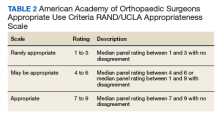
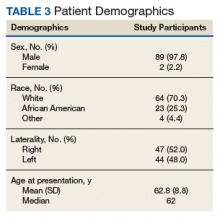
User login
New consensus recommendations on vaccination in hemophilia
New consensus statements, released by a panel of 11 experts, provide 27 specific recommendations related to the use of vaccines in patients with hemophilia.
The recommendations from the Italian Haemophilia and Vaccinations (HEVA) project were authored by an 11-member committee with expertise in both hemophilia and immunization. The authors reviewed 20 relevant studies published before August 30, 2017.
“Vaccination of patients with severe congenital bleeding disorders remains a challenge, and clinicians are often uncertain about immunization recommendations for these patients,” wrote Elena Santagostino, MD, PhD, of the Centro Emofilia e Trombosi Angelo Bianchi Bonomi in Milan, Italy, and her colleagues. The report is published in Haemophilia.
The statements were also validated by separate groups of clinicians at hemophilia treatment centers across Italy.
The key issues described included vaccination schedule, route of administration, vaccination of patients with antibodies that impede coagulation factor VIII inhibitors, and risk of inhibitor formation with vaccination.
In general, committee members agreed on the majority of statements, with the exception of those related to the possible links between vaccination and inhibitor formation. There were a total of five statements on which no consensus was reached.
Vaccinations in both adults and children with hemophilia should be provided in accordance with the institutional schedule for those without bleeding disorders, according to the recommendations.
“The only difference is that vaccination of patients with haemophilia requires comprehensive planning, taking into account disease severity, type and route of vaccination, and bleeding risk,” the experts wrote.
The panel also reported that current data suggest that vaccination timing is not affected by the timing of factor VIII replacement.
The authors acknowledged that the recommendations were formed by a group of experts from Italy using a specific consensus framework. As a result, they may not be applicable to all patient populations.
The manuscript was supported by Sobi. The authors reported financial relationships with Bayer, Bioverativ, CSL Behring, Grifols, Kedrion, Novo Nordisk, Pfizer, and other companies.
SOURCE: Santagostino E et al. Haemophilia. 2019 Apr 16. doi: 10.1111/hae.13756.
New consensus statements, released by a panel of 11 experts, provide 27 specific recommendations related to the use of vaccines in patients with hemophilia.
The recommendations from the Italian Haemophilia and Vaccinations (HEVA) project were authored by an 11-member committee with expertise in both hemophilia and immunization. The authors reviewed 20 relevant studies published before August 30, 2017.
“Vaccination of patients with severe congenital bleeding disorders remains a challenge, and clinicians are often uncertain about immunization recommendations for these patients,” wrote Elena Santagostino, MD, PhD, of the Centro Emofilia e Trombosi Angelo Bianchi Bonomi in Milan, Italy, and her colleagues. The report is published in Haemophilia.
The statements were also validated by separate groups of clinicians at hemophilia treatment centers across Italy.
The key issues described included vaccination schedule, route of administration, vaccination of patients with antibodies that impede coagulation factor VIII inhibitors, and risk of inhibitor formation with vaccination.
In general, committee members agreed on the majority of statements, with the exception of those related to the possible links between vaccination and inhibitor formation. There were a total of five statements on which no consensus was reached.
Vaccinations in both adults and children with hemophilia should be provided in accordance with the institutional schedule for those without bleeding disorders, according to the recommendations.
“The only difference is that vaccination of patients with haemophilia requires comprehensive planning, taking into account disease severity, type and route of vaccination, and bleeding risk,” the experts wrote.
The panel also reported that current data suggest that vaccination timing is not affected by the timing of factor VIII replacement.
The authors acknowledged that the recommendations were formed by a group of experts from Italy using a specific consensus framework. As a result, they may not be applicable to all patient populations.
The manuscript was supported by Sobi. The authors reported financial relationships with Bayer, Bioverativ, CSL Behring, Grifols, Kedrion, Novo Nordisk, Pfizer, and other companies.
SOURCE: Santagostino E et al. Haemophilia. 2019 Apr 16. doi: 10.1111/hae.13756.
New consensus statements, released by a panel of 11 experts, provide 27 specific recommendations related to the use of vaccines in patients with hemophilia.
The recommendations from the Italian Haemophilia and Vaccinations (HEVA) project were authored by an 11-member committee with expertise in both hemophilia and immunization. The authors reviewed 20 relevant studies published before August 30, 2017.
“Vaccination of patients with severe congenital bleeding disorders remains a challenge, and clinicians are often uncertain about immunization recommendations for these patients,” wrote Elena Santagostino, MD, PhD, of the Centro Emofilia e Trombosi Angelo Bianchi Bonomi in Milan, Italy, and her colleagues. The report is published in Haemophilia.
The statements were also validated by separate groups of clinicians at hemophilia treatment centers across Italy.
The key issues described included vaccination schedule, route of administration, vaccination of patients with antibodies that impede coagulation factor VIII inhibitors, and risk of inhibitor formation with vaccination.
In general, committee members agreed on the majority of statements, with the exception of those related to the possible links between vaccination and inhibitor formation. There were a total of five statements on which no consensus was reached.
Vaccinations in both adults and children with hemophilia should be provided in accordance with the institutional schedule for those without bleeding disorders, according to the recommendations.
“The only difference is that vaccination of patients with haemophilia requires comprehensive planning, taking into account disease severity, type and route of vaccination, and bleeding risk,” the experts wrote.
The panel also reported that current data suggest that vaccination timing is not affected by the timing of factor VIII replacement.
The authors acknowledged that the recommendations were formed by a group of experts from Italy using a specific consensus framework. As a result, they may not be applicable to all patient populations.
The manuscript was supported by Sobi. The authors reported financial relationships with Bayer, Bioverativ, CSL Behring, Grifols, Kedrion, Novo Nordisk, Pfizer, and other companies.
SOURCE: Santagostino E et al. Haemophilia. 2019 Apr 16. doi: 10.1111/hae.13756.
FROM HAEMOPHILIA
First North American clinical guidelines for hidradenitis suppurativa released
Rigorous evidence is unavailable for most interventions for treating hidradenitis suppurativa (HS), so management needs to be individualized, according to the
The guidelines were published in the Journal of the American Academy of Dermatology.
In an interview, Christopher Sayed, MD, cochair of the guidelines committee, of the department of dermatology at the University of North Carolina, Chapel Hill, said in an interview that the North American guidelines vary from the British Association of Dermatologists (Br J Dermatol. 2018 Dec 15. doi: 10.1111/bjd.17537) and European guidelines (J Eur Acad Dermatol Venereol. 2015 Apr;29[4]:619-44). For example, surgery is an active treatment option for various stages of the disorder in the North American guidelines, whereas surgery is considered a last-ditch effort in the British and European guidelines.
Surgical intervention is often needed “for patients to be the best they can be, and it can be difficult for medicine alone to fix [certain] patients,” Dr. Sayed said. This point that “using medical treatment alone or just surgical treatment alone often doesn’t lead to the best outcome” is stressed in the North American guidelines, he noted.
Limited evidence for high-level recommendations
Adalimumab (Humira), a tumor necrosis factor blocker, was the only therapy for which level 1A evidence is available, and this is because of its study in large-scale randomized controlled trials. (The Food and Drug Administration approved adalimumab in 2015 for treating moderate to severe HS.) Other biologic therapies such as infliximab, anakinra, and ustekinumab carry level 2B recommendations, which Dr. Sayed said will likely influence the availability of these therapies.
Similarly, Nd:YAG laser carries a level 2B recommendation, as does wide excision surgical intervention.
Many of the other treatment modalities explored in the guidelines are not well supported by the literature, according to Dr. Sayed . “The vast majority ... had category C recommendations,” he said. “Some things we tried to evaluate that, really, we can’t give any recommendation on at all because there was no evidence.” He noted that changes in lifestyle and dietary practices are issues patients with HS frequently bring up, but they carry very little evidence of benefit in the literature.
“Even things we use very commonly in HS are often supported by weak evidence because we have to rely on clinical experience. ... There’s just not funding for trials of older drugs or lifestyle interventions,” he said. Treatment mainstays such as tetracycline-class antibiotics, for example, similarly have no large-scale randomized controlled trials to support their use.
Consider comorbidity screening
Treatment is frequently complicated by patient comorbidities, including type 2 diabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), and impaired sexual health, said Dr. Sayed.
“The disease leads to scarring and disfigurement, and can [have a] higher impact on quality of life than almost any other dermatologic disease if you compare them side by side using quality of life measures,” he noted. “We know these patients are more likely to have depression, there are high rates of suicide among these patients. A lot of that has to do with the fact that it’s a chronic disease where there is pain and disfigurement, and patients often grow very, very frustrated in part due to the disease.”
He advised consistent follow-up to ensure patients are not frustrated because of lack of perceived progress.
No one-size-fits-all approach
Dr. Sayed said individualized management is one of the most important parts of taking care of a patient with HS. For example, patients with Hurley stage 1 and Hurley stage 2 variations of the disease may present very differently, and that is the reason why the guidelines do not offer a stepwise treatment algorithm for the disease.
“[The guidelines] have many treatments that may overlap different stages of disease, where those things might need to be used together,” he said. “It takes a lot of discussion with patients about their treatment preferences and listening to whether their disease is progressing or not, whether or not it’s stable, and then trying to figure out whether things like surgery fit into the treatment strategy.”
Despite these recommendations, there may be situations where the answer is not listed in the guidelines. “The guidelines are based on evidence that’s available at the time we review the literature,” he said. “For many patients, they may fail every treatment that’s recommended within the guidelines. There will be times where you have to be creative for patients and go beyond what the guidelines can currently recommend and give patients the treatment that they need even when it seems like all options have been exhausted.”
The authors report relationships with 3M, AbbVie, Adelphi Values, Amgen, BSN, Celgene, Chemocentryx, Coloplast, Galderma, Hidramed Solutions, Hollister, the HS Foundation, Incyte, InflaRx, Integra, Janssen, KCI Inc., Lenicura, Leo, Lilly, The Microdermis Corporation, Novartis, Pfizer, UCB, Valeant, and XBiotech both inside and outside the supported work.
SOURCES: Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.067; Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.068.
Rigorous evidence is unavailable for most interventions for treating hidradenitis suppurativa (HS), so management needs to be individualized, according to the
The guidelines were published in the Journal of the American Academy of Dermatology.
In an interview, Christopher Sayed, MD, cochair of the guidelines committee, of the department of dermatology at the University of North Carolina, Chapel Hill, said in an interview that the North American guidelines vary from the British Association of Dermatologists (Br J Dermatol. 2018 Dec 15. doi: 10.1111/bjd.17537) and European guidelines (J Eur Acad Dermatol Venereol. 2015 Apr;29[4]:619-44). For example, surgery is an active treatment option for various stages of the disorder in the North American guidelines, whereas surgery is considered a last-ditch effort in the British and European guidelines.
Surgical intervention is often needed “for patients to be the best they can be, and it can be difficult for medicine alone to fix [certain] patients,” Dr. Sayed said. This point that “using medical treatment alone or just surgical treatment alone often doesn’t lead to the best outcome” is stressed in the North American guidelines, he noted.
Limited evidence for high-level recommendations
Adalimumab (Humira), a tumor necrosis factor blocker, was the only therapy for which level 1A evidence is available, and this is because of its study in large-scale randomized controlled trials. (The Food and Drug Administration approved adalimumab in 2015 for treating moderate to severe HS.) Other biologic therapies such as infliximab, anakinra, and ustekinumab carry level 2B recommendations, which Dr. Sayed said will likely influence the availability of these therapies.
Similarly, Nd:YAG laser carries a level 2B recommendation, as does wide excision surgical intervention.
Many of the other treatment modalities explored in the guidelines are not well supported by the literature, according to Dr. Sayed . “The vast majority ... had category C recommendations,” he said. “Some things we tried to evaluate that, really, we can’t give any recommendation on at all because there was no evidence.” He noted that changes in lifestyle and dietary practices are issues patients with HS frequently bring up, but they carry very little evidence of benefit in the literature.
“Even things we use very commonly in HS are often supported by weak evidence because we have to rely on clinical experience. ... There’s just not funding for trials of older drugs or lifestyle interventions,” he said. Treatment mainstays such as tetracycline-class antibiotics, for example, similarly have no large-scale randomized controlled trials to support their use.
Consider comorbidity screening
Treatment is frequently complicated by patient comorbidities, including type 2 diabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), and impaired sexual health, said Dr. Sayed.
“The disease leads to scarring and disfigurement, and can [have a] higher impact on quality of life than almost any other dermatologic disease if you compare them side by side using quality of life measures,” he noted. “We know these patients are more likely to have depression, there are high rates of suicide among these patients. A lot of that has to do with the fact that it’s a chronic disease where there is pain and disfigurement, and patients often grow very, very frustrated in part due to the disease.”
He advised consistent follow-up to ensure patients are not frustrated because of lack of perceived progress.
No one-size-fits-all approach
Dr. Sayed said individualized management is one of the most important parts of taking care of a patient with HS. For example, patients with Hurley stage 1 and Hurley stage 2 variations of the disease may present very differently, and that is the reason why the guidelines do not offer a stepwise treatment algorithm for the disease.
“[The guidelines] have many treatments that may overlap different stages of disease, where those things might need to be used together,” he said. “It takes a lot of discussion with patients about their treatment preferences and listening to whether their disease is progressing or not, whether or not it’s stable, and then trying to figure out whether things like surgery fit into the treatment strategy.”
Despite these recommendations, there may be situations where the answer is not listed in the guidelines. “The guidelines are based on evidence that’s available at the time we review the literature,” he said. “For many patients, they may fail every treatment that’s recommended within the guidelines. There will be times where you have to be creative for patients and go beyond what the guidelines can currently recommend and give patients the treatment that they need even when it seems like all options have been exhausted.”
The authors report relationships with 3M, AbbVie, Adelphi Values, Amgen, BSN, Celgene, Chemocentryx, Coloplast, Galderma, Hidramed Solutions, Hollister, the HS Foundation, Incyte, InflaRx, Integra, Janssen, KCI Inc., Lenicura, Leo, Lilly, The Microdermis Corporation, Novartis, Pfizer, UCB, Valeant, and XBiotech both inside and outside the supported work.
SOURCES: Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.067; Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.068.
Rigorous evidence is unavailable for most interventions for treating hidradenitis suppurativa (HS), so management needs to be individualized, according to the
The guidelines were published in the Journal of the American Academy of Dermatology.
In an interview, Christopher Sayed, MD, cochair of the guidelines committee, of the department of dermatology at the University of North Carolina, Chapel Hill, said in an interview that the North American guidelines vary from the British Association of Dermatologists (Br J Dermatol. 2018 Dec 15. doi: 10.1111/bjd.17537) and European guidelines (J Eur Acad Dermatol Venereol. 2015 Apr;29[4]:619-44). For example, surgery is an active treatment option for various stages of the disorder in the North American guidelines, whereas surgery is considered a last-ditch effort in the British and European guidelines.
Surgical intervention is often needed “for patients to be the best they can be, and it can be difficult for medicine alone to fix [certain] patients,” Dr. Sayed said. This point that “using medical treatment alone or just surgical treatment alone often doesn’t lead to the best outcome” is stressed in the North American guidelines, he noted.
Limited evidence for high-level recommendations
Adalimumab (Humira), a tumor necrosis factor blocker, was the only therapy for which level 1A evidence is available, and this is because of its study in large-scale randomized controlled trials. (The Food and Drug Administration approved adalimumab in 2015 for treating moderate to severe HS.) Other biologic therapies such as infliximab, anakinra, and ustekinumab carry level 2B recommendations, which Dr. Sayed said will likely influence the availability of these therapies.
Similarly, Nd:YAG laser carries a level 2B recommendation, as does wide excision surgical intervention.
Many of the other treatment modalities explored in the guidelines are not well supported by the literature, according to Dr. Sayed . “The vast majority ... had category C recommendations,” he said. “Some things we tried to evaluate that, really, we can’t give any recommendation on at all because there was no evidence.” He noted that changes in lifestyle and dietary practices are issues patients with HS frequently bring up, but they carry very little evidence of benefit in the literature.
“Even things we use very commonly in HS are often supported by weak evidence because we have to rely on clinical experience. ... There’s just not funding for trials of older drugs or lifestyle interventions,” he said. Treatment mainstays such as tetracycline-class antibiotics, for example, similarly have no large-scale randomized controlled trials to support their use.
Consider comorbidity screening
Treatment is frequently complicated by patient comorbidities, including type 2 diabetes, metabolic syndrome, polycystic ovary syndrome (PCOS), and impaired sexual health, said Dr. Sayed.
“The disease leads to scarring and disfigurement, and can [have a] higher impact on quality of life than almost any other dermatologic disease if you compare them side by side using quality of life measures,” he noted. “We know these patients are more likely to have depression, there are high rates of suicide among these patients. A lot of that has to do with the fact that it’s a chronic disease where there is pain and disfigurement, and patients often grow very, very frustrated in part due to the disease.”
He advised consistent follow-up to ensure patients are not frustrated because of lack of perceived progress.
No one-size-fits-all approach
Dr. Sayed said individualized management is one of the most important parts of taking care of a patient with HS. For example, patients with Hurley stage 1 and Hurley stage 2 variations of the disease may present very differently, and that is the reason why the guidelines do not offer a stepwise treatment algorithm for the disease.
“[The guidelines] have many treatments that may overlap different stages of disease, where those things might need to be used together,” he said. “It takes a lot of discussion with patients about their treatment preferences and listening to whether their disease is progressing or not, whether or not it’s stable, and then trying to figure out whether things like surgery fit into the treatment strategy.”
Despite these recommendations, there may be situations where the answer is not listed in the guidelines. “The guidelines are based on evidence that’s available at the time we review the literature,” he said. “For many patients, they may fail every treatment that’s recommended within the guidelines. There will be times where you have to be creative for patients and go beyond what the guidelines can currently recommend and give patients the treatment that they need even when it seems like all options have been exhausted.”
The authors report relationships with 3M, AbbVie, Adelphi Values, Amgen, BSN, Celgene, Chemocentryx, Coloplast, Galderma, Hidramed Solutions, Hollister, the HS Foundation, Incyte, InflaRx, Integra, Janssen, KCI Inc., Lenicura, Leo, Lilly, The Microdermis Corporation, Novartis, Pfizer, UCB, Valeant, and XBiotech both inside and outside the supported work.
SOURCES: Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.067; Alikhan A et al. J Am Acad Dermatol. 2019. doi: 10.1016/j.jaad.2019.02.068.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
ASCO/ASH Clinical Practice Update: Erythropoiesis-stimulating agents
Erythropoiesis-stimulating agents (ESAs) may be offered to patients with chemotherapy-associated anemia whose cancer treatment is not curative in intent and whose hemoglobin (HgB) has declined to less than 10 g/dL, according to a clinical practice guideline update by the American Society of Clinical Oncology (ASCO) and American Society of Hematology (ASH).
Furthermore, ESAs should not be offered to patients with chemotherapy-associated anemia whose cancer treatment is curative in intent, wrote Julia Bohlius, MD, MScPH, of the University of Bern, Switzerland, along with her associates on the expert panel. The report is in the Journal of Clinical Oncology.
The panel members systematically reviewed the body of literature for evidence pertaining to the use of ESAs in patients with cancer. After the review, the team included 15 meta-analyses and 2 randomized controlled trials (RCTs).
“For biosimilar ESAs, the literature search was expanded to include meta-analyses and RCTs in patients with cancer or chronic kidney disease and cohort studies in patients with cancer,” they wrote.
The update addressed 10 key clinical questions and provided recommendations based on the available literature and clinical experience.
The addition of iron to treatment with an ESA may provide better hematopoietic response and lower the chances of RBC transfusion, according to the guidelines.
In addition, the review revealed that biosimilars of epoetin alfa could provide safety and efficacy similar to that of other reference products; however, the evidence in cancer is still unclear.
“ESAs (including biosimilars) may be offered to patients with chemotherapy-associated anemia whose cancer treatment is not curative in intent and whose hemoglobin has declined to less than 10 g/dL,” they recommended.
As an alternative to ESAs, they stated that “RBC transfusion is also an option.”
The panel acknowledged that ESAs should not be provided to the majority of patients with nonchemotherapy-related anemia, excluding certain patients with myelodysplastic syndromes.
More information on the guidelines is available on the ASCO and ASH websites.
The study was funded by the American Society of Clinical Oncology. The expert panel reported financial affiliations with AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Novartis, Takeda, and several others.
SOURCE: Bohlius J et al. J Clin Oncol. 2019 Apr 10. doi: 10.1200/JCO.18.02142.
Erythropoiesis-stimulating agents (ESAs) may be offered to patients with chemotherapy-associated anemia whose cancer treatment is not curative in intent and whose hemoglobin (HgB) has declined to less than 10 g/dL, according to a clinical practice guideline update by the American Society of Clinical Oncology (ASCO) and American Society of Hematology (ASH).
Furthermore, ESAs should not be offered to patients with chemotherapy-associated anemia whose cancer treatment is curative in intent, wrote Julia Bohlius, MD, MScPH, of the University of Bern, Switzerland, along with her associates on the expert panel. The report is in the Journal of Clinical Oncology.
The panel members systematically reviewed the body of literature for evidence pertaining to the use of ESAs in patients with cancer. After the review, the team included 15 meta-analyses and 2 randomized controlled trials (RCTs).
“For biosimilar ESAs, the literature search was expanded to include meta-analyses and RCTs in patients with cancer or chronic kidney disease and cohort studies in patients with cancer,” they wrote.
The update addressed 10 key clinical questions and provided recommendations based on the available literature and clinical experience.
The addition of iron to treatment with an ESA may provide better hematopoietic response and lower the chances of RBC transfusion, according to the guidelines.
In addition, the review revealed that biosimilars of epoetin alfa could provide safety and efficacy similar to that of other reference products; however, the evidence in cancer is still unclear.
“ESAs (including biosimilars) may be offered to patients with chemotherapy-associated anemia whose cancer treatment is not curative in intent and whose hemoglobin has declined to less than 10 g/dL,” they recommended.
As an alternative to ESAs, they stated that “RBC transfusion is also an option.”
The panel acknowledged that ESAs should not be provided to the majority of patients with nonchemotherapy-related anemia, excluding certain patients with myelodysplastic syndromes.
More information on the guidelines is available on the ASCO and ASH websites.
The study was funded by the American Society of Clinical Oncology. The expert panel reported financial affiliations with AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Novartis, Takeda, and several others.
SOURCE: Bohlius J et al. J Clin Oncol. 2019 Apr 10. doi: 10.1200/JCO.18.02142.
Erythropoiesis-stimulating agents (ESAs) may be offered to patients with chemotherapy-associated anemia whose cancer treatment is not curative in intent and whose hemoglobin (HgB) has declined to less than 10 g/dL, according to a clinical practice guideline update by the American Society of Clinical Oncology (ASCO) and American Society of Hematology (ASH).
Furthermore, ESAs should not be offered to patients with chemotherapy-associated anemia whose cancer treatment is curative in intent, wrote Julia Bohlius, MD, MScPH, of the University of Bern, Switzerland, along with her associates on the expert panel. The report is in the Journal of Clinical Oncology.
The panel members systematically reviewed the body of literature for evidence pertaining to the use of ESAs in patients with cancer. After the review, the team included 15 meta-analyses and 2 randomized controlled trials (RCTs).
“For biosimilar ESAs, the literature search was expanded to include meta-analyses and RCTs in patients with cancer or chronic kidney disease and cohort studies in patients with cancer,” they wrote.
The update addressed 10 key clinical questions and provided recommendations based on the available literature and clinical experience.
The addition of iron to treatment with an ESA may provide better hematopoietic response and lower the chances of RBC transfusion, according to the guidelines.
In addition, the review revealed that biosimilars of epoetin alfa could provide safety and efficacy similar to that of other reference products; however, the evidence in cancer is still unclear.
“ESAs (including biosimilars) may be offered to patients with chemotherapy-associated anemia whose cancer treatment is not curative in intent and whose hemoglobin has declined to less than 10 g/dL,” they recommended.
As an alternative to ESAs, they stated that “RBC transfusion is also an option.”
The panel acknowledged that ESAs should not be provided to the majority of patients with nonchemotherapy-related anemia, excluding certain patients with myelodysplastic syndromes.
More information on the guidelines is available on the ASCO and ASH websites.
The study was funded by the American Society of Clinical Oncology. The expert panel reported financial affiliations with AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Novartis, Takeda, and several others.
SOURCE: Bohlius J et al. J Clin Oncol. 2019 Apr 10. doi: 10.1200/JCO.18.02142.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
EULAR takes SLE guidance to the next level
The goal was “to update the EULAR recommendations for the management of systemic lupus erythematosus [SLE], based on literature review and expert consensus,” said authors led by Antonis Fanouriakis, MD, PhD, of the rheumatology and clinical immunology unit at Attikon University Hospital, Athens.
The team accomplished their aim in 33 recommendations – about twice as many as in 2008 – covering goals of therapy, treatment, specific manifestations, and comorbidities (Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089).
A lot has changed in the past 11 years, and the new guidelines reflect that. Biologics, for instance, were barely mentioned in 2008, except as a topic for future research. The new document makes a strong recommendation for add-on belimumab (Benlysta) to be considered in persistently active or flaring extrarenal disease, and rituximab (Rituxan) for organ-threatening, refractory disease.
The group now also makes a strong recommendation for hydroxychloroquine (Plaquenil) for all lupus patients, barring contraindications, at a dose not exceeding 5 mg per kg real body weight, with ophthalmologic screening performed at baseline, after 5 years, and yearly thereafter. It also calls for routine antiphospholipid antibody testing.
Calcineurin inhibitors weren’t mentioned at all in 2008, but they show up in the new document with a moderate recommendation as first-line topical options for skin disease, along with glucocorticoids. The authors also made a moderate recommendation for diagnostic kidney biopsy, calling it “essential” to catch renal involvement early; EULAR was less certain in 2008. Also new in 2019, and barely mentioned in 2008, there’s an entire section on hematologic manifestations, as well as advice on thalidomide for cutaneous disease.
For hematologic disease, the group makes a weak recommendation for pulsed intravenous methylprednisolone and/or intravenous immunoglobulin, with mycophenolate, azathioprine, or cyclosporine for maintenance. Cyclophosphamide, along with rituximab, are options for severe hematologic cases.
Cardiovascular disease, like biologics, was mentioned mostly in 2008 as a topic for future research; the new guidelines contain an entire section on the issue. There’s a strong recommendation for regular assessment of traditional and disease-related risk factors, including persistently active disease; increased disease duration; medium or high titers of antiphospholipid antibodies; renal involvement; and chronic glucocorticoid use. High-risk people, the document notes, “may be candidates for preventative strategies as in the general population,” including low-dose aspirin and statins.
The new guidance is also more certain about mycophenolate for renal disease, with a strong recommendation for use as an induction and maintenance agent, with azathioprine the other strong candidate for maintenance. EULAR also made a weak recommendation for mycophenolate with low-dose calcineurin inhibitors in severe nephrotic syndrome, in some circumstances.
For antiphospholipid antibody carriers, EULAR noted that a recent randomized, open-label trial comparing rivaroxaban against warfarin “was prematurely terminated due to an excess of thromboembolic events in the rivaroxaban arm. Thus, in patients with SLE-antiphospholipid syndrome, “use of novel oral anticoagulants for secondary prevention should be avoided.”
The group notes that management should aim at “remission of disease symptoms and signs, prevention of damage accrual, and minimization of drug side effects, as well as improvement of quality of life.” To that end, it said newly defined low disease-activity states, such as an SLE Disease Activity Index score of 3 or less on antimalarials, are useful to guide treatment, and have comparable rates of remission and flare prevention.
SOURCE: Fanouriakis A et al. Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089
The goal was “to update the EULAR recommendations for the management of systemic lupus erythematosus [SLE], based on literature review and expert consensus,” said authors led by Antonis Fanouriakis, MD, PhD, of the rheumatology and clinical immunology unit at Attikon University Hospital, Athens.
The team accomplished their aim in 33 recommendations – about twice as many as in 2008 – covering goals of therapy, treatment, specific manifestations, and comorbidities (Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089).
A lot has changed in the past 11 years, and the new guidelines reflect that. Biologics, for instance, were barely mentioned in 2008, except as a topic for future research. The new document makes a strong recommendation for add-on belimumab (Benlysta) to be considered in persistently active or flaring extrarenal disease, and rituximab (Rituxan) for organ-threatening, refractory disease.
The group now also makes a strong recommendation for hydroxychloroquine (Plaquenil) for all lupus patients, barring contraindications, at a dose not exceeding 5 mg per kg real body weight, with ophthalmologic screening performed at baseline, after 5 years, and yearly thereafter. It also calls for routine antiphospholipid antibody testing.
Calcineurin inhibitors weren’t mentioned at all in 2008, but they show up in the new document with a moderate recommendation as first-line topical options for skin disease, along with glucocorticoids. The authors also made a moderate recommendation for diagnostic kidney biopsy, calling it “essential” to catch renal involvement early; EULAR was less certain in 2008. Also new in 2019, and barely mentioned in 2008, there’s an entire section on hematologic manifestations, as well as advice on thalidomide for cutaneous disease.
For hematologic disease, the group makes a weak recommendation for pulsed intravenous methylprednisolone and/or intravenous immunoglobulin, with mycophenolate, azathioprine, or cyclosporine for maintenance. Cyclophosphamide, along with rituximab, are options for severe hematologic cases.
Cardiovascular disease, like biologics, was mentioned mostly in 2008 as a topic for future research; the new guidelines contain an entire section on the issue. There’s a strong recommendation for regular assessment of traditional and disease-related risk factors, including persistently active disease; increased disease duration; medium or high titers of antiphospholipid antibodies; renal involvement; and chronic glucocorticoid use. High-risk people, the document notes, “may be candidates for preventative strategies as in the general population,” including low-dose aspirin and statins.
The new guidance is also more certain about mycophenolate for renal disease, with a strong recommendation for use as an induction and maintenance agent, with azathioprine the other strong candidate for maintenance. EULAR also made a weak recommendation for mycophenolate with low-dose calcineurin inhibitors in severe nephrotic syndrome, in some circumstances.
For antiphospholipid antibody carriers, EULAR noted that a recent randomized, open-label trial comparing rivaroxaban against warfarin “was prematurely terminated due to an excess of thromboembolic events in the rivaroxaban arm. Thus, in patients with SLE-antiphospholipid syndrome, “use of novel oral anticoagulants for secondary prevention should be avoided.”
The group notes that management should aim at “remission of disease symptoms and signs, prevention of damage accrual, and minimization of drug side effects, as well as improvement of quality of life.” To that end, it said newly defined low disease-activity states, such as an SLE Disease Activity Index score of 3 or less on antimalarials, are useful to guide treatment, and have comparable rates of remission and flare prevention.
SOURCE: Fanouriakis A et al. Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089
The goal was “to update the EULAR recommendations for the management of systemic lupus erythematosus [SLE], based on literature review and expert consensus,” said authors led by Antonis Fanouriakis, MD, PhD, of the rheumatology and clinical immunology unit at Attikon University Hospital, Athens.
The team accomplished their aim in 33 recommendations – about twice as many as in 2008 – covering goals of therapy, treatment, specific manifestations, and comorbidities (Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089).
A lot has changed in the past 11 years, and the new guidelines reflect that. Biologics, for instance, were barely mentioned in 2008, except as a topic for future research. The new document makes a strong recommendation for add-on belimumab (Benlysta) to be considered in persistently active or flaring extrarenal disease, and rituximab (Rituxan) for organ-threatening, refractory disease.
The group now also makes a strong recommendation for hydroxychloroquine (Plaquenil) for all lupus patients, barring contraindications, at a dose not exceeding 5 mg per kg real body weight, with ophthalmologic screening performed at baseline, after 5 years, and yearly thereafter. It also calls for routine antiphospholipid antibody testing.
Calcineurin inhibitors weren’t mentioned at all in 2008, but they show up in the new document with a moderate recommendation as first-line topical options for skin disease, along with glucocorticoids. The authors also made a moderate recommendation for diagnostic kidney biopsy, calling it “essential” to catch renal involvement early; EULAR was less certain in 2008. Also new in 2019, and barely mentioned in 2008, there’s an entire section on hematologic manifestations, as well as advice on thalidomide for cutaneous disease.
For hematologic disease, the group makes a weak recommendation for pulsed intravenous methylprednisolone and/or intravenous immunoglobulin, with mycophenolate, azathioprine, or cyclosporine for maintenance. Cyclophosphamide, along with rituximab, are options for severe hematologic cases.
Cardiovascular disease, like biologics, was mentioned mostly in 2008 as a topic for future research; the new guidelines contain an entire section on the issue. There’s a strong recommendation for regular assessment of traditional and disease-related risk factors, including persistently active disease; increased disease duration; medium or high titers of antiphospholipid antibodies; renal involvement; and chronic glucocorticoid use. High-risk people, the document notes, “may be candidates for preventative strategies as in the general population,” including low-dose aspirin and statins.
The new guidance is also more certain about mycophenolate for renal disease, with a strong recommendation for use as an induction and maintenance agent, with azathioprine the other strong candidate for maintenance. EULAR also made a weak recommendation for mycophenolate with low-dose calcineurin inhibitors in severe nephrotic syndrome, in some circumstances.
For antiphospholipid antibody carriers, EULAR noted that a recent randomized, open-label trial comparing rivaroxaban against warfarin “was prematurely terminated due to an excess of thromboembolic events in the rivaroxaban arm. Thus, in patients with SLE-antiphospholipid syndrome, “use of novel oral anticoagulants for secondary prevention should be avoided.”
The group notes that management should aim at “remission of disease symptoms and signs, prevention of damage accrual, and minimization of drug side effects, as well as improvement of quality of life.” To that end, it said newly defined low disease-activity states, such as an SLE Disease Activity Index score of 3 or less on antimalarials, are useful to guide treatment, and have comparable rates of remission and flare prevention.
SOURCE: Fanouriakis A et al. Ann Rheum Dis. 2019 Mar 29. doi: 10.1136/annrheumdis-2019-215089
FROM ANNALS OF THE RHEUMATIC DISEASES
ASCO, CCO issue multiple myeloma treatment guidelines
New clinical practice guidelines, jointly released by two leading cancer organizations, provide nearly 50 specific recommendations for the management of newly diagnosed and relapsed multiple myeloma patients.
The guidelines from the American Society of Clinical Oncology (ASCO) and Cancer Care Ontario (CCO) were authored by a panel of 21 experts in medical oncology, surgery, radiation oncology, and advocacy who reviewed 124 relevant studies published between 2005 and 2018.
“The treatment of multiple myeloma has changed significantly in the last 5 years. Since 2015, four new drugs have been approved, thus providing more options and adding to the complexity of treatment options,” the expert panel wrote in the Journal of Clinical Oncology.
The recommendations are intended to put in context recent randomized trials and drug advances, according to the experts, led by cochairs Joseph Mikhael, MD, of City of Hope Cancer Center, Phoenix, and the International Myeloma Foundation, North Hollywood, Calif., and Tom Martin, MD, of the University of California, San Francisco.
Specifically, the recently approved agents include the proteasome inhibitor ixazomib, the histone deacetylase inhibitor panobinostat, and the monoclonal antibodies daratumumab and elotuzumab, directed at CD38 and SLAMF7, respectively.
There are 20 specific recommendations for newly diagnosed, transplant-eligible patients with multiple myeloma; 10 recommendations for newly diagnosed, transplant-ineligible patients; and 16 recommendations related to relapsed disease in the ASCO/CCO guidelines.
All transplant-eligible patients should be offered up-front autologous stem cell transplant (ASCT), according to the guidelines. By contrast, allogeneic transplant is not routinely recommended but “may be considered” in select high-risk patients, and tandem transplant “should not be routinely recommended,” the expert panelists said in their report.
Lenalidomide maintenance therapy should be routinely offered to standard-risk, transplant-eligible patients, according to the panel, whereas bortezomib maintenance could be considered in those who are intolerant of lenalidomide or can’t receive that immunomodulatory drug.
“Evidence is emerging for the use of ixazomib as maintenance therapy and may also be considered,” the panel members said, citing the TOURMALINE-MM3 study results presented at the 2018 annual meeting of the American Society of Hematology and recently published in the Lancet.
Although minimal residual disease (MRD)–negative status is linked to improved outcomes, there is insufficient evidence that MRD can be used today to modify maintenance therapy based on depth of response in transplant-eligible patients, according to the guidelines. Likewise, in transplant-ineligible patients, MRD shouldn’t be used to guide treatment goals in clinical practice, the authors said.
Triplet therapies such as bortezomib, lenalidomide, and dexamethasone (VRd) can be considered for transplant ineligible patients, as can the combination of daratumumab and bortezomib plus melphalan and prednisone that was approved by the Food and Drug Administration in May 2018.
For patients with biochemically relapsed myeloma and high-risk disease – defined as early relapse and presence of high-risk cytogenetics – treatment should begin immediately, whereas close observation may be appropriate for patients with asymptomatic, slowly progressive relapse.
Triplets containing two novel therapies should be administered on first relapse, and should continue until disease progression, the expert panel advised.
If it was not already done after primary induction, ASCT should be offered to relapsed, transplant-eligible myeloma patients.
The expert panel reported numerous financial relationships with industry, including Celgene, Sanofi, AbbVie, TeneoBio, Roche, June Therapeutics, and others.
SOURCE: Mikhael J et al. J Clin Oncol. 2019 Apr 1. doi: 10.1200/JCO.18.02096.
New clinical practice guidelines, jointly released by two leading cancer organizations, provide nearly 50 specific recommendations for the management of newly diagnosed and relapsed multiple myeloma patients.
The guidelines from the American Society of Clinical Oncology (ASCO) and Cancer Care Ontario (CCO) were authored by a panel of 21 experts in medical oncology, surgery, radiation oncology, and advocacy who reviewed 124 relevant studies published between 2005 and 2018.
“The treatment of multiple myeloma has changed significantly in the last 5 years. Since 2015, four new drugs have been approved, thus providing more options and adding to the complexity of treatment options,” the expert panel wrote in the Journal of Clinical Oncology.
The recommendations are intended to put in context recent randomized trials and drug advances, according to the experts, led by cochairs Joseph Mikhael, MD, of City of Hope Cancer Center, Phoenix, and the International Myeloma Foundation, North Hollywood, Calif., and Tom Martin, MD, of the University of California, San Francisco.
Specifically, the recently approved agents include the proteasome inhibitor ixazomib, the histone deacetylase inhibitor panobinostat, and the monoclonal antibodies daratumumab and elotuzumab, directed at CD38 and SLAMF7, respectively.
There are 20 specific recommendations for newly diagnosed, transplant-eligible patients with multiple myeloma; 10 recommendations for newly diagnosed, transplant-ineligible patients; and 16 recommendations related to relapsed disease in the ASCO/CCO guidelines.
All transplant-eligible patients should be offered up-front autologous stem cell transplant (ASCT), according to the guidelines. By contrast, allogeneic transplant is not routinely recommended but “may be considered” in select high-risk patients, and tandem transplant “should not be routinely recommended,” the expert panelists said in their report.
Lenalidomide maintenance therapy should be routinely offered to standard-risk, transplant-eligible patients, according to the panel, whereas bortezomib maintenance could be considered in those who are intolerant of lenalidomide or can’t receive that immunomodulatory drug.
“Evidence is emerging for the use of ixazomib as maintenance therapy and may also be considered,” the panel members said, citing the TOURMALINE-MM3 study results presented at the 2018 annual meeting of the American Society of Hematology and recently published in the Lancet.
Although minimal residual disease (MRD)–negative status is linked to improved outcomes, there is insufficient evidence that MRD can be used today to modify maintenance therapy based on depth of response in transplant-eligible patients, according to the guidelines. Likewise, in transplant-ineligible patients, MRD shouldn’t be used to guide treatment goals in clinical practice, the authors said.
Triplet therapies such as bortezomib, lenalidomide, and dexamethasone (VRd) can be considered for transplant ineligible patients, as can the combination of daratumumab and bortezomib plus melphalan and prednisone that was approved by the Food and Drug Administration in May 2018.
For patients with biochemically relapsed myeloma and high-risk disease – defined as early relapse and presence of high-risk cytogenetics – treatment should begin immediately, whereas close observation may be appropriate for patients with asymptomatic, slowly progressive relapse.
Triplets containing two novel therapies should be administered on first relapse, and should continue until disease progression, the expert panel advised.
If it was not already done after primary induction, ASCT should be offered to relapsed, transplant-eligible myeloma patients.
The expert panel reported numerous financial relationships with industry, including Celgene, Sanofi, AbbVie, TeneoBio, Roche, June Therapeutics, and others.
SOURCE: Mikhael J et al. J Clin Oncol. 2019 Apr 1. doi: 10.1200/JCO.18.02096.
New clinical practice guidelines, jointly released by two leading cancer organizations, provide nearly 50 specific recommendations for the management of newly diagnosed and relapsed multiple myeloma patients.
The guidelines from the American Society of Clinical Oncology (ASCO) and Cancer Care Ontario (CCO) were authored by a panel of 21 experts in medical oncology, surgery, radiation oncology, and advocacy who reviewed 124 relevant studies published between 2005 and 2018.
“The treatment of multiple myeloma has changed significantly in the last 5 years. Since 2015, four new drugs have been approved, thus providing more options and adding to the complexity of treatment options,” the expert panel wrote in the Journal of Clinical Oncology.
The recommendations are intended to put in context recent randomized trials and drug advances, according to the experts, led by cochairs Joseph Mikhael, MD, of City of Hope Cancer Center, Phoenix, and the International Myeloma Foundation, North Hollywood, Calif., and Tom Martin, MD, of the University of California, San Francisco.
Specifically, the recently approved agents include the proteasome inhibitor ixazomib, the histone deacetylase inhibitor panobinostat, and the monoclonal antibodies daratumumab and elotuzumab, directed at CD38 and SLAMF7, respectively.
There are 20 specific recommendations for newly diagnosed, transplant-eligible patients with multiple myeloma; 10 recommendations for newly diagnosed, transplant-ineligible patients; and 16 recommendations related to relapsed disease in the ASCO/CCO guidelines.
All transplant-eligible patients should be offered up-front autologous stem cell transplant (ASCT), according to the guidelines. By contrast, allogeneic transplant is not routinely recommended but “may be considered” in select high-risk patients, and tandem transplant “should not be routinely recommended,” the expert panelists said in their report.
Lenalidomide maintenance therapy should be routinely offered to standard-risk, transplant-eligible patients, according to the panel, whereas bortezomib maintenance could be considered in those who are intolerant of lenalidomide or can’t receive that immunomodulatory drug.
“Evidence is emerging for the use of ixazomib as maintenance therapy and may also be considered,” the panel members said, citing the TOURMALINE-MM3 study results presented at the 2018 annual meeting of the American Society of Hematology and recently published in the Lancet.
Although minimal residual disease (MRD)–negative status is linked to improved outcomes, there is insufficient evidence that MRD can be used today to modify maintenance therapy based on depth of response in transplant-eligible patients, according to the guidelines. Likewise, in transplant-ineligible patients, MRD shouldn’t be used to guide treatment goals in clinical practice, the authors said.
Triplet therapies such as bortezomib, lenalidomide, and dexamethasone (VRd) can be considered for transplant ineligible patients, as can the combination of daratumumab and bortezomib plus melphalan and prednisone that was approved by the Food and Drug Administration in May 2018.
For patients with biochemically relapsed myeloma and high-risk disease – defined as early relapse and presence of high-risk cytogenetics – treatment should begin immediately, whereas close observation may be appropriate for patients with asymptomatic, slowly progressive relapse.
Triplets containing two novel therapies should be administered on first relapse, and should continue until disease progression, the expert panel advised.
If it was not already done after primary induction, ASCT should be offered to relapsed, transplant-eligible myeloma patients.
The expert panel reported numerous financial relationships with industry, including Celgene, Sanofi, AbbVie, TeneoBio, Roche, June Therapeutics, and others.
SOURCE: Mikhael J et al. J Clin Oncol. 2019 Apr 1. doi: 10.1200/JCO.18.02096.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Prevention and Treatment of Traveler’s Diarrhea
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
Importance
The prevention and treatment of traveler’s diarrhea (TD) is a common reason that patients consult their physician prior to foreign travel. TD can result in lost time and opportunity, as well as overseas medical encounters and hospitalization. to providers regarding the use of antibiotic and nonantibiotic therapies for the prevention and treatment of TD.
Prophylaxis
The panel recommends that antimicrobial prophylaxis should not be used routinely in travelers, but it should be considered for travelers who are at high risk of health-related complications of TD (both strong recommendations, low/very low level of evidence [LOE]). High-risk individuals include those with a history of clinically significant long-term morbidity following an enteric infection or serious chronic illnesses that predisposes them for TD-related complications. Bismuth subsalicylate (BSS) may be considered for any traveler to prevent TD (3, strong recommendation, high LOE). Studies show that a lower dose of 1.05 g/day is preventive, although it is unclear whether it is as effective as higher doses of 2.1 g/day or 4.2 g/day. When prophylaxis is indicated, travelers should be prescribed rifaximin (strong recommendation, moderate LOE) based on susceptibility of most enteric pathogens and the drug’s extremely favorable safety profile. Fluoroquinolones (FQ) are no longer recommended for prophylaxis (strong recommendation, low/very low LOE) because of neurologic and musculoskeletal side effects that may outweigh benefits, as well as emerging resistance of enteric pathogens (70%-80% in Campylobacter spp. from Nepal and Thailand and 65% in Enterotoxigenic Escherichia coli [ETEC] and Enteroaggregative E. coli [EAEC] in India).
Treatment
The following treatment recommendations are based on the classification of TD using functional effects of severity; therefore, the panel made new definitions for TD severity. This is a change from previous definitions that utilized a traditional frequency-based algorithm in order to tailor therapy for the individual. Individuals can be prescribed antibiotics and antimotility agents to take with them during travel, along with advice regarding how to judge when to use each agent.
Mild: diarrhea that is tolerable, is not distressing, and does not interfere with planned activities.
Encourage supportive measures such as rehydration and nonantibiotic, antimotility drugs, such as loperamide or BSS (both strong recommendations, moderate LOE).
Moderate: diarrhea that is distressing or interferes with planned activities.
Antibiotics may be used (weak recommendation, moderate LOE) as early and effective treatment may mitigate the well-described chronic health consequences including irritable bowel syndrome. Three options exist. FQs may be used outside of Southeast and South Asia (strong recommendation, moderate LOE), but their potential for adverse effects and musculoskeletal consequences must be considered. Azithromycin may be used (strong recommendation, high LOE) because studies show no significant differences in efficacy between it and FQs, limited resistance to common TD pathogens (although concerns exist in Nepal), and good side effect profile. Another choice is rifaximin (weak recommendation, moderate LOE), although one should exercise caution for empirical therapy in regions in which being at high risk of invasive pathogens is anticipated.
Loperamide may be used as adjunctive therapy for moderate to severe TD (strong recommendation, high LOE) to add symptomatic relief with curative treatment or as monotherapy in moderate TD (strong recommendation, high LOE). This is specifically true in children aged 2-11 years, in whom loperamide is beneficial without causing severe side effects.
Severe: diarrhea that is incapacitating or completely prevents planned activities; all dysentery (passage of grossly bloody stools).
Antibiotics should be used (strong recommendation, high LOE). Azithromycin is the preferred choice and is first-line for dysentery or febrile diarrhea (strong recommendation, moderate LOE) because of the likelihood of FQ-resistant bacteria being the cause of dysentery. FQs and rifaximin are also choices that can be used to treat severe, nondysenteric TD (both weak recommendations, moderate LOE).
Furthermore, single-dose antibiotics may be used to treat moderate or severe TD (strong recommendation, high LOE) because studies have shown equivalent efficacy for treatment of watery noninvasive diarrhea among FQs (3 days, single dose), azithromycin (3 days, single dose), and rifaximin (3 days, three times daily).
Persistent: diarrhea lasting longer than 2 weeks.
Functional bowel disease (FBD) may occur after bouts of TD and may meet Rome III or IV criteria for irritable bowel syndrome. Thus, in a traveler without pretravel GI disease, in whom the evaluation for microbial etiologies and underlying GI disease is negative, postinfectious FBD must be considered.
Follow-up and diagnostic testing
The panel recommends microbiological testing in returning travelers with severe or persistent symptoms, bloody/mucousy diarrhea, or in those who fail empiric therapy (strong recommendation, low/very low LOE). Molecular testing, aimed at a broad range of clinically relevant pathogens, is preferred when rapid results are clinically important or nonmolecular tests have failed to establish a diagnosis. Furthermore, molecular testing may, in some cases, detect colonization rather than infection.
The bottom line
The expert panel made 20 graded recommendations to help guide the provider with nonantibiotic and antibiotic prophylaxis and treatment of TD. The main take-home points include:
- Prophylaxis should be considered only in high-risk groups; rifaximin is the first choice, and BSS is a second option.
- All travelers should be provided with loperamide and an antibiotic for self-treatment if needed.
- Mild diarrhea should be treated with increased fluid intake and loperamide or BSS.
- Moderate to severe diarrhea should be treated with single-dose antimicrobial therapy of FQ or azithromycin or with rifaximin dosing three times a day.
- Instead of antibiotics, loperamide may be considered as monotherapy for moderate diarrhea; loperamide can be used with antibiotics for both moderate and severe TD.
Dr. Shrestha is a second-year resident in the Family Medicine Residency Program at Abington (Pa.) - Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington - Jefferson Health.
Reference:
AGA Clinical Practice Update: Switching between biologics and biosimilars in inflammatory bowel disease
Patients with inflammatory bowel disease (IBD) will soon have access to new biosimilars to infliximab, adalimumab, and other monoclonal antibodies, experts wrote in an American Gastroenterological Association clinical practice update.
“It is anticipated that biosimilars for IBD are here to stay,” wrote Laura E. Raffals, MD, of the Mayo Clinic in Rochester, Minn., and her associates in Clinical Gastroenterology and Hepatology. “Provided that the regulatory pathway remains rigorous and postmarketing surveillance is performed adequately, clinicians and patients can be reassured that these agents will provide the same well-described effectiveness for moderate to severe Crohn’s disease and ulcerative colitis, without new safety concerns.”
Evidence supports the use of biosimilars in IBD, but switching patients in stable remission on infliximab (Remicade) to a biosimilar, namely infliximab-dyyb (Inflectra), should remain a case-by-case choice, according to an AGA clinical practice update. Pending more safety data, the update’s authors recommended against nonmedical switches during pregnancy and urge special attention when considering whether to switch children.
Biologics have revolutionized IBD treatment, but at a steep price. As patents expire, companies have developed biosimilar agents that aim to conserve safety and efficacy at lower cost. Studies support this idea, although whether initiating or switching to biosimilars will save patients (versus hospitals or payers) money “remains to be seen,” the practice update states.
The FDA approval process for biosimilars is more rigorous than that for generics, but it skips the multiple phases of clinical trials required to approve reference biologics. Instead, the FDA requires robust evidence that the biosimilar has comparable structure, function, immunogenicity, animal toxicity, pharmacokinetics and pharmacodynamics, and clinical safety and efficacy in humans. Under U.S. law, a biosimilar cannot be FDA approved if its clinically active components differ from the reference product or it shows clinically meaningful differences in safety, potency, or purity.
So far, five biosimilars have been approved by the FDA for use in IBD, although not all are on the market yet: infliximab-dyyb (Inflectra), adalimumab-atta (Amjevita), infliximab-abda (Renflexis), adalimumab-adbm (Cyltezo), and infliximab-qbtx (Ixifi). Most postmarketing studies of their use involved patients on stable doses of Remicade who switched to biosimilar infliximab-dyyb (Inflectra).
The best known of these studies is the double-blind, randomized NOR-SWITCH trial, in which patients with Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, or chronic plaque psoriasis on Remicade either continued it or switched to biosimilar infliximab-dyyb (Inflectra). At week 52, both safety and the likelihood of worsening disease activity were similar regardless of treatment randomization. The study was not powered to assess subgroup outcomes in Crohn’s disease or ulcerative colitis, the practice update notes.
More recently, the results of the 16-week SECURE trial also indicated that switching to infliximab-dyyb (Inflectra) was safe and well tolerated by patients with remitted IBD. However, the FDA has not yet designated any biosimilar as “interchangeable” with an approved biologic confirmed safe in multiple switches, the practice update notes. As a result, state laws prohibit patients from being switched to a biosimilar without notification. Both the NOR-SWITCH and SECURE trials were done in Europe.
Clinicians also must understand that antidrug antibodies to originator and biosimilar infliximab cross-react with each other, the experts emphasized. Switching patients with antibodies to Remicade or a biosimilar to the other product therefore risks an immediate hypersensitivity reaction, including life-threatening anaphylaxis.
The authors disclosed no external funding sources. One author disclosed ties to AbbVie, Janssen, Pfizer, Merck, Samsung Bioepis, and Amgen. The rest reported having no conflicts of interest.
SOURCE: Raffals LA et al. Clin Gastroenterol Hepatol. 2018 Sep 6. doi: 10.1016/j.cgh.2018.08.064.
Patients with inflammatory bowel disease (IBD) will soon have access to new biosimilars to infliximab, adalimumab, and other monoclonal antibodies, experts wrote in an American Gastroenterological Association clinical practice update.
“It is anticipated that biosimilars for IBD are here to stay,” wrote Laura E. Raffals, MD, of the Mayo Clinic in Rochester, Minn., and her associates in Clinical Gastroenterology and Hepatology. “Provided that the regulatory pathway remains rigorous and postmarketing surveillance is performed adequately, clinicians and patients can be reassured that these agents will provide the same well-described effectiveness for moderate to severe Crohn’s disease and ulcerative colitis, without new safety concerns.”
Evidence supports the use of biosimilars in IBD, but switching patients in stable remission on infliximab (Remicade) to a biosimilar, namely infliximab-dyyb (Inflectra), should remain a case-by-case choice, according to an AGA clinical practice update. Pending more safety data, the update’s authors recommended against nonmedical switches during pregnancy and urge special attention when considering whether to switch children.
Biologics have revolutionized IBD treatment, but at a steep price. As patents expire, companies have developed biosimilar agents that aim to conserve safety and efficacy at lower cost. Studies support this idea, although whether initiating or switching to biosimilars will save patients (versus hospitals or payers) money “remains to be seen,” the practice update states.
The FDA approval process for biosimilars is more rigorous than that for generics, but it skips the multiple phases of clinical trials required to approve reference biologics. Instead, the FDA requires robust evidence that the biosimilar has comparable structure, function, immunogenicity, animal toxicity, pharmacokinetics and pharmacodynamics, and clinical safety and efficacy in humans. Under U.S. law, a biosimilar cannot be FDA approved if its clinically active components differ from the reference product or it shows clinically meaningful differences in safety, potency, or purity.
So far, five biosimilars have been approved by the FDA for use in IBD, although not all are on the market yet: infliximab-dyyb (Inflectra), adalimumab-atta (Amjevita), infliximab-abda (Renflexis), adalimumab-adbm (Cyltezo), and infliximab-qbtx (Ixifi). Most postmarketing studies of their use involved patients on stable doses of Remicade who switched to biosimilar infliximab-dyyb (Inflectra).
The best known of these studies is the double-blind, randomized NOR-SWITCH trial, in which patients with Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, or chronic plaque psoriasis on Remicade either continued it or switched to biosimilar infliximab-dyyb (Inflectra). At week 52, both safety and the likelihood of worsening disease activity were similar regardless of treatment randomization. The study was not powered to assess subgroup outcomes in Crohn’s disease or ulcerative colitis, the practice update notes.
More recently, the results of the 16-week SECURE trial also indicated that switching to infliximab-dyyb (Inflectra) was safe and well tolerated by patients with remitted IBD. However, the FDA has not yet designated any biosimilar as “interchangeable” with an approved biologic confirmed safe in multiple switches, the practice update notes. As a result, state laws prohibit patients from being switched to a biosimilar without notification. Both the NOR-SWITCH and SECURE trials were done in Europe.
Clinicians also must understand that antidrug antibodies to originator and biosimilar infliximab cross-react with each other, the experts emphasized. Switching patients with antibodies to Remicade or a biosimilar to the other product therefore risks an immediate hypersensitivity reaction, including life-threatening anaphylaxis.
The authors disclosed no external funding sources. One author disclosed ties to AbbVie, Janssen, Pfizer, Merck, Samsung Bioepis, and Amgen. The rest reported having no conflicts of interest.
SOURCE: Raffals LA et al. Clin Gastroenterol Hepatol. 2018 Sep 6. doi: 10.1016/j.cgh.2018.08.064.
Patients with inflammatory bowel disease (IBD) will soon have access to new biosimilars to infliximab, adalimumab, and other monoclonal antibodies, experts wrote in an American Gastroenterological Association clinical practice update.
“It is anticipated that biosimilars for IBD are here to stay,” wrote Laura E. Raffals, MD, of the Mayo Clinic in Rochester, Minn., and her associates in Clinical Gastroenterology and Hepatology. “Provided that the regulatory pathway remains rigorous and postmarketing surveillance is performed adequately, clinicians and patients can be reassured that these agents will provide the same well-described effectiveness for moderate to severe Crohn’s disease and ulcerative colitis, without new safety concerns.”
Evidence supports the use of biosimilars in IBD, but switching patients in stable remission on infliximab (Remicade) to a biosimilar, namely infliximab-dyyb (Inflectra), should remain a case-by-case choice, according to an AGA clinical practice update. Pending more safety data, the update’s authors recommended against nonmedical switches during pregnancy and urge special attention when considering whether to switch children.
Biologics have revolutionized IBD treatment, but at a steep price. As patents expire, companies have developed biosimilar agents that aim to conserve safety and efficacy at lower cost. Studies support this idea, although whether initiating or switching to biosimilars will save patients (versus hospitals or payers) money “remains to be seen,” the practice update states.
The FDA approval process for biosimilars is more rigorous than that for generics, but it skips the multiple phases of clinical trials required to approve reference biologics. Instead, the FDA requires robust evidence that the biosimilar has comparable structure, function, immunogenicity, animal toxicity, pharmacokinetics and pharmacodynamics, and clinical safety and efficacy in humans. Under U.S. law, a biosimilar cannot be FDA approved if its clinically active components differ from the reference product or it shows clinically meaningful differences in safety, potency, or purity.
So far, five biosimilars have been approved by the FDA for use in IBD, although not all are on the market yet: infliximab-dyyb (Inflectra), adalimumab-atta (Amjevita), infliximab-abda (Renflexis), adalimumab-adbm (Cyltezo), and infliximab-qbtx (Ixifi). Most postmarketing studies of their use involved patients on stable doses of Remicade who switched to biosimilar infliximab-dyyb (Inflectra).
The best known of these studies is the double-blind, randomized NOR-SWITCH trial, in which patients with Crohn’s disease, ulcerative colitis, spondyloarthritis, rheumatoid arthritis, psoriatic arthritis, or chronic plaque psoriasis on Remicade either continued it or switched to biosimilar infliximab-dyyb (Inflectra). At week 52, both safety and the likelihood of worsening disease activity were similar regardless of treatment randomization. The study was not powered to assess subgroup outcomes in Crohn’s disease or ulcerative colitis, the practice update notes.
More recently, the results of the 16-week SECURE trial also indicated that switching to infliximab-dyyb (Inflectra) was safe and well tolerated by patients with remitted IBD. However, the FDA has not yet designated any biosimilar as “interchangeable” with an approved biologic confirmed safe in multiple switches, the practice update notes. As a result, state laws prohibit patients from being switched to a biosimilar without notification. Both the NOR-SWITCH and SECURE trials were done in Europe.
Clinicians also must understand that antidrug antibodies to originator and biosimilar infliximab cross-react with each other, the experts emphasized. Switching patients with antibodies to Remicade or a biosimilar to the other product therefore risks an immediate hypersensitivity reaction, including life-threatening anaphylaxis.
The authors disclosed no external funding sources. One author disclosed ties to AbbVie, Janssen, Pfizer, Merck, Samsung Bioepis, and Amgen. The rest reported having no conflicts of interest.
SOURCE: Raffals LA et al. Clin Gastroenterol Hepatol. 2018 Sep 6. doi: 10.1016/j.cgh.2018.08.064.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA publishes care pathway for IBD in pregnancy
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
Ideally, pregnant women with inflammatory bowel disease (IBD) should receive coordinated care from gastroenterologists and maternal-fetal medicine specialists, plus additional input from nutritionists, lactation counselors, and colorectal surgeons as needed, states a new report from the American Gastroenterological Association.
But in reality, these women often receive scant and conflicting advice from health care providers, writes Uma Mahadevan, MD, of the University of California, San Francisco, with her associates in Gastroenterology.
An “explosion” of new treatments in the past 15 years has given hope to many women with IBD who wish to be healthy enough to conceive, the experts noted. But in a recent AGA survey, more than 40% of obstetrician/gynecologist (OB/GYN) providers felt that women with IBD received inadequate information about pregnancy, compared with patients with other immune-mediated diseases. Strikingly, 94% of surveyed clinicians said they had patients stop taking their IBD medications during pregnancy because they feared harm to the fetus. In doing so, these patients actually risked greater disease activity, perinatal flares, and adverse pregnancy outcomes.
Therefore, the AGA, in partnership with the Crohn’s & Colitis Foundation, the Society for Maternal-Fetal Medicine, and Girls With Guts, crafted a standardized, evidence-based care pathway for health care providers from diverse disciplines who treat women with IBD in all stages of family planning. Its authors recommended that a maternal-fetal medicine specialist oversee obstetric care whenever possible. A gastroenterologist should continue IBD care by seeing the patient once during the first or second trimester and thereafter depending on IBD severity. The patient should receive a “clear and easily understandable consensus plan” for managing complex care during and after pregnancy, according to the pathway.
Aminosalicylates, biologics, and immunomodulators can be continued during pregnancy and delivery. Biologics have not shown teratogenicity in large studies, but monotherapy is preferred to reduce infection risk in infants. Clinicians should calculate weight-based doses according to prepregnancy weight. Doses can be tweaked to achieve minimal trough levels near delivery.
During pregnancy, patients should stop antidiarrheal therapy with loperamide and diphenoxylate when possible. Proinflammatory mediators are known to damage hippocampal neurogenesis and neuronal cytoarchitecture during brain development, so patients should understand the need for good inflammatory control during pregnancy. However, biologic therapy is preferred, and patients should only use corticosteroids adjunctively if needed for flares.
The usual indications guide the choice between a vaginal or cesarean delivery, the pathway states. Vaginal delivery often is possible for patients without active perineal disease, while cesarean is recommended for women with prior perineal surgery or active perineal disease or rectovaginal fistulas. The perineal area can be examined for active disease during the routine visit for group B streptococcus screening culture at 35-37 weeks’ gestation. For women who have had ileal-pouch anal anastomosis surgery, mode of delivery does not seem to affect pouch function, but cesarean delivery is thought to prevent anal sphincter injury and the accompanying risk of incontinence.
For ostomy patients, stretching of the abdominal wall during pregnancy can lead to stomal problems, such as displacement, enlargement, retraction, stenosis, and prolapse. A nutritionist can help ostomy patients avoid excess weight gain, and a colorectal surgeon and ostomy/wound nurse can help coordinate postpartum care. If cesarean delivery is needed, simply covering the ostomy with gauze sufficiently protects the operative field.
Since IBD increases the risk of venous thromboembolism, clinicians should consider prophylactic anticoagulation after cesarean delivery and during a hospitalization for IBD flares, according to the care pathway. Breastfeeding women can receive unfractionated heparin, low-molecular-weight heparin, or warfarin up to 3-6 weeks post partum, but they should not receive oral direct thrombin or factor Xa inhibitors.
In addition, most IBD medications are either undetectable in breast milk or are secreted at such low concentrations that they pose no known risk to infants. Therefore, patients can continue IBD medications after delivery – except methotrexate, which has not been sufficiently studied to assess its safety. Breastfeeding women with IBD should avoid using fenugreek to increase milk production, since it can cause diarrhea and bleeding.
Finally, infants should not receive live vaccines during the first 6 months after birth if their mothers received biologics besides certolizumab during the third trimester, the pathway notes. In the United States, this applies only to the oral rotavirus vaccine.
For more information about the care pathway and resources for your patients, visit IBDParenthoodProject.org.
SOURCE: Mahadevan U et al. Gastroenterology. 2019 Jan 15. doi: 10.1053/j.gastro.2018.12.022.
FROM THE AMERICAN GASTROENTEROLOGICAL ASSOCIATION
New postmenopausal osteoporosis guidelines emphasize patient priorities
NEW ORLEANS – In new guidelines for the pharmacologic management of osteoporosis, bisphosphonates have been identified as the first-line therapy with denosumab (Prolia) listed as an acceptable alternative that is particularly well suited for high-risk patients, according to a presentation at the annual meeting of the Endocrine Society.
“We hope our guideline will not only improve patient care but provide confidence in treatment,” reported guideline writing committee member Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough.
The new guidelines are evidence based, relying on randomized, controlled trials to evaluate the data quality of treatment options with GRADE methodology, but Dr. Rosen said that the guideline writing committee also considered patient preferences because of concerns about the abundant evidence that adherence to pharmacologic therapies for osteoporosis is poor.
“There is a considerable gap in the treatment of osteoporosis. Most women will not take anti-osteoporosis therapies despite their efficacy, and those who do often stop,” Dr. Rosen observed. He said it was the intention of the writing committee to provide acceptable recommendations with a clear outline of benefits and risks in order to enlist patients more successfully in understanding and participating in fracture prevention.
The Endocrine Society guidelines, which are available online and will soon appear in print (J Clin Endocrinol Metab. 2019;104:1-28), are focused on pharmacologic management and therefore differ from guidelines on diagnosis and treatment published previously by the American Association of Clinical Endocrinologists (AACE) (Endocr Pract. 2016;22[Suppl 4]:1-42).
The AACE guidelines, which devote considerable space to prevention, indicated that bisphosphonates should “be generally considered as initial options for most patients who are candidates for treatment.” The AACE guidelines identify denosumab as the “treatment of choice” in patients with renal insufficiency (although not in those on dialysis or with end-stage renal disease).
In outlining some of the key features of the new guidelines at ENDO 2019, Dr. Rosen drew attention to a call for reevaluation of the need for bisphosphonates after patients have been on this therapy for 3 or more years. For those found at this time to be at low or moderate risk of fracture, a drug holiday is recommended based on guideline-cited evidence that bisphosphonates offer a residual therapeutic effect after stopping.
However, stopping is not recommended in those who remain at high risk. In these patients, bone density should be monitored at regular intervals for the goal of switching or intensifying therapy if needed. This includes use of teriparatide (Forteo) or abaloparatide (Tymlos) for periods of up to 2 years in patients with a history of severe or multiple fractures. These and other choices are included in a detailed algorithm covering both low- and high-risk patients.
Although many postmenopausal women hope to avoid pharmacologic therapy with high dietary intake of calcium and vitamin D, Dr. Rosen stressed the limited benefit of these nutrients in preventing fracture for those with established osteoporosis. While acknowledging that calcium and vitamin D enhance mineralization and maintenance of bone mass, he characterized them as “supplements” once pharmacologic therapies are indicated.
Unsurprisingly, patients prefer oral therapies that are effective but with a low burden of adverse events, according to a review of evidence undertaken by the guideline committee. Cost was a less important consideration. Dr. Rosen indicated that recognizing patient goals and priorities while explaining relative risks might engage patients in selecting a therapy to which they are willing to adhere.
He reported having no relevant financial relationships.
NEW ORLEANS – In new guidelines for the pharmacologic management of osteoporosis, bisphosphonates have been identified as the first-line therapy with denosumab (Prolia) listed as an acceptable alternative that is particularly well suited for high-risk patients, according to a presentation at the annual meeting of the Endocrine Society.
“We hope our guideline will not only improve patient care but provide confidence in treatment,” reported guideline writing committee member Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough.
The new guidelines are evidence based, relying on randomized, controlled trials to evaluate the data quality of treatment options with GRADE methodology, but Dr. Rosen said that the guideline writing committee also considered patient preferences because of concerns about the abundant evidence that adherence to pharmacologic therapies for osteoporosis is poor.
“There is a considerable gap in the treatment of osteoporosis. Most women will not take anti-osteoporosis therapies despite their efficacy, and those who do often stop,” Dr. Rosen observed. He said it was the intention of the writing committee to provide acceptable recommendations with a clear outline of benefits and risks in order to enlist patients more successfully in understanding and participating in fracture prevention.
The Endocrine Society guidelines, which are available online and will soon appear in print (J Clin Endocrinol Metab. 2019;104:1-28), are focused on pharmacologic management and therefore differ from guidelines on diagnosis and treatment published previously by the American Association of Clinical Endocrinologists (AACE) (Endocr Pract. 2016;22[Suppl 4]:1-42).
The AACE guidelines, which devote considerable space to prevention, indicated that bisphosphonates should “be generally considered as initial options for most patients who are candidates for treatment.” The AACE guidelines identify denosumab as the “treatment of choice” in patients with renal insufficiency (although not in those on dialysis or with end-stage renal disease).
In outlining some of the key features of the new guidelines at ENDO 2019, Dr. Rosen drew attention to a call for reevaluation of the need for bisphosphonates after patients have been on this therapy for 3 or more years. For those found at this time to be at low or moderate risk of fracture, a drug holiday is recommended based on guideline-cited evidence that bisphosphonates offer a residual therapeutic effect after stopping.
However, stopping is not recommended in those who remain at high risk. In these patients, bone density should be monitored at regular intervals for the goal of switching or intensifying therapy if needed. This includes use of teriparatide (Forteo) or abaloparatide (Tymlos) for periods of up to 2 years in patients with a history of severe or multiple fractures. These and other choices are included in a detailed algorithm covering both low- and high-risk patients.
Although many postmenopausal women hope to avoid pharmacologic therapy with high dietary intake of calcium and vitamin D, Dr. Rosen stressed the limited benefit of these nutrients in preventing fracture for those with established osteoporosis. While acknowledging that calcium and vitamin D enhance mineralization and maintenance of bone mass, he characterized them as “supplements” once pharmacologic therapies are indicated.
Unsurprisingly, patients prefer oral therapies that are effective but with a low burden of adverse events, according to a review of evidence undertaken by the guideline committee. Cost was a less important consideration. Dr. Rosen indicated that recognizing patient goals and priorities while explaining relative risks might engage patients in selecting a therapy to which they are willing to adhere.
He reported having no relevant financial relationships.
NEW ORLEANS – In new guidelines for the pharmacologic management of osteoporosis, bisphosphonates have been identified as the first-line therapy with denosumab (Prolia) listed as an acceptable alternative that is particularly well suited for high-risk patients, according to a presentation at the annual meeting of the Endocrine Society.
“We hope our guideline will not only improve patient care but provide confidence in treatment,” reported guideline writing committee member Clifford J. Rosen, MD, director of the Center for Clinical and Translational Research at Maine Medical Center Research Institute, Scarborough.
The new guidelines are evidence based, relying on randomized, controlled trials to evaluate the data quality of treatment options with GRADE methodology, but Dr. Rosen said that the guideline writing committee also considered patient preferences because of concerns about the abundant evidence that adherence to pharmacologic therapies for osteoporosis is poor.
“There is a considerable gap in the treatment of osteoporosis. Most women will not take anti-osteoporosis therapies despite their efficacy, and those who do often stop,” Dr. Rosen observed. He said it was the intention of the writing committee to provide acceptable recommendations with a clear outline of benefits and risks in order to enlist patients more successfully in understanding and participating in fracture prevention.
The Endocrine Society guidelines, which are available online and will soon appear in print (J Clin Endocrinol Metab. 2019;104:1-28), are focused on pharmacologic management and therefore differ from guidelines on diagnosis and treatment published previously by the American Association of Clinical Endocrinologists (AACE) (Endocr Pract. 2016;22[Suppl 4]:1-42).
The AACE guidelines, which devote considerable space to prevention, indicated that bisphosphonates should “be generally considered as initial options for most patients who are candidates for treatment.” The AACE guidelines identify denosumab as the “treatment of choice” in patients with renal insufficiency (although not in those on dialysis or with end-stage renal disease).
In outlining some of the key features of the new guidelines at ENDO 2019, Dr. Rosen drew attention to a call for reevaluation of the need for bisphosphonates after patients have been on this therapy for 3 or more years. For those found at this time to be at low or moderate risk of fracture, a drug holiday is recommended based on guideline-cited evidence that bisphosphonates offer a residual therapeutic effect after stopping.
However, stopping is not recommended in those who remain at high risk. In these patients, bone density should be monitored at regular intervals for the goal of switching or intensifying therapy if needed. This includes use of teriparatide (Forteo) or abaloparatide (Tymlos) for periods of up to 2 years in patients with a history of severe or multiple fractures. These and other choices are included in a detailed algorithm covering both low- and high-risk patients.
Although many postmenopausal women hope to avoid pharmacologic therapy with high dietary intake of calcium and vitamin D, Dr. Rosen stressed the limited benefit of these nutrients in preventing fracture for those with established osteoporosis. While acknowledging that calcium and vitamin D enhance mineralization and maintenance of bone mass, he characterized them as “supplements” once pharmacologic therapies are indicated.
Unsurprisingly, patients prefer oral therapies that are effective but with a low burden of adverse events, according to a review of evidence undertaken by the guideline committee. Cost was a less important consideration. Dr. Rosen indicated that recognizing patient goals and priorities while explaining relative risks might engage patients in selecting a therapy to which they are willing to adhere.
He reported having no relevant financial relationships.
REPORTING FROM ENDO 2019
Evaluation of the American Academy of Orthopaedic Surgeons Appropriate Use Criteria for the Nonarthroplasty Treatment of Knee Osteoarthritis in Veterans
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
Knee osteoarthritis (OA) affects almost 9.3 million adults in the US and accounts for $27 billion in annual health care expenses.1,2 Due to the increasing cost of health care and an aging population, there has been renewed interest in establishing criteria for nonarthroplasty treatment of knee OA.
In 2013, using the RAND/UCLA Appropriateness method, the American Academy of Orthopaedic Surgeons (AAOS) developed an appropriate use criteria (AUC) for nonarthroplasty management of primary OA of the knee, based on orthopaedic literature and expert opinion.3 Interventions such as activity modification, weight loss, prescribed physical therapy, nonsteroidal anti-inflammatory drugs, tramadol, prescribed oral or transcutaneous opioids, acetaminophen, intra-articular corticosteroids, hinged or unloading knee braces, arthroscopic partial menisectomy or loose body removal, and realignment osteotomy were assessed. An algorithm was developed for 576 patients scenarios that incorporated patient-specific, prognostic/predictor variables to assign designations of “appropriate,” “may be appropriate,” or “rarely appropriate,” to treatment interventions.4,5 An online version of the algorithm (orthoguidelines.org) is available for physicians and surgeons to judge appropriateness of nonarthroplasty treatments; however, it is not intended to mandate candidacy for treatment or intervention.
Clinical evaluation of the AAOS AUC is necessary to determine how treatment recommendations correlate with current practice. A recent examination of the AAOS Appropriateness System for Surgical Management of Knee OA found that prognostic/predictor variables, such as patient age, OA severity, and pattern of knee OA involvement were more heavily weighted when determining arthroplasty appropriateness than was pain severity or functional loss.6 Furthermore, non-AAOS AUC prognostic/predictor variables, such as race and gender, have been linked to disparities in utilization of knee OA interventions.7-9 Such disparities can be costly not just from a patient perceptive, but also employer and societal perspectives.10
The Department of Veterans Affairs (VA) health care system represents a model of equal-access-to care system in the US that is ideal for examination of issues about health care utilization and any disparities within the AAOS AUC model and has previously been used to assess utilization of total knee arthroplasty.9 The aim of this study was to characterize utilization of the AAOS AUC for nonarthroplasty treatment of knee OA in a VA patient population. We asked the following questions: (1) What variables are predictive of receiving a greater number of AAOS AUC evaluated nonarthroplasty treatments? (2) What variables are predictive of receiving “rarely appropriate” AAOS AUC evaluated nonarthroplasty treatment? (3) What factors are predictive of duration of nonarthroplasty care until total knee arthroplasty (TKA)?
Methods
The institutional review board at the Louis Stokes Cleveland VA Medical Center in Ohio approved a retrospective chart review of nonarthroplasty treatments utilized by patients presenting to its orthopaedic section who subsequently underwent knee arthroplasty between 2013 and 2016. Eligibility criteria included patients aged ≥ 30 years with a diagnosis of unilateral or bilateral primary knee OA. Patients with posttraumatic OA, inflammatory arthritis, and a history of infectious arthritis or Charcot arthropathy of the knee were excluded. Patients with a body mass index (BMI) > 40 or a hemoglobin A1c > 8.0 at presentation were excluded as nonarthroplasty care was the recommended course of treatment above these thresholds.
Data collected included race, gender, duration of nonarthroplasty treatment, BMI, and Kellgren-Lawrence classification of knee OA at time of presentation for symptomatic knee OA.11 All AAOS AUC-evaluated nonarthroplasty treatments utilized prior to arthroplasty intervention also were recorded (Table 1).
Statistical Analysis
Statistical analysis was completed with GraphPad Software Prism 7.0a (La Jolla, CA) and Mathworks MatLab R2016b software (Natick, MA). Univariate analysis with Student t tests with Welch corrections in the setting of unequal variance, Mann-Whitney nonparametric tests, and Fisher exact test were generated in the appropriate setting. Multivariable analyses also were conducted. For continuous outcomes, stepwise multiple linear regression was used to generate predictive models; for binary outcomes, binomial logistic regression was used.
Factors analyzed in regression modeling for the total number of AAOS AUC evaluated nonarthroplasty treatments utilized and the likelihood of receiving a rarely appropriate treatment included gender, race, function-limiting pain, range of motion (ROM), ligamentous instability, arthritis pattern, limb alignment, mechanical symptoms, BMI, age, and Kellgren-Lawrence grade. Factors analyzed in timing of TKA included the above variables plus the total number of AUC interventions, whether the patient received an inappropriate intervention, and average appropriateness of the interventions received. Residual analysis with Cook’s distance was used to identify outliers in regression. Observations with Cook’s distance > 3 times the mean Cook’s distance were identified as potential outliers, and models were adjusted accordingly. All statistical analyses were 2-tailed. Statistical significance was set to P ≤ .05 for all outputs.
Results
In the study, 97.8% of participants identified as male, and the mean age was 62.8 years (Table 3).
Appropriate Use Criteria Interventions
Patients received a mean of 5.2 AAOS AUC evaluated interventions before undergoing arthroplasty management at a mean of 32.3 months (range 2-181 months) from initial presentation. The majority of these interventions were classified as either appropriate or may be appropriate, according to the AUC definitions (95.1%). Self-management and physical therapy programs were widely utilized (100% and 90.1%, respectively), with all use of these interventions classified as appropriate.
Hinged or unloader knee braces were utilized in about half the study patients; this intervention was classified as rarely appropriate in 4.4% of these patients. Medical therapy was also widely used, with all use of NSAIDs, acetaminophen, and tramadol classified as appropriate or may be appropriate. Oral or transcutaneous opioid medications were prescribed in 14.3% of patients, with 92.3% of this use classified as rarely appropriate. Although the opioid medication prescribing provider was not specifically evaluated, there were no instances in which the orthopaedic service provided an oral or transcutaneous opioid prescriptions. Procedural interventions, with the exception of corticosteroid injections, were uncommon; no patient received realignment osteotomy, and only 12.1% of patients underwent arthroscopy. The use of arthroscopy was deemed rarely appropriate in 72.7% of these cases.
Factors Associated With AAOS AUC Intervention Use
There was no difference in the number of AAOS AUC evaluated interventions received based on BMI (mean [SD] BMI < 35, 5.2 [1.0] vs BMI ≥ 35, 5.3 [1.1], P = .49), age (mean [SD] aged < 60 years, 5.4 [1.0] vs aged ≥ 60 years, 5.1 [1.2], P = .23), or Kellgren-Lawrence arthritic grade (mean [SD] grade ≤ 2, 5.5 [1.0] vs grade > 2, 5.1 [1.1], P = .06). These variables also were not associated with receiving a rarely appropriate intervention (mean [SD] BMI < 35, 0.27 [0.5] vs BMI > 35, 0.2 [0.4], P = .81; aged > 60 years, 0.3 [0.5] vs aged < 60 years, 0.2 [0.4], P = .26; Kellgren-Lawrence grade < 2, 0.4 [0.6] vs grade > 2, 0.2 [0.4], P = .1).
Regression modeling to predict total number of AAOS AUC evaluated interventions received produced a significant model (R2 = 0.111, P = .006). The presence of ligamentous instability (β coefficient, -1.61) and the absence of mechanical symptoms (β coefficient, -0.67) were negative predictors of number of AUC interventions received. Variance inflation factors were 1.014 and 1.012, respectively. Likewise, regression modeling to identify factors predictive of receiving a rarely appropriate intervention also produced a significant model (pseudo R2= 0.06, P = .025), with lower Kellgren-Lawrence grade the only significant predictor of receiving a rarely appropriate intervention (odds ratio [OR] 0.54; 95% CI, 0.42 -0.72, per unit increase).
Timing from presentation to arthroplasty intervention was also evaluated. Age was a negative predictor (β coefficient -1.61), while positive predictors were reduced ROM (β coefficient 15.72) and having more AUC interventions (β coefficient 7.31) (model R2= 0.29, P = < .001). Age was the most significant predictor. Variance inflations factors were 1.02, 1.01, and 1.03, respectively. Receiving a rarely appropriate intervention was not associated with TKA timing.
Discussion
This single-center retrospective study examined the utilization of AAOS AUC-evaluated nonarthroplasty interventions for symptomatic knee OA prior to TKA. The aims of this study were to validate the AAOS AUC in a clinical setting and identify predictors of AAOS AUC utilization. In particular, this study focused on the number of interventions utilized prior to knee arthroplasty, whether interventions receiving a designation of rarely appropriate were used, and the duration of nonarthroplasty treatment.
Patients with knee instability used fewer total AAOS AUC evaluated interventions prior to TKA. Subjective instability has been reported as high as 27% in patients with OA and has been associated with fear of falling, poor balance confidence, activity limitations, and lower Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) physical function scores.12 However, it has not been found to correlate with knee laxity.13 Nevertheless, significant functional impairment with the risk of falling may reduce the number of nonarthroplasty interventions attempted. On the other hand, the presence of mechanical symptoms resulted in greater utilization of nonarthroplasty interventions. This is likely due to the greater utilization of arthroscopic partial menisectomy or loose body removal in this group of patients. Despite its inclusion as an AAOS AUC evaluated intervention, arthroscopy remains a contentious treatment for symptomatic knee pain in the setting of OA.14,15
For every unit decrease in Kellgren-Lawrence OA grade, patients were 54% more likely to receive a rarely appropriate intervention prior to knee arthroplasty. This is supported by the recent literature examining the AAOS AUC for surgical management of knee OA. Riddle and colleagues developed a classification tree to determine the contributions of various prognostic variables in final classifications of the 864 clinical vignettes used to develop the appropriateness algorithm and found that OA severity was strongly favored, with only 4 of the 432 vignettes with severe knee OA judged as rarely appropriate for surgical intervention.6
Our findings, too, may be explained by an AAOS AUC system that too heavily weighs radiographic severity of knee OA, resulting in more frequent rarely appropriate interventions in patients with less severe arthritis, including nonarthroplasty treatments. It is likely that rarely appropriate interventions were attempted in this subset of our study cohort based on patient’s subjective symptoms and functional status, both of which have been shown to be discordant with radiographic severity of knee OA.16
Oral or transcutaneous prescribed opioid medications were the most frequent intervention that received a rarely appropriate designation. Patients with preoperative opioid use undergoing TKA have been shown to have a greater risk for postoperative complications and longer hospital stay, particularly those patients aged < 75 years. Younger age, use of more interventions, and decreased knee ROM at presentation were predictive of longer duration of nonarthroplasty treatment. The use of more AAOS AUC evaluated interventions in these patients suggests that the AAOS AUC model may effectively be used to manage symptomatic OA, increasing the time from presentation to knee arthroplasty.
Interestingly, the use of rarely appropriate interventions did not affect TKA timing, as would be expected in a clinically effective nonarthroplasty treatment model. The reasons for rarely appropriate nonsurgical interventions are complex and require further investigation. One possible explanation is that decreased ROM was a marker for mechanical symptoms that necessitated additional intervention in the form of knee arthroscopy, delaying time to TKA.
Limitations
There are several limitations of this study. First, the small sample size (N = 90) requires acknowledgment; however, this limitation reflects the difficulty in following patients for years prior to an operative intervention. Second, the study population consists of veterans using the VA system and may not be reflective of the general population, differing with respect to gender, racial, and socioeconomic factors. Nevertheless, studies examining TKA utilization found, aside from racial and ethnic variability, patient gender and age do not affect arthroplasty utilization rate in the VA system.17
Additional limitations stem from the retrospective nature of this study. While the Computerized Patient Record System and centralized care of the VA system allows for review of all physical therapy consultations, orthotic consultations, and medications within the VA system, any treatments and intervention delivered by non-VA providers were not captured. Furthermore, the ability to assess for confounding variables limiting the prescription of certain medications, such as chronic kidney disease with NSAIDs or liver disease with acetaminophen, was limited by our study design.
Although our study suffers from selection bias with respect to examination of nonarthroplasty treatment in patients who have ultimately undergone TKA, we feel that this subset of patients with symptomatic knee OA represents the majority of patients evaluated for knee OA by orthopaedic surgeons in the clinic setting. It should be noted that although realignment osteotomies were sometimes indicated as appropriate by AAOS AUC model in our study population, this intervention was never performed due to patient and surgeon preference. Additionally, although it is not an AAOS AUC evaluated intervention, viscosupplementation was sporadically used during the study period; however, it is now off formulary at the investigation institution.
Conclusion
Our study suggests that patients without knee instability use more nonarthroplasty treatments over a longer period before TKA, and those patients with less severe knee OA are at risk of receiving an intervention judged to be rarely appropriate by the AAOS AUC. Such interventions do not affect timing of TKA. Nonarthroplasty care should be individualized to patients’ needs, and the decision to proceed with arthroplasty should be considered only after exhausting appropriate conservative measures. We recommend that providers use the AAOS AUC, especially when treating younger patients with less severe knee OA, particularly if considering opiate therapy or knee arthroscopy.
Acknowledgments
The authors would like to acknowledge Patrick Getty, MD, for his surgical care of some of the study patients. This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center in Ohio.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.
1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330.
2. Losina E, Walensky RP, Kessler CL, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113-1121; discussion 1121-1122.
3. Members of the Writing, Review, and Voting Panels of the AUC on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee, Sanders JO, Heggeness MH, Murray J, Pezold R, Donnelly P. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria on the Non-Arthroplasty Treatment of Osteoarthritis of the Knee. J Bone Joint Surg Am. 2014;96(14):1220-1221.
4. Sanders JO, Murray J, Gross L. Non-arthroplasty treatment of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):256-260.
5. Yates AJ Jr, McGrory BJ, Starz TW, Vincent KR, McCardel B, Golightly YM. AAOS appropriate use criteria: optimizing the non-arthroplasty management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2014;22(4):261-267.
6. Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic Surgeons appropriateness rating system. Osteoarthritis Cartilage. 2017;25(12):1994-1998.
7. Morgan RC Jr, Slover J. Breakout session: ethnic and racial disparities in joint arthroplasty. Clin Orthop Relat Res. 2011;469(7):1886-1890.
8. O’Connor MI, Hooten EG. Breakout session: gender disparities in knee osteoarthritis and TKA. Clin Orthop Relat Res. 2011;469(7):1883-1885.
9. Ibrahim SA. Racial and ethnic disparities in hip and knee joint replacement: a review of research in the Veterans Affairs Health Care System. J Am Acad Orthop Surg. 2007;15(suppl 1):S87-S94.
10. Karmarkar TD, Maurer A, Parks ML, et al. A fresh perspective on a familiar problem: examining disparities in knee osteoarthritis using a Markov model. Med Care. 2017;55(12):993-1000.
11. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886-1893.
12. Nguyen U, Felson DT, Niu J, et al. The impact of knee instability with and without buckling on balance confidence, fear of falling and physical function: the Multicenter Osteoarthritis Study. Osteoarthritis Cartilage. 2014;22(4):527-534.
13. Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506-1516.
14. Laupattarakasem W, Laopaiboon M, Laupattarakasem P, Sumananont C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst Rev. 2008;(1):CD005118.
15. Lamplot JD, Brophy RH. The role for arthroscopic partial meniscectomy in knees with degenerative changes: a systematic review. Bone Joint J. 2016;98-B(7):934-938.
16. Whittle R, Jordan KP, Thomas E, Peat G. Average symptom trajectories following incident radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. RMD Open. 2016;2(2):e000281.
17. Jones A, Kwoh CK, Kelley ME, Ibrahim SA. Racial disparity in knee arthroplasty utilization in the Veterans Health Administration. Arthritis Rheum. 2005;53(6):979-981.