User login
Choose your steps for treating chronic spontaneous urticaria
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
REPORTING FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
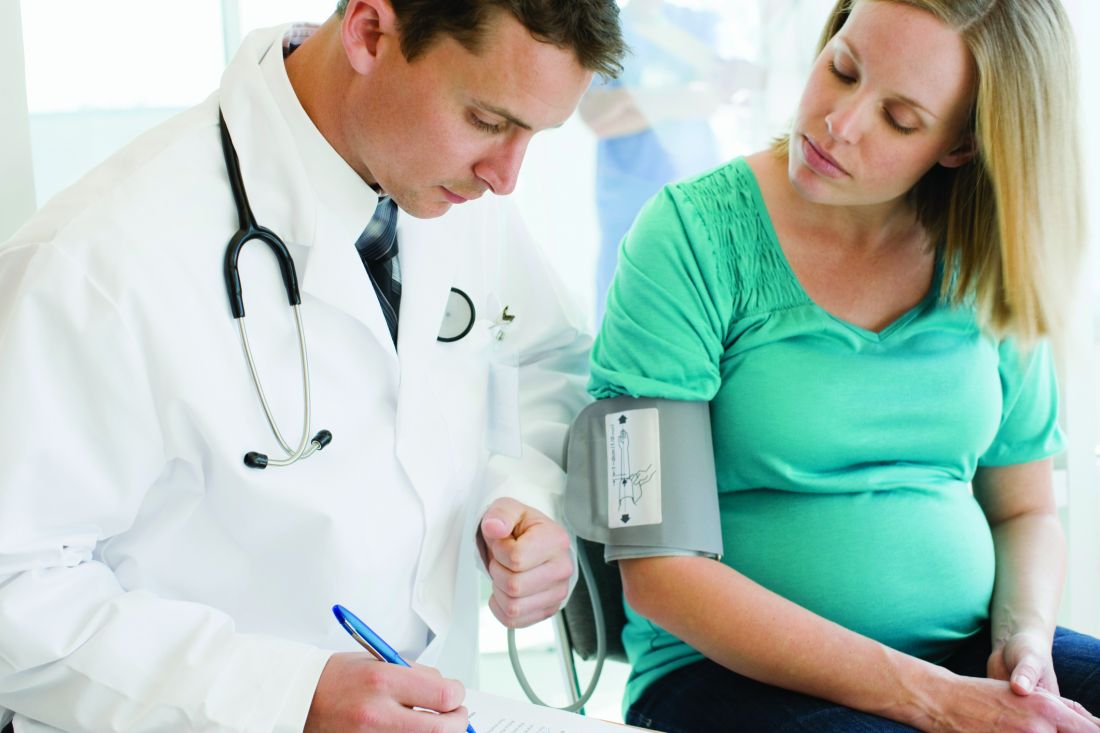
ACOG updates guidance on chronic hypertension in pregnancy, gestational hypertension
Ob.gyns. will need to focus more on individualized care as they use the two new practice bulletins, one on chronic hypertension in pregnancy and one on gestational hypertension and preeclampsia, released by the American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics.
The bulletins will replace the 2013 ACOG hypertension in pregnancy task force report and are published in the January issue of Obstetrics & Gynecology.
“The task force was a tour de force in creating a comprehensive view of hypertensive diseases of pregnancy, including research,” Christian M. Pettker, MD, who helped develop both practice bulletins, stated in a press release. “The updated guidance provides clearer recommendations for the management of gestational hypertension with severe-range blood pressure, an emphasis on and instructions for timely treatment of acutely elevated blood pressures, and more defined recommendations for the management of pain in postoperative patients with hypertension.”
“Ob.gyns. will need to focus more on individualized care and may find it’s best to err on the side of caution because the appropriate treatment of hypertensive diseases in pregnancy may be the most important focus of our attempts to improve maternal mortality and morbidity in the United States,” he said.*
Gestational hypertension or preeclampsia
For women with gestational hypertension or preeclampsia at 37 weeks of gestation or later without severe features, the guidelines recommend delivery rather than expectant management.
Those patients with severe features of gestational hypertension or preeclampsia or eclampsia should receive magnesium sulfate to prevent or treat seizures.
Patients should receive low-dose aspirin (81 mg/day) for preeclampsia prophylaxis between 12 weeks and 28 weeks of gestation if they have high-risk factors of preeclampsia such as multifetal gestation, a previous pregnancy with preeclampsia, renal disease, autoimmune disease, type 1 or type 2 diabetes mellitus, chronic hypertension, or a previous pregnancy with preeclampsia; or more than one moderate risk factor such as a family history of preeclampsia, maternal age greater than 35 years, first pregnancy, body mass index greater than 30, personal history factors, or sociodemographic characteristics.
NSAIDs should continue to be used in preference to opioid analgesics.
The guidance also discusses mode of delivery, antihypertensive drugs and thresholds for treatment, management of acute complications for preeclampsia with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, the optimal treatment for eclampsia, and postpartum hypertension and headache.
Chronic hypertension
Pregnant women with chronic hypertension also should receive low-dose aspirin between 12 weeks and 28 weeks of gestation. Antihypertensive therapy should be initiated for women with persistent chronic hypertension at systolic pressure of 160 mm Hg or higher and/or diastolic pressure of 110 mm Hg or higher. Consider treating patients at lower blood pressure (BP) thresholds depending on comorbidities or underlying impaired renal function.
ACOG has recommended treating pregnant patients as chronically hypertensive according to recently changed criteria from the American College of Cardiology and the American Heart Association, which call for classifying blood pressure into the following categories:
- Normal. Systolic BP less than 120 mm Hg; diastolic BP less than 80 mm Hg.
- Elevated. Systolic BP greater than or equal to 120-129 mm Hg; diastolic BP greater than 80 mm Hg.
- Stage 1 hypertension. Systolic BP, 130-139 mm Hg; diastolic BP, 80-89 mm Hg.
- Stage 2 hypertension. Systolic BP greater than or equal to 140 mm Hg; diastolic BP greater than or equal to 90 mm Hg.
“The new blood pressure ranges for nonpregnant women have a lower threshold for hypertension diagnosis compared to ACOG’s criteria,” Dr. Pettker said. “This will likely cause a general increase in patients classified as chronic hypertensive and will require shared decision making by the ob.gyn. and the patient regarding appropriate management in pregnancy.”
The guideline also discusses chronic hypertension with superimposed preeclampsia; tests for baseline evaluation of chronic hypertension in pregnancy; common oral antihypertensive agents to use in pregnancy and those to use for urgent blood pressure control in pregnancy; control of acute-onset severe-range hypertension; and postpartum considerations in patients with chronic hypertension.
SOURCE: Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 202. Obstet Gynecol. 2019;133:e1-25; Chronic hypertension in pregnancy. ACOG Practice Bulletin No. 203. Obstet Gynecol. 2019;133:e26-50.
This article was updated 1/11/19 and 11/19/19.
Ob.gyns. will need to focus more on individualized care as they use the two new practice bulletins, one on chronic hypertension in pregnancy and one on gestational hypertension and preeclampsia, released by the American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics.
The bulletins will replace the 2013 ACOG hypertension in pregnancy task force report and are published in the January issue of Obstetrics & Gynecology.
“The task force was a tour de force in creating a comprehensive view of hypertensive diseases of pregnancy, including research,” Christian M. Pettker, MD, who helped develop both practice bulletins, stated in a press release. “The updated guidance provides clearer recommendations for the management of gestational hypertension with severe-range blood pressure, an emphasis on and instructions for timely treatment of acutely elevated blood pressures, and more defined recommendations for the management of pain in postoperative patients with hypertension.”
“Ob.gyns. will need to focus more on individualized care and may find it’s best to err on the side of caution because the appropriate treatment of hypertensive diseases in pregnancy may be the most important focus of our attempts to improve maternal mortality and morbidity in the United States,” he said.*
Gestational hypertension or preeclampsia
For women with gestational hypertension or preeclampsia at 37 weeks of gestation or later without severe features, the guidelines recommend delivery rather than expectant management.
Those patients with severe features of gestational hypertension or preeclampsia or eclampsia should receive magnesium sulfate to prevent or treat seizures.
Patients should receive low-dose aspirin (81 mg/day) for preeclampsia prophylaxis between 12 weeks and 28 weeks of gestation if they have high-risk factors of preeclampsia such as multifetal gestation, a previous pregnancy with preeclampsia, renal disease, autoimmune disease, type 1 or type 2 diabetes mellitus, chronic hypertension, or a previous pregnancy with preeclampsia; or more than one moderate risk factor such as a family history of preeclampsia, maternal age greater than 35 years, first pregnancy, body mass index greater than 30, personal history factors, or sociodemographic characteristics.
NSAIDs should continue to be used in preference to opioid analgesics.
The guidance also discusses mode of delivery, antihypertensive drugs and thresholds for treatment, management of acute complications for preeclampsia with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, the optimal treatment for eclampsia, and postpartum hypertension and headache.
Chronic hypertension
Pregnant women with chronic hypertension also should receive low-dose aspirin between 12 weeks and 28 weeks of gestation. Antihypertensive therapy should be initiated for women with persistent chronic hypertension at systolic pressure of 160 mm Hg or higher and/or diastolic pressure of 110 mm Hg or higher. Consider treating patients at lower blood pressure (BP) thresholds depending on comorbidities or underlying impaired renal function.
ACOG has recommended treating pregnant patients as chronically hypertensive according to recently changed criteria from the American College of Cardiology and the American Heart Association, which call for classifying blood pressure into the following categories:
- Normal. Systolic BP less than 120 mm Hg; diastolic BP less than 80 mm Hg.
- Elevated. Systolic BP greater than or equal to 120-129 mm Hg; diastolic BP greater than 80 mm Hg.
- Stage 1 hypertension. Systolic BP, 130-139 mm Hg; diastolic BP, 80-89 mm Hg.
- Stage 2 hypertension. Systolic BP greater than or equal to 140 mm Hg; diastolic BP greater than or equal to 90 mm Hg.
“The new blood pressure ranges for nonpregnant women have a lower threshold for hypertension diagnosis compared to ACOG’s criteria,” Dr. Pettker said. “This will likely cause a general increase in patients classified as chronic hypertensive and will require shared decision making by the ob.gyn. and the patient regarding appropriate management in pregnancy.”
The guideline also discusses chronic hypertension with superimposed preeclampsia; tests for baseline evaluation of chronic hypertension in pregnancy; common oral antihypertensive agents to use in pregnancy and those to use for urgent blood pressure control in pregnancy; control of acute-onset severe-range hypertension; and postpartum considerations in patients with chronic hypertension.
SOURCE: Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 202. Obstet Gynecol. 2019;133:e1-25; Chronic hypertension in pregnancy. ACOG Practice Bulletin No. 203. Obstet Gynecol. 2019;133:e26-50.
This article was updated 1/11/19 and 11/19/19.
Ob.gyns. will need to focus more on individualized care as they use the two new practice bulletins, one on chronic hypertension in pregnancy and one on gestational hypertension and preeclampsia, released by the American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics.
The bulletins will replace the 2013 ACOG hypertension in pregnancy task force report and are published in the January issue of Obstetrics & Gynecology.
“The task force was a tour de force in creating a comprehensive view of hypertensive diseases of pregnancy, including research,” Christian M. Pettker, MD, who helped develop both practice bulletins, stated in a press release. “The updated guidance provides clearer recommendations for the management of gestational hypertension with severe-range blood pressure, an emphasis on and instructions for timely treatment of acutely elevated blood pressures, and more defined recommendations for the management of pain in postoperative patients with hypertension.”
“Ob.gyns. will need to focus more on individualized care and may find it’s best to err on the side of caution because the appropriate treatment of hypertensive diseases in pregnancy may be the most important focus of our attempts to improve maternal mortality and morbidity in the United States,” he said.*
Gestational hypertension or preeclampsia
For women with gestational hypertension or preeclampsia at 37 weeks of gestation or later without severe features, the guidelines recommend delivery rather than expectant management.
Those patients with severe features of gestational hypertension or preeclampsia or eclampsia should receive magnesium sulfate to prevent or treat seizures.
Patients should receive low-dose aspirin (81 mg/day) for preeclampsia prophylaxis between 12 weeks and 28 weeks of gestation if they have high-risk factors of preeclampsia such as multifetal gestation, a previous pregnancy with preeclampsia, renal disease, autoimmune disease, type 1 or type 2 diabetes mellitus, chronic hypertension, or a previous pregnancy with preeclampsia; or more than one moderate risk factor such as a family history of preeclampsia, maternal age greater than 35 years, first pregnancy, body mass index greater than 30, personal history factors, or sociodemographic characteristics.
NSAIDs should continue to be used in preference to opioid analgesics.
The guidance also discusses mode of delivery, antihypertensive drugs and thresholds for treatment, management of acute complications for preeclampsia with HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, the optimal treatment for eclampsia, and postpartum hypertension and headache.
Chronic hypertension
Pregnant women with chronic hypertension also should receive low-dose aspirin between 12 weeks and 28 weeks of gestation. Antihypertensive therapy should be initiated for women with persistent chronic hypertension at systolic pressure of 160 mm Hg or higher and/or diastolic pressure of 110 mm Hg or higher. Consider treating patients at lower blood pressure (BP) thresholds depending on comorbidities or underlying impaired renal function.
ACOG has recommended treating pregnant patients as chronically hypertensive according to recently changed criteria from the American College of Cardiology and the American Heart Association, which call for classifying blood pressure into the following categories:
- Normal. Systolic BP less than 120 mm Hg; diastolic BP less than 80 mm Hg.
- Elevated. Systolic BP greater than or equal to 120-129 mm Hg; diastolic BP greater than 80 mm Hg.
- Stage 1 hypertension. Systolic BP, 130-139 mm Hg; diastolic BP, 80-89 mm Hg.
- Stage 2 hypertension. Systolic BP greater than or equal to 140 mm Hg; diastolic BP greater than or equal to 90 mm Hg.
“The new blood pressure ranges for nonpregnant women have a lower threshold for hypertension diagnosis compared to ACOG’s criteria,” Dr. Pettker said. “This will likely cause a general increase in patients classified as chronic hypertensive and will require shared decision making by the ob.gyn. and the patient regarding appropriate management in pregnancy.”
The guideline also discusses chronic hypertension with superimposed preeclampsia; tests for baseline evaluation of chronic hypertension in pregnancy; common oral antihypertensive agents to use in pregnancy and those to use for urgent blood pressure control in pregnancy; control of acute-onset severe-range hypertension; and postpartum considerations in patients with chronic hypertension.
SOURCE: Gestational hypertension and preeclampsia. ACOG Practice Bulletin No. 202. Obstet Gynecol. 2019;133:e1-25; Chronic hypertension in pregnancy. ACOG Practice Bulletin No. 203. Obstet Gynecol. 2019;133:e26-50.
This article was updated 1/11/19 and 11/19/19.
FROM OBSTETRICS & GYNECOLOGY
Clinical Guidance: Thiopurine agents for the treatment of IBD
A new clinical practice update recommends combination therapy with tumor necrosis factor (TNF) inhibitors and thiopurines, as opposed to either therapy alone, for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD). The commentary was published in Gastroenterology.
Clinicians should also note that while several clinical trials use weight-based dosing to monitor clinical response following thiopurine therapy, 6-thioguanine levels have inevitably shown to better predict prognosis, wrote Stephen B. Hanauer, MD, AGAF, of Northwestern University in Chicago and his colleagues.
The thiopurine drug class is composed of many different agents, including thioguanine, azathioprine, and mercaptopurine. Methotrexate, a folate antagonist affecting thymidylate production, is commonly used alongside thiopurines as steroid-sparing agents for patients with UC and CD. Among these therapies, various different dosing strategies and routes of administration are used to manage active disease.
Initially, thiopurines were studied exclusively as monotherapy for the treatment of patients with steroid-intractable CD; however, results showed only marginal benefit when using these agents alone. As a result, combination trials were performed subsequently, and these revealed modest efficacy for use as maintenance therapies in both UC and CD. Further studies reported that methotrexate is beneficial only as a maintenance therapy for CD given that trial evidence confirmed treatment limitations in patients with UC.
“Thiopurines also have the potential to reduce postoperative recurrence of Crohn’s disease, in particular when administered with imidazole antibiotics,” the experts wrote. “There is currently no controlled data regarding the efficacy of methotrexate as maintenance therapy in ulcerative colitis,” they added.
Despite its limitations in UC, 25 mg of methotrexate administered intramuscularly once weekly in combination with oral steroids has shown benefits for inducing disease remission and limiting steroid use in the management of active CD. Comparatively, other trials have failed to show the same benefits with oral methotrexate. In addition, a number of clinical case series have reported benefit for use of methotrexate as a maintenance therapy for CD in patients who initially responded to methotrexate induction therapy.
Consequently, Dr. Hanauer and his colleagues recommended that methotrexate only be given in combination with biologics if being used for the treatment of UC.
“Thiopurines and methotrexate can be used in combination with anti-TNF biologics, in particular infliximab, to reduce immunogenicity and increase blood levels,” they stated.
One agent in particular, thioguanine, exhibits unique therapeutic efficacy in patients allergic to azathioprine or mercaptopurine. Despite this benefit, thioguanine use has been linked with an increased risk of developing hepatic nodular regenerative hyperplasia, as well as venoocclusive disease. Given these limitations, long-term use of thioguanine was not recommended by the authors.
With respect to safety, routine laboratory monitoring for both liver and hematologic adverse effects is recommended. In rare cases, patients may develop secondary lymphomas in response to thiopurine treatment. Moreover, regular follow-up is essential because of the higher prevalence of nonmelanoma skin cancers seen with thiopurines use.
“Patients using thiopurines for the treatment of IBD, particularly Caucasian patients, should avoid excessive sun exposure and use high-strength sun block,” the experts wrote. “Health care deliverers should ensure patients undergo appropriate dermatologic evaluations and investigate suspicious skin lesions in these patients,” they further reported.
Another important monitoring consideration is ongoing infection risk, in particular with opportunistic and viral pathogens. Because of the immunosuppressive effects of therapy, both methotrexate and thiopurine use are linked with a greater chance of developing these infections. Accordingly, Dr. Hanauer and his colleagues recommended that, before initiation of these therapies, applicable preventative measures should be taken, including administration of influenza, human papillomavirus, varicella zoster virus, pneumococcus, and hepatitis B vaccines.
“Live vaccines are contraindicated once therapy has begun; however, zoster vaccination can be given while patients are receiving azathioprine at less than 2 mg/kg,” they stated.
The experts went on to report that withdrawal of thiopurine agents, when used in combination therapy, has the potential to reduce therapeutic levels of infliximab and promote development of antidrug antibodies. However, the experts did not suggest a method to manage these complications. Further studies are needed to answer these and other remaining questions regarding thiopurine use in the setting of IBD.
SOURCE: Hanauer SB et al. Gastroenterology. 2018 Sep 6. doi: 10.1053/j.gastro.2018.08.043.
*This story was updated on January 4, 2019.
A new clinical practice update recommends combination therapy with tumor necrosis factor (TNF) inhibitors and thiopurines, as opposed to either therapy alone, for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD). The commentary was published in Gastroenterology.
Clinicians should also note that while several clinical trials use weight-based dosing to monitor clinical response following thiopurine therapy, 6-thioguanine levels have inevitably shown to better predict prognosis, wrote Stephen B. Hanauer, MD, AGAF, of Northwestern University in Chicago and his colleagues.
The thiopurine drug class is composed of many different agents, including thioguanine, azathioprine, and mercaptopurine. Methotrexate, a folate antagonist affecting thymidylate production, is commonly used alongside thiopurines as steroid-sparing agents for patients with UC and CD. Among these therapies, various different dosing strategies and routes of administration are used to manage active disease.
Initially, thiopurines were studied exclusively as monotherapy for the treatment of patients with steroid-intractable CD; however, results showed only marginal benefit when using these agents alone. As a result, combination trials were performed subsequently, and these revealed modest efficacy for use as maintenance therapies in both UC and CD. Further studies reported that methotrexate is beneficial only as a maintenance therapy for CD given that trial evidence confirmed treatment limitations in patients with UC.
“Thiopurines also have the potential to reduce postoperative recurrence of Crohn’s disease, in particular when administered with imidazole antibiotics,” the experts wrote. “There is currently no controlled data regarding the efficacy of methotrexate as maintenance therapy in ulcerative colitis,” they added.
Despite its limitations in UC, 25 mg of methotrexate administered intramuscularly once weekly in combination with oral steroids has shown benefits for inducing disease remission and limiting steroid use in the management of active CD. Comparatively, other trials have failed to show the same benefits with oral methotrexate. In addition, a number of clinical case series have reported benefit for use of methotrexate as a maintenance therapy for CD in patients who initially responded to methotrexate induction therapy.
Consequently, Dr. Hanauer and his colleagues recommended that methotrexate only be given in combination with biologics if being used for the treatment of UC.
“Thiopurines and methotrexate can be used in combination with anti-TNF biologics, in particular infliximab, to reduce immunogenicity and increase blood levels,” they stated.
One agent in particular, thioguanine, exhibits unique therapeutic efficacy in patients allergic to azathioprine or mercaptopurine. Despite this benefit, thioguanine use has been linked with an increased risk of developing hepatic nodular regenerative hyperplasia, as well as venoocclusive disease. Given these limitations, long-term use of thioguanine was not recommended by the authors.
With respect to safety, routine laboratory monitoring for both liver and hematologic adverse effects is recommended. In rare cases, patients may develop secondary lymphomas in response to thiopurine treatment. Moreover, regular follow-up is essential because of the higher prevalence of nonmelanoma skin cancers seen with thiopurines use.
“Patients using thiopurines for the treatment of IBD, particularly Caucasian patients, should avoid excessive sun exposure and use high-strength sun block,” the experts wrote. “Health care deliverers should ensure patients undergo appropriate dermatologic evaluations and investigate suspicious skin lesions in these patients,” they further reported.
Another important monitoring consideration is ongoing infection risk, in particular with opportunistic and viral pathogens. Because of the immunosuppressive effects of therapy, both methotrexate and thiopurine use are linked with a greater chance of developing these infections. Accordingly, Dr. Hanauer and his colleagues recommended that, before initiation of these therapies, applicable preventative measures should be taken, including administration of influenza, human papillomavirus, varicella zoster virus, pneumococcus, and hepatitis B vaccines.
“Live vaccines are contraindicated once therapy has begun; however, zoster vaccination can be given while patients are receiving azathioprine at less than 2 mg/kg,” they stated.
The experts went on to report that withdrawal of thiopurine agents, when used in combination therapy, has the potential to reduce therapeutic levels of infliximab and promote development of antidrug antibodies. However, the experts did not suggest a method to manage these complications. Further studies are needed to answer these and other remaining questions regarding thiopurine use in the setting of IBD.
SOURCE: Hanauer SB et al. Gastroenterology. 2018 Sep 6. doi: 10.1053/j.gastro.2018.08.043.
*This story was updated on January 4, 2019.
A new clinical practice update recommends combination therapy with tumor necrosis factor (TNF) inhibitors and thiopurines, as opposed to either therapy alone, for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD). The commentary was published in Gastroenterology.
Clinicians should also note that while several clinical trials use weight-based dosing to monitor clinical response following thiopurine therapy, 6-thioguanine levels have inevitably shown to better predict prognosis, wrote Stephen B. Hanauer, MD, AGAF, of Northwestern University in Chicago and his colleagues.
The thiopurine drug class is composed of many different agents, including thioguanine, azathioprine, and mercaptopurine. Methotrexate, a folate antagonist affecting thymidylate production, is commonly used alongside thiopurines as steroid-sparing agents for patients with UC and CD. Among these therapies, various different dosing strategies and routes of administration are used to manage active disease.
Initially, thiopurines were studied exclusively as monotherapy for the treatment of patients with steroid-intractable CD; however, results showed only marginal benefit when using these agents alone. As a result, combination trials were performed subsequently, and these revealed modest efficacy for use as maintenance therapies in both UC and CD. Further studies reported that methotrexate is beneficial only as a maintenance therapy for CD given that trial evidence confirmed treatment limitations in patients with UC.
“Thiopurines also have the potential to reduce postoperative recurrence of Crohn’s disease, in particular when administered with imidazole antibiotics,” the experts wrote. “There is currently no controlled data regarding the efficacy of methotrexate as maintenance therapy in ulcerative colitis,” they added.
Despite its limitations in UC, 25 mg of methotrexate administered intramuscularly once weekly in combination with oral steroids has shown benefits for inducing disease remission and limiting steroid use in the management of active CD. Comparatively, other trials have failed to show the same benefits with oral methotrexate. In addition, a number of clinical case series have reported benefit for use of methotrexate as a maintenance therapy for CD in patients who initially responded to methotrexate induction therapy.
Consequently, Dr. Hanauer and his colleagues recommended that methotrexate only be given in combination with biologics if being used for the treatment of UC.
“Thiopurines and methotrexate can be used in combination with anti-TNF biologics, in particular infliximab, to reduce immunogenicity and increase blood levels,” they stated.
One agent in particular, thioguanine, exhibits unique therapeutic efficacy in patients allergic to azathioprine or mercaptopurine. Despite this benefit, thioguanine use has been linked with an increased risk of developing hepatic nodular regenerative hyperplasia, as well as venoocclusive disease. Given these limitations, long-term use of thioguanine was not recommended by the authors.
With respect to safety, routine laboratory monitoring for both liver and hematologic adverse effects is recommended. In rare cases, patients may develop secondary lymphomas in response to thiopurine treatment. Moreover, regular follow-up is essential because of the higher prevalence of nonmelanoma skin cancers seen with thiopurines use.
“Patients using thiopurines for the treatment of IBD, particularly Caucasian patients, should avoid excessive sun exposure and use high-strength sun block,” the experts wrote. “Health care deliverers should ensure patients undergo appropriate dermatologic evaluations and investigate suspicious skin lesions in these patients,” they further reported.
Another important monitoring consideration is ongoing infection risk, in particular with opportunistic and viral pathogens. Because of the immunosuppressive effects of therapy, both methotrexate and thiopurine use are linked with a greater chance of developing these infections. Accordingly, Dr. Hanauer and his colleagues recommended that, before initiation of these therapies, applicable preventative measures should be taken, including administration of influenza, human papillomavirus, varicella zoster virus, pneumococcus, and hepatitis B vaccines.
“Live vaccines are contraindicated once therapy has begun; however, zoster vaccination can be given while patients are receiving azathioprine at less than 2 mg/kg,” they stated.
The experts went on to report that withdrawal of thiopurine agents, when used in combination therapy, has the potential to reduce therapeutic levels of infliximab and promote development of antidrug antibodies. However, the experts did not suggest a method to manage these complications. Further studies are needed to answer these and other remaining questions regarding thiopurine use in the setting of IBD.
SOURCE: Hanauer SB et al. Gastroenterology. 2018 Sep 6. doi: 10.1053/j.gastro.2018.08.043.
*This story was updated on January 4, 2019.
FROM GASTROENTEROLOGY
Key clinical point: Best clinical practices surrounding the use of thiopurines in patients with inflammatory bowel disease (IBD) were summarized by a group of experts.
Major finding:
Study details: Expert opinion consensus–based review of current evidence surrounding thiopurine therapy for IBD, without complete systematic review of the literature.
Disclosures: The authors reported no conflicts of interest.
Source: Hanauer SB et al. Gastroenterology. 2018 Sep 6. doi: 10.1053/j.gastro.2018.08.043.
AGA Clinical Practice Update: Endoscopic submucosal dissection
The surgical technique published in Clinical Gastroenterology and Hepatology.
Clinicians should recognize ESD as one of the main treatment modalities for GI cancer enclosed within the superficial esophageal mucosa, which includes squamous cell dysplasia, wrote Peter V. Draganov, MD, of the University of Florida in Gainesville with his fellow experts.
Endoscopic resection is a surgical method used to treat both malignant and nonmalignant GI lesions. Over the past several years, the technique has advanced significantly, progressing from snare polypectomy to endoscopic mucosal resection, with current practice now ESD. The minimally invasive technique is considered first-line therapy in patients with colorectal lesions lacking invasive cancer.
While the technique is widely used in Asian countries, and as practice continues to rise throughout Europe, uptake in the United States has been slow. Several factors may be responsible for this delay, including a lack of ESD experts and training centers, underestimation of the benefits associated with ESD, and a likely bias of American oncologists toward treatment with surgical resection. In recent years, extensive improvements have occurred in ESD technique, such as incorporation of pocket and tunnel strategies, which have significantly contributed to the overall safety and efficacy of the procedure.
“With low thresholds for performing endoscopy for upper GI symptoms and the promotion of screening colonoscopy for colon cancer prevention, more precancerous lesions and early cancers are being detected that may be amenable to endoscopic resection by ESD,” the experts wrote.
For mucosal lesions too large to be removed by standard endoscopic resection, or lesions at high risk of being deemed malignant, the guidelines recommend using ESD to remove these lesions. Dr. Draganov and his colleagues acknowledged that the probability of lymph node metastasis is marginally higher when the procedure is used for these widened indications; however, the risk of metastasis remains sufficiently low. Along those lines, several additional recommendations were made related to the expanded indications for ESD, including use in certain patients with Barrett’s esophagus, colorectal neoplasia, and other forms of superficial gastric cancer.
“Expanded indications for gastric ESD include moderately and well-differentiated superficial cancers that are [more than] 2 cm, lesions [up to] 3 cm with ulceration or that contain early submucosal invasion, and poorly differentiated superficial cancers [up to] 2 cm in size,” the experts stated.
With respect to cost, endoscopic resection was found to provide significant savings in comparison to surgical techniques for the removal of colorectal lesions. The economic analysis revealed that using a lesion-specific ESD model for high-risk patients could allow for notable cost reductions.
“Although some insurers have begun preapproving and covering their members who might benefit from ESD, the hurdles preventing other patients from being covered for this innovative and potentially cost-saving procedure should be removed,” they added.
Other recommendations were made in regards to effective implementation of a stepwise ESD educational model to train American endoscopists on how to properly perform the procedure. The proposed strategy involves completion of a formal training program, independent study, self-practice using animal models, and live viewing of cases by ESD experts. In addition, they recommend that newly trained endoscopists complete their first procedures on patients with absolute indications for ESD.
“At present, there is no standardized approach for ESD training in the United States,” the experts wrote. They further explained that “the usual starting point is to attend an ESD course or series of courses that provide increasingly more in-depth exposure.” And they concluded, “a guiding principle should be that our patients’ interests and welfare stand above all else and that patients must not be used as an opportunity for practice or skills acquisition.”
The practice update also recommends that endoscopists avoid the use of techniques that have the ability to produce submucosal fibrosis. Dr. Draganov and his colleagues warn that these practices, such as “tattooing in close proximity to or beneath a lesion for marking” and “partial snare resection of a portion of a lesion for histopathology,” can impede subsequent endoscopic procedures.
Dr. Draganov and several coauthors disclosed financial affiliations with AbbVie, Boston Scientific Corporation, Cook Medical, Olympus America, and others.
SOURCE: Draganov PV et al. Clin Gastroenterol Hepatol. 2018 Aug 2. doi: 10.1016/j.cgh.2018.07.041.
The surgical technique published in Clinical Gastroenterology and Hepatology.
Clinicians should recognize ESD as one of the main treatment modalities for GI cancer enclosed within the superficial esophageal mucosa, which includes squamous cell dysplasia, wrote Peter V. Draganov, MD, of the University of Florida in Gainesville with his fellow experts.
Endoscopic resection is a surgical method used to treat both malignant and nonmalignant GI lesions. Over the past several years, the technique has advanced significantly, progressing from snare polypectomy to endoscopic mucosal resection, with current practice now ESD. The minimally invasive technique is considered first-line therapy in patients with colorectal lesions lacking invasive cancer.
While the technique is widely used in Asian countries, and as practice continues to rise throughout Europe, uptake in the United States has been slow. Several factors may be responsible for this delay, including a lack of ESD experts and training centers, underestimation of the benefits associated with ESD, and a likely bias of American oncologists toward treatment with surgical resection. In recent years, extensive improvements have occurred in ESD technique, such as incorporation of pocket and tunnel strategies, which have significantly contributed to the overall safety and efficacy of the procedure.
“With low thresholds for performing endoscopy for upper GI symptoms and the promotion of screening colonoscopy for colon cancer prevention, more precancerous lesions and early cancers are being detected that may be amenable to endoscopic resection by ESD,” the experts wrote.
For mucosal lesions too large to be removed by standard endoscopic resection, or lesions at high risk of being deemed malignant, the guidelines recommend using ESD to remove these lesions. Dr. Draganov and his colleagues acknowledged that the probability of lymph node metastasis is marginally higher when the procedure is used for these widened indications; however, the risk of metastasis remains sufficiently low. Along those lines, several additional recommendations were made related to the expanded indications for ESD, including use in certain patients with Barrett’s esophagus, colorectal neoplasia, and other forms of superficial gastric cancer.
“Expanded indications for gastric ESD include moderately and well-differentiated superficial cancers that are [more than] 2 cm, lesions [up to] 3 cm with ulceration or that contain early submucosal invasion, and poorly differentiated superficial cancers [up to] 2 cm in size,” the experts stated.
With respect to cost, endoscopic resection was found to provide significant savings in comparison to surgical techniques for the removal of colorectal lesions. The economic analysis revealed that using a lesion-specific ESD model for high-risk patients could allow for notable cost reductions.
“Although some insurers have begun preapproving and covering their members who might benefit from ESD, the hurdles preventing other patients from being covered for this innovative and potentially cost-saving procedure should be removed,” they added.
Other recommendations were made in regards to effective implementation of a stepwise ESD educational model to train American endoscopists on how to properly perform the procedure. The proposed strategy involves completion of a formal training program, independent study, self-practice using animal models, and live viewing of cases by ESD experts. In addition, they recommend that newly trained endoscopists complete their first procedures on patients with absolute indications for ESD.
“At present, there is no standardized approach for ESD training in the United States,” the experts wrote. They further explained that “the usual starting point is to attend an ESD course or series of courses that provide increasingly more in-depth exposure.” And they concluded, “a guiding principle should be that our patients’ interests and welfare stand above all else and that patients must not be used as an opportunity for practice or skills acquisition.”
The practice update also recommends that endoscopists avoid the use of techniques that have the ability to produce submucosal fibrosis. Dr. Draganov and his colleagues warn that these practices, such as “tattooing in close proximity to or beneath a lesion for marking” and “partial snare resection of a portion of a lesion for histopathology,” can impede subsequent endoscopic procedures.
Dr. Draganov and several coauthors disclosed financial affiliations with AbbVie, Boston Scientific Corporation, Cook Medical, Olympus America, and others.
SOURCE: Draganov PV et al. Clin Gastroenterol Hepatol. 2018 Aug 2. doi: 10.1016/j.cgh.2018.07.041.
The surgical technique published in Clinical Gastroenterology and Hepatology.
Clinicians should recognize ESD as one of the main treatment modalities for GI cancer enclosed within the superficial esophageal mucosa, which includes squamous cell dysplasia, wrote Peter V. Draganov, MD, of the University of Florida in Gainesville with his fellow experts.
Endoscopic resection is a surgical method used to treat both malignant and nonmalignant GI lesions. Over the past several years, the technique has advanced significantly, progressing from snare polypectomy to endoscopic mucosal resection, with current practice now ESD. The minimally invasive technique is considered first-line therapy in patients with colorectal lesions lacking invasive cancer.
While the technique is widely used in Asian countries, and as practice continues to rise throughout Europe, uptake in the United States has been slow. Several factors may be responsible for this delay, including a lack of ESD experts and training centers, underestimation of the benefits associated with ESD, and a likely bias of American oncologists toward treatment with surgical resection. In recent years, extensive improvements have occurred in ESD technique, such as incorporation of pocket and tunnel strategies, which have significantly contributed to the overall safety and efficacy of the procedure.
“With low thresholds for performing endoscopy for upper GI symptoms and the promotion of screening colonoscopy for colon cancer prevention, more precancerous lesions and early cancers are being detected that may be amenable to endoscopic resection by ESD,” the experts wrote.
For mucosal lesions too large to be removed by standard endoscopic resection, or lesions at high risk of being deemed malignant, the guidelines recommend using ESD to remove these lesions. Dr. Draganov and his colleagues acknowledged that the probability of lymph node metastasis is marginally higher when the procedure is used for these widened indications; however, the risk of metastasis remains sufficiently low. Along those lines, several additional recommendations were made related to the expanded indications for ESD, including use in certain patients with Barrett’s esophagus, colorectal neoplasia, and other forms of superficial gastric cancer.
“Expanded indications for gastric ESD include moderately and well-differentiated superficial cancers that are [more than] 2 cm, lesions [up to] 3 cm with ulceration or that contain early submucosal invasion, and poorly differentiated superficial cancers [up to] 2 cm in size,” the experts stated.
With respect to cost, endoscopic resection was found to provide significant savings in comparison to surgical techniques for the removal of colorectal lesions. The economic analysis revealed that using a lesion-specific ESD model for high-risk patients could allow for notable cost reductions.
“Although some insurers have begun preapproving and covering their members who might benefit from ESD, the hurdles preventing other patients from being covered for this innovative and potentially cost-saving procedure should be removed,” they added.
Other recommendations were made in regards to effective implementation of a stepwise ESD educational model to train American endoscopists on how to properly perform the procedure. The proposed strategy involves completion of a formal training program, independent study, self-practice using animal models, and live viewing of cases by ESD experts. In addition, they recommend that newly trained endoscopists complete their first procedures on patients with absolute indications for ESD.
“At present, there is no standardized approach for ESD training in the United States,” the experts wrote. They further explained that “the usual starting point is to attend an ESD course or series of courses that provide increasingly more in-depth exposure.” And they concluded, “a guiding principle should be that our patients’ interests and welfare stand above all else and that patients must not be used as an opportunity for practice or skills acquisition.”
The practice update also recommends that endoscopists avoid the use of techniques that have the ability to produce submucosal fibrosis. Dr. Draganov and his colleagues warn that these practices, such as “tattooing in close proximity to or beneath a lesion for marking” and “partial snare resection of a portion of a lesion for histopathology,” can impede subsequent endoscopic procedures.
Dr. Draganov and several coauthors disclosed financial affiliations with AbbVie, Boston Scientific Corporation, Cook Medical, Olympus America, and others.
SOURCE: Draganov PV et al. Clin Gastroenterol Hepatol. 2018 Aug 2. doi: 10.1016/j.cgh.2018.07.041.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The American Gastroenterological Association (AGA) has released clinical guidance regarding the use of endoscopic submucosal dissection (ESD).
Major finding: ESD should be established as an endoscopic technique that allows for total removal of malignant lesions that could otherwise lead to future complications for patients.
Study details: Expert review focused on the current and upcoming role of ESD in clinical gastroenterology practice in the United States.
Disclosures: Dr. Draganov and several coauthors disclosed financial affiliations with AbbVie, Boston Scientific, Cook Medical, Olympus America, and others.
Source: Draganov PV et al. Clin Gastroenterol Hepatol. 2018 Aug 2. doi: 10.1016/j.cgh.2018.07.041.
Joint guidelines offer recommendations for treating peripheral artery disease
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
New diabetes guidelines downgrade insulin as first-line injectable treatment
The American Diabetes Association is out with new standard-of-care guidelines that – among other things – reject injectable insulin as the main first-line treatment for type 2 diabetes mellitus (T2DM), debut a cardiac risk calculator, and offer new recommendations regarding medications for patients with kidney disease, clogged arteries, and heart failure.
The ADA’s newly released 2019 Standards of Medical Care in Diabetes “emphasize a patient-centered approach that considers the multiple health and life factors of each person living with diabetes,” said William T. Cefalu, MD, the ADA’s chief scientific, medical, and mission officer, in a statement.
The 193-page guidelines are now available online at the Diabetes Care website and will be available via an app and the print edition of the journal.
Here’s a closer look at a few of the many new and revised recommendations in the 2019 Standards of Care.
Diabetes treatment
In a new guideline, the standards of care says glucagonlike peptide–1 (GLP-1) receptor agonists should be “a first-line treatment” – ahead of insulin – “for most [type 2] patients who need the greater efficacy of an injectable medication.”
However, the recommendations note that the “high costs and tolerability issues are important barriers to the use of GLP-1 receptor agonists.”
A new recommendation suggests the use of sodium-glucose cotransporter 2 inhibitors or GLP-1 receptor agonists “with demonstrated cardiovascular disease benefit” in patients with type 2 diabetes who have confirmed atherosclerotic cardiovascular disease.
A related new recommendation says sodium-glucose cotransporter 2 inhibitors are the preferred treatment for these patients who have heart failure or are at high risk of developing it.
In a new recommendation, the ADA suggests that patients with type 2 diabetes and chronic kidney disease potentially take a sodium-glucose cotransporter 2 inhibitor or a GLP-1 receptor agonist, which has been shown to reduce the risk of chronic kidney disease progression, cardiac events, or both.
There’s a greater focus on insulin as the preferred treatment for hyperglycemia in gestational diabetes mellitus “as it does not cross the placenta to a measurable extent.” The ADA also warns against metformin and glyburide as first-line agents because they “both cross the placenta to the fetus.”
Diabetes monitoring and screening
The ADA now recommends use of the American College of Cardiology’s atherosclerotic cardiovascular disease risk calculator, the ASCVD Risk Estimator Plus. The calculator assesses the risk of this disease over 10 years and is “generally a useful tool.”
The ACA recommends screening for cardiac risk factors at least once a year in patients with diabetes.
Physicians are no longer advised to check the feet of patients with diabetes at every visit; now the recommendation is for those at high risk of ulceration only. However, an annual examination of feet is recommended for all patients with diabetes.
The ADA now recommends that patients with type 2 diabetes or prediabetes undergo screening for nonalcoholic steatohepatitis and liver fibrosis if they have elevated liver enzymes or an ultrasound examination shows signs of fatty liver.
Gabapentin is now listed along with pregabalin and duloxetine as first-line drug treatments for neuropathic pain in diabetes.
The American Diabetes Association is out with new standard-of-care guidelines that – among other things – reject injectable insulin as the main first-line treatment for type 2 diabetes mellitus (T2DM), debut a cardiac risk calculator, and offer new recommendations regarding medications for patients with kidney disease, clogged arteries, and heart failure.
The ADA’s newly released 2019 Standards of Medical Care in Diabetes “emphasize a patient-centered approach that considers the multiple health and life factors of each person living with diabetes,” said William T. Cefalu, MD, the ADA’s chief scientific, medical, and mission officer, in a statement.
The 193-page guidelines are now available online at the Diabetes Care website and will be available via an app and the print edition of the journal.
Here’s a closer look at a few of the many new and revised recommendations in the 2019 Standards of Care.
Diabetes treatment
In a new guideline, the standards of care says glucagonlike peptide–1 (GLP-1) receptor agonists should be “a first-line treatment” – ahead of insulin – “for most [type 2] patients who need the greater efficacy of an injectable medication.”
However, the recommendations note that the “high costs and tolerability issues are important barriers to the use of GLP-1 receptor agonists.”
A new recommendation suggests the use of sodium-glucose cotransporter 2 inhibitors or GLP-1 receptor agonists “with demonstrated cardiovascular disease benefit” in patients with type 2 diabetes who have confirmed atherosclerotic cardiovascular disease.
A related new recommendation says sodium-glucose cotransporter 2 inhibitors are the preferred treatment for these patients who have heart failure or are at high risk of developing it.
In a new recommendation, the ADA suggests that patients with type 2 diabetes and chronic kidney disease potentially take a sodium-glucose cotransporter 2 inhibitor or a GLP-1 receptor agonist, which has been shown to reduce the risk of chronic kidney disease progression, cardiac events, or both.
There’s a greater focus on insulin as the preferred treatment for hyperglycemia in gestational diabetes mellitus “as it does not cross the placenta to a measurable extent.” The ADA also warns against metformin and glyburide as first-line agents because they “both cross the placenta to the fetus.”
Diabetes monitoring and screening
The ADA now recommends use of the American College of Cardiology’s atherosclerotic cardiovascular disease risk calculator, the ASCVD Risk Estimator Plus. The calculator assesses the risk of this disease over 10 years and is “generally a useful tool.”
The ACA recommends screening for cardiac risk factors at least once a year in patients with diabetes.
Physicians are no longer advised to check the feet of patients with diabetes at every visit; now the recommendation is for those at high risk of ulceration only. However, an annual examination of feet is recommended for all patients with diabetes.
The ADA now recommends that patients with type 2 diabetes or prediabetes undergo screening for nonalcoholic steatohepatitis and liver fibrosis if they have elevated liver enzymes or an ultrasound examination shows signs of fatty liver.
Gabapentin is now listed along with pregabalin and duloxetine as first-line drug treatments for neuropathic pain in diabetes.
The American Diabetes Association is out with new standard-of-care guidelines that – among other things – reject injectable insulin as the main first-line treatment for type 2 diabetes mellitus (T2DM), debut a cardiac risk calculator, and offer new recommendations regarding medications for patients with kidney disease, clogged arteries, and heart failure.
The ADA’s newly released 2019 Standards of Medical Care in Diabetes “emphasize a patient-centered approach that considers the multiple health and life factors of each person living with diabetes,” said William T. Cefalu, MD, the ADA’s chief scientific, medical, and mission officer, in a statement.
The 193-page guidelines are now available online at the Diabetes Care website and will be available via an app and the print edition of the journal.
Here’s a closer look at a few of the many new and revised recommendations in the 2019 Standards of Care.
Diabetes treatment
In a new guideline, the standards of care says glucagonlike peptide–1 (GLP-1) receptor agonists should be “a first-line treatment” – ahead of insulin – “for most [type 2] patients who need the greater efficacy of an injectable medication.”
However, the recommendations note that the “high costs and tolerability issues are important barriers to the use of GLP-1 receptor agonists.”
A new recommendation suggests the use of sodium-glucose cotransporter 2 inhibitors or GLP-1 receptor agonists “with demonstrated cardiovascular disease benefit” in patients with type 2 diabetes who have confirmed atherosclerotic cardiovascular disease.
A related new recommendation says sodium-glucose cotransporter 2 inhibitors are the preferred treatment for these patients who have heart failure or are at high risk of developing it.
In a new recommendation, the ADA suggests that patients with type 2 diabetes and chronic kidney disease potentially take a sodium-glucose cotransporter 2 inhibitor or a GLP-1 receptor agonist, which has been shown to reduce the risk of chronic kidney disease progression, cardiac events, or both.
There’s a greater focus on insulin as the preferred treatment for hyperglycemia in gestational diabetes mellitus “as it does not cross the placenta to a measurable extent.” The ADA also warns against metformin and glyburide as first-line agents because they “both cross the placenta to the fetus.”
Diabetes monitoring and screening
The ADA now recommends use of the American College of Cardiology’s atherosclerotic cardiovascular disease risk calculator, the ASCVD Risk Estimator Plus. The calculator assesses the risk of this disease over 10 years and is “generally a useful tool.”
The ACA recommends screening for cardiac risk factors at least once a year in patients with diabetes.
Physicians are no longer advised to check the feet of patients with diabetes at every visit; now the recommendation is for those at high risk of ulceration only. However, an annual examination of feet is recommended for all patients with diabetes.
The ADA now recommends that patients with type 2 diabetes or prediabetes undergo screening for nonalcoholic steatohepatitis and liver fibrosis if they have elevated liver enzymes or an ultrasound examination shows signs of fatty liver.
Gabapentin is now listed along with pregabalin and duloxetine as first-line drug treatments for neuropathic pain in diabetes.
FROM DIABETES CARE
New PTSD prevention guidelines released
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Hydrocortisone is only drug rated as an ‘intervention with emerging evidence of efficacy’
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
Barcelona – New evidence-based guidelines on posttraumatic stress disorder prevention and treatment from the International Society for Traumatic Stress Studies (ISTSS) highlight an uncomfortable truth: Namely, the basis for early formal intervention of any sort is sorely lacking.
“I’m acutely aware that a lot of people in the mental health field are not aware of the evidence base as it stands at the moment,” Jonathan I. Bisson, MD, said at the annual congress of the European College of Neuropsychopharmacology. “There’s something very human about trying to do something. I think we find it very hard to do nothing following a traumatic event.”
Dr. Bisson, a professor of psychiatry at Cardiff (Wales) University and the chair of the ISTSS guidelines committee, provided an advance look at the ISTSS guidelines, which have since been released.
Secondary prevention of PTSD can entail either blocking development of symptoms after exposure to trauma or treating early emergent PTSD symptoms. Dr. Bisson emphasized that, although multiple exciting prospects are on the horizon for secondary prevention, those interventions need further work before implementation. The ISTSS guidelines, based on the group’s meta-analyses of 361 randomized controlled trials, rated most of the diverse psychosocial, psychological, and pharmacologic interventions that have been proposed or are now actually being used in clinical practice as either “low effect,” “interventions with emerging evidence,” or “insufficient evidence to recommend.” Those interventions are not backed by sufficient evidence of efficacy to be ready for prime time use in clinical practice.
Morever, the potential for iatrogenic harm is very real.
to a trauma,” the psychiatrist observed. “It’s normal to cry after a bereavement, for example. But should we be pathologizing that, or is that the body’s way of actually bringing itself to terms with something that’s very extreme?
“So we’ve got to be careful in our efforts to shape emotional processing, which might do absolutely nothing – which I’d argue is a problem when we’ve got limited resources because we should be focusing those resources on things that make a difference. Or it could minimize or prevent prolonged distress or pathology, which is what we’re after. Or it could interfere with the adaptive acute stress response – and that’s a real problem and one we’ve got to be very careful about,” Dr. Bisson said. “So ‘primum non nocere’ – first do no harm – should be a principle we adhere to.”
Neurobiology of PTSD
The accepted view of the neurobiology of PTSD is that it represents a failure of the medial prefrontal/anterior cingulate network to regulate activity in the amygdala, with resultant hyperreactivity to threat. Enhanced negative feedback of cortisol occurs. The brain’s response to low cortisol is to increase levels of corticotropin-releasing factor, which has the unwanted consequence of increased locus coeruleus activity and noradrenaline release. The resultant adrenergic surge facilitates the laying down and consolidation of traumatic memories.
Also, low cortisol levels disinhibit retrieval of traumatic memories, so the affected individual thinks more about the trauma. All of this elicits an uncontrolled sympathetic response, so the patient remains in a constant state of hyperarousal characteristic of PTSD.
“In theory we should have some really simple ways to prevent PTSD from occurring if we get in there soon enough: reducing noradrenergic overactivity via alpha2-adrenergic receptor agonism with an agent such as clonidine; postsynaptic beta-adrenergic blocking with a drug such as propranolol; or alpha1-adrenergic receptor blocking, as with prazosin. All of these approaches reduce noradrenergic tone and therefore should be effective, in theory, to prevent PTSD.
“We should also be able to use indirect strategies to reduce noradrenergic overactivity: GABA agents like benzodiazepines, alcohol, and gabapentin oppose noradrenaline action in the amygdala. I’m not suggesting drinking all the time to prevent PTSD, but there’s a strong association in several studies, with about a 50% reduction in rates of PTSD in those who are intoxicated at the time of the trauma,” according to Dr. Bisson.
Unfortunately, to date, none of those pharmacologic approaches have been effective when studied in randomized trials.
One pharmacologic intervention
Only one drug, hydrocortisone, was rated an “intervention with emerging evidence of efficacy” for prevention of PTSD symptoms in adults when given within the first 3 months after a traumatic event. Three placebo-controlled, randomized trials have shown a positive effect.
“It should be said that most of the studies of hydrocortisone have been done in individuals following extreme physical illness, such as septic shock sufferers, so the generalizability is a bit of a question. Nevertheless, it’s the one agent that has meta-analytic evidence of being effective at preventing PTSD, although more research is needed,” Dr. Bisson said.
Results of randomized trials featuring those agents have been “really disappointing” in light of what seems a sound theoretic rationale, he continued.
“We’re really struggling from a pharmacologic perspective to know what to do. I would say we are still at the experimental stage, and there’s no real good evidence that we should give any medication to prevent PTSD,” Dr. Bisson said.
Early psychosocial interventions
The ISTSS guidelines rate only two single-session interventions for prevention as rising to the promising level of “emerging evidence” of clinically important benefit: single-session eye movement desensitization and reprocessing (EMDR), which in its multisession format is a well-established treatment with strong evidence of efficacy in established PTSD, and a program known as Group 512 PM, which combines group debriefing with group cohesion–building exercises.
“Group 512 PM was done in groups of Chinese army personnel helping in recovery efforts following a 2008 earthquake in China that killed 80,000 people. It resulted in nearly a 50% reduction in PTSD versus no debriefing. This cohesion training might be a clue to us as something to work on in the future,” Dr. Bisson said.
The ISTSS guidelines deem there is insufficient evidence to recommend single-session group debriefing, group stress management, heart stress management, group education, trauma-focused counselling, computerized visuospatial task, individual psychoeducation, or individual debriefing.
“In six randomized controlled trials over nearly the last 20 years, we see a strong signal that individual psychological debriefing isn’t effective. So, certainly, going into a room with an individual or a couple and talking about what they’ve been through in great detail and getting them to express their emotions and advising them that’s a normal reaction doesn’t seem to be enough. And rather worryingly, the people who tend to do worse with that sort of intervention are the people who’ve got the most symptoms when they started, so they’re the ones at highest risk of developing PTSD,” Dr. Bisson said.
Multisession prevention interventions such as brief dyadic therapy and self-guided Internet interventions are supported by emerging evidence. Less promising, and with insufficient evidence to recommend, according to the ISTSS, are brief interpersonal therapy, brief individual trauma processing therapy, telephone-based cognitive-behavioral therapy (CBT), and nurse-led intensive care recovery programs.
For multisession early treatment interventions for patients with emerging traumatic stress symptoms within the first 3 months, the new ISTSS guidelines recommend as standard therapy CBT with a trauma focus, EMDR, or cognitive therapy. Stepped or collaborative care is rated as having “low effect.” There is emerging evidence for structured writing interventions and Internet-based guided self-help. And there is insufficient evidence to recommend behavioral activation, Internet virtual reality therapy, telephone-based CBT with a trauma focus, computerized neurobehavioral training, or supportive counseling.
Treating adults with established PTSD
Pharmacotherapy, including fluoxetine, sertraline, paroxetine, and venlafaxine is rated in the guidelines as a low-effect treatment. Quetiapine has emerging evidence of efficacy. Everything else has insufficient evidence.
Psychological therapies such as EMDR, CBT with a trauma focus, prolonged exposure, cognitive therapy, and cognitive processing therapy received strong recommendations. In fact, those are the only interventions in the entire ISTSS guidelines that received a “strong recommendation” rating. A weaker “standard recommendation” is given to CBT without a trauma focus, narrative exposure therapy, present-centered therapy, group CBT with a trauma focus, and guided Internet-based therapy with a trauma focus. Interventions with emerging evidence of efficacy include virtual reality therapy, reconsolidation of traumatic memories, and couples CBT with a trauma focus.
Best-practice approach to prevention
“In my view, and what I tell people, is that after a traumatic event I think practical pragmatic support in an empathic manner is the best first step,” Dr. Bisson said. “And it doesn’t have to be provided by a mental health professional. In fact, your family and friends are the best people to provide that. And then, we watchfully wait to see if traumatic stress symptoms emerge. If they do, and particularly if their trajectory is going up, then at about 1 month, I would get in there and deliver a therapy, either CBT with a trauma focus, EMDR, or cognitive therapy with a trauma focus. All of those have a significant positive effect for this group.”
Although he restricted his talk to secondary prevention of PTSD in adults, the ISTSS guidelines also address early intervention in children and adolescents.
Dr. Bisson reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
ACR, NPF unveil new psoriatic arthritis treatment guideline
The American College of Rheumatology and the National Psoriasis Foundation have released a joint treatment guideline for psoriatic arthritis that, for the first time, includes a conditional recommendation to use tumor necrosis factor–inhibitor(TNFi) biologics over methotrexate and other oral small molecules as a first-line therapy in patients with active disease.
“The available low-quality evidence is inconclusive regarding the efficacy of OSMs [oral small molecules] in management of PsA, whereas there is moderate-quality evidence of the benefits of TNFi biologics, in particular regarding their impact on the prevention of disease progression and joint damage,” wrote the panel of authors, who were led by Jasvinder A. Singh, MD, of the University of Alabama at Birmingham. “In making their recommendation, the panel recognized the cost implications, but put considerations of quality of evidence for benefit over other considerations. This guideline provides recommendations for early and aggressive therapy in patients with newly diagnosed PsA.”
The 28-page guideline, published online Nov. 30 in the Journal of Psoriasis and Psoriatic Arthritis and also in Arthritis Care & Research and Arthritis & Rheumatology, is the first set of PsA-specific recommendations to be assembled using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology that the ACR has used for RA and other conditions. GRADE uses systematic reviews of the scientific literature available to evaluate and grade the quality of evidence in a particular domain. The evidence reviews are then used to create guideline recommendations for or against particular therapy options that range from strong to conditional, depending on the quality of evidence available.
Based on the GRADE methodology and consensus building, the guideline authors crafted recommendations for eight different clinical scenarios, including the initial treatment of patients with active PsA who have not received either OSMs or other treatments; treatment of patients with active PsA despite treatment with an OSM; treatment of patients with active PsA despite treatment with a TNFi biologic either as monotherapy or in combination with methotrexate; treatment of patients with active PsA despite treatment with an interleukin (IL)-17 inhibitor or IL-12/23 inhibitor monotherapy; treatment of patients with active PsA including treat-to-target, active axial disease, enthesitis, or active inflammatory bowel disease; treatment of patients with active PsA and comorbidities, including concomitant diabetes and recurrent serious infections; vaccination in patients with active PsA; and treatment of patients with active PsA with nonpharmacologic interventions such as yoga and weight loss. Most of the treatment recommendations are conditional based on very low to moderate quality evidence. “Health care providers and patients must take into consideration all active disease domains, comorbidities, and the patient’s functional status in choosing the optimal therapy for an individual at a given point in time,” the authors emphasized.
Only five of the recommendations are listed as strong, including smoking cessation. Three of the strong recommendations concern adult patients with active PsA and concomitant active inflammatory bowel disease despite treatment with an OSM. They are “switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic,” “switch to a TNFi monoclonal antibody biologic over an IL-7i biologic,” and “switch to an IL-12/23i biologic over switching to an IL-17i biologic.”
The process of creating the guideline included input from a panel of nine adults who provided the authors with perspective on their values and preferences. “The concept of treat-to-target was challenging for patients,” the authors noted. “Although they saw value in improved outcomes, they also thought this strategy could increase costs to the patient (e.g., copayments, time traveling to more frequent appointments, etc.) and potentially increase adverse events. Therefore, a detailed conversation with the patient is needed to make decisions regarding treat-to-target.”
The authors concluded the guideline by calling for more comparative data to inform treatment selection in the future. “Several ongoing trials, including a trial to compare a TNFi biologic combination therapy with a TNFi biologic monotherapy and MTX monotherapy, will inform treatment decisions,” they wrote. “We anticipate future updates to the guideline when new evidence is available.”
Dr. Singh, who is also a staff rheumatologist at the Birmingham (Ala.) Veterans Affairs Medical Center, led development of the 2012 and 2015 ACR treatment guidelines for RA. He has received consulting fees from a variety of companies marketing rheumatologic drugs as well as research support from Takeda and Savient. The other guideline authors reported having numerous financial ties to industry.
SOURCE: Singh J et al. Arthritis Care Res. 2018 Nov 30. doi: 10.1002/acr.23789.
The American College of Rheumatology and the National Psoriasis Foundation have released a joint treatment guideline for psoriatic arthritis that, for the first time, includes a conditional recommendation to use tumor necrosis factor–inhibitor(TNFi) biologics over methotrexate and other oral small molecules as a first-line therapy in patients with active disease.
“The available low-quality evidence is inconclusive regarding the efficacy of OSMs [oral small molecules] in management of PsA, whereas there is moderate-quality evidence of the benefits of TNFi biologics, in particular regarding their impact on the prevention of disease progression and joint damage,” wrote the panel of authors, who were led by Jasvinder A. Singh, MD, of the University of Alabama at Birmingham. “In making their recommendation, the panel recognized the cost implications, but put considerations of quality of evidence for benefit over other considerations. This guideline provides recommendations for early and aggressive therapy in patients with newly diagnosed PsA.”
The 28-page guideline, published online Nov. 30 in the Journal of Psoriasis and Psoriatic Arthritis and also in Arthritis Care & Research and Arthritis & Rheumatology, is the first set of PsA-specific recommendations to be assembled using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology that the ACR has used for RA and other conditions. GRADE uses systematic reviews of the scientific literature available to evaluate and grade the quality of evidence in a particular domain. The evidence reviews are then used to create guideline recommendations for or against particular therapy options that range from strong to conditional, depending on the quality of evidence available.
Based on the GRADE methodology and consensus building, the guideline authors crafted recommendations for eight different clinical scenarios, including the initial treatment of patients with active PsA who have not received either OSMs or other treatments; treatment of patients with active PsA despite treatment with an OSM; treatment of patients with active PsA despite treatment with a TNFi biologic either as monotherapy or in combination with methotrexate; treatment of patients with active PsA despite treatment with an interleukin (IL)-17 inhibitor or IL-12/23 inhibitor monotherapy; treatment of patients with active PsA including treat-to-target, active axial disease, enthesitis, or active inflammatory bowel disease; treatment of patients with active PsA and comorbidities, including concomitant diabetes and recurrent serious infections; vaccination in patients with active PsA; and treatment of patients with active PsA with nonpharmacologic interventions such as yoga and weight loss. Most of the treatment recommendations are conditional based on very low to moderate quality evidence. “Health care providers and patients must take into consideration all active disease domains, comorbidities, and the patient’s functional status in choosing the optimal therapy for an individual at a given point in time,” the authors emphasized.
Only five of the recommendations are listed as strong, including smoking cessation. Three of the strong recommendations concern adult patients with active PsA and concomitant active inflammatory bowel disease despite treatment with an OSM. They are “switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic,” “switch to a TNFi monoclonal antibody biologic over an IL-7i biologic,” and “switch to an IL-12/23i biologic over switching to an IL-17i biologic.”
The process of creating the guideline included input from a panel of nine adults who provided the authors with perspective on their values and preferences. “The concept of treat-to-target was challenging for patients,” the authors noted. “Although they saw value in improved outcomes, they also thought this strategy could increase costs to the patient (e.g., copayments, time traveling to more frequent appointments, etc.) and potentially increase adverse events. Therefore, a detailed conversation with the patient is needed to make decisions regarding treat-to-target.”
The authors concluded the guideline by calling for more comparative data to inform treatment selection in the future. “Several ongoing trials, including a trial to compare a TNFi biologic combination therapy with a TNFi biologic monotherapy and MTX monotherapy, will inform treatment decisions,” they wrote. “We anticipate future updates to the guideline when new evidence is available.”
Dr. Singh, who is also a staff rheumatologist at the Birmingham (Ala.) Veterans Affairs Medical Center, led development of the 2012 and 2015 ACR treatment guidelines for RA. He has received consulting fees from a variety of companies marketing rheumatologic drugs as well as research support from Takeda and Savient. The other guideline authors reported having numerous financial ties to industry.
SOURCE: Singh J et al. Arthritis Care Res. 2018 Nov 30. doi: 10.1002/acr.23789.
The American College of Rheumatology and the National Psoriasis Foundation have released a joint treatment guideline for psoriatic arthritis that, for the first time, includes a conditional recommendation to use tumor necrosis factor–inhibitor(TNFi) biologics over methotrexate and other oral small molecules as a first-line therapy in patients with active disease.
“The available low-quality evidence is inconclusive regarding the efficacy of OSMs [oral small molecules] in management of PsA, whereas there is moderate-quality evidence of the benefits of TNFi biologics, in particular regarding their impact on the prevention of disease progression and joint damage,” wrote the panel of authors, who were led by Jasvinder A. Singh, MD, of the University of Alabama at Birmingham. “In making their recommendation, the panel recognized the cost implications, but put considerations of quality of evidence for benefit over other considerations. This guideline provides recommendations for early and aggressive therapy in patients with newly diagnosed PsA.”
The 28-page guideline, published online Nov. 30 in the Journal of Psoriasis and Psoriatic Arthritis and also in Arthritis Care & Research and Arthritis & Rheumatology, is the first set of PsA-specific recommendations to be assembled using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology that the ACR has used for RA and other conditions. GRADE uses systematic reviews of the scientific literature available to evaluate and grade the quality of evidence in a particular domain. The evidence reviews are then used to create guideline recommendations for or against particular therapy options that range from strong to conditional, depending on the quality of evidence available.
Based on the GRADE methodology and consensus building, the guideline authors crafted recommendations for eight different clinical scenarios, including the initial treatment of patients with active PsA who have not received either OSMs or other treatments; treatment of patients with active PsA despite treatment with an OSM; treatment of patients with active PsA despite treatment with a TNFi biologic either as monotherapy or in combination with methotrexate; treatment of patients with active PsA despite treatment with an interleukin (IL)-17 inhibitor or IL-12/23 inhibitor monotherapy; treatment of patients with active PsA including treat-to-target, active axial disease, enthesitis, or active inflammatory bowel disease; treatment of patients with active PsA and comorbidities, including concomitant diabetes and recurrent serious infections; vaccination in patients with active PsA; and treatment of patients with active PsA with nonpharmacologic interventions such as yoga and weight loss. Most of the treatment recommendations are conditional based on very low to moderate quality evidence. “Health care providers and patients must take into consideration all active disease domains, comorbidities, and the patient’s functional status in choosing the optimal therapy for an individual at a given point in time,” the authors emphasized.
Only five of the recommendations are listed as strong, including smoking cessation. Three of the strong recommendations concern adult patients with active PsA and concomitant active inflammatory bowel disease despite treatment with an OSM. They are “switch to a monoclonal antibody TNFi biologic over a TNFi biologic soluble receptor biologic,” “switch to a TNFi monoclonal antibody biologic over an IL-7i biologic,” and “switch to an IL-12/23i biologic over switching to an IL-17i biologic.”
The process of creating the guideline included input from a panel of nine adults who provided the authors with perspective on their values and preferences. “The concept of treat-to-target was challenging for patients,” the authors noted. “Although they saw value in improved outcomes, they also thought this strategy could increase costs to the patient (e.g., copayments, time traveling to more frequent appointments, etc.) and potentially increase adverse events. Therefore, a detailed conversation with the patient is needed to make decisions regarding treat-to-target.”
The authors concluded the guideline by calling for more comparative data to inform treatment selection in the future. “Several ongoing trials, including a trial to compare a TNFi biologic combination therapy with a TNFi biologic monotherapy and MTX monotherapy, will inform treatment decisions,” they wrote. “We anticipate future updates to the guideline when new evidence is available.”
Dr. Singh, who is also a staff rheumatologist at the Birmingham (Ala.) Veterans Affairs Medical Center, led development of the 2012 and 2015 ACR treatment guidelines for RA. He has received consulting fees from a variety of companies marketing rheumatologic drugs as well as research support from Takeda and Savient. The other guideline authors reported having numerous financial ties to industry.
SOURCE: Singh J et al. Arthritis Care Res. 2018 Nov 30. doi: 10.1002/acr.23789.
FROM ARTHRITIS CARE & RESEARCH
PAD guidelines: Consensus needed between U.S. and Europe
Recent advances in the management of peripheral artery disease (PAD) have resulted in new guideline creation in both the United States and Europe.
While there is considerable consensus between the guidelines, there are multiple differences in emphasis and a differing approach to the types and quality of evidence used to back recommendations, according to a comparative review published in the Journal of the American College of Cardiology. The American Heart Association and American College of Cardiology, together with other organizations, issued an update to their previous guidelines on the management and diagnosis of lower extremity PAD in 2016. In 2017, the European Society of Cardiology in conjunction with the European Society for Vascular Surgery updated their own comprehensive guidelines.
Both the U.S. and the European guidelines stress the importance of lowering risk factors for PAD. This includes stopping smoking, lipid and blood pressure management, and controlling glucose, according to Aaron P. Kithcart, MD, of Brigham and Women’s Hospital, Boston, and Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn.
However, the U.S. guidelines focus more on moderating lifestyle factors, including the pursuit of regular physical activity and the use of supervised exercise, whereas the European guidelines focus considerable attention on recommendations for revascularization in patients with limb-threatening ischemia.
Perhaps the major source of variation between the two sets of guidelines, according to the reviewers, are based upon the intended audiences: “The American document limits its focus to PAD but is applicable to practitioners of every background, whereas the European guideline extends the discussion to all PADs to include carotid and vertebral, upper extremities, mesenteric, and renal arteries in addition to lower-extremity artery disease; but is designed to be a source for cardiologists.”
Accordingly, the ESC/ESVS guidelines approach medical therapy with a more holistic flavor, whereas the ACC/AHA guidelines are specific to the lower-extremity complications of atherosclerosis, according to the reviewers.
Both sets of guidelines come to the conclusion that there is a need for more evidence to identify patients who are at the greatest risk of tissue loss, but overall they differ in their approach to available data. The ACC/AHA is more inclusive of smaller, well-done nonrandomized studies, whereas the ESC/ESVS relegates small studies to Level of Evidence: C. “We believe this difference drives the variation of therapeutic recommendations more than any other factor,” the authors note.
More randomized studies would align recommendations across both organizations, according to Dr. Kithcart and Dr. Beckman (JACC 2018;72:2789-801).
“The management of PAD has progressed a great deal over the last decade. ... Several clinical trials over the coming years should help clarify how revascularization should be approached, and which patients are most likely to benefit. Until then, maintaining good cardiovascular health, including regular physical activity, smoking cessation, lipid-lowering therapy, blood pressure management, and glucose control have the most benefit in patients with PAD,” the researchers concluded.
Dr. Beckman served as a consultant for several pharmaceutical companies, and on the data and safety monitoring board for Bayer and Novartis. Dr. Kithcart reported that he has no relevant conflicts.
SOURCE: Kithcart, AP et al. JACC 2018;72:2789-801.
Recent advances in the management of peripheral artery disease (PAD) have resulted in new guideline creation in both the United States and Europe.
While there is considerable consensus between the guidelines, there are multiple differences in emphasis and a differing approach to the types and quality of evidence used to back recommendations, according to a comparative review published in the Journal of the American College of Cardiology. The American Heart Association and American College of Cardiology, together with other organizations, issued an update to their previous guidelines on the management and diagnosis of lower extremity PAD in 2016. In 2017, the European Society of Cardiology in conjunction with the European Society for Vascular Surgery updated their own comprehensive guidelines.
Both the U.S. and the European guidelines stress the importance of lowering risk factors for PAD. This includes stopping smoking, lipid and blood pressure management, and controlling glucose, according to Aaron P. Kithcart, MD, of Brigham and Women’s Hospital, Boston, and Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn.
However, the U.S. guidelines focus more on moderating lifestyle factors, including the pursuit of regular physical activity and the use of supervised exercise, whereas the European guidelines focus considerable attention on recommendations for revascularization in patients with limb-threatening ischemia.
Perhaps the major source of variation between the two sets of guidelines, according to the reviewers, are based upon the intended audiences: “The American document limits its focus to PAD but is applicable to practitioners of every background, whereas the European guideline extends the discussion to all PADs to include carotid and vertebral, upper extremities, mesenteric, and renal arteries in addition to lower-extremity artery disease; but is designed to be a source for cardiologists.”
Accordingly, the ESC/ESVS guidelines approach medical therapy with a more holistic flavor, whereas the ACC/AHA guidelines are specific to the lower-extremity complications of atherosclerosis, according to the reviewers.
Both sets of guidelines come to the conclusion that there is a need for more evidence to identify patients who are at the greatest risk of tissue loss, but overall they differ in their approach to available data. The ACC/AHA is more inclusive of smaller, well-done nonrandomized studies, whereas the ESC/ESVS relegates small studies to Level of Evidence: C. “We believe this difference drives the variation of therapeutic recommendations more than any other factor,” the authors note.
More randomized studies would align recommendations across both organizations, according to Dr. Kithcart and Dr. Beckman (JACC 2018;72:2789-801).
“The management of PAD has progressed a great deal over the last decade. ... Several clinical trials over the coming years should help clarify how revascularization should be approached, and which patients are most likely to benefit. Until then, maintaining good cardiovascular health, including regular physical activity, smoking cessation, lipid-lowering therapy, blood pressure management, and glucose control have the most benefit in patients with PAD,” the researchers concluded.
Dr. Beckman served as a consultant for several pharmaceutical companies, and on the data and safety monitoring board for Bayer and Novartis. Dr. Kithcart reported that he has no relevant conflicts.
SOURCE: Kithcart, AP et al. JACC 2018;72:2789-801.
Recent advances in the management of peripheral artery disease (PAD) have resulted in new guideline creation in both the United States and Europe.
While there is considerable consensus between the guidelines, there are multiple differences in emphasis and a differing approach to the types and quality of evidence used to back recommendations, according to a comparative review published in the Journal of the American College of Cardiology. The American Heart Association and American College of Cardiology, together with other organizations, issued an update to their previous guidelines on the management and diagnosis of lower extremity PAD in 2016. In 2017, the European Society of Cardiology in conjunction with the European Society for Vascular Surgery updated their own comprehensive guidelines.
Both the U.S. and the European guidelines stress the importance of lowering risk factors for PAD. This includes stopping smoking, lipid and blood pressure management, and controlling glucose, according to Aaron P. Kithcart, MD, of Brigham and Women’s Hospital, Boston, and Joshua A. Beckman, MD, of Vanderbilt University, Nashville, Tenn.
However, the U.S. guidelines focus more on moderating lifestyle factors, including the pursuit of regular physical activity and the use of supervised exercise, whereas the European guidelines focus considerable attention on recommendations for revascularization in patients with limb-threatening ischemia.
Perhaps the major source of variation between the two sets of guidelines, according to the reviewers, are based upon the intended audiences: “The American document limits its focus to PAD but is applicable to practitioners of every background, whereas the European guideline extends the discussion to all PADs to include carotid and vertebral, upper extremities, mesenteric, and renal arteries in addition to lower-extremity artery disease; but is designed to be a source for cardiologists.”
Accordingly, the ESC/ESVS guidelines approach medical therapy with a more holistic flavor, whereas the ACC/AHA guidelines are specific to the lower-extremity complications of atherosclerosis, according to the reviewers.
Both sets of guidelines come to the conclusion that there is a need for more evidence to identify patients who are at the greatest risk of tissue loss, but overall they differ in their approach to available data. The ACC/AHA is more inclusive of smaller, well-done nonrandomized studies, whereas the ESC/ESVS relegates small studies to Level of Evidence: C. “We believe this difference drives the variation of therapeutic recommendations more than any other factor,” the authors note.
More randomized studies would align recommendations across both organizations, according to Dr. Kithcart and Dr. Beckman (JACC 2018;72:2789-801).
“The management of PAD has progressed a great deal over the last decade. ... Several clinical trials over the coming years should help clarify how revascularization should be approached, and which patients are most likely to benefit. Until then, maintaining good cardiovascular health, including regular physical activity, smoking cessation, lipid-lowering therapy, blood pressure management, and glucose control have the most benefit in patients with PAD,” the researchers concluded.
Dr. Beckman served as a consultant for several pharmaceutical companies, and on the data and safety monitoring board for Bayer and Novartis. Dr. Kithcart reported that he has no relevant conflicts.
SOURCE: Kithcart, AP et al. JACC 2018;72:2789-801.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Sickle cell disease guidelines release set for early 2019
, according to Robert Liem, MD, chair of the American Society of Hematology coordination panel for the initiative.
The new clinical practice recommendations will expand on 2014 guidelines published by the National Heart, Lung, and Blood Institute in a way that will help both hematologists and nonhematologists who take care of patients with sickle cell disease, Dr. Liem said in a video interview at the annual meeting of the American Society of Hematology.
Five different guidelines are under development to cover different aspects of acute and chronic complications of sickle cell disease, including pain, cardiopulmonary and kidney disease, cerebrovascular disease, transfusion support, and stem cell transplantation.
Watch the video to learn more about the guideline effort from the perspective of Dr. Liem, who is also the director of the Comprehensive Sickle Cell Program at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
, according to Robert Liem, MD, chair of the American Society of Hematology coordination panel for the initiative.
The new clinical practice recommendations will expand on 2014 guidelines published by the National Heart, Lung, and Blood Institute in a way that will help both hematologists and nonhematologists who take care of patients with sickle cell disease, Dr. Liem said in a video interview at the annual meeting of the American Society of Hematology.
Five different guidelines are under development to cover different aspects of acute and chronic complications of sickle cell disease, including pain, cardiopulmonary and kidney disease, cerebrovascular disease, transfusion support, and stem cell transplantation.
Watch the video to learn more about the guideline effort from the perspective of Dr. Liem, who is also the director of the Comprehensive Sickle Cell Program at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
, according to Robert Liem, MD, chair of the American Society of Hematology coordination panel for the initiative.
The new clinical practice recommendations will expand on 2014 guidelines published by the National Heart, Lung, and Blood Institute in a way that will help both hematologists and nonhematologists who take care of patients with sickle cell disease, Dr. Liem said in a video interview at the annual meeting of the American Society of Hematology.
Five different guidelines are under development to cover different aspects of acute and chronic complications of sickle cell disease, including pain, cardiopulmonary and kidney disease, cerebrovascular disease, transfusion support, and stem cell transplantation.
Watch the video to learn more about the guideline effort from the perspective of Dr. Liem, who is also the director of the Comprehensive Sickle Cell Program at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
FROM ASH 2018