User login
Don’t give up on influenza vaccine
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Systems biology – A primer
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.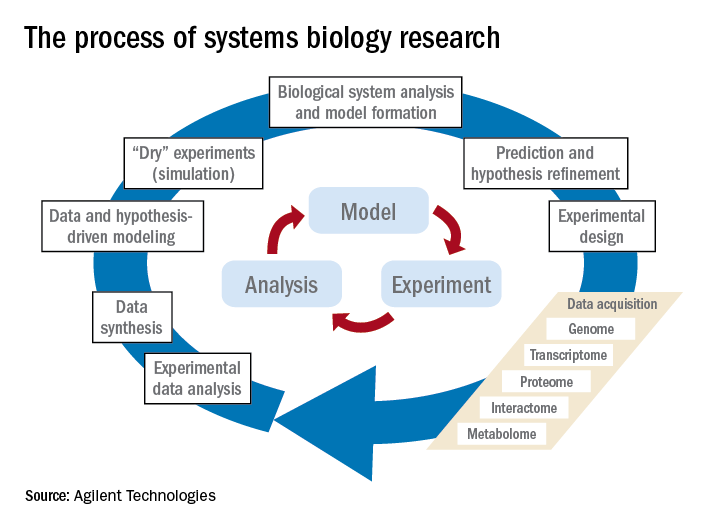
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at [email protected].
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at [email protected].
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at [email protected].
Group B streptococcus
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Vaccine renaissance
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.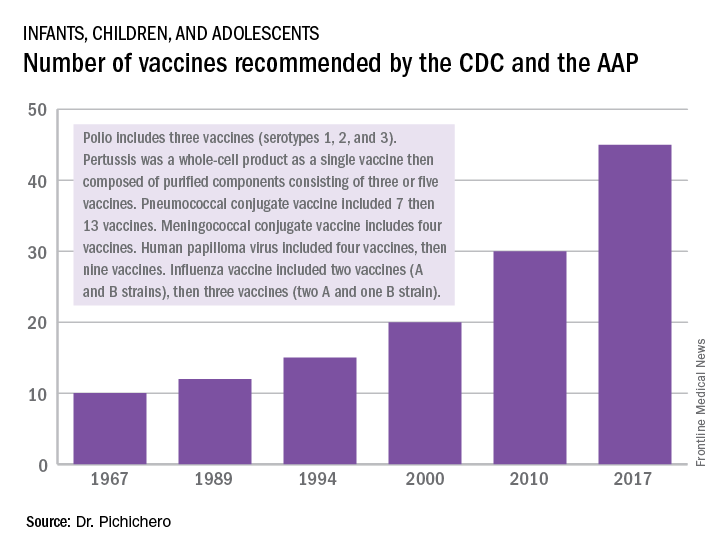
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at [email protected].
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at [email protected].
In 1967, pediatric patients were vaccinated routinely against eight diseases with 10 vaccines: smallpox; diphtheria; tetanus and pertussis; polio serotypes 1, 2, and 3; measles; rubella; and mumps. Then in 1989, vaccine discovery took a dramatic upward trend. For the physicians and scientists involved in vaccine discovery, the driving force may have been a passion for scientific discovery and a humanitarian motivation, but what drove this major change in pediatric infectious diseases was economics.
I believe The hiatus of more than 20 years between the introduction of the mumps vaccine in 1967 and that of the Hib vaccine in 1989 in my view was because the economic incentives to develop vaccines were absent. In fact, in the 1970s and early 1980s, vaccine manufacturers were drawing back from making vaccines because they were losing money selling them at a few dollars per dose.
A trailblazing path had been created, and more and more vaccines have been discovered and come to market since then. Combination vaccines and vaccines for adolescents and adults have followed. The biggest blockbuster is Prevnar13 (actually 13 vaccines contained in a single combination), now with annual sales in excess of $7 billion worldwide and growing. Other vaccines with sales of a billion dollars or more are also on the market; anything in excess of $1 billion is considered a blockbuster in the pharmaceutical industry and gets the attention of CEOs (and investors) in a big way.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has received funding awarded to his institution for vaccine research from GlaxoSmithKline, Merck, Pfizer, and Sanofi Pasteur. Email him at [email protected].
Artemisinin: Its global impact on the treatment of malaria
Malaria remains a major international public health concern. In 2015, the World Health Organization estimated that 212 million individuals were infected and that there were 429,000 deaths. This represents a 21% decline in incidence globally and a 29% decline in global mortality between 2010 and 2015. In 2016, malaria was endemic in 91 countries and territories, down from 108 in 2000. Although malaria has been eliminated from the United States since the early 1950s, approximately 1,700 cases are reported annually, most of which occur in returned travelers, according to the Centers for Disease Control and Prevention.
Five species of Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale, and, more recently, P. knowelsi) account for most of the infections in humans and are transmitted by the bite of an infected female Anopheles mosquito. The disease is rarely acquired by blood transfusion, by needle sharing, by organ transplantation, or congenitally. Once diagnosed, malaria can be treated; however, delay in initiating therapy can lead to both serious and fatal outcomes.
Treatment
Historically, drug development was driven by the need to protect the military. While quinine was isolated from the bark of the cinchona tree in 1820, chloroquine, proguanil, mefloquine, and atovaquone each were developed during or after a military conflict during 1945-1985. Tetracycline/doxycycline and clindamycin also have antimalarial activity. Use of any of these agents as monotherapy has led to drug resistance and treatment failure.
Artemisinin
Artemisinin (also known as qinghao su) and its derivatives are a new class of antimalarials derived from the sweet wormwood plant Artemisia annua. Initially developed in China in the 1970s, this class gained global attention in the 1990s. and have the fastest parasite clearance time, rapid resolution of symptoms, and an excellent safety profile. They have activity against all Plasmodium species.
Because of artemisinins’ rapid elimination, they are used in combination with an agent that also kills blood parasites but has a slower elimination rate and a different mechanism of action. The goal is to prevent and delay the development of resistance and reduce recrudescence. The superiority of artemisinin-based combination therapy (ACT) over monotherapies has been documented.
Resistance, always a concern, has remained limited to specific areas in Southeast Asia since reported in 2008. Monitoring drug efficacy, safety, quality of antimalarials is ongoing, as is discouraging monotherapy use of these agents. Globally, artemisinins are the mainstay of treatment. Spread of resistance would be a major setback for both malaria control and elimination.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Malaria remains a major international public health concern. In 2015, the World Health Organization estimated that 212 million individuals were infected and that there were 429,000 deaths. This represents a 21% decline in incidence globally and a 29% decline in global mortality between 2010 and 2015. In 2016, malaria was endemic in 91 countries and territories, down from 108 in 2000. Although malaria has been eliminated from the United States since the early 1950s, approximately 1,700 cases are reported annually, most of which occur in returned travelers, according to the Centers for Disease Control and Prevention.
Five species of Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale, and, more recently, P. knowelsi) account for most of the infections in humans and are transmitted by the bite of an infected female Anopheles mosquito. The disease is rarely acquired by blood transfusion, by needle sharing, by organ transplantation, or congenitally. Once diagnosed, malaria can be treated; however, delay in initiating therapy can lead to both serious and fatal outcomes.
Treatment
Historically, drug development was driven by the need to protect the military. While quinine was isolated from the bark of the cinchona tree in 1820, chloroquine, proguanil, mefloquine, and atovaquone each were developed during or after a military conflict during 1945-1985. Tetracycline/doxycycline and clindamycin also have antimalarial activity. Use of any of these agents as monotherapy has led to drug resistance and treatment failure.
Artemisinin
Artemisinin (also known as qinghao su) and its derivatives are a new class of antimalarials derived from the sweet wormwood plant Artemisia annua. Initially developed in China in the 1970s, this class gained global attention in the 1990s. and have the fastest parasite clearance time, rapid resolution of symptoms, and an excellent safety profile. They have activity against all Plasmodium species.
Because of artemisinins’ rapid elimination, they are used in combination with an agent that also kills blood parasites but has a slower elimination rate and a different mechanism of action. The goal is to prevent and delay the development of resistance and reduce recrudescence. The superiority of artemisinin-based combination therapy (ACT) over monotherapies has been documented.
Resistance, always a concern, has remained limited to specific areas in Southeast Asia since reported in 2008. Monitoring drug efficacy, safety, quality of antimalarials is ongoing, as is discouraging monotherapy use of these agents. Globally, artemisinins are the mainstay of treatment. Spread of resistance would be a major setback for both malaria control and elimination.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Malaria remains a major international public health concern. In 2015, the World Health Organization estimated that 212 million individuals were infected and that there were 429,000 deaths. This represents a 21% decline in incidence globally and a 29% decline in global mortality between 2010 and 2015. In 2016, malaria was endemic in 91 countries and territories, down from 108 in 2000. Although malaria has been eliminated from the United States since the early 1950s, approximately 1,700 cases are reported annually, most of which occur in returned travelers, according to the Centers for Disease Control and Prevention.
Five species of Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale, and, more recently, P. knowelsi) account for most of the infections in humans and are transmitted by the bite of an infected female Anopheles mosquito. The disease is rarely acquired by blood transfusion, by needle sharing, by organ transplantation, or congenitally. Once diagnosed, malaria can be treated; however, delay in initiating therapy can lead to both serious and fatal outcomes.
Treatment
Historically, drug development was driven by the need to protect the military. While quinine was isolated from the bark of the cinchona tree in 1820, chloroquine, proguanil, mefloquine, and atovaquone each were developed during or after a military conflict during 1945-1985. Tetracycline/doxycycline and clindamycin also have antimalarial activity. Use of any of these agents as monotherapy has led to drug resistance and treatment failure.
Artemisinin
Artemisinin (also known as qinghao su) and its derivatives are a new class of antimalarials derived from the sweet wormwood plant Artemisia annua. Initially developed in China in the 1970s, this class gained global attention in the 1990s. and have the fastest parasite clearance time, rapid resolution of symptoms, and an excellent safety profile. They have activity against all Plasmodium species.
Because of artemisinins’ rapid elimination, they are used in combination with an agent that also kills blood parasites but has a slower elimination rate and a different mechanism of action. The goal is to prevent and delay the development of resistance and reduce recrudescence. The superiority of artemisinin-based combination therapy (ACT) over monotherapies has been documented.
Resistance, always a concern, has remained limited to specific areas in Southeast Asia since reported in 2008. Monitoring drug efficacy, safety, quality of antimalarials is ongoing, as is discouraging monotherapy use of these agents. Globally, artemisinins are the mainstay of treatment. Spread of resistance would be a major setback for both malaria control and elimination.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
A ‘game changer’ for pediatric HIV
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at [email protected].
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at [email protected].
In memory of Anne Marie Regan, CPNP, senior research coordinator, Pediatric HIV Program, Boston City Hospital
Our first child with perinatal HIV presented in 1985 at age 4 weeks with failure to thrive, vomiting, diarrhea, and thrush. Over the next several years, the number of HIV-infected infants grew exponentially, and by 1991, we were caring for more than 50 infants and children at Boston City Hospital.
Antiretrovirals were marginally effective for HIV-infected infants and children at this time. Subsequently, we embarked on a national effort to prevent vertical transmission. We participated first in the study of pharmacokinetics of zidovudine (AZT) in newborns. We enrolled patients in ACTG 076 to test the hypothesis that treatment with AZT during pregnancy and labor, and in the infant, would reduce the risk of vertical transmission. Fifty U.S. and nine French sites enrolled 473 women between April 1991 and December 20, 1993. The results were spectacular; 8 of 100 infants in the AZT treatment group, compared with 25 out of 100 infants in the control group, developed HIV. By 1995, HIV testing was offered to all women at Boston Medical Center (formerly Boston City Hospital), and the promise of prevention of vertical transmission was reaching fruition. Between 1996 and 2016, approximately 500 HIV-infected women delivered at Boston Medical Center with vertical transmission identified in only 6 (1.2%) infants; without ACTG 076, we would have expected 125! In 2013, the Centers for Disease Control and Prevention reported that 70% of pregnant HIV-infected women received the complete 076 regimen, and 93% of mothers or infants received some part of the regimen. In 1992, 900 HIV-infected infants were diagnosed in the United States, and as many as 2,000 newborns were estimated to have been born infected with HIV; in 2015, 86 vertical transmissions were identified. This was, and remains, a remarkable accomplishment.
Dr. Pelton is chief of pediatric infectious diseases and coordinator of the maternal-child HIV program at Boston Medical Center. Ms. Moloney is a certified pediatric nurse practitioner in the division of pediatric infectious diseases. Dr. Pelton said he had no relevant financial disclosures, and Ms. Moloney is a speaker (on vaccines) for Sanofi Pasteur. Email them at [email protected].
Salmonella infections: The source may be as close as your patient’s backyard
I recently received a group text from a friend voicing her frustration that her neighbor had acquired chickens, and she shared a photo of some roaming freely in the front yard. Naturally, my response was related to the potential infectious disease exposure and infections. Another friend chimed in “fresh eggs, and these are free range chickens. They don’t get sick. ... Many people in my area have chickens.” Unbeknownst to my friends, they had helped me select the ID Consult topic for this month.
Nontyphoidal Salmonella bacteria are associated with a wide spectrum of infections which range from asymptomatic gastrointestinal carriage to bacteremia, meningitis, osteomyelitis, and focal infections. Invasive disease is seen most often in children younger than 5 years of age, persons aged 65 years or older, and individuals with hemoglobinopathies including sickle cell disease and those with immunodeficiencies. Annually, the Centers for Disease Control and Prevention estimates that nontyphoidal salmonellosis is responsible for 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths in the United States. Gastroenteritis is the most common manifestation of the disease and is characterized by abdominal cramps, diarrhea, and fever that develops 12-72 hours after exposure. It is usually self-limited. As previously reported in this column (June, 2017), Salmonella is one of the top two foodborne pathogens in the United States, and most outbreaks have been associated with consumption of contaminated food. But wait, contaminated food is not the only cause of some of our most recent outbreaks.
Live poultry-associated salmonellosis (LPAS)
LPAS was first reported in the 1950s. More recent epidemiologic data was published by C. Basler et al. (Emerging Infect Dis. 2016;22[10]:1705-11). LPAS was defined as two or more culture confirmed human Salmonella infections with a combination of epidemiologic, laboratory, or traceback evidence linking illnesses to contact with live poultry. The median outbreak size involved 26 cases (range, 4-363) and 77% (41 of 53) were multistate. The median age of the patients was 9 years (range, less than 1 to 92 years), and 31% were aged 5 years or younger. Exposure to chicks and ducklings was reported in 85% and 38%, respectively. High-risk practices included keeping poultry inside of the home (46%), snuggling baby birds (49%), and kissing baby birds (13%). The median time from purchase of poultry to onset of illness was 17 days (range, 1-672), and 66% reported onset of illness less than 30 days after purchase. Almost 52% reported owning poultry for less than 1 year.
The number of outbreaks continued to increase. From 1990 to 2005, there were a total of 17 outbreaks, compared with 36 between 2006 and 2014. Historically, outbreaks occurred in children around Easter when brightly colored dyed chicks were purchased. In the above review, 80% of outbreaks began in February, March, or April with an average duration of 4.9 months (range, 1-12).
Salmonella isolates
Backyard flocks and LPAS
More recently outbreaks have been associated with backyard flocks occurring year round and affecting both adults and children in contrast to seasonal peaks. The first multistate backyard flock outbreak was documented in 2007. Currently, the CDC is investigating 10 separate multistate outbreaks that began on Jan. 4, 2017. It involves 48 states, 961 infected individuals, 215 hospitalizations, and 1 death. At least 5 salmonella serotypes have been isolated.
What about the hatcheries?
It’s estimated that 50 million live poultry are sold annually. Birds are shipped within 24 hours after hatching via the U.S. Postal Service in boxes containing up to 100 chicks. Delivery occurs within 72 hours of hatching. Approximately 20 mail order hatcheries provide the majority of poultry sold to the general public. The National Poultry Improvement Plan (NPIP) is a voluntary state and federal testing and certification program whose goal is to eliminate poultry disease from breeder flocks to prevent egg-transmitted and hatchery-disseminated diseases. All hatcheries may participate. They also may participate in the voluntary Salmonella monitoring program. Note participation is not mandatory.
Preventing future outbreaks: patient/parental education is mandatory
1. Make sure your parents know about the association of Salmonella and live poultry. Reinforce these are farm animals, not pets. Purchase birds from hatcheries that participate in NPIP and the Salmonella monitoring programs.
2. Chicks, ducklings, or other live poultry should not be taken to schools, day care facilities, or nursing homes. Poultry should not be allowed in the home or in areas where food or drink is being prepared or consumed.
3. Poultry should not be snuggled, kissed, or allowed to touch one’s mouth. Hand washing with soap and water should occur after touching live poultry or any object touched in areas where they live or roam.
4. Contact with live poultry should be avoided in those at risk for developing serious infections including persons aged 5 years or younger, 65 years or older, immunocompromised individuals, and those with hemoglobinopathies.
5. All equipment used to care for live birds should be washed outdoors. Owners should have designated shoes when caring for poultry which should never be worn inside the home.
Hopefully, the next time you see a patient with fever and diarrhea you will recall this topic and ask about their contact with live poultry.
Additional resources to facilitate discussions can be found at www.cdc.gov/salmonella.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
I recently received a group text from a friend voicing her frustration that her neighbor had acquired chickens, and she shared a photo of some roaming freely in the front yard. Naturally, my response was related to the potential infectious disease exposure and infections. Another friend chimed in “fresh eggs, and these are free range chickens. They don’t get sick. ... Many people in my area have chickens.” Unbeknownst to my friends, they had helped me select the ID Consult topic for this month.
Nontyphoidal Salmonella bacteria are associated with a wide spectrum of infections which range from asymptomatic gastrointestinal carriage to bacteremia, meningitis, osteomyelitis, and focal infections. Invasive disease is seen most often in children younger than 5 years of age, persons aged 65 years or older, and individuals with hemoglobinopathies including sickle cell disease and those with immunodeficiencies. Annually, the Centers for Disease Control and Prevention estimates that nontyphoidal salmonellosis is responsible for 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths in the United States. Gastroenteritis is the most common manifestation of the disease and is characterized by abdominal cramps, diarrhea, and fever that develops 12-72 hours after exposure. It is usually self-limited. As previously reported in this column (June, 2017), Salmonella is one of the top two foodborne pathogens in the United States, and most outbreaks have been associated with consumption of contaminated food. But wait, contaminated food is not the only cause of some of our most recent outbreaks.
Live poultry-associated salmonellosis (LPAS)
LPAS was first reported in the 1950s. More recent epidemiologic data was published by C. Basler et al. (Emerging Infect Dis. 2016;22[10]:1705-11). LPAS was defined as two or more culture confirmed human Salmonella infections with a combination of epidemiologic, laboratory, or traceback evidence linking illnesses to contact with live poultry. The median outbreak size involved 26 cases (range, 4-363) and 77% (41 of 53) were multistate. The median age of the patients was 9 years (range, less than 1 to 92 years), and 31% were aged 5 years or younger. Exposure to chicks and ducklings was reported in 85% and 38%, respectively. High-risk practices included keeping poultry inside of the home (46%), snuggling baby birds (49%), and kissing baby birds (13%). The median time from purchase of poultry to onset of illness was 17 days (range, 1-672), and 66% reported onset of illness less than 30 days after purchase. Almost 52% reported owning poultry for less than 1 year.
The number of outbreaks continued to increase. From 1990 to 2005, there were a total of 17 outbreaks, compared with 36 between 2006 and 2014. Historically, outbreaks occurred in children around Easter when brightly colored dyed chicks were purchased. In the above review, 80% of outbreaks began in February, March, or April with an average duration of 4.9 months (range, 1-12).
Salmonella isolates
Backyard flocks and LPAS
More recently outbreaks have been associated with backyard flocks occurring year round and affecting both adults and children in contrast to seasonal peaks. The first multistate backyard flock outbreak was documented in 2007. Currently, the CDC is investigating 10 separate multistate outbreaks that began on Jan. 4, 2017. It involves 48 states, 961 infected individuals, 215 hospitalizations, and 1 death. At least 5 salmonella serotypes have been isolated.
What about the hatcheries?
It’s estimated that 50 million live poultry are sold annually. Birds are shipped within 24 hours after hatching via the U.S. Postal Service in boxes containing up to 100 chicks. Delivery occurs within 72 hours of hatching. Approximately 20 mail order hatcheries provide the majority of poultry sold to the general public. The National Poultry Improvement Plan (NPIP) is a voluntary state and federal testing and certification program whose goal is to eliminate poultry disease from breeder flocks to prevent egg-transmitted and hatchery-disseminated diseases. All hatcheries may participate. They also may participate in the voluntary Salmonella monitoring program. Note participation is not mandatory.
Preventing future outbreaks: patient/parental education is mandatory
1. Make sure your parents know about the association of Salmonella and live poultry. Reinforce these are farm animals, not pets. Purchase birds from hatcheries that participate in NPIP and the Salmonella monitoring programs.
2. Chicks, ducklings, or other live poultry should not be taken to schools, day care facilities, or nursing homes. Poultry should not be allowed in the home or in areas where food or drink is being prepared or consumed.
3. Poultry should not be snuggled, kissed, or allowed to touch one’s mouth. Hand washing with soap and water should occur after touching live poultry or any object touched in areas where they live or roam.
4. Contact with live poultry should be avoided in those at risk for developing serious infections including persons aged 5 years or younger, 65 years or older, immunocompromised individuals, and those with hemoglobinopathies.
5. All equipment used to care for live birds should be washed outdoors. Owners should have designated shoes when caring for poultry which should never be worn inside the home.
Hopefully, the next time you see a patient with fever and diarrhea you will recall this topic and ask about their contact with live poultry.
Additional resources to facilitate discussions can be found at www.cdc.gov/salmonella.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
I recently received a group text from a friend voicing her frustration that her neighbor had acquired chickens, and she shared a photo of some roaming freely in the front yard. Naturally, my response was related to the potential infectious disease exposure and infections. Another friend chimed in “fresh eggs, and these are free range chickens. They don’t get sick. ... Many people in my area have chickens.” Unbeknownst to my friends, they had helped me select the ID Consult topic for this month.
Nontyphoidal Salmonella bacteria are associated with a wide spectrum of infections which range from asymptomatic gastrointestinal carriage to bacteremia, meningitis, osteomyelitis, and focal infections. Invasive disease is seen most often in children younger than 5 years of age, persons aged 65 years or older, and individuals with hemoglobinopathies including sickle cell disease and those with immunodeficiencies. Annually, the Centers for Disease Control and Prevention estimates that nontyphoidal salmonellosis is responsible for 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths in the United States. Gastroenteritis is the most common manifestation of the disease and is characterized by abdominal cramps, diarrhea, and fever that develops 12-72 hours after exposure. It is usually self-limited. As previously reported in this column (June, 2017), Salmonella is one of the top two foodborne pathogens in the United States, and most outbreaks have been associated with consumption of contaminated food. But wait, contaminated food is not the only cause of some of our most recent outbreaks.
Live poultry-associated salmonellosis (LPAS)
LPAS was first reported in the 1950s. More recent epidemiologic data was published by C. Basler et al. (Emerging Infect Dis. 2016;22[10]:1705-11). LPAS was defined as two or more culture confirmed human Salmonella infections with a combination of epidemiologic, laboratory, or traceback evidence linking illnesses to contact with live poultry. The median outbreak size involved 26 cases (range, 4-363) and 77% (41 of 53) were multistate. The median age of the patients was 9 years (range, less than 1 to 92 years), and 31% were aged 5 years or younger. Exposure to chicks and ducklings was reported in 85% and 38%, respectively. High-risk practices included keeping poultry inside of the home (46%), snuggling baby birds (49%), and kissing baby birds (13%). The median time from purchase of poultry to onset of illness was 17 days (range, 1-672), and 66% reported onset of illness less than 30 days after purchase. Almost 52% reported owning poultry for less than 1 year.
The number of outbreaks continued to increase. From 1990 to 2005, there were a total of 17 outbreaks, compared with 36 between 2006 and 2014. Historically, outbreaks occurred in children around Easter when brightly colored dyed chicks were purchased. In the above review, 80% of outbreaks began in February, March, or April with an average duration of 4.9 months (range, 1-12).
Salmonella isolates
Backyard flocks and LPAS
More recently outbreaks have been associated with backyard flocks occurring year round and affecting both adults and children in contrast to seasonal peaks. The first multistate backyard flock outbreak was documented in 2007. Currently, the CDC is investigating 10 separate multistate outbreaks that began on Jan. 4, 2017. It involves 48 states, 961 infected individuals, 215 hospitalizations, and 1 death. At least 5 salmonella serotypes have been isolated.
What about the hatcheries?
It’s estimated that 50 million live poultry are sold annually. Birds are shipped within 24 hours after hatching via the U.S. Postal Service in boxes containing up to 100 chicks. Delivery occurs within 72 hours of hatching. Approximately 20 mail order hatcheries provide the majority of poultry sold to the general public. The National Poultry Improvement Plan (NPIP) is a voluntary state and federal testing and certification program whose goal is to eliminate poultry disease from breeder flocks to prevent egg-transmitted and hatchery-disseminated diseases. All hatcheries may participate. They also may participate in the voluntary Salmonella monitoring program. Note participation is not mandatory.
Preventing future outbreaks: patient/parental education is mandatory
1. Make sure your parents know about the association of Salmonella and live poultry. Reinforce these are farm animals, not pets. Purchase birds from hatcheries that participate in NPIP and the Salmonella monitoring programs.
2. Chicks, ducklings, or other live poultry should not be taken to schools, day care facilities, or nursing homes. Poultry should not be allowed in the home or in areas where food or drink is being prepared or consumed.
3. Poultry should not be snuggled, kissed, or allowed to touch one’s mouth. Hand washing with soap and water should occur after touching live poultry or any object touched in areas where they live or roam.
4. Contact with live poultry should be avoided in those at risk for developing serious infections including persons aged 5 years or younger, 65 years or older, immunocompromised individuals, and those with hemoglobinopathies.
5. All equipment used to care for live birds should be washed outdoors. Owners should have designated shoes when caring for poultry which should never be worn inside the home.
Hopefully, the next time you see a patient with fever and diarrhea you will recall this topic and ask about their contact with live poultry.
Additional resources to facilitate discussions can be found at www.cdc.gov/salmonella.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
Alternative birthing practices increase risk of infection
All three have been associated with sporadic, serious neonatal infections.
The U.S. prevalence of water births – delivering a baby underwater – is currently unknown, but in the United Kingdom the practice is common. According to a 2015 National Health Service maternity survey, approximately 9% of women who underwent vaginal delivery opted for water birth (Arch Dis Child Fetal Neonatal Ed. 2016 Jul;101[4]:F357-65). Both the Royal College of Obstetricians and Gynaecologists and the Royal College of Midwives endorse this practice for healthy women with uncomplicated term pregnancies. According to a 2009 Cochrane Review, immersion during the first phase of labor reduces the use of epidural/spinal analgesia (Cochrane Database Syst Rev. 2009. doi: 10.1002/14651858.CD000111.pub3). The maternal benefits of delivery under water, though, have not been clearly defined.
Legionella pneumophila is an uncommon pathogen in children, but cases of neonatal Legionnaires’ disease have been reported after water birth. Two affected babies born in Arizona in 2016 were successfully treated and survived (MMWR Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6622a4). A baby born in Texas in 2014 died of sepsis and respiratory failure (Emerg Infect Dis. 2015. doi: 10.3201/eid2101.140846). Canadian investigators have reported fatal disseminated herpes simplex virus infection in an infant after water birth; the mother had herpetic whitlow and a recent blister concerning for HSV on her thigh (J Pediatric Infect Dis Soc. 2017 May 16. doi: 10.1093/jpids/pix035).
Admittedly, each of these cases might have been prevented by adherence to recommended infection control practices, and the absolute risk of infection after water birth is unknown and likely to be small. Still, neither the American Academy of Pediatrics nor the American College of Obstetricians and Gynecologists currently recommend the practice. ACOG suggests that “births occur on land, not in water” and has called for well-designed, prospective studies of the maternal and perinatal benefits and risks associated with immersion during labor and delivery (Obstet Gynecol. 2016;128:1198-9).
Placentophagia – consuming the placenta after birth – has been promoted by celebrity moms, including Katherine Heigl and Kourtney Kardashian. Placenta can be cooked, blended raw into a smoothie, or dehydrated and encapsulated.
Proponents of placentophagia claim health benefits of this practice, including improved mood and energy, and increased breast milk production. There are few published data to support these claims. A recent case report suggests the practice has the potential to harm the baby. In June 2017, Oregon public health authorities described a neonate with recurrent episodes of group B streptococcal (GBS) bacteremia. An identical strain of GBS was cultured from capsules containing the mother’s dehydrated placenta – she had consumed six of the capsules daily beginning a few days after the baby’s birth. According to the Morbidity and Mortality Weekly Report communication, “no standards exist for processing placenta for consumption” and the “placenta encapsulation process does not eradicate infectious pathogens per se. … Placenta capsule ingestion should be avoided”(MMWR Morb Mortal Wkly Rep. 2017;66:677-8. doi: 10.15585/mmwr.mm6625a4).
Finally, the ritual practice of umbilical cord nonseverance or lotus birth deserves a mention. In a lotus birth, the umbilical cord is left uncut, allowing the placenta to remain attached to the baby until the cord dries and naturally separates, generally 3-10 days after delivery. Describing a spiritual connection between the baby and the placenta, proponents claim lotus birth promotes bonding and allows for a gentler transition between intra- and extrauterine life.
A review of PubMed turned up no formal studies of this practice, but case reports describe complications such as neonatal idiopathic hepatitis and neonatal sepsis. The Royal College of Obstetricians and Gynaecologists has issued a warning about lotus births, advising that babies be monitored closely for infection. RCOG spokesperson Dr. Patrick O’Brien said in a 2008 statement, “If left for a period of time after the birth, there is a risk of infection in the placenta which can consequently spread to the baby. The placenta is particularly prone to infection as it contains blood. Within a short time after birth, once the umbilical cord has stopped pulsating, the placenta has no circulation and is essentially dead tissue.”
Interestingly, a quick scan of Etsy, the popular e-commerce website, turned up a number of lotus birth kits for sale. These generally contain a decorative cloth bag as well as an herb mix containing lavender and eucalyptus to promote drying and mask the smell of the decomposing placenta.
In contrast, many pediatricians, me included, are not well informed about these practices and don’t routinely ask expectant moms about their plans. I propose that we can advocate for our patients-to-be by learning about these practices so that we can engage in an honest, respectful discussion about potential risks and benefits. For me, for now, the risks outweigh the benefits.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
All three have been associated with sporadic, serious neonatal infections.
The U.S. prevalence of water births – delivering a baby underwater – is currently unknown, but in the United Kingdom the practice is common. According to a 2015 National Health Service maternity survey, approximately 9% of women who underwent vaginal delivery opted for water birth (Arch Dis Child Fetal Neonatal Ed. 2016 Jul;101[4]:F357-65). Both the Royal College of Obstetricians and Gynaecologists and the Royal College of Midwives endorse this practice for healthy women with uncomplicated term pregnancies. According to a 2009 Cochrane Review, immersion during the first phase of labor reduces the use of epidural/spinal analgesia (Cochrane Database Syst Rev. 2009. doi: 10.1002/14651858.CD000111.pub3). The maternal benefits of delivery under water, though, have not been clearly defined.
Legionella pneumophila is an uncommon pathogen in children, but cases of neonatal Legionnaires’ disease have been reported after water birth. Two affected babies born in Arizona in 2016 were successfully treated and survived (MMWR Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6622a4). A baby born in Texas in 2014 died of sepsis and respiratory failure (Emerg Infect Dis. 2015. doi: 10.3201/eid2101.140846). Canadian investigators have reported fatal disseminated herpes simplex virus infection in an infant after water birth; the mother had herpetic whitlow and a recent blister concerning for HSV on her thigh (J Pediatric Infect Dis Soc. 2017 May 16. doi: 10.1093/jpids/pix035).
Admittedly, each of these cases might have been prevented by adherence to recommended infection control practices, and the absolute risk of infection after water birth is unknown and likely to be small. Still, neither the American Academy of Pediatrics nor the American College of Obstetricians and Gynecologists currently recommend the practice. ACOG suggests that “births occur on land, not in water” and has called for well-designed, prospective studies of the maternal and perinatal benefits and risks associated with immersion during labor and delivery (Obstet Gynecol. 2016;128:1198-9).
Placentophagia – consuming the placenta after birth – has been promoted by celebrity moms, including Katherine Heigl and Kourtney Kardashian. Placenta can be cooked, blended raw into a smoothie, or dehydrated and encapsulated.
Proponents of placentophagia claim health benefits of this practice, including improved mood and energy, and increased breast milk production. There are few published data to support these claims. A recent case report suggests the practice has the potential to harm the baby. In June 2017, Oregon public health authorities described a neonate with recurrent episodes of group B streptococcal (GBS) bacteremia. An identical strain of GBS was cultured from capsules containing the mother’s dehydrated placenta – she had consumed six of the capsules daily beginning a few days after the baby’s birth. According to the Morbidity and Mortality Weekly Report communication, “no standards exist for processing placenta for consumption” and the “placenta encapsulation process does not eradicate infectious pathogens per se. … Placenta capsule ingestion should be avoided”(MMWR Morb Mortal Wkly Rep. 2017;66:677-8. doi: 10.15585/mmwr.mm6625a4).
Finally, the ritual practice of umbilical cord nonseverance or lotus birth deserves a mention. In a lotus birth, the umbilical cord is left uncut, allowing the placenta to remain attached to the baby until the cord dries and naturally separates, generally 3-10 days after delivery. Describing a spiritual connection between the baby and the placenta, proponents claim lotus birth promotes bonding and allows for a gentler transition between intra- and extrauterine life.
A review of PubMed turned up no formal studies of this practice, but case reports describe complications such as neonatal idiopathic hepatitis and neonatal sepsis. The Royal College of Obstetricians and Gynaecologists has issued a warning about lotus births, advising that babies be monitored closely for infection. RCOG spokesperson Dr. Patrick O’Brien said in a 2008 statement, “If left for a period of time after the birth, there is a risk of infection in the placenta which can consequently spread to the baby. The placenta is particularly prone to infection as it contains blood. Within a short time after birth, once the umbilical cord has stopped pulsating, the placenta has no circulation and is essentially dead tissue.”
Interestingly, a quick scan of Etsy, the popular e-commerce website, turned up a number of lotus birth kits for sale. These generally contain a decorative cloth bag as well as an herb mix containing lavender and eucalyptus to promote drying and mask the smell of the decomposing placenta.
In contrast, many pediatricians, me included, are not well informed about these practices and don’t routinely ask expectant moms about their plans. I propose that we can advocate for our patients-to-be by learning about these practices so that we can engage in an honest, respectful discussion about potential risks and benefits. For me, for now, the risks outweigh the benefits.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
All three have been associated with sporadic, serious neonatal infections.
The U.S. prevalence of water births – delivering a baby underwater – is currently unknown, but in the United Kingdom the practice is common. According to a 2015 National Health Service maternity survey, approximately 9% of women who underwent vaginal delivery opted for water birth (Arch Dis Child Fetal Neonatal Ed. 2016 Jul;101[4]:F357-65). Both the Royal College of Obstetricians and Gynaecologists and the Royal College of Midwives endorse this practice for healthy women with uncomplicated term pregnancies. According to a 2009 Cochrane Review, immersion during the first phase of labor reduces the use of epidural/spinal analgesia (Cochrane Database Syst Rev. 2009. doi: 10.1002/14651858.CD000111.pub3). The maternal benefits of delivery under water, though, have not been clearly defined.
Legionella pneumophila is an uncommon pathogen in children, but cases of neonatal Legionnaires’ disease have been reported after water birth. Two affected babies born in Arizona in 2016 were successfully treated and survived (MMWR Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6622a4). A baby born in Texas in 2014 died of sepsis and respiratory failure (Emerg Infect Dis. 2015. doi: 10.3201/eid2101.140846). Canadian investigators have reported fatal disseminated herpes simplex virus infection in an infant after water birth; the mother had herpetic whitlow and a recent blister concerning for HSV on her thigh (J Pediatric Infect Dis Soc. 2017 May 16. doi: 10.1093/jpids/pix035).
Admittedly, each of these cases might have been prevented by adherence to recommended infection control practices, and the absolute risk of infection after water birth is unknown and likely to be small. Still, neither the American Academy of Pediatrics nor the American College of Obstetricians and Gynecologists currently recommend the practice. ACOG suggests that “births occur on land, not in water” and has called for well-designed, prospective studies of the maternal and perinatal benefits and risks associated with immersion during labor and delivery (Obstet Gynecol. 2016;128:1198-9).
Placentophagia – consuming the placenta after birth – has been promoted by celebrity moms, including Katherine Heigl and Kourtney Kardashian. Placenta can be cooked, blended raw into a smoothie, or dehydrated and encapsulated.
Proponents of placentophagia claim health benefits of this practice, including improved mood and energy, and increased breast milk production. There are few published data to support these claims. A recent case report suggests the practice has the potential to harm the baby. In June 2017, Oregon public health authorities described a neonate with recurrent episodes of group B streptococcal (GBS) bacteremia. An identical strain of GBS was cultured from capsules containing the mother’s dehydrated placenta – she had consumed six of the capsules daily beginning a few days after the baby’s birth. According to the Morbidity and Mortality Weekly Report communication, “no standards exist for processing placenta for consumption” and the “placenta encapsulation process does not eradicate infectious pathogens per se. … Placenta capsule ingestion should be avoided”(MMWR Morb Mortal Wkly Rep. 2017;66:677-8. doi: 10.15585/mmwr.mm6625a4).
Finally, the ritual practice of umbilical cord nonseverance or lotus birth deserves a mention. In a lotus birth, the umbilical cord is left uncut, allowing the placenta to remain attached to the baby until the cord dries and naturally separates, generally 3-10 days after delivery. Describing a spiritual connection between the baby and the placenta, proponents claim lotus birth promotes bonding and allows for a gentler transition between intra- and extrauterine life.
A review of PubMed turned up no formal studies of this practice, but case reports describe complications such as neonatal idiopathic hepatitis and neonatal sepsis. The Royal College of Obstetricians and Gynaecologists has issued a warning about lotus births, advising that babies be monitored closely for infection. RCOG spokesperson Dr. Patrick O’Brien said in a 2008 statement, “If left for a period of time after the birth, there is a risk of infection in the placenta which can consequently spread to the baby. The placenta is particularly prone to infection as it contains blood. Within a short time after birth, once the umbilical cord has stopped pulsating, the placenta has no circulation and is essentially dead tissue.”
Interestingly, a quick scan of Etsy, the popular e-commerce website, turned up a number of lotus birth kits for sale. These generally contain a decorative cloth bag as well as an herb mix containing lavender and eucalyptus to promote drying and mask the smell of the decomposing placenta.
In contrast, many pediatricians, me included, are not well informed about these practices and don’t routinely ask expectant moms about their plans. I propose that we can advocate for our patients-to-be by learning about these practices so that we can engage in an honest, respectful discussion about potential risks and benefits. For me, for now, the risks outweigh the benefits.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
PANS and PANDAS – A step forward?
In the Journal of Child and Adolescent Psychopharmacology’s July 2017 issue, a group of respected individuals representing diverse expertise published “guidelines” to support clinical management of pediatric acute-onset neuropsychiatric syndrome (PANS) and its subclass PANDAS (those associated with streptococcal infection). PANS represents an enigmatic clinical syndrome that includes abrupt onset of obsessive-compulsive disorder (OCD) or eating restriction in combination with anxiety, attention deficit, hyperkinesia, emotional lability, irritability, aggressive or oppositional behavior, or academic decline. Neurologic findings also may be present; these are most often motor or vocal tics, but choreiform movements of the finger (repetitive motions that are rapid, jerky, and involuntary), deteriorating penmanship, sleep disruptions, or urinary frequency also may be present. The clinical course most often is relapsing and remitting with overall improvement over months or years.
(J Child Adolesc Psychopharmacol. 2017 Apr 7. doi: 10.1089/cap.2016.0151).
Specific recommendations include:
1. Searching for a coexisting infectious etiology with history, exam, and appropriate laboratory testing (including ASO and ADB antibodies), and, when present, treating accordingly. Even in the absence of definitive evidence of GAS infection, they recommend an initial course of antimicrobial therapy such as that given to patients with rheumatic fever.
2. For children with PANDAS (PANS with either culture or serologic evidence of GAS), consider instituting long-term streptococcal prophylaxis. The data on its value is mixed; however, most studies find more than 40% (and as many as 75%) of exacerbations are associated with GAS, and at least one study reports a reduction in neuropsychiatric exacerbations in children on penicillin or azithromycin prophylaxis for a 1-year period. Such decisions should be individualized: In children with strong evidence of exacerbations linked to GAS, there was thought to be greater likelihood of benefit, while, in those with no evidence for prior GAS infection, the potential for benefit was thought to be insufficient to justify prophylaxis. Furthermore, the optimal duration of prophylaxis is unknown. The guidelines recommend up to 2 years, but individualization is appropriate since severe cases may warrant prolonged prophylaxis.
3. In children who present with PANDAS and a positive throat culture for GAS, follow-up should be the same as that given for rheumatic fever, with reculture at 2-7 days and retreatment if there is persistence of GAS.
4. Vigilance for GAS infection in family members is appropriate, including obtaining throat cultures from persons with pharyngitis and treating them promptly when results are positive.
5. When GAS infection is not identified, the clinician should search for alternative infectious agents, such as Mycoplasma pneumoniae (using polymerase chain reaction on throat or nasopharyngeal swab), influenza virus, or alternative infections such as sinusitis, and treat accordingly.
6. Children with PANS and PANDAS should be immunized according to Advisory Committee of Immunization Practices recommendations, which includes annual influenza immunization. The committee reported that symptom flares after immunization were uncommon, brief, and manageable with NSAIDs.
7. The committee suggested that optimization of serum vitamin D levels among children with PANS and PANDAS could be of benefit, despite limited evidence. The committee members recommended treating children with PANS/PANDAS with vitamin D3 as needed to maintain serum 25-hydroxy vitamin D levels above 30 ng/mL. No benefit for adenotonsillectomy was identified. The committee recommended that tonsillectomy and/or adenoidectomy should limited to those with traditional indications (sleep apnea, failure to thrive, and abnormally large tonsils, etc.). The committee also found no evidence to suggest that probiotics modulate this condition.
These guidelines come with an important caveat. They represent a practical clinical approach for the management of infection in the context of PANS or PANDAS and rely heavily on the clinical experience of the members of the PANS/PANDAS Consortium. They provide criteria for the retrospective diagnosis of GAS infection and recommend treatment of GAS in all patients with newly diagnosed PANS. The suggested guidelines are supported by limited data and recognize that further prospective study of the mechanistic link between infection and PANS, clarification of the risk factors for development of PANS, and definitive study of the risks and benefits of antimicrobial prophylaxis are needed.
The consortium also has published two accompanying guidelines that address psychiatric (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0145) and immunomodulatory management (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0148) in the same issue of the Journal of Child and Adolescent Psychopharmacology.
Proposed criteria for documenting GAS infection in PANS pediatric patients
- A rise in serial antibody level, regardless of rapid test or culture result. This definition does not require clinical pharyngitis.
- Acute pharyngitis with a positive GAS throat culture, with or without a rising antibody level.
- Pharyngitis with characteristic palatal petechiae.
- Pharyngitis with a characteristic scarlatiniform rash.
- Pharyngitis without a throat swab or serology, but intimate (usually household) exposure to a proven GAS case.
- Asymptomatic pharyngeal colonization documented after an intimate exposure.
- Asymptomatic pharyngeal colonization after a negative throat swab documented within the prior 3-4 months.
- Single ASO or ADB antibody level within 6 months after the initial onset of neuropsychiatric symptoms may be accepted as positive if it is more than 95th percentile, using the laboratory’s normal standard for children of comparable age, or provisionally ASO greater than or equal to 1:480 or ADB greater than or equal to 1:1280.
- Both ASO and ADB are elevated at more than 80% percentile for age in the same serum sample within 6 months after the initial onset of neuropsychiatric symptoms.
- Culture-documented streptococcal dermatitis.
Source: J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0151.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
In the Journal of Child and Adolescent Psychopharmacology’s July 2017 issue, a group of respected individuals representing diverse expertise published “guidelines” to support clinical management of pediatric acute-onset neuropsychiatric syndrome (PANS) and its subclass PANDAS (those associated with streptococcal infection). PANS represents an enigmatic clinical syndrome that includes abrupt onset of obsessive-compulsive disorder (OCD) or eating restriction in combination with anxiety, attention deficit, hyperkinesia, emotional lability, irritability, aggressive or oppositional behavior, or academic decline. Neurologic findings also may be present; these are most often motor or vocal tics, but choreiform movements of the finger (repetitive motions that are rapid, jerky, and involuntary), deteriorating penmanship, sleep disruptions, or urinary frequency also may be present. The clinical course most often is relapsing and remitting with overall improvement over months or years.
(J Child Adolesc Psychopharmacol. 2017 Apr 7. doi: 10.1089/cap.2016.0151).
Specific recommendations include:
1. Searching for a coexisting infectious etiology with history, exam, and appropriate laboratory testing (including ASO and ADB antibodies), and, when present, treating accordingly. Even in the absence of definitive evidence of GAS infection, they recommend an initial course of antimicrobial therapy such as that given to patients with rheumatic fever.
2. For children with PANDAS (PANS with either culture or serologic evidence of GAS), consider instituting long-term streptococcal prophylaxis. The data on its value is mixed; however, most studies find more than 40% (and as many as 75%) of exacerbations are associated with GAS, and at least one study reports a reduction in neuropsychiatric exacerbations in children on penicillin or azithromycin prophylaxis for a 1-year period. Such decisions should be individualized: In children with strong evidence of exacerbations linked to GAS, there was thought to be greater likelihood of benefit, while, in those with no evidence for prior GAS infection, the potential for benefit was thought to be insufficient to justify prophylaxis. Furthermore, the optimal duration of prophylaxis is unknown. The guidelines recommend up to 2 years, but individualization is appropriate since severe cases may warrant prolonged prophylaxis.
3. In children who present with PANDAS and a positive throat culture for GAS, follow-up should be the same as that given for rheumatic fever, with reculture at 2-7 days and retreatment if there is persistence of GAS.
4. Vigilance for GAS infection in family members is appropriate, including obtaining throat cultures from persons with pharyngitis and treating them promptly when results are positive.
5. When GAS infection is not identified, the clinician should search for alternative infectious agents, such as Mycoplasma pneumoniae (using polymerase chain reaction on throat or nasopharyngeal swab), influenza virus, or alternative infections such as sinusitis, and treat accordingly.
6. Children with PANS and PANDAS should be immunized according to Advisory Committee of Immunization Practices recommendations, which includes annual influenza immunization. The committee reported that symptom flares after immunization were uncommon, brief, and manageable with NSAIDs.
7. The committee suggested that optimization of serum vitamin D levels among children with PANS and PANDAS could be of benefit, despite limited evidence. The committee members recommended treating children with PANS/PANDAS with vitamin D3 as needed to maintain serum 25-hydroxy vitamin D levels above 30 ng/mL. No benefit for adenotonsillectomy was identified. The committee recommended that tonsillectomy and/or adenoidectomy should limited to those with traditional indications (sleep apnea, failure to thrive, and abnormally large tonsils, etc.). The committee also found no evidence to suggest that probiotics modulate this condition.
These guidelines come with an important caveat. They represent a practical clinical approach for the management of infection in the context of PANS or PANDAS and rely heavily on the clinical experience of the members of the PANS/PANDAS Consortium. They provide criteria for the retrospective diagnosis of GAS infection and recommend treatment of GAS in all patients with newly diagnosed PANS. The suggested guidelines are supported by limited data and recognize that further prospective study of the mechanistic link between infection and PANS, clarification of the risk factors for development of PANS, and definitive study of the risks and benefits of antimicrobial prophylaxis are needed.
The consortium also has published two accompanying guidelines that address psychiatric (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0145) and immunomodulatory management (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0148) in the same issue of the Journal of Child and Adolescent Psychopharmacology.
Proposed criteria for documenting GAS infection in PANS pediatric patients
- A rise in serial antibody level, regardless of rapid test or culture result. This definition does not require clinical pharyngitis.
- Acute pharyngitis with a positive GAS throat culture, with or without a rising antibody level.
- Pharyngitis with characteristic palatal petechiae.
- Pharyngitis with a characteristic scarlatiniform rash.
- Pharyngitis without a throat swab or serology, but intimate (usually household) exposure to a proven GAS case.
- Asymptomatic pharyngeal colonization documented after an intimate exposure.
- Asymptomatic pharyngeal colonization after a negative throat swab documented within the prior 3-4 months.
- Single ASO or ADB antibody level within 6 months after the initial onset of neuropsychiatric symptoms may be accepted as positive if it is more than 95th percentile, using the laboratory’s normal standard for children of comparable age, or provisionally ASO greater than or equal to 1:480 or ADB greater than or equal to 1:1280.
- Both ASO and ADB are elevated at more than 80% percentile for age in the same serum sample within 6 months after the initial onset of neuropsychiatric symptoms.
- Culture-documented streptococcal dermatitis.
Source: J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0151.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
In the Journal of Child and Adolescent Psychopharmacology’s July 2017 issue, a group of respected individuals representing diverse expertise published “guidelines” to support clinical management of pediatric acute-onset neuropsychiatric syndrome (PANS) and its subclass PANDAS (those associated with streptococcal infection). PANS represents an enigmatic clinical syndrome that includes abrupt onset of obsessive-compulsive disorder (OCD) or eating restriction in combination with anxiety, attention deficit, hyperkinesia, emotional lability, irritability, aggressive or oppositional behavior, or academic decline. Neurologic findings also may be present; these are most often motor or vocal tics, but choreiform movements of the finger (repetitive motions that are rapid, jerky, and involuntary), deteriorating penmanship, sleep disruptions, or urinary frequency also may be present. The clinical course most often is relapsing and remitting with overall improvement over months or years.
(J Child Adolesc Psychopharmacol. 2017 Apr 7. doi: 10.1089/cap.2016.0151).
Specific recommendations include:
1. Searching for a coexisting infectious etiology with history, exam, and appropriate laboratory testing (including ASO and ADB antibodies), and, when present, treating accordingly. Even in the absence of definitive evidence of GAS infection, they recommend an initial course of antimicrobial therapy such as that given to patients with rheumatic fever.
2. For children with PANDAS (PANS with either culture or serologic evidence of GAS), consider instituting long-term streptococcal prophylaxis. The data on its value is mixed; however, most studies find more than 40% (and as many as 75%) of exacerbations are associated with GAS, and at least one study reports a reduction in neuropsychiatric exacerbations in children on penicillin or azithromycin prophylaxis for a 1-year period. Such decisions should be individualized: In children with strong evidence of exacerbations linked to GAS, there was thought to be greater likelihood of benefit, while, in those with no evidence for prior GAS infection, the potential for benefit was thought to be insufficient to justify prophylaxis. Furthermore, the optimal duration of prophylaxis is unknown. The guidelines recommend up to 2 years, but individualization is appropriate since severe cases may warrant prolonged prophylaxis.
3. In children who present with PANDAS and a positive throat culture for GAS, follow-up should be the same as that given for rheumatic fever, with reculture at 2-7 days and retreatment if there is persistence of GAS.
4. Vigilance for GAS infection in family members is appropriate, including obtaining throat cultures from persons with pharyngitis and treating them promptly when results are positive.
5. When GAS infection is not identified, the clinician should search for alternative infectious agents, such as Mycoplasma pneumoniae (using polymerase chain reaction on throat or nasopharyngeal swab), influenza virus, or alternative infections such as sinusitis, and treat accordingly.
6. Children with PANS and PANDAS should be immunized according to Advisory Committee of Immunization Practices recommendations, which includes annual influenza immunization. The committee reported that symptom flares after immunization were uncommon, brief, and manageable with NSAIDs.
7. The committee suggested that optimization of serum vitamin D levels among children with PANS and PANDAS could be of benefit, despite limited evidence. The committee members recommended treating children with PANS/PANDAS with vitamin D3 as needed to maintain serum 25-hydroxy vitamin D levels above 30 ng/mL. No benefit for adenotonsillectomy was identified. The committee recommended that tonsillectomy and/or adenoidectomy should limited to those with traditional indications (sleep apnea, failure to thrive, and abnormally large tonsils, etc.). The committee also found no evidence to suggest that probiotics modulate this condition.
These guidelines come with an important caveat. They represent a practical clinical approach for the management of infection in the context of PANS or PANDAS and rely heavily on the clinical experience of the members of the PANS/PANDAS Consortium. They provide criteria for the retrospective diagnosis of GAS infection and recommend treatment of GAS in all patients with newly diagnosed PANS. The suggested guidelines are supported by limited data and recognize that further prospective study of the mechanistic link between infection and PANS, clarification of the risk factors for development of PANS, and definitive study of the risks and benefits of antimicrobial prophylaxis are needed.
The consortium also has published two accompanying guidelines that address psychiatric (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0145) and immunomodulatory management (J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0148) in the same issue of the Journal of Child and Adolescent Psychopharmacology.
Proposed criteria for documenting GAS infection in PANS pediatric patients
- A rise in serial antibody level, regardless of rapid test or culture result. This definition does not require clinical pharyngitis.
- Acute pharyngitis with a positive GAS throat culture, with or without a rising antibody level.
- Pharyngitis with characteristic palatal petechiae.
- Pharyngitis with a characteristic scarlatiniform rash.
- Pharyngitis without a throat swab or serology, but intimate (usually household) exposure to a proven GAS case.
- Asymptomatic pharyngeal colonization documented after an intimate exposure.
- Asymptomatic pharyngeal colonization after a negative throat swab documented within the prior 3-4 months.
- Single ASO or ADB antibody level within 6 months after the initial onset of neuropsychiatric symptoms may be accepted as positive if it is more than 95th percentile, using the laboratory’s normal standard for children of comparable age, or provisionally ASO greater than or equal to 1:480 or ADB greater than or equal to 1:1280.
- Both ASO and ADB are elevated at more than 80% percentile for age in the same serum sample within 6 months after the initial onset of neuropsychiatric symptoms.
- Culture-documented streptococcal dermatitis.
Source: J Child Adolesc Psychopharmacol. 2017. doi: 10.1089/cap.2016.0151.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. Email him at [email protected].
Hepatitis C is a pediatric disease now
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].