User login
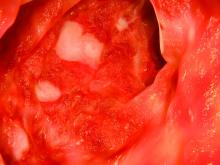
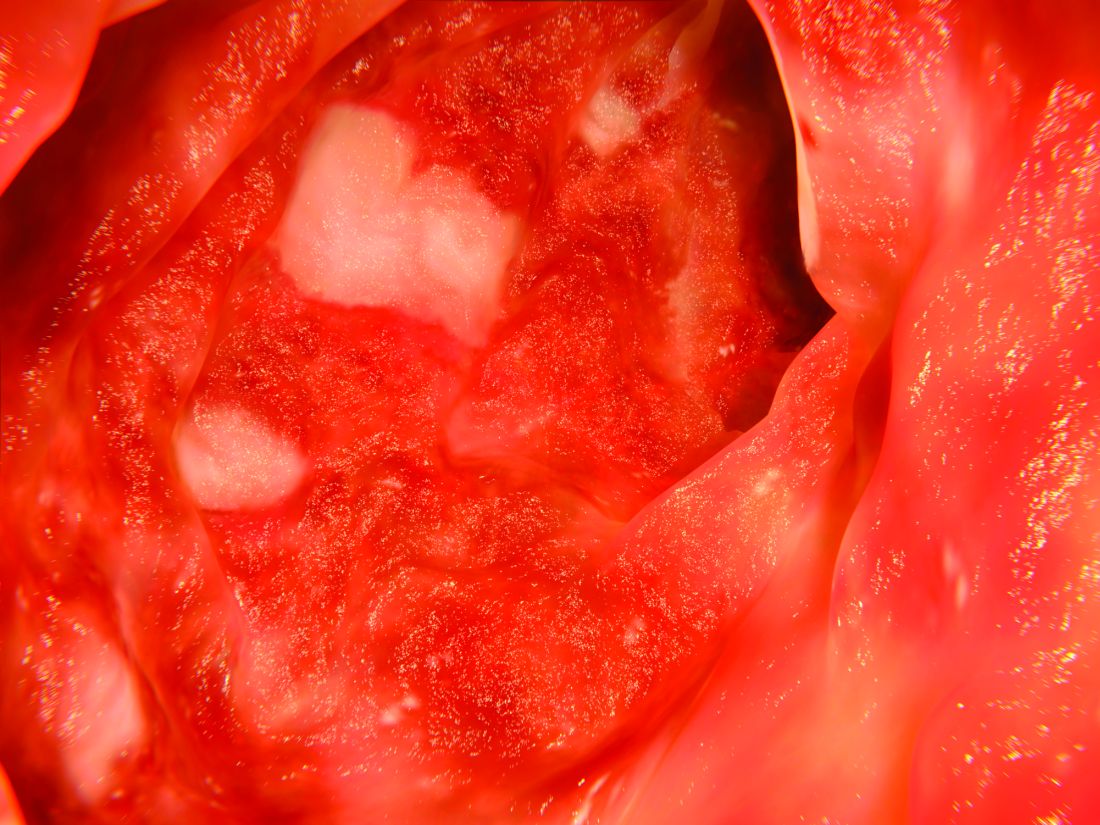
ASCO publishes new guideline for treatment, follow-up of early-stage colorectal cancer
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
An expert panel appointed by the American Society of Clinical Oncology has issued a new guideline for the treatment and follow-up of patients with early-stage colorectal cancer.
The multidisciplinary, multinational panel identified and reviewed previous guidelines from 12 different developers to create the new ASCO guideline; of these, recommendations from six guidelines were adapted into the evidence base. All recommendations have a consensus rate of at least 75%.
For patients with basic, nonobstructing stage I-IIA colon cancer, open resection is recommended; those with enhanced disease should receive laparoscopic or minimally invasive surgery. For nonobstructing stage IIB-IIC colon cancer, recommended treatment for basic disease is open resection; emergency surgical resection is recommended in enhanced disease.
Treatment for basic, obstructing IIB-IIC disease is resection and/or diversion and is emergency surgical resection in enhanced disease. In left-sided, stage IIB-IIC disease, colonic stent placement is recommended. In high-risk, obstructing stage II disease or in T4N0/T3N0 disease with high-risk features, adjuvant chemotherapy is recommended.
In cT1N0 and cT2n0 rectal cancer, total mesorectal excision is recommended; for cT3n0, total mesorectal excision is recommended in basic and limited cases, with diversion recommended in other cases. For resectable cT3N0 rectal cancer, patients should receive base neoadjuvant chemotherapy.
For follow-up, patients should receive a combination of medical history, physical examination, carcinoembryonic antigen testing, imaging, and endoscopy, with the frequency depending on patient setting.
More information, including a data supplement with additional evidence tables, a methodology supplement with information about evidence quality and strength of recommendations, slide sets, and clinical tools and resources is available at www.asco.org/resource-stratified-guidelines, according to the guideline.
Several members of the expert panel reported conflicts of interest.
SOURCE: Costas-Chavarri A et al. J Glob Oncol. 2019 Feb 25. doi: 10.1200/JGO.18.00214.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
AAP: Treating patients during disasters raises liability risks
In a 2019 technical report, the AAP outlines common claims that can arise when treating children during disasters and how certain circumstances can force you to deviate from routine medical practices. In an accompanying policy statement by the AAP committee on medical liability and risk management, recommendations are offered for how to prepare for and prevent such legal risks.
During disasters, liability dangers can increase when circumstances “devolve into an environment of limited choices for both patients and providers,” and you have fewer treatment options available to you, according to the guidance authored by New York pediatrician Dr. Robin L. Altman and her associates.
Common claims that stem from treating patients during disasters are negligence, abandonment, and lack of informed consent. The AAP technical report offers examples about how these accusations can occur, including:
- When during a disaster, you are forced to alter treatment because of scarce medical supplies or equipment, you may later be accused of negligence if the patient’s outcome is negatively affected by the modified treatment.
- When a disaster progresses to overwhelming conditions, and you must practice in an altered health care environment that demands atypical actions, such actions may later be questioned and be accused of providing suboptimal care. Documentation of medical decision making for instance, a primary defense for one’s actions, may be compromised because of an inoperable electronic medical record. Similarly, past medical history may be unavailable, which may impact the appropriateness of care provision.
- In chaotic conditions, you may have to stop treating some patients to focus their time and resources elsewhere, which may lead to an abandonment claim, defined as unilateral termination of a physician-patient relationship – without proper patient notice – when treatment is still required. An abandonment claim also may arise when you have to make decisions in extreme conditions about which patients to transfer or evacuate first and whom to leave behind.
- When providing medical care to children during disasters, a lack of informed consent claim can arise if adequate parental permission is unattainable. This may result from families that are separated or displaced children in need of medical care.
Other claims that can arise from providing care during disasters include HIPAA breaches, licensing violations, discrimination claims, and Emergency Medical Treatment and Labor Act (EMTALA) violations, among others.
To reduce liability risks, you should strive to understand liability risks and limitations during disasters and take steps to mitigate them by crafting a disaster readiness plan, according to the AAP policy statement. The plan should include provider and staff education on improving medical care during disasters and how best to document medical decisions made in disaster-affected health care environments. Proactively identifying obstacles to care during disasters also is key. You can use the AAP division of state government affairs as a resource; it can provide current information on disaster liability in the different states.
You also should understand potential limits to your medical malpractice insurance coverage during disasters and take steps to add coverage for identified gaps, according to the AAP guidelines.
AAP recommends that you advocate for your health center to have active disaster plans that cover children’s needs and for your hospital to conduct regular drills that test pediatric capabilities. Throughout the guidelines, the AAP calls on the U.S. Department of Health and Human Services to review current state and federal liability laws, and for the agency to recommend new laws that address disaster-response liability protections for doctors. HHS also should assess the liability coverage needs of physicians during crisis times and take action to reduce inconsistencies in state malpractice protections for volunteer physicians and nonvolunteer physicians, according to AAP.
The AAP policy statement is timely because of the number of recent disasters in the United States, said Dr. Altman, lead author of the two papers.
Citing the Federal Emergency Management Agency, Dr. Altman said there were 59 major disaster declarations and 16 emergency declarations in 2017, along with more than 300 mass shooting incidents and more than 110 other man-made disasters such as fires and industrial accidents.
“Disaster conditions can result in pediatric health care providers being faced with the need to address medical conditions outside of their scope of training and experience, without access to the usual fund of patient history and background information, without the usual input or consent from parents or guardians, without the usual assistance of data such as laboratory values or physiologic monitoring, and without knowledge of how long dire conditions will last,” Dr. Altman said in an AAP News statement. “In addition, this can occur within the backdrop of one’s own physical exhaustion, concerns for the safety of one’s own family members, and the risk of loss of valuable and expensive professional property and supplies.”
The AAP guidance can help pediatricians understand the unique professional liability risks that may occur when caring for pediatric patients and families during a disaster, she said.
“It is the hope that this will raise awareness, improve preparedness, and reduce potential deficiencies in professional liability protections for health care providers trying to do their best to care for patients during these infrequent, yet debilitating, events,” Dr. Altman said in the statement.
There was no external funding, and the authors indicated they had no relevant financial disclosures.
SOURCES: Pediatrics. 2019. doi: 10.1542/peds.2018-3892; Pediatrics. 2019. doi: 10.1542/peds.2018-3893.
In a 2019 technical report, the AAP outlines common claims that can arise when treating children during disasters and how certain circumstances can force you to deviate from routine medical practices. In an accompanying policy statement by the AAP committee on medical liability and risk management, recommendations are offered for how to prepare for and prevent such legal risks.
During disasters, liability dangers can increase when circumstances “devolve into an environment of limited choices for both patients and providers,” and you have fewer treatment options available to you, according to the guidance authored by New York pediatrician Dr. Robin L. Altman and her associates.
Common claims that stem from treating patients during disasters are negligence, abandonment, and lack of informed consent. The AAP technical report offers examples about how these accusations can occur, including:
- When during a disaster, you are forced to alter treatment because of scarce medical supplies or equipment, you may later be accused of negligence if the patient’s outcome is negatively affected by the modified treatment.
- When a disaster progresses to overwhelming conditions, and you must practice in an altered health care environment that demands atypical actions, such actions may later be questioned and be accused of providing suboptimal care. Documentation of medical decision making for instance, a primary defense for one’s actions, may be compromised because of an inoperable electronic medical record. Similarly, past medical history may be unavailable, which may impact the appropriateness of care provision.
- In chaotic conditions, you may have to stop treating some patients to focus their time and resources elsewhere, which may lead to an abandonment claim, defined as unilateral termination of a physician-patient relationship – without proper patient notice – when treatment is still required. An abandonment claim also may arise when you have to make decisions in extreme conditions about which patients to transfer or evacuate first and whom to leave behind.
- When providing medical care to children during disasters, a lack of informed consent claim can arise if adequate parental permission is unattainable. This may result from families that are separated or displaced children in need of medical care.
Other claims that can arise from providing care during disasters include HIPAA breaches, licensing violations, discrimination claims, and Emergency Medical Treatment and Labor Act (EMTALA) violations, among others.
To reduce liability risks, you should strive to understand liability risks and limitations during disasters and take steps to mitigate them by crafting a disaster readiness plan, according to the AAP policy statement. The plan should include provider and staff education on improving medical care during disasters and how best to document medical decisions made in disaster-affected health care environments. Proactively identifying obstacles to care during disasters also is key. You can use the AAP division of state government affairs as a resource; it can provide current information on disaster liability in the different states.
You also should understand potential limits to your medical malpractice insurance coverage during disasters and take steps to add coverage for identified gaps, according to the AAP guidelines.
AAP recommends that you advocate for your health center to have active disaster plans that cover children’s needs and for your hospital to conduct regular drills that test pediatric capabilities. Throughout the guidelines, the AAP calls on the U.S. Department of Health and Human Services to review current state and federal liability laws, and for the agency to recommend new laws that address disaster-response liability protections for doctors. HHS also should assess the liability coverage needs of physicians during crisis times and take action to reduce inconsistencies in state malpractice protections for volunteer physicians and nonvolunteer physicians, according to AAP.
The AAP policy statement is timely because of the number of recent disasters in the United States, said Dr. Altman, lead author of the two papers.
Citing the Federal Emergency Management Agency, Dr. Altman said there were 59 major disaster declarations and 16 emergency declarations in 2017, along with more than 300 mass shooting incidents and more than 110 other man-made disasters such as fires and industrial accidents.
“Disaster conditions can result in pediatric health care providers being faced with the need to address medical conditions outside of their scope of training and experience, without access to the usual fund of patient history and background information, without the usual input or consent from parents or guardians, without the usual assistance of data such as laboratory values or physiologic monitoring, and without knowledge of how long dire conditions will last,” Dr. Altman said in an AAP News statement. “In addition, this can occur within the backdrop of one’s own physical exhaustion, concerns for the safety of one’s own family members, and the risk of loss of valuable and expensive professional property and supplies.”
The AAP guidance can help pediatricians understand the unique professional liability risks that may occur when caring for pediatric patients and families during a disaster, she said.
“It is the hope that this will raise awareness, improve preparedness, and reduce potential deficiencies in professional liability protections for health care providers trying to do their best to care for patients during these infrequent, yet debilitating, events,” Dr. Altman said in the statement.
There was no external funding, and the authors indicated they had no relevant financial disclosures.
SOURCES: Pediatrics. 2019. doi: 10.1542/peds.2018-3892; Pediatrics. 2019. doi: 10.1542/peds.2018-3893.
In a 2019 technical report, the AAP outlines common claims that can arise when treating children during disasters and how certain circumstances can force you to deviate from routine medical practices. In an accompanying policy statement by the AAP committee on medical liability and risk management, recommendations are offered for how to prepare for and prevent such legal risks.
During disasters, liability dangers can increase when circumstances “devolve into an environment of limited choices for both patients and providers,” and you have fewer treatment options available to you, according to the guidance authored by New York pediatrician Dr. Robin L. Altman and her associates.
Common claims that stem from treating patients during disasters are negligence, abandonment, and lack of informed consent. The AAP technical report offers examples about how these accusations can occur, including:
- When during a disaster, you are forced to alter treatment because of scarce medical supplies or equipment, you may later be accused of negligence if the patient’s outcome is negatively affected by the modified treatment.
- When a disaster progresses to overwhelming conditions, and you must practice in an altered health care environment that demands atypical actions, such actions may later be questioned and be accused of providing suboptimal care. Documentation of medical decision making for instance, a primary defense for one’s actions, may be compromised because of an inoperable electronic medical record. Similarly, past medical history may be unavailable, which may impact the appropriateness of care provision.
- In chaotic conditions, you may have to stop treating some patients to focus their time and resources elsewhere, which may lead to an abandonment claim, defined as unilateral termination of a physician-patient relationship – without proper patient notice – when treatment is still required. An abandonment claim also may arise when you have to make decisions in extreme conditions about which patients to transfer or evacuate first and whom to leave behind.
- When providing medical care to children during disasters, a lack of informed consent claim can arise if adequate parental permission is unattainable. This may result from families that are separated or displaced children in need of medical care.
Other claims that can arise from providing care during disasters include HIPAA breaches, licensing violations, discrimination claims, and Emergency Medical Treatment and Labor Act (EMTALA) violations, among others.
To reduce liability risks, you should strive to understand liability risks and limitations during disasters and take steps to mitigate them by crafting a disaster readiness plan, according to the AAP policy statement. The plan should include provider and staff education on improving medical care during disasters and how best to document medical decisions made in disaster-affected health care environments. Proactively identifying obstacles to care during disasters also is key. You can use the AAP division of state government affairs as a resource; it can provide current information on disaster liability in the different states.
You also should understand potential limits to your medical malpractice insurance coverage during disasters and take steps to add coverage for identified gaps, according to the AAP guidelines.
AAP recommends that you advocate for your health center to have active disaster plans that cover children’s needs and for your hospital to conduct regular drills that test pediatric capabilities. Throughout the guidelines, the AAP calls on the U.S. Department of Health and Human Services to review current state and federal liability laws, and for the agency to recommend new laws that address disaster-response liability protections for doctors. HHS also should assess the liability coverage needs of physicians during crisis times and take action to reduce inconsistencies in state malpractice protections for volunteer physicians and nonvolunteer physicians, according to AAP.
The AAP policy statement is timely because of the number of recent disasters in the United States, said Dr. Altman, lead author of the two papers.
Citing the Federal Emergency Management Agency, Dr. Altman said there were 59 major disaster declarations and 16 emergency declarations in 2017, along with more than 300 mass shooting incidents and more than 110 other man-made disasters such as fires and industrial accidents.
“Disaster conditions can result in pediatric health care providers being faced with the need to address medical conditions outside of their scope of training and experience, without access to the usual fund of patient history and background information, without the usual input or consent from parents or guardians, without the usual assistance of data such as laboratory values or physiologic monitoring, and without knowledge of how long dire conditions will last,” Dr. Altman said in an AAP News statement. “In addition, this can occur within the backdrop of one’s own physical exhaustion, concerns for the safety of one’s own family members, and the risk of loss of valuable and expensive professional property and supplies.”
The AAP guidance can help pediatricians understand the unique professional liability risks that may occur when caring for pediatric patients and families during a disaster, she said.
“It is the hope that this will raise awareness, improve preparedness, and reduce potential deficiencies in professional liability protections for health care providers trying to do their best to care for patients during these infrequent, yet debilitating, events,” Dr. Altman said in the statement.
There was no external funding, and the authors indicated they had no relevant financial disclosures.
SOURCES: Pediatrics. 2019. doi: 10.1542/peds.2018-3892; Pediatrics. 2019. doi: 10.1542/peds.2018-3893.
ACOG: Avoid inductions before 39 weeks unless medically necessary
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
FROM OBSTETRICS & GYNECOLOGY
AGA Clinical Practice Update: Changing utility of serology and histologic measures in celiac disease
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
For children and adolescents with strong clinical suspicion for celiac disease, repeated transglutaminase-2-IgA (TG2-IgA) levels that are more than 10 times higher than the upper limit of normal often suffices for diagnosis, according to an American Gastroenterological Association clinical practice update and expert review.
This approach precludes the need for esophagogastroduodenoscopy (EGD) in about 30%-50% of cases, wrote Steffen Husby, MD, PhD, of Odense University Hospital (Denmark), together with his associates in Gastroenterology. “When such a strongly positive TG2-IgA is combined with a positive endomysial antibody in a second blood sample, the positive predictive value for celiac disease is virtually 100%.” But for adults, they recommend confirmatory histologic analysis of duodenal biopsies with Marsh classification, counting of lymphocytes per high-power field, and morphometry.
Transglutaminase-2 is the major autoantigen present in celiac disease and can now be assessed with accurate, convenient, high-throughput tests, such as enzyme-linked immunosorbent assays. To maximize test TG2-IgA accuracy, Dr. Husby and his associates recommend testing patients who have compatible signs and symptoms of celiac disease or are asymptomatic but have other risk factors, such as confirmed autoimmune diseases (type 1 diabetes, autoimmune thyroid or liver diseases), chromosome abnormalities (Down or Turner syndrome), or first-degree relatives with celiac disease.
Several other serologic tests are available but have a more limited role in diagnosing celiac disease, according to the practice update. Perhaps most useful is the endomysial antibody (EMA) test, which evaluates tissue-bound TG2-IgA. This test is highly specific but labor-intensive and user-sensitive and thus is best used to confirm a positive TG2-IgA result. Deamidated gliadin peptide antibody assays are less accurate than TG2-IgA, while HLA-DQ2/DQ8 testing is best reserved for cases where the diagnosis is complicated by a prior gluten-free diet or inconclusive antibody titers or histology.
For adults from populations with less than a 5% prevalence of celiac disease, all guidelines recommend following serology with confirmatory biopsy, and the experts concur. If biopsy was part of the initial work-up, they recommend performing confirmatory serology before starting a gluten-free diet. If the biopsy was negative but celiac disease is strongly suspected, they recommend TG2-IgA testing followed by repeat biopsies, when possible, either at the same time or in the future.
For children with suspected celiac disease, the North American Society for Pediatric Gastroenterology Hepatology and Nutrition recommends starting with biopsy, while the European Society for Paediatric Gastroenterology Hepatology and Nutrition suggests starting with quantitative TG2-IgA testing, followed by TG2-IgA, EMA, or HLA-DQ2/DQ8 assays if TG2-IgA is 10 times higher than the upper limit of normal. However, EGD with biopsies and even a gluten challenge may be needed if serology results are unclear, the experts state. They recommend against gluten-free or low-gluten diets prior to diagnosis, since these can lower the sensitivity of both histology and serology. If a patient has unclear test results and is already on a gluten-free diet, they suggest resuming eating three slices of wheat bread daily for 1-3 months, followed by TG2-IgA testing.
A small but important subgroup of patients have strong suspicion for celiac disease but are negative on IgA isotype tests because of IgA deficiency. In such suspected cases, the experts recommend measuring total IgA, IgG deamidated gliadin antibodies, and TG2-IgG levels. They note that IgG isotype testing for TG2 antibodies is not celiac specific outside the setting of IgA deficiency.
Serology has a useful but more limited role in managing celiac disease, according to the practice update. Negative TG2-IgA and other serology does not guarantee that the intestinal mucosa has healed, so patients with ongoing or relapsing symptoms without another obvious cause should have repeat biopsies. However, serology that stays positive over time usually indicates ongoing mucosal damage and gluten exposure, so these follow-up tests are appropriate 6 and 12 months after diagnosing celiac disease and yearly thereafter.
Dr. Husby reported receiving grant support from the University of Southern Denmark, the Region of Southern Denmark, and the Novo Nordisk Research Fund. He also reported receiving payments from Thermo Fisher Scientific and an advisory relationship with Inova. Two coauthors reported ties to Alba Therapeutics, Celimmune, Intrexon, GlaxoSmithKline, and several other pharmaceutical companies.
SOURCE: Husby S et al. Gastroenterology. 2018 Dec 19. doi: 10.1053/j.gastro.2018.12.010.
FROM GASTROENTEROLOGY
AHA: Consider obesity as CVD risk factor in children
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
FROM CIRCULATION
AACE/ACE algorithm provides practical clinical guidance on managing diabetes
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
FROM ENDOCRINE PRACTICE
AGA Guideline: Treatment of mild to moderate ulcerative colitis
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
FROM GASTROENTEROLOGY
American Heart Association guideline on the management of blood cholesterol
The purpose of this guideline is to provide direction for the management of patients with high blood cholesterol to decrease the incidence of atherosclerotic vascular disease. The update was undertaken because new evidence has emerged since the publication of the 2013 ACC/AHA cholesterol guideline about additional cholesterol-lowering agents including ezetimibe and PCSK9 inhibitors.
Measurement and therapeutic modalities
In adults aged 20 years and older who are not on lipid-lowering therapy, measurement of a lipid profile is recommended and is an effective way to estimate atherosclerotic cardiovascular disease (ASCVD) risk and documenting baseline LDL-C.
Statin therapy is divided into three categories: High-intensity statin therapy aims for lowering LDL-C levels by more than 50%, moderate-intensity therapy by 30%-49%, and low-intensity therapy by less than 30%.
Cholesterol management groups
In all individuals at all ages, emphasizing a heart-healthy lifestyle, meaning appropriate diet and exercise, to decrease the risk of developing ASCVD should be advised.
Individuals fall into groups with distinct risk of ASCVD or recurrence of ASCVD and the recommendations are organized according to these risk groups.
Secondary ASCVD prevention: Patients who already have ASCVD by virtue of having had an event or established diagnosis (MI, angina, cerebrovascular accident, or peripheral vascular disease) fall into the secondary prevention category:
- Patients aged 75 years and younger with clinical ASCVD: High-intensity statin therapy should be initiated with aim to reduce LDL-C levels by 50%. In patients who experience statin-related side effects, a moderate-intensity statin should be initiated with the aim to reduce LDL-C by 30%-49%.
- In very high-risk patients with an LDL-C above 70 mg/dL on maximally tolerated statin therapy, it is reasonable to consider the use of a non–statin cholesterol-lowering agent with an LDL-C goal under 70 mg/dL. Ezetimibe (Zetia) can be used initially and if LDL-C remains above 70 mg/dL, then consideration can be given to the addition of a PCSK9-inhibitor therapy (strength of recommendation: ezetimibe – moderate; PCSK9 – strong). The guideline discusses that, even though the evidence supports the efficacy of PCSK9s in reducing the incidence of ASCVD events, the expense of PCSK9 inhibitors give them a high cost, compared with value.
- For patients more than age 75 years with established ASCVD, it is reasonable to continue high-intensity statin therapy if patient is tolerating treatment.
Severe hypercholesterolemia:
- Patients with LDL-C above 190 mg/dL do not need a 10-year risk score calculated. These individuals should receive maximally tolerated statin therapy.
- If patient is unable to achieve 50% reduction in LDL-C and/or have an LDL-C level of 100 mg/dL, the addition of ezetimibe therapy is reasonable.
- If LDL-C is still greater than 100mg/dL on a statin plus ezetimibe, the addition of a PCSK9 inhibitor may be considered. It should be recognized that the addition of a PCSK9 in this circumstance is classified as a weak recommendation.
Diabetes mellitus in adults:
- In patients aged 40-75 years with diabetes, regardless of 10-year ASCVD risk, should be prescribed a moderate-intensity statin (strong recommendation).
- In adults with diabetes mellitus and multiple ASCVD risk factors, it is reasonable to prescribe high-intensity statin therapy with goal to reduce LDL-C by more than 50%.
- In adults with diabetes mellitus and 10-year ASCVD risk of 20% or higher, it may be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce LDL-C levels by 50% or more.
- In patients aged 20-39 years with diabetes that is either of long duration (at least 10 years, type 2 diabetes mellitus; at least 20 years, type 1 diabetes mellitus), or with end-organ damage including albuminuria, chronic renal insufficiency, retinopathy, neuropathy, or ankle-brachial index below 0.9, it may be reasonable to initiate statin therapy (weak recommendation).
Primary prevention in adults: Adults with LDL 70-189 mg/dL and a 10-year risk of a first ASCVD event (fatal and nonfatal MI or stroke) should be estimated by using the pooled cohort equation. Adults should be categorized according to calculated risk of developing ASCVD: low risk (less than 5%), borderline risk (5% to less than 7.5%), intermediate risk (7.5% and higher to less than 20%), and high risk (20% and higher) (strong recommendation:
- Individualized risk and treatment discussion should be done with clinician and patient.
- Adults in the intermediate-risk group (7.5% and higher to less than 20%), should be placed on moderate-intensity statin with LDL-C goal reduction of more than 30%; for optimal risk reduction, especially in high-risk patients, an LDL-C reduction of more than 50% (strong recommendation).
- Risk-enhancing factors can favor initiation of intensification of statin therapy.
- If a decision about statin therapy is uncertain, consider measuring coronary artery calcium (CAC) levels. If CAC is zero, statin therapy may be withheld or delayed, except those with diabetes as above, smokers, and strong familial hypercholesterolemia with premature ASCVD. If CAC score is 1-99, it is reasonable to initiate statin therapy for patients older than age 55 years; If CAC score is 100 or higher or in the 75th percentile or higher, it is reasonable to initiate a statin.
Statin safety: Prior to initiation of a statin, a clinician-patient discussion is recommended detailing ASCVD risk reduction and the potential for side effects/drug interactions. In patients with statin-associated muscle symptoms (SAMS), a detailed account for secondary causes is recommended. In patients with true SAMS, it is recommended to check a creatine kinase level and hepatic function panel; however, routine measurements are not useful. In patients with statin-associated side effects that are not severe, reassess and rechallenge patient to achieve maximal lowering of LDL-C with a modified dosing regimen.
The bottom line
Lifestyle modification is important at all ages, with specific population-guided strategies for lowering cholesterol in subgroups as discussed above. Major changes to the AHA/ACC Cholesterol Clinical Practice Guidelines now mention new agents for lowering cholesterol and using CAC levels as predictability scoring.
Reference
Grundy SM et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018 Nov 10.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Palko is a second-year resident in the family medicine residency program at Abington Jefferson Hospital.
The purpose of this guideline is to provide direction for the management of patients with high blood cholesterol to decrease the incidence of atherosclerotic vascular disease. The update was undertaken because new evidence has emerged since the publication of the 2013 ACC/AHA cholesterol guideline about additional cholesterol-lowering agents including ezetimibe and PCSK9 inhibitors.
Measurement and therapeutic modalities
In adults aged 20 years and older who are not on lipid-lowering therapy, measurement of a lipid profile is recommended and is an effective way to estimate atherosclerotic cardiovascular disease (ASCVD) risk and documenting baseline LDL-C.
Statin therapy is divided into three categories: High-intensity statin therapy aims for lowering LDL-C levels by more than 50%, moderate-intensity therapy by 30%-49%, and low-intensity therapy by less than 30%.
Cholesterol management groups
In all individuals at all ages, emphasizing a heart-healthy lifestyle, meaning appropriate diet and exercise, to decrease the risk of developing ASCVD should be advised.
Individuals fall into groups with distinct risk of ASCVD or recurrence of ASCVD and the recommendations are organized according to these risk groups.
Secondary ASCVD prevention: Patients who already have ASCVD by virtue of having had an event or established diagnosis (MI, angina, cerebrovascular accident, or peripheral vascular disease) fall into the secondary prevention category:
- Patients aged 75 years and younger with clinical ASCVD: High-intensity statin therapy should be initiated with aim to reduce LDL-C levels by 50%. In patients who experience statin-related side effects, a moderate-intensity statin should be initiated with the aim to reduce LDL-C by 30%-49%.
- In very high-risk patients with an LDL-C above 70 mg/dL on maximally tolerated statin therapy, it is reasonable to consider the use of a non–statin cholesterol-lowering agent with an LDL-C goal under 70 mg/dL. Ezetimibe (Zetia) can be used initially and if LDL-C remains above 70 mg/dL, then consideration can be given to the addition of a PCSK9-inhibitor therapy (strength of recommendation: ezetimibe – moderate; PCSK9 – strong). The guideline discusses that, even though the evidence supports the efficacy of PCSK9s in reducing the incidence of ASCVD events, the expense of PCSK9 inhibitors give them a high cost, compared with value.
- For patients more than age 75 years with established ASCVD, it is reasonable to continue high-intensity statin therapy if patient is tolerating treatment.
Severe hypercholesterolemia:
- Patients with LDL-C above 190 mg/dL do not need a 10-year risk score calculated. These individuals should receive maximally tolerated statin therapy.
- If patient is unable to achieve 50% reduction in LDL-C and/or have an LDL-C level of 100 mg/dL, the addition of ezetimibe therapy is reasonable.
- If LDL-C is still greater than 100mg/dL on a statin plus ezetimibe, the addition of a PCSK9 inhibitor may be considered. It should be recognized that the addition of a PCSK9 in this circumstance is classified as a weak recommendation.
Diabetes mellitus in adults:
- In patients aged 40-75 years with diabetes, regardless of 10-year ASCVD risk, should be prescribed a moderate-intensity statin (strong recommendation).
- In adults with diabetes mellitus and multiple ASCVD risk factors, it is reasonable to prescribe high-intensity statin therapy with goal to reduce LDL-C by more than 50%.
- In adults with diabetes mellitus and 10-year ASCVD risk of 20% or higher, it may be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce LDL-C levels by 50% or more.
- In patients aged 20-39 years with diabetes that is either of long duration (at least 10 years, type 2 diabetes mellitus; at least 20 years, type 1 diabetes mellitus), or with end-organ damage including albuminuria, chronic renal insufficiency, retinopathy, neuropathy, or ankle-brachial index below 0.9, it may be reasonable to initiate statin therapy (weak recommendation).
Primary prevention in adults: Adults with LDL 70-189 mg/dL and a 10-year risk of a first ASCVD event (fatal and nonfatal MI or stroke) should be estimated by using the pooled cohort equation. Adults should be categorized according to calculated risk of developing ASCVD: low risk (less than 5%), borderline risk (5% to less than 7.5%), intermediate risk (7.5% and higher to less than 20%), and high risk (20% and higher) (strong recommendation:
- Individualized risk and treatment discussion should be done with clinician and patient.
- Adults in the intermediate-risk group (7.5% and higher to less than 20%), should be placed on moderate-intensity statin with LDL-C goal reduction of more than 30%; for optimal risk reduction, especially in high-risk patients, an LDL-C reduction of more than 50% (strong recommendation).
- Risk-enhancing factors can favor initiation of intensification of statin therapy.
- If a decision about statin therapy is uncertain, consider measuring coronary artery calcium (CAC) levels. If CAC is zero, statin therapy may be withheld or delayed, except those with diabetes as above, smokers, and strong familial hypercholesterolemia with premature ASCVD. If CAC score is 1-99, it is reasonable to initiate statin therapy for patients older than age 55 years; If CAC score is 100 or higher or in the 75th percentile or higher, it is reasonable to initiate a statin.
Statin safety: Prior to initiation of a statin, a clinician-patient discussion is recommended detailing ASCVD risk reduction and the potential for side effects/drug interactions. In patients with statin-associated muscle symptoms (SAMS), a detailed account for secondary causes is recommended. In patients with true SAMS, it is recommended to check a creatine kinase level and hepatic function panel; however, routine measurements are not useful. In patients with statin-associated side effects that are not severe, reassess and rechallenge patient to achieve maximal lowering of LDL-C with a modified dosing regimen.
The bottom line
Lifestyle modification is important at all ages, with specific population-guided strategies for lowering cholesterol in subgroups as discussed above. Major changes to the AHA/ACC Cholesterol Clinical Practice Guidelines now mention new agents for lowering cholesterol and using CAC levels as predictability scoring.
Reference
Grundy SM et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018 Nov 10.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Palko is a second-year resident in the family medicine residency program at Abington Jefferson Hospital.
The purpose of this guideline is to provide direction for the management of patients with high blood cholesterol to decrease the incidence of atherosclerotic vascular disease. The update was undertaken because new evidence has emerged since the publication of the 2013 ACC/AHA cholesterol guideline about additional cholesterol-lowering agents including ezetimibe and PCSK9 inhibitors.
Measurement and therapeutic modalities
In adults aged 20 years and older who are not on lipid-lowering therapy, measurement of a lipid profile is recommended and is an effective way to estimate atherosclerotic cardiovascular disease (ASCVD) risk and documenting baseline LDL-C.
Statin therapy is divided into three categories: High-intensity statin therapy aims for lowering LDL-C levels by more than 50%, moderate-intensity therapy by 30%-49%, and low-intensity therapy by less than 30%.
Cholesterol management groups
In all individuals at all ages, emphasizing a heart-healthy lifestyle, meaning appropriate diet and exercise, to decrease the risk of developing ASCVD should be advised.
Individuals fall into groups with distinct risk of ASCVD or recurrence of ASCVD and the recommendations are organized according to these risk groups.
Secondary ASCVD prevention: Patients who already have ASCVD by virtue of having had an event or established diagnosis (MI, angina, cerebrovascular accident, or peripheral vascular disease) fall into the secondary prevention category:
- Patients aged 75 years and younger with clinical ASCVD: High-intensity statin therapy should be initiated with aim to reduce LDL-C levels by 50%. In patients who experience statin-related side effects, a moderate-intensity statin should be initiated with the aim to reduce LDL-C by 30%-49%.
- In very high-risk patients with an LDL-C above 70 mg/dL on maximally tolerated statin therapy, it is reasonable to consider the use of a non–statin cholesterol-lowering agent with an LDL-C goal under 70 mg/dL. Ezetimibe (Zetia) can be used initially and if LDL-C remains above 70 mg/dL, then consideration can be given to the addition of a PCSK9-inhibitor therapy (strength of recommendation: ezetimibe – moderate; PCSK9 – strong). The guideline discusses that, even though the evidence supports the efficacy of PCSK9s in reducing the incidence of ASCVD events, the expense of PCSK9 inhibitors give them a high cost, compared with value.
- For patients more than age 75 years with established ASCVD, it is reasonable to continue high-intensity statin therapy if patient is tolerating treatment.
Severe hypercholesterolemia:
- Patients with LDL-C above 190 mg/dL do not need a 10-year risk score calculated. These individuals should receive maximally tolerated statin therapy.
- If patient is unable to achieve 50% reduction in LDL-C and/or have an LDL-C level of 100 mg/dL, the addition of ezetimibe therapy is reasonable.
- If LDL-C is still greater than 100mg/dL on a statin plus ezetimibe, the addition of a PCSK9 inhibitor may be considered. It should be recognized that the addition of a PCSK9 in this circumstance is classified as a weak recommendation.
Diabetes mellitus in adults:
- In patients aged 40-75 years with diabetes, regardless of 10-year ASCVD risk, should be prescribed a moderate-intensity statin (strong recommendation).
- In adults with diabetes mellitus and multiple ASCVD risk factors, it is reasonable to prescribe high-intensity statin therapy with goal to reduce LDL-C by more than 50%.
- In adults with diabetes mellitus and 10-year ASCVD risk of 20% or higher, it may be reasonable to add ezetimibe to maximally tolerated statin therapy to reduce LDL-C levels by 50% or more.
- In patients aged 20-39 years with diabetes that is either of long duration (at least 10 years, type 2 diabetes mellitus; at least 20 years, type 1 diabetes mellitus), or with end-organ damage including albuminuria, chronic renal insufficiency, retinopathy, neuropathy, or ankle-brachial index below 0.9, it may be reasonable to initiate statin therapy (weak recommendation).
Primary prevention in adults: Adults with LDL 70-189 mg/dL and a 10-year risk of a first ASCVD event (fatal and nonfatal MI or stroke) should be estimated by using the pooled cohort equation. Adults should be categorized according to calculated risk of developing ASCVD: low risk (less than 5%), borderline risk (5% to less than 7.5%), intermediate risk (7.5% and higher to less than 20%), and high risk (20% and higher) (strong recommendation:
- Individualized risk and treatment discussion should be done with clinician and patient.
- Adults in the intermediate-risk group (7.5% and higher to less than 20%), should be placed on moderate-intensity statin with LDL-C goal reduction of more than 30%; for optimal risk reduction, especially in high-risk patients, an LDL-C reduction of more than 50% (strong recommendation).
- Risk-enhancing factors can favor initiation of intensification of statin therapy.
- If a decision about statin therapy is uncertain, consider measuring coronary artery calcium (CAC) levels. If CAC is zero, statin therapy may be withheld or delayed, except those with diabetes as above, smokers, and strong familial hypercholesterolemia with premature ASCVD. If CAC score is 1-99, it is reasonable to initiate statin therapy for patients older than age 55 years; If CAC score is 100 or higher or in the 75th percentile or higher, it is reasonable to initiate a statin.
Statin safety: Prior to initiation of a statin, a clinician-patient discussion is recommended detailing ASCVD risk reduction and the potential for side effects/drug interactions. In patients with statin-associated muscle symptoms (SAMS), a detailed account for secondary causes is recommended. In patients with true SAMS, it is recommended to check a creatine kinase level and hepatic function panel; however, routine measurements are not useful. In patients with statin-associated side effects that are not severe, reassess and rechallenge patient to achieve maximal lowering of LDL-C with a modified dosing regimen.
The bottom line
Lifestyle modification is important at all ages, with specific population-guided strategies for lowering cholesterol in subgroups as discussed above. Major changes to the AHA/ACC Cholesterol Clinical Practice Guidelines now mention new agents for lowering cholesterol and using CAC levels as predictability scoring.
Reference
Grundy SM et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018 Nov 10.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Palko is a second-year resident in the family medicine residency program at Abington Jefferson Hospital.
Revised U.S. A fib guidelines revamp anticoagulation
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
Imaging, radiotherapy clarified in new PMBCL guidelines
Fertility preservation, imaging and radiotherapy guidelines, and best practices in relapse or salvage therapy for primary mediastinal B-cell lymphoma (PMBCL) are all highlighted in a new good practice paper from the British Society for Haematology.
Though PMBCL was previously thought of as a subtype of diffuse large B-cell lymphoma, “gene expression profiling data has shown it to be a separate clinicopathological entity with evidence of an overlap with classic Hodgkin lymphoma,” said Kate Cwynarski, MD, PhD, of University College London Hospitals NHS Foundation Trust in England, and her coauthors. The recommendations were published in the British Journal of Haematology.
PMBCL makes up 2%-4% of non-Hodgkin lymphomas, they said; a bulky anterior mediastinal mass is the usual initial presentation. PMBCL does not usually spread beyond the thoracic cavity.
Biopsy, which should be reviewed by a hematopathologist, is required for a histological diagnosis of PMBCL. A multidisciplinary team should review the clinical presentation, pathology, and management plan, according to the good practice paper authors. This was a strong recommendation backed by a high level of evidence.
In addition, patients should receive positron emission tomography–computed tomography (PET/CT) at diagnosis, before steroids are administered, if possible, as standard of care. Results from the PET/CT should be reported in accordance with international guidelines. These strong recommendations are backed by high-quality evidence.
If PET/CT is performed, then “a bone marrow biopsy is not considered essential,” said Dr. Cwynarski and her coauthors. However, if the findings would influence management, such as when there is extranodal disease that presents central nervous system opportunities, then bone marrow biopsy should be performed. It should also be performed when cytotoxic therapy was initiated before PET/CT could be done. This is a weak recommendation supported by moderate evidence.
Since patients with PMBCL are usually young adults at presentation, it’s important to consider fertility preservation in the face of chemotherapy. For males, semen preservation should be offered. Female patients may not be able to postpone treatment long enough to accomplish egg harvesting. The risk of infertility and premature ovarian failure will depend on the treatment regimen, so “the risks of each individual therapeutic regimen should be discussed with the patient,” Dr. Cwynarski and her colleagues said.
If a patient is diagnosed with PMBCL while pregnant, treatment should be managed in conjunction with high-risk obstetrics and anesthesia specialists. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been used in pregnancy, and immunotherapy without antimetabolites can be considered in the second and third trimesters, according to the good practice paper. These are strong fertility and pregnancy recommendations, backed by moderate to low-quality evidence.
If superior vena cava obstruction causes thrombosis, local standard of care for anticoagulation should be used, but therapy-induced thrombocytopenia should be taken into consideration.
There is a lack of prospective, randomized studies to guide treatment decisions in PMBCL, according to the paper. Still, adding rituximab improves both response rates and duration of remission, they noted.
The standard of care for treatment is six cycles of R-CHOP and involved site radiotherapy (ISRT). If the patient is being cared for at a site that can manage the complexities of dose adjustment and monitoring, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) without ISRT is an alternative, according to the good practice paper.
All patients should be offered clinical trial participation when feasible, a strong recommendation based on high-quality evidence.
To assess the response to therapy, R-CHOP and ISRT recipients not participating in a clinical trial should receive a PET-CT scan 2-3 months after treatment is completed, and DA-EPOCH-R patients should receive their scan 6 weeks after the end of therapy. For all patients, Deauville criteria should be used in reporting response scan results. These strong recommendations about posttherapy imaging are based on moderate-quality evidence.
The rate of relapse and refractory disease is relatively low at about 10%-30%, Dr. Cwynarski and her colleagues said. Relapse usually happens within the first year and is rare after 2 years; extranodal disease is common, but usually spares the central nervous system and bone marrow. The good practice paper authors strongly recommend, based on high-quality evidence, that biopsy and fluorodeoxyglucose-PET/CT should be performed with relapse.
Radiotherapy can be considered if the relapse is localized and the patient didn’t receive initial radiotherapy, a strong recommendation with moderate evidence to support it.
Salvage regimens for patients who have not previously achieved complete metabolic response lack a disease-specific evidence base, noted Dr. Cwynarski and her colleagues. Taking this into consideration, a PMBCL salvage regimen should be the same as that offered to patients with relapsed diffused large B-cell lymphoma. High-dose therapy and autologous stem cell transplantation is appropriate for responsive disease.
If radiotherapy had not been given previously, it should be considered either pre- or post transplant. This, along with the other salvage therapy guidance, is a weak recommendation, backed by moderate evidence.
For longer-term follow-up, asymptomatic patients should not have routine imaging, a strong recommendation with moderate evidence. “[P]atients who remain in remission may be considered for discharge back to primary care,” Dr. Cwynarski and her coauthors said, making a weak recommendation based on low-quality evidence. Patients and their primary care providers should know about the potential for such long-term complications as cardiac toxicities and second malignancies.
SOURCE: Cwynarski K et al. Br J Haematol. 2019 Jan 4. doi:10.1111/bjh.15731
Fertility preservation, imaging and radiotherapy guidelines, and best practices in relapse or salvage therapy for primary mediastinal B-cell lymphoma (PMBCL) are all highlighted in a new good practice paper from the British Society for Haematology.
Though PMBCL was previously thought of as a subtype of diffuse large B-cell lymphoma, “gene expression profiling data has shown it to be a separate clinicopathological entity with evidence of an overlap with classic Hodgkin lymphoma,” said Kate Cwynarski, MD, PhD, of University College London Hospitals NHS Foundation Trust in England, and her coauthors. The recommendations were published in the British Journal of Haematology.
PMBCL makes up 2%-4% of non-Hodgkin lymphomas, they said; a bulky anterior mediastinal mass is the usual initial presentation. PMBCL does not usually spread beyond the thoracic cavity.
Biopsy, which should be reviewed by a hematopathologist, is required for a histological diagnosis of PMBCL. A multidisciplinary team should review the clinical presentation, pathology, and management plan, according to the good practice paper authors. This was a strong recommendation backed by a high level of evidence.
In addition, patients should receive positron emission tomography–computed tomography (PET/CT) at diagnosis, before steroids are administered, if possible, as standard of care. Results from the PET/CT should be reported in accordance with international guidelines. These strong recommendations are backed by high-quality evidence.
If PET/CT is performed, then “a bone marrow biopsy is not considered essential,” said Dr. Cwynarski and her coauthors. However, if the findings would influence management, such as when there is extranodal disease that presents central nervous system opportunities, then bone marrow biopsy should be performed. It should also be performed when cytotoxic therapy was initiated before PET/CT could be done. This is a weak recommendation supported by moderate evidence.
Since patients with PMBCL are usually young adults at presentation, it’s important to consider fertility preservation in the face of chemotherapy. For males, semen preservation should be offered. Female patients may not be able to postpone treatment long enough to accomplish egg harvesting. The risk of infertility and premature ovarian failure will depend on the treatment regimen, so “the risks of each individual therapeutic regimen should be discussed with the patient,” Dr. Cwynarski and her colleagues said.
If a patient is diagnosed with PMBCL while pregnant, treatment should be managed in conjunction with high-risk obstetrics and anesthesia specialists. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been used in pregnancy, and immunotherapy without antimetabolites can be considered in the second and third trimesters, according to the good practice paper. These are strong fertility and pregnancy recommendations, backed by moderate to low-quality evidence.
If superior vena cava obstruction causes thrombosis, local standard of care for anticoagulation should be used, but therapy-induced thrombocytopenia should be taken into consideration.
There is a lack of prospective, randomized studies to guide treatment decisions in PMBCL, according to the paper. Still, adding rituximab improves both response rates and duration of remission, they noted.
The standard of care for treatment is six cycles of R-CHOP and involved site radiotherapy (ISRT). If the patient is being cared for at a site that can manage the complexities of dose adjustment and monitoring, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) without ISRT is an alternative, according to the good practice paper.
All patients should be offered clinical trial participation when feasible, a strong recommendation based on high-quality evidence.
To assess the response to therapy, R-CHOP and ISRT recipients not participating in a clinical trial should receive a PET-CT scan 2-3 months after treatment is completed, and DA-EPOCH-R patients should receive their scan 6 weeks after the end of therapy. For all patients, Deauville criteria should be used in reporting response scan results. These strong recommendations about posttherapy imaging are based on moderate-quality evidence.
The rate of relapse and refractory disease is relatively low at about 10%-30%, Dr. Cwynarski and her colleagues said. Relapse usually happens within the first year and is rare after 2 years; extranodal disease is common, but usually spares the central nervous system and bone marrow. The good practice paper authors strongly recommend, based on high-quality evidence, that biopsy and fluorodeoxyglucose-PET/CT should be performed with relapse.
Radiotherapy can be considered if the relapse is localized and the patient didn’t receive initial radiotherapy, a strong recommendation with moderate evidence to support it.
Salvage regimens for patients who have not previously achieved complete metabolic response lack a disease-specific evidence base, noted Dr. Cwynarski and her colleagues. Taking this into consideration, a PMBCL salvage regimen should be the same as that offered to patients with relapsed diffused large B-cell lymphoma. High-dose therapy and autologous stem cell transplantation is appropriate for responsive disease.
If radiotherapy had not been given previously, it should be considered either pre- or post transplant. This, along with the other salvage therapy guidance, is a weak recommendation, backed by moderate evidence.
For longer-term follow-up, asymptomatic patients should not have routine imaging, a strong recommendation with moderate evidence. “[P]atients who remain in remission may be considered for discharge back to primary care,” Dr. Cwynarski and her coauthors said, making a weak recommendation based on low-quality evidence. Patients and their primary care providers should know about the potential for such long-term complications as cardiac toxicities and second malignancies.
SOURCE: Cwynarski K et al. Br J Haematol. 2019 Jan 4. doi:10.1111/bjh.15731
Fertility preservation, imaging and radiotherapy guidelines, and best practices in relapse or salvage therapy for primary mediastinal B-cell lymphoma (PMBCL) are all highlighted in a new good practice paper from the British Society for Haematology.
Though PMBCL was previously thought of as a subtype of diffuse large B-cell lymphoma, “gene expression profiling data has shown it to be a separate clinicopathological entity with evidence of an overlap with classic Hodgkin lymphoma,” said Kate Cwynarski, MD, PhD, of University College London Hospitals NHS Foundation Trust in England, and her coauthors. The recommendations were published in the British Journal of Haematology.
PMBCL makes up 2%-4% of non-Hodgkin lymphomas, they said; a bulky anterior mediastinal mass is the usual initial presentation. PMBCL does not usually spread beyond the thoracic cavity.
Biopsy, which should be reviewed by a hematopathologist, is required for a histological diagnosis of PMBCL. A multidisciplinary team should review the clinical presentation, pathology, and management plan, according to the good practice paper authors. This was a strong recommendation backed by a high level of evidence.
In addition, patients should receive positron emission tomography–computed tomography (PET/CT) at diagnosis, before steroids are administered, if possible, as standard of care. Results from the PET/CT should be reported in accordance with international guidelines. These strong recommendations are backed by high-quality evidence.
If PET/CT is performed, then “a bone marrow biopsy is not considered essential,” said Dr. Cwynarski and her coauthors. However, if the findings would influence management, such as when there is extranodal disease that presents central nervous system opportunities, then bone marrow biopsy should be performed. It should also be performed when cytotoxic therapy was initiated before PET/CT could be done. This is a weak recommendation supported by moderate evidence.
Since patients with PMBCL are usually young adults at presentation, it’s important to consider fertility preservation in the face of chemotherapy. For males, semen preservation should be offered. Female patients may not be able to postpone treatment long enough to accomplish egg harvesting. The risk of infertility and premature ovarian failure will depend on the treatment regimen, so “the risks of each individual therapeutic regimen should be discussed with the patient,” Dr. Cwynarski and her colleagues said.
If a patient is diagnosed with PMBCL while pregnant, treatment should be managed in conjunction with high-risk obstetrics and anesthesia specialists. Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has been used in pregnancy, and immunotherapy without antimetabolites can be considered in the second and third trimesters, according to the good practice paper. These are strong fertility and pregnancy recommendations, backed by moderate to low-quality evidence.
If superior vena cava obstruction causes thrombosis, local standard of care for anticoagulation should be used, but therapy-induced thrombocytopenia should be taken into consideration.
There is a lack of prospective, randomized studies to guide treatment decisions in PMBCL, according to the paper. Still, adding rituximab improves both response rates and duration of remission, they noted.
The standard of care for treatment is six cycles of R-CHOP and involved site radiotherapy (ISRT). If the patient is being cared for at a site that can manage the complexities of dose adjustment and monitoring, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) without ISRT is an alternative, according to the good practice paper.
All patients should be offered clinical trial participation when feasible, a strong recommendation based on high-quality evidence.
To assess the response to therapy, R-CHOP and ISRT recipients not participating in a clinical trial should receive a PET-CT scan 2-3 months after treatment is completed, and DA-EPOCH-R patients should receive their scan 6 weeks after the end of therapy. For all patients, Deauville criteria should be used in reporting response scan results. These strong recommendations about posttherapy imaging are based on moderate-quality evidence.
The rate of relapse and refractory disease is relatively low at about 10%-30%, Dr. Cwynarski and her colleagues said. Relapse usually happens within the first year and is rare after 2 years; extranodal disease is common, but usually spares the central nervous system and bone marrow. The good practice paper authors strongly recommend, based on high-quality evidence, that biopsy and fluorodeoxyglucose-PET/CT should be performed with relapse.
Radiotherapy can be considered if the relapse is localized and the patient didn’t receive initial radiotherapy, a strong recommendation with moderate evidence to support it.
Salvage regimens for patients who have not previously achieved complete metabolic response lack a disease-specific evidence base, noted Dr. Cwynarski and her colleagues. Taking this into consideration, a PMBCL salvage regimen should be the same as that offered to patients with relapsed diffused large B-cell lymphoma. High-dose therapy and autologous stem cell transplantation is appropriate for responsive disease.
If radiotherapy had not been given previously, it should be considered either pre- or post transplant. This, along with the other salvage therapy guidance, is a weak recommendation, backed by moderate evidence.
For longer-term follow-up, asymptomatic patients should not have routine imaging, a strong recommendation with moderate evidence. “[P]atients who remain in remission may be considered for discharge back to primary care,” Dr. Cwynarski and her coauthors said, making a weak recommendation based on low-quality evidence. Patients and their primary care providers should know about the potential for such long-term complications as cardiac toxicities and second malignancies.
SOURCE: Cwynarski K et al. Br J Haematol. 2019 Jan 4. doi:10.1111/bjh.15731
FROM BRITISH JOURNAL OF HAEMATOLOGY