Psychopharmacol. 2011;31(3):377-379.
8. Bodén R, Lundgren M, Brandt L, et al. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry. 2012;69(7):715-721.
9. Maáková E, Hubicˇková L. Antidepressant drug exposure during pregnancy. CZTIS small prospective study. Neuro Endocrinol Lett. 2011;32(suppl 1):53-56.
10. Bristol-Myers Squibb (Ed.). Aripiprazole: Drugdex drug evaluations, 1974-2003. Princeton, NJ: Thomson Micromedex; 2003.
11. U.S. Food and Drug Administration. FDA datasheet: Aripiprazole. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21436slr006_abilify_lbl.pdf. Accessed April 8, 2013.
12. Nguyen T, Teoh S, Hackett LP, et al. Placental transfer of aripiprazole. Aust N Z J Psychiatry. 2011;45(6):500-501.
13. Newport DJ, Calamaras MR, DeVane CL, et al. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164(8):1214-1220.
14. Barnas C, Bergant A, Hummer M, et al. Clozapine concentrations in maternal and fetal plasma, amniotic fluid, and breast milk. Am J Psychiatry. 1994;151(6):945.
15. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
16. Lin H, Chen I, Chen Y, et al. Maternal schizophrenia and pregnancy outcome: does the use of antipsychotics make a difference? Schizophr Res. 2010;116(1):55-60.
17. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):
381-390.
18. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
19. Gilad O, Merlob P, Stahl B, et al. Outcome of infants exposed to olanzapine during breastfeeding. Breastfeed Med. 2011;6(2):55-58.
20. Klier CM, Mossaheb N, Saria A, et al. Pharmacokinetics and elimination of quetiapine, venlafaxine, and trazodone during pregnancy and postpartum. J Clin Psychopharmacol. 2007;27(6):720-722.
21. U.S. Food and Drug Administration. FDA datasheet: quetiapine. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20639se1-017,016_seroquel_lbl.pdf. Accessed April 11, 2013.
22. Coppola D, Russo LJ, Kwarta RF Jr, et al. Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes. Drug Saf. 2007;30(3):247-264.
23. Mackay FJ, Wilton LV, Pearce GL, et al. The safety of risperidone: a post-marketing study on 7,684 patients. Hum Psychopharmacol. 1998;13(6):413-418.
24. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2012.
25. U.S. Food and Drug Administration. FDA datasheet: risperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020272s056,020588s044,021346s033,021444s03lbl.pdf. Accessed April 11, 2013.
26. Katz E, Adashi EY. Hyperprolactinemic disorders. Clin Obstet Gynecol. 1990;33(3):622-639.
27. Davis JR. Prolactin and reproductive medicine. Curr Opin Obstet Gynecol. 2004;16(4):331-337.
28. Johnson KC, Laprairie JL, Brennan PA, et al. Prenatal antipsychotic exposure and neuromotor performance during infancy. Arch Gen Psychiatry. 2012;69(8):787-794. doi: 10.1001/archgenpsychiatry.2012.160.
29. U.S. Food and Drug Administration. FDA datasheet: ziprasidone. http://www.fda.gov/downloads/Advisory
Committees/CommitteesMeetingMaterials/Pediatric AdvisoryCommittee/UCM191883.pdf. Accessed March 15, 2013.
30. U.S. Food and Drug Administration. FDA datasheet: lurasidone. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200603Orig1s000PharmR.pdf. Accessed March 15, 2013.
31. U.S. Food and Drug Administration. FDA datasheet: asenapine. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022117s000_OtherR.pdf. Accessed March 15, 2013.
32. U.S. Food and Drug Administration. FDA datasheet: iloperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022192lbl.pdf. Accessed March 15, 2013.
33. U.S. Food and Drug Administration. FDA datasheet: paliperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021999s018lbl.pdf. Accessed March 15, 2013.
34. Weggelaar NM, Keijer WJ, Janssen PK. A case report of risperidone distribution and excretion into human milk: how to give good advice if you have not enough data available. J Clin Psychopharmacol. 2011;31(1):129-131.
Although clinicians and patients generally are cautious when prescribing or using antipsychotics during pregnancy, inadequately controlled psychiatric illness poses risks to both mother and child. Calculating the risks and benefits of antipsychotic use during pregnancy is limited by an incomplete understanding of the true effectiveness and full spectrum of risks of these medications. Ethical principles prohibit the type of rigorous research that would be needed to achieve clarity on this issue. This article reviews studies that might help guide clinicians who are considering prescribing an atypical antipsychotic to manage psychiatric illness in a pregnant woman.
Antipsychotic efficacy in pregnancy
All atypical antipsychotics available in the United States are FDA-approved for treating schizophrenia; some also have been approved for treating bipolar disorder, unipolar depression, or symptoms associated with autism (Table 1). Atypical antipsychotics frequently are used off-label for these and other categories of psychiatric illness, including unipolar depression, generalized anxiety disorder, and obsessive-compulsive disorder.
Studies of pharmacotherapy in pregnant women tend to focus more on safety rather than efficacy. Clinical decisions for an individual patient are best made based on knowledge about which medications have been effective for that patient in the past (Algorithm).
However, safety concerns in pregnancy may require modifying an existing regimen. In other cases, new symptoms arise during pregnancy and necessitate new medications. Additionally, a drug’s effectiveness may be affected by physiologic changes of pregnancy that can alter drug metabolism,1 potentially necessitating dose changes (Box 1).
Risks of treatment vs illness
Complete safety data on the use of any psychotropic medication during pregnancy are not available. To date, studies of atypical antipsychotics do not support any increased risk for congenital malformations large enough to be detected in medium-sized samples,2-4 although it is possible that there are increases in risk that are below the detection limit of these studies. Data regarding delivery outcomes are conflicting and difficult to interpret.
Several studies2-4 have yielded inconsistent results, including:
• risks for increased birth weight and large for gestational age3
• risks for low birth weight and small for gestational age2
• no significant differences from controls.4
Atypical antipsychotics increase the risk of gestational diabetes, whereas typical antipsychotics do not appear to increase this risk.4
Until recently, research has been limited by difficulties in separating the effects of treatment from the effects of psychiatric illness, which include intrauterine growth retardation, prematurity, preterm birth, low Apgar scores, and congenital defects.5 In addition, most studies address early and easily measurable outcomes such as preterm labor, birth weight, and congenital malformations. Researchers are just beginning to investigate more subtle and long-term potential behavioral effects.
Several recent studies have explored outcomes associated with antipsychotic use during pregnancy while attempting to separate the effects of treatment from those of disease (Box 2).
Data on atypicals
Aripiprazole. Case reports of aripiprazole use during pregnancy have reported difficulties including transient unexplained fetal tachycardia that required emergent caesarean section6 and transient respiratory distress.7 Several small case series were not powered to detect risks related to aripiprazole.8,9
Animal data suggest teratogenic potential at dosages 3 and 10 times the maximum recommended human dose.10,11 Two studies7,12 that measured placental transfer of aripiprazole found cord-to-maternal serum concentration ratios ranging from 0.47 to 0.63, which is similar to the ratios for quetiapine and risperidone and lower than those for olanzapine and haloperidol.13
There are insufficient data to identify risks related to aripiprazole compared with other drugs in its class, and fewer reports are available than for other atypical antipsychotics such as quetiapine and olanzapine. Placental transfer appears to be on the lower end of the spectrum for drugs in this class. Aripiprazole would be an acceptable choice for a woman who had a history of response to aripiprazole but likely would not be a first choice for a woman requiring a new medication during pregnancy.
Clozapine. In case reports, adverse effects associated with clozapine exposure during pregnancy include major malformations, gestational metabolic complications, poor pregnancy outcome, and perinatal adverse reactions. In one case, neonatal plasma clozapine concentrations were found to be twice that found in maternal plasma.14 Animal data have shown no evidence of increased teratogenicity at 2 to 4 times the maximum recommended human dosages.15 Boden et al8 found an increased risk for gestational diabetes and macrocephaly with clozapine (11 exposures). Four other series2-4,16 were underpowered to detect concerns related specifically to clozapine.
There are insufficient data to identify risks related specifically to clozapine use during pregnancy. However, the rare but severe adverse effects associated with clozapine in other patient populations—including agranulocytosis and severe constipation17—could be devastating in a pregnant patient, which suggests this medication would not be a first-line treatment.
Olanzapine. In postmarketing surveillance studies and case reports, there have been have anecdotal cases of fetal malformations related to olanzapine use during pregnancy. Several larger studies2-4,8,16 did not find higher rates of congenital malformations or any pattern of malformation types, although none were designed or powered to examine rare events. Animal data show no evidence of teratogenicity.18 A study comparing rates of placental passage of antipsychotics13 found higher rates for olanzapine than for quetiapine and risperidone, as well as higher prevalence of low birth weight and perinatal complications. A neonatal withdrawal syndrome has also been reported.19 Boden et al8 found an increased risk for gestational diabetes and macrocephaly with olanzapine.
Data suggest that olanzapine may be associated with somewhat higher rates of the adverse effects attributable to atypical antipsychotics (gestational diabetes and possibly macrocephaly), which could be related to olanzapine’s relatively higher rate of placental passage. Olanzapine could be a reasonable choice in a woman who had a history of good response to this medication, but would be lower priority than quetiapine when a new drug is indicated during pregnancy.
Quetiapine. In clinical trials, quetiapine had lower rates of placental passage compared with risperidone and olanzapine.13 One case report found only small changes in quetiapine serum levels during pregnancy.20 Prospective studies (90 exposures,8 36 exposures,2 7 exposures,16 4 exposures,3 and 4 exposures4) show no increase in fetal malformations or adverse neonatal health outcomes related to quetiapine, and manufacturer safety data reveal no teratogenic effect, although delays in fetal skeletal ossification were seen in rats and rabbits at doses comparable to the recommended human range.21
Quetiapine is a reasonable first choice when a new atypical antipsychotic is indicated for a pregnant patient.
Risperidone. Rates of placental passage of risperidone are higher compared with quetiapine.13 Postmarketing surveillance data (265 exposures22 and 10 exposures23) and prospective studies (including 72 exposures,8 49 exposures,2 51 exposures,4 16 exposures,16 and 5 exposures3) suggest risperidone has no major teratogenic effect. When malformations were present, they were similar to expected rates and types of malformations, and no specific malformation type was overrepresented. However, in some cases, researchers noted a withdrawal-emergent syndrome that included various combinations of tremors, irritability, poor feeding, and somnolence.22 Animal data are similarly reassuring, although increases in early fetal mortality and (potentially related) changes in maternal behavior have been observed in rats.24,25 A major caveat with risperidone is its propensity to cause hyperprolactinemia, which is detrimental to efforts to conceive and maintain a pregnancy.26,27
Risperidone is not associated with higher rates of adverse events in pregnancy than other atypical antipsychotics. It would not be a first choice for a woman trying to conceive or in the early stages of pregnancy, but would be a reasonable choice for a woman already well into pregnancy.
Ziprasidone. Available reports are few and generally do not report findings on ziprasidone separately.8,28 Manufacturer data includes 5 spontaneous abortions, one malformation, and one stillbirth among 57 exposures,4 and available animal data suggest significant developmental toxicity and impaired fertility.29 In pregnant rats, ziprasidone dosed as low as 0.5 times the maximum human recommended dose resulted in delayed fetal skeletal ossification, increased stillbirths, and decreased fetal weight and postnatal survival, and ziprasidone dosed as low as 0.2 times the maximum recommended human dose resulted in developmental delays and neurobehavioral impairments in offspring. In pregnant rabbits, ziprasidone dosed at 3 times the maximum recommended human dose resulted in cardiac and renal malformations.29
Although available data are too sparse to draw reliable conclusions, the small amount of human data plus animal data suggest that ziprasidone should be less preferred than other atypical antipsychotics during pregnancy.
Lurasidone. No data addressing lurasidone use in humans during pregnancy are available. Material submitted to the FDA includes no evidence of teratogenicity or embryo-fetal toxicity in rat and rabbit studies using 3 and 12 times the maximum recommended human dose (80 mg) based on a body surface area comparison.30
Asenapine. No data specifically addressing asenapine use in humans during pregnancy are available. Studies in rats and rabbits found no increase in teratogenicity, but did find increases in postimplantation loss and decreases in pup survival and weight gain with maternal doses equivalent to less than the maximum recommended human dose.31
Iloperidone. No data specifically addressing iloperidone use in humans during pregnancy are available. Animal studies of iloperidone found multiple developmental toxicities when iloperidone was administered during gestation.32 In one study, pregnant rats were given up to 26 times the maximum recommended human dose of 24 mg/d during the period of organogenesis. The highest dose caused increased early intrauterine deaths, decreased fetal weight and length, decreased fetal skeletal ossification, and increased minor fetal skeletal anomalies and variations. In a similar study using pregnant rabbits, the highest dose caused increased early intrauterine deaths and decreased fetal viability at term.
Paliperidone. In animal studies, there were no increases in fetal abnormalities when pregnant rats and rabbits were treated with up to 8 times the maximum recommended human dose of paliperidone during the period of organogenesis.33
A single case report34 measured levels of risperidone and its 9-hydroxy metabolite, paliperidone, in the breast milk of a mother who had taken risperidone during pregnancy and in the serum of her child. 9-OH-risperidone dose in breast milk was calculated as 4.7% of the weight-adjusted maternal dose, and serum levels in the infant were undetectable. No ill effects on the child were observed.
It is not possible to draw solid conclusions about atypical antipsychotics’ potential effects on human development from animal studies. Because of the lack of human data for the newer atypical antipsychotics—asenapine, iloperidone, lurasidone, paliperidone—in general these agents would not be advisable as first-line medications for treating pregnant women.
A few caveats
All atypical antipsychotics share the propensity to trigger or worsen glucose intolerance, which can have significant negative consequences in a pregnant patient. When deciding to use an atypical antipsychotic during pregnancy, blood glucose should be monitored carefully and regularly.
Because all atypical antipsychotics (except clozapine) are FDA pregnancy class C—indicating that animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks—the decision to use these medications must be based on an individualized assessment of risks and benefits. Patients and their providers together should make a fully informed decision.
There is an urgent need for larger and better-designed investigations that will be sufficiently powered to detect differences in outcomes—particularly major malformations, preterm delivery, adverse events in labor and delivery, metabolic and anthropometric effects on the newborn, and neurodevelopmental and psychiatric outcomes for individuals exposed in utero—between women without mental illness, untreated women with mental illness, and women receiving atypical antipsychotics during pregnancy. Further research into the pharmacokinetics and clinical efficacy of antipsychotics in pregnant women also would be useful. Clinicians can assist with these efforts by submitting their patient data to a pregnancy registry maintained by the Massachusetts General Hospital (see Related Resources).
Bottom Line
Treatment with atypical antipsychotics during pregnancy may increase the risk of adverse birth outcomes, but inadequately controlled mental illness also carries some degree of risk. The decision to use any atypical antipsychotic during pregnancy must be based on an individualized assessment of risks and benefits and made by the pregnant patient and her provider.
Related Resources
- Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36(3):518-544. www.ncbi.nlm.nih.gov/pmc/articles/PMC2879689.
- Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. www.womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Paliperidone • Invega
Clozapine • Clozaril Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Iloperidone • Fanapt Ziprasidone • Geodon
Lurasidone • Latuda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Although clinicians and patients generally are cautious when prescribing or using antipsychotics during pregnancy, inadequately controlled psychiatric illness poses risks to both mother and child. Calculating the risks and benefits of antipsychotic use during pregnancy is limited by an incomplete understanding of the true effectiveness and full spectrum of risks of these medications. Ethical principles prohibit the type of rigorous research that would be needed to achieve clarity on this issue. This article reviews studies that might help guide clinicians who are considering prescribing an atypical antipsychotic to manage psychiatric illness in a pregnant woman.
Antipsychotic efficacy in pregnancy
All atypical antipsychotics available in the United States are FDA-approved for treating schizophrenia; some also have been approved for treating bipolar disorder, unipolar depression, or symptoms associated with autism (Table 1). Atypical antipsychotics frequently are used off-label for these and other categories of psychiatric illness, including unipolar depression, generalized anxiety disorder, and obsessive-compulsive disorder.
Studies of pharmacotherapy in pregnant women tend to focus more on safety rather than efficacy. Clinical decisions for an individual patient are best made based on knowledge about which medications have been effective for that patient in the past (Algorithm).
However, safety concerns in pregnancy may require modifying an existing regimen. In other cases, new symptoms arise during pregnancy and necessitate new medications. Additionally, a drug’s effectiveness may be affected by physiologic changes of pregnancy that can alter drug metabolism,1 potentially necessitating dose changes (Box 1).
Risks of treatment vs illness
Complete safety data on the use of any psychotropic medication during pregnancy are not available. To date, studies of atypical antipsychotics do not support any increased risk for congenital malformations large enough to be detected in medium-sized samples,2-4 although it is possible that there are increases in risk that are below the detection limit of these studies. Data regarding delivery outcomes are conflicting and difficult to interpret.
Several studies2-4 have yielded inconsistent results, including:
• risks for increased birth weight and large for gestational age3
• risks for low birth weight and small for gestational age2
• no significant differences from controls.4
Atypical antipsychotics increase the risk of gestational diabetes, whereas typical antipsychotics do not appear to increase this risk.4
Until recently, research has been limited by difficulties in separating the effects of treatment from the effects of psychiatric illness, which include intrauterine growth retardation, prematurity, preterm birth, low Apgar scores, and congenital defects.5 In addition, most studies address early and easily measurable outcomes such as preterm labor, birth weight, and congenital malformations. Researchers are just beginning to investigate more subtle and long-term potential behavioral effects.
Several recent studies have explored outcomes associated with antipsychotic use during pregnancy while attempting to separate the effects of treatment from those of disease (Box 2).
Data on atypicals
Aripiprazole. Case reports of aripiprazole use during pregnancy have reported difficulties including transient unexplained fetal tachycardia that required emergent caesarean section6 and transient respiratory distress.7 Several small case series were not powered to detect risks related to aripiprazole.8,9
Animal data suggest teratogenic potential at dosages 3 and 10 times the maximum recommended human dose.10,11 Two studies7,12 that measured placental transfer of aripiprazole found cord-to-maternal serum concentration ratios ranging from 0.47 to 0.63, which is similar to the ratios for quetiapine and risperidone and lower than those for olanzapine and haloperidol.13
There are insufficient data to identify risks related to aripiprazole compared with other drugs in its class, and fewer reports are available than for other atypical antipsychotics such as quetiapine and olanzapine. Placental transfer appears to be on the lower end of the spectrum for drugs in this class. Aripiprazole would be an acceptable choice for a woman who had a history of response to aripiprazole but likely would not be a first choice for a woman requiring a new medication during pregnancy.
Clozapine. In case reports, adverse effects associated with clozapine exposure during pregnancy include major malformations, gestational metabolic complications, poor pregnancy outcome, and perinatal adverse reactions. In one case, neonatal plasma clozapine concentrations were found to be twice that found in maternal plasma.14 Animal data have shown no evidence of increased teratogenicity at 2 to 4 times the maximum recommended human dosages.15 Boden et al8 found an increased risk for gestational diabetes and macrocephaly with clozapine (11 exposures). Four other series2-4,16 were underpowered to detect concerns related specifically to clozapine.
There are insufficient data to identify risks related specifically to clozapine use during pregnancy. However, the rare but severe adverse effects associated with clozapine in other patient populations—including agranulocytosis and severe constipation17—could be devastating in a pregnant patient, which suggests this medication would not be a first-line treatment.
Olanzapine. In postmarketing surveillance studies and case reports, there have been have anecdotal cases of fetal malformations related to olanzapine use during pregnancy. Several larger studies2-4,8,16 did not find higher rates of congenital malformations or any pattern of malformation types, although none were designed or powered to examine rare events. Animal data show no evidence of teratogenicity.18 A study comparing rates of placental passage of antipsychotics13 found higher rates for olanzapine than for quetiapine and risperidone, as well as higher prevalence of low birth weight and perinatal complications. A neonatal withdrawal syndrome has also been reported.19 Boden et al8 found an increased risk for gestational diabetes and macrocephaly with olanzapine.
Data suggest that olanzapine may be associated with somewhat higher rates of the adverse effects attributable to atypical antipsychotics (gestational diabetes and possibly macrocephaly), which could be related to olanzapine’s relatively higher rate of placental passage. Olanzapine could be a reasonable choice in a woman who had a history of good response to this medication, but would be lower priority than quetiapine when a new drug is indicated during pregnancy.
Quetiapine. In clinical trials, quetiapine had lower rates of placental passage compared with risperidone and olanzapine.13 One case report found only small changes in quetiapine serum levels during pregnancy.20 Prospective studies (90 exposures,8 36 exposures,2 7 exposures,16 4 exposures,3 and 4 exposures4) show no increase in fetal malformations or adverse neonatal health outcomes related to quetiapine, and manufacturer safety data reveal no teratogenic effect, although delays in fetal skeletal ossification were seen in rats and rabbits at doses comparable to the recommended human range.21
Quetiapine is a reasonable first choice when a new atypical antipsychotic is indicated for a pregnant patient.
Risperidone. Rates of placental passage of risperidone are higher compared with quetiapine.13 Postmarketing surveillance data (265 exposures22 and 10 exposures23) and prospective studies (including 72 exposures,8 49 exposures,2 51 exposures,4 16 exposures,16 and 5 exposures3) suggest risperidone has no major teratogenic effect. When malformations were present, they were similar to expected rates and types of malformations, and no specific malformation type was overrepresented. However, in some cases, researchers noted a withdrawal-emergent syndrome that included various combinations of tremors, irritability, poor feeding, and somnolence.22 Animal data are similarly reassuring, although increases in early fetal mortality and (potentially related) changes in maternal behavior have been observed in rats.24,25 A major caveat with risperidone is its propensity to cause hyperprolactinemia, which is detrimental to efforts to conceive and maintain a pregnancy.26,27
Risperidone is not associated with higher rates of adverse events in pregnancy than other atypical antipsychotics. It would not be a first choice for a woman trying to conceive or in the early stages of pregnancy, but would be a reasonable choice for a woman already well into pregnancy.
Ziprasidone. Available reports are few and generally do not report findings on ziprasidone separately.8,28 Manufacturer data includes 5 spontaneous abortions, one malformation, and one stillbirth among 57 exposures,4 and available animal data suggest significant developmental toxicity and impaired fertility.29 In pregnant rats, ziprasidone dosed as low as 0.5 times the maximum human recommended dose resulted in delayed fetal skeletal ossification, increased stillbirths, and decreased fetal weight and postnatal survival, and ziprasidone dosed as low as 0.2 times the maximum recommended human dose resulted in developmental delays and neurobehavioral impairments in offspring. In pregnant rabbits, ziprasidone dosed at 3 times the maximum recommended human dose resulted in cardiac and renal malformations.29
Although available data are too sparse to draw reliable conclusions, the small amount of human data plus animal data suggest that ziprasidone should be less preferred than other atypical antipsychotics during pregnancy.
Lurasidone. No data addressing lurasidone use in humans during pregnancy are available. Material submitted to the FDA includes no evidence of teratogenicity or embryo-fetal toxicity in rat and rabbit studies using 3 and 12 times the maximum recommended human dose (80 mg) based on a body surface area comparison.30
Asenapine. No data specifically addressing asenapine use in humans during pregnancy are available. Studies in rats and rabbits found no increase in teratogenicity, but did find increases in postimplantation loss and decreases in pup survival and weight gain with maternal doses equivalent to less than the maximum recommended human dose.31
Iloperidone. No data specifically addressing iloperidone use in humans during pregnancy are available. Animal studies of iloperidone found multiple developmental toxicities when iloperidone was administered during gestation.32 In one study, pregnant rats were given up to 26 times the maximum recommended human dose of 24 mg/d during the period of organogenesis. The highest dose caused increased early intrauterine deaths, decreased fetal weight and length, decreased fetal skeletal ossification, and increased minor fetal skeletal anomalies and variations. In a similar study using pregnant rabbits, the highest dose caused increased early intrauterine deaths and decreased fetal viability at term.
Paliperidone. In animal studies, there were no increases in fetal abnormalities when pregnant rats and rabbits were treated with up to 8 times the maximum recommended human dose of paliperidone during the period of organogenesis.33
A single case report34 measured levels of risperidone and its 9-hydroxy metabolite, paliperidone, in the breast milk of a mother who had taken risperidone during pregnancy and in the serum of her child. 9-OH-risperidone dose in breast milk was calculated as 4.7% of the weight-adjusted maternal dose, and serum levels in the infant were undetectable. No ill effects on the child were observed.
It is not possible to draw solid conclusions about atypical antipsychotics’ potential effects on human development from animal studies. Because of the lack of human data for the newer atypical antipsychotics—asenapine, iloperidone, lurasidone, paliperidone—in general these agents would not be advisable as first-line medications for treating pregnant women.
A few caveats
All atypical antipsychotics share the propensity to trigger or worsen glucose intolerance, which can have significant negative consequences in a pregnant patient. When deciding to use an atypical antipsychotic during pregnancy, blood glucose should be monitored carefully and regularly.
Because all atypical antipsychotics (except clozapine) are FDA pregnancy class C—indicating that animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks—the decision to use these medications must be based on an individualized assessment of risks and benefits. Patients and their providers together should make a fully informed decision.
There is an urgent need for larger and better-designed investigations that will be sufficiently powered to detect differences in outcomes—particularly major malformations, preterm delivery, adverse events in labor and delivery, metabolic and anthropometric effects on the newborn, and neurodevelopmental and psychiatric outcomes for individuals exposed in utero—between women without mental illness, untreated women with mental illness, and women receiving atypical antipsychotics during pregnancy. Further research into the pharmacokinetics and clinical efficacy of antipsychotics in pregnant women also would be useful. Clinicians can assist with these efforts by submitting their patient data to a pregnancy registry maintained by the Massachusetts General Hospital (see Related Resources).
Bottom Line
Treatment with atypical antipsychotics during pregnancy may increase the risk of adverse birth outcomes, but inadequately controlled mental illness also carries some degree of risk. The decision to use any atypical antipsychotic during pregnancy must be based on an individualized assessment of risks and benefits and made by the pregnant patient and her provider.
Related Resources
- Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36(3):518-544. www.ncbi.nlm.nih.gov/pmc/articles/PMC2879689.
- Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. www.womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry.
Drug Brand Names
Aripiprazole • Abilify Olanzapine • Zyprexa
Asenapine • Saphris Paliperidone • Invega
Clozapine • Clozaril Quetiapine • Seroquel
Haloperidol • Haldol Risperidone • Risperdal
Iloperidone • Fanapt Ziprasidone • Geodon
Lurasidone • Latuda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30(3):781-789.
2. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4): 444-449; quiz 546.
3. Newham JJ, Thomas SH, MacRitchie K, et al. Birth weight of infants after maternal exposure to typical and atypical antipsychotics: prospective comparison study. Br J Psychiatry. 2008;192(5):333-337.
4. Reis M, Kallen B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28(3):279-288.
5. Matevosyan NR. Pregnancy and postpartum specifics in women with schizophrenia: a meta-study. Arch Gynecol Obstet. 2011;283(2):141-147.
6. Mendhekar DN, Sunder KR, Andrade C. Aripiprazole use in a pregnant schizoaffective woman. Bipolar Disord. 2006;8(3):299-300.
7. Watanabe N, Kasahara M, Sugibayashi R, et al. Perinatal use of aripiprazole: a case report. J Clin Psychopharmacol. 2011;31(3):377-379.
8. Bodén R, Lundgren M, Brandt L, et al. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry. 2012;69(7):715-721.
9. Maáková E, Hubicˇková L. Antidepressant drug exposure during pregnancy. CZTIS small prospective study. Neuro Endocrinol Lett. 2011;32(suppl 1):53-56.
10. Bristol-Myers Squibb (Ed.). Aripiprazole: Drugdex drug evaluations, 1974-2003. Princeton, NJ: Thomson Micromedex; 2003.
11. U.S. Food and Drug Administration. FDA datasheet: Aripiprazole. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21436slr006_abilify_lbl.pdf. Accessed April 8, 2013.
12. Nguyen T, Teoh S, Hackett LP, et al. Placental transfer of aripiprazole. Aust N Z J Psychiatry. 2011;45(6):500-501.
13. Newport DJ, Calamaras MR, DeVane CL, et al. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164(8):1214-1220.
14. Barnas C, Bergant A, Hummer M, et al. Clozapine concentrations in maternal and fetal plasma, amniotic fluid, and breast milk. Am J Psychiatry. 1994;151(6):945.
15. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
16. Lin H, Chen I, Chen Y, et al. Maternal schizophrenia and pregnancy outcome: does the use of antipsychotics make a difference? Schizophr Res. 2010;116(1):55-60.
17. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):
381-390.
18. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
19. Gilad O, Merlob P, Stahl B, et al. Outcome of infants exposed to olanzapine during breastfeeding. Breastfeed Med. 2011;6(2):55-58.
20. Klier CM, Mossaheb N, Saria A, et al. Pharmacokinetics and elimination of quetiapine, venlafaxine, and trazodone during pregnancy and postpartum. J Clin Psychopharmacol. 2007;27(6):720-722.
21. U.S. Food and Drug Administration. FDA datasheet: quetiapine. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20639se1-017,016_seroquel_lbl.pdf. Accessed April 11, 2013.
22. Coppola D, Russo LJ, Kwarta RF Jr, et al. Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes. Drug Saf. 2007;30(3):247-264.
23. Mackay FJ, Wilton LV, Pearce GL, et al. The safety of risperidone: a post-marketing study on 7,684 patients. Hum Psychopharmacol. 1998;13(6):413-418.
24. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2012.
25. U.S. Food and Drug Administration. FDA datasheet: risperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020272s056,020588s044,021346s033,021444s03lbl.pdf. Accessed April 11, 2013.
26. Katz E, Adashi EY. Hyperprolactinemic disorders. Clin Obstet Gynecol. 1990;33(3):622-639.
27. Davis JR. Prolactin and reproductive medicine. Curr Opin Obstet Gynecol. 2004;16(4):331-337.
28. Johnson KC, Laprairie JL, Brennan PA, et al. Prenatal antipsychotic exposure and neuromotor performance during infancy. Arch Gen Psychiatry. 2012;69(8):787-794. doi: 10.1001/archgenpsychiatry.2012.160.
29. U.S. Food and Drug Administration. FDA datasheet: ziprasidone. http://www.fda.gov/downloads/Advisory
Committees/CommitteesMeetingMaterials/Pediatric AdvisoryCommittee/UCM191883.pdf. Accessed March 15, 2013.
30. U.S. Food and Drug Administration. FDA datasheet: lurasidone. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200603Orig1s000PharmR.pdf. Accessed March 15, 2013.
31. U.S. Food and Drug Administration. FDA datasheet: asenapine. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022117s000_OtherR.pdf. Accessed March 15, 2013.
32. U.S. Food and Drug Administration. FDA datasheet: iloperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022192lbl.pdf. Accessed March 15, 2013.
33. U.S. Food and Drug Administration. FDA datasheet: paliperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021999s018lbl.pdf. Accessed March 15, 2013.
34. Weggelaar NM, Keijer WJ, Janssen PK. A case report of risperidone distribution and excretion into human milk: how to give good advice if you have not enough data available. J Clin Psychopharmacol. 2011;31(1):129-131.
1. Chang J, Streitman D. Physiologic adaptations to pregnancy. Neurol Clin. 2012;30(3):781-789.
2. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4): 444-449; quiz 546.
3. Newham JJ, Thomas SH, MacRitchie K, et al. Birth weight of infants after maternal exposure to typical and atypical antipsychotics: prospective comparison study. Br J Psychiatry. 2008;192(5):333-337.
4. Reis M, Kallen B. Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol. 2008;28(3):279-288.
5. Matevosyan NR. Pregnancy and postpartum specifics in women with schizophrenia: a meta-study. Arch Gynecol Obstet. 2011;283(2):141-147.
6. Mendhekar DN, Sunder KR, Andrade C. Aripiprazole use in a pregnant schizoaffective woman. Bipolar Disord. 2006;8(3):299-300.
7. Watanabe N, Kasahara M, Sugibayashi R, et al. Perinatal use of aripiprazole: a case report. J Clin Psychopharmacol. 2011;31(3):377-379.
8. Bodén R, Lundgren M, Brandt L, et al. Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Arch Gen Psychiatry. 2012;69(7):715-721.
9. Maáková E, Hubicˇková L. Antidepressant drug exposure during pregnancy. CZTIS small prospective study. Neuro Endocrinol Lett. 2011;32(suppl 1):53-56.
10. Bristol-Myers Squibb (Ed.). Aripiprazole: Drugdex drug evaluations, 1974-2003. Princeton, NJ: Thomson Micromedex; 2003.
11. U.S. Food and Drug Administration. FDA datasheet: Aripiprazole. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21436slr006_abilify_lbl.pdf. Accessed April 8, 2013.
12. Nguyen T, Teoh S, Hackett LP, et al. Placental transfer of aripiprazole. Aust N Z J Psychiatry. 2011;45(6):500-501.
13. Newport DJ, Calamaras MR, DeVane CL, et al. Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. Am J Psychiatry. 2007;164(8):1214-1220.
14. Barnas C, Bergant A, Hummer M, et al. Clozapine concentrations in maternal and fetal plasma, amniotic fluid, and breast milk. Am J Psychiatry. 1994;151(6):945.
15. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013.
16. Lin H, Chen I, Chen Y, et al. Maternal schizophrenia and pregnancy outcome: does the use of antipsychotics make a difference? Schizophr Res. 2010;116(1):55-60.
17. Young CR, Bowers MB Jr, Mazure CM. Management of the adverse effects of clozapine. Schizophr Bull. 1998;24(3):
381-390.
18. Zyprexa [package insert]. Indianapolis, IN: Eli Lilly and Company; 1997.
19. Gilad O, Merlob P, Stahl B, et al. Outcome of infants exposed to olanzapine during breastfeeding. Breastfeed Med. 2011;6(2):55-58.
20. Klier CM, Mossaheb N, Saria A, et al. Pharmacokinetics and elimination of quetiapine, venlafaxine, and trazodone during pregnancy and postpartum. J Clin Psychopharmacol. 2007;27(6):720-722.
21. U.S. Food and Drug Administration. FDA datasheet: quetiapine. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20639se1-017,016_seroquel_lbl.pdf. Accessed April 11, 2013.
22. Coppola D, Russo LJ, Kwarta RF Jr, et al. Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes. Drug Saf. 2007;30(3):247-264.
23. Mackay FJ, Wilton LV, Pearce GL, et al. The safety of risperidone: a post-marketing study on 7,684 patients. Hum Psychopharmacol. 1998;13(6):413-418.
24. Risperdal [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2012.
25. U.S. Food and Drug Administration. FDA datasheet: risperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020272s056,020588s044,021346s033,021444s03lbl.pdf. Accessed April 11, 2013.
26. Katz E, Adashi EY. Hyperprolactinemic disorders. Clin Obstet Gynecol. 1990;33(3):622-639.
27. Davis JR. Prolactin and reproductive medicine. Curr Opin Obstet Gynecol. 2004;16(4):331-337.
28. Johnson KC, Laprairie JL, Brennan PA, et al. Prenatal antipsychotic exposure and neuromotor performance during infancy. Arch Gen Psychiatry. 2012;69(8):787-794. doi: 10.1001/archgenpsychiatry.2012.160.
29. U.S. Food and Drug Administration. FDA datasheet: ziprasidone. http://www.fda.gov/downloads/Advisory
Committees/CommitteesMeetingMaterials/Pediatric AdvisoryCommittee/UCM191883.pdf. Accessed March 15, 2013.
30. U.S. Food and Drug Administration. FDA datasheet: lurasidone. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200603Orig1s000PharmR.pdf. Accessed March 15, 2013.
31. U.S. Food and Drug Administration. FDA datasheet: asenapine. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022117s000_OtherR.pdf. Accessed March 15, 2013.
32. U.S. Food and Drug Administration. FDA datasheet: iloperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022192lbl.pdf. Accessed March 15, 2013.
33. U.S. Food and Drug Administration. FDA datasheet: paliperidone. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021999s018lbl.pdf. Accessed March 15, 2013.
34. Weggelaar NM, Keijer WJ, Janssen PK. A case report of risperidone distribution and excretion into human milk: how to give good advice if you have not enough data available. J Clin Psychopharmacol. 2011;31(1):129-131.