User login
How useful is random biopsy when no colposcopic lesions are seen?
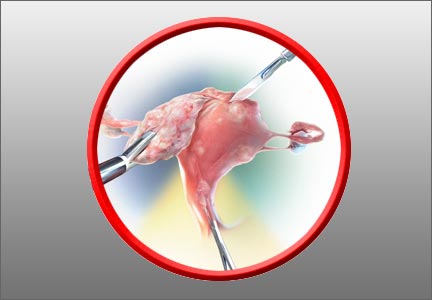
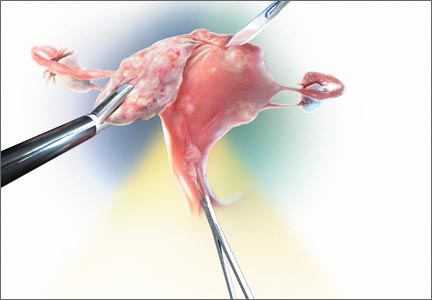
When performing colposcopy for abnormal cytology results or high-risk human papillomavirus (HPV), clinicians often are faced with an absence of visible lesions. This situation raises the following question in his or her mind: “Should I perform a random biopsy?”
Details of the study
In a multicenter US study of more than 47,000 women, performed to assess HPV diagnostics between May 2008 and August 2009, colposcopy was performed in nonpregnant women aged 25 or older with an intact uterus due to atypical squamous cells of undetermined significance or greater cytology results or high-risk HPV. The study participants and the colposcopists were blinded to the test results. In women with satisfactory colposcopy results but in whom no colposcopic lesions were noted, one random biopsy of the squamocolumnar junction was performed.
Among 2,796 women (mean age, 39.5 years) with a random biopsy performed, the findings were: normal, cervical intraepithelial neoplasia (CIN)−1, CIN2, and CIN3 in 90.0%, 5.7%, 1.3%, and 1.4%, respectively. Among all participants aged 25 and older, random biopsies accounted for 20.9% and 18.9% of the CIN2 or worse and CIN3 or worse cases, respectively. Among women positive for HPV 16 or 18, the likelihood of the random biopsy detecting CIN2 or worse was 24.7% and 8.6% for those with abnormal cytology or normal cytology, respectively.
What this evidence means for your practice
Consistent with other reports, the results of this post hoc analysis underscore the limitations of colposcopy. Just as results of a prior study indicated that taking two or more biopsies increases diagnostic yield,1 this large study points out the substantial benefit gained from performing a random biopsy of the squamocolumnar junction when no colposcopic lesions are identified.
—Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Gage JC, Hanson VW, Abbey K, et al; SCUS LSIL Triage Study (ALTS) Group. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108(2):264–272.
When performing colposcopy for abnormal cytology results or high-risk human papillomavirus (HPV), clinicians often are faced with an absence of visible lesions. This situation raises the following question in his or her mind: “Should I perform a random biopsy?”
Details of the study
In a multicenter US study of more than 47,000 women, performed to assess HPV diagnostics between May 2008 and August 2009, colposcopy was performed in nonpregnant women aged 25 or older with an intact uterus due to atypical squamous cells of undetermined significance or greater cytology results or high-risk HPV. The study participants and the colposcopists were blinded to the test results. In women with satisfactory colposcopy results but in whom no colposcopic lesions were noted, one random biopsy of the squamocolumnar junction was performed.
Among 2,796 women (mean age, 39.5 years) with a random biopsy performed, the findings were: normal, cervical intraepithelial neoplasia (CIN)−1, CIN2, and CIN3 in 90.0%, 5.7%, 1.3%, and 1.4%, respectively. Among all participants aged 25 and older, random biopsies accounted for 20.9% and 18.9% of the CIN2 or worse and CIN3 or worse cases, respectively. Among women positive for HPV 16 or 18, the likelihood of the random biopsy detecting CIN2 or worse was 24.7% and 8.6% for those with abnormal cytology or normal cytology, respectively.
What this evidence means for your practice
Consistent with other reports, the results of this post hoc analysis underscore the limitations of colposcopy. Just as results of a prior study indicated that taking two or more biopsies increases diagnostic yield,1 this large study points out the substantial benefit gained from performing a random biopsy of the squamocolumnar junction when no colposcopic lesions are identified.
—Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
When performing colposcopy for abnormal cytology results or high-risk human papillomavirus (HPV), clinicians often are faced with an absence of visible lesions. This situation raises the following question in his or her mind: “Should I perform a random biopsy?”
Details of the study
In a multicenter US study of more than 47,000 women, performed to assess HPV diagnostics between May 2008 and August 2009, colposcopy was performed in nonpregnant women aged 25 or older with an intact uterus due to atypical squamous cells of undetermined significance or greater cytology results or high-risk HPV. The study participants and the colposcopists were blinded to the test results. In women with satisfactory colposcopy results but in whom no colposcopic lesions were noted, one random biopsy of the squamocolumnar junction was performed.
Among 2,796 women (mean age, 39.5 years) with a random biopsy performed, the findings were: normal, cervical intraepithelial neoplasia (CIN)−1, CIN2, and CIN3 in 90.0%, 5.7%, 1.3%, and 1.4%, respectively. Among all participants aged 25 and older, random biopsies accounted for 20.9% and 18.9% of the CIN2 or worse and CIN3 or worse cases, respectively. Among women positive for HPV 16 or 18, the likelihood of the random biopsy detecting CIN2 or worse was 24.7% and 8.6% for those with abnormal cytology or normal cytology, respectively.
What this evidence means for your practice
Consistent with other reports, the results of this post hoc analysis underscore the limitations of colposcopy. Just as results of a prior study indicated that taking two or more biopsies increases diagnostic yield,1 this large study points out the substantial benefit gained from performing a random biopsy of the squamocolumnar junction when no colposcopic lesions are identified.
—Andrew M. Kaunitz, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Gage JC, Hanson VW, Abbey K, et al; SCUS LSIL Triage Study (ALTS) Group. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108(2):264–272.
Reference
- Gage JC, Hanson VW, Abbey K, et al; SCUS LSIL Triage Study (ALTS) Group. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108(2):264–272.
VIDEO: Emory’s medical director offers advice on managing an Ebola patient
PHILADELPHIA – How would your hospital handle an Ebola patient? Ask Dr. Bruce Ribner.
At ID Week 2014 in Philadelphia, Dr. Ribner, medical director of Emory University Hospital’s serious communicable diseases unit in Atlanta, gave a detailed account of his hospital’s management of the first two American patients treated at Emory after contracting the disease while working in Africa.
PHILADELPHIA – How would your hospital handle an Ebola patient? Ask Dr. Bruce Ribner.
At ID Week 2014 in Philadelphia, Dr. Ribner, medical director of Emory University Hospital’s serious communicable diseases unit in Atlanta, gave a detailed account of his hospital’s management of the first two American patients treated at Emory after contracting the disease while working in Africa.
PHILADELPHIA – How would your hospital handle an Ebola patient? Ask Dr. Bruce Ribner.
At ID Week 2014 in Philadelphia, Dr. Ribner, medical director of Emory University Hospital’s serious communicable diseases unit in Atlanta, gave a detailed account of his hospital’s management of the first two American patients treated at Emory after contracting the disease while working in Africa.
AT ID WEEK 2014
Global Ebola—Are We Prepared?
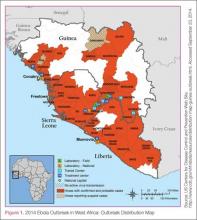
In this age of globalization, just a few hours of air travel separates even the most remote places in our world. Given this reality, the recent epidemic of Ebola virus disease (EVD) in West Africa (Figure 1) has arrived on the doorstep of Texas Health Presbyterian Hospital in Dallas. Ill and potentially infected US healthcare workers and missionaries brought home for treatment and quarantine, plus the travel of the general population through the affected countries of Sierra Leone, Liberia, Guinea, and Nigeria, require all acute-care providers to be cognizant that this deadly disease may present in any community ED in the United States. Awareness and knowledge of the appropriate steps to manage care safely and effectively, while mindfully preventing the potential for viral transmission, is paramount.
Hemorrhagic Fevers
Viral hemorrhagic fevers are manifestations of four distinct families of RNA viruses: arenaviruses, bunyaviruses, flaviviruses, and filoviruses. All of these families of viruses depend on a natural insect or animal (nonhuman) host and are thus restricted geographically to the regions where the endemic hosts reside. The viruses can only infect a human when one comes into direct contact with an infected host; this human becomes an infectious host when symptoms of disease develop and subsequently, the possibility of transmission to other close direct human contacts exists.
Ebola Virus Species
The family within which the Ebola virus species are classified is the filoviruses. Five species of Ebola filovirus have been isolated to date: Ebola virus (Zaire ebolavirus), Sudan virus (Sudan ebolavirus), Taï Forest virus (Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus), and Bundibugyo virus (Bundibugyo ebolavirus). The fifth, Reston virus (Reston ebolavirus), has caused disease in nonhuman primates, but not in humans. This epidemic has been attributed to a variant of the Zaire species.2 Transmission through direct contact with body fluids of febrile live infected patients and the postmortem period continues in communities and healthcare sites, as lack of adequate personal protective equipment (PPE) and meticulous environmental hygiene remains a challenge in many of these settings.
Clinical Presentation
In EVD, the onset of symptoms typically occurs abruptly at an average of 8 to 10 days postexposure and includes fever, headache, myalgia, and malaise; in some patients, an erythematous maculopapular rash involving the face, neck, trunk, and arms erupts by days 5 to 7.1 The nonspecific nature of these early signs and symptoms warrants caution in any patient known to have traveled in an endemic country with potential exposure to body fluids of infected patients—underscoring the importance of both obtaining a complete travel history to determine the potential for disease exposure in patients presenting with infectious-disease symptoms and effectively communicating this information to all ED staff.
In addition, such caution includes healthcare mission workers caring for Ebola patients, those involved in butchering infected animals for meat, and persons participating in traditional funeral rituals for those deceased from Ebola without the use of adequate PPE and/or environmental hygiene. Other more common infectious diseases with shared features of EVD must also be considered at this stage and include malaria, meningococcemia, measles, and typhoid fever, among others.
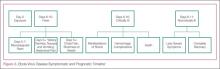
After the first 5 days of exposure, progression of symptoms may include severe watery diarrhea, nausea and vomiting, abdominal pain, shortness of breath, chest pain, headache, and/or confusion. Conjunctival injection may also develop. Not all patients will have signs of hemorrhagic fever with bleeding from the mouth, eyes, ears, in stool, or from internal organs, but petechiae, ecchymosis, and oozing from venipuncture sites may develop. Those at the highest risk of death show signs of sepsis, such as shock and multiorgan system failure, which may include hemorrhagic manifestations, early in the course of their illness. Patients with these complications typically expire between days 6 to16.1 Survivors of the disease tend to have fever with less severe symptoms for a period of several more days, and then begin to improve clinically between days 6 to 11 after onset of symptoms1 (Figure 3).
Ecology
A zoonotic filovirus transmissible from animal populations to humans causes EVD. Research strongly suggests that fruit bats are the reservoir and hosts for this filovirus. Direct human contact with bats or with wild animals that have been infected by bats initiates the human-to-human transmission of EVD.3
Pathogenesis
Through direct contact with mucous membranes, a break in the skin, or parenterally, Ebola enters and infects multiple cell types. Incubation periods appear shorter in infections acquired through direct injection (6 days) than for contact transmission (10 days).1 Emesis, urine, stool, sweat, semen, cerebrospinal fluid, breast milk, and saliva are actively capable of viral transmission. From point of entry, the virus migrates to the lymph nodes, then to liver, spleen, and adrenal glands. Hepatocellular necrosis leads to clotting-factor derangement and dysfunction resulting in coagulopathy and bleeding and potential liver failure. Necrosis of adrenal tissue may be present and results in impaired steroid synthesis and hypotension. The presence of the virus appears to incite a cytokine inflammatory storm causing microvascular leakage, with the end effect of multiorgan system failure, shock, coagulopathy, and lymphocytopenia from cellular apoptosis.1 With cellular death, immune system function is further disabled, more viral particles are released into the infected host, and body fluids remain infectious postmortem.
Laboratory Findings
Laboratory findings in viral hemorrhagic fevers can vary depending on the exact viral cause and the stage in the disease process. Leukocyte counts in early stages can reveal leukopenia and specifically lymphopenia, while in later stages leukocytosis with a left shift of neutrophils can predominate. Hemoglobin and hematocrit can show relative hemoconcentration, especially if renal manifestations of the disease occur. Thrombocytopenia also develops with viral hemorrhagic fevers, although in late stages thrombocytosis has been seen. Blood urea nitrogen (BUN) and creatinine (Cr) levels will rise with the occurrence of acute renal failure in late stages of the disease. Liver function studies, aspartate aminotransferase (AST) in particular, and alanine aminotransferase (ALT) have been found to rise in severe disease and in late stages due to multifocal hepatic necrosis, with AST typically greater than ALT. An association between elevated AST (~900 IU/L), BUN, Cr, albumin levels, and mortality has been statistically confirmed by McElroy et al4 in the Ebola outbreak in Uganda in 2000 to 2001; findings previously confirmed in the same geographic and temporal outbreak by Rollin et al.5 Survivors did not have nearly the same degree of elevation in liver enzymes, with AST levels averaging ~150 IU/L.5 The authors suggested that normalizing AST levels was perhaps indicative of acute recovery, but some patients still succumbed to complications of the illness.5
Coagulation studies, including prothrombin time, partial thromboplastin time, d-dimer, and fibrin split products will reflect disseminated intravascular coagulation (DIC) in those patients who develop hemorrhagic manifestations, which is common in late stages of the disease. Direct infection of vascular endothelial cells with damage to these cells has been shown to occur in the course of infection, yet nonhuman primate experimental studies and pathology examinations of Ebola victims have implied that DIC plays an important part in the hemorrhagic disease leading to the fatal shock syndrome seen in the most severe cases.6 Observed in 2000 during the care of Sudan species EVD patients in Uganda reported by Rollin et al,5 a distinct difference has been noted in quantitative d-dimer levels between survivors and fatal cases. Case fatalities showed a 4-fold increase in quantitative d-dimer levels (140,000 ng/mL) compared with survivors (44,000 ng/mL) during the acute phase of infection 6 to 8 days postsymptom onset.5
Acute phase reactants, high nitric oxide levels, cytokines, and higher viral loads have also been associated with fatal outcomes.4 McElroy et al4 measured the common acute phase reactant biomarker ferritin in patients of the 2000 Uganda outbreak and found levels to be higher in samples from patients who died and from patients with hemorrhagic complications and higher viral loads. Thus, these authors postulate ferritin is a potential marker for EVD severity.4
Commercially available assays for detection of viral particles are still in development and no point-of-care rapid detection testing is available. No test can reliably be used to diagnose viral hemorrhagic fever prior to symptom onset.7 Enzyme-linked immunosorbent assay and reverse transcriptase-polymerase chain reaction (RT-PCR) of viral particles or tissue cell cultures are available only through the CDC, and are the current most reliable methods to confirm the diagnosis of viral hemorrhagic fevers, including Ebola.
Patient Management in the ED
Standard infection-control procedures in place in US hospitals, when meticulously practiced, should be adequate to prevent transmission of EVD. As Ebola virus is only transmitted through direct contact with infected body fluids and secretions, PPE including mask, gloves, gown, shoe covers, and eye protection (goggles or face shield), should be appropriate. In donning PPE, remember that gloves should be the last item to pull in place and to pull off, turning the gown and gloves inside out. One’s hands should be washed before removing the mask and face shield/goggles, and they should be rewashed after completion of PPE removal. A tight fit of the glove over the elastic wristband of the gown is preferable and can ensure a better barrier to any biohazard. Meticulous hand hygiene after removal and proper disposal of PPE is paramount to successful contact protection. Diligent care should be taken in any procedure that might expose a healthcare provider to body fluids, such as blood draws, central line insertion, lumbar puncture and other invasive procedures. Standard contact isolation methods with the patient in a single room with the door closed is sufficient. Appropriate use of standard hospital disinfectants, including bleach solutions or hospital grade ammonium cleaners are already standard practice and easily implemented. It is recommended that procedures that produce aerosol particles should be avoided in patients with suspected infection; yet in some circumstances, the course of care may require such procedures. In this case, to minimize potential airborne spread, airborne and droplet precautions should also be initiated by placing the patient in a negative pressure room and implementing the use of properly fitted N-95 respirators for all present in the room.8
The mainstay of treatment for viral hemorrhagic fevers in the ED begins with initiation of contact isolation of the patient to prevent spread to the healthy. Supportive care involving aggressive intravenous fluid resuscitation to maintain blood pressure, oxygen support as needed, blood products as indicated for DIC, correction of electrolyte abnormalities, including dialysis for renal failure, is the only treatment widely available at this time. In patients who exhibit generalized edema as a result of hypoproteinemia from liver damage and third spacing, serum protein monitoring and replacement is indicated.9
An experimental treatment, ZMapp, which is a monoclonal antibody that is derived from mice and blocks Ebola from entering cells, has recently been used in combination with supportive treatment in six individuals infected with Ebola. However, ZMapp is still in early stages of development and testing is not widely available. No clinical trials have begun, but are planned. Two individuals treated with ZMapp have recovered in the United States; three healthcare workers are recovering in West Africa10,11; and one patient has died. It is not clear whether this medication is responsible wholly or in part for their recovery.
According to Dr Bruce Ribner, Director of Emory University Hospital’s Infectious Disease unit in an interview with Scientific American’s Dina Fine Maron on August 27, 2014, the two patients cared for at Emory have developed immunity to Zaire Ebolavirus.11 Continued outpatient monitoring is allowing study to help understand immunity to Ebola, and may lead to further treatment and vaccination development. Thus far, cross-protection against other Ebola viral strains for these recovered individuals is not as robust, indicating that this family of viruses are different enough that recovery from infection with one species may not be enough to confer immunity to a different species exposure. Blood transfusions from recovered patients have been described, but there is no clinical evidence to support any benefit from this therapy at this time.9
Personal protective equipment is a mainstay in the collection of specimens for viral-specific testing by the CDC, including full-face shield or goggles, masks to cover nose and mouth, gowns, and gloves. For routine laboratory testing and patient care, all of the above PPE is recommended, along with use of a biosafety cabinet or plexiglass splash guard, which is in accordance with Occupational Safety and Health Administration bloodborne pathogens standards.12
Specimens should be collected for Ebola testing only after the onset of symptoms, such as fever (see Table for supplemental information). In patients suspected of having viral exposure, it may take up to 3 days for the virus to reach detectable levels with RT-PCR. Consultation per hospital procedure with the local and/or state health department prior to specimen transport for testing to the CDC is mandatory. Public health officials will help ensure appropriate patient selection and proper procedures for specimen collection, and make arrangements for testing, which is only available through the CDC. The CDC will not accept any specimen without appropriate local/state health department consultation.
Ideally, preferred specimens for Ebola testing include 4 mL of whole blood properly preserved with EDTA, clot activator, sodium polyanethol sulfonate, or citrate in plastic collection tubes stored at 4˚C or frozen.12 Specimens should be placed in a durable, leak-proof container for transport within a facility; pneumatic tube systems should be avoided due to concerns for glass breakage or leaks. Hospitals should follow state or local health department policies and procedures for Ebola testing prior to notifying the CDC and initiating sample transportation.
The Dallas Index Case
Deplaning a flight from Liberia in Dallas, Texas on September 20, this index patient had no outward signs of illness, and thus no reason to cause any health concern. Joining in the community with friends and family, it was not until 4 days later that he reportedly developed a fever. Yet 2 more days passed before this patient initially sought ambulatory care at Dallas Health Presbyterian Hospital Emergency Department, during which time additional close contacts were exposed to infection. After evaluation, antibiotics were prescribed and the patient was released. Though this case is under investigation, according to a CNN report,13 the patient did inform a member of the ED nursing staff of his travel history, but this information was not communicated to the rest of the healthcare team. The presence and recognition of this patient’s travel history with disease symptoms heightens the level of suspicion for the possibility of EVD, and is the cornerstone of patient selection for Ebola testing.14
After the patient was discharged, another 2 days passed, during which time his condition deteriorated at home. Emergency medical services (EMS) transport was summoned to take the patient back to the hospital, expanding exposure to first responders, who appropriately utilized masks and gloves during transport. During this ED visit, his travel history was obtained and communicated, and he was appropriately isolated, supported, and admitted. These are early reported details of the case, and local public health officials continue to work with a team from the CDC to trace, isolate, and monitor all contacts with this patient (including the transporting emergency medical technicians) for evidence of further cases.15
This index case illustrates valuable lessons for all emergency care workers going forward. Ebola virus disease is now global, in the sense that it has proven its ability to present in a community far from its endemic home. Infectious diseases in their early stages present in nonspecific constellations of symptoms, and the key to rapid identification of EVD lies in careful attention to the recent travel history and exposure potential. Since patients may not offer this information for various reasons (eg, degree of symptoms, language barriers, fear, denial), it must be sought out lest more index patients be released into the public. As CDC Director Thomas Frieden related to CNN, “If someone’s been in West Africa within 21 days and they’ve got a fever, immediately isolate them and get them tested for Ebola.”13
Concerning the EMS transport of this patient, the ambulance used was disinfected per standard local protocols and remained in service for 2 days after this patient was transported. Though local officials are confident in the disinfection technique, it was pulled from service after the diagnosis was confirmed to ensure its full sterilization from Ebola virus16 before returning to full service. The rapid and robust public health response in progress will undoubtedly reveal further information over the coming days to weeks. To quote Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, “We are going to see more cases show up around the world.”15
In addition to the index patient, it is important to keep in mind that secondary cases may present. If this occurs, the travel question must be altered to reflect the possibility that a secondary patient may not have been the traveler but rather one who was in close contact with an ill person who was in contact with him or her. Such a scenario would have a huge impact if that traveler did not seek treatment and would in turn require ED personnel to seek-out the information and report it to local public health officials.
Quelling the Panic
Even though Ebola is only spread through direct contact with infected bodily secretions, there is still significant public fear of the disease due to the high mortality rate and the graphic nature of symptoms in late stages of the disease. Daily monitoring of contacts of symptomatic Ebola patients for evidence of disease development—mainly fever—is sensible. Asymptomatic persons need not be confined or hospitalized unless fever develops, in which case contact isolation should occur until formal Ebola testing can rule-out the disease. Personal protective equipment, standardized hospital cleaning protocols with meticulous adherence, as well as quickly burying the deceased with adequate contact precautions, can all limit potential exposure and spread of the disease. Public health discussion and education about the virus and methods of transmission are needed so that individuals are not denied proper treatment or scared away from medical centers.
Importantly, communication by public health and medical experts on local and national levels should be with news media that embrace honest and careful reporting to avoid sensationalism and foster appropriate concern—ensuring that content is fundamental to curtailing panic and undue public fear.
For Internationally Traveling Clinicians
At present, the area endemic for Ebola remains confined to sub-Saharan Africa and West Africa. Clinicians should remain alert when traveling or treating patients in these areas. However, with the ease of international air travel, the potential for the spread of disease is recognized with many bordering nations now screening passengers from affected countries and some closing their borders to travelers from endemic areas. If a clinician encounters febrile patients in endemic areas, the differential diagnosis for any febrile illness must include Ebola, as well as malaria and other more common infectious agents. A thorough history about recent travel, ill contacts, and possible exposures should be sufficient in categorizing the risk of Ebola, but a high index of suspicion is necessary for prompt and proper treatment of those affected and to curtail spread of disease.
Despite the efforts of the national and local health systems and many nongovernmental organizations, including the World Health Organization, this epidemic continues to hold strong in the affected West African countries. Methods of containment of the virus are seemingly simple by modern standards, yet tragically beyond access for many on the ground. Lack of clean water sources in affected communities is a significant barrier to basic personal and environmental hygiene. Inadequate safe food sources and poaching encourages the hunt for primate bushmeat and thus presents a formidable local challenge.17 Lack of adequate PPE for healthcare workers, for those responsible for facility environmental hygiene, and for family members participating in traditional funeral rites for Ebola victims compounds the problem. Illness and deaths among exposed healthcare workers have led to the loss of significant numbers of nurses and doctors. This has caused legitimate fear in qualified individuals who subsequently decline to accept jobs caring for Ebola patients, which in turn increases the burden on those who remain. Additionally, some nongovernmental organizations have canceled scheduled aid trips to West Africa in response to the epidemic out of concern for the health of their workers. Meticulous management of environmental hygiene including sharps, surfaces, soiled linens, reusable medical equipment, waste products, and the preparations for burial of the deceased pose definite challenges to containment and prevention of transmission. Strict adherence to the use of PPE and hand hygiene is essential for all in contact with Ebola patients, pre- and postmortem. The lack of layperson comprehension and community understanding of the illness itself and the mechanism of viral transmission along with fear and mistrust for healthcare providers and nongovernmental medical missionaries are all serious barriers to the containment of disease spread. In fact, rumors that the virus does not truly exist, and that the illness is a result of biological warfare, cannibalistic rituals, or witchcraft add to the complexity of the situation.11
For these reasons, it is essential that efforts to control this epidemic in endemic healthcare facilities include effective health surveillance, infection-control programs, and community outreach fostering mutual trust-building, honest communication, and education. Success will require a multifaceted approach, and a global response will be needed to quiet this global threat. On September 16, the United States announced a robust response to deploy military engineers and medical personnel to assist in training healthcare workers and building care centers in Liberia. The United Nations, France, and the United Kingdom are also supporting this important effort to build stronger healthcare infrastructures in these vulnerable countries.16
Conclusion
This 2014 epidemic of EVD raises justifiable concerns regarding the impact of globalization. Though unlikely to pose a direct threat of epidemic proportion on US soil, the unanticipated occurrence of an index case may trigger a terrifying outbreak in any community, as it already has in the city of Dallas. Given that the early stages of EVD are indistinguishable from most other viral syndromes, the importance of reflection on individual and general healthcare facility adherence to standard infection control precautions and procedures warrant merit. Eliciting accurate travel histories and possible exposures are germane to narrowing the scope of possible etiologies of all infectious diseases. As an opportunity for improvement, this epidemic should incite elevated caution in the everyday handling of all patients with febrile illnesses and contact-transmissible infections including methicillin-resistant Staphylococcus aureus and Clostridium difficile, which affect a great number of US patients on a daily basis. Not only could this strategy prevent additional local outbreaks of EVD, but it would promote the safety of healthcare workers and the community served through attention to better infection preventive measures at the point of care, every time.
Dr McCammon is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Chidester is an instructor, department of emergency medicine, and a fellow in international wilderness medicine, Eastern Virginia Medical School, Norfolk.
- Ebola virus disease information for clinicians in U.S. Healthcare Settings. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/clinician-information-us-healthcare-settings.html. Updated September 5, 2014. Accessed September 23, 2014.
- 2014 Ebola outbreak in West Africa. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/outbreaks/guinea/. Updated September 18, 2014. Accessed September 23, 2014.
- Virus ecology graphic. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/resources/virus-ecology.html. Updated August 1, 2014. Accessed September 23, 2014.
- McElroy AK, Erickson BR, Flietstra TD, et al. Ebola hemorrhagic fever: novel biomarker correlates of clinical outcome. J Infect Dis. 2014;210(4):558-566.
- Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S364-S371.
- Geisbert TW, Young HA, Jahrling PB, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163(6): 2371-2382.
- Blumberg L, Enria D, Bausch DG. Viral haemorrhagic fevers. In: Farrar J, Hotez, PJ, Junghanss T, Kang G, Lalloo D, White N, eds. Manson’s Tropical Diseases: Expert Consult. 23rd ed. Philadelphia, PA: Elsevier Saunders; 2014:171-194.
- Safe management of patients with Ebola virus disease (EVD) in U.S. hospitals. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/patient-management-us-hospitals.html. Updated September 5, 2014. Accessed September 23, 2014.
- Maron DF. Ebola doctor reveals how infected Americans were cured. Scientific American. http://www.scientificamerican.com/article/ebola-doctor-revealshow-infected-americans-were-cured/.
- Tribune wire reports. American Ebola patients treated with ZMapp experimental drug. Chicago Tribune. August 21, 2014. http://www.chicagotribune.com/lifestyles/health/chi-ebola-zmapp-20140821-story.html. Accessed September 23, 2014.
- Ebola crisis: doctors in Liberia ‘recovering after taking ZMapp’ [transcript]. BBC News. http://www.bbc.com/news/world-africa-28860204. August 19, 2014. Accessed September 23, 2014.
- Interim guidance for specimen collection, transport, testing, and submission for persons under investigation for Ebola virus disease in the United States. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimen-collection-submission-patients-suspected-infection-ebola.html. Updated September 8, 2014. Accessed September 23, 2014.
- Catherine E. Shoichet CE, Fantz A, Yan H. Hospital ‘dropped the ball’ with Ebola patient’s travel history, NIH official says. CNN News. http://www.cnn.com/2014/10/01/health/ebola-us/index.html. Accessed October 2, 2014.
- Ebola (Ebola Virus Disease): Diagnosis. Centers for Disease Control and Prevention Web Site. http://www.cdc.gov/vhf/ebola/diagnosis/. Updated September 19, 2014. Accessed October 1, 2014.
- Gilblom K, Langreth R. Dallas hospital initially let Ebola patient go with drugs. Bloomberg News Web Site. http://www.bloomberg.com/news/2014-09-30/first-ebola-case-is-diagnosed-in-the-u-s-cdc-reports.html. Accessed October 1, 2014.
- Bates D, Szathmary Z and Boyle L. Up to twelve Americans could have Ebola: Fears grow in Dallas after first victim of deadly virus to reach U.S. remained at large for a week. September 30, 2014. Mail Online. http://www.dailymail.co.uk/news/article-2775608/CDC-confirms-Dallas-patient-isolation-testing-returning-region-plagued-Ebola-HAS-deadly-virus.html. Updated October 1, 2014. Accessed October 1, 2014.
- International affairs: bushmeat. U.S. Fish & Wildlife Service Web site. http://www.fws.gov/international/wildlife-without-borders/global-program/bushmeat.html. Accessed September 23, 2014.
In this age of globalization, just a few hours of air travel separates even the most remote places in our world. Given this reality, the recent epidemic of Ebola virus disease (EVD) in West Africa (Figure 1) has arrived on the doorstep of Texas Health Presbyterian Hospital in Dallas. Ill and potentially infected US healthcare workers and missionaries brought home for treatment and quarantine, plus the travel of the general population through the affected countries of Sierra Leone, Liberia, Guinea, and Nigeria, require all acute-care providers to be cognizant that this deadly disease may present in any community ED in the United States. Awareness and knowledge of the appropriate steps to manage care safely and effectively, while mindfully preventing the potential for viral transmission, is paramount.
Hemorrhagic Fevers
Viral hemorrhagic fevers are manifestations of four distinct families of RNA viruses: arenaviruses, bunyaviruses, flaviviruses, and filoviruses. All of these families of viruses depend on a natural insect or animal (nonhuman) host and are thus restricted geographically to the regions where the endemic hosts reside. The viruses can only infect a human when one comes into direct contact with an infected host; this human becomes an infectious host when symptoms of disease develop and subsequently, the possibility of transmission to other close direct human contacts exists.
Ebola Virus Species
The family within which the Ebola virus species are classified is the filoviruses. Five species of Ebola filovirus have been isolated to date: Ebola virus (Zaire ebolavirus), Sudan virus (Sudan ebolavirus), Taï Forest virus (Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus), and Bundibugyo virus (Bundibugyo ebolavirus). The fifth, Reston virus (Reston ebolavirus), has caused disease in nonhuman primates, but not in humans. This epidemic has been attributed to a variant of the Zaire species.2 Transmission through direct contact with body fluids of febrile live infected patients and the postmortem period continues in communities and healthcare sites, as lack of adequate personal protective equipment (PPE) and meticulous environmental hygiene remains a challenge in many of these settings.
Clinical Presentation
In EVD, the onset of symptoms typically occurs abruptly at an average of 8 to 10 days postexposure and includes fever, headache, myalgia, and malaise; in some patients, an erythematous maculopapular rash involving the face, neck, trunk, and arms erupts by days 5 to 7.1 The nonspecific nature of these early signs and symptoms warrants caution in any patient known to have traveled in an endemic country with potential exposure to body fluids of infected patients—underscoring the importance of both obtaining a complete travel history to determine the potential for disease exposure in patients presenting with infectious-disease symptoms and effectively communicating this information to all ED staff.
In addition, such caution includes healthcare mission workers caring for Ebola patients, those involved in butchering infected animals for meat, and persons participating in traditional funeral rituals for those deceased from Ebola without the use of adequate PPE and/or environmental hygiene. Other more common infectious diseases with shared features of EVD must also be considered at this stage and include malaria, meningococcemia, measles, and typhoid fever, among others.
After the first 5 days of exposure, progression of symptoms may include severe watery diarrhea, nausea and vomiting, abdominal pain, shortness of breath, chest pain, headache, and/or confusion. Conjunctival injection may also develop. Not all patients will have signs of hemorrhagic fever with bleeding from the mouth, eyes, ears, in stool, or from internal organs, but petechiae, ecchymosis, and oozing from venipuncture sites may develop. Those at the highest risk of death show signs of sepsis, such as shock and multiorgan system failure, which may include hemorrhagic manifestations, early in the course of their illness. Patients with these complications typically expire between days 6 to16.1 Survivors of the disease tend to have fever with less severe symptoms for a period of several more days, and then begin to improve clinically between days 6 to 11 after onset of symptoms1 (Figure 3).
Ecology
A zoonotic filovirus transmissible from animal populations to humans causes EVD. Research strongly suggests that fruit bats are the reservoir and hosts for this filovirus. Direct human contact with bats or with wild animals that have been infected by bats initiates the human-to-human transmission of EVD.3
Pathogenesis
Through direct contact with mucous membranes, a break in the skin, or parenterally, Ebola enters and infects multiple cell types. Incubation periods appear shorter in infections acquired through direct injection (6 days) than for contact transmission (10 days).1 Emesis, urine, stool, sweat, semen, cerebrospinal fluid, breast milk, and saliva are actively capable of viral transmission. From point of entry, the virus migrates to the lymph nodes, then to liver, spleen, and adrenal glands. Hepatocellular necrosis leads to clotting-factor derangement and dysfunction resulting in coagulopathy and bleeding and potential liver failure. Necrosis of adrenal tissue may be present and results in impaired steroid synthesis and hypotension. The presence of the virus appears to incite a cytokine inflammatory storm causing microvascular leakage, with the end effect of multiorgan system failure, shock, coagulopathy, and lymphocytopenia from cellular apoptosis.1 With cellular death, immune system function is further disabled, more viral particles are released into the infected host, and body fluids remain infectious postmortem.
Laboratory Findings
Laboratory findings in viral hemorrhagic fevers can vary depending on the exact viral cause and the stage in the disease process. Leukocyte counts in early stages can reveal leukopenia and specifically lymphopenia, while in later stages leukocytosis with a left shift of neutrophils can predominate. Hemoglobin and hematocrit can show relative hemoconcentration, especially if renal manifestations of the disease occur. Thrombocytopenia also develops with viral hemorrhagic fevers, although in late stages thrombocytosis has been seen. Blood urea nitrogen (BUN) and creatinine (Cr) levels will rise with the occurrence of acute renal failure in late stages of the disease. Liver function studies, aspartate aminotransferase (AST) in particular, and alanine aminotransferase (ALT) have been found to rise in severe disease and in late stages due to multifocal hepatic necrosis, with AST typically greater than ALT. An association between elevated AST (~900 IU/L), BUN, Cr, albumin levels, and mortality has been statistically confirmed by McElroy et al4 in the Ebola outbreak in Uganda in 2000 to 2001; findings previously confirmed in the same geographic and temporal outbreak by Rollin et al.5 Survivors did not have nearly the same degree of elevation in liver enzymes, with AST levels averaging ~150 IU/L.5 The authors suggested that normalizing AST levels was perhaps indicative of acute recovery, but some patients still succumbed to complications of the illness.5
Coagulation studies, including prothrombin time, partial thromboplastin time, d-dimer, and fibrin split products will reflect disseminated intravascular coagulation (DIC) in those patients who develop hemorrhagic manifestations, which is common in late stages of the disease. Direct infection of vascular endothelial cells with damage to these cells has been shown to occur in the course of infection, yet nonhuman primate experimental studies and pathology examinations of Ebola victims have implied that DIC plays an important part in the hemorrhagic disease leading to the fatal shock syndrome seen in the most severe cases.6 Observed in 2000 during the care of Sudan species EVD patients in Uganda reported by Rollin et al,5 a distinct difference has been noted in quantitative d-dimer levels between survivors and fatal cases. Case fatalities showed a 4-fold increase in quantitative d-dimer levels (140,000 ng/mL) compared with survivors (44,000 ng/mL) during the acute phase of infection 6 to 8 days postsymptom onset.5
Acute phase reactants, high nitric oxide levels, cytokines, and higher viral loads have also been associated with fatal outcomes.4 McElroy et al4 measured the common acute phase reactant biomarker ferritin in patients of the 2000 Uganda outbreak and found levels to be higher in samples from patients who died and from patients with hemorrhagic complications and higher viral loads. Thus, these authors postulate ferritin is a potential marker for EVD severity.4
Commercially available assays for detection of viral particles are still in development and no point-of-care rapid detection testing is available. No test can reliably be used to diagnose viral hemorrhagic fever prior to symptom onset.7 Enzyme-linked immunosorbent assay and reverse transcriptase-polymerase chain reaction (RT-PCR) of viral particles or tissue cell cultures are available only through the CDC, and are the current most reliable methods to confirm the diagnosis of viral hemorrhagic fevers, including Ebola.
Patient Management in the ED
Standard infection-control procedures in place in US hospitals, when meticulously practiced, should be adequate to prevent transmission of EVD. As Ebola virus is only transmitted through direct contact with infected body fluids and secretions, PPE including mask, gloves, gown, shoe covers, and eye protection (goggles or face shield), should be appropriate. In donning PPE, remember that gloves should be the last item to pull in place and to pull off, turning the gown and gloves inside out. One’s hands should be washed before removing the mask and face shield/goggles, and they should be rewashed after completion of PPE removal. A tight fit of the glove over the elastic wristband of the gown is preferable and can ensure a better barrier to any biohazard. Meticulous hand hygiene after removal and proper disposal of PPE is paramount to successful contact protection. Diligent care should be taken in any procedure that might expose a healthcare provider to body fluids, such as blood draws, central line insertion, lumbar puncture and other invasive procedures. Standard contact isolation methods with the patient in a single room with the door closed is sufficient. Appropriate use of standard hospital disinfectants, including bleach solutions or hospital grade ammonium cleaners are already standard practice and easily implemented. It is recommended that procedures that produce aerosol particles should be avoided in patients with suspected infection; yet in some circumstances, the course of care may require such procedures. In this case, to minimize potential airborne spread, airborne and droplet precautions should also be initiated by placing the patient in a negative pressure room and implementing the use of properly fitted N-95 respirators for all present in the room.8
The mainstay of treatment for viral hemorrhagic fevers in the ED begins with initiation of contact isolation of the patient to prevent spread to the healthy. Supportive care involving aggressive intravenous fluid resuscitation to maintain blood pressure, oxygen support as needed, blood products as indicated for DIC, correction of electrolyte abnormalities, including dialysis for renal failure, is the only treatment widely available at this time. In patients who exhibit generalized edema as a result of hypoproteinemia from liver damage and third spacing, serum protein monitoring and replacement is indicated.9
An experimental treatment, ZMapp, which is a monoclonal antibody that is derived from mice and blocks Ebola from entering cells, has recently been used in combination with supportive treatment in six individuals infected with Ebola. However, ZMapp is still in early stages of development and testing is not widely available. No clinical trials have begun, but are planned. Two individuals treated with ZMapp have recovered in the United States; three healthcare workers are recovering in West Africa10,11; and one patient has died. It is not clear whether this medication is responsible wholly or in part for their recovery.
According to Dr Bruce Ribner, Director of Emory University Hospital’s Infectious Disease unit in an interview with Scientific American’s Dina Fine Maron on August 27, 2014, the two patients cared for at Emory have developed immunity to Zaire Ebolavirus.11 Continued outpatient monitoring is allowing study to help understand immunity to Ebola, and may lead to further treatment and vaccination development. Thus far, cross-protection against other Ebola viral strains for these recovered individuals is not as robust, indicating that this family of viruses are different enough that recovery from infection with one species may not be enough to confer immunity to a different species exposure. Blood transfusions from recovered patients have been described, but there is no clinical evidence to support any benefit from this therapy at this time.9
Personal protective equipment is a mainstay in the collection of specimens for viral-specific testing by the CDC, including full-face shield or goggles, masks to cover nose and mouth, gowns, and gloves. For routine laboratory testing and patient care, all of the above PPE is recommended, along with use of a biosafety cabinet or plexiglass splash guard, which is in accordance with Occupational Safety and Health Administration bloodborne pathogens standards.12
Specimens should be collected for Ebola testing only after the onset of symptoms, such as fever (see Table for supplemental information). In patients suspected of having viral exposure, it may take up to 3 days for the virus to reach detectable levels with RT-PCR. Consultation per hospital procedure with the local and/or state health department prior to specimen transport for testing to the CDC is mandatory. Public health officials will help ensure appropriate patient selection and proper procedures for specimen collection, and make arrangements for testing, which is only available through the CDC. The CDC will not accept any specimen without appropriate local/state health department consultation.
Ideally, preferred specimens for Ebola testing include 4 mL of whole blood properly preserved with EDTA, clot activator, sodium polyanethol sulfonate, or citrate in plastic collection tubes stored at 4˚C or frozen.12 Specimens should be placed in a durable, leak-proof container for transport within a facility; pneumatic tube systems should be avoided due to concerns for glass breakage or leaks. Hospitals should follow state or local health department policies and procedures for Ebola testing prior to notifying the CDC and initiating sample transportation.
The Dallas Index Case
Deplaning a flight from Liberia in Dallas, Texas on September 20, this index patient had no outward signs of illness, and thus no reason to cause any health concern. Joining in the community with friends and family, it was not until 4 days later that he reportedly developed a fever. Yet 2 more days passed before this patient initially sought ambulatory care at Dallas Health Presbyterian Hospital Emergency Department, during which time additional close contacts were exposed to infection. After evaluation, antibiotics were prescribed and the patient was released. Though this case is under investigation, according to a CNN report,13 the patient did inform a member of the ED nursing staff of his travel history, but this information was not communicated to the rest of the healthcare team. The presence and recognition of this patient’s travel history with disease symptoms heightens the level of suspicion for the possibility of EVD, and is the cornerstone of patient selection for Ebola testing.14
After the patient was discharged, another 2 days passed, during which time his condition deteriorated at home. Emergency medical services (EMS) transport was summoned to take the patient back to the hospital, expanding exposure to first responders, who appropriately utilized masks and gloves during transport. During this ED visit, his travel history was obtained and communicated, and he was appropriately isolated, supported, and admitted. These are early reported details of the case, and local public health officials continue to work with a team from the CDC to trace, isolate, and monitor all contacts with this patient (including the transporting emergency medical technicians) for evidence of further cases.15
This index case illustrates valuable lessons for all emergency care workers going forward. Ebola virus disease is now global, in the sense that it has proven its ability to present in a community far from its endemic home. Infectious diseases in their early stages present in nonspecific constellations of symptoms, and the key to rapid identification of EVD lies in careful attention to the recent travel history and exposure potential. Since patients may not offer this information for various reasons (eg, degree of symptoms, language barriers, fear, denial), it must be sought out lest more index patients be released into the public. As CDC Director Thomas Frieden related to CNN, “If someone’s been in West Africa within 21 days and they’ve got a fever, immediately isolate them and get them tested for Ebola.”13
Concerning the EMS transport of this patient, the ambulance used was disinfected per standard local protocols and remained in service for 2 days after this patient was transported. Though local officials are confident in the disinfection technique, it was pulled from service after the diagnosis was confirmed to ensure its full sterilization from Ebola virus16 before returning to full service. The rapid and robust public health response in progress will undoubtedly reveal further information over the coming days to weeks. To quote Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, “We are going to see more cases show up around the world.”15
In addition to the index patient, it is important to keep in mind that secondary cases may present. If this occurs, the travel question must be altered to reflect the possibility that a secondary patient may not have been the traveler but rather one who was in close contact with an ill person who was in contact with him or her. Such a scenario would have a huge impact if that traveler did not seek treatment and would in turn require ED personnel to seek-out the information and report it to local public health officials.
Quelling the Panic
Even though Ebola is only spread through direct contact with infected bodily secretions, there is still significant public fear of the disease due to the high mortality rate and the graphic nature of symptoms in late stages of the disease. Daily monitoring of contacts of symptomatic Ebola patients for evidence of disease development—mainly fever—is sensible. Asymptomatic persons need not be confined or hospitalized unless fever develops, in which case contact isolation should occur until formal Ebola testing can rule-out the disease. Personal protective equipment, standardized hospital cleaning protocols with meticulous adherence, as well as quickly burying the deceased with adequate contact precautions, can all limit potential exposure and spread of the disease. Public health discussion and education about the virus and methods of transmission are needed so that individuals are not denied proper treatment or scared away from medical centers.
Importantly, communication by public health and medical experts on local and national levels should be with news media that embrace honest and careful reporting to avoid sensationalism and foster appropriate concern—ensuring that content is fundamental to curtailing panic and undue public fear.
For Internationally Traveling Clinicians
At present, the area endemic for Ebola remains confined to sub-Saharan Africa and West Africa. Clinicians should remain alert when traveling or treating patients in these areas. However, with the ease of international air travel, the potential for the spread of disease is recognized with many bordering nations now screening passengers from affected countries and some closing their borders to travelers from endemic areas. If a clinician encounters febrile patients in endemic areas, the differential diagnosis for any febrile illness must include Ebola, as well as malaria and other more common infectious agents. A thorough history about recent travel, ill contacts, and possible exposures should be sufficient in categorizing the risk of Ebola, but a high index of suspicion is necessary for prompt and proper treatment of those affected and to curtail spread of disease.
Despite the efforts of the national and local health systems and many nongovernmental organizations, including the World Health Organization, this epidemic continues to hold strong in the affected West African countries. Methods of containment of the virus are seemingly simple by modern standards, yet tragically beyond access for many on the ground. Lack of clean water sources in affected communities is a significant barrier to basic personal and environmental hygiene. Inadequate safe food sources and poaching encourages the hunt for primate bushmeat and thus presents a formidable local challenge.17 Lack of adequate PPE for healthcare workers, for those responsible for facility environmental hygiene, and for family members participating in traditional funeral rites for Ebola victims compounds the problem. Illness and deaths among exposed healthcare workers have led to the loss of significant numbers of nurses and doctors. This has caused legitimate fear in qualified individuals who subsequently decline to accept jobs caring for Ebola patients, which in turn increases the burden on those who remain. Additionally, some nongovernmental organizations have canceled scheduled aid trips to West Africa in response to the epidemic out of concern for the health of their workers. Meticulous management of environmental hygiene including sharps, surfaces, soiled linens, reusable medical equipment, waste products, and the preparations for burial of the deceased pose definite challenges to containment and prevention of transmission. Strict adherence to the use of PPE and hand hygiene is essential for all in contact with Ebola patients, pre- and postmortem. The lack of layperson comprehension and community understanding of the illness itself and the mechanism of viral transmission along with fear and mistrust for healthcare providers and nongovernmental medical missionaries are all serious barriers to the containment of disease spread. In fact, rumors that the virus does not truly exist, and that the illness is a result of biological warfare, cannibalistic rituals, or witchcraft add to the complexity of the situation.11
For these reasons, it is essential that efforts to control this epidemic in endemic healthcare facilities include effective health surveillance, infection-control programs, and community outreach fostering mutual trust-building, honest communication, and education. Success will require a multifaceted approach, and a global response will be needed to quiet this global threat. On September 16, the United States announced a robust response to deploy military engineers and medical personnel to assist in training healthcare workers and building care centers in Liberia. The United Nations, France, and the United Kingdom are also supporting this important effort to build stronger healthcare infrastructures in these vulnerable countries.16
Conclusion
This 2014 epidemic of EVD raises justifiable concerns regarding the impact of globalization. Though unlikely to pose a direct threat of epidemic proportion on US soil, the unanticipated occurrence of an index case may trigger a terrifying outbreak in any community, as it already has in the city of Dallas. Given that the early stages of EVD are indistinguishable from most other viral syndromes, the importance of reflection on individual and general healthcare facility adherence to standard infection control precautions and procedures warrant merit. Eliciting accurate travel histories and possible exposures are germane to narrowing the scope of possible etiologies of all infectious diseases. As an opportunity for improvement, this epidemic should incite elevated caution in the everyday handling of all patients with febrile illnesses and contact-transmissible infections including methicillin-resistant Staphylococcus aureus and Clostridium difficile, which affect a great number of US patients on a daily basis. Not only could this strategy prevent additional local outbreaks of EVD, but it would promote the safety of healthcare workers and the community served through attention to better infection preventive measures at the point of care, every time.
Dr McCammon is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Chidester is an instructor, department of emergency medicine, and a fellow in international wilderness medicine, Eastern Virginia Medical School, Norfolk.
In this age of globalization, just a few hours of air travel separates even the most remote places in our world. Given this reality, the recent epidemic of Ebola virus disease (EVD) in West Africa (Figure 1) has arrived on the doorstep of Texas Health Presbyterian Hospital in Dallas. Ill and potentially infected US healthcare workers and missionaries brought home for treatment and quarantine, plus the travel of the general population through the affected countries of Sierra Leone, Liberia, Guinea, and Nigeria, require all acute-care providers to be cognizant that this deadly disease may present in any community ED in the United States. Awareness and knowledge of the appropriate steps to manage care safely and effectively, while mindfully preventing the potential for viral transmission, is paramount.
Hemorrhagic Fevers
Viral hemorrhagic fevers are manifestations of four distinct families of RNA viruses: arenaviruses, bunyaviruses, flaviviruses, and filoviruses. All of these families of viruses depend on a natural insect or animal (nonhuman) host and are thus restricted geographically to the regions where the endemic hosts reside. The viruses can only infect a human when one comes into direct contact with an infected host; this human becomes an infectious host when symptoms of disease develop and subsequently, the possibility of transmission to other close direct human contacts exists.
Ebola Virus Species
The family within which the Ebola virus species are classified is the filoviruses. Five species of Ebola filovirus have been isolated to date: Ebola virus (Zaire ebolavirus), Sudan virus (Sudan ebolavirus), Taï Forest virus (Taï Forest ebolavirus, formerly Côte d’Ivoire ebolavirus), and Bundibugyo virus (Bundibugyo ebolavirus). The fifth, Reston virus (Reston ebolavirus), has caused disease in nonhuman primates, but not in humans. This epidemic has been attributed to a variant of the Zaire species.2 Transmission through direct contact with body fluids of febrile live infected patients and the postmortem period continues in communities and healthcare sites, as lack of adequate personal protective equipment (PPE) and meticulous environmental hygiene remains a challenge in many of these settings.
Clinical Presentation
In EVD, the onset of symptoms typically occurs abruptly at an average of 8 to 10 days postexposure and includes fever, headache, myalgia, and malaise; in some patients, an erythematous maculopapular rash involving the face, neck, trunk, and arms erupts by days 5 to 7.1 The nonspecific nature of these early signs and symptoms warrants caution in any patient known to have traveled in an endemic country with potential exposure to body fluids of infected patients—underscoring the importance of both obtaining a complete travel history to determine the potential for disease exposure in patients presenting with infectious-disease symptoms and effectively communicating this information to all ED staff.
In addition, such caution includes healthcare mission workers caring for Ebola patients, those involved in butchering infected animals for meat, and persons participating in traditional funeral rituals for those deceased from Ebola without the use of adequate PPE and/or environmental hygiene. Other more common infectious diseases with shared features of EVD must also be considered at this stage and include malaria, meningococcemia, measles, and typhoid fever, among others.
After the first 5 days of exposure, progression of symptoms may include severe watery diarrhea, nausea and vomiting, abdominal pain, shortness of breath, chest pain, headache, and/or confusion. Conjunctival injection may also develop. Not all patients will have signs of hemorrhagic fever with bleeding from the mouth, eyes, ears, in stool, or from internal organs, but petechiae, ecchymosis, and oozing from venipuncture sites may develop. Those at the highest risk of death show signs of sepsis, such as shock and multiorgan system failure, which may include hemorrhagic manifestations, early in the course of their illness. Patients with these complications typically expire between days 6 to16.1 Survivors of the disease tend to have fever with less severe symptoms for a period of several more days, and then begin to improve clinically between days 6 to 11 after onset of symptoms1 (Figure 3).
Ecology
A zoonotic filovirus transmissible from animal populations to humans causes EVD. Research strongly suggests that fruit bats are the reservoir and hosts for this filovirus. Direct human contact with bats or with wild animals that have been infected by bats initiates the human-to-human transmission of EVD.3
Pathogenesis
Through direct contact with mucous membranes, a break in the skin, or parenterally, Ebola enters and infects multiple cell types. Incubation periods appear shorter in infections acquired through direct injection (6 days) than for contact transmission (10 days).1 Emesis, urine, stool, sweat, semen, cerebrospinal fluid, breast milk, and saliva are actively capable of viral transmission. From point of entry, the virus migrates to the lymph nodes, then to liver, spleen, and adrenal glands. Hepatocellular necrosis leads to clotting-factor derangement and dysfunction resulting in coagulopathy and bleeding and potential liver failure. Necrosis of adrenal tissue may be present and results in impaired steroid synthesis and hypotension. The presence of the virus appears to incite a cytokine inflammatory storm causing microvascular leakage, with the end effect of multiorgan system failure, shock, coagulopathy, and lymphocytopenia from cellular apoptosis.1 With cellular death, immune system function is further disabled, more viral particles are released into the infected host, and body fluids remain infectious postmortem.
Laboratory Findings
Laboratory findings in viral hemorrhagic fevers can vary depending on the exact viral cause and the stage in the disease process. Leukocyte counts in early stages can reveal leukopenia and specifically lymphopenia, while in later stages leukocytosis with a left shift of neutrophils can predominate. Hemoglobin and hematocrit can show relative hemoconcentration, especially if renal manifestations of the disease occur. Thrombocytopenia also develops with viral hemorrhagic fevers, although in late stages thrombocytosis has been seen. Blood urea nitrogen (BUN) and creatinine (Cr) levels will rise with the occurrence of acute renal failure in late stages of the disease. Liver function studies, aspartate aminotransferase (AST) in particular, and alanine aminotransferase (ALT) have been found to rise in severe disease and in late stages due to multifocal hepatic necrosis, with AST typically greater than ALT. An association between elevated AST (~900 IU/L), BUN, Cr, albumin levels, and mortality has been statistically confirmed by McElroy et al4 in the Ebola outbreak in Uganda in 2000 to 2001; findings previously confirmed in the same geographic and temporal outbreak by Rollin et al.5 Survivors did not have nearly the same degree of elevation in liver enzymes, with AST levels averaging ~150 IU/L.5 The authors suggested that normalizing AST levels was perhaps indicative of acute recovery, but some patients still succumbed to complications of the illness.5
Coagulation studies, including prothrombin time, partial thromboplastin time, d-dimer, and fibrin split products will reflect disseminated intravascular coagulation (DIC) in those patients who develop hemorrhagic manifestations, which is common in late stages of the disease. Direct infection of vascular endothelial cells with damage to these cells has been shown to occur in the course of infection, yet nonhuman primate experimental studies and pathology examinations of Ebola victims have implied that DIC plays an important part in the hemorrhagic disease leading to the fatal shock syndrome seen in the most severe cases.6 Observed in 2000 during the care of Sudan species EVD patients in Uganda reported by Rollin et al,5 a distinct difference has been noted in quantitative d-dimer levels between survivors and fatal cases. Case fatalities showed a 4-fold increase in quantitative d-dimer levels (140,000 ng/mL) compared with survivors (44,000 ng/mL) during the acute phase of infection 6 to 8 days postsymptom onset.5
Acute phase reactants, high nitric oxide levels, cytokines, and higher viral loads have also been associated with fatal outcomes.4 McElroy et al4 measured the common acute phase reactant biomarker ferritin in patients of the 2000 Uganda outbreak and found levels to be higher in samples from patients who died and from patients with hemorrhagic complications and higher viral loads. Thus, these authors postulate ferritin is a potential marker for EVD severity.4
Commercially available assays for detection of viral particles are still in development and no point-of-care rapid detection testing is available. No test can reliably be used to diagnose viral hemorrhagic fever prior to symptom onset.7 Enzyme-linked immunosorbent assay and reverse transcriptase-polymerase chain reaction (RT-PCR) of viral particles or tissue cell cultures are available only through the CDC, and are the current most reliable methods to confirm the diagnosis of viral hemorrhagic fevers, including Ebola.
Patient Management in the ED
Standard infection-control procedures in place in US hospitals, when meticulously practiced, should be adequate to prevent transmission of EVD. As Ebola virus is only transmitted through direct contact with infected body fluids and secretions, PPE including mask, gloves, gown, shoe covers, and eye protection (goggles or face shield), should be appropriate. In donning PPE, remember that gloves should be the last item to pull in place and to pull off, turning the gown and gloves inside out. One’s hands should be washed before removing the mask and face shield/goggles, and they should be rewashed after completion of PPE removal. A tight fit of the glove over the elastic wristband of the gown is preferable and can ensure a better barrier to any biohazard. Meticulous hand hygiene after removal and proper disposal of PPE is paramount to successful contact protection. Diligent care should be taken in any procedure that might expose a healthcare provider to body fluids, such as blood draws, central line insertion, lumbar puncture and other invasive procedures. Standard contact isolation methods with the patient in a single room with the door closed is sufficient. Appropriate use of standard hospital disinfectants, including bleach solutions or hospital grade ammonium cleaners are already standard practice and easily implemented. It is recommended that procedures that produce aerosol particles should be avoided in patients with suspected infection; yet in some circumstances, the course of care may require such procedures. In this case, to minimize potential airborne spread, airborne and droplet precautions should also be initiated by placing the patient in a negative pressure room and implementing the use of properly fitted N-95 respirators for all present in the room.8
The mainstay of treatment for viral hemorrhagic fevers in the ED begins with initiation of contact isolation of the patient to prevent spread to the healthy. Supportive care involving aggressive intravenous fluid resuscitation to maintain blood pressure, oxygen support as needed, blood products as indicated for DIC, correction of electrolyte abnormalities, including dialysis for renal failure, is the only treatment widely available at this time. In patients who exhibit generalized edema as a result of hypoproteinemia from liver damage and third spacing, serum protein monitoring and replacement is indicated.9
An experimental treatment, ZMapp, which is a monoclonal antibody that is derived from mice and blocks Ebola from entering cells, has recently been used in combination with supportive treatment in six individuals infected with Ebola. However, ZMapp is still in early stages of development and testing is not widely available. No clinical trials have begun, but are planned. Two individuals treated with ZMapp have recovered in the United States; three healthcare workers are recovering in West Africa10,11; and one patient has died. It is not clear whether this medication is responsible wholly or in part for their recovery.
According to Dr Bruce Ribner, Director of Emory University Hospital’s Infectious Disease unit in an interview with Scientific American’s Dina Fine Maron on August 27, 2014, the two patients cared for at Emory have developed immunity to Zaire Ebolavirus.11 Continued outpatient monitoring is allowing study to help understand immunity to Ebola, and may lead to further treatment and vaccination development. Thus far, cross-protection against other Ebola viral strains for these recovered individuals is not as robust, indicating that this family of viruses are different enough that recovery from infection with one species may not be enough to confer immunity to a different species exposure. Blood transfusions from recovered patients have been described, but there is no clinical evidence to support any benefit from this therapy at this time.9
Personal protective equipment is a mainstay in the collection of specimens for viral-specific testing by the CDC, including full-face shield or goggles, masks to cover nose and mouth, gowns, and gloves. For routine laboratory testing and patient care, all of the above PPE is recommended, along with use of a biosafety cabinet or plexiglass splash guard, which is in accordance with Occupational Safety and Health Administration bloodborne pathogens standards.12
Specimens should be collected for Ebola testing only after the onset of symptoms, such as fever (see Table for supplemental information). In patients suspected of having viral exposure, it may take up to 3 days for the virus to reach detectable levels with RT-PCR. Consultation per hospital procedure with the local and/or state health department prior to specimen transport for testing to the CDC is mandatory. Public health officials will help ensure appropriate patient selection and proper procedures for specimen collection, and make arrangements for testing, which is only available through the CDC. The CDC will not accept any specimen without appropriate local/state health department consultation.
Ideally, preferred specimens for Ebola testing include 4 mL of whole blood properly preserved with EDTA, clot activator, sodium polyanethol sulfonate, or citrate in plastic collection tubes stored at 4˚C or frozen.12 Specimens should be placed in a durable, leak-proof container for transport within a facility; pneumatic tube systems should be avoided due to concerns for glass breakage or leaks. Hospitals should follow state or local health department policies and procedures for Ebola testing prior to notifying the CDC and initiating sample transportation.
The Dallas Index Case
Deplaning a flight from Liberia in Dallas, Texas on September 20, this index patient had no outward signs of illness, and thus no reason to cause any health concern. Joining in the community with friends and family, it was not until 4 days later that he reportedly developed a fever. Yet 2 more days passed before this patient initially sought ambulatory care at Dallas Health Presbyterian Hospital Emergency Department, during which time additional close contacts were exposed to infection. After evaluation, antibiotics were prescribed and the patient was released. Though this case is under investigation, according to a CNN report,13 the patient did inform a member of the ED nursing staff of his travel history, but this information was not communicated to the rest of the healthcare team. The presence and recognition of this patient’s travel history with disease symptoms heightens the level of suspicion for the possibility of EVD, and is the cornerstone of patient selection for Ebola testing.14
After the patient was discharged, another 2 days passed, during which time his condition deteriorated at home. Emergency medical services (EMS) transport was summoned to take the patient back to the hospital, expanding exposure to first responders, who appropriately utilized masks and gloves during transport. During this ED visit, his travel history was obtained and communicated, and he was appropriately isolated, supported, and admitted. These are early reported details of the case, and local public health officials continue to work with a team from the CDC to trace, isolate, and monitor all contacts with this patient (including the transporting emergency medical technicians) for evidence of further cases.15
This index case illustrates valuable lessons for all emergency care workers going forward. Ebola virus disease is now global, in the sense that it has proven its ability to present in a community far from its endemic home. Infectious diseases in their early stages present in nonspecific constellations of symptoms, and the key to rapid identification of EVD lies in careful attention to the recent travel history and exposure potential. Since patients may not offer this information for various reasons (eg, degree of symptoms, language barriers, fear, denial), it must be sought out lest more index patients be released into the public. As CDC Director Thomas Frieden related to CNN, “If someone’s been in West Africa within 21 days and they’ve got a fever, immediately isolate them and get them tested for Ebola.”13
Concerning the EMS transport of this patient, the ambulance used was disinfected per standard local protocols and remained in service for 2 days after this patient was transported. Though local officials are confident in the disinfection technique, it was pulled from service after the diagnosis was confirmed to ensure its full sterilization from Ebola virus16 before returning to full service. The rapid and robust public health response in progress will undoubtedly reveal further information over the coming days to weeks. To quote Michael Osterholm, PhD, MPH, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, “We are going to see more cases show up around the world.”15
In addition to the index patient, it is important to keep in mind that secondary cases may present. If this occurs, the travel question must be altered to reflect the possibility that a secondary patient may not have been the traveler but rather one who was in close contact with an ill person who was in contact with him or her. Such a scenario would have a huge impact if that traveler did not seek treatment and would in turn require ED personnel to seek-out the information and report it to local public health officials.
Quelling the Panic
Even though Ebola is only spread through direct contact with infected bodily secretions, there is still significant public fear of the disease due to the high mortality rate and the graphic nature of symptoms in late stages of the disease. Daily monitoring of contacts of symptomatic Ebola patients for evidence of disease development—mainly fever—is sensible. Asymptomatic persons need not be confined or hospitalized unless fever develops, in which case contact isolation should occur until formal Ebola testing can rule-out the disease. Personal protective equipment, standardized hospital cleaning protocols with meticulous adherence, as well as quickly burying the deceased with adequate contact precautions, can all limit potential exposure and spread of the disease. Public health discussion and education about the virus and methods of transmission are needed so that individuals are not denied proper treatment or scared away from medical centers.
Importantly, communication by public health and medical experts on local and national levels should be with news media that embrace honest and careful reporting to avoid sensationalism and foster appropriate concern—ensuring that content is fundamental to curtailing panic and undue public fear.
For Internationally Traveling Clinicians
At present, the area endemic for Ebola remains confined to sub-Saharan Africa and West Africa. Clinicians should remain alert when traveling or treating patients in these areas. However, with the ease of international air travel, the potential for the spread of disease is recognized with many bordering nations now screening passengers from affected countries and some closing their borders to travelers from endemic areas. If a clinician encounters febrile patients in endemic areas, the differential diagnosis for any febrile illness must include Ebola, as well as malaria and other more common infectious agents. A thorough history about recent travel, ill contacts, and possible exposures should be sufficient in categorizing the risk of Ebola, but a high index of suspicion is necessary for prompt and proper treatment of those affected and to curtail spread of disease.
Despite the efforts of the national and local health systems and many nongovernmental organizations, including the World Health Organization, this epidemic continues to hold strong in the affected West African countries. Methods of containment of the virus are seemingly simple by modern standards, yet tragically beyond access for many on the ground. Lack of clean water sources in affected communities is a significant barrier to basic personal and environmental hygiene. Inadequate safe food sources and poaching encourages the hunt for primate bushmeat and thus presents a formidable local challenge.17 Lack of adequate PPE for healthcare workers, for those responsible for facility environmental hygiene, and for family members participating in traditional funeral rites for Ebola victims compounds the problem. Illness and deaths among exposed healthcare workers have led to the loss of significant numbers of nurses and doctors. This has caused legitimate fear in qualified individuals who subsequently decline to accept jobs caring for Ebola patients, which in turn increases the burden on those who remain. Additionally, some nongovernmental organizations have canceled scheduled aid trips to West Africa in response to the epidemic out of concern for the health of their workers. Meticulous management of environmental hygiene including sharps, surfaces, soiled linens, reusable medical equipment, waste products, and the preparations for burial of the deceased pose definite challenges to containment and prevention of transmission. Strict adherence to the use of PPE and hand hygiene is essential for all in contact with Ebola patients, pre- and postmortem. The lack of layperson comprehension and community understanding of the illness itself and the mechanism of viral transmission along with fear and mistrust for healthcare providers and nongovernmental medical missionaries are all serious barriers to the containment of disease spread. In fact, rumors that the virus does not truly exist, and that the illness is a result of biological warfare, cannibalistic rituals, or witchcraft add to the complexity of the situation.11
For these reasons, it is essential that efforts to control this epidemic in endemic healthcare facilities include effective health surveillance, infection-control programs, and community outreach fostering mutual trust-building, honest communication, and education. Success will require a multifaceted approach, and a global response will be needed to quiet this global threat. On September 16, the United States announced a robust response to deploy military engineers and medical personnel to assist in training healthcare workers and building care centers in Liberia. The United Nations, France, and the United Kingdom are also supporting this important effort to build stronger healthcare infrastructures in these vulnerable countries.16
Conclusion
This 2014 epidemic of EVD raises justifiable concerns regarding the impact of globalization. Though unlikely to pose a direct threat of epidemic proportion on US soil, the unanticipated occurrence of an index case may trigger a terrifying outbreak in any community, as it already has in the city of Dallas. Given that the early stages of EVD are indistinguishable from most other viral syndromes, the importance of reflection on individual and general healthcare facility adherence to standard infection control precautions and procedures warrant merit. Eliciting accurate travel histories and possible exposures are germane to narrowing the scope of possible etiologies of all infectious diseases. As an opportunity for improvement, this epidemic should incite elevated caution in the everyday handling of all patients with febrile illnesses and contact-transmissible infections including methicillin-resistant Staphylococcus aureus and Clostridium difficile, which affect a great number of US patients on a daily basis. Not only could this strategy prevent additional local outbreaks of EVD, but it would promote the safety of healthcare workers and the community served through attention to better infection preventive measures at the point of care, every time.
Dr McCammon is an assistant professor, department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Chidester is an instructor, department of emergency medicine, and a fellow in international wilderness medicine, Eastern Virginia Medical School, Norfolk.
- Ebola virus disease information for clinicians in U.S. Healthcare Settings. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/clinician-information-us-healthcare-settings.html. Updated September 5, 2014. Accessed September 23, 2014.
- 2014 Ebola outbreak in West Africa. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/outbreaks/guinea/. Updated September 18, 2014. Accessed September 23, 2014.
- Virus ecology graphic. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/resources/virus-ecology.html. Updated August 1, 2014. Accessed September 23, 2014.
- McElroy AK, Erickson BR, Flietstra TD, et al. Ebola hemorrhagic fever: novel biomarker correlates of clinical outcome. J Infect Dis. 2014;210(4):558-566.
- Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S364-S371.
- Geisbert TW, Young HA, Jahrling PB, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163(6): 2371-2382.
- Blumberg L, Enria D, Bausch DG. Viral haemorrhagic fevers. In: Farrar J, Hotez, PJ, Junghanss T, Kang G, Lalloo D, White N, eds. Manson’s Tropical Diseases: Expert Consult. 23rd ed. Philadelphia, PA: Elsevier Saunders; 2014:171-194.
- Safe management of patients with Ebola virus disease (EVD) in U.S. hospitals. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/patient-management-us-hospitals.html. Updated September 5, 2014. Accessed September 23, 2014.
- Maron DF. Ebola doctor reveals how infected Americans were cured. Scientific American. http://www.scientificamerican.com/article/ebola-doctor-revealshow-infected-americans-were-cured/.
- Tribune wire reports. American Ebola patients treated with ZMapp experimental drug. Chicago Tribune. August 21, 2014. http://www.chicagotribune.com/lifestyles/health/chi-ebola-zmapp-20140821-story.html. Accessed September 23, 2014.
- Ebola crisis: doctors in Liberia ‘recovering after taking ZMapp’ [transcript]. BBC News. http://www.bbc.com/news/world-africa-28860204. August 19, 2014. Accessed September 23, 2014.
- Interim guidance for specimen collection, transport, testing, and submission for persons under investigation for Ebola virus disease in the United States. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimen-collection-submission-patients-suspected-infection-ebola.html. Updated September 8, 2014. Accessed September 23, 2014.
- Catherine E. Shoichet CE, Fantz A, Yan H. Hospital ‘dropped the ball’ with Ebola patient’s travel history, NIH official says. CNN News. http://www.cnn.com/2014/10/01/health/ebola-us/index.html. Accessed October 2, 2014.
- Ebola (Ebola Virus Disease): Diagnosis. Centers for Disease Control and Prevention Web Site. http://www.cdc.gov/vhf/ebola/diagnosis/. Updated September 19, 2014. Accessed October 1, 2014.
- Gilblom K, Langreth R. Dallas hospital initially let Ebola patient go with drugs. Bloomberg News Web Site. http://www.bloomberg.com/news/2014-09-30/first-ebola-case-is-diagnosed-in-the-u-s-cdc-reports.html. Accessed October 1, 2014.
- Bates D, Szathmary Z and Boyle L. Up to twelve Americans could have Ebola: Fears grow in Dallas after first victim of deadly virus to reach U.S. remained at large for a week. September 30, 2014. Mail Online. http://www.dailymail.co.uk/news/article-2775608/CDC-confirms-Dallas-patient-isolation-testing-returning-region-plagued-Ebola-HAS-deadly-virus.html. Updated October 1, 2014. Accessed October 1, 2014.
- International affairs: bushmeat. U.S. Fish & Wildlife Service Web site. http://www.fws.gov/international/wildlife-without-borders/global-program/bushmeat.html. Accessed September 23, 2014.
- Ebola virus disease information for clinicians in U.S. Healthcare Settings. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/clinician-information-us-healthcare-settings.html. Updated September 5, 2014. Accessed September 23, 2014.
- 2014 Ebola outbreak in West Africa. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/outbreaks/guinea/. Updated September 18, 2014. Accessed September 23, 2014.
- Virus ecology graphic. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/resources/virus-ecology.html. Updated August 1, 2014. Accessed September 23, 2014.
- McElroy AK, Erickson BR, Flietstra TD, et al. Ebola hemorrhagic fever: novel biomarker correlates of clinical outcome. J Infect Dis. 2014;210(4):558-566.
- Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S364-S371.
- Geisbert TW, Young HA, Jahrling PB, et al. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163(6): 2371-2382.
- Blumberg L, Enria D, Bausch DG. Viral haemorrhagic fevers. In: Farrar J, Hotez, PJ, Junghanss T, Kang G, Lalloo D, White N, eds. Manson’s Tropical Diseases: Expert Consult. 23rd ed. Philadelphia, PA: Elsevier Saunders; 2014:171-194.
- Safe management of patients with Ebola virus disease (EVD) in U.S. hospitals. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/patient-management-us-hospitals.html. Updated September 5, 2014. Accessed September 23, 2014.
- Maron DF. Ebola doctor reveals how infected Americans were cured. Scientific American. http://www.scientificamerican.com/article/ebola-doctor-revealshow-infected-americans-were-cured/.
- Tribune wire reports. American Ebola patients treated with ZMapp experimental drug. Chicago Tribune. August 21, 2014. http://www.chicagotribune.com/lifestyles/health/chi-ebola-zmapp-20140821-story.html. Accessed September 23, 2014.
- Ebola crisis: doctors in Liberia ‘recovering after taking ZMapp’ [transcript]. BBC News. http://www.bbc.com/news/world-africa-28860204. August 19, 2014. Accessed September 23, 2014.
- Interim guidance for specimen collection, transport, testing, and submission for persons under investigation for Ebola virus disease in the United States. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/vhf/ebola/hcp/interim-guidance-specimen-collection-submission-patients-suspected-infection-ebola.html. Updated September 8, 2014. Accessed September 23, 2014.
- Catherine E. Shoichet CE, Fantz A, Yan H. Hospital ‘dropped the ball’ with Ebola patient’s travel history, NIH official says. CNN News. http://www.cnn.com/2014/10/01/health/ebola-us/index.html. Accessed October 2, 2014.
- Ebola (Ebola Virus Disease): Diagnosis. Centers for Disease Control and Prevention Web Site. http://www.cdc.gov/vhf/ebola/diagnosis/. Updated September 19, 2014. Accessed October 1, 2014.
- Gilblom K, Langreth R. Dallas hospital initially let Ebola patient go with drugs. Bloomberg News Web Site. http://www.bloomberg.com/news/2014-09-30/first-ebola-case-is-diagnosed-in-the-u-s-cdc-reports.html. Accessed October 1, 2014.
- Bates D, Szathmary Z and Boyle L. Up to twelve Americans could have Ebola: Fears grow in Dallas after first victim of deadly virus to reach U.S. remained at large for a week. September 30, 2014. Mail Online. http://www.dailymail.co.uk/news/article-2775608/CDC-confirms-Dallas-patient-isolation-testing-returning-region-plagued-Ebola-HAS-deadly-virus.html. Updated October 1, 2014. Accessed October 1, 2014.
- International affairs: bushmeat. U.S. Fish & Wildlife Service Web site. http://www.fws.gov/international/wildlife-without-borders/global-program/bushmeat.html. Accessed September 23, 2014.
Tissue extraction during minimally invasive Gyn surgery. Second of 2 Parts: Counseling the patient
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
Dr. Robert L. Barbieri’s Editor’s Picks September 2014
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
Editor in Chief Robert L. Barbieri, MD, provides an overview of three articles appearing in OBG Management’s September 2014 issue. Listen to his take on why these articles are of particular importance to women’s health professionals.
Access all of the articles in the September 2014 issue here.
Tissue extraction during minimally invasive Gyn surgery. First of 2 parts: Best practices for an environment in flux
The world of minimally invasive gynecologic surgery has been transformed over the past 10 months—specifically in regard to the option of open power morcellation. From individual hospital bans of the procedure to an official warning from the US Food and Drug Administration (FDA)1 and the potential for further government action, the change has been swift and certain. Johnson & Johnson has recalled all power morcellators, many institutions now have bans in place, and one major insurer has announced its plan to discontinue coverage of power morcellation in three states.
What effect have these actions had on the availability of minimally invasive approaches to benign hysterectomy and myomectomy? And given new information on the risk of occult malignancy during these surgeries, how has patient selection and preoperative assessment changed? To address these and other questions, OBG Management convened a panel of experts in minimally invasive gynecology and asked them to share their perspective. In this case-based discussion, they offer their views on the morcellation controversy and their current approach to hysterectomy, myomectomy, and tissue extraction. Next month, in Part 2 of their discussion, they address patient counseling and FDA actions.
What is your preferred approach?
OBG Management: In light of the morcellation controversy, what is your preferred approach for benign hysterectomy?
Kimberly Kho, MD, MPH: Whenever possible and appropriate, vaginal hysterectomy is my preferred route. However, many surgical cases require evaluation of the abdominal cavity for pain, endometriosis, or a concerning adnexal mass. In such cases, and in cases involving a very large uterus, I prefer laparoscopic hysterectomy—either laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy (TLH). I tend to perform TLH more frequently in these cases if the uterus lacks descent or the patient’s anatomy restricts vaginal access. Even in these cases, and with very large myomas and uteri, I have been successful removing the uterus vaginally, although this approach frequently involves vaginal morcellation with a scalpel.
Arnold P. Advincula, MD: My preferred approach for both benign hysterectomy and myomectomy is robot-assisted laparoscopy. I have used this approach over the past 13 years. In my hands, it is reproducible, safe, efficient, and cost-effective and affords me the ability to tackle a wide range of complex cases.
Cheryl Iglesia, MD: Like Dr. Kho, I prefer vaginal hysterectomy.
Jason D. Wright, MD: I also prefer the vaginal approach. In fact, I believe it should be the preferred approach for hysterectomy for benign gynecologic disease whenever it is feasible. And the laparoscopic and robot-assisted approaches carry less perioperative morbidity than abdominal hysterectomy.
Given the recent concerns about open power morcellation, I prefer to perform either vaginal hysterectomy or minimally invasive hysterectomy without morcellation. If neither approach is feasible, given anatomic considerations, I counsel the patient about the risks and benefits of abdominal hysterectomy, compared with minimally invasive hysterectomy with morcellation.
Linda D. Bradley, MD: For women who meet minimally invasive surgical criteria, I prefer the laparoscopic approach because of its many benefits, including a shorter hospital stay (which reduces the risk of hospital-acquired infection and iatrogenic complications of hospitalization), lower risk of incisional infection, lower requirement for pain medications, and faster return to work.
OBG Management: What about myomectomy? Would your approach be different?
Dr. Bradley: Many myomectomy cases can be done hysteroscopically. I would like to point out, however, that when we talk about hysteroscopy, the morcellation issue is moot. Although there are hysteroscopic surgical devices that have used the word “morcellator” in their names, hysteroscopic morcellation is performed within a closed system—the uterine cavity—and so carries none of the risks of laparoscopic morcellation.
I prefer to perform nonhysteroscopic cases using a laparoscopic approach, creating a small mini-laparotomy to remove the fibroid intact or using a knife to morcellate the tissue outside of the peritoneal cavity.
Dr. Kho: I use a similar laparoscopic approach for myomectomy, using laparoscopy to assess the uterus and fibroids, enucleate the fibroid and remove it from the uterus, and then creating a mini-laparotomy incision
3 cm to 4 cm in length to manually remove or morcellate the fibroid and reapproximate the myometrium.
Dr. Iglesia: I rarely perform myomectomy but would likely do it laparoscopically or robotically to achieve minimally invasive benefits such as fewer adhesions and less postoperative pain.
How do you manage tissue extraction?
OBG Management: What methods of tissue extraction do you currently use during hysterectomy and myomectomy?
Dr. Advincula: I currently utilize a contained, extracorporeal, transumbilical, manual scalpel-morcellation technique for all myomectomy cases, as well as hysterectomy cases not amenable to transvaginal extraction.
Dr. Iglesia: I rely on vaginal removal of tissue and vaginal morcellation.
Dr. Kho: I infrequently perform supracervical hysterectomy, so almost all the hysterectomies I do are total hysterectomies. I remove the uterus through the vagina. In addition, because the size of the specimen frequently is too large to remove through a colpotomy intact, I morcellate the uterus manually with a scalpel using coring, wedge resection, and myomectomy. I find this to be an efficient and controlled method for tissue removal, with minimal tissue scattering. I also have begun to perform the same type of vaginal morcellation with the specimen enclosed in a bag.
That being said, the spread of occult malignancy has been reported after all types of morcellation—not just with power morcellation but also with vaginal and abdominal morcellation. So we are increasingly performing tissue extraction in an enclosed fashion using manual morcellation in a containment bag through a mini-laparotomy or posterior colpotomy to minimize the risk of leaving tissue fragments behind.
Dr. Wright: Although different methods of tissue extraction, including morcellation within a bag, are commonly discussed, data documenting the safety of these methods are extremely limited and patients should be counseled accordingly.
Similarly, the risk of adverse pathology increases substantially with age, and morcellation should be considered with great caution—if at all—in older women.
Given the risks associated with power morcellation, I try to avoid uterine disruption at the time of hysterectomy and perform either vaginal or minimally invasive total hysterectomy. In older women, because of the higher risk of underlying pathology, I prefer laparotomy if anatomic considerations preclude a vaginal or minimally invasive total hysterectomy. Younger women can be counseled about the risks and benefits of various routes of extraction. Patients with any suspicious findings during preoperative evaluation or surgery itself should have their uterus removed without disruption or fragmentation.
In regard to myomectomy specifically, a significant portion of the data we have on the risks of power morcellation derives from studies of hysterectomy. There are minimal data describing the risk of occult pathology at the time of minimally invasive myomectomy. Although younger patients likely are at relatively low risk for occult malignancy, they should be counseled that population-based estimates of cancer at the time of myomectomy are lacking.
Dr. Bradley: Since the controversy over morcellation arose, the Cleveland Clinic not only has banned the procedure but also removed all morcellators from its shelves, and it is unclear whether the option will be revisited after the FDA renders its final verdict. So my approach to tissue extraction is either vaginal morcellation or using a mini-laparotomy to remove the whole specimen intact or put it in a bag and morcellate it with a knife.
Case 1: A premenopausal patient scheduled for myomectomy
OBG Management: Let’s move on to a specific case. Let’s say the patient is a 35-year-old woman with a large fibroid, to be removed by myomectomy. How would you quantify her risk of occult malignancy? And what would preoperative assessment entail?
Dr. Iglesia: This patient’s risk of occult malignancy is low. I would obtain pelvic ultrasonography and endometrial biopsy, with cervical cytology included. Preoperative magnetic resonance imaging (MRI) would be indicated if there is a possibility that power morcellation will be performed. If power morcellation were selected, I would perform it using a bag.
Dr. Bradley: At the Cleveland Clinic, we now utilize the FDA risk estimates for occult malignancy of 1 in 300 to 1 in 350 women,1 and I counsel patients using these figures. In the past several years, we have begun to use MRI with and without contrast to determine the size, number, and location of the fibroids, to determine our surgical approach, and to guide our discussion with the patient of what we will be able to do—for example, laparoscopy versus laparotomy.
OBG Management: Would the FDA figures you give be applicable to a young woman such as this 35-year-old?
Dr. Bradley: We’re using those figures with all of our premenopausal patients.
OBG Management: And does the MRI pick up sarcomas?
Dr. Bradley: No imaging is 100% sensitive in detecting sarcoma. We do MRI, and if the fibroid has any areas of necrosis, irregularity, or poor tissue planes that would arouse our suspicion of adenomyoma or sarcoma, we perform the myomectomy via laparotomy. But as I mentioned earlier, we don’t use power morcellation at all anymore—so this patient you describe would likely undergo laparoscopic removal using a bag and a knife to extract it to the skin level.
Although every patient is different, in general, if we have a patient with a single large fibroid 10 cm or less in size, we try to remove it laparoscopically or with robot assistance rather than via laparotomy. We also perform endometrial biopsy.
Dr. Advincula: First, it’s important to define prevalence and incidence when discussing risks. Prevalence would be the number of patients with a leiomyosarcoma per 100,000 women, whereas incidence is the number of patients given a diagnosis of leiomyosarcoma within a year per 100,000 women. In this case, a 35-year-old woman would have a prevalence of leiomyosarcoma, in the general population, of 3 to 7 per 100,000 women and an incidence of less than 1%.
My preoperative assessment would involve MRI of the pelvis with T2 weighted images to better characterize her uterus. Although there has been much discussion lately about the use of lactate dehydrogenase (LDH) isoenzyme panels in combination with MRI to detect occult leiomyosarcoma, the reliability and reproducibility of that combined approach are not fully vetted and, as yet, are not a standard part of my workup. Endometrial sampling would certainly be warranted with any associated history of abnormal uterine bleeding.
Dr. Wright: As I mentioned earlier, most data on power morcellation have been derived from studies of women undergoing hysterectomy. To date, accurate estimates to predict the risk of occult cancer in this patient planning to undergo myomectomy are largely lacking. For women undergoing hysterectomy using power morcellation, advanced age is the strongest risk factor for occult malignancy. Although this patient’s risk of cancer likely is relatively low, she should be counseled that precise estimates are lacking.
Preoperatively, she should undergo endometrial sampling if she has abnormal bleeding. However, the reliability of endometrial sampling, as well as imaging, is limited in the detection of uterine sarcomas.
Case 2: Perimenopausal patient undergoing hysterectomy
OBG Management: How would your approach to preoperative assessment change if this patient were a 47-year-old perimenopausal woman with a single large fibroid to be removed by hysterectomy?
Dr. Bradley: It would be the same preoperative assessment—an MRI and an endometrial biopsy.
Dr. Iglesia: The risk of occult malignancy would be greater than with the first patient. Again, I would use pelvic ultrasound, endometrial biopsy, and cervical cytology to assess her, and I would perform vaginal or TLH. MRI would be indicated if there is a possibility of performing intraperitoneal morcellation. I would prefer doing any morcellation in a bag or via laparotomy.
Dr. Wright: Based on age alone, this perimenopausal patient’s risk for an underlying cancer is 0.2%.2 If the patient has any abnormal bleeding, she should undergo endometrial sampling preoperatively. The diagnostic modalities currently available—which include endometrial sampling as well as imaging, even MRI—are unreliable in the diagnosis of uterine sarcomas, and the patient should be counseled accordingly if she is considering power morcellation.
If it is technically feasible, vaginal hysterectomy or a minimally invasive hysterectomy without power morcellation are preferred. If neither modality is feasible and the patient is considering power morcellation, she should be carefully counseled about the underlying risk not only of uterine cancer but also of other adverse pathologic abnormalities.
Case 3: Postmenopausal woman scheduled for hysterectomy
OBG Management: Let’s change the details of the case again. This time she’s 55 years old and postmenopausal. She, too, has a large fibroid to be removed via hysterectomy. What is her risk of occult cancer? How would you assess her preoperatively?
Dr. Bradley: My approach would be the same as in the first two cases. However, because this patient is menopausal, morcellation would be off the table. (And it already is off the table—for any patient—at the Cleveland Clinic.) I usually prefer open hysterectomy for these patients, which is very different from what we were doing 1 year ago.
I want to expand on Dr. Wright’s comments about other pathologic abnormalities. As a woman ages, her cancer risk becomes greatest for malignancy of the endometrium rather than cancer in a fibroid. If this were my 55-year-old patient, and I had been seeing her for 20 years, and her fibroids had remained the same size but she was now having bleeding, I’d be more concerned about an endometrial problem—hyperplasia, a polyp, or cancer.
If the patient were having bulk symptoms, new pain, and imaging that shows, over 10 years between perimenopause and postmenopause, that there has been growth of the fibroids, I would be concerned about a sarcoma.
Some women who present with postmenopausal bleeding have ovarian cancer, and some studies show that a significant percentage of women with ovarian cancer present with bleeding as a primary symptom.3 So in a postmenopausal patient, I really want to know about the health of the ovaries. Are they enlarged on imaging?
There is also a bimodal distribution of human papillomavirus (HPV) infection and cervical cancer, with peaks of infection at ages 26 to 30 years and again at 46 to 50 years in some populations. The second age peak is followed by an increase in cervical intraepithelial neoplasia (CIN) 2 and 3 and invasive cervical cancer 20 years later.4 So I also want to consider the possibility of cervical cancer in this population.
I tell my patients that pretty much everybody has fibroids. Because they are so common, I need to look at the whole picture.
Dr. Wright: For the perimenopausal and postmenopausal patients we are discussing, preoperative evaluation and counseling would be similar to that for the premenopausal woman. However, given recent data, it is important to note that the prevalence ratio for a uterine malignancy increases with increasing age.2 Clinicians need to be mindful of red flags in perimenopausal and postmenopausal women.
Dr. Kho: I agree that, for each of the cases we have discussed, we need to consider more than just the presence of the fibroid. Risk stratification based on clinical factors like age, menopausal status, BMI, the indication for surgery, and response to any other therapy is extremely important and should guide decision-making regarding the surgical approach.
Although we lack reliable methods to differentiate fibroids from leiomyosarcomas, there are other malignant conditions of the uterus, as Dr. Bradley pointed out. Premalignant conditions of the uterus and cervix and endometrial adenocarcinomas occur far more frequently in the population than sarcomas, and we may be increasing risks by morcellating unsuspected cancers of any type.
At this time, whenever I am considering morcellation in any patient, I obtain pelvic imaging, endometrial biopsy, and current cervical cancer screening. If any of these studies suggest a malignant or premalignant condition, I avoid morcellation. Similarly, if a patient’s clinical history raises suspicion of a potential underlying malignant process, such as new symptoms of an enlarging myoma in a postmenopausal woman, I will try to find an alternative to morcellation.
Why we need a national surgery registry
The controversy surrounding open power morcellation was precipitated by the reporting of a single case—that of a prominent physician who had an unsuspected cancer morcellated during the course of a hysterectomy and was later upgraded to Stage 4 leiomyosarcoma as a result. But it wasn’t a gynecologic surgeon who reported the case—it was the patient herself. And we all know she was by no means the first case of an inadvertently morcellated sarcoma.
I would wager that few physicians are well versed in how to contact the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. And not many are familiar with the other specialized registries in the United States, let alone know how to report to them. Another important but often unaddressed issue: Is it hassle-free to make a report? The answer: A resounding “No.”
Who do we inform about our experiences—successful or not—in the operating room? And who pays for data collection? At present, the system is piecemeal or scattershot at best.
Sorely needed is a system for reporting that is easy to use, broad, and deep. A nationally funded system would be best. Otherwise, who is going to maintain the database? Who will filter the data? Who will ensure that the information that is entered is correct so that outcomes can be followed accurately?
For those of us employed by a hospital or other institution, it tends to be the institution itself that gathers the data—when it is gathered. But what about surgeons in private practice? How do they monitor themselves? And what about privately owned outpatient surgery centers, where patients sojourn no longer than 23 hours? Is it reasonable to expect them to add the burden and expense of data collection without a national mandate?
I know firsthand some of the skewed information that results when reporting is piecemeal or manipulated. When I was a resident, for example, in some localities, it was not uncommon for sexually transmitted diseases to go unreported when the patient had private insurance. The result: Only those in the lower socioeconomic ranks appeared to experience this problem.
Clearly, we need national standards and a national protocol. And to achieve that we need leaders strong enough to argue that the expense represents dollars well spent.
- Linda D. Bradley, MD
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page and then see how your peers have voted.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Accessed August 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
3. Lurie G, Thompson PJ, McDuffie KE, et al. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol Oncol. 2009;114(2):231–236.
4. Chan PK, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126(1):297–301.
The world of minimally invasive gynecologic surgery has been transformed over the past 10 months—specifically in regard to the option of open power morcellation. From individual hospital bans of the procedure to an official warning from the US Food and Drug Administration (FDA)1 and the potential for further government action, the change has been swift and certain. Johnson & Johnson has recalled all power morcellators, many institutions now have bans in place, and one major insurer has announced its plan to discontinue coverage of power morcellation in three states.
What effect have these actions had on the availability of minimally invasive approaches to benign hysterectomy and myomectomy? And given new information on the risk of occult malignancy during these surgeries, how has patient selection and preoperative assessment changed? To address these and other questions, OBG Management convened a panel of experts in minimally invasive gynecology and asked them to share their perspective. In this case-based discussion, they offer their views on the morcellation controversy and their current approach to hysterectomy, myomectomy, and tissue extraction. Next month, in Part 2 of their discussion, they address patient counseling and FDA actions.
What is your preferred approach?
OBG Management: In light of the morcellation controversy, what is your preferred approach for benign hysterectomy?
Kimberly Kho, MD, MPH: Whenever possible and appropriate, vaginal hysterectomy is my preferred route. However, many surgical cases require evaluation of the abdominal cavity for pain, endometriosis, or a concerning adnexal mass. In such cases, and in cases involving a very large uterus, I prefer laparoscopic hysterectomy—either laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy (TLH). I tend to perform TLH more frequently in these cases if the uterus lacks descent or the patient’s anatomy restricts vaginal access. Even in these cases, and with very large myomas and uteri, I have been successful removing the uterus vaginally, although this approach frequently involves vaginal morcellation with a scalpel.
Arnold P. Advincula, MD: My preferred approach for both benign hysterectomy and myomectomy is robot-assisted laparoscopy. I have used this approach over the past 13 years. In my hands, it is reproducible, safe, efficient, and cost-effective and affords me the ability to tackle a wide range of complex cases.
Cheryl Iglesia, MD: Like Dr. Kho, I prefer vaginal hysterectomy.
Jason D. Wright, MD: I also prefer the vaginal approach. In fact, I believe it should be the preferred approach for hysterectomy for benign gynecologic disease whenever it is feasible. And the laparoscopic and robot-assisted approaches carry less perioperative morbidity than abdominal hysterectomy.
Given the recent concerns about open power morcellation, I prefer to perform either vaginal hysterectomy or minimally invasive hysterectomy without morcellation. If neither approach is feasible, given anatomic considerations, I counsel the patient about the risks and benefits of abdominal hysterectomy, compared with minimally invasive hysterectomy with morcellation.
Linda D. Bradley, MD: For women who meet minimally invasive surgical criteria, I prefer the laparoscopic approach because of its many benefits, including a shorter hospital stay (which reduces the risk of hospital-acquired infection and iatrogenic complications of hospitalization), lower risk of incisional infection, lower requirement for pain medications, and faster return to work.
OBG Management: What about myomectomy? Would your approach be different?
Dr. Bradley: Many myomectomy cases can be done hysteroscopically. I would like to point out, however, that when we talk about hysteroscopy, the morcellation issue is moot. Although there are hysteroscopic surgical devices that have used the word “morcellator” in their names, hysteroscopic morcellation is performed within a closed system—the uterine cavity—and so carries none of the risks of laparoscopic morcellation.
I prefer to perform nonhysteroscopic cases using a laparoscopic approach, creating a small mini-laparotomy to remove the fibroid intact or using a knife to morcellate the tissue outside of the peritoneal cavity.
Dr. Kho: I use a similar laparoscopic approach for myomectomy, using laparoscopy to assess the uterus and fibroids, enucleate the fibroid and remove it from the uterus, and then creating a mini-laparotomy incision
3 cm to 4 cm in length to manually remove or morcellate the fibroid and reapproximate the myometrium.
Dr. Iglesia: I rarely perform myomectomy but would likely do it laparoscopically or robotically to achieve minimally invasive benefits such as fewer adhesions and less postoperative pain.
How do you manage tissue extraction?
OBG Management: What methods of tissue extraction do you currently use during hysterectomy and myomectomy?
Dr. Advincula: I currently utilize a contained, extracorporeal, transumbilical, manual scalpel-morcellation technique for all myomectomy cases, as well as hysterectomy cases not amenable to transvaginal extraction.
Dr. Iglesia: I rely on vaginal removal of tissue and vaginal morcellation.
Dr. Kho: I infrequently perform supracervical hysterectomy, so almost all the hysterectomies I do are total hysterectomies. I remove the uterus through the vagina. In addition, because the size of the specimen frequently is too large to remove through a colpotomy intact, I morcellate the uterus manually with a scalpel using coring, wedge resection, and myomectomy. I find this to be an efficient and controlled method for tissue removal, with minimal tissue scattering. I also have begun to perform the same type of vaginal morcellation with the specimen enclosed in a bag.
That being said, the spread of occult malignancy has been reported after all types of morcellation—not just with power morcellation but also with vaginal and abdominal morcellation. So we are increasingly performing tissue extraction in an enclosed fashion using manual morcellation in a containment bag through a mini-laparotomy or posterior colpotomy to minimize the risk of leaving tissue fragments behind.
Dr. Wright: Although different methods of tissue extraction, including morcellation within a bag, are commonly discussed, data documenting the safety of these methods are extremely limited and patients should be counseled accordingly.
Similarly, the risk of adverse pathology increases substantially with age, and morcellation should be considered with great caution—if at all—in older women.
Given the risks associated with power morcellation, I try to avoid uterine disruption at the time of hysterectomy and perform either vaginal or minimally invasive total hysterectomy. In older women, because of the higher risk of underlying pathology, I prefer laparotomy if anatomic considerations preclude a vaginal or minimally invasive total hysterectomy. Younger women can be counseled about the risks and benefits of various routes of extraction. Patients with any suspicious findings during preoperative evaluation or surgery itself should have their uterus removed without disruption or fragmentation.
In regard to myomectomy specifically, a significant portion of the data we have on the risks of power morcellation derives from studies of hysterectomy. There are minimal data describing the risk of occult pathology at the time of minimally invasive myomectomy. Although younger patients likely are at relatively low risk for occult malignancy, they should be counseled that population-based estimates of cancer at the time of myomectomy are lacking.
Dr. Bradley: Since the controversy over morcellation arose, the Cleveland Clinic not only has banned the procedure but also removed all morcellators from its shelves, and it is unclear whether the option will be revisited after the FDA renders its final verdict. So my approach to tissue extraction is either vaginal morcellation or using a mini-laparotomy to remove the whole specimen intact or put it in a bag and morcellate it with a knife.
Case 1: A premenopausal patient scheduled for myomectomy
OBG Management: Let’s move on to a specific case. Let’s say the patient is a 35-year-old woman with a large fibroid, to be removed by myomectomy. How would you quantify her risk of occult malignancy? And what would preoperative assessment entail?
Dr. Iglesia: This patient’s risk of occult malignancy is low. I would obtain pelvic ultrasonography and endometrial biopsy, with cervical cytology included. Preoperative magnetic resonance imaging (MRI) would be indicated if there is a possibility that power morcellation will be performed. If power morcellation were selected, I would perform it using a bag.
Dr. Bradley: At the Cleveland Clinic, we now utilize the FDA risk estimates for occult malignancy of 1 in 300 to 1 in 350 women,1 and I counsel patients using these figures. In the past several years, we have begun to use MRI with and without contrast to determine the size, number, and location of the fibroids, to determine our surgical approach, and to guide our discussion with the patient of what we will be able to do—for example, laparoscopy versus laparotomy.
OBG Management: Would the FDA figures you give be applicable to a young woman such as this 35-year-old?
Dr. Bradley: We’re using those figures with all of our premenopausal patients.
OBG Management: And does the MRI pick up sarcomas?
Dr. Bradley: No imaging is 100% sensitive in detecting sarcoma. We do MRI, and if the fibroid has any areas of necrosis, irregularity, or poor tissue planes that would arouse our suspicion of adenomyoma or sarcoma, we perform the myomectomy via laparotomy. But as I mentioned earlier, we don’t use power morcellation at all anymore—so this patient you describe would likely undergo laparoscopic removal using a bag and a knife to extract it to the skin level.
Although every patient is different, in general, if we have a patient with a single large fibroid 10 cm or less in size, we try to remove it laparoscopically or with robot assistance rather than via laparotomy. We also perform endometrial biopsy.
Dr. Advincula: First, it’s important to define prevalence and incidence when discussing risks. Prevalence would be the number of patients with a leiomyosarcoma per 100,000 women, whereas incidence is the number of patients given a diagnosis of leiomyosarcoma within a year per 100,000 women. In this case, a 35-year-old woman would have a prevalence of leiomyosarcoma, in the general population, of 3 to 7 per 100,000 women and an incidence of less than 1%.
My preoperative assessment would involve MRI of the pelvis with T2 weighted images to better characterize her uterus. Although there has been much discussion lately about the use of lactate dehydrogenase (LDH) isoenzyme panels in combination with MRI to detect occult leiomyosarcoma, the reliability and reproducibility of that combined approach are not fully vetted and, as yet, are not a standard part of my workup. Endometrial sampling would certainly be warranted with any associated history of abnormal uterine bleeding.
Dr. Wright: As I mentioned earlier, most data on power morcellation have been derived from studies of women undergoing hysterectomy. To date, accurate estimates to predict the risk of occult cancer in this patient planning to undergo myomectomy are largely lacking. For women undergoing hysterectomy using power morcellation, advanced age is the strongest risk factor for occult malignancy. Although this patient’s risk of cancer likely is relatively low, she should be counseled that precise estimates are lacking.
Preoperatively, she should undergo endometrial sampling if she has abnormal bleeding. However, the reliability of endometrial sampling, as well as imaging, is limited in the detection of uterine sarcomas.
Case 2: Perimenopausal patient undergoing hysterectomy
OBG Management: How would your approach to preoperative assessment change if this patient were a 47-year-old perimenopausal woman with a single large fibroid to be removed by hysterectomy?
Dr. Bradley: It would be the same preoperative assessment—an MRI and an endometrial biopsy.
Dr. Iglesia: The risk of occult malignancy would be greater than with the first patient. Again, I would use pelvic ultrasound, endometrial biopsy, and cervical cytology to assess her, and I would perform vaginal or TLH. MRI would be indicated if there is a possibility of performing intraperitoneal morcellation. I would prefer doing any morcellation in a bag or via laparotomy.
Dr. Wright: Based on age alone, this perimenopausal patient’s risk for an underlying cancer is 0.2%.2 If the patient has any abnormal bleeding, she should undergo endometrial sampling preoperatively. The diagnostic modalities currently available—which include endometrial sampling as well as imaging, even MRI—are unreliable in the diagnosis of uterine sarcomas, and the patient should be counseled accordingly if she is considering power morcellation.
If it is technically feasible, vaginal hysterectomy or a minimally invasive hysterectomy without power morcellation are preferred. If neither modality is feasible and the patient is considering power morcellation, she should be carefully counseled about the underlying risk not only of uterine cancer but also of other adverse pathologic abnormalities.
Case 3: Postmenopausal woman scheduled for hysterectomy
OBG Management: Let’s change the details of the case again. This time she’s 55 years old and postmenopausal. She, too, has a large fibroid to be removed via hysterectomy. What is her risk of occult cancer? How would you assess her preoperatively?
Dr. Bradley: My approach would be the same as in the first two cases. However, because this patient is menopausal, morcellation would be off the table. (And it already is off the table—for any patient—at the Cleveland Clinic.) I usually prefer open hysterectomy for these patients, which is very different from what we were doing 1 year ago.
I want to expand on Dr. Wright’s comments about other pathologic abnormalities. As a woman ages, her cancer risk becomes greatest for malignancy of the endometrium rather than cancer in a fibroid. If this were my 55-year-old patient, and I had been seeing her for 20 years, and her fibroids had remained the same size but she was now having bleeding, I’d be more concerned about an endometrial problem—hyperplasia, a polyp, or cancer.
If the patient were having bulk symptoms, new pain, and imaging that shows, over 10 years between perimenopause and postmenopause, that there has been growth of the fibroids, I would be concerned about a sarcoma.
Some women who present with postmenopausal bleeding have ovarian cancer, and some studies show that a significant percentage of women with ovarian cancer present with bleeding as a primary symptom.3 So in a postmenopausal patient, I really want to know about the health of the ovaries. Are they enlarged on imaging?
There is also a bimodal distribution of human papillomavirus (HPV) infection and cervical cancer, with peaks of infection at ages 26 to 30 years and again at 46 to 50 years in some populations. The second age peak is followed by an increase in cervical intraepithelial neoplasia (CIN) 2 and 3 and invasive cervical cancer 20 years later.4 So I also want to consider the possibility of cervical cancer in this population.
I tell my patients that pretty much everybody has fibroids. Because they are so common, I need to look at the whole picture.
Dr. Wright: For the perimenopausal and postmenopausal patients we are discussing, preoperative evaluation and counseling would be similar to that for the premenopausal woman. However, given recent data, it is important to note that the prevalence ratio for a uterine malignancy increases with increasing age.2 Clinicians need to be mindful of red flags in perimenopausal and postmenopausal women.
Dr. Kho: I agree that, for each of the cases we have discussed, we need to consider more than just the presence of the fibroid. Risk stratification based on clinical factors like age, menopausal status, BMI, the indication for surgery, and response to any other therapy is extremely important and should guide decision-making regarding the surgical approach.
Although we lack reliable methods to differentiate fibroids from leiomyosarcomas, there are other malignant conditions of the uterus, as Dr. Bradley pointed out. Premalignant conditions of the uterus and cervix and endometrial adenocarcinomas occur far more frequently in the population than sarcomas, and we may be increasing risks by morcellating unsuspected cancers of any type.
At this time, whenever I am considering morcellation in any patient, I obtain pelvic imaging, endometrial biopsy, and current cervical cancer screening. If any of these studies suggest a malignant or premalignant condition, I avoid morcellation. Similarly, if a patient’s clinical history raises suspicion of a potential underlying malignant process, such as new symptoms of an enlarging myoma in a postmenopausal woman, I will try to find an alternative to morcellation.
Why we need a national surgery registry
The controversy surrounding open power morcellation was precipitated by the reporting of a single case—that of a prominent physician who had an unsuspected cancer morcellated during the course of a hysterectomy and was later upgraded to Stage 4 leiomyosarcoma as a result. But it wasn’t a gynecologic surgeon who reported the case—it was the patient herself. And we all know she was by no means the first case of an inadvertently morcellated sarcoma.
I would wager that few physicians are well versed in how to contact the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. And not many are familiar with the other specialized registries in the United States, let alone know how to report to them. Another important but often unaddressed issue: Is it hassle-free to make a report? The answer: A resounding “No.”
Who do we inform about our experiences—successful or not—in the operating room? And who pays for data collection? At present, the system is piecemeal or scattershot at best.
Sorely needed is a system for reporting that is easy to use, broad, and deep. A nationally funded system would be best. Otherwise, who is going to maintain the database? Who will filter the data? Who will ensure that the information that is entered is correct so that outcomes can be followed accurately?
For those of us employed by a hospital or other institution, it tends to be the institution itself that gathers the data—when it is gathered. But what about surgeons in private practice? How do they monitor themselves? And what about privately owned outpatient surgery centers, where patients sojourn no longer than 23 hours? Is it reasonable to expect them to add the burden and expense of data collection without a national mandate?
I know firsthand some of the skewed information that results when reporting is piecemeal or manipulated. When I was a resident, for example, in some localities, it was not uncommon for sexually transmitted diseases to go unreported when the patient had private insurance. The result: Only those in the lower socioeconomic ranks appeared to experience this problem.
Clearly, we need national standards and a national protocol. And to achieve that we need leaders strong enough to argue that the expense represents dollars well spent.
- Linda D. Bradley, MD
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page and then see how your peers have voted.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The world of minimally invasive gynecologic surgery has been transformed over the past 10 months—specifically in regard to the option of open power morcellation. From individual hospital bans of the procedure to an official warning from the US Food and Drug Administration (FDA)1 and the potential for further government action, the change has been swift and certain. Johnson & Johnson has recalled all power morcellators, many institutions now have bans in place, and one major insurer has announced its plan to discontinue coverage of power morcellation in three states.
What effect have these actions had on the availability of minimally invasive approaches to benign hysterectomy and myomectomy? And given new information on the risk of occult malignancy during these surgeries, how has patient selection and preoperative assessment changed? To address these and other questions, OBG Management convened a panel of experts in minimally invasive gynecology and asked them to share their perspective. In this case-based discussion, they offer their views on the morcellation controversy and their current approach to hysterectomy, myomectomy, and tissue extraction. Next month, in Part 2 of their discussion, they address patient counseling and FDA actions.
What is your preferred approach?
OBG Management: In light of the morcellation controversy, what is your preferred approach for benign hysterectomy?
Kimberly Kho, MD, MPH: Whenever possible and appropriate, vaginal hysterectomy is my preferred route. However, many surgical cases require evaluation of the abdominal cavity for pain, endometriosis, or a concerning adnexal mass. In such cases, and in cases involving a very large uterus, I prefer laparoscopic hysterectomy—either laparoscopic-assisted vaginal hysterectomy or total laparoscopic hysterectomy (TLH). I tend to perform TLH more frequently in these cases if the uterus lacks descent or the patient’s anatomy restricts vaginal access. Even in these cases, and with very large myomas and uteri, I have been successful removing the uterus vaginally, although this approach frequently involves vaginal morcellation with a scalpel.
Arnold P. Advincula, MD: My preferred approach for both benign hysterectomy and myomectomy is robot-assisted laparoscopy. I have used this approach over the past 13 years. In my hands, it is reproducible, safe, efficient, and cost-effective and affords me the ability to tackle a wide range of complex cases.
Cheryl Iglesia, MD: Like Dr. Kho, I prefer vaginal hysterectomy.
Jason D. Wright, MD: I also prefer the vaginal approach. In fact, I believe it should be the preferred approach for hysterectomy for benign gynecologic disease whenever it is feasible. And the laparoscopic and robot-assisted approaches carry less perioperative morbidity than abdominal hysterectomy.
Given the recent concerns about open power morcellation, I prefer to perform either vaginal hysterectomy or minimally invasive hysterectomy without morcellation. If neither approach is feasible, given anatomic considerations, I counsel the patient about the risks and benefits of abdominal hysterectomy, compared with minimally invasive hysterectomy with morcellation.
Linda D. Bradley, MD: For women who meet minimally invasive surgical criteria, I prefer the laparoscopic approach because of its many benefits, including a shorter hospital stay (which reduces the risk of hospital-acquired infection and iatrogenic complications of hospitalization), lower risk of incisional infection, lower requirement for pain medications, and faster return to work.
OBG Management: What about myomectomy? Would your approach be different?
Dr. Bradley: Many myomectomy cases can be done hysteroscopically. I would like to point out, however, that when we talk about hysteroscopy, the morcellation issue is moot. Although there are hysteroscopic surgical devices that have used the word “morcellator” in their names, hysteroscopic morcellation is performed within a closed system—the uterine cavity—and so carries none of the risks of laparoscopic morcellation.
I prefer to perform nonhysteroscopic cases using a laparoscopic approach, creating a small mini-laparotomy to remove the fibroid intact or using a knife to morcellate the tissue outside of the peritoneal cavity.
Dr. Kho: I use a similar laparoscopic approach for myomectomy, using laparoscopy to assess the uterus and fibroids, enucleate the fibroid and remove it from the uterus, and then creating a mini-laparotomy incision
3 cm to 4 cm in length to manually remove or morcellate the fibroid and reapproximate the myometrium.
Dr. Iglesia: I rarely perform myomectomy but would likely do it laparoscopically or robotically to achieve minimally invasive benefits such as fewer adhesions and less postoperative pain.
How do you manage tissue extraction?
OBG Management: What methods of tissue extraction do you currently use during hysterectomy and myomectomy?
Dr. Advincula: I currently utilize a contained, extracorporeal, transumbilical, manual scalpel-morcellation technique for all myomectomy cases, as well as hysterectomy cases not amenable to transvaginal extraction.
Dr. Iglesia: I rely on vaginal removal of tissue and vaginal morcellation.
Dr. Kho: I infrequently perform supracervical hysterectomy, so almost all the hysterectomies I do are total hysterectomies. I remove the uterus through the vagina. In addition, because the size of the specimen frequently is too large to remove through a colpotomy intact, I morcellate the uterus manually with a scalpel using coring, wedge resection, and myomectomy. I find this to be an efficient and controlled method for tissue removal, with minimal tissue scattering. I also have begun to perform the same type of vaginal morcellation with the specimen enclosed in a bag.
That being said, the spread of occult malignancy has been reported after all types of morcellation—not just with power morcellation but also with vaginal and abdominal morcellation. So we are increasingly performing tissue extraction in an enclosed fashion using manual morcellation in a containment bag through a mini-laparotomy or posterior colpotomy to minimize the risk of leaving tissue fragments behind.
Dr. Wright: Although different methods of tissue extraction, including morcellation within a bag, are commonly discussed, data documenting the safety of these methods are extremely limited and patients should be counseled accordingly.
Similarly, the risk of adverse pathology increases substantially with age, and morcellation should be considered with great caution—if at all—in older women.
Given the risks associated with power morcellation, I try to avoid uterine disruption at the time of hysterectomy and perform either vaginal or minimally invasive total hysterectomy. In older women, because of the higher risk of underlying pathology, I prefer laparotomy if anatomic considerations preclude a vaginal or minimally invasive total hysterectomy. Younger women can be counseled about the risks and benefits of various routes of extraction. Patients with any suspicious findings during preoperative evaluation or surgery itself should have their uterus removed without disruption or fragmentation.
In regard to myomectomy specifically, a significant portion of the data we have on the risks of power morcellation derives from studies of hysterectomy. There are minimal data describing the risk of occult pathology at the time of minimally invasive myomectomy. Although younger patients likely are at relatively low risk for occult malignancy, they should be counseled that population-based estimates of cancer at the time of myomectomy are lacking.
Dr. Bradley: Since the controversy over morcellation arose, the Cleveland Clinic not only has banned the procedure but also removed all morcellators from its shelves, and it is unclear whether the option will be revisited after the FDA renders its final verdict. So my approach to tissue extraction is either vaginal morcellation or using a mini-laparotomy to remove the whole specimen intact or put it in a bag and morcellate it with a knife.
Case 1: A premenopausal patient scheduled for myomectomy
OBG Management: Let’s move on to a specific case. Let’s say the patient is a 35-year-old woman with a large fibroid, to be removed by myomectomy. How would you quantify her risk of occult malignancy? And what would preoperative assessment entail?
Dr. Iglesia: This patient’s risk of occult malignancy is low. I would obtain pelvic ultrasonography and endometrial biopsy, with cervical cytology included. Preoperative magnetic resonance imaging (MRI) would be indicated if there is a possibility that power morcellation will be performed. If power morcellation were selected, I would perform it using a bag.
Dr. Bradley: At the Cleveland Clinic, we now utilize the FDA risk estimates for occult malignancy of 1 in 300 to 1 in 350 women,1 and I counsel patients using these figures. In the past several years, we have begun to use MRI with and without contrast to determine the size, number, and location of the fibroids, to determine our surgical approach, and to guide our discussion with the patient of what we will be able to do—for example, laparoscopy versus laparotomy.
OBG Management: Would the FDA figures you give be applicable to a young woman such as this 35-year-old?
Dr. Bradley: We’re using those figures with all of our premenopausal patients.
OBG Management: And does the MRI pick up sarcomas?
Dr. Bradley: No imaging is 100% sensitive in detecting sarcoma. We do MRI, and if the fibroid has any areas of necrosis, irregularity, or poor tissue planes that would arouse our suspicion of adenomyoma or sarcoma, we perform the myomectomy via laparotomy. But as I mentioned earlier, we don’t use power morcellation at all anymore—so this patient you describe would likely undergo laparoscopic removal using a bag and a knife to extract it to the skin level.
Although every patient is different, in general, if we have a patient with a single large fibroid 10 cm or less in size, we try to remove it laparoscopically or with robot assistance rather than via laparotomy. We also perform endometrial biopsy.
Dr. Advincula: First, it’s important to define prevalence and incidence when discussing risks. Prevalence would be the number of patients with a leiomyosarcoma per 100,000 women, whereas incidence is the number of patients given a diagnosis of leiomyosarcoma within a year per 100,000 women. In this case, a 35-year-old woman would have a prevalence of leiomyosarcoma, in the general population, of 3 to 7 per 100,000 women and an incidence of less than 1%.
My preoperative assessment would involve MRI of the pelvis with T2 weighted images to better characterize her uterus. Although there has been much discussion lately about the use of lactate dehydrogenase (LDH) isoenzyme panels in combination with MRI to detect occult leiomyosarcoma, the reliability and reproducibility of that combined approach are not fully vetted and, as yet, are not a standard part of my workup. Endometrial sampling would certainly be warranted with any associated history of abnormal uterine bleeding.
Dr. Wright: As I mentioned earlier, most data on power morcellation have been derived from studies of women undergoing hysterectomy. To date, accurate estimates to predict the risk of occult cancer in this patient planning to undergo myomectomy are largely lacking. For women undergoing hysterectomy using power morcellation, advanced age is the strongest risk factor for occult malignancy. Although this patient’s risk of cancer likely is relatively low, she should be counseled that precise estimates are lacking.
Preoperatively, she should undergo endometrial sampling if she has abnormal bleeding. However, the reliability of endometrial sampling, as well as imaging, is limited in the detection of uterine sarcomas.
Case 2: Perimenopausal patient undergoing hysterectomy
OBG Management: How would your approach to preoperative assessment change if this patient were a 47-year-old perimenopausal woman with a single large fibroid to be removed by hysterectomy?
Dr. Bradley: It would be the same preoperative assessment—an MRI and an endometrial biopsy.
Dr. Iglesia: The risk of occult malignancy would be greater than with the first patient. Again, I would use pelvic ultrasound, endometrial biopsy, and cervical cytology to assess her, and I would perform vaginal or TLH. MRI would be indicated if there is a possibility of performing intraperitoneal morcellation. I would prefer doing any morcellation in a bag or via laparotomy.
Dr. Wright: Based on age alone, this perimenopausal patient’s risk for an underlying cancer is 0.2%.2 If the patient has any abnormal bleeding, she should undergo endometrial sampling preoperatively. The diagnostic modalities currently available—which include endometrial sampling as well as imaging, even MRI—are unreliable in the diagnosis of uterine sarcomas, and the patient should be counseled accordingly if she is considering power morcellation.
If it is technically feasible, vaginal hysterectomy or a minimally invasive hysterectomy without power morcellation are preferred. If neither modality is feasible and the patient is considering power morcellation, she should be carefully counseled about the underlying risk not only of uterine cancer but also of other adverse pathologic abnormalities.
Case 3: Postmenopausal woman scheduled for hysterectomy
OBG Management: Let’s change the details of the case again. This time she’s 55 years old and postmenopausal. She, too, has a large fibroid to be removed via hysterectomy. What is her risk of occult cancer? How would you assess her preoperatively?
Dr. Bradley: My approach would be the same as in the first two cases. However, because this patient is menopausal, morcellation would be off the table. (And it already is off the table—for any patient—at the Cleveland Clinic.) I usually prefer open hysterectomy for these patients, which is very different from what we were doing 1 year ago.
I want to expand on Dr. Wright’s comments about other pathologic abnormalities. As a woman ages, her cancer risk becomes greatest for malignancy of the endometrium rather than cancer in a fibroid. If this were my 55-year-old patient, and I had been seeing her for 20 years, and her fibroids had remained the same size but she was now having bleeding, I’d be more concerned about an endometrial problem—hyperplasia, a polyp, or cancer.
If the patient were having bulk symptoms, new pain, and imaging that shows, over 10 years between perimenopause and postmenopause, that there has been growth of the fibroids, I would be concerned about a sarcoma.
Some women who present with postmenopausal bleeding have ovarian cancer, and some studies show that a significant percentage of women with ovarian cancer present with bleeding as a primary symptom.3 So in a postmenopausal patient, I really want to know about the health of the ovaries. Are they enlarged on imaging?
There is also a bimodal distribution of human papillomavirus (HPV) infection and cervical cancer, with peaks of infection at ages 26 to 30 years and again at 46 to 50 years in some populations. The second age peak is followed by an increase in cervical intraepithelial neoplasia (CIN) 2 and 3 and invasive cervical cancer 20 years later.4 So I also want to consider the possibility of cervical cancer in this population.
I tell my patients that pretty much everybody has fibroids. Because they are so common, I need to look at the whole picture.
Dr. Wright: For the perimenopausal and postmenopausal patients we are discussing, preoperative evaluation and counseling would be similar to that for the premenopausal woman. However, given recent data, it is important to note that the prevalence ratio for a uterine malignancy increases with increasing age.2 Clinicians need to be mindful of red flags in perimenopausal and postmenopausal women.
Dr. Kho: I agree that, for each of the cases we have discussed, we need to consider more than just the presence of the fibroid. Risk stratification based on clinical factors like age, menopausal status, BMI, the indication for surgery, and response to any other therapy is extremely important and should guide decision-making regarding the surgical approach.
Although we lack reliable methods to differentiate fibroids from leiomyosarcomas, there are other malignant conditions of the uterus, as Dr. Bradley pointed out. Premalignant conditions of the uterus and cervix and endometrial adenocarcinomas occur far more frequently in the population than sarcomas, and we may be increasing risks by morcellating unsuspected cancers of any type.
At this time, whenever I am considering morcellation in any patient, I obtain pelvic imaging, endometrial biopsy, and current cervical cancer screening. If any of these studies suggest a malignant or premalignant condition, I avoid morcellation. Similarly, if a patient’s clinical history raises suspicion of a potential underlying malignant process, such as new symptoms of an enlarging myoma in a postmenopausal woman, I will try to find an alternative to morcellation.
Why we need a national surgery registry
The controversy surrounding open power morcellation was precipitated by the reporting of a single case—that of a prominent physician who had an unsuspected cancer morcellated during the course of a hysterectomy and was later upgraded to Stage 4 leiomyosarcoma as a result. But it wasn’t a gynecologic surgeon who reported the case—it was the patient herself. And we all know she was by no means the first case of an inadvertently morcellated sarcoma.
I would wager that few physicians are well versed in how to contact the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database. And not many are familiar with the other specialized registries in the United States, let alone know how to report to them. Another important but often unaddressed issue: Is it hassle-free to make a report? The answer: A resounding “No.”
Who do we inform about our experiences—successful or not—in the operating room? And who pays for data collection? At present, the system is piecemeal or scattershot at best.
Sorely needed is a system for reporting that is easy to use, broad, and deep. A nationally funded system would be best. Otherwise, who is going to maintain the database? Who will filter the data? Who will ensure that the information that is entered is correct so that outcomes can be followed accurately?
For those of us employed by a hospital or other institution, it tends to be the institution itself that gathers the data—when it is gathered. But what about surgeons in private practice? How do they monitor themselves? And what about privately owned outpatient surgery centers, where patients sojourn no longer than 23 hours? Is it reasonable to expect them to add the burden and expense of data collection without a national mandate?
I know firsthand some of the skewed information that results when reporting is piecemeal or manipulated. When I was a resident, for example, in some localities, it was not uncommon for sexually transmitted diseases to go unreported when the patient had private insurance. The result: Only those in the lower socioeconomic ranks appeared to experience this problem.
Clearly, we need national standards and a national protocol. And to achieve that we need leaders strong enough to argue that the expense represents dollars well spent.
- Linda D. Bradley, MD
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page and then see how your peers have voted.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Accessed August 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
3. Lurie G, Thompson PJ, McDuffie KE, et al. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol Oncol. 2009;114(2):231–236.
4. Chan PK, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126(1):297–301.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Accessed August 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi: 10.1001/jama.2014.9005.
3. Lurie G, Thompson PJ, McDuffie KE, et al. Prediagnostic symptoms of ovarian carcinoma: a case-control study. Gynecol Oncol. 2009;114(2):231–236.
4. Chan PK, Chang AR, Yu MY, et al. Age distribution of human papillomavirus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2010;126(1):297–301.
Put Key Principles, Characteristics of Effective Hospital Medicine Groups to Work
I hope you’re already familiar with “The Key Principles and Characteristics of an Effective Hospital Medicine Group: An Assessment Guide for Hospitals and Hospitalists” [www.hospitalmedicine.org/keychar] and have spent at least a few minutes reviewing the list of 10 “principles” and 47 “characteristics” thought to be associated with effective hospital medicine groups (HMGs). (Full disclosure: I was one of the authors of the article published in February 2014 in the Journal of Hospital Medicine.) Most of us are very busy, so the temptation might be high to set the article aside and risk forgetting it. But I hope many in our field, both clinicians and administrators, will look at it more carefully. There are a number of ways you could use the guide to stimulate thinking or change in your practice.
Grading Our Specialty
I just returned from a meeting of about 10 hospitalist leaders from different organizations around the country. Attendees represented the diversity of our field, including hospital-employed HMGs, large hospitalist management companies, and academic programs. We spent a portion of the meeting discussing what grade we as a group would give the whole specialty of hospital medicine on each of the 10 “principles.” Essentially, we generated a report card for the U.S. hospitalist movement.
This wasn’t a rigorous scientific exercise; instead, it was a robust and thought-provoking discussion around what grade to assign. Opinions regarding the appropriate grade varied significantly, but a common theme was that our specialty really “owns” the importance of pursuing many or most of the principles listed in the article and is devoting time and resources to them even if many individual HMGs might have a long way to go to perform optimally.
For example, meeting attendees thought our field has for a long time worked diligently to “support care coordination across the care continuum” (Principle 6). No one thought that all HMGs do this optimally, but the consensus was that most HMGs have invested effort to do it well. And most were concerned that many HMGs still lack “adequate resources” (Principle 3) and sufficiently “engaged hospitalists” (Principle 2)—and that the former contributes to the latter.
The opinion of the hospitalist leaders who happened to attend the meeting where this conversation took place doesn’t represent the final word on how our specialty is performing, but I think all involved found value in having the conversation, hearing different perspectives about what we’re doing well and where we should focus energy and resources to improve.
Grading Your HM Group
You might want to do something similar within your own group, but make it more relevant by grading how your own practice performs on each of the 10 principles. You could do this on your own just to stimulate your thinking, or you could have each member of your HMG generate a report card of your group’s performance—then discuss where there is agreement or disagreement within the group.
You could structure this sort of individual or group assessment simply as an exercise to generate ideas and conversation about the practice, or your group could take a more formal approach and use it as part of a planning process to determine future practice management-related goals. I know of some groups that scheduled strategic planning meetings specifically to discuss which of the elements to make a priority.
Discussion Document for Leadership
In addition to using the article to generate conversation among hospitalists within your group, it can be a really valuable tool in guiding conversations with hospital leaders and the entity that employs the hospitalists. For example, you could use the article to generate or update the job description of the lead hospitalist or practice manager. Or during annual budgeting for the hospitalist practice, the guide could be used as a checklist to think about whether there are important areas that would benefit from more resources.
Of course, there is a risk that hospital leaders or those who employ the hospitalists could use the article primarily to criticize a hospitalist group and its leader for not already having excellent performance on every one of the principles and characteristics listed. That would be pretty unfortunate; there probably isn’t a single group that performs well on every domain, and the real value of the article is to “be aspirational, helping to raise the bar” for each HMG and our specialty as a whole.
And, as discussed in the article, an HMG doesn’t need to be a stellar performer on all 47 characteristics to be effective. Some of the characteristics listed in the article may not apply to all groups, so all involved in the management of any individual HMG should think about whether to set some aside when assessing their own group.
Where to Go from Here
The article is based on expert opinion, with the help of many more people than those listed as author, and I’m hopeful it will stimulate researchers to study some of these principles and characteristics. For many reasons, we will probably never have robust data, but I’d be happy for whatever we can get.
There is a pretty good chance that the evolution in the work we do and the nature of the hospital setting mean that the principles and characteristics may need to be revised periodically. I would love to know how they might be different in 10 or 20 years.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
I hope you’re already familiar with “The Key Principles and Characteristics of an Effective Hospital Medicine Group: An Assessment Guide for Hospitals and Hospitalists” [www.hospitalmedicine.org/keychar] and have spent at least a few minutes reviewing the list of 10 “principles” and 47 “characteristics” thought to be associated with effective hospital medicine groups (HMGs). (Full disclosure: I was one of the authors of the article published in February 2014 in the Journal of Hospital Medicine.) Most of us are very busy, so the temptation might be high to set the article aside and risk forgetting it. But I hope many in our field, both clinicians and administrators, will look at it more carefully. There are a number of ways you could use the guide to stimulate thinking or change in your practice.
Grading Our Specialty
I just returned from a meeting of about 10 hospitalist leaders from different organizations around the country. Attendees represented the diversity of our field, including hospital-employed HMGs, large hospitalist management companies, and academic programs. We spent a portion of the meeting discussing what grade we as a group would give the whole specialty of hospital medicine on each of the 10 “principles.” Essentially, we generated a report card for the U.S. hospitalist movement.
This wasn’t a rigorous scientific exercise; instead, it was a robust and thought-provoking discussion around what grade to assign. Opinions regarding the appropriate grade varied significantly, but a common theme was that our specialty really “owns” the importance of pursuing many or most of the principles listed in the article and is devoting time and resources to them even if many individual HMGs might have a long way to go to perform optimally.
For example, meeting attendees thought our field has for a long time worked diligently to “support care coordination across the care continuum” (Principle 6). No one thought that all HMGs do this optimally, but the consensus was that most HMGs have invested effort to do it well. And most were concerned that many HMGs still lack “adequate resources” (Principle 3) and sufficiently “engaged hospitalists” (Principle 2)—and that the former contributes to the latter.
The opinion of the hospitalist leaders who happened to attend the meeting where this conversation took place doesn’t represent the final word on how our specialty is performing, but I think all involved found value in having the conversation, hearing different perspectives about what we’re doing well and where we should focus energy and resources to improve.
Grading Your HM Group
You might want to do something similar within your own group, but make it more relevant by grading how your own practice performs on each of the 10 principles. You could do this on your own just to stimulate your thinking, or you could have each member of your HMG generate a report card of your group’s performance—then discuss where there is agreement or disagreement within the group.
You could structure this sort of individual or group assessment simply as an exercise to generate ideas and conversation about the practice, or your group could take a more formal approach and use it as part of a planning process to determine future practice management-related goals. I know of some groups that scheduled strategic planning meetings specifically to discuss which of the elements to make a priority.
Discussion Document for Leadership
In addition to using the article to generate conversation among hospitalists within your group, it can be a really valuable tool in guiding conversations with hospital leaders and the entity that employs the hospitalists. For example, you could use the article to generate or update the job description of the lead hospitalist or practice manager. Or during annual budgeting for the hospitalist practice, the guide could be used as a checklist to think about whether there are important areas that would benefit from more resources.
Of course, there is a risk that hospital leaders or those who employ the hospitalists could use the article primarily to criticize a hospitalist group and its leader for not already having excellent performance on every one of the principles and characteristics listed. That would be pretty unfortunate; there probably isn’t a single group that performs well on every domain, and the real value of the article is to “be aspirational, helping to raise the bar” for each HMG and our specialty as a whole.
And, as discussed in the article, an HMG doesn’t need to be a stellar performer on all 47 characteristics to be effective. Some of the characteristics listed in the article may not apply to all groups, so all involved in the management of any individual HMG should think about whether to set some aside when assessing their own group.
Where to Go from Here
The article is based on expert opinion, with the help of many more people than those listed as author, and I’m hopeful it will stimulate researchers to study some of these principles and characteristics. For many reasons, we will probably never have robust data, but I’d be happy for whatever we can get.
There is a pretty good chance that the evolution in the work we do and the nature of the hospital setting mean that the principles and characteristics may need to be revised periodically. I would love to know how they might be different in 10 or 20 years.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
I hope you’re already familiar with “The Key Principles and Characteristics of an Effective Hospital Medicine Group: An Assessment Guide for Hospitals and Hospitalists” [www.hospitalmedicine.org/keychar] and have spent at least a few minutes reviewing the list of 10 “principles” and 47 “characteristics” thought to be associated with effective hospital medicine groups (HMGs). (Full disclosure: I was one of the authors of the article published in February 2014 in the Journal of Hospital Medicine.) Most of us are very busy, so the temptation might be high to set the article aside and risk forgetting it. But I hope many in our field, both clinicians and administrators, will look at it more carefully. There are a number of ways you could use the guide to stimulate thinking or change in your practice.
Grading Our Specialty
I just returned from a meeting of about 10 hospitalist leaders from different organizations around the country. Attendees represented the diversity of our field, including hospital-employed HMGs, large hospitalist management companies, and academic programs. We spent a portion of the meeting discussing what grade we as a group would give the whole specialty of hospital medicine on each of the 10 “principles.” Essentially, we generated a report card for the U.S. hospitalist movement.
This wasn’t a rigorous scientific exercise; instead, it was a robust and thought-provoking discussion around what grade to assign. Opinions regarding the appropriate grade varied significantly, but a common theme was that our specialty really “owns” the importance of pursuing many or most of the principles listed in the article and is devoting time and resources to them even if many individual HMGs might have a long way to go to perform optimally.
For example, meeting attendees thought our field has for a long time worked diligently to “support care coordination across the care continuum” (Principle 6). No one thought that all HMGs do this optimally, but the consensus was that most HMGs have invested effort to do it well. And most were concerned that many HMGs still lack “adequate resources” (Principle 3) and sufficiently “engaged hospitalists” (Principle 2)—and that the former contributes to the latter.
The opinion of the hospitalist leaders who happened to attend the meeting where this conversation took place doesn’t represent the final word on how our specialty is performing, but I think all involved found value in having the conversation, hearing different perspectives about what we’re doing well and where we should focus energy and resources to improve.
Grading Your HM Group
You might want to do something similar within your own group, but make it more relevant by grading how your own practice performs on each of the 10 principles. You could do this on your own just to stimulate your thinking, or you could have each member of your HMG generate a report card of your group’s performance—then discuss where there is agreement or disagreement within the group.
You could structure this sort of individual or group assessment simply as an exercise to generate ideas and conversation about the practice, or your group could take a more formal approach and use it as part of a planning process to determine future practice management-related goals. I know of some groups that scheduled strategic planning meetings specifically to discuss which of the elements to make a priority.
Discussion Document for Leadership
In addition to using the article to generate conversation among hospitalists within your group, it can be a really valuable tool in guiding conversations with hospital leaders and the entity that employs the hospitalists. For example, you could use the article to generate or update the job description of the lead hospitalist or practice manager. Or during annual budgeting for the hospitalist practice, the guide could be used as a checklist to think about whether there are important areas that would benefit from more resources.
Of course, there is a risk that hospital leaders or those who employ the hospitalists could use the article primarily to criticize a hospitalist group and its leader for not already having excellent performance on every one of the principles and characteristics listed. That would be pretty unfortunate; there probably isn’t a single group that performs well on every domain, and the real value of the article is to “be aspirational, helping to raise the bar” for each HMG and our specialty as a whole.
And, as discussed in the article, an HMG doesn’t need to be a stellar performer on all 47 characteristics to be effective. Some of the characteristics listed in the article may not apply to all groups, so all involved in the management of any individual HMG should think about whether to set some aside when assessing their own group.
Where to Go from Here
The article is based on expert opinion, with the help of many more people than those listed as author, and I’m hopeful it will stimulate researchers to study some of these principles and characteristics. For many reasons, we will probably never have robust data, but I’d be happy for whatever we can get.
There is a pretty good chance that the evolution in the work we do and the nature of the hospital setting mean that the principles and characteristics may need to be revised periodically. I would love to know how they might be different in 10 or 20 years.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Does PTSD during pregnancy increase the likelihood of preterm birth?
The decision about whether to treat any mental health condition during pregnancy is complex, and this study adds to the growing body of knowledge that will inform clinicians and patients about the risks associated with the symptoms of mental health conditions such as posttraumatic stress disorder (PTSD), major depression, and anxiety.
Psychosocial stress during pregnancy has been associated with preterm delivery, possibly related to activation of the hypothalamic-pituitary-adrenal axis, which can result in elevations in maternal glucocorticoids and corticotropin-releasing hormone (CRH). The literature describing the association between stress-related conditions, including PTSD, with preterm birth (PTB), has been inconsistent and limited with regard to power, assessment of the burden of symptoms, and confounding variables including psychiatric comorbidity and psychotropic medications that also have been linked with PTB. Disentangling the risks for PTB associated with psychiatric illness from those associated with psychotropic medication will have a significant impact on decision-making regarding the treatment of psychiatric illness during pregnancy.
In this study, investigators sought to determine whether a likely diagnosis of PTSD or use of antidepressants or benzodiazepines during pregnancy is associated with the risk of PTB.
Details of the trial
A total of 2,654 women from 137 obstetric practices were interviewed prior to 17 weeks of pregnancy and classified as positive or negative for major depressive episode (MDE) in the past 5 years, antidepressant treatment, or PTSD symptoms. Information regarding prior pregnancies as well as medication use (focusing on benzodiazepines and serotonin reuptake inhibitors [SRIs]), smoking, alcohol and drug use, and pregnancy complications was collected.
Recursive partitioning, simple, and multivariable logistic regression analysis was used to analyze the data.
The researchers found that for each point increase on the Modified PTSD Symptom Scale, the risk of PTB increased by 1% to 2%, suggesting that the presence of PTSD symptoms (even if insufficient to fulfill criteria for a PTSD diagnosis) or a history of trauma is linked to PTB. The greatest risk of PTB in women with likely PTSD was found among those who also reported symptoms of MDE. Women with both conditions (n = 51) had a risk of PTB nearly as high as the risk conferred by having had a previous PTB (adjusted odds ratio [OR], 4.08; 95% confidence interval [CI], 1.27−13.15).
The estimated risk of this pattern of psychiatric comorbidity was much larger than that for either benzodiazepine (n = 67) or SRI (n = 291) treatment, although a risk for PTB was associated with each medication (adjusted OR, 1.99; 95% CI, 0.98−4.03 for benzodiazepines and adjusted OR, 1.55; 95% CI, 1.02−2.36 for SRIs).
This study has considerable strengths, not the least of which is its longitudinal prospective design, which allowed for multiple time points of assessment, as well as the large sample size of patients experiencing PTSD symptoms. In addition, the investigators were able to evaluate PTSD symptoms in a dimensional analysis with varying levels of severity, rather than a single time point with a single categorical assessment.
Some limitations include the study’s inability to consider the role of anxiety disorders other than PTSD, because of the relatively small numbers of those patients. In addition, the assessment of confounding variables did not include psychotropic agents other than SSRIs and benzodiazepines. Increasingly, medications such as mood stabilizers, anticonvulsants, and second-generation antipsychotics are used for primary or adjunctive treatment of mood and trauma-related disorders during pregnancy, and misuse of prescribed medications like opioids can be associated with maternal and fetal stress through withdrawal. The s
tudy’s authors also pointed out that they did not measure biomarkers such as CRH to correlate the stress experienced by patients with likely diagnoses of PTSD with the PTB outcomes.
What this evidence means for practice.
The presence of untreated or unremitting psychiatric symptoms must be viewed as an exposure during pregnancy, along with consideration of the risks associated with treatment for psychiatric conditions. This study adds to the growing body of knowledge that untreated symptoms of anxiety and mood disorders, in this case likely PTSD and likely major depression, during pregnancy can have a significant effect on pregnancy outcome.This study demonstrates that while serotonin reuptake inhibitors and benzodiazepines do increase the risk for PTB, the combination of PTSD symptoms and major depressive symptoms independently increases the risk of PTB—to the same magnitude as a prior history of PTB. —Leena P. Mittal, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The decision about whether to treat any mental health condition during pregnancy is complex, and this study adds to the growing body of knowledge that will inform clinicians and patients about the risks associated with the symptoms of mental health conditions such as posttraumatic stress disorder (PTSD), major depression, and anxiety.
Psychosocial stress during pregnancy has been associated with preterm delivery, possibly related to activation of the hypothalamic-pituitary-adrenal axis, which can result in elevations in maternal glucocorticoids and corticotropin-releasing hormone (CRH). The literature describing the association between stress-related conditions, including PTSD, with preterm birth (PTB), has been inconsistent and limited with regard to power, assessment of the burden of symptoms, and confounding variables including psychiatric comorbidity and psychotropic medications that also have been linked with PTB. Disentangling the risks for PTB associated with psychiatric illness from those associated with psychotropic medication will have a significant impact on decision-making regarding the treatment of psychiatric illness during pregnancy.
In this study, investigators sought to determine whether a likely diagnosis of PTSD or use of antidepressants or benzodiazepines during pregnancy is associated with the risk of PTB.
Details of the trial
A total of 2,654 women from 137 obstetric practices were interviewed prior to 17 weeks of pregnancy and classified as positive or negative for major depressive episode (MDE) in the past 5 years, antidepressant treatment, or PTSD symptoms. Information regarding prior pregnancies as well as medication use (focusing on benzodiazepines and serotonin reuptake inhibitors [SRIs]), smoking, alcohol and drug use, and pregnancy complications was collected.
Recursive partitioning, simple, and multivariable logistic regression analysis was used to analyze the data.
The researchers found that for each point increase on the Modified PTSD Symptom Scale, the risk of PTB increased by 1% to 2%, suggesting that the presence of PTSD symptoms (even if insufficient to fulfill criteria for a PTSD diagnosis) or a history of trauma is linked to PTB. The greatest risk of PTB in women with likely PTSD was found among those who also reported symptoms of MDE. Women with both conditions (n = 51) had a risk of PTB nearly as high as the risk conferred by having had a previous PTB (adjusted odds ratio [OR], 4.08; 95% confidence interval [CI], 1.27−13.15).
The estimated risk of this pattern of psychiatric comorbidity was much larger than that for either benzodiazepine (n = 67) or SRI (n = 291) treatment, although a risk for PTB was associated with each medication (adjusted OR, 1.99; 95% CI, 0.98−4.03 for benzodiazepines and adjusted OR, 1.55; 95% CI, 1.02−2.36 for SRIs).
This study has considerable strengths, not the least of which is its longitudinal prospective design, which allowed for multiple time points of assessment, as well as the large sample size of patients experiencing PTSD symptoms. In addition, the investigators were able to evaluate PTSD symptoms in a dimensional analysis with varying levels of severity, rather than a single time point with a single categorical assessment.
Some limitations include the study’s inability to consider the role of anxiety disorders other than PTSD, because of the relatively small numbers of those patients. In addition, the assessment of confounding variables did not include psychotropic agents other than SSRIs and benzodiazepines. Increasingly, medications such as mood stabilizers, anticonvulsants, and second-generation antipsychotics are used for primary or adjunctive treatment of mood and trauma-related disorders during pregnancy, and misuse of prescribed medications like opioids can be associated with maternal and fetal stress through withdrawal. The s
tudy’s authors also pointed out that they did not measure biomarkers such as CRH to correlate the stress experienced by patients with likely diagnoses of PTSD with the PTB outcomes.
What this evidence means for practice.
The presence of untreated or unremitting psychiatric symptoms must be viewed as an exposure during pregnancy, along with consideration of the risks associated with treatment for psychiatric conditions. This study adds to the growing body of knowledge that untreated symptoms of anxiety and mood disorders, in this case likely PTSD and likely major depression, during pregnancy can have a significant effect on pregnancy outcome.This study demonstrates that while serotonin reuptake inhibitors and benzodiazepines do increase the risk for PTB, the combination of PTSD symptoms and major depressive symptoms independently increases the risk of PTB—to the same magnitude as a prior history of PTB. —Leena P. Mittal, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The decision about whether to treat any mental health condition during pregnancy is complex, and this study adds to the growing body of knowledge that will inform clinicians and patients about the risks associated with the symptoms of mental health conditions such as posttraumatic stress disorder (PTSD), major depression, and anxiety.
Psychosocial stress during pregnancy has been associated with preterm delivery, possibly related to activation of the hypothalamic-pituitary-adrenal axis, which can result in elevations in maternal glucocorticoids and corticotropin-releasing hormone (CRH). The literature describing the association between stress-related conditions, including PTSD, with preterm birth (PTB), has been inconsistent and limited with regard to power, assessment of the burden of symptoms, and confounding variables including psychiatric comorbidity and psychotropic medications that also have been linked with PTB. Disentangling the risks for PTB associated with psychiatric illness from those associated with psychotropic medication will have a significant impact on decision-making regarding the treatment of psychiatric illness during pregnancy.
In this study, investigators sought to determine whether a likely diagnosis of PTSD or use of antidepressants or benzodiazepines during pregnancy is associated with the risk of PTB.
Details of the trial
A total of 2,654 women from 137 obstetric practices were interviewed prior to 17 weeks of pregnancy and classified as positive or negative for major depressive episode (MDE) in the past 5 years, antidepressant treatment, or PTSD symptoms. Information regarding prior pregnancies as well as medication use (focusing on benzodiazepines and serotonin reuptake inhibitors [SRIs]), smoking, alcohol and drug use, and pregnancy complications was collected.
Recursive partitioning, simple, and multivariable logistic regression analysis was used to analyze the data.
The researchers found that for each point increase on the Modified PTSD Symptom Scale, the risk of PTB increased by 1% to 2%, suggesting that the presence of PTSD symptoms (even if insufficient to fulfill criteria for a PTSD diagnosis) or a history of trauma is linked to PTB. The greatest risk of PTB in women with likely PTSD was found among those who also reported symptoms of MDE. Women with both conditions (n = 51) had a risk of PTB nearly as high as the risk conferred by having had a previous PTB (adjusted odds ratio [OR], 4.08; 95% confidence interval [CI], 1.27−13.15).
The estimated risk of this pattern of psychiatric comorbidity was much larger than that for either benzodiazepine (n = 67) or SRI (n = 291) treatment, although a risk for PTB was associated with each medication (adjusted OR, 1.99; 95% CI, 0.98−4.03 for benzodiazepines and adjusted OR, 1.55; 95% CI, 1.02−2.36 for SRIs).
This study has considerable strengths, not the least of which is its longitudinal prospective design, which allowed for multiple time points of assessment, as well as the large sample size of patients experiencing PTSD symptoms. In addition, the investigators were able to evaluate PTSD symptoms in a dimensional analysis with varying levels of severity, rather than a single time point with a single categorical assessment.
Some limitations include the study’s inability to consider the role of anxiety disorders other than PTSD, because of the relatively small numbers of those patients. In addition, the assessment of confounding variables did not include psychotropic agents other than SSRIs and benzodiazepines. Increasingly, medications such as mood stabilizers, anticonvulsants, and second-generation antipsychotics are used for primary or adjunctive treatment of mood and trauma-related disorders during pregnancy, and misuse of prescribed medications like opioids can be associated with maternal and fetal stress through withdrawal. The s
tudy’s authors also pointed out that they did not measure biomarkers such as CRH to correlate the stress experienced by patients with likely diagnoses of PTSD with the PTB outcomes.
What this evidence means for practice.
The presence of untreated or unremitting psychiatric symptoms must be viewed as an exposure during pregnancy, along with consideration of the risks associated with treatment for psychiatric conditions. This study adds to the growing body of knowledge that untreated symptoms of anxiety and mood disorders, in this case likely PTSD and likely major depression, during pregnancy can have a significant effect on pregnancy outcome.This study demonstrates that while serotonin reuptake inhibitors and benzodiazepines do increase the risk for PTB, the combination of PTSD symptoms and major depressive symptoms independently increases the risk of PTB—to the same magnitude as a prior history of PTB. —Leena P. Mittal, MD
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Recurrent urinary tract infection: Who is at risk and best options for management
STOP relying on 2D ultrasound for IUD localization
Several decades ago, the negative publicity associated with the Dalkon Shield brand of intrauterine device (IUD) caused a decline in the use of this form of long-acting reversible contraception (LARC). However, there has been a resurgence in the use of IUDs in the past few years;1 the current opinion of the American College of Obstetricians and Gynecologists encourages first-line use of LARC, and the IUD is popular with patients.2
Related articles:
Let's increase our use of IUDs and improve contraceptive effectiveness in this country. Robert L. Barbieri, MD (Editorial; August 2012)
5 IUD myths dispelled. Anne A. Moore, DNP, APN (September 2-13)
Some patients with IUDs will experience more painful periods, intramenstrual cramps or bleeding, or heavier menses. Until recently, many clinicians (including us) believed these possible adverse effects were not unexpected and often warned patients that it was not abnormal if one or more of these occurred.
More recently, however, results of an important study showed that patients with part of their IUD not totally located within the endometrial cavity (eg, protruded into the cervix or partially piercing the myometrium) had an increased rate of pain and bleeding.3 Of patients with any abnormally located part of their IUD, 36% had abnormal bleeding and 39% had pain, compared with 15% of women who reported abnormal bleeding and 19% who reported pain when their IUD was totally positioned within the endometrial cavity (P = .02 and .03, respectively).
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
A 3D ultrasound can reveal malpositioning not identified on 2D
Prior to the widespread availability of ultrasonography, some practitioners will remember placing a sound in the uterus taped to a tenaculum and obtaining a flat plate and cross table lateral abdominal x-ray to ensure an IUD was indeed “intrauterine.” With the advent of transvaginal ultrasonography, a long-axis view with a centrally located IUD was thought to definitively locate the device as intrauterine (FIGURES 1A, 2A, AND 3A).
Related articles:
How to identify and localize IUDs on ultrasound. Michelle Stalnaker, MD, and Andrew Kaunitz, MD (Images in GYN ultrasound; August 2014)
Update on Contraception. Melissa Chen, MD, MPH, and Mitchell Creinin, MD (August 2014)
Now, with the advent of 3D transvaginal ultrasonography and the ability to construct a coronal plane, some IUDs, which appear to be totally normal on 2D sonography, actually show an arm that pierces the myometrium or protrudes into the cervix (FIGURES 1B, 2B, AND 3B). This in fact is probably the location that must exist at insertion for so-called “migration” to occur through the myometrium as uterine contractions, especially with menses, occur.
So the next time a patient with an IUD reports pain or bleeding, STOP doing only 2D ultrasound and START obtaining a 3D coronal reconstructive view.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD [published correction appears in J Ultrasound Med. 2010;29(12):1848]. J Ultrasound Med. 2010;29(10):1453–1456.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114(6):1434–1438.
3. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110–115.
Several decades ago, the negative publicity associated with the Dalkon Shield brand of intrauterine device (IUD) caused a decline in the use of this form of long-acting reversible contraception (LARC). However, there has been a resurgence in the use of IUDs in the past few years;1 the current opinion of the American College of Obstetricians and Gynecologists encourages first-line use of LARC, and the IUD is popular with patients.2
Related articles:
Let's increase our use of IUDs and improve contraceptive effectiveness in this country. Robert L. Barbieri, MD (Editorial; August 2012)
5 IUD myths dispelled. Anne A. Moore, DNP, APN (September 2-13)
Some patients with IUDs will experience more painful periods, intramenstrual cramps or bleeding, or heavier menses. Until recently, many clinicians (including us) believed these possible adverse effects were not unexpected and often warned patients that it was not abnormal if one or more of these occurred.
More recently, however, results of an important study showed that patients with part of their IUD not totally located within the endometrial cavity (eg, protruded into the cervix or partially piercing the myometrium) had an increased rate of pain and bleeding.3 Of patients with any abnormally located part of their IUD, 36% had abnormal bleeding and 39% had pain, compared with 15% of women who reported abnormal bleeding and 19% who reported pain when their IUD was totally positioned within the endometrial cavity (P = .02 and .03, respectively).
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
A 3D ultrasound can reveal malpositioning not identified on 2D
Prior to the widespread availability of ultrasonography, some practitioners will remember placing a sound in the uterus taped to a tenaculum and obtaining a flat plate and cross table lateral abdominal x-ray to ensure an IUD was indeed “intrauterine.” With the advent of transvaginal ultrasonography, a long-axis view with a centrally located IUD was thought to definitively locate the device as intrauterine (FIGURES 1A, 2A, AND 3A).
Related articles:
How to identify and localize IUDs on ultrasound. Michelle Stalnaker, MD, and Andrew Kaunitz, MD (Images in GYN ultrasound; August 2014)
Update on Contraception. Melissa Chen, MD, MPH, and Mitchell Creinin, MD (August 2014)
Now, with the advent of 3D transvaginal ultrasonography and the ability to construct a coronal plane, some IUDs, which appear to be totally normal on 2D sonography, actually show an arm that pierces the myometrium or protrudes into the cervix (FIGURES 1B, 2B, AND 3B). This in fact is probably the location that must exist at insertion for so-called “migration” to occur through the myometrium as uterine contractions, especially with menses, occur.
So the next time a patient with an IUD reports pain or bleeding, STOP doing only 2D ultrasound and START obtaining a 3D coronal reconstructive view.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Several decades ago, the negative publicity associated with the Dalkon Shield brand of intrauterine device (IUD) caused a decline in the use of this form of long-acting reversible contraception (LARC). However, there has been a resurgence in the use of IUDs in the past few years;1 the current opinion of the American College of Obstetricians and Gynecologists encourages first-line use of LARC, and the IUD is popular with patients.2
Related articles:
Let's increase our use of IUDs and improve contraceptive effectiveness in this country. Robert L. Barbieri, MD (Editorial; August 2012)
5 IUD myths dispelled. Anne A. Moore, DNP, APN (September 2-13)
Some patients with IUDs will experience more painful periods, intramenstrual cramps or bleeding, or heavier menses. Until recently, many clinicians (including us) believed these possible adverse effects were not unexpected and often warned patients that it was not abnormal if one or more of these occurred.
More recently, however, results of an important study showed that patients with part of their IUD not totally located within the endometrial cavity (eg, protruded into the cervix or partially piercing the myometrium) had an increased rate of pain and bleeding.3 Of patients with any abnormally located part of their IUD, 36% had abnormal bleeding and 39% had pain, compared with 15% of women who reported abnormal bleeding and 19% who reported pain when their IUD was totally positioned within the endometrial cavity (P = .02 and .03, respectively).
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
A 3D ultrasound can reveal malpositioning not identified on 2D
Prior to the widespread availability of ultrasonography, some practitioners will remember placing a sound in the uterus taped to a tenaculum and obtaining a flat plate and cross table lateral abdominal x-ray to ensure an IUD was indeed “intrauterine.” With the advent of transvaginal ultrasonography, a long-axis view with a centrally located IUD was thought to definitively locate the device as intrauterine (FIGURES 1A, 2A, AND 3A).
Related articles:
How to identify and localize IUDs on ultrasound. Michelle Stalnaker, MD, and Andrew Kaunitz, MD (Images in GYN ultrasound; August 2014)
Update on Contraception. Melissa Chen, MD, MPH, and Mitchell Creinin, MD (August 2014)
Now, with the advent of 3D transvaginal ultrasonography and the ability to construct a coronal plane, some IUDs, which appear to be totally normal on 2D sonography, actually show an arm that pierces the myometrium or protrudes into the cervix (FIGURES 1B, 2B, AND 3B). This in fact is probably the location that must exist at insertion for so-called “migration” to occur through the myometrium as uterine contractions, especially with menses, occur.
So the next time a patient with an IUD reports pain or bleeding, STOP doing only 2D ultrasound and START obtaining a 3D coronal reconstructive view.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD [published correction appears in J Ultrasound Med. 2010;29(12):1848]. J Ultrasound Med. 2010;29(10):1453–1456.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114(6):1434–1438.
3. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110–115.
1. Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD [published correction appears in J Ultrasound Med. 2010;29(12):1848]. J Ultrasound Med. 2010;29(10):1453–1456.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114(6):1434–1438.
3. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110–115.