User login
Experts address barriers to genetic screening
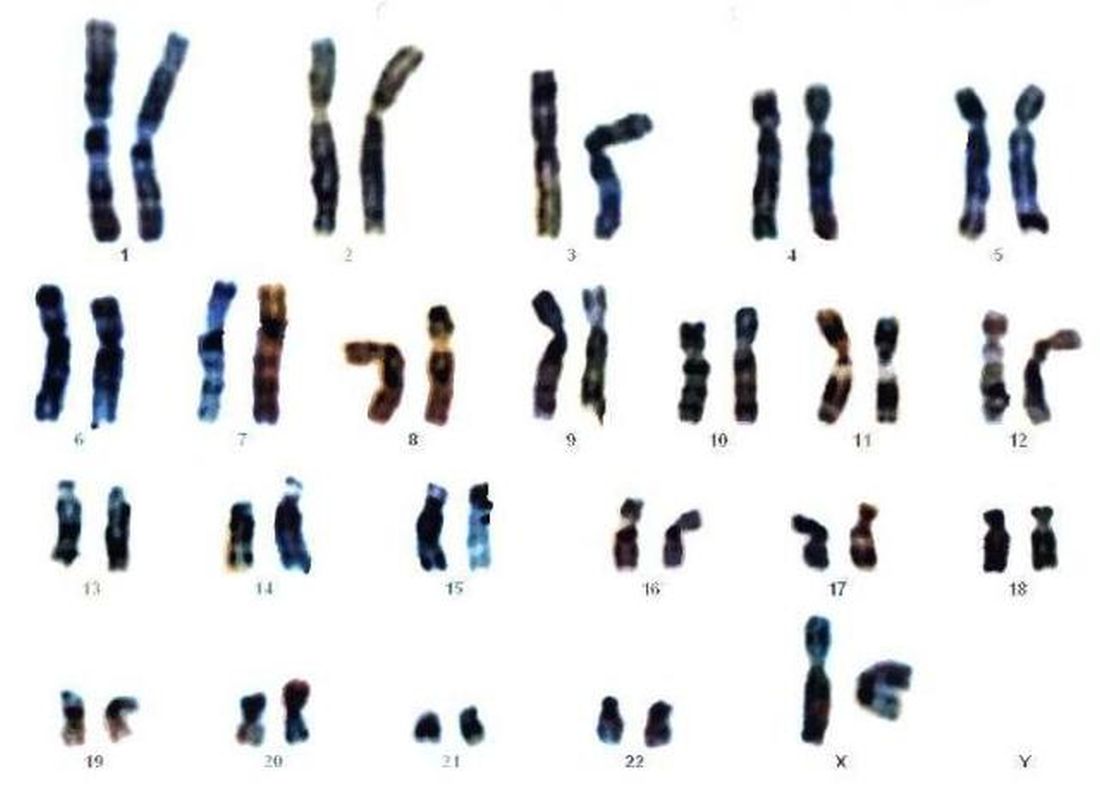
WASHINGTON – Early diagnosis and intervention for genetic diseases using the latest carrier screening can allow families to be prepared and informed prior to pregnancy, said Aishwarya Arjunan, MS, MPH, a clinical product specialist for carrier screening at Myriad Women’s Health, part of a diagnostic testing company based in Salt Lake City, Utah.
“Rare diseases are responsible for 35% of deaths in the first year of life,” she said in a panel discussion at the Rare Diseases and Orphan Products Breakthrough Summit sponsored by the National Organization for Rare Disorders.
Most patients with rare diseases go through a “diagnostic odyssey” lasting an average of 8 years before they receive an accurate diagnosis, she said. During this time, data suggest that they have likely been misdiagnosed three times and have seen more than 10 specialists, she added.
Barriers to genetic screening include limited access to genetics professionals, lack of patient and provider education about screening, issues of insurance coverage and reimbursement, coding challenges, and misperceptions about the perceived impact of screening, noted Jodie Vento, manager of the Center for Rare Disease Therapy at the Children’s Hospital of Pittsburgh.
The genetic carrier screening options, often referred to as panethnic expanded carrier screening, represents a change from previous screening protocols based on ethnicity, said Ms. Arjunan. However, guidelines for screening based on ethnicity “misses a significant percentage of pregnancies affected by serious conditions and widens the health disparity gap,” she said.
By contrast, expanded carrier screening allows for standardization of care that gives couples and families information to make decisions and preparations.
Current genetic testing strategies include single gene testing, in which a single gene of interest is tested; multigene panel testing, in which a subset of clinically important genes are tested; whole-exome sequencing, in which the DNA responsible for coding proteins is tested; and whole-genome sequencing, in which the entire human genome is tested for genetic disorders.
Improving access to genetic testing involves a combination of provider education, changes in payer policies, action by advocacy groups, and adjustment of societal guidelines, said Ms. Arjunan. However, the advantages of expanded carrier screening are many and include guiding patients to expert care early and setting up plans for long-term care and follow-up, she noted. In addition, early identification through screening can help patients reduce or eliminate the diagnostic odyssey and connect with advocacy and community groups for support, she concluded.
The presenters had no financial conflicts to disclose.
WASHINGTON – Early diagnosis and intervention for genetic diseases using the latest carrier screening can allow families to be prepared and informed prior to pregnancy, said Aishwarya Arjunan, MS, MPH, a clinical product specialist for carrier screening at Myriad Women’s Health, part of a diagnostic testing company based in Salt Lake City, Utah.
“Rare diseases are responsible for 35% of deaths in the first year of life,” she said in a panel discussion at the Rare Diseases and Orphan Products Breakthrough Summit sponsored by the National Organization for Rare Disorders.
Most patients with rare diseases go through a “diagnostic odyssey” lasting an average of 8 years before they receive an accurate diagnosis, she said. During this time, data suggest that they have likely been misdiagnosed three times and have seen more than 10 specialists, she added.
Barriers to genetic screening include limited access to genetics professionals, lack of patient and provider education about screening, issues of insurance coverage and reimbursement, coding challenges, and misperceptions about the perceived impact of screening, noted Jodie Vento, manager of the Center for Rare Disease Therapy at the Children’s Hospital of Pittsburgh.
The genetic carrier screening options, often referred to as panethnic expanded carrier screening, represents a change from previous screening protocols based on ethnicity, said Ms. Arjunan. However, guidelines for screening based on ethnicity “misses a significant percentage of pregnancies affected by serious conditions and widens the health disparity gap,” she said.
By contrast, expanded carrier screening allows for standardization of care that gives couples and families information to make decisions and preparations.
Current genetic testing strategies include single gene testing, in which a single gene of interest is tested; multigene panel testing, in which a subset of clinically important genes are tested; whole-exome sequencing, in which the DNA responsible for coding proteins is tested; and whole-genome sequencing, in which the entire human genome is tested for genetic disorders.
Improving access to genetic testing involves a combination of provider education, changes in payer policies, action by advocacy groups, and adjustment of societal guidelines, said Ms. Arjunan. However, the advantages of expanded carrier screening are many and include guiding patients to expert care early and setting up plans for long-term care and follow-up, she noted. In addition, early identification through screening can help patients reduce or eliminate the diagnostic odyssey and connect with advocacy and community groups for support, she concluded.
The presenters had no financial conflicts to disclose.
WASHINGTON – Early diagnosis and intervention for genetic diseases using the latest carrier screening can allow families to be prepared and informed prior to pregnancy, said Aishwarya Arjunan, MS, MPH, a clinical product specialist for carrier screening at Myriad Women’s Health, part of a diagnostic testing company based in Salt Lake City, Utah.
“Rare diseases are responsible for 35% of deaths in the first year of life,” she said in a panel discussion at the Rare Diseases and Orphan Products Breakthrough Summit sponsored by the National Organization for Rare Disorders.
Most patients with rare diseases go through a “diagnostic odyssey” lasting an average of 8 years before they receive an accurate diagnosis, she said. During this time, data suggest that they have likely been misdiagnosed three times and have seen more than 10 specialists, she added.
Barriers to genetic screening include limited access to genetics professionals, lack of patient and provider education about screening, issues of insurance coverage and reimbursement, coding challenges, and misperceptions about the perceived impact of screening, noted Jodie Vento, manager of the Center for Rare Disease Therapy at the Children’s Hospital of Pittsburgh.
The genetic carrier screening options, often referred to as panethnic expanded carrier screening, represents a change from previous screening protocols based on ethnicity, said Ms. Arjunan. However, guidelines for screening based on ethnicity “misses a significant percentage of pregnancies affected by serious conditions and widens the health disparity gap,” she said.
By contrast, expanded carrier screening allows for standardization of care that gives couples and families information to make decisions and preparations.
Current genetic testing strategies include single gene testing, in which a single gene of interest is tested; multigene panel testing, in which a subset of clinically important genes are tested; whole-exome sequencing, in which the DNA responsible for coding proteins is tested; and whole-genome sequencing, in which the entire human genome is tested for genetic disorders.
Improving access to genetic testing involves a combination of provider education, changes in payer policies, action by advocacy groups, and adjustment of societal guidelines, said Ms. Arjunan. However, the advantages of expanded carrier screening are many and include guiding patients to expert care early and setting up plans for long-term care and follow-up, she noted. In addition, early identification through screening can help patients reduce or eliminate the diagnostic odyssey and connect with advocacy and community groups for support, she concluded.
The presenters had no financial conflicts to disclose.
EXPERT ANALYSIS FROM NORD 2019
New Registry Offers Insight Into Opsoclonus-Myoclonus Syndrome
WASHINGTON – a rare disease affecting only 1 in 5,000,000 individuals, mostly aged 1-5 years, based on data from a new patient registry.
In partnership with the National Organization of Rare Disorders (NORD) the nonprofit OMSLife Foundation has created a patient registry to better understand the disease experience in patients, wrote Mike Michaelis, chairman of OMSLife, and his colleagues. Early data from 275 enrolled patients were presented in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
The registry patients were mainly born in the United States (86%) and white (74%); approximately half were female. Of 150 patients who indicated symptoms at onset, 87% reported ataxia. Additional symptoms at onset were myoclonus (61%), opsoclonus (59%), tremors (46%), sleep disturbances (45%), temper tantrums (38%), vomiting (27%), fever (13%), headache (9%) and other symptoms (13%).
The researchers reviewed associations of symptoms at onset to determine the frequency of other symptoms for each individual symptom. Ataxia was present with 89% or higher instances of the other reported symptoms. Of note, some symptoms occurred more frequently than expected, such as temper tantrums and tremors in approximately 70% of patients with sleep disturbances. Myoclonus and opsoclonus, as well as fever and vomiting, also were significantly associated with the presence of other symptoms.
Two-thirds of the registry patients (69%) were diagnosed within 3 months of symptom onset, and 83% of these were diagnosed by a neurologist. Based on the Mitchell-Pike OMS severity scale, 59% of the patients met criteria for severe disease, 34% were classified as moderate, and 7% were mild. The registry is ongoing, but the current data provide insight on the clinical picture and common symptoms of OMS, the researchers said.
OMS Life was established in 2012 to support patients, caregivers, and researchers in raising awareness of opsoclonus-myoclonus syndrome as well as funds for research.
The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
WASHINGTON – a rare disease affecting only 1 in 5,000,000 individuals, mostly aged 1-5 years, based on data from a new patient registry.
In partnership with the National Organization of Rare Disorders (NORD) the nonprofit OMSLife Foundation has created a patient registry to better understand the disease experience in patients, wrote Mike Michaelis, chairman of OMSLife, and his colleagues. Early data from 275 enrolled patients were presented in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
The registry patients were mainly born in the United States (86%) and white (74%); approximately half were female. Of 150 patients who indicated symptoms at onset, 87% reported ataxia. Additional symptoms at onset were myoclonus (61%), opsoclonus (59%), tremors (46%), sleep disturbances (45%), temper tantrums (38%), vomiting (27%), fever (13%), headache (9%) and other symptoms (13%).
The researchers reviewed associations of symptoms at onset to determine the frequency of other symptoms for each individual symptom. Ataxia was present with 89% or higher instances of the other reported symptoms. Of note, some symptoms occurred more frequently than expected, such as temper tantrums and tremors in approximately 70% of patients with sleep disturbances. Myoclonus and opsoclonus, as well as fever and vomiting, also were significantly associated with the presence of other symptoms.
Two-thirds of the registry patients (69%) were diagnosed within 3 months of symptom onset, and 83% of these were diagnosed by a neurologist. Based on the Mitchell-Pike OMS severity scale, 59% of the patients met criteria for severe disease, 34% were classified as moderate, and 7% were mild. The registry is ongoing, but the current data provide insight on the clinical picture and common symptoms of OMS, the researchers said.
OMS Life was established in 2012 to support patients, caregivers, and researchers in raising awareness of opsoclonus-myoclonus syndrome as well as funds for research.
The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
WASHINGTON – a rare disease affecting only 1 in 5,000,000 individuals, mostly aged 1-5 years, based on data from a new patient registry.
In partnership with the National Organization of Rare Disorders (NORD) the nonprofit OMSLife Foundation has created a patient registry to better understand the disease experience in patients, wrote Mike Michaelis, chairman of OMSLife, and his colleagues. Early data from 275 enrolled patients were presented in a poster at the NORD Rare Summit, held by the National Organization for Rare Disorders.
The registry patients were mainly born in the United States (86%) and white (74%); approximately half were female. Of 150 patients who indicated symptoms at onset, 87% reported ataxia. Additional symptoms at onset were myoclonus (61%), opsoclonus (59%), tremors (46%), sleep disturbances (45%), temper tantrums (38%), vomiting (27%), fever (13%), headache (9%) and other symptoms (13%).
The researchers reviewed associations of symptoms at onset to determine the frequency of other symptoms for each individual symptom. Ataxia was present with 89% or higher instances of the other reported symptoms. Of note, some symptoms occurred more frequently than expected, such as temper tantrums and tremors in approximately 70% of patients with sleep disturbances. Myoclonus and opsoclonus, as well as fever and vomiting, also were significantly associated with the presence of other symptoms.
Two-thirds of the registry patients (69%) were diagnosed within 3 months of symptom onset, and 83% of these were diagnosed by a neurologist. Based on the Mitchell-Pike OMS severity scale, 59% of the patients met criteria for severe disease, 34% were classified as moderate, and 7% were mild. The registry is ongoing, but the current data provide insight on the clinical picture and common symptoms of OMS, the researchers said.
OMS Life was established in 2012 to support patients, caregivers, and researchers in raising awareness of opsoclonus-myoclonus syndrome as well as funds for research.
The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
REPORTING FROM NORD SUMMIT 2018
Key clinical point: Most patients with OMS experienced multiple symptoms at disease onset; ataxia was the most common.
Major finding: Approximately 87% of patients with OMS reported ataxia at disease onset and 59% experienced severe disease.
Study details: The data come from a registry including 275 OMS patients.
Disclosures: The study was supported by the OMSLife Foundation, NORD, and Trio Health Analytics. The researchers are employed by these organizations.
Aplastic Anemia and MDS International Foundation (AAMDSIF)
AAMDSIF will be hosting seven “Living with Aplastic Anemia, MDS, PNH” patient and family conferences in cities around the country this year. Each free event offers opportunities to learn from leading medical experts and to connect with other patients and caregivers. More.
AAMDSIF will be hosting seven “Living with Aplastic Anemia, MDS, PNH” patient and family conferences in cities around the country this year. Each free event offers opportunities to learn from leading medical experts and to connect with other patients and caregivers. More.
AAMDSIF will be hosting seven “Living with Aplastic Anemia, MDS, PNH” patient and family conferences in cities around the country this year. Each free event offers opportunities to learn from leading medical experts and to connect with other patients and caregivers. More.
Cure SMA
Cure SMA is conducting a survey of pediatricians to assess baseline awareness regarding spinal muscular atrophy. Support Cure SMA and take the survey! More.
Cure SMA is conducting a survey of pediatricians to assess baseline awareness regarding spinal muscular atrophy. Support Cure SMA and take the survey! More.
Cure SMA is conducting a survey of pediatricians to assess baseline awareness regarding spinal muscular atrophy. Support Cure SMA and take the survey! More.
CurePSP
The First International Symposium on PSP and CBD will take place in London Oct. 25-26, 2018, in conjunction with CurePSP and the PSP Association. The latest research updates will be shared through scientific and clinical presentations and poster sessions. View a draft agenda and register now. CurePSP sponsors and organizes family conferences around the US, providing opportunities to learn more about PSP, CBD, and MSA. Join CurePSP and the Mayo Clinic in Rochester, MD, on June 29-30, 2018. More.
The First International Symposium on PSP and CBD will take place in London Oct. 25-26, 2018, in conjunction with CurePSP and the PSP Association. The latest research updates will be shared through scientific and clinical presentations and poster sessions. View a draft agenda and register now. CurePSP sponsors and organizes family conferences around the US, providing opportunities to learn more about PSP, CBD, and MSA. Join CurePSP and the Mayo Clinic in Rochester, MD, on June 29-30, 2018. More.
The First International Symposium on PSP and CBD will take place in London Oct. 25-26, 2018, in conjunction with CurePSP and the PSP Association. The latest research updates will be shared through scientific and clinical presentations and poster sessions. View a draft agenda and register now. CurePSP sponsors and organizes family conferences around the US, providing opportunities to learn more about PSP, CBD, and MSA. Join CurePSP and the Mayo Clinic in Rochester, MD, on June 29-30, 2018. More.
Ehlers-Danlos Society
The Ehlers-Danlos Society will hold an International Symposium Sept. 26-29, 2018, in Ghent, Belgium. Clinicians and researchers are encouraged to attend to discuss the molecular, pathogenic, clinical, and management aspects of all types of Ehlers-Danlos syndromes. More.
The Ehlers-Danlos Society will hold an International Symposium Sept. 26-29, 2018, in Ghent, Belgium. Clinicians and researchers are encouraged to attend to discuss the molecular, pathogenic, clinical, and management aspects of all types of Ehlers-Danlos syndromes. More.
The Ehlers-Danlos Society will hold an International Symposium Sept. 26-29, 2018, in Ghent, Belgium. Clinicians and researchers are encouraged to attend to discuss the molecular, pathogenic, clinical, and management aspects of all types of Ehlers-Danlos syndromes. More.
Osteogenesis Imperfecta Foundation (OIF)
The OIF will host its biennial national conference in Baltimore July 13-15, 2018. The program will feature sessions on medical care and practical living, forums with leading experts in osteogenesis imperfect care and research, and other opportunities. More.
The OIF will host its biennial national conference in Baltimore July 13-15, 2018. The program will feature sessions on medical care and practical living, forums with leading experts in osteogenesis imperfect care and research, and other opportunities. More.
The OIF will host its biennial national conference in Baltimore July 13-15, 2018. The program will feature sessions on medical care and practical living, forums with leading experts in osteogenesis imperfect care and research, and other opportunities. More.
Morgan Leary Vaughan Foundation
Register now for the inaugural Speaking of NEC: Unplugged on June 11, 2018, in Cromwell Connecticut, a one-day regional conference focused on reducing the devastating effects of necrotizing enterocolitis (NEC) on premature infants and their families. More.
Register now for the inaugural Speaking of NEC: Unplugged on June 11, 2018, in Cromwell Connecticut, a one-day regional conference focused on reducing the devastating effects of necrotizing enterocolitis (NEC) on premature infants and their families. More.
Register now for the inaugural Speaking of NEC: Unplugged on June 11, 2018, in Cromwell Connecticut, a one-day regional conference focused on reducing the devastating effects of necrotizing enterocolitis (NEC) on premature infants and their families. More.
MDS Foundation
Join the MDS Foundation for a Patient and Family/Caregiver forum on May 5, 2018, in Salt Lake City. This one-day forum provides new patients, long-term survivors, and caregivers the opportunity to learn from experts about treatments and strategies for living with MDS. More.
Join the MDS Foundation for a Patient and Family/Caregiver forum on May 5, 2018, in Salt Lake City. This one-day forum provides new patients, long-term survivors, and caregivers the opportunity to learn from experts about treatments and strategies for living with MDS. More.
Join the MDS Foundation for a Patient and Family/Caregiver forum on May 5, 2018, in Salt Lake City. This one-day forum provides new patients, long-term survivors, and caregivers the opportunity to learn from experts about treatments and strategies for living with MDS. More.
International Pemphigus and Pemphigoid Foundation
In May 2018, the IPPF will bring together pemphigus and pemphigoid patients, clinicians, researchers, and industry partners to focus on ongoing or future clinical trials and their underlying science. This will take place prior to the International Investigative Dermatology meeting in Orlando. More.
In May 2018, the IPPF will bring together pemphigus and pemphigoid patients, clinicians, researchers, and industry partners to focus on ongoing or future clinical trials and their underlying science. This will take place prior to the International Investigative Dermatology meeting in Orlando. More.
In May 2018, the IPPF will bring together pemphigus and pemphigoid patients, clinicians, researchers, and industry partners to focus on ongoing or future clinical trials and their underlying science. This will take place prior to the International Investigative Dermatology meeting in Orlando. More.