User login
Approach to the asymptomatic adnexal mass: When to operate, refer, or observe
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Complex atypical hyperplasia: When is it appropriate to refer?
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Complex atypical hyperplasia (CAH) of the endometrium is considered the precursor for endometrioid endometrial cancer, the most common gynecologic cancer in the United States. This disease is most frequently diagnosed by gynecologists who are evaluating symptoms of abnormal uterine bleeding in premenopausal women or in postmenopausal women who experience new bleeding. Medical therapies, typically progestin-based treatments, can be employed, particularly when fertility preservation is desired or among patients who are poor surgical candidates. However, the most definitive therapy remains surgery with total hysterectomy for two reasons: CAH is associated with a 28% risk for the development of invasive cancer, and occult invasive cancer frequently coexists with CAH.1,2 This raises a question for gynecologists: Given the risk for occult endometrial cancer, should patients be referred to a gynecologic oncologist for their surgery?
What is the risk for cancer?
What is the significance of occult malignancy with CAH?
If surgeons are aware of endometrial cancer preoperatively or intraoperatively, decisions can be made about staging, particularly the need for lymphadenectomy. The virtues of staging in endometrial cancer is a controversial and frequently debated topic. No survival (therapeutic) benefit from lymphadenectomy has been observed in prospective trials when the information from staging results is not used to guide adjuvant therapy.4 However, the administration of adjuvant chemotherapy is associated with improved survival for patients with lymph node metastases.5 Therefore, if there is a benefit to staging with lymphadenectomy, it is its ability to identify patients who most need this life-saving systemic therapy.
Not all patients with endometrial cancer are at equal risk for harboring lymph node metastases and the majority may not benefit from lymphadenectomy. Patients with tumors that are deeply invasive, moderate or high grade, larger than 2 cm, or that have lymphovascular space invasion are at higher risk for lymph node metastases. Women with low grade, minimally invasive tumors that are smaller than 2 cm have extremely low risk for metastases.6 These criteria are commonly employed to stratify women at lowest risk and minimize unnecessary lymphadenectomy procedures. It should be noted that all three of these low risk features must be present to convey that negligible risk profile. The finding of a grade 1 invasive tumor alone is not enough to exclude potential lymph node metastases, particularly in the case of large or deeply invasive cancers.
How can the diagnosis be made preoperatively or intraoperatively?
The gold standard for discriminating between CAH and endometrial cancer is definitive surgical pathology. However, if surgeons wait until these results are available, they have lost the opportunity to stage the patient without subjecting them to a second surgery. The preoperative discovery of cancer may be increased by performing diagnostic curettage rather than relying on office endometrial biopsy sampling.7 This is likely due to the increased volume of tissue removed with dilation and curettage, and a reduction in the risk for sampling error. The addition of hysteroscopy to curettage does not improve upon the detection of cancer. Preoperative MRI to evaluate for depth of myometrial invasion has been described in cases of known endometrial cancer; however, its role in discriminating between CAH and invasive cancer is not well studied.
Intraoperative frozen section is commonly employed to evaluate the hysterectomy specimen for cancer in order to triage patients to staging during that same surgery. However, the accuracy of frozen section with definitive pathology is only approximately 50%.8 This means that at least half of women with CAH will have a false negative frozen section result and will have lost the opportunity for staging at the same procedure. The inaccuracy of frozen section is often overlooked by surgeons who may feel that it is a very straightforward diagnostic procedure. In reality, the characterization of CAH and invasive cancer is technically challenging and relies on multiple sectioning and significant experience in gynecologic pathology.9
Should all patients with CAH be referred and staged?
An alternative to relying on the frozen section process and its inherent inaccuracies would be to routinely stage all women with CAH, knowing that approximately 40% of them have occult cancer, and more than a third of those will have high risk features for lymph node metastases. However, due to the risks associated with lymphadenectomy, particularly lymphedema, most gynecologic oncologists do not routinely stage patients with preoperative CAH with complete lymphadenectomy.
An alternative to the all (complete lymphadenectomy) or none (hysterectomy alone) approach is to perform sentinel lymph node (SLN) biopsy for patients with CAH. SLN biopsy involves removing scant, but high yield lymphatic tissue, and has been shown to be extremely sensitive in detecting metastatic disease.10 This approach is commonly employed by surgeons in the treatment of ductal carcinoma in situ of the breast which, like CAH, is a stage 0 cancer that can be associated with invasive carcinoma on final pathology. In the case of ductal carcinoma in situ, the risk for upstaging is actually substantially lower (25%) than what is observed in CAH.11 Therefore, it would seem even more compelling to apply this approach for endometrial pathologies. The ability to apply the SLN technique is lost after hysterectomy is performed, as there is no longer the target organ into which tracer can be injected; therefore, if SLN biopsy is to be offered to these patients, it needs to be performed using only the preoperative diagnosis of CAH. In this approach, there will be overtreatment of approximately two-thirds of patients, albeit with a less radical and morbid staging procedure.
Making the decision to refer
Ultimately, decisions to refer or not are guided by comprehensive discussions between patient and provider that outline the potential risks and benefits of various approaches. Patients frequently have strong relationships with confidence in their gynecologists who may have cared for them for many years, and may be motivated to have them perform their surgery. For others, the uncertainty and possibility of an unstaged cancer and the potential of a second surgery drives their decision to seek an oncology consultation. Clinicians should discuss the inherent uncertainties in the diagnosis of CAH and the potential for underlying cancer and lymph node metastases, and help patients determine the balance of their underlying competing concerns regarding the risk for inadequate surgery versus the risk of unnecessary surgical procedures.
Summary of recommendations
Invasive endometrial cancer will be identified in the hysterectomy specimens of approximately 40% of women with a preoperative diagnosis of complex endometrial hyperplasia. Preoperative dilation and curettage may reduce the potential for missed occult cancer. Frozen section is an option for determining which patients might benefit from staging but is associated with significant inaccuracies. Failure to diagnose malignancy pre- or intraoperatively handicaps postoperative decision making regarding the necessity of adjuvant chemotherapy, and prevents the ability to offer patients potentially less morbid staging techniques such as SLN biopsy. When gynecologists without oncology training perform these hysterectomies, they should discuss these scenarios to patients and consider referral to gynecologic oncology for patients who desire the potential for comprehensive staging if necessary.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. She reports no relevant financial disclosures.
References
1. J Clin Oncol. 2010 Feb;28:788-92.
2. Cancer. 2006 Feb;106:812-9.
3. Int J Gynecol Cancer. 2005 Jan-Feb;15:127-31.
4. Lancet. 2009 Jan;373(9658):125-36.
5. J Clin Oncol. 2006 Jan;24:36-44.
6. Gynecol Oncol 2008 Apr;109:11-8.
7. Am J Obstet Gynecol. 2010 Oct;203(4):349. e1-6.
8. Am J Obstet Gynecol. 2007 May;196(5):e40-2.
9. Obstet Gynecol. 2012 Nov;120(5):1160-75.
10. Lancet Oncol. 2017 Mar;18(3):384-92.
11. Radiology. 2011 Jul;260:119-28.
Strategies to evaluate postmenopausal bleeding
Postmenopausal bleeding is a symptom that can announce the presence of a gynecologic malignancy. In this column, we will discuss the important considerations to make in the work-up of this symptom.
Roughly 10% of women will present for evaluation of postmenopausal bleeding.1 More than a third of these women will have benign pathology, with the incidence of endometrial cancer in this group at only about 5%.2 Other gynecologic malignancies should be considered as well, including cervical, vaginal, vulvar, and more rarely, those of the fallopian tubes or ovaries.
Use of ultrasound
Ultrasound is a commonly performed initial approach to work-up because of its noninvasive nature. Transvaginal ultrasound has a high negative predictive value of 99.4%-100% in ruling out malignancy.3 Among women with postmenopausal bleeding, the risk of cancer is 7.3% if their endometrial lining is 5 mm or greater and less than 0.07% risk if their lining is 4 mm or less. Therefore, this cutoff dimension is typically used to triage patients to additional sampling.
If ultrasound is performed on postmenopausal women who are asymptomatic (no bleeding), then an endometrial stripe of greater than 11 mm is considered justification for further work-up and is associated with a 6.7% risk of endometrial cancer.4 If the ultrasound reveals intracavitary lesions, a sonohysterogram would be preferred to characterize intrauterine pathology. In fact, sonohysterography is superior to transvaginal ultrasound (with a sensitivity of 80% vs. 49%, respectively) in detecting endometrial polypoid lesions.5 Preoperative identification of an intracavitary lesion may assist in selecting the best sampling technique (blind vs. hysteroscopy-guided approach).
Endometrial sampling
If an ultrasound reveals a thickened or unevaluable endometrial stripe or if the clinician chooses to proceed directly with diagnostic confirmation, several options for endometrial sampling exist, including office-based or operative procedures, as well as blind or visually guided ones. Endometrial pipelle biopsy, D&C without hysteroscopy, endometrial lavage, and endometrial brush biopsy all constitute “blind” sampling techniques. Targeted biopsy techniques include hysteroscopy D&C and saline infusion sonohysterography–guided biopsy.
Blind D&C
Although D&C may be considered the gold standard of diagnostic sampling techniques, it should be noted that 60% of these procedures sample less than half of the endometrium.6 When used in conjunction with hysteroscopy, the sensitivity in detecting cancer is high at 97% with a specificity of 93%-100%.7
While some patients are candidates for office-based procedures, D&C often requires regional or general anesthesia and is frequently performed in a hospital-based environment or surgical center. This may be most appropriate for patients who have had failed office attempts at sampling, have multiple medical comorbidities that limit the feasibility of office-based procedures (such as morbid obesity), or have severe cervical stenosis. D&C is associated with an increased risk for uterine perforation, compared with outpatient sampling procedures.
The need to go to the operating room rather than to an ambulatory setting also may increase the costs borne by the patient. The advantages of D&C include the potential for large-volume sampling and the potentially therapeutic nature of the procedure in cases of benign pathology.
Office-based procedures
Office-based sampling techniques include those using a pipelle, those employing an endometrial brush, and those guided by saline infusion sonohysterography. If performed in the office, they require minimal or no cervical dilation, are associated with a lower risk of perforation or adverse reaction to anesthesia, and usually have lower costs for patients.
Endometrial pipelle biopsies are a very effective diagnostic tool when there is global, endometrial pathology; they have a sensitivity of 83% in confirming cancer.8 It is an inexpensive and technically straightforward technique that can be easily performed in an office setting.
However, when the endometrial lining is atrophied, alternative tools may provide superior results. Endometrial brushes have been shown to be 33% more successful in collecting adequate samples,compared with pipelles, because they sample a larger endometrial surface area.9
There is ongoing development of sampling techniques, such as endometrial lavage or the combination of saline infusion sonohysterography and endometrial biopsy.10 However, future studies regarding accuracy, cost, and patient acceptability are needed before these techniques are translated to the clinical setting.
Targeted endometrial sampling
Targeted or visually guided sampling, such as hysteroscopy, has been shown to be very accurate in identifying benign pathology, although the sensitivity of hysteroscopic diagnosis of cancer is significantly lower at approximately 50%.11 Therefore, the benefit of hysteroscopy is in complementing the blind nature of D&C by guiding sampling of intracavitary lesions, should they exist.
Hysteroscopy is safe in endometrial cancer and is not associated with upstaging the cancer from transtubal extirpation of malignant cells.12
The addition of hysteroscopy contributes some cost and equipment to the blind D&C procedure; therefore, it might be best applied in cases where there is known intracavitary pathology or inadequate prior sampling. In well-selected patients, hysteroscopy often can be used in an office setting, which improves the practicality of the procedure. Smaller and, in some cases, disposable equipment aids in the feasibility of adding visual guidance to office sampling.
Optimizing sampling
Postmenopausal women have a higher risk for sampling failure, compared with younger women. Obesity also is a risk for failed sampling.13 Cervical ripening with misoprostol may increase access to the endometrial cavity, and ultrasound guidance may decrease the risk of uterine perforation in a stenotic cervix.
Clinicians should ensure that histology results are concordant with clinical data. Discordant results should be reevaluated. For example, if an ultrasound demonstrates a thickened endometrial stripe, but the sampling reveals “scant atrophic tissue,” then there is unexplained pathology to address. Further work-up, such as more comprehensive sampling with hysteroscopy, should be considered in such cases. Additionally, persistent postmenopausal bleeding, despite a benign endometrial biopsy, should be reevaluated over time to rule out occult disease missed during prior sampling.
Clinicians are now equipped with multiple ways of obtaining clinical data, and patients have options that may decrease barriers to their care. Hysteroscopy does not improve upon D&C in the diagnosis of endometrial cancer, although it may be helpful in distinguishing and treating nonmalignant lesions.
Dr. Cotangco is a resident in the department of obstetrics and gynecology at the University of Illinois, Chicago. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Acta Obstet Gynecol Scand. 2004 Feb;83(2):203-7.
2. Menopause Int. 2010 Mar;16(1):5-8.
3. Obstet Gynecol. 2009 Aug;114(2 Pt 1):409-11.
4. Ultrasound Obstet Gynecol. 2004 Oct;24(5):558-65.
5. Ultrasound Obstet Gynecol. 2001 Aug;18(2):157-62.
6. Am J Obstet Gynecol. 2009 Jul;201(1):5-11.
7. Obstet Gynecol Clin North Am. 2000 Jun;27(2):235-44.
8. J Reprod Med. 1995 Aug;40(8):553-5.
9. BJOG. 2008 Jul;115(8):1028-36.
10. PLoS Med. 2016 Dec. doi: 10.1371/journal.pmed.1002206.
11. Arch Gynecol Obstet. 2012 Mar;285(3):839-43.
12. Am J Obstet Gynecol. 2012 Jul;207(1):71.e1-5.
13. Gynecol Oncol. 2017 Feb;144(2):324-8.
Postmenopausal bleeding is a symptom that can announce the presence of a gynecologic malignancy. In this column, we will discuss the important considerations to make in the work-up of this symptom.
Roughly 10% of women will present for evaluation of postmenopausal bleeding.1 More than a third of these women will have benign pathology, with the incidence of endometrial cancer in this group at only about 5%.2 Other gynecologic malignancies should be considered as well, including cervical, vaginal, vulvar, and more rarely, those of the fallopian tubes or ovaries.
Use of ultrasound
Ultrasound is a commonly performed initial approach to work-up because of its noninvasive nature. Transvaginal ultrasound has a high negative predictive value of 99.4%-100% in ruling out malignancy.3 Among women with postmenopausal bleeding, the risk of cancer is 7.3% if their endometrial lining is 5 mm or greater and less than 0.07% risk if their lining is 4 mm or less. Therefore, this cutoff dimension is typically used to triage patients to additional sampling.
If ultrasound is performed on postmenopausal women who are asymptomatic (no bleeding), then an endometrial stripe of greater than 11 mm is considered justification for further work-up and is associated with a 6.7% risk of endometrial cancer.4 If the ultrasound reveals intracavitary lesions, a sonohysterogram would be preferred to characterize intrauterine pathology. In fact, sonohysterography is superior to transvaginal ultrasound (with a sensitivity of 80% vs. 49%, respectively) in detecting endometrial polypoid lesions.5 Preoperative identification of an intracavitary lesion may assist in selecting the best sampling technique (blind vs. hysteroscopy-guided approach).
Endometrial sampling
If an ultrasound reveals a thickened or unevaluable endometrial stripe or if the clinician chooses to proceed directly with diagnostic confirmation, several options for endometrial sampling exist, including office-based or operative procedures, as well as blind or visually guided ones. Endometrial pipelle biopsy, D&C without hysteroscopy, endometrial lavage, and endometrial brush biopsy all constitute “blind” sampling techniques. Targeted biopsy techniques include hysteroscopy D&C and saline infusion sonohysterography–guided biopsy.
Blind D&C
Although D&C may be considered the gold standard of diagnostic sampling techniques, it should be noted that 60% of these procedures sample less than half of the endometrium.6 When used in conjunction with hysteroscopy, the sensitivity in detecting cancer is high at 97% with a specificity of 93%-100%.7
While some patients are candidates for office-based procedures, D&C often requires regional or general anesthesia and is frequently performed in a hospital-based environment or surgical center. This may be most appropriate for patients who have had failed office attempts at sampling, have multiple medical comorbidities that limit the feasibility of office-based procedures (such as morbid obesity), or have severe cervical stenosis. D&C is associated with an increased risk for uterine perforation, compared with outpatient sampling procedures.
The need to go to the operating room rather than to an ambulatory setting also may increase the costs borne by the patient. The advantages of D&C include the potential for large-volume sampling and the potentially therapeutic nature of the procedure in cases of benign pathology.
Office-based procedures
Office-based sampling techniques include those using a pipelle, those employing an endometrial brush, and those guided by saline infusion sonohysterography. If performed in the office, they require minimal or no cervical dilation, are associated with a lower risk of perforation or adverse reaction to anesthesia, and usually have lower costs for patients.
Endometrial pipelle biopsies are a very effective diagnostic tool when there is global, endometrial pathology; they have a sensitivity of 83% in confirming cancer.8 It is an inexpensive and technically straightforward technique that can be easily performed in an office setting.
However, when the endometrial lining is atrophied, alternative tools may provide superior results. Endometrial brushes have been shown to be 33% more successful in collecting adequate samples,compared with pipelles, because they sample a larger endometrial surface area.9
There is ongoing development of sampling techniques, such as endometrial lavage or the combination of saline infusion sonohysterography and endometrial biopsy.10 However, future studies regarding accuracy, cost, and patient acceptability are needed before these techniques are translated to the clinical setting.
Targeted endometrial sampling
Targeted or visually guided sampling, such as hysteroscopy, has been shown to be very accurate in identifying benign pathology, although the sensitivity of hysteroscopic diagnosis of cancer is significantly lower at approximately 50%.11 Therefore, the benefit of hysteroscopy is in complementing the blind nature of D&C by guiding sampling of intracavitary lesions, should they exist.
Hysteroscopy is safe in endometrial cancer and is not associated with upstaging the cancer from transtubal extirpation of malignant cells.12
The addition of hysteroscopy contributes some cost and equipment to the blind D&C procedure; therefore, it might be best applied in cases where there is known intracavitary pathology or inadequate prior sampling. In well-selected patients, hysteroscopy often can be used in an office setting, which improves the practicality of the procedure. Smaller and, in some cases, disposable equipment aids in the feasibility of adding visual guidance to office sampling.
Optimizing sampling
Postmenopausal women have a higher risk for sampling failure, compared with younger women. Obesity also is a risk for failed sampling.13 Cervical ripening with misoprostol may increase access to the endometrial cavity, and ultrasound guidance may decrease the risk of uterine perforation in a stenotic cervix.
Clinicians should ensure that histology results are concordant with clinical data. Discordant results should be reevaluated. For example, if an ultrasound demonstrates a thickened endometrial stripe, but the sampling reveals “scant atrophic tissue,” then there is unexplained pathology to address. Further work-up, such as more comprehensive sampling with hysteroscopy, should be considered in such cases. Additionally, persistent postmenopausal bleeding, despite a benign endometrial biopsy, should be reevaluated over time to rule out occult disease missed during prior sampling.
Clinicians are now equipped with multiple ways of obtaining clinical data, and patients have options that may decrease barriers to their care. Hysteroscopy does not improve upon D&C in the diagnosis of endometrial cancer, although it may be helpful in distinguishing and treating nonmalignant lesions.
Dr. Cotangco is a resident in the department of obstetrics and gynecology at the University of Illinois, Chicago. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Acta Obstet Gynecol Scand. 2004 Feb;83(2):203-7.
2. Menopause Int. 2010 Mar;16(1):5-8.
3. Obstet Gynecol. 2009 Aug;114(2 Pt 1):409-11.
4. Ultrasound Obstet Gynecol. 2004 Oct;24(5):558-65.
5. Ultrasound Obstet Gynecol. 2001 Aug;18(2):157-62.
6. Am J Obstet Gynecol. 2009 Jul;201(1):5-11.
7. Obstet Gynecol Clin North Am. 2000 Jun;27(2):235-44.
8. J Reprod Med. 1995 Aug;40(8):553-5.
9. BJOG. 2008 Jul;115(8):1028-36.
10. PLoS Med. 2016 Dec. doi: 10.1371/journal.pmed.1002206.
11. Arch Gynecol Obstet. 2012 Mar;285(3):839-43.
12. Am J Obstet Gynecol. 2012 Jul;207(1):71.e1-5.
13. Gynecol Oncol. 2017 Feb;144(2):324-8.
Postmenopausal bleeding is a symptom that can announce the presence of a gynecologic malignancy. In this column, we will discuss the important considerations to make in the work-up of this symptom.
Roughly 10% of women will present for evaluation of postmenopausal bleeding.1 More than a third of these women will have benign pathology, with the incidence of endometrial cancer in this group at only about 5%.2 Other gynecologic malignancies should be considered as well, including cervical, vaginal, vulvar, and more rarely, those of the fallopian tubes or ovaries.
Use of ultrasound
Ultrasound is a commonly performed initial approach to work-up because of its noninvasive nature. Transvaginal ultrasound has a high negative predictive value of 99.4%-100% in ruling out malignancy.3 Among women with postmenopausal bleeding, the risk of cancer is 7.3% if their endometrial lining is 5 mm or greater and less than 0.07% risk if their lining is 4 mm or less. Therefore, this cutoff dimension is typically used to triage patients to additional sampling.
If ultrasound is performed on postmenopausal women who are asymptomatic (no bleeding), then an endometrial stripe of greater than 11 mm is considered justification for further work-up and is associated with a 6.7% risk of endometrial cancer.4 If the ultrasound reveals intracavitary lesions, a sonohysterogram would be preferred to characterize intrauterine pathology. In fact, sonohysterography is superior to transvaginal ultrasound (with a sensitivity of 80% vs. 49%, respectively) in detecting endometrial polypoid lesions.5 Preoperative identification of an intracavitary lesion may assist in selecting the best sampling technique (blind vs. hysteroscopy-guided approach).
Endometrial sampling
If an ultrasound reveals a thickened or unevaluable endometrial stripe or if the clinician chooses to proceed directly with diagnostic confirmation, several options for endometrial sampling exist, including office-based or operative procedures, as well as blind or visually guided ones. Endometrial pipelle biopsy, D&C without hysteroscopy, endometrial lavage, and endometrial brush biopsy all constitute “blind” sampling techniques. Targeted biopsy techniques include hysteroscopy D&C and saline infusion sonohysterography–guided biopsy.
Blind D&C
Although D&C may be considered the gold standard of diagnostic sampling techniques, it should be noted that 60% of these procedures sample less than half of the endometrium.6 When used in conjunction with hysteroscopy, the sensitivity in detecting cancer is high at 97% with a specificity of 93%-100%.7
While some patients are candidates for office-based procedures, D&C often requires regional or general anesthesia and is frequently performed in a hospital-based environment or surgical center. This may be most appropriate for patients who have had failed office attempts at sampling, have multiple medical comorbidities that limit the feasibility of office-based procedures (such as morbid obesity), or have severe cervical stenosis. D&C is associated with an increased risk for uterine perforation, compared with outpatient sampling procedures.
The need to go to the operating room rather than to an ambulatory setting also may increase the costs borne by the patient. The advantages of D&C include the potential for large-volume sampling and the potentially therapeutic nature of the procedure in cases of benign pathology.
Office-based procedures
Office-based sampling techniques include those using a pipelle, those employing an endometrial brush, and those guided by saline infusion sonohysterography. If performed in the office, they require minimal or no cervical dilation, are associated with a lower risk of perforation or adverse reaction to anesthesia, and usually have lower costs for patients.
Endometrial pipelle biopsies are a very effective diagnostic tool when there is global, endometrial pathology; they have a sensitivity of 83% in confirming cancer.8 It is an inexpensive and technically straightforward technique that can be easily performed in an office setting.
However, when the endometrial lining is atrophied, alternative tools may provide superior results. Endometrial brushes have been shown to be 33% more successful in collecting adequate samples,compared with pipelles, because they sample a larger endometrial surface area.9
There is ongoing development of sampling techniques, such as endometrial lavage or the combination of saline infusion sonohysterography and endometrial biopsy.10 However, future studies regarding accuracy, cost, and patient acceptability are needed before these techniques are translated to the clinical setting.
Targeted endometrial sampling
Targeted or visually guided sampling, such as hysteroscopy, has been shown to be very accurate in identifying benign pathology, although the sensitivity of hysteroscopic diagnosis of cancer is significantly lower at approximately 50%.11 Therefore, the benefit of hysteroscopy is in complementing the blind nature of D&C by guiding sampling of intracavitary lesions, should they exist.
Hysteroscopy is safe in endometrial cancer and is not associated with upstaging the cancer from transtubal extirpation of malignant cells.12
The addition of hysteroscopy contributes some cost and equipment to the blind D&C procedure; therefore, it might be best applied in cases where there is known intracavitary pathology or inadequate prior sampling. In well-selected patients, hysteroscopy often can be used in an office setting, which improves the practicality of the procedure. Smaller and, in some cases, disposable equipment aids in the feasibility of adding visual guidance to office sampling.
Optimizing sampling
Postmenopausal women have a higher risk for sampling failure, compared with younger women. Obesity also is a risk for failed sampling.13 Cervical ripening with misoprostol may increase access to the endometrial cavity, and ultrasound guidance may decrease the risk of uterine perforation in a stenotic cervix.
Clinicians should ensure that histology results are concordant with clinical data. Discordant results should be reevaluated. For example, if an ultrasound demonstrates a thickened endometrial stripe, but the sampling reveals “scant atrophic tissue,” then there is unexplained pathology to address. Further work-up, such as more comprehensive sampling with hysteroscopy, should be considered in such cases. Additionally, persistent postmenopausal bleeding, despite a benign endometrial biopsy, should be reevaluated over time to rule out occult disease missed during prior sampling.
Clinicians are now equipped with multiple ways of obtaining clinical data, and patients have options that may decrease barriers to their care. Hysteroscopy does not improve upon D&C in the diagnosis of endometrial cancer, although it may be helpful in distinguishing and treating nonmalignant lesions.
Dr. Cotangco is a resident in the department of obstetrics and gynecology at the University of Illinois, Chicago. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Acta Obstet Gynecol Scand. 2004 Feb;83(2):203-7.
2. Menopause Int. 2010 Mar;16(1):5-8.
3. Obstet Gynecol. 2009 Aug;114(2 Pt 1):409-11.
4. Ultrasound Obstet Gynecol. 2004 Oct;24(5):558-65.
5. Ultrasound Obstet Gynecol. 2001 Aug;18(2):157-62.
6. Am J Obstet Gynecol. 2009 Jul;201(1):5-11.
7. Obstet Gynecol Clin North Am. 2000 Jun;27(2):235-44.
8. J Reprod Med. 1995 Aug;40(8):553-5.
9. BJOG. 2008 Jul;115(8):1028-36.
10. PLoS Med. 2016 Dec. doi: 10.1371/journal.pmed.1002206.
11. Arch Gynecol Obstet. 2012 Mar;285(3):839-43.
12. Am J Obstet Gynecol. 2012 Jul;207(1):71.e1-5.
13. Gynecol Oncol. 2017 Feb;144(2):324-8.
Approaching intraoperative bowel injury
Enterotomy can be a serious complication in abdominopelvic surgery, particularly if it is not immediately recognized and treated. Risk of visceral injury increases when complex dissection is required for treatment of cancer, resection of endometriosis, and extensive lysis of adhesions.
In a retrospective review from 1984 to 2003, investigators assessed intestinal injuries at the time of gynecologic operations. Of the 110 cases reported, about 37% occurred during the opening of the peritoneal cavity, 38% during adhesiolysis and pelvic dissection, 9% during laparoscopy, 9% during vaginal surgery, and 8% during dilation and curettage. Of the bowel injuries, more than 75% were minor.1 Mortality from unrecognized bowel injury is significant, and as such, appropriate recognition and management of these injuries is critical.2
Some basic principles are critical when surgeons face a bowel injury:
1. Recognize the extent of the injury, including the size of the breach, the depth (full or partial thickness), and the nature of the injury (thermal or cold).
2. Assess the integrity of the bowel, including adequacy of blood supply, prior bowel damage from radiation, and absence of downstream obstruction.
3. Ensure no other occult injuries exist in other segments.
4. Obtain adequate exposure and mobilization of the bowel beyond the site of injury, including the adjacent bowel. This involves releasing other adhesions so that adequate bowel length is available for a tension-free repair.
Methods of repair
The decision to employ each is influenced by multiple factors. Primary closure is best suited to small lesions (1 cm or less) that are a result of cold or sharp injury. However, thermal injury sustained via electrosurgical devices induces delayed tissue damage beyond the visible edges of the immediate defect, and surgeons should consider a resection of bowel to at least 1 cm beyond the immediately apparent injury site. Additionally, resection and re-anastamosis should also be considered if the damaged segment of bowel has poor blood supply, integrity, or the repair would result in tension along the suture/staple line or luminal narrowing.
Simple small bowel closures
Serosal abrasions need not be repaired; however, small tears of the serosa and muscularis can be managed with a single layer of interrupted 3-0 absorbable or permanent silk suture on a tapered needle. The suture line should be perpendicular to the longitudinal axis of the bowel at 2-mm to 3-mm intervals in order to prevent narrowing of the lumen. The suture should pass through serosal and muscular layers in an imbricating (Lembert) stitch. For smaller defects of less than 6 mm, a single layer closure is typically adequate.
Small bowel resection
Some larger defects, thermal injuries, and segments with multiple enterotomies may be best repaired with resection and re-anastamosis technique. A segment of resectable bowel is chosen such that the afferent and efferent limbs to be re-anastamosed can be reapproximated in a tension-free fashion. A mesenterotomy is made at the proximal and distal portions of the involved bowel. A gastrointestinal anastomotic stapler is then inserted perpendicularly across the bowel. The remaining wedge of connected mesentery can then be efficiently excised with an electrothermal bipolar coagulator device ensuring that maximal mesentery and blood supply are preserved to the remaining limbs of intestine. The proximal and distal segments are then aligned at the antimesenteric sides.
Large bowel repair
Defects in the serosa and small lacerations can be managed with a primary closure, similar to the small intestine. For more extensive injuries that may require resection, diversion, or complicated repair, consultation with a gynecologic oncologist or general or colorectal surgeon may be indicated as colotomy repairs are associated with higher rates of breakdown and fistula. If fecal contamination is present, copious irrigation should be performed and placement of a peritoneal drain to reduce the likelihood of abscess formation should be considered. If appropriate antibiotic prophylaxis for colonic surgery has not been given prior to skin incision, it should be administered once the colotomy is identified.
Standard prophylaxis for hysterectomy (such as a first-generation cephalosporin like cefazolin) is not adequate for large bowel surgery, and either metronidazole should be added or a second-generation cephalosporin such as cefoxitin should be given. For patients with penicillin allergy, clindamycin or vancomycin with either gentamicin or a fluoroquinolone should be administered.6
Postoperative management
The potential for postoperative morbidity must be understood for appropriate management following bowel surgery. Ileus is common and the clinician should understand how to diagnose and manage it. Additionally, intra-abdominal abscess, anastomotic leak, fistula formation, and mechanical obstruction are complications that may require surgical intervention and must be vigilantly managed.
The routine use of postoperative nasogastric tube (NGT) does not hasten return of bowel function or prevent leak from sites of gastrointestinal repair. In fact, early feeding has been associated with reduced perioperative complications and earlier return of bowel function has been observed without the use of NGT.7 In general, for small and large intestinal injuries, early feeding is considered acceptable.8
Prolonged antibiotic prophylaxis, beyond 24 hours, is not recommended.6
Avoiding injury
Gynecologic surgeons should adhere to surgical principles with sharp dissection for adhesions, gentle tissue handling, adequate exposure, and light retraction to prevent bowel injury or minimize their extent. Laparoscopic entry sites should be chosen based on the likelihood of abdominal adhesions. When the patient’s history predicts a high likelihood of intraperitoneal adhesions, the left upper quadrant site should be strongly considered as the entry site. The likelihood of gastrointestinal injury is not influenced by open versus closed laparoscopic entry and surgeons should use the technique with which they have the greatest experience and skill.9 However, in patients who have had prior laparotomies, there is an increased risk of periumbilical adhesions, and consideration should be made for a nonumbilical entry site.10 Methodical sharp dissection and sparing use of thermal energy should be used with adhesiolysis. When injury occurs, prompt recognition, preparation, and methodical management can mitigate the impact.
Dr. Staley is a gynecologic oncology fellow at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
References
1. Int Surg. 2006 Nov-Dec;91(6):336-40.
2. J Am Coll Surg. 2001 Jun;192(6):677-83.
3. Doherty, G. Current Diagnosis and Treatment: Surgery. Thirteenth Edition. New York: McGraw Hill, 2010.
4. Hoffman B. Williams Gynecology. Third Edition. New York: McGraw Hill, 2016.
5. Berek J, Hacker N. Berek & Hacker’s Gynecologic Oncology. Sixth Edition. Philadelphia: Wolters Kluwer, 2015.
6. Surg Infect (Larchmt). 2013 Feb;14(1):73-156.
7. Br J Surg. 2005 Jun;92(6):673-80.
8. Am J Obstet Gynecol. 2001 Jul;185(1):1-4.
9. Cochrane Database Syst Rev. 2015 Aug 31;8:CD006583.
10. Br J Obstet Gynaecol. 1997 May;104(5):595-600.
Enterotomy can be a serious complication in abdominopelvic surgery, particularly if it is not immediately recognized and treated. Risk of visceral injury increases when complex dissection is required for treatment of cancer, resection of endometriosis, and extensive lysis of adhesions.
In a retrospective review from 1984 to 2003, investigators assessed intestinal injuries at the time of gynecologic operations. Of the 110 cases reported, about 37% occurred during the opening of the peritoneal cavity, 38% during adhesiolysis and pelvic dissection, 9% during laparoscopy, 9% during vaginal surgery, and 8% during dilation and curettage. Of the bowel injuries, more than 75% were minor.1 Mortality from unrecognized bowel injury is significant, and as such, appropriate recognition and management of these injuries is critical.2
Some basic principles are critical when surgeons face a bowel injury:
1. Recognize the extent of the injury, including the size of the breach, the depth (full or partial thickness), and the nature of the injury (thermal or cold).
2. Assess the integrity of the bowel, including adequacy of blood supply, prior bowel damage from radiation, and absence of downstream obstruction.
3. Ensure no other occult injuries exist in other segments.
4. Obtain adequate exposure and mobilization of the bowel beyond the site of injury, including the adjacent bowel. This involves releasing other adhesions so that adequate bowel length is available for a tension-free repair.
Methods of repair
The decision to employ each is influenced by multiple factors. Primary closure is best suited to small lesions (1 cm or less) that are a result of cold or sharp injury. However, thermal injury sustained via electrosurgical devices induces delayed tissue damage beyond the visible edges of the immediate defect, and surgeons should consider a resection of bowel to at least 1 cm beyond the immediately apparent injury site. Additionally, resection and re-anastamosis should also be considered if the damaged segment of bowel has poor blood supply, integrity, or the repair would result in tension along the suture/staple line or luminal narrowing.
Simple small bowel closures
Serosal abrasions need not be repaired; however, small tears of the serosa and muscularis can be managed with a single layer of interrupted 3-0 absorbable or permanent silk suture on a tapered needle. The suture line should be perpendicular to the longitudinal axis of the bowel at 2-mm to 3-mm intervals in order to prevent narrowing of the lumen. The suture should pass through serosal and muscular layers in an imbricating (Lembert) stitch. For smaller defects of less than 6 mm, a single layer closure is typically adequate.
Small bowel resection
Some larger defects, thermal injuries, and segments with multiple enterotomies may be best repaired with resection and re-anastamosis technique. A segment of resectable bowel is chosen such that the afferent and efferent limbs to be re-anastamosed can be reapproximated in a tension-free fashion. A mesenterotomy is made at the proximal and distal portions of the involved bowel. A gastrointestinal anastomotic stapler is then inserted perpendicularly across the bowel. The remaining wedge of connected mesentery can then be efficiently excised with an electrothermal bipolar coagulator device ensuring that maximal mesentery and blood supply are preserved to the remaining limbs of intestine. The proximal and distal segments are then aligned at the antimesenteric sides.
Large bowel repair
Defects in the serosa and small lacerations can be managed with a primary closure, similar to the small intestine. For more extensive injuries that may require resection, diversion, or complicated repair, consultation with a gynecologic oncologist or general or colorectal surgeon may be indicated as colotomy repairs are associated with higher rates of breakdown and fistula. If fecal contamination is present, copious irrigation should be performed and placement of a peritoneal drain to reduce the likelihood of abscess formation should be considered. If appropriate antibiotic prophylaxis for colonic surgery has not been given prior to skin incision, it should be administered once the colotomy is identified.
Standard prophylaxis for hysterectomy (such as a first-generation cephalosporin like cefazolin) is not adequate for large bowel surgery, and either metronidazole should be added or a second-generation cephalosporin such as cefoxitin should be given. For patients with penicillin allergy, clindamycin or vancomycin with either gentamicin or a fluoroquinolone should be administered.6
Postoperative management
The potential for postoperative morbidity must be understood for appropriate management following bowel surgery. Ileus is common and the clinician should understand how to diagnose and manage it. Additionally, intra-abdominal abscess, anastomotic leak, fistula formation, and mechanical obstruction are complications that may require surgical intervention and must be vigilantly managed.
The routine use of postoperative nasogastric tube (NGT) does not hasten return of bowel function or prevent leak from sites of gastrointestinal repair. In fact, early feeding has been associated with reduced perioperative complications and earlier return of bowel function has been observed without the use of NGT.7 In general, for small and large intestinal injuries, early feeding is considered acceptable.8
Prolonged antibiotic prophylaxis, beyond 24 hours, is not recommended.6
Avoiding injury
Gynecologic surgeons should adhere to surgical principles with sharp dissection for adhesions, gentle tissue handling, adequate exposure, and light retraction to prevent bowel injury or minimize their extent. Laparoscopic entry sites should be chosen based on the likelihood of abdominal adhesions. When the patient’s history predicts a high likelihood of intraperitoneal adhesions, the left upper quadrant site should be strongly considered as the entry site. The likelihood of gastrointestinal injury is not influenced by open versus closed laparoscopic entry and surgeons should use the technique with which they have the greatest experience and skill.9 However, in patients who have had prior laparotomies, there is an increased risk of periumbilical adhesions, and consideration should be made for a nonumbilical entry site.10 Methodical sharp dissection and sparing use of thermal energy should be used with adhesiolysis. When injury occurs, prompt recognition, preparation, and methodical management can mitigate the impact.
Dr. Staley is a gynecologic oncology fellow at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
References
1. Int Surg. 2006 Nov-Dec;91(6):336-40.
2. J Am Coll Surg. 2001 Jun;192(6):677-83.
3. Doherty, G. Current Diagnosis and Treatment: Surgery. Thirteenth Edition. New York: McGraw Hill, 2010.
4. Hoffman B. Williams Gynecology. Third Edition. New York: McGraw Hill, 2016.
5. Berek J, Hacker N. Berek & Hacker’s Gynecologic Oncology. Sixth Edition. Philadelphia: Wolters Kluwer, 2015.
6. Surg Infect (Larchmt). 2013 Feb;14(1):73-156.
7. Br J Surg. 2005 Jun;92(6):673-80.
8. Am J Obstet Gynecol. 2001 Jul;185(1):1-4.
9. Cochrane Database Syst Rev. 2015 Aug 31;8:CD006583.
10. Br J Obstet Gynaecol. 1997 May;104(5):595-600.
Enterotomy can be a serious complication in abdominopelvic surgery, particularly if it is not immediately recognized and treated. Risk of visceral injury increases when complex dissection is required for treatment of cancer, resection of endometriosis, and extensive lysis of adhesions.
In a retrospective review from 1984 to 2003, investigators assessed intestinal injuries at the time of gynecologic operations. Of the 110 cases reported, about 37% occurred during the opening of the peritoneal cavity, 38% during adhesiolysis and pelvic dissection, 9% during laparoscopy, 9% during vaginal surgery, and 8% during dilation and curettage. Of the bowel injuries, more than 75% were minor.1 Mortality from unrecognized bowel injury is significant, and as such, appropriate recognition and management of these injuries is critical.2
Some basic principles are critical when surgeons face a bowel injury:
1. Recognize the extent of the injury, including the size of the breach, the depth (full or partial thickness), and the nature of the injury (thermal or cold).
2. Assess the integrity of the bowel, including adequacy of blood supply, prior bowel damage from radiation, and absence of downstream obstruction.
3. Ensure no other occult injuries exist in other segments.
4. Obtain adequate exposure and mobilization of the bowel beyond the site of injury, including the adjacent bowel. This involves releasing other adhesions so that adequate bowel length is available for a tension-free repair.
Methods of repair
The decision to employ each is influenced by multiple factors. Primary closure is best suited to small lesions (1 cm or less) that are a result of cold or sharp injury. However, thermal injury sustained via electrosurgical devices induces delayed tissue damage beyond the visible edges of the immediate defect, and surgeons should consider a resection of bowel to at least 1 cm beyond the immediately apparent injury site. Additionally, resection and re-anastamosis should also be considered if the damaged segment of bowel has poor blood supply, integrity, or the repair would result in tension along the suture/staple line or luminal narrowing.
Simple small bowel closures
Serosal abrasions need not be repaired; however, small tears of the serosa and muscularis can be managed with a single layer of interrupted 3-0 absorbable or permanent silk suture on a tapered needle. The suture line should be perpendicular to the longitudinal axis of the bowel at 2-mm to 3-mm intervals in order to prevent narrowing of the lumen. The suture should pass through serosal and muscular layers in an imbricating (Lembert) stitch. For smaller defects of less than 6 mm, a single layer closure is typically adequate.
Small bowel resection
Some larger defects, thermal injuries, and segments with multiple enterotomies may be best repaired with resection and re-anastamosis technique. A segment of resectable bowel is chosen such that the afferent and efferent limbs to be re-anastamosed can be reapproximated in a tension-free fashion. A mesenterotomy is made at the proximal and distal portions of the involved bowel. A gastrointestinal anastomotic stapler is then inserted perpendicularly across the bowel. The remaining wedge of connected mesentery can then be efficiently excised with an electrothermal bipolar coagulator device ensuring that maximal mesentery and blood supply are preserved to the remaining limbs of intestine. The proximal and distal segments are then aligned at the antimesenteric sides.
Large bowel repair
Defects in the serosa and small lacerations can be managed with a primary closure, similar to the small intestine. For more extensive injuries that may require resection, diversion, or complicated repair, consultation with a gynecologic oncologist or general or colorectal surgeon may be indicated as colotomy repairs are associated with higher rates of breakdown and fistula. If fecal contamination is present, copious irrigation should be performed and placement of a peritoneal drain to reduce the likelihood of abscess formation should be considered. If appropriate antibiotic prophylaxis for colonic surgery has not been given prior to skin incision, it should be administered once the colotomy is identified.
Standard prophylaxis for hysterectomy (such as a first-generation cephalosporin like cefazolin) is not adequate for large bowel surgery, and either metronidazole should be added or a second-generation cephalosporin such as cefoxitin should be given. For patients with penicillin allergy, clindamycin or vancomycin with either gentamicin or a fluoroquinolone should be administered.6
Postoperative management
The potential for postoperative morbidity must be understood for appropriate management following bowel surgery. Ileus is common and the clinician should understand how to diagnose and manage it. Additionally, intra-abdominal abscess, anastomotic leak, fistula formation, and mechanical obstruction are complications that may require surgical intervention and must be vigilantly managed.
The routine use of postoperative nasogastric tube (NGT) does not hasten return of bowel function or prevent leak from sites of gastrointestinal repair. In fact, early feeding has been associated with reduced perioperative complications and earlier return of bowel function has been observed without the use of NGT.7 In general, for small and large intestinal injuries, early feeding is considered acceptable.8
Prolonged antibiotic prophylaxis, beyond 24 hours, is not recommended.6
Avoiding injury
Gynecologic surgeons should adhere to surgical principles with sharp dissection for adhesions, gentle tissue handling, adequate exposure, and light retraction to prevent bowel injury or minimize their extent. Laparoscopic entry sites should be chosen based on the likelihood of abdominal adhesions. When the patient’s history predicts a high likelihood of intraperitoneal adhesions, the left upper quadrant site should be strongly considered as the entry site. The likelihood of gastrointestinal injury is not influenced by open versus closed laparoscopic entry and surgeons should use the technique with which they have the greatest experience and skill.9 However, in patients who have had prior laparotomies, there is an increased risk of periumbilical adhesions, and consideration should be made for a nonumbilical entry site.10 Methodical sharp dissection and sparing use of thermal energy should be used with adhesiolysis. When injury occurs, prompt recognition, preparation, and methodical management can mitigate the impact.
Dr. Staley is a gynecologic oncology fellow at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures.
References
1. Int Surg. 2006 Nov-Dec;91(6):336-40.
2. J Am Coll Surg. 2001 Jun;192(6):677-83.
3. Doherty, G. Current Diagnosis and Treatment: Surgery. Thirteenth Edition. New York: McGraw Hill, 2010.
4. Hoffman B. Williams Gynecology. Third Edition. New York: McGraw Hill, 2016.
5. Berek J, Hacker N. Berek & Hacker’s Gynecologic Oncology. Sixth Edition. Philadelphia: Wolters Kluwer, 2015.
6. Surg Infect (Larchmt). 2013 Feb;14(1):73-156.
7. Br J Surg. 2005 Jun;92(6):673-80.
8. Am J Obstet Gynecol. 2001 Jul;185(1):1-4.
9. Cochrane Database Syst Rev. 2015 Aug 31;8:CD006583.
10. Br J Obstet Gynaecol. 1997 May;104(5):595-600.
Low malignant potential tumors of the ovary: A review
Low malignant potential tumors of the ovary – otherwise known as borderline tumors – include ovarian tumors with atypical cellularity, which lack stromal invasion that differentiates them from low grade and high grade invasive carcinomas. They can coexist with extraovarian disease, however, in the setting of borderline tumors these foci are referred to as “implants” rather than metastases. As discussed below, these implants can exhibit the presence of invasion or not.
Classification
The two most common histologic categories of borderline tumors are serous and mucinous cell types. Rarer histologic types such as endometrioid, clear cell, and Brenner also exist. However, these are so infrequent that they will not be covered in this discussion as there are very limited data to make generalizations about these histologies.
Serous borderline tumors contain cellularity similar to that of fallopian tubal epithelium. Approximately 25% of all serous ovarian tumors exhibit borderline features. Compared with mucinous tumors, they are more commonly bilateral and smaller in size (mean size of 12 cm) at the time of diagnosis and they are more likely to be associated with extraovarian implants (typically peritoneal). In fact, up to 25% of serous borderline tumors have concomitant extraovarian implants. Cancer antigen (CA) 125 is commonly a tumor marker for these tumors (elevated in 45% of early stage disease and 80% of advanced stage disease).1
Incidence
The incidence of borderline ovarian tumors is 2.5 per 100,000 woman years in the United States. About 70% are diagnosed at stage I.3 They arise in a younger population compared with invasive ovarian carcinomas. Risk factors for development of borderline tumors are similar to those of invasive ovarian carcinomas (such as nulliparity) but there may be a stronger association between the development of borderline ovarian tumors and infertility, as well as prior use of infertility treatment.4
Diagnosis
The diagnosis of borderline tumors of the ovaries occurs almost exclusively at the time of surgical pathology (either frozen section or definitive pathology).
Preoperative assessments with imaging and tumor markers – usually CA 125 and carcinoembryonic antigen (CEA) – are nonspecific for this tumor type. Preoperative imaging will typically reveal complex ovarian cysts with papillations and vascularity. However, in the case of mucinous borderline tumors, unilocular cysts are common.1 The presence of ascites and peritoneal implants can be observed on preoperative imaging of serous borderline tumors with extraovarian disease. However, it is not possible for this imaging to accurately differentiate borderline tumors with implants from low grade and high grade carcinomas with metastases.
Surgical management
Borderline tumors are commonly diagnosed in women of reproductive age and decisions need to be made regarding fertility sparing surgery, ovarian sparing surgery, and whether staging is performed. The recommended surgery for women who have completed child bearing is complete hysterectomy with bilateral salpingo-oophorectomy. However, cystectomy or unilateral salpingo-oophorectomy can be considered for women who desire fertility preservation. Conservative fertility preserving surgery is associated with an increased risk of recurrence, but with no negative impact on survival.1
Staging – with at least omentectomy and comprehensive evaluation of the peritoneal cavity, with or without peritoneal biopsies – can be considered, though staging is not associated with improved survival. Lymphadenectomy is also not associated with improved oncologic outcomes and routine lymphadenectomy is not recommended for borderline tumors.1 However, about a quarter of patients with gross evidence of extraovarian disease have implants within lymph nodes. Bulky lymph nodes should be removed, particularly in this group of patients.
Complete removal of extraovarian implants is the surgical intervention that is most important for survival and recurrence.1 This requires that surgeons thoroughly evaluate the peritoneal cavity and retroperitoneum, and possess the capability to completely resect all sites of disease.
Historically appendectomy was part of surgical staging of mucinous borderline tumors in order to identify a primary appendiceal lesion, but only 1% of patients with a grossly normal appearing appendix have significant pathology identified. This is no longer recommended.2
Treatment
The primary treatment for borderline tumors of the ovary is surgery. A minimally invasive approach is appropriate when feasible, though it may be associated with an increased risk of cyst rupture, particularly if cystectomy is attempted. Outcomes are best when extraovarian implants are completely resected. Adjuvant chemotherapy is not associated with improved survival and is not routinely recommended, though the guidelines from the National Comprehensive Cancer Network include this as an option for patients with advanced stage disease that is either completely or incompletely resected.5
Prognosis
In general, prognosis is excellent for borderline tumors with 5- and 10-year survival of 99% and 97%, 98% and 90%, and 96% and 88% for stages I, II and III tumors, respectively.1 However, several pathologic, molecular, and anatomic features are important in predicting who is at highest risk for recurrence.
Serous borderline tumors with invasive implants (as opposed to desmoplastic implants) and incompletely resected extraovarian implants are associated with increased recurrence and poor prognosis.Micropapillary features and stromal invasion are histologic features that have historically been associated with worse prognosis, but it is unclear if these are independent risk factors, or instead associated with invasive implants. For mucinous borderline tumors, intraepithelial carcinoma has been inconclusively associated with poor prognosis.1,6
Surveillance
Recurrences do occur in patients with a history of borderline tumors of the ovary, however these typically occur late. For this reason, surveillance is important and should continue for many years after diagnosis. Most recurrences are within the peritoneal cavity and are treated with surgical excision and patients should be counseled regarding symptoms of recurrence that include gastrointestinal symptoms, bloating, and pain.
In accordance with guidelines from the Society of Gynecologic Oncology, surveillance examinations can take place annually as there is no evidence that more frequent evaluations improve outcomes. These visits should include physical examinations (with pelvic examinations), symptom assessment, and, if elevated preoperatively, assessment of relevant tumor markers (typically CA 125 and/or CEA).7 Surveillance should continue for at least 10 years postoperatively.
Routine imaging is not recommended for all patients in surveillance. However, for patients who have had fertility-sparing surgery, imaging with pelvic ultrasound is recommended, particularly for women with a history of cystectomy or serous borderline tumor (who are at increased risk for bilateral tumors).
Prognosis is most closely associated with the presence of invasive implants and residual disease following surgery. Surgeons who manage these tumors can safely consider fertility-sparing procedures but should be equipped to completely resect all gross disease.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Lancet Oncol. 2012 Mar;13(3):e103-15.
2. Arch Gynecol Obstet. 2016 Nov;294(6):1283-9.
3. Cancer. 2002 Dec 1;95(11):2380-9.
4. Am J Epidemiol. 2002 Feb 1;155(3):217-24.
5. J Natl Compr Canc Netw. 2016 Sep;14(9):1134-63.
6. BJOG. 2016 Mar;123(4):498-508.
7. Gynecol Oncol. 2017 Jul;146(1):3-10.
Low malignant potential tumors of the ovary – otherwise known as borderline tumors – include ovarian tumors with atypical cellularity, which lack stromal invasion that differentiates them from low grade and high grade invasive carcinomas. They can coexist with extraovarian disease, however, in the setting of borderline tumors these foci are referred to as “implants” rather than metastases. As discussed below, these implants can exhibit the presence of invasion or not.
Classification
The two most common histologic categories of borderline tumors are serous and mucinous cell types. Rarer histologic types such as endometrioid, clear cell, and Brenner also exist. However, these are so infrequent that they will not be covered in this discussion as there are very limited data to make generalizations about these histologies.
Serous borderline tumors contain cellularity similar to that of fallopian tubal epithelium. Approximately 25% of all serous ovarian tumors exhibit borderline features. Compared with mucinous tumors, they are more commonly bilateral and smaller in size (mean size of 12 cm) at the time of diagnosis and they are more likely to be associated with extraovarian implants (typically peritoneal). In fact, up to 25% of serous borderline tumors have concomitant extraovarian implants. Cancer antigen (CA) 125 is commonly a tumor marker for these tumors (elevated in 45% of early stage disease and 80% of advanced stage disease).1
Incidence
The incidence of borderline ovarian tumors is 2.5 per 100,000 woman years in the United States. About 70% are diagnosed at stage I.3 They arise in a younger population compared with invasive ovarian carcinomas. Risk factors for development of borderline tumors are similar to those of invasive ovarian carcinomas (such as nulliparity) but there may be a stronger association between the development of borderline ovarian tumors and infertility, as well as prior use of infertility treatment.4
Diagnosis
The diagnosis of borderline tumors of the ovaries occurs almost exclusively at the time of surgical pathology (either frozen section or definitive pathology).
Preoperative assessments with imaging and tumor markers – usually CA 125 and carcinoembryonic antigen (CEA) – are nonspecific for this tumor type. Preoperative imaging will typically reveal complex ovarian cysts with papillations and vascularity. However, in the case of mucinous borderline tumors, unilocular cysts are common.1 The presence of ascites and peritoneal implants can be observed on preoperative imaging of serous borderline tumors with extraovarian disease. However, it is not possible for this imaging to accurately differentiate borderline tumors with implants from low grade and high grade carcinomas with metastases.
Surgical management
Borderline tumors are commonly diagnosed in women of reproductive age and decisions need to be made regarding fertility sparing surgery, ovarian sparing surgery, and whether staging is performed. The recommended surgery for women who have completed child bearing is complete hysterectomy with bilateral salpingo-oophorectomy. However, cystectomy or unilateral salpingo-oophorectomy can be considered for women who desire fertility preservation. Conservative fertility preserving surgery is associated with an increased risk of recurrence, but with no negative impact on survival.1
Staging – with at least omentectomy and comprehensive evaluation of the peritoneal cavity, with or without peritoneal biopsies – can be considered, though staging is not associated with improved survival. Lymphadenectomy is also not associated with improved oncologic outcomes and routine lymphadenectomy is not recommended for borderline tumors.1 However, about a quarter of patients with gross evidence of extraovarian disease have implants within lymph nodes. Bulky lymph nodes should be removed, particularly in this group of patients.
Complete removal of extraovarian implants is the surgical intervention that is most important for survival and recurrence.1 This requires that surgeons thoroughly evaluate the peritoneal cavity and retroperitoneum, and possess the capability to completely resect all sites of disease.
Historically appendectomy was part of surgical staging of mucinous borderline tumors in order to identify a primary appendiceal lesion, but only 1% of patients with a grossly normal appearing appendix have significant pathology identified. This is no longer recommended.2
Treatment
The primary treatment for borderline tumors of the ovary is surgery. A minimally invasive approach is appropriate when feasible, though it may be associated with an increased risk of cyst rupture, particularly if cystectomy is attempted. Outcomes are best when extraovarian implants are completely resected. Adjuvant chemotherapy is not associated with improved survival and is not routinely recommended, though the guidelines from the National Comprehensive Cancer Network include this as an option for patients with advanced stage disease that is either completely or incompletely resected.5
Prognosis
In general, prognosis is excellent for borderline tumors with 5- and 10-year survival of 99% and 97%, 98% and 90%, and 96% and 88% for stages I, II and III tumors, respectively.1 However, several pathologic, molecular, and anatomic features are important in predicting who is at highest risk for recurrence.
Serous borderline tumors with invasive implants (as opposed to desmoplastic implants) and incompletely resected extraovarian implants are associated with increased recurrence and poor prognosis.Micropapillary features and stromal invasion are histologic features that have historically been associated with worse prognosis, but it is unclear if these are independent risk factors, or instead associated with invasive implants. For mucinous borderline tumors, intraepithelial carcinoma has been inconclusively associated with poor prognosis.1,6
Surveillance
Recurrences do occur in patients with a history of borderline tumors of the ovary, however these typically occur late. For this reason, surveillance is important and should continue for many years after diagnosis. Most recurrences are within the peritoneal cavity and are treated with surgical excision and patients should be counseled regarding symptoms of recurrence that include gastrointestinal symptoms, bloating, and pain.
In accordance with guidelines from the Society of Gynecologic Oncology, surveillance examinations can take place annually as there is no evidence that more frequent evaluations improve outcomes. These visits should include physical examinations (with pelvic examinations), symptom assessment, and, if elevated preoperatively, assessment of relevant tumor markers (typically CA 125 and/or CEA).7 Surveillance should continue for at least 10 years postoperatively.
Routine imaging is not recommended for all patients in surveillance. However, for patients who have had fertility-sparing surgery, imaging with pelvic ultrasound is recommended, particularly for women with a history of cystectomy or serous borderline tumor (who are at increased risk for bilateral tumors).
Prognosis is most closely associated with the presence of invasive implants and residual disease following surgery. Surgeons who manage these tumors can safely consider fertility-sparing procedures but should be equipped to completely resect all gross disease.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Lancet Oncol. 2012 Mar;13(3):e103-15.
2. Arch Gynecol Obstet. 2016 Nov;294(6):1283-9.
3. Cancer. 2002 Dec 1;95(11):2380-9.
4. Am J Epidemiol. 2002 Feb 1;155(3):217-24.
5. J Natl Compr Canc Netw. 2016 Sep;14(9):1134-63.
6. BJOG. 2016 Mar;123(4):498-508.
7. Gynecol Oncol. 2017 Jul;146(1):3-10.
Low malignant potential tumors of the ovary – otherwise known as borderline tumors – include ovarian tumors with atypical cellularity, which lack stromal invasion that differentiates them from low grade and high grade invasive carcinomas. They can coexist with extraovarian disease, however, in the setting of borderline tumors these foci are referred to as “implants” rather than metastases. As discussed below, these implants can exhibit the presence of invasion or not.
Classification
The two most common histologic categories of borderline tumors are serous and mucinous cell types. Rarer histologic types such as endometrioid, clear cell, and Brenner also exist. However, these are so infrequent that they will not be covered in this discussion as there are very limited data to make generalizations about these histologies.
Serous borderline tumors contain cellularity similar to that of fallopian tubal epithelium. Approximately 25% of all serous ovarian tumors exhibit borderline features. Compared with mucinous tumors, they are more commonly bilateral and smaller in size (mean size of 12 cm) at the time of diagnosis and they are more likely to be associated with extraovarian implants (typically peritoneal). In fact, up to 25% of serous borderline tumors have concomitant extraovarian implants. Cancer antigen (CA) 125 is commonly a tumor marker for these tumors (elevated in 45% of early stage disease and 80% of advanced stage disease).1
Incidence
The incidence of borderline ovarian tumors is 2.5 per 100,000 woman years in the United States. About 70% are diagnosed at stage I.3 They arise in a younger population compared with invasive ovarian carcinomas. Risk factors for development of borderline tumors are similar to those of invasive ovarian carcinomas (such as nulliparity) but there may be a stronger association between the development of borderline ovarian tumors and infertility, as well as prior use of infertility treatment.4
Diagnosis
The diagnosis of borderline tumors of the ovaries occurs almost exclusively at the time of surgical pathology (either frozen section or definitive pathology).
Preoperative assessments with imaging and tumor markers – usually CA 125 and carcinoembryonic antigen (CEA) – are nonspecific for this tumor type. Preoperative imaging will typically reveal complex ovarian cysts with papillations and vascularity. However, in the case of mucinous borderline tumors, unilocular cysts are common.1 The presence of ascites and peritoneal implants can be observed on preoperative imaging of serous borderline tumors with extraovarian disease. However, it is not possible for this imaging to accurately differentiate borderline tumors with implants from low grade and high grade carcinomas with metastases.
Surgical management
Borderline tumors are commonly diagnosed in women of reproductive age and decisions need to be made regarding fertility sparing surgery, ovarian sparing surgery, and whether staging is performed. The recommended surgery for women who have completed child bearing is complete hysterectomy with bilateral salpingo-oophorectomy. However, cystectomy or unilateral salpingo-oophorectomy can be considered for women who desire fertility preservation. Conservative fertility preserving surgery is associated with an increased risk of recurrence, but with no negative impact on survival.1
Staging – with at least omentectomy and comprehensive evaluation of the peritoneal cavity, with or without peritoneal biopsies – can be considered, though staging is not associated with improved survival. Lymphadenectomy is also not associated with improved oncologic outcomes and routine lymphadenectomy is not recommended for borderline tumors.1 However, about a quarter of patients with gross evidence of extraovarian disease have implants within lymph nodes. Bulky lymph nodes should be removed, particularly in this group of patients.
Complete removal of extraovarian implants is the surgical intervention that is most important for survival and recurrence.1 This requires that surgeons thoroughly evaluate the peritoneal cavity and retroperitoneum, and possess the capability to completely resect all sites of disease.
Historically appendectomy was part of surgical staging of mucinous borderline tumors in order to identify a primary appendiceal lesion, but only 1% of patients with a grossly normal appearing appendix have significant pathology identified. This is no longer recommended.2
Treatment
The primary treatment for borderline tumors of the ovary is surgery. A minimally invasive approach is appropriate when feasible, though it may be associated with an increased risk of cyst rupture, particularly if cystectomy is attempted. Outcomes are best when extraovarian implants are completely resected. Adjuvant chemotherapy is not associated with improved survival and is not routinely recommended, though the guidelines from the National Comprehensive Cancer Network include this as an option for patients with advanced stage disease that is either completely or incompletely resected.5
Prognosis
In general, prognosis is excellent for borderline tumors with 5- and 10-year survival of 99% and 97%, 98% and 90%, and 96% and 88% for stages I, II and III tumors, respectively.1 However, several pathologic, molecular, and anatomic features are important in predicting who is at highest risk for recurrence.
Serous borderline tumors with invasive implants (as opposed to desmoplastic implants) and incompletely resected extraovarian implants are associated with increased recurrence and poor prognosis.Micropapillary features and stromal invasion are histologic features that have historically been associated with worse prognosis, but it is unclear if these are independent risk factors, or instead associated with invasive implants. For mucinous borderline tumors, intraepithelial carcinoma has been inconclusively associated with poor prognosis.1,6
Surveillance
Recurrences do occur in patients with a history of borderline tumors of the ovary, however these typically occur late. For this reason, surveillance is important and should continue for many years after diagnosis. Most recurrences are within the peritoneal cavity and are treated with surgical excision and patients should be counseled regarding symptoms of recurrence that include gastrointestinal symptoms, bloating, and pain.
In accordance with guidelines from the Society of Gynecologic Oncology, surveillance examinations can take place annually as there is no evidence that more frequent evaluations improve outcomes. These visits should include physical examinations (with pelvic examinations), symptom assessment, and, if elevated preoperatively, assessment of relevant tumor markers (typically CA 125 and/or CEA).7 Surveillance should continue for at least 10 years postoperatively.
Routine imaging is not recommended for all patients in surveillance. However, for patients who have had fertility-sparing surgery, imaging with pelvic ultrasound is recommended, particularly for women with a history of cystectomy or serous borderline tumor (who are at increased risk for bilateral tumors).
Prognosis is most closely associated with the presence of invasive implants and residual disease following surgery. Surgeons who manage these tumors can safely consider fertility-sparing procedures but should be equipped to completely resect all gross disease.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Lancet Oncol. 2012 Mar;13(3):e103-15.
2. Arch Gynecol Obstet. 2016 Nov;294(6):1283-9.
3. Cancer. 2002 Dec 1;95(11):2380-9.
4. Am J Epidemiol. 2002 Feb 1;155(3):217-24.
5. J Natl Compr Canc Netw. 2016 Sep;14(9):1134-63.
6. BJOG. 2016 Mar;123(4):498-508.
7. Gynecol Oncol. 2017 Jul;146(1):3-10.
Optimizing HPV vaccination
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Human papillomavirus (HPV) is the most common sexually transmitted infection. Exposure is widespread and most individuals clear the infection without symptoms or development of disease. However, a subset of individuals experience persistent infection, a state which can lead to carcinogenesis of lower genital tract malignancies, particularly cervical cancer.1
Vaccine coverage
Persistent infection with high-risk (oncogenic) HPV is well known to be the cause of cervical cancer. There are two HPV vaccines manufactured for the purposes of cervical cancer, anal cancer, and genital wart prevention (Cervarix and Gardasil). The Cervarix vaccine covers high-risk HPV subtypes 16 and 18 and the Gardasil vaccine prevents both low-risk HPV subtypes 6 and 11, which can cause genital warts, and high-risk HPV subtypes 16, 18, 31, 33, 45, 52 and 58, which cause cervical dysplasia and cancer.
High-risk HPV is also associated with head and neck, vulvar, vaginal, and penile cancers, though the vaccines are not approved by the Food and Drug Administration for prevention of these diseases.2
Vaccination indications
Since vaccination prevents multiple subtypes of HPV, an individual who has already been exposed will still benefit from protection from other subtypes of HPV through vaccination. HPV vaccination is not approved during pregnancy but can be initiated in the postpartum period when women are engaged in their health care and receiving other vaccinations, such as varicella or the MMR vaccine.
Recommended schedule
Until October 2016, the vaccination schedule was based on a three-dose series (0, 2, and 6 months). Currently, the CDC recommends that children aged under 15 years at the time of first dose may opt for a two-dose series (0 and 6-12 months). For those aged 15-26 years, the three-dose schedule remains the recommended course.
The benefits of two-dose schedule are convenience, cost, and increased likelihood of completion. Data presented at the 2017 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer showed that rates of cervical dysplasia were equivalent for women who completed a two-dose schedule versus a three-dose schedule.4
Efficacy
A recent meta-analysis of clinical trials of the HPV vaccines describe efficacy of 95%-97% in prevention of CIN 1-3.5 While its greatest efficacy is in its ability to prevent primary HPV infection, there still is some benefit for individuals who already were exposed to HPV prior to vaccination. As stated previously, women with a history of prior HPV vaccination have lower rates of recurrence of cervical dysplasia after treatment. Additionally, recent research has shown that women who received HPV vaccinations after a LEEP procedure for CIN 2 or 3 experience significantly lower recurrence rates, compared with women who did not receive vaccinations after LEEP (2.5% vs. 8.5%).6 This raises the possibility of a therapeutic role for HPV vaccination in women infected with HPV. Prospective studies are currently evaluating this question.
Myths
The most common side effects of the HPV vaccine are pain, redness, or swelling at the injection site. Other known side effects include fever, headache or malaise, nausea, syncope, or muscle/joint pain – similar to other vaccinations. Anaphylaxis is a rare complication.
Some parents and pediatricians report concerns that vaccination could lead to earlier sexual activity. Multiple studies have shown that girls who receive HPV vaccination are no more likely to become pregnant or get a sexually transmitted infection (proxies for intercourse) than are girls who were not vaccinated.7,8
Maximizing vaccination rates
HPV vaccination rates in the United States lag significantly behind rates in countries with national vaccine programs, such as Australia and Denmark.9 Early data from Australia already have shown a decrease in genital warts and CIN 2+ incidence within the 10 years of starting its school-based vaccine program, with approximately 73% of 12- to 15-year-olds having completed the vaccine series.2 In contrast, just 40% and 22% of 13- to 17-year-old girls and boys in the United States, respectively, had completed the vaccine series in 2014, according to the CDC.10
Vaccination gaps between girls and boys are narrowing, and more teens will be able to complete the series with the new two-dose recommendation for those younger than 15 years. However, our current rates of vaccination are significantly lower for HPV than for other routinely recommended adolescent vaccines (such as Tdap and meningococcal) and more must be done to encourage vaccination.
Studies have shown that parents are more likely to vaccinate their children if providers recommend the vaccine.11 As women’s health care providers, we do not always see children during the time period that is ideal for vaccination. However, we take care of many women who are presenting for routine gynecologic care, pregnancy, or with abnormal Pap smear screenings. These are ideal opportunities to educate and offer HPV vaccination to women in the approved age groups, as well as to encourage parents to vaccinate their children.
As with other vaccines, the recommendation should be clear and focused on the cancer prevention benefit. Using methods in which the recommendation is “announced” in a brief statement assuming parents/patients are ready to vaccinate versus open-ended conversations, has been studied as a potentially successful method to increase uptake of HPV vaccination.12 Additionally, documentation of HPV vaccination status should be built into electronic medical record templates to prompt clinicians to ask and offer HPV vaccination at visits, including postpartum visits.
Dr. Rahangdale is an associate professor of ob.gyn. at the University of North Carolina, Chapel Hill, and is director of the North Carolina Women’s Hospital Cervical Dysplasia Clinic. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Epidemiol. 2008 Jul 15;168(2):123-37.
2. Int J Cancer. 2012 Nov 1;131(9):1969-82.
3. BMJ. 2012 Mar 27;344:e1401. doi: 10.1136/bmj.e1401.
4. Gynecol Oncol. 2017 Jun. doi. org/10.1016/j.ygyno.2017.03.031.
5. Int J Prev Med. 2017 Jun 1;8:44. doi: 10.4103/ijpvm.IJPVM_413_16.
6. Gynecol Oncol. 2013 Aug;130(2):264-8.
7. Pediatrics. 2012 Nov;130(5):798-805.
8. JAMA Intern Med. 2015 Apr;175(4):617-23.
9. Clin Pediatr (Phila). 2016 Sep;55(10):904-14.
10. MMWR Morb Mortal Wkly Rep. 2015 Jul 31;64(29):784-92.
11. Vaccine. 2016 Feb 24;34(9):1187-92.
12. Pediatrics. 2017 Jan;139(1). pii:e20161764. doi: 10.1542/peds.2016-1764.
Understanding the human papillomavirus
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
Human papillomavirus (HPV) is the most prevalent sexually transmitted disease globally. It is causally related to the development of several malignancies, including cervical, anal, and oropharyngeal ones, because of its integration and dysregulation of the genome of infected cells. Fortunately, vaccination is available to prevent development of HPV-related diseases. Understanding this virus, its carcinogenic role, and the importance of prevention through vaccination are critically important for ob.gyns. This column reviews the fundamentals of HPV biology, epidemiology, and carcinogenesis.
Viral anatomy
HPV are members of the A genus of the family Papovaviridae. They contain between 7,800 and 7,900 base pairs. They are nonenveloped, double-stranded DNA viruses with a circular structure. The viral DNA is contained within an icosahedral capsid that measures 45 nm-55 nm. The HPV genome has three critical regions: the long control region, otherwise known as the upstream regulatory region; the early region; and the late region.1
Capsid proteins are similar between groups. Therefore, HPV are categorized into “types” and “subtypes” based on the extent of DNA similarity. There are more than 100 types of HPV in humans.2 The type of HPV is determined by the gene sequences of E6/E7 and L1 and must show more than 10% difference between types. The gene sequences between different subtypes differ by 2%-5%.
Epidemiology of HPV infection
HPV are widely distributed among mammalian species but are species specific. Their tissue affinity varies by type. HPV types 1, 2, and 4 cause common or plantar warts. HPV types 6 and 11 cause condyloma acuminata (genital warts) and low grade dysplasia. HPV types 16 and 18 – in addition to 31 and 52 – are of particular interest to oncologists because they are associated with lower genital tract high grade dysplasia and invasive carcinoma. Infection with HPV 16 is present in about half of invasive cervical cancers, with HPV type 18 present in 20% of cervical cancers. Adenocarcinomas of the cervix are more commonly associated with HPV 18. Anal cancer and oropharyngeal cancer are more commonly associated with HPV 16.3
HPV infections are acquired through cutaneous touching (including hand to genital) and HPV positivity is most commonly present within the first 10 years after sexual debut.4 However, most individuals who acquire HPV do so as a transient infection, which is cleared without sequelae. Those who fail to rapidly clear HPV infection, and in whom it becomes chronic, face an increasing risk of development of dysplasia and invasive carcinoma. The incidence of HPV infection increases again at menopause, but, for these older women, the new finding of HPV detection may be related to reactivation of an earlier infection rather than exclusively new exposure to the virus.5
Diagnosis and testing
HPV infection can be detected through DNA testing, RNA testing, and cellular markers.6
HPV DNA testing was the original form of testing offered. It improved the sensitivity over cytology alone in the detection of precursors to malignancy but had relatively poor specificity, resulting in a high false positive rate and unnecessary referral to colposcopy. The various tests approved by the Food and Drug Administration – Hybrid Capture 2 (HC2), Cervista, and Cobas 4800 – differ in the number and nature of HPV types that they detect.
HPV RNA testing has developed and involves measuring the expression of E6 and E7 RNA. This testing is FDA approved and has the potential to improve upon the specificity of DNA testing procedures by decreasing false positives.
Measurement of cellular markers is currently considered experimental/exploratory and is not yet FDA approved for diagnostic purposes in screening or confirmation of HPV infection or coexisting dysplasia. It involves measuring the downstream cellular targets (such as p16) of E6 or E7 activity.
The mechanism of carcinogenesis
The early region of the HPV genome is downstream from the upstream regulatory region. It codes for proteins involved in viral infection and replication. The two most important genes in the early region are E6 and E7. When integrated into the human genome of the lower genital tract cell, the viral genes E6 and E7 negatively interfere with cell cycle control and mechanisms to halt dysregulation.7
E6 and E7 are considered oncogenes because they cause loss of function of the critical tumor suppressor proteins p53 and the retinoblastoma protein. The p53 protein is typically responsible for controlling cell cycling through the G0/G1 to S phases. It involves stalling cellular mitosis in order to facilitate DNA repair mechanisms in the case of damaged cells, thereby preventing replication of DNA aberrations. The retinoblastoma protein also functions to inhibit cells that have acquired DNA damage from cycling and induces apoptosis in DNA damaged cells. When protein products of E6 and E7 negatively interact with these two tumor suppressor proteins they overcome the cell’s safeguard arrest response.
In the presence of other carcinogens, such as products of tobacco exposure, the increased DNA damage sustained by the genital tract cell is allowed to go relatively unchecked by the HPV coinfection, which has disabled tumor suppressor function. This facilitates immortality of the damaged cell, amplification of additional DNA mutations, and unchecked cellular growth and dysplastic transformation. E6 and E7 are strongly expressed in invasive genital tract lesions to support its important role in carcinogenesis.
HIV coinfection is another factor that promotes carcinogenesis following HPV infection because it inhibits clearance of the virus through T-cell mediated immunosuppression and directly enhances expression of E6 and E7 proteins in the HIV and HPV coinfected cell.8 For these reasons, HIV-positive women are less likely to clear HPV infection and more likely to develop high grade dysplasia or invasive carcinomas.
Prevention and vaccination
HPV vaccinations utilize virus-like particles (VLPs). These VLPs are capsid particles generated from the L1 region of the HPV DNA. The capsid proteins coded for by L1 are highly immunogenic. VLPs are recombinant proteins created in benign biologic systems (such as yeast) and contain no inner DNA core (effectively empty viral capsids) and therefore are not infectious. The L1 gene is incorporated into a plasmid, which is inserted into the nucleus of a eukaryotic cell. Transcription and translation of the L1 gene takes place, creating capsid proteins that self-assemble into VLPs. These VLPs are retrieved and inoculated into candidate patients to illicit an immune response.
Quadrivalent, nine-valent, and bivalent vaccines are available worldwide. However, only the nine-valent vaccine – protective against types 6, 11, 16, 18, 31, 33, 45, 52, and 58 – is available in the United States. This theoretically provides more comprehensive coverage against cervical cancer–causing HPV types, as 70% of cervical cancer is attributable to HPV 16 and 18, but an additional 20% is attributable to HPV 31, 33, 45, 52, and 58. This vaccine also provides protection against the HPV strains that cause genital warts and low-grade dysplastic changes.9
HPV, in most instances, is a transient virus with no sequelae. However, if not cleared from the cells of the lower genital tract, anus, or oropharynx it can result in the breakdown of cellular correction strategies and culminate in invasive carcinoma. Fortunately, highly effective and safe vaccinations are available and should be broadly prescribed.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):415-28.
2. Gynecol Oncol. 2011 Apr;121(1):32-42.
3. Cancer Epidemiol Biomarkers Prev. 2008 Jul;17(7):1611-22.
4. JAMA. 2007 Feb 28;297(8):813-9.
5. J Infect Dis. 2013; 207(2): 272-80.
6. J Natl Cancer Inst. 2011 Mar 2;103(5):368-83.
7. J Natl Cancer Inst. 2000 May 3;92(9):690-8.
8. Lancet. 2002 Jan 12;359(9301):108-13.
9. Obstet Gynecol 2017;129:e173–8.
PARP inhibitors: New developments in ovarian cancer treatment
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 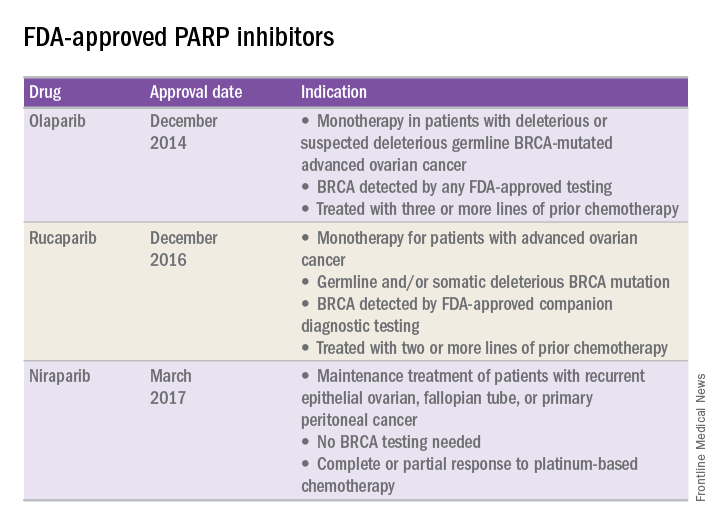
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Optimizing surveillance for gynecologic cancers
Gynecologic cancers contribute to approximately 15% of cancer survivorship care for women. Many patients share surveillance visits between their gynecologic oncologist and their ob.gyn. or primary care physician to capitalize on preexisting relationships and ensure the provision of comprehensive wellness care. Providing high quality surveillance care is challenging because it requires vigilance in the detection of recurrence but also avoidance of unnecessary, costly, and inaccurate testing.
The oncologic benefits for various surveillance guidelines are not well established by prospective studies. However, in updated surveillance recommendations, the Society of Gynecologic Oncology (SGO) takes available data, costs, and benefits into consideration.1 The guidelines, authored by Ritu Salani, MD, provide an excellent resource for understanding appropriate testing and evaluation during surveillance care.
The cornerstone of a surveillance visit is a thorough symptom assessment. Positive reporting of symptoms remains the most sensitive method for detecting recurrences; therefore, patients should be educated and quizzed on common recurrence symptoms. For example, endometrial cancer most commonly recurs in the vagina with symptoms of vaginal bleeding or discharge. Lower extremity swelling can signify pelvic sidewall recurrences and abdominal bloating or pain can signify peritoneal recurrence of ovarian or endometrial cancer.
All women who are undergoing surveillance for gynecologic cancers should receive physical examinations that include a pelvic exam with a speculum and bimanual exam with rectovaginal exam. Many locoregional recurrences are salvageable for most gynecologic cancers, which is not true for most distant recurrences, emphasizing the importance of pelvic examinations.
In addition to surveillance of recurrence, these visits should focus on risk modification – tobacco, obesity, bone demineralization – as well as preventive health strategies, such as vaccinations, nongynecologic cancer screenings, and cardiovascular disease intervention. Clinicians should also ask about sequelae to cancer therapy, such as neuropathy, lymphedema, sexual dysfunction, depression, and fatigue.
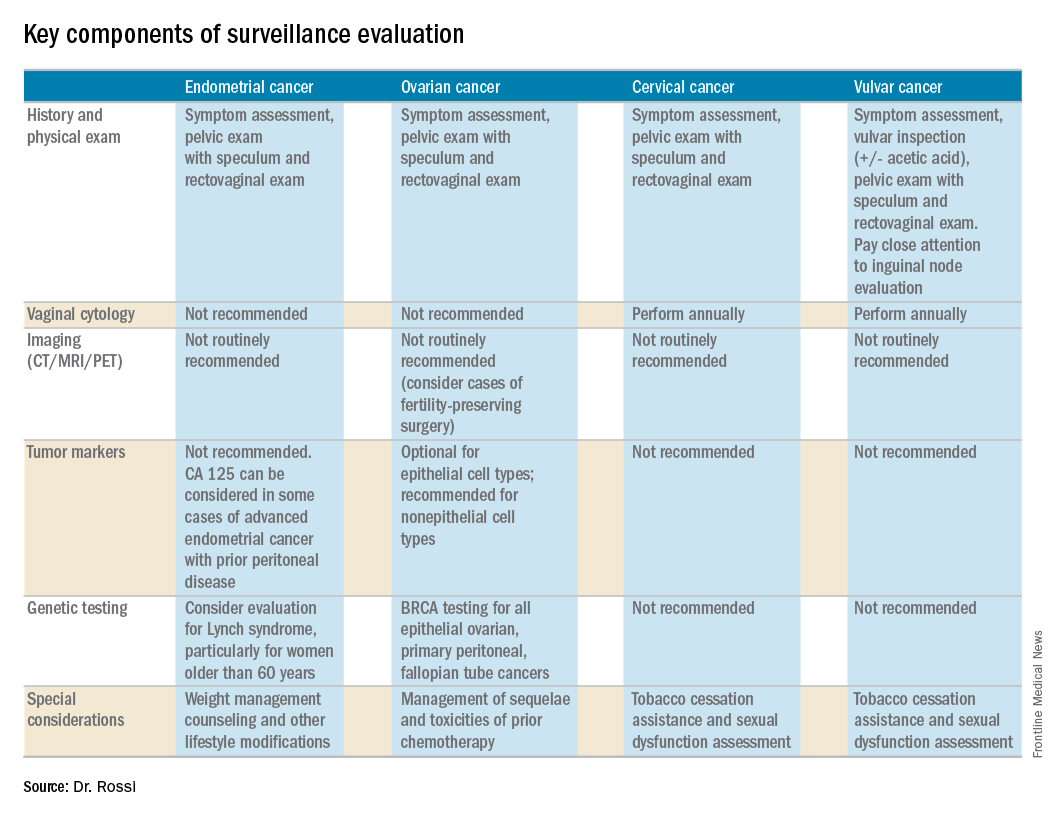
Endometrial cancer
Endometrial cancer recurs most commonly among women with a history of advanced stage cancer or early stage disease with high/intermediate risk factors, but all survivors should be evaluated regularly for recurrence. The vagina is the most common site for recurrence. Fortunately, many vaginal recurrences can be cured with salvage therapies.
Women with the lowest risk for recurrence (stage IA, grades 1 and 2 disease) who did not originally qualify for adjuvant therapy can be followed every 6 months for 2 years and then annually.
Vaginal cytology is no longer recommended for the routine surveillance of endometrial cancer because of its poor sensitivity in detecting recurrence and low positive predictive value (particularly after vaginal radiation).2 Any suspicious lesions identified on speculum examination should be biopsied, rather than sampled with cytologic smear. Routine imaging (with CT or PET/CT) and cancer antigen (CA) 125 tumor marker assessment is not supported unless the initial stage was advanced. These tests should be reserved for confirmation of concerning symptoms or examination findings.
This group of patients has particular survivorship needs with respect to obesity interventions. Obesity is associated with poor prognosis from endometrial cancer, and patients should be counseled about this and offered strategies for weight loss and lifestyle modification. Lynch syndrome testing and colon cancer screening are also an important consideration in this population.
Ovarian cancer
Ovarian cancer recurrence rates are high, and, while salvage therapies are rarely curative, enduring responses may be achieved in some patients, making surveillance visits critical. The SGO recommends surveillance visits every 3 months in the first 2 years, every 4 months in year 3, and then every 6 months for an additional 2 years. At these visits, patients should be queried about symptoms with particular emphasis on peritoneal signs (bloating, distension, gastrointestinal disturbance, and abdominal pain) as most recurrences will be within the peritoneal cavity.
CA 125 tumor marker elevation during the surveillance phase may signal recurrence prior to the development of symptoms but initiating chemotherapy early because of elevations in CA 125 does not improve survival.3 However, in the platinum-sensitive population with a longer disease-free interval, earlier detection of recurrence by CA 125 may identify patients in whom complete secondary cytoreduction is more attainable and is associated with improved survival.4 Therefore, the SGO suggests that CA 125 assessment is optional. The benefits and limitations of earlier detection of recurrence should be discussed with each patient. This recommendation differs for survivors of nonepithelial ovarian cancers (such as germ-cell or sex-cord stromal), in which case the measurement of the appropriate tumor markers (such as alpha-fetoprotein, human chorionic gonadotropin, and inhibin) should be performed routinely as part of surveillance evaluation.
Evidence does not support routine imaging (such as CT or PET). It should be reserved as a confirmatory measure for patients with concerning symptoms, examination findings, or elevations in tumor markers. When ovarian cancer has been treated with fertility-preserving surgery in women of younger reproductive ages, pelvic ultrasounds may be used as part of their surveillance care to monitor retained ovaries and pelvic structures.
BRCA-gene testing should be offered to all women with epithelial ovarian, fallopian tube, and primary peritoneal cancer as it impacts future cancer risk, as well as chemotherapy selection.5
Cervical cancer
In the first 2 years after completing primary treatment for cervical cancer, those at high risk for recurrence (including those who were recommended to adjuvant therapy) should be evaluated every 3 months for 2 years, followed by visits at 6-month intervals for an additional 3 years. Low-risk patients can be followed every 6 months for 2 years, followed by annual visits thereafter.
Pap testing should be performed annually, rather than at each surveillance visit. It should not to detect recurrence – for which it has poor sensitivity and specificity – but rather to detect new HPV-related dysplasia.6
Many patients with cervical cancer have a tobacco use history, placing them at risk for other cancers. Educate patients about the risk and provide cessation assistance.
Vulvar cancer
Prognosis for early stage vulvar cancer is very good; however, local recurrences are common (as much as 40%) in the 10 years following diagnosis.7 It is important to thoroughly inspect the vulva, vagina, and cervix at each surveillance visit. In high-risk patients, examinations should take place every 3 months for the first 2 years after completing primary treatment and every 6 months until 5 years post treatment. Low-risk patients can be followed every 6 months for 2 years and annually thereafter.
Identification and early treatment of dysplasia is important. Careful attention should also be made to palpation of the inguinal nodal regions. One in 10 women will have a late recurrence (greater than 5 years), so vulvar inspections should continue at least annually for the remainder of a woman’s life.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Gynecol Oncol. 2017 Mar 31. doi: 10.1016/j.ygno.2017.03.022.
2. Obstet Gynecol. 2013 Jan;121(1):129-35.
3. Lancet. 2010 Oct 2;376(9747):1155-63.
4. Gynecol Oncol. 2009 Jan;112(1):265-74.
5. Gynecol Oncol. 2015 Jan;136(1):3-7.
6. Obstet Gynecol. 2011 Sep;118(3):548-53.
7. Gynecol Oncol. 2016 Jan;140(1):8-14.
Gynecologic cancers contribute to approximately 15% of cancer survivorship care for women. Many patients share surveillance visits between their gynecologic oncologist and their ob.gyn. or primary care physician to capitalize on preexisting relationships and ensure the provision of comprehensive wellness care. Providing high quality surveillance care is challenging because it requires vigilance in the detection of recurrence but also avoidance of unnecessary, costly, and inaccurate testing.
The oncologic benefits for various surveillance guidelines are not well established by prospective studies. However, in updated surveillance recommendations, the Society of Gynecologic Oncology (SGO) takes available data, costs, and benefits into consideration.1 The guidelines, authored by Ritu Salani, MD, provide an excellent resource for understanding appropriate testing and evaluation during surveillance care.
The cornerstone of a surveillance visit is a thorough symptom assessment. Positive reporting of symptoms remains the most sensitive method for detecting recurrences; therefore, patients should be educated and quizzed on common recurrence symptoms. For example, endometrial cancer most commonly recurs in the vagina with symptoms of vaginal bleeding or discharge. Lower extremity swelling can signify pelvic sidewall recurrences and abdominal bloating or pain can signify peritoneal recurrence of ovarian or endometrial cancer.
All women who are undergoing surveillance for gynecologic cancers should receive physical examinations that include a pelvic exam with a speculum and bimanual exam with rectovaginal exam. Many locoregional recurrences are salvageable for most gynecologic cancers, which is not true for most distant recurrences, emphasizing the importance of pelvic examinations.
In addition to surveillance of recurrence, these visits should focus on risk modification – tobacco, obesity, bone demineralization – as well as preventive health strategies, such as vaccinations, nongynecologic cancer screenings, and cardiovascular disease intervention. Clinicians should also ask about sequelae to cancer therapy, such as neuropathy, lymphedema, sexual dysfunction, depression, and fatigue.

Endometrial cancer
Endometrial cancer recurs most commonly among women with a history of advanced stage cancer or early stage disease with high/intermediate risk factors, but all survivors should be evaluated regularly for recurrence. The vagina is the most common site for recurrence. Fortunately, many vaginal recurrences can be cured with salvage therapies.
Women with the lowest risk for recurrence (stage IA, grades 1 and 2 disease) who did not originally qualify for adjuvant therapy can be followed every 6 months for 2 years and then annually.
Vaginal cytology is no longer recommended for the routine surveillance of endometrial cancer because of its poor sensitivity in detecting recurrence and low positive predictive value (particularly after vaginal radiation).2 Any suspicious lesions identified on speculum examination should be biopsied, rather than sampled with cytologic smear. Routine imaging (with CT or PET/CT) and cancer antigen (CA) 125 tumor marker assessment is not supported unless the initial stage was advanced. These tests should be reserved for confirmation of concerning symptoms or examination findings.
This group of patients has particular survivorship needs with respect to obesity interventions. Obesity is associated with poor prognosis from endometrial cancer, and patients should be counseled about this and offered strategies for weight loss and lifestyle modification. Lynch syndrome testing and colon cancer screening are also an important consideration in this population.
Ovarian cancer
Ovarian cancer recurrence rates are high, and, while salvage therapies are rarely curative, enduring responses may be achieved in some patients, making surveillance visits critical. The SGO recommends surveillance visits every 3 months in the first 2 years, every 4 months in year 3, and then every 6 months for an additional 2 years. At these visits, patients should be queried about symptoms with particular emphasis on peritoneal signs (bloating, distension, gastrointestinal disturbance, and abdominal pain) as most recurrences will be within the peritoneal cavity.
CA 125 tumor marker elevation during the surveillance phase may signal recurrence prior to the development of symptoms but initiating chemotherapy early because of elevations in CA 125 does not improve survival.3 However, in the platinum-sensitive population with a longer disease-free interval, earlier detection of recurrence by CA 125 may identify patients in whom complete secondary cytoreduction is more attainable and is associated with improved survival.4 Therefore, the SGO suggests that CA 125 assessment is optional. The benefits and limitations of earlier detection of recurrence should be discussed with each patient. This recommendation differs for survivors of nonepithelial ovarian cancers (such as germ-cell or sex-cord stromal), in which case the measurement of the appropriate tumor markers (such as alpha-fetoprotein, human chorionic gonadotropin, and inhibin) should be performed routinely as part of surveillance evaluation.
Evidence does not support routine imaging (such as CT or PET). It should be reserved as a confirmatory measure for patients with concerning symptoms, examination findings, or elevations in tumor markers. When ovarian cancer has been treated with fertility-preserving surgery in women of younger reproductive ages, pelvic ultrasounds may be used as part of their surveillance care to monitor retained ovaries and pelvic structures.
BRCA-gene testing should be offered to all women with epithelial ovarian, fallopian tube, and primary peritoneal cancer as it impacts future cancer risk, as well as chemotherapy selection.5
Cervical cancer
In the first 2 years after completing primary treatment for cervical cancer, those at high risk for recurrence (including those who were recommended to adjuvant therapy) should be evaluated every 3 months for 2 years, followed by visits at 6-month intervals for an additional 3 years. Low-risk patients can be followed every 6 months for 2 years, followed by annual visits thereafter.
Pap testing should be performed annually, rather than at each surveillance visit. It should not to detect recurrence – for which it has poor sensitivity and specificity – but rather to detect new HPV-related dysplasia.6
Many patients with cervical cancer have a tobacco use history, placing them at risk for other cancers. Educate patients about the risk and provide cessation assistance.
Vulvar cancer
Prognosis for early stage vulvar cancer is very good; however, local recurrences are common (as much as 40%) in the 10 years following diagnosis.7 It is important to thoroughly inspect the vulva, vagina, and cervix at each surveillance visit. In high-risk patients, examinations should take place every 3 months for the first 2 years after completing primary treatment and every 6 months until 5 years post treatment. Low-risk patients can be followed every 6 months for 2 years and annually thereafter.
Identification and early treatment of dysplasia is important. Careful attention should also be made to palpation of the inguinal nodal regions. One in 10 women will have a late recurrence (greater than 5 years), so vulvar inspections should continue at least annually for the remainder of a woman’s life.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Gynecol Oncol. 2017 Mar 31. doi: 10.1016/j.ygno.2017.03.022.
2. Obstet Gynecol. 2013 Jan;121(1):129-35.
3. Lancet. 2010 Oct 2;376(9747):1155-63.
4. Gynecol Oncol. 2009 Jan;112(1):265-74.
5. Gynecol Oncol. 2015 Jan;136(1):3-7.
6. Obstet Gynecol. 2011 Sep;118(3):548-53.
7. Gynecol Oncol. 2016 Jan;140(1):8-14.
Gynecologic cancers contribute to approximately 15% of cancer survivorship care for women. Many patients share surveillance visits between their gynecologic oncologist and their ob.gyn. or primary care physician to capitalize on preexisting relationships and ensure the provision of comprehensive wellness care. Providing high quality surveillance care is challenging because it requires vigilance in the detection of recurrence but also avoidance of unnecessary, costly, and inaccurate testing.
The oncologic benefits for various surveillance guidelines are not well established by prospective studies. However, in updated surveillance recommendations, the Society of Gynecologic Oncology (SGO) takes available data, costs, and benefits into consideration.1 The guidelines, authored by Ritu Salani, MD, provide an excellent resource for understanding appropriate testing and evaluation during surveillance care.
The cornerstone of a surveillance visit is a thorough symptom assessment. Positive reporting of symptoms remains the most sensitive method for detecting recurrences; therefore, patients should be educated and quizzed on common recurrence symptoms. For example, endometrial cancer most commonly recurs in the vagina with symptoms of vaginal bleeding or discharge. Lower extremity swelling can signify pelvic sidewall recurrences and abdominal bloating or pain can signify peritoneal recurrence of ovarian or endometrial cancer.
All women who are undergoing surveillance for gynecologic cancers should receive physical examinations that include a pelvic exam with a speculum and bimanual exam with rectovaginal exam. Many locoregional recurrences are salvageable for most gynecologic cancers, which is not true for most distant recurrences, emphasizing the importance of pelvic examinations.
In addition to surveillance of recurrence, these visits should focus on risk modification – tobacco, obesity, bone demineralization – as well as preventive health strategies, such as vaccinations, nongynecologic cancer screenings, and cardiovascular disease intervention. Clinicians should also ask about sequelae to cancer therapy, such as neuropathy, lymphedema, sexual dysfunction, depression, and fatigue.

Endometrial cancer
Endometrial cancer recurs most commonly among women with a history of advanced stage cancer or early stage disease with high/intermediate risk factors, but all survivors should be evaluated regularly for recurrence. The vagina is the most common site for recurrence. Fortunately, many vaginal recurrences can be cured with salvage therapies.
Women with the lowest risk for recurrence (stage IA, grades 1 and 2 disease) who did not originally qualify for adjuvant therapy can be followed every 6 months for 2 years and then annually.
Vaginal cytology is no longer recommended for the routine surveillance of endometrial cancer because of its poor sensitivity in detecting recurrence and low positive predictive value (particularly after vaginal radiation).2 Any suspicious lesions identified on speculum examination should be biopsied, rather than sampled with cytologic smear. Routine imaging (with CT or PET/CT) and cancer antigen (CA) 125 tumor marker assessment is not supported unless the initial stage was advanced. These tests should be reserved for confirmation of concerning symptoms or examination findings.
This group of patients has particular survivorship needs with respect to obesity interventions. Obesity is associated with poor prognosis from endometrial cancer, and patients should be counseled about this and offered strategies for weight loss and lifestyle modification. Lynch syndrome testing and colon cancer screening are also an important consideration in this population.
Ovarian cancer
Ovarian cancer recurrence rates are high, and, while salvage therapies are rarely curative, enduring responses may be achieved in some patients, making surveillance visits critical. The SGO recommends surveillance visits every 3 months in the first 2 years, every 4 months in year 3, and then every 6 months for an additional 2 years. At these visits, patients should be queried about symptoms with particular emphasis on peritoneal signs (bloating, distension, gastrointestinal disturbance, and abdominal pain) as most recurrences will be within the peritoneal cavity.
CA 125 tumor marker elevation during the surveillance phase may signal recurrence prior to the development of symptoms but initiating chemotherapy early because of elevations in CA 125 does not improve survival.3 However, in the platinum-sensitive population with a longer disease-free interval, earlier detection of recurrence by CA 125 may identify patients in whom complete secondary cytoreduction is more attainable and is associated with improved survival.4 Therefore, the SGO suggests that CA 125 assessment is optional. The benefits and limitations of earlier detection of recurrence should be discussed with each patient. This recommendation differs for survivors of nonepithelial ovarian cancers (such as germ-cell or sex-cord stromal), in which case the measurement of the appropriate tumor markers (such as alpha-fetoprotein, human chorionic gonadotropin, and inhibin) should be performed routinely as part of surveillance evaluation.
Evidence does not support routine imaging (such as CT or PET). It should be reserved as a confirmatory measure for patients with concerning symptoms, examination findings, or elevations in tumor markers. When ovarian cancer has been treated with fertility-preserving surgery in women of younger reproductive ages, pelvic ultrasounds may be used as part of their surveillance care to monitor retained ovaries and pelvic structures.
BRCA-gene testing should be offered to all women with epithelial ovarian, fallopian tube, and primary peritoneal cancer as it impacts future cancer risk, as well as chemotherapy selection.5
Cervical cancer
In the first 2 years after completing primary treatment for cervical cancer, those at high risk for recurrence (including those who were recommended to adjuvant therapy) should be evaluated every 3 months for 2 years, followed by visits at 6-month intervals for an additional 3 years. Low-risk patients can be followed every 6 months for 2 years, followed by annual visits thereafter.
Pap testing should be performed annually, rather than at each surveillance visit. It should not to detect recurrence – for which it has poor sensitivity and specificity – but rather to detect new HPV-related dysplasia.6
Many patients with cervical cancer have a tobacco use history, placing them at risk for other cancers. Educate patients about the risk and provide cessation assistance.
Vulvar cancer
Prognosis for early stage vulvar cancer is very good; however, local recurrences are common (as much as 40%) in the 10 years following diagnosis.7 It is important to thoroughly inspect the vulva, vagina, and cervix at each surveillance visit. In high-risk patients, examinations should take place every 3 months for the first 2 years after completing primary treatment and every 6 months until 5 years post treatment. Low-risk patients can be followed every 6 months for 2 years and annually thereafter.
Identification and early treatment of dysplasia is important. Careful attention should also be made to palpation of the inguinal nodal regions. One in 10 women will have a late recurrence (greater than 5 years), so vulvar inspections should continue at least annually for the remainder of a woman’s life.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Gynecol Oncol. 2017 Mar 31. doi: 10.1016/j.ygno.2017.03.022.
2. Obstet Gynecol. 2013 Jan;121(1):129-35.
3. Lancet. 2010 Oct 2;376(9747):1155-63.
4. Gynecol Oncol. 2009 Jan;112(1):265-74.
5. Gynecol Oncol. 2015 Jan;136(1):3-7.
6. Obstet Gynecol. 2011 Sep;118(3):548-53.
7. Gynecol Oncol. 2016 Jan;140(1):8-14.
Risk for obstetric complications when treating cervical dysplasia
Cervical dysplasia is a condition commonly encountered by the gynecologist. It is either treated (with excision or ablation) or monitored, depending on the lesion grade, cytologic history, medical history, and reproductive goals. Cervical dysplasia commonly arises in women of reproductive age. Therefore, consider reproductive effects when deciding whether to treat or monitor, as well as when choosing the treatment modality.
Background
Approximately two-thirds of human papillomavirus infections resolve within a year, and more than 90% resolve within 2 years. Similarly, low-grade cervical intraepithelial neoplasia (CIN 1) lesions frequently resolve. High-grade (CIN 2 and CIN 3) lesions regress less commonly, with 5% and 12%-40% progressing to invasive cancer, respectively. Therefore, treatment is typically recommended.
Obstetric implications
Potential obstetric risks of treatment for CIN include infertility, spontaneous abortion, preterm premature rupture of membranes (PPROM), preterm delivery, and perinatal/neonatal mortality. These risks are discussed individually below. Mechanisms that have been suggested for such complications include decreased cervical mucous, cervical scarring impeding conception or dilation, loss of cervical volume, collagen breakdown, and immunologic processes due to decreased physical defenses or microbiome shifts.
Fertility
Studies have shown that treatment does not appear to impede conception. The overall pregnancy rate is higher among treated women than untreated women. Pregnancy rates are not different among women intending to conceive or among women attempting conception for more than 12 months, with the caveat being that these studies are heterogenous.2,3
Miscarriage
No difference has been observed in total (less than 24 weeks) miscarriage rate or first trimester (less than 12 weeks) miscarriage rate among treated and untreated women. However, the second trimester miscarriage rate is significantly higher among treated women (risk ratio, 2.60).2 This risk is most notable following laser conization or LEEP.4 There may also be an association between ablation and pregnancy loss.
Preterm birth and PPROM
Several studies and meta-analyses show an association between preterm birth and treatment for CIN using LEEP or CKC. There is an increased risk of severe preterm delivery (relative risk, 2.78), extreme preterm delivery (relative risk, 5.33), and low birth weight (relative risk, 2.86) with CKC.5 LEEP is associated with the same outcomes, albeit the risk is lower than with CKC.6 The risk of preterm birth is even lower for ablation.7
The risk of PPROM is approximately two times higher among those treated with LEEP, and PPROM rates are higher among those treated with CKC, compared with LEEP.9,10
Other complications
Ectopic pregnancy and termination rates may be higher in treated women, compared with untreated women.2 However, there does not appear to be an increased risk for perinatal/neonatal mortality, cesarean section, or neonatal intensive care unit admission among women treated with excisional procedures.6
Pointers for practice
- Due to the potential for adverse obstetric complications following excisional procedures for cervical dysplasia, gynecologists should closely adhere to the American Society for Colposcopy and Cervical Pathology guidelines when determining the appropriateness of dysplasia interventions. The decision to treat, versus monitor, dysplasia in a woman who plans future childbearing should be made with the patient after thorough discussion of the risks and benefits of each path.
- Women younger than age 30 years should not be screened for high-risk human papillomavirus because of both its high incidence and its high rate of spontaneous resolution.
- For reproductive-aged women with CIN 2 and adequate colposcopy, the American Society for Colposcopy and Cervical Pathology supports either monitoring with cytology and colposcopy every 6 months for a year or excisional treatment. However, women with CIN 3, inadequate colposcopy, prior cervical cancer, diethylstilbestrol exposure, or decreased immunity should undergo excisional treatment.
- When selecting an excisional method (LEEP or CKC), surgeons should choose the most appropriate technique for the patient’s pathology but should acknowledge the observed higher rates of PPROM, preterm birth, and low-birth-weight infants among those receiving CKC, and tailor the size of the excision to the specific lesion.
- Consider recommending a 12-month interval between treatment and pregnancy to ensure resolution of high-grade dysplasia. Furthermore, obstetric risk may be increased within 12 months following treatment.
Dr. Robbins is a resident in the department of obstetrics and gynecology at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Obstet Gynecol. 2011 Jul;205(1):19-27.
2. Cochrane Database Syst Rev. 2015 Sep 29;(9):CD008478.
3. BMJ. 2014 Oct 28;349:g6192.
4. Obstet Gynecol. 2016 Dec;128(6):1265-73.
5. BMJ. 2008 Sep 18;337:a1284.
6. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
7. BJOG. 2011 Aug;118(9):1031-41.
8. Obstet Gynecol. 2013 May;121(5):1063-8.
9. Lancet. 2006 Feb 11;367(9509):489-98.
10. Gynecol Obstet Invest. 2014;77(4):240-4.
Cervical dysplasia is a condition commonly encountered by the gynecologist. It is either treated (with excision or ablation) or monitored, depending on the lesion grade, cytologic history, medical history, and reproductive goals. Cervical dysplasia commonly arises in women of reproductive age. Therefore, consider reproductive effects when deciding whether to treat or monitor, as well as when choosing the treatment modality.
Background
Approximately two-thirds of human papillomavirus infections resolve within a year, and more than 90% resolve within 2 years. Similarly, low-grade cervical intraepithelial neoplasia (CIN 1) lesions frequently resolve. High-grade (CIN 2 and CIN 3) lesions regress less commonly, with 5% and 12%-40% progressing to invasive cancer, respectively. Therefore, treatment is typically recommended.
Obstetric implications
Potential obstetric risks of treatment for CIN include infertility, spontaneous abortion, preterm premature rupture of membranes (PPROM), preterm delivery, and perinatal/neonatal mortality. These risks are discussed individually below. Mechanisms that have been suggested for such complications include decreased cervical mucous, cervical scarring impeding conception or dilation, loss of cervical volume, collagen breakdown, and immunologic processes due to decreased physical defenses or microbiome shifts.
Fertility
Studies have shown that treatment does not appear to impede conception. The overall pregnancy rate is higher among treated women than untreated women. Pregnancy rates are not different among women intending to conceive or among women attempting conception for more than 12 months, with the caveat being that these studies are heterogenous.2,3
Miscarriage
No difference has been observed in total (less than 24 weeks) miscarriage rate or first trimester (less than 12 weeks) miscarriage rate among treated and untreated women. However, the second trimester miscarriage rate is significantly higher among treated women (risk ratio, 2.60).2 This risk is most notable following laser conization or LEEP.4 There may also be an association between ablation and pregnancy loss.
Preterm birth and PPROM
Several studies and meta-analyses show an association between preterm birth and treatment for CIN using LEEP or CKC. There is an increased risk of severe preterm delivery (relative risk, 2.78), extreme preterm delivery (relative risk, 5.33), and low birth weight (relative risk, 2.86) with CKC.5 LEEP is associated with the same outcomes, albeit the risk is lower than with CKC.6 The risk of preterm birth is even lower for ablation.7
The risk of PPROM is approximately two times higher among those treated with LEEP, and PPROM rates are higher among those treated with CKC, compared with LEEP.9,10
Other complications
Ectopic pregnancy and termination rates may be higher in treated women, compared with untreated women.2 However, there does not appear to be an increased risk for perinatal/neonatal mortality, cesarean section, or neonatal intensive care unit admission among women treated with excisional procedures.6
Pointers for practice
- Due to the potential for adverse obstetric complications following excisional procedures for cervical dysplasia, gynecologists should closely adhere to the American Society for Colposcopy and Cervical Pathology guidelines when determining the appropriateness of dysplasia interventions. The decision to treat, versus monitor, dysplasia in a woman who plans future childbearing should be made with the patient after thorough discussion of the risks and benefits of each path.
- Women younger than age 30 years should not be screened for high-risk human papillomavirus because of both its high incidence and its high rate of spontaneous resolution.
- For reproductive-aged women with CIN 2 and adequate colposcopy, the American Society for Colposcopy and Cervical Pathology supports either monitoring with cytology and colposcopy every 6 months for a year or excisional treatment. However, women with CIN 3, inadequate colposcopy, prior cervical cancer, diethylstilbestrol exposure, or decreased immunity should undergo excisional treatment.
- When selecting an excisional method (LEEP or CKC), surgeons should choose the most appropriate technique for the patient’s pathology but should acknowledge the observed higher rates of PPROM, preterm birth, and low-birth-weight infants among those receiving CKC, and tailor the size of the excision to the specific lesion.
- Consider recommending a 12-month interval between treatment and pregnancy to ensure resolution of high-grade dysplasia. Furthermore, obstetric risk may be increased within 12 months following treatment.
Dr. Robbins is a resident in the department of obstetrics and gynecology at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Obstet Gynecol. 2011 Jul;205(1):19-27.
2. Cochrane Database Syst Rev. 2015 Sep 29;(9):CD008478.
3. BMJ. 2014 Oct 28;349:g6192.
4. Obstet Gynecol. 2016 Dec;128(6):1265-73.
5. BMJ. 2008 Sep 18;337:a1284.
6. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
7. BJOG. 2011 Aug;118(9):1031-41.
8. Obstet Gynecol. 2013 May;121(5):1063-8.
9. Lancet. 2006 Feb 11;367(9509):489-98.
10. Gynecol Obstet Invest. 2014;77(4):240-4.
Cervical dysplasia is a condition commonly encountered by the gynecologist. It is either treated (with excision or ablation) or monitored, depending on the lesion grade, cytologic history, medical history, and reproductive goals. Cervical dysplasia commonly arises in women of reproductive age. Therefore, consider reproductive effects when deciding whether to treat or monitor, as well as when choosing the treatment modality.
Background
Approximately two-thirds of human papillomavirus infections resolve within a year, and more than 90% resolve within 2 years. Similarly, low-grade cervical intraepithelial neoplasia (CIN 1) lesions frequently resolve. High-grade (CIN 2 and CIN 3) lesions regress less commonly, with 5% and 12%-40% progressing to invasive cancer, respectively. Therefore, treatment is typically recommended.
Obstetric implications
Potential obstetric risks of treatment for CIN include infertility, spontaneous abortion, preterm premature rupture of membranes (PPROM), preterm delivery, and perinatal/neonatal mortality. These risks are discussed individually below. Mechanisms that have been suggested for such complications include decreased cervical mucous, cervical scarring impeding conception or dilation, loss of cervical volume, collagen breakdown, and immunologic processes due to decreased physical defenses or microbiome shifts.
Fertility
Studies have shown that treatment does not appear to impede conception. The overall pregnancy rate is higher among treated women than untreated women. Pregnancy rates are not different among women intending to conceive or among women attempting conception for more than 12 months, with the caveat being that these studies are heterogenous.2,3
Miscarriage
No difference has been observed in total (less than 24 weeks) miscarriage rate or first trimester (less than 12 weeks) miscarriage rate among treated and untreated women. However, the second trimester miscarriage rate is significantly higher among treated women (risk ratio, 2.60).2 This risk is most notable following laser conization or LEEP.4 There may also be an association between ablation and pregnancy loss.
Preterm birth and PPROM
Several studies and meta-analyses show an association between preterm birth and treatment for CIN using LEEP or CKC. There is an increased risk of severe preterm delivery (relative risk, 2.78), extreme preterm delivery (relative risk, 5.33), and low birth weight (relative risk, 2.86) with CKC.5 LEEP is associated with the same outcomes, albeit the risk is lower than with CKC.6 The risk of preterm birth is even lower for ablation.7
The risk of PPROM is approximately two times higher among those treated with LEEP, and PPROM rates are higher among those treated with CKC, compared with LEEP.9,10
Other complications
Ectopic pregnancy and termination rates may be higher in treated women, compared with untreated women.2 However, there does not appear to be an increased risk for perinatal/neonatal mortality, cesarean section, or neonatal intensive care unit admission among women treated with excisional procedures.6
Pointers for practice
- Due to the potential for adverse obstetric complications following excisional procedures for cervical dysplasia, gynecologists should closely adhere to the American Society for Colposcopy and Cervical Pathology guidelines when determining the appropriateness of dysplasia interventions. The decision to treat, versus monitor, dysplasia in a woman who plans future childbearing should be made with the patient after thorough discussion of the risks and benefits of each path.
- Women younger than age 30 years should not be screened for high-risk human papillomavirus because of both its high incidence and its high rate of spontaneous resolution.
- For reproductive-aged women with CIN 2 and adequate colposcopy, the American Society for Colposcopy and Cervical Pathology supports either monitoring with cytology and colposcopy every 6 months for a year or excisional treatment. However, women with CIN 3, inadequate colposcopy, prior cervical cancer, diethylstilbestrol exposure, or decreased immunity should undergo excisional treatment.
- When selecting an excisional method (LEEP or CKC), surgeons should choose the most appropriate technique for the patient’s pathology but should acknowledge the observed higher rates of PPROM, preterm birth, and low-birth-weight infants among those receiving CKC, and tailor the size of the excision to the specific lesion.
- Consider recommending a 12-month interval between treatment and pregnancy to ensure resolution of high-grade dysplasia. Furthermore, obstetric risk may be increased within 12 months following treatment.
Dr. Robbins is a resident in the department of obstetrics and gynecology at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Obstet Gynecol. 2011 Jul;205(1):19-27.
2. Cochrane Database Syst Rev. 2015 Sep 29;(9):CD008478.
3. BMJ. 2014 Oct 28;349:g6192.
4. Obstet Gynecol. 2016 Dec;128(6):1265-73.
5. BMJ. 2008 Sep 18;337:a1284.
6. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
7. BJOG. 2011 Aug;118(9):1031-41.
8. Obstet Gynecol. 2013 May;121(5):1063-8.
9. Lancet. 2006 Feb 11;367(9509):489-98.
10. Gynecol Obstet Invest. 2014;77(4):240-4.