User login
Respiratory infection– and asthma-prone children
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).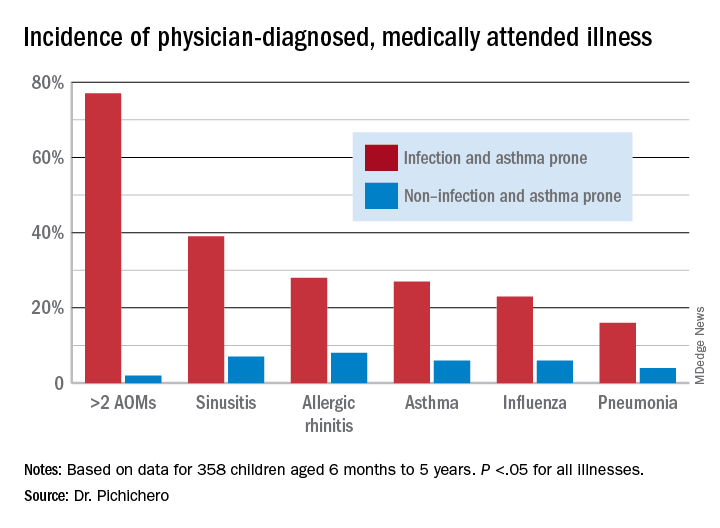
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Some children are more susceptible to viral and bacterial respiratory infections in the first few years of life than others. However, the factors contributing to this susceptibility are incompletely understood. The pathogenesis, development, severity, and clinical outcomes of respiratory infections are largely dependent on the resident composition of the nasopharyngeal microbiome and immune defense.1
Respiratory infections caused by bacteria and/or viruses are a leading cause of death in children in the United States and worldwide. The well-recognized, predominant causative bacteria are Streptococcus pneumoniae (pneumococcus), nontypeable Haemophilus influenzae (Hflu), and Moraxella catarrhalis (Mcat). Respiratory infections caused by these pathogens result in considerable morbidity, mortality, and account for high health care costs. The clinical and laboratory group that I lead in Rochester, N.Y., has been studying acute otitis media (AOM) etiology, epidemiology, pathogenesis, prevention, and treatment for over 3 decades. Our research findings are likely applicable and generalizable to understanding the pathogenesis and immune response to other infectious diseases induced by pneumococcus, Hflu, and Mcat since they are also key pathogens causing sinusitis and lung infections.
Previous immunologic analysis of children with AOM by our group provided clarity in differences between infection-prone children manifest as otitis prone (OP; often referred to in our publications as stringently defined OP because of the stringent diagnostic requirement of tympanocentesis-proven etiology of infection) and non-OP children. We showed that about 90% of OP children have deficient immune responses following nasopharyngeal colonization and AOM, demonstrated by inadequate innate responses and adaptive immune responses.2 Many of these children also showed an increased propensity to viral upper respiratory infection and 30% fail to produce protective antibody responses after injection of routine pediatric vaccines.3,4
In this column, I want to share new information regarding differences in the nasopharyngeal microbiome of children who are respiratory infection prone versus those who are non–respiratory infection prone and children with asthma versus those who do not exhibit that clinical phenotype. We performed a retrospective analysis of clinical samples collected from 358 children, aged 6 months to 5 years, from our prospectively enrolled cohort in Rochester, N.Y., to determine associations between AOM and other childhood respiratory illnesses and nasopharyngeal microbiota. In order to define subgroups of children within the cohort, we used a statistical method called unsupervised clustering analysis to see if relatively unique groups of children could be discerned. The overall cohort successfully clustered into two groups, showing marked differences in the prevalence of respiratory infections and asthma.5 We termed the two clinical phenotypes infection and asthma prone (n = 99, 28% of the children) and non–infection and asthma prone (n = 259, 72% of the children). Infection- and asthma-prone children were significantly more likely to experience recurrent AOM, influenza, sinusitis, pneumonia, asthma, and allergic rhinitis, compared with non–infection- and asthma-prone children (Figure).
The two groups did not experience significantly different rates of eczema, food allergy, skin infections, urinary tract infections, or acute gastroenteritis, suggesting a common thread involving the respiratory tract that did not cross over to the gastrointestinal, skin, or urinary tract. We found that age at first nasopharyngeal colonization with any of the three bacterial respiratory pathogens (pneumococcus, Hflu, or Mcat) was significantly associated with the respiratory infection– and asthma-prone clinical phenotype. Specifically, respiratory infection– and asthma-prone children experienced colonization at a significantly earlier age than nonprone children did for all three bacteria. In an analysis of individual conditions, early Mcat colonization significantly associated with pneumonia, sinusitis, and asthma susceptibility; Hflu with pneumonia, sinusitis, influenza, and allergic rhinitis; and pneumococcus with sinusitis.
Since early colonization with the three bacterial respiratory pathogens was strongly associated with respiratory illnesses and asthma, nasopharyngeal microbiome analysis was performed on an available subset of samples. Bacterial diversity trended lower in infection- and asthma-prone children, consistent with dysbiosis in the respiratory infection– and asthma-prone clinical phenotype. Nine different bacteria genera were found to be differentially abundant when comparing respiratory infection– and asthma-prone and nonprone children, pointing the way to possible interventions to make the respiratory infection– and asthma-prone child nasopharyngeal microbiome more like the nonprone child.
As I have written previously in this column, recent accumulating data have shed light on the importance of the human microbiome in modulating immune homeostasis and disease susceptibility.6 My group is working toward generating new knowledge for the long-term goal of identifying new therapeutic strategies to facilitate a protective, diverse nasopharyngeal microbiome (with appropriately tuned intranasal probiotics) to prevent respiratory pathogen colonization and/or subsequent progression to respiratory infection and asthma. Also, vaccines directed against colonization-enhancing members of the microbiome may provide a means to indirectly control respiratory pathogen nasopharyngeal colonization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Contact him at [email protected]
References
1. Man WH et al. Nat Rev Microbiol. 2017;15(5):259-70.
2. Pichichero ME. J Infect. 2020;80(6):614-22.
3. Ren D et al. Clin Infect Dis. 2019;68(9):1566-74.
4. Pichichero ME et al. Pediatr Infect Dis J. 2013;32(11):1163-8.
5. Chapman T et al. PLoS One. 2020 Dec 11;15(12).
6. Blaser MJ. The microbiome revolution. J Clin Invest. 2014;124:4162-5.
Extensive limb swelling after vaccines – including SARS-CoV-2 vaccine
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.
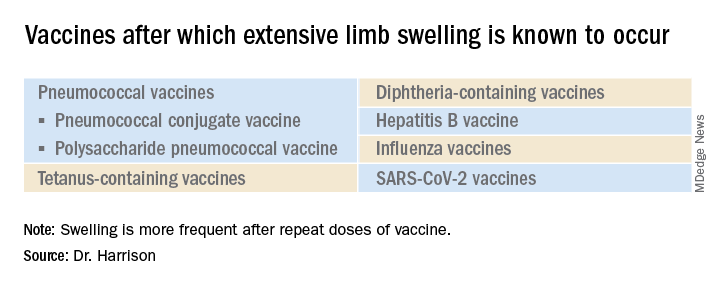
What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
A 19-month-old boy comes to the office with a large firm erythematous swelling of his anterior left thigh that reaches from just below the inguinal crease to the patella. He got his routine immunizations 2 days prior to this visit including the fourth DTaP dose in his left thigh. Clinicians who care for children and who give routine immunizations occasionally see such an adverse effect following immunization (AEFI). These large local reactions have been described for many decades and occur after many vaccines.

What is extensive limb swelling (ELS)? ELS is defined as erythema/swelling crossing a joint or extending mostly joint to joint. It is a subset of large local AEFIs. ELS is generally firm and often erythematous with varying degrees of pain. ELS is now most frequent after pneumococcal conjugate vaccines (PCV) and DTaP, with a 1%-4% rate after DTaP boosters.1-3 ELS and other large local swelling reactions occur at nearly any age.1 And yet there is still much that is not known about their true pathogenesis. Likewise, there are no accurate predictors of which vaccinees will develop large inflammatory processes at or near the site of immunization.
ELS after standard vaccines
The largest report to date on AEFI of all ages, including ELS, covered 1990-2003.1 Two upfront caveats are: This study evaluated ELS before PCVs were available, and in adults, repeat 23-valent pneumococcal polysaccharide vaccine was the most common cause of ELS in this study, comprising 45% of all adult ELS.
Considering all ages, ELS onset was nearly always greater than 1 hour and was less than 24 hours post vaccine in almost 75% of patients. However, for those aged under 2 years, onset in less than 24 hours was even more frequent (84%). Interestingly, concomitant fever occurred in less than 25% regardless of age. In adults, ELS after tetanus- and diphtheria-containing vaccines occurred mostly in women (75%); whereas for ELS under 8 years of age, males predominated (about 60%). Of note, tetanus- and diphtheria-containing vaccines were the most frequent ELS-inducing vaccines in children, that is, 75% aged under 8 years and 55% for those aged 8-17 years. Focusing on pediatric ELS after DTaP by dose, 33% were after the fourth, 31% after the fifth, 12% after the second, 10% after the first, and 3% after the third dose. In the case above, ELS was after the fourth dose.
Clinicians caring for children know how to manage ELS after DTaP or PCVs. They understand that ELS looks scary and is uncomfortable but is not dangerous and requires no specific treatment. Supportive management, that is, pain reliever, cool compresses, and TLC, are warranted. ELS is not a contraindication to subsequent immunization with the same vaccine. That said, large local reactions or ELS do occur with subsequent doses of that same vaccine at varying rates up to 66% of the time. Management is the same with repeat episodes, and no sequelae are expected. Supportive management only is standard unless one suspects a very rare Arthus reaction. If central necrosis occurs or swelling evolution/resolution is not per expectations, referral to a vaccine expert can sort out if it is an Arthus reaction, in which case, subsequent use of the same vaccine in not recommended.
ELS and SARS-CoV-2 vaccines
With SARS-CoV-2 vaccines now authorized for adolescents and expected in a few months for younger children, large local AEFI reactions related to pediatric SARS-CoV-2 vaccines are expected, given that “COVID arm” is now well described in adults.4 Overall, ELS/large local reactions have been reported more frequently with the Moderna than Pfizer mRNA vaccine.4 In the almost 42% of adults having ELS post first dose, repeat ELS post second dose often appears sooner but also resolves more quickly, with no known sequelae.5
Some biopsies have shown delayed-type hypersensitivity reactions (DTH) (superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells),6,7 while others show no DTH but these patients have findings of immediate hypersensitivity findings and negative skin testing to the vaccine.8 With regard to sex, Dutch ELS data in White adults reveal 90% occur in females – higher than the 75% female rate after standard vaccines.7 Onset of ELS data show that Pfizer mRNA vaccinees had onset on average at 38 hours (range, <1 hr to 12 days). Boston data mostly in White adults reveal later onset (median, 6 days; range, 2-12 days).4 In contrast, adults of color appear to have later onset (mean, 8 days; range, 4-14 days).9
In addition to the local swelling, patients had concurrent injection-site AEFIs of pain (65%), warmth (63%), and pruritus (26%), plus myalgia (51%), headache (48%), malaise (45%), fatigue (43%), chills (33%), arthralgia (30%), and fever (28%).7
What should we tell families about pediatric ELS before we give SARS-CoV-2 vaccines to children? Clinical pediatric SARS-CoV-2 vaccine trials are smaller “immunologic bridging” studies, not requiring proof of efficacy. So, the precise incidence of pediatric ELS (adult rate is estimated under 1/100,000) may not be known until months after general use. Nevertheless, part of our counseling of families will need to include ELS/large local reactions. Unless new data show otherwise, the spiel that clinicians have developed to counsel about the rare chance of ELS after routine vaccines should also be useful to inform families of the rare chance of ELS post SARS-CoV-2 vaccine.
The bottom line is that the management of pediatric ELS after SARS-CoV-2 vaccines should be the same as after standard vaccines. And remember, whether the reactions are DTH or not, neither immediate local injection-site reactions nor DTH reactions are contraindications to subsequent vaccination unless anaphylaxis or Arthus reaction is suspected.10,11
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Woo EJ and the Vaccine Adverse Event Reporting System Working Group. Clin Infect Dis 2003;37:351-8.
2. Rennels MB et al. Pediatrics 2000;105:e12.
3. Huber BM, Goetschel P. J Pediatr. 2011;158:1033.
4. Blumenthal KG et al. N Engl J Med. 2021;384:1273-7.
5. McMahon DE et al. J Amer Acad Dermatol. 2021;85(1):46-55. 6. Johnston MS et al. JAMA Dermatol. 2021;157(6):716-20 .
7. ELS associated with the administration of Comirnaty®. WHO database Vigilyze (cited 2021 Feb 22). Available from https://vigilyze.who-umc.org/.
8. Baeck M et al. N Engl J Med. 2021 Jun. doi: 10.1056/NEJMc2104751.
9. Samarakoon U et al. N Eng J Med. 2021 Jun 9. doi: 10.1056/NEJMc2108620.
10. Kelso JM et al. J Allergy Clin Immunol. 2012;130:25-43.
11. Zafack JG et al. Pediatrics. 2017;140(3):e20163707.
COVID-19 in children and adolescents: Disease burden and severity
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
My first thought on this column was maybe Pediatric News has written sufficiently about SARS-CoV-2 infection, and it is time to move on. However, the agenda for the May 12th Advisory Committee on Immunization Practice includes a review of the Pfizer-BioNTech COVID-19 vaccine safety and immunogenicity data for the 12- to 15-year-old age cohort that suggests the potential for vaccine availability and roll out for early adolescents in the near future and the need for up-to-date knowledge about the incidence, severity, and long-term outcome of COVID-19 in the pediatric population.
Updating and summarizing the pediatric experience for the pediatric community on what children and adolescents have experienced because of SARS-CoV-2 infection is critical to address the myriad of questions that will come from colleagues, parents, and adolescents themselves. A great resource, published weekly, is the joint report from the American Academy of Pediatrics and the Children’s Hospital Association.1 As of April 29, 2021, 3,782,724 total child COVID-19 cases have been reported from 49 states, New York City (NYC), the District of Columbia, Guam, and Puerto Rico. Children represent approximately 14% of cases in the United States and not surprisingly are an increasing proportion of total cases as vaccine impact reduces cases among older age groups. Nearly 5% of the pediatric population has already been infected with SARS-CoV-2. Fortunately, compared with adults, hospitalization, severe disease, and mortality remain far lower both in number and proportion than in the adult population. Cumulative hospitalizations from 24 states and NYC total 15,456 (0.8%) among those infected, with 303 deaths reported (from 43 states, NYC, Guam, and Puerto Rico). Case fatality rate approximates 0.01% in the most recent summary of state reports. One of the limitations of this report is that each state decides how to report the age distribution of COVID-19 cases resulting in variation in age range; another is the data are limited to those details individual states chose to make publicly available.
Although children do not commonly develop severe disease, and the case fatality is low, there are still insights to be learned from understanding risk features for severe disease. Preston et al. reviewed discharge data from 869 medical facilities to describe patients 18 years or younger who had an inpatient or emergency department encounter with a primary or secondary COVID-19 discharge diagnosis from March 1 through October 31, 2020.2 They reported that approximately 2,430 (11.7%) children were hospitalized and 746, nearly 31% of those hospitalized, had severe COVID disease. Those at greatest risk for severe disease were children with comorbid conditions and those less than 12 years, compared with the 12- to 18-year age group. They did not identify race as a risk for severe disease in this study. Moreira et al. described risk factors for morbidity and death from COVID in children less than 18 years of age3 using CDC COVID-NET, the Centers for Disease Control and Prevention COVID-19–associated hospitalization surveillance network. They reported a hospitalization rate of 4.7% among 27,045 cases. They identified three risk factors for hospitalization – age, race/ethnicity, and comorbid conditions. Thirty-nine children (0.19%) died; children who were black, non-Hispanic, and those with an underlying medical condition had a significantly increased risk of death. Thirty-three (85%) children who died had a comorbidity, and 27 (69%) were African American or Hispanic/Latino. The U.S. experience in children is also consistent with reports from the United Kingdom, Italy, Spain, Germany, France, and South Korea.4 Deaths from COVID-19 were uncommon but relatively more frequent in older children, compared with younger age groups among children less than 18 years of age in these countries.
Acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) do not predominantly target the neurologic systems; however, neurologic complications have been reported, some of which appear to result in long-lasting disability. LaRovere et al. identified 354 (22%) of 1,695 patients less than 21 years of age with acute COVID or MIS-C who had neurologic signs or symptoms during their illness. Among those with neurologic involvement, most children had prior neurologic deficits, mild symptoms, that resolved by the time of discharge. Forty-three (12%) were considered life threatening and included severe encephalopathy, stroke, central nervous system infection/demyelination, Guillain-Barre syndrome or variant, or acute cerebral edema. Several children, including some who were previously healthy prior to COVID, had persistent neurologic deficits at discharge. In addition to neurologic morbidity, long COVID – a syndrome of persistent symptoms following acute COVID that lasts for more than 12 weeks without alternative diagnosis – has also been described in children. Buonsenso et al. assessed 129 children diagnosed with COVID-19 between March and November 2020 in Rome, Italy.5 Persisting symptoms after 120 days were reported by more than 50%. Symptoms like fatigue, muscle and joint pain, headache, insomnia, respiratory problems, and palpitations were most common. Clearly, further follow-up of the long-term outcomes is necessary to understand the full spectrum of morbidity resulting from COVID-19 disease in children and its natural history.
The current picture of COVID infection in children younger than 18 reinforces that children are part of the pandemic. Although deaths in children have now exceeded 300 cases, severe disease remains uncommon in both the United States and western Europe. Risk factors for severe disease include comorbid illness and race/ethnicity with a disproportionate number of severe cases in children with underlying comorbidity and in African American and Hispanic/Latino children. Ongoing surveillance is critical as changes are likely to be observed over time as viral evolution affects disease burden and characteristics.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University schools of medicine and public health and senior attending physician in pediatric infectious diseases, Boston Medical Center. Email him at [email protected].
References
1. Children and COVID-19: State-Level Data Report. Services AAP.org.
2. Preston LE et al. JAMA Network Open. 2021;4(4):e215298. doi:10.1001/jamanetworkopen.2021.5298
3. Moreira A et al. Eur J Pediatr. 2021;180:1659-63.
4. SS Bhopal et al. Lancet 2021. doi: 10.1016/ S2352-4642(21)00066-3.
5. Buonsenso D et al. medRxiv preprint. doi: 10.1101/2021.01.23.21250375.
Tick talk for families and pediatricians
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.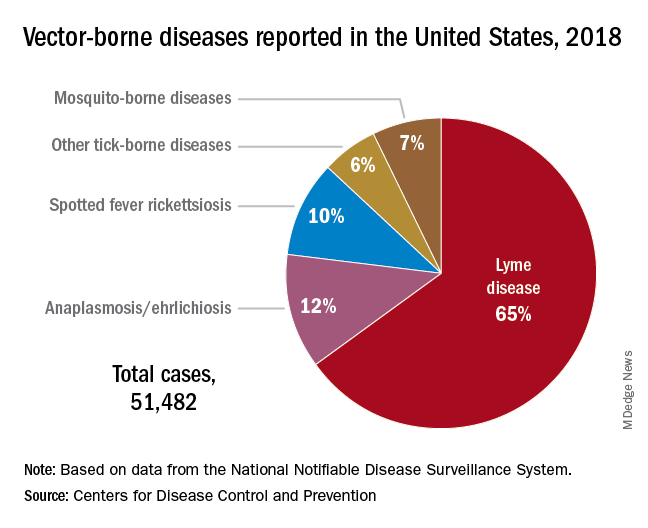
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Spring 2021 has arrived with summer quickly approaching. It is our second spring and summer during the pandemic. Travel restrictions have minimally eased for vaccinated adults. However, neither domestic nor international leisure travel is encouraged for anyone. Ironically, air travel is increasing. For many families, it is time to make decisions regarding summer activities. Outdoor activities have been encouraged throughout the pandemic, which makes it a good time to review tick-borne diseases. Depending on your location, your patients may only have to travel as far as their backyard to sustain a tick bite.
Ticks are a group of obligate, bloodsucking arthropods that feed on mammals, birds, and reptiles. There are three families of ticks. Two families, Ixodidae (hard-bodied ticks) and Argasidae (soft-bodied ticks) are responsible for transmitting the most diseases to humans in the United States. Once a tick is infected with a pathogen it usually survives and transmits it to its next host. Ticks efficiently transmit bacteria, spirochetes, protozoa, rickettsiae, nematodes, and toxins to humans during feeding when the site is exposed to infected salivary gland secretions or regurgitated midgut contents. Pathogen transmission can also occur when the feeding site is contaminated by feces or coxal fluid. Sometimes a tick can transmit multiple pathogens. Not all pathogens are infectious (e.g., tick paralysis, which occurs after exposure to a neurotoxin and red meat allergy because of alpha-gal). Ticks require a blood meal to transform to their next stage of development (larva to nymph to adult). Life cycles of hard and soft ticks differ with most hard ticks undergoing a 2-year life cycle and feeding slowly over many days. In contrast, soft ticks feed multiple times often for less than 1 hour and are capable of transmitting diseases in less than 1 minute.
Rocky Mountain spotted fever was the first recognized tick-borne disease (TBD) in humans. Since then, 18 additional pathogens transmitted by ticks have been identified with 40% being described since 1980. The increased discovery of tickborne pathogens has been attributed to physician awareness of TBD and improved diagnostics. The number of cases of TBD has risen yearly. Ticks are responsible for most vector-transmitted diseases in the United States with Lyme disease most frequently reported.
Mosquito transmission accounts for only 7% of vector-borne diseases. Three species of ticks are responsible for most human disease: Ixodes scapularis (Black-legged tick), Amblyomma americanum (Lone Star tick), and Dermacentor variabilis (American dog tick). Each is capable of transmitting agents that cause multiple diseases.
Risk for acquisition of a specific disease is dependent upon the type of tick, its geographic location, the season, and duration of the exposure.
Humans are usually incidental hosts. Tick exposure can occur year-round, but tick activity is greatest between April and September. Ticks are generally found near the ground, in brushy or wooded areas. They can climb tall grasses or shrubs and wait for a potential host to brush against them. When this occurs, they seek a site for attachment.
In the absence of a vaccine, prevention of TBD is totally dependent upon your patients/parents understanding of when and where they are at risk for exposure and for us as physicians to know which pathogens can potentially be transmitted by ticks. Data regarding potential exposure risks are based on where a TBD was diagnosed, not necessarily where it was acquired. National maps that illustrate the distribution of medically significant ticks and presence or prevalence of tick-borne pathogens in specific areas within a region previously may have been incomplete or outdated. The Centers for Disease Control and Prevention initiated a national tick surveillance program in 2017; five universities were established as regional centers of excellence to help prevent and rapidly respond to emerging vector-borne diseases across the United States. One goal is to standardize tick surveillance activities at the state level. For state-specific activity go to https://www.cdc.gov/ncezid/dvbd/vital-signs/index.html.
Prevention: Here are a few environmental interventions you can recommend to your patients
- Remove leaf litter, clear tall brush, and grass around the home and at edge of lawns. Mow the lawn frequently.
- Keep playground equipment, decks, and patios away from yard edges and trees.
- Live near a wooded area? Place a 3-ft.-wide barrier of gravel or wood chips between the areas.
- Put up a fence to keep unwanted animals out.
- Keep the yard free of potential hiding place for ticks (e.g., mattresses or furniture).
- Stack wood neatly and in a dry area.
- Use pesticides, but do not rely on them solely to prevent ticks exposure.
Personal interventions for patients when outdoors
- Use Environmental Protection Agency–registered insect repellents. Note: Oil of lemon-, eucalyptus-, and para-menthane-diol–containing products should not be used in children aged3 years or less.
- Treat clothing and gear with products containing 0.5% permethrin to repel mosquitoes and ticks.
- Check cloths for ticks. Drying clothes on high heat for 10 minutes will kill ticks. If washing is needed use hot water. Lower temperatures will not kill ticks.
- Do daily body checks for ticks after coming indoors.
- Check pets for ticks.
Tick removal
- Take tweezers, grasp the tick as close to the skin’s surface as possible.
- Pull upward. Do not twist or jerk the tick. Place in a container. Ideally submit for species identification.
- After removal, clean the bite area with alcohol or soap and water.
- Never crush a tick with your fingers.
When should you include TBD in your differential for a sick child?
Headache, fever, arthralgia, and rash are symptoms for several infectious diseases. Obtaining a history of recent activities, tick bite, or travel to areas where these diseases are more prevalent is important. You must have a high index of suspicion. Clinical and laboratory clues may help.
Delay in treatment is more detrimental. If you suspect rickettsia, ehrlichiosis, or anaplasmosis, doxycycline should be started promptly regardless of age. Consultation with an infectious disease specialist is recommended.
The United States recognizes it is not adequately prepared to address the continuing rise of vector-borne diseases. In response, on Jan. 20, 2021, the CDC’s division of vector-borne diseases with input from five federal departments and the EPA developed a joint National Public Health Framework for the Prevention and Control of Vector-Borne Diseases in Humans to tackle issues including risk, detection, diagnosis, treatment, prevention and control of TBD. Stay tuned.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
The lost year – even for common respiratory viruses
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.
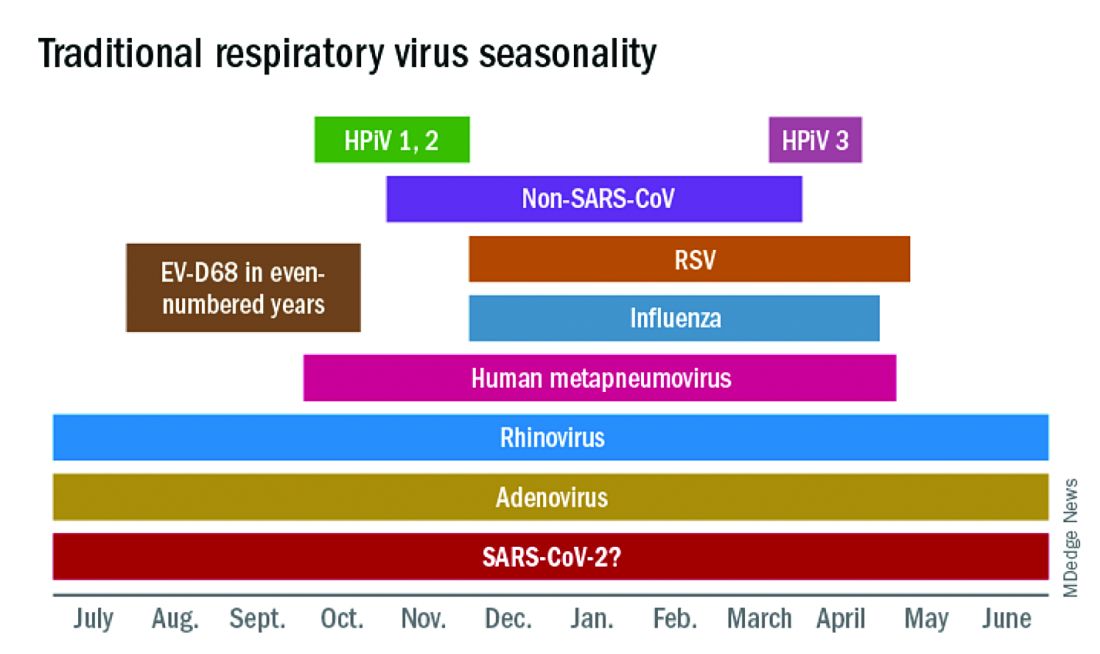
The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).
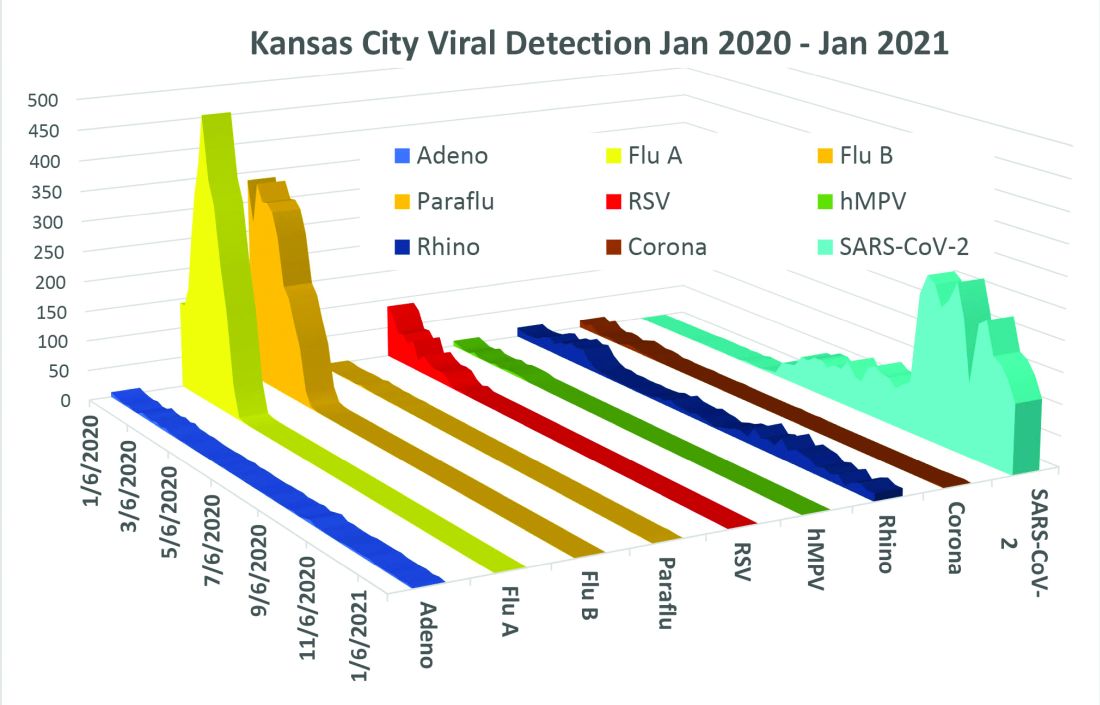
What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
In this column in September 2020, you read how common respiratory viruses’ seasons are usually so predictable, each virus arising, peaking, and then dying out in a predictable virus parade (Figure 1).1 Well, the predictable virus seasonal pattern was lost in 2020. Since March of 2020, it is striking how little activity was detected for the usual seasonal viruses in Kansas City after mid-March 2020 (Figure 2).2 So, my concern in September 2020 for possible rampant coinfections of common viruses with or in tandem with SARS-CoV-2 did not pan out. That said, the seasons for non–SARS-CoV-2 viruses did change; I just didn’t expect they would nearly disappear.

The 2020 winter-spring. In the first quarter (the last part of the overall 2019-2020 respiratory viral season), viral detections were chugging along as usual up to mid-March (Figure 2); influenza, respiratory syncytial virus (RSV), and rhinovirus were the big players.
Influenza. In most years, influenza type B leads off and is quickly replaced by type A only to see B reemerge to end influenza season in March-April. In early 2020, both influenza type A and influenza type B cocirculated nearly equally, but both dropped like a rock in mid-March (Figure 2).2 Neither type has been seen since with the exception of sporadic detections – perhaps being false positives.
RSV. In the usual year in temperate mid-latitudes of the northern hemisphere, RSV season usually starts in early December, peaks in January-March, and declines gradually until the end of RSV season in April (Figure 1). In southern latitudes, RSV is less seasonal, being present most of the year, but peaking in “winter” months.3 But in 2020, RSV also disappeared in mid-March and has yet to reappear.
Other viruses. Small bumps in detection of parainfluenza of varying types usually frame influenza season, one B bump in early autumn and another in April-May. In most years, human metapneumovirus is detected on and off, with worse years at 2- to 3-year intervals. Adenovirus occurs year-round with bumps as children get back to school in autumn. Yet in 2020, almost no parainfluenza, adenovirus, common coronaviruses, or human metapneumovirus were detected in either spring or autumn. This was supposed to be a banner summer-autumn for EV-D68 – but almost none was detected. Interestingly, the cockroach of viruses, rhinovirus, has its usual year (Figure 2).

What happened? Intense social mitigation interventions, including social distancing and closing daycares and schools, were likely major factors.4 For influenza, vaccine may have helped but uptake was not remarkably better than most prior years. There may have been “viral competition,”where a new or highly transmissible virus outcompetes less-transmissible viruses with lower affinity for respiratory receptors.5,6 Note that SARS-CoV-2 has very high affinity for the ACE2 receptor and has been highly prevalent. So, SARS-CoV-2 could fit the theoretical mold for a virus that outcompetes others.
Does it matter for the future? Blunted 2019-2020 and nearly absent 2020-2021 respiratory virus season may have set the stage for intense 2021-2022 rebounds for the non–SARS-CoV-2 viruses. We now have two whole and one partial birth cohort with no experience with seasonal respiratory viruses, including EV-D68 (and nonrespiratory viruses too – like norovirus, parechovirus, and other enteroviruses). Most viruses have particularly bad seasons every 2-3 years, thought to be caused by increasing accumulation of susceptible individuals in consecutive birth cohorts until a critical mass of susceptible individuals is achieved. The excess in susceptible individuals means that each contagious case is likely to expose one or more susceptible individuals, enhancing transmission and infection numbers in an ever-extending ripple effect. We have never had this many children aged under 3 years with no immunity to influenza, RSV, etc. So unless mother nature is kind (when has that happened lately?), expect rebound years for seasonal viruses as children return to daycare/schools and as social mitigation becomes less necessary in the waning pandemic.
Options? If you ramped up telehealth visits for the pandemic, that may be a saving grace, i.e., more efficiency so more “visits” can be completed per day, and less potential contact in reception rooms between well and ill children. And if there was ever a time to really intensify efforts to immunize all our pediatric patients, the next two seasons are just that. Adding a bit of a warning to families with young children also seems warranted. If they understand that, while 2021-2022 will be better for SARS-CoV-2, it is likely going to be worse for the other viruses.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Harrison CJ. 2020-2021 respiratory viral season: Onset, presentations, and testing likely to differ in pandemic, Pediatric News: September 17, 2020.
2. Olsen SJ et al. MMWR Morb Mortal Wkly Rep. 2020;69:1305-9.
3. Respiratory Syncytial Virus Surveillance. http://www.floridahealth.gov/diseases-and-conditions/respiratory-syncytial-virus/_documents/2021-w4-rsv-summary.pdf
4. Baker RE et al. PNAS. Dec 2020 117;(48):30547-53.
5. Sema Nickbakhsh et al. PNAS. Dec 2019 116;(52):27142-50.
6. Kirsten M et al. PNAS. Mar 2020 117;(13):6987.
Waiting for the COVID 19 vaccine, or not?
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 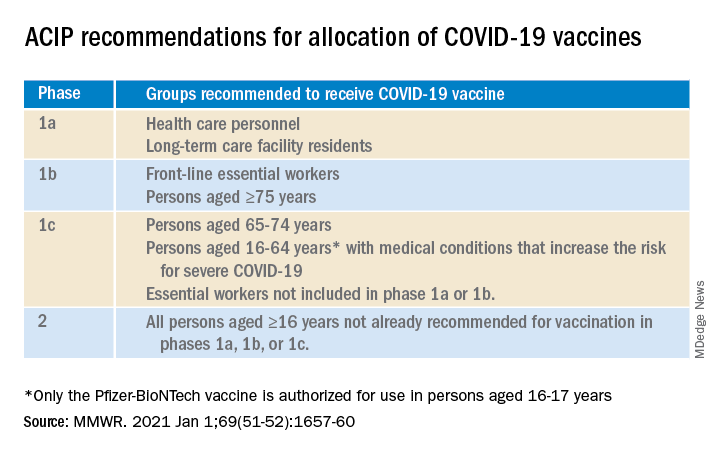
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
A shot of relief. A shot of hope. Those are the words used to describe COVID-19 vaccines on a television commercial running in prime time in Kentucky.
“We all can’t get the vaccine at once,” the announcer says solemnly, “but we’ll all get a turn.”
For some of us, that turn came quickly. In December, the Advisory Committee on Immunization Practices recommended that health care personnel (HCP) and long-term care facility residents be the first to be immunized with COVID-19 vaccines (see table). 
On Dec. 14, 2020, Sandra Lindsay, a nurse and director of patient care services in the intensive care unit at Long Island Jewish Medical Center, was the first person in the United States to receive a COVID-19 vaccine outside a clinical trial.
In subsequent days, social media sites were quickly flooded with photos of HCP rolling up their sleeves or flashing their immunization cards. There was jubilation ... and perhaps a little bit of jealousy. There were tears of joy and some tears of frustration.
There are more than 21 million HCP in the United States and to date, there have not been enough vaccines nor adequate infrastructure to immunize all of them. According to the Centers for Disease Control and Prevention Data Tracker, as of Jan. 7, 2021, 21,419,800 doses of vaccine had been distributed to states to immunize everyone identified in phase 1a, but only 5,919,418 people had received a first dose. Limited supply has necessitated prioritization of subgroups of HCP; those in the front of the line have varied by state, and even by hospital or health care systems within states. Both the American Academy of Pediatrics and the American Academy of Family Physicians have noted that primary care providers not employed by a hospital may have more difficulty accessing vaccine.
The mismatch between supply and demand has created an intense focus on improving supply and distribution. Soon though, we’re going to shift our attention to how we increase demand. We don’t have good data on those who being are offered COVID-19 vaccine and declining, but several studies that predate the Emergency Use Authorization for the Pfizer-BioNTech and Moderna vaccines suggest significant COVID-19 vaccine hesitancy among adults in the United States.
One large, longitudinal Internet-based study of U.S. adults found that the proportion who reported they were “somewhat or very likely” to receive COVID-19 vaccine declined from 74% in early April to 56% in early December.
In the Understanding America Study, self-reported likelihood of being vaccinated with COVID-19 vaccine was lower among Black compared to White respondents (38% vs. 59%; aRR, 0.7 [95% confidence interval, 0.6-0.8]), and lower among women compared to men (51% vs. 62%; aRR, 0.9 [95% CI, 0.8-0.9]). Those 65 years of age and older were more likely to report a willingness to be vaccinated than were those 18-49 years of age, as were those with at least a bachelor’s degree compared to those with a high school education or less.
A study conducted by the Pew Research Center in November – before any COVID-19 vaccines were available – found that only 60% of American adults said they would “definitely or probably get a vaccine for coronavirus” if one were available. That was an increase from 51% in September, but and overall decrease of 72% in May. Of the remaining 40%, just over half said they did not intend to get vaccinated and were “pretty certain” that more information would not change their minds.
Concern about acquiring a serious case of COVID-19 and trust in the vaccine development process were associated with an intent to receive vaccine, as was a personal history of receiving a flu shot annually. Willingness to be vaccinated varied by age, race, and family income, with Black respondents, women, and those with a lower family incomes less likely to accept a vaccine.
To date, few data are available about HCP and willingness to receive COVID-19 vaccine. A preprint posted at medrxiv.org reports on a cross-sectional study of more than 3,400 HCP surveyed between Oct. 7 and Nov. 9, 2020. In that study, only 36% of respondents voiced a willingness to be immunized as soon as vaccine is available. Vaccine acceptance increased with increasing age, income level, and education. As in other studies, self-reported willingness to accept vaccine was lower in women and Black individuals. While vaccine acceptance was higher in direct medical care providers than others, it was still only 49%.
So here’s the paradox: Even as limited supplies of vaccine are available and many are frustrated about lack of access, we need to promote the value of immunization to those who are hesitant. Pediatricians are trusted sources of vaccine information and we are in a good position to educate our colleagues, our staff, the parents of our patients and the community at-large.
A useful resource for those ready to take that step it is the CDC’s COVID-19 Vaccination Communication Toolkit. While this collection is designed to build vaccine confidence and promote immunization among health care providers, many of the strategies will be easily adapted for use with patients.
It’s not clear when we might have a COVID 19 vaccine for most children. The Pfizer-BioNTech vaccine emergency use authorization includes those as young as 16 years of age, and 16- and 17-year-olds with high risk medical conditions are included in phase 1c of vaccine allocation. Pfizer is currently enrolling children as young as 12 years of age in clinical trials, and Moderna and Janssen are poised to do the same. It is conceivable but far from certain that we could have a vaccine for children late this year. Are parents going to be ready to vaccinate their children?
Limited data about parental acceptance of vaccine for their children mirrors what was seen in the Understanding America Study and the Pew Research Study. In December 2020, the National Parents Union surveyed 1,008 parents of public school students enrolled in kindergarten through 12th grade. Sixty percent of parents said they would allow their children to receive a COVID-19 vaccine, while 25% would not and 15% were unsure. This suggests that now is the time to begin building vaccine confidence with parents. One conversation starter might be, “I am going to be vaccinated as soon as the vaccine is available.” Ideally, many of you will soon be able to say what I do: “I am excited to tell you that I have been immunized with the COVID-19 vaccine. I did this to protect myself, my family, and our community. I’m hopeful that vaccine will soon be available for all of us.”
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
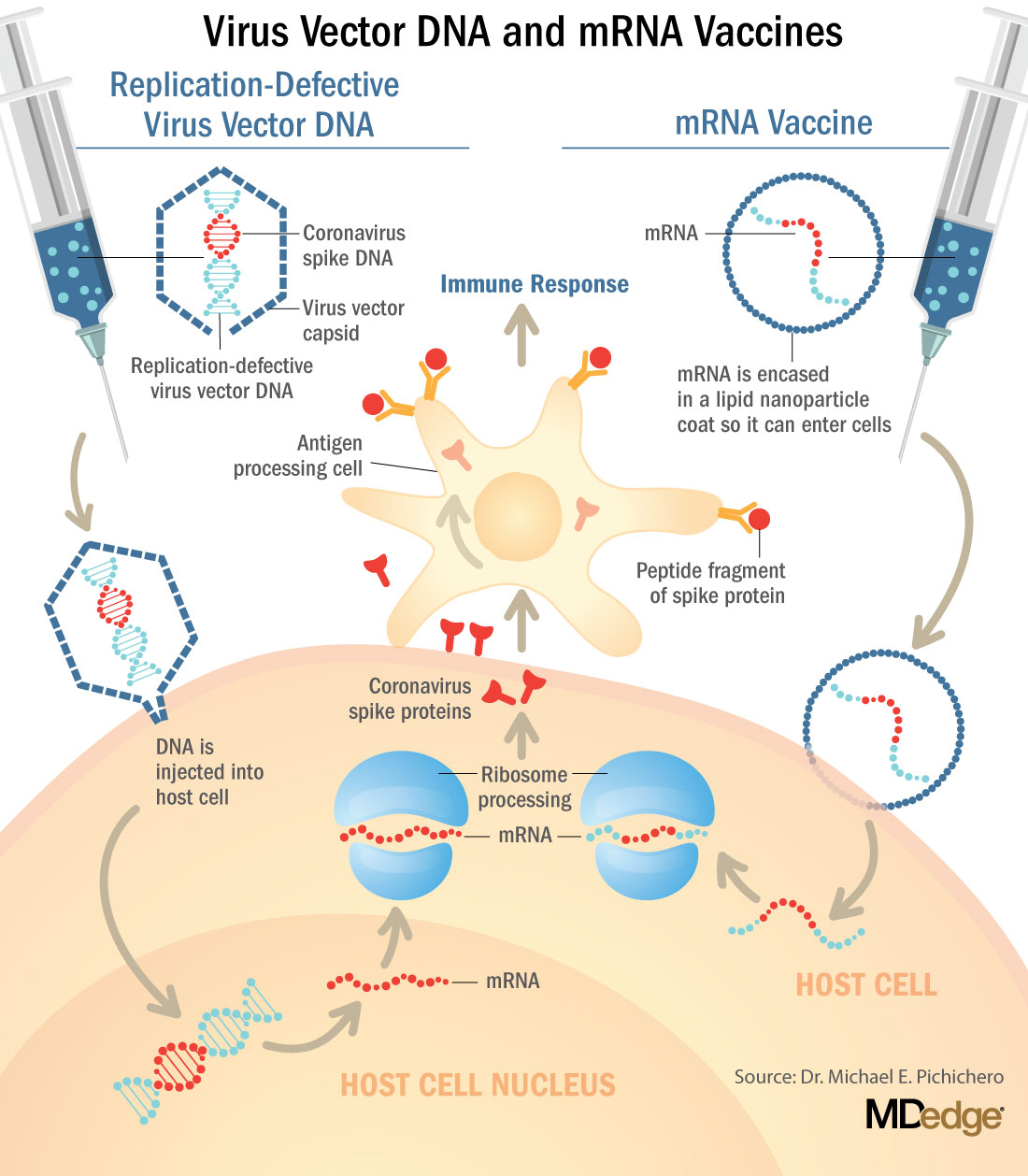
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Should our patients really go home for the holidays?
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Direct-acting agents cure hepatitis C in children
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at [email protected].
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at [email protected].
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.
Between 23,000 and 46,000 U.S. children live with chronic hepatitis C virus with a prevalence of 0.17% anti–hepatitis C virus (HCV) antibody positivity in those aged 6-11 years and 0.39% among children aged 12-19 years. In the United States, genotype 1 is most frequent, followed by genotypes 2 and 3. About 99% of cases result from vertical transmission; transfusion-related cases have not been observed in recent decades.Only viremic mothers are at risk of transmission as those who have spontaneously cleared HCV viremia or have been treated successfully do not risk transmission. Maternal HCV viral load appears to be a risk factor for HCV transmission, however transmission is reported at all levels of viremia.
In conjunction with the opioid epidemics, the prevalence of HCV infection has increased over the last decade. The Centers for Disease Control and Prevention reported that, between 2009 and 2014, the prevalence of HCV infection increased from 1.8 to 3.4 per 1,000 live births. They identified substantial state-to-state variation with the highest rate in West Virginia (22.6 per 1,000 live births), and the lowest in Hawaii (0.7 per 1,000 live births). The implications are clear that increasing numbers of newborns are exposed to HCV and, if transmission rates are between 1% and 5%, 80-400 U.S. infants each year acquire HCV infection.
HCV in children
HCV in children is almost always associated with persistent transaminitis. Chronic infection is defined as the persistence of HCV RNA for at least 6 months, and clearance of HCV infection is determined by the persistent disappearance of HCV RNA. Regardless of infection status, an infant may have detectable maternal anti-HCV antibody in serum until 18 months of age, resulting from passive transfer. In addition, prolonged infection can lead to cirrhosis, hepatocellular carcinoma, or decompensated liver disease. Potential extrahepatic manifestations including reduced physical and psychosocial health also are linked to chronic HCV. Autoimmune disease also has been reported in children with HCV. As well, the stigma of HCV elicits fear in school and child care settings that is a result of public misunderstanding regarding routes of hepatitis C transmission. No restriction of regular childhood activities is required in the daily life of HCV-infected children.
Taken together, increasing rates of HCV infection in pregnant women, increasing numbers of exposed and infected infants annually, potential for both short- and long-term morbidity, and curative nontoxic treatment,
Screening for HCV
There is considerable discussion about which strategy for screening of at-risk infants is more appropriate. Some groups advocate for HCV-RNA testing within the first year of life. Proponents argue the use of a highly sensitive RNA assay early in life has potential to increase detection of infected infants while a negative result allows the conclusion the infant is not infected. Advocates hypothesize that early identification has potential to improve continued follow-up.
Opponents argue that early testing does not change the need for repeat testing after 18 months to confirm diagnosis. They also argue that HCV RNA is more expensive than an antibody-based testing; and treatment will not begin prior to age 3 as there is still opportunity for viremia to spontaneously clear.
Direct acting agents licensed
Ledipasvir/sofosbuvir (Harvoni) was initially demonstrated as curative for genotype 1, 4, 5, or 6 infection in a phase 2, multicenter, open-label study of 100 adolescents with genotype 1 treated for 12 weeks. Sustained virologic response (SVR) was documented in 98% of participants.The regimen was safe and well tolerated in this population, and the adult dosage formulation resulted in pharmacokinetic characteristics similar to those observed in adults. Two clinical trials supported the efficacy of ledipasvir/sofosbuvir in the pediatric population aged 3-11 years. This regimen also is recommended for interferon-experienced (± ribavirin, with or without an HCV protease inhibitor) children and adolescents aged 3 years or older with genotype 1 or 4. A 12-week course is recommended for patients without cirrhosis; 24 weeks is recommended for those with compensated cirrhosis. The combination of ledipasvir/sofosbuvir is the only treatment option for children aged 3-6 years with genotype 1, 4, 5, or 6 infection.
The efficacy of sofosbuvir/velpatasvir (Epclusa) once daily for 12 weeks was first evaluated in an open-label trial among children aged 6 years and older with genotype 1, 2, 3, 4, or 6 infection, without cirrhosis or with compensated cirrhosis. Subsequently, the “cocktail” was evaluated in children aged 6-12 years, with 76% genotype 1, 3% genotype 2, 15% genotype 3, and 6% genotype 4. SVR12 rates were 93% (50/54) in children with genotype 1, 91% (10/11) in those with genotype 3, and 100% in participants with genotype 2 (2/2) or genotype 4 (4/4). Sofosbuvir/velpatasvir was approved in March 2020 by the Food and Drug Administration for pediatric patients aged 6 years and older. Given its pangenotypic activity, safety, and efficacy, sofosbuvir/velpatasvir is currently recommended as a first choice for HCV treatment in children and adolescents aged at least 6 years.
The daily fixed-dose combination of glecaprevir/pibrentasvir (Mavyret) was approved in April 2019 for adolescents aged 12-17 years, and weighing at least 45 kg.Treatment is for 8 weeks, and includes treatment-naive patients without cirrhosis or those with compensated cirrhosis. SVR12 rates for Mavyret have ranged from 91% to 100 % across clinic trials. FDA approval and HCV guideline treatment recommendations for direct-acting antiviral (DAA)–experienced adolescents are based on clinical trial data from adults. Given its pangenotypic activity, safety, and efficacy record in adult patients, glecaprevir/pibrentasvir is recommended as a first choice for adolescent HCV treatment. Glecaprevir/pibrentasvir once approved for children less than 3 years of age will be safe and efficacious as a pangenotypic treatment option in children with chronic HCV infection.
Current recommendations
Tools for identifying HCV infected infants as early as a few months of age are available, yet studies demonstrate that a minority of at-risk children are tested for HCV using either an HCV polymerase chain reaction strategy early in life or an anti-HCV antibody strategy after 18 months of age.
Therapy with direct-acting agents is now licensed to those aged 3 years and offers the potential for cure, eliminating concern for possible progression after prolonged infection. Such therapy offers the potential to eliminate the stigma faced by many children as well as the hepatic and extrahepatic manifestations observed in children. Medication formulation and the child’s abilities to take the medication needs to be considered when prescribing DAAs. It is important to assess if the child can successfully swallow pills. Currently, Harvoni is the only medication that comes in both pellet and pill formulations. The dose is based on weight. The pellets need to be given in a small amount of nonacidic food; they cannot be chewed.
All children with chronic HCV infection are candidates for treatment. When significant fibrosis and/or cirrhosis is present treatment should not be delayed once the child is age 3 years; when only transaminitis is present, treatment can be delayed. In our experience, parents are eager to complete treatment before starting kindergarten.
Liver biopsy for obtaining liver tissue for histopathologic examination is not routinely indicated in children with chronic HCV infection but should be evaluated case by case. Noninvasive tests of hepatic fibrosis have been used in children, these include serologic markers (i.e., FibroSure) and radiologic tests such as ultrasound-based transient elastography (i.e., Fibroscan). Validation for pediatric patients is variable for the different serologic tests. Studies have shown that Fibroscan using the M probe is feasible for a wide range of ages, but poor patient cooperation may make measurement difficult.
Further details regarding dosing and choice of formulation is available at https://www.hcvguidelines.org/unique-populations/children.
Dr. Sabharwal is assistant professor of pediatrics at Boston University and attending physician in pediatric infectious diseases at Boston Medical Center. Ms. Moloney is an instructor in pediatrics at Boston University and a pediatric nurse practitioner in pediatric infectious diseases at Boston Medicine Center. Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. Boston Medical Center received funding from AbbVie for study of Harvoni in Children 3 years of age and older. Email them at [email protected].
References
MMWR Morb Mortal Wkly Rep. 2017 May 12;66(18):470-3. Hepatol Commun. 2017 March 23. doi: 10.1002/hep4.1028. Hepatology. 2020 Feb;71(2):422-30. Lancet Gastroenterol Hepatol. 2019 Apr 11. doi: 10.1016/S2468-1253(19)30046-9. Arch Dis Child. 2006 Sep;91(9):781-5. J Pediatr Gastroenterol Nutr. 2010 Feb;50(2):123-31.